Abstract
Background
There remains a serious need to prevent the progression of invasive prostate cancer (PCa). We previously showed that secreted extracellular nicotinamide phosphoribosyltransferase (eNAMPT) is a multifunctional innate immunity regulator via TLR4 ligation which has been implicated in PCa progression. Here we investigate the role of eNAMPT as a diagnostic biomarker and therapeutic target in the progression of PCa.
Methods
Tumor NAMPT expression and plasma eNAMPT level were evaluated in human subjects with various PCa tumor stages and high risk subjects followed-up clinically for PCa. The genetic regulation of NAMPT expression in PCa cells and the role of eNAMPT in PCa invasion were investigated utilizing in vitro and in vivo models.
Findings
Marked NAMPT expression was detected in human extraprostatic-invasive PCa tissues compared to minimal expression of organ-confined PCa. Plasma eNAMPT levels were significantly elevated in PCa subjects compared to male controls, and significantly greater in subjects with extraprostatic-invasive PCa compared to subjects with organ-confined PCa. Plasma eNAMPT levels showed significant predictive value for diagnosing PCa. NAMPT expression and eNAMPT secretion were highly upregulated in human PCa cells in response to hypoxia-inducible factors and EGF. In vitro cell culture and in vivo preclinical mouse model studies confirmed eNAMPT-mediated enhancement of PCa invasiveness into muscle tissues and dramatic attenuation of PCa invasion by weekly treatment with an eNAMPT-neutralizing polyclonal antibody.
Interpretation
This study suggests that eNAMPT is a potential biomarker for PCa, especially invasive PCa. Neutralization of eNAMPT may be an effective therapeutic approach to prevent PCa invasion and progression.
Keywords: Nicotinamide phosphoribosyltransferase, eNAMPT, Prostate cancer, Tumor progression, Biomarker, Targeted therapy
Research in Context.
Evidence before this study
Our research team has long been investigating NAMPT functions and roles in diverse human diseases especially related to innate inflammatory responses. Our system biology approach and genomic-intensive strategies identified the secreted extracellular eNAMPT as a novel upstream regulator in innate immunity through binding to TLR-4 and activation of NFκB signaling pathways. Innate immunity pathways and locally-produced or circulating chemokines/cytokines are now known to be intimately involved in cancer progression. Our interrogation of published microarray datasets of four cancer types defined a 39-gene NAMPT-driven signature (N39) predicting a poor prognosis in different cancers. The NAMPT knockdown-influenced dysregulated genes were strongly enriched in cancer-related KEGG terms and cancer biology signaling pathways such as in apoptosis and prostate cancer. Silencing NAMPT expression sensitizes tumors to chemotherapeutic agents while knowledge regarding eNAMPT involvement in cancer pathobiology continues to evolve. To explore the role of NAMPT in tumorigenesis, we screened 10 different common human cancers for an association with NAMPT expression and found significant correlation between human prostate adenocarcinoma and high NAMPT expression. The cBioportal for Cancer Genomics database (http://www.cbioportal.org/study) shows that NAMPT is amplified rather than being missense-mutated in prostate cancer. This study further investigates the role of NAMPT/eNAMPT as a diagnostic and therapeutic target in different stages of prostate cancer.
Added value of this study
We have found that plasma eNAMPT levels have significant predictive value for diagnosis of clinically significant prostate cancer and for risk stratification of invasive prostate cancer. We have also demonstrated that eNAMPT promotes aggressive prostate cancer cell invasion significantly prevented by neutralizing eNAMPT. These findings suggest that plasma eNAMPT is a novel biomarker for risk assessment in prostate cancer and a critically important and novel therapeutic target.
Implications of all the available evidence
Currently, there is an urgent need to prevent prostate cancer progression due to high incidence and untreatable nature of the disease after progression. Under selective pressure or an altered microenvironment, prostate cancer evolves from an androgen-driven cancer to autocrine- or paracrine-driven cancer within the tumor microenvironment. This transition often signals tumor invasion and dissemination, and eventually leads to an autonomous castrate-resistant prostate cancer which is incurable with current standard therapies. Thus, a novel therapy targeting the autocrine or paracrine mediators may be potentially a potent mechanism to prevent prostate cancer progression and reduce prostate cancer lethality. As the present study suggests eNAMPT as an upstream mediator promoting prostate cancer cell transition to an invasive phenotype, these findings indicate a potential for innovative therapies, such as a humanized eNAMPT-neutralizing monoclonal antibody, that address the urgent need to prevent prostate cancer progression. Our study may lead to development of a novel biomarker for risk assessment and a novel therapeutic approach for preventing prostate cancer progression thereby significantly impacting the clinical management of prostate cancer patients. As NAMPT has been implicated in a variety of human cancers, our findings in prostate cancer may well apply to other cancers as well. Given our prior reports that NAMPT genetic variants significantly influence NAMPT expression and eNAMPT secretion in several pathological conditions, this study may lead to development of precise personalized medicine for management of cancer patients.
Alt-text: Unlabelled box
1. Introduction
Prostate cancer (PCa) is the second most common male cancer and there exists a serious unmet need for prevention of PCa progression and recurrence. Targeting the transition from organ-confined PCa (95% 5-year survival) to metastatic cancer (30% 5-year survival) is paramount to influencing PCa lethality [1,2]. In the majority of cases, the PCa exhibits an indolent tumor phenotype. Unfortunately, in a subset of patients, progression to an aggressive, metastatic PCa phenotype is triggered [3]. To reduce PCa morbidity and mortality requires the identification of risk factors that influence PCa invasive progression, requires the identification of biomarkers that herald this progression, and mandates the development of novel therapeutic approaches to halt or attenuate this progression [4,5].
Innate immunity pathways involving locally-produced and circulating chemokines/cytokines are known to be intimately involved in the progression to aggressive and advanced PCa [6,7]. Nicotinamide phosphoribosyltransferase (NAMPT) is an inflammatory cytokine whose intracellular enzymatic activity (iNAMPT) is the rate-limiting step in nicotinamide adenine dinucleotide (NAD) biosynthesis [8]. NAMPT-mediated NAD biosynthesis controls the functions of mammalian sirtuin family members, as well as other NAD-consuming enzymes, such as PARPs, in each subcellular compartment, therefore, involving in a variety of critical biological processes, including cell metabolism and stress response [8, 9]. In contrast to iNAMPT, we previously have shown that the extracellularly-secreted NAMPT (eNAMPT) is a key innate immunity regulator and potent damage-associated molecular pattern protein (DAMP) responding to potentially injurious danger signals [10], [11], [12] via ligation of Toll-like receptor 4 (TLR4) and activation of this evolutionarily-conserved inflammatory cascade [10].
Although knowledge regarding eNAMPT involvement in cancer pathobiology is continuously evolving [13, 14], the role of eNAMPT in PCa is largely unexplored [15, 16]. We have shown that eNAMPT ligation of TLR4 and subsequent NF-κB signaling pathways facilitate generation of tumor-supporting M2 macrophages [17], cells required for PCa escape from the capsule [18]. TLR4 is expressed on both PCa cells and tumor -infiltrating lymphocytes, macrophages and is linked to PCa tumorigenesis and progression [19, 20]. Increased TLR4 expression and increased TLR4 responsiveness have been observed in the PCa microenvironment [21]. Inflammation through TLR4 signaling promotes the development of an immune-suppressive microenvironment with recruitment of myeloid-derived suppressor cells [22]. Gene knockout or silencing of TLR4 in PCa cells decreases tumor cell migration, invasion and survival [23]. In the present study, we now demonstrate eNAMPT as a significant biomarker for invasive PCa and an effective biologic target for reducing/preventing aggressive PCa invasion and potentially the lethality of late stage PCa.
2. Methods
2.1. Patient subjects
Surgically-resected tissues from twenty-three subjects with primary prostate adenocarcinoma (January to November 2013) were retrospectively collected and studied. The research protocol and retrospective analysis were approved by the Institutional Review Board of University of Arizona College of Medicine and Banner-University Medical Center (IRB# 1907781076) and formal written consent obtained from study subjects. Patient information was anonymized and de-identified prior to analysis. All patients underwent radical prostatectomy without neoadjuvant therapy with processed surgical specimens reviewed by specialized PCa pathologists. Ten patients exhibited organ-confined pathology stage 2 (pT2) PCa and 13 patients showed extraprostatic extension pathology stage 3 (pT3) PCa (9 with pT3a and 4 with pT3b) (Table 1). All patients were free of metastases to regional lymph nodes or distal organs. The prostate specific antigen (PSA) levels at the time of pre-surgical evaluation were recorded and blood samples collected from the study patients (n = 20, 10-pT2, 10-pT3 PCa) before surgery was biobanked (−70°C, University of Arizona Cancer Center). Archived prostate tissue paraffin blocks were collected from the twenty-three study patients and from age-matched subjects (n = 10) with normal prostate tissue or with benign prostatic hyperplasia (prostate biopsies or transurethral prostatectomy).
TABLE 1.
PCa patient clinical information, PSA levels, eNAMPT levels, tumor stages (pTNM)* and histologic grades.
| Patients | Age | pTNM | Gleason Score | Grade Group | PSA (ng/ml) | eNAMPT (ng/ml) |
|---|---|---|---|---|---|---|
| 1 | 73 | pT2N0M0 | 3 + 3 = 6 | 1 | 9.4 | 29.69 |
| 2 | 63 | pT2N0M0 | 3 + 3 = 6 | 1 | 5 | 25.55 |
| 3 | 70 | pT2N0M0 | 3 + 4 = 7 | 2 | 10.8 | 30.15 |
| 4 | 65 | pT2N0M0 | 3 + 4 = 7 | 2 | 4.4 | 31.07 |
| 5 | 62 | pT2N0M0 | 3 + 4 = 7 | 2 | 10.3 | 19.96 |
| 6 | 52 | pT2N0M0 | 3 + 4 = 7 | 2 | 2.8 | 21.83 |
| 7 | 64 | pT2N0M0 | 3 + 4 = 7 | 2 | 19.1 | 26.93 |
| 8 | 79 | pT2N0M0 | 3 + 4 = 7 | 2 | 10.8 | 30.15 |
| 9 | 72 | pT2N0M0 | 4 + 3 = 7 | 3 | 5.2 | 23.23 |
| 10 | 71 | pT2N0M0 | 4 + 3 = 7 | 3 | 9.6 | 17.61 |
| 11 | 50 | pT3aN0M0 | 3 + 3 = 6 | 1 | 6.8 | 31.99 |
| 12 | 62 | pT3aN0M0 | 3 + 4 = 7 | 2 | 4.5 | 23.69 |
| 13 | 71 | pT3aN0M0 | 3 + 4 = 7 | 2 | 14 | 26.93 |
| 14 | 79 | pT3bN0M0 | 3 + 4 = 7 | 2 | 12.5 | ? |
| 15 | 76 | pT3aN0M0 | 4 + 3 = 7 | 3 | 8.4 | 26.47 |
| 16 | 63 | pT3bN0M0 | 4 + 4 = 8 | 3 | 9.2 | ? |
| 17 | 67 | pT3aN0M0 | 4 + 4 = 8 | 4 | 9.1 | 22.77 |
| 18 | 72 | pT3aN0M0 | 4 + 4 = 8 | 4 | 12.6 | 45.69 |
| 19 | 68 | pT3aN0M0 | 3 + 5 = 8 | 4 | 2.2 | 52.09 |
| 20 | 68 | pT3aN0M0 | 5 + 4 = 9 | 5 | 6.6 | 41.58 |
| 21 | 70 | pT3aN0M0 | 5 + 5 = 10 | 5 | 14 | 97.12 |
| 22 | 71 | pT3bN0M0 | 4 + 5 = 9 | 5 | 13 | ? |
| 23 | 65 | pT3bN0M0 | 4 + 5 = 9 | 5 | 13.5 | 24.62 |
*Staging based on the American Joint Committee on Cancer (AJCC) 8th edition 2018 tumor pathologic staging system.
