Key Points
Question
What is the effect of time-restricted eating on weight loss and metabolic health in patients with overweight and obesity?
Findings
In this prospective randomized clinical trial that included 116 adults with overweight or obesity, time-restricted eating was associated with a modest decrease (1.17%) in weight that was not significantly different from the decrease in the control group (0.75%).
Meaning
Time-restricted eating did not confer weight loss or cardiometabolic benefits in this study.
This randomized clinical trial examines the effect of 16:8-hour time-restricted eating on weight loss and metabolic risk markers.
Abstract
Importance
The efficacy and safety of time-restricted eating have not been explored in large randomized clinical trials.
Objective
To determine the effect of 16:8-hour time-restricted eating on weight loss and metabolic risk markers.
Interventions
Participants were randomized such that the consistent meal timing (CMT) group was instructed to eat 3 structured meals per day, and the time-restricted eating (TRE) group was instructed to eat ad libitum from 12:00 pm until 8:00 pm and completely abstain from caloric intake from 8:00 pm until 12:00 pm the following day.
Design, Setting, and Participants
This 12-week randomized clinical trial including men and women aged 18 to 64 years with a body mass index (BMI, calculated as weight in kilograms divided by height in meters squared) of 27 to 43 was conducted on a custom mobile study application. Participants received a Bluetooth scale. Participants lived anywhere in the United States, with a subset of 50 participants living near San Francisco, California, who underwent in-person testing.
Main Outcomes and Measures
The primary outcome was weight loss. Secondary outcomes from the in-person cohort included changes in weight, fat mass, lean mass, fasting insulin, fasting glucose, hemoglobin A1c levels, estimated energy intake, total energy expenditure, and resting energy expenditure.
Results
Overall, 116 participants (mean [SD] age, 46.5 [10.5] years; 70 [60.3%] men) were included in the study. There was a significant decrease in weight in the TRE (−0.94 kg; 95% CI, −1.68 to −0.20; P = .01), but no significant change in the CMT group (−0.68 kg; 95% CI, -1.41 to 0.05, P = .07) or between groups (−0.26 kg; 95% CI, −1.30 to 0.78; P = .63). In the in-person cohort (n = 25 TRE, n = 25 CMT), there was a significant within-group decrease in weight in the TRE group (−1.70 kg; 95% CI, −2.56 to −0.83; P < .001). There was also a significant difference in appendicular lean mass index between groups (−0.16 kg/m2; 95% CI, −0.27 to −0.05; P = .005). There were no significant changes in any of the other secondary outcomes within or between groups. There were no differences in estimated energy intake between groups.
Conclusions and Relevance
Time-restricted eating, in the absence of other interventions, is not more effective in weight loss than eating throughout the day.
Trial Registration
ClinicalTrials.gov Identifiers: NCT03393195 and NCT03637855
Introduction
The prevalence of overweight (body mass index [BMI, calculated as weight in kilograms divided by height in meters squared], 25 to 30) and obesity (BMI greater than 30) has increased dramatically recently1 and is associated with increased risk for chronic diseases.2 Even modest weight reduction can improve cardiovascular disease risk.3 However, long-term adherence to lifestyle changes is difficult. Therefore, it is important to find novel lifestyle-modification interventions that are (1) effective in reducing weight and (2) accessible and straightforward to enhance adherence.
Intermittent fasting (IF) has gained attention as a simple weight loss method. Intermittent fasting refers to eating windows separated by defined periods of fasting (>12 hours and up to 48 hours, or more). Most of the reported benefits of IF are either untested or undertested in humans.4 Time-restricted eating (TRE) is a specific IF protocol involving consistent fasting and eating periods within a 24-hour cycle.
Time restricted feeding (TRF) prevents weight gain in mice challenged with an isocaloric high-fat diet (HFD)5 and reduces weight and metabolic outcomes in already obese mice.6 Weight loss without a decrease in calorie intake suggests that TRF could affect energy expenditure to achieve a negative calorie balance.
Prior small studies in humans with overweight or obesity demonstrate that TRE can result in reduced calorie intake and is associated with a decrease in body weight and/or fat mass.7,8,9,10 We conducted a randomized clinical trial (RCT) designed to determine the effect of TRE on weight and comprehensive metabolic outcomes in overweight and obese patients. We hypothesized that 8-hour TRE prescribed to individuals with overweight and obesity would lead to weight loss and improvements in metabolic markers compared with individuals following a standard 3-meals-per-day diet (consistent meal timing [CMT]).
Methods
Experimental Model and Participant Details
This study was conducted with approvals from the institutional review board at the University of California, San Francisco (UCSF) and the University of Hawai’i Cancer Center (UHCC). The trial protocol is available in Supplement 1. The clinical trial was registered on ClinicalTrials.gov (NCT03393195).
Participants were recruited between August 2018 and June 2019 and data collection was completed in October 2019. Overall, 141 participants were enrolled in the study and were randomized. We randomized 25 participants for whom we never received any data. Data were collected from 116 participants; and 105 completed the 12-week protocol. The study was conducted on a custom mobile study application (app) on the Eureka Research Platform. Participants received study surveys through the study app. Participants were given a bluetooth weight scale to use daily, which was connected through the study app. Participants were randomized to 1 of 2 interventions. The study intervention only included recommendations to the timing of food intake (no recommendation for calorie and macronutrient intake or physical activity), and participants received daily reminders about their eating windows through the app. The CMT group was instructed to eat 3 structured meals per day. Snacking between meals was permitted. The TRE group was instructed to eat ad libitum from 12:00 pm until 8:00 pm and completely abstain from caloric intake from 8:00 pm until 12:00 pm the following day (16 hours fast:8 hours eat). Only noncaloric beverages were permitted outside of the eating widow. Participants provided consent through the app, and received a $50 Visa gift card for participating in the study.
Weight Measurements
All participants received an iHealth Lite Bluetooth scale (Model HS4S) to use at home. Participant accounts were linked to the Eureka Research platform. Participants were instructed to use the scale daily in the morning before eating or drinking and prior to structured physical activity.
In-Person Metabolic Testing
Participants who lived within 60 miles of UCSF were eligible to undergo extensive in-person metabolic testing at the UCSF Clinical Research Center and the UCSF Body Composition Laboratory as detailed by Ng et al.11 Enrollment was capped at 50 participants, and 50 participants opted into the in-person testing. A total of 46 participants completed all 4 in-person visits.
