Abstract
Background:
Neighborhood deprivation adversely effects neurodevelopment and cognitive function; however, mechanisms remain unexplored. Neighborhood deprivation could be particularly impactful in late childhood/early adolescence, in neural regions with protracted developmental trajectories, e.g., prefrontal cortex (PFC).
Methods:
The Adolescent Brain Cognitive Development (ABCD) study recruited 10,205 youth. Geocoded residential history was used to extract individual neighborhood characteristics. A general cognitive ability index and MRI scans were completed. Associations with neurocognition were examined. The relation of PFC surface area and cortical thickness to neighborhood deprivation was tested. PFC subregions and asymmetry, with putative differential environmental susceptibility during key developmental periods, were explored. Analyses tested PFC area as a possible mediating mechanism.
Results:
Neighborhood deprivation predicted neurocognitive performance (β = −0.11), even after accounting for parental education and household income (β = −0.07). Higher neighborhood deprivation related to greater overall PFC surface area (η2p = 0.003), and differences in leftward asymmetry were observed for area (η2p = 0.001), and thickness (η2p = 0.003). Subregion analyses highlighted differences among critical areas that are actively developing in late childhood/early adolescence and are essential to modulating high order cognitive function. These included orbitofrontal, superior frontal, rostral middle frontal, and frontal pole regions (Cohen’s d = 0.03–0.09). PFC surface area partially mediated the relation between neighborhood deprivation and neurocognition.
Discussion:
Neighborhood deprivation related to cognitive function (a foundational skill tied to a range of lifetime outcomes) and PFC morphology, with evidence found for partial mediation of PFC on neurocognitive function. Results inform public health conceptualizations of development and environmental vulnerability.
Keywords: Neighborhood deprivation, Prefrontal cortex, Neurodevelopment, Environmental vulnerability, Cognition
1. Introduction
In recent years, seminal investigations have aimed to characterize phenomenologically distinct environmental influences on neurocognition during development (Farah et al., 2006; Laus et al., 2011; McLaughlin et al., 2014a; Noble et al., 2005; Pechtel and Pizzagalli, 2011; Sirin, 2005; Wu et al., 2015). Exposure to deprivation, defined as environments that do not provide needs or resources necessary for healthy development, has been identified as a critical environmental vulnerability factor (Cubbin and Winkleby, 2005; Jessop, 1992; Krishnadas et al., 2013; Matheson et al., 2008; McLaughlin et al., 2014b; Verhaeghe and Tampubolon, 2012). Animal and human studies on exposure to deprivation at the individual-level have yielded compelling evidence to its impact on both cognitive function and brain morphology (Akman et al., 2004; Beckett et al., 2006; Gee et al., 2013; Mackes et al., 2020; McLaughlin et al., 2014b; Mehta et al., 2009; Sheridan et al., 2012; Wiesel and Hubel, 1965). Largely these studies have examined individual cases of deprivation (e.g., parental neglect), but broad societal environments are also potential sources of deprivation (e.g., neighborhood poverty). Fewer studies, have broadened the scope of deprivation to considering structural, or systems level exposure (Wu et al., 2015). Local, neighborhood level, and even country-level structural characteristics (such as neighborhood socioeconomic status and national income inequality) have been consistently shown to impact individual health and development (Forsberg et al., 2018; Jaffe et al., 2005; Matheson et al., 2008; Tuliani et al., 2017). Yet, few investigations have sought to clarify whether brain morphology neural gray matter features can be impacted as a result of systems-level exposure to environmental vulnerability factors such as deprivation. Likewise, associations with general and specific functioning, such as cognitive performance, have yet to be integrated into these conceptualizations for key neurodevelopmental stages. The present investigation utilized a large, geographically diverse, nationally representative sample of youth to clarify possible relations between structural factors, cognitive and neural features. Identifying mechanisms through which neighborhood deprivation could be impactful is a necessary step toward identifying targets for epidemiological and public health models of healthy development.
Deprivation environments theoretically comprise settings in environmental resources are lacking (McLaughlin et al., 2014a); these can include scarcity of educational, cognitive, economic or health resources, which could lead to negative effects on neurodevelopment (Akman et al., 2004; Diamond et al., 1972; Globus et al., 1973). Animal and human research on deprivation environments has to date largely focused on individual-level exposure to deprivation (i.e. individual socioeconomic status in human studies, experimental manipulations of enriched versus deprived environments in animal studies) (Akman et al., 2004; Diamond et al., 1972; Farah et al., 2006; Globus et al., 1973; Hackman et al., 2010; Lawson et al., 2017; Mackes et al., 2020; Mackey et al., 2015; Noble et al., 2005). Although these investigations have yielded valuable insights as to the possible effect of deprivation on neural function, deprivation can exist at a number of levels of a social structure. Exposure can range from individual-level deprivation (e.g. deprivation inside the immediate home), to deprivation of the broader environmental context (e.g. neighborhood features).
Research on the broader environmental and social context (i.e. structural characteristics) has long highlighted the necessity of taking into account systems-level factors potentially impacting healthy development (Bronfenbrenner, 1994; Glass and McAtee, 2006). This structural approach complements the valuable literature on individual-level factors (e.g., childhood institutionalization), generalizing the scope of what we consider to be influential to relatively distal factors (e.g., neighborhood resource scarcity). High deprivation environments occur at the systems level when various socioeconomic needs or resources are lacking, which may possibly hinder species-normative development. Existing literature exploring neighborhood deprivation shows evidence that it could be associated with a host of adverse health outcomes, including increased mortality rates (Forsberg et al., 2018; Jaffe et al., 2005; Matheson et al., 2008; Tuliani et al., 2017). Exposure during childhood could have particularly pervasive effects lasting throughout the lifetime (Mensah and Hobcraft, 2008). Neighborhood deprivation could be impactful on functional outcomes (such as neurocognitive function) and neural features, though these questions remain unexplored.
Lower neurocognitive function constitutes a foundational marker strongly related to a host of life outcomes (Aichele et al., 2016), and has a detrimental impact on health that is observable as early as adolescence (Sörberg et al., 2014). Research looking into individual-level deprivation exposure supports the notion that deprivation relates to lower cognitive function in the case of childhood institutionalization (Beckett et al., 2006) and socioeconomic status (Noble et al., 2005). Although evidence exists that neighborhood deprivation impacts cognition in older adults (Lang et al., 2008; McCann et al., 2018; Sheffield and Peek, 2009; Wu et al., 2015; Zeki Al Hazzouri et al., 2011), it has not been examined in earlier development, where individual-level deprivation has a demonstrated impact.
Exposure to deprived environments could affect neural regions with a protracted neurodevelopmental trajectory, such as the prefrontal cortex (PFC) (Mackes et al., 2020; McLaughlin et al., 2011, 2014a; Pechtel and Pizzagalli, 2011). PFC development undergoes a critical developmental milestone preceding puberty and adolescence, during which synaptic pruning, trophic glial and vascular changes, cell shrinkage and neuronal specialization begins to take place at a large scale (Gogtay et al., 2004; Tamnes et al., 2017). Prefrontal surface area and thickness, which follow unique developmental trajectories and thus may reflect differences in timing of impact, would be particularly informative (Lyall et al., 2015). Despite the fact, studies tying together neural mechanisms and neurocognition in the context of neighborhood deprivation are, to our knowledge, lacking in the literature. An association of neighborhood deprivation to PFC development and neurocognitive function could pinpoint larger community enrichment as a valuable strategy alongside targeted individual approaches to care at the population level.
The current study comprised a nationally representative sample of children aged 9–11 years old, an age range that has been established as a critical period of healthy PFC development (Gogtay et al., 2004; Tamnes et al., 2017). First, the study related neighborhood deprivation to neurocognition over and above proximal individual-level factors (such as parental education and household income). Then, the study examined neighborhood deprivation relations to PFC surface area and cortical thickness. Given their unique neurodevelopmental trajectories, surface area and cortical thickness were honed in on in order to gauge possibly distinct influences of neighborhood deprivation on PFC neurodevelopment (Raznahan et al., 2011; Shaw et al., 2008; Wierenga et al., 2014). The final, third aim explored whether PFC area would mediate an existing relation between neighborhood deprivation and neurocognitive function as a potential, contributing mechanism.
2. Materials and methods
The multisite Adolescent Brain Cognitive Development (ABCD) study aims to better understand adolescent development through a multimodal perspective (Volkow et al., 2018). The ABCD study utilized a school-based recruitment strategy, collecting cognitive and neuroimaging data from 9 to 1 1 year old children (Garavan et al., 2018). Written informed consent was obtained from participants, and data collection was approved by respective institutional review boards. Recruitment was conducted in a way that ensured the sample was representative of the U.S. population. The current study extracted data from the ABCD Release 2.0 (March 2019; DOI:10.15154/506121). Data is available as part of the Adolescent Brain Cognitive Development (ABCD) study. Permission to access the data can be applied for at nda.gov.
