Objectives:
Cannabis is a known teratogen. Data availability addressing both major congenital anomalies and cannabis use allowed us to explore their geospatial relationships.
Methods:
Data for the years 1998 to 2009 from Canada Health and Statistics Canada was analyzed in R. Maps have been drawn and odds ratios, principal component analysis, correlation matrices, least squares regression and geospatial regression analyses have been conducted using the R packages base, dplyr, epiR, psych, ggplot2, colorplaner and the spml and spreml functions from package splm.
Results:
Mapping showed cannabis use was more common in the northern Territories of Canada in the Second National Survey of Cannabis Use 2018. Total congenital anomalies, all cardiovascular defects, orofacial clefts, Downs syndrome and gastroschisis were all found to be more common in these same regions and rose as a function of cannabis exposure. When Canada was dichotomized into high and low cannabis use zones by Provinces v Territories the Territories had a higher rate of total congenital anomalies 450.026 v 390.413 (O.R. = 1.16 95%C.I. 1.08-1.25, P = 0.000058; attributable fraction in exposed 13.25%, 95%C.I. 7.04–19.04%). In geospatial analysis in a spreml spatial error model cannabis was significant both alone as a main effect (P < 2.0 × 10−16) and in all its first and second order interactions with both tobacco and opioids from P < 2.0 × 10−16.
Conclusion:
These results show that the northern Territories of Canada share a higher rate of cannabis use together with elevated rates of total congenital anomalies, all cardiovascular defects, Down's syndrome and gastroschisis. This is the second report of a significant association between cannabis use and both total defects and all cardiovascular anomalies and the fourth published report of a link with Downs syndrome and thereby direct major genotoxicity. The correlative relationships described in this paper are confounded by many features of social disadvantage in Canada's northern territories. However, in the context of a similar broad spectrum of defects described both in animals and in epidemiological reports from Hawaii, Colorado, USA and Australia they are cause for particular concern and indicate further research.
Keywords: cannabis, cardiovascular defects, congenital defect, geospatial analysis, teratology, total congenital defects
Cannabis is used by over 180 million people worldwide (Degenhardt et al., 2013). Several national surveys in Canada demonstrate that its use is concentrated mainly in young adults 18 to 44 in the reproductive age group (Statistics Canada, 2018; Statistics Canada, 2019a,b). Nationally representative Canadian surveys show that cannabis use by females has risen nationally from 78.5% from 7% to 12.5% in the period 2013 to 2018 (Health Canada, 2016; Statistics Canada, 2019a,b). And in some parts of Canada, such as parts of Nunavut, cannabis use is said to be largely holoendemic with rates as high as 85% of males less than 45 years stating that they had used cannabis in the past year, falling to 43% thereafter, and 70% of females 15 to 19 years, falling to 50% for females 25 to 44 years (Nunavut Government, 2019). Epidemiological evidence from other parts shows that 24% of Californian teenagers recently tested positive to cannabinoids, a trend which is rising with time (Young-Wolff et al., 2017).
Drugs of addiction were estimated to cost the Canadian Government CAD$38billion in 2014 alone in a recently released University of Victoria report of which CAD$2,660,000,000 (7%) was attributed to cannabis (Canadian Substance Use Costs and Harms Working Group et al., 2018).
Cannabis of course has a very benign public image and the common perception is that it is relatively harmless “soft” drug (Murphy et al., 2018). As a result, cannabis was recommended to 69% of pregnant patients by cannabis dispensaries in Colorado, often to alleviate morning sickness (Dickson et al., 2018).
In parallel research investigators have long had an interest in the teratological actions of cannabis and cannabinoids. Detailed cellular and animal studies date back to the 1960's when many serious defects including spina bifida, phocomelia, omphalocele and myelocoele were described in New Zealand white rabbits and hamsters which are the experimental model animals which most reliably model patterns of human teratology (Geber and Schramm, 1969a,1969b; Graham, 1976).
Canada presents an ideal opportunity to study the teratological implications of prenatal cannabis exposure (PCE) in human populations. Not only does it have an advanced system of birth defects registries and detailed data on birth numbers in their population, but it also has survey data on cannabis use across Provinces and Territories on several occasions now, all of which are in the public domain.
For these reasons we wished to investigate the extent to which cannabis use in that nation explains the observed patterns of teratology across several organ systems. The primary resource for this work was the publication by Canada Health (Public Health Agency of Canada and Health Canada, 2013). As data pertaining to neural tube defects is highly affected by therapeutic practices such as termination for defect and also impinged upon by universal folic acid supplementation, Canadian neural tube defects have been treated separately and are the subject of a separate report (Reece and Hulse, 2019a,2020a).
METHODS
Data
Congenital anomaly data was taken from tabulations from the Canadian Congenital Anomalies Surveillance System (CCASS) covering the period 1998 to 2009 in (Public Health Agency of Canada, 2013; Public Health Agency of Canada and Health Canada, 2013). This sophisticated surveillance system captures the majority of congenital anomalies born across the country. It is standardized against all births recorded in the National Register of Births. Gastroschisis incidence data was supplemented with raw data unadjusted for age and age-adjusted data from (Bassil et al., 2016) which covered a similar period. The percent of the population which acknowledged cannabis use in the previous 3 months was taken from (Statistics Canada, 2018).
Regional Drug Use Data. Data on per capita drug costs in 2007 by Canadian Province and Territory was taken from a recent University of Victoria report (Canadian Substance Use Costs and Harms Working Group et al., 2018). Missing data for Quebec was estimated as described in the text. Higher resolution spatial or temporal data for drug use and birth defects data has not been made publicly available.
Median household income data for the year 2005 was downloaded from Statistics Canada (2019a,b).
Statistics
Data was analyzed in “R” from Central R Archive Network version 3.6.1 and R Studio 1.2.1335 2019. Parameters were scaled by z-transformation (subtracted from their mean and divided by their standard deviation) to equalize the scales and distributions of the different variables. Parameters were also log transformed where appropriate as guided by the Shapiro test in order to optimize normality assumptions. The arcsinh hyperbolic transformation was used to log numeric vectors which included zero and negative numbers. Graphs and maps were drawn in ggplot2. The map projection employed was the Lambert Conformal Conical Projection (Environmental Systems Research Institute (ESRI) and European Petroleum Research Group (EPSG) No. 102002) which is the projection most favored by Statistics Canada. The R package “colorplaner” was used to plot bivariate colorplanes mapping 2 variables onto a single field. Principal component analysis was conducted using the psych package in R. The number of principal components to use was decided using a Scree plot also plotted in psych. Odds ratios, attributable fractions in the exposed and attributable fractions in the population were calculated using the epi.2by2 function from the R package epiR. Geospatial analysis was conducted with the spml and spreml functions in the R package splm by Millo and Piras (2012). Spatial weights and neighbor connectivity were derived originally from the poly2 nb function from the R package spdep and subsequently corrected manually. All initial regression models were reduced to the final models which have been presented by the classical method of manual deletion of the least significant term. P < 0.05 was considered significant.
Ethics
Ethical approval was granted to this study from the Human Ethics Research Committees of the Southcity Medical Centre in Brisbane and the University of Western Australia in Perth.
RESULTS
Figure 1 redraws the pediatric epidemiology data from (Public Health Agency of Canada and Health Canada, 2013) and presents data on total congenital anomalies, cardiovascular defects, orofacial defects, Downs Syndrome, gastroschisis, age-unadjusted, age-adjusted gastroschisis data (from Bassil et al., 2016) and limb deficiency data. In most cases the incidence data obviously peaks in the northern Territories of Nunavut and / or Northwest Territory.
FIGURE 1.
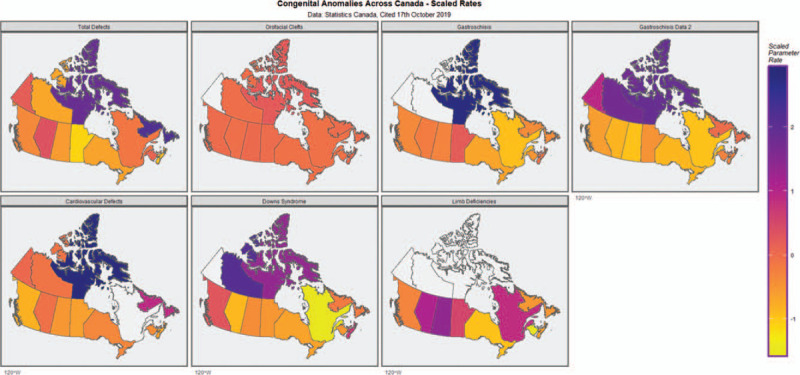
Choropleth maps of various congenital anomaly rates across Canada.
Figure 2 presents this same data as a paneled bivariate plot of the rates of various anomalies by the cannabis exposure rate taken from a recent nationally representative survey of Canadian cannabis use conducted by Statistics Canada (Statistics Canada, 2018). This survey has been employed measuring cannabis use as it was the first survey which we could identify which covered the whole of Canada. Interpretation of these map-graphs is straightforward in that provinces or Territories which are pink and purple indicate regions where both cannabis use and the various congenital anomalies are elevated. It is readily apparent from inspection of these graphs that most of the nominated defects are elevated in the northern Territories of Canada with the exception of limb deficiencies where there is no data for the Territories Yukon, Northwest and Nunavut.
FIGURE 2.
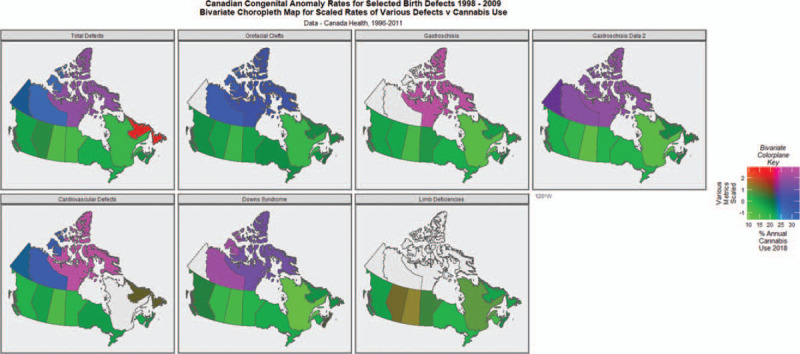
Bivariate choropleth maps of cannabis use against various congenital anomaly rates as indicated.