Statistical Analysis
The statistical analysis plan is available in Supplement 2. The primary outcome was change in weight since baseline, measured daily via iHealth scales, in the overall cohort of 116 participants. To estimate the intention-to-treat effect of treatment assignment, we used a linear mixed model with fixed effects for treatment assignment, days since baseline, and their interaction, and random effects for participant and day, with unstructured covariance matrix, accommodating any nonlinearity in the trajectories using 3-knot cubic splines. The treatment effect was estimated by the fitted between-group difference at day 90, net of any baseline difference. In sensitivity analyses, we repeated the analysis after Winsorizing outliers, which was defined as points more than 1.5 times the interquartile range below the 25th or above the 75th percentile of the overall distribution. No adjustments were made to P values or confidence intervals for multiple comparisons for the primary outcome.
Results
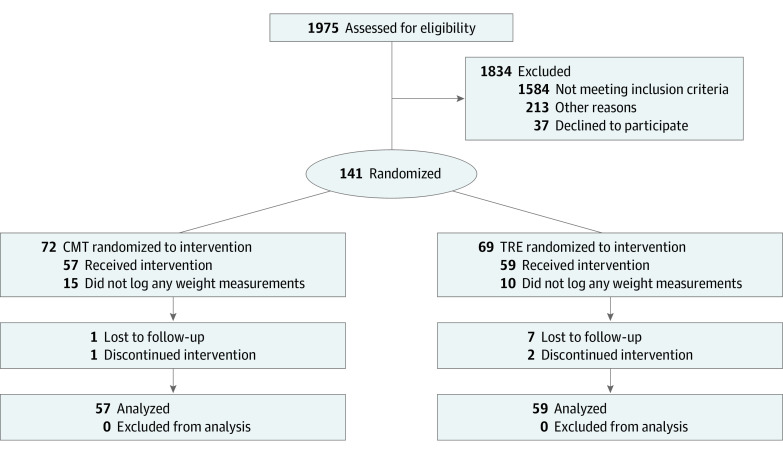
Of the 141 participants who were randomized to 1 of the 2 interventions, 105 (74.5%) completed the entire 12-week intervention (Figure 1). Of the 36 randomized participants who did not complete the study, 25 never recorded weight measurements (TRE n = 10, CMT n = 15), 8 were lost to follow-up (TRE n = 7, CMT n = 1), and 3 discontinued intervention (TRE n = 2, CMT n = 1). Participants had a mean (SD) age of 46.5 (10.5) years and a mean (SD) weight of 99.2 (16.0) kg (Table 1).
Figure 1. CONSORT Flow Diagram.
CMT indicates consistent meal timing group; TRE, time-restricted eating group. CONSORT flow diagram describing process of participant recruitment, enrollment, randomization, and data analysis. Participants were excluded from participating if they (1) were older than 64 years (n = 5), (2) had a body mass index (calculated as weight in kilograms divided by height in meters squared) less than 27 (n = 348) or greater than 43 (n = 72), (3) did not regularly consume breakfast (n = 566), (4) were unwilling or unable to skip breakfast (n = 761), (5) had a current or past cancer diagnosis (n = 21), (6) were breastfeeding, pregnant, or planned to be pregnant within 6 months (n = 21), (7) had current diagnosis of type 1 or type 2 diabetes mellitus (n = 177), (8) were taking glucose-lowering drugs (n = 133) or weight loss pills (n = 116), (9) had a history of gastric bypass or any weight-loss surgery (n = 66), (10) had a weight fluctuation of more than 15% in past 5 years (n = 467), (11) had a history of anorexia or bulimia (n = 39), (12) frequently traveled across time zones (n = 99) or worked unusual work hours (n = 182), or (13) were unable to fast for prolonged periods (n = 168).
Table 1. Baseline Characteristics.
| Characteristic | No. (%) | ||
|---|---|---|---|
| Total | CMT | TRE | |
| Total cohort | |||
| No. | 116 | 57 | 59 |
| Age, mean (SD), y | 46.5 (10.5) | 46.1 (10.3) | 46.8 (10.8) |
| Female | 46 (39.7) | 22 (38.6) | 24 (40.7) |
| Male | 70 (60.3) | 35 (61.4) | 35 (59.3) |
| Weight, mean (SD), kg | 99.2 (16.0) | 99.1 (15.1) | 99.3 (16.9) |
| BMI, mean (SD) | 32.7 (4.2) | 32.6 (3.4) | 32.9 (4.9) |
| In-person cohort | |||
| No. | 50 | 25 | 25 |
| Age, mean (SD), y | 43.8 (11.2) | 44.4 (10.7) | 43.3(11.8) |
| Female | 22 (44.0) | 10 (40.0) | 12 (48.0) |
| Male | 28 (56.0) | 15 (60.0) | 13 (52.0) |
| Black | 2 (4.0) | 0 | 2 (8.0) |
| White | 25 (50) | 16 (64.0) | 9 (36.0) |
| Latinx | 7 (14.0) | 3 (12.9) | 4 (16.0) |
| Asian | 12 (24.0) | 5 (20.0) | 7 (28.0) |
| Other/multi | 4 (8.0) | 1 (4.0) | 3 (12.0) |
| Weight, mean (SD), kg | 92.8 (14.2) | 93.0 (13.3) | 92.6 (15.2) |
| BMI, mean (SD), | 31.4 (4.0) | 31.3 (3.5) | 31.5 (4.5) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CMT, consistent meal timing group; TRE, time-restricted eating group.
Self-reported adherence to the diets was 1002 of 1088 (92.1%) in the CMT group (did not miss any meals) and 1128 of 1351 (83.50%) in the TRE group (ate only within the 8-hour window) (Figure 2A).
Figure 2. Adherence and Weight Change in the Total Cohort.
