Abstract
Background and Purpose-
Inflammation has emerged as a key component of the pathophysiology of intracranial aneurysms. Mast cells have been detected in human intracranial aneurysm tissues, and their presence was associated with intramural microhemorrhage and wall degeneration. We hypothesized that mast cells play a critical role in the development of aneurysmal rupture and that mast cells can be used as a therapeutic target for the prevention of aneurysm rupture.
Methods-
Intracranial aneurysms were induced in adult mice using a combination of induced systemic hypertension and a single injection of elastase into the cerebrospinal fluid. Aneurysm formation and rupture were assessed over 3 weeks. Roles of mast cells were assessed using a mast cell stabilizer (cromolyn), a mast cell activator (C48/80), and mice that are genetically lacking mature mast cells (KitW-sh/W-sh mice).
Results-
Pharmacological stabilization of mast cells with cromolyn markedly decreased the rupture rate of aneurysms (80% vs. 19%, n = 10 vs. n =16) without affecting the aneurysm formation. The activation of mast cells with C48/80 significantly increased the rupture rate of aneurysms (25% vs. 100%, n = 4 vs. n = 5) without affecting the overall rate of aneurysm formation. Furthermore, the genetic deficiency of mast cells significantly prevented aneurysm rupture (80% vs. 25%, n = 10 vs. n = 8, wild type vs. KitW-sh/W-sh mice).
Conclusions-
These results suggest that mast cells play a key role in promoting aneurysm rupture but not formation. Stabilizers of mast cells may have a potential therapeutic value in preventing intracranial aneurysm rupture in patients.
Keywords: Intracranial aneurysm, mast cells, inflammation, mice
Keywords: Cerebral aneurysm, cerebrovascular disease/stroke
Graphical Abstract
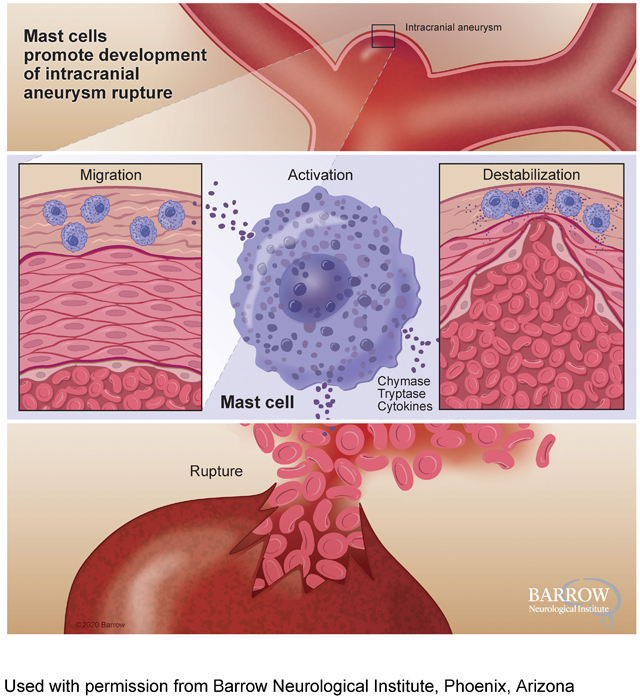
Introduction
Rupture of intracranial aneurysms causes aneurysmal subarachnoid hemorrhage. The 30-day mortality rate after aneurysmal subarachnoid hemorrhage can be as high as 45%.1 Therefore, surgical clipping or endovascular coiling are offered to patients with unruptured aneurysms for the prevention of aneurysmal rupture. Significant technical advancements and refinements have been made in these invasive treatments. However, the adverse outcome rates resulting from the clipping and coiling of unruptured aneurysms are still not negligible.2 Therefore, pharmacological prevention of aneurysmal rupture may be an attractive alternative approach in patients with unruptured aneurysms.3
Inflammation is increasingly recognized as a critical component in the pathophysiology of intracranial aneurysms.4–9 Observational studies have shown the presence of inflammatory cells and inflammatory markers in human intracranial aneurysm tissues and serum samples.5, 10 Mast cells have been detected in human intracranial aneurysm tissues,7, 11 and the presence of mast cells was associated with intramural microhemorrhage and wall degeneration of human intracranial aneurysms.11
Mast cells, classically known as key regulators of allergic reactions, have emerged as integral players in cardiovascular diseases.12–17 By releasing cytokines, including tryptase, chymases, cathepsins, and interleukins, mast cells can affect vascular inflammation and remodeling.12, 18–20 Blocking the cytokine release from mast cells reduces the development and progression of atherosclerosis and abdominal aortic aneurysm in animals.14, 16 Although previous studies suggested an association between mast cell activation and pathological remodeling of aneurysm walls,21, 22 the direct link between mast cell activation and the development of aneurysmal rupture has not been established. Therefore, we tested whether mast cells contribute to the development of aneurysmal rupture utilizing the genetic and pharmacological tools in a mouse model of intracranial aneurysm.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Mouse model of intracranial aneurysm
Experiments were conducted in accordance with the guidelines approved by the Institutional Animal Care and Use Committee. We used C57BL/6J and KitW-sh/W-sh transgenic male mice (Jackson Laboratory, Bar Harbor, Maine). Intracranial aneurysms were induced by combining induced systemic hypertension and a single injection of elastase (35.0 milli-units, unless indicated otherwise) into the cerebrospinal fluid at the right basal cistern as we previously described.3, 23
A flow chart of all mice used in the current study is included in online supplemental materials (Supplemental Figure I, please see https://www.ahajournals.org/journal/str).
Evaluation of aneurysm formation and rupture
To detect aneurysmal rupture, two blinded observers performed a daily neurological examination as previously described.3 Aneurysms are defined as a localized outward bulging of the vascular wall (>150% of the control artery).24 When mice develop neurological symptoms associated with aneurysmal rupture (neurological score: 1–5), we euthanize them immediately (within 4 hours). In both euthanized and dead mice, we inspect the brain samples and verify the presence of aneurysm and hematoma from subarachnoid hemorrhage by examining the Circle of Willis and its major branches under a dissecting microscope (10X).3 Our study confirmed the specificity and sensitivity of this approach in detecting aneurysmal rupture.3 All remaining mice were euthanized four weeks after aneurysm induction.3, 25, 26 Mice were transcardially perfused with phosphate-buffered saline, followed by a gelatin-containing blue dye to visualize cerebral arteries.
Dosing of drugs
The doses of cromolyn (12.5 or 25 mg/kg/day, intraperitoneally) and C48/80 (2 or 4 mg/kg/day, intraperitoneally) were chosen according to previous published studies in mice.27, 28 Cromolyn is a clinical used drug against diseases such as asthma and systemic mastocytosis. The dose used in the current study is below the dose used in humans according to surface area conversion.29
Tissue collection and immunohistochemistry
Mast cells from representative aneurysms were stained as previously described.7, 16 We collected aneurysms from mice at seven days after aneurysm induction, a time point before aneurysmal rupture begins to occur.3 The brain tissues were fixed with 4% paraformaldehyde for 24 hours and then immersed in 15% sucrose for 24 hours and 30% sucrose for another 24 hours. Then, the tissues were embedded in optimal cutting temperature compound (Tissue-Tek) at −80˚C. Sections were immunohistochemically stained with monoclonal antibodies to mast cells using anti-mast cell tryptase clone AA1 (DakoCytomation, Carpentaria, CA). Sections were incubated in primary antibody overnight at 4°C, followed by incubation with corresponding biotinylated secondary antibodies (Vector Laboratories) and with a complex of avidin-biotin-horseradish peroxidase (Vector Laboratories). Immunoreactivity was visualized by incubating the sections with 0.05% 3,3’-diaminobenzidine (Vector Laboratories). Nuclei were visualized by counterstaining with aqueous hematoxylin.
