Abstract
A compelling set of links between chemotherapy- or radiation-induced intestinal inflammation and microbial dysbiosis has emerged. It is the proportional imbalance between pathogenic and beneficial bacteria that aggravates intestinal mucositis. Bacteria that ferment fibers and produce short-chain fatty acids (SCFAs), (such as acetate, propionate, and butyrate) are typically reduced in the mucosa and feces of patients undergoing cancer therapy. In contrast, increasing lipopolysaccharide-producing bacteria result in proinflammatory events by interacting with Toll-like receptors. A collective acceptance is that bacterial metabolites are critical in recovering intestinal homeostasis. We herein review evidence supporting the positive roles carried out by SCFAs. SCFAs, acting as signaling molecules, directly activate G-coupled-receptors and inhibit histone deacetylases. Thus, SCFAs are able to strengthen the gut barrier and regulate immunomodulatory functions. Furthermore, it is possible to reverse intestinal microbial dysbiosis and subsequently suppress the secretion of proinflammatory cytokines by directly applying SCFA-producing bacteria. In addition, anticancer effects of SCFAs have proved in the colorectal cancer. In this review, we discuss microbial dysbiosis and its impact on chemotherapy- or radiation-induced intestinal mucositis. Moreover, we summarize the mechanisms of SCFA production and its effects on intestinal mucositis. This review suggests the therapeutic potential of SCFAs for the management of chemotherapy- or radiation-induced intestinal inflammation.
Keywords: Short-chain fatty acids, chemotherapy, radiotherapy, intestinal inflammation, dysbiosis
Introduction
Alteration of the gastrointestinal mucosa is the main lesion that occurs after radiotherapy or chemotherapy [1]. Both radiation and drugs are directly involved in intestinal crypt cell apoptosis and villous atrophy [2,3], which breaks down the intestinal barrier. Patients suffering from chemotherapy- or radiation-induced intestinal inflammation present with vomiting, abdominal pain, diarrhea, malnutrition, fatigue, electrolyte imbalance, and infections [1]. Additionally, fatal complications, such as fistula formation, obstruction, or perforation, may appear along with the late-onset toxicities of chemicals and irradiation [4].
The gut microbiota plays a complex and dynamic role that is integral to the immune system and human health [5]. It is currently understood that, besides the impact of gut microbiota on the response to diverse forms of cancer therapy, tumor treatments may in turn affect the microbiota (that is, induce dysbiosis) [6,7]. Intestinal dysbiosis is distinguished by an overwhelming disbalance in the relative abundance of beneficial bacteria and pathogenic bacteria, even presenting a conspicuous dearth of beneficial bacteria and an overgrowth of harmful bacteria [1]. Published studies [8,9] have suggested that intestinal microbial dysbiosis may aggravate the inflammation provoked by radiation and chemical reagents. For example, the decreased proportions of butyrate-producing bacteria, such as Roseburia, Coprococcus, and Faecalibacterium, can disrupt the mucus layer. At molecular level, the mechanism by which microbiota affects mucositis is its capacity to manufacture either pathogenic metabolites or the salutary that protect it from diseases. Evidence suggests that SCFAs can suppress the secretion of the proinflammatory cytokine IL-6 by inhibiting the nuclear factor-κB (NF-κB) signaling pathway [10].
There are neither standardized therapeutic nor potential prophylactic to relieve mucositis symptoms or allow a safe dose of radiation and chemical reagents for superior cancer control. Glutamine, antibiotics, granulocyte-macrophage colony-stimulating factor, and sucralfate did not show any clinical benefit in this regard [1]. This review will then focus on SCFAs derived from microbial fermentation of dietary fibers and their therapeutic potential in versatile aspects of pathology processes. SCFAs, as signaling molecules, are capable of mitigating proinflammation through the conversion of the intestinal epithelium and permeability [11]. Furthermore, SCFAs will restore homeostasis by ascending beneficial bacteria and decreasing the pathogenic [11]. Based on the attenuation of chronic inflammation and interaction with microbiota, SCFAs can contribute to anticancer therapeutic efficacy. In conclusion, the use of SCFAs is an optional strategy for managing enteritis. In this review, we present the composition and function of symbiotic bacteria in the healthy intestinal tract. Subsequently, we describe the specifics of chemotherapy- or radiation-induced intestinal mucositis associated with microbial dysbiosis. A better understanding of the molecular mechanism of SCFAs is required to develop and implement optimal preventive and curative approaches for patient care. By reviewing the active mechanism of regulation of dysbiosis, anti-inflammatory responses, and SCFAs inhibition of tumor growth, we suggest considering SCFAs along with anticancer therapies.
Composition and functions of symbiotic microbiota in healthy human intestinal tract
There is a distinct raise in the understanding of the completeness and sophistication of the host-microbiota relationship and its effects on human health [5,12,13]. The number of commensal bacterial species in the robust gut ranges between 500 and 1,000 [14], which translates into a ratio of bacteria to human cells close to 1:1 [15]. The gut microbiota, referring to bacteria as well as fungi, viruses, archaea, phages, and protozoa, reside in the human intestine [16]. The constituent bacterial species have been identified predominantly to five phyla by the sequencing of 16S-rRNA-encoding genes: Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, and Verrucomicrobia [16]. Among these, Bacteroidetes and Firmicutes are roughly represent about 98%of the gut microbiota [17,18]. Owing to the acidic and anaerobic environment in the intestinal canal, anaerobes are much more abundant than aerobes [19]. Recent research [20] revealed that the proximal gastrointestinal tract is dominated by Firmicutes and that the colon is enriched by Bacteroidetes. Precisely because the majority is bacteria, the main research on gut microbiota is limited to this domain. In order to uncover the composition and coordination of commensal bacteria, large-scale endeavors is necessary to be launched [21]: the US National Institutes of Health (NIH)-funded Human Microbiome Project (HMP), the European Metagenomics of the Human Intestinal Tract (MetaHIT), and the American Gut Project (Figure 1).
Figure 1.
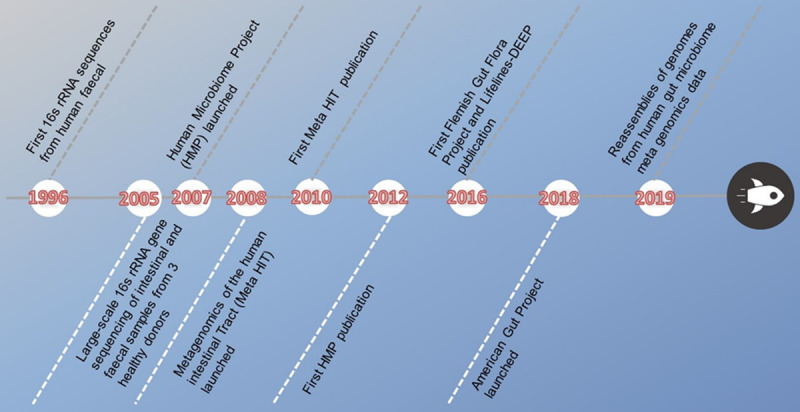
Schematic diagram of sequencing development.
The interactions between the host and commensal bacteria extend beyond the local enteric environment [22] in, for instance, inflammatory bowel disease (IBD), autoimmune disease [23], cancer ([24]), metabolic syndrome [12,25] and neurodegenerative disorders [26]. Microbiome-wide studies have documented significant correlations between commensal bacteria and enteric homeostasis, exemplified by nutrient metabolism upon dietary intake [22], promoting effects of physiological and biochemical processes [27], maintenance of the gut barrier function and the immune system [28], as well as protection against translocation of intestinal pathogens [29,30]. To prevent the intestinal barrier function from being breached, Lactobacillus forms a biofilm covering the enterocytes so that the pathogen-associated receptors are split away from pathogenic bacteria in the intestinal milieu [31]. Furthermore, it is substantiated that microbiota-associated metabolites have effects on modulating NLRP6 inflammasome signaling and secretion of IL-18. The activation of inflammasome signaling is crucial for hindering it from gut dysbiosis and intestinal inflammation provoked by barrier damage [32]. Moreover, symbiotic bacteria help consolidate the integrity of the enterocytes. For example, to trigger off an increased tolerance of epithelium to foreign stimuli, Lactobacillus can stimulate the biosynthesis of heat-shock protein 72 within enterocytes in a p38 mitogen-activated protein kinase (p38/MAPK)-dependent manner [33]. Analogously, Streptococcus thermophiles will manufacture lactic acid to suppress pathogenic bacteria by decreasing the pH of the gut environment [34]. Beyond physical isolation and production of inhibitory metabolites, beneficial bacteria is able to inhibit the growth of harmful bacterial species through metabolic competition or contact-dependent killing [35]. For example, colonization by bacteriocin-producing Enterococcus faecalis limits the number of indigenous E. faecalis as well as infection by vancomycin-resistant E. faecalis [36]. Under iron-starving circumstance, microcins produced by the probiotic Escherichia coli strain Nissle 1917 hamper intestinal colonization by other symbiotic E. coli and Salmonella [37].
Mutuality between chemotherapy- or radiation-induced intestinal inflammation and gut microbial dysbiosis
Chemotherapy- or radiation-induced enteritis associated with gut microbial dysbiosis
The incidence of chemotherapy- or radiation-induced enteritis has risen rapidly in recent years. The risk of diarrhea is approximately 10% in patients receiving standard chemotherapy for colorectal cancer with FOLFOX (folinic acid, 5-fluorouracil, and oxaliplatin) and about 20% in patients receiving the FOLFOXIRI regimen (FOLFOX, and irinotecan) [38]. Approximately 50% of patients suffer from gastrointestinal mucositis after pelvic or abdominal radiation treatment and the incidence is higher in patients undergoing concurrent chemotherapy [39]. The occurrence of such side effects may impair patients’ quality of life, increase healthcare costs, and result in late-onset toxicities, eventually interrupting therapy. This review aims to reveal the underlying mechanisms of the intestinal microbiota in intestinal inflammation and discuss potential implications for the clinical treatment of gastrointestinal mucositis.
