Abstract
Soluble interleukin (IL)-15 exists under two forms: as monomer (sIL-15) or as heterodimeric complex in association with sIL-15Rα (sIL-15/IL-15Rα). Both forms have been successfully tested in experimental tumor murine models and are currently undergoing investigation in phase I/II clinical trials. Despite more than 20 years research on IL-15, some controversial issues remain to be addressed. A first point concerns the detection of the sIL-15/IL-15Rα in plasma of healthy donors or patients with cancer and its biological significance. The second and third unsolved question regards the protumorigenic role of the IL-15/IL-15Rα complex in human cancer and the detrimental immunological consequences associated to prolonged exposure of natural killer (NK) cells to both forms of soluble IL-15, respectively. Data suggest that in vivo prolonged or repeated exposure to monomeric sIL-15 or the soluble complex may lead to NK hypo-responsiveness through the expansion of the CD8+/CD44+ T cell subset that would suppress NK cell functions. In vitro experiments indicate that soluble complex and monomeric IL-15 may cause NK hyporesponsiveness through a direct effect caused by their prolonged stimulation, suggesting that this mechanism could also be effective in vivo. Therefore, a better knowledge of IL-15 and a more appropriate use of both its soluble forms, in terms of concentrations and time of exposure, are essential in order to improve their therapeutic use. In cancer, the overproduction of sIL-15/IL-15Rα could represent a novel mechanism of immune escape. The soluble complex may act as a decoy cytokine unable to efficiently foster NK cells, or could induce NK hyporesponsiveness through an excessive and prolonged stimulation depending on the type of IL-15Rα isoforms associated. All these unsolved questions are not merely limited to the knowledge of IL-15 pathophysiology, but are crucial also for the therapeutic use of this cytokine. Therefore, in this review, we will discuss key unanswered issues on the heterogeneity and biological significance of IL-15 isoforms, analyzing both their cancer-related biological functions and their therapeutic implications.
Keywords: cytokines, receptors, immunologic, tumor escape, tumor microenvironment, killer cells, natural
Introduction
Soluble monomeric Interleukin-15 (sIL-15) and its super agonist isoform sIL-15/IL-15Rα, owing to their target cell specificity, seem much more suitable than other cytokines to enhance antitumor and antiviral activity of effector cells and are currently under investigation in phase I/II clinical trials. Nevertheless, in our opinion, some question marks still have to be debated and deserve an accurate analysis in order to reach a safe and efficient therapeutic use.
In this review, we will analyze: (1) the complex biological properties of the IL-15/IL-15Rα system, (2) the type of circulating IL-15 detected in healthy donors (HD) and patients with cancer and its biological significance, (3) the detection in leukemic and solid tumor patients of IL-15 and IL-15Rα and their impact in cancer progression, (4) the employment of IL-15 or sIL-15/IL-15Rα soluble complex as cancer treatment both in pre-clinical and clinical trials, (5) the impact of the prolonged stimulation with IL-15 and the sIL-15/IL-15Rα on NK cells and the possible emergence of a novel immune escape mechanism by cancer cells.
The IL-15/IL-15 receptor system
Interleukin-15 (IL-15) is a pleiotropic cytokine, initially characterized as a T-cell growth factor1 2 that links in vivo innate and adaptive immune responses.3 IL-15 belongs to the four α-helix bundle cytokine family and is produced constitutively by different cell types such as monocytes, macrophages, dendritic cells, stromal cells, epithelial cells (for review see ref. 4 and muscle cells).5 Rather complex biological features characterize IL-15. Regarding IL-15, it exists under several functional forms: (1) a soluble monomeric form (sIL-15), (2) the soluble complex sIL-15/IL-15Rα, (3) the transpresented form (tp-IL-15) and (4) the transmembrane form (tmb-IL-15) (figure 1A).
Figure 1.
Different membrane-bound IL-15 and sIL-15/IL-15Rα complexes defined by the IL-15Rα isoform present in the complex. (A) IL-15 exists under several functional forms: a soluble monomeric form (sIL-15), the soluble complex sIL-15/IL-15Rα (a), the tp-IL-15 and the tmb-IL-15 (b). sIL-15 binds either the intermediate affinity dimeric IL-2Rβ/γc receptor or the high affinity trimeric IL-15Rα/IL-2Rβ/γc receptor, whereas the sIL-15/IL-15Rα complex binds only the IL-2Rβ/γc receptor (a). The tp-IL-15 is bound to membrane IL-15Rα chains and the tmb-IL-15, is anchored to the cell membrane via an IL-15Rα–independent mechanism: both signals in trans to surrounding cells expressing the dimeric IL-2Rβ/γc receptor (transpresentation). tp-IL-15 is either assembled within the cells before emerging to the cell surface, or sIL-15 may bind cytokine-free membrane-bound IL-15Rα to promote consequently IL-15 trans-presentation to IL-2Rβ/γc receptor expressing cells (b). (B) Uncleavable tp-IL-15/IL-15Rα complex bearing the IL-15Rα EX2 isoforms, competent for signaling in cis and trans (a). sIL-15/IL-15Rα complex bearing the NPR-IL-15Rα without biological activity (b). Uncleavable IL-15/IL-15Rα complex bearing IL-15Rα-IC-EX2a-AID isoforms, competent for reverse signaling (c). sIL-15/IL-15Rα complex bearing the IL-15Rα-IC3 isoform provided of high biological activity (d). IL-15, interleukin-15; NPR, natural proteolytic release.
IL-15 functions on binding to the IL-2Rβ/γc heterodimer, shared with IL-2, formed by IL-2Rβ and γc chains. IL-2Rβ/γc dimeric receptor binds IL-15 with an intermediate affinity (Kd=0.27 to 2.5 nM)6and transduce the signal within the cells by activating the Jak/Stat pathway (Jak 1 and 3, Stat3 and 5). The IL-15Rα chain confers specificity to IL-15 and binds the cytokine with a high affinity (Kd=10–80 pM). IL-15Rα forms a high affinity trimeric receptor with IL-2Rβ and γc chains, allowing the cells to respond to low concentrations of IL-15. IL-15Rα is also competent for signal transduction by itself7 8 (figure 1A).
The IL-15 modes of action
Soluble free IL-15, secreted at very low concentrations, is capable of activating cells expressing the high affinity IL-15Rα/IL-2Rβ/γc receptor.9 In this respect, some cell types present in the peripheral blood (PB) could act in vivo as ‘cellular sinks’ thus rendering free sIL-15 almost undetectable.10–13 The soluble complex sIL-15/IL-15Rα, first described by our laboratories,14 15 displays more extended half-life and greater bioavailability in comparison with monomeric sIL-15 and interacts primarily with cells expressing the intermediate affinity IL-2Rβ/γc receptor.16–18 In addition to the soluble forms, IL-15 could be associated to the cell membrane acting via cell-cell contacts. Two membrane-bound forms of IL-15 have been reported: the transpresented form of IL-15 bound to membrane IL-15Rα chains (tp-IL-15)14 16 19 and the IL-15 anchored to the cell membrane via an IL-15Rα-independent mechanism (tmb-IL-15).20–22 IL-15 bound to IL-15Rα is competent for signaling in cis,23 but mainly it delivers signals in trans to surrounding cells expressing the dimeric IL-2Rβ/γc receptor. This novel mode of action, called IL-15 transpresentation,19 has been demonstrated to be the dominant mechanism by which IL-15 delivers its signal in vivo to support the development and the functions of NK and CD8 T cells.24 25 IL-15 transpresentation requires that IL-15 binds to IL-15Rα within the cells before emerging to the cell surface14 26 (figure 1A). This signaling mechanism requires the cleavage of the tp-IL-15 complex from presenting cells and its internalization by responding cells that store and use cleaved tp-IL-15 for their own proliferation and survival.16 However, we cannot exclude that sIL-15 could bind cytokine-free membrane-bound IL-15Rα to promote consequently IL-15 trans-presentation to IL-2Rβ/γc receptor expressing cells. On the other hand, tmb-IL-15 is not only competent for signaling in trans to neighboring cells, but it can also deliver a reverse signal to the tmb-IL-15 positive cell on stimulation with recombinant sIL-15Rα (sIL-15Rα) chain or anti-IL-15 antibodies.20 21 27 Despite different kinetics of activation, all IL-15 forms transduce a similar signal to cells, activating the same pathways.28 However, we have demonstrated in some conditions, that the tmb-IL-15, but not the soluble form, selectively activates Stat-6 in hematopoietic progenitors.14
Circulating forms of IL-15
Difficulties have been encountered to measure the amount of sIL-15, inherent to the presence of several forms. More commonly, researchers used ELISA methods to detect IL-15 proteins in cell cultures, serum or biological fluids. These methods are highly depend on the antibodies used as some of them target the interface of IL-15 facing IL-15Rα rendering IL-15 undetectable. By contrast, other combination of antibodies are able to detect IL-15 bound or not to IL-15Rα preventing to conclude on the involvement of one form from the other. Moreover, the existence of several isoforms of IL-15 and IL-15Rα could also impact in the detection of the protein impairing the binding of the antibody. There is no commercially available detection kit to measure every forms of IL-15 at once, rendering the analysis of the presence of IL-15 in biological fluids and their relevance difficult. For instance, in order to measure exclusively the sIL-15/IL-15Rα heterodimers in human sera: Bouchaud et al 29 developed a sandwich radioimmunoassay, while Bergamaschi et al 30 developed a chemiluminescent immunoassay. Both used for capture and detection different commercialy available reagents chosen for assuring specific quantification. In figure 2, we employed the hIL-15/IL-15Rα complex DuoSet ELISA from R&D Systems.
