Supplemental Digital Content is available in the text.
Keywords: per- and polyfluoroalkyl substances, geographic, pregnancy, birth outcomes
Background:
Per- and polyfluoroalkyl substances (PFAS) are ubiquitous in the serum of the general US population, and were detected in public water systems serving approximately 16.5 million US residents during 2013–2015. Low birthweight was associated with PFAS exposures in previous studies.
Methods:
Birthweights for singleton births during 2013–2015 were obtained from CDC WONDER, multiply stratified by county, maternal age, race, education, smoking status, and parity. PFAS water concentrations were obtained from EPA UCMR3 database and aggregated by county. Multiple regression weighted by inverse variance was used to produce effect estimates equivalent to those that would be obtained from individual-level data on birthweight and confounders.
Results:
Adjusting for stratification demographic confounders (maternal age, race, education, smoking status, and parity), we found an average change in birthweight of 0.9 g (95% confidence interval [CI] = −0.5, 2.2), −1.3 g (−1.6, −0.9), −3.8 g (−4.9, −2.7), and −3.8 g (−4.3, −3.3) per ng/L increase in the population-weighted average perfluorooctanoic acid, perfluorooctane sulfonate, perfluoroheptanoic acid, and perfluorohexane sulfonic acid in public water supplies by county, respectively. We found an average change in birthweight of −1.0 g (95% CI = −1.2, −0.8) per ng/L increase in the sum of perfluorooctanoic acid, perfluorooctane sulfonate, perfluoroheptanoic acid, and perfluorohexane sulfonic acid concentrations in public water supplies.
Conclusions:
The direction and magnitude of association between PFAS and birthweight varied by PFAS chemical in this study. Conclusions are tempered by inherent limitations of the 2 public-use datasets, and by the sensitivity of our results to alternative methods such as mutual adjustment for co-exposures.
What this study adds
In this manuscript, we examine associations between PFAS detections in public water supplies and birthweight in the US on the county-level during 2013–2015. PFAS water concentrations used in this study are free of reverse causality and/or physiological confounding compared to PFAS serum measurements. We show that using county-level multiple-stratified average birthweights in weighted regression models produces effect estimates equivalent to those that would be obtained from using individual-level data on birthweight and confounders. In addition, we explore the association between PFAS and birthweight more comprehensively by including some understudied PFAS chemicals (i.e., PFHxS and perfluoroheptanoic acid [PFHpA]) and accounting for co-exposure to other contaminants and number of drinking water violations. Overall, our work is the first nation-wide statistical analysis in the US on PFAS in public water supplies and birthweight.
Introduction
Per- and polyfluoroalkyl substances (PFAS) are synthetic chemicals consisting of a fully fluorinated carbon chain. Due to their chemical and thermal stability, PFAS have been widely used in consumer products and industrial process since the 1940s, such as stain repellents, lubricants, paints, textiles, firefighting foams, nonstick cookware, and food packaging.1 Long-chain PFAS can persist indefinitely in the environment, and bioaccumulate in humans and other organisms.2–4 Food sources, drinking water, dust, and air are the main exposure routes to humans.5,6
According to the National Health and Nutrition Examination Survey in 2015–2016, the geometric means of perfluorooctanoic acid (PFOA), perfluorooctane sulfonate (PFOS), perfluorononanoic acid (PFNA), and perfluorohexane sulfonic acid (PFHxS) in the serum samples of the general US population were 1.56, 4.72, 0.58, and 1.18 ng/ml, respectively.7 The half-lives for serum or plasma elimination of PFAS in humans range from 2 to more than 10 years in previous studies.8–11 For pregnant women, the PFAS concentrations in maternal serum, umbilical cord serum, and breast milk are strongly associated with each other.12
Some studies suggest that exposure to PFAS could cause adverse reproductive health effects in humans,13–17 while others found little association.18–20 Due to physiological changes during pregnancy, including increased glomerular filtration rate and parallel expansion of blood volume,21,22 the observed inverse association between PFAS concentrations and birthweight could be due to reverse causality or uncontrolled confounding factors such as glomerular filtration rate.23,24 A recent meta-analysis found an inverse association between PFOA and birthweight when the blood was sampled late in the pregnancy; however, little association was found when blood was sampled at times less susceptible to physiological confounding or reverse causation (i.e., shortly before conception or early in pregnancy).24
Under the third Unregulated Contaminant Monitoring Rule (UCMR3), the US Environmental Protection Agency (US EPA) tested thirty contaminants, including 6 PFAS, in public water systems (PWSs) during 2013–2015.25 Based on samples collected from multiple points in a PWS, UCMR3 provides scientifically valid data on the occurrence of unregulated contaminants. It is the most comprehensive dataset of PFAS occurrence in public drinking water in the US.26,27
Few epidemiological studies on PFAS and birthweight have accounted for or use study designs that are resistant to reverse causality/physiological confounding, or have considered co-exposure to PFAS other than PFOA and PFOS, or co-exposure with other pollutants. Based on UCMR3 and the birthweight data from CDC WONDER, we conducted a county-level study of PFAS and birthweight in the US while adjusting for maternal age, race, education, smoking status, and parity, a similar set of adjustment variables to previous studies.19,28,29 In addition, we were able to investigate co-exposures to other UCMR3 contaminants and overall water quality when examining the association between a specific PFAS and birthweight. Although use of county-level exposure measures likely introduces some degree of Berkson measurement error, this study is free of reverse causality/physiological confounding due to our use of an external exposure metric, PFAS concentrations in public water, rather than an exposure metric potentially influenced by physiological processes, that is, serum concentrations during pregnancy.30 In addition, we show that our use of county-level multiple-stratified average birthweights in weighted regression models produces effect estimates equivalent to those that would be obtained from using individual-level data on birthweight and the stratification variables.
