Abstract
Background.
Men represent a small proportion of breast cancer diagnoses, and they are often excluded from clinical trials. Current treatments are largely extrapolated from evidence in women. We compare practice patterns between men and women with breast cancer following the publication of several landmark clinical trials in surgery.
Patients and Methods.
Patients with invasive breast cancer (2004–2015) from the National Cancer Data Base were identified; subcohorts were created based on eligibility for NSABP-B06, CALGB 9343, and ACOSOG Z0011. Practice patterns were stratified by gender and compared. Cox proportional hazards regression analyses were utilized to estimate the association between OS and gender.
Results.
Of the 1,664,746 patients identified, 99% were women and 1% were men. Among NSABP-B06 eligible men, mastectomy rates did not change (consistently ~ 80%), and their adjusted OS was minimally worse compared with women (HR 1.19, 95% CI 1.11–1.28). Following publication of CALGB 9343, omission of radiation after lumpectomy was less likely in men and lagged behind that of women, despite similar OS (male HR 0.92, 95% CI 0.59–1.44). Application of ACOSOG Z0011 findings resulted in deescalation of axillary surgery for men and women with comparable OS (male HR 0.69, 95% CI 0.33–1.45).
Conclusions.
Uptake of clinical trial results for men with breast cancer often mirrors that for women, despite exclusion from these studies. Furthermore, when study findings were applied to eligible patients, men and women demonstrated similar survival. Observational studies can help inform the potential application of study findings to this unique population and improve patient enrollment in clinical trials.
Keywords: Male breast cancer, Breast surgery, Breast radiation, Local–regional therapy
INTRODUCTION
Men with breast cancer account for < 1% of all breast cancer patients, and they are often excluded from clinical trials.1,2 Furthermore, most studies related to male breast cancer are based on older data and may not accurately reflect contemporary practice patterns.1–5 Current treatment strategies for men with breast cancer are largely extrapolated from evidence in women, as demonstrated in the recent guidelines published by the American Society of Clinical Oncology (ASCO).6 For women with breast cancer, three important practice-changing trials around local–regional management include National Surgical Adjuvant Breast and Bowel Project B-06 (NSABP-B06), Cancer and Leukemia Group B 9343 (CALGB 9343), and American College of Surgeons Oncology Group Z0011 (ACOSOG Z0011). For NSABP-B06, women with small (< 4 cm) breast cancers were randomized to total mastectomy, lumpectomy, or lumpectomy and radiation.7,8 Women who underwent mastectomy had similar outcomes to those who underwent lumpectomy and radiation.7 For CALGB 9343, elderly women with small, clinically node negative, estrogen-receptor-positive (ER+) breast cancer who underwent lumpectomy and received tamoxifen were randomized to receive either adjuvant radiation or no radiation.9,10 Although radiation receipt significantly decreased local recurrence rates, the absolute rates of recurrence were low in both groups. Regardless, there was no difference in overall survival.9 For ACOSOG Z0011, women with small (cT1–2), clinically node negative breast cancer who underwent lumpectomy and were found to have 1–2 positive sentinel nodes were randomized to receive an axillary lymph node dissection (ALND) or no further axillary surgery.11,12 Final analyses revealed no difference in survival in these two groups.11
Importantly, all of these studies excluded men, and although it has been assumed that the same data would apply to men, it has not been prospectively studied. It is also unclear whether providers have adopted these findings into their management strategy for men with breast cancer. As such, in an era where women with breast cancer are managed based on high-level evidence, we seek to explore how these same treatments are being applied to men with breast cancer. Specifically, we aim to compare contemporary practice patterns over time between men and women with breast cancer following the publication of three landmark local–regional clinical trials (NSABP-B06, CALGB 9343, and ACOSOG Z0011).
PATIENTS AND METHODS
Patients diagnosed with breast cancer between 2004 and 2015 from the National Cancer Data Base (NCDB, 2015 Participant User File) were selected. Patients with non-WHO-defined histology,13 noninvasive, or T0/Tis breast cancer were excluded. Anatomic and prognostic stage groups were based on the American Joint Committee on Cancer Staging Manual, 8th edition.14 The cohort was stratified by gender, and subcohorts were created generally based on trial eligibility for NSABP-B068 CALGB 9343,10 and ACOSOG Z0011.11 Additional exclusion criteria were applied to each subgroup analysis as specified below, in alignment with those used for the original clinical trial inclusion/exclusion criteria.
For the NSABP-B06 subgroup analysis, we included those with clinical stage T1–2, N0–1, M0 invasive breast cancer; those who underwent lumpectomy must have received adjuvant radiation; those who received neoadjuvant therapies were excluded; any type of axillary nodal surgery was allowed. For the CALGB 9343 subgroup analysis, we included those aged ≥ 70 years with clinical stage T1, N0, M0, ER+ invasive breast cancer who underwent lumpectomy and received adjuvant endocrine therapy; those with missing radiation information or who received neoadjuvant therapies were excluded; any type of axillary nodal surgery was allowed. For the ACOSOG Z0011 subgroup analysis, we included those with clinical stage T1–2, N0, M0 invasive breast cancer who underwent lumpectomy and had at least 1 lymph node removed at the time of surgery; those who were found to have 1–2 positive lymph nodes at the time of surgery were included; those who received neoadjuvant therapies were excluded. Based on prior work,15 sentinel lymph node biopsy (SLNB) was defined as removal of ≤ 5 lymph nodes; ALND was defined as removal of ≥ 10 lymph nodes; and those with 6–9 lymph nodes removed were excluded from this subgroup analysis to minimize assumptions regarding the surgeon’s intent of the axillary procedure.
Patient characteristics were summarized, with N (%) for categorical variables and median (interquartile range) for continuous variables for all patients. The Chi square test was used to compare categorical variables, and t-tests were used to compare continuous variables. Patient characteristics were also compared within stage I–III, stage IV, and within each subcohort for the three trials, separately. After stratifying by gender, practice patterns were compared over time.
