Abstract
Catecholamine neurotransmission plays a key role in regulating a variety of behavioral and physiologic processes, and its dysregulation is implicated in both neurodegenerative and neuropsychiatric disorders. Over the last four decades, in vivo electrochemistry has enabled the discovery of contrasting catecholamine regulation in the brain. These rapid and spatially resolved measurements have been conducted in brain slices, and in anesthetized and freely behaving animals. In this review, we describe the methods enabling in vivo measurements of dopamine and norepinephrine, and subsequent findings regarding their release and regulation in intact animals. We thereafter discuss key studies in awake animals, demonstrating that these catecholamines are not only differentially regulated, but are released in opposition of each other during appetitive and aversive stimuli.
I. Introduction
The catecholamine neurotransmitters dopamine and norepinephrine modulate a variety of behavioral and physiologic processes through their actions on postsynaptic receptors, and their dysregulation underlies the pathophysiology of many disease states. Deficits in dopamine and norepinephrine are a hallmark of neurodegenerative disorders such as Alzheimer’s (Weinshenker, 2008) and Parkinson’s disease (Schapira, 2009), and adaptations in catecholamine signaling are thought to contribute to the development of psychiatric disorders such as depression and drug addiction (Koob and Volkow, 2010; Chaudhury et al., 2015). Despite making up a relatively small proportion of synapses (Brady et al., 2005), catecholamine neurons can signal through volume transmission (Garris et al., 1994; Cragg et al., 2001) to affect a wide variety of downstream targets and influence communication throughout the entire brain. Over 40 years ago, Ralph Adams envisioned using electrochemistry in vivo to measure oxidizable neurotransmitters (Kissinger et al., 1973). This technique has enabled researchers to measure neurotransmitter release and uptake in brain slices, anesthetized, and freely behaving animals to more precisely determine their actions on neural communication and behavior. Although several electrochemical techniques have been applied to the challenge, fast-scan cyclic voltammetry (FSCV) has been more widely applied due to its fast time resolution and chemical selectivity over other measurements.
It is impossible to cover the last four decades of in vivo electrochemistry in a single review. Therefore, this review has three goals. First, we will provide a general overview of FSCV and the pioneering work in brain slices that led to measurements in intact animals. Second, we will focus on the use of anesthetized animals combined with pharmacology to reveal differences in regulation of dopamine and norepinephrine. Third, we will highlight studies in freely moving animals that reveal contrasting roles for these catecholamines in modulating behavior.
II. Building the Foundation for In Vivo Recordings
A. Electrochemical Methods for Catecholamine Measurements
Electrochemical methods have enabled researchers to measure dynamic fluctuations of catecholamines in brain tissue (Robinson et al., 2008). These methods rely on the oxidation or reduction of the molecule of interest at a solid electrode, and the resultant current provides a quantitative measure of catecholamine concentrations. Electrochemical measurements have a time resolution sufficient to study both release and uptake, and can be combined with pharmacology to probe local mechanisms governing extracellular neurotransmitter concentrations. The two most commonly employed methods are FSCV and constant-potential amperometry.
In FSCV, a potential sweep is applied to an electrode at a rapid scan rate (100–1000 V/s) to oxidize and reduce electroactive species. The current from this potential sweep results in a characteristic cyclic voltammogram (Fig. 1) and can be converted into concentration estimates for the species of interest with in vitro calibration factors and multivariate analysis techniques (Keithley et al., 2009; Rodeberg et al., 2015). FSCV measurements are typically conducted at glass-encased, carbon-fiber microelectrodes in which the sensor is 5–10 μm in diameter, and with an active length of 50–150 μm. The small size of the sensor allows for minimal tissue damage, as well as excellent spatial selectivity (Peters et al., 2004). Voltammetric measurements are typically conducted at fast sampling frequencies by repeating the potential sweep every 20–200 ms, allowing for the detection of single release and uptake events.
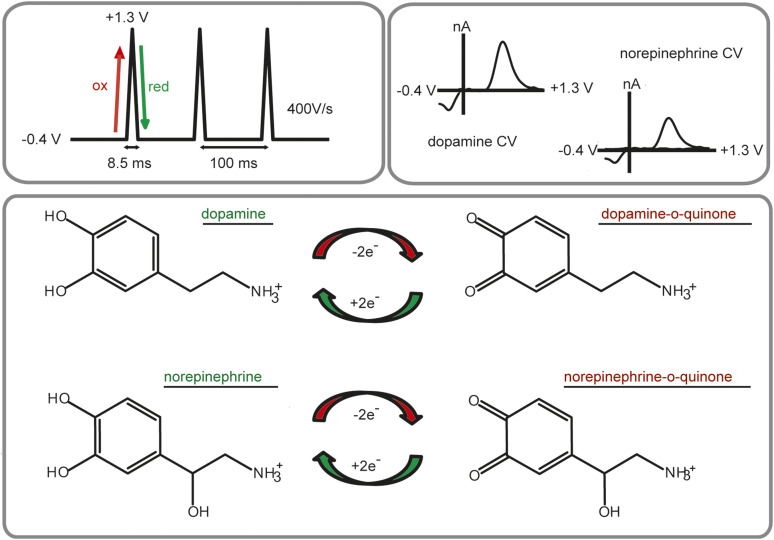
Fig. 1.
FSCV for the detection of catecholamines. The most commonly used waveform sweeps from −0.4 to +1.3 V at a scan rate of 400 V/s. The positive-going scan oxidizes dopamine and norepinephrine to their ortho-quinone form, and the negative-going scan reduces them back to dopamine or norepinephrine. Plotting the resultant current versus potential results in identical characteristic cyclic voltammograms (CVs) for both dopamine and norepinephrine.
Constant-potential amperometry (CPA) measurements are also conducted at carbon-fiber microelectrodes; however, this method differs from FSCV in that it uses a single potential to oxidize or reduce the molecule of interest. The simplicity of this approach results in better time resolution than FSCV because it is only limited by the sampling frequency. Similar to CPA, carbon-fiber microelectrodes are used in chronoamperometry, which also relies on a single electrolysis potential. However, chronoamperometry differs from CPA in that the potential is stepped periodically instead of constantly applied. It is important to note that in both CPA and chronoamperometry, any molecule that is electroactive at that given potential will be detected at the electrode. Thus, amperometric measurements have poor chemical selectivity as compared with FSCV. To combat this, researchers have turned to chemically treated electrodes to enhance analyte selectivity (Gerhardt et al., 1984). For a detailed comparison of FSCV, chronoamperometry, and CPA, we refer the reader to a recent review (Bucher and Wightman, 2015).
In addition to electrochemical detection of endogenous catecholamine release, catecholamines can also be exogenously applied to examine the kinetics of neurotransmitter clearance. This approach has been used in brain slices (Falkenburger et al., 2001), as well as in anesthetized (Cass et al., 1992; Zahniser et al., 1999) and freely moving animals (Gerhardt et al., 1999; Sabeti et al., 2002). Exogenous application is often used in amperometric methods because it confirms the identity of the measured species. However, the disappearance of applied catecholamines reflects information about the diffusion of the analyte away from the electrode surface, as well as clearance due to uptake. Therefore, care must be taken when interpreting data obtained by this methodology.
Due to the chemical selectivity of FSCV over amperometry, FSCV has been the more widely applied electrochemical technique for measuring both release and uptake of catecholamines. A commonly used voltammetric waveform for detection of catecholamines sweeps from −0.4 to +1.3 V at 400 V/s, applied every 100 ms (Fig. 1). However, it is important to note that dopamine and norepinephrine differ structurally by a single hydroxyl group, and current voltammetric waveforms cannot distinguish between cyclic voltammograms of the two catecholamines in vivo. Thus, we and others have turned to a histologic and pharmacological signal validation method that is discussed further in Pharmacological Validation. Although this method ensures the catecholamine signal is purely dopamine or norepinephrine, it typically precludes measurements in regions containing mixed catecholamines, such as the prefrontal cortex. However, recent advances in optogenetics (Deisseroth, 2015) and chemogenetics (Roth, 2016) allow for the excitation or inhibition of specific cell populations. Although dopamine and norepinephrine cannot be differentiated electrochemically, it is possible that these selective stimulation methods will further extend the reach of FSCV for catecholamine measurements throughout the brain. For the time being, the electrochemical signal needs to be validated pharmacologically to claim that the measured current is solely dopaminergic or noradrenergic in nature.
B. Regulation of Extracellular Catecholamines in Brain Slices
1. Release.
In brain slice recordings, a carbon-fiber electrode is positioned ∼100 µm into the tissue near a stimulating electrode. The release and subsequent uptake of neurotransmitters are measured while directly depolarizing nearby terminals (Kelly and Wightman, 1987; Palij et al., 1990). Early studies focused on dopamine, and FSCV in brain slices allowed researchers to determine that extracellular concentrations were a balance of release and uptake, with metabolism operating on a slower time scale (Near et al., 1988; Sulzer et al., 2016). Dopamine release per stimulation pulse ranges from ∼15 nM in the basolateral amygdala to ∼90 nM in the dorsal striatum (Garris and Wightman, 1994a, 1995). These values are in stark contrast to norepinephrine release, which reaches only ∼2 nM in the dorsal lateral geniculate, and ∼7 nM in the anteroventral thalamus (Mitchell et al., 1994; Garris and Wightman, 1995). Additionally, maximal norepinephrine efflux only weakly depends on stimulation current above 250 µA, unlike striatal dopamine, which does not approach saturation even with stimulation currents of 450 µA (Kennedy et al., 1992; Miles et al., 2002). Longer pulse trains are required to elicit norepinephrine release in slices containing the bed nucleus of the stria terminalis (BNST), and the kinetics of norepinephrine release are slower as compared with dopamine (Kennedy et al., 1992; Miles et al., 2002). These differential release profiles may be due to the differences in vesicular release rate between norepinephrine and dopamine (Chiti and Teschemacher, 2007), or differential dependence on N-type calcium channel activity (Mitchell and Adams, 1993). Despite evidence that both catecholamines are released from similar sized vesicles (Bergquist and Ludwig, 2008; Papke et al., 2012), the mechanism underlying differential release kinetics in slices is unknown.
2. Uptake.
After catecholamines are released, they are cleared from the extracellular space by neuronal transporters. The rate of uptake is often approximated using t1/2, the time it takes to clear half of the neurotransmitter concentration from the extracellular space. As measured by t1/2, uptake rates differ vastly between dopamine and norepinephrine. For dopamine, t1/2 values are <0.1 second in both dorsal and ventral striatum; for norepinephrine t1/2 values exceed 1.0 second (Garris and Wightman, 1994a, 1995; Mitchell et al., 1994).
The primary clearance mechanism for dopamine is mediated by the dopamine transporter (DAT), which obeys Michaelis–Menten kinetics (Wightman et al., 1988). Uptake rates are heterogeneous in subregions of the striatum (Trout and Kruk, 1992; Jones et al., 1996; Siciliano et al., 2014; Salinas et al., 2016), which might be attributed to differences in striosome and matrix compartments (Salinas et al., 2016). Researchers have manipulated DAT expression to determine its contribution to extracellular dopamine concentrations in brain slice preparations, and genetic deletion of DAT prolongs the life of extracellular striatal dopamine by 300-fold (Jones et al., 1998). Conversely, overexpression of DAT results in a 50% faster dopamine uptake rate, and an overall reduction in evoked dopamine concentrations (Salahpour et al., 2008).
The primary clearance mechanism for norepinephrine is the norepinephrine transporter (NET), but non-NET mechanisms may play a larger role in norepinephrine clearance. The lifetime of ventral BNST (vBNST) norepinephrine clearance is only prolonged sixfold in NET knockout mice (Xu et al., 2000; Miles et al., 2002), and both organic cation transporters (OCTs) and DAT are expressed in the vBNST (Miles et al., 2002; Gasser et al., 2009). Although DAT has a higher affinity for dopamine over norepinephrine, DAT knockout mice exhibit a slight reduction in vBNST norepinephrine clearance rate (Miles et al., 2002). However, DAT is not likely a major contributor to norepinephrine clearance in animals with normal NET function because pharmacological blockade of DAT in rats does not affect norepinephrine clearance rate in the vBNST (Palij and Stamford, 1992). However, in cases of prolonged signaling, DAT or OCTs may serve as an additional mechanism for norepinephrine clearance.
Although the main substrates for DAT and NET are dopamine and norepinephrine, respectively, the role of catecholamine transporters is further complicated in regions receiving both dopaminergic and noradrenergic innervation. Catecholamine transporters are notoriously promiscuous (Daws, 2009), and NET serves as the primary clearance mechanism for both dopamine and norepinephrine in the cortex (Moron et al., 2002). Thus, care must be taken when choosing pharmacological agents for signal validation, as discussed below in Pharmacological Validation.
3. Autoreceptors.
Extracellular catecholamine concentrations are also governed by autoreceptor control of release. Circuitry is mostly severed in a slice preparation, which serves as an advantage when examining regulation of release at catecholamine terminals. Any effects on release exerted by receptor agonists/antagonists can thus be attributed to direct actions on terminals. The principal autoreceptor for dopamine is the D2 (Beaulieu and Gainetdinov, 2011). D2 receptors maximally inhibit dopamine release within 500–1000 ms, and the time course varies between dorsal and ventral striatum (Phillips et al., 2002). D2 receptors become markedly desensitized after knockout of DAT due to persistent elevation of extracellular dopamine concentrations (Jones et al., 1999). The principal autoreceptor controlling norepinephrine efflux is the α2, and it operates on a similar time course as D2 receptors (Palij and Stamford, 1993; Trendelenburg et al., 2001). Despite having similar mechanisms in place to control extracellular concentrations, the fundamental differences in dopamine and norepinephrine release and uptake position them to influence neuronal communication in diverse ways.
