Abstract
Aim
In 2014, Memorial Sloan Kettering Cancer Center was identified as an outlier for increased length of stay (LOS) after colorectal surgery. We subsequently implemented a comprehensive Enhanced Recovery After Surgery (ERAS) program in January 2016, which is continually monitored to target areas for improvement. The primary aim of this study was to evaluate the impact of a newly established ERAS program in a high-volume colorectal center over time.
Method
This was a retrospective cohort study, comparing 3000 sequential cancer patients who underwent elective colorectal surgery before and after ERAS implementation. Patients were divided into three groups (Pre-, Early, and Late ERAS). Adherence to ERAS process measures and outcomes (LOS, complications, and 30-day readmission) were compared among the three time periods.
Results
Adherence to ERAS metrics significantly increased over time, from a median of 25% Pre-ERAS to 67% Early and 75% Late ERAS (p < 0.0001). Mean LOS decreased from 5.2 days Pre-ERAS to 4.5 Early and 4.0 Late ERAS (p < 0.0001). There were no differences in rates of complications or readmissions, and patients with shorter LOS had lower readmission rates. With ERAS, the readmission rate was 4.4% for patients discharged within 3 days, versus >10% for LOS ≥5 days (p < 0.0001).
Conclusion
Initiation of an ERAS program at a high-volume colorectal center was associated with decreased LOS, without increasing morbidity. Increased ERAS adherence was associated with a further decrease in LOS. Multidisciplinary monitoring to promote protocol adherence is necessary for maintaining a safe and effective ERAS program.
Keywords: enhanced recovery after surgery, enhanced recovery program, adherence, colorectal surgery
INTRODUCTION
Enhanced Recovery After Surgery (ERAS) programs or Enhanced Recovery Programs (ERP) are a series of evidence-based, multidisciplinary interventions implemented throughout the perioperative period to achieve early postoperative recovery. ERAS programs have been shown to decrease complications and hospital length of stay (LOS), with associated advantages of improved quality of life, decreased nosocomial infections and exposures, reduced hospital costs, and more efficient cancer care delivery [1–12].
However, these benefits are closely linked to protocol adherence, as previously reported in smaller longitudinal studies [6, 13, 14]. Achieving and maintaining ERAS adherence is a significant challenge when developing a multidisciplinary program, which includes frequent education and managing patient and clinician expectations, weighed against the real world nuances of patient-directed clinical care. This is especially true in the oncologic patient population, who often harbor additional high-risk features, such as older age, comorbidities, immunocompromised status, and poor nutrition, which increase perioperative morbidity and the complexity of postoperative care.
While ERAS principles were often incorporated into the colorectal surgical practices at Memorial Sloan Kettering Cancer Center (MSKCC), a formalized colorectal ERAS program was not established until 2016. We regularly contribute surgical cases to the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP), the risk-adjusted, outcomes-based initiative for improving the quality of surgical care. In 2014, it was identified that while the MSKCC colorectal surgery service compared favorably with other NSQIP institutions with regards to postoperative morbidity, mortality, urinary tract infections, surgical site infections, and reoperation rates, we remained a consistent outlier for longer LOS (NSQIP site summary, 4/1/2012 – 3/31/2013: mean 6.8 days; odds ratio 1.90, confidence interval [C.I.] 1.32 – 2.74).
A collaborative task force was therefore established to address this concern, which included creation of a formal colorectal ERAS program. The main objective was reducing LOS, without compromising overall outcomes, including complication and readmission rates. Following extensive multidisciplinary collaboration and expert consultation from other institutions, we designed and implemented a colorectal ERAS program at MSKCC that began in January 2016, with regular multidisciplinary meetings to monitor ongoing protocol adherence and outcomes. This process of routine evaluation has been integral for identifying not only areas of improvement but also successes to provide continual feedback to the multidisciplinary team.
Here we present an analysis of our early experience developing an ERAS program for a high-volume, tertiary cancer center. We compare the 1000 colorectal patients prior to ERAS implementation to the subsequent 2000 patients in the 3-year post-intervention period. We compare adherence to process measures and postoperative outcomes (LOS, complications, and 30-day readmission) across three time periods of intervention (Pre-ERAS, Early ERAS, and Late ERAS). We hypothesized that the ongoing programmatic and educational improvements would be reflected in clinical differences between the Early and Late intervention periods.
METHOD
ERAS program
Starting in January 2016, a colorectal ERAS program was implemented at MSKCC. The perioperative program focuses on adequate preoperative hydration, multimodal analgesia to minimize narcotic use, goal-directed perioperative fluid administration [15], early ambulation, early removal of urinary catheters, and early feeding after surgery (Supplementary Table 1). Interventions are then modified as clinically appropriate for each patient. An electronic dashboard was developed to prospectively collect data from the colorectal ERAS program and measure adherence metrics and outcomes. Approximately once per quarter, the Multidisciplinary Enhanced Recovery after Colorectal Surgery Team meets to review recent performance and provide feedback to target areas for improvement. Members of the multidisciplinary team include representatives and leadership from colorectal surgery, anesthesiology, nursing (from the inpatient, outpatient, presurgical, operative, and postoperative care settings), advanced practice providers, house staff, case management, nutrition, physical therapy, occupational therapy, and informatics teams.
