Abstract
Opioid peptides and receptors are broadly expressed throughout peripheral and central nervous systems and have been the subject of intense long-term investigations. Such studies indicate that some endogenous neuropeptides, called anti-opioids, participate in a homeostatic system that tends to reduce the effects of endogenous and exogenous opioids. Anti-opioid properties have been attributed to various peptides, including melanocyte inhibiting factor (MIF)-related peptides, cholecystokinin (CCK), nociceptin/orphanin FQ (N/OFQ), and neuropeptide FF (NPFF). These peptides counteract some of the acute effects of opioids, and therefore, they are involved in the development of opioid tolerance and addiction. In this work, the anti-opioid profile of endogenous peptides was described, mainly taking into account their inhibitory influence on opioid-induced effects. However, the anti-opioid peptides demonstrated complex properties and could show opioid-like as well as anti-opioid effects. The aim of this review is to detail the phenomenon of crosstalk taking place between opioid and anti-opioid systems at the in vivo pharmacological level and to propose a cellular and molecular basis for these interactions. A better knowledge of these mechanisms has potential therapeutic interest for the control of opioid functions, notably for alleviating pain and/or for the treatment of opioid abuse.
Keywords: anti-opioids, NPFF, cholecystokinin, nociceptin, MIF-1, kissorphin
1. Introduction
The discovery of the endogenous opioid system in the 1970s initiated a new understanding of the mechanisms involved in the activity of both extracted and synthetic opium compounds. In 1973, the specific opioid binding sites in the brain were established, which became referred to as μ (μ1, μ2, μ3), δ (δ1, δ2, δ3), and κ (κ1, κ2, κ3) opioid receptors (also known, respectively, as MOR, DOR and KOR) [1,2]. The opioid receptors are classified as membrane receptors with a seven-transmembrane topology and belong to the large G protein-coupled receptor superfamily [3]. Of note, the nociceptin/orphanin FQ (N/OFQ) receptor, discovered in 1995, is currently considered to be a non-opioid branch of the opioid receptor family [1] (Table 1).
Table 1.
Opioid receptors and their functions.
| Opioid Receptor | Subtypes | Previous and Unofficial Names | Effects of Activation |
|---|---|---|---|
| µ | µ1, µ2, µ3 | Mu receptor/MOP/OP3/MOPr/opioid receptor, mu 1 |
|
| δ | δ1, δ2 | DOP/DOR/OP1/Delta receptor/DOR-1/DOPr |
|
| κ | κ1, κ2, κ3 | KOR-1/Kappa receptor/OP2/KOP/KOPr |
|
| Nociceptin receptor | ORL1 | N/OFQ receptor/OP4/KOR-3/NOCIR/kappa3-related opioid receptor/MOR-C/nociceptin receptor ORL1/XOR1/NOP-r/nociceptin/orphanin FQ receptor/NOPr |
|
Binding sites for the main three opioid receptors overlap in most structures, but some exhibit higher expression of one receptor over the others. Opioid receptors are located in areas involved in the following: (1) pain transmission, such as the thalamus, rostroventral medulla, periaqueductal grey area, pons, or in the spinal cord of the dorsal horn; (2) the rewarding system, such as the nucleus accumbens (NAc), ventral tegmental area (VTA), or the prefrontal cortex; (3) other brain areas, such as the hypothalamus, amygdala, ventral pallidum, globus pallidus, nucleus raphe, hippocampus, and olfactory bulb [4]. However, they are also presented in peripheral tissues, for example, in the gastrointestinal and respiratory tract [4,5]. Through the characteristic distribution of opioid receptors, the opioid system plays a central role in nociception and analgesia [6,7], and it is implicated in the motivational and rewarding effects of natural rewards and the drugs of abuse. Moreover, it regulates numerous physiological actions, including responses to stress, respiration, gastrointestinal transit, as well as endocrine and immune functions [1].
In reference to the discovery of opioid receptors, several endogenous ligands forming the opioid peptide family were characterized [8]. Enkephalins, dynorphins, endorphins, and endomorphins are produced by the proteolytic cleavage of large protein precursors known as preproenkephalin (PENK), preprodynorphin (PDYN), and proopiomelanocortin (POMC), respectively. However, the exact pre-propeptide precursors of endomorphins have not yet been identified. All opioid peptides share a common NH2-terminal Tyr-Gly-Gly-Phe signature sequence, which interacts with opioid receptors [9]. Enkephalins ([Met]-enkephalin and [Leu]-enkephalin) produce the effect mainly by DOR activation, but they also have an affinity for MOR. Dynorphins (Dynorphin A and Dynorphin B) exert their effects primarily through the KOR and have less affinity for the MOR and DOR. In contrast, endorphins (α-endorphin, β-endorphin, and γ-endorphin) and endomorphins (Endomorphin-1 and Endomorphin-2) demonstrate the highest affinity and selectivity for the MOR [10] (Table 2).
Table 2.
Endogenous opioid peptides, their precursors, and receptor affinity.
| Precursor | Endogenous Opioid Peptide | Relative Opioid Receptor Affinity |
|---|---|---|
| Proenkephalin (PENK) | [Met]-enkephalin [Leu]-enkephalin |
µ, δ (δ >> µ) |
| Proopiomelanocortin (POMC) | β-endorphin | µ and δ (δ = µ) |
| Prodynorphin (PDYN) | Dynorphin A Dynorphin A (1–8) Dynorphin B α-neoendorphin β-neoendorphin |
κ, µ, δ (κ >> µ, δ) |
| Prepronociceptin (PNOC) | Nociceptin/Orphanin FQ | ORL-1 |
| Unknown | Endomorphin-1 Endomorphin-2 |
µ |
There is also evidence for the existence of an endogenous anti-opioid system that balances the actions of endogenous opioids and acts as part of an endogenous homeostatic system. The first demonstration of peptide with an anti-opioid activity in 1979 and prediction of the existence of other endogenous anti-opioid peptides in the central nervous system have prompted extensive research. Presently, this group includes melanocyte inhibiting factor (MIF)-related peptides, cholecystokinin (CCK), nociceptin/orphanin (N/OFQ), neuropeptide FF (NPFF), and several others.
2. Opioid System and Reward
The endogenous opioid system is not only the main target for the reinforcing and rewarding effects of opioids. It is also the target of non-opioid drugs such as ethanol, cocaine, and nicotine. Research has established that by attaching to the MOR, drugs of abuse indirectly stimulate the dopaminergic neurons by inhibiting GABAergic neurons that normally maintain the mesolimbic dopamine system under an inhibitory control [11]. DOR and their endogenous ligands (to a lesser extent) are implicated in indirect positive reinforcement and the regulation of drug consumption by improving emotional states or facilitating drug-context association [12]. Likewise, the important role of KOR/dynorphin in the dysphoric effects of drugs of abuse has been thoroughly reviewed [13,14]. The obtained data indicate that PDYN-derived peptides interact with KOR mainly to limit drug reward and to conciliate the dysphoric aspects of some drugs (e.g., cannabinoids, nicotine). Moreover, under stressful conditions, KOR/dynorphin activity increases sensitivity to cocaine reward and regulates ethanol intake. Therefore, it can be concluded that the KOR activation opposes MOR signaling in the control of hedonic homeostasis and mediates the aversive effects of abused drugs observed as dysphoria, hallucinations, or malaise. Conversely, the inhibition of MOR and DOR suppresses the positive reinforcing properties of natural rewards, opiates, or non-opioid drugs, whereas the activation of KOR facilitates these effects [15,16].
3. Opioid System and Drugs of Abuse
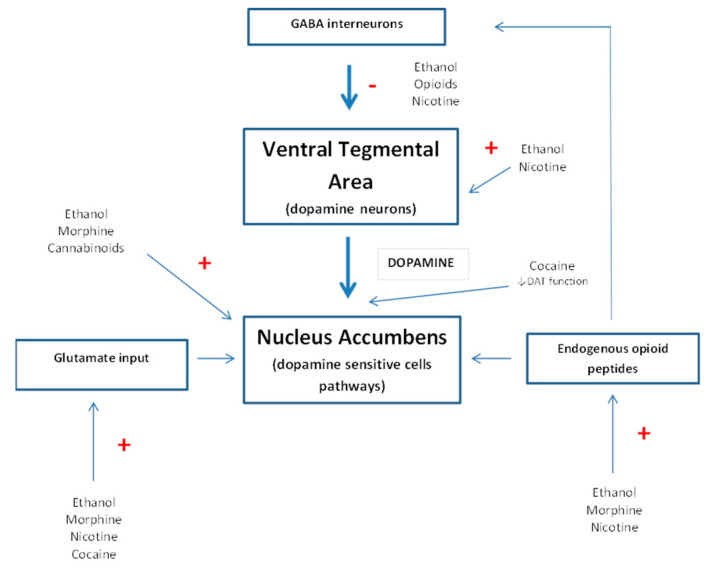
The endogenous opioid system is central to addiction. Research on this system has substantially contributed to our understanding of the brain substrates and molecular mechanisms of drugs of abuse. Early studies focused on the role of opioid receptors as a mediator of the pharmacological effects of morphine and other opiates [17]. Subsequently, it was noticed that opioid receptors are also involved in the multiple effects of non-opioid drugs [18] (Figure 1).
Figure 1.
Proposed model for the pharmacological action of drugs of abuse in the ventral tegmental area–nucleus accumbens pathway. DAT: dopamine transporter.
Ethanol, in contrast, does not have a specific receptor, but it interacts with various receptors, neurotransmitters, and neuromodulators. However, the participation of opioid receptors in ethanol reinforcement has generated considerable interest [19,20]. As a result of such research, it was recognized that (1) opioid receptors are an important target of ethanol action in the central nervous system, (2) indirect effects of ethanol on opioid receptors contribute to its behavioral effects in animals and humans, (3) ethanol increases β-endorphin and enkephalin levels in the NAc, (4) ethanol administration causes alterations in the number and activity of opioid receptors, and (5) ethanol intake depends on the activity of the endogenous opioid system [21,22,23,24,25,26,27]. However, a growing body of evidence suggests that for the most part, MORs [28] and the activation of β-endorphinergic neurons of the arcuate nucleus are involved in the rewarding (addictive) effects of ethanol [23]. The available experimental data accord well with clinical studies in which ethanol abuse has come to be interpreted as a compensation for inherent deficits in the endogenous opioid system, and opioid antagonists have been found useful in preventing relapse in alcoholics [29,30].
Multiple studies have also pointed out the role of opioid receptors and their endogenous ligands in psychostimulant addictions such as that of cocaine. Cocaine influences the endogenous opioid system through increase of (1) the β-endorphin [31], (2) striatal preprodynorphin mRNA [32,33,34], and (3) striatonigral dynorphin [35,36] levels in rats. In addition, the repeated administration of cocaine enhances the expression and function of opioid receptors observed as increases in the levels of MOR and KOR [37,38,39,40] without an influence on DOR density [41]. The involvement of the endogenous opioid system in the effects of cocaine was also confirmed by behavioral studies that clearly indicate that KORs are involved in the motivational and rewarding effects of cocaine [42,43,44,45]. In contrast, the data about MOR contribution in the rewarding effects of cocaine are conflicting.
Some studies [46,47] have reported that the motivational effects of cocaine measured in the locomotor sensitization paradigm were abolished in MOR knockout mice. In contrast, Becker et al. [48] observed no change, while Hall et al. [49] noted even enhanced cocaine-induced locomotor sensitization in this group of animals. Differences were also observed for the rewarding effects of cocaine measured in the conditioned place preference (CPP)test, and they presumably were caused by variations in dose and experimental conditions (number of pairings or number and duration of conditioning sessions). Most published experiments with MOR knockout mice have indicated the maintenance of cocaine CPP in this group [49,50]. The exception is the reduction of cocaine CPP observed by Hall et al. [49]. More conclusive results were obtained in experiments with MOR antagonists that attenuated the rewarding [51,52], but not motivational effects of cocaine [51]. The data regarding the role of DOR in cocaine effects are also conflicting. It has been shown that high doses of DOR antagonists block [53] or reduce [54,55,56] the rewarding effects of cocaine, while low doses did not modify both the effects of cocaine [57,58] or blocked entirely the development (but not expression) of locomotor sensitization and the conditioned rewarding effects of cocaine [59,60].
As with cocaine, pharmacological and genetic studies have demonstrated the critical role of the opioid system in several aspects of nicotine addiction. Most published studies have indicated that primarily MOR and KOR are involved in nicotine’s rewarding effects, but with opposite impact. Similar to other drugs of abuse, nicotine enhances the dopamine levels in the NAc by the activation of MOR in the VTA, and it has no rewarding effects in MOR [61], PENK gene [62], or β-endorphin [63] knockout mice. Instead, KOR and their endogenous ligands modulate the rewarding effects of nicotine in an opposing manner, which is observed as an enhancement of sensitivity to nicotine self-administration [64]. Nicotine may also indirectly activate the endogenous opioid system by increasing the release of some endogenous opioid peptides. Furthermore, it has been observed that acute nicotine administration increases plasma concentrations of β-endorphin in rats [65,66,67]. However, this effect may be rather related to a peripheral response to stress after nicotine administration than the activation of the endogenous opioid system [68,69]. In addition, chronic nicotine treatment increases the expression of mRNA for PDYN [70], while the influence on the level of PENK depends on the animal species and the regiment of administration [71,72]. The involvement of the endogenous opioid system in the effects of nicotine has also been demonstrated for withdrawal rodents and humans. Researchers have observed that the administration of an opioid receptor antagonist precipitates the nicotine withdrawal syndrome [73,74]—a decrease in MOR [61] and PENK knockout mice [62]—but not in the absence of PDYN [64] or β-endorphin [63].
The addictive properties of cannabinoids, one of the most popular stimulants, are directly related to the activation of CB1 receptors [75]. However, both pharmacological studies and genetic approaches demonstrate that cannabinoid and endogenous opioid systems interact to regulate both the neurochemical and behavioral responses to cannabinoids [76,77]. Although mechanisms underlying these interactions remain still unclear, receptors from these two systems show overlapping distribution in various brain structures. Moreover, they may form heterodimers that control GABA and glutamate release [78,79,80], and through these complexes, they regulate various behavioral responses, including drug addiction. The endogenous opioid system is involved in all phases of cannabinoid addiction—the development, maintenance [81,82,83,84], reinstatement [85], and abstinence syndrome [86]. However, Castañé et al. [81] suggest that a cooperative action between MOR and DOR is essential for the expression of cannabinoid dependence.
The endogenous opioid system plays a key role in several aspects of the addictive properties of drugs of abuse and could be an important element in identifying new possible therapeutic goals in the treatment of addiction. Moreover, anti-opioid substances may become a promising tool in the treatment of drug addictions. The introduction of all peptides with anti-opioid properties would be largely beyond the scope of this paper; thus, we will focus only on the four most commonly described: (melanocyte-inhibiting factor (MIF-1), cholecystokinin (CCK), nociceptin/rphanin FQ (N/OFQ) and neuropeptide FF (NPFF).
4. Anti-Opioids
4.1. MIF-1
The first endogenous peptide with anti-opioid properties—melanocyte-inhibiting factor (MIF-1) (Pro-Leu- Gly-NH2) was reported by Kastin et al. in 1979 [87]. Alternate forms of this peptide create a family called Tyr-MIF-1 instead of MIF-1, since Tyr-MIF-1 has been found to have its own high affinity to non-opiate binding sites in the brain [88,89,90,91]. This family of peptides includes MIF-1 (Pro-Leu-Gly-NH2), Tyr-MIF-1 (Tyr-Pro-Leu-Gly-NH2), Tyr-W-MIF-1 (Tyr-Pro-Trp-Gly-NH2), and Tyr-K-MIF-1 (Tyr-Pro-Lys-Gly-NH2), and it has been isolated from bovine hypothalamus and human brain cortex. Unlike most other putative anti-opioid peptides, they bind to MOR (with the exception of MIF-1 and Tyr-K-MIF-1) and to their own non-opioid receptors [92,93,94]. However, they are able to interact with specific receptors with about 50-fold higher affinity than with MOR [95]. This is one of the most important differences between the Tyr-MIF-1 family and other anti-opioid peptides such as CCK or NPFF—both of which possess no affinity to opioid receptors.
