Abstract
Innovative solutions are required to effectively address the unprecedented surge of demand on our healthcare systems created by the COVID-19 pandemic. Home treatment and monitoring of patients who are asymptomatic or mildly symptomatic can be readily implemented to ameliorate the health system burden while maintaining safety and effectiveness of care. Such endeavor requires careful triage and coordination, telemedicine and technology support, workforce and education, as well as robust infrastructure. In the understandable paucity of evidence-based, protocolized approaches toward HOT for COVID-19 patients, our group has created the current document based on the cumulative experience of members of the Joint ACAIM-WACEM COVID-19 Clinical Management Taskforce. Utilizing available evidence-based resources and extensive front-line experience, the authors have suggested a pragmatic pathway for providing safe and effective home oxygen therapy in the community setting.
Keywords: COVID-19, COVID-HOT, home monitoring, oxygen therapy, outpatient therapy, triage
Introduction
In many acutely affected hot spots of COronaVIrus Disease 2019 (COVID-19), the capacity of local and regional healthcare systems, and in particular the availability of emergency department (ED) and inpatient beds, may be insufficient during epidemic surge conditions.[1,2] To adequately manage the high volume of patient encounters and hospital admissions in the setting of exaggerated imbalances within critical resource availability, special considerations and unconventional measures must be entertained.[3,4,5,6] Furthermore, given our increasing knowledge of the somewhat unpredictable COVID-19 clinical progression, it is prudent to institute an active observation regimen to detect early signs of deterioration that can occur in a non-trivial proportion of patients who may not initially require hospital admission.[7,8,9] As the current COVID-19 inpatient strategy relies heavily on managing oxygenation, selected patients could be discharged home if oxygen administration could be addressed in a safe manner, under well-designed and appropriately implemented regimens.[10,11,12,13]. The need for diligent and close monitoring of COVID-19 patients who are discharged home arises due to the occurrence of clinically silent and unpredictable hypoxia, and thus an increased risk of potentially preventable mortality.[7,14,15] Consequently and understandably, there is some degree of controversy surrounding this topic, mandating a properly structured and highly regimented approach.[11,16]
Clinical Rationale
Given the rapidly evolving pandemic, there is an acute need for a standardized, evidence-based, algorithmic approach to home oxygen therapy (HOT) and monitoring for COVID-19 infection (COVID-HOT) to adequately address both patient care requirements and current healthcare resource limitations. The Joint ACAIM-WACEM COVID-19 Clinical Management Taskforce (CCMT) presents the comprehensive COVID-HOT protocol [Figures 1 and 2][17,18,19,20,21] along with important risk stratification definitions, such as the SCRB-60 score [Table 1],[22] as well as the Breathlessness Screening Tool [Table 2].[23,24] As with any other clinical assessment platform, the evaluation of each patient should always begin with, and be based on, a careful medical history and a detailed clinical examination. It is recognized herein that telepresence may not provide as robust of a clinical assessment as an in-person visit; however, we must acknowledge that the overall risk-benefit equation of in-person encounters in the midst of a pandemic is generally unfavourable.[8,9,25] Building upon the foundation of a well-structured and reliable telemedicine service, the managing healthcare provider (HCP) should be familiar with essential remote patient assessment tools and their limitations [Tables 1 and 2].[22,23,24,25,26,27,28,29,30]
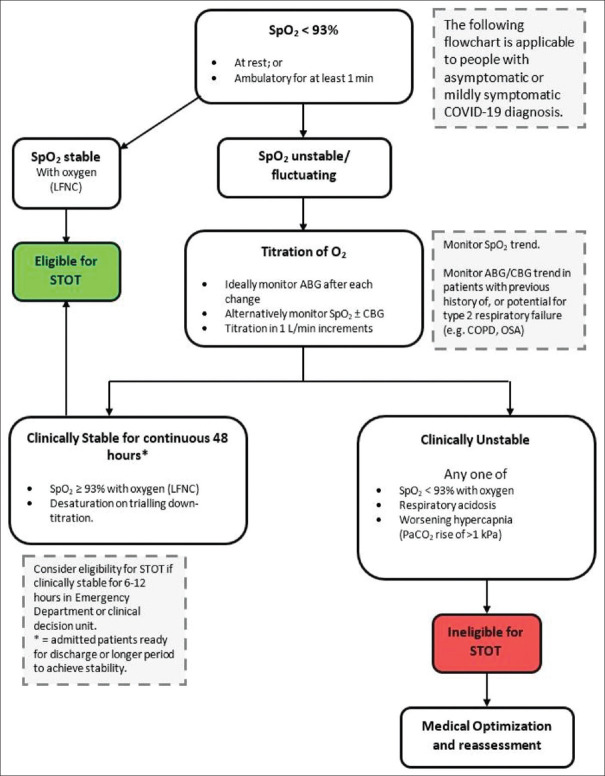
Figure 1.
