Abstract
1-Ally-3-methylimidazolium chloride ([Amim]Cl), dimethyl sulfoxide (DMSO), and CaCl2 were selected to construct dissolution systems to produce value-added products from pretreatment of waste corrugated cardboards (P-WCCs). The dissolution behaviors of P-WCCs before and after ball milling were studied in different dissolution systems. The regenerated cellulose films were quickly and efficiently prepared via dissolving, regenerating, and pressurized drying. When 4 wt % waste corrugated cardboard was dissolved in [Amim]Cl for 4 h at 90 °C, the regenerated cellulose films featured tensile strengths as high as 59.00 MPa. Adding 40% DMSO and 2 wt % CaCl2 increased the tensile strength of the film to a maximum value of 85.86 MPa. This demonstrates that DMSO improves the ability of WCC to dissolve in ionic liquids; Ca2+ improves the tensile strength and thermal stability of the regenerated cellulose film but reduces its transparency. This work provides a new, simple, and highly efficient way to use WCCs for packaging and wrapping.
1. Introduction
In 2018, China’s express industry generated about 50.7 billion packages,1 80% of which used corrugated boxes as packaging materials but the recovery rate of these packaging materials was less than one-tenth, This resulted in a large number of paper resources being wasted and the main component of the corrugated board being cellulose. Cellulose is a type of polymer with high strength, degradability, and renewability, making it a promising material in biomass refining. However, there are hydrogen bond network supramolecular structures in the macromolecular structure of cellulose, which makes cellulose insoluble in water and most common organic solvents, severely limiting its applications.2 A specific range of the DP of cellulose can be dissolved in a NaOH aqueous solution, as the NMMO solvent system has high energy consumption, poor thermal stability, and may be accompanied by side reactions because of oxidation.3,4 At present, the LiCl/DMAc solvent system is mainly limited to the laboratory because of LiCl is expensive, the cellulose solution is not stable, and cellulose molecular chain easily gather.5 Ionic liquids have proven to be excellent green solvents for cellulose and have great potential in the dissolution and processing of lignocellulose.6 1-Butyl-3-methylimidazolium chloride ([Bmim]Cl), 1-ethyl-3-methylimidazolium acetate ([Emim]OAc), and 1-allyl-3-methylimidazolium chloride ([Amim]Cl) have been used for homogeneous esterification of cellulose as well as for the formation of films and fibers.7 Ionic liquids can destroy the hydrogen bonding between spiral cellulose molecular chains, due to the high concentration of anionic intrusions in the cellulose intramolecular and intermolecular hydrogen bonds, forming new hydrogen bonds to divide cellulose.8 The main factor affecting the solubility in ionic liquids is the structure of ionic liquids. It has been found that the stronger the hydrogen bond acceptance capacity of anions in the ionic liquid structure, or the short alkyl chain, smaller central group, or strong electron-withdrawing groups such as carbon–carbon double bond and hydroxyl group in the cationic group structure, the stronger the solubility.9 It was reported that [Bmim]Cl can dissolve 10% cellulose,10 [Amim]Cl can dissolve 14.5% cellulose,11 and [Emim]OAc can dissolve 20% of untreated cellulose.12 But both [Amim]Cl and [Emim]OAc have a lower melting point and viscosity and greater solubility with regard to cellulose.13,14 [Emim]OAc has a complex synthesis procedure than those of [Amim]Cl and [Bmim]Cl; [Bmim]Cl and [Emim]OAc are so hygroscopic that the viscosity determination measurements with the air contact are not reliable.15 The solubility of imidazolium ionic liquids is because of the strong hydrogen bonding that arises from their alkalinity and low solvent viscosity. Cl–, [OAC]−, and other strong basic anions are strong electron donors, as they can easily form a strong hydrogen bond with H in a natural polysaccharide molecule −OH and cooperate with the hydrogen bond between the imidazole cation and O in the natural polysaccharide molecule −OH to form a electron donor–receptor complex, so as to dissolve the natural polysaccharide.16−18 Recently, it has been found that cationic structures also play an important role in this behavior during cellulose dissolution.19
The regenerated cellulose films can be prepared by dissolving, settling, and precipitating lignocellulose (cellulose, hemicellulose, and lignin) with ionic liquids.20 Moreover, it is easy to prepare these films21 and their chemical properties are fairly robust.22 The dissolution of cellulose in ionic liquids involves not only interactions between the solute and the solvent23 but also mass transfer in the ionic liquid.7,15 Ionic liquids are relatively viscous,24 so it is often necessary to add organic cosolvents such as dimethyl sulfoxide (DMSO) to improve the dissolution rate and reduce the viscosity of the solution system and save time.25−27 Xu28 studied how Ca2+ promotes interactions between cellulose chains, which can improve the tensile strength of the regenerated cellulose films. The regenerated cellulose films have been widely used in various fields due to their high performance, such as in wastewater prevention,29 groundwater pollution treatment,30 medical equipment,31 industrial pervaporation,32 and bioreactors.33 Cellulose can also adsorb heavy metals,34 adsorb organic pollutants,35 perform antibacterial functions,36 and act as drug carriers,37 after grafting functional groups onto cellulose or performing other physical modifications. In recent years, the regenerated cellulose films have also received extensive attention in green packaging,38,39 coating,40 screen display devices,41 optoelectronic materials,42 and electronic flexible screens.43 Sixta44 presented a novel regenerated cellulose fiber process of the Lyocell type using [DBNH][OAc] as a solvent, and the resultant cellulose solution has a high dissolution power and low viscosity. Its excellent properties make the Ioncell-F fibers suitable as a reinforcing material in composite structures and other technical applications.
In this work, we seek to achieve high-value utilization of waste corrugated cardboard using a [Amim]Cl/DMSO/CaCl2 dissolution system. DMSO can improve the dissolution of cellulose in [Amim]Cl and CaCl2 can improve the mechanical property of the films. In addition, the recovery of [Amim]Cl is pretty high and the recovered [Amim]Cl still has a good ability to dissolve fiber, and the performance of the cellulose film (R-WCC film) regenerated from the recovered [Amim]Cl is also very good.
2. Results and Discussion
2.1. Composition of Fibers
Waste corrugated cardboard is mainly composed of cellulose, hemicellulose, and lignin, accounting for 52.02, 6.79, and 10.43% of the total composition, respectively.45 From Table 1, it can be inferred that the cellulose ratio of P-WCC is increased to 82.19%, the amounts of ash and impurities are greatly reduced, the ratios of lignin and hemicellulose are reduced by the NaOH solution treatment, and the pulp can be bleached by H2O2. Finally, after the KOH solution treatment, the WCC became fluffy, which facilitated the dissolution.20
Table 1. Composition of WCC, P-WCC, and P-WCC Film.
| cellulose (%) | hemicellulose (%) | lignin (%) | ash (%) | other additives (%) | |
|---|---|---|---|---|---|
| WCC | 52.02 | 6.79 | 10.43 | 15.71 | 15.05 |
| P-WCC | 82.19 | 7.11 | 7.65 | 0.92 | 2.13 |
| P-WCC film | 81.62 | 6.22 | 7.03 | 1.24 | 3.89 |
Moreover, the DP of cellulose in WCC and P-WCC is 1635 and 600, respectively, and the DP of cellulose in the P-WCC film decreased to 485, according to GB/T 1548-2016. This may be due to the protonation of Cl– with water molecules to form HCl, resulting in the acid drop of polysaccharide molecules in the dissociation reaction.46
2.2. Viscosity Analysis
The addition of a cosolvent can have a strong influence on the viscosity,47 polarity,48 solvation,49 and other physical properties of an ionic liquid. Measuring the viscosity of cellulose in an ionic liquid system can help to clarify the dissolution mechanism of such a mixture.50
As shown in Figure 1, DMSO can greatly improve the rheological properties of the solvent, and adding just 10% DMSO at 70 °C can reduce the solution viscosity by 58.94%. Therefore, adding DMSO at a lower temperature has a greater overall impact on the viscosity of [Amim]Cl. The viscosity of [Amim]Cl decreases with the increase of DMSO content over 40%, but the change is negligible. With the addition of the cosolvent DMSO, the viscosity of the dissolving system decreases, the internal friction of the ionic liquid decreases, and the hydrocarbon binding force decreases. This makes the cellulose molecular chains disperse better in [Amim]Cl, thereby improving the solubility of cellulose.
