Abstract
Loss of ovarian function in women is associated with sleep disturbances and cognitive decline, which suggest a key role for estrogens and/or progestins in modulating these symptoms. The effects of ovarian hormones on sleep and cognitive processes have been studied in separate research fields that seldom intersect. However, sleep has a considerable impact on cognitive function. Given the tight connections between sleep and cognition, ovarian hormones may influence selective aspects of cognition indirectly, via the modulation of sleep. In support of this hypothesis, a growing body of evidence indicates that the development of sleep disorders following menopause contributes to accelerated cognitive decline and dementia in older women. This paper draws from both the animal and human literature to present an integrated view of the effects of ovarian hormones on sleep and cognition across the adult female lifespan.
Keywords: estrogens, progesterone, menopause, hormone replacement therapy, sleep loss, memory, executive function, primate, rodent, human
Introduction
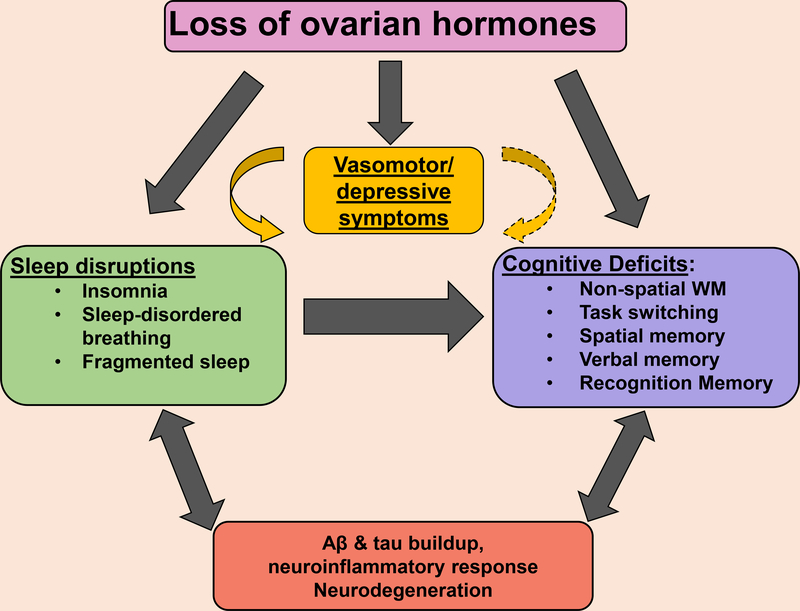
Several lines of research indicate that ovarian hormones, including estrogens (E) and progesterone (P), affect brain structure, cognition, and behavior in females across the lifespan. Most studies focus on cognitive and behavioral changes that occur following the menopausal transition, when levels of E and P gradually decline. Interestingly, this period is accompanied by sleep disruptions, hot flashes (HFs), mood changes, cognitive decline and increased risk of dementia, suggesting that the loss of circulating ovarian hormones is a key contributor to these symptoms. However, the hypothesis that ovarian hormones have a causal role in these symptoms has most often been examined in distinct lines of research focusing on a single domain (e.g. ovarian hormones and cognition) with little cross-talk among research fields. In this paper, we argue that a more integrated approach incorporating multiple behavioral outcomes may provide a better understanding of the effects on ovarian hormones on behavior. This review focuses on two of these domains, sleep and cognition. We first show that both sleep (reviewed in section 1) and selective cognitive abilities (reviewed in section 2) are independently sensitive to ovarian hormones in females. Further, we discuss a growing body of evidence demonstrating tight connections between sleep and several cognitive domains, including attention, executive function, and spatial memory (reviewed in section 3). Since the literature on sleep and cognition is particularly vast, we discuss specific studies on abilities shown to be sensitive to both ovarian hormones and sleep in adult females. In synthesizing these findings (Section 4), we suggest that ovarian hormones may play an important role in regulating the impact of sleep on cognitive function in females. Although limited data are currently available to support this hypothesis, as most of the studies on the interplay between sleep and cognition were conducted in mixed-sex samples where biological sex was not taken into consideration, or in male-only samples, we believe that it is an important area for future research with potential for therapeutic intervention.
1. Sleep is sensitive to ovarian hormones
1.1. Overview of sleep
Sleep is a behavior observed in most organisms, and in contrast to wakefulness, involves reduced responsiveness to external stimuli (Campbell & Tobler, 1984; Hendricks et al., 2000). Two regulatory mechanisms influence the sleep-wake cycle; biological clocks (i.e. circadian rhythms), which synchronize the timing of sleep over an ~24 hr cycle, and homeostatic drive, or the need for sleep, which increases proportionally with the time spent awake (Borbély, 1982). Changes in cortical activity that occurs during the sleep/wake cycle can be monitored using electroencephalography (EEG) and used to quantify the different vigilance states. These states include 1) wake, characterized by high frequency, low amplitude EEGs, 2) rapid-eye movement (REM), with EEGs that are similar to wake (high frequency/ low amplitude), and 3) non-rapid eye movement (NREM), which is further divided into three stages (N1-N3). The deepest stage, N3, is also known as slow-wave sleep (SWS), which involves low frequency, high amplitude EEGs (i.e. delta waves). The sleep-wake cycle typically progresses from wake, to the lightest stage of NREM (N1), then to deeper stages (N2-N3) before transitioning to REM. In adult humans, each cycle takes approximately 90 min, with a total of 3–5/night (Armitage & Hoffmann, 2001). Sleep can become disrupted by frequent awakenings (i.e. fragmented sleep), extended periods of arousals, and diminished SWS (Guilleminault et al., 1988).
1.1. Sleep quality and sleep architecture in women
Excellent reviews describing the role of ovarian hormones on sleep are available (Lord et al., 2014; Moline et al., 2004; Mong et al., 2011, 2016; Polo-Kantola, 2011), and we only briefly discuss this topic here. Sex differences have been reported in both subjective and objective sleep measures. Women are 1.3–1.8 times more likely to report sleep problems than men (Polo-Kantola, 2011), including more disrupted and insufficient sleep (Groeger et al., 2004; Lindberg et al., 1997; Middelkoop et al., 1996; Reyner et al., 1995; Zhang & Wing, 2006), poorer sleep quality, as well as difficulty falling and staying asleep (Lindberg et al., 1997; Zhang & Wing, 2006). Although these data suggest that sleep quality is poorer in women than men, studies using objective polysomnographic (PSG) recordings do not corroborate these findings. Indeed, compared to men, women across a wide range of ages sleep for longer (Bixler et al., 2009; Ohayon, Carskadon, Guilleminault, & Vitiello, 2004; Polo-Kantola et al., 2016; Tonetti, Fabbri, & Natale, 2008; Ursin, Bjorvatn, & Holsten, 2005), have a shorter sleep onset latency, spend less time transitioning between wakefulness and sleep (Goel et al., 2005), and have more SWS and slow-wave activity (SWA; delta, ~0.5– 4 Hz) during sleep (Bixler et al., 2009; Carrier, Land, Buysse, Kupfer, & Monk, 2001; Dijk et al., 1989; Ehlers & Kupfer, 1997; Latta et al., 2005; Mourtazaev et al., 1995). In addition, the decline in SWS that occurs with age (Mander et al., 2016) has been reported to be attenuated in women compared to men (Ehlers & Kupfer, 1997; but see Carrier et al., 2001). Two other studies show that objective sleep is more resistant to sleep disruptions in young women than in young men. Vgontas et al (2004) found that changes in inflammation markers and maximum cortisol values during restricted sleep were greater in men than in women. In addition, objective sleep measures (sleep efficiency and percentage of N1) were less affected by night blood draws in women than in men in another study (Blixter et al., 2009). Overall, these findings suggest that women’s objective sleep is better and more resistant to disturbances than men’s. However, a few studies observe more SWA in women following sleep deprivation (SD; Armitage & Hoffmann, 2001; Manber et al., 1999), suggesting a greater accumulation of sleep debt in women than in men (Webb, 1982).
Given that many aspects of objective sleep quality appear superior in women, additional factors must contribute to the subjective experience of sleep quality. One potential factor is circadian timing, which is also influenced by sex (for a review, see Bailey & Silver, 2014). While women go to bed earlier, and wake up earlier than men (Adan & Natale, 2002; Roenneberg et al., 2007; Tonetti et al., 2008), there is some evidence that their circadian period is shorter than men’s (Cain et al., 2010; Duffy et al., 2011), and that their sleep timing occurs later than their circadian timing. For instance, Cain and colleagues (2010) compared both sleep timing and circadian timing in men and women matched on age and habitual wake time. Circadian timing was estimated based on diurnal fluctuations in core body temperature and endogenous melatonin levels. The authors observed that sleep timing was equivalent across sex, but that the circadian timing was earlier in women, suggesting that sleep may be occurring at a later biological time in women. This might explain the discrepancy between subjective and objective sleep measures in women, as well as greater susceptibility to insomnia in women than in men (Zhang & Wing, 2006). However, other factors affect subjective sleep measures including depressed mood (Toffol et al., 2014), which is also more common in women (e.g., Altemus et al, 2014). Therefore, more research is necessary to fully understand why women report more sleep problems than men.
Reproductive hormones have been shown to modulate women’s sleep across adulthood. Not only do sleep problems including insomnia emerge at puberty when levels of these hormones increase (Zhang & Wing, 2006), increases in sleep complaints typically coincide with periods involving large fluctuations of ovarian hormones, including puberty, pregnancy, and the menopausal transition (as reviewed by Mong et al., 2016). There is also evidence for sleep changes across the menstrual cycle. In general, sleep appears to be poorest during the mid-to-late luteal phase, when ovarian hormones begin to decline (as reviewed by Baker & Driver, 2007; Lord et al., 2014; Moline et al., 2003). Compared to the follicular phase, the luteal phase is associated with increased reports of nighttime awakenings and arousal during sleep and decreased SWS (De Zambotti et al., 2015b). In addition, sleep spindles, which are short bursts of activity, are more frequent, longer in duration and occur in higher EEG spectral frequency (14– 17 Hz) during the luteal than the follicular phase (Baker, Kahan et al., 2007; de Zambotti et al., 2015b; Driver et al., 1996). Differences in objective sleep measures are also observed in women taking oral contraceptives as indicated by increased N2, REM, and reduced SWS relative to naturally cycling women (Baker et al., 2001a,b; Burdick, Hoffman, and Armitage, 2002). However, other studies either find limited (e.g. Romans et al., 2015) or no differences (Baker & Colrain, 2010) for sleep changes across the menstrual cycle.
The menopausal transition, which is characterized by erratic fluctuations in reproductive hormone levels followed by an eventual decline (Harlow et al, 2012) is associated with poor sleep. Forty to 60% of perimenopausal women report sleep disturbances and insomnia (Baker et al. 2015; Joffe et al., 2010; Moline et al., 2004; Polo-Kantola 2011). The menopausal transition is also associated with increased frequency of self-reported problems, such as falling and staying asleep (Kravitz et al., 2008), and reduced total sleep time (Lampio, Saaresranta, Polo, & Polo-Kantola, 2013). Levels of follicular stimulating hormone may play a role in sleep quality, as they are positively related to waking after sleep onset, number of awakenings, and arousals in perimenopausal women without sleep complaints (De Zambotti et al., 2015a). This association is independent of age, body mass index and HFs. However, studies using PSG recordings do not consistently report objectively measured sleep disruptions in menopausal women. For example, Young and colleagues (2003a) observed lower sleep satisfaction in peri- and post-menopausal women compared to pre-menopausal women, but demonstrated better sleep architecture, as indicated by better sleep efficiency (% time in bed actually asleep), more SWS, and less N2. Similarly, other studies either report better sleep patterns in postmenopausal women (e.g. Sharkey et al., 2003), or no disruptions in PSG recordings in peri- and postmenopausal women (Shaver et al., 1988). One possibility is that objectively-measured sleep disruptions during menopause are related to the occurrence of vasomotor symptoms, including HFs. Hot flashes are characterized by an intense sensation of heat, followed by sweating and skin vasodilation (De Zambotti et al., 2014; Freedman, 2014). Menopausal women with insomnia are more likely to have HFs, and the presence of HFs predicts the number of PSG-awakenings (Baker et al., 2015). De Zambotti and colleagues (2014) reported that menopausal women with insomnia exhibit an average of 3.5 HFs per night, with the majority (64%) resulting in awakenings that lasted about 16 min. The time women spent awake following a HF is negatively associated with sleep efficiency (time spent in sleep over time spent in bed), and positively associated with waking after sleep onset. These findings are consistent with previous reports (e.g. Kravitz et al., 2008), including one demonstrating an association between severe HFs and chronic insomnia in peri and postmenopausal women (Ohayon, 2006). There is also evidence that the menopausal transition is associated with an increased risk of developing sleep-disordered breathing (Young et al., 2003b).
The most convincing evidence for a role of ovarian hormones in sleep comes from hormone therapy (HRT) studies. Postmenopausal women with HRT have a reduced latency to fall asleep (Moe et al., 2001; Schiff et al., 1979), and fewer nighttime awakenings (Erlik et al., 1981; Polo-Kantola et al., 1999; Scharf et al., 1997; Thompson & Oswald, 1977) and wakefulness (Montplaisir et al., 2001; Thompson and Oswald, 1977). E2 given for 8 weeks to peri- and postmenopausal women is associated with reduced self-reported insomnia symptoms and improved sleep quality (Ensrud et al., 2015). In addition, Moe et al (2001) reported that night blood draws result in greater sleep disruption for postmenopausal women without HRT compared to women on HRT. However, a number of studies reported no benefit of HRT on sleep (Young et al. 2003; Pickett et al., 1989; Purdie et al., 1995; Kalleinen et al., 2008). It is possible that HRT’s beneficial effects on sleep are only observed in some women. For instance, HRT alleviates sleep complaints in women that experience HFs. Joffe and colleagues (2013) examined the impact of pharmacologically-induced menopause on HFs and sleep in young women (~27 years old). Administration of a gonatrotropin-releasing hormone agonist (GnRHa, leuprolide acetate) reduced levels of E2 to those observed in postmenopausal women, and resulted in HFs in 69% of participants, with the number of HFs correlating with sleep disturbances, as measured by waking after sleep onset, number of awakenings, and percent time spent in N1. Other studies also support these findings (e.g. Kravitz et al., 2008; Polo-Kantola et al., 1998a, 1999). Further, HRT might improve sleep in women experiencing depressive symptoms, as was demonstrated in a recent study (Lampio, Saaresranta, Engblom, Polo, & Polo-Kantola, 2016). This is a particularly important point, given that risk for depression is 30% to three times greater during the menopausal transition compared to the premenopausal stage (as reviewed by Maki et al., 2010), and poor sleep has been shown to be a predictor of depressive symptoms during perimenopause (Avis et al., 2001; Freeman et al., 2004; Joffe et al., 2011).
1.3. Sleep in female animals
There is considerable evidence that sex and ovarian hormones influence sleep in rodents. However, while studies in humans suggest that women sleep longer than men (Bixler et al., 2009; Ohayon et al., 2004; Tonetti et al., 2008), female rodents appear to spend less time in sleep states than males, including less NREM in mice (Ehlen et al., 2013; Franken et al., 2006; Paul et al., 2006), and REM in rats (Fang and Fishbein, 1996; Yamaoka, 1980). Rodents also demonstrate sex differences in sleep patterns, as female rodents experience higher delta power (Paul et al., 2006) than males. Several studies have shown that ovarian hormones modulate sleep behavior in female rodents and contribute to this sex difference. For example, proestrous, the phase when both E2 and P are elevated, is associated with decreased time in both REM and SWS (Hadjimarkou et al., 2008), and increased fragmented sleep (Schwierin et al., 1998) during the dark phase of the light-dark cycle. Ovariectomy abolishes both the fluctuations in sleep behavior across the estrous cycle (Mong et al., 2016), and the sex difference (Koehl, Battle, & Meerlo, 2006; Paul et al., 2006). E2 replacement in ovariectomized (OVX) rats reduces REM (Cusmano et al., 2014; Deurveilher et al., 2013; Schwartz and Mong 2011, 2013) and SWS (Deurveilher et al., 2013) during the dark phase. Similar results are reported in mice (Koehl, Battle, & Turek, 2003; Paul et al., 2006, 2009). E2 also influences recovery from SD. When using an acute total SD procedure limited to the first 6 hr of the light phase, increased SWA and reduced sleep fragmentation was observed during the subsequent 6 hr (Schwierin et al., 1998). While recovery from SD is unaffected by estrous phase (Schwierin et al., 1998), it is enhanced by E2. Deurveilher and colleagues (2009) demonstrated enhanced REM during recovery from SD in rats that received continuous E2 replacement. A subsequent study demonstrated that continuous E2 and P replacement resulted in increased duration of REM and NREM sleep episodes, and decrease in brief awakenings during recovery sleep (i.e. during dark phase; Deurveilher, Rusak, & Semba, 2011). In middle-aged OVX rats, these hormones increase NREM-associated delta power during recovery from SD, suggesting that ovarian hormones enhance sleep intensity (Deurveilher et al., 2013). While these data suggest that E2 replacement facilitates recovery from SD, Schwartz and Mong (2013) showed that this enhancement is restricted to sleep occuring during the light phase of the light-dark cycle. In that study, estradiol benzoate (EB) replacement enhanced REM during the light phase, when rats spent most of their time asleep, but suppressed REM during the dark phase. Together, these data provide strong evidence that estrogens modulate sleep in female rats. While much of the evidence suggests that sleep is reduced by E2, the direction of the effect depends on the time of day, with E2 facilitating sleep during the light phase, when rats spend most of their time asleep, and increasing wakefulness during the dark phase when rats sleep less. Since rats are nocturnal with polyphasic sleep patterns, which differ from the consolidated sleep patterns of humans, research in diurnal animals may better model the effects of E2 on sleep in humans. We recently conducted a preliminary study (Gervais, Viechweg, et al., 2016) on the effects of E2 on sleep patterns in the common marmoset (Callithrix jacchus), a small primate with consolidated sleep patterns, who spends 85% of the dark phase of the light/dark cycle in sleep (Hoffmann et al., 2012). We found that E2 replacement was associated with improved sleep quality and reduced number of arousals in two middle-aged OVX marmosets, suggesting that E2 enhances sleep in this diurnal primate. These preliminary results need to be confirmed with a larger sample size, but suggest that the female marmoset is a useful model for further investigating the mechanisms by which ovarian hormones may improve sleep in women.
