Abstract
Background:
This study is the first to examine the association between plasma levels of brain-derived neurotrophic factor (BDNF) and the antisuicidal effects of repeated ketamine infusions in depressed patients with suicidal ideation.
Methods:
Fifty-seven depressed patients with suicidal ideation received six ketamine infusions (0.5 mg/kg) during a 12 days period. Suicidality was measured with the Scale for Suicidal Ideations (SSI-part 1), item 10 of the Montgomery–Åsberg Depression Rating Scale (MADRS), and item 3 of the Hamilton Depression Rating Scale (HAMD) at baseline, 1 day after the first infusion (1 day), 1 day after the sixth infusion (13 days), and at 2 weeks after the last infusion (26 days). Plasma levels of BDNF were measured by enzyme-linked immunosorbent assay at baseline, 13 days, and 26 days.
Results:
Overall, 46 (80.7%) depressed patients with suicidal ideation had an antisuicidal response at 13 days. Despite a significant reduction in suicidal symptoms over time, no changes in plasma levels of BDNF were found after ketamine treatment when compared with baseline. Correlation analysis showed that no significant association was observed between the plasma levels of BDNF and the changes in the severity of suicidal symptoms as measured by SSI-part 1, item 10 of the MADRS, or item 3 of the HAMD at 1 day, 13 days, and 26 days (all p < 0.05).
Conclusion:
Our results indicated that plasma levels of BDNF may not serve as a biomarker for determining the antisuicidal effects of six ketamine infusions in depressed patients with suicidal ideation.
Keywords: antisuicidal effects, BDNF, depression, ketamine, suicidal ideation
Introduction
Suicide is a substantial public health concern that accounts for more than 0.8 million deaths each year1 and ranks as one of the three leading causes of death among individuals aged between 15 and 44 years.2 A previous study found that suicide is prevalent among individuals suffering from some forms of psychiatric disorders, especially mood disorders.3 Up to 66% of depressed patients suffer from suicidal ideation at some point, and depressed patients are approximately 20 times more likely to die by suicide than the general population.1,4 However, treatment strategies for depressed patients with acute suicidal ideation and behavior are limited.5,6 Although numerous studies have found that serotonin and norepinephrine reuptake inhibitors,7 selective serotonin reuptake inhibitors,8 and mood stabilizers (e.g. lithium)9 could effectively reduce suicidal ideation, these treatments generally take 2–4 weeks. Therefore, novel treatment approaches that could rapidly and effectively reduce suicidal ideation are urgently needed, particularly for patients with treatment-refractory depression (TRD).
Subanesthetic doses of the N-methyl-D-aspartate glutamate receptor antagonist ketamine have been found to lead to rapid and robust reductions in both depressive symptoms and suicidal ideation in depressed patients.10–13 A single ketamine infusion at 0.5 mg/kg over 40 min demonstrated greater reductions in depressive symptoms and suicidal ideation than midazolam among depressed patients who had expressed clinically significant suicidal ideation within the past 24 h.13 After controlling for the improvement of depressive symptoms, the antisuicidal effects of a single ketamine infusion remained significant.10 Repeated infusions of ketamine at subanesthetic doses have been increasingly utilized to extend the time to relapse,14,15 and Murrough et al. found that the median time to relapse after the completion of six ketamine infusions was 18 days.14 Zhan et al. found that 49 (57.0%) depressed patients with suicidal ideation had a relief of suicidal ideation after the first ketamine infusion, and 56 patients (65.1%) experienced a relief of suicidal ideation after completing the last ketamine infusion.6 Although ketamine has been shown to be strongly associated with the relief of suicidal ideation for depressed patients, some depressed patients do not respond to single or repeated infusions of ketamine, and the reason is not clear.6
Numerous studies have examined the possible pretreatment predictors of the effects of ketamine in depressed patients. For example, high body mass index,16,17 family history of alcohol dependence,16–18 baseline cognitive function,19 baseline left hippocampal volumes,20 early changes in kynurenine (KYN) and kynurenic acid/KYN,21 and adiponectin22 could predict the effects of ketamine in depressed patients. Neurotrophic factors, particularly brain-derived neurotrophic factor (BDNF), are implicated in the pathophysiology of depressed patients,23,24 suggesting that BDNF may be considered a biomarker of the antidepressant effects of ketamine. However, very few studies have explored the role of BDNF levels in the antisuicidal effect of a single ketamine infusion in depressed patients, and the existing findings are inconsistent.25,26 Unfortunately, no studies have explored the relationship between plasma levels of BDNF and the antisuicidal effect of repeated ketamine infusions in depressed patients with suicidal ideation.