2.1. Neighborhood deprivation
Residential history was established by collecting information on addresses where the participants had lived. Addresses were used to establish the Census tracts corresponding to each address. Publicly available Census data was then used to calculate the area deprivation index (ADI). The ADI was calculated based on the American Community Survey 2015 5-year summary, which has been successfully adapted in numerous investigations to assess deprivation at the neighborhood level (Kind et al., 2014). The ADI metric is compiled for each individual’s tract, representing Census-delineated neighborhoods of each participant. The area deprivation index has 17 subscores, including percentage of population aged ≥25 years with <9 years of education, percentage of population aged ≥25 years with at least a high school diploma, percentage of employed persons aged ≥16 years in white collar occupations, median family income, income disparity (log of 100 x ratio of the number of households with 50000 annual income), median home value, median gross rent, median monthly mortgage, percentage of home owners, percentage of occupied housing units with >1 person per room (crowding), percentage of civilian labor force population aged ≥16 years unemployed (unemployment rate), percentage of families below the poverty level, percentage of population below 138% of the poverty threshold, percentage of single parent households with children aged <18 years, percentage of occupied housing units without a motor vehicle, percentage of occupied housing units without a telephone, and percentage of occupied housing units without complete plumbing. A composite ADI was computed for each participant based on the average of each address in which they had lived. Quintiles were then created based on ADI scores. ADI quintiles are predominantly used in the literature, their utility having been demonstrated across relevant socioeconomic domains (Knighton et al., 2016). Quintiles were numbered such that the first quintile has the lowest degree of deprivation, and the fifth quintile has the highest degree of deprivation (see Table 1).
Table 1.
Demographic characteristics.
| Q1 |
Q2 |
Q3 |
Q4 |
Q5 |
Group Comparison | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | ||
| Sex (Female) | 977 | 47.80 | 935 | 45.90 | 956 | 46.70 | 996 | 49.00 | 990 | 48.50 | χ2 (4,10204) = 5.33, p = 0.026* |
| Household income | χ2 (36,9375) = 3008.68, p < 0.001* | ||||||||||
| Less than $5000 | 24 | 1.3 | 32 | 1.7 | 47 | 2.5 | 61 | 3.3 | 170 | 9.5 | |
| $5000–11999 | 19 | 1 | 30 | 1.6 | 49 | 2.6 | 83 | 4.4 | 172 | 9.6 | |
| $12000–15999 | 20 | 1.1 | 19 | 1 | 30 | 1.6 | 66 | 3.5 | 101 | 5.7 | |
| $16000–24999 | 26 | 1.4 | 42 | 2.2 | 67 | 3.5 | 89 | 4.8 | 203 | 11.4 | |
| $25000–34999 | 43 | 2.3 | 67 | 3.5 | 78 | 4.1 | 140 | 7.5 | 234 | 13.1 | |
| $35000–49999 | 72 | 3.8 | 95 | 4.9 | 143 | 7.5 | 231 | 12.4 | 249 | 13.9 | |
| $50000–74000 | 144 | 7.6 | 196 | 10.2 | 310 | 16.3 | 348 | 18.6 | 311 | 17.4 | |
| $75000–99999 | 186 | 9.8 | 315 | 16.4 | 354 | 18.6 | 342 | 18.3 | 180 | 10.1 | |
| $100000–199999 | 750 | 39.6 | 856 | 44.5 | 690 | 36.2 | 454 | 24.3 | 162 | 9.1 | |
| $200000+ | 608 | 32.1 | 271 | 14.1 | 136 | 7.1 | 55 | 2.9 | 5 | 0.3 | |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age (months) | 119.34 | 7.49 | 119.44 | 7.44 | 118.88 | 7.58 | 119.06 | 7.53 | 118.49 | 7.41 | F(4, 10200) = 5.27, p < 0.001* |
| Weight (inches) | 80.42 | 21.12 | 81.65 | 22.11 | 83.49 | 23.18 | 86.09 | 25.81 | 90.08 | 29.61 | F(4, 10200) = 50.25, p < 0.001* |
| Cognition total scorea | 46.76 | 16.86 | 46.19 | 15.88 | 45.08 | 16.36 | 44 | 15.46 | 41.82 | 15.24 | β = 0.11, t = 10.62, p < 0.001* |
| Parental education | 17.71 | 2.17 | 17.23 | 2.32 | 16.57 | 2.6 | 16 | 2.54 | 14.79 | 2.52 | F(4,10043) = 436.04, p < 0.001* |
| ADI index score | 55.22 | 27.05 | 89.52 | 3.5 | 98.4 | 2.15 | 105.83 | 2.31 | 115.63 | 3.72 | F(4,10200) = 7120.82, p < 0.001* |
2.2. Cognitive function
The NIH Toolbox Cognition domain comprises seven measures measuring the constructs of executive function, episodic memory, language, processing speed, working memory, and attention (Weintraub et al., 2013). NIH Toolbox instruments were validated in a sample of 476 participants ranging in age from 3 to 85 years, with representative sex, racial/ethnic categories, and education levels, and have been found to have appropriate test-retest reliability, as well as convergent and discriminant construct validity (Gershon et al., 2010, 2013; Weintraub et al., 2013). The current analyses utilized the NIH Toolbox composite score for Cognition. Scores were converted to T-scores derived from the NIH Toolbox nationally representative normative sample. Demographic variables were adjusted for in the corrected scores, including age, gender, race/ethnicity and educational attainment.
2.3. Structural imaging
Participants completed a high-resolution T1-weighted structural MRI scan (1-mm isotropic voxels) using scanners from GE Healthcare (Waukesha, Wisconsin), Philips Healthcare (Andover, Massachusetts), or Siemens Healthcare (Erlangen, Germany) (Casey et al., 2018). Structural MRI data was processed using FreeSurfer version 5.3.0 (http://surfer.nmr.mgh.harvard.edu/) (Dale et al., 1999; Fischl et al., 1999) according to standard processing pipelines (Casey et al., 2018). Processing included removal of nonbrain tissue, segmentation of gray and white matter structures (Fischl et al., 2002) and cortical parcellation (Fischl et al., 2004). All scan sessions underwent radiological review whereby scans with incidental findings were identified. Quality control for the structural images comprised visual inspection of T1 images and FreeSurfer outputs for quality (Hagler et al., 2019). Quality review was conducted by the ABCD team. Subjects whose scans failed inspection (due to severe artifacts or irregularities) were excluded. The Desikan-Killiany Atlas was used for cortical parcellation (Hagler et al., 2019). Regions of interest included caudal middle frontal, lateral orbitofrontal, medial orbitofrontal, rostral middle frontal, superior frontal, and frontal pole.
2.4. Parental education and household income
Data was collected on how many years of education the participant’s parent(s) had completed. In the case that the participant lived in a oneguardian household, the number of years for that parent was used. In the case that the participant lived in a two-guardian household, the number of years of education for each was averaged for both guardians. For household income, information was collected according to household income in the past 12 months (see Table 1). Of the original sample, 9375 subjects had data on both parental education and household income.
2.5. Data analysis
SPSS 25 was used for analyses. Chi-squares/One-way ANOVAS were used as appropriate to test for demographic differences among quintiles. The first aim was to determine whether neighborhood deprivation relates to cognitive function (using the composite NIH toolbox cognitive score). The second aim, in turn, was to determine whether neighbourhood deprivation would relate to PFC area and thickness, which exhibit distinct developmental trajectories and structural features (Wierenga et al., 2014). The third aim sought to determine whether the relationship with neurocognition, if present, would be mediated by PFC. For the first aim, a general linear model examined if composite cognitive scores would be predicted by composite ADI quintiles beyond the variance related to individual SES and parental education. To address the second aim, two repeated-measures ANCOVAS were run separately for deprivation quintiles (between-groups) predicting area and thickness (within groups, including subregions), respectively accounting for the variance related to age, sex, weight, parental education, household income, and a whole brain correction. Examining overall PFC with subregions as a repeated measure allows for detection of general, global PFC differences between groups (main effect of quintile) as well as for subregion-specific differences between groups (quintile by subregion interactions). For area analyses, variance related to total surface area was accounted for in the model. For thickness analyses, variance related to mean total thickness was accounted for in the model. Within-subject factors included hemisphere (left and right) as well as PFC regions (caudal middle frontal, lateral orbitofrontal, medial orbitofrontal, rostral middle frontal, superior frontal, and frontal pole). The model included tests for main effects, as well as interactions of quintiles by hemisphere, quintiles by region, and quintiles by hemisphere by region. In the case of interactions by quintile, Bonferroni-corrected, post-hoc tests were run to better understand relations between quintiles.