Most of the accumulated data for the anti-opioid properties of this group involve MIF-1 and Tyr-MIF-1, which antagonize the actions of endo- and exogenous opiates, with a higher effect observed for Tyr-MIF-1 than MIF-1 [87,90,96,97,98]. Moreover, they showed inhibitory actions not only against opioid antinociception, but also against hypothermia and opioid-induced ingestive behavior [87] with no effect on endogenous opioid independent analgesia [99]. In addition, Zadina et al. [100] have indicated that the chronic administration of opioids results in an enhancement of Tyr-MIF-1 activity. Such effect is observed only after prolonged administration and may be involved in the expression of opioid withdrawal. Similarly, Tyr-MIF-1 triggers an abstinence syndrome in morphine-dependent rodents, but not in opioid-naïve animals [93] (Table 3).
Table 3.
Attenuation of opioid effects by selected endogenous neuropeptides.
| Peptide | Effect | References |
|---|---|---|
MIF-1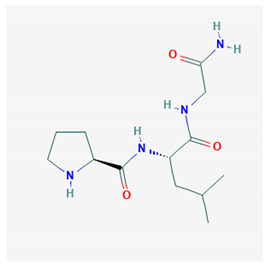 PubChem Identifier: CID 92910 URL: https://pubchem.ncbi.nlm.nih.gov/compound/92910 |
Attenuates morphine antinociception | [87,96,97,101,102,103,104] |
| Attenuates stress-induced antinociception | [87,99,104,105,106] | |
| Attenuates enkephalinergic analgesia | [105] | |
| Attenuates morphine-induced hypothermia and inhibit guinea pig ileum contractions | [106] | |
| Precipitate morphine withdrawal symptoms | [93] | |
| Inhibition of hypothermia and hypomotility produced by morphine | [106] | |
CCK-8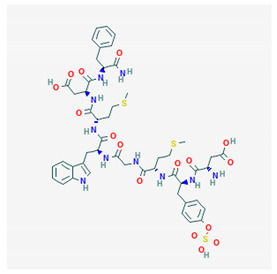 PubChem Identifier: CID 9833444 URL: https://pubchem.ncbi.nlm.nih.gov/compound/9833444 |
Attenuates morphine antinociception | [107,108,109,110] |
| Attenuates foot shock antinociception | [107,110] | |
| CCK-8 antagonist attenuates morphine tolerance | [111,112] | |
| CCK-8 antagonist potentiates analgesia morphine | [111] | |
| CCK-8 antagonist does not block morphine dependence | [113] | |
| Attenuates β-endorphin (1-31) catalepsy | [113] | |
Nociceptin/Orphanin FQ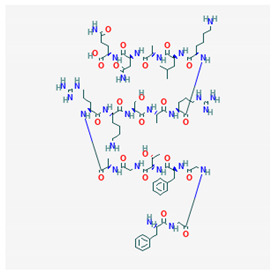 PubChem Identifier: CID 16131448 URL: https://pubchem.ncbi.nlm.nih.gov/compound/16131448 |
Attenuates morphine antinociception | [114] |
| Inhibits morphine-induced CPP | [115,116,117,118] | |
| Inhibits ethanol-induced CPP | [105,115] | |
| Blockade or deprivation of NOP receptors potentiate rewarding effects of morphine | [119] | |
NPFF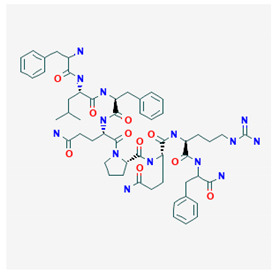 PubChem Identifier: CID 123797 URL: https://pubchem.ncbi.nlm.nih.gov/compound/123797 |
Precipitates opioid withdrawal syndrome | [118] |
| Attenuates morphine antinociception | [120] | |
| Chronic morphine increases NPFF levels in cerebrospinal fluid | [118,121,122] | |
| Anti-NPFF IgG attenuates naloxone-induced | [118] | |
| Anti-NPFF IgG reverses morphine tolerance in the rat | [123] | |
| Putative NPFF antagonist attenuates morphine | [124] | |
| Suppresses DAMGO-induced inhibition of withdrawal abstinence syndrome | [125] |
Subsequent research suggests that the anti-opioid properties of Tyr-MIF-1 seem to be mediated by MOR since this peptide shows low, but significant, affinity and selectivity for MOR. Particularly under conditions of reduced receptor reserve, e.g., in a morphine tolerant state, Tyr-MIF-1 may act as an MOR receptor antagonist. It should be emphasized that other agonists with relatively low efficiency to receptors are known to become antagonists when the efficiency of the stimulus–response coupling is reduced [126]. Moreover, correlating with their low affinity to MOR, their inhibitory effects against morphine-like activity are relatively weaker than those of traditional MOR antagonists, such as naloxone or CTOP [112].
Additionally, MIF’s peptides can act directly at opioid receptors and behave as antagonists at low concentration and as agonists at high concentrations. As expected, most of these peptides were reported to have agonistic properties for MOR in several experimental paradigms, including analgesia [101]. Here, Tyr-W-MIF-1 primarily induces dose-dependent short-lasting analgesia [127,128] and modulates gastric emptying and gastrointestinal motility [129,130]. The mentioned agonistic effects are relatively lower than those observed for morphine and are completely attenuated by MOR, but not DOR and KOR antagonists.
Nonetheless, while only a few studies have targeted Tyr-K-MIF-1 and its biological activity, its antinociceptive properties are unlikely to be related to interactions with opioid receptors, as might be for Tyr-W-MIF-1 and Tyr-MIF-1. Based on cited research, it can be clearly stated that physiologically, these peptides may biphasically, both negatively and positively, control the MOR system. Therefore, they may be considered not as anti-opioid but rather opioid-modulating peptides.
4.2. Cholecystokinin
Cholecystokinin (CCK) is a peptide hormone present in the gastrointestinal tract [131], but which also serves as a neuropeptide [132]. It is composed of varying numbers of amino acids depending on the post-translational modification of the 150-amino acid precursor, preprocholecystokinin. Thus, the CCK peptide hormone exists in several forms, each identified by the number of amino acids in the structure, e.g., CCK58, CCK33, CCK22, or CCK8. However, in the brain, sulfated CCK8 (CCK8S) [133] is the most abundant. This possesses one of the strongest endogenous anti-opioid properties [109]. It acts through binding to specific CCK receptors, CCKA (A for alimentary) and CCKB (B for brain) (also designated as CCK1 and CCK2) [134,135,136], and it possesses no affinity for opioid receptors [137].
CCK1 receptors were first characterized in peripheral organs such as the gallbladder, the pancreas, and the gastrointestinal tract, but they can also be found in many brain areas, mainly the interpeduncular nucleus, nucleus tractus solitarius, and area postrema [138,139]. It has been observed that they are principally implicated in the regulation of food and liquid intake. CCK2 receptors are localized in the gastrointestinal tract and in structures of the limbic system such as the cerebral cortex, NAc, striatum, hippocampus and amygdala, which are crucial for the control of anxiety, emotions, pain, memory, and satiety [140,141].
Initially as a result of a similarity to gastrin, interest in CCK has been focused on its role in the control of food intake. Subsequently, the discovery of the various effects of CCK on the central nervous system increased the interest in the brain and led to a number of studies associating with the central effects of this peptide. The anti-opioid activity of CCK was first reported by Faris et al. [107]. Afterward, it was confirmed and characterized in a large number of behavioral studies (Table 3). However, the cellular and molecular mechanisms underlying the CCK anti-opioid action are still poorly understood. The presence of opioid receptors on CCK-containing neurons suggests the direct effect of opioids on the release of CCK [142] with differences at the central nervous system and spinal cord level. In the central nervous system, chronic opioid administration increases CCK release and CCK2 receptor expression [136,143,144]. In contrast, at the spinal cord level, both selective MOR (DAMGO) and low doses of DOR (DTLET) agonist result in a reduction of CCK release in a naloxone-reversible manner [145]. Furthermore, the antagonism of endogenous CCK at the spinal cord level may enhance the effects of exogenously administered opioid receptor agonists [146]. The observed effect may indicate that endogenously released CCK tonically inhibits opioid activity and reduces the binding affinity of MOR ligands [137,147]. It also has been suggested that endogenous CCK modulates the potency of spinal opioids observed in inflammatory or neuropathic pain [146,148]. Moreover, in contrast to NPFF and N/OFQ, after central and peripheral administration, CCK reduces the antinociception evoked by exogenous, as well as endogenous opioids, presumably by the activation of CCK2 receptors [113]. However, unexpectedly, mice lacking the CCK2 receptor showed a lower pain threshold than wild-type animals and are less sensitive to the analgesic effects of endogenous opioids or morphine [113]. It should also be emphasized that the stimulation of CCK1 receptors facilitates pain control by the opioid system [135].
It is increasingly apparent that CCK regulates a variety of physiological processes, including cognition, reward, and learning or memory [149,150]. Based on the localization of CCK receptors within the brain reward system, experiments clearly indicate that endogenous CCK is necessary for the morphine rewarding effects [151] and is upregulated after chronic morphine treatment. This has been confirmed by subsequent studies that have demonstrated that CCK receptor blockade or knockout inhibits the development of opioid tolerance, dependence, withdrawal syndrome [134,152], and the reinstatement of morphine reward [153,154]. At the same time, it has been noticed that the administration of exogenous CCK-8 prevents the development of morphine addiction and reduces withdrawal symptoms [155]. Crespi et al. have reported that CCK is also involved in cocaine [156] and ethanol [157] intake. Some researchers explain these effects as the result of CCK-mediated regulation of anxiety and stress [158] or food and liquid consumption [159]. However, mostly, it is concluded that this effect points out a direct implication in the drug-dependence phenomenon by interaction with other neurotransmitter systems. It is indeed well documented that neurotransmitters such as dopamine and serotonin are largely involved in drug abuse and that these systems can interact with central CCK functions. However, in current thinking, the main focus of the anti-opioid action of CKK remains the CCK–opioid interaction that at least in the rodent is mediated by the CCK2 receptors [160,161,162].
4.3. Nociceptin/Orphanin FQ (N/OFQ)
In 1994, soon after the cloning of classical opioid receptors, several research groups identified a new G protein–coupled receptor with high homology to opioid receptors, but which was not able to bind opioid ligands. On this basis, it was named opioid receptor-like-1 (ORL-1) [163]. One year after the cloning of the NOP receptor, two groups independently identified the endogenous ORL1-ligand, named “nociceptin/orphanin FQ” (N/OFQ). The name “nociceptin” was determined on the basis of its ability to elicit hyperalgesia after supraspinal administration in mice, and “orphanin FQ” was chosen for its ability to recognize a previously known orphan receptor, and for its first and last amino acid residues (F and Q) [164,165]. Following the identification of N/OFQ as the endogenous agonist of ORL-1, the receptor was renamed “nociceptin opioid peptide receptor” and abbreviated as “NOP receptor”. International Union of Basic and Clinical Pharmacology (IUPHAR) considers it a subcategory of the opioid peptide receptor family [166].
N/OFQ displays a primary sequence very similar to that of opioid peptides, especially Dynorphin A. However, Phe being presented in position 1 instead of Tyr makes this peptide highly selective for its own receptor over classical opioid receptors [167], and its pharmacological effects are not reversed by the non-selective opioid antagonist—naloxone [114]. Since its discovery, N/OFQ has been of great interest to researchers as a result of its wide distribution in the nervous system. It is found in the cortex, anterior olfactory nucleus, lateral septum, hypothalamus, hippocampus, amygdala, central gray, pontine nuclei, interpeduncular nucleus, substantia nigra, raphe complex, locus coeruleus, and spinal cord [168].
The available results show that N/OFQ evokes complex pharmacological effects in animals, eliciting either an anti-opioid/anti-hyperalgesic action or analgesic effect depending on the dose and route of administration, testing paradigm, and even animal species. Functionally, the N/OFQ is considered to have an anti-opioid action (Table 3). When injected intracerebroventricularly (i.c.v), it antagonizes opioid analgesia, including opioid-mediated stress-induced analgesia and the analgesic effects of MOR, DOR, and KOR agonists [115]. In contrast to i.c.v. injection, spinal (i.t.) administration of this peptide produces an opioid-like, antinociceptive effect [169]. However, the synthetic NOP agonist, Ro65-6570, given systemically, did not produce statistically significant effects in the mouse tail withdrawal assay, but it was effective in inhibiting the nociceptive effect in inflammatory pain [170,171]. This antinociceptive effect was observed at a dose that induced severe motor side effects [170]. In turn, systemic administration Ro 65-6570 to non-human primates exerted potent and efficacious antinociception with the absence of motor and sedative side effects [152]. More importantly, this agonist did not produce reinforcing effects that were per se comparable with opioid agonists [172]. Additionally, mice knockout for the NOP gene displayed increased nociceptive behaviors in some assays (i.e., formalin test) [171].
Furthermore, N/OFQ administrated i.t. enhances morphine—or electroacupuncture—induced analgesia [169,173,174], and it produces antinociceptive synergy with morphine in experimental neuropathy [119] in rodents. Moreover, the systemic or i.t. coactivation of MOP and NOP receptors produced synergistic antinociception in primates [116,175] without other side effects. These studies strongly support the therapeutic potential of mixed MOP/NOP agonists as innovative analgesics.
Therefore, the consequence of this research was the introduction of cebranopadol, a first-in-class potent analgesic agent with agonistic activity that targets NOP and opioid receptors [176]. This compound displays analgesic, antiallodynic, and antihyperalgesic properties in several rat models of acute nociceptive, inflammatory, cancer, and neuropathic pain [177,178]. In contrast to classic opioids, it has a higher analgesic potency in models of neuropathic pain than in acute nociceptive pain [179]. In addition, even at higher doses, cebranopadol does not induce motor coordination deficits or respiratory depression [177] and has limited potential to produce opioid-type physical dependence in rodents [180]. Hence, it seems to possess a broader therapeutic window than classical opioids. Therefore, this compound is particularly interesting as a novel, potent bifunctional agonist of NOP/opioid receptors.
Research indicates that the interactions between N/OFQ and the opioid system are responsible for maintaining homeostasis in the body. Indeed, Yuan et al. show that the chronic administration of high doses of morphine accelerates the release and biosynthesis of N/OFQ in the rat brain. This effect is presumably involved in the blockade of the opioid analgesic effect and promotes the development of morphine tolerance. Surprisingly, the chronic administration of exogenous N/OFQ, concomitantly with morphine, attenuates the development of morphine tolerance, without impact on the basal and morphine nociceptive responses after single administration [181]. These observations may indicate that N/OFQ is not implicated in the early phase of the development of tolerance.
