Abstract
This clinical review paper discusses the pathophysiology of the pulmonary and cardiovascular manifestations of a SARS-CoV-2 infection and the ensuing implications on acute cardiovascular care in patients presenting with a severe COVID-19 syndrome admitted to an intensive acute cardiac care unit. The high prevalence of old age, obesity, diabetes, hypertension, heart failure, and ischaemic heart disease in patients who develop a severe to critical COVID-19 syndrome suggests shared pathophysiological mechanisms. Pre-existing endothelial dysfunction and an impaired innate immune response promote the development by the viral infection of an acute endothelialitis in the pulmonary microcirculation complicated by abnormal vasoconstrictor responses, luminal plugging by inflammatory cells, and intravascular thrombosis. This endothelialitis extends into the systemic circulation what may lead to acute myocardial injury, myocarditis, and thromboembolic complications both in the arterial and venous circulation. Ever since the first case reports from the city of Wuhan in China in December of 2019, coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) continues as a raging pandemic causing significant morbidity and mortality [1]. In this paper we provide a review of the epidemiological characteristics and the pathophysiology of SARS-CoV-2 infection, and formulate the hypothesis that endothelialitis may play a key role in the pathogenesis of the pulmonary and cardiovascular complications of COVID-19.
Keywords: COVID-19, SARS-CoV-2, endothelium, endothelialitis, microcirculation, thrombosis, diabetes, obesity, adult respiratory distress syndrome, cytokine storm, ventilation
Age and case fatality rate
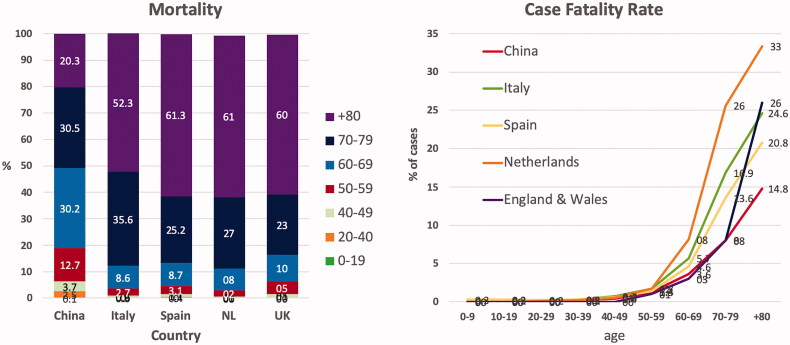
Early reports of the outbreak of the COVID-19 pandemic in China showed that the severity of the condition and mortality increased exponentially above the age of 60 years [2–4]. The epidemiological observations on the COVID-19 pandemic in Europe confirm the importance of advanced age as a significant risk factor for COVID-19 mortality [5–10] (Figure 1). In contrast to China, where only 20% of the deaths were older than 80 years, octogenarians and nonagenarians account in Europe for almost two-thirds of the mortality. Differences in the age pyramid and the more prominent contribution of people over 80 years, can probably explain the higher mortality rates in Europe [11].
Figure 1.
Mortality and case fatality rates due to COVID-19. Left: relative contribution of different age groups to the mortality related to COVID-19 in China, Italy, Spain, the Netherlands (NL) and the UK. Right: case fatality rate for different age groups in China, Italy, Spain, the Netherlands and England and Wales.
The case fatality rate is almost nonexistent in children and very low in adults up to 50 years but rises exponentially from 1.0% for patients aged 50 to 59 years to more than 20% for patients over 80 years. Compared to China, the case fatality in patients aged 80 years or older is markedly higher In Europe (Figure 1). Statistics from the COVID-19 pandemic in Europe [6,8] show that a high number of octo- and nona-genarians were not admitted to a hospital in case of COVID-19 suspicion, which may explain in part the high case fatality rate in this age group.
Comorbidities
Epidemiological studies at the start of the COVID-19 pandemic in China reported a high prevalence of arterial hypertension, diabetes, cardiovascular disease, cerebrovascular disease, and chronic obstructive lung disease among hospitalised individuals [4,12–15]. Meta-analyses of these first epidemiological reports showed that these comorbidities were associated with a significantly higher risk for a more severe form of COVID-19 infection and requiring intensive care treatment [16,17].
The first report on the COVID-19 pandemic in Italy confirmed that similar to the observations in China, deaths occurred mainly among older, male patients with multiple comorbidities [5]. Of the 27,955 deceased patients, 60% had three or more comorbidities. Arterial hypertension (68%), diabetes mellitus type II (31%), atrial fibrillation (22%), chronic renal failure (20%), and chronic obstructive lung disease (17%) were the top 5 most prevalent comorbidities in the fatal cases [18]. Reports from the pandemic in Spain also reported a significantly higher prevalence of pre-existing cardiovascular diseases (60% vs. 25%), diabetes (32% vs. 14%), and respiratory diseases (20% vs. 9%) in deceased patients than in survivors [6].
Observations from a large hospital in New York show that obesity in patients under 60 is a significant risk factor for COVID-19 hospital and critical care admission: patients under 60 with a BMI between 30 and 34 were two times more likely, and patients with a BMI ≥35 were 3.6 times more likely to be admitted to critical care than patients with a BMI <30 [19]. Another report from a network of outpatient offices and acute care hospitals in New York confirmed advanced age (>75 years), BMI > 40, and heart failure as the most potent independent risk factors for hospitalisation [20]. The recognition of obesity as an independent risk factor is consistent with an earlier report from China, showing that obesity, especially in men, significantly increases the risk of developing severe pneumonia due to COVID-19 [21]. A recent extensive study based on the hospital records of a large number of COVID-19 patients in the United Kingdom confirmed that obesity (as recognised by clinical staff) is associated with in-hospital mortality after adjustment for other comorbidities, age, and sex [22].
Most studies reporting on the predisposing role of comorbidities on mortality in the COVID-19 pandemic are small and based on hospital records. Several of the identified risk factors correlate with age, but correction for age was not possible in many studies. Recently, an extensive cohort study using primary care electronic health record data of 17.5 million patients linked to in-hospital COVID-19 mortality data showed that death from COVID-19 was strongly associated with male sex, older age and deprivation, obesity, uncontrolled diabetes, severe asthma, and various other prior medical conditions (chronic cardiovascular diseases, chronic respiratory diseases, hematological malignancies, among others) [23]. In contrast with earlier studies, arterial hypertension was, after adjustment for age, not an independent risk factor for COVID-19 hospital death. Hypertension is strongly associated with increasing age, and therefore their relative contribution to the COVID-19 mortality is difficult to unravel.
Another population-wide study performed in England using primary care electronic patient records confirmed the importance of diabetes mellitus as a risk factor for COVID-19 mortality independent of age, ethnicity, deprivation, and cardiovascular comorbidities [24]. In that study, patients with diabetes type 1 had3.5 times, and patients with diabetes type 2 had two times the risk for in-hospital death compared to non-diabetic patients.
In summary, although advanced age (≥80 years) is the strongest predictor of mortality related to an infection with COVID-19, there is extensive evidence from reports on the COVID-19 pandemic that diabetes mellitus and pre-existing cardiovascular diseases are independent major risk factors for hospitalisation at an ICU and mortality. Many of these comorbidities are linked to obesity and the related impairment of metabolic and cardiovascular physiology [25].