Figure 1.
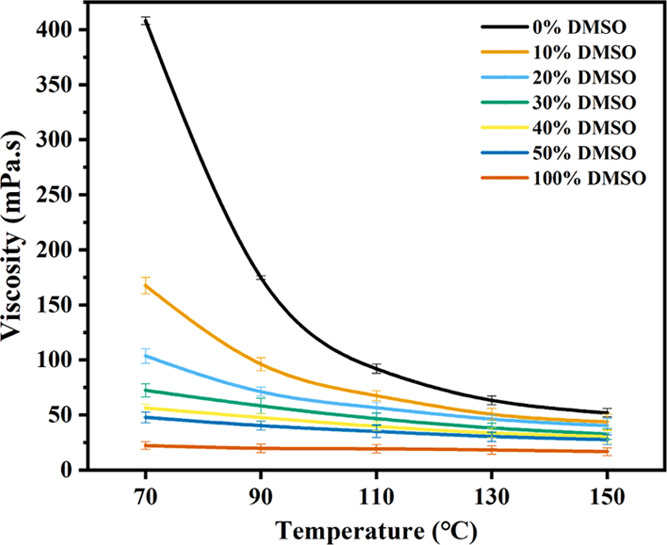
Effect of addition of DMSO on the viscosity of [Amim]Cl at different temperatures.
2.3. Solubility Properties
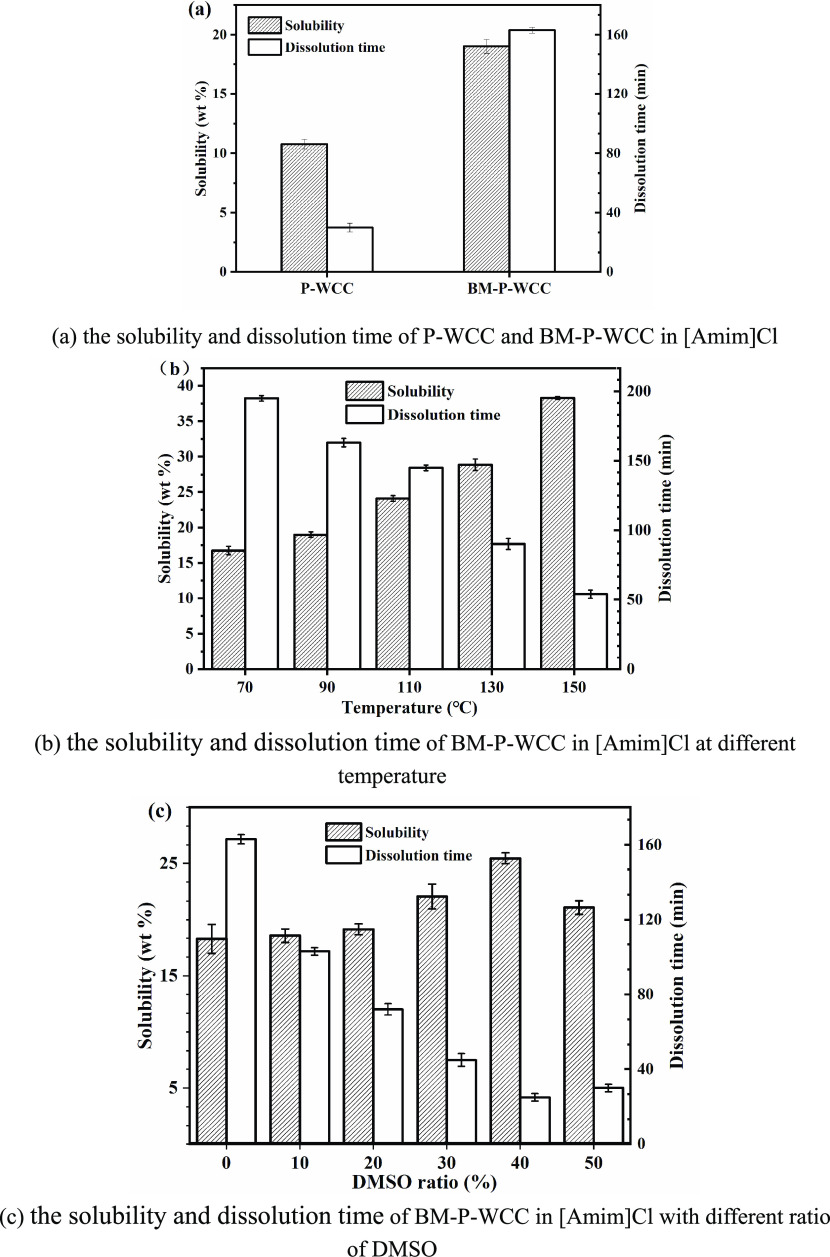
The polarizing micrographs of P-WCC in [Amim]Cl at 90 °C is shown in Figure S1. Figure 2a shows the solubility and dissolution time of P-WCC and BM-P-WCC in [Amim]Cl at 90 °C. The solubility of the fiber after ball milling increases from 10.79 to 19.01 wt %. This increase in solubility is due to the breaking of hydrogen bonds between cellulose molecules under mechanical force, which destroys the crystalline regions. Ball milling treatment reduces the fiber size and increases the specific surface area, thus improving the solubility as observed by Bodvik.51 Cellulose absorbs the mechanical energy during the ball milling process as shown in the X-ray diffraction (XRD) data in Figure S2; the crystallinity of the P-WCC fiber was 72.19%, while that of the BM-P-WCC fiber was reduced to 55.42%, such that the crystalline structure of cellulose changes from a metastable cellulose I type structure to a stable cellulose II type structure. Further, the cross-linking between cellulose molecules in the II type structure is closer, decreasing the dissolution time.
Figure 2.
Solubility and dissolution time of P-WCC and BM-P-WCC in ionic liquids.
Figure 2b shows that as the temperature increases, the solubility and dissolution time increase continuously. In this experiment, 4 wt % BM-P-WCC was added to [Amim]Cl to examine the effect of the dissolution temperature on its solubility and dissolution time. The solubility is 16.73 wt %; when the temperature increases to 150 °C, the solubility increases to 38.31 wt % and the dissolution time decreases from 195 to 54 min.
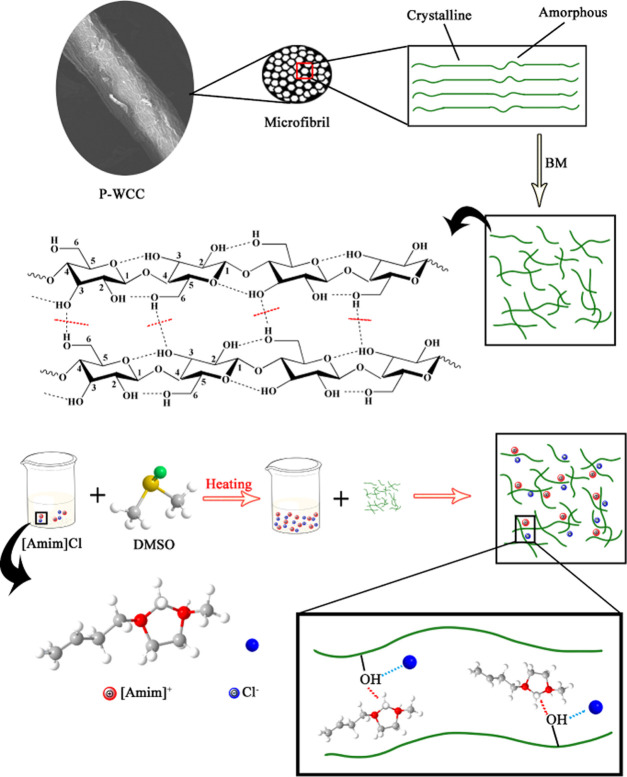
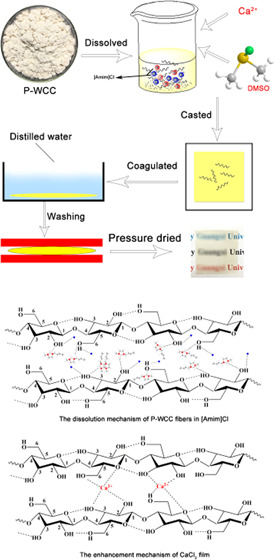
Adding DMSO can reduce the viscosity of the cellulose solution and effectively improve the solubility of cellulose in an ionic liquid.25 BM-P-WCC (4 wt %) was added to 5 g of [Amim]Cl and dissolved at 90 °C for 4 h. Figure 2c shows that as the amount of DMSO increases, the solubility of BM-P-WCC in the ionic liquid first increases and then decreases. When the DMSO content is more than 20%, the solubility begins to increase slowly; the solubility reaches a maximum of 25.47 wt % when the DMSO content is 40% and then begins to decrease. In other words, the time required for 5 g of [Amim]Cl to dissolve 0.2 g of BM-P-WCC decreases from 163 to 25 min. These results show that the addition of DMSO reduces the viscosity of the solvent and effectively improves the solubility of WCC fibers in [Amim]Cl. Under the action of a certain temperature and proper cosolvent, the internal friction force of the ionic liquid decreases and the binding force of the hydrocarbon also decreases, which manifests as a decrease in viscosity. The diagram of the dissolution mechanism of WCC fibers in ionic liquids is shown in Figure 3. The cellulose molecular chains can be better dispersed in [Amim]Cl, and thus the solubility of WCC fibers in [Amim]Cl improves under such conditions, a conclusion also reached by Andanson26 and Lv.52
Figure 3.