This study aims to examine the potential association between plasma levels of BDNF and ketamine’s antisuicidal effects in depressed patients with suicidal ideation. We hypothesized that six ketamine infusions could increase plasma levels of BDNF in antisuicidal responders compared with antisuicidal non-responders, and plasma levels of BDNF would be associated with the severity of suicidal ideation after the last ketamine infusion.
Methods
Inclusion criteria and study procedure
Data analyzed in this study were derived from a clinical trial (registration number of Chinese Clinical Trial Registry: ChicCTR-OOC-17012239) that investigated the antidepressant and antisuicidal effects of adjunctive ketamine for depressed patients and was conducted from November 2016 to December 2017 in the Affiliated Brain Hospital of Guangzhou Medical University.15,27 The detailed study design and clinical findings of this single-arm open-label trial have been published elsewhere.15,27 Fifty-seven depressed patients aged between 18 and 65 years with suicidal ideation, defined as a score of ⩾2 on the first five items of the Scale for Suicidal Ideations (SSI-part 1),5,28 were treated by a thrice-weekly treatment regimen of ketamine intravenously infused at subanesthetic doses (0.5 mg/kg, each lasting 40 min) for 2 weeks. All subjects received antidepressant medications and continued to receive the same regimen and dosage of psychiatric medications during the study period. The enrolled depressed patients with suicidal ideation had no history of drug or alcohol abuse or major medical or neurological diseases. The severity of depressive symptoms was assessed using the 17-item Hamilton Depression Rating Scale (HAMD)29,30 and the Montgomery–Åsberg Depression Rating Scale (MADRS).31,32 This study was approved by the Affiliated Brain Hospital of Guangzhou Medical University Institutional Review Board and was conducted in accordance with the Declaration of Helsinki (Ethical Application Ref: 2016030).
Antisuicidal response
Suicidal ideation was assessed by the SSI, the HAMD suicide item, and the MADRS suicide item at baseline, 1 day after the first infusion (1 day), 1 day after the sixth infusion (13 days), and 14 days after the completion of six ketamine infusions (26 days). As recommended by previous studies,6,33 an antisuicidal response to ketamine at 13 days was defined as a score of <2 on the SSI-part 1.
Plasma levels of BDNF
Plasma was collected from depressed patients with suicidal ideation at baseline, 13 days, and 26 days and stored at −80°C. According to the manufacturer’s instructions, a commercially available enzyme-linked immunosorbent assay kit (EMD Millipore Corporation, MA, USA) was used to examine plasma levels of BDNF. The contents of BDNF were determined using absorbance at a wavelength of 450 nm and optical density values following the standard curve values.
Statistical analysis
Data of the intent-to-treat (ITT) or modified ITT sample of 57 depressed patients with suicidal ideation were analyzed using SPSS 24.0 statistical software. Baseline sociodemographic and clinical characteristics and plasma levels of BDNF for each group (antisuicidal responders and non-responders, as determined by the SSI-part 1 score at 13 days) were analyzed using the Mann–Whitney U test for non-normally distributed continuous data, Student’s t-tests for normally distributed continuous data, the chi-squared test or Fisher’s exact test for categorical data. Linear mixed models were used to assess the effects of repeated ketamine infusions on suicidality (SSI-part 1, item 3 of the HAMD, and item 10 of the MADRS) and on plasma levels of BDNF over time between responders and non-responders at 13 days. Correlation analyses were performed to examine the relationship between plasma BDNF levels and the antisuicidal effects of repeated ketamine infusions. In clinical practice, TRD patients with suicidal ideation commonly suffer from the more severe deleterious effects of delayed responses. Thus, an additional analysis was conducted focusing on TRD patients with suicidal ideation in this study. Statistical significance was set at p < 0.05 (two-tailed).
