Abstract
Astrocytes control multiple processes in the nervous system in health and disease. It is now clear that specific astrocyte subsets or activation states are associated with specific genomic programs and functions. The advent of novel genomic technologies has enabled rapid progress in the characterization of astrocyte heterogeneity and its control by astrocyte interactions with other cells in the central nervous system (CNS). In this review, we provide an overview of the multifaceted roles of astrocytes in the context of CNS inflammation, highlighting recent discoveries on astrocyte subsets and their regulation. We explore mechanisms of crosstalk between astrocytes and other cells in the CNS in the context of neuroinflammation and neurodegeneration, and discuss how these interactions shape pathological outcomes.
Keywords: astrocyte, neuroinflammation, glial cells, multiple sclerosis, cell interactions, trained immunity
Introduction
Astrocytes are abundant central nervous system (CNS) glial cells, which have emerged as key players in health and disease. Developmentally, astrocytes arise from neural progenitor cells (NPCs) within the subventricular zone (SVZ) and populate the entire brain by migrating along radial glia processes (Ge et al., 2012; Molofsky and Deneen, 2015). Once astrocytes reach their final destination, axis patterning cues guide the maturation of astrocytes into regionally defined subgroups with a high degree of functional specialization, laying the foundation for their multifaceted roles in health and disease (Deneen et al., 2006; Ge et al., 2012; Hochstim et al., 2008; Molofsky and Deneen, 2015; Molofsky et al., 2014; Tsai et al., 2012). Astrocytes are key constituents of the glia limitans, and thus actively contribute to the formation and maintenance of the blood brain barrier (BBB), which separates the peripheral blood circulation from the highly controlled CNS microenvironment (Abbott et al., 2006; Sofroniew, 2015a). Furthermore, astrocytes secrete neurotrophic factors to regulate synaptogenesis, neuronal differentiation, and neuronal survival (Allen et al., 2012b; Christopherson et al., 2005; Chung et al., 2015; Molofsky et al., 2014; Wheeler and Quintana, 2019). Once neuronal synapses are established, astrocytes actively modulate synaptic transmission through the release and clearance of neurotransmitters, and the regulation of extracellular ion concentration (Haim and Rowitch, 2017; Santello and Volterra, 2009; Walz, 2000).
Increased resolution into complex cellular networks enabled by new methodologies such as single-cell RNA-sequencing (scRNA-seq), spatial transcriptomics, and cell-specific CRISPR-based perturbation studies suggests that astrocytes actively contribute to the pathogenesis of multiple neurological disorders. Indeed, astrocytes respond to inflammatory signals and can themselves promote inflammation, which makes them important players in neurologic diseases like multiple sclerosis (MS), Alzheimer disease (AD), Parkinson disease (PD), Huntington disease (HD) and amyotrophic lateral sclerosis (ALS) (Di Giorgio et al., 2007; Diaz-Castro et al., 2019; Gu et al., 2010; Habib et al., 2020; Molofsky and Deneen, 2015; Solano et al., 2008; Tong et al., 2014; Wheeler et al., 2020a; Wheeler and Quintana, 2019). Thus, it is not surprising that the communication between astrocytes and CNS-resident or CNS-infiltrating cells plays a central role in tissue physiology and pathology (Anderson et al., 2016; Anderson et al., 2018; Chao et al., 2019; Faulkner et al., 2004; Liddelow et al., 2017; Lin et al., 2017; Nagai et al., 2019; Rothhammer et al., 2016; Wheeler et al., 2020b; Wheeler et al., 2019). However, the mechanisms involved are not completely understood. In this review, we explore the communication between reactive astrocytes and other cells in the CNS, and discuss how these bidirectional signaling events regulate CNS inflammation and pathological outcomes.
Astrocytes in CNS Inflammation
It is now clear that astrocyte reactivity is a heterogeneous process resulting in a spectrum of molecular, cellular, and functional changes which have provided the basis for the study of astrocyte heterogeneity in health and disease (Anderson et al., 2014; Ben Haim and Rowitch, 2017; Khakh and Deneen, 2019). Although the outcome and individual aspects of astrogliosis might differ, scientists have traditionally viewed the increased expression of glial fibrillary acidic protein (GFAP) as a marker of astrocyte activation and a hallmark of multiple CNS pathologies (Sofroniew, 2009). Interestingly, GFAP was first isolated from demyelinated MS plaques, providing one of the first molecular links between astrocytes and MS (Eng and Vanderhaegen, 1970). Over 50 years later, the repertoire of disease-associated astrocyte markers has grown. Indeed, advancements in genomics and spatial transcriptomics have fueled the identification of novel pathways that control astrocyte function in health and neuroinflammation (Bayraktar et al., 2020; Liddelow and Barres, 2017; Saunders et al., 2018; Wheeler et al., 2020b; Zeisel et al., 2018; Zeisel et al., 2015). Based on these studies, it is now clear that different astrocyte subpopulations either promote, or limit disease pathogenesis in a highly context-dependent manner (Bayraktar et al., 2020; Liddelow and Barres, 2017; Liddelow et al., 2017; Lin et al., 2017; Molofsky et al., 2014; Rothhammer et al., 2016; Wheeler et al., 2020b; Wheeler and Quintana, 2019).
CNS inflammation in MS is associated with BBB breakdown and the recruitment of peripheral immune cells. In this context, astrocytes sense and respond to pro-inflammatory cytokines secreted by CNS-resident and CNS-recruited peripheral immune cells, thereby modulating the responses of neighboring cells throughout the CNS (Rothhammer and Quintana, 2015; Sofroniew, 2015b). In vivo ablation studies in the mouse experimental autoimmune encephalomyelitis (EAE) model of MS suggest that astrocytes limit disease development in early disease stages (Liedtke et al., 1998; Mayo et al., 2014; Toft-Hansen et al., 2011; Voskuhl et al., 2009). In contrast, selective ablation of reactive astrocytes during the chronic phase of EAE ameliorated disease, decreasing microglial activation and monocyte infiltration (Mayo et al., 2014).
Astrocyte signaling pathways seem to converge on common downstream transcriptional regulators during inflammation. For example, nuclear translocation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) heterodimer is a central step in astrocyte activation and contributes to the progression of EAE and other CNS pathologies (Blank and Prinz, 2014; Brambilla et al., 2005; Brambilla et al., 2012; Brambilla et al., 2014; Brambilla et al., 2009; Loo et al., 2006; Rothhammer and Quintana, 2019). Selective blockade of astrocyte NF-κB signaling in models of CNS inflammation/injury including EAE (Brambilla et al., 2014; Brambilla et al., 2009; Loo et al., 2006; Wang et al., 2013a), spinal cord injury (SCI) (Brambilla et al., 2005) and optic neuritis (Brambilla et al., 2012) improves clinical outcomes and is associated with decreased levels of pro-inflammatory cytokines and oxidative stress, pointing to a central role for NF-κB in the response of astrocytes to inflammatory stimuli and other insults. The nuclear translocation of NF-κB is regulated by multiple pathways that can broadly be divided into drivers and suppressors of NF-κB activation.
Nuclear translocation of NF-κB in astrocytes is triggered by pro-inflammatory stimuli such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-17 (Qian et al., 2007), reactive oxygen species (ROS) (Chandel et al., 2000), phagocytosed myelin (Ponath et al., 2017), Toll-like receptor (TLR) engagement (Kawai and Akira, 2007) and other factors associated with CNS inflammation (Fig. 1) (Filippi et al., 2018; Reich et al., 2018). In addition, sphingolipids like sphingosine 1-phosphate (S1P) also drive NF-κB activation and play important roles in the regulation MS and EAE (Rosen and Goetzl, 2005; Rothhammer et al., 2017) (Fig. 1). S1P is a biologically active phospholipid generated from ceramide which controls proliferation of several cell types and leukocyte trafficking from lymphoid tissues into circulation (Rivera et al., 2008). Interestingly, astrocytes upregulate the expression of the sphingolipid receptor S1PR1 upon activation, and conditional ablation of S1PR1 in astrocytes results in diminished EAE severity and increased neuronal survival (Choi et al., 2011). Modulation of S1PR1 by the FDA-approved agonist fingolimod suppresses NF-κB translocation in astrocytes, reducing the production of IL-6, TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), chemokine (C-C motif) ligand 2 (CCL2) and nitric oxide (NO) levels (Rothhammer et al., 2017).
Figure 1. Astrocyte signaling in the context of CNS inflammation.
The transcription factor NF-κB controls multiple aspects of astrocyte pro-inflammatory responses. NF-κB signaling is triggered/boosted by cytokines released by microglia and other cells in the inflamed CNS, and by the sphingolipids S1P and LacCer. AHR activation by dietary components, environmental factors or metabolites derived from the commensal flora limits NF-κB activation. Additional inflammatory pathways are triggered by the binding of environmental toxins to S1R/IRE1α and downstream signaling through XBP1.
Lactosylceramide (LacCer), another ceramide-derived sphingolipid, has also been implicated in the regulation of astrocyte responses during CNS inflammation (Mayo et al., 2014). LacCer synthesis is catalyzed by β−1,4-galactosyltransferase 6 (B4GALT6), an enzyme whose expression is controlled by NF-κB (Chatterjee et al., 2008), and which is upregulated in astrocytes during the progressive phase of EAE in non-obese diabetic mice (Mayo et al., 2014) (Fig. 1). LacCer produced by activated astrocytes enhances IRF-1 and NF-κB recruitment to the promoter regions of Ccl2, Csf2 and Nos2, which subsequently induces cytokine production, microglial activation, and pro-inflammatory monocyte recruitment. Selective inactivation of B4GALT6 in astrocytes suppressed this effect and reduced the infiltration of pro-inflammatory monocytes into the CNS (Mayo et al., 2014), collectively highlighting the relevance of astrocytic sphingolipid signaling in the context of CNS inflammation.
Based on the potential deleterious consequences of its dysregulated activation, it is not surprising that multiple mechanisms limit NF-κB activation in astrocytes. One of these mechanisms involves the ligand- activated transcription factor aryl hydrocarbon receptor (AHR), whose activity is modulated by small molecules provided, for example, by cellular and commensal flora metabolism (Gutiérrez-Vázquez and Quintana, 2018; Wheeler et al., 2017) (Fig. 1). Indeed, the metabolism of dietary tryptophan by the intestinal microbiota is an important physiologic source of AHR agonists (Rothhammer and Quintana, 2019). Following activation by its agonists, AHR can limit NF-κB signaling through several mechanisms including those mediated by the suppressor of cytokine signaling 2 (SOCS2) (Rothhammer et al., 2016; Yeste et al., 2016), and also AHR direct dimerization with the NF-κB subunits RelA and RelB (Rothhammer and Quintana, 2019; Salisbury and Sulentic, 2015; Vogel et al., 2014; Vogel et al., 2007). Accordingly, dietary metabolites derived from tryptophan suppress NF-κB signaling in astrocytes and limit CNS inflammation in an AHR-dependent manner (Rothhammer et al., 2016). Specific inactivation of Ahr in astrocytes worsens EAE and increases the expression of pro-inflammatory cytokines (IL-6, IL-12, IL-23, GM-CSF, NO), chemokines (CCL2, CCL20, CXCL10) and other molecules associated with astrocyte reactivity (vimentin, GFAP).
