Abstract
Objectives
We aimed to assess the effect of metabolic syndrome (MetS) on incident oral potentially malignant disorder (OPMD).
Design
We conducted a prospective cohort study of the Changhua community-based integrated screening (CHCIS) programme and nationwide oral cancer screening programme during the period between 2005 and 2014.
Setting
CHCIS, Taiwan.
Participants
We enrolled 17 590 participants aged 30 years and older.
Main outcomes and measures
We assessed the impact of MetS on the outcome measured by incident OPMD.
Results
The incidences of OPMD among subjects with and without MetS were 7.68 ‰ and 5.38 ‰, respectively. After adjusting for confounders, subjects with MetS exhibited a statistically greater risk of developing OPMD compared with those who were free of MetS by 33% (adjusted rate ratio, aRR=1.33, 95% CI 1.14 to 1.55). Individual components of MetS still remained significant, including central obesity (aRR=1.22, 95% CI 1.04 to 1.44), hypertriglyceridaemia (aRR=1.26, 95% CI 1.07 to 1.49) and hyperglycaemia (aRR=1.20, 95% CI 1.02 to 1.41). Central obesity and hypertriglyceridaemia were also statistically associated with a subtype of OPMD, namely, leukoplakia.
Conclusion
The temporal influence of MetS on the risk of incident OPMD was noted in our prospective cohort study. Therefore, promoting an MetS prevention and control programme might reduce the occurrence of OPMD and oral cancer.
Keywords: oral medicine, oncology, epidemiology
Strengths and limitations of this study.
A large population-based prospective cohort study was conducted to examine the impact of metabolic syndrome (MetS) on incident oral potentially malignant disorder (OPMD).
This is the first study to investigate the effect of MeTS on incidence of OPMD as well as subtypes of OPMD, especially leukoplakia and oral submucous fibrosis.
Investigations into other subtypes of OPMD are limited due to the rarity of other OPMD cases in our population.
The results of our study are based on a Taiwanese population 30 years and older, so the generalisation of our results to other regions would be limited especially given ethnic, genetic and dietary features.
Introduction
Oral potentially malignant disorder (OPMD) is an disorder that has potential for subsequent progression to oral cancer.1 Thus, a better understanding of the risk factors for the occurrence of OPMD is important for the primary prevention of oral cancer.2 Evidence on tobacco use, betel quid chewing and alcohol drinking has well documented these major risk factors for OPMD.3 4 Metabolic syndrome (MetS) is associated with the increased risk of several cancers, including oral cancer.5 6 MetS is also associated with OPMD.7 8 Such an association due to common shared underlying pathways (such as chronic inflammation) could be attributed to OPMD. Several studies have proposed the possible biological linkage between OPMD and MetS, which may have proinflammatory markers and insulin resistance in common.9 10 However, the true biological causes accounting for such an association between MetS and OPMD remain elusive. In spite of this, it is still very worthwhile to study how MetS is associated with OPMD by clarifying the temporal relationship between MetS and OPMD. A prospective cohort study is, therefore, required.
In the Changhua community-based integrated screening (CHCIS) programme, a routine health check-up that embraces biomarker tests for MetS has been conducted annually since 2005.11 The early detection of OPMD and oral cancer has been provided under the instruction of nationwide oral cancer screening programme.12 This screened cohort provides an opportunity to elucidate the effect of MetS on the incidence of OPMD with a normal cohort at baseline and followed over time until 2014.
Using empirical data from a large population-based integrated screening programme in combination with a nationwide oral cancer screening programme with oral visual inspection, the major aim of this study was to assess the temporal influence of MetS on OPMD.
Materials and methods
Study design
Our study design consists of two main steps. The first step is tailored for prevalence (cross-sectional design), and the second step is a longitudinal follow-up for incident cases of OPMD (figure 1). We conducted cross-sectional analysis to determine the prevalence of OPMD among the MetS and MetS-free groups at baseline (identified at the first screening round) to create a normal cohort by excluding those who were diagnosed with OPMD or oral cancer before or at the first screening. Subjects in the normal cohort have undergone repeated screening continuously.
Figure 1.
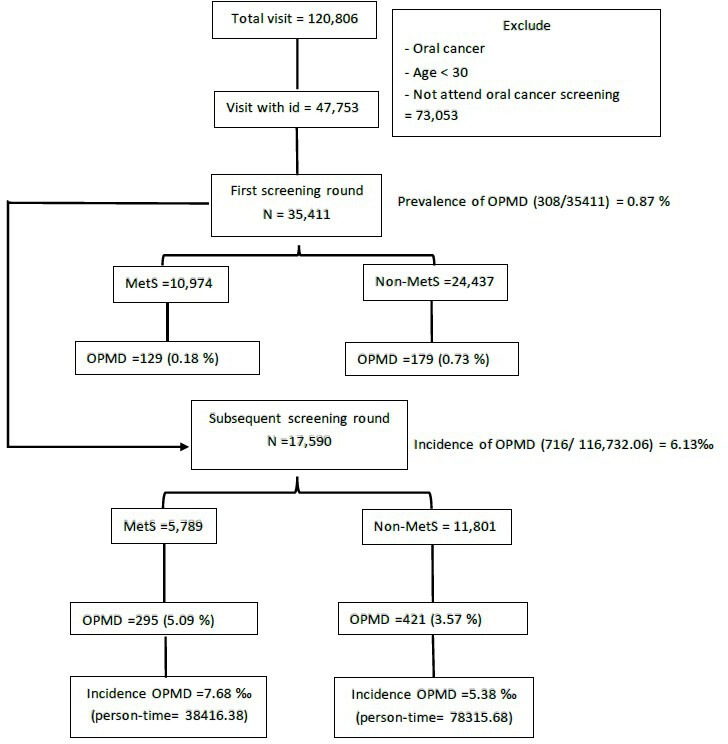
Flow chart for prospective normal cohort study design. MetS, metabolic syndrome; OPMD, oral potentially malignant disorder.
To address our initial hypothesis that MetS plays a role in the aetiology of OPMD, a prospective follow-up study was adopted. We followed the OPMD-free cohort who attended subsequent screenings in the nationwide oral cancer screening programme to identify those with an OPMD diagnosis in subsequent screening rounds. It should be noted that subjects may attend the CHCIS and nationwide oral cancer screening programme at different times. We defined the status of MetS of participants using the first screen in CHCIS and the first diagnosis of OPMD in the nationwide oral cancer screening programme.
