Abstract
Objective
Although the prognostic role of human papilloma virus (HPV) status in oropharyngeal head and neck squamous cell carcinoma (SCC) is well established, growing evidence shows that there may be a prognostic role for HPV status in hypopharyngeal SCC. The objective of this study was to determine the prognostic role of HPV status in hypopharyngeal SCC.
Methods
We performed a retrospective, population‐based analysis of 1934 adult patients with HNSCC diagnosed between 2010‐2016 and treated with a combination of surgery and/or radiotherapy, with or without chemotherapy, and a subset of 641 patients with hypopharyngeal SCC and known HPV status included in the Surveillance, Epidemiology, and End Results (SEER) Head and Neck with HPV Status Database. Patient data were used to determine the adjusted 2‐year cancer‐specific survival (CSS) and overall survival (OS) for the entire cohort and the specific subgroup of hypopharyngeal cancer patients with known HPV status.
Results
Of the 1934 hypopharynx SCC cases, HPV status was unknown in 1294 (66.9%), and 167 (8.6%) were HPV positive; among hypopharynx cases with known HPV status, 21.6% were HPV positive. In models adjusting for sex, age, race/ethnicity, marital status and stage, patients with HPV‐positive hypopharyngeal tumors had improved CSS compared with patients with HPV‐negative tumors (CSS: HR: .57, 95% CI = .38 to .86, P = .008; OS: HR: .49, 95% CI = .34 to .71, P = <.001).
Conclusion
Our findings in a large cohort of hypopharyngeal SCC with known HPV status and cancer‐specific survival support the hypothesis that HPV has a prognostic role in hypopharyngeal cancer. Consideration should be given to increased testing for HPV in hypopharyngeal SCC.
Level of Evidence
4
Keywords: clinical research, head and neck, human papilloma virus, hypopharynx/esophagus

We performed a population‐based analysis to determine the adjusted 2‐year cancer‐specific survival and overall survival of 641 patients with hypopharyngeal SCC and known HPV status included in the Surveillance, Epidemiology, and End Results Head and Neck with HPV Status Database. In models adjusting for sex, age, race/ethnicity, marital status and stage, patients with HPV‐positive hypopharyngeal tumors had improved CSS compared with patients with HPV‐negative tumors (CSS: HR: .57, 95% CI=.38 to .86, P=.008; OS: HR: .49, 95% CI=.34 to .71, P= < .001), which supports the hypothesis that HPV has a prognostic role hypopharyngeal cancer. Consideration should be given to increased testing for HPV in hypopharyngeal HNSCC.
1. INTRODUCTION
Human papilloma virus (HPV) infection is a well‐known risk factor in the development of head and neck squamous cell carcinoma, although risk associated with this infection varies by anatomic tumor site. 1 , 2 , 3 , 4 The prognostic role of HPV infection specifically in oropharyngeal head and neck squamous cell carcinoma (HNSCC) is well established, 5 , 6 , 7 , 8 , 9 and has been incorporated into recent prognostic staging groups. 10 Accordingly, clinical treatment guidelines now include HPV testing for oropharyngeal SCC, 11 and efforts are ongoing to explore whether treatment can be tailored to HPV status.
Limited data exist about the role of HPV in nonoropharyngeal SCC, in part because these cancers are less common. Proportions of HPV or p16 (a surrogate marker for HPV infection) positivity have been estimated at 13% to 24% in hypopharyngeal cancers, 9 , 12 , 13 , 14 lower than those observed in nonoropharyngeal sites. Existing data regarding the prognostic value of HPV or p16 status 9 , 12 , 13 , 15 , 16 , 17 , 18 , 19 in hypopharyngeal SCC is conflicting. A recent large study of US Veterans suggested that there may be a prognostic role for p16 status in nonoropharyngeal SCC, including patients with hypopharyngeal cancers, 13 although the sample size for that analysis was small.
The National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) program has collected data on the HPV status of patients with head and neck squamous carcinoma diagnosed between 2010 and 2016, representing the largest known US database of HPV status that includes cancer‐specific survival for patients with hypopharyngeal cancers. The aim of this study was to investigate whether HPV status has a prognostic role in patients with hypopharyngeal squamous cell carcinoma.
2. MATERIALS AND METHODS
2.1. Study population
We performed a population‐based analysis of patients included in the Surveillance, Epidemiology, and End Results (SEER) Head and Neck with HPV Status Database. Patients who were over 18 with a pathologically confirmed diagnosis of invasive squamous cell carcinoma, available cause‐specific survival and staging, nonoverlapping primary tumor sites, and nonmetastatic disease, whose treatment included any standard therapy (including any surgery and/or radiation) were selected (eFigure 1). We selected patients treated with standard therapies because untreated patients or patients treated with a nonstandard therapy, such as chemotherapy alone, likely have poor overall prognosis due to unmeasured noncancer factors that may obscure the relationship between HPV status and survival. Given that survival outcomes included cancer death related to this diagnosis, our sample included patients with this cancer diagnosis indicated as the first of two or more cancer diagnoses.