C. Catecholaminergic Plasticity
A number of studies have used brain slice preparations to investigate adaptations to release and uptake mechanisms. For example, self-administration of psychostimulants produces variable adaptations in dopamine regulation. Following 5 days of amphetamine self-administration, evoked dopamine release is increased in brain slices containing the nucleus accumbens (NAc) and is accompanied by decreased D2 autoreceptor function (Calipari et al., 2014b). Following cocaine self-administration, electrically stimulated dopamine release is instead attenuated in brain slices, and cocaine is less effective at elevating dopamine concentrations (Mateo et al., 2005; Ferris et al., 2011, 2012; Calipari et al., 2014a). However, when cocaine self-administering animals are given a single infusion of amphetamine, dopamine terminal function in slices is restored; that is, evoked release magnitudes and cocaine inhibition of DAT more closely resemble that of drug-naive animals (Ferris et al., 2015). Interestingly, increasing surface DAT density increases the potency of the psychostimulants amphetamine and methamphetamine, without altering the effect of uptake blockers such as cocaine (Salahpour et al., 2008; Calipari et al., 2013, 2015). DAT-overexpressing animals have faster uptake rates, exhibit a threefold increase in amphetamine-potentiated dopamine release as compared with controls, and develop a preference for amphetamine at lower doses as compared with wild-type animals (Salahpour et al., 2008). Similar to DAT overexpression, animals that self-administer methylphenidate have increased dopamine uptake rates, and amphetamine and methamphetamine potency is increased in these animals; that is, the psychostimulants become more effective at inhibiting dopamine uptake (Calipari et al., 2013). However, neither methylphenidate self-administration nor DAT overexpression alters uptake inhibition by uptake blockers cocaine and nomifensine (Calipari et al., 2013, 2015). In addition, the potency of amphetamine differs between striatal subregions, and is closely linked with uptake rates; that is, elevated uptake rates are associated with increased uptake inhibition by amphetamine (Siciliano et al., 2014). Although both cocaine and amphetamine act at DAT, it is clear from these functional measurements that the potency of amphetamine, but not cocaine, is dependent on DAT expression levels.
Catecholamine receptors are also regulated by G protein–coupled receptor kinases, and deletion of G protein–coupled receptor kinase 2 (GRK2) from D1- or D2-containing neurons produces contrasting effects on dopamine regulation in brain slices (Daigle et al., 2014). GRK2 deletion in D1-containing neurons enhances evoked dopamine release and uptake rates. Conversely, GRK2 deletion in D2-containing neurons enhances D2 autoreceptor activity and depresses baseline dopamine release without changing dopamine uptake rate (Daigle et al., 2014). These adaptations are paralleled by bidirectional sensitivity to cocaine: in D1-containing neurons, GRK2 deletion enhances cocaine-amplified dopamine release without altering cocaine-mediated uptake inhibition. Conversely, lack of GRK2 in D2 neurons reduces cocaine-amplified dopamine release and cocaine-mediated inhibition of uptake rates and is accompanied by reduced behavioral sensitivity to a cocaine challenge (Daigle et al., 2014). This hypodopaminergic state is similar to that of animals with a history of cocaine self-administration (Mateo et al., 2005; Ferris et al., 2011, 2012; Calipari et al., 2014a ). Indeed, chronic cocaine administration reduces GRK2 expression in the NAc (Schroeder et al., 2009). Together, these findings suggest that GRK2 expression in D2-containing neurons is important for cocaine-mediated dopamine release and uptake inhibition.
Brain slices have also been used to uncover changes to dopamine regulation in rats reared in social isolation. These animals exhibit enhanced dopamine release and uptake as compared with group-reared rats (Yorgason et al., 2016). Interestingly, social isolation amplifies methamphetamine, but not cocaine inhibition of DAT (Yorgason et al., 2016). Because methamphetamine and cocaine both act at DAT, these findings suggest other adaptations may play a role in the plasticity. The stress of social isolation most likely facilitates release of corticosterone, which has a profound impact on other transport mechanisms, such as OCTs (Gasser et al., 2006). In agreement with this hypothesis, corticosterone decreases dopamine clearance in the NAc, presumably by inhibiting OCTs expressed in the NAc (Graf et al., 2013).
Although fewer studies have used slice voltammetry to assay changes in noradrenergic function, work from the Stamford laboratory demonstrated adaptations in somatodendritic norepinephrine release in the locus coeruleus (LC). Bath application of the analgesic tramadol reduces norepinephrine clearance in slices (Halfpenny et al., 1999), but chronic treatment does not affect uptake mechanisms. Instead, chronic tramadol appears to sensitize α2 function, and in a manner that resembles the actions of an antidepressant (Hopwood et al., 2001). Although tramadol is an opioid analgesic, these functional measurements of its pharmacological effects lend support to its antidepressant activity in mice (Rojas-Corrales et al., 1998). In another study, researchers asked how presynaptic norepinephrine regulation is changed in mice lacking the metabolic enzyme monoamine oxidase A. In monoamine oxidase A knockout mice, peak LC norepinephrine efflux is higher and is accompanied by decreased clearance rates and α2 control over release in brain slices (Owesson et al., 2002, 2003). Similar adaptations to vBNST norepinephrine were found in anesthetized rats following stressors, as discussed below in Adaptations in Catecholamine Function.
D. Other Modulators of Catecholamine Release
Terminal catecholamine release can be modulated by other signaling molecules. For example, acetylcholine’s actions through cholinergic receptors modulate dopamine concentrations dependent on stimulation frequency (Zhang et al., 2009). In a study designed to mimic the tonic (<5 Hz) and phasic (20 Hz) firing patterns of dopamine neurons found in vivo, Zhang et al. (2009) used slice voltammetry to measure dopamine release during differing stimulation frequencies. In this work, blockade of β2-containing nicotinic acetylcholine receptors (nAChRs) predominantly suppressed dopamine evoked from tonic (<5 Hz) stimulation trains (Zhang et al., 2009). However, decreased dopamine release after nAChR blockade was frequency dependent, and dopamine release increased under nAChR blockade at frequencies over 10 Hz (Zhang et al., 2009). Acetylcholine can also modulate dopamine release independent of midbrain dopamine neuron activity, and optogenetic activation of cholinergic interneurons alone is sufficient to elicit dopamine release in brain slices containing the striatum, as well as in intact animals (Cachope et al., 2012; Threlfell et al., 2012). Acetylcholine released from cholinergic interneurons acts directly on dopamine terminals in the NAc to evoke release that is dependent on β2-containing nAChRs, and this cholinergic activity does not require activation of midbrain dopamine neurons (Cachope et al., 2012; Threlfell et al., 2012). In addition to optogenetic or electrical stimulations, insulin also activates cholinergic interneurons to enhance dopamine release in striatal slices (Stouffer et al., 2015). However, in slices containing the ventral tegmental area (VTA), insulin instead suppresses somatodendritic release and enhances dopamine reuptake (Mebel et al., 2012).
Nitric oxide (NO) donors also modulate dopamine in a manner dependent on cholinergic activity. Under reduced acetylcholine, NO modulates dopamine release in slices in a frequency-independent manner and acts directly on dopamine terminals, in contrast to its frequency-dependent modulations in the presence of nicotinic receptor activity (Hartung et al., 2011). We could find no reports detailing how norepinephrine release is influenced by other signaling molecules in slices. Because infusion of NO donors into the BNST produces anxiety (Faria et al., 2016), and norepinephrine in the BNST regulates the stress axis (Forray and Gysling, 2004), NO donors may potentiate norepinephrine release in the BNST to produce elevated stress/anxiety, but this has not yet been shown. Additionally, a host of neuropeptides is known to regulate BNST activity (Kash et al., 2015) and may, in turn, regulate norepinephrine release. By combining slice voltammetry with the diverse toolbox of genetic manipulations, specific cell-type activation, and selective pharmacology, new modulators of catecholamine release will be identified that will contribute to a better understanding of how these neurotransmitters are regulated and aid in the development of more effective pharmacotherapies.
III. In Vivo Recordings in Anesthetized Animals
A. Pharmacological Validation
Voltammetric measurements in anesthetized animals allow for precise measurements of release and uptake in the intact brain. Because neural activity is suppressed in anesthetized animals, neurotransmitter release is typically elicited by stimulating neurons or their axon bundles directly. Early studies used direct electrical stimulation, although recent optogenetic strategies provide an opportunity to excite or inhibit discrete cell types (Witten et al., 2011; McCutcheon et al., 2014). Dopamine and norepinephrine differ structurally by only a hydroxyl group, and their voltammograms in vivo are indistinguishable (Park et al., 2009). Thus, we have turned to a multistep approach to validate the origin of the signal at the electrode. First, we limit our measurements to regions containing predominantly dopamine or norepinephrine. Tissue homogenate studies have confirmed that norepinephrine is the primary catecholamine in the vBNST and the anteroventral thalamus; thus, our first in vivo norepinephrine measurements were restricted to those regions (Park et al., 2009). Many dopamine-rich regions lie adjacent to the vBNST, and, without the visual confirmation of electrode placement afforded by a slice preparation, we turned to a pharmacological approach to rule out contributions by dopamine (Fig. 2). Voltammetric signals are only considered noradrenergic if they respond to adrenergic agents (e.g., α2 antagonist idazoxan), but not dopaminergic agents (e.g., D2 antagonist raclopride). Finally, a constant current is applied to the carbon-fiber electrode to make a lesion in the brain for subsequent histologic validation of electrode placement in the target region.
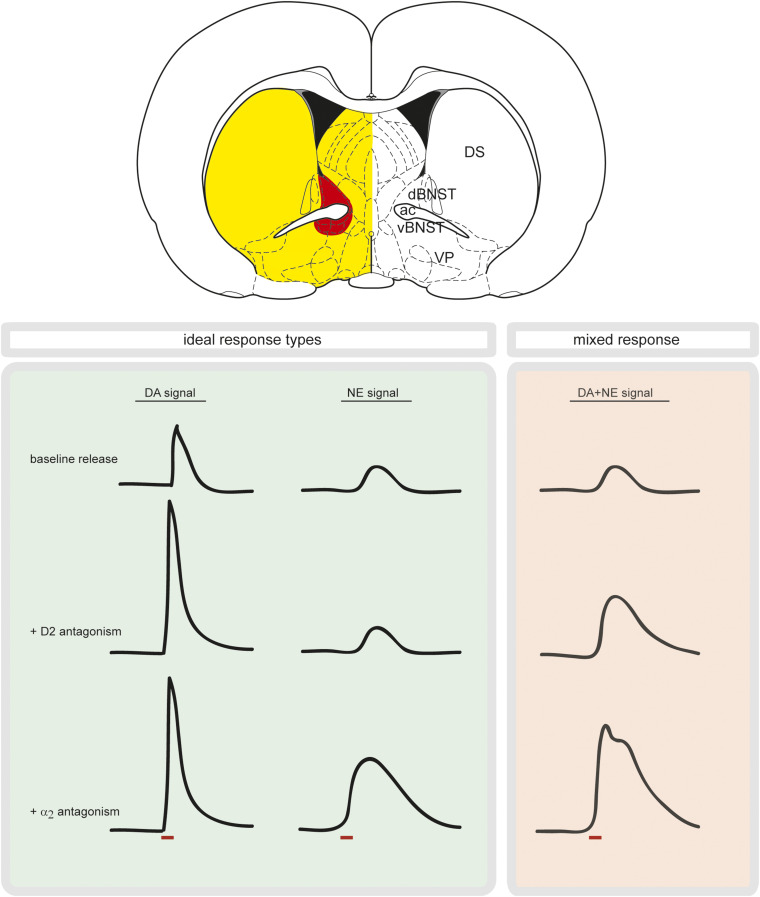
Fig. 2.
Spatially resolved measurements combined with pharmacology ensure either dopamine or norepinephrine measurements. Brain slice, adapted from the atlas of Paxinos and Watson showing norepinephrine terminals (red) surrounded by dopamine terminals (yellow), highlighting the need for a small sensor. Boxes show mock electrically stimulated response types to different drugs. Red bar denotes stimulation. In the green box, a pure dopamine signal increases with D2 antagonism and remains elevated with α2 antagonism; a pure norepinephrine signal does not increase following D2 antagonism and only responds to α2 antagonism. In the red box, a mixed dopamine/norepinephrine signal responds to both D2 and α2 antagonists. ac, anterior commissure; dBNST, dorsal bed nucleus of the stria terminalis; DS, dorsal striatum; VP, ventral pallidum.
B. Differential Release of Catecholamines in Anesthetized Animals
1. Dopamine.
Dopamine release is typically evoked by stimulating neurons in the VTA/substantia nigra (SN) or the medial forebrain bundle (MFB). The excellent spatial selectivity afforded by microelectrodes allows for characterization of regulation mechanisms in discrete structures or microdomains of a given region. By carefully lowering the carbon-fiber and stimulating electrodes, it is possible to generate a functional map of the neurons and terminals that support catecholamine release. Early studies used voltammetry in anesthetized rats to reveal an apparent heterogeneity of release and uptake in compartments of the striatum and basolateral amygdala (BLA) (May and Wightman, 1989; Garris and Wightman, 1994b). Dopamine regulation also differs between the NAc and the olfactory tubercle (OT) (Wakabayashi et al., 2016). Dopamine release reaches smaller concentrations in the OT as compared with the NAc, and DAT inhibition produces smaller increases in OT dopamine compared with NAc (Wakabayashi et al., 2016).
Recent work in our laboratory has used this classic mapping approach combined with multiple electrodes and pharmacology to reveal an unexpected population of dopamine neurons that release dopamine into the contralateral striatum (Fox et al., 2016a). Stimulations of the VTA elicit dopamine release in the NAc both ipsilateral and contralateral to the stimulation, although release is ∼20× higher following ipsilateral versus contralateral VTA stimulations. Contralaterally projecting dopamine neurons originating from the VTA are also differentially regulated by D2 receptors because they are more sensitive to the D2 antagonist raclopride than ipsilateral VTA projections (Fox et al., 2016a). Dopamine is also released in the dorsal striatum following stimulations of the contralateral SN. In contrast to the NAc, dopamine release in the dorsomedial striatum exhibits hemispheric equivalence, that is, dopamine release is of similar magnitude following ipsilateral or contralateral SN stimulations (Fox et al., 2016a). Hemispherically equivalent dopamine release in the dorsomedial striatum is also found after stimulating the ipsilateral or contralateral pedunculopontine tegmental nucleus (PPTg), an excitatory input to the SN. Furthermore, hemispherically equivalent release is accompanied by similar D2 control over contralateral and ipsilateral SN projections (Fox et al., 2016a). These findings suggest that differential D2 receptor control may underlie the differences between contralateral dopamine release in the NAc and striatum. Although anatomic (Geisler and Zahm, 2005) and behavioral (Steinberg et al., 2014) data support cross-hemispheric dopamine projections, voltammetric measurements confirm their functional nature and precipitate new areas of inquiry regarding contralateral catecholamine projections.
