Herpes simplex virus 1 (HSV-1) is very successful in establishing acute and latent infections in humans by counteracting host antiviral innate immune responses. HSV-1 has evolved various strategies to evade host antiviral innate immunity and some cellular survival-associated pathways. Since there is still no vaccine available for HSV-1, a continuous update of information regarding the interaction between HSV-1 infection and the host antiviral innate immunity will provide novel insights to develop new therapeutic strategies for HSV-1 infection and its associated diseases.
KEYWORDS: DDR, NLR, RIG-I/MDA5, TLR, antiviral immunity, apoptosis, cGAS-STING, herpes simplex virus, immune evasion
SUMMARY
Herpes simplex virus 1 (HSV-1) is very successful in establishing acute and latent infections in humans by counteracting host antiviral innate immune responses. HSV-1 has evolved various strategies to evade host antiviral innate immunity and some cellular survival-associated pathways. Since there is still no vaccine available for HSV-1, a continuous update of information regarding the interaction between HSV-1 infection and the host antiviral innate immunity will provide novel insights to develop new therapeutic strategies for HSV-1 infection and its associated diseases. Here, we update recent studies about how HSV-1 evades the host antiviral innate immunity, specifically how HSV-1 proteins directly or indirectly target the adaptors in the antiviral innate immunity signaling pathways to downregulate the signal transduction. Additionally, some classical intracellular stress responses, which also play important roles in defense of viral invasion, will be discussed here. With a comprehensive review of evasion mechanisms of antiviral innate immunity by HSV-1, we will be able to develop potential new targets for therapies and a possible vaccine against HSV-1 infections.
INTRODUCTION
Herpes simplex virus 1 (HSV-1) is a member of the Alphaherpesvirinae subfamily. The viral particle is composed of the linear double-stranded DNA (dsDNA) genome of approximately 152 kb, an icosahedral capsid 100 to 110 nm in diameter, tegument, and a spikey envelope. To date, more than 80 genes encoded by HSV-1 have been identified (1). The pathogenesis of HSV-1 infection follows a cycle of primary infection of epithelial cells in the oral mucosa or genital mucosa, lifelong latency in neurons, and reactivation (2). HSV-1 primary infection usually occurs at young ages, mostly with mild clinical symptoms in immunocompetent individuals, except for a considerable fatality rate in neonates (3). HSV-1 infection is responsible for establishing primary and recurrent vesicular eruptions, primarily in the orolabial and genital mucosa (4). Approximately 3.7 billion people aged under 50 are suffering from HSV-1 infection and its associated diseases worldwide (5).
HSV-1 has existed for millennia restricted in human beings, and the battle between the host and the virus has never ceased. Innate immunity represents the first line of host defense that limits viral spread and regulates the activation of the ensuing adaptive response. After the detection of viral pathogen-associated molecular patterns (PAMPs) by host pathogen recognition receptors (PRRs), the associated signaling pathways trigger the activation of the interferon (IFN) regulatory factor (IRF) family members and the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). These transcription factors modulate the expression of type I interferons (IFN-I), including IFN-β and 13 subtypes of IFN-α in humans, which subsequently induce the expression of IFN-stimulated genes (ISGs). Theoretically, the host-virus interaction upon the primary infection could evoke the host immune responses, leading to the control and restriction of viral infection and replication. However, HSV-1 has evolved numerous strategies to evade and subvert host immune surveillance and ultimately establish a latent infection, indicating a powerful immune evasion capability for HSV-1.
During HSV-1 infection, viral glycoproteins and nucleic acids, including the abundant unmethylated CpG motifs in viral genomic DNA and some double-stranded RNA (dsRNA) intermediates, are genuine PAMPs for PRRs (6, 7). To date, at least four families of PRRs that recognize HSV-1 PAMPs have been well characterized. The first subfamily is Toll-like receptors (TLRs), whose signal transduction is dependent on adaptor protein myeloid differentiation factor 88 (MyD88) (like TLR1, 2, 7, and 9) or Toll/interleukin-1 (IL-1) receptor domain-containing adaptor inducing IFN-β (TRIF) (like TLR3 and 4) (8). Evidence indicates that the chaperone Unc-93 homolog B1 (UNC93B1) is not only essential for intracellular trafficking of these endosome-associated TLRs, including TLR3, 7, 8, and 9, exiting from endoplasmic reticulum (ER) via a direct interaction (9, 10), but is also involved in TLR stability independent of trafficking (11). Upon HSV-1 infection, the peripheral blood mononuclear cells (PBMCs) from two UNC93B1 mutant patients produce markedly lower levels of IFN-I and IFN-λ than those of healthy individuals (12), indicating that UNC93B1 may be involved in host antiviral immune responses. The second subfamily is retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), including RIG-I, melanoma differentiation-associated gene 5 (MDA5), and laboratory of genetics and physiology 2 (LGP2), via recognizing dsRNA intermediates during HSV-1 replication to trigger IFN-I production (13–15). Beside TLRs and RLRs, the cytosolic DNA sensor stimulator of interferon genes (STING) and the nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) are another two subfamilies of PRRs, which have been recently discovered to participate in sensing HSV-1 during the host’s innate immune responses (16, 17). As a dsDNA virus, HSV-1 can be recognized by the host via numerous DNA sensors and many associated proteins upstream of STING, including DNA-dependent activator of IFN regulatory factors (DAI), absent in melanoma 2 (AIM2), gamma-IFN-inducible protein 16 (IFI16), and cyclic GMP-AMP synthase (cGAS) (18–20).
HSV-1 has coexisted and coevolved with its human host for a long time (21); therefore, this tricky virus has developed multitudinous mighty immune evasion mechanisms to evade the host’s sensing and subsequent antiviral innate immunity. In this review, we will summarize and update recent findings about the strategies of HSV-1 to evade host innate immunosurveillance, including the canonical PRR-mediated induction of IFN-I and its downstream ISGs as well as some other cellular defense and protection responses, such as DNA damage response (DDR), autophagy, ER stress, and stress granule (SG)-mediated antiviral innate immunity. A comprehensive evaluation of host antiviral innate immunity and viral immune evasion strategies in the battle between the host and virus contributes to our understanding of viral pathogenesis and provides information that may help to find novel drug targets and potential immunotherapeutic treatments against HSV-1 infection-associated diseases.
HSV-1 EVADES PATHOGEN RECOGNITION RECEPTOR-MEDIATED ANTIVIRAL INNATE IMMUNITY
The expression of PRRs endows the host cells with the capacity to detect and respond to viral infections. Once engaged with viral ligands, TLRs and other cytosolic receptors transmit signals to downstream adaptor proteins to stimulate the production of IFN-I and its downstream ISGs. To survive and replicate in the host cells, viruses develop multiple strategies to counteract the host antiviral innate immunity. The classical innate immune responses initiated by TLRs, RLRs, DNA sensors, nucleotide-binding oligomerization domain-like receptor (NLR) sensors, and the downstream IFNAR-JAK-STAT signaling pathway will be discussed in this section. However, we will focus on the corresponding countermeasures by HSV-1 to restrain the host antiviral innate immunity.
TLR Signaling Pathway
TLRs play a critical role in early defense against viruses, which are initiated by recognizing viral components leading to the activation of a spectrum of innate immune signaling pathways, with a result of the induction of IFN-I, proinflammatory factors, cytokines, and chemokines (22). According to the subcellular location of PRRs, TLRs can be divided into cellular surface and intracellular endosomal receptors, among which TLR1/TLR2 and TLR4 are the main cellular surface TLRs, while TLR3, TLR7, TLR8, and TLR9 are mainly expressed in the endosomes (23, 24).
Membrane recognition of viral proteins and endosomal recognition of viral nucleic acids by TLRs are critical for controlling HSV-1 infection. To date, TLR2, TLR3, TLR4, TLR7, and TLR9 have been shown to participate in recognition of HSV-1 and induction of IFN-I during viral entry and replication (25–28). TLR2 recognizes structures from pathogens, such as bacterial proteins, yeast cell walls, and viral envelope glycoproteins. For HSV-1, TLR2 recognizes glycoproteins gB and gH/gL (29–31). In addition to priming IFN-I production, TLR2 also mediates the induction of proinflammatory cytokines in the brains of neonatal mice in vivo and microglial cells from mice upon HSV-1 infection (32, 33), leading to the overzealous neuroimmune responses in the central nervous system (CNS), which may exacerbate the mortality caused by viral encephalitis. TLR4 is a receptor for lipopolysaccharide (LPS), preferably activated by a bacterial infection, whereas TLR4 is also activated during HSV-1 infection in primary mouse astrocytes (27). However, no definite viral ligand has been identified for TLR4 until now.
The viral dsRNA intermediates produced during HSV-1 replication (34, 35) presumably serve as the ligands for TLR3 (6). In humans, TLR3-deficient individuals are prone to suffer from HSV-1 encephalitis in childhood (36, 37). A previous study with a murine model shows that TLR3 is capable of resisting the invasion of HSV-2 into the CNS in an astrocyte-mediated IFN-dependent manner in vivo (38). Interestingly, TLR3-mediated IFN-I production upon HSV-1 infection is cell type-dependent. In mouse fibroblasts and CNS resident cells, such as neurons, astrocytes, microglia, and oligodendrocytes, TLR3 is demonstrated to limit the replication of HSV-1, paralleling with IFN-I production, implicating a protective role of TLR3 for the host (38, 39). However, in human PBMCs (36) and murine macrophages (40), upon infection with HSV-1, TLR3 seems not necessary for IFN-I production. All of these studies together demonstrate that TLR3 may be dispensable for antiviral immunity in the peripheral system but plays a specific immune protective role in controlling HSV infection in the CNS. The pretreatment of the TLR7 agonists in animals markedly decreases viral load in the genital tract after HSV-1/2 infection, implying a protective role for TLR7 in HSV-1/2 infection-associated diseases (41).
In addition, the genomic DNA of HSV-1/2 is characterized by the unmethylated CpG motifs, which are the only natural ligands for TLR9 (42, 43). During HSV-1/2 infection in vivo, TLR9 stimulates the production of IFN-I in a cell-type- and time-dependent manner. In parental mice, a significant amount of IFN-I is produced in splenic plasmacytoid dendritic cells (pDCs) but not conventional dendritic cells (cDCs) upon HSV-2 infection at the early stage. Meanwhile, in TLR9 knockout (KO) mice, both pDCs and cDCs fail to produce IFN-I only at the early but not late stage of infection, indicating that TLR9 contributes to the initial but not late stage production of IFN-I upon HSV infection. However, in macrophages and fibroblasts, the production of IFN-I is dependent on viral replication but not TLR9 (44). In TLR2/TLR9 double-KO mice, the viral loads in the brain are significantly higher than those of their parental or single-gene KO mice, indicating synergetic protection for TLR2 and TLR9 in the brain (25). A recent study also confirms the cooperative role for TLR2 and TLR9 to control viral replication and dissemination in the CNS (45). To date, both canonical NF-κB/IRFs and noncanonical NF-κB pathways have been demonstrated to be involved in the IFN-I production mediated by TLR9 (46, 47).
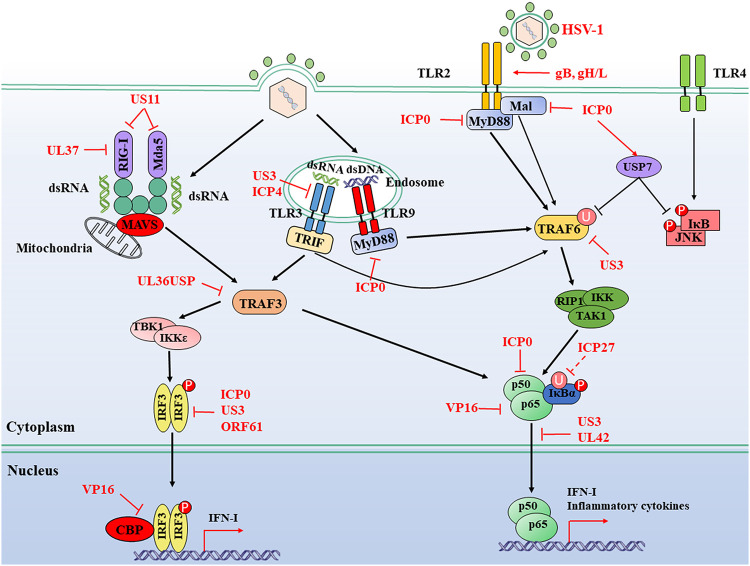
HSV-1 carries multiple ligands detected by TLRs yet persists in millions of infected individuals, indicating a breakdown in host defense against the virus. HSV-1 has evolved numerous measures to manipulate TLR signaling and evade the antiviral innate immune responses to facilitate viral replication (Fig. 1).
FIG 1.
HSV-1-mediated evasion of the RLR and TLR signaling pathway. TLRs are located at both the plasma membrane and endosomes. They sense different ligands like viral dsRNA, dsDNA, and glycoproteins; transduce signals through TRIF and MyD88; and then lead the activation of IRFs and NF-κB. RIG-I and MDA5 detect distinct RNA structures and signal through the adaptor protein MAVS protein to trigger IRF3 and NF-κB activation. The IFN-I and inflammatory cytokines are induced for antiviral immunity. HSV-1 proteins can hijack multiple steps downstream of RLR and TLR signaling pathways. Solid lines indicate confirmed interactions between adaptors and HSV-1 proteins. Dashed lines indicate uncertain interactions or that the underlying mechanism is unknown. CBP, CREB-binding protein; P, phosphate; U, ubiquitin.
HSV-1 engaged in the TLR2 signaling pathway is involved in the induction of both IFN-I and inflammatory cytokines. ICP0 is an immediate early (IE) protein carried by HSV-1 and a viral E3 ligase (48). Upon infection with wild-type (WT) HSV-1, the expression of IL-6 is relatively lower than that of ICP0-null mutants, suggesting an antagonistic role for ICP0 to inhibit host inflammatory responses (49). Ectopic expression of ICP0 is sufficient to abolish the TLR-2-driven NF-κB signaling pathway by targeting adaptor proteins MyD88 and Mal for degradation (49). Also, upon HSV-1 infection, the deubiquitinating enzyme ubiquitin-specific peptidase 7 (USP7) is hijacked by ICP0 to shuttle from the nucleus to the cytoplasm, leading to the deubiquitination of tumor necrosis factor receptor-associated factor 6 (TRAF6) and IκB kinase (IKK) γ, both of which are downstream adaptors of TLR signaling pathways (50). ICP0 also recruits USP7 to suppress the phosphorylation of Jun N-terminal protein kinase (JNK) and IκB, which are downstream of TLR4 signal activation (50). At the very early stage of infection, the virally encoded kinase and tegument protein US3 inhibits the TLR2 signaling pathway by blocking the nuclear accumulation of NF-κB, consequently leading to a reduction of inflammatory cytokines like IL-6, IL-8, and chemokine CCL2 (51). Ectopic expression of US3 inhibits TLR2 signaling at or downstream of MyD88 and upstream of p65. Although the ubiquitination of endogenous TRAF6 is inhibited in the presence of US3, its protein expression level does not change, indicating that US3 may block the TLR2 signaling pathway at or upstream of the ubiquitination of TRAF6 (51). More studies are needed to explain the exact immune evasion mechanism of the TLR2 signaling pathway by US3 and other viral proteins.
In human monocytic U937 cells, infection with a US3-null or US3/ICP4-double-null mutant, but not WT HSV-1, enhances both the transcription and the expression of TLR3 as well as IFN-I-inducible myxovirus resistance protein A (MxA) (52). Moreover, the activated dimeric IRF3 is observed in US3-null and US3/ICP4-double-null HSV-1 but not in WT or US3-rescued virus-infected U937 cells (52). These results demonstrate at least that US3 shows the capability to evade the host TLR3 signaling pathway. Recently, mammalian target of rapamycin complex 1 (mTORC1) and mTORC2 have been found to be involved in multiple TLR-mediated IFN-I inductions in vitro and in vivo. In an HSV-1-infected murine model, mTORC2 is essential for the initiation of TLR3-dependent IFN-I production in cultured primary mouse neurons (53). It could be interesting to determine whether HSV-1 is capable of inhibiting the activity of mTORC2 and evading TLR3-mediated antiviral immune responses. The tuberous sclerosis complex (TSC) is a heterodimer composed of TSC1 and TSC2 subunits. When the amino acid residues T1462 and S939 in TSC2 are phosphorylated by protein kinase B (PKB), also named Akt, the activity of TSC will be inhibited, which subsequently promotes the activation of mTORC1 (54).
Interestingly, US3, a conserved viral Ser/Thr kinase in alphaherpesviruses, is capable of masquerading as Akt to activate mTORC1. In WT HSV-1-infected primary normal human dermal fibroblast (NHDF) cells, the phosphorylation of TSC2 can still be observed even with the pretreatment of Akt inhibitor; however, in US3-null virus-infected cells, the phosphorylation of TSC2 is inhibited when treated with Akt inhibitor (55). Therefore, during HSV-1 infection, US3 may induce the activation of mTORC1, which further stimulates viral gene translation and replication in vitro (55). However, some evidence demonstrates that the activation of the TANK-binding kinase 1 (TBK1)-mTORC1 axis seems essential for the activation of TLR3/TLR4 and TLR7/TLR9 in macrophages and pDCs, respectively (56–58). These data emphasize the immune protective role of mTORC1 in activating the production of IFN-I. But, to date, no evidence has shown the immune evasion of US3 in immunocytes during viral infection. It is unknown whether mTORC1 will be activated by HSV-1 for its survival or to stimulate the antiviral response. It would be an interesting project. More evidence is needed to clarify the role of mTORC1 in the interaction between HSV-1 and host innate immune response during viral infection in different cell types.