Patients
From January 2016, when the ERAS program was initiated, through December 2018, we performed 2000 ERAS-eligible colorectal surgeries at MSKCC. ERAS-eligible cases were defined as elective operations (colon or rectal resection, stoma creation, and stoma reversal), excluding urgent or emergent cases, multi-visceral resections, and cytoreductive surgeries. Case types were identified based on Current Procedural Terminology (CPT) codes. We divided this post-intervention cohort into the first sequential 1000 cases (Early ERAS group) and second 1000 cases (Late ERAS group). These were compared to a control group of the 1000 consecutive ERAS-eligible colorectal surgeries performed immediately prior to ERAS implementation (Pre-ERAS group). Adherence to ERAS process measures and postoperative outcomes (LOS, complications, and 30-day readmission) were compared across the three time periods (Pre-ERAS, Early ERAS, and Late ERAS). This study was approved by the institutional review board (MSKCC IRB #16-1265).
ERAS interventions
Adherence to ERAS interventions were measured in the preoperative, intraoperative, and postoperative settings. Preoperative interventions included consumption of the carbohydrate drink ClearFast and administration of alvimopan and gabapentin. Perioperative pain management included regional anesthetic options, either an epidural or a transversus abdominis plane (TAP) block. Nonopioid infusions (ketamine or dexmedetomidine) were administered intraoperatively.
Postoperative ERAS interventions included use of acetaminophen, non-steroidal anti-inflammatory drugs (NSAID), and gabapentin. Of note, no narcotics are included in the ERAS order sets; rescue opioids were selectively prescribed only as clinically indicated per clinician discretion, after appropriate nonopioid analgesics had been maximized. Epidural and urinary catheter duration, as well as urinary catheter reinsertion rates, were measured. Time to ambulation and time to regular diet were measured in days. As outlined in Supplementary Table 1, the standard was removal of urinary catheter, ambulation, and advancement to a regular diet all by postoperative day 1, with modifications as clinically appropriate.
For each patient, overall adherence was calculated as the percentage of preoperative, intraoperative, and postoperative ERAS interventions achieved. Adherence to the continuous variables (urinary catheter duration, time to ambulation, and time to regular diet) were defined by the threshold < 24 hours from surgery.
Postoperative complications and outcomes
Postoperative outcomes examined were LOS, 30-day complications, and 30-day readmission. LOS was calculated as time between date of discharge and date of surgery. Patients were evaluated for readmission within 30 days from the time of discharge. Complications within 30 days of surgery were interrogated from the institutionally maintained surgical database, including grade and category. Additionally, rates of surgical site infections (SSI) were calculated from the rigorously maintained and audited SSI surveillance program as reported to the National Healthcare Safety Network (NHSN). Types of SSI (superficial incisional primary, deep incisional primary, and intraabdominal infection) were categorized as per NHSN standardized definitions.
Statistical Analysis
Descriptive statistics for nominal or categorical variables were represented as percentages and continuous variables as means with standard deviations or medians with interquartile ranges (i.q.r.). The chi-square test was used for categorical variables. Continuous variables were analyzed with Studenťs t test or analysis of variance, with Tukey’s multiple comparisons test for pairwise comparisons between time periods, or Kruskal-Wallis with Dunn’s multiple comparisons test for nonparametric distributions. All statistical tests were two-sided, with a designated p-value threshold of 0.05 to indicate statistical significance. Statistical analyses were performed using R (www.R-project.org) and GraphPad Prism 8.
RESULTS
We compared 3000 sequential patients who underwent ERAS-eligible colorectal surgery, distributed equally across three time periods: Pre-ERAS (May 9, 2014 – December 30, 2015), Early ERAS (January 5, 2016 – June 20, 2017), and Late ERAS (June 21, 2017 – December 20, 2018). Patient demographics and surgery characteristics were as listed in Table 1. There were no differences in age, sex, American Society of Anesthesiologists (ASA) class, and body mass index. Of note, the majority of our patients were ASA class P3, consistent with an oncologic patient population.
Table 1.
Patient demographics and operation characteristics: All cases.
| Pre-ERAS | ERAS (early) | ERAS (late) | p | |
|---|---|---|---|---|
| n | 1000 | 1000 | 1000 | |
| Age [mean, years (SD)] | 58.2 (13.5) | 58.8 (13.3) | 58.6 (13.1) | 0.62 |
| Sex, male (%) | 530 (53) | 554 (55) | 536 (54) | 0.53 |
| Race/ethnicity (%) | <0.001 | |||
| Asian or Indian subcontinental | 58 (5.8) | 62 (6.2) | 92 (9.2) | |
| Black or African American | 71 (7.1) | 55 (5.5) | 42 (4.2) | |
| Hispanic or Latino | 35 (3.5) | 46 (4.6) | 55 (5.5) | |
| Native American – American Indian/Alaskan | 0 (0) | 3 (0.3) | 0 (0) | |
| Native Hawaiian or Pacific Islander | 1 (0.1) | 3 (0.3) | 0 (0) | |
| White | 779 (77.9) | 785 (78.5) | 781 (78.1) | |
| Unknown | 56 (5.6) | 46 (4.6) | 30 (3.0) | |
| ASA class (%) | 0.55 | |||
| P1 | 4 (0.4) | 2 (0.2) | 2 (0.2) | |
| P2 | 295 (29.5) | 289 (28.9) | 286 (28.6) | |
| P3 | 685 (68.5) | 682 (68.2) | 695 (69.5) | |
| P4 | 16 (1.6) | 27 (2.7) | 17 (1.7) | |
| BMI [mean, kg/m2 (SD)] | 28.5 (6.0) | 28.3 (6.0) | 27.9 (6.1) | 0.12 |
| Surgical approach, open (%) | 377 (38) | 299 (30) | 303 (30) | <0.001 |
| Surgery description | 0.001 | |||
| Colon resection (%) | 595 (59.5) | 584 (58.4) | 527 (52.7) | |
| Rectal resection (%) | 171 (17.1) | 201 (20.1) | 210 (21.0) | |
| Ostomy reversal (%) | 195 (19.5) | 163 (16.3) | 191 (19.1) | |
| Other (%) | 39 (3.9) | 52 (5.2) | 72 (7.2) | |
| Stomal diversion | 0.70 | |||
| Colostomy (%) | 70 (7.0) | 75 (7.5) | 87 (8.7) | |
| Ileostomy (%) | 115 (11.5) | 118 (11.8) | 113 (11.3) | |
| None (%) | 815 (81.5) | 807 (80.7) | 800 (80.0) | |
| EBL [median, mL (i.q.r)] | 100 (40 – 200) | 90 (40 – 200) | 90 (40 – 200) | 0.11 |
| Duration of surgery [mean, hours (SD)] | 3.4 (1.9) | 3.4 (1.9) | 3.5 (1.9) | 0.55 |
ERAS, Enhanced Recovery after surgery. SD, standard deviation. ASA, American Society of Anesthesiologists. BMI, body mass index. EBL, estimated blood loss. i.q.r., interquartile range.