Commonly, chemotherapy- or radiation-related intestinal microbial dysbiosis is characterized by an imbalanced proportion between beneficial and pathogenic bacteria (Figure 2). Explicit modifications of the microbiota constitution have been observed during therapy in clinical studies (Table 1). Facts reflected by these clinical studies are that patients receiving standard anticancer treatments exhibit conspicuous alteration in their intestinal bacteria. Although some changes may be secondary, no matter whether to the underlying diagnosis or to inflammation, it is confessed that they conduce to mucositis pathogenesis. In a pediatric and adolescent population receiving chemotherapy, a marked reduction of Firmicutes phylum and an increase of Bacteroidetes (i.e., Bacteroides and Parabacteroides) in relative abundance were reported. The authors [40] concluded that the disturbed balance in the intestinal microbiota (specifically for the mucolytic gram-positive anaerobic bacteria, including Ruminococcus gnavus and R. torques) may contribute to the development of gastrointestinal complications in children with acute lymphocytic leukemia following chemotherapy. In intestinal microbial dysbiosis associated with radiation enteritis, patients presented a higher abundance of Bacteroides, Serratia, Prevotella, Megamonas, and Novosphingobium in their fecal specimens [2]. Furthermore, 454 high-throughput pyrosequencing analyses were performed in feces of patients undergoing conditioning chemotherapy, which provided detection of low-abundance species and description of rarefaction dimensions about the more thorough understanding of chemotherapy-associated modifications of gut microbes [1]. At genera level, there was a marked increase in Escherichia and a profound decrease in butyrate-producing bacteria (Roseburia, Faecalibacterium etc.). Thus, patients experienced conditioning chemotherapy exhibit regulations of the gut microbiota characterized by a significant establishment of Escherichia genus bacteria, the most frequently isolated pathogens from blood culture in patients with cancer and bacteremia [9]. In addition, a decline in the Firmicutes phylum in fecal samples is a typical feature of gut microbial dysbiosis associated with chemotherapy [9] and radiotherapy [41]. Moreover, there was a decreased relative abundance of Lactobacillus and Bifidobacterium in the feces of patients after chemotherapy [42]. However, evidences from a recent meta-analysis [43] showed no benefits for the utilization of probiotics in radiotherapy-induced diarrhea and suggested that future research focused on pairing gastrointestinal toxicities with certain microbial phenotypes to allow targeted microbiota manipulation.
Figure 2.
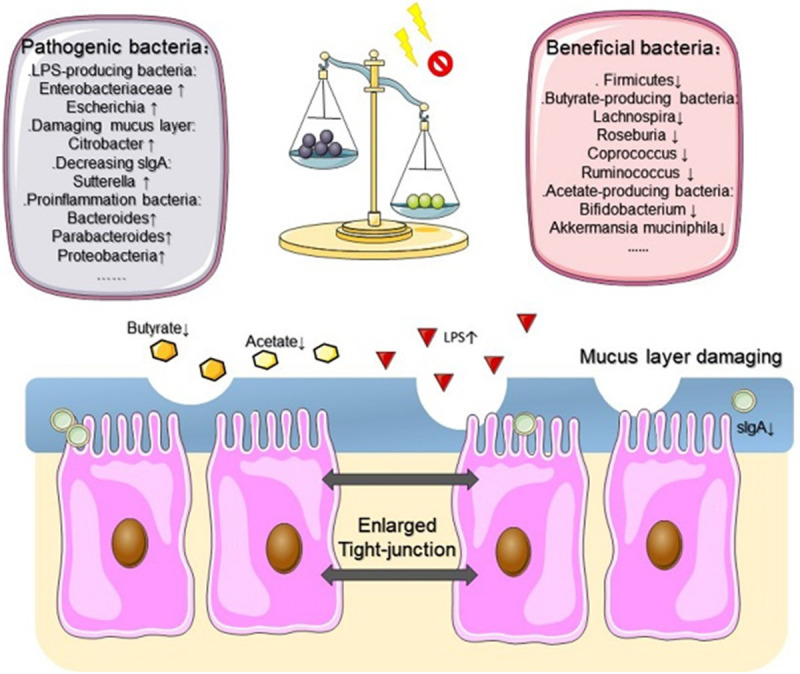
The overwhelming imbalance between beneficial and pathogenic bacteria is the main characteristic of chemotherapy- or radiotherapy-associated intestinal microbial dysbiosis. In this context, SCFAs concentration is decreased by a large margin. It also disrupts the intestinal barrier. In case of increased permeability or interrupted mucus layer, intestinal inflammation is more severe in this chaos milieu after chemotherapy or radiotherapy. SCFAs, short chain fatty acids; ↑, increased; ↓, decreased.
Table 1.
Intestinal microbiota alternation during anticancer treatment in clinical studies
| Study year (ref) | Number of patients | Anticancer treatment | Bacteria-detecting techniques | Samples | Bacteria Alteration |
|---|---|---|---|---|---|
| Rajagopala et al., 2019 [40] | 32 pediatric and adolescent acute lymphoblastic leukemia (ALL) patients | Chemotherapy | qPCR and 16S rRNA gene sequencing | Feces | Relative abundance: |
| Phylum level: | |||||
| Firmicutes↓; Bacteroidetes↑ | |||||
| Genus level: Bacteroides↑ | |||||
| Alistipes↓; Parabacteroides↑ | |||||
| Lachnospiraceae-UCG-005↑ | |||||
| Faecalibacterium↓ | |||||
| Pseudobutyrivibrio↓ | |||||
| Lachnoclostridium↑; Fusicatenibacter↓ | |||||
| Wang el at., 2019 [2] | Eighteen patients with stage II-IV cervical cancer (CCa) | Pelvic radiotherapy DT: 50.4 Gy in 28 fractions | DNA extraction; 16S rRNA gene sequencing and bioinformatics | Feces | Relative abundance: |
| Phylum level: Bacteroidetes↑ | |||||
| Proteobacteria↑ Firmicutes↓ | |||||
| Class level: Gammaproteobacteria↑ | |||||
| Order level: Enterobacteriales↑ | |||||
| Oceanospirillales↑ | |||||
| Family level: Enterobacteriaceae↑ | |||||
| Phyllobacteriaceae↑ | |||||
| Beijerinckiaceae↑ | |||||
| Ruminococcaceae↓; Bacteroidaceae↓ | |||||
| Genus level: Serratia↑ | |||||
| Bacteroides↑ Prevotella↑ | |||||
| Megamonas↑, Novosphingobium↑ | |||||
| Prevotella↑ | |||||
| Montassier et al., 2015 [9] | 28 patients with non-Hodgkin lymphoma | 5 consecutive chemotherapy days: high-dose carmustine (bis-chloroethylnitrosourea), etoposide, aracytine and melphalan | 16S rRNA gene sequencing and 454 high-throughput pyrosequencing | Feces | Relative abundance: |
| Phylum level: | |||||
| Firmicutes↓; Actinobacteria↓ | |||||
| Proteobacteria↑ | |||||
| Family level: Enterococcaceae↑ | |||||
| Enterobacteriaceae↑ | |||||
| Genus level: Ruminococcus↓ | |||||
| Oscillospira↓, Blautia↓ | |||||
| Lachnospira↓, Roseburia↓ | |||||
| Dorea↓, Coprococcus↓ | |||||
| Anaerostipes↓, Clostridium↓ | |||||
| Collinsella↓; Adlercreutzia↓ | |||||
| Bifidobacterium↓; Citrobacter↑ | |||||
| Klebsiella↑, Enterococcus↑ | |||||
| Megasphaera↑, Parabacteroides↑ | |||||
| Wang et al., 2015 [8] | 8 females with cervical cancer, 1 female with anal cancer and 2 males with colorectal cancer | Conventional radiotherapy at a dosage of 1.8-2.0 Gy/day, five times a week during the 5-week period (a cumulative dosage of 44-50 Gy) | 454 high-throughput pyrosequencing | Feces | Relative abundant: |
| Phylum level: | |||||
| Firmicutes/Bacteroidetes ratio↓ | |||||
| Family level: | |||||
| Lachnospiraceae↓ | |||||
| Genus level: Faecalibacterium↓, | |||||
| Oscillibacter↓, Roseburia↓, | |||||
| Streptococcus↓; Clostridium↑ | |||||
| Bacteroides↑ | |||||
| Stringer et al., 2013 [42] | 11 patients with colorectal cancer; 2 with breast cancer; 1 with melanoma, and 2 healthy individuals | Different chemotherapy treatment protocols with or without concomitant antibiotics | qPCR | Feces | Relative abudance: |
| Genus level: Lactobacillus↓, | |||||
| Bacteroides↓, Bifidobacterium↓, | |||||
| Enterococcus↓, Staphylococcus↑ | |||||
| Species level: Escherichia coli↑ | |||||
| Nam et al., 2013 [41] | 9 gynecological cancer patients | Pelvic radiotherapy DT: 50.4 Gy in 28 fractions | 454 high-throughput pyrosequencing | Feces | Relative abundant: |
| Phylum level: | |||||
| Firmicutes↓, Fusobacterium↑ | |||||
| Family level: Eubacteriaceae↓, | |||||
| Fusobacteriaceae↑; Streptococcacea↑ |
*↑, increase; ↓, decrease.
A comprehensive review [44] proposed five pathways through which intestinal microbial dysbiosis may impact the pathophysiology of intestinal mucositis: (i) inflammation and oxidative stress, (ii) gastrointestinal permeability destruction, (iii) mucus layer formation alteration, (iv) epithelial repair, and (v) secretion of immune factors. Availing to these overlapping steps, the intestinal microbial dysbiosis enables proinflammatory responses to be sustained. As for the changes in epithelial permeability or barrier, a recent in vitro study [2] showed that incubating enterocytes with fecal bacteria from patients with severe radiation enteritis impaired cell layer integrity, increased permeability, and stimulated cytokine secretion and NF-κB pathway activation. Hakansson et al. [45] showed that leucocytes infiltrated into irradiated normal cells when cells were exposed to radiation.
Sustaining proinflammatory responses induced by chemotherapy- or radiation-associated intestinal microbial dysbiosis
The formation of reactive oxygen (ROS), nitrogen (RNS), and sulfur species (RSS) activate the NF-κB pathway implicated with mucositis, promoting the production of proinflammatory cytokines such as IL-1β, IL-6, and TNF-α and subsequently promoting key inflammatory responses [46]. These inflammatory responses and ROS lead to mitochondrial dysfunction, which triggers the amplification of ROS production from impaired mitochondria.
