Abstract
In gestational diabetes mellitus (GDM), women are unable to compensate for the increased insulin resistance during pregnancy. Data are limited regarding the pharmacodynamic effects of metformin and glyburide during pregnancy. This study characterized insulin sensitivity (SI), β-cell responsivity, and disposition index (DI) in women with GDM utilizing a mixed-meal tolerance test (MMTT) before and during treatment with GLY monotherapy (GLY, n=38), metformin monotherapy (MET, n=34), or glyburide and metformin combination therapy (COMBO; n=36). GLY significantly decreased dynamic β-cell responsivity (31%). MET and COMBO significantly increased SI (121% and 83%, respectively). While GLY, MET, and COMBO improved DI, metformin (MET and COMBO) demonstrated a larger increase in DI (p=0.05) and a larger decrease in MMTT peak glucose concentrations (p=0.03) than subjects taking only GLY. Maximizing SI with MET followed by increasing β-cell responsivity with GLY or supplementing with insulin might be a more optimal strategy for GDM management than monotherapy.
Keywords: gestational diabetes mellitus, glyburide, metformin, pregnancy, pharmacodynamics, insulin sensitivity, β-cell responsivity, disposition index, insulin, glucose, OCT2, MATE, PMAT
Introduction
Insulin resistance and compensatory increase in insulin concentrations occur during normal pregnancy. Although insulin secretion does increase across gestation in women with gestational diabetes mellitus (GDM), there is an overall decreased ability to compensate for the degree of insulin resistance that occurs, leading to elevated blood glucose.(1, 2) If inadequately treated, GDM poses significant risks to the mother, fetus, and neonate.(3, 4) The American College of Obstetricians and Gynecologists (ACOG) recommends that all pregnant women be screened for GDM between 24-28 weeks gestation and on entry to prenatal care for those with risk factors.(5)
Treatments for women with GDM include dietary changes and pharmacotherapy. Historically, insulin has been the first-line therapy, but oral glucose-lowering drugs such as metformin and glyburide have increased in popularity because of their ease of administration, lower cost, and comparable efficacy to insulin.(6, 7) Recent GDM recommendations from ACOG(4) exclude glyburide from first line oral treatment due to concerns regarding neonatal hypoglycemia and macrosomia. Glyburide, a second-generation sulfonylurea, increases insulin release.(8) Metformin, an insulin sensitizer, lowers glucose concentrations by suppressing hepatic glucose production, decreasing glucose absorption and increasing peripheral glucose uptake and utilization.(9) A meta-analysis reported that metformin was associated with more rapid glucose control and lower postprandial glucose concentrations, whereas glyburide was associated with lower fasting glucose concentrations and hemoglobin A1Cs.(10) Data are limited regarding the pharmacodynamics (PD) of glyburide and metformin during pregnancy.
Biomathematical models, such as the oral minimal model of glucose and C-peptide kinetics, have been developed to describe the dynamic temporal relationship between serum glucose, insulin, and C-peptide concentrations in response to the oral glucose tolerance test (OGTT) and the mixed meal tolerance test (MMTT).(11–13) Insulin sensitivity (SI), β-cell responsivity and disposition index (DI) have been utilized as quantitative indices to characterize the overall metabolic state in pregnant and non-pregnant individuals.(11,14,15) Many factors contribute to glucose homeostasis; however, insulin secretion and action are the primary elements that determine response to glucose exposure and thereby glucose tolerance. The insulin secretory pathway is complex and regulated by multiple factors. Pancreatic β-cell function encompasses those processes leading to the release of insulin in response to elevated glucose concentrations. Exposure of β-cells to an abrupt increase of glucose elicits biphasic insulin secretion. The first phase involves the release of immediately available insulin-filled vesicles through exocytosis, the rate of which is defined as dynamic β-cell responsivity. It describes insulin secretion driven by the rate of change in glucose concentrations. The second phase involves the mobilization of reserved insulin granules to the cell membrane and their subsequent release, the rate of which is defined as static β-cell responsivity. It describes insulin secretion primarily driven by glucose concentrations. Total β-cell responsivity is controlled by both the static and dynamic components, as well as baseline β-cell responsivity, which represents the basal, non-stimulated index of insulin secretion. SI is a measure of the cascade of insulin actions needed to increase glucose utilization and suppress hepatic glucose production. Insulin resistance is a state of reduced SI, which can stem from defects in glucose transport as a result of impairments in translocation, fusion, exposure or activation of GLUT-4 glucose transporters. DI describes the overall metabolic state and is a product of SI and total β-cell responsivity. It is an index of β-cell function, which takes into account the degree of insulin resistance. SI and total β-cell responsivity are hyperbolically related, i.e., reflecting the regulated feedback mechanisms that exist between blood glucose and insulin.(16) These indices provide a mechanistic understanding of patients’ underlying disease pathology and response to pharmacotherapies.
Most oral GDM treatment strategies utilize either glyburide or metformin alone. However, the failure rates for individual agents are high (14-21% for glyburide and ~40% for metformin).(6,17–22) Considering the heterogeneous pathology of GDM (differing insulin resistance and/or β-cell dysfunction) and the different mechanisms of action for glyburide and metformin, some individuals might benefit from glyburide or metformin monotherapies and some might require combination therapy to optimize glycemic control. The objectives of this study were to 1) characterize the PD effects of GLY, MET and COMBO in the management of GDM as determined by response to the oral MMTT; 2) evaluate the effects of gestational age on SI, β-cell responsivity and overall DI in healthy pregnant women; and 3) evaluate the effects of genotype on metformin PD response during pregnancy.
Materials and Methods
Subjects.
This was a multicenter, prospective, randomized, non-blinded Phase I/II longitudinal PD study (clinicaltrials.gov identifier NCT01329016). The study was approved by the institutional review boards at the University of Washington, Madigan Army Medical Center, University of Texas Medical Branch in Galveston, University of Pittsburgh, Indiana University, University of Utah Health Care, University of Alabama at Birmingham and RTI International and conducted in accordance with their guidelines. All subjects gave written, informed consent. There were two groups of women recruited for this study: pregnant women with a diagnosis of GDM (n=109) and healthy pregnant women (n=30).
Entry Criteria.
GDM entry criteria included: pregnant women prior to 32 weeks gestation, singleton pregnancy, 18-45 years of age, failed diet therapy and required drug treatment. GDM diagnosis was made in one of 3 ways: 1) 3-hour OGTT (100 Gm glucose orally with 2 or more values meeting or exceeding targets: fasting ≥95 mg/dL, 1-hour ≥180 mg/dL, 2-hour ≥155 mg/dL and 3-hour ≥140 mg/dL) , 2) 2-hour OGTT (75 Gm glucose orally with 1 or more values meeting or exceeding targets: fasting ≥92 mg/dL, 1-hour glucose ≥180 mg/dL, 2-hour glucose >153 mg/dL) or 3) 1-hour OGTT (50 Gm glucose orally with 1-hour glucose ≥185). Exclusion criteria for women with GDM included: medications expected to interact with glyburide or metformin, medications expected to alter blood glucose concentrations, serum creatinine >1.2 mg/dL; hematocrit <28%; allergy to glyburide, metformin, or sulfa-drugs; significant liver disease; congestive heart failure or history of myocardial infarction; moderate to severe pulmonary disease; and adrenal or pituitary insufficiency. Healthy pregnant women entry criteria included: singleton pregnancy, 18-45 years of age, between 20-32 weeks gestation and a normal 1-hour or 2-hour OGTT. Exclusion criteria for healthy pregnant women included: hematocrit <28% or known kidney, liver, heart, pulmonary, adrenal or pituitary disease as well as drugs that alter glucose concentrations.
Diagnosis and Treatment.