A mediation analysis was conducted using PROCESS v3.4 Model 4 (Hayes, 2017). In the model, age, sex, total surface area, parental education and household income were controlled for. Given the observed main effect from aim 1, PFC surface area was tested as a mediator. All analyses were re-run having identified outliers (Schwertman et al., 2004), these values were then Winsorized to the next non-outlier value. Given that this did not significantly alter observed findings, results are presented using the original data.
3. Results
3.1. Demographic characteristics and neurocognition
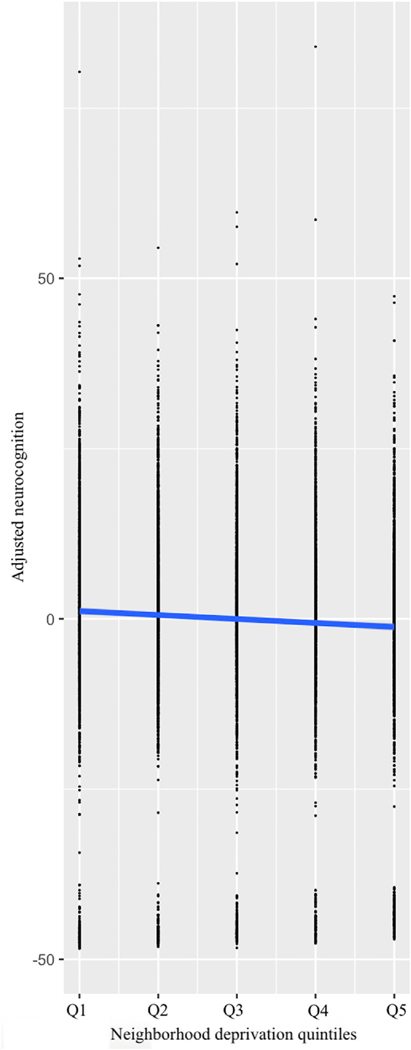
The current study comprised 10,205 participants. Quintiles did not differ by sex, χ2(4, 10204) = 5.33, p = 0.26. Significant differences in months of age F(4, 10200) = 5.27, p < 0.001 and weight F(4, 10200) = 50.25, p < 0.001 were detected between quintiles. As expected, significant differences were detected between quintiles with regards to parental education, F(4,10043) = 436.04, p < 0.001 and household income, χ2(36, 9375) = 3008.68, p < 0.001. The association of neighborhood deprivation and neurocognitive function was significant, β = −0.11, t = 10.62, p < 0.001 (and persisted when accounting for parental education and household income, β = −0.07, t = 5.60, p < 0.001). Greater neighborhood deprivation related to lower neurocognitive performance (Fig. 1).
Fig. 1.
Association between neurocognitive performance and neighborhood deprivation quintiles. Increasing levels of neighborhood deprivation relate to lower neurocognitive performance, p < 0.001. Neurocognition values are adjusted for age, sex, parental education, and household income.
4. PFC surface area
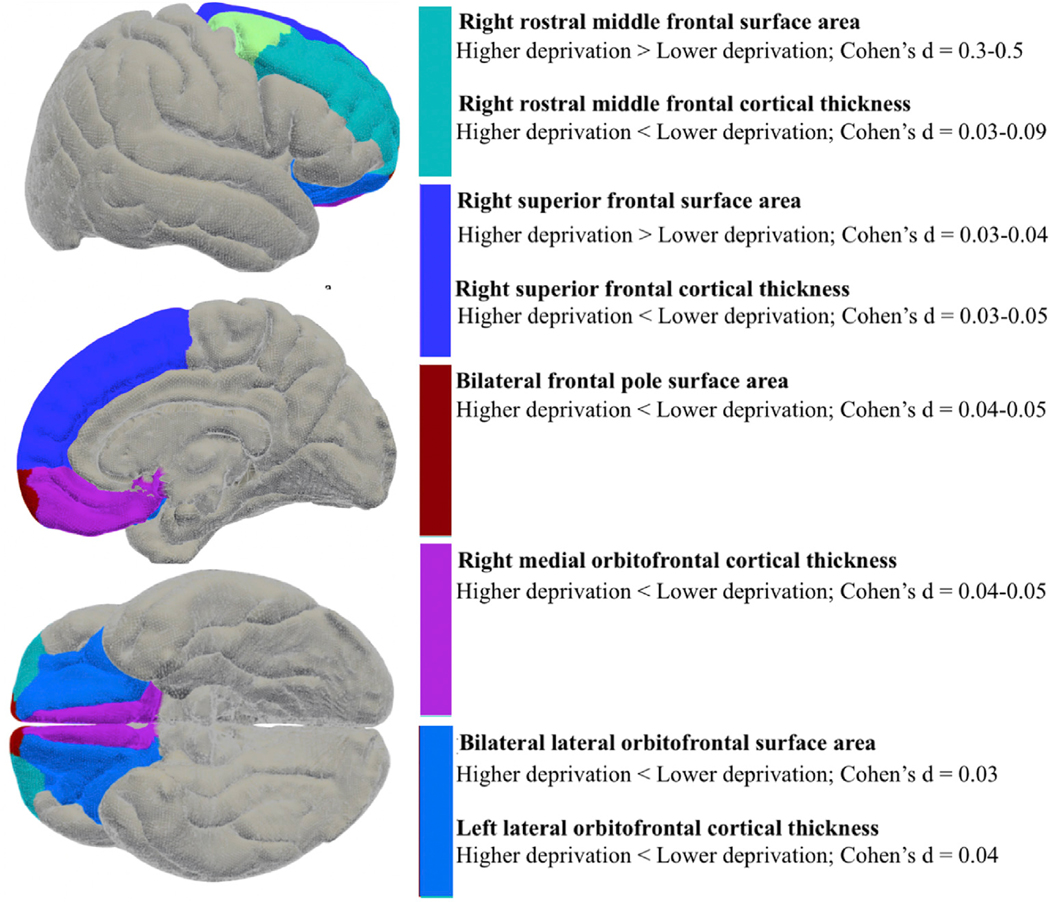
Within a nested repeated-measures general linear model, within-subject factors were defined as frontal regions (6) nested within hemisphere (right and left), and between-subject factors were defined as quintiles, with the model accounting for variance related to total area, age, sex, weight, parental education, household income and site. As for between-subject effects, there was a main effect of quintiles on PFC surface area, F(4,9262) = 6.29, p < 0.001, η2p = 0.003. Quintile 5 showed greater total PFC area than Quintile 1 (mean difference = 22.52, SE = 4.72, p < 0.001, 95% CI 9.26–35.78) and Quintile 2 (mean difference = 16.18, SE = 4.56, p = 0.004, 95% CI 3.38–28.98). Quintile 3 also showed greater total PFC area than Quintile 1, mean difference = 13.48, SE = 4.19, p = 0.01, 95% CI 1.72–25.24. For within subjects effects, there was no main effect of hemisphere, Greenhouse-Geisser F(1, 9262) = 2.64, p = 0.10, though there was an effect of hemisphere by quintiles, Greenhouse-Geisser F(4, 9262) = 2.47, p = 0.04, η2p = 0.001. Across quintiles the left hemisphere exhibited greater surface area (Table 2). Quintile 1 (exhibiting the lowest levels of deprivation in the current samples) had greatest leftward asymmetry with confidence intervals that did not overlap with quintiles 2–5. As expected, there was a main effect of area, Greenhouse-Geisser F(2.99, 27714.73) = 109.33, p > 0.001, η2p = 0.012, and an area by quintiles interaction, Greenhouse-Geisser F(11.97, 27714.73) = 3.70, p > 0.001, η2p = 0.002. An interaction of hemisphere by area by quintiles was also observed, Greenhouse-Geisser F(11.93, 27616.31) = 2.03, p = 0.02, η2p = 0.001. For the frontal pole, greater neighborhood deprivation related to lower surface area. For right rostral middle frontal and superior frontal regions, greater neighborhood deprivation related to greater surface area (Figs. 2 and 3; Table 3).
Table 2.
Bonferroni-corrected significant mean differences between quintiles of leftward asymmetry for frontal area and thickness.
| Surface area |
|||||
|---|---|---|---|---|---|
| Quintile | Cohen’s d | Mean difference | SE | pBonferroni | 95% CI |
| 5 | 0.069 | 23.123 | 3.504 | <0.001 | 16.254–29.992 |
| 4 | 0.075 | 22.795 | 3.153 | <0.001 | 16.614–28.977 |
| 3 | 0.073 | 21.834 | 3.106 | <0.001 | 15.746–27.922 |
| 2 | 0.088 | 26.584 | 3.138 | <0.001 | 20.433–32.734 |
| 1 | 0.110 | 34.515 | 3.250 | <0.001 | 28.145–40.885 |
| Thickness | |||||
| Quintile | Mean difference | Mean difference | SE | pBonferroni | 95% CI |
| 5 | 0.109 | 0.021 | 0.002 | <0.001 | 0.016–0.025 |
| 4 | 0.119 | 0.023 | 0.002 | <0.001 | 0.019–0.027 |
| 3 | 0.088 | 0.017 | 0.002 | <0.001 | 0.013–0.021 |
| 2 | 0.062 | 0.012 | 0.002 | <0.001 | 0.008–0.016 |
| 1 | 0.047 | 0.009 | 0.002 | <0.001 | 0.005–0.013 |
Fig. 2.
