Abstract
Second-generation antipsychotic–related weight gain and metabolic disturbances are a major public health issue given the widespread prescribing of these medications. The lack of clearly known mechanisms of cardiometabolic adverse effects and the relevance of cardiometabolic health for survival make this an important area for research. While nonpharmacologic and some pharmacologic treatments have shown benefits vs control conditions or placebo, the effects are modest and long-term benefits are less clear. Therefore, new approaches to mitigate second-generation antipsychotic–associated cardiometabolic burden are sorely needed.
Keywords: Tat-3L4F, second-generation antipsychotics, cardiometabolic risk, gut microbiota
Antipsychotic Treatment and Cardiometabolic Risk
The use of the second-generation antipsychotics (SGAs) carries significant risk of cardiovascular disease (CVD) not only by association but also causation (García-Tornadú et al., 2010; Scigliano and Ronchetti, 2013). SGA use has been associated with weight gain, dyslipidemia, and type 2 diabetes (Maayan and Correll, 2011; De Hert et al., 2012). Their negative cardiometabolic impact is of major concern in light of increased worldwide off-label use in the last decades (Correll et al., 2011; Hirsch and Pringsheim, 2016). CVD significantly affects severe mentally ill (SMI) (De Hert et al., 2018; Taipale et al., 2018) and thus their overall quality of life (Reininghaus et al., 2015; Hayes et al., 2017).
The mechanism via which SGAs induce adverse effects is complex and poorly understood, however intensively studied (De Hert et al., 2012). Glucometabolic side effects may directly induce insulin resistance acting on insulin-dependent organs and hypothalamic-liver circuit and dysregulate hypothalamic food intake centers, resulting in increased energy intake and adiposity (Ballon et al., 2014). SGA-induced obesity elevates the concentration of free fatty acids and inflammatory markers, both diminishing the insulin sensitivity (Chen et al., 2017). Moreover, SGAs may negatively disrupt gut microbiota (Skonieczna-Żydecka et al., 2019), conferring disadvantages to the host metabolism (Heiss and Olofsson, 2018).
Main Strategies to Diminish Sga Side Effects Include Both Pharmacological and Lifestyle Interventions
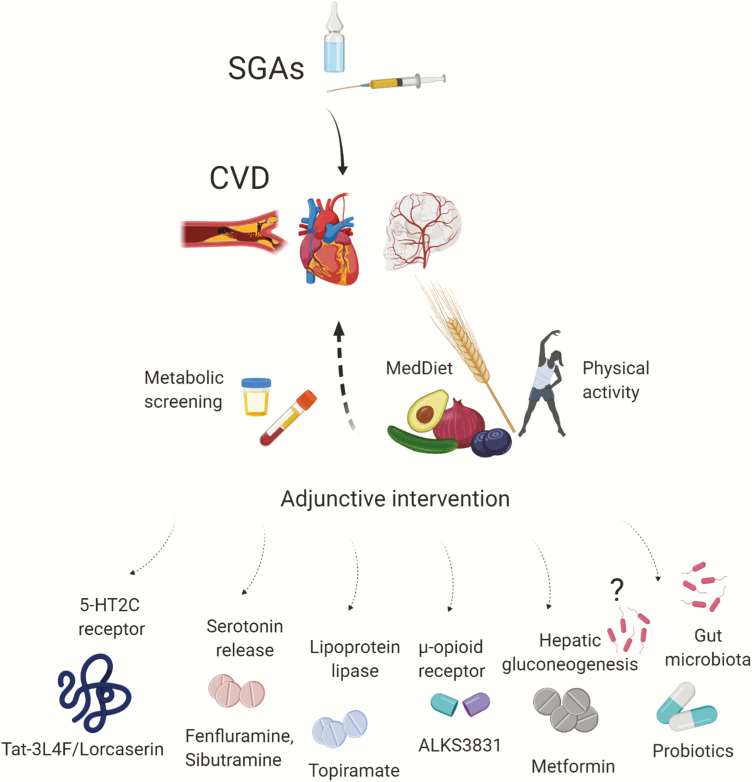
Every patient (and her or his family physician), shortly after SGA initiation, should be informed about the risk of cardiovascular adverse effects but also receive means for intervention to counteract potential side effects, even in case of unclear efficacy (Figure 1; Stroup and Gray, 2018). The SMI population requiring SGAs should be regularly assessed for diabetes, body mass index (BMI), waist circumference, fasting glucose (or hemoglobin A1C) and lipid levels, and blood pressure. However, monitoring rates in clinical practices have been suboptimal but stable over time (Mitchell et al., 2012).
