Abstract
Background:
Dexmedetomidine is only approved for use in humans as an intravenous medication. An oral formulation may broaden the use and benefits of dexmedetomidine to numerous care settings. We hypothesized that oral dexmedetomidine (300-mcg to 700-mcg) would result in plasma concentrations consistent with sedation while maintaining hemodynamic stability.
Methods:
We performed a single-site, open-label, phase I dose-escalation study of a solid oral dosage formulation of dexmedetomidine in healthy volunteers (n = 5, 300-mcg; followed by n = 5, 500-mcg; followed by n = 5, 700-mcg). The primary study outcome was hemodynamic stability defined as lack of hypertension, hypotension, or bradycardia. We assessed this outcome by analyzing raw hemodynamic data. Plasma dexmedetomidine concentrations were determined by liquid chromatograph-tandem mass spectrometry. Nonlinear mixed effect models were used for pharmacokinetic and pharmacodynamic analyses.
Results:
Oral dexmedetomidine was associated with plasma concentration-dependent decreases in heart rate and mean arterial pressure. All, but one subject in the 500-mcg group, met our criteria for hemodynamic stability. The plasma concentration profile was adequately described by a 2-compartment, weight allometric, first-order absorption, first-order elimination pharmacokinetic model. The standardized estimated parameters for an individual of 70 kg was V1 = 35.6 [95% CI: 23.8–52.8] L; V2 = 54.7 [34.2–81.7] L; CL = 0.56 [0.49–0.64] L/min; and F = 7.2 [4.7–14.4]%. Linear models with effect sites adequately described the decreases in mean arterial pressure and heart rate associated with oral dexmedetomidine administration. However, only the 700-mcg group reached plasma concentrations that have previously been associated with sedation (>0.2 ng/ml).
Conclusions:
Oral administration of dexmedetomidine in doses between 300–700 mcg was associated with decreases in heart rate and mean arterial pressure. Despite low oral absorption, the 700-mcg dose scheme reached clinically relevant concentrations for possible use as a sleep-enhancing medication.
Keywords: dexmedetomidine, oral formulation, non-rapid eye movement sleep, PK-PD
Introduction
Dexmedetomidine is an alpha-2 adrenergic agonist sedative that promotes sleep neurophysiology.1–10 It is widely administered to patients in intensive care units as a pharmacological aid to reduce the incidence and duration of delirium.11–15 Dexmedetomidine is only approved for use in humans as an intravenous medication. An oral formulation of dexmedetomidine is expected to broaden its sedation and delirium-sparing benefits to numerous care settings (e.g., general medical and surgical units). We recently reported that a single night time loading dose of intravenous dexmedetomidine promoted non-rapid eye movement stage 3 sleep, preserved regular sleep cycling, and may confer the cognitive benefits of sleep to healthy subjects.6 This result suggests that continuous infusions of dexmedetomidine may not be necessary for sleep promotion, and that dexmedetomidine could be developed as an oral sleep-enhancing medication.
Whether a solid oral formulation of dexmedetomidine will reach plasma concentrations, and by proxy, brain concentrations necessary to promote sleep neurophysiology is unclear. In humans, plasma concentrations of dexmedetomidine between 0.2 ng/mg and 0.3 ng/ml result in rousable sedation,16 while plasma concentrations above 1.9 ng/ml may be necessary for unarousable sedation.17 Intravenous dexmedetomidine has been associated with a biphasic mean arterial blood pressure (MAP) response.18,16,17 Low dexmedetomidine plasma concentrations (<~ 1.9 ng/ml) are associated with decreased MAP and heart rate (HR),17 while high plasma concentrations (>~ 1.9 ng/ml) are associated with increased MAP.17 These MAP and HR findings are likely mediated through drug activity on peripheral vascular endothelium and in the central nervous system, respectively.16,19,20 However, MAP and HR changes associated with oral dexmedetomidine have not been characterized.
Therefore, we performed a phase I dose escalation study (n = 5, 300-mcg; followed by n = 5, 500-mcg; followed by n = 5, 700-mcg) of oral dexmedetomidine. We hypothesized that oral dexmedetomidine would be associated with hemodynamic stability (see methods section for definition) and plasma concentrations that are consistent with rousable sedation. We constructed a pharmacokinetic-pharmacodynamic (PKPD) model to characterize the relationship between plasma concentrations of dexmedetomidine, MAP and HR data.
Methods
The Partners Human Research Committee approved this study, which was conducted at the Massachusetts General Hospital, Boston, MA, and was registered on clinicaltrials.gov (NCT02818569) on June 29, 2016 (Principal Investigator: Oluwaseun Akeju). This study was conducted under a Food and Drug Administration Investigator-Initiated Investigational New Drug Application (IND129461). A data and safety monitoring board was charged with the safety of study subjects and to ensure that the scientific goals of the study were met.
Recruitment for this study occurred between October 2016 and April 2017. A study flyer was disseminated through the Partners Public Affairs distribution list. Potential study participants contacted a clinical research coordinator who administered a questionnaire to confirm that the study inclusion and exclusion criteria were met. The primary inclusion criterion was meeting the American Society of Anesthesiology Physical Status I. The following information was also verified by self-report: regular sleep-wake cycles, absence of naps, or consumption of alcohol or caffeinated beverages before sleep, drug-free, and non-smoking status. Before potential enrollment, subjects underwent a complete medical history and standard pre-anesthesia assessment. Other procedures included a toxicology screen to rule out prohibited drug use, a pregnancy test for females, and an electrocardiogram to rule out cardiac conduction abnormalities. None of the subjects had any known history of sleep disorders or any physical or psychiatric illness. Written informed consent was obtained from all subjects before any study-related procedures. There was no evidence of abnormal liver or renal function blood tests at baseline screening.
This was a single-site, open-label, phase I dose escalation study (n = 5, male = 2, 300-mcg; followed by n = 5, male = 4, 500-mcg; followed by n = 5, male = 1, 700-mcg) of a solid oral dosage formulation of dexmedetomidine in 15 healthy subjects. The primary outcome of the study was hemodynamic stability. We defined hemodynamic instability as 1) hypotension: systolic blood pressure (SBP) < 60 mmHg, diastolic blood pressure (DBP) < 40 mmHg or decrease of SBP by ≥ 50 % from baseline which was sustained over two consecutive minutes; 2) hypertension: SBP > 180 mmHg, DBP > 100 mmHg, or increase of SBP by > 50% from baseline which was sustained over two minutes; or, 3) bradycardia: HR < 40 bpm or decrease by > 50% from baseline sustained over two consecutive minutes.
Study subjects were instructed to arrive at 7:30 AM to prepare for study-related procedures. A urine toxicology screen was performed to rule out the use of prohibited substances, and a urine pregnancy test was also performed to rule out pregnancy in all female subjects. An anesthesiologist placed arterial and intravenous lines, and blood samples were obtained at the following intervals from the arterial line: baseline, 10, 20, 30, 45 minutes, and 1, 2, 3, 4, 5, 6, 7 hours after the oral administration of dexmedetomidine. Blood samples were centrifuged within 2 hours of collection at 3000 revolutions per minute for 30 minutes at 24° C, stored in a −20° C freezer for 24 hours and then transferred to a −80° freezer for long-term storage (9 to 15 months) prior to transfer to an outside laboratory for plasma concentration analysis. The arterial line was also used to monitor blood pressure. Physiological measurements included 5-lead electrocardiogram, pulse oximetry, end-tidal CO2, and 32-channel electroencephalogram data. Dexmedetomidine hydrochloride, USP, purchased from Jiangsu Hengrui Medicine Company Limited (Jiangsu Province, China) was weighed in a laminar flow hood and packaged in oral capsules with lactose powder. The capsules were stored according to recommendations in airtight containers at room temperature, and for less than 6 months. All capsules were administered prior to the drug retest date on the certificate of analysis. A board-certified anesthesiologist monitored all subjects throughout the study.