Regions (for area and thickness) associated with neighborhood deprivation in Bonferroni-corrected analyses (pBonferroni < 0.05). Analyses adjust for age, sex, a whole brain correction, weight, parental education, and household income.
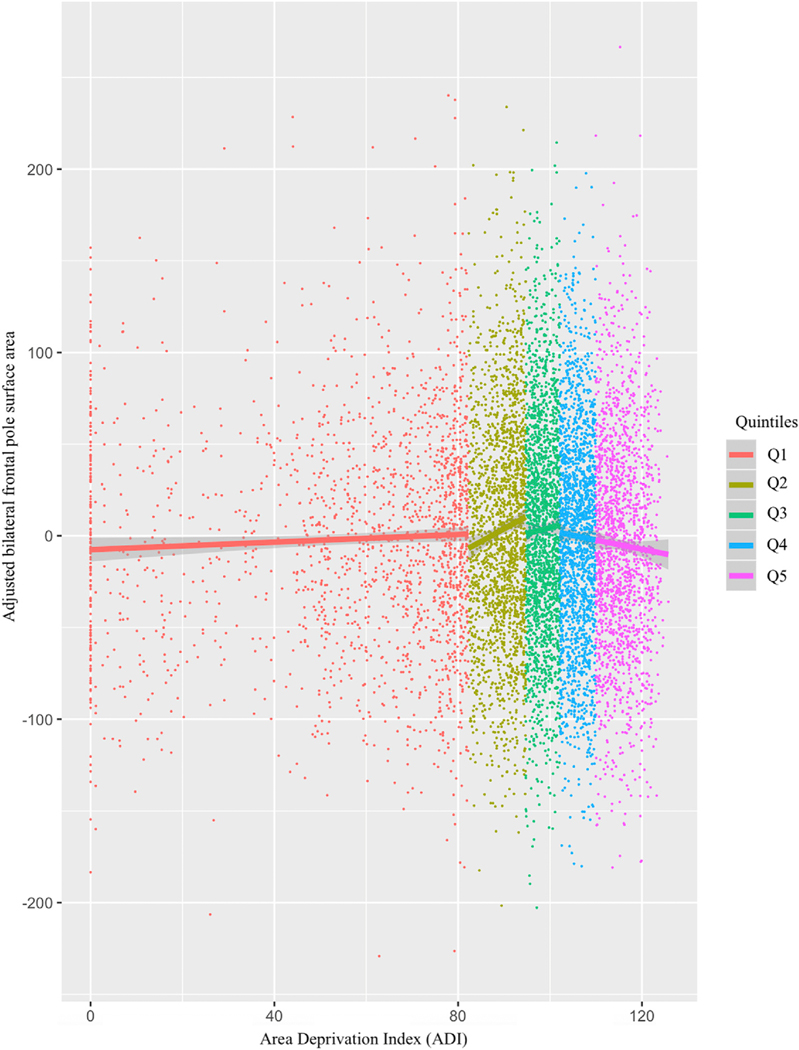
Fig. 3.
Bilateral frontal pole surface area plotted according to individual Area Deprivation Index (ADI) values used to sort participants into quintiles (color-coded to the right). The highest deprivation quintile (Q5) shows smaller surface area relative to Q2 and Q3, pBonferroni < 0.05. Adjusted area values constitute estimated marginal means partialling out age, sex, a whole brain correction, weight, parental education, and household income.
Table 3.
Bonferroni-corrected significant associations between quintiles and prefrontal subregion frontal area/thickness.
| Surface area PFC region | Hemisphere | Summary | Cohen’s d | Mean difference | SE | pBonferroni | 95% CI | ||
|---|---|---|---|---|---|---|---|---|---|
| Caudal Middle Frontal | – | no difference | – | – | – | – | |||
| Lateral Orbitofrontal | Left | 5 < 3 = 2 > 1; 5 < 2 | [5 < 3] −0.031; | [5 < 3] −22.369; | [5 < 3] 7.457; | [5 < 3] .027; | [5 < 3] −43.307 to −1.432; | ||
| [5 < 2] −0.031; | [5 < 2] −23.100; | [5 < 2] 7.668; | [5 < 2] .026; | [5 < 2] −44.629 to −1.571; | |||||
| [3 > 1] 0.031; | [3 > 1] 20.740; | [3 > 1] 7.045; | [3 > 1] .032; | [3 > 1] .960–40.519; | |||||
| [2 > 1] 0.032; | [2 > 1] 21.470; | [2 > 1] 6.960; | [2 > 1] .020; | [2 > 1] 1.928–41.012; | |||||
| Right | 5 = 4 < 2 = 1 | [5 < 2] −0.031; | [5 < 2] −23.773; | [5 < 2] 8.022; | [5 < 2] .031; | [5 < 2] −46.296 to −1.249; | |||
| [4 < 2] −0.031; | [4 < 2] −22.064; | [4 < 2] 7.408; | [4 < 2] .029; | [4 < 2] −42.863 to −1.265; | |||||
| Medial Orbitofrontal | – | no difference | – | – | – | – | |||
| Rostral Middle Frontal | Right | 5 > 4 = 3 = 2 = 1 | [5 > 4] 0.031; | [5 > 4] 55.909; | [5 > 4] 18.770; | [5 > 4] .029; | [5 > 4] 3.209–108.610; | ||
| [5 > 3] 0.040; | [5 > 3] 74.410; | [5 > 3] 19.263; | [5 > 3] .001; | [5 > 3] 20.324–128.496; | |||||
| [5 > 2] 0.045; | [5 > 2] 85.697; | [5 > 2] 19.808; | [5 > 2] .000; | [5 > 2] 30.082–141.311; | |||||
| [5 > 1] 0.054; | [5 > 1] 107.621; | [5 > 1] 20.519; | [5 > 1] .000; | [5 > 1] 50.010–165.232; | |||||
| Superior Frontal | Right | 5 = 4 = 3 > 2; 5 > 1; 3 > 1 | [5 > 1] 0.032; | [5 > 1] 57.487; | [5 > 1] 18.692; | [5 > 1] .021; | [5 > 1] 5.005–109.970; | ||
| [3 > 2] 0.032; | [3 > 2] 50.650; | [3 > 2] 16.295; | [3 > 2] .019; | [3 > 2] 4.899–96.402; | |||||
| [3 > 1] 0.039; | [3 > 1] 62.569; | [3 > 1] 16.579; | [3 > 1] .002; | [3 > 1] 16.020–109.117; | |||||
| Frontal Pole | Left | 5 = 4 < 3 = 2 = 1; 5 < 2 | [5 < 3] −0.037; | [5 < 3] −4.228; | [5 < 3] 1.175; | [5 < 3] .003; | [5 < 3] −7.527 to −.928; | ||
| [5 < 2] −0.045; | [5 < 2] −5.239; | [5 < 2] 1.208; | [5 < 2] .000; | [5 < 2] −8.631 to −1.846; | |||||
| Right | 5 < 3 = 2 = 1 | [5 < 3] −0.036; | [5 < 3] −5.405; | [5 < 3] 1.547; | [5 < 3] .005; | [5 < 3] −9.749 to −1.061; | |||
| Thickness PFC region | Hemisphere | Summary | Mean difference | SE | pBonferroni | 95% CI | |||
| Caudal Middle Frontal | – | ||||||||
| Lateral Orbitofrontal | Left | 5 < 3 | [5 < 3] −0.036; | [5 < 3] −.014; | [5 < 3] .004; | [5 < 3] .009; | [5 < 3] −.026 to −.002; | ||
| Medial Orbitofrontal | Right | 5 < 3 = 2; 4 < 2 | [5 < 3] −0.035; | [5 < 3] −.020; | [5 < 3] .006; | [5 < 3] .003; | [5 < 3] −.036 to −.005; | ||
| [5 < 2] −0.050; | [5 < 2] −.029; | [5 < 2] .006; | [5 < 2] .000; | [5 < 2] −.045 to −.012; | |||||
| [4 < 2] −0.039; | [4 < 2] −.019; | [4 < 2] .005; | [4 < 2] .004; | [4 < 2] −.034 to −.004; | |||||
| Rostral Middle Frontal | Right | 5 < 4 < 3 = 2 = 1; 4 < 2 = 1 | [5 < 4] −0.039; | [5 < 4] −.019; | [5 < 4] .005; | [5 < 4] .004; | [5 < 4] −.034 to −.004; | ||
| [5 < 3] −0.069; | [5 < 3] −.020; | [5 < 3] .003; | [5 < 3] .000; | [5 < 3] −.029 to −.010; | |||||
| [5 < 2] −0.090; | [5 < 2] −.026; | [5 < 2] .003; | [5 < 2] .000; | [5 < 2] −.036 to −.016; | |||||
| [5 < 1] −0.057; | [5 < 1] −.022; | [5 < 1] .004; | [5 < 1] .000; | [5 < 1] −.032 to −.011; | |||||
| [4 < 3] −0.031; | [4 < 3] −.009; | [4 < 3] .003; | [4 < 3] .037; | [4 < 3] −.018 - .000; | |||||
| [4 < 2] −0.052; | [4 < 2] −.015; | [4 < 2] .003; | [4 < 2] .000; | [4 < 2] −.024 to −.006; | |||||
| [4 < 1] −0.038; | [4 < 1] −.011; | [4 < 1] .003; | [4 < 1] .008; | [4 < 1] −.020 to −.002; | |||||
| Superior Frontal | Right | 5 < 2 = 1; 4 < 2 = 1 | [5 < 2] −0.045; | [5 < 2] −.013; | [5 < 2] 0.003; | [5 < 2] .002; | [5 < 2] −.022 to −.003; | ||
| [5 < 1] −0.029; | [5 < 1] −.011; | [5 < 1] 0.004; | [5 < 1] .016; | [5 < 1] −.021 to −.001; | |||||
| [4 < 2] −0.042; | [4 < 2] −.012; | [4 < 2] 0.003; | [4 < 2] .001; | [4 < 2] −.021 to −.003; | |||||
| [4 < 1] −0.035; | [4 < 1] −.010; | [4 < 1] 0.003; | [4 < 1] .013; | [4 < 1] −.020 to −.001; | |||||