Toll-like receptors and gastrointestinal mucositis
Research [47] found that microbiota dysbiosis in mice gut following the administration of broad-spectrum antibiotics has been accompanied by increased susceptibility to methotrexate-induced intestinal injury, which is suppressed by Toll-like receptors (TLR) 2 ligands. The potential mechanism by which TLR affects the intestinal proinflammatory response in chemotherapy- or radiation-induced epithelial damage is the robust activation of the NF-κB pathway [44,48]. For instance, in mice subjected to chemotherapy or radiotherapy, TLR4 drives the secretion of proinflammatory cytokines (IL-1β, TNF-α, and IL-6) corresponding to lipopolysaccharide (LPS)-producing bacteria through the TLR4-MyD88-NF-κB signaling pathway, which has a crucial impact on tumor response to anticancer treatments [49-52]. This significant mechanism is shown in Figure 3. Conversely, mitigation of irinotecan-associated pain and gut toxicity appeared in TLR4-knockout mice [48]. Egan et al. [53] showed that the NF-κB pathway is essential for radiation-induced apoptosis and inflammation by altering downstream transcriptional factors that ultimately produce inflammatory cytokines. This study showed that activation of NF-κB also mediates LPS proinflammatory function. More specifically, Gerassy-Vainberg et al. [54] highlighted that the imbalance proportion of propionate-producing bacteria (Akkermansia) is highly associated with increased secretion of IL-1β by the host. Analogously, the inflammatory events may be multiple partially due to the lack of anti-inflammatory bacteria (Faecalibacterium prausnitzii and Bifidobacterium) [55,56]. Such occurrences will induce the secretion of IL-10 [54] and antagonize the inhibitory-κB kinase degradation by producing nonlipophilic compounds [55]. Furthermore, SCFAs have been reported to inhibit the activation of the NF-κB signaling pathway in stressed enterocytes. Hence, if SCFA-producing bacteria (Ruminococcus, Coprococcus, and Roseburia) were absent after chemotherapy or radiotherapy, gut inflammation could potentially increase.
Figure 3.
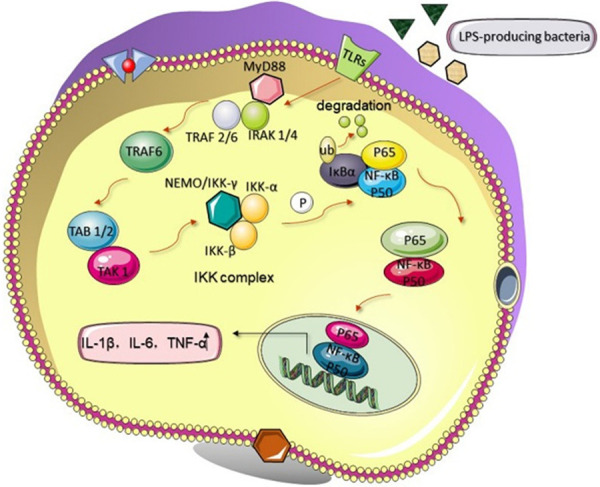
LPS-producing bacteria interact with TLRs through the NF-κB signaling pathway. For instance, TLR4 drives the secretion of proinflammatory cytokines in enterocytes corresponding to LPS-producing bacteria through the TLR4-MyD88-NF-κB signaling pathway. Then, TRAF6 is activated. After that, the compound of TAB1/2 and TAK1 is activated, and subsequently promotes the IKK complex to phosphorylate the IκB molecule, which ultimately undergoes ubiquitination and degradation. Then, NF-κB, including p50 and p65, will translocate into the nucleus to regulate the expression of genes encoding IL-1β, IL-6, and TNF-α. LPS, lipopolysaccharide; TLRs, Toll-like receptors; MyD88, myeloid differentiation factor 88; IRAK, IL-1 receptor-associated kinase; TRAF6, TNF receptor-associated factor 6; TAK1, TGF-β-activated kinase 1; IKK, inhibitor of κB kinase; NEMO, NF-κB essential modulator; I-κB, inhibitor of κB; P, phosphorylation; P50/P60, subunits of NF-κB; Ub, ubiquitination; ↑, increase.
Oxidative stress
After chemotherapy or radiotherapy, DNA is damaged directly or indirectly via the formation of free radicals such as ROS, RNS, and RSS [57]. Intestinal inflammation prolongs the chemotherapy or radiation response by producing more free radicals, cytokines, and growth factors [58], which means that oxidative stress occurs after the proinflammatory events, in both tumor cell death and normal tissue toxicity. For example, patients exposed to the standard radiotherapy dose can develop immunogenic cell death and radiation enteritis, associated with the release of proinflammatory cytokines (IL-1β, IL-6, and TNF-α) [2,59,60]. These proinflammatory cytokines are capable of triggering the amplification of free radicals production by stimulating ROS- and RNS-producing enzymes such as cyclooxygenase-2, nicotinamide adenine dinucleotide phosphate oxidase, and nitric oxide synthase (NOS) [61]. Overproduction of ROS causes oxidative damage to the intestinal epithelium and contributes to acute and chronic complications [62]. For instance, IL-1β is able to induce neutrophils to release superoxide through activation of the p38/MAPK signaling pathway [63]. Moreover, IL-1β is capable of upregulating the expression of the gene encoding inducible NO synthase (iNOS), thus increasing capillary permeability to induce inflammation by enriching the concentration of NO within the endothelium [64]. Likewise, TNF-α could clear lesioned cells or bacterial infection by inducing ROS. Therefore, the oxidative stress nearly synchronized with inflammation responses aggravates the microenvironment in the lesioned gut during/after chemotherapy or radiotherapy.
Intestinal barrier
Microbial homeostasis has some protective effects on the intestinal barrier, particularly in the lesioned gut milieu. However, chemotherapy or radiotherapy can break the balance of commensal bacteria, impairing the barrier function by increasing intestinal permeability and interrupting the mucus layer (Figures 2 and 4).
Figure 4.
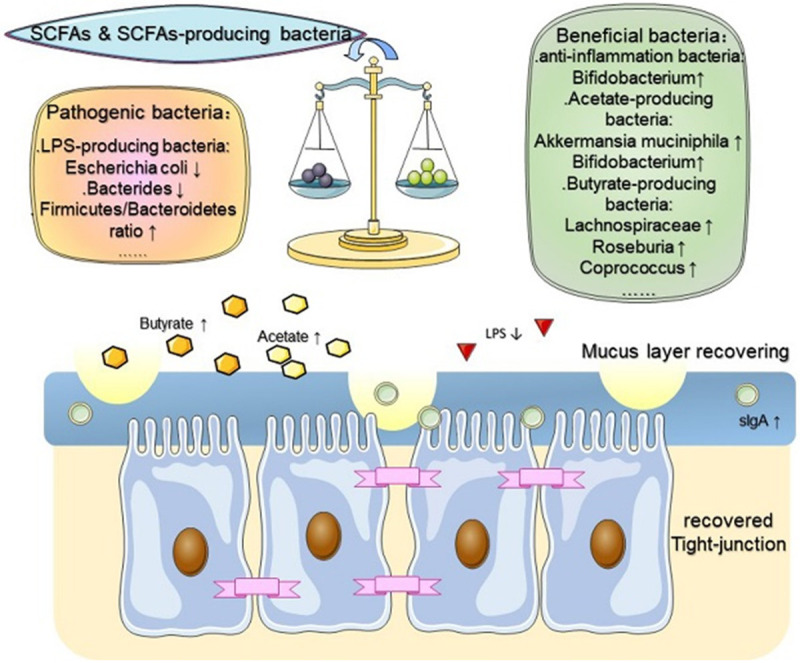
SCFAs regulate chemotherapy- or radiotherapy-associated intestinal microbial dysbiosis and repair the intestinal barrier. Applying SCFAs directly or SCFA-producing bacteria regained intestinal homeostasis and ordered the milieu by increasing the proportions of beneficial bacteria and reducing the number of pathogenic bacteria. Thereupon, the recovered mucus layer and reduced intestinal permeability also emerged. SCFAs, short chain fatty acids; ↑, increased; ↓, decreased.
To facilitate an antitumor effect, cyclophosphamide increases intestinal permeability by allowing microbial translocation to trigger the maturation of T helper 17 (Th17) cells in the lamina propria [65]. Th17 cells can aggravate oxidative stress by promoting the secretion of G-CSF. However, microbial translocation enhances intestinal tumor progression and subsequently leads to intestinal barrier dysfunction [66]. Modulating the tight junction proteins is also a remarkable way to regulate intestinal permeability. For example, Bifidobacterium can upregulate the expression of genes encoding claudin (a tight junction protein) [67]. The relative abundance of such bacteria is always decreased after chemo- or radiotherapy. Furthermore, LPS-producing bacteria are able to interact with TLRs and subsequently activate the NF-κB signaling pathway. In this context, IL-1β is capable of widening the intercellular spaces and disrupting the tight junctions.
The epithelial mucus layer is another protective factor that prevents enteritis contributing to intestinal integrity and is regulated by gut bacteria. It consists of glycoproteins, mucins, immunoglobulins, and butyrate. The main reason for the mucus layer interruption induced by chemo- or radiotherapy is the decrease in butyrate-producing bacteria [8,9]. In addition, several bacteria can damage the mucus layer directly. For instance, Enterobacteriaceae members, which increase in the gut after chemotherapy, impair the absorption of cysteine, proline, and methionine [9], leading to a reduction in mucin synthesis. Importantly, the gut microbiota may also damage the intestinal mucosa by altering the absorption of bile salts and changing stool frequency after radiation therapy [54]. Furthermore, secretory immunoglobulin A (sIgA), a vital antibody, is able to neutralize toxins and suppress pathogens in the mucus layer. However, sIgA concentration may be declined after radiotherapy due to intestinal microbial dysbiosis. In an animal model [68], it was found that an increase in Sutterella spp. modulates an sIgA-low phenotype, which empowers the hosts to be predisposed to dextran sulfate sodium (DSS)-induced colitis.
