Abstract
Objective
To analyze the correlations of saliva production and pH value with disease activity, disease severity, and oral health-related quality of life in patients with diffuse cutaneous systemic sclerosis (dcSSc) without concomitant Sjögren’s syndrome (SS) or SS-related antibodies.
Methods
This cross-sectional study included 28 patients with dcSSc and matching healthy controls. Sialometric assessment and caries status were compared between the two groups. Clinical and laboratory parameters were used to evaluate disease severity, in accordance with the Medsger Severity Scale.
Results
In patients with dsSSc, reduced saliva production and higher pH value were associated with disease activity and severity; moreover, caries status was correlated with SSc disease characteristics, including disease duration and disease severity. Oral health-related quality of life was negatively correlated with mean salivary flow rate.
Conclusions
These findings contradict the existing notion that reduced saliva production in patients with SSc is linked to SS-related antibodies or caused by underlying SS. In addition, patients with dcSSc exhibit elevated risk of cardiovascular disease and invasive dental treatment has been shown to enhance the rates of stroke and heart attack in the general population; therefore, oral health is particularly important in patients with SSc.
Keywords: Diffuse cutaneous systemic sclerosis, salivary flow, caries, tooth status, activity, severity
Introduction
Systemic sclerosis (SSc) is a rare connective tissue disease, characterized by inflammation, vascular dysfunction, and excessive fibrosis of connective tissue supporting the skin and visceral organs.1 The most widely used classification system divides SSc into limited cutaneous and diffuse cutaneous (dcSSc) subtypes.2 The prevalence of SSc appears to be lower in Europe than in the United States; in Croatia, the estimated prevalence of SSc is 15.6 per 100,000.3
The orofacial region is affected in approximately 80% of patients with SSc. Management of SSc mainly involves treatment of organ-specific complications. Orofacial symptoms are typically overlooked owing to the severity of systemic manifestations.4 When orofacial symptoms are diagnosed, treatment is generally based on prevention of infections and caries. Xerostomia is a disease caused by reduced salivary flow due to various factors.5 Medications include salivary substitutes and sialagogue drugs. Approximately 30% to 40% of patients with SSc exhibit xerostomia.6,7 The etiology of xerostomia includes salivary gland fibrosis or concomitant Sjögren’s syndrome (SS). A prospective study of consecutive patients with SSc revealed that the prevalence of SS was 68%.8 Further investigation of labial salivary gland biopsies in that study revealed that 58% of patients with SSc and SS had glandular fibrosis; 23% had concomitant SS, indicated by lymphocytic sialadenitis. Notably, patients with SSc have lower salivary flow rates and salivary pH values, compared with healthy individuals.9 Reduced saliva production in patients with SSc has been associated with SS-related antibodies.10 However, reduced saliva production has not been associated with disease severity in patients with SSc.10,11
Quality of life is severely impaired in patients with SSc.12 An investigation of oral health-related quality of life (HRQoL), using the Oral Health Impact Profile, showed that HRQoL is lower in patients with SSc than in the general population.13 However, patients with SSc and concomitant SS or SS-related antibodies were included in most previous studies, which greatly influenced the conclusions of those studies. A recent study involving patients with SSc, without concomitant SS or SS-related antibodies, confirmed the impairment of unstimulated and stimulated salivary flow, regardless of disease subtype.14 Importantly, direct saliva measurement and possible correlations with disease characteristics were not evaluated in prior studies. In this study, we hypothesized that saliva production and pH value may be related to disease activity, disease severity, and oral HRQoL in patients with dcSSc, without concomitant SS or SS-related antibodies.
Materials and methods
Participants
This cross-sectional study was conducted from August 2015 to February 2017 at University Hospital Center (UHC) Split (Split, Croatia). Patients who met the 2013 American College of Rheumatology/European League Against Rheumatism classification criteria for definite SSc were eligible to participate in the study.15 The distinction between limited cutaneous SSc and dcSSc was made in accordance with the criteria described by LeRoy et al.2 Disease duration was calculated from the onset of initial manifestations of disease, without Raynaud’s phenomenon. Patients with dcSSc were included regardless of whether they had missing dentures; included patients also did not take medication that could influence salivary gland function (within 4 weeks of enrollment) and did not smoke. Patients were excluded if they had acute or chronic infections (mucositis or candidiasis); they were also excluded if they had other systemic and periodontal diseases (by assessment of periodontal pocket depth and clinical attachment level). Secondary SS was a key exclusion criterion; patient assessment included questionnaires for xerostomia and xeropthalmia, Schirmer’s I test, unstimulated whole salivary flow, minor salivary gland biopsy, and assessment of anti-Ro/La antibodies. In patients with another well-defined connective tissue disease, the presence of one symptom (ocular or oral) plus two of the three objective criteria (i.e., ocular signs, histopathology, or oral signs) was considered indicative of secondary SS.16 Patients with dsSSc were matched for sex and age with healthy controls for comparisons.