Methods
Data collection
Multiple-stratified birthweight data
We obtained the average birthweight from singleton births from 2013 to 2015, multiple-stratified by county, maternal age, bridged-race (race), education, tobacco use (smoking status), and live birth order (parity) from CDC WONDER.31 CDC suppresses data for groups with less than 10 births. Within each state, counties with less than the population of 100,000 persons were de-identified and combined under the label of “Unidentified Counties” in the dataset, thus we excluded them from our analysis as they could not be linked with the UCMR3 data. Equivalent subdivisions to “County” included “Parish” in Louisiana, “Borough” in Alaska, and “Independent City” in Virginia, Maryland, and Missouri. Overall, there were 580 US counties with populations greater than 100,000. In the states and years that applied the 1989 US Standard Certificate of Live Birth (Alabama, Arizona, Arkansas, Hawaii, Maine, Michigan, and West Virginia in 2013; Rhode Island during 2013–2014; Connecticut and New Jersey during 2013–2015), education and smoking status of the mothers were coded by CDC as “Excluded” and “Not Reported,” respectively, as they were not comparable to the data that used the 2003 revision of the birth certificate. We excluded the groups with “Unknown or Not Stated,” “Excluded,” or “Not Reported” information in education, smoking status, and parity, which accounted for 9.6% of the singleton births in the 580 large US counties (Figure S1, Supplemental Digital Content I; http://links.lww.com/EE/A101). After the exclusion, the birthweight data covered 552 counties in the US. Overall, these counties could represent the US counties with populations greater than 100,000 that applied the 2003 revision of the birth certificate. For crude (unadjusted) epidemiological analysis, we obtained the average birthweight from singleton births from 2013 to 2015 stratified by county only, excluding the “Unknown or Not Stated,” “Excluded,” and “Not Reported” categories in education, smoking status, and parity.
PFAS and other water quality indicators
Under UCMR3, 30 contaminants, including 6 PFAS (PFOA, PFOS, perfluorobutane sulfonic acid [PFBS], PFNA, PFHpA, and PFHxS) were monitored using analytical methods developed by the US EPA.32 PFAS were monitored using EPA Method 537 at 4,908 US PWSs during 2013–2015, including almost all PWSs serving >10,000 people and a representative sample of around 800 PWSs serving ≤10,000 people. The number of UCMR3 water samples collected at each PWS during 2013–2015 ranged from 1 to 484. In total, 1,928 counties were monitored in UCMR3 over the 3 years, covering the 50 US states, District of Columbia, and some of the other US territories. Multiple PWSs could serve the same county, and different counties could also share a common PWS. In our analyses, the number of water samples taken within a county was the sum of water samples from all the PWSs that serve this county; if a PWS served 2 counties, then the water samples were counted in the number of water samples for both counties. The distribution of the number of water samples taken per county is shown in Table S1 in Supplemental Digital Content I; http://links.lww.com/EE/A101. The minimum reporting level (MRL) was 10 ng/L for PFHpA, 20 ng/L for PFOA and PFNA, 30 ng/L for PFHxS, 40 ng/L for PFOS, and 90 ng/L for PFBS. Overall, around 16.5 million people in the US were served by PWSs containing at least 1 of the 6 PFAS at concentrations exceeding the MRLs. Proxy indicators for PFAS exposure in this study include the percentage of water measurements with PFAS detection by county and the population-weighted average PFAS water concentrations by county. We merged the UCMR3 PFAS data with the CDC birthweight data by county, and excluded Hampton City in Virginia as it was not monitored under UCMR3. The final datasets include 551 counties in the US (see Figure S1 and Table S2 in Supplemental Digital Content I; http://links.lww.com/EE/A101), covering 47 US states and District of Columbia.
Because only a limited number of contaminants were monitored under UCMR3, and might not be indicative of general water quality, we also obtained the count of violations by PWS for the 551 counties during 2013–2015 from the US EPA (SDWIS Federal Reports Advanced Search) and used the average count of violations per PWS by county under the US EPA rules33 as an indicator of the overall water quality. Because only 4 counties had detections for PFBS and only 10 counties had detections for PFNA, we excluded both PFBS and PFNA from the analysis. PFOA, PFOS, PFHpA, and PFHxS are all moderately or highly correlated with each other (correlation coefficients range from 0.49 to 0.74), which is expected as they often have shared sources. Other water quality indicators including the number of violations and other UCMR3 contaminants except for 1,4-dioxane are weakly associated with PFAS (r < 0.3), and thus were not adjusted for in our analyses (Figure S2, Supplemental Digital Content I; http://links.lww.com/EE/A101). Among the 551 counties in the data, 87 counties had detection for at least 1 of PFOA, PFOS, PFHpA, and PFHxS. In comparison, among the 1,928 counties monitored under UCMR3 during 2013–2015, 162 counties had detection for at least 1 of the 4 PFAS.
For analyses using continuous PFAS concentrations, we only used data for the 87 counties with at least 1 detection of PFOA, PFOS, PFHpA, or PFHxS. For each UCMR3 water measurement in these 87 counties, we substituted values that were below the MRL with 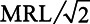 . After the substitution, for each PFAS chemical we first averaged the concentrations by PWS, and then averaged across PWSs by county, weighting by the average population served by each PWS during 2013–2015. The distribution of the number of water samples taken from the 87 counties is shown in Table S3 in Supplemental Digital Content I; http://links.lww.com/EE/A101.
. After the substitution, for each PFAS chemical we first averaged the concentrations by PWS, and then averaged across PWSs by county, weighting by the average population served by each PWS during 2013–2015. The distribution of the number of water samples taken from the 87 counties is shown in Table S3 in Supplemental Digital Content I; http://links.lww.com/EE/A101.