Figure 2.
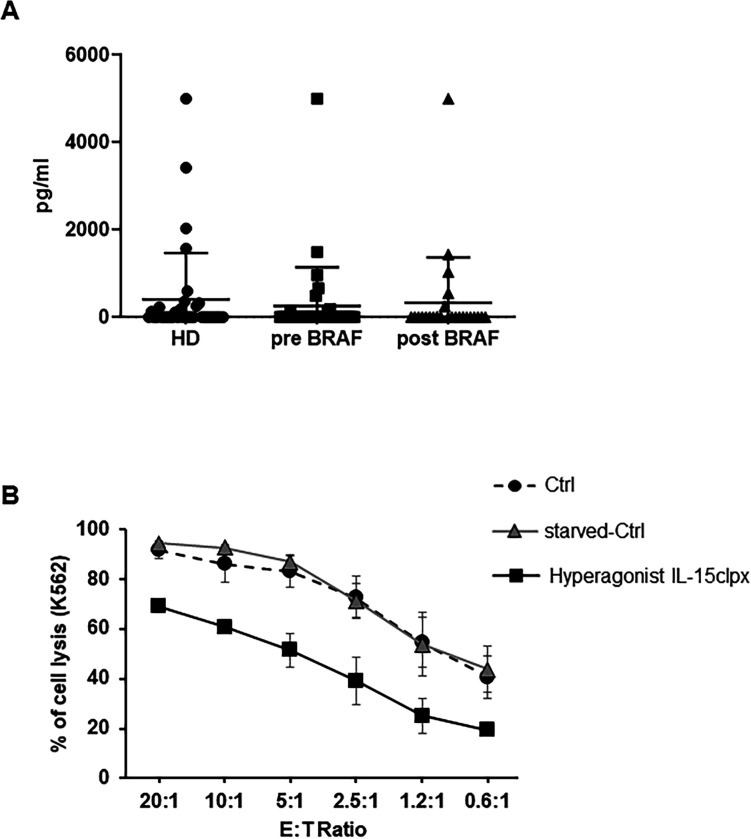
Detection of sIL-15/IL-15Rα complex in human plasmas and effect of long-lasting stimulation of NK cells with the hyperagonist RLI. (A) Detection of sIL-15/IL-15Rα complex in the plasma of HD and metastatic melanoma patients. Dot-Plot analysis of the levels of sIL-15/IL-15Rα complex in the plasma from 35 HD and from 35 metastatic melanoma patients before and after anti-BRAF treatment. Quantification was performed by ELISA assay (for additional information see online supplemental material). (B) Analysis of the long-term stimulation on the cytotoxic potential of activated NK (aNK) cells with the Hyperagonist RLI. Percentage of K-562 cell lysis resulted by ANK cell cytotoxicity assays. Allogeneic ANK cells were used as effector cells against cell Tracker green labeled K-562 cells used as targets at different E:T ratios. Freshly NK cells were amplified over 12 days with rhIL-2 at 600 UI/mL. Thereafter, NK cells were either continuously fed with rhIL-2 (Ctrl) or starved for 36 hours and then restimulated over additional 8 days with rhIL-2 at 600 UI/mL (starved-Ctrl) or with Hyperagonist IL-15clpx at 1 ng/mL. Data were expressed as mean±SD (n=3). HD, healthy donors; NK, natural killer; rhIL-2, human recombinant interleukin; sIL, soluble IL.
jitc-2020-001428supp003.pdf (98.4KB, pdf)
Finally, IL-15 has been demonstrated to mainly deliver its signal by transpresentation, thus biologically active IL-15 in its membrane-bound form would be undetectable by ELISA methods, underestimating the role of IL-15 in physiology as well as in pathology. However, measuring circulating IL-15 could give insights for abnormal production and aberrant target cell activations.
In mice
A first point to clarify is to identify which form of circulating IL-15 is present in the serum of HD. Indeed, it has been postulated that all circulating IL-15 found in the serum of human and mice exists as heterodimeric complex associated with the sIL-15Rα, thus excluding the presence of circulating cytokine in its monomeric form.30 This assertion, in our opinion, is only partially plausible in mice in which sera there is a constant detection of sIL-15Rα that binding to circulating IL-15 may generate, at least in part, a fraction of the sIL-15/IL-15Rα complex.20 30 31 In addition, a recent paper shows that, in murine sera, the free monomeric sIL-15 form is more abundant than the sIL-15/IL-15Rα complex, this latter representing only 14% of the circulating cytokine.5 By contrast, in mice stimulated with lipopolysaccharide or polyinosinic-polycytidylic acid (pI:C), sIL-15 was mostly associated to IL-15Rα, as a sIL-15/IL-15Rα complex.26 These data strongly suggest that in ‘resting mice’ only a minor fraction of IL-15 is represented by the soluble complex, which may be necessary to feed some lymphoid subsets. On the contrary, in inflammatory and infectious settings most of circulating IL-15 is converted to the soluble complex isoform thus acting as a rapid alert response to pathological situations.
In humans
In human HD sera, the monomeric IL-15 serum concentration ranges between 1 and 6 pg/mL32–35 and its role is to foster specific lymphoid subsets.10–13 Indeed, in pathological conditions, such as lymphodepleted patients, serum level of monomeric sIL-15 rapidly raises to supraphysiological concentrations due to the absence of the natural cellular sinks. The cut-off point, defining elevated supraphysiological concentrations, is 20 pg/mL.35 These values can be detected in several inflammatory diseases29 32–35 and in arthritis patients, where they are associated to more severe disease.32
Concerning the detection of sIL-15/IL-15Rα in human sera, the few papers that have investigated this point showed that sIL-15/IL-15Rα has been detected at concentrations ranging between 43 and 231 pg/mL in the plasma of eight out of eight metastatic melanoma patients undergoing lymphodepletion30 which, per se, could amplify the phenomenon. By contrast, Bouchaud et al have studied 37 HD showing that their serum levels of the sIL-15/IL-15Rα were barely detectable.29
In figure 2A, we have investigated both the presence and the level of the sIL-15/IL-15Rα complex in plasmas of 35 HD: about one in three of the plasmas resulted positive with values ranging from 0.3 ng/mL up to 5 ng/mL. Plasmatic absence of the soluble complex may simply reflect a constant consumption by PB natural cellular sinks for this cytokine. Alternatively, the complex is not secreted in order to avoid the excessive stimulation of immune cells in comparison with that induced by monomeric sIL-15.14–18 On the other hand, plasmatic detection in one third of the ‘HD’ could be due to benign unapparent inflammatory conditions at the time of the blood withdrawal. Indeed, in animal models, the transient secretion of the sIL-15/IL-15Rα complex is elicited by inflammatory stimuli and viral infections thus acting as response to specific situations requiring rapid and powerful activation of cytolytic subsets.36 Interestingly, two of the enrolled HD, subsequently resulted to be affected by chronic renal diseases.
Different forms of human IL-15/IL-15Rα complexes
These data point out two major questions about the origin of the soluble complex in humans and about its physiological and pathological significance. Differently from what happens in rodents,20 31 in healthy humans spontaneous secretion of sIL-15Rα cannot be detected.36–39 Therefore, the soluble complex seems not to derive by the coupling of free monomeric IL-15 with sIL-15Rα. This mechanism may be at play in a limited number of neoplastic or inflammatory diseases characterized by the presence of detectable amounts of sIL-15Rα in patients.29 37 40 In humans, the likely origin of the soluble complex is the intracellular assembly, in specialized cells, of IL-15 with specific isoforms of IL-15Rα, generated by alternative splicing, that could results in different types of soluble complex displaying specific properties.
In this context, eight isoforms of IL-15Rα have been initially described and resulted from alternative splicing of exon 3 and/or alternate usage of exon 7 or 7’7 and the alternative splicing of exon 2.38 All these isoforms were characterized by their high binding affinity to IL-157 38 and for their capacity to migrate to cell membrane or to the nuclear membrane.38 Interestingly, the isoforms lacking exon 2, unable to bind IL-15, were detected at the plasma membrane at high density and were supposed to display regulatory functions competing for recruitment of the IL-2Rβ and γc transducing subunits.38 Subsequently, we discoverd the natural proteolytic release (NPR) of human sIL-15Rα acting as a specific high affinity IL-15 antagonist acting through the IL-15Rα/IL-2Rβ/γc trimeric receptor, thus showing for the first time the existence of an IL-15Rα isoform with a specific function.39 More recently, Müller et al detected eight new N-terminal and C-terminal splice variants of human IL-15Rα influencing whether IL-15 would be secreted or expressed on cell membrane.41 The isoforms containing the Ex2A domain, such as the wild type (WT) IL-15Rα, prevent the release of the IL-15/IL-15Rα heterodimers from cell membranes thereby facilitating its transpresentation function. In addition, some C-terminal Ex2A+ isoforms, expressing alternative intracellular domains (AID), would form mbIL-15/IL-15Rα-IC-AID competent for triggering a reverse signaling within the IL-15/IL-15Rα-producing cells. Finally, the IC3 isoform that does not contain the Ex2A domain functions as a soluble secreted cytokine that is more efficient in stimulating CD8+ T-cells and NK cells proliferation, than the IL-15/IL-15Rα WT heterodimer.41 Thus, it appears increasingly likely that each IL-15Rα isoform displays specific distinct functions.