Other work in intact animals has revealed NAc dopamine release is differentially modulated by α6-containing nAChRs. Infusion of the α6 nAChR antagonist α-conotoxin MII into the VTA decreases evoked dopamine in the NAc (Wickham et al., 2013). However, similar α6 antagonism in slice preparations containing the NAc results in enhanced dopamine release (Exley et al., 2008). These findings suggest that nAChRs can modulate dopamine release in a site-dependent manner and highlight the importance of making measurements in an intact brain. The high spatial resolution afforded by microelectrodes combined with site-specific pharmacological approaches will continue to enable functional characterization of dopaminergic circuits in intact animals.
2. Norepinephrine.
In anesthetized animals, norepinephrine release is typically measured by stimulating neurons in the nucleus of the solitary tract, or its axon bundles. Although most in vivo norepinephrine studies are conducted in the vBNST, both the dorsal BNST and the NAc shell receive some norepinephrine innervation in addition to dopamine. Selective pharmacology indicates that norepinephrine in the NAc is restricted to the more caudal portion of the shell (Park et al., 2010), and norepinephrine in the dorsal BNST is contained to the medial portion (Herr et al., 2012). In a study designed to compare norepinephrine responses between dorsal and ventral BNST, researchers found norepinephrine release in the dorsomedial BNST is ∼50% of vBNST release (Herr et al., 2012). Accompanying reduced release amplitudes in the dorsomedial BNST are slower clearance rates and reduced α2 autoreceptor function as compared with regulation in the vBNST (Herr et al., 2012).
To directly compare evoked catecholamines, Park et al. (2011) used a dual-electrode approach to measure dopamine and norepinephrine release simultaneously in the NAc and vBNST with stimulations that targeted both noradrenergic axons and the VTA/SN. As previously demonstrated in slices, release and uptake of norepinephrine in the vBNST are slower as compared with dopamine in the NAc, even with identical stimulation location (Park et al., 2011). The two catecholamines are also differentially regulated. Tyrosine hydroxylase inhibition depletes dopamine release faster than norepinephrine, and basal levels of dopamine increase when D2 receptors are antagonized and uptake is blocked with amphetamine (Park et al., 2011). In contrast, there are no elevations in basal norepinephrine in the vBNST following uptake inhibition with amphetamine and concomitant α2 autoreceptor inhibition (Park et al., 2011). This contrasting regulation is also found in studies using more selective uptake inhibition. When dopamine D2 autoreceptors and DAT are blocked in anesthetized animals, dopamine concentrations fluctuate spontaneously in the striatum (Venton and Wightman, 2007). However, similar blockade of noradrenergic α2 autoreceptors and NET does not elicit spontaneous norepinephrine fluctuations in the vBNST (Park et al., 2015). Although norepinephrine and dopamine have similar regulation mechanisms, they signal in distinct ways when control mechanisms are blocked. These findings hint at unknown mechanisms controlling norepinephrine release beyond that of noradrenergic autoreceptors and transporters. Similar to dopaminergic modulation by nAChRs, norepinephrine release is most likely influenced by other receptor types, but further work is needed to identify their contributions. Additionally, the larger stimulations required to elicit release of norepinephrine suggest that norepinephrine is only released endogenously under extreme physiologic conditions, in stark contrast to dopamine.
3. Mixed Catecholamines.
A few studies have made measurements in regions with mixed catecholamines, despite their indistinguishable voltammograms. The prefrontal cortex (PFC) receives both dopaminergic and noradrenergic innervation, and early work shows dopamine is the predominant catecholamine released in the medial PFC following VTA stimulations (Garris et al., 1993). This finding was confirmed in a recent report; however, D2 receptor antagonism paradoxically attenuates dopamine release in this region (Shnitko and Robinson, 2014). Pharmacology in cortical regions must thus be selected carefully for signal validation, particularly because NET takes up dopamine in the PFC (Moron et al., 2002). In future endeavors, selective stimulation methods, combined with anatomic and pharmacological validation, will enable additional voltammetric characterization of catecholamine signaling in regions receiving mixed innervation.
C. Adaptations in Catecholamine Function
Voltammetric measurements in anesthetized animals allow for researchers to identify how different manipulations interact with intact circuitry to produce functional adaptations in catecholamine release. For example, researchers used anesthetized animals to determine the role of N-methyl-D-aspartate receptors (NMDARs) expressed specifically in dopamine neurons (Zweifel et al., 2009). This study found genetic inactivation of NMDARs in dopamine neurons disrupts evoked dopamine release in a stimulation site-dependent manner. Whereas dopamine release is unchanged in NMDAR knockout mice following MFB stimulations, dopamine release elicited by the PPTg is blunted as compared with controls (Zweifel et al., 2009). Without the intact circuity afforded by an anesthetized preparation, the site specificity of glutamatergic modulation of dopamine release may not have been uncovered. Another study found altered dopamine release in mice overexpressing the catecholamine metabolic enzyme catechol-o-methyl transferase (Simpson et al., 2014). In mice overexpressing catechol-o-methyl transferase, striatal dopamine release capacity is increased despite unchanged levels of tyrosine hydroxylase or DAT (Simpson et al., 2014). It is clear that measuring the functional consequence of genetic manipulation on dopamine release provides more information than markers of dopaminergic activity alone.
In addition to genetic alteration, recent work in our laboratory has examined how baseline genetic differences impact catecholamine regulation mechanisms. In these studies, norepinephrine release was compared in the vBNST of Sprague-Dawley (SD), Lewis, and Wistar-Kyoto (WKY) rats (McElligott et al., 2013; Fox et al., 2015). Whereas release and uptake were similar between SD and WKY rats (Fox et al., 2015), there were marked differences in uptake and autoreceptor control in Lewis rats (McElligott et al., 2013). Although the amplitude of norepinephrine release was similar in all three strains, Lewis rats showed slower norepinephrine uptake rates as compared with SD or WKY rats, despite no difference in apparent NET expression (McElligott et al., 2013). Similarly, α2 function, but not expression was attenuated in Lewis rats, because elevation of evoked norepinephrine was blunted in Lewis rats after α2 antagonism with idazoxan as compared with SD rats (McElligott et al., 2013). Additionally, depletion of LC norepinephrine with DSP-4 produced adaptations to α2 receptors and uptake in SD, but not WKY rats, without changing norepinephrine release magnitude (Fox et al., 2015). These findings underscore the importance of voltammetric catecholamine measurements in intact systems, because differential release and uptake most likely contribute to the phenotypic variations observed in genetically diverse animal models.
Dysregulations in catecholamine signaling are implicated in the development of addiction and numerous neuropsychiatric conditions (Koob and Volkow, 2010). Several studies have used anesthetized preparations to uncover adaptations to catecholamine circuits following administration of drugs of abuse. Chronic administration of cocaine, heroin, or a “speedball” cocktail of the two produces variable adaptations to NAc dopamine in rats. In animals with a history of chronic cocaine, heroin, or speedball self-administration, evoked dopamine is reduced compared with animals receiving a single drug dose (Pattison et al., 2012). Dopamine reuptake rate is also greater in speedball-administering animals compared with drug-naive animals, or animals that self-administered cocaine or heroin alone (Pattison et al., 2012). This hypofunction of the dopamine system after cocaine self-administration was confirmed in another recent report (Siciliano et al., 2015b). However, 1-day pretreatment with cocaine does not alter the dopamine response to a subsequent cocaine challenge (Addy et al., 2010).
Another study examined the effect of repeated cocaine treatment on dopamine release, and found that 7 days of cocaine exposure instead potentiated the effect of a cocaine challenge on dopamine signaling in the NAc. This increase in elevated dopamine after cocaine was accompanied by an increase in apparent Km of DAT (Addy et al., 2010). This study appears to contradict numerous reports describing blunted dopaminergic responses to cocaine after cocaine self-administration (Mateo et al., 2005; Ferris et al., 2012; Calipari et al., 2014a; Siciliano et al., 2015a,b). However, it is important to note that Addy et al. (2010) used once per day experimenter-delivered cocaine (in contrast to the 6-hour self-administration sessions used in Siciliano et al., 2015b). Furthermore, electrochemical measurements were made after >24 hours of withdrawal in Addy et al. (2010), as opposed to ∼18 hours in Siciliano et al. (2015b). It is possible that the differences in dopamine release in response to cocaine challenge reflect the different dosing regimen between the two studies, or adaptations to dopamine regulation following a longer withdrawal period. In support of the prolonged cocaine withdrawal hypothesis, Cameron et al. (2016) found that after 30 days of forced abstinence, a cocaine challenge increased NAc dopamine release in animals with a history of self-administration. Future work should address the mechanisms underlying dopaminergic adaptations after variable periods of cocaine withdrawal because this plasticity appears to be time-course dependent.
There is some evidence that κ opioid receptors may play a role in differential cocaine-potentiated dopamine release after withdrawal. The endogenous ligand for κ opioid receptors is dynorphin, which is released in response to stressful events (Chavkin, 2013), such as drug withdrawal. The κ activation alone inhibits evoked dopamine in the NAc, and, on a short time scale, pretreatment with a κ agonist attenuates the dopamine response to cocaine in the NAc (Ehrich et al., 2014). However, on a longer time scale, pretreatment with a κ agonist increases the cocaine-induced increase in NAc dopamine (Ehrich et al., 2014), similar to potentiated dopamine release after >24-hour withdrawal, or 30 days of forced abstinence (Addy et al., 2010; Cameron et al., 2016). Further work is needed to determine whether κ opioid receptor activation alone can explain the differences between these studies (Addy et al., 2010; Siciliano et al., 2015b; Cameron et al., 2016). Regardless, the time-course dependence of κ opioid modulation of dopamine is interesting in the context of stress-induced cocaine use. It is possible that dynorphin released in response to stress promotes a dysphoric state that drives drug use. On a short time scale, this stress may decreases cocaine’s actions on mesolimbic dopamine, resulting in escalation of drug intake to compensate for cocaine’s attenuated effect. However, on a longer time scale, κ activation may potentiate cocaine’s effects on dopamine, further driving its reinforcing properties. Although cocaine increases extracellular dopamine concentrations, it is clear that there are other signaling mechanisms outside of elevated dopamine that drive persistent drug use.
Adaptations to dopamine signaling have also been studied in anesthetized animals after other drugs of abuse. An acute dose of ethanol decreases evoked dopamine and slows clearance in the medial PFC (Shnitko et al., 2014). Release magnitudes are likewise suppressed in the medial PFC when ethanol is infused directly into the VTA (Shnitko et al., 2014), and a systemic ethanol challenge also reduces NAc dopamine release (Shnitko et al., 2016). In animals with adolescent alcohol exposure, tonic levels of NAc dopamine are reduced in adulthood, and these animals exhibit increased risk-taking behavior (Schindler et al., 2016). Although tonic NAc dopamine is reduced, phasic NAc dopamine release is increased in animals with adolescent alcohol exposure, in a manner dependent on stimulation location: PPTg- but not MFB-evoked phasic dopamine release is increased compared with alcohol-naive animals (Schindler et al., 2016). In agreement with this, another study found adolescent alcohol exposure enhances VTA-stimulated dopamine release in the NAc, and an ethanol challenge produces larger increases in stimulated dopamine release compared with alcohol-naive animals (Shnitko et al., 2016). Interestingly, the administration of an allosteric GABAA agonist attenuates both increased dopamine release and increased risk-taking behavior in animals with adolescent alcohol exposure (Schindler et al., 2016). These findings suggest that there is increased inhibitory tone after alcohol exposure that drives changes in dopaminergic signaling through a disinhibitory mechanism, which may contribute to the behavioral changes. Although acute ethanol reduces evoked dopamine in both the NAc and medial PFC, alcohol exposure in adolescence clearly leads to dopaminergic plasticity that may increase the reinforcing properties of alcohol later in life.
In addition to alcohol, large doses of methamphetamine have also been shown to reduce evoked striatal dopamine and decrease DAT uptake rates (Howard et al., 2011). These large doses are considered neurotoxic and result in decreased striatal DAT levels (Howard et al., 2013). Pretreatment with neurotoxic doses of methamphetamine reduces the concentrations of both pharmacologically induced (Robinson et al., 2014) and naturally occurring dopamine transients (Howard et al., 2013). Although drugs of abuse can increase dopaminergic transmission (Covey et al., 2014), it is clear that this is dependent on dosing and prior drug exposure.
Work investigating adaptations to norepinephrine signaling has been limited, but two recent studies from our laboratory examined the effects of stress and drug exposure on norepinephrine release and regulation. Three days of naloxone-precipitated morphine withdrawal dysregulates norepinephrine signaling in the vBNST in a strain-dependent manner (McElligott et al., 2013; Fox et al., 2015). In morphine-withdrawn SD rats, α2 receptors become desensitized, norepinephrine clearance rate is slowed, and these animals exhibit increased anxiety-like behavior (McElligott et al., 2013; Fox et al., 2015). In morphine-withdrawn WKY rats, norepinephrine clearance rate is unchanged, but these animals exhibit increased anxiety-like behavior and decreased α2 receptor function (Fox et al., 2015). Lewis rats exhibit elevated anxiety-like behavior at baseline, and morphine withdrawal does not further elevate anxiety, nor depress α2 function or norepinephrine clearance in these animals (McElligott et al., 2013). Furthermore, following 2 weeks of social isolation stress, stressed SD rats resemble morphine-dependent rats, that is, elevated anxiety-like behavior, desensitized α2 receptors, and slow norepinephrine clearance in the vBNST (McElligott et al., 2013; Fox et al., 2015). In contrast, WKY rats do not change anxiety-like behavior or vBNST norepinephrine regulation in response to social isolation stress (Fox et al., 2015). Similar to drug exposure, Lewis rats do not alter noradrenergic synaptic function following social isolation, suggesting their regulation mechanisms are maximally disrupted (unpublished data). These findings underscore the importance of genetic factors in susceptibility to catecholamine dysregulation after stress exposure or drug withdrawal.
Researchers have also used anesthetized animals to investigate the mechanisms of nonabused drugs on catecholamine regulation. For example, the dopamine precursor, levodopa (L-DOPA), enhances dopamine release in both dorsal and ventral striatum, but causes a delayed inhibition of dopamine release in the dorsal striatum (Harun et al., 2015). L-DOPA also reduces uptake rates by decreasing Vmax of DAT (Harun et al., 2015). Because dopamine is the metabolic precursor to norepinephrine, L-DOPA may also affect norepinephrine concentrations, but this area is currently unexplored. Noradrenergic deficits are also a key component in the development of Alzheimer’s disease, and the way pharmacotherapies alter catecholaminergic function should be a topic of ongoing investigation. Electrochemical measurements in anesthetized animals allow researchers to examine discrete circuits and how they become functionally altered after a variety of treatments. Continued efforts should focus on changes in norepinephrine function in addition to dopamine due to their different patterns of release and uptake in vivo.
