Abstract
Regulation of axonal dopamine release by local microcircuitry is at the hub of several biological processes that govern the timing and magnitude of signaling events in reward-related brain regions. An important characteristic of dopamine release from axon terminals in the striatum is that it is rapidly modulated by local regulatory mechanisms. These processes can occur via homosynaptic mechanisms - such presynaptic dopamine autoreceptors and dopamine transporters - as well heterosynaptic mechanisms such as retrograde signaling from postsynaptic cholinergic and dynorphin systems, among others. Additionally, modulation of dopamine release via diffusible messengers such as nitric oxide and hydrogen peroxide, allows for various metabolic factors to quickly and efficiently regulate dopamine release and subsequent signaling. Here we review how these mechanisms work in concert to influence the timing and magnitude of striatal dopamine signaling, independent of action potential activity at the level of dopaminergic cell bodies in the midbrain, thereby providing a parallel pathway by which dopamine can be modulated. Understanding the complexities of local regulation of dopamine signaling is required for building comprehensive frameworks of how activity throughout the dopamine system is integrated to drive signaling and control behavior.
Regulation of axonal dopamine release by local microcircuitry is at the hub of several biological processes that govern the timing and magnitude of signaling events in reward-related brain regions. Dopamine release at terminals in the striatum can be modulated by a variety of mechanisms, including homosynaptic mechanisms intrinsic to the cell itself and heterosynaptic mechanisms that integrate activity of local microcircuitry. Together, these mechanisms work in concert to dynamically regulate dopamine release, uptake and repackaging for future release events, and thus exert precise control over dopaminergic activity and drive subsequent behaviors. Here we review how these mechanisms work together to influence the timing and magnitude of striatal dopamine signaling, independent of action potential activity at the level of dopaminergic cell bodies in the midbrain, thereby providing a parallel pathway by which dopamine can be modulated.
Introduction
Dopamine cell bodies in the midbrain send long-range projections to many targets in cortical and limbic structures allowing for dopaminergic modulation of neuronal function across the brain (Chen & Bonci 2017; Russo & Nestler 2013). The timing and magnitude of dopamine release is thought to relay information fundamental for guiding organismal behavior, with theories ranging from hypothesizing that phasic dopamine and accompanying release encodes reward prediction error, to incentive value or even representations of an internal clock (Schultz et al. 1997; Meck 2006; Berridge 2007). These facets of release are tightly controlled by a collection of receptor and transporter systems expressed at the presynaptic membrane. In the mesolimbic dopamine system, where dopamine is released from terminals in the nucleus accumbens (NAc), these include dopamine transporters (Richardson et al. 2016), dopamine type-2 autoreceptors (D2Rs) (Benoit-Marand et al. 2011), heteroreceptors (Zhang & Sulzer 2012) including channels regulating ion flux (Brimblecombe et al. 2015; Martel et al. 2011) and nicotinic acetylcholine receptors (Grady et al. 2007), and other signaling molecules such as nitric oxide (NO) (West et al. 2002) as well as steroid and sex hormones (Becker & Chartoff 2019). Direct regulation of dopamine release at the level of the terminal - through effectors located directly within or on dopamine terminals – is a critical component of dopamine release regulation and associated signaling.
The role of dopamine release is often discussed as an all-or-nothing process that compares how much dopamine is released relative to the baseline, ignoring the granularity of the signal regarding how the magnitude, spread and duration is shaped by the local microenvironment. These properties likely contain critical information that extends far beyond just the relative peak concentration of release events. The contribution of local microcircuitry is fundamental in regulating the timing of signaling, both pre- and post-synaptic, as well as the interactions between basal dopamine levels and stimulus-dependent signaling downstream. When accounting for these factors in sum, dopaminergic microcircuitry can exhibit a robust dynamic range, and therefore, there is likely more complex information within these signaling domains that allows for more nuance in what is being encoded. Here we review the current research on the complex mechanisms regulating the dynamics of dopamine release at its projection targets. Ultimately, mechanisms that elicit and shape dopamine release at the terminal may represent novel avenues for therapeutic interventions targeting striatal dopamine dysregulation in disease and as such an understanding of these complex regulatory mechanisms is critical to moving the field forward.
1. Homosynaptic Regulation at the Terminal
Dopamine terminals in reward-related brain regions, such as the NAc are regulated by a number of transmitters supplied by locally-projecting neurons (heterosynaptic - see section 2) or through responses to dopamine itself (homosynaptic - discussed in the present section). We will discuss the three following critical processes that govern release magnitude and temporal dynamics: (1) alterations in release probability and release pool dynamics (2) autoreceptor-mediated feedback mechanisms, and (3) changes in the function and expression levels of transporters controlling dopamine reuptake and repackaging. These processes all work to modulate the magnitude, onset, and duration of dopamine levels in the extracellular space.
1.1. Regulation of dopamine release
Early observations demonstrated that a single vesicle filled with neurotransmitter molecules represents the elementary unit of synaptic transmission (Katz 1971). Recent work has shown that the magnitude of release events the central nervous system can be modulated by a number of factors, including neurotrophic factors and neurotransmitter synthesis (Pothos et al. 2000; Pothos et al. 1998). Thus, the regulation of dopamine release is a potent mechanism by which dopamine terminals can be regulated. One common mechanism that impacts the magnitude and onset of dopamine release is related to changes in release mechanisms within the terminal itself, including changes in the distribution of various vesicle pools (section 1.1.1) and changes in the conductance or expression of calcium channels on presynaptic dopamine terminals (section 1.1.2) (Figure 1A). Plasticity in these mechanisms may allow for differential transformations between upstream action potential activity and downstream release, both across time/experience and between distinct terminals arising from the same soma.
Figure 1. Homosynaptic and heterosynaptic regulators of presynaptic dopamine release in the striatum.
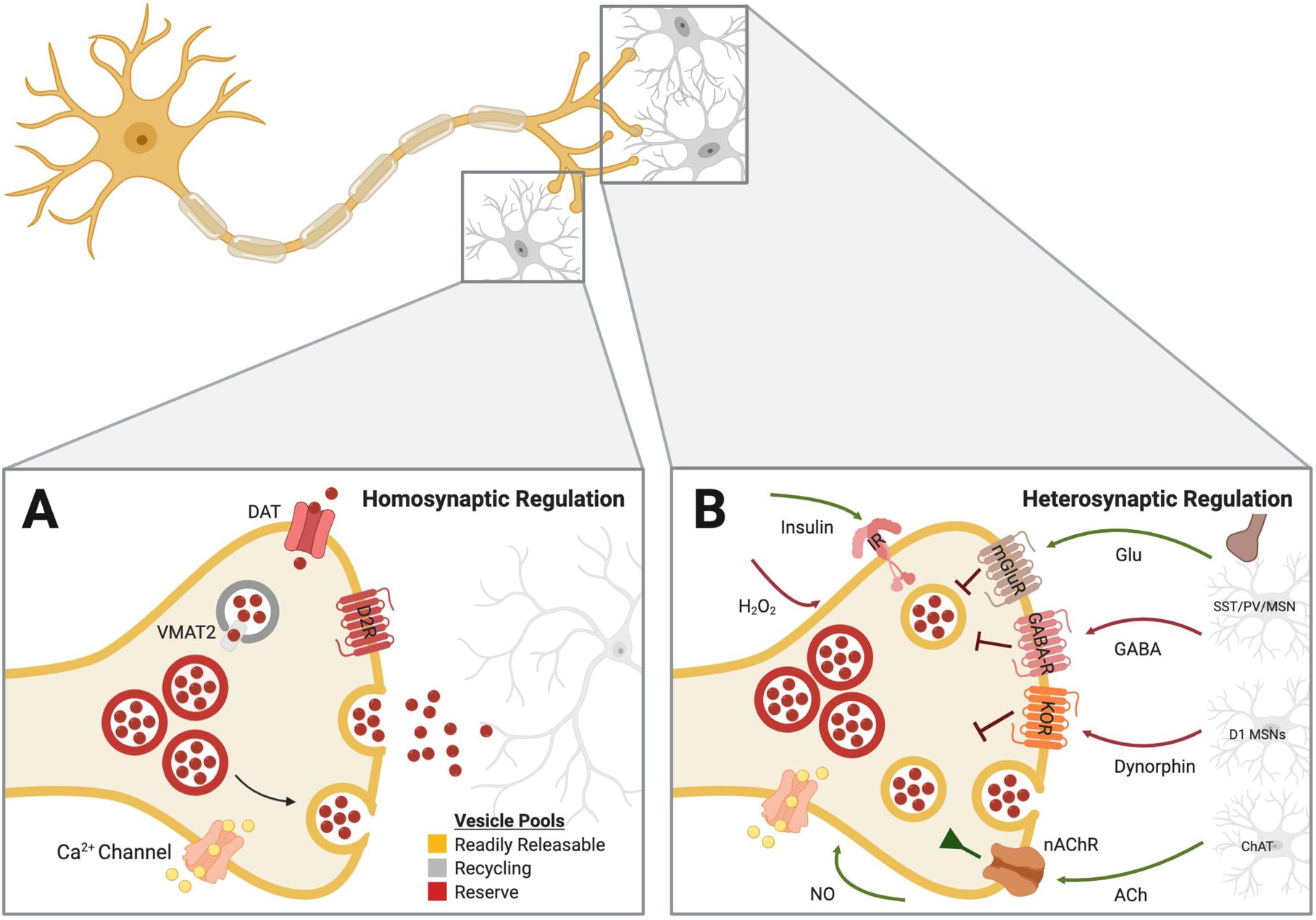
Dopamine release at the terminal can be elicited and modulated through mechanisms intrinsic to the cell itself, as well as via substrates released from postsynaptic cells and presynaptic inputs from non-dopaminergic systems. A. Fluctuations in the functionality of transporters, changes in the size of the various vesicle pools and activity of autoreceptors, all termed homosynaptic regulators, each have established roles in determining the amplitude of evoked dopamine release events. B. Several neurotransmitters from local microcircuitry, such as glutamate (Zhang and Sulzer 2003), GABA (Brog et al. 1993; Pennartz et al. 1994), dynorphin (Britt and McGehee 2008; Thompson et al. 2008), and acetylcholine (Collins et al. 2016; Kosillo et al. 2016; Shin et al. 2017; Yorgason et al., 2017), as well as diffusible retrograde signaling molecules like hydrogen peroxide (Chen et al. 2001) and nitric oxide (Kiss et al. 1999) can all exert influences on dopamine release at the axon terminal. ACh = acetylecholine; ChAt = choline acetyltransferase; DAT = dopamine transporter; GABA-R = GABA receptor ; Glu = glutamate; IR = insulin receptor; KOR = kappa opioid receptor; nAChR = nicotinic acetylcholine receptor; NO = nitric oxide; mGluR = metabotropic glutamate receptor; MSN = medium spiny neuron; SST = somatostatin; PV = paralbumin; VMAT = vesicular monoamine transporter.
1.1.1. Vesicle pool effects
Dopamine is released from multiple separate pools or compartments (Ewing et al. 1983; Javoy & Glowinski 1971; del Castillo & Katz 1954). These pools can most simply be divided into three distinct pools: the readily releasable pool, the recycling pool and the reserve pool (Rizzoli & Betz 2005).
-
Readily releasable pool. The readily releasable pool contains recently synthesized dopamine and is released first following activation by action potentials (Besson et al. 1969). The structure of this pool ensures immediate exocytosis of neurotransmitter in response to depolarization in the fast-acting synapse, though in response to continued activity the magnitude of this pool declines. However, this pool can be quickly refilled from the other pools of dopamine (Yavich & MacDonald 2000). Plasticity in this refilling process does indeed occur, as evidence showed that high-frequency stimulation repeated at 5 second intervals significantly delayed the decline in dopamine (Wang & Kaczmarek 1998; Stevens & Wesseling 1998). This effect is perhaps due to increased rate of refill from the reserve compartment, which has been proposed for other types of synapses (Richards et al. 2003). Previous work has shown that multiple psychostimulants can elicit increased dopamine release via mobilization of secondary reserves of dopamine (Venton et al. 2006; Shore 1976). Further, the distribution of dopamine between the different pools may be related to individual differences in responding for cocaine (Verheij & Cools 2011; Verheij et al. 2008). This dopamine mobilization by cocaine is synapsin - and dopamine transporter (DAT) -dependent (Venton et al. 2006; Kile et al. 2010). Importantly, in non-dopaminergic systems, calcium signaling has been demonstrated to play a role in the upregulation of release magnitude due to docking of reserve pool vesicles following priming events (Wang et al. 2016; Thanawala & Regehr 2013) an effect that may play a similar role in dopaminergic terminals.
