Abstract
Physical activity (PA) is one of the most important modifiable factors associated with fracture risk. However, the association between interval changes in PA and the risk of fracture remains unknown. We investigated the risk of fracture development according to interval changes in PA in middle aged and older individuals. In this nationwide cohort study of adults aged ≥ 40 years, more than 4.9 million individuals without fractures within the last year who underwent two consecutive national health screenings in Korea from 2009 to 2012 were identified. The risk of fracture between 2013 and 2016 according to interval changes in regular PA was prospectively analyzed. Compared to individuals with a continuous lack of PA, those with a decrease in PA (0.41/1000 person-years (PY) decrease in incidence rate (IR); adjusted hazard ratio (aHR) 0.975; 95% confidence interval (CI) 0.964–0.987), increase in PA (1.8/1000 PY decrease in IR; aHR 0.948; 95% CI 0.937–0.959), and continuous PA (3.58/1000 PY decrease in IR; aHR 0.888; 95% CI 0.875–0.901) had a significantly reduced risk of fracture. Interval changes in regular PA were associated with risk of fracture. Individuals who engaged in continuous regular PA exhibited the maximum protective benefit against fracture.
Subject terms: Health care, Medical research, Risk factors
Introduction
Fractures are one of the most important public health priorities, especially in older individuals. The risk of fracture increases in individuals aged ≥ 50 years; moreover, fractures are associated with serious disorders and diseases, as well as increased mortality1,2. Indeed, more than half of patients with hip fractures are unable to return to their pre-fracture states, and the mortality rate rises to 33% within 1 year of fracture3–5. Therefore, fractures are an important contributor to socioeconomic loss.
Physical activity (PA) is one of the most important modifiable factors associated with fracture risk, along with alcohol intake and smoking6. Previous studies have demonstrated that exercise can help prevent fractures; however, the majority of these studies have included only elderly populations and none have assessed how changes in PA affect fracture prevention7,8. Thus far, there remains no clear consensus regarding the frequency and intensity of PA recommended to prevent fractures. One study found that the minimum amount of exercise that affected bone mineral density (BMD) was 2.1–2.5 sessions per week; however, it did not explore how changes in exercise habits affect fracture occurrence9.
Here, we investigated a group of middle-aged and older patients with demographic data, health survey information, medical tests, and medical claims from the Korean National Health Insurance Service (K-NHIS) in South Korea. The primary aim of this study was to identify the relationship between interval changes in PA and the risk of fracture. The secondary aim was to identify differences in fracture incidence according to PA in different sex, age, and previous fracture history subgroups.
Materials and methods
Data sources
This nationwide population-based study was conducted in South Korea using data obtained from the K-NHIS. Two data sets were used: the Korean National Health Examination (K-NHE) database and the K-NHIS claims database. The K-NHE database was used to select the participants and obtain information regarding confounding variables. The linked K-NHIS claims database for the same participants was then used to evaluate the occurrence of fractures. Insured Korean adults aged > 40 years and employees aged > 20 years undergo regular health checkups provided by the K-NHIS every 1–2 years. The K-NHE data obtained through these checkups provide information concerning anthropometric measurements, smoking and alcohol consumption status, and medical history through self-reported questionnaires and laboratory findings10. This database and the aforementioned nationwide medical records were combined and analyzed to construct a cohort to investigate health problems with NHIS research approval. The K-NHIS is a mandatory national health insurance system that covers approximately 97% of the South Korean population for all medical procedures, except cosmetic surgery and treatment for injuries sustained in road traffic accidents. The remaining 3% of the population is included in the Medical Aid program. Patients enrolled in the K-NHIS pay for 30% of their total medical expenses, and medical service providers submit claims for reimbursement from the K-NHIS for the remaining costs. Thus, medical information from almost all patients treated by a healthcare institution is prospectively integrated into the database. The K-NHIS claims database includes extensive health information from approximately 50,000,000 South Koreans. Indeed, it contains data from all clinics and hospitals regarding diagnoses and comorbidities (coded in accordance with the 10th revision of the International Statistical Classification of Diseases and Related Health Problems [ICD-10]), demographic characteristics, prescriptions, medical services (i.e., treatments and procedures), and costs for inpatients (i.e., those admitted to hospital) and outpatients (i.e., those who received ambulatory care). Since 2015, the K-NHIS has provided a dynamic, nationally representative, retrospective cohort database that includes information from nearly the entire South Korean population, and is open to all researchers whose study protocols are approved by the official review committee.
Study design and participants
In this study, relevant information in the K-NHIS cohort database was collected to determine key variables used for adjustment and subgroup analyses11. Sociodemographic variables were obtained including sex, age, and perceived family economic status. Respondents were asked to self-record their height and weight; their body mass index (BMI, kg/m2) was calculated based on these data. All BMI and body weight data were measured by trained examiners. The study population was subdivided into two groups according to age: (1) middle-aged group, 40–64 years; and (2) elderly group, age ≥ 65 years. The study population was also divided based on income, with the low-income level defined as income below the 20th percentile. Current drinker was defined as individuals who drank > 30 g of alcohol per day. Individuals with diabetes were defined as either patients prescribed anti-diabetic drugs who had ICD-10 codes (E11–E14) or as patients with fasting glucose levels > 126 mg/dL, based on data from the K-NHE database. The presence of hypertension was defined as a systolic/diastolic blood pressure ≥ 140/90 mmHg from the K-NHE database, or the presence of ≥ 1 claim per year for a prescription of antihypertensive agents using ICD-10 codes (I10–I13, I15). The presence of dyslipidemia was defined as a total cholesterol level ≥ 240 mg/dL in the K-NHE database, or the presence of ≥ 1 claim per year for the prescription of antihyperlipidemic agents using ICD-10 codes (E78). Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate < 60 mL/min/1.73 m2. These definitions based on ICD codes have been validated in previous studies12,13. Blood pressure was measured using a blood pressure monitor after 5 min of rest, and all blood tests were performed after 8 h of overnight fasting.