2.2. Immunohistochemistry staining and semi-quantitative analysis
Paraffin-embedded blocks of PCa and benign prostate tissues were processed to 4 µm paraffin tissue sections. Three representative tissue blocks and six sequential tissue sections per tissue block were processed for each subject. After rehydration and serum blocking, the paraffin sections were sequentially incubated with a rabbit anti-human NAMPT polyclonal antibody with dilution of 1:1000 (Bethyl Laboratories, Inc, Cat #A300-A375A, Montgomery, TX), HRP-conjugated ABC kit (VECTASTAIN ABC HRP kit, VECTOR Laboratories, Burlingame, CA) and followed using DAB as detection reagent (VECTASTAIN DAB kit, VECTOR Laboratories, Burlingame, CA). One set of stained slides from each tissue block were counterstained with hematoxylin for morphologic examination, and one parallel set of stained slides were processed without counterstain for the analysis of NAMPT immunoreactivity using Image J described below. Negative controls and negative absorption controls were utilized without use of primary antibody or pre-absorbed primary antibody solution which was produced by incubation of the primary antibody with NAMPT peptide at 4 °C overnight. Normal human placenta tissues were used as positive controls. Immunohistochemistry staining studies were repeated three times.
The intensity of NAMPT immunostaining in PCa tissue sections was determined using ImageJ Fiji software (version 1.2; WS Rasband, National Institute of Health, Bethesda, MD). The H&E slides, NAMPT-immunostained with counterstain slides, and NAMPT-immunostained without counterstain slides were reviewed by PCa-specialized pathologist and regionally matched to identify the adenocarcinoma areas and the normal/benign gland areas. To quantify the areas of cells expressing NAMPT, 200 × mosaic images of at least six region-matched prostate adenocarcinoma areas and normal/benign gland areas were randomly selected from three stained slides of each tissue sample. The images were captured using binocular Leica light microscope (Leica™ DM2500) at bright field and CCD color video camera (Leica DFC320) attached to a computer system and uploaded to ImageJ software. At least 6 random areas of pure carcinoma or benign gland tissue of each image were selected using the Selecting tool in ImageJ software. Following the standard recommended protocol [24, 25], the mean Gray Value at each selected area was measured [25] using the Measurement tool in ImageJ to represent the NAMPT immunostaining intensity. The acellular areas of each slide were selected and measured as background Gray Value which was used to normalize the NAMPT-immunostaining intensity. The gray values of NAMPT immunostaining were quantified and presented as mean Gray Value ± s.e.m.. Data were compared between normal/benign glands and prostate cancers, and between prostate cancers with organ-confined tumor (pathology stage 2, pT2) and prostate cancers with extra-prostatic tumor extension (pathology stage 3, pT3a and pT3b).
2.3. ELISA detection of secreted eNAMPT in plasma
Levels of eNAMPT in plasma were detected in a total of 104 PCa patients, 180 high risk subjects without PCa and 105 healthy male controls from the University of Arizona Health Sciences Biorepository (Table 2). The high risk group were men with PSA levels >4 ng/ml, a suspicious digital rectal examination, or PSA velocity >0.75 ng/ml/year (Table 2). Within this cohort of 253 men, seventy-three subjects were subsequently diagnosed with PCa by prostate biopsies and the remainder of the cohort (n = 180) remained PCa -free for up to a 5 year follow up period [26]. A previously reported non-commercial in-house ELISA assay [11] utilizing a proprietary goat α-NAMPT polyclonal antibody, a rabbit polyclonal antibody (Thermo Scientific cat. PA534858) and a secondary donkey anti-rabbit IgG antibody-HRP (Life Technologies cat. A16035) [11] was used to asses eNAMPT level in the plasma samples of 34 PCa, 130 high risk Not-PCa and 105 healthy control subjects. This ELISA assay was also utilized to assess eNAMPT levels in supernatants from PC3 and DU145 cultures exposed to a variety of stimuli.
TABLE 2.
PCa cohort, high risk cohort without PCa, healthy male control cohort and age-matched healthy male control cohort characteristics.
| PCa Subjects | High Risk Without PCa | Healthy Male Controls | Age-Matched Healthy Male Controls | |
|---|---|---|---|---|
| Total Number | 104 | 180 | 105 | 27 |
| Age (mean±SD) | 67.4 ± 6.7 | 67±8 | 46±18 | 68.5 ± 10.4 |
| PSA (ng/ml) (mean±SD) | 9.4 ± 6.5 | 6.7 ± 3.9 | NA | NA |
| eNAMPT (ng/ml) (mean±SD) | 35.6 ± 4.03 | 15.7 ± 1.21 | 14.9 ± 1.26 | 15.6 ± 1.4 |
To validate the in-house results, plasma eNAMPT levels were measured in 70 PCa subjects and 50 high risk subjects without PCa using commercial Human NAMPT/Visfatin ELISA Kit (EH482RB, Thermo Fisher Scientific, Waltham, MA) according to the manufacture's protocol. Briefly, the diluted standards and samples were added to NAMPT antibody coated wells, sequentially incubated with NAMPT biotin conjugate, streptavidin-HRP, TMB substrate and stop solution, measured at 450 nm with iMark™ microplate absorbance reader (Bio-Rad, Hercules, CA). This ELISA assay was also utilized to assess eNAMPT levels in the plasma samples of PCa xenograft mice.
2.4. Western blot
As we described previously [11], protein extracts from PC3 and DU145 cells were separated by 10% SDS-PAGE, transferred to nitrocellulose membranes (100 V for 1.5 h), and immunoreacted with a rabbit anti-human NAMPT polyclonal antibody (1:10,000, Bethyl Laboratories, Inc, Cat #A300-A375A, Montgomery, TX), or mouse anti-human β-actin monoclonal antibody. Immunoreactive proteins were detected with the enhanced chemiluminescent detection system according to the manufacturer's directions (Amersham, Little Chalfont, UK). Intensities of immunoreactive protein bands were quantified using ImageQuant software (Molecular Dynamics, Sunnyvale, CA). All experiments were repeated a minimum of three times.
2.5. Human PCa cell cultures
The PC3 human PCa cell line (NCI-DTP Cat# PC-3, RRID:CVCL_0035) is derived from a bone metastasis of a primary prostate adenocarcinoma (ATCC® CRL-1435™) and the DU145 cell line (NCI-DTP Cat# DU-145, RRID:CVCL_0105) from a central nervous system metastasis of a primary prostate adenocarcinoma [27]. When transplanted into nude or severe combined immunodeficient (SCID) mice, the cells lines are tumorigenic [28]. PCa cell lines were maintained in IDMEM (Gibco, Grand Island, NY) with 10% fetal bovine serum (Gibco), penicillin (500 μg/ml), and streptomycin sulfate (500 μg/ml) at 37 °C in humidified air with 5% CO2. All cell lines were validated by the STR profiling and showed >85% match. All populations tested negative for the presence of mycoplasma and murine viruses. For each experiment, triplicates were set up and repeated at a minimal of three times.
2.6. Matrix metallopeptidases (MMPs) activity assay
The activity of MMPs were assessed in the supernatants of serum-deprived DMEM medium from PC3 and Du-145 cell cultures with eNAMPT 100 ng/ml incubation. The MMP activity assay kit (#ab112146, Abcam, Cambridge, MA, USA) was used to analyze the activity of MMPs from cell supernatants by incubating with Green substrate. The fluorescence (Ex/Em=480/520 nm) was measured using a GloMax®-Multi Detection System Fluorometer (Promega, Madison, WI) to calculate the enzymatic activity of MMPs.
2.7. Chromatin immunoprecipitation (ChIP) assay and real-time qPCR
ChIP were performed using ChIP assay kit (#9003, Cell Signaling Technology, Denver, MA) following manufacture protocol. Briefly, PC3 and DU145 cells were transfected by On-Target Plus siRNAs against HIF1α and HIF2α or siCONTROL#2 (Dharmacon Co., Lafayette, CO) with FuGENE HD (Promega) as previously mentioned [29]. Cells were exposed to normoxia (21% O2) or hypoxia (5% O2) for 24 hrs. Chromatin was crosslinked by 1% formaldehyde, digested by Micrococcal Nuclease, and immunoprecipitated by anti-HIF1α, anti-HIF2α (ab2185 and ab199, Abcam, Cambridge, MA) or normal rabbit IgG with protein G magnetic beads. The purified DNA was quantitated by Real-Time Quantitative PCR using SsoFast EvaGreen Supermix (BioRad, Hercules, CA) following the manufacturer's protocol. Primer sequences were as follow: 5′-AGAGCTGGCGTCTGGGAG-3′; and 5′-GCCTTCACCCCGTCACCC-3′. The controls included the same samples incubated with normal rabbit IgG as the primary antibody.
2.8. NAMPT gene promoter activity luciferase reporter assay
The plasmid constructs containing a 3-kb segment of the NAMPT gene promoter (−3028 bp to +1 ATG) and the Renilla luciferase reporter have been previously described [30, 31]. These plasmid constructs were transfected into PCa cell cultures (PC3 and DU145) with Renilla luciferase reporter pRL-TK co-transfection as an internal control. Transfected cells were exposed to epidermal growth factor (EGF) (100 ng/ml), the prolyl hydroxylase PHD2 inhibitor, FG-4592 (100 µM, to increase HIF1/2α), or testosterone (100 nM) for 4 or 24 h. Luciferase activity was measured by Dual-Luciferase Assay Kits.