COVID-19 and the respiratory system: morphological and ventilatory changes in the lungs
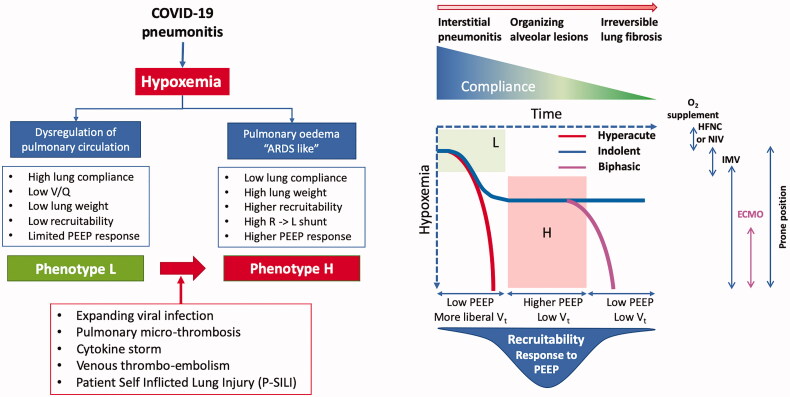
Patients with COVID-19 pneumonia show significant variability in clinical presentation with a broad and time-related spectrum with at least two different phenotypes with distinct morphological and physiological characteristics [26] (Figure 2), potentially related to the duration and progression of pulmonary disease.
Figure 2.
COVID-19 and the lung: functional ventilatory phenotypes and clinical course. HFNC (high flow nasal canula); NIV (non-invasive ventilation); IMV (invasive mechanical ventilation); ECMO (extracorporeal membrane oxygenation), PEEP (positive end-expiratory pressure). Figure in part redrawn and adapted from [37].
Although incompletely defined, it appears that initially pneumonic infiltrates are relatively small and confined most often to subpleural segments. Microscopic study shows in this phase, Infiltration of alveolar walls by numerous lymphocytes and interstitial edema, the typical lesions of a viral pneumonia [27,28]. When requiring mechanical ventilation these patients have near-normal pulmonary compliance, a low ventilation-perfusion ratio with normal pulmonary artery pressures, and low lung recruitability, requiring low expiratory pressure [26]. The marked hypoxaemia that patients with this L-phenotype may show may be related to impaired regional pulmonary vasoreactivity with loss of the normal hypoxic vasoconstrictor response leading to an increased shunt fraction [26]. Further, a thrombotic micro-angiopathy [29,30] contributes significantly to the pathophysiology of the COVID-19 pulmonary infection: fibrin thrombi are present both in capillaries and small arterioles; there also is activation of megakaryocytes, possibly native to the lung, with platelet aggregation and platelet-rich clot formation, in addition to fibrin deposition [31,32]. Entrapment of neutrophils in fibrin nets may additionally contribute to vascular obstruction.
Patients may transition from the L-phenotype to the H-phenotype of COVID-19 pneumonia characterised by variable (heterogeneous) compliance, a high right-to-left shunt, large confluent pulmonary infiltrates, and potentially high lung recruitability [26], more typical of ARDS. Histologic examination of patients who died at this later stage shows acute fibrinous and organising pneumonic lesions with extensive intra-alveolar fibrin deposition, intraluminal loose connective tissue formation in the alveolar ducts, and bronchioles and moderate interstitial lymphocytic infiltrates [27]. There is a significant vascular injury with cytoplasmic vacuolisation and detachment of endothelial cells.
Several factors besides expansion of the viral pneumonia and its immune response may contribute to the pathogenesis of the worsening respiratory failure. The intravascular micro- and macro-thrombosis within the pulmonary circulation will lead to increased dead-space ventilation [33]. This, combined with increased metabolic demand may result in a marked increase of the respiratory drive during spontaneous breathing increased rate and augmented transpulmonary pressure and strain. The combination of high negative inspiratory intrathoracic pressures and increased lung permeability can exacerbate interstitial and pulmonary edema leading to worsening of pulmonary infiltrates and respiratory failure. The consequent in part patient self inflicted lung injury that may occur during high-flow oxygen therapy or non-invasive ventilation, may be attenuated by intubation, potentially indicated once deep negative swings in intra-esophageal pressure are observed [34,35], but has to be balanced against the risks of intubation and ventilation, as well as ventilator-induced lung injury. At a later stage, dense consolidation and progressive fibroproliferation will result in decreased recruitability by positive end-expiratory pressure (PEEP) ventilation or positioning of the patient in a prone position [36].
The clinical course may follow three main patterns: a hyper-acute course, with severe hypoxaemia and dyspnoea necessitating immediate intubation; a more indolent course with moderate or severe hypoxaemia but with only moderate work of breathing and a biphasic course, in which patients have an initial indolent course followed after 5–7 days by an acute deterioration with fever, and worsening respiratory failure with consolidating bilateral infiltrates [37]. The underlying pathological, physiological and ventilatory characteristics remain to be completely characterised, and are clearly complex, heterogeneous and demand an individualised approach to management.
COVID-19 and the cardiovascular system: the role of angiotensin-converting enzyme 2 in the renin-angiotensin system
The COVID-19 pandemic has led to a renewed interest in angiotensin-converting enzyme 2 (ACE2), which – besides being a counteractive regulator of the renin-angiotensin system (RAS) – has an additional biological role: function as the receptor for cell entry by coronaviruses [38,39]. Similar to SARS-COV1, the coronavirus that caused the severe acute respiratory syndrome (SARS) with whom it shows high genomic identity, SARS-COV-2 uses the ACE2 cell membrane-bound receptor for cell entry [40,41]. The vast majority of the knowledge about the molecular pathways involved in the cellular infection by SARS-CoV-2, as will be discussed below, is based on experimental studies using SARS-CoV1.
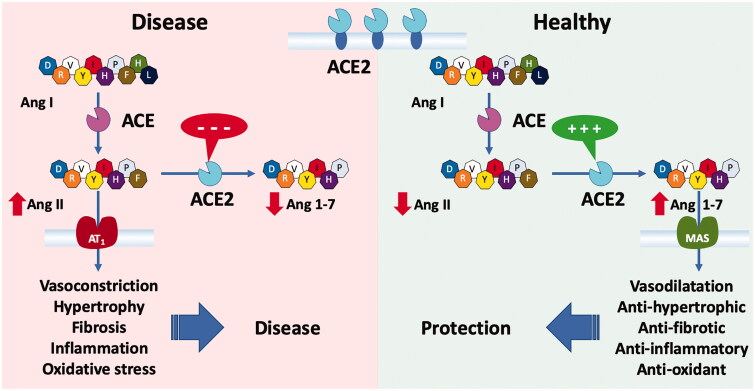
ACE2, a homolog of the angiotensin-converting enzyme (ACE), was discovered 20 years ago [39,42]. This membrane-bound carboxypeptidase is universally present in the cardiovascular system as well as in the lung, intestine, and kidney. It hydrolyses angiotensin II (Ang II) to angiotensin 1–7 (Ang 1–7), which has vasodilator and cardioprotective effects through activation of the MAS receptor, coded by the MAS1 gene (mitochondrial assembly 1) [43,44] (Figure 3). By hydrolysing Ang II to Ang 1–7, ACE2 counterbalances the vasoconstriction induced by activation of the ACE-Ang II-angiotensin receptor 1 (AT1) axis of the RAS. In various diseases where the ACE-Ang II-AT1 axis is activated, ACE2 may mitigate the detrimental effects of Ang II [44]. As a consequence, impairment of the cardioprotective effects the ACE2-Ang [1-7]-MAS axis will accelerate the disease-promoting actions of an activated ACE-Ang II-AT1 axis. Furthermore, excess of Ang II in disease will further weaken the protective role of ACE2 by activating disintegrin and metalloprotease 17 (ADAM17), an enzyme that leads to shedding of the ectodomain of ACE2 from the cell membrane into the circulation [45,46]. A high concentration of this soluble ACE2 is a known marker of an unfavourable prognosis in patients with cardiovascular disease [47].
Figure 3.
Role of ACE2 in the renin-angiotensin system (RAS). ACE2 balances the two axes of the RAS. Normal activity of ACE2 promotes the protective ACE2/Ang 1-7/Mas receptor axis and loss of ACE2 results in overactivity in the ACE/Ang II/AT1 receptor axis causing a shift towards diseased states.