Plasma dexmedetomidine concentrations were determined by liquid chromatograph-tandem mass spectrometry (LC-MS/MS). To each (0.1 mL) plasma sample and appropriate calibration standards, dexmedetomidine-D4 was added as an internal standard. Samples were extracted by protein precipitation by the addition of 0.5 mL of acetonitrile. The samples were centrifuged, and the supernatant transferred to autosampling vials. The analytic instrument was an AB Sciex API Qtrap 5500 quadrupole mass spectrometer equipped with QJet ion guide and accelerated by a LINAC collision cell (AB Sciex, Foster City, CA, U.S.A.) with an atmospheric pressure chemical ionization probe in a Turbo Vion source, interfaced with a Waters Corporation (Milford, MA, U.S.A.) Acquity ultra pressure liquid chromatograph. Analyst software 1.6.2 (AB Sciex, Foster City, CA, U.S.A.) was used for system control and data processing. The liquid chromatography system was equipped with an Acquity UPLC HSS T3 1.8 μm, 2.1 × 50 mm HPLC column, and an Acquity UPLC HSS T3 1.84 μm VanGuard precolumn (Milford, MA, U.S.A.). The mobile phase consisted of a mixture of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B), with a flow rate of 0.5 ml/min and run time of 2 min. Solvents A and B were combined in a gradient: 0–1 min: 85% A; 1–1.5 min: 50% A; 1.6–2 min: return to initial conditions and hold for 3 min. The electrospray source was operated in the positive ionization mode, using collision gas (CAD) 12, curtain gas (CUR) 20, ion source gas 40, and ion spray voltage 5500 V with temperature 500 °C. The instrument was operated in the multiple reaction monitoring (M.R.M.) mode. The following MRM transitions of precursor ions to product ions were selected: dexmedetomidine, m/z 337.2->188.2 (collision energy, CE 24 V); dexmedetomidine-D4, m/z 342.2 −> 188.2 (collision energy, CE 35 V). The scan time was 100 ms for all analyses. The concentration range in calibration standards was 0.05 to 5 ng/ml of dexmedetomidine. Thus, the lower limit of sensitivity was 0.05 ng/ml of plasma using a 0.1 mL sample. The within- and between-day variability did not exceed 10%.
The hemodynamic dataset contained systolic, diastolic and heart rate measurements collected every 5 seconds per session for all subjects. MAP was calculated based on systolic and diastolic blood pressure, as previously described.21 We assessed our primary study endpoint by analyzing the raw hemodynamic data in MATLAB (MathWorks, Natick, MA). Briefly, to detect episodes of hemodynamic instability, we first defined baseline as the mean of the first 5 minutes (60 data points). We assessed for hemodynamic instability (as defined above) by iteratively scanning the data over 24 data points using a sliding window of 1 data point (i.e., 0 to 2 mins, 5 seconds-2mins 5 secs, etc.,).
We recorded electroencephalogram data using a standard 32-channel electroencephalogram cap (ANT Neuro, Netherlands) with average referencing. We down sampled the electroencephalogram data to 256 Hz and analyzed data from C3–C4 and P3–P4 bipolar electrodes. We used the Chronux toolbox in MATLAB (MathWorks, Natick, MA) to compute the multitaper spectral estimates. The spectral parameters were: TW or time-bandwidth product=3, K or number of tapers= 5 and T or window size= 4 seconds with 3 seconds of overlapping windows.
For PKPD modeling, we performed data reduction by retaining raw hemodynamic records 10 minutes apart. After data reduction, we checked the data to reduce the influence of artifacts associated with arterial line handling (e.g., blood sample draws, saline flushes). For missing values or outlying values, we replaced the data using a 60-second median filter. For illustrative purposes, we calculated the mean MAP and mean HR and fitted a smooth function to the data (‘rloess’ method) in MATLAB (MathWorks, Natick, MA).
One and two-compartment, first-order absorption, and first-order elimination, PK models were used to fit the dexmedetomidine plasma concentration (Cp) data. Population parameter estimates (V1, central volume of distribution (L); V2, peripheral volume of distribution (L); CL, elimination clearance (L/min); Q, distribution clearance (L/min);Tabs, absorption rate half-time (min) and F, bioavailability) were obtained using nonlinear mixed effects modeling performed in NONMEM v7.4 (ICON Development Solutions, USA). Population and individual parameters were estimated using the first-order conditional estimation (FOCE-I) method that included interaction options.22 The convergence criterion was 3 significant digits. Between-individual variability in model parameters was assumed to be log-normal. Residual variability was characterized by a proportional error model. Because our study subjects did not receive intravenous dexmedetomidine, we did not have a reference to estimate the fraction of the administered dose that reached the systemic circulation (F, bioavailability). Thus, to estimate bioavailability, we pooled our study data with the data from a previous study of intravenous dexmedetomidine with 13 healthy normal weight adults.23 Data were selected from 13 nonobese healthy patients, aged 29–58 years old, and weighing 47–91 kg that received a loading dose of dexmedetomidine of 0.5 mcg/kg over 10 min followed by a continuous infusion of 0.5 mcg/kg/h during general anesthesia. Venous blood samples of 6 ml were drawn at 0, 5, 10, 20, 30, 45, 60, 90, 120, 240 and 360–480 minutes after the onset of dexmedetomidine administration. Dexmedetomidine serum concentrations were measured by high-performance liquid chromatography coupled with tandem mass spectrometric. The lower limit of quantification in this study was 0.01 ng/ml.
Parameter values were standardized to 70-kg total body weight, as expressed in equation 1,
| Equation 1 |
where Pi is the parameter in the ith subject, PTVSt is the population parameter estimate standardized for a 70 kg subject,Wi is the weight of the ith subject, and PWR is the exponent for the allometric model, accounting for a value of 1 for volume of distribution, 0.25 for absorption rate half-time and 0.75 for clearance.
Individual PK model predictions were directly linked to HR and MAP data. Additionally, an effect compartment model was used to characterize the time delay between dexmedetomidine Cp and measured hemodynamic responses. This model was parameterized with a single parameter keo, the plasma effect-site equilibration rate constant, as expressed in equation 2,
| Equation 2 |
where Ce is the predicted dexmedetomidine effect-site concentration.
A linear model (Equation 3) and a sigmoidal Emax model (Equation 4) were used to fit hemodynamic response data.
| Equation 3 |
| Equation 4 |
where E0 is the heart rate value or mean arterial blood pressure value before dexmedetomidine administration. SLOPE describes the steepness of the linear concentration-response curve. Emax is the response value at the maximum drug effect. Ce50 is the effect-site concentration at half of Emax. γ is the coefficient describing the steepness of the concentration-response curve in the Emax model.
PK parameters estimated for each individual (post hoc Bayesian estimates) in the previous PK step were used as input for the PD part of the analysis (Sequential approach). Between-individual variability in model parameters was assumed to be log-normal. Residual variability for hemodynamic response data was characterized by an additive error model.
Model selection was based on the inspection of goodness-of-fit plots, visual predictive check plots (VPC), precision, plausibility of the estimated parameters, and minimum value of the objective function (-2·log(likelihood) [-2LL]) estimated by NONMEM. For 2 nested models, a decrease in −2LL of 3.84, or 6.63 points for an added parameter, is considered significant at 0.05 or 0.01, respectively (X2-distribution). Likelihood profiles, implemented in PLT Tools version 6 (a graphical interface for the NONMEM system),24 provided a means to evaluate parameter uncertainty. VPC plots were simulated in R with the ‘vpc’ package. VPC plots are modelling tools that estimate the concentration prediction intervals and graphically superimpose these intervals on observed concentrations after a standardized dose.