Diagram of the dissolution mechanism of WCC fibers in ionic liquids.
2.4. Mechanical Characterization
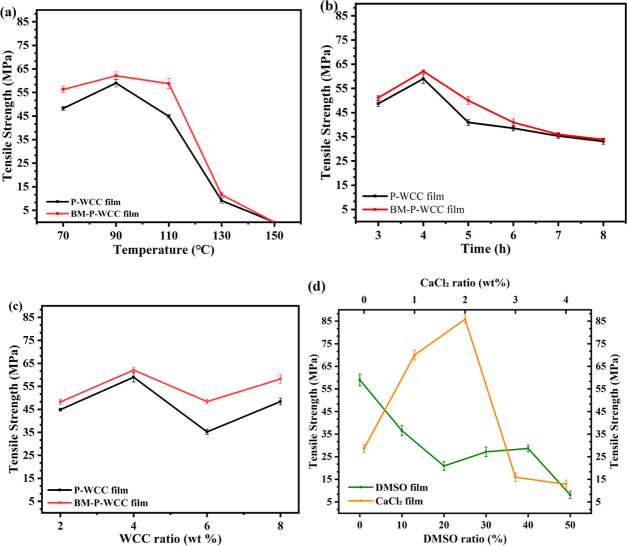
The regenerated cellulose films (named as P-WCC film and BM-P-WCC film) were prepared by adding 4 wt % P-WCC or BM-P-WCC to [Amim]Cl and dissolving for 4 h at variable dissolution temperatures. As shown in Figure 4a, the tensile strength of the film first increases and then decreases as the dissolution temperature increases, and the tensile strength of the BM-P-WCC film is slightly higher than that of the P-WCC film. The tensile strengths of the P-WCC and BM-P-WCC films had maximum values of 59.00 and 62.10 MPa, respectively at 90 °C. When the temperature increased above 110 °C, the tensile strengths of these films became poor. At temperatures higher than 150 °C, the film formation became noticeably difficult and the tensile strengths were recorded as 0 MPa. The WCC fiber dissolves in [Amim]Cl through a nonderivative form, which can better retain the original mechanical strength of lignocellulose in the fiber. When the temperature is too high, the cellulosic macromolecules oxidize and degrade in ionic liquids, resulting in poor tensile strength. According to Figure 4b, when the dissolution time increases from 3 to 4 h, the tensile strengths of the P-WCC and BM-P-WCC films increase from 48.69 and 51.16 MPa to 59.00 and 62.10 MPa, respectively. The tensile strengths of the films begin to decrease when the dissolution time continues to increase beyond this point, denoting an optimal dissolution time of 4 h; this is consistent with the research results of Zhang.53 Because lignocellulose is easy to oxidize and degrade in ionic liquids,54−57 when the dissolution time is too long, the tensile strength of the regenerated cellulose film decreases.
Figure 4.
Effect of the dissolution temperature (a), dissolution time (b), fibers ratio (c), DMSO ratio, and CaCl2 ratio (d) on the tensile strength of the films.
When the amount of the added fiber is close to the maximum solubility of [Amim]Cl, the viscosity of the ionic liquid will be very high and the viscosity of the film-forming solution has a great influence on the film-forming effect. Therefore, we studied the influence of different added WCC ratios on the tensile strength of the P-WCC and BM-P-WCC films. In Figure 4c, the regenerated cellulose films were prepared by dissolving BM-P-WCC or P-WCC at variable ratios in [Amim]Cl at 90 °C for 4 h. When 4 wt % WCC was added, the tensile strength of the resulting P-WCC and BM-P-WCC films reached their maximum values of 59.00 and 62.10 MPa, respectively, and then decreased as more WCC fibers were added. When the fiber ratio is greater than 8 wt %, a cellulose hydrogel forms due to the high viscosity of the film-forming solution. In addition, the tensile strength of the BM-P-WCC film is slightly higher than that of the P-WCC film; crystallinity may affect the tensile strengths of the films, as shown in Figure S2, which is consistent with the results of Zheng.14
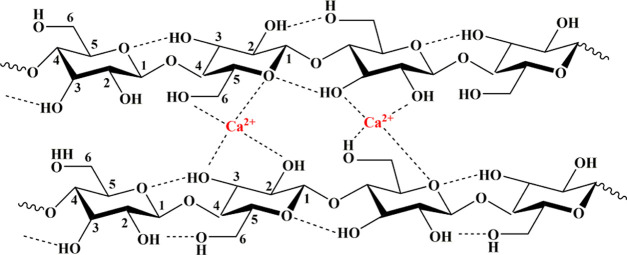
As BM-P-WCC was proved to be too difficult to be regenerated into a film using the [Amim]Cl/DMSO system, only the tensile strength of the regenerated cellulose film prepared using P-WCC was measured here. DMSO film was prepared by adding 4 wt % P-WCC, different DMSO ratios, and dissolving at 90 °C for 4 h. As shown in Figure 4d, the tensile strength of the film decreases as the DMSO ratio increases and at a ratio of 40%, the tensile strength of the P-WCC film is 28.65 MPa, after which the tensile strength sharply decreases. DMSO can prevent the oxidation and decomposition of fibers in ionic liquids and improve the dissolution efficiency of fibers in [Amim]Cl. However, too much DMSO will promote the acid-catalyzed water depolymerization of cellulose in [Amim]Cl58 and will reduce the tensile strength of the film. It shows the change in the tensile strength of the regenerated cellulose film (named as CaCl2 film) in Figure 4d prepared by adding 4 wt % P-WCC and 40% DMSO at 90 °C at 4 h of dissolution, using varying CaCl2 additions of 0–4 wt %. The tensile strength of the CaCl2 film first increases and then decreases as the amount of CaCl2 increases. At a CaCl2 addition of 2 wt %, the tensile strength of the film reaches a maximum value of 85.86 MPa, which is 45.53% higher than that of the P-WCC film (59.00 MPa) and 199.86% higher than that of the DMSO film (28.65 MPa). The tensile strength of common commercial polyolefin films (PE, PP) is typically 20–40 MPa,59 and the tensile strength of the cellulose film regenerated from ionic liquids ([EMIMO]Ac) and ([Bmim]Cl) is, respectively, about 7060 and 34 MPa.61 The regenerated porous cellulose films were prepared by dissolving in the LiCl/DMAc solvent; its tensile strength reached 29.22 MPa62 and the tensile strength of all-cellulose films regenerated from 7 wt % NaOH/12 wt % urea aqueous solution change from 30 to 135 MPa.63 Thus, this CaCl2 film has the potential to replace petroleum-based plastic bags as packaging materials. The same anion of CaCl2 and [Amim]Cl improves the tensile strength of the regenerated cellulose film, which can promote the dissolution and regeneration of cellulose in ionic liquids. Meanwhile, Ca2+ is cross-linked with the cellulose molecular chains to enhance the tensile strength of the film via the mechanism shown in Figure 5. The excess CaCl2 will also adhere to the surface of cellulose and reduce the tensile strength of the CaCl2 film.