Determination of eligibility for short-term home oxygen therapy in COVID-19.[17] Legend: LFNC = Low flow nasal cannula, CBG = Capillary blood gas, STOT = Short term oxygen therapy, SpO2 = Peripheral capillary oxygen saturation, ABG = Arterial blood gas, COPD = Chronic obstructive pulmonary disease, OSA = obstructive sleep apnea)
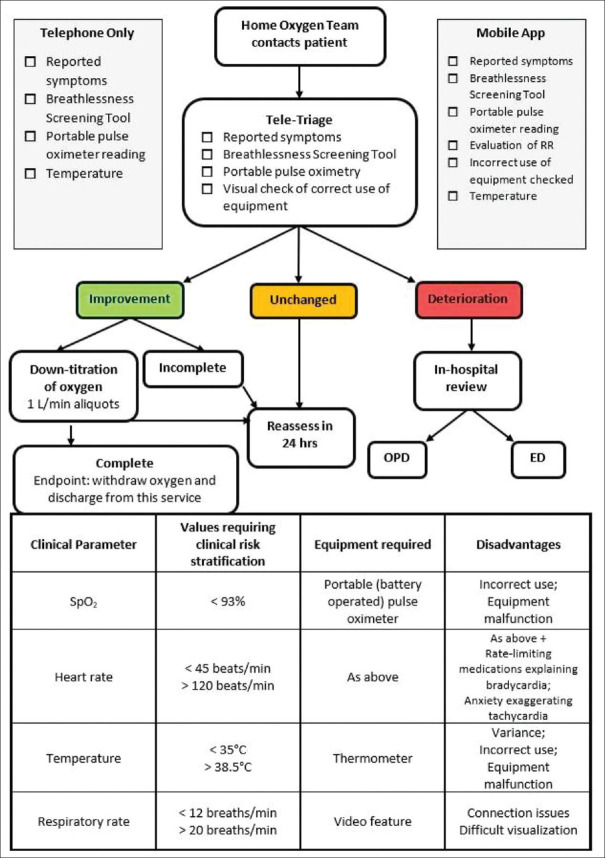
Figure 2.
Tele-follow up of patients on home oxygen therapy in COVID-19.[18,19,20,21] Legend: RR = Respiratory Rate, OPD = Out-patient Department, ED = Emergency Department
Table 1.
Clinical severity classification for COVID-19
| Feature | Mild Risk Features | Moderate Risk Features | Severe Risk Features |
|---|---|---|---|
| Symptoms | As these can be significantly variable, consider an absence of moderate and severe symptoms. | Abdominal symptoms, syncope, falls, seizures, confusion. | Moderate symptoms + hypoxic agitation, coma, respiratory fatigue/failure, cardiac arrest. |
| Risk stratification score | SCRB-60 score of O-1 | SCRB-60 score of 2-3 | SCRUB-60 score of 4-5 |
| Labs | Normal or mild derangement (lymphopenia expected) | Elevated liver enzymes (greater than twice normal value), elevated CRP (>50), AKI grade MI. | Moderate labs + AKI grade III or more, elevated hs-troponin and/or d-dimer, respiratory and/or metabolic acidosis, Type 2 respiratory failure, elevated lactate |
| Imaging (X-ray, POCUS, CT-scan) | No changes/minimal changes | Evidence of small regions of pneumonia (early) or fibrosis (late). Patchy or multifocal B-lines on POCUS. | Multi-lobar pneumonia or pulmonary infarction. Confluent B-lines on POCUS. |
| Oxygen requirement at rest | No | Managed with titration through LFNC or facemask | Requirement for HFNC/ reservoir bag mask/assisted ventilation/ invasive ventilation/ ECLS |
| Oxygen requirement on ambulation (for l minute or longer) | No or ≤2 L/min to maintain saturation ≥ 93% | As above to maintain saturation ≥ 93% | As above to maintain saturation above ≥ 93% |
| Presence of moderate to severe infection will require admission and treatment based on the above, thus though the presence of comorbidities may be significant to disease progression and prognosis, it will not change decision for admission. On the other hand, in the presence of mild disease as described above, due consideration should be given to the impact of comorbidities when deciding appropriate and safe disposition. | |||
|
S Sp02 < 93% C Confusion R Respiratory Rate > 30 breaths/min B Blood pressure: systolic < 90 or diastolic < 60 mmHg 60 Age > 60 years | |||
CRP = C reactive protein, AKI = Acute kidney injury, POCUS = Point of care ultrasound, LFNC = Low flow nasal cannula, HFNC = High flow nasal cannula, ECLS = Extracorporeal life support, hs troponin = high sensitivity troponin, SCRB-60 (modified risk stratification tool)[22]
Table 2.
The breathlessness screening tool (BST) where the patient counts from 1 to 30 in their native language[23,24]
| The Breathlessness Screening Tool (BST) | ||
|---|---|---|
| Method: 1)Ask the patient to take a deep breath in. 2)Then count aloud in native language from 1 to 30 in a single breath, as rapidly as possible. 3)Record the "maximum count." 4)Record the "counting time" using a stopwatch. |
Maximum number of counted numbers on single breath < 7 OR
Time between consecutive breaths < 5 sec Corresponds to Sp02 < 90% (on room air) with sensitivity of 87% and specificity of 82% |
Caveats: 1)Tool has not been fully validated in the telehealth setting. 2)Tool correlates oxygen saturations on room air rather than on supplemental oxygen; thus, in the context of this algorithm, it can be used after the patient has been off oxygen > 1 hr*. 3)Effort-dependent, thus variable. Repeated testing may by more reliable. 4)To be used in conjunction with other indices and monitoring criteria. |
|
Maximum number of counted numbers on single breath < 10 OR
Time between consecutive breaths < 7 sec Corresponds to Sp02 < 95% (on room air) with sensitivity of 91% and specificity of 93% | ||
SpO2 = Peripheral capillary oxygen saturation, * = an arbitrary time interval, which can be modified by decision of home oxygen team
Point-of-Care Capabilities
