Abstract
Objective:
Despite effective psychological and pharmacological treatments, there is a large unmet burden of illness in post-traumatic stress disorder (PTSD). Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive intervention and a putative treatment strategy for PTSD. The evidence base to date suggests that rTMS targeting the dorsolateral prefrontal cortex (DLPFC), in particular the right DLPFC, leads to improvements in PTSD symptoms. However, optimal stimulation parameters have yet to be determined. In this study, we examine the efficacy of high- and low-frequency rTMS of the right DLPFC using a randomized, double-blind, sham-controlled design in civilian PTSD.
Methods:
We conducted a 2-week single-site randomized sham-controlled trial of rTMS targeting the right DLPFC. We recruited civilians aged 19 to 70 with PTSD and randomized subjects with allocation concealment to daily 1-Hz rTMS, 10-Hz rTMS, or sham rTMS. The primary outcome was improvement in Clinician Administered PTSD Scale–IV (CAPS-IV). Secondary outcomes included change in depressive and anxiety symptoms.
Results:
We recruited 31 civilians with PTSD. One 1-Hz-treated patient developed transient suicidal ideation. Analyses revealed significant improvement in CAPS-IV symptoms in the 1-Hz group relative to sham (Hedges’ g = −1.07) but not in the 10-Hz group. This was not attributable to changes in anxious or depressive symptomatology. Ten-Hz stimulation appeared to improve depressive symptoms compared to sham.
Conclusion:
Low-frequency rTMS is efficacious in the treatment of civilian PTSD. Our data suggest that high-frequency rTMS of the right DLPFC is worthy of additional investigation for the treatment of depressive symptoms comorbid with PTSD.
Keywords: repetitive transcranial magnetic stimulation, rTMS, post-traumatic stress disorder, PTSD, civilian PTSD, depression, randomized clinical trial, RCT
Abstract
Objectif :
Malgré les traitements psychologiques et pharmacologiques efficaces, il y a une lourde charge de maladie non comblée dans le trouble de stress post-traumatique (TSPT). La stimulation magnétique transcrânienne répétitive (SMTr) est une intervention non invasive et une stratégie de traitement réputée pour le TSPT. La base des données probantes jusqu’ici suggère que la SMTr qui cible le cortex préfrontal dorsolatéral (CPFDL), en particulier le CPFDL droit, entraîne des améliorations des symptômes du TSPT. Cependant, les paramètres de stimulation optimale demeurent à déterminer. Dans la présente étude, nous examinons l’efficacité de la SMTr à haute et à basse fréquence du CPFDL droit à l’aide d’une méthode randomisée, à double aveugle, simulée contrôlée dans le TSPT chez des civils.
Méthodes :
Nous avons mené un essai randomisé simulé contrôlé de deux semaines à site unique de SMTr axée sur le CPFDL droit. Nous avons recruté des civils âgés de 19 à 70 ans souffrant de TSPT et des sujets randomisés pour qui la répartition dissimulée consistait quotidiennement en une SMTr de 1-Hz, une SMTr de 10-Hz, ou une SMTr simulée. Le résultat principal était l’amélioration de l’échelle du TSPT IV administrée par un clinicien (ETAC-IV). Les résultats secondaires comprenaient un changement des symptômes dépressifs et anxieux.
Résultats :
Nous avons recruté 31 civils souffrant de TSPT. Un patient traité à 1-Hz a développé une idéation suicidaire transitoire. Des analyses ont révélé une amélioration significative des symptômes à l’ETAC-IV dans le groupe 1-Hz relatif à la simulation (g de Hedges = −1.07), mais pas dans le groupe 10-Hz. Cela n’était pas attribuable aux changements de la symptomatology ieanxieuse ou dépressive. La stimulation 10-Hz semblait améliorer les symptômes dépressifs comparativement à la simulation.
Conclusion :
La SMTr de basse fréquence est efficace dans le traitement du TSPT chez des civils. Nos données suggèrent que la SMTr de haute fréquence du CPFDL droit mérite plus d’investigation pour le traitement des symptômes dépressifs comorbides du TSPT.
Introduction
Post-traumatic stress disorder (PTSD) is a debilitating condition that develops in up to a third of individuals after trauma.1 These individuals are at an increased risk for other mental disorders and for suicide, at great direct and indirect cost to society.2,3 While certain groups, such as military personnel, have rates of PTSD as high as 15%, PTSD remains common in civilian populations, with a lifetime prevalence of 7.5% in the United States4 and 9.2% in Canada.5 Current treatments are efficacious, with clinically meaningful effect sizes in psychotherapy6 and psychopharmacological trials.7 Yet, there remains a large unmet treatment need, and novel intervention strategies are needed. Noninvasive neurostimulation treatments, such as repetitive transcranial magnetic stimulation (rTMS), hold particular promise.