RLR Signaling Pathway
RLRs are highly homologous in structure, sharing the same DExD/H-box RNA helicase core domain in the center and a zinc-binding domain at the C terminus. Apart from LGP2, both RIG-I and MDA5 contain two N-terminal caspase activation and recruitment domains (CARDs) (59). The CARD domain recruits the downstream adaptor mitochondrial antiviral signaling (MAVS) protein residing at the outer mitochondrial membrane and facilitates the activation of the MAVS protein to mediate signal transduction (60). Subsequently, the activated MAVS protein recruits downstream proteins TBK1/IKKε, which phosphorylate and activate IRF3 or IRF7, accompanying some other transcription factors to stimulate the expression of IFN-I and proinflammatory cytokines (61–63).
It is interesting to understand how infection with HSV-1, as a DNA virus, activates the host cellular RNA sensors. To date, some potential mechanisms have been revealed. On the one hand, during viral replication, HSV-1 generates RNA intermediates, which could be nucleic acid ligands for RLRs. For example, upon HSV-1 infection in primary murine glial cells, the viral dsDNA acts as a template for RNA polymerase III to generate 5′-ppp-RNA recognized by RIG-I followed by the activation of antiviral immune responses (64). On the other hand, host-derived RNAs produced upon HSV-1 infection could also initiate the RLR signaling pathway. Evidence shows that the recently discovered host-derived RNA named 5S rRNA pseudogene 141 (RNA5SP141) transcript could bind to RIG-I during HSV-1 infection. In uninfected cells, RNA5SP141 mainly localizes in the nucleus. However, upon HSV-1 infection, RNA5SP141 is relocalized from the nucleus to the cytoplasm, in which it can be recognized by RIG-I (65). The silencing of RNA5SP141 strongly blocks the antiviral response to HSV-1, Epstein-Barr virus (EBV), as well as influenza A virus (IAV) (65). These studies have extended RIG-I recognition from exogenous RNAs to host-derived RNAs and expanded our knowledge of the molecular mechanisms of RIG-I recognition. However, the underlying mechanisms driving the relocation of RNA5SP141 from the nucleus to the cytoplasm and whether HSV-1 activates RIG-I or MDA5 through hijacking other intracellular proteins are not yet clarified. It is still a considerable task to illuminate the underlying mechanism of RIG-I activation by HSV-1. Currently, significant progress has been made for the complex interaction between HSV-1 infection and the RLR signaling pathway (Fig. 1).
RIG-I and MDA5.
US11, an RNA-binding tegument protein encoded by HSV-1, interrupts both MDA5 and RIG-I-mediated antiviral signaling pathways. Both in ectopic expression and HSV-1-infected cell models, US11 directly targets the C-terminal domain of RIG-I and MDA5, resulting in a blockade of signal transduction. The C-terminal RNA-binding domain is required for US11 to interact directly with RIG-I and MDA5, indicating a novel role for US11 to help HSV-1 evade host innate immune surveillance (66). Upon infection with WT HSV-1, viral tegument protein UL37, which also acts as a deamidase, binds to RIG-I and mediates its deamidation, leading to an impairment of function for RIG-I and a blockade of downstream antiviral immune responses (67).
TRAF3.
TNF receptor-associated factor 3 (TRAF3) is an adaptor protein belonging to the TRAF family. It plays an essential role in both MyD88- and TRIF-dependent TLR-mediated signaling pathways, which are also implicated in the signaling activated by RIG-I and MDA5 via transmitting signals from the MAVS protein to TBK1. The K63-linked polyubiquitination of TRAF3 is indispensable for MAVS protein signaling to recruit TBK1 and IKKε (68, 69). UL36 is the largest tegument protein in the Herpesviridae; it is conserved across this family and is essential for viral replication. It contains a deubiquitinase (DUB) motif at its N terminus, also known as UL36 ubiquitin-specific protease (UL36USP) (70, 71). UL36USP abrogates Sendai virus (SeV)-induced IRF3 dimerization, promoter activation, and transcription of IFN-β by interacting with and deubiquitinating TRAF3 to prevent the recruitment of the downstream adaptor TBK1. Cells infected with UL36USP-mutated virus produce more IFN-β than those infected with WT HSV-1. These findings demonstrate that UL36USP counteracts the RLR signaling pathway via removing polyubiquitin chains on TRAF3 (72).
DNA-Sensing Signaling Pathway
The endosomal TLR9 is the first identified DNA sensor recognizing CpG DNA (43). Then, DAI, together with AIM2, RNA polymerase III, IFI16, DDX41, and some proteins involved in the DNA damaged responses (DDR) have been demonstrated to sense DNA and induce IFN-I production, playing an essential role in defense against infection with DNA viruses (73–75). cGAS has been identified as the only universal cytoplasmic DNA sensor in various cell types (76). cGAS recognizes dsDNA via a sequence-independent interaction. As shown in Fig. 2, when exposed to dsDNA, dimeric cGAS forms ladder-like networks between two separate stretches of dsDNA or on one long crooked dsDNA helix (77). Afterward, cGAS catalyzes the synthesis of cyclic GMP-AMP (cGAMP), a second messenger to activate STING through direct binding. STING polymerization and translocation through COPII-mediated vesicles via Golgi are the critical processes after binding with cGAMP (78). Later, Golgi-located STING undergoes palmitoylation and recruits TBK1 for phosphorylation transition, promoting the nuclear importation of IRF3 and NF-κB, resulting in IFN-I transcription and production (79–81). Interestingly, the adaptor protein TRIF mainly for TLR signaling is also crucial for cGAS-STING pathway-mediated antiviral responses in a cell type-dependent manner (82).
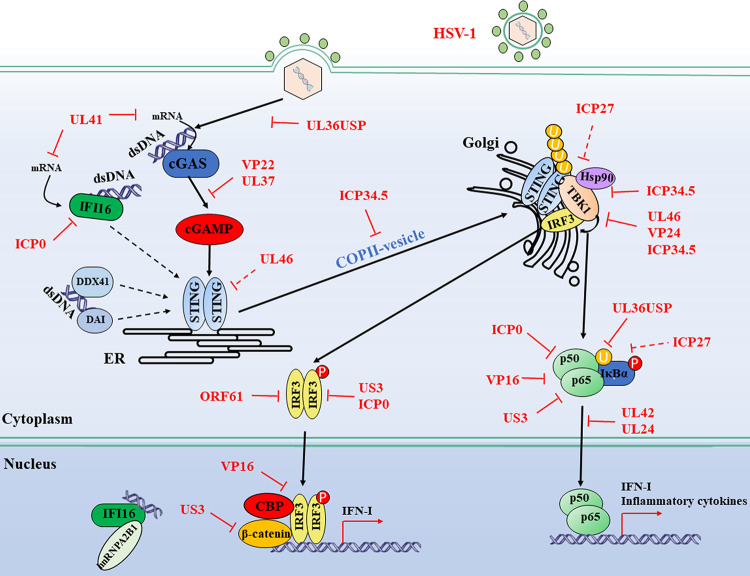
FIG 2.
Evasion of the DNA sensor-mediated IFN-I signaling pathway by HSV-1. Cytosolic DNA sensors, such as cGAS, IFI16, DDX41, and DAI, recognize double-stranded DNA in the cytosol and trigger IFN-I production through the transmission of a series of signals. Multiple steps in the DNA-sensing signaling pathway can be targeted by HSV-1 proteins, including both DNA sensor-mediated viral recognition and subsequent signaling. Many adaptors and transcription factors further downstream in the DNA sensor-mediated signaling pathway, such as TBK1, IRF3, p65, and p50, are shared by the RLR-mediated IFN-I signaling pathway. Therefore, it is plausible to consider that viral proteins targeting these molecules in the RLR-mediated signaling pathway may inhibit cytosolic DNA-sensing signals through a similar mechanism. Solid lines indicate confirmed interactions between adaptors and HSV-1 proteins. Dashed lines indicate uncertain interactions or that the underlying mechanism is not known. CBP, CREB-binding protein; P, phosphate; U, ubiquitin. (Based on data from reference 92.)
HSV-1 is a dsDNA enveloped virus. Upon infection, viral capsids are conveyed along the cytoskeleton in the cytoplasm, and then the genome is injected into the nucleus through the nuclear pore complex (83). During early infection, a certain amount of HSV-1 capsids are ubiquitinated in the cytosol and degraded by the proteasome. Hence, the genomic DNA is released into the cytoplasm and subsequently detected by DNA sensors (84). Moreover, dsDNA amplified during HSV-1 replication and assembly is released into the cytoplasm, where it is recognized and catalyzed by cGAS, leading to the activation of STING and downstream signal adaptors (84). DNA sensor-mediated innate immune pathways play an important role in restricting HSV-1 infection. Viral evasion mechanisms involved in cGAS, STING, IFI16, and the downstream signaling TBK1-IRF3 axis and NF-κB by HSV-1 components are discussed in this section and summarized in Fig. 2.
cGAS.
cGAS is activated by binding with dsDNA to generate the second message cGAMP depending on its enzymatic activity, which then activates STING to initiate the antiviral immune responses. Therefore, the enzymatic activity is indispensable for cGAS to exert its antiviral effect. However, HSV-1 can inhibit the enzymatic activity of cGAS. Infection with WT HSV-1 but not the VP22-null virus inhibits the immunostimulatory DNA (ISD)-induced activation of the IFN-I signaling pathway. The relevant mechanism indicates that VP22 can directly interact with cGAS to suppress its enzymatic activity, followed by an inhibition of IFN-I production and downstream ISGs (85). In C57BL/6J and cGAS KO mouse in vivo models, the HSV-1 tegument protein UL37 is critical for viral survival and the inhibition of IFN-β production. The underlying mechanisms reveal that, in WT HSV-1-infected human THP-1 cells, UL37 could inhibit the activation of cGAS via its deamidase activity, resulting in loss of cGAMP synthesis and blockade of downstream signaling, but not in UL37 mutant HSV-1-infected cells (86). Recently, UL41 has been shown to significantly reduce the accumulation of cGAS mRNA in HSV-1-infected human foreskin fibroblasts cells (HFF) cells and then abrogate the cGAS-STING-mediated IFN-I signaling pathway dependent on its RNase activity (87).
STING.
cGAMP binding, phosphorylation, dimerization, and trafficking are the critical steps for STING activation in response to cytoplasmic DNA. Therefore, HSV-1 is very likely to target these steps for immune evasion. ICP34.5 is a virulence factor carried by HSV-1. Upon HSV-1 infection, ICP34.5 targets STING to prevent its transport from the ER to the Golgi apparatus via direct interaction, followed by inhibition of STING activation and downstream antiviral signaling pathway (88). UL46 is a tyrosine-phosphorylated tegument protein. It is reported that the stable ectopic expression of UL46 could decrease the accumulation of transcripts and expression of both STING and IFI16. However, in WT or UL46-null HSV-1-infected cells, the expression of STING seems unaffected (89), which is not a biologically relevant phenomenon. Interestingly, our unpublished data indicate that the transient ectopic expression of UL46 does not affect the expression of STING at all.
IFI16.
IFI16 is a novel DNA sensor for both abnormal self dsDNA and viral dsDNA to induce the production of IFN-I and is involved in the inflammasome and inflammatory responses (90); it belongs to the PYHIN protein family, whose members contain a pyrin domain and two DNA binding HIN domains. The recognition of HSV-1 genomic DNA in the nucleus by IFI16 leads to the acetylation of IFI16, which is essential for its cytoplasmic redistribution and recruiting STING to activate IRF3 and NF-κB, followed by the induction of IFN-β and IL-6 to restrict viral replication and initiate an inflammatory response, respectively (91, 92). In addition, IFI16 exhibits a repressive effect on viral gene expression via a direct binding on all gene class transcription sites. Histone modifications associated with WT HSV-1 promoters are also observed in HFF by IFI16 during HSV-1 infection, leading to a restriction of genomic DNA replication. Therefore, the accumulation of IFI16 on the HSV-1 genome restricts gene expression and viral replication, independent of the inflammasome responses (93).
Although the IFI16-mediated DNA-sensing pathway can be activated during HSV-1 infection, HSV-1 has developed multiple mechanisms to evade its antiviral immune responses to facilitate viral infection and replication. During early HSV-1 infection, IFI16 mediates IFN-β induction, while at later times postinfection, ICP0 targets IFI16 for rapid degradation depending on its E3 ubiquitin ligase activity (17, 94). UL41 reduces the expression of IFI16 by degrading its mRNA (95). More studies are guaranteed to thoroughly investigate the countermeasures by HSV-1 to evade the antiviral responses mediated by IFI16 during viral infections.
TBK1-IRF3 Axis
TBK1 is a serine/threonine protein kinase that mediates the phosphorylation of IRF3, which is one of the essential transcription factors for IFN-I production. The TBK1-IRF3 axis plays a central role in almost all types of PRR-mediated antiviral innate immune responses. In that case, HSV-1 has evolved many mechanisms to interrupt the signal transduction at the level of the TBK1-IRF3 axis for survival, and many viral proteins are involved in it.
ICP0.
The viral ICP0 is a multifunctional viral protein. It contains a zinc-stabilized RING finger domain in the N terminus that confers E3 ubiquitin ligase activity, which is essential for proteasome-dependent degradation of several cellular proteins. In the TBK1-IRF3 axis, ICP0 also plays a critical role in dampening the production of IFN and ISGs during HSV-1 infection. A lot of evidence demonstrates that ICP0 inhibits the nuclear accumulation of activated IRF3 induced by SeV infection and targets it for degradation in a proteasome-dependent manner (96, 97). In SeV and HSV-1 coinfected models, ICP0 sequesters IRF3 and CBP/p300 away from the host chromatin in the nucleus and accelerates the degradation of activated IRF3 (97). Interestingly, an ICP0 homologous protein, ORF61, a viral E3 ligase encoded by another alphaherpesvirus varicella-zoster virus, targets the activated but not the resting IRF3 for degradation, showing similar effects with ICP0 (98).
Although the RING finger domain and E3 ubiquitin ligase activity are demonstrated to be important for ICP0 to inhibit IRF3- and IRF7-mediated production of ISGs, no degradation of known IRF3 components is observed in WT HSV-1-infected fibroblasts (99). Besides, studies from another group demonstrate that the cytoplasmic localization is a requisite for ICP0 to mediate the degradation of IRF3. At the early stage of viral infection, ICP0 mainly localizes in the nucleus, but with the infection progressing, the de novo translated ICP0 shuttles mostly to the cytoplasm and inhibits the IRF3 activation in a proteasome-independent manner (100). The involved mechanisms are still unclear. The distinct effects of ICP0 on IRF3 from different groups may stem from different experimental systems, cell lines, virus strains, infection time, and ectopic expressions of ICP0. Undeniably, ICP0 is a multifunctional protein to facilitate viral replication and immune evasion.
ICP27.
ICP27 is a conserved multifunctional regulatory protein among all herpesviruses. In WT HSV-1-infected THP-1 cells, the phosphorylated IRF3 is significantly reduced 18 h postinfection (hpi), while the ICP27-null virus fails to impede the activation of IRF3 during the viral infection at all time points. ICP27 has been shown to interact with TBK1 and STING in a manner depending on its RGG motif, resulting in an impairment of IRF3 activation and IFN-I production (101). It is noteworthy that ICP27, as an IE protein, controls the transcription of many viral early and late genes (102). Therefore, it is very likely that ICP27 may interfere with the IFN-I signaling pathway in an indirect way by regulating the expression of other viral proteins to facilitate immune evasion.
ICP34.5.
In human lung fibroblast (HEL) cells, the viral tegument protein ICP34.5-null HSV-1 but not WT HSV-1 is capable of evoking the activation of IRF3 both 3 and 6 hpi, indicating that ICP34.5 is a potential viral protein to block IRF3 signaling. Ectopic expression of ICP34.5 can sequester TBK1 and interfere with the interaction between TBK1 and IRF3, ultimately leading to the inactivation of IRF3 and the abrogation of IFN-I production, which may facilitate viral replication and neuroinvasion (103). The lack of TBK1-binding domain (TBD) within ICP34.5 fails to affect the replication and virulence of HSV-1 and the activation of IRF3 in HFF cells. However, infection with ICP34.5-null HSV-1 leads to a significantly higher level of phosphorylation of IRF3 than that of the WT virus, indicating that TBD does not impact TBK1 function during infection (104). Also, ICP34.5 inhibits the IRF3 activation indirectly. It restrains the phosphorylation of eukaryotic initiation factor-2α (eIF-2α) and facilitates the expression of viral proteins, such as ICP0, which act as antagonistic proteins against IFN-I production at the IRF3 level. However, the exact underlying molecular mechanism is still elusive (104).
US3.
