ABSTRACT
Multiple studies have identified changes within the gut microbiome in response to diarrheal-inducing bacterial pathogens. However, examination of the microbiome in response to viral pathogens remains understudied. Compounding this, many studies use fecal samples to assess microbiome composition; which may not accurately mirror changes within the small intestine, the primary site for most enteric virus infections. As a result, the functional significance of small intestinal microbiome shifts during infection is not well defined. To address these gaps, rotavirus-infected neonatal mice were examined for changes in bacterial community dynamics, host gene expression, and tissue recovery during infection. Profiling bacterial communities using 16S rRNA sequencing suggested significant and distinct changes in ileal communities in response to rotavirus infection, with no significant changes for other gastrointestinal (GI) compartments. At 1-d post-infection, we observed a loss in Lactobacillus species from the ileum, but an increase in Bacteroides and Akkermansia, both of which exhibit mucin-digesting capabilities. Concomitant with the bacterial community shifts, we observed a loss of mucin-filled goblet cells in the small intestine at d 1, with recovery occurring by d 3. Rotavirus infection of mucin-producing cell lines and human intestinal enteroids (HIEs) stimulated release of stored mucin granules, similar to in vivo findings. In vitro, incubation of mucins with Bacteroides or Akkermansia members resulted in significant glycan degradation, which altered the binding capacity of rotavirus in silico and in vitro. Taken together, these data suggest that the response to and recovery from rotavirus-diarrhea is unique between sub-compartments of the GI tract and may be influenced by mucin-degrading microbes.
KEYWORDS: Rotavirus, microbiome, mucus, Muc2, Akkermansia, Bacteroides, glycans
Introduction
The intestinal microbiome represents a vast population of microorganisms that broadly impacts human health.1-3 In general, the gut microbiome consists of the major phyla Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, and Verrucomicrobia.1,4-9 The composition of these bacterial members varies along the length of the intestine.4,6,10 Disruption of the normal microbiome structure has been associated with a number of intestinal disorders, including inflammatory bowel diseases (IBD),11-22 irritable bowel syndrome (IBS),23-30 obesity,31-39 and enteric infections.40-50 It is well documented that bacterial pathogens that cause diarrhea significantly alter the microbiome. These agents include Clostridium difficile, Clostridium perfringens, enterotoxigenic, enteroaggregative, and diffuse-adhering Escherichia coli (ETEC, EAEC, and DAEC), Vibrio cholera, Campylobacter, Salmonella, and Shigella.40-50 In addition, diarrhea-inducing parasites, such as Giardia, Entamoeba, and Strongyloides can alter the microbiome.41,51 However, compared to work on bacterial pathogens, there are fewer studies examining the effects of diarrhea-inducing viral pathogens on the gut microbiome. Studies that do focus on viral-induced changes in the microbiome rely largely on stool samples, which may not accurately reflect changes in the microbiome in the small intestine, a key site for chloride and water secretion that drive diarrhea.4-6,52-54 Thus, further investigation is needed to understand how the small intestinal microbiome is altered following enteric viral infections.
Rotavirus (RV) is a non-enveloped RNA virus that is transmitted via the fecal – oral route and primarily infects small intestinal enterocytes.55,56 Rotavirus is the leading cause of diarrheal diseases in children, with an estimated 258 million cases and 128,500 deaths per year.57 Though four vaccines are in use, they have varying degrees of efficacy, especially in developing countries where available vaccines do not elicit robust protective immune responses.58,59 Some researchers speculate that the gut microbiota may influence rotavirus infection and vaccine immunogenicity.60,61 However, an in-depth analysis of the intestinal microbiome following rotavirus infection in the small intestine has yet to be undertaken. As such, the role of the microbiome in modulating rotavirus infection remains poorly understood. We speculated that viral infections disrupt the balance of the small intestinal gut microbiota and that the microbiota plays a key role in regulating rotavirus infection. Understanding the alterations that occur in the bacterial gut communities during rotavirus may aid in development of novel, more targeted, therapeutics.
In this study, we examined the effects of rotavirus infection on the microbial population along the length of the small intestine and colon. We used neonatal mice that, similar to human infants, develop diarrhea when infected with rotavirus. Using this well-characterized neonatal model, we examined 16S rRNA profiles of the stomach, jejunum, ileum, and colon for PBS-control and rotavirus-infected mice. Rotavirus infection was found to correlate with a time-dependent increase in Bacteroides and Akkermansia genera and a decrease in Lactobacillus in the ileum. Interestingly, no changes were observed in other gastrointestinal segments. Rotavirus infection was also associated with increased secretion of the dominant mucin protein Muc2 in the small intestine. Using mucin-producing cell lines and human jejunal enteroids, we confirmed that rotavirus infection results in secretion of mucus. In vitro and in silico analyses of Bacteroides thetaiotaomicron and Akkermansia mucinphila demonstrated that these microbes could degrade glycans that normally act as decoy receptors for rotavirus. Incubation of purified Muc2 from germ-free mice with rotavirus prior to infection of MA104 cells reduced rotavirus infectivity. However, pre-treatment of germ-free Muc2 with B. thetaiotaomicron and A. mucinphila prevented this response. These findings indicate that B. thetaiotaomicron and A. mucinphila may participate in rotavirus infection during the acute phase. Our work is among the first to demonstrate that rotavirus-infection is associated with a shift in the gut microbiota which may impact rotavirus pathogenesis.
Results
Rotavirus infection results in diarrhea and weight loss in BALB/c pups
To examine the effects of acute rotavirus infection on the gut microbiome, neonatal BALB/c mice were orally gavaged with rhesus rotavirus or PBS as the negative control. Previous work using BALB/c mice and rhesus rotavirus has established that diarrhea peaks 3–4 days post-infection (dpi) and returns to baseline on d 7.62 Diarrhea severity and weight gain were monitored for 3-days post-infection to assess the peak of diarrhea.63,64 None of the PBS-treated control mice exhibited any signs of diarrhea, but 88.5% of rotavirus-infected pups experienced diarrhea on at least 1 d during the experiment (Figure 1a, b). The rotavirus-infected pups exhibited an average diarrhea score of 3.0 ± 0.9 on 1 dpi compared to 1.0 ± 0.2 in PBS controls (Figure 1b). Additionally, rotavirus-infected pups gained weight more slowly than the control animals, with significant differences occurring at 2 dpi as diarrhea started to become more severe (Figure 1c). Peak diarrhea severity occurred on 3 dpi, so as a result, we choose to focus on 1 and 3 dpi to study changes in the gut microbiome.
Figure 1.
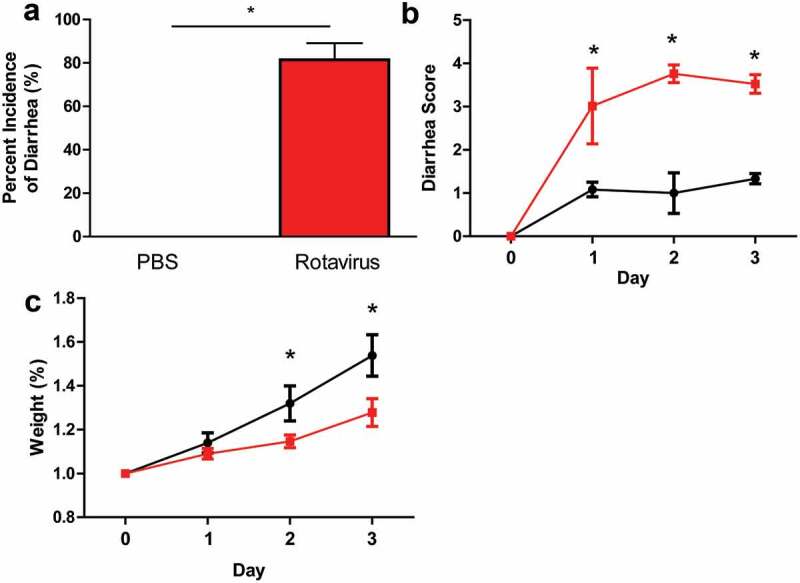
Rotavirus-infected BALB/c pups exhibit hallmarks of rotavirus-associated disease. a. The average percentage of BALB/c pups with diarrhea as recorded every 24 h for PBS-treated (n = 11; black bar) or rotavirus-infected (n = 12; red bar). One-Way ANOVA, *p < .05. b. Diarrhea scores over a 3-d period from PBS-treated (black) and rotavirus-infected (red) mice. One-Way Repeated Measures ANOVA, *p < .05. c. The average weight change of PBS-treated or rotavirus-infected BALB/c pups as measured every 24 h. All data presented as the average ± standard deviation. One-Way Repeated Measures ANOVA, *p < .05.
In addition to diarrhea severity, we also examined the effect of rotavirus infection histologically by H&E stain of intestinal sections (Figure 2). Consistent with other studies, rotavirus-infected mice at 1 dpi exhibited villi with swelling, vacuolization of epithelial cells, necrosis, and lymphocytic infiltration in the small intestine (duodenum-ileum) (Figure 2). These pronounced changes were most obvious in the jejunum and ileum, and no histological changes observed within the colon. By 3 dpi, we observed fewer swollen villi and less severe vacuolization in the small intestine of the rotavirus-infected mice than that observed on 1 dpi. Further, consistent with previous studies using the neonatal mouse model of rotavirus infection, no morphological changes were observed in the colon of pups at 1 or 3 dpi.
Figure 2.
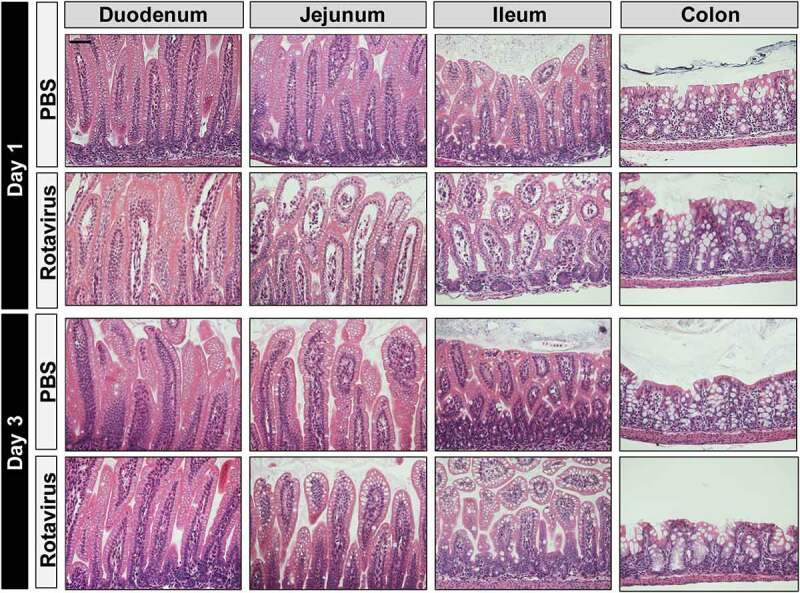
Rotavirus-infected BALB/c Pups Exhibit Histopathology in the Small Intestine. H&E staining of intestinal segments (duodenum, jejunum, ileum, and colon) from PBS-treated or rotavirus-infected at 1 or 3 dpi (day post-infection). H&E images were acquired on an upright Nikon Eclipse 90i microscope using a 20X Plan Apo (NA 0.75) DIC objective with a DS-Fi1-U2 camera and Nikon Elements software. Scale bar = 50 µm.
Rotavirus-infection shifts the ileal microbiome
To identify changes within the intestinal microbiome, 16S rRNA sequencing was performed on samples throughout the GI tract (stomach, jejunum, ileum, and colon). At 1 dpi, no effect of rotavirus was detected in alpha diversity by for either the stomach, jejunum, or colonic communities by Shannon Diversity (Supplemental Figure 1A) or Simpson Diversity Indices (Supplemental Figure 1B); however, there was a statistically significant difference in diversity between treatment groups in the ileum. At 3 dpi, rotavirus infection had no effect on the diversity of any compartment, despite some ongoing diarrhea in the infected animals (Supplemental Figure 1A,B).
Principal coordinates analysis (PCoA) plot on the weighted UniFrac distance matrix generated from rarefied taxon abundances showed that the composition of the rotavirus-infected populations became more divergent than those from the parallel PBS controls at 1 dpi only in the ileum (Supplemental Figure 2), with no changes observed in the other compartments (Supplemental Figure 2A, B, D). Consistent with the diversity indices, these divergent populations became indistinguishable by 3 dpi (Supplemental Figure 2E-G). Examination of the microbial population at the phylum level demonstrated significant changes in the ileum at 1 dpi (Figure 3c), while other GI compartments remained unchanged (Figure 3a, b, d). In the ileum, Bacteroidetes, Verricomicrobia, and Proteobacteria phlya increased with corresponding decreases in Firmicutes compared with PBS-treated controls (Figure 3c). This change was present at 1 dpi, but microbial distribution returned to PBS-control levels by 3 dpi (Figure 3c). Exploring these changes at the family level, we found that differences were driven by increased abundance of Bacteroidales, Verrucomicrobiales, and Enterobacteriales and decreased abundance of Lactobacillales (Supplemental Figure 3). Consistent with the phylum distribution, no changes were observed at the family level in the stomach, jejunum, or colon at 1 or 3 dpi (Supplemental Figure 3A-D). Genus level identify confirmed that the neonatal GI tract is normally dominated by Lactobacillus species (Figure 4). However, in the ileum at 1 dpi, decreased levels of Lactobacillus and Enterococcus species were accompanied by increases in Bacteroides, Akkermansia, Enterobacter, Ezakiella, and Faecalibacterium compared with controls (Figure 4c). No changes in microbiome composition were observed in any compartment at 3 dpi (Figure 4a, b, d).