We did not perform a formal sample size calculation. However, our sample size is consistent with a previous dexmedetomidine PKPD study in 10 healthy volunteers.25
Results
The mean ± SD ages and weights were: 31.0 ± 11.0 years and 71.2 ± 9.4 kg for the 300-mcg group; 25.0 ± 6.0 years and 82.0 ± 7.6 kg for the 500-mcg group; and 24.0 ± 5.0 years and 74 ± 17.9 kg for the 700-mcg group.
The maximum mean plasma concentrations of dexmedetomidine were 0.18 ±0.18 ng/mL, 300-mcg group; 0.12 ± 0.09 ng/mL, 500-mcg group; and 0.38 ± 0.44 ng/mL, 700-mcg group. These data are summarized in figure 1A. MAP and mean HR data are presented in figure 1B, C. We found that the maximum plasma concentration in the 500-mcg group was lower than the 300-mcg group. However, measured plasma concentrations were consistent with hemodynamic changes. Dexmedetomidine plasma concentration, electroencephalogram and HR data of an illustrative subject from each dose group (300 mcg, 500 mcg, and 700 mcg) are presented in supplemental figures 1–3.
Fig. 1.
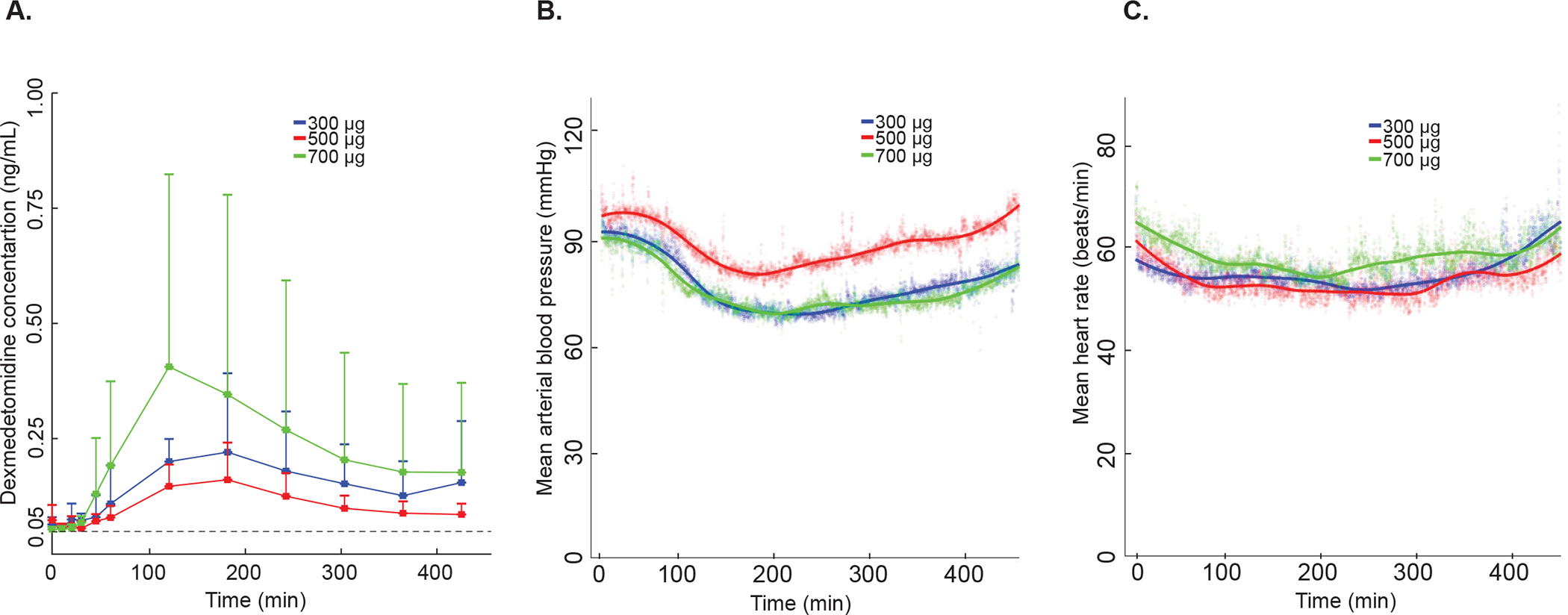
Dexmedetomidine plasma concentration, mean arterial blood pressure, and mean heart rate. (A) Dexmedetomidine plasma concentration measured by liquid chromatograph-tandem mass spectrometry. The mean plasma concentration for the 500-mcg group was lower than the 300-mcg and 700-mcg groups at all time points beginning 30 minutes after drug administration. Vertical error bars represent the standard deviation. Dotted black line at 0.05 ng/ml represents the lower limit of sensitivity (B) Group mean arterial blood pressure. The line represents a smoothed best fit line to the data. Shaded colored bubbles represent the mean of arterial pressure over each group. (C) Group mean heart rate. The line represents a smoothed best-fit line to the data. Shaded colored bubbles represent the mean of heart rate over each group. Blue color represents the 300-mcg group (n = 5), red color represents the 500-mcg group (n = 5), and the green color represents the 700-mcg (n = 5). Mcg, micrograms.
One subject in the 500-mcg group did not satisfy our criteria for hemodynamic stability. This subject, with a baseline HR in the 50’s, exhibited HRs < 40 bpm that lasted 2.5 minutes. This event began 295 minutes after the administration of dexmedetomidine when the subject was sleeping (electroencephalogram slow-delta and spindle oscillations were observed; Supplemental Fig. 2) and resolved spontaneously. No other periods in this, nor other subjects, met our definition of hemodynamic instability. No other adverse events were reported during this study.
We observed that the measured plasma concentrations were not consistent with the increase in dexmedetomidine dose, i.e. the maximum plasma concentration in the 500-mcg group was lower than the 300-mcg group (Fig. 1A). Therefore, in order to estimate the PKPD parameters reliably, we excluded the 500-mcg group data from our modelling analysis.
Dexmedetomidine plasma concentration data were better described by a 2-compartment than a 1-compartment, first-order absorption, and first-order elimination, PK model. (ΔOBJ 215.128, p<0.001) The body weight in our study ranged between 63–103 kg. Considering the sample size and observed inconsistencies in dose-concentration relationships, we decided to use a weight scaled allometric model as our base structural model. This model assumes robust previous knowledge regarding the effect of size in model parameters.26 An additional parameter to represent elapsed time between drug intake and the beginning of the absorption process (time lag), elicited an improvement in model fit (ΔOBJ 93.347, p<0.001). No other covariates were tested. The 2-compartment model with time lag was selected as our final PK model. Final population parameter estimates, coefficient of variation, standard errors, and 95% confidence intervals are presented in Table 1. Diagnostic plots confirmed the adequacy of model fit in the oral study data (Fig. 2A, Suppl. Fig. 4 A, C, Suppl. Fig. 5 A, C, E and Suppl. Fig. 6 A), and the pooled intravenous study data (Fig. 2B, Suppl. Fig. 4 B, D, Suppl. Fig. 5 B, D, F and Suppl. Fig. 6 B).27
Table 1.