US3 is also capable of abolishing the dimerization and nuclear translocation of IRF3 and subsequently abrogating IFN-I production induced by SeV infection. The kinase activity is essential for US3 to mediate the hyperphosphorylation of IRF3 at Ser175, an atypical phosphorylation site for IRF3. Cells and mice infected with both US3-null and kinase activity mutated viruses produced remarkably more massive amounts of IFN-β than those infected with WT HSV-1 (105). In the nucleus, β-catenin acts as a coactivator for IRF3-mediated transcription and is essential for IFN production following viral infection (106). Recently, β-catenin was shown to be required for the cGAS-STING signaling pathway to produce IFN-I during HSV-1 infection but is antagonized by viral kinase US3. Compared to the WT HSV-1 infection, the US3-null virus fails to downregulate the production of IFN-I and ISGs induced by β-catenin. The underlying mechanism is that US3 interacts with and hyperphosphorylates β-catenin at Thr556 to block its nuclear translocation (107).
US11.
The heat shock protein 90 (Hsp90) is a chaperone involved in the activation and stability of TBK1 in both RNA- and DNA-sensing signaling pathways (108, 109). Infection with the EUS11 virus, in which the promoter of US11 is deleted and replaced with the α47 promoter, could remarkably suppress the expression of IFN-β, RANTES, and ISGs like WT HSV-1. During WT HSV-1 and EUS11 viral infection, the expression level of IRF3 is not affected, but TBK1 is downregulated. The underlying mechanism reveals that, upon viral infection, US11 could interact with Hsp90 to form a complex to displace TBK1, subsequently leading to the degradation of TBK1. Correspondingly, the ectopic expression of US11 also demonstrates that US11 could disrupt the interaction between Hsp90 and TBK1 and subsequent degradation of TBK1 in a proteasome-dependent manner (110).
VP16.
VP16 is an abundant virion phosphoprotein that is synthesized in the later stage of infection and then packaged into virions (111). It is demonstrated that the ectopic expression of VP16 could significantly block the production of IFN-β induced by SeV infection in HEK 293T cells. In addition, VP16 could selectively inhibit IRF3- but not IRF7-mediated IFN-stimulated response element (ISRE) promoter activation. The underlying mechanisms reveal that VP16 binds to IRF3 and inhibits the formation of the IRF3-CBP (CREB binding protein) complex in the nucleus upon SeV infection (112).
VP24.
In HFF cells with stable knockdown of VP24, infection with HSV-1 prompts more transcription of IFN-I mRNA than that of parent cells. Evidence shows that VP24 interacts with TBK1 to abolish the interaction between TBK1 and IRF3 and inhibit the subsequent activation of IRF3 and IFN-I signaling (113).
UL46.
The infection of WT HSV-1 in vitro and ectopic expression of UL46 can significantly decrease the activation of TBK1 and abolish the interaction between TBK1 and IRF3, leading to the inhibition of IFN-I production (114). Meanwhile, in UL46-null HSV-1-infected HFF cells, the treatment of ISD could successfully induce the activation of IRF3 and TBK1, which are inhibited upon infection with WT HSV-1, indicating that UL46 is critical for HSV-1 to evade TBK1-mediated signaling (114).
NF-κB
NF-κB is a critical transcription factor involved in antiviral innate immune responses. In the resting state, IκBα combines with the NF-κB p50/p65 heterodimer to form an inactivation complex. When upstream signals activate the IKK, IκBα is phosphorylated by activated IKK and degraded by the K48-linked ubiquitin-proteasome pathway. Then, the activated NF-κB p50/p65 heterodimer is imported into the nucleus, leading to the production of IFN-I and inflammatory chemokines (115–118).
Evidence from the UL36USP mutant and WT HSV-1 shows that UL36USP has a potential role in blocking ISD-induced NF-κB activation by deubiquitinating IκBα to prevent its degradation in virally infected HFF cells (119). UL24 is conserved among the Herpesviridae family and plays a critical role in viral reactivation from latency. Recently, HSV-1 UL24 also shows the potential to target cGAS-STING-mediated NF-κB signaling pathway selectively. The ectopic expression of UL24 and WT HSV-1 infection can inhibit the transcription or production of IFN-β and IL-6 via direct binding to subunits p65 and p50, leading to a reduction of their nuclear translocation. However, compared with WT HSV-1 infection, both transcription and production of IFN-I induced by ISD are significantly rescued by the UL24 mutant (UL24X) virus (120).
ICP27 is involved in the regulation of viral gene transcription and protein translation (102). Ectopic expression of ICP27 stabilizes IκBα by blocking its phosphorylation and ubiquitination and subsequently suppresses NF-κB activation, which may contribute to the early evasion of HSV-1 infection. However, it remains unclear whether ICP27 works similarly during viral infection (121). ICP0 binds to the p65/p50 complex and degrades p50 through its E3 ligase activity and the ubiquitin-proteasome pathway, resulting in the inhibition of NF-κB activation (122). US3 interacts with and hyperphosphorylates p65 at Ser75 depending on its kinase activity, causing the blockade of its nuclear translocation and a decreased expression of inflammatory chemokine IL-8 (123). Besides interfering with IRF3 functions, ectopic expression of VP16 also blocks the activation of the NF-κB induced by SeV or tumor necrosis factor alpha (TNF-α) through direct interaction with p65 (112). UL42 is a viral DNA polymerase processivity factor and is essential for viral replication. The ectopic expression of UL42 suppresses the NF-κB activation induced by TNF-α and SeV. The underlying mechanism is that the N terminus of UL42 is required and sufficient to bind to p65 and p50 to retain them in the cytoplasm (124).
NLR Signaling Pathway
The NLR family consists of specific cytoplasmic sensors responsible for the detection of invading pathogens and endogenous danger signals. The NLR family contains more than 20 members, including AIM2, IFI16, and NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3), etc., among which, NLRP3 is one of the best-characterized inflammasome-associated DNA sensors (125). The NLRP3-mediated inflammasome pathway plays a critical role in antiviral innate immunity by inducing the production of proinflammatory cytokines, such as IL-18 and IL-1β.
The NLRP3 inflammasome is responsible for the host inflammatory responses upon viral infection and damage stress. It is an oligomeric complex comprised of a core protein NLRP3 and an adaptor protein apoptosis-associated speck-like molecule containing a caspase recruitment domain (ASC), both of which are required for the activation of pro-caspase-1 to produce two main effectors, IL-1β and IL-18 (126). Mounting evidence suggests that NLRP3 can be activated by RNA and DNA viruses, including herpesviruses (127). Viral components and danger signals from the cytosol, such as protein accumulation or mitochondrial damage caused by a viral infection, can be sensed by NLRP3 (128–130). Herpetic keratitis is a common disease caused by HSV-1 corneal infection driven by local inflammation (131). A previous study demonstrated that NLRP3 KO mice tend to be more susceptible to HSV-1 infection and develop more severe keratitis symptoms than their parental mice, indicating that NLRP3 may play a protective role (132). Surprisingly, IL-1β secretion in NLRP3 KO mice increases at all time points postinfection, but there is no significant difference for IL-18, suggesting that IL-1β and IL-18 induced by HSV-1 infection in animals function in an NLRP3 inflammasome-independent manner (132).
Appropriate activation of inflammasomes could trigger the host's antiviral inflammatory responses, but the aberrant activation may lead to the overwhelming inflammation that accelerates the infected tissue's inflammatory damage. A study of B6 mouse infection models shows that the virulent viral factors may determine the type (protective or harmful) and the inflammasome activation level (133). Compared with the less-virulent HSV-1 strains RE, F, KOS, and KOS63, the corneal infection of virulent HSV-1 strains McKrae, 17, and KOS79 in vivo induce a higher level of proinflammatory cytokines and recruit more inflammatory monocytes into the inflamed cornea, which is irrespective of viral replication (133). The exaggerated inflammatory response results in clinical corneal inflammatory herpetic disease. However, the underlying mechanisms of how virulent HSV-1 strains trigger high-level inflammatory damage are still unknown, which would be an interesting project in the future.
AIM2 and IFI16 are localized in the cytoplasm and the nucleus, respectively. In HFF cells, HSV-1 infection promotes the inflammasome activation via NLRP3 and IFI16 (17), while in keratinocytes, NLRP3, IFI16, and AIM2 are all required (134). However, in a human macrophage cell line, THP-1, HSV-1 infection can evoke inflammasome activation in a manner dependent on NLRP3-ASC-caspase-1 but not AIM2 (135). These specific cell type-dependent events illuminate that HSV-1 infection may elicit different inflammasome signaling pathways in different cell types. Various mechanisms may be evolved by HSV-1 to inhibit these pathways due to the specific inflammasome activation in different cells. During viral infection, compared with ICP0 mutant HSV-1, WT HSV-1 could induce the degradation of IFI16 in an ICP0- and proteasome-dependent manner (17), while viral RNase UL41 degrades the IFI16 mRNA depending on its RNase activity (95). VP22 can block the release of proinflammatory cytokines mediated by AIM2 via targeting the pro-caspase-1 activation as well as block the oligomerization of AIM2 (19).
IFNAR-JAK-STAT Signaling Pathway and Its Downstream ISGs
IFNAR-JAK-STAT signaling pathway.
The antiviral activities of IFN-I are initiated by binding to their cognate receptor IFNAR1 and IFNAR2 to trigger a signaling cascade, namely, the Janus kinase (JAK) signal transducer, and activation of transcription (STAT) pathways. Firstly, IFNAR1/IFNAR2 recruit and phosphorylate tyrosine kinase 2 (Tyk2) and JAK1, leading to the phosphorylation and heterodimerization of STAT1/STAT2 (136). Secondly, the heteromeric STAT1/STAT2 bind to IRF9 and form a complex known as IFN-stimulated gene factor 3 (ISGF3) to translocate into the nucleus. Lastly, the ISGF3 complex binds to the ISRE to stimulate the transcription of various ISGs. IFN-I can induce more than 300 ISG proteins, most of which may participate in the antiviral responses (137). IFNAR1/IFNAR2 KO cells permit widespread dissemination of HSV-1 to visceral organs and the nervous system, causing significantly higher viral replication and different transmissions in the host, indicating the vital role of IFN-I in limiting the systemic spread of HSV-1 (138).
As discussed above, much progress has been made to dissect the evasion mechanisms of innate immune responses to reduce IFN-I production by HSV-1. While a certain amount of IFN-I is still produced during viral entry and early infection, it will activate its downstream signaling pathway to produce ISGs. However, HSV-1 also evolves multiple mechanisms to interfere with the IFNAR-JAK-STAT signaling pathway to reduce ISG production and further evade the antiviral innate immune responses (75). Several HSV-1 proteins have been shown to exert inhibitory effects on the IFNAR-JAK-STAT signaling pathway and downstream ISGs (Fig. 3). Meanwhile, HSV-1 develops strategies to block the production of ISGs. HSV-1 mutants deficient in all IE proteins (139) or only ICP0 (140, 141), but not WT viruses, induce many ISGs, like ISG54, ISG56, ISG15, and myxovirus resistance gene 1, indicating that HSV-1 IE proteins or their regulated early or late proteins play a vital role in abolishing ISG production. ICP0 seems to inhibit the activation of STAT1. In STAT1 KO mice, host repression of ICP0-null HSV-1 is alleviated, indicating that the in vivo elimination of ICP0-null viruses is STAT dependent. ICP0 can antagonize the STAT1-dependent restriction of HSV-1 in vivo (142). Ectopic expression of ICP27 downregulates STAT1 phosphorylation and produces a small, heat-stable, unknown IFN-I antagonizing protein that impedes STAT1 nuclear accumulation. The restraint of IFN-I-activated STAT1 phosphorylation by ICP27 occurs at or upstream of JAK1 phosphorylation, suggesting that ICP27 plays a role at or before JAK1 to inhibit its downstream signaling (143, 144). However, it is not clear whether ICP27 works identically during viral infection.
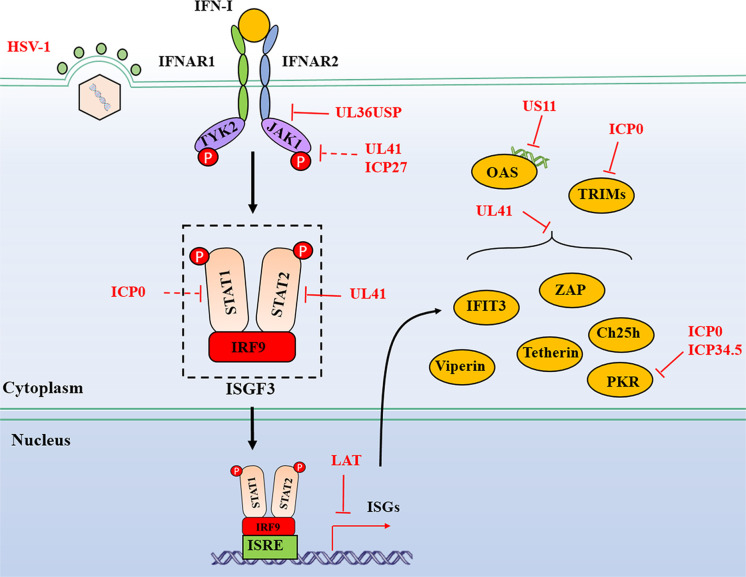
FIG 3.
HSV-1-mediated evasion of the IFNAR-JAK-STAT signaling pathway. The antiviral activities of IFN-I are initiated by binding to their cognate receptor IFNAR1 and IFNAR2 to trigger a signaling cascade, namely, JAK-STAT pathways. Viral proteins from HSV-1 interact with adaptors to block the signal transduction, and some proteins target ISGs directly for evasion of host antiviral immunity. Dashed lines indicate uncertain interactions that need to be studied further, or the underlying mechanism is unknown. P, phosphate.
Moreover, upon infection with HSV-1, UL41 partially downregulates the accumulation of JAK1 and STAT2 at a relatively high multiplicity of infection (145). During ectopic expression of UL36USP as well as during WT HSV-1 infection, UL36USP specifically binds to IFNAR2 and blocks its interaction with JAK1, which is interestingly independent of its DUB activity, to antagonize the activation of the IFN-JAK-STAT signaling pathway (146). Nevertheless, our current understanding of the countermeasures by HSV-1 against IFN-I-mediated antiviral immunity is still limited. More potential antagonists of HSV-1 on the IFNAR-JAK-STAT pathway remain to be discovered.
ISGs.
ISGs can be induced in an IFN-dependent or -independent manner upon HSV-1 infection. They play a central role in limiting viral infection and replication. Viral proteins may directly target ISGs to establish various immune evasion strategies (Fig. 3). Zinc-finger antiviral protein (ZAP), viperin, tetherin, cholesterol 25-hydroxylase (Ch25h), PKR, 2′ to 5′ oligoadenylate synthetase (OAS), the interferon-induced protein with tetratricopeptide repeat 3 (IFIT3), and tripartite motifs (TRIMs) will be discussed in this section.
(i) ZAP.
ZAP mediates the inhibition of viruses by preventing the accumulation of viral mRNA by interacting with ZAP responsive elements in viral RNA (147). ZAP is shown to be unable to inhibit HSV-1 infection (148). UL41 is responsible for the viral abrogation of ZAP antiviral activity by targeting ZAP mRNA for degradation, consequently, downregulating its expression via endonuclease activity of UL41 (149).
(ii) Viperin.
Viperin, a well-studied ISG, is highly induced through both IFN-I-dependent and -independent manners; however, viperin could barely be detected in cells infected with WT HSV-1. Ectopic expression of viperin restricts the replication of UL41-null HSV-1, indicating that viperin antiviral activity is counteracted by UL41 to reduce its mRNA accumulation via RNase activity of UL41 (150).
(iii) Tetherin.
Tetherin is also known as BST2 and CD317. HSV-1 glycoprotein M (gM), but not other glycoproteins, efficiently eliminates tetherin from the plasma membrane to antagonize its viral restriction activity (151). HSV-1 also depletes tetherin from virally infected cells by degrading its mRNA via RNase activity of UL41, and that knockdown of tetherin enhances the replication and release of UL41-null HSV-1 (152).
(iv) Ch25h.
Ch25h encodes an ER located enzyme that catalyzes the cholesterol's oxidation to generate a soluble element, 25-hydroxycholesterol (25HC) (153). Ch25h displays a critical antiviral role with its enzymatic product 25HC (154). UL41 downregulates the expression of Ch25h via its endonuclease activity and abrogates its antiviral activity (155).
(v) PKR.
Upon viral infection activation, PKR is activated by dsRNA and subsequently phosphorylates the eukaryotic translation initiation factor eIF-2α, resulting in the restraint of cellular mRNA translation and, thereby, hindering viral protein synthesis and subsequent viral replication (156). At the same time, HSV-1 infection significantly decreases phosphorylation of PKR, JAK1, and STAT1, suggesting a possible immune evasion mechanism of PKR by HSV-1 (157). ICP34.5 dephosphorylates eIF-2α to abolish the antiviral activity of PKR and is required for HSV-1 to inhibit IFN production (158, 159). During HSV-1 infection, US11 directly interacts with PKR at the C terminus. An amino acid fragment from residues 88 to 155 is critical for US11 binding to PKR (160). Then, the short stretch of amino acids 91 to 121 has been mapped to be enough to mediate the interaction between US11 and PKR in an RNA-dependent manner (161). Interestingly, US11 can effectively compensate for the function of ICP34.5 to block the phosphorylation of eIF-2α in ICP34.5-null-infected cells (162). Unlike US11, during early infection, the viral RNase UL41 targets RNAs for degradation to block the activation of PKR (163).