Ethical considerations
All procedures performed in the study were in accordance with the ethical standards of UHC Split (local Ethics Committee, reference number 2181-198-03-04-16-0022) and complied with the tenets of the 1964 Helsinki declaration and its later amendments. A biopsy in the healthy control group was not performed, in accordance with the ethics committee guidelines. All participants provided written informed consent, prior to inclusion in the study.
Assessments
Each participant underwent rheumatological examination and comprehensive laboratory evaluation. Indirect immunofluorescence on HEp-2 cells was used to determine the presence of anticentromere antibodies, and an enzyme-linked immunosorbent assay (Cat. No. BI-5000; Biomedica, Vienna, Austria) was used to test for SSc- and SS-related antibodies (i.e., anti-Scl70, anti-Ro 52/TRIM-21, anti-SSA/Ro 60, and anti-SSB/La antibodies).
Glandular saliva was collected in a standardized manner. To minimize circadian fluctuations of salivary secretion, assessments of salivary flow rate were performed at a pre-defined time of day.17 Salivary pH was measured with a pH meter. A single examiner (K.P.) assessed periodontal and caries statuses. Interincisal distance was measured as the distance from the incisal edge of the lower central incisor tooth to the incisal edge of the upper central incisor.18 Oral mucosal, periodontal, and tooth conditions were examined. Periodontal disease status was assessed with respect to periodontal pocket depth and clinical attachment level.10 The decayed, missing, and filled teeth (DMFT) index was used to evaluate tooth status.
The Croatian version of the Oral Health Impact Profile 49 was used to evaluate oral HRQoL; the questionnaire was used without any modifications relative to the published version.19 The European Scleroderma Trials and Research 2016 revised standard was used to assess disease activity, with a score ≥2.5 defined as active disease.20 The modified Rodnan skin score (mRSS) was used to evaluate skin involvement, with skin thickness as a surrogate for disease severity.21,22 Severity scores for each of the nine organ systems were established, in accordance with the modified Medsger Disease Severity Scale.23
Statistical analysis
All analyses were performed with IBM SPSS Statistics, version 22.0 (IBM Corp., Armonk, NY, USA). P-values <0.05 were considered statistically significant for all data analyses. Means ± standard deviations were calculated for continuous variables in both groups; the Mann–Whitney U test was used for all comparisons of continuous variables between the two groups. Associations between qualitative variables were analyzed with the chi-squared test, chi-squared test for linear trend, or Fisher’s exact test when appropriate. Spearman rank coefficients were calculated for all linear correlations between continuous variables.
Results
Twenty-eight consecutive patients with dsSSc (93% women; mean age, 51 years; median disease duration, 6 years) were enrolled in this study (Table 1). Six patients with dcSSc were excluded from the study due to the presence of secondary SS. Comparisons of sialometric assessments (pH, unstimulated salivary flow [USF] rate and stimulated salivary flow [SSF] rate) and caries status (DMFT index) between patients with dcSSc and healthy controls are provided in Table 2. Resting and stimulated pH saliva values were significantly lower in patients with dcSSc than in healthy controls (P < 0.001 and P < 0.01, respectively); moreover, oral HRQoL (i.e., Oral Health Impact Profile 49 score) was significantly lower (P < 0.001) and DMFT index was significantly higher (P < 0.01) in patients with dcSSc (indicating worse caries status).
Table 1.