Based on these results, figure 1B illustrates 4 forms of the IL-15/IL-15Rα complex related to the isoform of the IL-15Rα chain involved in the complex: (1) membrane bound uncleavable forms bearing the IL-15Rα EX2 isoforms competent for signaling in cis and trans (2) a soluble complex bearing the NPR-IL-15Rα without biological activity (3) membrane bound uncleavable forms bearing IL-15Rα-IC-AID isoforms competent for reverse signaling (4 a soluble complex consisting of the IL-15Rα-IC3 isoform with high biological activity. The existence of the form without biological activity well documented in Crohn Disease (CD), a disorder characterized by elevated levels of inflammatory cytokines such as TNFα, IL-6, IL-1β and IL-15. CD patients, who responded to treatment with Infliximab, show an increase in serum level of sIL-15Rα and the IL-15/sIL-15Rα complex in concomitance with a decrease of IL-15. Infliximab induced the proteolytic release of IL-15Rα from epithelial cells, thus neutralizing IL-15 through its soluble receptor.29 Accordingly, in each pathological context, it will be necessary to identify the cells responsible for the production of sIL-15/sIL-15Rα. In a physiological setting, expression of IL-15 displays hierarchical expression among myeloid cells, while in a neoplastic setting, sIL-15/sIL-15Rα is produced by particular subsets of myeloid cells present in the TME and, in some instances, by tumor cells themselves. Subsequently, it will be necessary to identify within the producer cells the predominant IL-15Rα isoforms. This will allow to deduce which type of soluble complex is present in the plasma and to determine its biological activity, analyzing, for instance, the signal transduction activated in resting NK by the soluble complex purified from the sera.
IL-15 and IL-15Rα in cancer
Even if IL-15 is a promising therapeutic tool, it must be stated that the expression of IL-15 and/or IL-15 receptors can be detected in several leukemia-derived and solid tumor-derived cell lines, where they display protumorigenic properties.
Hematological Malignacies
In humans, differently from mice,29 30 sIL-15Rα is not detectable or it is present at very low levels in the sera of HD but it is present in patients affected by different diseases.37 40 For instance, T-cell large granular lymphocyte leukemia (LGL) is characterized by increased serum levels of sIL-15Rα, composed by the natural shed form of sIL-15Rα as well as the alternatively spliced form of sIL-15Rα suggesting different cellular sources. In addition, IL-15Rα expression is upregulated in patients' PB mononuclear cells as well. Upregulation of IL-15Rα could protect IL-15 from proteolysis, decrease the IL-15 response threshold of the cells, and sIL-15Rα complexed with monomeric circulating IL-15 (sIL-15) may generate the soluble complex. Both events may participate to progression of the disease.37 Moreover, LGL cells express tp-IL-15,42 that via signaling in cis and in trans14 18 22, could facilitate leukemic cells expansion.42
IL-15 can act as growth and viability factor for malignant T cells in lymphoma patients. Skin lesions and PB T-cells of cutaneous T-cell lymphoma patients show over-expression of both IL-15 mRNA and protein.43 44 In these patients, survival of malignant CD4+ T-cells is dependent, in the early stages, on IL-15 supplied from the microenvironment, but during disease progression, malignant cells, through autocrine IL-15 production, may autonomously support their growth and survival.43 In human T-cell lymphocytic virus leukemia, viral Tax protein induces the production of both IL-15 and IL-15Rα and this IL-15 autocrine loops leads to leukemia progression.44 45 In multiple myeloma, malignant plasma cells display functional IL-15 receptor with increased expression of the IL-15Rα chain and autocrine production of IL-15, these phenomena favor survival and proliferation of malignant cells independently from their microenvironment.46 All these data are summarized in (online supplemental table 1).
jitc-2020-001428supp001.pdf (202.2KB, pdf)
Solid cancer
Increased levels of sIL-15Rα in the serum of patients with head and neck cancer and high intratumoral IL-15 concentration, are highly correlated with poor clinical outcome.40 47 Clinical studies report a correlation between high intratumoral IL-15 concentrations and poor clinical outcome also in patients with lung cancer.48
Concerning human melanomas, we had previously shown that IL-15 produced by primary melanoma cell cultures, was involved in tumor escape mechanisms and in the activation of pro-inflammatory signals.8 49 In analogy, injection of small concentrations of human recombinant IL-15 (rhIL-15) in nude mice caused the development of much more aggressive tumors on subcutaneous implantations of human melanoma cells.50 The above-mentioned data,8 40 47–50 together with the detection of high levels of sIL-15/IL-15Rα in the serum of metastatic melanoma patients,30 strongly suggests that the generation of intratumoral and/or circulating sIL-15/IL-15Rα complexes may contribute to the development of a tumor microenvironment favorable to tumor progression and immune escape.
However, there are exceptions to the above described behavior since, in some solid tumors, the IL-15/IL-15R complex may also play an antineoplastic role. For instance, in mice implanted with various tumor cell lines, the sIL-15/IL-15Rα complexes are abundant in the interstitial fluid of freshly implanted tumors and their expression anticipates the infiltration of tumor infiltrating lymphocytes that play a major role in tumor regression. By contrast, the relative levels of sIL-15/IL-15Rα complexes are low in mice with advanced tumors and are associated with tumor progression. However, in advanced tumors the sIL-15/IL-15Rα complexes can be rescued by intratumoral activation of stimulator of interferon genes leading to tumor regression.51 These are intriguing results, even if they have been obtained in mice implanting a limited number of cell lines. It could be interesting to evaluate these parameters studying the development of chemically or virus induced tumors, looking for the density of stromal cells producing the sIL-15/IL-15Rα complexes, together with the quali-quantitative characteristics of infiltrating immunocompetent cells. Concerning human tumors, triple negative breast cancer cells overexpress IL-15Rα, and the concomitant expression of IL-15 in IL-15Rα-expressing cells, stimulates an autocrine signaling cascade in the absence of IL-2Rβ- and IL-2Rγc chains, promoting cell proliferation, migration and blocking apoptosis. Nevertheless, in this subset of high-grade breast tumors, coexpression of IL-15 and IL-15Rα is associated with better survival outcomes. This is likely, due to the fact that IL-15 and IL-15Rα coexpressed on cancer cells could paracrinely activate infiltrating mononuclear cells potentially leading to an anti-tumor immune response.52
Intrarenal IL-15, produced by tubular and cortical cells, plays a major role in the preservation of epithelial homeostasis (for review see53). By contrast, renal clear carcinoma cells (RCCs) do not secrete IL-15,27 but express tmb-IL-1554 55 and do not express neither in vitro nor in vivo the γc chain and JAK3, its signaling partner.56 Thus, in RCCs, both recombinant IL-15 (rhIL-15) and tmb-IL-IL5 transduce an unbalanced signal promoting the epithelial mesenchymal transition, thus behaving as protumorigenic factors.54 56 By contrast, renal cancer stem cells express the IL-15Rα/IL-2Rβ/γc heterotrimeric receptor. This receptor, when stimulated by rhIL-15, promotes the generation of polarized, non-tumorigenic and IL-15 secreting epithelial cells that behave as ‘normal cells’ being capable of autocrine IL-15 production.53 57 Thus, IL-15 may display at the same time pro-tumor and antitumor effects depending on the subset of renal cancer cell involved. It is tempting to speculate that the induction of γc chain obtained in vitro in RCC cells using ERK1/2 inhibitors,53 in vivo could induce a modified microenvironment more suitable for IL-15 therapeutic use.