2. Selective aspects of cognition are sensitive to ovarian hormones
Ovarian hormones also affect cognitive function in females across the adult lifespan. Several extensive reviews are already available on this topic (Frick et al., 2015; Galea et al., 2016; Hamson, Roes, & Galea, 2016; Maki & Henderson, 2012; Maki, 2013; Sherwin & Henry, 2008). Our goal here is not to replicate this work but rather to emphasize main aspects of cognition that are both sensitive to ovarian hormones and modulated by sleep (as described in the next section). While several studies examine the impact of ovarian hormones loss on global measures of cognitive impairment (e.g. Mini Mental Status Exam), we focus on studies that examine specific cognitive abilities, as the impact of ovarian hormones on cognition is domain specific. We review findings from humans, non-human primates (NHP), and rodents implicating E2 in prefrontal cortex (PFC)-dependent cognition before switching to abilities that involve the medial temporal lobe (MTL), which includes the hippocampus (HPC), perirhinal cortex (PRh), entorhinal cortex (EC), and parahippocampal cortex. For many abilities described below, age and reproductive stage influence the direction of results and efforts were made to review such findings separately when possible. A summary of the findings for each ability are presented in Table 1.
Table 1.
Summary of the effects of ovarian hormones on cognition
| Ability | Sample Characteristics | Younger | Older | References |
|---|---|---|---|---|
| Attention | ||||
| Humans | ||||
| Premenopause | F > L | Pletzer et al., 2014 | ||
| Postmenopause (55–65 years) | HRT > no HRT | Castonguay et al., 2015 | ||
| Postmenopause | HRT > no HRT | Fedor-Feyberg, 1977; | ||
| Postmenopause (∼60 years) | ERT > no ERT | Schmidt et al., 1996 | ||
| Postmenopause (∼65 years) | HRT > no HRT | Smith et al., 2001 | ||
| Postmenopause (∼53 years) | HRT = no HRT | Gleason et al., 2015 | ||
| Postmenopause (47–65 years) | ERT = no ERT | Polo-Kantola et al., 1998b | ||
| Postmenopause (53–72 years) | HRT = no HRT | Alhola et al., 2006 | ||
| Postmenopause (∼53 years) | HRT = no HRT | Keenan et al., 2001 | ||
| Postmenopause (58–75 years) | HRT = no HRT | Wolf et al., 2005 | ||
| Animals | ||||
| OVX macaques (6–11 years) | OVX < intact E2 > vehicle |
Voyko 2002 | ||
| OVX macaques (7–16 years) | E2 = Vehicle | Lacreuse et al., 2009 | ||
| OVX macaques (∼20 years) | E2 > No E2 | Voytko et al., 2009 | ||
| OVX macaques (18–25 years) | E2 > No E2 | Kohama et al., 2016 | ||
| 17-mo OVX rats | E2 > No E2 | Bohacek & Daniel, 2010 | ||
| Intact rats | Proestrous < Estrous/Diestrous | Quinlan et al., 2010 | ||
| E2 < Vehicle | Almey et al., 2013 | |||
| Non-spatial working memory | ||||
| Humans | ||||
| Maintenance | ||||
| Digit Span forward: | Postmenopause | HRT = no HRT | Alhola et al., 2006; Castonguay et al., 2015; Duff & Hampson, 2000; Gleason et al., 2015; Keenan et al., 2001; Polo-Kantola et al., 1998b; Schmidt et al., 1996 | |
| Manipulation/Updating | ||||
| Early F = Late F | Joseph et al., 2012 | |||
| Postmenopause (∼56 years) | HRT > no HRT | Duff & Hampson, 2000 | ||
| Postmenopause (55–65 years) | HRT = no HRT HRT > no HRT |
Castonguay et al., 2015 | ||
| Postmenopause (∼53 years) | HRT = no HRT | Gleason et al., 2015 | ||
| Postmenopause (∼53 years) | HRT > no HRT | Keenan et al., 2001 | ||
| Postmenopause (50–65 years) | 3 days ERT > no ERT | Krug et al., 2006 | ||
| Postmenopause (∼69 years) | HRT = no HRT | Janowsky et al., 2000 | ||
| Postmenopause (∼70 years) | ERT > no ERT | Baker et al., 2012 | ||
| Animals | ||||
|
Object-DRST Face-DRST |
OVX macaques (6–9 years) | EE2 = no EE2 EE2 < no EE2 |
Lacreuse & Herndon, 2003 | |
| Object-DRST | OVX macaques (7–16 years) | EB = no EB | Lacreuse et al., 2009 | |
| OVX rats | Low EB > no EB Mod-high EB < no EB |
Wide et al., 2004 | ||
| OVX rats | High E2 < no E2 | Wang et al., 2008 | ||
| OVX rats | High E2 < no E2 | High E2 < no E2 | Wang et al., 2009 | |
| Task switching | ||||
| Humans | ||||
| Premenopause | GnRHa = GnRHa + E/P GnRHa: ↓ DLPFC GnRHa + E/P: ↑ DLPFC |
Berman et al., 1997 | ||
| Postmenopause (∼60 years) | ERT > no ERT | Schmidt et al., 1996 | ||
| Postmenopause (55–65 years) | HRT > no HRT | Castonguay et al., 2015 | ||
| Early postmenopause (∼52 years) | ↑DLPFC in HRT users; correlated with performance | Girard et al., 2017 | ||
| Postmenopause (∼65 years) | 3-mo ERT = no ERT | Duka et al., 2000 | ||
| Animals | ||||
| OVX macaques (∼20 years) | HRT > no HRT | Voytko et al., 2009 | ||
| OVX macaques (∼24 years) | EE2 = no EE2 | Lacreuse et al., 2004 | ||
| Young OVX rats | E2 > no E2 | Lipatova et al., 2016 | ||
| reversal learning | OVX marmosets (3–5 years) | High E2 < No E2 | Lacreuse et al., 2014 | |
| reversal learning | OVX rats (21–22 mo) | E2 < no E2 | Gibbs et al., 2011 | |
| Response inhibition | ||||
| Humans | ||||
| Go/No-Go, Stop-Signal | Premenopause | F < L | Colzato et al., 2010; Reimers et al., 2014 | |
| Stroop Interference | Postmenopause (50–65 years) | 3 days ERT > no ERT | Krug et al., 2006 | |
| Stroop Interference | Postmenopause | HRT = no HRT | Alhola et al., 2006; Baker et al., 2012; Castonguay et al., 2015; Duka et al., 2000; Polo-Kantola et al., 1998b; Wolf et al., 2005 | |
| Animals | ||||
| Stop Signal | Premenopausal baboons | O = L | Lacreuse et al., 2016 | |
| DRL | OVX rats | High E2 < no E2 | Wang et al., 2008 | |
| DRL | OVX rats | High E2 < no E2 | E2 = no E2 | Wang et al., 2011 |
| Decision making | ||||
| Humans | ||||
| Premenopause & postmenopause (∼55 years) | Menses = mid L | ERT = no ERT | Reavis & Overman, 2001 | |
| Premenopause | F > menses | Smith et al., 2014 | ||
| Animals | ||||
| OVX rats | High EB > no EB | Uban et al., 2012 | ||
| Spatial Working Memory | ||||
| Humans | ||||
| Premenopause | High E2 > Low E2 E2 correlates with performance |
Hampson & Morley 2013 | ||
| Postmenopause (∼56 years) | HRT > no HRT | Duff & Hampson, 2000 | ||
| Postmenopause (∼71 years) | 3-mo ERT = no ERT | Schiff et al., 2005 | ||
| Animals | ||||
| Premenopause | O < F=L | Lacreuse et al., 2001 | ||
| OVX macaques (6–9 years) | EE2 = no EE2 | Lacreuse et al., 2003 | ||
| OVX macaques (7–16 years) | E2 = Vehicle | Lacreuse et al., 2009 | ||
| Macaques (6–10 years) | OVX = intact E2 = no E2 |
Voytko, 2000 | ||
| Macaques (∼ 10 years) | E2 = no E2 | Hao et al., 2007 | ||
| Macaques (20–27 years) | Premenopause > peri/postmenopause | Roberts et al., 1997 | ||
| OVX macaques (∼22 years) | E2 > no E2 | Rapp et al., 2003 | ||
| OVX macaques (18–25 years) | E2 > E2/P > No E2 | Kohama et al., 2016 | ||
| OVX macaques (21–24 years) | EE2 > no EE2 | Lacreuse et al., 2002 | ||
| OVX macaques (∼20 years) | E2 = E2/P = no E2 | Voytko et al., 2008 | ||
| OVX marmosets (3–5 years) | High E2 < No E2 | Lacreuse et al., 2014 | ||
| OVX macaques (19–27 years) | Long-term OVX > intact | Lacreuse et al., 2000 | ||
| Intact rats | Proestrous > estrous | Pompili et al., 2010 | ||
| OVX rats | E2 > no E2 | Gibbs, 2007; Holmes et al., 2002; Luine et al., 1998; Sandstrom & Williams, 2001; Velàzquez-Zamora et al., 2012 | ||
| OVX rats | Win-stay: E2 = no E2 Win-shift: E2 < no E2 |
Galea et al., 2001 | ||
| OVX rats | Intra-PFC/HPC E2 > vehicle | Sinopoli et al., 2006 | ||
| 12-mo old OVX rats | E2 > no E2 | Daniel et al., 2006 | ||
| 21–25-mo old OVX rats | E2+P > no E2+P | Gibbs, 2000 | ||
| 13–22-mo old OVX rats | OVX < intact E2 > no E2 | Markowska & Savonenko, 2002 | ||
| Spatial reference memory | ||||
| Animals | ||||
| Intact rats | Proestrous = estrous | Berry & McMahan, 1997; | ||
| Intact rats | Watermaze: Proestrous < estrous RAM: Proestrous = estrous |
Pompili et al., 2010 | ||
| Proestrous < estrous | Warren & Juraska, 1997 | |||
| OVX rats | Watermaze: E2 > no E2 Y-maze: E2 = no E2 |
McLaughlin, 2008 | ||
| E2 > no E2 Intra-HPC E2 > vehicle |
Packard & Teather, 1997a,b | |||
| OVX rats | E2 < no E2 | Galea et al., 2001 | ||
| OVX rats | E2 = no E2 | Fader et al., 1999; Holmes et al., 2002; Luine et al., 1998; | ||
| 4, 16, & 24-mo old OVX rats | E2 > no E2 | E2 = no E2 | Talboom et al., 2008 | |
| 13–22-mo old OVX rats | OVX = intact E2 = no E2 |
Markowska & Savonenko, 2002 | ||
| Object placement/NOIP test | ||||
| Animals | ||||
| OVX rats | E2 > no E2 | As reviewed by Tuscher et al., 2014; McLaughlin et al., 2008 | ||
| Spatial strategy | ||||
| Humans | ||||
| Premenopause | Late L > Early F/O | Hussain et al., 2016 | ||
| Animals | ||||
| Rats | Proestrous > estrous E2 > no E2 |
E2 > no E2 | As reviewed by Korol & Pisani, 2015; Korol et al., 2004 | |
| OVX rats | Intra-HPC E2 > Vehicle | Zurkovsky et al., 2007 | ||
| Verbal memory | ||||
| Humans | ||||
| Premenopause | Inconsistent reports, or F = L = Menses | As reviewed by Poromaa & Gingnell, 2014 | ||
| Premenopause | GnRHa < cycling | Craig et al., 2008 | ||
| GnRHa = cycling | Guerrieri et al., 2016; Owens et al., 2002 | |||
| Age: ∼45–52 years | Peri-/postmenopause/ < premenopause | Greendale et al., 2009; Weber et al., 2013 | ||
| Surgically menopausal women (36–45 years) | BL > post-surgery | Sherwin, 1988 | ||
| Postmenopause | HRT > no HRT | Baker et al., 2012; Castonguay et al., 2015; Jacobs et al., 1998; Kampen & Sherwin, 1994; Keenan et al., 2001; Maki, Zonderman, & Resnick, 2001; Phillips & Sherwin, 1992b; Resnick et al., 1998 | ||
| Postmenopause | HRT = no HRT | Baker et al., 2012; Gleason et al., 2015; Henderson et al., 2016 | ||
| Visual recognition memory | ||||
| Humans | ||||
| Premenopause | GnRHa < cycling | Craig et al., 2010 | ||
| Postmenopause (∼65–67 years) | ERT > no ERT | Resnick et al., 1998 | ||
| Postmenopause (∼64 years) | HRT = no HRT | Alhola et al., 2006 | ||
| Older AD patients (∼74 years) | ERT = no ERT | Sundermann et al., 2006 | ||
| Animals | ||||
| Premenopause |
No/brief retention delay: F = L 30-s retention delay: O < F=L |
Kromrey, Czoty, & Nader, 2015; Lacreuse et al., 2001 | ||
| OVX macaques (6–9 years) | EE2 = no EE2 | Lacreuse & Herndon, 2003 | ||
| OVX macaques (7–16 years) | E2 = Vehicle | Lacreuse et al., 2009 | ||
| OVX macaques (∼20–24 years) | HRT > no HRT | Lacreuse et al., 2002; Rapp et al., 2003; Voytko et al., 2008 | ||
| OVX rats with low E2 | Intra-PRh E2 < vehicle | Gervais et al., 2013, 2016 | ||
| Novel object preference | ||||
| Rats | Proestrous > estrous E2 > no E2 Intra-HPC > vehicle Intra-PRh > vehicle |
Intra-HPC E2 = vehicle | As reviewed by: Galea, Frick, Hampson, Sohrabji, & Choleris, 2016; Luine, 2015; Tuscher et al., 2014 | |
Note: AD = Alzheimer’s Disease; DLPFC = dorsolateral prefrontal cortex; DRST = delayed recognition span test; E2 = 17β-estradiol; EB = estradiol benzoate; EE2 = ethinyl estradiol; ERT = estrogen replacement therapy; F = follicular phase; GnRHa = gonadotropin releasing hormone agonist; HPC = hippocampus; HRT = hormone replacement therapy; L = luteal; mo = month; O = Ovulatory; P = progesterone; PRh = perirhinal cortex; OVX = ovariectomized.
2.1. Attention
2.1.1. Women.
Studies in either naturally or surgically postmenopausal women report inconsistent effects of HRT on attention, with some observing no effect (Alhola et al., 2006; Gleason et al., 2015; Keenan et al., 2001; Polo-Kantola et al., 1998b; Wolf et al., 2005), while others report improved performance on at least one measure of attention in HRT users (Castonguay et al., 2015; Smith, Giordani, Lajiness-O’Neill, & Zubieta, 2001; Schmidt et al., 1996; Fedor-Feyberg, 1977). In premenopausal women, divided attention appears unaffected by menstrual phase. However, sustained attention is reduced during the luteal relative to the follicular phase, and is negatively correlated with P levels (Pletzer et al., 2014).
2.1.2. Female animals.
Inconsistent effects of hormones are also seen in animal studies. Continuous E2 replacement was found to enhance selective attention as measured by the 5-choice serial reaction time test (5-CSRTT), which requires animals to identify which of 5 locations on a screen was briefly illuminated. E2 was beneficial in young OVX rats (Bohacek and Daniel, 2010) and in older rats when given immediately, but not 5 months following OVX (Bohacek & Daniel, 2010). Another method for indexing selective attention in rats (Escobar et al., 2002), is latent inhibition (LI), which occurs when pre-exposure to a conditioned stimulus (CS) impairs subsequent learning of the CS- unconditioned stimulus (US). Latent inhibition is impaired when conditioning occurs during proestrous (Quinlan et al., 2010) or when OVX rats are treated with continuous (17–37 pg/ml) or cyclic (implant: 28 pg/ml, serum, and acute E2:10 mg/kg, s.c. every 4 days) E2 replacement (Almey et al., 2013). While these results appear paradoxical, it is possible that the latent inhibition impairment is due to enhanced Pavlovian fear conditioning rather than impaired attention to the CS during pre-exposure.
A few studies have examined the effects of ovarian hormones on attention in NHPs. While one study in young OVX monkeys found no effect of EB replacement on a Cued Search task, where they had to select a target stimulus among distractors in the presence of valid or invalid cues (Lacreuse et al., 2009), other studies using a modified Posner task, that measures the ability to shift visual spatial attention, provided consistent evidence for positive effects of HRT, in young (Voytko, 2002), middle-aged (Voytko et al., 2009) or older OVX macaques (Kohama et al., 2016). Taken together, the results from human and animal studies indicate that the effects of ovarian hormones may depend on the type of attentional process being investigated.
2.2. Executive function
Executive function encompasses a wide range of processes which require the maintenance and manipulation of working memory (WM) and which can be conceptualized as updating, task switching, response inhibition, and decision making (Chudasama & Robbins, 2006). Given the multifaceted aspect of executive functioning, this section reviews each ability separately.
2.2.1. Updating (non-spatial) working memory
2.2.1.1. Women.
Evidence from postmenopausal women taking HRT suggests that ovarian hormones modulate non-spatial WM, so long as the task involves manipulating or updating information in WM. Estrogen replacement therapy (ERT) has no effect on simple WM tasks, like the Digit Span-forward test in postmenopausal women (Alhola et al., 2006; Castonguay et al., 2015; Duff and Hampson, 2000; Gleason et al., 2015; Keenan et al., 2001; Polo-Kantola et al., 1998b; Schmidt et al., 1996), and inconsistent findings are reported for the backward subtest, with some reporting benefits (e.g. Duff and Hampson, 2000), and others finding no effect (Castonguay et al., 2015; Gleason et al., 2015). Beneficial effects of ERT are observed on more challenging non-spatial WM tasks, including the N-back test & Digit Ordering tests (Castonguay et al., 2015; Duff and Hampson, 2000; Keenan et al., 2001; Krug et al., 2006). If HRT begins later in life, the benefits are not always observed. For example, on the self-ordered pointing test, one study reported no benefit of HRT in older postmenopausal women (Janowsky, Chavez, & Orwoll, 2000), while a second study reported superior performance following 2 months of ERT (Baker et al., 2012). Verbal N-back performance is unaffected by menstrual phase, although there is evidence for differences in patterns of activity during the task, with reduced left hemispheric activity during the late follicular phase (Joseph, Swearingen, Corbly, Curry, & Kelly, 2012).
2.2.1.2. Female animals.
Complex WM tasks used with animals typically involve functioning of both the HPC and PFC and so any impact of hormones can reflect effects on either region. The delayed recognition span test (DRST, Moss, Killiany, Lai, Rosene, & Herndon, 1997) requires monkeys to track a new stimulus in an increasing array of serially presented stimuli and can be administered in a spatial (identical stimuli, see section 2.3.1) or non-spatial form (color, object or face stimuli). In two studies using young OVX rhesus monkeys, E2 replacement was ineffective in modulating object-DRST performance (Lacreuse & Herndon, 2003; Lacreuse et al., 2009), but impaired performance on the face version (Lacreuse & Herndon, 2003), consistent with an effect of E2 on the processing of socioemotional information (e.g., Derntl et al., 2008).
