Abstract
Cichorium intybus L., (chicory) is employed in various traditional medicines to treat a wide range of diseases and disorders. In the current investigation, two new naphthalane derivatives viz., cichorins D (1) and E (2), along with one new anthraquinone cichorin F (3), were isolated from Cichorium intybus. In addition, three previously reported compounds viz., β-sitosterol (4), β-sitosterol β-glucopyranoside (5), and stigmasterol (6) were also isolated from Cichorium intybus. Their structures were established via extensive spectroscopic data, including 1D (1H and 13C) and 2D NMR (COSY, HSQC and HMBC), and ESIMS. Cichorin E (2) has a weak cytotoxic effect on breast cancer cells (MDA-MB-468: IC50: 85.9 µM) and Ewing’s sarcoma cells (SK-N-MC: IC50: 71.1 µM); cichorin F (3) also illustrated weak cytotoxic effects on breast cancer cells (MDA-MB-468: IC50: 41.0 µM and MDA-MB-231: IC50: 45.6 µM), and SK-N-MC cells (IC50: 71.9 µM). Moreover compounds 1–3 did not show any promising anthelmintic effects.
Keywords: Cichorium intybus, Asteraceae, naphthalene, anthraquinone, natural product, cytotoxic effects
1. Introduction
Cichorium intybus L. (chicory) (Figure 1) is a herb of the family Asteraceae. This plant is employed in Uighur folk medicine as a diuretic agent and cholagogic because of its wide range of biological effects viz., antioxidant, anti-inflammatory, and antibacterial [1,2,3]. Additionally, it is also used in traditional medicine to treat diabetes, malaria, gastric ulcers, digestive disorder, and stomachic ailments [4,5]. Moreover, its leaves and roots are reported to be used as an appetizer, digestive, depurative, diuretic, cholagogue, hypoglycemic, and laxative. In addition, a root decoction of C. intybus has been employed to treat liver enlargement, jaundice, and gout [4].
Figure 1.
C. intybus photographed by the authors.
Chicory is considered safe to be used in food or in medicine [6]. In addition, the Food and Drug Administration (FDA) has categorized C. intybus extract as “generally to be regarded as safe” and included the plant in the “Everything Added to Food in the United States” category [7]. Chicory is used as a tonic in Indian traditional medicine and is commonly used to treat diarrhea and enlarged spleen problems [4]. Moreover, various C. intybus extracts have demonstrated a wide range of biological and pharmacological properties viz., anti-hyperuricemia, anti-inflammatory, and antidiabetic, antinematodal, antioxidant and antiproliferative, hepatoprotective, antibacterial, and anti-protozoal effects [4,8,9,10,11,12,13,14,15,16,17,18].
It has been reported that C. intybus extracts have a cytotoxic effect on breast cancer (MCF-7) [19], amelanotic melanoma (C32), prostate cancer (LNCaP), renal adenocarcinoma (ACHN) [20], leukemia cells [21], Ehrlich ascites carcinoma [22], prostate cancer (PC-3) [23], breast cells (T47D and SKBR3) [23,24], and cervical cells (HeLa) [25]. Moreover sesquiterpenes reported from C. intybus have shown cytotoxic effects on ovarian cancer cells [26], murine lymphoma [27], and leukemia cells [28]. C. intybus is a nutritious forb employed to enhance the nutritive value for grazing ruminants [29]. It has been reported that gastrointestinal parasites infected small ruminants after eating (Puna) chicory [29]. Numerous reports have shown direct evidence of the anthelmintic effects of C. intybus viz., decreased worm egg numbers in feces [29,30], decreased abomasal worm burdens [29,31], decreased male worms in parasitized animals [29,32], and decreased ability of infective larvae [29,32]. Some reports have demonstrated that sesquiterpene lactones are responsible for anthelmintic effects because these compounds are reported to have significant anthelmintic effects [29,33].
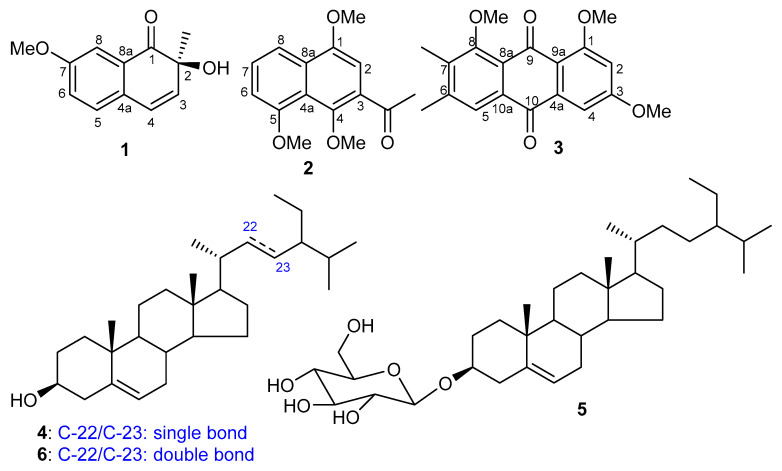
The literature has revealed that various natural products with a diverse range of structural skeletons have been reported from C. intybus viz., caffeoylquinic acids [34], flavonenes and their glycosides [34], anthocyanins and their glycosides [34], sesquiterpenes [35], steroids [36], triterpenes [37], and benzo-isochromenes [37,38]. In this investigation, we isolated and characterized three new compounds viz., cichorins D–F (1–3) (Figure 2) along with three reported compounds viz., β-sitosterol (4), β-sitosterol β-glucopyranoside (5), and stigmasterol (6). In the current study, three new compounds 1–3 and three known compounds 4–6 were isolated from C. intybus. Compounds 1–3 were evaluated for cytotoxic and anthelmintic effects.
Figure 2.
Structures of compounds isolated from C. intybus.
2. Results and Discussion
C. intybus was initially extracted with EtOH and ethanolic extract was subjected to repeated column chromatography, providing three new compounds viz., 1–3 (Figure 2), along with three known compounds. Cichorin D (1) was isolated as a white solid and the IR spectrum demonstrated the presence of a benzene ring (1580 and 1420 cm−1), hydroxyl (3390 cm−1), and carbonyl group (1630 cm−1) (see Experimental section). The molecular formula of compound 1 was established to be C12H12O3 based on the HRESIMS, along with 1H and 13C NMR spectral analyses. The 1H NMR spectrum (Table 1) demonstrated the presence of an ABM spin system of an aromatic ring at δH 7.62 (d, J = 8.0 Hz, 1H, H-5), 6.98 (dd, J = 8.0, 2.6 Hz, H-6), and 6.82 (d, J = 2.6 Hz, 1H, H-8). The COSY correlations further confirmed that two protons viz., δH 7.62 (H-5) and 6.98 (H-6) were coupled together through ortho coupling (J = 8.0 Hz), while the two protons viz., δH 6.98 (H-6) and 6.82 (H-8) were coupled to each other via meta coupling (J = 2.6 Hz) as well as ortho coupling (J = 8.0 Hz).
Table 1.