Figure 5.
Existence of Ca2+ in cellulose molecules.
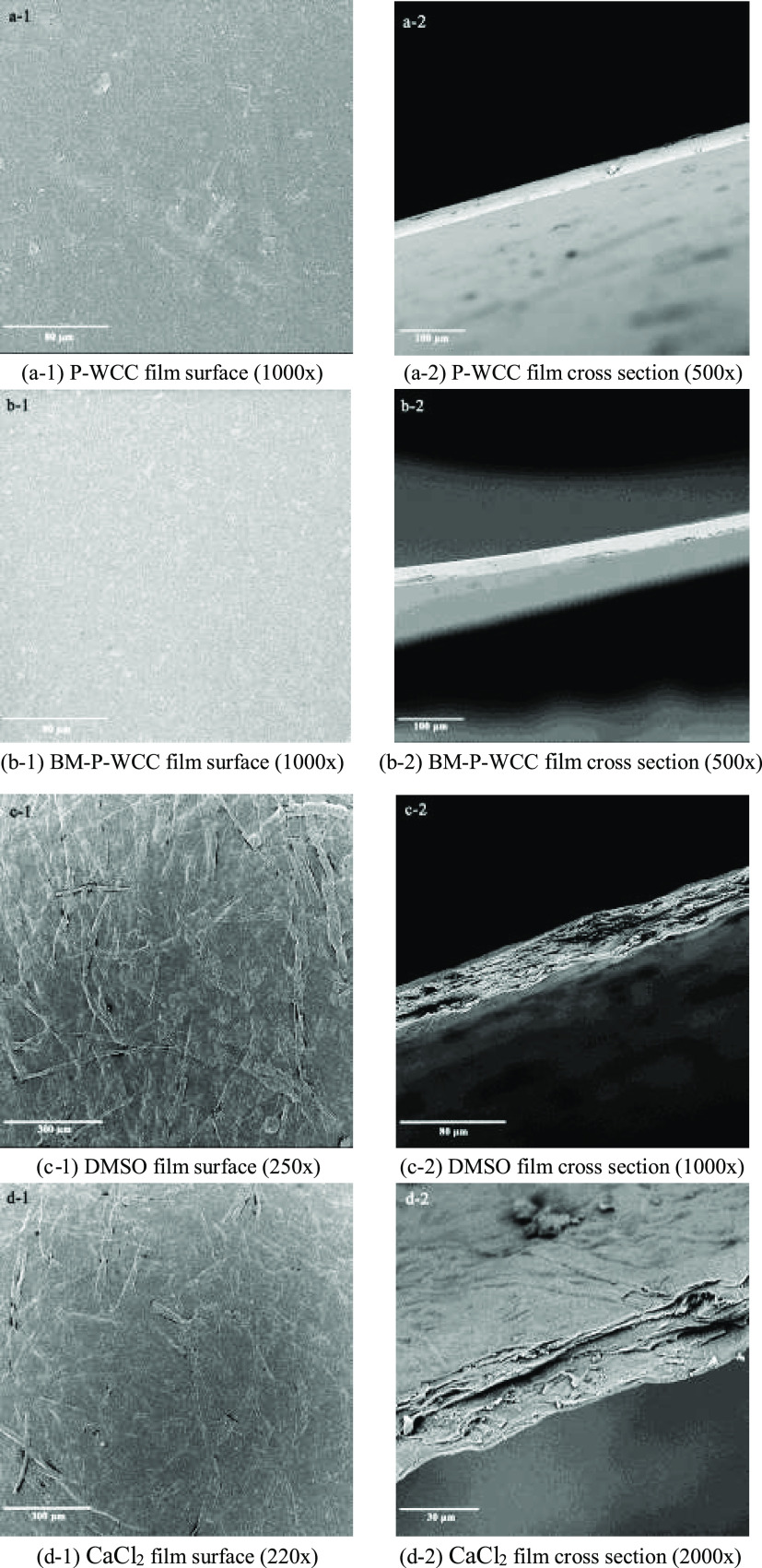
2.5. Micromorphology Analysis
Figure 6 shows the scanning electron microscopy (SEM) images of the surface and cross section of the films prepared under different conditions. There are a few particles on the surface (Figure 6a-1) and cross section (Figure 6a-2) of the P-WCC film and a few protrusions and holes on the cross section, which may be caused by the removal or fracturing of larger fibers when the film is quenched. In contrast, the surface (Figure 6b-1) of the BM-P-WCC film is more smooth, compact, and bright owing to ball milling, and the cross-sectional structure (Figure 6b-2) is even and smooth without a grainy appearance. The surface of the DMSO film is rough but the fiber structures are exposed and are interlaced and stacked to form a layered structure (Figure 6c-2). The surface of the CaCl2 film is still relatively rough with obvious fiber structures (Figure 6d-1) and the cross section features some pores (Figure 6d-2). The inner fibers are closely interwoven into a thin network structure but this structure is slightly flat and dense compared with that of the DMSO film (Figure 6c-1). The addition of CaCl2 inhibited the production of protons from the active hydrogen at the second position on [Amim]+ in the ionic liquid, thus weakening the acid-catalyzed water depolymerization of cellulose. This allows Ca2+ to cross-link on the cellulose molecular chain, thus enhancing the tensile strength of the film. Xu28 demonstrated that such nonrigid fiber structures can be effectively cross-linked by Ca2+.
Figure 6.
SEM images of the surface, and cross section of the regenerated cellulose films.
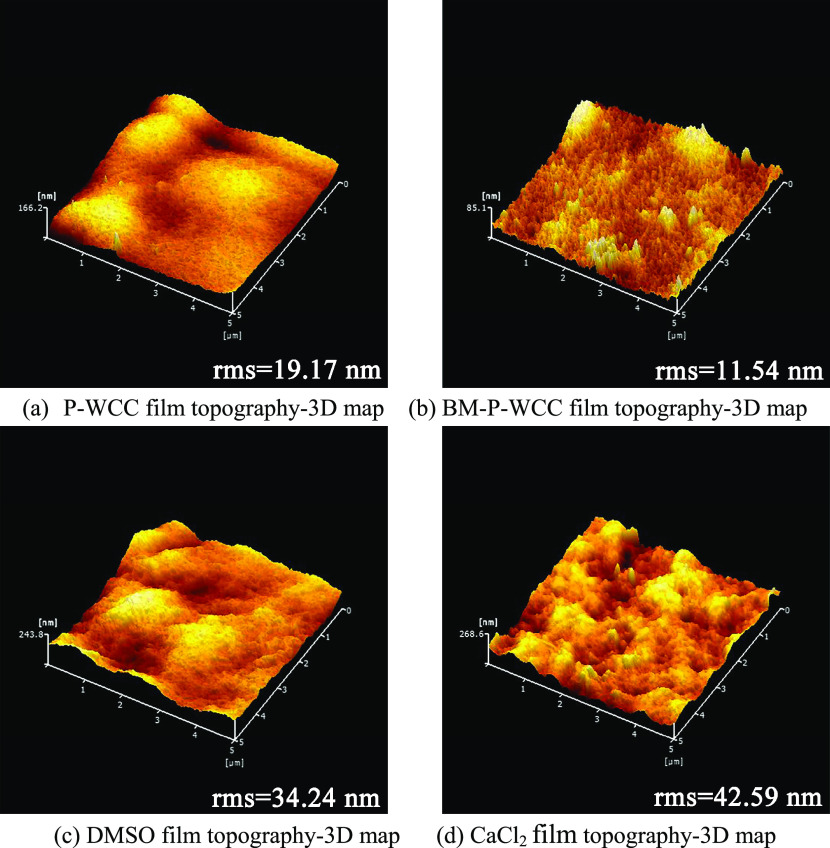
2.6. Roughness Analysis
Figure 7 shows the three-dimensional (3D) topographic maps of the P-WCC, BM-P-WCC, DMSO, and CaCl2 films using atomic force microscopy (AFM). The roughness value of the BM-P-WCC film (Figure 7b) is 11.54 nm, which is consistent with our SEM results. The size of the milled fiber in BM-P-WCC is uniform, making this film relatively smooth. The roughness value of the P-WCC film (Figure 7a) is slightly higher. With the addition of DMSO and CaCl2, the roughness values increase to 34.24 nm (Figure 7c) and 42.59 nm (Figure 7d), respectively. However, the roughness values of the regenerated cellulose films under different conditions are all less than 50 nm, indicating that the films have good surface structures.