Figure 3 presents a paneled plot of the major per capita costs of various addictive drugs across Canada in 2007 and includes median household income data derived from Statistics Canada (2019a,b). Whist there are many surveys and samples of drug use across Canada we were only able to identify 1 medico-economic study which included data on all Canadian Provinces and Territories (Canadian Substance Use Costs and Harms Working Group et al., 2018). Hence in the present work the per capita cost to Government has been taken as a proxy of drug use. Given that this study is constrained to a sample size of only 13 Provinces and Territories it is important from an analytical perspective that missing data be minimized as far as possible.
FIGURE 3.
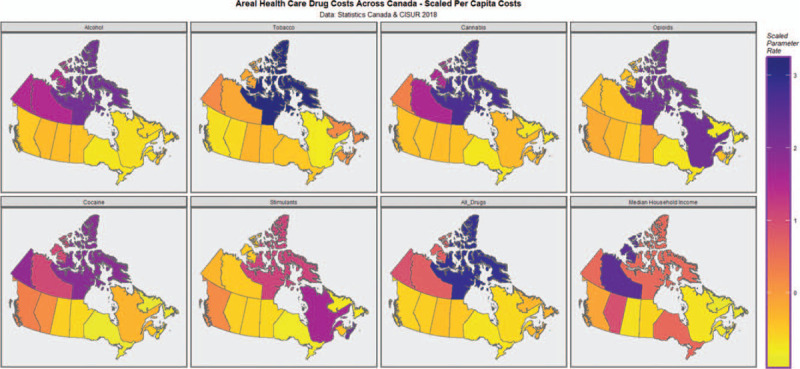
Choropleth maps of drug related health care costs across Canada including median household income.
Figure 4 presents bivariate choropleth maps of drug use or median household income along the bivariate colorplane ordinate as a function of cannabis exposure along the abscissa.
FIGURE 4.
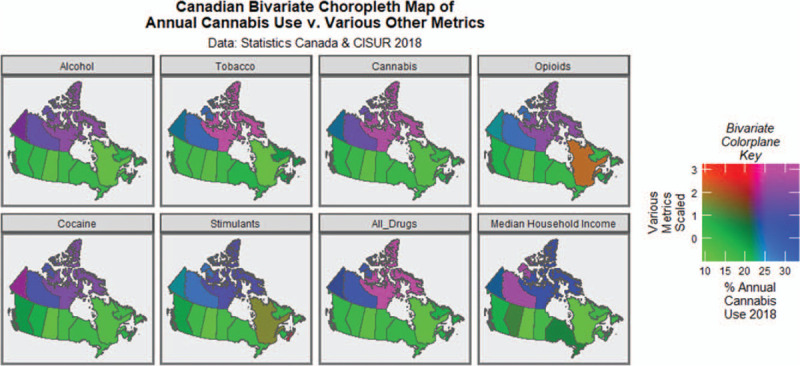
Bivariate choropleth map of cannabis use rate against the use of various other addictive drugs and median household income.
Indeed if one groups the Provinces into a low risk group and the three northern Territories as a high risk group, one notes that 774 anomalous births occurred amongst 17,199 births (450.03 / 10,000) compared to 109,494 anomalies amongst 2,821,764 births in the Provinces (390.41/10,000) which is significantly elevated (O.R. = 1.16, 95%C.I. 1.08–1.25; Chi Sq. = 16.019, df = 1, P = 0.0000577, attributable fraction in exposed 13.25%, 95%C.I. 7.04–19.04%).
These data can be combined by principal component analysis. Hence cardiovascular, orofacial, gastroschisis and downs syndrome were combined into principal components. Scree plot analysis showed that one principal component (PC1) captured 78% of the variance between these four defect classes.
Figure 5 presents the various defects against the rate of use of cannabis use in the last 3 months for each of the 9 congenital anomaly classes discussed above. One notes that in 8 of the 9 cases the slope of the regression line appears to be positive. That is the incidence of the defect appears to be positively correlated with the cannabis use incidence. The single exception is the limb defects where the data is grossly incomplete, as noted above.
FIGURE 5.
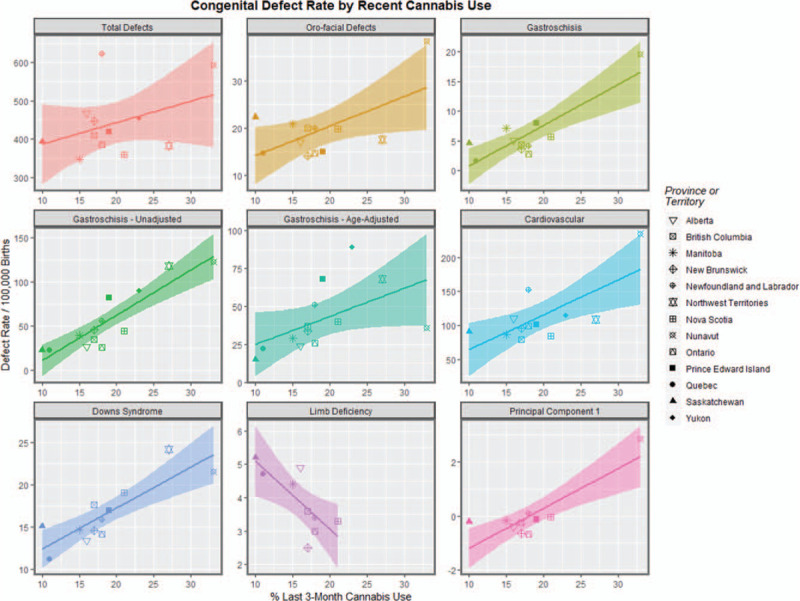
Scatterplot of the rate of various defects by cannabis use rates including regression lines.
Figure 6 shows (A) a quantitative correlogram including correlation coefficients and confidence intervals, for the various organ-specific defects and cannabis use together with (B) a qualitative correlogram illustrating these relationships. The correlogram is listed in order of its principal components. This figure starkly highlights the close correlations of all the defect classes with cannabis use, the sole exception being limb deficiencies where the data is notably lacking.
FIGURE 6.
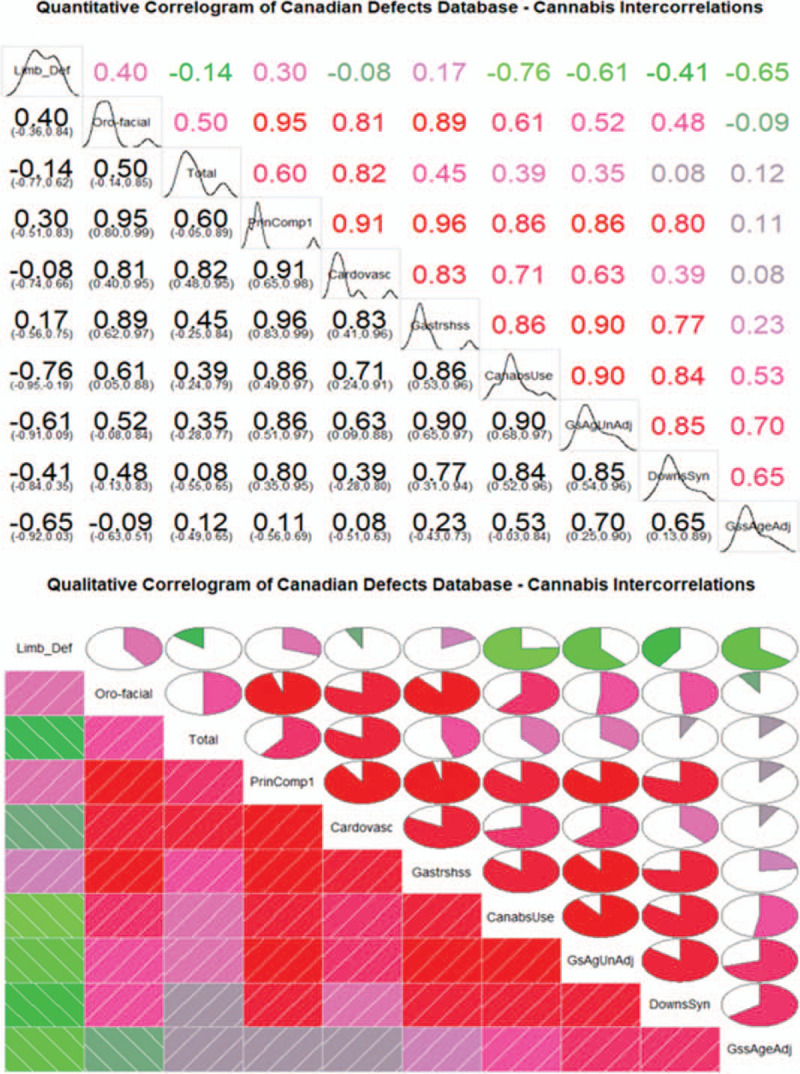
Correlograms of cannabis use rates against various congenital anomalies.
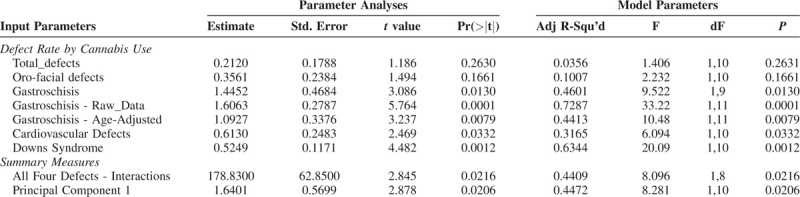
Table 1 shows the ordinary least squares (ols) linear regression of each of the defect classes against the cannabis use rate. The first 7 lines of the table present the data as discussed above. One notes that 5 of these lines are significant. Two summary measures are presented in the last 2 lines of this table. The first line applies to a multivariate model with a 4-way interaction between the 4 defects gastroschisis, orofacial, cardiovascular and Down's syndrome regressed against cannabis use. The second line is the above described Principal Component 1.