The diagnosis has evolved since its introduction in 1980, reflecting greater appreciation of the forms of trauma that can result in PTSD. For instance, the experience of helplessness or horror during the event is not critical to its development, and direct experience of the trauma is not required. This has supported a view of PTSD as a disorder of neural circuitry,8 rather than strictly a product of exposure to experiences outside of the normative range of human experience,9 with important genetic10,11 and epigenetic components.12 Pathological reactions to trauma ultimately manifest as a complex interplay of circuit alterations resulting in intrusive symptoms, altered arousal and reactivity, altered cognition and mood, and avoidance behaviors.1,4
rTMS is a noninvasive neurostimulation intervention and a putative treatment for disorders of altered circuitry by depolarizing neuronal membranes in targeted regions of cortex through electromagnetic induction. In PTSD, altered prefrontal cortex (PFC) function has repeatedly emerged in neuroimaging studies.13–15 The majority of rTMS studies to date have targeted the dorsolateral PFC (DLPFC) with sham-controlled trials16–21 and meta-analyses22,23 suggesting clinical efficacy. Yet, it remains unclear what are the optimal treatment parameters. More specifically, rTMS using various frequencies have been studied.23 Some studies have found evidence for superior efficacy of low-frequency rTMS,18 high-frequency rTMS,16 and no difference between high and low frequency.19 Others have found no difference in benefit between right versus left DLPFC high-frequency stimulation.17,24 Sham-controlled studies utilizing the novel intermittent theta-burst protocol have indicated that while PTSD symptoms may not improve after active stimulation, social and occupational functioning can improve.25 Studies on bilateral26 or left DLPFC27–29 stimulation have reported transient improvements in PTSD symptoms; however, stimulation characteristics have been inconsistent. Here, we examine the efficacy of high- and low-frequency rTMS of the right DLPFC using a randomized, double-blind, sham-controlled design in civilian PTSD.
Materials and Methods
Trial Design
We conducted a 2-week, double-blind (participants and raters) randomized sham-controlled study of rTMS in the treatment of PTSD (NCT01806168). The duration of the trial reflected the weight of evidence at the time of conception and continues to reflect modern randomized controlled trial design in noninvasive neurostimulation in this population.25 Participants were randomized by random sequence generation 2:2:1:1 with allocation concealment by the envelope method as follows: active 1-Hz rTMS to the right DLPFC, active 10-Hz rTMS to the right DLPFC, sham 1-Hz rTMS to the right DLPFC, and sham 10 Hz to the right DLPFC. Individuals randomized to the sham groups were pooled in all analyses. This study was approved by the Clinical Research Ethics Board of the University of British Columbia. Participants provided written informed consent.
Participants
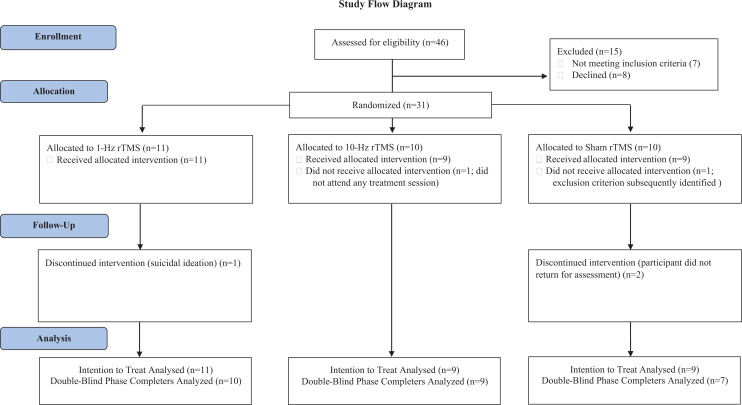
Participants were recruited from the psychiatry outpatient and community programs of Vancouver Coastal Health between 2014 and 2018 (Figure 1). Some subjects were recruited after attending an outpatient psychoeducation group prior to rTMS but had not received formal group therapy. All assessments and treatments took place at Vancouver General Hospital. Inclusion criteria for this study were as follows: male and female participants aged 19 to 70 with a primary diagnosis of non-combat-related PTSD. This was confirmed with the Mini-International Neuropsychiatric Interview (MINI).30 Participants had to have stable psychotropic medications for 4 weeks before starting rTMS and subsequently had no changes in medications or psychotherapy until the completion of the rTMS trial.
Figure 1.
CONSORT diagram.
Exclusion criteria for the study included diagnoses of psychotic illnesses, bipolar disorder type 1, substance use disorder within the last 3 months (excepting nicotine), borderline personality disorder, or antisocial personality disorder. Those with active suicidal ideation were excluded, along with individuals who had unstable medical illnesses and individuals with neurological disorders including previous stroke. rTMS exclusion factors also included a history of seizure, intracranial ferromagnetic objects, and implantable devices in the head or neck region.
Sample Size
Based on the standardized effect size for the primary outcome, as found in a previous meta-analysis published contemporaneously to trial design (Hedges’ g = 1.65)22 and with alpha set to 0.05 and 80% power, a sample size of 9 subjects would be required for each condition.
