Abstract
Pseudomonas aeruginosa developed its biocontrol agent property through the production of antifungal derivatives, with the phenazine among them. In this study, the applications of crude phenazine synthesized by Pseudomonas aeruginosa UPMP3 and hexaconazole were comparatively evaluated for their effectiveness to suppress basal stem rot infection in artificially G. boninense-challenged oil palm seedlings. A glasshouse experiment under the randomized completely block design was set with the following treatments: non-inoculated seedlings, G. boninense inoculated seedlings, G. boninense inoculated seedlings with 1 mg/ml phenazine application, G. boninense inoculated seedlings with 2 mg/ml phenazine application and G. boninense inoculated seedlings with 0.048 mg/ml hexaconazole application. Seedlings were screened for disease parameters and plant vigour traits (plant height, plant fresh weight, root fresh, and dry weight, stem diameter, and total chlorophyll) at 1-to-4 month post-inoculation (mpi). The application of 2 mg/ml phenazine significantly reduced disease severity (DS) at 44% in comparison to fungicide application (DS = 67%). Plant vigour improved from 1 to 4 mpi and the rate of disease reduction in seedlings with phenazine application (2 mg/ml) was twofold greater than hexaconazole. At 4, 6 and 8 wpi, an up-regulation of chitinase and β-1,3 glucanase genes in seedlings treated with phenazine suggests the involvement of induced resistance in G. boninense-oil palm pathosystem.
Subject terms: Plant sciences, Plant immunity, Plant molecular biology
Introduction
Bacteria are primary producers of natural phenazines. The nitrogen containing heterocyclic phenazines are vital for the development of biofilm, also a key structure that supports the survival of bacteria under adverse environmental conditions. Phenazines function as cell signals for regulation of gene expression patterns and as electron shuttles for the modification of cellular redox states. The phenazine-producing Eubacteria includes Burkholderia, Ervinia, Sorangium, Nocardia and a wide variety of both gram-positive and gram-negative species1. A considerable number of Pseudomonas biocontrol strains with antifungal activity produce phenazines2,3. Phenazines are toxic to a wide range of microorganisms and its physical properties have been reported to influence in vivo activities at the cellular level. The antimicrobial activity of phenazine is affected by the rate of oxidative-reductive process, transformation rate of the compound and the degree at which toxic superoxide radicals are accumulated in the target cells4. The action of phenazine has been studied in barley, oat, rice, wheat and bean for the development of biocontrol agents targeting a wide array of phytopathogens5,6.
The soil-inhabitant Pseudomonas aeruginosa produces a number of antifungal factors such as the pyocyanin (5-n-methyl-1-hydroxy-phenazine), phenazine-1-carboxylic acid and phenazine-1-carboxamide7–10. These bright coloured pigments have a broad-spectrum antibiotic activity and therefore their efficiency for biocontrol applications such as biological pesticides have been extensively studied4,11. The axenic culture of P. aeruginosa UPMP3 has shown to produce core phenazine (PHZ), phenazine-l-carboxylic acid (PCA) and pyocyanin (PYO) antibiotics at variable concentrations. On an individual evaluation, each antibiotic showed in vitro antagonistic activity against Ganoderma boninense at a minimal inhibition ratio of 1: 10: 15/PHZ: PCA: PYO. The PHZ extracted from P. aeruginosa UPMP3 crude extract demonstrated the best antifungal efficiency against Ganoderma boninense amongst the other phenazine derivatives evaluated at a laboratory scale experiment12.
The basidiomycetous G. boninense causes basal stem rot (BSR) of oil palms (OPs). Over the years, BSR has been severely affecting OP productivity especially in South East Asian countries such as Malaysia and Indonesia13–15. A continuous prevalence of BSR suggests that effective disease control strategies are still lacking. The pathogenic fungus penetrates into host cells by using thin-like needle structures, also known as the micro-hyphae16 prior to colonization (biotrophic nutrition). During colonization, the host plant remains asymptomatic until the pathogen switches into necrotrophic nutrition. At this stage, white cellulose of the wood component becomes exposed upon the successful degradation of lignin component. Later, the OP trunks form fruiting bodies and eventually rot before death. In large-scale oil palm plantations, disease symptoms of BSR include stunted and unopened spear leaves, yellowing and senescence of upper fronds and ‘one-sided mottling’ of the canopy17,18. Likewise, under controlled environment, artificially G. boninense challenged oil palm seedlings have shown the emergence of fruiting bodies followed by yellowing and browning of leaves prior to seedling necrotic death19.
The plant induced resistance re-conditions host defensive capacity upon elicitation by a specific biotic challenge; systemic acquired resistance (SAR) and induced systemic resistance (ISR). The perception of pathogen by host cells activates a concert of biochemical and metabolic activities which includes the burst of reactive oxygen species, production of secondary metabolites and pathogenesis-related (PR) proteins. These compounds are co-ordinately synthesized and accumulated by host plant for resistance enhancement. The host plant PR genes encode for PR proteins during defense response against microbial attack20. These low-molecular weight proteins of about 6–43 kDa in size have been observed in numerous host–pathogen interactions particularly during fungal infection21. Both β-1,3-glucanase (EC 3.2.1.39) and chitinase (EC 3.2.1.14) are PR proteins widely characterized for fungal pathogen resistance enhancement in crop plants. The β-1,3-glucanase catalyzes the hydrolytic cleavage of (1,3)-β-D-glucosidic linkages present in (1,3)-β-glucans of fungal cell walls while chitinase acts on the breakdown of chitin (β-1,4-linked N-acetyl glucosamine) homopolymer. The involvement of induced resistance has been correlated with the increase activity of PR proteins22.
In Southeast Asia region, BSR of OPs is managed mainly by fungicidal treatments. Application of fungicides effectively prolongs the life span of the infected palms and their subsequent productivity over time. In Malaysia, the hexaconazole application is the most preferred method for BSR management as the systemic fungicide is reasonably effective at a low concentration (EC50 = 0.026 µg ml−1) and has convenient application strategies such as the soil drenching and soil injection methods23. In this study, a BSR disease trial was performed to compare the effect of phenazine and hexaconazole on the expression of PR genes and disease development (disease severity and plant vigour traits). A basal stem rot disease trial consists of G. boninense inoculated seedlings treated with phenazine (P. aeruginosa derivatives) and hexaconazole was conducted. Root-specific expression of PR genes at the early stage of infection was quantified by reverse-transcriptase PCR to identify involvement of induced resistance in the treated seedlings. Our findings suggest that phenazine isolated from P. aeruginosa could be efficiently exploited for biological pesticide formulation as an alternative to chemical pesticides. Phenazine-based biological pesticide would offer an environmentally sound solution to BSR management apart being highly effective for disease suppression and oil palm growth enhancement.
