Abstract
Objective
To describe the intraindividual changes of heart biomarker levels during and after pregnancy and to evaluate existing cut-off levels for heart failure or myocardial ischaemia in pregnant women.
Method
A total of 196 healthy pregnant women were recruited from maternal outpatient clinics and included in the study. Blood samples were obtained on four occasions: at 10–12 gestational weeks (gw), 20–25 gw, after delivery and 6 months postpartum and analysed for N-terminal pro-brain natriuretic peptide (NTproBNP) and high sensitive cardiac troponin T (hs-cTNT). Echocardiography ruled out existing cardiac disease. Estimated glomerular filtration rate (eGFR) was calculated.
Results
There were significant changes in NTproBNP between the measurements with the highest NTproBNP at 10–12 gw and the lowest value being at 20–25 gw, (with eGFR being the highest). Hs-cTNT was significantly higher at the peripartum measurement compared with the other measurements (p<0.05). Regardless, the 95th percentile for both biomarkers was below cut-off levels of 300 ng/L for NTproBNP and 14 ng/L for hs-cTNT. There was an association between NTproBNP above the upper limit of normal of 125 ng/L and eGFR (p=0.04) and between hs-cTNT >5.0 ng/L and time from delivery to blood sample (p=0.001) at the peripartum measurement. Both were associated with the use of oxytocin.
Conclusion
Existing cut-off values for ruling out heart failure (NTproBNP <300 ng/L) and myocardial ischaemia (hs-cTNT <14 ng/L) are applicable during pregnancy and after delivery. Elevated levels mandate further attention on cardiac symptoms and renal function.
Keywords: myocardial disease, heart failure, cardiac function, emergency medicine, chest pain clinic
Key questions.
What is already known about this subject?
Pregnancy and delivery is associated with cardiovascular changes. Heart biomarkers N-terminal pro-brain natriuretic peptide (NTproBNP) and high sensitive cardiac troponin T (hs-cTNT) are used as diagnostic tools for heart failure and myocardial ischaemia. NTproBNP levels in healthy women during pregnancy have been described previously with comparison to a cohort of non-pregnant blood donors and were found to be higher in early pregnancy.
What does this study add?
Descriptive data of normal intraindividual change in NTproBNP and hs-cTNT during pregnancy, delivery and postpartum. This is the first study with consecutive measurements of both heart biomarkers in the same women during and after pregnancy. We relate our findings to cut-off values used in clinical practice.
How might this impact on clinical practice?
Cardiovascular symptoms in pregnant women need attention. We have shown that mild elevations of heart biomarkers are prevalent, but existing cut-off values for ruling out heart failure (NTproBNP <300 ng/L) and myocardial ischaemia (hs-cTNT <14 ng/L) are applicable during pregnancy and in the peripartum period. The consecutive levels of heart biomarkers in healthy individuals can serve as a reference for women with known or suspected heart disease.
Introduction
Pregnancy is a state of volume overload and cause changes in the circulatory systems.1–4 Delivery is a strenuous physical effort and pain, and the use of oxytocin for labour augmentation, anaesthesia, operative delivery and postpartum haemorrhage may affect the circulation. When acute cardiac symptoms occur in previously healthy individuals, heart biomarkers are used to discriminate between benign cause and cardiac-related diagnoses. NTproBNP (N-terminal pro-brain natriuretic peptide) is used for diagnosis, prognosis and risk stratification with a high negative predictive value for ruling out heart failure, a condition associated with volume overload of the ventricles.5 In patients with heart failure, NTproBNP is a strong predictor of mortality.6 High-sensitive cardiac troponin T (hs-cTNT) is a commonly used biomarker for detecting cardiac myocyte damage and is elevated in ischaemic heart disease and also increases after extreme exercise, such as running a marathon, or even minor exertion, such as spinning for 1 hour.7–9 Pregnancy is associated with changes in physical and haemodynamic parameters in healthy women.10 11 The increase in plasma volume and renal excretion during normal pregnancy may affect the levels of heart biomarkers used for ruling out heart failure (NTproBNP) or myocardial ischaemia (hs-cTNT).12 Both biomarkers are affected by age and renal function.13 14 NTproBNP is also related to body mass index (BMI), sexual hormone binding globulin and ethnicity.15–17 The aim of this prospective observational study was to consecutively evaluate normal changes in levels of NTproBNP and hs-cTNT during and after pregnancy in prepregnancy healthy women and compare them to proposed cut-off levels.
Methods
From September 2015 to April 2017, all healthy pregnant women in the catchment area of four maternal outpatient clinics with mixed socioeconomic structure in the Gothenburg area were asked to participate. Inclusion criteria were prepregnancy healthy women, age 18–44 years, no heart, lung or kidney disease, no regular medication, good understanding of the Swedish language and an intention to deliver the child at an obstetric clinic in Gothenburg. A total of 199 women with both ethnic Swedish and foreign background, the majority being Caucasian, chose to participate after informed consent. Data were obtained on four occasions:
Gestational week (gw) 10–12 at the maternal outpatient clinic. History, including previous pregnancies and smoking habit, was noted. Pregnancies conceived by in vitro fertilisation were not excluded. Data on height, weight and blood pressure were measured. Blood samples to determine haemoglobin (Hb), erythrocyte volume fraction/haematocrit (EVF), creatinine, NTproBNP and hs-cTNT and urine dipstick were drawn.
Gestational week 20–25 at the cardiology clinic in Sahlgrenska University Hospital/Östra. History, smoking habit and medications (ie, supplementary iron, vitamins and levothyroxine) were noted. Height and weight was measured. A physical examination was made, during which blood pressure was measured, and ECG and transthoracic echocardiography (TTE) were performed. Blood samples as in ‘A’ were drawn. A urine dipstick test was used to determine presence of proteinuria.