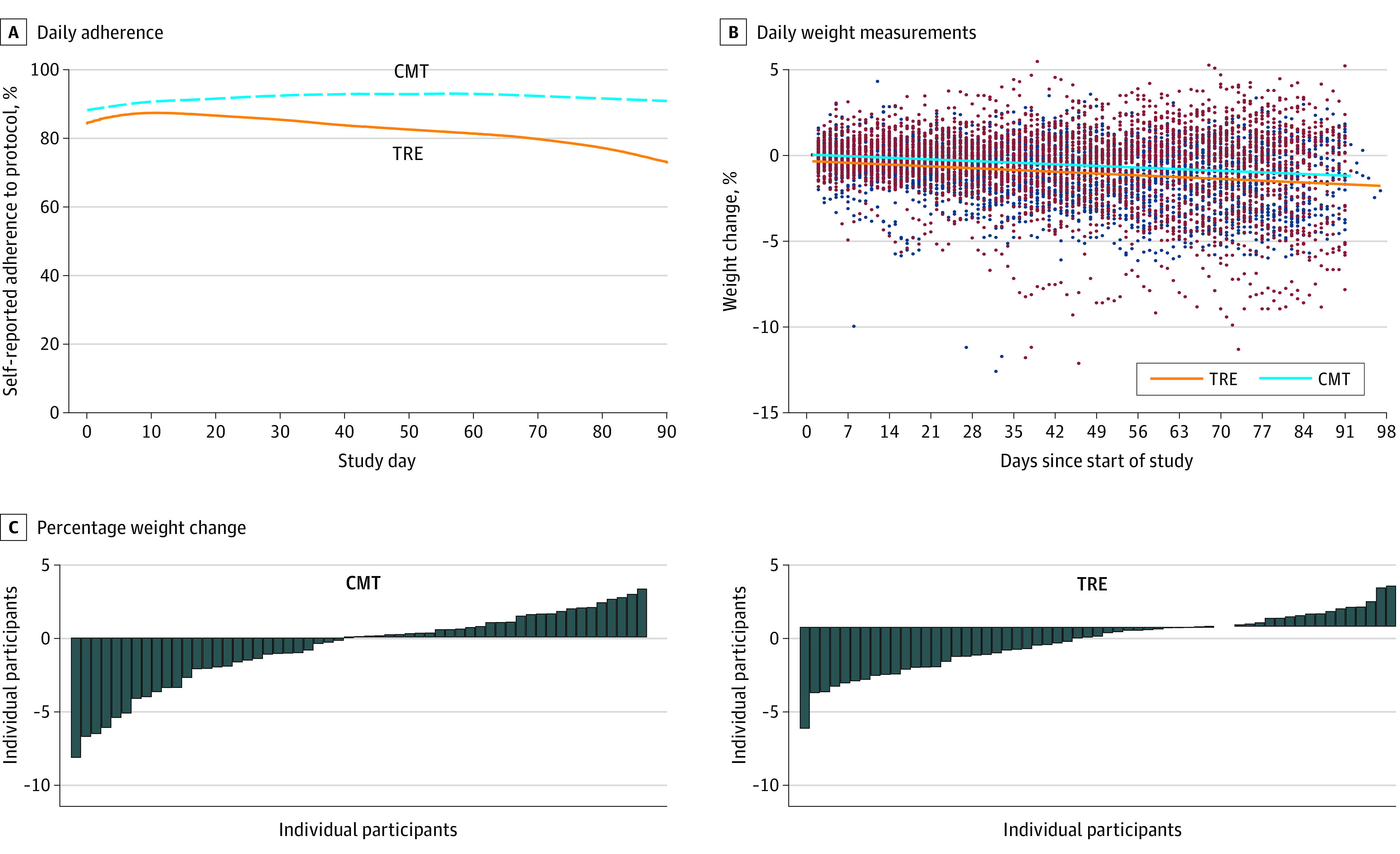
A, Participants were sent daily adherence surveys through the study application (“Did you adhere to your eating plan on the previous day?” Yes/No). Responses from all completed surveys were analyzed. The percent adherence to protocol is shown over time for consistent meal timing group (CMT) (dashed blue line; n = 41) and time-restricted eating (TRE) participants (solid orange line; n = 44). B, Individual daily weight measurements as recorded from the at-home scale are shown for each participant over time throughout the duration of the study. The individual weight measurements are show as maroon dots for the CMT group (n = 57) and blue dots for TRE group (n = 59). The solid lines represent weight over time as determined from the linear mixed model. C, Waterfall plot showing percent weight change for each participant from the total cohort in the CMT group (left) and TRE group (right).
Of the 141 participants randomized in the study, we invited persons living within 60 miles of San Francisco (enrollment was first come, first served and was capped at 50) to undergo comprehensive in-person metabolic testing (referred to as in-person cohort). Overall, 46 of 50 participants completed the entire in-person testing protocol (CMT n = 24, TRE n = 22). Baseline characteristics of both cohorts are shown in Table 1.
Weight
There was a significant decrease in weight in the TRE group (−0.94 kg; 95% CI, −1.68 kg to −0.20 kg; P = .01) and a nonsignificant decrease in weight in the CMT group (−0.68 kg; 95% CI, −1.41 kg to 0.05 kg; P = .07). Importantly, there was no significant difference in weight change between groups (−0.26 kg; 95% CI, −1.30 kg to 0.78 kg; P = .63) (Figure 2, B and C) (Table 2). There was a significant decrease in percentage of baseline weight in the TRE group (−1.17%; 95% CI, −1.89% to −0.45%; P = .002) and in the CMT group (−0.75%; 95% CI, −1.47% to −0.04%, P = .04); however, there was no significant difference between groups (−0.41%; 95% CI, −1.43% to 0.60%; P = .43) (Table 2). There were no statistically significant changes in estimated energy intake or energy expenditure between groups (eFigure 1A and 1B in Supplement 3).
Table 2. Weight Change in the Total Cohort.
| Total Cohort (iHealth weight measurements) | CMT (n = 57 included in analysis) | ∆CMT | ∆CMT P value | TRE (n = 59 included in analysis) | ∆TRE | ∆TRE P value | Difference between groups | P value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Preintervention | Postintervention | Preintervention | Postintervention | |||||||
| iHealth weight, mean (SD), kg | 99.2 (95.1 to 103.3) | 98.5 (94.3 to 102.7) | −0.68 (−1.41 to 0.05) | .07 | 99.2 (95.1 to 103.2) | 98.2 (94.1 to 102.4) | −0.94 (−1.68 to −0.20) | .01 | −0.26 (−1.30 to 0.78) | .63 |
| Weight change, mean (SD), % | NA | NA | −0.75 (−1.47 to −0.04) | .04 | NA | NA | −1.17 (−1.89 to −0.45) | .002 | −0.41 (−1.43 to 0.60) | .43 |
Abbreviations: CMT, consistent meal timing group; NA, not applicable; TRE, time-restricted eating group.
