Abstract
Circadian rhythms are a very exquisite mechanism to influence on transcriptional levels and physiological activities of various molecules that affect cell metabolic pathways. Long-term alteration of circadian rhythms increases the risk of cardiovascular diseases, hypertension, hypertriglyceridemia, and metabolic syndrome. A drastic change in dietary patterns can affect synchronizing the circadian clock within the metabolic system. Therefore, the interaction between the host and the bacterial community colonizing the mammalian gastrointestinal tract has a great impact on the circadian clock in diurnal programs. Here, we propose that the microbiota regulates body composition through the transcriptional oscillation of circadian regulators. The transcriptional regulator, NFIL3 (also called E4BP4) is a good example. Compositional change of the commensal bacteria influences the rhythmic expression of NFIL3 in the epithelium, which subsequently controls obesity and insulin resistance. Therefore, control of circadian regulators would be a promising therapeutic target for metabolic diseases.
Keywords: circadian rhythms, microbiota, metabolic diseases, cytokine, obesity
Introduction
Obesity is a major risk factor for several co-occurring diseases, including type II diabetes mellitus, non-alcoholic fatty liver disease, and ischemic cardiovascular disease, and the prevalence of these diseases has increased at an astounding rate in the past decades (1). About 44% of the global population is overweight, and more than 300 million individuals are affected by morbid obesity (2). This is thought to be the result of dramatic changes in the human lifestyle, ranging from a drastic change in dietary patterns, improved hygiene, and altered sleep cycles. Therefore, there is an urgent need to identify host and environmental factors that regulate human metabolism and energy homeostasis. In considering these two aspects, the intestinal flora is an environmental factor that greatly affects the body composition of mammals (3). The gut flora facilitates energy collection when energy derived from the diet is stored in adipose tissue (4). Recently, there is some evidence indicating a role for temporal and spatial dynamics in the community of microorganisms that inhabit the gastrointestinal tract. The circadian clock evolved in most species to adjust the physiology of the organism to daily environmental fluctuations (5–7). Epidemiological and experimental evidence has demonstrated that clock disturbances are linked to metabolic diseases, including obesity and hyperglycemia (8, 9). In understanding how regulation of microbial host metabolic pathways affects energy storage and body composition, we propose that the microbiota regulates body composition through the clock regulating transcription factor NFIL3 (also called E4BP4), which influences the circadian clock in intestinal epithelial cells through the regulation of group 3 innate lymphocyte cells (ILC3). This review describes how NFIL3 regulates body composition and establishes an essential network between the circadian clock and host metabolism.
Circadian Clock and Metabolic Disorders
Circadian rhythms are a very exquisite mechanism by which organisms can adapt their behaviors to the 24-h light-dark (LD) cycle change in the external environment evoked by the rotation of the earth around the sun (10). Transcriptional levels and physiological activities of various molecules that affect cell metabolic pathways and organ functions have their own periodicity, which is known to be very consistent with the LD cycle of the external environment. Synchronizing the circadian clock with the metabolic system is necessary to make dietary substrates available for metabolic pathways that are energetically expensive and plays an important role in optimizing energy use. The intrinsic circadian clock is entrained by LD cycles, and the mammalian master clock resides in the suprachiasmatic nucleus (SCN), a small area of the anterior hypothalamus. The clock plays a role to drive oscillators distributed in various peripheral tissues through behavioral and neuroendocrine signals (11). Peripheral tissues have functional clock oscillators that are self-sustained and can operate independently of the central pacemaker and SCN rhythms (12). For instance, the temporal pattern of food intake influences the quality and quantity of the circadian transcriptome in the mouse liver. Therefore, while the LD cycle resets the master clock in the SCN, the timing of food intake can be a potent synchronizer of peripheral clocks.
Disruption of the circadian clock due to a genetic defect has been shown to have a strong causal relationship with metabolic diseases (13, 14). Indeed, sleep restriction or a 28-h LD cycle reduces insulin sensitivity and glucose tolerance (15). This has been demonstrated in epidemiological studies indicating that long-term alteration of sleep patterns increases the risk of obesity and metabolic disorders (16). Furthermore, the prevalence of cardiovascular diseases, hypertension, hypertriglyceridemia, and metabolic syndrome is higher in shift workers compared to day-time workers, and restriction of sleep time has been shown to increase the risk of obesity and diabetes. In this context, the temporal and spatial dynamics of the microbial flora may have a profound effect on host metabolism by tightly associating with the circadian clock.
