Abstract
Background
The objective of our study was to assess the analytical performance of a multiplex assay (Oncuria™) to quantify protein biomarkers towards a bladder cancer associated diagnostic signature in voided urine.
Method
ology: Using Luminex xMAP technology, a custom immunoassay was developed to measure the concentrations of 10 urinary analytes (angiogenin, ANG; apolipoprotein E, APOE; alpha-1 antitrypsin, A1AT; carbonic anhydrase 9, CA9; interleukin 8, IL8; matrix metallopeptidase 9, MMP9; matrix metallopeptidase 10, MMP10; plasminogen activator inhibitor 1, PAI1; syndecan 1, SDC1; vascular endothelial growth factor, VEGF). Selectivity, sensitivity, specificity, precision, linearity, dynamic range, and detection threshold were assessed using recombinant proteins and human urine samples. Analytical variability with respect to batch size, run, day, operator, and interference were also evaluated.
Results
Analytical evaluation demonstrated a) all antigen cross-reactivity was noted to be <1% of the tested concentration, b) minimal detected dose ranged from 0.295 pg/mL in IL8 to 31.1 pg/mL in APOE, c) highly reproducible and accurate noting coefficient of variation (CV) and relative error (RE) values below 15% for all analytes and d) minimal interference. The assay can be completed in <5 h using as little as 150 μL of voided urine.
Conclusion
To our knowledge, this is the first multiplex bead-based immunoassay for the non-invasive detection of bladder cancer that has been analytically validated as a tool with the potential to help clinicians manage patients at risk of harboring bladder cancer.
Highlights
-
•
Cytology has changed little since its inception in 1947 and suffers from low detection sensitivity for bladder cancer.
-
•
Oncuria™ is a multiplex bead-based immunoassay.
-
•
Oncuria™ has limited antigen cross-reactivity and interference and favorable detected dose and reproducibility.
-
•
Oncuria ™, an accurate multiplex detection assay for bladder cancer, can be performed on <200 ul of urine in <5 hrs.
1. Introduction
Currently, voided urinary cytology (VUC), which has not changed in 70 years since its inception in 1947 [1], is still considered the gold-standard for the non-invasive detection of bladder cancer (BCa). Cancer of the urinary bladder has two unique features: 1) the tumor is continuously ‘bathed’ in a urine and 2) the tumor is able to shed cells or release tumor associated products (e.g., protein, DNA, RNA) directly into urine, which is easily collected and assessed. Though urine is largely considered a harsh environment with varying pH and high salt levels, products associated with bladder tumors within the urine have been shown to correlate with their presence within the actual bladder tumor. Understandably, a good deal of research has focused on identifying urine-based tumor biomarkers with a potential benefit over VUC. Promising diagnostic biomarkers included NMP-22 (nuclear matrix protein 22) and BTA (bladder tumor antigen). Unfortunately, these tests suffer from high false-positive rates. Thus, no single marker has sufficient predictive power to be applied to the management of individual patients [2,3].
With this in mind, we have developed a multiplex bead-based immunoassay that simultaneously monitors the absolute concentrations of 10 analytes (angiogenin, ANG; apolipoprotein E, APOE; alpha-1 antitrypsin, A1AT; carbonic anhydrase 9, CA9; interleukin 8, IL8; matrix metallopeptidase 9, MMP9; matrix metallopeptidase 10, MMP10; plasminogen activator inhibitor 1, PAI1; syndecan 1, SDC1; vascular endothelial growth factor, VEGF). Reflecting the abundance of these proteins in voided urine from patients with BCa. In addition, the concentration of each of the 10 analytes is incorporated into a weighted algorithm, which is used to generate a risk score. To date, we have validated this signature in over 2100 individuals [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]]. As this population grows with the accrual of additional patients, data interpretation and detection power will increase, thereby rendering the application more robust with the ability to support clinical decision making in the context of a) evaluating patients with hematuria and b) evaluating patients with a history of BCa on tumor surveillance. To meet clinical requirements, we have previously demonstrated that these proteins are present and stable in voided urine and thus are an adequate starting material for the assay [[4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17]].
To further demonstrate the utility of this multiplex bead-based immunoassay in clinical settings, a strict development, optimization, and validation strategy was implemented at Nonagen Bioscience Corporation in collaboration with Bio-Techne (Minneapolis, MN), which could be performed in a clinical molecular pathology laboratory certified by Clinical Laboratory Improvement Amendments (CLIA). The main objectives of this strategy were to optimize the components of the assay into an integrated workflow and to develop a standard operating protocol of sample collection, processing, assay run, quality assurance, quality control, and data generation. Multiple sets of actual samples were used to validate the robustness of the multiplex bead-based immunoassay (Oncuria™) and to generate appropriate run controls to routinely monitor assay performance. The validation specifically addressed analytical variables (i.e., assay-related) such as calibration curves, selectivity, specificity, sensitivity, accuracy, intra-assay precision, inter-assay precision, linearity, recovery and stability. Here, we provide an overview of the analytical performance of the multiplex bead-based immunoassay.