Overall survival (OS) was defined as the time from diagnosis to death or last follow-up. Kaplan–Meier (K–M) curves were used to visualize unadjusted OS, and the log-rank test was used to test for a difference between genders. Cox proportional hazards regression analyses were utilized to estimate the association between OS and gender, after adjustment for known covariates, including year of diagnosis (2004–2009 vs 2010–2014), facility type/location, patient age, insurance status, race/ethnicity, Charlson/Deyo comorbidity score, tumor histology, size, grade, number of positive lymph nodes, ER status, progesterone receptor (PR) status, human-epidermal-growth-factor-receptor-2 (HER2) status, type of breast surgery, chemotherapy receipt, radiation receipt, and endocrine therapy receipt. The analysis was repeated for different stages and for each clinical trial subcohort.
A p value < 0.05 was considered statistically significant, and no adjustments were made for multiple comparisons. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.5.0. Due to use of deidentified data, our institutional review board granted the study exempt status.
RESULTS
Of the 1,664,746 patients identified, 99% were women (N = 1,648,070) and 1% were men (N = 16,676) (Supplementary Fig. 1). The median follow-up was 68.1 months. Compared with women, men were diagnosed at an older age (median 66 years vs 61 years, p < 0.001), and correspondingly, men tended to have higher Charlson/Deyo comorbidity scores (score ≥ 1: 20.1% in men vs 14.9% in women, p < 0.001). Men presented with larger tumors (median 2 vs 1.6 cm, p < 0.001), higher nodal stage (clinically node positive 24% vs 19.6%, p < 0.001), and higher grade tumors (grade 2/3, 85.7% vs 77.7%, p < 0.001), despite the finding that triple-negative breast cancer (TNBC) and HER2+ cancers were less common in men (TNBC 4.7% vs 12.7%; HER2+ 12.4% vs 14.8%; p < 0.001). Interestingly however, median Oncotype DX recurrence scores were similar (15 vs 16, p = 0.02). Men were more likely to be diagnosed with de novo metastatic disease (11.4% vs 7.6%, p < 0.001) (Table 1; Supplementary Table 1).
TABLE 1.
Summary of select patient demographics and tumor/disease characteristics. Analysis based on a cohort of men and women with invasive breast cancer from the National Cancer Data Base (2004–2015). Data presented as N (%) or median (IQR)
| All patients N = 1,664,746 100.0% |
Female N = 1,648,070 99.0% |
Male N = 16,676 1.0% |
p-Value | |
|---|---|---|---|---|
| Follow-up (months) | ||||
| Median (IQR) | 68.1 (40.0–100.3) | 68.1 (40.0–100.4) | 67.0 (38.8–98.7) | |
| Age (years) | <0.001 | |||
| Median (IQR) | 61 (51–71) | 61 (51–71) | 66 (56–75) | |
| Race/ethnicity | <0.001 | |||
| Non-Hispanic White | 1,240,882 (79.5%) | 1,228,247 (79.5%) | 12,635 (81.2%) | |
| Non-Hispanic Black | 176,016 (11.3%) | 174,093 (11.3%) | 1,923 (12.4%) | |
| Non-Hispanic other | 60,061 (3.8%) | 59,660 (3.9%) | 401 (2.6%) | |
| Hispanic | 83,566 (5.4%) | 82,969 (5.4%) | 597 (3.8%) | |
| Charlson/deyocomorbidityscore | <0.001 | |||
| 0 | 1,406,310 (85.0%) | 1,393,146 (85.1%) | 13,164 (79.9%) | |
| 1 | 208,768 (12.6%) | 206,106 (12.6%) | 2662 (16.2%) | |
| ≥ 2 | 38,508 (2.3%) | 37,868 (2.3%) | 640 (3.9%) | |
| Histology | <0.001 | |||
| Ductal | 1,465,280 (88.0%) | 1,449,381 (87.9%) | 15,899 (95.3%) | |
| Lobular | 174,046 (10.5%) | 173,464 (10.5%) | 582 (3.5%) | |
| Other | 25,420 (1.5%) | 25,225 (1.5%) | 195 (1.2%) | |
| Tumorsize(mm) | <0.001 | |||
| Median (IQR) | 17 (10–27) | 16 (10–27) | 20 (14–30) | |
| PositiveLNs | <0.001 | |||
| Median (IQR) | 2 (1–4) | 2 (1–4) | 2 (1–5) | |
| Oncotype DX recurrence score | 0.02 | |||
| Median (IQR) | 16 (11–22) | 16 (11–22) | 15 (9–24) | |
| Oncotype DX recurrence score with patients diagnosed after 2010 0.02 | 0.02 | |||
| Median (IQR) | 16 (11–22) | 16 (11–22) | 15 (9–24) | |
| Grade | <0.001 | |||
| 1 | 344,002 (22.2%) | 341,769 (22.3%) | 2233 (14.3%) | |
| 2 | 669,072 (43.2%) | 661,283 (43.1%) | 7789 (50.0%) | |
| 3 | 537,317 (34.7%) | 531,751 (34.6%) | 5566 (35.7%) | |
| Molecularsubtypes by receptors | <0.001 | |||
| HR+/HER2− | 638,490 (72.6%) | 631,178 (72.5%) | 7312 (82.8%) | |
| HER2+ | 129,920 (14.8%) | 128,822 (14.8%) | 1098 (12.4%) | |
| TNBC | 110,822 (12.6%) | 110,403 (12.7%) | 419 (4.7%) | |
| Anatomicstagegroups | ||||
| I | 691,354 (56.3%) | 686,273 (56.4%) | 5081 (42.8%) | <0.001 |
| II | 361,612 (29.4%) | 357,125 (29.4%) | 4487 (37.8%) | |
| III | 95,644 (7.8%) | 94,445 (7.8%) | 1199 (10.1%) | |
| IV | 79,818 (6.5%) | 78,715 (6.5%) | 1103 (9.3%) | |
| Prognosticstagegroup | ||||
| I | 781,087 (74.6%) | 774,586 (74.7%) | 6501 (66.9%) | <0.001 |
| II | 129,997 (12.4%) | 128,478 (12.4%) | 1519 (15.6%) | |
| III | 55,756 (5.3%) | 55,167 (5.3%) | 589 (6.1%) | |
| IV | 79,818 (7.6%) | 78,715 (7.6%) | 1103 (11.4%) |
Frequency may not add up to total because of missing values. Percentages are of the total reported and are based on the non-missing cohort of each variable
IQR interquartile range, LN lymph nodes, HR hormone receptor, HER2 human epidermal growth factor receptor 2, TNBC triple-negative breast cancer
Men were significantly more likely to undergo mastectomy (70.1% vs 39.7%, p < 0.001) and more extensive axillary surgery (≥ 10 lymph nodes examined, 36.3% vs 24.9%, p < 0.001). Men were less likely to receive postlumpectomy radiation (66.4% vs 81.9%), although rates of postmastectomy radiation were similar between men and women (27.9% vs 28.2%). Rates of chemotherapy (42.5% vs 45%) and endocrine therapy if hormone-receptor-positive (HR+ 48.8% vs 51.9%) were also similar between genders, but men were less likely to undergo neoadjuvant chemotherapy (6.8% vs 11.1%, p < 0.001) (Table 2).