A layer of intestinal epithelial cells (IECs) provides the physical barrier that separates commensal bacteria living in the intestinal lumen from the body. Interactions between commensal bacteria and Toll-like receptors (TLRs) members of the pattern recognition receptors (PRRs) are known to be mandatory for IEC homeostasis maintained by host-commensal symbiosis. Several functions in IECs, including nutrient absorption, cell proliferation, motility, and metabolic activities are regulated in a circadian rhythm. The circadian variation in the host intestine is tightly associated with diurnal oscillations of the intestinal microbiota colonizing the mammalian gastrointestinal tract, thus the oscillations of the circadian clock in IELs are controlled by the timing of food intake and the composition of the diet, which affects the expression pattern of some TLR genes. Moreover, the functional feature generated by the microbiota oscillations feeds into the circadian clock network not only in the intestine but also in the system as a whole (17).
Recent evidence indicates that alterations in the composition of the microbiome change the susceptibility to obesity of the host (18). Obesity and diabetes are known to cause chronic hyperglycemia in IELs, leading to a breakdown of barrier function that facilitated the alteration of intestinal bacterial components (19). Therefore, a chronic increase of glucose levels in obesity contributes to a detrimental effect in the maintenance of a barrier function in the IELs as a consequence of the functional reprogramming of metabolism and transcription. Subsequently, the perturbation in the coordinated daily interplay between the microbiota oscillations and the circadian transcriptional program in IELs enhances the risk for the development of obesity and systemic inflammation.
The composition and function of the bacterial community colonizing the mammalian gastrointestinal tract also undergo oscillations, which are affected by the dietary condition (14, 20, 21). The interaction between the host and gut microbiome can affect the circadian clock in different tissues (22, 23). Systemic metabolite rhythms and programming of transcriptional oscillations impact the homeostatic diurnal variation in the liver (22). These observations indicate that the microbiome is a major source of clock-modifying metabolites.
Molecular Architecture of the Circadian Clock in the Gut
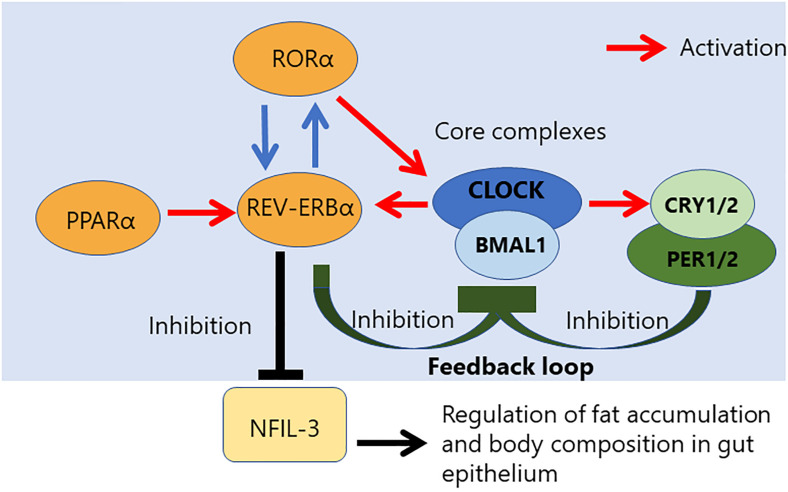
Circadian rhythm is controlled by a core loop composed of the heterodimeric complex of two transcriptional activators, the circadian locomotor output cycles kaput (CLOCK), and brain and muscle ARNT-like 1 (BMAL1) (Figure 1) (24). The CLOCK and BMAL1 form the heterodimer via their HLH-PAS domains, and the heterodimer of transcriptional activators subsequently results in translocation to the nucleus, where it binds to an E-box sequence (CACGTG) in the promoter regions of repressors of CLOCK/BMAL1-mediated transcription, such as Period (PER 1 to 3) and Cryptochrome (CRY 1 and 2) genes. CRY inhibits the histone acetyltransferase p300, leading to a decrease of CLOCK/BMAL1-mediated transcription (25). The stability of the PER and CRY proteins is regulated by specific E3 ubiquitin ligase complexes, and the CLOCK/BMAL1 vs. CRY/PER mutually regulates each other via the central autoregulatory feedback loop (Figure 1). Therefore, this feedback loop is important to determine the periodicity of the circadian oscillation (26, 27).
FIGURE 1.