Peripartum period, after delivery, at the obstetric clinic at Sahlgrenska University Hospital. Gestational age and delivery mode, use of oxytocin as labour augmentation or postpartum haemorrhage prophylaxis and use of epidural anaesthesia were noted. Maternal and neonatal weights were measured. Complications from obstetric records, including diagnosis of preeclampsia, pregnancy induced hypertension, fever and postpartum haemorrhage >1000 mL were noted. Time (hours) from delivery to blood samples was noted. Blood samples as in ‘A’ were drawn.
Postpartum 6 months. Time (months) from delivery. History, smoking habit and postpartum complications were noted. Maternal height and weight was measured. A physical examination was administered, during which blood pressure was measured, and ECG and TTE were performed. Blood samples as in ‘A’ were drawn. Urine dipstick was taken.
We analysed the heart biomarkers in the entire cohort of prepregnancy healthy women and selected a subgroup of women consisting of women with spontaneous conception, BMI <35 at first antenatal visit, a normal pregnancy and delivery that is, no miscarriage, no treatment with levothyroxine, no hypertensive disorders of pregnancy, not more than 1+proteinuria on one occasion and a vaginal non-assisted delivery at term without complications and no postpartum haemorrhage >1000 mL. The non-selected group was prepregnancy healthy women with any of the above conditions. No participants were involved in the design, or conduct, or reporting, or dissemination of the study. All participants signed informed consent forms.
Blood samples were analysed at Sahlgrenska University Hospital, Gothenburg by an accredited central lab (ISO 15189). Hb was measured using an ADVIA 2120i (Siemens Medical Diagnostics, Dublin, Ireland) and reported as mmol/L. The EVF ratio was calculated using an ADVIA 2120i. Creatinine was analysed using a CREP 2 Cobas c111 (Roche Diagnostics, Mannheim, Germany) and reported as µmol/L. NTproBNP was analysed using an Elecsys proBNPII (Roche Diagnostics, Mannheim, Germany) and reported as ng/L. High-sensitive cardiac TNT (Hs-cTnT) was measured using an Elecsys hs-cTnT immunoassay (Roche Diagnostics, Mannheim, Germany) and reported as ng/L. BMI was calculated as weight (kg)/height2 (m). Body Surface Area (BSA) was calculated using the Du Bois formula: weight0.425 (kg) * height0.725 (cm) * 0.007184. Estimated glomerular filtration (eGFR) was calculated using the Lund-Malmö formula and reported as mL/min/1.73 m2.18 Echocardiography was recorded by two experienced physicians using General Electric Healthcare Vivid7 Dimension and subsequently re-evaluated by one experienced physician. Cardiac output (CO) was calculated according to echocardiography guidelines19 and the cardiac index (CI) was determined by CO/BSA.
Statistical analysis
Descriptive results are reported as median with IQR, mean with SD or numbers and percentage as appropriate. Comparison between the measurements from the four time points were analysed using repeated measures analysis of variance (ANOVA). Continuous variables were natural log-transformed because of non-normality. Point-biserial correlation coefficients for dichotomous variables were calculated to examine associations between cut-off values for NTproBNP and hs-cTNT and the patient characteristics. A T-test or the Mann-Whitney-U test was used for significance testing between cut-off levels and continuous variables, and Fisher’s exact test was used between categorical variables and cut-off values. A two-way between subjects repeated measures ANOVA was conducted to compare the selected subgroup from the non-selected. Bonferroni adjusted p values were used when appropriate for multiple variables. Cut-off values were chosen based on guidelines and the literature. Comparisons between hypertensive complication and not were Bonferroni-adjusted significance tested for pairwise comparisons using the Kruskal-Wallis mean rank tests. Statistical analysis was performed using IBM SPSS V.25 (Armonk, New York, USA).
Results
Included participants and missing data are shown in figure 1. A total of 196 prepregnancy healthy women form the basis for the present analysis. Two patients with twin pregnancies were excluded from analysis and one patient was excluded because of a previously undetected bicuspid aortic valve with aortic dilatation. There were 12 miscarriages between the first and second visit; three of these participants accepted a later appointment for physical examination, ECG and TTE with normal findings. No women withdrew consent. Two women experienced blood sampling distress and declined further participation, one woman moved from the area and the remaining missing visits were because lack of time or participants not showing up. The first patient was included on 18 November 2015 and the last postpartum visit was on 5 June 2018.
Figure 1.
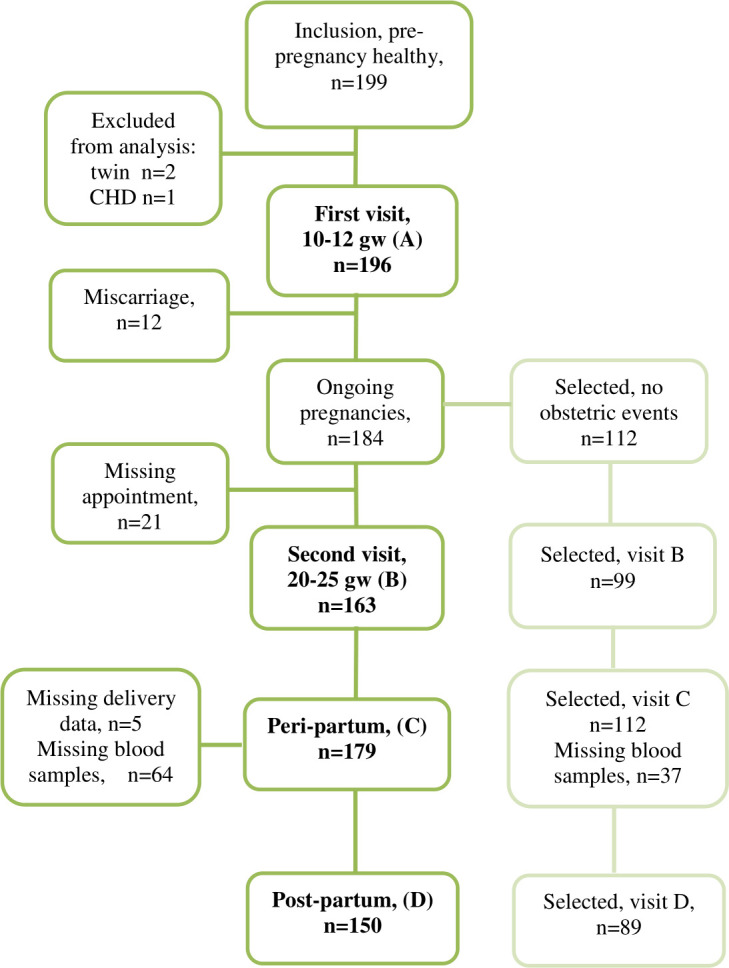
Inclusion and missing data. Inclusion criteria were prepregnancy healthy women. Selected subgroup was women without any obstetric events. CHD, congenital heart disease.