(vi) OAS.
OAS is a dsRNA-responsive effector. It undergoes a conformational change after binding to dsRNA, resulting in 2′ to 5′ OAS synthesis. Subsequently, it activates latent RNase L, leading to degradation of viral and cellular RNA and suppressing viral replication (164). US11 could sufficiently block OAS activation through its dsRNA-binding domain and by sequestering available dsRNA produced during infection (165). Additionally, during HSV-1 infection, ICP0 prevents the cleavage of rRNA in an RNase L-independent manner, indicating that some other unidentified RNase may restrict viral infection (166).
(vii) IFIT3.
The IFIT3, also known as ISG60, is an ISG that restricts the replication of many DNA and RNA viruses (167). However, it does not affect the replication of WT HSV-1, and the underlying molecular mechanism is that UL41 decreases the accumulation of IFIT3 mRNA via its endonuclease activity to abolish the antiviral activity of IFIT3 (168).
(viii) TRIMs.
TRIMs belong to the RING E3 ligase family. There are around 70 members of the TRIM family proteins in humans, some of which, such as TRIM56, TRIM32, and TRIM38, can inhibit HSV-1 infection by ubiquitination and activation of STING and potentiating IFN-I production (169–171). TRIM5α is the first identified TRIM member to bind with viral capsids leading to premature disassembly and inactivation of viruses (172). An unbiased proteomics approach has demonstrated the interaction between ICP0 and TRIM27. The accumulation of TRIM27 is significantly reduced during WT HSV-1 infection. However, it is recovered when interrupting the RING domain of ICP0 or impeding the proteasome, suggesting that TRIM27 is a novel target of ICP0 for degradation (173).
Furthermore, TRIM27 KO enhances viral infection of ICP0-null HSV-1, suggesting an antiviral role of TRIM27 on viral replication despite being targeted for degradation by ICP0 (173). TRIM43 was recently shown to restrict herpesvirus infection by regulating nuclear lamina integrity (174). Ectopic expression of WT TRIM43, but not its mutant form with the RING-finger domain deletion, can suppress HSV-1 replication, indicating that the antiviral activity of TRIM43 is dependent on its RING E3 ligase activity (174).
HSV-1 EVADES DDR, ER STRESS, AUTOPHAGY, STRESS GRANULES, AND APOPTOSIS/NECROSIS
Besides eliciting the classical IFN-I signaling pathway, HSV-1 infection could elicit other immune responses that contribute to restricting viral infection. As an acellular organism, the life activities of HSV-1 are all dependent on the host replication and translation system. Therefore, as foreign substances to the host, viral components including proteins and nucleic acids are potent to stimulate the host stress responses, including DDR, ER stress, autophagy, SGs, apoptosis/necrosis, and inflammasome responses (Fig. 4).
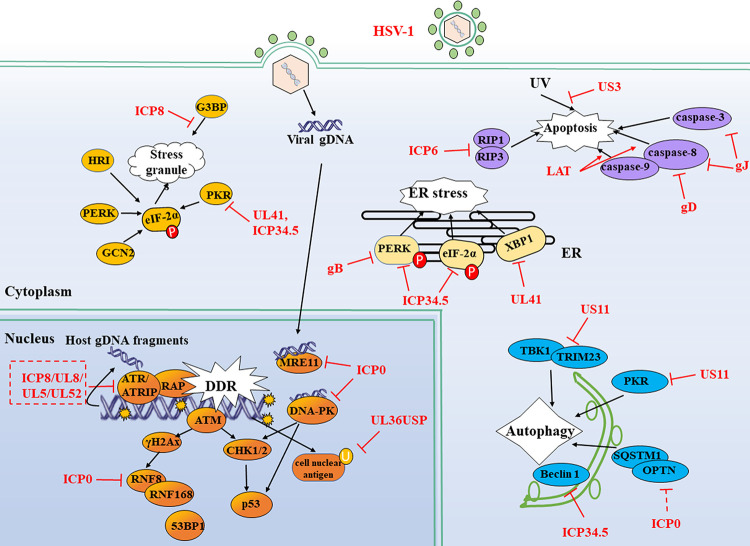
FIG 4.
HSV-1 evades DDR, ER stress, autophagy, stress granules (SGs), and apoptosis. HSV-1 infection could elicit some other immune responses like SGs, ER stress, apoptosis, DDR, and autophagy that contribute to restricting viral infection. However, these immune responses are also manipulated by viral proteins. Solid lines indicate confirmed interactions between adaptors and HSV-1 proteins. P, phosphate; U, ubiquitin.
DDRs
Environmental exposure, replication stresses, and virus invasion usually mediate DNA damage, resulting in chromosomal double-strand breaks (DSBs), which further induce the host DDR responses. Generally, upon the DDR, cell cycle checkpoint events will be initiated to stop the replication of damaged DNA through the ATM (ataxia telangiectasia mutated) and ATR (ATM and Rad3 related) pathway. It is a self-protection mechanism for the cells. During infection, the HSV-1 genome injected into the nucleus with DNA gaps and nicks in the ends is a natural “ligand” for DDR sensors, further stimulating the activation of cellular DDR signaling (175). The DDR sensors, such as DNA-dependent protein kinase (DNA-PK) and meiotic recombination 11 homolog A (MRE11), have recently been demonstrated to induce some antiviral cytokines, including IFN-I (176). As for HSV-1, the DDR signaling could be evoked by naked viral DNA in ATM- and DNA-PK-dependent manners, promoting the antiviral innate immune responses to restrict viral replication.
However, HSV-1 has evolved evasion strategies to inhibit DDR signaling pathways for its survival. Evidence from various ICP0 mutant HSV-1 and other IE-null viruses demonstrates that ICP0 alone is sufficient to induce the degradation of DNA-PK dependent on its RING finger domain or E3 ligase activity (177). Human DNA-PK recognizes exogenous exposed DNA ends and activates a STING-independent DNA-sensing antiviral pathway, which results in IFN-I production. Compared to ICP0-null HSV-1, infection with WT HSV-1 blocks DNA-activated phosphorylation of heat shock protein A8, a downstream adaptor of DNA-PK in STING KO HEK 293 cells, suggesting that DNA-PK stimulates a STING-independent DNA-sensing pathway. It remains unclear whether ICP0 abrogates this response through degrading DNA-PKs dependent on its E3 ligase activity (178). The E3 ubiquitin ligase RNF8 ubiquitinates cellular histone and facilitates the accumulation of repair factors at DSBs with the help of RNF168, which binds to and further modifies the ubiquitinated histones (179, 180). ICP0 prevents the accumulation of DNA repair factors by decreasing the expression of RNF8 and RNF168 and enhances viral replication (181). Phosphorylation of ICP0 T67 by cellular kinase 1 (CK1) recruits and targets the RNF8 forkhead-associated domain for degradation, thereby countering antiviral defense and promoting viral replication (182). Thus, ICP0 abrogates antiviral restriction by several mechanisms, including at least the inhibition of DDR factor-mediated innate immune signaling or direct degradation of DDR proteins.
In addition, HSV-1 early protein ICP8, a multifunctional single-stranded DNA (ssDNA) binding protein, is essential for viral genome replication in all stages and participates in multiple steps of the HSV life cycle (183, 184). During HSV-1 infection, a complex comprising of ICP8 and the helicase/primase complex (UL8/UL5/UL52) is formed and distributes in the DNA damage sites in the nucleus, colocalizing with ATR/ATRIP and RPA to inhibit ATR signaling (185). UL36USP deubiquitinates proliferating cell nuclear antigen in vivo and in vitro, which participates in DNA damage-caused replication fork stalling and supports viral replication during HSV-1 entry into the cells (186).
Evidence demonstrates that HSV-1 navigates different host DDR factors to facilitate viral proliferation, and ICP0 is shown with multifunctional features. More studies are guaranteed to comprehensively investigate whether other viral proteins are involved in the immune evasion of DDR factor-mediated antiviral restriction.
ER Stress
The ER is a multifunctional organelle essential for signal transduction and folding protein secretion and the transport of Ca2+. It can sense the intracellular stress from various stimuli, such as hypoxia, misfolded or unfolded proteins, heat shock, and viral infection (187). Once the unfolded proteins are monitored, the ER will correct the misfolded proteins through the unfolded protein response (UPR) mediated by inositol requiring protein 1 (IRE1) or activating transcription factor 6. Meanwhile, a transient translational arrest may also be triggered through activating PKR-like ER kinase (PERK), which is an essential kinase for eIF-2α (188). Therefore, the ER is critical for cellular homeostasis maintenance. During HSV-1 infection, the accumulation of viral proteins could stimulate the ER stress responses by phosphorylated PERK. Although, during productive infection, HSV-1 translates plenty of viral proteins depending on the host translational system (189, 190). Therefore, HSV-1 has made great efforts to prevent translation interruption events.
ICP34.5 is a multifunctional protein and plays an essential role during HSV-1 infection. During productive infection of WT but not ICP34.5-null HSV-1, the dephosphorylation of endoplasmic reticulum resident kinase PERK and eIF-2α is observed, resulting in a shutoff of the ER responses (189). In HSV-1-infected cells, the viral glycoprotein gB could physically bind to PERK and then regulate the accumulation of viral protein in the ER in a PERK-dependent manner, leading to the inhibition of ER responses (191). Interestingly, ICP0 seems to have its unique way of affecting cellular ER stress. In a reporter gene assay in HEK 293 cells, the ectopic expression of ICP0 would activate the ER stress-responsive reporter plasmids. In addition, compared with the infection of the WT virus, the ICP0-null HSV-1 severely diminishes the activation of ER stress-responsive reporter plasmids (190). Upon the treatment of various cellular stress stimulators, including thapsigargin, tunicamycin, hydrogen peroxide, dithiothreitol, and brefeldin A, the ICP0 promoter's response is similar to that of ER stress-responsive reporters. Moreover, the infection of WT HSV-1 promotes the activation of the ICP0 promoter in HeLa cells (190). These data indicate that ICP0 may act as an early ER stress sensor to monitor the status of the cellular stress response, and this may well explain why the reestablishment of lytic HSV-1 infection is attributed to the receipt of stress-induced signaling by ICP0 (192, 193).
IRE1/X box-binding protein 1 (XBP1) is a branch of UPR that evokes the ER stress response. In both transfection and viral infection models, UL41 can downregulate the expression of endogenous or ectopic XBP1 (194). A reduction of XBP1 mRNA accumulation induced by thapsigargin is observed in WT but not in UL41-null HSV-1-infected cells, indicating a transcriptional modification role for UL41 to reduce XBP1 expression and block the host ER stress response (194). HSV-1 has evolved multiple mechanisms to prevent the host ER stress response from the translation of viral proteins, one of the most important events during its life cycle (194).
Autophagy
Autophagy is a conserved cellular lysosomal degradation process that is critical for cell survival and homeostasis. It serves as an efficient cleaner to “eat” and recycle damaged cellular organelles, which protects the host from pathogenic infections, aging, and cancer cells. The dysfunction of autophagy contributes to some human diseases, including viral diseases (195). During HSV-1 infection, partial virions are targeted and captured by intracellular autophagosomes for degradation in a PKR-dependent way (196). Furthermore, autophagy is also involved in the delivery of viral nucleic acids to endosomal TLRs in plasmacytoid dendritic cells, leading to the activation of the TLR-IFN-I antiviral signaling pathway (197). These results indicate the contributions of autophagy in the priming of host innate antiviral responses.
HSV-1 has also developed strategies to block the autophagy process. In viral infection murine models, the interaction between ICP34.5 and Beclin 1 is essential for HSV-1 efficient replication in peripheral tissues at late infection. The viral titer of ICP34.5 mutant virus lacking the Beclin-binding domain (BBD) is significantly lower than that of its rescued virus in the cornea, trigeminal ganglia (TG), periocular skin, and brain tissues (198). Furthermore, both in yeast and mammalian cells, ectopic expression of ICP34.5 is demonstrated to inhibit Beclin 1-dependent autophagy via direct interaction with Beclin 1, which is also confirmed in HSV-1 WT and ICP34.5 mutant infection cell models. Although it is dispensable for ICP34.5 to block the host-cell shutoff, the BBD domain is critical for HSV-1 to restrain the autophagy process in neurons and maintain neurovirulence in vivo (199). The tripartite motif (TRIM)-TBK1 axis is essential for the activation of the autophagy pathway triggered by HSV-1 (200). In HSV-1-infected cells, US11 protein could interrupt the TRIM23-TBK1 complex by direct binding to the ARF domain of TRIM23, which subsequently suppresses autophagy and autophagy-mediated viral restriction (201). In addition, ectopic expression of US11 interacts with PKR to inhibit cellular autophagy and the autophagosome formation in both HeLa cells and fibroblasts induced by poly(I·C) treatment or starvation (202). During HSV-1 early infection, ICP0 has recently been demonstrated to exhibit a novel mechanism to modulate cellular autophagy by downregulating the expression of two autophagy adaptor proteins, sequestosome (SQSTM1) and optineurin (OPTN) in a proteasome-dependent manner (203). Surprisingly, the E3 ubiquitin ligase activity of ICP0 does not seem to be required for the degradation of SQSTM1 and OPTN, suggesting that an unknown mechanism may be involved in it.
Stress Granules
SGs are non-membrane-bound assemblies of proteins and mRNA located in the cytoplasm. These are induced when translation initiation is blocked in response to cellular stress, such as heat shock, oxidative stress, and glucose deprivation. The formation of SGs is dependent on the inactivated eIF-2α in a phosphorylation state, which is regulated by one of the four kinases, including PKR, PKR-like ER-localized eIF-2α kinase (PERK), heme-regulated inhibitor (HRI) kinase, as well as general control nonrepressed 2 (GCN2) kinase. The SG assembly is a novel concept in the antiviral innate immune response. Recently, two groups have demonstrated that the RNA sensors MDA5 and RIG-I localize to SGs upon viral infection. The colocalization of RIG-I and SGs stimulates IFN-I production but, interestingly, not for MDA5 (204, 205). Some viral infections induce the assembly of SGs, while some other viruses inhibit this process (206–209). For HSV-1 infection, some strains like F or KOS are unable to stimulate the accumulation of SGs, but in some SG components or its related protein KO mouse embryo fibroblast cell lines, the H129 strain could grow faster than that in parental cells (210), which provides at least two tips. First, viral replication of HSV-1 could be restricted by SG responses; second, the reasons for the undetectable accumulation of SGs may result from the counteracting strategies by HSV-1. No doubt, the SG assembly is observed in UL41-null HSV-1-infected cells, in which the PKR activation and IFN-β production are also observed, implying that HSV-1 could block the SG-mediated antiviral innate immune responses by UL41 (211). Compelling evidence shows that during HSV-2 infection in HeLa cells, UL41 disrupts the accumulation of SGs depending on its endoribonuclease activity and facilitates viral growth in vitro (212–214), highlighting the critical role of UL41 in the resistance of host antiviral responses mediated by the SG assembly. The Ras-GAP SH3 domain-binding protein (G3BP) is an RNA-binding protein that acts as an effector for the formation of SGs. HSV-1 major DNA binding protein ICP8 has been found to interrupt SG formation by binding to G3BP when ectopically expressed (215).
Apoptosis/Necrosis
Apoptosis and necrosis are two primary cell death forms that maintain host homeostasis in mammalian cells in response to internal stress or external pathogenic infections (216). Apoptosis is a programmed cell death characterized by the formation of cell shrinkage and membrane blebbing, which is a moderate cell death pattern. Relatively, it is a passive death, as cell necrosis is more violent with organelles swelling and plasma membrane rupture (217). Both cell apoptosis and necrosis are host defense mechanisms to restrict viral replication and transmission. In general, cell apoptosis can be initiated by the following three different pathways: the mitochondria pathway, cell-death receptors like the Fas/FasL pathway, and the ER stress pathway, in which caspases are the main exercisers. Considerable evidence suggested that, when caspase activity is inhibited, the cell death process can still proceed in a caspase-independent manner, which is defined as “necroptosis” (218, 219). Necroptosis is a programmed form of necrosis, which is also involved in host defense against pathogenic infections.
HSV-1 could induce and block the apoptosis signaling pathway at multiple steps. ICP0 shows the capability to elicit the activation of apoptosis with an unknown mechanism during HSV-1 infection in vitro (220). Cell necroptosis is tightly regulated by the receptor-interacting protein (RIP) kinase family and their substrates. Viral ICP6 encoded by the UL39 gene, the large subunit of ribonucleotide reductase, exhibits multiple mechanisms to affect host apoptosis and necrosis. On the one hand, it can trigger RIP3/lineage kinase domain-like protein (MLKL)-dependent necrosis through directly promoting the kinase activity of RIP3 in mouse cells during HSV-1 infection in vivo (221). On the other hand, in HSV-1-infected human cells, viral ribonucleotide reductase large subunit UL39/ICP6 acts as an inhibitor to block the interaction between RIP1 and RIP3, resulting in a suppression of cell necroptosis (222).