In prostate cancer (PC), IL-15 overexpression is associated with recurrence-free survival suggesting that IL-15 could represent a potential prognostic biomarker.58 Subsequently, it has been shown that IL-15 decreases PC cells migration and invasion in vitro and in vivo. In addition, the presence of IL-15 in the tumor microenvironment decreases proliferation and blood vessel formation, suggesting that IL-15 may display antitumor effects inducing inflammation and shaping the stroma.59 Immunostaining of 70 colon cancer biopsies and implantation of human colon cancer cells in nude mice show that IL-15 is produced by metastatic colon carcinoma cells and can induce hyperplasia in the mucosa adjacent to colon cancer, thus contributing to angiogenesis and tumor progression.60 In addition, IL-15 promotes in vitro the proliferation, motility, and invasiveness of human colon cancer cells as well as increase their resistance to apoptosis.61 By contrast, in human patients, it has been recently shown that loss of IL-15 expression in about 30% of metastatic patients correlates with lower T-cell density, decreased T-cell proliferation, high risk of relapse and decreased survival.62
The above-mentioned results (summarized in online supplemental table 2), show how the role of intratumoral IL-15 is complex, ambivalent and dependent on several, not fully elucidated, factors such as the type of IL-15 produced, the IL-15-Rα chain isoforms involved in sIL-15/IL-15Rα complexes, the presence of functional IL-15 receptor on tumor cells as well as their response to stromal and endogenous IL-15. Thus, it is difficult to foresee if circulating and intratumoral IL-15 will behave as friend or foe, at present we suspect that on the long term the role of foe will emerge.
jitc-2020-001428supp002.pdf (199KB, pdf)
IL-15, sIL-15/IL-15Rα complex and cancer treatment
Preclinical models
Given the target cell specificity, IL-15 looks more promising than other cytokines in enhancing the efficacy of effector cells against tumors and viral infections.63–65 Indeed, rhIL-15 or the sIL-15/IL-15Rα used either alone or in combination with other treatments, have shown efficacy in several experimental tumor models66–69 (data summarized in table 1). However, these results should be revisited taking into account which species has been employed as source of IL-15 in the experiments. An underestimated point concerns the type of soluble complex employed in the different mouse models. Indeed, human IL-15/IL-15Rα heterodimers are highly effective without causing side effects16 17 70–75 By contrast, mouse sIL-15/IL-15Rα or human sIL-15/IL-15Rα in humanized mice are associated with important detrimental effects or generation of inefficient NK cells, highlighting the existence of negative feedbacks counteracting excessive NK stimulation.76–78 The above-mentioned discrepancies indicate that mouse cells are more sensitive to murine IL-15 than to human IL-15 and suggest the existence of species-related differences in the activity of IL-15 in mouse models. This must be taken into account when comparing the effects of IL-15 across species.79
Table 1.
IL-15, sIL15/IL15Rα complex and cancer treatment: preclinical models
| Tumor type | IL-15 isotype employed | Outcome | Reference |
| B-16 melanoma | rhIL-15+adoptive cell transfer | Tumor regression | 65 |
| Metastatic colon carcinoma | Murine recombinant IL-15 (mrIL-15)+blockade of checkpoints inhibition | Longer survival of tumor-bearing animals | 66 |
| Colon carcinoma | mrIL-15+anti-CD40 Abs | Longer survival of tumor-bearing mice | 67 |
| Lung cancer | DC transduced with viral vectors based on simian virus 40 expressing IL-15 (rSVIL-15) | Complete tumor remission in 73% of mice | 68 |
| MC38 colon carcinoma and TC-1 epithelial carcinoma | Superagonist het IL-15 | delayed primary tumor growth+Increased NK and CD8+T cell tumor infiltration | 74 |
| Colon carcinoma | Hyperagonist RLI+PD1 blockade | Complete tumor remission in 100% mice | 73 |
| B-16 melanoma | Superagonist het IL-15+adoptive T cell transfer | Decreased of tumor size | 69 |
| B-16 melanoma | Hyperagonist RLI | Reduced no | 70 |
| B-16 melanoma | CD11c-driven IL-15 | Total lung metastasis inhibition | 80 |
| 4T1 mouse mammary carcinoma | Hyperagonist RLI | Lung metastasis inhibition | 72 |
Hyperagonist RLI, IL15Rα-linker-IL15. Is a fusion protein linking the NH2-terminal domains of IL15Rα to IL15 through a 20-amino acid linker
DC, dendritic cell; IL-15, interleukin 15; mrIL-15, murine recombinant IL-15; NK, natural killer; rhIL-15, human recombinant IL-15; rSVIL-15, simian virus 40 expressing IL-15.
Another point to consider is the target specificity of the different IL-15 isoforms. In this context, using a new series of transgenic mice delivering IL-15 as sIL-15 or complexed to IL-15Rα, distinct effects have been identified on NK or CD8+ T-cells.80 Thus, mature NK cells are rescued exclusively by monomeric IL-15 (sIL-15) that also mediates a powerful antimetastatic response in a model of mouse melanoma.80 These data underline the specific role of NK cells in the control of metastatic spread.80–82 In this model, even if the levels of IL-1580 are clearly supra physiological, it seems that, at least in mice, each IL-15 form could have specific targets.
Finally, we should also consider the IL-15-dosing regimens.
A first study employing transgenic mouse engineered to constitutively overexpress sIL-15 (mean±SEM, 186.7±41.8 pg/mL) exhibited early expansions of NK and CD8+ T-cells. Later, these mice developed fatal lymphocytic leukemia with a T-NK phenotype.83 These data suggest that in vivo prolonged exposure to supra physiological concentrations of IL-15 may be highly detrimental. More recently, two other studies investigated the impact of transient and prolonged in vivo stimulation by the sIL-15/IL-15Rα complex on NK and CD8+ T cells. Elpek and coll. have shown that 2 weeks stimulation of CD8+ T cells with sIL-15/IL-15Rα induced an activated phenotype, robust T-cell receptor stimulation and effector function. By contrast, this regimen caused an accumulation of mature NK cells with considerably impaired activation, cytotoxicity and proliferative activity.78 More recently, Frutoso et al, have shown in vivo that NK cells respond to a single stimulation cycle with sIL-15 or the sIL-15/IL-15Rα complexe, while they become hyporesponsive and fail to expand after a second cycle of stimulation. This could be due to the contemporary expansion of CD44+CD8+ T cells. These data highlights as an excessive stimulation of NK cells by sIL-15 and sIL-15/IL-15Rα complexes may induce NK hyporesponsiveness.84 Indeed, prolonged NK cell activation using ionomycin as well as repeated exposure to Toll-like receptors (TLRs) agonists and85 repeated injections of interferon (IFN)-α86 induced decreased cytolytic function and cytokine production.87 Both TLRs and IFN-α stimulation increase production of IL-15, IL-15Rα and/or of the soluble complex36 85 86 88 89 that are potentially responsible of NK cells exhaustion. These data would suggest that regimens based on prolonged stimulation with IL-15 and IL-15 superagonist should be avoided.
Clinical trials
rhIL-15 is currently under investigation in several Phase I clinical trials (NCT02395822, NCT01385423, NCT03388632, NCT01572493 NCT01021059 NCT01369888. NCT01875601) in which the cytokine is administered employing different dosing regimens, including intravenous bolus and subcutaneous and continuous infusion of monomeric sIL-15 both in adult and pediatric solid tumors.90–94 There is also a number of clinical trials (NCT01727076, NCT01885897, NCT02523469), based on the utilization of an IL-15N72D/IL-15Rα-Fc super-agonist complex, termed N-803 (formerly Alt-803, from NantKwest, Culver City, California, USA) supposed to maximize expansion and priming of immune cells (NK and CD8+ T-cells).95 96 However, in the latter setting a drawback rises from the rapid consumption of the injected IL-15 agonists due to the rapid and huge expansion of the target immune cells.97 The above-mentioned clinical trials are summarized in table 2.
Table 2.
IL-15-based clinical trial
| Clinical trial | Dosing regimen | Tumor type | Phase | Efficiency | Side effects | Reference |
| NCT02359822 | Subcutaneous rhIL-15 | Acute myeloid leukemia | Phase 1/2 | 40% remission | CRS 56% Neurotoxicity 33% |
90 |
| NCT01385423 | Intravenous rhIL-15 | Acute myeloid leukemia | Phase 1/2 | 32% complete remission | No | 90 |
| NTC03388632 | Intravenous Bolus rhIL-15 | Metastatic malignancies | Phase 1 | Stable Disease | 3 µg/kg thrombocytopenia +hypotension 0.3 µg/kg no side effects |
91 |
| NCT01572493 | Continuous intravenous infusion rhIL-15 | Metastatic malignancies | Phase 1 | Significant expansion of CD8+ and NK cell | 30% serious adverse events | 92 |
| NCT01021059 | Bolus infusion rhIL-15 | Metastatic melanoma and RCC | Phase 1 | Dramatic expansion of NK and γδ T cells | 3 µg/kg thrombocytopenia +hypotension 0.3 µg/mL no side effects |
93 |
| NCT01727076 | Subcutaneous rhIL-15 | Metastatic malignancies | Phase 1 | Stable disease dramatic NK expansion and discrete CD8+T cells expansion | Grade 2: Pancreatits Grade 3: cardiac chest pain |
98 |
| NCT01369888 | CY+Fludarabine+TILs+rhIL-15 | Melanoma | Phase 1 | autoimmune toxicity | 101 | |
| NCT01875601 | Intravenous autologous NK cell infusion+rhIL-15 | Solid tumors, brain tumors, sarcoma, pediatric cancers, neuroblastoma | Phase 1 | Safety | 94 | |
| NCT01727076 | Subcutaneous N-803 | Metastatic malignancies | Phase 1 | NK expansion | Injection skin rash | 95 |
| NTC01727076 | Intravenous N-803 | Metastatic malignancies | Phase 1 | NK expansion | Nausea, fatigue | 95 |
| NTC01885897 | Intravenous N-803 | Hematologic malignancies relapsing after allo-hematopoietic cell transplantation (HCT) | Phase 1 | Clearance of lung metastasis in two patients. Modest expansion of NK and CD8+T cells |
Fever chills and rigors | 96 |
| NCT01885897 | Subcutaneous H-803 | Hematologic malignancies relapsing after allo-HCT | Phase 1 | Sustained expansion of NK and CD8+T cells | Injection skin rash | 96 |
| NCT02523469 | Subcutaneous H-803+PD1 Blockade | Non-small-cell lung carcinoma | Phase 1b | Reinduction of response to PD-1 blockade | Injection skin rash | 100 |
HCT, allo-hematopoietic cell transplantation; NK, natural killer; RCC, renal clear carcinoma cell; rhIL-15, human recombinant interleukin-15.