Study cohort establishment
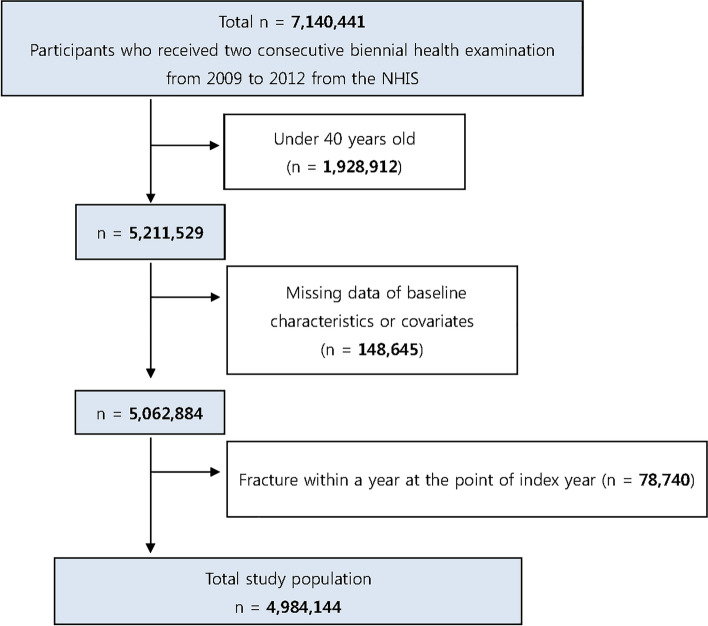
A total of 7,140,441 individuals who underwent two consecutive biennial K-NHEs from 2009 to 2010 and 2011 to 2012 were initially enrolled. Individuals aged < 40 years (n = 1,928,912) and those with any missing baseline characteristic data or health examination survey covariates (n = 148,645) were excluded. The linked K-NHIS claims database was then requested to determine the incidence of fracture in this cohort. To exclude pre-existing cases, individuals diagnosed with a fracture within 1 year of the index year were excluded (n = 78,740). A total of 4,984,144 participants were included in the final study population (Fig. 1).
Figure 1.
Flowchart of inclusion and exclusion criteria, based on the dataset of the Korean National Health Insurance Service (K-NHIS).
Classification of interval change in physical activity
During each K-NHE period, the participants in the K-NHIS cohort responded to a series of self-administered questionnaires regarding PA and other lifestyle behaviors. PA was measured using the Korean version of the International Physical Activity Questionnaire (IPAQ)-short version, which was designed for use in PA surveillance studies13. The IPAQ-short version consists of seven questions intended to determine the frequencies and durations of walking, moderate PA, and vigorous PA during the previous 7 days. The frequency of moderate PA was assessed with the following question: “In the past week, on how many days did you engage in 30 min or more of PA that increased your heart rate or breathing rate (e.g., brisk walking, playing doubles tennis, or cycling at a regular pace)?”. The frequency of vigorous PA was assessed with the following question: “In the past week, on how many days did you engage in 20 min or more of PA that was so vigorous it left you soaked with perspiration or breathless (e.g., running, aerobics, hiking, or fast cycling)?”. The response options ranged from 1 (none) to 6 (> 5 days per week).
Regular PA was defined using either of the two following criteria, based on the IPAQ Scoring Protocol: (1) vigorous intensity activity ≥ 3 days per week, or (b) moderate intensity activity ≥ 5 days per week14. The interval change in regular PA was evaluated among the participants at the two consecutive biennial K-NHEs. Categories were created as follows for change in regular PA at the second examination (2011–2012), compared to the first examination (2009–2010): (i) continuous lack of PA (regular PA (−) to (−)); (ii) decrease in PA (regular PA (+) to (−)); (iii) increase in PA (regular PA (−) to (+)); and (iv) continuous PA (regular PA (+) to (+))15.
Follow-up for fracture outcomes
Information regarding medical claims records of the K-NHIS was collected to identify fracture events during the follow-up period, between January 1, 2013 and December 31, 2016. ICD-10 codes and hospitalization records from the K-NHIS system were used to identify the causes for total fracture events. Fractures were defined using ICD-10 codes, as follows: vertebral, S12.0, S12.1, S12.2, S22.0, S22.1, S32.0, M48.4, and M48.5; hip, S72.0, S72.1, and S72.2; and other fractures, including upper arm, S42.0, S42.2, and S42.3; forearm, S52.5 and S52.6; and lower leg, S82.3, S82.5, and S82.6. Individuals were deemed to have vertebral or other fractures when they had ≥ 2 outpatient visits within 12 months associated with the relevant ICD-10 codes. Hip fracture was defined as one hospitalization with a relevant diagnosis code. A fracture was defined as an instance of ≥ 1 of the above fracture types in an individual. If the participant died during the follow-up period, they were censored at the time of death. For the participants who experienced multiple incidences of fractures, first occurrence of fracture was considered as an event and follow-up was stopped.
Statistical analysis
Baseline participant characteristics were calculated using numbers and percentages for categorical variables, and means and standard deviations for continuous variables. The characteristics of groups were compared using the chi-squared test for categorical variables and the t-test for continuous variables. Because the size of the total study population was large, nearly all variables showed significant differences according to the presence or absence of the outcome. The incidence rate (IR) was computed based on the prevalence of the outcome per 1,000 person-years (PY) from the number of total fracture events and the PY in each group, according to the change in PA status. Changes in IR were also assessed.