1H NMR (400 MHz) and 13C NMR (100 MHz) data of cichorin D (1) in CDCl3.
| No | 1H NMR | 13C NMR |
|---|---|---|
| 1 | - | 205.2 |
| 2 | - | 76.5 |
| 3 | 6.27 (d, J = 9.0 Hz, 1H) | 122.9 |
| 4 | 7.37 (d, J = 9.0 Hz, 1H) | 145.7 |
| 4a | 129.3 | |
| 5 | 7.62 (d, J = 8.0 Hz, 1H) | 126.8 |
| 6 | 6.98 (dd, J = 8.5, 2.6 Hz, 1H) | 115.8 |
| 7 | - | 159.1 |
| 8 | 6.82 (d, J = 2.6 Hz, 1H) | 114.6 |
| 8a | - | 136.9 |
| 7-OMe | 3.83 (s, 3H) | 55.4 |
| 2-Me | 1.52 (s, 3H) | 33.0 |
| 2-OH | 3.61 (s, OH, 1H) |
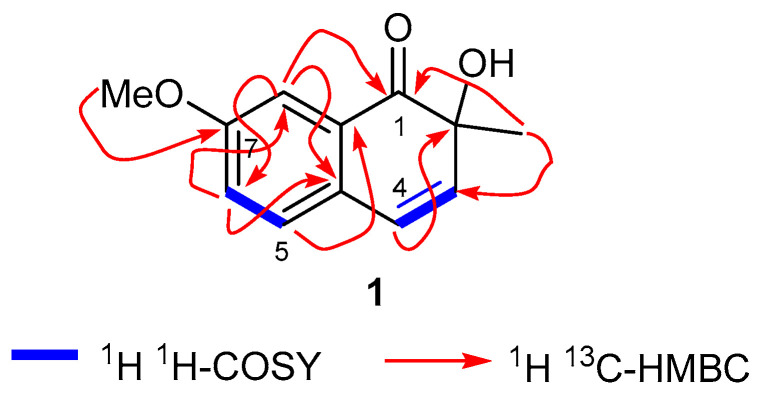
The 1H NMR spectrum also illustrated the presence of an AB olefinic spin system at δH 7.37 (d, J = 9.0 Hz, 1H, H-4) and 6.27 (d, J = 9.0 Hz, H-3); this is also evident from COSY correlations (Figure 3). In addition, the 1H NMR spectrum revealed the presence of one methoxy group (δH 3.83, s, 3H, OMe), and one methyl group (δH 1.52, s, 3H, 2-Me), and this was further confirmed from 13C NMR peaks at δC 55.4 and 33.0, respectively. The 13C NMR spectrum along with DEPT experiments illustrated 1 to possess 12 signals attributed to 5 methine, one methyl, one methoxy, and five quaternary carbons. The 13C NMR spectrum displayed signals for a ketone at δC 205.2 along with a saturated quaternary carbon at δC 76.5 and from these peaks, the proposed structure for this compound is one that possesses a 1,2-dihydronaphthalane skeleton.
Figure 3.
Key COSY and HMBC correlations of cichorin D (1).
The HMBC spectral correlations include the signal at δH 3.83 to C-7, which established the methoxy group to be at C-7. The regiochemistry at ring A was also confirmed via HMBC correlations (Figure 3) as follows: δH 6.82 (H-8) to C-6, C-7, C-4a, and C-8a; 6.98 (H-6) to C-4a, C-5, C-7, and C-8; 7.62 (H-5) to C-4a, C-6, and C-7. Moreover the keto group at C-1 was established via HMBC corrections between H-8 (δH 6.82) to δC 205.2, along with the correlation of the methyl group (δH 1.52) to δC 205.2. In turn, the methyl group at C-2 was evident based on HMBC correlations of this methyl group (δH 1.52) to C-1, C-2, and C-3. Based on NMR analysis, the compound was found to be a naphthalenone derivative and a literature survey illustrated that the naphthalene analog, berryammone B, reported from the plant Berrya ammonilla, also comprises of a keto group at C-1 and a similar C-2 chiral centre as our new compound 1 [39].
Furthermore, the AB olefin spin system protons at δH 7.37, d, J = 9.0 Hz and at 6.27 d, J = 9.0 Hz represent H-4 and H-3, respectively, of ring B, which is evident from HMBC correlations depicted in Figure 3. Moreover, the saturated quaternary carbon at δC 87.1 suggested that the hydroxyl group is attached to C-2. Moreover, the tentative assignment of absolute configuration of compound 1 was established by comparing specific rotation data with a similar published compound, berryammone B [39]. As a result, the chemical structure of compound 1 was confirmed to be 2-hydroxy-7-methoxy-2-methylnaphthalen-1(2H)-one, named cichorin D based on the producing plant.
Cichorin E (2) was obtained as a light yellow solid and its molecular formula was established to be C15H16O4 via HRESIMS, and 1D and 2D NMR techniques. The IR spectrum showed the presence of a benzene ring (1610 and 1420 cm−1) and a carbonyl 1610 (cm−1). The 1H NMR spectrum (Table 2) illustrates the presence of three methoxy groups (δH 4.04, 3.99, and 3.82) and a C-acetyl group (2.78 (s, 3H, COMe). Additionally, the 1H NMR spectrum (Table 2) illustrated the presence of four aromatic signals at δH 7.89 (dd, J = 1.5, 8.0 Hz, 1H, H-8), 7.51 (t, J = 8.0 Hz, 1H, H-7), 7.06 (s, 1H, H-2), 6.98 (dd, J = 1.5, 8.0 Hz, H-6). Furthermore COSY correlations, illustrated that the three protons (δH 7.98, 7.51, and 6.98) were coupled to each other through meta (J = 1.5 Hz) and ortho (J = 8.0 Hz) coupling. On the other hand, the fourth aromatic proton at δH 7.06 (s, 1H, H-2) appeared as a singlet. Based on these spectroscopic findings, compound 2 has a naphthalene skeleton bearing a monosubstituted A ring and trisubstituted B ring [40,41,42,43,44].
Table 2.
1H NMR (400 MHz) and 13C NMR (100 MHz) data of cichorins E (2) and F (3) in CDCl3.