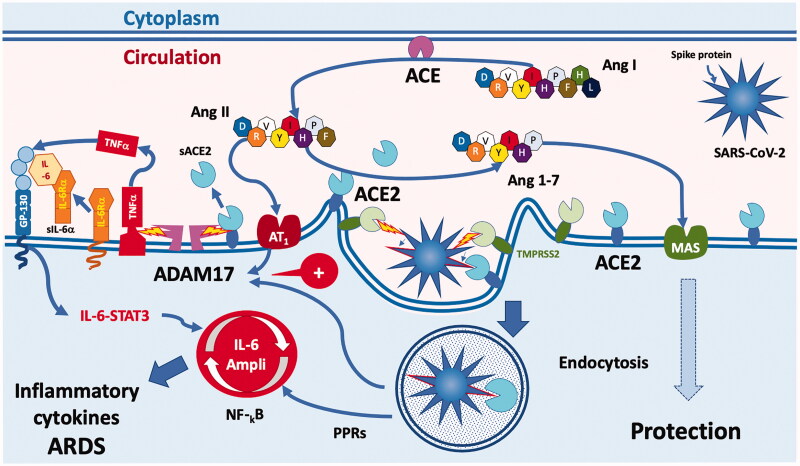
ACE2 is also abundant in the lung alveolar epithelial cells and the endothelial cells of the lung capillaries [39]. Coronaviruses invade the cell after binding of the viral spike glycoprotein to the ectodomain of ACE2 [41,48–50] (Figure 4). Priming of the spike protein by transmembrane serine protease 2 (TMPRSS2), a cell membrane-bound protease, is an essential first step for cell entry by the virus [41]. Endocytosis of ACE2 bound to the virus particle reduces the number of ACE2 enzymes on the cell surface and thus weakens ACE2-mediated tissue protection [49]. Several positive feedforward mechanisms reduce further ACE2 activity. For instance, virus uptake in the cell stimulates activation of ADAM17 leading to shedding of ACE2 [51]. ADAM17 also induces the release of cytokines, which in turn activate other proteolytic enzymes leading to further shedding of ACE2 from the cell membrane. Due to all of these interlocking and mutually enhancing mechanisms, coronavirus infection in tissues progressively weakens the protective ACE2-Ang [1-7]-MAS axis, thus shifting the balance towards the ACE-Ang II-AT1 axis and its disease-promoting actions. Indeed, Ang II acts not only as a vasoconstrictor but also stimulates the production of pro-inflammatory cytokines via AT1 [52,53]. The activation of ADAM17 by Ang II generates TNFα and soluble IL-6R, which may lead to activation of the transcription factor STAT3. Coronavirus infection may lead to the activation of Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-kB) via pattern recognition receptors (PRRs) [54]. The combined activation of NF-kB and STAT3 leads to the activation of the IL-6 amplifier, causing a massive production of cytokines and the development of ARDS [52].
Figure 4.
COVID-19 uses angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) as cell entry receptors. Viral spike glycoprotein activated by TMPRSS2 interacts with cell surface ACE2 and both are internalised through endocytosis, resulting in decreased surface ACE2 expression. Endocytosis upregulates ADAM17 activity, which cleaves ACE2 from the cell membrane, perpetuating the loss of ACE2 from tissue RAS. Loss of ACE2 leads to the accumulation of Ang II, which through AT1 receptors also upregulates ADAM17, resulting in further cleavage of cell surface ACE2. The activation of ADAM17 by Ang II also generates TNFα and soluble IL-6R, which can lead to activation of the transcription factor STAT3. SARS-CoV-2 itself leads to the activation of NF-kB via pattern recognition receptors (PPRs). The combined activation of NF-kB and STAT3 leads to the activation of the IL-6 amplifier, causing a massive production of cytokines and the development of ARDS.
In the lungs, preclinical research shows that diffuse alveolar lung damage, occurring after aspiration or after infection with corona or influenza viruses, is promoted by activation of the ACE-Ang II-AT1 axis of the RAS whereas ACE2 exerts a protective effect [55]. Infection with SARS-CoV1 results through the binding of the viral spike protein with membrane-bound ACE2 in a downregulation of the protective effect of the ACE2-Ang [1-7]-MAS axis, which leads to increased lung injury and edema promoted by the unopposed ACE-AngII-AT1 axis of the RAS [49,56]. It is important to note that the administration of the SARS-CoV1 spike protein alone is sufficient to significantly worsen the acute lung injury induced by acid aspiration in wild type mice through the downregulation of ACE2 [49]. Based on these preclinical studies and considering that the spike protein of SARS-CoV-2 has a ten times greater affinity for binding to ACE2 than SARS-COV1 [57], it becomes clear that ACE2 deficiency may play a pivotal role in ARDS caused by a COVID-19 infection.
Animal studies have shown an increased myocardial expression of ACE2 mRNA, as well an increased ACE2 activity, by treatment with an ACE-inhibitor (ACE-I) or an angiotensin receptor blocker (ARB) [58]. Hypertensive patients treated with the ARB olmesartan showed an increased urinary secretion of ACE2, suggesting that up-regulation of ACE2 may also occur in humans [59]. Based on these studies, it has been suggested that patients with diabetes or cardiovascular disease treated with these "ACE2 stimulants", facilitating a COVID-19 infection, have an increased risk of developing a severe and fatal form of the disease [60,61]. However, in animal studies, treatment with an ARB had a rather protective effect against the experimental lung damage elicited by aspiration of acid [55], the spike protein of the coronavirus [49], or by endotoxin/lipopolysaccharide (LPS) [62]. Moreover, the administration of recombinant ACE2 was protective against lung damage elicited by acid aspiration in mice [55] and improved ventilation parameters by ARDS induced by LPS in rats [62]. Therefore, preclinical research indicates that is unlikely that treatment with ACE-I or ARB may predispose to the development of more severe forms of COVID-19.
Several observational studies have already addressed the question if treatment with ACE-I and ARB may be associated with a higher risk to develop severe COVID-19 infection or may have an impact on clinical outcomes [63–67]. One has to be cautious when drawing conclusions from these studies, because either the study population was small – based on a single hospital cohort – or extensive but based on the retrospective analysis of medical records. A first extensive observational study on this topic was performed using data from 169 hospitals in Asia, Europe, and North America on patients hospitalised with COVID-19 infection [68] but was retracted because the raw data were not completely available to the investigators [68]. Two studies in the United States performed a propensity score-matched analysis on a large number of patients tested for COVID-19. In a first study, performed at the NYU Langone health system, the median difference in the likelihood of testing positive or having severe disease because of ACE-I or ARB use was not significant after propensity score matching [69]. In a second US study performed at Cleveland Clinic Health System in Ohio and Florida, the frequency of a COVID-19 positive test result was not significantly different in patients taking either ACE-I or ARB at the time of testing. However, the likelihood of hospital and ICU admission was higher compared to non-use after propensity score matching for a limited number of classic confounding variables [70]. Two studies from Europe performed an extensive population-based case-control study using administrative prescription data. A case-control study from Milan found no independent risk-association between ACE-I or ARB use and development of COVID-19 or severe COVID-19 [71]. Use of ACE-I or ARB was more common in patients with COVID-19 than among controls because of their higher prevalence of cardiovascular disease. In a case-population study from Madrid, treatment with an ACE-I or ARB was not associated with increased risk for hospitalisation of patients with a documented COVID-19 infection [72]. Moreover, in diabetic patients under treatment with an ACE-I or ARB, the risk for hospital admission was significantly decreased, suggesting a protective effect. In a large case-control study from Denmark prior use of ACE-I/ARB was not significantly associated with COVID-19 diagnosis or with mortality among patients diagnosed as having COVID-19 [73].
In summary, although solid scientific evidence from prospective controlled trials is missing, the results of the present studies do no point to an increased risk association between treatment with ACE-I or ARB and COVID-19. Therefore, these medications should not be discontinued to prevent severe lung complications of COVID-19. Several scientific associations have published statements recommending to continue ACEI or ARB therapy in patients with hypertension and COVID-19 infection [74–76].