TABLE 2.
Summary of treatments and trial eligibility. Analysis based on a cohort of men and women with invasive breast cancer from the National Cancer Data Base (2004–2015). Data presented as N (%)
| All patients N = 1,664,746 100.0% |
Female N = 1,648,070 99.0% |
Male N = 16,676 1.0% |
p-Value | |
|---|---|---|---|---|
| Breast surgerytype | <0.001 | |||
| Lumpectomy | 874,135 (52.5%) | 870,630 (52.8%) | 3505 (21.0%) | |
| Mastectomy | 666,636 (40%) | 654,947 (39.7%) | 11,689 (70.1%) | |
| No surgery | 118,956 (7.1%) | 117,522 (7.1%) | 1434 (8.6%) | |
| Other surgery | 177 (0.0%) | 177 (0.0%) | 0 (0%) | |
| Unknown | 4842 (0.3%) | 4794 (0.3%) | 48 (0.3%) | |
| HadLNsexamined | <0.001 | |||
| Yes | 1,460,780 (88.4%) | 1,446,372 (88.4%) | 14,408 (87.1%) | |
| No or unknown | 191,919 (11.6%) | 189,785 (11.6%) | 2134 (12.9%) | |
| Extentofaxillarysurgery | <0.001 | |||
| LNs1–5 | 900,727 (63.2%) | 893,665 (63.3%) | 7062 (50.2%) | |
| LNs6–9 | 168,497 (11.8%) | 166,600 (11.8%) | 1897 (13.5%) | |
| ≥ 10 LNs | 356,933 (25.0%) | 351,818 (24.9%) | 5115 (36.3%) | |
| Receivedany radiationtherapy | <0.001 | |||
| No | 723,975 (44.0%) | 713,398 (43.7%) | 10,577 (64.4%) | |
| Yes | 923,166 (56.0%) | 917,318 (56.3%) | 5848 (35.6%) | |
| Local-regionaltherapy | <0.001 | |||
| Lumpectomy alone | 152,680 (10.0%) | 151,553 (10.0%) | 1127 (7.5%) | |
| Mastectomy alone | 470,784 (30.8%) | 462,519 (30.6%) | 8265 (55.2%) | |
| Lumpectomy + radiation | 715,406 (46.9%) | 713,079 (47.2%) | 2327 (15.5%) | |
| Mastectomy + radiation | 187,969 (12.3%) | 184,704 (12.2%) | 3265 (21.8%) | |
| Receivedany chemotherapy | <0.001 | |||
| No | 888,740 (55.1%) | 879,526 (55%) | 9214 (57.5%) | |
| Yes | 725,593 (44.9%) | 718,775 (45%) | 6818 (42.5%) | |
| Timing of chemotherapy | <0.001 | |||
| Adjuvant chemo only | 476,110 (31.0%) | 471,071 (31.0%) | 5039 (33.0%) | |
| Neoadjuvant chemo (± adjuvant) | 169,378 (11.0%) | 168,345 (11.1%) | 1033 (6.8%) | |
| No chemo | 888,740 (57.9%) | 879,526 (57.9%) | 9214 (60.3%) | |
| Received endocrine therapy based on HR status | <0.001 | |||
| Endocrine if HR+ | 837,139 (51.9%) | 829,361 (51.9%) | 7778 (48.8%) | |
| No endocrine if HR+ | 474,588 (29.4%) | 467,577 (29.3%) | 7011 (44%) | |
| Endocrine if HR− | 8768 (0.5%) | 8726 (0.5%) | 42 (0.3%) | |
| No endocrine if HR− | 293,787 (18.2%) | 292,679 (18.3%) | 1108 (7%) | |
| NASBP-B06 eligibility | 0.26 | |||
| No | 947,818 (56.9%) | 938,252 (56.9%) | 9566 (57.4%) | |
| Yes | 716,928 (43.1%) | 709,818 (43.1%) | 7110 (42.6%) | |
| CALGB 9343eligibility | <0.001 | |||
| No | 1,578,355 (94.8%) | 1,561,943 (94.8%) | 16,412 (98.4%) | |
| Yes | 86,391 (5.2%) | 86,127 (5.2%) | 264 (1.6%) | |
| ACOSOG Z0011eligibility | <0.001 | |||
| No | 1,617,977 (97.2%) | 1,601,474 (97.2%) | 16,503 (99%) | |
| Yes | 46,769 (2.8%) | 46,596 (2.8%) | 173 (1.0%) |
Frequency may not add up to total because of missing values. Percentages are of the total reported and are based on the nonmissing cohort of each variable
LN lymph node, HR hormone receptor
Compared with women, men had a worse unadjusted OS within each clinical anatomic stage, although the difference for those with stage IV disease was small (Supplementary Fig. 2). Similar findings were noted when stratified by clinical prognostic stages (men generally had a worse unadjusted OS), except for those with stage III disease, whose OS was not significantly different (Supplementary Fig. 3).