| Compound 2 | Compound 3 | |||
|---|---|---|---|---|
| No | 1H NMR | 13C NMR | 1H NMR | 13C NMR |
| 1 | – | 151.3 | 161.7 | |
| 2 | 7.06 (s, 1H) | 103.2 | 6.76 (d, J = 2.0 Hz, 1H) | 105.0 |
| 3 | – | 129.0 | 163.8 | |
| 4 | – | 152.0 | 7.34 (d, J = 2.0 Hz, 1H) | 101.9 |
| 4a | – | 120.1 | 136.6 | |
| 5 | – | 156.8 | 7.81 (s, 1H) | 123.7 |
| 6 | 6.98 (dd, J = 1.5, 8.0 Hz, 1H) | 107.3 | 143.7 | |
| 7 | 7.51 (t, J = 8.0 Hz, 1H) | 128.0 | 139.6 | |
| 8 | 7.89 (dd, J = 1.5, 8.0 Hz, 1H) | 114.8 | 158.1 | |
| 8a | – | 131.1 | 131.5 | |
| 9 | – | – | 181.8 | |
| 9a | – | – | 118.1 | |
| 10 | – | – | 183.8 | |
| 10a | – | – | 125.6 | |
| 1-OMe | 3.99 (s, 3H) | 55.7 | 3.97 (s, 3H) | 56.5 |
| 3-OMe | – | – | 3.95 (s, 3H) | 55.8 |
| 4-OMe | 3.82 (s, 3H) | 63.8 | ||
| 5-Ome | 4.04 (s, 3H) | 56.1 | ||
| 8-OMe | – | – | 3.92 (s, 3H) | 61.5 |
| 6-Me | – | – | 2.39 (s, 3H) | 20.7 |
| 7-Me | – | – | 2.31 (s, 3H) | 12.1 |
| COMe | 2.78 (s, 3H) | 31.4 | ||
| COMe | – | 201.2 |
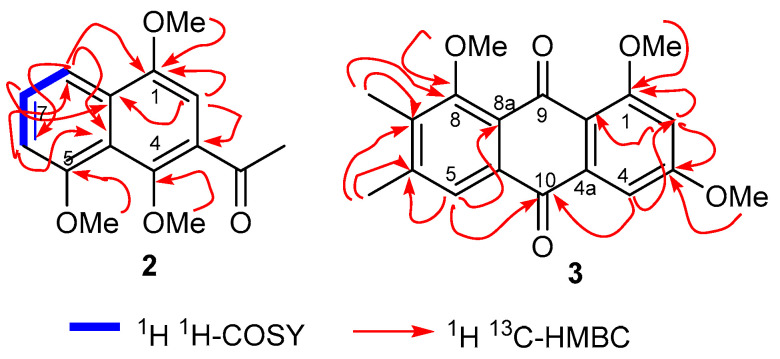
The 13C NMR and DEPT spectra (see Experimental section) demonstrated that 2 comprised of fifteen carbon signals attributed to four aromatic methane carbon signals, three methoxy, one C-acetyl and its carbonyl, and six quaternary carbons. The regiochemistry of the two methoxy groups and one C-acetyl group in ring A was established from the HMBC correlations: 1-OMe to C-1; H-2 to C-1, C-3, C-4, COMe, and C-8a; COMe to C-3; 4-OMe to C-4. Moreover, HMBC correlations of H-8 to C-8a, C-7, and C-6; H-6 to C-5, C-4a, C-7, C-8, and; H-7 to C-5, C-6, C-8, and C-8a; 5-OMe to C-5 established the ring B regiochemistry (Figure 4). The 1H NMR spectral data was similar to that of the synthetic compound 3-acetyl-1,5-dimethoxy-4-naphthol [45], which lacks a methoxy group at C-4. The additional methoxy signal at C-4 i.e., at δH 3.82 (s, 3H; δc: 63.8) further confirmed the structure of naphthalene analog 2. Consequently, the cichorin E (2) structure was established to be 1-(1,4,8-trimethoxynaphthalen-2-yl) ethan-1-one (2).
Figure 4.
Key COSY and HMBC correlations of cichorin E (2) and F (3).
Cichorin F (3) was isolated as a yellow solid, and this molecule has a molecular formula of C19H18O5, as established by the HRESIMS, and 1H and 13C NMR analysis. The 1H NMR spectrum of compound 3 (Table 2) possesses three aromatic protons at δH 7.81 (s, 1H), 7.34 (d, J = 2.0 Hz, 1H), 6.76 (d, J = 2.0 Hz), three methoxy groups at δH 3.97 (s, 3H), 3.95 (s, 3H), 3.92 (s, 3H), and two aromatic methyl groups at δH 2.39 (s, 3H, Me), and 2.31 (s, 3H, Me). Moreover, the 13C NMR spectrum displayed 19 carbon signals, comprising two carbonyls [δC 181.8 (C-9) and 183.8 (C-10)], nine aromatic quaternary carbons along with three aromatic methine, two methyl, and three methoxy carbons.
These spectroscopic characteristics along with the chemical shifts strongly suggest that compound 3 has a highly substituted anthraquinone skeleton [46], possessing three methoxy carbons at δC 55.8, 56.5, 61.5 and two methyl groups at δC 20.7 (6-Me), 12.1 (7-Me). The HMBC spectrum revealed that the proton signals of H-4 (δH 7.34) and H-5 (δH 7.81) possess correlations to the C-10 carbonyl moiety at δC 183.8 (Figure 3) as well as show connectivity to C-4 and C-5, respectively. In addition, the signal at δH 7.34 (H-4) is meta-coupled (J = 2.0 Hz) with the proton signal at δH 6.76 and thus this signal is assigned to H-2 based on the COSY correlation as well. Furthermore, the H-2 HMBC correlations to C-1, C-3, and C-4 lend further credence to this assignment. In addition, the HMBC correlations of the 1-OMe, 3-OMe and 8-OMe to C-1, C-3 and C-8 demonstrated that these three methoxy groups were located at C-1, C-3, and C-8 respectively. Correlations between the methyl signals at δH 2.39 (s, 3H, Me) and 2.31 (s, 3H, Me) to C-6 and C-7 demonstrated that these methyl groups were located at C-6 and C-7, respectively. The NMR data of anthraquinone 3 is similar to the natural 8-demethylated anthraquinone, 1-hydroxy-6,8-dimethoxy-2,3-dimethyl-anthraquinone [47], the synthesis of which has been reported [48]. The additional C-8 methoxy signal at δH 3.92 (s, 3H; δc: 61.5) further confirmed the structure of anthraquinone 3. The 1H,1H-COSY, HSQC, and HMBC spectra (Figure 4) established a complete assignment for anthraquinone 3. As a result, the structure of the new cichorin F (3) was determined to be 1,6,8-trimethoxy-2,3-dimethylanthracene-9,10-dione.
The literature revealed that only a few 1,2-dihydronaphthalane derivatives have been reported to bear a keto group at C-1 and a chiral centre at C-2 (similar as compound 1) having a hydroxyl and alkyl group. Compounds with similar structures include 7-dihydroxy-3(4H)-isocadalen-4-one [49], Berryammone B [39], trichbenzoisochromen A [50], and bombamalone A [51]. Moreover, various naphthalene compounds (aglycones and glycosides) have been reported from plants that have similar substitution patterns, as in compound 2. These compounds are dianellin [52], 5-hydroxydianellin [52], stellalderol [52], parvinaphthols A and B [53], penthosides A and B [54], Eucleanal [40], Eucleanal A and B [41]. Interestingly, anthraquinone 3 has an additional methyl group at C-7 in comparison to trimethoxylated emodin. Moreover few anthraquinones have been reported to have methyl groups at C-6 and C-7 and these compounds are ventinone A [55], 1-hydroxy-6,8-dimethoxy-2,3-dimethylanthraquinone [47], and 1,5-Dihydroxy-8-methoxy-2,3-dimethylanthraquinone [56]. In addition, 2-ethyl-1-hydroxy-8-methoxy-3-methyl anthraquinone and 2-ethyl-1,8-dihydroxy-3-methyl-anthraquinone also have been reported as natural products bearing methyl and ethyl groups at C-6 and C-7 [57]. The three reported natural products viz., β-sitosterol (4) [58], β-sitosterol glucopyranoside (5) [59], and stigmasterol (6) [58] were identified by comparing their NMR data with published data.
