Abstract
The FMR1 gene contains a polymorphic CGG trinucleotide sequence within its 5′ untranslated region. More than 200 CGG repeats (termed a full mutation) underlie the severe neurodevelopmental condition fragile X syndrome, while repeat lengths that range between 55 and 200 (termed a premutation) result in the conditions fragile X-associated tremor/ataxia syndrome and fragile X-associated premature ovarian insufficiency (FXPOI). Premutations in FMR1 are the most common monogenic cause of premature ovarian insufficiency and are routinely tested for clinically; however, the mechanisms that contribute to the pathology are still largely unclear. As studies in this field move towards unravelling the molecular mechanisms involved in FXPOI aetiology, we review the evidence surrounding the two main theories which describe an RNA toxic gain-of-function mechanism, resulting in the loss of function of RNA-binding proteins, or a protein-based mechanism, where repeat-associated non-AUG translation leads to the formation of an abnormal polyglycine containing protein, called FMRpolyG.
Keywords: FXPOI, molecular mechanisms, RNA gain-of-function, RAN translation
Introduction
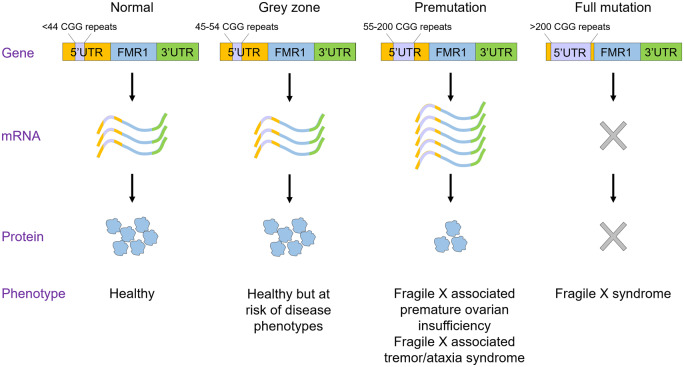
Fragile X-associated premature ovarian insufficiency (FXPOI) is among a family of disorders caused by the expansion of a CGG trinucleotide repeat sequence located in the 5′ untranslated region (UTR) of the FMR1 gene on the X chromosome. This repeat sequence is polymorphic in length, with categorization into four different size ranges (Fig. 1). Healthy individuals have a normal repeat length with fewer than 44 CGG repeats, while having between 45 and 54 repeats is classified as intermediate, or grey zone. Although this sequence length is not directly associated with disease phenotypes, some level of CGG repeat instability has been reported, which results in variable repeat expansion during mother-offspring transmission (Nolin et al., 1996). The range of 55–200 CGG repeats is considered a premutation, and more than 200 CGG repeats are categorized as a full mutation. Full mutations underlie the severe neurodevelopmental condition fragile X syndrome (Kronquist et al., 2008), which is the most common cause of inherited intellectual disability and autism in males, with patients suffering from a wide range of clinical, cognitive and behavioural dysfunctions. While premutation carriers were originally thought to be clinically unaffected, it was noted that some females in families with a fragile X male experienced a premature menopause, now characterized as FXPOI (Cronister et al., 1991; Conway et al., 1998). Premutations also result in the more recent described fragile X-associated tremor/ataxia syndrome (FXTAS), a multisystem neurological disorder with tremor and ataxia as its principal features, which was initially recognized in ageing carriers but with clinical features potentially also present in children (Hagerman et al., 2001). Much of the research into the molecular mechanisms underlying the pathology of FMR1-associated conditions has focused on the neurological aspects: the aim of this review is to critically appraise putative mechanisms underlying the pathogenesis of FXPOI, specifically the evidence investigating the RNA gain-of-function hypothesis and the contribution of repeat-associated non-AUG (RAN) translation, drawing on parallels in FXTAS and advances made in that disease.
Figure 1.
CGG repeat length is polymorphic. Normal individuals have fewer than 44 CGG repeats (mean 29) and CGG repeat lengths between 45 and 54 are termed grey zone, as they are at risk of developing disease phenotypes due to CGG repeat instability. Individuals with a premutation have between 55 and 200 CGG repeats, elevated FMR1 mRNA levels and reduced FMRP protein levels, and the premutation causes the conditions fragile X-associated premature ovarian insufficiency and fragile X-associated tremor/ataxia syndrome. Individuals with more than 200 CGG repeats have a full mutation, where the FMR1 gene is transcriptionally silenced, leading to an absence of FMR1 mRNA and FMRP protein. The full mutation causes fragile X syndrome. Figure adapted from Hagerman and Hagerman (2002).
Fragile X-associated premature ovarian insufficiency
Premature ovarian insufficiency (POI) is defined as the loss of ovarian hormonal function with evidence of follicle depletion before the age of 40 years (Welt, 2008). It thus has a direct impact on fertility and secondary consequences arising from oestrogen deficiency, such as osteoporosis and bone fractures (Gallagher, 2007), impaired endothelial function (Kalantaridou et al., 2004), earlier onset of coronary heart disease and increased cardiovascular mortality (Mondul et al., 2005) (see Webber et al., 2016 for a comprehensive review). In addition, women who have a premature menopause are reported to suffer from more anxiety, depression and psychological distress than women with normal ovarian function (van der Stege et al., 2008; Hunter et al., 2010). Identifiable causes of POI include iatrogenic causes, often secondary to the treatment of cancer, and autoimmune causes. Genetic causes remain largely enigmatic, despite the identification of a substantial number of genes necessary for normal ovarian function from murine knock-out studies (Matzuk, 2000). A clear familial pattern is found in some cases even without syndromic conditions such as blepharophimosis/ptosis/epicanthus inversus syndrome, which is associated with mutations in the granulosa cell-specific gene FOXL2 (Crisponi et al., 2001). The X chromosome carries many genes that govern follicular maturation and overall ovarian function, and numerical and structural changes in this chromosome, as in Turner syndrome or triple X syndrome, are associated with POI (reviewed in Fortuño and Labarta, 2014). Abnormalities in the X-linked FMR1 gene were the first causative genetic abnormality identified and are the only monogenic cause currently tested for in routine clinical practice, as POI occurs in about 20% of FMR1 premutation carriers compared with approximately 1% of all women in the general population (Sherman et al., 2014). CGG repeat length shows a strong non-linear association with penetrance for POI (Sullivan et al., 2005; Ennis et al., 2006; Allen et al., 2007; Tejada et al., 2008). In the general population, 29 CGG repeats are the most frequent length observed (Fu et al., 1991). Women carrying midrange repeats (approximately 70–90 repeats) have the highest risk for POI, while carriers of smaller and larger premutation repeat lengths have an increased risk of POI compared to the general population, but not to the same extent as mid-range carriers. It remains contentious whether normal or grey zone CGG repeat lengths are correlated with ovarian reserve parameters, age at natural menopause and IVF outcome (Gleicher et al., 2009; Gleicher et al., 2011; Voorhuis et al., 2013; Gustin et al., 2015; Banks et al., 2016; Pastore et al., 2019). In addition, X chromosome inactivation (Sullivan et al., 2005; Tejada et al., 2008; Spath et al., 2010), background genes (Hunter et al., 2008; Spath et al., 2011) and smoking (Spath et al., 2011) have all been investigated to explain the incomplete penetrance of POI among premutation carriers.