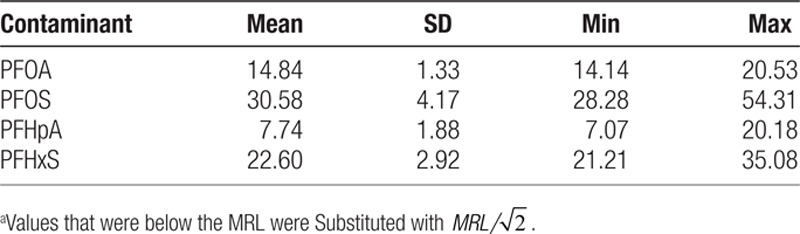
Descriptive statistics for the percentage of water measurements with detection for PFOA, PFOS, PFHpA, and PFHxS are shown in Table 1, and the population-weighted average water concentrations of PFAS in the counties with detection of at least 1 of the 4 PFAS are shown in Table 2. The average percentages in Table 1 are all less than 1% due to the fact that over 80% of the counties did not have detection for PFAS. Because our study only includes a small part of the counties monitored by UCMR3, we also show the descriptive statistics of water measurements for all 1,928 counties in UCMR3 and the 162 counties with detection of at least 1 of the 4 PFAS in UCMR3 in Tables S1.1 and S1.2 in Supplemental Digital Content I; http://links.lww.com/EE/A101. The average values of the 2 proxy indicators for PFAS exposure in all UCMR3 counties are similar to those of the counties covered by our study.
Table 1.
Percentage of water measurements with detection (%) of PFAS in 551 counties in the US, 2013–2015.
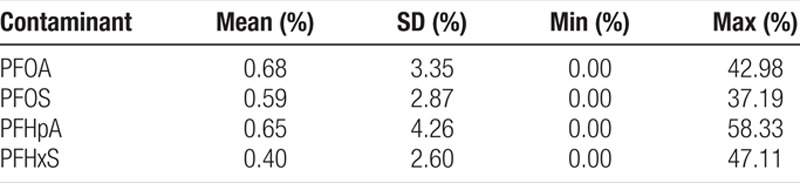
Table 2.
Population-weighted average of UCMR3 water concentrationsa (ng/L) of PFAS in 87 counties in the US with detection of at least 1 of PFOA, PFOS, PFHpA, or PFHxS, 2013–2015.
Weighted linear regression models
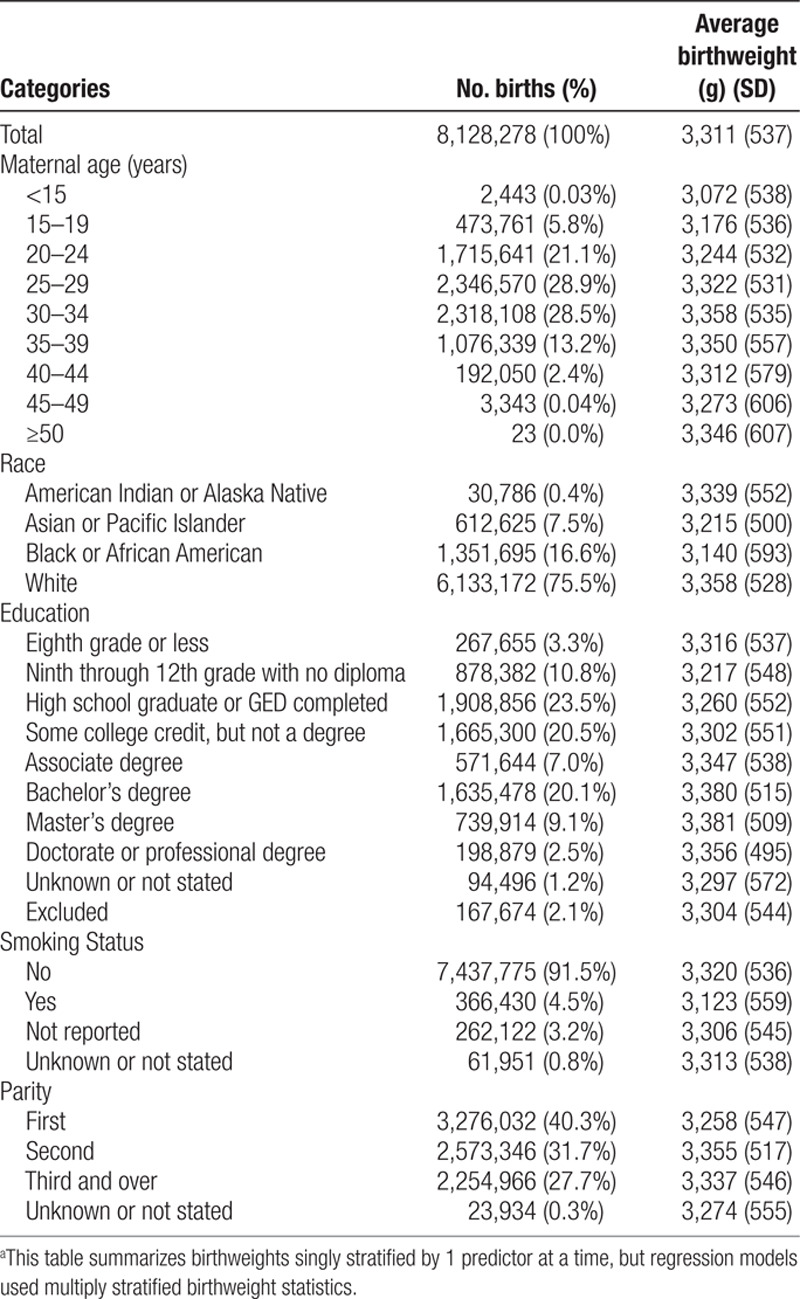
We obtained multiple-stratified county-level birthweight statistics (mean and SD) from the CDC. We calculated the aggregated county-level birthweight statistics (Table 3) for the 551 counties, for which the distributions of age, race, education, smoking status, and parity are similar to that of the entire US population (Table S4, Supplemental Digital Content I; http://links.lww.com/EE/A101). In the Supplemental Digital Content II; http://links.lww.com/EE/A102, we show that using county-level multiple-stratified average birthweights in weighted regression models produces equivalent results to those that would be obtained from individual-level data on birthweight and confounders. In particular, using the number of births in each stratum for the weights produces the same regression slope and variance of the effect estimate that would be obtained from unweighted multiple linear regression with the individual-level data, and using the inverse variance34 of the average birthweight for the weights produces the same regression slope and variance of the effect estimate that would be obtained from individual-level weighted regression allowing for heteroscedasticity. This is a very useful result for avoiding aggregation bias (also known as ecological fallacy) for analysis of public-use birthweight data and other datasets that multiply stratify on key confounding variables. However, results will only be identical when adjusting for covariates that are available with multiple stratification at the county-level; adjustment for covariates that are not multiply stratified (e.g., US Census poverty rates) could result in different parameter estimates than those that would be obtained using individual-level data (e.g., personal socioeconomic status). Because of this mathematical result and the strong negative correlation between poverty and education level based on 2013, 2014, and 2015 American Community Survey 1-year estimates (r = −0.82), we did not include the county-level percentage of poverty in the primary analyses. Additional adjustment for the county-level poverty percentage was only conducted as sensitivity analyses. In addition, analyses using individual-level exposure measurements (e.g., tap water PFAS concentrations measured at each participant’s home) may produce different results than analyses using group-level exposure assignments.