Study population and data collection
The CHCIS programme is a population-based screening programme that followed the service model of the Keelung community-based integrated screening (KCIS) programme.13 These programmes provided screening services of multiple cancers (liver cancer, breast cancer, colorectal cancer, oral cancer and cervical cancer), chronic diseases (hyperlipidaemia, hypertension, hyperglycaemia and MetS) and anthropometric measurements.11 The population in this study consists of dwellers aged 30 years or older that have been participated in both CHCIS and the nationwide oral cancer screening programme between 2005 and 2014. Subjects who had a diagnosis of oral cancer before the first attendance to the CHCIS programme were excluded.
All participants were instructed to follow an 8-hour fasting before blood draw. Biochemical examination of fasting glucose and lipid profiles was performed. The anthropometric measures for body height, body weight and circumferences of waist and hip were measured by either public health nurses or well-trained volunteer social workers in the community settings. All participants in the CHCIS programme were interviewed to obtain information on education level, oral habits (including betel nut chewing, cigarette smoking and alcohol drinking), dietary habits, personal disease history and family disease history. For oral habits, we classified the habit as never, quit or current user. Quitting in our study refers to participants who reported habitual use of chewing betel quid, smoking cigarettes or drinking alcohol; however, at the time of interview, they reported no regular consumption of betel quid, cigarettes or alcohol. Dietary factors, including meat, vegetable and fruit consumption, were classified as seldom (including never), infrequent and frequent. Infrequent meat consumption was defined as having 1–2 units per day, and frequent meat consumption was defined as 3–4 units per day. Infrequent vegetable consumption was defined as having a half or one bowl per day, and frequent vegetable consumption was defined as 3–4 bowls per day. Infrequent fruit consumption was defined as 1–4 times per week, and frequent fruit consumption was defined as more than five times per week.
Instruction on informed consent was first given and approved by those who expressed the willingness of participating in the study.
OPMD detection
Since 2005, the oral visual inspection for all eligible participants was performed in Changhua County. In each on-site screening centre, trained dentists or physicians examined all participants. For those who were clinically diagnosed with oral leukoplakia, erythroleukoplakia, erythroplakia, oral submucous fibrosis (OSF) and verrucous hyperplasia were recorded as positive for OPMD.
Metabolic syndrome
MetS was defined according to the Epidemiology Task Force Consensus Group criteria (2005)14 in which participants presented at least three or more of the five components including: (1) central obesity (waist circumference ≥80 cm for females and ≥90 cm for males), (2) hypertriglyceridaemia (≥150 mg/dL), (3) low level of high-density lipoprotein cholesterol (<50 mg/dL for females and <40 mg/dL for males), (4) elevated blood pressure (systolic blood pressure ≥130 mm Hg or diastolic blood pressure ≥85 mm Hg) and (5) hyperglycaemia (fasting glucose ≥100 mg/dL).
Patient and public involvement
Participants in our study were recruited through the CHCIS programme. Participants were not involved in the design and conduct of the study. Staff in the Changhua County Public Health Bureau and local health centres were responsible for preparation and implementation of the screening service in the community.
The results of our study will be disseminated to the public through the Changhua County Public Health Bureau.
Statistical analysis
Prevalence of OPMD was presented as cases per 100 persons. The OPMD incidence rate was presented as cases per 1000 person-years. The univariate Poisson regression model was first used to estimate the rate ratio (RR) for MetS and factors in association with the risk for developing OPMD. The adjusted RR (aRR) was further estimated using the multivariable Poisson regression model when significant confounding factors from the univariate analyses and other factors reported of having significant association with OPMD in previous studies were retained in the model. In addition to the dichotomous variable of MetS or not, we also examined the effect of each individual component of MetS and also the MetS score in separate models with both univariate and multivariate analyses. The magnitude of the effect between MetS and subtypes of OPMD was estimated in separate multivariable Poisson regression models. Statistical significance was defined as p<0.05. All analyses were conducted with SAS V.9.4 (SAS).
Results
A total of 35 411 subjects aged 30 years or older were included in this study from 2005 to 2014 in Changhua. The prevalence of OPMD was 0.87% (=306/35 411). The prevalence of MetS was 31% (=10 974/35 411) (figure 1). Subjects with MetS had a statistically significantly 1.44-fold (95% CI 1.14 to 1.82) increased risk to develop the risk for OPMD compared with those without MetS (see online supplemental table 1).
bmjopen-2020-041971supp001.pdf (130.2KB, pdf)
The incidence of OPMD varies based on demographic and lifestyle factors (table 1). The incidence of OPMD in subjects with MetS (7.68 per 1000 person-years) was increased compared with those who were free of MetS (5.38 per 1000 person-years). Male subjects aged between 40 and 59 years and those with increased body mass index, increased blood pressure and elevated lipid profiles tended to exhibit an increased risk of OPMD compared with their complementary groups. A previous habit of betel quid chewing, smoking and alcohol drinking was associated with an increased incidence of OPMD. High consumption of meat and lower consumption of vegetables and fruit were also related to higher risk of OPMD.
Table 1.