For many women, the most immediate and significant consequences of POI are amenorrhoea and an inability to conceive, however, some women present with intermittent ovarian function and conceive naturally (Rebar et al., 1982; Fraison et al., 2019); thus women with FXPOI may have a child with fragile X syndrome due to CGG repeat instability (Nelson et al., 2005). Female relatives may also be asymptomatic carriers of expanded repeat number, with consequences for themselves and future potential offspring. Although normal-length repeats are usually transmitted in a stable manner, the risk of expansion increases with the length of the repeat, with grey zone-length repeats expanding to full mutation within several generations, and premutations having the highest risk, with expansion to full mutation in one generation (Nolin et al., 2003; Fernandez-Carvajal et al., 2009). Here, the sex of the transmitting parent is important; while the risk of expansion of grey zone alleles is higher for fathers than mothers, the expansion of premutation alleles is limited to mothers only (Sullivan et al., 2002). Furthermore, the number and position of AGG nucleotides, which usually interrupt the CGG repeat tract (CGG repeats are not ‘pure’) have also been explored. In their absence, the length of pure CGG is increased and is more prone to replication slippage and subsequent expansion (Yrigollen et al., 2012; Nolin et al., 2015). AGG interruptions have also been directly correlated with parameters of the ovarian reserve, with patients having longer uninterrupted CGG repeats post-AGG interruptions having the lowest anti-Müllerian hormone levels and antral follicle counts (Lekovich et al., 2018).
Clues to the aetiology of FXPOI: physiological and molecular mechanisms
The physiological mechanisms that underlie compromised follicular function preceding the full development of FXPOI are unclear, but it is proposed these insults could occur at various stages of follicular development. These include (i) inadequate formation of the ovarian reserve during foetal life and (ii) alterations in follicle recruitment and maturation that cause abnormally extensive or fast depletion of the ovarian reserve during post-natal (neonatal, childhood and adult) life. While information from women is scarce, the development and use of model systems has gone some way towards addressing this, with data available from three published knock-in mouse strains, harbouring 130 (here, ‘130R’) (Hoffman et al., 2012), 90 (here, ‘90R’) (Lu et al., 2012) and 100-199 (here, ‘Dutch’) (Bontekoe et al., 2001) CGG repeats in the FMR1 5′UTR, respectively (see Berman et al., 2014; Sherman et al., 2014 for comprehensive reviews on these models). There is mostly consensus in the reproductive physiology between the 130R and 90R strains, which show that the premutation allele does not hinder the establishment of the primordial follicle pool, but rather affects ovarian follicle growth and survival, with a parallel decrease in litter size (Pastore and Johnson, 2014). Additionally, the growth of ovarian follicles is slower due to an elevation in the follicular apoptotic index (Uslu et al., 2017), with a decrease in the number of cumulus granulosa cells associated with mature and ovulatory follicles. Both 130R and 90R models are associated with increased levels of follicular atresia and this follicular decline appears to affect each follicle stage equally (Hoffman et al., 2012; Lu et al., 2012), in contrast to what is seen in many mutant mice with impaired fertility where specific follicle stages are often affected. This compromised follicle function was found to correlate with reduced mitochondrial DNA copy number, total mitochondrial mass and altered expression of genes that control mitochondrial function (Conca Dioguardi et al., 2016), and while mitochondrial abnormalities have been detected in premutation carriers (Alvarez-Mora et al., 2017), causal links between the premutation, mitochondria and follicle function remain to be elucidated. While granulosa cell abnormalities may be one source of reduced follicle viability, oocytes from premutation mice have aberrant localization of the fragile X mental retardation protein (FMRP), with much higher FMRP levels in the nucleus than in the cytoplasm compared with wildtype mice (Hoffman et al., 2012). Given that FMRP has been suggested to have a function in the nucleus (Bagni and Greenough, 2005), increased expression within the nucleus may be deleterious if it causes dysregulation of this function. Furthermore, oocytes of premutation mice also have increased ubiquitin levels, which may indicate a reduced efficiency of the proteasome system for protein degradation or an increased accumulation of abnormal proteins targeted for degradation by ubiquitin (Hoffman et al., 2012). Interestingly, an ovarian phenotype has also been observed in the full mutation knockout mouse model, with FMR1 knockout mice having an increased number of follicles and larger ovaries than wildtype mice, with corpora lutea indicating ovulation (Ascano et al., 2012). Here, the authors suggest that loss of FMRP may lead to precocious follicular development, although while this model may have the potential to recapitulate ovarian insufficiency through follicle over-activation; whether this mechanism is associated with FXPOI is an unanswered question. However, women who carry the full mutation do not appear to be at increased risk of POI; whether this is because they are heterozygous for the loss of FMRP is unknown (Sherman et al., 2014).
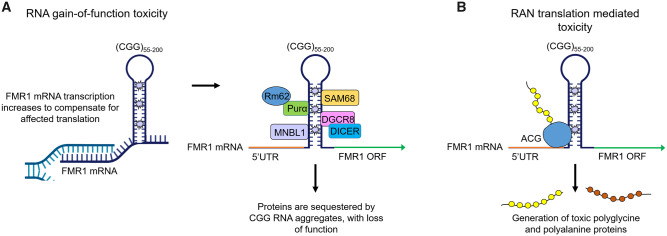
Indeed, and perhaps paradoxically, clues to the aetiology of FXPOI at the molecular level may lie in the fact that women carrying full mutations do not develop POI. In FMR1 full mutations, the expanded CGG repeat is recognized as a CpG island resulting in methylation, chromatin condensation and transcriptional silencing of FMR1 and thus a loss of FMRP (Warren, 2007). Two novel regions named FREE1 and FREE2 have also been identified as having a role in the epigenetic regulation of FMR1 (Godler et al., 2010). Largely located within intron 1, FREE1 and FREE2 are hypermethylated in fragile X individuals but unmethylated in carriers of smaller expansions. FMRP is a ribosome-associated RNA-binding protein that interacts with the coding region of transcripts encoding pre- and post-synaptic proteins implicated in autism spectrum disorders. FMRP reversibly stalls ribosomes on its target mRNAs, and loss of this translational brake on a subset of synaptic proteins contributes to fragile X syndrome (Darnell et al., 2011). Indeed, some authors (Gleicher et al., 2009; Schuettler et al., 2011) speculate that reductions in FMRP expression in granulosa cells as a result of the premutation might directly impact the ovarian reserve through increased follicle activation, as FMRP may translationally regulate paracrine signals and signalling pathways that are important for the regulation of the initiation of follicle growth. In particular, data from the ‘90R’ premutation mouse showed reduced expression of phosphorylated mTOR (Lu et al., 2012), while the Fmr1 knockout model had elevated mTOR levels (Ascano et al., 2012). Dysregulation of mTOR signalling can result in ovarian dysfunction, with inhibition of mTOR causing reduced granulosa cell proliferation (Yu et al., 2011), which is a notable phenotype in the premutation mouse models. Furthermore, experiments in human granulosa cell line COV434 allude to potential interactions between AKT/mTOR signalling and FMR1 (Rehnitz et al., 2017). While significant efforts have been made to uncover and validate the mRNA targets of FMRP in the brain (Darnell et al., 2005; Darnell et al., 2011; Bagni et al., 2012; Suhl et al., 2014; Maurin et al., 2018), there has been little progress regarding the identification of the direct translational targets of FMRP within the ovary. However, in premutation and grey zone carriers, FMR1 is transcriptionally active and mRNA levels are actually elevated (Tassone et al., 2007), with reports describing a correlation between increasing repeat length, increasing FMR1 transcript levels, and decreasing FMRP levels. Thus a key hypothesis is that FMR1 mRNA gain-of-function toxicity may underlie FXPOI, as is thought to be the case in the other premutation associated disorder, FXTAS (Hagerman and Hagerman, 2002) (Fig. 2A). An alternative hypothesis has also been proposed, based on the observation that repeat sequences can increase the frequency at which translation initiates at non-AUG start codons, a process known as RAN translation. These false start sites result in polyglycine and/or polyalanine-containing proteins that have been shown to be neurotoxic and have been detected in the brains of FXTAS patients (Todd et al., 2013) (Fig. 2B).
Figure 2.