Interestingly, some gut commensal bacteria known to produce AHR agonists are ampicillin-sensitive (Zelante et al., 2013); their elimination by ampicillin administration at late stages of EAE worsens disease outcome. This disease worsening and the concomitant increased expression of pro-inflammatory genes in astrocytes is reversed by the administration of indoxyl-3-sulfate (I3S), an AHR agonist whose levels in circulation are controlled by the gut flora (Rothhammer et al., 2016), overall highlighting how AHR participates in the control of NF-kB activation and CNS inflammation by the gut-CNS axis.
Astrocyte Interactions with Microglia during Neuroinflammation
Astrocytes and other glial cells, including microglia and oligodendrocytes, form and maintain a highly controlled microenvironment essential for efficient neuron function within the CNS (Allen and Lyons, 2018). While glial cells were initially thought to merely provide trophic support for neurons, it is now clear that a closely intertwined neuron-glia network is a key prerequisite for adequate CNS function (Chung et al., 2015; Fields and Stevens-Graham, 2002; Keren-Shaul et al., 2017; Liddelow and Barres, 2017; Wallace and Raff, 1999; Wheeler et al., 2020b).
Astrocytes, microglia, and their interactions control CNS physiology in health and disease (Colombo and Farina, 2016; Glass and Saijo, 2010; Goldmann and Prinz, 2013; Keren-Shaul et al., 2017; Liddelow and Barres, 2017; Prinz et al., 2019; Rothhammer et al., 2018; Rothhammer and Quintana, 2019; Sofroniew, 2009; Vainchtein et al., 2018; Wheeler et al., 2020b). The molecular tête-à-tête between astrocytes and microglia begins early during their colonization of the CNS parenchyma and plays an essential role in CNS health and neuronal transmission (Pfrieger and Barres, 1997). Bidirectional communication between astrocytes and microglia modulates CNS inflammation through the secretion of multiple cytokines and inflammatory mediators. For example, Liddelow and colleagues (2017) reported that LPS-activated microglia induce a neurotoxic phenotype in reactive astrocytes. Specifically, the authors found that microglial secretion of IL-1α, TNF-α, and complement component 1q (C1q) induces a transcriptional response in astrocytes characterized by the production of an as-yet unidentified neurotoxic factor, decreased phagocytic activity, and the reduced expression of neurotrophic factors (Fig. 2A). Based on the expression of complement component 3 (C3), a putative marker of these neurotoxic astrocytes, the authors detected this neurotoxic astrocyte subset in several neurodegenerative disorders like HD, AD, and MS, suggesting that common mechanisms mediate microglia- astrocyte crosstalk in these diseases.
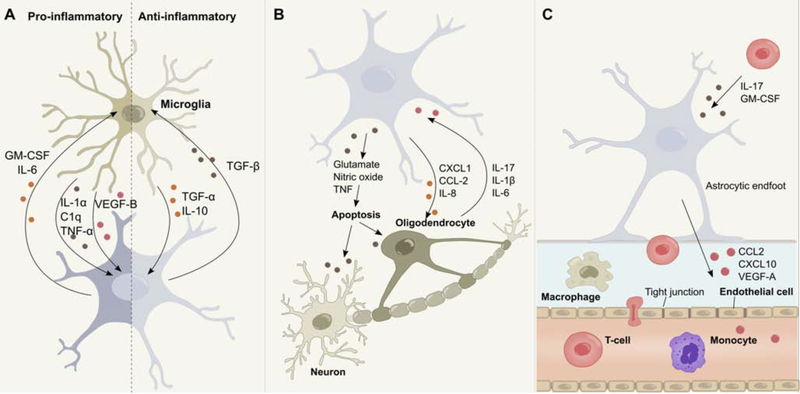
Figure 2. Astrocyte interactions in the context of neuroinflammation.
(A) Bidirectional communication between astrocytes and microglia regulates their responses during CNS inflammation. (B) Upon activation, astrocytes release neurotoxic amounts of NO, glutamate or downregulate the uptake of extracellular neurotransmitter, ultimately leading to neuronal and oligodendrocyte death. Astrocytes furthermore control the recruitment of oligodendrocytes through the secretion of multiple cytokines. In addition, astrocytes respond to pro-inflammatory mediators secreted by activated oligodendrocytes. (C) Astrocytes control the recruitment of leukocytes into perivascular spaces and the CNS parenchyma through the secretion of multiple molecules. Interactions between activated astrocytes and endothelial cells increase BBB permeability and facilitate leukocyte infiltration, while bidirectional communication between astrocytes and peripheral immune cells potentiates CNS inflammation and contributes to disease progression.
Astrocyte-microglia communication likely involves multiple mechanisms. One recent study identified positive and negative microglial regulators of astrocyte pathogenic responses. Rothhammer et al (2018) reported that AHR signaling in microglia regulates the expression of pro-inflammatory genes (Ccl2, Illb, Nos2) in astrocytes by modulating the microglial expression of vascular endothelial growth factor (VEGF)-B and transforming growth factor (TGF)-α (Fig. 2A). Microglial VEGF-B boosts NF-κB translocation in astrocytes via FLT-1 signaling to drive their pathogenic activities during EAE, while TGF-a acts via the ErbB1 receptor in astrocytes to limit EAE progression and induce the production of neuroprotective factors (White et al., 2008). Interestingly, the production of these factors in microglia is regulated by the intestinal flora via metabolites that cross the BBB and control microglial transcriptional programs (2016).
Other mechanisms by which microglia actively control astrocyte functions in neuroinflammation include stromal cell-derived factor (SDF)-1a signaling through C-X-C chemokine receptor type 4 (CXCR4), reported to drive glutamate release by astrocytes. Bezzi and colleagues (2001) showed that SDF1α-CXCR4 signaling in the presence of TNF-α leads to increased intracellular Ca2+ levels and astrocyte glutamate release. Microglial TNF-α production promotes astrocyte glutamate release, which boosts neuron excitotoxicity (Fig. 2B). In summary, these findings illustrate how microglial factors modulate astrocyte responses, and consequently CNS pathology.
The interactions mentioned above highlight the importance of microglia to astrocyte communication in the regulation of CNS inflammation and neurodegeneration. Indeed, is important to consider the reciprocal regulation of microglia by astrocytes. GM-CSF is a known regulator of microglial activation, participating in numerous pro-inflammatory processes essential for EAE development (Hamilton, 2008; McQualter et al., 2001; Wheeler et al., 2020b). Interestingly, GM-CSF is produced by astrocytes as determined by GM-CSF fate-mapping and other studies (Komuczki et al., 2019; Wheeler et al., 2019). Along these lines, Mayo et al. demonstrated that B4GALT6/LacCer-dependent signaling in reactive astrocytes modulates transcriptional programs in microglia and CNS-infiltrating monocytes via the production of GM-CSF (Mayo et al., 2014) (Fig. 2A).
Similar observations have been made for IL-6, a potent mediator of CNS inflammation with important roles in EAE development (Atreya et al., 2000; Gruol and Nelson, 1997; Heink et al., 2017; Samoilova et al., 1998). So far, only a few studies have examined the direct role of astrocyte-derived IL-6 on neuroinflammation, but recent data by Sanchis and colleagues (2020) demonstrates that conditional ablation of IL-6 in astrocytes ameliorates EAE in a sex-dependent manner. This effect may partially be mediated by the reduced activation of microglia, as global IL-6 depletion studies demonstrated reduced expression of MHC-II and pro-inflammatory genes (Garner et al., 2018; Savarin et al., 2015). The notion that astrocytes promote microglia pro-inflammatory functions through the secretion of IL-6, GM-CSF and other signaling factors is further supported by a recent study that investigated the contribution of environmental factors to the pathogenic activities of astrocytes during neuroinflammation (Wheeler et al., 2019). This study identified a novel signaling pathway driven by sigma receptor 1 (SigmaR1) and inositol-requiring enzyme-1α (IRE1α), leading to the activation of the transcription factor X-box binding protein 1 (XBP1) (Fig. 1), which enhances astrocyte-driven CNS inflammation and controls monocyte and microglia responses during EAE. CRISPR/Cas9-based inactivation of Sigmar1, Ern1 (encoding IRE1α), or Xbp1 in astrocytes, decreased pro-inflammatory gene expression in microglia and monocytes, suggesting that SigmaR1-IRE1α-XBP1 signaling modulates astrocyte-microglia crosstalk (Wheeler et al., 2019). The activation of the unfolded protein response in astrocytes also contributes to prion-induced neurodegeneration, suggesting that therapeutic targeting of this pathway may be broadly applicable to multiple neurologic diseases (Smith et al., 2020).
The complexity of the bidirectional communication between microglia and astrocytes during neuroinflammation was further highlighted by a study investigating the effects of IL-10/TGF-β signaling in CNS-resident cells during inflammation (Norden et al., 2014). In the context of EAE, astrocytes produce TGF-β in response to microglia-derived IL-10, which acts on microglia to limit pro-inflammatory gene expression, while it upregulates the expression of anti-inflammatory genes (Fig. 2A). Collectively, these data suggest that astrocytes modulate microglial transcriptional states as part of a bidirectional dialogue that coordinates the responses of CNS-resident cells. Similar mechanisms of astrocyte-microglia crosstalk participate in CNS development and neural circuit formation, highlighting the relevance of glial communication during development and under healthy conditions. For example, astrocyte-derived IL-33 has been proposed to function as a rheostat, modulating microglial synapse engulfment through mechanisms that control NF-κB signaling and immune functions (Tnf, Cxcl2, Cxcl10) (Nguyen et al., 2020; Vainchtein et al., 2018).
Astrocyte Interactions with Oligodendrocytes during Neuroinflammation
Oligodendrocytes had classically been viewed as immunologically inert—bystanders of pathogenic glial and immune cell responses (Zeis et al., 2016). However, this idea has been recently challenged by several studies which demonstrate that oligodendrocytes play an active role in CNS immunomodulation (Falcão et al., 2018; Gibson et al., 2019; Huynh et al., 2014; Jakel et al., 2019; Moyon et al., 2015; Peferoen et al., 2014; Zeis et al., 2016; Zeis and Schaeren-Wiemers, 2008). Bidirectional communication between astrocytes and oligodendrocytes plays a vital role in this process, as oligodendrocytes express a wide array of receptors responsive to inflammatory stimuli secreted by astrocytes (Moyon et al., 2015; Omari et al., 2005; Zeis and Schaeren-Wiemers, 2008) and vice versa (Moyon et al., 2015; Ramesh et al., 2012; Tzartos et al., 2008). The exact mechanisms underlying the interrelationship between astrocytes and oligodendrocytes remain largely elusive, but accumulating evidence derived from in vitro and in vivo models of neuroinflammation suggests that their functions are closely intertwined. For instance, it has been shown that activated astrocytes promote apoptosis of oligodendrocytes via TNF (Gomez-Rivera et al., 2017; Kim et al., 2011; Valentin-Torres et al., 2018), Fas ligand (FasL) (Li et al., 2002) and the secretion of glutamate (Bezzi et al., 2001), leading to reduced remyelination, myelin clearance and subsequent neuronal death (Butts et al., 2008) (Fig. 2B). This process has been postulated to amplify, or even initiate CNS autoimmunity (Traka et al., 2016), although inconsistent results call for the reevaluation of this “inside-out” hypothesis of the origin of CNS inflammation (Locatelli et al., 2012). Conversely, astrocytes can also promote neuroprotective oligodendrocyte functions through the recruitment of oligodendrocyte progenitor cells (OPCs) to sites of inflammation via the secretion of CXCL1, IL-8 and CCL-2 (Moyon et al., 2015; Omari et al., 2005) (Fig. 2B). Together with the production of ciliary neurotrophic factor (CNTF), these astrocyte activities promote the differentiation of OPCs into mature myelinating cells, which increases remyelination in areas of CNS inflammation and, consequently, help restore nerve conduction (Domingues et al., 2016).