IV. Catecholamine Function in Awake Animals
A. Spontaneous Fluctuations
The first awake-animal FSCV dopamine measurements were conducted nearly 20 years ago, and the signals that researchers found were closely associated with a novel stimulus or environment (Garris et al., 1997; Rebec et al., 1997; Robinson et al., 2001). However, dopamine concentrations were also found to fluctuate in the absence of any external stimuli in awake animals at rest (Robinson et al., 2002) (Table 1). Spontaneous dopamine fluctuations, or transients, have been measured in the dorsal striatum, nucleus accumbens, and olfactory tubercle (Robinson et al., 2002), and they display heterogeneity in their frequency, amplitude, and duration in subregions of the NAc (Wightman et al., 2007). Dopamine transients in the NAc originate from phasic cell firing in the VTA (Sombers et al., 2009), are increased by cannabinoid receptor activation (Cheer et al., 2004), and are the main source of average extracellular NAc dopamine levels (Owesson-White et al., 2012). DAT blockade with nomifensine increases spontaneous and stimuli-related dopamine transients (Robinson and Wightman, 2004), and acute phenylalanine/tyrosine depletion reduces the frequency, but not amplitude, of spontaneous transients (Shnitko et al., 2016). Additionally, cocaine increases the magnitude and duration of spontaneous dopamine transients in the NAc core and shell, but increases in dopamine transient frequency after cocaine are only found in the NAc shell (Aragona et al., 2008).
TABLE 1.
Opposing catecholamine responses in awake animals
Upward arrows reflect increases, and downward arrows reflect decreases in phasic release. Superscripted letters correspond to the following references. The literature cited in this table is meant to provide notable examples of contrasting signaling and is by no means comprehensive.
| Stimulus |
NAc Dopamine |
vBNST Norepinephrine |
|---|---|---|
| At rest (transients) | Presenta | Absentb |
| ICSS | ||
| Stimulation | ↑c | ↑d |
| ICSS-predictive cue | ↑c | No effectd |
| ICSS-extinction | ↓d | ↑d |
| Food reward | ||
| Unexpected food | ↑e | No effectf |
| Food-predictive cue | ↑g | Unknown |
| Food omission | ↓h | Unknown |
| Drugs of abuse | ||
| Drug exposure | ↑i | No effectj unknown |
| Drug-predictive cue | ↑k | Unknown |
| Drug withdrawal | ↓j | ↑j, Unknown |
| Noxious/Aversive | ||
| Quinine | ↓l | ↑m |
| Fear cues | ↑↓n | Unknown |
| Tail pinch | ↑↓o | ↑o |
Recent work employing a dual-electrode approach has revealed that, at rest, spontaneous dopamine transients in the NAc shell synchronize ∼75% of the time between hemispheres (Fox et al., 2016a). Importantly, FSCV measurements of dopamine transients provide a clearer picture of dopaminergic activity, as the time course of dopamine transients as measured by FSCV is more closely linked with uptake inhibition-induced stereotypy than microdialysis measurements (Budygin et al., 2000). In contrast to striatal dopamine, norepinephrine concentrations are not known to fluctuate spontaneously in the vBNST of animals at rest (Park et al., 2012, 2013; Fox et al., 2016b), further illustrating the differences in endogenous catecholamine signaling (Table 1).
B. Intracranial Self-Stimulation
Intracranial self-stimulation (ICSS) was first described by Olds and Milner (1954), and, through extensive mapping studies, it was determined that sites that supported the best self-stimulation were centered around the medial forebrain bundle, implicating catecholamine signaling as a principal mediator of the behavior. In this paradigm, an animal is trained to respond instrumentally (e.g., lever press) to deliver an electrical stimulation to its brain. The presentation of the lever is traditionally preceded by a cue that predicts reward availability. Dopamine is released following direct electrical stimulation of the VTA/SN or MFB, but, as animals become well trained, the NAc dopamine release moves to the cue in a time-locked fashion and is accompanied by decreases in stimulation-evoked dopamine (Table 1) (Owesson-White et al., 2008). To this end, the dopamine response elicited by a reward-predicting cue, but not the reward, provides strong support for dopamine’s involvement in reward prediction error (Schultz, 2013). Furthermore, cue-associated dopamine release increases when the cue predicts a greater stimulation magnitude, and this increase is associated with a decreased latency to lever press for the stimulation (Beyene et al., 2010).
In early work from Garris et al. (1999), rats were trained to self-stimulate the VTA in a continuous manner, that is, the lever was not retracted after each lever press. These prolonged periods of self-stimulation sessions diminish the magnitude of NAc dopamine release, which the authors described as a dissociation of dopamine release from ICSS. More recent work in our laboratory has revisited this idea (Rodeberg et al., 2016). Although dopamine concentrations are markedly attenuated following continuous ICSS, the disappearance of dopamine during ICSS can instead be attributed to dopamine concentrations falling below the limit of detection (Rodeberg et al., 2016). Thus, although prolonged periods of stimulation diminish dopamine release magnitudes, dopamine is still an important mediator of VTA self-stimulation behavior.
Further work has uncovered how dopamine interacts with specific postsynaptic receptors in the NAc to drive ICSS (Owesson-White et al., 2016). In this study, Owesson-White et al. (2016) employed a multimodal sensor that combines voltammetric dopamine measurements with single-unit activity. This method allows for the real-time characterization of dopaminergic modulation of cell firing in awake animals (Takmakov et al., 2011), and can be paired with iontophoresis to probe local receptor activity (Belle et al., 2013). In this study, rats were trained to press a lever for electrical stimulation of the VTA (Owesson-White et al., 2016). Dopamine was released in the NAc following cue presentation and lever press, and unit activity was associated with either the cue or the lever press, but not both. For cue-responsive cells, increased dopamine release occurred with broad increases in firing rate. For lever press– responsive cells, activity either increased or decreased, and the changes in firing rate to the press were shorter in duration than elevations in dopamine. Locations without changes in unit activity were also without dopamine release; thus, changes in activity could be attributed to dopamine’s actions at its receptors (Owesson-White et al., 2016).
To identify the receptor-level mechanisms behind dopaminergic modulation of cell firing, the authors coupled iontophoresis barrels to the multimodal sensor (Owesson-White et al., 2016). Cell firing was altered following delivery of specific pharmacological agents; thus, neurons could be chemotyped as containing D1 or D2 dopamine receptors (Belle et al., 2013). One population of lever press–responsive cells was identified as D1-containing, and two-thirds of these D1 cells were excited during the lever press. The remaining lever-press cells were identified as D2-containing, and 90% of these cells responded with an inhibition. Remarkably, when the authors identified the cue-responsive cells, they were all excitatory and D2-containing. Taken together, this study reveals dopamine responses to the cue exclusively activate D2-containing neurons in the NAc, whereas dopamine’s actions through both D1 and D2 receptors modulate the activity of lever press–responsive cells (Owesson-White et al., 2016). The coupling of iontophoresis with electrochemical and electrophysiological recordings provides a technical advantage over previous drug delivery techniques because large volumes of drugs can disrupt lever pressing for ICSS (Cheer et al., 2007a). Future work employing this discrete drug delivery technique will allow for receptor-level mechanisms to be uncovered in real time, without necessitating the use of transgenic animals. Furthermore, this technique may be extended into regions containing mixed catecholamines for signal validation prior to making measurements during behavior.
Typical ICSS stimulations also target noradrenergic axons, and, in one study, dopamine and norepinephrine responses were recorded in the dorsolateral and ventral BNST during ICSS (Park et al., 2013). Both norepinephrine and dopamine were evoked by the electrical stimulation delivered following the lever press (Table 1) (Park et al., 2013). Similar to the NAc, cues that predict lever presentation elicited dopamine release in the dorsolateral BNST; however, cues did not elicit norepinephrine release in the vBNST even during NET inhibition (Park et al., 2013). When animals underwent extinction, that is, a lever press no longer resulted in electrical stimulation, norepinephrine and dopamine overflow switched. Whereas norepinephrine was released in the vBNST at the time of stimulation anticipation, dopamine concentrations in both NAc and dorsolateral BNST decreased (Park et al., 2013) (Table 1). Although norepinephrine release is not elicited by cues predicting ICSS, its release during extinction may serve as a signal to guide action selection. When effort no longer results in reward or positive outcome, norepinephrine signaling may facilitate learning about the negative outcome. Indeed, animals lacking LC norepinephrine lever press longer and with more vigor during ICSS extinction (Mason and Iversen, 1979), and LC lesions impair attention (Selden et al., 1990). Future work should address the involvement of norepinephrine in extinction from other reward paradigms, which may provide insight into how action selection is shaped in the context of negative or unanticipated outcomes.
C. Natural Rewards
A number of studies have measured dopamine release in response to natural rewards, such as food pellets or sucrose. In general, unexpected delivery of food reward elicits dopamine release in the NAc, and the magnitude of dopamine release is greater in animals previously food-restricted (Roitman et al., 2008; Cone et al., 2014) (Table 1). Glutamatergic inputs to dopamine neurons are important for dopamine release in response to food, because mice lacking NMDARs in dopamine neurons exhibit reduced dopamine release in the NAc after unexpected food delivery (Parker et al., 2010). Additionally, dopamine release to unexpected food is potentiated by infusions of grehlin in the lateral ventricle or intra-VTA orexin-A (Cone et al., 2014).
Dietary changes can also cause adaptations in dopaminergic signaling, and a prolonged high-fat diet reduces uptake without altering DAT gene expression (Cone et al., 2013). Diet can also alter insulin sensitivity, and insulin-deficient rats have reduced DAT surface expression and dopamine uptake (Williams et al., 2007). Insulin can suppress somatodendritic dopamine concentrations in the VTA through increased uptake (Mebel et al., 2012); however, in the NAc, insulin signaling enhances dopamine release by exciting cholinergic interneurons (Stouffer et al., 2015). Importantly, the effect of insulin on striatal dopamine release is diet-dependent: rats fed an obesogenic diet exhibit a complete loss of insulin-potentiated dopamine release (Stouffer et al., 2015). It is clear that diet and insulin can alter dopaminergic function, but further work is needed to more precisely elucidate the mechanism by which diet-altered dopamine function contributes to maladaptive eating behavior.
In contrast to dopamine, little is known regarding norepinephrine signaling during caloric rewards or changes to diet, which is of particular interest given norepinephrine’s involvement in anorexia and feeding behavior (Janhunen et al., 2013; Nedelescu et al., 2016). Only one study examined norepinephrine release during food administration, and found unexpected sucrose delivery does not elicit vBNST norepinephrine release (Park et al., 2012) (Table 1). Norepinephrine release in the vBNST may instead coincide with food omission as it is during omission of ICSS reward (Park et al., 2013); however, this remains to be shown. Norepinephrine release during food omission has interesting implications in the context of feeding behavior. Because vBNST norepinephrine can influence the hypothalamic–pituitary–adrenal axis (Forray and Gysling, 2004), it may engage the brain’s stress centers to suppress feeding and promote anorexia. Future work should address the role of norepinephrine signaling during delivery and omission of high-calorie foods to determine how it contributes to dysregulated food consumption.
Food-predictive cues also elicit dopamine release in the NAc (Roitman et al., 2004) and dorsolateral striatum (Shnitko and Robinson, 2015). In the NAc, sucrose-predictive cues evoke greater dopamine release than those that predict saccharin (McCutcheon et al., 2012) (Table 1). There is some evidence that inputs from the BLA modulate NAc dopamine release to food-predictive cues, because inactivation of the BLA with baclofen/muscimol attenuates NAc dopamine evoked by sucrose-paired cues (Jones et al., 2010). Interestingly, this manipulation does not diminish VTA-stimulated dopamine release in the NAc, and suggests the glutamatergic inputs from the BLA modulate NAc dopamine through a terminally mediated mechanism (Jones et al., 2010). In support of glutamatergic influence over NAc dopamine responses, mice lacking NMDARs release smaller concentrations of dopamine in the NAc during delivery of a food reward (Parker et al., 2010). Dopamine released in response to a food-predictive cue selectively modulates cells in the NAc that are excited during the cue, but not those that are inhibited during the cue (Cacciapaglia et al., 2011). Additionally, dopamine responses vary based on NAc subregion; although cue-evoked dopamine is observed in both NAc core and shell, it is of greater magnitude and duration in the shell as compared with the core (Cacciapaglia et al., 2012). When rats must press one lever to extend a second lever for sucrose delivery, dopamine responses also vary between the NAc core and shell. In the core, dopamine release is greatest after presentation of the first lever, or the “seeking lever,” and less for subsequent presentation of the “taking lever” and reward delivery. In the shell, dopamine release is robust to both levers as well as to reward delivery (Saddoris et al., 2015a).
When animals are asked to choose between immediate and delayed food reward, dopamine responses in the shell scale with the interval between cue and reward delivery, and with an animal’s preferred reward, that is, dopamine release is greater when the interval between cue and reward is shorter, and is greater during delivery of an animal’s preferred reward (Day et al., 2010; Sugam et al., 2012; Saddoris et al., 2015b). Interestingly, optogenetic enhancement of dopamine release in the NAc during the cue alters the decisions rats make regarding which lever to press for varying reward magnitude, and they alter choice based on delay, but not reward magnitude (Saddoris et al., 2015b). Norepinephrine may be released in the vBNST during presentation of a lesser-magnitude reward than anticipated by the animal, but no studies have investigated norepinephrine signaling in paradigms involving different food reward magnitude.
Dopamine release is also elicited during delivery of a noncaloric reward. In sodium-depleted animals, NAc dopamine signaling increases when animals are given a salt solution. Over time, dopamine release moves to the salt-predictive cue in sodium-depleted rats (Cone et al., 2016). We could find no similar studies of phasic norepinephrine response to noncaloric reward.