Real-time polymerase chain reaction detection of cytokines and mast cell-originated enzymes
We collected total RNA samples from cerebral arteries (Circle of Willis, including aneurysms) 14 days after aneurysm induction as previously described.30, 31 We measured mRNA expression levels of inflammation-related cytokines (angiotensin II type I receptor, interleukin-6, matrix metallopeptidase 9, and tumor necrosis factor-α), cathepsin G, chymase, and tryptase. RNA was extracted using the RNeasy Mini Kit (Qiagen, CA) and transcribed to complementary DNA using the QuantiTect reverse transcription kit (Qiagen). The mRNA expression levels were determined using SYBR Green technology (Applied Biosystems, CA). Quantitative values were obtained from the threshold cycle value (CT), and the data were analyzed by the 2−◿◿CT method. glyceraldehyde-3-phosphate dehydrogenase expression was quantified and used as an internal RNA control.
Statistical analysis
Fisher’s exact test was used to analyze the incidences of aneurysm formation and rupture rates. P-values < 0.05 were considered statistically significant. Data are expressed as means ± SD.
Results
The presence of mast cells in intracranial aneurysms in the mouse model
To confirm the presence of mast cells in intracranial aneurysms in this model, we stained representative aneurysms for mast cells as previously described.16 We collected aneurysms from mice at seven days after aneurysm induction, a time point before aneurysmal rupture begins to occur.3 Consistent with clinical studies that used human intracranial aneurysm tissues,7, 11 intracranial aneurysms in the mouse model exhibited infiltration of mast cells into the adventitial layers and the surrounding tissues (Figure 1).
Figure 1. Presence of mast cells in mouse intracranial aneurysms.
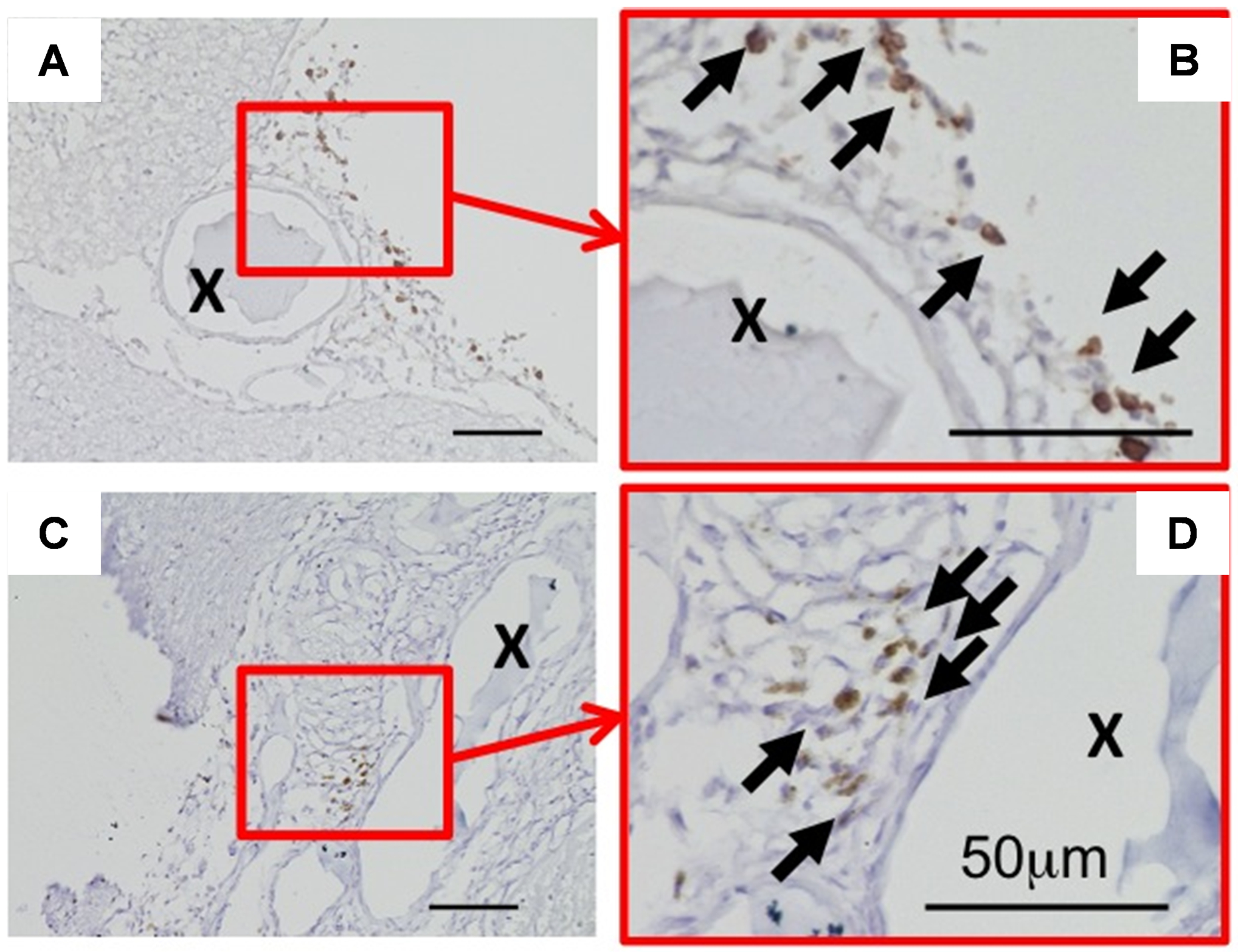
Representative images of immunohistochemical staining of mast cells in mouse intracranial aneurysms. Brown color indicates positive staining for mast cells. Arrows indicate mast cells. “X” indicates the lumen of aneurysms. Scale bar: 50 μm.
Pharmacological stabilization of mast cells prevented the aneurysmal rupture.