Figure 7.
Surface roughness of the regenerated cellulose films.
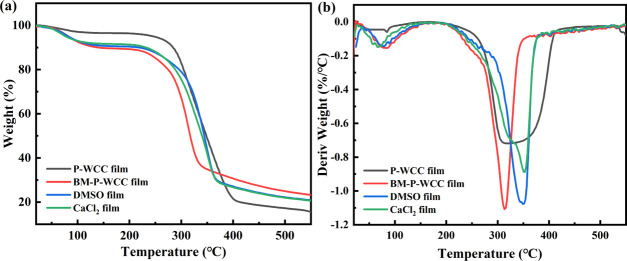
2.7. Thermogravimetric Analysis (TGA)
Figure 8 shows TGA and derivative thermogravimetric (DTG) data of the P-WCC, BM-P-WCC, DMSO, and CaCl2 films. There are two periods of weight loss in these films (Figure 8a): the first time is near 100 °C, which is the weight loss peak of water, while the second time is the weight loss of the thermal decomposition of the films. Figure 8b shows that the thermal decomposition temperatures of the BM-P-WCC, DMSO, and CaCl2 films are 347, 313, 349, and 351 °C, respectively; the thermal stability of the BM-P-WCC film is lower than that of the P-WCC film but the thermal stability of the regenerated cellulose film was improved after adding DMSO and CaCl2.
Figure 8.
TGA (a) and DTG (b) curves of the regenerated films.
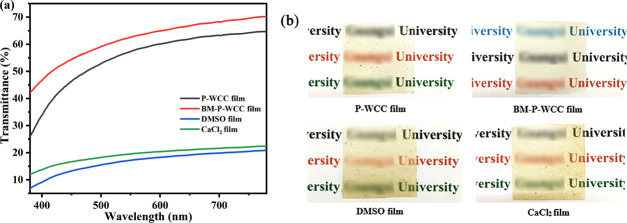
2.8. Optical Performance
Figure 9a shows that the smooth BM-P-WCC film shows the highest transmittance of all films studied here, in the wavelength range of 380–780 nm. This is because the small-sized BM-P-WCC fibers gather closely within the film, resulting in low porosity and less light scattering on the film surface. As shown in Figure 9b, the P-WCC film is slightly yellowish with lower transmittance than the BM-P-WCC film. DMSO film has the lowest transmittance and appears dark yellow and foggy translucent due to light scattering within the interlaced fiber network structure of the film, decreasing both the transmittance and transparency. The transmittance of the CaCl2 film increased by approximately 7% compared to the DMSO film and became more transparent due to the presence of Ca2+, which is consistent with our SEM and AFM results. The transmittance of the regenerated cellulose films varies when prepared under different conditions, which could be useful in different applications.
Figure 9.
Transmittance (a) and visual pattern (b) of the regenerated cellulose films.
2.9. Recovered Ionic Liquids
It was proved that [Amim]Cl was recovered completely by 1H NMR spectra and FTIR spectra (Figures S3 and S4). The recovery rate of the ionic liquid is approximately 85%, and an R-WCC film was prepared from the recycled ionic liquid in the same manner as the BM-P-WCC film for comparison (4 wt % BM-P-WCC fibers dissolved in the recovered [Amim]Cl at 90 °C for 4 h); the properties of this film are shown in Table 2.
Table 2. Effect of the Recovery of Ionic Liquids on Some Properties of the R-WCC Films.
| film | tensile strength (MPa) | rms (nm) | transmittance (%) |
|---|---|---|---|
| BM-P-WCC | 62.10 | 11.54 | 70.18 |
| R-WCC | 60.24 | 13.64 | 69.30 |
The tensile strength, surface roughness (Figure S5), and transmittance (Figure S6) of the BM-P-WCC and R-WCC films are relatively close, indicating that the recovered ionic liquid still has a good ability to dissolve the fiber and that this change has little effect on the physical properties of the film.
3. Conclusions
Four kinds of regenerated cellulose films with good mechanical properties were successfully prepared using low-cost waste corrugated cardboard (WCC) instead of high-grade pulp as a raw material. Adding 4 wt % fiber in [Amim]Cl and dissolving for 4 h at 90 °C yielded the best results. The regenerated cellulose films were smooth, compact, and transparent or semi-transparent, depending on the DMSO ratio, CaCl2 ratio, and whether or not ball milling was used. The films exhibited good mechanical properties, such as a maximum tensile strength of 85.86 MPa for the CaCl2 film. These films also had good thermal stability and could be used in fields with low transparency requirements. The BM-P-WCC film had a smooth and uniform surface and high transmittance and could be applied in fields requiring higher requirements. The transmittance of the P-WCC film was slightly lower than that of the BM-P-WCC film. With the addition of the cosolvent DMSO, the tensile strength of the DMSO film decreased, whereas the DMSO film prepared with 40% DMSO had low tensile strength, low transmittance, and poor transparency.
[Amim]Cl was a nonderivatizing solvent for the WCC fiber. Moreover, a deeper analysis has led to a greater understanding of this route. The XRD patterns indicate a transformation in the crystal structure (from cellulose I to cellulose II) that occurred during the dissolution and regeneration processes. TGA results demonstrated that the DMSO and CaCl2 films possessed high thermal stability similar to that of the P-WCC film. Therefore, the present work provides a simple and effective method to convert low-grade cellulose resources into high-value cellulose-based products.
4. Materials and Methods
4.1. Materials
Waste corrugated cardboard (C-type corrugated box), [Amim]Cl (C7H11N2Cl, ≥99%, Lanzhou Institute of Chemical Technology, Chinese Academy of Sciences), DMSO (AR, ≥99.8%, Tianjin Fuyu Fine Chemical Co., Ltd.), CaCl2 (AR, ≥96.0%, Chengdu Kelong Chemical Reagent Plant), NaOH (AR, ≥96.0%, Chengdu Kelong Chemical Reagent Plant), KOH (AR, ≥85.0%, Tianjin Zhiyuan Chemical Reagent Co., Ltd.), H2O2 (AR, ≥30%, Tianjin Hengxing Chemical Reagent Manufacturing Co., Ltd.), and phosphotungstic acid (AR, H3O40PW12·xH2O, Shanghai Aladdin Biochemical Technology Co., Ltd.) were used in this study.
4.2. Pretreatment of Waste Corrugated Cardboard
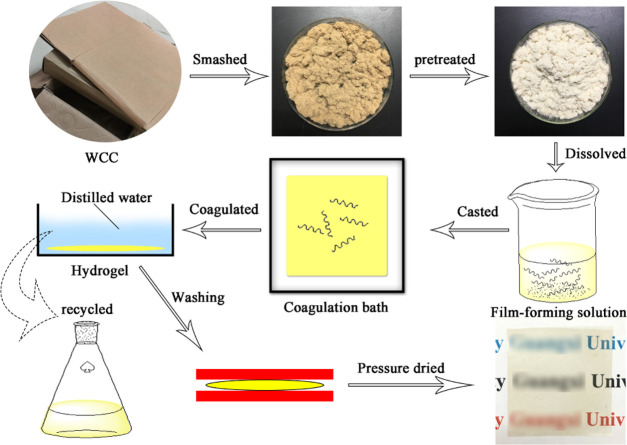
As shown in Figure 10, waste corrugated cardboard was cut into small pieces (1–2 cm), crushed and passed through a 700 μm sieve, treated with a 2 wt % NaOH solution in the ratio 1:10 (g/mL), and then stirred at 90 °C for 2 h. Subsequently, the samples were treated with a 20% H2O2 solution in the ratio 1:20 (g/mL) and stirred at 70 °C for 1 h. Finally, this sample was treated with a 4 wt % KOH solution in the ratio 1:10 (g/mL) and stirred at 90 °C for 2 h. The precipitate was washed with deionized water to a neutral pH and dried under vacuum at 40 °C for 48 h to obtain the waste corrugated board (named as P-WCC), which was kept sealed when not in use. One-half of the above-mentioned P-WCC was used to prepare BM-P-WCC via ball milling with a rotation speed of 4000 rpm for 10 h and kept sealed when not in use.