Baseline characteristics and delivery outcomes for the entire study population are shown in table 1. At the postpartum visits, no women were pregnant again. Preeclampsia complicated three pregnancies (1.7 %) and pregnancy induced hypertension complicated four pregnancies (2.2 %). Eleven women (6 %) had other peripartum complications (eg, fever). There were no cardiac complications and no perinatal deaths.
Table 1.
Characteristics of study population
| Variables | Median, (IQR) or n, (%) | N |
| Age (years) | 31 (28–34) | 196 |
| Nulliparous | 93 (48%) | 196 |
| Multiparous | 103 (52%) | 196 |
| Smoking before pregnancy | 14 (7%) | 192 |
| Non-smoking at first antenatal visit | 178 (93%) | 192 |
| IVF | 11 (6%) | 183 |
| Height at first antenatal visit, cm | 168 (163–173) | 196 |
| Weight at first antenatal visit, kg | 67 (61–75) | 195 |
| BMI at first antenatal visit | 24 (22–27) | 196 |
| BP systolic at first antenatal visit, mm Hg | 112 (107–120) | 195 |
| BP diastolic at first antenatal visit, mm Hg | 65 (60–70) | 195 |
| Gestational age at first antenatal visit, weeks | 10 (10–11) | 196 |
| Gestational age at second visit, weeks | 22 (21–24) | 163 |
| Gestational age at delivery, weeks | 40 (39–41) | 179 |
| Epidural anaesthesia | 77 (43%) | 179 |
| Spontaneous vaginal delivery | 148 (83%) | 179 |
| Elective caesarean delivery | 14 (8%) | 179 |
| Emergency caesarean delivery | 12 (7%) | 179 |
| Assisted vaginal delivery | 5 (3%) | 179 |
| Oxytocin as labour augmentation | 69 (39%) | 178 |
| Oxytocin postpartum only | 96 (54%) | 178 |
| Postpartum haemorrhage >1000 mL | 25 (14%) | 177 |
| Birth weight (g) | 3618 (3322–4020) | 178 |
| Time delivery to blood sample (hours) | 26 (17–34) | 122 |
| Time delivery to postpartum visit (months) | 7 (6–8) | 150 |
BMI, body mass index; BP, blood pressure; IVF, in vitro fertilisation.
Blood pressure, height, weight and urine samples were obtained at 10–12 gw, at 20–25 gw and postpartum. There was a statistically significant difference between the three blood pressure measurements, with the lowest systolic and diastolic being at 10–12 gw and the highest at postpartum. There was no significant change in BMI and BSA between 10–12 gw and postpartum, but the two measurements were significantly higher at 20–25 gw compared with the measurements taken on the two previous occasions.
Urine analysis showed 1+proteinuria in three women at 10–12 gw, in four women at 20–25 gw and in two women postpartum. One woman had 4+proteinuria at the postpartum visit. None of the women with proteinuria had hypertension ≥140/90 mm Hg on those occasions or NTproBNP above the upper limit of normal (ULN) of 125 ng/L or hs-cTNT >5 ng/L, except for one woman with 1+proteinuria at 20–25 gw and blood pressure 130/80 mm Hg, NTproBNP of 265 ng/L with hs-cTNT <5. This woman later developed pregnancy induced hypertension. Hb was significantly lower at 20–25 gw and peripartum compared with 10–12 gw and postpartum, and EVF showed the same pattern.
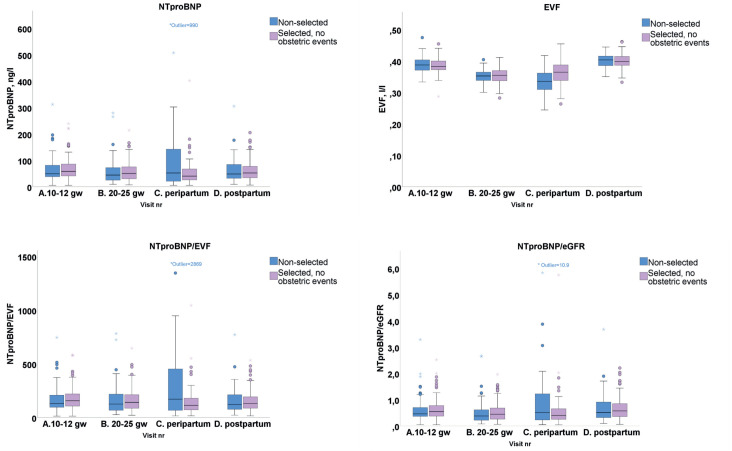
Boxplots for the consecutive measurements of NTproBNP, EVF, NTproBNP/EVF and NTproBNP/eGFR are shown in figure 2. The ULN for NTproBNP proposed by the ESC guidelines for heart failure is 125 ng/L.5 Table 2 shows NTproBNP for the four measurements, and the statistically significant changes between the time points for the entire cohort and for the selected subgroup of women without obstetric events. There was no significant difference between the selected and the non-selected subgroup. Because pregnancy is associated with an increase in plasma volume, with 13% lower EVF at 20–25 gw and peripartum compared with the postpartum levels, we related the NTproBNP values with EVF. There was no statistically significant difference in NTproBNP/EVF between the four measurements. Estimated glomerular filtration rate (eGFR) was increased during pregnancy with statistically significant changes between the four measurements, with a 23% higher median value at 20–25 gw compared with postpartum levels. When relating NTproBNP to eGFR, there was a significantly lower NTproBNP at 20–25 gw corresponding with higher glomerular filtration rate compared with first visit and postpartum, with no difference between the subgroups.