| Frontal Pole | no difference | – | – | – | – | ||||
4.1. PFC thickness
Within a nested repeated-measures general linear model, within-subject factors were defined as frontal regions nested within hemispheres, and between-subject factors were defined as quintiles, with the model accounting for variance related to total average thickness, age, sex, weight, parental education, household income and site. For between-subject effects, there was no main effect of quintiles on PFC thickness, F(4,9262) = 1.07, p = 0.37. For within-subject effects, there was a significant main effect of hemisphere exhibiting leftward asymmetry, Greenhouse-Geisser F(1, 9262) = 4.53, p = 0.03, η2p = 0.001. In addition, there was an interaction of hemisphere by quintiles, Greenhouse-Geisser F(4, 9262) = 6.58, p < 0.001, η2p = 0.003. Quintiles 3, 4 and 5 (exhibiting greater levels of deprivation in the current samples) had greater leftward asymmetry with confidence intervals that did not overlap with quintile 1, the quintile with the lowest deprivation levels. As expected, there was a main effect of thickness (Greenhouse-Geisser F(2.66, 24672.84) = 334.06, p < 0.001, η2p = 0.035) and an interaction of thickness by quintiles (Greenhouse-Geisser F(10.66, 24672.84) = 2.40, p = 0.01, η2p = 0.001). Finally, an interaction of hemisphere by region by quintiles was observed, Greenhouse-Geisser F(8.69, 20114.09) = 3.71, p < 0.001, η2p = 0.002. For the left lateral orbitofrontal, right medial orbitofrontal, right superior frontal and right rostral middle frontal regions, greater neighborhood deprivation related to lower thickness (Table 3).
4.2. Mediation of PFC area
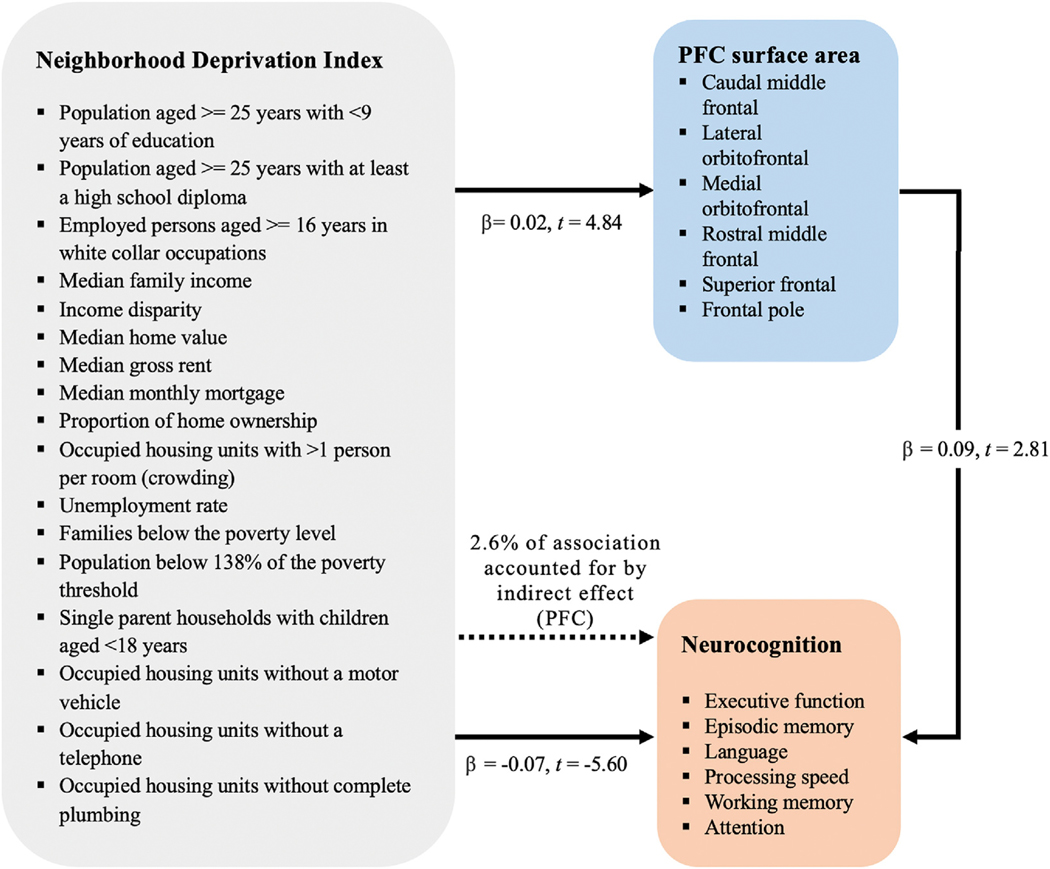
In line with the main effect observed in aim 1, the regression measuring the association of neighborhood deprivation and PFC area was significant, β = 0.02, t = 4.84, p < 0.001. Greater deprivation related to greater PFC area. While controlling for neighborhood deprivation, the relationship of the mediator (PFC area) with neurocognitive function was significant, β = 0.09, t = 2.81, p = 0.005. Greater PFC area predicted higher neurocognitive performance. Further, analyses showed that while controlling for the mediator (PFC area), neighborhood deprivation remained a significant predictor of neurocognitive function, β = −0.06, t = −5.50, p < 0.001. The Sobel test for indirect effects had a 95% confidence interval of −0.0031 to −0.0004, calculated using 5000 bootstrap samples. The model suggests partial mediation; 2.6% of the association between neighborhood deprivation and neurocognitive function is accounted for by PFC area (β = 0.002, SE = 0.001) (Fig. 4).
Fig. 4.
Association between neighborhood deprivation and neurocognitive function, with prefrontal area as a partial mediator. Greater neighborhood deprivation predicts lower neurocognitive performance. Independently, greater prefrontal surface area predicts higher neurocognitive performance. The association between neighborhood deprivation and neurocognitive performance is partially mediated by prefrontal surface area.
5. Discussion
Exposure to neighborhood level deprivation was explored in relation to cognitive function and protracted brain development (within PFC) in a nationally representative sample of youth. First, greater levels of neighborhood level deprivation predicted lower neurocognitive performance. Second, neighborhood deprivation related to distinct prefrontal gray matter features. Greater PFC area was observed with increased neighborhood deprivation levels. Additionally, neighborhood deprivation impacted the frontal asymmetry of gray matter area and thickness; the normative leftward bias was significantly different between the high and low deprivation groups. Further, the effect of neighborhood deprivation varied by PFC metric. Both surface area and thickness showed regional specificity; neighborhood deprivation particularly impacted critical regions including orbitofrontal, superior, rostral medial frontal, and frontal pole regions. To examine relevance of PFC area as a possible mechanism underlying cognitive deficits, associations between neighborhood deprivation and neurocognitive function were examined, with PFC area tested as a potential mediator. Analyses showed PFC surface area independently predicted neurocognitive performance; strikingly, the relation between neighborhood deprivation and neurocognition was found to be partially mediated by PFC surface area. Taken together, findings suggest that exposure to deprivation at the neighborhood level relates to both functional outcomes (i.e. neurocognition) and neurodevelopment (i.e. PFC). Results suggest that systems level environmental features are relevant and ought to be accounted for in models of environmental effects during critical periods of human neurodevelopment.