In young OVX rodents, enhanced performance on non-spatial versions of the delayed spatial alternation task has been observed following continuous low E2 replacement (Wide, Hanratty, Ting, & Galea, 2004). However, impaired performance is seen following higher doses in young, middle-aged and older OVX rats (Wang, et al., 2008, 2009; Wide et al., 2004).
These studies indicate some evidence for beneficial effects of E2 on challenging non-spatial WM tasks in postmenopausal women and female rodents, but the effects depend on the E2 dose (rats), and age of subjects (women).
2.2.2. Task switching.
2.2.2.1. Women.
Task switching involves shifting attention from an initially important feature to another that subsequently conveys information about where to respond correctly to obtain a reward (Butts et al., 2013). There is accumulating evidence that ovarian hormones improve performance and/or modulate neural activation associated with the Wisconsin Card Sorting Test (WCST). A seminal study used PET to examine brain activation patterns (regional cerebral blood flow) associated with WCST performance in premenopausal women treated with a GnRHa and add-back of E or P. While no change in performance was observed, the normal dorsolateral PFC (DLPFC) activation was suppressed with GnRHa treatment and restored following E or P administration (Berman et al., 1997). Similarly, a recent study using fMRI demonstrated increased activity of the DLPFC during task switching in early postmenopausal women taking cyclic HRT. In addition, higher activity was associated with better performance (Girard et al., 2017). Another study in postmenopausal women (~60 years old), found that ERT was associated with an increased number of categories completed on the WCST (Schmidt et al., 1996) and HRT use improved performance on the number-letter task (Castonguay et al., 2015). Yet, at least one study in an elderly sample reported no benefit of 3 weeks ERT on set shifting (e.g. Duka et al., 2000).
2.2.2.2. Female animals.
E2 has beneficial effects on WCST-like performance in aged female macaques when treatment occurs soon after OVX. E2 with or without P given immediately following OVX improves performance in macaques (~20 years old; Voytko et al., 2009), but no benefit is observed 9 years after OVX (Lacreuse, Chhabra, Hall, & Herndon, 2004). Benefits are also seen on set shifting in female rats (Lipatova et al., 2016). However, E2 does not improve all aspects of task switching, as it has been shown to impair reversal learning in middle-aged marmosets (Lacreuse et al., 2014), and aged OVX rats (Gibbs et al., 2011). These studies demonstrate task-specific benefits of P or E2 on WCST and set-shifting in women and in animals, so long as treatment occurs soon after hormone loss. Overall, studies in women indicate positive effects of P or E2 on task switching, but animal studies have been sparse and inconsistent.
2.2.3. Response inhibition.
2.2.3.1. Women.
This ability can be measured by tasks that require inhibiting a prepotent response, such as the Stroop test, the Go/No-Go task or the Stop-Signal task. Improved performance on the Stroop test was observed in postmenopausal women given acute ERT (Krug et al., 2006). However, other studies do not report an effect (Alhola et al., 2006; Baker et al., 2012; Castonguay et al., 2015; Duka et al., 2000; Polo-Kantola et al., 1998b; Wolf et al., 2005). In the Go/No-Go task, subjects must produce a response for one stimulus type and inhibit responding for other stimuli, whereas in the Stop-Signal task they must inhibit responding following the appearance of a Stop signal after the response is already initiated. Impaired response inhibition was observed during the follicular phase in premenopausal women on both the Stop-Signal and Go/No-Go tasks (Colzato et al., 2010; Reimers et al., 2014), with higher E2 levels predicting poorer response inhibition (Colzato et al., 2010).
2.2.3.2. Female animals.
The effect of E2 replacement in female rodents suggests that age modulates the impact of E2 on response inhibition. Using the differential reinforcement of low rates of responding (DRL) task, which requires withholding a response for a specified duration, Wang and colleagues (2008, 2011) reported deficits in young and middle-aged OVX rats taking E2 replacement. E2 had no effect in older rats (Wang et al., 2011). One study in baboons reported a female advantage but no effect of menstrual phase on the Stop-Signal task (Lacreuse, Gullstrand, & Fagot, 2016). Overall, there is little evidence for a benefit of HRT on response inhibition in menopausal women or older female rats. High E2 appears detrimental to response inhibition in both premenopausal women and young female rats.
2.2.4. Decision making.
There is also limited evidence that ovarian hormones modulate decision making in humans. The delayed discounting task, which assesses the ability to forego an immediate small reward for a larger delayed reward, is sensitive to the menstrual cycle: the tendency to prefer smaller immediate rewards drops from menses (low E2) to the follicular phase (high E2). The change in preference was negatively correlated with E2 levels, suggesting that higher E2 levels are associated with a decreased bias for immediate reward (Smith, Sierra, Oppler, & Boettiger, 2014). Delayed discounting performance in rats is also enhanced by higher E2 levels (Uban et al., 2012). These benefits of high E2 levels on decision making appear task specific, as no effect is seen in young or postmenopausal women on the Iowa Gambling task, in which participants must select the advantageous deck in a set of 4 decks with different pay-offs (Reavis & Overman, 2001). Additional studies are needed to draw firm conclusions about the effects of E2 on decision making.
2.3. Spatial memory
2.3.1. Spatial working memory.
2.3.1.1. Women.
There is convincing evidence that ovarian hormones are important for spatial memory, including spatial WM in many species. Although spatial WM involves both PFC and HPC function, the effects are often attributed to local effects of E2 on HPC function (Frick et al., 2015; Hamson et al., 2016). Elevated E2 levels are associated with better performance on a spatial WM task in premenopausal women (Hampson and Morley, 2013). HRT use is associated with improved spatial WM performance in one study of postmenopausal women (age: 45–65 years old; Duff & Hampson, 2000), but not in another study of older hysterectomized women (mean age 71; Schiff, Bulpitt, Wesnes, & Rajkumar, 2005).
2.3.1.2. Female animals.
Data from NHPs generally demonstrate beneficial effects of ovarian hormones on spatial WM, but point to a clear effect of age. Although young female macaques show decrements in spatial-DRST performance during the periovulatory period of the cycle (high E2; Lacreuse, Verreault, & Herndon, 2001), E2 replacement has no effect in young OVX monkeys (Lacreuse & Herndon, 2003; Lacreuse et al., 2009). Delayed Response (DR) performance is also unaffected by OVX (up to 24 month post-surgery; Voytko, 2000), and E2 replacement in young monkeys (Voytko, 2000; Hao et al., 2007). Opposing effects are observed in older macaques. One of the first observations that endocrine status modulated cognitive performance in older macaques was that peri/postmenopausal females were impaired on the DR relative to age-matched premenopausal females (Roberts et al, 1997). Performance also correlated with estrogen metabolite levels, providing more direct support for the role of estrogens in this memory task. Later studies confirmed that HRT benefits DR (Rapp et al., 2003; Kohama et al, 2016), and spatial-DRST performance in older OVX macaques (Lacreuse et al., 2002). Conflicting results are reported in some studies, however, with either no effect of HRT in middle-aged macaques (Voytko, Higgs, & Murray, 2008), impairments following E2 replacement in OVX marmosets (Lacreuse et al., 2014) or enhanced performance in long-term OVX compared to age-matched intact females (~12 years; Lacreuse, Herndon, & Moss, 2000).
There is strong evidence for the beneficial effects of E2 on spatial WM in rodents, and unlike NHPs, this effect is independent of age. Enhanced spatial WM in young rats is observed during proestrous on the win-stay version of the radial-arm maze (RAM) task (Pompili et al., 2010). Spatial WM of young rats is also improved following E2 replacement in a number of studies using the delayed matching-to-position (DMP) task (Gibbs, 2007; Sandstrom & Williams, 2001; Velázquez-Zamora et al., 2012), the win-stay version of the RAM at a low dose (Holmes et al., 2002) or the win-shift version of the RAM following retention delays between 3–5 hr (Luine et al., 1998; but see Galea et al., 2001). In addition, Intra-cranial infusions of E2 into the medial PFC or dorsal HPC enhances win-shift performance in OVX rats (Sinopoli et al., 2006). In middle- and older-aged rats (12 & 17-month old), continuous E2 replacement given immediately, but not 5 months post OVX is associated with better performance on the win-shift version of RAM test with a 2.5 hr retention delay (Daniel et al., 2006). Other studies using cyclic administration of E2 alone, or combined with P report comparable results on the delayed spatial alternation, and D(N)MP tasks (Gibbs, 2000; Markowska & Savonenko, 2002). Thus, there is strong evidence that E2 enhances spatial WM in both humans, rodents, and older NHPs.
2.3.2. Spatial reference memory.
Spatial reference memory in rodents has most often been assessed with the fixed platform condition of the watermaze, where they have to remember the location of the hidden platform across trials. Fixed platform watermaze performance is not improved by endogenous fluctuations of ovarian hormones (Berry & McMahan, 1997; Pompili et al., 2010; Warren & Juraska, 1997), yet systemic and intra-HPC infusions of E2 following OVX enhances both the acquisition and consolidation of the task (McLaughlin, 2008; Packard & Teather, 1997a,b), so long as the treatment is given either before or immediately after acquisition. Indeed, E2 replacement reduces swimming distance in young and middle-aged rats and E2 levels correlate with performance, but E2 has no effect in old OVX rats (Talboom et al., 2008). Fixed platform acquisition is also unaffected by long-term OVX in older rats (Markowska & Savonenko, 2002).
E2 replacement also protects performance on the novel-object-in-place preference test (NOIP, aka object placement test, as reviewed by Tuscher, Fortress, Kim, & Frick, 2014), which tests rat’s ability to detect a novel object and improves place learning in both young, middle-aged, and old rats (as reviewed by Korol & Pisani, 2015) by acting locally in the HPC (Zurkovsky et al., 2007). While a bias to use a spatial strategy when solving a maze is associated with proestrous in rats (Korol et al., 2004), spatial bias was associated with the late luteal phase, when P levels are high in a recent study in premenopausal women (Hussain et al., 2016).
Elevated physiological E2 levels have no effect on reference memory errors on the RAM (Fader, Johnson, & Dohanich, 1999; Holmes et al., 2002; Luine et al., 1998; Pompili et al., 2010), and long-term spatial memory (6 & 24 hr; McLaughlin et al., 2008), and but supraphysiological levels of E2 (10 μg/day) appear to increase the number of reference memory errors on the RAM (Galea et al., 2001).
2.4. Verbal memory in women
Verbal memory is also dependent on HPC function (Sherwin and Grigorova, 2011). There is a reliable female advantage in this ability (Maki, 2015), and modest effects of E2 are seen (for a review, see Maki, 2015; Sherwin, 2011). Studies examining verbal memory across the menstrual cycle provide inconsistent results (for a review, see Poromaa & Gingnell, 2014). Conflicting findings are also observed in premenopausal women who have experienced ovarian failure. For example, GnRHa given to premenopausal women resulted in verbal memory deficits in one study (Craig et al., 2008), but had no effect in others (Guerrieri et al., 2016; Owens et al., 2002). However, perimenopausal women demonstrate impaired verbal memory (Greendale et al., 2009; Weber et al., 2013), as do women that experienced surgical menopause (Sherwin, 1988). E2 replacement given to naturally (Baker et al., 2012; Castonguay et al., 2015; Jacobs et al., 1998; Kampen & Sherwin, 1994; Keenan et al., 2001; Maki, Zonderman, & Resnick, 2001; Resnick et al., 1998) and surgically menopausal women (Phillips & Sherwin, 1992b) improves verbal memory performance in some, but not all studies (e.g. Baker et al., 2012; Gleason et al., 2015; Henderson et al., 2016). Therefore, while verbal memory is affected during the menopausal transition, HRT does not have consistent effects on this ability.
2.6. Visual recognition memory
Visual recognition memory (RM) tasks assess the ability to recognize a familiar from a novel object. There is some support for a modulatory role of E2 in visual recognition memory (RM). Premenopausal women with acute ovarian suppression (via GnRHa) are impaired on the delayed matching-to-sample (DMS) task (Craig et al., 2010). In a sample of postmenopausal women, Resnick and colleagues (1998) examined the effect of ERT on regional cerebral blood flow during a visual RM test. ERT users had better RM and greater blood flow in the right parahippocampal gyrus (including the EC, PRh, and parahippocampal cortex) and inferior parietal regions, left visual association, and anterior thalamic regions. However, not all studies report benefits of HRT use on visual RM (e.g. Alhola et al., 2006; Sundermann et al., 2006). In one study, enhancements in olfactory, but not visual, RM were observed in women with AD taking ERT (Sundermann et al., 2006).
In NHPs, limited support exists for a role of E2 in RM. In younger NHPs, DMS task performance is not influenced by endogenous ovarian hormones following brief retention delays (Kromrey, Czoty, & Nader, 2015; Lacreuse et al., 2001), although a small decrement in performance is observed during the periovulatory stage following a 30-s delay (Lacreuse et al., 2001). E2 replacement in young OVX macaques also has no effect on D(N)MS task performance (Lacreuse & Herndon, 2003; Lacreuse et al., 2009), and only small beneficial effects are found in older females (Lacreuse et al., 2002; Rapp et al., 2003; Voytko et al., 2008).
A larger body of evidence supporting a role of E2 in RM comes from rodent studies. Most studies use the novel-object preference test (NOP test; also referred to as the Novel-Object Recognition task, the Object Recognition Memory task, and the Spontaneous Object Recognition task). Visual RM is indexed by the animals’ exploration time. When rodents spend more time investigating novel than a previously-presented object, it is assumed that they recognize the familiar object. Several studies demonstrate that increased exploration of novel objects (i.e. novelty preference) following retention delays ranging from 5-min to 3 days is associated with elevated levels of E2, and that OVX eliminates this novelty preference (as reviewed by Galea et al., 2016; Luine, 2015, Tuscher et al., 2014). This beneficial effect of E2 on novelty preference is robust, and is observed in naturally cycling animals, following acute, cyclic, and continuous E2 replacement (for a review, see Galea, Frick, Hampson, Sohrabji, & Choleris, 2016; Luine, 2015; Tuscher et al., 2014), and following intra-HPC (Boulware, Heisler, & Frick, 2013; Fernandez et al., 2008; Frick et al., 2015; Lewis, Kerr, Orr, & Frick, 2008; Phan et al., 2012; Tuscher et al., 2016) and intra-PRh (Gervais et al., 2013, Gervais, Hamel et al., 2016) infusions of E2. However, novelty preference and DNMS tasks may measure different aspects of RM. In the same group of rats that demonstrated enhanced novelty preference following cyclic and intra-PRh infusion of E2 (Gervais et al., 2013, Gervais, Hamel et al., 2016), reduced accuracy on the DNMS task was observed. The vehicle-treated animals in these studies failed to demonstrate novelty preference, yet performed better on the DNMS task, indicating that rats can have intact RM and still fail to demonstrate a preference for the novel object. Thus, findings from NOP tasks should be interpreted with caution. Taken together, these studies indicate that the beneficial effects of E2 on visual RM are small or inexistent in primates, but consistently positive in rodents, unless when acting locally in the PRh.
2.6. Summary
Spatial memory and strategy are shown to be sensitive to E2 across species. However, these effects depend on task parameters (Korol & Pisani, 2015), dose (Galea et al., 2016; Hamson et al., 2016; Sherwin, 2011), age (Galea et al., 2016; Hao et al., 2007, Lacreuse, 2006), and the timing of treatment initiation (Galea et al., 2016; Hamson et al., 2016; Maki, 2013; Sherwin & Henry, 2008. Studies in rats demonstrate that this enhancement is likely due to local effects of E2 on the dorsal HPC. However, the PFC also contributes to spatial WM, and so the beneficial effects of E2 can result in local effects in either region. In addition to the strong evidence for the benefits of E2 on spatial WM, reference memory and spatial strategy, studies also show important morphological and physiological effects on the HPC that can promote these abilities, including increased volume (Lord et al., 2008), synaptogenesis, neurogenesis, protection after brain injury, and increased excitatory neurotransmission (as reviewed by Galea et al., 2016; Frick et al., 2015; McEwen & Milner, 2017).
There is also evidence that ovarian hormones modulate certain abilities dependent on the PFC, including complex nonspatial WM, and task switching (but not reversal learning). However, age, timing and type of hormone regimen, and task demands influence whether benefits are observed. While there are inconsistent findings regarding the impact on attention, it may be due to task parameters. Ovarian hormones do not appear to influence simple WM tasks, and only a limited number of studies have examined decision making. It is currently unclear why these hormones might impact some of these abilities and not others, so additional studies, particularly in animals, are needed to clarify these differences. Emerging evidence shows the beneficial effects of E2 on the mPFC of rodents (as reviewed by Frick et al., 2015; Frankfurt & Luine, 2015), and PFC in NHPs (Bailey et al., 2011; Hara et al., 2015; Hao et al., 2006, 2007; Wang et al., 2013), consistent with the idea that E2 may influence some of these ability through direct effects on the PFC. E2 is also an important modulator of neurotransmitters such as dopamine, acetylcholine, serotonin, glutamate and GABA, all of which can influence PFC-dependent cognition. For instance, E2 has enhancing or impairing effects on non-spatial WM depending on dopamine levels (Jacobs and D’Esposito, 2011), and dopaminergic drugs can influence the effect of E2 on LI (Almey et al., 2013). Clearly, more attention is needed to understand the discrepant results reported in the literature.
A small number of studies show that the PRh is also impacted by E2 (Fonseca et al., 2013; Gervais et al., 2015). This structure, which is important for visual RM (Brown and Aggleton, 2001), is emerging as an important early marker for AD, as neurofibrillary tangles develop in the PRh before moving onto the HPC (Braak & Braak, 1991), and atrophy has been shown to occur in preclinical AD (Wolk et al., 2017). Thus, additional focus is needed on extrahippocampal structures and the abilities that depend on them, particularly in the context of understanding E2’s role in cognitive aging.
Next, we present evidence that the cognitive abilities that are modulated by ovarian hormones are also impacted by sleep. Unfortunately, most of these studies were conducted in either mixed-sex samples where biological sex was not factored in, or in the case of animal studies, male-only samples. Therefore, it is currently unknown whether the impact of sleep on cognitive function might be sex-specific. A summary of findings is presented in Table 2.
Table 2.