The incidence (per 1000) of oral potentially malignant disorders by demographic features, status of metabolic syndrome and other associated risk factors
| N | Person-years | OPMD | OSF | Leukoplakia | Verrucous hyperplasia | Erythroplakia+ erythroleukoplakia |
||||||
| No | ‰ | No | ‰ | No | ‰ | No | ‰ | No | ‰ | |||
| Overall | 17 590 | 116 732.06 | 716 | 6.13 | 149 | 1.28 | 521 | 4.46 | 20 | 0.17 | 26 | 0.22 |
| Metabolic syndrome | ||||||||||||
| Yes | 5789 | 38 416.38 | 295 | 7.68 | 58 | 1.51 | 219 | 5.7 | 7 | 0.18 | 11 | 0.29 |
| No | 11 801 | 78 315.68 | 421 | 5.38 | 91 | 1.16 | 302 | 3.86 | 13 | 0.17 | 15 | 0.19 |
| Age | ||||||||||||
| 30–39 | 1178 | 8296.07 | 47 | 5.67 | 13 | 1.57 | 28 | 3.38 | 1 | 0.12 | 5 | 0.6 |
| 40–49 | 4359 | 29 193.98 | 210 | 7.19 | 42 | 1.44 | 154 | 5.28 | 8 | 0.27 | 6 | 0.21 |
| 50–59 | 5538 | 35 137.59 | 267 | 7.6 | 48 | 1.37 | 205 | 5.83 | 6 | 0.17 | 8 | 0.23 |
| 60–69 | 4176 | 27 778.33 | 160 | 5.76 | 37 | 1.33 | 115 | 4.14 | 4 | 0.14 | 4 | 0.14 |
| 70+ | 2339 | 16 326.09 | 32 | 1.96 | 9 | 0.55 | 19 | 1.16 | 1 | 0.06 | 3 | 0.18 |
| Sex | ||||||||||||
| Male | 15 619 | 104 569.65 | 703 | 6.72 | 146 | 1.4 | 511 | 4.89 | 20 | 0.19 | 26 | 0.25 |
| Female | 1971 | 12 162.41 | 13 | 1.07 | 3 | 0.25 | 10 | 0.82 | 0 | 0 | 0 | 0 |
| Education | ||||||||||||
| University | 2140 | 13 691.15 | 53 | 3.87 | 4 | 0.29 | 47 | 3.43 | 1 | 0.07 | 1 | 0.07 |
| Senior high school | 4173 | 26 814.93 | 174 | 6.49 | 39 | 1.45 | 126 | 4.7 | 3 | 0.11 | 6 | 0.22 |
| Junior high school or lower | 11 228 | 75 877.21 | 487 | 6.42 | 106 | 1.4 | 347 | 4.57 | 16 | 0.21 | 18 | 0.24 |
| Betel quid chewing | ||||||||||||
| Never | 11 925 | 79 006.46 | 256 | 3.24 | 38 | 0.48 | 203 | 2.57 | 10 | 0.13 | 5 | 0.06 |
| Quit* | 3544 | 23 719.97 | 236 | 9.95 | 62 | 2.61 | 162 | 6.83 | 6 | 0.25 | 6 | 0.25 |
| Current | 2110 | 13 920.02 | 224 | 16.09 | 49 | 3.52 | 156 | 11.21 | 4 | 0.29 | 15 | 1.08 |
| Smoking | ||||||||||||
| Never | 6976 | 46 286.91 | 101 | 2.18 | 21 | 0.45 | 75 | 1.62 | 1 | 0.02 | 4 | 0.09 |
| Quit* | 3656 | 24 678.95 | 126 | 5.11 | 36 | 1.46 | 82 | 3.32 | 3 | 0.12 | 5 | 0.2 |
| Current | 6947 | 45 680.37 | 489 | 10.7 | 92 | 2.01 | 364 | 7.97 | 16 | 0.35 | 17 | 0.37 |
| Alcohol drinking | ||||||||||||
| Never | 8041 | 53 484.46 | 212 | 3.96 | 48 | 0.9 | 155 | 2.9 | 4 | 0.07 | 5 | 0.09 |
| Quit* | 1009 | 6798.76 | 58 | 8.53 | 10 | 1.47 | 44 | 6.47 | 2 | 0.29 | 2 | 0.29 |
| Current | 8529 | 56 365.96 | 446 | 7.91 | 91 | 1.61 | 322 | 5.71 | 14 | 0.25 | 19 | 0.34 |
| BMI (kg/m2) | ||||||||||||
| <18.5 | 422 | 2852.29 | 9 | 3.16 | 5 | 1.75 | 3 | 1.05 | 0 | 0 | 1 | 0.35 |
| 18.5–24.9 | 8844 | 58 824.11 | 313 | 5.32 | 66 | 1.12 | 221 | 3.76 | 13 | 0.22 | 13 | 0.22 |
| >25 | 8324 | 55 055.66 | 394 | 7.16 | 78 | 1.42 | 297 | 5.39 | 7 | 0.13 | 12 | 0.22 |
| Triglyceride (mg/dL) | ||||||||||||
| <150 | 12 178 | 81 399.38 | 405 | 4.98 | 87 | 1.07 | 289 | 3.55 | 14 | 0.17 | 15 | 0.18 |
| ≥150 | 5412 | 35 332.68 | 311 | 8.8 | 62 | 1.75 | 232 | 6.57 | 6 | 0.17 | 11 | 0.31 |
| HDL-C (mg/dL) † | ||||||||||||
| Abnormal | 5684 | 37 372.54 | 268 | 7.17 | 50 | 1.34 | 204 | 5.46 | 5 | 0.13 | 9 | 0.24 |
| Normal | 11 781 | 78 407.84 | 441 | 5.62 | 98 | 1.25 | 312 | 3.98 | 14 | 0.18 | 17 | 0.22 |
| Blood pressure (mm Hg)‡ | ||||||||||||
| Normal | 10 869 | 71 713.89 | 440 | 6.14 | 94 | 1.31 | 321 | 4.48 | 12 | 0.17 | 13 | 0.18 |
| Elevated risk | 2858 | 19 152.31 | 127 | 6.63 | 23 | 1.2 | 91 | 4.75 | 7 | 0.37 | 6 | 0.31 |
| Hypertension | 3863 | 25 865.86 | 149 | 5.76 | 32 | 1.24 | 109 | 4.21 | 1 | 0.04 | 7 | 0.27 |
| Glucose (mg/dL) | ||||||||||||
| <100 | 11 974 | 78 755.06 | 454 | 5.76 | 90 | 1.14 | 332 | 4.22 | 13 | 0.17 | 19 | 0.24 |
| 100–125 | 3907 | 26 462.49 | 165 | 6.24 | 37 | 1.4 | 120 | 4.53 | 5 | 0.19 | 3 | 0.11 |
| >125 | 1709 | 11 514.51 | 97 | 8.42 | 22 | 1.91 | 69 | 5.99 | 2 | 0.17 | 4 | 0.35 |
| Meat | ||||||||||||
| Seldom | 4820 | 31 984.38 | 171 | 5.35 | 38 | 1.19 | 127 | 3.97 | 3 | 0.09 | 3 | 0.09 |
| Infrequent | 11 904 | 78 845.33 | 488 | 6.19 | 94 | 1.19 | 360 | 4.57 | 13 | 0.16 | 21 | 0.27 |
| Frequent | 829 | 5625.25 | 56 | 9.96 | 17 | 3.02 | 33 | 5.87 | 4 | 0.71 | 2 | 0.36 |
| Vegetable | ||||||||||||
| Seldom | 3679 | 24 216.53 | 172 | 7.1 | 42 | 1.73 | 124 | 5.12 | 1 | 0.04 | 5 | 0.21 |
| Infrequent | 13 469 | 89 529.87 | 527 | 5.89 | 105 | 1.17 | 384 | 4.29 | 19 | 0.21 | 19 | 0.21 |
| Frequent | 308 | 2045.5 | 6 | 2.93 | 0 | 0 | 6 | 2.93 | 0 | 0 | 0 | 0 |
| Fruit | ||||||||||||
| Seldom | 1608 | 10 685.41 | 102 | 9.55 | 20 | 1.87 | 75 | 7.02 | 2 | 0.19 | 5 | 0.47 |
| Infrequent | 7190 | 47 575.85 | 333 | 7 | 74 | 1.56 | 233 | 4.9 | 10 | 0.21 | 16 | 0.34 |
| Frequent | 8773 | 58 318.08 | 280 | 4.8 | 55 | 0.94 | 212 | 3.64 | 8 | 0.14 | 5 | 0.09 |
*Quit: quit betel quid chewing, quit smoking or quit alcohol drinking defined as those who once participated in these oral habits but no longer participate in these habit on the day of interview.