To evaluate the association between fracture risk and interval change in PA status between the two biennial K-NHE periods (2009–2010 and 2011–2012), Cox proportional hazards regression models were used; hazard ratios (HR) and 95% confidence intervals (CI) were calculated for each PA status, relative to the reference (continuous lack of PA). In Model 1, an unadjusted analysis was conducted by considering a range of covariates potentially associated with the development of fractures. In Model 2, an analysis was performed with adjustment for age and sex. In Model 3, further adjustments were made for lifestyle variables, including current smoking and alcohol consumption, as well as household income levels. In Model 4, a fully adjusted model was developed using BMI, dyslipidemia, and previous fracture within 3 years as the adjustment variables. To evaluate the robustness of the influence of clinical conditions on the association between interval change in PA status and fracture, the HRs for fracture in various subgroups were analyzed using Cox regression analyses with P-values for interaction. Subgroup analyses stratified by age (< 65 and ≥ 65 years), sex (male and female), BMI (< 25 and ≥ 25 kg/m2), household income level (income < 20th percentile or ≥ 20th percentile), cigarette smoking (current smoker or never smoker), current alcohol consumption (none or heavy), comorbidities (diabetes, hypertension, dyslipidemia, and CKD), and previous fracture (no or yes; within 3 years) were conducted. All statistical analyses were performed using SAS software (version 9.3; SAS Institute, Cary, NC, USA). Two-sided P-values < 0.05 were considered to indicate statistical significance for all analyses.
Ethics statement
The study protocol was approved by the K-NHIS institutional review board. Anonymized and de-identified information was used for analyses; therefore, informed consent was not required. The study protocol was also approved by the Institutional Review Board of Korea University Hospital (Ansan, South Korea; approval no. 2020AS0030), and the study was conducted in accordance with the principles of the Declaration of Helsinki. All study procedures were performed in accordance with the relevant guidelines and regulations.
Results
Cohort characteristics
The study sample comprised 3,303,504 participants (66.28%) with a continuous lack of PA (regular PA (−) to (−)), 583,561 participants with a decrease in PA (regular PA (+) to (−)), 650,888 participants with an increase in PA (regular PA (−) to (+)), and 446,191 participants with continuous PA (regular PA (+) to (+)).
The demographic and clinical characteristics of the participants are compared in Table 1. The proportions of men, age subgroups, high BMI, and current smokers and drinkers, as well as mean age, BMI participants with low incomes, and participants with comorbidities (i.e., diabetes, hypertension, dyslipidemia, or CKD) were significantly different among the groups (P < 0.0001). Because the size of the total study population was large, all variables showed significant differences according to the interval changes in PA status. During the follow-up period, the prevalences of newly diagnosed fracture were 5.99% (n = 197,858) in the continuous lack of PA group, 5.84% (n = 34,066) in the decrease in PA group, 5.26% (n = 34,261) in the increase in PA group, and 4.52% (n = 20,188) in the continuous PA group. The incidence of fracture was highest in the continuous lack of PA group, which included participants who did not consistently exercise. Compared to the decrease in PA group, which included participants who had exercised continuously in the past but stopped, the incidence of fracture in the increase in PA group was significantly lower. The incidence of fracture was lowest (< 5%) in the continuous PA group, which included participants who had continued to exercise from the past to the present.
Table 1.
Baseline characteristics of participants according to interval changes in physical activity status.
| Interval changes in regular PA (from 2009–2010 to 2011–2012) | Continuous lack of PA (N = 3,303,504) | Decrease in PA (N = 583,561) | Increase in PA (N = 650,888) | Continuous PA (N = 446,191) | P-value |
|---|---|---|---|---|---|
| PA (−) to (−) | PA (+) to (−) | PA (−) to (+) | PA (−) to (−) | ||
| Demographics | |||||
| Male sex | 1,687,642 (51.09) | 322,733 (55.3) | 360,244 (55.35) | 288,410 (64.64) | < 0.0001 |
| Age (years) | 54.71 ± 10.53 | 55.95 ± 10.18 | 55.02 ± 9.91 | 55.14 ± 9.67 | < 0.0001 |
| Age distribution | |||||
| ≥ 65 years | 623,067 (18.86) | 124,656 (21.36) | 120,002 (18.44) | 81,934 (18.36) | < 0.0001 |
| BMI (kg/m2) | 23.94 ± 3.05 | 24.16 ± 2.93 | 24.04 ± 2.88 | 24.16 ± 2.76 | < 0.0001 |
| High BMI (≥ 25 kg/m2) | 1,120,785 (33.93) | 212,040 (36.34) | 223,493 (34.34) | 159,046 (35.65) | < 0.0001 |
| Current smoker | 701,111 (21.22) | 105,362 (18.06) | 116,059 (17.83) | 76,412 (17.13) | < 0.0001 |
| Current drinker | 1,391,493 (42.12) | 250,991 (43.01) | 293,956 (45.16) | 231,673 (51.92) | < 0.0001 |
| Low-income | 2095 (29.84) | 2095 (29.84) | 8347 (23.78) | 8347 (23.78) | < 0.0001 |
| Comorbidities | |||||
| Diabetes | 378,394 (11.45) | 79,911 (13.69) | 82,294 (12.64) | 59,516 (13.34) | < 0.0001 |
| Hypertension | 1,094,622 (33.14) | 212,878 (36.48) | 224,188 (34.44) | 158,971 (35.63) | < 0.0001 |
| Dyslipidemia | 820,600 (24.84) | 156,172 (26.76) | 166,169 (25.53) | 114,208 (25.6) | < 0.0001 |
| Chronic kidney disease | 203,313 (6.15) | 38,170 (6.54) | 40,453 (6.22) | 28,153 (6.31) | < 0.0001 |
| Fracture | |||||
| Previous fracture (within 3 years) | 99,987 (3.03) | 17,532 (2.69) | 17,376 (2.98) | 10,148 (2.27) | < 0.0001 |
| Incidence | 197,858 (5.99) | 34,066 (5.84) | 34,261 (5.26) | 20,188 (4.52) | < 0.0001 |
Means ± standard deviation.