Summary of dexmedetomidine individual pharmacokinetic parameter estimates
| Parameters | Typical Value of the Population (Coefficient of Variation, %) | Standard Error | 95% CI |
|---|---|---|---|
| Central Volume of distribution, V1 (L) | 35.6 (19.4) | 6.92 | 23.8, 52.8 |
| Peripheral Volume of distribution, V2 (L) | 54.7 (17.9) | 9.83 | 34.2, 81.7 |
| Elimination clearance, CL (L/min) | 0.56 (6.3) | 0.03 | 0.49, 0.64 |
| Distribution clearance, Q (L/min) | 2.18 (11.4) | 0.24 | 1.76, 2.69 |
| Absorption rate half-time, Tabs (min) | 54.7 (17.9) | 11.6 | 26.1, 96.5 |
| Lag Time (min) | 41.6 (2.4) | 0.99 | 39.3, 47.6 |
| Bioavailability, F (%) | 7.2 (26.1) | 0.02 | 4.7, 14.4 |
| Additive residual error (ng/ml) | 0.02 | - | - |
| Proportional residual error (%) | 27 | - | - |
Between subject variability expressed as an apparent coefficient of variation; CI, confidence interval of the parameter estimated by likelihood profile.
Fig. 2.
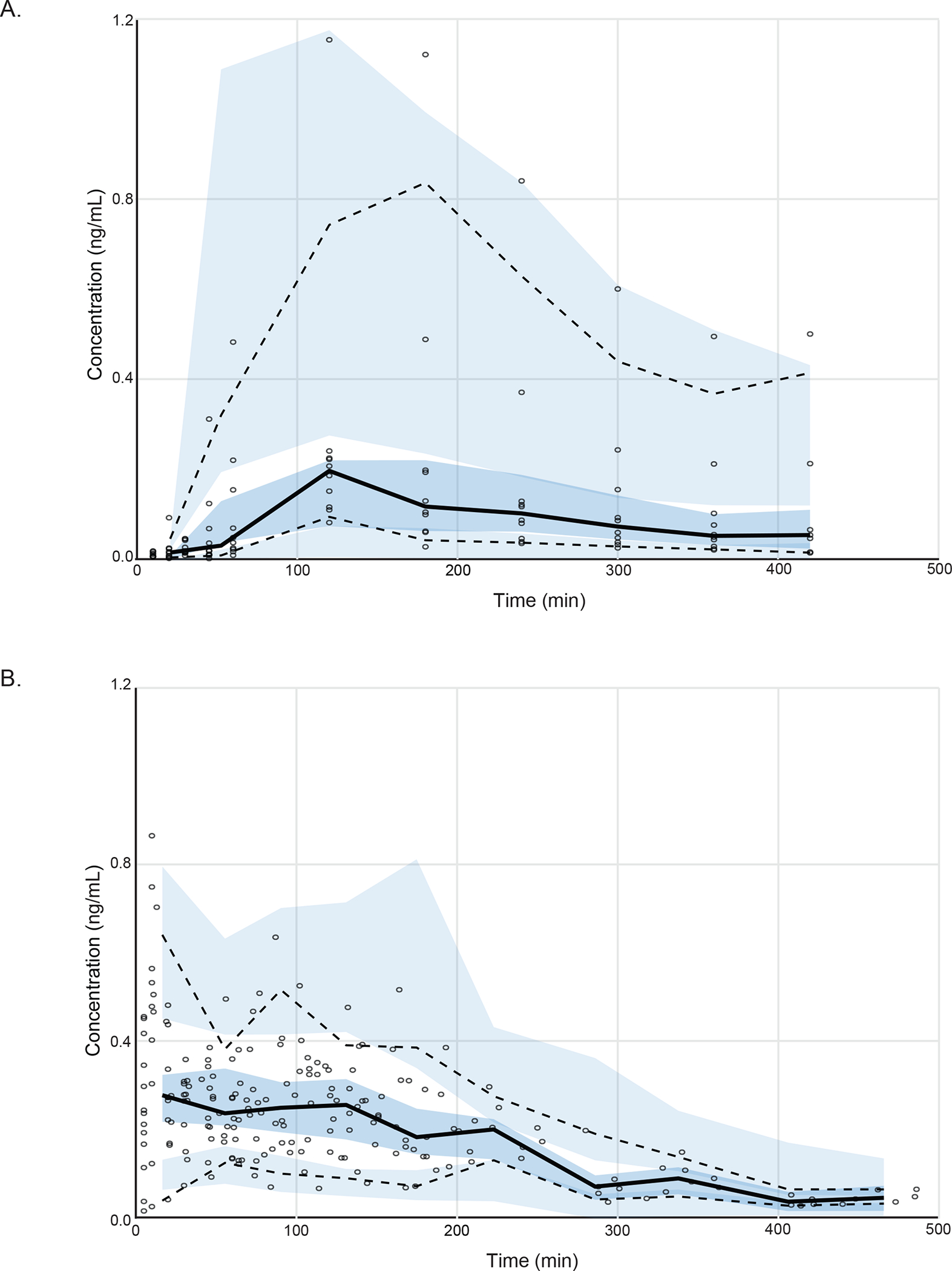
Visual predictive check (VPC) plots. Dexmedetomidine plasma concentration of (A) our oral study group (n=10) and (B) pooled intravenous study group (n=13). Solid black line indicates 50 percentiles and dashed black lines indicate 2.5 and 97.5 percentiles of observation. Shaded area indicates 95% prediction interval. The VPC plot confirms the adequacy of model predictions, showing no apparent deviations between model and data. The 95% confidence interval and median for observed data lies within the predicted intervals obtained by simulation.
The observed decrease in MAP and HR after the administration of oral dexmedetomidine were adequately described by linear models. We also tested the sigmoidal inhibitory Emax model. However, with both responses, the Emax and C50 parameters were estimated with poor precision. The linear model was selected as our final structural model for MAP and HR. In our modeling approach we first, directly related dexmedetomidine plasma concentrations and response data. Then, we incorporated an effect site model. The effect-site model improved the fit of the MAP data (ΔOBJ 159, p<0.001) and the HR data (ΔOBJ 71.332, p<0.001). The likelihood profile analysis confirmed that E0, Slope and keo parameters were estimated with good precision for both responses. Final population parameter mean (coefficients of variation %), standard error, and 95% confidence interval are presented in Table 2 and Table 3 for MAP and HR, respectively. Diagnostic plots (visual predictive check and goodness of fit plots) confirmed the model adequantely, describing the median observed MAP (Fig. 3A, Suppl. Fig. 7 A, C, E and Suppl. Fig. 8 A, C)27 and HR (Fig. 3B, Suppl. Fig. 7 B, D, F and Suppl. Fig. 8 B, D)27 in the dataset. For MAP model, variability in the dataset is overpredicted.
Table 2.
Dexmedetomidine population pharmacodynamic parameter estimates for the effect in mean arterial blood pressure.
| Parameters | Mean (Coefficient of Variation, %) | Standard Error | 95% CI |
|---|---|---|---|
| Effect at zero concentration, E0 (mmHg) | 90 (10) | 3.1 | 84, 96 |
| Slope | −704 (70) | 159 | −1168, −426 |
| Rate constant for elimination from the effect compartment, KE0 (min−1) | 0.005 (20) | 0.0007 | 0.0007, 0.006 |
| Additive Error (mmHg) | 5.44 | - | - |
CI, Confidence interval of the parameter estimated by likelihood profile analysis.
Table 3.
Dexmedetomidine population pharmacodynamic parameter estimates for the effect in mean arterial heart rate.
| Parameters | Mean (Coefficient of Variation, %) | Standard Error | 95% CI |
|---|---|---|---|
| Effect at zero concentration, E0 (mmHg) | 60 (11) | 2.3 | 56, 65 |
| Slope | −160 (56) | 48 | −273, −89 |
| Rate constant for elimination from the effect compartment, KE0 (min−1) | 0.011 (69) | 0.005 | 0.008, 0.029 |
| Additive Error (mmHg) | 5.7 | - | - |
CI, Confidence interval of the parameter estimated by likelihood profile analysis.
Fig. 3.