Figure 2.
Levels of NTproBNP, EVF, NTproBNP/EVF and NTproBNP/eGFR on four timepoints. One extreme outlier on the peripartum measurement (with fever during vaginal delivery but no hypertensive disease) with NTproBNP 990 ng/L. eGFR, estimated glomerular filtration rate; EVF, erythrocyte volume fraction; NTproBNP, N-terminal pro-brain natriuretic peptide.
Table 2.
NTproBNP and hs-cTNT at four time points; total prepregnancy healthy cohort and subgroups
| NTproBNP (ng/l) | ||||
| Total cohort | A (10-12 gw) n=196 | B (20-25 gw) n=163 | C (peripartum) n=120 | D (postpartum) n=150 |
| Median (range) | 56 (5–312) | 47 (7–279) | 44 (5–990) | 51 (6–305) |
| Mean, (SD) | 67 (45) | 58 (45) | 75 (112) | 62 (44) |
| 95th percentile | 155 | 140 | 207 | 149 |
| n above 300 ng/l | 1 | 0 | 4 | 1 |
| p < 0.05 vs sample | B | A, D | n.s. | B |
| Selected vs non-selected | p=0.103 | p=0.935 | p=0.164 | p=0.431 |
| Selected cohort (no events) | A n=111 | B n=98 | C n=74 | D n=89 |
| Median (range) | 57 (5–239) | 50 (7–214) | 42 (5–402) | 52 (6–205) |
| Mean (SD) | 69 (42) | 58 (40) | 55 (53) | 62 (40) |
| 95th percentile | 155 | 139 | 155 | 151 |
| n above 300 ng/l | 0 | 0 | 1 | 0 |
| p <0.05 vs sample | B, C | A, D | A | B |
| Non-selected cohort | A n=85 | B n=65 | C n=46 | D n=61 |
| Median (range) | 49 (5–312) | 44 (9–279) | 53 (5–990) | 48 (9–305) |
| Mean (SD) | 65 (48) | 57 (52) | 107 (164) | 63 (48) |
| 95th percentile | 165 | 153 | 435 | 139 |
| n above 300 ng/l | 1 | 0 | 3 | 1 |
| p <0.05 vs sample | n.s. | n.s. | n.s. | n.s. |
| Total cohort | A n=196 | B n=163 | C n=119 | D n=150 |
| Median (range) | <5 (<5–6.1) | <5 (<5–10.5) | 5.21 (<5– 30.6) | <5 (<5–11.7) |
| Mean (SD) | 5.02 (0.13) | 5.03 (0.43) | 6.67 (3.32) | 5.08 (0.65) |
| 95th percentile | <5 | <5 | 12 | <5 |
| n above 14 ng/l | 0 | 0 | 3 | 0 |
| p < 0.05 vs sample | C | C | A, B, D | C |
| Selected vs non-selected | p=0.731 | p=0.222 | p=0.171 | p=0.362 |
| Selected cohort (no events) | A n=111 | B n=98 | C n=74 | D n=89 |
| Median (range) | <5 (5–6.1) | <5 (<5–<5) | 5.2 (<5– 22.1) | <5 (<5–11.7) |
| Mean (SD) | 5.02 (0.13) | <5 (0.00) | 6.3 (2.48) | 5.13 (0.84) |
| 95th percentile | <5 | <5 | 10.6 | <5 |
| n above 14 ng/l | 0 | 0 | 1 | 0 |
| p <0.05 vs sample | C | C | A, B, D | C |
| Non-selected cohort | A n=85 | B n=65 | C n=45 | D n=61 |
| Median (range) | <5 (<5–5.9) | <5 (<5–10.5) | 5.65 (<5–30.6) | <5 (<5–<5) |
| Mean (SD) | 5.02 (0.13) | 5.08 (0.68) | 7.32 (4.33) | <5 (0) |
| 95th percentile | <5 | <5 | 13.95 | <5 |
| n above 14 ng/l | 0 | 0 | 2 | 0 |
| p <0.05 vs sample | C | C | A, B, D | C |
Hs-cTNT levels below 5.0 are reported from lab as <5.0 without further grading. ‘P<0.05 vs sample’ shows time points with significant differences between the measurements. The selected subgroup consisted of prepregnancy healthy women with spontaneous conception, BMI <35 at first antenatal visit, a normal pregnancy and delivery that is, no miscarriage, no treatment with levothyroxine, no hypertensive disorders of pregnancy, not more than 1+proteinuria on one occasion and a vaginal non-assisted delivery at term without complications and no postpartum haemorrhage >1000 mL. The non-selected group were prepregnancy healthy women with any of the above conditions.
BMI, body mass index; hs-cTNT, high sensitive cardiac troponin T; n.s., non-significant; NTproBNP, N-terminal pro-brain natriuretic peptide.