Results
Disease assessment of the infected oil palm seedlings
Oil palm seedlings artificially infected with G. boninense developed the BSR disease symptoms over a period of 4 months. Apparent foliar symptoms observed includes yellowing followed by browning of lower leaves and formation of sporophore/fruiting bodies. The disease incidence (DI%) computed based on the foliar symptoms gradually increased over the months post-inoculation (mpi). The non-inoculated seedlings from the negative control treatment (T1) showed absence of any visible disease symptoms. Generally, the DI% of the inoculated + treated seedlings (T3, T4 and T5) was lower than the control seedlings (T2) at each mpi. A lower DI corresponds to the effectiveness of the treatment to suppress the disease progression. Among the treatment groups, seedlings inoculated + treated with phenazine at 2 mg/ml (T4) showed the lowest DI value throughout 1-to-4 mpi. Interestingly, at 1 mpi, the DI was zero for T4 in comparison to other treatment groups: T2, DI = 22.22%; T3, DI = 11.11%; T5 DI = 11.11%. At 2 mpi, the DI of each treatment groups increased steeply as following: T2, DI = 55.56%; T3, DI = 33.33%; T4, DI = 22.22%; T5, DI = 33.33%. At 3 mpi, the DI continued to show slight increment at 77.78, 44.45, 33.33 and 66.67 for T2, T3, T4 and T5, respectively. At 4 mpi, the DI of the phenazine treated seedlings showed the lowest values: DI = 44.44%, for T4 and DI = 55.56% for T3. This was followed by the inoculated + hexaconazole treated seedlings (T5) at DI = 66.67% and T2 at DI = 88.89% (Fig. 1).
Figure 1.
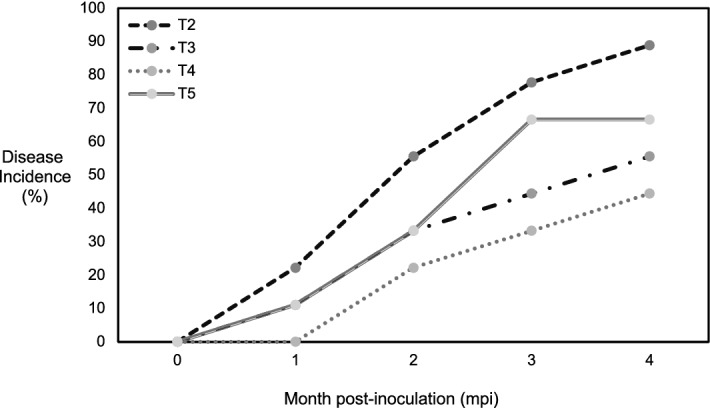
Disease incidence (DI) of oil palm seedlings challenged with Ganoderma boninense and treated with phenazine and hexaconazole. T2–T5 denote the different treatments: T2, G. boninense inoculated oil palm seedlings; T3, G. boninense inoculated oil palm seedlings treated with 1 mg/ml phenazine; T4, G. boninense inoculated oil palm seedlings treated with 2 mg/ml phenazine; T5, G. boninense inoculated oil palm seedlings treated with hexaconazole. The DI values are recorded at monthly intervals (1–4 mpi). All values are expressed as the means of three biological replicates.
Further, the basal stem rot disease progression was evaluated by the following parameters: area under the disease progress curve (AUDPC), epidemic rate (ER) and disease reduction (DR). The effectiveness of the treatment to delay or to suppress the onset of BSR symptoms was expressed as DR whereas the ER reflected on the progress of disease development under the different treatment per se. The AUDPC, ER and DR values among the treatments showed significant differences at p < 0.05. The AUPDC of the untreated seedlings (T2) was significantly highest at 116.3 followed by the inoculated + hexaconazole treated seedlings (T5) at 86.90. Seedlings inoculated + treated with phenazine at 1 mg/ml showed AUPDC = 77.65 and seedlings inoculated + treated with phenazine at 2 mg/ml showed the least value at AUPDC = 54.93. Similar trend was obtained for the ER scores: Treatment with the least AUPDC scores obtained the smallest ER and vice versa. The ER values of each treatment corroborated to the DR values. Among the treatments, at ER = 0.111 and DR = 52.76, the application of phenazine at 2 mg/ml (T4) showed the best efficiency to control BSR development whereas the application of hexaconazole was found least efficient at ER = 0.1666 and DR = 25.27 (Table 1).
Table 1.
Effect of phenazine and hexaconazole applications on oil palm seedlings artificially infected with Ganoderma boninense.
| Treatment | AUDPC1 | ER1 | DR1,2 |
|---|---|---|---|
| T1 (negative control)—Gb | |||
| T2 (positive control) + Gb | 116.30a | 0.22221a | 0.00 |
| T3 (phenazine 1 mg/ml) + Gb | 77.65c | 0.1388c | 33.23b |
| T4 (phenazine 2 mg/ml) + Gb | 54.93d | 0.1111d | 52.76a |
| T5 (hexaconazole 0.048 mg/ml ) + Gb | 86.90b | 0.1666b | 25.27c |
Means within columns followed by different letters are significantly different at p < 0.05 according to Least Significant Difference (LSD) test. Gb represents the Ganoderma boninense inoculum and + /− sign denotes with/without the artificial infection of oil palm seedlings. Each treatment (T1–T5) are described in parentheses: T1 and T2; control samples T3–T5; treated samples.
1Means of 3 biological replicates.
2% of disease reduction.
Effect of treatment conditions on oil palm seedlings’ vigor traits
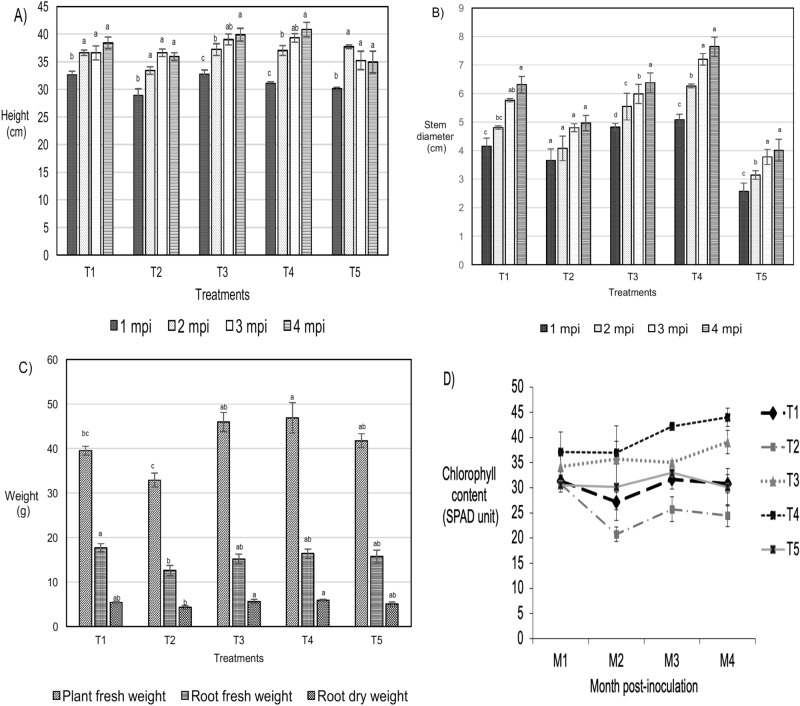
The plant height, stem diameter and chlorophyll content were evaluated at 1-to-4 month post-inoculation (mpi) whilst the plant fresh weight and root biomass was recorded at 4 mpi only. In general, the application of phenazine at both 1 mg/ml (T3) and 2 mg/ml (T4) on Ganoderma boninense inoculated oil palm seedlings improved the plant height, stem diameter, plant fresh weight, root fresh weight and root dry weight throughout the disease trial as compared to hexaconazole treated seedlings. The plant height for seedlings subjected to all treatments ranged at 28.9–40.8 cm and increased with the mpi. At 1, 2 and 3 mpi, seedlings treated with phenazine (T3 and T4) showed significantly the highest growth performance at 39.9 and 40.8 cm, respectively. At 4 mpi, the plant height of all treatments showed no significant difference.