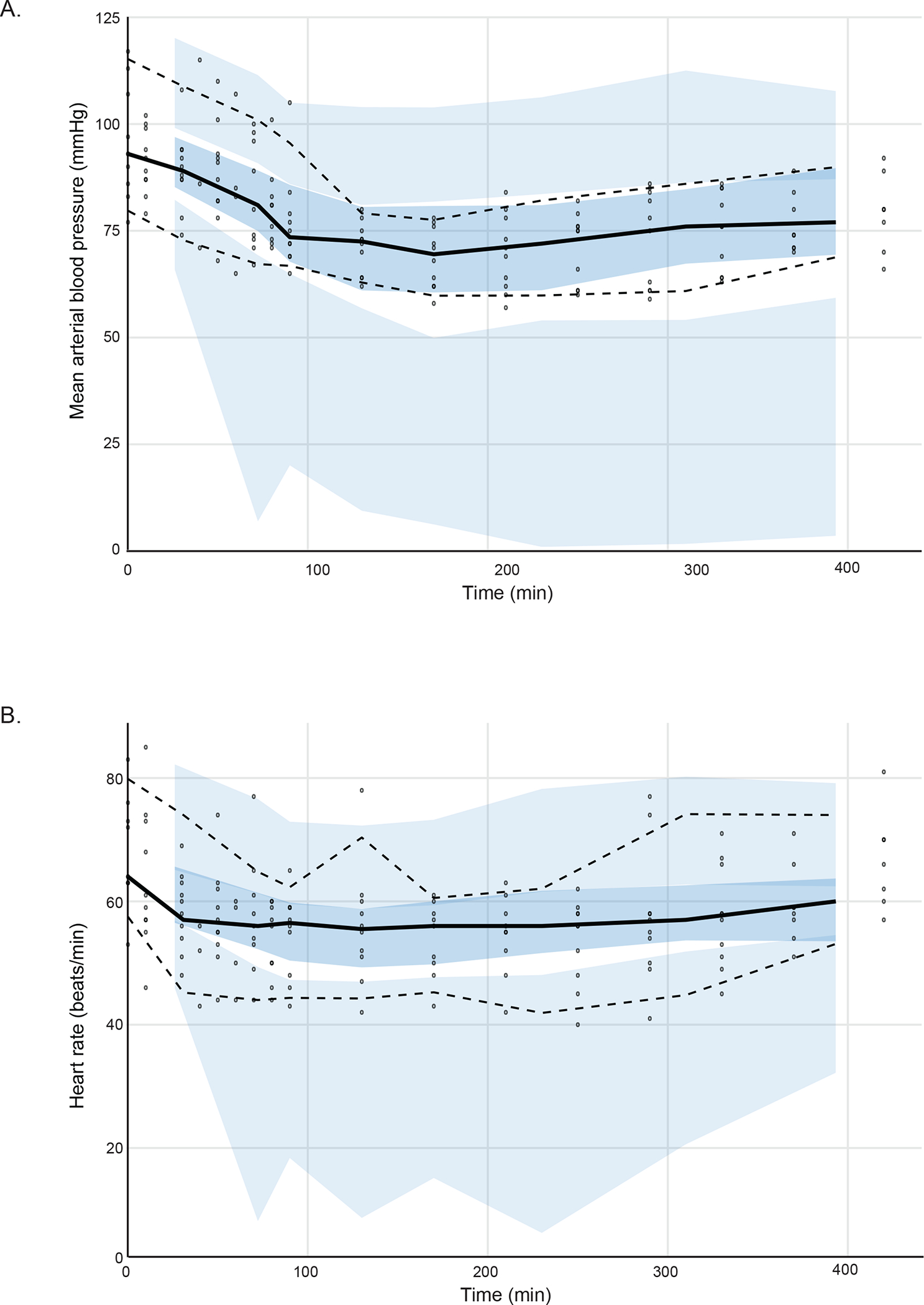
Visual predictive check (VPC) plots. (A) Mean arterial blood pressure and (B) heart rate versus time. Solid black line indicates 50 percentiles and dashed black lines indicate 2.5 and 97.5 percentiles of observation. Shaded area indicates 95% prediction interval. The VPC plot confirms the adequacy of model predictions, showing no apparent deviations between model and data. The 95% confidence interval and median for observed data lies within the predicted intervals obtained by simulation.
Discussion
In this phase I study, we administered a solid oral formulation of dexmedetomidine to healthy human subjects and assessed for hemodynamic stability. Oral dexmedetomidine (300-mcg, 500-mcg, and 700-mcg) resulted in maximum mean plasma concentrations (0.12 to 0.38 ng/mL) that occurred 2 to 3 hours after drug administration. These doses were associated with low plasma concentration-dependent decreases in MAP and HR that were adequately described by linear effect-site models. However, only the 700-mcg group was associated with plasma concentrations that have previously been associated with sedation (>0.2 ng/ml).16
The primary outcome of this study was hemodynamic stability. We did not intervene with vasoactive medications to maintain hemodynamic stability in any study subject because the MAP and HR decreases associated with oral dexmedetomidine were not deemed clinically significant. We did not observe the known biphasic effect of dexmedetomidine on the MAP (decrease followed by increase) in our study. This is likely because plasma concentrations in our study were much lower than those associated with the hypertensive effect of dexmedetomidine (onset of hypertension ranges from 1.9 ng/mL17 to 2.4 ng/mL16).
One subject in the 500-mcg group experienced an episode of bradycardia with HR less than 40 bpm that lasted for 2.5 minutes prior to spontaneous resolution (Suppl. Fig. 2). This episode occurred approximately 5 hours after the administration of dexmedetomidine, at a relatively low plasma concentration (0.05 to 0.08 ng/mL), and approximately 3 hours after we measured the peak plasma concentration of 0.15 ng/mL. During this episode, delta (0.1 to 4 Hz) and spindle (13 to 16 Hz) oscillations, which were consistent with rapid eye movement sleep, were visible on the electroencephalogram. Thus, it is likely that this HR finding was normotypical of sleep in this subject.
We previously found that a nightime 1 mcg/kg loading dose of intravenous dexmedetomidine biased the sleep architecture during the first half of the night to non-rapid eye movement stage 3 sleep,6 which is also known as restorative sleep. The plasma concentration profile of oral dexmedetomdine in our study did not approximate those previously reported for a 1 mcg/kg loading dose of intravenous dexmedetomidine.16,28 Thus, it is unlikely that oral dexmedetomidine will bias the sleep architecture to restorative sleep. However, in humans, relatively low doses of dexmedetomidine have been associated with non-rapid eye movement stage 2 sleep in numerous studies.29–31 Thus, although the bioavailability of oral dexmedetomidine reported in this manuscript was low, a clinical implication of our finding is that 700-mcg of oral dexmedetomidine is likely to promote non-rapid eye movement stage 2 sleep. Further, the nocturnal administration of low dose dexmedetomidine has been associated with decreased incidence and duration of delirium.15,32 Thus, another clinical implication of our finding is that the nocturnal administration of oral dexmedetomidine may generalize its delirium sparing benefits to non-intensive care unit settings.