Figure 3.
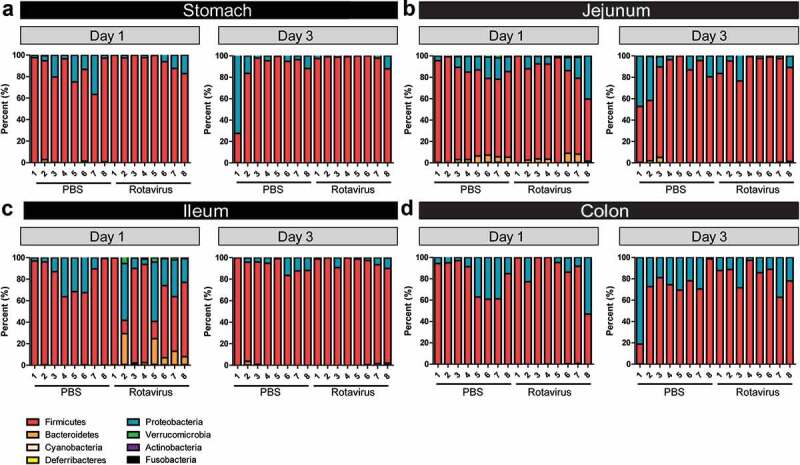
Phylum shifts occur in the ileum at day 1 post-rotavirus infection. 16S rRNA sequencing analysis of the bacterial community at the level of phylum. Eight phyla are presented: Actinobacteria (purple), Bacteroidetes (orange), Cyanobacteria (peach), Deferribacteres (yellow), Firmicutes (red), Fusobacteria (black), Proteobacteria (blue), and Verrucomicrobia (green). Data is represented for the stomach (a), jejunum (b), ileum (c), and colon (d) of PBS-treated (black) and rotavirus-infected (red) BALB/c pups at 1 and 3 dpi. Only statistical significance was reached in the ileum at 1 dpi (p < .05) One-Way ANOVA.
Figure 4.
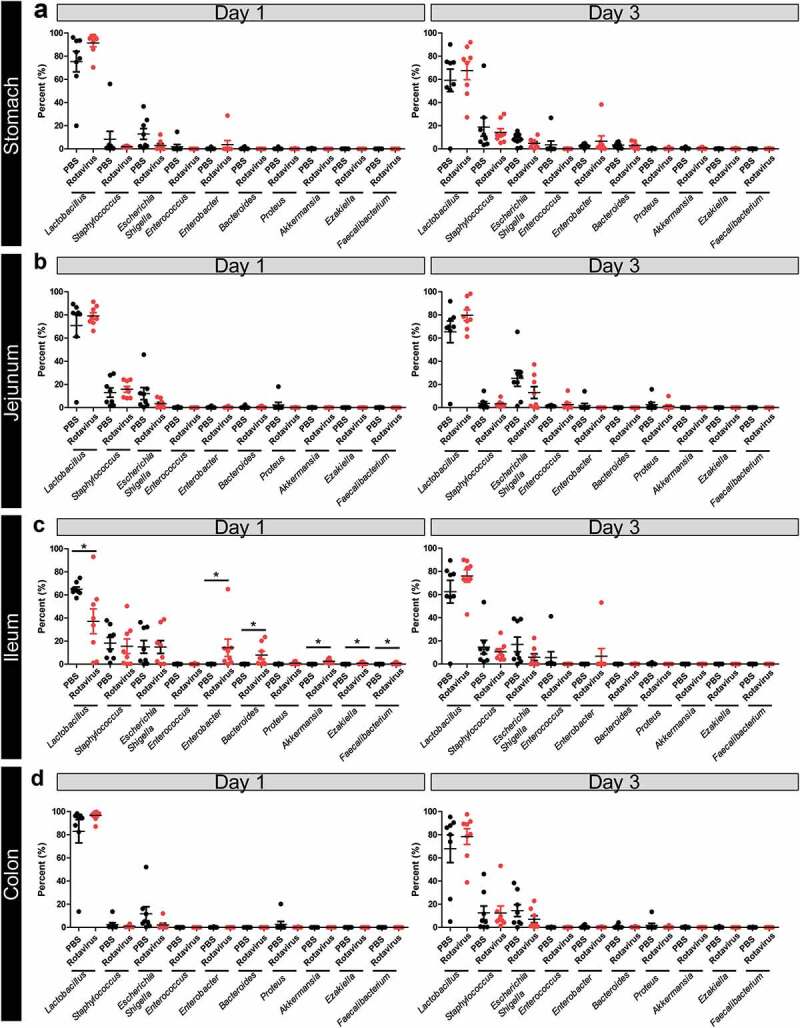
Shifts in bacterial genera are observed in the ileum at 1 dpi. Relative abundances of the top 10 genera present in the expressed contents of the stomach (a), jejunum (b), ileum (c), and colon (d) of PBS-treated (black) and rotavirus-infected (red) BALB/c pups at 1 and 3 dpi as determined by 16S rRNA gene sequencing. Only statistical significance was reached in the ileum at 1 dpi (p < .05) One-Way ANOVA.
Mucin stores are depleted in the small intestine of rotavirus-infected mice
Since we observed changes in a number of mucin-digesting microbes in the rotavirus-infected animals, we next examined the intestinal mucus layer in PBS or rotavirus-inoculated mice. Under normal conditions, the secreted mucus layer and cell surface mucins of the glycocalyx form a network that helps maintain the microbiota at a safe distance from the host. First, Periodic Acid Schiff-Alcian Blue (PAS-AB) staining was used to identify acidic (purple) and neutral (blue) mucins in PBS-control and rotavirus-infected mice at 1 and 3 dpi (Figure 5). At 1 dpi, rotavirus-infected mice exhibited fewer mucin-filled goblet cells in the duodenum, jejunum, and ileum compared to PBS-control mice. At 3 dpi, rotavirus-infected mice maintained fewer mucin-filled goblet cells in the duodenum and jejunum but exhibited normal levels of goblet cells stained by PAS-AB in the ileum. No changes were observed in colonic mucin levels. Immunostaining for Muc2, the dominant-secreted intestinal mucin, confirmed our PAS-AB results and showed that Muc2 levels are significantly decreased in rotavirus-infected duodenum, jejunum, and ileum at 1 dpi and rotavirus-infected duodenum and jejunum at 3 dpi compared to PBS-controls (Figure 6). Although we observed Muc2 secretion and goblet cell depletion in the duodenum and jejunum, we only observed increased levels of mucin-degrading bacteria in the ileum. We speculate that this phenomenon reflects the oxygen sensitivity and niche localization of Bacteroides and Akkermansia, which typically reside within the terminal ileum and colon. We next sought to define the localization of the bacteria within the mucus layer by fluorescent in situ hybridization (FISH) with a universal bacteria probe (Supplemental Figure 4). In concordance with diminished Muc2 staining in rotavirus-infected ileum, the microbes were in closer proximity to the host epithelium compared with microbes from PBS-control mice, but this distance returned to baseline by 3 dpi.
Figure 5.
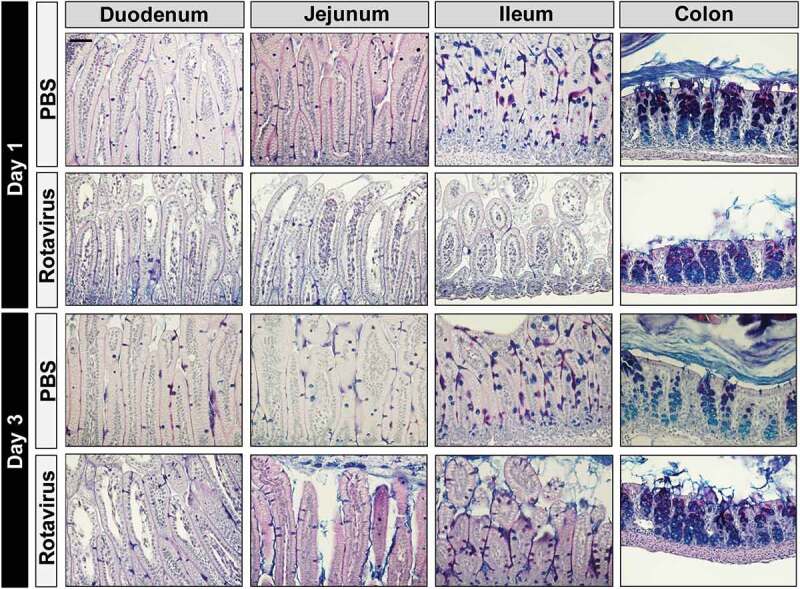
Infection with rotavirus depletes mucus-filled goblet cells. Periodic Acid-Schiffs Alcian Blue (PAS-AB) stains at 1 and 3 dpi of intestinal segments (stomach, jejunum, ileum, and colon) from PBS-treated or rotavirus-infected mice. Acidic mucins are denoted by their purple staining. PAS-AB images were acquired on an upright Nikon Eclipse 90i microscope using a 20X Plan Apo (NA 0.75) DIC objective with a DS-Fi1-U2 camera and Nikon Elements software. Scale bar = 50 µm.
Figure 6.
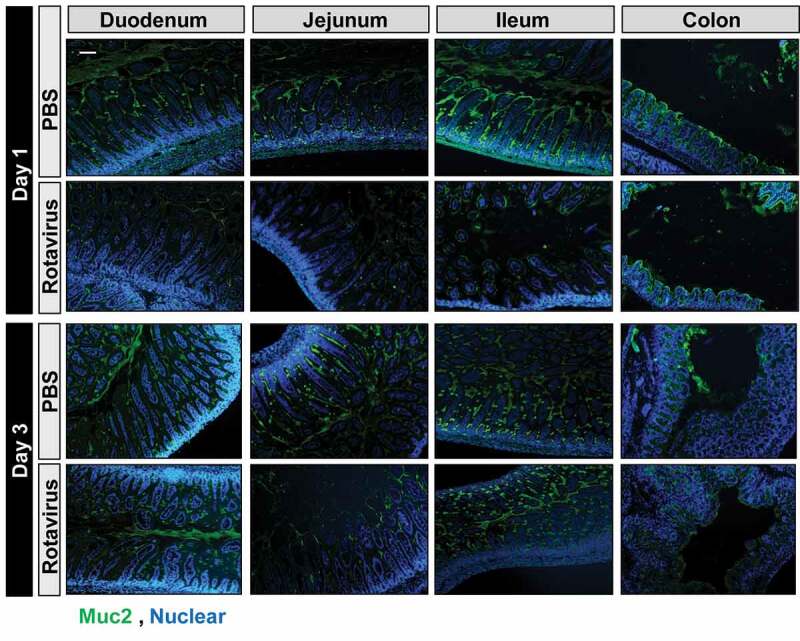
Infection with rotavirus depletes Muc2-filled goblet cells. Muc2 immunostaining at 1 and 3 dpi of intestinal segments (stomach, jejunum, ileum, and colon) from PBS-treated or rotavirus-infected mice (Muc2: green, nuclear Hoechst: blue). Fluorescent images were acquired on an upright Nikon Eclipse 90i microscope using a 10X Plan Fluor (NA 0.3) phase contrast objective with an ORCA-Flash 4.0 sCMOS camera and Nikon Elements software. Scale bar = 100 µm.
Since the jejunum and ileum displayed lower numbers of mucin-filled goblet cells at 1 and 3 dpi, we questioned whether this lack of mucin staining was due to depletion of stored Muc2 granules or loss of goblet cells themselves. To address this question, we examined several goblet cell markers by qPCR from the jejunum-ileal junction (Figure 7a–e). Although we observed decreased levels of Muc2-positive goblet cells by immunostaining, we found no change in Muc2 mRNA levels at 1 and 3 dpi (Figure 7a), indicating that the goblet cells were still present. Goblet cells also secrete trefoil factors (TFFs) and the antimicrobial RELM-β into the intestinal mucus layer.65-69 Similar to our Muc2 gene expression, no changes were observed in Tff3 or Relm-β expression in the rotavirus-infected mice compared to control mice at either 1 or 3 dpi (Figure 7b, c). These data point to granule expulsion from the goblet cells, rather than a loss of goblet cells by differentiation or apoptosis. Although the markers of goblet cell-secreted factors were unchanged, changes were observed in two key transcription factors that drive goblet cell differentiation. The goblet cell transcription factor Klf4 was increased on 1 dpi in rotavirus-infected mice and decreased on 3 dpi (Figure 7d). Additionally, the transcription factor SPEF was increased on 3 dpi in rotavirus-infected mice compared to PBS-control mice (Figure 7e). These data indicated a response of the host to produce more goblet cells. We speculate that this increase in goblet cell transcription may be a compensatory mechanism in response to the decreased mucus layer and increased localization of microbes to the epithelium.