In contrast, the binding of RIP1/RIP3 to ICP6 in mouse cells initiates the cell necroptosis as a host-defense response (223). The exact opposite role of ICP6 in response to cell necroptosis in human and mouse cells may result from an evolution outcome for HSV-1, as the mouse is not the natural host for HSV-1, which could not develop a strategy to evade the cell necroptosis response, suggesting that HSV-1 may manipulate cell necroptosis in a species-specific manner via ICP6 (224). Although viral infection may trigger and block cell apoptosis and necrosis, in most cases, HSV-1 protects host cells from death to meet its own needs. Therefore, more strategies are employed.
US3 is well-studied in blocking apoptosis induced by HSV-1. Some recombinant viruses first demonstrate the antiapoptotic activity of US3. The apoptosis is induced in Vero cells infected with R7041, which lacks most of the US3 gene, whereas no apoptosis is observed in the recombinant R7306 virus, in which the US3 gene has been repaired (225). However, another study shows that the virus deletion of US3 still retains some antiapoptotic activity, showing that US3 and US5 work concomitantly to inhibit apoptosis during HSV-1 infection in Vero and Jurkat cells (226). Mechanism studies reveal that US3 inhibits cell apoptosis through posttranslational modification of the Bcl-2 antagonist of cell death (BAD) (227, 228) as well as through blocking the activity of caspase-3 (229). US5 encodes a viral glycoprotein gJ, which blocks cell apoptosis induced by Fas ligation, UV, or granzyme B through inhibiting caspase-3 and caspase-8 activity (226). Another glycoprotein gD also shows great potential to inhibit cell apoptosis. HSV-1 lacking gD can induce apoptosis in infected cells, indicating an antiapoptosis function for gD (230). The underlying mechanism is that gD can suppress caspase-8 activity, leading to an inhibition of cell apoptosis (231).
Furthermore, US3 encoded by HSV-2 is also reported to block the corneal epithelial apoptosis in vivo 2 days postinfection in murine models (232). However, the apoptotic pathway in human corneal epithelial cells during HSV-1 infection has not been studied thoroughly (233). The latency-associated transcript (LAT), a marker of latent infection in TG, plays a protective role in caspase-8- and caspase-9-induced apoptosis in neurons, which might be beneficial to neuronal survival from latent infection (234, 235). Viral IE protein ICP27 is also essential for blocking apoptosis during infection in human HEp-2 cells (236, 237). In ICP27-null virus-infected cells but not WT-infected cells, the apoptotic phenomenon is observed with a feature of membrane blebbing and the formation of apoptotic bodies, which is in a caspase-3-dependent manner (237).
CONCLUDING REMARKS
HSV-1 infection is highly prevalent and pathogenic at all ages. After primary infection, the latent infection may persist for a lifetime in the nervous system and is ready to reactivate under stress to cause recurrent disease. The innate immune system is the first line of host defense and is characterized by the production of IFN-I and ISGs to restrict viral infection and transmission. During the long struggle between viruses and host innate immune systems, HSV-1 has employed corresponding immune evasion strategies to favor their survival. These mechanisms involve multiple viral proteins, especially IE proteins, tegument proteins, and other functional proteins to target cellular antiviral signaling pathways. Most viral proteins are capable of counteracting more than one signal molecule, such as ICP0, ICP34.5, and UL41, etc., and some are focused on the same signals, such as IRF3 and NF-κB, etc. Recently, substantial progress has been made in identifying early antiviral components elicited and blocked by HSV-1. Here, we have outlined and discussed the currently known countermeasures employed by HSV-1 (summarized in Fig. 1 and 4). However, more of these evasion mechanisms will undoubtedly be revealed in the near future.
These findings will hopefully provide new insights for immunotherapies and targeted therapies to interfere with viral infection. Remarkably, the findings involved in cGAS-STING, apoptosis/necrosis, and autophagy pathways evaded by HSV-1 may significantly contribute to the discovery of effective drugs for treatment, as many targets have been applied in the drug development of cancer therapies. Some STING agonists for cancer treatment have been carried out in clinical trials. Apoptosis/necrosis and autophagy are also hot topics in cancer therapy. TLR2 has been demonstrated to play a critical role in the pathogenesis of HSV-induced encephalitis, which may trigger several cellular responses, including NF-κB activation and inflammatory cytokine and chemokine secretion, especially in neonates (238). Therefore, the treatment of small molecule inhibitors of TLR2 for HSV-1 encephalitis is supposed to be beneficial for patients. To date, several inhibitors of TLR2 signaling have been developed (239–241). However, none of them is licensed for human use. Recently, the pocket within the TLR2 TIR domain was predicted to be an efficient target to develop small molecule inhibitors to block the TLR2 signaling pathway. Additionally, a potent inhibitor C16H15NO4 (C29) and its derivative have been identified to inhibit both mouse and human TLR2 signaling pathways without cytotoxicity (242). In HSV-1 encephalitis animal models, we might try these small molecules to determine whether the production of inflammatory cytokines can be blocked. Some other TLRs, like TLR9 and TLR3, etc., are reported to play protective roles for the host to defend against infection with HSVs (25, 36). In that case, agonists for these TLRs would be beneficial for controlling HSV infection and replication. The application of TLR9 agonists is demonstrated to provide promising adjuvants for HSV-2 vaccines, which protect the host from symptomatic disease and reduce the latent viral reservoir in the guinea pig challenge model (243).
Furthermore, additional studies on the interaction between HSV-1 and host defense immune signaling are needed to better understand the progression from latency to reactivation and which phase is always accompanied by severe outcomes. These studies may provide novel antiviral therapies to target viral components or cellular immune signals.
ACKNOWLEDGMENTS
We apologize to all colleagues whose contributions were not discussed and cited owing to space constraints. We are grateful to Sisilia Zheng for a critical review of the manuscript. We thank previous lab members who have contributed papers for this review.
Current work in the Zheng laboratory relevant to this article was supported by grants from the Science and Technology Program of Fujian (2018Y9064).
Biographies

Huifang Zhu received her bachelor's degree in biotechnology from Shenyang Pharmaceutical University, China, in 2008, and then received her master's degree in biochemistry and molecular biology from Wuhan Institute of Virology, Chinese Academy of Sciences, in 2011. She joined the Cancer Stem Cell group as a research assistant in Kunming Institute of Zoology, Chinese Academy of Sciences, from 2011 to 2012. She received her Ph.D. in pharmacology from Kunming Institute of Botany, Chinese Academy of Sciences, in 2019. She is currently an associate research fellow in the First Affiliated Hospital of Gannan Medical University and a postdoctoral fellow at the Fujian Medical University. Her research interests focus on the immune evasion mechanisms of RSV. She also develops screening models of natural products to inhibit the inflammatory damage induced by RSV infection.

Chunfu Zheng received his Ph.D. in infectious diseases from Chongqing Medical University, China, in 2001, followed by postdoctoral training from 2001 to 2007 at Saskatchewan University and University of Calgary, Canada, in molecular virology and cellular immunology. Then, he worked at Wuhan Institute of Virology, Chinese Academy of Sciences as a Senior Scientist and Soochow University as a Professor from 2007 to 2017. He has engaged in researches on molecular virology and viral immunology since 2001. He is currently a Professor at Fujian Medical University, China, and an adjunct professor at University of Calgary, Canada, with research interests in HSV-1-mediated immune evasion strategies for host antiviral innate immunity. He serves as an editorial board member for the Journal of Virology, Frontiers in Immunology, and Journal of Medical Virology, an Associate Editor for Virology Journal and Frontiers in Microbiology, and a guest editor for Frontiers in Immunology.
REFERENCES
- 1.Davison AJ. 2010. Herpesvirus systematics. Vet Microbiol 143:52–69. doi: 10.1016/j.vetmic.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denes CE, Everett RD, Diefenbach RJ. 2020. Tour de herpes: cycling through the life and biology of HSV-1. Methods Mol Biol 2060:1–30. doi: 10.1007/978-1-4939-9814-2_1. [DOI] [PubMed] [Google Scholar]
- 3.Looker KJ, Magaret AS, May MT, Turner KME, Vickerman P, Newman LM, Gottlieb SL. 2017. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob Health 5:e300–e309. doi: 10.1016/S2214-109X(16)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saleh D, Sharma S. 2020. Herpes simplex type 1. StatPearls, Treasure Island, FL. [Google Scholar]
- 5.Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, Newman LM. 2015. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS One 10:e0140765. doi: 10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma Y, He B. 2014. Recognition of herpes simplex viruses: Toll-like receptors and beyond. J Mol Biol 426:1133–1147. doi: 10.1016/j.jmb.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Full F, Ensser A. 2019. Early nuclear events after herpesviral infection. J Clin Med 8:1408. doi: 10.3390/jcm8091408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowie AG, Unterholzner L. 2008. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol 8:911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. 2008. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature 452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 10.Itoh H, Tatematsu M, Watanabe A, Iwano K, Funami K, Seya T, Matsumoto M. 2011. UNC93B1 physically associates with human TLR8 and regulates TLR8-mediated signaling. PLoS One 6:e28500. doi: 10.1371/journal.pone.0028500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelka K, Bertheloot D, Reimer E, Phulphagar K, Schmidt SV, Christ A, Stahl R, Watson N, Miyake K, Hacohen N, Haas A, Brinkmann MM, Marshak-Rothstein A, Meissner F, Latz E. 2018. The chaperone UNC93B1 regulates Toll-like receptor stability independently of endosomal TLR transport. Immunity 48:911–922. doi: 10.1016/j.immuni.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casrouge A, Zhang SY, Eidenschenk C, Jouanguy E, Puel A, Yang K, Alcais A, Picard C, Mahfoufi N, Nicolas N, Lorenzo L, Plancoulaine S, Senechal B, Geissmann F, Tabeta K, Hoebe K, Du X, Miller RL, Heron B, Mignot C, de Villemeur TB, Lebon P, Dulac O, Rozenberg F, Beutler B, Tardieu M, Abel L, Casanova JL. 2006. Herpes simplex virus encephalitis in human UNC-93B deficiency. Science 314:308–312. doi: 10.1126/science.1128346. [DOI] [PubMed] [Google Scholar]
- 13.Melchjorsen J. 2012. Sensing herpes: more than toll. Rev Med Virol 22:106–121. doi: 10.1002/rmv.716. [DOI] [PubMed] [Google Scholar]
- 14.Rasmussen SB, Jensen SB, Nielsen C, Quartin E, Kato H, Chen ZJ, Silverman RH, Akira S, Paludan SR. 2009. Herpes simplex virus infection is sensed by both Toll-like receptors and retinoic acid-inducible gene- like receptors, which synergize to induce type I interferon production. J Gen Virol 90:74–78. doi: 10.1099/vir.0.005389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melchjorsen J, Rintahaka J, Soby S, Horan KA, Poltajainen A, Ostergaard L, Paludan SR, Matikainen S. 2010. Early innate recognition of herpes simplex virus in human primary macrophages is mediated via the MDA5/MAVS-dependent and MDA5/MAVS/RNA polymerase III-independent pathways. J Virol 84:11350–11358. doi: 10.1128/JVI.01106-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phelan T, Little MA, Brady G. 2020. Targeting of the cGAS-STING system by DNA viruses. Biochem Pharmacol 174:113831. doi: 10.1016/j.bcp.2020.113831. [DOI] [PubMed] [Google Scholar]
- 17.Johnson KE, Chikoti L, Chandran B. 2013. Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J Virol 87:5005–5018. doi: 10.1128/JVI.00082-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furr SR, Chauhan VS, Moerdyk-Schauwecker MJ, Marriott I. 2011. A role for DNA-dependent activator of interferon regulatory factor in the recognition of herpes simplex virus type 1 by glial cells. J Neuroinflammation 8:99. doi: 10.1186/1742-2094-8-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maruzuru Y, Ichinohe T, Sato R, Miyake K, Okano T, Suzuki T, Koshiba T, Koyanagi N, Tsuda S, Watanabe M, Arii J, Kato A, Kawaguchi Y. 2018. Herpes simplex virus 1 VP22 inhibits AIM2-dependent inflammasome activation to enable efficient viral replication. Cell Host Microbe 23:254–265. doi: 10.1016/j.chom.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Orzalli MH, Broekema NM, Diner BA, Hancks DC, Elde NC, Cristea IM, Knipe DM. 2015. cGAS-mediated stabilization of IFI16 promotes innate signaling during herpes simplex virus infection. Proc Natl Acad Sci U S A 112:E1773–E1781. doi: 10.1073/pnas.1424637112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGeoch DJ, Dolan A, Ralph AC. 2000. Toward a comprehensive phylogeny for mammalian and avian herpesviruses. J Virol 74:10401–10406. doi: 10.1128/jvi.74.22.10401-10406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 23.Takeda K, Akira S. 2015. Toll-like receptors. Curr Protoc Immunol 109:14.12.1–14.12.10. doi: 10.1002/0471142735.im1412s109. [DOI] [PubMed] [Google Scholar]
- 24.Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, Ludwig H, Sutter G, Suzuki K, Hemmi H, Sato S, Yamamoto M, Uematsu S, Kawai T, Takeuchi O, Akira S. 2006. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol 7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. 2008. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J Immunol 181:8604–8612. doi: 10.4049/jimmunol.181.12.8604. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Guan Y, Sun X, Shi L, Liang R, Lv X, Xin W. 2013. HSV-1 activates NF-kappaB in mouse astrocytes and increases TNF-alpha and IL-6 expression via Toll-like receptor 3. Neurol Res 35:755–762. doi: 10.1179/016164113X13703372991516. [DOI] [PubMed] [Google Scholar]
- 27.Villalba M, Hott M, Martin C, Aguila B, Valdivia S, Quezada C, Zambrano A, Concha MI, Otth C. 2012. Herpes simplex virus type 1 induces simultaneous activation of Toll-like receptors 2 and 4 and expression of the endogenous ligand serum amyloid A in astrocytes. Med Microbiol Immunol 201:371–379. doi: 10.1007/s00430-012-0247-0. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Zhang J, Kumar A, Zheng M, Atherton SS, Yu FS. 2006. Herpes simplex virus 1 infection induces the expression of proinflammatory cytokines, interferons and TLR7 in human corneal epithelial cells. Immunology 117:167–176. doi: 10.1111/j.1365-2567.2005.02275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leoni V, Gianni T, Salvioli S, Campadelli-Fiume G. 2012. Herpes simplex virus glycoproteins gH/gL and gB bind Toll-like receptor 2, and soluble gH/gL is sufficient to activate NF-kappaB. J Virol 86:6555–6562. doi: 10.1128/JVI.00295-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai M, Li M, Wang K, Wang S, Lu Q, Yan J, Mossman KL, Lin R, Zheng C. 2013. The herpes simplex virus 1-encoded envelope glycoprotein B activates NF-kappaB through the Toll-like receptor 2 and MyD88/TRAF6-dependent signaling pathway. PLoS One 8:e54586. doi: 10.1371/journal.pone.0054586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai MS, Li ML, Zheng CF. 2012. Herpesviral infection and Toll-like receptor 2. Protein Cell 3:590–601. doi: 10.1007/s13238-012-2059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. 2004. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc Natl Acad Sci U S A 101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aravalli RN, Hu S, Rowen TN, Palmquist JM, Lokensgard JR. 2005. Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol 175:4189–4193. doi: 10.4049/jimmunol.175.7.4189. [DOI] [PubMed] [Google Scholar]