Relationship between NFIL-3 and the core complex controlling the circadian clock. Circadian rhythm is regulated by a transcriptional feedback loop composed of three core complexes. CLOCK/NPAS2/BMAL1 complex. NFIL-3 is a part of accessory loop, which regulates fat accumulation and body composition in gut epithelium.
Additional transcriptional feedback loops that are composed of members of the retinoic acid-related orphan nuclear receptor family, REV-ERBα/β repressor and RORα activator, ensure the stability and fidelity of the molecular clock (28). The antiphasic expression of the RORα and RevErbα controls a rhythmic expression of TLR in IELs (22). REV-ERB and its antagonist receptor RORα also competitively bind to the promoter region of the Bmal1 gene to control rhythmic chromatin dynamics (29–32). The transcription regulator of lipid metabolism, peroxisome proliferator-activated receptor a (PPARα) is a critical activator of RevErbα expression (22). Activation of PPARα is known to promote many aspects of fatty acid metabolism. On the other hand, BMAL1 controls the rhythmic expression of short-chain fatty acids (SCFA) receptor, Ffar2/3, in the colonic muscle layer. Moreover, some evidence is indicating that diurnal microbial SCFA levels also influence on intestinal motility (33, 34).
The circadian rhythm associated genes directly contribute to the pathogenesis of intestinal diseases. Sleep disruption and chronic fatigue are the major complaints of patients with inflammatory bowel disease (IBD), and these symptoms affect the inflammatory process of the disease (35–37). The loss of BMAL1 disrupts both the circadian clock and the timing of regeneration in the mouse intestine controlled by TNF (38). Deficiency of Per1/2 results in not only decreased proliferation of intestinal stem cells (39) but also increased cell death of intestinal epithelial cells in the lower hemicrypts (40). In humans, a polymorphism in Per3 has been associated with increased susceptibility to and disease severity of IBD (41). Moreover, deficiency of Per1/2 controlled Wee1 plays a role in mitotic cell cycle arrest, resulting in increased susceptibility of the gut epithelium to inflammatory processes (42).
In the intestinal immune response, it has been reported that an essential role of gut-resident macrophages, particularly residents in lamina propria, contributes to host defense (43). The transcriptional profile in self-maintaining macrophage has a great impact on their localization in peripheral tissue. Therefore, impairment in diurnal rhythmicity programs of transcription may result in a reduction of intestinal functions.
Role of the Clock Regulator NFIL3 and the Circadian Clock in the Immune System
NFIL3 was originally identified as a transcriptional repressor that binds to the E-box that controls the circadian clock (44); it is located in an auxiliary loop that exists outside the core loop (45). NFIL3 is a basic leucine zipper transcriptional factor that is mainly expressed in DC, T cells, and various other immune cells. This factor is required for the differentiation of the conventional dendritic cell 1 (cDC1) subset involved in cross-presentation (46, 47). Recently, NFIL3 expression was found to be required for the transition stage of cDC1 progenitors through the Nfil3–Zeb2–Id2 pathway that controls the Irf8 enhancer switch (48). NFIL3 is expressed in common lymphoid progenitors (CLPs) and regulates the expression of Id2 and Eomes genes that are important for NK cell development (49, 50). Indeed, NFIL3 deficiency causes an intrinsic defect in NK cell development. NFIL3 also controls the differentiation of several other innate lymphoid cells (ILCs), including group 2 ILC (ILC2) and ILC3 cells, through the repression of Id2 in CLPs (51, 52). NFIL3 is a common regulator directing the development of CLPs that differentiate into all ILC lineages (53).
Regulation by circadian clocks has been described in the innate immune system because the CLOCK/BMAL1 complex regulates the expression of Toll-like receptor 9 (TLR9) and represses the expression of REV-ERBα, suppressing the induction of interleukin-6 (54). In contrast, the role of the circadian clock in the adaptive immune system seems to be controversial. Many previous studies indicated that the cell-intrinsic circadian clock is involved in different aspects of adaptive immune function. But, mice with a T-cell-specific deletion of Bmal1 had normal differentiation of TH17 cells (55), suggesting no intrinsic role of circadian clocks in the T cell response of the intestinal tract. On the other hand, NFIL3 was suggested to act as a repressor of a key driver of nuclear receptor RORgt, which is an essential intrinsic transcriptional factor for TH17 cell differentiation. Therefore, the diurnal expression of NFIL3 is regulated by the circadian network through direct repression of REV-ERBa, which binds to the consensus sequence of the Nfil3 locus and represses NFIL3 expression (Figure 1). Therefore, NFIL3 expression in T cells plays a role in controlling the intestinal immune response regulated by TH17 cells (45). For TH1 cell immune function, NFIL3 has a different role to induce IL-10 and IL-13 expression (56) and, in this case, the expression of NFIL3 is induced by excessive IFN-γ stimulation to induce IL-10 and IL-13.