p = chi-square test for categorical variables; analysis of variance / t-test or Kruskal-Wallis test (nonparametric) for continuous variables.
The surgical approach was less likely to be Open in the ERAS periods (30% Early and Late), as compared to Pre-ERAS (38%, p < 0.001). The types of operations differed (p = 0.001), with slightly more rectal resections performed in the ERAS periods. There were otherwise no differences in the rates of stoma creation, estimated blood loss, or duration of surgery. Patient characteristics and demographics demonstrated similar trends when analyzed by surgical approach, either minimally invasive (MIS) or Open (Supplementary Tables 2–3).
ERAS interventions
Preoperative ERAS interventions included the use of ClearFast, alvimopan, and gabapentin. Adherence with these process measures increased significantly across all three time periods (p < 0.001, Table 2). Overall, patients were more likely to receive a TAP block or epidural in the ERAS periods (85% Early, 93% Late), as compared to Pre-ERAS (31%, p < 0.001). Patients were also more likely to receive intraoperative nonopioid infusions (ketamine or dexmedetomidine) in the ERAS era (p < 0.001). The improved adherence trends were especially pronounced for MIS cases, with significantly increased rates of ClearFast, alvimopan, gabapentin, TAP block or epidural, and intraoperative nonopioid infusion rates (Supplementary Table 4).
Table 2.
ERAS adherence: All cases.
| Pre-ERAS | ERAS (early) | ERAS (late) | p | ||
|---|---|---|---|---|---|
| n | 1000 | 1000 | 1000 | ||
| Preoperative | ClearFast (%) | 0 (0) | 82 (8.2) | 702 (70.2) | <0.001abc |
| Alvimopan (%) | 0 (0) | 835 (83.5) | 890 (89.0) | <0.001abc | |
| Gabapentin (%) | 0 (0) | 866 (86.6) | 917 (91.7) | <0.001abc | |
| Preoperative/Intraoperative | TAP block or Epidural (%) | 314 (31.4) | 849 (84.9) | 925 (92.5) | <0.001abc |
| Intraoperative | Nonopioid infusion (%) | 197 (19.7) | 759 (75.9) | 786 (78.6) | <0.001ab |
| Postoperative | Acetaminophen (%) | 983 (98.3) | 983 (98.3) | 969 (96.9) | 0.046 |
| NSAID (%) | 652 (65.2) | 865 (86.5) | 748 (74.8) | <0.001abc | |
| Gabapentin (%) | 61 (6.1) | 330 (33.0) | 495 (49.5) | <0.001abc | |
| No rescue opioid (%) | 19 (1.9) | 365 (36.5) | 468 (46.8) | <0.001abc | |
| Urinary catheter duration [mean, days (SD)] | 2.1 (1.3) | 1.6 (1.3) | 1.4 (1.0) | <0.001abc | |
| Time to ambulation [mean, days (SD)] | 0.6 (0.7) | 0.6 (0.6) | 0.5 (0.5) | 0.024b | |
| Time to regular diet [mean, days (SD)] | 3.3 (1.9) | 2.4 (2.2) | 2.0 (1.7) | <0.001abc | |
| Overall | Adherence [median, % (i.q.r.)] | 25 (25 – 33) | 67 (58 – 75) | 75 (67 – 83) | <0.0001abc |
ERAS, Enhanced Recovery after surgery. TAP, transversus abdominis plane. NSAID, non-steroidal anti-inflammatory. SD, standard deviation. i.q.r., interquartile range.
p = chi-square test for categorical variables; analysis of variance / t-test with Tukey’s multiple comparisons test or Kruskal-Wallis (nonparametric) with Dunn’s multiple comparisons test for continuous variables.
significant for Pre- vs Early ERAS.
significant for Pre- vs Late ERAS.
significant for Early vs Late ERAS.