TABLE 1.
Relationship of Various Defects to Cannabis Use
In addition to showing a bivariate correlation between cannabis use and several classes of congenital anomalies it was considered also of interest to explore the relative role of cannabis in relation to the use of other addictive drugs and to major socioeconomic covariates such as poverty as quantified by the median household income.
The rate of total congenital defects for Quebec was not reported in the major Health Canada Report on this subject (Public Health Agency of Canada and Health Canada, 2013) so it was estimated at 429 by mean substitution from the other Provinces excluding Prince Edward Island (which is which is atypical on many metrics).
As noted above the cost of drug use in Quebec was not available to the authors at the time their report was compiled (Canadian Substance Use Costs and Harms Working Group et al., 2018). However, drug cost related to failure of productivity data was available. Since the ratio of lost productivity to health costs lies in a narrow band for the Provinces (excluding Prince Edward Island; 1.2567 ± 0.0502 mean ± standard error of the mean, ie, ±4.00%) as do the per capita drug-related health care costs (0.3263 ± 0.0086, ie, ±2.65%) and productivity losses (0.4075 ± 0.0092. ie, ±2.26%), it is possible to estimate the health related costs and the total drug related costs as shown in Supplementary Tables 1 and 2. This data is the basis for Figures 3 and 4 and for the regression studies which follow.
A correlation matrix of the various total drug-related costs with the total congenital anomaly rate is presented in Supplementary Table 3. In this analysis the total congenital anomaly rate for Quebec (404) was derived by mean substitution for the Provinces omitting Newfoundland and Labrador which is an outlier. The significance of these various correlations is shown in Supplementary Table 4. Total per capita cannabis cost is noted to relate significantly to total per capita drug use costs, total per capita alcohol costs and total per capita cocaine costs.
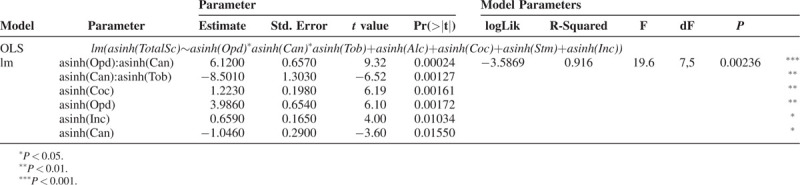
If one regresses the arcsinh of the total scaled defects against the arcsinh scaled per capita costs of drug use for tobacco, alcohol, cannabis, cocaine, amphetamines and also median household income including interactive terms between tobacco, cannabis and opioids by ordinary least squares (ols) linear regression one obtains the highly significant results reported in Table 2 in a final reduced model. In this model cannabis is noted to be highly significant on its own as a main effect (P = 0.01550) and in interaction with opioids and tobacco from P = 0.00024. One notes from Table 2 that the adjusted R-squared value is very high at 0.9160 and the value at which the log likelihood is optimized is LogLik = −3.5869.
TABLE 2.
Linear Regression of Total Defects Against Major Addictive Drugs
However, the data in question are not independent and identically normally distributed (iid) data as they exist in geospatial proximity to each other in the nation of Canada. This implies that this data is well suited to the modern advanced techniques of geospatial analysis. Within the R computing environment sophisticated software exists for undertaking such analyses including the spdep, spatialreg, and splm packages. To accomplish this one first needs to establish the geospatial relationships between the various Provinces and Territories. These links have been identified (Fig. 7A) and adjusted (Fig. 7B) so that the geospatial neighbor links shown in Figure 7C are derived. When 2 areas touch either their side or their corners they are said to share “Queen” contiguity by analogy with the moves of the queen piece in Chess. Figure 7C illustrates the first order queen relationships which have been identified and were employed in the analysis.
FIGURE 7.
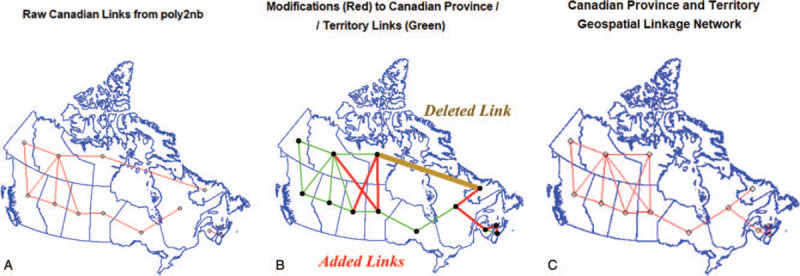
Canadian Province and Territory Geospatial linkage network connectivity map: (A) Automatic (software derived) links, (B) manual corrections to links and (C) corrected links. Note the areal centroids are defined as being the centre of the largest polygon in the case of those Territories which include island elements.
When one runs a spatial panel maximum likelihood (spml) analysis on the same model as in Table 2 using a SARAR (spatial autocorrelation with spatial correlation on the error terms, or combined spatial error and spatial lag model) structure one notes the results shown in the upper part of Table 3. Indeed in this remarkable model all the terms are seen to be highly significant from very low P values (P < 2 × 10−16). The LogLik value of this model is 36.7754 as shown, or significantly greater than the first ols model. Use of the spatial Hausman test confirms that the SARAR model is the correct model structure specification to employ.
TABLE 3.
Geospatial Regression of Total Defects Against Major Addictive Drugs
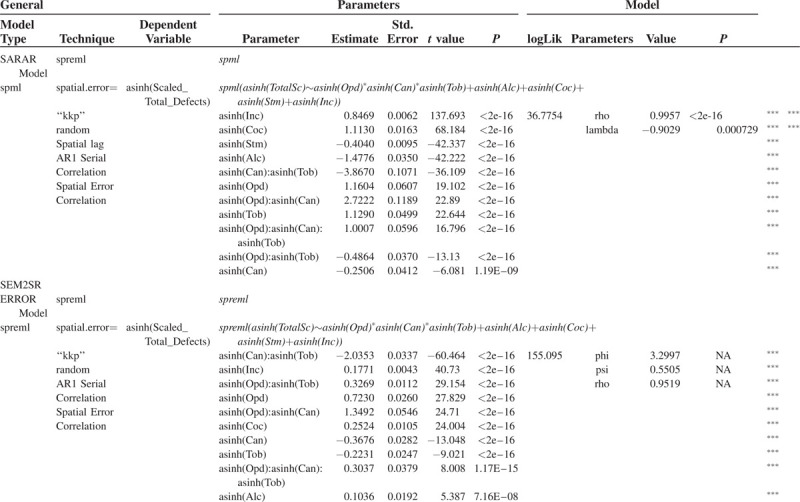
One can then progress to the impressive new spreml (spatial panel random effects maximum likelihood) function in R recently described and implemented by Millo (2014) which allows one to more accurately specify the error structure of the spatial model. Spatial Hausman tests indicate on this occasion that the spatial error model with spatial random errors (following Kapoor et al., 2007, autocorrelation and spatial error correlation) is the most appropriate model to use.
The same model formula as previously is again employed within this framework. As shown in the lower half of Table 3 highly significant results are again obtained. Cannabis is significant by itself as a main effect from P < 2.0 × 10−16 and also in all of its interactions with tobacco and opioids from P = 2.0 × 10−16. The LogLik parameter at model optimization is now noted to be much higher at 155.095 and this model well out-performs the previous spml model (spatial Hausman test: χ2 = 79,146, df = 11, P < 2 × 10−16).
If the same model is run without using any arcsinh transformations the same results are obtained but the overall model LogLik parameter falls drastically to LogLik = 53.522 (data not shown).
DISCUSSION
This study presents the strikingly similar distribution of many of the major congenital anomalies and the cannabis use rate across Canada graphically using maps and quantifies this relationship using regression analyses which shows that gastroschisis, age-adjusted gastroschisis data, age-unadjusted gastroschisis data, cardiovascular defects, Downs syndrome, an interactive term between these three defects and orofacial defects and their first Principal Component, all show statistically significant correlational and associational relationships with the amount of cannabis smoked in the last three months. Hence many of the most common groups of congenital anomalies demonstrate significant statistical relationships with cannabis consumption in Canada. Geospatial regression of total congenital defects showed that all drug exposure and particularly cannabis use was highly related to the incidence of congenital anomalies with high levels of statistical significance P < 2.0 × 10−16.
These elegant findings have far-reaching implications in many respects. As noted the present report describes statistical associations only. Cannabinoids however have been linked to multiple cellular pathways and pathophysiological mechanisms. This data suggests that molecular and cellular pathways described experimentally may impact the neonatal and pediatric epidemiological disease profile of a country and become prominent at the national level. Some of these mechanisms and pathways are described below.
Moreover the fact that a similar spectrum of congenital defects was recently found in a detailed review of the teratological experience in Colorado during and after the process of cannabis legalization there (Reece and Hulse, 2019a,2020a) and has also been reported from Hawaii (Forrester and Merz, 2007) and USA (Reece and Hulse, 2020d) means that generally similar findings have now been replicated in several jurisdictions.