At glutamatergic synapses, plasticity processes like LTD have been shown to be related to decreases in the readily releasable pool (Goda & Stevens 1998). Additionally, inhibition of the mammalian target of rapamycin (mTOR), which plays a role in activity-dependent plasticity, within the presynaptic bouton has been shown to induce the formation of autophagic vacuoles which sequester and degrade dopamine-containing vesicles, thereby providing another mechanism for decreasing the size of the pool and subsequently release (Schmitz et al. 2003). Ultimately, the releasable pool is maintained by both synthesis and reuptake-dependent vesicular repackaging, and therefore is inextricably linked with other presynaptic agents, such as transporters and synthesis/degradation enzymes (sections 1.2 and 1.3).
Recycling pool. The recycling pool consists of a mobile pool of dopamine, composing roughly 10–20% of available dopamine (Rizzoli & Betz 2005; Denker & Rizzoli 2010). This pool exists to meet the high demand of the cell, as de novo synthesis of vesicles from the soma is a much slower process (Chanaday et al. 2019). However, it should be noted that more recent studies indicate that the distinctions between the recycling pool and the reserve pool are less clear, proposing that vesicles eventually mature from the recycling pool to the reserve pool over a discrete period of time (Kamin et al. 2010; Denker & Rizzoli 2010). This maturation process then sets up the possibility that this recycling process may then be involved in aspects of terminal plasticity.
Reserve pool. Finally, it is also important to consider the reserve pool. The reserve pool represents the largest pool of dopamine, consisting of roughly 80–90% of the available dopamine. These reserve pools are regulated by the synapsin protein family, the loss of which prevents cocaine augmentation of dopamine release following long simulations (Venton et al. 2006). Facilitation of shifting from the reserve pool to the active zone of the terminal is thought to be dependent on calcium influx, as well as actin polymerization status via interactions with alpha-synuclein (Bellani et al. 2010). The size of the reserve pool is a key determinant in establishing release magnitude following long stimulation paradigms. Moreover, effects on the reserve pool may underlie part of the physiological response to psychostimulants, as long duration stimulation of the dorsal striatum has been shown to elicit reduced dopamine release after administration of amphetamine (Covey et al. 2013).
Together, these studies demonstrate that the distribution of dopamine between the different vesicle pools is one of several dynamic processes that allows for regulation of release. Indeed, shifts in the release pool distribution that are important for release have been shown to occur when there are perturbations in dopamine clearance mechanisms (Jones et al. 1999), and may in fact serve as a compensatory mechanism following persistent stimulation and/or uptake blockade (as would be seen with stimulant use (Calipari et al. 2014)), or simply a way to shift release efficiency following different experiences. However, the precise conditions that result in release pool distribution shifts are not well understood and require further study.
1.1.2. Calcium regulation of dopamine release
Calcium entry into the bouton through voltage-gated calcium channels bears the primary responsibility of initiating synaptic transmission at conventional synapses. These channels interact functionally with other ion channels to modulate release (Martel et al. 2011). This is a key process that can be regulated via numerous mechanisms to enhance release. For example, calcium channels can be enhanced via upregulated expression levels or via changes in conductance. Further, the relationship between terminal depolarization and neurotransmitter release can change over repeated stimulations, an effect that is driven by changes in the sensitivity of calcium binding proteins for calcium itself, also altering the relationship between calcium and exocytotic release (Hsu et al. 1996). Together, these mechanisms allow for experience-dependent plasticity in calcium systems that are critical for neurotransmitter release.
Significant heterogeneity exists in the presence of channel types and function at the dopaminergic terminal across various brain areas (Brimblecombe et al. 2015). There are multiple types of calcium channels, many of which have differential effects on dopamine terminals and dopamine release. Regarding dopamine regulation, N-Type (CAV2.2) has the largest effect on dopamine release where blocking these channels almost completely eliminates evoked dopamine release from terminals in the ventral striatum (Phillips & Stamford 2000; Brimblecombe et al. 2015). P/Q type (CAV2.1) calcium channels are also a critical regulators of dopamine release, where blocking these channels significantly reduces release, but does not completely abolish it. Blocking T-type channels modestly reduces release, but much smaller than the previously mentioned subtypes. Finally, it is likely that L-type calcium channels (CAV1.2/1.3/1.4) are present on dopamine terminals in the NAc, though pharmacological blockade has no effect on dopamine release in slice voltammetry experiments (Brimblecombe et al. 2015), which calls into question their functional role in direct release regulation as well as presence directly on dopamine terminals. Importantly, all calcium channels present on the membrane of terminals are capable of regulating calcium flux through posttranslational modifications, such as those that can alter conductance or more broadly via expression; thus, implicating these channels as potential mediators of experience-dependent plasticity at dopamine synapses. In addition to CAV 1.2, 1.3, 1.4, 2.1, and 2.2, TRP channels are also potent regulators of calcium signaling and have been suggested to have a role in plasticity within this circuit (Wescott et al. 2013; Fowler et al. 2007). The ability to regulate calcium following experience-dependent plasticity is critical for release, given the clear relationship between the size of the readily releasable pool and calcium (Thanawala & Regehr 2013).
Calcium regulation of release through voltage gated calcium channels is a critical step in action potential-dependent release; however, calcium regulation of intracellular signaling and release can also occur through not only calcium channels, but a variety of ligand-gated receptors that are permeable to calcium. For example, some nicotinic acetylcholine receptors are highly calcium permeable (Séguéla et al. 1993; Lendvai & Vizi 2008). Therefore, it is conceivable that any changes in nicotinic receptor density or subunit composition resulting from plasticity mechanisms or sex-dependent effects may be related to changes in calcium influx in the terminal that results from acetylcholine binding and associated channel opening. Broadly, these heterosynaptic receptor mechanisms are described in more detail below (section 2), and these findings indicate that they may serve as a critical component of release regulation via modulation of calcium influx into dopamine terminals.
Together, interactions between releasable pool organization, calcium dynamics, and release machinery all interact to alter the magnitude of dopamine release events directly at terminal projections in the striatum. This is a critical component allowing for release to scale, allowing for a dynamic system that can change on a rapid or prolonged time scale.
1.2. Autoreceptor-mediated feedback mechanisms
In addition to calcium channels, release can also be regulated via G-protein coupled receptors that are located on dopamine terminals. This group of receptors is comprised of both heteroreceptors (described in detail in section 2) and autoreceptors. D2Rs are autoreceptors that provide inhibitory feedback to curtail signaling at times of heightened extracellular dopamine levels, thereby profoundly regulating both the temporal dynamics of extracellular dopamine levels and the magnitude of evoked dopamine release events. Of the receptors expressed on dopamine terminals in the striatum, D2Rs are among the most studied and have been well characterized. They are located both at the level of the cell-body in the VTA as well as directly on terminals in striatal regions where they act to downregulate tyrosine hydroxylase (TH) expression and cellular activity, leading to decreases in dopamine production and release (Ford 2014; Palij et al. 1990; Kennedy et al. 1992; Benoit-Marand et al. 2001; Benoit-Marand et al. 2011; Phillips et al. 2002; Rouge-Pont et al. 2002; Anzalone et al. 2012; Schmitz et al. 2002). Mechanistically, these receptors are Gi-coupled and early work showed that D2R-mediated decreases in dopamine neuron excitability occur via activation of inwardly rectifying potassium (K+) channels – both GIRK and Kv channels - and via inhibiting P/Q and N-type calcium channels (Cardozo & Bean 1995). However, more recent work has shown that while D2R-activation may alter calcium currents, though these effects on calcium currents are downstream of initial K+ channel effects (Congar et al. 2002). Indeed, there is a wealth of evidence demonstrating that the ability of D2Rs to reduce terminal dopamine release occurs through functional interactions with K+ channels, as D2R-mediated reductions in dopamine overflow can be attenuated by bath application of Kv1.1/1.2/1.6 blockers, with a smaller role played by interactions with GIRK channels (Fulton et al. 2011; Martel et al. 2011; Congar et al. 2002). Additionally, D2R activation results in observable increases in Kv1.2 mediated currents, and germline loss of Kv1.2 as well as selective blockage of Kv1.2 prevent D2R-mediated dopaminergic release regulation further highlighting the role that potassium channels play in this process (Fulton et al. 2011).
Aside from acute regulation of terminal excitability and associated release, activation of D2Rs has been shown to drive posttranslational modifications to other regulators, such as DAT and vesicular monoamine transporter-2 (VMAT2), leading to lasting alterations in transporter function and associated repackaging efficiency (Meiergerd et al. 1993; Belin et al. 2007), an effect that alters the timing of dopamine release. Similarly, chronic activation of D2Rs can also induce morphological changes in dopaminergic axon growth and alterations in plasticity across multiple striatal subregions (Fasano et al. 2010; Parish et al. 2001; Parish et al. 2002; Tripanichkul et al. 2003; Giguère et al. 2019). These changes have important implications for subsequent terminal release events and dopamine-mediated plasticity of other cell types across the brain as well.
Region-specific D2 regulation.
D2Rs are critical regulators of dopamine release across the entire striatum; however, their efficacy in reducing dopamine release has been shown to differ depending on the striatal subregion. D2Rs are more efficacious at reducing dopamine release in dorsal striatal regions and their efficacy reduces in a stepwise function from dorsolateral striatum down to medial shell (Rothblat & Schneider 1997). However, it should be noted that this observation has been contested by more recent studies, which demonstrate that D2 agonists have similar inhibitory effects in the dorsal striatum, NAc core and NAc shell (Maina & Mathews 2010; Holloway et al. 2019). Region-specificity in function may be due to interactions with other regulators mentioned here, as recent evidence has highlighted that activation of D2Rs in the dorsal striatum, but not the ventral striatum, increases DAT phosphorylation and surface trafficking (Gowrishankar et al. 2018). Taken together, this potential heterogeneity provides a region-specific regulatory mechanism allowing for different relationships between release dynamics and timing between regions – an effect that may allow for region-specific behavioral regulation.
1.3. Changes in the function and expression levels of transporters
One of the most critical regulators of dopamine signaling is the speed at which it is cleared from the extracellular space and repackaged for future release events. Below we outline how transport regulation plays a key role in dopamine terminal regulation through the major regulators of this process: DAT and VMAT2.
1.3.1. Dopamine transporter (DAT)
Mounting evidence has demonstrated that the DAT represents a critical regulator of terminal release and plasticity via regulation of the temporal dynamics of dopamine in the extracellular space (Condon et al. 2019). DAT is a low capacity, high affinity transporter expressed on dopamine neurons and can be found on dopamine cell bodies, their dendrites, and their axonal projections (Ciliax et al. 1995; Freed et al. 1995). It is comprised of 12 transmembrane domains with both the N- and C-terminal located intracellularly (Giros & Caron 1993; Uhl & Kitayama 1993). DAT is a member of the Na+/Cl- transporters and that uses the Na+ concentration gradient created by the Na+/K+ ATPase to transport dopamine from the extracellular space to the intracellular space. In the striatum, the DAT is the primary method of clearing out dopamine from the extracellular space (Jones et al. 1998; Chen & Reith 2000; Giros et al. 1996). As such, any changes in DAT expression or function can have profound consequences on the timing and duration of dopamine’s interactions with receptors after release events.
DAT is a target of many psychotropic drugs such as cocaine, methamphetamine, and amphetamine (Baumann et al. 2012; Rothman et al. 2001; Belovich et al. 2019; Cartier et al. 2015; Hamilton et al. 2014) and therefore has been well-studied for its role in substance use disorder, motivation, and dopamine signaling. Impaired clearance resulting from inhibition of the DAT by compounds such as cocaine produce elevated levels of dopamine in the extracellular space for prolonged periods of time, and consequently increases in postsynaptic dopamine receptor activation on target cells downstream. Similar findings have been reported with other approaches such as genetic downregulation of DAT, where mice lacking the DAT showed elevated extracellular dopamine and an extended half-life of dopamine in the extracellular space. Importantly, these mice also had a decreased releasable pool of dopamine despite an elevated rate of dopamine synthesis (Jones et al. 1998), highlighting the critical role that DAT plays in vesicular repackaging. Thus, DAT is involved not only in clearance rates, but also serves as an important step in packaging dopamine into vesicles by bringing released dopamine back into the presynaptic cell and facilitating its repackaging into vesicles through interactions between specific synaptic vesicle proteins and the N-terminus of the DAT (Egaña et al. 2009).