2. Materials and methods
Reagents and assay. Previously, we reported on a prototype of a multiplex, Magnetic Luminex® Performance Assay designed to detect the 10 protein analytes associated with the urine-based BCa associated signature (Alpha 1 anti-trypsin = A1AT, Apolipoprotein E = APOE, Angiogenin = ANG, Carbonic Anhydrase 9 = CA9, Interleukin 8 = IL8, Matrix metalloproteinase 9 = MMP9, Matrix metalloproteinase 10 = MMP10, Plasminogen Activator Inhibitor 1 = PAI1, Syndecan 1 = SDC1 and Vascular Epithelial Growth Factor = VEGF) using the Luminex® MAGPIX® CCD Imager, Luminex® 100/200TM or Bio-Rad® Bio-Plex®, dual laser, flow-based sorting and detection platforms [4]. The final monoclonal antibody pairs (capture and detection) for the multiplex assay were selected based on sensitivity, specificity, physical properties, and recognition of native proteins. Subsequently, a panel of diluents, dilution concentrations and physical conditions of the multiplex assay were reviewed and optimized. The optimized assay is the subject of the current analytical studies.
Standards and Controls. Diluent RD2-1 along with the following calibrator diluents; RD6-62 and RD5-10 (R&D Systems Inc, Minnepolis, MN) were incorporated into the assay. Recombinant proteins of each of the 10 analytes were available and included in the multiplex assay as standards, though this had some constraints. Each standard was used to generate a calibration curve (Supplemental Fig. 1). A cocktail comprised of the 10 proteins was generated and is referred to as the master calibrator. Furthermore, low, medium and high concentrations for each protein were incorporated into a control cocktail (low, mid and high controls). Negative control was only the diluent without the addition of the recombinant proteins. For each assay run, the standards and controls were run in duplicate. Specifically, three unique standard vials and six unique control vials (low, medium and high) were analyzed across three operators for each of the 10 proteins. Average (minimal and maximal) analyte levels are reported along with coefficient of variation (%CV) for the different levels of the measured condition.
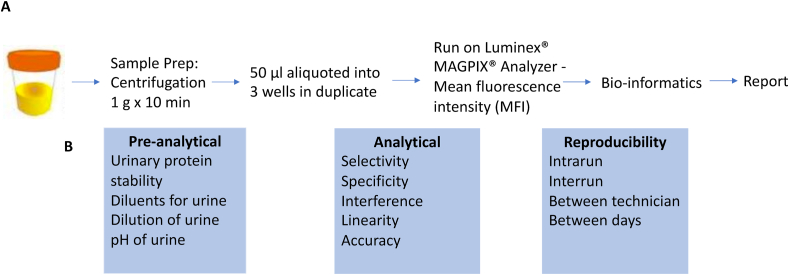
Assay. Microparticle cocktail, consisting of 10 protein-specific antibodies (capture) pre-coated onto color-coded magnetic microparticles (Luminex Corp) and biotinylated antibody cocktail, consisting of 10 protein-specific biotinylated antibodies (detection) were available. Briefly, microparticles (50 μl) along with standard, controls or samples (50 μl) were pipetted into wells within a 96-well plate and incubated for 2 h at room temperature. Using a magnet, the microparticles were immobilized, the wells were decanted and then washed with 100 μl of wash buffer. After washing away any unbound substances, the biotinylated antibody cocktail (50 μl) was added into wells of a 96-well plate and incubated for 1 h at room temperature. Following immobilization of the beads with a magnet, decanting and another wash with 100 μl of wash buffer to remove any unbound biotinylated antibody, 50 μl of streptavidin-phycoerythrin conjugate (Streptavidin-PE), which binds to the biotinylated antibody, was added to each well. Lastly, microparticles were immobilized, the wells were decanted and then washed with 100 μl of wash buffer, which was decanted. The microparticles were resuspended in wash buffer and read using the Luminex® MAGPIX® Analyzer. Two spectrally distinct Light Emitting Diodes (LEDs) illuminated the microparticles. One LED identified the analyte that is being detected and the second LED determined the magnitude of the PE-derived signal, which is in direct proportion to the amount of analyte bound. Each well was imaged with a CCD camera. Mean fluorescence intensity (MFI) was calculated (Fig. 1, workflow).
Fig. 1.
Overview of the assay validation. The assay relies on a multiplex immunoassay platform to simultaneously measure absolute concentrations of 10 urinary proteins from a bladder cancer associated diagnostic signature. A) The simplified workflow consist of a 10 min centrifugation of the voided urine sample, after which time, the sample is aliquoted into the 96 well plate for analysis. B) Performance is evaluated for several preanalytical and analytical parameters by a series of reproducibility studies and by experiments that demonstrate selectivity, specificity, interference, linearity and accuracy.