Successful implementation of the COVID-HOT protocol is heavily reliant on the availability and applicability of various point-of-care (POC) tools. From the perspective of the COVID-19 pandemic, such tools may include POC diagnostic testing; point-of-care ultrasonography (POCUS); as well as some form of telemetry capability consisting of pulse oximetry (SpO2) and potentially heart rate and blood pressure monitoring.[31,32,33] Of utmost importance is the availability of family members or other reliable caretakers who are available to assist with most of the fundamental, non-emergency home care scenarios. Such individuals require education about staying safe and reducing their own risk of contracting the infection while providing support needed to care for their loved one,[34,35] including the on-going management of comorbid health conditions and maintenance of some level of physical activity.[36] Both the patients and their closest contacts need to be aware of the importance of recognizing ‘silent hypoxia’, which represents a difficult-to-detect disease acuity escalation,[14,37] as well as the logistical considerations associated with supplemental oxygen therapy and respiratory maneuvers such as awake proning.[5,35] As always, patient safety is of paramount importance, and thus detailed HOT safety education must be provided to all stakeholders [Figure 3].[38] Finally, when available, increasingly sophisticated artificial intelligence (AI)-based systems should be utilized to provide both diagnostic assistance and decision-making support in the home care setting, especially under the “stay-at-home” paradigm.[5,33,39,40,41]
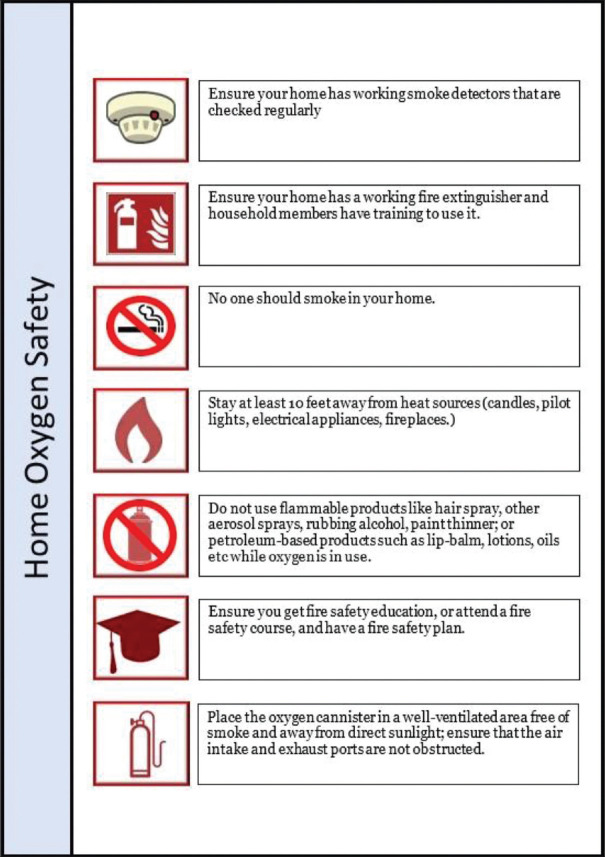
Figure 3.
Home oxygen safety poster
Risk Assessment and Determination of Safe Transition to COVID-HOT Status
Patient should undergo a thorough clinical risk assessment before the decision to implement the COVID-HOT protocol is made. Table 1 provides a highly granular framework for the determination of relative risk regarding the decision to admit to a hospital, observe temporarily, or discharge to home under the COVID-HOT algorithm. The decision tool is relatively complex and requires careful analysis of each component, with the likelihood of potentially serious consequences of mis-triage increased by rushing through the process. Non-COVID-19 diagnoses must be carefully considered, including seasonally appropriate testing for influenza and other viral and non-viral illnesses.[42] Careful physical exam, COVID-19-specific biomarker testing and appropriate confirmatory viral testing constitute an essential part of this assessment.
The ACAIM-WACEM CCMT emphasizes strongly that the COVID-HOT protocol should only be used with utmost caution, fully leveraging the collective clinical team experience and hardwired secondary confirmatory assessment by a senior provider before proceeding, and never in the setting of a single HCP acting in isolation. It is strongly recommended that one of the two certifying providers be trained in either Critical Care or Emergency Medicine. Consequently, the COVID-HOT protocol should never be employed in a single-provider setting or a setting where second-provider confirmation is not feasible. Moreover, the protocol should only be applied in situ ations where patients had already undergone a non-trivial period of observation (e.g., ≥6 hours), thus resulting in sufficient levels of HCP confidence regarding case-specific illness acuity “trajectory”. Finally, the COVID-HOT protocol should not be implemented in health-care systems without an understanding of, and experience in, managing ambulatory home oxygen treatments at the community level. This, in turn, involves appropriate expertise and understanding of supply chains, pertinent safety measures and system dynamics (beyond the scope of this article).[43,44,45,46,47]
It is important to recognize that COVID-19 can produce a fairly heterogeneous array of signs and symptoms, and thus may mimic a variety of different diseases and/or syndromes (and vice-versa).[48,49,50] Furthermore, COVID-19 can co-exist with other acute illnesses.[9,42] Therefore, careful consideration is critical when discharging patients from ED, limited-duration observation, or inpatient environments to receive active, on-going treatment at home.
Initiation of At-Home Services, Including Technological and Logistical Considerations
Providers must assume that patients and/or their families may not be familiar with modern tele-presence tools. Consequently, appropriate education should be provided to all stakeholders. It is recommended that “practice runs” are conducted, where experimentation with the platform is encouraged and the user has the opportunity to “break the system” in a simulated setting. Appropriate, easy-to-access technical support should be available around-the-clock, with clearly defined and readily accessible primary and back-up communication channels.[51,52,53,54]
It is important that patients and their caregivers be instructed on monitoring clinical parameters as outlined in this paper [Figure 2],[18,19,20,21] and that any modifications to the protocol (s) presented herein are carefully and thoughtfully vetted at appropriate institutional clinical leadership level(s). Of note, telemedicine platforms should be deployed and utilized on devices/platforms/device types already familiar to the patient and/or the caregiver, such as their smart phone, tablet, laptop, or desktop computer. Graphical interfaces, on-screen buttons, and in-app navigation should be intuitive, with very clearly written instructions provided to patients and their caregiver (s).