As a first step, we tested whether the stabilization of mast cells can prevent aneurysmal rupture. We utilized cromolyn (disodium cromoglycate, 25 mg/kg/day) as a mast cell stabilizer as previously described.16, 27, 28 We initiated a daily treatment with either cromolyn or vehicle six days after aneurysm induction for a total treatment course of three weeks (Figure 2A). Previous studies by ours and others found that aneurysm formation occurs during the first 6–7 days after aneurysm induction in this model and that aneurysmal rupture begins approximately 7 days after aneurysm induction.3, 25, 32, 33, 34, 35 By treating mice with an experimental agent starting from 6 days after aneurysm induction, it can be tested whether the experimental agent prevents aneurysmal rupture after aneurysms are formed, mimicking the clinical setting.3, 25, 26
Figure 2. Pharmacological stabilization of mast cells by cromolyn after aneurysm formation prevented aneurysmal rupture,
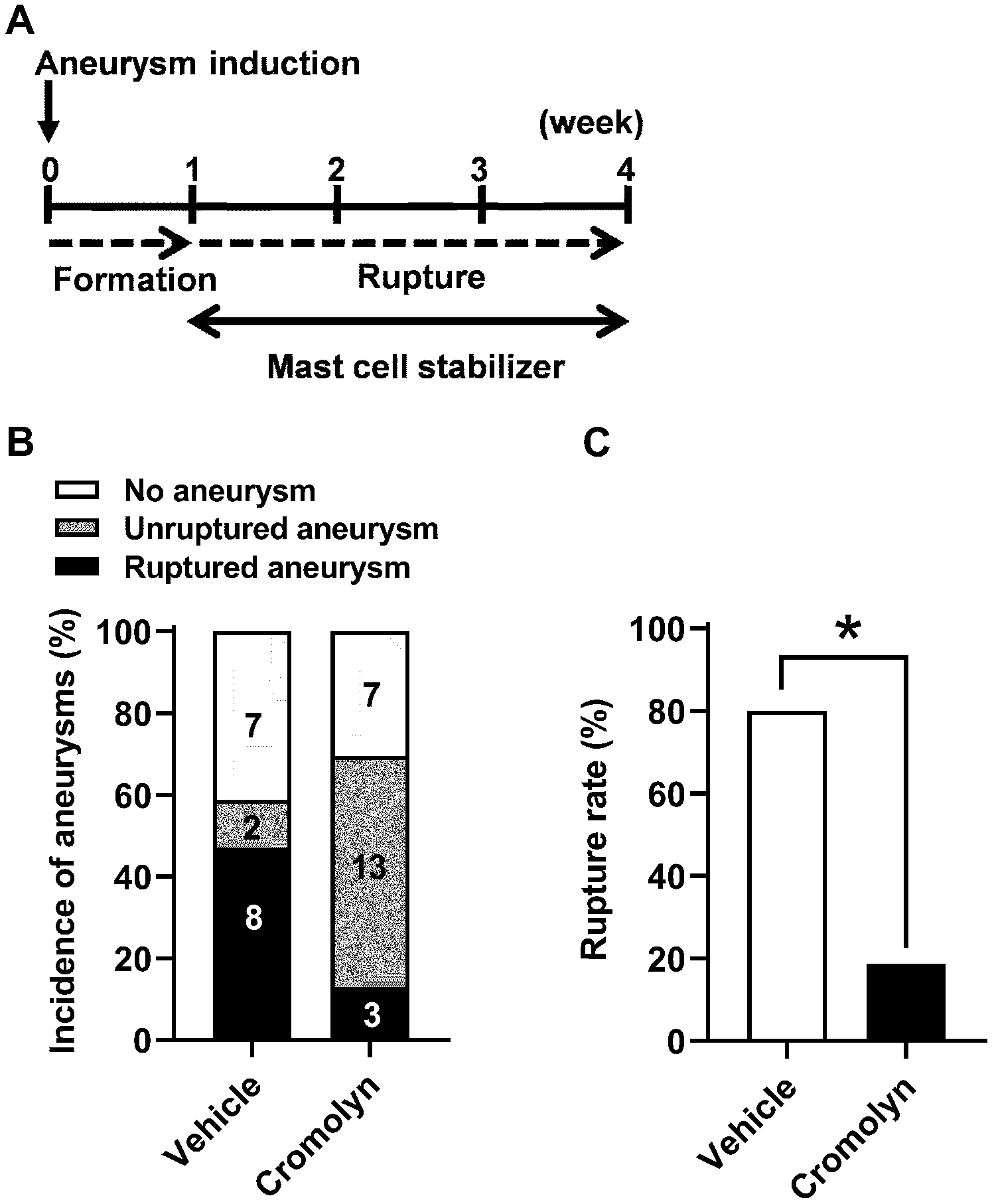
A. Time window of the mast cell stabilization treatment by cromolyn. B. No difference in the incidence of aneurysm between cromolyn-treated and vehicle-treated mice. C. Aneurysm rupture rate was significantly decreased in cromolyn-treated as compared to vehicle-treated mice. * P < 0.05.
As shown in Figure 2, although there was no difference in the overall incidence of aneurysm formation (including both ruptured and unruptured aneurysms) between the vehicle and cromolyn group (59% vs. 70%; n = 17 vs. n = 23; P = 0.52, Figure 2B), the stabilization of mast cells by cromolyn after aneurysmal formation significantly reduced the rupture rate (80% vs. 19%); n = 10 vs. n =16; P < 0.05, Figure 2C). To test the dose-dependency of cromolyn’s protective effect against aneurysm rupture, in a separate experiment, we reduced the dose of cromolyn by 50% (12.5 mg/kg/day). As expected, half-dose cromolyn treatment also reduced the rupture rate as compared to the vehicle-treated group, although this did not reach statistical significance (80% vs. 50%; n = 10 vs. n =12; P = 0.16, Supplemental Figure II, please see https://www.ahajournals.org/journal/str).
As a next step, we tested whether mast cell stabilization affects the formation of aneurysms by administering cromolyn, a mast cell stabilizer, during aneurysm formation. Cromolyn treatment was started one day before aneurysm induction and continued for a week (Figure 3A).
Figure 3. The mast cell stabilization during aneurysmal formation did not affect aneurysm rupture.
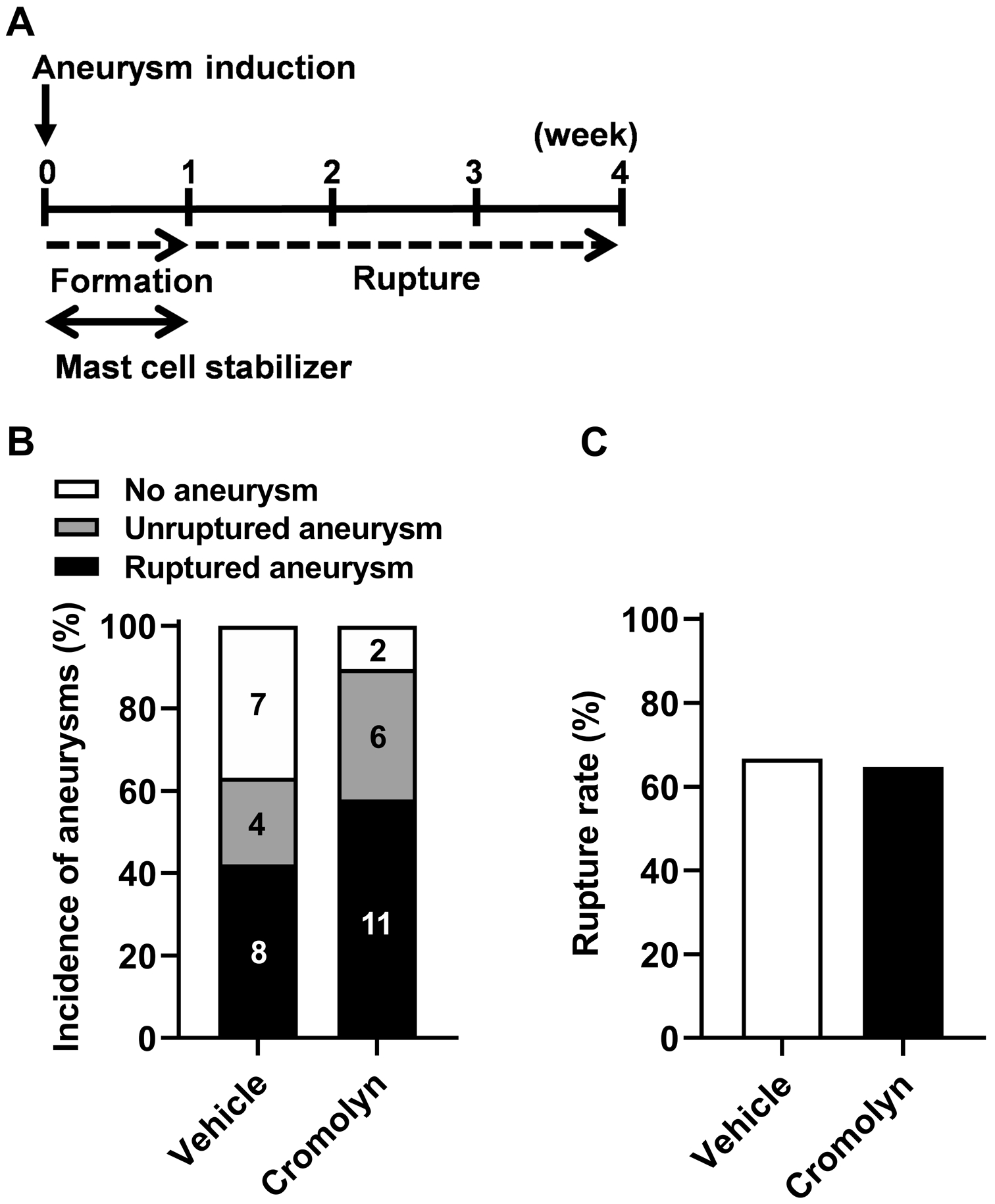
A. Time window of the mast cell stabilization treatment by cromolyn. B, C. No difference in the incidence of aneurysms or rupture rate was found between cromolyn-treated and vehicle-treated mice.