In addition, the repeated stimulation with rhIL-15 in patients with cancer may result detrimental. Indeed, a recent study has shown that one rhIL-15 infusion led to an increase in NK cell expansion and proliferation in the PB of patients, whereas a second infusion of rhIL-15 resulted in a decrease in NK cell responses.98 Moreover, two recent studies also show that repeated cycles of N-803 injection may lead to reduced biological responsiveness in NK cells of SIV+ macaque99 and, in a phase 1b trial, in patients with metastatic non-small cell lung cancer.100
These data open up two questions: NK hyporesponsiveness observed in cancer patients receiving repeated cycles of IL-15 or soluble complex is induced by the contemporary expansion of an inhibitory T cell subset as reported in mice84 or may be directly induced by IL-15 and/or the super-agonist complex? If the second hypothesis holds true, ex vivo protocols for the expansion of NK cells intended to be reinjected in patients with cancer as a complementary immunologic treatment should be revisited. Felices and coll. have investigated this point and their data indicate that continuous treatment of NK cells, over a 9-day period, with rhIL-15 at 20 ng/mL resulted in decreased viability, cell cycle arrest gene expression pattern, decreased function both in vitro and in vivo, and reduced tumor control.101 These data are truly alarming, since the IL-15 concentration employed (20 ng/mL) thought being supra-physiologic, is in the range of those employed by dozens of laboratories working on NK cells. These data are difficult to explain, but it is possible that this behavior occurs commonly at different degrees and may be underestimated in the routinely practice. An alternative explanation could reside in the proneness of recombinant IL-15, suspended in classical buffers, to rapidly undergo self-aggregation with loss of biological activity.102 Thus IL-15 suspended in classical buffers maintained for even few days at 4°C, may lose biological activity in an unpredictable way generating incertitude concerning the real concentration employed that could result partially inefficient in stimulating NK cells. In this respect, the use of an anti-cytokine aggregation buffer solves the above mentioned problems (unpublished results).
The presence of high level of the sIL-15/IL-15Rα complex in lymphodepleted melanoma metastatic patients could be essentially due to the lymphodepletion that would cause the loss of natural sinks leading to temporary increase of the plasmatic sIL-15/IL-15Rα complex. In order to solve this question, we analyzed the plasma from 35 non-lymphodepleted metastatic melanoma patients. We observed that one third of them resulted highly positive before anti-BRAF treatment and maintained this positivity after therapy showing long-lasting supra-physiological plasmatic levels of sIL-15/IL-15Rα only in a subset of patients (figure 2A). Since NK cells are major players in the control of the metastatic evolution,82 we wondered whether the high levels of the sIL-15/IL-15Rα complex, detected in the plasma of metastatic melanoma patients undergoing30 or not lymphodepletion, would display or not biological activity on NK cells. In the latter instance, high level of a soluble complex lacking biological activity29 39 could act as a decoy cytokine unable to provide homeostatic signals to their cellular sinks present in the PB.10–13 By contrast, constant high plasmatic levels of a soluble complex at high biological activity could induce suboptimal NK activation of: (1) PB-NK cells for instance in metastatic patients, or (2) NK cells pre-expanded ex vivo and destined to NK cell-based anti-tumor therapies.96 97 The latter issue could be tentatively solved by the in vivo silencing of the IL-15Rα-IC3 isoform responsible for the assembly of the soluble complex at high biological activity.41 In order to investigate the latter point we developed an in vitro protocol for mimicking this situation. For this purpose, NK cells were expanded during 12 days with IL-2, shortly starved and then refed for 8 days either with the hyperagonist IL-15clpx15 or with IL-2 (starved-Ctrl). At the same time, NK cells were continuously maintained in IL-2 without starvation and served as control (Ctrl). NK cells fed with Hyperagonist IL-15clpx exhibited a statistically significant reduction of cytotoxic effects on K-562 target cells as compared with Ctrl and starved-Ctrl NK cells (figure 2B), suggesting that Hyperagonist IL-15clpx could really cause direct functional NK impairment.
Upcoming IL-15-based combined immunotherapies
Finding a single agent-based therapy effective in causing complete tumor regression and long-lasting anti-tumor responses represents a real challenge owing to the multiple immunological barriers/escape mechanisms present in tumors. While having some efficacy in inducing tumor regression as a monotherapy, IL-15 isoforms show a greater potential when used in combination with other immunotherapies. Indeed, very efficient anti-tumor effect was detected in the preclinical pulmonary metastasis model using CT26 colon carcinoma cells, when N-803 was combined with the checkpoint inhibitors anti-CTLA-4 and anti-PD-L1. This treatment markedly expanded subsets of NK and memory CD8+ T cells. The improved NK cells function played a fundamental role in reducing tumor metastases and mice survival.103 A marked antitumor effect was obtained in combination therapies using sIL-15 or IL-15/IL-15Rα with checkpoint blocking antibodies targeting either PD-L1 or CTLA-4. In addition, combination of IL-15 superagonists (hyperagonist RLI and N-803) and blocking antibodies against PD-L1 and CTLA-4 resulted in remarkable improvement in antitumor responses leading to increased survival and reduced tumor growth in several preclinical models (for review see ref. 94). Moreover, in MHC class I-deficient melanoma, refractory to CD8+ T cell-mediated immunity, combination of IL-15 with TIGIT blockade could rescue antitumor NK cell-mediated cytotoxic activity and inhibit tumor metastasis in two mouse melanoma models.104 Recently, the group of Huntington identified the cytokine-inducible SH2-containing protein (CIS) as a critical negative regulator of IL-15 signaling in NK cells. Targeting CIS improved NK cell ex vivo proliferation and anti-tumor functions without any apparent secondary or off-target effects. Thus, the inhibition of CIS constitutes a novel method of unleashing the NK cell antitumor response with important perpesctives in clinical immunotherapy.105 Finally, an important synergistic antitumor activity was shown after administration of agonistic anti-CD40 Abs in combination with IL-15 in preclinical models of colon, prostatic and pancreatic cancers.67 106 107 These novel preclinical data support the scientific basis for clinical trials employing this combination immunotherapy strategy for the treatment of patients with cancer. The promising results of pre-clinical models led to the development of human protocols. For instance, the combination of ex vivo IL-15-activated NK cells with anti-NKG2A blocking mAbs revealed an effective strategy for the treatment of multiple myeloma patients.108
Overall, these data strongly support the notion that IL-15-based harnessing of NK cells, in combination with checkpoint inhibitors, may effectively unleash NK cells thus representing an effective therapeutic strategy, particularly in HLA-class I tumors that are ‘invisible’ to CD8+ T lymphocytes. This concept has relevance not only in adult malignancies, but also in the setting of several refractory or recurrent high-risk pediatric tumors.94
Concluding remarks
Plasmatic IL-15 in humans is present either as monomeric form or as soluble complex (sIL-15/IL-15Rα), whose function likely depends on the IL-15Rα isoform involved. Thus, a better knowledge of IL-15 and a correct use of both sIL-15 forms, in terms of concentrations, time of exposure and rapid consumption by target cellular sinks, is necessary in order to improve their therapeutic use, adopting for instance combinational protocols. Finally, the generation of high concentrations of circulating sIL-15/IL-15Rα in some patients with cancer could represent a novel mechanism of immune escape.
jitc-2020-001428supp004.pdf (203.2KB, pdf)
Footnotes
Contributors: Conception and design: BA, SDM, EM, LM and PFF. Development of methodology: EM, NT, FRM and SDM. Analysis and interpretation of data: SDM, PFF, NT, FRM, BB, EM and BA. Writing and revision of the manuscript: BA, SDM, EM, SN, BB, GP, PFF and LM. Administrative, technical or material support: BB, SDM, GP, SO, SN, EM and NT. Study supervision: BA, EM and LM.