In the in-person cohort (n = 50), there was a significant decrease in weight in the TRE group using the in-person weight measurements (−1.70 kg; 95% CI, −2.56 kg to −0.83 kg; P < .001) but not in the CMT group (−0.57 kg; 95% CI, −1.40 kg to 0.26 kg; P = .18) (Table 3) (eFigure 2 in Supplement 3). There was a nonsignificant difference in weight loss between groups (−1.13 kg; 99.7% CI, −2.33% to 0.07%; P = .07) (Table 3). There was a significant decrease in percentage of baseline weight in the TRE group (−1.81%; 95% CI, −2.85% to 0.78%; P < .001) but not in the CMT group (−0.65%; 95% CI, −1.64% to 0.34%; P = .19) or between groups (−1.16%; 95% CI, −2.59% to 0.27%; P = .11). There was strong agreement between in-person weight measurements and at-home weight measurements as determined by a Bland-Altman analysis (eFigure 3 in Supplement 3).
Table 3. Body Composition and Energy Expenditure Measurements in the In-Person Cohorta.
| In-person cohort | CMT | ∆CMT | ∆CMT P value | TRE | ∆TRE | ∆TRE P value | Difference between groups | P value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Preintervention (n = 25) | Postintervention (n = 24) | Preintervention (n = 25) | Postintervention (n = 22) | |||||||
| Weight, kgb | 93.0 (87.4 to 98.5) | 92.4 (86.9 to 97.9) | −0.57 (−1.40 to 0.26) | .18 | 92.6 (87.0 to 98.1) | 90.9 (85.3 to 96.4) | −1.70 (−2.56 to −0.83) | <.001b | −1.13 (−2.33 to 0.07) | .07 |
| Weight change, % | −0.65 (−1.64 to 0.34) | .19 | −1.81 (−2.85 to −0.78) | <.001b | −1.16 (−2.59 to 0.27) | .11 | ||||
| Fat mass, kgb | 30.7 (27.7 to 33.7) | 30.6 (27.6 to 33.6) | −0.03 (−0.66 to 0.60) | .93 | 30.3 (27.3 to 33.3) | 29.8 (26.8 to 32.8) | −0.51 (−1.17 to 0.15) | .13 | −0.48 (−1.75 to 0.79) | .3 |
| Fat mass, % | 33.0 (30.4 to 35.7) | 32.9 (30.3 to 35.6) | −0.07 (−0.55 to 0.42) | .78 | 32.9 (30.3 to 35.6) | 32.8 (30.2 to 35.5) | −0.09 (−0.59 to 0.42) | .74 | −0.02 (−0.72 to 0.68) | .96 |
| Visceral fat mass, kg | 0.625 (0.529 to 0.721) | 0.634 (0.537 to 0.730) | 0.0088 (−0.0188 to 0.0364) | .53 | 0.58 (0.48 to 0.67) | 0.576 (0.480 to 0.673) | −0.0026 (−0.0314 to 0.0263) | .86 | −0.0114 (−0.0513 to 0.0285) | .58 |
| Subcutaneous fat mass, kg | 1.95 (1.74 to 2.17) | 1.94 (1.72 to 2.16) | −0.013 (−0.066 to 0.040) | .63 | 1.87 (1.66 to 2.09) | 1.84 (1.62 to 2.06) | −0.038 (−0.093 to 0.017) | .17 | −0.025 (−0.101 to 0.051) | .51 |
| Lean mass, kgb | 59.7 (55.3 to 64.1) | 59.3 (55.0 to 63.7) | −0.35 (−0.95 to 0.25) | .25 | 60.0 (55.6 to 64.4) | 58.9 (54.5 to 63.3) | −1.10 (−1.73 to −0.48) | <.001b | −0.75 (−1.96 to 0.45) | .09 |
| Trunk lean mass, kg | 30.5 (28.3 to 32.6) | 30.3 (28.2 to 32.5) | −0.15 (−0.54 to 0.24) | .45 | 30.4 (28.3 to 32.6) | 30.0 (27.8 to 32.1) | −0.47 (−0.88 to −0.06) | .024c | −0.32 (−0.89 to 0.25) | .27 |
| Appendicular lean mass, kg | 25.8 (23.6 to 28.0) | 25.6 (23.4 to 27.8) | −0.17 (−0.41 to 0.07) | .16 | 26.1 (24.0 to 28.3) | 25.5 (23.3 to 27.7) | −0.64 (−0.89 to −0.39) | <.001b | −0.47 (−0.82 to −0.12) | .009 |
| Appendicular lean mass index, kg/m2 | 8.62 (8.10 to 9.14) | 8.56 (8.04 to 9.08) | −0.058 (−0.136 to 0.020) | .14 | 8.80 (8.28 to 9.32) | 8.58 (8.06 to 9.10) | −0.220 (−0.301 to −0.139) | <.001b | −0.162 (−0.274 to −0.050) | .005 |
| Total body water, kgc | 42.7 (39.6 to 45.8) | 42.1 (39.0 to 45.2) | −0.59 (−1.06 to −0.13) | .01 | 41.9 (38.6 to 45.1) | 41.5 (38.3 to 44.7) | −0.36 (−0.85 to 0.13) | .14 | 0.23 (−0.44 to 0.91) | .5 |
| Bone mineral content, g | 2541.9 (2388.3 to 2695.5) | 2546.9 (2393.3 to 2700.5) | 5.00 (−8.33 to 18.33) | .46 | 2511.3 (2357.7 to 2664.9) | 2523.2 (2369.6 to 2676.9) | 11.95 (−1.97 to 25.87) | .09 | 6.95 (−12.32 to 26.23) | .48 |
| Circumference, cm | ||||||||||
| Waist | 106.6 (102.3 to 110.8) | 105.9 (101.6 to 110.2) | −0.69 (−4.28 to 2.90) | .71 | 106.3 (102.1 to 110.5) | 104.5 (100.1 to 108.9) | −1.81 (−5.53 to 1.92) | .34 | −1.12 (−6.29 to 4.05) | .67 |
| Hip | 109.5 (106.2 to 112.8) | 109.5 (106.2 to 112.8) | 0.01 (−2.16 to 2.18) | .99 | 111.5 (108.2 to 114.7) | 110.2 (106.8 to 113.6) | −1.28 (−3.53 to 0.98) | .27 | −1.29 (−4.42 to 1.85) | .42 |