As shown in Figure 3, the stabilization of mast cells during aneurysm formation did not affect the formation of aneurysms (63% vs. 89%; n = 19 vs. n = 19; P = 0.13, Figure 3B). In addition, the mast cell stabilization during aneurysm formation stage did not alter the rupture rate (67% vs. 65%; n = 12 vs. n = 17; P = 0.39, Figure 3C), indicating the unique role of mast cells in promoting aneurysmal rupture after aneurysms are formed. Mast cell stabilization during or after aneurysm formation did not significantly affect blood pressure or body weight (data not shown).
To explore the potential mechanisms underlying the protective effect of cromolyn on aneurysm rupture, we conducted a separate set of experiments to investigate the molecules that may be responsible for the mast cells-mediated effect on aneurysm rupture. We used Real-Time polymerase chain reaction to quantify the mRNA of inflammatory cytokines, and enzymes with mast cell origin (angiotensin II type I receptor, interleukin-6, matrix metallopeptidase 9, tumor necrosis factor-α, cathepsin G, chymase, and tryptase) in vessels of the Circle of Willis. We found that cromolyn treatment significantly reduced the expression of tryptase as compared to the vehicle-treated group (vehicle vs. cromolyn, 1.0 ± 1.09 vs. 0.096 ± 0.099, n = 4 for both groups, P < 0.05) (Supplemental Figure III, please see https://www.ahajournals.org/journal/str).
Pharmacological activation of mast cells after aneurysm formation promoted aneurysmal rupture without affecting the aneurysmal formation.
To further confirm the role of mast cells in the promotion of aneurysmal rupture, we tested whether activation of mast cells promotes aneurysm rupture. Compound 48/80 (C48/80) (4 mg/kg/day) was used as a mast cell activator as previously described.28, 36 C48/80 promotes mast cell activation and degranulation by activating phospholipase D via heterotrimeric guanosine 5’-Triphosphate-binding proteins.28, 37 We used 17.5 milli-units of elastase so that the rupture rate in the vehicle group would be relatively low and that the promotion of rupture by the mast cell activator can be tested.38
As shown in Figure 4, while the mast cell activation did not affect the overall incidence of intracranial aneurysms (44% vs. 45%; n = 9 vs. n = 11; P = 1.00, Figure 4B), it significantly increased the rupture rate (25% vs. 100%; n = 4 vs. n = 5; P < 0.05, Figure 4C), further confirming the role of mast cells in promoting aneurysmal rupture.
Figure 4. Pharmacological activation of mast cells after aneurysm formation promoted aneurysmal rupture without affecting the aneurysmal formation.
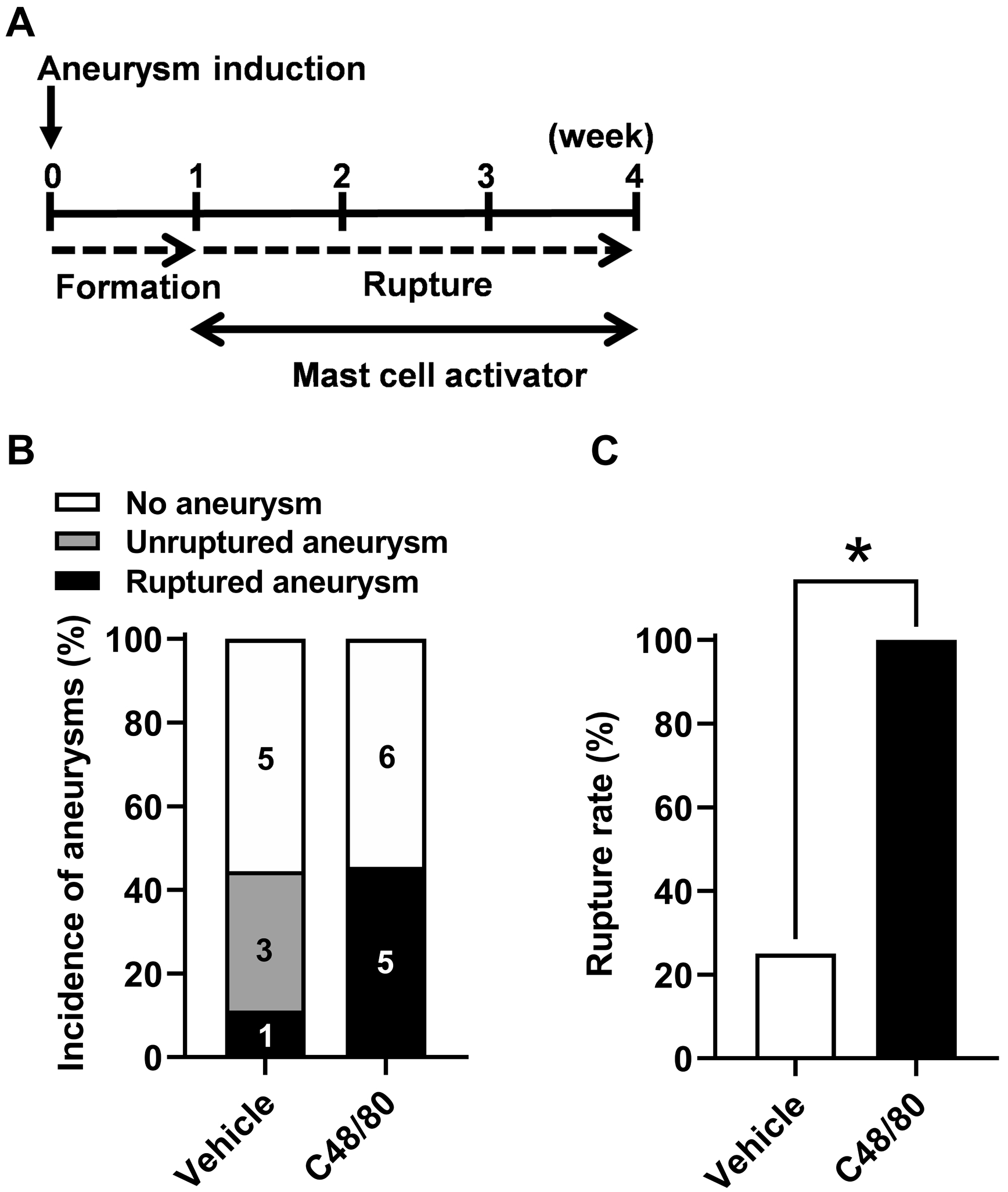
A. Time window of the mast cell activation treatment by C48/80. B. No difference in the incidence of aneurysm between C48/80-treated and vehicle-treated mice. C. Aneurysm rupture rate was significantly increased in C48/80-treated as compared to vehicle-treated mice. * P < 0.05.