Funding: This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC)-Special Program Metastatic disease: the key unmet need in oncology 5 per mille 2018 Id. 21 147. Italian Ministry of Health Id: RF-2016–02362288 (to GP); 5×1000 Italian Ministry of Health 2015 and 2016; RC-2019 IRCCS Ospedale Policlinico San Martino (to GP). Region Pays de la Loire PIRAMID 2015–08206, Fondation pour la Recherche Médicale DEQ201708 39118, Ligue Contre le Cancer R20034NN (to EM). FRM is recipient of a Fondazione Umberto Veronesi fellowship. SDM and NT were supported by AIRC fellowships for Italy.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The use of human overflow plasmas and the research protocol were approved by Internal Ethics Boards of Humanitas, Rozzano, Italy (STEPSI18) Clinical and Research Center and of the National Cancer Institute (IST, Genoa, Italy (OMA12.007). Buffy coats were collected from volunteer blood donors admitted to the blood transfusion service of OPBG after obtaining informed consent. The Ethical Committee of OPBG approved the study (825/2014) and it was conducted in accordance with the ethical principles stated in the Declaration of Helsinki.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Burton JD, Bamford RN, Peters C, et al. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A 1994;91:4935–9. 10.1073/pnas.91.11.4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grabstein KH, Eisenman J, Shanebeck K, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science 1994;264:965–8. 10.1126/science.8178155 [DOI] [PubMed] [Google Scholar]
- 3.Waldmann TA. Interleukin-15 in the treatment of cancer. Expert Rev Clin Immunol 2014;10:1689–701. 10.1586/1744666X.2014.973856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood 2001;97:14–32. 10.1182/blood.V97.1.14 [DOI] [PubMed] [Google Scholar]
- 5.Anderson BG, Quinn LS. Free IL-15 is more abundant than IL-15 complexed with soluble IL-15 receptor-α in murine serum: implications for the mechanism of IL-15 secretion. Endocrinology 2016;157:1315–20. 10.1210/en.2015-1746 [DOI] [PubMed] [Google Scholar]
- 6.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med 1994;180:1395–403. 10.1084/jem.180.4.1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson DM, Kumaki S, Ahdieh M, et al. Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL15RA and IL2RA genes. J Biol Chem 1995;270:29862–9. 10.1074/jbc.270.50.29862 [DOI] [PubMed] [Google Scholar]
- 8.Pereno R, Giron-Michel J, Gaggero A, et al. IL-15/IL-15Ralpha intracellular trafficking in human melanoma cells and signal transduction through the IL-15Ralpha. Oncogene 2000;19:5153–62. 10.1038/sj.onc.1203873 [DOI] [PubMed] [Google Scholar]
- 9.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol 2006;6:595–601. 10.1038/nri1901 [DOI] [PubMed] [Google Scholar]
- 10.Dudley ME, Yang JC, Sherry R, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 2008;26:5233–9. 10.1200/JCO.2008.16.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyiadzis M, Memon S, Carson J, et al. Up-Regulation of NK cell activating receptors following allogeneic hematopoietic stem cell transplantation under a lymphodepleting reduced intensity regimen is associated with elevated IL-15 levels. Biol Blood Marrow Transplant 2008;14:290–300. 10.1016/j.bbmt.2007.12.490 [DOI] [PubMed] [Google Scholar]
- 12.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 2005;202:907–12. 10.1084/jem.20050732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anthony SM, Rivas SC, Colpitts SL, et al. Inflammatory signals regulate IL-15 in response to Lymphodepletion. J Immunol 2016;196:4544–52. 10.4049/jimmunol.1600219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giron-Michel J, Giuliani M, Fogli M, et al. Membrane-Bound and soluble IL-15/IL-15Ralpha complexes display differential signaling and functions on human hematopoietic progenitors. Blood 2005;106:2302–10. 10.1182/blood-2005-01-0064 [DOI] [PubMed] [Google Scholar]
- 15.Mortier E, Quéméner A, Vusio P, et al. Soluble interleukin-15 receptor alpha (IL-15R alpha)-sushi as a selective and potent agonist of IL-15 action through IL-15R beta/gamma. Hyperagonist IL-15 x IL-15R alpha fusion proteins. J Biol Chem 2006;281:1612–9. 10.1074/jbc.M508624200 [DOI] [PubMed] [Google Scholar]
- 16.Tamzalit F, Barbieux I, Plet A, et al. IL-15.IL-15Rα complex shedding following trans-presentation is essential for the survival of IL-15 responding NK and T cells. Proc Natl Acad Sci U S A 2014;111:8565–70. 10.1073/pnas.1405514111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubinstein MP, Kovar M, Purton JF, et al. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha}. Proc Natl Acad Sci U S A 2006;103:9166–71. 10.1073/pnas.0600240103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stoklasek TA, Schluns KS, Lefrançois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol 2006;177:6072–80. 10.4049/jimmunol.177.9.6072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubois S, Mariner J, Waldmann TA, et al. Il-15Ralpha recycles and presents IL-15 in trans to neighboring cells. Immunity 2002;17:537–47. 10.1016/S1074-7613(02)00429-6 [DOI] [PubMed] [Google Scholar]
- 20.Bulfone-Paus S, Bulanova E, Budagian V, et al. The interleukin-15/interleukin-15 receptor system as a model for juxtacrine and reverse signaling. Bioessays 2006;28:362–77. 10.1002/bies.20380 [DOI] [PubMed] [Google Scholar]
- 21.Neely GG, Epelman S, Ma LL, et al. Monocyte surface-bound IL-15 can function as an activating receptor and participate in reverse signaling. J Immunol 2004;172:4225–34. 10.4049/jimmunol.172.7.4225 [DOI] [PubMed] [Google Scholar]
- 22.Bo H, Wei X-Q, Dong H, et al. Elevated expression of transmembrane IL-15 in immune cells correlates with the development of murine lupus: a potential target for immunotherapy against SLE. Scand J Immunol 2009;69:119–29. 10.1111/j.1365-3083.2008.02197.x [DOI] [PubMed] [Google Scholar]
- 23.Olsen SK, Ota N, Kishishita S, et al. Crystal structure of the interleukin-15.interleukin-15 receptor alpha complex: insights into trans and cis presentation. J Biol Chem 2007;282:37191–204. 10.1074/jbc.M706150200 [DOI] [PubMed] [Google Scholar]
- 24.Burkett PR, Koka R, Chien M, et al. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med 2004;200:825–34. 10.1084/jem.20041389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortier E, Advincula R, Kim L, et al. Macrophage- and dendritic-cell-derived interleukin-15 receptor alpha supports homeostasis of distinct CD8+ T cell subsets. Immunity 2009;31:811–22. 10.1016/j.immuni.2009.09.017 [DOI] [PubMed] [Google Scholar]
- 26.Mortier E, Woo T, Advincula R, et al. Il-15Ralpha chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med 2008;205:1213–25. 10.1084/jem.20071913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khawam K, Giron-Michel J, Gu Y, et al. Human renal cancer cells express a novel membrane-bound interleukin-15 that induces, in response to the soluble interleukin-15 receptor alpha chain, epithelial-to-mesenchymal transition. Cancer Res 2009;69:1561–9. 10.1158/0008-5472.CAN-08-3198 [DOI] [PubMed] [Google Scholar]
- 28.Perdreau H, Mortier E, Bouchaud G, et al. Different dynamics of IL-15R activation following IL-15 cis- or trans-presentation. Eur Cytokine Netw 2010;21:29–307. 10.1016/j.cyto.2010.07.129 [DOI] [PubMed] [Google Scholar]
- 29.Bouchaud G, Mortier E, Flamant M, et al. Interleukin-15 and its soluble receptor mediate the response to infliximab in patients with Crohn's disease. Gastroenterology 2010;138:2378–87. 10.1053/j.gastro.2010.02.044 [DOI] [PubMed] [Google Scholar]
- 30.Bergamaschi C, Bear J, Rosati M, et al. Circulating IL-15 exists as heterodimeric complex with soluble IL-15Rα in human and mouse serum. Blood 2012;120:e1–8. 10.1182/blood-2011-10-384362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinn LS, Anderson BG, Strait-Bodey L, et al. Serum and muscle interleukin-15 levels decrease in aging mice: correlation with declines in soluble interleukin-15 receptor alpha expression. Exp Gerontol 2010;45:106–12. 10.1016/j.exger.2009.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamana A, Ortiz AM, Alvaro-Gracia JM, et al. Characterization of serum interleukin-15 in healthy volunteers and patients with early arthritis to assess its potential use as a biomarker. Eur Cytokine Netw 2010;21:186–94. 10.1684/ecn.2010.0203 [DOI] [PubMed] [Google Scholar]
- 33.Suzuki J, Morimoto S, Amano H, et al. Serum levels of interleukin 15 in patients with rheumatic diseases. J Rheumatol 2001;28:2389–91. [PubMed] [Google Scholar]