3. Biological Activities
3.1. Cytotoxic Effects
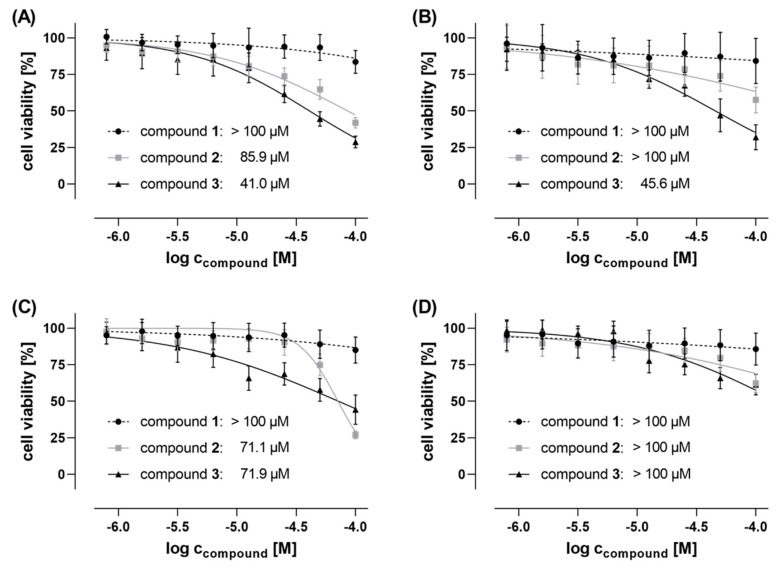
Compounds 1, 2 and 3 were screened for cytotoxic effects on the viability of four different human cancer cell lines, namely prostate adenocarcinoma cells (PC-3), triple-negative breast adenocarcinoma (MDA-MB-468 and MDA-MB-231) cells, and Ewing’s sarcoma SK-N-MC cells. Cell viability and cytotoxicity assays were conducted by using tetrazolium salt MTT and crystal violet (CV), respectively, both with colorimetric read-out after 48 h cell treatment. The saponin digitonin (100 µM), was used as a cytotoxic positive control that was subsequently used for the IC50 analyses to normalize the raw data to 0% remaining cell viability, while untreated cells (negative control) were used for normalization, complying 100% cell viability. Compounds 1, 2, and 3 were tested with concentrations up to 100 µM in order to determine IC50 values. Whereas compound 1 was not active (IC50s > 100 µM) in all tested cell lines, compound 2 exhibited anti-proliferative effects in the triple-negative breast cancer (TNBC) cell line MDA-MB-468 (IC50 = 85.9 µM and 80.0 µM when determined by MTT and CV assay, respectively) and the Ewing’s sarcoma cell line SK-N-MC (IC50 = 71.1 µM and 56.4 µM using MTT and CV assay, respectively) (see Figure 5; Figure S1, and Table 3). Contrarily, other tested cell lines, the second TNBC cell line MDA-MB-231 and the prostate cancer PC-3 cells, were detected to be insensitive against compound 2, with non-detectable IC50 values higher than 100 µM. Highest anti-proliferative and cytotoxic activity, respectively, was found for compound 3. IC50 values in the lower micromolar concentration range were detected in both TNBC cell lines, MDA-MB-468, and MDA-MB-231, as well as in Ewing’s sarcoma SK-N-MC cells (41.0 µM, 45.6 µM, and 71.9 µM, respectively, determined by MTT assay; and 47.1 µM, 49.1 µM and 52.9 µM, respectively, determined by CV assay).
Figure 5.
Cell viability of (A) breast cancer MDA-MB-468 cells, (B) breast cancer MDA-MB-231 cells, (C) Ewing’s sarcoma SK-N-MC cells, and (D) prostate cancer PC-3 cells treated for 48 h with compounds 1 (●), 2 (■), and 3 (▲), respectively, as determined by MTT assay. Data has been normalized to cell viabilities between 0% (represented by digitonin-treated cells; cytotoxic positive control) and 100% (represented by untreated cells; negative control). IC50 values of the anti-proliferative/cytotoxic compound activities have been calculated by using GraphPad Prism 8.0.2 software and are given in micromole concentrations. Data has been collected in two independent biological replicates each with technical quadruplicates.
Table 3.
IC50 values of the anti-proliferative and cytotoxic effects of compounds 1, 2, and 3 as determined after 48 h treatment of breast cancer MDA-MB-468 and MDA-MB-231 cells, Ewing’s sarcoma SK-N-MC cells, and prostate cancer PC-3 cells by using MTT and CV (crystal violet) assays, respectively.
| IC50 Values (after 48 h Treatment) | Assay | Compound 1 | Compound 2 | Compound 3 |
|---|---|---|---|---|
| MDA-MB-468 (breast cancer) | MTT | >100 µM | 85.9 µM | 41.0 µM |
| CV | >100 µM | 80.0 µM | 47.7 µM | |
| MDA-MB-231 (breast cancer) | MTT | >100 µM | >100 µM | 45.6 µM |
| CV | >100 µM | >100 µM | 49.1 µM | |
| SK-N-MC (Ewing’s sarcoma) | MTT | >100 µM | 71.1 µM | 71.9 µM |
| CV | >100 µM | 56.4 µM | 52.9 µM | |
| PC-3 (prostate cancer) | MTT | >100 µM | >100 µM | >100 µM |
| CV | >100 µM | >100 µM | >100 µM |
In all cases, the IC50 values determined by both MTT (assaying the metabolic activity of vital cells) and crystal violet (CV) assay (quantifying the total DNA of the remaining population of viable cells) were in the same concentration range, supporting the validity of these data. Interestingly, the detected anticancer activities are remarkable since both compounds 2 and 3 are much less active in PC-3 cells, a cell line with higher sensitivity against a broader panel of anticancer agents, but show activities (IC50s) in the lower micromolar range against breast cancer cell lines MDA-MB-468 and, at least compound 3, MDA-MB-231, as well as against SK-N-MC cells. This indicates to some extent a cancer cell line selectivity of compounds 2 and 3, which has to be investigated in more depth in future studies. Moreover, anticancer activities against triple-negative breast cancer (TNBC) cells and Ewing’s sarcoma cells are of particular pharmaceutical interest, since TNBCs and orphan Ewing’s sarcoma still lack satisfactory therapeutic options.
3.2. Anthelmintic Effects
C. intybus has attracted interest owing to its anthelmintic effects on gastrointestinal (GI) nematodes in monogastrics and ruminants [60]. Scientific evidence has demonstrated that chicory-rich diets can potently decrease infections with GI abomasal nematodes in cattle and sheep in vivo [60]. For this reason, compounds 1–3 have been tested for their anthelmintic effects on Caenorhabditis elegans. The results showed that compound 1 reduced motility of all worms, even though mortality (10%) was low. On the other hand, compound 2 did not reduce the motility of all worms as 1 did, but it had a higher mortality (24%). Compound 3 had no noticeable effects on worms.
4. Conclusions
Phytochemical investigations of C. intybus provided two new naphthalane derivatives viz., cichorins D (1) and E (2) along with one new anthraquinone, cichorin F (3). Their structures were established via extensive spectroscopic investigations, including 1D and 2D NMR, and ESIMS techniques. Compound 1 is a 1,2-dihydronapthalane derivative bearing a C-1 keto group; compound 2 is a trimethoxy substituted naphthalene derivative; and cichorin F (3) is a highly substituted anthraquinone having three methoxy and two aromatic methyl groups. Cichorin E (2) was shown to possess weak cytotoxic effects on MDA-MB-468 and SK-N-MC cancer cells, while cichorin F (3) demonstrated weak cytotoxic effects on MDA-MB-468, MDA-MB-231, and SK-N-MC.