2.9. In vitro PCa invasion assay
The Cultrex invasion assay was performed as previously described [32, 33]. Prostate smooth muscle cells (PrSMC; Lonza, catalog no. CC-2587), were placed into tissue culture Transwell inserts pre-coated with collagen (24-well plate, 40,000 per well, Corning Inc, Catalog No. 351,152) and incubated for 1 week. A differentiated layer of PrSMCs was detected either by direct observation or by staining for the smooth muscle marker, desmin. PC3 cells were serum-starved overnight and a total of 500,000 PC3 cells were placed into each insert. The upper chamber contained serum-free media, whereas the lower chamber contained media with 150 μl/well of control vehicle or conditioned media containing eNAMPT (100 ng/ml, human recombinant, Enzo Life Sciences Inc., Farmingdale, NY). To assess PC3 cell invasion (24 h, 37 °C), the top and bottom chambers were aspirated, washed twice, and invading PC3 cells detected using calcein-AM-containing cell dissociation buffer and immunofluorescence reader at 485/520 (manufacturer instructions). To visualize the PC3 invasion via immunofluorescent microscopy, inserts were washed in PBS, fixed in paraformaldehyde, stained with nuclear dye DAPI and stained for desmin to exclude PrSMC cells migrating through the collagen layer.
2.10. Preclinical PCa xenograft models and PCa invasion in vivo
Our previously established PCa human xenograft model [34] was utilized to assess tumor invasion analysis in vivo. SCID (C.B-Igh−1b/IcrTac-Prkdcscid) mice consist of BALB-c/B-17 mice (Jackson Laboratories, Bar Harbor, ME) were maintained in a pathogen-free environment (in compliance with USPHS guidelines and ethical approval). Male SCID mice 5–6 weeks old were inoculated intraperitoneally with 1 × l06 PC3 cells suspended in 0.1 ml of phosphate-buffered saline (PBS) using a tuberculin syringe with a 25-gage needle. The anti-eNAMPT antibody treatment group (n = 5) consisted of mice treated with intraperitoneal injection of a proprietary goat anti-eNAMPT polyclonal antibody (pAb) (1 mg/kg, 3 times/week, 3 weeks). The control group (n = 5) received intraperitoneal injected of PBS (3 times/week, 3 weeks). Both groups of mice were monitored closely, weighted twice a week and were sacrificed within a CO2 chamber 21 days after inoculation. The thoracic and abdominal cavities were opened separately, leaving the diaphragm intact. Thoracic cavity contents were removed and the chest ribcage with the diaphragm remaining attached, was next removed. The diaphragms were next fixed in 10% buffered formalin for 48 h and then carefully dissected from the ribcage using a razor blade. Serial perpendicular sections within 2 mm interval were embedded in paraffin for light microscopy. The thin diaphragm tissues were oriented vertically in the embedding paraffin to allow for the preparation of transverse sections of the diaphragm showing tumor colonies and the underlying tissues of the diaphragm. An average of 3 tissue blocks per diaphragm were generated and three tissue sections (4 μM) per tissue block produced.
2.11. Assessment of PCa cell diaphragmatic invasion
The architecture of diaphragm provided a convenient platform for analyzing tumor cell muscle invasion in vivo. The tumor colonies grossly observed on the diaphragmatic surface were measured in area size using an electronic digital caliper. Each H&E slide of diaphragm from each animal was analyzed using an Olympus DP2-SAL camera system and ImageJ software as follow: 1) The PC3 cell colonies with invasive components were counted as the number of invasive sites. 2) The area size of each PC3 cell colony on and within the diaphragmatic muscle (total tumor size) and each invasive component (invasive tumor size) were measured and calculated for the percentage of the invasive tumor size to the total tumor size. 3) The deepest depth of tumor invasion of each invasive site was measured. Immunohistochemistry staining for Ki67 (rabbit monoclonal antibody, clone 30–9, Ventana Medical Systems, Inc., Tucson, AZ) was performed on the diaphragm paraffin tissue sections. The number of invasive sites, the percentage of invasive tumor, the depth of tumor invasion and the Ki67 proliferation index were compared between control PCa-challenged SCID mice and eNAMPT pAb- treated mice.
2.12. Statistical analysis
The student t- tests and ANOVA were performed using GraphPad Prism version 6.0 for the comparisons of NAMPT immunostaining intensity among benign, organ-confined PCa and extra-prostatic invasive PCa, the depth of tumor invasion, the percentage of invasive tumor and the number of invasive sites between untreated and eNAMPT pAb- treated animal groups, MMPs activity, eNAMPT secretion levels in supernatants of PCa cells. The Mann-Whitney test U test for independent samples or non-parametric test was used for the comparison of eNAMPT plasma levels among controls, high risk subjects without PCa and PCa subjects, and between organ-confined PCa and extra-prostatic invasive PCa subjects. The correlations between eNAMPT plasma levels and PSA level, and between iNAMPT and eNAMPT production in PCa cells were assessed using Rho score. A p value <0.05 was taken to be statistically significant.
Role of funding source: BLS is supported by the University of Arizona Health Sciences Career Development Award. This funding source has no role in this study design, data collection, data analyses, interpretation, or writing of report.
3. Results
3.1. NAMPT is highly expressed in invasive PCa with extraprostatic extension
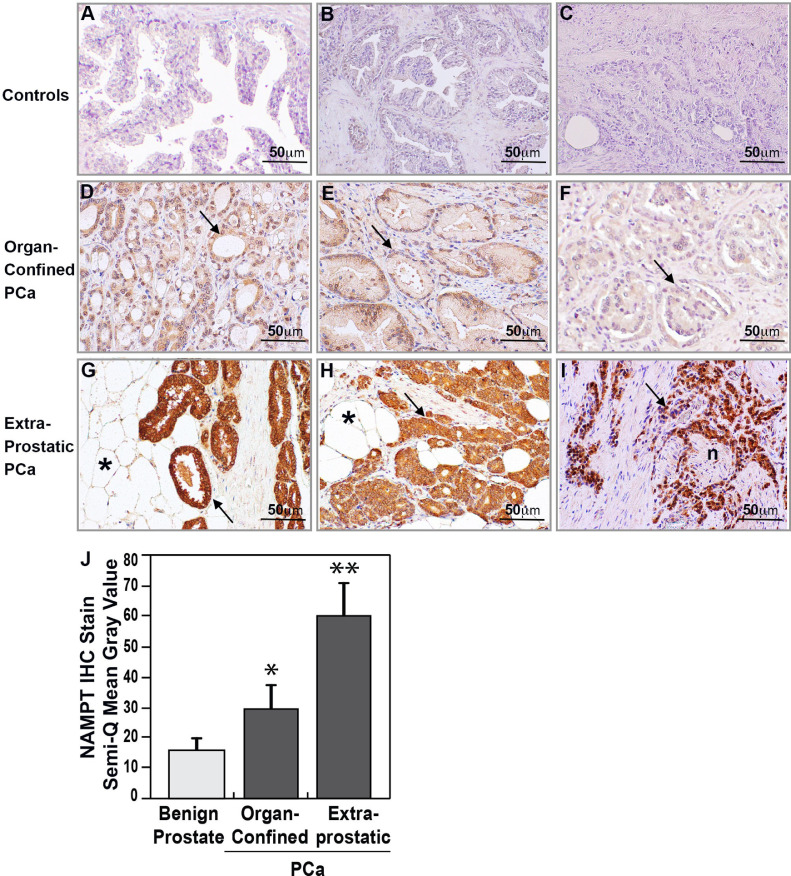
Prostatic tissues from 23 subjects (Table 1) with primary PCa who underwent radical prostatectomy surgery without neoadjuvant therapy, were analyzed. Ten subjects had organ-confined, Stage 2 (pT2) PCa and 13 subjects had extraprostatic extension, Stage 3a/b (pT3) PCa. Immunohistochemistry staining of prostate tissue from ten age-matched control subjects with normal or benign prostate hyperplasia (Fig. 1A and 1B) demonstrated barely detectable NAMPT expression compared to negative control (Fig. 1C). In contrast, NAMPT tissue immunoreactivity was significantly increased in each PCa tissue sample (23/23) (p<0.05). The collected PCa tissues were next grouped into organ-confined PCa (pT2) and extraprostatic PCa (pT3a and pT3b) based on pathologic stage. Representative NAMPT staining images from three organ-confined PCa subjects are shown in Figs. 1D-F with all 10 organ-confined PCa subjects (n = 10) demonstrating moderate NAMPT expression in the cytoplasm of neoplastic epithelium. Interestingly, NAMPT immunoreactivity in the organ-confined group was of moderate intensity even in high morphologic grade PCa (Gleason grade 4 + 4 = 8, Group 4) (Fig. 1F). In contrast, each extraprostatic invasive PCa tissue evaluated (n = 13) showed markedly enhanced NAMPT expression (Fig. 1G-I), was significantly higher than organ-confined PCa (p<0.05), and consistently high regardless of histologic Gleason grades. Fig. 1G illustrates that a PCa subject with low Gleason score (3 + 3 = 6, Group 1) but high pathologic stage pT3 where NAMPT was strongly expressed in the low grade well-formed neoplastic glands especially in the extraprostatic-invasive PCa invading through prostate capsule into peri‑prostate fat tissue. In PCa subjects with Gleason scores 3 + 4 = 7 (Group 2) to 5 + 5 = 10 (Group 5) with high pathology stage pT3, NAMPT expression was consistently high in the high grade poorly-formed glands and solid sheet-like PCa cells. Same as shown in Fig. 1G, high density of NAMPT expression was detected in a PCa subject with Gleason score 3 + 4 = 7, Group 2 PCa (Fig. 1H), as well as a PCa subject with Gleason grade 5 + 5 = 10, Group 5 PCa (Fig. 1I). Quantitation of NAMPT immunostaining intensity showed significantly higher NAMPT expression levels in PCa tissues compared to benign prostate tissues (p<0.05) and were significantly increased in extraprostatic-invasive PCa compared to organ-confined PCa (p<0.05). Together, these data indicate that prostate cancers with extraprostatic extension exhibit high NAMPT expression regardless of Gleason score grade suggesting that NAMPT expression may be closely related to the invasive status of PCa.
Fig. 1.
NAMPT expression is significantly increased in PCa with extraprostatic invasion. Immunohistochemistry revealed minimal NAMPT detection in normal (A) and benign hyperplastic (B) prostate tissues. Antibody negative controls (C) showed no staining in PCa tissue. NAMPT immunostaining (arrows) was weak to moderate in histologic Group 1 (D, E) or Group 3 (F) organ-confined PCa (Gleason scores 3 + 3 = 6 or 4 + 3 = 7) but was markedly increased in extraprostatic invasive PCa with Gleason score 3 + 3 = 6, Group 1 (G), 3 + 4 = 7, Group 2 (H) or 5 + 5 = 10, Group 5 (I) and invasion into periprostatic fat (*) and nerve (n). ImageJ software semi-quantitation of NAMPT immunostaining intensity (J) represented as mean Gray Value confirmed significantly higher NAMPT staining in PCa tissues compared to benign prostate tissues (n = 10) (* p<0.05) with further significant increased expression in extraprostatic-invasive PCa (n = 13) compared to organ-confined PCa (n = 10) (** p<0.05). A-I, x 200.