The finding that all cardiovascular anomalies rise in parallel with cannabis use is novel and original and may well be important epidemiologically. Cardiovascular anomalies are the leading cause of congenital anomalies which themselves are the leading cause of death in the first 5 years of life in developed nations. Both the American Academy of Pediatrics and the American Heart Association have previously issued a position statement which notes increased rates of ventricular septal defect and Ebstein's anomaly in association with cannabis use (Jenkins et al., 2007). Colorado has recently reported a tripling of the rate of atrial septal defect in that state (Colorado: Department of Public Health and the Environment, 2018; Reece and Hulse, 2019a,2019d), and indeed atrial septal defect is noted to be more common in cannabis liberal states across USA based on the National Birth Defects Prevention Network publicly available dataset (National Birth Defects Prevention Network, 2018; Reece and Hulse, 2020d). A large Hawaiian study of over 300,000 births found that hypoplastic left heart, tetralogy of Fallot, pulmonary valve atresia and or stenosis, ventricular septal defect and atrial septal defect were all increased after prenatal cannabis exposure (Forrester and Merz, 2007). Transposition of the great arteries has previously been linked with paternal cannabis use (Wilson et al., 1998). The present finding may have arisen either because most of the cardiac defects are higher in high cannabis using areas, or because some of the commoner defects are elevated and these suffice to increase the whole group significantly. This latter was the pattern recently observed in Colorado where atrial septal defect (ASD), ventricular septal defect (VSD) and patent ductus arteriosus (PDA) were all found to be dramatically increased in a temporal pattern closely tracking the increased availability of cannabis in that state (Colorado: Department of Public Health and the Environment, 2018; Reece and Hulse, 2019a,2019c, 2019d). Moreover, in the most recent Colorado dataset all cardiovascular anomalies have also risen both against time and against cannabis use (Colorado: Department of Public Health and the Environment, 2018; Reece and Hulse, 2019a,2020a). It may be that the larger numbers afforded by the present national level study confers higher statistical power to observe a difference overall. Now that the commonest class of congenital defects, all cardiovascular defects, have been linked with cannabis use in Hawaii, Colorado and Canada the implications are likely to be very far reaching indeed. The large subject of epigenomics was shown in a recent review to be a particularly important factor in cardiovascular morphogenesis and presumably teratogenesis (Reece et al., 2018; Reece and Hulse, 2019a,2019c, 2019d) and was recently shown to widely implicated in cannabinoid molecular and cellular pathways (Reece and Hulse, 2016; Murphy et al., 2018; Reece and Hulse, 2019a,2019c, 2019d).
Gastroschisis was also shown to be elevated in the present study. This was observed both in the general rate reported by the CCASS (Public Health Agency of Canada and Health Canada, 2013) and in the age-adjusted and age-unadjusted data reported by (Bassil et al., 2016). This result is consistent both with the uniform findings of all seven studies which have examined this question (Torfs et al., 1994; Werler et al., 2003; Forrester and Merz, 2007; Draper et al., 2008; van Gelder et al., 2009a; David et al., 2014; Skarsgard et al., 2015) including several prior reports from the Canadian Surgical Surveillance Group on this subject (Alshehri et al., 2013; Butler et al., 2015; Skarsgard et al., 2015; Bassil et al., 2016; Puligandla et al., 2017) presumably reporting on cases which overlap in part with some of the subjects reported herein by CCASS (Moore et al., 2013). This uniformity of the literature in this subject is indeed noteworthy. Although there is some discussion as to the most likely causal mechanism for gastroschisis it is thought that vascular mechanisms may be in effect partly because vasoactive drugs such as cocaine and amphetamines have been previously implicated. It is less well known that cannabis is frequently both pro-inflammatory and vasoactive in humans and acts through many receptors with the cannabinoid type 1 receptor CB1R usually the dominant receptor (Cutando et al., 2017; Menahem, 2017). CB1R is widely distributed both in brain and in endovascular and endocardial tissues including within the endocardial tissues of the fetus from early in embryonic life (Pacher et al., 2018).
Downs syndrome was also found to be elevated. Down's syndrome has been reported in one previously published epidemiological study in association with prenatal cannabis exposure which was in Hawaii (Forrester and Merz, 2007). It was also found to be rising in the Colorado dataset both against time and against cannabis use (Colorado: Department of Public Health and the Environment, 2018; Reece and Hulse, 2019a,2019c, 2019d). It was similarly found to be elevated across USA (Reece and Hulse, 2020d). It is however well established that cannabis tests positive in the micronucleus assay which is a test for major genetic damage which is accepted as having cross-species implications (Zimmerman and Zimmerman, 1990). Cannabinoids interfere with tubulin metabolism, the monomer from which polymeric microtubules are made both at the proteomic level and by interfering with stathmin which is an important guidance molecule for microtubular growth, neurogenesis, spinogenesis and axon guidance (Wang et al., 2011; Tortoriello et al., 2014; Martel et al., 2016). Microtubules form both the mitotic spindle along which chromosomes slide at the time of anaphase during cell division, and form the structural basis of the nerve axon. Hence it is easy to see how chromosomes can become separated from the main body of the chromosomal mass at the time of the anaphase division, and then become isolated in small diminutive micronuclei by themselves where, lacking the normal large complement of DNA accessory molecules needed for genomic stability and DNA replication and repair, they frequently fall prey to major disruption, breaking up, and even advanced pulverization by mechanisms known as chromothripsis, chromoplexy, and collectively chromoanagenesis (Zhang et al., 2015). Hence there is a direct link from micronucleus formation to chromosomal mis-segregation errors including the chromosomal trisomies. Interestingly Downs syndrome has been shown to be associated with a 20-fold increase in B-cell acute lymphocytic leukaemia risk related to constitutive activation by triplication of the gene HMGN1 at chromosome 21q22 which specifies an immature B-cell fate and also modifies the epigenome, nucleosome binding, and the accessibility of the transcription machinery genome-wide (Mowery et al., 2018). Further implications of these biological pathways are developed below.
The demonstrated links between prenatal cannabis exposure and Downs syndrome in Hawaii, Colorado, Canada and USA are strong direct clinical evidence of major genotoxicity (Forrester and Merz, 2007; Reece and Hulse, 2019a,2020a; Reece and Hulse, 2020d).
Impacts of impaired neurogenesis were mentioned above. The endocannabinoid system has been shown to be critically involved in synapse formation and pruning, axonal growth and pathfinding, progenitor cell proliferation and neuronal differentiation (Frau et al., 2019). Whilst the hippocampus is known to be involved in memory formation it is believed to form an index to cortically stored memories, much like a library card system indexes a book on a shelf. Incorporation of adult born hippocampal granule cells into the neuronal network over a prolonged maturation and incorporation period bestows vital plasticity on the network and greatly advances memory formation, recall, differentiation and assists higher cortical processing of advanced executive function (Miller and Sahay, 2019). Autism spectrum disorder has been linked with thousands of genes which are themselves organized into networks which affect brain development including synapse formation and function, neurite growth and neuronal proliferation and migration (Gazestani et al., 2019) and many of which are perturbed by cannabinoids. Key amongst these pathways is the critical Wnt -β-catenin system signaling (Gazestani et al., 2019) which is a key body and brain morphogen and is powerfully perturbed by cannabinoids (Xian et al., 2018; McKenzie et al., 2019; Nalli et al., 2019; Reece and Hulse, 2019a,2019c, 2019d,2020b,2020c).
Orofacial defects were not found to be statistically associated with cannabis use in this study but were found to be linked in the large Hawaiian study (Forrester and Merz, 2007).
Limb defects was not shown to be elevated in the present study perhaps because data for the Territories, which use more cannabis, was absent. Arm reduction defects were identified as being linked with prenatal cannabis exposure by the Hawaiian investigators (Forrester and Merz, 2007). And one also notes press reports from a birth defects registry in Ain in France near the Swiss border where a 58-fold elevation in the rate of babies born without arms was recently reported (Agence France-Presse in Paris, 2018; Willsher, 2018) together with cannabis in the food chain in Europe, and cattle born without legs. In this context it is highly pertinent just a few miles away across the Swiss border there is no such elevation in the rate of phocomelia and cannabis is not allowed to be included in the food chain. Interestingly a new report from Germany has just identified three cases of phocomelia presenting in a birthing suite in just three months in a medium sized hospital there (Robinson, 2019). Whether subsequent research in Canada will confirm such a relationship will require further data.
Clearly the presence of a variety of cannabinoids in the food chain is of particular concern. We were therefore concerned to learn while this paper was in review that cannabis edibles including drinks and creams will become available across Canada from 17th October 2019 (Canada Broadcasting Commission, 2019).
The confirmation that all cardiovascular congenital anomalies and Down's syndrome along with gastroschisis are linked epidemiologically with cannabis use potentially broadens the scope of cannabis-related defects. Previously one could argue that there is good evidence that the following seven defects are linked with prenatal cannabis exposure: atrial septal defect, Ebstein's anomaly (Jenkins et al., 2007), gastroschisis, anencephaly, oesophageal atresia with or without tracheo-esophageal fistula, diaphragmatic hernia (Van Gelder et al., 2014;Van Gelder et al., 2009b) and long lasting cerebral executive cortical dysfunction (Smith et al., 2006; Smith et al., 2010; Smith et al., 2016; Brents, 2017). The findings of the Forrester 2007 report from Hawaii (Forrester and Merz, 2007) are clearly an outlier in the literature and so how much of its findings that in fact 21 defects were increased among individuals consuming cannabis should be accepted is a moot point. However this paper has very impressive predictive power for many defects such as tripling atrial septal defect rates in Colorado, rising atrial septal defect rates in high cannabis states across USA, the pattern of congenital defects in high cannabis US states such as Alaska, Colorado, Massachusetts and Oregon, and the high frequency of chromosomal defects in Colorado, the phocomelia outbreak in humans and cows in parts of France but not in nearby Switzerland mentioned above and the increased Downs syndrome in Canada in the present report - amongst others. On the basis of such impressive predictive accuracy it may well be argued that much of the defect list of this paper should be included as putatively or potentially cannabis related.
Irrespective of what one considers about the Forrester 2007 paper it is clear that cannabis is now linked by association with many congenital disorders. The inclusion of all cardiovascular disorders and chromosomal mis-segregation disorders as suggested by the present report clearly adds two major categories of congenital anomalies to this list and powerfully amplifies teratological concerns.
This data has implications for Nunavut and the other northern Territories of Canada. Nunavut has a broad spectrum of socioeconomic disadvantage including high rates of homelessness, poverty, hunger, violent crime, sexual disease, dietary inadequacy, and premature and underweight birth (Kilpatrick, 2015; Nunavut Government, 2019). Nunavut also has a high rate of total congenital anomalies (Public Health Agency of Canada and Health Canada, 2013). Formal dissection of cannabis exposure compared to the many other adverse social and health situations will require much further research. However, it is also obvious that with all of the adversity inherent in the harsh arctic environment an avoidable and increased burden of cannabis-related congenital defects would not be conducive to improved pediatric public health.
One consideration which is of particular importance to the present primarily epidemiological enquiry relates to the possible molecular and cellular mechanisms responsible for the observed changes. The issue is of particular significance since the existence of plausible biological mechanisms is a key factor in many algorithms which consider criteria of causality such as that of Hill (1965).