Interventions
Using a Magstim Super Rapid2 (Magstim Company Ltd, United Kingdom) with a Double 70 mm Air Film Coil model 3910-00, we determined each individual’s resting motor threshold (RMT) according to the visualization method of the abductor pollicis brevis.31 The right DLPFC was determined using the “6 cm rule” by measuring 6 cm anteriorly to the area on the parasagittal line where RMT was found.32 Stimulation intensity was 120% of RMT for all participants. Individuals randomly assigned to 1-Hz stimulation received 2,250 pulses over 37.5 min, whereas those assigned to 10-Hz stimulation received 3,000 pulses over 37.5 min (4 s stimulation train with 26 s intertrain interval). The sham condition involved either 1-Hz or 10-Hz sham stimulation with the above parameters by utilizing a sham Magstim D70 Air Film Coil model 3950-00, which was identical in appearance and produced a similar sound as the active coil as well as mimicked the vibratory somatosensory effect of active stimulation. The rTMS was administered solely by the clinic’s rTMS nurse (S.W.) who was unblinded to the protocol. High- and low-frequency stimulation of the right DLPFC were chosen as the active interventions reflecting the weight of the evidence at the time of trial design.16–18,20
The double-blind phase of this study involved 2 weeks of daily treatments (10 treatments) followed by assessment. While some more recent rTMS in PTSD trial designs have delivered more treatments,19,21 we aligned our trial design with the foundational trials in this area published prior to design and implementation of our trial,16–18 as have other recent trials in PTSD.25
Outcomes
Interested participants were screened by telephone or through a referral from their treating psychiatrist and then assessed in person using the MINI to confirm the diagnosis and eligibility. All clinical assessments were performed by psychiatrists (K.L. and P.C.).
The primary outcome of this study was a change in severity of PTSD symptoms at treatment end as assessed by the Clinician Administered PTSD Scale–IV (CAPS-IV).33 This semistructured instrument was administered by a blinded rater at baseline, at the conclusion of the blinded treatment phase, and at 3-month follow-up.
Secondary outcomes for this trial were also defined as changes at treatment end and were assessed at baseline, at treatment end, and at 3-month follow-up. These included the Hamilton Depression Rating Scale–21 Items34,35 and a clinician-administered semistructured assessment of depressive symptoms. Participants also completed self-report measures including the PTSD Checklist for Civilians (PCL-C36; a self-reported PTSD symptom scale), the Quick Inventory of Depressive Symptomatology, the Beck Anxiety Inventory (BAI), and the Generalized Anxiety Disorder Assessment (GAD-7).37
Data Analysis
Statistical analyses were performed using SPSS v24 (IBM Corporation). Demographics and baseline characteristics were analyzed with Student t test in the case of continuous variables and chi-square test for dichotomous variables. To test primary and secondary outcomes, we utilized intention to treat data and linear mixed models with a group random effect. Three-month follow-up data are reported in the table; however, there was significant and uneven attrition preventing analysis. Significance was set as α ≤ 0.05.
Results
From 2014 to 2018, we recruited 31 civilian participants with a primary diagnosis of PTSD based on Diagnostic and Statistical Manual of Mental Disorders, 4th Edition. The most common type of trauma was sexual violence reported by 16 participants, exposure to actual or threatened death or serious injury reported by 17 participants, and witnessing such incidents in 2 participants. Multiple traumatic events were reported by 8 participants. An additional 5 participants reported significant histories of emotional trauma. All but 2 participants concurrently met criteria for major depressive disorder. There were no significant differences between baseline demographic or clinical characteristics (Table 1).
Table 1.
Demographic Data and Analysis.