The N and C termini of DAT can both be post-translationally modified to alter its membrane stability and function (Sweeney et al. 2017; Hamilton et al. 2014; Belovich et al. 2019; Cartier et al. 2015). Protein kinase C (PKC) has been shown to phosphorylate the DAT, cause internalization, and decrease transport kinetics (Moritz et al. 2015; Foster et al. 2008; Loder & Melikian 2003). PKC activity is also involved in amphetamine-mediated dopamine efflux along with CaMKII, Akt, and PI-3 kinase (Fog et al. 2006; Wei et al. 2007). While phosphorylation of DAT by PKC can decrease DAT activity, phosphorylation by other kinases such as ERK1/2 can increase DAT activity (Morón et al. 2003). The phosphatases PP1 and PP2A associate with DAT and are responsible for dephosphorylation of specific phosphorylation sites on the DAT (Foster et al. 2012). Further, inhibition of PP2A activity decreases dopamine uptake (Bauman et al. 2000). All of the above effectors - ERK, PKC, PP1, PP2A, CaMKII, Akt, and PI3K - are directly linked to activity-dependent signaling, providing a potential mechanism by which changes in terminal excitability can regulate clearance on a rapid timescale. Indeed, changes in membrane potential alter DAT membrane expression and dopamine clearance via rapid DAT trafficking on a second timescale, through interactions Rit2 (Richardson et al. 2016; Fagan et al. 2020; Sweeney et al. 2020). Additionally, increasing dopamine neuron activity increased phosphorylated-ERK, DAT phosphorylation and threonine53 (known to be phosphorylated by ERK) as well as functionally increased in the maximal rate of reuptake (Vmax) (Foster et al. 2012; Calipari et al. 2017).
Several receptors are also involved in regulating DAT function and expression. For example, DAT has direct protein-protein interactions with the D2R and co-expression of D2Rs increases DAT membrane expression and subsequent uptake in cultured neurons (Mayfield & Zahniser 2001; Lee et al. 2007). However, conditional loss of the D2R in dopamine neurons does not alter dopamine kinetics (Giguère et al. 2019). These data suggest that the D2R-mediated mechanisms can regulate DAT function quickly and transiently, but D2Rs are not necessary for basal DAT activity. Activation of κ-opioid receptors (KORs) also increases dopamine uptake through DAT regulation (Thompson et al. 2000) while activation of mGluR5 receptors decreases the maximal rate of dopamine update (Vmax)(Page et al. 2001), suggesting that Gi (KOR) and Gq (mGluR5) mediated signaling exert opposing actions on DAT-mediated clearance rates.
Region-specific DAT regulation.
DAT expression, like D2Rs, shows region-specific effects on dopamine signaling. Dopamine clearance is highest in dorsal striatal regions and clearance rates decrease across a gradient from dorsolateral striatum down to medial shell. This region-specific DAT regulation is important as psychostimulant drugs, which inhibit transporter function, can have region-specific efficacy. For example, the effects of amphetamine are largest in the dorsal striatum due to the higher density of DAT in the region (Ramsson et al. 2011; Siciliano et al. 2014).
1.3.2. Vesicular monoamine transporter-2 (VMAT2)
While DAT clears dopamine from the extracellular space, VMAT2 clears dopamine from the cytosolic space and sequesters it into synaptic vesicles (Erickson et al. 1992; Liu et al. 1992). This serves two important functions: 1) by sequestering dopamine in vesicles it protects the cell from free radicals formed by the oxidation of dopamine during its enzymatic degradation by monoamine oxidases in the cytosol (Liu & Edwards 1997; Liu et al. 1992) and 2) it allows the dopamine to be recycled and to be prepared for re-release. This transporter has 12 transmembrane domains with N- and C- terminals in the cytosol and several potential sites for posttranslational modification, including phosphorylation and glycosylation sites (Schuldiner et al. 1995). This transporter relies on the H+ gradient created by the H+ ATP-ase, which works to sequester H+ ions in the vesicles and create a proton gradient. Conversely, VMAT2 functions as an antiporter, transporting 2 H+ molecules out of the vesicle for each dopamine molecule it transports into the vesicle [VMAT2 function reviewed in (Eiden & Weihe 2011)]. Inhibition of VMAT2 function via pharmacological methods, such as application of reserpine, leads to decreases in quantal dopamine release (Floor et al. 1995; Pothos et al. 1998). Increases in VMAT2 expression or function result in increased dopamine loading into vesicles which leads to increases in quantal release of dopamine as well as increases in release events, providing a critical role for VMAT2 in regulating the amount of dopamine released in the extracellular space (Pothos et al. 2000; Eiden & Weihe 2011; Lohr et al. 2014; Lohr et al. 2015). Along similar lines, multiple studies have shown that expression of VMAT2 is required for vesicular dopamine storage, with VMAT2 deficient mice showing significantly reduced dopamine release depending on the relative levels of VMAT expression (Patel et al. 2003; Mooslehner et al. 2001; Wang et al. 1997). Acute blockade of VMAT2 function with reserpine also diminishes dopamine release as measured by voltammetry, suggesting that this blockade significantly prevents pool refilling (Kuhr et al. 1986). Additionally, expression of VMAT2 can be regulated in an activity-dependent manner and function can be altered by N-terminal glycosylation by CREB (Watson et al. 2001; Yao et al. 2004)(Watson et al. 2001; Yao, Erickson, and Hersh 2004). Thus, VMAT2 regulation also represents a critical factor that dictates the amount of stimulus-dependent dopamine release and can allow for dynamic changes as cellular activity changes.
Taken together, transporters exert important effects on the functioning of the dopamine terminals. They exert strong influence over extracellular and intracellular dopamine levels, releasable pool volume, release magnitude, and duration of dopamine’s presence in the extracellular space.
2. Heterosynaptic Regulation at the Terminal
In addition to homosynaptic mechanisms that regulate dopamine release independent of other local circuitry, there is complex microcircuitry in the NAc whereby ligands can be released from non-dopaminergic populations and bind to receptors located directly on the dopamine terminal (Figure 1B). In addition to the dopamine terminals originating from the VTA that are described above, the striatum contains several types of postsynaptic neurons including GABAergic medium spiny projection neurons as well as a diverse population of interneurons (e.g. parvalbumin, somatostatin, cholinergic). Regulation of the levels of released ligands - via changes in the excitability of interneuron populations - along with regulation of the expression and function of the receptors for these ligands can dynamically influence the magnitude and frequency of synaptic dopamine release. Below we review the regulators that directly influence release at dopamine terminals through monosynaptic connections – i.e. via transmitters/molecules that signal through receptors/effectors that are located directly on or within dopamine terminals.
2.1. G-protein coupled receptors (GPCRs) and ligand-gated ion channels
Dopamine terminals in the striatum express several classes of heteroreceptors that can be classified as ligand-gated ionotropic receptors or G-protein coupled receptors (GPCRs). Ligand-gated receptors – such as nicotinic acetylcholine receptors (nAChRs) (Quik et al. 2007), and GABA-A receptors (Brodnik et al. 2019) - bind to neurotransmitter an allow for the passage of ions that can increase or decrease release probability and terminal excitability. Similarly, GPCRs located on terminals can also alter release, membrane excitability, function of transporters, and dopamine synthesis (Huang & Thathiah 2015) through initiation of intracellular cascades that can cause a variety of effects, including increases or decreases in cAMP production, opening of inward rectifying potassium channels that cause hyperpolarization, changes to kinase function and increases or decreases in gene expression. Dopamine terminals express many classes of GPCRs, including: D2Rs (Ford 2014; Chesselet 1984), the kappa opioid receptors (KOR) (Svingos et al. 2001; Ronken et al. 1993a), GABA-B receptors (Lopes et al. 2019; Ronken et al. 1993a), as well as metabotropic glutamate receptors (mGluR) (Kuwajima et al. 2007; Manzoni et al. 1997). Generally, these receptors can be classified as excitatory or inhibitory based on the 2nd messenger system they activate. Receptors that couple to excitatory G proteins - Gs or Gq - tend to increase activity of the cell by increasing cAMP production, increasing calcium levels and increasing gene expression (Huang & Thathiah 2015). Conversely, receptors that couple to inhibitory G proteins - Gi/o - tend to decrease the activity of the cell by inhibiting cAMP production, activating GIRK channels (and/or other inwardly rectifying channels), inhibiting calcium channels, and increasing or decreasing gene expression. Below we outline ligand-gated receptors and GPCRs that have been shown to directly regulate dopamine release at terminals:
2.1.1. K-opioid receptors (KORs)
Another major regulator of dopamine release occurs via KORs. These GPCRs are located on dopamine terminals and act as a negative regulator of dopamine release through direct Gi signaling cascades that affect release probability and dopamine synthesis, as well as through interactions with the DAT that alter clearance rates (Britt & McGehee 2008; Thompson et al. 2000). Dynorphin, the endogenous ligand of KORs, is released from postsynaptic D1 receptor containing medium spiny neurons (MSNs) and acts to reduce dopamine release when high levels of D1 MSN activation occur. To this end, terminals can be regulated via changes in local dynorphin tone in an experience-dependent fashion. For example, repeated drug exposure and stress have both been shown to alter both dynorphin levels as well as KOR regulation - effects that converge to regulate tonic dopamine levels within the synapse (Karkhanis et al. 2015; Karkhanis et al. 2016; Bruchas et al. 2010; Land et al. 2008; Crowley & Kash 2015).
Region-specific KOR regulation.
Additionally, like with other regulatory mechanisms, expression of these receptors follows a region specificity, such that the NAc shell displays a “patchy” or clustered expression pattern (Svingos et al. 1999; Mansour et al. 1994), while the caudate-putamen shows dense expression in the medial and ventral portion (Steiner & Gerfen 1996; Steiner & Gerfen 1998). When total receptor availability or functional effects on dopamine release are compared, studies have found greater KOR availability/function within the NAc compared to dorsal regions of the striatum, an effect that has been demonstrated in both rodents and non-human primates (Siciliano et al. 2015; Le Merrer et al. 2009).
2.1.2. Metabotropic glutamate receptors (mGluRs)
While it is well-known that glutamate has robust actions on dopamine signaling within the striatum, how exactly glutamate exerts these actions has been a matter of controversy. Early work showed that exogenous application of L-glutamic acid increased spontaneous release of dopamine in striatal slices, though whether this was mediated through ionotropic or metabotropic receptors was unknown (Giorguieff et al. 1977). Local infusion of a glutamate reuptake inhibitor into the NAc resulted in increased extracellular dopamine in the NAc of rats as measured by microdialysis (Segovia & Mora 2001), which is consistent with human imaging studies (Gleich et al. 2015). Exactly which receptors were present on dopamine terminals was a matter of much debate until electron microscopy confirmed the presence of mGluR1a on dopaminergic terminals (Paquet & Smith 2003). In line with this evidence, previous work has shown that intrastriatal injection of the mGluR group I and II agonist 1S,3R‐ACPD results in a behavioral phenotype that can be directly correlated with increased tissue levels of dopamine and its metabolites (Sacaan et al. 1992). However, there are limited studies that have outlined the precise mechanisms by which these receptors directly regulate dopamine release. Work from Sulzer and colleagues has shown that blockade of glutamatergic uptake in slice decreases dopamine release through mGluR1 activation, involving a mechanism involving phospholipase C and activation of calcium-dependent potassium channels (Zhang & Sulzer 2003). Interestingly, this glutamate appears to come from spillover from nearby corticostriatal glutamatergic synapses (Zhang & Sulzer 2003). The localization of these receptors on dopamine terminals and subsequent activation provides yet another potential mechanism by which glutamatergic inputs into the striatum may be able to directly regulate dopamine terminals independent of local microcircuitry.
2.1.3. GABA receptors
There are a variety of sources of GABA within the striatum such as parvalbumin and somatostatin interneurons, and GABAergic MSNs (Brog et al. 1993; Pennartz et al. 1994). Locally released GABA acts to reduce dopamine release, either through Gi-coupled GABA-B receptors or ionotropic GABA-A receptors (Pitman et al. 2014; Ronken et al. 1993b; Brodnik et al. 2019). Indeed, these effects on dopamine release can occur through either GABA-A and GABA-B receptors as agonism of either receptor subtype reduces dopamine release (Pitman et al. 2014; Brodnik et al. 2019). However, GABA-A receptor-mediated reductions in dopamine release can be blocked with a GABA-B receptor antagonist, suggesting that GABA-A effects occur through a polysynaptic mechanism, rather than through direct effects on terminals (Brodnik et al. 2019). Thus, striatal GABA can inhibit dopamine release through both GABA-A and GABA-B receptors; however, there is some debate as to the localization of these receptors on local circuitry and terminals (Lopes et al. 2019; Pitman et al. 2014; Brodnik et al. 2019) .
2.1.4. Nicotinic acetylcholine receptors (nAChRs)
One of the most studied circuits in regard to local striatal regulation of dopamine release is acetylcholine release from cholinergic interneurons and the associated activation of nAChRs. nAchRs are pentameric ligand-gated iononotropic receptors that are activated by the endogenous ligand acetylcholine and are also the primary site of action for the exogenous ligand nicotine. As such these local circuit regulation mechanisms have been of interest in our understanding of substance use disorder, and much of our basic understanding of this process has originated from studies investigating how nicotinic agonists and antagonists affect dopamine release (Fennell et al. 2019; Lim et al. 2014; Yorgason et al. 2017; Kosillo et al. 2016; Shin et al. 2017; Collins et al. 2016; Cachope et al. 2012; Threlfell et al. 2012). In the striatum, dopamine is released in tonic (slow and regular) and phasic (short, burst/spikes) frequency patterns (Liss & Roeper 2010) that are subject to heavy modulation by these cholinergic systems (Rice & Cragg 2004). Normally, when increasing electrical stimulation frequencies are applied to dopamine terminals in brain slices, the total amount of dopamine release stays relatively stable; however, when acetylcholine is blocked by a nAChR antagonist, dopamine release is robustly responsive to stimulation frequency (Rice & Cragg 2004; Zhang & Sulzer 2004). These results have led to the hypothesis that acetylcholine in the striatum acts as a low-pass filter at dopamine terminals. In other words, in basal conditions where tonic acetylcholine is present high frequencies stimulations are “filtered out”, but when acetylcholine is blocked, or reduced via endogenous mechanisms, this filter is lifted (Rice & Cragg 2004; Zhang & Sulzer 2004).