Stem diameter increased throughout the disease trial in all treatments at various pattern. The stem diameter of seedlings inoculated + treated with 1 mg/ml and 2 ml/ml phenazine (T3 and T4) was higher than the non-inoculated (T1), inoculated + non-treated (T2) and inoculated + hexaconazole (T5) treated seedlings. The T4 seedlings showed the best performance consistently from 1 to 4 mpi with stem diameter ranging at 5–7.6 cm. The stem diameter of the T4 seedlings was the greatest while the inoculated + hexaconazole seedlings (T5) showed the least growth increment (2.6–4 cm).
All the inoculated + treated seedlings (T3, T4 and T5) showed a higher biomass (plant fresh weight, root fresh weight and root dry weight) in comparison to the inoculated + non-treated seedlings (T2). Plant fresh weight at 4 mpi was significantly highest in the inoculated + phenazine treated seedlings (T4) at 46.9 kg. Root fresh weight was highest in the non-inoculated seedlings (T1) at 17.7 kg whilst the root dry weight was significantly highest in the T4 seedlings. Among the treatments, seedlings inoculated + treated with 2 mg/ml of phenazine (T4) showed the best growth performance and the inoculated + hexaconazole treated seedlings (T5) was the poorest.
Chlorophyll content of the non-inoculated seedlings (T1) ranged from 27.2 to 31.6 throughout the disease trial. At 1 mpi, the chlorophyll content of the inoculated seedlings (T2) showed the least value at 30.7, followed by an apparent decline at 2 mpi (20.8) and slight increment in the subsequent sampling intervals (3 and 4 mpi). The results of T2 may suggest that disease development progressively reduced the chlorophyll content. For seedlings inoculated + treated with phenazine (T3 and T4), the chlorophyll content increased gradually with the sampling intervals: T3; 34.2–39.1 and T4; 37–44. The seedlings inoculated + treated with hexaconazole (T5) showed no apparent trend: decreased at 2 mpi (30.2) followed by an increase (33.0) at 3 mpi and a decrease (30.1) at 4 mpi (Fig. 2).
Figure 2.
Plant vigour assessment of oil palm seedlings under various treatments under a disease trial: T1; non-inoculated oil palm seedlings without application, T2; Ganoderma boninense inoculated oil palm seedlings without application, T3; G. boninense inoculated oil palm seedlings treated with 1 mg/mL of phenazine, T4; G. boninense inoculated oil palm seedlings treated with 2 mg/ml of phenazine, and T5; G. boninense inoculated oil palm seedlings treated with 1 mg/ml hexaconazole. Treatments were evaluated at monthly intervals from 1-to-5 month post-inoculation (mpi). (A) Height, (B) stem diameter, (C) weight and (D) chlorophyll content expressed in SPAD units. All values are indicated as average means of three biological replicates and error bar represents the standard error.
Histological analysis of the non-treated and phenazine treated root tissues
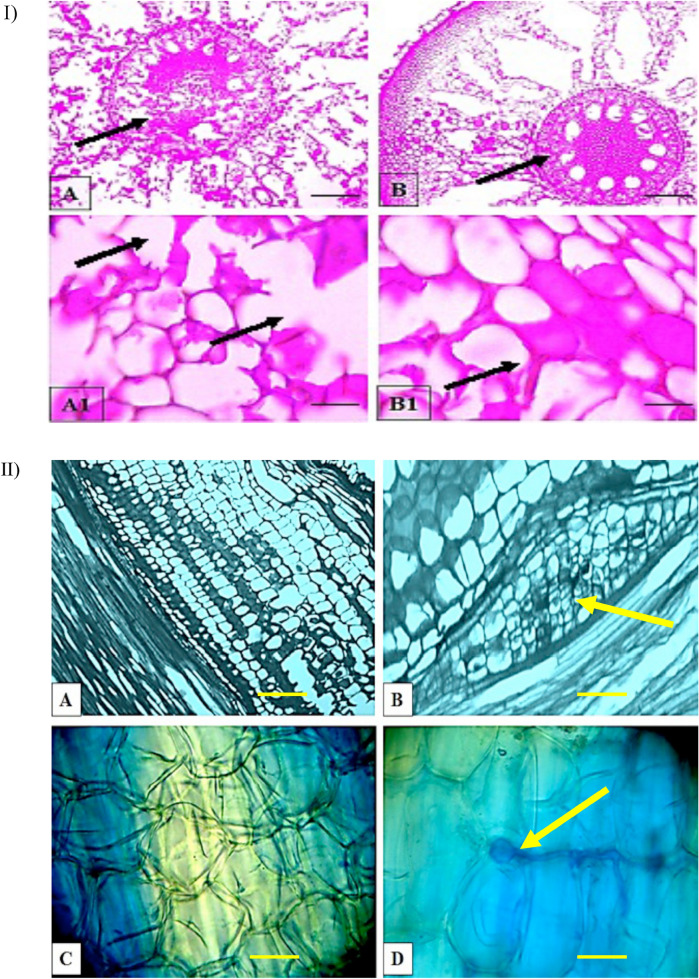
A histological analysis was conducted on root tissues obtained from the inoculated + non-treated and inoculated + phenazine treated oil palm seedlings (4 month post-inoculation). All cross-sections of the seedlings showed three distinct layers which include the epidermis (outermost), cortex (middle) and vascular bundle (inner-most). The inoculated + phenazine treated root tissues were found healthy in comparison to the inoculated + non-treated seedlings. The inoculated + non-treated seedlings showed clear formation of disintegrated parenchyma cells at the vascular bundle while the inoculated + treated seedlings had intact cells, free from any form of malformations (Fig. 3).
Figure 3.
Histological observation of control and infected (at 4 month post-inoculation) oil palm root tissues. (I) Horizontal sections (A) Infected root tissues showing degraded vascular bundle (black arrow) (× 10 magnification), (A1) disintegrated parenchyma cells (black arrow) ( × 40 magnification). (B) Phenazine treated root tissues showing an intact vascular bundle (black arrow) ( × 10 magnification) and (B1) parenchyma cells (black arrow) ( × 40 magnification). (II) Longitudinal sections. (A) Healthy root tissue ( × 10 magnification), (B) infected root tissue showing Ganoderma colonization evident by formation of bulb like structure ( × 10 magnification) (yellow arrow), (C) phenazine treated root tissue ( × 40 magnification), and (D) Appressorium (yellow arrow) penetrating into cortex region ( × 40 magnification). Scale bars are equivalent to 30 µm.