Figure 1.
Potential add-on interventions to counteract second-generation antipsychotic (SGA)-induced metabolic dysregulation.
Lifestyle Interventions
Lifestyle interventions should be initiated before pharmacological treatments and, if insufficient alone, be continued together (Cooper et al., 2016). As meta-analyzed by Speyer et al. (Speyer et al., 2019), a group of 4267 psychotic patients benefited statistically from physical activity and dietary approaches supported by behavioral counselling. The overall effect size on BMI was −0.63 (95% confidence interval [CI] = −1.02,−0.23; P = .002) with identical but nonsignificant effect size of −0.63 at follow up. The chance to decrease weight by at least 5% of initial body weight increased by one-half (relative risk [RR] = 1.51, 95% CI = 1.07, 2.13; P = .02). However, given the high degree of obesity in the SMI, the overall effects were interpreted as clinically insufficient (Speyer et al., 2019). The Mediterranean diet is recommended in lipid disorders (Bagetta et al., 2020). It includes fiber source and the reduction in the intake of proinflammatory nutrients, such as trans and saturated fatty acids (Ruiz-Núñez et al., 2016) or simple sugars, especially fructose, which enhances intrahepatic fat synthesis and insulin resistance (Rippe and Angelopoulos, 2016). The desired goal is to lose about 5% to 10% of the initial body weight over a 6-month period, which can increase insulin sensitivity by 30% to 60% (Hoyas and Leon-Sanz, 2019). The Mediterranean diet lifestyle includes moderate physical activity (Alvarez-Alvarez et al., 2018). In a recent study, 4 trainings per week, 45 minutes each, improved the cardiometabolic fitness in schizophrenia patients (Curcic et al., 2017). As recommended by the European Psychiatric Association (Stubbs et al., 2018), both aerobic and anaerobic activities (heart rate ≤85% of maximum) should be advised.
Pharmacological Interventions
To ameliorate SGA-induced cardiometabolic impairments, lowering the dose and switching to a lower risk antipsychotic can be used (Keks et al., 2019). The latter might significantly decrease BMI (z-score change from baseline: –0.11 ± 0.04, P = .001) compared with controls continuing a baseline SGA regimen (Correll et al., 2020). Furthermore, implementing adjunctive pharmacologic agents is frequently utilized, although, overall, such trials have had modest success (Vancampfort et al., 2019). To counteract SGA-induced weight changes, metformin has been the most extensively studied adjunctive medication inhibiting hepatic gluconeogenesis and thus improving the sensitivity of insulin in skeletal muscles (Zheng et al., 2017). The drug was demonstrated to impact the BMI z-score change equal to –0.09 ± 0.03 (P = .002) compared with a control group of patients with no add-on drug (Correll et al., 2020).
Other effective options for weight loss include the choice of fenfluramine, sibutramine, topiramate, and reboxetine (Mizuno et al., 2014; Correll et al., 2016) acting as appetite inhibitors via the enhancement of serotonin release stimulation of lipoprotein lipase and inhibition of norepinephrine reuptake, respectively (Dayabandara et al., 2017). However, these pharmacologic interventions may trigger adverse effects, such as lactic acidosis or gastrointestinal complaints in metformin therapy or cognitive blunting during topiramate use (Maayan and Correll, 2011). Among a dozen new drugs that are currently being studied to treat schizophrenia (Krogmann et al., 2019), ALKS3831, the combination of olanzapine plus samidorphan, a μ-opioid antagonist, has been found to significantly reduce the weight gain associated with olanzapine (Martin et al., 2019), which is one of the most weight gain–producing antipsychotics. ALKS-3831 is currently under review with the Food and Drug Administration.