†HDL-C: abnormal defined as (male with 0<HDL<40) or (female with 0<HDL<50). Normal defined as (male with 40≤HDL) or (female with 50≤HDL).
‡Hypertension: normal defined as systolic blood pressure (SBP) <130 or diastolic blood pressure (DBP) <85. Elevated risk defined as 130≤SBP<140 or 85≤DBP<90. Hypertension defined as SBP ≥140 or DBP ≥90.
BMI, body mas index; HDL-C, high-density lipoprotein cholesterol; OPMD, oral potentially malignant disorder; OSF, oral submucosa fibrosis.
Table 2 shows the effect of MetS on the risk of OPMD. In univariate analysis, participants with MetS had a 42% increased risk of developing OPMD compared with those who were MetS free (RR=1.42, 95% CI 1.22 to 1.66). Other factors were also associated with increased risks of developing OPMD, including male, age less than 70, betel nut chewing, cigarette smoking, alcohol drinking, meat consumption and lower education level. In multivariable analysis, after adjusting for potential confounding factors, including age, sex, education level, betel nut chewing, cigarette smoking, meat consumption, vegetable consumption, the intake of fruit and alcohol drinking, the association of MetS with an elevated risk of OPMD remained significant (aRR=1.33, 95% CI 1.14 to 1.55).
Table 2.
The association between Mets, other factors and oral potentially malignant disorders (Mets → OPMD)
| RR | 95% CI | aRR | 95% CI | |
| Metabolic syndrome | ||||
| Yes vs no | 1.42 | 1.22 to 1.66 | 1.33 | 1.14 to 1.55 |
| Sex | ||||
| Male vs female | 7.14 | 3.94 to 12.94 | 3.49 | 1.89 to 6.44 |
| Age groups (vs 70+) | ||||
| 30–39 | 2.89 | 1.85 to 4.52 | 2.17 | 1.35 to 3.47 |
| 40–49 | 3.53 | 2.43 to 5.12 | 2.63 | 1.79 to 3.85 |
| 50–59 | 3.63 | 2.52 to 5.24 | 3.1 | 2.14 to 4.49 |
| 60–69 | 2.85 | 1.95 to 4.16 | 2.53 | 1.73 to 3.71 |
| Betel nut chewing (vs never) | ||||
| Quit* | 3.03 | 2.54 to 3.63 | 2 | 1.62 to 2.47 |
| Current | 4.92 | 4.10 to 5.89 | 2.68 | 2.16 to 3.33 |
| Cigarette smoking (vs never) | ||||
| Quit* | 2.32 | 1.78 to 3.03 | 1.31 | 0.96 to 1.78 |
| Current | 4.9 | 3.94 to 6.09 | 2.47 | 1.90 to 3.20 |
| Alcohol drinking (vs never) | ||||
| Quit* | 2.18 | 1.62 to 2.92 | 1.23 | 0.90 to 1.68 |
| Current | 1.95 | 1.65 to 2.30 | 1.03 | 0.86 to 1.23 |
| Meat (vs seldom) | ||||
| Infrequent | 1.13 | 0.95 to 1.35 | 0.95 | 0.79 to 1.13 |
| Frequent | 1.77 | 1.30 to 2.41 | 1.23 | 0.90 to 1.68 |
| Vegetable (vs seldom) | ||||
| Infrequent | 0.83 | 0.70 to 0.99 | 0.92 | 0.77 to 1.10 |
| Frequent | 0.36 | 0.15 to 0.87 | 0.46 | 0.19 to 1.11 |
| Fruit (vs seldom) | ||||
| Infrequent | 0.74 | 0.59 to 0.93 | 0.91 | 0.72 to 1.15 |
| Frequent | 0.51 | 0.40 to 0.64 | 0.79 | 0.62 to 1.00 |
| Education level (vs junior high school or lower) | ||||
| Senior high school | 1 | 0.84 to 1.19 | 0.97 | 0.80 to 1.17 |
| University | 0.6 | 0.45 to 0.81 | 0.84 | 0.62 to 1.14 |
*Quit: quit betel quid chewing, quit smoking or quit alcohol drinking defined as those who once participated in these oral habits but no longer participate in these habits on the day of interview.
aRR, adjusted rate ratio; Mets, metabolic syndrome; OPMD, oral potentially malignant disorder; RR, risk ratio.