RNA gain-of-function toxicity and RAN translation-mediated toxicity. (A) RNA gain-of-function toxicity. FMR1 transcription increases to compensate for affected translation. Subsequently, premutation CGG repeat lengths form intranuclear aggregates that can sequester RNA binding proteins, inhibiting them from carrying out their normal roles, leading to cell dysfunction. (B) Repeat-associated non-AUG (RAN) translation-mediated toxicity. Translation of FMR1 mRNA is initiated from a near cognate ACG start codon, resulting in the production of polyglycine and/or polyalanine-containing proteins that interfere with normal cell function or might be directly toxic. Figure adapted from Berman et al. (2014).
RNA gain-of-function toxicity: does it underlie FXPOI?
Several pathologies, such as Huntington’s disease and myotonic dystrophy, are caused by trinucleotide expansions, and these expansions can result in either a loss or gain-of-function of the translated protein. Fragile X syndrome is an example of where the expansion has a loss-of-function mechanism at the protein level, as the trinucleotide repeat results in lower or absent levels of the gene product, FMRP (Warren, 2007). When there is a gain-of-function, mRNA or protein are thought to acquire a new cellular function depending on the extent and localization of the expansion. In FXTAS, ubiquitin-positive intranuclear inclusions comprised of mRNA and protein are observed in the brain (Galloway and Nelson, 2009), and FMR1 mRNA can be found within these inclusions (Tassone et al., 2004). This, together with the elevated expression of expanded FMR1 mRNA in premutation carriers (Tassone et al., 2000a), gave rise to the hypothesis that a toxic mRNA gain-of-function effect might be responsible for the development of FXTAS (Tassone et al., 2000a,b; Hagerman et al., 2001; Greco et al., 2002). This concept originated from the pathogenesis of myotonic dystrophy, where a CUG triplet expansion in the promoter of the DMPK gene leads to the formation of intranuclear inclusions. These inclusions sequester the RNA-binding protein MBNL (muscleblind-like), leading to dysregulated splicing of MBNL targets and thus clinical symptoms through a deficit of affected proteins (Fardaei et al., 2001; Kanadia et al., 2003). If this mechanism does contribute to FXTAS pathogenesis, its involvement in FXPOI may also be plausible.
According to this hypothesized model for FMR1 premutations, expanded CGG-containing transcripts are exported from the nucleus in messenger ribonucleoprotein complexes, however, these transcripts do not join the 40S ribosomal subunit and consequently translation is affected, reducing FMRP levels. To compensate, FMR1 transcription is augmented and consequently, there is an increase in FMR1 mRNA levels (Oostra and Willemsen, 2003). Cells attempt to eliminate surplus FMR1 transcripts via the ubiquitin-proteasome degradation pathway (Barasoain et al., 2016) however when this is not achieved, intranuclear inclusions containing CGG-repeat mRNA are formed. Simultaneously, this CGG-repeat mRNA is known to adopt secondary structures, such as intramolecular hairpins (Zumwalt et al., 2007), thus RNA-binding proteins could bind to these non-canonical RNA structures, forming RNA-protein aggregates in cells (Fig. 2A). Sequestration of these RNA-binding proteins inhibits them from their normal functions, compromising cell integrity and potentially causing cell death. It is important to note that in this model, toxicity arises as a result of the expanded CGG repeat itself, and not of overexpression of FMR1 protein product, as overexpression of FMR1 mRNA without a CGG repeat expansion does not trigger neuronal death or produce behavioural deficits (Fernández et al., 2012). While it was initially suggested that the toxic effect of the elevated levels of FMR1 mRNA could lead to a diminished ovarian reserve before birth (Conway et al., 1995), and indeed FMR1 is expressed in germ cells in the human foetal ovary (Rosario et al., 2016), it has since been proposed that ovarian dysfunction associated with premutation alleles could be considered a late age-of-onset disease, with mRNA toxic effects causing increased atresia of follicles throughout the lifetime (Sullivan et al., 2005). Indeed, evidence from premutation mouse models is in line with this idea. Comparably, women with type 1 myotonic dystrophy have evidence of a diminished ovarian reserve (Srebnik et al., 2014), thus the accumulation of RNA-protein complexes thought to underlie that disease could perhaps also occur in the ovary.
Alternative mechanisms proposed to describe the toxicity of premutation RNA have suggested that expanded-repeat RNA may separately elicit a stress response, evidence of which has been observed both in brain tissue of premutation carriers (Chen et al., 2010) and a Drosophila model of repeat RNA toxicity, where the innate immune response is activated (Samaraweera et al., 2013). It is thought that RNA secondary structures may arise in mid-range CGG repeat carriers with reduced AGG interruptions, which could cause cell dysfunction within the ovaries resulting in the diminished ovarian reserve observed in these specific carriers (Lekovich et al., 2018). Indeed, hairpin secondary structures formed from repeat CGG RNA can act as a substrate for the enzyme Dicer (Handa et al., 2003), which is responsible for generating small RNAs that act via the RNA interference pathway post-transcriptionally to reduce the expression of complement target genes. While small RNAs that are toxic to cultured neuronal cells have been generated from expanded CAG repeats (Samaraweera et al., 2013), it remains to be seen whether repeat-CGG RNA can be toxic in the same way. Similarly, increased amounts of FMR1 mRNA in granulosa cells could also lead to a rise in R-loop formation, a secondary DNA-RNA hybrid structure formed by the CGG repeats. The presence of R-loops could lead to increased DNA damage within the cell, because in this structure DNA is more unstable and susceptible to mutagenesis and genomic instability (Aguilera, 2002; Gan et al., 2011; Man et al., 2017). While these structures have been observed in cultured human dermal fibroblasts containing the premutation (Loomis et al., 2014), no R-loops have been detected in human granulosa cells.
Nevertheless, in support of the FMR1 premutation model and RNA gain-of-function hypothesis, increased expression of FMR1 mRNA has been observed in the ovaries of all premutation mouse models, with expression being localized to both granulosa cells and oocytes (Sherman et al., 2014). Furthermore, although FMRP levels differ among the premutation mouse models, likely due to their genetic construction, both the 130R and 90R mice have reduced FMRP expression (Sherman et al., 2014). In humans, FMRP is expressed in oocytes of foetal ovaries and in granulosa cells of mature follicles (Rife et al., 2004; Rosario et al., 2016), and increased FMR1 transcript levels have been reported in granulosa cells of premutation carriers (Elizur et al., 2014). In that work, the authors also describe a significant negative linear correlation between granulosa cell FMR1 expression and the number of oocytes retrieved after ovarian stimulation for preimplantation genetic diagnosis, with the most obvious effects observed in women carrying mid-range (80–120) repeat lengths. While these data are only correlative, these findings suggest that FMR1 mRNA accumulation in granulosa cells may be an important cause of follicle dysfunction and loss. A preliminary report of in vitro-based studies further demonstrated that transfection of the human granulosa cell tumour line COV434 with plasmid DNA expressing 88 CGG repeats decreased cell viability after 48 h (Hubayter et al., 2009), also supporting a proposed toxic RNA gain-of-function mechanism.