Table 3.
Predictors of birthweighta among singleton pregnancies in 551 counties in the US, 2013–2015.
In secondary analyses, we employed lasso regression to account for exposure mixtures, penalizing the coefficients for each PFAS chemical and 1,4-dioxane.
We used statistical software R, version 3.6.0 for statistical analyses.
Results
In Figures 1 and 2, we display the relation between average birthweight and the 2 proxies for PFAS exposure while using inverse-variance weights in the regression models.
Figure 1.
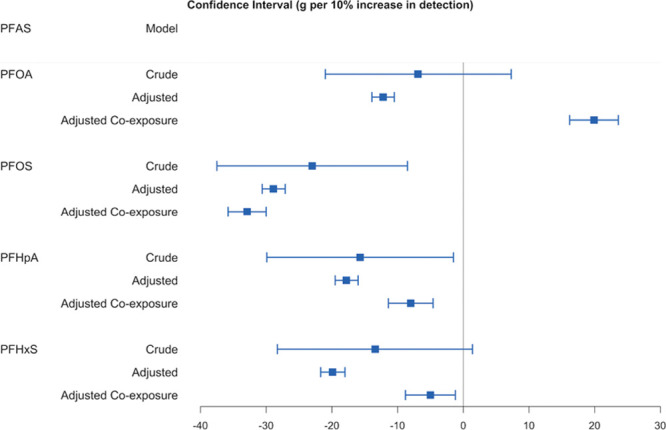
The change of average birthweight (g) for 10% increase in the detection of PFAS: MLE, 95% CI. Using regressions weighted by inverse variance of average birthweight. Crude model: association between PFAS and birthweight only. Adjusted model: adjusted for maternal age (<15, 15–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, ≥50), race (American Indian or Alaska Native, Asian or Pacific Islander, Black or African American, White), education (eighth grade or less; ninth through 12th grade with no diploma; High school graduate or GED completed; Some college credit, but not a degree; Associate degree; Bachelor’s degree; Master’s degree; Doctorate or professional degree), smoking status (yes, no), and parity (first, second, third and over). Adjusted co-exposure model: adjusted for the other 3 PFAS, 1,4-dioxane, and all covariates in the adjusted model.
Figure 2.
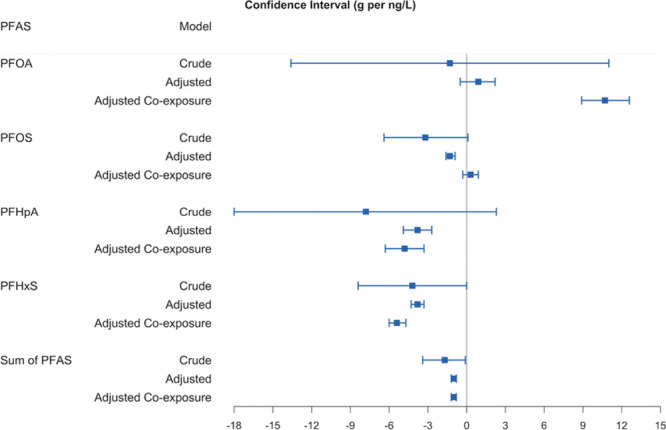
The change of average birthweight (g) for 1 ng/L increase in the population-weighted average PFAS water concentration: MLE, 95% CI (1 g per ng/L = 1 g per ppt = 1,000 g per ng/ml). Using 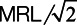 substitution for the non-detections and regressions weighted by inverse variance of average birthweight. Crude model: association between PFAS and birthweight only. Adjusted model: adjusted for maternal age (<15, 15–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, ≥50), race (American Indian or Alaska Native, Asian or Pacific Islander, Black or African American, White), education (eighth grade or less; ninth through 12th grade with no diploma; high school graduate or GED completed; some college credit, but not a degree; associate degree; bachelor’s degree; master’s degree; doctorate or professional degree), smoking status (yes, no), and parity (first, second, third and over). Adjusted co-exposure model: adjusted for the co-exposures (the other 3 PFAS and 1,4-dioxane; or 1,4-dioxane only for the model includes the sum of PFAS), and all covariates in the adjusted model.
substitution for the non-detections and regressions weighted by inverse variance of average birthweight. Crude model: association between PFAS and birthweight only. Adjusted model: adjusted for maternal age (<15, 15–19, 20–24, 25–29, 30–34, 35–39, 40–44, 45–49, ≥50), race (American Indian or Alaska Native, Asian or Pacific Islander, Black or African American, White), education (eighth grade or less; ninth through 12th grade with no diploma; high school graduate or GED completed; some college credit, but not a degree; associate degree; bachelor’s degree; master’s degree; doctorate or professional degree), smoking status (yes, no), and parity (first, second, third and over). Adjusted co-exposure model: adjusted for the co-exposures (the other 3 PFAS and 1,4-dioxane; or 1,4-dioxane only for the model includes the sum of PFAS), and all covariates in the adjusted model.