In addition to exclusively focusing on MetS outcome, we also investigated the effects of individual components of MetS (table 3). The results show that central obesity (aRR=1.22, 95% CI 1.04 to 1.44), hypertriglyceridaemia (aRR=1.26, 95% CI 1.07 to 1.49) and hyperglycaemia (aRR=1.20, 95% CI 1.02 to 1.41) led to a statistically significant increased risk of OPMD. However, the effects of MetS components were different with respect to OPMD subtypes (table 4). For leukoplakia, only central obesity (aRR=1.30, 95% CI 1.07 to 1.57) and hypertriglyceridaemia (aRR=1.29, 95% CI 1.06 to 1.57) remained significant. Only hyperglycaemia (aRR=1.43, 95% CI 0.99 to 2.05) exhibited a borderline association with an increased risk for OSF. MetS led to a 33% elevated risk of verrucous hyperplasia, but it was not statistically significant due to the small number (aRR=1.33, 95% CI 0.51 to 3.46). Same phenomenon was noted for erythroplakia and erythroleukoplakia (aRR=1.59, 95% CI 0.67 to 3.75). We also provide detailed results on the effects of dichotomous MetS, individual components of MetS and MetS score for all OPMD cases (see online supplemental tables 2–3), leukoplakia (see online supplemental tables 4–6), OSF (see online supplemental table 7–9), verrucous hyperplasia (see online supplemental tables 10–12 and erythroplakia and erythroleukoplakia (see online supplemental tables 13–15).
Table 3.
The effect of metabolic syndrome components on oral potentially malignant disorders
| All OPMD | |||
| aRR* | 95% CI | P value | |
| Component of metabolic syndrome | |||
| Central obesity | 1.22 | 1.04 to 1.44 | 0.0162 |
| Hypertriglyceridaemia | 1.26 | 1.07 to 1.49 | 0.0066 |
| Low HDL-C | 1.12 | 0.95 to 1.32 | 0.1851 |
| Elevated blood pressure | 0.93 | 0.79 to 1.09 | 0.3586 |
| Hyperglycaemia | 1.20 | 1.02 to 1.41 | 0.0297 |
| Metabolic syndrome score | 1.14 | 1.08 to 1.20 | <0.0001 |
*aRR for components of metabolic syndrome and metabolic syndrome score were treated in different models with adjustment of age, sex, education level, betel nut chewing, cigarette smoking, alcohol drinking, meat, vegetable and fruit consumption.
aRR, adjusted rate ratio; HDL-C, high-density lipoprotein cholesterol; OPMD, oral potentially malignant disorder.
Table 4.
The association between metabolic syndrome and subtypes of oral potentially malignant disorders using multivariable Poisson regression
| Leukoplakia | OSF | Verrucous hyperplasia | Erythroplakia | |||||
| aRR* | 95% CI | aRR† | 95% CI | aRR‡ | 95% CI | aRR‡ | 95% CI | |
| Metabolic syndrome | ||||||||
| Yes vs no | 1.37 | 1.14 to 1.64 | 1.22 | 0.87 to 1.71 | 1.33 | 0.51 to 3.46 | 1.59 | 0.67 to 3.75 |
| Component of metabolic syndrome | ||||||||
| Central obesity | 1.3 | 1.07 to 1.57 | 1.06 | 0.74 to 1.52 | 1.17 | 0.47 to 2.89 | 0.94 | 0.37 to 2.36 |
| Hypertriglyceridaemia | 1.29 | 1.06 to 1.57 | 1.21 | 0.83 to 1.76 | 0.98 | 0.40 to 2.40 | 1.39 | 0.54 to 3.58 |
| Low HDL-C | 1.17 | 0.97 to 1.42 | 0.94 | 0.64 to 1.38 | 0.79 | 0.31 to 1.99 | 1.18 | 0.47 to 2.97 |
| Elevated blood pressure | 0.9 | 0.75 to 1.09 | 0.95 | 0.66 to 1.37 | 1.34 | 0.46 to 3.85 | 1.22 | 0.50 to 3.00 |
| Hyperglycaemia | 1.16 | 0.96 to 1.41 | 1.43 | 0.99 to 2.05 | 1.28 | 0.52 to 3.19 | 0.99 | 0.37 to 2.64 |
| Metabolic syndrome score | 1.16 | 1.09 to 1.24 | 1.1 | 0.98 to 1.24 | 1.02 | 0.68 to 1.54 | 1.13 | 0.83 to 1.55 |
*aRR for metabolic syndrome, components of metabolic syndrome and metabolic syndrome score were treated in different models with adjustment of age, sex, education level, betel nut chewing, cigarette smoking, alcohol drinking, meat, vegetable and fruit consumption.
†aRR for metabolic syndrome, components of metabolic syndrome and metabolic syndrome score were treated in different models with adjustment of age, sex, education level, betel nut chewing, cigarette smoking, alcohol drinking, meat and fruit consumption.
‡aRR for metabolic syndrome, components of metabolic syndrome and metabolic syndrome score were treated in different models with adjustment of betel nut chewing and cigarette smoking.
aRR, adjusted rate ratio; HDL-C, high-density lipoprotein cholesterol; OSF, oral submucous fibrosis.
Discussion
In contrast to previous studies that place emphasis on the association between MetS and OPMD, the main objective of the present study, in addition to corroborating the association studies, was to investigate a temporal sequence pertaining to the effect of MetS on incident OPMD based on a longitudinal cohort study. A statistically significant impact of MetS on incident OPMD was observed. We used a longitudinal follow-up study design to address the limitation of the cross-sectional study design given that it cannot elucidate the temporal relationship between MetS and OPMD.
The association between MetS and OPMD has been elucidated in several previous cross-sectional studies conducted in KCIS and in Yunlin county, and MetS increased the risk of OPMD by 68% and 39%, respectively,7 8 which has been also confirmed in our current study. We also found that MetS led to a 44% increased risk associated with MetS for the presence of OPMD.
Furthermore, given its prospective cohort study design, our study further demonstrated the temporal effect of MetS and individual components on incident OPMD. Such a causal relationship between MetS and the risk for OPMD is independent of two well-established risk factors for oral premalignant lesions, namely smoking and betel quid chewing.3 15 16 Applying such information to oral cancer screening would provide additional value for identifying a high-risk category of OPMD.