To test the dose-dependency of C48/80’s promoting effect on aneurysm rupture, in a separate experiment, we reduced the dose of C48/80 by 50% (2 mg/kg/day). As expected, the mice treated with 2 mg/kg/day C48/80 had a rupture rate that fell between the groups receiving 4 mg/kd/day and vehicle (44% vs. 80%; n = 9 vs. n =15; P = 0.07, Supplemental Figure II, please see https://www.ahajournals.org/journal/str).
In the Real-Time polymerase chain reaction quantification of the mRNA of inflammatory cytokines, and enzymes with mast cell origin (angiotensin II type I receptor, interleukin-6, matrix metallopeptidase 9, tumor necrosis factor-α, cathepsin G, chymase, and tryptase), we did not detect any statistically significant difference between the vehicle-treated and C48/80-treated groups on these molecules (Supplemental Figure IV, please see https://www.ahajournals.org/journal/str).
Mast cell activation did not significantly affect blood pressure or body weight (data not shown).
Genetic deficiency of mast cells reduces aneurysmal rupture.
To complement the pharmacological experiments, we conducted an experiment using KitW-sh/W-sh mice, mast cell-deficient mice. KitW-sh/W-sh mice genetically lack mature mast cells.16, 39 KitW-sh/W-sh mice in the C57BL/6 background are not anemic or sterile, and they have normal bone marrow and blood neutrophil counts.19
Consistent with our findings on pharmacological stabilization of mast cells, mast cell-deficient mice (KitW-sh/W-sh mice) had a significantly lower rupture rate than wild-type littermate mice, indicating that a lack of mast cells prevents aneurysmal rupture (80% for control mice and 25% for KitW-sh/W-sh mice; n = 10 vs. n = 8; P < 0.05) (Figure 5B). There was no difference in the overall incidence of aneurysms between mast cell-deficient mice and wild-type mice (71% vs. 73%; n = 14 vs. n = 11; P = 1.00, Figure 5A). There was no difference in the systolic blood pressure at any time point between the KitW-sh/W-sh mice and the wild-type mice, either (data not shown).
Figure 5. Genetic deficiency of mast cells decreased the aneurysmal rupture rate without affecting the aneurysmal formation.
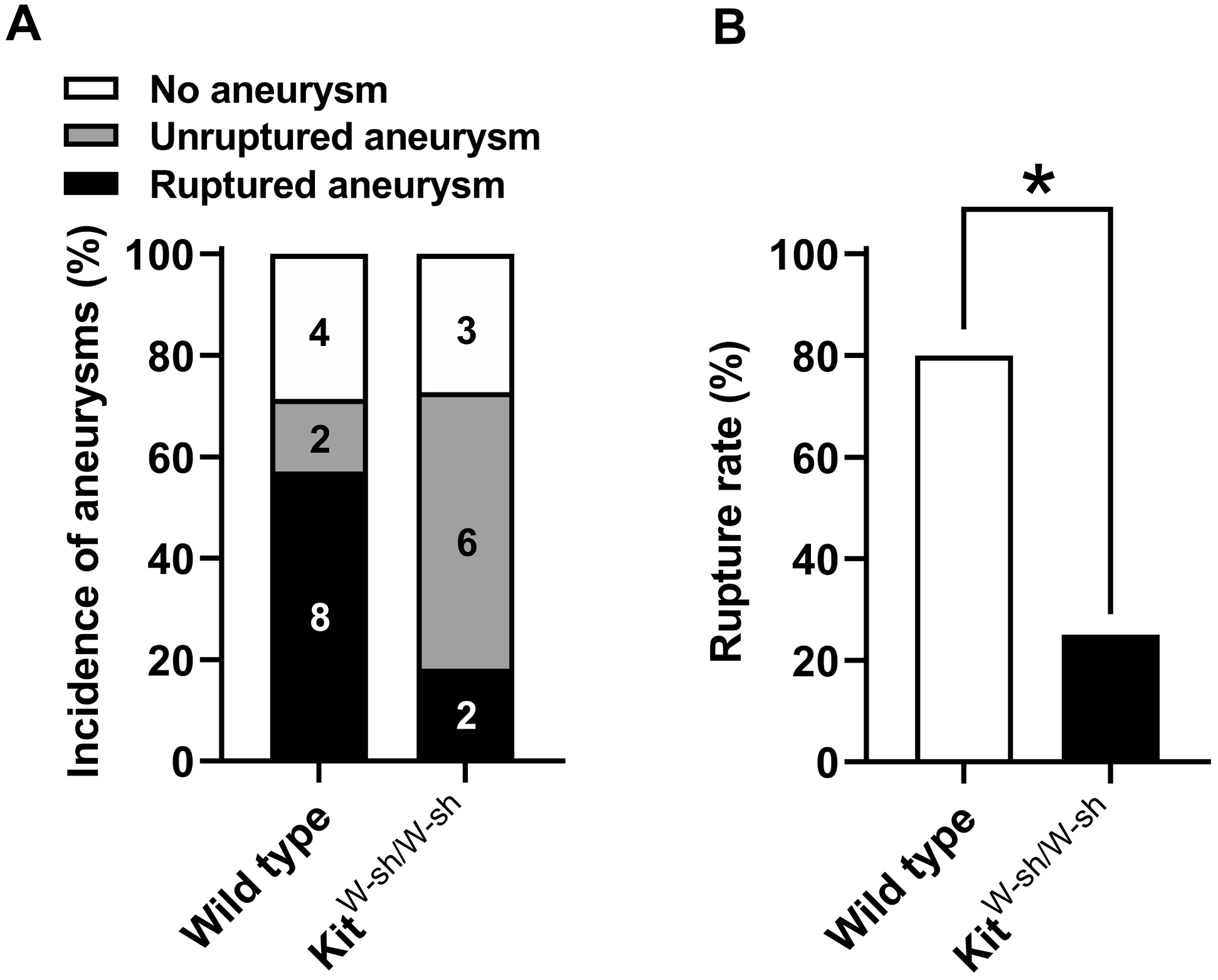
A. No difference in the incidence of aneurysm between the KitW-sh/W-sh mice and the wild-type mice. B. Aneurysm rupture rate was significantly decreased in the KitW-sh/W-sh mice as compared to the wild-type mice. * P < 0.05.
As an additional experiment, we confirmed the specificity of cromolyn’s stabilization by treating KitW-sh/W-sh mice with cromolyn. As shown in Figure 6, the treatment with cromolyn did not affect the incidence of aneurysms in KitW-sh/W-sh mice (69% vs. 50%; n = 16 vs. n = 8; P = 0.41, Figure 6B). However, more importantly, the protective effect of cromolyn against the development of aneurysmal rupture was abolished, as evidenced by the lack of difference in the rupture rate between KitW-sh/W-sh mice treated with the vehicle and KitW-sh/W-sh mice treated with cromolyn (27% vs. 25%; n = 11 vs. n = 4; P = 0.28, Figure 6C).
Figure 6. Genetic deficiency of mast cells eliminated cromolyn treatment effects.