Phenazine application induces the expression of pathogenesis-related (PR) genes
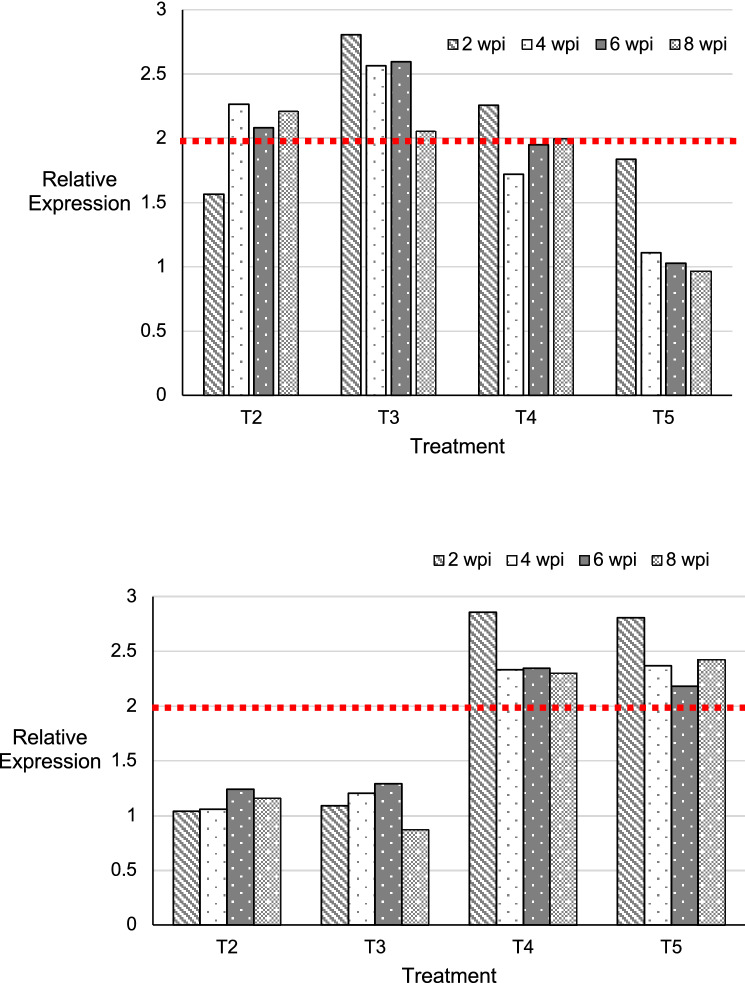
The expression of PR genes (chitinase and β-1,3 glucanase) were found to be up-regulated in oil palm root tissues with the presence of G. boninense and phenazine application throughout the following sampling period: 2, 4, 6, and 8 weeks post-inoculation (wpi). The PCR product of chitinase and β-1, 3 glucanase genes from oil palm seedlings were 820 bp and 419 bp, respectively (Fig. 4). For quantification of PR genes, the relative quantification of the corresponding to target PCR products was calculated against a housekeeping gene, the GADPH 109 bp. Since the identification of two PR gene products along with house-keeping gene product were done simultaneously in a PCR cocktail, formation of primer dimer was observed during the gel view (S3). In this study, a relative expression level of > 2 was assumed significant.
Figure 4.

Amplification of pathogenesis related genes from root tissues of oil palm seedlings: chitinase (i), β- 1,3 glucanase (ii) and a housekeeping GAPDH gene (iii). Red arrow indicate 500 bp. Each lane is labelled as following: M; 100 bp DNA ladder (marker), P; positive control comprised of PCR product of target gene at the following sizes: (i) 891 bp, (ii) 491 bp and (iii) 100 bp, N; negative control and S = samples at 4 weeks-post-inoculation S1; G. boninense inoculated + phenazine 1 mg/ml treated oil palm seedlings, S2; G. boninense inoculated + phenazine 2 mg/ml treated oil palm seedlings and S3; G. boninense inoculated + hexaconazole treated oil palm seedlings.
In the inoculated seedlings (T2), the expression level of chitinase was low at 2 wpi (1.56) before a gradual increase of > 2 in the subsequent sampling periods (4, 6 and 8 wpi). Among the treated seedling groups, only the inoculated + phenazine (1 mg/ml) treated seedlings (T3) showed a significant up-regulation of chitinase gene at all sampling intervals (2, 4, 6 and 8 wpi). The expression level was highest at 2 wpi (2.8) and decreased at 8 wpi (2.1). The inoculated + hexaconazole treated seedlings (T5) showed the lowest expression levels throughout the sampling periods. At 2 wpi, the expression level was 1.84 declined gradually to 1.11, 1.03 and 0.97 at 4, 6, 8 wpi, respectively (Fig. 5).
Figure 5.
Relative quantification of chitinase (top) and β-1,3 glucanase (bottom) gene in oil palm root tissues subjected to different treatment condition using RT-PCR method: T2; Ganoderma boninense inoculated oil palm seedlings, T3; G. boninense inoculated + phenazine 1 mg/ml treated oil palm seedlings, T4; G. boninense inoculated + phenazine 2 mg/ml treated oil palm seedlings and T5; G. boninense inoculated + hexaconazole 1 mg/ml treated oil palm seedlings. Bars with different patterns represent the following sampling intervals: 1, 2, 3 and 4 weeks post-inoculation (wpi). Red dotted line at relative expression > 2 indicates significant difference.
The expression of β-1,3 glucanase at 2, 4, 6 and 8 wpi was significant at > 2 in the inoculated + phenazine (2 mg/ml) treated seedlings (T4) and inoculated + hexaconazole treated seedlings (T5). The β-1,3 glucanase expression was highest at 2 wpi at 2.86 and 2.81 in T4 and T5, respectively. The expression pattern in the inoculated seedlings (T2) increased from 2-to-6 wpi followed by a decline at 8 wpi (1.15). Similar trend was observed in the inoculated + phenazine (1 mg/ml) treated seedlings (T3); expression pattern increased (1.09–1.30) from 2-to-6 wpi before a decline at 8 wpi (0.87) (Fig. 5).
Discussion
World production and consumption of oils and fats have been growing steadily over the decades; a 4% annual increase over the past 32 years24 with palm oil among them. The palm oil has been extensively marketed for the biofuel industry, forage feed and in the production of a wide range of chemical products such as the soaps and cosmetics. An oil palm tree grows well in a hot climate and has an average life span of 25–30 years. The tree is able to yield one tonne of oil on about 0.26 hectares of land; about 8–10 folds higher than soybean, sunflower and rapeseed. In the South-east Asian region, the oil palm industry shapes the social-economic development of the nation with an annual export revenue of 65 billion. The highly efficient oil-seed crop is affected by numerous diseases; basal stem rot (BSR), leaf spot disease, algae leaf spot, fruit rot, stem wet rot and charcoal base rot25. Amongst these field emerging diseases, the BSR of oil palms has gained enormous attention as significant yield losses are ongoing and reliable control strategies remain scarce. In Malaysia, the oil palm covers about 5.85 million hectares and represents 60% of the nation’s agricultural land. In a survey conducted throughout 1995–2017, the BSR disease incidence reported a gradual increase from 1.51% (1995) to 3.17% (2012) and to 7.4% (2017). The BSR disease incidence was affected by oil palm age, planting density and soil properties25,26.
The BSR of oil palms is caused by the basidiomycetous Ganoderma boninese, a hembitrophic white fungus which colonizes the host by biotrophic nutrition during the early stage infection and later evolves into a necrotroph as the disease progresses to cause host death19. The pathogen spreads the disease through root contact between healthy and infected tissue and by a vector species; Episcapha 4-maculata (Episcapha/tiger beetle) carries the G. boninense basidiospores in its mouthparts26. To date, BSR management tools include sanitation/cultural practices, application of fungicides, fumigants and biocontrol agents. Popularly employed fungicides applied via the hand-knock injector and trunk drenching methods are dazomet, hexaconazole, thiram, benomyl, triadimefon, triadimenol, tridemorph and tetraconazole24. Among these fungicidal treatments, the application of hexaconazole for G. boninense diseased palm trees has demonstrated the best efficiency as the infected palms could remain fertile upon a prolong use27,28. The hexaconazole is a member of the triazole group which carries the systemic demethylation inhibitors that acts selectively to impede growth of the fungal tissues; vegetative mycelium29,30. However, with a continuous application of fungicide, the notorious G. boninense evolves to acquire resistance. Alternative approaches are increasingly sought to replace the use of fungicides which are becoming inefficient over a long term.