Postoperative NSAID and gabapentin use significantly increased after ERAS intervention, while acetaminophen usage remained consistently high. As compared to Pre-ERAS, NSAID usage was higher in both the Early and Late ERAS periods, though there was a decrease between the Early and Late ERAS periods. Conversely, the rate of gabapentin administration increased significantly across all three time periods (p < 0.001). With these non-narcotic interventions, the rates of rescue opioid administration decreased significantly. Excluding the intraoperative and post-anesthesia care settings, the percentage of patients who did not receive rescue opioids increased from only 1.9% Pre-ERAS to 36.5% in the Early ERAS and 46.8% Late ERAS periods (p < 0.001). This trend was even stronger among MIS cases, where the rate of rescue opioid avoidance significantly increased from 2.7% Pre-ERAS to 55.1% Late ERAS (p < 0.001, Supplementary Table 4).
Urinary catheters were removed sooner (p < 0.001), with no difference in the rates of urinary catheter reinsertion (8.3% Pre-ERAS, 6.6% Early, 7.3% Late ERAS, p = 0.46). Times to ambulation and regular diet also significantly decreased across these time periods (p = 0.024 and < 0.001, respectively).
Overall, adherence rates significantly increased across all three time periods, from median 25% (i.q.r. 25 – 33%) Pre-ERAS to 67% (58 – 75%) Early and 75% (67 – 83%) Late ERAS (p < 0.0001). Adherence rates significantly increased not only in comparison between the Pre-ERAS and ERAS periods but also between the Early and Late ERAS periods (Table 2 and Figure 1). This trend was significant in both MIS and Open subgroup analyses (Supplementary Tables 4–5 and Supplementary Figure 1a–b).
Figure 1. Adherence to ERAS metrics significantly increased over time: All cases.
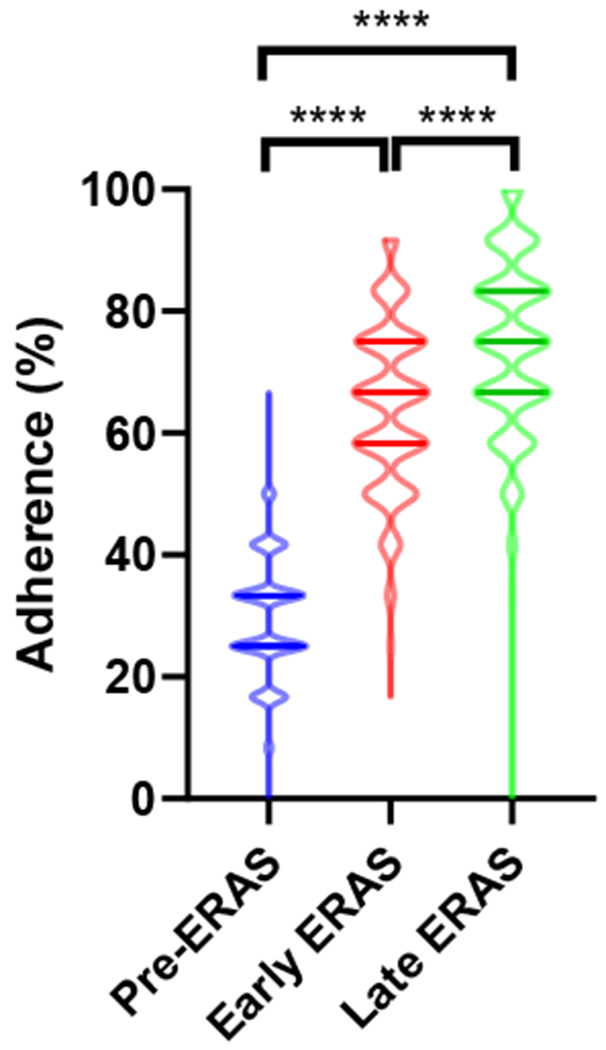
Violin plots. ERAS, Enhanced Recovery after surgery. p = Kruskal-Wallis, Dunn’s multiple comparisons test.
Complications
As captured in our institutionally maintained database of surgical complications, there were no differences in the overall rates of 30-day complications (9 – 10%) among the Pre-ERAS, Early ERAS, and Late ERAS periods (Table 3a). In addition, there were no differences in the severity of complications (p = 0.24) or types of complications (p = 0.21).
Table 3a.
Complications: All cases.
| Pre-ERAS | ERAS (early) | ERAS (late) | p | |
|---|---|---|---|---|
| n | 1000 | 1000 | 1000 | |
| Grade | 0.24 | |||
| No complication | 903 (90.3) | 893 (89.3) | 903 (90.3) | |
| 1 | 23 (2.3) | 33 (3.3) | 28 (2.8) | |
| 2 | 45 (4.5) | 35 (3.5) | 35 (3.5) | |
| 3 | 29 (2.9) | 32 (3.2) | 32 (3.2) | |
| 4 | 0 (0) | 6 (0.6) | 2 (0.2) | |
| 5 | 0 (0) | 1 (0.1) | 0 (0) | |
| Category | 0.21 | |||
| Cardiovascular system | 2 (0.2) | 6 (0.6) | 2 (0.2) | |
| Endocrine system | 0 (0) | 1 (0.1) | 0 (0) | |
| Gastrointestinal system | 34 (3.4) | 41 (4.1) | 49 (4.9) | |
| General | 7 (0.7) | 5 (0.5) | 6 (0.6) | |
| Genitourinary system | 7 (0.7) | 3 (0.3) | 8 (0.8) | |
| Hematologic or vascular system | 11 (1.1) | 13 (1.3) | 3 (0.3) | |
| Infection | 19 (1.9) | 25 (2.5) | 14 (1.4) | |
| Metabolic | 0 (0) | 1 (0.1) | 0 (0) | |
| Musculoskeletal system | 0 (0) | 2 (0.2) | 2 (0.2) | |
| Nervous system | 2 (0.2) | 0 (0) | 0 (0) | |
| Pulmonary system | 2 (0.2) | 1 (0.1) | 2 (0.2) | |
| Wound or skin | 13 (1.3) | 9 (0.9) | 11 (1.1) | |
ERAS, Enhanced Recovery after surgery. p = chi-square test.