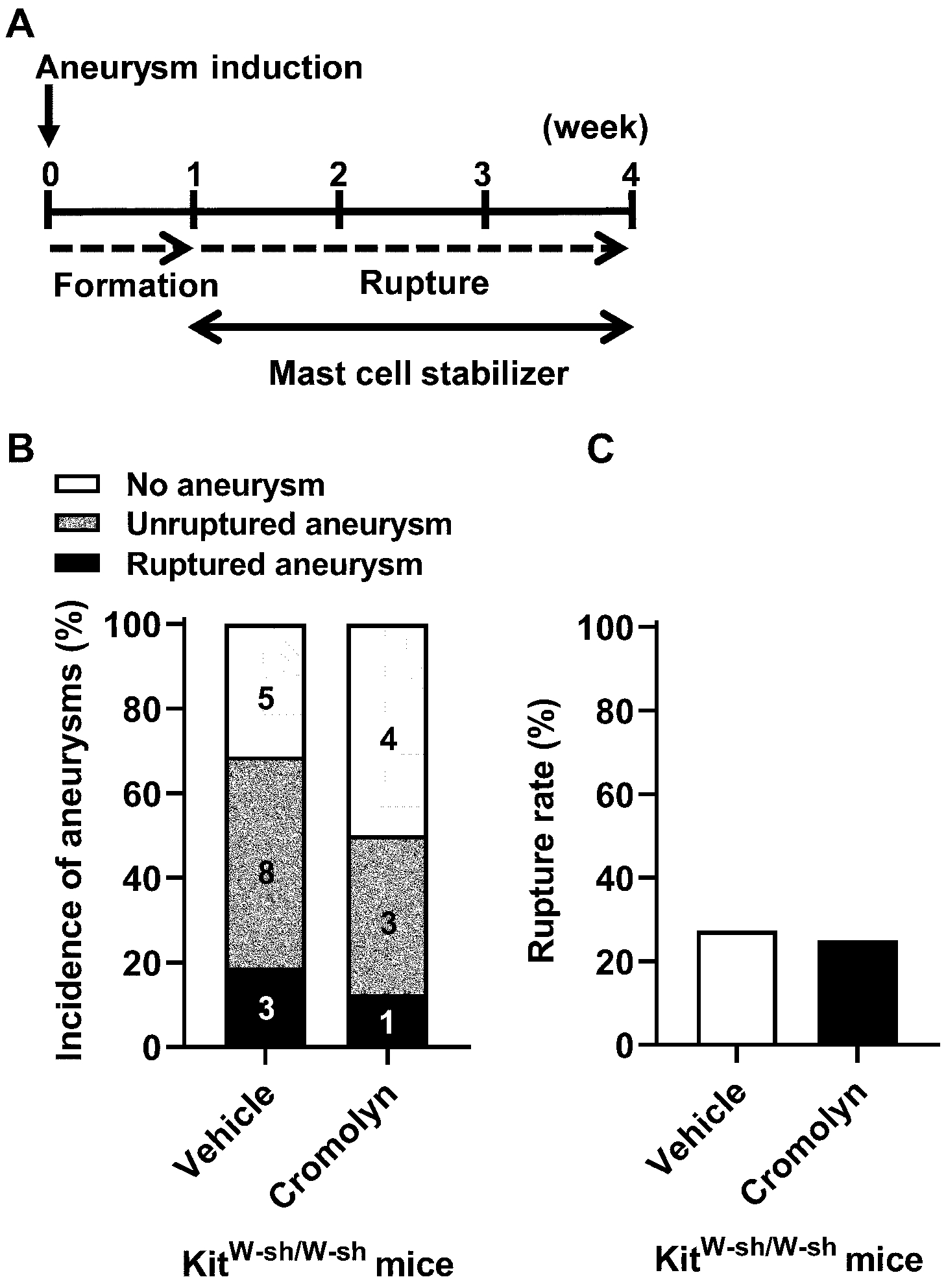
A. Time window of the mast cell stabilization treatment by cromolyn in the KitW-sh/W-sh mice. B, C. No difference in the incidence of aneurysm or rupture rate was found between cromolyn-treated and vehicle-treated groups in the KitW-sh/W-sh mice.
Discussion
Mast cells have been detected in human intracranial aneurysm tissues.7, 11, 40 Mast cells were more abundant in ruptured aneurysms than in unruptured aneurysms in humans,7 and the presence of mast cells was associated with the degeneration and microhemorrhage with the aneurysmal walls.11 However, the causal relationship between mast cell infiltration and development of aneurysmal rupture has not been established.
In this study, using both pharmacological and genetic approaches, we demonstrated that mast cells can promote the development of aneurysmal rupture. For the pharmacological studies, we used both activator and stabilizer of mast cells and showed the link between mast cell activation and the development of aneurysmal rupture. In addition, our results revealed the unique role of mast cells in the development of aneurysmal rupture. While mast cells promote aneurysmal rupture, they do not seem to play any significant role in the formation of aneurysms. The unique role of mast cells in the development of aneurysmal rupture was confirmed by our experiment that utilized the mouse that genetically lacks mature mast cells.
Previously, in a rat model of aneurysm initiation that employed the continuous administration of lysyl oxidase inhibitor (beta-aminopropyl nitrile), renal hypertension, and unilateral carotid artery ligation, the pharmacological stabilization of mast cells that started the day of aneurysm induction surgery reduced mast cell infiltration and inflammation within the cerebral arteries without affecting the formation of aneurysm.41 These findings were consistent with our findings that showed no effect of mast cell activation or stabilization on the formation of aneurysms. However, in the study that used the rat model, the potential role of mast cells in the development of aneurysmal rupture could not be assessed.41 In the mouse model that was used in the current study, spontaneous aneurysmal rupture occurs with a predictable time course, and the aneurysmal rupture can be easily detected by assessing neurological symptoms.3, 23, 24, 33 This model provided us with a unique opportunity to study the mechanisms of aneurysmal rupture as well as the potential roles of mast cells in the development of aneurysmal rupture. Our findings on the promotion of aneurysmal rupture by mast cells are consistent with our previous studies that showed that mesenchymal stem cells and their microvesicles prevented aneurysmal rupture through the stabilization of mast cells.21, 22 In the current study, we firmly established the link between mast cells and aneurysmal rupture by directly manipulating mast cells using both pharmacological and genetic approaches.
Mast cells can release numerous cytokines and chemokines upon their activation and degranulation. At the same time, a number of cytokines and chemokines can activate mast cells. Many of these cytokines are reported to be involved in the pathophysiology of intracranial aneurysm. For example, tumor necrosis factor-α and hepatocyte growth factor, cytokines that can be released by mast cell, have been shown to play a key role in the promotion of aneurysmal rupture.42, 43 Stromal cell-derived factor-1, a known chemoattractant for mast cells, seem to induce pathological remodeling of aneurysmal walls in animals.44 Moreover, the conversion of angiotensin I to angiotensin II by mast cell-derived chymases in the aneurysm wall may cause the activation of the vascular renin-angiotensin system that was reported to promote aneurysmal rupture.25, 32 In addition, matrix metalloproteinases can be activated by mast cell-derived chymases and cathepsins.18
Tryptase is the major proteinase in mast cells and has been used as a marker for mast cells activation.45, 46 Our data showed that cromolyn treatment reduced the expression of tryptase. This may provide a direct mechanistic explanation for the protective effect of the mast cell stabilizer. It is also likely that concerted effects of multiple cytokines and chemokines mediate mast cell’s promotion of aneurysmal rupture. Future studies that utilize mast cell-specific deletion of cytokines or chemokines may be needed to further elucidate the underlying molecular mechanism. Nevertheless, considering the clinical availability of mast cell stabilizers such as cromolyn and tranilast,41 our current study may provide the basis for future clinical pilot studies to test mast cell stabilization for the prevention of aneurysmal rupture in humans.