D. Aversion
A number of studies have examined the effects of aversive stimuli on dopaminergic transmission. Systemic delivery of the aversive agent lithium chloride blunts phasic dopamine release in the NAc (Fortin et al., 2016), and oral administration of the aversive tastant quinine suppresses dopamine release in the NAc (Roitman et al., 2008) and dorsolateral BNST (Park et al., 2012). Quinine reduces dopamine tone in the NAc by reducing release frequency and is dependent on corticotropin-releasing factor (CRF) signaling (Twining et al., 2015). Blocking CRF receptors in the VTA blocks the inhibitory effect of quinine on NAc dopamine, suggesting that CRF release during aversive stimuli can act directly on VTA neurons to suppress NAc dopamine release (Twining et al., 2015). During quinine delivery, decreased dopamine in the NAc is accompanied by increased vBNST norepinephrine (Park et al., 2012). This reciprocal catecholamine signaling during negative stimuli was also found in a study examining the noxious stimulus of a tail pinch. The predominant response during tail-pinch delivery is a suppression of dopamine overflow in the NAc, whereas the same stimulus elicits norepinephrine release in the vBNST (Park et al., 2015). Although the tail-pinch study was conducted in anesthetized animals, one might suspect that norepinephrine overflow increases in awake animals during delivery of a noxious stimulus such as a foot shock. Indeed, markers of noradrenergic activity increase following foot shock (Rassnick et al., 1998; Passerin et al., 2000).
Due to electrical interference, it is difficult to make electrochemical measurements of catecholamine release during delivery of a foot shock. However, recent work has focused on contrasting dopamine signaling in the NAc during fear-predictive cues (Badrinarayan et al., 2012). Cues that predict foot shock decrease dopaminergic transmission in the NAc core by decreasing the probability of dopamine release. In the NAc shell, the same cues elicit increases in dopaminergic transmission by enhancing the amplitude of dopamine release (Badrinarayan et al., 2012). However, neither increases nor decreases in NAc dopamine were strictly associated with freezing behavior elicited by the fear-predictive cue (Badrinarayan et al., 2012). We could find no similar studies on phasic norepinephrine responses during fear-predictive cues. Norepinephrine may be released in the vBNST during a cue that predicts foot shock, as it is during the delivery of aversive quinine (Park et al., 2012), or during a tail pinch (Park et al., 2015). However, because no work has found norepinephrine responses to cues in general, further work is needed to investigate this possibility.
E. Social Interaction
Social interactions also elicit phasic dopamine responses, and the introduction of an unfamiliar rat or conspecific increases the frequency of dopamine transients (Robinson et al., 2002). Dopamine is also released in the NAc in response to prosocial ultrasonic vocalizations (Willuhn et al., 2014). However, increased dopamine release to both prosocial vocalizations and conspecific interaction declines rapidly following habituation (Robinson et al., 2002; Willuhn et al., 2014). NAc dopamine is also modulated by reward delivery to another rat, as stronger dopamine release is measured during conspecific reward receipt versus an empty box. Similar to other prosocial interactions, this response attenuates, and even becomes reversed in repeated trials, with reductions in dopamine during conspecific reward delivery (Kashtelyan et al., 2014).
In rats subjected to social defeat stress, interaction with an aggressive rat increases burst firing in the VTA and elevates the frequency of NAc dopamine transients (Anstrom et al., 2009). However, changes in norepinephrine signaling during an aggressive encounter have not been studied. Social defeat stress increases norepinephrine synthesis and NET expression in the LC (Chen et al., 2012; Fan et al., 2013), suggesting negative social interaction may alter norepinephrine release. Furthermore, when Long Evans rats are subjected to social defeat stress, some rats become aggressive. These aggressive rats have increased anxiety-like behavior, exhibit decreased latency to attack, and have increased amygdalar norepinephrine content as compared with nonaggressors (Patki et al., 2015). Given that norepinephrine influences the hypothalamic-pituitary-adrenal axis, it is tempting to hypothesize norepinephrine is released in the vBNST during an animal’s decision to fight or flee during aggressive social interactions, and this should be a topic of future investigation.
F. Drugs of Abuse
1. Psychostimulants.
Drugs of abuse have variable actions on catecholamine neurons, and those that interfere with reuptake (e.g., cocaine, psychostimulants) are traditionally classified as either blockers or releasers. Both categories can suppress firing rates through global elevation of dopamine and subsequent activation of D2 autoreceptors, although this effect varies (Shi et al., 2000, 2004; Koulchitsky et al., 2012). Blockers, such as cocaine, bind the transporter and allosterically inhibit reuptake (Torres et al., 2003), whereas releasers, such as amphetamine, reverse the transporter and release dopamine in an action-potential–independent manner (Sulzer, 2011). However, recent work challenges the action-potential independence of amphetamine-evoked dopamine release. Abolishing cell firing in dopamine neurons also abolishes amphetamine-elicited dopamine transients, suggesting that, in awake animals, amphetamine exerts its effects via an action-potential–dependent manner (Covey et al., 2016). A large number of studies have examined the effects of psychostimulants on spontaneous dopamine transients. To avoid redundant coverage, we direct the reader to a recent review (Covey et al., 2014).
2. Cannabanoids.
Emerging evidence supports cannabinoid modulation of dopamine signaling and drug reward (Cheer et al., 2007b; Loewinger et al., 2012; Oleson et al., 2012; Hernandez et al., 2014), and cannabinoid’s effects on the dopaminergic system have been recently reviewed in detail (Oleson and Cheer, 2012; Covey et al., 2015). Despite reports that cannabinoids increase LC norepinephrine activity (Oropeza et al., 2005; Page et al., 2007), how cannabinoid receptor activation influences phasic norepinephrine release has not been investigated.
3. Alcohol.
Electrophysiological data show that ethanol stimulates dopaminergic transmission (Brodie et al., 1999), and tonic activation of VTA dopamine neurons suppresses voluntary alcohol drinking by elevating basal dopamine efflux (Bass et al., 2013). However, there is disparity in alcohol’s effects on neurochemistry. Microdialysis data support a biphasic dopaminergic response, in which low doses produce increases in NAc dopamine (Yoshimoto et al., 1992) and decreases at higher doses (Blanchard et al., 1993). As measured by FSCV, acute ethanol dose-dependently decreases evoked dopamine in the dorsal striatum (Budygin et al., 2001), but there is an apparent heterogeneity of dopamine response in the NAc. In some recording locations, ethanol increases dopamine transient frequency, whereas in some the frequency is decreased or even unaffected (Robinson et al., 2009). Interestingly, cues that predict a sweetened ethanol reward are time locked to dopamine release in the dorsolateral striatum and NAc, but not in the dorsomedial striatum (Shnitko and Robinson, 2015). Although alcohol-predictive cues elicit dopamine release, the amplitude of release does not differ between rats consuming sweetened alcohol versus those consuming the sweetened solution alone (Shnitko and Robinson, 2015). This apparent heterogeneity in dopamine response to ethanol should be addressed because alcohol exposure has circuit-specific effects on dopaminergic transmission (Schindler et al., 2016). Although ethanol can enhance stimulated dopamine, its effects on endogenous dopamine release are conflicting.
It is currently unknown how ethanol impacts phasic norepinephrine release. One microdialysis study showed that ethanol dose-dependently increases basal NAc norepinephrine in animals reared in social isolation, but not those reared in group housing (Karkhanis et al., 2014). Future work should address alcohol’s effect on phasic norepinephrine signaling, particularly in the BNST, because the BNST can modulate ethanol-seeking behavior (Pina et al., 2015) and is an important structure for the development of alcohol use disorders (Kash, 2012).
4. Opiates.
In addition to alcohol, recent work has examined the effect of an acute i.v. delivery of opiates on catecholamine release. In one study, researchers delivered the opiates oxycodone and morphine to freely moving rats (Vander Weele et al., 2014). Intravenous oxycodone increases dopamine transient frequency and magnitude in the NAc for ∼1 hour; however, i.v. morphine produces a much shorter (∼1 minute) increase in phasic dopamine transmission (Vander Weele et al., 2014). In our laboratory, we extended this work to determine the impact of drug withdrawal in addition to drug exposure on dopamine release. In contrast to the previous report, s.c. administration of morphine produces a persistent (>1 hour) increase in dopamine transient frequency; however, the average magnitude of dopamine transients is similar between animals given morphine or saline (Fox et al., 2016b). This difference may reflect the differential time course of drug delivery between the two studies (Vander Weele et al., 2014; Fox et al., 2016b). Interestingly, when animals undergo naloxone-precipitated withdrawal, dopaminergic transmission decreases in the NAc (Fox et al., 2016b). In morphine-withdrawn animals, naloxone treatment reduces dopamine transient frequency back to baseline or saline conditions. Furthermore, naloxone decreases the average concentration per transient only in animals undergoing morphine withdrawal (Fox et al., 2016b). However, a single morphine withdrawal episode is insufficient to elicit persistent adaptations in dopaminergic signaling because after treatment, electrically evoked dopamine concentrations reach similar amplitudes in animals treated with naloxone after either saline or morphine (Fox et al., 2016b). Thus, the decreases in dopaminergic transmission reflect alterations of dopamine signaling specifically during the withdrawal period.
In this study, we also examined norepinephrine responses in the vBNST during morphine and precipitated withdrawal (Fox et al., 2016b). In contrast to NAc dopamine, morphine exposure does not elicit norepinephrine responses in the vBNST. However, robust norepinephrine release events occur during precipitated withdrawal that coincide with specific somatic withdrawal behaviors (Fox et al., 2016b). Interestingly, norepinephrine release during drug withdrawal persists for 10 seconds, in contrast to the brief time course (∼1 second) of spontaneous dopamine transients. These persistent norepinephrine elevations during withdrawal occur in a manner similar to norepinephrine released during ICSS extinction or quinine infusions (Park et al., 2012, 2013). Furthermore, a single withdrawal episode is sufficient to reduce evoked norepinephrine in withdrawn animals (Fox et al., 2016b), further highlighting the contrast between dopaminergic and noradrenergic signaling. Importantly, this reduction in releasable norepinephrine, but not dopamine, is in agreement with tissue content in the NAc and vBNST after repeated episodes of opiate withdrawal (McElligott et al., 2013). Together with the opposing responses highlighted in Table 1, opposing dopamine and norepinephrine signaling during drug exposure and withdrawal support reciprocal roles for catecholamines during appetitive and aversive stimuli.
G. Norepinephrine–Dopamine Interactions
Due to the opposing nature of NAc dopamine and BNST norepinephrine, it is tempting to hypothesize that these reciprocal responses reflect feedback between the two catecholaminergic systems, and that norepinephrine release may influence the reduction of dopaminergic transmission during aversive stimuli. Indeed, glutamatergic inputs from the vBNST exert excitatory influence over VTA dopamine neurons (Georges and Aston-Jones, 2002), and norepinephrine’s actions through α2A receptors decrease excitatory transmission in the vBNST (Egli et al., 2005). Norepinephrine can also act through β receptors to increase GABAA inhibition of VTA-projecting BNST neurons (Dumont and Williams, 2004), leading to increased inhibition of the VTA. In further support for noradrenergic modulation of dopamine transient concentrations, elevation of norepinephrine with systemic α2 antagonism suppresses the magnitude of spontaneous dopamine transient concentrations in the NAc (Fox et al., 2016b). The reciprocal actions of dopamine and norepinephrine during drug exposure and withdrawal (Fox et al., 2016b), reward-learning and extinction (Park et al., 2013), and appetitive and aversive tastants (Park et al., 2012) may be generalized to other paradigms; however, additional work is needed. Future work should address how these opposing responses develop longitudinally to shape learning about rewarding and aversive stimuli.
V. Clinical Implications
Voltammetric catecholamine measurements in models of human disease have provided new insights into their pathogenesis. To model Parkinson’s disease, researchers have turned to animals that express the mutant proteins found in human patients to uncover how these mutations lead to catecholaminergic deficits. For example, researchers expressed mutant human leucine-rich repeat kinase 2 in rats and found impaired striatal dopamine release. The adaptations to circuit function appear in the absence of neurodegeneration, and suggest that dopaminergic dysfunction might precede measureable markers of neurodegeneration and cell death (Sloan et al., 2016). In another study, expression of mutant α-synuclein in tyrosine–hydroxylase neurons produces differential catecholamine deficits that are regionally specific: evoked dopamine is reduced in the dorsal striatum, but not in the NAc, and vBNST norepinephrine release is unchanged (Taylor et al., 2014). These genetic manipulations afford ways to test early circuit function, and might become a useful preclinical model for testing Parkinson’s therapeutics.
Researchers have also used voltammetry to investigate aberrant dopamine signaling in Angelman syndrome. Angelman syndrome is a neurodevelopmental disorder characterized by ataxic movements, developmental delay, and excessive exuberance (Williams, 2010). Patients with Angelman syndrome lack ubiquitin ligase E3A (UBE3A), due to mutations or deletions of the maternal allele UBE3A, and recent work has used mice lacking maternal UBE3A to model the syndrome (Riday et al., 2012; Berrios et al., 2016). Mice lacking maternal UBE3A are more sensitive to VTA self-stimulation; that is, the mice acquire robust self-stimulation behavior to lower electrical stimulation currents (Riday et al., 2012), and deliver more optogenetic self-stimulations as compared with controls (Berrios et al., 2016). Despite the behavioral similarities between these two studies, they are in apparent contrast with one another with respect to changes in dopamine signaling. Riday et al. (2012) found differentially altered dopamine signaling in UBE3A-deficient mice without adaptations to the number of dopaminergic cells: whereas NAc dopamine release is increased in these mice, nigrostriatal dopamine release is attenuated. In contrast, Berrios et al. (2016) used optogenetics to elicit dopamine release in brain slices containing the NAc, and found no differences in dopamine release magnitude between UBE3A-deficient mice or their controls. However, there are important methodological differences that may contribute to the apparent differences in dopamine release between the two studies. First, direct depolarization of dopamine terminals in a brain slice is not necessarily indicative of the magnitude of dopamine release in an intact brain. Second, electrical stimulations do not provide the cell-type specificity of optogenetic approaches. Third, adaptations in afferent, nondopaminergic neurons may facilitate dysregulated signaling that may not be apparent in a slice preparation. Moving forward, we need to consider the evidence from both slice and intact animal preparations to integrate how circuit function becomes disrupted in disease models.
Recent advances have enabled researchers to also conduct electrochemical measurements in humans in parallel with deep brain stimulation probes. Human recordings typically employ a fused-silica microelectrode assembly that houses reference and recording electrodes and is positioned within or nearby deep brain stimulation probes. In the first human recordings, striatal dopamine fluctuations were measured during a decision-making task (Kishida et al., 2011). More recent work asked how dopamine encodes reward prediction errors in Parkinson’s patients (Kishida et al., 2016). Although this study found dopamine integrates reward prediction error with counterfactual prediction error, it is difficult to determine whether this is a normal property of dopamine neurons, given the relatively low dopamine concentrations and possible adaptations following pharmacological intervention in Parkinson’s patients. Human measurements are further complicated by the calibration methodology often employed. Although standardized training sets for principal component analysis may predict the identity of a given analyte (e.g., dopamine), they are notoriously poor at concentration prediction, which may lead to inappropriate interpretation of the data (Johnson et al., 2016). Nevertheless, information gleaned from preclinical disease models with recordings in humans will provide new mechanistic insight for the progression and treatment of neurodegenerative diseases.