Identification of the proteins that bind and are sequestered by CGG RNA within intranuclear inclusions in the brain has been an important step towards understanding the contribution of RNA gain-of-function toxicity to the pathogenesis of FXTAS. Several proteins have been identified that bind to CGG repeats in FXTAS-affected cells, unlike other disorders associated with unstable repeat expansions, which usually have a principle protein deposit (Loesch and Hagerman, 2012). RNA binding proteins that have been shown to interact directly with CGG-repeat RNA include hnRNP A2/B1, a protein involved in pre-mRNA processing (Jin et al., 2007; Sellier et al., 2010), Purα, which is associated with transcription regulation in neuronal development (Sellier et al., 2010), and the RNA splicing protein MBNL1, a protein also implicated in the pathogenesis of myotonic dystrophy (Loesch and Hagerman, 2012). The RNA binding protein DGCR8 has also been demonstrated to bind to expanded CGG repeats, resulting in its partial sequestration with its protein partner DROSHA, a key enzyme that executes the initiation step of miRNA processing in the nucleus (Sellier et al., 2013). Consequently, miRNA processing was reduced, as evidenced by decreased levels of mature miRNAs in neuronal cells expressing CGG repeats and brain tissue from FXTAS patients, suggesting that the dysregulation of miRNAs may be involved in premutation-related toxicity. Overexpression of hnRNP (Sofola et al., 2007), Purα (Jin et al., 2007) and DROSHA (Sellier et al., 2013) can rescue neurodegeneration in a fly model of FXTAS, however, whether they can rescue the mammalian phenotype remains to be seen. Importantly, CGG RNA aggregates have been shown to be dynamic structures that expand in size and number over time (Sellier et al., 2010), thus while some proteins are initially sequestered by RNA aggregates in a specific manner, these proteins in turn are able to recruit additional proteins. These include CUGBP1 in the case of hnRNP A2/B1 (Kuyumcu-Martinez et al., 2007) and the RNA helicase Rm62 in the case of Purα (Qurashi et al., 2011), ultimately causing widespread cellular dysfunction. With regards to relevance for FXPOI, the identification of SAM68 in CGG RNA aggregates is particularly meaningful. Although the authors used a cell line model of the premutation to demonstrate sequestration of SAM68, and consequent loss of its normal alternate splicing function (Sellier et al., 2010), SAM68 has been suggested to regulate the mRNA splicing of FSH and LH receptors (Bianchi et al., 2010). Sam68−/− female mice are severely subfertile and morphological analyses of the ovary indicated a significant reduction in the number of secondary and pre-antral follicles. Crosslinking/immunoprecipitation experiments showed that Sam68 directly binds FSH and LH receptor mRNAs, which were down-regulated in ovaries of adult knockout mice. Indeed, altered splicing of these proteins in FXPOI-affected granulosa cells could lead to FSH and LH resistance at the receptor level, preventing follicle maturation (albeit that primordial follicle loss rather than gonadotrophin resistance is the ovarian phenotype in FXPOI). However, although the proteins discussed here are predicted to be also relevant in the pathogenesis of FXPOI, there has been no validation as yet of these proteins in ovary-specific premutation models. Furthermore, while a degree of overlap is expected given the parallels with FXTAS, it is probable that some deregulated proteins will be specific to granulosa cells, thus identification of these ovarian targets will be essential as they are likely to be key in understanding the RNA gain-of-function mechanism that may potentially contribute to FXPOI.
Finally, the discovery of long non-coding (lnc) RNAs originating from the FMR1 locus also supports RNA toxic gain-of-function as one of the possible pathophysiologic mechanisms underlying FXPOI. Using genomic approaches (Khalil et al., 2008), and more recently, ‘Deep-RACE’ methodology (Pastori et al., 2014), the FMR1 gene locus was interrogated for the occurrence of novel lncRNAs, leading to the discovery of FMR4, FMR5 and FMR6. While FMR5 appeared to show no differential expression between unaffected, premutation and full mutation carriers (Pastori et al., 2014), FMR4 was silenced in full mutation carriers and up-regulated in premutation carriers, similar to FMR1 (Khalil et al., 2008). Unexpectedly though, FMR6 was silenced in both full and premutation mutation carriers, suggesting abnormal transcription and/or chromatin remodelling prior to transition to the full mutation (Khalil et al., 2008). Both FMR4 and FMR6 are thought to regulate FMR1 stability, splicing, subcellular localization and translational efficiency in FXTAS, and have been proposed to be useful biomarkers allowing for early detection and therapeutic intervention in fragile X syndrome and FXTAS. FMR6, in particular, may also be useful as a biomarker for FXPOI. In a study investigating the relationship between lncRNA accumulation and the pathophysiology of FXPOI, there was a non-linear association between the CGG-repeat number and FMR6 expression in granulosa cells, with the highest levels of FMR6 observed in women with mid-size CGG repeats (80–120) (Elizur et al., 2016). In addition, the authors showed that increased FMR6 expression had a negative relationship with the number of oocytes retrieved; no correlations were observed with FMR4. Thus the production of lncRNAs from the FMR1 locus may be an additional pathway by which RNA can cause toxicity in FXPOI.
Repeat-associated non-AUG translation: does it contribute to FXPOI?
Translation initiation is highly complex process requiring the step-wise assembly of elongation-competent 80S ribosomes at start codons of mRNA. In the canonical ribosome scanning model, successful translation of eukaryotic mRNA is thought to require components of the preinitiation complex to scan through the 5′UTR in the 5′ to 3′ direction, until it encounters an AUG codon in a good Kozak context, at which point, base-pairing between the AUG codon and the CAU anti-codon on tRNAMet takes place. The 40S ribosome is now committed to its selection of start codon and is joined by the 60S ribosomal subunit, allowing the first peptide bond to be formed, thus beginning translation elongation (see Dever and Green, 2012 for a comprehensive review). In particular, dependency on the RNA helicase activity of eIF4A to resolve RNA secondary and tertiary structures is of significance, as these structures can impact translation initiation both positively and negatively depending on their location (Pestova and Kolupaeva, 2002). Furthermore, these structures can cause multiple atypical modes of initiation, one of which is the translation of mRNA initiated at non-AUG start codons.
RAN translation enables initiation and elongation in the absence of an AUG start codon, resulting in the production of multiple homopolymer-containing proteins, depending on the reading frame (Green et al., 2016). Repeat sequences can drive RAN translation whether they are located within 5′UTRs, protein-coding reading frames, introns or non-coding RNAs. This non-canonical translational initiation process was discovered through the study of CAG trinucleotide expansions in the ATXN8 gene, which causes the neurodegenerative disorder spinocerebellar ataxia type 8 (SCA8) (Zu et al., 2011). Unexpectedly, mutation of the only AUG codon upstream of the coding sequence did not abolish translation, rather a series of homopolymeric proteins were generated with glutamine, serine or alanine repeats. This phenomenon was shown to be dependent on the stability of the RNA secondary structures formed as a result of the expanded CAG repeats, as decreasing the number of the repeats stopped RAN translation. Importantly, polyalanine-ATXN8 proteins have been observed in the cerebella of SCA8 human patients and mouse models, and a similar approach has provided evidence of a polyglutamine RAN product from the DMPK gene with expanded CAG repeats in myotonic dystrophy (Zu et al., 2011). More recently it has been demonstrated that the CGG-repeats found in the 5′UTR of FMR1 also support RAN translation initiation, thus this may be a potential protein-based mechanism that underlies the development of FXTAS and FXPOI (Todd et al., 2013).
