Abstract
Introduction
Real-time continuous glucose monitoring (rt-CGM) informs users about current interstitial glucose levels and allows early detection of glycaemic excursions and timely adaptation by behavioural change or pharmacological intervention. Randomised controlled studies adequately powered to evaluate the impact of long-term application of rt-CGM systems on the reduction of adverse obstetric outcomes in women with gestational diabetes (GDM) are missing. We aim to assess differences in the proportion of large for gestational age newborns in women using rt-CGM as compared with women with self-monitored blood glucose (primary outcome). Rates of neonatal hypoglycaemia, caesarean section and shoulder dystocia are secondary outcomes. A comparison of glucose metabolism and quality of life during and after pregnancy completes the scope of this study.
Methods and analysis
Open-label multicentre randomised controlled trial with two parallel groups including 372 female patients with a recent diagnosis of GDM (between 24+0 until 31+6 weeks of gestation): 186 with rt-CGM (Dexcom G6) and 186 with self-monitored blood glucose (SMBG). Women with GDM will be consecutively recruited and randomised to rt-CGM or control (SMBG) group after a run-in period of 6–8 days. The third visit will be scheduled 8–10 days later and then every 2 weeks. At every visit, glucose measurements will be evaluated and all patients will be treated according to the standard care. The control group will receive a blinded CGM for 10 days between the second and third visit and between week 36+0 and 38+6. Cord blood will be sampled immediately after delivery. 48 hours after delivery neonatal biometry and maternal glycosylated haemoglobin A1c (HbA1c) will be assessed, and between weeks 8 and 16 after delivery all patients receive a re-examination of glucose metabolism including blinded CGM for 8–10 days.
Ethics and dissemination
This study received ethical approval from the main ethic committee in Vienna. Data will be presented at international conferences and published in peer-reviewed journals.
Trial registration number
NCT03981328; Pre-results.
Keywords: diabetes in pregnancy, fetal medicine, maternal medicine, clinical trials
Strengths and limitations of this study.
This is a randomised controlled trial recruiting 372 pregnant women after the gestational diabetes (GDM) diagnosis at five sites in Austria, Germany, Sweden and Switzerland.
The study uses the newest version of a real-time continuous glucose monitoring (rt-CGM) system which enables the user rapidly to identify glycaemic excursions and allows timely adaptation by behavioural change or pharmacological intervention.
The study will increase knowledge about possible limitations of self-monitored blood glucose (SMBG; routine care), such as undetected hyperglycaemia or hypoglycaemia.
The study might show possible improvement of adverse perinatal outcome and particularly fetal macrosomia in offspring of mothers with GDM monitored by rt-CGM versus SMBG.
Introduction
The incidence of obesity and diabetes is rising worldwide even in younger populations. With a rise in maternal obesity also gestational diabetes mellitus (GDM) becomes more prevalent with a prevalence of up to 18% of pregnancies.1 2 Previous studies found hyperglycaemia in pregnancy to be associated with gestational complications including macrosomia and neonatal hyperinsulinaemic hypoglycaemia3 and an increased long-term risk for obesity or diabetes in the offspring’s later life.4 Large interventional trials provided evidence that obstetric and neonatal complications such as large for gestational age offspring (LGA, defined as birth weight >90th pctl) or shoulder dystocia can be significantly reduced by intensified treatment of even mild forms of maternal hyperglycaemia (eg, by lifestyle modification or pharmacotherapy).5–7
Continuous glucose monitoring (CGM) has been shown to improve glycaemic control without increasing the risk of hypoglycaemia in patients with type 1 and 2 diabetes.8 9 In 2003, a study compared the glycaemic profile reflected by CGM and self-monitored blood glucose (SMBG) in 34 gravid patients with type 1 diabetes over a period of 3 days and found that on average more than 3 hours of hyperglycaemic episodes per day were undetected by SMBG and nocturnal hypoglycaemic episodes could be revealed by CGM 1–4 hours before showing clinical manifestations or being detected by SMBG.10 However, only a small number of studies evaluated the use of CGM in pregnancies affected by GDM: in the setting of a larger non-randomised observational study, Yu et al11 found that mothers in the CGM group (use over 72 hours every 2–4 weeks) had improved glycaemic control as well as a lower amount of glycaemic variability as compared with a control group using SMBG. In addition, the CGM group showed lower birth weight percentiles associated with a lower risk for LGA offspring (13.7 vs 25.8%) or neonatal hypoglycaemia (5.5 vs 14%). Also a second observational study including 57 pregnant women with GDM indicated that CGM was more effectively detecting hyperglycaemic episodes as well as nocturnal hypoglycaemia than SMBG.12 A study in 73 pregnant women with GDM, randomly assigned to either SMBG or CGM for a duration of 48 hours after diagnosis, found that CGM detected a markedly higher proportion of women requiring glucose lowering pharmacotherapy (31 vs 8%).13 Another randomised controlled trial on 106 women with GDM observed significantly lower weight gain associated with CGM. LGA cases were more often observed in the SMBG group (52.7 vs 35.3%). However, the difference failed statistical significance as the study was not powered for obstetrical outcomes.14
Unfortunately, both randomised controlled studies used older versions of a blinded CGM device, where glucose values were not directly visible for patients. In contrast, more recently developed ‘real-time’ CGM (rt-CGM) systems provide users with information about current glucose levels and alert the patient before the upper or lower glucose threshold is reached or when glucose levels change rapidly. Hence, glycaemic excursions can be rapidly identified and accordingly adapted by behavioural change or pharmacological intervention. A number of studies including non-pregnant patients showed superiority of rt-CGM over older blinded CGM versions in order to effectively empower and educate patients with diabetes to better understand how dietary habits, exercise or pharmacotherapy affects their glucose levels.15 A beneficial effect of rt-CGM in pregnancy was also supported by the continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT) trial for pregnant women with type 1 diabetes.16 Only one recent study compared SMBG with rt-CGM in women with GDM using a single application for 3–7 days within 2 weeks after diagnosis but it failed to demonstrate improvements in glycosylated haemoglobin A1c (HbA1c) or pregnancy outcomes, which was, however, likely due to the sample size and the short duration of intervention (single application).17
Taken together, larger randomised controlled studies adequately powered to evaluate the impact of long-term application of rt-CGM systems on the risk reduction of adverse obstetric outcomes are missing.18 Of note, such studies are of high clinical relevance because of their guideline-changing potential. In addition, rt-CGM has the potential to reduce reported barriers to SMBG (such as inconvenience, pain or stigma of testing in public places) in order to improve poor reliability and adherence to glucose monitoring, which is a non-negligible problem in the treatment of GDM.19
Hypotheses
The main hypothesis of the proposed study is that rt-CGM can effectively reduce the risk for LGA newborns (primary outcome) and other neonatal and obstetric complications. It is further hypothesised that rt-CGM can improve maternal glycaemic control, body weight gain during pregnancy and (as rt-CGM potentially improves self-management strategies) has beneficial effects on maternal metabolism after pregnancy.