Surgical site infections are also reported to the NHSN in a subset of colorectal cases, which are routinely audited for accuracy. In this database, the rates and types of SSIs also did not differ among the different time periods, before and after ERAS intervention (Table 3b). In addition, there were no differences in overall rates, grades, and types of complications in both MIS (Supplementary Table 6a–b) and Open (Supplementary Table 7a–b) approach subgroup analyses.
Table 3b.
Surgical site infections of all SSI surveillance cases reported to National Healthcare Safety Network.
| Pre-ERAS | ERAS (early) | ERAS (late) | p | |
|---|---|---|---|---|
| n reported (%) | 500 (50) | 737 (73.7) | 702 (70.2) | |
| SSI type | ||||
| SIP (%) | 6 (1.2) | 7 (0.9) | 3 (0.4) | 0.31 |
| DIP (%) | 1 (0.2) | 1 (0.1) | 0 (0) | 0.53 |
| IAB (%) | 10 (2.0) | 17 (2.3) | 17 (2.4) | 0.89 |
ERAS, Enhanced Recovery after surgery. SSI, surgical site infection. SIP, superficial incisional primary. DIP, deep incisional primary. IAB, intraabdominal infection. p = chi-square test.
Outcomes
With these ERAS interventions, postoperative LOS significantly decreased from a mean of 5.2 days Pre-ERAS, to 4.5 days Early ERAS and 4.0 days Late ERAS (p < 0.0001, Table 4). These differences were significant not only between the Pre-ERAS and ERAS periods, but also between the Early and Late ERAS periods (Figure 2). Among MIS cases, LOS significantly decreased from a mean of 4.9 days Pre-ERAS to 3.7 days Late ERAS, while LOS for Open cases decreased from a mean of 5.5 to 4.6 days (Table 4 and Supplementary Figure 2a–b).
Table 4.
Outcomes: All, MIS, and Open cases.
| Pre-ERAS | ERAS (early) | ERAS (late) | p | |
|---|---|---|---|---|
| All cases (n) | 1000 | 1000 | 1000 | |
| LOS (days) | <0.0001abc | |||
| Mean (SD) | 5.2 (2.8) | 4.5 (4.3) | 4.0 (3.7) | |
| Median (i.q.r) | 4.6 (3.8 – 5.9) | 3.2 (2.7 – 4.9) | 3.1 (2.2 – 4.1) | |
| 30-day readmission (%) | 79 (7.9) | 76 (7.6) | 77 (7.7) | 0.97 |
| MIS cases (n) | 623 | 701 | 697 | |
| LOS (days) | <0.0001abc | |||
| Mean (SD) | 4.9 (2.5) | 4.2 (4.2) | 3.7 (3.2) | |
| Median (i.q.r) | 4.2 (3.8 – 5.3) | 3.1 (2.3 – 4.2) | 3.0 (2.1 – 4.0) | |
| 30-day readmission (%) | 46 (7.4) | 45 (6.4) | 52 (7.5) | 0.70 |
| Open cases (n) | 377 | 299 | 303 | |
| LOS (days) | 0.02b | |||
| Mean (SD) | 5.5 (3.2) | 5.2 (4.5) | 4.6 (4.6) | |
| Median (i.q.r) | 4.9 (3.2 – 6.3) | 4.0 (2.9 – 5.9) | 3.1 (2.2 – 5.7) | |
| 30-day readmission (%) | 33 (8.8) | 31 (10.4) | 25 (8.3) | 0.64 |
ERAS, Enhanced Recovery after surgery. LOS, length of stay. i.q.r., interquartile range.
p = chi-square test (categorical variables) or ordinary one-way ANOVA, Tukey’s multiple comparisons test (continuous variables).
significant for Pre- vs Early ERAS.
significant for Pre- vs Late ERAS.
significant for Early vs Late ERAS.
Figure 2. Length of stay decreased after ERAS intervention and between early and late ERAS periods: All cases.