Based on FDA’s guidance on analytical testing [18], the following experiments were undertaken: selectivity, specificity or antigen cross reactivity, interference, sensitivity (minimal detected dose = MDD), precision intra-assay and inter-assay and linearity.
Selectivity. The selectivity of the assay was evaluated by assessing the biological matrix from voided urine samples. Briefly, the master calibrator was serially diluted 1:2 in urine from 6 pooled healthy controls over 6 dilution ranges and subjected to the multiplex assay. In addition, 44 clinical samples were available to measure the analytes in its natural medium, urine. Mean, standard deviation, % detectable and minimum and maximum were reported.
Specificity or antigen cross reactivity. Each standard was spiked into calibrator diluent at 50 ng/mL or 3x the high standard, whichever is greater, and then run in triplicate on the multiplex assay. Cross reactivity between the antibodies was defined as observed concentration of protein/spiked concentration of a protein with a target of <1% increase in signal due to cross reactivity and shift of <3 SD of the observed concentration due to interference.
Interference. A panel of 14 potential interfering substances were identified; L-Ascorbic acid (Sigma A0278 Stock @ 10 g/L), bilirubin (Sigma B4126 Stock @ 0.2 mg/mL), human hemoglobin (Sigma H7379 Stock @ 20 mg/mL), IgG from human serum (Sigma I4506 Stock @ 25 mg/mL), NaCl (Sigma S3014 Stock @ 350.5 mg/mL), Uric Acid (Sigma U2625 Stock @ 6 mg/dL), Urea (Sigma U4883 Stock @ 3200 mg/dL), Acetylsalicylic Acid (Cayman 70260 Stock @ 3 mg/mL), Phenazopyridine (Sigma 34076 Stock @ 1 mg/mL), Thiotepa (Sigma T6069 Stock @ 20 mg/mL), Trimethoprim (Sigma T7883 Stock @ 400 mg/L), Sodium Azide (Sigma71289 Stock @ 100 mg/mL), Glucose (Sigma G5767 Stock @ 100 mg/mL) and recombinant human albumin (Sigma A9731 Stock @ 200 mg/mL) (Supplemental Fig. 2). Each of the potential interfering substances was tested over a range of 5 serial dilutions and individually spiked into voided urine from 6 pooled healthy controls and run in triplicate on the multiplex assay. Pooled urines without spiked interferents served as a control. When the interfering substances resulted in an elevated concentration of the analyte of > 3x SD from pooled unspiked urines (ideally in a dose dependent manner), this was noted as a false positive and defined as positive interference.
Precision. Precision is determined by the replicate analysis of samples containing known amounts of the biomarkers. Precision was measured using a minimum of five determinations per concentration with a minimum of three concentrations in the range of expected study sample concentrations. The mean CV should be <20% except at lower limit of quantitation (LLOQ), when it should be <25%.
Sample Stability. Sample handling prior to analysis has the potential to dramatically influence the results of a measurement. For this reason, it is important to investigate if different storage conditions contribute to systematic errors in order to provide the clinicians with adequate sample collection and transport instructions. The information gathered will also be useful once the sample reaches the laboratory, i.e., how it should be stored until analysis or pending a possible need for a re-run. Pooled urine samples were analyzed at the following time points 0 h, 24 h, 72 h and 30 days at 4 °C.
Linearity. Natural and spiked linearity were tested. Two separate 1:2 dilutions was created for each of the 6 voided samples from healthy controls and label “a" and “b" (190 μl sample + 190 μl diluent). Next, 20 μl diluent was added to samples labeled as “a” (Control), while 20 μl of recombinant protein was added to samples labeled as “b”. Then, a 2-fold dilution series for 3 additional points was performed. Criteria which needed to be met for the assay to pass included to be between 65 and 135% detection from control.
Data Analysis. Descriptive statistics, mean, standard deviation, % detectable, minimum dose, maximum dose, minimum Median Fluorescent Intensity (MFI), maximum MFI and %CV, were used to report analytical testing.
Patients and specimen processing. Ethical review and approval of the study was performed by Western Institutional Review Board (20141019). After informed consent, voided urine samples were collected prior to any instrumentation. Six healthy controls each provided 500 mL of mid-stream voided urine. The urines were pooled, aliquoted and used for the analytical testing. An additional 44 banked voided urine samples (38 controls and 6 BCa) were available for analytical testing. Banked urines were allowed to thaw and then centrifuged at 1000 g for 10 min to remove any debris prior to testing in duplicate on the multiplex assay.
3. Results
Calibration curves. Supplemental Fig. 1 shows a six-point calibration curve across the 4 log dynamic range for each of the 10 biomarkers. The measuring range of the curves illustrate the lower and upper limits of quantification (LLOQ and ULOQ, respectively).