Cellular Pathways and Molecular Mechanisms
Cannabis has been shown to work on developing organisms by a number of complex pathways which have been summarized elsewhere (Reece et al., 2016; Reece and Hulse, 2016; Reece, 2018; Reece and Hulse, 2019b,2020b, 2020c). Briefly stated cannabinoids have been shown to work via: interference with synapse formation by interruption of neurexin / neuroligin scaffolding (Foldy et al., 2013; Anderson et al., 2015; Wang, 2016); impeding notch signaling (Lu et al., 2006; Newton et al., 2009; Tanveer et al., 2012; Kim et al., 2014) an important morphogen for cardiac, vascular, brain, and hemopoietic tissues (Carlson, 2014); impeding robo/slit signaling with effects on human neocortical exuberant outgrowth, nerve and blood vessel guidance, tissue development in kidney, breast, lung and muscle (Alpar et al., 2014; Blockus and Chedotal, 2016), spinal cord midline guidance, several neurodevelopmental disorders including dyslexia (Galaburda et al., 2006) and psychopathy (Viding et al., 2010); impeding axonal guidance by interference with stathmin signaling (Tortoriello et al., 2014); cytoskeletal impairment affecting the actin cytoskeleton (Wang et al., 2011; Miller et al., 2019) and microtubule structure and function (Wang et al., 2011; Miller et al., 2019); defects on egg and sperm development including gross sperm deformities involving head and tail malformations (Morishima, 1984; Hembree et al., 1999; Szutorisz and Hurd, 2016; Johnson et al., 2017; Murphy et al., 2018); impairment of mitochondrial function (Sarafian et al., 2003; Sarafian et al., 2006); impairment of sperm mitochondrial function (Rossato et al., 2005); impairment of replacement of sperm histones by protamines (Chioccarelli et al., 2010); epigenetic effects (Yang et al., 2014) and micronucleus effects (Van Went, 1978; Piatti et al., 1989; Parolini and Binelli, 2014; Reece and Hulse, 2016) including cytoplasmic bridges and nuclear blebbing (Morishima, 1984; Huang et al., 1999; Russo et al., 2018).
Whilst many of these pathways carry wide ranging implications for derangement of multiple organ systems recent work on the epigenetic effects of prenatal cannabinoids and the micronuclear induction activities of several cannabinoids are particularly noteworthy.
It has been known since 1990 that tetrahydrocannabinol, cannabidiol and cannabinol are associated with micronucleus formation at physiologically relevant doses in rodents (Zimmerman and Raj, 1980; Zimmerman and Zimmerman, 1990). Both classical and more recent studies show additional evidence of nuclear blebs and cytoplasmic bridges being formed between dividing daughter cells (Stefanis and Issidorides, 1976; Morishima, 1984; Russo et al., 2018). The mitotic spindle is formed of microtubules which are polymerized tubulin monomers. Chromosomes slide along these “microtubular rails” at the end of anaphase to cluster into two groups, the forming pro-nuclei of the nascent daughter cells. Any chromosomes which become detached from this system can be left behind and are surrounded by a new nuclear envelope. However the mitotic spindle inhibits the proper assembly of the micronuclear envelop which it forms close to the spindle so that lamin B, which is required for proper strength of the nuclear envelop, and the nuclear pore complexes which are required for importing of the host of enzymes used in normal DNA replication and transcription, cannot occur (Liu et al., 2018). For these reasons the micronuclear nuclear envelopes are very fragile and prone to rupture.
This premature rupture of the micronuclear envelope releases DNA into the cytoplasm where it is taken up by various cytosolic innate immune system Pattern Recognition Receptors (PRR's) which trigger an interferon type 1 response. The principal DNA sensor is cGAS (cyclic AMP-GMP Synthase) which binds to the signalling scaffold STING (Stimulator of Interferon Genes) which transmits a signal directly to type I interferon genes and signals via the non-canonical pathway to Nuclear Factor κB (NF-κB) which powerfully activates cell-autonomous innate immune signaling (Bartsch et al., 2017; Harding et al., 2017; Mackenzie et al., 2017). The presence of blebs in the nuclear envelope is a sign of nuclear ageing (Gluck et al., 2017), and also a source of more nuclear DNA which further fuels this process. Immune stimulation is a signal to the immune system to deal with the genomically disrupted cell. Interestingly Morishima described both cytoplasmic bridges and blebbing in ova in association with a high loss rate after the first meiotic division following cannabinoid exposure long ago (Morishima, 1984). It has therefore become apparent that the nucleoplasmic bridges and blebs are the cause of the high rate of ova loss (Johnson et al., 2017).
This cGAS-STING pathway has been shown to be key to the generation of the Senescence-Associated Secretory Phenotype (SASP), made up of cytokines, chemokines, growth factors and proteases, which enforces senescent cell arrest both in the cell of origin and in nearby cells (ie, induces both autocrine and paracrine signaling) (Gluck et al., 2017; Mackenzie et al., 2017; Yang et al., 2017; Ablasser, 2019; Ablasser and Chen, 2019). This pathway is stimulated by chronic inflammation, chronic stress including tobacco smoking and aging (Niwa and Ushijima, 2010; Issa, 2014; Maegawa et al., 2014; Vaz et al., 2017). It is also implicated in cell transformation and carcinogenesis, in spreading the ageing phenotype to nearby cells, in reinforcing cellular senescence cell autonomously (Ablasser, 2019; Shang et al., 2019; Zhang et al., 2019), and in the process of cancer metastasis (Bakhoum et al., 2018). Since inflammation is highly oxidative this can damage the bases of DNA including re-arrange the methylation marks and its machinery from gene body to gene promoter, a change which is characteristic of ageing (Niwa and Ushijima, 2010; O’Hagan et al., 2011; Issa, 2014). This change has at least three principal effects in that it reduces gene expression overall by suppressing promoters, it releases gene and particularly non-coding intergenic repeat elements and gene bodies from methylation suppression, and it transfers some elements from histone control to DNA methylation and relatively fixed suppression.
Epigenetic studies are receiving increased attention in addictive and neuropsychiatric disorders with well-known implications for transgenerational transmission of environmental traits through the principal epigenetic mechanisms of histone post-translational modifications, DNA methylation and non-coding RNA's (Manikkam et al., 2012; Hughes, 2014). Indeed transmission of key behavioral and neurological traits between generations has recently been demonstrated by both histone modifications and DNA methylation routes (DiNieri et al., 2011; Szutorisz et al., 2014; Watson et al., 2015; Szutorisz and Hurd, 2016). Severe and premature pruning of dendritic arbors and synaptic boutons by adolescent cannabis exposure has been shown to be epigenetically mediated (Miller et al., 2019). Overlap between frontal cortex transcriptomes of prenatal cannabis exposure and schizophrenia and autism has been demonstrated (Guennewig et al., 2018). Indeed perturbations of the epigenetic machinery itself (histone modification, covalent chromatin modification, histone lysine modification, histone methyltransferase Jade 2, histone arginine methyl transferase Prmt5) by adolescent cannabis exposure described by the authors as “vast”, “marked” and “profound” has been shown (Miller et al., 2019).
One careful study of sperm epigenetic alterations in humans and rate showed 6640 differentially methylated sites by more than 10% across the genome in humans and 3979 differentially methylated CpG islands (Murphy et al., 2018). Overall a globally lower level of methylation was seen across both human and rat genomes. Pathways in cancer and the hippo pathway (also involved in cancer and morphogenesis) were consistently upregulated in both species. For 177 affected genes there was a significant dose-response relationship between gene expression levels and cannabis use.
Using a well-established method of biological age assessment based on arterial stiffness adult patients exposed to cannabis were shown to be have increased arterial stiffness and so to be biologically older (Reece et al., 2016). This finding is consistent with pro-inflammatory actions of cannabis (Sarafian et al., 1999; Bindukumar et al., 2008; Mukhopadhyay et al., 2010; Rajesh et al., 2012; Wolff et al., 2015; Pacher et al., 2018) which are also linked with advancing biological age (Issa et al., 2001; Hahn et al., 2008; Chiba et al., 2012). It was recently shown in advanced cellular senescence that LINE-1 mobile transposable elements, so-called “jumping genes” or retrotransposons, which comprise 17% of the genome, can become mobilized and re-insert into the genome in a random manner using endogenous reverse transcriptases (De Cecco et al., 2019). Not only is this destructive to the genomic sequence with downstream consequences including teratogenesis, carcinogenesis, aging and age-related degenerative disease, but this also activates cytoplasmic cGAS-STING signaling and autocrine and paracrine senescence programs (Dou et al., 2017; Bakhoum et al., 2018; De Cecco et al., 2019; Shang et al., 2019; Zhang et al., 2019). This novel aging mechanism is yet to be evaluated following cannabis exposure.
The above brief overview demonstrates several major points. There are numerous described mechanistic pathways by which prenatal cannabinoid exposure can impact fetal morphogenetic outcomes. There are many interactions between epigenetic and genetic mechanisms by which perturbation at the epigenetic level can have serious and widespread effects at the genetic level including alteration of the genetic sequence. Not only do the above alterations collectively describe numerous degenerative changes which closely phenocopy the aging process, but the gametes themselves, namely the ovum and sperm, may be said in many respects to be aged even prior to conception. In such a scenario widespread teratogenic effects across multiple systems is not unexpected. Indirect evidence related to this was recently provided by workers in adults showing that a wide variety of common brain disorders are associated with an acceleration of brain aging including changes widely reported in cannabis users including minimal cognitive dysfunction and schizophrenia (Kaufmann et al., 2019).