Animal studies have demonstrated that aging is associated with a significant reduction in ACE2 expression in the lung, a finding that was more pronounced in male than in female rats [77]. In cerebral arteries, ACE2 deficiency caused impairment of endothelium-dependent vasodilation and augmented oxidative stress, both most marked in old mice [78]. Endothelial cells in which aging was induced by Ang II, display increased senescence, apoptosis, and oxygen radical production, and decreased ACE2 expression [79]. Analysis of gene expression in 30 different human tissues showed significantly higher ACE2 expression in female than in male patients, an age-dependent decrease in ACE2 expression, and a highly significant decrease in type II diabetic patients [80]. Preclinical and clinical research has further shown that the ACE2-Ang [1-7]-MAS axis plays an essential protective role in the cardiovascular system and that its impairment contributes to the development of various forms of heart failure due to arterial hypertension, ischaemic heart injury, diabetes and obesity [44], which all are highly prevalent in older ages.
Although counterintuitive, ACE2-deficiency associated with aging may play a pivotal role in the pathogenesis of severe lung injury during COVID-19 [81]. A possible protective effect of a moderate ACE2 deficiency against viral cellular infection is highly unlikely because of the very high affinity of SARS-CoV-2 to the ACE2 receptors. In patients with ACE2 deficiency, one may expect that the ACE2 downregulation induced by viral entry will magnify the imbalance between the protective ACE2-Ang [1-7]-MAS axis and the lung injury promoting ACE-Ang II-AT1 axis of the RAS.
Cardiovascular complications of COVID-19
Myocarditis
Experiences with the Middle Eastern Respiratory Syndrome (MERS), SARS-CoV1 and other non-SARS coronaviruses demonstrate that this virus family is capable to cause symptomatic myocarditis [82–84]. It might therefore be hypothesised that many COVID-19 positive patients presenting with the clinical suspicion of an acute coronary syndrome actually exhibit myocarditis-associated myocardial injury [85]. In fact, a considerable amount of critically ill COVID-19 patients requiring ICU admission are characterised by elevated troponin levels in terms of myocardial injury, as shown in several published case series. In an early Chinese study which included 52 critically ill patients, about one third of patients showed elevated troponin levels [86]. A considerably larger analysis on 416 patients confirmed these results, by showing that approximately 20% of these patients had signs of myocardial injury. Patients with myocardial injury were older, sicker and suffered from a higher mortality even after adjusting for age and comorbidities [87]. Another study from China confirmed this association between elevated troponin levels and mortality, 59% of patients that died displayed elevated troponin levels, as compared to only 1% of the survivors [13]. Similar data were reported from North America, in an analysis from New York 36% of the nearly 3000 patients studied where characterised by elevated troponin levels [88]. While most troponin elevations were mild, those with a more significant myocardial injury showed a tripling of the mortality risk. Finally, a meta-analysis including 22 studies and 3684 COVID-19 positive patients, confirmed the association between myocardial injury and increased mortality [89].
Most case reports included patients with clinically suspected myocarditis, lacking a definite diagnosis using pathology, histology or even imaging [85,90]. From those studies it could not be concluded whether SARS-CoV-2 can actually infiltrate the myocardium causing myocardial injury or whether the observed myocardial injury is a side effect of the cytokine storm characterising severe COVID-19 cases [91]. Other potential mechanisms leading to cardiac injury include microvascular damage due to a pro-thrombotic milieu, hypoxaemia caused by critical illness and acute coronary syndrome. A recent case series including 18 patients admitted for ST-elevating myocardial infarction (STEMI) showed a high prevalence of non-coronary myocardial injury. Of interest, those patients had a higher mortality rate as compared to those with ischaemic MI [92].
The first described case suggesting the presence of SARS-CoV-2 within the myocardium came from Pavia, one of the epicentres of the Italian COVID-19 outbreak. The histology of a 69-year old patient with COVID-19 associated cardiogenic shock requiring veno-arterial extracorporeal membrane oxygenation (VA-ECMO) showed virus-positive myocarditis and the described virus resembled coronavirus in terms of size and morphology [93]. An autopsy study from Germany, including 39 deceased COVID-19 patients showed presence of SARS-CoV-2 in 61.5% with 41% showing a high viral load. Interestingly, while those patients were characterised by a cytokine response, no inflammatory cell influx could be detected [94]. These reports suggest that virus entry causing acute myocarditis might be an important mechanism for the high rate of myocardial injury. In this regard it is of major interest that the myocardium is characterised by a significant ACE-2 expression [95,96].
Still, as a cytokine storm often characterises critically ill COVID-19 patients, it remains unclear whether direct viral infiltration or the systemic reaction to the virus infection plays a more determining role in the high incidence of myocardial injury. In addition, hemodynamic changes, initiation of a strong systemic inflammatory reaction and the pro-thrombotic milieu characterising critically ill infectious disease patients may contribute to those observations [97,98]. In a German cardiac MRI study including unselected patients from a regional testing centre that recently recovered from COVID-19 infection, of whom only one third needed hospitalisation, 78% of patients showed MRI evidence of cardiac involvement and 60% had evidence of ongoing myocardial inflammation [99]. Moreover, on the day of the CMR, which took place on average 71 days after time of diagnosis (IQR 64–92 days), high sensitivity Troponin T was detectable in 71 patients and significantly elevated in 5 patients. Forty-one percent of the patients with cardiac involvement had delayed gadolinium enhancement. Cardiac findings on MRI were irrespective of pre-existing conditions, the severity of the infection and the presence of cardiac symptoms. Also an earlier Chinese CMR study had revealed similar findings [100]. The exact meaning of this myocardial involvement in a mildly affected population remains unclear as of today.
Thrombotic complications
Thrombo-embolism affecting both the arterial and venous system is an additional common and potentially dangerous complication in COVID-19 patients [101–104]. Early on into the pandemic it has been hypothesised that an overactive immune activation, ongoing platelet activity and endothelial dysfunction or stasis might be contributing factors [105]. Elevated D-Dimer levels reflecting ongoing fibrinolysis were identified early as a prognostic marker for thromboembolic complications and poor outcome [13,106]. In a pooled analysis of 4 ICUs treating 150 COVID-19 patients, a high rate of thromboembolic complications, primarily of pulmonary embolisms, was observed despite prophylactic and in some cases therapeutic anticoagulation [107]; Nearly all patients experiencing thromboembolic events had elevated D-Dimer levels. When comparing these results with a historic ARDS population, a considerably higher rate of thromboembolic complications and alterations in routine coagulation parameters was seen. Another observational study from Lombardy, Italy showed a 22% rate of thromboembolic complications, despite prophylactic anticoagulation with low molecular weight heparin (LMWH) [108]. Of interest, a small observational study suggested that patients already on oral anticoagulation when infected with SARS-CoV-2 were protected against thromboembolic complications [109].