The plasma concentration time profile of oral and intravenous dexmedetomidine was adequately described by a 2-compartment, first-order absorption, and first-order elimination, PK model. The elimination clearances and volume of distribution in our study are largely consistent with previous studies.16,33 A relevant finding from the current pharmacokinetic analysis is that overall bioavailability after oral administration was low (7.2% [CI95%: 4.7–14]). To allow estimation of bioavailability, we pooled the current data with a subset of data from a previous study that administered dexmedetomidine intravenously. We note that our estimate could be biased because intravenous data were based on venous rather than arterial sampling, and drug concentrations were measured using a different assay. However, our findings are consistent with a previous study that reported a 16% oral bioavailability of dexmedetomidine compared to an 82% buccal bioavailability, suggesting an extensive first-pass effect.34
The major limitation of our study is that the measured plasma concentrations of the 500-mcg group were lower than concentrations observed in the 300-mcg group. We reviewed study records with the trial pharmacist and confirmed that this finding was not secondary to dispensing errors and might be due to poor dexmedetomidine content uniformity in capsules. To eliminate the inconsistencies observed in the dose-concentration relationship between the 300 mcg and 500 mcg doses, we decided to exclude the 500-mcg group from our modeling analysis. The fact that current PK estimates are consistent with those of previous studies supports our modeling approach.16,33,34 Other limitations are that we studied only healthy subjects between 20 to 47 years of age, lacked a placebo group, and had a relatively low sample size. The over-prediction of the variability in our MAP model is a likely consequence of the limited sample size used to develop the model. Further, we did not study hemodynamic stability after the administration of anesthetic drugs that are known to significantly affect physiology.35–40 Thus, future studies in various populations are necessary.
We conclude that oral administration of dexmedetomidine in doses between 300–700 mcg was associated with decreases in heart rate and mean arterial pressure. Despite low oral absorption, the 700-mcg dose scheme reached clinically relevant concentrations for possible use as a sleep-enhancing medication.
Supplementary Material
Suppl. Fig. 1. Dexmedetomidine plasma concentration, central spectrogram, occipital spectrogram and heart rate from an illustrative subject during the oral dexmedetomidine (300-mcg) study visit. (A) The dexmedetomidine plasma concentrations were sampled at the following intervals: baseline, 10, 20, 30, 45 minutes, and 1, 2, 3, 4, 5, 6, 7 hours after the drug administration. (B) Central spectrogram and (C) occipital spectrogram. The presence of delta (0.1 to 4 Hz), theta (4 to 8 Hz), and spindle (13 to 16 Hz) oscillations are suggestive of a dexmedetomidine-induced effect. (D) Raw heart rate data.
Suppl. Fig. 2. Dexmedetomidine plasma concentration, central spectrogram, occipital spectrogram and heart rate from an illustrative subject during the oral dexmedetomidine (500-mcg) study visit. (A) The dexmedetomidine plasma concentrations were sampled at the following intervals: baseline, 10, 20, 30, 45 minutes, and 1, 2, 3, 4, 5, 6, 7 hours after the drug administration. (B) Central spectrogram and (C) occipital spectrogram. The presence of delta (0.1 to 4 Hz), theta (4 to 8 Hz), and spindle (13 to 16 Hz) oscillations are suggestive of a dexmedetomidine-induced effect. (D) Raw heart rate data. Red oval repesents episode with HR <40 bpm that lasted for 2.5 minutes.
Suppl. Fig. 3. Dexmedetomidine plasma concentration, central spectrogram, occipital spectrogram and heart rate from an illustrative subject during the oral dexmedetomidine (700-mcg) study visit. (A) The dexmedetomidine plasma concentrations were sampled at the following intervals: baseline, 10, 20, 30, 45 minutes, and 1, 2, 3, 4, 5, 6, 7 hours after the drug administration. (B) Central spectrogram and (C) occipital spectrogram. The presence of delta (0.1 to 4 Hz), theta (4 to 8 Hz), and spindle (13 to 16 Hz) oscillations are suggestive of a dexmedetomidine-induced effect. (D) Raw heart rate data.
Suppl. Fig. 4. Goodness-of-fit plots. (A) Population observed/predicted and (C) Post hoc individual observed/predicted dexmedetomidine concentrations versus time for our oral dexmedetomidine study group data (n=10). (B) Population observed/predicted and (D) Post hoc individual observed/predicted dexmedetomidine concentrations versus time for pooled intravenous dexmedetomidine study group (n=13). COBS = observed plasma concentration; CPRED = population predicted plasma concentration. Each blue line represents a subject. The red line represent the smoother (loess) for all patients. Black line shows the perfect fit.
Suppl. Fig. 5. Conditional Weighted Residual (CWRES) goodness-of-fit plots. Conditional Weighted Residual versus (A) time, (C) Population predicted, and (E) Post hoc predicted for our oral dexmedetomidine study group (n=10). Conditional Weighted Residual versus (B) time, (D) Population predicted, and (F) Post hoc predicted for pooled intravenous dexmedetomidine study group (n=13). Blue points represents the CWRES distribution. The red line is a super smoother (loess) for all patients. The horizontal black line at y=0 represents a perfect fit. In the plots, the CWRES are homogeneously distributed above and below the horizontal line at y=0.
Suppl. Fig. 6. Goodness-of-fit plots. Population observed/predicted for (A) our oral dexmedetomidine study group data (n=10) and pooled intravenous dexmedetomidine study group (n=13). (B) Post hoc individual observed/predicted for (B) our oral dexmedetomidine study group data (n=10) and pooled intravenous dexmedetomidine study group (n=13). Each blue dot represents the data from pooled intravenous dexmedetomidine study group and green dot represents the data from our oral dexmedetomidine study group. The green line is a super smoother (loess) for oral study group and the blue line is a super smoother (loess) for intravenous study group. Black line shows the perfect fit.
Suppl. Fig. 7. Conditional Weighted Residual (CWRES) goodness-of-fit plots for our oral dexmedetomidine study group (n=10). Conditional Weighted Residual versus (A) time, (C) population prediciton, and (E) post hoc individual prediciton for mean arterial pressure. Conditional Weighted Residual versus (B) time, (D) population prediciton, and (F) post hoc individual prediciton for heart rate. Blue points represents the CWRES distribution. The red line is a super smoother (loess) for all patients. The horizontal black line at y=0 represents a perfect fit. In the plots, the CWRES are homogeneously distributed above and below the horizontal line at y=0.
Suppl. Fig. 8. Goodness-of-fit plots. (A) Population observed/predicted and (C) Post hoc individual observed/predicted mean arterial pressure versus time for our oral dexmedetomidine study group data (n=10). (B) Population observed/predicted and (D) Post hoc individual observed/predicted heart rate versus time for pooled intravenous dexmedetomidine study group (n=13). COBS, MAP = observed mean arterial pressure; CPRED, MAP = population predicted mean arterial pressure. COBS, HR = observed heart rate; CPRED, HR = population predicted heart rate. Each blue line represents a subject. The red line represent the smoother (loess) for all patients. Black line shows the perfect fit.
Acknowledgments:
Lorenzo Berra, M.D., Associate Professor, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts, U.S.A, served on the Data Safety and Monitoring Board.
Amaka Eneanya, M.D., M.P.H., Assistant Professor, Department of Medicine, Perelman School of Medicine, Philadelphia, Pennsylvania, U.S.A, served on the Data Safety and Monitoring Board.
Oleg Evgenov, M.D., Ph.D., Associate Professor, Department of Anesthesiology, Perioperative Care, and Pain Medicine, New York University School of Medicine, New York, New York, U.S.A, served on the Data Safety and Monitoring Board.
James Rhee, M.D., Ph.D., Assistant Professor, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts, U.S.A, served on the Data Safety and Monitoring Board.
Kenneth Shelton, M.D., Assistant Professor, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts, U.S.A, served on the Data Safety and Monitoring Board.
Funding Statement: NIH NIA RO1AG053582 to O.A.; and, Innovation funds from the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital to O.A.
Footnotes
Clinical Trial Number: NCT02818569
Conflicts of Interest: O.A. has received speaker’s honoraria from Masimo Corporation, and is listed as an inventor on a patent on brain monitoring during general anesthesia and sedation that is assigned to Massachusetts General Hospital. O.A. has received institutionally distributed royalty from this licensed patent. Funds from División de Anestesiología, Escuela de Medicina, Pontificia Universidad Católica de Chile to JP and LC. All other authors declare that no competing interests exist.