Patient education can be challenging and relies on multiple factors including but not limited to provider training, patient and/or caregiver level of technological knowledge and education, as well as a diverse number of cultural factors and expectations.[55,56,57,58,59,60,61] The safety and quality of home-based care and self-monitoring depend on patient and/or caregiver understanding of the disease and the ability to practically and efficiently use the tele-presence tools at their disposal. In this context, good patient education requires a supportive learning environment, and the ability to individualize instructions to the patient's needs, level of understanding, general comfort with applicable technology, among other factors and expectations.[59,60] There must also be an established system that facilitates continuing/on-going education and support.[60,62] If there is lack of assurance that the patient or their caregiver(s) can effectively comply with the requirements of HOT and associated monitoring needs, or it is clear that they are unable or unwilling to cooperate with the requirements of tele-monitoring and appropriate escalation of care, then HOT should not be implemented and an alternative strategy pursued. It is vital for the patient and their care provider(s) to be fully engaged in the overall process in order to optimize both safety and quality of the care delivered.
Community-Based Management
Home oxygen therapy is a method of home or community-based care. Though we propose a specialist team to implement and coordinate the delivery of this care, we believe that the patient's primary care team must also be involved. The primary care team is ultimately the central point of contact whenever multiple disciplines are involved, and they are often the first point of contact for patients seeking medical care. They may be the primary referring team for patients with silent hypoxia who are otherwise well, or mildly unwell patients. Patients who are sent home from hospital or specialist clinics are also discharged back to primary care teams. These teams can continue to actively monitor, recognize the need for readmission or referral to the specialist teams, or recognize and resolve care needs that do not require specialist input while managing the patient holistically. Additionally, primary care teams and family physicians have a personal bond and rapport with their patients, fostering sustainable long-term healthcare interactions that are known to enhance treatment compliance and effective care delivery.
COVID-19 has resulted in a reorganization and restructuring of care provision in and out of hospital. Telemedicine has become a routine part of healthcare delivery. Furthermore, now that its benefits have been fully realized, the elevated level of utilization may continue well beyond the current pandemic. This paper addresses telemonitoring in COVID-19 patients, but can also serve to generate ideas regarding the future of telemedicine across various settings. Family physicians and primary care teams may modify their practices to include telemedicine as part of their healthcare delivery and follow-up.
Long-Term Versus Short-Term Oxygen Therapy
Long-term oxygen therapy (LTOT) is an established safe and effective treatment for patients with chronic pulmonary conditions. Its primary aims include the improvement of quality of life and reduction in morbidity and mortality.[17,63,64] In contrast, short-term oxygen therapy (STOT) is generally less well studied, and its applications are fairly heterogeneous.[65,66,67] More specifically, the use of STOT in COVID-19 patients is based on the primarily hypoxic pathophysiology of SARS-CoV-2 pulmonary damage.[14,22] The eligibility criteria for referral to the HOT team for STOT administration have been extrapolated from existing LTOT regimens, to create a novel treatment algorithm [Figure 1].[17] We therefore propose STOT as novel therapy in COVID-19, and while management objectives remain the same, there is an added benefit of providing safe care while reducing hospital burden in the context of an on-going pandemic.[5]
Establishment of a Specialty Follow-Up Team
Due to the specific needs for follow-up of this cohort of patients, as well as the elevated risk of at-home adverse events, institutions are strongly advised to establish a dedicated HOT follow-up team. Such teams should be empowered to systematically implement the current recommendations, act as point of contact for patients, organize HCP and patient education, oversee referrals to other specialities or teams (e.g., smoking cessation services, rehabilitation teams, physiotherapy, occupational therapy, etc.), and serve to liaise with respective teams for any concerns to HOT logistics, including equipment issues or patient non-compliance. This follow-up team can further develop dedicated institutional referral documentation, necessary educational materials, and patient information contact points. Assigning clear roles and responsibilities within the follow-up team will reduce confusion and streamline communication for both HCPs and patients.
Oxygen Sources: Concentrators, Cylinders, Liquid Oxygen
A dedicated team should be responsible for deciding the mode of oxygen delivery and dosing. Concentrators are commonly used for LTOT delivery and can either be stationary at home, or portable with the patient.[68,69,70] An oxygen concentrator is an electrically powered device which filters room air, removing nitrogen, to provide an oxygen-enriched gas mixture. Home concentrators require installation and regular maintenance by specialized vendors. In the context of the current pandemic, this is not ideal as it potentially increases infectious exposure risk to both household members and the company personnel. Transportable and portable concentrators are similar to home concentrators, but generally smaller in size and weight.[71,72,73] Cylinder oxygen comes in a reinforced metal container with compressed gas under high pressure which is safely and steadily released via its regulator (tap). Liquid oxygen is oxygen that is cooled such that it condenses from gaseous to liquid form and can be stored in appropriately insulated containers; however, this approach requires training to reduce problems with gas leakage and burns.[74] In the context of the COVID-HOT algorithm, we recommend the use of transportable or portable oxygen concentrators at flow rates of 4 L/min or less.[17] At all times, users of any concentrated oxygen must keep in mind the so-called “fire triangle” and be mindful of the dangers of any potential co-presence of fuel (e.g., alcohol, textiles, bedding materials); heat (e.g., electrical equipment, space heaters); and oxygen in close proximity.[75]
Patients with Assisted Ventilation at Home
Special consideration should be made for oxygen use in patients who already use continuous positive airway pressure therapy (CPAP) for conditions such as obstructive sleep apnoea (OSA), obesity hypoventilation syndrome (OHS), chronic obstructive pulmonary disease (COPD) or overlap syndrome (a combination of the above pathologies). This is because patients who rely on baseline respiratory support may be at higher risk of developing hypoxemia and hypercapnia. There is paucity of clinical research supporting HOT in the treatment of OHS or overlap syndrome. Oxygen has been used as an add-on therapy to non-invasive ventilation (NIV).[17]
Venous Thromboembolism (VTE) Prophylaxis
A comprehensive discussion of VTE in COVID-19 is beyond the scope of this manuscript as it is a complex topic.[3] We encourage that there is due consideration for VTE prophylaxis in this group of patients. Any implementations of VTE prophylaxis should be consistent with established local/institutional policies and should be evaluated in the context of risk-benefit equation associated with observed epidemiological patterns.