Tat-3L4f: The New Kid on the Block to Counteract Sga-Associated Risk of Cvd
In the present issue of IJNP, Wang et al. (2020) described a new intervention with a molecule, Tat-3L4F, whose mode of action includes the agonism-like (allosteric) effects on the serotonin 5-HT2C receptor, which has previously been linked to alleviation of weight gain and hyperphagia (Lord et al., 2017). Interestingly, the suppression of weight gain and appetite together with marked differences in metabolic profile (e.g., increased bile synthesis) came along with phosphatase tensin homolog (PTEN) downregulation in the hypothalamus, which was shown to counteract increased appetite (Sumita et al., 2014). The use of PTEN resulted in reduced body weight, food intake, and blood glucose and lipids in tested rodents. This study is promising due to the unrestricted dopamine influx, as reduced dopamine tone can contribute to cognitive deficits and negative symptoms in mentally ill patients during antidopaminergic therapy (Sullivan et al., 2015). Nevertheless, more studies should elucidate the mechanism of action, potential adverse effects, and clinical efficacy of PTEN. To date, lorcarserin, another 5-HT2C receptor agonist, has been successfully introduced into clinical care. A meta-analysis (Kuo et al., 2020) including 9452 patients indicated that lorcarserin was superior vs placebo, significantly improving anthropometric (weight, BMI, waist circumference), carbohydrate (fasting glucose, HbA1C), and lipid (low-density lipoprotein cholesterol, triacylglycerols) indices along with blood pressure and heart rate. However, long-term stability of these important outcomes is still to be explored, especially regarding other drugs modulating the serotonergic pathway (Singh and Singh, 2020).
Gut Microbiota–Focused Therapy: New Frontier in the Old Battlefield?
An emerging concept within the interventions aimed at improving cardiometabolic health in the SMI has focused on gut microbiota, stimulated by SGA antimicrobial activity, resembling antibiotics (Kristiansen, 1979; Thorsing et al., 2013). The microbial alterations have been linked to cardiometabolic risk (Heiss and Olofsson, 2018; Omer and Atassi, 2017), and SGA-induced changes within gut microbiota could potentially contribute to metabolic adverse events in psychiatric patients (Kanji et al., 2018). As systematically reviewed by us, SGAs were demonstrated to elevate Firmicutes phylum abundance relative to Bacteroidetes, a pattern that is typical for obesity and metabolic malfunctions (Abenavoli et al., 2019). Olanzapine was found to induce adiposity and had an unfavorable impact on serotonin receptors expression, short chain fatty acid synthesis, and inflammation, while risperidone suppressed resting metabolic rate (Skonieczna-Żydecka et al., 2019). Moreover, a favorable effect of prebiotic and probiotic administration on the risk of metabolic and weight disturbances has been reported (Kao et al., 2018; Szulińska et al., 2018). We suggest considering co-administration of pro-/pre-/synbiotic therapy concomitant to SGAs. Based on the emerging data, adding gut microbiome–stabilizing treatment might positively affect host metabolism modulating of (1) fasting glucose, (2) glycated haemoglobin, (3) dyslipidemia, and (4) hypertension in overweight individuals. Target probiotic strains including Collinsella aerofaciens were found to be increased in children treated with risperidone but who did not experience weight gain (Bahr et al., 2015). Potential next-generation probiotics include Akkermansia, Bacteroides spp., and Eubacterium halli as well as bacterial structural elements (cell wall proteins) and metabolites (short chain fatty acids) (Romaní-Pérez et al., 2017). Moreover, adjunctive metformin was found to modulate microbiota, resulting in improved propionate and butyrate production and thus glucose homeostasis (Forslund et al., 2015), and to decrease low-grade inflammation in obese individuals (Zhou et al., 2017). In mice receiving metformin, adipose tissue macrophages were found to be polarized to an antiinflammatory M2 phenotype subsequent to AMPK activation (Jing et al., 2018), supporting the hypothesis that inflammation may be crucial for weight gain secondary to SGAs. Metformin was capable of modulating low-grade inflammation in obese animals (Lee et al., 2018). Furthermore, fecal transplants from metformin-treated mice significantly improved metabolic parameters in treated donors. Other agents (e.g., the GLP-1 agonist-liraglutide) not only decreased fasting blood glucose, waist circumference, and HbA1C in schizophrenia patients (Larsen et al., 2017; Khera et al., 2018; Siskind et al., 2019) but also affected gut Akkermansia in schizophrenia patients with elevated body mass due to clozapine and olanzapine therapy (Wang et al., 2017). Moreover, dysbiosis is associated with GLP-1 resistance responsible for glucose intolerance and impairment of the brain-gut axis function (Yamane and Inagaki, 2018). Nevertheless, more research is needed to prove the efficacy of microbiome-based therapies in the clinic.