SEER site codes were used to identify oropharyngeal, nasopharyngeal, and hypopharyngeal cancers. Tumor HPV status was determined through submission per the SEER CS Collaborative Stage Data Collection System, version 02.02‐02.05 schemas. HPV status was determined using the applicable Collaborative Stage site‐specific factor 10 for each disease site schema throughout the data collection period. 20 HPV status included any testing done on surgical specimens (HPV in situ hybridization [ISH], tissue PCR, ISH for E6/7 RNA, real time‐PCR for E6/7 RNA) and excluded blood or serology testing. Of note, individuals with only a p16 immunohistochemistry (IHC) marker are were reported as unknown HPV status. 21
Demographic variables were obtained, including age (continuous), sex (male/female), marital status (married/not married/unknown), race/ethnicity (White, Black, Hispanic, Asian/Pacific islander, and other/unknown), overall stage per AJCC 7 (as determined by TNM status), and treatment modality (surgery, radiation, surgery/radiation, surgery/chemotherapy, or surgery/radiation/chemotherapy).
Patient survival time was calculated using time from diagnosis to death (classified as head and neck cancer related or not) or November 2016, whichever came first. Using this information, we determined the unadjusted 2‐year cancer‐specific survival (CSS) and overall survival (OS) for the entire hypopharyngeal SCC cohort and Kaplan‐Meier estimates with log‐rank tests were used to evaluate differences in survival by HPV status.
We then performed the analysis in the specific subgroup of hypopharyngeal cancer patients with known HPV status. In the hypopharyngeal SCC subgroup, unadjusted cox regression models were used to test for associations between patient characteristics and survival, including HPV status, age, sex, marital status, race/ethnicity, stage, and treatment modality.
Cox regression models was used to examine the relationship between HPV status and CSS and OS after adjusting for age, sex, race/ethnicity, marital status, and stage. Then we performed a competing risks analysis (with nonhead and neck cancer death as our competing outcome) using the cumulative incidence function 22 and plotted cumulative incidence curves to confirm our survival findings, testing for differences in the curves using the Pepe‐Mori method. 23
Two‐sided P values with an α level < .05 were applied to all statistical tests. The statistical analysis was performed R v3.3 (R Core Team, Vienna, Austria). The Mount Sinai Hospital Institutional Review Board determined that the study was exempt.
3. RESULTS
3.1. Survival estimates in patients with hypopharyngeal SCC by HPV status
There were 1934 adult patients with nonmetastatic HNSCC of the hypopharynx pathologically diagnosed between 2010 and 2016 and treated with surgery and/or radiotherapy, with or without chemotherapy. Overall and cause‐specific survival by HPV status (negative, positive, and unknown) are summarized in Table 1 and survival curves are shown in eFigure 2.
TABLE 1.
Characteristics and survival of SEER hypopharyngeal SCC patients
| Overall | HPV− | HPV+ | HPV unknown | P value | |
|---|---|---|---|---|---|
| Hypopharynx SCC patients [N (%)] | 1934 (8.3) | 473 (24.4) | 167 (8.6) | 1294 (66.9) | – |
| 2 year cancer‐specific survival (95% CI) | 64.0 (61.6, 66.5) | 68.6 (63.8, 73.8) | 79.1 (72.1, 86.7) | 60.8 (57.9, 63.8) | <.001 a |
| 2 year overall survival (95% CI) | 57.2 (54.8, 59.7) | 61.8 (56.9, 67.1) | 78.1 (71.1, 85.9) | 53.5 (50.7, 56.5) | <.001 a |
Abbreviations: CI, confidence interval; HPV, human papillomavirus; SCC, squamous cell carcinoma.
P value for log‐rank (Mantel Cox) test. α = .05, CI 95% for all tests. Percentages may not add to 100 due to rounding. HPV status determined by any testing done on surgical specimens (HPV in situ hybridization [ISH], tissue PCR, ISH for E6/7 RNA, real time‐PCR for E6/7 RNA) and excluded blood or serology testing.
Median follow up was 17 months (interquartile range [IQR]: 8, 35). HPV status was unknown in 67% of hypopharynx patients. There were significant differences in cause‐specific and overall survival for hypopharynx patients by HPV status (Table 1).
3.2. Baseline characteristics and survival estimates in patients with hypopharyngeal SCC with known HPV status
We then analyzed the subset of 640 patients with hypopharyngeal SCC and known HPV status. Of the 640 hypopharynx patients with known HPV status, 167 (26.1%) were HPV positive. Baseline characteristics (including HPV status, age, sex, race, marital status, stage, and initial treatment) and cause specific and overall survival are summarized in Table 2. There were greater proportions of HPV‐positive patients who were White or Hispanic; otherwise there were no significant differences in demographics, tumor stage or treatment by HPV status.
TABLE 2.