- 34.Kozak M, Roizman B. 1975. RNA synthesis in cells infected with herpes simplex virus. IX. Evidence for accumulation of abundant symmetric transcripts in nuclei. J Virol 15:36–40. doi: 10.1128/JVI.15.1.36-40.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jacquemont B, Roizman B. 1975. RNA synthesis in cells infected with herpes simplex virus. X. Properties of viral symmetric transcripts and of double-stranded RNA prepared from them. J Virol 15:707–713. doi: 10.1128/JVI.15.4.707-713.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Y, Audry M, Ciancanelli M, Alsina L, Azevedo J, Herman M, Anguiano E, Sancho-Shimizu V, Lorenzo L, Pauwels E, Philippe PB, Perez de Diego R, Cardon A, Vogt G, Picard C, Andrianirina ZZ, Rozenberg F, Lebon P, Plancoulaine S, Tardieu M, Valerie D, Jouanguy E, Chaussabel D, Geissmann F, Abel L, Casanova JL, Zhang SY. 2011. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med 208:2083–2098. doi: 10.1084/jem.20101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang SY, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, Plancoulaine S, Titeux M, Cognet C, von Bernuth H, Ku CL, Casrouge A, Zhang XX, Barreiro L, Leonard J, Hamilton C, Lebon P, Heron B, Vallee L, Quintana-Murci L, Hovnanian A, Rozenberg F, Vivier E, Geissmann F, Tardieu M, Abel L, Casanova JL. 2007. TLR3 deficiency in patients with herpes simplex encephalitis. Science 317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 38.Reinert LS, Harder L, Holm CK, Iversen MB, Horan KA, Dagnæs-Hansen F, Ulhøi BP, Holm TH, Mogensen TH, Owens T, Nyengaard JR, Thomsen AR, Paludan SR. 2012. TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. J Clin Invest 122:1368–1376. doi: 10.1172/JCI60893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lafaille FG, Pessach IM, Zhang SY, Ciancanelli MJ, Herman M, Abhyankar A, Ying SW, Keros S, Goldstein PA, Mostoslavsky G, Ordovas-Montanes J, Jouanguy E, Plancoulaine S, Tu E, Elkabetz Y, Al-Muhsen S, Tardieu M, Schlaeger TM, Daley GQ, Abel L, Casanova JL, Studer L, Notarangelo LD. 2012. Impaired intrinsic immunity to HSV-1 in human iPSC-derived TLR3-deficient CNS cells. Nature 491:769–773. doi: 10.1038/nature11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malmgaard L, Melchjorsen J, Bowie AG, Mogensen SC, Paludan SR. 2004. Viral activation of macrophages through TLR-dependent and -independent pathways. J Immunol 173:6890–6898. doi: 10.4049/jimmunol.173.11.6890. [DOI] [PubMed] [Google Scholar]
- 41.Miller RL, Imbertson LM, Reiter MJ, Gerster JF. 1999. Treatment of primary herpes simplex virus infection in guinea pigs by imiquimod. Antiviral Res 44:31–42. doi: 10.1016/s0166-3542(99)00052-2. [DOI] [PubMed] [Google Scholar]
- 42.Krug A, Luker GD, Barchet W, Leib DA, Akira S, Colonna M. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 103:1433–1437. doi: 10.1182/blood-2003-08-2674. [DOI] [PubMed] [Google Scholar]
- 43.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. 2003. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med 198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rasmussen SB, Sorensen LN, Malmgaard L, Ank N, Baines JD, Chen ZJ, Paludan SR. 2007. Type I interferon production during herpes simplex virus infection is controlled by cell-type-specific viral recognition through Toll-like receptor 9, the mitochondrial antiviral signaling protein pathway, and novel recognition systems. J Virol 81:13315–13324. doi: 10.1128/JVI.01167-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uyangaa E, Choi JY, Patil AM, Hossain FMA, Park SO, Kim B, Kim K, Eo SK. 2018. Dual TLR2/9 recognition of herpes simplex virus infection is required for recruitment and activation of monocytes and NK cells and restriction of viral dissemination to the central nervous system. Front Immunol 9:905. doi: 10.3389/fimmu.2018.00905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iversen MB, Ank N, Melchjorsen J, Paludan SR. 2010. Expression of type III interferon (IFN) in the vaginal mucosa is mediated primarily by dendritic cells and displays stronger dependence on NF-κB than type I IFNs. J Virol 84:4579–4586. doi: 10.1128/JVI.02591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zyzak J, Mitkiewicz M, Leszczynska E, Reniewicz P, Moynagh PN, Siednienko J. 2020. HSV-1/TLR9-mediated IFNβ and TNFα induction is Mal-dependent in macrophages. J Innate Immun 12:387–398. doi: 10.1159/000504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hagglund R, Roizman B. 2002. Characterization of the novel E3 ubiquitin ligase encoded in exon 3 of herpes simplex virus-1-infected cell protein 0. Proc Natl Acad Sci U S A 99:7889–7894. doi: 10.1073/pnas.122246999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Lint AL, Murawski MR, Goodbody RE, Severa M, Fitzgerald KA, Finberg RW, Knipe DM, Kurt-Jones EA. 2010. Herpes simplex virus immediate-early ICP0 protein inhibits Toll-like receptor 2-dependent inflammatory responses and NF-κB signaling. J Virol 84:10802–10811. doi: 10.1128/JVI.00063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daubeuf S, Singh D, Tan Y, Liu H, Federoff HJ, Bowers WJ, Tolba K. 2009. HSV ICP0 recruits USP7 to modulate TLR-mediated innate response. Blood 113:3264–3275. doi: 10.1182/blood-2008-07-168203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sen J, Liu X, Roller R, Knipe DM. 2013. Herpes simplex virus US3 tegument protein inhibits Toll-like receptor 2 signaling at or before TRAF6 ubiquitination. Virology 439:65–73. doi: 10.1016/j.virol.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peri P, Mattila RK, Kantola H, Broberg E, Karttunen HS, Waris M, Vuorinen T, Hukkanen V. 2008. Herpes simplex virus type 1 Us3 gene deletion influences Toll-like receptor responses in cultured monocytic cells. Virol J 5:140. doi: 10.1186/1743-422X-5-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato R, Kato A, Chimura T, Saitoh SI, Shibata T, Murakami Y, Fukui R, Liu K, Zhang Y, Arii J, Sun-Wada GH, Wada Y, Ikenoue T, Barber GN, Manabe T, Kawaguchi Y, Miyake K. 2018. Combating herpesvirus encephalitis by potentiating a TLR3-mTORC2 axis. Nat Immunol 19:1071–1082. doi: 10.1038/s41590-018-0203-2. [DOI] [PubMed] [Google Scholar]
- 54.Ma XM, Blenis J. 2009. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 55.Chuluunbaatar U, Roller R, Feldman ME, Brown S, Shokat KM, Mohr I. 2010. Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication. Genes Dev 24:2627–2639. doi: 10.1101/gad.1978310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. 2008. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol 9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bodur C, Kazyken D, Huang K, Ekim Ustunel B, Siroky KA, Tooley AS, Gonzalez IE, Foley DH, Acosta-Jaquez HA, Barnes TM, Steinl GK, Cho KW, Lumeng CN, Riddle SM, Myers MG Jr, Fingar DC. 2018. The IKK-related kinase TBK1 activates mTORC1 directly in response to growth factors and innate immune agonists. EMBO J 37:19–38. doi: 10.15252/embj.201696164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saitoh SI, Abe F, Kanno A, Tanimura N, Mori Saitoh Y, Fukui R, Shibata T, Sato K, Ichinohe T, Hayashi M, Kubota K, Kozuka-Hata H, Oyama M, Kikko Y, Katada T, Kontani K, Miyake K. 2017. TLR7 mediated viral recognition results in focal type I interferon secretion by dendritic cells. Nat Commun 8:1592. doi: 10.1038/s41467-017-01687-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brisse M, Ly H. 2019. Comparative structure and function analysis of the RIG-I-like receptors: RIG-I and MDA5. Front Immunol 10:1586. doi: 10.3389/fimmu.2019.01586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou F, Sun L, Zheng H, Skaug B, Jiang Q-X, Chen ZJ. 2011. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146:448–461. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seth RB, Sun L, Ea C-K, Chen ZJ. 2005. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF 3. Cell 122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 62.Fitzgerald KA, McWhirter SM, Faia KL, Rowe DC, Latz E, Golenbock DT, Coyle AJ, Liao S-M, Maniatis T. 2003. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 63.Fang R, Jiang Q, Zhou X, Wang C, Guan Y, Tao J, Xi J, Feng J-M, Jiang Z. 2017. MAVS activates TBK1 and IKKε through TRAFs in NEMO dependent and independent manner. PLoS Pathog 13:e1006720. doi: 10.1371/journal.ppat.1006720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crill EK, Furr-Rogers SR, Marriott I. 2015. RIG-I is required for VSV-induced cytokine production by murine glia and acts in combination with DAI to initiate responses to HSV-1. Glia 63:2168–2180. doi: 10.1002/glia.22883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiang JJ, Sparrer KMJ, van Gent M, Lassig C, Huang T, Osterrieder N, Hopfner KP, Gack MU. 2018. Viral unmasking of cellular 5S rRNA pseudogene transcripts induces RIG-I-mediated immunity. Nat Immunol 19:53–62. doi: 10.1038/s41590-017-0005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xing J, Wang S, Lin R, Mossman KL, Zheng C. 2012. Herpes simplex virus 1 tegument protein US11 downmodulates the RLR signaling pathway via direct interaction with RIG-I and MDA-5. J Virol 86:3528–3540. doi: 10.1128/JVI.06713-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao J, Zeng Y, Xu S, Chen J, Shen G, Yu C, Knipe D, Yuan W, Peng J, Xu W, Zhang C, Xia Z, Feng P. 2016. A viral deamidase targets the helicase domain of RIG-I to block RNA-induced activation. Cell Host Microbe 20:770–784. doi: 10.1016/j.chom.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oganesyan G, Saha SK, Guo B, He JQ, Shahangian A, Zarnegar B, Perry A, Cheng G. 2006. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 69.Häcker H, Redecke V, Blagoev B, Kratchmarova I, Hsu L-C, Wang GG, Kamps MP, Raz E, Wagner H, Häcker G, Mann M, Karin M. 2006. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 70.Abaitua F, O'Hare P. 2008. Identification of a highly conserved, functional nuclear localization signal within the N-terminal region of herpes simplex virus type 1 VP1-2 tegument protein. J Virol 82:5234–5244. doi: 10.1128/JVI.02497-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kattenhorn LM, Korbel GA, Kessler BM, Spooner E, Ploegh HL. 2005. A deubiquitinating enzyme encoded by HSV-1 belongs to a family of cysteine proteases that is conserved across the family Herpesviridae. Mol Cell 19:547–557. doi: 10.1016/j.molcel.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 72.Wang S, Wang K, Li J, Zheng C. 2013. Herpes simplex virus 1 ubiquitin-specific protease UL36 inhibits beta interferon production by deubiquitinating TRAF3. J Virol 87:11851–11860. doi: 10.1128/JVI.01211-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiu YH, Macmillan JB, Chen ZJ. 2009. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138:576–591. doi: 10.1016/j.cell.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ. 2011. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol 12:959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin Y, Zheng C. 2019. A tug of war: DNA-sensing antiviral innate immunity and herpes simplex virus type I infection. Front Microbiol 10:2627. doi: 10.3389/fmicb.2019.02627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun L, Wu J, Du F, Chen X, Chen ZJ. 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andreeva L, Hiller B, Kostrewa D, Lassig C, de Oliveira Mann CC, Jan Drexler D, Maiser A, Gaidt M, Leonhardt H, Hornung V, Hopfner KP. 2017. cGAS senses long and HMGB/TFAM-bound U-turn DNA by forming protein-DNA ladders. Nature 549:394–398. doi: 10.1038/nature23890. [DOI] [PubMed] [Google Scholar]
- 78.Zhang C, Shang G, Gui X, Zhang X, Bai XC, Chen ZJ. 2019. Structural basis of STING binding with and phosphorylation by TBK1. Nature 567:394–398. doi: 10.1038/s41586-019-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cai X, Chiu YH, Chen ZJ. 2014. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell 54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 80.Abe T, Barber GN. 2014. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1. J Virol 88:5328–5341. doi: 10.1128/JVI.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mukai K, Konno H, Akiba T, Uemura T, Waguri S, Kobayashi T, Barber GN, Arai H, Taguchi T. 2016. Activation of STING requires palmitoylation at the Golgi. Nat Commun 7:11932. doi: 10.1038/ncomms11932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X, Majumdar T, Kessler P, Ozhegov E, Zhang Y, Chattopadhyay S, Barik S, Sen GC. 2016. STING requires the adaptor TRIF to trigger innate immune responses to microbial infection. Cell Host Microbe 20:329–341. doi: 10.1016/j.chom.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shahin V, Hafezi W, Oberleithner H, Ludwig Y, Windoffer B, Schillers H, Kuhn JE. 2006. The genome of HSV-1 translocates through the nuclear pore as a condensed rod-like structure. J Cell Sci 119:23–30. doi: 10.1242/jcs.02705. [DOI] [PubMed] [Google Scholar]
- 84.Horan KA, Hansen K, Jakobsen MR, Holm CK, Soby S, Unterholzner L, Thompson M, West JA, Iversen MB, Rasmussen SB, Ellermann-Eriksen S, Kurt-Jones E, Landolfo S, Damania B, Melchjorsen J, Bowie AG, Fitzgerald KA, Paludan SR. 2013. Proteasomal degradation of herpes simplex virus capsids in macrophages releases DNA to the cytosol for recognition by DNA sensors. J Immunol 190:2311–2319. doi: 10.4049/jimmunol.1202749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang J, You H, Su C, Li Y, Chen S, Zheng C. 2018. Herpes simplex virus 1 tegument protein VP22 abrogates cGAS/STING-mediated antiviral innate immunity. J Virol 92:e00841-18. doi: 10.1128/JVI.00841-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang J, Zhao J, Xu S, Li J, He S, Zeng Y, Xie L, Xie N, Liu T, Lee K, Seo GJ, Chen L, Stabell AC, Xia Z, Sawyer SL, Jung J, Huang C, Feng P. 2018. Species-specific deamidation of cGAS by herpes simplex virus UL37 protein facilitates viral replication. Cell Host Microbe 24:234–248. doi: 10.1016/j.chom.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Su C, Zheng C. 2017. Herpes simplex virus 1 abrogates the cGAS/STING-mediated cytosolic DNA-sensing pathway via its virion host shutoff protein, UL41. J Virol 91:e02414-16. doi: 10.1128/JVI.02414-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan S, Liu X, Ma Y, Cao Y, He B. 2018. Herpes simplex virus 1 γ134.5 protein inhibits STING activation that restricts viral replication. J Virol 92:e01015-18. doi: 10.1128/JVI.01015-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deschamps T, Kalamvoki M. 2017. Evasion of the STING DNA-sensing pathway by VP11/12 of herpes simplex virus 1. J Virol 91:e00535-17. doi: 10.1128/JVI.00535-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowie AG. 2010. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol 11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ansari MA, Dutta S, Veettil MV, Dutta D, Iqbal J, Kumar B, Roy A, Chikoti L, Singh VV, Chandran B. 2015. Herpesvirus genome recognition induced acetylation of nuclear IFI16 is essential for its cytoplasmic translocation, inflammasome and IFN-β responses. PLoS Pathog 11:e1005019. doi: 10.1371/journal.ppat.1005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zheng C. 2018. Evasion of cytosolic DNA-stimulated innate immune responses by herpes simplex virus 1. J Virol 92:e00099-17. doi: 10.1128/JVI.00099-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson KE, Bottero V, Flaherty S, Dutta S, Singh VV, Chandran B. 2014. IFI16 restricts HSV-1 replication by accumulating on the HSV-1 genome, repressing HSV-1 gene expression, and directly or indirectly modulating histone modifications. PLoS Pathog 10:e1004503. doi: 10.1371/journal.ppat.1004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Orzalli MH, DeLuca NA, Knipe DM. 2012. Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proc Natl Acad Sci U S A 109:E3008–E3017. doi: 10.1073/pnas.1211302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Orzalli MH, Broekema NM, Knipe DM. 2016. Relative contributions of herpes simplex virus 1 ICP0 and vhs to loss of cellular IFI16 vary in different human cell types. J Virol 90:8351–8359. doi: 10.1128/JVI.00939-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Melroe GT, DeLuca NA, Knipe DM. 2004. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J Virol 78:8411–8420. doi: 10.1128/JVI.78.16.8411-8420.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Melroe GT, Silva L, Schaffer PA, Knipe DM. 2007. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: potential role in blocking IFN-beta induction. Virology 360:305–321. doi: 10.1016/j.virol.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu H, Zheng C, Xing J, Wang S, Li S, Lin R, Mossman KL. 2011. Varicella-zoster virus immediate-early protein ORF61 abrogates the IRF3-mediated innate immune response through degradation of activated IRF3. J Virol 85:11079–11089. doi: 10.1128/JVI.05098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin R, Noyce RS, Collins SE, Everett RD, Mossman KL. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J Virol 78:1675–1684. doi: 10.1128/jvi.78.4.1675-1684.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paladino P, Collins SE, Mossman KL. 2010. Cellular localization of the herpes simplex virus ICP0 protein dictates its ability to block IRF3-mediated innate immune responses. PLoS One 5:e10428. doi: 10.1371/journal.pone.0010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Christensen MH, Jensen SB, Miettinen JJ, Luecke S, Prabakaran T, Reinert LS, Mettenleiter T, Chen ZJ, Knipe DM, Sandri-Goldin RM, Enquist LW, Hartmann R, Mogensen TH, Rice SA, Nyman TA, Matikainen S, Paludan SR. 2016. HSV-1 ICP27 targets the TBK1-activated STING signalsome to inhibit virus-induced type I IFN expression. EMBO J 35:1385–1399. doi: 10.15252/embj.201593458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sandri-Goldin RM. 2011. The many roles of the highly interactive HSV protein ICP27, a key regulator of infection. Future Microbiol 6:1261–1277. doi: 10.2217/fmb.11.119. [DOI] [PubMed] [Google Scholar]