Summary of the effect of sleep loss on cognition
| Ability | Sample Characteristics | Younger | Older | References |
|---|---|---|---|---|
| Attention | ||||
| Humans | ||||
| Meta-analysis | Deficits seen after 24–48 hr SD | As reviewed by Lim & Dinges, 2010 | ||
| Mixed sex (∼20 years) | 1 night PSR < no restriction | Rossa et al., 2014 | ||
| Young (∼22 years) & old (∼66 years) men | Young more impaired than older after 40 hr SD | Adam et al., 2006 | ||
| Young (∼21 years) & old (∼66 years) mixed-sex | Young more impaired than older after 24 hr SD | Duffy et al., 2009 | ||
| Young (∼23 years) & older (∼66 years) men | Young more impaired than older after 40 hr SD | Sagaspe et al., 2012 | ||
| Animals | ||||
| Age & sex not indicated | 24 hr SD < BL | Christie et al., 2008 | ||
| Male rats (∼2-mo old) | 28 hr PSR < BL 58–148 hr PSR = BL |
Deurveilher et al., 2015 | ||
| Male rats | 4–10 hr SD < no SD | Córdova et al., 2006 | ||
| Non-spatial working memory | ||||
| Humans | ||||
| Meta-analysis | Deficits seen after 24–48 hr SD | As reviewed by Lim & Dinges, 2010 | ||
| Mixed-sex (22–38 years) | 51–54 hr SD = BL | Tucker et al., 2010, 2011 | ||
| Task switching | ||||
| Humans | ||||
| Patients with sleep-related breathing disorders < controls | As reviewed by Fulda & Schulz, 2003 | |||
| Mixed sex (∼21 years) | 34–36 hr SD = controls | Binks et al., 1999 | ||
| Mixed sex (18–23 years) | 5 days PSR < BL | Herscovitch et al., 1980 | ||
| Male (19–32 years) | 1 night SD increases shift cost | Heuer et al., 2004 | ||
| Mixed sex (∼22 years) | 1 night SD impairs preparatory bias | Jennings et al., 2003 | ||
| Animals | ||||
| Young male rats | 3/24 hr fragmented sleep < controls | McCoy et al., 2007 | ||
| Response inhibition | ||||
| Humans | ||||
| Mixed sex (∼24 years) | 23/32/55 hr SD < BL | Drummond et al., 2006 | ||
| Mixed sex (∼37 years) | 4 nights of PSR < control | Demos et al., 2016 | ||
| Young (∼23 years) & older (∼66 years) men | 40 hr SD < BL for both groups equally | Sagaspe et al., 2012 | ||
| Mixed sex (∼20 years) | 1 night PSR = no restriction | Rossa et al., 2014 | ||
| Animals | ||||
| Male rats | 7 days of PSR < BL | Kamphuis et al., 2016 | ||
| 3-mo old male rats | SD < sleep | Borquez et al., 2014 | ||
| Decision making | ||||
| Humans | ||||
| Mixed sex (∼25 years) | 49/75 hr SD < BL | Killgore et al., 2006, 2007 | ||
| Mixed sex (∼21 years) | 1 night SD < Rested | Fraser et al., 2013 | ||
| Mixed sex (∼25 years) | 51 hr SD = BL 75 hr SD < BL |
Killgore et al., 2011 | ||
| Male (18–28 years) | 36 hr SD < RW | Lei et al., 2016 | ||
| Mixed sex (∼20 years) | 1 night PSR < no restriction | Rossa et al., 2014 | ||
| Mixed sex (∼37 years) | 4 nights of PSR = control | Demos et al., 2016 | ||
| Review | Some complex tasks: SD = control | As reviewed by Harrison & Horne, 1980 | ||
| Review | Majority of studies: SD < control 5 studies: SD = control |
As reviewed by Womack et al., 2013 | ||
| Mixed sex (18–30 years) | ED: 1 night SD < RM DD: 1 night SD < RM |
Libedinsky et al. 2013, | ||
| Spatial memory | ||||
| Humans | ||||
| Male (18–30 years) | HPC activity during SWS resembled patterns during encoding of learning task | Peigneux et al., 2004 | ||
| Mixed sex (20–30 years) | SWS and not REM involved in spatial memory consolidation | Rasch et al., 2007 | ||
| Animals | ||||
| 9-mo old male rats | HPC activity during SWS resembled patterns during encoding of novel environment | Wilson & McNaughton, 1994 | ||
| Female macaques (19–25 years) | 4 hr PSD | Performance negatively related to sleep latency/wake bouts | Haley et al., 2009 | |
| Male mouse lemurs (2–3 years) | Spatial learning: 8 hr SD = control Spatial retrieval: 8 hr SD < control |
Rahman et al., 2013 | ||
| Male rats (6–7 mo old) | Reference memory: 4 hr PSD 0–4 hr post-learning < control Working memory: 4 hr PSD 0–4 hr post-learning = control |
Smith et al., 1998 | ||
| Male rats | Reference memory: 24 hr sleep fragmentation < controls Working memory: 24 hr sleep fragmentation = controls |
Ward et al., 2009 | ||
| Male rats | Working memory: 5–7 days of intermittent hypoxia < controls | Row et al., 2007 | ||
| Male rats (1–1.5 mo) | 4 hr SD immediately post-learning < control 4 hr SD 4–8 hr post-learning = controls |
Ishikawa et al., 2014 | ||
| Male rats | Sleep following encoding enhances spatial memory | Inostroza et al., 2013 | ||
| Male mice | 6 hr SD immediately post-learning = control 6 hr SD 4–8 hr post-learning = controls |
Palchykova et al., 2006 | ||
| Verbal memory | ||||
| Humans | ||||
| 21–35 years, sex not indicated | Free recall: 35 hr SD < rested state Recognition: 35 hr SD = rested state |
Drummond et al., 2000 | ||
| Mixed sex (∼61 years) sample with obstructive sleep apnea | No association between objective sleep and word recall | Lutsey et al., 2016 | ||
| Mixed sex (∼23 years) | Sleep immediately after learning > wake | Sheth et al., 2012 | ||
| 18–22 years. Sex unspecified | Sleep immediately after learning > wake | Ellenbogen et al., 2009 | ||
| Female (∼53 years) | Sleep duration & HFs predict delayed LM, but not CVLT | Maki et al., 2008 | ||
| Mixed sex sample (20–30 years) | Recollection: SWS > REM Familiarity: SWS = REM |
Daurat et al., 2007 | ||
| Mixed sex sample (19–28 years) | Recollection: SWS > wake Familiarity: SWS/REM = Wake |
Drosopoulos et al., 2005 | ||
| University students. Age & sex unspecified | Sleep immediately post learning = Delayed sleep | Nesca & Koulak, 1994 | ||
| Visual recognition memory | ||||
| Humans | ||||
| Mixed sex (∼22 years) | 1 night SD < control | Mograss et al., 2009 | ||
| Mixed sex (18–36 years) | 1 night SD < control | Acheson et al., 2007 | ||
| Animals | ||||
| Male rats (1–1.5 mo) | 4 hr SD immediately post-learning = control 4 hr SD 4–8 hr post-learning = controls |
Ishikawa et al., 2014 | ||
| Male mice | 6 hr SD immediately post-learning < control 6 hr SD 4–8 hr post-learning = controls |
Palchykova et al., 2006 | ||
Note: BL = baseline; CVLT = California verbal learning test; DD = delay discounting; DLPFC = dorsolateral prefrontal cortex; DRST = delayed recognition span test; ED = effort discounting; HF = hot flash; hr = hour; LM = Logical memory; mo = month; PSD = paradoxical sleep deprivation; PSR = partial sleep restriction; SD = sleep deprivation; REM = rapid-eye movement; RW = rested wakefulness; SOP = self-ordered pointing test; SWS = slow-wave sleep.
3. Impact of sleep on cognitive functioning
The relation between sleep and cognition has been extensively studied in humans, as described in several excellent reviews (Alhola & Polo-Kantola, 2007; Killgore, 2010; Walker, 2008; Walker, 2009). The impact of chronic sleep loss on neural, and glial function in the CNS, which is described elsewhere (Zhao et al., 2017), provides potential mechanisms through which sleep may impact cognition. Sleep disturbances produce deficits in a wide range of cognitive domains including attention, executive function, and memory. This section provides a brief review of the literature on the impact of sleep on these specific cognitive domains, as they have also been shown to be sensitive to ovarian hormones. The goal of this section is to link the evidence that sleep impacts the same abilities that are enhanced by ovarian hormones. This will provide a basis for the next section, which discusses the few studies that have addressed whether ovarian hormones may impact cognition in part via effects on sleep.
First, we review data demonstrating the impact of SD on abilities that depend on the PFC (including WM, attention, and executive function). Unless otherwise stated, SD procedures below will involve acute total SD, which is the most common experimental model of sleep loss (Alhola and Polo-Kantola, 2007). In the second section, we cover the importance of sleep in memory consolidation of abilities that dependent on structures of the MTL. Whenever possible, we characterize the associated neural substrates, emphasize aging effects and incorporate studies in animal models (reviewed in McCoy & Strecker, 2011). It is important to note that animal studies examining the relationships between sleep and cognition are still very sparse, and particularly lacking in NHPs. The mechanisms by which sleep affects cognition have been reviewed in detail elsewhere (Abel et al., 2013; Tononi and Cerelli, 2014) and will not be emphasized here. One influential theory, the synaptic homeostasis hypothesis, proposes that the fundamental function of sleep is the restoration of synaptic and cellular homeostasis, by which the costly synaptic strengthening that is required during wake is normalized during sleep (Tononi and Cirelli, 2014). Synaptic renormalization during sleep, largely through SWA, could explain many of the benefits of sleep on learning and memory.
3.1. Sleep and Attention
Attention regulation is a key function of sleep. It is well known than one effect of SD is the inability to pay attention effectively the following day. Multiple aspects of attention are affected, including vigilant (sustained) attention, reaction time, divided attention and selective attention, but the effects on sustained attention, which are the most robust and reliable, are thought to account for most of the deficits.
In the psychomotor vigilance test (PVT), a classic test to measure vigilant attention, SD results in an overall slowing of responses, increases of errors of commission and long lapses, as well as unpredictable behavior (as reviewed by Lim and Dinges, 2008). Even one night of partial sleep restriction (PSR) results in slower responses on the PVT (Rossa et al., 2014). Interestingly, male rats exposed to 24h of SD (Christie et al., 2008), or 28 hr of PSR (Deurveilher, Bush, Rusak, Eskes, & Semba, 2015) show strikingly similar results. However, attention appears to recover following longer PSR (52–148 hr; Deurveilher et al., 2015). The attentional performance of rats is also impaired on the 5-CSRTT, as shown by longer response latencies and increased number of errors and omissions after 4, 7, and 10 h of SD (Cόrdova et al., 2006).
Studies on divided attention, the ability to perform concurrent tasks, or on selective attention, the ability to focus cognitive on particular targets (locations, objects, or features) while ignoring irrelevant distracters, have produced more mixed results. Lim and Dinges’ (2010) meta-analysis of 70 studies revealed that short term (< 48h) SD causes deficits in a wide range of cognitive tasks, but that most deficits can be attributed to impairments in sustained attention. Not unexpectedly, neuroimaging studies focusing on brain activation after acute SD show decreased brain activation in the fronto-parietal attention network (PFC and intraparietal sulcus) and in the salience network (insula and medial frontal cortex), as well as increased thalamic activation (meta-analysis of 11 neuroimaging studies Ma, Dinges, Basner, & Rao, 2015). With regard to aging, consistent reports indicate that the effects of SD on attentional tasks are actually greater in young adults than older adults (Adam et al., 2006; Duffy et al., 2009; Sagaspe et al., 2012) perhaps due to the effective recruitment of compensatory mechanisms in the aged brain (Sagaspe et al., 2012).
3.2. Sleep and Executive Function
It remains unclear whether tasks that require higher level cognitive capacities, such as tasks of executive function are specifically affected by sleep disturbances or whether the deficits can be explained by deficits in attentional resources.
3.2.1. Non-spatial working memory (WM).
Both accuracy and response times are significantly affected by SD in several WM tasks (Lim and Dinges, 2010). However, there is evidence that the non-executive aspects of the tasks, such as simple reaction time, can account for most of the deficits (Tucker et al., 2010, 2011).
3.2.3. Task switching.
Some studies report profound deficits on tasks requiring mental flexibility and attention shifts, such as the WCST in patients with sleep disorders (as reviewed by Fulda and Schulz, 2003). While one night of SD does not affect WCST in a healthy population (Binks et al., 1999), 5 nights of PSR do (Herscovitch et al., 1980), suggesting that a minimum amount of sleep loss is necessary to detect effects on task switching. Other studies find that sleep-deprived individuals showed greater switch costs, indicating that sleep loss reduces the capacity to modify behavior rapidly and flexibly to changing demands (Heuer et al., 2004; Jennings et al., 2003). Task switching in rats, as measured by the attentional set shifting task, is also sensitive to sleep loss (i.e. 3 and 24 hr of sleep fragmentation; McCoy et al., 2007).
3.2.4. Response inhibition.
Drummond and colleagues (2006) reported impaired ability to withhold a prepotent response (No-Go stimuli) on a Go/No-Go task following 23, 32 and 55 hr of SD. Interestingly, only the 55 hr test session also impaired the ability to respond correctly to “Go” stimuli, suggesting that inhibitory control was specifically impaired despite relatively intact attention. In a mixed-sex sample, 4 nights of PSR (6 hr/night) negatively impacted the rate of false alarms on the Go/No-Go task (Demos et al., 2016). The impact of sleep loss on response inhibition has been shown in both young and older people (Sagaspe et al, 2012). A cumulative effect of several nights of PSR may be necessary to detect an effect, as one night of PSR did not alter performance on an emotional version of the Go/No-Go task (Rossa et al., 2014).
Response inhibition is also disrupted by sleep loss in rats. Kamphuis and colleagues (2016) showed that PSR (4 hr sleep/day) for 7 days resulted in decreased behavioral control and increased bursts of responses on the DRL task. The importance of sleep on response inhibition in rats is also observed using the Go/No-go task (Borquez et al., 2014).
3.2.5. Decision making.
The impact of SD on decision making is described in detail in a recent review. For example, Killgore and colleagues (2006) used the Iowa gambling task to compare patterns of decision making at baseline and following 49 hr of SD. Under normal conditions, subjects learned to avoid large payoff high-risk decks in favor of decks providing modest but more consistent payoffs. After two nights of sleep loss, however, these same subjects tended to prefer riskier selections, despite the long-term losses that resulted. This same pattern was replicated in a second study with SD extended to 75 hr (Killgore et al., 2007). Interestingly, administration of caffeine did not reverse the deficit, suggesting that the impairments were not simply due to deficits in alertness and sustained attention. Performance during a gambling task was also influenced by SD in a young mixed-sex sample (Fraser, Conduit, & Phillips, 2013), with participants failing to reduce their bets when risk increased and time to respond decreased. Other studies also show detrimental effects of sleep loss on decision making and risk taking (e.g. Killgore, Kamimori, & Balkin, 2011; Lei et al., 2016; Rossa et al., 2014), although some studies fail to find a difference (for a review, see Womack et al., 2013, Harrison & Horne, 2000). For example, 4 nights of PSR had no effect on performance on the Balloon Analogue Risk Task (Demos et al., 2016). Others argue for dissociable effects on different decision-making tasks (e.g. Womack et al., 2013), including Libedinsky and colleagues (2013), who observed impairments on effort discounting, but not delay discounting in the same subjects.
3.3. Sleep and Medial Temporal Lobe-dependent Memory
Sleep impacts all aspects of long-term memory (Mander et al., 2016), including acquisition, consolidation, and retrieval. In humans, SWS is important for the consolidation of declarative memories (Peigneux, et al., 2001; Rauchs, Desgranges, Foret, & Eustache, 2005; Smith, 2001), which involves the HPC and other structures of the MTL (Preston and Eichenbaum, 2013). The consolidation process allows for initially unstable memories to be integrated into a network of more stable memory representations.
3.3.1. Spatial Memory
Numerous studies indicate an important role of SWS in spatial memory consolidation. HPC neurons that are active while rodents explore a novel environment are called place cells (O’Keefe and Nadel, 1978). Wilson and McNaughton (1994) recorded the activity of place cells in rats during acquisition of a spatial task, and again during subsequent sleep. The same cells that fired together during encoding tended to fire together during subsequent SWS, and cells that did not fire during training tended to not fire during SWS. This pattern of activity is consistent with the idea that HPC activity during SWS may be involved in spatial memory consolidation. Using cerebral blood flow measurements, Peigneux and colleagues (2004) demonstrated similar results in humans, in that hippocampal areas that were activated during a virtual route learning task were re-activated during subsequent SWS. They also showed that this re-activation during SWS correlated positively with the degree of improvement on the retention test. A subsequent study provides further support for a role of HPC activity during SWS in spatial memory consolidation. Rasch and colleagues (2007) trained participants on a spatial (HPC-dependent) and a finger sequence tapping (HPC-independent) task during simultaneous presentation of an odor cue as a contextual stimulus. Participants then slept in a MRI scanner, and functional images and sleep parameters were taken. The contextual odor or a control odor were presented during SWS or REM. Significant HPC activation was revealed during SWS when the contextual odor was presented. There was no effect during REM, nor during presentation of the control odor. Re-presentation of the contextual odor during SWS led to enhanced performance on the spatial memory, but had no effect on the finger tapping task. Together, these studies support the role of SWS in spatial memory consolidation.
Very few studies have examined the relationships between sleep and spatial memory in NHPs, but they support a beneficial effect of sleep in spatial performance. For example, Haley and colleagues (2009) reported a negative association between sleep latency/wake bouts and performance on a spatial navigational task in older female macaques. Similar results were obtained by Rahman and colleagues (2013) in a study examining the impact of SD on spatial memory in mouse lemurs. They trained male mouse lemurs on a modified version of the Barnes maze, and tested them 24 hr later. Subjects received 8 hr of SD either before training, or immediately before testing. Only those that received SD immediately before testing demonstrated impaired performance. However, SD following learning may also disrupt spatial memory consolidation. Smith and colleagues (1998) selectively disrupted REM sleep in male rats following training on the win-stay version of the RAM. Rats received 4 hr of SD either immediately, 4 hr, or 8 hr after training. Only those that received SD immediately following training demonstrated impaired performance, although the deficits were restricted to reference memory errors. Spatial reference memory impairments are also observed following sleep loss in other studies (e.g. Ward et al., 2009), while spatial WM deficits can be seen following 5–7 days of intermittent hypoxia (Row et al., 2007). NOIP performance of male rodents is impacted by SD occurring soon after encoding in some (e.g. Inostroza et al., 2013; Ishikawa et al., 2014), but not all studies (i.e. Palchykova et al., 2006). Taken together, these studies demonstrate that sleep is important for spatial memory, with some evidence suggesting that reference memory may be more sensitive to sleep loss than working memory.