Categorical variables: n (%).
Bold indicates a statistically significant result (P < 0.05).
PA physical activity; regular PA: high-intensity exercise ≥ 3 days per week for at least 30 min or moderate-intensity exercise ≥ 5 days per week for at least 20 min; BMI body mass index; current drinker: alcohol consumption > 30 g per day; low income: household income < 20% of median.
Risk of fracture according to interval changes in PA status
Statistically adjusted Cox proportional hazards regression analyses were performed (Model 2, adjusted for age and sex; Model 3, adjusted for age, sex, smoking, alcohol consumption, and household income; and Model 4, adjusted for age, sex, smoking, alcohol consumption, household income, BMI, dyslipidemia, and previous fracture) to calculate HRs for newly diagnosed fractures in participants according to interval changes in PA (Table 2). The reference for fracture event HRs was participants with a continuous lack of PA. Participants with an increase in PA (regular PA (−) to (+)) and those with continuous PA (regular PA (+) to (+)) showed markedly lower risks of subsequent fracture development, despite adjustment for several potentially confounding variables (increase in PA group: adjusted HR 0.948; 95% CI 0.937–0.959; continuous PA group: adjusted HR 0.888, 95% CI 0.875–0.901) (Table 2). Therefore, the current study demonstrates a preventive effect of continuous PA or an increase in PA on fracture development.
Table 2.
Calculated hazard ratios for fracture according to changes in physical activity status.
| Changes in regular PA | Event (n) (fracture) | Total FU duration (PY) | IR (per 1000 PY) | Hazard ratio (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | ||||
| Continuous lack of PA (PA (−) to (−)) | 197,858 | 14,014,911.3 | 14.12 | 1 | 1 | 1 | 1 |
| Decrease in PA (PA (+) to (−)) | 34,066 | 2,483,882.1 | 13.71 | 0.971 (0.96–0.982) | 0.965 (0.954–0.976) | 0.971 (0.96–0.982) | 0.975 (0.964–0.987) |
| Increase in PA (PA (−) to (+)) | 34,261 | 2,779,982.8 | 12.32 | 0.873 (0.864–0.884) | 0.936 (0.925–0.947) | 0.942 (0.931–0.953) | 0.948 (0.937–0.959) |
| Continuous PA (PA (+) to (+)) | 20,188 | 1,914,666.5 | 10.54 | 0.748 (0.737–0.759) | 0.867 (0.854–0.879) | 0.877 (0.865–0.89) | 0.888 (0.875–0.901) |
Incidence rate = event (fracture) / total follow-up duration.
Model 1: Non-adjusted.
Model 2: Adjusted for age and sex.
Model 3: Adjusted for age, sex, smoking, alcohol consumption, and household income.
Model 4: Adjusted for age, sex, smoking, alcohol consumption, household income, body mass index, dyslipidemia, and previous fracture.
PA physical activity; regular PA: performing high-intensity exercise ≥ 3 days per week for at least 30 min or moderate-intensity exercise ≥ 5 days per week for at least 20 min; FU follow-up; PY person-year; IR incidence rate; CI confidence interval.
Subgroup analyses for the risk of fracture according to interval changes in PA
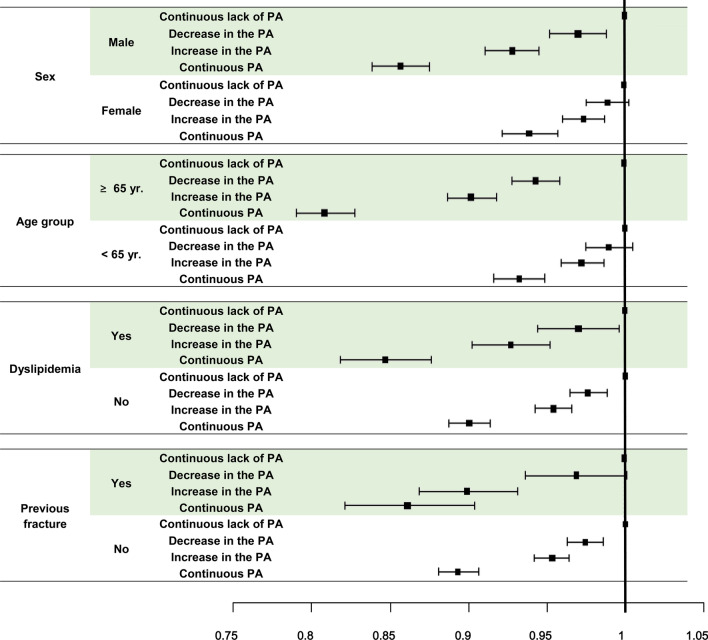
Multivariable Cox proportional hazards regression analyses adjusted for confounding variables (age, sex, smoking, alcohol consumption, household income, BMI, diabetes, and hypertension) were used to estimate adjusted HRs for fracture, based on demographic characteristics and chronic comorbidities (Table 3 and Fig. 2). In subgroup analyses, the effect of continuous PA on fracture prevention was significantly higher among men (HR 0.85) than among women (HR 0.94) (P < 0.0001). Among the age subgroups, a significant prevention in the incidence of fracture was observed in the ≥ 65-year-old group (HR 0.80) compared to the < 65-year-old group (HR 0.93) (P < 0.0001). Moreover, subgroup analyses revealed significantly reduced HRs for the incidence of fracture in participants with previous fracture (HR 0.86) and dyslipidemia (HR 0.84), compared to participants without previous fracture (HR 0.89) or dyslipidemia (HR 0.90) (P = 0.0111 and P = 0.041) (Table 3). The increase in PA group and decrease in PA group also showed consistent trends. There were no significant differences in other subgroup analyses, which included BMI, smoking drinking, household income, diabetes, hypertension, and CKD (P > 0.05). In Fig. 2, the 95% CIs between categories overlap because the HRs for each category are represented by a single graph. Each subgroup category was analyzed independently.