Using inverse-variance weights and covariate-adjusted models, we found for the 551 counties significant negative associations between birthweight and PFAS detection; while adjusting for co-exposures to other PFAS and 1,4-dioxane reversed the effect estimate for PFOA (Figure 1). In addition, we compared 2 different weights in regression: group size (number of births), and inverse variance of average birthweight. The results of analyses using these 2 sets of weights in different models are similar, but adjustment for confounders produces somewhat different results from the crude analyses (Tables S5–S8, Supplemental Digital Content I; http://links.lww.com/EE/A101).
For the 87 counties with detection of at least 1 of the 4 PFAS, we examined the association between the population-weighted average PFAS water concentrations and birthweight, using inverse-variances as regression weights (Tables S9–S12, Supplemental Digital Content I; http://links.lww.com/EE/A101). We also summed the population-weighted average water concentrations of the 4 PFAS to determine the overall association of PFAS with birthweight (Table S13, Supplemental Digital Content I; http://links.lww.com/EE/A101). Using inverse-variance weights and 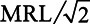 substitution in the covariate-adjusted models, we found no association between birthweight and PFOA concentration (0.9 [−0.5, 2.2] g per ng/L, Table S9), and significant negative associations between birthweight and PFOS (−1.3 [−1.6, −0.9] g per ng/L, Table S10), PFHpA (−3.8 [−4.9, −2.7] g per ng/L, Table S11), and PFHxS (−3.8 [−4.3, −3.3] g per ng/L, Table S12) concentrations. Additionally adjusting for co-exposures to other PFAS and 1,4-dioxane greatly impacted the effect estimate for PFOA. Overall, the sum of 4 PFAS was negatively associated with birthweight (−1.0 [−1.2, −0.8] g per ng/L, Table S13). We also conducted sensitivity analyses with zero substitution and MRL substitution to compare to the results from
substitution in the covariate-adjusted models, we found no association between birthweight and PFOA concentration (0.9 [−0.5, 2.2] g per ng/L, Table S9), and significant negative associations between birthweight and PFOS (−1.3 [−1.6, −0.9] g per ng/L, Table S10), PFHpA (−3.8 [−4.9, −2.7] g per ng/L, Table S11), and PFHxS (−3.8 [−4.3, −3.3] g per ng/L, Table S12) concentrations. Additionally adjusting for co-exposures to other PFAS and 1,4-dioxane greatly impacted the effect estimate for PFOA. Overall, the sum of 4 PFAS was negatively associated with birthweight (−1.0 [−1.2, −0.8] g per ng/L, Table S13). We also conducted sensitivity analyses with zero substitution and MRL substitution to compare to the results from 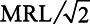 substitution. For PFOA, PFHpA, PFHxS, and the sum of 4 PFAS, the results were consistent regardless of the substitution methods (Table S9 and Tables S11–S13). For PFOS, the 3 substitution methods produced noticeably different results when adjusting for other PFAS (Table S10).
substitution. For PFOA, PFHpA, PFHxS, and the sum of 4 PFAS, the results were consistent regardless of the substitution methods (Table S9 and Tables S11–S13). For PFOS, the 3 substitution methods produced noticeably different results when adjusting for other PFAS (Table S10).
Discussions
We used the public-use dataset of UCMR3 and Natality data from CDC WONDER to conduct a county-level study of birthweight and PFAS concentrations in drinking water in the US, with multiple stratification by key confounding variables to yield unbiased estimation of the individual-level associations. There are several advantages of using the 2 datasets in the study. First, epidemiological associations using PFAS water concentrations are free of reverse causality and/or physiological confounding, which may have biased the epidemiological associations reported by other studies using PFAS serum measurements, especially when collected late in pregnancy. Second, we explore the association between PFAS and birthweight more comprehensively by including some understudied PFAS chemicals, that is, PFHxS and PFHpA. Third, the availability of multiple-stratified birthweight data from CDC allows us to control for maternal age, education, race, smoking status, and parity, producing equivalent results to those that would be obtained from individual-level data on birthweight and these confounding variables. In this study, weighting by group size (equivalent to ordinary multiple regression using individual-level data) or inverse variance (equivalent to weighted multiple regression for heteroscedasticity using individual-level data) produced similar results.