Figure 10.
Preparation procedure of the regenerated cellulose films from waste corrugated cardboard.
4.3. Preparation of the Regenerated Cellulose Films
The preparation procedure of the regenerated cellulose films is shown in Figure S1. After pretreatment, 0.8 g of P-WCC, 0.2 g of CaCl2, and 40% DMSO (to 20 g of [Amim]Cl) were dispersed into 20 g of [Amim]Cl. The mixture was then heated at 90 °C for 4 h under magnetic stirring, so that a homogeneous 4 wt % P-WCC/[Amim]Cl solution was obtained. The resulting solution was cast onto a poly(tetrafluoroethylene) plate with a spreader to obtain a 0.5 mm thick layer after 30 min setting time and immediately immersed into a coagulation bath (deionized water) to form a cellulose-based hydrogel, which was labeled as Hydrogel. The cellulose-based hydrogel was then washed at least five times with distilled water until the residual [Amim]Cl was completely removed (the scrubbing solution was reserved for later use). Finally, applying 900 MPa at 50 °C for 20 min using a Kaiser rapid sheet former (2DA, Estanit GmbH, Germany), the regenerated cellulose films were obtained. The films were dried at 60 °C for 24 h for characterization.
4.4. Chemical Compositions of WCC and P-WCC
The mass fraction of cellulose, hemicelluloses, lignin, and ash content in WCC and P-WCC were characterized by the Fan method, as shown in Table 1.64
4.5. Viscosity of [Amim]Cl/DMSO
The viscosity of [Amim]Cl/DMSO was measured for 0.5 min using a modular advanced rheometer (HAAKE MARS 40, HAAKE Technology Co., Ltd., Germany), with an FL22 4B/ss Vane rotor and CCB25 measuring cup. For each sample, at least three specimens were tested, and the average value was reported.
4.6. Solubility Properties of Fibers in [Amim]Cl
The dissolving property of P-WCC and BM-P-WCC in [Amim]Cl was observed using a polarizing microscope (Nikon E200, Nikon Co., Ltd., Japan). The solubility was calculated following Liu,65 where the dissolution time refers to the time required to completely dissolve 0.2 g of P-WCC or BM-P-WCC in 5 g of [Amim]Cl.
4.7. Mechanical Characterization
The tensile strength of the regenerated cellulose film was measured on an electronic universal material testing machine (3367, Instron, U.K.) at a crosshead speed of 5 mm/min. Specimens 50 mm in length and 10 mm in width were used for these tests, and a gauge length of 25 mm was maintained. For each sample, at least five specimens were tested, and the average value was reported. The mechanical tests were done by following the ASTM D-882 standard.
4.8. Micromorphology Analysis
Samples (cut into squares of 10 mm × 10 mm) were lifted with tweezers and attached to the stage with a conductive paste. Then, they were sputter-coated with a thin layer of platinum before being observed by scanning electron microscopy (SEM) (Phenom-World Pro, FEI). The film was placed in liquid nitrogen for 10 s and quenched to obtain the fracture surface; the cross-sectional structures of the films were observed at 10 V and the surface structures of the films were observed at 5 V.
4.9. AFM Analysis
Films were cut into squares of 10 mm × 10 mm for observation, and the samples were measured by atomic force microscopy (AFM) (5100N, Hitachi High-Tech Co., Ltd.) at 8.5 V, 299 kHz, tapping mode, C = 32 N/m, tip diameter = 7 nm, and probe type = SI-DFP2.
4.10. TGA Analysis
The thermal decomposition behavior of the samples was investigated using a synchronous thermal analyzer (STA 449 F5, Netzsch Instruments Manufacturing Co., Ltd.) under a nitrogen atmosphere at a heating rate of 10 °C/min, gas flow of 20 mL/min, and scanning temperature of 20–550 °C.
4.11. Optical Performance
Films were cut into specimens of 40 mm × 9 mm before testing, and the transmittance of a film laid on the inner wall of the colorimetric vessel was measured by UV spectrophotometry (Lambda950, PerkinElmer Instruments) over the wavelength range of 380–780 nm.
4.12. Recovery Rate of Ionic Liquids
The scrubbing solution reserved after preparing the regenerated cellulose films was filtered with a Nylon Millipore filter (Φ = 0.45 μm), and the water solvent was removed by rotary evaporation (RE-3000 B, Shanghai Xiande Experimental Instrument Co., Ltd.) at 105 °C under a 0.1 MPa vacuum.66 3A zeolite was added to remove impurities, and the material was vacuum-dried at 80 °C for 24 h to obtain the recovered ionic liquid.
Acknowledgments
The authors acknowledge financial support from the Basic Ability Improvement Project of Young and Middle-Aged Teachers in Guangxi Colleges and Universities, 2020 (2020KY01012), the Nanning Scientific Research and Technological Development Plan Project (20195215), the Guangxi Key Laboratory of Clean Pulp Papermaking and Pollution Control (ZR201806-6), and the Guangxi Science and Technology Project (Guangxi Science AB18221126).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02713.
Data of the polarizing micrographs of P-WCC in [Amim]Cl; XRD spectrum of P-WCC and BM-P-WCC; FTIR and 1H NMR of [Amim]Cl and recovered [Amim]Cl; AFM graphic and optical performance figure of the R-WCC film (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ran L. Express business of double 11 Shopping Festival. People’s Transportation 2018, 12, 32–36. [Google Scholar]
- Minnick D. L.; Flores R. A.; DeStefano M. R.; Scurto A. M. Cellulose Solubility in Ionic Liquid Mixtures: Temperature, Cosolvent, and Antisolvent Effects. J. Phys. Chem. B 2016, 120, 7906–7919. 10.1021/acs.jpcb.6b04309. [DOI] [PubMed] [Google Scholar]
- Koganti N.; Mitchell J. R.; Ibbett R. N.; Foster T. J. Solvent Effects on Starch Dissolution and Gelatinization. Biomacromolecules 2011, 12, 2888–2893. 10.1021/bm200390a. [DOI] [PubMed] [Google Scholar]
- Koganti N.; Mitchell J.; MacNaughtan W.; Hill S.; Foster T. Effect of granule organisation on the behaviour of starches in the NMMO (N-methyl morpholine N-oxide) solvent system. Carbohydr. Polym. 2015, 116, 103–110. 10.1016/j.carbpol.2014.06.060. [DOI] [PubMed] [Google Scholar]