- 34.Kakumu S, Okumura A, Ishikawa T, et al. Serum levels of IL-10, IL-15 and soluble tumour necrosis factor-alpha (TNF-alpha) receptors in type C chronic liver disease. Clin Exp Immunol 1997;109:458–63. 10.1046/j.1365-2249.1997.4861382.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gangemi S, Basile G, Monti D, et al. Age-related modifications in circulating IL-15 levels in humans. Mediators Inflamm 2005;2005:245–7. 10.1155/MI.2005.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anthony SM, Howard ME, Hailemichael Y, et al. Soluble interleukin-15 complexes are generated in vivo by type I interferon dependent and independent pathways. PLoS One 2015;10:e0120274. 10.1371/journal.pone.0120274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Petrus M, Bamford R, et al. Increased serum soluble IL-15Rα levels in T-cell large granular lymphocyte leukemia. Blood 2012;119:137–43. 10.1182/blood-2011-04-346759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubois S, Magrangeas F, Lehours P, et al. Natural splicing of exon 2 of human interleukin-15 receptor alpha-chain mRNA results in a shortened form with a distinct pattern of expression. J Biol Chem 1999;274:26978–84. 10.1074/jbc.274.38.26978 [DOI] [PubMed] [Google Scholar]
- 39.Mortier E, Bernard J, Plet A, et al. Natural, proteolytic release of a soluble form of human IL-15 receptor alpha-chain that behaves as a specific, high affinity IL-15 antagonist. J Immunol 2004;173:1681–8. 10.4049/jimmunol.173.3.1681 [DOI] [PubMed] [Google Scholar]
- 40.Badoual C, Bouchaud G, Agueznay NEH, et al. The soluble alpha chain of interleukin-15 receptor: a proinflammatory molecule associated with tumor progression in head and neck cancer. Cancer Res 2008;68:3907–14. 10.1158/0008-5472.CAN-07-6842 [DOI] [PubMed] [Google Scholar]
- 41.Müller JR, Waldmann TA, Kruhlak MJ, et al. Paracrine and transpresentation functions of IL-15 are mediated by diverse splice versions of IL-15Rα in human monocytes and dendritic cells. J Biol Chem 2012;287:40328–38. 10.1074/jbc.M112.378612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zambello R, Facco M, Trentin L, et al. Interleukin-15 triggers the proliferation and cytotoxicity of granular lymphocytes in patients with lymphoproliferative disease of granular lymphocytes. Blood 1997;89:201–11. 10.1182/blood.V89.1.201 [DOI] [PubMed] [Google Scholar]
- 43.Döbbeling U, Dummer R, Laine E, et al. Interleukin-15 is an autocrine/paracrine viability factor for cutaneous T-cell lymphoma cells. Blood 1998;92:252–8. 10.1182/blood.V92.1.252.413k08_252_258 [DOI] [PubMed] [Google Scholar]
- 44.Azimi N, Brown K, Bamford RN, et al. Human T cell lymphotropic virus type I Tax protein trans-activates interleukin 15 gene transcription through an NF-kappaB site. Proc Natl Acad Sci U S A 1998;95:2452–7. 10.1073/pnas.95.5.2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mariner JM, Lantz V, Waldmann TA, et al. Human T cell lymphotropic virus type I Tax activates IL-15R alpha gene expression through an NF-kappa B site. J Immunol 2001;166:2602–9. 10.4049/jimmunol.166.4.2602 [DOI] [PubMed] [Google Scholar]
- 46.Tinhofer I, Marschitz I, Henn T, et al. Expression of functional interleukin-15 receptor and autocrine production of interleukin-15 as mechanisms of tumor propagation in multiple myeloma. Blood 2000;95:610–8. 10.1182/blood.V95.2.610 [DOI] [PubMed] [Google Scholar]
- 47.Nguyen ST, Hasegawa S, Tsuda H, et al. Identification of a predictive gene expression signature of cervical lymph node metastasis in oral squamous cell carcinoma. Cancer Sci 2007;98:740–6. 10.1111/j.1349-7006.2007.00454.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seike M, Yanaihara N, Bowman ED, et al. Use of a cytokine gene expression signature in lung adenocarcinoma and the surrounding tissue as a prognostic classifier. J Natl Cancer Inst 2007;99:1257–69. 10.1093/jnci/djm083 [DOI] [PubMed] [Google Scholar]
- 49.Barzegar C, Meazza R, Pereno R, et al. Il-15 is produced by a subset of human melanomas, and is involved in the regulation of markers of melanoma progression through juxtacrine loops. Oncogene 1998;16:2503–12. 10.1038/sj.onc.1201775 [DOI] [PubMed] [Google Scholar]
- 50.Doucet C, Meazza R, Pottin-Clemenceau C, et al. Role of interleukin (IL)-2 and IL-15 in the tumour progression of a melanoma cell line MELP, derived from an IL-2 progressor patient. Melanoma Res 1997;7:S19–17. 10.1097/00008390-199708001-00004 [DOI] [PubMed] [Google Scholar]
- 51.Santana Carrero RM, Beceren-Braun F, Rivas SC, et al. Il-15 is a component of the inflammatory milieu in the tumor microenvironment promoting antitumor responses. Proc Natl Acad Sci U S A 2019;116:599–608. 10.1073/pnas.1814642116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marra P, Mathew S, Grigoriadis A, et al. IL15RA drives antagonistic mechanisms of cancer development and immune control in lymphocyte-enriched triple-negative breast cancers. Cancer Res 2014;74:4908–21. 10.1158/0008-5472.CAN-14-0637 [DOI] [PubMed] [Google Scholar]
- 53.Giron-Michel J, Azzi S, Ferrini S, et al. Interleukin-15 is a major regulator of the cell-microenvironment interactions in human renal homeostasis. Cytokine Growth Factor Rev 2013;24:13–22. 10.1016/j.cytogfr.2012.08.006 [DOI] [PubMed] [Google Scholar]
- 54.Azzi S, Gallerne C, Romei C, et al. Human renal normal, tumoral, and cancer stem cells express membrane-bound interleukin-15 isoforms displaying different functions. Neoplasia 2015;17:509–17. 10.1016/j.neo.2015.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yuan H, Meng X, Guo W, et al. Transmembrane-Bound IL-15-Promoted epithelial-mesenchymal transition in renal cancer cells requires the Src-dependent Akt/GSK-3β/β-catenin pathway. Neoplasia 2015;17:410–20. 10.1016/j.neo.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giron-Michel J, Azzi S, Khawam K, et al. Interleukin-15 plays a central role in human kidney physiology and cancer through the γC signaling pathway. PLoS One 2012;7:e31624. 10.1371/journal.pone.0031624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azzi S, Bruno S, Giron-Michel J, et al. Differentiation therapy: targeting human renal cancer stem cells with interleukin 15. J Natl Cancer Inst 2011;103:1884–98. 10.1093/jnci/djr451 [DOI] [PubMed] [Google Scholar]
- 58.Blum DL, Koyama T, M'Koma AE, et al. Chemokine markers predict biochemical recurrence of prostate cancer following prostatectomy. Clin Cancer Res 2008;14:7790–7. 10.1158/1078-0432.CCR-08-1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rohena-Rivera K, Sánchez-Vázquez MM, Aponte-Colón DA, et al. Il-15 regulates migration, invasion, angiogenesis and genes associated with lipid metabolism and inflammation in prostate cancer. PLoS One 2017;12:e0172786. 10.1371/journal.pone.0172786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuniyasu H, Ohmori H, Sasaki T, et al. Production of interleukin 15 by human colon cancer cells is associated with induction of mucosal hyperplasia, angiogenesis, and metastasis. Clin Cancer Res 2003;9:4802–10. [PubMed] [Google Scholar]
- 61.Kuniyasu H, Oue N, Nakae D, et al. Interleukin-15 expression is associated with malignant potential in colon cancer cells. Pathobiology 2001;69:86–95. 10.1159/000048761 [DOI] [PubMed] [Google Scholar]
- 62.Mlecnik B, Bindea G, Angell HK, et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci Transl Med 2014;6:228ra37. 10.1126/scitranslmed.3007240 [DOI] [PubMed] [Google Scholar]
- 63.Ahmad A, Ahmad R, Iannello A, et al. Il-15 and HIV infection: lessons for immunotherapy and vaccination. Curr HIV Res 2005;3:261–70. 10.2174/1570162054368093 [DOI] [PubMed] [Google Scholar]
- 64.Diab A, Cohen AD, Alpdogan O, et al. Il-15: targeting CD8+ T cells for immunotherapy. Cytotherapy 2005;7:23–35. 10.1016/S1465-3249(05)70786-6 [DOI] [PubMed] [Google Scholar]
- 65.Klebanoff CA, Gattinoni L, Palmer DC, et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res 2011;17:5343–52. 10.1158/1078-0432.CCR-11-0503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu P, Steel JC, Zhang M, et al. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res 2010;16:6019–28. 10.1158/1078-0432.CCR-10-1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang M, Yao Z, Dubois S, et al. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc Natl Acad Sci U S A 2009;106:7513–8. 10.1073/pnas.0902637106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vera M, Razquin N, Prieto J, et al. Intratumoral injection of dendritic cells transduced by an SV40-based vector expressing interleukin-15 induces curative immunity mediated by CD8+ T lymphocytes and NK cells. Mol Ther 2005;12:950–9. 10.1016/j.ymthe.2005.03.030 [DOI] [PubMed] [Google Scholar]
- 69.Bergamaschi C, Pandit H, Nagy BA, et al. Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-γ, CXCL9 and CXCL10. J Immunother Cancer 2020;8:e000599. 10.1136/jitc-2020-000599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bessard A, Solé V, Bouchaud G, et al. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Mol Cancer Ther 2009;8:2736–45. 10.1158/1535-7163.MCT-09-0275 [DOI] [PubMed] [Google Scholar]