Table 3.
Subgroup analyses of risk for fracture according to changes in physical activity status.
| Changes in regular PA | n | Event (n) (Fracture) | Total FU duration (PY) | IR (per 1,000 PY) | Hazard ratio (95% CI) | P-value | |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | Continuous lack of PA | 1,687,642 | 60,572 | 7,200,983.0 | 8.41 | 1 | < 0.0001 |
| Decrease in PA | 322,733 | 12,366 | 1,380,043.0 | 8.96 | 0.97 (0.95–0.99) | ||
| Increase in PA | 360,244 | 12,578 | 1,546,174.6 | 8.13 | 0.93 (0.91–0.94) | ||
| Continuous PA | 288,410 | 9205 | 1,242,733.0 | 7.41 | 0.85 (0.83–0.87) | ||
| Female | Continuous lack of PA | 1,615,862 | 137,286 | 6,813,928.4 | 20.15 | 1 | |
| Decrease in PA | 260,828 | 21,700 | 1,103,839.1 | 19.66 | 0.99 (0.98–1.00) | ||
| Increase in PA | 290,644 | 21,683 | 1,233,808.2 | 17.57 | 0.97 (0.96–0.99) | ||
| Continuous PA | 157,781 | 10,983 | 671,933.5 | 16.35 | 0.94 (0.92–0.96) | ||
| Age group | |||||||
| ≥ 65 yr | Continuous lack of PA | 623,067 | 94,244 | 2,516,088.0 | 37.46 | 1 | < 0.0001 |
| Decrease in PA | 124,656 | 15,327 | 512,563.4 | 29.90 | 0.94 (0.93–0.96) | ||
| Increase in PA | 120,002 | 13,636 | 496,338.8 | 27.47 | 0.90 (0.90–0.92) | ||
| Continuous PA | 81,934 | 7215 | 343,105.2 | 21.03 | 0.80 (0.78–0.82) | ||
| < 65 yr | Continuous lack of PA | 2,680,437 | 103,614 | 11,498,823.3 | 9.01 | 1 | |
| Decrease in PA | 458,905 | 18,739 | 1,971,318.7 | 9.51 | 0.99 (0.97–1.01) | ||
| Increase in PA | 530,886 | 20,625 | 2,283,644.0 | 9.03 | 0.97 (0.96–0.99) | ||
| Continuous PA | 364,257 | 12,973 | 1,571,561.3 | 8.25 | 0.93 (0.91–0.95) | ||
| Dyslipidemia | |||||||
| Yes | Continuous lack of PA | 378,394 | 31,662 | 1,574,149.7 | 20.11 | 1 | 0.041 |
| Decrease in PA | 79,911 | 6065 | 334,950.7 | 18.11 | 0.97 (0.94–1.00) | ||
| Increase in PA | 82,294 | 5641 | 346,689.0 | 16.27 | 0.92 (0.90–0.95) | ||
| Continuous PA | 59,516 | 3317 | 252,542.1 | 13.13 | 0.84 (0.81–0.87) | ||
| No | Continuous lack of PA | 2,925,110 | 166,196 | 12,440,761.6 | 13.36 | 1 | |
| Decrease in PA | 503,650 | 28,001 | 2,148,931.4 | 13.03 | 0.98 (0.96–0.99) | ||
| Increase in PA | 568,594 | 28,620 | 2,433,293.8 | 11.76 | 095 (0.94–0.96) | ||
| Continuous PA | 386,675 | 16,871 | 1,662,124.4 | 10.15 | 0.90 (0.88–0.91) | ||
| Previous fracture | |||||||
| Yes | Continuous lack of PA | 99,987 | 23,284 | 379,208.7 | 61.40 | 1 | 0.0111 |
| Decrease in PA | 17,376 | 3662 | 67,266.5 | 54.44 | 0.97 (0.93–1.00) | ||
| Increase in PA | 17,532 | 3249 | 68,833.1 | 47.20 | 0.90 (0.86–0.93) | ||
| Continuous PA | 10,148 | 1643 | 40,487.4 | 40.58 | 0.86 (0.81–0.90) | ||
| No | Continuous lack of PA | 3,203,517 | 174,574 | 13,635,702.6 | 12.80 | 1 | |
| Decrease in PA | 566,185 | 30,404 | 2,416,615.6 | 12.58 | 0.97 (0.96–0.99) | ||
| Increase in PA | 633,356 | 31,012 | 2,711,149.6 | 11.44 | 0.95 (0.94–0.96) | ||
| Continuous PA | 436,043 | 18,545 | 1,874,179.1 | 9.90 | 0.89 (0.88–0.90) | ||
Incidence rate = event (fracture) / total follow-up duration.
Adjusted for age, sex, smoking, drinking alcohol, household income, body mass index, and diabetes mellitus.