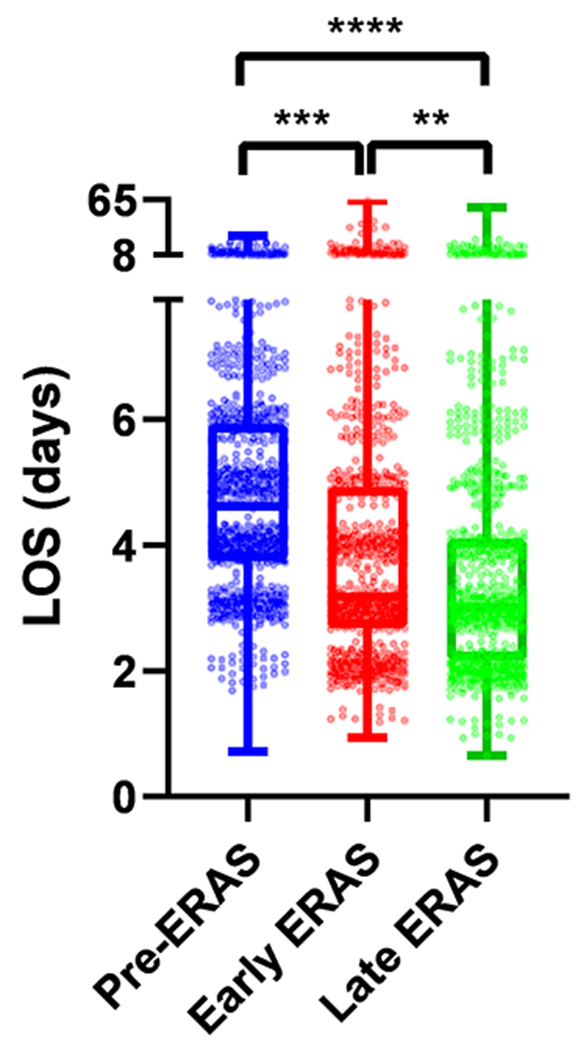
Box and whisker plots. LOS, length of stay. ERAS, Enhanced Recovery after surgery. p = ordinary one-way ANOVA, Tukey’s multiple comparisons test.
Additional subgroup analysis was performed by type of surgery. For MIS colectomies, LOS decreased from a mean 4.7 days Pre-ERAS to 3.9 days Early and 3.3 days Late ERAS (p < 0.0001 Pre- vs Early and Pre- vs Late ERAS; p = 0.002 Early vs Late ERAS). For MIS proctectomies with stomal diversion, LOS decreased from a mean 6.1 days Pre-ERAS to 5.6 days Early and 4.6 days Late ERAS (p = 0.03 Pre- vs Late ERAS). LOS for Open stoma reversals also decreased from a mean 4.4 days Pre-ERAS to 3.4 days Early and 3.2 days Late ERAS (p = 0.002 Pre- vs Early ERAS; p < 0.0001 Pre- vs Late ERAS). Of note, there were no differences in LOS for open colectomy and proctectomy; however, this may represent a cohort of patients with clinically significant factors that necessitated selection of an open surgical approach.
Importantly, there were no differences in the rates of 30-day readmission overall, as well as in MIS and Open approach subgroup analyses (Table 4). In addition, patients with shorter LOS had lower 30-day readmission rates, both before (Figure 3a) and after (Figure 3b) ERAS intervention. This association was stronger in the ERAS period; patients discharged within 3 days had a mean readmission rate of 4.4% (C.I. 3.2 – 5.9%), as compared to 7.1% for LOS of 4 days and >10% for LOS ≥5 days (Figure 3b, p < 0.0001). This trend was again significant in surgical approach subgroup analysis; since ERAS implementation, patients discharged within 3 days had lower 30-day readmission rates for both MIS (4.2%, C.I. 3.0-6.1%, Supplementary Figure 3) and Open cases (4.7%, C.I. 2.6-8.2%, Supplementary Figure 4), as compared to those patients with longer LOS.
Figure 3. Shorter length of stay was associated with lower 30-day readmission rates, before (A) and after (B) ERAS intervention: All cases.
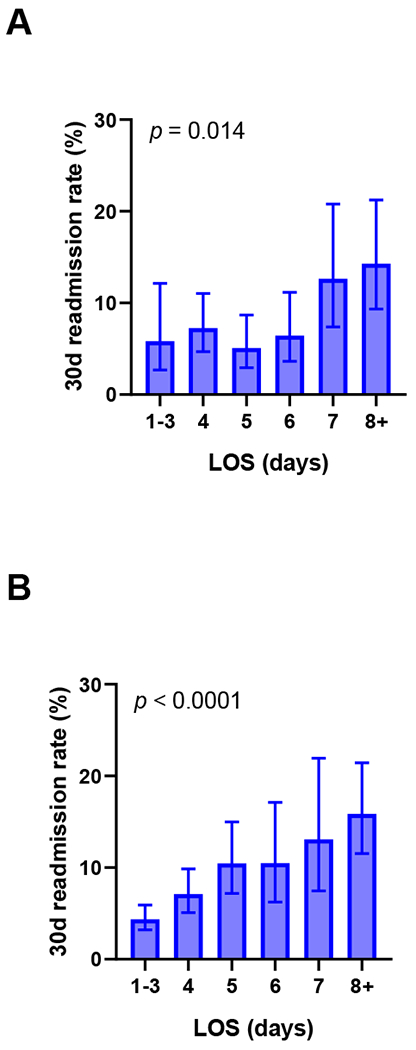
LOS, length of stay. Estimated 95% confidence intervals. p = chi-square test.
DISCUSSION
The implementation of a multimodal ERAS program was associated with a decreased LOS for patients undergoing elective colorectal surgery in a high-volume, tertiary cancer center. As ERAS protocol adherence increased over time, this was associated with a further decrease in LOS. A particular highlight of this program has been the significantly decreased use of postoperative rescue narcotics, with the benefits of avoiding opioid-associated side effects, toxicities, and long-term dependence [4, 16, 17]. This was most striking in the MIS cohort, with only 2.7% patients avoiding rescue narcotics Pre-ERAS, up to 55% in the Late ERAS period and most recently, sustained at 75% (data not shown).