- 71.Guo Y, Luan L, Rabacal W, et al. Il-15 superagonist–mediated immunotoxicity: role of NK cells and IFN-γ. J Immunol 2015;195:2353–64. 10.4049/jimmunol.1500300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Desbois M, Béal C, Charrier M, et al. Il-15 superagonist RLI has potent immunostimulatory properties on NK cells: implications for antimetastatic treatment. J Immunother Cancer 2020;8:e000632. 10.1136/jitc-2020-000632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Desbois M, Le Vu P, Coutzac C, et al. Il-15 trans-signaling with the superagonist RLI promotes Effector/Memory CD8+ T cell responses and enhances antitumor activity of PD-1 antagonists. J Immunol 2016;197:168–78. 10.4049/jimmunol.1600019 [DOI] [PubMed] [Google Scholar]
- 74.Chertova E, Bergamaschi C, Chertov O, et al. Characterization and favorable in vivo properties of heterodimeric soluble IL-15·IL-15Rα cytokine compared to IL-15 monomer. J Biol Chem 2013;288:18093–103. 10.1074/jbc.M113.461756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ng SSM, Nagy BA, Jensen SM, et al. Heterodimeric IL15 treatment enhances tumor infiltration, persistence, and effector functions of adoptively transferred tumor-specific T cells in the absence of Lymphodepletion. Clin Cancer Res 2017;23:2817–30. 10.1158/1078-0432.CCR-16-1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patil NK, Luan L, Bohannon JK, et al. Il-15 superagonist expands mCD8+ T, NK and NKT cells after burn injury but fails to improve outcome during burn wound infection. PLoS One 2016;11:e0148452. 10.1371/journal.pone.0148452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wege AK, Weber F, Kroemer A, et al. Il-15 enhances the anti-tumor activity of trastuzumab against breast cancer cells but causes fatal side effects in humanized tumor mice (hTM). Oncotarget 2017;8:2731–44. 10.18632/oncotarget.13159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elpek KG, Rubinstein MP, Bellemare-Pelletier A, et al. Mature natural killer cells with phenotypic and functional alterations accumulate upon sustained stimulation with IL-15/IL-15Ralpha complexes. Proc Natl Acad Sci U S A 2010;107:21647–52. 10.1073/pnas.1012128107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eisenman J, Ahdieh M, Beers C, et al. Interleukin-15 interactions with interleukin-15 receptor complexes: characterization and species specificity. Cytokine 2002;20:121–9. 10.1006/cyto.2002.1989 [DOI] [PubMed] [Google Scholar]
- 80.Polansky JK, Bahri R, Divivier M, et al. High dose CD11c-driven IL15 is sufficient to drive NK cell maturation and anti-tumor activity in a trans-presentation independent manner. Sci Rep 2016;6:19699. 10.1038/srep19699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tallerico R, Conti L, Lanzardo S, et al. Nk cells control breast cancer and related cancer stem cell hematological spread. Oncoimmunology 2017;6:e1284718. 10.1080/2162402X.2017.1284718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.López-Soto A, Gonzalez S, Smyth MJ, et al. Control of metastasis by NK cells. Cancer Cell 2017;32:135–54. 10.1016/j.ccell.2017.06.009 [DOI] [PubMed] [Google Scholar]
- 83.Fehniger TA, Suzuki K, VanDeusen JB, et al. Fatal leukemia in interleukin-15 transgenic mice. Blood Cells Mol Dis 2001;27:223–30. 10.1006/bcmd.2001.0379 [DOI] [PubMed] [Google Scholar]
- 84.Frutoso M, Morisseau S, Tamzalit F, et al. Emergence of NK cell hyporesponsiveness after two IL-15 stimulation cycles. J Immunol 2018;201:493–506. 10.4049/jimmunol.1800086 [DOI] [PubMed] [Google Scholar]
- 85.Maluish AE, Ortaldo JR, Conlon JC, et al. Depression of natural killer cytotoxicity after in vivo administration of recombinant leukocyte interferon. J Immunol 1983;131:503–7. [PubMed] [Google Scholar]
- 86.Mattei F, Schiavoni G, Belardelli F, et al. Il-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol 2001;167:1179–87. 10.4049/jimmunol.167.3.1179 [DOI] [PubMed] [Google Scholar]
- 87.Romera-Cárdenas G, Thomas LM, Lopez-Cobo S, et al. Ionomycin treatment renders NK cells hyporesponsive. PLoS One 2016;11:e0150998. 10.1371/journal.pone.0150998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Colpitts SL, Stoklasek TA, Plumlee CR, et al. Cutting edge: the role of IFN-α receptor and MyD88 signaling in induction of IL-15 expression in vivo. J.i. 2012;188:2483–7. 10.4049/jimmunol.1103609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu Q, Egelston C, Gagnon S, et al. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J Clin Invest 2010;120:607–16. 10.1172/JCI39293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cooley S, He F, Bachanova V, et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv 2019;3:1970–80. 10.1182/bloodadvances.2018028332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waldmann TA, Dubois S, Miljkovic MD, et al. Il-15 in the combination immunotherapy of cancer. Front Immunol 2020;11:868. 10.3389/fimmu.2020.00868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Conlon KC, Potter EL, Pittaluga S, et al. Il15 by continuous intravenous infusion to adult patients with solid tumors in a phase I trial induced dramatic NK-cell subset expansion. Clin Cancer Res 2019;25:4945–54. 10.1158/1078-0432.CCR-18-3468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Conlon KC, Lugli E, Welles HC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol 2015;33:74–82. 10.1200/JCO.2014.57.3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robinson TO, Schluns KS. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol Lett 2017;190:159–68. 10.1016/j.imlet.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Margolin K, Morishima C, Velcheti V, et al. Phase I trial of ALT-803, a novel recombinant IL15 complex, in patients with advanced solid tumors. Clin Cancer Res 2018;24:5552–61. 10.1158/1078-0432.CCR-18-0945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Romee R, Cooley S, Berrien-Elliott MM, et al. First-In-Human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood 2018;131:2515–27. 10.1182/blood-2017-12-823757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hangasky JA, Waldmann TA, Santi DV. Interleukin 15 pharmacokinetics and consumption by a dynamic cytokine sink. Front Immunol 2020;11:1813. 10.3389/fimmu.2020.01813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miller JS, Morishima C, McNeel DG, et al. A first-in-human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin Cancer Res 2018;24:1525–35. 10.1158/1078-0432.CCR-17-2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ellis-Connell AL, Balgeman AJ, Zarbock KR, et al. ALT-803 transiently reduces simian immunodeficiency virus replication in the absence of antiretroviral treatment. J Virol 2018;92. 10.1128/JVI.01748-17. [Epub ahead of print: 01 Feb 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wrangle JM, Velcheti V, Patel MR, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1B trial. Lancet Oncol 2018;19:694–704. 10.1016/S1470-2045(18)30148-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Felices M, Lenvik AJ, McElmurry R, et al. Continuous treatment with IL-15 exhausts human NK cells via a metabolic defect. JCI Insight 2018;3. 10.1172/jci.insight.96219. [Epub ahead of print: 08 Feb 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hanick NA, Rickert M, Varani L, et al. Elucidation of the interleukin-15 binding site on its alpha receptor by NMR. Biochemistry 2007;46:9453–61. 10.1021/bi700652f [DOI] [PubMed] [Google Scholar]
- 103.Kim PS, Kwilas AR, Xu W, et al. Il-15 superagonist/IL-15RαSushi-Fc fusion complex (IL-15SA/IL-15RαSu-Fc; ALT-803) markedly enhances specific subpopulations of NK and memory CD8+ T cells, and mediates potent anti-tumor activity against murine breast and colon carcinomas. Oncotarget 2016;7:16130–45. 10.18632/oncotarget.7470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chauvin J-M, Ka M, Pagliano O, et al. Il15 stimulation with TIGIT blockade reverses CD155-mediated NK-cell dysfunction in melanoma. Clin Cancer Res 2020;26:5520–33. 10.1158/1078-0432.CCR-20-0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Delconte RB, Kolesnik TB, Dagley LF, et al. Cis is a potent checkpoint in NK cell-mediated tumor immunity. Nat Immunol 2016;17:816–24. 10.1038/ni.3470 [DOI] [PubMed] [Google Scholar]
- 106.Zhang M, Ju W, Yao Z, et al. Augmented IL-15Rα expression by CD40 activation is critical in synergistic CD8 T cell-mediated antitumor activity of anti-CD40 antibody with IL-15 in TRAMP-C2 tumors in mice. J.i. 2012;188:6156–64. 10.4049/jimmunol.1102604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Audenaerde JR, Marcq E, von Scheidt B, et al. Novel combination immunotherapy for pancreatic cancer: potent anti-tumor effects with CD40 agonist and interleukin-15 treatment. Clin Transl Immunology 2020;9:e1165. 10.1002/cti2.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tognarelli S, Wirsching S, von Metzler I, et al. Enhancing the activation and releasing the brakes: a double hit strategy to improve NK cell cytotoxicity against multiple myeloma. Front Immunol 2018;9:2743. 10.3389/fimmu.2018.02743 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-001428supp003.pdf (98.4KB, pdf)
jitc-2020-001428supp001.pdf (202.2KB, pdf)
jitc-2020-001428supp002.pdf (199KB, pdf)
jitc-2020-001428supp004.pdf (203.2KB, pdf)