Characteristics and survival of SEER nonmetastatic hypopharyngeal SCC patients with known HPV status
| Overall | HPV− | HPV+ | P value | |
|---|---|---|---|---|
| Patients [N (%)] | 640 (100) | 473 (73.9) | 167 (26.1) | |
| 2 year cause‐specific survival [% (95%CI)] | 71.3 (67.2, 75.6) | 68.6 (63.8, 73.8) | 79.1 (72.1, 86.7) | .005 a |
| 2 year overall survival (95% CI) | 65.9 (61.8, 70.4) | 61.8 (56.9, 67.1) | 78.1 (71.1, 85.9) | <.001 a |
| Age | ||||
| Mean (SD) | 62.8 (9.8) | 63.0 (10.0) | 62.4 (9.2) | .50 b |
| Sex | .73 c | |||
| Male [N (%)] | 533 (83.3) | 392 (82.9) | 141 (84.4) | |
| Female [N (%)] | 107 (16.7) | 81 (17.1) | 26 (15.6) | |
| Marital status | .06 c | |||
| Married [N (%)] | 299 (46.7) | 214 (45.2) | 85 (50.9) | |
| Not married [N (%)] | 307 (48.0) | 238 (50.3) | 69 (41.3) | |
| Unknown [N (%)] | 34 (5.3) | 21 (0.04) | 13 (7.8) | |
| Race/ethnicity | .02 c | |||
| White [N (%)] | 447 (69.8) | 318 (67.2) | 129 (77.2) | |
| Black [N (%)] | 77 (12.0) | 67 (14.2) | 10 (6.0) | |
| Hispanic [N (%)] | 56 (8.8) | 39 (8.2) | 17 (10.2) | |
| Asian/Pacific islander [N (%)] | 49 (7.7) | 41 (8.7) | 8 (4.8) | |
| Other/unknown [N (%)] | 11 (1.7) | 8 (1.7) | 3 (1.8) | |
| Overall stage (AJCC 7) | .37 c | |||
| I [N(%)] | 25 (3.9) | 22 (4.7) | 3 (1.8) | |
| II [N (%)] | 60 (9.4) | 47 (9.9) | 13 (7.8) | |
| III [N (%)] | 112 (17.5) | 82 (17.3) | 30 (18.0) | |
| IVX [N (%)] | 21 (3.3) | 13 (2.7) | 8 (4.8) | |
| IV A [N (%)] | 349 (54.5) | 258 (54.5) | 91 (54.5) | |
| IV B [N (%)] | 73 (11.4) | 51 (10.8) | 22 (13.2) | |
| Treatment modality | .43 c | |||
| Surgery [N (%)] | 20 (3.1) | 14 (3.0) | 6 (3.6) | |
| Radiation [N (%)] | 70 (10.9) | 57 (12.1) | 13 (7.8) | |
| Surgery and radiation [N (%)] | 39 (6.1) | 30 (6.3) | 9 (5.4) | |
| Radiation and chemotherapy [N (%)] | 432 (67.4) | 310 (65.5) | 121 (72.5) | |
| Surgery and chemotherapy [N (%)] | 4 (6.2) | 4 (0.8) | 0 (0) | |
| Surgery, radiation and chemotherapy [N (%)] | 76 (11.9) | 58 (12.3) | 18 (10.8) |
Notes: HPV status determined by any testing done on surgical specimens (HPV in situ hybridization [ISH], tissue PCR, ISH for E6/7 RNA, real time‐PCR for E6/7 RNA) and excluded blood or serology testing. Percentages may not add to 100 due to rounding.
Abbreviations: HPV, human papillomavirus; SCC, squamous cell carcinoma.
a P value for log‐rank (Mantel Cox) test.
b P value for one‐way ANOVA.
c P value for Pearson's chi‐squared test.
Median follow up in the hypopharynx SCC subgroup with known HPV status was 17 months (IQR: 8, 35). In unadjusted models (Table 3, Figure 1), patients with HPV positivity had improved 2‐year cancer‐specific survival and overall survival.
TABLE 3.