Subjects with GDM were randomized to: GLY, MET, or COMBO and initial dosage and escalation were determined per treatment algorithms as seen in Supplemental Figures S2–S4. Provider discretion was allowed. Blood glucose concentrations were considered controlled when ≥75% of fasting glucose concentrations were ≤95 mg/dL and ≥75% of either 1-hour postprandial glucose concentrations were <140 mg/dL or 2-hour postprandial glucose concentrations were <120 my/dL. Glyburide initial dosage was 2.5 mg orally twice daily. Doses were titrated until glucose concentrations were considered controlled with maximum dosage of 8.75 mg orally three times daily. Metformin initial dosage was 500 mg twice daily and titrated to clinical control. In the COMBO group, subjects received 2.5 mg of glyburide and 500 mg of metformin twice daily initially and titrated to clinical control. If subjects did not achieve glycemic control by titration of dosage according to their treatment algorithm, then subjects completed SD2 and medications were switched or adjusted per provider’s preference. Treatment was initiated at ≤32 weeks of gestation. Glyburide and metformin administrations were not controlled for fasting or fed conditions except on the SD2. On SD2, glyburide and/or metformin were administered simultaneously with initiation of the MMTT. Subjects were determined to be non-adherent if they did not adhere to their treatment regimen based on study pill count or physician clinical impression or did not follow study protocol.
MMTT.
PD parameters were estimated prior to (SD1) and during-treatment (SD2) utilizing a MMTT consisting of one can of Boost Plus® energy drink, two slices of whole wheat toast, and two teaspoons of margarine, which was consumed within ten minutes. SD2 took place once subjects achieved clinical control or prior to switching therapy if they failed to achieve glycemic control. Serial blood samples were collected pre-MMTT (time=0), and 10, 20, 30, 60, 90, 120, 150, 180, 210, and 240 minutes following the initiation of the MMTT to measure serum glucose, insulin, and C-peptide concentrations. Glucose concentrations were measured using a glucose oxidase/peroxidase assay (23). Insulin and C-peptide concentrations were measured using previously described radioimmunoassays.(24,25)
MMTT Parameter Estimation.
SI, β-cell responsivity and DI were estimated as previously described.(11–13,15,26–28) Model parameters were estimated for individual subjects by nonlinear least squares regression using the SAAM II software (version 2.3, The Epsilon Group, Charlottesville, VA). AUCs for glucose, C-peptide, and insulin were calculated utilizing trapezoidal rule in R.(29) PD response was defined as an increase in PD parameter estimates on SD2 relative to SD1 based on the known mechanisms of action for the drug, i.e., GLY increases total β-cell responsivity, MET increases SI and COMBO therapy increases either or both parameters. Gestational age-matched healthy pregnant subjects were included in this study to estimate and correct for gestational age-dependent changes in PD parameters between SD1 and SD2. The correction for gestational age-dependent effects was accomplished by subtracting the average difference between SD2 and SD1 in the healthy pregnant subjects from individual GDM subjects’ SD2 parameters.
Genotyping.
DNA was isolated from whole blood, and genotypes were determined using validated TaqMan assays. Maternal samples were assayed for OCT1: SLC22A1 (rs622342); OCT2: SLC22A2c.808G>T polymorphism (rs316019); MATE1: SLC47A1 (rs2289668), and (rs8065082); MATE2-K: −130G>A polymorphism (rs12943590); as well as PMAT (rs2685753) and (rs6971788).
Statistical analyses.
Differences in PD parameter estimates between SD1 and SD2 were estimated using a paired Student’s t test or between arms of the study using an unpaired Student’s t test or ANOVA. A chi-squared test was used to compare race and ethnicity between study arms. Results are reported as mean ± standard deviation (95% confidence interval). No adjustments for multiple testing were performed. All statistical analyses and graphs were done in R.(29,30)
Results
Demographics.
Demographics for adherent subjects who completed the study are reported in Table 1. Notably, healthy pregnant women were significantly younger and weighed less than those with GDM. Demographics for all subjects can be found in Supplemental Table S1. Nineteen subjects with GDM completed study day 1 (SD1), but not study day 2 (SD2). Reasons for withdrawal included: 3 lost to follow-up, 7 early delivery, 1 scheduling difficulties, 1 medication non-adherence, 1 became ineligible, 3 started alternate therapy, 1 anxiety with blood draws, 1 transferred care and 1 unknown. Results are reported for adherent subjects that completed the study.
Table 1.
Demographics and study drug dosing for adherent subjects with gestational diabetes and gestational age-matched healthy pregnant subjects who completed the study
| All GDM | COMBO | GLY | MET | HP | |
|---|---|---|---|---|---|
| n | 89 | 32 | 32 | 25 | 28 |
| Race/Ethnicity: | |||||
| White (%) | 81.2 | 78.1 | 80.0 | 82.1 | |
| Black (%) | 9.4 | 21.9 | 16.0 | 17.9 | |
| Hispanic (%) | 34.4 | 40.6 | 36.0 | 32.1 | |
| Asian (%) | 5.6 | 0 | 4.0 | 0 | |
| Native American (%) | 2.8 | 0 | 0 | 0 | |
| Age SD1 (years) | 30 ± 5 (19 to 42) | 29 ± 4 (19 to 37) | 30 ± 6 (20 to 42) | 31 ± 4 (22 to 39) | 25 ± 5 (18 to 38) (p<0.001)* |
| Height SD1 (cm) | 162 ± 6 (147 to 179) | 162 ± 7 (153 to 175) | 161 ± 6 (147 to 173) | 162 ± 6 (147 to 179) | 162 ± 8 (147 to 178) |
| Body weight SD1 (kg) | 90 ± 20 (60 to 200) | 100 ± 20 (60 to 200) | 90 ± 20 (60 to 200) | 90 ± 10 (70 to 100) | 80 ± 10 (50 to 100) (p<0.001)* |
| BMI Pre-pregnancy (kg/m2) | 33 ± 7 (20 to 55) | 34 ± 8 (22 to 55) | 33 ± 6 (20 to 53) | 31 ± 6 (21 to 43) | 27 ± 5 (20 to 40) (p<0.001)* |
| GA, SD1 (weeks) | 30 ± 2 (19 to 33) | 30 ± 3 (19 to 33) | 30 ± 2 (24 to 33) | 31 ± 2 (20 to 33) | 30 ± 1 (28 to 33) |
| GA, SD2 (weeks) | 35 ± 2 (26 to 39) | 35 ± 3 (26 to 38) | 36 ± 2 (32 to 39) | 35 ± 1 (32 to 38) | 36 ± 1 (34 to 38) |
| Glyburide dose SD2 (mg/day) | 8 ± 6 (1 to 30) | 6 ± 4 (1 to 20) | 10 ± 6 (2 to 30) | NA | NA |
| Metformin dose SD2 (mg/day) | 1,300 ± 500 (1,000 to 2,600) | 1,200 ± 500 (1,000 to 2,600) | NA | 1,400 ± 500 (1,000 to 2,000) | NA |
BMI = Body Mass Index, COMBO = glyburide/metformin combination, GA = gestational age, GDM = gestational diabetes mellitus, HP = Healthy pregnant subjects, GLY = glyburide monotherapy; MET = metformin monotherapy, COMBO = glyburide plus metformin combination therapy, SD1 = study day 1, SD2 = study day 2, * = significantly different than all GDM subjects, results reported as mean ± SD (range).
Glucose, Insulin and C-Peptide Concentrations.
Average serum concentration-time profiles for glucose, insulin, and C-peptide during a four-hour MMTT on SD1 and SD2 (during treatment for subjects with GDM) are shown in Figure 1 for GDM and HP groups. Mean glucose AUCs were lower on SD2 in the COMBO (p<0.001) and MET (p=0.004) groups, and not significantly different in the GLY (p=0.5) and HP (p=0.8) groups. Mean insulin AUCs were lower for the MET group on SD2 than SD1 (p=0.02) but not significantly different in any other groups. Mean C-peptide AUCs were similar before and with treatment in the COMBO (p=0.3) and MET groups (p=0.2). However, GLY and HP groups had higher C-peptide AUCs on SD2 than SD1 (GLY p=0.01; HP p<0.001).
Figure 1. Glucose, insulin and C-peptide concentration-time profiles for all subjects with gestational diabetes mellitus (GDM) and healthy pregnant subjects who completed the study and were adherent to study procedures.