Primary and secondary outcomes
Primary objective
To assess differences in the proportion of LGA newborns (birth weight >90 th pctl) in women with GDM using rt-CGM as compared with women with GDM using SMBG.
Secondary objectives
To assess differences in further obstetric or neonatal complications, neonatal hypoglycaemia, rate of caesarean section, shoulder dystocia and neonatal anthropometry will be assessed as secondary objectives. Further secondary outcomes are: differences in neonatal hyperinsulinaemia, rt-CGM measures such as mean interstitial glucose, glycaemic variability, time in target (65–140 mg/dL (3.6 to 7.8 mmol/L)) as well as time spent in hyperglycaemia and hypoglycaemia (time above and below range) (daytime: 07:01 to 22:59 hours and night time: 23:00 to 07:00 hour), duration and frequency postprandial hyperglycaemic excursions, start and amount of glucose lowering therapy, HbA1c, glycosylated fibronectin, change in bodyweight during pregnancy and after delivery as well as glucose disposal at postpartum (markers of insulin sensitivity, insulin secretion and β-cell function assessed by a postpartum oral glucose tolerance test (OGTT)). Health-related quality of life (HRQoL) is a patient-reported outcome which has become as important in the evaluation of interventions as patient-relevant clinical outcomes. Therefore, HRQoL will be elicited. In addition, preferences will be assessed, and a health economic evaluation in terms of cost-effectiveness (CEA) and cost-utility analysis (CUA) will be performed.
Expected effects on the advancement of clinical practice
The aim of this proposal is to assess the ability of rt-CGM to improve glycaemic control (reduction of mean glucose, hyperglycaemic episodes and duration, improvement of glycaemic variability) in order to prevent adverse pregnancy outcomes and neonatal complications in women with GDM. The results of this study will contribute to:
The improvement of clinical monitoring and management of glucose metabolism during pregnancy with GDM.
Increased knowledge about possible limitations of SMBG (routine care), such as undetected hyperglycaemia or hypoglycaemia, as well as to determine if comprehensive glucose data (as derived from rt-CGM) results in more or fewer women needing pharmacotherapy.
Possible improvement of adverse perinatal outcome and particularly fetal macrosomia in offspring of mothers with GDM.
Methods and analysis
Participants and recruitment; inclusion criteria
This study is designed as an open-label multicentre randomised controlled trial with two parallel groups including a total of 372 female patients (n=186 with rt-CGM, n=186 with SMBG) with a recent diagnosis of GDM. Diagnosis of GDM (ie, diabetes first diagnosed in the second and third trimester and not clearly type 1 or type 2 diabetes20) is made in accordance with the International Association of Diabetes in Pregnancy Study Groups (IADPSG) criteria after 24+0 weeks of gestation by a 2 hours 75 g OGTT.21 The study will be conducted at five academic hospitals in Austria, Switzerland, Sweden and Germany. All pregnant women (aged between 18 and 55 years) will be consecutively recruited after diagnosis of GDM between 24+0 and 31+6 weeks of gestation among women visiting the pregnancy outpatient departments (Division of Obstetrics and feto-maternal Medicine, Medical University of Vienna, Austria; Division of Obstetrics, University Hospital Basel, Switzerland; Department of Obstetrics, Charité‐Universitätsmedizin Berlin, Germany) or the diabetes outpatient departments (Division of Endocrinology and Metabolic Diseases at the Heinrich Heine University, Düsseldorf, Germany; Department of Medicine, University Hospital, Örebro, Sweden).
Exclusion criteria
Overt diabetes (ie, pregestationally known type 1 or type 2 diabetes or fasting plasma glucose during the OGTT ≥126 mg/dL (7.0 mmol/L) or HbA1c≥6.5% (44 mmol/L) or 2 hours post-load OGTT levels ≥200 mg/dL (11.1 mmol/L) assessed before 24+0 weeks of gestation, whereby results need to be confirmed by repeated testing in the absence of unequivocal hyperglycaemia according to the American Diabetes Association (ADA) standards20), history of bariatric surgery or other surgeries that induce malabsorption, long-term use (>2 weeks) of systemic steroids prior to enrolment, multiple pregnancy, patients already using glucose lowering medications (metformin or insulin) before study entry, fetal growth restriction due to placental dysfunction at study entry, inpatient psychiatric treatment up to 1 year before enrolment, participation in this study in previous pregnancy.
Study visits during pregnancy
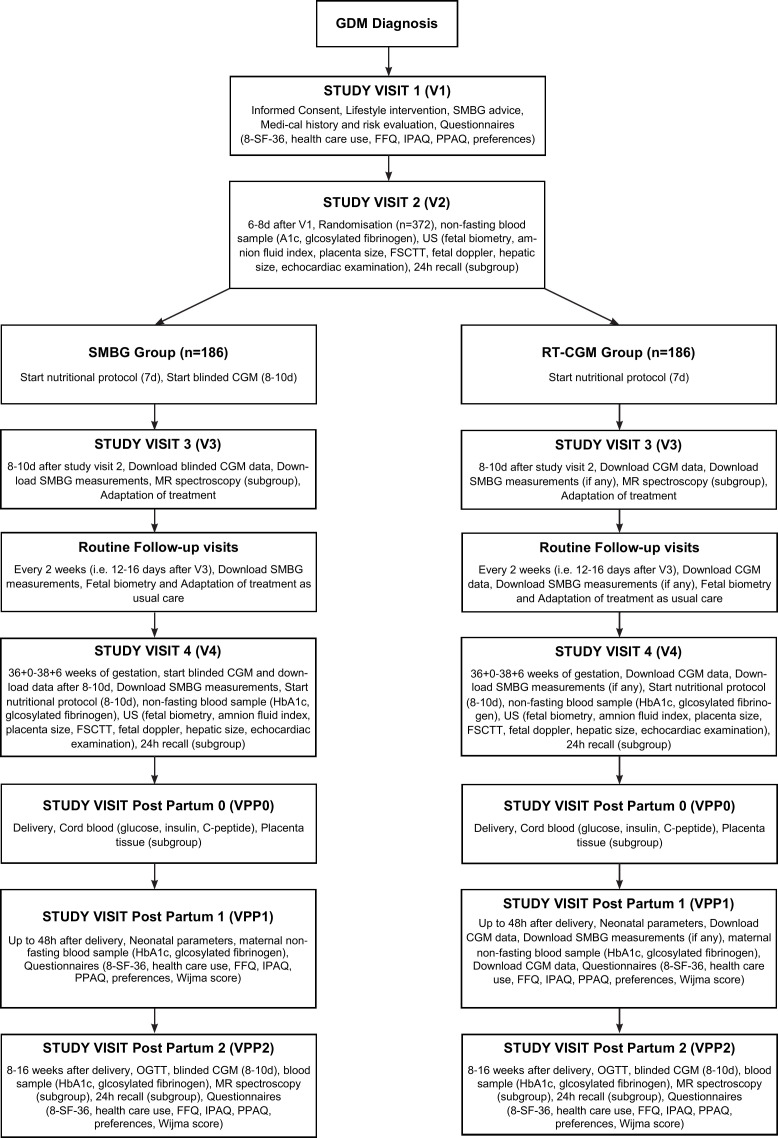
A flow diagram of the study visits is provided in figure 1. A broad risk evaluation will be performed in participating women at the initial contact (V1) including: evaluation of maternal age, parity, history of GDM in previous pregnancies, detailed family history, ethnicity, preconceptional diseases, obstetric history. Height (stadiometer measured to the nearest centimetre) and actual weight (calibrated scales, light indoor clothing) will be additionally assessed. Moreover, an evaluation of preconceptional weight (self-reported) and body mass index (BMI) as well as measurement of blood pressure will be performed. All patients receive medical advice for nutrition (isocaloric diet containing 40%–50% carbohydrates, 20% proteins and 30%–35% fat, divided into three meals and three snacks) and regular physical exercising for 30 min per day following international recommendations. In addition, participants are advised on capillary blood glucose measurement (fasting as well as 1 hour after