These seemingly opposing outcomes of astrocyte-oligodendrocyte interactions highlight the need for studies focused on the identification of the specific astrocyte and oligodendrocyte subsets that participate in these interactions. Indeed, it was recently shown that reactive astrocytes contribute to oligodendrocyte cell death in a microglia-dependent manner in a mouse model of chemotherapy (Gibson et al., 2019). These data, coupled with new tools that enable the dissection of oligodendrocyte networks (Mount et al., 2019), promise to lend new insight into the interactions between astrocytes and oligodendrocytes. Other studies suggest that oligodendrocytes contribute to CNS inflammation through additional mechanisms beyond myelination. For example, Falcão and colleagues (2018) reported that oligodendrocytes participate in phagocytosis, antigen presentation, and the activation of memory and effector CD4 T cells. Oligodendrocytes also secrete the pro-inflammatory cytokines IL-1β, CCL-2, IL-17 and IL-6 (Moyon et al., 2015; Ramesh et al., 2012; Tzartos et al., 2008), which induce NF-κB signaling and pro-inflammatory functions in astrocytes (Fig. 2B). In addition, oligodendrocytes reportedly contribute to CNS inflammation by disrupting BBB integrity (Niu et al., 2019), at least partially through the competition of oligodendrocytes with astrocyte endfeet for cerebral vasculature, which subsequently leads to the downregulation of tight-junction integrity (Niu et al., 2019). Finally, single nuclei RNA-sequencing (snRNA-seq) studies of the white matter of MS patients suggest these pathogenic functions might be associated to distinctive oligodendrocyte sub-populations (Jakel et al., 2019). These findings highlight the importance of bi-directional communication between specific astrocyte and oligodendrocyte populations for the pathogenesis of neurological disorders and set the stage for future investigations of their interactions.
Astrocyte Interactions with Neurons during Neuroinflammation
Astrocytes provide critical trophic support for neurons and play essential roles in health and development (Allen et al., 2012a; Allen and Barres, 2009; Allen and Eroglu, 2017; Christopherson et al., 2005; Chung et al., 2015). While initially considered as ‘brain glue’, providing a structural scaffold necessary for neuronal function, it is now clear that astrocyte-neuron interactions reach far beyond the simplistic idea of structural aid (Allen and Barres, 2009; Liddelow and Barres, 2017). Indeed, astrocyte-neuron interactions contribute to the pathogenesis of multiple neurologic diseases, and the neurotoxic capabilities of reactive astrocytes have been widely discussed (Anderson et al., 2014; Liddelow and Barres, 2017; Wheeler et al., 2020b; Zamanian et al., 2012), although a bona fide astrocytic neurotoxic factor remains to be discovered.
In this context, multiple studies have shown that the activation of NF-κB signaling in astrocytes during CNS inflammation triggers the production of NO (Locatelli et al., 2018; Rothhammer and Quintana, 2015; Wheeler et al., 2019), which shows detrimental effects on neurons when in excess (Calabrese et al., 2007). Indeed, a study by Colombo et al. (2012) presents an interesting conundrum of neurotrophin-induced NO neurotoxicity in astrocytes. While BDNF and NO foster neuronal survival under healthy conditions, elevated BDNF levels and the upregulation of its receptor, tropomyosin-related kinase B (TrkB) on activated astrocytes result in excessive NO production in vitro and, consequently, NO-driven neurotoxicity (Figs. 1,2B) (Colombo et al., 2012). Accordingly, the astrocyte-specific inactivation of TrkB ameliorates EAE and decreases neurodegeneration. In addition to NO-driven neurotoxicity, reactive astrocytes can also promote neuronal death through deficits in the control of neurotransmitter uptake and release. As described above, this effect is partially regulated by microglial CXCR4-dependent release of excessive amounts of glutamate during neuroinflammation, ultimately resulting in excitotoxicity and neuronal loss (Bezzi et al., 2001) (Fig. 2B). In a model of HD, Tong and colleagues demonstrated that accumulation of mutant huntingtin (mHTT) in striatal astrocytes was associated with the reduced expression of the inward rectifying K+ channel Kir4.1, resulting in increased extracellular K+ levels and consequently, neuronal excitotoxicity (Tong et al., 2014). Other roles of astrocytes in excitotoxicity include the reduced expression of the glutamate uptake transporters GLAST and GLT-1 in models of neuroinflammation (Pitt et al., 2000), AD (Matos et al., 2008) and ALS (Pardo et al., 2006), as well as reduced GABAergic neurotransmission in HD (Yu et al., 2018), highlighting the role of altered glial neurotransmitter recycling as a common mechanism of neurodegeneration in multiple diseases.
Besides the excessive secretion of cytotoxic species and the dysregulation of neurotransmitter uptake and release, alterations in the metabolic crosstalk between astrocytes and neurons also contribute to neurodegeneration in MS and other diseases. Chao et al (Chao et al., 2019) demonstrated that LacCer-induced activation of cytosolic phospholipase A2 (cPLA2) and its interaction with the mitochondrial antiviral signaling protein (MAVS) alters astrocyte metabolism, impairing the metabolic support of neurons via the lactate shuttle (Suzuki et al., 2011). Specifically, the LacCer-induced binding of cPLA2 to MAVS disrupts the complex of MAVS with hexokinase-2 (HK2), decreasing glycolysis and lactate production (Fig. 1). Under healthy conditions, lactate provided by astrocytes supports the metabolic needs of neurons (Magistretti and Allaman, 2018). However, the decreased metabolic support of neurons by astrocytes amplifies neurodegeneration. Similar observations of dysfunctional metabolic coupling between astrocytes and neurons have been made in the context of AD (Merlini et al., 2011), PD (Öhman and Forsgren, 2015), HD (Diaz-Castro et al., 2019; Polyzos et al., 2019), and ALS (Ferraiuolo et al., 2011). Although much of these data are derived from in vitro work, these findings strongly suggest that astrocytes can promote neuronal death by multiple mechanisms including excitotoxicity and dysfunctional metabolism. This astrocyte-driven neurotoxicity could synergize with deficits in the regulation of behavior by astrocytes in specific neural circuits, which may also contribute to and amplify neurologic disability (Adamsky et al., 2018; Nagai et al., 2019; Yu et al., 2018). New approaches will be needed to validate our current understanding of astrocyte-induced neurotoxicity in vivo and examine how therapeutic strategies could be used to reprogram reactive astrocytes in a neuroprotective state.
Astrocyte Interactions with Endothelial Cells during Neuroinflammation
Astrocyte foot processes form the glia limitans, which together with capillary endothelial cells, pericytes, and the basal lamina constitute the BBB that separates the CNS from the peripheral blood circulation (Abbott et al., 2006; Chow et al., 2020; Obermeier et al., 2013; Sofroniew, 2015b). Under healthy conditions, the BBB regulates the influx of hydrophilic substances, protects the brain from circulating pathogens, and contributes to the partial ‘immune privilege’ of the CNS (Abbott et al., 2006; Kipnis, 2016; Louveau et al., 2015b; Obermeier et al., 2013; Sofroniew, 2015b). Astrocytes and endothelial cells act as gatekeepers of the CNS and their crosstalk is essential to restrict leukocyte trafficking into the parenchyma. In the context of neuroinflammation, bidirectional communication between astrocytes and endothelial cells promotes BBB leakiness and allows for the infiltration of peripheral immune cells. Astrocyte-derived VEGF plays a central role in this process and participates in a signaling cascade that eventually results in increased BBB permeability (Sofroniew, 2015b). Indeed, Argaw and colleagues (2012) demonstrated that VEGF-A production in astrocytes is upregulated in response to IL-1β, a cytokine produced by activated microglia during neuroinflammation (Liddelow et al., 2017; Prinz et al., 2019; Wheeler et al., 2020b). VEGF-A induces the endothelial nitric oxide synthase (eNOS)-dependent downregulation of tight-junction proteins claudin-5 (Cldn5) and occludin (Ocln) in endothelial cells, which eventually disrupts tight-junctions and BBB integrity. In line with these findings, conditional ablation of Vegfa in astrocytes ameliorates EAE, and is characterized by a reduction in leukocyte infiltration, demyelination, and oligodendrocyte loss (Argaw et al., 2012).
Astrocytes also produce factors that boost BBB integrity during inflammatory conditions. For example, astrocytes promote BBB stability via the production of sonic-hedgehog (Shh) (Alvarez et al., 2011), a morphogen that has numerous roles during development and adulthood (Fuccillo et al., 2006; Ihrie et al., 2011). Stimulation of human astrocytes with TNF-α and IFN-γ leads to increased expression of Shh and subsequent upregulation of endothelial cell tight-junctions. Of note, SHH+ immunoreactive astrocytes are detected around MS demyelinating lesions, and SHH receptor expression is upregulated in endothelial cells (Alvarez et al., 2011). Collectively, these data highlight the importance of the control of the BBB by astrocyte-endothelial cell interactions during CNS inflammation, as well as the need to identify astrocyte subsets with seemingly opposing roles on BBB integrity.
Astrocyte Interactions with Peripheral Immune Cells during Neuroinflammation
The CNS parenchyma is usually considered an ‘immune privileged’ compartment under healthy conditions, which provides limited access to peripheral antibodies and leukocytes (Louveau et al., 2015a; Ransohoff and Engelhardt, 2012). However, astrocytes are amongst the first CNS-resident cells encountered by CNS-infiltrating leukocytes during neuroinflammation. Moreover the anatomical location of astrocyte endfeet enables them to react to soluble factors in the meningeal space (Iliff et al., 2012; Sofroniew, 2015b). As activated immune cells migrate from the periphery into the CNS, bidirectional interactions with astrocytes actively shape the recruitment, diapedesis and extravasation of leukocytes past endothelial barriers into perivascular spaces and the CNS parenchyma. CCL2 and CXCL10 are chemokines secreted by activated astrocytes that control the recruitment of perivascular leukocytes into the CNS (Fig. 2C) (Kim et al., 2014; Mills Ko et al., 2014; Paul et al., 2014). Interestingly, both CCL2 and CXCL10 are controlled by NF-κB (Brambilla et al., 2012; Brambilla et al., 2009), and have been shown to play important roles during CNS inflammation. For example, astrocyte-derived CCL2 is critical for both the onset (Huang et al., 2001) and the progression of EAE (Kim et al., 2014; Moreno et al., 2014), as its deletion is associated with decreased leukocyte infiltration and a transcriptional shift of macrophages towards an anti-inflammatory phenotype. Similar observations have been made for astrocyte-derived CXCL10, as the conditional ablation of CXCL10 results in decreased accumulation of leukocytes in perivascular spaces (Mills Ko et al., 2014). While CCL2 and CXCL10 represent signaling molecules through which astrocytes control the recruitment of monocytes, macrophages and T cells to the CNS (Gerard and Rollins, 2001), astrocyte-derived CXCL12 regulates the recruitment of pathogenic B-cells (Krumbholz et al., 2006).