5. Material and Methods
5.1. General Experimental Procedures
IR spectra were recorded using the Nicolet-510P spectrophotometerc (Thermo Fisher Scientific, Waltham, MA, USA); vmax in cm−1. The 1H NMR spectra were recorded on Bruker AMX-400 (Bruker Biospin Corp., Billerica, MA, USA) instruments using TMS as an internal reference. The chemical shifts are reported in parts per million (δ) while the coupling constants (J) are reported in hertz. The 13C NMR spectra were recorded at 100 MHz on the same instrument. Column chromatography (CC) was carried out using silica gel (70–230 and 230–400 mesh; E-Merck, Darmstadt, Germany) and Sephadex LH-20 (Amersham Biosciences AB, Uppsala, Sweden). Aluminum sheets precoated with silica gel 60 F 254 (0.2 mm thick; E-Merck) were used for TLC to check the purity of the compounds and were visualized under UV light (254 and 366 nm), followed by ceric sulfate as the spray reagent.
5.2. Plant Material
Whole plant material of C. intybus was collected from Parachinar, KPK, Pakistan, in July 2017, and identified by Dr Wahid Hussain (plant taxonomist) at the Department of Botany, Post Graduate College, Parachinar, Pakistan. A voucher specimen (No. B.MF Khan 20. GPGC PCR) has been deposited at the herbarium of the Botany Department, Post Graduate College, Parachinar, Pakistan.
5.3. Extraction and Isolation
The air-dried whole plant amterials (3 kg) of C. intybus was extracted with EtOH at room temperature. The whole ethanolic extract was evaporated to dryness, yielding 20 g of residue. The whole extract was subjected to Column Chromatography (CC) (silica gel, n-hexane, n-hexane–EtOAc and EtOAc) yielding 7 fractions (F1-7). Fraction F4 (230 mg) was rechromatographed and eluted with a mixture of n-hexane–EtOAc (1.5:8.5) providing cichorins D (1; 3.0 mg) and E (2; 5.0 mg). Fraction F3 (98 mg) was rechromatographed and eluted with n-hexane–EtOAc (7.5:2.5) to afford cichorin F (3; 5.4 mg) and β-sitosterol β-glucopyranoside (5; 11.3 mg). On the other hand, β-sitosterol (4) and stigmasterol (6) were purified from fraction F2 by rechromatographing this fraction and eluting with n-hexane–EtOAc (1.2:8.8).
5.3.1. Cichorin D (1)
While solid; [α]D25 = +11.2 (c 0.26, CH2Cl2); IR (KBr) vmax: 3410, 2950, 1600, 1420, 1000 cm−1; For 1H 13C NMR: see Table 1; ESI-MS (m/z): 227.2 [M + Na]+, C12H12NaO3; HRESIMS: m/z 205.0862 [M + H]: (calcd for C12H13O3, 205.0865).
5.3.2. Cichorin E (2)
Slightly Yellow gummy solid; IR (KBr) vmax: 1610, 1580, 1425, 1000 cm−1; For 1H 13C NMR: see Table 2; ESI-MS (m/z): 283.1 [M + Na]+, C15H16NaO4; HRESIMS: m/z 261.1137 [M + H]: (calcd for C15H17O4, 261.1127).
5.3.3. Cichorin F (3)
Yellow solid; IR (KBr) vmax: 1600, 1420, 1000 cm−1; For 1H 13C NMR: see Table 2; ESI-MS (m/z): 349.2 [M + Na]+, C19H18NaO5; HRESIMS: m/z 327.1238 [M + H]: (calcd for C19H19O5, 327.1232).
5.4. Cell Culture
Four human cancer cell lines were used to investigate the cytotoxicity and anticancer activity of compounds 1–3. All cell lines were purchased from ATCC (Manassas, VA, USA). The prostate adenocarcinoma cell line PC-3 and triple-negative breast adenocarcinoma (TNBC) cell line MDA-MB-231 were cultured in RPMI 1640 supplemented with 2 mM L-glutamine and 10% heat-inactivated FCS. DMEM supplemented with 2 mM L-glutamine and 10% heat-inactivated FCS was used for the triple-negative breast adenocarcinoma cell line MDA-MB-468 and the Ewing’s sarcoma cell line SK-N-MC. The cells were routinely cultured in T-75 culture flasks in a humidified atmosphere with 5% CO2 at 37 °C to reach subconfluency (~70–80%) prior to subsequent usage or subculturing. The adherent cells were rinsed with PBS and detached by using trypsin/EDTA (0.05% in PBS) prior to cell passaging and seeding. Basal cell culture media, FCS, l-glutamine, PBS and trypsin/EDTA for cell culturing were purchased from Capricorn Scientific GmbH (Ebsdorfergrund, Germany). The culture flasks, multi-well plates, and further cell culture plastics were purchased from TPP (Trasadingen, Switzerland) and Greiner Bio-One GmbH (Frickenhausen, Germany), respectively.
Cytotoxic Activity—In Vitro Cell Viability Assays
Anti-proliferative and cytotoxic effects of the compounds were investigated by performing colorimetric MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) and CV (crystal violet)-based cell viability assays (Sigma-Aldrich, Taufkirchen, Germany), respectively [61,62]. For this purpose, cells were seeded in low densities in 96-well plates (6000 cells/100 µL/well for both PC3 and MDA-MB-468; 4000 cells/100 µL/well for MDA-MB-231; and 12,000 cells/100 µL/well for SK-N-MC) using the aforementioned cell culture media. The cells were allowed to adhere for 24 h, followed by the 48 h compound treatment. Based on 20 mM DMSO stock solutions, the compounds were serially diluted in standard growth media to reach final concentrations of 100, 50, 25, 12.5, 6.25, 3.125, 1.56, and 0.78 µM for cell treatment. For control measures, cells were treated in parallel with 100 µM digitonin (positive control, for data normalization set to 0% cell viability). Each data point was determined in technical quadruplicates and two independent biological replicates. As soon as the 48 h incubation was finished, cell viability was measured.
For the MTT assay, cells were washed once with PBS, followed by incubation with MTT working solution (0.5 mg/mL MTT in culture medium) for 1 h under standard growth conditions. After discarding the MTT solution, DMSO was added in order to dissolve the formed formazan, followed by measuring formazan absorbance at 570 nm, and additionally at the reference/background wavelength of 670 nm, by using a SpectraMax M5 multi-well plate reader (Molecular Devices, San Jose, CA, USA).
For the CV assay, cells were washed once with PBS and fixed with 4% paraformaldehyde (PFA) for 20 min at room temperature (RT). After discarding the PFA solution, the cells were left to dry for 10 min and then stained with 1% crystal violet solution for 15 min at RT. The cells were washed with water and were dried overnight at RT. Afterwards, acetic acid (33% in aqua bidest) was added to the stained cells and absorbance was measured at 570 nm and 670 nm (reference wavelength) using a SpectraMax M5 multi-well plate reader (Molecular Devices, San Jose, CA, USA). For data analyses, GraphPad Prism version 8.0.2 and Microsoft Excel 2013 were used.