SCFA biosynthesis, absorption, and distribution
SCFAs are dated from microbial fermentation of dietary fibers and have versatile implications for the human body. Unlike microbiota-accessible carbohydrates that are digested by host enzymes in jejunum and ileum, dietary fibers are slaked by the microbial fermentative activity in the colon [69]. The major metabolites of such activities are SCFAs, including acetate, propionate, butyrate, isobutyrate, 2-methylbutyrate, and isovalerate [70,71]. In particular, keeping a fixed proportion with each other, acetate, propionate, and butyrate are taking up approximately 86% of the total human SCFAs in the gut [70]. Relatively minor amounts of branched-chain fatty acids, such as isobutyrate, 2-methylbutyrate, and isovalerate exclusively originate from branched-chain amino acids valine, isoleucine, and leucine [71]. Acetate production pathways are widely distributed among bacterial groups, whereas pathways for propionate, butyrate, and lactate production appear more highly conserved and substrate specific. Further supplementation of diets rich in dietary fiber increases SCFAs and then restores the beneficial gut microbiota and feathers out the microbial metabolites [72].
SCFAs are the major end products of the saccharolytic fermentation mediated by the enzymatic repertoire of specific members of the gut microbes escape digestion and absorption [73]. SCFA production pathways are relatively well understood and have been recently described in detail (Table 2). Biosynthesis of acetate is from pyruvate either via the acetyl coenzyme A (CoA) pathway or via the Wood-Ljungdahl pathway [74]. Propionate, is synthesized via three branches: (i) via the succinate pathway; (ii) via acrylate pathway [75], and (iii) via the propanediol pathway [76]. Another major SCFA, butyrate, is synthesized in multiple ways: (i) via the so-called classical pathway: butyryl-CoA can be transformed to butyrate by phosphotransbutyrylase and butyrate kinase [77]; (ii) acetate CoA-transferase route [78]; (iii) the microbe-induced condensation of lactate and acetate; (iv) via the lysine pathway [79].
Table 2.
SCFA production by microbes in the gut
| Study year (ref) | SCFAs | Producers | Pathway | Distribution |
|---|---|---|---|---|
| Louis et al., 2014 [142]; Rey et al., 2010 [143] | Acetate | Most of the enteric bacteria, e.g., Akkermansia muciniphila, Bacteroides spp., Bifidobacterium spp., Prevotella spp., Ruminococcus spp. | From pyruvate via acetyl-CoA | Intestinal |
| Blautia hydrogenotrophica, Clostridium spp., Streptococcus spp. | Wood-Ljungdahl pathway | Circulation | ||
| Louis et al., 2014; Scott et al., 2006 [76] | Propionate | Bacteroides spp., Phascolarctobacterium succinatutens, Dialister spp., Veillonella spp. | Succinate pathway | Intestinal |
| Megasphaera elsdenii, Coprococcus catus | Acrylate pathway | |||
| Salmonella spp., Roseburia inulinivorans, Ruminococcus obeum | Propanediol pathway | Circulation | ||
| Louis et al., 2014; Duncan et al., 2002 [78] | Butyrate | Coprococcus comes, Coprococcus eutactus | Phosphotransbutyrylase/Butyrate kinase intestinal pathway | Intestinal |
| Roseburia spp., Coprococcus catus, Faecalibacterium | Acetate CoA-transferase pathway | Intestinal |
Moreover, the biological gradient of SCFAs varies across different downstream tissues along the gut lumen. The highest levels occur in the cecum and proximal colon and the concentration declines toward the distal colon [70]. Butyrate is the primary energy source for colonocytes. It is metabolized in the epithelium mucosa and is partly consumed in the liver [80,81]. Acetate and propionate are absorbed in the portal vein. Acetate is released as the most abundant SCFA in the peripheral circulation [82]. Propionate is metabolized in the liver and thus the hepatic capacity to utilize SCFA interacts with gut SCFA production, leading to non-significant splanchnic propionate and butyrate output and high peripheral concentrations of acetate [81,83].
SCFAs as signaling molecules
It is necessary to elucidate the potential molecular mechanisms involving SCFAs (Figure 5) before illustrating the mechanisms through which SCFAs repair chemotherapy- or radiation-related metabolic dysfunction and inflammation.
Figure 5.
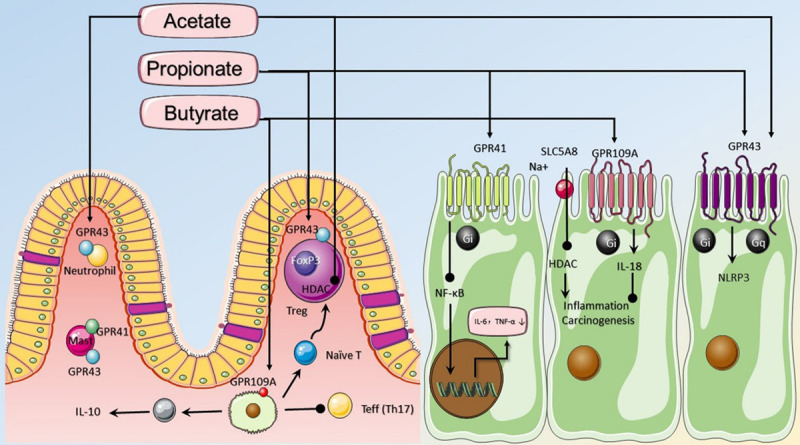
SCFAs, including acetate, propionate, and butyrate, have broad impacts on various aspects of host physiology or pathology. In the distal gut, sensed by GPR41, GPR43, and GPR109a, luminal SCFAs are able to attenuate inflammation by stimulating the chemotaxis of neutrophils, inhibiting NF-κB, increasing the secretion of IL-18, and activating the NLRP3 inflammasome pathway. SCFAs can enter cells through diffusion or SLC5A8-mediated transport and act as an energy source or an HDAC inhibitor. SCFAs promote Foxp3 and inhibit HDAC to regulate Tregs SCFAs can also increase the concentration of IL-10 and decrease the number of Th17 cells to reduce inflammation and tumorigenesis. SCFAs, short chain fatty acids; ↑, direct action; ♩, inhibit action.
SCFA as a ligand of the g protein-coupled receptor
G protein-coupled receptor (GPCR) is a family of superproteins in the human body, and the human genome encodes for approximately 800 GPCRs [84]. SCFAs activate GPCR in the extracellular environment and have anti-inflammatory effects by binding to three GPCRs in the intestine, namely: free fatty acid receptor 2 (GPR43 or FFAR2), free fatty acid receptor 3 (GPR41 or FFAR3), and hydroxycarboxylic acid receptor 2 (GPR109A or HCAR2). GPR43/FFAR2 is a Gi/o- and Gq-dual-coupled GPCR [85]; however, GPR41/FFAR3 and GPR109A couple only to Gi [86]. Propionate is the most potent activator of GPR41/FFAR3, while the most sensing affinity of GPR43/FFAR2 is to acetate. At present, it is unsealed whether GPR43 is expressed on the apical or basolateral side of the cell [86]. GPR109A is highly activated by butyrate and expressed on the lumen-facing apical membrane of colonic and small intestinal epithelial cells.
One pathway for SCFAs activating GPCR is associated with intestinal epithelial cells (IECs). In inflammation-induced colon cancer mouse models, SCFAs are able to stimulate efflux and hyperpolarization of K+ by interacting with GPR43 and GPR41 on IECs, which results in an increase in the secretion of IL-18 and NLRP3 inflammasomes, both of which are critical factors for intestinal homeostasis [87]. Similarly, butyrate can relieve intestinal inflammation through the homologous pathway via GPR109A in IECs [88]. In addition, SCFAs may influence GPCR by affecting the immune system. A study [89] showed that GPR43 (-/-) mice are more likely to develop DSS-induced IBD. In this context, GPR43 is necessary for the chemotaxis of neutrophils; simultaneously, inflammatory factors stimulated by LPS bacteria are decreased by SCFAs in neutrophilic granulocytes [90]. Besides, in neutrophils, it has been reported that butyrate and propionate are able to inhibit the maturation and function of dendritic cells (DCs) via GPR43 and GPR109A [88,91]. Moreover, Wu el at. [92] reported that acetate is beneficial for IECs against harmful bacteria by promoting intestinal IgA response which was mediated by “metabolite-sensing” GPR43 in DCs.
SCFA as an inhibitor of histone deacetylase
In addition to anti-inflammatory effects in the extracellular space, SCFAs can also act as signaling molecules that enter cells by sodium-coupled monocarboxylate-transporter 1 (SMCT1/SLC5A8) [93]. Acetyl groups derived from acetyl-CoA decorated histone tails by histone acetyltransferases (HATs) to promote gene transcription. In contrast, repressive chromatin structures are formed through the removal of acetyl groups induced by histone deacetylases (HDACs) [94]. Thus, histone acetylation plays a crucial role in gene expression. Due to the anticancer and immunosuppressive effects of HDAC inhibitors, SCFAs may emerge as triggers of cancer and immune homeostasis because of the collective acceptance that butyrate and, to a lesser extent, propionate are both HDAC inhibitors [95,96].
Acting as an efficient HDAC inhibitor, butyrate has been the most investigated SCFA in altering gene expression, including cell proliferation, apoptosis, and differentiation [97]. The butyrate concentration may emerge as a critical modulator that allows tautomerism between being an HAT activator and an HDAC inhibitor in a cell- and environment-specific context. For example, butyrate has up to 3 times more in cancer cells than in normal colonocyte [80].
One of the mechanisms by which SCFAs inhibit HDAC is to regulate the innate immune system. For example, LPS-stimulated butyrate restrains proinflammatory effectors in lamina propria macrophages, such as NO, IL-6, and IL-12. In this way, our intestinal immune system becomes hyporesponsive to beneficial symbionts [10]. Furthermore, butyrate and propionate enable the inhibition of the differentiation of bone marrow stem cells to DCs induced by the granulocyte-macrophage colony stimulating factor via HDAC1 and HDAC3 [98,99]. In addition, SCFAs regulate the generation of regulatory T cells (Tregs) and suppress the activation of effector T cells (Th1, Th2, and Th17) through HDAC inhibition. SCFAs increase the expression of Foxp3 and IL-10, both key factors limiting intestinal inflammation in colonic Tregs (cTregs), to promote microbiota-induced cTreg development associated with de novo generation of inducible Tregs (iTregs) by inhibiting HDAC in DCs [42]. Further research [100] reveals that SCFAs as HDAC inhibitors alleviate the inflammatory effect by increasing the content of p70 S6 kinase (S6K) and enhancing the acetylation of phosphorylated ribosomal protein S6 (rS6). Besides, there is another role SCFAs play in regulating gene expression within B cells to promote antibodies, by interacting with HDAC [101].