3.2. Plasma eNAMPT level is elevated in PCa and greater in invasive PCa with extraprostatic extension
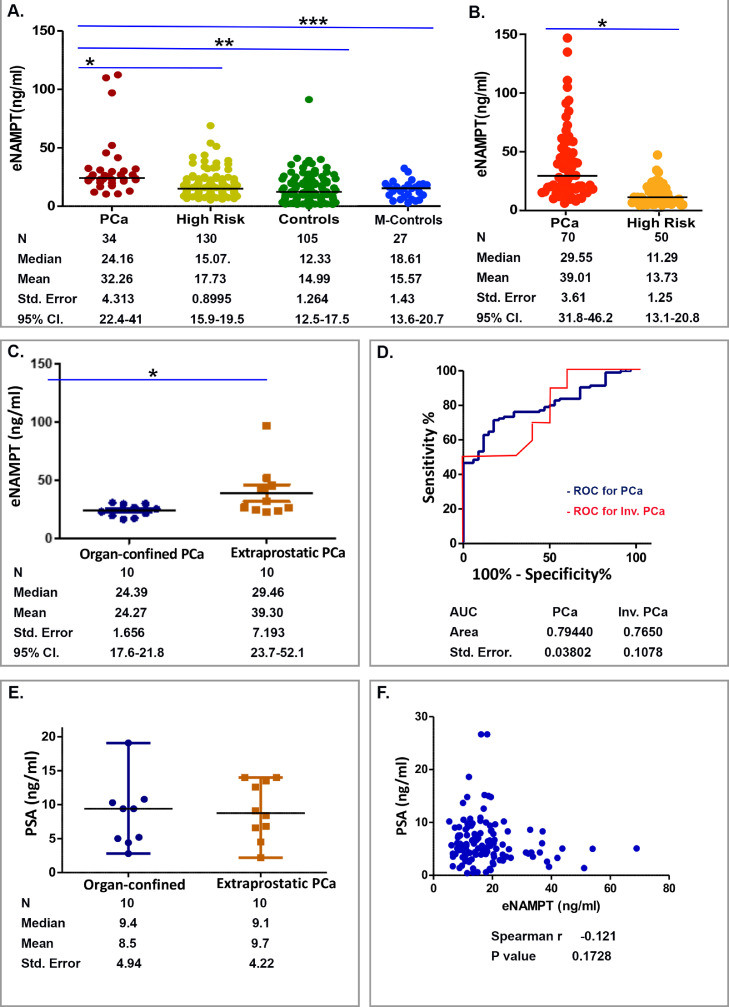
The mean average plasma eNAMPT levels in total 34 PCa subjects was 32.3 ± 4.3 ng/ml with a median of 24.2 ng/ml which was significantly higher compared with 130 high risk subjects without PCa (mean 17.7 ± 0.9 ng/ml; median 15.1 ng/ml), 105 healthy male controls (mean 14.9 ± 1.3 ng/ml; median 12.3 ng/ml) and 27 age-matched healthy male controls (mean 15.6 ± 1.4 ng/ml; median 18.6 ng/ml) (Fig. 2A). A commercial ELISA kit used to validate elevated plasma eNAMPT levels in 70 PCa subjects revealed a mean plasma eNAMPT level of 39.0 ± 3.6 ng/ml (median 29.5 ng/ml) which were significantly more elevated than plasma eNAMPT levels in 50 high risk subjects (mean 13.7 ± 1.2 ng/ml; median 11.3 ng/ml) (Fig. 2B, Table 2). These data further confirmed plasma eNAMPT levels to be significantly increased in PCa subjects.
Fig. 2.
Plasma eNAMPT levels track with human PCa and its invasion. A. Compared to healthy male controls (Controls), age-matched healthy male controls (M-Controls) and subjects with high PSA levels but without PCa (High risk), plasma eNAMPT levels were significantly higher in subjects developed PCa (PCa) (*, **, ***p<0.0001). B. Plasma eNAMPT levels were measured using the NAMPT ELISA detection kit from Thermo Fisher Scientific. This confirmed the significant increase in plasma eNAMPT levels in PCa (n = 70) compared to subjects without PCa (n = 50) (* p<0.05). C. Plasma eNAMPT levels were significantly higher in extraprostatic invasive PCa compared to organ-confined PCa (*p<0.05). D. Calculated by MedCal, the plasma eNAMPT levels (n = 34 PCa, 130 high risk, and 105 healthy male control) had significant (AUC 0.79, p<0.0001) predictive value for diagnosing PCa. Calculated by MedCal, the plasma eNAMPT levels (n = 10 organ-confined PCa, 13 extraprostatic-invasive PCa) had significant (AUC 0.76, p<0.05) predictive value for diagnosing invasive PCa. E. In contrast, there was no significant difference in presurgical blood PSA levels of subjects with organ-confined versus extraprostatic-invasive PCa (p>0.05). F. There was no significant correlation among the PSA levels and eNAMPT levels (n = 164, p>0.05).
In our PCa study cohort, twenty subjects with definitive pathologic stages (10 organ-confined pT2 PCa, and 10 extraprostatic-invasive pT3 PCa) had blood collected for plasma eNAMPT test. Plasma eNAMPT levels in extraprostatic-invasive PCa were significantly higher than organ-confined PCa (mean 39.3 ± 7.2 ng/ml vs 24.3 ± 1.7 ng/ml; median 29.5 ng/ml vs 24.4 ng/ml, p = 0.028) (Fig. 2C). In the extraprostatic-invasive PCa group, subject #21 (Table 1) had a high-grade, poorly differentiated PCa (Gleason score 5 + 5 = 10, group 5) and a very high plasma eNAMPT level of 97.1 ng/ml which explained well the nature of disease but appeared as a “statistical outlier”. We excluded this outlier from the dataset, and were reassured as eNAMPT levels remained significantly different between organ-confined versus extraprostatic-invasive group with a re-calculated p value of 0.019 (p<0.05).
Plasma eNAMPT levels appear to successfully identify subjects developing PCa from those who were suspected for PCa based on PSA levels and clinical examination. To test plasma eNAMPT as a diagnostic tool, receiver operating characteristic (ROC) curve was generated to show the relationship between sensitivity and specificity in determining the predictive value of plasma eNAMPT for diagnosing PCa. As shown in Fig. 2D, the area under the empirical ROC curve (AUC) was 0.79 which was significant (p<0.05). The sensitivity and specificity for high plasma eNAMPT >20 ng/ml (set by the upper level of 95% confidence interval in 130 high risk Not-PCa subjects as 19.5 ng/ml) were 79% and 82%, respectively. These results suggest the potential for plasma eNAMPT levels as an adjunct for PCa screening and risk stratification. We generated the receiver operating characteristic (ROC) curve to show the predictive value of plasma eNAMPT for diagnosing extraprostatic-invasive PCa. Although the number of samples was limited in this PCa cohort, as shown in Fig. 2D, the area under the empirical ROC curve (AUC) was 0.76 which was significant (p<0.05). Spearman's Rho correlation calculations in these 20 PCa subjects with different stages of PCa showed significant correlation between plasma eNAMPT levels and PCa tumor stages (organ-confined versus extraprostatic-invasive, p = 0.041). These data suggest that plasma eNAMPT levels may serve as a potential biomarker for diagnosis of PCa and a high level of eNAMPT may link to extraprostatic PCa invasion.
The PSA serum test is currently the only clinically-utilized biomarker to assess PCa risk. PSA levels in men with extraprostatic-invasive PCa (n = 13) did not significantly differ from men with organ-confined PCa (n = 10) (mean 9.2 ± 4.2 ng/ml vs 8.5 ± 4.9 ng/ml; median 9.4 ng/ml vs 8.7 ng/ml, p = 0.25) (Fig. 2E), consistent with previous observations that serum PSA levels correlate poorly with PCa tumor staging [35]. Spearman's Rho correlation calculations in 164 subjects with or without PCa showed no significant correlation between PSA levels and eNAMPT levels (p = 0.17) (Fig. 2F). In addition, the plasma eNAMPT levels were not correlated with age (r(105) =0.11, p>0.05).
3.3. NAMPT expression is upregulated in PCa cells by hypoxia-inducible factor 1/2α and EGF
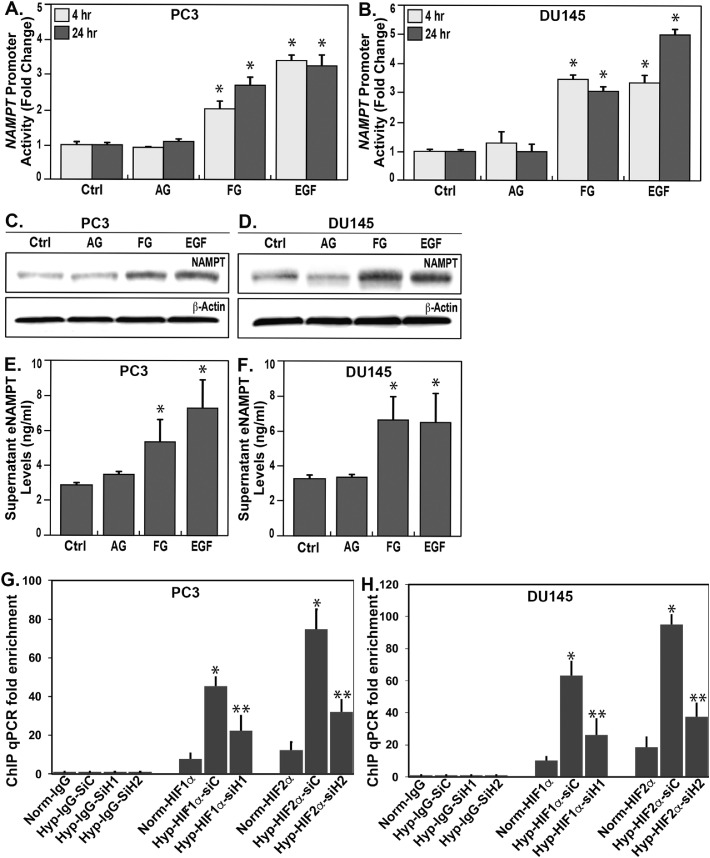
We next examined the molecular mechanisms potentially contributing to regulation of NAMPT expression in PCa. As cellular adaptation to hypoxic microenvironment correlates with PCa tumor invasion, metastasis, and resistance to chemoradiation [36], we assessed the role of hypoxia-inducible factors (HIF) in driving NAMPT expression as well as PCa cell activation by the epidermal growth factor (EGF) signaling pathway known to drive PCa metastatic progression and tumor growth [37, 38]. NAMPT expression was detected utilizing NAMPT promoter and luciferase reporters [30] in two transfected human PCa metastatic cell lines, PC3 (Fig. 3A) and DU145 cells (Fig. 3B). Significant increases in PC3 and DU145 NAMPT transcriptional activities were observed in response to the prolyl hydroxylase inhibitor, FG-4592, which increases protein expression of HIF1α and HIF2α, as well as in response to EGF stimulation. In contrast, NAMPT promoter activities were not significantly modified by androgen testosterone in either PCa cell line (Fig. 3A and 3B), consistent with the known androgen-independent phenotype of PC3 and DU145 cell lines [39]. Immunoblots for protein expression confirmed FG-4592- and EGF-induced NAMPT protein expression in PC3 (Fig. 3C) and DU145 cells (Fig. 3D) whereas testosterone did not. FG-4592 and EGF also induced eNAMPT secretion into the media from PC3 cells (Fig. 3E) and DU145 cells (Fig. 3F) indicating that hypoxia and EGF increase eNAMPT bioavailability to potentially impact PCa progression (Pearson's Correlation Coefficient r(24) =0.94, p<0.01). We utilized the ChIP assay to confirm HIF1α and HIF2α binding the NAMPT promoter which was significantly increased by hypoxia and decreased by siRNAs for HIF1α and HIF2α (Fig. 3G and 3H).