Increasing evidence suggests that this peptide may also play a crucial role in the regulation of reward mechanisms and drug abuse processes. As with other anti-opioid peptides, receptors for N/OFQ are widely represented in areas implicated in drug abuse and reward. The injection of N/OFQ results in the blockade of the rewarding properties of several common drugs of abuse such as morphine, cocaine, amphetamines, or ethanol [120,182,183,184,185,186,187,188] without side effects per se [189]. Interestingly, although N/OFQ has been proposed to be a functional “anti-opioid peptide”, it does not exhibit the aversive properties typical for opioid receptor antagonists. Based upon the ability of N/OFQ to block extracellular dopamine levels and block the CPP of so many abused drugs, it is surprising that N/OFQ was ineffective in attenuating heroin self-administration in rats [118] but was successful in blocking ethanol self-administration [190,191,192]. However, some studies found N/OFQ agonists decrease the self-administration of ethanol only in rats with a history of ethanol dependence, but not in naïve rats [193,194]. There is also no information demonstrating the influence of N/OFQ on the self-administration of cocaine or nicotine. It also should be noted that the effect of N/OFQ receptor activation was found to be different in two standard “drug abuse” paradigms, CPP and self-administration [195]. Presumably, N/OFQ receptor agonists are effective in attenuating acquisition but not the expression of the drug reward effect that underlies the self-administration paradigm. This suggests that different mechanisms may control these two paradigms. There is also evidence that N/OFQ can modulate the opioid withdrawal syndrome, thus decreasing the risk of relapse, and it may facilitate the treatment of opioid addiction. This statement is supported by behavioral studies that have uncovered that N/OFQ inhibits naloxone precipitated morphine withdrawal [196] without any change in behavior of the morphine-dependent animals when given alone [169]. The effect N/OFQ on the locomotor activity in rodents is still unclear, since neither a reduction nor stimulation has been observed. Moreover, the results were dependent on the dose and whether the animals were habituated or not to the test environment [197,198]. However, it has been reported that N/OFQ does not alter locomotor sensitization induced by cocaine and morphine [199]. Therefore, this outcome supports the idea that N/OFQ does not interfere with the motivational effects of drugs of abuse.
The data discussed in this review clearly indicate that N/OFQ behaves as a functional anti-opioid peptide especially in regard to the rewarding effects of opioids and ethanol. Interestingly, its influence on other physiological functions is observed after the administration of higher doses. Thus, the anti-opioid action of N/OFQ on the rewarding properties of drugs is more potent than that evoked on pain processes.
4.4. NPFF
Neuropeptides FF (NPFF), AF (NPAF), and SF(NPSF) are homologous, amidated peptides that were originally identified on the basis of sequence similarity to the neuropeptide FMRF-amide [184]. They have been hypothesized to have broad functions in the mammalian central nervous system, including the modulation of pain [200,201] or opiate functions [202,203], as well as the regulation of cardiovascular [204] and neuroendocrine functions [121]. One of these sequences, NPFF (FLFQPQRF-NH2), has been characterized as an endogenous anti-opioid peptide [122,124]. This peptide evokes pharmacological effects by interacting with the G-protein-coupled receptors, NPFF1 and NPFF2 [205,206], which are widely expressed in spinal cord and brain regions regulating emotional functions (fear, anxiety, reward) [207,208]. It should be emphasized that NPFF does not bind to opioid receptors, and conversely, opioids have no measurable affinity toward NPFF receptors [123,209]. However, many studies suggest that the NPFF system is functionally coupled to the opioid system, and each can mutually interact [210]. Furthermore, remarkable similarities in several key anti-opioid actions between CCK and NPFF were noted. Both peptides administrated alone cause no significant pronociceptive effects; rather, they exert a dose-dependent antagonism of opioid-induced analgesia. Furthermore, both peptides and their receptors strategically overlap with the opioid receptor distribution in many brain regions related to pain perception. Finally, the NPFF and CCK system can be upregulated and mobilized to exert an anti-opioid action in response to enhanced opioidergic transmission [112].
It has been observed that NPFFs possess a bimodal effect on pain perception: an anti-opioid effect after supraspinal administration and an opioid-like effect at the spinal level [211], similarly to N/OFQ. In general, NPFF reverses morphine- and stress-induced analgesia after i.c.v. administration [200,203,212]. In addition, pretreatment with anti-NPFF-IgG restores the analgesic effects of morphine in morphine-tolerant rats [213] and potentiates opioid analgesia [214]. These findings, along with the observations that long-term NPFF administration decreases the density of the MOR [124], suggests that these receptors are under the tonic inhibitory control of NPFF. NPFF also seems to play a role in opiate tolerance, since it has been observed that anti-NPFF antibodies can reverse the development of tolerance to morphine [215], as well as reduce the symptoms of naloxone-induced withdrawal syndrome [213,216]. Moreover, the chronic administration of morphine increases concentrations of NPFF in the cerebrospinal fluid [217,218] and presumably may underlie the development of opioid tolerance that is associated with increased NPFF activity. Furthermore, its action does not directly depend on opioid receptors. This effect also appears to be responsible for the occurrence of opioid withdrawal syndrome [202]. On the other hand, NPFF and its analogs, which are administered i.t., induce long-lasting analgesia, probably by increasing opioid peptides release in the spinal cord through the blockade of DOR autoreceptors [219], and they also potentiate morphine-induced analgesia [201,211].
Nociception is the physiological function in which this interaction between NPFF and opioids has been the most extensively studied; notwithstanding that reward, locomotion, feeding and intestinal motility are also affected. The presence of both NPFF-like immunoreactive material and NPFF binding sites in the mesolimbic structures [209,220] may point toward the involvement of NPFF in drug addiction processes. In fact, it also suggests that NPFF may be a good candidate for directly or indirectly regulating mesocorticolimbic system activity. This is because the supraspinal administration of NPFF or analogs antagonizes the rewarding effect of morphine by inhibiting dopamine release in the mesolimbic reward system [221,222]. In addition, Kotlinska et al. found that NPFF is able to block the expression of the rewarding effects of cocaine and amphetamine in the CPP test [223].
In contrast to the above results, NPFF is not able to inhibit the expression of ethanol-induced CPP. However, it should be noted that NPFF neither induced place preference nor aversion, although a tendency to aversive effect was seen after the administration of NPFF at a high dose. Several studies also showed the influence of NPFF on the motivational effects of drugs of abuse. This is observed as an inhibition of the hiperlocomotor effect. In fact, NPFF, administrated i.c.v. reduces the locomotor activity triggered by exposure to novelty and decreases dose-dependently the potentiation of novelty-induced locomotor response degradation [207]. Furthermore, NPFF i.c.v. administration reduces the sensitization to hyperlocomotor effects that is crucial in the development of morphine [220,222], heroin [224], cocaine [225], as well as amphetamine [223] and ethanol dependence [222]. These data show that NPFF receptors may be involved not only in the rewarding, but also in the motivational effects of many drugs of abuse. However, it should be emphasized that NPFF is unable to inhibit the rewarding effects of ethanol. It is probable that this effect is due to the fact that ethanol reward is a more complex process, and apart from the role of opioids, many other neurotransmitters are also involved in this effect.
In addition, one of the newly discovered kisspeptin derivatives, kissorphin (KSO) (Tyr-Asn-Trp-Asn-Ser-Phe-NH2), also evokes an anti-opioid effect. This peptide does not possess any activity on the GPR-54 receptor and does not affect the release of GnRH [226]. However, it activates the receptors for the anti-opioid peptide, NPFF. The NPFF-like anti-opioid activity of KSO was supported by Simonin et al. [226] and Milton [227], who showed that the biological activity of the KSO peptide is antagonized by the NPFF receptor antagonist RF9 but not the GRP-54 receptor antagonist KP234 [227]. Recent data [228,229,230] also confirmed the NPFF-like activity of KSO and provide a support for the anti-opioid character of KSO which, in contrast to NPFF, inhibits rewarding and motivational effects of ethanol.
5. Anti-Opioid Peptides: Potential Therapeutic Interest
The treatment of drug addiction still remains a medical need due to the dearth of effective and approved pharmacotherapies. The assessment of current addiction treatment has revealed the stark reality of the limited options to treat opioid and psychostimulant abuse. A growing problem is the elimination of withdrawal symptoms, as these often lead to a return to addiction even after a long period of abstinence. It is considered that the discovery of a group of substances with anti-opioid activity creates advanced therapeutic possibilities, and their use may result in the introduction of completely new and safer methods of addiction treatment.
In fact, the ability of N/OFQ to block the rewarding effects of opioids and some other psychostimulants, as well as its anxiolytic profile, underlines its therapeutically beneficial profile for drug abuse and relapse treatment. This is particularly important from a therapeutic point of view, since opioid antagonists such as naltrexone, which is currently used for the treatment of ethanol and opioid addiction, possess many drawbacks. Among these are anxiogenic activity and the precipitation of withdrawal symptoms. In contrast, N/OFQ does not exhibit the aversive properties at doses that block the rewarding properties of morphine and ethanol. Moreover, in contrast to opioid antagonists, the activation of brain NOP receptors attenuates alcohol [231] and opioid [232] withdrawal symptoms in rats.
Preclinical studies in rodents and non-human primates have shown that non-peptide, small-molecule N/OFQ receptor agonists demonstrate efficacy in attenuating the rewarding effects of various abused drugs. These compounds (AT-312, Ro 64-6198) show promising impact on the rewarding effects of many drugs of abuse and may have potential as pharmacotherapy even for treating polydrug addiction [192,233]. However, the stimulation of NOP receptors in the mesolimbic circuitry may lead, for example, to a hypodopaminergic and hypohedonic state that can increase the motivation for drugs of abuse. In fact, published data show that the activation of NOP following intra-ventral tegmental area (VTA) administration of N/OFQ attenuates dopamine release in the nucleus accumbens [184]. On the other hand, the administration of NOP agonists may depress N/OFQ transmission through receptor desensitization [234], and, in such a way, it may result in paradoxical antagonistic effects.
Thus, another strategy to attenuate drug abuse is to focus on NOP blockade. For instance, BTRX-246040 (also known as LY2940094), a selective and potent NOP antagonist [235], was found to reduce alcohol consumption in two different lines of genetically selected alcohol preferring rats [236] and in clinical trials with patients diagnosed with alcohol use disorders [237]. The putative therapeutic potential of NOP antagonists comes from studies in genetically modified NOP knockout rats. These animals self-administered significantly smaller amounts of alcohol, cocaine, and heroin, but showed unimpaired motivation for saccharin, which is a natural reward [238]. Hence, this group of compounds is more effective in preventing drug abuse [239] than NOP agonists and has promising utility for treating drug addiction.
Finally, a potentially effective alternative strategy for treatment of drug abuse is the coactivation of NOP and MOP receptors. Ciccocioppo et al. [240] found that buprenorphine, a partial agonist at MOP [240] and NOP receptors [241,242], has the ability to attenuate ethanol consumption in rats at high concentrations. Moreover, unlike buprenorphine, certain recently developed bifunctional NOP/MOP partial agonists (i.e., BU08028, BU10038, and AT-121) do not exhibit reinforcing effects in non-human primates [243,244,245]. These compounds selectively attenuate alcohol intake (BU08028) [246] and significantly attenuate oxycodone-induced reinforcing effects (daily systemic AT-121 pretreatment) [244] without disrupting food-maintained operant behavior. Furthermore, recently, two independent studies have demonstrated that cebranopadol, another NOP and MOP receptor agonist, is efficacious in attenuating the motivation for cocaine in drug self-administration studies, while leaving unaffected (or slightly increased) the consumption of natural rewards [247,248]. Therefore, it is possible that mixed NOP/MOP partial agonists may have great therapeutic potential for treating substance use disorders and/or polydrug addiction [249] with fewer side effects.
The broad spectrum of actions of N/OFQ has provoked the interest of academic and industrial researchers and generated a large panel of N/OFQ receptor ligands useful for target validation studies. In fact, N/OFQ receptor agonists have been proposed as innovative drugs for treating hypertension [250] and urinary incontinence [251], whereas N/OFQ receptor selective antagonists have been suggested as novel treatment for Parkinson’s disease [252], depression (including BTRX-246040, an NOP antagonist already tested in humans) [253,254], and possible drug abuse [239]. BTRX-246040 was also found to reduce depression symptoms in a second trial with heavy alcohol drinkers. Given the comorbidity of major depression and alcohol use disorder, a compound with such effects in patients could be a valuable addition to the medications available [255]. In addition, NOP antagonists have already been used to identify novel conditions/diseases for which the blockade of NOP receptor is associated with beneficial effects (traumatic injuries of the central nervous system, post-traumatic stress disorders, sepsis) [255,256,257,258,259].
In turn, some behavioral experiments have revealed that opioid-N/OFQ hybrids such as SR16435, which behaves as a mixed MOP–NOP receptor partial agonist [260], are highly promising analgesics for the treatment of acute and even neuropathic pain, devoid of respiratory depression [261]. For example, a mixed opioid/NOP agonist, cebranopadol, is in early phase clinical development in treating diabetic neuropathy, cancer pain, and lower back pain [262,263,264].
N/OFQ appears to have the widest potential clinical use, which is also directly related to its pleiotropic/multidirectional activities; however, likewise, CCK2 receptor antagonists have displayed a preventive or therapeutic effect on morphine dependence and withdrawal [265]. Moreover, observation that the CCK2 receptor plays a crucial role in the induction and persistence of anxiety and major depression has led to using CCK-4 routinely to induce anxiety in behavioral models. Additionally, CCK compounds, especially the selective CCKB antagonists, may be interesting drugs in the management of pain. Indeed, even if they do not induce analgesic responses alone, they are able to potentiate the antinociceptive effects of the opioids and reduce the eventual side effects of opioid chronic treatment. Thus, a concomitant administration of morphine and a CCK antagonist may permit reduction of the dose and frequency of morphine administration required for pain relief [266,267]. CCKB antagonists may also be useful in reducing the morphine withdrawal effect [268].
Regarding MIF-1, its high stability in human blood, combined with its long-lasting biological activity, makes this peptide a valuable candidate for a human therapeutic agent [269,270]. However, presently, this peptide is rather considered as a prototype for the group of anti-opioid peptides.
Other promising therapeutic candidates for pain and abuse treatment are NPFF and KSO. The last is a novel NPFF receptor agonist. In fact, previous studies using these peptides have provided a better understanding of the processes involved in ethanol, morphine, cocaine, and amphetamine dependence. However, they are still used mainly as reference substances in behavioral experiments and are the basis for the search for new drugs.
The use of anti-opioid peptides in behavioral studies has significantly contributed to a better understanding of the processes that lead to the development, maintenance, and relapse of drug addiction. However, for most of them, a restriction to widespread use is the difficulty of administration, since most of these compounds are unable to penetrate the blood–brain barrier after peripheral administration and thus cannot induce central effects. In order to overcome this limitation, further research is needed to develop new compounds of a non-peptide nature with anti-opioid activity.
6. Conclusions
The pharmacological effects of acute and chronic opioid administration could be modulated or even blocked by several endogenous peptides. All these peptides are referred to as anti-opioid or to a lesser extent, as opioid-modulating peptides, since some of them also possess pharmacological opioid-like properties. However, it should be emphasized that no single peptide possesses naloxone-like effects to all systems. Indeed, a compound that would be capable of modulating the multiple behavioral effects of the endogenous opioids should itself contain many elements. The variety of endogenous opiates strengthens our idea that endogenous anti-opioids constitute a numerous and diverse group, and the MIF-1 related peptides CCK, N/OFQ, and NPFF represent distinct neuronal systems.
The confirmation that endogenous opioid-modulating peptides may control the pharmacological effects of morphine and other drugs of abuse opens new, broad prospects for their therapeutic use, notably for alleviating pain and for treating opioid abuse. These compounds possess high efficacy, and some of them are already at the clinical trial stage. However, the precise mechanisms involved in these effects are still not completely characterized, and anti-opioids still remain a relatively underexplored system with potential therapeutic importance.