Conclusion
Novel and future-oriented solutions are needed to effectively address the unprecedented pressure on the healthcare systems created by the COVID-19 pandemic. Home treatment and monitoring of patients who are asymptomatic or mildly symptomatic can be readily implemented to ameliorate the health system burden without sacrificing safety or effectiveness. As a result, carefully implemented HOT paradigm may help optimize the utilization of scarce resources in response to a surge in patients requiring urgent medical attention. Due to the paucity of evidence-based, protocolized approaches toward home oxygen therapy for COVID-19 patients, our group created the current document. Based on the cumulative experience of members of the Joint ACAIM-WACEM CCMT, combined with available evidence-based resources, the authors created a pathway for providing safe and effective HOT care in the community setting. Effective implementations of this approach require a combination of excellent clinical judgement on the part of the treating HCP, availability of POC tools, real-time remote patient monitoring, and ongoing education of HCPs, patients, and their caretakers.
Cautionary Note
The ACAIM-WACEM CCMT emphasizes strongly that the COVID-HOT protocol should only be used with utmost caution, fully leveraging the collective clinical team experience and hardwired secondary confirmatory assessment by a senior provider before proceeding, and never in the setting of a single HCP acting in isolation. Consequently, the COVID-HOT protocol should never be employed in a single-provider setting or a setting where second-provider confirmation is not feasible. It is strongly recommended that one of the two determining providers be trained in Critical Care and/or Emergency Medicine. In all cases, each team decision should be thoroughly documented in the medical record, with all involved/responsible providers clearly identified and a justification provided for proceeding with the COVID-HOT care pathway. Such documentation should include the following mandatory components: (a) patient identification, clinical status, and diagnosis; (b) provider identification, specialty background (e.g., Critical Care), and appropriate seniority/training level (e.g., attending physician); (c) availability of required resources prior to discharge; (d) clear documentation of patient and caretaker information, including detailed instructions on when to seek further assistance/escalate care; (e) required oxygen safety training; and (f) appropriate treatment consent documentation, with clearly documented risk-benefit-alternative discussion. Finally, it is strongly recommended that regularly scheduled reviews of all COVID-HOT determinations are conducted at the institutional level, with focus on protocol compliance, clinical outcomes, any unexpected events, and patient safety.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hogg W, Lemelin J, Huston P, Dahrouge S. Increasing epidemic surge capacity with home-based hospital care. Can Fam Physician. 2006;52:563–4. [PMC free article] [PubMed] [Google Scholar]
- 2.Yang J-R, Hsu S-Z, Kuo C-Y, Huang H-Y Huang T-Y, Wang H-C, et al. An epidemic surge of influenza A (H3N2) virus at the end of the 2016–2017 season in Taiwan with an increased viral genetic heterogeneity. J Clin Virol. 2018;99:15–21. doi: 10.1016/j.jcv.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Remuzzi A, Remuzzi G. COVID-19 and Italy: What next? Lancet. 2020;395:1225–8. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, Yao W, Wang Y, Long C, Fu X. Wuhan and Hubei COVID-19 mortality analysis reveals the critical role of timely supply of medical resources. J Infect. 2020;81:147–78. doi: 10.1016/j.jinf.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stawicki SP, Jeanmonod R, Miller AC, Paladino L, Gaieski DF, Yaffee AQ, et al. The 2019–2020 novel coronavirus (severe acute respiratory syndrome coronavirus 2) pandemic: A joint American college of academic international medicine-world academic council of emergency medicine multidisciplinary COVID-19 working group statement. J Global Infect Dis. 2020;12:47–93. doi: 10.4103/jgid.jgid_86_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mareiniss DP. The impending storm: COVID-19, pandemics and our overwhelmed emergency departments. Am J Emerg Med. 2020;38:1293–4. doi: 10.1016/j.ajem.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annis T, Pleasants S, Hultman G, Lindemann E, Thompson JA, Billecke S, et al. Rapid implementation of a COVID-19 remote patient monitoring program. J Am Med Inform Assoc. 2020 doi: 10.1093/jamia/ocaa097. doi: 10.1093/jamia/ocaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doshi A, Platt Y, Dressen JR, Mathews BK, Siy JC. Keep calm and log on: Telemedicine for COVID-19 pandemic response. J Hosp Med. 2020;15:302–4. doi: 10.12788/jhm.3419. [DOI] [PubMed] [Google Scholar]