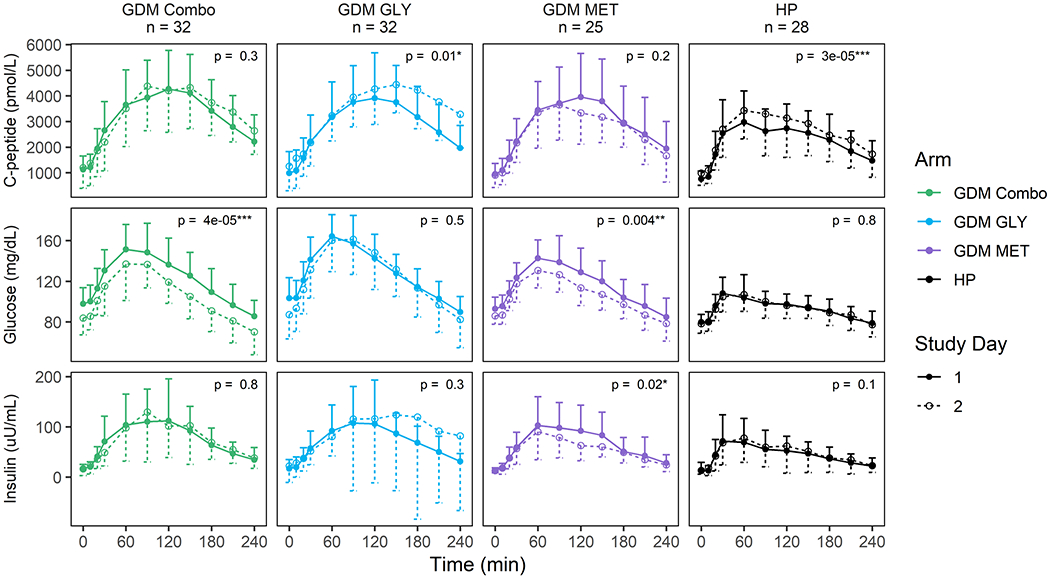
Shown are mean concentrations at each time point with the standard deviation represented by unidirectional error bars. Time since initiation of the MMTT and dose in minutes is listed on the x axis and the concentration of C-peptide (pmol/L), glucose (mg/dL), or insulin (μU/mL) is on the y axis. Data for SD1 are shown with filled circles (means), solid lines, and upper error bars (standard deviation); data for SD2 are shown with open circles, dashed lines, and lower error bars. Treatment arms are represented by COMBO = metformin/glyburide combination therapy group (green), MET = metformin monotherapy group (purple), GLY = glyburide monotherapy group (blue) and HP = healthy pregnancy group (black). Significance is indicated by asterisks.
Gestational Age-Dependent Changes.
The hyperbolic relationship between total β-cell responsivity and SI in healthy pregnant women is shown in Figure 2. From SD1 (30±1 weeks gestation) to SD2 (36±1 weeks gestation), the hyperbolic mean DI curve shifted down and to the left. Baseline β-cell responsivity increased by an average of 31% (p<0.001) and overall DI decreased by 6% (p=0.04) between SD1 and SD2 (Table 2). Other parameters were not significantly different between study days.
Figure 2. Effect of gestational age on the mean disposition index in healthy pregnant subjects.
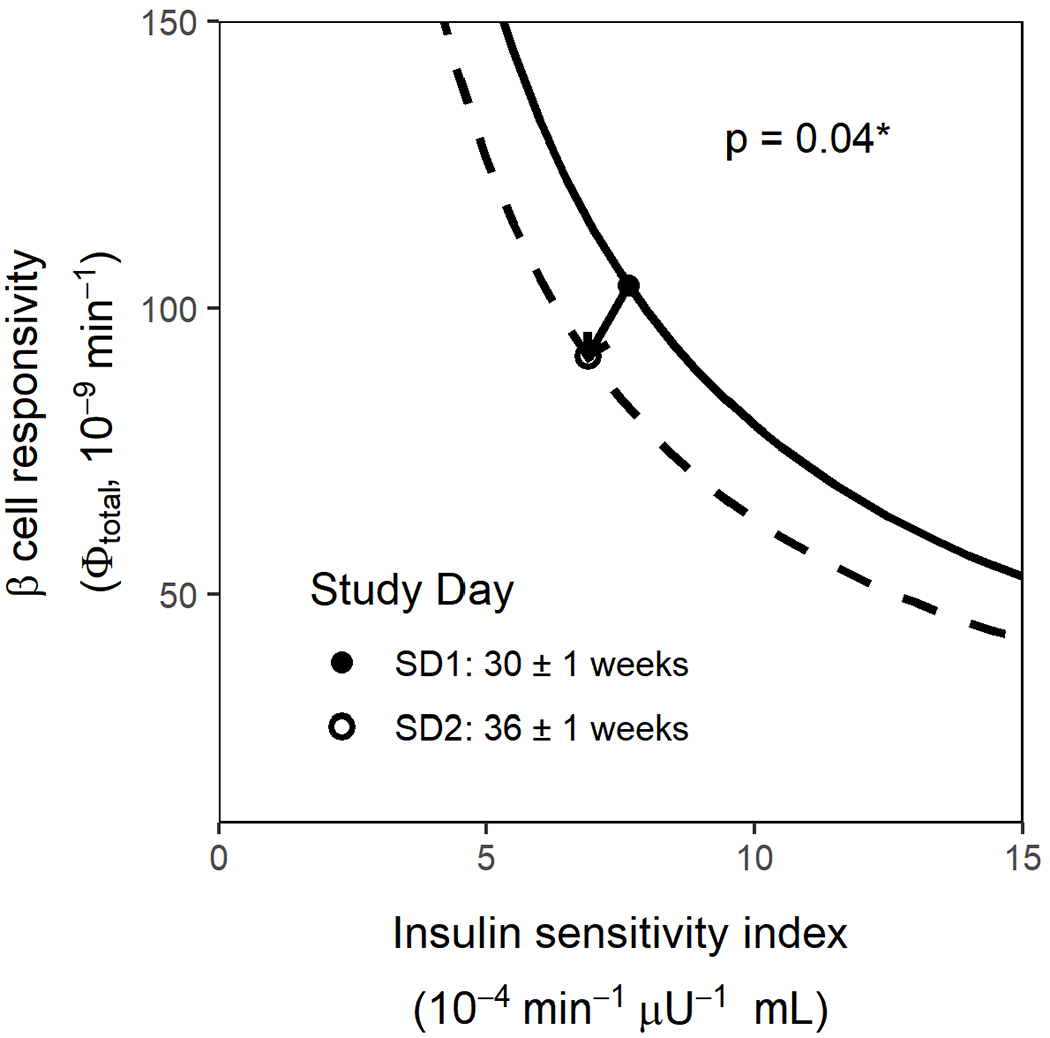
Only paired data for healthy pregnant subjects who completed study day 1 (SD1) and study day 2 (SD2) are included (n=28). Insulin sensitivity is depicted on the x axis, and total β cell responsivity is depicted on the y, and points depict the mean total β-cell responsivity and mean SI on SD1 (filled circle) and SD2 (open circle) for the HP group. The hyperbolas shown depict the calculated total β-cell responsivity given the range of SI values shown for HP on SD1 (solid line) and SD2 (dashed line) where total β-cell responsivity and the DI value used was the mean total β-cell responsivity times the mean SI.
Table 2.
Mixed meal tolerance test parameters for healthy pregnant control subjects (HP) study day 1 (SD1, baseline) and study day 2 (SD2)
| HP (n = 28) | |||
|---|---|---|---|
| Parameter | SD1 | SD2 | Δ |
| SI (10−4 min−1 μU−1 mL) | 8 ± 6 (2 to 20) | 7 ± 4 (1 to 20) | −0.8 ± 4 (−9 to 6) (p=0.6) |
| Φtotal (10−9 min−1) | 120 ± 60 (51 to 280) | 110 ± 40 (46 to 190) | −10 ± 50 (−100 to 20) (p=0.1) |
| Φstatic (10−9 min−1) | 100 ± 50 (30 to 200) | 80 ± 30 (30 to 200) | −10 ± 40 (−100 to 30) (p=0.1) |
| Φdynamic 10−9) | 3,000 ± 2,000 (1,000 to 7,000) | 3,000 ± 1,000 (1,000 to 5,000) | 2 ± 1,000 (−2,000 to 2,000) (p=1) |
| Φbaseline (10−9 min−1) | 10 ± 4 (4.3 to 20) | 14 ± 6 (6.2 to 26) | 3 ± 3 (−0.2 to 9) (p < 0.001) |
| DI (10−13 min−2 μU−1 mL) | 800 ± 500 (200 to 2,000) | 600 ± 300 (200 to 1,000) | −200 ± 400 (−1,000 to 400) (p=0.04) |
| Peak Glucose (mg/dL) | 120 ± 20 (94 to 150) | 120 ± 10 (96 to 150) | −0.7 ± 10 (−30 to 10) (p=0.8) |
Results reported as mean ± SD (95% confidence interval)
Δ = average change between study day 1 and study day 2, SI = Insulin sensitivity, Φ = β-cell responsivity and DI = Disposition index.