There are other limitations to this study. First, the animal model may not completely replicate biological events that lead to aneurysm formation and growth, as aneurysms were induced, but not spontaneously formed. While many studies indicated the critical roles of vascular inflammation in the pathophysiology of intracranial aneurysms, there may be significant differences in the triggering factors of vascular inflammation between human aneurysms and this model. In addition, the time course of aneurysmal formation and rupture in this model is shorter than that of the human aneurysm. However, the phenotypes of intracranial aneurysms in the model closely mimic that of intracranial aneurysms in humans.3, 23 More importantly, human intracranial aneurysms and aneurysms in this model share the end phenotypes, aneurysmal rupture and associated neurological symptoms, possibly indicating the common biological processes between human intracranial aneurysms and this mouse model of intracranial aneurysm.3, 25
Second, we cannot completely exclude potentially confounding effects of unknown development abnormalities of KitW-sh/W-sh mice. We used the KitW-sh/W-sh mice in the C57BL/6 background because their genetic defects are reported to be limited to mast cells.19 These mice are not anemic or sterile, and they have normal numbers of bone marrow and blood neutrophils.19 The KitW-sh/W-sh mice in the C57BL/6 background have been successfully used to study the roles of mast cells in various disease conditions.14, 16, 28
Third, there may be off-target effects of pharmacological agents we used for this study. For example, the mast cell activator, C48/80, may cause mast cell apoptosis.47 These limitations of using transgenic mice and pharmacological agents underscore the importance of employing both genetic and pharmacological approaches to firmly establish the role of mast cells in the development of aneurysmal rupture.
Lastly, as an initial step to study the contribution of mast cells to aneurysmal rupture, we used only male mice. Sex-dependent differences in the rupture rate of intracranial aneurysms have been observed in both humans and animals.26, 48, 49 Further studies will be needed to explore sex-dependent differences in the contribution of mast cells to the development of aneurysmal rupture.
This study showed the role of mast cells in the promotion of the rupture of intracranial aneurysm. Mast cells may serve as a potential therapeutic target for the prevention of intracranial aneurysmal rupture. Further mechanistic and clinical studies are needed to further establish the contribution of mast cells to the development of aneurysmal rupture and subarachnoid hemorrhage.
Supplementary Material
Acknowledgments
The authors thank Cindy Giljames and the staff of Neuroscience Publications at Barrow Neurological Institute for assistance with preparation of the visual abstract.
Sources of funding
The project was supported by grant number R01NS082280 (TH), R01NS109382 (TH), and R01NS109584 (TH) from the National Institute of Neurological Disorders and Stroke (NIH/NINDS), Cami Clark Chair of Research and Fight Like Frank Chair of Research from Brain Aneurysm Foundation (HS), and Barrow Neurological Foundation (TH). The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Non-standard Abbreviations and Acronyms
- CT
The threshold cycle value
Footnotes
Part of this work has been presented at the following meetings
The American Society of Anesthesiologists Annual Meeting 2012, October 13–17, 2012, Washington D.C.
The American Society of Anesthesiologists Annual Meeting 2013, October 12–16, 2013, San Francisco, CA
Disclosures
None.
References
- 1.Bederson JB, Awad IA, Wiebers DO, Piepgras D, Haley EC Jr., Brott T, Hademenos G, Chyatte D, Rosenwasser R, Caroselli. Recommendations for the management of patients with unruptured intracranial aneurysms: A statement for healthcare professionals from the stroke council of the american heart association. Stroke. 2000;31:2742–2750 [DOI] [PubMed] [Google Scholar]
- 2.Zacharia BE, Ducruet AF, Hickman ZL, Grobelny BT, Badjatia N, Mayer SA, Berman MF, Solomon RA, Connolly ES Jr. Technological advances in the management of unruptured intracranial aneurysms fail to improve outcome in new york state. Stroke. 2011;42:2844–2849 [DOI] [PubMed] [Google Scholar]
- 3.Makino H, Tada Y, Wada K, Liang EI, Chang M, Mobashery S, Kanematsu Y, Kurihara C, Palova E, Kanematsu M, et al. Pharmacological stabilization of intracranial aneurysms in mice: A feasibility study. Stroke. 2012;43:2450–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto T, Meng H, Young WL. Intracranial aneurysms: Links among inflammation, hemodynamics and vascular remodeling. Neurol Res. 2006;28:372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frosen J, Tulamo R, Paetau A, Laaksamo E, Korja M, Laakso A, Niemela M, Hernesniemi J. Saccular intracranial aneurysm: Pathology and mechanisms. Acta Neuropathol. 2012;123:773–786 [DOI] [PubMed] [Google Scholar]
- 6.Can A, Rudy RF, Castro VM, Yu S, Dligach D, Finan S, Gainer V, Shadick NA, Savova G, Murphy S, et al. Association between aspirin dose and subarachnoid hemorrhage from saccular aneurysms: A case-control study. Neurology. 2018;91:e1175–e1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasan D, Chalouhi N, Jabbour P, Hashimoto T. Macrophage imbalance (m1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: Preliminary results. J Neuroinflammation. 2012;9:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meng H, Metaxa E, Gao L, Liaw N, Natarajan SK, Swartz DD, Siddiqui AH, Kolega J, Mocco J. Progressive aneurysm development following hemodynamic insult. J Neurosurg. 2011;114:1095–1103 [DOI] [PubMed] [Google Scholar]
- 9.Gounis MJ, Vedantham S, Weaver JP, Puri AS, Brooks CS, Wakhloo AK, Bogdanov AA, Jr. Myeloperoxidase in human intracranial aneurysms: Preliminary evidence. Stroke. 2014;45:1474–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frosen J, Piippo A, Paetau A, Kangasniemi M, Niemela M, Hernesniemi J, Jaaskelainen J. Remodeling of saccular cerebral artery aneurysm wall is associated with rupture: Histological analysis of 24 unruptured and 42 ruptured cases. Stroke. 2004;35:2287–2293 [DOI] [PubMed] [Google Scholar]
- 11.Ollikainen E, Tulamo R, Frosen J, Lehti S, Honkanen P, Hernesniemi J, Niemela M, Kovanen PT. Mast cells, neovascularization, and microhemorrhages are associated with saccular intracranial artery aneurysm wall remodeling. Journal of neuropathology and experimental neurology. 2014;73:855–864 [DOI] [PubMed] [Google Scholar]
- 12.Iadecola C, Anrather J. The immunology of stroke: From mechanisms to translation. Nat Med. 2011;17:796–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun J, Zhang J, Lindholt JS, Sukhova GK, Liu J, He A, Abrink M, Pejler G, Stevens RL, Thompson RW, et al. Critical role of mast cell chymase in mouse abdominal aortic aneurysm formation. Circulation. 2009;120:973–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, MacFarlane LA, Mallen-St Clair J, Shi GP. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13:719–724 [DOI] [PubMed] [Google Scholar]