Results
A total of 57 depressed patients with suicidal ideation who provided a blood sample at baseline were enrolled in this trial. Among them, 77.2% (44/57) suffered from TRD. Plasma levels of BDNF at baseline ranged from 0.9 ng/ml to 27.0 ng/ml, with a mean value of 9.7 ng/ml. Sociodemographic characteristics, clinical characteristics and plasma levels of BDNF did not differ significantly between responders and non-responders at baseline (Table 1).
Table 1.
Baseline characteristics of antisuicidal responders and non-responders calculated by SSI-part 1 scores at 13 days.
| Variables | Total sample (N = 57) | Antisuicidal responders (n = 46) | Antisuicidal non-responders (n = 11) | Statistics | |||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | χ2 | df | p | |
| Male | 26 | 45.6 | 21 | 45.7 | 5 | 45.5 | 0.0 | 1 | 0.99 |
| Employed | 22 | 38.6 | 20 | 43.5 | 2 | 18.2 | 1.4 | 1 | 0.23 |
| Married | 32 | 56.1 | 25 | 54.3 | 7 | 63.6 | 0.05 | 1 | 0.83 |
| Patients with TRD | 44 | 77.2 | 35 | 76.1 | 9 | 81.8 | 0.0 | 1 | 0.99 |
| Mean | SD | Mean | SD | Mean | SD | T/Z | df | p | |
| Age (years) | 35.0 | 12.0 | 34.4 | 11.4 | 37.4 | 14.4 | −0.7 | 55 | 0.47 |
| BMI (kg/m2) | 22.2 | 3.5 | 22.1 | 3.3 | 22.8 | 4.5 | −0.6 | 55 | 0.54 |
| Illness duration (months) | 89.7 | 79.4 | 78.7 | 67.1 | 135.9 | 110.2 | —a | —a | 0.11 |
| Education levels (years) | 12.1 | 3.6 | 12.4 | 3.5 | 10.7 | 3.9 | 1.4 | 55 | 0.17 |
| Baseline plasma levels of BDNF (ng/ml) | 9.7 | 6.5 | 9.2 | 5.9 | 11.8 | 8.6 | —a | —a | 0.56 |
| Baseline MADRS scores | 33.6 | 7.9 | 33.0 | 7.1 | 36.2 | 10.6 | −1.2 | 55 | 0.23 |
| Baseline SSI-part 1 scores | 5.0 | 2.4 | 4.9 | 2.4 | 5.1 | 2.2 | −0.3 | 55 | 0.74 |
| Baseline scores on item 10 of the MADRS | 2.9 | 1.3 | 2.7 | 1.3 | 3.6 | 1.5 | −1.9 | 55 | 0.06 |
| Baseline scores on item 3 of the HAMD | 2.2 | 0.8 | 2.2 | 0.8 | 2.6 | 0.8 | −1.4 | 55 | 0.15 |
Mann–Whitney U test.
BDNF, brain derived neurotrophic factor; BMI, body mass index; df, degrees of freedom; HAMD, Hamilton Depression Rating Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; SD, standard deviation; SSI, Beck’s Scale for Suicide Ideation; TRD, treatment refractory depression; T/Z, T value/Z value.
Antisuicidal response and BDNF
In 57 depressed patients with suicidal ideation, the antisuicidal response rates at 1 day, 13 days, and 26 days were 61.4%, 80.7%, and 73.7%, respectively. Antisuicidal response rates for TRD patients with suicidal ideation (n = 44) were 61.4%, 79.5%, and 72.7% at 1 day, 13 days, and 26 days, respectively.