Astrocytes also control peripheral leukocytes once they have been recruited to the CNS. Following activation, astrocytes upregulate the expression of FasL to induce cell death in infiltrating lymphocytes via caspase signaling (Choi et al., 1999; Wang et al., 2013b). Conditional ablation of FasL expression by astrocytes worsens EAE, further supporting an anti-inflammatory role of astrocyte-induced apoptosis (Wang et al., 2013b). In contrast, Krumbholz and colleagues (2005) defined a mechanism through which astrocyte-derived BAFF supports B-cell survival in inflammatory diseases and primary B cell lymphoma. In support of these findings, BAFF is expressed by human astrocytes and increased BAFF levels are detected in the cerebral spinal fluid of MS patients. These opposing effects of astrocytes on T- and B-cell survival are in agreement with astrocyte targeting studies in early versus late stages of EAE, therefore highlighting the time-dependency (likely associated to the dominance of different activation states during the disease course) of their functions in disease (Liedtke et al., 1998; Mayo et al., 2014; Toft-Hansen et al., 2011; Voskuhl et al., 2009). While ablation of astrocytes during early stages potentially prevent astrocyte-induced cell death in infiltrating leukocytes and consequently allows for the spread of peripheral immune cells in the CNS, loss of astrocytes during late stages might in turn hinder pathogenic B-cell functions and reduce CNS inflammation.
In addition, astrocytes can sense and respond to various inflammatory cues once leukocytes are engrafted within the CNS parenchyma. For example, TH1 cell-derived IFN-γ upregulates astrocyte expression of IFN-g receptor 1 (IFNGR1) and MHC-II, allowing them to act as nonprofessional antigen-presenting cells (APCs) (Fontana et al., 1984; Gold et al., 1996; Hashioka et al., 2010; Soos et al., 1998; Sun and Wekerle, 1986; Yang et al., 2012). Furthermore IL-17, the signature cytokine of pathogenic TH17 cells, and GM-CSF, produced by pathogenic TH1 and TH17 cells, activate pro-inflammatory transcriptional programs in astrocytes (Elain et al., 2014; Kang et al., 2010; Wheeler et al., 2020b; Yi et al., 2014) (Fig. 2C). Taken together, these findings highlight the important functions of astrocytes in the potentiation of inflammatory cascades in the CNS. In a positive feedback loop, astrocytes can respond to pro-inflammatory cytokines secreted by infiltrating lymphocytes with the production of chemokines that further attract peripheral immune cells to the CNS, ultimately leading to chronic CNS inflammation and neurodegeneration.
Epigenetic Alterations in Astrocytes during CNS Inflammation
The chronic exposure to inflammatory signals and crosstalk with CNS-resident and non-resident cells alters astrocyte transcriptional programs, inducing “memory” responses stabilized by epigenetic modifications (Saijo et al., 2009; Wheeler et al., 2020b). In the context of inflammation, “memory” is usually associated with the response of T cells and B cells to repetitive encounters with the same antigen. Thus, a first encounter with a stimulus (“priming”) elicits transcriptional modifications, which lead to a different response in terms of quantity and quality, upon later encounter with the same stimulus. Immune memory can manifest as “immune training”, which describes an enhanced and diversified response to re-stimulation; or “immune tolerance,” which refers to a dampened response upon secondary stimulation (Neher and Cunningham, 2019; Nott and Glass, 2018; Wendeln et al., 2018).
Pioneering studies have extended the concept of memory to the innate immune response, to describe “trained” innate immunity associated to extensive epigenetic and metabolic remodeling (Netea et al., 2020). Indeed, it has been recently reported that microglia can also undergo training (Nott and Glass, 2018; Nott et al., 2019; Wendeln et al., 2018). This is an important finding in the context of neurological diseases, as epigenetic alterations may stabilize pathogenic activation states in CNS-resident cells, and might therefore offer novel therapeutic targets. Indeed, multiple reports suggest an important role for epigenetic modifications in the control of glial responses during neurologic disorders (Ayata et al., 2018; Baranzini et al., 2010; Huynh et al., 2014; Koch et al., 2013; Kular et al., 2018; Staszewski and Prinz, 2014; Wendeln et al., 2018).
Most work on trained immunity in glial cells has focused on microglia, as they express a multitude of immune receptors (Ayata et al., 2018; Colonna and Butovsky, 2017; Heneka et al., 2015; Nott et al., 2019; Prinz et al., 2019; Saijo et al., 2009; Wendeln et al., 2018). In a recent study, Wendeln and colleagues (2018) explored the question of whether microglia are trained in the context of neurologic diseases, and potentially contribute to the progression of AD and stroke. These studies established that peripherally applied pro-inflammatory stimuli drive immune training and tolerance mechanisms in microglia through extensive epigenetic reprogramming, inducing changes in microglial responses that persist for at least six months. Furthermore, these studies established that microglia trained by a single injection of lipopolysaccharide (LPS) exacerbate cerebral β-amyloidosis, while repeated LPS stimulation leads to immune tolerance and reduced Aβ-accumulation. Interestingly, these studies also detected reduced numbers of astrocytes in LPS-primed mice. Indeed, pro-inflammatory stimuli have been shown to induce epigenetic alterations in astrocytes, with long-term consequences for neuroimmune crosstalk (Beurel, 2011; Correa et al., 2011; Suh et al., 2010; Wheeler et al., 2020b). Furthermore, studies on the epigenetic control of astrocytes during differentiation and development support potential roles for epigenetic modifications in the regulation of astrocyte responses during neuroinflammation (Hatada et al., 2008; O’Callaghan et al., 2014; Takizawa et al., 2001); future studies should determine whether bona fide astrocyte trained responses contribute to the pathogenesis of neurologic disorders.
Histone Modifications
The epigenetic processes supporting trained immunity can broadly be divided into histone modifications and DNA methylation. Histone methylation and acetylation have been linked to the control of astrocytes in CNS inflammation. In a study investigating the effects of aging on histone methylation in astrocytes following ischemia, Chisholm et al. (2015) found that cerebral artery occlusion resulted in an increase of the H3K4me3 histone methylation mark (transcriptional enhancer), while levels of H3K9me3 (transcriptional repressor) were reduced, therefore suggesting an overall increase in transcriptional activity. Interestingly, pathway analysis detected increased VEGF signaling associated to higher H3K4me3 levels and decreased histone deacetylase (HDAC) expression, supporting a long-term effect of astrocyte-derived VEGF production on endothelial cell function and BBB permeability. HDACs have long been targeted in neurological disorders (Falkenberg and Johnstone, 2014) and multiple reports support a role of HDACs in astrocyte activation and neuroimmune crosstalk (Beurel, 2011; Correa et al., 2011; Saijo et al., 2009; Suh et al., 2010).
In another study, Ayata and colleagues (2018) reported that environmental stimuli drive the epigenetic regulation of region-specific clearance activity in microglia. The authors found that exposure to apoptotic cells induce the expression of KDM6A and KDM6B, histone demethylases that catalyze the removal of the transcriptional repressor H3K27me3 histone methylation marks in microglia, leading to the induction of inflammatory gene expression. Similar mechanisms may operate in astrocytes, as soluble factors derived from microglia boost HDAC activity in astrocytes (Correa et al., 2011). Although the specific signals through which microglia control histone modifications in astrocytes are not yet known, these initial findings suggest long-term effects of astrocyte-microglia crosstalk.
A pioneering study by Saijo and colleagues (2009) defined a microglia-astrocyte trans-repression pathway that negatively regulates pro-inflammatory responses and reduces neurotoxicity in the context of PD. The authors demonstrated that microglia and astrocyte activation induces the recruitment of the nuclear receptor related-1 (Nurr1) transcription factor, the co-repressor complex CoREST and NF-κB to target sites that control pro-inflammatory gene expression. The resulting transcriptional suppression of pro-inflammatory mediator expression in microglia and astrocytes prevented the synergistic amplification of pro-inflammatory responses and neurotoxicity. HDAC1 is a component of the CoREST complex. Thus, a Nurr1-mediated negative feedback loop between astrocytes and microglia might participate in the long-term suppression of pro-inflammatory responses. This hypothesis is in line with the findings of Beurel et al (Beurel, 2011), who reported that repeated stimulation of astrocytes with LPS results in HDAC6-induced histone deacetylation and tolerance characterized by decreased IL-6 production. Collectively, these results suggest that histone modifications induced by the crosstalk with other cell types, trigger long-term changes in astrocyte responses.
DNA Methylation
DNA methylation represses gene expression through the addition of methyl groups that impair interactions with transcriptional regulators (Greenberg and Bourc’his, 2019). DNA methylation is tightly regulated during astrocyte development (Fan et al., 2005; Hatada et al., 2008). Similarly, DNA methylation was recently reported to control astrocyte pro-inflammatory responses (Wheeler et al., 2020b), and has been proposed to contribute to CNS pathology in MS (Huynh et al., 2014). Single-cell RNA sequencing identified a subset of astrocytes expanded in EAE and MS. This astrocyte subset was characterized by decreased activation of NRF2, a transcription factor linked to neuroprotective astrocyte functions (Filippi et al., 2018). Follow up genomic analyses identified MAFG, a basic region and leucine zipper (bZIP)-type transcription factor of the family of small MAF proteins, as a negative regulator of NRF2 signaling in activated astrocytes. MAFG heterodimerizes with NRF2 to induce NRF2-driven gene expression, but MAFG homodimers compete for MAFG/NRF2 responsive elements to suppress NRF2-signaling (Katsuoka and Yamamoto, 2016) (Fig. 3). In addition, MAFG cooperates with the methionine adenosyltransferase IIα (MAT2α), which participates in the synthesis of substrates for DNA methylation and has been shown to cooperate with small MAF proteins to act as a transcriptional repressor (Katoh et al., 2011) (Fig. 3). In line with these findings, increased DNA méthylation was detected in NRF2-responsive elements of this astrocyte subset during EAE. Moreover, MAFG- and MAT2α-dependent CRISPR/Cas9-driven inactivation of Mat2α or Mafg ameliorated EAE.
Figure 3. Pro-inflammatory cytokine-induced epigenetic suppression of NRF2 signaling during CNS inflammation.
During initial exposure to inflammatory signals, astrocytes upregulate the formation of MAFG homodimers, which outcompete MAFG/NRF2 heterodimer binding to transcriptional response elements that control NRF2 signaling. In addition, MAT2α cooperates with MAFG and induces DNA methylation marks that restrict chromatin accessibility. Collectively, these epigenetic modifications suppress NRF2-driven inhibition of NF-κB signaling and lead to sustained inflammation.