There were women who had NTproBNP levels that exceeded the ULN in non-pregnant individuals of 125 ng/L (see table 3). One woman had levels above ULN on all four occasions, 23 women had levels above ULN on one occasion, 9 women had levels above ULN on two occasions and 4 women had levels above ULN on three occasions. There were five women, without signs of heart failure with NTproBNP levels above 300 ng/L, which is above the cut-off with a high negative predictive value for ruling out heart failure. Associations with NTproBNP-levels>125 ng/L at the peripartum measurement are shown in table 4. The women who were administered oxytocin as postpartum haemorrhage prophylaxis only, more often had NTproBNP below 125 ng/L than above at the peripartum measurement (63% vs 22 %, p=0.001). Spontaneous vaginal delivery was associated with NTproBNP <125 ng/L compared with assisted or caesarean delivery. The women with elevated NTproBNP had lower eGFR compared with women with NTproBNP <125 ng/L.
Table 3.
Women with high levels of NT-proBNP (ng/L) and hs-cTNT (ng/L)
| NTproBNP | A, (10–12 gw), n=196 | B, (20–25 gw), n=163 | C (peripartum), n=120 | D (postpartum), n=150 |
| Level >125, n (%) | 17 (9) | 13 (8) | 18 (15) | 12 (8) |
| Range>125 | 129–312 | 131–279 | 130–990 | 129–305 |
| eGFR, mean (SD) | 104 (9.7) | 106 (5.2) | 96 (14.2) | 91 (9.1) |
| Age, mean (SD) | 29 (4.8) | 29 (5.5) | 32 (5.0) | 32 (3.0) |
| BMI, median (IQR) | 23 (21–28) | 23 (21–27) | 23 (20–28) | 23 (20–29) |
| Level >300, n | 1 | 0 | 4 | 1 |
| Range>300 | 312 | n.a. | 302–990 | 305 |
| eGFR, range | 95 | n.a. | 70–91 | 83 |
| Age, range | 37 | n.a. | 26–33 | 37 |
| BMI, range | 36 | n.a. | 19–30 | 36 |
| Spontaneous vaginal delivery, n | 2 | |||
| Complication*, n | 0 | n.a. | 2 | 0 |
| C.I. post, range | 2.1 | n.a. | 1.6–3.0 | 2.1 |
| Hs-cTNT | A, n=196 | B, n=163 | C, n=119 | D, n=150 |
| Level >5.0, n (%) | 4 (2) | 1 (0.6) | 65 (55) | 3 (2) |
| Range>5.0 | 5.8–6.1 | 10.5 | 5.1–30.6 | 5.4–11.7 |
| eGFR, mean (SD) | 111 (8.6) | 105 (n.a) | 101 (10.6) | 88 (4.7) |
| Age, mean (SD) | 31 (6.4) | 37 (n.a.) | 31 (4.2) | 30 (2.1) |
| BMI, median (IQR) | 24 (23–25) | 36 (n.a) | 23 (22–28) | 24 (23–28) |
| Level >14.0, n | 0 | 0 | 3 | 0 |
| Range>14.0 | n.a. | n.a. | 14.4–30.6 | n.a. |
| eGFR, range | n.a. | n.a. | 84–103 | n.a. |
| Age, range | n.a. | n.a. | 28–37 | n.a. |
| BMI, range | n.a. | n.a. | 23–30 | n.a. |
| Spontaneous vaginal delivery, n | 3 | |||
| Complication†, n | 0 | 0 | 1 | 0 |
| CI post, range | n.a. | n.a. | 1.6–2.2 | n.a. |
*One woman with fever during delivery, one woman with pregnancy induced hypertension and postpartum haemorrhage.
†One woman with fever during delivery.
BMI, body mass index; CI post, Cardiac Index at the postpartum visit; eGFR, estimated glomerular filtration rate (mL/min/1.73) measured at the same time point as NTproBNP or hs-cTNT; gw, gestational week; hs-cTNT, high sensitive cardiac troponin T; n.a., not applicable; NTproBNP, N-terminal pro-brain natriuretic peptide.
Table 4.
Peripartum measurements. Descriptives of women with hs-cTNT > 5 ng/l (n= 65) and women with NTproBNP > 125 ng/l (n=18) at the peripartum measurement compared to women with low levels.
| Variable | Hs-cTNT≤5 | Hs-cTNT>5 | NTproBNP≤125 | NTproBNP>125 | ||
| (n=54) | (n=65) | p-value | (n=102) | (n=18) | p-value | |
| Age, (mean, SD) | 31.19 (4.74) | 30.78 (4.22) | 0.627 | 30.90 (4.36) | 31.56 (5.03) | 0.568 |
| eGFR, (mean, SD) | 103.00 (12.58) | 100.77 (10.56) | 0.291 | 102.48 (10.81) | 96.50 (14.23) | 0.042 |
| Time to blood test, (median, IQR) | 29.00 (18.00) | 22.25 (15.50) | 0.001 | 26.00 (16.00) | 26.50 (20.75) | 0.906 |
| Systolic BP,* (mean, SD) | 116.66 (7.36) | 118.58 (9.13) | 0.222 | 117.79 (8.57) | 116.69 (7.11) | 0.627 |
| Diastolic BP,* (mean, SD) | 67.81 (6.72) | 68.68 (7.96) | 0.534 | 68.51 (7.57) | 67.00 (5.99) | 0.449 |
| Cardiac Index,* (mean, SD) | 3.08 (.49) | 3.05 (.52) | 0.779 | 3.07 (.52) | 3.02 (.39) | 0.7330 |
| IVF (%) | 0.454 | 1.000 | ||||
| No | 52 (96) | 60 (92) | 96 (94) | 17 (94) | ||
| Yes | 2 (4) | 5 (8) | 6 (6) | 1 (6) | ||
| Peripartum oxytocin, n (%) | 0.116 | 0.003† | ||||
| No | 5 (9) | 5 (8) | 0.735 | 7 (7) | 3 (17) | 0.170 |
| Yes, labour augmentation | 13 (25) | 28 (43) | 0.035 | 30 (30) | 11 (61) | 0.010 |
| Yes, prophylaxis only | 35 (66) | 32 (49) | 0.067 | 64 (63) | 4 (22) | 0.001 |
| Delivery Mode, n (%) | 0.116 | 0.001 | ||||
| Spontaneous vaginal | 43 (80) | 58 (89) | 0.150 | 92 (90) | 10 (56) | 0.001 |
| Other | 11 (20) | 7 (11) | 0.130 | 10 (10) | 8 (44) | 0.001 |
*measured at time point B (20-25 gw). “Other” delivery mode was assisted vaginal or cesarean section
†Bonferroni-adjusted comparisons were calculated between hs-cTNT and peripartum medications and NTproBNP and peripartum medications. The Bonferroni-adjusted p-value was set to.0083.