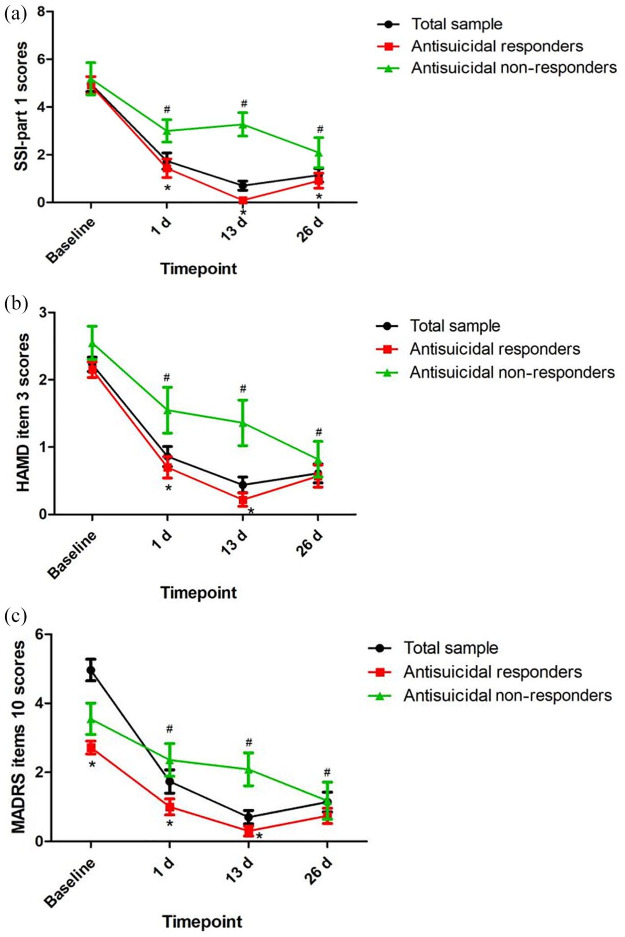
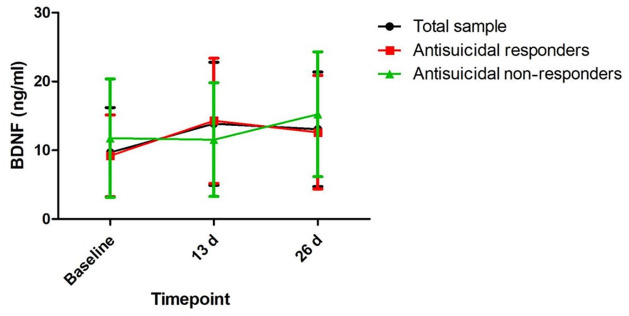
Table 2 shows the significant main effects of time, group, and group-by-time interactions on the SSI-part 1 score, item 3 of the HAMD, and item 10 of the MADRS (all p < 0.05). Antisuicidal responders had a significantly lower SSI-part 1 score, a lower score for item 10 of the MADRS, and lower scores for item 3 of the HAMD than antisuicidal non-responders at 1 day and 13 days (all p < 0.05) (Figure 1). However, no significant main effects of group, time or group-by-time interaction were found on plasma levels of BDNF (Table 2). Similarly, plasma levels of BDNF did not differ between antisuicidal responders and non-responders (Figure 2).
Table 2.
Comparison of suicidal ideation scores and plasma levels of BDNF between antisuicidal responders and non-responders in depressed patients with suicidal ideation using linear mixed model analysis.
| Variables | Group-by-time interactions | Time main effect | Group main effect | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| HAMD item 3 scores | 3.9 | 0.013 | 32.2 | <0.001 | 7.4 | 0.009 |
| MADRS item 10 scores | 4.4 | 0.007 | 22.0 | <0.001 | 8.8 | 0.004 |
| SSI-part 1 scores | 7.8 | <0.001 | 23.6 | <0.001 | 16.7 | <0.001 |
| Plasma levels of BDNF (ng/ml) | 1.3 | 0.295 | 1.8 | 0.177 | 0.2 | 0.666 |
Bold values are p < 0.05.
BDNF, brain-derived neurotrophic factor; HAMD, Hamilton Depression Rating Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; SSI, Beck’s Scale for Suicide Ideation.
Figure 1.
The antisuicidal effects of ketamine as measured by SSI-part 1 (A), item 3 of the HAMD (B), and item 10 of the MADRS (C).
#Significant difference was found when compared with baseline scores at the indicated times (p < 0.05).*Significant difference was found between responders and non-responders at the indicated times (p < 0.05).d, day; HAMD, Hamilton Depression Rating Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; SSI, Beck’s Scale for Suicide Ideation.
Figure 2.
Change in plasma BDNF levels in depressed patients with suicidal ideation at the indicated times.
No significant difference at the indicated times was found when compared with baseline levels across the total sample, including both responders and non-responders, and no significant difference at the indicated times was found between responders and non-responders.