Transcriptional Oscillation of NFIL3 in Intestinal Epithelial Cells
The defense at barrier surfaces by the gut epithelium plays a role in the containment of commensal bacteria. The barrier function of the gut epithelium is regulated by CD4+ T cell responses against commensal bacteria (57) and by antibacterial proteins derived from gut epithelial cells (58). NFIL3 is expressed by the small intestine epithelium under an LD cycle change, and this circadian expression is significantly altered in antibiotic-treated or germ-free mice (33). The expression of NFIL3 in the epithelium influences the response to commensal bacteria. Interestingly, intestinal epithelial cell-specific deficiency of NFIL3 has a great impact on the regulation of lipid storage and epididymal fat weight. Therefore, the expression of NFIL3 in the gut epithelium plays an important role in the regulation of lipid storage and body composition.
NFIL3 expression is known to be regulated by the core circadian clock transcriptional repressor REV-ERBα which binds to the Nfil3 locus, resulting in circadian expression of NFIL3 (45, 59). Intestinal epithelial cells sense commensal bacteria via TLRs and the signaling adapter molecule, Myeloid differentiation primary response 88 (MyD88), and these innate signaling pathways promote NFIL3 expression via suppression of REV-ERBα expression in the epithelium. Interestingly, the expression pattern of REV-ERBα and NFIL3 in Zeitgeber time 4 (ZT4) are mutually exclusive in epithelial cells.
The DC-ILC3 network can be activated by flagellin or lipopolysaccharide (LPS) present in the outer membrane of Gram-negative bacteria (60, 61). The penetration of gram-negative bacteria into the intestinal epithelial barrier allows them to contact lamina propria DC and activates CD11c+ DC to produce interleukin 23 (IL-23). This process subsequently leads to further activation of group 3 innate lymphoid cells (ILC3s). ILC3s are reported to play an important role in defense at intestinal barrier surfaces via IL-17 and IL-22 production (62). The DC-derived IL-23 promotes IL-22 production by ILC3s, subsequently leading to the up-regulation of NFIL3 in the intestinal epithelium (63). In this case, NFIL3 plays a role to regulate lipid absorption and export in intestinal epithelial cells via promoting the expression of several molecules controlling lipid metabolism, including a member of the class B scavenger receptor family, CD36, which is a transporter of dietary fatty acids, stearoyl-coenzyme A-desaturase 1 (SCD1), a fatty acid hydroxylase, CYP2E1, and a fatty acid-binding protein 4 (FABP4) (64–67). This finding is consistent with the observation in a loss of function mutant of NFIL3 that lipid metabolism is partially altered (68).
Therefore, network regulation of the microbiota and the circadian clock in the intestinal tract is a critical process to control obesity and insulin resistance (18). Transcriptional oscillation of circadian regulators like NFIL3, which are controlled by the diurnal rhythmicity of the microbiota, is an important program for host metabolism.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
- BMAL1
brain and muscle ARNT-like 1
- cDC1
conventional dendritic cell 1
- CLOCK
circadian locomotor output cycles kaput
- CLPs
common lymphoid progenitors
- CRY
Cryptochrome
- IBD
inflammatory bowel disease
- ILCs
innate lymphoid cells
- LD cycle
light-dark cycle
- LPS
lipopolysaccharide
- MYD88
Myeloid differentiation primary response 88
- PER
period
- ROR
retinoic acid receptor-related orphan receptor
- SCN
suprachiasmatic nucleus
- TLR9
toll-like receptor 9
- ZT4
Zeitgeber time 4.