Bibliography
- 1.Correa-Sales C, Rabin BC, Maze M: A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology 1992; 76: 948–52 [DOI] [PubMed] [Google Scholar]
- 2.Lu J, Nelson LE, Franks N, Maze M, Chamberlin NL, Saper CB: Role of endogenous sleep-wake and analgesic systems in anesthesia. J Comp Neurol 2008; 508: 648–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizobe T, Maghsoudi K, Sitwala K, Tianzhi G, Ou J, Maze M: Antisense technology reveals the alpha2A adrenoceptor to be the subtype mediating the hypnotic response to the highly selective agonist, dexmedetomidine, in the locus coeruleus of the rat. J Clin Invest 1996; 98: 1076–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nacif-Coelho C, Correa-Sales C, Chang LL, Maze M: Perturbation of ion channel conductance alters the hypnotic response to the alpha 2-adrenergic agonist dexmedetomidine in the locus coeruleus of the rat. Anesthesiology 1994; 81: 1527–34 [DOI] [PubMed] [Google Scholar]
- 5.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M: The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 2003; 98: 428–36 [DOI] [PubMed] [Google Scholar]
- 6.Akeju O, Brown EN: Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr Opin Neurobiol 2017; 44: 178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akeju O, Kim SE, Vazquez R, Rhee J, Pavone KJ, Hobbs LE, Purdon PL, Brown EN: Spatiotemporal Dynamics of Dexmedetomidine-Induced Electroencephalogram Oscillations. PLoS One 2016; 11: e0163431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song AH, Kucyi A, Napadow V, Brown EN, Loggia ML, Akeju O: Pharmacological Modulation of Noradrenergic Arousal Circuitry Disrupts Functional Connectivity of the Locus Ceruleus in Humans. J Neurosci 2017; 37: 6938–6945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Ferretti V, Guntan I, Moro A, Steinberg EA, Ye Z, Zecharia AY, Yu X, Vyssotski AL, Brickley SG, Yustos R, Pillidge ZE, Harding EC, Wisden W, Franks NP: Neuronal ensembles sufficient for recovery sleep and the sedative actions of alpha2 adrenergic agonists. Nat Neurosci 2015; 18: 553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashmi JA, Loggia ML, Khan S, Gao L, Kim J, Napadow V, Brown EN, Akeju O: Dexmedetomidine Disrupts the Local and Global Efficiencies of Large-scale Brain Networks. Anesthesiology 2017; 126: 419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, Whitten P, Margolis BD, Byrne DW, Ely EW, Rocha MG, Group SS: Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009; 301: 489–99 [DOI] [PubMed] [Google Scholar]
- 12.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, Stiles RA, Dittus RS, Bernard GR, Ely EW: Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007; 298: 2644–53 [DOI] [PubMed] [Google Scholar]
- 13.Reade MC, Eastwood GM, Bellomo R, Bailey M, Bersten A, Cheung B, Davies A, Delaney A, Ghosh A, van Haren F, Harley N, Knight D, McGuiness S, Mulder J, O’Donoghue S, Simpson N, Young P, Dah LIAI, the A, New Zealand Intensive Care Society Clinical Trials G: Effect of Dexmedetomidine Added to Standard Care on Ventilator-Free Time in Patients With Agitated Delirium: A Randomized Clinical Trial. JAMA 2016; 316: 773–4 [DOI] [PubMed] [Google Scholar]
- 14.Maldonado JR, Wysong A, van der Starre PJ, Block T, Miller C, Reitz BA: Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics 2009; 50: 206–17 [DOI] [PubMed] [Google Scholar]
- 15.Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, Zhu X, Zhu SN, Maze M, Ma D: Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016; 388: 1893–1902 [DOI] [PubMed] [Google Scholar]
- 16.Weerink MAS, Struys M, Hannivoort LN, Barends CRM, Absalom AR, Colin P: Clinical Pharmacokinetics and Pharmacodynamics of Dexmedetomidine. Clin Pharmacokinet 2017; 56: 893–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD: The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 2000; 93: 382–94 [DOI] [PubMed] [Google Scholar]
- 18.Bloor BC, Ward DS, Belleville JP, Maze M: Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology 1992; 77: 1134–42 [DOI] [PubMed] [Google Scholar]
- 19.Figueroa XF, Poblete MI, Boric MP, Mendizabal VE, Adler-Graschinsky E, Huidobro-Toro JP: Clonidine-induced nitric oxide-dependent vasorelaxation mediated by endothelial alpha(2)-adrenoceptor activation. Br J Pharmacol 2001; 134: 957–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talke P, Lobo E, Brown R: Systemically administered alpha2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology 2003; 99: 65–70 [DOI] [PubMed] [Google Scholar]
- 21.Sesso HD, Stampfer MJ, Rosner B, Hennekens CH, Gaziano JM, Manson JE, Glynn RJ: Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in Men. Hypertension 2000; 36: 801–7 [DOI] [PubMed] [Google Scholar]
- 22.Bauer RJ: NONMEM Tutorial Part I: Description of Commands and Options, with Simple Examples of Population Analysis. CPT Pharmacometrics Syst Pharmacol 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolle A, Paredes S, Cortinez LI, Anderson BJ, Quezada N, Solari S, Allende F, Torres J, Cabrera D, Contreras V, Carmona J, Ramirez C, Oliveros AM, Ibacache M: Dexmedetomidine metabolic clearance is not affected by fat mass in obese patients. Br J Anaesth 2018; 120: 969–977 [DOI] [PubMed] [Google Scholar]
- 24.Fisher D, Shafer S: PLT tools, 2017
- 25.Talke P, Anderson BJ: Pharmacokinetics and pharmacodynamics of dexmedetomidine-induced vasoconstriction in healthy volunteers. Br J Clin Pharmacol 2018; 84: 1364–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holford N, Heo YA, Anderson B: A pharmacokinetic standard for babies and adults. J Pharm Sci 2013; 102: 2941–52 [DOI] [PubMed] [Google Scholar]
- 27.Nguyen TH, Mouksassi MS, Holford N, Al-Huniti N, Freedman I, Hooker AC, John J, Karlsson MO, Mould DR, Perez Ruixo JJ, Plan EL, Savic R, van Hasselt JG, Weber B, Zhou C, Comets E, Mentre F, Model Evaluation Group of the International Society of Pharmacometrics Best Practice C: Model Evaluation of Continuous Data Pharmacometric Models: Metrics and Graphics. CPT Pharmacometrics Syst Pharmacol 2017; 6: 87–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li A, Yuen VM, Goulay-Dufay S, Sheng Y, Standing JF, Kwok PCL, Leung MKM, Leung AS, Wong ICK, Irwin MG: Pharmacokinetic and pharmacodynamic study of intranasal and intravenous dexmedetomidine. Br J Anaesth 2018; 120: 960–968 [DOI] [PubMed] [Google Scholar]
- 29.Alexopoulou C, Kondili E, Diamantaki E, Psarologakis C, Kokkini S, Bolaki M, Georgopoulos D: Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology 2014; 121: 801–7 [DOI] [PubMed] [Google Scholar]
- 30.Wu XH, Cui F, Zhang C, Meng ZT, Wang DX, Ma J, Wang GF, Zhu SN, Ma D: Low-dose Dexmedetomidine Improves Sleep Quality Pattern in Elderly Patients after Noncardiac Surgery in the Intensive Care Unit: A Pilot Randomized Controlled Trial. Anesthesiology 2016; 125: 979–991 [DOI] [PubMed] [Google Scholar]
- 31.Oto J, Yamamoto K, Koike S, Onodera M, Imanaka H, Nishimura M: Sleep quality of mechanically ventilated patients sedated with dexmedetomidine. Intensive Care Med 2012; 38: 1982–9 [DOI] [PubMed] [Google Scholar]
- 32.Skrobik Y, Duprey MS, Hill NS, Devlin JW: Low-Dose Nocturnal Dexmedetomidine Prevents ICU Delirium. A Randomized, Placebo-controlled Trial. Am J Respir Crit Care Med 2018; 197: 1147–1156 [DOI] [PubMed] [Google Scholar]