3.3.2. Verbal memory
Studies have addressed the impact of sleep on verbal memory. For example, Drummond and colleagues (2000) showed that 35 hr of SD impaired free recall, but not recognition relative to a rested state. While these results suggest that sleep loss negatively impacts recall, Lutsey and colleagues (2016) reported no association between objective sleep parameters (sleep fragmentation and sleep duration) on changes in performance on recall in individuals with obstructive sleep apnea. Positive effects of sleep was reported in two studies, where sleep immediately after verbal learning of word pairs protected memory from interference (Sheth and colleagues 2012; Ellenbogen et al., 2009). In addition, Maki and colleagues (2008) found that sleep duration and objective measures of HFs predicted performance on the delayed logical memory test, but not the California Verbal Learning Test, in midlife women. Another study demonstrated that SWS and not REM influenced word recognition judgements. A mixed-sex sample was presented with a word list prior to sleep dominated by either SWS, which occurs during early sleep or REM, which occurs during late stages of sleep. PSG recordings were measured and confirmed differences in SWS and REM during the early and late sleep phases. The authors demonstrated that SWS-dominated early sleep improved recollection-based judgements (recognition with episodic memory), but had no effect on familiarity-based judgements (recognition without episodic memory; Daurat et al., 2007). Similar effects were reported by Drosopoulos and colleagues (2005). One important limitation to the procedures used in these studies is that the time of day varied between the learning and test phases for the early and late sleep conditions. Nesca and Koulak (1994) demonstrated that sleep immediately following learning improved word recognition compared to delayed sleep. However, when the learning and testing phases were matched by time of day, no differences emerged, suggesting that the effect of sleep on performance may result from effects of circadian timing.
3.3.3. Visual recognition Memory
There are limited studies examining the impact of sleep on object RM. While 4 hr of SD is insufficient to impact object recognition memory in male mice (Ishikawa et al., 2014), 6 hr immediately following object exploration does prevent novelty preference (Palchykova et al., 2006). Studies in humans (mixed-sex) also provides support for the idea that sleep impacts visual RM (e.g. Acheson et al., 2007; Mograss et al., 2009). Additional studies, particularly in NHPs are needed to further investigate potential effects of sleep on visual RM.
3.4. Summary
SD has detrimental effects on abilities dependent on the PFC, including sustained attention, task switching, and response inhibition. Decision-making is impaired by short-term SD, but there is some suggestion that it can recover following chronic PSR. While deficits are also observed on non-spatial WM tasks, they likely result from reduced sustained attention.
Memory abilities dependent on structures in the MTL are influenced by sleep. SWS appears to be particularly important for spatial memory consolidation. Too few studies have been conducted on verbal memory and visual RM to draw any firm conclusions.
A few limitations from these studies are worth noting. In most cases, an acute total SD procedure was used, when chronic PSR and chronic sleep fragmentation are more representative of sleep problems experienced by individuals with sleep complaints/disorders. Chronic PSR is common, affecting approximately 30% of adults in the US (Olds et al., 2010). As such, it is crucial to correctly model these sleep patterns to strengthen our understanding of the impact sleep on these abilities. A second limitation is the paucity of animal studies, especially in NHPs. Such studies are needed to clarify the effects of sleep on specific cognitive abilities and identify the underlying mechanisms. A third limitation concerns the lack of attention paid to the biological sex of the participants. Among the studies reviewed above, most animal studies used exclusively males. In human studies, only a handful reported the biological sex of participants and even when they did, the effect of sex was not analyzed. As described by Hamson and colleagues (2016), there are sex differences in many cognitive abilities, with a male advantage emerging for many spatial abilities and a female advantage for many verbal abilities. These sex differences can obscure the effects of sleep on these abilities, making the omission of sex as a biological variable a major problem of these studies.
4. Ovarian hormones, sleep and cognition in females
Sleep disturbances may have a differential impact on cognitive function in men and women, due in part to the influence of sex hormones. However, very few studies to date have addressed this issue. The few reports available point to a sex difference in the impact of sleep on cognition. Genzel and colleagues (2012) provide evidence that sleep may enhance HPC-dependent memory in men, but not in women. They tested a young mixed-sex sample on a paired-associates test following a nap and compared performance to a group that did not take a nap. The women in the sample were in the menses phase of their cycle. Improved memory performance, and increased spindle activity during the nap was observed in men, but not women (Genzel et al., 2012). Studies using SD suggest that selective aspects of cognition are more sensitive to sleep disturbances in young men than women. In one study (Binks et al., 1999), estimated IQ (as measured by a short-form of Wechsler Adult Intelligence Scale-Revised) following 35 hr of SD was better in young women than men following SD, despite men scoring higher than women in the non-SD condition. However, sex differences were not observed for any other ability assessed, including sustained attention, word fluency, and cognitive flexibility. Another study reported poorer sustained attention (as indicated by slower response times) in young men than women following 38 hr of SD (Corsi-Cabrera et al., 2003). To our knowledge, the role of sex hormones in these sex differences has not been objectively investigated. However, the results are consistent with a protective role of ovarian hormones against SD induced-cognitive dysfunction in adult women.
Consequently, the loss of ovarian hormones following menopause could have a greater impact on sleep and thereby cognition in women than in men. Unfortunately, very few data are available to test the validity of this hypothesis. In a recent study in participants over 65, Chiu, and colleagues ( 2016) reported greater impairment in global functioning (as measured by the Mini Mental Status Exam) in women than men but the impact of sleep on cognition was not greatly affected by biological sex. Among men that had cognitive impairment, snoring, difficulty breathing during sleep, and prolonged sleep were related to scores on the Mini Mental Status Exam, whereas in women, only prolonged sleep was associated with cognitive scores. Additional studies are needed to determine whether the relationships between sleep and cognition differ between men and women and whether they change with age.
Findings from studies examining the effects of ovarian hormones on sleep and cognition in women have been inconsistent. Alhola and colleagues (2005) compared postmenopausal (58–72 years old) HRT users to non-users on attention, visual episodic memory, and visuomotor performance. Rebound sleep following SD enhanced performance on all abilities, however HRT use did not impact the effect of sleep on performance. Similarly, HRT use in postmenopausal women (59–72 years old) did not influence sustained attention following a longer 40 hr of SD (Karakorpi et al., 2006). In contrast, a study by Saletu (2003) reported that HRT (E valerate alone or in combination with a progestin) for two months improved subjective and objective sleep quality (trend) as well as sustained attention and processing speed in postmenopausal women. Cognitive processing capacity and perceptual processing resources were also improved by the combination therapy. The studies reporting negative results used small sample sizes and focused on a limited set of cognitive domains. While sleep is important for sustained attention, which was assessed in these studies, other abilities including response inhibition, task switching, decision making, and spatial memory are also sensitive to sleep. Further research is needed to address whether ovarian hormones regulate the impact of sleep on these abilities.
There is a paucity of animal studies focusing on the relations between ovarian hormones, sleep and cognition. A recent study in female rats suggests that ovarian hormones do regulate the effect of SD on MTL-dependent cognition, as assessed by performance on the fixed-platform watermaze task. Subjects included female rats that were OVX or gonadally intact. One set of subjects received 72 hr of SD, whereas the rest slept normally. SD impaired performance, but only in the OVX animals (Esmaeilpour et al., 2015). These results suggest that circulating levels of ovarian hormones may protect against the detrimental effects of SD on spatial memory consolidation. Despite these interesting results, no other study to our knowledge has addressed the impact of ovarian hormone loss on the association between sleep and memory in animals.
If ovarian hormones benefit sleep and protect the young female brain against the detrimental effects of sleep disturbances on cognition, the loss of ovarian hormones at menopause would be expected to have adverse consequences, first by increasing sleep disturbances and second by potentiating their impact on cognitive function. As reviewed in section 2, increased sleep disturbances are observed at menopause. In addition, accumulating evidence suggests that sleep dysfunction may accelerate age-related cognitive decline and potentially conversion to AD (Mander et al, 2016; Musiek & Holtzman, 2016).
Sleep dysfunction, characterized by reduced SWS quality, duration and increased sleep fragmentation, becomes more prevalent with advancing age (Ohayon et al, 2004). However, there are large individual differences in the type and severity of age-related sleep deterioration (Vitiello, 2009), which suggest the existence of underlying pathology such as AD in certain individuals (Mander et al, 2016). People with mild cognitive impairment or AD have much more severe sleep impairments than cognitively normal older adults (Hita-Yanez et al, 2012; Prinz et al, 1982, Westerberg et al, 2012). Importantly, sleep dysfunction may occur in the very early stages of AD, often before the appearance of any other symptoms (Mander et al, 2016; Lim et al, 2013) and the magnitude of sleep disruption correlates with disease severity. Cerebrospinal fluid (CSF) Tau and β-amyloid (Aβ) protein levels can predict the degree of decreased SWS, sleep efficiency and REM sleep in AD patients (Liguori et al, 2012). In addition, CSF orexin levels, which promote wakefulness, are associated with sleep deterioration, Tau levels, and cognitive decline in AD patients (Liguori et al, 2014). This and parallel evidence from murine models of AD (e.g., Kang et al, 2009, Roh et al, 2014) have led to the hypothesis that sleep dysfunction may have a causal role in the pathophysiology of AD (Mander, 2016; Musiek and Holtzman, 2016). Importantly, recent data indicate that SWS sleep increases the rate of Aβ clearance by twofold in the mouse brain, suggesting one mechanism by which sleep may benefit brain function (Xie et al, 2013).
Postmenopausal women are at greater risk for AD than men of the same age, and sex differences in longevity only partially explain this higher risk (Snyder et al., 2016). Further, surgical removal of the ovaries prior to natural menopause increases the risk of dementia (as reviewed by Au et al., 2016). Ovarian hormone loss during the menopausal transition has been hypothesized to contribute to the increased risk for AD (Janicki and Schupf, 2010). The data reviewed in sections 2, 3 and 4, suggest that sleep may be a key component linking estrogen loss to dementia risk. Whether the loss of ovarian hormones during menopause increases the risk of dementia in vulnerable women by specifically disrupting sleep certainly warrants experimental investigation. A proposed model illustrating potential pathways through which ovarian hormone loss may contribute to specific cognitive impairments and dementia is presented in Figure 1.
Figure 1.
Schematic model illustrating proposed effects of hormone deprivation resulting from menopause on sleep disruptions and selective cognitive deficits. Sleep disruptions may include reduced sleep quality/efficiency, or fragmented sleep, and in more severe cases, development of sleep disorders (i.e. insomnia or sleep disordered breathing). Since sleep is also known to modulate these cognitive abilities, it is proposed that loss of ovarian hormones can further exacerbate cognitive impairments via development of sleep disorders/fragmented sleep. There is some evidence that symptomatic women are more vulnerable. Extended periods of sleep disruptions could increase inflammation and synaptic damage leading to further cognitive deficits. Note: Aβ = β-amyloid; SDB = sleep-disordered breathing; WM = working memory.
Conclusion
Strong support exists for a modulatory role of ovarian hormones on sleep across species. Sleep problems in women emerge during puberty and fluctuate across the menstrual cycle. The menopausal transition is associated with decreased sleep quality and among those that also experience HFs or depressive symptoms, more PSG-awakenings. HRT improves sleep in some, but not all studies. More robust evidence comes from animal studies, where E2 modulates both spontaneous sleep, and recovery from SD.
Ovarian hormones also modulate certain cognitive abilities in adult females, and the direction of effects is influenced by several factors including age, duration of hormone loss, hormone regimen (acute, continuous, cyclic), and task demands (Galea et al., 2016; Hamson et al., 2016; Korol & Pisani, 2015). Generally, elevated levels of ovarian hormones are beneficial for sustained attention, manipulation of WM, task switching, and spatial, verbal, and recognition memory. Local effects of E2 in certain brain regions may also influence whether performance is enhanced or impaired.
Numerous studies demonstrate that sleep impacts cognition, including sustained attention, task switching, response inhibition, decision making, and memory. Most of these studies include male-only samples or mixed-sex samples where biological sex was not considered as a variable. The few studies that have examined sex differences suggest that sleep has a greater impact on cognition in young men than in young women. These as well as rodent data suggest that circulating ovarian hormones may protect the female brain from the detrimental effects of sleep disruptions.
Such a relationship would have important implications for age-related cognitive decline and dementia in postmenopausal women. Indeed, sleep disturbances increase during the menopausal transition. In vulnerable women, sleep disruptions may exacerbate cognitive decline, increase inflammation and synaptic damage and eventually set in motion a cascade of events leading to neurodegeneration (Mander et al, 2016; Musiek and Holzman, 2016). Although it is now well established that HRT does not improve cognitive symptoms in women with AD (Henderson, 2014), it is still unclear whether HRT given decades before the disease onset may provide cognitive benefits later in life (Henderson, 2014). Importantly, the onset of sleep dysfunction may precede AD symptomology and pathology by several years. Sleep may therefore provide a new target for therapeutic intervention. The development of appropriate animal models will be crucial to determine whether interventions that restore sleep patterns may protect the female brain from neurodegeneration later in life and whether HRT may provide therapeutic benefits.
Highlights.
Sleep is modulated by ovarian hormones in females across adult lifespan
Ovarian hormones also influence certain cognitive domains
Sleep loss impacts similar abilities, but biological sex often not considered
Animal models are crucial for understanding the benefits of sleep on cognition
List of abbreviations
- 5-CSRTT
5-choice serial reaction time test
- Aβ
β-amyloid
- AD
Alzheimer’s Disease
- DMP
delayed matching-to-position
- DNMP
delayed nonmatching-to-position
- DMS
delayed matching-to-sample
- DNMS
delayed nonmatching-to-sample
- DR
delayed response
- DRL
differential low rates of responding task
- DRST
delayed recognition span test
- E
estrogens
- E2
17β-estradiol
- EB
estradiol benzoate
- EC
entorhinal cortex
- EEG
electroencephalography
- ERT
estrogen replacement therapy
- FSH
Follicular stimulating hormone
- GnRHa
gonadotropin-releasing hormone agonist
- HFs
hot flashes
- HPC
hippocampus
- HRT
hormone replacement therapy
- MTL
medial temporal lobe
- N1-N3
stages 1–3 non-rapid eye movement
- NHP
non-human primate
- NOIP
novel object-in-place preference
- NOP
novel-object preference
- NREM
non-rapid eye movement
- OVX
ovariectomized
- P
progesterone
- PFC
prefrontal cortex
- PRh
perirhinal cortex
- PSG
polysomnogram
- PSR
Partial sleep restriction
- PVT
Psychomotor vigilance test
- RAM
radial-arm maze
- REM
rapid eye movement
- RM
recognition memory
- SD
sleep deprivation
- SWA
slow-wave activity
- SWS
slow-wave sleep
- WCST
Wisconsin card sorting test
- WM
working memory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Havekes R, Saletin JM, Walker MP, 2013. Sleep, Plasticity and Memory from Molecules to Whole-Brain Networks. Current Biology, 23(17), R774–R788. 10.1016/j.cub.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam M, Retey JV, Khatami R, Landolt HP, 2006. Age-related changes in the time course of vigilant attention during 40 hours without sleep in men. Sleep 29, 55–57. [DOI] [PubMed] [Google Scholar]
- Adan A, Natale V, 2002. Gender differences in morningness– eveningness preference. Chronobiol. Int. 19, 709–720. doi: 10.1081/CBI-120005390. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW 1999, Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav. Brain. Sci. 22(3), 425–444. [PubMed] [Google Scholar]
- Alhola P, Polo-Kantola P, 2007. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr. Dis. Treat. 3, 553–567. [PMC free article] [PubMed] [Google Scholar]
- Alhola P, Tallus M, Kylmala M, Portin R, Polo-Kantola P, 2005. Sleep deprivation, cognitive performance, and hormone therapy in postmenopausal women. Menopause-the J. North Am. Menopause Soc. 12, 149–155. doi: 10.1097/01.GME.0000142439.30302.9A. [DOI] [PubMed] [Google Scholar]
- Almey A, Hafez NM, Hantson A, Brake WG, 2013. Deficits in latent inhibition induced by estradiol replacement are ameliorated by haloperidol treatment. Front. Behav. Neurosci. 7, 136. doi: 10.3389/fnbeh.2013.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M, Sarvaiya N, Epperson NC, 2014. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocrinol. 35(3), 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au A, Feher A, McPhee L, Jessa A, Oh S, Einstein G, 2016. Estrogens, inflammation and cognition. Front. Neuroendocrinol. 40, 87–100. doi: 10.1016/j.yfrne.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Avis NE, Crawford S, Stellato R, Longcope C. Longitudinal study of hormone levels and depression among women transitioning through menopause. Climacteric 2001;4(3):243–249. [PubMed] [Google Scholar]