Selectivity. Selectivity is defined as the ability to measure and differentiate the biomarkers in the presence of components that may be expected to be present, i.e., in this case the ability to detect the biomarkers in voided human urine samples. Utilizing 44 clinical samples, we reported the mean +SD of each biomarker in this cohort along with percentage of samples in which the analyte was detectable and the minimum and maximum amounts of each biomarker (Supplemental Table 1). The only analytes that were detected in <50% of the samples were MMP10 (30%) and PAI1 (36%).
Antigen cross reactivity. We assessed whether the biomarkers adversely interfered with the performance of each of the antibody pair. Specifically, we examined reactivity of each antibody pair to each of the other 9 biomarkers in the assay. ANG was noted to interfere with IL8, MMP10, MMP9, SDC1, VEGF and CA9. Similarly VEGF was noted to interfere with ANG, IL8, MMP10, MMP9, SDC1 and CA9, while MMP10 was noted to interfere with CA9. Because of these noted interference, the multiplex plate design was noted to be – panel #1 CA9, IL8, MMP9 and VEGF, panel #2 ANG, APOE, PAI1, A1AT, SDC1 and panel #3 MMP10. All antigen cross-reactivity was noted to be <1% of the tested concentration (Supplemental Table 2).
Interference. In addition, we sought to identify any potential interfering substances in a biological matrix including endogenous matrix components, metabolites; decomposition products, and concomitant medication. Of the 10 analytes, false positive results were noted with ANG when NaCl levels were >75 mEq/L and CA9 when Thiotepa levels were >1000 ng/mL. Furthermore, the addition of urea resulted in a false positive result with IL8 when urea levels were >20 mg/dL, in CA9 when urea was >80 mg/dL, in MMP10 when urea was >160 mg/dL and in MMP9 at any level. Similarly, the addition of hemoglobin at any level resulted in a false positive result with MMP10, CA9, A1AT and MMP10. Since, the levels for CA9 and MMP10 were extremely low, these biomarkers noted greater varability when subjected to the interfering agents (Supplemental Table 3). In addition, we manipulated the pH of the pooled urine samples from 5.5 to 8.5 using HCl or NaOH, respectively. We did not noted a false positive readout of the multiplex assay when the pH varied (data not shown).
Sensitivity. Sensitivity is defined as the lowest analyte concentration that can be measured with acceptable accuracy and precision (i.e., minimal detected dose, MDD). The MDD ranged from 0.295 pg/mL in IL8 to 31.1 pg/mL in APOE, Table 1.
Table 1.
Minimal detected dose (Sensitivity) of 10 biomarkers.
| Analyte | Mean |
SD |
Min |
Max |
Low Std |
Low Delta |
1/2 Low Std (pg/mL) | Low Std (pg/mL) | MDD |
MDD/Low Std |
|---|---|---|---|---|---|---|---|---|---|---|
| (MFI) | (MFI) | (MFI) | (MFI) | (MFI) | (pg/mL) | |||||
| ANG | 18.1 | 0.888 | 17 | 20 | 58.3 | 40.2 | 7.86 | 15.7 | 0.695 | 4.42% |
| APOE | 59.1 | 3.07 | 52 | 64 | 119.5 | 60.4 | 153 | 306 | 31.1 | 10.20% |
| CA9 | 11.5 | 0.769 | 10 | 13 | 18.5 | 7.04 | 4.86 | 9.71 | 2.122 | 21.90% |
| IL8 | 13.3 | 0.653 | 12 | 15 | 30.5 | 17.2 | 1.94 | 3.87 | 0.295 | 7.61% |
| MMP10 | 18.5 | 0.871 | 16.5 | 20 | 40.5 | 22 | 11.7 | 23.3 | 1.85 | 7.93% |
| MMP9 | 8.3 | 0.598 | 7 | 10 | 25.8 | 17.5 | 24.5 | 48.9 | 3.35 | 6.84% |
| PAI1 | 20 | 0.805 | 19 | 22 | 37 | 17 | 1.79 | 3.58 | 0.339 | 9.47% |
| SDC1 | 20.2 | 0.686 | 19 | 21 | 43 | 22.8 | 65.5 | 131 | 7.89 | 6.02% |
| VEGF | 33.2 | 1.98 | 28 | 38.5 | 47 | 13.8 | 4.26 | 8.52 | 2.434 | 28.60% |
| A1AT | 13.2 | 0.697 | 12 | 15 | 40 | 26.8 | 203 | 405 | 21.1 | 5.20% |
MDD = 2 standard deviation (0 Std MFI) x Low Standard Concentration/Low Delta (MFI). Twenty replicates of the 0 Std MFI (Blank) are run on each assay.
Precision. Within-run (also known as intra-batch precision or repeatability) is an assessment of the precision during a single analytical run while between-run (also known as interbatch precision or repeatability) is an assessment with time, and may involve different analysts, equipment, reagents, and laboratories. The mean CV should be <20% except at lower limit of quantitation (LLOQ), when it should be <25%. All biomarkers achieved these requirements for intra-precision (Table 2), whereas only APOE was noted to have slightly elevated %CV at the low and mid control for inter-precision. This variation was not noted in the high control for APOE (Table 3).