- 33.Hannivoort LN, Eleveld DJ, Proost JH, Reyntjens KM, Absalom AR, Vereecke HE, Struys MM: Development of an Optimized Pharmacokinetic Model of Dexmedetomidine Using Target-controlled Infusion in Healthy Volunteers. Anesthesiology 2015; 123: 357–67 [DOI] [PubMed] [Google Scholar]
- 34.Anttila M, Penttila J, Helminen A, Vuorilehto L, Scheinin H: Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol 2003; 56: 691–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavone KJ, Su L, Gao L, Eromo E, Vazquez R, Rhee J, Hobbs LE, Ibala R, Demircioglu G, Purdon PL, Brown EN, Akeju O: Lack of Responsiveness during the Onset and Offset of Sevoflurane Anesthesia Is Associated with Decreased Awake-Alpha Oscillation Power. Front Syst Neurosci 2017; 11: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akeju O, Hamilos AE, Song AH, Pavone KJ, Purdon PL, Brown EN: GABAA circuit mechanisms are associated with ether anesthesia-induced unconsciousness. Clin Neurophysiol 2016; 127: 2472–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavone KJ, Akeju O, Sampson AL, Ling K, Purdon PL, Brown EN: Nitrous oxide-induced slow and delta oscillations. Clin Neurophysiol 2016; 127: 556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akeju O, Pavone KJ, Westover MB, Vazquez R, Prerau MJ, Harrell PG, Hartnack KE, Rhee J, Sampson AL, Habeeb K, Gao L, Pierce ET, Walsh JL, Brown EN, Purdon PL: A comparison of propofol- and dexmedetomidine-induced electroencephalogram dynamics using spectral and coherence analysis. Anesthesiology 2014; 121: 978–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chamadia S, Pedemonte JC, Hahm EY, Mekonnen J, Ibala R, Gitlin J, Ethridge BR, Qu J, Vazquez R, Rhee J, Liao ET, Brown EN, Akeju O: Delta oscillations phase limit neural activity during sevoflurane anesthesia. Commun Biol 2019; 2: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JM, Akeju O, Terzakis K, Pavone KJ, Deng H, Houle TT, Firth PG, Shank ES, Brown EN, Purdon PL: A Prospective Study of Age-dependent Changes in Propofol-induced Electroencephalogram Oscillations in Children. Anesthesiology 2017; 127: 293–306 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig. 1. Dexmedetomidine plasma concentration, central spectrogram, occipital spectrogram and heart rate from an illustrative subject during the oral dexmedetomidine (300-mcg) study visit. (A) The dexmedetomidine plasma concentrations were sampled at the following intervals: baseline, 10, 20, 30, 45 minutes, and 1, 2, 3, 4, 5, 6, 7 hours after the drug administration. (B) Central spectrogram and (C) occipital spectrogram. The presence of delta (0.1 to 4 Hz), theta (4 to 8 Hz), and spindle (13 to 16 Hz) oscillations are suggestive of a dexmedetomidine-induced effect. (D) Raw heart rate data.
Suppl. Fig. 2. Dexmedetomidine plasma concentration, central spectrogram, occipital spectrogram and heart rate from an illustrative subject during the oral dexmedetomidine (500-mcg) study visit. (A) The dexmedetomidine plasma concentrations were sampled at the following intervals: baseline, 10, 20, 30, 45 minutes, and 1, 2, 3, 4, 5, 6, 7 hours after the drug administration. (B) Central spectrogram and (C) occipital spectrogram. The presence of delta (0.1 to 4 Hz), theta (4 to 8 Hz), and spindle (13 to 16 Hz) oscillations are suggestive of a dexmedetomidine-induced effect. (D) Raw heart rate data. Red oval repesents episode with HR <40 bpm that lasted for 2.5 minutes.
Suppl. Fig. 3. Dexmedetomidine plasma concentration, central spectrogram, occipital spectrogram and heart rate from an illustrative subject during the oral dexmedetomidine (700-mcg) study visit. (A) The dexmedetomidine plasma concentrations were sampled at the following intervals: baseline, 10, 20, 30, 45 minutes, and 1, 2, 3, 4, 5, 6, 7 hours after the drug administration. (B) Central spectrogram and (C) occipital spectrogram. The presence of delta (0.1 to 4 Hz), theta (4 to 8 Hz), and spindle (13 to 16 Hz) oscillations are suggestive of a dexmedetomidine-induced effect. (D) Raw heart rate data.
Suppl. Fig. 4. Goodness-of-fit plots. (A) Population observed/predicted and (C) Post hoc individual observed/predicted dexmedetomidine concentrations versus time for our oral dexmedetomidine study group data (n=10). (B) Population observed/predicted and (D) Post hoc individual observed/predicted dexmedetomidine concentrations versus time for pooled intravenous dexmedetomidine study group (n=13). COBS = observed plasma concentration; CPRED = population predicted plasma concentration. Each blue line represents a subject. The red line represent the smoother (loess) for all patients. Black line shows the perfect fit.
Suppl. Fig. 5. Conditional Weighted Residual (CWRES) goodness-of-fit plots. Conditional Weighted Residual versus (A) time, (C) Population predicted, and (E) Post hoc predicted for our oral dexmedetomidine study group (n=10). Conditional Weighted Residual versus (B) time, (D) Population predicted, and (F) Post hoc predicted for pooled intravenous dexmedetomidine study group (n=13). Blue points represents the CWRES distribution. The red line is a super smoother (loess) for all patients. The horizontal black line at y=0 represents a perfect fit. In the plots, the CWRES are homogeneously distributed above and below the horizontal line at y=0.
Suppl. Fig. 6. Goodness-of-fit plots. Population observed/predicted for (A) our oral dexmedetomidine study group data (n=10) and pooled intravenous dexmedetomidine study group (n=13). (B) Post hoc individual observed/predicted for (B) our oral dexmedetomidine study group data (n=10) and pooled intravenous dexmedetomidine study group (n=13). Each blue dot represents the data from pooled intravenous dexmedetomidine study group and green dot represents the data from our oral dexmedetomidine study group. The green line is a super smoother (loess) for oral study group and the blue line is a super smoother (loess) for intravenous study group. Black line shows the perfect fit.
Suppl. Fig. 7. Conditional Weighted Residual (CWRES) goodness-of-fit plots for our oral dexmedetomidine study group (n=10). Conditional Weighted Residual versus (A) time, (C) population prediciton, and (E) post hoc individual prediciton for mean arterial pressure. Conditional Weighted Residual versus (B) time, (D) population prediciton, and (F) post hoc individual prediciton for heart rate. Blue points represents the CWRES distribution. The red line is a super smoother (loess) for all patients. The horizontal black line at y=0 represents a perfect fit. In the plots, the CWRES are homogeneously distributed above and below the horizontal line at y=0.
Suppl. Fig. 8. Goodness-of-fit plots. (A) Population observed/predicted and (C) Post hoc individual observed/predicted mean arterial pressure versus time for our oral dexmedetomidine study group data (n=10). (B) Population observed/predicted and (D) Post hoc individual observed/predicted heart rate versus time for pooled intravenous dexmedetomidine study group (n=13). COBS, MAP = observed mean arterial pressure; CPRED, MAP = population predicted mean arterial pressure. COBS, HR = observed heart rate; CPRED, HR = population predicted heart rate. Each blue line represents a subject. The red line represent the smoother (loess) for all patients. Black line shows the perfect fit.