In FXTAS, RAN translation initiated within the 5′UTR can occur in at least two reading frames, yielding either a polyglycine product, named FMRpolyG, or a polyalanine product (FMRpolyA) (Todd et al., 2013); however, it was demonstrated that translation predominantly occurs in the glycine frame through initiation at a near cognate ACG codon located upstream of the expanded CGG repeats (Fig. 2B) (Sellier et al., 2017). In accordance with this, FMRpolyG protein deposits are found to accumulate in ubiquitin-positive inclusions in the brain tissue of humans with FXTAS (Todd et al., 2013); it is unclear at present whether or not FMRpolyA is expressed in vivo. Furthermore, it should be noted that RAN translation can also occur on non-expanded repeats, thus it may have normal and pathogenic roles (Todd et al., 2013). FMRpolyG expression is observed in the ‘Dutch’ premutation mouse, and turning off FMR1 transgene expression in this model reverses the formation of neuronal FMRpolyG-positive inclusions and FXTAS behavioural deficits (Hukema et al., 2015). It is also proposed that similar to toxic RNA aggregates, FMRpolyG can sequester specific proteins required for viable cell function through protein–protein interaction. Indeed, FMRpolyG has been shown to interact with LAP2B, a protein essential in anchoring lamina proteins to the inner nuclear membrane (Sellier et al., 2017); overexpression of LAP2B rescues neuronal cell death induced by the expression of FMRpolyG. Furthermore, experiments in Drosophila and premutation cell line models suggest that FMRpolyG interferes with the ubiquitin proteasome system, and prevention of RAN translation can attenuate this impairment (Oh et al., 2015). With regards to FXPOI, ubiquitin-positive inclusions were frequently observed at a high density within ovarian stromal cell nuclei from five women with the condition compared to stromal cells of four control ovaries, where these ubiquitin-positive inclusions were much rarer (Chang et al., 2011). Furthermore, double labelling of ubiquitin-positive inclusions found in the stromal cells of one FXPOI patient revealed that the vast majority (>90%) of the inclusions also stained positive for FMRpolyG (Buijsen et al., 2016). As the ovarian tissue of this woman with FXPOI did not contain any follicles, analyses of the ovaries of the ‘Dutch’ mouse model were undertaken; while the ovaries of 20-week-old ‘Dutch’ mice contained a few ubiquitin-FMRpolyG-positive inclusions, in 40-week-old ‘Dutch’ mice, similar to the FXPOI ovarian tissue, numerous intranuclear inclusions were detected in the ovarian stromal cells, both with ubiquitin and FMRpolyG antibodies (Buijsen et al., 2016). Interestingly, in these studies, oocytes, granulosa and theca cells of follicles of all stages were negative for these inclusions, which is surprising as these are thought to be the primary cell types affected in FXPOI. It may be the case that loss of affected follicles may occur too quickly for the pathological inclusions to be visualized, analogous to the situation observed in Purkinje cells of FXTAS patients (Greco et al., 2002). In a more recent study, however, FMRpolyG aggregates have been identified in mural granulosa cells from six FMR1 premutation carriers, and these aggregates showed varying levels of co-localization with ubiquitin (Friedman-Gohas et al., 2020). None of the granulosa cells from the four control women expressed FMRpolyG. Additionally, in vitro work transfecting plasmids expressing premutation-range CGG repeats into COV434 cells resulted in the formation of FMRpolyG aggregates (Friedman-Gohas et al., 2020). Therefore, while this may provide some evidence that RAN translation and FMRpolyG expression might be linked to this premutation disease, significantly more data are necessary to establish conclusively whether FMRpolyG-inclusions are a pathological characteristic of FXPOI, and of mechanistic importance.
Concluding remarks
The molecular mechanism underlying FXPOI is enigmatic, and while the study of FXTAS and other expanded-repeat disorders has provided some insight, the discovery of RAN translation adds an additional complexity as to whether CGG-repeats in the 5′UTR of FMR1 elicit toxicity via RNA gain-of-function or protein-based means, or a combination of both. Transgenic mice expressing both CGG repeat RNA and FMRpolyG protein, but not mice expressing only the mutant RNA containing expanded CGG repeats, exhibit inclusion formation and motor phenotypes reminiscent of FXTAS (Sellier et al., 2017), implying that RAN protein products play a role in the disease phenotype. However, the ‘130R’ mouse, which is unable to make polyglycine and polyalanine proteins due to the position of a stop codon in the upstream sequence, still has evidence of ovarian dysfunction, suggesting that at least some pathology must arise independently of RAN translation and could be related to the elevated levels of premutation FMR1 mRNA. Furthermore, although data from the premutation mouse models show there is no detrimental effect on the ovarian reserve at birth, it is still a possibility that gonadotoxic RNA or protein aggregates may form during this crucial period, with the effects only becoming apparent in post-natal life. However, establishing the presence of RNA or protein aggregates in follicle structures may be difficult, given the limited access to such clinical specimens and the rapid loss of affected follicles in POI: only very limited ovarian histological evaluation has been reported in women with premutations (Chang et al., 2011). Typical intranuclear inclusion formation has also been observed in neurons of the anterior pituitary with degenerative changes in gonadotropic cells (Greco et al., 2007), raising the possibility of abnormal signalling via the hypothalamic–pituitary–gonadal axis. However, women with FXPOI have the characteristic elevated levels of FSH and LH (Murray et al., 1999) as in other causes of POI, so these inclusions may not have any immediate relevance. Finally, it has been shown that FMR1 mRNA in human and mouse brain is expressed as a combination of multiple isoforms that use alternative transcriptional start sites and different polyadenylation sites, and specific regulation of the UTRs is observed in the brains of premutation carriers (Tassone et al., 2011; Tseng et al., 2017). This suggests that transcript variants may play a role in pre-mutation related pathologies. Therefore, understanding the expression of these different isoforms will be fundamental as imbalances in their expression could underlie disease progression. Thus, while using FXTAS as a model to study FXPOI pathogenesis has had some benefit, the central questions which remain unanswered are the relative contributions of RNA gain-of-function and protein-based pathology to the overall disease phenotype, and the timing and location (at the cellular and sub-cellular level) of this insult in the ovary which results in follicle atresia and POI. Development of specific cell line and animal models to explore these questions will be key in illuminating the disease biology of FXPOI.
Authors’ roles
R.R. wrote the manuscript; R.A.A. edited the manuscript; both authors approved the final version.
Funding
The authors’ work in this field is support by grants from the Medical Research Council (G1100357 to R.A.A., MR/N022556/1 to the MRC Centre for Reproductive Health) and Wellbeing of Women (PRF005 to R.R.).
Conflict of interest
R.R. declares no conflict of interest. R.A.A. reports grants and personal fees from Roche Diagnostics and personal fees from Ferring Pharmaceuticals, IBSA, Merck Serono, outside the submitted work.