- 9.Chauhan V, Galwankar S, Arquilla B, Garg M, Di Somma S, El-Menyar A, et al. Novel coronavirus (COVID-19): Leveraging telemedicine to optimize care while minimizing exposures and viral transmission. J Emerg Trauma Shock. 2020;13:20–4. doi: 10.4103/JETS.JETS_32_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hick JL, Hanfling D, Wynia MK, Pavia AT. Washington, DC: National Academy of Medicine; 2020. Duty to plan: Health care, crisis standards of care, and novel coronavirus SARS-CoV-2. NAM perspectives. Discussion paper. doi: 10.31478/202003b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirby JJ, Alexander S, Bonthu M, Calaway N, Cornwell A, Davis J, et al. ACEP COVID-19 Field Guide. [accessed 20/06/2020];Updated. 2020 Jun 17; https://www.acep.org/corona/covid-19-field-guide/publishers-notice/ [Google Scholar]
- 12.Fisher B, Seese L, Sultan I, Kilic A. The importance of repeat testing in detecting coronavirus disease 2019 (COVID-19) in a coronary artery bypass grafting patient. J Cardiac Surg. 2020;35:1342–4. doi: 10.1111/jocs.14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnold FW, Mahmood K, Prabhu A, Aden D, Raghuram A, Burns M, et al. COPD exacerbation caused by SARS-CoV-2: A case report from the Louisville COVID-19 surveillance program. Univ Louisville J Respir Infect. 2020;4:5. [Google Scholar]
- 14.Galwankar SC, Paladino L, Gaieski DF, Nanayakkara KDPWB, Di Somma S, Grover J, et al. Management algorithm for subclinical hypoxemia in COVID-19 patients: Intercepting the ‘silent killer’. J Emerg Trauma Shock. 2020;13:110–3. doi: 10.4103/JETS.JETS_72_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson AR, Wah R, Thamman R. The Value of Remote Monitoring for the COVID-19 Pandemic. Telemed e-Health. 2020 doi: 10.1089/tmj.2020.0134. doi: 10.1089/tmj. 2020.0134. [DOI] [PubMed] [Google Scholar]
- 16.England N, Improvement N. Primary Care and Community Respiratory Resource Pack for Use During Covid-19. Updated 16/04/2020. [accessed 20/06/2020]. Avaliable from: https://www.pcrs-uk.org/sites/pcrs-uk.org/files/resources/COVID19/NHS-London-Primary-and-Community-Care-Respiratory-Resource-Pack-during-COVID-19-V3final-160420.pdf .
- 17.Hardinge M, Annandale J, Bourne S, Cooper B, Evans A, Freeman D, et al. British thoracic society guidelines for home oxygen use in adults. Thorax. 2015;70:i1–43. doi: 10.1136/thoraxjnl-2015-206865. [DOI] [PubMed] [Google Scholar]
- 18.Buekers J, Theunis J, De Boever P, Vaes AW, Koopman M, Janssen EV, et al. Wearable finger pulse oximetry for continuous oxygen saturation measurements during daily home routines of patients with chronic obstructive pulmonary disease (COPD) over one week: Observational study. JMIR Mhealth Uhealth. 2019;7:e12866. doi: 10.2196/12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaney JC, Jones K, Grathwohl K, Olivier KN. Implementation of an oxygen therapy clinic to manage users of long-term oxygen therapy. Chest. 2002;122:1661–7. doi: 10.1378/chest.122.5.1661. [DOI] [PubMed] [Google Scholar]
- 20.Buekers J, De Boever P, Vaes AW, Aerts J-M, Wouters EFM, Spruit MA, et al. Oxygen saturation measurements in telemonitoring of patients with COPD: A systematic review. Expert Rev Respir Med. 2018;12:113–23. doi: 10.1080/17476348.2018.1417842. [DOI] [PubMed] [Google Scholar]
- 21.Lacasse Y, Tan A-YM, Maltais F, Krishnan JA, et al. Home oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197:1254–64. doi: 10.1164/rccm.201802-0382CI. [DOI] [PubMed] [Google Scholar]
- 22.Sinha S, Sardesai I, Galwankar SC, Nanayakkara PWB, Narasimhan DR, Grover J, et al. Optimizing respiratory care in COVID-19: A comprehensive, protocolized, evidence-based, algorithmic approach. Int J Crit Illn Inj Sci. 2020;10:56–63. doi: 10.4103/IJCIIS.IJCIIS_69_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chorin E, Padegimas A, Havakuk O, Birati EY, Shacham Y, Milman A, et al. Assessment of respiratory distress by the roth score. Clin Cardiol. 2016;39:636–9. doi: 10.1002/clc.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenhalgh T, Kotze K, Van Der Westhuizen H-M. Are there any evidence-based ways of assessing dyspnoea (breathlessness) by telephone or video. 2020. 2020. May 6, Available from: https://www.cebm.net/covid-19/are-there-anyevidence-based-ways-of-assessing-dyspnoea - breathlessness-by-telephone-or-video/
- 25.Nitzkin JL, Zhu N, Marier RL. Reliability of telemedicine examination. Telemed J. 1997;3:141–57. doi: 10.1089/tmj.1.1997.3.141. [DOI] [PubMed] [Google Scholar]
- 26.Shindo Y, Sato S, Maruyama E, Ohashi T, Ogawa M, Imaizumi K, et al. Comparison of severity scoring systems A-DROP and CURB-65 for community-acquired pneumonia. Respirology. 2008;13:731–5. doi: 10.1111/j.1440-1843.2008.01329.x. [DOI] [PubMed] [Google Scholar]
- 27.Parsonage M, Nathwani D, Davey P, Barlow G. Evaluation of the performance of CURB-65 with increasing age. Clin Microbiol Infect. 2009;15:858–64. doi: 10.1111/j.1469-0691.2009.02908.x. [DOI] [PubMed] [Google Scholar]
- 28.Mulrennan S, Tempone SS, Ling ITW, Williams SH, Gan GC, Murray RJ, et al. Pandemic influenza (H1N1) 2009 pneumonia: CURB-65 score for predicting severity and nasopharyngeal sampling for diagnosis are unreliable. PLoS One. 2010;5:e12849. doi: 10.1371/journal.pone.0012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalita J, Kumar M, Misra UK. Serial single breath count is a reliable tool for monitoring respiratory functions in Guillain-Barré Syndrome. J Clin Neurosci. 2020;72:50–6. doi: 10.1016/j.jocn.2020.01.032. [DOI] [PubMed] [Google Scholar]