| Characteristic | 1-Hz Group (n = 11): Mean ± SD/n | 10-Hz Group (n = 9): Mean ± SD/n (%) | Sham Group (n = 9): Mean ± SD/n (%) | Statistic | P |
|---|---|---|---|---|---|
| Gender (% female) | 10 F/1 M | 7 F/2 M | 7 F/2 M | χ2 = 0.82 | 0.66 |
| Age (years) | 39.2 ± 13.5 | 43.5 ± 12.4 | 49.5 ± 6.9 | F(2, 28) = 1.97 | 0.15 |
| Study completers | 10 (90.9%) | 9 (100%) | 8 (88.8%) | χ2 = 0.66 | 0.71 |
| CAPS-IV score baseline | 72.2 ± 25.3 | 69.44 ± 18.29 | 55.2 ± 13.17 | F(2, 28) = 1.96 | 0.16 |
| PCL-C | 59.4 ± 16.4 | 65.3 ± 11.4 | 61.6 ± 7.9 | F(2, 26) = 0.51 | 0.60 |
| HDRS-21 score | 15.9 ± 10.0 | 17.1 ± 7.7 | 14.4 ± 5.4 | F(2, 27) = 0.24 | 0.78 |
| GAD-7 | 14.4 ± 5.3 | 12.3 ± 7.5 | 12.1 ± 4.7 | F(2, 23) = 0.37 | 0.69 |
| BAI | 34.6 ± 18.4 | 28.9 ± 19.8 | 35.1 ± 10.8 | F(2, 26) = 0.36 | 0.69 |
| Psychiatric comorbidities | |||||
| Major depressive disorder | 9 (81%) | 9 (100%) | 9 (100%) | χ2 = 3.51 | 0.17 |
| Generalized anxiety disorder | 6 (54.5%) | 3 (33.3%) | 3 (33.3%) | χ2 = 1.26 | 0.53 |
| Social phobia | 4 (36.3%) | 3 (33.3%) | 2 (22.2%) | ||
| Panic disorder | 9 (81%) | 5 (55.5%) | 6 (66.6%) | χ2 = 0.49 | 0.78 |
| Obsessive compulsive disorder | 2 (18.1%) | 1 (11.1%) | 0 (0.0%) | χ2 = 1.77 | 0.41 |
| Eating disorder | 0 (0.0% | 1 (11.1%) | 1 (11.1%) | χ2 = 1.31 | 0.51 |
| Attention deficit hyperactivity disorder | 1 (9.0%) | 0 (0.0%) | 0 (0.0%) | χ2 = 1.69 | 0.42 |
| Medications | |||||
| SSRI | 1 (9.0%) | 5 (55.5%) | 3 (33.3%) | χ2 = 5.02 | 0.08 |
| SNRI | 4 (36.3%) | 1 (11.1%) | 2 (22.2%) | χ2 = 1.75 | 0.41 |
| Antipsychotic | 3 (27.2%) | 2 22.2%) | 0 (0.0%) | χ2 = 2.80 | 0.24 |
| Prazosin | 2 (18.1%) | 1 (11.1%) | 2 (22.2%) | χ2 = 0.40 | 0.81 |
| Benzodiazepine | 3 (27.2%) | 2 (22.2%) | 3 (33.3%) | χ2 = 0.27 | 0.87 |
| Number of psychiatric medications | 2.18 ± 1.47 | 2.33 ± 1.65 | 1.66 ± 1.22 | F(2, 38) = 0.52 | 0.60 |
Note. BAI = Beck Anxiety Inventory; CAPS-IV = Clinically Administered PTSD Scale; F = female; GAD-7 = Generalized Anxiety Disorder 7-item; HDRS-21 = Hamilton Rating Scale for Depression (21 items); M = male; PCL-C = PTSD Checklist—Civilian version; SD = standard deviation; SNRI = serotonin noradrenalin reuptake inhibitor; SSRI = Selective serotonin reuptake inhibitor.
Although 3-month follow-up data were acquired, there was disproportionate attrition in the sham-treated group (χ2 = 9.01, df = 2; P = 0.011), and therefore, analyses of these data are not presented.
Adverse Events
A participant in the active 1-Hz group experienced suicidal ideation requiring brief hospitalization after 1 session and was withdrawn from the study. The subject had been experiencing flu-like symptoms prior to starting rTMS. No other serious adverse events occurred.
Primary Outcome—CAPS-IV Score
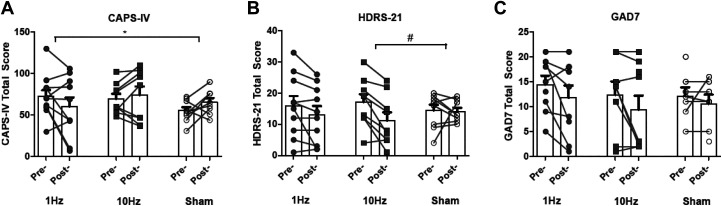
Analyses pertaining to clinician-rated PTSD symptoms are illustrated in Figure 2A and detailed in Table 2. This revealed a significant Time × Treatment effect at the conclusion of treatment: Time F(1, 29.19) = 0.02, P = 0.87; Treatment F(2, 18.95) = 0.73, P = 0.49; Time × Treatment F(2, 29.06) = 3.43, P = 0.046. Compared to sham treatment, PTSD symptoms among those receiving 1-Hz stimulation treatment significantly improved, t(29.43) = 2.43, P = 0.021, corresponding to a Hedges’ g of −1.07, whereas those receiving 10-Hz stimulation did not, t(29.50) = 0.45, P = 0.65.
Figure 2.
Change in clinical symptomatology. (A) CAPS-IV, (B) HDRS-21, and (C) GAD-7. CAPS-IV = Clinically Administered PTSD Scale; GAD-7 = Generalized Anxiety Disorder 7-item; HDRS-21 = Hamilton Rating Scale for Depression (21 items).
Table 2.