Pharmacodynamic Parameters.
All SD2 PD parameters for subjects with GDM are adjusted for gestational age. The mean hyperbolic relationships between total β-cell responsivity and SI for healthy pregnant women and women with GDM on SD1 are depicted in Figure 3. The vectors on the graph depict the mean PD effects of GLY, MET and COMBO therapy in subjects with GDM. MMTT PD parameters for subjects with GDM are reported in Table 3.
Figure 3. Pharmacodynamic effects of GLY, MET and GLY/MET Combo therapies.
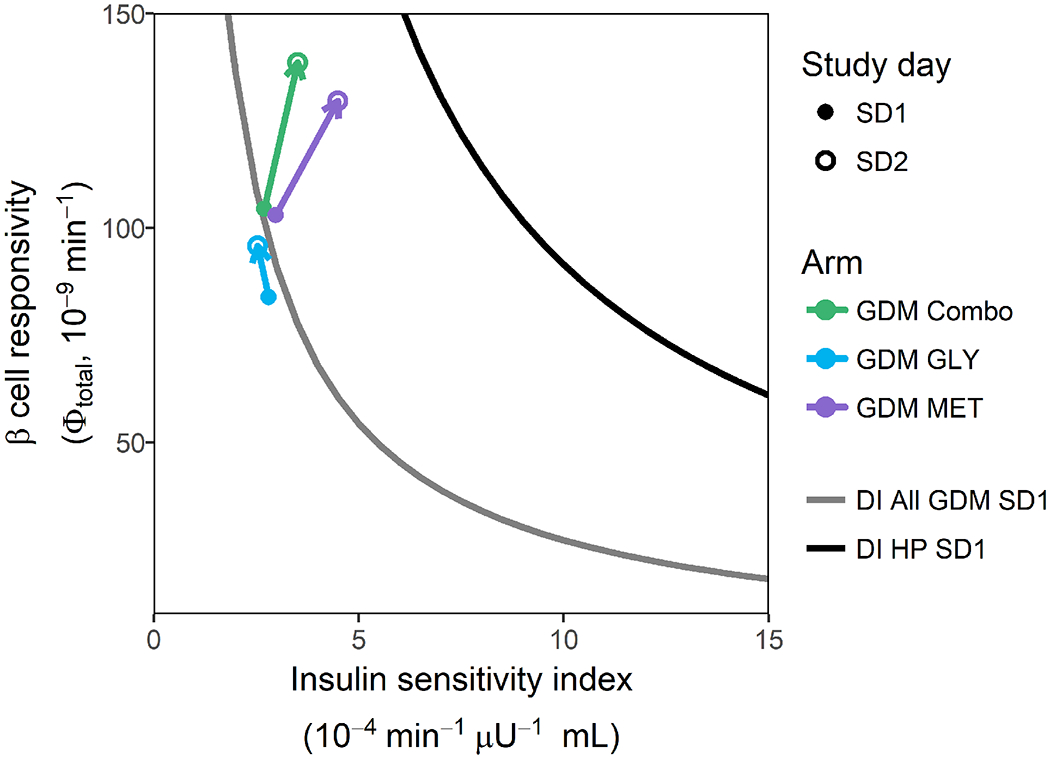
Gray line depicts the mean baseline disposition index for all adherent subjects with GDM who completed the study. The black line depicts the mean baseline disposition index for all healthy pregnant subjects who completed the study. The vectors depict the mean pharmacodynamic effect of GLY (blue arrow), MET (purple arrow) and GLY/MET combination therapy (green arrow). Solid dots represent mean baseline disposition index (study day 1) and open circles represent study day 2 mean disposition index adjusted for mean gestational age-dependent change.
Table 3.
Pharmacodynamic parameters following a MMTT on SD1 and SD2 adjusted for gestational-age-dependent changes in adherent subjects with GDM who completed the study.
| Parameter | COMBO (n = 32) | GLY (n = 32) | MET (n = 25) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SD1 | SD2 | Δ | SD1 | SD2 | Δ | SD1 | SD2 | Δ | |
| SI (10−4 min−1 μU−1 mL) | 3 ± 2 (2 to 3) | 4 ± 2 (3 to 4) | 0.9 ± 2 (0.1 to 2) (p=0.03*) | 3 ± 3 (2 to 4) | 3 ± 2 (2 to 3) | −0.3 ± 2 (−1 to 0.6) (p=0.5) | 3 ± 2 (2 to 4) | 4 ± 2 (4 to 5) | 2 ± 2 (0.5 to 3) (p=0.005**) |
| Φtotal (10−9 min−1) | 100 ± 60 (81 to 130) | 140 ± 80 (110 to 170) | 40 ± 80 (20 to 70) (p=0.004**) | 80 ± 30 (70 to 90) | 100 ± 40 (80 to 100) | 10 ± 50 (−6 to 30) (p=0.2) | 100 ± 50 (83 to 120) | 130 ± 70 (99 to 160) | 30 ± 60 (1 to 50) (p=0.04*) |
| Φstatic (10−9 min−1) | 90 ± 60 (70 to 100) | 130 ± 70 (100 to 160) | 40 ± 70 (20 to 70) (p=0.002**) | 70 ± 30 (70 to 80) | 90 ± 40 (80 to 100) | 10 ± 50 (−3 to 30) (p=0.09) | 90 ± 40 (70 to 100) | 120 ± 70 (87 to 150) | 30 ± 60 (1 to 50) (p=0.04*) |
| Φdynamic (10−9) | 2,000 ± 1,000 (2,000 to 2,000) | 2,000 ± 1,000 (1,000 to 2,000) | −400 ± 1,000 (−800 to 60) (p=0.09) | 1,400 ± 1,000 (1,100 to 1,800) | 800 ± 500 (600 to 900) | −600 ± 800 (−900 to −300) (p=1e-04***) | 2,000 ± 1,000 (1,000 to 2,000) | 2,000 ± 1,000 (2,000 to 3,000) | 100 ± 1,000 (−300 to 600) (p=0.5) |
| Φbaseline (10−9 min−1) | 13 ± 6 (11 to 15) | 13 ± 10 (9 to 16) | −0.5 ± 7 (−3 to 2) (p=0.7) | 10 ± 6 (8 to 12) | 10 ± 10 (9 to 20) | 3 ± 8 (0.3 to 6) (p=0.03*) | 11 ± 5 (9.3 to 14) | 9 ± 6 (6 to 10) | −3 ± 4 (−4 to −0.9) (p=0.004**) |
| DI (10−13 min−2 μU−1 mL) | 300 ± 400 (200 to 400) | 500 ± 400 (400 to 700) | 300 ± 300 (100 to 400) (p=2e-04***) | 200 ± 200 (200 to 300) | 300 ± 300 (200 to 500) | 100 ± 300 (5 to 200) (p=0.04*) | 300 ± 300 (200 to 400) | 600 ± 400 (500 to 800) | 300 ± 500 (100 to 500) (p=0.003**) |
| Peak glucose (mg/dL) | 160 ± 30 (150 to 170) | 150 ± 20 (140 to 160) | −9 ± 30 (−20 to 0.3) (p=0.06) | 170 ± 20 (160 to 180) | 170 ± 30 (160 to 180) | 2 ± 30 (−7 to 10) (p=0.7) | 150 ± 20 (140 to 160) | 140 ± 20 (130 to 140) | −10 ± 20 (−20 to −4) (p=0.006**) |
COMBO = glyburide/metformin combination therapy group; DI = disposition index (SI • Φtotal); GDM = gestational diabetes mellitus; GLY = glyburide monotherapy group; HP = healthy pregnant; MET = metformin monotherapy group; MMTT = mixed-meal tolerance test; Φ = β-cell responsivity; SD = standard deviation; SI = insulin sensitivity; Δ = average change between study day 1 and study day 2. Results reported as mean ± SD (95% confidence interval).