Author Contributions
Conceptualization, E.G.-T. and J.H.K.; validation, E.G.-T.; formal analysis, E.G.-T. and J.H.K.; writing—E.G.-T.; writing—review and editing, E.G.-T. and J.H.K.; visualization, E.G.-T.; supervision, E.G.-T.; project administration, E.G.-T. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bodnar R.J. Endogenous opiates and behavior: 2014. Peptides. 2016;75:18–70. doi: 10.1016/j.peptides.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Waldhoer M., Bartlett S.E., Whistler J.L. Opioid receptors. Annu. Rev. Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 3.Dhawan B.N., Cesselin F., Raghubir R., Reisine T., Bradley P.B., Portoghese P.S., Hamon M. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol. Rev. 1996;48:567–592. [PubMed] [Google Scholar]
- 4.Gray A.C., Coupar I.M., White P.J. Comparison of opioid receptor distributions in the rat ileum. Life Sci. 2006;78:1610–1616. doi: 10.1016/j.lfs.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 5.Wittert G., Hope P., Pyle D. Tissue distribution of opioid receptor gene expression in the rat. Biochem. Biophys. Res. Commun. 1996;218:877–881. doi: 10.1006/bbrc.1996.0156. [DOI] [PubMed] [Google Scholar]
- 6.Dickenson A.H., Kieffer B.L. Opiates: Basic mechanisms. In: McMahon S.B., Koltzenburg M., editors. Text-Book of Pain. 5th ed. Elsevier; London, UK: 2005. pp. 427–442. [Google Scholar]
- 7.Zöllner C., Stein C. Opioids. Handb. Exp. Pharmacol. 2007;177:31–63. doi: 10.1007/978-3-540-33823-9_2. [DOI] [PubMed] [Google Scholar]
- 8.Akil H., Owens C., Gutstein H., Taylor L., Curran E., Watson S. Endogenous opioids: Overview and current issues. Drug Alcohol Depend. 1998;51:127–140. doi: 10.1016/S0376-8716(98)00071-4. [DOI] [PubMed] [Google Scholar]
- 9.Kieffer B.L. Recent advances in molecular recognition and signal transduction of active peptides: Receptors for opioid peptides. Cell Mol. Neurobiol. 1995;15:615–635. doi: 10.1007/BF02071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mansour A., Khachaturian H., Lewis M.E., Akil H., Watson S.J. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J. Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 11.Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutz P.E., Kieffer B.L. The multiple facets of opioid receptor function: Implications for addiction. Curr. Opin. Neurobiol. 2013;23:473–479. doi: 10.1016/j.conb.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shippenberg T.S., Zapata A., Chefer V.I. Dynorphin and the pathophysiology of drug addiction. Pharmacol. Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wee S., Koob G.F. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology. 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shippenberg T.S., Elmer G.I. The neurobiology of opiate reinforcement. Crit. Rev. Neurobiol. 1998;12:267–303. doi: 10.1615/CritRevNeurobiol.v12.i4.10. [DOI] [PubMed] [Google Scholar]
- 16.Van Ree J.M., Niesink R.J., Van Wolfswinkel L., Ramsey N.F., Kornet M.M., Van Furth W.R., Vanderschuren L.J., Gerrits M.A., Van den Berg C.L. Endogenous opioids and reward. Eur. J. Pharmacol. 2000;405:89–101. doi: 10.1016/S0014-2999(00)00544-6. [DOI] [PubMed] [Google Scholar]
- 17.Kieffer B.L., Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog. Neurobiol. 2002;66:285–306. doi: 10.1016/S0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 18.Shippenberg T.S., LeFevour A., Chefer V.I. Targeting endogenous mu- and delta-opioid receptor systems for the treatment of drug addiction. CNS Neurol. Disord. Drug Targets. 2008;7:442–453. doi: 10.2174/187152708786927813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Chiara G., Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur. J. Pharmacol. 1985;115:131–132. doi: 10.1016/0014-2999(85)90598-9. [DOI] [PubMed] [Google Scholar]
- 20.Fibiger H.C., Phillips A.G. Mesocorticolimbic dopamine systems and reward. Ann. N. Y. Acad. Sci. 1988;537:206–215. doi: 10.1111/j.1749-6632.1988.tb42107.x. [DOI] [PubMed] [Google Scholar]
- 21.Bechtholt A.J., Cunningham C.L. Ethanol-induced conditioned place preference is expressed through a ventral tegmental area dependent mechanism. Behav. Neurosci. 2005;119:213–223. doi: 10.1037/0735-7044.119.1.213. [DOI] [PubMed] [Google Scholar]
- 22.Gianoulakis C. The effect of ethanol on the biosynthesis and regulation of opioid peptides. Experientia. 1989;45:428–435. doi: 10.1007/BF01952024. [DOI] [PubMed] [Google Scholar]
- 23.Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology. 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- 24.Heyser C.J., Roberts A.J., Schulteis G., Koob G.F. Central administration of an opiate antagonist decreases oral ethanol self-administration in rats. Alcohol Clin. Exp. Res. 1999;23:1468–1476. doi: 10.1111/j.1530-0277.1999.tb04669.x. [DOI] [PubMed] [Google Scholar]
- 25.Hyytia P., Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin. Exp. Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 26.June H.L., Cummings R., Eiler W.J., 2nd, Foster K.L., McKay P.F., Seyoum R., Garcia M., McCane S., Grey C., Hawkins S.E., et al. Central opioid receptors differentially regulate the nalmefene-induced suppression of ethanol-and saccharin-reinforced behaviors in alcohol-preferring (P) rats. Neuropsychopharmacology. 2004;29:285–299. doi: 10.1038/sj.npp.1300338. [DOI] [PubMed] [Google Scholar]
- 27.Margolis E.B., Fields H.L., Hjelmstad G.O., Mitchell J.M. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J. Neurosci. 2008;28:12672–12681. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Contet C., Kieffer B.L., Befort K. Mu opioid receptor: A gateway to drug addiction. Curr. Opin. Neurobiol. 2004;14:370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr. Top. Med. Chem. 2009;9:999–1015. doi: 10.2174/156802609789630956. [DOI] [PubMed] [Google Scholar]
- 30.Koob G.F. Neuroadaptive mechanisms of addiction: Studies on the extended amygdala. Eur. Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Moldow R.L., Fischman A.J. Cocaine induced secretion of ACTH, beta-endorphin, and corticosterone. Peptides. 1987;8:819–822. doi: 10.1016/0196-9781(87)90065-9. [DOI] [PubMed] [Google Scholar]
- 32.Daunais J.B., Roberts D.C., McGinty J.F. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport. 1993;4:543–546. doi: 10.1097/00001756-199305000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Daunais J.B., McGinty J.F. Cocaine binges differentially alter striatal preprodynorphin and zif/268 mRNAs. Brain Res. Mol. Brain Res. 1995;29:201–210. doi: 10.1016/0169-328X(94)00246-B. [DOI] [PubMed] [Google Scholar]
- 34.Hurd Y.L., Brown E.E., Finlay J.M., Fibiger H.C., Gerfen C.R. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Brain Res. Mol. Brain Res. 1992;13:165–170. doi: 10.1016/0169-328X(92)90058-J. [DOI] [PubMed] [Google Scholar]
- 35.Sivam S.P. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J. Pharmacol. Exp. Ther. 1989;250:818–824. [PubMed] [Google Scholar]
- 36.Smiley P.L., Johnson M., Bush L., Gibb J.W., Hanson G.R. Effects of Cocaine on Extrapyramidal and Limbic Dynorphin Systems. J. Pharmacol. Exp. Ther. 1990;253:938–943. [PubMed] [Google Scholar]
- 37.Hammer R.P. Cocaine alters opiate receptor binding in critical brain reward regions. Synapse. 1989;3:55–60. doi: 10.1002/syn.890030108. [DOI] [PubMed] [Google Scholar]
- 38.Izenwasser S., Heller B., Cox B.M. Continuous cocaine administration enhances mu- but not delta-opioid receptor-mediated inhibition of adenylyl cyclase activity in nucleus accumbens. Eur. J. Pharmacol. 1996;297:187–191. doi: 10.1016/0014-2999(95)00828-4. [DOI] [PubMed] [Google Scholar]
- 39.Schroeder J.A., Niculescu M., Unterwald E.M. Cocaine alters mu but not delta or kappa opioid receptor-stimulated in situ [35S]GTPgammaS binding in rat brain. Synapse. 2003;47:26–32. doi: 10.1002/syn.10148. [DOI] [PubMed] [Google Scholar]
- 40.Unterwald E.M., Rubenfeld J.M., Kreek M.J. Repeated cocaine administration upregulates κ and µ, but not δ, opioid receptors. Neuroreport. 1994;5:1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- 41.Unterwald E.M., Cox B.M., Kreek M.J., Cote T.E., Izenwasser S. Chronic repeated cocaine administration alters basal and opioid-regulated adenylyl cyclase activity. Synapse. 1993;15:33–38. doi: 10.1002/syn.890150104. [DOI] [PubMed] [Google Scholar]
- 42.Heidbreder C.A., Goldberg S.R., Shippenberg T.S. The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res. 1993;616:335–338. doi: 10.1016/0006-8993(93)90228-F. [DOI] [PubMed] [Google Scholar]
- 43.Shippenberg T.S., LeFevour A., Heidbreder C. kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J. Pharmacol. Exp. Ther. 1996;276:545–554. [PubMed] [Google Scholar]
- 44.Shippenberg T.S., Rea W. Sensitization to the behavioral effects of cocaine: Modulation by dynorphin and kappa-opioid receptor agonists. Pharmacol. Biochem. Behav. 1997;57:449–455. doi: 10.1016/S0091-3057(96)00450-9. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Butelman E.R., Schlussman S.D., Ho A., Kreek M.J. Effect of the endogenous kappa opioid agonist dynorphin A(1-17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology. 2004;172:422–429. doi: 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]
- 46.Mathon D.S., Lesscher H.M., Gerrits M.A., Kamal A., Pintar J.E., Schuller A.G., Spruijt B.M., Burbach J.P., Smidt M.P., van Ree J.M., et al. Increased GABAergic input to ventral tegmental area dopaminergic neurons associated with decreased cocaine reinforcement in mu-opioid receptor knockout mice. Neuroscience. 2005;130:359–367. doi: 10.1016/j.neuroscience.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Yoo J.H., Yang E.M., Lee S.Y., Loh H.H., Ho I.K., Jang C.G. Differential effects of morphine and cocaine on locomotor activity and sensitization in mu-opioid receptor knockout mice. Neurosci. Lett. 2003;344:37–40. doi: 10.1016/S0304-3940(03)00410-5. [DOI] [PubMed] [Google Scholar]
- 48.Becker A., Grecksch G., Kraus J., Loh H.H., Schroeder H., Hollt V. Rewarding effects of ethanol and cocaine in µ opioid receptor-deficient mice. Naunyn Schmiedebergs. Arch. Pharmacol. 2002;365:296–302. doi: 10.1007/s00210-002-0533-2. [DOI] [PubMed] [Google Scholar]
- 49.Hall F.S., Goeb M., Li X.F., Sora I., Uhl G.R. mu-Opioid receptor knockout mice display reduced cocaine conditioned place preference but enhanced sensitization of cocaine-induced locomotion. Brain Res. 2004;121:123–130. doi: 10.1016/j.molbrainres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 50.Contarino A., Kitchener P., Vallée M., Papaleo F., Piazza P.V. CRF1 receptor-deficiency increases cocaine reward. Neuropharmacology. 2017;117:41–48. doi: 10.1016/j.neuropharm.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 51.Rademacher D.J., Steinpreis R.E. Effects of the selective mu(1)-opioid recep-tor antagonist, naloxonazine, on cocaine-induced conditioned place preferenceand locomotor behavior in rats. Neurosci. Lett. 2002;332:159–162. doi: 10.1016/S0304-3940(02)00950-3. [DOI] [PubMed] [Google Scholar]
- 52.Ward H.G., Simansky K.J. Chronic prevention of μ-opioid receptor (MOR) G-protein coupling in the pontine parabrachial nucleus persistently decreases consumption of standard but not palatable food. Psychopharmacology. 2006;187:435–446. doi: 10.1007/s00213-006-0463-7. [DOI] [PubMed] [Google Scholar]
- 53.Menkens K., Bilsky E.J., Wild K.D., Portoghese P.S., Reid L.D., Porreca F. Cocaine place preference is blocked by the delta-opioid receptor antagonist, naltrindole. Eur. J. Pharmacol. 1992;219:345–346. doi: 10.1016/0014-2999(92)90319-Y. [DOI] [PubMed] [Google Scholar]
- 54.Kotlinska J.H., Gibula-Bruzda E., Pachuta A., Kunce D., Witkowska E., Chung N.N., Schiller P.W., Izdebski J. Influence of new deltorphin analogues on reinstatement of cocaine-induced conditioned place preference in rats. Behav. Pharmacol. 2010;21:638–648. doi: 10.1097/FBP.0b013e32833e7e97. [DOI] [PubMed] [Google Scholar]
- 55.Reid L.D., Glick S.D., Menkens K.A., French E.D., Bilsky E.J., Porreca F. Cocaine self-administration and naltrindole, a delta-selective opioid antagonist. Neuroreport. 1995;6:1409–1412. doi: 10.1097/00001756-199507100-00012. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki T., Mori T., Tsuji M., Misawa M., Nagase H. The role of delta-opioidreceptor subtypes in cocaine- and methamphetamine-induced place prefer-ences. Life Sci. 1994;55:L339–L344. doi: 10.1016/0024-3205(94)00774-8. [DOI] [PubMed] [Google Scholar]
- 57.Jones D.N., Bowen W.D., Portoghese P.S., Holtzman S.G. Delta-opioid receptor antagonists attenuate motor activity induced by amphetamine but not cocaine. Eur. J. Pharmacol. 1993;249:167–177. doi: 10.1016/0014-2999(93)90429-L. [DOI] [PubMed] [Google Scholar]
- 58.de Vries T.J., Babovic-Vuksanovic D., Elmer G., Shippenberg T.S. Lack of involvement of delta-opioid receptors in mediating the rewarding effects of cocaine. Psychopharmacology. 1995. 120:442–448. doi: 10.1007/BF02245816. [DOI] [PubMed] [Google Scholar]
- 59.Heidbreder C., Shoaib M., Shippenberg T.S. Differential role of delta-opioid receptors in the development and expression of behavioral sensitization to cocaine. Eur. J. Pharmacol. 1996;298:207–216. doi: 10.1016/0014-2999(95)00815-2. [DOI] [PubMed] [Google Scholar]
- 60.Shippenberg T.S., Heidbreder C. Sensitization to the conditioned rewarding effects of cocaine: Pharmacological and temporal characteristics. J. Pharmacol. Exp. Ther. 1995;273:808–815. [PubMed] [Google Scholar]