Although acetate is generally considered to have little connection with HDAC inhibitors, recent research [102] reported that it may alter the expression of FoxP3 and IL-17 by increasing the aromatic hydrocarbon receptor and enhancing histone acetylation in Caco-2 cells. This implies that acetate is a potential inhibitor of HDAC, but its pathway may be different from that of butyrate and propionate. However, a study [103] found that butyrate plays a dominant role in the early stage of inflammation by promoting the expression of T-bet and IFN-γ in T cells. This suggests that butyrate may activate the collective immune response by increasing inflammatory factors in the early stage of inflammation to alleviate the aggravation of the cancer.
Positive roles played by SCFAs
Accumulating evidence suggests that the SCFAs, butyrate in particular, play positive roles in regulating metabolic dysfunction of the host (Figure 6).
Figure 6.
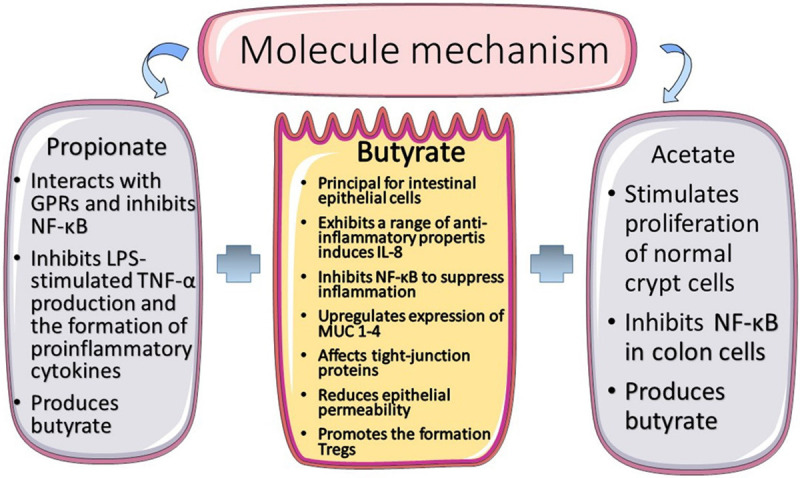
The summary of the short chain fatty acids mechanism.
SCFAs and immunity
Since the physical and biochemical barriers anatomically divide the immune cell from complicated bacterial milieu, our intestine is a standalone immunological site where perturbation of the equilibrium is mainly modulated by microbiota [104]. Nevertheless, chemotherapy and radiotherapy, cancer treatment modalities, can lead to intestinal microbial dysbiosis, thus provoking persistent inflammation and damaged gut homeostasis. In this review, we focus on SCFAs exerting anti-inflammatory effects by acting as signaling molecules toward a delicate balance after chemotherapy or radiotherapy.
Since the immune system can be persistently impacted, it is important to stay hyporesponsive to pathogenic bacteria and launch immunosuppressive mechanisms under the stimulation of radio- or chemotherapy. The suppressive activity is achieved by the full reciprocity between SCFAs and their targets in the host (GPCR and/or HDAC). Accumulating evidence has suggested the mechanism of SCFAs in Treg cells. Arpaia et al. [105] reported that providing acetate, propionate, and butyrate is able to alleviate the apparent decrease of colonic Tregs, which indicates that these three SCFAs independently affect Treg cells. Acetate and propionate stimulate the expansion of Treg cells and increase the expression of IL-10 by activating GPR43 to upregulate the expression of Foxp3 [106]. Furthermore, inflammation is attenuated by increasing Tregs and IL-10 via the signaling of GPR109A. Butyrate blocks the translocation of NF-κB pathway signal into the nucleus to suppress subsequent activation of downstream gene expression and generation of IL-6 [88].
Considering the high SCFA receptors expression in immune cells [88,107,108], we speculate that SCFAs play significant roles in T cell function. The description of the role SCFAs played in the T cells differentiation has revealed a fact that this process typically hinges on immunological milieu [100]. Unlike a previous study showing high expression of GPR43 in myeloid and Tregs cells [106], Park et al. [100] revealed that T cells express GPR43 inconspicuously and that cytokine expression is adjusted by SCFA-mediated HDAC inhibition other than GPR43. In addition, they also suggested that, if the host was eliminating pathogens, the differentiation from naive T cells into Th1 and Th17 cells to boost immunity. To summarize, SCFAs is able to regulate T cell function, but further research is desire for pinpointing the underlying mechanism. When it comes to human monocytes, SCFAs promote secretion of prostaglandin E2 through pertussis toxin-sensitive GPCRs, thereby inhibiting inflammatory responses [109].
The inhibitory effect of SCFAs on HDAC activity has been discussed above, including promoting histone acetylation, affecting gene expression and inflammatory response, and regaining intestinal homeostasis and cancer protection [109].
SCFAs and chemotherapy- or radiation-associated intestinal microbial dysbiosis
Previous research [8,9] suggest that, after chemotherapy or radiotherapy, patients with enteritis present low levels of SCFA-producing bacteria in their gut. Hence, SCFAs concentration is often found to be decreased in fecal samples of such patients. SCFAs have antagonistic effects on intestinal microbial dysbiosis, resulting in an increase in the number of beneficial bacteria and a decrease in the quantity of pathogenic bacterium in the gut (Figure 4). For example, Haenen et al. [110] found that pigs fed a resistant starch-supplemented diet exhibited higher proportions of butyrate- or propionate-producing bacteria, whereas potentially pathogenic bacteria (E. coli and Pseudomonas spp.) were decreased. Relevant results [111] showed that mice with DSS-induced colitis fed a high-fiber diet exhibited more Bacteroidetes (Porphyromonadaceae and Rikenellaceae) and Firmicutes (Lachnospiraceae) in their feces accompanied with less moderate enteritis than mice fed diets containing zero fiber, which reflects the potential therapeutic effects of SCFAs in attenuating inflammation. Similarly, dietary fibers enriched Bacteroidaceae and Bifidobacteriaceae and decreased potentially pathogenic bacteria such as Erysipelotrichaceae [109]. There is a natural synergy between butyrate and Bifidobacterium in anti-inflammatory function by downregulating the expression of IL-8 [112] and upregulating the number of Tregs at injured sites [113]. In addition, Bifidobacterium can inhibit LPS-induced autophagy to maintain intestinal barrier [114].
Specifically, butyrate exclusively activates the peroxisome proliferator-activated receptor gamma, a nuclear receptor that impairs the gene encoding iNOS [115], an interaction that has been confirmed to hinder the production of nitrate in IECs. Nitrate, a respiratory electron acceptor, is hanker for the reproduction of some pathogenic bacteria (Escherichia and Salmonella spp.) [116]. Once nitrate is lacking, the ratio of these harmful bacteria decline. Therefore, SCFAs are candidates for restoring gut homeostasis and managing lesioned intestinal mucosa.
However, direct application of SCFAs is not as valid as the direct administration of alive SCFA-producing bacteria to the mucosa recovery and intestinal homeostasis. For example, butyrate had lesser impact on attenuating inflammation in mice with 2,4,6-trinitrobenzene sulfonic acid-induced colitis than injections with alive F. prausnitzii or F. prausnitzii supernatant. However, both approaches increased IL-10 and declined IL-12 and TNF-α [56]. This may be due to the fact that a constant production and delivery of SCFAs to the mucosa is necessary to exert various effects.
SCFAs and the intestinal barrier
When suffering from chemotherapy- or radiotherapy-related intestinal inflammation, the permeability of enterocytes is increased, and the mucus layer is destroyed on account of intestinal microbial dysbiosis. Thus, the mucositis after the above cancer treatment is always accompanied by impaired barrier function. In the intestinal mucosa, SCFAs exert beneficial effects on IECs and immune cells through the induction of intracellular or extracellular processes (Figures 4 and 5).
First, the SCFA butyrate restores the epithelial barrier function by settling the hypoxia-inducible factor 1 (HIF-1, a transcription factor coordinating barrier protection), profiting from the low O2 concentrations in the colon. Although the reduction of HIF-1α expression and butyrate levels is greatly apparent in antibiotic-treated or germ-free (GF) mice, the expression of HIF-1α is regained after supplementing butyrate administration [117]. Importantly, rescue experiments demonstrated that butyrate plays a critical role towards HIF-1 in maintaining barrier integrity because it seldom induces the barrier function without HIF-1β in T84 cells [117]. Second, butyrate influences the expression of genes encoding tight-junction components and protein reassembly through the activation of certain transcription factors, including the signal transducer and activator of transcription 3 and specificity protein 1 [109]. Several in vitro studies have revealed that transepithelial electrical resistance is increased by butyrate in inflammatory conditions in mice and humans [118-121]. Hence, butyrate has a paramount contribution to recover tight junctions, which is an important therapeutic target. Third, butyrate promotes epithelial barrier function by consolidating the mucus layer, where sIgA can neutralize toxins and pathogens after chemo- or radiotherapy. Butyrate is capable of promoting mucin compound by upregulating the expression of the MUC1-4 genes [122]. Another important mechanism is the synthesis of antimicrobial peptides (AMPs) by IECs. Recent studies have shown that the expression of the AMPs RegIIIγ and β-defensins is distinctly suppressed in Gpr43 KO mice, while Gpr43 activation induced by butyrate impairs AMP secretion, including cathelicidin, in experimental models [123,124]. As a result, SCFAs can improve the microenvironment of epithelium by maintaining the physiologic composition of mucus and upregulating the secretion of AMPs.
SCFAs and anticancer effects
Recent compelling evidence suggests that the gastrointestinal microbiota plays a role in modulating responses to cancer immunotherapy [125-127], and data demonstrate that the microbial communities within the tumor microenvironment can contribute to therapeutic efficacy [128]. Generally speaking, patients suffering solid tumors usually treated with chemotherapy or radiotherapy. In this context, chronic inflammation within a tumor bed is a well-established risk factor for tumor remission after chemotherapy or radiotherapy, partially owing to the infiltration of some cancer-facilitating cells such as M2 macrophages and IL-17-producing cells [129,130]. Research from preclinical models shows that oral administration of feces from patients with colorectal cancer to GF mice can induce polyp formation, produce carcinogenic signals, and alter the local immune milieu to promote intestinal carcinogenesis through metabolites of oncogenic gut bacteria [131].