Clinical and laboratory characteristics of patients with dcSSc.
| Characteristic | ||
|---|---|---|
| Age (years)a | 56.45±13.60 | |
| Sex | Male | 2 (7.14) |
| Female | 26 (92.86) | |
| SSc duration (years)b | 6 (1–25) | |
| Autoantibody pattern | Antinuclearc | 2 (7.14) |
| Anticentromere | 8 (28.57) | |
| Anti-Scl70 | 18 (64.29) | |
| Hemoglobin (g/dL)a | 13.8±2.2 | |
| Creatinine (mg/dL)a | 0.83±0.21 | |
| Erythrocyte sedimentation rate(normal >24 mm/hour) | Normal | 16 (38.1) |
| High | 26 (61.9) | |
| mRSS | Normal | 0 (0) |
| Mild/moderate | 20 (71.42) | |
| Severe/end-stage | 8 (28.58) | |
| Gastrointestinal tract | Absent | 8 (28.58) |
| Mild/moderate | 19 (67.85) | |
| Severe/end-stage | 1 (3.57) | |
| Joint/tendon | Normal | 4 (14.28) |
| Mild/moderate | 23 (82.14) | |
| Severe/end-stage | 1 (3.57) | |
| Muscle | Normal | 7 (25.01) |
| Mild/moderate | 20 (71.42) | |
| Severe/end-stage | 1 (3.57) | |
| Digital ulcers | Absent | 26 (92.85) |
| Present | 2 (7.15) | |
| Peripheral vascular involvement | Absent | 4 (14.28) |
| Mild/moderate | 24 (85.72) | |
| Severe/end-stage | 0 (0.00) | |
| Lung involvement | Absent | 3 (10.71) |
| Mild/moderate | 24 (85.72) | |
| Severe/end-stage | 1 (3.57) | |
| Chest radiography | Normal | 19 (67.85) |
| Interstitial lung disease | 9 (32.15) | |
| Heart involvement | Absent | 7 (25.01) |
| Mild/moderate | 20 (71.42) | |
| Severe/end-stage | 1 (3.57) |
Data expressed as absolute number (%) unless otherwise indicated.
aValues expressed as mean ± SD.
bValues expressed as median (range).
cWithout anticentromere antibodies and anti-Scl70 antibodies.
Abbreviations: dcSSc, diffuse cutaneous systemic sclerosis; mRSS, modified Rodnan skin score; SSc, systemic sclerosis; SD, standard deviation.
Table 2.
Sialometric assessment and selected oral features between patients with dcSSc and healthy controls.
| Characteristic | Patients with dcSSc (n = 28) | Healthy controls (n = 28) | P value |
|---|---|---|---|
| Age | 59.50 (49.00–69.00) | 53.50 (48.00–61.50) | >0.05 |
| Number of teeth | 18 (12–21.50) | 26.00 (22.50–28.00) | <0.001 |
| Interincisal distance (mm) | 3.80 (3.40–4.50) | 4.80 (4.40–5.10) | <0.001 |
| Unstimulated salivary flow rate (mL/min) | 1.90 (0.70–2.40) | 4.55 (4.00–5.10) | <0.001 |
| Stimulated salivary flow rate (mL/min) | 3.50 (2.10–410) | 8.20 (7.15–9.30) | <0.001 |
| Resting salivary pH value | 6.25 (5.75–6.50) | 7.00 (4.00–15.50) | <0.001 |
| Stimulated salivary pH value | 6.75 (6.00–7.00) | 7.50 (5.25–16.00) | <0.01 |
| DMFT index | 28.50 (24.00–32.00) | 20.00 (17.00–23.50) | <0.01 |
| Oral Health Impact Profile 49 score | 45.50 (32.50–55.50) | 7.00 (4.00–15.00) | <0.001 |
Data expressed as mean (interquartile range).
*Age is not a confounder for sialometric assessment and selected oral features between groups.
Abbreviations: dcSSc, diffuse cutaneous systemic sclerosis; DMFT, decayed, missing, filled teeth.
There were significant negative correlations between caries status and mean salivary flow rates (USF and SSF) (ρ = −0.392, P = 0.02 and ρ = −0.316, P = 0.048) in patients with dcSSc. There were also significant negative correlations between oral HRQoL (i.e., Oral Health Impact Profile 49 score) and mean salivary flow rates (USF and SSF) (ρ = −0.560, P = 0.001 and ρ = −0.543, P = 0.001).