As hypothesized, increased levels of deprivation related to lower cognitive function. These results are consistent with, and of a similar effect size to those observed in older adults (50 years and older) (Drukker and van Os, 2003; Lang et al., 2008). The current study extends the existing literature on older adults (McCann et al., 2018; Sheffield and Peek, 2009; Wu et al., 2015; Zeki Al Hazzouri et al., 2011) to youth in late childhood to early adolescence undergoing critical normative neurodevelopmental processes. Results suggest that while the cognitive developmental literature has largely focused on individual-level factors, more attention to structural factors such as neighborhood characteristics may be warranted. Structural level trends may be present even after accounting for the impact of individual-level features; future investigations will be necessary to determine whether systems level policy initiatives addressing structural characteristics such as neighborhood features (even in the absence of individual-targeted initiatives) could have a protective impact on neurocognitive development at the aggregate level.
The age of our current sample, directly preceding puberty and adolescence, is critical for PFC development, with synaptic pruning, cell shrinkage, neuronal specialization, trophic glial and vascular changes starting to take place at a broad scale (Gogtay et al., 2004; Pechtel and Pizzagalli, 2011; Tamnes et al., 2017). These processes make the PFC particularly susceptible to environmental influence at this stage of neurodevelopment. In the present study, greater levels of deprivation predicted increased PFC surface area. Results support the broader literature on individual-level early life stress and effects on neural development (Gee et al., 2013; McLaughlin et al., 2014a, 2014b; Tottenham et al., 2010; Tottenham and Sheridan, 2010). The fact that an effect was not observed with regards to total PFC cortical thickness could mean the effect at this age is specific to surface area. Surface area and cortical thickness follow distinct developmental trajectories throughout childhood and early adolescence (Alemán-Gómez et al., 2013; Raznahan et al., 2011; Shaw et al., 2008; Tamnes et al., 2017). For example, while both cortical thickness and surface area show normative decreases throughout late childhood and adolescence, cortical thinning happens at a much greater degree compared to surface area (Tamnes et al., 2017). The observed difference relating to deprivation could be due to a delayed trajectory of synaptic pruning and dendritic arborisation (Alemán-Gómez et al., 2013; Bourgeois and Rakic, 1993; Huttenlocher and Dabholkar, 1997; Klein et al., 2014; Petanjek et al., 2011; Raznahan et al., 2011; Tamnes et al., 2017; White et al., 2010).
For surface area, decreased leftward asymmetry was observed as deprivation levels increased. Interestingly, for cortical thickness an opposing pattern was observed–increasing leftward asymmetry as deprivation levels increased. Perhaps divergences in leftward asymmetry at this age relate to altered developmental processes due to exposure to adverse environmental factors (Lawson et al., 2013; Luders et al., 2005); indeed, there is evidence that individual differences in cerebral lateralization may be vastly influenced by environmental factors occurring during early neurodevelopment (Bishop, 2013; Raj and van Oudenaarden, 2008). Further, asymmetry has been associated with impactful outcomes including psychopathology risk and cognitive function (Avnit et al., 2019; Damme et al., 2020; Keune et al., 2015). Future studies will be needed to further enrich and corroborate these hypotheses and incidental findings.
Subregion specific analyses yielded compelling results. Superior frontal and rostral middle frontal areas were larger in quintiles with greater neighborhood deprivation. By contrast, more ventral prefrontal areas including orbitofrontal and frontal pole regions showed lower area/thickness in quintiles with greater deprivation. Results are partially consistent with investigations on individual institutionalization conferring childhood deprivation (Mackes et al., 2020). Given these region’s involvement in fundamental cognitive functions (Bahlmann et al., 2015; Lopatina et al., 2017), and the lack of precedent in the literature, future studies will benefit from further assessing relations to environmental influence. On the contrary, bilateral frontal pole surface area decreased as neighborhood deprivation exposure increased; this could be due to it being among the first prefrontal regions to fully develop (Gogtay et al., 2004). Perhaps the region is more susceptible to early deprivation, with less compensatory mechanisms or developmental flexibility occurring; these processes could be functionally relevant given frontal pole relations to complex cognition (Burke et al., 2013; Gilbert et al., 2006; Liu et al., 2013; Semendeferi et al., 2001).
Decreases in left lateral orbitofrontal, right medial orbitofrontal, right superior frontal, and right rostral middle frontal regions related to greater neighborhood deprivation. Orbitofrontal thickness results are consistent with previous investigations on children exposed to maltreatment (Kelly et al., 2013) and institutionalization (Mackes et al., 2020; Sheridan et al., 2012). Subregion analyses are also partially consistent with an investigation in children exposed to maltreatment (Kelly et al., 2013) and another examining relations between children’s socioeconomic status and prefrontal cortical thickness (Lawson et al., 2013). Future studies will be needed to further flesh out relations between neighborhood deprivation and prefrontal cortical subregions.
Finally, analyses tested PFC area as a relevant mechanism. The model found evidence for PFC area partially mediating the relation between neighborhood deprivation and neurocognitive function. Results suggest that the association between distal exposure to deprivation and neurocognitive function becomes apparent at an early age, and is robust to related individual-level factors (i.e. household income and parental education). The proportion of the effect that PFC area explained was modest and is important to note; nonetheless, given the milieu of environmental, genetic, and neurodevelopmental factors that may each uniquely impact and be impacted by neighborhood deprivation, this is to be reasonably expected. PFC area is one of many candidate neurodevelopmental processes through which neighborhood deprivation could impact neurocognition. The model showed increased PFC area related to greater neurocognitive function in the current sample. Perhaps increased PFC surface area serves as a developed protective mechanism attenuating the established relation between increased deprivation and decreased neurocognitive function. Given these are novel questions, future investigations are needed to further build on these initial findings.
It is critical to highlight that effect sizes were rather modest for neighborhood deprivation predicting PFC brain metrics and neurocognition. These effect sizes are in line with studies of distal neighborhood-level characteristics predicting biological outcomes (Laraia et al., 2012; Lopez, 2007), as well as gray matter MRI pooled samples research (Sacher et al., 2012), which typically observe small effect sizes. Further, these findings were robust to accounting for more proximal characteristics relating to deprivation (parental education and household income), which are typically impactful to a greater magnitude. Although observed effects are small according to convention, it will be paramount for future investigations to ascertain clinical significance and possible relevance to public health initiatives. There is reason to think this pursuit could be fruitful. Indeed, public health and medicine research has successfully based treatment decisions on comparable effect sizes that would conventionally be viewed as small. For example, low-dose aspirin remains recommended to reduce the risk of heart attacks for those under 70 years old without bleeding risk, based on a 0.03 correlation (Steering Committee of the Physicians’ Health Study Research Group, 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989), and fruit and vegetable consumption is recommended to address weight and abdominal obesity based on an effect size of −0.05 (Schwingshackl et al., 2015). However, it is also noteworthy that while the initial policy impact of the aspirin studies was initially broader, it was eventually scaled back to include a more circumscribed set of circumstances (Arnett, 2019). Thus, while there is good reason to consider the present effects as an important step in clarifying our understanding in this critical area, it should not be forgotten that the effects were small. Future investigations would benefit from clarifying and identifying whether there are practical consequences to the presently observed effects, which will aid with interpreting and understanding their magnitude (Funder; Ozer, 2019). Perhaps targeting these individually small effects at the societal level through policy initiatives for neighborhood features has potential to provide public health improvements at the aggregate level—future study will be critical in determining these possibilities.
Overall, this study’s observations suggest that more attention to environmental characteristics may be warranted in public health and neurodevelopmental models; a general approach to addressing deprivation (including both individual-level and neighborhood/systems-level exposure) could hold widespread benefits to communities. Although the current study provides promising introductory evidence, there are several lines of inquiry that remain unanswered. First, it will be necessary to tease apart exposure during several different developmental periods, in order to confirm critical periods of impact for systems level exposures such as neighborhood deprivation. The timing of the most critical developmental periods may vary within-subjects, and so it will be essential to allow for multiple time points across the lifespan. In addition, gauging more detailed information on different environments that individuals are exposed to beyond their home environment could provide valuable information for developing interventions. Further fleshing out the intricate interactions between proximal exposure to environmental adversity and distal exposure at the systems level is also a much-needed area of inquiry. In all, results suggest that larger community enrichment could be a valuable tool alongside targeted individual approaches to care, and follow up investigations exploring this notion would be valuable for treatment and intervention.