The above examples focus on how signaling through nAchRs can alter dopamine release; however, these effects are beginning to be further characterized based on the type and location of nAchRs in the striatum. nAChRs have a number of subtypes, and as such, the expression and subunit composition of nAChRs on dopamine terminals is of vast importance to activity-dependent regulation of dopamine neurotransmission (Threlfell & Cragg 2011). Neuronal nAChRs are composed of combinations of any of the 10 alpha (α2 – α10) and 3 beta (β2 -β4) subunits (McGehee & Role 1995; Karlin 2002; Karlin 1993) . Each receptor is composed of 5 of these subunits, typically in a heteromeric composition, though homomeric arrangements also exist. Thus, there are a large number of possible compositions which can vary greatly in their physiological and biophysical properties (Lim et al. 2014) . Within the NAc, the most commonly expressed receptors contain α4, α6, α7, β2 and β3 (Quik et al. 2007) and are most often broadly divided into two groups that are either α6 or non- α6 containing (Threlfell & Cragg 2011; Salminen et al. 2004).
These subtypes of nAchRs can confer different biophysical properties, which adds further complexity in addition to differences in cell-type specific expression in the striatum. nAChRs have been shown to be located on cholinergic interneurons (Azam et al. 2003), glutamatergic terminals in the NAc (Marchi et al. 2002), cell bodies of GABAergic interneuronal populations (Inoue et al. 2016), and dopaminergic terminals (Lim et al. 2014; Champtiaux et al. 2003). Their location on dopamine terminals permits complex local circuit regulation through cholinergic regulation depending on spatial localization and timing. The β2 subunit is thought to be expressed in all nAChRs on dopamine terminals within the striatum, and has been shown to be important for the rewarding effects of nicotine (Picciotto et al. 1998). Moreover, activation of these specific receptors enhances dopamine release via a calcium-dependent mechanism, showing their ability to directly alter effectors necessary for dopamine release and associated plasticity (Turner 2004). Importantly, α6β2 subunit containing nAChRs located on dopamine terminals have been shown to mediate differential behavioral and physiological responses to nicotine (Siciliano et al. 2017; Fennell et al. 2019) . Therefore, the type of nAChR subunits present play a particularly important and critical role in the direct modulation of dopamine release via acetylcholine release.
2.1.5. Heterosynaptic regulation of calcium flux
While we discussed calcium channels as homosynaptic regulators of dopamine release above, it is important to note that many ligand-gated ion channels also often are highly permeable to calcium. Thus, calcium regulation via either homosynaptic or heterosynaptic mechanisms plays a significant role in activity-dependent dopamine release dynamics. In particular, nAChRs and NMDA, another ligand gated receptor, have both been demonstrated to be permeable to calcium (MacDermott et al. 1986). In fact, recent work showed that calcium entry through nAChRs leads to enhanced size of the ready releasable pool, which can subsequently result in increased quantal dopamine release (Turner 2004). Similarly, early work has shown that NMDA receptor activation can trigger dopamine release in the striatum, which may be mediated by increased calcium flux into the terminal, rather than other direct mechanisms (discussed in section 3.1) (Krebs et al. 1989; Krebs et al. 1991; Cheramy et al. 1996). Thus, modifications in calcium entry and synaptic regulation of this process can also play a critical role in regulating dopamine release dynamics across the striatum.
2.2. Heterosynaptic transport mechanisms: Organic cation transporter (OCT3)
The organic cation transporter 3 (OCT3) is a high capacity, low affinity transporter located on dopamine neurons (Mayer et al. 2018), surrounding non-dopaminergic neurons and astrocytes (Cui et al. 2009) that plays a smaller role than DAT in clearing dopamine from the extracellular space, but nonetheless does participate in dopamine temporal dynamics. OCT3 is a low affinity, high capacity system meaning that it does not efficiently bind dopamine but has the capacity to transport large amounts of substrate. Functionally, this transporter works to reduce excess extracellular dopamine to prevent toxicity and oxidative stress (Cui et al. 2009). Inhibition of this transporter by the steroid hormone corticosterone can affect dopamine transients in vivo and can extend effects of DAT-inhibitor drugs like cocaine (Graf et al. 2013). Other hormones, including gonadal hormones like 17β-estradiol (E2) and progesterone, may inhibit this transporter (Iversen & Salt 1970) but in vivo studies have not yet been conducted to determine if these effects occur in live animals. These hormonal effects point to the possibility of potential sex differences, however there is a surprising paucity of data regarding hormonal action on microcircuit regulation of dopamine dynamics.
Importantly, the ability of both steroid and sex hormones to selectively alter clearance through modulation of secondary uptake mechanisms provides an avenue for dopamine regulation in a context-specific fashion. Because low affinity transporters like OCT3 are only engaged in situations where there is high concentration of extracellular dopamine, this likely only plays a strong role in regulating dopamine signaling in certain situations (e.g. high intensity stimuli or drug effects) but not others (basal dopamine release). Thus, these mechanisms are likely more complicated than simply increasing or decreasing extracellular dopamine levels, and therefore understanding dopamine regulation moving forward will require an understanding of context-specific processes.
2.3. Non-canonical regulatory mechanisms: diffusible transmitters and hormones
In addition to modulation by the neurotransmitter mediated- and clearance based-mechanisms, there are several diffusible signaling mechanisms that play potent roles in modulating dopamine release, including nitric oxide, hydrogen peroxide (H2O2), and insulin. These signaling molecules, especially for hormonal control, allow for state-dependent regulation of dopamine release to modify signal transduction in different cellular environments.
2.3.1. Nitric oxide regulation of dopamine transmission
Nitric oxide is produced by a variety of neurons throughout the brain and its production is stimulated by elevated intracellular calcium levels through a calmodulin-dependent process [reviewed in (Vincent 2010)]. However, these neurons densely innervate neighboring neurons, supporting potential involvement of nitric oxide in the regulation of many systems, including dopamine terminals in the NAc. Indeed, sodium nitroprusside, which generates nitric oxide, dose-dependently increases the amount of [3H]-dopamine released from pre-loaded dopamine terminals (Hanbauer et al. 1992), and slows [3H]-dopamine uptake rates measured in striatal synaptosomes (Pogun et al. 1994). Further, administration of nitric oxide synthase inhibitors, which reduce nitric oxide production, decreases striatal dopamine release (Kiss et al. 1999), showing that the effects of nitric oxide are bidirectional. This relationship remains true for [3H]-dopamine electrically evoked from intact terminals in striatal slices (Sandor et al. 1995). It has been suggested that some of the effects of nitric oxide occur via direct inhibition of DAT, thereby slowing clearance to increase synaptic dopamine levels (Kiss et al. 1999; Kiss 2000). Indeed, the effects of nitric oxide on dopamine release can be blocked by nomifensine, a potent DAT inhibitor (Kiss et al. 1999). Together this provides a potent mechanism by which activity-dependent regulation of postsynaptic neurons can cause significant plasticity to dopamine terminals to alter feedforward signaling in this circuit following activity – and associated calcium influx – in postsynaptic cells.
2.3.2. Hydrogen peroxide (H2O2) modulation of dopamine dynamics
The enzymatic breakdown of dopamine and other monoamine neurotransmitters results in the production of several by-products, including reactive oxygen species (ROS) such hydrogen peroxide (H2O2). While atypical production of ROS is considered a harmful effect of oxidative cellular stress, under normal physiological conditions these species can also serve as important diffusible chemical messengers. Early work in the hippocampus deduced that H2O2 may inhibit release via inactivation of presynaptic ion channels (Pellmar 1987). Similarly, within the dopaminergic terminal, endogenous production of H2O2 inhibits presynaptic dopamine release through a calcium-dependent mechanism (Chen et al. 2001). H2O2 is generated from enzymatic reactions within medium spiny neurons and results in retrograde signaling that inhibits terminal dopamine release through ATP-sensitive potassium channels (Rice 2011). More recent work, leveraging approaches to monitor fluctuations in H2O2 and dopamine release concurrently, has also demonstrated H2O2-induced inhibition of dopamine release in dorsal striatum (Spanos et al. 2013). Thus, H2O2 along with nitric oxide represent two diffusible molecules that are generated in the postsynaptic cells in the striatum that function as potent retrograde regulators of dopamine terminals.
2.3.3. Insulin
In addition to the other receptors mentioned within this review, the NAc also expresses a high density of insulin receptors which function to regulate the reinforcing aspects of food intake through direct modulation of dopamine terminals (Werther et al. 1987; Fordahl & Jones 2017; Stouffer et al. 2015). Circulating levels of insulin have indeed been shown to bidirectionally modify dopamine dynamics, through interactions with DAT and through DAT trafficking mechanisms. In normal/basal states, insulin activates PI3 kinase (PI3K), leading to increases in dopamine uptake rates (Carvelli et al. 2002), an effect that leads to more efficient repackaging of dopamine. However in low-glucose states, for example following chronic food restriction, low circulating insulin levels are concomitant with reduced dopamine uptake rates – thus prolonging the time that dopamine is present in the synaptic cleft following release (Zhen et al. 2006). Dietary manipulations that lead to insulin resistance have shown a similar phenotype (Fordahl & Jones 2017). Similarly, glucose clearance rate is negatively correlated with dopamine clearance rates (Fordahl & Jones 2017) and this effect can be reversed by dose-dependent bath application of insulin (Fordahl & Jones 2017), indicating that the effects of insulin on dopamine dynamics are rapid and direct. Other lines of research have shown that insulin also reduces somatodendritic dopamine release in the VTA, providing a mechanism that also influences terminal DA release (Mebel et al. 2012; Naef et al. 2019).
Thus, in addition to canonical regulatory mechanisms through neurotransmitter systems, a number of diffusible signaling molecules and hormones act directly on dopamine terminals to alter dopamine signaling in response to both internal states (hunger, satiety) and following experience-dependent post-synaptic plasticity. These mechanisms serve as a process by which dopamine release, repackaging, and clearance can be modulated on either a rapid or more prolonged timescale to influence how incoming upstream signals induce dopamine release and associated downstream signaling.
3. Other candidate direct mechanisms
The sections above have outlined the receptor systems present directly on dopamine terminals that allow for direct monosynaptic regulation of dopamine release dynamics. However, there are a number of other systems that show robust dopamine terminal regulation independent of VTA activity - and as such may exert direct control; yet many of these effects have been debated to be through other indirect polysynaptic mechanisms and require further analysis to definitively define their circuit localization.
One important candidate mechanism concerns ionotropic glutamate receptors, such as the NMDA receptor, and whether they can directly modulate presynaptic dopaminergic terminal. Indeed, early work using push-pull cannulae and superfusion slice methods showed that activation of NMDA receptors resulted in increased dopamine release in the striatum (Krebs et al. 1989; Krebs et al. 1991; Cheramy et al. 1996). More recent work using in vivo voltammetric recordings demonstrated that intra-striatal infusion of an NMDA receptor antagonist reduces dopamine release (Borland & Michael 2004; Kulagina et al. 2001). However, ex vivo application of NMDA receptors agonists also reduced evoked accumbal dopamine release (Yavas & Young 2017; Wu et al. 2000), an effect that is blocked by application of an NMDAR antagonist. These opposing effects – where in vivo and ex vivo application of an antagonist and agonists have the same effect on dopamine release - suggest that there may be multiple circuit-based mechanisms responsible for these effects, and it has been suggested that these effects are unlikely to be mediated by direct modulation of terminal excitability (Yavas & Young 2017). A prominent theory is that NMDAR activation increases local glutamate spillover and leads to subsequent regulation of dopamine terminals through either activation of metabotropic glutamate receptors and/or cholinergic interneurons (Zhang & Sulzer 2003). Data suggests that both metabotropic glutamate receptors and nAChRs are key mediators of NMDAR-mediated effects, as antagonism of both receptors blocked NMDAR effects (Zhang & Sulzer 2003; Marchi & Grilli 2010). However, it is also possible that NMDAR activation increases in the activity of local cholinergic interneurons and acetylcholine release then activates nAChRs directly on dopamine terminals. However, activation of nAChRs negatively regulates the effect of NMDAR agonists on dopamine release via triggering internalization of NMDA receptors that are located directly on the dopaminergic terminals themselves, suggesting NMDA effects likely wok through other mechanisms (Salamone et al. 2014).