- 30.Kumari A, Malik S, Narkeesh K, Samuel AJ. Single breath count: A simple pulmonary function test using a mobile app. Indian J Thorac Cardiovasc Surg. 2017;33:369–70. [Google Scholar]
- 31.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area [published online ahead of print, 2020 Apr 22] [published correction appears in doi: 10.1001/jama.2020.7681] JAMA. 2020;323:2052–9. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicola M, O’Neill N, Sohrabi C, Khan M, Agha M, Agha R. Evidence based management guideline for the COVID-19 pandemic-review article. Int J Surg. 2020;77:206–16. doi: 10.1016/j.ijsu.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tavakoli M, Carriere J, Torabi A. Robotics, smart wearable technologies, and autonomous intelligent systems for healthcare during the COVID-19 pandemic: An analysis of the state of the art and future vision. Adv Intell Syst. 2020 doi: 10.1002/aisy. 202000071. [Google Scholar]
- 34.Greenhalgh T, Koh GCH, Car J. Covid-19: A remote assessment in primary care. BMJ. 2020;368:m1182. doi: 10.1136/bmj.m1182. [DOI] [PubMed] [Google Scholar]
- 35.Cieloszczyk A, Lewko A, Śliwka A, Wtoch T, Pyszora A. Koronawirus : SARS-Cov-2 : Zalecenia do prowadzenia fitjoterapii doroslych pacjentów z COVID-19 = Coronavirus : SARS-Cov-2 : Recommendations for physiotherapy of adult patients with COVID-19. (Documentation) Warsaw, Poland : Krajowa Izba Fizjoterapeutów = The Polish Chamber of Physiotherapists. 2020:17. [Google Scholar]
- 36.Middleton A, Simpson KN, Bettger JP, Bowden MG. COVID-19 pandemic and beyond: Considerations and costs of telehealth exercise programs for older adults with functional impairments living at home—Lessons learned from a pilot case study. Phys Ther. 2020 doi: 10.1093/ptj/pzaa089. doi: 10.1093/ptj/pzaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levitan R. The infection that's silently killing coronavirus patients. 2020 April 28, 2020. Available from: https://www.nytimes.com/2020/04/20/opinion/sunday/coronavirus-testing-pneumonia.html .
- 38.Services D.o.F. Using Home Oxygen Safely: What Everyone Needs to Know. 2018. Available from: https://www.mass.gov/files/documents/2018/10/23/2018%20HomeO2_with%20video.pdf .
- 39.Rao ASS, Vazquez JA. Identification of COVID-19 can be quicker through artificial intelligence framework using a mobile phone–based survey when cities and towns are under quarantine. Infect Control Hosp Epidemiol. 2020 doi: 10.1017/ice.2020.61. doi: 10.1017/ice. 2020.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaishya R, Javaid M, Haleem Khan I, Haleem A. Artificial intelligence (AI) applications for COVID-19 pandemic. Diabetes Metab Syndr: Clin Res Rev. 2020;14:337–9. doi: 10.1016/j.dsx.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bullock J, Luccioni A, Pham KH, Lam CSN, Luengo-Oroz M. Mapping the landscape of artificial intelligence applications against COVID-19. arXiv preprint arXiv: 2003.11336. 2020 [Google Scholar]
- 42.Chauhan V, Galwankar SC, Yellapu V, Perez-Figueroa IJ, Stawicki SP. The state of the globe: The trials and tribulations of the COVID-19 pandemic: Separated but together, telemedicine revolution, frontline struggle against ‘Silent Hypoxia,’ the relentless search for novel therapeutics and vaccines, and the daunting prospect of ‘COVI-Flu’. J Global Infect Dis. 2020;12:39–43. doi: 10.4103/jgid.jgid_96_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carchiolo V, Compagno L, Malgeri M, Trapani N, Previti ML, Loria MP, et al. An efficient real-time monitoring to manage home-based oxygen therapy. In World Conference on Information Systems and Technologies. Springer. 2018 [Google Scholar]
- 44.Kobayashi S, Hanagama M, Yamanda S, Yanai M. Home oxygen therapy during natural disasters: Lessons from the great East Japan earthquake. Eur Respir J. 2012;39:1047–8. doi: 10.1183/09031936.00149111. [DOI] [PubMed] [Google Scholar]
- 45.Flett KB, Breslin K, Braun PA, Hambidge SJ. Outpatient course and complications associated with home oxygen therapy for mild bronchiolitis. Pediatrics. 2014;133:769–75. doi: 10.1542/peds.2013-1872. [DOI] [PubMed] [Google Scholar]
- 46.Lynes D, Kelly C. Domiciliary oxygen therapy: Assessment and management. Nurs Stand. 2009;23:50–6. doi: 10.7748/ns2009.01.23.20.50.c6747. [DOI] [PubMed] [Google Scholar]
- 47.Tomio J, Sato H. Emergency and disaster preparedness for chronically ill patients: A review of recommendations. Open Access Emerg Med. 2014;6:69–79. doi: 10.2147/OAEM.S48532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gulati A, Pomeranz C, Qamar Z, Thomas S, Frisch D, George G, et al. A comprehensive review of manifestations of novel coronaviruses in the context of deadly COVID-19 global pandemic. Am J Med Sci. 2020 doi: 10.1016/j.amjms.2020.05.006. doi: 10.1016/j.amjms.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu G, et al. Coronavirus disease 2019 (COVID-19): A perspective from China. Radiology. 2020 doi: 10.1148/radiol.2020200490. doi: 10.1148/radiol. 2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nickel CH, Bingisser R. Mimics and chameleons of COVID-19. Swiss Med Wkly. 2020;150:w20231. doi: 10.4414/smw.2020.20231. [DOI] [PubMed] [Google Scholar]