- 103.Verpooten D, Ma Y, Hou S, Yan Z, He B. 2009. Control of TANK-binding kinase 1-mediated signaling by the γ134.5 protein of herpes simplex virus 1. J Biol Chem 284:1097–1105. doi: 10.1074/jbc.M805905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manivanh R, Mehrbach J, Knipe DM, Leib DA. 2017. Role of herpes simplex virus 1 γ34.5 in the regulation of IRF3 signaling. J Virol 91:e01156-17. doi: 10.1128/JVI.01156-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang S, Wang K, Lin R, Zheng C. 2013. Herpes simplex virus 1 serine/threonine kinase US3 hyperphosphorylates IRF3 and inhibits beta interferon production. J Virol 87:12814–12827. doi: 10.1128/JVI.02355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu J, Coyne CB, Sarkar SN. 2011. PKC alpha regulates Sendai virus-mediated interferon induction through HDAC6 and beta-catenin. EMBO J 30:4838–4849. doi: 10.1038/emboj.2011.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.You H, Lin Y, Lin F, Yang M, Li J, Zhang R, Huang Z, Shen Q, Tang R, Zheng C. 2020. β-catenin is required for the cGAS/STING signaling pathway but antagonized by the herpes simplex virus 1 US3 protein. J Virol 94:e01847-19. doi: 10.1128/JVI.01847-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang K, Shi H, Qi R, Sun S, Tang Y, Zhang B, Wang C. 2006. Hsp90 regulates activation of interferon regulatory factor 3 and TBK-1 stabilization in Sendai virus-infected cells. Mol Biol Cell 17:1461–1471. doi: 10.1091/mbc.e05-09-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee MN, Roy M, Ong SE, Mertins P, Villani AC, Li W, Dotiwala F, Sen J, Doench JG, Orzalli MH, Kramnik I, Knipe DM, Lieberman J, Carr SA, Hacohen N. 2013. Identification of regulators of the innate immune response to cytosolic DNA and retroviral infection by an integrative approach. Nat Immunol 14:179–185. doi: 10.1038/ni.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu X, Main D, Ma Y, He B. 2018. Herpes simplex virus 1 inhibits TANK-binding kinase 1 through formation of the Us11-Hsp90 complex. J Virol 92:e00402-18. doi: 10.1128/JVI.00402-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McLean G, Rixon F, Langeland N, Haarr L, Marsden H. 1990. Identification and characterization of the virion protein products of herpes simplex virus type 1 gene UL47. J Gen Virol 71:2953–2960. doi: 10.1099/0022-1317-71-12-2953. [DOI] [PubMed] [Google Scholar]
- 112.Xing J, Ni L, Wang S, Wang K, Lin R, Zheng C. 2013. Herpes simplex virus 1-encoded tegument protein VP16 abrogates the production of beta interferon (IFN) by inhibiting NF-κB activation and blocking IFN regulatory factor 3 to recruit its coactivator CBP. J Virol 87:9788–9801. doi: 10.1128/JVI.01440-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang D, Su C, Zheng C. 2016. Herpes simplex virus 1 serine protease VP24 blocks the DNA-sensing signal pathway by abrogating activation of interferon regulatory factor 3. J Virol 90:5824–5829. doi: 10.1128/JVI.00186-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.You H, Zheng S, Huang Z, Lin Y, Shen Q, Zheng C. 2019. Herpes simplex virus 1 tegument protein UL46 inhibits TANK-binding kinase 1-mediated signaling. mBio 10:e00919-19. doi: 10.1128/mBio.00919-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iwai K. 2014. Diverse roles of the ubiquitin system in NF-κB activation. Biochim Biophys Acta 1843:129–136. doi: 10.1016/j.bbamcr.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 116.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. 1997. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-alpha and NIK. Science 278:866–870. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 117.Wu C-J, Conze DB, Li T, Srinivasula SM, Ashwell JD. 2006. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-κB activation. Nat Cell Biol 8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 118.Hayden MS, Ghosh S. 2008. Shared principles in NF-κB signaling. Cell 132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 119.Ye R, Su C, Xu H, Zheng C. 2017. Herpes simplex virus 1 ubiquitin-specific protease UL36 abrogates NF-κB activation in DNA sensing signal pathway. J Virol 91:e02417-16. doi: 10.1128/JVI.02417-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu H, Su C, Pearson A, Mody CH, Zheng C. 2017. Herpes simplex virus 1 UL24 abrogates the DNA sensing signal pathway by inhibiting NF-κB activation. J Virol 91:e00025-17. doi: 10.1128/JVI.00025-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim JC, Lee SY, Kim SY, Kim JK, Kim HJ, Lee HM, Choi MS, Min JS, Kim MJ, Choi HS, Ahn JK. 2008. HSV-1 ICP27 suppresses NF-κB activity by stabilizing IκBα. FEBS Lett 582:2371–2376. doi: 10.1016/j.febslet.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 122.Zhang J, Wang K, Wang S, Zheng C. 2013. Herpes simplex virus 1 E3 ubiquitin ligase ICP0 protein inhibits tumor necrosis factor alpha-induced NF-κB activation by interacting with p65/RelA and p50/NF-κB1. J Virol 87:12935–12948. doi: 10.1128/JVI.01952-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang K, Ni L, Wang S, Zheng C. 2014. Herpes simplex virus 1 protein kinase US3 hyperphosphorylates p65/RelA and dampens NF-κB activation. J Virol 88:7941–7951. doi: 10.1128/JVI.03394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang J, Wang S, Wang K, Zheng C. 2013. Herpes simplex virus 1 DNA polymerase processivity factor UL42 inhibits TNF-α-induced NF-κB activation by interacting with p65/RelA and p50/NF-κB1. Med Microbiol Immunol 202:313–325. doi: 10.1007/s00430-013-0295-0. [DOI] [PubMed] [Google Scholar]
- 125.Franchi L, McDonald C, Kanneganti TD, Amer A, Nunez G. 2006. Nucleotide-binding oligomerization domain-like receptors: intracellular pattern recognition molecules for pathogen detection and host defense. J Immunol 177:3507–3513. doi: 10.4049/jimmunol.177.6.3507. [DOI] [PubMed] [Google Scholar]
- 126.Martinon F, Burns K, Tschopp J. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol Cell 10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 127.Zhao C, Zhao W. 2020. NLRP3 inflammasome—a key player in antiviral responses. Front Immunol 11:211. doi: 10.3389/fimmu.2020.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen IY, Ichinohe T. 2015. Response of host inflammasomes to viral infection. Trends Microbiol 23:55–63. doi: 10.1016/j.tim.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 129.Zhi X, Zhang Y, Sun S, Zhang Z, Dong H, Luo X, Wei Y, Lu Z, Dou Y, Wu R, Jiang Z, Weng C, Seong Seo H, Guo H. 2020. NLRP3 inflammasome activation by foot-and-mouth disease virus infection mainly induced by viral RNA and non-structural protein 2B. RNA Biol 17:335–349. doi: 10.1080/15476286.2019.1700058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ichinohe T, Yamazaki T, Koshiba T, Yanagi Y. 2013. Mitochondrial protein mitofusin 2 is required for NLRP3 inflammasome activation after RNA virus infection. Proc Natl Acad Sci U S A 110:17963–17968. doi: 10.1073/pnas.1312571110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Holland EJ, Schwartz GS. 1999. Classification of herpes simplex virus keratitis. Cornea 18:144–154. doi: 10.1097/00003226-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 132.Gimenez F, Bhela S, Dogra P, Harvey L, Varanasi SK, Jaggi U, Rouse BT. 2016. The inflammasome NLRP3 plays a protective role against a viral immunopathological lesion. J Leukoc Biol 99:647–657. doi: 10.1189/jlb.3HI0715-321R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Coulon PG, Dhanushkodi N, Prakash S, Srivastava R, Roy S, Alomari NI, Nguyen AM, Warsi WR, Ye C, Carlos-Cruz EA, Mai UT, Cruel AC, Ekmekciyan KM, Pearlman E, BenMohamed L. 2019. NLRP3, NLRP12, and IFI16 inflammasomes induction and caspase-1 activation triggered by virulent HSV-1 strains are associated with severe corneal inflammatory herpetic disease. Front Immunol 10:1631. doi: 10.3389/fimmu.2019.01631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Strittmatter GE, Sand J, Sauter M, Seyffert M, Steigerwald R, Fraefel C, Smola S, French LE, Beer HD. 2016. IFN-gamma primes keratinocytes for HSV-1-induced inflammasome activation. J Invest Dermatol 136:610–620. doi: 10.1016/j.jid.2015.12.022. [DOI] [PubMed] [Google Scholar]
- 135.Karaba AH, Figueroa A, Massaccesi G, Botto S, DeFilippis VR, Cox AL. 2020. Herpes simplex virus type 1 inflammasome activation in proinflammatory human macrophages is dependent on NLRP3, ASC, and caspase-1. PLoS One 15:e0229570. doi: 10.1371/journal.pone.0229570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Raftery N, Stevenson NJ. 2017. Advances in anti-viral immune defence: revealing the importance of the IFN JAK/STAT pathway. Cell Mol Life Sci 74:2525–2535. doi: 10.1007/s00018-017-2520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sadler AJ, Williams BR. 2008. Interferon-inducible antiviral effectors. Nat Rev Immunol 8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Luker GD, Prior JL, Song J, Pica CM, Leib DA. 2003. Bioluminescence imaging reveals systemic dissemination of herpes simplex virus type 1 in the absence of interferon receptors. J Virol 77:11082–11093. doi: 10.1128/jvi.77.20.11082-11093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nicholl MJ, Robinson LH, Preston CM. 2000. Activation of cellular interferon-responsive genes after infection of human cells with herpes simplex virus type 1. J Gen Virol 81:2215–2218. doi: 10.1099/0022-1317-81-9-2215. [DOI] [PubMed] [Google Scholar]
- 140.Mossman KL, Macgregor PF, Rozmus JJ, Goryachev AB, Edwards AM, Smiley JR. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J Virol 75:750–758. doi: 10.1128/JVI.75.2.750-758.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Eidson KM, Hobbs WE, Manning BJ, Carlson P, DeLuca NA. 2002. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J Virol 76:2180–2191. doi: 10.1128/jvi.76.5.2180-2191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Halford WP, Weisend C, Grace J, Soboleski M, Carr DJ, Balliet JW, Imai Y, Margolis TP, Gebhardt BM. 2006. ICP0 antagonizes Stat 1-dependent repression of herpes simplex virus: implications for the regulation of viral latency. Virol J 3:44. doi: 10.1186/1743-422X-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Johnson KE, Knipe DM. 2010. Herpes simplex virus-1 infection causes the secretion of a type I interferon-antagonizing protein and inhibits signaling at or before Jak-1 activation. Virology 396:21–29. doi: 10.1016/j.virol.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Johnson KE, Song B, Knipe DM. 2008. Role for herpes simplex virus 1 ICP27 in the inhibition of type I interferon signaling. Virology 374:487–494. doi: 10.1016/j.virol.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chee AV, Roizman B. 2004. Herpes simplex virus 1 gene products occlude the interferon signaling pathway at multiple sites. J Virol 78:4185–4196. doi: 10.1128/jvi.78.8.4185-4196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yuan H, You J, You H, Zheng C. 2018. Herpes simplex virus 1 UL36USP antagonizes type I interferon-mediated antiviral innate immunity. J Virol 92:e01161-18. doi: 10.1128/JVI.01161-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gao G, Guo X, Goff SP. 2002. Inhibition of retroviral RNA production by ZAP, a CCCH-type zinc finger protein. Science 297:1703–1706. doi: 10.1126/science.1074276. [DOI] [PubMed] [Google Scholar]
- 148.Bick MJ, Carroll JW, Gao G, Goff SP, Rice CM, MacDonald MR. 2003. Expression of the zinc-finger antiviral protein inhibits alphavirus replication. J Virol 77:11555–11562. doi: 10.1128/jvi.77.21.11555-11562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Su C, Zhang J, Zheng C. 2015. Herpes simplex virus 1 UL41 protein abrogates the antiviral activity of hZAP by degrading its mRNA. Virol J 12:203. doi: 10.1186/s12985-015-0433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shen G, Wang K, Wang S, Cai M, Li ML, Zheng C. 2014. Herpes simplex virus 1 counteracts viperin via its virion host shutoff protein UL41. J Virol 88:12163–12166. doi: 10.1128/JVI.01380-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Blondeau C, Pelchen-Matthews A, Mlcochova P, Marsh M, Milne RS, Towers GJ. 2013. Tetherin restricts herpes simplex virus 1 and is antagonized by glycoprotein M. J Virol 87:13124–13133. doi: 10.1128/JVI.02250-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zenner HL, Mauricio R, Banting G, Crump CM. 2013. Herpes simplex virus 1 counteracts tetherin restriction via its virion host shutoff activity. J Virol 87:13115–13123. doi: 10.1128/JVI.02167-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Reboldi A, Dang EV, McDonald JG, Liang G, Russell DW, Cyster JG. 2014. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science 345:679–684. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu SY, Aliyari R, Chikere K, Li G, Marsden MD, Smith JK, Pernet O, Guo H, Nusbaum R, Zack JA, Freiberg AN, Su L, Lee B, Cheng G. 2013. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 38:92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.You H, Yuan H, Fu W, Su C, Wang W, Cheng T, Zheng C. 2017. Herpes simplex virus type 1 abrogates the antiviral activity of Ch25h via its virion host shutoff protein. Antiviral Res 143:69–73. doi: 10.1016/j.antiviral.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 156.Garcia MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M. 2006. Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiol Mol Biol Rev 70:1032–1060. doi: 10.1128/MMBR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Low-Calle AM, Prada-Arismendy J, Castellanos JE. 2014. Study of interferon-beta antiviral activity against herpes simplex virus type 1 in neuron-enriched trigeminal ganglia cultures. Virus Res 180:49–58. doi: 10.1016/j.virusres.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 158.He B, Gross M, Roizman B. 1997. The γ134.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1α to dephosphorylate the α subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A 94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cheng G, Yang K, He B. 2003. Dephosphorylation of eIF-2α mediated by the γ134.5 protein of herpes simplex virus type 1 is required for viral response to interferon but is not sufficient for efficient viral replication. J Virol 77:10154–10161. doi: 10.1128/jvi.77.18.10154-10161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Poppers J, Mulvey M, Khoo D, Mohr I. 2000. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J Virol 74:11215–11221. doi: 10.1128/jvi.74.23.11215-11221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cassady KA, Gross M. 2002. The herpes simplex virus type 1 US11 protein interacts with protein kinase R in infected cells and requires a 30-amino-acid sequence adjacent to a kinase substrate domain. J Virol 76:2029–2035. doi: 10.1128/jvi.76.5.2029-2035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cassady KA, Gross M, Roizman B. 1998. The herpes simplex virus US11 protein effectively compensates for the γ134.5 gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2. J Virol 72:8620–8626. doi: 10.1128/JVI.72.11.8620-8626.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sciortino MT, Parisi T, Siracusano G, Mastino A, Taddeo B, Roizman B. 2013. The virion host shutoff RNase plays a key role in blocking the activation of protein kinase R in cells infected with herpes simplex virus 1. J Virol 87:3271–3276. doi: 10.1128/JVI.03049-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Floyd-Smith G, Slattery E, Lengyel P. 1981. Interferon action: RNA cleavage pattern of a (2'-5')oligoadenylate–dependent endonuclease. Science 212:1030–1032. doi: 10.1126/science.6165080. [DOI] [PubMed] [Google Scholar]
- 165.Sanchez R, Mohr I. 2007. Inhibition of cellular 2'-5' oligoadenylate synthetase by the herpes simplex virus type 1 Us11 protein. J Virol 81:3455–3464. doi: 10.1128/JVI.02520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sobol PT, Mossman KL. 2006. ICP0 prevents RNase L-independent rRNA cleavage in herpes simplex virus type 1-infected cells. J Virol 80:218–225. doi: 10.1128/JVI.80.1.218-225.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhou X, Michal JJ, Zhang L, Ding B, Lunney JK, Liu B, Jiang Z. 2013. Interferon induced IFIT family genes in host antiviral defense. Int J Biol Sci 9:200–208. doi: 10.7150/ijbs.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jiang Z, Su C, Zheng C. 2016. Herpes simplex virus 1 tegument protein UL41 counteracts IFIT3 antiviral innate immunity. J Virol 90:11056–11061. doi: 10.1128/JVI.01672-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Di Rienzo M, Romagnoli A, Antonioli M, Piacentini M, Fimia GM. 2020. TRIM proteins in autophagy: selective sensors in cell damage and innate immune responses. Cell Death Differ 27:887–902. doi: 10.1038/s41418-020-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Seo GJ, Kim C, Shin WJ, Sklan EH, Eoh H, Jung JU. 2018. TRIM56-mediated monoubiquitination of cGAS for cytosolic DNA sensing. Nat Commun 9:613. doi: 10.1038/s41467-018-02936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cui H, Liu Y, Huang Y. 2017. Roles of TRIM32 in corneal epithelial cells after infection with herpes simplex virus. Cell Physiol Biochem 43:801–811. doi: 10.1159/000481563. [DOI] [PubMed] [Google Scholar]