5.5. Anthelmintic Activity
Anthelmintic activity was performed according to a previously published procedure [63].
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No. (RG-1441-486).
Supplementary Materials
The following are available online: copies of 1H, 13C and 2D NMR spectra of compounds 1–3.
Author Contributions
Conceptualization, M.F.K. and H.H.; methodology, M.F.K., F.A.N. and O.M.N.; validation, I.A.; resources, W.H.; writing—original draft preparation, M.F.K.; writing—review and editing, I.R.G.; supervision, H.H.; project administration, M.F.K. and H.H.; funding acquisition, M.F.K.; R.R. and M.S. did the cytotoxic activity and M.D. worked on the anthelmintic effects. O.A.M.B., R.U. and S.H.A.: data curation and writing—review and editing. N.A.A.: investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia through research group No. (RG-1441-486).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Subhash C., Mukesh K., Pradeep D., Ku A. Studies on Industrial Importance and Medicinal Value of Chicory Plant (Cichorium intybus L.) Int. J. Adv. Res. 2016;4:1060–1071. [Google Scholar]
- 2.Liu Q., Chen Y., Shen C., Xiao Y., Wang Y., Liu Z., Liu X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-B. Faseb J. 2017;31:1494–1507. doi: 10.1096/fj.201601071R. [DOI] [PubMed] [Google Scholar]
- 3.El-Sayed Y.S., Lebda M.A., Mohammed H., Neoman S.A. Chicory (Cichorium intybus L.) Root Extract Regulates the Oxidative Status and Antioxidant Gene Transcripts in CCl4-Induced Hepatotoxicity. PLoS ONE. 2015;10:e0121549. doi: 10.1371/journal.pone.0121549. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Pushparaj P.N., Low H.K., Manikandan J., Tan B.K.H., Tan C.H. Anti-diabetic effects of Cichorium intybus in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2007;111:430–434. doi: 10.1016/j.jep.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Hussain W., Badshah L., Ullah M., Ali M., Ali A., Hussain F. Quantitative study of medicinal plants used by the communities residing in Koh-e-Safaid Range, northern Pakistani-Afghan borders. J. Ethnobiol. Ethnomed. 2018;14:30. doi: 10.1186/s13002-018-0229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rammal H., Younos C., Bouayed J., Chakou A., Bedouhene S., Soulimani R. Aperçu ethnobotanique et phytopharmacologique sur Cichorium intybus L. Phytotherapie. 2008;6:184–186. doi: 10.1007/s10298-008-0313-3. [DOI] [Google Scholar]
- 7.Wang Y., Lin Z., Zhang B., Jiang Z., Guo F., Yang T. Cichorium intybus L. Extract Suppresses Experimental Gout by Inhibiting the NF-κB and NLRP3 Signaling Pathways. Int. J. Mol. Sci. 2019;20:4921. doi: 10.3390/ijms20194921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Migliorini A.A., Piroski C.S., Daniel T.G., Cruz T.M., Escher G.B., do Carmo M.A.V., Azevedo L., Marques M.B., Granato D., Rosso N.D. Red Chicory (Cichorium intybus) Extract Rich in Anthocyanins: Chemical Stability, Antioxidant Activity, and Antiproliferative Activity In Vitro. J. Food Sci. 2019;84:990–1001. doi: 10.1111/1750-3841.14506. [DOI] [PubMed] [Google Scholar]
- 9.Chandra K., Jain V., Jabin A., Dwivedi S., Joshi S., Ahmad S., Jain S.K. Effect of Cichorium intybus seeds supplementation on the markers of glycemic control, oxidative stress, inflammation, and lipid profile in type 2 diabetes mellitus: A randomized, double-blind placebo study. Phytother. Res. 2020;34:1609–1618. doi: 10.1002/ptr.6624. [DOI] [PubMed] [Google Scholar]
- 10.Imam K.M.S.U., Xie Y., Liu Y., Wang F., Xin F. Cytotoxicity of Cichorium intybus L. metabolites (review) Oncol. Rep. 2019;42:2196–2212. doi: 10.3892/or.2019.7336. [DOI] [PubMed] [Google Scholar]
- 11.Kashani L.M.T., Majdzadeh M., Khanavi M., Taghizadeh M., Sadati N., Kahkeshani N., Vatankhah M., Ostadecfbd S.N. Cytotoxic activity of selected Iranian traditional medicinal plants on colon, colorectal and breast cancer cell lines. Arch. Breast Cancer. 2014;1:95–98. [Google Scholar]
- 12.Pena-Espinoza M., Valente A.H., Bornancin L., Simonsen H.T., Thamsborg S.M., Williams A.R., Lopez-Munoz R. Anthelmintic and metabolomic analyses of chicory (Cichorium intybus) identify an industrial by-product with potent in vitro antinematodal activity. Vet. Parasitol. 2020;280:109088. doi: 10.1016/j.vetpar.2020.109088. [DOI] [PubMed] [Google Scholar]
- 13.Sinkovic L., Jamnik P., Korosec M., Vidrih R., Meglic V. In-vitro and in-vivo antioxidant assays of chicory plants (Cichorium intybus L.) as influenced by organic and conventional fertilizers. BMC Plant Biol. 2020;20:36. doi: 10.1186/s12870-020-2256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma M., Afaque A., Dwivedi S., Jairajpuri Z.S., Shamsi Y., Khan M.F., Khan M.I., Ahmed D. Cichorium intybus attenuates streptozotocin induced diabetic cardiomyopathy via inhibition of oxidative stress and inflammatory response in rats. Interdiscip. Toxicol. 2019;12:111–119. doi: 10.2478/intox-2019-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arshad A., Hussain A., Pervaiz S. Evaluation of hepatoprotective effects of silybum marianum and Cichorium intybus extracts. Indo Am. J. Pharm. Sci. 2019;6:12176–12186. [Google Scholar]
- 16.Rahimullah T.G., Shah S.T., Rehman M., Hayat A. Phytochemical and antibacterial screening of Cichorium intybus seeds use in traditional medicine systems in Pakistan. Int. J. Basic Med. Sci. Pharm. 2018;8:46–49. [Google Scholar]
- 17.Woolsey I.D., Valente A.H., Williams A.R., Thamsborg S.M., Simonsen H.T., Enemark H.L. Anti-protozoal activity of extracts from chicory (Cichorium intybus) against Cryptosporidium parvum in cell culture. Sci. Rep. 2019;9:20414. doi: 10.1038/s41598-019-56619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jasim R.S. Antioxidant, antimicrobial activities and phytochemical constituents of Cichorium intybus L. aerial parts. Int. J. Bot. 2018;14:24–29. doi: 10.3923/ijb.2018.24.29. [DOI] [Google Scholar]