Figure 7.
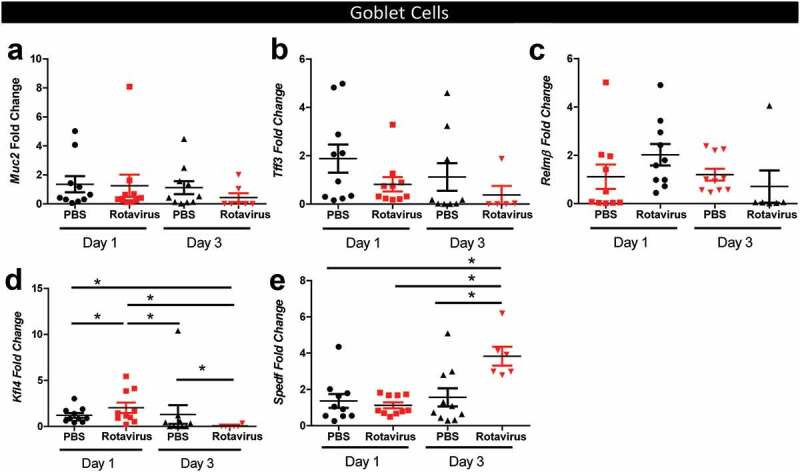
Quantitative Real-time PCR (qPCR) analysis reveals no changes in goblet cell secreted factors. qPCR of goblet cell-secreted factors Mucin 2 (Muc2) (a), Trefoil factor 3 (Tff3) (b), Relm-β (c), and goblet cell transcription factors Krüppel-like factor 4 (Kfl4) (d) and SAM pointed domain-containing Ets transcription factor (SPEDF) (e). All data is presented for PBS-treated (black) and rotavirus-infected (red) mice on 1 and 3 dpi. One-Way ANOVA *p < .05.
Goblet cells are known to secrete, or expel, Muc2 granules in response to stimuli. Release of stored mucin granules and subsequent depletion of goblet cell mucus is also associated with infection by the enteric protozoan parasite Entamoeba histolytica, and microbial pathogens C. difficile, C. rodentium, Salmonella, and V. cholera.70-78 Based on our observations of decreased Muc2 immunostaining, but normal Muc2 message levels in rotavirus-infected mice, we sought to determine if decreased Muc2 levels in goblet cells were due to mucin expulsion. We mock- or rotavirus-infected the MUC2-producing cell lines HT29-MTX and T84 and examined secreted mucin content after 16 hr incubation by Alcian blue/glacial acetic acid staining (Figure 8). Rotavirus infection increased expulsion of mucin from both mucin-producing cell types, resulting in an accumulation of mucus. Moreover, rotavirus infection of jejunual human intestinal enteroids (HIEs), a more biologically relevant system of small intestinal epithelium, also increased mucin expulsion compared to mock-inoculated cells (Figure 8). These data indicate that rotavirus infection triggers the release of stored mucin granules.
Figure 8.
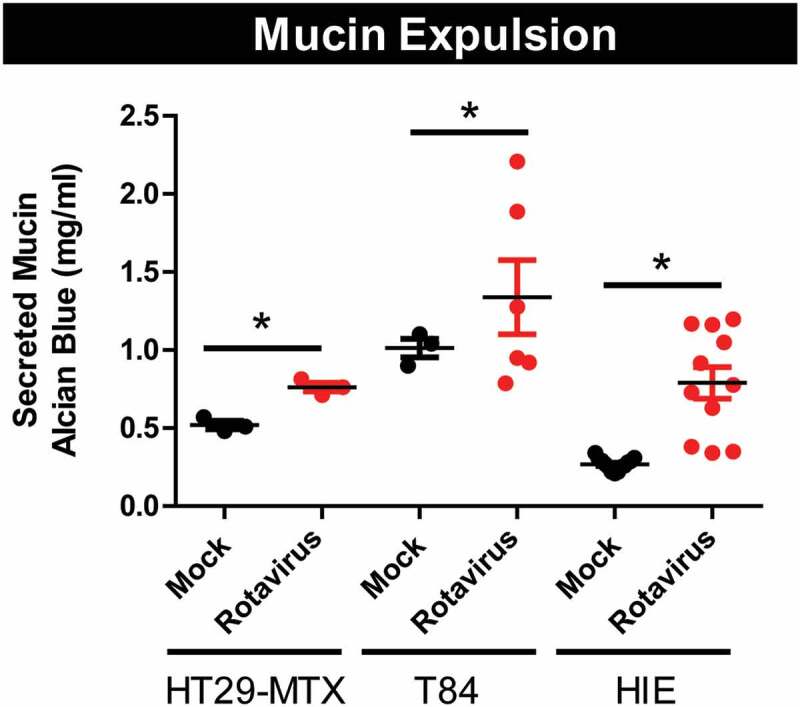
Rotavirus stimulates release of stored mucin-granules in vitro. MUC2-producing human cell lines, HT29-MX and T84 cells, as well human intestinal enteroids (HIEs) derived from jejunum were infected with Ito rotavirus in serum-free DMEM in the presence of 10 µg/mL Worthington’s Trypsin MA104 lysates. Supernatant was collected after overnight infection (16 h) and mucin-content was examined by PAS staining. Rotavirus infection (red) stimulated release of mucin compared to mock treatment (black). One-Way ANOVA *p < .05.
Bacteroides and Akkermansia species participate in rotavirus infection
Mucin glycans mirror structures found on epithelial cells. As a result, mucus has been hypothesized to serve as a decoy for enteric viruses. In theory, viruses adhere to mucus rather than enterocytes and may be expelled from the intestine. Rotavirus binds mucus, particularly mucin cores 2, 4, and 6 that harbor complex glycan structures.79 Select microbes can enzymatically cleave mucin glycans using glycosyl hydrolase enzymes. Cleaved glycan oligosaccharides benefit the bacteria since they can be used as a primary energy source. However, degradation of mucin glycans may also affect the ability of mucus to serve as decoy for pathogens.
To confirm the glycan-degrading capacity of the bacteria identified in the ileum of rotavirus-infected mice, we queried publicly available annotated genomes of Bacteroides and Akkermansia to identify glycosyl hydrolase enzymes specifically involved in mucin degradation. In silico analysis of the glycosyl hydrolases of Bacteroides and Akkermanisa species suggested that both groups harbor a large number of glycosyl hydrolases (GH) families that are involved in mucin degradation (Figure 9a–g). All annotated Bacteroides (n = 21) and Akkermanisa (n = 3) harbored high copy numbers of glycosyl hydrolase family 33 (GH 33), members of which remove sialic acid residues from mucin glycans (Figure 9a). The removal of sialic acid is considered the first step in mucin-glycan degradation. The Bacteroides and Akkermansia strains also possess homologs of fucosidases (GH 29, GH 95), which removes terminal fucose residues (Figure 9b, c), galactosidases (GH 2, GH 20), which remove galactose residues and N-acetylglucosaminidase (GH 84 and 89) (Figure 9d, e), which remove GlcNAC (Figure 9g, i). Interestingly, only Bacteroides members harbored homologs of the GH 42 family of galactosidase, indicating the potential for Bacteroides to remove more galactose residues than Akkermansia (figure 9f).
Figure 9.
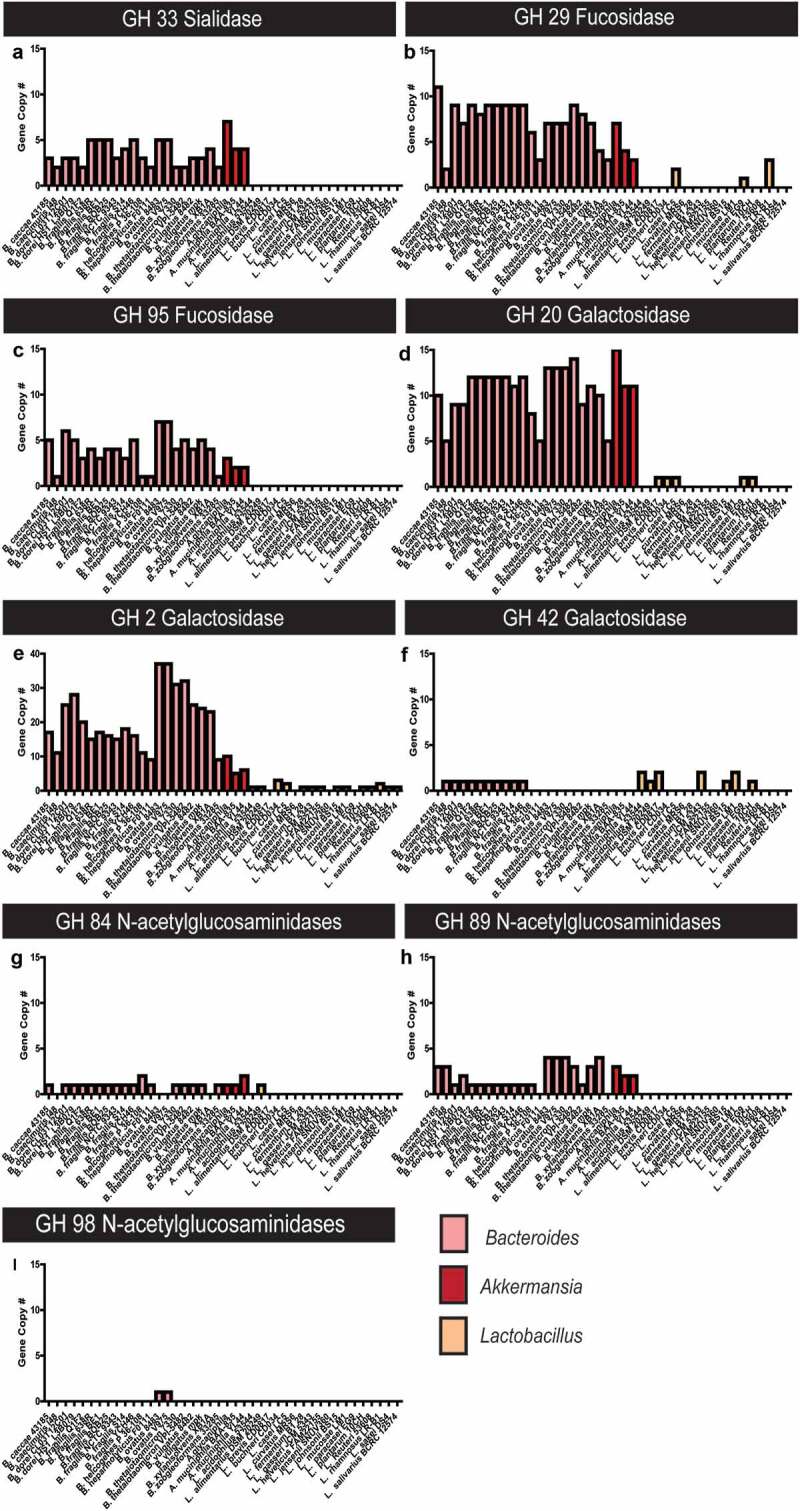
Bacteroides and Akkermansia species harbor extensive glycosyl hydrolases for mucin glycan degradation. All annotated Bacteroides and Akkermanisa genomes as well as selected intestinal Lactobacilli genomes were screened for glycosyl hydrolases using the CAZY database. Data presented in terms of copy number for glycosyl hydrolase (GH) families predicted to be involved in O-linked (A–I) mucin degradation. Glycosyl hydrolases include: a. sialidase GH 33, b. Fucosidase GH 29, c. Fucosidase GH 95, d.Galactosidase GH 20, e. Galactosidase GH 2, f. Galactosidase GH 42, g.N-acetylglucosaminidase GH 84, h. N-acetylglucosaminidase GH 89, and I. N-acetylglucosaminidase GH 98.
In contrast to the mucin glycan-degrading Bacteroides and Akkermansia members, analysis of the GHs in intestinal Lactobacillus species suggested that these members harbored few mucin-degrading GHs. No annotated Lactobacillus species contained GH 33 (sialidase), GH 95 (fucosidase), GH 89, or 98 (N-acetyglucosaminidase) (Figure 9). Three Lactobacillus species contained GH 29, homologs for fucosidase (Figure 9b), five species contained GH 20, homologs for galactosidase (Figure 9d), and one species contained GH 84, homologs for N-acetylglucosaminidase (Figure 9g). Lactobacilli were also found to harbor GH 2 and 42 (potential galactosidase) gene copies (Figure 9e, f). However, Lactobacillus species harbored far fewer gene copies of mucin-degrading genes compared to Bacteroides and Akkermansia. These analyses reveal the potential capacity of Bacteroides and Akkermansia to extensively degrade mucin glycans.