| Waist-to-hip ratio | 0.980 (0.957 to 1.004) | 0.970 (0.946 to 0.994) | −0.0107 (−0.0287 to 0.0074) | .25 | 0.953 (0.929 to 0.977) | 0.948 (0.924 to 0.973) | −0.0047 (−0.0234 to 0.0140) | .62 | 0.0060 (−0.0200 to 0.0320) | .65 |
| Circumference, cm | ||||||||||
| Bicep | 35.4 (34.4 to 36.4) | 35.3 (34.3 to 36.4) | −0.04 (−0.46 to 0.38) | .86 | 35.6 (34.6 to 36.6) | 35.3 (34.2 to 36.3) | −0.30 (−0.74 to 0.14) | .19 | −0.26 (−0.87 to 0.35) | .41 |
| Thigh | 57.5 (55.9 to 59.2) | 57.8 (56.2 to 59.5) | 0.27 (−0.37 to 0.90) | .41 | 57.7 (56.1 to 59.4) | 57.6 (55.9 to 59.2) | −0.16 (−0.82 to 0.51) | .64 | −0.42 (−1.35 to 0.50) | .37 |
| Handgrip strength, kg | 30.8 (26.8 to 34.8) | 31.1 (27.1 to 35.1) | 0.31 (−1.21 to 1.83) | .69 | 28.3 (24.3 to 32.3) | 28.8 (24.8 to 32.8) | 0.49 (−1.09 to 2.08) | .54 | 0.18 (−2.02 to 2.38) | .87 |
| Leg extension peak torque, ft-lb | 109.3 (97.1 to 121.4) | 100.9 (88.5 to 113.3) | −8.39 (−17.60 to 0.81) | .07 | 105.8 (93.6 to 117.9) | 105.9 (93.4 to 118.4) | 0.15 (−9.24 to 9.53) | .98 | 8.54 (−4.60 to 21.69) | .20 |
| Respiratory quotient | 0.741 (0.717 to 0.765) | 0.776 (0.752 to 0.801) | 0.0348 (0.0119 to 0.0577) | .003 | 0.765 (0.741 to 0.789) | 0.767 (0.742 to 0.792) | 0.0028 (−0.0209 to 0.0265) | .82 | −0.0320 (−0.0649 to 0.0009) | .06 |
| Resting metabolic rate, kcal/db | 1909.7 (1781.2 to 2038.2) | 1866.6 (1737.6 to 1995.6) | −43.1 (−104.2 to 18.0) | .17 | 1920.4 (1791.9 to 2048.9) | 1892.3 (1762.0 to 2022.5) | −28.1 (−91.8 to 35.5) | .39 | 15.0 (−108.1 to 138.0) | .74 |
| Total energy expenditure, kcal/db,c | 2772.1 (2563.4 to 2980.7) | 2644.7 (2436.1 to 2853.4) | −127.3 (−230.7 to −23.9) | .02 | 2718.3 (2500.4 to 2936.3) | 2540.5 (2322.6 to 2758.4) | −177.9 (−285.9 to −69.9) | .001 | −50.6 (−259.2 to 158.1) | .51 |
Abbreviations: CMT, consistent meal timing group; TRE, time-restricted eating group.
All data are presented as means (95% CI). For secondary outcome measures, Bonferroni-corrected confidence intervals are presented and Bonferroni-adjusted critical α of 0.006 is used. For data with statistical outliers, Winsorised data was used to generate P values.
Secondary outcome using a Bonferroni-corrected α of 0.006 and presenting 99.7% CI for between group differences.
Data analyzed only for completers (CMT n = 24, TRE n = 22).
Body Composition and Energy Expenditure
As measured by dual-energy x-ray absorptiometry (DXA), there was no significant change in whole body fat mass (FM) in the TRE (−0.51 kg; 95% CI, −1.17 kg to 0.15 kg; P = .13) or the CMT groups (−0.03 kg; 95% CI, −0.66 kg to 0.60 kg; P = .93), and there was no significant difference between groups (−0.48 kg; 99.7% CI, −1.75 kg to 0.79 kg; P = .30) (Table 3). There was a significant decrease in lean mass (calculated as fat-free mass minus bone mineral content) in the TRE (−1.10 kg; 95% CI, −1.73 kg to −0.48 kg; P < .001) but not in the CMT group (−0.35 kg; 95% CI, −0.95 kg to 0.25 kg; P = .25). There was no significant difference in lean mass between groups (−0.75 kg; 99.7% CI, −1.96 kg to 0.45 kg; P = .09). Appendicular lean mass (ALM) was decreased significantly in the TRE group (−0.64 kg; 95% CI, −0.89 kg to −0.39 kg; P < .001) but not in the CMT group (−0.17 kg; 95% CI, −0.41 kg to 0.07 kg; P = .16), and there was a significant difference between groups (−0.47 kg; 95% CI, −0.82 kg to −0.12 kg; P = .009). There was a significant decrease in appendicular lean mass index (ALMI) in the TRE group (−0.22 kg/m2; 95% CI, −0.30 kg/m2 to −0.14 kg/m2; P < .001) but not in the CMT group (−0.06 kg/m2; 95% CI, −0.14 kg/m2 to 0.02 kg/m2; P = .14). The difference in ALMI between groups was also significant (−0.16 kg/m2; 95% CI, −0.27 kg/m2 to −0.05 kg/m2; P = .005). Trunk lean mass significantly decreased in the TRE group (−0.47 kg; 95% CI, −0.88 kg to −0.06 kg; P = .02). There was no significant change in trunk lean mass in the CMT group (−0.15 kg; 95% CI, −0.54 kg to 0.24 kg; P = .45) or between groups (−0.32 kg; 95% CI, −0.89 kg to 0.25 kg; P = .27). For a comprehensive list of all body composition variables analyzed, see eTable 2 in Supplement 3.