Continued exposure to relatively low PFAS concentrations in drinking water can substantially increase serum concentrations, with reported steady-state serum: drinking water ratios of about 114:1 for PFOA, 125:1 for PFOS, and 194:1 for PFHxS.35–38 After long-term consumption of contaminated drinking water, the population-weighted average water concentrations of 14.84, 30.58, and 22.60 ng/L (1 ng/L = 10−3 ng/ml) for the 87 counties in the US during 2013–2015 (Table 2) are expected to increase serum concentrations by about 1.7, 3.8, and 4.4 ng/ml for PFOA, PFOS, and PFHxS, respectively. The estimates are similar to the medians (interquartile range [IQR]) of 1.6 (1.1–2.5) ng/ml and 4.8 (2.8–8.1) ng/ml for PFOA and PFOS, respectively, and higher than the median (IQR) of 1.2 (0.7–2.1) ng/ml for PFHxS for the general US population, suggesting that these exposures may have had measurable impacts on serum PFAS concentrations in these communities. The estimated effect sizes for PFAS serum concentrations would be 1/114, 1/125, and 1/194 of that of the water concentrations for PFOA, PFOS, and PFHxS, respectively. So, after adjusting for potential demographic confounders, our estimated effects are equivalent to an average change in birthweight of 7.6 g (95% confidence interval [CI] = −4.3, 19.4) per ng/ml increase in serum PFOA; −10.1 g (95% CI = −13.0, −7.2) per ng/ml increase in serum PFOS; and −19.5 g (95% CI = −21.9, −17.1) per ng/ml increase in serum PFHxS. In comparison, a recent meta-analysis by Steenland et al.24 reported a change in birthweight of −10.5 g (−16.7, −4.4) per ng/ml increase of PFOA in maternal or cord blood; and −3.3 g (−9.6, 3.0) per ng/ml when restricting to studies where blood was sampled early in pregnancy or shortly before conception, similar to the null association we found between PFOA and birthweight while adjusting for demographic confounders. Nevertheless, this is just a preliminary comparison without accounting for any uncertainty for the water to serum conversion factor, which is beyond the scope of this paper and should be addressed in future research. In addition, with the above estimates after water to serum conversions, we can expect a change in birthweight of 10.6 g (95% CI = −6.0, 27.2) for an IQR change of serum PFOA (1.4 ng/ml); −53.5 g (95% CI = −68.9, 38.2), for an IQR change of serum PFOS (5.3 ng/ml), and −27.3 g (95% CI = −30.7, −23.9) for an IQR change of serum PFHxS (1.4 ng/ml) in the general US population.
Our study also has a number of limitations. First, we attempted to identify the causal effects of PFAS on birthweight, but our interpretations are limited by the observational nature of the data and limited availability of multiple-stratified variables that had been collected on birth certificates at the individual level, which makes it difficult to rule out measurement error and uncontrolled confounding. Although effect estimates for the percentage of water measurements with detection for each PFAS became slightly larger in the negative direction after adjustment for known multiple-stratified confounders, suggesting that further adjustment using more accurate confounder measures would only increase the absolute effect sizes, we cannot guarantee the absence of an unidentified confounder strong enough to reverse the association. In sensitivity analyses, additional adjustment for the county-level percentage of poverty did not substantially change the results in Figures 1 and 2 (see Figure S3, Figure S4, Table S14, and Table S15 in Supplemental Digital Content I; http://links.lww.com/EE/A101), with the exception of population-weighted average PFOA water concentration, for which the effect was changed from null (0.9 g per ng/L, 95% CI = −0.5, 2.2; Table S9) to negative (−2.4 g per ng/L, 95% CI = −3.8, −1.1; Table S15) in the model adjusted for demographic confounders. Nevertheless, the percentage of poverty was barely correlated with the 2 proxy indicators for PFAS exposure on the county-level (Tables S16 and S17, Supplemental Digital Content I; http://links.lww.com/EE/A101); therefore it was unlikely to confound the associations observed in Figures 1 and 2. The change in effect estimate for PFOA from Figure 2 to Figure S4 by additionally adjusting for the county-level percentage of poverty highlights the difficulty in interpreting multi-level studies, and potential cross level bias in ecological inference when including a county-level variable (i.e., the percentage of poverty) that is highly correlated with an individual-level variable (i.e., education level).39 The increases in precision from crude models to adjusted models in Figures 1 and 2 can be explained by the well-established result of decreased residual standard error for multiple regression after adjusting for strong predictors of the outcome.40 This setting is quite different from logistic regression, for which adjustment for covariates can result in a loss or at best no gain of precision.41 Adjustment for co-exposure to other PFAS and 1,4-dioxane changed the effect estimates differently for the percentage of water measurements with detection for each PFAS (Figure 1), reversing the effect from negative to positive for PFOA, increasing the effect size in the negative direction for PFOS, and attenuating the negative associations towards the null for the other 2 PFAS. This highlights some of the difficulties in fitting and interpreting statistical models with correlated exposure mixtures, even using a large dataset. In particular, bias amplification could occur due to residual confounding while including co-exposures with a common source,42 which could explain why the adjustment for the other PFAS had such a strong effect on the regression parameter for PFOA. However, the direction and magnitude of bias amplification are not readily predictable in this setting. Overall, we believe that the results from adjusted models that do not include co-exposures are more reliable because they are less susceptible to bias amplification.42 In secondary analysis, we used lasso regression (with 10-fold cross-validation to obtain the optimal shrinkage parameter) to penalize the coefficients for 4 PFAS and 1,4-dioxane in inverse-variance weighted models. In this analysis, the coefficients for PFOA, PFHpA, and PFHxS are attenuated towards the null; while the coefficient for PFOS does not change significantly and the coefficient for 1,4-dioxane is zeroed out. In the models using population-weighted average water concentrations of the chemicals (PFOA, PFOS, PFHpA, PFHxS, and 1,4-dioxane) as proxy exposure for the 87 counties with at least 1 detection for PFOA, PFOS, PFHpA, or PFHxS, adjustment for co-exposure increases the effect size in the positive direction for PFOA, attenuates the negative association towards the null for PFOS, increases the effect size in the negative directions for PFHpA and PFHxS (Figure 2). In the lasso regression using inverse-variance weights and 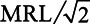 substitution, the coefficient for population-weighted average water concentration of PFOS is zeroed out, and the coefficients for population-weighted average water concentrations of the other PFAS are attenuated towards the null.
substitution, the coefficient for population-weighted average water concentration of PFOS is zeroed out, and the coefficients for population-weighted average water concentrations of the other PFAS are attenuated towards the null.