Fig. 3.
Influence of PCa-relevant transcription regulators on NAMPT promoter and eNAMPT secretion. PC3 and Du145 cell cultures were treated with FG-4592 100 uM, or EGF 100 ng/ml, or testosterone 100 nM for 4 or 24 h. The NAMPT promoter activities (using dual-luciferase assay) were significantly increased in response to FG-4592 and EGF (p<0.05) as early as 4 h and were sustained for up to 24 h (A, B), results confirmed by western blots of NAMPT protein levels in cells at 24 h (C, D).
NAMPT promoter activities and expression were unchanged in response to testosterone challenge (A-D), Measurement of secreted eNAMPT into culture media of PC3 and Du145 cells (ELISA) were significantly increased in response to FG-4592 and EGF (p<0.05) but not to testosterone for 24 h (E, F). Detected by ChIP assay, HIF1α and HIF2α significantly bind to NAMPT promoter region under hypoxia (Hyp) compared to normoxia (Norm), and the bindings were significantly decreased by transfection of specific HIF1α SiRNA (siH1) or HIF2α SiRNA (siH2) in both PC3 and DU145 cells (*, ** p<0.05). Non-specific IgG and siRNA (siC) were used as internal controls (G-H).
3.4. eNAMPT accelerates PCa cell invasion of smooth muscle in vitro
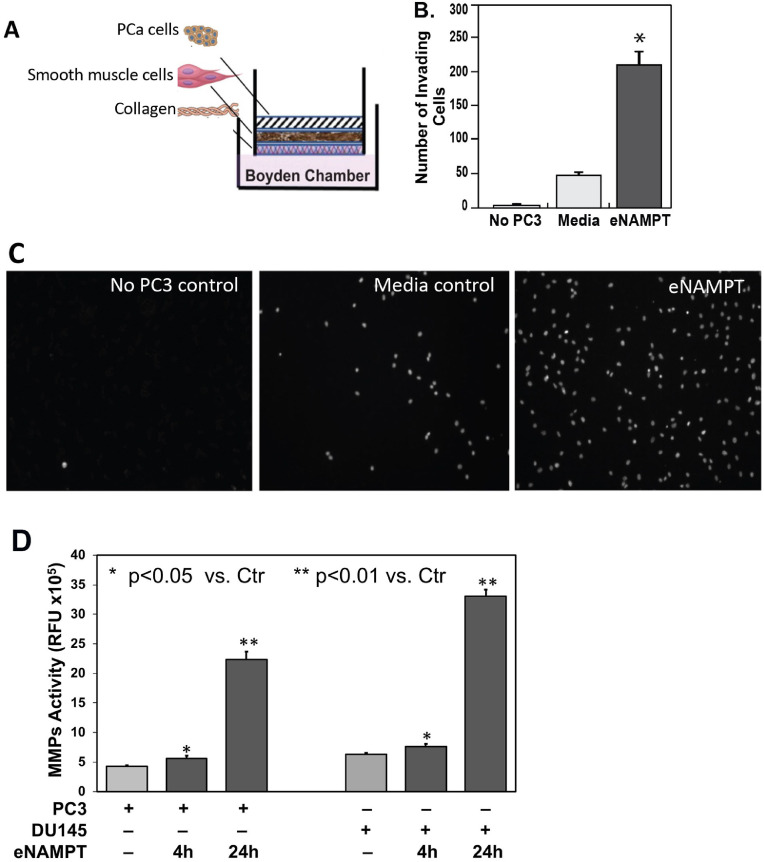
Prostate cancer dissemination requires invasion of smooth muscle tissues during vascular intravasation, extravasation and prostatic extracapsular extension [40]. A 3-D in vitro cell culture model of PCa cell invasion was used [33] involving placement of PCa cells on a layer of prostate smooth muscle cells (PrSMC) (Fig. 4A). eNAMPT (100 ng/ml) was added into the lower chamber and PC3 cells migrating through the PrSMC layer detected by directly cell counts in the lower chamber (Fig. 4B) or by observing PC3 cells attached to the abluminal aspect of Transwell insert (Fig. 4C). In the control group with media alone in the abluminal chamber, PC3 cells migration through the PrSMC and collagen layers was minimal (24 h). In contrast, PC3 cell migration was significantly increased when eNAMPT was present in the lower chamber media. Fig. 4B and 4C showed results representative of at least 3 technical replicates. These data demonstrated that eNAMPT accelerates PC3 invasiveness through smooth muscle cells. To further explore the possible mechanisms by which eNAMPT promotes PCa cell invasion, we investigated MMP production by PC3 and DU145 PCa cells in response to eNAMPT challenge. eNAMPT significantly increased MMPs release from both PC3 and DU145 detected as MMPs activity in cell supernatants as early as 4 h and much greater after 24 h of eNAMPT treatment (Fig. 4D).
Fig. 4.
Effect of eNAMPT on in vitro PCa invasion assays. PC3 cells were placed atop a 2–3 cell layer of prostate smooth muscle cells (PrSMCs) on a Boyden chamber insert as shown in the PCa invasion in vitro model (A). eNAMPT (100 ng/mL) added in the solution compartment was a chemoattractant stimulating PC3 cells to migrate through PrSMCs compared to control culture media over 24hr period shown by counting cells in the Boyden chamber (B, p<0.05) or by observing under microscope (C). MMPs activity was significantly increased in both PC3 and DU145 cell cultures in response to eNAMPT (100 ng/ml) stimulation as early as within 4 hrs and much greater after 24 hrs (D). B, C and D data were representative of triplicate repeats.
3.5. PCa cell muscle invasion in vivo is attenuated by an eNAMPT-neutralizing polyclonal antibody (pAb)
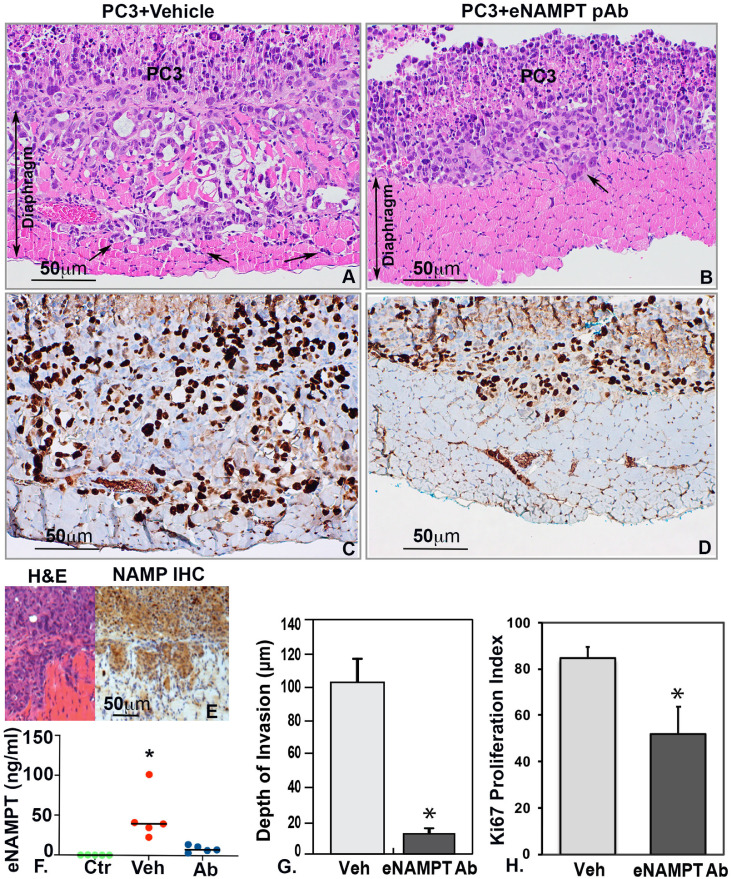
We next utilized a in vivo preclinical model of PCa invasion [33] with the inferior diaphragmatic muscle of the SCID mouse as the PCa invasion platform. PC3 cells were intraperitoneally injected into 2 groups of SCID mice with both groups sacrificed at 4 weeks. One PC3 group was administered an eNAMPT-neutralizing polyclonal antibody (pAb, 1 mg/kg) by intraperitoneal injection (3 times/week, 3 weeks). The second group received IP injection of PBS (control, 3 times/week, 3 weeks). The diaphragms from control and eNAMPT pAb-treated groups were harvested and examined grossly and microscopically. Control group mice exhibited a significantly greater size of tumor colonies growing on the inferior surface of the diaphragm as well as extensive deep invasion when compared to eNAMPT pAb-treated mice (tumor colony size control 14.3 ± 4.6 mm2 versus pAb-treated 6.9 ± 2.9 mm2, p<0.05). Histologic examination showed an aggressively invasive PC3 cell phenotype with robust diaphragmatic smooth muscle invasion (Fig. 5A). Tumor colonies in eNAMPT pAb-treated mice were smaller-sized than untreated mice and exhibited markedly reduced superficial invasion or no invasion (Fig. 5B). Immunohistochemistry staining for Ki67 showed abundant PC3 cell nuclei positive for Ki67 in untreated mice (Fig. 5C) and significantly fewer positive PC3 cell nuclei in eNAMPT pAb-treated mice (Fig. 5D). The mean Ki67 proliferation index in untreated mice was significantly higher than eNAMPT pAb-treated mice (84.6% ± 4.7% versus 51.9% ± 11.6%) (Fig. 5H). The diaphragm-invading PC3 cells in vivo demonstrated high NAMPT expression by immunohistochemistry (Fig. 5E) and mice with PC3 xenografts exhibited significantly higher plasma eNAMPT levels compared to mice with PC3 xenografts treated with eNAMPT-neutralizing antibody (Fig. 5F). Furthermore, a significantly greater number of invasion sites (5.0 ± 1.3) were observed in untreated control mice, compared to eNAMPT pAb-treated mice (2.0 ± 0.9, p<0.05). Consistent with these findings, for each tumor foci, the deepest depth of tumor invasion was significantly greater in the untreated control group (102.5 ± 13.9 μm) when compared to the eNAMPT pAb-treated group (11.8 ± 3.3 μm, p<0.05) (Fig. 5G). The percentage of invasive component within the tumor colonies were also calculated by measuring the area size of the invasive tumor within the diaphragm and the area size of the entire tumor colony. The percentage of invasive tumor was significantly higher in the untreated control group (32.2% ± 6.8%) compared to the eNAMPT pAb-treated group (1.25% ± 1%, p<0.05). Together, these results showed that weekly eNAMPT-neutralizing pAb treatment significantly inhibited PC3 cell invasion in vivo without significant weight changes or adverse effects. These data support eNAMPT-mediated promotion of PCa invasiveness and eNAMPT targeting (neutralizing antibody) as a potentially effective therapeutic strategy to prevent the development of an aggressively invasive PCa phenotype.