BDNF, brain-derived neurotrophic factor; d, day.
Additional analysis found that six ketamine infusions were also effective at reducing suicidal symptoms among TRD patients with suicidal ideation (Supplemental Table 1 and Supplemental Figure 1) but did not lead to different plasma levels of BDNF between antisuicidal responders or non-responders (Supplemental Table 1 and Supplemental Figure 2).
Relationship between BDNF and antisuicidal effects of ketamine
Changes in plasma levels of BDNF following ketamine treatment were not significantly associated with a reduction in SSI-part 1 scores, the scores of item 10 of the MADRS, or scores of item 3 of the HAMD at 1 day, 13 days, and 26 days in the whole sample (Table 3) or among only patients with TRD (Supplemental Table 2). Similarly, there was no significant association between baseline plasma levels of BDNF and suicidal scores (SSI-part 1, item 10 of the MADRS, and item 3 of the HAMD) at 1 day, 13 days, or 26 days in the entire sample (Table 3) or among only patients with TRD (Supplemental Table 2).
Table 3.
Correlation analysis between suicidal scores and plasma levels of BDNF in depressed patients with suicidal ideation at the indicated times.
| Variables | Reduction in SSI-part 1 scores | Reduction in scores on item 10 of the MADRS | Reduction in scores on item 3 of the HAMD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| At 1 day | At 13 days | At 26 days | At 1 day | At 13 days | At 26 days | At 1 day | At 13 days | At 26 days | ||
| Change in plasma levels of BDNF (ng/ml) | r | −0.037 | −0.032 | 0.165 | −0.136 | −0.011 | 0.041 | −0.121 | 0.049 | 0.069 |
| p | 0.810 | 0.836 | 0.285 | 0.379 | 0.943 | 0.792 | 0.433 | 0.752 | 0.659 | |
| Variables | SSI-part 1 scores | Scores on item 10 of the MADRS | Scores on item 3 of the HAMD | |||||||
| At 1 day | At 13 days | At 26 days | At 1 day | At 13 days | At 26 days | At 1 day | At 13 days | At 26 days | ||
| Plasma levels of BDNF at baseline (ng/ml) | r | 0.010 | 0.001 | 0.046 | 0.123 | −0.045 | −0.076 | 0.072 | −0.011 | 0.024 |
| p | 0.939 | 0.997 | 0.733 | 0.360 | 0.740 | 0.576 | 0.596 | 0.936 | 0.862 | |
BDNF, brain-derived neurotrophic factor; HAMD, Hamilton Depression Rating Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; r, Pearson coefficient of correlation; SSI, Beck’s Scale for Suicide Ideation.
Discussion
To our knowledge, this is the first study to assess the association between plasma levels of BDNF and the antisuicidal effects of six ketamine infusions in Chinese depressed patients with suicidal ideation and the first study to distinguish the effects between antisuicidal response and non-response groups. The main findings of this single-arm open-label trial are that (1) adjunctive ketamine at 0.5 mg/kg over 40 min induced a significant and rapid reduction in suicidal ideation, consistent with previous studies;10,13 (2) plasma levels of BDNF showed no significant changes at 13 days and 26 days when compared with baseline, even among antisuicidal responders and antisuicidal non-responders; (3) no significant association was found between plasma levels of BDNF and the observed antisuicidal effects of ketamine as measured by SSI-part 1, item 10 of the MADRS, and item 3 of the HAMD.