Plant-beneficial pseudomonads are excellent biocontrol agents for the fungal pathogen inhibition and subsequent disease suppression. Pseudomonas spp. are able to promote plant growth and suppress soil-borne diseases in many economically important crops31. The antifungal phenazine compounds secreted by Pseudomonas spp. are pivotal determinants for the suppression of soil-borne diseases. A large number of Pseudomonas spp. such as the Pseudomonas chlororaphis32, Pseudomonas putida33, Pseudomonas fluorescens34,35 were shown to positively impact the suppression of root take-all disease of wheat and barley, chrysanthemum yellows phytoplasma infection of daisy and Fusarium wilt of radish. Similarly, the Pseudomonas aeruginosa 7NSK2 showed induced resistance to Botrytis cinerea infecting beans36. In a study conducted on oil palms, the positive effects of plant growth promoting rhizobacteria (PGPR) on plant overall growth and development has shown remarkable impact on the root morphology: significant increase in lateral root length, root hair number, shoot length and yield. The application of endophytic bacteria evident both an improved vegetative growth and suppression of BSR in oil palm seedlings37 In others, the bacterial endophytes P. aeruginosa strain UPMP3 independently increased oil palm seedlings’ growth38 and effectively suppressed the BSR incidence39,40. The P. aeruginosa UPMP3 suppresses G. boninense infection via two distinct ways. First, the fungal penetration into host is blocked through antibiosis establishment by P. aeruginosa found colonizing the root tissues. Later, the induction of defence-related metabolites in host aids the hydrolyses of the G. boninense cell wall and subsequently destroy the invading pathogen38,41.
Under a controlled environment, various factors collectively contribute to the success of P. aeruginosa UPMP3 colonization onto oil palm roots. In comparison to fungicides, the direct application of endophytes onto oil palm roots aimed for priming the plants is certainly a cost-effective measure because formulation procedure could be omitted virtually. However, the efficiency for these endophytes to establish their population and remain stable under adverse environmental conditions is highly uncertain. The P. aeruginosa UPMP3 thrives well in rhizosphere planes subjected to an optimal light, temperature, and soil physico-chemical properties, all of which that does not describe the real condition of any oil palm plantations. In Malaysia, endophytes-based commercial augmentative biological control agents for BSR are available. Nevertheless, the efficiency of these biocontrol agent, especially P. aeruginosa UPMP3-based is limited by the following constraints: (1) may pose risk to human health as P. aeruginosa is an opportunistic human pathogen, (2) the population establishment of P. aeruginosa in the soil rhizosphere fluctuates with soil properties, and (3) population size/fitness of the P. aeruginosa is affected by weather. With climate change followed by an unpredictable weather pattern, application of P. aeruginosa UPMP3 endophytes for disease management becomes particularly difficult since the bacterial population demands an optimal condition for growth, sustenance and subsequent mode of action.
In this study, phenazine extracted from P. aeruginosa UPMP3 played a dual role on the overall physiology of oil palm seedlings: (1) suppressed BSR development and, (2) promoted plant growth. Application of phenazine onto oil palm root tissues reduced the BSR disease incidence significantly throughout the disease trial. The seedlings treated with phenazine (T4: G. boninense inoculated + phenazine at 2 mg/ml) showed the lowest disease score, at AUDPC = 54.93 unit. Application of naturally synthesized phenazine was far more effective than fungicide (T5: G. boninense + hexaconazole) which scored AUDPC = 86.90 units. Phenazine was applied at two different concentrations on a monthly basis and our results suggest that BSR suppression is most effective at a concentration of 2 mg/ml. The expression of chitinase and β-1,3 glucanase, hydrolytic enzymes which act on fungal cell wall components such as the glucan, chitin and peptidoglycans is an indicator of invoked systemic resistance in the host tissues. In the untreated plants, the BSR disease symptoms become more severe as the expression levels of chitinases and β-1,3 glucanase (T2: positive control + G. boninense) decline. An increase in the chitinase and β-1,3 glucanase gene expression levels were probably a general response of host plant to fungal contact, irrespective of its nature; beneficial or pathogenic. In oil palm-G. boninense treated with phenazine, an up-regulated expression of chitinase and β-1,3 glucanase genes indicated induced oil palm defense responses. These results suggest that chitinases alone or in combination with β-1,3-glucanase, may play a significant role in limiting Ganoderma infection of oil palm root tissues. In the present study no external disease symptoms were observed up to 3 months of the antibiotic treatment, while at the end of 4 months the DI and DS percentage were found to be significantly lower than the positive control (plants treated with G. boninense) and the hexaconazole treated plants. The application of phenazine demonstrated bio-fertilizing ability as the stem diameter of the phenazine treated seedlings (T3 and T4) were significantly higher than the Ganoderma-inoculated alone seedlings (T2). Application of hexaconazole (T5) inhibited growth of the stem diameter as the T5 was significantly lower than T2. These findings may suggest that the ability of hexaconazole to supress basal stem rot disease is slightly traded off for the optimal growth of stem diameter.
Phenazine is an antibiotic metabolite which is produced by the microbial population after reaching a threshold level42,43. Further studies on the quorum-sensing phenomenon of P. aeruginosa UPMP3 along with genomic information on gene sequences with potentials for manipulation via genetic engineering is required for large-scale phenazine biosynthesis and production. To our knowledge, this is the first study to demonstrate that phenazine extract from P. aeruginosa can alter the transcriptional activity of PR-related genes in oil palm-Ganoderma boninense pathosystem. The phenazine extract holds promising potentials in controlling basal stem rot disease development and oil palm growth enhancement. Displaying both the bio-fertilizing and biocontrol capabilities, future strategies directed towards commercialization of phenazine extract is necessary for basal stem rot management. Additional studies are required to optimize the timing of applications while making sure that the application protocol is fully compatible with the standard oil palm agricultural practices.
Material and methods
Planting materials
A total of 120 3-month-old oil palm seedlings (Dura × Pisifera) were obtained from Sime Darby Seeds and Agricultural Services Sdn. Bhd. (Banting, Malaysia). The seedlings were transferred into polyvinyl bags (10 cm × 10 cm) loaded with approximately 3 kg of soil mixture. The soil mixture (soil: peat: sand at 3:2:1) purchased from Miba-Mansura Trading Sdn Bhd, Selangor, Malaysia was sterilized for 2 h at 100 °C and allowed to cool for 3 days before use. All transplanted seedlings were watered on a daily basis and supplementary fertilizers were not applied throughout the experiment.
Experimental design
The experiment was conducted in glasshouse 1C, Field 10, Universiti Putra Malaysia. The seedlings were arranged under a completely randomized design (CRD) with five different treatments represented as three biological replicates, each. Description on each treatment is given in Table 2.
Table 2.
Treatment conditions of the Ganoderma boninense-inoculated oil palm seedlings.
| Treatment | Description |
|---|---|
| T1 | Oil palm seedlings (negative control) |
| T2 | G. boninense inoculated seedlings (positive control) |
| T3 | G. boninense inoculated seedlings + phenazine (1 mg/ml) |
| T4 | G. boninense inoculated seedlings + phenazine (2 mg/ml) |
| T5 | G. boninense inoculated seedlings + hexaconazole (0.048 mg/ml) |
Each treatment is represented by three biological replicates.