starting each meal) at the initial visit (V1). Randomisation will be done after a run-in period of 6–8 days when patients get used to SMBG (V2). The third visit (V3) will be scheduled 8–10 days after V2 and further follow-up visits every 2 weeks (ie, 12–16 days after each visit). HbA1c and glycosylated fibronectin will be assessed at V2 as well as at the first visit between 36+0 and 38+6 weeks of gestation (12 mL, non-fasting state) (V4). Detailed fetal ultrasound examinations, a detailed examination of dietary intake as well as a blinded CGM (control group only) will be performed at V2 and V4. Body weight change and use of glucose lowering medications (amount of insulin units) will be examined at every visit. At every follow-up visit, glucose measurements (SMBG or rt-CGM) and routine ultrasound examinations (fetal biometry and umbilical artery doppler) will be evaluated by the medical staff and all patients will be treated according to the standard of care for patients with GDM. This includes lifestyle modification and insulin therapy if recommended thresholds are exceeded. Both groups will be treated to be in the target range between 65 and 140 mg/dL (3.6 to 7.8 mmol/L) with at least 8 hours fasting glucose levels equal or below 95 mg/dL (5.3 mmol/L) and 1 hour postprandial glucose measurements equal or below 140 mg/dL (7.8 mmol/L) in accordance with the CONCEPTT study16 and the ADA recommendations,22 respectively. Intermediate acting neutral protamine Hagedorn (NPH) insulin is started in the evening if ≥2 measurements of fasting glucose are equal or above 95 mg/dL (5.3 mmol/L) in a period of 1 week and rapid acting insulin analogues (Aspart or Lispro) if ≥2 measurements of 1 hour postprandial glucose (either after breakfast, lunch or dinner) are equal or above 140 mg/dL (7.8 mmol/L) in a period of 1 week. NPH is started with 6–10 IU and increased by 4 IU (or in case of higher doses, ie, >25 IU by 20%) and rapid acting insulin (bolus insulin) is started with 2–4 IU and increased by 2–4 IU if thresholds are not achieved within 3 days. Long-acting insulin analogues such as glargine (U100/U300) or detemir can be used as an alternative to NPH. Patients are trained on insulin management and titration according to their glucose levels. Metformin can be used according to local practice guidelines (recommended in Sweden but not in Austria, Germany or Switzerland as first-line pharmacological intervention).
Figure 1.
Patient flow diagram. CGM, continuous glucose monitoring; FSCTT, fetal subcutanous tissue thickness; FFQ, food frequency questionnaire; GDM, gestational diabetes mellitus; IPAQ, international physical activity questionnaire; PPAQ, pregnancy physical activity questionnaire; RT-CGM, real-time CGM; SF-36, short form 36; SMBG, self-monitored bloodglucose.
Study visits postpartum
Cord blood will be sampled and stored (at −80°C) immediately after delivery (VPP0). A postpartum examination will be scheduled within 48 hours after delivery (VPP1) for assessment of neonatal parameters and maternal HbA1c and glycosylated fibronectin (12 mL, non-fasting state), as well as between 8 and 16 weeks after delivery (VPP2) in all patients for a detailed re-examination of glucose homeostasis postpartum (including lifestyle and dietary pattern as well as HbA1c, glycosylated fibronectin as well as a blinded CGM for 10 days and an OGTT to assess the presence of prediabetic conditions after pregnancy with GDM). The postpartum OGTT is further used to provide estimates of insulin sensitivity, β-cell function and hepatic insulin extraction, the major physiological components of impaired glucose tolerance.
Randomisation
Participants will be randomised to either treatment (rt-CGM augmented glucose monitoring) or control group (routine care SMBG) in a 1:1 ratio. The minimisation method23 with a 0.85 assignment probability will be used to minimise the imbalance between the groups according to week of gestation at study entry, that is, at V1 (three strata: 24+0 to 25+6, 26+0 to 27+6, 28+0 to 29+6, 30+0 to 31+6), previous pregnancy with GDM (two strata: yes or no) and preconceptional overweight/obesity status with three strata: (1) normal weight (ie, BMI below 25.0 kg/m²); (2) overweight (BMI 25.0–29.9 kg/m²); (3) obesity (BMI equal or above 30.0 kg/m²). Randomisation will be performed at the second study visit (V2) by using a randomisation software provided by the Medical University of Vienna.
Intervention
Patients randomised to the intervention group will be equipped with a rt-CGM sensor (Dexcom G6 sensor, a small flexible device that records interstitial glucose levels every 5 min) at V2. The sensor will be inserted into the subcutaneous tissue of the anterior abdominal wall (if this location is not tolerated by the pregnant patients, the upper buttock or posterior upper arm may be used instead). Additionally, patients will be advised to record capillary blood glucose values if glucose alerts or readings do not match with symptoms or expectations. Participants will be educated on how to exchange the sensor (has to be exchanged every ten days) and will be equipped with a real-time CGM monitor and instructed in its use. The monitor provides the user with information about current glucose levels and notifies the patient before her upper or lower glucose threshold are reached and when glucose levels change rapidly. All patients in the intervention group will be specially trained in the use of the system. As an alternative to the real-time monitor, the patients’ smartphone with an anonymised access to the CLARITY mobile app can be used (for details, see the Intervention: device description section).