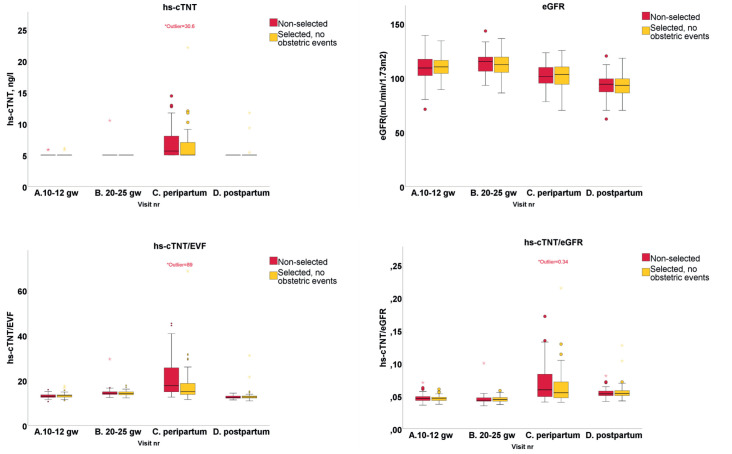
Boxplots for hs-cTNT, eGFR, hs-cTNT/EVF and hs-cTNT/eGFR on the four occasions are shown in figure 3. Table 2 shows hs-cTNT for the four measurements and the statistically significant changes between the time points for the entire cohort and for the selected subgroup of women without obstetric events. There was no significant difference between the selected and the non-selected subgroups. The analysing lab reported values equal to or above a cut-off of 5 ng/L without further grading. Healthy young individuals are expected to have hs-cTNT below 5 ng/L. The peripartum measurements were significantly higher than on all other occasions, whereas there was no difference between the other three measurements. Because renal function affects the hs-cTNT levels, we related hs-cTNT with eGFR and found a significant difference between all measurements with the highest level peripartum, but no difference between women with and without obstetric events.
Figure 3.
Levels of hs-cTNT, eGFR, hs-cTNT/EVF and hs-cTNT/eGFR on four timepoints. One extreme outlier on the peripartum measurement (with fever during vaginal delivery but no hypertensive disease) with hs-cTNT 30.6. eGFR, estimated glomerular filtration rate; EVF, erythrocyte volume fraction; hs-cTNT, high sensitive cardiac troponin T.
Table 3 shows women with hs-cTNT above 5 ng/L on the four occasions. The majority of the elevated hs-cTNT levels were on the peripartum measurement. There were three women with elevated hs-cTNT both on the first visit and at peripartum, one woman with elevated hs-cTNT on the second visit and at peripartum and two women with elevated hs-cTNT at peripartum and 6 months postpartum. Time (hours) from delivery to measurement was shorter in women with hs-cTNT above 5 ng/L (p=0.001). There was a trend but no statistically significant difference in the use of oxytocin between women with levels above or below 5 ng/L, and mode of delivery did not affect hs-cTNT (table 4). A widely used cut-off value for ruling out myocardial ischaemia is an hs-cTNT of 14 ng/L.7 There were three asymptomatic women with hs-cTNT above 14 ng/L, all at the peripartum measurement (see table 3). None of the women had hypertensive disease or other peripartum or postpartum cardiovascular complications, but one had fever during delivery.
There were seven prepregnancy healthy women with hypertensive complication (see the online supplementary appendix). Their NTproBNP at the peripartum measurement ranged from 19 to 302, hs-cTNT ranged from 5.0 to 11.7 and eGFR ranged from 78 to 112. There was no correlation between NTproBNP or hs-cTNT levels at the first visit and later hypertensive disease. There were 12 women with miscarriage who had blood samples taken at the first visit, all at 10–11 gw, and all before diagnosis of miscarriage. All of these women had heart biomarkers within the upper limits of normal.