Neurotensin is a signaling peptide that binds to the G-protein coupled receptor neurotensin receptor 1 (NTSR1) and plays a role in regulating synaptic plasticity in the dopaminergic system (Binder et al. 2001; White et al. 2012). These receptors are present on both cell bodies in the VTA and SN, as well as presynaptically in the dorsal and ventral portions of the striatum (Nicot et al. 1994; Palacios & Kuhar 1981; Schotte & Leysen 1989; Quirion et al. 1985). With respect to presynaptic release regulation, exogenous application of neurotensin has been shown to potentiate evoked dopamine release in NAc slices (Hetier et al. 1988). More recent voltammetric studies showed that phasic stimulation of dopaminergic terminal concurrent with exogenous neurotensin application results in augmented dopamine release (Fawaz et al. 2009). However, the effects are only observed with voltammetric recordings following multiple pulse, but not single pulse, stimulations. This is important to note as multiple pulse stimulation paradigms in ex vivo voltammetric studies allow for the recruitment of indirect circuit mechanisms, while single pulse stimulations largely isolate direct terminal effects. Thus, it is clear that neurotensin has potent regulatory effects on dopamine terminals in striatal regions; however, it is unclear whether this effect is monosynaptic or polysynaptic.
4. Expanding our understanding of dopamine release regulation
Taken together there are complex regulatory mechanisms that control dopamine release at the level of the terminal. The microcircuit mechanisms described here may allow for encoding of important information that extends computations beyond just the amount of relative release and may control dopamine release in microdomains. Indeed, there is evidence for differential dopamine release regulation and clearance kinetics within genetically defined patch and matrix compartments, even within a single defined region like the NAc core (Shu et al. 2013; Brimblecombe & Cragg 2017; Nastuk & Graybiel 1985; Jiménez-Castellanos & Graybiel 1989). Thus, moving forward it will be critical to understand how different release regulation that is driven by these monosynaptic connections alters behavior and signaling in unique ways.
Another important caveat is that a large majority of this work was conducted in male animals, despite seminal work showing significant local effects of gonadal hormones within mesolimbic systems (Becker 1999; Walker et al. 2006; Walker et al. 2000; Bazzett & Becker 1994; Cummings et al. 2014; Yoest et al. 2018; Lynch et al. 2002; Carroll & Smethells 2015; Anker & Carroll 2011). It should be noted that much of the work in males was conducted in ex vivo preparations which isolates the local circuits, and as such is advantageous for investigating direct mechanisms without confounding influences of long-range projection systems and other whole animal systems such as hormonal influences. However, it is difficult to disentangle how the organizational effects of sex may influence local microcircuitry and interact with the mechanisms described above. Along these lines, there is significant evidence of differences in distribution and size of dopamine cell bodies between the sexes, even early in development, suggesting that dopaminergic systems are at least in part differentially organized (Ovtscharoff et al. 1992). However, studies to explicitly test whether the reviewed mechanisms are present in females, the microcircuits are organized the same way between the sexes, or whether they affect dopamine release to a similar extent in both sexes have not been completed to date. Further, while work has focused on how sex interacts with aspects of substance use (Townsend et al. 2019; Siciliano 2019), depression (Ma et al. 2019), motivation and the dopaminergic mechanisms underlying these phenotypes (Becker & Koob 2016; Brady et al. 2019), it remains mostly unclear how sex interacts with the specific local terminal regulatory mechanisms outlined in this review to drive these behaviors. Taken together, lack of understanding of how terminal dopamine is modulated in both males and females will further impede the development of pharmaceuticals to many neuropsychiatric conditions involving dopamine dysfunction, including substance use disorder (Correa-De-Araujo 2006).
Finally, there has been a significant body of work that has sought to link dopamine to its role in motivated behavior, and as such, has supported dopamine’s role in reinforcement learning, motivation, and disorders associated with these behaviors (Dayan 2009; Berridge 2012; Berke 2018; Watabe-Uchida et al. 2017; Keiflin & Janak 2015; Ahmed 2018). However, discerning the exact contributions of the above described microcircuit mechanisms to dopamine release events during learning has remained elusive due to technical limitations. Early studies were conducted using microdialysis methods, which provided valuable insights but lacked the resolution to dissociate effects on dopamine release that occurred directly at the dopamine terminal from effects upstream in the VTA. Still other studies have utilized transgenic mouse lines (Lammel et al. 2015) or blocked entire receptor systems (Roberts et al. 1977; Mahler et al. 2019), both of which make it difficult to disentangle cell body mechanisms from terminal regulation. While the VTA to NAc projection is intimately involved with reward learning, activity in this circuit can result in an array of dopamine release mechanisms – reviewed above-, and how these distinct mechanisms contribute to learning processes has yet to be established. Moreover, the variety of mechanisms and resulting dopamine signal may differentially encode aspects of contingencies, and as such, achieving a precise understanding how these microcircuits evoke dopamine and in what specific contexts they are invoked is critical to understanding how this release controls downstream signaling and achieves behavioral control.
Acknowledgements:
The authors have no conflicts to report. We would like to thank Drs. Mark J. Ferris, Paul M. Jenkins, and Drew D. Kiraly, MD, for their helpful comments during the preparation of this manuscript. This work was supported by NIH grants DA042111 and DA048931 to E.S.C., DA045103 to C.A.S., GM07628 (J.E.Z.), and MH065215 (S.O.N.) as well as by funds DA047777 to A.R.J, Brain and Behavior Research Foundation to E.S.C and C.A.S., the Whitehall Foundation to E.S.C., and the Edward Mallincrodt Jr. Foundation to E.S.C. The figure was re-drawn in BioRender (biorender.com) by Marco Bazelmans based on a draft provided by the authors.
List of Abbreviations
- ACh
acetylecholine
- ChAT
choline acetyltransferase
- DAT
dopamine transporter
- D2R
dopamine type-2 atuoreceptor
- ERK
extracellular signal-related kinase
- E2
17β-estradiol
- Glu
glutamate
- GABAR
GABA receptor
- GPCR
G-protein coupled receptor
- H2O2
hydrogen peroxide
- IR
insulin receptor
- KOR
K-opioid receptor
- MSN
medium spiny neuron
- mTOR
mammalian target of rapamycin
- mGluR
metabotropic glutamate receptor
- NAc
nucleus accumbens
- nAChR
nicotininc acetylcholine receptor
- NO
nitric oxide
- NT
neurotensin
- NTSR1
neurotensin receptor 1
- OCT3
organic cation transporter 3
- PI3K
phosphoinositide 3-kinase
- PKC
protein kinase C
- PPAR
peroxisome proliferator-activated receptor
- PV
paralbumin
- ROS
reactive oxygen species
- SN
substantia nigra
- SST
somatostatin
- VMAT
vesicular monoamine transporter
- Vmax
maximal rate of dopamine reuptake
- VTA
ventral tegmental area
References
- Ahmed SH (2018) Trying to make sense of rodents’ drug choice behavior. Progress in Neuro-Psychopharmacology and Biological Psychiatry 87, 3–10. [DOI] [PubMed] [Google Scholar]
- Anker JJ and Carroll ME (2011) Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Current topics in behavioral neurosciences 8, 73–96. [DOI] [PubMed] [Google Scholar]
- Anzalone A, Lizardi-Ortiz JE, Ramos M et al. (2012) Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J. Neurosci 32, 9023–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan U and Leslie FM (2003) Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience 119, 965–977. [DOI] [PubMed] [Google Scholar]
- Bauman AL, Apparsundaram S, Ramamoorthy S, Wadzinski BE, Vaughan RA and Blakely RD (2000) Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J. Neurosci 20, 7571–7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA Jr., Partilla JS et al. (2012) The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37, 1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzett TJ and Becker JB (1994) Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 637, 163–172. [DOI] [PubMed] [Google Scholar]
- Becker JB (1999) Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol. Biochem. Behav 64, 803–812. [DOI] [PubMed] [Google Scholar]
- Becker JB and Chartoff E (2019) Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 44, 166–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB and Koob GF (2016) Sex Differences in Animal Models: Focus on Addiction. Pharmacol. Rev 68, 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V and Jaber M (2007) Cocaine-induced sensitization is associated with altered dynamics of transcriptional responses of the dopamine transporter, tyrosine hydroxylase, and dopamine D2 receptors in C57Bl/6J mice. Psychopharmacology 193, 567–578. [DOI] [PubMed] [Google Scholar]
- Bellani S, Sousa VL, Ronzitti G, Valtorta F, Meldolesi J and Chieregatti E (2010) The regulation of synaptic function by α-synuclein. Commun. Integr. Biol 3, 106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belovich AN, Aguilar JI, Mabry SJ et al. (2019) A network of phosphatidylinositol (4,5)-bisphosphate (PIP(2)) binding sites on the dopamine transporter regulates amphetamine behavior in Drosophila Melanogaster. Mol Psychiatry [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Marand M, Ballion B, Borrelli E, Boraud T and Gonon F (2011) Inhibition of dopamine uptake by D2 antagonists: an in vivo study. J. Neurochem 116, 449–458. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E and Gonon F (2001) Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J. Neurosci 21, 9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD (2018) What does dopamine mean? Nature neuroscience 21, 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191, 391–431. [DOI] [PubMed] [Google Scholar]
- Berridge KC (2012) From prediction error to incentive salience: mesolimbic computation of reward motivation. European Journal of Neuroscience 35, 1124–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson MJ, Cheramy A, Feltz P and Glowinski J (1969) Release of newly synthesized dopamine from dopamine-containing terminals in the striatum of the rat. Proc. Natl. Acad. Sci. U. S. A 62, 741–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ and Nemeroff CB (2001) Neurotensin and dopamine interactions. Pharmacol Rev 53, 453–486. [PubMed] [Google Scholar]
- Borland LM and Michael AC (2004) Voltammetric study of the control of striatal dopamine release by glutamate. Journal of Neurochemistry 91, 220–229. [DOI] [PubMed] [Google Scholar]
- Brady LJ, Johnson AR, Tat J and Calipari ES (2019) Sex Differences in Local Nucleus Accumbens Circuitry and Motivated Behavior. The FASEB Journal 33, 805.807–805.807. [Google Scholar]
- Brimblecombe KR and Cragg SJ (2017) The Striosome and Matrix Compartments of the Striatum: A Path through the Labyrinth from Neurochemistry toward Function. ACS Chem. Neurosci 8, 235–242. [DOI] [PubMed] [Google Scholar]
- Brimblecombe KR, Gracie CJ, Platt NJ and Cragg SJ (2015) Gating of dopamine transmission by calcium and axonal N-, Q-, T- and L-type voltage-gated calcium channels differs between striatal domains. J. Physiol 593, 929–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt JP and McGehee DS (2008) Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens. J. Neurosci 28, 1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodnik ZD, Batra A, Oleson EB and España RA (2019) Local GABAA Receptor-Mediated Suppression of Dopamine Release within the Nucleus Accumbens. ACS Chem. Neurosci 10, 1978–1985. [DOI] [PubMed] [Google Scholar]
- Brog JS, Salyapongse A, Deutch AY and Zahm DS (1993) The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J. Comp. Neurol 338, 255–278. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB and Chavkin C (2010) The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Research 1314, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang H-L, Morales M, Lovinger DM and Cheer JF (2012) Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep. 2, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Melchior JR, Bermejo K, Salahpour A, Roberts DC and Jones SR (2014) Methylphenidate and cocaine self‐administration produce distinct dopamine terminal alterations. Addiction biology 19, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Juarez B, Morel C et al. (2017) Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun 8, 13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo DL and Bean BP (1995) Voltage-dependent calcium channels in rat midbrain dopamine neurons: modulation by dopamine and GABAB receptors. J. Neurophysiol 74, 1137–1148. [DOI] [PubMed] [Google Scholar]