Summary and Conclusions
As the number of SGA prescriptions increases, the SMI population will experience more cardiometabolic effects. Thus, new approaches to mitigate this burden are sorely needed.
Statement of Interest
Christoph U. Correll received speaker’s fees, consultancy honoraria, equity ownership, profit‐sharing agreements, royalties, patents, and research grants from industry for which he received compensation for professional services in any of the previous 3 years or from whom he anticipates receiving such compensation in the near future.
References
- Abenavoli L, Scarpellini E, Colica C, Boccuto L, Salehi B, Sharifi-Rad J, Aiello V, Romano B, De Lorenzo A, Izzo AA, Capasso R (2019) Gut microbiota and obesity: a role for probiotics. Nutrients 11:2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Alvarez I, Zazpe I, Pérez de Rojas J, Bes-Rastrollo M, Ruiz-Canela M, Fernandez-Montero A, Hidalgo-Santamaría M, Martínez-González MA (2018) Mediterranean diet, physical activity and their combined effect on all-cause mortality: The Seguimiento Universidad de Navarra (SUN) cohort. Prev Med 106:45–52. [DOI] [PubMed] [Google Scholar]
- Bagetta D, Maruca A, Lupia A, Mesiti F, Catalano R, Romeo I, Moraca F, Ambrosio FA, Costa G, Artese A, Ortuso F, Alcaro S, Rocca R (2020) Mediterranean products as promising source of multi-target agents in the treatment of metabolic syndrome. Eur J Med Chem 186:111903. [DOI] [PubMed] [Google Scholar]
- Bahr SM, Tyler BC, Wooldridge N, Butcher BD, Burns TL, Teesch LM, Oltman CL, Azcarate-Peril MA, Kirby JR, Calarge CA (2015) Use of the second-generation antipsychotic, risperidone, and secondary weight gain are associated with an altered gut microbiota in children. Transl Psychiatry 5:e652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballon JS, Pajvani U, Freyberg Z, Leibel RL, Lieberman JA (2014) Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol Metab 25:593–600. [DOI] [PubMed] [Google Scholar]
- Chen J, Huang XF, Shao R, Chen C, Deng C (2017) Molecular mechanisms of antipsychotic drug-induced diabetes. Front Neurosci 11:643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SJ, Reynolds GP, Barnes T, England E, Haddad PM, Heald A, Holt R, Lingford-Hughes A, Osborn D, McGowan O, Patel MX, Paton C, Reid P, Shiers D, Smith J; and co-authors. (2016) BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J Psychopharmacol 30:717–748. [DOI] [PubMed] [Google Scholar]
- Correll CU, Kratochvil CJ, March JS (2011) Developments in pediatric psychopharmacology: focus on stimulants, antidepressants, and antipsychotics. J Clin Psychiatry 72:655–670. [DOI] [PubMed] [Google Scholar]
- Correll CU, Maayan L, Kane J, Hert MD, Cohen D (2016) Efficacy for psychopathology and body weight and safety of topiramate-antipsychotic cotreatment in patients with schizophrenia spectrum disorders: results from a meta-analysis of randomized controlled trials. J Clin Psychiatry 77:e746–e756. [DOI] [PubMed] [Google Scholar]
- Correll CU, Sikich L, Reeves G, Johnson J, Keeton C, Spanos M, Kapoor S, Bussell K, Miller L, Chandrasekhar T, Sheridan EM, Pirmohamed S, Reinblatt SP, Alderman C, Scheer A, Borner I, Bethea TC, Edwards S, Hamer RM, Riddle MA (2020) Metformin add-on vs. antipsychotic switch vs. continued antipsychotic treatment plus healthy lifestyle education in overweight or obese youth with severe mental illness: results from the IMPACT trial. World Psychiatry 19:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcic D, Stojmenovic T, Djukic-Dejanovic S, Dikic N, Vesic-Vukasinovic M, Radivojevic N, Andjelkovic M, Borovcanin M, Djokic G (2017) Positive impact of prescribed physical activity on symptoms of schizophrenia: randomized clinical trial. Psychiatr Danub 29:459–465. [DOI] [PubMed] [Google Scholar]