In the GLY group, dynamic β-cell responsivity decreased by an average of 31% (p<0.001), whereas baseline β-cell responsivity increased by 62% (p=0.03), and DI 119%, (p=0.04). No significant effects were seen in other PD parameters. In the MET group, SI increased by 121% (p=0.005); DI 203% (p=0.003); total β-cell responsivity 31% (p=0.04); and static β-cell responsivity 33% (p=0.04); whereas baseline β-cell responsivity decreased 28% (p=0.004), and MMTT peak glucose concentration 7% (p=0.006). There was no significant effect on dynamic β-cell responsivity. In the COMBO group, SI increased by 83% (p=0.03), total β-cell responsivity 57% (p=0.004), static β-cell responsivity 72% (p=0.002), and DI 224% (p<0.001). No significant effects were seen in other PD parameters. The change in DI for all GDM subjects taking metformin, combining those in the MET and COMBO groups, was greater than for the GLY group (p=0.05).
Distributions for changes in PD parameters are depicted in Supplemental Figure S1. In the subjects with GDM, 56% of subjects in the GLY and 74% of subjects in the COMBO group exhibited some pharmacologic response to glyburide (increase in total β-cell responsivity). In addition, 84% of subjects in the MET group and 74% of subjects in the COMBO group exhibited pharmacologic response to metformin (increase in SI). In the COMBO group, pharmacologic response to either glyburide and/or metformin (increase in total β-cell responsivity and/or SI) was seen in 90% of subjects and pharmacologic response to both glyburide and metformin (increase in total β-cell responsivity and SI) was seen in 58%.
Dosage.
Table 1 includes average glyburide and metformin doses/day on SD2 for subjects with GDM in the COMBO, GLY and MET groups. Mean glyburide dose/day was higher in the GLY group than in the COMBO group (p=0.005). The mean metformin dose/day was numerically higher in the MET group than in the COMBO group but failed to achieve significance (p=0.1).
Effect of genotype on MET pharmacodynamic response and metformin dose.
Figure 4A–C depict the association between metformin transporters — MATE2-K, PMAT and OCT2 genotypes and metformin response or dose.
Figure 4. Effects of a splice variant of multidrug and toxin extrusion transporter 2 (MATE2-K) and organic cation transporter 2 (OCT2) genotype on metformin response and dose in women with GDM who were adherent and completed the study.
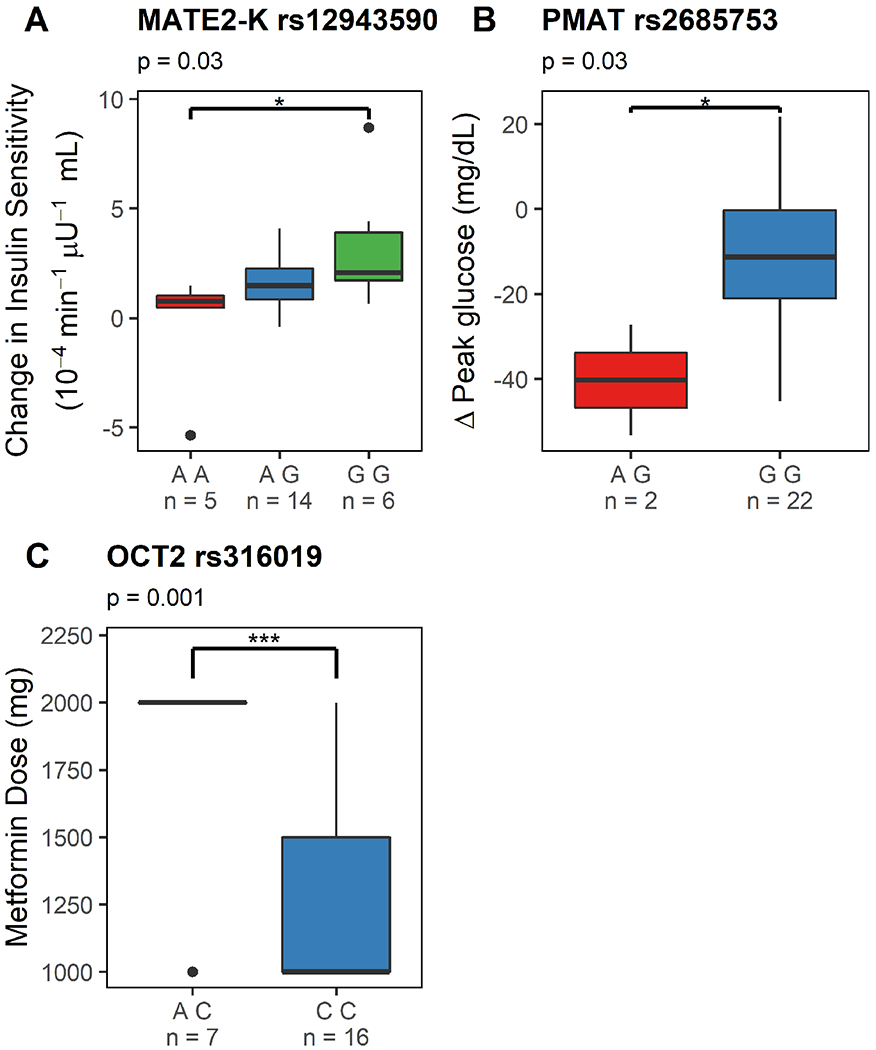
Figure 4A depicts a Tukey boxplot of the effects of MATE2-K genotype (rs12943590; AA, AG and GG) on metformin pharmacologic activity (i.e. change in insulin sensitivity between study day 1 and study day 2, adjusted for gestational age dependent changes) in women receiving metformin monotherapy. Figure 4B depicts a Tukey boxplot of the effects of PMAT genotype (rs2685753; AG and GG) on change in peak glucose concentrations in women receiving metformin monotherapy. Figure 4C depicts a Tukey boxplot of the effects of OCT2 genotype (rs316019, AC vs CC) on metformin dose on study day 2 in women receiving metformin monotherapy. *: p<0.05, **: p<0.01 and ***: p<0.001.
MATE2-K and Metformin’s Effect on Insulin Sensitivity.
Women with GDM with MATE2-K (rs12943590; G>A) AA genotype had a smaller change in insulin sensitivity with MET than those with MATE2-K GG genotype (Figure 4A, p=0.03).
PMAT and MET Effect on Peak Glucose Concentration.
PMAT (rs2685753 G>A) AG genotype was associated with a larger decrease in MMTT peak glucose concentrations than GG genotype (Figure 4B, p=0.03).
OCT2 Effect on Metformin Dose.
Women with OCT2 (rs316019) AC genotype on average required a higher metformin dose than those with OCT2 CC genotype (Figure 4C, p=0.001). There were no subjects with AA genotype.
In this study, MATE1, PMAT, OCT1 and OCT2 genotypes were not associated with metformin pharmacologic effect (change in SI); MATE1, MATE2-K, PMAT and OCT1 genotypes were not found to significantly affect metformin dose; and MATE1, MATE2-K, OCT1 and OCT2 genotypes did not alter metformin’s effect on MMTT peak glucose concentrations.
Discussion
The majority of medication prescribing during pregnancy is based on clinical trials in the non-pregnant population. The mechanism and magnitude of pharmacologic response to glyburide and metformin have not been described during pregnancy despite the significant alterations in glucose and insulin handling that occur during normal pregnancy. This study is the first to 1) quantify and compare the PD effects of GLY, MET and COMBO treatment in pregnant women with GDM and 2) report the effects of MATE2-K on metformin’s PD response (change in SI), OCT2 on metformin dose and PMAT on metformin’s effect on peak glucose concentrations during pregnancy.