- Tan X.Study of Dissolution and Gelatinization Behaviors of Starch/Cellulose in Ionic Liquid and Fabrication of Related Gastric Floating Drug Delivery System. In Doctor of Philosophy; South China University of Technology, 2017. [Google Scholar]
- Cao Y.; Zhang R.; Cheng T.; Guo J.; Xian M.; Liu H. Imidazolium-based ionic liquids for cellulose pretreatment: recent progresses and future perspectives. Appl. Microbiol. Biotechnol. 2017, 101, 521–532. 10.1007/s00253-016-8057-8. [DOI] [PubMed] [Google Scholar]
- Gericke M.; Schlufter K.; Liebert T.; Heinze T.; Budtova T. Rheological Properties of Cellulose/Ionic Liquid Solutions: From Dilute to Concentrated States. Biomacromolecules 2009, 10, 1188–1194. 10.1021/bm801430x. [DOI] [PubMed] [Google Scholar]
- Swatloski R. P.; Spear S. K.; Holbrey J. D.; Rogers R. D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. 10.1021/ja025790m. [DOI] [PubMed] [Google Scholar]
- Gupta K. M.; Jiang J. Cellulose dissolution and regeneration in ionic liquids: A computational perspective. Chem. Eng. Sci. 2015, 121, 180–189. 10.1016/j.ces.2014.07.025. [DOI] [Google Scholar]
- Isik M.; Sardon H.; Mecerreyes D. Ionic Liquids and Cellulose: Dissolution, Chemical Modification and Preparation of New Cellulosic Materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. 10.3390/ijms150711922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y.; Peng S.; He J.; Fan M.; Wang K. Effects of Inorganic Salts /Ionic Liquids on the Structure and Properties of Regenerated Cellulose. Polym. Eng. Sci. 2017, 33, 39–44. [Google Scholar]
- Hermanutz F.; Gähr F.; Uerdingen E.; Meister F.; Kosan B. New Developments in Dissolving and Processing of Cellulose in Ionic Liquids. Macromol. Symp. 2008, 262, 23–27. 10.1002/masy.200850203. [DOI] [Google Scholar]
- Zhang H.; Wu J.; Zhang J.; He J. 1-Allyl-3-methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. 10.1021/ma0505676. [DOI] [Google Scholar]
- Zheng X.; Huang F.; Chen L.; Huang L.; Cao S.; Ma X. Preparation of transparent film via cellulose regeneration: Correlations between ionic liquid and film properties. Carbohydr. Polym. 2019, 203, 214–218. 10.1016/j.carbpol.2018.09.060. [DOI] [PubMed] [Google Scholar]
- Sescousse R.; Le K. A.; Ries M. E.; Budtova T. Viscosity of Cellulose–Imidazolium-Based Ionic Liquid Solutions. J. Phys. Chem. B 2010, 114, 7222–7228. 10.1021/jp1024203. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Zhang H.; Wu J.; Zhang J.; He J.; Xiang J. NMR spectroscopic studies of cellobiose solvation in EmimAc aimed to understand the dissolution mechanism of cellulose in ionic liquids. Phys. Chem. Chem. Phys. 2010, 12, 1941–1947. 10.1039/b920446f. [DOI] [PubMed] [Google Scholar]
- Li J.; Lu Y.; Yang D.; Sun Q.; Liu Y.; Zhao H. Lignocellulose aerogel from wood-ionic liquid solution (1-allyl-3-methylimidazolium chloride) under freezing and thawing conditions. Biomacromolecules 2011, 12, 1860–1867. 10.1021/bm200205z. [DOI] [PubMed] [Google Scholar]
- Pinkert A.; Marsh K. N.; Pang S. Reflections on the solubility of cellulose. Ind. Eng. Chem. Res. 2010, 49, 11121–11130. 10.1021/ie1006596. [DOI] [Google Scholar]
- Lu B.; Xu A.; Wang J. Cation does matter: how cationic structure affects the dissolution of cellulose in ionic liquids. Green Chem. 2014, 16, 1326–1335. 10.1039/C3GC41733F. [DOI] [Google Scholar]
- Xia G.; Wan J.; Zhang J.; Zhang X.; Xu L.; Wu J.; He J.; Zhang J. Cellulose-based films prepared directly from waste newspapers via an ionic liquid. Carbohydr. Polym. 2016, 151, 223–229. 10.1016/j.carbpol.2016.05.080. [DOI] [PubMed] [Google Scholar]
- Sundberg J.; Toriz G.; Gatenholm P. Moisture induced plasticity of amorphous cellulose films from ionic liquid. Polymer 2013, 54, 6555–6560. 10.1016/j.polymer.2013.10.012. [DOI] [Google Scholar]
- Rahatekar S. S.; Rasheed A.; Jain R.; Zammarano M.; Koziol K. K.; Windle A. H.; Gilman J. W.; Kumar S. Solution spinning of cellulose carbon nanotube composites using room temperature ionic liquids. Polymer 2009, 50, 4577–4583. 10.1016/j.polymer.2009.07.015. [DOI] [Google Scholar]
- Lovell C. S.; Walker A.; Damion R. A.; Radhi A.; Tanner S. F.; Budtova T.; Ries M. E. Influence of Cellulose on Ion Diffusivity in 1-Ethyl-3-Methyl-Imidazolium Acetate Cellulose Solutions. Biomacromolecules 2010, 11, 2927–2935. 10.1021/bm1006807. [DOI] [PubMed] [Google Scholar]
- Gardas R. L.; Coutinho J. A. P. A group contribution method for viscosity estimation of ionic liquids. Fluid Phase Equilib. 2008, 266, 195–201. 10.1016/j.fluid.2008.01.021. [DOI] [Google Scholar]
- Kostag M.; Liebert T.; El Seoud O. A.; Heinze T. Efficient cellulose solvent: quaternary ammonium chlorides. Macromol. Rapid Commun. 2013, 34, 1580–1584. 10.1002/marc.201300497. [DOI] [PubMed] [Google Scholar]
- Andanson J. M.; Bordes E.; Devemy J.; Leroux F.; Padua A. A. H.; Gomes M. F. C. Understanding the role of co-solvents in the dissolution of cellulose in ionic liquids. Green Chem. 2014, 16, 2528–2538. 10.1039/c3gc42244e. [DOI] [Google Scholar]
- Ries M. E.; Radhi A.; Keating A. S.; Parker O.; Budtova T. Diffusion of 1-ethyl-3-methyl-imidazolium acetate in glucose, cellobiose, and cellulose solutions. Biomacromolecules 2014, 15, 609–617. 10.1021/bm401652c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q.; Chen C.; Rosswurm K.; Yao T.; Janaswamy S. A facile route to prepare cellulose-based films. Carbohydr. Polym. 2016, 149, 274–281. 10.1016/j.carbpol.2016.04.114. [DOI] [PubMed] [Google Scholar]
- Li H.; Cao Y.; Qin J.; Jie X.; Wang T.; Liu J.; Yuan Q. Development and characterization of anti-fouling cellulose hollow fiber UF membranes for oil–water separation. J. Membr. Sci. 2006, 279, 328–335. 10.1016/j.memsci.2005.12.025. [DOI] [Google Scholar]
- Imbrigiotta T. E.; Trotsky J. S.. Demonstration and Validation of a Regenerated Cellulose Dialysis Membrane Diffusion Sampler for Monitoring Ground Water Quality and Remediation Progress at DoD Sites for Perchlorate and Explosives Compounds, Naval Facilities Engineering Command Port Hueneme Ca Engineering Service Center, 2010.