- 19.Dahab R., Afifi F. Antiproliferative activity of selected medicinal plants of Jordan against a breast adenocarcinoma cell line (MCF7) Sci. Pharma. 2007;75:121–136. doi: 10.3797/scipharm.2007.75.121. [DOI] [Google Scholar]
- 20.Conforti F., Ioele G., Statti G., Marrelli M., Ragno G., Menichini F. Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem. Toxicol. 2008;46:3325–3332. doi: 10.1016/j.fct.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Saleem M., Abbas K., Naseer F., Ahmad M., Syed1 N.H., Javed F., Hussain K., Asima S. Anticancer activity of n-hexane extract of Cichorium intybus on lymphoblastic leukemia cells (Jurkat cells) Afr. J. Plant Sci. 2014;8:315–319. [Google Scholar]
- 22.Hazra B., Sarkar R., Bhattacharyya S., Roy P. Tumour inhibitory activity of chicory root extract against Ehrlich ascites carcinoma in mice. Fitoterapia. 2002;73:730–733. doi: 10.1016/S0367-326X(02)00232-0. [DOI] [PubMed] [Google Scholar]
- 23.Nawab A., Yunus M., Mahdi A.A., Gupta S. Evaluation of anti-cancer properties of medicinal plants from the Indiansub-continent. Mol. Cell. Pharmacol. 2011;3:21–29. [Google Scholar]
- 24.Mehrandish R., Awsat-Mellati A., Rahimipour A., Dehghan-Nayeri N. Anti-cancer activity of methanol extracts of Cichorium intybus on human breast cancer SKBR3 cell line. Razavi. Int. J. Med. 2017;5:e38369. [Google Scholar]
- 25.Esmaeilbeig M., Kouhpayeh S.A., Amirghofran Z. An inves-tigation of the growth inhibitory capacity of several medicinal plants from Iran on tumor cell lines. Iran J. Cancer Prev. 2015;8:e4032. doi: 10.17795/ijcp-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou C.X., Zou L., Zhao Z.Z., Zhu H., He Q.J., Yang B., Gan L.S. Terpenoids from Cichorium intybus. Nat. Prod. Commun. 2012;7:971–972. doi: 10.1177/1934578X1200700801. [DOI] [PubMed] [Google Scholar]
- 27.Seto M., Miyase T., Umehara K., Ueno A., Hirano Y., Otani N. Sesquiterpene lactones from Cichorium endivia L. and C. intybus L. and cytotoxic activity. Chem. Pharm. Bull. 1988;36:2423–2429. doi: 10.1248/cpb.36.2423. [DOI] [PubMed] [Google Scholar]
- 28.Lee K.T., Kim J.I., Park H.J., Yoo K.O., Han Y.N., Miyamoto K. Differentiation-inducing effect of magnolialide, a 1 beta-hydroxyeudesmanolide isolated from Cichorium intybus, on human leukemia cells. Biol. Pharm. Bull. 2000;23:1005–1007. doi: 10.1248/bpb.23.1005. [DOI] [PubMed] [Google Scholar]
- 29.Foster J.G., Cassida K.A., Turner K.E. In vitro analysis of the anthelmintic activity of forage chicory (Cichorium intybus L.) sesquiterpene lactones against a predominantly Haemonchus contortus egg population. Vet. Parasitol. 2011;180:298–306. doi: 10.1016/j.vetpar.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Athanasiadou S., Gray D., Younie D., Tzamaloukas O., Jackson F., Kyriazakis I. The use of chicory for parasite control in organic ewes and their lambs. Parasitology. 2007;134:299–307. doi: 10.1017/S0031182006001363. [DOI] [PubMed] [Google Scholar]
- 31.Marley C.L., Cook R., Keatinge R., Barrett J., Lampkin N.H. The effect of birdsfoot trefoil (Lotus corniculatus) and chicory (Cichorium intybus) on parasite intensities and performance of lambs naturally infected with helminth parasites. Vet. Parasitol. 2003;112:147–155. doi: 10.1016/S0304-4017(02)00412-0. [DOI] [PubMed] [Google Scholar]
- 32.Tzamaloukas O., Athanasiadou S., Kyriazakis I., Jackson F., Coop R.L. The consequences of short-term grazing of bioactive forages on established adult and incoming larvae populations of Teladorsagia circumcincta in lambs. Int. J. Parasitol. 2005;35:329–335. doi: 10.1016/j.ijpara.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Molan A.L., Duncan A.J., Barry T.N., McNabb W.C. Effects of condensed tannins and crude sesquiterpene lactones extracted from chicory on the motility of larvae of deer lungworm and gastrointestinal nematodes. Parasitol. Int. 2003;52:209–218. doi: 10.1016/S1383-5769(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 34.Carazzone C., Mascherpa D., Gazzani G., Papetti A. Iden-tification of phenolic constituents in red chicory salads (Cichorium intybus) by high-performance liquid chromatographywith diode array detection and electrospray ionisation tandemmass spectrometry. Food Chem. 2013;138:1062–1071. doi: 10.1016/j.foodchem.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 35.Kisiel W., Zielinska K. Guaianolides from Cichorium intybus and structure revision of Cichorium sesquiterpene lactones. Phytochemistry. 2001;57:523–527. doi: 10.1016/S0031-9422(01)00072-3. [DOI] [PubMed] [Google Scholar]
- 36.Krebsky E.O., Geuns J.M.C., de Proft M. Polyamines and sterois in Cichorium heads. Phytochemistry. 1999;50:549–553. doi: 10.1016/S0031-9422(98)00555-X. [DOI] [Google Scholar]
- 37.Hussain H., Hussain J., Saleem M., Miana G.A., Riaz M., Krohn K., Anwar S. Cichorin A: A new benzo-isochromene from Cichorium intybus. J. Asian Nat. Prod. Res. 2011;13:566–569. doi: 10.1080/10286020.2011.573789. [DOI] [PubMed] [Google Scholar]
- 38.Hussain H., Hussain J., Ali S., Al-Harrasi A., Saleem M., Miana G.A., Riaz M., Anwar S., Hussain S., Ali L. Cichorins B and C: Two new benzo-isochromenes from Cichorium intybus. J. Asian Nat. Prod. Res. 2012;14:297–300. doi: 10.1080/10286020.2011.652953. [DOI] [PubMed] [Google Scholar]
- 39.Chou T.H., Chien S.K., Hwang T.L., Wei D.C., Chen I.S., Sung P.J., Cheng M.J., Yang S.Z., Chang K.M., Chen J.J. Orthoquinone and naphthalenone derivatives from Berrya ammonilla and their anti-inflammatory activity. Planta Med. 2012;78:919–925. doi: 10.1055/s-0031-1298460. [DOI] [PubMed] [Google Scholar]
- 40.Ng’ang’a M.M., Hussain H., Chhabra S., Langat-Thoruwa C., Krohn K., Hussain J., Al-Harrasi A., Green I. Eucleanal: A newnaphthalene derivative from Euclea divinorum. Nat. Prod. Commun. 2012;7:193–194. [PubMed] [Google Scholar]