For the NSABP-B06 subgroup analysis, a similar proportion of men and women were eligible based on the inclusion criteria (42.6% vs 43.1%, p = 0.26; Table 2). However, the men tended to be older than the women (median age 66 years vs 61 years, p < 0.001), had larger tumors (median tumor size 19 mm vs 15 mm, p < 0.001), and had more lymph node involvement (median number of positive nodes 4 vs 3, p < 0.001; Supplementary Table 2). Among eligible men, mastectomy rates did not significantly change following publication of the 20-year follow-up results in 2002 (consistently ~ 80%; Fig. 1a). When stratified by gender, the unadjusted OS rates were slightly worse for men with breast cancer compared with women (Fig. 2a). However, when stratified by gender and local–regional management (surgery ± radiation), the unadjusted OS was similar for men and women undergoing breast conservation (5 years OS 0.93 vs 0.93; Fig. 2b). After adjustment, men eligible for NSABP-B06 had a slightly worse OS compared with women (HR 1.19, 95% CI 1.11–1.28, p < 0.001; Table 3).
FIG. 1.
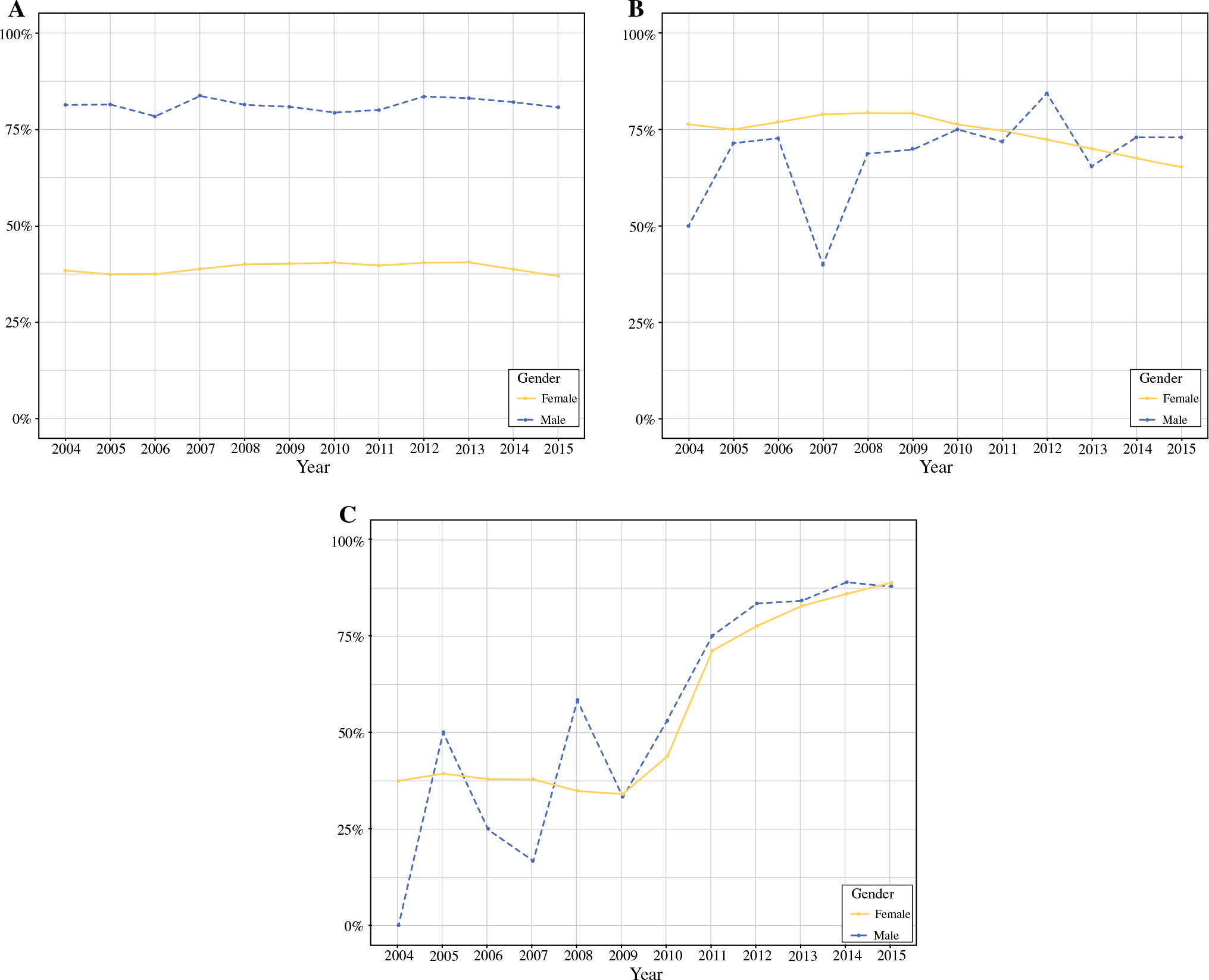
Uptake of three landmark clinical trials in surgery and comparison of practice patterns over time between men and women with invasive breast cancer who were potentially eligible for a NSABP-B06 (initial results published 1985, longer follow-up published 2002), b CALGB 9343 (initial results published 2004, longer follow-up published 2013), and ≥ ACOSOG Z0011 (initial results published 2011, longer follow-up published 2017). Analysis based on a cohort of men and women with invasive breast cancer from the National Cancer Data Base (2004–2015). SLNB sentinel lymph node biopsy
FIG. 2.