Table 2.
Intrarun assay.
| Panel 1 |
Diluent Set 1 |
Diluent Set 2 |
High Control | Low Control | Mid Control | High Control |
|---|---|---|---|---|---|---|
| CA9 | Low Control | Mid Control | ||||
| MEAN Dose (pg/mL) | 15 | 57 | 246 | 16 | 51 | 245 |
| Std. Deviation | 0.97 | 3 | 8.1 | 0.67 | 2 | 6.1 |
| % CV | 6.6% | 4.6% | 3.3% | 4.3% | 4.7% | 2.5% |
| IL8 |
Diluent Set 1 |
Diluent Set 2 |
High Control | Low Control | Mid Control | High Control |
|
Low Control |
Mid Control |
|||||
| MEAN Dose (pg/mL) | 17 | 67 | 282 | 18 | 59 | 272 |
| Std. Deviation | 0.89 | 3 | 7.6 | 0.72 | 2 | 5.3 |
| % CV | 5.4% | 3.8% | 2.7% | 4.0% | 4.0% | 2.0% |
| MMP9 |
Diluent Set 1 |
Diluent Set 2 |
High Control | Low Control | Mid Control | High Control |
|
Low Control |
Mid Control |
|||||
| MEAN Dose (pg/mL) | 270 | 1114 | 4643 | 296 | 994 | 4601 |
| Std. Deviation | 11.99 | 71 | 103.6 | 10.36 | 37 | 109.2 |
| % CV | 4.4% | 6.3% | 2.2% | 3.5% | 3.7% | 2.4% |
| VEGF |
Diluent Set 1 |
Diluent Set 2 |
High Control | Low Control | Mid Control | High Control |
|
Low Control |
Mid Control |
|||||
| MEAN Dose (pg/mL) | 38 | 162 | 713 | 41 | 126 | 626 |
| Std. Deviation | 1.74 | 6 | 20.4 | 1.45 | 4 | 18.6 |
| % CV | 4.5% | 3.5% | 2.9% | 3.6% | 3.2% | 3.0% |
|
Panel 2 |
Diluent Set 1 |
Diluent Set 2 |
High Control | Low Control | Mid Control | High Control |
|
ANG |
Low Control |
Mid Control |
||||
| MEAN Dose (pg/mL) | 57 | 226 | 910 | 55 | 226 | 911 |
| Std. Deviation | 5.30 | 6 | 21.0 | 1.70 | 5 | 36.3 |
| % CV | 9.3% | 2.5% | 2.3% | 3.1% | 2.3% | 4.0% |
| APOE |
Diluent Set 1 |
Diluent Set 2 |
High Control | Low Control | Mid Control | High Control |
|
Low Control |
Mid Control |
|||||
| MEAN Dose (pg/mL) | 1308 | 5042 | 17833 | 1021 | 4124 | 16924 |
| Std. Deviation | 131.33 | 152 | 506.0 | 32.30 | 155 | 589.7 |
| % CV | 10.0% | 3.0% | 2.8% | 3.2% | 3.7% | 3.5% |
| A1AT |
Diluent Set 1 |
Diluent Set 2 |
High Control | Low Control | Mid Control | High Control |
|
Low Control |
Mid Control |
|||||
| MEAN Dose (pg/mL) | 1211 | 4527 | 18254 | 1144 | 4459 | 18669 |
| Std. Deviation | 187.72 | 124 | 353.8 | 82.00 | 146 | 432.7 |
| % CV | 15.5% | 2.7% | 1.9% | 7.2% | 3.3% | 2.3% |
| PAI1 |
Diluent Set 1 |
Diluent Set 2 |
High Control | Low Control | Mid Control | High Control |
|
Low Control |
Mid Control |
|||||
| MEAN Dose (pg/mL) | 62 | 239 | 949 | 57 | 225 | 920 |
| Std. Deviation | 6.78 | 7 | 20.9 | 1.40 | 5 | 25.0 |
| % CV | 11.0% | 2.7% | 2.2% | 2.5% | 2.2% | 2.7% |
| SDC1 |
Diluent Set 1 |
Diluent Set 2 |
High Control | Low Control | Mid Control | High Control |
|
Low Control |
Mid Control |
|||||
| MEAN Dose (pg/mL) | 632 | 2471 | 9977 | 596 | 2365 | 9465 |
| Std. Deviation | 67.36 | 73 | 260.3 | 17.80 | 65 | 361.0 |
| % CV | 10.7% | 2.9% | 2.6% | 3.0% | 2.7% | 3.8% |
|
Panel 3 |
Diluent Set 1 |
Diluent Set 2 |
High Control | Low Control | Mid Control | High Control |
|
MMP10 |
Low Control |
Mid Control |
||||
| MEAN Dose (pg/mL) | 89 | 363 | 1529 | 107 | 403 | 1536 |
| Std. Deviation | 4.69 | 18 | 80.9 | 3.40 | 12 | 29.0 |
| % CV | 5.3% | 5.0% | 5.3% | 3.2% | 2.9% | 1.9% |
Table 3.