Intervention: device description
The Dexcom G6 intended use is for the management of diabetes in persons aged 2 years and older. The Dexcom G6 System is intended to replace fingerstick blood glucose testing for diabetes treatment decisions. Interpretation of the Dexcom G6 System results should be based on the glucose trends and several sequential readings over time. The Dexcom G6 System also aids in the detection of episodes of hyperglycaemia and hypoglycaemia, facilitating both acute and long-term therapy adjustments. The Dexcom G6 System can be used alone or in conjunction with digitally connected medical devices for the purpose of managing diabetes.
The system consists of a sensor, transmitter, receiver and mobile app. The sensor is a small, flexible wire inserted into subcutaneous tissue where it converts glucose into electrical current. The sensor incorporates an interferent layer that minimises the effect of potential electroactive interferents, such as acetaminophen, by preventing it from reaching the sensor wire surface. The benefit of this interferent layer in blocking the effects of acetaminophen prevents falsely high glucose readings. Thus, users may ingest acetaminophen while wearing the G6 CGM system. The transmitter, which is connected to the sensor and worn on the body, samples the electrical current produced by the sensor and converts the measurement into a glucose reading using an onboard algorithm. The receiver and/or the app displays the glucose reading along with a rate of change arrow and a trend graph. Additionally, the receiver and/or app issues alarms and alerts to notify the patient of glucose level changes and other important system conditions. Also, alarms will be provided if the receiver detects loss of connection to the sensor. The app provides the additional capability to share data with ‘followers’ using the Dexcom Share service. The receiver can be put into a blinded mode using CLARITY software. In this mode, users are unable to see the CGM data or receive CGM alerts.
CGM Ancillary Devices Dexcom CLARITY is an accessory for users of the Dexcom CGM system. It is a software programme that allows the transfer of glucose data from the CGM system to Dexcom remote servers for data management to allow the use of the CGM data by the user and study clinicians. Target ranges of 65 to 140 mg/dL (3.6 to 7.8 mmol/L) will be set and the patients will be introduced in the use of alarm settings. Both participants and study sites will use CLARITY to transfer glucose data between user and study site, whether CGM is used in blinded or real-time mode. A CLARITY mobile app can be used for a retrospective review of glucose data on the smart device and can also be set up to allow receipt of push notifications of CGM data facilitating weekly data review. For all patients (intervention and control group), an anonymised CLARITY account will be created by using a sequential study number which is allocated at randomisation (sex will be female and birth date for each account will be set to 1 January 1990 for all accounts). CLARITY also provides metrics to check for patient compliance.
Intervention: study proceedings
For participants who have a supported phone, the G6 CGM app will be installed on participant’s smartphone.
An anonymised CLARITY mobile account will be set up and linked to the research site.
Participants will use CGM data for their diabetes management.
A high alert threshold will be set at 140 mg/dL (7.8 mmol/L). Low alert threshold and urgent low soon alerts will be turned off. If participants require insulin, the low alert will be turned on and the threshold set at 65 mg/dL (3.6 mmol/L). In addition, the urgent low alert (55 mg/dL (3.1 mmol/L)), the urgent low soon alert (when glucose levels are falling fast and will be below 55 mg/dL (3.1 mmol/L) in less than 20 min) as well as alerts for rise and fall rate (3 mg/dL (0.17 mmol/L)) in addition to alerts for signal loss and no readings for more than 20 min will be enabled.
Participants with applicable smartphones may have CLARITY push notifications on the CLARITY mobile app about weekly time in range comparison enabled during the study.
For app users, the ‘Share and Follow’ functionality will be discussed and encouraged (ie, the study participants are able to invite followers to review their glucose levels).
For participants using the receiver only, the receiver will be downloaded into the CLARITY clinic account at each visit.
For participants using real-time CGM data summary will be downloaded for documentation at V3 and V4 (between 36+0 and 38+6) as well as after delivery (VPP1).
The research team will review the CGM in CLARITY to inform lifestyle and therapy recommendations.
The Dexcom G6 system does not require calibration during the study period.
Control group
The participants of the control group will perform self-monitored blood glucose testing with a study-provided blood glucose metre, including testing supplies. They will perform capillary blood glucose monitoring as routinely used for patients with GDM, that is, at least four capillary blood glucose values daily including measurements in a fasting state as well as 1 hour after starting each meal by using a routinely available blood glucose measurement device. The study participants will keep a logbook of their glucose values, which will be reviewed by clinicians from the study team at each visit and used for lifestyle and dietary recommendations as is routinely done in clinical practice. From V2 to V3 as well as once for ten days between gestational week 36+0 and 38+6, the control group receive blinded CGM; neither patients nor the treating medical staff will have access to the data recorded by the CGM sensor at this point in time. Instead, patients will control blood glucose levels based on SMBG, as is the routine procedure in current GDM treatment. Otherwise, the control group will receive the same study assessments as the intervention group. The blinded CGM will be removed and returned to Dexcom after the 10 days wear period after CGM data are uploaded to CLARITY by an unblinded investigator who must not communicate about the results with patients or medical staff.
Each participant of the control group will be assigned a study blood glucose metre to measure and store their blood glucose values during the study. Therefore, the Contour Next One system will be used. The metre has CE Mark clearance and is commercially available in Europe. Participants will receive an ample supply of metre test materials based on quantities routinely used. A commercially available desktop software (Diabass Pro) used in conjunction with Contour Next One system glucose metre for blood glucose monitoring will be used for downloading the metre data by the sites at V3 and V4 after checking that dates and times are correct.
Blood glucose metres used by the control group will be assessed to establish frequency of testing (overall and per week) as well as percentage of days with less than four measurements per day.
Analyses of CGM data
Rt-CGM data allow a detailed examination of the percentage of time in which glucose levels are in target range (time in target) (65–140 mg/dL (3.6–7.8 mmol/L)), hyperglycaemic episodes (glucose ≥140 mg/dL (7.8 mmol/L)) as well as mild (<65 mg/dL (3.6 mmol/L)), moderate (≤54 mg/dL (3.0 mmol/L)) or severe hypoglycaemic episodes (requiring third party assistance) and their duration. To this purpose, several indices of the glucose control quality will be calculated, such as Glycaemic Risk Assessment Diabetes Equation some indices of hypoglycaemia and hyperglycaemia, such as the High Blood Glucose Index (HBGI) and Low Blood Glucose Index, and indices assessing the risk associated to both low and high glycaemic values, such as Index of Glycaemic Control and Average Daily Risk Range. Glycaemic variability will also be assessed, which can be quantified by SD of the CGM data, or by more sophisticated indices, such as Mean Amplitude Glucose Excursions, Continuous Overlapping Net Glycaemic Action, Lability Index,24 25 as well as further indices that we developed internally, such as the Shape Index.26 These will be compared between real-time CGM users and controls (ie, from data obtained during the blinded CGM wear).
