GI endoscopy, particularly when performed through the oral route, is considered an aerosol-generating procedure. Such procedures may put endoscopists, anesthetists, and other healthcare personnel at risk.1,2 Transmission of infection can be interrupted by the proper use of personal protective equipment and by performing endoscopy in a negative pressure room.3 However, these rooms may not always be readily available. Aerosol chambers have been used recently to reduce the spread of COVID-19 during endotracheal intubation4 and as endoscopic shields during endoscopy.5
A prototype aerosol chamber has been designed by Mahindra and Mahindra (Mumbai, India) with a provision for both safe endoscopy and intubation. Essentially, the chamber consists of 4 medical-grade 4-mm-thick polycarbonate sheets, which can be assembled easily into an airtight box with 4 sides closed (Fig. 1). The chamber has 4 holes: 2 on the head side of the chamber and 1 each on the side walls. These holes are covered with replaceable stickers made of thin plastic film, which are easily pierceable (Fig. 2). The holes at the head side are used for endotracheal intubation (Fig. 3) and the hole on the left for endoscope introduction (Fig. 4). The height of the chamber allows manipulation of the laryngoscope to facilitate endoscopic intubation. During endoscopy, holes on the head end can also be used for stabilizing the patient (Fig. 5).
Figure 1.
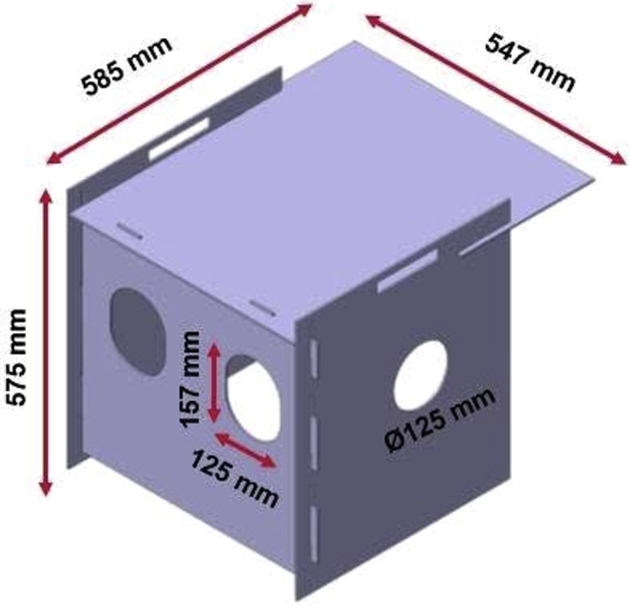
Design and dimensions of the dual-purpose aerosol chamber.
Figure 2.

Aerosol chamber demonstrating the different holes.
Figure 3.
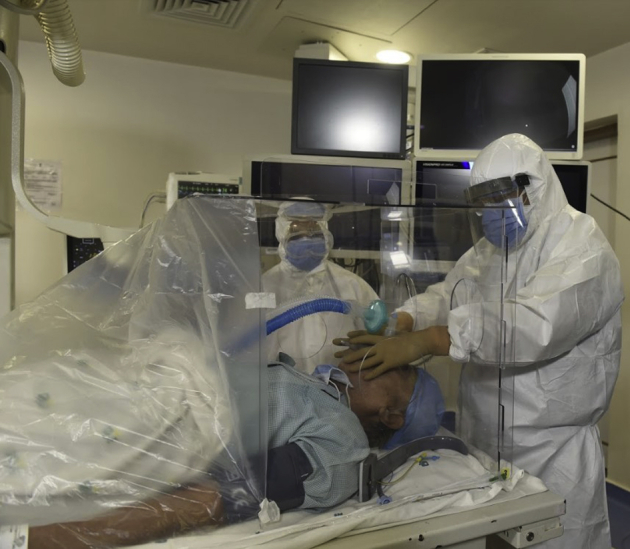
Endotracheal intubation done through the 2 holes at the head end.
Figure 4.
Endoscope piercing the hole in the left side wall.
Figure 5.
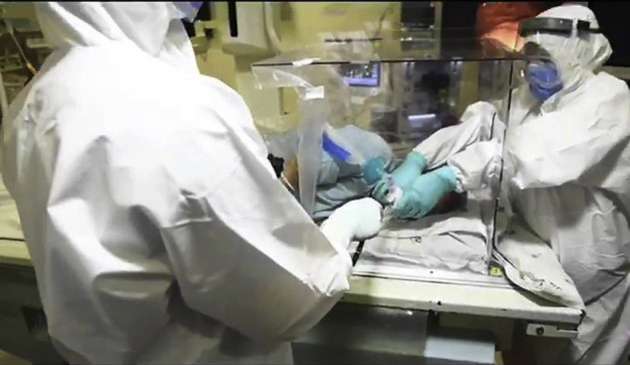
Endoscopist performing the procedure through the hole in the left side wall and the assistant stabilizing the patient with his hands through the 2 holes in the head end.
After endoscopy is complete, the chamber can be dismantled and the sheets can be sanitized before reuse. New plastic stickers are then used to cover the holes in the chamber. Our technique (Video 1, available online at www.VideoGIE.org) is simple, safe, and quick. For patients not requiring endotracheal intubation, we use a modified face mask on the patient after creating a small opening in its center to allow placement of a mouthguard through it (Fig. 6). Approval from the institutional review board was received on May 9, 2020.
Figure 6.
Modified mask to allow positioning of the mouth guard.
This aerosol chamber has the advantage of low cost (U.S.$50), easy transportability, rapid reassembly, reusability after sanitization, and ease of use for endotracheal intubation and endoscopy.
Disclosure
All authors disclose no financial relationships.
Supplementary data
Design, dimensions, and technique of use of the dual-purpose aerosol chamber.
References
- 1.Peng P.W., Ho P.L., Hota S.S. Outbreak of a new coronavirus: what anaesthetists should know. Br J Anesth. 2020;124:497–501. doi: 10.1016/j.bja.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chou R., Dana T., Buckley D.I. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Inter Med. 2020:M20–1632. doi: 10.7326/M20-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook T.M., El-Boghdadly K., McGuire B. Consensus guidelines for managing the airway in patients with COVID-19. Anaesthesia. 2020;75:785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canelli R., Connor C.W., Gonzalez M. Barrier enclosure during endotracheal intubation. N Engl J Med. 2020;382:1957–1958. doi: 10.1056/NEJMc2007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sagami R, Nishikiori H, Sato T, et al. Endoscopic shield: barrier enclosure during the endoscopy to prevent aerosol droplets during the COVID-19 pandemic. VideoGIE. Epub 2020 May 11. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Design, dimensions, and technique of use of the dual-purpose aerosol chamber.