openhrt-2020-001293supp001.pdf (102.8KB, pdf)
Discussion
The main findings are that the intraindividual descriptive analysis of normal biomarker levels during pregnancy show that established cut-off levels can be used during pregnancy. We found changes in NTproBNP levels within the same women between their pregnant and non-pregnant state, but, in the four measurements, the 75th percentile levels were below the ULN of 125 ng/L according to the 2016 ESC guidelines on heart failure.5 20 The 95th percentile of NTproBNP was below the 300 ng/L proposed for ruling out heart failure in all measurements. The highest median NTproBNP levels during pregnancy were at gw 10–12. This accords with Franz et al who found the highest levels at gw 11–15 in 94 women who were followed with four NTproBNP measurements during pregnancy and compared with a separate cohort of non-pregnant blood donors.21 22 We found significantly lower NTproBNP levels at gw 20–25 compared with postpartum levels, but no significant difference between gw 10–12 and postpartum levels. In our study, the 6-month postpartum levels were higher than in the non-pregnant control group of blood donors, in which the timing of measurements, number of participants and choice of controls may explain the different findings between our study and those of Franz et al. In the study of the blood donors, there were no data on BMI, which is why comparison is difficult because a higher BMI is associated with a lower NTproBNP.23 24 Burlingame et al compared brain natriuretic peptide (BNP) and NTproBNP during and after pregnancy in a predominantly Asian/mixed cohort with 34% of the women delivering via caesarean section compared with our 15%. In contrast to our findings, Burlingame et al found no difference between NTproBNP at gw 18–24 and postpartum 6–12 months. They found higher levels of NTproBNP than in our cohort at the peripartum measurement, but the 75th percentile was still below 300 ng/L. There was no information on renal function in the Burlingame et al study.25 In an earlier study on BNP, another heart failure biomarker, the authors found no difference between trimesters, but their comparison was not interindividual.26
The EVF changed but NTproBNP/EVF did not change between the four measurements, and when correcting NTproBNP for renal function, we found a lower NTproBNP with increasing glomerular filtration. We hypothesised that NTproBNP production increases with the increased cardiac output during pregnancy, but in the context of plasma volume changes and increased renal filtration, these changes seem to be in proportion to each other. We found that routine cut-off levels can be used during pregnancy as long as there is no renal disease or dehydration.
Cardiac troponin T, but not hs-cTNT, was studied in 50 women with preeclampsia and compared with pregnant women with diabetes or hypertension and the researchers found the levels to be higher in the preeclampsia women.27 Three women in our study had preeclampsia and also had the same levels of hs-cTNT as non-preeclampsia women, which is in contrast to a study on hs-cTroponin I (TNI) with higher levels in hypertensive women.28 TNI has also been studied during and after delivery with the highest levels being at 24 hours after delivery, but still within cut-off values for myocardial infarction.29 We found hs-cTNT to be below cut-off values for myocardial infarction during pregnancy, with or without adjustment for eGFR; none of our participants had an eGFR below 60 mL/min/1.73 m2. We found significant changes between all four measurements when relating hs-cTNT to eGFR, but those results merely reflect the changes in eGFR. However, at the peripartum measurement, three women (2.5 %) without renal impairment had hs-cTNT levels>14 ng/L without a diagnosis of myocardial infarction, which indicates that physical effort, medication and other factors may affect hs-cTNT after labour. The impact on future prognosis of slightly elevated NTproBNP and hs-cTNT in the peripartum setting is unknown.30
Limitations and strengths
This study had some limitations. We did not analyse sexual hormone binding globulin, which was positively associated with NTproBNP in a previous study.31 We chose to analyse creatinine to calculate eGFR rather than using cystatin which has been used by other authors. We used 6 months postpartum levels of biomarkers as a proxy for prepregnancy levels. However, we used echocardiography to rule out previously undetected heart disease, which is a strength of this study. In addition, we were able to compare the changes in biomarkers during pregnancy because the women were their own controls.
Conclusion
Existing cut-off values for ruling out heart failure (NTproBNP <300 ng/L) and myocardial ischaemia (hs-cTNT <14 ng/L) are applicable during pregnancy and in the peripartum period. Elevated levels mandate further attention to cardiac symptoms and renal function.
Acknowledgments
We thank Elias Johannesson, PhD, the West University, for statistical analysis, Maternal Outpatient Clinics Partille, Gamlestan, Krokslätt, Angered for including participants and taking blood samples, Helena Dellborg, research coordinator, and Görel Hultsberg-Olsson R.N. for taking blood samples and maintaining the database, the Department of Obstetrics and Gynaecology, Sahlgrenska University Hospital for taking peripartum blood samples and the participating women.
Footnotes
Contributors: All authors have contributed with design, conduct and reporting and are accountable for all aspects of the work. All authors, with EF as guarantor, take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Funding: The study was funded by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (grant number 236611). The study was also funded by The Swedish Heart-Lung Foundation (grant number 20150774).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was conducted according to the World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects and was approved by the Regional Ethical Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available.
References
- 1.Robson SC, Hunter S, Boys RJ, et al. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol 1989;256:H1060–5. 10.1152/ajpheart.1989.256.4.H1060 [DOI] [PubMed] [Google Scholar]
- 2.Yeomans ER, Gilstrap LC. Physiologic changes in pregnancy and their impact on critical care. Crit Care Med 2005;33:S256–8. 10.1097/01.CCM.0000183540.69405.90 [DOI] [PubMed] [Google Scholar]