Numerous randomized controlled studies13,34,35 and meta-analyses10 have reported a rapid and robust antisuicidal response to ketamine. Importantly, the Food and Drug Administration has approved esketamine (a form of ketamine) nasal spray for patients with TRD.36 Sinyor et al. found that depressed patients with suicidal ideation benefitted from six infusions of 0.5 mg/kg ketamine under “real-world” conditions.37 A recent meta-analysis found that ketamine led to a greater reduction in suicidal ideation than normal saline or midazolam within 1 day and for up to 7 days in depressed patients with suicidal ideation.10 Ballard et al. found that 29% of depressed patients had no or minimal antisuicidal response to a low-dose ketamine (0.5 mg/kg) infusion, which is similar to our findings.25
Interestingly, this study found no baseline sociodemographic or clinical characteristics that reliably distinguished antisuicidal responders and non-responders. The reasons for no differences in baseline sociodemographic and clinical characteristics between antisuicidal responders and non-responders were unclear. In general, some phenotypic characteristics were uniquely and closely correlated with antisuicidal non-responders, indicating that a long-standing history of suicidal ideation may suggest resistance to a rapid reduction in suicidal ideation. For example, Ionescu et al. found that repeated doses of intravenously infused ketamine did not produce significantly better antisuicidal effects than placebo in patients with TRD and current chronic suicidal ideation.38 Compared with the current study, that of Ionescu et al.38 recruited a relatively larger percentage of patients with family history of suicide and failed electroconvulsive therapy. Thus, future studies with large sample sizes are warranted to systematically examine which demographic characteristics and clinical symptom profiles are most likely to have a rapid and robust antisuicidal response to low-dose infusions of ketamine and to examine which patients may benefit from other interventions.25
Somewhat surprisingly, in this study, plasma levels of BDNF were not correlated with the antisuicidal effects of repeated ketamine infusions. A few studies have investigated the role of BDNF levels in the antisuicidal effects of a single ketamine infusion, but the findings have been inconsistent.25,26 For example, Ballard et al. found that plasma levels of BDNF were not associated with antisuicidal response to a single ketamine infusion.25 However, another study reported that a pre- to postinfusion decrease in serum levels of BDNF was associated with a reduction in suicidal ideation from baseline to 1 day after a single ketamine infusion.26 Chen et al. found that the BDNF Val66Met polymorphism may predict the antisuicidal response to a single ketamine infusion.4 Similarly, the findings of studies exploring the association of BDNF and the antidepressant effects of single ketamine infusions were also mixed.39,40 For instance, Haile et al. found that plasma levels of BDNF at 240 min after a single ketamine infusion were strongly and negatively correlated with MADRS scores at 240 min.39 Another study reported that the rapid initial antidepressant effects of ketamine are not mediated by BDNF.40 Ketamine might have a particular effect on exon-specific BDNF transcript levels and the rapid and sustained antidepressant and antisuicidal effects of ketamine may attribute to different BDNF transcript regulation.40 Only the rapid initial effects were evaluated in this study, possibly through fast activation of exons associated with the induction of immediate early genes. BDNF levels could change at later time points after completing six ketamine infusions. Thus, BDNF levels might play an important role in ketamine’s sustained, rather than acute, antidepressant and antisuicidal effects.
Several study limitations should be addressed. First, the lack of a control group limited our capability to interpret the efficacy of the treatment regimen. Second, the psychiatric medications used by depressed patients with suicidal ideation were not discontinued during ketamine treatment, and these medications might have potentially impacted the plasma levels of BDNF. Thus, the findings of this study may not be applied to individuals who are medication free. However, ketamine as an adjunctive therapy to other antidepressants in depressed patients with suicidal ideation has been increasingly used in clinical trials,41 particularly in larger “real-world” samples. Third, although the cortical levels of BDNF were not directly examined in this study, BDNF can cross the blood–brain barrier, and the plasma levels of BDNF can potentially reflect cortical levels of BDNF.42,43
Conclusion
In summary, our results indicated that plasma levels of BDNF may not serve as a biomarker for determining the antisuicidal effects of six ketamine infusions in depressed patients with suicidal ideation.
Supplemental Material
Supplemental material, sj-pdf-1-tpp-10.1177_2045125320973794 for Association between plasma levels of BDNF and the antisuicidal effects of repeated ketamine infusions in depression with suicidal ideation by Wei Zheng, Yan-Ling Zhou, Cheng-Yu Wang, Xiao-Feng Lan, Bin Zhang, Su-Miao Zhou, Su Yan, Ming-Zhe Yang, Sha Nie and Yu-Ping Ning in Therapeutic Advances in Psychopharmacology
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81801343, 81801345), Guangdong Basic and Applied Basic Research Foundation (2019A1515011366), the National Key Research and Development Program of China (2016YFC0906300), Science and Technology Department of Guangdong Province major science and technology (2016B010108003) and Guangzhou Municipal Psychiatric Disease Clinical Transformation Laboratory (201805010009). The funding source had no role in the study design, analysis or interpretation of data or in the preparation of the report or decision to publish.