Covid-19: pathophysiological conundrum
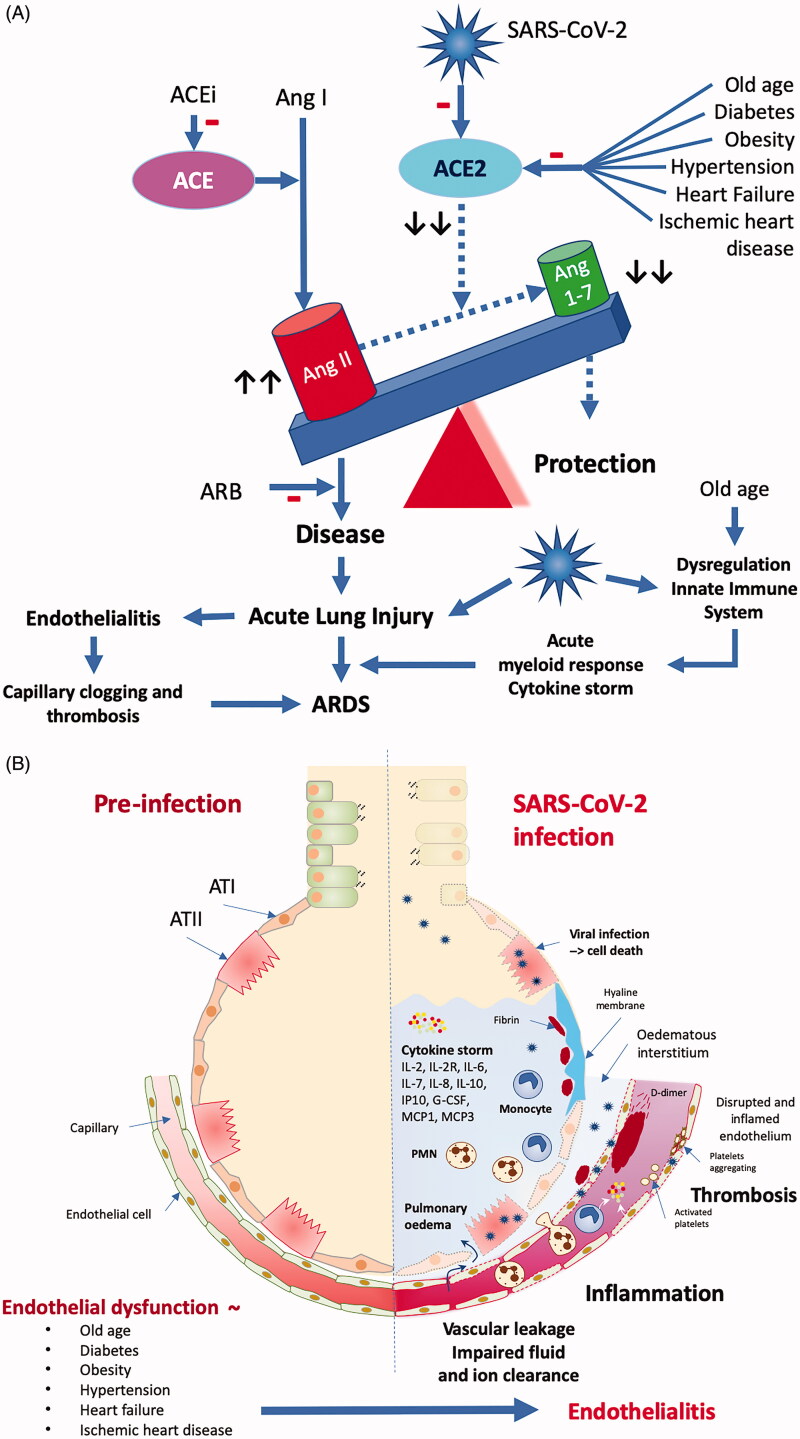
The wide variety of clinical presentations of a COVID-19 infection, from asymptomatic or mild respiratory symptoms in young children, an influenza-like syndrome with persistent, non-productive cough in young adults to critical, frequently lethal respiratory failure in elderly patients, is very intriguing. The high prevalence of old age, obesity, diabetes, hypertension, heart failure, and ischaemic heart disease in patients who develop a severe to critical COVID-19 syndrome suggests shared pathophysiological mechanisms (Figure 5). The COVID-19 pandemic becomes deadliest in the intersection with the equally pandemically spreading obesity, diabetes, and cardiovascular disease [110].
Figure 5.
Pathophysiological conundrum. Top: In older patients with obesity, diabetes, pre-existing cardiovascular diseases and associated endothelial dysfunction, SARS-CoV-2 infection critically unbalances the fragile equilibrium between the protective ACE2-Ang [1-7]-MAS -axis and the inflammation- and disease-promoting ACE -Ang II-AT1 axis of the RAS. Viral infection worsens the age-related dysregulation of the innate immune system slowing down the early immune response allowing increased and prolonged viral replication in the lung. The acute lung injury caused by the viral infection involves also the endothelium of the peri-alveolar capillaries. The ensuing endothelialitis promotes alveolar edoema formation due to increased vascular leakage through increased gap formation between the inflamed endothelial cells. Clogging and occlusion by inflammatory cells and intravascular thrombus formation within the lung capillaries worsens the ventilation-perfusion mismatch causing the impaired oxygen uptake by the lung. The acute lung injury triggers a later on-setting but overwhelming acute myeloid immune response and associated cytokine storm leading to further lung injury with the development of ARDS. Bottom: Viral infection extends into the peri-alveolar capillaries turning the pre-existent endothelial dysfunction into a severe endothelialitis with vascular leakage and impaired fluid and ion clearance causing pulmonary edoema, intense inflammation and intravascular and intra-alveolar thrombosis. ATI: alveolar epithelial cell type I; ATII: alveolar epithelial cell type II. Figure redrawn and adapted from [126,155].
It is not entirely unexpected that obese and diabetic patients are more prone to develop severe lung lesions and are more likely to die from a COVID-19 infection. In obese and diabetic patients with an influenza viral infection, a disturbed balance between pro-inflammatory (leptin) and anti-inflammatory (adiponectin) adipokines [111] and a delayed and blunted response of both the innate and adaptive immune system [112] – which is exacerbated by a sedentary lifestyle [113] – all promote the development of severe lung lesions [114]. Moreover, obese patients show a prolonged shedding of the virus [115], which may lead to prolongation of the spreading of the virus in the population [116] but also may increase the risk of differentiation into more virulent virus strains [117] that may increase the intensity of the illness and overall mortality during the pandemic. The high prevalence of obesity and diabetes in patients with a severe form of a COVID-19 infection and the terrifying high mortality observed during the current pandemic in regions with a high prevalence of obesity, suggests that the same mechanisms are at work [114,118,119].
Aging is associated with a progressive dysregulation of the innate immune system – also called immunosenescence – leading to a persistent basal inflammation that contributes to the development of chronic diseases such as atherosclerosis and Alzheimer's disease but also an impaired and delayed immune response to viral infections [120,121]. Coronaviruses encode several structural and non-structural proteins that antagonise the antiviral immune response and replicate rapidly, leading to enhanced production of cytokines and chemokines by infected epithelial cells. Together with a delayed and dysregulated antiviral immune response, this results at a later stage of the infection in a massive infiltration of inflammatory monocyte-macrophages and neutrophils, which are the predominant source of cytokines and chemokines in lethal pneumonia caused by coronaviruses [122]. Excessive production of cytokines may evolve into a "cytokine storm" causing extensive lung injury and the development of ARDS-like respiratory dysfunction [123].
The age at which the prevalence of severe COVID-19 lung lesions and its associated mortality increases exponentially coincides with the onset of the similarly age-related increase in the prevalence of vascular endothelial dysfunction [124]. This epidemiological association and other rapidly emerging clinical, pathological and molecular data on COVID-19 patients have led many researchers to postulate a more vascular-centric pathophysiology of COVID-19. It is hypothesised that dysfunction of the pulmonary microvascular endothelial cells, an integral part of the alveolar-capillary barrier, may play a key role in the pathogenesis of COVID-19 pneumonia and its associated acute respiratory failure [125–128]. A viral endothelialitis may contribute to the pathophysiology of the pulmonary and systemic microcirculatory changes in a COVID-19 infection. Post-mortem examination of COVID-19 patients showed viral inclusions in the endothelial cells with accumulation of inflammatory cells and evidence of endothelial apoptosis in the lung, heart, kidney, and small intestine [129]. Observation of increased numbers of circulating endothelial cells associated with elevated levels of soluble endothelial cell adhesion molecules and inflammatory cytokines in patients hospitalised with severe COVID-19 provides in vivo evidence that a viral and inflammatory endothelialitis may play a key role in the pathogenesis of COVID-19 [130]. The associated thrombo-inflammation or immunothrombosis may explain the coagulopathy observed in COVID-19 patients [131–133] and the high incidence of micro- and macrothrombosis observed in the lungs [32,104,134,135], arteries and veins [103,104,135]. The microvascular endothelium of the alveolar capillaries and precapillary arterioles functions as a signalling pathway that triggers hypoxic pulmonary vasoconstriction, the normal physiological response to alveolar hypoxia that provides essential protection against ventilation/perfusion mismatch in the lung [136]. Dysregulation of the pulmonary perfusion and loss of the hypoxic pulmonary vasoconstriction caused by the COVID-19 related endothelial dysfunction may explain the remarkable dissociation between the frequently severe hypoxaemia and the preserved lung mechanics in patients with an L-type phenotype COVID-19 pneumonia [137,138]. The impairment of hypoxic pulmonary vasoconstriction response in COVID-19 patients results in an increased intrapulmonary shunt leading to marked arterial hypoxaemia. Many patients with pronounced arterial hypoxaemia present initially without proportional signs of respiratory distress, referred to as silent or ‘happy’ hypoxaemia, due to the absence of an increased breathing work [139]. Notably, the degree of arterial hypoxaemia may be underestimated by pulse oximetry due to a leftward shift of the oxyhaemoglobin dissociation curve [139].