VI. Summary
Superficially, dopaminergic and noradrenergic signaling are similar. Both catecholamines are released from comparably sized vesicles (Bergquist and Ludwig, 2008; Papke et al., 2012) and are taken up by neuronal transporters, and their release is governed by autoreceptors that operate on similar time courses (Palij and Stamford, 1993; Trendelenburg et al., 2001; Phillips et al., 2002). Despite strikingly similar mechanisms in place to control their extracellular concentrations, voltammetric measurements have uncovered contrasting release and uptake profiles for the two catecholamines. Dopamine is released faster, reaches higher concentrations, and is cleared from the extracellular space more rapidly as compared with norepinephrine (Garris and Wightman, 1995). These distinct release profiles were first appreciated in brain slices, where striatal dopamine and BNST norepinephrine release had differential dependence on stimulation intensity (Kennedy et al., 1992; Miles et al., 2002). Dopamine and norepinephrine release are similarly divergent in anesthetized animals, where identical stimulations produce markedly different concentrations of the two catecholamines (Park et al., 2011). Brain slice measurements revealed that dopamine reuptake rate is faster than that of norepinephrine, and DAT appears to play a stronger role in dopamine clearance compared with NET’s role in norepinephrine clearance (Jones et al., 1998; Xu et al., 2000; Miles et al., 2002). In anesthetized animals, clearance rates for dopamine and norepinephrine are similarly divergent (Park et al., 2011), and simultaneous blockade of DAT and dopamine D2 autoreceptors elicits spontaneous dopamine fluctuations in the striatum (Venton and Wightman, 2007). This is in contrast to simultaneous NET inhibition and adrenergic α2 antagonism, which does not produce spontaneous norepinephrine release in the vBNST of anesthetized animals (Park et al., 2011, 2015). Despite having similar mechanisms in place to control release and uptake, dopamine and norepinephrine signal in markedly different ways.
Contrasting catecholamine signaling has also been described in awake and behaving animals. Dopamine concentrations fluctuate spontaneously in the absence of overt stimuli, whereas norepinephrine concentrations do not (Robinson et al., 2002; Sombers et al., 2009; Park et al., 2012; Fox et al., 2016b). In awake animals, the time course of dopamine release is more rapid than vBNST norepinephrine, with norepinephrine release persisting for 10 seconds compared with ∼1-second dopamine fluctuations (Park et al., 2012, 2013; Fox et al., 2016b). Furthermore, current evidence suggests that striatal dopamine and vBNST norepinephrine signal in opposition of each other during appetitive and aversive stimuli (Table 1). Whereas dopamine is released during presentation of reward-predicting cues, norepinephrine is instead released during reward omission (Park et al., 2013). Dopaminergic transmission typically increases during a positive stimulus, whereas increased noradrenergic transmission is associated with a negative stimulus (Park et al., 2012). Drugs of abuse increase the frequency of dopamine transients, in contrast to norepinephrine released during drug withdrawal (Fox et al., 2016b). However, due to the limited number of phasic norepinephrine measurements, it is impossible to make definitive claims about the roles of dopamine and norepinephrine in shaping behavior. Although there is strong evidence in support of reciprocal catecholamine signaling during positive and negative stimuli, some reports show dopamine release in response to aversive stimuli (Anstrom et al., 2009; Badrinarayan et al., 2012). There may be a similarly unexpected role for phasic norepinephrine during rewarding stimuli, but further work is needed to examine this possibility.
Great strides have been made in understanding the dynamic processes controlling release and uptake of catecholamines, but there are still unresolved questions. Notably, contrasting regulation of dopamine and norepinephrine has been described in regions receiving solely dopaminergic or noradrenergic innervation, yet it is unknown whether this pattern is maintained in regions receiving mixed catecholamine innervation. Due to the indistinguishable voltammograms of dopamine and norepinephrine, it has been difficult to make voltammetric measurements in regions receiving mixed inputs. However, the application of more selective stimulation methods, combined with pharmacological signal validation, should enable voltammetric characterization of catecholamine signaling throughout the brain. The contrasting regulation of dopamine and norepinephrine, combined with their opposing nature during rewarding and aversive stimuli, may not hold true in all brain regions or behavioral paradigms, and should be a topic of ongoing investigations.
Abbreviations
- BLA
basolateral amygdala
- BNST
bed nucleus of the stria terminalis
- CPA
constant-potential amperometry
- CRF
corticotropin-releasing factor
- DAT
dopamine transporter
- FSCV
fast-scan cyclic voltammetry
- GRK2
G protein–coupled receptor kinase 2
- ICSS
intracranial self-stimulation
- LC
locus coeruleus
- L-DOPA
levodopa
- MFB
medial forebrain bundle
- NAc
nucleus accumbens
- nAChR
nicotinic acetylcholine receptor
- NET
norepinephrine transporter
- NMDAR
N-methyl-D-aspartate receptor
- NO
nitric oxide
- OCT
organic cation transporter
- OT
olfactory tubercle
- PFC
prefrontal cortex
- PPTg
pedunculopontine tegmental nucleus
- SD
Sprague-Dawley
- SN
substantia nigra
- UBE3A
ubiquitin ligase E3A
- vBNST
ventral BNST
- VTA
ventral tegmental area
- WKY
Wistar-Kyoto
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Fox, Wightman.
Footnotes
References
- Addy NA, Daberkow DP, Ford JN, Garris PA, Wightman RM. (2010) Sensitization of rapid dopamine signaling in the nucleus accumbens core and shell after repeated cocaine in rats. J Neurophysiol 104:922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstrom KK, Miczek KA, Budygin EA. (2009) Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience 161:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. (2008) Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci 28:8821–8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badrinarayan A, Wescott SA, Vander Weele CM, Saunders BT, Couturier BE, Maren S, Aragona BJ. (2012) Aversive stimuli differentially modulate real-time dopamine transmission dynamics within the nucleus accumbens core and shell. J Neurosci 32:15779–15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass CE, Grinevich VP, Gioia D, Day-Brown JD, Bonin KD, Stuber GD, Weiner JL, Budygin EA. (2013) Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Front Behav Neurosci 7:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63:182–217. [DOI] [PubMed] [Google Scholar]
- Belle AM, Owesson-White C, Herr NR, Carelli RM, Wightman RM. (2013) Controlled iontophoresis coupled with fast-scan cyclic voltammetry/electrophysiology in awake, freely moving animals. ACS Chem Neurosci 4:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist F, Ludwig M. (2008) Dendritic transmitter release: a comparison of two model systems. J Neuroendocrinol 20:677–686. [DOI] [PubMed] [Google Scholar]
- Berrios J, Stamatakis AM, Kantak PA, McElligott ZA, Judson MC, Aita M, Rougie M, Stuber GD, Philpot BD. (2016) Loss of UBE3A from TH-expressing neurons suppresses GABA co-release and enhances VTA-NAc optical self-stimulation. Nat Commun 7:10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyene M, Carelli RM, Wightman RM. (2010) Cue-evoked dopamine release in the nucleus accumbens shell tracks reinforcer magnitude during intracranial self-stimulation. Neuroscience 169:1682–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, Glick SD. (1993) Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res 17:968–973. [DOI] [PubMed] [Google Scholar]
- Brady S, Siegel G, Albers RW, Price D. (2005) Basic Neurochemistry: Molecular, Cellular and Medical Aspects, Elsevier, Burlington, MA. [Google Scholar]
- Brodie MS, Pesold C, Appel SB. (1999) Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res 23:1848–1852. [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. (2011) Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci 34:1997–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher ES, Wightman RM. (2015) Electrochemical analysis of neurotransmitters. Annu Rev Anal Chem 8:239–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, Kilpatrick MR, Gainetdinov RR, Wightman RM. (2000) Correlation between behavior and extracellular dopamine levels in rat striatum: comparison of microdialysis and fast-scan cyclic voltammetry. Neurosci Lett 281:9–12. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. (2001) Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J Pharmacol Exp Ther 297:27–34. [PubMed] [Google Scholar]
- Cacciapaglia F, Saddoris MP, Wightman RM, Carelli RM. (2012) Differential dopamine release dynamics in the nucleus accumbens core and shell track distinct aspects of goal-directed behavior for sucrose. Neuropharmacology 62:2050–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciapaglia F, Wightman RM, Carelli RM. (2011) Rapid dopamine signaling differently modulates distinct microcircuits within the nucleus accumbens during sucrose-directed behavior. J Neurosci 31:13860–13869. DOI: 10.1523/JNEUROSCI.1340-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM, Cheer JF. (2012) Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Reports 2:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Jones SR. (2014a) Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem 128:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Salahpour A, Caron MG, Jones SR. (2013) Methylphenidate amplifies the potency and reinforcing effects of amphetamines by increasing dopamine transporter expression. Nat Commun 4:2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Siciliano CA, Jones SR. (2015) Differential influence of dopamine transport rate on the potencies of cocaine, amphetamine, and methylphenidate. ACS Chem Neurosci 6:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Sun H, Eldeeb K, Luessen DJ, Feng X, Howlett AC, Jones SR, Chen R. (2014b) Amphetamine self-administration attenuates dopamine D2 autoreceptor function. Neuropsychopharmacology 39:1833–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CM, Wightman RM, Carelli RM. (2014) Dynamics of rapid dopamine release in the nucleus accumbens during goal-directed behaviors for cocaine versus natural rewards. Neuropharmacology 86:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CM, Wightman RM, Carelli RM. (2016) One month of cocaine abstinence potentiates rapid dopamine signaling in the nucleus accumbens core. Neuropharmacology DOI: 10.1016/neuropharm.2016.09.006 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, Gerhardt GA, Mayfield RD, Curella P, Zahniser NR. (1992) Differences in dopamine clearance and diffusion in rat striatum and nucleus accumbens following systemic cocaine administration. J Neurochem 59:259–266. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Liu H, Han MH. (2015) Neuronal correlates of depression. Cell Mol Life Sci 72:4825–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C. (2013) Dynorphin: still an extraordinarily potent opioid peptide. Mol Pharmacol 83:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Aragona BJ, Heien ML, Seipel AT, Carelli RM, Wightman RM. (2007a) Coordinated accumbal dopamine release and neural activity drive goal-directed behavior. Neuron 54:237–244. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. (2004) Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci 24:4393–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. (2007b) Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci 27:791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Fan Y, Li Y, Sun Z, Bissette G, Zhu MY. (2012) Chronic social defeat up-regulates expression of norepinephrine transporter in rat brains. Neurochem Int 60:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiti Z, Teschemacher AG. (2007) Exocytosis of norepinephrine at axon varicosities and neuronal cell bodies in the rat brain. FASEB J 21:2540–2550. [DOI] [PubMed] [Google Scholar]
- Cone JJ, Chartoff EH, Potter DN, Ebner SR, Roitman MF. (2013) Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS One 8:e58251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone JJ, Fortin SM, McHenry JA, Stuber GD, McCutcheon JE, Roitman MF. (2016) Physiological state gates acquisition and expression of mesolimbic reward prediction signals. Proc Natl Acad Sci USA 113:1943–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone JJ, McCutcheon JE, Roitman MF. (2014) Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci 34:4905–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Bunner KD, Schuweiler DR, Cheer JF, Garris PA. (2016) Amphetamine elevates nucleus accumbens dopamine via an action potential-dependent mechanism that is modulated by endocannabinoids. Eur J Neurosci 43:1661–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Roitman MF, Garris PA. (2014) Illicit dopamine transients: reconciling actions of abused drugs. Trends Neurosci 37:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey DP, Wenzel JM, Cheer JF. (2015) Cannabinoid modulation of drug reward and the implications of marijuana legalization. Brain Res 1628(Pt A):233-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Nicholson C, Kume-Kick J, Tao L, Rice ME. (2001) Dopamine-mediated volume transmission in midbrain is regulated by distinct extracellular geometry and uptake. J Neurophysiol 85:1761–1771. [DOI] [PubMed] [Google Scholar]
- Daigle TL, Ferris MJ, Gainetdinov RR, Sotnikova TD, Urs NM, Jones SR, Caron MG. (2014) Selective deletion of GRK2 alters psychostimulant-induced behaviors and dopamine neurotransmission. Neuropsychopharmacology 39:2450–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC. (2009) Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Ther 121:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Jones JL, Wightman RM, Carelli RM. (2010) Phasic nucleus accumbens dopamine release encodes effort- and delay-related costs. Biol Psychiatry 68:306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. (2015) Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci 18:1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. (2004) Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci 24:8198–8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. (2005) Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology 30:657–668. [DOI] [PubMed] [Google Scholar]
- Ehrich JM, Phillips PE, Chavkin C. (2014) Kappa opioid receptor activation potentiates the cocaine-induced increase in evoked dopamine release recorded in vivo in the mouse nucleus accumbens. Neuropsychopharmacology 39:3036–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Clements MA, Hartung H, McIntosh JM, Cragg SJ. (2008) Alpha6-containing nicotinic acetylcholine receptors dominate the nicotine control of dopamine neurotransmission in nucleus accumbens. Neuropsychopharmacology 33:2158–2166. [DOI] [PubMed] [Google Scholar]
- Falkenburger BH, Barstow KL, Mintz IM. (2001) Dendrodendritic inhibition through reversal of dopamine transport. Science 293:2465–2470. [DOI] [PubMed] [Google Scholar]
- Fan Y, Chen P, Li Y, Zhu MY. (2013) Effects of chronic social defeat on expression of dopamine β-hydroxylase in rat brains. Synapse 67:300–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria MP, Miguel TT, Gomes KS, Nunes-de-Souza RL. (2016) Anxiety-like responses induced by nitric oxide within the BNST in mice: role of CRF1 and NMDA receptors. Horm Behav 79:74–83. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Mateo Y, Melchior JR, Roberts DC, Jones SR. (2012) Cocaine self-administration produces pharmacodynamic tolerance: differential effects on the potency of dopamine transporter blockers, releasers, and methylphenidate. Neuropsychopharmacology 37:1708–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Rose JH, Siciliano CA, Sun H, Chen R, Jones SR. (2015) A single amphetamine infusion reverses deficits in dopamine nerve-terminal function caused by a history of cocaine self-administration. Neuropsychopharmacology 40:1826–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris MJ, Mateo Y, Roberts DCS, Jones SR. (2011) Cocaine-insensitive dopamine transporters with intact substrate transport produced by self-administration. Biol Psychiatry 69:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forray MI, Gysling K. (2004) Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Brain Res Rev 47:145–160. [DOI] [PubMed] [Google Scholar]