- Baddeley AD, Hitch GJ, 1974. Working memory In: Bower GA (ed.) The Psychology of Learning and Motivation: Advances in Research and Theory. pp. 47–89. New York: Academic Press. [Google Scholar]
- Baker FC, Mitchell D, Driver HS, 2001. Oral contraceptives alter sleep and raise body temperature in young women. Pflugers Arch. 442, 729–737. doi: 10.1007/s004240100582. [DOI] [PubMed] [Google Scholar]
- Baker FC, Waner JI, Vieira EF, Taylor SR, Driver HS, Mitchell D, 2001. Sleep and 24 hour body hormonal contraceptives. J. Physiol. 530, 565–574. doi: 10.1111/j.1469-7793.2001.0565k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FC, Driver HS, 2007. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 8, 613–622. .doi: 10.1016/j.sleep.2006.09.011). [DOI] [PubMed] [Google Scholar]
- Baker FC, Kahan TL, Trinder J, Colrain IM, 2007. Sleep quality and the sleep electroencephalogram in women with severe premenstrual syndrome. Sleep. 30, 1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker FC, Willoughby AR, Sassoon SA, Colrain IM, de Zambotti M, 2015. Insomnia in women approaching menopause: Beyond perception. Psychoneuroendocrinology 60, 96–104. doi.org/10.1016/j.psyneuen.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Voytko ML, 1996. Spatial orienting of attention in adult and aged rhesus monkeys. Behav. Neurosci. 110, 898–904. [DOI] [PubMed] [Google Scholar]
- Baker FC, Willoughby AR, Sassoon SA, Colrain IM, de Zambotti M, 2015. Insomnia in women approaching menopause: Beyond perception. Psychoneuroendocrinology 60, 96–104. doi: 10.1016/j.psyneuen.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR, 1997. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A 94, 8836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry B, McMahan R, 1997. Spatial learning and memory at defined points of the estrous cycle: Effects on performance of a.. Behav. Neurosci. 111, 267. doi: 10.1037//0735-7044.111.2.267. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm ACE, 2003. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp. Neurol. 181, 301–312. doi: 10.1016/S0014-4886(03)00061-X. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm A-CE, 2004. Ovarian hormones and cognition in the aged female rat: II. progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav. Neurosci. 118, 707–714. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- Binks PG, Waters WF, Hurry M, 1999. Short-term total sleep deprivations does not selectively impair higher cortical functioning. Sleep 22, 328–334. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Papaliaga MN, Vgontzas AN, Lin HM, Pejovic S, Karataraki M, Vela-Bueno A, Chrousos GP, 2009. Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: Effects of age and menopause. J. Sleep Res. 18, 221–228. doi: 10.1111/j.1365-2869.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T, Yaffe K, Ancoli-Israel S, Schneider JL, Cauley JA, Hillier TA, Fink HA, Stone KL, 2006. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J. Gerontol. A. Biol. Sci. Med. Sci. 61, 405–410. doi:61/4/405 [pii]. [DOI] [PubMed] [Google Scholar]
- Borbély AA. A two process model of sleep regulation. Hum Neurobiol 1982;1:195–204. [PubMed] [Google Scholar]
- Bohacek J, Daniel JM, 2010. The beneficial effects of estradiol on attentional processes are dependent on timing of treatment initiation following ovariectomy in middle-aged rats. Psychoneuroendocrinology 35, 694–705. doi: 10.1016/j.psyneuen.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Borquez M, Born J, Navarro V, Betancourt R, Inostroza M, 2014. Sleep enhances inhibitory behavioral control in discrimination learning in rats. Exp. Brain Res. 232, 1469–1477. doi: 10.1007/s00221-013-3797-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM, 2013. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J. Neurosci. 33, 15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP, 2001. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2, 51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Burdick RS, Hoffmann R, Armitage R, 2002. Short note: oral contraceptives and sleep in depressed and healthy women. Sleep 25, 347–349. [PubMed] [Google Scholar]
- Butts KA, Floresco SB, Phillips AG, 2013. Acute stress impairs set-shifting but not reversal learning. Behav. Brain Res. 252, 222–229. doi: 10.1016/j.bbr.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SBS, Santhi N, Schoen MW, Czeisler CA, Duffy JF, 2010. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J. Biol. Rhythms 25, 288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SS, Tobler I, 1984. Animal sleep: A review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 8, 269–300. [DOI] [PubMed] [Google Scholar]
- Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH, 2001. The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20–60 years old). Psychophysiology 38, 232–242. doi: 10.1111/1469-8986.3820232. [DOI] [PubMed] [Google Scholar]
- Cassaday HJ, Nelson AJD, Pezze MA, 2014. From attention to memory along the dorsal-ventral axis of the medial prefrontal cortex: some methodological considerations. Front. Syst. Neurosci. 8, 160. doi: 10.3389/fnsys.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellini N, de Zambotti M, Covassin N, Sarlo M, Stegagno L, 2014. Working memory impairment and cardiovascular hyperarousal in young primary insomniacs. Psychophysiology 51, 206–214. doi: 10.1111/psyp.12167. [DOI] [PubMed] [Google Scholar]
- Chiu HY, Lai FC, Chen PY, Tsai PS, 2016. Differences between men and women aged 65 and older in the relationship between self-reported sleep and cognitive impairment: A nationwide survey in Taiwan. J. Am. Geriatr. Soc. 64, 2051–2058. doi: 10.1111/jgs.14316. [DOI] [PubMed] [Google Scholar]
- Christie MA, Mckenna JT, Connolly NP, Mccarley RW, Strecker RE, 2008. 24 Hours of sleep deprivation in the rat increases sleepiness and decreases vigilance: Introduction of the rat-psychomotor vigilance task. J. Sleep Res. 17, 376–384. doi: 10.1111/j.1365-2869.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, Hertsig G, van den Wildenberg WPM, Hommel B, 2010. Estrogen modulates inhibitory control in healthy human females: evidence from the stop-signal paradigm. Neuroscience 167, 709–715. [DOI] [PubMed] [Google Scholar]
- Cordova CA, Said BO, McCarley RW, Baxter MG, Chiba AA, Strecker RE, 2006. Sleep deprivation in rats produces attentional impairments on a 5-choice serial reaction time task. Sleep 29, 69–76. [PMC free article] [PubMed] [Google Scholar]
- Corsi-Cabrera M, Sánchez AI, Del-Río-Portilla Y, Villanueva Y, Pérez-Garci E, 2003. Effect of 38 h of total sleep deprivation on the waking EEG in women: Sex differences. Int. J. Psychophysiol. 50, 213–224. doi: 10.1016/S0167-8760(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Craig MC, Brammer M, Maki PM, Fletcher PC, Daly EM, Rymer J, Giampietro V, Picchioni M, Stahl D, Murphy DGM, 2010. The interactive effect of acute ovarian suppression and the cholinergic system on visuospatial working memory in young women. Psychoneuroendocrinology 35, 987–1000. doi: 10.1016/j.psyneuen.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Craig MC, Fletcher PC, Daly EM, Rymer J, Brammer M, Giampietro V, Maki PM, Murphy DGM, 2008. Reversibility of the effects of acute ovarian hormone suppression on verbal memory and prefrontal function in pre-menopausal women. Psychoneuroendocrinology 33, 1426–1431. doi: 10.1016/j.psyneuen.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Cusmano DM, Hadjimarkou MM, Mong JA, 2014. Gonadal steroid modulation of sleep and wakefulness in male and female rats is sexually differentiated and neonatally organized by steroid exposure. Endocrinology 155, 204–214. doi: 10.1210/en.2013-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Lee CD, 2004. Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiol Learn Mem 82, 142–149. [DOI] [PubMed] [Google Scholar]
- Daurat A, Terrier P, Foret J, Tiberge M, 2007. Slow wave sleep and recollection in recognition memory. Conscious. Cogn. 16, 445–455. doi: 10.1016/j.concog.2006.06.011. [DOI] [PubMed] [Google Scholar]
- De Zambotti M, Colrain IM, Baker FC, 2015a. Interaction between reproductive hormones and physiological sleep in women. J. Clin. Endocrinol. Metab. 100, 1426–1433. doi: 10.1210/jc.2014-3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zambotti M, Colrain IM, Javitz HS, Baker FC, 2014. Magnitude of the impact of hot flashes on sleep in perimenopausal women. Fertil. Steril. 102, 1708–1715. doi: 10.1016/j.fertnstert.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zambotti M, Willoughby AR, Sassoon SA, Colrain IM, Baker FC, 2015b. Menstrual cycle-related variation in physiological sleep in women in the early menopausal transition. J. Clin. Endocrinol. Metab. 100, 2918–2926. doi: 10.1210/jc.2015-1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Lamplmayr E, Kryspin-Exner I, Gur RC, Moser E, Habel U, 2008. Facial emotion recognition and amygdala activation are associated with menstrual cycle phase. Psychoneuroendocrinology 33, 1031–1040. doi: 10.1016/j.psyneuen.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurveilher S, Rusak B, Semba K, 2011. Female reproductive hormones alter sleep architecture in ovariectomized rats. Sleep 34, 519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deurveilher S, Rusak B, Semba K, 2009. Estradiol and progesterone modulate spontaneous sleep patterns and recovery from sleep deprivation in ovariectomized rats. Sleep 32, 865–77. [PMC free article] [PubMed] [Google Scholar]
- Deurveilher S, Seary ME, Semba K, 2013. Ovarian hormones promote recovery from sleep deprivation by increasing sleep intensity in middle-aged ovariectomized rats. Horm. Behav. 63, 566–576. doi: 10.1016/j.yhbeh.2013.02.011. [DOI] [PubMed] [Google Scholar]
- Devore EE, Grodstein F, Duffy JF, Stampfer MJ, Czeisler CA, Schernhammer ES, 2014. Sleep duration in midlife and later life in relation to cognition. J. Am. Geriatr. Soc. 62, 1073–1081. doi: 10.1111/jgs.12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Beersma DG, Bloem GM, 1989. Sex differences in the sleep EEG of young adults: visual scoring and spectral analysis. Sleep 12, 500–507. [DOI] [PubMed] [Google Scholar]
- Driver HS, Dijk DJ, Werth E, Biedermann K, Borbely AA., 1996. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J.Clin. Endocrinol. Metab. 81, 728–735. [DOI] [PubMed] [Google Scholar]
- Drosopoulos S, Wagner U, Born J, 2005. Sleep enhances explicit recollection in recognition memory. Learn. Mem. 12, 44–51. doi: 10.1101/lm.83805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond SPA, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB, 2000. Altered brain response to verbal learning following sleep deprivation. Nature 403, 655–657. [DOI] [PubMed] [Google Scholar]
- Drummond SPA, Paulus MP, Tapert SF, 2006. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J. Sleep Res. 15, 261–265. doi: 10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, 2004. An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 28, 699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E, 2000. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Horm Behav 38, 262–276. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang AM, Phillips AJK, Münch MY, Gronfier C, Wyatt JK, Dijk DJ, Wright KP, Czeisler CA, 2011. Quantification of behavior Sackler Colloquium: sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc. Natl Acad. Sci. 108, 15 602–15 608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Willson HJ, Wang W, Czeisler C. a, 2009. Healthy older adults better tolerate sleep deprivation than young adults. J. Am. Geriatr. Soc. 57, 1245–1251. doi: 10.1111/j.1532-5415.2009.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlen JC, Hesse S, Pinckney L, Paul KN, 2013. Sex chromosomes regulate nighttime sleep propensity during recovery from sleep loss in mice. PLoS ONE, 8, e62205. doi: 10.1371/journal.pone.0062205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Kupfer DJ, 1997. Slow-wave sleep: do young adult men and women age differently? J. Sleep Res. 6, 211–215. doi: 10.1046/j.1365-2869.1997.00041.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C, 2007. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 30, 123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen JM, Hulbert JC, Jiang Y, Stickgold R, 2009. The sleeping brain’s influence on verbal memory: Boosting resistance to interference. PLoS One 4, 2–5. doi: 10.1371/journal.pone.0004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensrud KE, Guthrie KA, Hohensee C, Caan B, Carpenter JS, Freeman EW, LaCroix AZ, Landis CA, Manson J, Newton KM, Otte J, Reed SD, Shifren JL, Sternfeld B, Woods NF, Joffe H, 2015. Effects of estradiol and venlafaxine on insomnia symptoms and sleep quality in women with hot flashes. Sleep 38, 97–108. doi: 10.5665/sleep.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlik Y, Tataryn IV, Meldrum DR, Lomax P, Bajorek JG, Judd HL, 1981. Association of waking episodes with menopausal hot flushes. JAMA 245:1741–4. [PubMed] [Google Scholar]
- Escobar M, Oberling P, Miller RR, 2002. Associative deficit accounts of disrupted latent inhibition and blocking in schizophrenia. Neurosci. Biobehav. Rev. 26, 203–216. doi: 10.1016/S0149-7634(01)00067-7 [DOI] [PubMed] [Google Scholar]
- Esmaeilpour K, Sheibani V, Saadati H, 2015. Caffeine improved spatial learning and memory deficit in sleep deprived female rat. Physiol. Pharmacol. 19, 121–129. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PE, Dohanich GP, 1999. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine of a radial-arm maze. Pharmacol Biochem Behav 62, 711–717. [DOI] [PubMed] [Google Scholar]
- Fang J, Fishbein W, 1996. Sex differences in paradoxical sleep: influences of estrus cycle and ovariectomy. Brain Res. 734, 275–285. doi: 10.1016/0006-8993(96)00652-X. [DOI] [PubMed] [Google Scholar]
- Fedor-Freybergh P, 1977. The influence of estrogens on the wellbeing and mental performance in climacteric and postmenopausal women. Acta Obstet. Gynaecol. Scand. 64:5–64. [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM, 2008. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J. Neurosci. 28, 8660–7. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier-Brochu É, Beaulieu-Bonneau S, Ivers H, Morin CM, 2012. Insomnia and daytime cognitive performance: A meta-analysis. Sleep Med. Rev. 16, 83–94. doi: 10.1016/j.smrv.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Franken P, Dudley CA, Estill SJ, Barakat M, Thomason R, O’Hara BF, McKnight SL, 2006. NPAS2 as a transcriptional regulator of non-rapid eye movement sleep: genotype and sex interactions. Proc. Natl Acad. Sci. 103, 7118–7123. doi: 10.1073/pnas.0602006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman RR, 2014. Menopausal hot flashes: Mechanisms, endocrinology, treatment. J. Steroid Biochem. Mol. Biol. 142, 115–120. doi: 10.1016/j.jsbmb.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Archives of General Psychiatry 2004;61(1):62–70. [PubMed: 14706945] [DOI] [PubMed] [Google Scholar]
- Frick KM, Kim J, Tuscher JJ, Fortress AM, 2015. Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn Mem 22, 472–493. doi: 10.1101/lm.037267.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Schulz H, 2003. Cognitive dysfunction in sleep-related breathing disorders: A meta-analysis. Sleep Res. Online 5, 13–43. [Google Scholar]
- Galea LA, Frick KM, Hampson E, Sohrabji F, Choleris E, 2016. Why estrogens matter for behavior and brain health. Neurosci. Biobehav. Rev. 36–44. doi: 10.1016/j.actamat.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LAM, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB, 2001. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav. Brain Res. 126, 115–126. doi: 10.1016/S0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Genzel L, Kiefer T, Renner L, Wehrle R, Kluge M, Grözinger M, Steiger A, Dresler M, 2012. Sex and modulatory menstrual cycle effects on sleep related memory consolidation. Psychoneuroendocrinology 37, 987–998. doi: 10.1016/j.psyneuen.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Gervais NJ, Hamel LM, Brake WG, Mumby DG, 2016. Intra-perirhinal cortex administration of estradiol, but not an ERβ agonist, modulates object-recognition memory in ovariectomized rat. [DOI] [PubMed]
- Gervais NJ, Jacob S, Brake WG, Mumby DG, 2013. Systemic and intra-rhinal-cortical 17-β estradiol administration modulate object-recognition memory in ovariectomized female rats. Horm. Behav. 64, 642–652. doi: 10.1016/j.yhbeh.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Gervais NJ, Viechweg SS, Mong JA, Lacreuse A, 2016. The middle-aged ovariectomized marmoset (Callithrix jacchus) as a model of menopausal symptoms: Preliminary evidence. Neuroscience 337, 1–8. doi: 10.1016/j.neuroscience.2016.08.056. [DOI] [PubMed] [Google Scholar]
- Gervais NJ, Workman KP, LaClair M, Mong JA, Remage-Healey L, Lacreuse A, 2016. Aromatase inhibition impairs cognition and thermoregulation in male and female gonadectomised middle-aged marmosets (Callithrix jacchus). Poster presented at the 46th annual meeting of the Society for Neuroscience, San Diego CA, USA. [Google Scholar]
- Gibbs RB, 2007. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm. Behav. 52, 352–359. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, 2000. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging 21, 107–116. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Chipman AM, Hammond R, Nelson D, 2011. Galanthamine plus estradiol treatment enhances cognitive performance in aged ovariectomized rats. Horm. Behav. 60, 607–616. doi: 10.1016/j.yhbeh.2011.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Kim H, Lao RP, 2005. Gender differences in polysomnographic sleep in young healthy sleepers. Chronobiol. Int. 22, 905–915. doi: 10.1080/07420520500263235. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, 1996. The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philos. Trans. R. Soc. Lond. B. 351, 1445–1453. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Huang MH, Wight RG, Seeman T, Luetters C, Avis NE, Johnston J, Karlamangla AS, 2009. Effects of the menopause transition and hormone use on cognitive performance in midlife women. Neurology 72, 1850–1857. doi: 10.1212/WNL.0b013e3181a71193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger JA, Zijlstra FR, Dijk DJ, 2004. Sleep quantity, sleep difficulties and their perceived consequences in a representative sample of some 2000 British adults. J. Sleep Res. 13, 359–371. doi: 10.1111/j.1365-2869.2004.00418.x. [DOI] [PubMed] [Google Scholar]