References
- 1.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. (2006) 444:881–7. 10.1038/nature05488 [DOI] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the global burden of disease study 2013. Lancet. (2014) 384:766–81. 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. (2004) 101:15718–23. 10.1073/pnas.0407076101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. (2006) 444:1027–31. 10.1038/nature05414 [DOI] [PubMed] [Google Scholar]
- 5.Hurd MW, Ralph MR. The significance of circadian organization for longevity in the golden hamster. J Biol Rhythms. (1998) 13:430–6. 10.1177/074873098129000255 [DOI] [PubMed] [Google Scholar]
- 6.Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci USA. (1998) 95:8660–4. 10.1073/pnas.95.15.8660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pittendrigh CS, Minis DH. Circadian systems: longevity as a function of circadian resonance in Drosophila melanogaster. Proc Natl Acad Sci USA. (1972) 69:1537–9. 10.1073/pnas.69.6.1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. (2012) 4:129ra43. 10.1126/scitranslmed.3003200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. (2009) 106:4453–8. 10.1073/pnas.0808180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. (2002) 418:935–41. 10.1038/nature00965 [DOI] [PubMed] [Google Scholar]
- 11.Brown SA, Azzi A. Peripheral circadian oscillators in mammals. Handb Exp Pharmacol. (2013) 217:45–66. 10.1007/978-3-642-25950-0_3 [DOI] [PubMed] [Google Scholar]
- 12.Mendoza-Cózatl D, Loza-Tavera H, Hernández-Navarro A, Moreno-Sánchez R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol Rev. (2005) 29:653–71. 10.1016/j.femsre.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 13.Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. (2014) 159:514–29. 10.1016/j.cell.2014.09.048 [DOI] [PubMed] [Google Scholar]
- 14.Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe. (2015) 17:681–9. 10.1016/j.chom.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. (1997) 18:716–38. 10.1210/edrv.18.5.0317 [DOI] [PubMed] [Google Scholar]
- 16.Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. (2011) 14:402–12. 10.1097/MCO.0b013e3283479109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thaiss CA, Levy M, Korem T, Dohnalová L, Shapiro H, Jaitin DA, et al. Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell. (2016) 167:1495–510. 10.1016/j.cell.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 18.Thaiss CA, Itav S, Rothschild D, Meijer MT, Levy M, Moresi C, et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. (2016) 540:544–51. 10.1038/nature20796 [DOI] [PubMed] [Google Scholar]
- 19.Thaiss CA. Microbiome dynamics in obesity. Science. (2018) 362:903–4. 10.1126/science.aav6870 [DOI] [PubMed] [Google Scholar]
- 20.Liang X, Bushman FD, FitzGerald GA. Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proc Natl Acad Sci USA. (2015) 112:10479–84. 10.1073/pnas.1501305112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab. (2014) 20:1006–17. 10.1016/j.cmet.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. (2013) 153:812–27. 10.1016/j.cell.2013.04.020 [DOI] [PubMed] [Google Scholar]
- 23.Murakami M, Tognini P, Liu Y, Eckel-Mahan KL, Baldi P, Sassone-Corsi P. Gut microbiota directs PPARγ-driven reprogramming of the liver circadian clock by nutritional challenge. EMBO Rep. (2016) 17:1292–303. 10.15252/embr.201642463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holtz Vitaterna M, Turek FW. Chapter 12. Circadian clock genes. Principles and Practice of Sleep Medicine (Fifth Edition). ed. Kruger M., Roth T., Dement W. Amsterdam: Elsevier; (2011). p. 141–50. [Google Scholar]
- 25.Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. (2003) 421:177–82. 10.1038/nature01314 [DOI] [PubMed] [Google Scholar]
- 26.Duguay D, Cermakian N. The crosstalk between physiology and circadian clock proteins. Chronobiol Int. (2009) 26:1479–513. 10.3109/07420520903497575 [DOI] [PubMed] [Google Scholar]
- 27.Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. (2011) 13:125–37. 10.1016/j.cmet.2011.01.006 [DOI] [PubMed] [Google Scholar]
- 28.Guillaumond F, Dardente H, Giguère V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. (2005) 20:391–403. 10.1177/0748730405277232 [DOI] [PubMed] [Google Scholar]