To determine if the rotavirus-associated microbial population had a functional shift toward mucin-glycan degradation, representative species B. thetaiotaomicron, A. mucinphila, and L. acidophilus were examined for their ability to degrade mucin glycans. Bacteria were incubated with germ-free, purified mouse mucin Muc2 and the phenol-sulfuric acid method was used to quantify cleaved mucin oligosaccharides (Figure 10A). Consistent with their GH profile, B. thetaiotaomicron and A. muciniphila exhibited an increased ability to enzymatically cleave mucin glycans compared to L. acidophilus. Mucin glycans have been speculated to serve as a decoy molecule for viral proteins and sequences of the VP8 domain of the rotavirus spike protein VP4 have been shown to bind to mucin core 2 glycans.79-81 To determine if Bacteroides and Akkermansia species could change the decoy potential of Muc2, plaque assays were performed in the presence of intact or degraded Muc2. As a negative control, we included methylcellulose, a polysaccharide commonly used to mimic the viscosity of a mucus environment, but which lacks glycan structures.82 Addition of intact, untreated Muc2 to rotavirus-infected wells resulted in decreased plaque formation [Plaque-Forming Units per milliliter of virus stock (PFU/ml)] (Figure 10B). Pre-incubation of Muc2 with B. thetaiotaomicron or A. muciniphila diminished the ability of Muc2 to prevent rotavirus-infection. Pre-incubation of Muc2 with L. acidophilus had no effect on the ability of Muc2 to prevent rotavirus-infection; an effect that mirrors the GH profiles of Lactobacili. Addition of methylcellulose also had no effect on rotavirus-infection. These data demonstrate that mucin glycans may act as a decoy binding mucin for rotavirus. These findings also point to the potential for mucin-degrading bacteria to influence the molecular mimicry of mucins and promote rotavirus infection.
Figure 10.
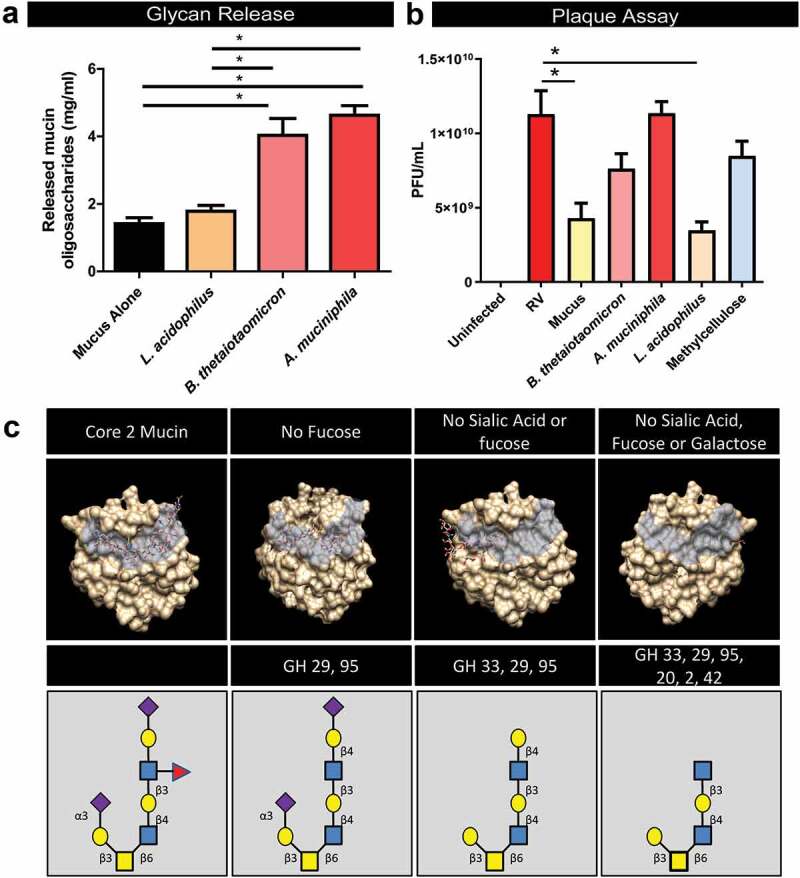
Bacteroides and Akkermanisa degrades mucin glycans in a manner which diminishes the binding capacity of rotavirus VP4. a. Incubation of Bacteroidetes thetaiotaomicron and Akkermansia mucinphila with purified germ-free cecal Muc2 result in release mucin oligosaccharides, as determined by the phenol-sulfuric acid method. Incubation of Muc2 with Lactobacillus acidophilus had no effect on oligosaccharide concentration. b. Plaque Assays quantification (plaque-forming units per milliliter of virus (PFU/ml)) of rotavirus added to MA104 African green monkey kidney in the presence of 10 mg/ml intact Muc2 or Muc2 pre-treated with B. thetaiotaomicron, A. mucinphila or L. acidophilus. Methylcellulose, which mirrors the viscosity of mucus was added as another control. One-Way ANOVA *p < .05. c. In silico analysis of the VP8 domain of the spike protein of rotavirus VP4 binding to mucin core 2 glycans (constructed using SWEET II software).
In an in silico approach, we constructed a typical mucin core 2 glycan structure and removed the glycan groups that would enzymatically cleaved based on the GHs profiles of Bacteroides and Akkermanisa (Figure 10C). Using molecular docking tools, we found that the VP8* domain of rotavirus has a diminished capacity to bind glycans lacking sialic acid, fucose, or galactose. These findings indicate that degradation of mucin glycans by B. thetaiotaomicron or A. muciniphila diminishes the protective role of mucus in minimizing rotavirus infection.
Discussion
Multiple studies have focused on the pathophysiology of rotavirus infection, but few studies examine the alteration of the intestinal bacterial composition, especially at the site of the small intestine. This study has provided an in-depth examination of the changes in the gut microbiota as a result of rotavirus infection and diarrhea using a neonatal mouse model of disease. The 16S rRNA data presented herein demonstrates that rotavirus-infection is capable of shifting alpha and beta diversity and diverging the composition of the microbiota from PBS-controls in the ileum at 1 dpi. In the ileum, increased representation of Bacteroides, Akkermansia, and Enterobacter was observed with decreased Lactobacillus species. These shifts in the microbiome were restored by 3 dpi, indicating the elasticity of the gut microbiota. At 1 dpi, we also observed significant decreases in Muc2-filled goblet cells, with no changes in Muc2 mRNA levels, indicating that Muc2 granules were expelled. Rotavirus alone was found to stimulate release of stored mucin granules from mucin-producing cell lines and HIEs. Finally, Bacteroides and Akkermansia species were shown to harbor an extensive network of glycosyl hydrolases (GH) which could degrade mucin glycans. We demonstrate using in vitro plaque assays that Muc2 complex glycans diminish rotavirus plaque formation, suggesting that mucin glycans can serve as a decoy for rotavirus. Muc2 mucins degraded by Bacteroides and Akkermansia were unable to prevent plaque formation. Consistent with our in vitro data, in silico analysis of mucin glycans confirmed that truncated glycans were unable to dock with rotavirus proteins. These findings point to a potential role of the ileal microbiome in promoting rotavirus infection.
A growing body of literature demonstrates that the resident microbiome participates in host susceptibility to viruses.83 The microbiome has been implicated in the pathogenesis of poliovirus, reovirus, norovirus, and mouse mammary tumor virus.84-89 In these models, depletion of the microbiota with antibiotics leads to a substantial attenuation of infection and reduced levels of viral replication compared to mice with an intact gut microbiota. Moreover, in poliovirus infection, a fecal microbiota transplant from untreated mice to antibiotic-treated mice is sufficient to restore pathogenesis.85 Similar to other enteric viruses, antibiotic treatment also suppresses rotavirus infection and reduces infectivity by 42%.90 Recently, Shi et al. demonstrated that a microbiome possessing high levels of Akkermansia supports rotavirus infections, while a microbiome containing segmented filamentous bacteria (SFB) can protect again rotavirus infection.91 Although rotavirus can infect germ-free mice, rotavirus infection persists longer (5 additional days post-infection) in conventional mice and has increased paracellular permeability at later time points.92 These data point to the intestinal microbiota in dictating the timing, magnitude, and duration of rotavirus infection. Together, these findings highlight the unique role that intestinal microbes have in promoting rotavirus infection.
Our work demonstrates that in neonatal mice, Bacteroides (phylum Bacteroidetes), Akkermanisa (phylum Verrucomicrobia), and Enterobacter (phylum Proteobacteria) are elevated in the ileum at 1 dpi, with corresponding decreases in Lactobacilli (phylum Firmicutes). Consistent with our findings, several groups have shown large-scale changes in Bacteroidetes in response to rotavirus infection. In neonatal gnotobiotic pigs, rotavirus infection increases the levels of Bacteroidetes and decrease levels of Firmicutes.93 Increased Bacteroidetes members are also present in children with rotavirus-induced gastroenteritis,94 and Zhang et al. demonstrated that three fecal Bacteroides OTUs were able to discriminate healthy children from rotavirus-infected children.95 In addition to Bacteroides, a positive correlation has also been found in children between Akkermansia and susceptibility to rotavirus.96 Moreover, infants receiving the oral rotavirus vaccine, Rotarix (RV1) demonstrated a modest enrichment of the phyla Bacteroidetes and Verrucomicrobia at 10 weeks of age; which correlated to shed vaccine rotavirus levels.97 These findings appear similar to our neonatal mouse ileum data, which suggests changes in Bacteroides and Akkermansia may influence rotavirus infection. Future studies using small intestinal samples are warranted to confirm these findings in humans.
Rotavirus infection has a profound effect on small intestinal epithelial homeostasis. Rotavirus infection induces villus atrophy, enhances epithelial cell turnover, promotes apoptosis, and is hallmarked by the presence of large vacuoles in enterocytes98-102. Work by Boshuizen and colleagues also identified that mouse rotavirus (EDIM) in BALB/c mice induced loss of mucin-filled goblet cells.103 Consistent with these findings, we also observed large vacuoles, villus atrophy, and loss of Muc2-positive goblet cells. The gold standard for identifying goblet cells by staining is secreted factors such as Muc2 and its glycans, Tff3, Relmβ, etc. As a result, it is difficult to rule out whether the goblet cells are absent or simply devoid of secreted components. Based on our in vitro assays using mucin-producing cell lines and HIEs where we observed secretion of MUC2 into the supernatant, we speculated that rotavirus infection stimulates the release of stored mucin granules and other secreted products, leaving the goblet cells unfilled rather than absent. This hypothesis is supported by our qPCR data, which indicated no differences in the expression of goblet cell-secreted compounds (Muc2, Tff3, and Relmβ) between PBS and rotavirus-infected mice. We believe this most likely indicates that our goblet cells are still functional and present after rotavirus infection. Interestingly, we also observed increased expression of transcription factors that determine goblet cell fate; which may indicate that the cell populations may be shifting toward increased goblet cell number. While we think our data indicates that the goblet cell population is maintained, but empty following rotavirus infection, we cannot eliminate the possibility that there are fewer goblet cells with increased Muc2 expression. In the future, mining of single-cell RNAseq may point to potential markers for staining. These are important considerations for future studies.
Rotaviruses attach to the membrane of host enterocytes in a process that requires the presence of specific glycans.104 It has been shown that rotavirus uses the VP8* domain of the spike protein VP4 to attach to cell surface glycans.79,105 Rotaviruses in general are known to adhere to sialic acid and galactose residues,106 oligosaccharides that are also found on mucin glycans. Rotavirus binds to mucin glycans, particularly core 2 and core 4 glycan structures which are commonly found in the intestine.79,105,107,108 Importantly, the adherence to mucin glycans appears to be conserved across multiple rotavirus strains.79,105 Crude and purified intestinal mucins from suckling and adult mice have been shown to be potent inhibitors of replication of rhesus rotavirus.109 Our research indicates that the addition of murine intestinal mucin inhibited adherence of rhesus rotavirus to MA104 cells, suggesting that mucins possess molecular mimicry and can prevent virus-cell attachment. In this model, removal of murine sialic acid by neuraminidase suppressed the ability of mucins to prevent rotavirus-cell adhesion, implying the functional importance of mucins’ polymeric structure and sialic acid content in its decoy function. Our in silico and in vitro data support the importance of intact mucin glycans in promoting anti-rotavirus infection effects. Consistent with our findings, mucus isolated from control mice was also able to neutralize infection by the human Wa rotavirus in Caco-2 cells.103 However, mucin isolated from mice infected with the murine rotavirus (EDIM) were less efficient in inhibiting rotavirus infection than the control mucus. The authors speculated that this decreased capacity to neutralize rotavirus was related to mucin glycosylation. Our work takes this observation step further by identifying that degradation of mucin glycans by the gut microbes is sufficient to suppress the protective effects of mucins.
Our data suggest that microbial nutrient availability shifts during rotavirus infection, thereby favoring mucus-degrading microbes. Dietary nutrients are known to decrease during rotavirus infection due to reduced dietary intake, partial loss of appetite, less nutritious liquid diets, and shorter GI transit time.110 Since luminal nutrients are depleted and rotavirus stimulates goblet cells to release stored mucin granules, it is likely that mucosa-associated microbes that harbor mucin-degrading glycosyl hydrolases can thrive. In the ileum, the rotavirus-induced glycan-availability favors the increased abundance of glycan-degrading bacteria, which in turn, decreases mucin-protection against rotavirus infection. These data suggest that rotavirus may stimulate mucin secretion to promote infection.