Second, the public-use birthweight data from CDC WONDER has several notable drawbacks, as shown by the flow chart of data processing in Figure S1, Supplemental Digital Content I; http://links.lww.com/EE/A101. (1) CDC suppresses data for the groups with less than 10 births; when we multiply stratified the data by county, maternal age, race, education, smoking status, and parity, we lost 5.3% of births compared to the data that was only stratified by county. (2) Counties with less than 100,000 population are de-identified in the dataset, which cannot be linked to the UCMR3 dataset, thus restricting the scope of the study to less than 580 counties. (3) There is likely some underreporting of maternal smoking status, which is difficult to obtain reliable data from birth certificate.43 (4) We excluded the missing values from the data, which accounted for 9.6% of the singleton births in the 580 counties. The “Excluded” category in education and “Not Reported” category in smoking status were missing at random, dependent only on the version of birth certificate used in the state in a specific year, rather than the value of the variables (education and smoking status) that are missing; and conditional on the version of birth certificate, the probability of missingness does not depend on the value of the variables. However, the “Unknown or Not Stated” in parity, education, and smoking status could depend on the actual values of these variables thus could be missing not at random. We lost all the births from 28 counties and 5.6% of the remaining 552 counties due to the exclusion. In all, the suppression, de-identification, and missingness reduced the number of births in our analysis by 32.9%; therefore our results based on the 551 counties may not be generalizable to the whole US. For the 551 counties, the missingness rate is 5.3%. With CDC permission and security clearances it is possible to obtain access to unsuppressed and fully identified birthweight data at secure federal facilities; we are currently taking steps to apply for access.
Third, our estimates for PFAS exposure solely relied on the UCMR3 data, which has several limitations: (1) we were unable to account for other sources of exposure to PFAS and other chemicals, such as food, dust, air pollution, non-UCMR3 chemicals in water, and/or unmeasured PFAS chemicals may be associated with both UCMR3 PFAS exposure and birthweight, in which case they may contribute uncontrolled confounding to our results. (2) The detection thresholds (MRLs) for the 6 PFAS measured in UCMR3 were as much as 16 times higher than the detection limits for the current standard testing method (method 537), so only the highest levels of PFAS contamination were reflected in the data; thus, it is likely that the percentages of water measurements with PFAS detection for each county underestimate the true extent of exposure. (3) Because UCMR3 only provides the PFAS concentrations in drinking water during 2013–2015 and the PFAS concentrations assigned for each county in our study may not reflect historical water exposure or total body burden for each individual, likely adding some degree of nondifferential exposure measurement error, predominantly of the Berkson type. (4) The number of samples taken from each PWS varied substantially across different counties but was often small, limiting the precision of the average concentrations at the PWS level. (5) One PWS could serve several counties, but we only know the total population served by each PWS rather than the population served by each PWS within each county, thus the population-weighted average water concentrations of PFAS by county could also be inaccurate. (6) UCMR3 was designed to monitor water quality by PWS, not to measure county-level exposure or exposure to specific subgroups defined by race, education or other characteristics that might be associated with residential location and water supply within a county; however, we assumed that everyone within a county had the same average PFAS exposure level and assign the population-weighted average water concentrations of PFAS to all the groups within the same county. (7) We also did not account for the fact that some people in these counties may be using private wells or smaller water systems not included in UCMR3, and thus may have had different exposures than their neighbors. We understand that USGS is developing information on PFAS in private wells in the US; incorporating that information into birthweight analyses would be a valuable future direction.
Conflicts of interest statement
S.M.B. has served as a compensated expert witness for PFOA medical monitoring lawsuits in New Hampshire. The terms of this arrangement were reviewed and approved by the University of California Irvine in accordance with its conflict of interest policies. S.M.B. and Y.Z. receive salary support from a CDC/ATSDR cooperative agreement on PFAS exposure and human health (#U01-TS000308).
Supplementary Material
Footnotes
Published online 5 August 2020
The data used in this study are all publicly available from the CDC and US EPA. The R code is available on request.
SDC Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.environepidem.com).
References
- 1.Kissa E. Fluorinated Surfactants and Repellents. 20012nd edNew York: Marcel Dekker [Google Scholar]
- 2.Giesy JP, Kannan K. Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol. 2001; 35:1339–1342 [DOI] [PubMed] [Google Scholar]
- 3.Buck RC, Franklin J, Berger U, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011; 7:513–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ATSDR (U.S. Department of Health and Human Services Agency for Toxic Substances and Disease Registry) Toxicological Profile for Perfluoroalkyls. 2018Atlanta, GA: Centers for Disease Control and Prevention; https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf. [PubMed] [Google Scholar]
- 5.Egeghy PP, Lorber M. An assessment of the exposure of Americans to perfluorooctane sulfonate: a comparison of estimated intake with values inferred from NHANES data. J Expo Sci Environ Epidemiol. 2011; 21:150–168 [DOI] [PubMed] [Google Scholar]
- 6.Jian JM, Guo Y, Zeng L, et al. Global distribution of perfluorochemicals (PFCs) in potential human exposure source-A review. Environ Int. 2017; 108:51–62 [DOI] [PubMed] [Google Scholar]
- 7.CDC (Centers for Disease Control and Prevention) Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019. Page 399, 403, 407, and 415. Atlanta, GA: US Centers for Disease Control and Prevention; https://www.cdc.gov/exposurereport/index.html. Accessed 23 March 2020. [Google Scholar]