- Dayabandara M, Hanwella R, Ratnatunga S, Seneviratne S, Suraweera C, de Silva VA (2017) Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat 13:2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M, Detraux J, van Winkel R, Yu W, Correll CU (2012) Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 8:114–126. [DOI] [PubMed] [Google Scholar]
- De Hert M, Detraux J, Vancampfort D (2018) The intriguing relationship between coronary heart disease and mental disorders. Dialogues Clin Neurosci 20:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslund K, et al. ; MetaHIT Consortium (2015) Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Tornadú I, Ornstein AM, Chamson-Reig A, Wheeler MB, Hill DJ, Arany E, Rubinstein M, Becu-Villalobos D (2010) Disruption of the dopamine d2 receptor impairs insulin secretion and causes glucose intolerance. Endocrinology 151:1441–1450. [DOI] [PubMed] [Google Scholar]
- Hayes JF, Marston L, Walters K, King MB, Osborn DPJ (2017) Mortality gap for people with bipolar disorder and schizophrenia: UK-based cohort study 2000–2014. Br J Psychiatry 211:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss CN, Olofsson LE (2018) Gut microbiota-dependent modulation of energy metabolism. J Innate Immun 10:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch LE, Pringsheim T (2016) Aripiprazole for autism spectrum disorders (ASD). Cochrane Database of Syst Rev Available at: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD009043.pub3/full. Accessed March 6, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyas I, Leon-Sanz M (2019) Nutritional challenges in metabolic syndrome. J Clin Med 8:1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y, Wu F, Li D, Yang L, Li Q, Li R (2018) Metformin improves obesity-associated inflammation by altering macrophages polarization. Mol Cell Endocrinol 461:256–264. [DOI] [PubMed] [Google Scholar]
- Kanji S, Fonseka TM, Marshe VS, Sriretnakumar V, Hahn MK, Müller DJ (2018) The microbiome-gut-brain axis: implications for schizophrenia and antipsychotic induced weight gain. Eur Arch Psychiatry Clin Neurosci 268:3–15. [DOI] [PubMed] [Google Scholar]
- Kao AC-C, Spitzer S, Anthony DC, Lennox B, Burnet PWJ (2018) Prebiotic attenuation of olanzapine-induced weight gain in rats: analysis of central and peripheral biomarkers and gut microbiota. Transl Psychiatry 8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keks N, Schwartz D, Hope J (2019) Stopping and switching antipsychotic drugs. Aust Prescr 42:152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khera R, Pandey A, Chandar AK, Murad MH, Prokop LJ, Neeland IJ, Berry JD, Camilleri M, Singh S (2018) Effects of weight-loss medications on cardiometabolic risk profiles: a systematic review and network meta-analysis. Gastroenterology 154:1309–1319.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen JE. (1979) Experiments to illustrate the effect of chlorpromazine on the permeability of the bacterial cell wall. Acta Pathol Microbiol Scand B 87:317–319. [DOI] [PubMed] [Google Scholar]
- Krogmann A, Peters L, von Hardenberg L, Bödeker K, Nöhles VB, Correll CU (2019) Keeping up with the therapeutic advances in schizophrenia: a review of novel and emerging pharmacological entities. CNS Spectr 24:38–69. [DOI] [PubMed] [Google Scholar]
- Kuo HH, Wang KT, Lee YH, Lin PL, Liu ME, Lin CY, Liu LY (2020) Effects of lorcaserin on cardiometabolic risk factors in overweight and obese patients: a systematic review and meta-analysis. J Clin Pharm Ther 45:35–44. [DOI] [PubMed] [Google Scholar]