In addition to our proposed mechanism of enzymatic glycan cleavage, the intestinal microbiota may influence viral pathogenesis through several other mechanisms. Poliovirus can directly bind to the Gram-negative bacterial outer-membrane component lipopolysaccharide (LPS).85,111 Binding of poliovirus to LPS or N‑acetylglucosamine containing polysaccharides enhanced virion stability and promoted the environmental fitness of the virus.85,111 Picornaviruses (Enterovirus genus members: CVB3-H3, CVB3-Nancy, and PV) and reovirus also bind to Gram-negative bacteria, which increases virus stability in the gut.112,113 Rotavirus can bind to Gram-negative E. coli Nissile,114 so it is also possible that rotavirus may bind to Gram-negative Bacteroides or Akkermansia. Adhesion of viruses to Gram-negative bacteria has also been proposed as a mechanism to promote attachment to target cells. Adhesion of poliovirus via LPS-mediated attachment to HeLa cells111 and bacteria-mediated enhancement of norovirus infection stimulated viral attachment to B cells.88 As mucin-degrading bacteria, Akkermansia and Bacteroides can get into closer proximity to the host epithelium than non-mucin-degrading bacteria, thus possibly aiding rotavirus in entry to enterocytes. Finally, the microbiota could also indirectly enhance infection by skewing the antiviral immune response toward tolerance. Recent work has demonstrated that immunostimulatory bacterial antigen flagellin can prevent rotavirus infection in mice,115 pointing to the potential role of the immune system in mediating rotavirus clearance. A. muciniphila as a pathobiont has been shown to attenuate pro-inflammatory cytokines116-118 and thus may participate in immune skewing during rotavirus infection. While we cannot rule out these alternative mechanisms in vivo, our in vitro assays are performed without bacteria, and still provide evidence that mucin degradation decreases the “decoy” ability of mucin to prevent rotavirus infection. Thus, the mucin-glycan structure appears to be an important host barrier to viruses.
Finally, these findings bring to light the potential for mucus to be an important selective pressure in transmissible enteric viruses. By serving as a decoy, some rotavirus is prevented from reaching the epithelium and expelled rotavirus could go on to infect other hosts. Therefore, the presence of mucus could also play a role in rotavirus dissemination. Collectively this work points to an interplay between the gut microbiota, host goblet cells, and rotavirus. A deeper understanding of mucus–virus interactions will likely provide further insights into rotavirus transmission, host restriction, and viral adaptation; information that could be invaluable for creating targeted therapeutics for rotavirus infection.
Materials & methods
Mouse model of rotavirus infection
BALB/c dams with natural litters were purchased from Charles River Laboratory (STRAIN 028). Mice were maintained on a normal mouse diet (7922 NIH-07 Mouse diet, Harlan Laboratories, Indianapolis, IN) and autoclaved deionized water, and housed with nestlets in microisolator cages. Pups were acclimated to the BSL2 facility for 24–48 h prior to treatment, and then allocated at random into two groups (n = 11 to 14) at d 7 or 8 of life. Pups were then orally gavaged with either 50 µl of 109 pfu/ml rhesus rotavirus (RRV) or the same volume of phosphate-buffered saline (PBS), monitored daily for weight and diarrhea severity, and finally euthanized at d 1 or 3 post-infection. To assess diarrhea, mouse pups’ abdomens were gently palpated to express and visualize feces. Stools were assessed on a scale between 1 and 4 for volume, color, and consistency as described previously,119 and an average score of ≥2.5 signified diarrhea. Percentage of pups with diarrhea was calculated as the number of stools with a diarrhea score ≥2.5 divided by the number of stools collected per cage per day. Pups from which a stool sample could not be collected on that day were not counted for percent diarrhea or mean diarrhea severity calculations. Following euthanasia, mice were dissected with sterile surgical instruments to remove the GI tract en masse from the pyloric sphincter to the distal colon, and individual GI segments collected with care to minimize cross-contamination from other mouse tissues. All animal experiments were performed with approval by the Institutional Animal Care and Use Committee (IACUC) at Baylor College of Medicine, Houston, TX, in accordance with U.S. National Institutes of Health guidelines.
Tissue fixing and staining
Excised intestinal segments used for 16S rRNA in-situ and immunofluorescence studies were fixed in Caronys fixative (Fisher, #S25237) or 10% formalin (Sigma Aldrich, #HT501128). Tissue was collected n = 6 mice irrespective of diarrhea scores. Intestinal segments were dehydrated through a series of ethanol and xylene and embedded in paraffin for sectioning. In addition, segments of ileum/ jejunum were dissected and stored in TRIZOL at −80⁰C for RNA isolation. Hemotoxylin & Eosin (H&E), Periodic Acid-Schiffs Alcian Blue (PAS-AB) and immunostaining were performed on 7-µm sections. Briefly, H&E stains were performed by a series of incubations with alum hematoxylin, 0.3% acid alcohol, Scott’s tap water, and eosin. Acidic mucins were identified using Alcian blue (AB) at pH 2.5 followed by periodic acid-Schiff's reagent (PAS). Following staining, slides were dehydrated, and a coverslip applied with mounting media (ThermoFisher, #14-390-5). For immunostaining, slides were dehydrated, and antigens were exposed by incubating slides in Vector Labs Antigen Unmasking Solution Citrate Buffer pH 6 (Vector labs, #F4680) in a steamer for 20 min. Sections were blocked for 1 h at room temperature in 10% donkey serum. Sections were incubated with anti-Muc2 antibody (dilution: 1:200, Rabbit anti-Muc2, Santa Cruz, #sc-15334) overnight at 4°C, followed by donkey-anti-rabbit-ALEXA 488 (dilution 1:300, Life Technologies, #A-21206) for 1 hr at room temperature. Nuclear stains were achieved by incubation with Hoechst 33342 (Sigma, #B2261) at room temperature for 10 min. All sections were cover-slipped with Fluoromount Aqueous Mounting Medium (Sigma Aldrich, #F4680) and imaged on a Nikon Eclipse 90i (Nikon). Semi-quantitative analysis of fluorescent stains was accomplished using FIJI (Formerly ImageJ) software by tabulating mean pixel intensity (National Institutes of Health) as previously described.4,75,120 The distance of the bacteria from the host was examined by fluorescent in situ hybridization (FISH) staining using a universal bacterial probe (5ʹ- [Cyanine3]-GCTGCCTCCCGTAGGAGT-3ʹ Integrated DNA Technologies, IDT) and Muc2 staining. Briefly, 7-µm sections were dehydrated in ethanol and incubated with the universal bacteria probe at 51°C in a humidifying dark chamber. Slides were hybridized for 45 min and counterstained with Hoechst for 10 min at room temperature.
All slides were imaged with widefield epifluorescence on a Nikon TiE inverted microscope and an upright Nikon Eclipse 90i microscope using a SPECTRA X LED light source (Lumencor). The following objectives were used: 10X Plan Fluor (NA 0.3) phase contrast objective, 20X Plan Apo (NA 0.75) differential interference contrast (DIC) objective, or a 40X Apo DIC water objective. Color images for H&E and PAS-AB sections were recorded using a DS-Fi1-U2 camera (Nikon). Fluorescence images were recorded using either an ORCA-Flash 4.0 sCMOS camera (Hamamatsu). Nikon Elements Advanced Research v4.5 software was used for data acquisition and image analysis.
16S rRNA gene sequencing and compositional analysis
16S rRNA gene sequencing methods were adapted from the methods developed for the NIH-Human Microbiome Project.121,122 Briefly, bacterial genomic DNA was extracted using MO BIO PowerSoil DNA Isolation Kit (MO BIO Laboratories). The 16S rRNA V4 region was amplified by PCR and sequenced on the MiSeq platform (Illumina) using the 2 × 250 bp paired-end protocol yielding pair-end reads that overlap almost completely. The primers used for amplification contain adapters for MiSeq sequencing and single-end barcodes allowing pooling and direct sequencing of PCR products. The 16S rRNA gene pipeline data incorporates phylogenetic and alignment-based approaches to maximize data resolution. The read pairs were demultiplexed based on the unique molecular barcodes, and reads were merged using USEARCH v7.0.1090.123 The pipeline for 16S rRNA analysis leverages custom analytic packages and pipelines were developed at the center for Metagenomics and Microbiome Research (CMMR) at Baylor College of Medicine. 16S rRNA gene sequences were clustered into Operational Taxonomic Units (OTUs) at a similarity cutoff value of 97% using the UPARSE algorithm. OTUs were mapped to an optimized version of the SILVA Database containing only the 16S v4 region to determine taxonomies. Abundances were recovered by mapping the demultiplexed reads to the UPARSE OTUs.124,125 A custom script constructed an OTU table from the output files generated in the previous two steps for downstream analyses of alpha-diversity, beta diversity, and phylogenetic trends.126 ATIMA (Agile Toolkit for Incisive Microbial Analyses), a software developed by the CMMR, was used to analyze and visualize microbiome data sets. Statistical analyses were also performed using ATIMA.
Gene expression analysis
Mouse jejunum/ileum was stored in TRIZOL and RNA was extracted according to manufacturer details (Invitrogen, # 15596026). The SensiFAST cDNA synthesis kit (Bioline USA Inc., # BIO-65053) was used to synthesize cDNA from 1 µg of mouse intestinal RNA. Gene expression was examined by quantitative real-time PCR (qPCR) using Fast SYBR Green (Thermo-Fisher, #4385617) and primers designed with PrimerDesign Software on a Qunatstudio3 qPCR Machine (ThermoFisher). Relative fold change was calculated using the ΔΔCT with the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Cell line cultures
Human colon derived T84 (ATCC CCL-248) cells were obtained from ATCC and maintained in Gibco Dulbecco’s Modified Eagle Medium (DMEM) F-12 (ThermoFisher) containing 2 mM GlutaMax (ThermoFisher) and 10% fetal bovine serum (FBS) at 37°C, 5% CO2. African green monkey kidney (MA104) cells were obtained from ATCC and HT29-MTX (12040401) cells were obtained from Sigma Aldrich. MA104 and HT29-MTX cells were maintained in DMEM with 10% fetal bovine serum (FBS) at 37°C, 5% CO2. Cell lines were routinely tested for Mycoplasma using the Mycoplasma Detection Kit (Lonza, cat# LT07-518). Cells were seeded at 5 × 105 cells/cm2 in 6-well tissue culture-treated plates (Corning) and grown to confluence.
Human intestinal enteroid cultures
Jejunum human intestinal enteroid (HIE) cultures were generated previously and deposited into the Texas Medical Center Digestive Diseases Center (TMC-DDC) Gastrointestinal Experimental Model Systems (GEMS) Core. We used jejunal cultures from patient J3, grown in complete media with growth factors (CMGF+), and maintained in culture in Matrigel (Corning, #354248) as described previously.127,128 HIE monolayers were prepared from three-dimensional cultures and seeded into flat 96-well plates as described previously.129,130 After 24 h in CMGF+ and 10 µM Y-27632 Rock inhibitor (Tocris Biosciences, CAT# 1254), the medium was changed to differentiation media, prepared as described previously.127,131 Differentiation medium was changed every day for 4 d to differentiate cells.
Rotavirus infection
Mucin-producing cell lines (HT29-MTX and T84) were grown to confluence in 6-well plates and then infected with rotavirus. Briefly, rotavirus strain SA114 F was propagated in African green monkey kidney MA104 cells in serum-free DMEM in the presence of 1 µg/mL Worthington’s Trypsin (Worthington Biochemical). Harvested cells were then subjected to three freeze/thaw cycles. Mock MA104 cell lysates and Ito rotavirus were treated with 10 µg/mL Worthington’s trypsin, incubated for 30 min at 37°C, then used to inoculate HT29-MTX and T84 cell monolayers. Inoculum was removed after 1 hr and replaced with fresh media. Monolayers were incubated overnight at 37°C with 5% CO2. Following incubation, the supernatants were removed and used for mucin quantification.
For HIE cultures, HIEs were grown in 96-well plates and differentiated for 4–5 d. Confluent differentiated HIE monolayers were washed once with CMGF- and then inoculated with MA104 cell lysate or rotavirus in CMGF-. Inoculum was then removed after 2 h and replaced with differentiation media for overnight incubation at 37°C with 5% CO2. Following incubation, the supernatants were removed and stored for mucin quantification.
HIE and cell line mucus expulsion assay
Following incubation (16–18 hr) with rotavirus, supernatant was collected and incubated with Alcian blue as previously described.132 Briefly, supernatant was incubated with equal volume 1% Alcian blue, 3% glacial acetic acid for 2 h at room temperature. Mucin proteins were pelleted by centrifugation at 12,000 x g for 10 min and mucin-containing pellets were washed three times with PBS. Pellets were then resuspended in PBS containing 10% SDS, sonicated to release the Alcian blue color and absorbance was measured at 620 nm on a spectrophotometer. A standard curve of pig stomach mucus was included and used to calculate mucin concentrations.