PA physical activity; regular PA: performing high-intensity exercise ≥ 3 days per week for at least 30 min or moderate-intensity exercise ≥ 5 days per week for at least 20 min; FU follow-up; PY person-year; IR incidence rate; CI confidence interval; yr. years.
Figure 2.
Fracture risk subgroup analyses according to changes in physical activity status.
Discussion
In this large population-based cohort study using the K-NHIS database, the relationship between interval changes in regular PA and fractures was assessed. Continuous regular PA, the key target, was associated with a significantly reduced risk of fracture in the general population. In contrast, the risk of fracture tended to increase in groups with reduced regular PA or a continuous lack of PA.
Our results regarding the association between reduced risk of fracture and regular PA are consistent with the findings of previous studies, in which high PA frequency was reportedly related to a lower risk of fracture. However, these studies only assessed PA at a single time point, rather than interval change in PA7,9,16. In our study, regular continuous PA was associated with reduced fracture incidence, compared with physical inactivity. This may explain why fracture incidence is often higher in older individuals, who typically have lower levels of PA than other members of the population17. In our study, continuous regular PA had a significantly greater effect on fracture prevention in men than in women, which may be due to larger increases in muscle mass (with similar exercise) in men than in women18. Muscles play important roles in protecting bones from severe trauma, stress, and strain, and the risk of fracture decreases with greater muscle mass19.
Continuous regular PA was associated with a greater reduction in fracture incidence in elderly individuals, defined as those > 65 years, compared with younger individuals. Indeed, fractures tend to occur more frequently in elderly individuals who have low BMD, and regular PA has been shown to increase BMD17. Therefore, regular PA may be effective for preventing fractures in elderly individuals. In particular, in one network meta-analysis, exercise was the only intervention that prevented fall-related injuries20. Moreover, a Cochrane meta-analysis found that exercise can reduce fractures associated with falls21. While it has been proposed that fractures occur in elderly individuals during PA due to a greater propensity for falls, various meta-analyses have reported that exercise has an anti-fracture effect in elderly individuals20,22,23. One meta-analysis suggested that participating in ≥ 3 sessions of PA per week increased the risk of falls in elderly individuals, while 2–3 sessions of exercise per week (each lasting 30–60 min) were safe and effective24. Although our study defined regular PA as high-intensity exercise ≥ 3 days per week for at least 30 min, or moderate-intensity exercise ≥ 5 days per week for at least 20 min, these amounts of PA may be excessive for elderly individuals.
The risk of fracture may increase by twofold in individuals with previous fracture, compared to individuals without previous fracture25. This effect is reportedly caused by low BMD, as well as changes in cancellous microarchitecture and damage to cortical bone (due to the fractures themselves or immobilization secondary to fracture)26. In addition, the risk of falls increases and the protective response against damage becomes impaired in individuals with previous fractures27–30. In the current study, individuals with previous fractures had higher anti-fracture effects, compared to individuals without previous fractures, in the continuous regular PA group. Therefore, patients with fractures should exercise regularly after acute treatment is completed. Notably, in our study, the anti-fracture effect of continuous regular PA was greater in the group with dyslipidemia than in the group without dyslipidemia. This is presumably because patients with dyslipidemia have lower BMD and higher incidence of fracture due to alterations in lipid metabolism31. Therefore, exercise is more important in patients with hyperlipidemia.
Several mechanisms may explain our findings regarding the association between interval changes in regular PA and fracture risk. First, PA has been shown to increase bone size and BMD32,33. Moreover, because exercise can improve functional strength and balance, it can reduce the risk of falls and help to prevent fractures21. Lastly, moderate-to-high intensity exercise can increase muscle strength34. Notably, muscle strength is related to muscle mass, which is a strong determinant of bone size, volume, density and strength35,36. Therefore, if muscle strength is improved through exercise, the risk of fracture may be lowered.
The benefits of exercise likely outweigh its risks, and reports of fractures caused by exercise are rare. Joint pain, muscle pain, and sprains are the most common side effects associated with exercise37,38. In a previous study, there was only one report of a fall during exercise (in a patient with spinal fractures), which did not result in serious side effects. Additionally, a previous systematic review concluded that exercise is sufficiently effective to outweigh potential harm37. Our study also showed that the anti-fracture effect of PA was greater than the risk of exercise-related fractures. These findings will help clinicians encourage their patients to increase their levels of PA.
A notable strength of this study was that it performed repeated measures analysis to investigate the association between interval changes in PA and the risk of fracture in the general population, using a large population-based database. In this study, the incidence of fractures was based on medical claims records collected at the national level, which are considered to be more accurate than retrospective data. Moreover, the study included adjustments for potential confounding factors, such as current smoking, alcohol consumption, and fracture history; extensive subgroup analyses were also performed.
There are some clinically significant findings in the present study. Continuous regular PA was found to be related with a markedly reduced risk of fracture. In addition, the effect of continuous regular PA was observed to be greater in individuals older than 65 years, male, and individuals with dyslipidemia or previous fracture history.
However, this study had some limitations. First, our research may have included inaccuracies because it was based on self-reported questionnaire data, which may not accurately reflect actual levels of PA among the participants. Nevertheless, self-reported questionnaires approximate PA at the population level, and the effectiveness of the IPAQ-based self-reported PA questionnaire has been confirmed in several studies39,40. Secondly, there are no data in the K-NHIS database that enabled evaluation of patients’ BMD. Third, because the study was based on real-world data, causes of interval changes in PA could not be assessed. Fourth, we did not include individuals within long-term care and/or assisted living. Fifth, we only analyzed the risk of fractures according to changes in regular PA. We did not consider time spent in PA, such as in sedentary behavior, light PA, moderate PA and vigorous PA. Also, in our study, as regular PA included both moderate and vigorous intensity PA together, the fracture risk could not be analyzed per each intensity of PA. Finally, caution is needed when generalizing these results to countries other than South Korea, and multi-ethnic cohort studies are warranted in the future.