- Carroll ME and Smethells JR (2015) Sex Differences in Behavioral Dyscontrol: Role in Drug Addiction and Novel Treatments. Front. Psychiatry 6, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier E, Hamilton PJ, Belovich AN et al. (2015) Rare autism-associated variants implicate syntaxin 1 (STX1 R26Q) phosphorylation and the dopamine transporter (hDAT R51W) in dopamine neurotransmission and behaviors. EBioMedicine 2, 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Morón JA, Kahlig KM et al. (2002) PI 3-kinase regulation of dopamine uptake. Journal of Neurochemistry 81, 859–869. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M et al. (2003) Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J. Neurosci 23, 7820–7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanaday NL, Cousin MA, Milosevic I, Watanabe S and Morgan JR (2019) The Synaptic Vesicle Cycle Revisited: New Insights into the Modes and Mechanisms. J. Neurosci 39, 8209–8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Avshalumov MV and Rice ME (2001) H2O2 Is a Novel, Endogenous Modulator of Synaptic Dopamine Release. Journal of Neurophysiology 85, 2468–2476. [DOI] [PubMed] [Google Scholar]
- Chen BT and Bonci A (2017) The Mesocortical Dopaminergic System. The Human Frontal Lobes: Functions and Disorders, 13. [Google Scholar]
- Chen N and Reith ME (2000) Structure and function of the dopamine transporter. Eur. J. Pharmacol 405, 329–339. [DOI] [PubMed] [Google Scholar]
- Cheramy A, Godeheu G, L’Hirondel M and Glowinski J (1996) Cooperative contributions of cholinergic and NMDA receptors in the presynaptic control of dopamine release from synaptosomes of the rat striatum. The Journal of pharmacology and experimental therapeutics 276, 616–625. [PubMed] [Google Scholar]
- Chesselet MF (1984) Presynaptic regulation of neurotransmitter release in the brain: facts and hypothesis. Neuroscience 12, 347–375. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB and Levey AI (1995) The dopamine transporter: immunochemical characterization and localization in brain. J. Neurosci 15, 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AL, Aitken TJ, Greenfield VY, Ostlund SB and Wassum KM (2016) Nucleus Accumbens Acetylcholine Receptors Modulate Dopamine and Motivation. Neuropsychopharmacology 41, 2830–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon MD, Platt NJ, Zhang Y-F et al. (2019) Plasticity in striatal dopamine release is governed by release-independent depression and the dopamine transporter. Nat. Commun 10, 4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congar P, Bergevin A and Trudeau LE (2002) D2 receptors inhibit the secretory process downstream from calcium influx in dopaminergic neurons: implication of K+ channels. J Neurophysiol 87, 1046–1056. [DOI] [PubMed] [Google Scholar]
- Correa-De-Araujo R (2006) Serious gaps: how the lack of sex/gender-based research impairs health. Journal of women’s health (2002) 15, 1116–1122. [DOI] [PubMed] [Google Scholar]
- Covey DP, Juliano SA and Garris PA (2013) Amphetamine elicits opposing actions on readily releasable and reserve pools for dopamine. PLoS One 8, e60763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley NA and Kash TL (2015) Kappa opioid receptor signaling in the brain: Circuitry and implications for treatment. Progress in Neuro-Psychopharmacology and Biological Psychiatry 62, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Aras R, Christian WV et al. (2009) The organic cation transporter-3 is a pivotal modulator of neurodegeneration in the nigrostriatal dopaminergic pathway. Proc. Natl. Acad. Sci. U. S. A 106, 8043–8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JA, Jagannathan L, Jackson LR and Becker JB (2014) Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend. 135, 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan P (2009) Dopamine, reinforcement learning, and addiction. Pharmacopsychiatry 42 Suppl 1, S56–65. [DOI] [PubMed] [Google Scholar]
- del Castillo J and Katz B (1954) Quantal components of the end-plate potential. J. Physiol 124, 560–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker A and Rizzoli SO (2010) Synaptic vesicle pools: an update. Front. Synaptic Neurosci 2, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egaña LA, Cuevas RA, Baust TB et al. (2009) Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J. Neurosci 29, 4592–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden LE and Weihe E (2011) VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse. Ann. N. Y. Acad. Sci 1216, 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JD, Eiden LE and Hoffman BJ (1992) Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc. Natl. Acad. Sci. U. S. A 89, 10993–10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing AG, Bigelow JC and Wightman RM (1983) Direct in vivo monitoring of dopamine released from two striatal compartments in the rat. Science 221, 169–171. [DOI] [PubMed] [Google Scholar]
- Fagan RR, Kearney PJ, Sweeney CG et al. (2020) Dopamine transporter trafficking and Rit2 GTPase: Mechanism of action and in vivo impact. J Biol Chem. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano C, Kortleven C and Trudeau LE (2010) Chronic activation of the D2 autoreceptor inhibits both glutamate and dopamine synapse formation and alters the intrinsic properties of mesencephalic dopamine neurons in vitro. Eur J Neurosci 32, 1433–1441. [DOI] [PubMed] [Google Scholar]
- Fawaz CS, Martel P, Leo D and Trudeau L-E (2009) Presynaptic action of neurotensin on dopamine release through inhibition of D2 receptor function. BMC Neuroscience 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennell AM, Pitts EG, Sexton LL and Ferris MJ (2019) Phasic Dopamine Release Magnitude Tracks Individual Differences in Sensitization of Locomotor Response following a History of Nicotine Exposure. bioRxiv, 646844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floor E, Leventhal PS, Wang Y, Meng L and Chen W (1995) Dynamic storage of dopamine in rat brain synaptic vesicles in vitro. J. Neurochem 64, 689–699. [DOI] [PubMed] [Google Scholar]
- Fog JU, Khoshbouei H, Holy M et al. (2006) Calmodulin kinase II interacts with the dopamine transporter C terminus to regulate amphetamine-induced reverse transport. Neuron 51, 417–429. [DOI] [PubMed] [Google Scholar]
- Ford CP (2014) The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience 282, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordahl SC and Jones SR (2017) High-Fat-Diet-Induced Deficits in Dopamine Terminal Function Are Reversed by Restoring Insulin Signaling. ACS Chemical Neuroscience 8, 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Adkins SD, Lever JR and Vaughan RA (2008) Phorbol ester induced trafficking-independent regulation and enhanced phosphorylation of the dopamine transporter associated with membrane rafts and cholesterol. J. Neurochem 105, 1683–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JD, Yang J-W, Moritz AE, Challasivakanaka S, Smith MA, Holy M, Wilebski K, Sitte HH and Vaughan RA (2012) Dopamine transporter phosphorylation site threonine 53 regulates substrate reuptake and amphetamine-stimulated efflux. J. Biol. Chem 287, 29702–29712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler MA, Sidiropoulou K, Ozkan ED, Phillips CW and Cooper DC (2007) Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. PLoS One 2, e573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed C, Revay R, Vaughan RA, Kriek E, Grant S, Uhl GR and Kuhar MJ (1995) Dopamine transporter immunoreactivity in rat brain. J. Comp. Neurol 359, 340–349. [DOI] [PubMed] [Google Scholar]
- Fulton S, Thibault D, Mendez JA, Lahaie N, Tirotta E, Borrelli E, Bouvier M, Tempel BL and Trudeau L-E (2011) Contribution of Kv1.2 voltage-gated potassium channel to D2 autoreceptor regulation of axonal dopamine overflow. The Journal of biological chemistry 286, 9360–9372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère N, Delignat-Lavaud B, Herborg F et al. (2019) Increased vulnerability of nigral dopamine neurons after expansion of their axonal arborization size through D2 dopamine receptor conditional knockout. PLoS genetics 15, e1008352–e1008352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorguieff MF, Kemel ML and Glowinski J (1977) Presynaptic effect of L-glutamic acid on the release of dopamine in rat striatal slices. Neuroscience Letters 6, 73–77. [DOI] [PubMed] [Google Scholar]
- Giros B and Caron MG (1993) Molecular characterization of the dopamine transporter. Trends Pharmacol. Sci 14, 43–49. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM and Caron MG (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379, 606–612. [DOI] [PubMed] [Google Scholar]
- Gleich T, Deserno L, Lorenz RC et al. (2015) Prefrontal and Striatal Glutamate Differently Relate to Striatal Dopamine: Potential Regulatory Mechanisms of Striatal Presynaptic Dopamine Function? The Journal of Neuroscience 35, 9615–9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y and Stevens CF (1998) Readily releasable pool size changes associated with long term depression. Proc. Natl. Acad. Sci. U. S. A 95, 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar R, Gresch PJ, Davis GL, Katamish RM, Riele JR, Stewart AM, Vaughan RA, Hahn MK and Blakely RD (2018) Region-Specific Regulation of Presynaptic Dopamine Homeostasis by D2 Autoreceptors Shapes the In Vivo Impact of the Neuropsychiatric Disease-Associated DAT Variant Val559. The Journal of Neuroscience 38, 5302–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC and Marks MJ (2007) The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem. Pharmacol 74, 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf EN, Wheeler RA, Baker DA et al. (2013) Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J. Neurosci 33, 11800–11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PJ, Belovich AN, Khelashvili G et al. (2014) PIP2 regulates psychostimulant behaviors through its interaction with a membrane protein. Nature chemical biology 10, 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanbauer I, Wink D, Osawa Y, Edelman GM and Gally JA (1992) Role of nitric oxide in NMDA-evoked release of [3H]-dopamine from striatal slices. Neuroreport 3, 409–412. [DOI] [PubMed] [Google Scholar]
- Hetier E, Boireau A, Dubedat P and Blanchard J (1988) Neurotensin effects on evoked release of dopamine in slices from striatum, nucleus accumbens and prefrontal cortex in rat. Naunyn-Schmiedeberg’s archives of pharmacology 337, 13–17. [DOI] [PubMed] [Google Scholar]
- Holloway ZR, Freels TG, Comstock JF, Nolen HG, Sable HJ and Lester DB (2019) Comparing phasic dopamine dynamics in the striatum, nucleus accumbens, amygdala, and medial prefrontal cortex. Synapse 73, e22074. [DOI] [PubMed] [Google Scholar]
- Hsu S-F, Augustine GJ and Jackson MB (1996) Adaptation of Ca2+-Triggered Exocytosis in Presynaptic Terminals. Neuron 17, 501–512. [DOI] [PubMed] [Google Scholar]
- Huang Y and Thathiah A (2015) Regulation of neuronal communication by G protein-coupled receptors. FEBS Lett. 589, 1607–1619. [DOI] [PubMed] [Google Scholar]
- Inoue R, Suzuki T, Nishimura K and Miura M (2016) Nicotinic acetylcholine receptor-mediated GABAergic inputs to cholinergic interneurons in the striosomes and the matrix compartments of the mouse striatum. Neuropharmacology 105, 318–328. [DOI] [PubMed] [Google Scholar]
- Iversen LL and Salt PJ (1970) Inhibition of catecholamine Uptake2 by steroids in the isolated rat heart. Br. J. Pharmacol 40, 528–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javoy F and Glowinski J (1971) Dynamic characteristic of the ‘functional compartment’ of dopamine in dopaminergic terminals of the rat striatum. J. Neurochem 18, 1305–1311. [DOI] [PubMed] [Google Scholar]
- Jiménez-Castellanos J and Graybiel AM (1989) Compartmental origins of striatal efferent projections in the cat. Neuroscience 32, 297–321. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Hu X-T, Cooper DC, Wightman RM, White FJ and Caron MG (1999) Loss of autoreceptor functions in mice lacking the dopamine transporter. Nature Neuroscience 2, 649–655. [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM and Caron MG (1998) Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. U. S. A 95, 4029–4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamin D, Lauterbach MA, Westphal V, Keller J, Schönle A, Hell SW and Rizzoli SO (2010) High- and low-mobility stages in the synaptic vesicle cycle. Biophys. J 99, 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Huggins KN, Konstantopoulos JK and Jones SR (2015) Chronic intermittent ethanol exposure reduces presynaptic dopamine neurotransmission in the mouse nucleus accumbens. Drug Alcohol Depend. 150, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Weiner JL and Jones SR (2016) Early-Life Social Isolation Stress Increases Kappa Opioid Receptor Responsiveness and Downregulates the Dopamine System. Neuropsychopharmacology 41, 2263–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A (1993) Structure of nicotinic acetylcholine receptors. Curr. Opin. Neurobiol 3, 299–309. [DOI] [PubMed] [Google Scholar]
- Karlin A (2002) Ion channel structure: emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci 3, 102. [DOI] [PubMed] [Google Scholar]
- Katz B (1971) Quantal mechanism of neural transmitter release. Science 173, 123–126. [DOI] [PubMed] [Google Scholar]
- Keiflin R and Janak PH (2015) Dopamine prediction errors in reward learning and addiction: from theory to neural circuitry. Neuron 88, 247–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RT, Jones SR and Wightman RM (1992) Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J. Neurochem 59, 449–455. [DOI] [PubMed] [Google Scholar]
- Kile BM, Guillot TS, Venton BJ, Wetsel WC, Augustine GJ and Wightman RM (2010) Synapsins differentially control dopamine and serotonin release. J. Neurosci 30, 9762–9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JP, Hennings ECP, Zsilla G and Vizi ES (1999) A possible role of nitric oxide in the regulation of dopamine transporter function in the striatum. Neurochemistry International 34, 345–350. [DOI] [PubMed] [Google Scholar]