ORCID iDs: Wei Zheng  https://orcid.org/0000-0003-2371-4789
https://orcid.org/0000-0003-2371-4789
Yu-Ping Ning  https://orcid.org/0000-0002-5727-2782
https://orcid.org/0000-0002-5727-2782
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Wei Zheng, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Yan-Ling Zhou, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Cheng-Yu Wang, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Xiao-Feng Lan, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Bin Zhang, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Su-Miao Zhou, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Su Yan, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Ming-Zhe Yang, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Sha Nie, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Yu-Ping Ning, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, 510182, China; The First School of Clinical Medicine, Southern Medical University, Guangzhou, Guangdong, China.
References
- 1. Chesney E, Goodwin GM, Fazel S. Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry 2014; 13: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aleman A, Denys D. Mental health: a road map for suicide research and prevention. Nature 2014; 509: 421–423. [DOI] [PubMed] [Google Scholar]
- 3. Cavanagh JT, Carson AJ, Sharpe M, et al. Psychological autopsy studies of suicide: a systematic review. Psychol Med 2003; 33: 395–405. [DOI] [PubMed] [Google Scholar]
- 4. Chen MH, Lin WC, Wu HJ, et al. Antisuicidal effect, BDNF Val66Met polymorphism, and low-dose ketamine infusion: reanalysis of adjunctive ketamine study of Taiwanese patients with treatment-resistant depression (AKSTP-TRD). J Affect Disord 2019; 251: 162–169. [DOI] [PubMed] [Google Scholar]
- 5. Zhou Y, Liu W, Zheng W, et al. Predictors of response to repeated ketamine infusions in depression with suicidal ideation: an ROC curve analysis. J Affect Disord 2020; 264: 263–271. [DOI] [PubMed] [Google Scholar]
- 6. Zhan Y, Zhang B, Zhou Y, et al. A preliminary study of anti-suicidal efficacy of repeated ketamine infusions in depression with suicidal ideation. J Affect Disord 2019; 251: 205–212. [DOI] [PubMed] [Google Scholar]
- 7. Serafini G, Pompili M, Del Casale A, et al. Duloxetine versus venlafaxine in the treatment of unipolar and bipolar depression. Clin Ter 2010; 161: 321–327. [PubMed] [Google Scholar]
- 8. Khan A, Khan SR, Hobus J, et al. Differential pattern of response in mood symptoms and suicide risk measures in severely ill depressed patients assigned to citalopram with placebo or citalopram combined with lithium: role of lithium levels. J Psychiatr Res 2011; 45: 1489–1496. [DOI] [PubMed] [Google Scholar]
- 9. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ 2013; 346: f3646. [DOI] [PubMed] [Google Scholar]
- 10. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry 2018; 175: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanacora G, Frye MA, McDonald W, et al. A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 2017; 74: 399–405. [DOI] [PubMed] [Google Scholar]
- 12. Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry 2013; 170: 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grunebaum MF, Galfalvy HC, Choo TH, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry 2018; 175: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 2013; 74: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng W, Zhou YL, Liu WJ, et al. Rapid and longer-term antidepressant effects of repeated-dose intravenous ketamine for patients with unipolar and bipolar depression. J Psychiatr Res 2018; 106: 61–68. [DOI] [PubMed] [Google Scholar]
- 16. Niciu MJ, Luckenbaugh DA, Ionescu DF, et al. Clinical predictors of ketamine response in treatment-resistant major depression. J Clin Psychiatry 2014; 75: e417–e423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rong C, Park C, Rosenblat JD, et al. Predictors of response to ketamine in treatment resistant major depressive disorder and bipolar disorder. Int J Environ Res Public Health 2018; 15: 771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phelps LE, Brutsche N, Moral JR, et al. Family history of alcohol dependence and initial antidepressant response to an N-methyl-D-aspartate antagonist. Biol Psychiatry 2009; 65: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng W, Zhou YL, Liu WJ, et al. Neurocognitive performance and repeated-dose intravenous ketamine in major depressive disorder. J Affect Disord 2019; 246: 241–247. [DOI] [PubMed] [Google Scholar]