The present study has a number of strengths. It uses national census data taken over many years. Hence the sample size is large and the rates of congenital anomalies are relatively stable. The statistical analysis is both straight forward and geospatially sophisticated. Indeed this is the first application of geospatial spatial analysis in the published literature relating to congenital anomalies of which we are aware. The cannabis use data is reliable and was taken from a large national sample conducted by Statistics Canada. Since data on abortions for defect was not included in the present dataset, it is considered that its inclusion is likely to strengthen the present findings. The study also suffers from several limitations. An obvious extension of the present work to shorter time intervals and higher geospatial resolution as was recently achieved in Canada with gastroschisis by (Bassil et al., 2016) would further extend the power and significance of this approach. Individual level data was not available to the present research team. Many of these study design limitations could be corrected by the use of a case-control framework which is advocated for future studies. Virtually all of the studies in this area suffer from a common drawback which is the reliance on self-report to define drug exposure. As illustrated by the Californian experience this is notoriously unreliable (Young-Wolff et al., 2017). An objective measure such as hair analysis (David et al., 2014) or the recently proposed epigenomic-glycomic biomarker (Reece et al., 2018) would not only provide an objective and quantitative measure of the exposure, but as hair grows by 1 cm per month, it would also allow the all-important timing of exposure relative to the gestational periods to be objectively determined.
It should be noted that the present study is an ecological study only and thus is not able to control for numerous confounders. For this reason, the relationships reported are noted to be correlative only and cannot be used by themselves to imply causal relationships. Having said that the close geospatial correlation of the defects described in the present report with those observed in animals and in other human populations particularly in Hawaii, Colorado, USA, Europe and Australia (Forrester and Merz, 2007; Queensland Health, 2018; Reece and Hulse, 2019a; Reece and Hulse, 2020d), in the context of numerous cellular and biological mechanisms necessarily raises causal concerns and strongly indicates further research in this area.
In conclusion these data show a correlation between many drugs but particularly cannabis use across the Provinces and Territories of Canada with total congenital defects, gastroschisis, Downs syndrome, overall cardiovascular defects, a combined index of the interactions of these three anomalies together with orofacial clefts and their first principal component. Formal geospatial analysis indicates with high level statistical significance that the use of all the classes of addictive drugs examined and particularly cannabis is highly associated with spatially distributed rates of total congenital anomalies. As such these findings extend the range of defects which might reasonably be linked with prenatal cannabis exposure and imply a wider teratological spectrum of defects than has previously been appreciated. Such findings imply caution in terms of increasing the availability and use of cannabis across whole populations and particularly by males and females in the reproductive age group. Particular concern relates to the entry of numerous cannabinoids into the food chain in North America with potentially far reaching implications as is increasingly observed in Europe. A formal large case-control study including objective measures of drug exposure such as hair analysis is clearly required. Since cannabis legalization has been clearly shown to induce increased public cannabis consumption (Hasin et al., 2017; Canadian Centre on Substance Use and Addiction, 2019) and since use in pregnancy generally closely parallels that in the general community (Young-Wolff et al., 2017; Volkow et al., 2019) the present statistically very highly significant data in the context of the wider mechanistic and preclinical and human epidemiological literature argues strongly against increasing cannabis availability under any legislative regime either in Canada or elsewhere and most especially amongst impoverished and disadvantaged communities.
Supplementary Material
Footnotes
The authors have no conflicts of interests to disclose.
REFERENCES
- Ablasser A. Structures of STING protein illuminate this key regulator of inflammation. Nature 2019; 567:321–322. [DOI] [PubMed] [Google Scholar]
- Ablasser A, Chen ZJ. cGAS in action: Expanding roles in immunity and inflammation. Science 2019; 363:eaat8657. [DOI] [PubMed] [Google Scholar]
- Agence France-Presse in Paris. France to investigate cause of upper limb defects in babies. The Guardian [serial online]. 2018. Available at: https://www.theguardian.com/world/2018/oct/21/france-to-investigate-cause-of-upper-limb-defects-in-babies. Accessed November 3, 2018. [Google Scholar]
- Alpar A, Tortoriello G, Calvigioni D, et al. Endocannabinoids modulate cortical development by configuring Slit2/Robo1 signalling. Nat Commun 2014; 5:4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshehri A, Emil S, Laberge JM, et al. Outcomes of early versus late intestinal operations in patients with gastroschisis and intestinal atresia: results from a prospective national database. J Pediatr Surg 2013; 48:2022–2026. [DOI] [PubMed] [Google Scholar]
- Anderson GR, Aoto J, Tabuchi K, et al. Beta-neurexins control neural circuits by regulating synaptic endocannabinoid signaling. Cell 2015; 162:593–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhoum SF, Ngo B, Laughney AM, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018; 553:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch K, Knittler K, Borowski C, et al. Absence of RNase H2 triggers generation of immunogenic micronuclei removed by autophagy. Hum Mol Genet 2017; 26:3960–3972. [DOI] [PubMed] [Google Scholar]
- Bassil KL, Yang J, Arbour L, et al. Spatial variability of gastroschisis in Canada, 2006-2011: an exploratory analysis. Can J Public Health 2016; 107:e62–e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindukumar B, Mahajan SD, Reynolds JL, et al. Genomic and proteomic analysis of the effects of cannabinoids on normal human astrocytes. Brain Res 2008; 1191:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blockus H, Chedotal A. Slit-Robo signaling. Development 2016; 143:3037–3044. [DOI] [PubMed] [Google Scholar]
- Brents L. Preedy VR. Correlates and consequences of Prenatal Cannabis Exposure (PCE): identifying and characterizing vulnerable maternal populations and determining outcomes in exposed offspring. Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis and Treatment. London: Academic Press; 2017. 160–170. [Google Scholar]
- Butler AE, Puligandla PS, Skarsgard ED. The Canadian Pediatric Surgery Network (CAPSNet): lessons learned from a national registry devoted to the study of congenital diaphragmatic hernia and gastroschisis. Eur J Pediatr Surg 2015; 25:474–480. [DOI] [PubMed] [Google Scholar]
- Canada Broadcasting Commission. Cannabis Edibles: What's Now Legal to Use, 2019. Available at: https://www.youtube.com/watch?v=rupHqBYJixQ. Accessed October 19, 2019. [Google Scholar]
- Canadian Centre on Substance Use and Addiction. Cannabis Legalization: Year One Observations, 2019. Available at: https://www.ccsa.ca/sites/default/files/2019-10/CCSA-Synthesis-Canada-Cannabis-Legalization-First-Year-Policy-Brief-2019-en.pdf. Accessed October 19, 2019. [Google Scholar]
- Canadian Substance Use Costs and Harms Working Group, Stockwell T, Young M, et al. Canadian Substance Use Costs and Harms in the Provinces and Territories (2007-2014). In: Canadian Institute for Substance Use Research, UoVaHC., ed., Ottawa, Ontario: Canadian Institute for Substance Use Research, Canadian Centre on Substance Use, Addiction, University of Victoria, 2018:1–38. [Google Scholar]
- Carlson BM. Human Embryology and Developmental Biology. Philadelphia: Elsevier; 2014. [Google Scholar]
- Chiba T, Marusawa H, Ushijima T. Inflammation-associated cancer development in digestive organs: mechanisms and roles for genetic and epigenetic modulation. Gastroenterology 2012; 143:550–563. [DOI] [PubMed] [Google Scholar]
- Chioccarelli T, Cacciola G, Altucci L, et al. Cannabinoid receptor 1 influences chromatin remodeling in mouse spermatids by affecting content of transition protein 2 mRNA and histone displacement. Endocrinology 2010; 151:5017–5029. [DOI] [PubMed] [Google Scholar]
- Colorado: Department of Public Health and the Environment. Colorado Responds to Children with Special Needs - Birth Defect Data, Colorado. In: Environment CDoPHat ed. Denver Colorado, USA: Colorado: Department of Public Health and the Environment, 2018. Available at: http://www.chd.dphe.state.co.us/cohid/. Accessed April 15, 2018. [Google Scholar]
- Cutando L, Maldonado R, Ozaita A. Preedy V. Microglial activation and cannabis exposure. Handbook of Cannabis and Related Pathologies: Biology, Pharmacology, Diagnosis and Treatment. New York: Academic Press; 2017. 401–412. [Google Scholar]
- David AL, Holloway A, Thomasson L, et al. A case-control study of maternal periconceptual and pregnancy recreational drug use and fetal malformation using hair analysis. PLoS One 2014; 9:e111038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cecco M, Ito T, Petrashen AP, et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 2019; 566:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Whiteford HA, Ferrari AJ, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet 2013; 382:1564–1574. [DOI] [PubMed] [Google Scholar]
- Dickson B, Mansfield C, Guiahi M, et al. Recommendations from cannabis dispensaries about first-trimester cannabis use. Obstet Gynecol 2018; 131:1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNieri JA, Wang X, Szutorisz H, et al. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol Psychiatry 2011; 70:763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Z, Ghosh K, Vizioli MG, et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature 2017; 550:402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper ES, Rankin J, Tonks AM, et al. Recreational drug use: a major risk factor for gastroschisis? Am J Epidemiol 2008; 167:485–491. [DOI] [PubMed] [Google Scholar]
- Foldy C, Malenka RC, Sudhof TC. Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling. Neuron 2013; 78:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester MB, Merz RD. Risk of selected birth defects with prenatal illicit drug use Hawaii 1986–2002. J Toxicol Environ Health A 2007; 70:7–18. [DOI] [PubMed] [Google Scholar]