- Kosillo P, Zhang Y-F, Threlfell S and Cragg SJ (2016) Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons. Cereb. Cortex [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs MO, Kemel ML, Gauchy C, Desban M and Glowinski J (1989) Glycine potentiates the NMDA-induced release of dopamine through a strychnine-insensitive site in the rat striatum. Eur J Pharmacol 166, 567–570. [DOI] [PubMed] [Google Scholar]
- Krebs MO, Trovero F, Desban M, Gauchy C, Glowinski J and Kemel ML (1991) Distinct presynaptic regulation of dopamine release through NMDA receptors in striosome- and matrix-enriched areas of the rat striatum. J Neurosci 11, 1256–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhr WG, Bigelow JC and Wightman RM (1986) In vivo comparison of the regulation of releasable dopamine in the caudate nucleus and the nucleus accumbens of the rat brain. The Journal of Neuroscience 6, 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulagina N, Zigmond M and Michael A (2001) Glutamate regulates the spontaneous and evoked release of dopamine in the rat striatum. Neuroscience 102, 121–128. [DOI] [PubMed] [Google Scholar]
- Kuwajima M, Dehoff MH, Furuichi T, Worley PF, Hall RA and Smith Y (2007) Localization and expression of group I metabotropic glutamate receptors in the mouse striatum, globus pallidus, and subthalamic nucleus: regulatory effects of MPTP treatment and constitutive Homer deletion. J. Neurosci 27, 6249–6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Steinberg Elizabeth E., Földy C, Wall Nicholas R., Beier K, Luo L and Malenka Robert C. (2015) Diversity of Transgenic Mouse Models for Selective Targeting of Midbrain Dopamine Neurons. Neuron 85, 429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ and Chavkin C (2008) The Dysphoric Component of Stress Is Encoded by Activation of the Dynorphin κ-Opioid System. The Journal of Neuroscience 28, 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer J, Becker JAJ, Befort K and Kieffer BL (2009) Reward processing by the opioid system in the brain. Physiol. Rev 89, 1379–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FJS, Pei L, Moszczynska A, Vukusic B, Fletcher PJ and Liu F (2007) Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J. 26, 2127–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai B and Vizi ES (2008) Nonsynaptic chemical transmission through nicotinic acetylcholine receptors. Physiol. Rev 88, 333–349. [DOI] [PubMed] [Google Scholar]
- Lim SAO, Kang UJ and McGehee DS (2014) Striatal cholinergic interneuron regulation and circuit effects. Front. Synaptic Neurosci 6, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss B and Roeper J (2010) 3.4 Ion Channels and Regulation of Dopamine Neuron Activity. Dopamine handbook, 118. [Google Scholar]
- Liu Y and Edwards RH (1997) The role of vesicular transport proteins in synaptic transmission and neural degeneration. Annu. Rev. Neurosci 20, 125–156. [DOI] [PubMed] [Google Scholar]
- Liu Y, Peter D, Roghani A, Schuldiner S, Privé GG, Eisenberg D, Brecha N and Edwards RH (1992) A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell 70, 539–551. [DOI] [PubMed] [Google Scholar]
- Loder MK and Melikian HE (2003) The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J. Biol. Chem 278, 22168–22174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr KM, Bernstein AI, Stout KA et al. (2014) Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc. Natl. Acad. Sci. U. S. A 111, 9977–9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr KM, Stout KA, Dunn AR, Wang M, Salahpour A, Guillot TS and Miller GW (2015) Increased Vesicular Monoamine Transporter 2 (VMAT2; Slc18a2) Protects against Methamphetamine Toxicity. ACS Chem. Neurosci 6, 790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes EF, Roberts BM, Siddorn RE, Clements MA and Cragg SJ (2019) Inhibition of Nigrostriatal Dopamine Release by Striatal GABAA and GABAB Receptors. J. Neurosci 39, 1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME and Carroll ME (2002) Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology 164, 121–137. [DOI] [PubMed] [Google Scholar]
- Ma L, Xu Y, Wang G and Li R (2019) What do we know about sex differences in depression: A review of animal models and potential mechanisms. Prog Neuropsychopharmacol Biol Psychiatry 89, 48–56. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Mayer ML, Westbrook GL, Smith SJ and Barker JL (1986) NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature 321, 519–522. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Brodnik ZD, Cox BM et al. (2019) Chemogenetic Manipulations of Ventral Tegmental Area Dopamine Neurons Reveal Multifaceted Roles in Cocaine Abuse. The Journal of Neuroscience 39, 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina FK and Mathews TA (2010) A functional fast scan cyclic voltammetry assay to characterize dopamine D2 and D3 autoreceptors in the mouse striatum. ACS Chem. Neurosci 1, 450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H and Watson SJ (1994) Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J. Comp. Neurol 350, 412–438. [DOI] [PubMed] [Google Scholar]
- Manzoni O, Michel JM and Bockaert J (1997) Metabotropic glutamate receptors in the rat nucleus accumbens. European Journal of Neuroscience 9, 1514–1523. [DOI] [PubMed] [Google Scholar]
- Marchi M and Grilli M (2010) Presynaptic nicotinic receptors modulating neurotransmitter release in the Central Nervous System: Functional interactions with other coexisting receptors. Progress in Neurobiology 92, 105–111. [DOI] [PubMed] [Google Scholar]
- Marchi M, Risso F, Viola C, Cavazzani P and others (2002) Direct evidence that release‐stimulating α7* nicotinic cholinergic receptors are localized on human and rat brain glutamatergic axon terminals. Journal of. [DOI] [PubMed] [Google Scholar]
- Martel P, Leo D, Fulton S, Bérard M and Trudeau L-E (2011) Role of Kv1 potassium channels in regulating dopamine release and presynaptic D2 receptor function. PLoS One 6, e20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer FP, Schmid D, Owens WA et al. (2018) An unsuspected role for organic cation transporter 3 in the actions of amphetamine. Neuropsychopharmacology 43, 2408–2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield RD and Zahniser NR (2001) Dopamine D2 receptor regulation of the dopamine transporter expressed in Xenopus laevis oocytes is voltage-independent. Mol. Pharmacol 59, 113–121. [DOI] [PubMed] [Google Scholar]
- McGehee DS and Role LW (1995) Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu. Rev. Physiol 57, 521–546. [DOI] [PubMed] [Google Scholar]
- Mebel DM, Wong JCY, Dong YJ and Borgland SL (2012) Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake. The European journal of neuroscience 36, 2336–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH (2006) Neuroanatomical localization of an internal clock: a functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res 1109, 93–107. [DOI] [PubMed] [Google Scholar]
- Meiergerd SM, Patterson TA and Schenk JO (1993) D2 receptors may modulate the function of the striatal transporter for dopamine: kinetic evidence from studies in vitro and in vivo. J. Neurochem 61, 764–767. [DOI] [PubMed] [Google Scholar]
- Mooslehner KA, Chan PM, Xu W, Liu L, Smadja C, Humby T, Allen ND, Wilkinson LS and Emson PC (2001) Mice with very low expression of the vesicular monoamine transporter 2 gene survive into adulthood: potential mouse model for parkinsonism. Molecular and cellular biology 21, 5321–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz AE, Rastedt DE, Stanislowski DJ, Shetty M, Smith MA, Vaughan RA and Foster JD (2015) Reciprocal Phosphorylation and Palmitoylation Control Dopamine Transporter Kinetics. J. Biol. Chem 290, 29095–29105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morón JA, Zakharova I, Ferrer JV et al. (2003) Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J. Neurosci 23, 8480–8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naef L, Seabrook L, Hsiao J, Li C and Borgland SL (2019) Insulin in the ventral tegmental area reduces cocaine-evoked dopamine in the nucleus accumbens in vivo. Eur J Neurosci 50, 2146–2155. [DOI] [PubMed] [Google Scholar]
- Nastuk MA and Graybiel AM (1985) Patterns of muscarinic cholinergic binding in the striatum and their relation to dopamine islands and striosomes. J. Comp. Neurol 237, 176–194. [DOI] [PubMed] [Google Scholar]
- Nicot A, Rostene W and Berod A (1994) Neurotensin receptor expression in the rat forebrain and midbrain: a combined analysis by in situ hybridization and receptor autoradiography. J Comp Neurol 341, 407–419. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W, Eusterschulte B, Zienecker R, Reisert I and Pilgrim C (1992) Sex differences in densities of dopaminergic fibers and GABAergic neurons in the prenatal rat striatum. Journal of Comparative Neurology 323, 299–304. [DOI] [PubMed] [Google Scholar]
- Page G, Peeters M, Najimi M, Maloteaux J-M and Hermans E (2001) Modulation of the neuronal dopamine transporter activity by the metabotropic glutamate receptor mGluR5 in rat striatal synaptosomes through phosphorylation mediated processes. J. Neurochem 76, 1282–1290. [DOI] [PubMed] [Google Scholar]
- Palacios JM and Kuhar MJ (1981) Neurotensin receptors are located on dopamine-containing neurones in rat midbrain. Nature 294, 587–589. [DOI] [PubMed] [Google Scholar]
- Palij P, Bull DR, Sheehan MJ, Millar J, Stamford J, Kruk ZL and Humphrey PP (1990) Presynaptic regulation of dopamine release in corpus striatum monitored in vitro in real time by fast cyclic voltammetry. Brain Res. 509, 172–174. [DOI] [PubMed] [Google Scholar]
- Paquet M and Smith Y (2003) Group I Metabotropic Glutamate Receptors in the Monkey Striatum: Subsynaptic Association with Glutamatergic and Dopaminergic Afferents. The Journal of Neuroscience 23, 7659–7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CL, Finkelstein DI, Drago J, Borrelli E and Horne MK (2001) The role of dopamine receptors in regulating the size of axonal arbors. J Neurosci 21, 5147–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CL, Stanic D, Drago J, Borrelli E, Finkelstein DI and Horne MK (2002) Effects of long-term treatment with dopamine receptor agonists and antagonists on terminal arbor size. Eur J Neurosci 16, 787–794. [DOI] [PubMed] [Google Scholar]
- Patel J, Mooslehner KA, Chan PM, Emson PC and Stamford JA (2003) Presynaptic control of striatal dopamine neurotransmission in adult vesicular monoamine transporter 2 (VMAT2) mutant mice. Journal of neurochemistry 85, 898–910. [DOI] [PubMed] [Google Scholar]
- Pellmar TC (1987) Peroxide alters neuronal excitability in the CA1 region of guinea-pig hippocampus in vitro. Neuroscience 23, 447–456. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ and Lopes da Silva FH (1994) The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol 42, 719–761. [DOI] [PubMed] [Google Scholar]
- Phillips PE and Stamford JA (2000) Differential recruitment of N-, P- and Q-type voltage-operated calcium channels in striatal dopamine release evoked by ‘regular’ and ‘burst’ firing. Brain Res 884, 139–146. [DOI] [PubMed] [Google Scholar]
- Phillips PEM, Hancock PJ and Stamford JA (2002) Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse 44, 15–22. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K and Changeux JP (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391, 173–177. [DOI] [PubMed] [Google Scholar]
- Pitman KA, Puil E and Borgland SL (2014) GABA(B) modulation of dopamine release in the nucleus accumbens core. Eur. J. Neurosci 40, 3472–3480. [DOI] [PubMed] [Google Scholar]
- Pogun S, Baumann MH and Kuhar MJ (1994) Nitric oxide inhibits [3H]dopamine uptake. Brain Research 641, 83–91. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Davila V and Sulzer D (1998) Presynaptic recording of quanta from midbrain dopamine neurons and modulation of the quantal size. J. Neurosci 18, 4106–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN, Larsen KE, Krantz DE, Liu Y, Haycock JW, Setlik W, Gershon MD, Edwards RH and Sulzer D (2000) Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J. Neurosci 20, 7297–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Bordia T and O’Leary K (2007) Nicotinic receptors as CNS targets for Parkinson’s disease. Biochem. Pharmacol 74, 1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirion R, Chiueh CC, Everist HD and Pert A (1985) Comparative localization of neurotensin receptors on nigrostriatal and mesolimbic dopaminergic terminals. Brain Research 327, 385–389. [DOI] [PubMed] [Google Scholar]
- Ramsson ES, Howard CD, Covey DP and Garris PA (2011) High doses of amphetamine augment, rather than disrupt, exocytotic dopamine release in the dorsal and ventral striatum of the anesthetized rat. J. Neurochem 119, 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME (2011) H2O2: a dynamic neuromodulator. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry 17, 389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME and Cragg SJ (2004) Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci 7, 583–584. [DOI] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Rizzoli SO and Betz WJ (2003) Synaptic vesicle pools at the frog neuromuscular junction. Neuron 39, 529–541. [DOI] [PubMed] [Google Scholar]
- Richardson BD, Saha K, Krout D et al. (2016) Membrane potential shapes regulation of dopamine transporter trafficking at the plasma membrane. Nat. Commun 7, 10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli SO and Betz WJ (2005) Synaptic vesicle pools. Nat. Rev. Neurosci 6, 57–69. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Corcoran ME and Fibiger HC (1977) On the role of ascending catecholaminergic systems in intravenous self-administration of cocaine. Pharmacology Biochemistry and Behavior 6, 615–620. [DOI] [PubMed] [Google Scholar]