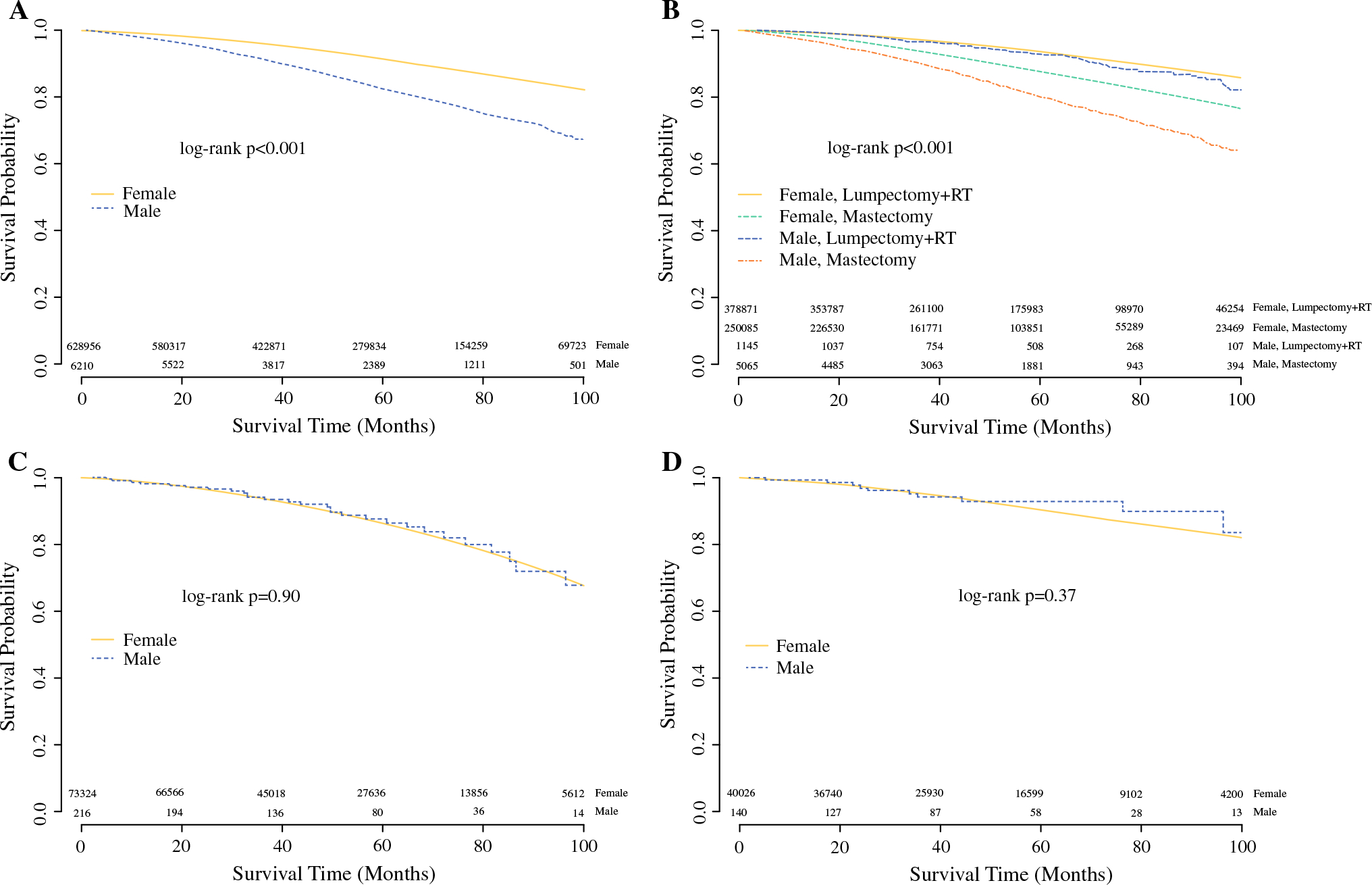
Overall survival for men and women “eligible” for landmark clinical trials: a NSABP-B06 eligible patients, b NSABP-B06 eligible patients stratified by local–regional treatment, c CALGB 9343 eligible patients, and d ACOSOG Z0011 eligible patients. Analysis based on a cohort of men and women with invasive breast cancer from the National Cancer Data Base (2004–2015). RT radiation therapy
TABLE 3.
Cox proportional hazard model of overall survival for three clinical trials (NSABP-B06 patients; N = 499,190, Event = 48,120); (CALGB 9343 patients; N = 54,644, Event = 7089); (ACOSOG Z0011; N = 32,204, Event = 3139). Models adjusted for year of diagnosis (2004–2009 or 2010–2014), facility type/location, patient age, insurance status, race/ethnicity, Charlson/Deyo comorbidity score, tumor histology, size, grade, number of positive lymph nodes, estrogen receptor status, progesterone receptor status, human epidermal growth factor receptor 2 status, type of breast surgery, chemotherapy receipt, radiation receipt, and endocrine therapy receipt
| HR (95% CI) | p value | Overall p value | |
|---|---|---|---|
| NSABP-B06 eligible patients | |||
| Female | -REF- | <0.001 | |
| Male | 1.19 (1.11–1.28) | <0.001 | |
| CALGB 9343 eligible patients | |||
| Female | -REF- | 0.72 | |
| Male | 0.92 (0.59–1.44) | 0.72 | |
| ACOSOG Z0011 eligible patients | |||
| Female | -REF- | 0.33 | |
| Male | 0.69 (0.33–1.45) | 0.33 | |
Men were less likely than women to meet inclusion criteria for the CALGB 9343 subgroup analysis (1.6% vs 5.2%, p < 0.001; Table 2). However, the median age, tumor size, and number of positive lymph nodes were similar between men and women (Supplementary Table 3). Following publication of the early results from CALGB 9343 in 2004, omission of radiation therapy after lumpectomy was less likely in men and lagged behind that of women (Fig. 1b). However, men and women had a similar unadjusted OS (5 years OS 0.88 vs 0.86; Fig. 2c). After adjustment, the OS remained similar for men and women [women: reference (REF); men: HR 0.92, 95% CI 0.59–1.44, p = 0.72; Table 3].
For the ACOSOG Z0011 subgroup analysis, men were less likely than women to meet the study inclusion criteria (1% vs 2.8%; p < 0.001; Table 2). The median age at diagnosis, tumor size, and number of positive lymph nodes were similar between men and women (Supplementary Table 4). The application of ACOSOG Z0011 trial findings resulted in deescalation of axillary surgery, a trend that was similar between men and women (Fig. 1c). Furthermore, the unadjusted OS was comparable between men and women (5 years OS 0.93 vs 0.90, p = 0.37; Fig. 2d), which remained true after adjustment (women: REF; men: HR 0.69, 95% CI 0.33–1.45, p = 0.33; Table 3).