Change in PTSD, Depressive, and Anxiety Symptoms over the Course of Treatment.
| 1-Hz Group (n = 11): Mean ± SD | 10-Hz Group (n = 9): Mean ± SD | Sham Group (n = 9): Mean ± SD | Treatment End | P | |
|---|---|---|---|---|---|
| Treatment × Time Effect | |||||
| CAPS-IV | |||||
| Baseline | 72.27 ± 25.34 | 69.44 ± 18.29 | 55.22 ± 13.17 | ||
| Treatment end | 59.80 ± 35.83 | 74.00 ± 30.97 | 65.12 ± 14.97 | F(2, 29.06) = 3.43 | 0.046 |
| 3-Month follow-up | 55.40 ± 29.20 (n = 10) | 70.55 ± 27.98 (n = 9) | 71.50 ± 19.97 (n = 4) | ||
| PCL-C | |||||
| Baseline | 59.40 ± 16.44 | 65.33 ± 11.40 | 61.62 ± 7.96 | ||
| Treatment end | 48.10 ± 23.54 | 53.44 ± 22.80 | 52.14 ± 10.05 | F(2, 27.64) = 0.064 | 0.93 |
| 3-Month follow-up | 48.66 ± 17.25 (n = 9) | 57.12 ± 14.77 (n = 8) | 52.66 ± 13.44 (n = 6) | ||
| HDRS-21 | |||||
| Baseline | 15.90 ± 10.01 | 17.11 ± 7.72 | 14.44 ± 5.45 | ||
| Treatment end | 12.30 ± 8.42 | 11.22 ± 7.99 | 14.44 ± 3.32 | F(2, 8.59) = 2.09 | 0.18 |
| 3-Month follow-up | 13.44 ± 8.48 (n = 9) | 14.11 ± 9.80 (n = 9) | 12.60 ± 8.53 (n = 5) | ||
| QIDS-SR | |||||
| Baseline | 14.80 ± 7.20 | 18.77 ± 5.99 | 15.14 ± 3.07 | ||
| Treatment end | 12.66 ± 6.63 | 15.00 ± 7.15 | 9.42 ± 4.68 | F(2, 15.39) = 0.63 | 0.54 |
| 3-Month follow-up | 12.55 ± 5.59 (n = 9) | 16.12 ± 7.12 (n = 8) | 12.40 ± 7.36 (n=5) | ||
| BAI | |||||
| Baseline | 34.60 ± 18.46 | 30.22 ± 20.63 | 35.14 ± 10.86 | ||
| Treatment end | 24.70 ± 18.19 | 22.77 ± 18.78 | 28.14 ± 10.97 | F(2, 17.15) = 0.14 | 0.86 |
| 3-Month follow-up | 26.55 ± 15.35 (n = 9) | 28.62 ± 19.33 (n = 8) | 33.20 ± 11.51 (n = 5) | ||
| GAD-7 | |||||
| Baseline | 14.80 ± 5.18 | 12.37 ± 7.55 | 11.00 ± 5.42 | ||
| Treatment end | 11.90 ± 7.03 | 8.50 ± 8.53 | 11.75 ± 5.62 | F(2,15.33) = 0.96 | 0.40 |
| 3-Month follow-up | 12.57 ± 6.82 (n = 7) | 11.14 ± 6.84 (n = 7) | 13.33 ± 4.04 (n = 3) | ||
Note. CAPS-IV = Clinically Administered PTSD Scale; BAI = Beck Anxiety Inventory; GAD-7 = Generalized Anxiety Disorder, 7 item; HDRS-21 = Hamilton Rating Scale for Depression (21 items); PCL-C = PTSD Checklist–Civilian version; PTSD = Post-traumatic stress disorder; QIDS-SR = Quick Inventory of Depressive Symptomatology (Self-report); SD = standard deviation.
We repeated this analysis controlling for anxiety symptoms as measured by the BAI to determine whether this effect was specific to PTSD symptoms or a nonspecific anxiolytic effect. This once again revealed a significant Time × Treatment effect at the conclusion of treatment: Time F(1, 25.67) = 0.10, P = 0.74; Treatment F(2, 26.22) = 0.64, P = 0.53; Time × Treatment F(2, 25.68) = 3.67, P = 0.039. Compared to sham treatment, PTSD symptoms among those receiving 1-Hz stimulation treatment significantly improved, t(25.43) = 2.53, P = 0.018, whereas those receiving 10-Hz stimulation did not, t(25.77) = 0.68, P = 0.49.
Secondary Outcomes
Clinician-rated Depressive Symptoms
Analyses pertaining to clinician-rated depressive symptoms are illustrated in Figure 2B and detailed in Table 2. This did not revealed a significant Time × Treatment effect at the conclusion of treatment: Time F(1, 8.60) = 7.05, P = 0.027; Treatment F(2, 6.89) = 0.00, P = 0.99; Time × Treatment F(2, 8.59) = 2.59, P = 0.18; however, compared to sham, the 10-Hz group demonstrated marginal improvement, t(8.65) = 2.04, P = 0.073.
Self-report Instruments
Analyses pertaining to self-report instruments are detailed in Table 2. Although the PCL-C strongly correlated with CAPS-IV (r = 0.76, P < 0.001), we did not observe any significant Time × Treatment interactions for self-reported PTSD symptoms. We similarly did not observe any statistically significant interactions for anxiety symptoms as quantified using the BAI or the GAD-7.