Assessment of dietary patterns
Dietary patterns will be assessed in all patients at V1, VPP1 and VPP2 via a published and validated Food Frequency Questionnaire (FFQ) proposed by the German Robert Koch Institute.27 It was also previously used for the German DEGS project (Studie zur Gesundheit Erwachsener in Deutschland) (www.degs-studie.de). Information from the FFQ will be analysed quantitatively or summarised by eating scores proposed in the literature (such as the Healthy Eating Index 2010 or Alternate Healthy Eating Index 2010) reflecting diet quality based on actual guidelines.28 29 In addition, all patients will be advised to conduct a nutritional protocol (7 days) from V2 to V3 as well as once at V4 (between 36+0 and 38+6 weeks of gestation). In a subgroup (only study site Vienna), dietary intake will also be assessed by performing 24 hours recalls by trained interviewers at V2, V4 and post partum (VPP2): one face-to-face interview (approx. 1 hour) and the others as telephone interviews (approx. 30 min) during which data are entered simultaneously in GloboDiet. GloboDiet is a computerised programme which was developed by the International Agency for Cancer Research within the framework of the European Prospective Investigation into Cancer and Nutrition Study for the conduction of harmonised and standardised 24 hours recalls.30 This open-ended software was used in numerous previous studies and was validated within the European Food Consumption Validation project.31–33 In brief, GloboDiet is an interview-based dietary assessment instrument that allows obtaining a very detailed description and quantification of foods, recipes and supplements consumed in the course of the preceding day and thus standardising data within and between countries. Probing questions and entering consumed foods in chronological order support the respondent’s memory. The standardised structure prescribes—on the food group level—possibilities of description and quantification of food items to choose from. Quantification of consumed foods is supported by the GloboDiet picture book that comprises coloured photographs of foods in different portion sizes, photographs of familiar household measures and schematic displays of forms (eg, bread, cake). The software provides an automatic coding of food items and recipe ingredients as well as a rough calculation of nutrient intake meant for quality control of the interview. GloboDiet is characterised by the obtained standardisation of dietary data within Europe, a large number of available foods and recipes, and a very detailed description of consumed foods. Currently, GloboDiet is one of the few dietary instruments providing comparable nutritional data within Europe. After finalisation of the interviews, GloboDiet will be linked to the local nutrition database—the Bundeslebenmittelschlüssel (BLS) enhanced by the Austrian Nutrition Table (Österreichische Nährwerttabelle, ÖNWT), containing typical Austrian foods and recipes—allowing analyses on food ingredients level and to conduct precise energy and risk assessment.
Assessment of physical activity
Physical activity will be assessed at V1, VPP1 and VPP2 via the International Physical Activity Questionnaire (IPAQ, long form). The IPAQ represents a well-accepted, validated instrument for monitoring population levels of physical activity in different settings and countries.34 It will be analysed via published guidelines for data processing and analysis at the IPAQ homepage Guidelines for data processing and analysis of the IPAQ35: in short, collected data will be summarised as median metabolic equivalent of task minutes per week, representing a continuous score for walking, moderate intensity activities, vigorous intensity activities and total activities, as recommended. In addition, the Pregnancy Physical Activity Questionnaire will be performed to capture information on physical activity participation and sedentary behaviour during pregnancy.36
Assessment of maternal intramyocellular and intrahepatocellular lipids
Intramyocellular (IMCL), and intrahepatocellular lipid contents (HCL) will be measured by using proton magnetic resonance spectroscopy (1H MRS) in a subgroup of 40 patients (20 rt-CGM, 20 SMBG) at V3 and after delivery (VPP2) according to previously described methods.37–39 The participants will be studied in supine position within a 3.0 Tesla whole-body magnet (Siemens or Philips). MRS is a non-invasive technique to evaluate tissue-specific metabolism and was shown to be safe and well tolerated by pregnant women in previous studies.40 41 Patients will be positioned with a left pelvic tilt to avoid pressure on the inferior vena cava according to other studies in pregnancy.41 For IMCL measurements, the calf muscle (right leg) will be positioned in a quadrature bird cage 1H volume coil. A circular 1H surface coil will be positioned over the liver for HCL measurement.
Fetal biometry
Parameters of fetal anthropometry as determined by ultrasound as well as neonatal data including length, weight, gestational age at delivery will be included in the final analysis. A detailed fetal ultrasound examination will be performed at V2 and repeated at V4 (between 36+0 to 38+6 weeks of gestation) to assess fetal growth parameters including head circumference, biparietal diameter and abdominal circumference and abdominal fat thickness, femur length (measured and expressed as standardised gestational age related fetal growth percentiles42), amnion fluid index as well as size and location of the placenta and fetal subcutaneous tissue thickness. Moreover, fetal growth symmetry will be assessed by fetal head to abdomen circumference ratio and fetal doppler measurements (mainly umbilical artery and middle cerebral artery43 and ductus venosus). Furthermore, fetal hepatic size (all hepatic diameters, such as area and volume) and umbilical venous volume flow and an echocardiography of the fetus will be performed in a subgroup (only study site Vienna).
Obstetric outcome
Obstetric outcome (caesarean section, birth injury, preterm birth before 37 completed weeks of gestation) stillbirth, small for gestational age (birth weight <10 th pctl), LGA infant (birth weight >90 th pctl), shoulder dystocia, admitted to neonatal intensive care unit umbilical cord blood pH, Apgar score) will be recorded immediately after delivery. Length of hospital stay for mothers and offspring as well the duration of high-level neonatal care, respiratory distress, fetal hyperbilirubinaemia and neonatal death ≤28 days will be further assessed. Calculations of age-adjusted and sex-adjusted percentiles will be performed by using international anthropometric standards according to those used in the CONCEPTT study.44 Neonatal hypoglycaemia is defined as local blood glucose ≤31 mg/dL (1.7 mmol/L) in the first 24 hours after delivery and ≤45 mg/dL (2.5 mmol/L) after the first 24 hours after delivery or treatment with glucose infusion according to the Hyperglycemia and Adverse Pregnancy Outcome study.3 Additional anthropometric measures of the offspring include head, shoulder and abdominal circumference, length, upper and lower arm and leg circumference and skinfold measurements (suprailiac and subscapular, triceps, quadriceps) in accordance with previous studies.45–47 Thereby, skinfold measurements will be performed by using a validated instrument (Harpenden Skinfold Caliper) within 48 hours after delivery (VPP1).
Assessment of cord blood
17 mL umbilical cord blood (1×8 mL serum and 1×9 mL EDTA) will be taken immediately after delivery to examine cord-blood glucose, insulin and C-peptide.