DISCUSSION
For men and women with breast cancer, we sought to compare changes in practice patterns following publication of three landmark local–regional clinical trials (NSABP-B06, CALGB 9343, and ACOSOG Z0011). After release of the long-term follow-up from NSABP-B06, men continued to undergo mastectomy at similar rates, despite a proven survival benefit, although this may be influenced by patient preferences as well. This contrasts with the observed changes in practice patterns after publication of CALGB 9343 and ACOSOG Z0011. Although practice patterns lagged for men, we did observe a similar change in radiation omission after lumpectomy for men and women eligible for CALGB 9343. For those eligible for ACOSOG Z0011, we noted a similar rate of deescalation of axillary surgery between men and women. Furthermore, our survival analysis showed no significant differences in OS between trial-eligible men and women for CALGB 9343 and ACOSOG Z0011. The analysis of the NSABP-B06 trials in the male breast cancer population demonstrated a slightly worse OS compared with women. Importantly, however, there did not appear to be a significant difference in OS between the breast-conserving therapy (BCT) cohorts. Overall, our findings highlight a potential larger issue—men with breast cancer are often excluded unnecessarily from potentially practice-changing and/or life-saving therapies until results are published. Furthermore, the routine uptake of evidence into clinical practice may take years in the best circumstances,16 and this may impact outcomes for men with breast cancer, although additional studies would be needed to explore this potential association.
Surgical resection is the most common treatment for nonmetastatic breast cancer in men and women.17 In our male cohort, 70.1% of men underwent mastectomy, which aligns with multiple studies reporting that men most often receive mastectomy versus BCT, despite ASCO and National Comprehensive Cancer Network (NCCN) guidelines that suggest the equivalence of mastectomy and BCT for many patients with breast cancer, regardless of gender.3,17–23 Notably different than would be expected for women with breast cancer, the results of the International Male Breast Cancer Program demonstrated that 45% of men who underwent lumpectomy did not receive adjuvant radiation (regardless of nodal status), and 30.7% of men who underwent mastectomy and had node positive disease did not receive radiation.20 Furthermore, a systematic review of breast-conservation surgery (BCS) noted that a wide range (12–100%) of men receive radiation following lumpectomy,24 while our data noted 67% of men received radiotherapy after lumpectomy. Similarly, a recent review of literature on radiotherapy compliance after breast-conserving surgery noted a range of 27–86% based on one prospective and nine retrospective cohort studies.25 To date, data comparing the benefits of mastectomy versus lumpectomy with radiation in the male breast cancer population remains lacking. In 2019, Bateni et al. retrospectively analyzed 8445 men with stage I–II breast cancer and aimed to determine whether the NSABP-B06 trial could be replicated in a male breast cancer population. In this study, BCT was associated with an improved OS compared with mastectomy, and further subanalysis revealed that this difference was only significant in men with T2 and stage II disease.26 Similarly, our analysis also revealed an OS benefit favoring BCT over mastectomy in the male population. However, our analysis of eligible men and women demonstrated that men had a slightly worse adjusted OS compared with women, although there were several important differences in the patient populations (men were older with higher comorbidity scores, larger tumors, and more positive nodes, similar to other studies),27 which may account for some of the survival differences and were not able to be fully adjusted for on the multivariable model. It is also important to note that fewer men may have had enough breast tissue to be considered for a lumpectomy, and more men may have chosen to undergo mastectomy (patient preference), neither of which could be accounted for in our data set. While the NCDB does not contain data related to the reasoning for treatment decisions, numerous reasons likely factored into why men consistently underwent mastectomy over time—concerns related to radiation compliance, cosmetic outcomes, recovery, etc.
Elderly men with breast cancer, as with elderly women with breast cancer, often present with larger tumors and more biologically favorable disease (ER/PR+) than their younger counterparts.28,29 For elderly women, the CALGB 9343 trial demonstrated no difference in OS for women ≥ 70 years with stage I disease who received lumpectomy and tamoxifen, regardless of radiotherapy receipt. In comparison, Tural et al. retrospectively reviewed 99 men with breast cancer, of which 51 were > 65 years. In the elderly population (> 65 years), only 33% received BCS, 82% received radiotherapy, and 34% received endocrine therapy.28 This suggests that many elderly men are receiving radiotherapy, which may be attributable to the large tumor size and more advanced disease. Looking at the male breast cancer population as a whole, Wang et al. Demonstrated that, in men with stage I–III, ER+/HER2− disease, radiotherapy did not improve survival.30 These results, although extended to those with stages II and III disease, align with the data from our analysis, where omission of radiotherapy after lumpectomy in men eligible for CALGB 9343 yielded similar survival outcomes to those observed in women. Some studies have equated male breast cancer to postmenopausal female breast cancer,31 which may explain the similar survival outcomes between elderly men and women on our analysis.
While the most common surgical procedure for men with breast cancer is still a modified radical mastectomy, SLNB is possible and preferred for many patients.32,33 Similar to those for women, the NCCN guidelines recommend SLNB be performed in men with a clinically node negative axilla.23 However, the International Male Breast Cancer Program noted that only 17.9% of men underwent a SLNB, although this did increase over time.20 For women, the ACOSOG Z0011 trial demonstrated that ALND could be omitted if tumors were small, clinically node negative, and SLNB only revealed 1–2 positive nodes.11,12 However, Vaysee et al. used two nomograms (validated in female populations) to demonstrate that the predictive factors of axillary lymph node metastasis used in female breast cancer were not valid in male breast cancer.34 Although this may be related to differences in the underlying tumor biology, it may not have implications for the surgical management of the axilla; For example, our analysis of ACOSOG Z0011 eligible patients showed that the OS for men was comparable to women, suggesting that, although the causes of nodal metastasis may differ by gender, the management may still be similar for similar stages of disease.