Bacterial culturing
Bacteroides thetaiotaomicron ATTC 29148, Akkermansia muciniphila ATCC BAA-83 and Lactobacillus acidophilus ATCC 4356 were used in this study. B. thetaiotamicron was cultured in Brain-heat-infusion medium supplemented with 2% yeast extract, 0.2% cysteine (BHIS). A. muciniphila was cultured in BHIS with 0.5% porcine stomach mucin (Sigma Aldrich CAT# M2378). L. acidophilus was cultured in De Man, Rogosa and Sharpe (MRS) medium. All cultures were grown in an Anaerobe Systems AS-580 anaerobic chamber supplied with a mixture of 10% CO2, 10% H2, and 80% N2 at 37°C.
Germ-free mouse mucus degradation assay
Germ-free mouse cecum contents were pooled together for mucus extraction (n = 10) as previously described methods.133,134 Briefly, diluted cecal were incubated with phenylmethylsulfonyl fluoride, EDTA, and iodoacetamide to prevent further degradation. Crude mucin protein was isolated by ethanol precipitation and purified by Guanidine chloride and cesium chloride isopycnic density gradient high-speed ultracentrifugation (70 Ti rotor, Beckman Coulter Inc.).135,136 Muc2 fractions were screened by dot blot with a rabbit anti-Muc2 antibody (Santa Cruz CAT # sc-15334). Pooled Muc2 fractions were dialyzed, lyophilized, and resuspended in HEPES-buffered Hanks salt solution (HH) at 10 mg/ml.
For mucin-degrading assays, cultures were grown in their appropriate rich media to exponential phase. Cultures were then adjusted to OD600 nm = 1.5 and bacteria were pelleted by centrifugation at 7,000 x g for 10 min. Pellets were washed thoroughly 3x with anaerobic PBS. After washing, bacterial pellets were resuspended in 10 mg/ml germ-free mouse Muc2 in defined media lacking glucose. Chemically Defined Minimal Medium (CDMM)137,138 was used for B. thetaioatomicron and A. muciniphilia, while Lactic Acid Bacteria Defined Media (LDMIV)139 was used for L. acidophilus. All cultures were incubated anaerobically at 37°C for 48 h. After incubation, Muc2 was precipitated by addition of ice-cold 100% ethanol and centrifugation at 9,000 x g for 30 min. The supernatant containing any carbohydrates was concentrated by vacuum centrifugation at 30⁰C overnight. The phenol-sulfuric acid method was used to quantitate cleaved carbohydrates as previously described140 with glucose as a standard. Briefly, 50 µl of sample was added into 96-well plate, followed by 150 µl concentrated H2SO4 and 30 µl of 5% phenol in PBS. Carbohydrates were measured colorimetrically at 490 nm on a 96-well plate reader. The ethanol-precipitated Muc2 pellet was washed 2x with PBS and resuspended in DMEM. The degraded Muc2 was used for rotavirus plaque assays.
Mucus plaque-inhibition assay
Prior to inoculation, rotavirus was trypsinized and incubated for 1 h with DMEM, germ-free mouse-derived Muc2, bacterial-treated Muc2, or methylcellulose (10 mg/ml). For plaque assays, MA104 cells monolayers were seeded in 6-well plates, and 2 d following seeding the cells were inoculated 1 hr with in-house rotavirus strain RRV at a dilution of 1 × 10−7) and the respective treatment. After 1 hr, the inoculum was removed and overlay media, comprised of equal parts of 2.4% Avicel (FMC Corporation) and 2x DMEM (Gibco), was added. Plaque assays were harvested 72 hr later and were fixed and stained with crystal violet to visualize plaques. Titer is represented as plaque-forming unites per milliliter (PFU/ml).
Identification of glycosyl hydrolases from bacteroides and Akkermansia species using the Carbohydrate-Active Enzymes database (CAZY)
Bacteroides and Akkermansia glycosyl hydrolases were examined using the Carbohydrate-Active Enzyme (CAZy) database (https//www.cazy.org) as previously described.141-144 Glycosyl hydrolase copy numbers were collected from all annotated genomes of Bacteroides and Akkermansia. Only glycosyl hydrolases known to be involved in mucin degradation were included for analysis (GH 2, 20, 29, 33, 42, 84, 85, 89, 95, 101, 129).107
Computational Modeling
Mucin glycans were constructed using SWEET II software (www.glycoscience.de). Sequences of the VP8 domain of the spike protein of rotavirus VP4, which are known to bind to mucin glycans,79 were obtained from PDB (http://www.rcsb.org) and docked with glycans using PatchDock (https://bioinfo3d.cs.tau.ac.il/PatchDock). Resulting structures were visualized using Chimera software (https://www.cgl.ucsf.edu/chimera).
Statistical analysis
Graphs were generated using GraphPad Prism software (version 8) (GraphPad Inc., La Jolla, CA). For statistical analysis of the microbiome data, the ATIMA software uses Mann-Whitney U test and Kruskal Wallis test to compare the difference between medians of 2 or more groups. For bacterial genus comparison, the data was found to be normally distributed and a One-Way ANOVA was used. PCoA plots employ the Monte Carlo permutation test to estimate p-values. All p-values are adjusted for multiple comparisons with the FDR algorithm. For all other statistical analyses, comparisons were made with either One-way ANOVA or a Repeated Measures One-Way ANOVA with the Bonferroni post-hoc test. The data are presented as mean ± SEM, with differences between the groups considered significant at P < .05 (*).
Supplementary Material
Funding Statement
This work was supported by the National Institutes of Health [R01DK115507]; National Institutes of Health [R21AI137710]; National Institutes of Health [P30DK56338]; National Institutes of Health [K12GM084897]; National Institutes of Health [T32DK007664-28]; National Institutes of Health [F30DK112563].
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norman JM, Handley SA, Virgin HW.. Kingdom-agnostic metagenomics and the importance of complete characterization of enteric microbial communities. Gastroenterology. 2014;146(6):1459–1469. doi: 10.1053/j.gastro.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown EM, Sadarangani M, Finlay BB.. The role of the immune system in governing host-microbe interactions in the intestine. Nat Immunol. 2013;14(7):660–667. doi: 10.1038/ni.2611. [DOI] [PubMed] [Google Scholar]
- 4.Engevik MA, Aihara E, Montrose MH, Shull GE, Hassett DJ, Worrell RT. Loss of NHE3 alters gut microbiota composition and influences bacteroides thetaiotaomicron growth. Am J Physiol Gastrointest Liver Physiol. 2013;305(10):G697–711. doi: 10.1152/ajpgi.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engevik MA, Faletti CJ, Paulmichl M, Worrell RT. Prebiotic properties of galursan HF 7K on mouse gut microbiota. Cell Physiol Biochem. 2013;32(7):96–110. doi: 10.1159/000356631. [DOI] [PubMed] [Google Scholar]
- 6.Engevik MA, Hickerson A, Shull GE, Worrell RT. Acidic conditions in the NHE2 -/- mouse intestine result in an altered mucosa-associated bacterial population with changes in mucus oligosaccharides. Cell Physiol Biochem. 2013;32(7):111–128. doi: 10.1159/000356632. [DOI] [PubMed] [Google Scholar]
- 7.D’Argenio V, Salvatore F. The role of the gut microbiome in the healthy adult status. Clin Chim Acta. 2015;451:97–102. doi: 10.1016/j.cca.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14(8):e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Human Microbiome Project C . Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerritsen J, Smidt H, Rijkers GT, de Vos WM. Intestinal microbiota in human health and disease: the impact of probiotics. Genes Nutr. 2011;6:209–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halfvarson J, Brislawn CJ, Lamendella R, Vazquez-Baeza Y, Walters WA, Bramer LM, D’Amato M, Bonfiglio F, McDonald D, Gonzalez A, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. 2017;2(5):17004. doi: 10.1038/nmicrobiol.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rehman A, Rausch P, Wang J, Skieceviciene J, Kiudelis G, Bhagalia K, Amarapurkar D, Kupcinskas L, Schreiber S, Rosenstiel P, et al. Geographical patterns of the standing and active human gut microbiome in health and IBD. Gut. 2016;65(2):238–248. doi: 10.1136/gutjnl-2014-308341. [DOI] [PubMed] [Google Scholar]
- 14.Eun CS, Kwak M-J, Han DS, Lee AR, Park DI, Yang S-K, Kim YS, Kim JF. Does the intestinal microbial community of Korean Crohn’s disease patients differ from that of western patients? BMC Gastroenterol. 2016;16(1):28. doi: 10.1186/s12876-016-0437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alipour M, Zaidi D, Valcheva R, Jovel J, Martinez I, Sergi C, Walter J, Mason AL, Wong GKS, Dieleman LA, et al. Mucosal barrier depletion and loss of bacterial diversity are primary abnormalities in paediatric ulcerative colitis. J Crohns Colitis. 2016;10(4):462–471. doi: 10.1093/ecco-jcc/jjv223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mar JS, Mar JS, LaMere BJ, Lin DL, Levan S, Nazareth M, Mahadevan U, Lynch SV. Disease severity and immune activity relate to distinct interkingdom gut microbiome states in ethnically distinct ulcerative colitis patients. MBio. 2016;7:e01072-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah R, Cope JL, Nagy-Szakal D, Dowd S, Versalovic J, Hollister EB, Kellermayer R. Composition and function of the pediatric colonic mucosal microbiome in untreated patients with ulcerative colitis. Gut Microbes. 2016;7(5):384–396. doi: 10.1080/19490976.2016.1190073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haberman Y, Tickle TL, Dexheimer PJ, Kim M-O, Tang D, Karns R, Baldassano RN, Noe JD, Rosh J, Markowitz J, et al. Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest. 2014;124(8):3617–3633. doi: 10.1172/JCI75436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong M, Li X, Wegener Parfrey L, Roth B, Ippoliti A, Wei B, Borneman J, McGovern DPB, Frank DN, Li E, et al. A modular organization of the human intestinal mucosal microbiota and its association with inflammatory bowel disease. PLoS One. 2013;8(11):e80702. doi: 10.1371/journal.pone.0080702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajilic-Stojanovic M, Shanahan F, Guarner F, de Vos WM. Phylogenetic analysis of dysbiosis in ulcerative colitis during remission. Inflamm Bowel Dis. 2013;19(3):481–488. doi: 10.1097/MIB.0b013e31827fec6d. [DOI] [PubMed] [Google Scholar]
- 21.Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng Z, Järnerot G, Tysk C, Jansson JK, Engstrand L, et al. A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139(6):1844–54 e1. doi: 10.1053/j.gastro.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 22.McIlroy J, Ianiro G, Mukhopadhya I, Hansen R, Hold GL. Review article: the gut microbiome in inflammatory bowel disease-avenues for microbial management. Aliment Pharmacol Ther. 2018;47(1):26–42. doi: 10.1111/apt.14384. [DOI] [PubMed] [Google Scholar]
- 23.Jeffery IB, O’Toole PW, Ohman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61(7):997–1006. doi: 10.1136/gutjnl-2011-301501. [DOI] [PubMed] [Google Scholar]
- 24.Krogius-Kurikka L, Lyra A, Malinen E, Aarnikunnas J, Tuimala J, Paulin L, Mäkivuokko H, Kajander K, Palva A. Microbial community analysis reveals high level phylogenetic alterations in the overall gastrointestinal microbiota of diarrhoea-predominant irritable bowel syndrome sufferers. BMC Gastroenterol. 2009;9(1):95. doi: 10.1186/1471-230X-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H-N, Wu H, Chen Y-Z, Chen Y-J, Shen X-Z, Liu -T-T. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig Liver Dis. 2017;49(4):331–337. doi: 10.1016/j.dld.2017.01.142. [DOI] [PubMed] [Google Scholar]
- 26.Kerckhoffs AP, Samsom M, van der Rest ME, de Vogel J, Knol J, Ben-Amor K, Akkermans LM. Lower bifidobacteria counts in both duodenal mucosa-associated and fecal microbiota in irritable bowel syndrome patients. World J Gastroenterol. 2009;15(23):2887–2892. doi: 10.3748/wjg.15.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ringel Y, Maharshak N. Intestinal microbiota and immune function in the pathogenesis of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2013;305(8):G529–41. doi: 10.1152/ajpgi.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crouzet L, Gaultier E, Del’Homme C, Cartier C, Delmas E, Dapoigny M, Fioramonti J, Bernalier-Donadille A. The hypersensitivity to colonic distension of IBS patients can be transferred to rats through their fecal microbiota. Neurogastroenterol Motil. 2013;25(4):e272–82. doi: 10.1111/nmo.12103. [DOI] [PubMed] [Google Scholar]
- 29.Giamarellos-Bourboulis E, Tang J, Pyleris E, Pistiki A, Barbatzas C, Brown J, Lee CC, Harkins TT, Kim G, Weitsman S, et al. Molecular assessment of differences in the duodenal microbiome in subjects with irritable bowel syndrome. Scand J Gastroenterol. 2015;50(9):1076–1087. doi: 10.3109/00365521.2015.1027261. [DOI] [PubMed] [Google Scholar]