Pathogenic bacteria but also commensal microbial elements have been validated to play a vital role in the response to inflammation and other pathological processes. Existing evidence inferred that chemotherapy and radiotherapy may influence microbial dysbiosis, as well as toxicity, via a variety of proposed mechanisms, although in-depth knowledge is needed. Preclinical models show that the gut microbiota is a double-edged sword to oxaliplatin, leading to either mechanical hyperalgesia or tumor cytotoxicity, the chemotherapy-related complications, by a way of increased ROS and proinflammatory factors in the dorsal root ganglion [132]. Ionizing irradiation has been reported to alter the constitutions of gut microbes in preclinical models too, reducing the abundance of Firmicutes and increasing the abundance of Proteobacteria and subsequently increasing susceptibility to colitis [54]. Furthermore, altered mucus quality is the main cause of increasing susceptibility to DSS-induced colitis in GF and antibiotic-treated mice [88].
To demonstrate the anticancer effects of SCFAs, the following mechanism is proposed. Based on early in vitro data, it seems that SCFAs inhibit HDACs to suppress cancer cells [133,134]. Nevertheless, this mechanism alone is unable to explain entirely the SCFAs effect on carcinogenesis in vivo. Accumulating evidence suggests that the suppressive effects of SCFAs on chronic inflammatory responses and intestinal carcinogenesis are mediated by SCFAs receptors [135], which function in an explicitly GPCR-associated manner rather than inhibition in HDACs. Activation of GPR43 by acetate plays a protective role against gut inflammation in mice [107], which suggests that SCFAs have a phylactic role in colonic inflammation. Likewise, the expression of the SCFA receptors GPR109A and GPR43 is markedly reduced in colon cancer [136], supporting the favorable role of SCFA signaling as well. Importantly, many mechanisms remain unclear and are the focus of studies currently investigating the causal links between tumor-associated microbiota and metabolites in inflammation response and cancer.
The SCFA receptors function in suppressing carcinogenesis is frequently linked to chronic inflammation. Particularly, butyrate appears to play a phylactic part based on the distinct decline butyrate-producing bacteria in an amelioration of experimental colitis via GPR109A [88]. Although the corresponding connection with GPR43 does not have a dominant state on colon cancer development [135], the SCFA-GPR43 axis is going to boost barrier immune responses, which limits persistent bacterial invasion, chronic inflammatory responses, and colon cancer development [137]. However, Coutzac et al. [138] observed that, in mice and patients, high serum levels of butyrate and propionate are associated with resistance to CTLA-4 blockade and a higher proportion of Treg cells.
Discussion and future perspective
Chemo- or radiotherapy induce major changes in the composition of the gut microbiota; subsequently, these random disruptions and the resulting metabolites are able to participate in the development of mucositis. Basic and clinical data suggest that SCFAs produced by microbial interactions with dietary polysaccharides are important energy resources and signaling molecules. In this review, we focus on the mechanism that SCFAs, as signaling molecules, adjust versatile class of intestinal immune system towards microenvironment homeostasis. It has become increasingly accepted that SCFA-producing bacteria are beneficial for human health, therefore, the therapeutic impacts of SCFAs in inflammation are important issues of concern and should be paid more attention from the public Unlike microbially produced metabolites, exogenously administered SCFAs are probably not as effective as the administration of live SCFA-producing bacteria to the mucosa [10,56]. Moreover, it needs to be a constant production and delivery of SCFAs to the mucosa for anti-inflammatory effects to occur [109]. Therefore, the success rules of SCFAs might be framed by enriching or recovering SCFA-producing bacteria in molding intestinal mucosa homeostasis from chemotherapy or radiotherapy-induced microbial dysbiosis. At molecular level, we discussed the anti-inflammatory mechanisms of SCFAs towards intestinal microbiota and immunity by acting as signaling molecules. SCFAs act as GPCR ligands in the extracellular milieu and HDAC inhibitors in the intracellular milieu. Both methods can combat intestinal inflammation and revolve the homeostasis by affecting the innate immune system and regulating Treg cells. Furthermore, recent studies prefer GPCR-associated receptors to HDAC inhibitors to explain the impaired effect of SCFAs. In addition, because of the increased expression of SLC5A8 and GPR109A, the host targets seem to exert a higher affinity for SCFAs than before [73]. The protective effect of SCFAs, as signal molecules, has been verified in animal models [87,110], but there is still a lack of interrelated data in the human. Advancements of SCFAs in clinic research are proceeding slowly, because of the collective acceptance that SCFAs seem to play different roles in various outside environment and human internal milieu rather than to work alone. It is necessary to consider the genetic background, bacterial metabolites interactions, and tumor microenvironment when we expect SCFAs to combat intestinal inflammation. This is why the opposite is observed in enteritis with similar amounts of SCFAs: resistance effect and aggravation [90,138,139].Since targets and SCFAs receptors is diverse between animal and the human body, in-depth studies are requisite for more details.
Although there currently are some challenges in the prevention of intestinal inflammation after chemotherapy or pelvic radiotherapy, moderate diet supplements of SCFAs lead to restoring bacterial homeostasis, attenuating inflammation, maintaining the barrier function, promoting antitumor effects, and mucosal repair after cancer treatments. Regrettably, few clinical trials explored the direct causality between SCFAs and enteritis. It’s worth noting that more investigators prefer focusing their attentions on the effect of different intaking-dietary fiber to uncovering the deep relationship of SCFAs and inflammation. Among their relevant preclinical trials [140,141], SCFAs are regarded as specific indicators to imply the extent of intestinal inflammation. Spatiotemporal concentration of SCFAs can indicate intestinal microbial imbalance and the subsequent occurrence of inflammation after undergoing chemo- or radiotherapy. Nevertheless, there is still a lack of a “gold standard” for the diagnosis of enteritis in clinical use. This suggests that SCFAs have the potential to be regarded as predictive markers for the risk of mucositis and could be a guide of precautionary measures. Lucubration of the gut microbiota and their metabolites by modern inspection techniques, such as metagenomic approaches, should contribute to personalized investigational strategies that can be ascertained in future clinical trials. Furthermore, improving identification techniques will make it possible to regard SCFAs as the biomarker in clinical detection index. SCFAs also inspire new ideas to treat other related intestinal diseases for the aspect of prophylaxis, diagnosis and intervention. Owing to deriving from microbial fermentation, SCFAs may be affected by food intake and microbial abundance. Thus, in order to mitigate enteritis associated with chemotherapy or radiotherapy, therapeutic approaches including adjusting food intake, regulating the proportion of gut bacteria and subsequently alter the concentration of SCFAs should be considered into clinical application. To achieve this prospective goal, further clinical research is indispensable in the future.
Acknowledgements
This work was supported by the Study on the relationship between chemoradiotherapy sensitivity and differential expression of micrornas in cervical cancer (2013106022), The combination of Chk-1SiRNA and caffeine sensitized liver cancer stem cells to radiation (2015Z009), CircRNA targeted mirNA-532-5P regulates the radiation resistance of CD109 to cervical cancer cells and its molecular mechanism (3D5197457429).
Disclosure of conflict of interest
None.