In general, the OS for men with breast cancer has been shown to be worse than the OS in women with breast cancer.1,4,35 In our study, we found that the adjusted OS for men eligible for CALGB 9343 and ACOSOG Z0011 did not significantly differ from similarly eligible women, although there was a slightly worse OS for men eligible for NSABP-B06. Some of the differences between other studies and our findings likely relate to differences in the populations being studied. Regardless, our findings generally suggest that men and women with breast cancer who have the same characteristics (demographics, tumor features, disease stage, and treatments received) will have similar outcomes. With male breast cancer often diagnosed at a later age and more advanced stage, questions arise as to whether the worse survival is due to lack of screening and early treatment or whether male breast cancer is biologically different.35 Proponents for male breast cancer being a different disease have shown that the distribution of tumor subtypes is different for men and women, and molecularly, these two cancers are different.35,36 However, Foerster et al. used a matched-pair analysis to conclude that the 5-year OS and disease-free survival were no different between men and women.37 In addition, recent studies have suggested that the distribution of scores from genomic testing may be similar between men and women with breast cancer.38 In contrast, others have demonstrated a significant difference in survival between men and women,39 which may be related to some of the consistently observed differences in disease stage at presentation in our study and others.27. To the best of the authors’ knowledge, the current study is one of the largest to date to compare survival outcomes between men and women with breast cancer, and we demonstrate that OS is comparable for most men and women with similar demographics and disease characteristics undergoing similar treatments.
As with any retrospective study using national data-bases, many of our study limitations are inherent to the NCDB.40 For example, the NCDB only captures OS and does not include breast cancer specific survival. The short follow-up may also limit the generalizability of our findings. In addition, the version of NCDB data (2015 PUF) used for this study did not specifically record the surgeon’s intent for axillary surgery (if the planned surgery was SLNB or ALND), and thus, the type of axillary surgery performed was based on the number of nodes removed, as done with prior research.15 Furthermore, we used current guidelines41 and data from prior studies on SLNB11,42 to support these definitions in particular. Although we did not know the surgeon’s intent for any of the surgeries (SLNB vs ALND), the specified ranges seemed reasonable for those with 1–5 and ≥ 10 nodes removed; however, for those with 6–9 nodes removed, we did not feel that they could be reasonably included in either subgroup, and thus, we elected to exclude these patients, which may have introduced some bias into our results. Data on adjuvant therapies, such as chemotherapy and radiation, are also not as accurately recorded.43. Furthermore, for patients with “missing” radiation information, those patients may or may not have received radiation, and excluding them from our analyses may have introduced unintended bias. Regardless, using the NCDB does allow for evaluation of a large population of men with breast cancer (> 16,000 in this study), and its data represents 70–80% of all breast cancer patients diagnosed in the USA.44.
In conclusion, the uptake of findings from landmark clinical trials in surgery for men with breast cancer often mirrors that for women, despite exclusion from these studies. Furthermore, when study findings are applied to similarly eligible patients, men and women demonstrate similar survival outcomes. Although retrospective studies are not the ideal research paradigm, prospective randomized controlled trials in men with breast cancer are typically not feasible or realistic. As such, observational studies can help inform or confirm the potential application of study findings to this unique population. Furthermore, the inclusion of men in clinical trials should be strongly considered for those patients meeting the other specified inclusion criteria, as delays in adopting new therapies for this population could potentially impact oncologic outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the deidentified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
FUNDING Dr. O. Fayanju is supported by the National Institutes of Health (NIH) under Award Number 1K08CA241390 (PI: Fayanju). This work was in part supported by Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan) for the Biostatistics Core.
Footnotes
DISCLOSURES : The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article. Dr. J. Plichta is a recipient of research funding by the Color Foundation (PI: Plichta). She serves on the NCCN Breast Cancer Screening Committee and the ASCO Clinical Practice Guideline Committee for the Management of Male Breast Cancer. Dr. E.S. Hwang serves on the NCI Breast Cancer Steering Committee and the NCCN Breast Cancer Prevention Committee. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Samantha Thomas: Consulting work with Abbvie, Inc, on biosimilar/bioequivalence. Unrelated to this work. Relationship complete effective January 2019. Jeremy Force: METAVIVOR Early Career Investigator Award.
Accepted for presentation at the American Society of Breast Surgeons’ Annual Meeting in May 2020.