- 20. Abdallah CG, Salas R, Jackowski A, et al. Hippocampal volume and the rapid antidepressant effect of ketamine. J Psychopharmacol 2015; 29: 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou Y, Zheng W, Liu W, et al. Antidepressant effect of repeated ketamine administration on kynurenine pathway metabolites in patients with unipolar and bipolar depression. Brain Behav Immun 2018; 74: 205–212. [DOI] [PubMed] [Google Scholar]
- 22. Machado-Vieira R, Gold PW, Luckenbaugh DA, et al. The role of adipokines in the rapid antidepressant effects of ketamine. Mol Psychiatry 2017; 22: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry 2006; 59: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 24. Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromol Med 2004; 5: 11–25. [DOI] [PubMed] [Google Scholar]
- 25. Ballard ED, Yarrington JS, Farmer CA, et al. Characterizing the course of suicidal ideation response to ketamine. J Affect Disord 2018; 241: 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grunebaum MF, Ellis SP, Keilp JG, et al. Ketamine versus midazolam in bipolar depression with suicidal thoughts: a pilot midazolam-controlled randomized clinical trial. Bipolar Disord 2017; 19: 176–183. [DOI] [PubMed] [Google Scholar]
- 27. Zheng W, Zhou YL, Liu WJ, et al. Investigation of medical effect of multiple ketamine infusions on patients with major depressive disorder. J Psychopharmacol 2019; 33: 494–501. [DOI] [PubMed] [Google Scholar]
- 28. Voort JLV, Ballard ED, Luckenbaugh DA, et al. Antisuicidal response following ketamine infusion is associated with decreased nighttime wakefulness in major depressive disorder and bipolar disorder. J Clin Psychiatry 2017; 78: 1068–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xie GR, Shen QJ. Use of the Chinese version of the Hamilton Rating Scale for Depression in general population and patients with major depression. Chin J Nerv Ment Dis 1984; 10: 364. [Google Scholar]
- 31. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–389. [DOI] [PubMed] [Google Scholar]
- 32. Zhong BL, Wang Y, Chen HH, et al. Reliability, validity and sensitivity of Montgomery-Åsberg Depression Rating Scale for patients with current major depressive disorder. Chin J Behav Med Brain Sci 2011; 20: 85–87. [Google Scholar]
- 33. Rasmussen KG, Lineberry TW, Galardy CW, et al. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol 2013; 27: 444–450. [DOI] [PubMed] [Google Scholar]
- 34. Murrough JW, Soleimani L, DeWilde KE, et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med 2015; 45: 3571–3580. [DOI] [PubMed] [Google Scholar]
- 35. Price RB, Iosifescu DV, Murrough JW, et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety 2014; 31: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng W, Cai DB, Xiang YQ, et al. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord 2020; 265: 63–70. [DOI] [PubMed] [Google Scholar]
- 37. Sinyor M, Williams M, Belo S, et al. Ketamine augmentation for major depressive disorder and suicidal ideation: preliminary experience in an inpatient psychiatry setting. J Affect Disord 2018; 241: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ionescu DF, Bentley KH, Eikermann M, et al. Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: a randomized, double blind, placebo controlled trial. J Affect Disord 2019; 243: 516–524. [DOI] [PubMed] [Google Scholar]
- 39. Haile CN, Murrough JW, Iosifescu DV, et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol 2014; 17: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Machado-Vieira R, Yuan P, Brutsche N, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry 2009; 70: 1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shiroma PR, Johns B, Kuskowski M, et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord 2014; 155: 123–129. [DOI] [PubMed] [Google Scholar]
- 42. Pillai A, Kale A, Joshi S, et al. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharmacol 2010; 13: 535–539. [DOI] [PubMed] [Google Scholar]
- 43. Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res 1996; 36: 280–286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tpp-10.1177_2045125320973794 for Association between plasma levels of BDNF and the antisuicidal effects of repeated ketamine infusions in depression with suicidal ideation by Wei Zheng, Yan-Ling Zhou, Cheng-Yu Wang, Xiao-Feng Lan, Bin Zhang, Su-Miao Zhou, Su Yan, Ming-Zhe Yang, Sha Nie and Yu-Ping Ning in Therapeutic Advances in Psychopharmacology