- 41.Ng’ang’a M.M., Hussain H., Chhabra S., Langat-Thoruwa C., Krohn K., Hussain J., Al-Harrasi A., Green I. Eucleanal A and B: Two newnapthalene derivatives from Euclea divinorum. Chin. Chem. Lett. 2012;23:576–578. doi: 10.1016/j.cclet.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 42.Mahabusarakam W., Hemtasin C., Chakthong S., Voravuthikunchai S.P., Olawumi I.B. Naphthoquinones, Anthraquinones and Naphthalene Derivatives from the Bulbs of Eleutherine americana. Planta Med. 2010;76:345–349. doi: 10.1055/s-0029-1186143. [DOI] [PubMed] [Google Scholar]
- 43.Lin C.N., Lu C.M., Lin H.C., Ko F.N., Teng C.M. Novel antiplatelet naphthalene from Rhamnus nakaharai. J. Nat. Prod. 1995;58:1934–1940. doi: 10.1021/np50126a023. [DOI] [PubMed] [Google Scholar]
- 44.Ganapaty S., Thomas P.S., Karagianis G., Waterman P.G., Brun R. Antiprotozoal and cytotoxic naphthalene derivatives from Diospyros assimilis. Phytochemistry. 2006;67:1950–1956. doi: 10.1016/j.phytochem.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 45.Uno H. Allylation of 2-Alkanoyl 1,4-Quinones with Allylsilanes and Allylstannanes. Efficient Synthesis of Pyranonaphthoquinone Antibiotics. J. Org. Chem. 1986;51:350–358. doi: 10.1021/jo00353a015. [DOI] [Google Scholar]
- 46.Zhou X.M., Zheng C.J., Chen G.Y., Song X.P., Han C.R., Li G.N., Fu Y.H., Chen W.H., Niu Z.G. Bioactive Anthraquinone Derivatives from the Mangrove-Derived Fungus Stemphylium sp. 33231. J. Nat. Prod. 2014;77:2021–2028. doi: 10.1021/np500340y. [DOI] [PubMed] [Google Scholar]
- 47.Verma R.P., Ambasta B.K., Prasad G., Sinha K.S. Isolation and characterization of a new anthraquinone derivative from Cassia grandis Linn. J. Indian Chem. Soc. 1997;74:428. [Google Scholar]
- 48.Jin H.S., Zhao L.M. A contribution to the study of the modified Marschalk reaction: Hydroxymethylation of 6,8-O-dimethyl emodin. Chin. Chem. Lett. 2010;21:568–571. doi: 10.1016/j.cclet.2010.01.013. [DOI] [Google Scholar]
- 49.Delgado G., Olivares M.S., Cha’vez M.I., Ramırez-Apan T., Linares E., Bye R., Espinosa-Garcıa F.J. Antiinflammatory Constituents from Heterotheca inuloides. J. Nat. Prod. 2001;64:861–864. doi: 10.1021/np0005107. [DOI] [PubMed] [Google Scholar]
- 50.Pang X., Lin X., Tian Y., Liang R., Wang J., Yang B., Zhou X., Kaliyaperumal K., Luo X., Tu Z., et al. Three new polyketides from the marine sponge-derived fungus Trichoderma sp. SCSIO41004. Nat. Prod. Res. 2018;32:105–111. doi: 10.1080/14786419.2017.1338286. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X., Zhu H., Zhang S., Yu Q., Xuan L. Sesquiterpenoids fromBombax malabaricum. J. Nat. Prod. 2007;70:1526–1528. doi: 10.1021/np070256j. [DOI] [PubMed] [Google Scholar]
- 52.Dias D.A., Silva C.A., Urban S. Naphthalene Aglycones and Glycosides from the Australian Medicinal Plant, Dianella callicarpa. Planta Med. 2009;75:1442–1447. doi: 10.1055/s-0029-1185724. [DOI] [PubMed] [Google Scholar]
- 53.Abdissa N., Pan F., Gruhonjic A., Gräfenstein J., Fitzpatrick P.A., Landberg G., Rissanen K., Yenesew A., Erdélyi M. Naphthalene Derivatives from the Roots of Pentas parvifolia and Pentas bussei. J. Nat. Prod. 2016;79:2181–2187. doi: 10.1021/acs.jnatprod.6b00178. [DOI] [PubMed] [Google Scholar]
- 54.Thanh N.V., Thao N.P., Dat L.D., Huong P.T.T., Lee S.H., Jang H.D., Cuong N.X., Nam N.H., Kiem P.V., Minh C.V., et al. Two new naphthalene glucosides and other bioactive compounds from the carnivorous plant Nepenthes mirabilis. Arch. Pharm. Res. 2015;38:1774–1782. doi: 10.1007/s12272-015-0576-9. [DOI] [PubMed] [Google Scholar]
- 55.Periyasamy K., Kaliyaperumal S. Ethnobotanical, Phytochemical and Pharmceutical Studies of Medicinal Plant, Ventilago Maderaspatana Gaertn (Red Creeper): A Review. Int. J. Curr. Pharm. Res. 2016;8:16–18. [Google Scholar]
- 56.Ambasta B.K., Prasad G., Sinha K.S., Verma R.P. An anthraquinone derivative from Cassia grandis Linn. Indian J. Chem. B. 1996;35:990–991. [Google Scholar]
- 57.Xuea C.M., Tianb L., Linab W.H., Deng Z.W. Anthraquinone derivatives from Micromonospora rhodorangea. Nat. Prod. Res. 2009;23:533–538. doi: 10.1080/14786410600898961. [DOI] [PubMed] [Google Scholar]
- 58.Habib M.R., Nikkon F., Rahman M., Haque M.E., Karim M.R. Isolation of stigmasterol and beta-sitosterol from methanolic extract of root bark of Calotropis gigantea (Linn) Pak. J. Biol. Sci. 2007;10:4174–4176. doi: 10.3923/pjbs.2007.4174.4176. [DOI] [PubMed] [Google Scholar]
- 59.Seo S., Tomita Y., Tori K., Yoshimura Y. Determination of the absolute configuration of a secondary hydroxy group in a chiral secondary alcohol using glycosidation shifts in carbon-13 nuclear magnetic resonance spectroscopy. J. Am. Chem. Soc. 1978;100:3331–3339. doi: 10.1021/ja00479a014. [DOI] [Google Scholar]
- 60.Peña-Espinoza M., Valente A., Thamsborg S.M., Simonsen H.T., Boas U., Enemark H.L., Lopez-Munoz R., Williams A. Antiparasitic activity of chicory (Cichorium intybus) and the role of its natural bioactive compounds: A review. Parasites Vectors. 2018;11:475. doi: 10.1186/s13071-018-3012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seixas N., Ravanello B.B., Morgan I., Kaluđerović G.N., Wessjohann L.A. Chlorambucil Conjugated Ugi Dendrimers with PAMAM-NH2 Core and Evaluation of Their Anticancer Activity. Pharmaceutics. 2019;11:59. doi: 10.3390/pharmaceutics11020059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smolko L., Smolková R., Samoľová E., Morgan I., Saoud M., Kaluđerović G.N. Two isostructural Co (II) flufenamato and niflumato complexes with bathocuproine: Analogues with a different cytotoxic activity. J. Inorg. Biochem. 2020;210:111160. doi: 10.1016/j.jinorgbio.2020.111160. [DOI] [PubMed] [Google Scholar]
- 63.Dos Santos C.H.C., de Carvalho M.G., Franke K., Wessjohann L. Dammarane-type triterpenoids from the stem of Ziziphus glaziovii Warm. (Rhamnaceae) Phytochemistry. 2019;162:250–259. doi: 10.1016/j.phytochem.2019.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.