- 15.Libby P, Shi GP. Mast cells as mediators and modulators of atherogenesis. Circulation. 2007;115:2471–2473 [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Sun J, Lindholt JS, Sukhova GK, Sinnamon M, Stevens RL, Adachi R, Libby P, Thompson RW, Shi GP. Mast cell tryptase deficiency attenuates mouse abdominal aortic aneurysm formation. Circ Res. 2011;108:1316–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei CC, Hase N, Inoue Y, Bradley EW, Yahiro E, Li M, Naqvi N, Powell PC, Shi K, Takahashi Y, et al. Mast cell chymase limits the cardiac efficacy of ang i-converting enzyme inhibitor therapy in rodents. J Clin Invest. 2010;120:1229–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Company C, Piqueras L, Naim Abu Nabah Y, Escudero P, Blanes JI, Jose PJ, Morcillo EJ, Sanz MJ. Contributions of ace and mast cell chymase to endogenous angiotensin ii generation and leucocyte recruitment in vivo. Cardiovascular research. 2011;92:48–56 [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Kuwabara A, Kamio Y, Hu S, Park J, Hashimoto T, Lee JW. Human mesenchymal stem cell-derived microvesicles prevent the rupture of intracranial aneurysm in part by suppression of mast cell activation via a pge2-dependent mechanism. Stem Cells. 2016;34:2943–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuwabara A, Liu J, Kamio Y, Liu A, Lawton MT, Lee JW, Hashimoto T. Protective effect of mesenchymal stem cells against the development of intracranial aneurysm rupture in mice. Neurosurgery. 2017;81:1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N, Lawton MT, Young WL, Liang EI, Nuki Y, et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tada Y, Wada K, Shimada K, Makino H, Liang EI, Murakami S, Kudo M, Kitazato KT, Nagahiro S, Hashimoto T. Roles of hypertension in the rupture of intracranial aneurysms. Stroke. 2014;45:579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tada Y, Wada K, Shimada K, Makino H, Liang EI, Murakami S, Kudo M, Shikata F, Pena Silva RA, Kitazato KT, et al. Estrogen protects against intracranial aneurysm rupture in ovariectomized mice. Hypertension. 2014;63:1339–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang M, Pang X, Karalis K, Theoharides TC. Stress-induced interleukin-6 release in mice is mast cell-dependent and more pronounced in apolipoprotein e knockout mice. Cardiovascular research. 2003;59:241–249 [DOI] [PubMed] [Google Scholar]
- 28.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, et al. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661 [DOI] [PubMed] [Google Scholar]
- 30.Shikata F, Shimada K, Sato H, Ikedo T, Kuwabara A, Furukawa H, Korai M, Kotoda M, Yokosuka K, Makino H, et al. Potential influences of gut microbiota on the formation of intracranial aneurysm. Hypertension. 2019;73:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimada K, Furukawa H, Wada K, Wei Y, Tada Y, Kuwabara A, Shikata F, Kanematsu Y, Lawton MT, Kitazato KT, et al. Angiotensin-(1–7) protects against the development of aneurysmal subarachnoid hemorrhage in mice. J Cereb Blood Flow Metab. 2015;35:1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimada K, Furukawa H, Wada K, Korai M, Wei Y, Tada Y, Kuwabara A, Shikata F, Kitazato KT, Nagahiro S, et al. Protective role of peroxisome proliferator-activated receptor-gamma in the development of intracranial aneurysm rupture. Stroke. 2015;46:1664–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makino H, Hokamura K, Natsume T, Kimura T, Kamio Y, Magata Y, Namba H, Katoh T, Sato S, Hashimoto T, et al. Successful serial imaging of the mouse cerebral arteries using conventional 3-t magnetic resonance imaging. J Cereb Blood Flow Metab. 2015;35:1523–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalouhi N, Starke RM, Correa T, Jabbour PM, Zanaty M, Brown RD Jr., Torner JC, Hasan DM. Differential sex response to aspirin in decreasing aneurysm rupture in humans and mice. Hypertension. 2016;68:411–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pascale CL, Martinez AN, Carr C, Sawyer DM, Ribeiro-Alves M, Chen M, O’Donnell DB, Guidry JJ, Amenta PS, Dumont AS. Treatment with dimethyl fumarate reduces the formation and rupture of intracranial aneurysms: Role of nrf2 activation. J Cereb Blood Flow Metab. 2019:271678X19858888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee-Rueckert M, Silvennoinen R, Rotllan N, Judstrom I, Blanco-Vaca F, Metso J, Jauhiainen M, Kovanen PT, Escola-Gil JC. Mast cell activation in vivo impairs the macrophage reverse cholesterol transport pathway in the mouse. Arterioscler Thromb Vasc Biol. 2011;31:520–527 [DOI] [PubMed] [Google Scholar]
- 37.Aridor M, Rajmilevich G, Beaven MA, Sagi-Eisenberg R. Activation of exocytosis by the heterotrimeric g protein gi3. Science. 1993;262:1569–1572 [DOI] [PubMed] [Google Scholar]
- 38.Kamio Y, Miyamoto T, Kimura T, Mitsui K, Furukawa H, Zhang D, Yokosuka K, Korai M, Kudo D, Lukas RJ, et al. Roles of nicotine in the development of intracranial aneurysm rupture. Stroke. 2018;49:2445–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tono T, Tsujimura T, Koshimizu U, Kasugai T, Adachi S, Isozaki K, Nishikawa S, Morimoto M, Nishimune Y, Nomura S, et al. C-kit gene was not transcribed in cultured mast cells of mast cell-deficient wsh/wsh mice that have a normal number of erythrocytes and a normal c-kit coding region. Blood. 1992;80:1448–1453 [PubMed] [Google Scholar]
- 40.Faleiro LC, Machado CR, Gripp A, Jr., Resende RA, Rodrigues PA. Cerebral vasospasm: Presence of mast cells in human cerebral arteries after aneurysm rupture. J Neurosurg. 1981;54:733–735 [DOI] [PubMed] [Google Scholar]
- 41.Ishibashi R, Aoki T, Nishimura M, Hashimoto N, Miyamoto S. Contribution of mast cells to cerebral aneurysm formation. Curr Neurovasc Res. 2010;7:113–124 [DOI] [PubMed] [Google Scholar]
- 42.Starke RM, Chalouhi N, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Wada K, Shimada K, Hasan DM, Greig NH, et al. Critical role of tnf-alpha in cerebral aneurysm formation and progression to rupture. J Neuroinflammation. 2014;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pena-Silva RA, Chalouhi N, Wegman-Points L, Ali M, Mitchell I, Pierce GL, Chu Y, Ballas ZK, Heistad D, Hasan D. Novel role for endogenous hepatocyte growth factor in the pathogenesis of intracranial aneurysms. Hypertension. 2015;65:587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoh BL, Hosaka K, Downes DP, Nowicki KW, Wilmer EN, Velat GJ, Scott EW. Stromal cell-derived factor-1 promoted angiogenesis and inflammatory cell infiltration in aneurysm walls. J Neurosurg. 2014;120:73–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohajeri M, Kovanen PT, Bianconi V, Pirro M, Cicero AFG, Sahebkar A. Mast cell tryptase - marker and maker of cardiovascular diseases. Pharmacol Ther. 2019;199:91–110 [DOI] [PubMed] [Google Scholar]
- 46.Payne V, Kam PC. Mast cell tryptase: A review of its physiology and clinical significance. Anaesthesia. 2004;59:695–703 [DOI] [PubMed] [Google Scholar]
- 47.Carvalho M, Benjamim C, Santos F, Ferreira S, Cunha F. Effect of mast cells depletion on the failure of neutrophil migration during sepsis. Eur J Pharmacol. 2005;525:161–169 [DOI] [PubMed] [Google Scholar]
- 48.de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: A systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoh BL, Rojas K, Lin L, Fazal HZ, Hourani S, Nowicki KW, Schneider MB, Hosaka K. Estrogen deficiency promotes cerebral aneurysm rupture by upregulation of th17 cells and interleukin-17a which downregulates e-cadherin. Journal of the American Heart Association. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tada Y, Kanematsu Y, Kanematsu M, Nuki Y, Liang EI, Wada K, Makino H, Hashimoto T. A mouse model of intracranial aneurysm: Technical considerations. Acta Neurochir Suppl. 2011;111:31–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tada Y, Makino H, Furukawa H, Shimada K, Wada K, Liang EI, Murakami S, Kudo M, Kung DK, Hasan DM, et al. Roles of estrogen in the formation of intracranial aneurysms in ovariectomized female mice. Neurosurgery. 2014;75:690–695; discussion 695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wada K, Makino H, Shimada K, Shikata F, Kuwabara A, Hashimoto T. Translational research using a mouse model of intracranial aneurysm. Translational stroke research. 2014;5:248–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss D, Taylor WR. Deoxycorticosterone acetate salt hypertension in apolipoprotein e−/− mice results in accelerated atherosclerosis: The role of angiotensin ii. Hypertension. 2008;51:218–224 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