Postpartum OGTT
The OGTT will be performed at VPP2 (ie, 8–16 weeks after delivery): after collecting blood samples for measurements of glucose (2 mL blood), insulin and C-peptide (3 mL blood) in the fasting state (at least 8 hours), participating women will receive a standardised 300 mL 75 g glucose. Further blood samples of glucose, insulin and C-peptide measurements will be taken at 30, 60, 90 and 120 min after intake of glucose. Insulin sensitivity during the OGTT will be assessed by the oral glucose insulin sensitivity index according to Mari et al48; this quantifies dynamic glucose clearance per unit change of insulin. The more recently developed PREDIM index will be used in addition.49 The new index provides excellent prediction of clamp-derived insulin sensitivity from OGTT or meal data. As an approximation for hepatic insulin resistance, the homeostasis model assessment of insulin resistance will be used. Insulin secretion will be calculated by using the C-peptide deconvolution method.50 β-cell function parameters, such as pancreatic glucose sensitivity and rate sensitivity, and potentiation of insulin secretion, will be computed by mathematical modelling.50
Assessment of HRQoL and patients’ preferences
HRQoL will be elicited using the SF-36 and the EQ-5D-5L.51 It can be expected that adherence to lifestyle and dietary recommendations are associated with individual risk preferences. Hence, risk and time preferences will be elicited based on a lottery approach.52 53 Participants will be asked to choose between two hypothetical lotteries that differ in expected outcomes which enables us to derive an individual classification of the risk type, that is, risk-averse, risk-neutral or risk-loving individuals. Quality of life as well as risk and time preferences will be assessed at V1, VPP1 and VPP2. Obstetrical patient’s satisfaction will be additionally assessed at VPP1 by using the Wijma score.54
Patient and public involvement
Patients and public were not involved in the study design and will not be involved in the study conduct, recruitment and dissemination.
Health economic evaluation
For the evaluation of a complex intervention, a health economic evaluation is recommended as well.55 56 In this study, a CEA and a CUA will be conducted from the perspective of the health insurance. The effect measure employed in the CEA will be the primary outcome of the main trial, that is, avoided cases of LGA newborns. Even if the effect parameter of the intervention group will not be superior to the control group, a health economic evaluation will be performed to inform about efficiency since costs might be lower in the intervention group.57 Due to the short intervention period quality-adjusted life weeks (QALWs) will be used in the CUA. QALWs will be calculated based on either the EQ-5D-5L or the SF-6D58 that derives preference-based scores from the SF-36. To receive utilities, quality of life will be evaluated by country-specific population-based preferences separately for each country involved in the trial. Similarly, intervention costs as well as healthcare costs (direct costs) will be calculated separately for each country using local prices and adjusted for local purchasing power parity. Healthcare use will be assessed by a validated instrument that is adapted to the requirements of the study.59 Healthcare use will comprise resource use dedicated to the mother but not the child, for example, clinical visits, outpatient contacts, contacts with therapists and medication. Intervention associated costs are costs of devices, software, test strips and costs due to education and training of study participants. Since the evaluation covers only the observation period alongside the trial, costs and effects will not be discounted. Comparing the outcomes and costs of the intervention group with the outcomes and costs of the control group yields the incremental cost-effectiveness ratio (ICER: additional cost per additional LGA newborn avoided) and the cost–utility ratio (ICUR: additional costs per additional QALW gained).
Reporting of adverse events, data and safety monitoring
Any (serious) adverse events (AE/SAE) are recorded by the investigator using the specific AE/SAE sheet of the clinical report form. All SAE are reported to the responsible ethics committee within an appropriate time frame.
Data safety and accuracy as well as patient safety will be monitored by local data and safety monitoring committees for clinical trials (eg, the KKS—competence centre for clinical trials—in Austria).
Sample size and statistical analysis
Sample size
With a sample size of n=338 (169 pregnant women per group), we will be able to detect a difference between two independent proportions of LGA of 13.7% versus 25.8% (according to the results of a previous study11) with a power of 80% and a two-sided type 1 error of α=0.05 (calculated for Pearson’s χ2 test). Considering a drop-out rate of 9%, a total sample size of n=372 (186 women per group) is necessary for this study. This is in line with the sample size suggested by Kestilä et al.13 A blinded sample size review (the proportion of LGA cases in the sample is reviewed) and adaptation is planned after 50% of the patients have been investigated. The sample-size calculation was performed by using the software G*Power (V.3.1.9.2).60
Analysis plan
Analyses should be conducted on the intention-to-treat principle. Categorical variables will be summarised by counts and proportions; continuous variables data will be summarised by means and SD or by median and IQR in the case of strong deviations from the normal distribution. Pearson’s χ2 test will be used to compare differences in the primary outcome (difference in proportion of LGA newborns) and for binary secondary outcomes (such as caesarean section rate, shoulder dystocia and neonatal hypoglycaemia). Bernard’s test will be used as an alternative if an expected frequency in contingence tables is equal or less than 5 and the Cochran-Mantel-Haenszel method will be used as sensitivity analysis to adjust for possible centre specific effects. Continuous secondary outcome parameters (such as mean glucose, duration and amount of hyperglycaemia, glycaemic variability and other rt-CGM measures, postpartum OGTT data, HbA1c, glycosylated fibronectin or anthropometric data of the newborn) will be compared by Student’s t-test. Rank based inference (such as the Brunner-Munzel test61) will be used as an alternative in case of skewed distributed parameters. The association between HbA1c, rt-CGM measures and delivery and risk of LGA offspring will be assessed by binary logistic regression. There are many possible objectives for which further exploratory analysis could be performed in this study (eg, functional principal components analysis for rt-CGM data). Hence, the present analysis plan represents only a selection of methods, which will be used for analysing the main objectives. Risk preferences will be analysed by non-parametric and parametric methods. In particular, we plan to classify study participants with respect to their risk tolerance (risk-aversion, risk-neutral and risk-loving) and deriving constant relative risk aversion utility functions. Associations between risk preferences and behaviour (dietary patterns and physical activity) will be investigated. For the health economic evaluation, ICER (additional cost per additional LGA newborn avoided) and ICUR (additional cost per additional quality-adjusted life year gained) will be calculated. 95% CIs will be analysed using bootstrap procedures.62 To consider uncertainty, cost-acceptability curves will be calculated.63 A two-sided p value≤0.05 is considered statistically significant. All analyses will be performed by using the statistic software R and contributing packages.64 No further adjustment for multiplicity is planned for this study.
Ethics and dissemination
This study received ethical approval from the main ethic committee in Vienna (1863/2018). Ethics approval will be obtained by the local institutional review boards in Basel, Berlin, Dusseldorf and Orebro. It was registered under www.ClinicalTrials.gov (NCT03981328). Data will be presented at international conferences and published in peer-reviewed journals.
Supplementary Material
Acknowledgments
Special thanks to all families who participate in this study.
Footnotes
Contributors: EAH, KW, JJ, AI, JS, AT, MM and CSG designed the study. MM, AT, AI, MV, GG, CSG and PR will perform statistical analysis and data interpretation. EAH, TL, DE, KW, CK, KW, KS, GY-S, IR, WH, KC, JS and CSG will be responsible for patient management. PH, WH, TL, IH, MR, PR, HF, MV, GG, BW, GY-S, CK and KS will make important contributions and critically reviewed this study protocol.