Unadjusted cancer‐specific and overall survival of SEER nonmetastatic hypopharyngeal SCC patients with known HPV status
| Cancer‐specific survival | Overall survival | |||||
|---|---|---|---|---|---|---|
| Covariable | Unadjusted HR | 95% CI | P value a | Unadjusted HR | 95% CI | P value a |
| HPV status | .005 | <.001 | ||||
| Negative | (1.00) | (1.00) | ||||
| Positive | 0.56 | (0.38, 0.84) | 0.48 | (0.33, 0.69) | ||
| .50 | .70 | |||||
| Sex | ||||||
| Male | (1.00) | (1.00) | ||||
| Female | 0.86 | (0.56, 1.33) | 1.08 | (0.76, 1.53) | ||
| Age (y) | 1.01 | (1.00, 1.03) | .09 | 1.02 | (1.01, 1.04) | .005 |
| Marital status | .004 | <.001 | ||||
| Not married | (1.00) | (1.00) | ||||
| Married | 1.45 | (1.04, 2.00) | 1.56 | (1.18, 2.07) | ||
| Unknown | 2.38 | (1.36, 4.17) | 2.25 | (1.36, 3.75) | ||
| Race/Ethnicity | .20 | .005 | ||||
| White | (1.00) | (1.00) | ||||
| Black | 1.54 | (0.99, 2.40) | 1.93 | (1.35, 2.77) | ||
| Hispanic | 1.25 | (0.71, 2.18) | 1.11 | (0.66, 1.87) | ||
| Asian/Pacific islander | 1.59 | (0.94, 2.69) | 1.54 | (0.96, 2.47) | ||
| Other/unknown | 1.37 | (0.43, 4.30) | 1.75 | (0.72, 4.27) | ||
| Overall stage (AJCC 7) | .007 | .09 | ||||
| I | (1.00) | (1.00) | ||||
| II | 2.51 | (0.29, 21.49) | 2.14 | (0.72, 6.35) | ||
| III | 6.71 | (0.91, 49.35) | 2.27 | (0.81, 6.36) | ||
| IVX | 7.23 | (0.84, 61.87) | 2.20 | (0.62, 7.79) | ||
| IV A | 9.32 | (1.30, 66.83) | 2.78 | (1.03, 7.53) | ||
| IV B | 10.49 | (1.42, 77.71) | 3.71 | (1.351, 10.49) | ||
| Treatment modality | .20 | .02 | ||||
| Surgery | (1.00) | (1.00) | ||||
| Radiation | 3.56 | (0.82, 15.53) | 4.48 | (1.36, 14.79) | ||
| Surgery and radiation | 1.32 | (0.26, 6.82) | 1.98 | (0.55, 7.10) | ||
| Radiation and chemotherapy | 2.72 | (0.67, 11.01) | 2.34 | (0.74, 7.33) | ||
| Surgery and chemotherapy | 7.16 | (0.65, 79.15) | 4.93 | (0.51, 47.45) | ||
| Surgery, radiation, and chemotherapy | 3.01 | (0.71, 12.78) | 2.40 | (0.73, 7.91) | ||
Abbreviations: CI, confidence interval; HPV, human papillomavirus; HR, hazard ratio; SCC, squamous cell carcinoma.
Using cox proportional hazards models, Wald test. α = .05, CI 95% for all tests. HPV status determined by any testing done on surgical specimens (HPV in situ hybridization [ISH], tissue PCR, ISH for E6/7 RNA, real time‐PCR for E6/7 RNA) and excluded blood or serology testing.
FIGURE 1.
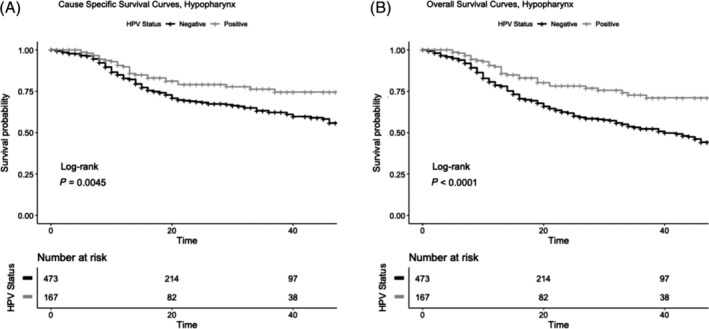
Cancer‐specific (A) and overall (B) survival of SEER nonmetastatic Hypopharyngeal SCC patients with known HPV status. HPV, human papillomavirus; SCC, squamous cell carcinoma. P value for log‐rank (Mantel Cox) test. α = .05, CI 95% for all tests. HPV status determined by any testing done on surgical specimens (HPV in situ hybridization [ISH], tissue PCR, ISH for E6/7 RNA, real time‐PCR for E6/7 RNA) and excluded blood or serology testing
In adjusted models (Table 4), patients with HPV‐positive hypopharyngeal tumors had improved CSS (HR: .57, 95% CI = .38 to .86, P = .008) and OS (HR: .49, 95% CI = .34 to .72, P = <.001) compared with patients with HPV‐negative tumors. Competing risks analysis also found that HPV‐positive tumors had lower risk of cancer‐related death after accounting for noncancer causes of death (P = .01, Figure 2).
TABLE 4.