Most health-care providers who prescribe oral glucose-lowering agents for women with GDM currently use either metformin or glyburide monotherapies, with significant failure rates.(22). This may occur for two reasons. First, evidence suggests glyburide and metformin exposures are reduced during pregnancy;(11,31) therefore, utilizing dosage strategies established in non-pregnant individuals might not be appropriate. For this reason, glyburide dosage up to 8.75 mg orally three times daily was allowed in this study. Second, the underlying pathology of GDM is heterogeneous with respect to β-cell dysfunction and insulin resistance. Monotherapies that treat only one facet of GDM pathology may not be as effective as a combination approach. The vectors depicted in Figure 3 demonstrated the average response to each approach. As expected, GLY (average dose: 10 mg/day) exhibited its effects primarily through β-cell responsivity and produced a small average DI vector. Although MET (average dose: 1400 mg/day) improved SI as expected, it also improved β-cell responsivity. The addition of glyburide (average dose: 6 mg/day) to metformin (average dose: 1200 mg/day) in the COMBO group boosted the average effect on β-cell responsivity, but had less effect on SI than the MET group, as the dose of metformin in the COMBO group was on average 200 mg/day lower. Interestingly, none of the drug treatment vectors moved the average DI back to what would be considered normal in pregnancy. Given the hyperbolic nature of the DI curve and where the women with GDM started at baseline, the treatments that improved SI most moved the subjects closer to a normal DI than those that primarily affected β-cell responsivity, i.e., the change in DI for women with GDM taking metformin in MET and COMBO groups was greater than in those taking GLY. The majority of women with GDM initiated treatment with a DI on the steep ascending portion of the hyperbolic DI curve, making GLY (i.e. altering β-cell responsivity alone) far less successful in normalizing overall DI. This suggests that maximizing metformin and thereby SI first and then adding glyburide to increase β-cell responsivity or adding insulin supplementation would be a more rational approach to managing patients with GDM. Further research is needed prior to making this a clinical recommendation. However, this is consistent with recent ACOG recommendations for women who decline insulin, to use metformin as the first-line alternative oral agent.(4)
In this study, the percent of subjects having some pharmacologic response (i.e. improvement in either SI and/or total β-cell responsivity) differed by treatment arm. Fifty-six percent of the GLY group, 84% of the MET group and 90% of the COMBO group had some pharmacologic response to glyburide and/or metformin. Both COMBO and MET were associated with significant reductions in MMTT average glucose exposure, whereas GLY on average was not (Figure 1). The Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) Study found a strong association between higher maternal glucose concentrations in response to an OGTT and higher risk of pregnancy complications from GDM.(32) This supports the merit of clinical trials exploring comparative efficacy of metformin either alone or in combination.
Consistent with the expected effect of GLY, average MMTT C-peptide concentrations were higher in the GLY group with treatment than before treatment (Figure 1). Although insulin concentrations were numerically higher with GLY, this did not reach statistical significance. Interestingly, in the COMBO group, neither average C-peptide nor insulin concentrations were significantly higher, perhaps due to the lower glyburide dosage in the COMBO group than GLY group (6 vs. 10 mg/day, p=0.005). Even so, average vector plots appear to show a small additional increase in β-cell responsivity in the COMBO group over the MET group, although this was not statistically significant (Figure 3). In the MET group, insulin concentrations were significantly lower with treatment than before treatment, reflecting metformin’s impact on SI and thus resulting in a lesser need for insulin and decreasing the demands on the pancreas.
Buchanan previously described the hyperbolic relationship between insulin secretion rate and SI in normal women and women with GDM in the 3rd trimester of pregnancy and postpartum. Similar to our findings, he depicted the population with GDM to have a lower-than-normal DI. Interestingly, Buchanan depicted both pregnant and non-pregnant DI on the same curve.(33) Subsequently, Buchanan’s group published a 4-year longitudinal study describing declining DI in women with and without GDM, noting a faster decline in DI in individuals with GDM.(34) In our study, “normal” pregnant women (i.e., those having normal values during their OGTT screening for GDM) had a 6% decline in their DI over an average of 5.5 weeks between SD1 and SD2 (mean difference: −200±400 × 10−13 min−2 μU−1 mL, p=0.04). This decreased ability to compensate for the degree of insulin resistance as pregnancy progresses, even among those who are “normal”, aligns with the continuum of GDM described in the HAPO study and calls for careful consideration and standardization of diagnosis.(32)
Multiple bi-directional drug transporters are involved in the disposition of metformin, including organic cation transporters 1–3 (OCT1, OCT2 and OCT3) as well as multidrug and toxin extrusion transporters 1, 2 and 2-K (MATE1, MATE2 and MATE2-K). OCT transporters and PMAT along with the MATE transporters work in series to move metformin across the cell.(35–37) MATE2-K (rs12943590 G>A) genetic variant is associated with increased promoter activity in vitro.(38,39) In vivo, some but not all studies have reported that MATE2-K rs12943590 G>A genetic variant has been associated with increased metformin renal clearance and decreased metformin efficacy. We are the first to report the effect of MATE2-K on metformin PD (i.e., the effect on SI following a MMTT). Consistent with reports of MATE2-K rs12943590 G>A genetic variant’s impact on transporter activity, we found that women with GDM and MATE2-K AA genotype had a smaller increase in insulin sensitivity with metformin than those with GG genotype (Figure 4A.(40,41)
In addition, women with GDM and OCT2 (rs316019) AC genotype required a higher metformin dose than those with OCT2 CC genotype. Previously, it had been reported that the OCT2–808T genetic variant was associated with increased activity compared to the OCT2 reference in stably transfected HEK-293 cells in vitro.(42) There are mixed reports on the effect of the OCT2 rs316019 A>C genetic variant on metformin disposition in vivo.(43–47) It has been previously reported that individuals heterozygous for the OCT2 variant allele (808G/T) had higher metformin renal clearance than those homozygous for the OCT2 reference allele (808G/G) in Caucasian volunteers.(35) In contrast, two studies in Asian volunteers reported the opposite effect (i.e. those heterozygous for OCT2 variant allele (808G/T) had lower metformin renal clearance than those homozygous for OCT2 reference allele (808G/G).(46,47) In this study we did not find an association with OCT2 genotype and insulin sensitivity, but did find a relationship between OCT2 genotype and metformin dose. This is somewhat surprising given that during pregnancy we found no association with OCT2 genotype and metformin renal clearance.(48) The explanation for this discrepancy is unclear. However, dosage is definitely a more confounded endpoint due to provider discretion than renal clearance or insulin sensitivity.
Last, there are conflicting results with respect to the effects of the PMAT (rs2685753 G>A) variant genotype and its association with metformin exposure.(49,50) Our results demonstrated that pregnant woman with GDM and the PMAT (rs2685753 G>A) AG genotype had a greater decrease in the MMTT peak glucose concentrations than those with GG genotype (p=0.03). PMAT was not significantly associated with changes in metformin PD (change in SI) or metformin pharmacokinetics, (48) which calls into question the mechanism by which PMAT genotype affected metformin’s effect on MMTT peak glucose concentrations.
Limitations.
With respect to the genetics portion of this study, the sample size is small to draw definitive conclusions with respect to the role of transporter genotypes in pharmacodynamic effects of metformin. Larger studies are needed to explore the genetic associations with the biology of response.
In summary, this study for the first time characterized the PD effects of GLY, MET and COMBO treatment in women with GDM. Individual PD responses were variable, and none of the treatment approaches fully normalize the overall glycemic response. However, those that included metformin shifted the overall DI closer to normal than those that primarily increased β-cell responsivity due to the hyperbolic shape of the DI curve and baseline parameters in the women with GDM. This suggests that maximizing metformin and thereby SI first and then adding glyburide to increase β-cell responsivity or adding insulin would be a more rational approach. Lastly in the pregnant women with GDM, associations were found between MATE2-K genotype and metformin’s pharmacologic effect, OCT2 and metformin dosage as well as PMAT and metformin’s effect on peak glucose concentrations.