Conclusion
Continuous regular PA is associated with fracture prevention. Specifically, it is more effective in men, individuals ≥ 65 years, and those with previous fractures and dyslipidemia.
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund.
Author contributions
S.H. and H.D.J. designed the study. H.D.J. performed the analysis and S.H. wrote the manuscript. S.C., G.D.K., K.H. and H.L. contributed to the interpretation of the results. B.K. and K.D.M. participated in the design and J.Y.H. conduct of the original cohort study as well as in critical reviewing of the manuscript. All authors approved the final version.
Data availability
Data are available from the Korea National Health Insurance Sharing Service Institutional Data Access/Ethics Committee (https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do) for researchers who meet the criteria for access to confidential data. However, in accordance with Korean law, the study authors are not permitted to transfer any data files to a third party.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Sangsoo Han and Hae-Dong Jang.
References
- 1.Odén A, McCloskey EV, Kanis JA, Harvey NC, Johansson H. Burden of high fracture probability worldwide: secular increases 2010–2040. Osteoporos Int. 2015;26:2243–2248. doi: 10.1007/s00198-015-3154-6. [DOI] [PubMed] [Google Scholar]
- 2.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 3.Bass E, French DD, Bradham DD, Rubenstein LZ. Risk-adjusted mortality rates of elderly veterans with hip fractures. Ann Epidemiol. 2007;17:514–519. doi: 10.1016/j.annepidem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Forsén L, Søgaard AJ, Meyer HE, Edna T, Kopjar B. Survival after hip fracture: short- and long-term excess mortality according to age and gender. Osteoporos Int. 1999;10:73–78. doi: 10.1007/s001980050197. [DOI] [PubMed] [Google Scholar]
- 5.Trombetti A, Herrmann F, Hoffmeyer P, Schurch MA, Bonjour JP, Rizzoli R. Survival and potential years of life lost after hip fracture in men and age-matched women. Osteoporos Int. 2002;13:731–737. doi: 10.1007/s001980200100. [DOI] [PubMed] [Google Scholar]
- 6.Coupland C, Wood D, Cooper C. Physical inactivity is an independent risk factor for hip fracture in the elderly. J Epidemiol. Community Health. 1993;47:441–443. doi: 10.1136/jech.47.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kemmler W, Häberle L, von Stengel S. Effects of exercise on fracture reduction in older adults: a systematic review and meta-analysis. Osteoporos Int. 2013;24:1937–1950. doi: 10.1007/s00198-012-2248-7. [DOI] [PubMed] [Google Scholar]
- 8.Kemmler W, Bebenek M, Kohl M, von Stengel S. Exercise and fractures in postmenopausal women. Final results of the controlled Erlangen Fitness and Osteoporosis Prevention Study (EFOPS) Osteoporos. Int. 2015;26:2491–2499. doi: 10.1007/s00198-015-3165-3. [DOI] [PubMed] [Google Scholar]
- 9.Kemmler W, von Stengel S, Kohl M. Exercise frequency and fracture risk in older adults—how often is enough? Curr. Osteoporos. Rep. 2017;15:564–570. doi: 10.1007/s11914-017-0407-7. [DOI] [PubMed] [Google Scholar]
- 10.Kwon KB, Choi Y, Sung KH, Chung CY, Lee KM, Kwon SS, et al. Correlation between accelerometer and questionnaire-based assessment of physical activity in patients with cerebral palsy. Clin. Orthop. Surg. 2020;12:107–112. doi: 10.4055/cios.2020.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JY, Park HK, Choi JH, Moon ES, Kim BS, Kim WS, et al. Prospective evaluation of the effectiveness of a home-based program of isometric strengthening exercises: 12-month follow-up. Clin. Orthop. Surg. 2010;2:173–178. doi: 10.4055/cios.2010.2.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park S, Chun J, Han KD, Soh H, Choi K, Kim JH, et al. Increased end-stage renal disease risk in patients with inflammatory bowel disease: a nationwide population-based study. World J. Gastroenterol. 2018;24:4798–4808. doi: 10.3748/wjg.v24.i42.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang EA, Han K, Chun J, Soh H, Park S, Im JP, et al. Increased risk of diabetes in inflammatory bowel disease patients: a nationwide population-based study in Korea. J. Clin. Med. 2019;8:E343. doi: 10.3390/jcm8030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IPAQ Research Committee. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ), Short Form, ver. 2.0. https://www.ipaq.ki.se. (2005).