- 8.Olsen GW, Burris JM, Ehresman DJ, et al. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007; 115:1298–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. Rate of decline in serum PFOA concentrations after granular activated carbon filtration at two public water systems in Ohio and West Virginia. Environ Health Perspect. 2010; 118:222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Beesoon S, Zhu L, Martin JW. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol. 2013; 47:10619–10627 [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Fletcher T, Mucs D, et al. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med. 2018; 75:46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SK, Lee KT, Kang CS, et al. Distribution of perfluorochemicals between sera and milk from the same mothers and implications for prenatal and postnatal exposures. Environ Pollut. 2011; 159:169–174 [DOI] [PubMed] [Google Scholar]
- 13.Apelberg BJ, Witter FR, Herbstman JB, et al. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ Health Perspect. 2007; 115:1670–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ Health Perspect. 2007; 115:1677–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M, Zeng XW, Qian ZM, et al. Isomers of perfluorooctanesulfonate (PFOS) in cord serum and birth outcomes in China: Guangzhou Birth Cohort Study. Environ Int. 2017; 102:1–8 [DOI] [PubMed] [Google Scholar]
- 16.Cao W, Liu X, Liu X, et al. Perfluoroalkyl substances in umbilical cord serum and gestational and postnatal growth in a Chinese birth cohort. Environ Int. 2018; 116:197–205 [DOI] [PubMed] [Google Scholar]
- 17.Kishi R, Nakajima T, Goudarzi H, et al. The association of prenatal exposure to perfluorinated chemicals with maternal essential and long-chain polyunsaturated fatty acids during pregnancy and the birth weight of their offspring: The Hokkaido Study. Environ Health Perspect. 2015; 123:1038–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Choi K, Ji K, et al. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ Sci Technol. 2011; 45:7465–7472 [DOI] [PubMed] [Google Scholar]
- 19.Savitz DA, Stein CR, Bartell SM, et al. Perfluorooctanoic acid exposure and pregnancy outcome in a highly exposed community. Epidemiology. 2012; 23:386–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Yang L, Li J, et al. Occurrence of perfluoroalkyl substances in cord serum and association with growth indicators in newborns from Beijing. Chemosphere. 2017; 169:396–402 [DOI] [PubMed] [Google Scholar]
- 21.Gibson HM. Plasma volume and glomerular filtration rate in pregnancy and their relation to differences in fetal growth. J Obstet Gynaecol Br Commonw. 1973; 80:1067–1074 [DOI] [PubMed] [Google Scholar]
- 22.Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis. 2013; 20:209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verner MA, Loccisano AE, Morken NH, et al. Associations of perfluoroalkyl substances (PFAS) with lower birth weight: an evaluation of potential confounding by glomerular filtration rate using a physiologically based pharmacokinetic model (PBPK). Environ Health Perspect. 2015; 123:1317–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steenland K, Barry V, Savitz D. Serum perfluorooctanoic acid and birthweight: an updated meta-analysis with bias analysis. Epidemiology. 2018; 29:765–776 [DOI] [PubMed] [Google Scholar]
- 25.US EPA (United States Environmental Protection Agency) Revisions to the unregulated contaminant monitoring rule (UCMR 3) for public water systems. Federal Register Rules Regulations. 2012; 77:26072–26101 [Google Scholar]
- 26.Hu XC, Andrews DQ, Lindstrom AB, et al. Detection of poly- and perfluoroalkyl substances (PFASs) in U.S. drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environ Sci Technol Lett. 2016; 3:344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurley S, Houtz E, Goldberg D, et al. Preliminary associations between the detection of perfluoroalkyl acids (PFAAs) in drinking water and serum concentrations in a sample of California women. Environ Sci Technol Lett. 2016; 3:264–269 [Google Scholar]
- 28.Thompson J, Lorber M, Toms LL, Kato K, Calafat AM, Mueller JF. Use of simple pharmacokinetic modeling to characterize exposure of Australians to perfluorooctanoic acid and perfluorooctane sulfonic acid. Environ Int. 2010; 36:390–397 [DOI] [PubMed] [Google Scholar]
- 29.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999-2008. Environ Sci Technol. 2011; 45:8037–8045 [DOI] [PubMed] [Google Scholar]
- 30.Weisskopf MG, Webster TF. Trade-offs of personal versus more proxy exposure measures in environmental epidemiology. Epidemiology. 2017; 28:635–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CDC. About natality, 2007-2018. https://wonder.cdc.gov/natality-current.html. Accessed 23 March 2020.
- 32.US EPA. Monitoring Unregulated Drinking Water Contaminants, UCMR 3 (2013-2015) Occurrence Data. Retrieved from https://www.epa.gov/dwucmr/occurrence-data-unregulated-contaminant-monitoring-rule. Accessed 23 March 2020.
- 33.US EPA. SDWIS Federal Reports Advanced Search. Retrieved from https://ofmpub.epa.gov/apex/sfdw/f?p=108:9:::NO::P9_REPORT:VIO. Accessed 23 March 2020.
- 34.Neter J, Kutner M, Nachtsheim C, Wasserman W. Applied Linear Statistical Models. 19964th edNew York: McGraw-Hill/Irwin [Google Scholar]
- 35.Bartell SM. Statistical methods for non-steady state exposure inference using biomarkers. PhD Dissertation. 2003Davis, CA: University of California [Google Scholar]
- 36.Hoffman K, Webster TF, Bartell SM, Weisskopf MG, Fletcher T, Vieira VM. Private drinking water wells as a source of exposure to perfluorooctanoic acid (PFOA) in communities surrounding a fluoropolymer production facility. Environ Health Perspect. 2011; 119:92–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartell SM. Online serum PFOA calculator for adults. Web-based software, version 0.9. https://www.ics.uci.edu/~sbartell/pfoacalc.html. Accessed 23 March 2020 [DOI] [PMC free article] [PubMed]
- 38.Lu S, Bartell SM. Serum PFAS calculator for adults, web-based software, version 1.1. www.ics.uci.edu/~sbartell/pfascalc.html. Accessed 23 March 2020
- 39.Blakely TA, Woodward AJ. Ecological effects in multi-level studies. J Epidemiol Community Health. 2000; 54:367–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fisher RA. Statistical Methods for Research Workers. 19345th edEdinburgh: Olivia and Boyd [Google Scholar]
- 41.Robinson LD, Jewell NP. Some surprising results about covariate adjustment in logistic regression models. Int Stat Rev. 1991; 59:227–240 [Google Scholar]
- 42.Weisskopf MG, Seals RM, Webster TF. Bias amplification in epidemiologic analysis of exposure to mixtures. Environ Health Perspect. 2018; 126:047003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Northam S, Knapp TR. The reliability and validity of birth certificates. J Obstet Gynecol Neonatal Nurs. 2006; 35:3–12 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