Interrun assay.
| Panel 1 | Low Control |
Mid Control |
High Control |
Low Control |
Mid Control |
High Control |
|---|---|---|---|---|---|---|
| Mean (pg/mL) | CV% | |||||
| CA9 | 14.21 | 55.30 | 236.38 | 15.2% | 11.1% | 6.4% |
| IL8 | 16.46 | 64.76 | 267.69 | 13.9% | 9.7% | 6.0% |
| MMP9 | 264.46 | 1069.56 | 4459.09 | 13.2% | 9.1% | 7.5% |
| VEGF | 40.20 | 157.07 | 692.83 | 14.7% | 12.5% | 5.7% |
| Panel 2 | Low Control | Mid Control | High Control | Low Control | Mid Control | High Control |
| Mean (pg/mL) | CV% | |||||
| ANG | 53.79 | 215.61 | 934.26 | 10.3% | 14.0% | 4.2% |
| APOE | 1131.06 | 4137.25 | 16709.26 | 22.4% | 22.6% | 13.3% |
| A1AT | 1198.11 | 4338.14 | 18875.43 | 8.6% | 13.8% | 3.8% |
| PAI1 | 57.29 | 222.17 | 940.58 | 9.0% | 13.6% | 4.2% |
| SDC1 | 594.70 | 2321.99 | 10206.49 | 10.0% | 14.2% | 4.3% |
| Panel 3 | Low Control | Mid Control | High Control | Low Control | Mid Control | High Control |
| Mean (pg/mL) | CV% | |||||
| MMP10 | 95.21 | 390.65 | 1564.85 | 11.6% | 7.2% | 3.7% |
Sample and Kit Stability. Pooled urine samples were placed at 4 °C and analyzed at 0 h, 24 h, 72 h and 30 days to evaluate stability of the biomarkers during sample collection and handling after short-term and long-term storage. Variation in storage time of urine samples did not adversely effect readout of the multiplex assay (data not shown). Furthermore, two lots of the multiplex assay was tested over 8 weeks. No difference in biomarker readout was noted, thus the kits are stable for at least 8 weeks (Supplemental Table 4). Additional stability testing out to 52 weeks is underway.
Recovery and dilutional linearity. As the proposed process does not require sample extraction, recovery or biomarker extraction efficiency is not required and was not performed. However, dilution linearity was performed to demonstrate that a sample with a spiked concentration above the ULOQ can be diluted to a concentration within the working range and still give a reliable result. In other words, it determines to which extent the dose–response of the analyte is linear in a particular diluent within the range of the standard curve. Thereby dilution of samples should not affect the accuracy and precision. At the same time, the presence of a hook effect, i.e., suppression of signal at concentrations above the ULOQ, is investigated. As it relates to natural sample linearity, CA9 and IL8 were non-detectable while MMP10 and A1AT were noted to be out of range. For spike linearity/recovery, only MMP10 was noted to be out of range (Supplemental Table 5) across the testing.
4. Discussion
Here, we outlined the analytical validation of Oncuria™ a multiplex bead-based immunoassay which possesses the ability to predict the presence of BCa in patients at risk, i.e., hematuria (gross or microscopic) and a history of BCa on tumor surveillance. Selectivity, specificity, interference, sensitivity, precision (intra and inter-assay) and linearity were evaluated to determine robustness. All of these parameters passed our internal quality assurance plan. Of note, the intent of the protein-based diagnostic assay (Oncuria™) is to measure the relative abundance of urine-based proteins associated with a BCa-associated diagnostic signature. Notably, only one multiplex protein-based assay is FDA approved, OVA1, which is for the early detection of ovarian cancer and measures absolute serum levels of CA125, apolipoprotein A1, beta 2 microglobulin, prealbumin, and transferrin to determine the risk for malignancy, and has an overall sensitivity of 92.2% as a stand-alone test and rises to 98.1% when accompanied by ultrasound imaging and physical examination [19,20]. No multiplex protein assay are on the horizon for the detection of BCa, however, Cxbladder is a urine-based multiplex RNA assay that is in early clinical testing/implementation [21].
To address the current limitations described above, we employed a stepwise approach associated with the derivation [[11], [12], [13], [14], [15], [16]], early validation [[8], [9], [10]] and development of a prototype multiplex protein assay [5,6]. Next, a meta-analysis was performed to re-evaluate and demonstrate the robustness and consistency of the diagnostic utility of the 10-plex urine-based diagnostic assay [22]. Recently, we transitioned the BCa-associated diagnostic signature to a multiplex bead-based immunoassay and noted improved operational characteristics of the multiplex bead-based immunoassay over the multiplex electrochemiluminescent assay [4].