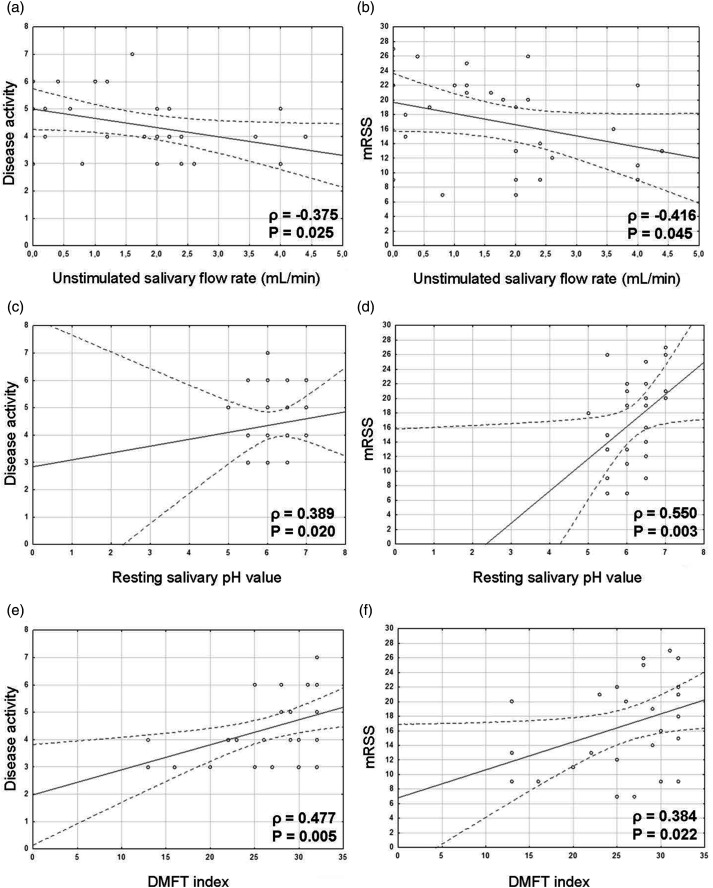
The USF rate was negatively correlated with disease activity (Figure 1a) and mRSS (Figure 1b). There were significant, positive correlations between mean resting pH value and disease activity (Figure 1c) and between mean resting pH value and mRSS (Figure 1d). These results suggested that the USF rate and pH value could partly reflect disease activity and skin involvement. Mean salivary flow rate was significantly positively associated with the severities of general (ρ = 0.327, P = 0.044), skin (ρ = 0.487, P = 0.004), and gastrointestinal (ρ = 0.323, P = 0.047) involvement in patients with dcSSc. The DMFT index was positively correlated with disease activity (Figure 1e) and mRSS (Figure 1f). Furthermore, caries status was correlated with disease duration (P = 0.005). However, caries status was not significantly correlated with oral HRQoL (i.e., Oral Health Impact Profile 49 score; data not shown). Caries status was significantly positively correlated with the severities of general, joint, and skin involvement (ρ = 0.391, P = 0.020; ρ = 0.364, P = 0.028; and ρ = 0.404, P = 0.016, respectively) in patients with dcSSc.
Figure 1.
Correlations in patients with dsSSc between (a) USF rate and disease activity (ρ = −0.375, P = 0.025), (b) USF rate and mRSS (ρ = −0.416, P = 0.045), (c) resting salivary pH value and disease activity (ρ = 0.389, P = 0.020), (d) resting salivary pH value and mRSS (ρ = 0.550, P = 0.003), (e) DMFT index and disease activity (ρ = 0.477, P = 0.005), and (f) DMFT index and mRSS (ρ = 0.384, P = 0.022). Abbreviations: dcSSc, diffuse cutaneous systemic sclerosis; DMFT, decayed, missing, filled teeth; mRSS, modified Rodnan skin score; USF, unstimulated salivary flow.
Discussion
In our previous study, we confirmed that low socioeconomic status and physician assessment of disease severity were positively associated with oral HRQoL in patients with SSc.24 In the present study, we found that reduced saliva production and higher pH values were associated with disease activity and severity in patients with dcSSc. Notably, the Canadian Systemic Sclerosis Oral Health Study did not find any association between reduced saliva production and disease characteristics, presumably due to the inclusion of patients with SSc who had concomitant SS or SS-related antibodies.10 Reduced saliva production was associated with the severities of general, skin, and gastrointestinal involvement in patients with dcSSc. To the best of our knowledge, this is the only study in which the findings contradict the existing notion that reduced saliva production in patients with SSc is linked to SS-related antibodies or presence of underlying SS.
SSc affects the defense systems in both unstimulated and stimulated human saliva.14 Generally, unstimulated saliva is considered to mainly consist of submandibular secretions, while the stimulated saliva is mainly composed of parotid fluid.25 Low SSF and USF rates have been observed in patients with dcSSc.14 Reduced salivary flow in patients with SSc may be related to considerable replacement of glandular tissue with fibrous tissue, as well as acinar atrophy.26 Reactive oxygen species have been shown to play a fundamental role in the pathogenesis of salivary gland dysfunction during the course of SSc.26,27 In a study of patients with predominantly cutaneous SSc, labial salivary gland fibrosis was not associated with any organ involvement.8 Moreover, a recent study showed that periodontal disease magnitude was strongly associated with mRSS and disease duration in a cohort mainly comprising patients with dcSSc.28 Therefore, we propose that limited cutaneous SSc and dcSSc should be regarded as separate subtypes of autoimmunity.