Respiratory quotient (RQ) did not change significantly in the TRE group (0.01; 95% CI, −0.02 to 0.03; P = .82); RQ increased in the CMT group (0.03; 95% CI, 0.01 to 0.06; P = .003), but there was no significant difference between groups (−0.03; 95% CI, −0.06 to −0.01, P = .06). There was no significant difference in resting metabolic rate (RMR) in the TRE (−28.1 kcal/d; 95% CI, −91.8 kcal/d to 35.5 kcal/d; P = .39) or the CMT group (−43.15 kcal/d; 95% CI, −104.2 kcal/d to 18.0 kcal/d; P = .17), and there was no significant difference between groups (15.0 kcal/d; 99.7% CI, −108.1 kcal/d to 138.0 kcal/d; P = .74) (Table 3). There was a significant decrease in total energy expenditure (TEE) in both groups (TRE: −177.9 kcal/d; 95% CI, −285.9 kcal/d to −69.9 kcal/d; P = .001; CMT: −127.3 kcal/d; 95% CI, −230.7 kcal/d to −23.9 kcal/d; P = .02). There was no significant difference between groups (−50.6 kcal/d; 99.7% CI, −259.2 kcal/d to 158.1 kcal/d; P = .51).
Blood Lipids, Glucose, Insulin, and Cardiometabolic Health Markers
There were no significant within-group or between-group differences in fasting glucose, fasting insulin, HOMA-IR, HbA1C, triglycerides, total cholesterol, LDL, or HDL levels (eTable 1 in Supplement 3). See eTable 3 in Supplement 3 for all other blood markers analyzed in the study.
There was no significant difference in systolic blood pressure in the TRE group (−1.69 mm Hg; 95% CI, −5.54 mm Hg to 2.15 mm Hg; P = .39), but there was a significant decrease in the CMT group (−3.86 mm Hg; 95% CI, −7.58 mm Hg to 0.14 mm Hg; P = .04) (eTable 1 in Supplement 3). There was no significant between-group difference in systolic blood pressure (2.17 mm Hg; 95% CI, −3.18 mm Hg to 7.52 mm Hg; P = .43). There was a significant change in diastolic blood pressure in the TRE group (−4.08 mm Hg; 95% CI, −8.11 mm Hg to −0.06 mm Hg; P = .047) but not in the CMT group (−3.01 mm Hg; 95% CI, −6.90 mm Hg to 0.89 mm Hg; P = .13) or between groups (−1.08 mm Hg; 95% CI, −6.67 mm Hg to 4.52 mm Hg; P = .71).
Sleep Quality Activity Tracking and Food Attitudes
There were no significant changes in any of the self-reported sleep measures in either group or between groups in the total cohort (eTable 5 in Supplement 3). However, Oura ring data revealed significant changes in sleep efficiency, sleep latency, and awake time in the TRE group and between groups (eTable 6 in Supplement 3).
The Oura ring data also revealed a significant reduction in daily movement in the TRE group (−2102.14 au; 95% CI, −3162.54 au to −1041.73 au; P < .001) and between groups (−1673.44 au; 95% CI, −3211.11 au to −135.7 au; P = .03) but not in the CMT group (−428.70 au; 95% CI, −1542.25 au to 684.85 au; P = .45). There was a significant decrease in step count in the TRE group (−2498.89 steps; 95% CI, −3939.91 to −1057.88; P < .001) and between groups (−2241.41 steps; 95% CI, −4320.51 to −162.31; P = .04) but not in the CMT group (−257.48 steps; 95% CI, −1756.20 to 1241.23; P = .74). The correlation between change in step count and change in TEE was 0.52 in the TRE group and 0.03 in the CMT group, but the 2 correlations did not differ significantly (eFigure 4 in Supplement 3).
Discussion
The TRE is attractive as a weight-loss option in that it does not require tedious, and time-consuming methods such as calorie-counting or adherence to complicated diets. Indeed, we found that self-reported adherence to the TRE schedule was high (Figure 2A); However, in contrast to our hypothesis, there was no greater weight loss with TRE compared with the CMT. In addition, we found among our secondary outcomes that there were few differences between the 2 groups. Specifically:, there were no significant differences in fat mass, fasting insulin, glucose, HbA1C, or blood lipids between the TRE and CMT groups.
Most humans eat throughout their waking hours.12 We prescribed an 8-hour eating window and did not prescribe calorie or macronutrient guidance so as to offer a simple, real-world recommendation to free-living individuals. We chose a 12 pm to 8 pm eating window because we reasoned that people would find it easier culturally to skip breakfast than dinner—a more social meal in most cultures.
Our results are consistent with a prior study demonstrating that a recommendation to skip breakfast does not affect weight outcomes in patients trying to lose weight13 but, contradict previous reports describing the beneficial effects of TRE on weight loss and other metabolic risk markers.7,10,14,15
Wilkinson et al10 found that TRE was associated with an approximately 3% weight loss and improvements in cardiovascular risk markers in patients with Metabolic Syndrome. This single-arm study was small (n = 19) and, importantly, did not have a control group.
Although the prescribed (12-8 pm) eating window is likely more attractive and more amenable to long-term adherence, it might not be optimal for the metabolic advantages of TRE. Sutton et al15 performed a 5-week RCT comparing early TRE (eTRE: 6-hour eating window with dinner before 3:00 pm) to a control diet (12-hour eating window). They found improved glycemic control and improvements in cardiovascular risk markers without changes in body weight in the eTRE group.