- 30.Tap J, Derrien M, Tornblom H, Brazeilles R, Cools-Portier S, Dore J, Störsrud S, Le Nevé B, Öhman L, Simrén M, et al. Identification of an intestinal microbiota signature associated with severity of irritable Bowel Syndrome. Gastroenterology. 2017;152(1):111–23 e8. doi: 10.1053/j.gastro.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 31.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 32.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto J-M, Kennedy S, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500(7464):541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 34.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liou AP, Paziuk M, Luevano JM Jr., Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5:178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94(1):58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87(3):534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106(7):2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong L-C, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot J-L, Zucker J-D, Doré J, Clément K. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98(1):16–24. doi: 10.3945/ajcn.113.058743. [DOI] [PubMed] [Google Scholar]
- 40.Gallardo P, Izquierdo M, Vidal RM, Chamorro-Veloso N, Rossello-Mora R, O’Ryan M, Farfán MJ. Distinctive gut microbiota is associated with diarrheagenic escherichia coli infections in Chilean children. Front Cell Infect Microbiol. 2017;7:424. doi: 10.3389/fcimb.2017.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Youmans BP, Ajami NJ, Jiang Z-D, Campbell F, Wadsworth WD, Petrosino JF, DuPont HL, Highlander SK. Characterization of the human gut microbiome during travelers‘ diarrhea. Gut Microbes. 2015;6(2):110–119. doi: 10.1080/19490976.2015.1019693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh P, Teal TK, Marsh TL, Tiedje JM, Mosci R, Jernigan K, Zell A, Newton DW, Salimnia H, Lephart P, et al. Intestinal microbial communities associated with acute enteric infections and disease recovery. Microbiome. 2015;3(1):45. doi: 10.1186/s40168-015-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monira S, Nakamura S, Gotoh K, Izutsu K, Watanabe H, Alam NH, Nakaya T, Horii T, Ali SI, Iida T, et al. Metagenomic profile of gut microbiota in children during cholera and recovery. Gut Pathog. 2013;5(1):1. doi: 10.1186/1757-4749-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsiao A, Ahmed AM, Subramanian S, Griffin NW, Drewry LL, Petri WA Jr., Haque R, Ahmed T, Gordon JI. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature. 2014;515(7527):423–426. doi: 10.1038/nature13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.David LA, Weil A, Ryan ET, Calderwood SB, Harris JB, Chowdhury F, Begum Y, Qadri F, LaRocque RC, Turnbaugh PJ, et al. Gut microbial succession follows acute secretory diarrhea in humans. MBio. 2015;6(3):e00381–15. doi: 10.1128/mBio.00381-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang JY, Antonopoulos DA, Kalra A, Tonelli A, Khalife WT, Schmidt TM, Young V. Decreased diversity of the fecal microbiome in recurrent clostridium difficile –associated diarrhea. J Infect Dis. 2008;197(3):435–438. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 47.De La Cochetiere MF, Durand T, Lalande V, Petit JC, Potel G, Beaugerie L. Effect of antibiotic therapy on human fecal microbiota and the relation to the development of Clostridium difficile. Microb Ecol. 2008;56(3):395–402. doi: 10.1007/s00248-007-9356-5. [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Yang C-L, Ge M-Y, Ibrahim M, Li B, Zhao W-J, Chen G-Y, Zhu B, Xie G-L. Regulatory role of tetR gene in a novel gene cluster of Acidovorax avenae subsp. avenae RS-1 under oxidative stress. Front Microbiol. 2014;5:335. doi: 10.3389/fmicb.2014.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L, Dong D, Jiang C, Li Z, Wang X, Peng Y. Insight into alteration of gut microbiota in Clostridium difficile infection and asymptomatic C. difficile colonization. Anaerobe. 2015;34:1–7. doi: 10.1016/j.anaerobe.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Daquigan N, Seekatz AM, Greathouse KL, Young VB, White JR. High-resolution profiling of the gut microbiome reveals the extent of Clostridium difficile burden. Npj Biofilms and Microbiomes. 2017;3(1):35. doi: 10.1038/s41522-017-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilchrist CA, Petri SE, Schneider BN, Reichman DJ, Jiang N, Begum S, Watanabe K, Jansen CS, Elliott KP, Burgess SL, et al. Role of the gut microbiota of children in diarrhea due to the protozoan parasite entamoeba histolytica. J Infect Dis. 2016;213(10):1579–1585. doi: 10.1093/infdis/jiv772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Engevik MA, Versalovic J. Biochemical features of beneficial microbes: foundations for therapeutic microbiology. Microbiol Spectr. 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu S, Chen D, Zhang J-N, Lv X, Wang K, Duan L-P, Nie Y, Wu X-L. Bacterial community mapping of the mouse gastrointestinal tract. PLoS One. 2013;8(10):e74957. doi: 10.1371/journal.pone.0074957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mu C, Yang Y, Su Y, Zoetendal EG, Zhu W. Differences in microbiota membership along the gastrointestinal tract of piglets and their differential alterations following an early-life antibiotic intervention. Front Microbiol. 2017;8:797. doi: 10.3389/fmicb.2017.00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–1416. doi: 10.1016/S0140-6736(13)60222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE, et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8:e72788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatrics. 2018;172(10):958–965. doi: 10.1001/jamapediatrics.2018.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen S-C, Tan L-B, Huang L-M, Chen K-T. Rotavirus infection and the current status of rotavirus vaccines. J Formos Med Assoc. 2012;111(4):183–193. doi: 10.1016/j.jfma.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 59.Babji S, Kang G. Rotavirus vaccination in developing countries. Curr Opin Virol. 2012;2(4):443–448. doi: 10.1016/j.coviro.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Desselberger U. Differences of rotavirus vaccine effectiveness by country: likely causes and contributing factors. Pathogens. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tissera MS, Cowley D, Bogdanovic-Sakran N, Hutton ML, Lyras D, Kirkwood CD, Buttery JP. Options for improving effectiveness of rotavirus vaccines in developing countries. Hum Vaccin Immunother. 2017;13(4):921–927. doi: 10.1080/21645515.2016.1252493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Knipping K, McNeal MM, Crienen A, van Amerongen G, Garssen J, Van’t Land B. A gastrointestinal rotavirus infection mouse model for immune modulation studies. Virol J. 2011;8(1):109. doi: 10.1186/1743-422X-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Estes MK, Morris AP. A viral enterotoxin. A new mechanism of virus-induced pathogenesis. Adv Exp Med Biol. 1999;473:73–82. [PubMed] [Google Scholar]
- 64.Osborne MP, Haddon SJ, Spencer AJ, Collins J, Starkey WG, Wallis TS, Clarke GJ, Worton KJ, Candy DCA, Stephen J, et al. An electron microscopic investigation of time-related changes in the intestine of neonatal mice infected with murine rotavirus. J Pediatr Gastroenterol Nutr. 1988;7(2):236–248. doi: 10.1097/00005176-198803000-00014. [DOI] [PubMed] [Google Scholar]
- 65.Paulsen FP, Woon C-W, Varoga D, Jansen A, Garreis F, Jager K, Amm M, Podolsky DK, Steven P, Barker NP, et al. Intestinal trefoil factor/TFF3 promotes re-epithelialization of corneal wounds. J Biol Chem. 2008;283(19):13418–13427. doi: 10.1074/jbc.M800177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Longman RJ, et al. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut. 2000;47(6):792–800. doi: 10.1136/gut.47.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taupin DR, Kinoshita K, Podolsky DK. Intestinal trefoil factor confers colonic epithelial resistance to apoptosis. Proc Natl Acad Sci U S A. 2000;97(2):799–804. doi: 10.1073/pnas.97.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wright NA, Poulsom R, Stamp G, Van Noorden S, Sarraf C, Elia G, Ahnen D, Jeffery R, Longcroft J, Pike C, et al. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993;104(1):12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]
- 69.Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996;110(2):489–497. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- 70.Meerovitch E, Chadee K. Entamoeba histolytica: early progressive pathology in the cecum of the gerbil (Meriones unguiculatus). Am J Trop Med Hyg. 1985;34(2):283–291. doi: 10.4269/ajtmh.1985.34.283. [DOI] [PubMed] [Google Scholar]
- 71.Gustafsson JK, Navabi N, Rodriguez-Pineiro AM, Alomran AH, Premaratne P, Fernandez HR, Banerjee D, Sjövall H, Hansson GC, Lindén SK, et al. Dynamic changes in mucus thickness and ion secretion during Citrobacter rodentium infection and clearance. PLoS One. 2013;8(12):e84430. doi: 10.1371/journal.pone.0084430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wlodarska M, Willing B, Keeney KM, Menendez A, Bergstrom KS, Gill N, Russell SL, Vallance BA, Finlay BB, et al. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated citrobacter rodentium -induced colitis. Infect Immun. 2011;79(4):1536–1545. doi: 10.1128/IAI.01104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhargava A, Clifton MS, Mhaske P, Liao M, Pothoulakis C, Leeman SE, Grady EF. Local injection of dsRNA targeting calcitonin receptor-like receptor (CLR) ameliorates Clostridium difficile toxin A-induced ileitis. Proc Natl Acad Sci U S A. 2013;110(2):731–736. doi: 10.1073/pnas.1219733110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cottrell GS, Amadesi S, Pikios S, Camerer E, Willardsen JA, Murphy BR, Caughey GH, Wolters PJ, Coughlin SR, Peterson A, et al. Protease-activated receptor 2, dipeptidyl peptidase I, and proteases mediate Clostridium difficile toxin A enteritis. Gastroenterology. 2007;132(7):2422–2437. doi: 10.1053/j.gastro.2007.03.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Engevik MA, Yacyshyn MB, Engevik KA, Wang J, Darien B, Hassett DJ, Yacyshyn BR, Worrell RT. Human Clostridium difficile infection: altered mucus production and composition. Am J Physiol Gastrointest Liver Physiol. 2015;308(6):G510–24. doi: 10.1152/ajpgi.00091.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fachi JL, Felipe JDS, Pral LP, da Silva BK, Correa RO, de Andrade MCP, da Fonseca DM, Basso PJ, Câmara NOS, de Sales E Souza ÉL, et al. Butyrate protects mice from clostridium difficile-induced colitis through an HIF-1-dependent mechanism. Cell Rep. 2019;27(3):750–61 e7. doi: 10.1016/j.celrep.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 77.Songhet P, Barthel M, Stecher B, Muller AJ, Kremer M, Hansson GC, Hardt W-D. Stromal IFN-γR-signaling modulates goblet cell function during salmonella typhimurium infection. PLoS One. 2011;6(7):e22459. doi: 10.1371/journal.pone.0022459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhowmick R, Ghosal A, Das B, Koley H, Saha DR, Ganguly S, Nandy RK, Bhadra RK, Chatterjee NS. Intestinal adherence of Vibrio cholerae involves a coordinated interaction between colonization factor GbpA and mucin. Infect Immun. 2008;76(11):4968–4977. doi: 10.1128/IAI.01615-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Y, Xu S, Woodruff AL, Xia M, Tan M, Kennedy MA, Jiang X. Structural basis of glycan specificity of P[19] VP8*: implications for rotavirus zoonosis and evolution. PLoS Pathog. 2017;13(11):e1006707. doi: 10.1371/journal.ppat.1006707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cohen M, Zhang X-Q, Senaati HP, Chen H-W, Varki NM, Schooley RT, Gagneux P. Influenza A penetrates host mucus by cleaving sialic acids with neuraminidase. Virol J. 2013;10(1):321. doi: 10.1186/1743-422X-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Isa P, Arias CF, Lopez S. Role of sialic acids in rotavirus infection. Glycoconj J. 2006;23(1–2):27–37. doi: 10.1007/s10719-006-5435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kavanaugh NL, Zhang AQ, Nobile CJ, Johnson AD, Ribbeck K, Berman J. Mucins suppress virulence traits of Candida albicans. MBio. 2014;5(6):e01911. doi: 10.1128/mBio.01911-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pfeiffer JK, Sonnenburg JL. The intestinal microbiota and viral susceptibility. Front Microbiol. 2011;2:92. doi: 10.3389/fmicb.2011.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pfeiffer JK, Virgin HW. Viral immunity. Transkingdom control of viral infection and immunity in the mammalian intestine. Science. 2016;351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science. 2011;334(6053):249–252. doi: 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kane M, Case LK, Kopaskie K, Kozlova A, MacDearmid C, Chervonsky AV, Golovkina TV. Successful transmission of a retrovirus depends on the commensal microbiota. Science. 2011;334(6053):245–249. doi: 10.1126/science.1210718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karst SM. The influence of commensal bacteria on infection with enteric viruses. Nat Rev Microbiol. 2016;14(4):197–204. doi: 10.1038/nrmicro.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jones MK, Watanabe M, Zhu S, Graves CL, Keyes LR, Grau KR, Gonzalez-Hernandez MB, Iovine NM, Wobus CE, Vinje J, et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science. 