- 29.Aguilar-Arnal L, Hakim O, Patel VR, Baldi P, Hager GL, Sassone-Corsi P. Cycles in spatial and temporal chromosomal organization driven by the circadian clock. Nat Struct Mol Biol. (2013) 20:1206–13. 10.1038/nsmb.2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. (2012) 338:349–54. 10.1126/science.1226339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perelis M, Ramsey KM, Marcheva B, Bass J. Circadian transcription from beta cell function to diabetes pathophysiology. J Biol Rhythms. (2016) 31:323–36. 10.1177/0748730416656949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vollmers C, Schmitz RJ, Nathanson J, Yeo G, Ecker JR, Panda S. Circadian oscillations of protein-coding and regulatory RNAs in a highly dynamic mammalian liver epigenome. Cell Metab. (2012) 16:833–45. 10.1016/j.cmet.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segers A, Desmet L, Thijs T, Verbeke K, Tack J, Depoortere I. The circadian clock regulates the diurnal levels of microbial short-chain fatty acids and their rhythmic effects on colon contractility in mice. Acta Physiol (Oxf). (2019) 225:e13193. 10.1111/apha.13193 [DOI] [PubMed] [Google Scholar]
- 34.Segers A, Desmet L, Sun S, Verbeke K, Tack J, Depoortere I. Night-time feeding of Bmal1-/- mice restores SCFA rhythms and their effect on ghrelin. J Endocrinol. (2020) 245:155–64. 10.1530/joe-20-0011 [DOI] [PubMed] [Google Scholar]
- 35.Ranjbaran Z, Keefer L, Farhadi A, Stepanski E, Sedghi S, Keshavarzian A. Impact of sleep disturbances in inflammatory bowel disease. J Gastroenterol Hepatol. (2007) 22:1748–53. 10.1111/j.1440-1746.2006.04820.x [DOI] [PubMed] [Google Scholar]
- 36.Ananthakrishnan AN, Long MD, Martin CF, Sandler RS, Kappelman MD. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol. (2013) 11:965–71. 10.1016/j.cgh.2013.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali T, Madhoun MF, Orr WC, Rubin DT. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis. (2013) 19:2440–3. 10.1097/MIB.0b013e3182a0ea54 [DOI] [PubMed] [Google Scholar]
- 38.Stokes K, Cooke A, Chang H, Weaver DR, Breault DT, Karpowicz P. The circadian clock gene BMAL1 coordinates intestinal regeneration. Cell Mol Gastroenterol Hepatol. (2017) 4:95–114. 10.1016/j.jcmgh.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karpowicz P, Zhang Y, Hogenesch JB, Emery P, Perrimon N. The circadian clock gates the intestinal stem cell regenerative state. Cell Rep. (2013) 3:996–1004. 10.1016/j.celrep.2013.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gavrila AM, Robinson B, Hoy J, Stewart J, Bhargava A, Amir S. Double-stranded RNA-mediated suppression of Period2 expression in the suprachiasmatic nucleus disrupts circadian locomotor activity in rats. Neuroscience. (2008) 154:409–14. 10.1016/j.neuroscience.2008.04.032 [DOI] [PubMed] [Google Scholar]
- 41.Mazzoccoli G, Palmieri O, Corritore G, Latiano T, Bossa F, Scimeca D, et al. Association study of a polymorphism in clock gene PERIOD3 and risk of inflammatory bowel disease. Chronobiol Int. (2012) 29:994–1003. 10.3109/07420528.2012.705935 [DOI] [PubMed] [Google Scholar]
- 42.Pagel R, Bär F, Schröder T, Sünderhauf A, Künstner A, Ibrahim SM, et al. Circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation in the intestine. FASEB J. (2017) 31:4707–19. 10.1096/fj.201700141RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schepper SD, Verheijden S, Lizarraga JA, Viola MF, Boesmans W, Stakenborg N, et al. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell. (2018) 175:400–15.e13. 10.1016/j.cell.2018.07.048 [DOI] [PubMed] [Google Scholar]
- 44.Mitsui S, Yamaguchi S, Matsuo T, Ishida Y, Okamura H. Antagonistic role of E4BP4 and PAR proteins in the circadian oscillatory mechanism. Genes Dev. (2001) 15:995–1006. 10.1101/gad.873501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, et al. TH17 cell differentiation is regulated by the circadian clock. Science. (2013) 342:727–30. 10.1126/science.1243884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kashiwada M, Pham NL, Pewe LL, Harty JT, Rothman PB. NFIL3/E4BP4 is a key transcription factor for CD8ɑ+ dendritic cell development. Blood. (2011) 117:6193–7. 10.1182/blood-2010-07-295873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seillet C, Jackson JT, Markey KA, Brady HJ, Hill GR, Macdonald KP, et al. CD8α+ DCs can be induced in the absence of transcription factors Id2, Nfil3, and Batf3. Blood. (2013) 121:1574–83. 10.1182/blood-2012-07-445650 [DOI] [PubMed] [Google Scholar]