Of note, GM-CSF produced by pro-inflammatory T-cells recruited to the CNS drives MAFG/MAT2α signaling in astrocytes, suggesting that the crosstalk between astrocytes and T cells regulates the epigenetic program that stabilizes and controls this astrocyte subset in the context of CNS inflammation (Fig. 3). Taken together, these observations shed new light onto the long-term epigenetic regulation of glial responses and its control by astrocyte-immune cell crosstalk (Ayata et al., 2018; Jakovcevski and Akbarian, 2012; Staszewski and Prinz, 2014). Novel tools, such as spatial transcriptomics (Eng et al., 2019; Moffitt et al., 2018; Rodriques et al., 2019; Wang et al., 2018), astrocyte-specific optogenetics (Adamsky et al., 2018; Nagai et al., 2019), and neuron-glia circuit mapping in virtual environments (Mu et al., 2019), will deepen our understanding of the epigenetic control of astrocyte responses, while identifying candidate targets for therapeutic intervention.
Concluding remarks
Astrocytes play multiple roles during CNS inflammation. On the one hand, they can restrict the influx of peripheral immune cells into the CNS, while producing neurotrophic factors to promote tissue repair. On the other hand, astrocytes can promote neurodegeneration and inflammation through the recruitment of peripheral inflammatory cells, the activation of CNS-resident microglia and their own intrinsic neurotoxic activities. The proper control of these diverse astrocyte responses requires the precise integration of signaling cues derived from CNS-resident and -recruited cells, highlighting the importance of regional location and cellular environment.
A central question in astrocyte biology has been the extent of astrocyte heterogeneity. The diversity of astrocyte subsets and activation states is now widely appreciated (Anderson et al., 2014; Ben Haim and Rowitch, 2017; Khakh and Deneen, 2019), but many questions still remain outstanding. Future studies should address important questions: (1) How many astrocyte subsets exist? (2) How are they regulated, how plastic are they, and what are their functions? (3) Where are they located? (4) With which cells do they communicate? (5) What are the correlates of these populations in humans? Clues to solving these problems could be gleaned from pioneering fate-mapping and cross-species studies of microglia (Geirsdottir et al., 2019; Ginhoux et al., 2010; Tay et al., 2017).
Novel technologies will also be needed to extensively characterize the astrocytic subsets involved, their cellular interactions, and what defines them in the context of their location in specific CNS microenvironments. For example, high throughput screens for the molecular characterization of astrocytes across brain region and disease states would greatly advance our knowledge of astrocyte heterogeneity. These screens may enable a thorough analysis of the interactions between astrocytes and other cells in the CNS, seeding additional questions: Which molecules are used by astrocytes to communicate with neighboring cells? On what timescale? How plastic are these interactions? Importantly, these analyses may lead to an understanding of how astrocyte interactions with other cell types could be exploited for therapeutic purposes.
In addition, it is important to understand astrocyte networks on a global level. Both functional and structural connectivity-mapping studies are needed to establish how astrocytes interact with neurons, other glial cells, and immune cells in health and disease (Fields, 2013). Analogous approaches have transformed our understanding of neural circuits (Chen et al., 2019; Huang et al., 2020; Kebschull et al., 2016; Oh et al., 2014). In summary, the future of glial biology will likely rest on the integration of multiple high throughput technologies to define the location, plasticity, connectivity, regulation and function of astrocyte subsets across the CNS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, Rönnbäck L, and Hansson E (2006). Astrocyte-endothelial interactions at the blood–brain barrier. Nature Reviews Neuroscience 7, 41–53. [DOI] [PubMed] [Google Scholar]
- Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T, Refaeli R, Horn H, Regev L, Groysman M, et al. (2018). Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 174, 59–71 e14. [DOI] [PubMed] [Google Scholar]
- Allen M, Zou F, Chai HS, Younkin C, Crook J, Pankratz V, Carrasquillo M, Rowley C, Nair A, Middha S, et al. (2012a). Novel Late-Onset Alzheimer’s Disease Loci Variants Associate with Brain Gene Expression (S54.001). Neurology 78, S54.001–S054.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, and Barres BA (2009). Neuroscience: Glia - more than just brain glue. Nature 457, 675–677. [DOI] [PubMed] [Google Scholar]
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, and Barres BA (2012b). Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486, 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, and Eroglu C (2017). Cell biology of astrocyte-synapse interactions. Neuron 96, 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ, and Lyons DA (2018). Glia as architects of central nervous system formation and function. Science 362, 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M, et al. (2011). The Hedgehog Pathway Promotes Blood-Brain Barrier Integrity and CNS Immune Quiescence. Science 334, 1727–1731. [DOI] [PubMed] [Google Scholar]
- Anderson MA, Ao Y, and Sofroniew MV (2014). Heterogeneity of reactive astrocytes. Neurosci Lett 565, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, and Sofroniew MV (2016). Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, O’Shea TM, Burda JE, Ao Y, Barlatey SL, Bernstein AM, Kim JH, James ND, Rogers A, Kato B, et al. (2018). Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature 561, 396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, et al. (2012). Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 122, 2454–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C, et al. (2000). Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: Evidence in Crohn disease and experimental colitis in vivo. Nature Medicine 6, 583–588. [DOI] [PubMed] [Google Scholar]
- Ayata P, Badimon A, Strasburger HJ, Duff MK, Montgomery SE, Loh Y-HE, Ebert A, Pimenova AA, Ramirez BR, Chan AT, et al. (2018). Epigenetic regulation of brain region-specific microglia clearance activity. Nature Neuroscience 21, 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranzini SE, Mudge J, van Velkinburgh JC, Khankhanian P, Khrebtukova I, Miller NA, Zhang L, Farmer AD, Bell CJ, Kim RW, et al. (2010). Genome, epigenome and RNA sequences of monozygotic twins discordant for multiple sclerosis. Nature 464, 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar OA, Bartels T, Holmqvist S, Kleshchevnikov V, Martirosyan A, Polioudakis D, Ben Haim L, Young AMH, Batiuk MY, Prakash K, et al. (2020). Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nature Neuroscience 23, 500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Haim L, and Rowitch DH (2017). Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18, 31–41. [DOI] [PubMed] [Google Scholar]
- Beurel E (2011). HDAC6 Regulates LPS-Tolerance in Astrocytes. PLOS ONE 6, e25804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, Clercq ED, Vescovi A, Bagetta G, Kollias G, Meldolesi J, and Volterra A (2001). CXCR4-activated astrocyte glutamate release via TNFα: amplification by microglia triggers neurotoxicity. Nature Neuroscience 4, 702–710. [DOI] [PubMed] [Google Scholar]
- Blank T, and Prinz M (2014). NF-κB signaling regulates myelination in the CNS. Front Mol Neurosci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu W-H, Frydel B, Bramwell A, Karmally S, Green EJ, and Bethea JR (2005). Inhibition of astroglial nuclear factor κB reduces inflammation and improves functional recovery after spinal cord injury. The Journal of Experimental Medicine 202, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Dvoriantchikova G, Barakat D, Ivanov D, Bethea JR, and Shestopalov VI (2012). Transgenic inhibition of astroglial NF-κB protects from optic nerve damage and retinal ganglion cell loss in experimental optic neuritis. Journal of Neuroinflammation 9, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, and Bethea JR (2014). Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia 62, 452–467. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, Ivanov D, Nathanson L, Barnum SR, and Bethea JR (2009). Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol 182, 2628–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts BD, Houde C, and Mehmet H (2008). Maturation-dependent sensitivity of oligodendrocyte lineage cells to apoptosis: implications for normal development and disease. Cell Death & Differentiation 15, 1178–1186. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, and Giuffrida Stella AM (2007). Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nature Reviews Neuroscience 8, 766–775. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Trzyna WC, McClintock DS, and Schumacker PT (2000). Role of Oxidants in NF-κB Activation and TNF-α Gene Transcription Induced by Hypoxia and Endotoxin. The Journal of Immunology 165, 1013–1021. [DOI] [PubMed] [Google Scholar]
- Chao C-C, Gutiérrez-Vázquez C, Rothhammer V, Mayo L, Wheeler MA, Tjon EC, Zandee SEJ, Blain M, de Lima KA, Takenaka MC, et al. (2019). Metabolic Control of Astrocyte Pathogenic Activity via cPLA2-MAVS. Cell 179, 1483–1498.e1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Kolmakova A, and Rajesh M (2008). Regulation of lactosylceramide synthase (glucosylceramide beta1-->4 galactosyltransferase); implication as a drug target. Curr Drug Targets 9, 272–281. [DOI] [PubMed] [Google Scholar]
- Chen X, Sun YC, Zhan H, Kebschull JM, Fischer S, Matho K, Huang ZJ, Gillis J, and Zador AM (2019). High-Throughput Mapping of Long-Range Neuronal Projection Using In Situ Sequencing. Cell 179, 772–786 e719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm NC, Henderson ML, Selvamani A, Park MJ, Dindot S, Miranda RC, and Sohrabji F (2015). Histone methylation patterns in astrocytes are influenced by age following ischemia. Epigenetics 10, 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Park JY, Lee J, Lim J-H, Shin E-C, Ahn YS, Kim C-H, Kim S-J, Kim J-D, Choi IS, and Choi I-H (1999). Fas Ligand and Fas Are Expressed Constitutively in Human Astrocytes and the Expression Increases with IL-1, IL-6, TNF-α, or IFN-γ. The Journal of Immunology 162, 1889–1895. [PubMed] [Google Scholar]
- Choi JW, Gardell SE, Herr DR, Rivera R, Lee C-W, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G, and Chun J (2011). FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proceedings of the National Academy of Sciences 108, 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow BW, Nunez V, Kaplan L, Granger AJ, Bistrong K, Zucker HL, Kumar P, Sabatini BL, and Gu C (2020). Caveolae in CNS arterioles mediate neurovascular coupling. Nature 579, 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CCA, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, and Barres BA (2005). Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433. [DOI] [PubMed] [Google Scholar]
- Chung W-S, Allen NJ, and Eroglu C (2015). Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harbor Perspectives in Biology 7, a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo E, Cordiglieri C, Melli G, Newcombe J, Krumbholz M, Parada LF, Medico E, Hohlfeld R, Meinl E, and Farina C (2012). Stimulation of the neurotrophin receptor TrkB on astrocytes drives nitric oxide production and neurodegeneration. The Journal of Experimental Medicine 209, 521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo E, and Farina C (2016). Astrocytes: Key Regulators of Neuroinflammation. Trends in Immunology 37, 608–620. [DOI] [PubMed] [Google Scholar]