- 172.Reszka N, Zhou C, Song B, Sodroski JG, Knipe DM. 2010. Simian TRIM5α proteins reduce replication of herpes simplex virus. Virology 398:243–250. doi: 10.1016/j.virol.2009.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Conwell SE, White AE, Harper JW, Knipe DM. 2015. Identification of TRIM27 as a novel degradation target of herpes simplex virus 1 ICP0. J Virol 89:220–229. doi: 10.1128/JVI.02635-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Full F, van Gent M, Sparrer KMJ, Chiang C, Zurenski MA, Scherer M, Brockmeyer NH, Heinzerling L, Sturzl M, Korn K, Stamminger T, Ensser A, Gack MU. 2019. Centrosomal protein TRIM43 restricts herpesvirus infection by regulating nuclear lamina integrity. Nat Microbiol 4:164–176. doi: 10.1038/s41564-018-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Smith S, Reuven N, Mohni KN, Schumacher AJ, Weller SK. 2014. Structure of the herpes simplex virus 1 genome: manipulation of nicks and gaps can abrogate infectivity and alter the cellular DNA damage response. J Virol 88:10146–10156. doi: 10.1128/JVI.01723-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ferguson BJ, Mansur DS, Peters NE, Ren H, Smith GL. 2012. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. Elife 1:e00047. doi: 10.7554/eLife.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Parkinson J, Lees-Miller SP, Everett RD. 1999. Herpes simplex virus type 1 immediate-early protein vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J Virol 73:650–657. doi: 10.1128/JVI.73.1.650-657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Burleigh K, Maltbaek JH, Cambier S, Green R, Gale M, James RC, Stetson DB. 2020. Human DNA-PK activates a STING-independent DNA sensing pathway. Sci Immunol 5:eaba4219. doi: 10.1126/sciimmunol.aba4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huen MSY, Grant R, Manke I, Minn K, Yu X, Yaffe MB, Chen J. 2007. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131:901–914. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Doil C, Mailand N, Bekker-Jensen S, Menard P, Larsen DH, Pepperkok R, Ellenberg J, Panier S, Durocher D, Bartek J, Lukas J, Lukas C. 2009. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell 136:435–446. doi: 10.1016/j.cell.2008.12.041. [DOI] [PubMed] [Google Scholar]
- 181.Lilley CE, Chaurushiya MS, Boutell C, Landry S, Suh J, Panier S, Everett RD, Stewart GS, Durocher D, Weitzman MD. 2010. A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses. EMBO J 29:943–955. doi: 10.1038/emboj.2009.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chaurushiya MS, Lilley CE, Aslanian A, Meisenhelder J, Scott DC, Landry S, Ticau S, Boutell C, Yates JR, Schulman BA, Hunter T, Weitzman MD. 2012. Viral E3 ubiquitin ligase-mediated degradation of a cellular E3: viral mimicry of a cellular phosphorylation mark targets the RNF8 FHA domain. Mol Cell 46:79–90. doi: 10.1016/j.molcel.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Weller SK, Lee KJ, Sabourin DJ, Schaffer PA. 1983. Genetic analysis of temperature-sensitive mutants which define the gene for the major herpes simplex virus type 1 DNA-binding protein. J Virol 45:354–366. doi: 10.1128/JVI.45.1.354-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gao M, Knipe DM. 1991. Potential role for herpes simplex virus ICP8 DNA replication protein in stimulation of late gene expression. J Virol 65:2666–2675. doi: 10.1128/JVI.65.5.2666-2675.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mohni KN, Smith S, Dee AR, Schumacher AJ, Weller SK. 2013. Herpes simplex virus type 1 single strand DNA binding protein and helicase/primase complex disable cellular ATR signaling. PLoS Pathog 9:e1003652. doi: 10.1371/journal.ppat.1003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dong X, Guan J, Zheng C, Zheng X. 2017. The herpes simplex virus 1 UL36USP deubiquitinase suppresses DNA repair in host cells via deubiquitination of proliferating cell nuclear antigen. J Biol Chem 292:8472–8483. doi: 10.1074/jbc.M117.778076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schwarz DS, Blower MD. 2016. The endoplasmic reticulum: structure, function and response to cellular signaling. Cell Mol Life Sci 73:79–94. doi: 10.1007/s00018-015-2052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hetz C. 2012. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 189.Cheng G, Feng Z, He B. 2005. Herpes simplex virus 1 infection activates the endoplasmic reticulum resident kinase PERK and mediates eIF-2α dephosphorylation by the γ134.5 protein. J Virol 79:1379–1388. doi: 10.1128/JVI.79.3.1379-1388.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Burnett HF, Audas TE, Liang G, Lu RR. 2012. Herpes simplex virus-1 disarms the unfolded protein response in the early stages of infection. Cell Stress Chaperones 17:473–483. doi: 10.1007/s12192-012-0324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mulvey M, Arias C, Mohr I. 2007. Maintenance of endoplasmic reticulum (ER) homeostasis in herpes simplex virus type 1-infected cells through the association of a viral glycoprotein with PERK, a cellular ER stress sensor. J Virol 81:3377–3390. doi: 10.1128/JVI.02191-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Everett RD. 2000. ICP0, a regulator of herpes simplex virus during lytic and latent infection. BioEssays 22:761–770. doi:. [DOI] [PubMed] [Google Scholar]
- 193.Halford WP, Schaffer PA. 2001. ICP0 is required for efficient reactivation of herpes simplex virus type 1 from neuronal latency. J Virol 75:3240–3249. doi: 10.1128/JVI.75.7.3240-3249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang P, Su C, Jiang Z, Zheng C. 2017. Herpes simplex virus 1 UL41 protein suppresses the IRE1/XBP1 signal pathway of the unfolded protein response via its RNase activity. J Virol 91:e02056-16. doi: 10.1128/JVI.02056-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Levine B, Deretic V. 2007. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol 7:767–777. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Talloczy Z, Jiang W, Virgin HWT, Leib DA, Scheuner D, Kaufman RJ, Eskelinen EL, Levine B. 2002. Regulation of starvation- and virus-induced autophagy by the eIF2α kinase signaling pathway. Proc Natl Acad Sci U S A 99:190–195. doi: 10.1073/pnas.012485299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. 2007. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 198.Leib DA, Alexander DE, Cox D, Yin J, Ferguson TA. 2009. Interaction of ICP34.5 with Beclin 1 modulates herpes simplex virus type 1 pathogenesis through control of CD4+ T-cell responses. J Virol 83:12164–12171. doi: 10.1128/JVI.01676-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, Burns D, Leib DA, Levine B. 2007. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 200.Sparrer KMJ, Gableske S, Zurenski MA, Parker ZM, Full F, Baumgart GJ, Kato J, Pacheco-Rodriguez G, Liang C, Pornillos O, Moss J, Vaughan M, Gack MU. 2017. TRIM23 mediates virus-induced autophagy via activation of TBK1. Nat Microbiol 2:1543–1557. doi: 10.1038/s41564-017-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu X, Matrenec R, Gack MU, He B. 2019. Disassembly of the TRIM23-TBK1 complex by the Us11 protein of herpes simplex virus 1 impairs autophagy. J Virol 93:e00497-19. doi: 10.1128/JVI.00497-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lussignol M, Queval C, Bernet-Camard MF, Cotte-Laffitte J, Beau I, Codogno P, Esclatine A. 2013. The herpes simplex virus 1 Us11 protein inhibits autophagy through its interaction with the protein kinase PKR. J Virol 87:859–871. doi: 10.1128/JVI.01158-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Waisner H, Kalamvoki M. 2019. The ICP0 protein of herpes simplex virus 1 (HSV-1) downregulates major autophagy adaptor proteins sequestosome 1 and optineurin during the early stages of HSV-1 infection. J Virol 93:e01258-19. doi: 10.1128/JVI.01258-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Langereis MA, Feng Q, van Kuppeveld FJ. 2013. MDA5 localizes to stress granules, but this localization is not required for the induction of type I interferon. J Virol 87:6314–6325. doi: 10.1128/JVI.03213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Onomoto K, Jogi M, Yoo JS, Narita R, Morimoto S, Takemura A, Sambhara S, Kawaguchi A, Osari S, Nagata K, Matsumiya T, Namiki H, Yoneyama M, Fujita T. 2012. Critical role of an antiviral stress granule containing RIG-I and PKR in viral detection and innate immunity. PLoS One 7:e43031. doi: 10.1371/journal.pone.0043031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lindquist ME, Mainou BA, Dermody TS, Crowe JE Jr. 2011. Activation of protein kinase R is required for induction of stress granules by respiratory syncytial virus but dispensable for viral replication. Virology 413:103–110. doi: 10.1016/j.virol.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Montero H, Rojas M, Arias CF, Lopez S. 2008. Rotavirus infection induces the phosphorylation of eIF2α but prevents the formation of stress granules. J Virol 82:1496–1504. doi: 10.1128/JVI.01779-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Qin Q, Hastings C, Miller CL. 2009. Mammalian orthoreovirus particles induce and are recruited into stress granules at early times postinfection. J Virol 83:11090–11101. doi: 10.1128/JVI.01239-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rao S, Hassine S, Monette A, Amorim R, DesGroseillers L, Mouland AJ. 2019. HIV-1 requires Staufen1 to dissociate stress granules and to produce infectious viral particles. RNA 25:727–736. doi: 10.1261/rna.069351.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Finnen RL, Banfield BW. 2016. Alphaherpesvirus subversion of stress-induced translational arrest. Viruses 8:81. doi: 10.3390/v8030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Burgess HM, Mohr I. 2018. Defining the role of stress granules in innate immune suppression by the herpes simplex virus 1 endoribonuclease VHS. J Virol 92:e00829-18. doi: 10.1128/JVI.00829-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Finnen RL, Pangka KR, Banfield BW. 2012. Herpes simplex virus 2 infection impacts stress granule accumulation. J Virol 86:8119–8130. doi: 10.1128/JVI.00313-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Finnen RL, Hay TJ, Dauber B, Smiley JR, Banfield BW. 2014. The herpes simplex virus 2 virion-associated ribonuclease vhs interferes with stress granule formation. J Virol 88:12727–12739. doi: 10.1128/JVI.01554-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Finnen RL, Zhu M, Li J, Romo D, Banfield BW. 2016. Herpes simplex virus 2 virion host shutoff endoribonuclease activity is required to disrupt stress granule formation. J Virol 90:7943–7955. doi: 10.1128/JVI.00947-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Panas MD, Schulte T, Thaa B, Sandalova T, Kedersha N, Achour A, McInerney GM. 2015. Viral and cellular proteins containing FGDF motifs bind G3BP to block stress granule formation. PLoS Pathog 11:e1004659. doi: 10.1371/journal.ppat.1004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nunez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G. 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Edinger AL, Thompson CB. 2004. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol 16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 218.Jaattela M, Tschopp J. 2003. Caspase-independent cell death in T lymphocytes. Nat Immunol 4:416–423. doi: 10.1038/ni0503-416. [DOI] [PubMed] [Google Scholar]
- 219.Lockshin RA, Zakeri Z. 2004. Caspase-independent cell death? Oncogene 23:2766–2773. doi: 10.1038/sj.onc.1207514. [DOI] [PubMed] [Google Scholar]
- 220.Sanfilippo CM, Blaho JA. 2006. ICP0 gene expression is a herpes simplex virus type 1 apoptotic trigger. J Virol 80:6810–6821. doi: 10.1128/JVI.00334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wang X, Li Y, Liu S, Yu X, Li L, Shi C, He W, Li J, Xu L, Hu Z, Yu L, Yang Z, Chen Q, Ge L, Zhang Z, Zhou B, Jiang X, Chen S, He S. 2014. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci U S A 111:15438–15443. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Guo H, Omoto S, Harris PA, Finger JN, Bertin J, Gough PJ, Kaiser WJ, Mocarski ES. 2015. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe 17:243–251. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Huang Z, Wu SQ, Liang Y, Zhou X, Chen W, Li L, Wu J, Zhuang Q, Chen C, Li J, Zhong CQ, Xia W, Zhou R, Zheng C, Han J. 2015. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe 17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 224.He S, Han J. 15 February 2020. Manipulation of host cell death pathways by herpes simplex virus. Curr Top Microbiol Immunol doi: 10.1007/82_2020_196. [DOI] [PubMed] [Google Scholar]
- 225.Leopardi R, Van Sant C, Roizman B. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci U S A 94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Jerome KR, Fox R, Chen Z, Sears AE, Lee H, Corey L. 1999. Herpes simplex virus inhibits apoptosis through the action of two genes, Us5 and Us3. J Virol 73:8950–8957. doi: 10.1128/JVI.73.11.8950-8957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Munger J, Roizman B. 2001. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc Natl Acad Sci U S A 98:10410–10415. doi: 10.1073/pnas.181344498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Cartier A, Komai T, Masucci MG. 2003. The Us3 protein kinase of herpes simplex virus 1 blocks apoptosis and induces phosporylation of the Bcl-2 family member Bad. Exp Cell Res 291:242–250. doi: 10.1016/s0014-4827(03)00375-6. [DOI] [PubMed] [Google Scholar]
- 229.Hagglund R, Munger J, Poon AP, Roizman B. 2002. U(S)3 protein kinase of herpes simplex virus 1 blocks caspase 3 activation induced by the products of U(S)1.5 and U(L)13 genes and modulates expression of transduced U(S)1.5 open reading frame in a cell type-specific manner. J Virol 76:743–754. doi: 10.1128/jvi.76.2.743-754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhou G, Roizman B. 2001. The domains of glycoprotein D required to block apoptosis depend on whether glycoprotein D is present in the virions carrying herpes simplex virus 1 genome lacking the gene encoding the glycoprotein. J Virol 75:6166–6172. doi: 10.1128/JVI.75.13.6166-6172.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Medici MA, Sciortino MT, Perri D, Amici C, Avitabile E, Ciotti M, Balestrieri E, De Smaele E, Franzoso G, Mastino A. 2003. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: role of nuclear factor κB. J Biol Chem 278:36059–36067. doi: 10.1074/jbc.M306198200. [DOI] [PubMed] [Google Scholar]
- 232.Asano S, Honda T, Goshima F, Watanabe D, Miyake Y, Sugiura Y, Nishiyama Y. 1999. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J Gen Virol 80:51–56. doi: 10.1099/0022-1317-80-1-51. [DOI] [PubMed] [Google Scholar]
- 233.Miles D, Athmanathan S, Thakur A, Willcox M. 2003. A novel apoptotic interaction between HSV-1 and human corneal epithelial cells. Curr Eye Res 26:165–174. doi: 10.1076/ceyr.26.3.165.14899. [DOI] [PubMed] [Google Scholar]
- 234.Ahmed M, Lock M, Miller CG, Fraser NW. 2002. Regions of the herpes simplex virus type 1 latency-associated transcript that protect cells from apoptosis in vitro and protect neuronal cells in vivo. J Virol 76:717–729. doi: 10.1128/jvi.76.2.717-729.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Henderson G, Peng W, Jin L, Perng GC, Nesburn AB, Wechsler SL, Jones C. 2002. Regulation of caspase 8- and caspase 9-induced apoptosis by the herpes simplex virus type 1 latency-associated transcript. J Neurovirol 8(Suppl 2):103–111. doi: 10.1080/13550280290101085. [DOI] [PubMed] [Google Scholar]
- 236.Aubert M, O'Toole J, Blaho JA. 1999. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J Virol 73:10359–10370. doi: 10.1128/JVI.73.12.10359-10370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Aubert M, Blaho JA. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J Virol 73:2803–2813. doi: 10.1128/JVI.73.4.2803-2813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kurt-Jones EA, Belko J, Yu C, Newburger PE, Wang J, Chan M, Knipe DM, Finberg RW. 2005. The role of toll-like receptors in herpes simplex infection in neonates. J Infect Dis 191:746–748. doi: 10.1086/427339. [DOI] [PubMed] [Google Scholar]
- 239.Cheng K, Wang X, Zhang S, Yin H. 2012. Discovery of small-molecule inhibitors of the TLR1/TLR2 complex. Angew Chem Int Ed Engl 51:12246–12249. doi: 10.1002/anie.201204910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Meng G, Rutz M, Schiemann M, Metzger J, Grabiec A, Schwandner R, Luppa PB, Ebel F, Busch DH, Bauer S, Wagner H, Kirschning CJ. 2004. Antagonistic antibody prevents toll-like receptor 2-driven lethal shock-like syndromes. J Clin Invest 113:1473–1481. doi: 10.1172/JCI20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Murgueitio MS, Henneke P, Glossmann H, Santos-Sierra S, Wolber G. 2014. Prospective virtual screening in a sparse data scenario: design of small-molecule TLR2 antagonists. ChemMedChem 9:813–822. doi: 10.1002/cmdc.201300445. [DOI] [PubMed] [Google Scholar]
- 242.Mistry P, Laird MH, Schwarz RS, Greene S, Dyson T, Snyder GA, Xiao TS, Chauhan J, Fletcher S, Toshchakov VY, MacKerell AD Jr, Vogel SN. 2015. Inhibition of TLR2 signaling by small molecule inhibitors targeting a pocket within the TLR2 TIR domain. Proc Natl Acad Sci U S A 112:5455–5460. doi: 10.1073/pnas.1422576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hensel MT, Marshall JD, Dorwart MR, Heeke DS, Rao E, Tummala P, Yu L, Cohen GH, Eisenberg RJ, Sloan DD. 2017. Prophylactic herpes simplex virus 2 (HSV-2) vaccines adjuvanted with stable emulsion and toll-like receptor 9 agonist induce a robust HSV-2-specific cell-mediated immune response, protect against symptomatic disease, and reduce the latent viral reservoir. J Virol 91:e02257-16. doi: 10.1128/JVI.02257-16. [DOI] [PMC free article] [PubMed] [Google Scholar]