Inoculum preparation and artificial infection of oil palm seedlings
Ganoderma boninense PER71 was grown on malt extract agar (MEA) at 28 °C. A 7-day-old mycelium culture was used for large-scale preparation of G. boninense inocula, hereafter termed Gano-wood block inocula. Freshly cut rubber (Hevea brasiliensis) wood blocks (RWBs) measuring 6 cm × 6 cm × 6 cm were dried at 80 °C for 3 days, washed briefly in tap water and soaked in distilled water for an overnight. The RWBs placed in polypropylene bags were autoclaved at 121 °C for 20 min. Approximately 100 ml of molten MEA was poured into each bag containing the RWB. The bags were closed by drawing its open end through a polyvinyl chloride (PVC) tubing (4 × 2 cm) and the remaining hole was plugged with cotton wool. The tubings of the polypropylene bags were covered with aluminium foil and the bags were autoclaved again for 30 min at 121 °C. Following a rest period for an overnight at room temperature, each bag of the RWB was inoculated with five plugs (5 mm diameter) of G. boninense mycelium and left for incubation in a dark chamber at 28 ± 2 °C. After 4 weeks, only Gano-wood block inocula that were fully covered with mycelia were selected for artificial infection of oil palm seedlings under the disease trial study (Fig. s1).
The oil palm seedlings were inoculated with Gano-wood block inocula as described by Khairuddin44. Briefly, oil palm seedlings were carefully excavated from the soil. The bole of the oil palm seedling was positioned directly on the Gano-wood block inoculum. The root tissues were dispersed around the sides of the Gano-wood block. Finally, the oil palm seedling seated onto the Gano-wood block was firmly re-planted into the soil (Fig. s2). At 2 week post-inoculation, each oil palm seedling from the relevant treatment groups, received 20 ml of phenazine and hexaconazole as described in Table 2 for three subsequent months. All applications were performed according to the soil drenching method (Fig. s3).
Large-scale isolation of phenazine from Pseudomonas auroeginosa UPMP3
The phenazine was isolated from P. aeruginosa UPMP3 population according to Chang and Blackwood’s method7 with slight modifications. The bacterial (approximately 108 cells/ml) population was inoculated onto King’s B broth and incubated at 30 °C with shaking at 150 rpm for 2 days. Following the bacterial exponential growth, cells were centrifuged at 3,500 rpm for 7 min. The resultant pellet was suspended into pigment production medium broth (5 ml) and re-incubated at 30 °C with shaking at 150 rpm for 4 days. The culture was adjusted to pH 2.0 by using concentrated HCl before extraction with an equal volume of benzene (analytical grade). The benzene layer was evaporated at 40 °C and the resultant residue was suspended into 1 ml of absolute methanol. To further confirm and quantify the isolated phenazine, a HPLC analysis was performed on a HPLC system (Waters) according to Watson et al. 2005 with the following modifications: the HPLC system was coupled to a 150 × 4.6 mm Ultracarb 5 µm ODS (30) column and solvent A (water: trifluoroacetic acid, 100:0.04, v/v) and solvent B (acetonitrile: water: trifluoroacetic acid, 90:10:0.04, v/v/v) were applied throughout the run at a sample injection volume of 10 μl. Solvent B was set as the mobile phase and the following condition was applied: solvent A was maintained for 15 min, followed by 90% solvent A and 10% solvent B solution for another 10 min. Next, a linear gradient of 70% solvent A and 30% solvent B solution was applied for 15 min before changing it to 64% solvent A and 34% solvent B solution, maintained till the end of the run (total time: 65 min). Phenazine was identified and quantified using authentic antibiotic standards prepared at various concentration. All samples were analysed thrice and concentration of phenazine antibiotics in each sample were expressed as μg/ml.
Disease assessment: plant vigour traits, disease incidence and disease severity
To determine the effect of the treatments on plant growth and disease suppression, a total of four sampling points was fixed throughout the disease trial; 1, 2, 3, and 4 month post-inoculation (mpi). At each sampling point, the following parameters were recorded: plant height, stem diameter, plant fresh weight, and root fresh weight, dry weight. Height was measured from one cm above the soil level (ground) to the tip of the leaves using a tape. The stem diameter was measured using a vernier digital calliper. Chlorophyll content was recorded at three random positions of a fully developed leaf in each seedling using a chlorophyll meter (SPAD-502-Minlota Co. Ltd. Japan). Plant fresh weight and root fresh weight were measured using a weighing scale while the dry weight was quantified similarly after drying at 50 °C for 72 h.
BSR disease development was recorded at each mpi according to a modified disease assessment scale by Breton et al.45. Symptoms that appear on the foliage were scored as following: 0, healthy plants show no visible symptoms; (1) yellowing of at least > 2 lower leaves and/or rhizomorph formation at the base of the bole; (2) necrosis of at least > 2 lower leaves with button-like sporophore at the base of the bole; (3) > 50% necrosis of leaves and sporophore at the base of the bole and (4) seedling complete necrosis and sporophore at the base of the bole. Symptoms on the internal root tissue were scored as following: (0) Healthy seedlings with no visible sign of internal rot; (1) 20% rotting of tissues; (2) 20–50% rotting of tissues; (3) > 50% rotting of tissues; (4) > 90% rotting of tissues. Foliar symptom scale was used to compute disease severity (external) according to Liu46 whereas the root internal symptoms scale was used to calculate internal disease severity according to Breton45 with slight modifications. For disease incidence (DI) % computation, the infected seedlings were described as following: chlorosis and/or necrosis of leaves, with or without production of sporophore and/or fruiting bodies. The DI% values of all the treatment groups at each mpi were computed to generate a disease progression curve according to Campbell and Madden47.
Histological analysis compares oil palm root tissues under different treatments
To understand the effect of the different treatments (phenazine and hexaconazole) on fungal pathogen colonization, root samples were collected at 4 mpi and prepared for histopathological views. Each root sample was fixed in FAA solution [70% ethanol: 5% glacial acetic acid: 5% formaldehyde at the ratio of 18: 1: 1 (v/v/v)] for 24 h at room temperature. Fixed tissues were dehydrated with a series of ethanol and butanol solutions and embedded in paraffin. Serial sections (10 μm) were performed using a rotary microtome (Leica RM2235, Germany) with a steel knife. The sections were floated in water drops, dried on a hot plate (37 °C), stained with acid fuchsin (0.1%), then counter stained with toluidine blue (0.05%). All steps used which include fixation, dehydration, embedding, sectioning staining and mounting of the root samples were carried out prior to observation under the light microscope (Motic BA300, China)48.
Quantification of pathogenesis-related (PR) genes by semi-quantitative reverse transcriptase PCR
Sampling time points for the quantification of PR-gene products were set as followings: 2, 4, 6, and 8 week post-inoculation (wpi). A total of three biological replicates were used to represent each treatment. At each sampling time point, the root tissues collected were washed thoroughly and ground into fine powder in liquid nitrogen. Total RNA was isolated according to the manufacturer’s protocol (Total RNA Mini Kit (Plant), Geneaid Biotech Ltd., Taiwan). The concentration and quality of the total RNA and subsequent cDNA yield was determined by Nanodrop 2000 cc (Thermo Scientific, USA). The integrity of the total RNA was evaluated using agarose gel electrophoresis. High quality total RNA samples were treated with DNase 1 (Fermentas, UAB, Lithuania) according to the manufacturer’s guideline before the first-strand cDNA synthesis. First-strand cDNA was synthesized using the RevertAid First strand cDNA synthesis kit (Thermo Scientific, USA) following the manufacture’s instruction.