- 3.Melchiorre K, Sharma R, Khalil A, et al. Maternal cardiovascular function in normal pregnancy: evidence of maladaptation to chronic volume overload. Hypertension 2016;67:754–62. 10.1161/HYPERTENSIONAHA.115.06667 [DOI] [PubMed] [Google Scholar]
- 4.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J 2018;39:3165–241. 10.1093/eurheartj/ehy340 [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 6.Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-Probnp testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International collaborative of NT-proBNP study. Eur Heart J 2006;27:330–7. 10.1093/eurheartj/ehi631 [DOI] [PubMed] [Google Scholar]
- 7.Hammarsten O, Fu MLX, Sigurjonsdottir R, et al. Troponin T percentiles from a random population sample, emergency room patients and patients with myocardial infarction. Clin Chem 2012;58:628–37. 10.1373/clinchem.2011.171496 [DOI] [PubMed] [Google Scholar]
- 8.Scharhag J, Herrmann M, Urhausen A, et al. Independent elevations of N-terminal pro-brain natriuretic peptide and cardiac troponins in endurance athletes after prolonged strenuous exercise. Am Heart J 2005;150:1128–34. 10.1016/j.ahj.2005.01.051 [DOI] [PubMed] [Google Scholar]
- 9.Duttaroy S, Thorell D, Karlsson L, et al. A single-bout of one-hour spinning exercise increases troponin T in healthy subjects. Scand Cardiovasc J 2012;46:2–6. 10.3109/14017431.2011.622783 [DOI] [PubMed] [Google Scholar]
- 10.Atkins AF, Watt JM, Milan P, et al. A longitudinal study of cardiovascular dynamic changes throughout pregnancy. Eur J Obstet Gynecol Reprod Biol 1981;12:215–24. 10.1016/0028-2243(81)90012-5 [DOI] [PubMed] [Google Scholar]
- 11.Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation 2014;130:1003–8. 10.1161/CIRCULATIONAHA.114.009029 [DOI] [PubMed] [Google Scholar]
- 12.Teasdale S, Morton A. Changes in biochemical tests in pregnancy and their clinical significance. Obstet Med 2018;11:160–70. 10.1177/1753495X18766170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maisel A, Mueller C, Adams K, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail 2008;10:824–39. 10.1016/j.ejheart.2008.07.014 [DOI] [PubMed] [Google Scholar]
- 14.Eggers KM, Al-Shakarchi J, Berglund L, et al. High-sensitive cardiac troponin T and its relations to cardiovascular risk factors, morbidity, and mortality in elderly men. Am Heart J 2013;166:541–8. 10.1016/j.ahj.2013.07.004 [DOI] [PubMed] [Google Scholar]
- 15.Krauser DG, Lloyd-Jones DM, Chae CU, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a proBNP investigation of dyspnea in the emergency department (pride) substudy. Am Heart J 2005;149:744–50. 10.1016/j.ahj.2004.07.010 [DOI] [PubMed] [Google Scholar]
- 16.Lam CSP, Cheng S, Choong K, et al. Influence of sex and hormone status on circulating natriuretic peptides. J Am Coll Cardiol 2011;58:618–26. 10.1016/j.jacc.2011.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta DK, Daniels LB, Cheng S, et al. Differences in natriuretic peptide levels by Race/Ethnicity (from the multi-ethnic study of atherosclerosis). Am J Cardiol 2017;120:1008–15. 10.1016/j.amjcard.2017.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Björk J, Grubb A, Sterner G, et al. Revised equations for estimating glomerular filtration rate based on the Lund-Malmö study cohort. Scand J Clin Lab Invest 2011;71:232–9. 10.3109/00365513.2011.557086 [DOI] [PubMed] [Google Scholar]
- 19.Quiñones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler quantification Task force of the nomenclature and standards Committee of the American Society of echocardiography. J Am Soc Echocardiogr 2002;15:167–84. 10.1067/mje.2002.120202 [DOI] [PubMed] [Google Scholar]
- 20.Gustafsson F, Steensgaard-Hansen F, Badskjaer J, et al. Diagnostic and prognostic performance of N-terminal proBNP in primary care patients with suspected heart failure. J Card Fail 2005;11:S15–20. 10.1016/j.cardfail.2005.04.022 [DOI] [PubMed] [Google Scholar]
- 21.Franz MB, Andreas M, Schiessl B, et al. NT-proBNP is increased in healthy pregnancies compared to non-pregnant controls. Acta Obstet Gynecol Scand 2009;88:234–7. 10.1080/00016340802596025 [DOI] [PubMed] [Google Scholar]
- 22.Hess G, Runkel S, Zdunek D, et al. Reference interval determination for N-terminal-B-type natriuretic peptide (NT-proBNP): a study in blood donors. Clin Chim Acta 2005;360:187–93. 10.1016/j.cccn.2005.04.031 [DOI] [PubMed] [Google Scholar]
- 23.Bionda C, Bergerot C, Ardail D, et al. Plasma BNP and NT-proBNP assays by automated immunoanalyzers: analytical and clinical study. Ann Clin Lab Sci 2006;36:299–306. [PubMed] [Google Scholar]
- 24.Sarzani R, Dessì-Fulgheri P, Paci VM, et al. Expression of natriuretic peptide receptors in human adipose and other tissues. J Endocrinol Invest 1996;19:581–5. 10.1007/BF03349021 [DOI] [PubMed] [Google Scholar]
- 25.Burlingame JM, Yamasato K, Ahn HJ, et al. B-type natriuretic peptide and echocardiography reflect volume changes during pregnancy. J Perinat Med 2017;45:577–83. 10.1515/jpm-2016-0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hameed AB, Chan K, Ghamsary M, et al. Longitudinal changes in the B-type natriuretic peptide levels in normal pregnancy and postpartum. Clin Cardiol 2009;32:E60–2. 10.1002/clc.20391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasupathi P, Manivannan U, Manivannan P, et al. Cardiac troponins and oxidative stress markers in non-pregnant, pregnant and preeclampsia women. Bangladesh Med Res Counc Bull 2010;36:4–9. 10.3329/bmrcb.v36i1.4806 [DOI] [PubMed] [Google Scholar]
- 28.Ravichandran J, Woon SY, Quek YS, et al. High-sensitivity cardiac troponin I levels in normal and hypertensive pregnancy. Am J Med 2019;132:362–6. 10.1016/j.amjmed.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 29.Shivvers SA, Wians FH, Keffer JH, et al. Maternal cardiac troponin I levels during normal labor and delivery. Am J Obstet Gynecol 1999;180:122–7. 10.1016/S0002-9378(99)70161-4 [DOI] [PubMed] [Google Scholar]
- 30.Manson JE, Bassuk SS. Biomarkers of cardiovascular disease risk in women. Metabolism 2015;64:S33–9. 10.1016/j.metabol.2014.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang AY, Abdullah SM, Jain T, et al. Associations among androgens, estrogens, and natriuretic peptides in young women: observations from the Dallas heart study. J Am Coll Cardiol 2007;49:109–16. 10.1016/j.jacc.2006.10.040 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2020-001293supp001.pdf (102.8KB, pdf)