The overall validation workflow for the assay utilized a combination of controls and samples to determine robustness, precision, accuracy, and potential confounders. From this evaluation, numerous run, sample, and protein level threshold levels that will serve as daily quality control (QC) parameters have been developed. Given the multiple biomarkers simultaneously evaluated, several controls were developed to monitor sample (preanalytical) and assay (analytical) parameters for potential variability. Previously, postanalytical bioinformatics pipeline and laboratory information system have been developed and validated to perform automatic data analysis from run QC to reporting, resulting in a <5-h turnaround time from specimen receipt to reporting. The analytical validation studies reported here demonstrated that when samples are adequately collected, processed and analyzed with suitable diluents and antibodies, common and at times harsh urine components have little impact on analyzing protein levels. Furthermore, when assessing the 10 analytes with the 14 interfering substances, CA9 and MMP10 analtyes typically expressed in lower concentrations, had greater varability, while ANG was effected by NaCl levels >75 mEq/L, IL8 and MMP9 were effected by urea levels >20 mg/dL and any level of urea, respectively, while A1AT were effected by any amount of hemoglobin. Thus, having a signature composed of a panel of analtyes, helps protect from such errors.
To our knowledge, this is the first multiplex bead-based immunoassay for the non-invasive detection of BCa that has been analytically validated as a tool with the potential to help clinicians manage patients at risk of harboring BCa. We believe that this assay, unlike most urine-based diagnostic assays, is well suited to identify patients who may require additional testing to rule in the presence of a bladder tumor. Implementing the assay clinically is a necessary first step to improve on current diagnostic approaches and to demonstrate the clinical utility of advanced molecular diagnostic testing.
Financial support
This work was supported by research grants from Weinman Foundation Fund USA (CJR) and NIH/NCI USA R01 CA1988887 (CJR).
Relationships
CJ Rosser is an officer of Nonagen BioScience Corp.
Declaration of competing interest
All other authors have NO conflict of interest to report.
Acknowledgement
Charles J. Rosser is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, HF Performed interfering substance testing, drafted and revised manuscript, LT Recruited subjects, processed and catalogued urine samples, RL Recruited subjects, processed and catalogued urine samples, PK Performed all validation experiments except interfering substances testing, MR Performed all validation experiments except interfering substances testing, YD Performed data analysis, RW Drafted and revised manuscript, CJR Developed concept, secured funding, overseer of entire project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.plabm.2020.e00189.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Papanicolaou Cytology of the urine sediment in neoplasms of the urinary tract. J. Urol. 1947 Feb;57(2):375–379. doi: 10.1016/S0022-5347(17)69643-5. [DOI] [PubMed] [Google Scholar]
- 2.Thomas L., Leyh H., Marberger M., Bombardieri E., Bassi P., Pagano F. Multicenter trial of the quantitative BTA TRAK assay in the detection of bladder cancer. Clin. Chem. 1999 Apr;45(4):472–477. [PubMed] [Google Scholar]
- 3.Chang Y.H., Wu C.H., Lee Y.L., Huang P.H., Kao Y.L., Shiau M.Y. Evaluation of nuclear matrix protein-22 as a clinical diagnostic marker for bladder cancer. Urology. 2004 Oct;64(4):687–692. doi: 10.1016/j.urology.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 4.Furuya H., Pagano I., Chee K., Kobayashi T., Wong R.S., Lee R., Rosser C.J. Comparison of commercial ELISA kits, a prototype multiplex electrochemoluminescent assay, and a multiplex bead-based immunoassay for detecting a urine-based bladder-cancer-associated diagnostic signature. Diagnostics. 2019 Oct 29;9(4):E166. doi: 10.3390/diagnostics9040166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodison S., Ogawa O., Matsui Y., Kobayashi T., Miyake M., Ohnishi S., Fujimoto K., Dai Y., Shimizu Y., Tsukikawa K., Furuya H., Rosser C.J. A multiplex urinary immunoassay for bladder cancer detection: analysis of a Japanese cohort. J. Transl. Med. 2016 Oct 7;14(1):287. doi: 10.1186/s12967-016-1043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu Y., Furuya H., Bryant Greenwood P., Chan O., Dai Y., Thornquist M.D., Goodison S., Rosser C.J. A multiplex immunoassay for the non-invasive detection of bladder cancer. J. Transl. Med. 2016;14:31. doi: 10.1186/s12967-016-0783-2. Jan 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang G., Gomes-Giacoia E., Dai Y., Lawton A., Miyake M., Furuya H., Goodison S., Rosser C.J. Validation and clinicopathologic associations of a urine-based bladder cancer biomarker signature. Diagn. Pathol. 