- Guerrieri GM, Wakim PG, Keenan PA, Schenkel LA, Berlin K, Gibson CJ, Rubinow DR, Schmidt PJ, 2016. Sex differences in visuospatial abilities persist during induced hypogonadism. Neuropsychologia 81, 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachul H, Frange C, Bezerra AG, Hirotsu C, Pires GN, Andersen ML, Bittencourt L, Tufika S, 2015. The effect of menopause on objective sleep parameters: Data from anepidemiologic study in São Paulo, Brazil. Maturitas 80, 170–178. doi: 10.1016/j.maturitas.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Hadjimarkou MM, Benham R, Schwarz JM, Holder MK, Mong JA, 2008. Estradiol suppresses rapid eye movement sleep and activation of sleep-active neurons in the ventrolateral preoptic area. Eur. J. Neurosci. 27, 1780–1792. doi: 10.1111/j.1460-9568.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- Haley GE, Landauer N, Renner L, Weiss A, Hooper K, Urbanski HF, Neuringer M, Raber J, 2009. Circadian activity associated with spatial learning and memory in aging rhesus monkeys 217, 55–62. doi: 10.1016/j.expneurol.2009.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson E, Morley EE, 2013. Estradiol concentrations and working memory performance in women of reproductive age. Psychoneuroendocrinology 38, 2897–2904. doi: 10.1016/j.psyneuen.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Hamson DK, Roes MM, Galea LAM, 2016. Sex Hormones and Cognition : Neuroendocrine Influences on Memory and Learning. Compr. Physiol. 6, 1295–1337. doi: 10.1002/cphy.c150031. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WGM, Lou W, Lasley BL, Hof PR, Morrison JH, 2007. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc. Natl. Acad. Sci. 104, 11465–70. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ, 2012. Executive summary of the Stages of Reproductive Aging Workshop +10: addressing the unfinished agenda of staging reproductive aging. Climacteric 15(2),105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heekeren HR, Marrett S, Ruff DA, Bandettini PA, Ungerleider LG, 2006. Involvement of human dorsolateral prefrontal cortex in perceptual decision making is independent of response modality. Proc. Natl. Acad. Sci 103, 10023–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW, 2014. Alzheimer’s disease: review of hormone therapy trials and implications for treatment and prevention after menopause. J. Steroid Biochem. Mol. Biol. 142, 99–106. 10.1016/j.jsbmb.2013.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks JC, Sehgal A, Pack AI, 2000. The need for a simple animal model to understand sleep. Prog. Neurobiol. 61, 339–351. [DOI] [PubMed] [Google Scholar]
- Heuer H, Kleinsorge T, Klein W, Kohlisch O, 2004. Total sleep deprivation increases the costs of shifting between simple cognitive tasks. Acta Psychol. (Amst). 117, 29–64. doi: 10.1016/j.actpsy.2004.04.005 [DOI] [PubMed] [Google Scholar]
- Hita-Yañez E, Atienza M, Cantero JL, 2013. Polysomnographic and subjective sleep markers of mild cognitive impairment. Sleep 36, 1327–34. doi: 10.5665/sleep.2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann K, Coolen A, Schlumbohm C, Meerlo P, Fuchs E, 2012. Remote long-term registrations of sleep-wake rhythms, core body temperature and activity in marmoset monkeys. Behav. Brain Res. 235, 113–123. doi: 10.1016/j.bbr.2012.07.033 [DOI] [PubMed] [Google Scholar]
- Holmes MM, Wide JK, Galea LA, 2002. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behav Neurosci 116, 928–934. [DOI] [PubMed] [Google Scholar]
- Hussain D, Hanafi S, Konishi K, Brake WG, Bohbot VD, 2016. Modulation of spatial and response strategies by phase of the menstrual cycle in women tested in a virtual navigation task. Psychoneuroendocrinology 70, 108–117. doi: 10.1016/j.psyneuen.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Yamada K, Pavlides C, Ichitani Y, 2014. Sleep deprivation impairs spontaneous object-place but not novel-object recognition in rats. Neurosci. Lett. 580, 114–118. doi: 10.1016/j.neulet.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Tang MX, Stern Y, Sano M, Marder K, Bell KL, Schofield P, Dooneief G, Gurland B, Mayeux R, 1998. Cognitive function in nondemented older women who took estrogen after menopause. Neurology 50, 368–373. [DOI] [PubMed] [Google Scholar]
- Jacobsen CF, 1936. The functions of the frontal association areas in monkeys. Comp. Psychol. Monogr.13, 1–60. [Google Scholar]
- Janicki S, Schupf N, 2010. Hormonal influences on cognition and risk for Alzheimer’s Disease. Curr Neurol Neurosci Rep 10, 359–366. doi: 10.1016/j.immuni.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky JS, Chavez B, Orwoll E, 2000. Sex steroids modify working memory. J. Cogn. Neurosci. 12, 407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Monk TH, Van der Molen MW, 2003. Sleep Deprivation Influences Some but Not All Processes of Supervisory Attention Author(s): Psychol. Sci. 14, 473–9. [DOI] [PubMed] [Google Scholar]
- Joffe H, Crawford S, Economou N, Kim S, Regan S, Hall JE, White D, 2013. A gonadotropin-releasing hormone agonist model demonstrates that nocturnal hot flashes interrupt objective sleep. Sleep 36, 1977–1985. doi: 10.5665/sleep.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K, Harrison Y, 2001. Frontal lobe function, sleep loss and fragmented sleep. Sleep Med. Rev. 5, 463–475. doi: 10.1053/smrv.2001.0203. [DOI] [PubMed] [Google Scholar]
- Kalleinen N, Polo-Kantola P, Himanen SL, Alhola P, Joutsen A, Urrila AS, Polo O, 2008. Sleep and the menopause - do postmenopausal women experience worse sleep than premenopausal women? Menopause Int. 14, 97–104. [DOI] [PubMed] [Google Scholar]
- Kampen DL, Sherwin BB, 1994. Estrogen use and verbal memory in healthy postmenopausal women. Obstet. Gynecol. 83, 979–983. [DOI] [PubMed] [Google Scholar]
- Kamphuis J, Baichel S, Lancel M, de Boer SF, Koolhaas JM, Meerlo P, 2016. Sleep restriction in rats leads to changes in operant behaviour indicative of reduced prefrontal cortex function. J. Sleep Res. doi: 10.1111/jsr.12455. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM, 2009. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science, 326(5955), 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakorpi M, Alhola P, Urrila AS, Kylmälä M, Portin R, Kalleinen N, Polo-Kantola P, 2006. Hormone treatment gives no benefit against cognitive changes caused by acute sleep deprivation in postmenopausal women. Neuropsychopharmacology 31, 2079–2088. doi: 10.1038/sj.npp.1301056. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Ezzat WH, Ginsburg K, Moore GJ, 2001. Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology 26, 577–590. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, 2010. Effects of sleep deprivation on cognition. Prog. Brain Res. 185, 105–129. doi: 10.1016/B978-0-444-53702-7.00007-5 [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Balkin TJ, Wesensten NJ, 2006. Impaired decision making following 49 hours of sleep deprivation. J. Sleep Res. 15, 7–13. doi: 10.1111/j.1365-2869.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Killgore WDS, Kahn-Greene ET, Grugle NL,. Killgore DB, Balkin TJ, 2009. Sustaining executive functions during sleep deprivation: A comparison of caffeine, dextroamphetamine, and modafinil. Sleep 32, 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WDS, Lipizzi EL, Kamimori GH, Balkin TJ, 2007. Caffeine effects on risky decision making after 75 hours of sleep deprivation. Aviat. Sp. Environ. Med. 78, 957–962. doi: 10.3357/ASEM.2106.2007 [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lee JH, Lee DY, Jhoo JH, Woo JI, 2011. Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am. J. Geriatr. Psychiatry 19, 374–381. doi: 10.1097/JGP.0b013e3181e9b976 [DOI] [PubMed] [Google Scholar]
- Koehl M, Battle S, Meerlo P, 2006. Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep 29, 1224–1231. [DOI] [PubMed] [Google Scholar]
- Koehl M, Battle SE, Turek FW, 2003. Sleep in female mice: a strain comparison across the estrous cycle. Sleep 26, 267–272. [DOI] [PubMed] [Google Scholar]
- Kohama SG, Renner L, Landauer N, Weiss AR, Urbanski HF, Park B, Voytko ML, Neuringer M, 2016. Effect of Ovarian Hormone Therapy on Cognition in the Aged Female Rhesus Macaque. J. Neurosci. 36, 10416–10424. doi: 10.1523/JNEUROSCI.0909-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL, 2004. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem 82, 309–323. [DOI] [PubMed] [Google Scholar]
- Korol DL, Pisani SL, 2015. Estrogens and cognition: Friends or foes?. An evaluation of the opposing effects of estrogens on learning and memory. Horm. Behav. 74, 105–115. doi: 10.1016/j.yhbeh.2015.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromrey SA, Czoty PW, Nader MA, 2015. Relationship between estradiol and progesterone concentrations and cognitive performance in normally cycling female cynomolgus monkeys. Horm. Behav. 72, 12–19. doi: 10.1016/j.yhbeh.2015.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R, Born J, Rasch B, 2006. A 3-day estrogen treatment improves prefrontal cortex-dependent cognitive function in postmenopausal women. Psychoneuroendocrinology 31, 965–975. doi: 10.1016/j.psyneuen.2006.05.007 [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Chhabra RK, Hall MJ, Herndon JG, 2004. Executive function is less sensitive to estradiol than spatial memory: performance on an analog of the card sorting test in ovariectomized aged rhesus monkeys. Behav. Processes 67, 313–319. doi: 10.1016/j.beproc.2004.05.004 [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Gullstrand J, Fagot J, 2016. Sex differences in inhibitory control in socially-housed baboons (Papio papio). Behav. Brain Res. 312, 231–237. doi: 10.1016/j.bbr.2016.06.032 [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG, 2003. Estradiol selectively affects processing of conspecifics’ faces in female rhesus monkeys. Psychoneuroendocrinology 28, 885–905. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Herndon JG, Moss MB, 2000. Cognitive function in aged ovariectomized female rhesus monkeys. Behav. Neurosci. 114, 506–513. doi: 10.1037//0735-7044.114.3.506 [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG, 2009. No effect of different estrogen receptor ligands on cognition in adult female monkeys. Physiol. Behav. 96, 448–456. doi: 10.1016/j.physbeh.2008.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG, 2002. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol. Aging 23, 589–600. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Chang J, Metevier CM, Laclair M, Meyer JS, Ferris CM, 2014. Oestradiol Modulation of Cognition in Adult Female Marmosets (Callithrix jacchus)., J. Neuroendocrinol. 19(3), 619–630. doi: 10.1111/jne.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Verreault M, Herndon JG, 2001. Fluctuations in spatial recognition memory across the menstrual cycle in female rhesus monkeys. Psychoneuroendocrinology 26, 623–639. doi:S0306–4530(01)00017–8 [pii]. [DOI] [PubMed] [Google Scholar]
- Latta F, Leproult R, Tasali E, Hofmann E, Van Cauter E, 2005. Sex differences in delta and alpha EEG activities in healthy older adults. Sleep 28, 1525–1534. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM, 2008. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav. Neurosci. 122, 716–21. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, Albanese M, Mercuri NB, Izzi F, Bernardini S, Nitti A, Sancesario GM, Sica F, Marciani MG, Placidi F, 2014. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurology 71(12), 1498–1505. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF, 2010. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol. Bull. 136(3), 136, 375–389. doi: 10.1037/a0018883.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Dinges DF, 2008. Sleep deprivation and vigilant attention. Ann. N. Y. Acad. Sci. 1129, 305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- Lindberg E, Janson C, Gislason T, Bjornsson E, Hetta J, Boman G, 1997. Sleep disturbances in a young adult population: can gender differences be explained by differences in psychological status? Sleep, 20, 381–387. [DOI] [PubMed] [Google Scholar]
- Lipatova O, Wiener N, Andrews K, Kirshenbaum AP, Green JT, Toufexis DJ, 2016. 17Β-Estradiol Replacement in Ovariectomized Female Rats Slows Set 1 Dorsolateral Striatial-Dependent Learning and Enhances Learning of Set 2 in an Extradimensional Set-Shifting Paradigm. Behav. Neurosci. 130, 44–49. doi: 10.1037/bne0000119 [DOI] [PubMed] [Google Scholar]
- Lord C, Sekerovic Z, Carrier J, 2014. Sleep regulation and sex hormones exposure in men and women across adulthood. Pathol. Biol. 62, 302–310. doi: 10.1016/j.patbio.2014.07.005 [DOI] [PubMed] [Google Scholar]
- Luine V, 2015. Recognition memory tasks in neuroendocrine research. Behav. Brain Res. 285, 158–164. doi: 10.1016/j.bbr.2014.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD, 1998. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav 34, 149–162. [DOI] [PubMed] [Google Scholar]
- Lutsey PL, Bengtson LGS, Punjabi NM, Shahar E, Mosley TH, 2016. Obstructive Sleep Apnea and 15-Year Cognitive Decline : The Atherosclerosis Risk in Communities ( ARIC ) Study. Sleep 39, 309–316. doi: 10.5665/sleep.5434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Dinges DF, Basner M, Rao H, 2015. How acute total sleep loss affects the attending brain: a meta-analysis of neuroimaging studies. Sleep 38, 233–40. doi: 10.5665/sleep.4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, 2015. Verbal memory and menopause. Maturitas 82, 288–289. doi: 10.1016/j.maturitas.2015.07.023 [DOI] [PubMed] [Google Scholar]
- Maki PM, Drogos LL, Rubin LH, Banuvar S, Shulman LP, Geller SE, 2008. Objective hot flashes are negatively related to verbal memory performance in midlife women 15, 848–856. doi: 10.1097/gme.0b013e31816d815e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Henderson VW, 2012. Hormone therapy, dementia, and cognition: the Women’s Health Initiative 10 years on. Climacteric 15, 256–62. doi: 10.3109/13697137.2012.660613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, 2013. Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause 20, 695–709. doi: 10.1097/GME.0b013e3182960cf8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Zonderman AB, Resnick SM, 2001. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am. J. Psychiatry 158, 227–233. doi: 10.1176/appi.ajp.158.2.227 [DOI] [PubMed] [Google Scholar]
- Mander BA, Winer JR, Jagust WJ, Walker MP, 2016. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends Neurosci. 39, 552–566. doi: 10.1016/j.tins.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV, 2002. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J. Neurosci. 22, 10985–10995. doi:22/24/10985 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon Maurice M., Carskadon Mary A., Guilleminault Christian, Vitiello Michael V., 2004. Meta-Analysis of Quantitative Sleep Parameters From Childhood to Old Age in Healthy Individuals: Developing Normative Sleep Values Across the Human Lifespan. Sleep 27, 1255–73. doi: 10.1080/16506073.2015.1026386 [DOI] [PubMed] [Google Scholar]
- McCoy JG, Strecker RE, 2011. The cognitive cost of sleep lost. Neurobiol. Learn. Mem. 96, 564–582. doi: 10.1016/j.nlm.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy JG, Tartar JL, Bebis AC, Ward CP, McKenna JT, Baxter MG, McGaughy J, McCarley RW, Strecker RE, 2007. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep 30, 52–60. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson H, Neisewander JL, Conrad CD, 2008. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: Evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Horm. Behav. 54, 386–395. doi: 10.1016/j.yhbeh.2008.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelkoop HA, Smilde-van den Doel DA, Neven AK, Kamphuisen HA, Springer CP, 1996. Subjective sleep characteristics of 1,485 males and females aged 50 – 93: effects of sex and age, and factors related to self-evaluated quality of sleep. J. Gerontol. A Biol. Sci. Med. Sci. 51, M108–M115. doi: 10.1093/gerona/51A.3.M108. [DOI] [PubMed] [Google Scholar]
- Moe KE, Larsen LH, Vitiello MV, Prinz PN, 2001. Estrogen replacement therapy moderates the sleep disruption associated with nocturnal blood sampling. Sleep 24, 886–894. [DOI] [PubMed] [Google Scholar]
- Mograss MA, Guillem F, Brazzini-Poisson V, Godbout R, 2009. The effects of total sleep deprivation on recognition memory processes: A study of event-related potential. Neurobiol. Learn. Mem. 91, 343–352. doi: 10.1016/j.nlm.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Moline ML, Broch L, Zak R, 2004. Sleep in women across the life cycle from adulthood through menopause. Med. Clin. North Am. 88, 705–736. doi: 10.1016/j.mcna.2004.01.009 [DOI] [PubMed] [Google Scholar]
- Mong JA, Cusmano DM, 2016. Sex differences in sleep: impact of biological sex and sex steroids. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 371, 20150110. doi: 10.1098/rstb.2015.0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, Silver R, 2011. Sleep, rhythms, and the endocrine brain: Influence of sex and gonadal hormones. J. Neurosci. 31, 16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montplaisir J, Lorrain J, Denesle R, Petit D 2001. Sleep in menopause: differential effects of two forms of hormone replacement therapy. Menopause 8, 10–16. [DOI] [PubMed] [Google Scholar]
- Moss MB, Killiany RJ, Lai ZC, Rosene DL, Herndon JG, 1997. Recognition memory span in rhesus monkeys of advanced age. Neurobiol. Aging 18, 13–9. doi:S0197458096002114 [pii]. [DOI] [PubMed] [Google Scholar]
- Musiek ES, Holtzman DM, 2016. Review: Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science, 354(6315), 1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesca M, Koulack D, 1994. Recognition memory, sleep, and circadian rhythms. Can. J. Sleep Circadian Rhythm. 48, 359–79. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L, 1978. The hippocampus as a cognitive map, Oxford University Press. [Google Scholar]
- Ohayon MM, 2006. Severe hot flashes are associated with chronic insomnia. Arch. Intern. Med. 166, 1262–1268. doi: 10.1001/archinte.166.12.1262 [DOI] [PubMed] [Google Scholar]
- Olds T, Maher C, Blunden S, Matricciani L. Normative data on the sleep habits of Australian children and adolescents. Sleep(2010) 33:1381–8. doi: 10.1093/sleep/33.10.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens JF, Matthews KA, Everson SA, 2002. Cognitive function effects of suppressing ovarian hormones in young women. Menopause 9, 227–235. doi: 10.1097/01.GME.0000015809.11124.C5 [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA, 1997a. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol. Learn. Mem. 68, 172–88. doi: 10.1006/nlme.1997.3785 [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA, 1997b. Intra-hippocampal estradiol infusion enhances memory in ovariectomized rats. Neuroreport 8, 3009–3013. doi: 10.1097/00001756-199709290-00004 [DOI] [PubMed] [Google Scholar]
- Palchykova S, Winsky-Sommerer R, Meerlo P, Dürr R, Tobler I, 2006. Sleep deprivation impairs object recognition in mice. Neurobiol. Learn. Mem. 85, 263–271. doi: 10.1016/j.nlm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Paul KN, Dugovic C, Turek FW, Laposky AD, 2006. Diurnal sex differences in the sleep – wake cycle of mice are dependent on gonadal function. Sleep, 29, 1211–1223. [DOI] [PubMed] [Google Scholar]
- Paul KN, Laposky AD, Turek FW., 2009. Reproductive hormone replacement alters sleep in mice. Neurosci. Lett. 463, 239–243. doi: 10.1016/j.neulet.2009.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK, 2002. Models of visuospatial and verbal memory across the adult life span. Psychol. Aging 17, 299–320. doi: 10.1037/0882-7974.17.2.299. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Delbeuck X and Maquet P, 2001. Sleeping brain, learning brain. The role of sleep for memory systems. Neuroreport 12, A111–A124. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, Luxen A, Maquet P, 2004. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron 44, 535–45. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, MacLusky NJ, Choleris E, 2012. Low doses of 17β-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology 37, 2299–309. doi: 10.1038/npp.2012.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippi N, Noblet V, Duron E, Cretin B, Boully C, Wisniewski I, Seux ML, Martin-Hunyadi C, Chaussade E, Demuynck C, Kremer S, Lehéricy S, Gounot D, Armspach JP, Hanon O, Blanc F, 2016. Exploring anterograde memory: a volumetric MRI study in patients with mild cognitive impairment. Alzheimers Res. Ther. 8, 26. doi: 10.1186/s13195-016-0190-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB 1992a. Variations in memory function and sex steroid hormones across the menstrual cycle. Psychoneuroendocrinology 17, 497–506. doi: 10.1016/0306-4530(92)90008-U. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB, 1992b. Effects of estrogen on memory function in surgically meopausal women. Psychoneuroendocrinology 17, 485–495. [DOI] [PubMed] [Google Scholar]