References
- 1.Touchefeu Y, Montassier E, Nieman K, Gastinne T, Potel G, Bruley des Varannes S, Le Vacon F, de La Cochetière MF. Systematic review: the role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis - current evidence and potential clinical applications. Aliment Pharmacol Ther. 2014;40:409–421. doi: 10.1111/apt.12878. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Wang Q, Wang X, Zhu L, Chen J, Zhang B, Chen Y, Yuan Z. Gut microbial dysbiosis is associated with development and progression of radiation enteritis during pelvic radiotherapy. J Cell Mol Med. 2019;23:3747–3756. doi: 10.1111/jcmm.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leibowitz BJ, Yang L, Wei L, Buchanan ME, Rachid M, Parise RA, Beumer JH, Eiseman JL, Schoen RE, Zhang L, Yu J. Targeting p53-dependent stem cell loss for intestinal chemoprotection. Sci Transl Med. 2018;10:eaam7610. doi: 10.1126/scitranslmed.aam7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauer-Jensen M, Denham JW, Andreyev HJ. Radiation enteropathy-pathogenesis, treatment and prevention. Nat Rev Gastroenterol Hepatol. 2014;11:470–479. doi: 10.1038/nrgastro.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 6.Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25:377–388. doi: 10.1038/s41591-019-0377-7. [DOI] [PubMed] [Google Scholar]
- 7.Qiu W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, Zambetti GP, Zhang L, Yu J. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2:576–583. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang A, Ling Z, Yang Z, Kiela PR, Wang T, Wang C, Cao L, Geng F, Shen M, Ran X, Su Y, Cheng T, Wang J. Gut microbial dysbiosis may predict diarrhea and fatigue in patients undergoing pelvic cancer radiotherapy: a pilot study. PLoS One. 2015;10:e0126312. doi: 10.1371/journal.pone.0126312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montassier E, Gastinne T, Vangay P, Al-Ghalith GA, des Varannes SB, Massart S, Moreau P, Potel G, de La Cochetiere MF, Batard E, Knights D. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther. 2015;42:515–528. doi: 10.1111/apt.13302. [DOI] [PubMed] [Google Scholar]
- 10.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiippala K, Jouhten H, Ronkainen A, Hartikainen A, Kainulainen V, Jalanka J, Satokari R. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. 2018;10:988. doi: 10.3390/nu10080988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sonnenburg JL, Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert JA, Quinn RA, Debelius J, Xu ZZ, Morton J, Garg N, Jansson JK, Dorrestein PC, Knight R. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature. 2016;535:94–103. doi: 10.1038/nature18850. [DOI] [PubMed] [Google Scholar]
- 14.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut Microbiota in Health and Disease. Physiological Reviews. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 15.Sender R, Fuchs S, Milo R. Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology. 2017;152:1671–1678. doi: 10.1053/j.gastro.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 17.Millar MR, Linton CJ, Cade A, Glancy D, Hall M, Jalal H. Application of 16S rRNA gene PCR to study bowel flora of preterm infants with and without necrotizing enterocolitis. J Clin Microbiol. 1996;34:2506–2510. doi: 10.1128/jcm.34.10.2506-2510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas F, Hehemann JH, Rebuffet E, Czjzek M, Michel G. Environmental and gut Bacteroidetes: the food connection. Front Microbiol. 2011;2:93. doi: 10.3389/fmicb.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maier E, Anderson RC, Roy NC. Understanding how commensal obligate anaerobic bacteria regulate immune functions in the large intestine. Nutrients. 2015;7:45–73. doi: 10.3390/nu7010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundin OH, Mendoza-Ladd A, Zeng M, Diaz-Arevalo D, Morales E, Fagan BM, Ordonez J, Velez P, Antony N, McCallum RW. The human jejunum has an endogenous microbiota that differs from those in the oral cavity and colon. Bmc Microbiology. 2017;17:160. doi: 10.1186/s12866-017-1059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vrancken G, Gregory AC, Huys GRB, Faust K, Raes J. Synthetic ecology of the human gut microbiota. Nat Rev Microbiol. 2019;17:754–763. doi: 10.1038/s41579-019-0264-8. [DOI] [PubMed] [Google Scholar]
- 22.Valdes AM, Walter L, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:9. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Longman RS, Littman DR. The functional impact of the intestinal microbiome on mucosal immunity and systemic autoimmunity. Current Opinion In Rheumatology. 2015;27:381–387. doi: 10.1097/BOR.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17:271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 25.Dabke K, Hendrick G, Devkota S. The gut microbiome and metabolic syndrome. J Clin Invest. 2019;129:4050–4057. doi: 10.1172/JCI129194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith PA. The tantalizing links between gut microbes and the brain. Neuroscientists are probing the idea that intestinal microbiota might influence brain development and behaviour. Nature. 2015;526:312–314. doi: 10.1038/526312a. [DOI] [PubMed] [Google Scholar]
- 27.Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science. 2018;362:776–780. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- 28.Eaton K, Pirani A, Snitkin ES Reproducibility Project: Cancer Biology. Iorns E, Tsui R, Denis A, Perfito N, Errington TM, Iorns E, Tsui R, Denis A, Perfito N, Errington TM. Replication study: intestinal inflammation targets cancer-inducing activity of the microbiota. Elife. 2018;7:e34364. doi: 10.7554/eLife.34364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jumpertz R, Duc Son L, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin R, Miquel S, Ulmer J, Langella P, Bermudez-Humaran LG. Gut ecosystem: how microbes help us. Benef Microbes. 2014;5:219–233. doi: 10.3920/BM2013.0057. [DOI] [PubMed] [Google Scholar]
- 32.Levy M, Thaiss CA, Zeevi D, Dohnalová L, Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig Y, Pevsner-Fischer M, Shapiro H, Christ A, Harmelin A, Halpern Z, Latz E, Flavell RA, Amit I, Segal E, Elinav E. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell. 2015;163:1428–1443. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018–C1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 34.Kolling GL, Wu M, Warren CA, Durmaz E, Klaenhammer TR, Timko MP, Guerrant RL. Lactic acid production by Streptococcus thermophilus alters Clostridium difficile infection and in vitro Toxin A production. Gut Microbes. 2012;3:523–529. doi: 10.4161/gmic.21757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rangan KJ, Hang HC. Biochemical mechanisms of pathogen restriction by intestinal bacteria. Trends Biochem Sci. 2017;42:887–898. doi: 10.1016/j.tibs.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kommineni S, Bretl DJ, Lam V, Chakraborty R, Hayward M, Simpson P, Cao Y, Bousounis P, Kristich CJ, Salzman NH. Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature. 2015;526:719–722. doi: 10.1038/nature15524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sassone-Corsi M, Nuccio SP, Liu H, Hernandez D, Vu CT, Takahashi AA, Edwards RA, Raffatellu M. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature. 2016;540:280–283. doi: 10.1038/nature20557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crino L, Benedetti G, Evangelista W, Fanchini L, Cortesi E, Picone V, Vitello S, Chiara S, Granetto C, Porcile G, Fioretto L, Orlandini C, Andreuccetti M, Masi G. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 2007;25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 39.Benson AB, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA, McCallum R, Mitchell EP, O’Dorisio TM, Vokes EE, Wadler S. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J. Clin. Oncol. 2004;22:2918–2926. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- 40.Rajagopala SV, Singh H, Yu Y, Zabokrtsky KB, Torralba MG, Moncera KJ, Frank B, Pieper R, Sender L, Nelson KE. Persistent gut microbial dysbiosis in children with acute lymphoblastic leukemia (ALL) during chemotherapy. Microb Ecol. 2020;79:1034–1043. doi: 10.1007/s00248-019-01448-x. [DOI] [PubMed] [Google Scholar]
- 41.Nam YD, Kim HJ, Seo JG, Kang SW, Bae JW. Impact of pelvic radiotherapy on gut microbiota of gynecological cancer patients revealed by massive pyrosequencing. PLoS One. 2013;8:e82659. doi: 10.1371/journal.pone.0082659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stringer AM, Al-Dasooqi N, Bowen JM, Tan TH, Radzuan M, Logan RM, Mayo B, Keefe DM, Gibson RJ. Biomarkers of chemotherapy-induced diarrhoea: a clinical study of intestinal microbiome alterations, inflammation and circulating matrix metalloproteinases. Support Care Cancer. 2013;21:1843–1852. doi: 10.1007/s00520-013-1741-7. [DOI] [PubMed] [Google Scholar]
- 43.Wardill HR, Van Sebille YZA, Ciorba MA, Bowen JM. Prophylactic probiotics for cancer therapy-induced diarrhoea: a meta-analysis. Curr Opin Support Palliat Care. 2018;12:187–197. doi: 10.1097/SPC.0000000000000338. [DOI] [PubMed] [Google Scholar]
- 44.van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010;6:e1000879. doi: 10.1371/journal.ppat.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hakansson A, Molin G. Gut microbiota and inflammation. Nutrients. 2011;3:637–682. doi: 10.3390/nu3060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 47.Frank M, Hennenberg EM, Eyking A, Ruenzi M, Gerken G, Scott P, Parkhill J, Walker AW, Cario E. TLR signaling modulates side effects of anticancer therapy in the small intestine. J Immunol. 2015;194:1983–1995. doi: 10.4049/jimmunol.1402481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wardill HR, Gibson RJ, Van Sebille YZ, Secombe KR, Coller JK, White IA, Manavis J, Hutchinson MR, Staikopoulos V, Logan RM, Bowen JM. Irinotecan-induced gastrointestinal dysfunction and pain are mediated by common TLR4-dependent mechanisms. Mol Cancer Ther. 2016;15:1376–1386. doi: 10.1158/1535-7163.MCT-15-0990. [DOI] [PubMed] [Google Scholar]
- 49.Riehl T, Cohn S, Tessner T, Schloemann S, Stenson WF. Lipopolysaccharide is radioprotective in the mouse intestine through a prostaglandin-mediated mechanism. Gastroenterology. 2000;118:1106–1116. doi: 10.1016/s0016-5085(00)70363-5. [DOI] [PubMed] [Google Scholar]
- 50.Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB. Perspectives on cancer therapy-induced mucosal injury - Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100:1995–2025. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]
- 51.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 52.McClure R, Massari P. TLR-dependent human mucosal epithelial cell responses to microbial pathogens. Front Immunol. 2014;5:1–13. doi: 10.3389/fimmu.2014.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Egan LJ, Eckmann L, Greten FR, Chae SW, Li ZW, Myhre GM, Robine S, Karin M, Kagnoff MF. I kappa B-kinase beta-dependent NF-kappa B activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci U S A. 2004;101:2452–2457. doi: 10.1073/pnas.0306734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerassy-Vainberg S, Blatt A, Danin-Poleg Y, Gershovich K, Sabo E, Nevelsky A, Daniel S, Dahan A, Ziv O, Dheer R, Abreu MT, Koren O, Kashi Y, Chowers Y. Radiation induces proinflammatory dysbiosis: transmission of inflammatory susceptibility by host cytokine induction. Gut. 2018;67:97–107. doi: 10.1136/gutjnl-2017-313789. [DOI] [PubMed] [Google Scholar]
- 55.Khokhlova EV, Smeianov VV, Efimov BA, Kafarskaia LI, Pavlova SI, Shkoporov AN. Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol Immunol. 2012;56:27–39. doi: 10.1111/j.1348-0421.2011.00398.x. [DOI] [PubMed] [Google Scholar]