- Fortin SM, Chartoff EH, Roitman MF. (2016) The aversive agent lithium chloride suppresses phasic dopamine release through central GLP-1 receptors. Neuropsychopharmacology 41:906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox ME, Mikhailova MA, Bass CE, Takmakov P, Gainetdinov RR, Budygin EA, Wightman RM. (2016a) Cross-hemispheric dopamine projections have functional significance. Proc Natl Acad Sci USA 113:6985–6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox ME, Rodeberg NT, Wightman RM. (2016b) Reciprocal catecholamine changes during opiate exposure and withdrawal. Neuropsychopharmacology DOI: 10.1038/npp.2016.135 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox ME, Studebaker RI, Swofford NJ, Wightman RM. (2015) Stress and drug dependence differentially modulate norepinephrine signaling in animals with varied HPA axis function. Neuropsychopharmacology 40:1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Christensen JRC, Rebec GV, Wightman RM. (1997) Real-time measurement of electrically evoked extracellular dopamine in the striatum of freely moving rats. J Neurochem 68:152–161. [DOI] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. (1994) Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci 14:6084–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Collins LB, Jones SR, Wightman RM. (1993) Evoked extracellular dopamine in vivo in the medial prefrontal cortex. J Neurochem 61:637–647. [DOI] [PubMed] [Google Scholar]
- Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. (1999) Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature 398:67–69. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. (1994a) Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci 14:442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. (1994b) In vivo voltammetric measurement of evoked extracellular dopamine in the rat basolateral amygdaloid nucleus. J Physiol 478:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Wightman R. (1995) Regional differences in dopamine release, uptake, and diffusion measured by fast-scan cyclic voltammetry, in Voltammetric Methods in Brain Systems (Boulton AA, Baker GB, Adams RN. eds) pp 179–220, Humana Press, Totowa, NJ. [Google Scholar]
- Gasser PJ, Lowry CA, Orchinik M. (2006) Corticosterone-sensitive monoamine transport in the rat dorsomedial hypothalamus: potential role for organic cation transporter 3 in stress-induced modulation of monoaminergic neurotransmission. J Neurosci 26:8758–8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser PJ, Orchinik M, Raju I, Lowry CA. (2009) Distribution of organic cation transporter 3, a corticosterone-sensitive monoamine transporter, in the rat brain. J Comp Neurol 512:529–555. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. (2005) Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol 490:270–294. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. (2002) Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci 22:5173–5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt GA, Ksir C, Pivik C, Dickinson SD, Sabeti J, Zahniser NR. (1999) Methodology for coupling local application of dopamine and other chemicals with rapid in vivo electrochemical recordings in freely-moving rats. J Neurosci Methods 87:67–76. [DOI] [PubMed] [Google Scholar]
- Gerhardt GA, Oke AF, Nagy G, Moghaddam B, Adams RN. (1984) Nafion-coated electrodes with high selectivity for CNS electrochemistry. Brain Res 290:390–395. [DOI] [PubMed] [Google Scholar]
- Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, Robble MA, Vranjkovic O, Wheeler DS, Mantsch JR, et al. (2013) Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci 33:11800–11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfpenny DM, Callado LF, Hopwood SE, Bamigbade TA, Langford RM, Stamford JA. (1999) Effects of tramadol stereoisomers on norepinephrine efflux and uptake in the rat locus coeruleus measured by real time voltammetry. Br J Anaesth 83:909–915. [DOI] [PubMed] [Google Scholar]
- Hartung H, Threlfell S, Cragg SJ. (2011) Nitric oxide donors enhance the frequency dependence of dopamine release in nucleus accumbens. Neuropsychopharmacology 36:1811–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harun R, Hare KM, Brough ME, Munoz MJ, Grassi CM, Torres GE, Grace AA, and Wagner AK (2015) Fast-scan cyclic voltammetry demonstrates that L-DOPA produces dose-dependent regionally selective, bimodal effects on striatal dopamine kinetics in vivo. J Neurochem DOI: 10.1111/jnc.13444 [published ahead of print]. [DOI] [PMC free article] [PubMed]
- Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. (2005) Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci USA 102:10023–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez G, Oleson EB, Gentry RN, Abbas Z, Bernstein DL, Arvanitogiannis A, Cheer JF. (2014) Endocannabinoids promote cocaine-induced impulsivity and its rapid dopaminergic correlates. Biol Psychiatry 75:487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr NR, Park J, McElligott ZA, Belle AM, Carelli RM, Wightman RM. (2012) In vivo voltammetry monitoring of electrically evoked extracellular norepinephrine in subregions of the bed nucleus of the stria terminalis. J Neurophysiol 107:1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood SE, Owesson CA, Callado LF, McLaughlin DP, Stamford JA. (2001) Effects of chronic tramadol on pre- and post-synaptic measures of monoamine function. J Psychopharmacol 15:147–153. [DOI] [PubMed] [Google Scholar]
- Howard CD, Daberkow DP, Ramsson ES, Keefe KA, Garris PA. (2013) Methamphetamine-induced neurotoxicity disrupts naturally occurring phasic dopamine signaling. Eur J Neurosci 38:2078–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard CD, Keefe KA, Garris PA, Daberkow DP. (2011) Methamphetamine neurotoxicity decreases phasic, but not tonic, dopaminergic signaling in the rat striatum. J Neurochem 118:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janhunen SK, la Fleur SE, Adan RA. (2013) Blocking alpha2A adrenoceptors, but not dopamine receptors, augments bupropion-induced hypophagia in rats. Obesity 21:E700–E708. [DOI] [PubMed] [Google Scholar]
- Johnson JA, Rodeberg NT, Wightman RM. (2016) Failure of standard training sets in the analysis of fast-scan cyclic voltammetry data. ACS Chem Neurosci 7:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Day JJ, Aragona BJ, Wheeler RA, Wightman RM, Carelli RM. (2010) Basolateral amygdala modulates terminal dopamine release in the nucleus accumbens and conditioned responding. Biol Psychiatry 67:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. (1999) Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci 2:649–655. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. (1998) Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA 95:4029–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SR, O’Dell SJ, Marshall JF, Wightman RM. (1996) Functional and anatomical evidence for different dopamine dynamics in the core and shell of the nucleus accumbens in slices of rat brain. Synapse 23:224–231. [DOI] [PubMed] [Google Scholar]
- Karkhanis AN, Locke JL, McCool BA, Weiner JL, Jones SR. (2014) Social isolation rearing increases nucleus accumbens dopamine and norepinephrine responses to acute ethanol in adulthood. Alcohol Clin Exp Res 38:2770–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL. (2012) The role of biogenic amine signaling in the bed nucleus of the stria terminals in alcohol abuse. Alcohol 46:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Pleil KE, Marcinkiewcz CA, Lowery-Gionta EG, Crowley N, Mazzone C, Sugam J, Hardaway JA, McElligott ZA. (2015) Neuropeptide regulation of signaling and behavior in the BNST. Mol Cells 38:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashtelyan V, Lichtenberg NT, Chen ML, Cheer JF, Roesch MR. (2014) Observation of reward delivery to a conspecific modulates dopamine release in ventral striatum. Curr Biol 24:2564–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley RB, Heien ML, Wightman RM. (2009) Multivariate concentration determination using principal component regression with residual analysis. Trends Analyt Chem 28:1127–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RS, Wightman RM. (1987) Detection of dopamine overflow and diffusion with voltammetry in slices of rat brain. Brain Res 423:79–87. [DOI] [PubMed] [Google Scholar]
- Kennedy RT, Jones SR, Wightman RM. (1992) Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J Neurochem 59:449–455. [DOI] [PubMed] [Google Scholar]
- Kishida KT, Saez I, Lohrenz T, Witcher MR, Laxton AW, Tatter SB, White JP, Ellis TL, Phillips PE, Montague PR. (2016) Subsecond dopamine fluctuations in human striatum encode superposed error signals about actual and counterfactual reward. Proc Natl Acad Sci USA 113:200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida KT, Sandberg SG, Lohrenz T, Comair YG, Sáez I, Phillips PE, Montague PR. (2011) Sub-second dopamine detection in human striatum. PLoS One 6:e23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissinger PT, Hart JB, Adams RN. (1973) Voltammetry in brain tissue: a new neurophysiological measurement. Brain Res 55:209–213. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulchitsky S, De Backer B, Quertemont E, Charlier C, Seutin V. (2012) Differential effects of cocaine on dopamine neuron firing in awake and anesthetized rats. Neuropsychopharmacology 37:1559–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewinger GC, Beckert MV, Tejeda HA, Cheer JF. (2012) Methamphetamine-induced dopamine terminal deficits in the nucleus accumbens are exacerbated by reward-associated cues and attenuated by CB1 receptor antagonism. Neuropharmacology 62:2192–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason ST, Iversen SD. (1979) Theories of the dorsal bundle extinction effect. Brain Res 180:107–137. [DOI] [PubMed] [Google Scholar]
- Mateo Y, Lack CM, Morgan D, Roberts DCS, Jones SR. (2005) Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology 30:1455–1463. [DOI] [PubMed] [Google Scholar]
- May LJ, Wightman RM. (1989) Heterogeneity of stimulated dopamine overflow within rat striatum as observed with in vivo voltammetry. Brain Res 487:311–320. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Beeler JA, Roitman MF. (2012) Sucrose-predictive cues evoke greater phasic dopamine release than saccharin-predictive cues. Synapse 66:346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Cone JJ, Sinon CG, Fortin SM, Kantak PA, Witten IB, Deisseroth K, Stuber GD, Roitman MF. (2014) Optical suppression of drug-evoked phasic dopamine release. Front Neural Circuits 8:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElligott ZA, Fox ME, Walsh PL, Urban DJ, Ferrel MS, Roth BL, Wightman RM. (2013) Noradrenergic synaptic function in the bed nucleus of the stria terminalis varies in animal models of anxiety and addiction. Neuropsychopharmacology 38:1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebel DM, Wong JC, Dong YJ, Borgland SL. (2012) Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. Eur J Neurosci 36:2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles PR, Mundorf ML, Wightman RM. (2002) Release and uptake of catecholamines in the bed nucleus of the stria terminalis measured in the mouse brain slice. Synapse 44:188–197. [DOI] [PubMed] [Google Scholar]
- Mitchell K, Adams RN. (1993) Comparison of the effects of voltage-sensitive calcium channel antagonism on the electrically stimulated release of dopamine and norepinephrine in vivo. Brain Res 604:349–353. [DOI] [PubMed] [Google Scholar]
- Mitchell K, Oke AF, Adams RN. (1994) In vivo dynamics of norepinephrine release-reuptake in multiple terminal field regions of rat brain. J Neurochem 63:917–926. [DOI] [PubMed] [Google Scholar]
- Morón JA, Brockington A, Wise RA, Rocha BA, Hope BT. (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 22:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Near JA, Bigelow JC, Wightman RM. (1988) Comparison of uptake of dopamine in rat striatal chopped tissue and synaptosomes. J Pharmacol Exp Ther 245:921–927. [PubMed] [Google Scholar]
- Nedelescu H, Chowdhury TG, Wable GS, Arbuthnott G, Aoki C. (2016) Cerebellar sub-divisions differ in exercise-induced plasticity of noradrenergic axons and in their association with resilience to activity-based anorexia. Brain Struct Funct DOI: 10.1007/s00429-016-1220-2 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds J, Milner P. (1954) Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 47:419–427. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Beckert MV, Morra JT, Lansink CS, Cachope R, Abdullah RA, Loriaux AL, Schetters D, Pattij T, Roitman MF, et al. (2012) Endocannabinoids shape accumbal encoding of cue-motivated behavior via CB1 receptor activation in the ventral tegmentum. Neuron 73:360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Cheer JF. (2012) A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harb Perspect Med 2:a012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oropeza VC, Page ME, Van Bockstaele EJ. (2005) Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res 1046:45–54. [DOI] [PubMed] [Google Scholar]
- Owesson CA, Hopwood SE, Callado LF, Seif I, McLaughlin DP, Stamford JA. (2002) Altered presynaptic function in monoaminergic neurons of monoamine oxidase-A knockout mice. Eur J Neurosci 15:1516–1522. [DOI] [PubMed] [Google Scholar]
- Owesson CA, Seif I, McLaughlin DP, Stamford JA. (2003) Different alpha(2) adrenoceptor subtypes control noradrenaline release and cell firing in the locus coeruleus of wildtype and monoamine oxidase-A knockout mice. Eur J Neurosci 18:34–42. [DOI] [PubMed] [Google Scholar]
- Owesson-White C, Belle AM, Herr NR, Peele JL, Gowrishankar P, Carelli RM, Wightman RM. (2016) Cue-evoked dopamine release rapidly modulates D2 neurons in the nucleus accumbens during motivated behavior. J Neurosci 36:6011–6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Cheer JF, Beyene M, Carelli RM, Wightman RM. (2008) Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc Natl Acad Sci USA 105:11957–11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Roitman MF, Sombers LA, Belle AM, Keithley RB, Peele JL, Carelli RM, Wightman RM. (2012) Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J Neurochem 121:252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Sparks SE, Qian Y, Menko AS, Van Bockstaele EJ. (2007) Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol Biochem Behav 86:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palij P, Bull DR, Sheehan MJ, Millar J, Stamford J, Kruk ZL, Humphrey PP. (1990) Presynaptic regulation of dopamine release in corpus striatum monitored in vitro in real time by fast cyclic voltammetry. Brain Res 509:172–174. [DOI] [PubMed] [Google Scholar]
- Palij P, Stamford JA. (1992) Real-time monitoring of endogenous noradrenaline release in rat brain slices using fast cyclic voltammetry: 1. Characterisation of evoked noradrenaline efflux and uptake from nerve terminals in the bed nucleus of stria terminalis, pars ventralis. Brain Res 587:137–146. [DOI] [PubMed] [Google Scholar]
- Palij P, Stamford JA. (1993) Real-time monitoring of endogenous noradrenaline release in rat brain slices using fast cyclic voltammetry. 2. Operational characteristics of the alpha 2 autoreceptor in the bed nucleus of stria terminalis, pars ventralis. Brain Res 607:134–140. [DOI] [PubMed] [Google Scholar]
- Papke JB, Moore-Dotson JM, Watson DJ, Wedell CD, French LR, Rendell SR, Harkins AB. (2012) Titration of synaptotagmin I expression differentially regulates release of norepinephrine and neuropeptide Y. Neuroscience 218:78–88. [DOI] [PubMed] [Google Scholar]