- Pickett CK, Regensteiner JG, Woodard WD, Hagerman DD, Weil JV, Moore LG, 1989. Progestin and estrogen reduce sleep-disordered breathing in postmenopausal women. J. Appl. Physiol. 66, 1656–1661. [DOI] [PubMed] [Google Scholar]
- Polo-Kantola P, Erkkola R, Irjala K, Pullinen S, Virtanen I, Polo O, 1999. Effect of short-term transdermal estrogen replacement therapy on sleep: a randomized, double-blind crossover trial in postmenopausal women. Fertil. Steril. 71, 873–80. [DOI] [PubMed] [Google Scholar]
- Polo-Kantola P, 2011. Sleep problems in midlife and beyond. Maturitas 68, 224–232. doi: 10.1016/j.maturitas.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Pompili A, Tomaz C, Arnone B, Tavares MC, Gasbarri A, 2010. Working and reference memory across the estrous cycle of rat: a long-term study in gonadally intact females. Behav. Brain Res. 213, 10–8. doi: 10.1016/j.bbr.2010.04.018 [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H, 2013. Interplay of hippocampus and prefrontal cortex in memory. Curr. Biol. 23, R764–R773. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, Gerber C, 1983. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol. Aging. 3(4), 361–370. [DOI] [PubMed] [Google Scholar]
- Purdie DW, Empson JAC, Crichton C, MacDonald L, 1995. Hormone replacement therapy, sleep quality and psychological wellbeing. Br. J. Obstet. Gynaecol. 102, 735–739. [DOI] [PubMed] [Google Scholar]
- Quinlan MG, Duncan A, Loiselle C, Graffe N, Brake WG, 2010. Latent inhibition is affected by phase of estrous cycle in female rats. Brain Cogn. 74, 244–248. doi: 10.1016/j.bandc.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Rahman A, Languille S, Lamberty Y, Babiloni C, Perret M, Bordet R, Blin OJ, Jacob T, Auffret A, Schenker E, Richardson J, Pifferi F, Aujard F, 2013. Sleep deprivation impairs spatial retrieval but not spatial learning in the non-human primate Grey Mouse Lemur. PLoS One 8, 1–9. doi: 10.1371/journal.pone.0064493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralls FM, Grigg-Damberger M, 2012. Roles of gender, age, race/ethnicity, and residential socioeconomics in obstructive sleep apnea syndromes. Curr. Opin. Pulm. Med. 18, 568–573. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts J. a, 2003. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J. Neurosci. 23, 5708–5714. doi:23/13/5708 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Büchel C, Gais S, Born J, 2007. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 315, 1426–9. doi: 10.1126/science.1138581 [DOI] [PubMed] [Google Scholar]
- Rauchs G, Desgranges B, Foret J, Eustache F, 2005. The relationship between memory systems and sleep stages. J. Sleep Res. 14, 123–140. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB, 1998. Effects of estrogen replacement therapy on PET cerebral blood flow and neuropsychological performance. Horm. Behav. 34, 171–182. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- Reyner LA, Horne JA, Reyner A, 1995. Gender- and age-related differences in sleep determined by home-recorded sleep logs and actimetry from 400 adults. Sleep, 18, 127–134. [PubMed] [Google Scholar]
- Robbins TW, 2002. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology 163, 362–380. [DOI] [PubMed] [Google Scholar]
- Robbins TW, 2007. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 362, 917–32. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. a, Gilardi KV, Lasley B, Rapp PR, 1997. Reproductive senescence predicts cognitive decline in aged female monkeys. Neuroreport 8, 2047–2051. doi: 10.1097/00001756-199705260-00048 [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT, 2014. Oophorectomy, estrogen, and dementia: A 2014 update. Mol. Cell. Endocrinol. 389, 7–12. doi: 10.1016/j.mce.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JH, Finn MB, Stewart FR, Mahan TE, Cirrito JR, Heda A., Snider BJ, Li M, Yanagisawa M, de Lecea L, Holtzman DM, 2014. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J. Exp. Med. 211(13), 2487–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M, 2007. Epidemiology of the human circadian clock. Sleep Med. Rev. 11, 429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Romans SE, Kreindler D, Einstein G, Laredo S, Petrovic MJ, Stanley J, 2015. Sleep quality and the menstrual cycle. Sleep Med. 16, 489–495. doi: 10.1016/j.sleep.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Park S, 2002. Verbal and spatial functions across the menstrual cycle in healthy young women. Psychoneuroendocrinology 27, 835–841. [DOI] [PubMed] [Google Scholar]
- Rune GM, Lohse C, Prange-Kiel J, Fester L, Frotscher M, 2006. Synaptic plasticity in the hippocampus: Effects of estrogen from the gonads or hippocampus? Neurochem. Res. 31, 145–155. doi: 10.1007/s11064-005-9004-8 [DOI] [PubMed] [Google Scholar]
- Sagaspe P, Taillard J, Ami??va H, Beck A, Rascol O, Dartigues JF, Capelli A, Philip P, 2012. Influence of age, circadian and homeostatic processes on inhibitory motor control: A Go/Nogo task study. PLoS One 7. doi: 10.1371/journal.pone.0039410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salentu B, 2003. Sleep, vigilance and cognition in postmenopausal women: Placebo-controlled studies with 2 mg estradiol valerate, with and without 3 mg dienogest. Climacteric, Suppl 2, 37–45. [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL, 2001. Memory retention is modulated by acute estradiol and progesterone replacement. Behav. Neurosci. 115, 384–393. doi: 10.1037/0735-7044.115.2.384 [DOI] [PubMed] [Google Scholar]
- Santhi N, Lazar AS, McCabe PJ, Lo JC, Groeger JA, Dijk D-J, 2016. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc. Natl. Acad. Sci. U. S. A. 1521637113-. doi: 10.1073/pnas.1521637113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf MB, McDannold MD, Stover R, Zaretsky N, Berkowitz DV, 1997. Effects of estrogen replacement therapy on rates of cyclic alternating patterns and hot flush events during sleep in postmenopausal women: a pilot study. Clin. Ther. 19, 3 in postmenopausal women: a pilot study. Clin. Ther. 19, 304–311. [DOI] [PubMed] [Google Scholar]
- Schiff I, Regestein Q, Tulchinsky D, Ryan KJ, 1979. Effects of estrogens on sleep and psychological state of hypogonadal women. JAMA 242, 2405–4. doi: 10.1001/jama.242.22.2405 [DOI] [PubMed] [Google Scholar]
- Schiff R, Bulpitt CJ, Wesnes KA, Rajkumar C, 2005. Short-term transdermal estradiol therapy, cognition and depressive symptoms in healthy older women. A randomised placebo controlled pilot cross-over study. Psychoneuroendocrinology 30, 309–315. doi: 10.1016/j.psyneuen.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Reinhart B, Kapellerm P, Fazekas G, Offenbacher H, Eber B, Schumacher M, Freidl W, 1996. Estrogen replacement therapy in older women: A neuropsychological and brain MRI study. J. Am. Geriatr. Soc. 44, 1307–1313. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Mong JA, 2013. Estradiol modulates recovery of REM sleep in a time-of-day-dependent manner. Am. J. Physiol. Regul. Integr. Comp. Physiol. 305, R271–80. doi: 10.1152/ajpregu.00474.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MD, Mong JA, 2011. Estradiol suppresses recovery of REM sleep following sleep deprivation in ovariectomized female rats. Physiol. Behav. 104, 962–971. doi: 10.1016/j.physbeh.2011.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwierin B, Borbely AA, Tobler I, 1998. Sleep homeostasis in the female rat during the estrous cycle. Brain Res. 811, 96–104. doi: 10.1016/S0006-8993(98)00991-3 [DOI] [PubMed] [Google Scholar]
- Seeman MV, 1997. Psychopathology in women and men: focus on female hormones. Am. J. Psychiatry 154, 1641–7. [DOI] [PubMed] [Google Scholar]
- Seo H, Barraclough DJ, Lee D, 2007. Dynamic signals related to choice and outcomes in the dorsolateral prefrontal cortex. Cerebral Cortex 17(Suppl 1), 110–117. [DOI] [PubMed] [Google Scholar]
- Shaver J, Giblin E, Lentz M, Lee K, 1988. Sleep patterns and stability in perimenopausal women. Sleep 11, 556–61. [DOI] [PubMed] [Google Scholar]
- Sherwin BB, 2012. Estrogen and cognitive functioning in women: lessons we have learned. Behav. Neurosci. 126, 123–7. doi: 10.1037/a0025539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB, 1988. Estrogen and or Androgen Replacement Therapy and Cognitive-Functioning in Surgically Menopausal Women. Psychoneuroendocrinology 13, 345–357. doi: 10.1016/0306-4530(88)90060-1 [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Grigorova M, 2011. Differential effects of estrogen and micronized progesterone or medroxyprogesterone acetate on cognition in postmenopausal women. Fertil. Steril. 96, 399–403. doi: 10.1016/j.fertnstert.2011.05.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF, 2008. Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: A critical review. Front. Neuroendocrinol. 29, 88–113. doi: 10.1016/j.yfrne.2007.08.002 [DOI] [PubMed] [Google Scholar]
- Sheth BR, Varghese R, Truong T, 2012. Sleep shelters verbal memory from different kinds of interference. Sleep 35, 985–96. doi: 10.5665/sleep.1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinopoli KJ, Floresco SB, Galea L. a M., 2006. Systemic and local administration of estradiol into the prefrontal cortex or hippocampus differentially alters working memory. Neurobiol. Learn. Mem. 86, 293–304. doi: 10.1016/j.nlm.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Smith C, 2001. Sleep states and memory processes in humans: procedural versus declarative memory systems. Sleep Med. Rev. 5, 491–506. [DOI] [PubMed] [Google Scholar]
- Smith CT, Conway JM, Rose GM, 1998. Brief paradoxical sleep deprivation impairs reference, but not working, memory in the radial arm maze task. Neurobiol. Learn. Mem. 69, 211–217. doi: 10.1006/nlme.1997.3809 [DOI] [PubMed] [Google Scholar]
- Smith CT, Sierra Y, Oppler SH, Boettiger CA, 2014. Ovarian Cycle Effects on Immediate Reward Selection Bias in Humans: A Role for Estradiol. J. Neurosci. 34, 5468–5476. doi: 10.1523/JNEUROSCI.0014-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Giordani B, Lajiness-O’Neill R, Zubieta JK, 2001. Long-term estrogen replacement is associated with improved nonverbal memory and attentional measures in postmenopausal women. Fertil. Steril. 76, 1101–1107. doi: 10.1016/S0015-0282(01)02902-02908. [DOI] [PubMed] [Google Scholar]
- Snyder HM, Asthana S, Bain L, Brinton R, Craft S, Dubal DB, Espeland MA, Gatz M, Mielke MM, Raber J, Rapp PR, Yaffe K, Carrillo MC, 2016. Sex biology contributions to vulnerability to Alzheimer’s disease: A think tank convened by the Women’s Alzheimer’s Research Initiative. Alzheimer’s Dement. 12, 1186–1196. doi: 10.1016/j.jalz.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire RF, Noudoost B, Schafer RJ, Moore T, 2013. Prefrontal contributions to visual selective attention. Annu. Rev. Neurosci. 36, 451–466. doi: 10.1146/annurev-neuro-062111-150439 [DOI] [PubMed] [Google Scholar]
- Stenuit P, Kerkhofs M, 2008. Effects of sleep restriction on cognition in women. Biol. Psychol. 77, 81–88. doi: 10.1016/j.biopsycho.2007.09.011 [DOI] [PubMed] [Google Scholar]
- Stranks EK, Crowe SF, 2016. The Cognitive Effects of Obstructive Sleep Apnea: An Updated Meta-analysis. Arch. Clin. Neuropsychol. 31, 186–93. doi: 10.1093/arclin/acv087 [DOI] [PubMed] [Google Scholar]
- Sufrinko A, Johnson EW, Henry LC, 2015. The influence of sleep duration and sleep-related symptoms on baseline neurocognitive performance among male and female high school athletes. Neuropsychology 30, 484–491. doi: 10.1037/neu0000250 [DOI] [PubMed] [Google Scholar]
- Sundermann E, Gilbert PE, Murphy C, 2006. Estrogen and performance in recognition memory for olfactory and visual stimuli in females diagnosed with Alzheimer’s disease. J. Int. Neuropsychol. Soc. 12, 400–404. doi: 10.1017/S1355617706060474 [DOI] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA, 2008. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol. Learn. Mem. 90, 155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J, Oswald I, 1977. Effect of oestrogen on the sleep, mood, and anxiety of menopausal women. Br. Med. J. 2, 1317–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toffol E, Kalleinen N, Urrila AS, Himanen S-L, Porkka-Heiskanen T, Partonen T, Polo-Kantola P, 2014. The relationship between mood and sleep in different female reproductive states. BMC Psychiatry 14, 177. doi: 10.1186/1471-244X-14-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti L, Fabbri M, Natale V, 2008. Sex difference in sleep-time preference and sleep need: a cross-sectional survey among Italian pre-adolescents, adolescents, and adults. Chronobiol. Int. 25, 745–759. doi: 10.1080/07420520802394191. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C, 2014. Sleep and the Price of Plasticity: From Synaptic and Cellular Homeostasis to Memory Consolidation and Integration. Neuron, 81(1), 12–34. 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HPA, 2010. Effects of sleep deprivation on dissociated components of executive functioning. Sleep 33, 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Fortress AM, Kim J, Frick KM, 2014. Regulation of object recognition and object placement by ovarian sex steroid hormones. Behav. Brain Res. 1–18. doi: 10.1016/j.bbr.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey J, Frick K., 2016. INhibition of location estrogen synthesius in the hippocampus impairs hippocampal memory consolidaiton in ovariectomized female mice. Horm. Behav. 6–11. doi: 10.1016/j.reaurg.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uban K. a, Rummel J, Floresco SB, Galea L. a M., 2012. Estradiol Modulates Effort-Based Decision Making in Female Rats. Neuropsychopharmacology 37, 390–401. doi: 10.1038/npp.2011.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haaren F, De Bruin JPC, Heinsbroek RPW, Van De Poll NE, 1985. Delayed spatial response alternation: Effects of delay-interval duration and lesions of the medial prefrontal cortex on response accuracy of male and female Wistar rats. Behav. Brain Res. 18, 41–49. doi: 10.1016/0166-4328(85)90167-6 [DOI] [PubMed] [Google Scholar]
- Velázquez-Zamora DA, Garcia-Segura LM, González-Burgos I, 2012. Effects of selective estrogen receptor modulators on allocentric working memory performance and on dendritic spines in medial prefrontal cortex pyramidal neurons of ovariectomized rats. Horm. Behav. 61, 512–517. doi: 10.1016/j.yhbeh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Vitiello MV, 2009. Recent advances in understanding sleep and sleep disturbances in older adults growing older does not mean sleeping poorly. Curr. Dir. Psychol. Sci. 18(6), 316–320. [Google Scholar]
- Voytko ML, 2000. The effects of long-term ovariectomy and estrogen replacement therapy on learning and memory in monkeys (Macaca fascicularis). Behav. Neurosci. 114, 1078–1087. doi: 10.1037//0735-7044.114.6.1078. [DOI] [PubMed] [Google Scholar]
- Voytko ML, 2002. Estrogen and the cholinergic system modulate visuospatial attention in monkeys (Macaca fascicularis). Behav. Neurosci. 116, 187–197. doi: 10.1037/0735-7044.116.2.187 [DOI] [PubMed] [Google Scholar]
- Voytko ML, Higgs CJ, Murray R, 2008. Differential effects on visual and spatial recognition memory of a novel hormone therapy regimen of estrogen alone or combined with progesterone in older surgically menopausal monkeys. Neuroscience 154, 1205–17. doi: 10.1016/j.neuroscience.2008.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytko ML, Murray R, Higgs CJ, 2009. Executive function and attention are preserved in older surgically menopausal monkeys receiving estrogen or estrogen plus progesterone. J. Neurosci. 29, 10362–10370. doi: 10.1523/JNEUROSCI.1591-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, 2009. The role of sleep in cognition and emotion. Ann. N. Y. Acad. Sci. 1156, 168–197. doi: 10.1111/j.1749-6632.2009.04416.x [DOI] [PubMed] [Google Scholar]
- Walker MP, 2008. Cognitive consequences of sleep and sleep loss. Sleep Med. 9 Suppl 1, S29–34. doi:S1389–9457(08)70014–5 [pii]\r10.1016/S1389–9457(08)70014–5 [DOI] [PubMed] [Google Scholar]
- Wang VC, Neese SL, Korol DL, Schantz SL, 2011. Estradiol impairs response inhibition in young and middle-aged, but not old rats. Neurotoxicol. Teratol. 33, 405–14. doi: 10.1016/j.ntt.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Neese SL, Korol DL, Schantz SL, 2009. Chronic estradiol replacement impairs performance on an operant delayed spatial alternation task in young, middle-aged, and old rats. Horm. Behav. 56, 382–90. doi: 10.1016/j.yhbeh.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Sable HJK, Ju YH, Allred CD, Helerich WG, Korol DL, Schantz SL, 2008. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behav. Neurosci. 122(4), 794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SG, Juraska JM, 1997. Spatial and nonspatial learning across the rat estrous cycle. Behav. Neurosci. 111, 259–266. doi: 10.1037/0735-7044.111.2.259 [DOI] [PubMed] [Google Scholar]
- Weber MT, Rubin LH, Maki PM, 2013. Cognition in perimenopause: the effect of transition stage. Menopause 20, 511–7. doi: 10.1097/gme.0b013e31827655e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Mander BA, Florczak SM, Weintraub S, Mesulam MM, Zee PC, Paller KA, 2012. Concurrent impairments in sleep and memory in amnestic mild cognitive impairment. J. Int. Neuropsychol. Soc. 18(03), 490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wide JK, Hanratty K, Ting J, Galea LA, 2004. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res 155, 45–53. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL, 1994. Reactivation of hippocampal ensemble memories during sleep. Science (80-. ). 265, 5–8. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Heinrich AB, Hanstein B, Kirschbaum C, 2005. Estradiol or estradiol/progesterone treatment in older women: No strong effects on cognition. Neurobiol. Aging 26, 1029–1033. doi: 10.1016/j.neurobiolaging.2004.09.012 [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, liff JJ, Takano T, 2013. Sleep drives metabolite clearance from the adult brain. Science 342(6156), 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Laffan AM, Harrison SL, Redline S, Spira AP, Ensrud KE, Ancoli-israel S, 2011. Sleep-disordered breathing, hypoxia, and risk of Mild Cognitive Impairment and dementia in older women. JAMA 306(6), 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka S, 1980. Modification of circadian sleep rhythms by gonadal steroids and the neural mechanisms involved. Brain Res. 185, 385–398. doi: 10.1016/0006-8993(80)91076-8. [DOI] [PubMed] [Google Scholar]
- Young T, Finn L, Austin D, Peterson A, 2003a. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am. J. Respir. Crit. Care Med. 167, 1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- Young T, Rabago D, Zgierska A, Austin D, Laurel F, 2003b. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep 26, 667–672. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wing YK, 2006. Sex differences in insomnia: a meta-analysis. Sleep, 29, 85–93. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL, 2007. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience 144, 26–37. doi: 10.1016/j.neuroscience.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]