Regarding an independent contributory cause of MetS accounting for OPMD, the association between MetS and tumour progression in OPMD and oral cancer might be attributable to the common underlying mechanism, an inflammatory process or immune response for both outcomes. To our knowledge, the exact pathway linking MetS and OPMD remains unclear. However, cytokines are often secreted by immune cells in response to inflammation. This process would lead an increased amount of C reactive protein (CRP).17 CRP is known as a biomarker for cardiovascular disease. Recently, CRP was found to increase oxygen radicals.18 These inflammatory factors can activate oncogenes and inactivate tumour suppressor genes and can potentially induce cell proliferation and prolong cell survival, which may result in genetic instability with an increased risk of cancer.19 Previous studies proposed common shared mechanisms between MetS and OPMD, including proinflammatory markers (tumor necrosis factor alpha (TNF-alpha), CRP and interleukin 6 (IL-6)) and insulin resistance.9 10 20 Therefore, MetS may affect cancer tumour cells through increased proliferation, angiogenesis and damage to the DNA molecule under chronic hyperglycaemia, insulin resistance and hyperinsulinaemia.21 22 In addition, MetS particularly with insulin resistance can overstimulate insulin growth factor-1 (IGF-1) and insulin receptor. An increasing and changing of IGF-1 signalling pathway and insulin receptor expression might also lead to an increased risk of cancer.17 In the present study, we found that central obesity, hyperglycaemia and hypertriglyceridaemia were significant individual components of MetS responsible for the development of OPMD. Previous studies revealed that central obesity can stimulate insulin resistance, dyslipidaemia and systematic inflammation. The individual components were considered to play a vital role in the pathogenesis of certain type of cancers.23 24 Moreover, insulin resistance was also associated with an increase in glucose and triglyceride production. Both were highly associated with the risk of developing OPMD in our analysis.
Betel quid’s substances (nitrosated and arecal alkaloid derivatives) increase the risk of oral cancer and OPMD. This effect was not restricted to their direct contact tissue. Lee et al found that betel quid chewing and components of MetS exhibit a positive correlation explained by oxidative stress and inflammation.25 An increase in the risk of oral cancer or OPMD by consuming betel quid and also cigarette smoking or alcohol drinking were noted in our study, even in patients who had quit these habits because they were exposed to these carcinogenesis components for a sufficient period. Our results were consistent with previous studies, which demonstrated that former or ex-consuming of these oral habits still had higher risk of oral cancer, leukoplakia and OSF compared with non-users.26 27
In addition to betel quid, foods were also of concern. Numerous studies unveiled that potential foods, such as red meat, were associated with increased IL-6,28 and vegetable and fruit could lowered CRP.29 In our study, we found that only high consumption of fruit was a protective factor of OPMD. Our findings were consistent with Fann et al and Maserejian et al who found that fruit decreased the risk of periodontal disease and OPMD, respectively.30 31 Interestingly, fruit also reduced the risk of MetS.32 Therefore, these findings support our hypothesis that inflammation is one of the potential mechanisms underlying the relationship between MetS and OPMD.
We examined the effect of MetS on OPMD subtypes and found that MetS was associated with an increased risk of leukoplakia but not other subtypes, including OSF, verrucous hyperplasia and erythleukoplakia, due to the limited number of cases. Regarding leukoplakia, among the components of MetS, only central obesity and hypertriglyceridaemia significantly elevated the risk of leukoplakia. These results were inconsistent with the previous study that found that only hypertriglyceridaemia and hyperglycaemia significantly increased the risk of leukoplakia.8 Regarding hypertriglyceridaemia in leukoplakia, a previous study reported significantly higher triglyceride levels in individuals with leukoplakia compared with healthy people.33 Increasing triglyceride levels were possibly due to the excessive release of free fatty acids, which resulted from insulin resistance. Moreover, insulin resistance can be stimulated by central obesity. In addition, Meisel et al reported that visceral obesity was more likely to be found in people with leukoplakia compared with those without.34 The aforementioned studies support our findings that two MetS components, including central obesity and hypertriglyceridaemia, are associated with leukoplakia. However, the mechanism remains unclear.
Although our study demonstrated that hyperglycaemia did not significantly increase the risk of OSF, the aRR exhibited the largest increased risk magnitude in OSF. Regarding OSF, it has been recognised that the development of fibrosis is pathologically responsible for tissue injury caused by chronic hyperglycaemia. The development of fibrosis was driven by the accumulation of extracellular matrix (ECM).35
One of the unique characteristics of OSF is the symptom of mouth opening restriction.36–38 A possible causation for restricted mouth opening might involve the dynamics of ECM deposited around muscle fibres in different stages of OSF, and these dynamics lead to the consequence of the loss of variety of ECM molecules, including elastin, and replacement with collagen type I muscle fibres.39 Notably, it has been shown that hyperglycaemia can alter the collagenolysis40 and also ECM’s components interaction through advanced glycation end products modification.41 42 These reasons mentioned above may support the borderline impact of hyperglycaemia on OSF and its symptom.
Another possibility of the discordance between these findings might be due to the differences in study approaches and communities with different dietary habits. However, both studies noted that hypertriglyceridaemia and hyperglycaemia were related to OPMD. In addition to these biological aspects, these results are supported by the strong epidemiological study design in which we followed up the OPMD-free study population until the occurrence of OPMD.
In the view of oral cancer control, primary prevention aims to reduce the exposure to risk factors. In Taiwan, several cessation campaigns have been launched, but most of these efforts only considered conventional risk factors, including cigarette smoking and betel nut chewing. Our study result showed that MetS was a risk factor for OPMD. In addition, a recent study also revealed that sweet beverage consumption elevated risk of overall cancer and breast cancer.43 The promotion of an MetS prevention programme after controlling for sugar-sweetened beverage or diet might reduce OPMD and oral cancer incidence in the future.
Several limitations existed in our study. First, several confounding factors that may link MetS and oral cancer, such as family history of oral cancer and history of chronic diseases other than MetS, were not considered. Second, the results of our study were derived from Taiwanese individuals older than 30 years, so external generalisation of our results to other regions would be limited especially on the grounds of ethnic, genetic and dietary backgrounds. Third, the association between MetS and verrucous hyperplasia, erythroplakia, and erythroleukoplakia should be interpreted with great caution given the limited number of cases in our population. Fourth, possible information bias exists for self-reported variables, especially oral habits. Betel nut chewing, smoking and alcohol drinking are behaviours that are deviant from social norms and regulations and can be possibly under-reported. Evidence on this phenomena has been demonstrated for reporting smoking behaviour.44 45 This notion might explain the 38 OSF subjects who reported never betel quid chewing, which contradicts the well-known association between OSF and betel quid chewing.