- 61.Berrendero F., Kieffer B.L., Maldonado R. Attenuation of nicotine-inducedantinociception, rewarding effects, and dependence in mu-opioid receptorknock-out mice. J. Neurosci. 2002;22:10935–10940. doi: 10.1523/JNEUROSCI.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berrendero F., Mendizabal V., Robledo P., Galeote L., Bilkei-Gorzo A., Zimmer A., Maldonado R. Nicotine-induced antinociception, rewarding effects, andphysical dependence are decreased in mice lacking the preproenkephalin gene. J. Neurosci. 2005;25:1103–1112. doi: 10.1523/JNEUROSCI.3008-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trigo J.M., Zimmer A., Maldonado R. Nicotine anxiogenic and rewarding effects are decreased in mice lacking beta-endorphin. Neuropharmacology. 2009;56:1147–1153. doi: 10.1016/j.neuropharm.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galeote L., Berrendero F., Bura S.A., Zimmer A., Maldonado R. Prodynorphin gene disruption increases the sensitivity to nicotine self-administration in mice. Int. J. Neuropsychopharmacol. 2009;12:615–625. doi: 10.1017/S1461145708009450. [DOI] [PubMed] [Google Scholar]
- 65.Conte-Devolx B., Oliver C., Giraud P., Gillioz P., Castanas E., Lissitzky J.C., Boudouresque F., Millet Y. Effect of nicotine on in vivo secretion of melanocorticotropic hormones in the rat. Life Sci. 1981;28:1067–1073. doi: 10.1016/0024-3205(81)90755-4. [DOI] [PubMed] [Google Scholar]
- 66.Backon J. Negative correlation of cigarette smoking and dysmenorrhea: Reduced prostaglandin synthesis due to beta-endorphin, nicotine, or acroleinantagonism. Med. Hypotheses. 1989;28:213–214. doi: 10.1016/0306-9877(89)90054-6. [DOI] [PubMed] [Google Scholar]
- 67.del Arbol J.L., Munoz J.R., Ojeda L., Cascales A.L., Irles J.R., Miranda M.T., RuizRequena M.E., Aguirre J.C. Plasma concentrations of beta-endorphinin smokers who consume different numbers of cigarettes per day. Pharmacol. Biochem. Behav. 2000;67:25–28. doi: 10.1016/S0091-3057(00)00291-4. [DOI] [PubMed] [Google Scholar]
- 68.Rasmussen N.A., Farr L.A. Beta-endorphin response to an acute pain stimulus. J. Neurosci. Methods. 2009;177:285–288. doi: 10.1016/j.jneumeth.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 69.Spaziante R., Merola B., Colao A., Gargiulo G., Cafiero T., Irace C., Rossi E., Oliver C., Lombardi G., Mazzarella B., et al. Beta-endorphin concentrations both in plasma and in cerebrospinal fluid in response to acute painful stimuli. J. Neurosurg. Sci. 1990;34:99–106. [PubMed] [Google Scholar]
- 70.Wewers M.E., Dhatt R.K., Snively T.A., Tejwani G.A. The effect of chronic administration of nicotine on antinociception, opioid receptor binding and met-enkelphalin levels in rats. Brain Res. 1999;822:107–113. doi: 10.1016/S0006-8993(99)01095-1. [DOI] [PubMed] [Google Scholar]
- 71.Dhatt R.K., Gudehithlu K.P., Wemlinger T.A., Tejwani G.A., Neff N.H., Hadji-constantinou M. Preproenkephalin mRNA and methionine-enkephalin content are increased in mouse striatum after treatment with nicotine. J. Neurochem. 1995;64:1878–1883. doi: 10.1046/j.1471-4159.1995.64041878.x. [DOI] [PubMed] [Google Scholar]
- 72.Houdi A.A., Dasgupta R., Kindy M.S. Effect of nicotine use and withdrawal on brain preproenkephalin A mRNA. Brain Res. 1998;799:257–263. doi: 10.1016/S0006-8993(98)00454-5. [DOI] [PubMed] [Google Scholar]
- 73.Balerio G.N., Aso E., Berrendero F., Murtra P., Maldonado R. Delta9-tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur. J. Neurosci. 2004;20:2737–2748. doi: 10.1111/j.1460-9568.2004.03714.x. [DOI] [PubMed] [Google Scholar]
- 74.Krishnan-Sarin S., Rosen M.I., O’Malley S.S. Naloxone challenge in smokers. Preliminary evidence of an opioid component in nicotine dependence. Arch. Gen. Psychiatry. 1999;56:663–668. doi: 10.1001/archpsyc.56.7.663. [DOI] [PubMed] [Google Scholar]
- 75.Maldonado R., Valverde O., Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 76.Trigo J.M., Martin-García E., Berrendero F., Robledo P., Maldonado R. The endogenous opioid system: A common substrate in drug addiction. Drug Alcohol Depend. 2010;108:183–194. doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 77.Viganò D., Rubino T., Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol. Biochem. Behav. 2005;81:360–368. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 78.Pickel V.M., Chan J., Kash T.L., Rodriguez J.J., MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–112. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 79.Salio C., Fischer J., Franzoni M.F., Mackie K., Kaneko T., Conrath M. CB1-cannabinoid and mu opioid receptor colocalization on postsynaptic target in the rat dorsal horn. Neuroreport. 2001;12:3689–3692. doi: 10.1097/00001756-200112040-00017. [DOI] [PubMed] [Google Scholar]
- 80.Castañé A., Robledo P., Matifas A., Kieffer B.L., Maldonado R. Cannabinoid withdrawal syndrome is reduced in double mu and delta opioid receptor knock-out mice. Eur. J. Neurosci. 2003;17:155–159. doi: 10.1046/j.1460-9568.2003.02409.x. [DOI] [PubMed] [Google Scholar]
- 81.Ghozland S., Matthes H.W., Simonin F., Filliol D., Kieffer B.L., Maldonado R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J. Neurosci. 2002;22:1146–1154. doi: 10.1523/JNEUROSCI.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Justinova Z., Tanda G., Munzar P., Goldberg S.R. The opioid antagonist naltrexone reduces the reinforcing effects of Delta 9 tetrahydrocannabinol (THC)in squirrel monkeys. Psychopharmacology. 2004;173:186–194. doi: 10.1007/s00213-003-1693-6. [DOI] [PubMed] [Google Scholar]
- 83.Navarro M., Carrera M.R., Fratta W., Valverde O., Cossu G., Fattore L., Chowen J.A., Gomez R., del Arco I., Villanua M.A., et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J. Neurosci. 2001;21:5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spano M.S., Fattore L., Cossu G., Deiana S., Fadda P., Fratta W. CB1 receptor agonist and heroin, but not cocaine, reinstate cannabinoid-seeking behaviour in the rat. Br. J. Pharmacol. 2004;143:343–350. doi: 10.1038/sj.bjp.0705932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Navarro M., Chowen J., Rocío A., Carrera M., del Arco I., Villanúa M.A., Martin Y., Roberts A.J., Koob G.F., de Fonseca F. R CB1 cannabinoid receptor antagonist-induced opiate withdrawal in morphine-dependent rats. Neuroreport. 1998;9:3397–3402. doi: 10.1097/00001756-199810260-00012. [DOI] [PubMed] [Google Scholar]
- 86.Kastin A.J., Olson R.D., Ehrensing R.H., Berzas M.C., Schally A.V., Coy D.H. MIF-I’s differential actions as an opiate antagonist. Pharmacol. Biochem. Behav. 1979;11:721–723. doi: 10.1016/0091-3057(79)90270-3. [DOI] [PubMed] [Google Scholar]
- 87.Erchegyi J., Kastin A.J., Zadina J.E. Isolation of a novel tetrapeptide with opiate and antiopiate activity from human brain cortex: Tyr-Pro-Trp-Gly-NH2 (Tyr-W-MIF-1) Peptides. 1992;13:623–631. doi: 10.1016/0196-9781(92)90165-Y. [DOI] [PubMed] [Google Scholar]
- 88.Galina Z.H., Kastin A.J. Existence of antiopiate systems as illustrated by MIF-I/Tyr-MIF-I. Minireview. Life Sci. 1986;39:2153–2159. doi: 10.1016/0024-3205(86)90391-7. [DOI] [PubMed] [Google Scholar]
- 89.Zadina J.E., Kastin A.J. Interactions between the antiopiate Tyr-MIF-1 and the mu opiate morphiceptin at their respective binding sites in brain. Peptides. 1985;6:965–970. doi: 10.1016/0196-9781(85)90329-8. [DOI] [PubMed] [Google Scholar]
- 90.Zadina J.E., Kastin A.J., Kersh D., Wyatt A. Tyr-MIF-1 and hemorphin can act as opiate agonists as well as antagonists in the guinea pig ileum. Life Sci. 1992;51:869–885. doi: 10.1016/0024-3205(92)90615-V. [DOI] [PubMed] [Google Scholar]
- 91.Hackler L., Kastin A.J., Zadina J.E. Isolation of a novel peptide with a unique binding profile from human brain cortex: Tyr-K-MIF-1 (Tyr-Pro-Lys-Gly-NH2) Peptides. 1994;15:945–950. doi: 10.1016/0196-9781(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 92.Malin D.R., Zadina J.E., Lake J.R., Rogillio R.B., Leyva J.E., Benson T.M., Corriere L.S., Handunge B.P., Kastin A.J. Tyr-MIF-I precipitates abstinence syndrome in morphine- dependent rats. Brain Res. 1993;610:169–171. doi: 10.1016/0006-8993(93)91233-I. [DOI] [PubMed] [Google Scholar]
- 93.Reed G.W., Olson G.A., Olson R.D. The Tyr-MIF-1 family of peptides. Neurosci. Biobehav. Rev. 1994;18:519–525. doi: 10.1016/0149-7634(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 94.Zadina J.E., Kastin A.J. Interaction of Tyr-MIF-I at opiate receptor sites. Pharmacol. Biochem. Behav. 1986;25:1303–1305. doi: 10.1016/0091-3057(86)90126-7. [DOI] [PubMed] [Google Scholar]
- 95.Kastin A.J., Stephens E., Ehrensing R.H., Fischman A.J. Tyr-MIF- 1 acts as an opiate antagonist in the tail-flick test. Pharmucol. Biochem. Behav. 1984;21:937–941. doi: 10.1016/S0091-3057(84)80076-3. [DOI] [PubMed] [Google Scholar]
- 96.Kastin A.J., Stephens E., Zadina J.E., Coy D.H., Fischman A.J. Tyr-MIF-I, identified in brain tissue, and its analogs are active in two models of antinociception. Pharmacol. Biochem. Behav. 1985;23:1045–1049. doi: 10.1016/0091-3057(85)90112-1. [DOI] [PubMed] [Google Scholar]
- 97.Kavaliers M., Hirst M. Inhibitory influences of MIF-1 (PLG) and Tyr-MIF-1 (YPLG) on aggression and defeat-induced analgesia in mice. Peptides. 1986;7:1007–1010. doi: 10.1016/0196-9781(86)90129-4. [DOI] [PubMed] [Google Scholar]
- 98.Galina Z.H., Kastin A.J. MIF-1 antagonizes warm-, but not cold-water stress-induced analgesia: Dissociation from immobility. Peptides. 1985;6:1109–1112. doi: 10.1016/0196-9781(85)90435-8. [DOI] [PubMed] [Google Scholar]
- 99.Zadina J.E., Kastin A.J., Ge L.J., Gulden H., Bungart K.J. Chronic, but not acute, administration of morphine alters antiopiate (Tyr-MIF-1) binding sites in rat brain. Life Sci. 1989;44:555–561. doi: 10.1016/0024-3205(89)90617-6. [DOI] [PubMed] [Google Scholar]
- 100.Kenakin T. Agonists, partial agonists, antagonists, inverse agonists and agonist/antagonists? Trends Pharmacol. Sci. 1987;8:423–426. doi: 10.1016/0165-6147(87)90229-X. [DOI] [Google Scholar]
- 101.Pan W., Kastin A.J. From MIF-1 to endomorphin: The Tyr-MIF-1 family of peptides. Peptides. 2007;28:2411–2434. doi: 10.1016/j.peptides.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 102.Ehrensing R.H., Kastin A.J., Michell G.F. Antagonism of morphine analgesia by prolyl-leucyl-glycinamide (MIF-1) in humans. Pharmacol. Biochem. Behav. 1984;21:975–978. doi: 10.1016/S0091-3057(84)80083-0. [DOI] [PubMed] [Google Scholar]
- 103.Kavaliers M., Innes D.G. Sex differences in the effects of Tyr-MIF-1 on morphine- and stress-induced analgesia. Peptides. 1992;13:1295–1297. doi: 10.1016/0196-9781(92)90038-5. [DOI] [PubMed] [Google Scholar]
- 104.Tesky G.C., Kavaliers M. Prolyl-leucyl-glycin-amide reduces aggression and blocks defeat-induced opioid analgesia in mice. Peptides. 1985;6:165–167. doi: 10.1016/0196-9781(85)90034-8. [DOI] [PubMed] [Google Scholar]
- 105.Ehrensing R.H., Michell G.F., Kastin A.J. Similar antagonism of morphine analgesia by MIF-1 and naloxone in Carassius auratus. Pharmacol. Biochem. Behav. 1982;17:757–761. doi: 10.1016/0091-3057(82)90358-6. [DOI] [PubMed] [Google Scholar]
- 106.Yehuda S., Kastin A.J., Coy D.H. Antagonistic actions of MIF-I on the hypothermia and hypomotility induced by beta-endorphin or morphine. Int. J. Neurosci. 1980;11:317–320. doi: 10.3109/00207458009147596. [DOI] [PubMed] [Google Scholar]
- 107.Yan Y.X., Hu W.L., Cong B., Ma C.L., Ni Z.Y., Niu Z.Q., Yu L. Expressions of μ opioid receptor and CCK receptor in rat primary hippocampal neurons and effect of chronic morphine exposure on them. J. Fourth MilMed. Univ. 2007;28:1214–1217. [Google Scholar]
- 108.Han J.S. Cholecystokinin octapeptide (CCK-8): A negative feedback control mechanism for opioid analgesia. Prog. Brain Res. 1995;105:263–271. doi: 10.1016/s0079-6123(08)63303-8. [DOI] [PubMed] [Google Scholar]
- 109.Han N.L., Luo F., Bian Z.P., Han J.S. Synergistic effect of cholecystokinin octapeptide and angiotensin II in reversal of morphine induced analgesia in rats. Pain. 2000;85:465–469. doi: 10.1016/S0304-3959(99)00294-8. [DOI] [PubMed] [Google Scholar]
- 110.Dourish C.T., O’Neill M.F., Coughlan J., Kitchener S.J., Hawley D., Iversen S.D. The selective CCK-B receptor antagonist L-365,260 enhances morphine analgesia and prevents morphine tolerance in the rat. Eur. J. Pharmacol. 1990;176:35–44. doi: 10.1016/0014-2999(90)90129-T. [DOI] [PubMed] [Google Scholar]
- 111.Magnuson D.S., Sullivan A.F., Simonnet G., Roques B.P., Dickenson A.H. Differential interactions of cholecystokinin and FLFQPQRF-NH2 with mu and delta opioid antinociception in the rat spinal cord. Neuropeptides. 1990;16:213–218. doi: 10.1016/0143-4179(90)90065-7. [DOI] [PubMed] [Google Scholar]
- 112.Watanabe H., Nakayama D., Ito K., Watanabe C., Mizoguchi H., Fujimura T., Murayama K., Kawamura S., Sato T., Sakurada C., et al. Tyr-W-MIF-1 analog containing D-Pro2 acts as a selective mu2-opioid receptor antagonist in the mouse. J. Pharmacol. Exp. Ther. 2005;312:1075–1081. doi: 10.1124/jpet.104.075697. [DOI] [PubMed] [Google Scholar]
- 113.Hebb A.L., Poulin J.F., Roach S.P., Zacharko R.M., Drolet G. Cholecystokin in andendogenous opioid peptides: Interactive influence on pain, cognition, and emotion. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1225–1238. doi: 10.1016/j.pnpbp.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 114.Calo’ G., Guerrini R., Rizzi A., Salvadori S., Regoli D. Pharmacology of nociceptin and its receptor: A novel therapeutic target. Br. J. Pharmacol. 2000;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tian J.H., Xu W., Fang Y., Mogil J.S., Grisel J.E., Grandy D.K., Han J.S. Bidirectional modulatory effect of orphanin FQ on morphine-induced analgesia: Antagonism in brain and potentiation in spinal cord of the rat. Br. J. Pharmacol. 1997;120:676–680. doi: 10.1038/sj.bjp.0700942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Byford A.J., Anderson A., Jones P.S., Palin R., Houghton A.K. The hypnotic, electroencephalographic, and antinociceptive properties of nonpeptide ORL1 receptor agonists after intravenous injection in rodents. Anesth. Analg. 2007;104:174–179. doi: 10.1213/01.ane.0000250403.88649.51. [DOI] [PubMed] [Google Scholar]