Caries is a pH-mediated disease in which lower salivary pH values contribute to the growth of cariogenic bacteria.29 In this study, we confirmed lower saliva production and lower pH values in patients with dcSSc, compared with healthy controls (Table 2). In the presence of enhanced disease activity, patients with dcSSc exhibited further reduction of saliva production, higher resting pH values, and greater numbers of caries (Figure 1). The reduced salivary flow rate in patients with dcSSc observed in this study could contribute to an elevated risk of caries; however, the buffering capacity of the saliva appeared to be preserved. The correlations of higher resting pH values and greater numbers of caries with enhanced disease severity warrant further research.
Poor oral health has also been linked to pulmonary diseases, particularly in individuals with a weakened immune system.30 We found strong relationships of caries status with overall disease severity, disease activity, and the extents of general, skin, and joint involvement. Therefore, caries prevention should be a treatment priority for patients with dcSSc. The rates of stroke and heart attack significantly increase in the first 4 weeks after an invasive dental treatment in the general population;31 notably, the long-term benefits of good oral health (with respect to general health) are of greater importance in patients with dcSSc, as these patients exhibit elevated cardiovascular risk.1 The negative correlation between mean salivary flow rate and oral HRQoL in this study indicates that reduced salivation could contribute to decline in oral HRQoL, as in patients with SS. In addition, this study revealed a tendency for a positive relationship between caries status and oral HRQoL; however, this was not statistically significant.
Regarding disease severity, mRSS was used as a surrogate measure because a validated measure was unavailable. This study was limited because of its small sample size, which limited the possibility of subgroup analysis; however, because this cohort included only patients with dcSSc, the sample size was considerable. Ideally, patients with limited cutaneous SSc of a similar disease duration would have been included as a control group, but enrollment of a sufficient number of such patients is difficult to achieve. Further longitudinal studies could also address the dynamics of changes in salivary flow and salivary pH values during the progression of disease.
Conclusion
Our results support the hypothesis that global measures of SSc severity and activity are correlated with salivary flow and tooth status in patients with dcSSc. Although these relationships need further investigation, clinicians should be aware of the enhanced prevalences of reduced salivation and caries in patients with dcSSc, as well as the potential systemic health problems related to tooth status.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Denton CP, Khanna D. Systemic sclerosis. Lancet 2017; 390: 1685–1699. [DOI] [PubMed] [Google Scholar]
- 2.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 1988; 15: 202–205. [PubMed] [Google Scholar]
- 3.Radic M, Martinovic Kaliterna D, Fabijanic D, et al. Prevalence of systemic sclerosis in Split-Dalmatia county in Southern Croatia. Clin Rheumatol 2010; 29: 419–421. [DOI] [PubMed] [Google Scholar]
- 4.Del Rosso A, Maddali-Bongi S. Oral health in patients with systemic sclerosis. Rheumatology (Oxford) 2014; 53: 1355–1356. [DOI] [PubMed] [Google Scholar]
- 5.Mortazavi H, Baharvand M, Movahhedian A, et al. Xerostomia due to systemic disease: a review of 20 conditions and mechanisms. Ann Med Health Sci Res 2014; 4: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajraktari IH, Kryeziu A, Sherifi F, et al. Oral manifestations of systemic sclerosis and correlation with anti-topoisomerase I antibodies (SCL-70). Med Arch 2015; 69: 153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crincoli V, Fatone L, Fanelli M, et al. Orofacial manifestations and temporomandibular disorders of systemic scleroderma: an observational study. Int J Mol Sci 2016; 17: pii: E1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avouac J, Sordet C, Depinay C, et al. Systemic sclerosis-associated Sjogren's syndrome and relationship to the limited cutaneous subtype: results of a prospective study of sicca syndrome in 133 consecutive patients. Arthritis Rheum 2006; 54: 2243–2249. [DOI] [PubMed] [Google Scholar]