Old age, obesity, diabetes, and all comorbidities that are prevalent in severe COVID-19 patients are all associated with endothelial dysfunction. The vascular endothelial cells in all these conditions have an altered pro-inflammatory and pro-adhesive phenotype [140] that may increase the susceptibility of the cells for infection by SARS-CoV-2. Cellular infection with SARS-CoV-2 causing direct inactivation of cell membrane-bound ACE2 receptor will critically unbalance the fragile equilibrium between the protective ACE2-Ang [1-7]-MAS -axis and the disease-promoting ACE -Ang II-AT1 axis of the local tissue RAS that exists in obese, old-aged patients with diabetes and pre-existing cardiovascular disease [141]. Combined with the aging-related dysregulated antiviral immune response, the acute disturbance in the RAS balance will explosively boost the development of a frequently lethal ARDS-like pneumonia. Overactivation of the ACE -Ang II-AT1 axis of the RAS and the endothelialitis caused by the viral infection will promote vasoconstriction, inflammation, and thrombosis in the micro- and macro-vascular bed of the lung [134], but also in the systemic circulation where it will cause myocardial injury and other cardiovascular complications [142–145] contributing to the high mortality of a SARS-CoV-2 infection. Immunosenescence [121,146] and inhibition of the antiviral immune response by SARS-CoV-2 [147] will extend viral replication and lung injury followed by a massive myeloid response and cytokine storm during the later phase of the disease-causing a lethal ARDS. (Figure 5).
Concluding remarks
In the pathophysiology of COVID-19 age-related changes in the immune response, unbalancing of the local tissue RAS due to direct and indirect inactivation of ACE2 activity and an acute endothelialitis all contribute to the severity and the evolution of the lung lesions, and also to the development of systemic multi-organ complications particularly in older patients with obesity, diabetes, and pre-existent cardiovascular disease.
Although clinical, laboratory, and autopsy evidence is mounting that endothelialitis plays a central role in the pathophysiology of COVID-19 [148], the therapy of this life-threatening syndrome remains mainly empirical. Based on these new insights, speculation on possible new therapeutic approaches may lead to the temptation to use innovative therapies on a compassionate basis before state-of-the-art clinical validation by high-quality randomised clinical trials. Although clinical research during a pandemic is difficult, lessons from previous pandemics learn that only obtaining firm scientific evidence through prospective clinical trials based on a global research and development strategy [149] leads to innovative therapies that can halt infectious disease outbreaks and improve the infected patients' outcome (e.g. Ebola-outbreak). It is laudable to learn from the ClinicalTrials.gov website that more than 2000 clinical trials are planned or underway, investigating new therapies and interventions in COVID-19 patients [150].
Extensive well-performed case-control studies demonstrated no increased risk of severe infection associated with ACE-I or ARB treatment nor did ACE-I or ARB interruption lead to improved survival rates at 30 days (BRACE CORONA trial). An eventual worse clinical outcome seems more related to pre-existing cardiovascular diseases than to its treatment by ACE-I and ARB, and preclinical research suggests a possible protective effect of ARB against diffuse alveolar damage after infection with corona or influenza viruses. Therefore, it appears wise not to discontinue treatments with ACE-I or ARB in patients with pre-existing cardiovascular diseases.
The management of acute respiratory failure in COVID-19 should be based on the new insights on its pathophysiology with at least two different phenotypes and three different clinical courses. Respiratory drive, intrapulmonary shunt and breathing work should be assessed on admission to the ICU and early intubation considered in order to prevent patient self-induced lung injury. When mechanical ventilation is needed, intubation and ventilation strategy should follow current practice guidelines [151] and updated recommendations [26,35,152].
Microcirculatory obstruction due to abnormal vasoconstrictor responses, luminal plugging by inflammatory cells, and intraluminal thrombosis related to the acute endothelialitis complicate the respiratory dysfunction in COVID-19 by increasing dead-space ventilation. We need more basic and clinical research on a possible beneficial effect of antithrombotic regimens on these microcirculatory changes. Further, it needs to be studied whether readily available coagulation markers can identify patients at high risk for thrombotic complications, especially of pulmonary thrombosis and whether these high-risk patients may benefit from therapeutic anticoagulation. Given the high incidence of thrombotic and thromboembolic complications, clinical practice guidelines recommend treating hospitalised patients with COVID-19 without evidence for disseminated intravascular coagulation with prophylactic doses of anticoagulation [105].
The detection of elevated biomarkers of myocardial injury needs to trigger the challenging differential diagnosis between myocardial injury due to supply-demand mismatch, myocarditis and acute coronary syndrome. Given the high prevalence of detectable levels of high-sensitivity troponin and MRI evidence of ongoing myocardial inflammation months after the acute infection [99], all convalescent COVID-19 patients should be monitored for de novo incidence of heart failure as a late cardiovascular complication [153].
Finally, after the current COVID-19 pandemic has ended, considerable attention will be paid to better preparation and organisation of health care systems for future infectious pandemics. In coping with the COVID-19 pandemic, we may however, not neglect to strive for more effective prevention and treatment of the other pandemic raging through our societies: the pandemic of obesity, diabetes, and cardiovascular diseases, a prime substrate for a deadly virus. In this regard, we may not forget that apart from pharmacological interventions, physical exercise is one of the most potent anti-oxidative and anti-inflammatory stimulus at the level of the endothelium, with a proven effect on halting the aging-related endothelial dysfunction, apart from its beneficial effects on the classical cardiovascular risk factors [154].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) . COVID-19 Map - Johns Hopkins Coronavirus Resource Center; 2020. [2020 Jun 24]. Available from: https://coronavirus.jhu.edu/map.html.
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Report of the WHO-China Joint Mission on Coronavirus Disease 2019. (COVID-19). 2020. Available from : https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf.
- 5.Onder G, Rezza G, Brusaferro S.. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. [DOI] [PubMed] [Google Scholar]
- 6.RENAVE . Informe sobre la situación de COVID-19 en España Informe COVID-19 no 30 . 11 de mayo de 2020. Available from: https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/InformesCOVID-19.aspx.
- 7.Istituto Superiore di Sanità. Epidemia COVID-19 Aggiornamento nazionale; 2020. Available from: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_26-marzo%202020.pdf.
- 8.Rijksinstituut voor Volksgezondheid en Milieu . Epidemiologische situatie COVID-19 in Nederland. 2020. [2020 May 29]. Available from: https://www.rivm.nl/documenten/epidemiologische-situatie-covid-19-in-nederland-28-mei-2020.
- 9.Office for National Statistics . Deaths registered weekly in England and Wales, provisional. 2020. [2020 May 29]. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/weeklyprovisionalfiguresondeathsregisteredinenglandandwales.
- 10.Natale F, Ghio D, Tarchi D, et al. COVID-19 cases and case fatality rate by age; 2020. [2020 May 21]. Available from: https://ec.europa.eu/knowledge4policy/publication/covid-19-cases-case-fatality-rate-age_en.