- Frau R, Miczán V, Traccis F, et al. Prenatal THC exposure produces a hyperdopaminergic phenotype rescued by pregnenolone. Nature Neurosci 2019; 22:1975–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, LoTurco J, Ramus F, et al. From genes to behavior in developmental dyslexia. Nat Neurosci 2006; 9:1213–1217. [DOI] [PubMed] [Google Scholar]
- Gazestani VH, Pramparo T, Nalabolu S, et al. A perturbed gene network containing PI3K-AKT, RAS-ERK and WNT-beta-catenin pathways in leukocytes is linked to ASD genetics and symptom severity. Nat Neurosci 2019; 22:1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geber WF, Schramm LC. Effect of marihuana extract on fetal hamsters and rabbits. Toxicol Appl Pharmacol 1969; 14:276–282. [DOI] [PubMed] [Google Scholar]
- Geber WF, Schramm LC. Teratogenicity of marihuana extract as influenced by plant origin and seasonal variation. Arch Int Pharmacodyn Ther 1969; 177:224–230. [PubMed] [Google Scholar]
- Gluck S, Guey B, Gulen MF, et al. Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence. Nat Cell Biol 2017; 19:1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JDP. Graham JDP. Cannabis and health. Cannabis and Health. London, New York, San Francisco: Academic Press; 1976. 271–320. [Google Scholar]
- Guennewig B, Bitar M, Obiorah I, et al. THC exposure of human iPSC neurons impacts genes associated with neuropsychiatric disorders. Transl Psychiatry 2018; 8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MA, Hahn T, Lee DH, et al. Methylation of polycomb target genes in intestinal cancer is mediated by inflammation. Cancer Res 2008; 68:10280–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding SM, Benci JL, Irianto J, et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017; 548:466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Cerda M, et al. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991–1992 to 2012–2013. JAMA Psychiatry 2017; 74:579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canada H. Health Canada. Canadian Tobacco Alcohol and Drugs (CTADS): 2015 Summary. Ottawa: Health Canada; 2016. 1. [Google Scholar]
- Hembree WC, Nahas GG, Zeidenberg P. Nahas GG, Sutin KM, Harvey DJ, et al. Changes in human spermatozoa associated with high dose marihuana smoking. Marijuana and Medicine. Totowa, New Jersey: Humana Press; 1999. 367–378. [Google Scholar]
- Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965; 58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HFS, Nahas GG, Hembree WC. Nahas GG, Sutin KM, Harvey DJ. Effects of marijuana inhalation on spermatogenesis of the rat. Marijuana and Medicine. Totowa, New Jersey: Humana Press; 1999. 359–366. [Google Scholar]
- Hughes V. Epigenetics: the sins of the father. Nature 2014; 507:22–24. [DOI] [PubMed] [Google Scholar]
- Issa JP. Aging and epigenetic drift: a vicious cycle. J Clin Invest 2014; 124:24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa JP, Ahuja N, Toyota M, et al. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res 2001; 61:3573–3577. [PubMed] [Google Scholar]
- Jenkins KJ, Correa A, Feinstein JA, et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge: a scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young: endorsed by the American Academy of Pediatrics. Circulation 2007; 115:2995–3014. [DOI] [PubMed] [Google Scholar]
- Johnson WL, Xie KT, Kwon M, et al. How the genome folds, divides, lives, and dies. Cold Spring Harb Symp Quant Biol 2017; 82:349–360. [DOI] [PubMed] [Google Scholar]
- Kapoor M, Kelejian HH, Prucha IR. Panel data models with spatially correlated error components. J Econom 2007; 140:97–130. [Google Scholar]
- Kaufmann T, van der Meer D, Doan NT, et al. Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat Neurosci 2019; 22:1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick JR. Judge OotS. A primer on nunavut. Nunavik, Nunavut 5th ed.Canada: Office of the Senior Judge; 2015. 1–46. [Google Scholar]
- Kim D, Lim S, Park M, et al. Ubiquitination-dependent CARM1 degradation facilitates Notch1-mediated podocyte apoptosis in diabetic nephropathy. Cell Signal 2014; 26:1774–1782. [DOI] [PubMed] [Google Scholar]
- Liu S, Kwon M, Mannino M, et al. Nuclear envelope assembly defects link mitotic errors to chromothripsis. Nature 2018; 561:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Newton C, Perkins I, et al. Cannabinoid treatment suppresses the T-helper cell-polarizing function of mouse dendritic cells stimulated with Legionella pneumophila infection. J Pharmacol Exp Ther 2006; 319:269–276. [DOI] [PubMed] [Google Scholar]
- Mackenzie KJ, Carroll P, Martin CA, et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 2017; 548:461–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegawa S, Gough SM, Watanabe-Okochi N, et al. Age-related epigenetic drift in the pathogenesis of MDS and AML. Genome Res 2014; 24:580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Guerrero-Bosagna C, Tracey R, et al. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One 2012; 7:e31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel G, Uchida S, Hevi C, et al. Genetic demonstration of a role for stathmin in adult hippocampal neurogenesis, spinogenesis, and NMDA receptor-dependent memory. J Neurosci 2016; 36:1185–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie MG, Cobbs LV, Dummer PD, et al. Non-canonical Wnt signaling through Ryk regulates the generation of somatostatin- and parvalbumin-expressing cortical interneurons. Neuron 2019; 103:853.e4–864.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menahem S. V.R. P. Cardiovascular effects of cannabis usage. Handbook of Cannabis and Related Pathologies: Biology, Pharmacology and Treatment. New York: Academic Press; 2017. 481–485. [Google Scholar]
- Miller ML, Chadwick B, Dickstein DL, et al. Adolescent exposure to Delta(9)-tetrahydrocannabinol alters the transcriptional trajectory and dendritic architecture of prefrontal pyramidal neurons. Mol Psychiatry 2019; 24:588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SM, Sahay A. Functions of adult-born neurons in hippocampal memory interference and indexing. Nat Neurosci 2019; 22:1565–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millo G. Maximum likelihood estimation of spatially and serially correlated panels with random effects. Comput Stat Data Anal 2014; 71:914–933. [Google Scholar]
- Millo G, Piras G. splm: spatial panel data models in R. J Stast Softw 2012; 47:1–38. [Google Scholar]
- Moore A, Roulean J, Skarsgard E. Public Health Agency of Canada HC Congenital anomalies in Canada, 2013. A perinatal health surveillance report. Chapter 7. Gastroschisis. Ottawa: Health Canada; 2013. 57–63. [Google Scholar]
- Morishima A. Effects of cannabis and natural cannabinoids on chromosomes and ova. NIDA Res Monogr 1984; 44:25–45. [PubMed] [Google Scholar]
- Mowery CT, Reyes JM, Cabal-Hierro L, et al. Trisomy of a down syndrome critical region globally amplifies transcription via HMGN1 overexpression. Cell Rep 2018; 25:1898.e5–1911.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Batkai S, et al. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res 2010; 85:773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SK, Itchon-Ramos N, Visco Z, et al. Cannabinoid exposure and altered DNA methylation in rat and human sperm. Epigenetics 2018; 13:1208–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalli Y, Dar MS, Bano N, et al. Analyzing the role of cannabinoids as modulators of Wnt/beta-catenin signaling pathway for their use in the management of neuropathic pain. Bioorg Med Chem Lett 2019; 29:1043–1046. [DOI] [PubMed] [Google Scholar]
- National Birth Defects Prevention Network. National Birth Defects Prevention Network, 2018. Available at: https://www.nbdpn.org/ar.php. Accessed July 15, 2018. [Google Scholar]
- Newton CA, Chou PJ, Perkins I, et al. CB(1) and CB(2) cannabinoid receptors mediate different aspects of delta-9-tetrahydrocannabinol (THC)-induced T helper cell shift following immune activation by Legionella pneumophila infection. J Neuroimmune Pharmacol 2009; 4:92–102. [DOI] [PubMed] [Google Scholar]
- Niwa T, Ushijima T. Induction of epigenetic alterations by chronic inflammation and its significance on carcinogenesis. Adv Genet 2010; 71:41–56. [DOI] [PubMed] [Google Scholar]
- Nunavut Government. Nunavut Births, 2019. Available at: http://www.stats.gov.nu.ca/en/Population%20births.aspx. Accessed March 31, 2019. [Google Scholar]
- O’Hagan HM, Wang W, Sen S, et al. Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell 2011; 20:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Steffens S, Hasko G, et al. Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol 2018; 15:151–166. [DOI] [PubMed] [Google Scholar]
- Parolini M, Binelli A. Oxidative and genetic responses induced by Delta-9-tetrahydrocannabinol (Delta-9-THC) to Dreissena polymorpha. Sci Total Environ 2014; 468–469:68–76. [DOI] [PubMed] [Google Scholar]
- Piatti E, Rizzi R, Re F, et al. Genotoxicity of heroin and cannabinoids in humans. Pharmacol Res 1989; 21: Suppl 1: 59–60. [DOI] [PubMed] [Google Scholar]
- Public Health Agency of Canada. Congenital Anomalies in Canada, 2013. A Perinatal Health Surveillance Report In: Public Health Agency of Canada HC ed. Ottawa: Health Canada, 2013:1–115. [Google Scholar]
- Public Health Agency of Canada, Health Canada. Congenital Anomalies in Canada, 2013. A Perinatal Health Surveillance Report. In: Public Health Agency of Canada, Health Canada eds. Ottawa: Health Canada, 2013:1–119. [Google Scholar]
- Puligandla PS, Baird R, Skarsgard ED, et al. Outcome prediction in gastroschisis - the gastroschisis prognostic score (GPS) revisited. J Pediatr Surg 2017; 52:718–721. [DOI] [PubMed] [Google Scholar]
- Queensland Health. Congenital Anomaly Linked Data Table and Notes. In: Queensland Health ed. Brisbane, Queensland: Queensland Health, 2018. Available at: https://www.health.qld.gov.au/hsu/dashboards/calf.xlsm. Accessed July 15, 2018. [Google Scholar]
- Rajesh M, Batkai S, Kechrid M, et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 2012; 61:716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS, Hulse GK. Cannabis teratology explains current patterns of Coloradan congenital defects: the contribution of increased cannabinoid exposure to rising teratological trends. Clin Pediatr 2019a; 58:1085–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS, Hulse GK. Explaining contemporary patterns of cannabis teratology. Clin Pediatr 2019b; 4:1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS, Hulse GK. Effect of cannabis legalization on us autism incidence and medium term projections. Clin Pediatr Open Access 2019c; 4:1–17. [Google Scholar]