References
- Aguilera A. The connection between transcription and genomic instability. EMBO J 2002;21:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, Charen K, He W, Taylor KC, Sherman SL.. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod 2007;22:2142–2152. [DOI] [PubMed] [Google Scholar]
- Alvarez-Mora MI, Rodriguez-Revenga L, Madrigal I, Guitart-Mampel M, Garrabou G, Mila M.. Impaired mitochondrial function and dynamics in the pathogenesis of FXTAS. Mol Neurobiol 2017;54:6896–6902. [DOI] [PubMed] [Google Scholar]
- Ascano M, Mukherjee N, Bandaru P, Miller JB, Nusbaum J, Corcoran DL, Langlois C, Munschauer M, Dewell S, Hafner M. et al. FMR1 targets distinct mRNA sequence elements to regulate protein expression. Nature 2012;492:382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagni C, Greenough WT.. From mRNP trafficking to spine dysmorphogenesis: the roots of fragile X syndrome. Nat Rev Neurosci 2005;6:376–387. [DOI] [PubMed] [Google Scholar]
- Bagni C, Tassone F, Neri G, Hagerman R.. Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. J Clin Invest 2012;122:4314–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks N, Patounakis G, Devine K, DeCherney AH, Widra E, Levens ED, Whitcomb BW, Hill MJ.. Is FMR1 CGG repeat length a predictor of in vitro fertilization stimulation response or outcome? Fertil Steril 2016;105:1537–1546.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barasoain M, Barrenetxea G, Huerta I, Telez M, Criado B, Arrieta I.. Study of the genetic etiology of primary ovarian insufficiency: FMR1 gene. Genes (Basel )2016;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RF, Buijsen RA, Usdin K, Pintado E, Kooy F, Pretto D, Pessah IN, Nelson DL, Zalewski Z, Charlet-Bergeurand N. et al. Mouse models of the fragile X premutation and fragile X-associated tremor/ataxia syndrome. J Neurodev Disord 2014;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi E, Barbagallo F, Valeri C, Geremia R, Salustri A, De Felici M, Sette C.. Ablation of the Sam68 gene impairs female fertility and gonadotropin-dependent follicle development. Hum Mol Genet 2010;19:4886–4894. [DOI] [PubMed] [Google Scholar]
- Bontekoe CJ, Bakker CE, Nieuwenhuizen IM, van der Linde H, Lans H, de Lange D, Hirst MC, Oostra BA.. Instability of a (CGG)98 repeat in the Fmr1 promoter. Hum Mol Genet 2001;10:1693–1699. [DOI] [PubMed] [Google Scholar]
- Buijsen RA, Visser JA, Kramer P, Severijnen EA, Gearing M, Charlet-Berguerand N, Sherman SL, Berman RF, Willemsen R, Hukema RK.. Presence of inclusions positive for polyglycine containing protein, FMRpolyG, indicates that repeat-associated non-AUG translation plays a role in fragile X-associated primary ovarian insufficiency. Hum Reprod 2016;31:158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, DeCaro JJ, Zheng M, Gearing M, Shubeck L, Sherman SL, Welt CK.. Ovarian histopathological and ubiquitin-immunophenotypic features in fragile X-associated primary ovarian insufficiency: a study of five cases and selected controls. Histopathology 2011;59:1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Tassone F, Berman RF, Hagerman PJ, Hagerman RJ, Willemsen R, Pessah IN.. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet 2010;19:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conca Dioguardi C, Uslu B, Haynes M, Kurus M, Gul M, Miao DQ, De Santis L, Ferrari M, Bellone S, Santin A. et al. Granulosa cell and oocyte mitochondrial abnormalities in a mouse model of fragile X primary ovarian insufficiency. Mol Hum Reprod 2016;22:384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway GS, Hettiarachchi S, Murray A, Jacobs PA.. Fragile X premutations in familial premature ovarian failure. Lancet 1995;346:309–310. [DOI] [PubMed] [Google Scholar]
- Conway GS, Payne NN, Webb J, Murray A, Jacobs PA.. Fragile X premutation screening in women with premature ovarian failure. Hum Reprod 1998;13:1184–1187. [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S. et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 2001;27:159–166. [DOI] [PubMed] [Google Scholar]
- Cronister A, Schreiner R, Wittenberger M, Amiri K, Harris K, Hagerman RJ.. Heterozygous fragile X female: historical, physical, cognitive, and cytogenetic features. Am J Med Genet 1991;38:269–274. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Mostovetsky O, Darnell RB.. FMRP RNA targets: identification and validation. Genes Brain Behav 2005;4:341–349. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW. et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 2011;146:247–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE, Green R.. The elongation, termination, and recycling phases of translation in eukaryotes. Cold Spring Harb Perspect Biol 2012;4:a013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizur SE, Dratviman-Storobinsky O, Derech-Haim S, Lebovitz O, Dor J, Orvieto R, Cohen Y.. FMR6 may play a role in the pathogenesis of fragile X-associated premature ovarian insufficiency. Gynecol Endocrinol 2016;32:334–337. [DOI] [PubMed] [Google Scholar]
- Elizur SE, Lebovitz O, Derech-Haim S, Dratviman-Storobinsky O, Feldman B, Dor J, Orvieto R, Cohen Y.. Elevated levels of FMR1 mRNA in granulosa cells are associated with low ovarian reserve in FMR1 premutation carriers. PLoS One 2014;9:e105121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis S, Ward D, Murray A.. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet 2006;14:253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardaei M, Larkin K, Brook JD, Hamshere MG.. In vivo co-localisation of MBNL protein with DMPK expanded-repeat transcripts. Nucleic Acids Res 2001;29:2766–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Carvajal I, Lopez Posadas B, Pan R, Raske C, Hagerman PJ, Tassone F.. Expansion of an FMR1 grey-zone allele to a full mutation in two generations. J Mol Diagn 2009;11:306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández JJ, Martínez R, Andújar E, Pérez-Alegre M, Costa A, Bonilla-Henao V, Sobrino F, Pintado C, Pintado E.. Gene expression profiles in the cerebellum of transgenic mice over expressing the human FMR1 gene with CGG repeats in the normal range. Genet Mol Res 2012;11:467–483. [DOI] [PubMed] [Google Scholar]
- Fortuño C, Labarta E.. Genetics of primary ovarian insufficiency: a review. J Assist Reprod Genet 2014;31:1573–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraison E, Crawford G, Casper G, Harris V, Ledger W.. Pregnancy following diagnosis of premature ovarian insufficiency: a systematic review. Reprod Biomed Online 2019;39:467–476. [DOI] [PubMed] [Google Scholar]
- Friedman-Gohas M, Elizur SE, Dratviman-Storobinsky O, Aizer A, Haas J, Raanani H, Orvieto R, Cohen Y.. FMRpolyG accumulates in FMR1 premutation granulosa cells. J Ovarian Res 2020;13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, Verkerk AJ, Holden JJ, Fenwick RG Jr, Warren ST. et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell 1991;67:1047–1058. [DOI] [PubMed] [Google Scholar]
- Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause 2007;14:567–571. [DOI] [PubMed] [Google Scholar]
- Galloway JN, Nelson DL.. Evidence for RNA-mediated toxicity in the fragile X-associated tremor/ataxia syndrome. Future Neurol 2009;4:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan W, Guan Z, Liu J, Gui T, Shen K, Manley JL, Li X.. R-loop-mediated genomic instability is caused by impairment of replication fork progression. Genes Dev 2011;25:2041–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Barad DH.. Defining ovarian reserve to better understand ovarian aging. Reprod Biol Endocrinol 2011;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleicher N, Weghofer A, Oktay K, Barad D.. Relevance of triple CGG repeats in the FMR1 gene to ovarian reserve. Reprod Biomed Online 2009;19:385–390. [DOI] [PubMed] [Google Scholar]
- Godler DE, Tassone F, Loesch DZ, Taylor AK, Gehling F, Hagerman RJ, Burgess T, Ganesamoorthy D, Hennerich D, Gordon L. et al. Methylation of novel markers of fragile X alleles is inversely correlated with FMRP expression and FMR1 activation ratio. Hum Mol Genet 2010;19:1618–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco CM, Hagerman RJ, Tassone F, Chudley AE, Del Bigio MR,, Jacquemont S, Leehey M, Hagerman PJ.. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain 2002;125:1760–1771. [DOI] [PubMed] [Google Scholar]