Fig. 5.
Effect of eNAMPT on in vivo PCa invasion assays. PC3 cells invaded through diaphragm muscle layer in untreated SICD mice (n = 5) (A); intraperitoneal injection with eNAMPT neutralizing antibody significantly inhibited the tumor invasion (n = 5) (B, G, *p<0.05). Immunohistochemistry showed abundant Ki67-positive PC3 cells in untreated group (C) compared to fewer Ki67-positive cells in eNAMPT antibody-treated PC3 cells (D). The percentages of Ki67-positive cells were significantly higher in untreated group (Veh) compared to eNAMPT pAb-treated group (eNAMPT ab) (H, *p<0.05). PC3 cells highly expressed NAMPT in vivo detected by immunohistochemistry (E) and the plasma eNAMPT levels were significantly higher in untreated group (Veh) compared to normal mice (Ctr) and eNAMPT pAb treated group (Ab) (F, *p<0.05). A-D x200; E x100.
4. Discussion
This study highlights several novel observations including the strong NAMPT expression in invasive prostate cancer tissues and the significant elevation of plasma eNAMPT in men with PCa, especially men with extraprostatic invasion. The American Urological Association recommends blood PSA screening for early PCa detection [41], however, this assay suffers from high false-positive results, high variability and poor correlation with PCa aggressiveness. Unlike plasma eNAMPT levels, in our limited cohort, PSA levels failed to distinguish stage 3 extraprostatic invasive PCa from stage 2 organ-confined PCa, and failed to identify PCa in high risk subjects. Prostate biopsies using the Gleason tumor grading system, in which a higher score correlates with a greater prognostic chance for metastatic disease [42], also failed to stratify cancer stages in PCa subjects with low Gleason scores [35]. For example, PCa cohort subject #11 (Table 1) exhibited a low Gleason score (3 + 3 = 6), and a low grade PSA level of 6.8 ng/ml, parameters that failed to predict the presence of stage III extraprostatic invasive PCa. In contrast, this subject exhibited a plasma eNAMPT level of 31.9 ng/ml, a level well above the range of eNAMPT levels in the organ-confined PCa group (range 16.7 to 31.07 ng/ml) potentially heralding suspicion for an aggressive invasive PCa. Elevated eNAMPT plasma levels may be associated with other risk factors including inflammation, obesity or genetic variance [29, 30, 43] and as a tumor marker is clearly not specific to PCa. However, a combination of eNAMPT and PSA plasma levels and biopsy may potentially provide a higher predictive value in risk stratification for PCa. Future studies evaluating a larger PCa cohort are needed to validate eNAMPT as a biomarker for diagnosis of PCa and stratification for high-risk invasive PCa.
The incidence of metastatic PCa has steadily increased 92% between years 2004 to 2013 in the United States [44]. Surgical, radiation, and hormonal therapies have reduced PCa mortality; however, curative options in advanced PCa remain extremely limited. Although the majority of patients respond to androgen-deprivation therapy initially, nearly all eventually develop metastatic castration-resistant PCa. Since 2004, multiple newly developed agents, androgen receptor-targeted agents, cellular immunotherapy, and radiopharmaceutical radium-223 have received FDA-approved for metastatic castration-resistant PCa [45]. Unfortunately, clinical progression remains extremely common despite the initial clinical effectiveness of these interventions [46]. Thus, targeting the transition from non-invasive organ-confined PCa, which has 95% five-year survival, to invasive metastatic cancer, with only 30% five-year survival, becomes critical to improve the outcome of this disease.
Intracellular NAMPT (iNAMPT) involvement has been demonstrated in PCa [16] and other human malignancies including colorectal, ovarian, breast, gastric, thyroid, endometrial carcinomas, myeloma, melanoma, astrocytoma, and malignant lymphoma [14, 16, 47, 48]. These studies validate NAMPT as a novel ‘‘oncogene’’ with a central role in carcinogenesis influencing human cancer pathology. NAMPT expression influences genes involved in cancer pathogenesis and survival. Our interrogation of microarray data sets derived from four cancer types defined a 39-gene NAMPT-driven signature (N39) that predicted a poor prognosis [18] with NAMPT-influenced genes strongly dysregulating cancer biology signaling pathways including those involved in PCa [18]. This signature is enriched in cancer-related KEGG terms, such as pathways in cancer, colorectal cancer, renal cell carcinoma, as well as prostate cancer. Interrogation of the cBioportal for Cancer Genomics (https://www.cbioportal.org) shows NAMPT to be amplified in TCGA public data sets of PCa, further validating NAMPT as a novel ‘‘oncogene’’ with influence on human cancer pathology. Silencing NAMPT expression sensitizes tumors to chemotherapeutic agents [49] supporting NAMPT as a clinically-relevant therapeutic target. Prior targeting of NAMPT for cancer treatment has been focused on developing agents that inhibit iNAMPT enzymatic activity and regulation of biosynthesis of NAD via a salvage pathway with the synthesis of nicotinamide mononucleotide (NMN) from nicotinamide (NAM) and phosphoribosyl pyrophosphate (PRPP) [12, 50]. iNAMPT enzymatic inhibitors (GMX-1776 or CHS-828, APO-866 aka Daporinad or FK866), have been developed as anti-cancer treatment options. However, based on the safety and efficacy reported from four phase I clinical trials, significant dose-limiting toxicities such as thrombocytopenia and various gastrointestinal symptoms were observed before treatment effects were reached [51, 52]. To date, a safe therapeutic window has not been achieved by inhibiting iNAMPT enzymatic activity.
In this study, we explored extracellular secreted eNAMPT as a specific therapeutic target for invasive PCa, demonstrating an effective treatment approach by neutralizing eNAMPT to prevent PCa invasive progression from organ-confined cancer to extraprostatic invasive cancer. We demonstrated that eNAMPT enhances tumor invasiveness and that neutralizing circulating eNAMPT with a pAb prevents PCa smooth muscle invasion. Based upon extensive studies in inflammatory preclinical models of disease [10, [53], [54], [55]], we speculate that the mechanism by which eNAMPT accelerates tumor invasiveness is via its function as a damage-associated molecular pattern protein (DAMP) responding to hypoxia and mechanical stress [30] and activating JNK1, p38, ERK [56] and Stat3 pathways [57] as a result of ligation of Toll-like receptor 4 (TLR4). TLR4 ligation markedly increases NFκB-dependent expression of inflammatory chemokines and cytokines [10, 11] and mediates cell growth/survival and angiogenesis. TLR4 is expressed on PCa cells, muscle cells, tumor infiltrating lymphocytes and M2 macrophages, and has been well linked to PCa tumorigenesis and invasion [19, 20, 58, 59]. As an immune effector molecule, eNAMPT is released by a variety of injurious stimuli, including hypoxia, and by diverse sources in the tumor microenvironment (macrophages, lymphocytes, PCa cells). Based on our earlier report, we speculate that eNAMPT/TLR4-mediated inflammatory signaling facilitates generation of tumor-supporting M2 macrophages recognized to be associated with PCa onset, PCa recurrence and transition to aggressive PCa metastases [11, 60]. We further speculate that a humanized eNAMPT-neutralizing mAb currently in development will dampen PCa inflammatory cascades and delay the transition from indolent PCa to aggressive, castration-resistant, metastatic PCa phenotype. We anticipate a much safer and more effective therapeutic approach by targeting extracellular eNAMPT rather than inhibiting intracellular iNAMPT in cancer therapy. Although the molecular mechanisms by which eNAMPT functions as a mediator to promote tumor cell invasion and the specific functioning form of eNAMPT secreted by PCa are still unclear and will be further investigated, our current study demonstrates the significant efficacy of anti-eNAMPT neutralizing antibodies in preventing aggressive PCa invasion in preclinical PCa mice models. Targeting eNAMPT appears to be a promising safe and effective approach for treatment of PCa invasive progression and potentially other cancers as well.
In summary, eNAMPT appears to be a druggable target amenable to eNAMPT-neutralizing biologic therapy in preventing PCa invasion. Therapeutic targeting of eNAMPT may antagonize and potentially delay the switch to aggressive invasive disease in androgen deprivation therapy-resistant PCa and prevent PCa progression. While speculative, this is consistent with prior studies demonstrating that uncoupling the dynamic reciprocity that occurs in androgen-independent tumor interaction in the metastatic microenvironment, results in a halt in progression to metastases. Clinical trials in men with PCa utilizing eNAMPT-neutralizing therapies, possibly selecting subjects via eNAMPT plasma levels or by NAMPT genotypes, may prove effective approach in halting PCa progression.
Author contributions statement
Belinda L Sun was responsible for conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology and manuscript writing. Xiaoguang Sun, Nancy Casanova, and Radu Oita performed investigation, data curation, formal analysis and methodology. Alexander N. Garcia, Amit M. Algotar, Sara M. Camp, Vivian Reyes Hernon, and Taylor Gregory assisted data curation. Anne E. Cress provided support and advice on conceptualization, funding acquisition, methodology, and edited the manuscript. Joe GN Garcia was responsible for supervision of overall investigation, conceptualization, funding acquisition, data interpretation, and reviewed and edited the manuscript to the final version.
Contributors
All authors read and approved the final version of the manuscript.
Data sharing statement
The related data and materials are available for sharing upon request to Dr. Belinda L Sun and Dr. Joe GN Garcia.
Declaration of Competing Interest
All authors declare that no conflicts of interest exist.
Acknowledgments
This study is supported by the University of Arizona Health Sciences Career Development Award to Belinda L Sun. The authors are grateful for the Department of Pathology for providing pathologic diagnosis of human prostate cancer tissues and the University of Arizona Cancer Center for sharing the bio-banked blood samples.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.103059.
Contributor Information
Belinda L Sun, Email: bsun@email.arizona.edu.
Joe G.N. Garcia, Email: skipgarcia@email.arizona.edu.