- 15.Park S. Associations of physical activity with sleep satisfaction, perceived stress, and problematic Internet use in Korean adolescents. BMC Public Health. 2014;14:1143. doi: 10.1186/1471-2458-14-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pihlajamäki H, Parviainen M, Kyröläinen H, Kautiainen H, Kiviranta I. Regular physical exercise before entering military service may protect young adult men from fatigue fractures. BMC Musculoskelet. Disord. 2019;20:126. doi: 10.1186/s12891-019-2513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202. doi: 10.1016/j.bone.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Alway SE, Grumbt WH, Gonyea WJ, Stray-Gundersen J. Contrasts in muscle and myofibers of elite male and female bodybuilders. J. Appl. Physiol. 1989;67:24–31. doi: 10.1152/jappl.1989.67.1.24. [DOI] [PubMed] [Google Scholar]
- 19.Giladi M, Milgrom C, Simkin A, Danon Y. Stress fractures. Identifiable risk factors. Am. J. Sports Med. 1991;19:647–652. doi: 10.1177/036354659101900617. [DOI] [PubMed] [Google Scholar]
- 20.Tricco AC, Thomas SM, Veroniki AA, Hamid JS, Cogo E, Strifler L, et al. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA. 2017;318:1687–1699. doi: 10.1001/jama.2017.15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherrington C, Fairhall NJ, Wallbank GK, Tiedemann A, Michaleff ZA, Howard K, et al. Exercise for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2019;1:CD012424. doi: 10.1002/14651858.CD012424.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2012;9:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Khoury F, Cassou B, Charles MA, Dargent-Molina P. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f6234. doi: 10.1136/bmj.f6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Souto BP, Rolland Y, Vellas B, Maltais M. Association of Long-term exercise training with risk of falls, fractures, hospitalizations, and mortality in older adults: a systematic review and meta-analysis. JAMA Intern. Med. 2019;179:394–405. doi: 10.1001/jamainternmed.2018.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanis JA, Johnell O, De Laet C, Johansson H, Odén A, Delmas P, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Crabtree N, Loveridge N, Parker M, Rushton N, Power J, Bell KL, et al. Intracapsular hip fracture and the region-specific loss of cortical bone: analysis by peripheral quantitative computed tomography. J. Bone Miner. Res. 2001;16:1318–1328. doi: 10.1359/jbmr.2001.16.7.1318. [DOI] [PubMed] [Google Scholar]
- 27.Gunnes M, Mellström D, Johnell O. How well can a previous fracture indicate a new fracture? A questionnaire study of 29,802 postmenopausal women. Acta Orthop. Scand. 1998;69:508–512. doi: 10.3109/17453679808997788. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson MK, Hasserius R, Obrant KJ. Individuals who sustain nonosteoporotic fractures continue to also sustain fragility fractures. Calcif. Tissue Int. 1993;53:229–231. doi: 10.1007/BF01320906. [DOI] [PubMed] [Google Scholar]
- 29.Ensrud KE, Nevitt MC, Yunis C, Cauley JA, Seeley DG, Fox KM, et al. Correlates of impaired function in older women. J. Am. Geriatr. Soc. 1994;42:481–489. doi: 10.1111/j.1532-5415.1994.tb04968.x. [DOI] [PubMed] [Google Scholar]
- 30.Nelson HD, Nevitt MC, Scott JC, Stone KL, Cummings SR, Cummings S, et al. Smoking, alcohol, and neuromuscular and physical function of older women. JAMA. 1994;272:1825–1831. doi: 10.1001/jama.1994.03520230035035. [DOI] [PubMed] [Google Scholar]
- 31.Yezerska I, Hernández Hernández J, Olmos Martínez J, González MJ. Dyslipidemia and bone metabolism. A common bond of the osteoporosis and the atherosclerosis? Rev. Osteoporos. Metab. Miner. 2011;3:41–50. [Google Scholar]
- 32.Gianoudis J, Bailey CA, Ebeling PR, Nowson CA, Sanders KM, Hill K, et al. Effects of a targeted multimodal exercise program incorporating high-speed power training on falls and fracture risk factors in older adults: a community-based randomized controlled trial. J. Bone Miner. Res. 2014;29:182–191. doi: 10.1002/jbmr.2014. [DOI] [PubMed] [Google Scholar]
- 33.Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;7:CD000333. doi: 10.1002/14651858.CD000333.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Borde R, Hortobágyi T, Granacher U. Dose–response relationships of resistance training in healthy old adults: a systematic review and meta-analysis. Sports Med. 2015;45:1693–1720. doi: 10.1007/s40279-015-0385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinonen A, McKay HA, Whittall KP, Forster BB, Khan KM. Muscle cross-sectional area is associated with specific site of bone in prepubertal girls: a quantitative magnetic resonance imaging study. Bone. 2001;29:388–392. doi: 10.1016/S8756-3282(01)00588-9. [DOI] [PubMed] [Google Scholar]
- 36.van Langendonck L, Claessens AL, Lysens R, Koninckx PR, Beunen G. Association between bone, body composition and strength in premenarcheal girls and postmenopausal women. Ann. Hum. Biol. 2004;31:228–244. doi: 10.1080/03014460310001638929. [DOI] [PubMed] [Google Scholar]
- 37.Liu CJ, Latham N. Adverse events reported in progressive resistance strength training trials in older adults: 2 sides of a coin. Arch. Phys. Med. Rehabil. 2010;91:1471–1473. doi: 10.1016/j.apmr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Chilibeck PD, Vatanparast H, Cornish SM, Abeysekara S, Charlesworth S. Evidence-based risk assessment and recommendations for physical activity: arthritis, osteoporosis, and low back pain. Appl. Physiol. Nutr. Metab. 2011;36:S49–S79. doi: 10.1139/h11-037. [DOI] [PubMed] [Google Scholar]
- 39.Loney T, Standage M, Thompson D, Sebire SJ, Cumming S. Self-report vs objectively assessed physical activity: which is right for public health? J. Phys. Act Health. 2011;8:62–70. doi: 10.1123/jpah.8.1.62. [DOI] [PubMed] [Google Scholar]
- 40.Armstrong ME, Green J, Reeves GK, Beral V, Cairns BJ, Million Women Study Collaborators Frequent physical activity may not reduce vascular disease risk as much as moderate activity: large prospective study of women in the United Kingdom. Circulation. 2015;131:721–729. doi: 10.1161/CIRCULATIONAHA.114.010296. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Korea National Health Insurance Sharing Service Institutional Data Access/Ethics Committee (https://nhiss.nhis.or.kr/bd/ay/bdaya001iv.do) for researchers who meet the criteria for access to confidential data. However, in accordance with Korean law, the study authors are not permitted to transfer any data files to a third party.