- 117.Murphy N.P., Lee Y., Maidment N.T. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/S0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- 118.Ciccocioppo R., Panocka I., Polidori C., Regoli D., Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology. 1999;141:220–224. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- 119.Cremeans C.M., Gruley E., Kyle D.J., Ko M.C. Roles of μ-opioid receptors and nociceptin/orphanin FQ peptide receptors in buprenorphine-induced physiological responses in primates. J. Pharmacol. Exp. Ther. 2012;343:72–81. doi: 10.1124/jpet.112.194308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Devine D.P., Reinscheid R.K., Monsma F.J., Jr., Civelli O., Akil H. The novel neuropeptide orphanin FQ fails to produce conditioned place preference or aversion. Brain Res. 1996;727:225–229. doi: 10.1016/0006-8993(96)00476-3. [DOI] [PubMed] [Google Scholar]
- 121.Cesselin F. Opioid and anti-opioid peptides. Fundam. Clin. Pharmacol. 1995;9:409–433. doi: 10.1111/j.1472-8206.1995.tb00517.x. [DOI] [PubMed] [Google Scholar]
- 122.Rothman R.B. A review of the role of anti-opioid peptides in morphine tolerance and dependence. Synapse. 1992;12:129–138. doi: 10.1002/syn.890120206. [DOI] [PubMed] [Google Scholar]
- 123.Panula P., Kalso E., Nieminen M., Kontinen V.K., Brandt A., Pertovaara A. Neuropeptide FF and modulation of pain. Brain Res. 1999;848:191–196. doi: 10.1016/S0006-8993(99)02044-2. [DOI] [PubMed] [Google Scholar]
- 124.Bonini J.A., Jones K.A., Adham N., Forray C., Artymyshyn R., Durkin M.M., Smith K.E., Tamm J.A., Boteju L.W., Lakhlani P.P., et al. Identification and characterization of two G protein-coupled receptors for neuropeptide FF. J. Biol. Chem. 2000;275:39324–39331. doi: 10.1074/jbc.M004385200. [DOI] [PubMed] [Google Scholar]
- 125.Morgan M.M., Grisel J.E., Robbins C.S., Grandy D.K. Antinociception mediated by the periaqueductal gray is attenuated by orphanin FQ. Neuroreport. 1997;8:3431–3434. doi: 10.1097/00001756-199711100-00003. [DOI] [PubMed] [Google Scholar]
- 126.Wang J.Q., Fibuch E.E., Sakurada S., Han J.S. Biologically Active Peptides. Elsevier; Amsterdam, The Netherlands: 2006. Anti-opioid peptides; pp. 1345–1350. Chapter 187. [Google Scholar]
- 127.Zamfirova R., Bocheva A., Dobrinova Y., Todorov S. Study on the antinociceptive action of Tyr-K-MIF-1, a peptide from the MIF family. Auton. Autacoid. Pharmacol. 2007;27:93–98. doi: 10.1111/j.1474-8673.2007.00394.x. [DOI] [PubMed] [Google Scholar]
- 128.Million M., Fioramonti J., Bueno L. Oral administration of Tyr-MIF-1 stimulates gastric emptying and gastrointestinal motility in rodents. Peptides. 1992;13:469–474. doi: 10.1016/0196-9781(92)90076-F. [DOI] [PubMed] [Google Scholar]
- 129.Million M., Fioramonti J., Bueno L. Central administration of Tyr-MIF-1 stimulates gastrointestinal motility in rats: Evidence for the involvement of dopamine, sigma and CCK receptors. Neuropeptides. 1994;26:77–85. doi: 10.1016/0143-4179(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 130.Dufresne M., Seva C., Fourmy D. Cholecystokinin and gastrin receptors. Physiol. Rev. 2006;86:805–847. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- 131.Ma K.T., Si J.Q., Zhang Z.Q., Zhao L., Fan P., Jin J.L., Li X.Z., Zhu L. Modulatory effect of CCK-8S on GABA-induced depolarization from rat dorsal root ganglion. Brain Res. 2006;1121:66–75. doi: 10.1016/j.brainres.2006.08.094. [DOI] [PubMed] [Google Scholar]
- 132.Rehfeld J.F., Friis-Hansen L., Goetze J.P., Hansen T.V. The biology of cholecystokinin and gastrin peptides. Curr. Top. Med. Chem. 2007;7:1154–1165. doi: 10.2174/156802607780960483. [DOI] [PubMed] [Google Scholar]
- 133.Dauge V., Sebret A., Beslot F., Matsui T., Roques B.P. Behavioral profile of CCK2 receptor-deficient mice. Neuropsychopharmacology. 2001;25:690–698. doi: 10.1016/S0893-133X(01)00291-3. [DOI] [PubMed] [Google Scholar]
- 134.Moran T.H., Schwartz G.J. Neurobiology of cholecystokinin. Crit. Rev. Neurobiol. 1994;9:1–28. [PubMed] [Google Scholar]
- 135.Pommier B., Beslot F., Simon A., Pophillat M., Matsui T., Dauge V., Roques B.P., Noble F. Deletion of CCK2 receptor in mice results in an upregulation of the endogenous opioid system. J. Neurosci. 2002;22:2005–2011. doi: 10.1523/JNEUROSCI.22-05-02005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang X.J., Fan S.G., Ren M.F., Han J.S. Cholecystokinin-8 suppressed 3H-etorphine binding to rat brain opiate receptors. Life Sci. 1989;45:117–123. doi: 10.1016/0024-3205(89)90285-3. [DOI] [PubMed] [Google Scholar]
- 137.Hill D.R., Campbell N.J., Shaw T.M., Woodruff G.N. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J. Neurosci. 1987;7:2967–2976. doi: 10.1523/JNEUROSCI.07-09-02967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moran T.H., Robinson P.H., Goldrich M.S., McHugh P.R. Two brain cholecystokinin receptors: Implications for behavioral actions. Brain Res. 1986;362:175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- 139.Crawley J.N. Modulation of mesolimbic dopaminergic behaviors by cholecystokinin. Ann. N. Y. Acad Sci. 1988;537:380–396. doi: 10.1111/j.1749-6632.1988.tb42121.x. [DOI] [PubMed] [Google Scholar]
- 140.Gaviraghi G., Feriani A., Marien M., Trist D. Central cholecystokinin receptors: Opportunity for drug discovery. Pharmacochem. Libr. 1992;18:345–365. [Google Scholar]
- 141.Faris P.L., Komisaruk B.R., Watkins L.R., Mayer D.J. Evidence for the neuropeptide cholecystokinin as an antagonist of opiate analgesia. Science. 1983;219:310–312. doi: 10.1126/science.6294831. [DOI] [PubMed] [Google Scholar]
- 142.Benoliel J.J., Mauborgne A., Bourgoin S., Legrand J.C., Hamon M., Cesselin F. Opioid control of the in vitro release of cholecystokinin-like material from the rat substantia nigra. J. Neurochem. 1992;58:916–922. doi: 10.1111/j.1471-4159.1992.tb09344.x. [DOI] [PubMed] [Google Scholar]
- 143.You Z.B., Tzschentke T.M., Brodin E., Wise R.A. Electrical stimulation of the prefrontal cortex increases cholecystokinin, glutamate, and dopamine release in the nucleus accumbens: An in vivo microdialysis study in freely moving rats. J. Neurosci. 1998;18:6492–6500. doi: 10.1523/JNEUROSCI.18-16-06492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rodríguez R.E., Sacristán M.P. In vivo release of CCK-8 from the dorsal horn of the rat: Inhibition by DAGOL. FEBS Lett. 1989;250:215–217. doi: 10.1016/0014-5793(89)80723-9. [DOI] [PubMed] [Google Scholar]
- 145.Stanfa L.C., Dickenson A.H. Cholecystokinin as a factor in the enhanced potency of spinal morphine following carrageenin inflammation. Br. J. Pharmacol. 1993;108:967–973. doi: 10.1111/j.1476-5381.1993.tb13493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang X.J., Han J.S. Modification by cholecystokinin octapeptide of the binding of mu-, delta-, and kappa-opioid receptors. J. Neurochem. 1990;55:1379–1382. doi: 10.1111/j.1471-4159.1990.tb03149.x. [DOI] [PubMed] [Google Scholar]
- 147.Verge V.M.K., Wiesenfeld-Hallin Z., Hokfelt T. Cholecystokinin in mammalian primary sensory neurons and spinal cord: In situ hybridization studies in rat and monkey. Eur. J. Neurosci. 1993;5:240–250. doi: 10.1111/j.1460-9568.1993.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 148.Itoh S., Katsuura G., Maeda Y. Caerulein and cholecystokinin suppress beta-endorphin- induced analgesia in the rat. Eur. J. Pharmacol. 1982;80:421–425. doi: 10.1016/0014-2999(82)90089-9. [DOI] [PubMed] [Google Scholar]
- 149.Sebret A., Léna I., Crété D., Matsui T., Roques B.P., Daugé V. Rat hippocampal neurons are critically involved in physiological improvement of memory processes induced by cholecystokinin-B receptor stimulation. J. Neurosci. 1999;19:7230–7237. doi: 10.1523/JNEUROSCI.19-16-07230.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mitchell J.M., Bergren L.J., Chen K.S., Fields H.L. Cholecystokinin is necessary for the expression of morphine conditioned place preference. Pharmacol. Biochem. Behav. 2006;85:787–795. doi: 10.1016/j.pbb.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 151.Alttoa A., Harro J. Effect of CCK1 and CCK2 receptor blockade on amphetamine-stimulated exploratory behavior and sensitization to amphetamine. Eur. Neuropsychopharmacol. 2004;14:324–331. doi: 10.1016/j.euroneuro.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 152.Lu L., Huang M., Ma L., Li J. Different role of cholecystokinin (CCK)-A and CCK-B receptors in relapse to morphine dependence in rats. Behav. Brain Res. 2001;120:105–110. doi: 10.1016/S0166-4328(00)00361-2. [DOI] [PubMed] [Google Scholar]
- 153.Lu L., Zhang B., Liu Z.Y., Zhang Z.Y. Reactivation of cocaine conditioned place preference induced by stress is reversed by cholecystokinin-B receptors antagonist in rats. Brain. Res. 2002;954:132–140. doi: 10.1016/S0006-8993(02)03359-0. [DOI] [PubMed] [Google Scholar]
- 154.Wen D., Ma C.L., Cong B., Zhang Y.J., Yang S.C., Meng Y.X., Yu F., Ni Z.Y., Li S.J. Effects of CCK-8 and its receptor antagonists given intracerebroventricularly on withdrawal symptom of morphine dependent rats. Chin. Pharmacol. Bull. 2011;27:1368–1373. [Google Scholar]
- 155.Crespi F. The role of cholecystokinin (CCK), CCK-A or CCK-B receptor antagonists in the spontaneous preference for drugs of abuse (alcohol or cocaine) in naive rats. Methods Find. Exp. Clin. Pharmacol. 1998;20:679–697. doi: 10.1358/mf.1998.20.8.487502. [DOI] [PubMed] [Google Scholar]
- 156.Crespi F., Corsi M., England T., Ratti E., Trist D.G., Gaviraghi G. Spontaneous preference for ethanol in naive rats is influenced by cholecystokinin A receptor antagonism. Alcohol. 1997;14:327–332. doi: 10.1016/S0741-8329(96)00179-6. [DOI] [PubMed] [Google Scholar]
- 157.Vasar E., Harro J., Pold A., Lang A. CCK receptors and anxiety in rats. In: Dourish C.T., Cooper S.J., Iversen S.D., Iversen. L.L., editors. Multiple Cholecystokinin Receptors in the CNS. Oxford University Press; Oxford, UK: 1992. pp. 143–148. [Google Scholar]
- 158.Kulkosky P.J., Clayborne Y.J., Sandoval S.L. Cholecystokinin and bombesin inhibit ethanol and food intake in rats selectively bred for ethanol sensitivity. Alcohol Clin. Exp. Res. 1993;17:545–551. doi: 10.1111/j.1530-0277.1993.tb00797.x. [DOI] [PubMed] [Google Scholar]
- 159.Noble F., Blommaert A., Fournie-Zaluski M.C., Roques B.P. A selective CCK-B receptor antagonist potentiates mu-, but not delta-opioid receptor-mediated antinociception in the formalin test. Eur. J. Pharmacol. 1995;273:145–151. doi: 10.1016/0014-2999(94)00688-4. [DOI] [PubMed] [Google Scholar]
- 160.Wiesenfeld-Hallin Z., Xu X.-J., Hughes J., Horwell D.C., Ho¨kfelt T. PD134308, a selective antagonist of cholecystokinin type-B receptor, enhances the analgesic effect of morphine and synergistically interacts with intrathecal galanin to depress spinal nociceptive reflexes. Proc. Natl. Acad. Sci. USA. 1990;87:7105–7109. doi: 10.1073/pnas.87.18.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou Y., Sun Y.H., Zhang Z.W., Han J.S. Increased release of immunoreactive cholecystokinin octapeptide by morphine and potentiation of m-opioid analgesia by CCKB receptor antagonist L365,260 in rat spinal cord. Eur. J. Pharmacol. 1993;234:147–154. doi: 10.1016/0014-2999(93)90948-H. [DOI] [PubMed] [Google Scholar]
- 162.Lambert D.G. The nociceptin/orphanin FQ receptor: A target with broad therapeutic potential. Nat. Rev. Drug Discov. 2008;7:694–710. doi: 10.1038/nrd2572. [DOI] [PubMed] [Google Scholar]
- 163.Meunier J.C., Mollereau C., Toll L., Suaudeau C., Moisand C., Alvinerie P., Butour J.L., Guillemot J.C., Ferrara P., Monsarrat B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- 164.Reinscheid R.K., Nothacker H.P., Bourson A., Ardati A., Henningsen R.A., Bunzow J.R., Grandy D.K., Langen H., Monsma F.J., Jr., Civelli O. Orphanin FQ: A neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 165.Cox B.M., Christie M.J., Devi L., Toll L., Traynor J.R. Challenges for opioid receptor nomenclature: IUPHAR Review 9. Br. J. Pharmacol. 2015;172:317–323. doi: 10.1111/bph.12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gintzler A.R., Adapa I.D., Toll L., Medina V.M., Wang L. Modulation of enkephalin release by nociceptin (orphanin FQ) Eur. J. Pharmacol. 1997;325:29–34. doi: 10.1016/S0014-2999(97)00103-9. [DOI] [PubMed] [Google Scholar]
- 167.Mogil J.S., Pasternak G.W. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol. Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- 168.Mogil J.S., Grisel J.E., Zhangs G., Belknap J.K., Grandy D.K. Functional antagonism of mu-, delta- and kappa-opioid antinociception by orphanin FQ. Neurosci. Lett. 1996;214:131–134. doi: 10.1016/0304-3940(96)12917-7. [DOI] [PubMed] [Google Scholar]
- 169.Rizzi A., Cerlesi M.C., Ruzza C., Malfacini D., Ferrari F., Bianco S., Tommaso Costa T., Guerrini R., Claudio Trapella C., Calo G. Pharmacological characterization of cebranopadol a novel analgesic acting as mixed nociceptin/orphanin FQ and opioid receptor agonist. Pharmacol. Res. Perspect. 2016;4:e00247. doi: 10.1002/prp2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rizzi A., Ruzza C., Bianco S., Trapella C., Calo C. Antinociceptive action of NOP and opioid receptor agonists in the mouse orofacial formalin test. Peptides. 2017;94:71–77. doi: 10.1016/j.peptides.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 171.Ko M.C., Woods J.H., Fantegrossi W.E., Galuska C.M., Wichmann J., Prinssen E.P. Behavioral effects of a synthetic agonist selective for nociceptin/orphanin FQ peptide receptors in monkeys. Neuropsychopharmacology. 2009;34:2088–2096. doi: 10.1038/npp.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hu E., Calò G., Guerrini R., Ko M.C. Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112. Pain. 2010;148:107–113. doi: 10.1016/j.pain.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Xu X., Grass S., Hao J., Xu I.S., Wiesenfeld-Hallin Z. Nociceptin/orphanin FQ in spinal nociceptive mechanisms under normal and pathological conditions. Peptides. 2000;21:1031–1036. doi: 10.1016/S0196-9781(00)00234-5. [DOI] [PubMed] [Google Scholar]