- Larsen JR, Vedtofte L, Jakobsen MSL, Jespersen HR, Jakobsen MI, Svensson CK, Koyuncu K, Schjerning O, Oturai PS, Kjaer A, Nielsen J, Holst JJ, Ekstrøm CT, Correll CU, Vilsbøll T, Fink-Jensen A (2017) Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry 74:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Lee Y, Kim J, An J, Lee S, Kong H, Song Y, Lee C-K, Kim K (2018) Modulation of the gut microbiota by metformin improves metabolic profiles in aged obese mice. Gut Microbes 9:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord CC, Wyler SC, Wan R, Castorena CM, Ahmed N, Mathew D, Lee S, Liu C, Elmquist JK (2017) The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor 2C. J Clin Invest 127:3402–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan L, Correll CU (2011) Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. J Child Adolesc Psychopharmacol 21:517–535. [DOI] [PubMed] [Google Scholar]
- Martin WF, Correll CU, Weiden PJ, Jiang Y, Pathak S, DiPetrillo L, Silverman BL, Ehrich EW (2019) Mitigation of olanzapine-induced weight gain with samidorphan, an opioid antagonist: a randomized double-blind phase 2 study in patients with schizophrenia. Am J Psychiatry 176:457–467. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Delaffon V, Vancampfort D, Correll CU, De Hert M (2012) Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med 42:125–147. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Suzuki T, Nakagawa A, Yoshida K, Mimura M, Fleischhacker WW, Uchida H (2014) Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull 40:1385–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer E, Atassi H (2017) The microbiome that shapes us: can it cause obesity? Curr Gastroenterol Rep 19:59. [DOI] [PubMed] [Google Scholar]
- Reininghaus U, Dutta R, Dazzan P, Doody GA, Fearon P, Lappin J, Heslin M, Onyejiaka A, Donoghue K, Lomas B, Kirkbride JB, Murray RM, Croudace T, Morgan C, Jones PB (2015) Mortality in schizophrenia and other psychoses: a 10-year follow-up of the ӔSOP first-episode cohort. Schizophr Bull 41:664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe JM, Angelopoulos TJ (2016) Relationship between added sugars consumption and chronic disease risk factors: current understanding. Nutrients 8:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaní-Pérez M, Agusti A, Sanz Y (2017) Innovation in microbiome-based strategies for promoting metabolic health. Curr Opin Clin Nutr Metab Care 20:484–491. [DOI] [PubMed] [Google Scholar]
- Ruiz-Núñez B, Dijck-Brouwer DAJ, Muskiet FAJ (2016) The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J Nutr Biochem 36:1–20. [DOI] [PubMed] [Google Scholar]
- Scigliano G, Ronchetti G (2013) Antipsychotic-induced metabolic and cardiovascular side effects in schizophrenia: a novel mechanistic hypothesis. CNS Drugs 27:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Singh R (2020) Efficacy and safety of lorcaserin in obesity: a systematic review and meta-analysis of randomized controlled trials. Expert Rev Clin Pharmacol 13:183–190. [DOI] [PubMed] [Google Scholar]
- Siskind D, Hahn M, Correll CU, Fink-Jensen A, Russell AW, Bak N, Broberg BV, Larsen J, Ishøy PL, Vilsbøll T, Knop FK, Kisely S, Ebdrup BH (2019) Glucagon-like peptide-1 receptor agonists for antipsychotic-associated cardio-metabolic risk factors: a systematic review and individual participant data meta-analysis. Diabetes Obes Metab 21:293–302. [DOI] [PubMed] [Google Scholar]
- Skonieczna-Żydecka K, Łoniewski I, Misera A, Stachowska E, Maciejewska D, Marlicz W, Galling B (2019) Second-generation antipsychotics and metabolism alterations: a systematic review of the role of the gut microbiome. Psychopharmacology (Berl) 236:1491–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speyer H, Jakobsen AS, Westergaard C, Nørgaard HCB, Pisinger C, Krogh J, Hjorthøj C, Nordentoft M, Gluud C, Correll CU, Jørgensen KB (2019) Lifestyle interventions for weight management in people with serious mental illness: a systematic review with meta-analysis, trial sequential analysis, and meta-regression analysis exploring the mediators and moderators of treatment effects. Psychother Psychosom 88:350–362. [DOI] [PubMed] [Google Scholar]