In analysis of secondary outcomes, we found a significant reduction in lean mass in the TRE group. In the in-person cohort, the average weight loss in the TRE group was 1.70 kg. Of this, 1.10 kg (approximately 65% of weight lost) was lean mass; only 0.51 kg of weight loss was fat mass. Loss of lean mass during weight loss typically accounts for 20% to 30% of total weight loss.16,17,18,19,20,21,22 The proportion of lean mass loss in this study (approximately 65%) far exceeds the normal range of 20% to 30%.22 In addition, there was a highly significant between-group difference in ALM. Appendicular lean mass is correlated with nutritional and physical status, and reduced ALM can lead to weakness, disability, and impaired quality of life.23,24,25,26 This serves as a caution for patient populations at risk for sarcopenia because TRE could exacerbate muscle loss.27 Finally, the extent of lean mass loss during weight loss has been positively correlated with weight regain.28
The effect of TRE on lean mass is largely unexplored. Prior studies show that TRE prevents gains in lean mass.29 A follow-up study showed that when calorie intake and protein intake were matched to prestudy consumption, no change in lean mass was observed.30 An RCT comparing TRE in overweight and obese patients demonstrated a significant loss of lean mass compared with controls, but no significant change in fat loss between groups.31 Ad libitum feeding during TRE leads to reduced calorie intake and might also reduce protein intake.9 Together, these data highlight the importance of adequate protein consumption while adhering to a TRE diet. Many studies have shown that adequate/excessive protein consumption during weight loss can mitigate losses in lean mass.16,28,32,33,34,35 National Health and Nutrition Examination Survey data show that most daily protein intake occurs during meals, and snacking accounts for a small portion of total daily protein intake.36 The loss of ALM during TRE could be mitigated by increasing the number of meals within the eating window or consuming protein supplements.16,28 Timing of protein consumption may also play a role in changes in lean mass.37,38,39
Strengths and Limitations
Strengths of the study include randomization, an easy to follow, real-world prescription-based intervention, and an appropriate control group. Although there was statistically significant weight loss in the TRE group, there was no difference between groups. This indicates that participation in a weight loss study alone (even in the control group) is sufficient to lead to short-term weight loss and highlights the importance of including a control arm in weight loss studies.
A limitation is we do not have self-reported measures of energy or macronutrient intake. Although we did not measure calorie intake, mathematical modeling of changes in energy intake suggests that calorie intake did not significantly differ between groups. This model has been validated to be more accurate than self-reported energy intake.40,41 We did not measure changes in protein intake. Given the loss of ALM in participants in the TRE arm and previous reports of decreased protein consumption from TRE,9,29 it is possible that protein intake was altered by TRE in this cohort, and this clearly warrants future study. Finally, the DXA analysis of lean mass did not factor in muscle hydration, so it is possible that changes in hydration could confound the lean mass calculations. To help control for this, participants fasted for more than 12 hours and voided their bladder prior to DXA scans. The change in lean mass in the TRE group was much greater than the loss of body water, so it is unlikely that differences in muscle hydration would account for all of the lean mass loss.
Conclusions
In this RCT, a prescription of TRE did not result in weight loss when compared with a control prescription of 3 meals per day. Time-restricted eating did not change any relevant metabolic markers. Finally, there was a decrease in ALM in the TRE group compared with CMT. Together, the results of this study (1) do not support the efficacy of TRE for weight loss, (2) highlight the importance of control interventions, and (3) offer caution about the potential effects of TRE on ALM. Future studies should be aimed at understanding the effects of early vs late TRE and protein intake or timing as a means to offset the loss in ALM.
Trial Protocol
Statistical Analysis Plan
eMethods and eReferences
eFigure 1. Estimated energy intake and energy expenditure derived from at-home weight measurements in total cohort
eFigure2. Weight change in in-person cohort
eFigure 3. Bland-Altman plot for in-person weights and at-home weights using the iHealth Bluetooth scale
eFigure 4. Correlation of change in step count and change in TEE
eTable 1. Insulin and glucose homeostasis and cardiometabolic health measurements in in-person subjects
eTable 2. All body composition and strength measures
eTable 3. Other blood markers in in-person cohort
eTable 4. MOCACARE at home blood pressure measurements in total cohort
eTable 5. Self-reported sleep measures
eTable 6. Sleep and activity measures from Oura ring in in-person cohort
Data Sharing Statement
References
- 1.Hales CM CM, Fryar CD, Ogden CL Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. National Center for Health Statistics. 2020. [Google Scholar]
- 2.National Heart L, and Blood Institute. Managing overweight and obesity in adults: Systematic evidence review from the Obesity Expert Panel. https://wwwnhlbinihgov/sites/default/files/media/docs/obesity-evidence-review.pdf. 2013. Accessed November 1, 2013.
- 3.Brown JD, Buscemi J, Milsom V, Malcolm R, O’Neil PM. Effects on cardiovascular risk factors of weight losses limited to 5-10. Transl Behav Med. 2016;6(3):339-346. doi: 10.1007/s13142-015-0353-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cioffi I, Evangelista A, Ponzo V, et al. . Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: a systematic review and meta-analysis of randomized controlled trials. J Transl Med. 2018;16(1):371. doi: 10.1186/s12967-018-1748-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hatori M, Vollmers C, Zarrinpar A, et al. . Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848-860. doi: 10.1016/j.cmet.2012.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991-1005. doi: 10.1016/j.cmet.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchison AT, Regmi P, Manoogian ENC, et al. . Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring). 2019;27(5):724-732. doi: 10.1002/oby.22449 [DOI] [PubMed] [Google Scholar]
- 8.Anton SD, Lee SA, Donahoo WT, et al. . The effects of time restricted feeding on overweight, older adults: a pilot study. Nutrients. 2019;11(7):E1500. doi: 10.3390/nu11071500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoni R RT, Robertson MD, Johnston JD. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. Journal of Nutritional Science. Published online August 30, 2018. doi: 10.1017/jns.2018.13 [DOI] [Google Scholar]