- 174.Courteix C., Coudoré-Civiale M.A., Privat A.M., Pélissier T., Eschalier A., Fialip J. Evidence for an exclusive antinociceptive effect of nociceptin/orphanin FQ, an endogenous ligand for the ORL1 receptor, in two animal models of neuropathic pain. Pain. 2004;110:236–245. doi: 10.1016/j.pain.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 175.Linz K., Christoph T., Tzschentke T.M., Koch T., Schiene K., Gautrois M., Schröder W., Kögel B.Y., Beier H., Englberger W., et al. Cebranopadol: A novel potent analgesic nociceptin/orphanin FQ peptide and opioid receptor agonist. J. Pharmacol. Exp. Ther. 2014;349:535–548. doi: 10.1124/jpet.114.213694. [DOI] [PubMed] [Google Scholar]
- 176.Salat K., Furgala A., Salat R. Evaluation of cebranopadol, a dually acting nociceptin/orphanin FQ and opioid receptor agonist in mouse models of acute, tonic, and chemotherapy-induced neuropathic pain. Inflammopharmacology. 2018;26:361–374. doi: 10.1007/s10787-017-0405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tzschentke T.M., Linz L., Frosch S., Christoph T. Antihyperalgesic, Antiallodynic, and Antinociceptive Effects of Cebranopadol, a Novel Potent Nociceptin/Orphanin FQ and Opioid Receptor Agonist, after Peripheral and Central Administration in Rodent Models of Neuropathic Pain. Pain Pract. 2017;17:1032–1041. doi: 10.1111/papr.12558. [DOI] [PubMed] [Google Scholar]
- 178.Calo G., Lambert D.G. Nociceptin/orphanin FQ receptor ligands and translational challenges: Focus on cebranopadol as an innovative analgesic. Br. J. Anaesth. 2018;121:1105–1114. doi: 10.1016/j.bja.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tzschentke T.M., Kögel B.Y., Frosch S., Linz K. Limited potential of cebranopadol to produce opioid-type physical dependence in rodents. Addict. Biol. 2018;23:1010–1019. doi: 10.1111/adb.12550. [DOI] [PubMed] [Google Scholar]
- 180.Lutfy K., Hossain S.M., Khaliq I., Maidment N.T. Orphanin FQ/nociceptin attenuates the development of morphine tolerance in rats. Br. J. Pharmacol. 2001;134:529–534. doi: 10.1038/sj.bjp.0704279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lutfy K., Do T., Maidment N.T. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology. 2001;154:1–7. doi: 10.1007/s002130000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Murphy N.P., Ly H.T., Maidment N.T. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience. 1996;75:1–4. doi: 10.1016/0306-4522(96)00322-3. [DOI] [PubMed] [Google Scholar]
- 183.Murphy N.P., Maidment N.T. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J. Neurochem. 1999;73:179–186. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- 184.Rutten K., De Vry J., Bruckmann W., Tzschentke T.M. Pharmacological blockade or genetic knockout of the NOP receptor potentiates the rewarding effect of morphine in rats. Drug Alcohol Depend. 2011;114:253–256. doi: 10.1016/j.drugalcdep.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 185.Ciccocioppo R., Angeletti S., Sanna P.P., Weiss F., Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur. J. Pharmacol. 2000;404:153–159. doi: 10.1016/S0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- 186.Kotlinska J., Wichmann J., Legowska A., Rolka K., Silberring J. Orphanin FQ/nociceptin but not Ro 65-6570 inhibits the expression of cocaine-induced conditioned place preference. Behav. Pharmacol. 2002;13:229–235. doi: 10.1097/00008877-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 187.Kotlinska J., Rafalski P., Biala G., Dylag T., Rolka K., Silberring J. Nociceptin inhibits acquisition of amphetamine-induced place preference and sensitization to stereotypy in rats. Eur. J. Pharmacol. 2003;474:233–239. doi: 10.1016/S0014-2999(03)02081-8. [DOI] [PubMed] [Google Scholar]
- 188.Zhao R.J., Woo R.S., Jeong M.S., Shin B.S., Kim D.G., Kim K.W. Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport. 2003;14:2383–2385. doi: 10.1097/00001756-200312190-00019. [DOI] [PubMed] [Google Scholar]
- 189.Walker J.R., Spina M., Terenius L., Koob G.F. Nociceptin fails to affect heroin self-administration in the rat. Neuroreport. 1998;9:2243–2247. doi: 10.1097/00001756-199807130-00017. [DOI] [PubMed] [Google Scholar]
- 190.Ciccocioppo R., Economidou D., Fedeli A., Angeletti S., Weiss F., Heilig M., Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the anti-opioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology. 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kuzmin A., Kreek M.J., Bakalkin G., Liljequist S. The nociceptin/orphanin FQ receptor agonist Ro 646198 reduces alcohol self-administration and prevents relapse-like alcohol drinking. Neuropsychopharmacology. 2007;32:902–910. doi: 10.1038/sj.npp.1301169. [DOI] [PubMed] [Google Scholar]
- 192.Ciccocioppo R., de Guglielmo G., Hansson A.C., Ubaldi M., Kallupi M., Cruz M.T., Oleata C.S., Heilig M., Roberto M. Restraint stress alters nociceptin/orphanin FQ and CRF systems in the rat central amygdala: Significance for anxiety-like behaviors. J. Neurosci. 2014;34:363–372. doi: 10.1523/JNEUROSCI.2400-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.de Guglielmo G., Martin-Fardon R., Teshima K., Ciccocioppo R., Weiss F. MT-7716, a potent NOP receptor agonist, preferentially reduces ethanol seeking and reinforcement in post-dependent rats. Addict. Biol. 2015;20:643–651. doi: 10.1111/adb.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cippitelli A., Schoch J., Debevec G., Zaveri N.T., Toll L. A key role for the N/OFQ-NOP receptor system in modulating nicotine taking in a model of nicotine and alcohol co-administration. Sci. Rep. 2016;6:26594. doi: 10.1038/srep26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kotlinska J., Suder P., Legowska A., Rolka K., Silberring J. Orphanin FQ/nociceptin inhibits morphine withdrawal. Life Sci. 2000;66:PL119–PL123. doi: 10.1016/S0024-3205(99)00648-7. [DOI] [PubMed] [Google Scholar]
- 196.Devine D.P., Taylor L., Reinscheid R.K., Monsma F.J., Jr., Civelli O., Akil H. Rats rapidly develop tolerance to the locomotor-inhibiting effects of the novel neuropeptide orphanin FQ. Neurochem. Res. 1996;21:1387–1396. doi: 10.1007/BF02532380. [DOI] [PubMed] [Google Scholar]
- 197.Florin S., Suaudeau C., Meunier J.C., Costentin J. Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur. J. Pharmacol. 1996;317:9–13. doi: 10.1016/S0014-2999(96)00707-8. [DOI] [PubMed] [Google Scholar]
- 198.Narayanan S., Maidment N.T. Orphanin FQ and behavioral sensitization to cocaine. Pharmacol. Biochem. Behav. 1999;63:271–277. doi: 10.1016/S0091-3057(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 199.Yang H.Y.T., Fratta W., Majane E.A., Costa E. Isolation, sequencing, synthesis and pharmacological characterization of two brain neuropeptides that modulate the action of morphine. Proc. Natl. Acud. Sci. USA. 1985;82:7757–7761. doi: 10.1073/pnas.82.22.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gouardères C., Sutak M., Zajac J.M., Jhamandas K. Antinociceptive effects of intrathecally administered FBFamide and FMRFamide in the rat. Eur. J. Pharmucol. 1993;237:73–81. doi: 10.1016/0014-2999(93)90095-Y. [DOI] [PubMed] [Google Scholar]
- 201.Malin D.H., Lake J.R., Hammond M.V., Fowler D.E., Rogillio R.B., Brown S.L., Sims J.L., Leecraft B.M., Yang H.Y. FMRF-NH2-like mammalian octapeptide: Possible role in opiate dependence and abstinence. Peptides. 1990;11:969–972. doi: 10.1016/0196-9781(90)90018-Z. [DOI] [PubMed] [Google Scholar]
- 202.Tang J., Yang H.Y., Costa E. Inhibition of spontaneous and opi- ate modifiate nociception by an endogenous neuropeptide with Phe-Met-Arg-Phe-NH2-like immunoreactivity. Proc. Natl. Acad. Sci. USA. 1984;81:5002–5005. doi: 10.1073/pnas.81.15.5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Panula P., Aarnisalo A.A., Wasowicz K. Neuropeptide FF, a mammalian neuropeptide with multiple functions. Prog. Neurobiol. 1996;48:461–487. doi: 10.1016/0301-0082(96)00001-9. [DOI] [PubMed] [Google Scholar]
- 204.Majane E.A., Zhu J., Aarnisalo A.A., Panula P., Yang H.Y. Origin of neurohypophyseal neuropeptide-FF (FLFQPQRF-NH2) Endocrinology. 1993;133:1578–1584. doi: 10.1210/endo.133.4.8404597. [DOI] [PubMed] [Google Scholar]
- 205.Kotani M., Detheux M., Vandenbogaerde A., Communi D., Vanderwinden J.M., Le Poul E., Brézillon S., Tyldesley R., Suarez-Huerta N., Vandeput F., et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 206.Cador M., Marco N., Stinus L., Simonnet G. Interaction between neuropeptide FF and opioids in the ventral tegmental area in the behavioral response to novelty. Neuroscience. 2002;110:309–318. doi: 10.1016/S0306-4522(01)00587-5. [DOI] [PubMed] [Google Scholar]
- 207.Liu Q., Guan X.M., Martin W.J., McDonald T.P., Clements M.K., Jiang Q., Zeng Z., Jacobson M., Williams D.L., Jr., Yu H., et al. Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J. Biol. Chem. 2001;276:36961–36969. doi: 10.1074/jbc.M105308200. [DOI] [PubMed] [Google Scholar]
- 208.Allard M., Geoffre S., Legendre P., Vincent J.D., Simonnet G. Characterization of rat spinal cord receptors to FLFQPQRFa-mide, a mammalian morphine modulating peptide: A binding study. Brain Res. 1989;500:169–176. doi: 10.1016/0006-8993(89)90311-9. [DOI] [PubMed] [Google Scholar]
- 209.Raffa R.B., Kim A., Rice K.C., De Costa B.R., Codd E.E., Rothman R.B. Low Affinity of FMRFamide and Four FaRPs (FMRFamide-related Peptides), Including the Mammalian-Derived FaRPs F-8-Famide (NPFF) and A-18-Famide, for Opioid Mu, Delta, Kappa 1, Kappa 2a, or Kappa 2b Receptors. Peptides. 1994;15:401–404. doi: 10.1016/0196-9781(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 210.Roumy M., Zajac J.M. Neuropeptide FF, Pain and Analgesia. Eur. J. Pharmacol. 1998;345:1–11. doi: 10.1016/S0014-2999(97)01604-X. [DOI] [PubMed] [Google Scholar]
- 211.Kavaliers M. Inhibitory influences of mammalian FMRF-amide (Phe-Met-Arg-Phe-amide)-related peptides on nociception and morphine- and stress-induced analgesia in mice. Neurosci. Lett. 1990;115:307–312. doi: 10.1016/0304-3940(90)90473-M. [DOI] [PubMed] [Google Scholar]
- 212.Lake J.R., Hammond M.V., Shaddox R.C., Hunsicker L.M., Yang H.Y., Malin D.H. IgG from neuropeptide FF antiserum reverses morphine tolerance in the rat. Neurosci. Lett. 1991;132:29–32. doi: 10.1016/0304-3940(91)90425-S. [DOI] [PubMed] [Google Scholar]
- 213.Kavaliers M., Yang H.Y. Effects of mammalian FMRF-NH2-related peptides and IgG from antiserum against them on aggression and defeat-induced analgesia in mice. Peptides. 1991;12:235–239. doi: 10.1016/0196-9781(91)90005-A. [DOI] [PubMed] [Google Scholar]
- 214.Kavaliers M., Yang H.Y. IgG from antiserum against endogenous mammalian FMRF-NH, -related peptides augments morphine- and stress-induced analgesia in mice. Peptides. 1989;10:741–745. doi: 10.1016/0196-9781(89)90106-X. [DOI] [PubMed] [Google Scholar]
- 215.Malin D.H., Lake J.R., Leyva J.E., Hammond M.V., Rogillio R.B., Arcangeli K.R., Ludgate K., Moore G.M., Payza K. Analog of neuropeptide FF attenuates morphine abstinence syndrome. Peptides. 1991;12:1011–1014. doi: 10.1016/0196-9781(91)90052-Q. [DOI] [PubMed] [Google Scholar]
- 216.Devillers J.P., Labrouche S.A., Castes E., Simonnet G. Release of neuropeptide FF, an anti-opioid peptide, in rat spinal cord slices is voltage- and Ca*+-sensitive. Possible involvement of P-type Ca2+ channels. J. Neurochem. 1995;64:1567–1575. doi: 10.1046/j.1471-4159.1995.64041567.x. [DOI] [PubMed] [Google Scholar]
- 217.Stinus L., Allard M., Gold L., Simonnet G. Changes in CNS neuropeptide FF-like material, pain sensitivity, and opiate dependence following chronic morphine treatment. Peptides. 1995;16:1235–1241. doi: 10.1016/0196-9781(95)02019-S. [DOI] [PubMed] [Google Scholar]
- 218.Ballet S., Mauborgne A., Gouardères C., Bourgoin A.S., Zajac J.M., Hamon M., Cesselin F. The neuropeptide FF analogue, 1DME, enhances in vivo met-enkephalin release from the rat spinal cord. Neuropharmacology. 1999;38:1317–1324. doi: 10.1016/S0028-3908(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 219.Marco N., Stinus L., Allard M., Le Moal M., Simonnet G. Neuro- peptide FF receptors within the ventral mesencephalon and dopaminergic terminal areas: Localization and functional anti-opioid involvement. Neuroscience. 1995;64:1035–1044. doi: 10.1016/0306-4522(94)00383-G. [DOI] [PubMed] [Google Scholar]
- 220.Kersanté F., Wang J.Y., Chen J.C., Mollereau C., Zajac J.M. Anti-opioid effects of neuropeptide FF receptors in the ventral tegmental area. Neurosci. Lett. 2011;488:305–309. doi: 10.1016/j.neulet.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 221.Kotlinska J., Pachuta A., Dylag T., Silberring J. Neuropeptide FF (NPFF) reduces the expression of morphine- but not of ethanol-induced conditioned place preference in rats. Peptides. 2007;28:2235–2242. doi: 10.1016/j.peptides.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 222.Kotlinska J.H., Gibula-Bruzda E., Koltunowska D., Raoof H., Suder P., Silberring J. Modulation of neuropeptide FF (NPFF) receptors influences the expression of amphetamine-induced conditioned place preference and amphetamine withdrawal anxiety-like behavior in rats. Peptides. 2012;33:156–163. doi: 10.1016/j.peptides.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 223.Simonnet G., Devillers J.P., Boisserie F. Pain facilitory systems activated by opiate receptor stimulation: Possible role of NPFF, an anti-opioid peptide Regulatory. Peptides. 1994;54:277–278. doi: 10.1016/0167-0115(94)90497-9. [DOI] [Google Scholar]