- Stroup TS, Gray N (2018) Management of common adverse effects of antipsychotic medications. World Psychiatry 17:341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs B, Vancampfort D, Hallgren M, Firth J, Veronese N, Solmi M, Brand S, Cordes J, Malchow B, Gerber M, Schmitt A, Correll CU, De Hert M, Gaughran F, Schneider F, Kinnafick F, Falkai P, Möller HJ, Kahl KG (2018) EPA guidance on physical activity as a treatment for severe mental illness: a meta-review of the evidence and Position Statement from the European Psychiatric Association (EPA), supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur Psychiatry 54:124–144. [DOI] [PubMed] [Google Scholar]
- Sullivan LC, Clarke WP, Berg KA (2015) Atypical antipsychotics and inverse agonism at 5-HT2 receptors. Curr Pharm Des 21:3732–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumita T, Ono H, Suzuki T, Sakai G, Inukai K, Katagiri H, Asano T, Katayama S, Awata T (2014) Mediobasal hypothalamic PTEN modulates hepatic insulin resistance independently of food intake in rats. Am J Physiol Endocrinol Metab 307:E47–E60. [DOI] [PubMed] [Google Scholar]
- Szulińska M, Łoniewski I, van Hemert S, Sobieska M, Bogdański P (2018) Dose-dependent effects of multispecies probiotic supplementation on the lipopolysaccharide (LPS) level and cardiometabolic profile in obese postmenopausal women: a 12-week randomized clinical trial. Nutrients 10:773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale H, Mittendorfer-Rutz E, Alexanderson K, Majak M, Mehtälä J, Hoti F, Jedenius E, Enkusson D, Leval A, Sermon J, Tanskanen A, Tiihonen J (2018) Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res 197:274–280. [DOI] [PubMed] [Google Scholar]
- Thorsing M, Klitgaard JK, Atilano ML, Skov MN, Kolmos HJ, Filipe SR, Kallipolitis BH (2013) Thioridazine induces major changes in global gene expression and cell wall composition in methicillin-resistant Staphylococcus aureus USA300. PLoS One 8:e64518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancampfort D, Firth J, Correll CU, Solmi M, Siskind D, De Hert M, Carney R, Koyanagi A, Carvalho AF, Gaughran F, Stubbs B (2019) The impact of pharmacological and non-pharmacological interventions to improve physical health outcomes in people with schizophrenia: a meta-review of meta-analyses of randomized controlled trials. World Psychiatry 18:53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Saha S, Van Horn S, Thomas E, Traini C, Sathe G, Rajpal DK, Brown JR (2017) Gut microbiome differences between metformin‐ and liraglutide‐treated T2DM subjects. Endocrinol Diabetes Metab 1:e00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang D, Chen Y, Fang X, Yu L, Zhang C (2020) A novel synthetic interfering peptide Tat-3L4F attenuates olanzapine-induced weight gain through disrupting crosstalk between serotonin receptor 2C and protein phosphatase and tensin homolog in rats. Int J Neuropsychoph 23:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane S, Inagaki N (2018) Regulation of glucagon-like peptide-1 sensitivity by gut microbiota dysbiosis. J Diabetes Investig 9:262–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Yang XH, Cai DB, Ungvari GS, Ng CH, Wang N, Ning YP, Xiang YT (2017) Adjunctive metformin for antipsychotic-related hyperprolactinemia: a meta-analysis of randomized controlled trials. J Psychopharmacol 31:625–631. [DOI] [PubMed] [Google Scholar]
- Zhou L, Cai X, Yang W, Han X, Ji L (2017) The magnitude of weight loss induced by metformin is independently associated with BMI at baseline in newly diagnosed type 2 diabetes: post-hoc analysis from data of a phase IV open-labeled trial. Adv Clin Exp Med 26:671–677. [DOI] [PubMed] [Google Scholar]