Adjusted cancer‐specific and overall survival of SEER nonmetastatic hypopharyngeal SCC patients with known HPV status
| Cancer‐specific survival | Overall survival | ||||||
|---|---|---|---|---|---|---|---|
| Covariable | Adjusted HR | 95% CI | P value a | Adjusted HR | 95% CI | P value a | |
| HPV status | |||||||
| Negative | (1.00) | (1.00) | |||||
| Positive | .57 | (.38, .86) | .008 | .49 | (.34, .72) | <.001 | |
| Sex | |||||||
| Male | (1.00) | (1.00) | |||||
| Female | 1.05 | (.67, 1.65) | .83 | 1.20 | (.83, 1.73) | .34 | |
| Age (y) | 1.02 | (1.01, 1.04) | .005 | 1.03 | (1.02, 1.05) | <.001 | |
| Marital status | |||||||
| Not married | (1.00) | (1.00) | |||||
| Married | 1.38 | (.98, 1.95) | .06 | 1.42 | (1.05, 1.91) | .02 | |
| Unknown | 3.04 | (1.74, 5.33) | <.001 | 2.64 | (1.56, 4.44) | <.001 | |
| Race/ethnicity | |||||||
| White | (1.00) | (1.00) | |||||
| Black | 1.46 | (.93, 2.29) | .13 | 1.80 | (1.24, 2.61) | .002 | |
| Hispanic | 1.11 | (.63, 1.95) | .71 | 1.01 | (.60, 1.71) | .96 | |
| Asian/Pacific islander | 1.55 | (.89, 2.68) | .12 | 1.36 | (.83, 2.21) | .22 | |
| Other/unknown | 1.00 | (.31, 3.21) | .99 | 1.30 | (.52, 3.24) | .57 | |
| Overall stage (AJCC 7) | |||||||
| I | (1.00) | (1.00) | |||||
| II | 2.16 | (.25, 18,55) | .48 | 1.91 | (.64, 5.74) | .25 | |
| III | 7.02 | (.95, 52.04) | .06 | 2.43 | (.86, 6.90) | .10 | |
| IV X | 7.62 | (.89, 65.71) | .06 | 2.47 | (.69, 8.83) | .16 | |
| IV A | 10.04 | (1.34, 72.62) | .02 | 3.14 | (1.14, 8.61) | .03 | |
| IV B | 12.59 | (1.68, 94.56) | .01 | 4.59 | (1.58, 13.30) | .005 | |
Abbreviations: CI, confidence interval; HR, hazard ratio; HPV, human papillomavirus; SCC, squamous cell carcinoma.
Using cox proportional hazards models, Wald test. α = .05, CI 95% for all tests. HPV status determined by any testing done on surgical specimens (HPV in situ hybridization [ISH], tissue PCR, ISH for E6/7 RNA, real time‐PCR for E6/7 RNA) and excluded blood or serology testing.
FIGURE 2.
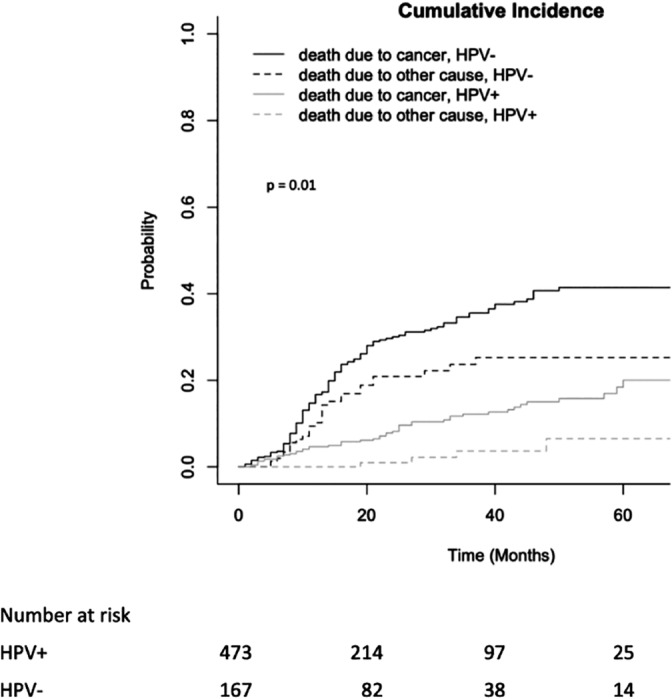
Cumulative incidence of death due to cancer and non‐cancer‐related death in SEER nonmetastatic Hypopharyngeal SCC patients with known HPV status. HPV, human papillomavirus; SCC, squamous cell carcinoma. P value for log‐rank (Mantel Cox) test. α = .05, CI 95% for all tests. HPV status determined by any testing done on surgical specimens (HPV in situ hybridization [ISH], tissue PCR, ISH for E6/7 RNA, real time‐PCR for E6/7 RNA) and excluded blood or serology testing
4. DISCUSSION
In this large population‐based study of patients with treated hypopharyngeal SCC we found that HPV‐positive tumors were associated with a better prognosis than HPV‐negative tumors. This finding supports consideration for HPV testing among all hypopharyngeal cancers. Additional testing coupled with careful staging are needed prior to interpreting how HPV status may impact management of hypopharyngeal SCC.