Funding: This study is supported by Dexcom grant project number OUS-2018-027.
Disclaimer: The funding source is not involved in study design, the collection, analysis and interpretation of data, the writing of the manuscript; and in the decision to submit the article for publication.
Competing interests: The authors declare that there are no further financial or personal relationships with other people or organisations that could inappropriately influence the work reported or the conclusions, implications or opinions stated.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ 2017;356:j1. 10.1136/bmj.j1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sacks DA, Hadden DR, Maresh M, et al. . Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the hyperglycemia and adverse pregnancy outcome (HAPO) study. Diabetes Care 2012;35:526–8. 10.2337/dc11-1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, et al. . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002. 10.1056/NEJMoa0707943 [DOI] [PubMed] [Google Scholar]
- 4.Dabelea D, Hanson RL, Lindsay RS, et al. . Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–11. 10.2337/diabetes.49.12.2208 [DOI] [PubMed] [Google Scholar]
- 5.Crowther CA, Hiller JE, Moss JR, et al. . Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477–86. 10.1056/NEJMoa042973 [DOI] [PubMed] [Google Scholar]
- 6.Landon MB, Spong CY, Thom E, et al. . A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339–48. 10.1056/NEJMoa0902430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horvath K, Koch K, Jeitler K, et al. . Effects of treatment in women with gestational diabetes mellitus: systematic review and meta-analysis. BMJ 2010;340:c1395. 10.1136/bmj.c1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: a review of the technology and clinical use. Diabetes Res Clin Pract 2017;133:178–92. 10.1016/j.diabres.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 9.Liebl A, Henrichs HR, Heinemann L, et al. . Continuous glucose monitoring: evidence and consensus statement for clinical use. J Diabetes Sci Technol 2013;7:500–19. 10.1177/193229681300700227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yogev Y, Chen R, Ben-Haroush A, et al. . Continuous glucose monitoring for the evaluation of gravid women with type 1 diabetes mellitus. Obstet Gynecol 2003;101:633–8. 10.1016/s0029-7844(02)02714-x [DOI] [PubMed] [Google Scholar]
- 11.Yu F, Lv L, Liang Z, et al. . Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: a prospective cohort study. J Clin Endocrinol Metab 2014;99:4674–82. 10.1210/jc.2013-4332 [DOI] [PubMed] [Google Scholar]
- 12.Chen R, Yogev Y, Ben-Haroush A, et al. . Continuous glucose monitoring for the evaluation and improved control of gestational diabetes mellitus. J Matern Fetal Neonatal Med 2003;14:256–60. 10.1080/jmf.14.4.256.260 [DOI] [PubMed] [Google Scholar]
- 13.Kestilä KK, Ekblad UU, Rönnemaa T. Continuous glucose monitoring versus self-monitoring of blood glucose in the treatment of gestational diabetes mellitus. Diabetes Res Clin Pract 2007;77:174–9. 10.1016/j.diabres.2006.12.012 [DOI] [PubMed] [Google Scholar]
- 14.Wei Q, Sun Z, Yang Y, et al. . Effect of a CGMS and SMBG on maternal and neonatal outcomes in gestational diabetes mellitus: a randomized controlled trial. Sci Rep 2016;6:19920. 10.1038/srep19920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn D, Pettus J, Edelman S. Unblinded CGM should replace blinded CGM in the clinical management of diabetes. J Diabetes Sci Technol 2016;10:793–8. 10.1177/1932296816632241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feig DS, Donovan LE, Corcoy R, et al. . Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. The Lancet 2017;390:2347–59. 10.1016/S0140-6736(17)32400-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alfadhli E, Osman E, Basri T. Use of a real time continuous glucose monitoring system as an educational tool for patients with gestational diabetes. Diabetol Metab Syndr 2016;8:48. 10.1186/s13098-016-0161-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polsky S, Garcetti R. CGM, pregnancy, and remote monitoring. Diabetes Technol Ther 2017;19:S-49–S-59. 10.1089/dia.2017.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cosson E, Baz B, Gary F, et al. . Poor reliability and poor adherence to self-monitoring of blood glucose are common in women with gestational diabetes mellitus and may be associated with poor pregnancy outcomes. Diabetes Care 2017;40:1181–6. 10.2337/dc17-0369 [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care 2018;41:S13–27. 10.2337/dc18-S002 [DOI] [PubMed] [Google Scholar]
- 21.International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, et al. . International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–82. 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Diabetes Association 13. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41:S137–43. 10.2337/dc18-S013 [DOI] [PubMed] [Google Scholar]
- 23.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–15. 10.2307/2529712 [DOI] [PubMed] [Google Scholar]
- 24.Rodbard D. Interpretation of continuous glucose monitoring data: glycemic variability and quality of glycemic control. Diabetes Technol Ther 2009;11 Suppl 1:S-55–S-67. 10.1089/dia.2008.0132 [DOI] [PubMed] [Google Scholar]
- 25.Moscardó V, Herrero P, Reddy M, et al. . Assessment of glucose control metrics by discriminant ratio. Diabetes Technol Ther 2020;22:719–26. 10.1089/dia.2019.0415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tura A, Morbiducci U, Sbrignadello S, et al. . Shape of glucose, insulin, C-peptide curves during a 3-H oral glucose tolerance test: any relationship with the degree of glucose tolerance? Am J Physiol Regul Integr Comp Physiol 2011;300:R941–8. 10.1152/ajpregu.00650.2010 [DOI] [PubMed] [Google Scholar]
- 27.Haftenberger M, Heuer T, Heidemann C, et al. . Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr J 2010;9:36. 10.1186/1475-2891-9-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guenther PM, Casavale KO, Reedy J, et al. . Update of the healthy eating index: HEI-2010. J Acad Nutr Diet 2013;113:569–80. 10.1016/j.jand.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiuve SE, Fung TT, Rimm EB, et al. . Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–18. 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slimani N, Ferrari P, Ocké M, et al. . Standardization of the 24-hour diet recall calibration method used in the European prospective investigation into cancer and nutrition (EPIC): general concepts and preliminary results. Eur J Clin Nutr 2000;54:900–17. 10.1038/sj.ejcn.1601107 [DOI] [PubMed] [Google Scholar]