- 48.Bagadia P, Huang X, Liu TT, Durai V, Grajales-Reyes GE, Nitschké M, et al. An Nfil3-Zeb2-Id2 pathway imposes Irf8 enhancer switching during cDC1 development. Nat Immunol. (2019) 20:1174–85. 10.1038/s41590-019-0449-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Male V, Nisoli I, Kostrzewski T, Allan DS, Carlyle JR, Lord GM, et al. The transcription factor E4bp4/Nfil3 controls commitment to the NK lineage and directly regulates Eomes and Id2 expression. J Exp Med. (2014) 211:635–42. 10.1084/jem.20132398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geiger TL, Abt MC, Gasteiger G, Firth MA, O’Connor MH, Geary CD, et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med. (2014) 211:1723–31. 10.1084/jem.20140212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seillet C, Rankin LC, Groom JR, Mielke LA, Tellier J, Chopin M, et al. Nfil3 is required for the development of all innate lymphoid cell subsets. J Exp Med. (2014) 211:1733–40. 10.1084/jem.20140145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu W, Domingues RG, Fonseca-Pereira D, Ferreira M, Ribeiro H, Lopez-Lastra S, et al. NFIL3 orchestrates the emergence of common helper innate lymphoid cell precursors. Cell Rep. (2015) 10:2043–54. 10.1016/j.celrep.2015.02.057 [DOI] [PubMed] [Google Scholar]
- 53.Yu X, Wang Y, Deng M, Li Y, Ruhn KA, Zhang CC, et al. The basic leucine zipper transcription factor NFIL3 directs the development of a common innate lymphoid cell precursor. eLife. (2014) 3:e04406. 10.7554/eLife.04406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LA. Circadian clock proteins and immunity. Immunity. (2014) 40:178–86. 10.1016/j.immuni.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 55.Hemmers S, Rudensky AY. The cell-intrinsic circadian clock is dispensable for lymphocyte differentiation and function. Cell Rep. (2015) 11:1339–49. 10.1016/j.celrep.2015.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Motomura Y, Kitamura H, Hijikata A, Matsunaga Y, Matsumoto K, Inoue H, et al. The transcription factor E4BP4 regulates the production of IL-10 and IL-13 in CD4+ T cells. Nat Immunol. (2011) 12:450–9. 10.1038/ni.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hepworth MR, Monticelli LA, Fung TC, Ziegler CG, Grunberg S, Sinha R, et al. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. (2013) 498:113–7. 10.1038/nature12240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. (2012) 12:503–16. 10.1038/nri3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duez H, van der Veen JN, Duhem C, Pourcet B, Touvier T, Fontaine C, et al. Regulation of bile acid synthesis by the nuclear receptor Rev-erbalpha. Gastroenterology. (2008) 135:689–98. 10.1053/j.gastro.2008.05.035 [DOI] [PubMed] [Google Scholar]
- 60.Pickard JM, Maurice CF, Kinnebrew MA, Abt MC, Schenten D, Golovkina TV, et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature. (2014) 514:638–41. 10.1038/nature13823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. (2015) 528:560–4. 10.1038/nature16460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. (2012) 336:1321–5. 10.1126/science.1222551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Kuang Z, Yu X, Ruhn KA, Kubo M, Hooper LV. The intestinal microbiota regulates body composition through NFIL3 and the circadian clock. Science. (2017) 357:912–6. 10.1126/science.aan0677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coburn CT, Knapp FF, Jr., Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. (2000) 275:32523–9. 10.1074/jbc.M003826200 [DOI] [PubMed] [Google Scholar]
- 65.Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, et al. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. (2002) 297:240–3. 10.1126/science.1071527 [DOI] [PubMed] [Google Scholar]
- 66.Zong H, Armoni M, Harel C, Karnieli E, Pessin JE. Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and insulin resistance. Am J Physiol Endocrinol Metab. (2012) 302:E532–9. 10.1152/ajpendo.00258.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Thaiss CA. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. (1996) 274:1377–9. 10.1126/science.274.5291.1377 [DOI] [PubMed] [Google Scholar]
- 68.Cai L, Wang Z, Ji A, Meyer JM, van der Westhuyzen DR. Scavenger receptor CD36 expression contributes to adipose tissue inflammation and cell death in diet-induced obesity. PLoS One. (2012) 7:e36785. 10.1371/journal.pone.0036785 [DOI] [PMC free article] [PubMed] [Google Scholar]