- 11.Dowd JB, Andriano L, Brazel DM, et al. Demographic science aids in understanding the spread and fatality rates of COVID-19. Proc Natl Acad Sci USA. 2020;117(18):9696–9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Sun W, Li J, et al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease. medRxiv. 2019;2020:2020.02.17.20024166. [Google Scholar]
- 15.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, Li R, Lu Z, et al. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY). 2020;12(7):6049–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SARS-CoV-2 Surveillance Group . Characteristics of SARS-CoV-2 patients dying in Italy, Report based on available data on May 7, 2020. 2020. Available from: https://www.epicentro.iss.it/en/coronavirus/bollettino/Report-COVID-2019_7_may_2020.pdf.
- 19.Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020;71(15):896–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. BMJ. 2020;369:m1966. DOI: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qingxian C, Chen F, Fang L, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China (3/13/2020); 2020. Available from: https://ssrn.com/abstract=3556658 or [DOI] [PubMed]
- 22.Docherty AB, Harrison EM, Green CA, et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barron E, Bakhai C, Kar P, et al. Type 1 and Type 2 diabetes and COVID-19 related mortality in England: a whole population study. 2020. Available from: https://www.england.nhs.uk/wp-content/uploads/2020/05/valabhji-COVID-19-and-Diabetes-Paper-1.pdf. [DOI] [PMC free article] [PubMed]
- 25.Stefan N, Birkenfeld AL, Schulze MB, et al. Obesity and impaired metabolic health in patients with COVID-19. Nat Rev Endocrinol. 2020;16(7):341–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Copin M-C, Parmentier E, Duburcq T, et al. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020;46(6):1124–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian S, Hu W, Niu L, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGonagle D, O'Donnell JS, Sharif K, et al. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020;2(7):e437–e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marongiu F, Grandone E, Barcellona D.. Pulmonary thrombosis in 2019-nCoV pneumonia? J Thromb Haemost. 2020;18(6):1511–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox SE, Akmatbekov A, Harbert JL, et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18(6):1517–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diehl JL, Peron N, Chocron R, et al. Respiratory mechanics and gas exchanges in the early course of COVID-19 ARDS: a hypothesis-generating study. Ann Intensive Care. 2020;10(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tonelli R, Fantini R, Tabbì L, et al. Early inspiratory effort assessment by esophageal manometry predicts noninvasive ventilation outcome in De Novo respiratory failure. A pilot study. Am J Respir Crit Care Med. 2020;202(4):558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Möhlenkamp S, Thiele H.. Ventilation of COVID-19 patients in intensive care units. Herz. 2020;45(4):329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gattinoni L, Chiumello D, Rossi S.. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camporota L, Vasques F, Sanderson B, et al. Identification of pathophysiological patterns for triage and respiratory support in COVID-19. Lancet Resp Med. 2020;8(8):752–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K, Gheblawi M, Oudit GY.. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020;142(5):426–428. [DOI] [PubMed] [Google Scholar]
- 39.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2 . Circ Res. 2020;126(10):1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tipnis SR, Hooper NM, Hyde R, et al. A Human homolog of angiotensin-converting enzyme. cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275(43):33238–33243. [DOI] [PubMed] [Google Scholar]
- 43.Iusuf D, Henning RH, van Gilst WH, et al. Angiotensin-(1-7): pharmacological properties and pharmacotherapeutic perspectives. Eur J Pharmacol. 2008;585(2–3):303–312. [DOI] [PubMed] [Google Scholar]
- 44.Patel VB, Zhong J-C, Grant MB, et al. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ Res. 2016;118(8):1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel VB, Clarke N, Wang Z, et al. Angiotensin II induced proteolytic cleavage of myocardial ACE2 is mediated by TACE/ADAM-17: a positive feedback mechanism in the RAS. J Mol Cell Cardiol. 2014;66:167–176. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Mukerjee S, Silva-Alves CRA, et al. A disintegrin and metalloprotease 17 in the cardiovascular and central nervous systems. Front Physiol. 2016;7:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varagic J, Ahmad S, Nagata S, et al. ACE2: angiotensin II/angiotensin-(1-7) balance in cardiac and renal injury. Curr Hypertens Rep. 2014;16(3):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H, Yang P, Liu K, et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18(2):290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haga S, Yamamoto N, Nakai-Murakami C, et al. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008;105(22):7809–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirano T, Murakami M.. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(5):731–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eguchi S, Kawai T, Scalia R, et al. Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension. 2018;71(5):804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Wit E, van Doremalen N, Falzarano D, et al. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicholls J, Peiris M.. Good ACE, bad ACE do battle in lung injury, SARS. Nat Med. 2005;11(8):821–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferrario CM, Jessup J, Gallagher PE, et al. Effects of renin-angiotensin system blockade on renal angiotensin-(1-7) forming enzymes and receptors. Kidney Int. 2005;68(5):2189–2196. [DOI] [PubMed] [Google Scholar]
- 59.Furuhashi M, Moniwa N, Mita T, et al. Urinary angiotensin-converting enzyme 2 in hypertensive patients may be increased by olmesartan, an angiotensin II receptor blocker. Am J Hypertens. 2015;28(1):15–21. [DOI] [PubMed] [Google Scholar]
- 60.Fang L, Karakiulakis G, Roth M.. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pal R, Bhansali A.. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020;162:108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wösten-van Asperen RM, Lutter R, Specht PA, et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1-7) or an angiotensin II receptor antagonist. J Pathol. 2011;225(4):618–627. [DOI] [PubMed] [Google Scholar]
- 63.Li J, Wang X, Chen J, et al. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bean D, Kraljevic Z, Searle T, et al. Angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID‐19 infection in a multi‐site UK acute hospital trust. Eur Heart J Fail. 2020;22:967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jung S-Y, Choi JC, You S-H, et al. Association of renin-angiotensin-aldosterone system inhibitors with COVID-19-related outcomes in Korea: a nationwide population-based cohort study. Clin Infect Dis. 2020:ciaa624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tedeschi S, Giannella M, Bartoletti M, et al. Clinical impact of renin-angiotensin system inhibitors on in-hospital mortality of patients with hypertension hospitalized for COVID-19. Clin Infect Dis. 2020;15:899–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mehra MR, Desai SS, Kuy S, et al. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med. 2020;382(25):e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Reynolds HR, Adhikari S, Pulgarin C, et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382(25):2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(9):1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mancia G, Rea F, Ludergnani M, et al. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382(25):2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de Abajo FJ, Rodríguez-Martín S, Lerma V, et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395(10238):1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fosbøl EL, Butt JH, Østergaard L, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020;324(2):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Simone G. Position statement of the ESC council on hypertension on ACE-inhibitors and angiotensin receptor blockers; 2020. [cited 2020 Mar 13]. Available from: https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang.
- 75.Patients taking ACE-i and ARBs who contract COVID-19 should continue treatment, unless otherwise advised by their physician Statement from the American Heart Association, the Heart Failure Society of America and the American College of Cardiology; 2020. [cited 2020 Mar 17]. Available from: https://newsroom.heart.org/news/patients-taking-ace-i-and-arbs-who-contract-covid-19-should-continue-treatment-unless-otherwise-advised-by-their-physician#.XnDMEt7Ot9I.twitter.
- 76.A statement from the International Society of Hypertension on COVID-19; 2020.. Available from: https://ish-world.com/news/a/A-statement-from-the-International-Society-of-Hypertension-on-COVID-19/.