- Park J, Aragona BJ, Kile BM, Carelli RM, Wightman RM. (2010) In vivo voltammetric monitoring of catecholamine release in subterritories of the nucleus accumbens shell. Neuroscience 169:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Bucher ES, Budygin EA, Wightman RM. (2015) Norepinephrine and dopamine transmission in 2 limbic regions differentially respond to acute noxious stimulation. Pain 156:318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Bucher ES, Fontillas K, Owesson-White C, Ariansen JL, Carelli RM, Wightman RM. (2013) Opposing catecholamine changes in the bed nucleus of the stria terminalis during intracranial self-stimulation and its extinction. Biol Psychiatry 74:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kile BM, Wightman RM. (2009) In vivo voltammetric monitoring of norepinephrine release in the rat ventral bed nucleus of the stria terminalis and anteroventral thalamic nucleus. Eur J Neurosci 30:2121–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Takmakov P, Wightman RM. (2011) In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. J Neurochem 119:932–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Wheeler RA, Fontillas K, Keithley RB, Carelli RM, Wightman RM. (2012) Catecholamines in the bed nucleus of the stria terminalis reciprocally respond to reward and aversion. Biol Psychiatry 71:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JG, Zweifel LS, Clark JJ, Evans SB, Phillips PE, Palmiter RD. (2010) Absence of NMDA receptors in dopamine neurons attenuates dopamine release but not conditioned approach during Pavlovian conditioning. Proc Natl Acad Sci USA 107:13491–13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passerin AM, Cano G, Rabin BS, Delano BA, Napier JL, Sved AF. (2000) Role of locus coeruleus in foot shock-evoked Fos expression in rat brain. Neuroscience 101:1071–1082. [DOI] [PubMed] [Google Scholar]
- Patki G, Atrooz F, Alkadhi I, Solanki N, Salim S. (2015) High aggression in rats is associated with elevated stress, anxiety-like behavior, and altered catecholamine content in the brain. Neurosci Lett 584:308–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattison LP, McIntosh S, Budygin EA, Hemby SE. (2012) Differential regulation of accumbal dopamine transmission in rats following cocaine, heroin and speedball self-administration. J Neurochem 122:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JL, Miner LH, Michael AC, Sesack SR. (2004) Ultrastructure at carbon fiber microelectrode implantation sites after acute voltammetric measurements in the striatum of anesthetized rats. J Neurosci Methods 137:9–23. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. (2003) Subsecond dopamine release promotes cocaine seeking. Nature 422:614–618. [DOI] [PubMed] [Google Scholar]
- Phillips PEM, Hancock PJ, Stamford JA. (2002) Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse 44:15–22. [DOI] [PubMed] [Google Scholar]
- Pina MM, Young EA, Ryabinin AE, Cunningham CL. (2015) The bed nucleus of the stria terminalis regulates ethanol-seeking behavior in mice. Neuropharmacology 99:627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassnick S, Hoffman GE, Rabin BS, Sved AF. (1998) Injection of corticotropin-releasing hormone into the locus coeruleus or foot shock increases neuronal Fos expression. Neuroscience 85:259–268. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Christensen JR, Guerra C, Bardo MT. (1997) Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res 776:61–67. [DOI] [PubMed] [Google Scholar]
- Riday TT, Dankoski EC, Krouse MC, Fish EW, Walsh PL, Han JE, Hodge CW, Wightman RM, Philpot BD, Malanga CJ. (2012) Pathway-specific dopaminergic deficits in a mouse model of Angelman syndrome. J Clin Invest 122:4544–4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Heien ML, Wightman RM. (2002) Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci 22:10477–10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Hermans A, Seipel AT, Wightman RM. (2008) Monitoring rapid chemical communication in the brain. Chem Rev 108:2554–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM. (2009) Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol Clin Exp Res 33:1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Phillips PE, Budygin EA, Trafton BJ, Garris PA, Wightman RM. (2001) Sub-second changes in accumbal dopamine during sexual behavior in male rats. Neuroreport 12:2549–2552. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Wightman RM. (2004) Nomifensine amplifies subsecond dopamine signals in the ventral striatum of freely-moving rats. J Neurochem 90:894–903. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Howard CD, Pastuzyn ED, Byers DL, Keefe KA, Garris PA. (2014) Methamphetamine-induced neurotoxicity disrupts pharmacologically evoked dopamine transients in the dorsomedial and dorsolateral striatum. Neurotox Res 26:152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeberg NT, Johnson JA, Bucher ES, Wightman RM. (2016) Dopamine dynamics during continuous intracranial self-stimulation: effect of waveform on fast-scan cyclic voltammetry data. ACS Chem Neurosci DOI: 10.1021/acschemneuro.6b00142 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodeberg NT, Johnson JA, Cameron CM, Saddoris MP, Carelli RM, Wightman RM. (2015) Construction of training sets for valid calibration of in vivo cyclic voltammetric data by principal component analysis. Anal Chem 87:11484–11491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. (2004) Dopamine operates as a subsecond modulator of food seeking. J Neurosci 24:1265–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. (2008) Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci 11:1376–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Corrales MO, Gibert-Rahola J, Micó JA. (1998) Tramadol induces antidepressant-type effects in mice. Life Sci 63:PL175–PL180. [DOI] [PubMed] [Google Scholar]
- Roth BL. (2016) DREADDs for neuroscientists. Neuron 89:683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J, Adams CE, Burmeister J, Gerhardt GA, Zahniser NR. (2002) Kinetic analysis of striatal clearance of exogenous dopamine recorded by chronoamperometry in freely-moving rats. J Neurosci Methods 121:41–52. [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Cacciapaglia F, Wightman RM, Carelli RM. (2015a) Differential dopamine release dynamics in the nucleus accumbens core and shell reveal complementary signals for error prediction and incentive motivation. J Neurosci 35:11572–11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM. (2015b) Mesolimbic dopamine dynamically tracks, and is causally linked to, discrete aspects of value-based decision making. Biol Psychiatry 77:903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, et al. (2008) Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc Natl Acad Sci USA 105:4405–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas AG, Davis MI, Lovinger DM, Mateo Y. (2016) Dopamine dynamics and cocaine sensitivity differ between striosome and matrix compartments of the striatum. Neuropharmacology 108:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AHV. (2009) Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol Sci 30:41–47. [DOI] [PubMed] [Google Scholar]
- Schindler AG, Soden ME, Zweifel LS, Clark JJ. (2016) Reversal of alcohol-induced dysregulation in dopamine network dynamics may rescue maladaptive decision-making. J Neurosci 36:3698–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JA, McCafferty MR, Unterwald EM. (2009) Regulation of dynamin 2 and G protein-coupled receptor kinase 2 in rat nucleus accumbens during acute and repeated cocaine administration. Synapse 63:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. (2013) Updating dopamine reward signals. Curr Opin Neurobiol 23:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden NR, Robbins TW, Everitt BJ. (1990) Enhanced behavioral conditioning to context and impaired behavioral and neuroendocrine responses to conditioned stimuli following ceruleocortical noradrenergic lesions: support for an attentional hypothesis of central noradrenergic function. J Neurosci 10:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhang XX, Jones MD, Bunney BS. (2000) Dual effects of D-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J Neurosci 20:3504–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhou Y. (2004) Psychostimulants induce low-frequency oscillations in the firing activity of dopamine neurons. Neuropsychopharmacology 29:2160–2167. [DOI] [PubMed] [Google Scholar]
- Shnitko TA, Kennerly LC, Spear LP, Robinson DL. (2014) Ethanol reduces evoked dopamine release and slows clearance in the rat medial prefrontal cortex. Alcohol Clin Exp Res 38:2969–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA, Robinson DL. (2014) Anatomical and pharmacological characterization of catecholamine transients in the medial prefrontal cortex evoked by ventral tegmental area stimulation. Synapse 68:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA, Robinson DL. (2015) Regional variation in phasic dopamine release during alcohol and sucrose self-administration in rats. ACS Chem Neurosci 6:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA, Spear LP, Robinson DL. (2016) Adolescent binge-like alcohol alters sensitivity to acute alcohol effects on dopamine release in the nucleus accumbens of adult rats. Psychopharmacology 233:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Ferris MJ, Jones SR. (2015a) Adaptations of presynaptic dopamine terminals induced by psychostimulant self-administration. ACS Chem Neurosci 6:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Jones SR. (2014) Amphetamine potency varies with dopamine uptake rate across striatal subregions. J Neurochem 131:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Ferris MJ, Jones SR. (2015b) Cocaine self-administration disrupts mesolimbic dopamine circuit function and attenuates dopaminergic responsiveness to cocaine. Eur J Neurosci 42:2091–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson EH, Morud J, Winiger V, Biezonski D, Zhu JP, Bach ME, Malleret G, Polan HJ, Ng-Evans S, Phillips PE, et al. (2014) Genetic variation in COMT activity impacts learning and dopamine release capacity in the striatum. Learn Mem 21:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan M, Alegre-Abarrategui J, Potgieter D, Kaufmann AK, Exley R, Deltheil T, Threlfell S, Connor-Robson N, Brimblecombe K, Wallings R, et al. (2016) LRRK2 BAC transgenic rats develop progressive, L-DOPA-responsive motor impairment, and deficits in dopamine circuit function. Hum Mol Genet 25:951–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. (2009) Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci 29:1735–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Boivin JR, Saunders BT, Witten IB, Deisseroth K, Janak PH. (2014) Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens. PLoS One 9:e94771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouffer MA, Woods CA, Patel JC, Lee CR, Witkovsky P, Bao L, Machold RP, Jones KT, de Vaca SC, Reith ME, et al. (2015) Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun 6:8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. (2005) Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology 30:853–863. [DOI] [PubMed] [Google Scholar]
- Sugam JA, Day JJ, Wightman RM, Carelli RM. (2012) Phasic nucleus accumbens dopamine encodes risk-based decision-making behavior. Biol Psychiatry 71:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D. (2011) How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69:628–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Cragg SJ, Rice ME. (2016) Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia 6:123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takmakov P, McKinney CJ, Carelli RM, Wightman RM. (2011) Instrumentation for fast-scan cyclic voltammetry combined with electrophysiology for behavioral experiments in freely moving animals. Rev Sci Instrum 82:074302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TN, Potgieter D, Anwar S, Senior SL, Janezic S, Threlfell S, Ryan B, Parkkinen L, Deltheil T, Cioroch M, et al. (2014) Region-specific deficits in dopamine, but not norepinephrine, signaling in a novel A30P α-synuclein BAC transgenic mouse. Neurobiol Dis 62:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. (2012) Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75:58–64. [DOI] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG. (2003) Plasma membrane monoamine transporters: structure, regulation and function. Nat Rev Neurosci 4:13–25. [DOI] [PubMed] [Google Scholar]
- Trendelenburg AU, Klebroff W, Hein L, Starke K. (2001) A study of presynaptic alpha2-autoreceptors in alpha2A/D-, alpha2B- and alpha2C-adrenoceptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol 364:117–130. [DOI] [PubMed] [Google Scholar]
- Trout SJ, Kruk ZL. (1992) Differences in evoked dopamine efflux in rat caudate putamen, nucleus accumbens and tuberculum olfactorium in the absence of uptake inhibition: influence of autoreceptors. Br J Pharmacol 106:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining RC, Wheeler DS, Ebben AL, Jacobsen AJ, Robble MA, Mantsch JR, Wheeler RA. (2015) Aversive stimuli drive drug seeking in a state of low dopamine tone. Biol Psychiatry 77:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Weele CM, Porter-Stransky KA, Mabrouk OS, Lovic V, Singer BF, Kennedy RT, Aragona BJ. (2014) Rapid dopamine transmission within the nucleus accumbens: dramatic difference between morphine and oxycodone delivery. Eur J Neurosci 40:3041–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Wightman RM. (2007) Pharmacologically induced, subsecond dopamine transients in the caudate-putamen of the anesthetized rat. Synapse 61:37–39. [DOI] [PubMed] [Google Scholar]
- Wakabayashi KT, Bruno MJ, Bass CE, Park J. (2016) Application of fast-scan cyclic voltammetry for the in vivo characterization of optically evoked dopamine in the olfactory tubercle of the rat brain. Analyst 141:3746–3755. [DOI] [PubMed] [Google Scholar]
- Weinshenker D. (2008) Functional consequences of locus coeruleus degeneration in Alzheimer’s disease. Curr Alzheimer Res 5:342–345. [DOI] [PubMed] [Google Scholar]
- Wickham R, Solecki W, Rathbun L, McIntosh JM, Addy NA. (2013) Ventral tegmental area α6β2 nicotinic acetylcholine receptors modulate phasic dopamine release in the nucleus accumbens core. Psychopharmacology 229:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. (1988) Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience 25:513–523. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Carelli RM. (2007) Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci 26:2046–2054. [DOI] [PubMed] [Google Scholar]
- Williams CA. (2010) The behavioral phenotype of the Angelman syndrome. Am J Med Genet C Semin Med Genet 154C:432–437. [DOI] [PubMed] [Google Scholar]
- Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, France CP, Gore JC, Daws LC, Avison MJ, et al. (2007) Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol 5:e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Tose A, Wanat MJ, Hart AS, Hollon NG, Phillips PE, Schwarting RK, Wöhr M. (2014) Phasic dopamine release in the nucleus accumbens in response to pro-social 50 kHz ultrasonic vocalizations in rats. J Neurosci 34:10616–10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, et al. (2011) Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron 72:721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG. (2000) Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci 3:465–471. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Calipari ES, Ferris MJ, Karkhanis AN, Fordahl SC, Weiner JL, Jones SR. (2016) Social isolation rearing increases dopamine uptake and psychostimulant potency in the striatum. Neuropharmacology 101:471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. (1992) Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol 9:17–22. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Larson GA, Gerhardt GA. (1999) In vivo dopamine clearance rate in rat striatum: regulation by extracellular dopamine concentration and dopamine transporter inhibitors. J Pharmacol Exp Ther 289:266–277. [PubMed] [Google Scholar]
- Zhang L, Doyon WM, Clark JJ, Phillips PE, Dani JA. (2009) Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol Pharmacol 76:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, et al. (2009) Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci USA 106:7281–7288. [DOI] [PMC free article] [PubMed] [Google Scholar]