- Takesawa S.; Satoh S.; Hidai H.; Sekiguchi M.; Sakai K. Degradation by gamma irradiation of regenerated cellulose membranes for clinical dialysis. ASAIO J. 1987, 33, 584–587. [PubMed] [Google Scholar]
- Wang W.; Bai Q.; Liang T.; Bai H.; Liu X. Preparation of amino-functionalized regenerated cellulose membranes with high catalytic activity. Int. J. Biol. Macromol. 2017, 102, 944–951. 10.1016/j.ijbiomac.2017.04.096. [DOI] [PubMed] [Google Scholar]
- Marichal-Gallardo P.; Pieler M. M.; Wolff M. W.; Reichl U. Steric exclusion chromatography for purification of cell culture-derived influenza A virus using regenerated cellulose membranes and polyethylene glycol. J. Chromatogr. 2017, 1483, 110–119. 10.1016/j.chroma.2016.12.076. [DOI] [PubMed] [Google Scholar]
- Wang J.; Wei L.; Ma Y.; Li K.; Li M.; Yu Y.; Wang L.; Qiu H. Collagen/cellulose hydrogel beads reconstituted from ionic liquid solution for Cu (II) adsorption. Carbohydr. Polym. 2013, 98, 736–743. 10.1016/j.carbpol.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Duri S.; Tran C. D. Supramolecular composite materials from cellulose, chitosan, and cyclodextrin: facile preparation and their selective inclusion complex formation with endocrine disruptors. Langmuir 2013, 29, 5037–5049. 10.1021/la3050016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Luo X.; Huang L.; Lin S.; Chen L. Development of ionic liquid-mediated antibacterial cellulose-chitosan films. J. Biobased Mater. Bioenergy 2015, 9, 389–395. 10.1166/jbmb.2015.1537. [DOI] [Google Scholar]
- Tran C. D.; Mututuvari T. M. Cellulose, chitosan, and keratin composite materials. Controlled drug release. Langmuir 2015, 31, 1516–1526. 10.1021/la5034367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G.; Lyu X.; Lee J.; Cui X.; Chen W. N. Biodegradable and transparent cellulose film prepared eco-friendly from durian rind for packaging application. Food Packag. Shelf Life 2019, 21, 100345 10.1016/j.fpsl.2019.100345. [DOI] [Google Scholar]
- Amalini A. N.; Noor Haida M. K.; Imran K.; Mohamad Haafiz M. K. Relationship between dissolution temperature and properties of oil palm biomass based-regenerated cellulose films prepared via ionic liquid. Mater. Chem. Phys. 2019, 221, 382–389. 10.1016/j.matchemphys.2018.09.028. [DOI] [Google Scholar]
- Zhang X. F.; Song L.; Wang Z.; Wang Y.; Wan L.; Yao J. Highly transparent graphene oxide/cellulose composite film bearing ultraviolet shielding property. Int. J. Biol. Macromol. 2020, 145, 663–667. 10.1016/j.ijbiomac.2019.12.241. [DOI] [PubMed] [Google Scholar]
- Jung Y. H.; Chang T. H.; Zhang H.; Yao C.; Zheng Q.; Yang V. W.; Mi H.; Kim M.; Cho S. J.; Park D. W.; Jiang H.; Lee J.; Qiu Y.; Zhou W.; Cai Z.; Gong S.; Ma Z. High-performance green flexible electronics based on biodegradable cellulose nanofibril paper. Nat. Commun. 2015, 6, 7170 10.1038/ncomms8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H.; Fang Z.; Wang Z.; Dai J.; Yao Y.; Shen F.; Preston C.; Wu W.; Peng P.; Jang N.; Yu Q.; Yu Z.; Hu L. Extreme Light Management in Mesoporous Wood Cellulose Paper for Optoelectronics. ACS Nano 2016, 10, 1369–1377. 10.1021/acsnano.5b06781. [DOI] [PubMed] [Google Scholar]
- Ye D.; Lei X.; Li T.; Cheng Q.; Chang C.; Hu L.; Zhang L. Ultrahigh Tough, Super Clear, and Highly Anisotropic Nanofiber-Structured Regenerated Cellulose Films. ACS Nano 2019, 13, 4843–4853. 10.1021/acsnano.9b02081. [DOI] [PubMed] [Google Scholar]
- Sixta H.; Michud A.; Hauru L.; Asaadi S.; Ma Y.; King A. W.; Kilpeläinen I.; Hummel M. Ioncell-F: a high-strength regenerated cellulose fibre. Nord. Pulp Pap. Res. J. 2015, 30, 43–57. 10.3183/npprj-2015-30-01-p043-057. [DOI] [Google Scholar]
- Sun N.; Rodriguez H.; Rahman M.; Rogers R. D. Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass?. Chem. Commun. 2011, 47, 1405–1421. 10.1039/C0CC03990J. [DOI] [PubMed] [Google Scholar]
- Kärkkäinen J.; Lappalainen K.; Joensuu P.; Lajunen M. HPLC-ELSD analysis of six starch species heat-dispersed in [BMIM] Cl ionic liquid. Carbohydr. Polym. 2011, 84, 509–516. 10.1016/j.carbpol.2010.12.011. [DOI] [Google Scholar]
- Li W.; Zhang Z.; Han B.; Hu S.; Xie Y.; Yang G. Effect of water and organic solvents on the ionic dissociation of ionic liquids. J. Phys. Chem. B 2007, 111, 6452–6456. 10.1021/jp071051m. [DOI] [PubMed] [Google Scholar]
- Seddon K. R.; Stark A.; Torres M.-J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. 10.1351/pac200072122275. [DOI] [Google Scholar]
- Remsing R. C.; Liu Z.; Sergeyev I.; Moyna G. Solvation and aggregation of N, N′-dialkylimidazolium ionic liquids: A multinuclear NMR spectroscopy and molecular dynamics simulation study. J. Phys. Chem. B 2008, 112, 7363–7369. 10.1021/jp800769u. [DOI] [PubMed] [Google Scholar]
- Cruz H.; Fanselow M.; Holbrey J. D.; Seddon K. R. Determining relative rates of cellulose dissolution in ionic liquids through in situ viscosity measurement. Chem. Commun. 2012, 48, 5620–5622. 10.1039/c2cc31487h. [DOI] [PubMed] [Google Scholar]
- Bodvik R.; Dedinaite A.; Karlson L.; Bergström M.; Bäverbäck P.; Pedersen J. S.; Edwards K.; Karlsson G.; Varga I.; Claesson P. M. Aggregation and network formation of aqueous methylcellulose and hydroxypropylmethylcellulose solutions. Colloids Surf., A 2010, 354, 162–171. 10.1016/j.colsurfa.2009.09.040. [DOI] [Google Scholar]
- Lv Y.; Wu J.; Zhang J.; Niu Y.; Liu C. Y.; He J.; Zhang J. Rheological properties of cellulose/ionic liquid/dimethylsulfoxide (DMSO) solutions. Polymer 2012, 53, 2524–2531. 10.1016/j.polymer.2012.03.037. [DOI] [Google Scholar]
- Zhang X.Preparation Technology and Properties of Cellulose Membran in Ionic Liqiud; Master, Guangxi University, 2013. [Google Scholar]
- Cheng L.; Zhu T.; Liu W.; Zhang Y.; Wang H.; Yu M. Investigation on Dissolution of Cellulose in Ionic Liquids. Synth. Fiber China 2008, 37, 9–13. [Google Scholar]
- Liu C.; Li W.; Sun R.; Ye J. Degradation and Homogeneous Derivatization of Cellulose in Room Temperature Ionic Liquid AmimCl. Paper Sci. Technol. 2007, 26, 37–40. [Google Scholar]
- Li Z.; Yan G. Investigation of the thermal stability of cellulose and the capability of regenerative cellulose. Appl. Sci. Technol. 2007, 034, 67–70. [Google Scholar]
- Fu F.; Deng Y.; Sun N.; Xiao Z. The Dissolution and Degradation of Cellulose in ionic liquids. Hangzhou Chem. Ind. 2010, 40, 22–25. [Google Scholar]
- Gazit O. M.; Katz A. Dialkylimidazolium ionic liquids hydrolyze cellulose under mild conditions. ChemSusChem 2012, 5, 1542–1548. 10.1002/cssc.201100803. [DOI] [PubMed] [Google Scholar]
- Cao Y.; Li H.; Zhang Y.; Zhang J.; He J. Structure and properties of novel regenerated cellulose films prepared from cornhusk cellulose in room temperature ionic liquids. J. Appl. Polym. Sci. 2010, 116, 547–554. 10.1002/app.31273. [DOI] [Google Scholar]
- Leppänen I.; Vikman M.; Harlin A.; Orelma H. Enzymatic Degradation and Pilot-Scale Composting of Cellulose-Based Films with Different Chemical Structures. J. Polym. Environ. 2020, 28, 458–470. 10.1007/s10924-019-01621-w. [DOI] [Google Scholar]
- Wei X.; Zhang L.; Wang J.; Li J.; Zhou W. Preparation of cellulose film in ionic liquid by high shearing and application in pineapple preservation. Mater. Res. Express 2020, 7, 025313 10.1088/2053-1591/ab73fd. [DOI] [Google Scholar]
- Teng G.; Lin S.; Xu D.; Heng Y.; Hu D. Renewable cellulose separator with good thermal stability prepared via phase inversion for high-performance supercapacitors. J. Mater. Sci.: Mater. Electron. 2020, 31, 7916–7926. [Google Scholar]
- Cheng G.; Zhu P.; Li J.; Cheng F.; Lin Y.; Zhou M. All-cellulose films with excellent strength and toughness via a facile approach of dissolution-regeneration. J. Appl. Polym. Sci. 2019, 136, 46925 10.1002/app.46925. [DOI] [Google Scholar]
- Van Soest P. J.; Robertson J. B.; Cornell U.. Animal Science. In Analysis of Forages and Fibrous Foods; Cornell University: Ithaca, N.Y., 1985. [Google Scholar]
- Liu Z.; Wang H.; Li Z.; Lu X.; Zhang X.; Zhang S.; Zhou K. Characterization of the regenerated cellulose films in ionic liquids and rheological properties of the solutions. Mater. Chem. Phys. 2011, 128, 220–227. 10.1016/j.matchemphys.2011.02.062. [DOI] [Google Scholar]
- Wang X.; Li H.; Cao Y.; Tang Q. Dissolution and regeneration of Chinese parasol sawdust in ionic liquid 1-ally-3-methylimidazolium chloride. CIESC J. 2011, 62, 2951–2957. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.