Supplementary Material
Figure S1. Changes in pharmacodynamic parameters with drug treatment of GDM. Histograms of the distribution of changes in total β-cell responsivity (Φtotal), SI, and DI for subjects receiving either COMBO (green), GLY (blue), or MET (purple) for the treatment of GDM adjusted for gestational age-dependent changes. Bar height indicates number of subjects. Zero, indicated with a vertical red line, represents no change.
Figure S3. Metformin Monotherapy Dosage Titration. This figure depicts the study guide for metformin monotherapy dosage titration. Starting dosage was metformin 500 mg orally twice daily and was titrated as high as 2000 mg/day in divided doses. Provider’s discretion was allowed. In addition, this figure depicts when study day 2 MMTT should be completed. po = orally, bid = twice daily, tid = three time daily, qid = four times daily, PD = pharmacodynamic.
Figure S2. Glyburide Monotherapy Dosage Titration. This figure depicts the study guide for glyburide monotherapy dosage titration. Starting dosage was glyburide 2.5 mg orally twice daily and was titrated as high as 8.75 mg orally three times daily. Provider’s discretion was allowed. In addition, this figure depicts when SD2 MMTT should be completed. po = orally, bid = twice daily, tid = three time daily, PD = pharmacodynamic.
Table S1. Demographics of all eligible subjects with gestational diabetes and gestational age-matched healthy pregnant subjects.
Figure S4. Glyburide and Metformin Combination Therapy Dosage Titration. This figure depicts the study guide for glyburide and metformin dosage titration in the combination therapy group. Starting glyburide dosage was 2.5 mg orally twice daily and titrated as high as 10 mg orally twice daily. Starting metformin dosage was 500 mg orally twice daily and titrated as high as 2000 mg/day in divided doses. Provider’s discretion was allowed. In addition, this figure depicts when study day 2 MMTT should be completed. po = orally, bid = twice daily, tid = three time daily, qid = four times daily, PD = pharmacodynamic.
Study Highlights.
What is the current knowledge on the topic?
There is no data describing and comparing the mechanism and magnitude of the clinical pharmacological effects of GLY, MET and COMBO therapy for GDM.
What question did this study address?
The objectives of this study were to characterize the PD effects of GLY, MET and COMBO therapy for GDM management; evaluate the effects of gestational age on SI, β-cell responsivity and DI; and evaluate the effects of genotype on metformin PD during pregnancy.
What does this study add to our knowledge?
This is the first study to describe the PD of GLY, MET and COMBO therapy in women with GDM as well as to report the effects of MATE2-K on metformin’s PD response (change in SI), OCT2 on metformin dose and PMAT on metformin’s effect on peak glucose concentrations during pregnancy.
How might this change clinical pharmacology or translational science?
Based on the clinical pharmacology gained in this study, maximizing metformin and thereby SI first and then adding glyburide to increase β-cell responsivity or adding insulin would be a more rational approach to GDM treatment. Further research is needed prior to making this a clinical recommendation.
Acknowledgements
In memoriam we acknowledge the tremendous contribution of David A. Flockhart, Ph.D., M.D. to the Obstetric-fetal Pharmacology Research Unit Network. We also thank the research coordinators and nurses including Alisha Bouge, Claudine Hernandez, Karen Hays, Ira Kantrowitz-Gordon, Anna Lemchen, Heather Follen, Holly West, Julie Croxford, Dawn Fisher, Wenchen Zhao, Becky Cypher and Janie Klank for their hard work in completing this study.
Funding Information
This research was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grant #U10HD063094, U10HD047892, U10HD097905, U10HD047891, U10HD057753 and the NIH National Center for Advancing Translational Science through the Clinical and Translational Science Awards Program grant # ULITR000423, TLITR000422, ULITR001108, National Institute of General Medical Sciences R01GM124264 and unrestricted research funds from the University of Washington. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institute of Health. In addition, the views expressed are those of the author(s) and do not reflect the official policy or position of the US Army Medical Department, Department of the Army, Department of Defense or the U.S. Government. The investigators have adhered to the policies for protection of human subjects as prescribed in 45 CFR 46.
Footnotes
Conflict of Interest/Disclosure
The authors declare that at the time of the conduct and analysis of the study, they had no competing interests for this work. However, since the completion of the study, D.L.S.’s affiliation has changed to PRAHealthSciences.
References
- (1).Jovanovic L, Pettitt DJ Gestational diabetes mellitus. J.A.M.A 286, 2516–2518 (2001). [DOI] [PubMed] [Google Scholar]
- (2).Paglia MJ, Coustan DR Gestational diabetes: evolving diagnostic criteria. Curr. Opin. Obste.t Gynecol. 23, 72–75 (2011). [DOI] [PubMed] [Google Scholar]
- (3).The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int J Gynaecol Obstet 78, 69–77 (2002). [DOI] [PubMed] [Google Scholar]
- (4).Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet. Gynecol 131(2):e49–e64 (2018). [DOI] [PubMed] [Google Scholar]
- (5).Committee on Practice, Bulletins-Obstetrics. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet. Gynecol 122, 406–416 (2013). [DOI] [PubMed] [Google Scholar]
- (6).Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales OA comparison of glyburide and insulin in women with gestational diabetes mellitus. N. Engl. .J Med 343, 1134–1138 (2000). [DOI] [PubMed] [Google Scholar]
- (7).Camelo Castillo W, Boggess K, Sturmer T, Brookhart MA, Benjamin DK Jr., Jonsson Funk M Trends in glyburide compared with insulin use for gestational diabetes treatment in the United States, 2000-2011. Obstetrics and gynecology 123, 1177–1184 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Feldman JM Glyburide: a second-generation sulfonylurea hypoglycemic agent. History, chemistry, metabolism, pharmacokinetics, clinical use and adverse effects. Pharmacotherapy. 5, 43–62 (1985). [DOI] [PubMed] [Google Scholar]
- (9).Kirpichnikov D, McFarlane SI, Sowers JR Metformin: an update. Ann. Intern. Med 137, 25–33 (2002). [DOI] [PubMed] [Google Scholar]
- (10).Liang H-L, Ma S-J, Xiao Y-N, Tan H-Z. Comparative efficacy and safety of oral antidiabetic drugs and insulin in treating gestational diabetes mellitus. An updated PRISMA-compliant network meta-analysis. Medicine (Baltimore). September;96(38):e7939 (2017). doi: 10.1097/MD.0000000000007939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hebert MF et al. Are we optimizing gestational diabetes treatment with glyburide? The pharmacologic basis for better clinical practice. Clin. Pharmacol. Ther 85, 607–614 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Dalla Man C, Caumo A, Cobelli C The oral glucose minimal model: estimation of insulin sensitivity from a meal test. IEEE Transactions on Bio-Medical Engineering. 49, 419–429 (2002). [DOI] [PubMed] [Google Scholar]
- (13).Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes. 50, 150–158 (2001). [DOI] [PubMed] [Google Scholar]
- (14).Bergman RN, Phillips LS, Cobelli C Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J. Clin. Invest 68, 1456–1467 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Cobelli C et al. Assessment of beta-cell function in humans, simultaneously with insulin sensitivity and hepatic extraction, from intravenous and oral glucose tests. Am. J. Phys. Endocrin. Metab 293, E1–E15 (2007). [DOI] [PubMed] [Google Scholar]
- (16).Bergman RN Minimal model: perspective from 2005. Hormone Res. 64 Suppl 3, 8–15 (2005). [DOI] [PubMed] [Google Scholar]
- (17).Jacobson GF, Ramos GA, Ching JY, Kirby RS, Ferrara A, Field DR Comparison of glyburide and insulin for the management of gestational diabetes in a large managed care organization. Am. J. Obstet. Gynecol 193, 118–124 (2005). [DOI] [PubMed] [Google Scholar]
- (18).Kremer CJ, Duff P Glyburide for the treatment of gestational diabetes. Am. J. Obstet. Gynecol 190, 1438–1439 (2004). [DOI] [PubMed] [Google Scholar]