- Colonna M, and Butovsky O (2017). Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annual Review of Immunology 35, 441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa F, Mallard C, Nilsson M, and Sandberg M (2011). Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: restoring effects of inhibitors of HDACs, p38 MAPK and GSK3β. Neurobiol Dis 44, 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneen B, Ho R, Lukaszewicz A, Hochstim CJ, Gronostajski RM, and Anderson DJ (2006). The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron 52, 953–968. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, and Eggan K (2007). Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci 10, 608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Castro B, Gangwani MR, Yu X, Coppola G, and Khakh BS (2019). Astrocyte molecular signatures in Huntington’s disease. Sci Transl Med 11. [DOI] [PubMed] [Google Scholar]
- Domingues HS, Portugal CC, Socodato R, and Relvas JB (2016). Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front Cell Dev Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elain G, Jeanneau K, Rutkowska A, Mir AK, and Dev KK (2014). The selective anti-IL17A monoclonal antibody secukinumab (AIN457) attenuates IL17A-induced levels of IL6 in human astrocytes. Glia 62, 725–735. [DOI] [PubMed] [Google Scholar]
- Eng C-HL, Lawson M, Zhu Q, Dries R, Koulena N, Takei Y, Yun J, Cronin C, Karp C, Yuan G, and Cai L (2019). Transcriptome-scale super-resolved imaging in tissues by RNA seqFISH. Nature 568, 235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng FL, and Vanderhaegen JJ (1970). A study of proteins in old multiple sclerosis plaques. Trans Am Soc Neurochem 1:42. [Google Scholar]
- Falcão AM, van Bruggen D, Marques S, Meijer M, Jäkel S, Agirre E, Samudyata, Floriddia EM, Vanichkina DP, ffrench-Constant C, et al. (2018). Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis. Nature Medicine 24, 1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg KJ, and Johnstone RW (2014). Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nature Reviews Drug Discovery 13, 673–691. [DOI] [PubMed] [Google Scholar]
- Fan G, Martinowich K, Chin MH, He F, Fouse SD, Hutnick L, Hattori D, Ge W, Shen Y, Wu H, et al. (2005). DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development 132, 3345–3356. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, and Sofroniew MV (2004). Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24, 2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraiuolo L, Higginbottom A, Heath PR, Barber S, Greenald D, Kirby J, and Shaw PJ (2011). Dysregulation of astrocyte-motoneuron cross-talk in mutant superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain: A Journal of Neurology 134, 2627–2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD (2013). Neuroscience: Map the other brain. Nature 501, 25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, and Stevens-Graham B (2002). New Insights into Neuron-Glia Communication. Science 298, 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, and Rocca MA (2018). Multiple sclerosis. Nat Rev Dis Primers 4, 43. [DOI] [PubMed] [Google Scholar]
- Fontana A, Fierz W, and Wekerle H (1984). Astrocytes present myelin basic protein to encephalitogenic T-cell lines. Nature 307, 273–276. [DOI] [PubMed] [Google Scholar]
- Fuccillo M, Joyner AL, and Fishell G (2006). Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nature Reviews Neuroscience 7, 772–783. [DOI] [PubMed] [Google Scholar]
- Garner KM, Amin R, Johnson RW, Scarlett EJ, and Burton MD (2018). Microglia priming by interleukin-6 signaling is enhanced in aged mice. J Neuroimmunol 324, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W-P, Miyawaki A, Gage FH, Jan YN, and Jan LY (2012). Local generation of glia is a major astrocyte source in postnatal cortex. Nature 484, 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geirsdottir L, David E, Keren-Shaul H, Weiner A, Bohlen SC, Neuber J, Balic A, Giladi A, Sheban F, Dutertre CA, et al. (2019). Cross-Species Single-Cell Analysis Reveals Divergence of the Primate Microglia Program. Cell 179, 1609–1622 e1616. [DOI] [PubMed] [Google Scholar]
- Gerard C, and Rollins BJ (2001). Chemokines and disease. Nature Immunology 2, 108–115. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L, et al. (2019). Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment. Cell 176, 43–55 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, and Saijo K (2010). Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nature Reviews Immunology 10, 365–376. [DOI] [PubMed] [Google Scholar]
- Gold R, Schmied M, Tontsch U, Hartung HP, Wekerle H, Toyka KV, and Lassmann H (1996). Antigen presentation by astrocytes primes rat T lymphocytes for apoptotic cell death. A model for T-cell apoptosis in vivo. Brain: A Journal of Neurology 119 ( Pt 2), 651–659. [DOI] [PubMed] [Google Scholar]
- Goldmann T, and Prinz M (2013). Role of microglia in CNS autoimmunity. Clin Dev Immunol 2013, 208093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rivera F, Raphael I, and Forsthuber T (2017). Signaling via TNFR2 mediates CNS remyelination in EAE through regulation of oligodendrocyte progenitor cells. The Journal of Immunology 198, 219.214–219.214. [Google Scholar]
- Greenberg MVC, and Bourc’his D (2019). The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol 20, 590–607. [DOI] [PubMed] [Google Scholar]
- Gruol DL, and Nelson TE (1997). Physiological and pathological roles of interleukin-6 in the central nervous system. Mol Neurobiol 15, 307–339. [DOI] [PubMed] [Google Scholar]
- Gu X-L, Long C-X, Sun L, Xie C, Lin X, and Cai H (2010). Astrocytic expression of Parkinson’s disease-related A53T alpha-synuclein causes neurodegeneration in mice. Mol Brain 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Vázquez C, and Quintana FJ (2018). Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity 48, 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib N, McCabe C, Medina S, Varshavsky M, Kitsberg D, Dvir-Szternfeld R, Green G, Dionne D, Nguyen L, and Marshall JL (2020). Disease-associated astrocytes in Alzheimer’s disease and aging. Nature Neuroscience, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim LB, and Rowitch DH (2017). Functional diversity of astrocytes in neural circuit regulation. Nature Reviews Neuroscience 18, 31–41. [DOI] [PubMed] [Google Scholar]
- Hamilton JA (2008). Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 8, 533–544. [DOI] [PubMed] [Google Scholar]
- Hashioka S, Klegeris A, Schwab C, Yu S, and McGeer PL (2010). Differential expression of interferon-y receptor on human glial cells in vivo and in vitro. Journal of Neuroimmunology 225, 91–99. [DOI] [PubMed] [Google Scholar]
- Hatada I, Namihira M, Morita S, Kimura M, Horii T, and Nakashima K (2008). Astrocyte-Specific Genes Are Generally Demethylated in Neural Precursor Cells Prior to Astrocytic Differentiation. PLOS ONE 3, e3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heink S, Yogev N, Garbers C, Herwerth M, Aly L, Gasperi C, Husterer V, Croxford AL, Möller-Hackbarth K, Bartsch HS, et al. (2017). Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic T(H)17 cells. Nat Immunol 18, 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Golenbock DT, and Latz E (2015). Innate immunity in Alzheimer’s disease. Nature Immunology 16, 229–236. [DOI] [PubMed] [Google Scholar]
- Hochstim C, Deneen B, Lukaszewicz A, Zhou Q, and Anderson DJ (2008). Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 133, 510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Wang J, Kivisakk P, Rollins BJ, and Ransohoff RM (2001). Absence of Monocyte Chemoattractant Protein 1 in Mice Leads to Decreased Local Macrophage Recruitment and Antigen-Specific T Helper Cell Type 1 Immune Response in Experimental Autoimmune Encephalomyelitis. The Journal of Experimental Medicine 193, 713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Kebschull JM, Furth D, Musall S, Kaufman MT, Churchland AK, and Zador AM (2020). BRICseq Bridges Brain-wide Interregional Connectivity to Neural Activity and Gene Expression in Single Animals. Cell 182, 177–188 e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh JL, Garg P, Thin TH, Yoo S, Dutta R, Trapp BD, Haroutunian V, Zhu J, Donovan MJ, Sharp AJ, and Casaccia P (2014). Epigenome-wide differences in pathology-free regions of multiple sclerosis–affected brains. Nature Neuroscience 17, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Shah JK, Harwell CC, Levine JH, Guinto CD, Lezameta M, Kriegstein AR, and Alvarez-Buylla A (2011). Persistent Sonic hedgehog Signaling in Adult Brain Determines Neural Stem Cell Positional Identity. Neuron 71, 250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4, 147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakel S, Agirre E, Mendanha Falcao A, van Bruggen D, Lee KW, Knuesel I, Malhotra D, Ffrench-Constant C, Williams A, and Castelo-Branco G (2019). Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski M, and Akbarian S (2012). Epigenetic mechanisms in neurological disease. Nature Medicine 18, 1194–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C, et al. (2010). Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity 32, 414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh Y, Ikura T, Hoshikawa Y, Tashiro S, Ito T, Ohta M, Kera Y, Noda T, and Igarashi K (2011). Methionine adenosyltransferase II serves as a transcriptional corepressor of Maf oncoprotein. Mol Cell 41, 554–566. [DOI] [PubMed] [Google Scholar]
- Katsuoka F, and Yamamoto M (2016). Small Maf proteins (MafF, MafG, MafK): History, structure and function. Gene 586, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, and Akira S (2007). Signaling to NF-κB by Toll-like receptors. Trends in Molecular Medicine 13, 460–469. [DOI] [PubMed] [Google Scholar]
- Kebschull JM, Garcia da Silva P, Reid AP, Peikon ID, Albeanu DF, and Zador AM (2016). High-Throughput Mapping of Single-Neuron Projections by Sequencing of Barcoded RNA. Neuron 91, 975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, et al. (2017). A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 169, 1276–1290 e1217. [DOI] [PubMed] [Google Scholar]
- Khakh BS, and Deneen B (2019). The Emerging Nature of Astrocyte Diversity. Annu Rev Neurosci 42, 187–207. [DOI] [PubMed] [Google Scholar]
- Kim RY, Hoffman AS, Itoh N, Ao Y, Spence R, Sofroniew MV, and Voskuhl RR (2014). Astrocyte CCL2 sustains immune cell infiltration in chronic experimental autoimmune encephalomyelitis. Journal of Neuroimmunology 274, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Steelman AJ, Koito H, and Li J (2011). Astrocytes promote TNF-mediated toxicity to oligodendrocyte precursors. Journal of Neurochemistry 116, 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J (2016). Multifaceted interactions between adaptive immunity and the central nervous system. Science 353, 766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MW, Metz LM, and Kovalchuk O (2013). Epigenetic changes in patients with multiple sclerosis. Nature Reviews Neurology 9, 35–43. [DOI] [PubMed] [Google Scholar]
- Komuczki J, Tuzlak S, Friebel E, Hartwig T, Spath S, Rosenstiel P, Waisman A, Opitz L, Oukka M, Schreiner B, et al. (2019). Fate-Mapping of GM-CSF Expression Identifies a Discrete Subset of InflammationDriving T Helper Cells Regulated by Cytokines IL-23 and IL-1β. Immunity 50, 1289–1304.e1286. [DOI] [PubMed] [Google Scholar]
- Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisäkk P, Ransohoff RM, Hofbauer M, Farina C, Derfuss T, Hartle C, et al. (2006). Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 129, 200–211. [DOI] [PubMed] [Google Scholar]