- Ronken E, Mulder A and Schoffelmeer A (1993a) Interacting presynaptic k‐opioid and GABAa receptors modulate dopamine release from rat striatal synaptosomes. Journal of neurochemistry 61, 1634–1639. [DOI] [PubMed] [Google Scholar]
- Ronken E, Mulder AH and Schoffelmeer AN (1993b) Interacting presynaptic kappa-opioid and GABAA receptors modulate dopamine release from rat striatal synaptosomes. J. Neurochem 61, 1634–1639. [DOI] [PubMed] [Google Scholar]
- Rothblat DS and Schneider JS (1997) Regionally specific effects of haloperidol and clozapine on dopamine reuptake in the striatum. Neurosci. Lett 228, 119–122. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI and Partilla JS (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39, 32–41. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV and Borrelli E (2002) Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. J. Neurosci 22, 3293–3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ and Nestler EJ (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14, 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacaan AI, Bymaster FP and Schoepp DD (1992) Metabotropic glutamate receptor activation produces extrapyramidal motor system activation that is mediated by striatal dopamine. J. Neurochem 59, 245–251. [DOI] [PubMed] [Google Scholar]
- Salamone A, Zappettini S, Grilli M et al. (2014) Prolonged nicotine exposure down-regulates presynaptic NMDA receptors in dopaminergic terminals of the rat nucleus accumbens. Neuropharmacology 79, 488–497. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC and Grady SR (2004) Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol. Pharmacol 65, 1526–1535. [DOI] [PubMed] [Google Scholar]
- Sandor NT, Brassai A, Pliskas A and Lendvai B (1995) Role of nitric oxide in modulating neurotransmitter release from rat striatum. Brain research bulletin 36, 483–486. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Benoit-Marand M, Gonon F and Sulzer D (2003) Presynaptic regulation of dopaminergic neurotransmission. J. Neurochem 87, 273–289. [DOI] [PubMed] [Google Scholar]
- Schmitz Y, Schmauss C and Sulzer D (2002) Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J. Neurosci 22, 8002–8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotte A and Leysen JE (1989) Autoradiographic evidence for the localization of high affinity neurotensin binding sites on dopaminergic nerve terminals in the nigrostriatal and mesolimbic pathways in rat brain. J Chem Neuroanat 2, 253–257. [PubMed] [Google Scholar]
- Schuldiner S, Shirvan A and Linial M (1995) Vesicular neurotransmitter transporters: from bacteria to humans. Physiol. Rev 75, 369–392. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P and Montague PR (1997) A Neural Substrate of Prediction and Reward. Science 275, 1593–1599. [DOI] [PubMed] [Google Scholar]
- Segovia G and Mora F (2001) Involvement of NMDA and AMPA/kainate receptors in the effects of endogenous glutamate on extracellular concentrations of dopamine and GABA in the nucleus accumbens of the awake rat. Brain Res Bull 54, 153–157. [DOI] [PubMed] [Google Scholar]
- Séguéla P, Wadiche J, Dineley-Miller K, Dani JA and Patrick JW (1993) Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J. Neurosci 13, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Adrover MF and Alvarez VA (2017) Distinctive Modulation of Dopamine Release in the Nucleus Accumbens Shell Mediated by Dopamine and Acetylcholine Receptors. J. Neurosci 37, 11166–11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore PA (1976) Actions of amfonelic acid and other non-amphetamine stimulants on the dopamine neuron. J. Pharm. Pharmacol 28, 855–857. [DOI] [PubMed] [Google Scholar]
- Shu Z, Taylor IM and Michael AC (2013) The dopamine patchwork of the rat nucleus accumbens core. Eur. J. Neurosci 38, 3221–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA (2019) Capturing the complexity of sex differences requires multidimensional behavioral models. Neuropsychopharmacology 44, 1997–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Cuzon Carlson VC, Helms CM, Lovinger DM, Grant KA and Jones SR (2015) Voluntary ethanol intake predicts κ-opioid receptor supersensitivity and regionally distinct dopaminergic adaptations in macaques. J. Neurosci 35, 5959–5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES and Jones SR (2014) Amphetamine potency varies with dopamine uptake rate across striatal subregions. J. Neurochem 131, 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, McIntosh JM, Jones SR and Ferris MJ (2017) α6β2 subunit containing nicotinic acetylcholine receptors exert opposing actions on rapid dopamine signaling in the nucleus accumbens of rats with high-versus low-response to novelty. Neuropharmacology 126, 281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanos M, Gras-Najjar J, Letchworth JM, Sanford AL, Toups JV and Sombers LA (2013) Quantitation of Hydrogen Peroxide Fluctuations and Their Modulation of Dopamine Dynamics in the Rat Dorsal Striatum Using Fast-Scan Cyclic Voltammetry. ACS Chemical Neuroscience 4, 782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H and Gerfen CR (1996) Dynorphin regulates D1 dopamine receptor-mediated responses in the striatum: relative contributions of pre- and postsynaptic mechanisms in dorsal and ventral striatum demonstrated by altered immediate-early gene induction. J. Comp. Neurol 376, 530–541. [DOI] [PubMed] [Google Scholar]
- Steiner H and Gerfen CR (1998) Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp. Brain Res 123, 60–76. [DOI] [PubMed] [Google Scholar]
- Stevens CF and Wesseling JF (1998) Activity-dependent modulation of the rate at which synaptic vesicles become available to undergo exocytosis. Neuron 21, 415–424. [DOI] [PubMed] [Google Scholar]
- Stouffer MA, Woods CA, Patel JC et al. (2015) Insulin enhances striatal dopamine release by activating cholinergic interneurons and thereby signals reward. Nat Commun 6, 8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Chavkin C, Colago EE and Pickel VM (2001) Major coexpression of kappa-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synapse 42, 185–192. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Colago EE and Pickel VM (1999) Cellular sites for dynorphin activation of kappa-opioid receptors in the rat nucleus accumbens shell. J. Neurosci 19, 1804–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney CG, Kearney PJ, Fagan RR et al. (2020) Conditional, inducible gene silencing in dopamine neurons reveals a sex-specific role for Rit2 GTPase in acute cocaine response and striatal function. Neuropsychopharmacology 45, 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney CG, Tremblay BP, Stockner T, Sitte HH and Melikian HE (2017) Dopamine Transporter Amino and Carboxyl Termini Synergistically Contribute to Substrate and Inhibitor Affinities. J Biol Chem 292, 1302–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanawala MS and Regehr WG (2013) Presynaptic calcium influx controls neurotransmitter release in part by regulating the effective size of the readily releasable pool. J. Neurosci 33, 4625–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AC, Zapata A, Justice JB and others (2000) κ-Opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. Journal of. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S and Cragg SJ (2011) Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front. Syst. Neurosci 5, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K and Cragg SJ (2012) Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75, 58–64. [DOI] [PubMed] [Google Scholar]
- Townsend EA, Negus SS, Caine SB, Thomsen M and Banks ML (2019) Sex differences in opioid reinforcement under a fentanyl vs. food choice procedure in rats. Neuropsychopharmacology 44, 2022–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripanichkul W, Stanic D, Drago J, Finkelstein DI and Horne MK (2003) D2 Dopamine receptor blockade results in sprouting of DA axons in the intact animal but prevents sprouting following nigral lesions. Eur J Neurosci 17, 1033–1045. [DOI] [PubMed] [Google Scholar]
- Turner TJ (2004) Nicotine enhancement of dopamine release by a calcium-dependent increase in the size of the readily releasable pool of synaptic vesicles. J. Neurosci 24, 11328–11336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR and Kitayama S (1993) A cloned dopamine transporter. Potential insights into Parkinson’s disease pathogenesis. Adv. Neurol 60, 321–324. [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PEM, Wetsel WC, Gitler D, Greengard P, Augustine GJ and Wightman RM (2006) Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J. Neurosci 26, 3206–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheij MMM and Cools AR (2011) Reserpine differentially affects cocaine-induced behavior in low and high responders to novelty. Pharmacol. Biochem. Behav 98, 43–53. [DOI] [PubMed] [Google Scholar]
- Verheij MMM, de Mulder ELW, De Leonibus E, van Loo KMJ and Cools AR (2008) Rats that differentially respond to cocaine differ in their dopaminergic storage capacity of the nucleus accumbens. J. Neurochem 105, 2122–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SR (2010) Nitric oxide neurons and neurotransmission. Progress in Neurobiology 90, 246–255. [DOI] [PubMed] [Google Scholar]
- Walker QD, Ray R and Kuhn CM (2006) Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology 31, 1193–1202. [DOI] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM and Kuhn CM (2000) Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience 95, 1061–1070. [DOI] [PubMed] [Google Scholar]
- Wang LY and Kaczmarek LK (1998) High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature 394, 384–388. [DOI] [PubMed] [Google Scholar]
- Wang X, Pinter MJ and Rich MM (2016) Reversible Recruitment of a Homeostatic Reserve Pool of Synaptic Vesicles Underlies Rapid Homeostatic Plasticity of Quantal Content. J. Neurosci 36, 828–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-M, Gainetdinov RR, Fumagalli F, Xu F, Jones SR, Bock CB, Miller GW, Wightman RM and Caron MG (1997) Knockout of the Vesicular Monoamine Transporter 2 Gene Results in Neonatal Death and Supersensitivity to Cocaine and Amphetamine. Neuron 19, 1285–1296. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida M, Eshel N and Uchida N (2017) Neural Circuitry of Reward Prediction Error. Annual Review of Neuroscience 40, 373–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson F, Kiernan RS, Deavall DG, Varro A and Dimaline R (2001) Transcriptional activation of the rat vesicular monoamine transporter 2 promoter in gastric epithelial cells: regulation by gastrin. J. Biol. Chem 276, 7661–7671. [DOI] [PubMed] [Google Scholar]
- Wei Y, Williams JM, Dipace C, Sung U, Javitch JA, Galli A and Saunders C (2007) Dopamine transporter activity mediates amphetamine-induced inhibition of Akt through a Ca2+/calmodulin-dependent kinase II-dependent mechanism. Mol. Pharmacol 71, 835–842. [DOI] [PubMed] [Google Scholar]
- Werther GA, Hogg A, Oldfield BJ, McKINLEY MJ, FIGDOR R, ALLEN AM and MENDELSOHN FA (1987) Localization and characterization of insulin receptors in rat brain and pituitary gland using in vitro autoradiography and computerized densitometry. Endocrinology 121, 1562–1570. [DOI] [PubMed] [Google Scholar]
- Wescott SA, Rauthan M and Xu XZS (2013) When a TRP goes bad: transient receptor potential channels in addiction. Life Sci. 92, 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Galloway MP and Grace AA (2002) Regulation of striatal dopamine neurotransmission by nitric oxide: effector pathways and signaling mechanisms. Synapse 44, 227–245. [DOI] [PubMed] [Google Scholar]
- White JF, Noinaj N, Shibata Y et al. (2012) Structure of the agonist-bound neurotensin receptor. Nature 490, 508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Pearl SM, Zigmond MJ and Michael AC (2000) Inhibitory glutamatergic regulation of evoked dopamine release in striatum. Neuroscience 96, 65–72. [DOI] [PubMed] [Google Scholar]
- Yao J, Erickson JD and Hersh LB (2004) Protein kinase A affects trafficking of the vesicular monoamine transporters in PC12 cells. Traffic 5, 1006–1016. [DOI] [PubMed] [Google Scholar]
- Yavas E and Young AMJ (2017) N-Methyl-d-aspartate Modulation of Nucleus Accumbens Dopamine Release by Metabotropic Glutamate Receptors: Fast Cyclic Voltammetry Studies in Rat Brain Slices in Vitro. ACS Chem. Neurosci 8, 320–328. [DOI] [PubMed] [Google Scholar]
- Yavich L and MacDonald E (2000) Dopamine release from pharmacologically distinct storage pools in rat striatum following stimulation at frequency of neuronal bursting. Brain Res. 870, 73–79. [DOI] [PubMed] [Google Scholar]
- Yoest KE, Quigley JA and Becker JB (2018) Rapid effects of ovarian hormones in dorsal striatum and nucleus accumbens. Horm. Behav 104, 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Zeppenfeld DM and Williams JT (2017) Cholinergic Interneurons Underlie Spontaneous Dopamine Release in Nucleus Accumbens. J. Neurosci 37, 2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H and Sulzer D (2003) Glutamate spillover in the striatum depresses dopaminergic transmission by activating group I metabotropic glutamate receptors. J. Neurosci 23, 10585–10592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H and Sulzer D (2004) Frequency-dependent modulation of dopamine release by nicotine. Nat. Neurosci 7, 581–582. [DOI] [PubMed] [Google Scholar]
- Zhang H and Sulzer D (2012) Regulation of striatal dopamine release by presynaptic auto- and heteroreceptors. Basal Ganglia 2, 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen J, Reith MEA and Carr KD (2006) Chronic food restriction and dopamine transporter function in rat striatum. Brain Research 1082, 98–101. [DOI] [PubMed] [Google Scholar]