The amplifications of chitinase, β-1, 3 glucanase and a housekeeping [glyceraldehyde phosphate dehydrogenase (GAPDH)] genes were performed in a PCR cocktail described as following: 2X PCR Master Mix (Fermentase, UAB, Lithuania), 100 ng cDNA, 1X PCR Master Mix, 1 μM primer oligonucleotides and nuclease free water. The primer pairs were designed according to Sathyapriya et al.44. Amplification was performed in a thermocycler (T3 Thermocycler, Biometra, Germany) with the following conditions: pre-denaturation for 2 min at 95 °C, followed by 40 cycles of denaturation (30 s) at 95 °C, annealing (30 s) at 56 °C, elongation for 1 min at 72 °C and final extension for 10 min at 72 °C. Target gene sequences and their corresponding amplicon products (bands) were viewed by a gel documentation system (Gel Doc 2000, BIO-RAD) after an electrophoresis at the following condition: 2% (w/v) agarose gel immersed in 1X TAE buffer subjected to 65 V for 45 min runtime. The intensity of each band was computationally scored by Gene Tool Software version 1.0 (Quality One 1-D Analysis Software, Version 4.6.9, BIO-RAD) (Fig. s4). The relative expression of each target gene was calculated as shown below:
Digital expression value of house-keeping gene = Digital band intensity value of house-keeping gene/expected amplicon length.
Digital expression value of target gene of interest (goi) = Digital band intensity value of target gene/expected amplicon length/Digital expression value of house-keeping gene.
Relative expression ratio of target gene = Digital expression value of infected (goi) plant/Digital expression value of non-infected control (goi) plant.
Statistical analysis
For normalization of the raw data, the disease incidence and disease severity values were subjected to arcsine transformation whereas the plant vigour data were subjected to log transformations. A one-way ANOVA was performed and any absence/presence of significant differences between the treatments for the different parameters in this study were determined by Least Significant Difference (LSD) at p < 0.05 using the SAS 9.3 statistical package.
Supplementary information
Acknowledgements
The authors would like to thank Ministry of Higher Education (MOHE) Malaysia for providing the Fundamental Research Grant Scheme (FRGS) to carry out this study.
Author contributions
W.P. and W.M.Y. conceived the study, W.P., N.G. and M.R. conducted the experiment; W.P. and N.G. analysed the data and wrote the manuscript, R.O. and H.J. coordinated and revised the research flow, W.M.Y. secured funding. All authors have approved the final content of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Waheeda Parvin, Email: waheeda_bfri@yahoo.com.
Mui-Yun Wong, Email: muiyun@upm.edu.my.
Supplementary information
is available for this paper at 10.1038/s41598-020-72156-7.
References
- 1.Pierson LS, Pierson EA. Metabolism and function of phenazines in bacteria: impacts on the behavior of bacteria in the environment and biotechnological processes. Appl. Microbiol. Biotechnol. 2010;86:1659–1670. doi: 10.1007/s00253-010-2509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell JF, Vargas JM, Jr, Nair MG, Detweiler AR, Chandra A. Management of dollar spot on creeping bentgrass with metabolites of Pseudomonas aureofaciens (TX-1) Plant Dis. 2000;84:19–24. doi: 10.1094/PDIS.2000.84.1.19. [DOI] [PubMed] [Google Scholar]
- 3.Tambong JT, Hofte M. Phenazines are involved in biocontrol of Pythiummyriotylum on cocoyam by Pseudomonas aeruginosa PNA1. Eur. J. Plant Pathol. 2001;107:511–521. doi: 10.1023/A:1011274321759. [DOI] [Google Scholar]
- 4.Thomas FC, Woeng CA, Guido VB, Ben JJL. Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol. 2003;157:503–523. doi: 10.1046/j.1469-8137.2003.00686.x. [DOI] [PubMed] [Google Scholar]
- 5.Zongwang M, Gia KHH, Marc O, Monica H. Role of phenazines and cyclic lipopeptides produced by pseudomonas sp. CMR12a in induced systemic resistance on rice and bean. Environ. Microbiol. Rep. 2016;8:896–904. doi: 10.1111/1758-2229.12454. [DOI] [PubMed] [Google Scholar]
- 6.Price-Whelan A, Dietrich LEP, Newman DK. Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nat. Chem. Biol. 2006;2:71–77. doi: 10.1038/nchembio764. [DOI] [PubMed] [Google Scholar]
- 7.Chang PC, Blackwood AC. Simultaneous production of three phenazine pigments by Pseudomonas aeruginosa Mac 436. Can. J. Microbiol. 1969;15:439–444. doi: 10.1139/m69-077. [DOI] [PubMed] [Google Scholar]
- 8.Mavrodi DV, et al. Diversity and evolution of the phenazine biosynthesis pathway. Appl. Environ. Microbiol. 2010;76:866–879. doi: 10.1128/AEM.02009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mentel M, et al. Of two make one: the biosynthesis of phenazines. Chem. Biol. Chem. 2009;10:2295–2304. doi: 10.1002/cbic.200900323. [DOI] [PubMed] [Google Scholar]
- 10.Mavrodi DV, et al. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J. Bacteriol. 2001;183:6454–6465. doi: 10.1128/JB.183.21.6454-6465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olorunleke FE, Hua GKH, Kieu NP, Ma Z, Hofte M. Interplay between orfamides, sessilins and phenazines in the control of Rhizoctonia diseases by Pseudomonas sp. CMR12a. Environ. Microbiol. Rep. 2015;7:774–781. doi: 10.1111/1758-2229.12310. [DOI] [PubMed] [Google Scholar]
- 12.Parvin W, Othman R, Jaafar H, Wong MY. Detection of phenazines from UPMP3 strain of Pseudomonas aeruginosa and its antagonistic effects against Ganoderma boninense. Int. J. Agric. Biol. 2016;18:483–488. doi: 10.17957/IJAB/15.0111. [DOI] [Google Scholar]
- 13.Pornsuriya C, et al. A survey of diseases and orders in oil palms of Southern Thailand. Plant Pathol. J. 2013;12(4):169–175. doi: 10.3923/ppj.2013.169.175. [DOI] [Google Scholar]
- 14.Idris, A. S., Mior, M. H. A. Z., Maizatul, S. M. & Kushairi, A. Survey on status of oil palm in Malysia 2009–2010. In Proceedings of the 1993 PORIM international palm oil congress fortifying and energizing world. Palm Oil Research Institute of Malaysia, Kuala Lumpur, Malaysia (2011).
- 15.Susanto, A. Basal stem rot in Indonesia. Biology, economic importance, epidemiology, detection and control. In Proceedings of international workshop on awareness, detection and control of oil palm devastating diseases. Kuala Lumpur Convention Center, Kuala Lumpur, Malaysia (2009).