2014 Nov 12;9:200. doi: 10.1186/s13000-014-0200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L.M., Chang M., Dai Y., Chai K.X., Dyrskjøt L., Sanchez-Carbayo M., Szarvas T., Zwarthoff E.C., Lokeshwar V., Jeronimo C., Parker A.S., Ross S., Borre M., Orntoft T.F., Jaeger T., Beukers W., Lopez L.E., Henrique R., Young P.R., Urquidi V., Goodison S., Rosser C.J. External validation of a multiplex urinary protein panel for the detection of bladder cancer in a multicenter cohort. Canc. Epidermiol. Biomark. Prev. 2014 Sep;23(9):1804–1812. doi: 10.1158/1055-9965.EPI-14-0029. Epub 2014 Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosser C.J., Chang M., Dai Y., Ross S., Mengual L., Alcaraz A., Goodison S. Urinary protein biomarker panel for the detection of recurrent bladder cancer. Canc. Epidermiol. Biomark. Prev. 2014 Jul;23(7):1340–1345. doi: 10.1158/1055-9965.EPI-14-0035. Epub 2014 Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosser C.J., Ross S., Chang M., Dai Y., Mengual L., Zhang G., Kim J., Urquidi V., Alcaraz A., Goodison S. Multiplex protein signature for the detection of bladder cancer in voided urine samples. J. Urol. 2013 Dec;190(6):2257–2262. doi: 10.1016/j.juro.2013.06.011. Epub 2013 Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urquidi V., Goodison S., Cai Y., Sun Y., Rosser C.J. A candidate molecular biomarker panel for the detection of bladder cancer. Canc. Epidermiol. Biomark. Prev. 2012 Dec;21(12):2149–2158. doi: 10.1158/1055-9965.EPI-12-0428. Epub 2012 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodison S., Chang M., Dai Y., Urquidi V., Rosser C.J. A multi-analyte assay for the non-invasive detection of bladder cancer. PloS One. 2012;7(10) doi: 10.1371/journal.pone.0047469. Epub 2012 Oct 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang N., Feng S., Shedden K., Xie X., Liu Y., Rosser C.J., Lubman D.M., Goodison S. Urinary glycoprotein biomarker discovery for bladder cancer detection using LC/MS-MS and label-free quantification. Clin. Canc. Res. 2011 May 15;17(10):3349–3359. doi: 10.1158/1078-0432.CCR-10-3121. Epub 2011 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodison S., Rosser C.J., Urquidi V. Urinary proteomic profiling for diagnostic bladder cancer biomarkers. Expert Rev. Proteomics. 2009 Oct;6(5):507–514. doi: 10.1586/epr.09.70.Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosser C.J., Liu L., Sun Y., Villicana P., McCullers M., Porvasnik S., Young P.R., Parker A.S., Goodison S. Bladder cancer-associated gene expression signatures identified by profiling of exfoliated urothelia. Canc. Epidermiol. Biomark. Prev. 2009 Feb;18(2):444–453. doi: 10.1158/1055-9965.EPI-08-1002. Epub 2009 Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreunin P., Zhao J., Rosser C., Urquidi V., Lubman D.M., Goodison S. Bladder cancer associated glycoprotein signatures revealed by urinary proteomic profiling. J. Proteome Res. 2007 Jul;6(7):2631–2639. doi: 10.1021/pr0700807. Epub 2007 May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreunin P., Zhao J., Rosser C., Urquidi V., Lubman D.M., Goodison S. Bladder cancer associated glycoprotein signatures revealed by urinary proteomic profiling. J. Proteome Res. 2007 Jul;6(7):2631–2639. doi: 10.1021/pr0700807. Epub 2007 May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidance for industry: bioanalytical method validation. Retrieved fromhttp://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm.
- 19.Longoria T.C., Ueland F.R., Zhang Z. Clinical performance of a multivariate index assay for detecting early-stage ovarian cancer. Am. J. Obstet. Gynecol. 2014;210:78. doi: 10.1016/j.ajog.2013.09.017. e1–78.e9. [DOI] [PubMed] [Google Scholar]
- 20.Ware Miller R., Smith A., DeSimone C.P. Performance of the American College of Obstetricians and Gynecologists’ ovarian tumor referral guidelines with a multivariate index assay. Obstet. Gynecol. 2011;117:1298–1306. doi: 10.1097/AOG.0b013e31821b1d80. [DOI] [PubMed] [Google Scholar]
- 21.Darling D., Luxmanan C., O’Sullivan P., Lough T., Suttie J. Clinical utility of cxbladder for the diagnosis of urothelial carcinoma. Adv. Ther. 2017 May;34(5):1087–1096. doi: 10.1007/s12325-017-0518-7. Epub 2017 Mar 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masuda N., Ogawa O., Park M., Liu A.Y., Goodison S., Dai Y., Kozai L., Furuya H., Lotan Y., Rosser C.J., Kobayashi T. Meta-analysis of a 10-plex urine-based biomarker assay for the detection of bladder cancer. Oncotarget. 2018 Jan 3;9(6):7101–7111. doi: 10.18632/oncotarget.23872. eCollection 2018 Jan 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.