2014;346(6210):755–759. doi: 10.1126/science.1257147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, Virgin HW, et al. Commensal microbes and interferon- determine persistence of enteric murine norovirus infection. Science. 2015;347(6219):266–269. doi: 10.1126/science.1258025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Uchiyama R, Chassaing B, Zhang B, Gewirtz AT. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J Infect Dis. 2014;210(2):171–182. doi: 10.1093/infdis/jiu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi Z, Zou J, Zhang Z, Zhao X, Noriega J, Zhang B, Zhao C, Ingle H, Bittinger K, Mattei LM, et al. Segmented filamentous bacteria prevent and cure rotavirus infection. Cell. 2019;179(3):644–58 e13. doi: 10.1016/j.cell.2019.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heyman M, Corthier G, Petit A, Meslin J-C, Moreau C, Desjeux J-F. Intestinal absorption of macromolecules during viral enteritis: an experimental study on rotavirus-infected conventional and germ-free mice. Pediatr Res. 1987;22(1):72–78. doi: 10.1203/00006450-198707000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Twitchell EL, Tin C, Wen K, Zhang H, Becker-Dreps S, Azcarate-Peril MA, Vilchez S, Li G, Ramesh A, Weiss M, et al. Modeling human enteric dysbiosis and rotavirus immunity in gnotobiotic pigs. Gut Pathog. 2016;8(1):51. doi: 10.1186/s13099-016-0136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen S-Y, Tsai C-N, Lee Y-S, Lin C-Y, Huang K-Y, Chao H-C, Lai M-W, Chiu C-H. Intestinal microbiome in children with severe and complicated acute viral gastroenteritis. Sci Rep. 2017;7(1):46130. doi: 10.1038/srep46130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X, Ma X, Wang N, Yao T, et al. New subgroup of Bacteroidetes and diverse microorganisms in Tibetan plateau glacial ice provide a biological record of environmental conditions. FEMS Microbiol Ecol. 2009;67(1):21–29. doi: 10.1111/j.1574-6941.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 96.Rodriguez-Diaz J, Garcia-Mantrana I, Vila-Vicent S, Gozalbo-Rovira R, Buesa J, Monedero V, Collado MC. Relevance of secretor status genotype and microbiota composition in susceptibility to rotavirus and norovirus infections in humans. Sci Rep. 2017;7(1):45559. doi: 10.1038/srep45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parker EPK, Praharaj I, Zekavati A, Lazarus RP, Giri S, Operario DJ, Liu J, Houpt E, Iturriza-Gómara M, Kampmann B, et al. Influence of the intestinal microbiota on the immunogenicity of oral rotavirus vaccine given to infants in south India. Vaccine. 2018;36(2):264–272. doi: 10.1016/j.vaccine.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boshuizen JA, Reimerink JH, Korteland-van Male AM, van Ham VJ, Koopmans MP, Büller HA, Dekker J, Einerhand AWC. Changes in small intestinal homeostasis, morphology,and gene expression during rotavirus infection of infant mice. J Virol. 2003;77(24):13005–13016. doi: 10.1128/JVI.77.24.13005-13016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Collins J, Starkey WG, Wallis TS, Clarke GJ, Worton KJ, Spencer AJ, Haddon SJ, Osborne MP, Candy DCA, Stephen J, et al. Intestinal enzyme profiles in normal and rotavirus-infected mice. J Pediatr Gastroenterol Nutr. 1988;7(2):264–272. doi: 10.1097/00005176-198803000-00017. [DOI] [PubMed] [Google Scholar]
- 100.Starkey WG, Collins J, Wallis TS, Clarke GJ, Spencer AJ, Haddon SJ, Osborne MP, Candy DCA, Stephen J. Kinetics, tissue specificity and pathological changes in murine rotavirus infection of mice. J Gen Virol. 1986;67(12):2625–2634. doi: 10.1099/0022-1317-67-12-2625. [DOI] [PubMed] [Google Scholar]
- 101.Katyal R, Rana SV, Vaiphei K, Ohja S, Singh K, Singh V. Effect of rotavirus infection on small gut pathophysiology in a mouse model. J Gastroenterol Hepatol. 1999;14(8):779–784. doi: 10.1046/j.1440-1746.1999.01948.x. [DOI] [PubMed] [Google Scholar]
- 102.Shepherd RW, Butler DG, Cutz E, Gall DG, Hamilton JR. The mucosal lesion in viral enteritis. Extent and dynamics of the epithelial response to virus invasion in transmissible gastroenteritis of piglets. Gastroenterology. 1979;76(4):770–777. doi: 10.1016/S0016-5085(79)80177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boshuizen JA, Reimerink JH, Korteland-van Male AM, van Ham VJ, Bouma J, Gerwig GJ, Koopmans MPG, Büller HA, Dekker J, Einerhand AWC, et al. Homeostasis and function of goblet cells during rotavirus infection in mice. Virology. 2005;337(2):210–221. doi: 10.1016/j.virol.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 104.Bomsel M, Alfsen A. Entry of viruses through the epithelial barrier: pathogenic trickery. Nat Rev Mol Cell Biol. 2003;4(1):57–68. doi: 10.1038/nrm1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pang -L-L, Wang M-X, Sun X-M, Yuan Y, Qing Y, Xin Y, Zhang J-Y, Li -D-D, Duan Z-J. Glycan binding patterns of human rotavirus P[10] VP8* protein. Virol J. 2018;15(1):161. doi: 10.1186/s12985-018-1065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jolly CL, Beisner BM, Holmes IH. Rotavirus infection of MA104 cells is inhibited by Ricinus lectin and separately expressed single binding domains. Virology. 2000;275(1):89–97. doi: 10.1006/viro.2000.0470. [DOI] [PubMed] [Google Scholar]
- 107.Tailford LE, Crost EH, Kavanaugh D, Juge N. Mucin glycan foraging in the human gut microbiome. Front Genet. 2015;6:81. doi: 10.3389/fgene.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun X, Li D, Qi J, Chai W, Wang L, Wang L, et al. Glycan binding specificity and mechanism of human and porcine P[6]/P[19] rotavirus VP8*s. J Virol. 2018;92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen CC, Baylor M, Bass DM. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology. 1993;105(1):84–92. doi: 10.1016/0016-5085(93)90013-3. [DOI] [PubMed] [Google Scholar]
- 110.Rahaman MM, Wahed MA. Direct nutrient loss and diarrhea. In: Chen LC, Scrimshaw NS, editors. Diarrhea and malnutrition: interactions, mechanisms, and interventions. Boston (MA): Springer US; 1983. p. 155–160. [Google Scholar]
- 111.Robinson CM, Jesudhasan PR, Pfeiffer JK. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe. 2014;15(1):36–46. doi: 10.1016/j.chom.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aguilera ER, Nguyen Y, Sasaki J, Pfeiffer JK. Bacterial stabilization of a panel of picornaviruses. mSphere. 2019;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berger AK, Yi H, Kearns DB, Mainou BA, Parker JSL. Bacteria and bacterial envelope components enhance mammalian reovirus thermostability. PLoS Pathog. 2017;13(12):e1006768. doi: 10.1371/journal.ppat.1006768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kandasamy S, Vlasova AN, Fischer D, Kumar A, Chattha KS, Rauf A, Shao L, Langel SN, Rajashekara G, Saif LJ, et al. Differential Effects of Escherichia coli Nissle and Lactobacillus rhamnosus Strain GG on Human Rotavirus Binding, Infection, and B Cell Immunity. J Immunol. 2016;196(4):1780–1789. doi: 10.4049/jimmunol.1501705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang B, Chassaing B, Shi Z, Uchiyama R, Zhang Z, Denning TL, Crawford SE, Pruijssers AJ, Iskarpatyoti JA, Estes MK, et al. Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science. 2014;346(6211):861–865. doi: 10.1126/science.1256999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu W, Lv L, Shi D, Ye J, Fang D, Guo F, Li Y, He X, Li L. Protective effect of Akkermansia muciniphila against immune-mediated liver injury in a mouse model. Front Microbiol. 2017;8:1804. doi: 10.3389/fmicb.2017.01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao S, Liu W, Wang J, Shi J, Sun Y, Wang W, Ning G, Liu R, Hong J. Akkermansia muciniphila improves metabolic profiles by reducing inflammation in chow diet-fed mice. J Mol Endocrinol. 2017;58(1):1–14. doi: 10.1530/JME-16-0054. [DOI] [PubMed] [Google Scholar]
- 118.Schneeberger M, Everard A, Gomez-Valades AG, Matamoros S, Ramirez S, Delzenne NM, Gomis R, Claret M, Cani PD. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. 2015;5(1):16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272(5258):101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 120.Engevik MA, Engevik KA, Yacyshyn MB, Wang J, Hassett DJ, Darien B, Yacyshyn BR, Worrell RT. Human Clostridium difficile infection: inhibition of NHE3 and microbiota profile. Am J Physiol Gastrointest Liver Physiol. 2015;308(6):G497–509. doi: 10.1152/ajpgi.00090.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(Suppl Supplement_1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 124.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 125.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2012;41(D1):D590–6. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, Ross MC, Lloyd RE, Doddapaneni H, Metcalf GA, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562(7728):583–588. doi: 10.1038/s41586-018-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, van Houdt WJ, Pronk A, van Gorp J, Siersema PD, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141(5):1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 128.Saxena K, Blutt SE, Ettayebi K, Zeng X-L, Broughman JR, Crawford SE, Karandikar UC, Sastri NP, Conner ME, Opekun AR, et al. Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J Virol. 2015;90(1):43–56. doi: 10.1128/JVI.01930-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, Ciorba MA, Stappenbeck TS. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015;64(6):911–920. doi: 10.1136/gutjnl-2013-306651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ettayebi K, Crawford SE, Murakami K, Broughman JR, Karandikar U, Tenge VR, Neill FH, Blutt SE, Zeng X-L, Qu L, et al. Replication of human noroviruses in stem cell-derived human enteroids. Science. 2016;353(6306):1387–1393. doi: 10.1126/science.aaf5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chang-Graham AL, Danhof HA, Engevik MA, Tomaro-Duchesneau C, Karandikar UC, Estes MK, Versalovic J, Britton RA, Hyser JM. Human intestinal enteroids with inducible neurogenin-3 expression as a novel model of gut hormone secretion. Cell Mol Gastroenterol Hepatol. 2019;8(2):209–229. doi: 10.1016/j.jcmgh.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ouwehand AC, Grasten S, Niemi P, Mykkanen H, Salminen S. Wheat or rye supplemented diets do not affect faecal mucus concentration or the adhesion of probiotic micro-organisms to faecal mucus. Lett Appl Microbiol. 2000;31(1):30–33. doi: 10.1046/j.1472-765x.2000.00758.x. [DOI] [PubMed] [Google Scholar]
- 133.Ouwehand AC, Isolauri E, Kirjavainen PV, Salminen SJ. Adhesion of four Bifidobacterium strains to human intestinal mucus from subjects in different age groups. FEMS Microbiol Lett. 1999;172(1):61–64. doi: 10.1111/j.1574-6968.1999.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 134.Juntunen M, Kirjavainen PV, Ouwehand AC, Salminen SJ, Isolauri E. Adherence of probiotic bacteria to human intestinal mucus in healthy infants and during rotavirus infection. Clin Diagn Lab Immunol. 2001;8(2):293–296. doi: 10.1128/CDLI.8.2.293-296.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tkachenko A, Da Silva L, Hearne J, Parveen S, Waguespack Y. An assay to screen bacterial adhesion to mucus biomolecules. Lett Appl Microbiol. 2013;56(1):79–82. doi: 10.1111/lam.12003. [DOI] [PubMed] [Google Scholar]
- 136.Starkey BJ, Snary D, Allen A. Fractionation of water-soluble pig gastric mucus by equilibrium density-gradient centrifugation in caesium chloride. Biochem J. 1972;128:123P. [PMC free article] [PubMed] [Google Scholar]
- 137.Karasawa T, Ikoma S, Yamakawa K, Nakamura S. A defined growth medium for Clostridium difficile. Microbiology. 1995;141(2):371– 375. doi: 10.1099/13500872-141-2-371. [DOI] [PubMed] [Google Scholar]
- 138.Yamakawa K, Kamiya S, Meng XQ, Karasawa T, Nakamura S. Toxin production by Clostridium difficile in a defined medium with limited amino acids. J Med Microbiol. 1994;41(5):319–323. doi: 10.1099/00222615-41-5-319. [DOI] [PubMed] [Google Scholar]
- 139.Engevik MA, Luk B, Chang-Graham AL, Hall A, Herrmann B, Ruan W, et al. Bifidobacterium dentium fortifies the intestinal mucus layer via autophagy and calcium signaling pathways. MBio. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S, Lee YC. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal Biochem. 2005;339(1):69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 141.Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 2009;37(Database):D233–8. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol. 2013;11(7):497–504. doi: 10.1038/nrmicro3050. [DOI] [PubMed] [Google Scholar]
- 143.Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42(D1):D490–5. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park BH, Karpinets TV, Syed MH, Leuze MR, Uberbacher EC. CAZymes Analysis Toolkit (CAT): web service for searching and analyzing carbohydrate-active enzymes in a newly sequenced organism using CAZy database. Glycobiology. 2010;20(12):1574–1584. doi: 10.1093/glycob/cwq106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