- Reece AS, Hulse GK. Impacts of Cannabinoid Epigenetics on Human Development: Reflections on Murphy et. al. ’Cannabinoid Exposure and Altered DNA Methylation in Rat and Human Sperm’ Epigenetics 2018; 13: 1208–1221. Epigenetics 2019; 14:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS, Hulse GK. Cannabis consumption patterns parallel the East-West gradient in Canadian neural tube defect incidence: an ecological study. Glob Pediatr Health 2020a;6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS, Hulse GK. Epidemiological associations of various substances and multiple cannabinoids with autism in USA. Clin Pediatr Open Access 2020b;4:1–20. [Google Scholar]
- Reece AS, Hulse GK. Gastroschisis and autism: dual canaries in the Californian coal mine. JAMA 2020c;154:366–367. [DOI] [PubMed] [Google Scholar]
- Reece AS, Hulse GK. Geotemporospatial Analysis of United States 38-44 Congenital Anomalies as a Function of Multiple Cannabinoid- and Substance- Exposure in the Decades of Cannabis Legalization: Phenocopying Thalidomide and Hundred Megabase-Scale Genotoxicity. In: Heritable Cannabinoid Epidemiology: Neurotoxicity, Genotoxicity, Teratogenicity and Carcinogenicity. New York: Springer, 2020d (in press). [Google Scholar]
- Reece AS, Norman A, Hulse GK. Cannabis exposure as an interactive cardiovascular risk factor and accelerant of organismal ageing – a longitudinal study. BMJ - Open 2016; 6:e011891–e011900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece AS. Known cannabis teratogenicity needs to be carefully considered. Br Med J 2018; 362:k3357. [Google Scholar]
- Reece AS, Hulse GK. Chromothripsis and epigenomics complete causality criteria for cannabis- and addiction-connected carcinogenicity, congenital toxicity and heritable genotoxicity. Mutat Res 2016; 789:15–25. [DOI] [PubMed] [Google Scholar]
- Reece AS, Wang W, Hulse GK. Pathways from epigenomics and glycobiology towards novel biomarkers of addiction and its radical cure. Med Hypotheses 2018; 116:10–21. [DOI] [PubMed] [Google Scholar]
- Robinson M. Babies born with deformed hands spark investigation in Germany, 2019. Available at: https://edition.cnn.com/2019/09/16/health/hand-deformities-babies-gelsenkirchen-germany-intl-scli-grm/index.html. Accessed October 5, 2019. [Google Scholar]
- Rossato M, Ion Popa F, Ferigo M, et al. Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J Clin Endocrinol Metab 2005; 90:984–991. [DOI] [PubMed] [Google Scholar]
- Russo C, Ferk F, Misik M, et al. Low doses of widely consumed cannabinoids (cannabidiol and cannabidivarin) cause DNA damage and chromosomal aberrations in human-derived cells. Arch Toxicol 2019; 93:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarafian TA, Habib N, Oldham M, et al. Inhaled marijuana smoke disrupts mitochondrial energetics in pulmonary epithelial cells in vivo. Am J Physiol 2006; 290:L1202–L1209. [DOI] [PubMed] [Google Scholar]
- Sarafian TA, Kouyoumjian S, Khoshaghideh F, et al. Delta 9-tetrahydrocannabinol disrupts mitochondrial function and cell energetics. Am J Physiol 2003; 284:L298–306. [DOI] [PubMed] [Google Scholar]
- Sarafian TA, Magallanes JA, Shau H, et al. Oxidative stress produced by marijuana smoke. An adverse effect enhanced by cannabinoids. Am J Respir Cell Mol Biol 1999; 20:1286–1293. [DOI] [PubMed] [Google Scholar]
- Shang G, Zhang C, Chen ZJ, et al. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP. Nature 2019; 567:389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarsgard ED, Meaney C, Bassil K, et al. Maternal risk factors for gastroschisis in Canada. Birth Defects Res A Clin Mol Teratol 2015; 103:111–118. [DOI] [PubMed] [Google Scholar]
- Smith AM, Fried PA, Hogan MJ, et al. Effects of prenatal marijuana on visuospatial working memory: an fMRI study in young adults. Neurotoxicol Teratol 2006; 28:286–295. [DOI] [PubMed] [Google Scholar]
- Smith AM, Longo CA, Fried PA, et al. Effects of marijuana on visuospatial working memory: an fMRI study in young adults. Psychopharmacology (Berl) 2010; 210:429–438. [DOI] [PubMed] [Google Scholar]
- Smith AM, Mioduszewski O, Hatchard T, et al. Prenatal marijuana exposure impacts executive functioning into young adulthood: an fMRI study. Neurotoxicol Teratol 2016; 58:53–59. [DOI] [PubMed] [Google Scholar]
- Statistics Canada. National Cannabis Survey, Second Quarter, 2018. Available at: https://www.facebook.com/StatisticsCanada/posts/1636405843137586:0. Accessed March 17, 2019. [Google Scholar]
- Statistics Canada. 2019a. Available at: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/hlt-fst/inc-rev/Table.cfm?Lang=Eng&T=101&S=99&O=A. Accessed December 18, 2019. [Google Scholar]
- Statistics Canada. Third Quarter National Cannabis Survey, Canada. In: Canada S, ed., Ottawa: Statistics Canada, 2019:1. [Google Scholar]
- Stefanis CN, Issidorides MR. Nahas GG. Cellular effects of chronic cannabis use in man. Marihuana: Chemistry, Biochemistry and Cellular Effects. New York: Springer; 1976. 533–550. [Google Scholar]
- Szutorisz H, DiNieri JA, Sweet E, et al. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology 2014; 39:1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, Hurd YL. Epigenetic effects of cannabis exposure. Biol Psychiatry 2016; 79:586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanveer R, Gowran A, Noonan J, et al. The endocannabinoid, anandamide, augments Notch-1 signaling in cultured cortical neurons exposed to amyloid-beta and in the cortex of aged rats. J Biol Chem 2012; 287:34709–34721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torfs CP, Velie EM, Oechsli FW, et al. A population-based study of gastroschisis: demographic, pregnancy, and lifestyle risk factors. Teratology 1994; 50:44–53. [DOI] [PubMed] [Google Scholar]
- Tortoriello G, Morris CV, Alpar A, et al. Miswiring the brain: Delta9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. EMBO J 2014; 33:668–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder MM, Reefhuis J, Caton AR, et al. Maternal periconceptional illicit drug use and the risk of congenital malformations. Epidemiology 2009; 20:60–66. [DOI] [PubMed] [Google Scholar]
- Van Gelder MMHJ, Donders ART, Devine O, et al. Using bayesian models to assess the effects of under-reporting of cannabis use on the association with birth defects, national birth defects prevention study, 1997-2005. Paediatr Perinat Epidemiol 2014; 28:424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gelder MMHJ, Reefhuis J, Caton AR, et al. Maternal periconceptional illicit drug use and the risk of congenital malformations. Epidemiology 2009; 20:60–66. [DOI] [PubMed] [Google Scholar]
- Van Went GF. Mutagenicity testing of 3 hallucinogens: LSD, psilocybin and delta 9-THC, using the micronucleus test. Experientia 1978; 34:324–325. [DOI] [PubMed] [Google Scholar]
- Vaz M, Hwang SY, Kagiampakis I, et al. Chronic cigarette smoke-induced epigenomic changes precede sensitization of bronchial epithelial cells to single-step transformation by KRAS mutations. Cancer Cell 2017; 32:360–376. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Hanscombe KB, Curtis CJ, et al. In search of genes associated with risk for psychopathic tendencies in children: a two-stage genome-wide association study of pooled DNA. J Child Psychol Psychiatry 2010; 51:780–788. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Han B, Compton WM, et al. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA 2019; 322:167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. Endocannabinoid mediates excitatory synaptic function of β-Neurexins. Commentary: β-neurexins control neural circuits by regulating synaptic endocannabinoid signaling. Front Neurosci 2016; 10:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yuan W, Li MD. Genes and pathways co-associated with the exposure to multiple drugs of abuse, including alcohol, amphetamine/methamphetamine, cocaine, marijuana, morphine, and/or nicotine: a review of proteomics analyses. Mol Neurobiol 2011; 44:269–286. [DOI] [PubMed] [Google Scholar]
- Watson CT, Szutorisz H, Garg P, et al. Genome-wide DNA methylation profiling reveals epigenetic changes in the rat nucleus accumbens associated with cross-generational effects of adolescent THC exposure. Neuropsychopharmacology 2015; 40:2993–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werler MM, Sheehan JE, Mitchell AA. Association of vasoconstrictive exposures with risks of gastroschisis and small intestinal atresia. Epidemiology 2003; 14:349–354. [PubMed] [Google Scholar]
- Willsher K. Baby arm defects prompt nationwide investigation in France. Guardian [serial online]. 2018. Available at: https://www.theguardian.com/world/2018/oct/31/baby-arm-defects-prompt-nationwide-investigation-france. Accessed November 3, 2018. [Google Scholar]
- Wilson PD, Loffredo CA, Correa-Villaseñor A, et al. Attributable fraction for cardiac malformations. Am J Epidemiol 1998; 148:414–423. [DOI] [PubMed] [Google Scholar]
- Wolff V, Schlagowski AI, Rouyer O, et al. Tetrahydrocannabinol induces brain mitochondrial respiratory chain dysfunction and increases oxidative stress: a potential mechanism involved in cannabis-related stroke. Biomed Res Int 2015; 2015:323706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian X, Tang L, Wu C, et al. miR-23b-3p and miR-130a-5p affect cell growth, migration and invasion by targeting CB1R via the Wnt/beta-catenin signaling pathway in gastric carcinoma. Onco Targets Ther 2018; 11:7503–7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wang H, Ren J, et al. cGAS is essential for cellular senescence. Proc Natl Acad Sci U S A 2017; 114:E4612–E4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Hegde VL, Rao R, et al. Histone modifications are associated with Delta9-tetrahydrocannabinol-mediated alterations in antigen-specific T cell responses. J Biol Chem 2014; 289:18707–18718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Tucker L, Alexeeff S, et al. Trends in self-reported and biochemically tested marijuana use among pregnant females in California from 2009-2016. JAMA 2017; 318:2490–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Shang G, Gui X, et al. Structural basis of STING binding with and phosphorylation by TBK1. Nature 2019; 567:394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CZ, Spektor A, Cornils H, et al. Chromothripsis from DNA damage in micronuclei. Nature 2015; 522:179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AM, Raj AY. Influence of cannabinoids on somatic cells in vivo. Pharmacology 1980; 21:277–287. [DOI] [PubMed] [Google Scholar]
- Zimmerman S, Zimmerman AM. Genetic effects of marijuana. Int J Addict 1990; 25:19–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