Prevalence of HPV or p16 positivity in hypopharyngeal SCC has been estimated to be 13% to 24%, 9 , 12 , 14 and our sample included ~9% HPV positive hypopharyngeal cancers among all patients. However, among those with HPV testing, the proportion of HPV‐positive tumors was higher in our sample, at ~26%. This suggests that the prevalence of HPV‐positivity may be higher than expected in hypopharyngeal SCC, although the large proportion of cases without HPV status and our lack of data regarding the reasons for HPV testing in the cancer cases limits this conclusion. For example, tumors adjacent or with extension to the oropharynx may have been more likely to be tested and it is possible that in a large registry that there may be misclassification of tumor origin. Furthermore, there may be variability in the detection assay results collected by SEER that account for this high prevalence, including nonquantitative PCR, that are less reliable and overestimate clinically significant HPV detection. 24 SEER required tissue HPV testing to establish tumor viral involvement as opposed to p16 IHC assessment, a commonly employed surrogate marker of HPV‐associated tumors. P16 has been found to have less concordance with HPV testing in nonoropharyngeal HNSCC sites, 5 , 9 , 12 , 18 , 25 also potentially explaining these differences. Nonetheless, our results are consistent with a global meta‐analysis showing the prevalence of laryngeal/hypopharyngeal high‐risk HPV positivity to be ~24%, 14 and these data likely reflect variable detection assays similar to SEER. However, misclassification of p16 positive tumors as HPV 16 positive for oropharyngeal patients was observed in a review of SEER registry data from Iowa, 26 and it is possible that the HPV variable in this dataset includes some tumors that were similarly misclassified. We do not yet know the accuracy of HPV testing in the hypopharynx subset, though SEER continues their quality assurance efforts. Despite this, other recent findings indicating the potential prognostic value of both p16 and HPV support the conclusion that testing both p16 and HPV to determine how hypopharyngeal cancers differ from other head and neck cancers with regard to the prognostic significance of each of these markers is warranted. 12 , 13
Other studies have shown results consistent with our findings. Chung et al. 12 evaluated p16 and HPV status of patients treated on Radiation Therapy Oncology Group (RTOG) 0129, 0234 and 9501 trials, finding that p16‐positive hypopharynx SCC patients had improved progression‐free survival and a trend toward improved overall survival in a small cohort of 61 patients. Improved overall survival outcomes in HPV‐positive hypopharyngeal SCC patients have been observed in single institution or database studies, although these studies do not include cancer‐specific outcomes, 16 , 17 , 27 were combined with other subsites and/or were limited to patients with locally advanced disease, 28 or included patients with metastatic disease and those who did not receive appropriate therapies. 17 In comparison, our analysis of cancer‐specific outcomes was limited to patients with nonmetastatic disease and those who likely received standard therapy, though our results remain consistent with these other studies. In particular, our study included cancer‐specific survival and our findings were consistent with Bryant and colleagues 13 who identified United States Veterans Affairs patients with HNSCC and found that p16 had a prognostic role in nonoropharyngeal cancers including both laryngeal and hypopharyngeal cancers, using competing risks models incorporating smoking and comorbidities. Our findings support their results in a larger, more representative sample including women.
However, our findings of improved cause‐specific and overall survival in HPV‐positive hypopharyngeal SCC are inconsistent with smaller studies looking at the role of p16 or HPV in nonoropharyngeal SCC. A study by Wilson et al. evaluated 27 hypopharyngeal patients by retrospective chart review, and found that there was no prognostic significance of p16 status. 15 Other small studies have shown similar results, 18 , 19 , 29 , 30 , 31 though most were limited by a small sample size given the lower frequency of HPV positivity in hypopharyngeal SCC. One of the larger studies evaluated cancer‐specific survival in pooled outcomes for larynx (62 patients) and hypopharynx (14 patients) cases which found no difference in survival outcomes based on HPV status, although it included only seven HPV+ patients thus limiting the interpretation of the results. 18 Similarly, in the pooled analysis of DAHANCA trials, Lassen and colleagues 9 found no association of p16 status and survival in hypopharyngeal patients, though their sample size was limited to 158 patients with only 21 (13%) being p16 positive. These studies were limited by small sample sizes, and classification by p16 positivity may have identified a different patient group than our study which utilized testing for viral antigens and nucleic acids.
Treatment de‐escalation in patients with HPV‐related oropharyngeal SCC based on improved prognosis has generated considerable interest with several ongoing trials. 32 , 33 Although recent studies show that chemotherapy deintensification for HPV‐related oropharyngeal SCC has not been successful, 34 , 35 efforts to deintensify radiation or surgery in oropharyngeal SCC 33 continue. Conversely, treatment escalation in patients with non‐HPV‐related oropharyngeal SCC patients is also being explored. 36 This framework may translate to cancers of the hypopharynx in an effort to reduce therapy‐related morbidity, though careful consideration of incorporating broad HPV testing and other clinical factors is required prior to exploring treatment modification in hypopharyngeal SCC.