- 77.Xudong X, Junzhu C, Xingxiang W, et al. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78(19):2166–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Silva RAP, Chu Y, Miller JD, et al. Impact of ACE2 deficiency and oxidative stress on cerebrovascular function with aging. Stroke. 2012;43(12):3358–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang C, Wang J, Ma X, et al. ACE2-EPC-EXs protect ageing ECs against hypoxia/reoxygenation-induced injury through the miR-18a/Nox2/ROS pathway. J Cell Mol Med. 2018;22(3):1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen J, Jiang Q, Xia X, et al. Individual variation of the SARS-CoV2 receptor ACE2 gene expression and regulation . Aging Cell. 2020;19(7):e13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verdecchia P, Cavallini C, Spanevello A, et al. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med. 2016;36(1):78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen J, Zhang HT, Xie YQ, et al. [Morphological study of severe acute respiratory syndrome (SARS)]. Zhonghua Bing li Xue za Zhi = Chin J Pathol. 2003;32(6):516–520. [PubMed] [Google Scholar]
- 84.Riski H, Hovi T, Frick MH.. Carditis associated with coronavirus infection. Lancet. 1980;2(8185):100–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial injury in patients hospitalized With COVID-19 infection. J Am Coll Cardiol. 2020;76(5):533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Parohan M, Yaghoubi S, Seraji A.. Cardiac injury is associated with severe outcome and death in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Eur Heart J Acute Cardiovasc Care. 2020. DOI: 10.1177/2048872620937165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu H, Ma F, Wei X, et al. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020:ehaa190. DOI: 10.1093/eurheartj/ehaa190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bangalore S, Sharma A, Slotwiner A, et al. ST-segment elevation in patients with Covid-19 - a case series. N Engl J Med. 2020;382(25):2478–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tavazzi G, Pellegrini C, Maurelli M, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lindner D, Fitzek A, Brauninger H, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020:e203551. DOI: 10.1001/jamacardio.2020.3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen L, Li X, Chen M, et al. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116(6):1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nicin L, Abplanalp WT, Mellentin H, et al. Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J. 2020;41(19):1804–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Davidson JA, Warren-Gash C.. Cardiovascular complications of acute respiratory infections: current research and future directions. Expert Rev anti Infect Ther. 2019;17(12):939–942. [DOI] [PubMed] [Google Scholar]
- 98.Smeeth L, Thomas SL, Hall AJ, et al. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. [DOI] [PubMed] [Google Scholar]
- 99.Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020:e203557. DOI: 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang L, Zhao P, Tang D, et al. Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. 2020;13(11):2330–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173(4):268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Klok FA, Kruip MJHA, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75(23):2950–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lippi G, Favaloro EJ.. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost. 2020;120(05):876–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tavazzi G, Civardi L, Caneva L, et al. Thrombotic events in SARS-CoV-2 patients: an urgent call for ultrasound screening. Intensive Care Med. 2020;46(6):1121–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lachant DJ, Lachant NA, Kouides P, et al. Chronic therapeutic anticoagulation is associated with decreased thrombotic complications in SARS-CoV-2 infection. J Thromb Haemost. 2020;18:2640–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang C, Jin Z.. An acute respiratory infection runs into the most common noncommunicable epidemic—COVID-19 and cardiovascular diseases. JAMA Cardiol. 2020;5(7):743. [DOI] [PubMed] [Google Scholar]
- 111.Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Honce R, Schultz-Cherry S.. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol. 2019;10:1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gleeson M, Bishop NC, Stensel DJ, et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. [DOI] [PubMed] [Google Scholar]
- 114.Luzi L, Radaelli MG.. Influenza and obesity: its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020;57(6):759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maier HE, Lopez R, Sanchez N, et al. Obesity increases the duration of influenza A virus shedding in adults. J Infect Dis. 2018;218(9):1378–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schultz-Cherry S. Beyond disease severity: the impact of obesity on influenza A virus shedding. J Infect Dis. 2018;218(9):1354–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Honce R, Karlsson EA, Wohlgemuth N, et al. Obesity-related microenvironment promotes emergence of virulent influenza virus strains. mBio. 2020;11(2):e03341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dietz W, Santos-Burgoa C.. Obesity and its implications for COVID-19 mortality. Obesity (Silver Spring). 2020;28(6):1005–1005. [DOI] [PubMed] [Google Scholar]
- 119.Lockhart SM, O’Rahilly S.. When two pandemics meet: why is obesity associated with increased COVID-19 mortality? Med. 2020. DOI: 10.1016/j.medj.2020.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shaw AC, Goldstein DR, Montgomery RR.. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ventura MT, Casciaro M, Gangemi S, et al. Immunosenescence in aging: between immune cells depletion and cytokines up-regulation. Clin Mol Allergy. 2017;15:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Channappanavar R, Fehr Anthony R, Vijay R, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Channappanavar R, Perlman S.. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Donato AJ, Machin DR, Lesniewski LA.. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ Res. 2018;123(7):825–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Teuwen L-A, Geldhof V, Pasut A, et al. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):448–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaur S, Tripathi DM, Yadav A.. The enigma of endothelium in COVID-19. Front Physiol. 2020;11:989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pons S, Fodil S, Azoulay E, et al. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS-CoV-2 infection. Crit Care. 2020;24(1):353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gustafson D, Raju S, Wu R, et al. Overcoming barriers: the endothelium as a linchpin of coronavirus disease 2019 pathogenesis? Arterioscler Thromb Vasc Biol. 2020;40(8):1818–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guervilly C, Burtey S, Sabatier F, et al. Circulating endothelial cells as a marker of endothelial injury in severe COVID -19. Int J Infect Dis. 2020;11:1789–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Connors JM, Levy JH.. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Abou-Ismail MY, Diamond A, Kapoor S, et al. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McFadyen JD, Stevens H, Peter K.. The emerging threat of (micro)thrombosis in COVID-19 and its therapeutic implications. Circ Res. 2020;127(4):571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grimmer B, Kuebler WM.. The endothelium in hypoxic pulmonary vasoconstriction. J Appl Physiol (1985). 2017;123(6):1635–1646. [DOI] [PubMed] [Google Scholar]
- 137.Gattinoni L, Coppola S, Cressoni M, et al. COVID-19 does not lead to a "typical" acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jain A, Doyle DJ.. Stages or phenotypes? A critical look at COVID-19 pathophysiology. Intensive Care Med. 2020;46(7):1494–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dhont S, Derom E, Van Braeckel E, et al. The pathophysiology of 'happy' hypoxemia in COVID-19. Respir Res. 2020;21(1):198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pober JS, Sessa WC.. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7(10):803–815. [DOI] [PubMed] [Google Scholar]
- 141.AlGhatrif M, Cingolani O, Lakatta EG.. The dilemma of coronavirus disease 2019, aging, and cardiovascular disease: insights from cardiovascular aging science. JAMA Cardiol. 2020;5(7):747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bonow RO, Fonarow GC, O’Gara PT, et al. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5(7):751. [DOI] [PubMed] [Google Scholar]
- 143.Zheng Y-Y, Ma Y-T, Zhang J-Y, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Atri D, Siddiqi HK, Lang JP, et al. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. JACC Basic Transl Sci. 2020;5(5):518–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tersalvi G, Vicenzi M, Calabretta D, et al. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J Card Fail. 2020;26(6):470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cunha LL, Perazzio SF, Azzi J, et al. Remodeling of the immune response with aging: immunosenescence and its potential impact on COVID-19 immune response. Front Immunol. 2020;11:1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Konno Y, Kimura I, Uriu K, et al. SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32(12):108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lowenstein CJ, Solomon SD.. Severe COVID-19 is a microvascular disease. Circulation. 2020;142(17):1609–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Organisation WH . R&D blueprint and COVID-19; 2020. [cited 2020 Oct 10]. Available from: https://www.who.int/teams/blueprint/covid-19.
- 150.Clinical trials on COVID-19. Available from: https://clinicaltrials.gov/ct2/results?type=Intr&cond=COVID-19.
- 151.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fan E, Beitler JR, Brochard L, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8(8):816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yancy CW, Fonarow GC.. Coronavirus disease 2019 (COVID-19) and the heart—is heart failure the next chapter? JAMA Cardiol. 2020. DOI: 10.1001/jamacardio.2020.3575 [DOI] [PubMed] [Google Scholar]
- 154.Gevaert AB, Lemmens K, Vrints CJ, et al. Targeting endothelial function to treat heart failure with preserved ejection fraction: the promise of exercise training. Oxid Med Cell Longev. 2017;2017:4865756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Matthay MA, , Zemans RL, , Zimmerman GA, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]