- Krumbholz M, Theil D, Derfuss T, Rosenwald A, Schrader F, Monoranu C-M, Kalled SL, Hess DM, Serafini B, Aloisi F, et al. (2005). BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. The Journal of Experimental Medicine 201, 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kular L, Liu Y, Ruhrmann S, Zheleznyakova G, Marabita F, Gomez-Cabrero D, James T, Ewing E, Linden M, Gornikiewicz B, et al. (2018). DNA methylation as a mediator of HLA-DRB1*15:01 and a protective variant in multiple sclerosis. Nature Communications 9, 2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Maeda Y, Ming X, Cook S, Chapin J, Husar W, and Dowling P (2002). Apoptotic death following Fas activation in human oligodendrocyte hybrid cultures. J Neurosci Res 69, 189–196. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, and Barres BA (2017). Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 46, 957–967. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC, et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Edelmann W, Chiu FC, Kucherlapati R, and Raine CS (1998). Experimental autoimmune encephalomyelitis in mice lacking glial fibrillary acidic protein is characterized by a more severe clinical course and an infiltrative central nervous system lesion. The American Journal of Pathology 152, 251–259. [PMC free article] [PubMed] [Google Scholar]
- Lin CCJ, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, et al. (2017). Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 20, 396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locatelli G, Theodorou D, Kendirli A, Jordao MJC, Staszewski O, Phulphagar K, Cantuti-Castelvetri L, Dagkalis A, Bessis A, Simons M, et al. (2018). Mononuclear phagocytes locally specify and adapt their phenotype in a multiple sclerosis model. Nat Neurosci 21, 1196–1208. [DOI] [PubMed] [Google Scholar]
- Locatelli G, Wörtge S, Buch T, Ingold B, Frommer F, Sobottka B, Krüger M, Karram K, Bühlmann C, Bechmann I, et al. (2012). Primary oligodendrocyte death does not elicit anti-CNS immunity. Nat Neurosci 15, 543–550. [DOI] [PubMed] [Google Scholar]
- Loo G.v., Lorenzi RD, Schmidt H, Huth M, Mildner A, Schmidt-Supprian M, Lassmann H, Prinz MR, and Pasparakis M (2006). Inhibition of transcription factor NF-κB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nature Immunology 7, 954–961. [DOI] [PubMed] [Google Scholar]
- Louveau A, Harris TH, and Kipnis J (2015a). Revisiting the concept of CNS immune privilege. Trends in Immunology 36, 569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al. (2015b). Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, and Allaman I (2018). Lactate in the brain: from metabolic end-product to signalling molecule. Nature Reviews Neuroscience 19, 235–249. [DOI] [PubMed] [Google Scholar]
- Matos M, Augusto E, Oliveira CR, and Agostinho P (2008). Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience 156, 898–910. [DOI] [PubMed] [Google Scholar]
- Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, Alvarez JI, Mascanfroni ID, Yeste A, Kivisäkk P, Kallas K, et al. (2014). Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nature Medicine 20, 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, and Bernard CCA (2001). Granulocyte Macrophage Colony-Stimulating FactorA New Putative Therapeutic Target in Multiple Sclerosis. The Journal of Experimental Medicine 194, 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlini M, Meyer EP, Ulmann-Schuler A, and Nitsch RM (2011). Vascular ß-amyloid and early astrocyte alterations impair cerebrovascular function and cerebral metabolism in transgenic arcAß mice. Acta Neuropathol 122, 293–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills Ko E, Ma JH, Guo F, Miers L, Lee E, Bannerman P, Burns T, Ko D, Sohn J, Soulika AM, and Pleasure D (2014). Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. Journal of Neuroinflammation 11, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt JR, Bambah-Mukku D, Eichhorn SW, Vaughn E, Shekhar K, Perez JD, Rubinstein ND, Hao J, Regev A, Dulac C, and Zhuang X (2018). Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, and Deneen B (2015). Astrocyte development: A Guide for the Perplexed. Glia 63, 1320–1329. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Kelley KW, Tsai H-H, Redmond SA, Chang SM, Madireddy L, Chan JR, Baranzini SE, Ullian EM, and Rowitch DH (2014). Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature 509, 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Bannerman P, Ma J, Guo F, Miers L, Soulika AM, and Pleasure D (2014). Conditional Ablation of Astroglial CCL2 Suppresses CNS Accumulation of M1 Macrophages and Preserves Axons in Mice with MOG Peptide EAE. J Neurosci 34, 8175–8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount CW, Yalcin B, Cunliffe-Koehler K, Sundaresh S, and Monje M (2019). Monosynaptic tracing maps brain-wide afferent oligodendrocyte precursor cell connectivity. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyon S, Dubessy AL, Aigrot MS, Trotter M, Huang JK, Dauphinot L, Potier MC, Kerninon C, Melik Parsadaniantz S, Franklin RJM, and Lubetzki C (2015). Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. J Neurosci 35, 4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Bennett DV, Rubinov M, Narayan S, Yang CT, Tanimoto M, Mensh BD, Looger LL, and Ahrens MB (2019). Glia Accumulate Evidence that Actions Are Futile and Suppress Unsuccessful Behavior. Cell 178, 27–43 e19. [DOI] [PubMed] [Google Scholar]
- Nagai J, Rajbhandari AK, Gangwani MR, Hachisuka A, Coppola G, Masmanidis SC, Fanselow MS, and Khakh BS (2019). Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell 177, 1280–1292 e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher JJ, and Cunningham C (2019). Priming Microglia for Innate Immune Memory in the Brain. Trends in Immunology 40, 358–374. [DOI] [PubMed] [Google Scholar]
- Netea MG, Dominguez-Andres J, Barreiro LB, Chavakis T, Divangahi M, Fuchs E, Joosten LAB, van der Meer JWM, Mhlanga MM, Mulder WJM, et al. (2020). Defining trained immunity and its role in health and disease. Nat Rev Immunol 20, 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, Taloma SE, Barron JJ, Molofsky AB, Kheirbek MA, and Molofsky AV (2020). Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell 182, 388–403 e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Tsai H-H, Hoi KK, Huang N, Yu G, Kim K, Baranzini SE, Xiao L, Chan JR, and Fancy SPJ (2019). Aberrant oligodendroglial-vascular interactions disrupt the Blood Brain Barrier triggering CNS inflammation. Nature Neuroscience 22, 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Fenn AM, Dugan A, and Godbout JP (2014). TGFβ produced by IL-10 redirected astrocytes attenuates microglial activation. Glia 62, 881–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott A, and Glass CK (2018). Immune memory in the brain. Nature 556, 312–313. [DOI] [PubMed] [Google Scholar]
- Nott A, Holtman IR, Coufal NG, Schlachetzki JCM, Yu M, Hu R, Han CZ, Pena M, Xiao J, Wu Y, et al. (2019). Brain cell type–specific enhancer-promoter interactome maps and disease-risk association. Science 366, 1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan JP, Kelly KA, VanGilder RL, Sofroniew MV, and Miller DB (2014). Early Activation of STAT3 Regulates Reactive Astrogliosis Induced by Diverse Forms of Neurotoxicity. PLOS ONE 9, e102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier B, Daneman R, and Ransohoff RM (2013). Development, maintenance and disruption of the blood-brain barrier. Nature medicine 19, 1584–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SW, Harris JA, Ng L, Winslow B, Cain N, Mihalas S, Wang Q, Lau C, Kuan L, Henry AM, et al. (2014). A mesoscale connectome of the mouse brain. Nature 508, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A, and Forsgren L (2015). NMR metabonomics of cerebrospinal fluid distinguishes between Parkinson’s disease and controls. Neurosci Lett 594, 36–39. [DOI] [PubMed] [Google Scholar]
- Omari KM, John GR, Sealfon SC, and Raine CS (2005). CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain 128, 1003–1015. [DOI] [PubMed] [Google Scholar]
- Pardo AC, Wong V, Benson LM, Dykes M, Tanaka K, Rothstein JD, and Maragakis NJ (2006). Loss of the astrocyte glutamate transporter GLT1 modifies disease in SOD1(G93A) mice. Exp Neurol 201, 120–130. [DOI] [PubMed] [Google Scholar]
- Paul D, Ge S, Lemire Y, Jellison ER, Serwanski DR, Ruddle NH, and Pachter JS (2014). Cell- selective knockout and 3D confocal image analysis reveals separate roles for astrocyte-and endothelial-derived CCL2 in neuroinflammation. J Neuroinflammation 11, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peferoen L, Kipp M, van der Valk P, van Noort JM, and Amor S (2014). Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 141, 302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfrieger FW, and Barres BA (1997). Synaptic Efficacy Enhanced by Glial Cells in Vitro. Science 277, 1684–1687. [DOI] [PubMed] [Google Scholar]
- Pitt D, Werner P, and Raine CS (2000). Glutamate excitotoxicity in a model of multiple sclerosis. Nature Medicine 6, 67–70. [DOI] [PubMed] [Google Scholar]
- Polyzos AA, Lee DY, Datta R, Hauser M, Budworth H, Holt A, Mihalik S, Goldschmidt P, Frankel K, Trego K, et al. (2019). Metabolic Reprogramming in Astrocytes Distinguishes Region-Specific Neuronal Susceptibility in Huntington Mice. Cell metabolism 29, 1258–1273.e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponath G, Ramanan S, Mubarak M, Housley W, Lee S, Sahinkaya FR, Vortmeyer A, Raine CS, and Pitt D (2017). Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain: A Journal of Neurology 140, 399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Jung S, and Priller J (2019). Microglia Biology: One Century of Evolving Concepts. Cell 179, 292–311. [DOI] [PubMed] [Google Scholar]
- Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-wit D, Xiao J, Lu Y, Giltiay N, Liu J, et al. (2007). The adaptor Act1 is required for interleukin 17–dependent signaling associated with autoimmune and inflammatory disease. Nature Immunology 8, 247–256. [DOI] [PubMed] [Google Scholar]
- Ramesh G, Benge S, Pahar B, and Philipp MT (2012). A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. Journal of Neuroinflammation 9, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, and Engelhardt B (2012). The anatomical and cellular basis of immune surveillance in the central nervous system. Nature Reviews Immunology 12, 623–635. [DOI] [PubMed] [Google Scholar]
- Reich DS, Lucchinetti CF, and Calabresi PA (2018). Multiple Sclerosis. N Engl J Med 378, 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Proia RL, and Olivera A (2008). The alliance of sphingosine-1-phosphate and its receptors in immunity. Nature Reviews Immunology 8, 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, Welch J, Chen LM, Chen F, and Macosko EZ (2019). Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science 363, 1463–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H, and Goetzl EJ (2005). Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nature Reviews Immunology 5, 560–570. [DOI] [PubMed] [Google Scholar]
- Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao C-C, Ardura-Fabregat A, Lima K.A.d., Gutiérrez-Vázquez C, Hewson P, Staszewski O, et al. (2018). Microglial control of astrocytes in response to microbial metabolites. Nature 557, 724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Kenison JE, Tjon E, Takenaka MC, Lima K.A.d., Borucki DM, Chao C-C, Wilz A, Blain M, Healy L, et al. (2017). Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proceedings of the National Academy of Sciences 114, 2012–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao C-C, Patel B, Yan R, Blain M, et al. (2016). Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and CNS inflammation via the aryl hydrocarbon receptor. Nature medicine 22, 586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, and Quintana FJ (2015). Control of autoimmune CNS inflammation by astrocytes. Semin Immunopathol 37, 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, and Quintana FJ (2019). The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol 19, 184–197. [DOI] [PubMed] [Google Scholar]
- Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, and Glass CK (2009). A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137, 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury RL, and Sulentic CEW (2015). The AhR and NF-κB/Rel Proteins Mediate the Inhibitory Effect of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin on the 3’ Immunoglobulin Heavy Chain Regulatory Region. Toxicol Sci 148, 443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoilova EB, Horton JL, Hilliard B, Liu TS, and Chen Y (1998). IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol 161, 6480–6486. [PubMed] [Google Scholar]
- Sanchis P, Fernádez-Gayol O, Comes G, Escrig A, Giralt M, Palmiter RD, and Hidalgo J (2020). Interleukin-6 Derived from the Central Nervous System May Influence the Pathogenesis of Experimental Autoimmune Encephalomyelitis in a Cell-Dependent Manner. Cells 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, and Volterra A (2009). Synaptic modulation by astrocytes via Ca2+-dependent glutamate release. Neuroscience 158, 253–259. [DOI] [PubMed] [Google Scholar]
- Saunders A, Macosko EZ, Wysoker A, Goldman M, Krienen FM, de Rivera H, Bien E, Baum M, Bortolin L, Wang S, et al. (2018). Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 174, 1015–1030.e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarin C, Hinton DR, Valentin-Torres A, Chen Z, Trapp BD, Bergmann CC, and Stohlman SA (2015). Astrocyte response to IFN-γ limits IL-6-mediated microglia activation and progressive autoimmune encephalomyelitis. Journal of Neuroinflammation 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HL, Freeman OJ, Butcher AJ, Holmqvist S, Humoud I, Schatzl T, Hughes DT, Verity NC, Swinden DP, Hayes J, et al. (2020). Astrocyte Unfolded Protein Response Induces a Specific Reactivity State that Causes Non-Cell-Autonomous Neuronal Degeneration. Neuron 105, 855–866 e855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2009). Molecular dissection of reactive astrogliosis and glial scar formation. Trends in Neurosciences 32, 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2015a). Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16, 249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV (2015b). Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16, 249–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano RM, Casarejos MJ, Menéndez-Cuervo J, Rodriguez-Navarro JA, García de Yébenes J, and Mena MA (2008). Glial Dysfunction in Parkin Null Mice: Effects of Aging. The Journal of Neuroscience 28, 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos JM, Morrow J, Ashley TA, Szente BE, Bikoff EK, and Zamvil SS (1998). Astrocytes Express Elements of the Class II Endocytic Pathway and Process Central Nervous System Autoantigen for Presentation to Encephalitogenic T Cells. The Journal of Immunology 161, 5959–5966. [PubMed] [Google Scholar]
- Staszewski O, and Prinz M (2014). Glial epigenetics in neuroinflammation and neurodegeneration. Cell Tissue Res 356, 609–616. [DOI] [PubMed] [Google Scholar]
- Suh H-S, Choi S, Khattar P, Choi N, and Lee SC (2010). Histone deacetylase inhibitors suppress the expression of inflammatory and innate immune response genes in human microglia and astrocytes. J Neuroimmune Pharmacol 5, 521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, and Wekerle H (1986). Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature 320, 70–72. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, and Alberini CM (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa T, Nakashima K, Namihira M, Ochiai W, Uemura A, Yanagisawa M, Fujita N, Nakao M, and Taga T (2001). DNA Methylation Is a Critical Cell-Intrinsic Determinant of Astrocyte Differentiation in the Fetal Brain. Developmental Cell 1, 749–758. [DOI] [PubMed] [Google Scholar]
- Tay TL, Mai D, Dautzenberg J, Fernandez-Klett F, Lin G, Sagar, Datta M, Drougard A, Stempfl T, Ardura-Fabregat A, et al. (2017). A new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci 20, 793–803. [DOI] [PubMed] [Google Scholar]
- Toft-Hansen H, Füchtbauer L, and Owens T (2011). Inhibition of reactive astrocytosis in established experimental autoimmune encephalomyelitis favors infiltration by myeloid cells over T cells and enhances severity of disease. Glia 59, 166–176. [DOI] [PubMed] [Google Scholar]
- Tong X, Ao Y, Faas GC, Nwaobi SE, Xu J, Haustein MD, Anderson MA, Mody I, Olsen ML, Sofroniew MV, and Khakh BS (2014). Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat Neurosci 17, 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traka M, Podojil JR, McCarthy DP, Miller SD, and Popko B (2016). Oligodendrocyte death results in immune-mediated CNS demyelination. Nature Neuroscience 19, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, et al. (2012). Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337, 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, and Fugger L (2008). Interleukin-17 Production in Central Nervous System-Infiltrating T Cells and Glial Cells Is Associated with Active Disease in Multiple Sclerosis. The American Journal of Pathology 172, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainchtein ID, Chin G, Cho FS, Kelley KW, Miller JG, Chien EC, Liddelow SA, Nguyen PT, Nakao-Inoue H, Dorman LC, et al. (2018). Astrocyte-derived Interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science (New York, NY) 359, 1269–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Torres A, Savarin C, Barnett J, and Bergmann CC (2018). Blockade of sustained tumor necrosis factor in a transgenic model of progressive autoimmune encephalomyelitis limits oligodendrocyte apoptosis and promotes oligodendrocyte maturation. Journal of Neuroinflammation 15, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CFA, Khan EM, Leung PSC, Gershwin ME, Chang WLW, Wu D, Haarmann-Stemmann T, Hoffmann A, and Denison MS (2014). Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-κB. J Biol Chem 289, 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel CFA, Sciullo E, Li W, Wong P, Lazennec G, and Matsumura F (2007). RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol 21, 2941–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LBJ, Tiwari-Woodruff S, and Sofroniew MV (2009). Reactive Astrocytes Form Scar-Like Perivascular Barriers to Leukocytes during Adaptive Immune Inflammation of the CNS. J Neurosci 29, 11511–11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VA, and Raff MC (1999). A role for Sonic hedgehog in axon-to-astrocyte signalling in the rodent optic nerve. Development 126, 2901–2909. [DOI] [PubMed] [Google Scholar]
- Walz W (2000). Role of astrocytes in the clearance of excess extracellular potassium. Neurochemistry International 36, 291–300. [DOI] [PubMed] [Google Scholar]
- Wang X, Allen WE, Wright MA, Sylwestrak EL, Samusik N, Vesuna S, Evans K, Liu C, Ramakrishnan C, Liu J, et al. (2018). Three-dimensional intact-tissue sequencing of single-cell transcriptional states. Science 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Deckert M, Xuan NT, Nishanth G, Just S, Waisman A, Naumann M, and Schlüter D (2013a). Astrocytic A20 ameliorates experimental autoimmune encephalomyelitis by inhibiting NF-κB- and STAT1-dependent chemokine production in astrocytes. Acta Neuropathol 126, 711–724. [DOI] [PubMed] [Google Scholar]
- Wang X, Haroon F, Karray S, Deckert M, and Schlüter D (2013b). Astrocytic Fas ligand expression is required to induce T-cell apoptosis and recovery from experimental autoimmune encephalomyelitis. European Journal of Immunology 43, 115–124. [DOI] [PubMed] [Google Scholar]
- Wendeln A-C, Degenhardt K, Kaurani L, Gertig M, Ulas T, Jain G, Wagner J, Häsler LM, Wild K, Skodras A, et al. (2018). Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556, 332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA, Clark IC, Tjon EC, Li Z, Zandee SEJ, Couturier CP, Watson BR, Scalisi G, Alkwai S, Rothhammer V, et al. (2020a). MAFG-driven astrocytes promote CNS inflammation. Nature 578, 593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA, Clark IC, Tjon EC, Li Z, Zandee SEJ, Couturier CP, Watson BR, Scalisi G, Alkwai S, Rothhammer V, et al. (2020b). MAFG-driven astrocytes promote CNS inflammation. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA, Jaronen M, Covacu R, Zandee SEJ, Scalisi G, Rothhammer V, Tjon EC, Chao C-C, Kenison JE, Blain M, et al. (2019). Environmental Control of Astrocyte Pathogenic Activities in CNS Inflammation. Cell 176, 581–596.e518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA, and Quintana FJ (2019). Regulation of Astrocyte Functions in Multiple Sclerosis. Cold Spring Harbor perspectives in medicine 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler MA, Rothhammer V, and Quintana FJ (2017). Control of immune-mediated pathology via the aryl hydrocarbon receptor. J Biol Chem 292, 12383–12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RE, Yin FQ, and Jakeman LB (2008). TGF-alpha increases astrocyte invasion and promotes axonal growth into the lesion following spinal cord injury in mice. Exp Neurol 214, 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Tao HQ, Liu YM, Zhan XX, Liu Y, Wang XY, Wang JH, Mu LL, Yang LL, Gao ZM, et al. (2012). Characterization of the interaction between astrocytes and encephalitogenic lymphocytes during the development of experimental autoimmune encephalitomyelitis (EAE) in mice. Clinical & Experimental Immunology 170, 254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeste A, Takenaka MC, Mascanfroni ID, Nadeau M, Kenison JE, Patel B, Tukpah A-M, Babon JAB, DeNicola M, Kent SC, et al. (2016). Tolerogenic nanoparticles inhibit T cell-mediated autoimmunity through SOCS2. Sci Signal 9, ra61–ra61. [DOI] [PubMed] [Google Scholar]
- Yi H, Bai Y, Zhu X, lin L, Zhao L, Wu X, Buch S, Wang L, Chao J, and Yao H (2014). IL-17A Induces MIP-1α Expression in Primary Astrocytes via Src/MAPK/PI3K/NF-kB Pathways: Implications for Multiple Sclerosis. J Neuroimmune Pharmacol 9, 629–641. [DOI] [PubMed] [Google Scholar]
- Yu X, Taylor AMW, Nagai J, Golshani P, Evans CJ, Coppola G, and Khakh BS (2018). Reducing Astrocyte Calcium Signaling In Vivo Alters Striatal Microcircuits and Causes Repetitive Behavior. Neuron 99, 1170–1187 e1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, and Barres BA (2012). Genomic analysis of reactive astrogliosis. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 32, 6391–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeis T, Enz L, and Schaeren-Wiemers N (2016). The immunomodulatory oligodendrocyte. Brain Res 1641, 139–148. [DOI] [PubMed] [Google Scholar]
- Zeis T, and Schaeren-Wiemers N (2008). Lame ducks or fierce creatures? The role of oligodendrocytes in multiple sclerosis. J Mol Neurosci 35, 91–100. [DOI] [PubMed] [Google Scholar]
- Zeisel A, Hochgerner H, Lönnerberg P, Johnsson A, Memic F, van der Zwan J, Häring M, Braun E, Borm LE, La Manno G, et al. (2018). Molecular Architecture of the Mouse Nervous System. Cell 174, 999–1014.e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Juréus A, Marques S, Munguba H, He L, Betsholtz C, et al. (2015). Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science (New York, NY) 347, 1138–1142. [DOI] [PubMed] [Google Scholar]
- Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, et al. (2013). Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385. [DOI] [PubMed] [Google Scholar]