Supplementary Material
Acknowledgments
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org ), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/Consortium_Members.pdf. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from [NIMH Data Archive Digital Object Identifier (DOI) 10.15154/1506121]. DOIs can be found at https://nda.nih.gov/general-query.html?q=query=studies%20%7Eand%7E%20orderBy=id%20%7Eand%7E%20orderDirection=Ascending. The research reported in this manuscript was also supported by the National Institute Of Mental Health of the National Institutes of Health under Award Number F31MH119776 (T.V.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of competing interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuroimage.2020.117086.
References
- Aichele S, Rabbitt P, Ghisletta P, 2016. Think fast, feel fine, live long: a 29-year study of cognition, health, and survival in middle-aged and older adults. Psychol. Sci. 27, 518–529. [DOI] [PubMed] [Google Scholar]
- Akman C, Zhao Q, Liu X, Holmes GL, 2004. Effect of food deprivation during early development on cognition and neurogenesis in the rat. Epilepsy Behav. 5, 446–454. [DOI] [PubMed] [Google Scholar]
- Alemán-Gómez Y, Janssen J, Schnack H, Balaban E, Pina-Camacho L, Alfaro-Almagro F, Castro-Fornieles J, Otero S, Baeza I, Moreno D, 2013. The human cerebral cortex flattens during adolescence. J. Neurosci. 33, 15004–15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett DK, Blumenthal Roger S., Albert Michelle A., Buroker Andrew B., Goldberger Zachary D., Hahn Ellen J., Himmelfarb, Cheryl Dennison, 2019. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 74, e177–e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avnit A, Alyagon U, Zibman S, Zangen A, 2019. Abnormal functional frontal asymmetry and behavioral correlates in adult ADHD: a TMS-EEG study. Brain Stimulation: Basic, Translational, and Clinical Research in Neuromodulation 12, 440. [Google Scholar]
- Bahlmann J, Beckmann I, Kuhlemann I, Schweikard A, Münte T, 2015. Transcranial magnetic stimulation reveals complex cognitive control representations in the rostral frontal cortex. Neuroscience 300, 425–431. [DOI] [PubMed] [Google Scholar]
- Beckett C, Maughan B, Rutter M, Castle J, Colvert E, Groothues C, Kreppner J, Stevens S, O’Connor TG, Sonuga-Barke EJ, 2006. Do the effects of early severe deprivation on cognition persist into early adolescence? Findings from the English and Romanian adoptees study. Child Dev. 77, 696–711. [DOI] [PubMed] [Google Scholar]
- Bishop DV, 2013. Cerebral asymmetry and language development: cause, correlate, or consequence? Science 340, 1230531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois J-P, Rakic P, 1993. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J. Neurosci. 13, 2801–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U, 1994. Ecological models of human development. Readings on the development of children 2, 37–43. [Google Scholar]
- Burke CJ, Brünger C, Kahnt T, Park SQ, Tobler PN, 2013. Neural integration of risk and effort costs by the frontal pole: only upon request. J. Neurosci. 33, 1706–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, 2018. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Developmental cognitive neuroscience 32, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubbin C, Winkleby MA, 2005. Protective and harmful effects of neighborhood-level deprivation on individual-level health knowledge, behavior changes, and risk of coronary heart disease. Am. J. Epidemiol. 162, 559–568. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- Damme KS, Vargas T, Calhoun V, Turner J, Mittal VA, 2020. Global and specific cortical volume Asymmetries in individuals with psychosis risk syndrome and schizophrenia: a mixed cross-sectional and longitudinal perspective. Schizophr. Bull. 46 (3), 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MC, Rosenzweig MR, Bennett EL, Lindner B, Lyon L, 1972. Effects of environmental enrichment and impoverishment on rat cerebral cortex. J. Neurobiol. 3, 47–64. [DOI] [PubMed] [Google Scholar]
- Drukker M, van Os J, 2003. Mediators of neighbourhood socioeconomic deprivation and quality of life. Soc. Psychiatr. Psychiatr. Epidemiol. 38, 698–706. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, Malmud EK, Hurt H, 2006. Childhood poverty: specific associations with neurocognitive development. Brain Res. 1110, 166–174. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM, 1999. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 8, 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, 2004. Automatically parcellating the human cerebral cortex. Cerebr. Cortex 14, 11–22. [DOI] [PubMed] [Google Scholar]
- Forsberg P-O, Ohlsson H, Sundquist K, 2018. Causal nature of neighborhood deprivation on individual risk of coronary heart disease or ischemic stroke: a prospective national Swedish co-relative control study in men and women. Health Place 50, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder DC, Ozer DJ, 2019. Evaluating effect size in psychological research: sense and nonsense. Advances in Methods and Practices in Psychological Science 2, 156–168. [Google Scholar]
- Garavan H, Bartsch H, Conway K, Decastro A, Goldstein R, Heeringa S, Jernigan T, Potter A, Thompson W, Zahs D, 2018. Recruiting the ABCD sample: design considerations and procedures. Developmental cognitive neuroscience 32, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N, 2013. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. Unit. States Am. 110, 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon RC, Cella D, Fox NA, Havlik RJ, Hendrie HC, Wagster MV, 2010. Assessment of neurological and behavioural function: the NIH Toolbox. Lancet Neurol. 9 (2), 138–139. 10.1016/S1474-4422(09)70335-7. [DOI] [PubMed] [Google Scholar]
- Gershon RC, Wagster MV, Hendrie HC, Fox NA, Cook KF, Nowinski CJ, 2013. NIH toolbox for assessment of neurological and behavioral function. Neurology 80, S2–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Spengler S, Simons JS, Frith CD, Burgess PW, 2006. Differential functions of lateral and medial rostral prefrontal cortex (area 10) revealed by brain–behavior associations. Cerebr. Cortex 16, 1783–1789. [DOI] [PubMed] [Google Scholar]
- Glass TA, McAtee MJ, 2006. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc. Sci. Med. 62, 1650–1671. [DOI] [PubMed] [Google Scholar]
- Globus A, Rosenzweig MR, Bennett EL, Diamond MC, 1973. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J. Comp. Physiol. Psychol. 82, 175. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. Unit. States Am. 101, 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ, 2010. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat. Rev. Neurosci. 11, 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler DJ Jr., Hatton S, Cornejo MD, Makowski C, Fair DA, Dick AS, Sutherland MT, Casey B, Barch DM, Harms MP, 2019. Image processing and analysis methods for the adolescent brain cognitive development study. Neuroimage 202, 116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, 2017. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Guilford Publications. [Google Scholar]
- Huttenlocher PR, Dabholkar AS, 1997. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 387, 167–178. [DOI] [PubMed] [Google Scholar]
- Jaffe DH, Eisenbach Z, Neumark YD, Manor O, 2005. Individual, household and neighborhood socioeconomic status and mortality: a study of absolute and relative deprivation. Soc. Sci. Med. 60, 989–997. [DOI] [PubMed] [Google Scholar]
- Jessop E, 1992. Individual morbidity and neighbourhood deprivation in a non-metropolitan area. J. Epidemiol. Community 46, 543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PA, Viding E, Wallace GL, Schaer M, De Brito SA, Robustelli B, McCrory EJ, 2013. Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: neural markers of vulnerability? Biol. Psychiatr. 74, 845–852. [DOI] [PubMed] [Google Scholar]
- Keune PM, Wiedemann E, Schneidt A, Schönenberg M, 2015. Frontal brain asymmetry in adult attention-deficit/hyperactivity disorder (ADHD): extending the motivational dysfunction hypothesis. Clin. Neurophysiol. 126, 711–720. [DOI] [PubMed] [Google Scholar]
- Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, Greenberg C, Smith M, 2014. Neighborhood socioeconomic disadvantage and 30 day rehospitalizations: an analysis of Medicare data. Ann. Intern. Med. 161, 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Rotarska-Jagiela A, Genc E, Sritharan S, Mohr H, Roux F, Han CE, Kaiser M, Singer W, Uhlhaas PJ, 2014. Adolescent brain maturation and cortical folding: evidence for reductions in gyrification. PloS One 9, e84914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton AJ, Savitz L, Belnap T, Stephenson B, VanDerslice J, 2016. Introduction of an area deprivation index measuring patient socioeconomic status in an integrated health system: implications for population health. eGEMs 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadas R, McLean J, Batty GD, Burns H, Deans KA, Ford I, McConnachie A, McLean JS, Millar K, Sattar N, 2013. Socioeconomic deprivation and cortical morphology: psychological, social, and biological determinants of ill health study. Psychosom. Med. 75, 616–623. [DOI] [PubMed] [Google Scholar]