Discussion
Our randomized sham-controlled trial examining high- and low-frequency rTMS of the right DLPFC in civilian PTSD suggests that low-frequency 1-Hz rTMS results in greater improvements in PTSD symptoms relative to sham. We did not observe any changes in general anxiety symptomatology, and improvement of PTSD symptoms in the 1-Hz group could not be accounted for by changes in anxiety symptoms. Moreover, we observed a trend toward improvement in depressive symptoms in the 10-Hz group relative to sham.
Previous studies have explored the effect of low-frequency rTMS on core symptoms of PTSD and have found that low-frequency rTMS could alleviate symptoms of PTSD such as alterations in arousal and reactivity,20 persistent avoidance,26 and intrusion symptoms38; however, this has not been consistent across trials.16,20 Compared with most of the previous studies that examined the effect of low-frequency rTMS in civilian PTSD,23 we utilized a higher intensity stimulus and more pulses in each treatment session. Our findings add to the body of evidence that low-frequency rTMS can have a significant impact on PTSD core symptoms even over a relatively brief treatment period of 2 weeks, with a large effect size in our 1-Hz group that is comparable to the effect sizes shown in similar previous 2-week trials.39 It is, however, possible that additional improvements would have been observed in both treatment conditions had our protocol extended beyond 2 weeks. This was notably the case in a recent 6-week randomized controlled trial targeting the right DLPFC with either 1-Hz or 10-Hz stimulation in combat-related PTSD that demonstrated large improvements in both PTSD and depressive symptoms in both groups.19 While our data add to the relative efficacy of target site and stimulation duty cycle, the treatment duration and dose remain important areas for future research to address.
Although the neuroimaging literature in PTSD has repeatedly identified the PFC as an important node in PTSD, these studies have highlighted the medial PFC (mPFC),13–15,40–42 consistent with data suggesting that the mPFC interacts with key emotional nodes implicated in PTSD including the hippocampus and amygdala. Studies examining the predictive utility of functional magnetic resonance imaging have similarly highlighted the importance of DMPFC, temporoparietal junction, and limbic connectivity in predicting outcomes to theta-burst stimulation,25 as well as the subgenual cingulate and decreased connectivity with the default mode network with high-frequency rTMS.43 TMS as a tool for probing neural networks and clinical response to intervention has revealed a strong relationship for the ventral attention network and the DMPFC,13 as well as DLPFC-amygdala connectivity.44 Where possible, future studies should consider combining mapping and functional characterization to optimize patient selection and stimulation parameter selection.
Deep rTMS targeting the mPFC bilaterally has shown some evidence for clinical benefit in PTSD when used in combination with exposure treatment.45 Other coil configurations permit stimulation of the dorsomedial PFC, and these have shown evidence of clinical benefit in major depression.16 Additional studies targeting the mPFC in PTSD in comparison to the DLPFC target are required in order to determine differential efficacy and potentially improve outcomes in this population.
With respect to safety and tolerability, rTMS was generally well tolerated. Both 1-Hz and 10-Hz protocols appeared acceptable to participants. One participant in the 1-Hz arm was withdrawn from the study after developing transient suicidal ideation with one active rTMS session. It is unclear whether this was rTMS related or not, as the participant had a remote history of hospitalizations for transient suicidal ideation and was developing flu-like symptoms prior to rTMS initiation. Nevertheless, the possibility of unanticipated psychiatric symptoms with noninvasive neurostimulation16 warrants careful consideration and clinical supervision.
Limitations
Several limitations to this study should be highlighted. The sample size was limited and retention within the sham-treated group further limited statistical power, which increases the possibility of both type 1 and type 2 error. This was most notable at the 3-month follow-up time point and may reflect a nonspecific effect of active rTMS. We did not verify the integrity of blinding, and we did not use imaging to define treatment targets. These are methodological considerations that should be included in future studies. Despite heterogeneity in the types of civilian trauma, comorbidities, and concomitant psychotropics, our sample is representative of clinical practice which increases the external validity and generalizability of our results. While medication classes were not differentially present in each group, we are underpowered to investigate the effects of specific agents and dosing and their interaction with specific stimulation protocols.
Conclusions
Low-frequency 1-Hz rTMS of the right DLPFC is efficacious and well tolerated in civilian PTSD and appears to result in reductions in PTSD symptoms following ten 1-Hz rTMS treatments when compared with sham stimulation. In this study, there was no significant difference between 1-Hz and 10-Hz outcome measures. While 10-Hz rTMS of the right DLPFC did not separate from sham-rTMS, this protocol appeared to improve depressive symptoms in this civilian PTSD sample. Confirmation in larger samples is required.
Footnotes
Authors’ Note: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declaration of Conflicting Interests: The author(s) declared potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Peter Chan is the co-owner and practitioner at Brainstim Healthcare, a private rTMS clinic in Vancouver, Canada. Other authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by a Vancouver Coastal Health Research Institute Team Grant.