In conclusion, our prospective cohort study design affirmed the notion that MetS elevated the risk of OPMD. This epidemiological evidence provides new insight for health policy-makers to promote MetS prevention to reduce OPMD and oral cancer in the future.
Supplementary Material
Acknowledgments
We are grateful to Taiwan Cancer registry for information on cancer registry data, Changhua County Public Health Bureau for screening activities, and Health and Welfare Data Science Center, Ministry of Health and Welfare for providing administrative and technical support.
Footnotes
PS and S-TW contributed equally.
Contributors: PSi, ST-W, YP-Y and AM-FY were responsible for conceptualisation and methodology. S-MP and YP-Y contributed to data curation and investigation. PSi, L-SC and AM-FY performed statistical analysis. PSi and ST-W wrote the original draft. This study was supervised by YP-Y and AM-FY. TH-HC and PSa participated in editing manuscript. All authors have reviewed.
Funding: This work was supported by Ministry of Science and Technology, Taiwan (MOST 108-2118-M-038-001-MY3 and MOST 108-2118-M-038-002-MY3).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Institutional Review Board of Taipei Medical University (TMU-JIRB: N201611014).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No additional data are available.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Warnakulasuriya S. Clinical features and presentation of oral potentially malignant disorders. Oral Surg Oral Med Oral Pathol Oral Radiol 2018;125:582–90. 10.1016/j.oooo.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 2.Sankaranarayanan R, Ramadas K, Amarasinghe H, et al. Oral cancer: prevention, early detection, and treatment : Gelband H, Jha P, Sankaranarayanan R, et al., Cancer: disease control priorities. Vol. 3 3rd edn The International Bank for Reconstruction and Development / The World Bank, 2015. http://www.ncbi.nlm.nih.gov/books/NBK343649/ [PubMed] [Google Scholar]
- 3.Axell T, Holmstrup P, Kramer IRH, et al. International seminar on oral leukoplakia and associated lesions related to tobacco habits. Community Dent Oral Epidemiol 1984;12:145–54. 10.1111/j.1600-0528.1984.tb01428.x [DOI] [Google Scholar]
- 4.Juntanong N, Siewchaisakul P, Bradshaw P, et al. Prevalence and factors associated with oral pre-malignant lesions in northeast Thailand. Asian Pac J Cancer Prev 2016;17:4175–9. [PubMed] [Google Scholar]
- 5.Esposito K, Chiodini P, Colao A, et al. Metabolic syndrome and risk of cancer. Diabetes Care 2012;35:2402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stocks T, Bjørge T, Ulmer H, et al. Metabolic risk score and cancer risk: pooled analysis of seven cohorts. Int J Epidemiol 2015;44:1353–63. 10.1093/ije/dyv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang C-C, Lin M-S, Chen Y-T, et al. Metabolic syndrome and health-related behaviours associated with pre-oral cancerous lesions among adults aged 20-80 years in Yunlin County, Taiwan: a cross-sectional study. BMJ Open 2015;5:e008788. 10.1136/bmjopen-2015-008788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yen AM-F, Chen SL-S, Chiu SY-H, et al. Association between metabolic syndrome and oral pre-malignancy: a community- and population-based study (KCIS No. 28). Oral Oncol 2011;47:625–30. 10.1016/j.oraloncology.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 9.Chiang CP, Wu HY, Liu BY, et al. Quantitative analysis of immunocompetent cells in oral submucous fibrosis in Taiwan. Oral Oncol 2002;38:56–63. 10.1016/S1368-8375(01)00026-4 [DOI] [PubMed] [Google Scholar]
- 10.Ujpál M, Matos O, Bíbok G, et al. Diabetes and oral tumors in Hungary: epidemiological correlations. Diabetes Care 2004;27:770–4. 10.2337/diacare.27.3.770 [DOI] [PubMed] [Google Scholar]
- 11.Yeh Y-P, Hu T-H, Cho P-Y, et al. Evaluation of abdominal ultrasonography mass screening for hepatocellular carcinoma in Taiwan. Hepatology 2014;59:1840–9. 10.1002/hep.26703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chuang S-L, Su WW-Y, Chen SL-S, et al. Population-based screening program for reducing oral cancer mortality in 2,334,299 Taiwanese cigarette smokers and/or betel quid chewers. Cancer 2017;123:1597–609. 10.1002/cncr.30517 [DOI] [PubMed] [Google Scholar]
- 13.Chen TH-H, Chiu Y-H, Luh D-L, et al. Community-based multiple screening model: design, implementation, and analysis of 42,387 participants. Cancer 2004;100:1734–43. 10.1002/cncr.20171 [DOI] [PubMed] [Google Scholar]
- 14.Alberti KGMM, Zimmet P, Shaw J, et al. The metabolic syndrome-a new worldwide definition. Lancet 2005;366:1059–62. 10.1016/S0140-6736(05)67402-8 [DOI] [PubMed] [Google Scholar]
- 15.Shiu M-N, Chen TH-H. Impact of betel quid, tobacco and alcohol on three-stage disease natural history of oral leukoplakia and cancer: implication for prevention of oral cancer. Eur J Cancer Prev 2004;13:39–45. 10.1097/00008469-200402000-00007 [DOI] [PubMed] [Google Scholar]
- 16.Yen AM-F, Chen S-C, Chen TH-H. Dose-response relationships of oral habits associated with the risk of oral pre-malignant lesions among men who CheW betel quid. Oral Oncol 2007;43:634–8. 10.1016/j.oraloncology.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 17.Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97. 10.1038/nri2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prasad K. C-Reactive protein increases oxygen radical generation by neutrophils. J Cardiovasc Pharmacol Ther 2016. [DOI] [PubMed] [Google Scholar]
- 19.Feller L, Altini M, Lemmer J. Inflammation in the context of oral cancer. Oral Oncol 2013;49:887–92. 10.1016/j.oraloncology.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 20.Hsu H-J, Yang Y-H, Shieh T-Y, et al. Role of cytokine gene (interferon-γ, transforming growth factor-β1, tumor necrosis factor-α, interleukin-6, and interleukin-10) polymorphisms in the risk of oral precancerous lesions in Taiwanese. Kaohsiung J Med Sci 2014;30:551–8. 10.1016/j.kjms.2014.09.003 [DOI] [PubMed] [Google Scholar]