Concerns have been raised that enhanced recovery and early discharge may lead to missed complications and need for readmission, especially in a high-risk cancer population. It is therefore important to highlight that despite shorter LOS, we observed no differences in the rates of complications, including urinary catheter reinsertion, surgical site infections, and 30-day readmission. In fact, patients discharged within 72 hours had lower rates of readmission (4.2% MIS and 4.7% Open cases), similar to previous reports [18]. This suggests that patients who recover well enough to be discharged early along an ERAS pathway fare better than those who require longer hospitalizations.
ERAS-associated benefits are closely linked to protocol adherence. Gustafsson et al. previously reported their experience of 953 colorectal cancer patients who underwent resection in the 6-year period after initiation of an ERAS program. As compared to patients with low ERAS adherence, those with high adherence had lower rates of complications, symptoms delaying discharge, and readmissions [14]. More recently, the prospective, multicenter Postoperative Outcomes Within Enhanced Recovery After Surgery Protocol (POWER) study examined 2084 patients who underwent elective colorectal surgery during a 2-month period. Regardless of whether an institution had an established ERAS program, adherence to ERAS metrics was associated with a lower rate of early postoperative complications [9]. In the Netherlands, Cakir et al. reviewed their initial experience for colon cancer patients resected in the 4 years after ERAS implementation [13]. While LOS initially decreased, this was not sustained when ERAS adherence rates fell. They subsequently instituted several improvements, including a dedicated nurse practitioner to track ERAS compliance. In their published update, they compared 759 colon cancer patients in the 8 years post-intervention to 57 patients in the year prior to ERAS intervention [6]. The programmatic changes significantly increased ERAS adherence, and patients who underwent surgery in years with high adherence had shorter LOS as compared to those with low adherence (5.7 vs 7.3 days, p < 0.001).
Recognizing the value of protocol adherence, we have emphasized routine multidisciplinary monitoring as an important component of our ERAS program. As a result, we increased adherence rates in the Late ERAS period through several improvements. Most significant was the creation of electronic ERAS order sets to establish baseline expectations and automate pathway advancements. This effort took a year of multidisciplinary collaboration, including clinician education to ensure that order sets would still be modified as clinically necessary to optimize ERAS adherence while maintaining patient safety. We also identified a significant barrier to adherence has been the culture shift for both patients and clinicians in their expectations for an enhanced postoperative recovery. This continues to be addressed with multidisciplinary education and frequent updates to patient educational resources, to align patient and clinician expectations and motivate postoperative progress. While many institutions may start ERAS programs, we have highlighted the importance of continual monitoring in order to improve and sustain this multidisciplinary effort.
In our ongoing evaluation of the ERAS program, we have also recognized the importance of focusing on trends of adherence and outcomes, rather than setting strict metrics of compliance. While there appears to be room for improving protocol adherence to further optimize patient outcomes, there will always be limitations to “perfect” compliance as dictated by appropriate clinical care. Side effects and toxicities must be carefully considered with the administration of any medication. Considerations with the routine use of NSAIDs include risks of bleeding and renal insufficiency, especially in the setting of fluid-restrictive ERAS measures. More recently, there have also been reports of an association with increased anastomotic leakage, especially with non-selective NSAIDs [19–21], though a correlation has not been demonstrated prospectively [22]. In our experience, we found no increase in the rates of bleeding or anastomotic leakage since implementation of the ERAS program, despite significantly increased NSAID use. However, we do note the utilization of NSAIDs is not universal (in fact, adherence rates slightly decreased from the Early to Late ERAS periods), which may reflect careful clinician assessment that has allowed us to avoid significant NSAID-related complications. It is critical that we continue to balance a cautious approach to optimizing adherence to the ERAS program with patient-directed care. This emphasizes the importance of ongoing monitoring for any ERAS program, to permit early recognition of patient outcomes adversely affected by overly strict interventions.
There are several limitations to this study. One is the retrospective, observational design. However, biases were avoided by selecting consecutive patients for review based on CPT codes and the ad hoc clinical criteria outlined above. This is also a single institution experience. While our patient population and demographics may not be universally representative, our study explores the overall effects of an early, perioperative ERAS program, including the challenges of implementation and multidisciplinary maintenance. Another limitation of this study is the data were queried from the electronic medical record, which may be limited in scope and documentation; we therefore cannot exclude the possibility of certain metrics being underreported. However, we did confirm the clinically relevant complications of infections and anastomotic leakage (i.e. intraabdominal infection) correlated well with the separately maintained and audited NHSN SSI surveillance database.
A significant strength of our study is the large number of cases examined as compared to prior studies. While our ERAS program was implemented recently in 2016, limiting the period of observation, we nonetheless demonstrated significantly improved adherence rates and outcomes for 3000 patients across three time periods of intervention. Future evaluation of our ongoing ERAS program will be important to confirm progress and sustainability.
CONCLUSIONS
Implementation of a multimodal ERAS program for patients undergoing colorectal surgery at a tertiary cancer center was associated with a clinically significant decrease in LOS without an associated increase in postoperative morbidity or readmission rates. Programmatic improvements increased adherence to the ERAS protocol and was associated with a further decrease in LOS. In our experience, ongoing evaluation and multidisciplinary collaboration result in a safe and effective ERAS program.
Supplementary Material
ACKNOWLEDGMENTS
We would like to acknowledge Traci Hedrick and Robert Thiele for their expertise in helping us design and implement our colorectal ERAS program. We would also like to thank all the members of the Memorial Sloan Kettering Cancer Center Multidisciplinary Enhanced Recovery After Colorectal Surgery Team and their innumerable contributions to the ongoing success and maintenance of the colorectal ERAS program.