Appendix. Supplementary materials
References
- 1.Kim M.M., Hoffman K.E., Levy L.B., Frank S.J., Pugh T.J., Choi S. Improvement in prostate cancer survival over time: a 20-year analysis. Cancer J. 2012;18(1):1–8. doi: 10.1097/PPO.0b013e3182467419. Epub 2012/02/01PubMed PMID: 22290249. [DOI] [PubMed] [Google Scholar]
- 2.Partin A.W., Kattan M.W., Subong E.N., Walsh P.C., Wojno K.J., Oesterling J.E. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997;277(18):1445–1451. Epub 1997/05/14. PubMed PMID: 9145716. [PubMed] [Google Scholar]
- 3.Sowalsky A.G., Ye H., Bhasin M., Van Allen E.M., Loda M., Lis R.T. Neoadjuvant-intensive androgen deprivation therapy selects for prostate tumor foci with diverse subclonal oncogenic alterations. Cancer Res. 2018;78(16):4716–4730. doi: 10.1158/0008-5472.CAN-18-0610. Epub 2018/06/21. doi: 0008-5472.CAN-18-0610 [pii]PubMed PMID: 29921690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latosinska A., Frantzi M., Merseburger A.S., Mischak H. Promise and implementation of proteomic prostate cancer biomarkers. Diagnostics (Basel) 2018;8(3) doi: 10.3390/diagnostics8030057. Epub 2018/08/31. doi: diagnostics8030057 [pii]PubMed PMID: 30158500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hendriks R.J., van Oort I.M., Schalken J.A. Blood-based and urinary prostate cancer biomarkers: a review and comparison of novel biomarkers for detection and treatment decisions. Prostate Cancer Prostatic Dis. 2018;20(1):12–19. doi: 10.1038/pcan.2016.59. Epub 2016/12/07. doi: pcan201659 [pii]PubMed PMID: 27922627. [DOI] [PubMed] [Google Scholar]
- 6.Taverna G., Pedretti E., Di Caro G., Borroni E.M., Marchesi F., Grizzi F. Inflammation and prostate cancer: friends or foe? Inflamm Res. 2015;64(5):275–286. doi: 10.1007/s00011-015-0812-2. Epub 2015/03/20PubMed PMID: 25788425. [DOI] [PubMed] [Google Scholar]
- 7.Ou T., Lilly M., Jiang W. The pathologic role of toll-like receptor 4 in prostate cancer. Front Immunol. 2018;9:1188. doi: 10.3389/fimmu.2018.01188. Epub 2018/06/22PubMed PMID: 29928275; PubMed Central PMCID: PMCPMC5998742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garten A., Schuster S., Penke M., Gorski T., de Giorgis T., Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11(9):535–546. doi: 10.1038/nrendo.2015.117. Epub 2015/07/29PubMed PMID: 26215259. [DOI] [PubMed] [Google Scholar]
- 9.Audrito V., Messana V.G., Deaglio S. NAMPT and NAPRT: two metabolic enzymes with key roles in inflammation. Front Oncol. 2020;10:358. doi: 10.3389/fonc.2020.00358. Epub 2020/04/09PubMed PMID: 32266141; PubMed Central PMCID: PMCPMC7096376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camp S.M., Ceco E., Evenoski C.L., Danilov S.M., Zhou T., Chiang E.T. Unique toll-like receptor 4 activation by NAMPT/PBEF induces NFkappaB signaling and inflammatory lung injury. Sci Rep. 2015;5:13135. doi: 10.1038/srep13135. PubMed PMID: 26272519; PubMed Central PMCID: PMC4536637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oita R.C., Camp S.M., Ma W., Ceco E., Harbeck M., Singleton P. Novel mechanism for nicotinamide phosphoribosyltransferase inhibition of TNF-alpha-mediated apoptosis in human lung endothelial cells. Am J Respir Cell Mol Biol. 2018;59(1):36–44. doi: 10.1165/rcmb.2017-0155OC. Epub 2018/01/18PubMed PMID: 29337590; PubMed Central PMCID: PMCPMC6039874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galli U., Colombo G., Travelli C., Tron G.C., Genazzani A.A., Grolla A.A. Recent advances in NAMPT inhibitors: a novel immunotherapic strategy. Front Pharmacol. 2020;11:656. doi: 10.3389/fphar.2020.00656. Epub 2020/06/02PubMed PMID: 32477131; PubMed Central PMCID: PMCPMC7235340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grolla A.A., Travelli C., Genazzani A.A., Sethi J.K. Extracellular nicotinamide phosphoribosyltransferase, a new cancer metabokine. Br J Pharmacol. 2016;173(14):2182–2194. doi: 10.1111/bph.13505. Epub 2016/04/30PubMed PMID: 27128025; PubMed Central PMCID: PMCPMC4919578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalamaga M., Christodoulatos G.S., Mantzoros C.S. The role of extracellular and intracellular Nicotinamide phosphoribosyl-transferase in cancer: diagnostic and therapeutic perspectives and challenges. Metabolism. 2018;82:72–87. doi: 10.1016/j.metabol.2018.01.001. Epub 2018/01/14PubMed PMID: 29330025. [DOI] [PubMed] [Google Scholar]
- 15.Keshari K.R., Wilson D.M., Van Criekinge M., Sriram R., Koelsch B.L., Wang Z.J. Metabolic response of prostate cancer to nicotinamide phophoribosyltransferase inhibition in a hyperpolarized MR/PET compatible bioreactor. Prostate. 2015;75(14):1601–1609. doi: 10.1002/pros.23036. Epub 2015/07/17PubMed PMID: 26177608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B., Hasan M.K., Alvarado E., Yuan H., Wu H., Chen W.Y. NAMPT overexpression in prostate cancer and its contribution to tumor cell survival and stress response. Oncogene. 2011;30(8):907–921. doi: 10.1038/onc.2010.468. Epub 2010/10/20PubMed PMID: 20956937. [DOI] [PubMed] [Google Scholar]
- 17.Sroka I.C., Sandoval C.P., Chopra H., Gard J.M., Pawar S.C., Cress A.E. Macrophage-dependent cleavage of the laminin receptor alpha6beta1 in prostate cancer. Mol Cancer Res. 2011;9(10):1319–1328. doi: 10.1158/1541-7786.MCR-11-0080. Epub 2011/08/10PubMed PMID: 21824975; PubMed Central PMCID: PMCPMC3196809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou T., Wang T., Garcia J.G. Expression of nicotinamide phosphoribosyltransferase-influenced genes predicts recurrence-free survival in lung and breast cancers. Sci Rep. 2014;4:6107. doi: 10.1038/srep06107. Epub 2014/08/26PubMed PMID: 25146220; PubMed Central PMCID: PMCPMC4141256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akira S., Takeda K., Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680. doi: 10.1038/90609. Epub 2001/07/31PubMed PMID: 11477402. [DOI] [PubMed] [Google Scholar]
- 20.Zhao S., Zhang Y., Zhang Q., Wang F., Zhang D. Toll-like receptors and prostate cancer. Front Immunol. 2014;5:352. doi: 10.3389/fimmu.2014.00352. Epub 2014/08/08.PubMed PMID: 25101092; PubMed Central PMCID: PMCPMC4107957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatti G., Rivero V., Motrich R.D., Maccioni M. Prostate epithelial cells can act as early sensors of infection by up-regulating TLR4 expression and proinflammatory mediators upon LPS stimulation. J Leukoc Biol. 2006;79(5):989–998. doi: 10.1189/jlb.1005597. Epub 2006/03/09PubMed PMID: 16522744. [DOI] [PubMed] [Google Scholar]
- 22.Almand B., Clark J.I., Nikitina E., van Beynen J., English N.R., Knight S.C. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–689. doi: 10.4049/jimmunol.166.1.678. Epub 2000/12/21PubMed PMID: 11123353. [DOI] [PubMed] [Google Scholar]
- 23.Hua D., Liu M.Y., Cheng Z.D., Qin X.J., Zhang H.M., Chen Y. Small interfering RNA-directed targeting of Toll-like receptor 4 inhibits human prostate cancer cell invasion, survival, and tumorigenicity. Mol Immunol. 2009;46(15):2876–2884. doi: 10.1016/j.molimm.2009.06.016. Epub 2009/08/01PubMed PMID: 19643479. [DOI] [PubMed] [Google Scholar]
- 24.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi: 10.1038/nmeth.2089. Epub 2012/08/30PubMed PMID: 22930834; PubMed Central PMCID: PMCPMC5554542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crowe A.R., Yue W. Semi-quantitative Determination of Protein Expression using Immunohistochemistry staining and analysis: an integrated protocol. Bio Protoc. 2019;9(24) doi: 10.21769/BioProtoc.3465. Epub 2019/12/24PubMed PMID: 31867411; PubMed Central PMCID: PMCPMC6924920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Algotar A.M., Stratton M.S., Ahmann F.R., Ranger-Moore J., Nagle R.B., Thompson P.A. Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer. Prostate. 2013;73(3):328–335. doi: 10.1002/pros.22573. Epub 2012/08/14PubMed PMID: 22887343; PubMed Central PMCID: PMCPMC4086804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stone K.R., Mickey D.D., Wunderli H., Mickey G.H., Paulson D.F. Isolation of a human prostate carcinoma cell line (DU 145) Int J Cancer. 1978;21(3):274–281. doi: 10.1002/ijc.2910210305. Epub 1978/03/15PubMed PMID: 631930. [DOI] [PubMed] [Google Scholar]
- 28.Namekawa T., Ikeda K., Horie-Inoue K., Inoue S. Application of prostate cancer models for preclinical study: advantages and limitations of cell lines, patient-derived xenografts, and three-dimensional culture of patient-derived cells. Cells. 2019;8(1) doi: 10.3390/cells8010074. Epub 2019/01/24PubMed PMID: 30669516; PubMed Central PMCID: PMCPMC6357050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun X., Sun B.L., Babicheva A., Vanderpool R., Oita R.C., Casanova N. Direct extracellular NAMPT involvement in pulmonary hypertension and vascular remodeling. Transcriptional regulation by SOX and HIF-2alpha. Am J Respir Cell Mol Biol. 2020;63(1):92–103. doi: 10.1165/rcmb.2019-0164OC. Epub 2020/03/07PubMed PMID: 32142369; PubMed Central PMCID: PMCPMC7328254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X., Elangovan V.R., Mapes B., Camp S.M., Sammani S., Saadat L. The NAMPT promoter is regulated by mechanical stress, signal transducer and activator of transcription 5, and acute respiratory distress syndrome-associated genetic variants. Am J Respir Cell Mol Biol. 2014;51(5):660–667. doi: 10.1165/rcmb.2014-0117OC. PubMed PMID: 24821571; PubMed Central PMCID: PMC4224084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adyshev D.M., Elangovan V.R., Moldobaeva N., Mapes B., Sun X., Garcia J.G. Mechanical stress induces pre-B-cell colony-enhancing factor/NAMPT expression via epigenetic regulation by miR-374a and miR-568 in human lung endothelium. Am J Respir Cell Mol Biol. 2014;50(2):409–418. doi: 10.1165/rcmb.2013-0292OC. Epub 2013/09/24PubMed PMID: 24053186; PubMed Central PMCID: PMCPMC3930953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sroka I.C., Chopra H., Das L., Gard J.M., Nagle R.B., Cress A.E. Schwann cells increase prostate and pancreatic tumor cell invasion using laminin binding A6 integrin. J Cell Biochem. 2016;117(2):491–499. doi: 10.1002/jcb.25300. Epub 2015/08/05PubMed PMID: 26239765; PubMed Central PMCID: PMCPMC4809241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubenstein C.S., Gard J.M.C., Wang M., McGrath J.E., Ingabire N., Hinton J.P., et al. Gene editing of alpha6 integrin inhibits muscle invasive networks and increases cell-cell biophysical properties in prostate cancer. Cancer Res. 79(18):4703–14. Epub 2019/07/25. doi: 0008-5472.CAN-19-0868 [pii]10.1158/0008-5472.CAN-19-0868. PubMed PMID: 31337652. [DOI] [PMC free article] [PubMed]