- 21.Bellastella G, Scappaticcio L, Esposito K, et al. Metabolic syndrome and cancer: “The common soil hypothesis”. Diabetes Res Clin Pract 2018;143:389–97. 10.1016/j.diabres.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 22.Yunusova NV, Spirina LV, Frolova AE, et al. Association of IGFBP-6 expression with metabolic syndrome and adiponectin and IGF-IR receptor levels in colorectal cancer. Asian Pac J Cancer Prev 2016;17:3963–9. [PubMed] [Google Scholar]
- 23.Cuilin Z, Rexrode Kathryn M, van Dam Rob M, et al. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality. Circulation 2008;117:1658–67. [DOI] [PubMed] [Google Scholar]
- 24.Owolabi EO, Ter Goon D, Adeniyi OV. Central obesity and normal-weight central obesity among adults attending healthcare facilities in buffalo City metropolitan Municipality, South Africa: a cross-sectional study. J Health Popul Nutr 2017;36:54. 10.1186/s41043-017-0133-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee B-J, Chan M-Y, Hsiao H-Y, et al. Relationship of oxidative stress, inflammation, and the risk of metabolic syndrome in patients with oral cancer. Oxid Med Cell Longev 2018;2018:1–7. 10.1155/2018/9303094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen P-H, Mahmood Q, Mariottini GL, et al. Adverse health effects of betel quid and the risk of oral and pharyngeal cancers. Biomed Res Int. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee C-H, Ko Y-C, Huang H-L, et al. The precancer risk of betel quid chewing, tobacco use and alcohol consumption in oral leukoplakia and oral submucous fibrosis in southern Taiwan. Br J Cancer 2003;88:366–72. 10.1038/sj.bjc.6600727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozawa M, Shipley M, Kivimaki M, et al. Dietary pattern, inflammation and cognitive decline: the Whitehall II prospective cohort study. Clin Nutr 2017;36:506–12. 10.1016/j.clnu.2016.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silveira BKS, Oliveira TMS, Andrade PA, et al. Dietary pattern and macronutrients profile on the variation of inflammatory biomarkers: scientific update. Cardiol Res Pract 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fann JC-Y, Lai H, Chiu SY-H, et al. A population-based study on the association between the intake of soft drinks and periodontal disease in Taiwanese adults aged 35-44 years (KCIS No. 33). Public Health Nutr 2016;19:1471–8. 10.1017/S1368980015002608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maserejian NN, Giovannucci E, Rosner B, et al. Prospective study of fruits and vegetables and risk of oral premalignant lesions in men. Am J Epidemiol 2006;164:556–66. 10.1093/aje/kwj233 [DOI] [PubMed] [Google Scholar]
- 32.Lee M, Lim M, Kim J. Fruit and vegetable consumption and the metabolic syndrome: a systematic review and dose-response meta-analysis. Br J Nutr 2019;122:723–33. 10.1017/S000711451900165X [DOI] [PubMed] [Google Scholar]
- 33.Granero Fernandez M, Lopez-Jornet P. Association between smoking, glycaemia, blood lipoproteins and risk of oral leukoplakia. Aust Dent J 2017;62:47–51. 10.1111/adj.12431 [DOI] [PubMed] [Google Scholar]
- 34.Meisel P, Dau M, Sümnig W, et al. Association between glycemia, serum lipoproteins, and the risk of oral leukoplakia: the population-based study of health in Pomerania (SHIP). Diabetes Care 2010;33:1230–2. 10.2337/dc09-1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag 2008;4:575–96. 10.2147/vhrm.s1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angadi PV, Rekha KP. Oral submucous fibrosis: a clinicopathologic review of 205 cases in Indians. Oral Maxillofac Surg 2011;15:15–19. 10.1007/s10006-010-0225-x [DOI] [PubMed] [Google Scholar]
- 37.Shih Y-H, Wang T-H, Shieh T-M, et al. Oral submucous fibrosis: a review on etiopathogenesis, diagnosis, and therapy. Int J Mol Sci 2019;20:2940. 10.3390/ijms20122940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang C-Y, Hsia S-M, Hsieh P-L, et al. Slug mediates myofibroblastic differentiation to promote fibrogenesis in buccal mucosa. J Cell Physiol 2019;234:6721–30. 10.1002/jcp.27418 [DOI] [PubMed] [Google Scholar]
- 39.Utsunomiya H, Tilakaratne WM, Oshiro K, et al. Extracellular matrix remodeling in oral submucous fibrosis: its stage-specific modes revealed by immunohistochemistry and in situ hybridization. J Oral Pathol Med 2005;34:498–507. 10.1111/j.1600-0714.2005.00339.x [DOI] [PubMed] [Google Scholar]
- 40.Stultz CM, Edelman ER. A structural model that explains the effects of hyperglycemia on collagenolysis. Biophys J 2003;85:2198–204. 10.1016/S0006-3495(03)74645-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh VP, Bali A, Singh N, et al. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol 2014;18:1–14. 10.4196/kjpp.2014.18.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pastino AK, Greco TM, Mathias RA, et al. Stimulatory effects of advanced glycation endproducts (AGEs) on fibronectin matrix assembly. Matrix Biol 2017;59:39–53. 10.1016/j.matbio.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chazelas E, Srour B, Desmetz E, et al. Sugary drink consumption and risk of cancer: results from NutriNet-Santé prospective cohort. BMJ 2019;366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gnambs T, Kaspar K. Disclosure of sensitive behaviors across self-administered survey modes: a meta-analysis. Behav Res Methods 2015;47:1237–59. 10.3758/s13428-014-0533-4 [DOI] [PubMed] [Google Scholar]
- 45.Connor Gorber S, Schofield-Hurwitz S, Hardt J, et al. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res 2009;11:12–24. 10.1093/ntr/ntn010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-041971supp001.pdf (130.2KB, pdf)