ORCID iDs: Kawai Leong, MD, MSc, FRCPC  https://orcid.org/0000-0001-7495-0613
https://orcid.org/0000-0001-7495-0613
Peter Chan, MD, FRCPC  https://orcid.org/0000-0002-6450-4326
https://orcid.org/0000-0002-6450-4326
Raymond W. Lam, MD, FRCPC  https://orcid.org/0000-0001-7142-4669
https://orcid.org/0000-0001-7142-4669
Alexander McGirr, MD, PhD, FRCPC  https://orcid.org/0000-0002-8425-3958
https://orcid.org/0000-0002-8425-3958
References
- 1. Shalev A, Liberzon I, Marmar C. Post-traumatic stress disorder. N Engl J Med. 2017;376(25):2459–2469. [DOI] [PubMed] [Google Scholar]
- 2. Ivanova JI, Birnbaum HG, Chen L, et al. Cost of post-traumatic stress disorder vs major depressive disorder among patients covered by Medicaid or private insurance. Am J Manag Care. 2011;17(8):e314–323. [PubMed] [Google Scholar]
- 3. Walker EA, Katon W, Russo J, Ciechanowski P, Newman E, Wagner AW. Health care costs associated with posttraumatic stress disorder symptoms in women. Arch Gene Psychiatry. 2003;60(4):369–374. [DOI] [PubMed] [Google Scholar]
- 4. Kassem MS, Lagopoulos J, Stait-Gardner T, et al. Stress-induced grey matter loss determined by MRI is primarily due to loss of dendrites and their synapses. Mol Neurobiol. 2013;47(2):645–661. [DOI] [PubMed] [Google Scholar]
- 5. Van Ameringen M, Mancini C, Patterson B, Boyle MH. Post-traumatic stress disorder in Canada. CNS Neurosci Ther. 2008;14(3):171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cusack K, Jonas DE, Forneris CA, et al. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2016;43(3):128–141. [DOI] [PubMed] [Google Scholar]
- 7. Cipriani A, Williams T, Nikolakopoulou A, et al. Comparative efficacy and acceptability of pharmacological treatments for post-traumatic stress disorder in adults: a network meta-analysis. Psychol Med. 2018;48(12):1975–1984. [DOI] [PubMed] [Google Scholar]
- 8. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. [DOI] [PubMed] [Google Scholar]
- 9. Fenster RJ, Lebois LAM, Ressler KJ, Suh J. Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci. 2018;19(9):535–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Logue MW, Amstadter AB, Baker DG, et al. The psychiatric genomics consortium posttraumatic stress disorder workgroup: posttraumatic stress disorder enters the age of large-scale genomic collaboration. Neuropsychopharmacology. 2015;40(10):2287–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Afifi TO, Asmundson GJ, Taylor S, Jang KL. The role of genes and environment on trauma exposure and posttraumatic stress disorder symptoms: a review of twin studies. Clin Psychol Rev. 2010;30(1):101–112. [DOI] [PubMed] [Google Scholar]
- 12. Lappalainen T, Greally JM. Associating cellular epigenetic models with human phenotypes. Nat Rev Genet. 2017;18(7):441–451. [DOI] [PubMed] [Google Scholar]
- 13. Etkin A, Maron-Katz A, Wu W, et al. Using fMRI connectivity to define a treatment-resistant form of post-traumatic stress disorder. Sci Transl Med. 2019;11(486):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. [DOI] [PubMed] [Google Scholar]
- 15. Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry. 2006;60(4):376–382. [DOI] [PubMed] [Google Scholar]
- 16. Cohen H, Kaplan Z, Kotler M, Kouperman I, Moisa R, Grisaru N. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2004;161(3):515–524. [DOI] [PubMed] [Google Scholar]
- 17. Boggio PS, Rocha M, Oliveira MO, et al. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J Clin Psychiatry. 2010;71(8):992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watts BV, Landon B, Groft A, Young-Xu Y. A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul. 2012;5(1):38–43. [DOI] [PubMed] [Google Scholar]
- 19. Kozel FA, Van Trees K, Larson V, et al. One hertz versus ten hertz repetitive TMS treatment of PTSD: a randomized clinical trial. Psychiatry Res. 2019;273(4):153–162. [DOI] [PubMed] [Google Scholar]
- 20. Osuch EA, Benson BE, Luckenbaugh DA, Geraci M, Post RM, McCann U. Repetitive TMS combined with exposure therapy for PTSD: a preliminary study. J Anxiety Disord. 2009;23(1):54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kozel FA, Motes MA, Didehbani N, et al. Repetitive TMS to augment cognitive processing therapy in combat veterans of recent conflicts with PTSD: a randomized clinical trial. J Affect Disord. 2018;229(5):506–514. [DOI] [PubMed] [Google Scholar]