- 10.Wilkinson MJ, Manoogian ENC, Zadourian A, et al. . Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92-104 e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng BK, Sommer MJ, Wong MC, et al. . Detailed 3-dimensional body shape features predict body composition, blood metabolites, and functional strength: the Shape Up! studies. Am J Clin Nutr. 2019;110(6):1316-1326. doi: 10.1093/ajcn/nqz218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789-798. doi: 10.1016/j.cmet.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhurandhar EJ, Dawson J, Alcorn A, et al. . The effectiveness of breakfast recommendations on weight loss: a randomized controlled trial. Am J Clin Nutr. 2014;100(2):507-513. doi: 10.3945/ajcn.114.089573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11(6):E1234. doi: 10.3390/nu11061234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutton EF, Beyl R, Early KS, et al. . Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212-1221 e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verreijen AM, Verlaan S, Engberink MF, et al. . A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;101(2):279-286. doi: 10.3945/ajcn.114.090290 [DOI] [PubMed] [Google Scholar]
- 17.Garrow JS, Summerbell CD. Meta-analysis: effect of exercise, with or without dieting, on the body composition of overweight subjects. Eur J Clin Nutr. 1995;49(1):1-10. doi: 10.1038/sj.ejcn.1600534 [DOI] [PubMed] [Google Scholar]
- 18.Magkos F, Fraterrigo G, Yoshino J, et al. . Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23(4):591-601. doi: 10.1016/j.cmet.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santanasto AJ, Glynn NW, Newman MA, et al. . Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley D, Conte C, Mittendorfer B, et al. . Gastric bypass and banding equally improve insulin sensitivity and β cell function. J Clin Invest. 2012;122(12):4667-4674. doi: 10.1172/JCI64895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson MJ, Friedl KE, Frykman PN, Moore RJ. Loss of muscle mass is poorly reflected in grip strength performance in healthy young men. Med Sci Sports Exerc. 1994;26(2):235-240. doi: 10.1249/00005768-199402000-00015 [DOI] [PubMed] [Google Scholar]
- 22.Cava E, Yeat NC, Mittendorfer B. Preserving healthy muscle during weight loss. Adv Nutr. 2017;8(3):511-519. doi: 10.3945/an.116.014506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. ; European Working Group on Sarcopenia in Older People . Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412-423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50(5):889-896. doi: 10.1046/j.1532-5415.2002.50216.x [DOI] [PubMed] [Google Scholar]
- 25.Rathnayake N, Alwis G, Lenora J, Lekamwasam S. Concordance between appendicular skeletal muscle mass measured with DXA and estimated with mathematical models in middle-aged women. J Physiol Anthropol. 2018;37(1):19. doi: 10.1186/s40101-018-0179-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JC, Harhay MO, Harhay MN. Appendicular lean mass and mortality among prefrail and frail older adults. J Nutr Health Aging. 2017;21(3):342-345. doi: 10.1007/s12603-016-0753-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasmi M, Sellami M, Denham J, et al. . Time-restricted feeding influences immune responses without compromising muscle performance in older men. Nutrition. 2018;51-52:29-37. doi: 10.1016/j.nut.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 28.Pasiakos SM, Cao JJ, Margolis LM, et al. . Effects of high-protein diets on fat-free mass and muscle protein synthesis following weight loss: a randomized controlled trial. FASEB J. 2013;27(9):3837-3847. doi: 10.1096/fj.13-230227 [DOI] [PubMed] [Google Scholar]
- 29.Tinsley GM, Forsse JS, Butler NK, et al. . Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur J Sport Sci. 2017;17(2):200-207. doi: 10.1080/17461391.2016.1223173 [DOI] [PubMed] [Google Scholar]
- 30.Moro T, Tinsley G, Bianco A, et al. . Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290. doi: 10.1186/s12967-016-1044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow LS, Manoogian ENC, Alvear A, et al. . Time-restricted eating effects on body composition and metabolic measures in humans who are overweight: a feasibility study. Obesity (Silver Spring). 2020;28(5):860-869. doi: 10.1002/oby.22756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Houston DK, Nicklas BJ, Ding J, et al. ; Health ABC Study . Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) study. Am J Clin Nutr. 2008;87(1):150-155. doi: 10.1093/ajcn/87.1.150 [DOI] [PubMed] [Google Scholar]
- 33.Kim JE, O’Connor LE, Sands LP, Slebodnik MB, Campbell WW. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta-analysis. Nutr Rev. 2016;74(3):210-224. doi: 10.1093/nutrit/nuv065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bopp MJ, Houston DK, Lenchik L, Easter L, Kritchevsky SB, Nicklas BJ. Lean mass loss is associated with low protein intake during dietary-induced weight loss in postmenopausal women. J Am Diet Assoc. 2008;108(7):1216-1220. doi: 10.1016/j.jada.2008.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim IY, Schutzler S, Schrader A, et al. . Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults. Am J Physiol Endocrinol Metab. 2015;308(1):E21-E28. doi: 10.1152/ajpendo.00382.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krok-Schoen JL, Jonnalagadda SS, Luo M, Kelly OJ, Taylor CA. Nutrient intakes from meals and snacks differ with age in middle-aged and older americans. Nutrients. 2019;11(6):E1301. doi: 10.3390/nu11061301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farsijani S, Morais JA, Payette H, et al. . Relation between mealtime distribution of protein intake and lean mass loss in free-living older adults of the NuAge study. Am J Clin Nutr. 2016;104(3):694-703. doi: 10.3945/ajcn.116.130716 [DOI] [PubMed] [Google Scholar]
- 38.Mamerow MM, Mettler JA, English KL, et al. . Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr. 2014;144(6):876-880. doi: 10.3945/jn.113.185280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Areta JL, Burke LM, Ross ML, et al. . Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol. 2013;591(9):2319-2331. doi: 10.1113/jphysiol.2012.244897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo J, Robinson JL, Gardner CD, Hall KD. Objective versus self-reported energy intake changes during low-carbohydrate and low-fat diets. Obesity (Silver Spring). 2019;27(3):420-426. doi: 10.1002/oby.22389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanghvi A, Redman LM, Martin CK, Ravussin E, Hall KD. Validation of an inexpensive and accurate mathematical method to measure long-term changes in free-living energy intake. Am J Clin Nutr. 2015;102(2):353-358. doi: 10.3945/ajcn.115.111070 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eMethods and eReferences
eFigure 1. Estimated energy intake and energy expenditure derived from at-home weight measurements in total cohort
eFigure2. Weight change in in-person cohort
eFigure 3. Bland-Altman plot for in-person weights and at-home weights using the iHealth Bluetooth scale
eFigure 4. Correlation of change in step count and change in TEE
eTable 1. Insulin and glucose homeostasis and cardiometabolic health measurements in in-person subjects
eTable 2. All body composition and strength measures
eTable 3. Other blood markers in in-person cohort
eTable 4. MOCACARE at home blood pressure measurements in total cohort
eTable 5. Self-reported sleep measures
eTable 6. Sleep and activity measures from Oura ring in in-person cohort
Data Sharing Statement