- 31.Crispim SP, de Vries JHM, Geelen A, et al. . Two non-consecutive 24 h recalls using EPIC-Soft software are sufficiently valid for comparing protein and potassium intake between five European centres--results from the European Food Consumption Validation (EFCOVAL) study. Br J Nutr 2011;105:447–58. 10.1017/S0007114510003648 [DOI] [PubMed] [Google Scholar]
- 32.de Boer EJ, Slimani N, van 't Veer P, et al. . The European food consumption validation project: conclusions and recommendations. Eur J Clin Nutr 2011;65 Suppl 1:S102–7. 10.1038/ejcn.2011.94 [DOI] [PubMed] [Google Scholar]
- 33.Slimani N, Casagrande C, Nicolas G, et al. . The standardized computerized 24-h dietary recall method EPIC-Soft adapted for pan-European dietary monitoring. Eur J Clin Nutr 2011;65 Suppl 1:S5–15. 10.1038/ejcn.2011.83 [DOI] [PubMed] [Google Scholar]
- 34.Craig CL, Marshall AL, Sjöström M, et al. . International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 35.Committee IR Guidelines for data processing and analysis of the International physical activity questionnaire (IPAQ)–short and long forms, 2005. Available: https://ci.nii.ac.jp/naid/10030318551/
- 36.Chasan-Taber L, Schmidt MD, Roberts DE, et al. . Development and validation of a pregnancy physical activity questionnaire. Med Sci Sports Exerc 2004;36:1750–60. 10.1249/01.MSS.0000142303.49306.0D [DOI] [PubMed] [Google Scholar]
- 37.Krssak M, Roden M. The role of lipid accumulation in liver and muscle for insulin resistance and type 2 diabetes mellitus in humans. Rev Endocr Metab Disord 2004;5:127–34. 10.1023/B:REMD.0000021434.98627.dc [DOI] [PubMed] [Google Scholar]
- 38.Prikoszovich T, Winzer C, Schmid AI, et al. . Body and liver fat mass rather than muscle mitochondrial function determine glucose metabolism in women with a history of gestational diabetes mellitus. Diabetes Care 2011;34:430–6. 10.2337/dc10-1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livingstone RS, Begovatz P, Kahl S, et al. . Initial clinical application of modified Dixon with flexible echo times: hepatic and pancreatic fat assessments in comparison with 1H MRS. Magn Reson Mater Phy 2014;27:397–405. 10.1007/s10334-013-0421-4 [DOI] [PubMed] [Google Scholar]
- 40.Hodson K, Man CD, Smith FE, et al. . Mechanism of insulin resistance in normal pregnancy. Horm Metab Res 2013;45:567–71. 10.1055/s-0033-1337988 [DOI] [PubMed] [Google Scholar]
- 41.Hodson K, Dalla Man C, Smith FE, et al. . Liver triacylglycerol content and gestational diabetes: effects of moderate energy restriction. Diabetologia 2017;60:306–13. 10.1007/s00125-016-4143-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadlock FP, Deter RL, Harrist RB, et al. . Estimating fetal age: computer-assisted analysis of multiple fetal growth parameters. Radiology 1984;152:497–501. 10.1148/radiology.152.2.6739822 [DOI] [PubMed] [Google Scholar]
- 43.Salomon LJ, Alfirevic Z, Berghella V, et al. . Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2011;37:116–26. 10.1002/uog.8831 [DOI] [PubMed] [Google Scholar]
- 44.Gestation Network Gestation network, 2018. Available: https://www.gestation.net/cc/about.htm
- 45.Brans YW, Sumners JE, Dweck HS, et al. . A noninvasive approach to body composition in the neonate: dynamic skinfold measurements. Pediatr Res 1974;8:215–22. 10.1203/00006450-197404000-00001 [DOI] [PubMed] [Google Scholar]
- 46.Dauncey MJ, Gandy G, Gairdner D. Assessment of total body fat in infancy from skinfold thickness measurements. Arch Dis Child 1977;52:223–7. 10.1136/adc.52.3.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jelsma JGM, van Poppel MNM, Galjaard S, et al. . DALI: Vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention: an European multicentre, randomised trial - study protocol. BMC Pregnancy Childbirth 2013;13:142. 10.1186/1471-2393-13-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mari A, Pacini G, Murphy E, et al. . A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001;24:539–48. 10.2337/diacare.24.3.539 [DOI] [PubMed] [Google Scholar]
- 49.Tura A, Chemello G, Szendroedi J, et al. . Prediction of clamp-derived insulin sensitivity from the oral glucose insulin sensitivity index. Diabetologia 2018;61:1135–41. 10.1007/s00125-018-4568-4 [DOI] [PubMed] [Google Scholar]
- 50.Mari A, Tura A, Gastaldelli A, et al. . Assessing insulin secretion by modeling in multiple-meal tests: role of potentiation. Diabetes 2002;51 Suppl 1:S221–6. 10.2337/diabetes.51.2007.S221 [DOI] [PubMed] [Google Scholar]
- 51.Hinz A, Kohlmann T, Stöbel-Richter Y, et al. . The quality of life questionnaire EQ-5D-5L: psychometric properties and normative values for the general German population. Qual Life Res 2014;23:443–7. 10.1007/s11136-013-0498-2 [DOI] [PubMed] [Google Scholar]
- 52.Holt CA, Laury SK. Risk aversion and incentive effects. American Economic Review 2002;92:1644–55. 10.1257/000282802762024700 [DOI] [Google Scholar]
- 53.Andersen S, Harrison GW, Lau MI, et al. . Eliciting risk and time preferences. Econometrica 2008;76:583–618. 10.1111/j.1468-0262.2008.00848.x [DOI] [Google Scholar]
- 54.Wijma K, Wijma B, Zar M. Psychometric aspects of the W-DEQ; a new questionnaire for the measurement of fear of childbirth. J Psychosom Obstet Gynaecol 1998;19:84–97. 10.3109/01674829809048501 [DOI] [PubMed] [Google Scholar]
- 55.Craig P, Dieppe P, Macintyre S, et al. . Developing and evaluating complex interventions: the new medical research council guidance. BMJ 2008;337:a1655. 10.1136/bmj.a1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Drummond MF. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press (Oxford medical publications), 2005. [Google Scholar]
- 57.Schöffski O, der SJ-MGvon, Gesundheitsökonomische evaluationen. 4th ed. Berlin Heidelberg: Springer-Verlag, 2012. [Google Scholar]
- 58.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271–92. 10.1016/S0167-6296(01)00130-8 [DOI] [PubMed] [Google Scholar]
- 59.Chernyak N, Ernsting C, Icks A. Pre-test of questions on health-related resource use and expenditure, using behaviour coding and cognitive interviewing techniques. BMC Health Serv Res 2012;12:303. 10.1186/1472-6963-12-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faul F, Erdfelder E, Lang A-G, et al. . G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 61.Brunner E, Munzel U. The nonparametric behrens-fisher problem: asymptotic theory and a Small-Sample approximation. Biom J 2000;42:17–25. [DOI] [Google Scholar]
- 62.Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327–40. [DOI] [PubMed] [Google Scholar]
- 63.Fenwick E, O'Brien BJ, Briggs A. Cost-effectiveness acceptability curves-facts, fallacies and frequently asked questions. Health Econ 2004;13:405–15. 10.1002/hec.903 [DOI] [PubMed] [Google Scholar]
- 64.R Core Team R: a language and environment for statistical computing, 2017. Available: https://www.R-project.org/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.