- Lang IA, Llewellyn DJ, Langa KM, Wallace RB, Huppert FA, Melzer D, 2008. Neighborhood deprivation, individual socioeconomic status, and cognitive function in older people: analyses from the English Longitudinal Study of Ageing. J. Am. Geriatr. Soc. 56, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laraia BA, Karter AJ, Warton EM, Schillinger D, Moffet HH, Adler N, 2012. Place matters: neighborhood deprivation and cardiometabolic risk factors in the Diabetes Study of Northern California (DISTANCE). Soc. Sci. Med. 74, 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laus MF, Duarte Manhas Ferreira Vales L, Braga Costa TM, Sousa Almeida S, 2011. Early postnatal protein-calorie malnutrition and cognition: a review of human and animal studies. Int. J. Environ. Res. Publ. Health 8, 590–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson GM, Camins JS, Wisse L, Wu J, Duda JT, Cook PA, Gee JC, Farah MJ, 2017. Childhood socioeconomic status and childhood maltreatment: distinct associations with brain structure. PloS One 12, e0175690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, Farah MJ, 2013. Associations between children’s socioeconomic status and prefrontal cortical thickness. Dev. Sci. 16, 641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Qin W, Li W, Fan L, Wang J, Jiang T, Yu C, 2013. Connectivity-based parcellation of the human frontal pole with diffusion tensor imaging. J. Neurosci. 33, 6782–6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatina N, Sadacca BF, McDannald MA, Styer CV, Peterson JF, Cheer JF, Schoenbaum G, 2017. Ensembles in medial and lateral orbitofrontal cortex construct cognitive maps emphasizing different features of the behavioral landscape. Behav. Neurosci. 131, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez RP, 2007. Neighborhood risk factors for obesity. Obesity 15, 2111–2119. ` [DOI] [PubMed] [Google Scholar]
- Luders E, Narr K, Thompson P, Rex D, Jancke L, Toga A, 2005. Hemispheric asymmetries in cortical thickness. Cerebr. Cortex 16, 1232–1238. [DOI] [PubMed] [Google Scholar]
- Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, Hamer RM, Shen D, Gilmore JH, 2015. Dynamic development of regional cortical thickness and surface area in early childhood. Cerebr. Cortex 25, 2204–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackes NK, Golm D, Sarkar S, Kumsta R, Rutter M, Fairchild G, Mehta MA, Sonuga-Barke EJ, 2020. Early childhood deprivation is associated with alterations in adult brain structure despite subsequent environmental enrichment. Proc. Natl. Acad. Sci. Unit. States Am. 117, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AP, Finn AS, Leonard JA, Jacoby-Senghor DS, West MR, Gabrieli CF, Gabrieli JD, 2015. Neuroanatomical correlates of the income-achievement gap. Psychol. Sci. 26, 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson FI, Moineddin R, Glazier RH, 2008. The weight of place: a multilevel analysis of gender, neighborhood material deprivation, and body mass index among Canadian adults. Soc. Sci. Med. 66, 675–690. [DOI] [PubMed] [Google Scholar]
- McCann A, McNulty H, Rigby J, Hughes CF, Hoey L, Molloy AM, Cunningham CJ, Casey MC, Tracey F, O’Kane MJ, 2018. Effect of area-level socioeconomic deprivation on risk of cognitive dysfunction in older adults. J. Am. Geriatr. Soc. 66, 1269–1275. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Fox NA, Zeanah CH, Nelson CA, 2011. Adverse rearing environments and neural development in children: the development of frontal electroencephalogram asymmetry. Biol. Psychiatr. 70, 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Lambert HK, 2014a. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci. Biobehav. Rev. 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA, 2014b. Widespread reductions in cortical thickness following severe early-life deprivation: a neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biol. Psychiatr. 76, 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, Rutter M, Sonuga-Barke EJ, 2009. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. JCPP (J. Child Psychol. Psychiatry) 50, 943–951. [DOI] [PubMed] [Google Scholar]
- Mensah F, Hobcraft J, 2008. Childhood deprivation, health and development: associations with adult health in the 1958 and 1970 British prospective birth cohort studies. J. Epidemiol. Community 62, 599–606. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, Farah MJ, 2005. Neurocognitive correlates of socioeconomic status in kindergarten children. Dev. Sci. 8, 74–87. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA, 2011. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology 214, 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petanjek Z, Judaš M, Šimić G, Rašin MR, Uylings HB, Rakic P, Kostović I, 2011. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc. Natl. Acad. Sci. Unit. States Am. 108, 13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj A, van Oudenaarden A, 2008. Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135, 216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, Clasen L, Gogtay N, Giedd JN, 2011. How does your cortex grow? J. Neurosci. 31, 7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Fünfstück T, Soliman A, Villringer A, Schroeter ML, 2012. Mapping the depressed brain: a meta-analysis of structural and functional alterations in major depressive disorder. J. Affect. Disord. 140, 142–148. [DOI] [PubMed] [Google Scholar]
- Schwertman NC, Owens MA, Adnan R, 2004. A simple more general boxplot method for identifying outliers. Comput. Stat. Data Anal. 47, 165–174. [Google Scholar]
- Schwingshackl L, Hoffmann G, Kalle-Uhlmann T, Arregui M, Buijsse B, Boeing H, 2015. Fruit and vegetable consumption and changes in anthropometric variables in adult populations: a systematic review and meta-analysis of prospective cohort studies. PloS One 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semendeferi K, Armstrong E, Schleicher A, Zilles K, Van Hoesen GW, 2001. Prefrontal cortex in humans and apes: a comparative study of area 10. Am. J. Phys. Anthropol.: The Official Publication of the American Association of Physical Anthropologists 114, 224–241. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, 2008. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 28, 3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield KM, Peek MK, 2009. Neighborhood context and cognitive decline in older Mexican Americans: results from the hispanic established populations for epidemiologic studies of the elderly. Am. J. Epidemiol. 169, 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA, 2012. Variation in neural development as a result of exposure to institutionalization early in childhood. Proc. Natl. Acad. Sci. Unit. States Am. 109, 12927–12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirin SR, 2005. Socioeconomic status and academic achievement: a meta-analytic review of research. Rev. Educ. Res. 75, 417–453. [Google Scholar]
- Sörberg A, Allebeck P, Hemmingsson T, 2014. IQ and somatic health in late adolescence. Intelligence 44, 155–162. [Google Scholar]
- Steering Committee of the Physicians’ Health Study Research Group, 1988. Preliminary report: findings from the aspirin component of the ongoing Physicians’ Health Study. N. Engl. J. Med. 318, 262–264. [DOI] [PubMed] [Google Scholar]
- Steering Committee of the Physicians’ Health Study Research Group, 1989. Final report on the aspirin component of the ongoing Physicians’ Health Study. N. Engl. J. Med. 321, 129–135. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Herting MM, Goddings A-L, Meuwese R, Blakemore S-J, Dahl RE, Güroğlu B, Raznahan A, Sowell ER, Crone EA, 2017. Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J. Neurosci. 37, 3402–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Eigsti IM, 2010. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 13, 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA, 2010. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 3, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuliani TA, Shenoy M, Parikh M, Jutzy K, Hilliard A, 2017. Impact of area deprivation index on coronary stent utilization in a medicare nationwide cohort. Popul. Health Manag. 20, 329–334. [DOI] [PubMed] [Google Scholar]
- Verhaeghe P-P, Tampubolon G, 2012. Individual social capital, neighbourhood deprivation, and self-rated health in England. Soc. Sci. Med. 75, 349–357. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, Pérez-Stable EJ, Riley WT, Bloch MH, Conway K, 2018. The conception of the ABCD study: from substance use to a broad NIH collaboration. Developmental cognitive neuroscience 32, 4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen K, 2013. Cognition assessment using the NIH Toolbox. Neurology 80, S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Su S, Schmidt M, Kao C-Y, Sapiro G, 2010. The development of gyrification in childhood and adolescence. Brain Cognit. 72, 36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S, 2014. Unique developmental trajectories of cortical thickness and surface area. Neuroimage 87, 120–126. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH, 1965. Extent of recovery from the effects of visual deprivation in kittens. J. Neurophysiol. 28, 1060–1072. [DOI] [PubMed] [Google Scholar]
- Wu Y-T, Prina AM, Brayne C, 2015. The association between community environment and cognitive function: a systematic review. Soc. Psychiatr. Psychiatr. Epidemiol. 50, 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki Al Hazzouri A, Haan MN, Osypuk T, Abdou C, Hinton L, Aiello AE, 2011. Neighborhood socioeconomic context and cognitive decline among older Mexican Americans: results from the Sacramento Area Latino Study on Aging. Am. J. Epidemiol. 174, 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.