- 51.Smith AC, Bensink M, Armfield N, Stillman J, Caffery L. Telemedicine and rural health care applications. J Postgrad Med. 2005;51:286–93. [PubMed] [Google Scholar]
- 52.Olson CA, Thomas JF. Telehealth: No longer an idea for the future. Adv Pediatr. 2017;64:347–70. doi: 10.1016/j.yapd.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Boots R, Widdicombe N, Lipman J. Quality management in intensive care: A practical guide. Cambridge University Press; 2016. Applications of telemedicine in the intensive care unit; pp. 235–46. [Google Scholar]
- 54.Lowery CL, Bronstein JM, Benton TL, Fletcher DA. Distributing medical expertise: The evolution and impact of telemedicine in arkansas. Health Aff. 2014;33:235–43. doi: 10.1377/hlthaff.2013.1001. [DOI] [PubMed] [Google Scholar]
- 55.Nwabueze SN, Meso PN, Mbarika VW, Kifle M, Okoli C, Chustz M. 2009 42nd Hawaii International Conference on System Sciences. IEEE; 2009. The effects of culture of adoption of telemedicine in medically underserved communities. [Google Scholar]
- 56.Mansouri-Rad P, Mahmood MA, Thompson SE, Putnam K. 2013 46th Hawaii International Conference on System Sciences. IEEE; 2013. Culture matters: Factors affecting the adoption of telemedicine. [Google Scholar]
- 57.Ziadlou D, Eslami A, Hassani H. 2008 Third International Conference on Broadband Communications, Information Technology & Biomedical Applications. IEEE; 2008. Telecommunication methods for implementation of telemedicine systems in crisis. [Google Scholar]
- 58.Darkins A, Cary M. Telemedicine and telehealth: Principles, policies, performances and pitfalls. Springer Publishing Company; 2000. [Google Scholar]
- 59.LeRouge C, Garfield MJ, Hevner AR. Proceedings of the 35th Annual Hawaii International Conference on System Sciences. IEEE; 2002. Quality attributes in telemedicine video conferencing. [Google Scholar]
- 60.Klonoff DC. SAGE Publications; 2012. Improved outcomes from diabetes monitoring: The benefits of better adherence, therapy adjustments, patient education, and telemedicine support. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gustke SS, Balch DC, West VL, Rogers LO. Patient satisfaction with telemedicine. Telemed J. 2000;6:5–13. [Google Scholar]
- 62.Svavarsdottir MH, Sigurethardottir AK, Steinsbekk A. How to become an expert educator: A qualitative study on the view of health professionals with experience in patient education. BMC Med Educ. 2015;15:87. doi: 10.1186/s12909-015-0370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koczulla AR, Schneeberger T, Jarosch I, Kenn K, Gloeckl R. Long-term oxygen therapy. Dtsch Arztebl Int. 2018;115:871–7. doi: 10.3238/arztebl.2018.0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stretton T. Provision of long term oxygen therapy. Thorax. 1985;40:801–5. doi: 10.1136/thx.40.11.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev. 2016;2016:CD006429. doi: 10.1002/14651858.CD006429.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hardinge M, Suntharalingam J, Wilkinson T. Guideline update: The British Thoracic Society Guidelines on home oxygen use in adults. Thorax. 2015;70:589–91. doi: 10.1136/thoraxjnl-2015-206918. [DOI] [PubMed] [Google Scholar]
- 67.O’Donohue WJ., Jr Home oxygen therapy. Clin Chest Med. 1997;18:535–45. doi: 10.1016/s0272-5231(05)70400-7. [DOI] [PubMed] [Google Scholar]
- 68.Petty TL, O’Donohue WJ., Jr Further recommendations for prescribing, reimbursement, technology development, and research in long-term oxygen therapy. Summary of the Fourth Oxygen Consensus Conference, Washington, DC, October 15-16, 1993. Am J Respir Crit Care Med. 1994;150:875–7. doi: 10.1164/ajrccm.150.3.8087365. [DOI] [PubMed] [Google Scholar]
- 69.Chang TT, Lipinski CA, Sherman HF. A hazard of home oxygen therapy. J Burn Care Rehabil. 2001;22:71–4. doi: 10.1097/00004630-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 70.Ringbæk TJ, Lange P, Viskum K. Are patients on long-term oxygen therapy followed up properly? Data from the Danish Oxygen Register. J Int Med. 2001;250:131–6. doi: 10.1046/j.1365-2796.2001.00865.x. [DOI] [PubMed] [Google Scholar]
- 71.Melani AS, Sestini P, Rottoli P. Home oxygen therapy: Re-thinking the role of devices. Exp Rev Clin Pharmacol. 2018;11:279–89. doi: 10.1080/17512433.2018.1421457. [DOI] [PubMed] [Google Scholar]
- 72.Masroor R, Iqbal A, Buland K, Kazi WA. Use of a portable oxygen concentrator and its effect on the overall functionality of a remote field medical unit at 3650 meters elevation. Anaesth Pain Intensive Care. 2013;17:45–50. [Google Scholar]
- 73.Murphie P. Oxygen delivery devices: exploring the options. Pract Nurs. 2014;25:124–8. [Google Scholar]
- 74.Uygur F, Sever C, Noyan N. Frostbite burns caused by liquid oxygen. J Burn Care Res. 2009;30:358–61. doi: 10.1097/BCR.0b013e318198a769. [DOI] [PubMed] [Google Scholar]
- 75.Saeed M, Swaroop M, Yanagawa FS, Buono A, Stawicki SP. Avoiding fire in the operating suite: An intersection of prevention and common sense. Vignettes in patient safety. 3 IntechOpen; 2018. [Google Scholar]