- Greco CM, Soontrapornchai K, Wirojanan J, Gould JE, Hagerman PJ, Hagerman RJ.. Testicular and pituitary inclusion formation in fragile X associated tremor/ataxia syndrome. J Urol 2007;177:1434–1437. [DOI] [PubMed] [Google Scholar]
- Green KM, Linsalata AE, Todd PK.. RAN translation-What makes it run? Brain Res 2016;1647:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin SL, Ding VY, Desai M, Leader B, Baker VL.. Evidence of an age-related correlation of ovarian reserve and FMR1 repeat number among women with "normal" CGG repeat status. J Assist Reprod Genet 2015;32:1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Hagerman PJ.. The fragile X premutation: into the phenotypic fold. Curr Opin Genet Dev 2002;12:278–283. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ, Leehey M, Heinrichs W, Tassone F, Wilson R, Hills J, Grigsby J, Gage B, Hagerman PJ.. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 2001;57:127–130. [DOI] [PubMed] [Google Scholar]
- Handa V, Saha T, Usdin K.. The fragile X syndrome repeats form RNA hairpins that do not activate the interferon-inducible protein kinase, PKR, but are cut by Dicer. Nucleic Acids Res 2003;31:6243–6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Le WW, Entezam A, Otsuka N, Tong ZB, Nelson L, Flaws JA, McDonald JH, Jafar S, Usdin K.. Ovarian abnormalities in a mouse model of fragile X primary ovarian insufficiency. J Histochem Cytochem 2012;60:439–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubayter ZR, Tong ZB, Popat V, Hagerman PJ, Troendle J, Nelson LM.. Fragile X associated primary ovarian insufficiency (FXPOI): ovarian phenotype and FMR1 RNA toxic gain of function in human granulosa cells. Fertil Steril 2009. S6–S7. 10.1016/j.fertnstert.2009.07.025. [Google Scholar]
- Hukema RK, Buijsen RA, Schonewille M, Raske C, Severijnen LA, Nieuwenhuizen-Bakker I, Verhagen RF, van Dessel L, Maas A, Charlet-Berguerand N. et al. Reversibility of neuropathology and motor deficits in an inducible mouse model for FXTAS. Hum Mol Genet 2015;24:4948–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Epstein MP, Tinker SW, Charen KH, Sherman SL.. Fragile X-associated primary ovarian insufficiency: evidence for additional genetic contributions to severity. Genet Epidemiol 2008;32:553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Rohr JK, Sherman SL.. Co-occurring diagnoses among FMR1 premutation allele carriers. Clin Genet 2010;77:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Duan R, Qurashi A, Qin Y, Tian D, Rosser TC, Liu H, Feng Y, Warren ST.. Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome. Neuron 2007;55:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantaridou SN, Naka KK, Papanikolaou E, Kazakos N, Kravariti M, Calis KA, Paraskevaidis EA, Sideris DA, Tsatsoulis A, Chrousos GP. et al. Impaired endothelial function in young women with premature ovarian failure: normalization with hormone therapy. J Clin Endocrinol Metab 2004;89:3907–3913. [DOI] [PubMed] [Google Scholar]
- Kanadia RN, Johnstone KA,, Mankodi A, Lungu C, Thornton CA, Esson D, Timmers AM, Hauswirth WW, Swanson MS.. A muscleblind knockout model for myotonic dystrophy. Science 2003;302:1978–1980. [DOI] [PubMed] [Google Scholar]
- Khalil AM, Faghihi MA,, Modarresi F, Brothers SP, Wahlestedt C.. A novel RNA transcript with antiapoptotic function is silenced in fragile X syndrome. PLoS One 2008;3:e1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronquist KE, Sherman SL, Spector EB.. Clinical significance of tri-nucleotide repeats in Fragile X testing: a clarification of American College of Medical Genetics guidelines. Genet Med 2008;10:845–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuyumcu-Martinez NM, Wang GS, Cooper TA.. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol Cell 2007;28:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekovich J, Man L, Xu K, Canon C, Lilienthal D, Stewart JD, Pereira N, Rosenwaks Z, Gerhardt J.. CGG repeat length and AGG interruptions as indicators of fragile X-associated diminished ovarian reserve. Genet Med 2018;20:957–964. [DOI] [PubMed] [Google Scholar]
- Loesch D, Hagerman R.. Unstable mutations in the FMR1 gene and the phenotypes. Adv Exp Med Biol 2012;769:78–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis EW, Sanz LA, Chedin F, Hagerman PJ.. Transcription-associated R-loop formation across the human FMR1 CGG-repeat region. PLoS Genet 2014;10:e1004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Lin L, Tan H, Wu H, Sherman SL, Gao F, Jin P, Chen D.. Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice. Hum Mol Genet. 2012;21:5039–5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man L, Lekovich J, Rosenwaks Z, Gerhardt J.. Fragile X-associated diminished ovarian reserve and primary ovarian insufficiency from molecular mechanisms to clinical manifestations. Front Mol Neurosci 2017;10:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzuk MM. Revelations of ovarian follicle biology from gene knockout mice. Mol Cell Endocrinol 2000;163:61–66. [DOI] [PubMed] [Google Scholar]
- Maurin T, Lebrigand K, Castagnola S, Paquet A, Jarjat M, Popa A, Grossi M, Rage F, Bardoni B.. HITS-CLIP in various brain areas reveals new targets and new modalities of RNA binding by fragile X mental retardation protein. Nucleic Acids Res 2018;46:6344–6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondul AM, Rodriguez C, Jacobs EJ, Calle EE.. Age at natural menopause and cause-specific mortality. Am J Epidemiol 2005;162:1089–1097. [DOI] [PubMed] [Google Scholar]
- Murray A, Webb J, MacSwiney F, Shipley EL, Morton NE, Conway GS.. Serum concentrations of follicle stimulating hormone may predict premature ovarian failure in FRAXA premutation women. Hum Reprod 1999;14:1217–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LM, Covington SN, Rebar RW.. An update: spontaneous premature ovarian failure is not an early menopause. Fertil Steril 2005;83:1327–1332. [DOI] [PubMed] [Google Scholar]
- Nolin SL, Brown WT, Glicksman A, Houck GE Jr, Gargano AD, Sullivan A, Biancalana V, Brondum-Nielsen K, Hjalgrim H, Holinski-Feder E. et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am J Hum Genet 2003;72:454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolin SL, Glicksman A, Ersalesi N, Dobkin C, Brown WT, Cao R, Blatt E, Sah S,, Latham GJ, Hadd AG.. Fragile X full mutation expansions are inhibited by one or more AGG interruptions in premutation carriers. Genet Med 2015;17:358–364. [DOI] [PubMed] [Google Scholar]
- Nolin SL, 3rd, Lewis FA, Ye LL, Houck GE Jr, Glicksman AE, Limprasert P, Li SY, Zhong N, Ashley AE, Feingold E. et al. Familial transmission of the FMR1 CGG repeat. Am J Hum Genet 1996;59:1252–1261. [PMC free article] [PubMed] [Google Scholar]
- Oh SY, He F, Krans A, Frazer M, Taylor JP, Paulson HL, Todd PK.. RAN translation at CGG repeats induces ubiquitin proteasome system impairment in models of fragile X-associated tremor ataxia syndrome. Hum Mol Genet 2015;24:4317–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostra BA, Willemsen R.. A fragile balance: FMR1 expression levels. Hum Mol Genet 2003;12 Spec No 2:R249–R257. [DOI] [PubMed] [Google Scholar]
- Pastore LM, Christianson MS, McGuinness B, Vaught KC, Maher JY, Kearns WG.. Does theFMR1 gene affect IVF success? Reprod Biomed Online 2019;38:560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore LM, Johnson J.. The FMR1 gene, infertility, and reproductive decision-making: a review. Front Genet 2014;5:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori C, Peschansky VJ, Barbouth D, Mehta A, Silva JP, Wahlestedt C.. Comprehensive analysis of the transcriptional landscape of the human FMR1 gene reveals two new long noncoding RNAs differentially expressed in Fragile X syndrome and Fragile X-associated tremor/ataxia syndrome. Hum Genet 2014;133:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, Kolupaeva VG.. The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev 2002;16:2906–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qurashi A, Li W, Zhou JY, Peng J, Jin P.. Nuclear accumulation of stress response mRNAs contributes to the neurodegeneration caused by Fragile X premutation rCGG repeats. PLoS Genet 2011;7:e1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebar RW, Erickson GF, Yen SS.. Idiopathic premature ovarian failure: clinical and endocrine characteristics. Fertil Steril 1982;37:35–41. [PubMed] [Google Scholar]