4.1. Limitations
Strengths of this study include the large sample size and representativeness of the patient population such that it is generalizable to the US population. However, our findings must be viewed in light of several limitations. First, the lack of smoking or other comorbidity data limit the interpretation of our findings. However, SEER does include cause of death information that allows us to account for mortality not related to head and neck cancer so we performed a competing risks analysis to account for noncancer mortality. Second, detailed treatment information on chemotherapy and radiotherapy is not available, nor are data regarding comorbidities that may influence treatment selection, thus we cannot interpret our data with respect to HPV status predicting response to treatment though treatment selection has been shown to be of importance in patients with hypopharyngeal cancer. 37 In addition, as discussed above, there may be classification errors (both in exact tumor site and HPV status), which is why we believe our findings ultimately support exploration of testing for both p16 and HPV status to determine the prognostic significance of both of these factors. The addition of p16 status to the SEER registry would provide additional important information. Our findings also suggest greater influence of HPV status with longer follow up, and a future analysis should be performed for later time points once the SEER data have matured to a greater extent. Lastly, caution must be used when considering treatment modification based on registry data, and these findings cannot support modification of current diagnostic or therapeutic practices without further validation.
5. CONCLUSION
Our findings in a large cohort of hypopharyngeal HNSCC with known HPV status and cancer‐specific outcomes support the hypothesis that HPV has a prognostic role hypopharyngeal cancer. Consideration should be given to increased testing for HPV in hypopharyngeal HNSCC.
CONFLICT OF INTEREST
Dr. Wisnivesky has received consulting honorarium from Sanofi, Galaxosmithkline, and Banook and research grants from Sanofi and Quorum unrelated to the manuscript. All other authors have no financial conflicts of interest to disclose.
Supporting information
Data S1 eFigure 1. Patient selection diagram.
eFigure 2. Cancer‐specific and overall survival of SEER nonmetastatic hypopharyngeal SCC patients. HPV, human papillomavirus; SCC, squamous cell carcinoma. P value for log‐rank (Mantel Cox) test. α = .05, CI 95% for all tests. HPV status determined by any testing done on surgical specimens (HPV in situ hybridization [ISH], tissue PCR, ISH for E6/7 RNA, real time‐PCR for E6/7 RNA) and excluded blood or serology testing.
ACKNOWLEDGMENTS
Funding: Supported in part by the National Institutes of Health (NIH) T32CA225617 (DCM). The NIH had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Marshall DC, Kao DD, Bakst R, et al. Prognostic role of human papilloma virus status in hypopharyngeal squamous cell carcinoma. Laryngoscope Investigative Otolaryngology. 2020;5:860–867. 10.1002/lio2.443
Funding information National Institutes of Health, Grant/Award Number: T32CA225617
BIBLIOGRAPHY
- 1. Gillison ML. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709‐720. 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 2. Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16‐positive and human papillomavirus type 16‐negative head and neck cancers. J Natl Cancer Inst. 2008;100:407‐420. 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 3. Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous‐cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125‐1131. 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 4. Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91:386‐396. 10.1016/j.mayocp.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 5. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24‐35. 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rischin D, Young RJ, Fisher R, et al. Prognostic significance of p16INK4Aand human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142‐4148. 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rietbergen MM, Brakenhoff RH, Bloemena E, et al. Human papillomavirus detection and comorbidity: critical issues in selection of patients with oropharyngeal cancer for treatment de‐escalation trials. Ann Oncol. 2013;24:2740‐2745. 10.1093/annonc/mdt319. [DOI] [PubMed] [Google Scholar]
- 8. Posner MR, Lorch JH, Goloubeva O, et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071‐1077. 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lassen P, Primdahl H, Johansen J, et al. Impact of HPV‐associated p16‐expression on radiotherapy outcome in advanced oropharynx and non‐oropharynx cancer. Radiother Oncol. 2014;113:310‐316. 10.1016/j.radonc.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 10. Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th ed. American College of Surgeons: Chicago, IL; 2017. 10.1007/978-3-319-40618-3 [DOI] [Google Scholar]
- 11. Al‐Sarraf M, Balaraman S. Head and neck cancers NCCN Guidelines Version 2.2019. National Comprehensive Cancer Network; 2019. 10.1016/s0921-4410(04)22018-x. [DOI] [Google Scholar]
- 12. Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32:3930‐3938. 10.1200/JCO.2013.54.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bryant AK, Sojourner EJ, Vitzthum LK, et al. Prognostic role of p16 in nonoropharyngeal head and neck cancer. J Natl Cancer Inst. 2018;110:1393‐1399. 10.1093/jnci/djy072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systemic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467‐475. 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 15. Wilson DD, Rahimi AS, Saylor DK, et al. p16 not a prognostic marker for hypopharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2012;138:556‐561. 10.1001/archoto.2012.950. [DOI] [PubMed] [Google Scholar]
- 16. Tian S, Switchenko JM, Jhaveri J, et al. Survival outcomes by high‐risk human papillomavirus status in nonoropharyngeal head and neck squamous cell carcinomas: a propensity‐scored analysis of the National Cancer Data Base. Cancer. 2019;125(16):2782–2793. 10.1002/cncr.32115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li H, Torabi SJ, Yarbrough WG, Mehra S, Osborn HA, Judson B. Association of human papillomavirus status at head and neck carcinoma subsites with overall survival. JAMA Otolaryngol Head Neck Surg. 2018;144:519‐525. 10.1001/jamaoto.2018.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salazar CR, Smith RV, Garg MK, et al. Human papillomavirus‐associated head and neck squamous cell carcinoma survival: a comparison by tumor site and initial treatment. Head Neck Pathol. 2014;8:77‐87. 10.1007/s12105-013-0486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stephen JK, Divine G, Chen KM, Chitale D, Havard S, Worsham MJ. Significance of p16 in site‐specific HPV positive and HPV negative head and neck squamous cell carcinoma. Cancer Clin Oncol. 2013;2:51‐61. 10.5539/cco.v2n1p51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collaborative Stage Data Collection System, Versions 02.02‐02.05. https://cancerstaging.org/cstage/coding/Pages/Version-02.05.aspx. Accessed November 20, 2019.