Electronic supplementary material The online version of this article (https://doi.org/10.1245/s10434-020-08901-z) contains supplementary material, which is available to authorized users.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Greif JM, Pezzi CM, Klimberg VS, Bailey L, Zuraek M. Gender differences in breast cancer: analysis of 13,000 breast cancers in men from the National Cancer Data Base. Ann Surg Oncol. 2012;19(10):3199–3204. [DOI] [PubMed] [Google Scholar]
- 2.Giordano SH. Breast cancer in men. N Engl J Med. 2018;378(24):2311–2320. [DOI] [PubMed] [Google Scholar]
- 3.Fields EC, DeWitt P, Fisher CM, Rabinovitch R. Management of male breast cancer in the United States: a surveillance, epidemiology and end results analysis. Int J Radiat Oncol Biol Phys. 2013;87(4):747–752. [DOI] [PubMed] [Google Scholar]
- 4.Liu N, Johnson KJ, Ma CX. Male breast cancer: an updated surveillance, epidemiology, and end results data analysis. Clin Breast Cancer. 2018;18(5):e997–e1002. [DOI] [PubMed] [Google Scholar]
- 5.Fentiman IS. Surgical options for male breast cancer. Breast Cancer Res Treat. 2018;172(3):539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassett MJ, Somerfield MR, Baker ER, et al. Management of male breast cancer: ASCO guideline. J Clin Oncol. 2020:Jco1903120. [DOI] [PubMed] [Google Scholar]
- 7.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312(11):665–673. [DOI] [PubMed] [Google Scholar]
- 9.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes KS, Schnaper LA, Berry D, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351(10):971–977. [DOI] [PubMed] [Google Scholar]
- 11.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO/IARC Classification of Tumours. Vol 4 4 ed: World Health Organization; 2012. [Google Scholar]
- 14.AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer International Publishing; 2016. [Google Scholar]
- 15.Park TS, Thomas SM, Rosenberger LH, et al. The association of extent of axillary surgery and survival in women with N2–3 invasive breast cancer. Ann Surg Oncol. 2018;25(10):3019–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuttle TM, Rueth NM, Abbott A, Virnig BA. United States trends in the surgical treatment of primary breast cancer. World J Surg. 2012;36(7):1475–1479. [DOI] [PubMed] [Google Scholar]
- 17.Sarmiento S, McColl M, Musavi L, et al. Male breast cancer: a closer look at patient and tumor characteristics and factors that affect survival using the National Cancer Database. Breast Cancer Res Treat. 2020;180(2):471–479. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman A, Ben Ishay O, Horesh N, et al. Breast cancer in men: a single center experience over a period of 22 years. Isr Med Assoc J. 2020;22(3):160–163. [PubMed] [Google Scholar]
- 19.Pellini F, Granuzzo E, Urbani S, et al. Male breast cancer: surgical and genetic features and a multidisciplinary management strategy. Breast Care (Basel). 2020;15(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardoso F, Bartlett JMS, Slaets L, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG international male breast cancer program. Ann Oncol. 2018;29(2):405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassett MJ, Somerfield MR, Giordano SH. Management of male breast cancer: ASCO guideline summary. J Oncol Pract. 2020:JOP.19.00792. [DOI] [PubMed] [Google Scholar]
- 22.Ruddy KJ, Winer EP. Male breast cancer: risk factors, biology, diagnosis, treatment, and survivorship. Ann Oncol. 2013;24(6):1434–1443. [DOI] [PubMed] [Google Scholar]
- 23.Network NCC. Breast Cancer (Version 3.2020). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 21 Apr 21 2020.
- 24.De La Cruz LM, Thiruchelvam PTR, Shivani J, Trina J, Blankenship SA, Fisher CS. Saving the male breast: a systematic literature review of breast-conservation surgery for male breast cancer. Ann Surg Oncol. 2019;26(12):3939–3944. [DOI] [PubMed] [Google Scholar]
- 25.Sauder CAM, Bateni SB, Davidson AJ, Nishijima DK. Breast conserving surgery compared with mastectomy in male breast cancer: a brief systematic review. Clin Breast Cancer. 2020;20(3):e309–e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bateni SB, Davidson AJ, Arora M, et al. Is breast-conserving therapy appropriate for male breast cancer patients+ A national cancer database analysis. Ann Surg Oncol. 2019;26(7):2144–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010;28(12):2114–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tural D, Selcukbiricik F, Aydogan F, et al. Male breast cancers behave differently in elderly patients. Jpn J Clin Oncol. 2013;43(1):22–27. [DOI] [PubMed] [Google Scholar]
- 29.Gennari R, Curigliano G, Rotmensz N, et al. Breast carcinoma in elderly women: features of disease presentation, choice of local and systemic treatments compared with younger postmenopasual patients. Cancer. 2004;101(6):1302–1310. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Sun Y, Qu J, et al. Survival analysis for male ductal and lobular breast cancer patients with different stages. Future oncol (London, England). 2019;15(2):167–180. [DOI] [PubMed] [Google Scholar]
- 31.Liukkonen S, Saarto T, Maenpaa H, Sjostrom-Mattson J. Male breast cancer: a survey at the Helsinki University Central Hospital during 1981–2006. Acta Oncol. 2010;49(3):322–327. [DOI] [PubMed] [Google Scholar]
- 32.Losurdo A, Rota S, Gullo G, et al. Controversies in clinico-pathological characteristics and treatment strategies of male breast cancer: a review of the literature. Crit Rev Oncol Hematol. 2017;113:283–291. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Marcuartu JJ, Alvarez-Perez RM, Sousa Vaquero JM, Jimenez-Hoyuela Garcia JM. Selective sentinel lymph node biopsy in male breast cancer. Rev Esp Med Nucl Imagen Mol. 2018;37(3):146–150. [DOI] [PubMed] [Google Scholar]
- 34.Vaysse C, Sroussi J, Mallon P, et al. Prediction of axillary lymph node status in male breast carcinoma. Ann Oncol. 2013;24(2):370–376. [DOI] [PubMed] [Google Scholar]
- 35.Gucalp A, Traina TA, Eisner JR, et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res Treat. 2019;173(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moelans CB, de Ligt J, van der Groep P, et al. The molecular genetic make-up of male breast cancer. Endocr Relat Cancer. 2019;26(10):779–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foerster R, Foerster FG, Wulff V, et al. Matched-pair analysis of patients with female and male breast cancer: a comparative analysis. BMC Cancer. 2011;11:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams AD, McGreevy CM, Tchou JC, De La Cruz LM. Utility of Oncotype DX in male breast cancer patients and impact on chemotherapy administration: a comparative study with female patients. Ann Surg Oncol. 2020. 10.1245/s10434-020-08473-y. [DOI] [PubMed] [Google Scholar]
- 39.Iorfida M, Bagnardi V, Rotmensz N, et al. Outcome of male breast cancer: a matched single-institution series. Clin Breast Cancer. 2014;14(5):371–377. [DOI] [PubMed] [Google Scholar]
- 40.Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3(12):1722–1728. [DOI] [PubMed] [Google Scholar]
- 41.Gradishar WJ, Anderson BO, Abraham J, et al. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Online 3/14/2019 2019. [Google Scholar]
- 42.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11(10):927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mallin K, Palis BE, Watroba N, et al. Completeness of American Cancer Registry Treatment Data: implications for quality of care research. J Am Coll Surg. 2013;216(3):428–437. [DOI] [PubMed] [Google Scholar]
- 44.Mallin K, Browner A, Palis B, et al. Incident cases captured in the National Cancer Database compared with those in U.S. population based central cancer registries in 2012–2014. Ann Surg Oncol. 2019;26(6):1604–1612. 10.1245/s10434-019-07213-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