- 9.Jung S, Martin T, Schmittbuhl M, et al. The spectrum of orofacial manifestations in systemic sclerosis: a challenging management. Oral Dis 2017; 23: 424–439. [DOI] [PubMed] [Google Scholar]
- 10.Baron M, Hudson M, Tatibouet S, et al. Relationship between disease characteristics and orofacial manifestations in systemic sclerosis: Canadian Systemic Sclerosis Oral Health Study III. Arthritis Care Res (Hoboken) 2015; 67: 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wangkaew S, Kasitanon N, Sivasomboon C, et al. Sicca symptoms in Thai patients with rheumatoid arthritis, systemic lupus erythematosus and scleroderma: a comparison with age-matched controls and correlation with disease variables. Asian Pac J Allergy Immunol 2006; 24: 213–221. [PubMed] [Google Scholar]
- 12.Almeida C, Almeida I, Vasconcelos C. Quality of life in systemic sclerosis. Autoimmun Rev 2015; 14: 1087–1096. [DOI] [PubMed] [Google Scholar]
- 13.Baron M, Hudson M, Tatibouet S, et al. The Canadian systemic sclerosis oral health study: orofacial manifestations and oral health-related quality of life in systemic sclerosis compared with the general population. Rheumatology (Oxford) 2014; 53: 1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knaś M, Zalewska A, Waszkiewicz N, et al. Salivary: flow and proteins of the innate and adaptive immunity in the limited and diffused systemic sclerosis. J Oral Pathol Med 2014; 43: 521–529. [DOI] [PubMed] [Google Scholar]
- 15.Van Den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013; 65: 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002; 61: 554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci 1993; 694: 72–77. [DOI] [PubMed] [Google Scholar]
- 18.Cox SC, Walker DM. Establishing a normal range for mouth opening: its use in screening for oral submucous fibrosis. Br J Oral Maxillofac Surg 1997; 35: 40–42. [DOI] [PubMed] [Google Scholar]
- 19.Petricević N, Celebić A, Papić M, et al. The Croatian version of the Oral Health Impact Profile Questionnaire. Coll Antropol 2009; 33: 841–847. [PubMed] [Google Scholar]
- 20.Valentini G, Iudici M, Walker UA, et al. The European Scleroderma Trials and Research group (EUSTAR) task force for the development of revised activity criteria for systemic sclerosis: derivation and validation of a preliminarily revised EUSTAR activity index. Ann Rheum Dis 2017; 76: 270–276. [DOI] [PubMed] [Google Scholar]
- 21.Clements P, Lachenbruch P, Siebold J, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol 1995; 22: 1281–1285. [PubMed] [Google Scholar]
- 22.Khanna D, Furst DE, Clements PJ, et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord 2017; 2: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medsger TA, Jr, Bombardieri S, Czirjak L, et al. Assessment of disease severity and prognosis. Clin Exp Rheumatol 2003; 21: S42–S46. [PubMed] [Google Scholar]
- 24.Parat K, Radić M, Borić K, et al. Association of low socioeconomic status and physician assessment of disease severity with oral health-related quality of life in patients with systemic sclerosis: a pilot study from Croatia, a country in transition. J Int Med Res 2018; 46: 5127–5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagler RM. Salivary glands and the aging process: mechanistic aspects, health-status and medicinal-efficacy monitoring. Biogerontology 2004; 5: 223–233. [DOI] [PubMed] [Google Scholar]
- 26.Zalewska A, Knaś M, Gińdzieńska-Sieśkiewicz E, et al. Salivary antioxidants in patients with systemic sclerosis. J Oral Pathol Med 2014; 43: 61–68. [DOI] [PubMed] [Google Scholar]
- 27.Su H, Baron M, Benarroch M, et al. Altered salivary redox homeostasis in patients with systemic sclerosis. J Rheumatol 2010; 37: 1858–1863. [DOI] [PubMed] [Google Scholar]
- 28.Isola G, Williams RC, Lo Gullo A, et al. Risk association between scleroderma disease characteristics, periodontitis, and tooth loss. Clin Rheumatol 2017; 36: 2733–2741. [DOI] [PubMed] [Google Scholar]
- 29.Valm AM. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J Mol Biol 2019; 431: 2957–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manger D, Walshaw M, Fitzgerald R, et al. Evidence summary: the relationship between oral health and pulmonary disease. Br Dent J 2017; 222: 527–533. [DOI] [PubMed] [Google Scholar]
- 31.Minassian C, D'Aiuto F, Hingorani AD, et al. Invasive dental treatment and risk for vascular events: a self-controlled case series. Ann Intern Med 2010; 153: 499–506. [DOI] [PubMed] [Google Scholar]