- 34.McCandless J., Cress A., Rabinovitz I., Payne C., Bowden G., Knox J. A human xenograft model for testing early events of epithelial neoplastic invasion. Int J Oncol. 1997;10(2):279–285. Epub 1997/02/01. PubMed PMID: 21533373; PubMed Central PMCID: PMCPMC5390482. [PMC free article] [PubMed] [Google Scholar]
- 35.Tosoian J.J., Chappidi M., Feng Z., Humphreys E.B., Han M., Pavlovich C.P., et al. Prediction of pathological stage based on clinical stage, serum prostate-specific antigen, and biopsy Gleason score: partin Tables in the contemporary era. BJU Int. 119(5):676–83. Epub 2016/07/02. doi: 10.1111/bju.13573. PubMed PMID: 27367645. [DOI] [PubMed]
- 36.Fraga A., Ribeiro R., Principe P., Lopes C., Medeiros R. Hypoxia and prostate cancer aggressiveness: a tale with many endings. Clin Genitourin Cancer. 2015;13(4):295–301. doi: 10.1016/j.clgc.2015.03.006. Epub 2015/05/27PubMed PMID: 26007708. [DOI] [PubMed] [Google Scholar]
- 37.Day K.C., Lorenzatti Hiles G., Kozminsky M., Dawsey S.J., Paul A., Broses L.J. HER2 and EGFR overexpression support metastatic progression of prostate cancer to bone. Cancer Res. 2017;77(1):74–85. doi: 10.1158/0008-5472.CAN-16-1656. Epub 2016/10/30PubMed PMID: 27793843; PubMed Central PMCID: PMCPMC5214538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montanari M., Rossetti S., Cavaliere C., D'Aniello C., Malzone M.G., Vanacore D. Epithelial-mesenchymal transition in prostate cancer: an overview. Oncotarget. 2017;8(21):35376–35389. doi: 10.18632/oncotarget.15686. Epub 2017/04/22PubMed PMID: 28430640; PubMed Central PMCID: PMCPMC5471062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sobel R.E., Sadar M.D. Cell lines used in prostate cancer research: a compendium of old and new lines–part 1. J Urol. 2005;173(2):342–359. doi: 10.1097/01.ju.0000141580.30910.57. Epub 2005/01/12. doi: S0022-5347(05)60467-3 [pii]PubMed PMID: 15643172. [DOI] [PubMed] [Google Scholar]
- 40.Harryman W.L., Hinton J.P., Rubenstein C.P., Singh P., Nagle R.B., Parker S.J. The cohesive metastasis phenotype in human prostate cancer. Biochim Biophys Acta. 2016;1866(2):221–231. doi: 10.1016/j.bbcan.2016.09.005. Epub 2016/10/25. doi: S0304-419X(16)30067-1 [pii]PubMed PMID: 27678419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carter H.B., Albertsen P.C., Barry M.J., Etzioni R., Freedland S.J., Greene K.L. Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419–426. doi: 10.1016/j.juro.2013.04.119. Epub 2013/05/11PubMed PMID: 23659877; PubMed Central PMCID: PMCPMC4020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooperberg M.R., Broering J.M., Carroll P.R. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009;101(12):878–887. doi: 10.1093/jnci/djp122. Epub 2009/06/11PubMed PMID: 19509351; PubMed Central PMCID: PMCPMC2697208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kieswich J., Sayers S.R., Silvestre M.F., Harwood S.M., Yaqoob M.M., Caton P.W. Monomeric eNAMPT in the development of experimental diabetes in mice: a potential target for type 2 diabetes treatment. Diabetologia. 2016;59(11):2477–2486. doi: 10.1007/s00125-016-4076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiner A.B., Matulewicz R.S., Eggener S.E., Schaeffer E.M. Increasing incidence of metastatic prostate cancer in the United States (2004-2013) Prostate Cancer Prostatic Dis. 2016;19(4):395–397. doi: 10.1038/pcan.2016.30. Epub 2016/07/20PubMed PMID: 27431496. [DOI] [PubMed] [Google Scholar]
- 45.Moreno-Vinasco L., Quijada H., Sammani S., Siegler J., Letsiou E., Deaton R. Nicotinamide phosphoribosyltransferase inhibitor is a novel therapeutic candidate in murine models of inflammatory lung injury. Am J Respir Cell Mol Biol. 2014;51(2):223–228. doi: 10.1165/rcmb.2012-0519OC. Epub 2014/03/05PubMed PMID: 24588101; PubMed Central PMCID: PMCPMC4148034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cornford P., Bellmunt J., Bolla M., Briers E., De Santis M., Gross T. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71(4):630–642. doi: 10.1016/j.eururo.2016.08.002. Epub 2016/09/07PubMed PMID: 27591931. [DOI] [PubMed] [Google Scholar]
- 47.Sampath D., Zabka T.S., Misner D.L., O'Brien T., Dragovich P.S. Inhibition of nicotinamide phosphoribosyltransferase (NAMPT) as a therapeutic strategy in cancer. Pharmacol Ther. 151:16–31. Epub 2015/02/25. doi: S0163-7258(15)00048-0 [pii]10.1016/j.pharmthera.2015.02.004. PubMed PMID: 25709099. [DOI] [PubMed]
- 48.Heske .CM. Beyond energy metabolism: exploiting the additional roles of NAMPT for cancer therapy. Front Oncol. 2019;9:1514. doi: 10.3389/fonc.2019.01514. Epub 2020/02/06PubMed PMID: 32010616; PubMed Central PMCID: PMCPMC6978772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh R.K., van Haandel L., Heruth D.P., Ye S.Q., Leeder J.S., Becker M.L. Nicotinamide phosphoribosyltransferase deficiency potentiates the antiproliferative activity of methotrexate through enhanced depletion of intracellular ATP. J Pharmacol Exp Ther. 2018;365(1):96–106. doi: 10.1124/jpet.117.246199. Epub 2018/02/09PubMed PMID: 29420256; PubMed Central PMCID: PMCPMC5830637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dalamaga M., Christodoulatos G.S., Mantzoros C.S. The role of extracellular and intracellular Nicotinamide phosphoribosyl-transferase in cancer: diagnostic and therapeutic perspectives and challenges. Metabolism. 82:72–87. Epub 2018/01/14. doi: S0026-0495(18)30005-2 [pii]10.1016/j.metabol.2018.01.001. PubMed PMID: 29330025. [DOI] [PubMed]
- 51.von Heideman A., Berglund A., Larsson R., Nygren P. Safety and efficacy of NAD depleting cancer drugs: results of a phase I clinical trial of CHS 828 and overview of published data. Cancer Chemother Pharmacol. 2010;65(6):1165–1172. doi: 10.1007/s00280-009-1125-3. Epub 2009/10/01PubMed PMID: 19789873. [DOI] [PubMed] [Google Scholar]
- 52.Roulston A., Shore G.C. New strategies to maximize therapeutic opportunities for NAMPT inhibitors in oncology. Mol Cell Oncol. 2016;3(1) doi: 10.1080/23723556.2015.1052180. Epub 2016/06/17PubMed PMID: 27308565; PubMed Central PMCID: PMCPMC4845202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen J., Sysol J.R., Singla S., Zhao S., Yamamura A., Valdez-Jasso D. Nicotinamide phosphoribosyltransferase promotes pulmonary vascular remodeling and is a therapeutic target in pulmonary arterial hypertension. Circulation. 2017;135(16):1532–1546. doi: 10.1161/CIRCULATIONAHA.116.024557. Epub 2017/02/17PubMed PMID: 28202489; PubMed Central PMCID: PMCPMC5400780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong S.B., Huang Y., Moreno-Vinasco L., Sammani S., Moitra J., Barnard J.W. Essential role of pre-B-cell colony enhancing factor in ventilator-induced lung injury. Am J Respir Crit Care Med. 2008;178(6):605–617. doi: 10.1164/rccm.200712-1822OC. PubMed PMID: 18658108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ye S.Q., Simon B.A., Maloney J.P., Zambelli-Weiner A., Gao L., Grant A. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am J Respir Crit Care Med. 2005;171(4):361–370. doi: 10.1164/rccm.200404-563OC. PubMed PMID: 15579727. [DOI] [PubMed] [Google Scholar]
- 56.Pillai V.B., Sundaresan N.R., Kim G., Samant S., Moreno-Vinasco L., Garcia J.G. Nampt secreted from cardiomyocytes promotes development of cardiac hypertrophy and adverse ventricular remodeling. Am J Physiol Heart Circ Physiol. 2013;304(3):H415–H426. doi: 10.1152/ajpheart.00468.2012. Epub 2012/12/04.PubMed PMID: 23203961; PubMed Central PMCID: PMCPMC3774498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y., Zhang Y., Dorweiler B., Cui D., Wang T., Woo C.W. Extracellular Nampt promotes macrophage survival via a nonenzymatic interleukin-6/STAT3 signaling mechanism. J Biol Chem. 2008;283(50):34833–34843. doi: 10.1074/jbc.M805866200. Epub 2008/10/24.PubMed PMID: 18945671; PubMed Central PMCID: PMCPMC2596403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schellekens W-Jan M., van Hees H.W.H., Vaneker M., Linkels M., Dekhuijzen P.N.R., Scheffer Gert J. Toll-like receptor 4 signaling in ventilator-induced diaphragm atrophy. Anesthesiology. 2012;117(2):329–338. doi: 10.1097/ALN.0b013e3182608cc0. [DOI] [PubMed] [Google Scholar]
- 59.Ghosh S., Lertwattanarak R., Garduño JdJ, Galeana J.J., Li J., Zamarripa F. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J Gerontol. 2014;70(2):232–246. doi: 10.1093/gerona/glu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Audrito V., Serra S., Brusa D., Mazzola F., Arruga F., Vaisitti T. Extracellular nicotinamide phosphoribosyltransferase (NAMPT) promotes M2 macrophage polarization in chronic lymphocytic leukemia. Blood. 2015;125(1):111–123. doi: 10.1182/blood-2014-07-589069. Epub 2014/11/05PubMed PMID: 25368373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.