- Rehnitz J, Alcoba DD, Brum IS, Hinderhofer K, Youness B, Strowitzki T, Vogt PH.. FMR1 and AKT/mTOR signalling pathways: potential functional interactions controlling folliculogenesis in human granulosa cells. Reprod Biomed Online 2017;35:485–493. [DOI] [PubMed] [Google Scholar]
- Rife M, Nadal A, Mila M, Willemsen R.. Immunohistochemical FMRP studies in a full mutated female fetus. Am J Med Genet A 2004;124a:129–132. [DOI] [PubMed] [Google Scholar]
- Rosario R, Filis P, Tessyman V, Kinnell H, Childs AJ, Gray NK, Anderson RA.. FMRP associates with cytoplasmic granules at the onset of meiosis in the human oocyte. PLoS One 2016;11:e0163987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaraweera SE, O'Keefe LV, van Eyk CL, Lawlor KT, Humphreys DT, Suter CM, Richards RI.. Modeling and analysis of repeat RNA toxicity in Drosophila. Methods Mol Biol 2013;1017:173–192. [DOI] [PubMed] [Google Scholar]
- Schuettler J, Peng Z, Zimmer J, Sinn P, von Hagens C, Strowitzki T, Vogt PH.. Variable expression of the Fragile X Mental Retardation 1 (FMR1) gene in patients with premature ovarian failure syndrome is not dependent on number of (CGG)n triplets in exon 1. Hum Reprod 2011;26:1241–1251. [DOI] [PubMed] [Google Scholar]
- Sellier C, Buijsen RAM, He F, Natla S, Jung L, Tropel P, Gaucherot A, Jacobs H, Meziane H, Vincent A. et al. Translation of expanded CGG repeats into FMRpolyG is pathogenic and may contribute to fragile X tremor ataxia syndrome. Neuron 2017;93:331–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellier C, Freyermuth F, Tabet R, Tran T, He F, Ruffenach F, Alunni V, Moine H, Thibault C, Page A. et al. Sequestration of DROSHA and DGCR8 by expanded CGG RNA repeats alters microRNA processing in fragile X-associated tremor/ataxia syndrome. Cell Rep 2013;3:869–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellier C, Rau F, Liu Y, Tassone F, Hukema RK, Gattoni R, Schneider A, Richard S, Willemsen R, Elliott DJ. et al. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J 2010;29:1248–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SL, Curnow EC, Easley CA, Jin P, Hukema RK, Tejada MI, Willemsen R, Usdin K. Use of model systems to understand the etiology of fragile X-associated primary ovarian insufficiency (FXPOI). J Neurodev Disord 2014;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofola OA, Jin P, Qin Y, Duan R, Liu H, de Haro M, Nelson DL, Botas J.. RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a Drosophila model of FXTAS. Neuron 2007;55:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spath MA, Feuth TB, Smits AP, Yntema HG, Braat DD, Thomas CM, van Kessel AG, Sherman SL, Allen EG.. Predictors and risk model development for menopausal age in fragile X premutation carriers. Genet Med 2011;13:643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spath MA, Nillesen WN, Smits AP, Feuth TB, Braat DD, van Kessel AG, Yntema HG.. X chromosome inactivation does not define the development of premature ovarian failure in fragile X premutation carriers. Am J Med Genet A 2010;152a:387–393. [DOI] [PubMed] [Google Scholar]
- Srebnik N, Margalioth EJ, Rabinowitz R, Varshaver I, Altarescu G, Renbaum P, Levi-Lahad E, Weintraub A, Eldar-Geva T.. Ovarian reserve and PGD treatment outcome in women with myotonic dystrophy. Reprod Biomed Online 2014;29:94–101. [DOI] [PubMed] [Google Scholar]
- Suhl JA, Chopra P, Anderson BR, Bassell GJ, Warren ST.. Analysis of FMRP mRNA target datasets reveals highly associated mRNAs mediated by G-quadruplex structures formed via clustered WGGA sequences. Hum Mol Genet 2014;23:5479–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AK, Crawford DC, Scott EH, Leslie ML, Sherman SL.. Paternally transmitted FMR1 alleles are less stable than maternally transmitted alleles in the common and intermediate size range. Am J Hum Genet 2002;70:1532–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL.. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod 2005;20:402–412. [DOI] [PubMed] [Google Scholar]
- Tassone F, Beilina A, Carosi C, Albertosi S, Bagni C, Li L, Glover K, Bentley D, Hagerman PJ.. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA 2007;13:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, De Rubeis S, Carosi C, La Fata G, Serpa G, Raske C, Willemsen R, Hagerman PJ, Bagni C.. Differential usage of transcriptional start sites and polyadenylation sites in FMR1 premutation alleles. Nucleic Acids Res 2011;39:6172–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ.. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet 2000. a;66:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Mills JB, Harris SW, Gane LW, Hagerman PJ.. Clinical involvement and protein expression in individuals with the FMR1 premutation. Am J Med Genet 2000. b;91:144–152. [DOI] [PubMed] [Google Scholar]
- Tassone F, Iwahashi C, Hagerman PJ.. FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS). RNA Biol 2004;1:103–105. [DOI] [PubMed] [Google Scholar]
- Tejada MI, Garcia-Alegria E, Bilbao A, Martinez-Bouzas C, Beristain E, Poch M, Ramos-Arroyo MA, Lopez B, Fernandez Carvajal I, Ribate MP. et al. Analysis of the molecular parameters that could predict the risk of manifesting premature ovarian failure in female premutation carriers of fragile X syndrome. Menopause 2008;15:945–949. [DOI] [PubMed] [Google Scholar]
- Todd PK, Oh SY, Krans A, He F, Sellier C, Frazer M, Renoux AJ, Chen KC, Scaglione KM, Basrur V. et al. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron 2013;78:440–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng E, Tang HT, AlOlaby RR, Hickey L, Tassone F.. Altered expression of the FMR1 splicing variants landscape in premutation carriers. Biochim Biophys Acta Gene Regul Mech 2017;1860:1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslu B, Dioguardi CC, Haynes M, Miao DQ, Kurus M, Hoffman G, Johnson J.. Quantifying growing versus non-growing ovarian follicles in the mouse. J Ovarian Res 2017;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stege JG, Groen H, van Zadelhoff SJ, Lambalk CB, Braat DD, van Kasteren YM, van Santbrink EJ, Apperloo MJ, Weijmar Schultz WC, Hoek A.. Decreased androgen concentrations and diminished general and sexual well-being in women with premature ovarian failure. Menopause 2008;15:23–31. [DOI] [PubMed] [Google Scholar]
- Voorhuis M, Onland-Moret NC, Fauser BC, Ploos van Amstel HK, van der Schouw YT, Broekmans FJ.. The association of CGG repeats in the FMR1 gene and timing of natural menopause. Hum Reprod 2013;28:496–501. [DOI] [PubMed] [Google Scholar]
- Warren ST. The epigenetics of fragile X syndrome. Cell Stem Cell 2007;1:488–489. [DOI] [PubMed] [Google Scholar]
- Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de Muinck Keizer-Schrama S, Hogervorst E, Janse F. et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod 2016;31:926–937. [DOI] [PubMed] [Google Scholar]
- Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf )2008;68:499–509. [DOI] [PubMed] [Google Scholar]
- Yrigollen CM, Durbin-Johnson B, Gane L, Nelson DL, Hagerman R, Hagerman PJ, Tassone F.. AGG interruptions within the maternal FMR1 gene reduce the risk of offspring with fragile X syndrome. Genet Med 2012;14:729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Yaba A, Kasiman C, Thomson T, Johnson J.. mTOR controls ovarian follicle growth by regulating granulosa cell proliferation. PLoS One 2011;6:e21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu T, Gibbens B, Doty NS, Gomes-Pereira M, Huguet A, Stone MD, Margolis J, Peterson M, Markowski TW, Ingram MA. et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci USA 2011;108:260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumwalt M, Ludwig A, Hagerman PJ, Dieckmann T.. Secondary structure and dynamics of the r(CGG) repeat in the mRNA of the fragile X mental retardation 1 (FMR1) gene. RNA Biol 2007;4:93–100. [DOI] [PubMed] [Google Scholar]