- 224.Kotlinska J., Pachuta A., Silberring J. Neuropeptide FF (NPFF) reduces the expression of cocaine-induced conditioned place preference and cocaine-induced sensitization in animals. Peptides. 2008;29:933–939. doi: 10.1016/j.peptides.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 225.Takino T., Koshikawa N., Miyamori H., Tanaka M., Sasaki T., Okada Y., Seiki M., Sato H. Cleavage of metastasis suppressor gene product KiSS-1 protein/metastin by matrix metalloproteinases. Oncogene. 2003;22:4617–4626. doi: 10.1038/sj.onc.1206542. [DOI] [PubMed] [Google Scholar]
- 226.Simonin F., Schmitt M., Laulin J.P., Laboureyras E., Jhamandas J.H., MacTavish D., Matifas A., Mollereau C., Laurent P., Parmentier M., et al. RF9, a potent and selective neuropeptide FF receptor antagonist, prevents opioid-induced tolerance associated with hyperalgesia. Proc. Natl. Acad. Sci. USA. 2006;103:466–471. doi: 10.1073/pnas.0502090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Milton N.G. In Vitro Activities of Kissorphin, a Novel Hexapeptide KiSS-1 Derivative, in Neuronal Cells. J. Amino Acids. 2012;2012:691463. doi: 10.1155/2012/691463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gibula-Bruzda E., Marszalek-Grabska M., Gawel K., Trzcinska R., Silberring J., Kotlinska J.H. The new kisspeptin derivative—Kissorphin (KSO)—Attenuates acute hyperlocomotion and sensitization induced by ethanol and morphine in mice. Alcohol. 2017;64:45–53. doi: 10.1016/j.alcohol.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 229.Gibula-Tarlowska E., Grochecki P., Silberring J., Kotlinska J.H. The kisspeptin derivative kissorphin reduces the acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in rats. Alcohol. 2019;81:11–19. doi: 10.1016/j.alcohol.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 230.Gibula-Tarlowska E., Kedzierska E., Piechura K., Silberring J., Kotlinska J.H. The influence of a new derivate of kisspeptin-10—Kissorphin (KSO) on the rewarding effects of morphine in the conditioned place preference (CPP) test in male rats. Behav. Brain Res. 2019;372:112043. doi: 10.1016/j.bbr.2019.112043. [DOI] [PubMed] [Google Scholar]
- 231.Economidou D., Cippitelli A., Stopponi S., Braconi S., Clementi S., Ubaldi M., Martin-Fardon R., Weiss F., Massi M., Ciccocioppo R. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin. Exp. Res. 2011;35:747–755. doi: 10.1111/j.1530-0277.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shoblock J.R., Wichmann J., Maidment N.T. The effect of a systemically active ORL-1 agonist, Ro 64-6198, on the acquisition, expression, extinction, and reinstatement of morphine conditioned place preference. Neuropharmacology. 2005;49:439–446. doi: 10.1016/j.neuropharm.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 233.Toll L., Bruchas M.R., Calo G., Cox B.M., Zaveri N.T. Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems. Pharmacol. Rev. 2016;68:419–457. doi: 10.1124/pr.114.009209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Toledo M.A., Pedregal C., Lafuente C., Diaz N., Martinez-Grau M.A., Jimenez A., Benito A., Torrado A., Mateos C., Joshi E.M., et al. Discovery of a novel series of orally active nociceptin/orphanin FQ (NOP) receptor antagonists based on a dihydrospiro(piperidine-4,7’-thieno[2,3-c]pyran) scaffold. J. Med. Chem. 2014;57:3418–3429. doi: 10.1021/jm500117r. [DOI] [PubMed] [Google Scholar]
- 235.Rorick-Kehn L.M., Ciccocioppo R., Wong C.J., Witkin J.M., Martinez-Grau M.A., Stopponi S., Adams B.L., Katner J.S., Perry K.W., Toledo M.A., et al. A Novel, Orally Bioavailable Nociceptin Receptor Antagonist, LY2940094, Reduces Ethanol Self-Administration and Ethanol Seeking in Animal Models. Alcohol Clin. Exp. Res. 2016;40:945–954. doi: 10.1111/acer.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Post A., Smart T.S., Jackson K., Mann J., Mohs R., Rorick-Kehn L., Statnick M., Anton R., O’Malley S.S., Wong C.J. Proof-of-Concept Study to Assess the Nociceptin Receptor Antagonist LY2940094 as a New Treatment for Alcohol Dependence. Alcohol Clin. Exp. Res. 2016;40:1935–1944. doi: 10.1111/acer.13147. [DOI] [PubMed] [Google Scholar]
- 237.Kallupi M., Scuppa G., de Guglielmo G., Calo G., Weiss F., Statnick M.A., Rorick-Kehn L.M., Ciccocioppo R. Genetic Deletion of the Nociceptin/Orphanin FQ Receptor in the Rat Confers Resilience to the Development of Drug Addiction. Neuropsychopharmacology. 2017;42:695–706. doi: 10.1038/npp.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ciccocioppo R., Borruto A.M., Domi A., Teshima K., Cannella N., Weiss F. NOP-related mechanisms in substance use disorders. Handb. Exp. Pharmacol. 2019;254:187–212. doi: 10.1007/164_2019_209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ciccocioppo R., Economidou D., Rimondini R., Sommer W., Massi M., Heilig M. Buprenorphine reduces alcohol drinking through activation of the nociceptin/orphanin FQ-NOP receptor system. Biol. Psychiatry. 2007;61:4–12. doi: 10.1016/j.biopsych.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Huang P., Kehner G.B., Cowan A., Liu-Chen L.Y. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: Norbuprenorphine is a potent opioid agonist. J. Pharmacol. Exp. Ther. 2001;297:688–695. [PubMed] [Google Scholar]
- 241.Wnendt S., Kruger T., Janocha E., Hildebrandt D., Englberger W. Agonistic effect of buprenorphine in a nociceptin/OFQ receptor-triggered reporter gene assay. Mol. Pharmacol. 1999;56:334–338. doi: 10.1124/mol.56.2.334. [DOI] [PubMed] [Google Scholar]
- 242.Ding H., Czoty P.W., Kiguchi N., Cami-Kobeci G., Sukhtankar D.D., Nader M.A., Husbands S.M., Ko M.C. A novel orvinol analog, BU08028, as a safe opioid analgesic without abuse liability in primates. Proc. Natl. Acad. Sci. USA. 2016;113:E5511–E5518. doi: 10.1073/pnas.1605295113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ding H., Kiguchi N., Yasuda D., Daga P.R., Polgar W.E., Lu J.J., Czoty P.W., Kishioka S., Zaveri N.T., Ko M.C. A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci. Transl. Med. 2018;10:eaar3483. doi: 10.1126/scitranslmed.aar3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kiguchi N., Ding H., Cami-Kobeci G., Sukhtankar D.D., Czoty P.W., DeLoid H.B., Hsu F.C., Toll L., Husbands S.M., Ko M.C. BU10038 as a safe opioid analgesic with fewer side-effects after systemic and intrathecal administration in primates. Br. J. Anaesth. 2019;122:e146–e156. doi: 10.1016/j.bja.2018.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Flynn S.M., Epperly P.M., Davenport A.T., Cami-Kobeci G., Husbands S.M., Ko M.C., Czoty P.W. Effects of stimulation of mu opioid and nociceptin/orphanin FQ peptide (NOP) receptors on alcohol drinking in rhesus monkeys. Neuropsychopharmacology. 2019;44:1476–1484. doi: 10.1038/s41386-019-0390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.de Guglielmo G., Matzeu A., Kononoff J., Mattioni J., Martin-Fardon R., George O. Cebranopadol Blocks the Escalation of Cocaine Intake and Conditioned Reinstatement of Cocaine Seeking in Rats. J. Pharmacol. Exp. Ther. 2017;362:378–384. doi: 10.1124/jpet.117.241042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Shen Q., Deng Y., Ciccocioppo R., Cannella N. Cebranopadol, a Mixed Opioid Agonist, Reduces Cocaine Self-administration through Nociceptin Opioid and Mu Opioid Receptors. Front. Psychiatry. 2017;8:234. doi: 10.3389/fpsyt.2017.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zaveri N.T. The nociceptin/orphanin FQ receptor (NOP) as a target for drug abuse medications. Curr. Top. Med. Chem. 2011;11:1151–1156. doi: 10.2174/156802611795371341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kantola I., Scheinin M., Gulbrandsen T., Meland N., Smerud K.T. Safety, tolerability, and antihypertensive effect of SER100, an opiate receptor-like 1 (ORL-1) partial agonist, in patients with isolated systolic hypertension. Clin. Pharmacol. Drug Dev. 2017;6:584–591. doi: 10.1002/cpdd.330. [DOI] [PubMed] [Google Scholar]
- 250.Angelico P., Barchielli M., Lazzeri M., Guerrini R., Caló G. Nociceptin/ orphanin FQ and urinary bladder. Handb. Exp. Pharmacol. 2019;254:347–365. doi: 10.1007/164_2018_182. [DOI] [PubMed] [Google Scholar]
- 251.Mercatelli D., Pisanò C.A., Novello S., Morari M. NOP receptor ligands and Parkinson’s disease. Handb. Exp. Pharmacol. 2019;254:213–232. doi: 10.1007/164_2018_199. [DOI] [PubMed] [Google Scholar]
- 252.Ferrari F., Rizzo S., Ruzza C., Calo G. Detailed In Vitro Pharmacological Characterization of the Clinically Viable Nociceptin/Orphanin FQ Peptide Receptor Antagonist BTRX-246040. J. Pharmacol. Exp. Ther. 2020;373:34–43. doi: 10.1124/jpet.119.262865. [DOI] [PubMed] [Google Scholar]
- 253.Gavioli E.C. Nociceptin/orphanin FQ receptor antagonists as innovative antidepressant drugs. Pharmacol. Ther. 2013;140:10–25. doi: 10.1016/j.pharmthera.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 254.Awwad H.O., Durand C.D., Gonzalez L.P., Tompkins P., Zhang Y., Lerner M.R., Brackett D.J., Sherry D.M., Awasthi V., Standifer K.M. Post-blast treatment with Nociceptin/Orphanin FQ peptide (NOP) receptor antagonist reduces brain injury- induced hypoxia and signaling proteins in vestibulomotor-related brain regions. Behav. Brain Res. 2018;340:183–194. doi: 10.1016/j.bbr.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 255.Witkin J.M., Wallace T.L., Martin W.J. Therapeutic Approaches for NOP Receptor Antagonists in Neurobehavioral Disorders: Clinical Studies in Major Depressive Disorder and Alcohol Use Disorder with BTRX-246040 (LY2940094) Handb. Exp. Pharmacol. 2019;254:399–415. doi: 10.1007/164_2018_186. [DOI] [PubMed] [Google Scholar]
- 256.Carvalho D., Petronilho F., Vuolo F., Machado R.A., Constantino L., Guerrini R., Calo G., Gavioli E.C., Streck E.L., Dal-Pizzol F. The nociceptin/orphanin FQ-NOP receptor antagonist effects on an animal model of sepsis. Intensive Care Med. 2008;34:2284–2290. doi: 10.1007/s00134-008-1313-3. [DOI] [PubMed] [Google Scholar]
- 257.Genovese R.F., Dobre S. Mitigation of adverse behavioral impact from predator exposure by the nociceptin/orphanin FQ peptide antagonist J-113397 in rats. Behav. Pharmacol. 2017;28:521–530. doi: 10.1097/FBP.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 258.Zhang Y., Simpson-Durand C.D., Standifer K.M. Nociceptin/orphanin FQ peptide receptor antagonist JTC-801 reverses pain and anxiety symptoms in a rat model of post-traumatic stress disorder. Br. J. Pharmacol. 2015;172:571–582. doi: 10.1111/bph.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Khroyan T.V., Polgar W.E., Jiang F., Zaveri N.T., Toll L. Nociceptin/orphanin FQ receptor activation attenuates antinociception induced by mixed nociceptin/orphanin FQ/mu-opioid receptor agonists. J. Pharmacol. Exp. Ther. 2009;331:946–953. doi: 10.1124/jpet.109.156711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Guillemyn K., Starnowska J., Lagard C., Dyniewicz J., Rojewska E., Mika J., Chung N.N., Utard V., Kosson P., Lipkowski A.W., et al. Bifunctional Peptide-Based Opioid Agonist-Nociceptin Antagonist Ligands for Dual Treatment of Acute and Neuropathic Pain. J. Med. Chem. 2016;59:3777–3792. doi: 10.1021/acs.jmedchem.5b01976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Christoph A., Eerdekens M.H., Kok M., Volkers G., Freynhagen R. Cebranopadol, a novel first-in-class analgesic drug candidate: First experience in patients with chronic low back pain in a randomized clinical trial. Pain. 2017;158:1813–1824. doi: 10.1097/j.pain.0000000000000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Eerdekens M.H., Kapanadze S., Koch E.D., Kralidis G., Volkers G., Ahmedzai S.H., Meissner W. Cancer-related chronic pain: Investigation of the novel analgesic drug candidate cebranopadol in a randomized, double-blind, noninferiority trial. Eur. J. Pain. 2019;23:577–588. doi: 10.1002/ejp.1331. [DOI] [PubMed] [Google Scholar]
- 263.Tzschentke T.M., Linz K., Koch T., Christoph T. Cebranopadol: A novel first- in-class potent analgesic acting via NOP and opioid receptors. Handb. Exp. Pharmacol. 2019;254:367–398. doi: 10.1007/164_2019_206. [DOI] [PubMed] [Google Scholar]
- 264.Lu L., Huang M., Liu Z., Ma L. Cholecystokinin-B receptor antagonists attenuate morphine dependence and withdrawal in rats. Neuroreport. 2000;11:829–832. doi: 10.1097/00001756-200003200-00034. [DOI] [PubMed] [Google Scholar]
- 265.Baber N.S., Dourish C.T., Hill D.R. The role of CCK, caerulein, and CCK antagonists in nociception. Pain. 1989;39:307–328. doi: 10.1016/0304-3959(89)90045-6. [DOI] [PubMed] [Google Scholar]
- 266.Wiesenfeld-Hallin Z., Xu X.-J. The role of cholecystokinin in nociception, neuropathic pain and opiate tolerance. Reg. Peptides. 1996;65:23–28. doi: 10.1016/0167-0115(96)00068-7. [DOI] [PubMed] [Google Scholar]
- 267.Maldonado R., Valverde O., Ducos B., Blommaert A.G., Fournie-Zaluski M.C., Roques B.P. Inhibition of morphine withdrawal by the association of RB 101, an inhibitor of enkephalin catabolism, and the CCKB antagonist PD-134,308. Br. J. Pharmacol. 1995;114:1031–1039. doi: 10.1111/j.1476-5381.1995.tb13309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Kastin A.J., Banks W.A., Hahn K., Zadina J.E. Extreme stability of Tyr-MIF-1 in CSF. Neurosci. Lett. 1994;174:26–28. doi: 10.1016/0304-3940(94)90110-4. [DOI] [PubMed] [Google Scholar]
- 269.Dourish C.T., Hawley D., Iversen S.D. Enhancement of morphine analgesia and prevention of morphine tolerance in the rat by the cholecystokinin antagonist L-364,718. Eur. J. Pharmacol. 1988;147:469–472. doi: 10.1016/0014-2999(88)90183-5. [DOI] [PubMed] [Google Scholar]
- 270.Bocheva A., Dzambazova-Maximova E. Antiopioid properties of the TYR-MIF-1 family. Methods Find. Exp. Clin. Pharmacol. 2004;26:673–677. doi: 10.1358/mf.2004.26.9.872564. [DOI] [PubMed] [Google Scholar]