- (19).Groop L et al. Comparison of pharmacokinetics, metabolic effects and mechanisms of action of glyburide and glipizide during long-term treatment. Diabetes Care. 10, 671–678 (1987). [DOI] [PubMed] [Google Scholar]
- (20).Chmait R, Dinise T, Moore T Prospective observational study to establish predictors of glyburide success in women with gestational diabetes mellitus. J. Perinatol 24, 617–622 (2004). [DOI] [PubMed] [Google Scholar]
- (21).Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, Mi GTI Metformin versus insulin for the treatment of gestational diabetes. N. Engl. J. Med 358, 2003–2015 (2008). [DOI] [PubMed] [Google Scholar]
- (22).Moore LE, Clokey D, Rappaport VJ, Curet LB Metformin compared with glyburide in gestational diabetes: a randomized controlled trial. Obstet. Gynecol 115, 55–59 (2010). [DOI] [PubMed] [Google Scholar]
- (23).Bandi ZL, Fuller JB, Bee DE, James GP Extended clinical trial and evaluation of glucose determination with the Eastman Kodak Ektachem GLU/BUN Analyzer. Clin. Chem 27, 27–34 (1981). [PubMed] [Google Scholar]
- (24).Haffner SM, Mykkanen L, Stern MP, Valdez RA, Heisserman JA & Bowsher RR Relationship of proinsulin and insulin to cardiovascular risk factors in nondiabetic subjects. Diabetes. 42, 1297–1302 (1993). [DOI] [PubMed] [Google Scholar]
- (25).Wiedmeyer HM et al. International comparison of C-peptide measurements. Clinical chemistry 53, 784–787 (2007). [DOI] [PubMed] [Google Scholar]
- (26).Caumo A, Bergman RN, Cobelli C Insulin sensitivity from meal tolerance tests in normal subjects: a minimal model index. J. Clin. Endocrinol. Metab 85, 4396–4402 (2000). [DOI] [PubMed] [Google Scholar]
- (27).Dalla Man C, Caumo A, Basu R, Rizza R, Toffolo G, Cobelli C Minimal model estimation of glucose absorption and insulin sensitivity from oral test: validation with a tracer method. Am. J. Physiol. Endocrinol. Metab 287, E637–E643 (2004). [DOI] [PubMed] [Google Scholar]
- (28).Dalla Man C et al. Two-hour seven-sample oral glucose tolerance test and meal protocol: minimal model assessment of beta-cell responsivity and insulin sensitivity in nondiabetic individuals. Diabetes. 54, 3265–3273 (2005). [DOI] [PubMed] [Google Scholar]
- (29).R Core Team (2018). R: A language and environment for statistical computing. R Foundation for StatisticalComputing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- (30).Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag New York, 2016. [Google Scholar]
- (31).Eyal S et al. Pharmacokinetics of metformin during pregnancy. Drug Metab. Dispos 38, 833–840 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).The HAPO Study Cooperative Research Group, Metzger BE, et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med 358(19):1991–2002 (2008). [DOI] [PubMed] [Google Scholar]
- (33).Buchanan TA Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J. Clin. Endocrinol. Metab 86:989–993 (2001). [DOI] [PubMed] [Google Scholar]
- (34).Xiang AH, et al. Longitudinal changes in insulin sensitivity and beta cell function between women with and without a history of gestational diabetes mellitus. Diabetologia. 56:2753–2760 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Nies AT, Damme K, Kruck S, Schaeffeler E, Schwab M Structure and function of multidrug and toxin extrusion proteins (MATEs) and their relevance to drug therapy and personalized medicine. Arch. Toxicol 90:1555–1584 (2016). [DOI] [PubMed] [Google Scholar]
- (36).Motohashi H, et al. Precise comparison of protein localization among OCT, OAT and MATE in human kidney. J. Pharm. Sci 102:3302–3308 (2013). [DOI] [PubMed] [Google Scholar]
- (37).Motohashi H, Inui K-I Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS. J 15:581–588 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Choi JH, et al. A common 5’-UTR variant in MATE2-K is associated with poor response to metformin. Clin. Pharmacol. Ther 90:674–684 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Yoon H, Cho HY, Yoo HD, Kim SM, Lee YB Influence of organic cation transporter polymorphisms on the population pharmacokinetics of metformin in healthy subjects. AAPS. J 15:571–580 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Chung J-Y., et al. Functional characterization of MATE2-K genetic variants and their effects on metformin pharmacokinetics. Pharmacogenet. Genomics 23:365–373 (2013). [DOI] [PubMed] [Google Scholar]
- (41).Stocker SL, et al. The effect of novel promoter varints in MATE1 and MATE2 on the pharmacokinetics and pharmacodynamics of metformin. Clin. Pharmacol. Ther 93:186–194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Chen Y, Zhang S, Sorani M, Giacomini KM Transport of paraquat by human organic cation transporters and multidrug and toxic compound extrusion family. J. Pharmacol. Exp. Ther 322:695–700 (2007). [DOI] [PubMed] [Google Scholar]
- (43).Christensen MMH, et al. A gene-gene interaction between polymorphisms in OCT2 and MATE1 genes influences the renal clearance of metformin. Pharmacogenet. Genom 23:526–534 (2013). [DOI] [PubMed] [Google Scholar]
- (44).Tkac I, et al. Pharmacogenomic association between a variant in SLC47A1 gene and therapeutic response to metformin in type 2 diabetes. Diabetes, Obesity Metab. 15:189–191 (2013). [DOI] [PubMed] [Google Scholar]
- (45).Chen Y, et al. Effect of genetic variation in organic cation transporter 2 on the renal elimination of metformin. Pharmacogenet. and Genom. 19:497–504 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Wang Z-J., Yin OQP, Tomlinson B, Chow MSS OCT2 polymorphisms and in vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet. Genom 18:637–645 (2008). [DOI] [PubMed] [Google Scholar]
- (47).Song IS, et al. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin. Pharmacol. Ther 84:559–562 (2008). [DOI] [PubMed] [Google Scholar]
- (48).Liao MZ, et al. Effects of Pregnancy on the Pharmacokinetics of Metformin. Drug. Metab. Dispos (2019) In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Moon SJ, Oh J, Lee SH, Choi Y, Yu K-S, Chung J-Y Effect of plasma membrane monoamine transporter genetic variants on pharmacokinetics of metformin in humans. Transl. Clin. Pharmacol 26:79–85 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Staiger H, Schaeffeler E, Schwab M, Haring H-U Pharmacogenetics: implications for modern type 2 diabetes therapy. Rev. Diabet. Stud 12:363–376 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Changes in pharmacodynamic parameters with drug treatment of GDM. Histograms of the distribution of changes in total β-cell responsivity (Φtotal), SI, and DI for subjects receiving either COMBO (green), GLY (blue), or MET (purple) for the treatment of GDM adjusted for gestational age-dependent changes. Bar height indicates number of subjects. Zero, indicated with a vertical red line, represents no change.
Figure S3. Metformin Monotherapy Dosage Titration. This figure depicts the study guide for metformin monotherapy dosage titration. Starting dosage was metformin 500 mg orally twice daily and was titrated as high as 2000 mg/day in divided doses. Provider’s discretion was allowed. In addition, this figure depicts when study day 2 MMTT should be completed. po = orally, bid = twice daily, tid = three time daily, qid = four times daily, PD = pharmacodynamic.
Figure S2. Glyburide Monotherapy Dosage Titration. This figure depicts the study guide for glyburide monotherapy dosage titration. Starting dosage was glyburide 2.5 mg orally twice daily and was titrated as high as 8.75 mg orally three times daily. Provider’s discretion was allowed. In addition, this figure depicts when SD2 MMTT should be completed. po = orally, bid = twice daily, tid = three time daily, PD = pharmacodynamic.
Table S1. Demographics of all eligible subjects with gestational diabetes and gestational age-matched healthy pregnant subjects.
Figure S4. Glyburide and Metformin Combination Therapy Dosage Titration. This figure depicts the study guide for glyburide and metformin dosage titration in the combination therapy group. Starting glyburide dosage was 2.5 mg orally twice daily and titrated as high as 10 mg orally twice daily. Starting metformin dosage was 500 mg orally twice daily and titrated as high as 2000 mg/day in divided doses. Provider’s discretion was allowed. In addition, this figure depicts when study day 2 MMTT should be completed. po = orally, bid = twice daily, tid = three time daily, qid = four times daily, PD = pharmacodynamic.