- 16.Govender N, Wong MY. Detection of oil palm root penetration by Agrobacterium-mediated transformed Ganoderma boninense, expressing green fluorescent protein. Phytopathology. 2017 doi: 10.1094/PHYTO-02-16-0062-R. [DOI] [PubMed] [Google Scholar]
- 17.Turner P. Oil Palm Diseases and Disorders. Kuala Lumpur: Oxford University Press; 1981. [Google Scholar]
- 18.Chung GF. Management of Ganoderma diseases in oil palm plantations. Planter. 2011;87:325–339. [Google Scholar]
- 19.Govender NT, Seman IA, Mahmood M, Wong MY. The phenylpropanoid pathway and lignin in defence against Ganoderma boninense colonized root tissues in oil palm (Elaeis guineensis Jacq.) Front. Plant Sci. 2017 doi: 10.3389/fpls.2017.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antoniw JF, Ritter CE, Pierpoint WS, Van Loon LC. Comparison of three pathogenesis-related proteins from plants of two cultivars of tobacco infected with TMV. J. Gen. Virol. 1980;47:79–87. doi: 10.1099/0022-1317-47-1-79. [DOI] [Google Scholar]
- 21.Gupta P, Ravi I, Sharma V. Induction of b-1,3-glucanase and chitinase activity in the defense response of Eruca sativa plants against the fungal pathogen Alternaria brassicicola. J. Plant Interact. 2013;8:155–161. doi: 10.1080/17429145.2012.679705. [DOI] [Google Scholar]
- 22.Idris, A. S., Arifurrahman R. & Kushairi, A. Hexaconazole as a preventive treatment for managing Ganoderma in oil palm. MPOB Information Series. ISSN 1511–7871 (2010).
- 23.Martin H. Induced systemic resistance (lSR) against pathogens—a promising field for ecological research. Perspect. Plant Ecol. Evol. Syst. 2001;4:65–79. doi: 10.1078/1433-8319-00015. [DOI] [Google Scholar]
- 24.Oil World Annual 2019. https://www.oilworld.biz/. Retrieved January 2020.
- 25.Idris, A. S., Rusli, M. H., Sundram, S., Madihah, A. Z. & Izzuddin, M. A. Breakthrough of Ganoderma research. In Malaysian Society of Applied Biology. Symposium on Ganoderma Research 2019. Adversity and Breakthrough Researches in Ganoderma. Universiti Kebangsaan Malaysia (2019).
- 26.Idris, A. S. & Norman, K. Control and management of Ganoderma disease in peat areas. In MPOB-SOPPOA Workshop Sibu, Sarawak, Malaysia (2016).
- 27.Idris A, Arifurrahman R, Kushairi A. Hexaconazole as a preventive treatment for managing Ganoderma in oil palm. MPOB TS Inf. Ser. 2010;75:533–534. [Google Scholar]
- 28.Idris, A., Arifurrahman, R. & Kushairi D. An evaluation of hexaconazole for controlling Ganoderma basal stem rot of oil palm in the field as a preventive treatment. PIPOC (Agric. Biotechnol. Sustain.) Malaysia (2009).
- 29.Khalfallah S, Menkissoglu-Spiroudi U, Constantinidou HA. Dissipation study of the fungicide tetraconazole in greenhouse-grown cucumbers. J. Agric. Food Chem. 1998;46:1614–1617. doi: 10.1021/jf9706540. [DOI] [Google Scholar]
- 30.Russell PA. Century of fungicide evolution. J. Agric. Sci. 2005;143:11–25. doi: 10.1017/S0021859605004971. [DOI] [Google Scholar]
- 31.Kohl J, Kolnaar R, Ravensberg WJ. Mode of action of microbiological control agents against plant diseases: relevance beyond efficacy. Front. Plant Sci. 2019;10:845. doi: 10.3389/fpls.2019.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu JM, Wang D, Pierson LS, III, Pierson EA. Effect of producing different phenazines on bacterial fitness and biological control in Pseudomonas chlororaphis. Plant Pathol. J. 2018;34:44–58. doi: 10.5423/PPJ.FT.12.2017.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gamalero E, et al. Effects of Pseudomonas putida S1Pf1Rif against chrysanthemum yellows phytoplasma infection. Phytopathology. 2010;100:xx. doi: 10.1094/PHYTO-100-8-0805. [DOI] [PubMed] [Google Scholar]
- 34.Leeman M, et al. Induction of systemic resistance against Fusarium wilt of radish by lipopolys lipopolysaccharides of Pseudomonas fluorescens. Phytopathology. 1995;85:1021–1027. doi: 10.1094/Phyto-85-1021. [DOI] [Google Scholar]
- 35.Weller DM, et al. Role of 2,4-diacetylphloroglucinol-producing fluorescent Pseudomonas spp. in the defense of plant roots. Plant Biol. 2007;9:4–20. doi: 10.1055/s-2006-924473. [DOI] [PubMed] [Google Scholar]
- 36.Meyer GD, Höfte M. Salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 induces resistance to leaf infection by Botrytis cinerea on bean. Phytopathology. 1997;87(6):588–593. doi: 10.1094/PHYTO.1997.87.6.588. [DOI] [PubMed] [Google Scholar]
- 37.Shamala, S. Assessment of Ganoderma infection in oil palm (Elaeis guineensis Jacq.) by pre-inoculation of Arbuscular Mycorrhiza Fungi and endophytic bacteria. Ph.D. thesis, Universiti Putra Malaysia (2012).
- 38.Sundram S, Meon S, Seman IA, Othman R. Application of arbuscular mycorrhizal fungi with Psedomonas aeruginosa UPMP3 reduces the development of Ganoderma basal stem rot disease in oil palm seedlings. Mycorrhiza. 2014;25(5):387–397. doi: 10.1007/s00572-014-0620-5. [DOI] [PubMed] [Google Scholar]
- 39.Zaiton S, Sariah M, Zainal-Abidin MA. Isolation and characterization of microbial endophytes from oil palm roots: Implication as biocontrol agents against Ganoderma. Planter. 2006;82:587–597. [Google Scholar]
- 40.Sapak Z, Meon S, Ahmad ZAM. Effect of endophytic bacteria on growth and suppression of Ganoderma infection in oil palm. Int. J. Agric. Biol. 2008;10:127–132. [Google Scholar]
- 41.Sathyapriya HM, Sariah A, Siti N, Wong MY. Root colonisation of Pseudomonas aeruginosa strain UPMP3 and induction of defence-related genes in oil palm (Elaeis guineensis) Ann. Appl. Biol. 2012;160:137–214. doi: 10.1111/j.1744-7348.2011.00525.x. [DOI] [Google Scholar]
- 42.Zaiton S, Sariah M, Zainal-Abidin MA. Effect of endophytic bacteria on growth and suppression of Ganoderma infection in oil palm. Int. J. Agric. Biol. 2008;10:127–132. [Google Scholar]
- 43.Raaijmakers JM, Mazzola M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 2012;50:403–424. doi: 10.1146/annurev-phyto-081211-172908. [DOI] [PubMed] [Google Scholar]
- 44.Khairuddin, H. Basal stem rot of oil palm: incidence, etiology and control. Master of Agriculture Science thesis, Universiti Pertanian Malaysia, Selangor, Malaysia (1990).
- 45.Breton, F., Hasan, Y., Hariadi, Y., Lubis, Z. & De Franqueville, H. Characterization of parameters for the development of an early screening test for Basal Stem Rot tolerance in oil palm progenies. In Proceedings of Agriculture, Biotechnology and Sustainability Conference. Technological Breakthroughs and Commercialization—The Way Forward, PIPOC 2005 MPOB International Palm Oil Congress, 25–29 September 2005. Kuala Lumpur, Malaysia (2005).
- 46.Liu L, Kloepper JW, Tuzun S. Induction of systemic resistance in cucumber against bacterial angular leaf spot by plant growth-promoting rhizobacteria. J. Phytopathol. 1995;85:843–847. doi: 10.1094/Phyto-85-843. [DOI] [Google Scholar]
- 47.Campbell CL, Madden LV. Introduction to Plant Disease Epidemiology. Hoboken: Wiley; 1990. [Google Scholar]
- 48.Delly JG. Teaching Microscopy. Chicago: Microscope Publications Ltd; 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.