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Disclosures: This study was approved by the institutional review board (MSKCC IRB #16-1265). AA is a consultant for Pacira Pharmaceuticals. The authors have no other commercial associations or financial disclosures to declare.
REFERENCES
- [1].Gustafsson UO, Scott MJ, Hubner M, et al. Guidelines for Perioperative Care in Elective Colorectal Surgery: Enhanced Recovery After Surgery (ERAS®) Society Recommendations: 2018. World J Surg 2019; 43: 659–695. [DOI] [PubMed] [Google Scholar]
- [2].Nygren J, Thacker J, Carli F, et al. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr 2012; 31: 801–816. [DOI] [PubMed] [Google Scholar]
- [3].Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg 2002; 183: 630–641. [DOI] [PubMed] [Google Scholar]
- [4].McEvoy MD, Scott MJ, Gordon DB, et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on optimal analgesia within an enhanced recovery pathway for colorectal surgery: part 1-from the preoperative period to PACU. Perioper Med (Lond) 2017; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moonesinghe SR, Grocott MPW, Bennett-Guerrero E, et al. American Society for Enhanced Recovery (ASER) and Perioperative Quality Initiative (POQI) joint consensus statement on measurement to maintain and improve quality of enhanced recovery pathways for elective colorectal surgery. Perioper Med (Lond) 2017; 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bakker N, Cakir H, Doodeman HJ, et al. Eight years of experience with Enhanced Recovery After Surgery in patients with colon cancer: Impact of measures to improve adherence. Surgery 2015; 157: 1130–1136. [DOI] [PubMed] [Google Scholar]
- [7].Vlug MS, Wind J, Hollmann MW, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 2011; 254: 868–875. [DOI] [PubMed] [Google Scholar]
- [8].Khoo CK, Vickery CJ, Forsyth N, et al. A Prospective Randomized Controlled Trial of Multimodal Perioperative Management Protocol in Patients Undergoing Elective Colorectal Resection for Cancer. Ann Surg 2007; 245: 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ripolles-Melchor J, Ramirez-Rodriguez JM, Casans-Frances R, et al. Association Between Use of Enhanced Recovery After Surgery Protocol and Postoperative Complications in Colorectal Surgery: The Postoperative Outcomes Within Enhanced Recovery After Surgery Protocol (POWER) Study. JAMA Surg 2019; 154: 725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marcus RK, Lillemoe HA, Rice DC, et al. Determining the Safety and Efficacy of Enhanced Recovery Protocols in Major Oncologic Surgery: An Institutional NSQIP Analysis. Ann Surg Oncol 2019; 26: 782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].McGugin CJ, Coopey SB, Smith BL, et al. Enhanced Recovery Minimizes Opioid Use and Hospital Stay for Patients Undergoing Mastectomy with Reconstruction. Ann Surg Oncol 2019; 26: 3464–3471. [DOI] [PubMed] [Google Scholar]
- [12].Webb C, Day R, Velazco CS, et al. Implementation of an Enhanced Recovery After Surgery (ERAS) Program is Associated with Improved Outcomes in Patients Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol 2020; 27: 303–312. [DOI] [PubMed] [Google Scholar]
- [13].Cakir H, Stijn MFM van, Cardozo AMFL, et al. Adherence to Enhanced Recovery After Surgery and length of stay after colonic resection. Colorectal Disease 2013; 15: 1019–1025. [DOI] [PubMed] [Google Scholar]
- [14].Gustafsson UO, Hausel J, Thorell A, et al. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 2011; 146: 571–577. [DOI] [PubMed] [Google Scholar]
- [15].Makaryus R, Miller TE, Gan TJ. Current concepts of fluid management in enhanced recovery pathways. British Journal of Anaesthesia 2018; 120: 376–383. [DOI] [PubMed] [Google Scholar]
- [16].Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. JAMA 2014; 311: 2110–2120. [DOI] [PubMed] [Google Scholar]
- [17].Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology 2006; 104: 570–587. [DOI] [PubMed] [Google Scholar]
- [18].Grass F, Hiibner M, Mathis KL, et al. Identification of patients eligible for discharge within 48 h of colorectal resection. Br J Surg. Epub ahead of print 7 January 2020. DOI: 10.1002/bjs.11399. [DOI] [PubMed] [Google Scholar]
- [19].Gorissen KJ, Benning D, Berghmans T, et al. Risk of anastomotic leakage with non-steroidal anti-inflammatory drugs in colorectal surgery. Br J Surg 2012; 99: 721–727. [DOI] [PubMed] [Google Scholar]
- [20].Klein M, Gögenur I, Rosenberg J. Postoperative use of non-steroidal anti-inflammatory drugs in patients with anastomotic leakage requiring reoperation after colorectal resection: cohort study based on prospective data. BMJ 2012; 345: e6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hakkarainen TW, Steele SR, Bastaworous A, et al. Nonsteroidal anti-inflammatory drugs and the risk for anastomotic failure: a report from Washington State’s Surgical Care and Outcomes Assessment Program (SCOAP). JAMA Surg 2015; 150: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].EuroSurg Collaborative. Safety and efficacy of non-steroidal anti-inflammatory drugs to reduce ileus after colorectal surgery. Br J Surg 2020; 107: el61–el69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