- 21. American Joint Committee on Cancer . CAnswer forum; Collaborative Stage; Pharynx; Oropharynx. http://cancerbulletin.facs/org/forums. Accessed November 20, 2019.
- 22. Gray RJ. A class of K‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141‐1154. [Google Scholar]
- 23. Pepe MS, Mori M. Kaplan‐Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993;12(8):737‐751. 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 24. Witt BL, Albertson DJ, Coppin MG, Horrocks CF, Post M, Gulbahce HE. Use of in situ hybridization for HPV in head and neck tumors: experience from a national reference laboratory. Head Neck Pathol. 2015;9(1):60‐64. 10.1007/s12105-014-0549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lingen MW, Xiao W, Schmitt A, et al. Low etiologic fraction for high‐risk human papillomavirus in oral cavity squamous cell carcinomas. Oral Oncol. 2013;49:1‐8. 10.1016/j.oraloncology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 26. Kahl A, Charlton M, Pagedar N, et al. Accuracy of the HPV status site‐specific factor 10 (SSF‐10) variable for patients with oropharyngeal cancers in the Iowa Cancer Registry, 2010‐2014. Head Neck. 2018;40(10):2199‐2209. 10.1002/hed.25314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bates JE, Morris CG, Hitchcock KE, Dziegielewski PT, Mendenhall WM, Amdur RJ. Locally advanced hypopharyngeal and laryngeal cancer: influence of HPV status. Radiother Oncol. 2019;140:6‐9. 10.1016/j.radonc.2019.05.013. [DOI] [PubMed] [Google Scholar]
- 28. Burr R, Harari M, Ko C, et al. HPV impacts survival of stage IVC non‐oropharyngeal HNSCC cancer patients. Otorhinolaryngol Neck Surg. 2017;3(1):1–7. 10.15761/ohns.1000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hughes RT, Beuerlein WJ, O'Neill SS, et al. Human papillomavirus‐associated squamous cell carcinoma of the larynx or hypopharynx: clinical outcomes and implications for laryngeal preservation. Oral Oncol. 2019;98:20‐27. 10.1016/j.oraloncology.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kachnic LA, Winter K, Myerson RJ, et al. NRG Oncology/RTOG 0529: long‐term outcomes of dose‐painted intensity modulated radiation therapy, 5‐fluorouracil, and mitomycin‐C in anal canal cancer. Int J Radiat Oncol. 2017;99(2):S64‐S65. 10.1016/j.ijrobp.2017.06.159. [DOI] [Google Scholar]
- 31. Dalianis T, Grün N, Koch J, et al. Human papillomavirus DNA and p16INK4a expression in hypopharyngeal cancer and in relation to clinical outcome, in Stockholm, Sweden. Oral Oncol. 2015;51:857‐861. 10.1016/j.oraloncology.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 32. O'Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus‐related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543‐550. 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 33. Kelly JR, Husain ZA, Burtness B. Treatment de‐intensification strategies for head and neck cancer. Eur J Cancer. 2016;68:125‐133. 10.1016/j.ejca.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus‐positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non‐inferiority trial. Lancet. 2019;393:40‐50. 10.1016/S0140-6736(18)32779-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low‐risk human papillomavirus‐positive oropharyngeal cancer (De‐ESCALaTE HPV): an open‐label randomised controlled phase 3 trial. Lancet. 2019;393:51‐60. 10.1016/S0140-6736(18)32752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. AF CRT +/− Nimorazole in HNSCC. http://clinicaltrials.gov/show/NCT01880359. Accessed February 15, 2020.
- 37. Hochfelder CG, McGinn AP, Mehta V, Castellucci E, Kabarriti R, Ow TJ. Treatment sequence and survival in locoregionally advanced hypopharyngeal cancer: a surveillance, epidemiology, and end results‐based study. Laryngoscope. 2019;1‐11. 10.1002/lary.28452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 eFigure 1. Patient selection diagram.
eFigure 2. Cancer‐specific and overall survival of SEER nonmetastatic hypopharyngeal SCC patients. HPV, human papillomavirus; SCC, squamous cell carcinoma. P value for log‐rank (Mantel Cox) test. α = .05, CI 95% for all tests. HPV status determined by any testing done on surgical specimens (HPV in situ hybridization [ISH], tissue PCR, ISH for E6/7 RNA, real time‐PCR for E6/7 RNA) and excluded blood or serology testing.