- 22. Berlim MT, Van Den Eynde F. Repetitive transcranial magnetic stimulation over the dorsolateral prefrontal cortex for treating posttraumatic stress disorder: an exploratory meta-analysis of randomized, double-blind and sham-controlled trials. Can J Psychiatry. 2014;59(9):487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yan T, Xie Q, Zheng Z, Zou K, Wang L. Different frequency repetitive transcranial magnetic stimulation (rTMS) for posttraumatic stress disorder (PTSD): a systematic review and meta-analysis. J Psychiatr Res. 2017;89:125–135. [DOI] [PubMed] [Google Scholar]
- 24. Fryml LD, Pelic CG, Acierno R, et al. Exposure therapy and simultaneous repetitive transcranial magnetic stimulation: a controlled pilot trial for the treatment of posttraumatic stress disorder. J ECT. 2019;35(1):53–60. [DOI] [PubMed] [Google Scholar]
- 25. Philip NS, Barredo J, Aiken E, et al. Theta-burst transcranial magnetic stimulation for posttraumatic stress disorder. Am J Psychiatry. 2019;176(11):939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grisaru N, Amir M, Cohen H, Kaplan Z. Effect of transcranial magnetic stimulation in posttraumatic stress disorder: a preliminary study. Biol Psychiatry. 1998;44(1):52–55. [DOI] [PubMed] [Google Scholar]
- 27. McCann UD, Kimbrell TA, Morgan CM, et al. Repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Arch Gene Psychiatry. 1998;55(3):276–279. [DOI] [PubMed] [Google Scholar]
- 28. Rosenberg PB, Mehndiratta RB, Mehndiratta YP, Wamer A, Rosse RB, Balish M. Repetitive transcranial magnetic stimulation treatment of comorbid posttraumatic stress disorder and major depression. J Neuropsychiatry Clin Neurosci. 2002;14(3):270–276. [DOI] [PubMed] [Google Scholar]
- 29. Carpenter LL, Conelea C, Tyrka AR, et al. 5 Hz repetitive transcranial magnetic stimulation for posttraumatic stress disorder comorbid with major depressive disorder. J Affect Disord. 2018;235(4):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33; quiz 34–57. [PubMed] [Google Scholar]
- 31. Pridmore S, Fernandes Filho JA, Nahas Z, Liberatos C, George MS. Motor threshold in transcranial magnetic stimulation: a comparison of a neurophysiological method and a visualization of movement method. J ECT. 1998;14(1):25–27. [PubMed] [Google Scholar]
- 32. McClintock SM, Reti IM, Carpenter LL, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. 2018;79(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-administered PTSD Scale. J Traum Stress. 1995;8(1):75–90. [DOI] [PubMed] [Google Scholar]
- 34. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gene Psychiatry. 1988;45(8):742–747. [DOI] [PubMed] [Google Scholar]
- 36. Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP. The PTSD checklist for DSM-5 (PCL-5). Scale available from the National Center for PTSD at www.ptsd.va.gov. 2013;10. [Google Scholar]
- 37. Lowe B, Decker O, Muller S, et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care. 2008;46(3):266–274. [DOI] [PubMed] [Google Scholar]
- 38. Nam DH, Pae CU, Chae JH. Low-frequency, repetitive transcranial magnetic stimulation for the treatment of patients with posttraumatic stress disorder: a double-blind, sham-controlled study. Clin Psychopharmacol Neurosci. 2013;11(2):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cirillo P, Gold AK, Nardi AE, et al. Transcranial magnetic stimulation in anxiety and trauma-related disorders: a systematic review and meta-analysis. Brain Behav. 2019;9(6):e01284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thome J, Terpou BA, McKinnon MC, Lanius RA. The neural correlates of trauma-related autobiographical memory in posttraumatic stress disorder: a meta-analysis. Depress Anxiety. 2020;37(4):321–345. [DOI] [PubMed] [Google Scholar]
- 41. Kamiya K, Abe O. Imaging of posttraumatic stress disorder. Neuroimaging Clin N Am. 2020;30(1):115–123. [DOI] [PubMed] [Google Scholar]
- 42. Kunimatsu A, Yasaka K, Akai H, Kunimatsu N, Abe O. MRI findings in posttraumatic stress disorder. J Magn Reson Imaging. 2019:9. [DOI] [PubMed] [Google Scholar]
- 43. Philip NS, Barredo J, van’t Wout-Frank M, Tyrka AR, Price LH, Carpenter LL. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 2018;83(3):263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fonzo GA, Goodkind MS, Oathes DJ, et al. PTSD psychotherapy outcome predicted by brain activation during emotional reactivity and regulation. Am J Psychiatry. 2017;174(12):1163–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Isserles M, Shalev AY, Roth Y, et al. Effectiveness of deep transcranial magnetic stimulation combined with a brief exposure procedure in post-traumatic stress disorder—a pilot study. Brain Stimul. 2013;6(3):377–383. [DOI] [PubMed] [Google Scholar]