- 56.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A. 2008;105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW. Biological response of cancer cells to radiation treatment. Front Mol Biosci. 2014;1:24–24. doi: 10.3389/fmolb.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim JH, Jenrow KA, Brown SL. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat Oncol J. 2014;32:103–115. doi: 10.3857/roj.2014.32.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Najafi M, Motevaseli E, Shirazi A, Geraily G, Rezaeyan A, Norouzi F, Rezapoor S, Abdollahi H. Mechanisms of inflammatory responses to radiation and normal tissues toxicity: clinical implications. Int J Radiat Biol. 2018;94:335–356. doi: 10.1080/09553002.2018.1440092. [DOI] [PubMed] [Google Scholar]
- 60.Yahyapour R, Amini P, Rezapour S, Cheki M, Rezaeyan A, Farhood B, Shabeeb D, Musa AE, Fallah H, Najafi M. Radiation-induced inflammation and autoimmune diseases. Mil Med Res. 2018;5:9. doi: 10.1186/s40779-018-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farhood B, Goradel NH, Mortezaee K, Khanlarkhani N, Salehi E, Nashtaei MS, Shabeeb D, Musa AE, Fallah H, Najafi M. Intercellular communications-redox interactions in radiation toxicity; potential targets for radiation mitigation. J Cell Commun Signal. 2019;13:3–16. doi: 10.1007/s12079-018-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yahyapour R, Motevaseli E, Rezaeyan A, Abdollahi H, Farhood B, Cheki M, Rezapoor S, Shabeeb D, Musa AE, Najafi M, Villa V. Reduction-oxidation (redox) system in radiationinduced normal tissue injury: molecular mechanisms and implications in radiation therapeutics. Clin Transl Oncol. 2018;20:975–988. doi: 10.1007/s12094-017-1828-6. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki K, Hino M, Kutsuna H, Hato F, Sakamoto C, Takahashi T, Tatsumi N, Kitagawa S. Selective activation of p38 mitogen-activated protein kinase cascade in human neutrophils stimulated by IL-1 beta. J Immunol. 2001;167:5940–5947. doi: 10.4049/jimmunol.167.10.5940. [DOI] [PubMed] [Google Scholar]
- 64.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Ann Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 65.Viaud S, Saccheri F, Mignot G, Yamazaki T, Daillere R, Hannani D, Enot DP, Pfirschke C, Engblom C, Pittet MJ, Schlitzer A, Ginhoux F, Apetoh L, Chachaty E, Woerther PL, Eberl G, Berard M, Ecobichon C, Clermont D, Bizet C, Gaboriau-Routhiau V, Cerf-Bensussan N, Opolon P, Yessaad N, Vivier E, Ryffel B, Elson CO, Dore J, Kroemer G, Lepage P, Boneca IG, Ghiringhelli F, Zitvogel L. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsieh CY, Osaka T, Moriyama E, Date Y, Kikuchi J, Tsuneda S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol Rep. 2015;3:17. doi: 10.14814/phy2.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moon C, Baldridge MT, Wallace MA, D CA, Burnham , Virgin HW, Stappenbeck TS. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature. 2015;521:90–93. doi: 10.1038/nature14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. J AOAC Int. 2012;95:50–60. doi: 10.5740/jaoacint.sge_macfarlane. [DOI] [PubMed] [Google Scholar]
- 70.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty-acids in human large-intestine, portal, hepatic and venous-blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smith EA, Macfarlane GT. Dissimilatory amino Acid metabolism in human colonic bacteria. Anaerobe. 1997;3:327–337. doi: 10.1006/anae.1997.0121. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez JI, Marzorati M, Grootaert C, Baran M, Van Craeyveld V, Courtin CM, Broekaert WF, Delcour JA, Verstraete W, Van de Wiele T. Arabinoxylan-oligosaccharides (AXOS) affect the protein/carbohydrate fermentation balance and microbial population dynamics of the Simulator of Human Intestinal Microbial Ecosystem. Microb Biotechnol. 2009;2:101–113. doi: 10.1111/j.1751-7915.2008.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 74.Ragsdale SW, Pierce E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim Biophys Acta. 2008;1784:1873–1898. doi: 10.1016/j.bbapap.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hetzel M, Brock M, Selmer T, Pierik AJ, Golding BT, Buckel W. Acryloyl-CoA reductase from Clostridium propionicumi - An enzyme complex of propionyl-CoA dehydrogenase and electron-transferring flavoprotein. Eur J Biochem. 2003;270:902–910. doi: 10.1046/j.1432-1033.2003.03450.x. [DOI] [PubMed] [Google Scholar]
- 76.Scott KP, Martin JC, Campbell G, Mayer CD, Flint HJ. Whole-genome transcription profiling reveals genes up-regulated by growth on fucose in the human gut bacterium “Roseburia inulinivorans”. J Bacteriol. 2006;188:4340–4349. doi: 10.1128/JB.00137-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Louis P, Duncan SH, McCrae SI, Millar J, Jackson MS, Flint HJ. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J Bacteriol. 2004;186:2099–2106. doi: 10.1128/JB.186.7.2099-2106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duncan SH, Barcenilla A, Stewart CS, Pryde SE, Flint HJ. Acetate utilization and butyryl coenzyme A (CoA): acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl Environ Microbiol. 2002;68:5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vital M, Howe AC, Tiedje JM. Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. Mbio. 2014;5:e00889. doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. The warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol Cell. 2012;48:612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van der Beek CM, Bloemen JG, van den Broek MA, Lenaerts K, Venema K, Buurman WA, Dejong CH. Hepatic uptake of rectally administered butyrate prevents an increase in systemic butyrate concentrations in humans. J Nutr. 2015;145:2019–2024. doi: 10.3945/jn.115.211193. [DOI] [PubMed] [Google Scholar]
- 82.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bloemen JG, Venema K, van de Poll MC, Olde Damink SW, Buurman WA, Dejong CH. Short chain fatty acids exchange across the gut and liver in humans measured at surgery. Clin Nutr. 2009;28:657–661. doi: 10.1016/j.clnu.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 84.Bjarnadottir TK, Gloriam DE, Hellstrand SH, Kristiansson H, Fredriksson R, Schioth HB. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics. 2006;88:263–273. doi: 10.1016/j.ygeno.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 85.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 87.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 88.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sina C, Gavrilova O, Forster M, Till A, Derer S, Hildebrand F, Raabe B, Chalaris A, Scheller J, Rehmann A, Franke A, Ott S, Hasler R, Nikolaus S, Folsch UR, Rose-John S, Jiang HP, Li J, Schreiber S, Rosenstiel P. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol. 2009;183:7514–7522. doi: 10.4049/jimmunol.0900063. [DOI] [PubMed] [Google Scholar]
- 90.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 92.Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y, Huang X, Xiao Y, Yao S, Zhao Q, Liu Z, Cong Y. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunology. 2017;10:946–956. doi: 10.1038/mi.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thangaraju M, Cresci G, Itagaki S, Mellinger J, Browning DD, Berger FG, Prasad PD, Ganapathy V. Sodium-coupled transport of the short chain fatty acid butyrate by SLC5A8 and its relevance to colon cancer. J Gastrointest Surg. 2008;12:1773–1781. doi: 10.1007/s11605-008-0573-0. [DOI] [PubMed] [Google Scholar]
- 94.Bose P, Dai Y, Grant S. Histone deacetylase inhibitor (HDACI) mechanisms of action: Emerging insights. Pharmacol Ther. 2014;143:323–336. doi: 10.1016/j.pharmthera.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 96.Jin UH, Cheng Y, Park H, Davidson LA, Callaway ES, Chapkin RS, Jayaraman A, Asante A, Allred C, Weaver EA, Safe S. Short chain fatty acids enhance aryl hydrocarbon (Ah) responsiveness in mouse colonocytes and caco-2 human colon cancer cells. Sci Rep. 2017;7:10163. doi: 10.1038/s41598-017-10824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 98.Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, Offermanns S, Ganapathy V. Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem. 2010;285:27601–27608. doi: 10.1074/jbc.M110.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu L, Li L, Min J, Wang J, Wu H, Zeng YJ, Chen S, Chu ZH. Butyrate interferes with the differentiation and function of human monocyte-derived dendritic cells. Cell Immunol. 2012;277:66–73. doi: 10.1016/j.cellimm.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 100.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunology. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim M, Qie YQ, Park J, Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host Microbe. 2016;20:202–214. doi: 10.1016/j.chom.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of foxp3 and il-17 expression and amelioration of experimental colitis. PLoS One. 2011;6:e23522. doi: 10.1371/journal.pone.0023522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kespohl M, Vachharajani N, Luu M, Harb H, Pautz S, Wolff S, Sillner N, Walker A, Schmitt-Kopplin P, Boettger T, Renz H, Offermanns S, Steinhoff U, Visekruna A. The microbial metabolite butyrate induces expression of Th1-associated factors in CD4(+) T cells. Front Immunol. 2017;8:12. doi: 10.3389/fimmu.2017.01036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 105.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–5. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic T-reg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 109.Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJM, Faber KN, Hermoso MA. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haenen D, Zhang J, Souza da Silva C, Bosch G, van der Meer IM, van Arkel J, van den Borne JJ, Pérez Gutiérrez O, Smidt H, Kemp B, Müller M, Hooiveld GJ. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J Nutr. 2013;143:274–283. doi: 10.3945/jn.112.169672. [DOI] [PubMed] [Google Scholar]
- 111.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, McKenzie CI, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- 112.Bai AP, Ouyang Q, Zhang W, Wang CH, Li SF. Probiotics inhibit TNF-alpha-induced interleukin-8 secretion of HT29 cells. World J Gastroenterol. 2004;10:455–457. doi: 10.3748/wjg.v10.i3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O’Mahony C, Scully P, O’Mahony D, Murphy S, O’Brien F, Lyons A, Sherlock G, MacSharry J, Kiely B, Shanahan F, O’Mahony L. Commensal-induced regulatory T cells mediate protection against pathogen-stimulated NF-kappa B activation. PLoS Pathog. 2008;4:e1000112. doi: 10.1371/journal.ppat.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han C, Ding Z, Shi H, Qian W, Hou X, Lin R. The role of probiotics in lipopolysaccharide-induced autophagy in intestinal epithelial cells. Cell Physiol Biochem. 2016;38:2464–2478. doi: 10.1159/000445597. [DOI] [PubMed] [Google Scholar]
- 115.Russo R, De Caro C, Avagliano C, Cristiano C, La Rana G, Raso GM, Canani RB, Meli R, Calignano A. Sodium butyrate and its synthetic amide derivative modulate nociceptive behaviors in mice. Pharmacol Res. 2016;103:279–291. doi: 10.1016/j.phrs.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 116.Byndloss MX, Olsan EE, Rivera-Chavez F, Tiffany CR, Cevallos SA, Lokken KL, Torres TP, Byndloss AJ, Faber F, Gao Y, Litvak Y, Lopez CA, Xu G, Napoli E, Giulivi C, Tsolis RM, Revzin A, Lebrilla CB, Baumler AJ. Microbiota-activated PPAR-gamma signaling inhibits dysbiotic Enterobacteriaceae expansion. Science. 2017;357:570–575. doi: 10.1126/science.aam9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A, Weir TL, Ehrentraut SF, Pickel C, Kuhn KA, Lanis JM, Vu N, Taylor CT, Colgan SP. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig Dis Sci. 2012;57:3126–3135. doi: 10.1007/s10620-012-2259-4. [DOI] [PubMed] [Google Scholar]
- 119.Zheng L, Kelly CJ, Battista KD, Schaefer R, Lanis JM, Alexeev EE, Wang RX, Onyiah JC, Kominsky DJ, Colgan SP. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of claudin-2. J Immunol. 2017;199:2976–2984. doi: 10.4049/jimmunol.1700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miao W, Wu XJ, Wang K, Wang WJ, Wang YM, Li ZG, Liu JJ, Li L, Peng LY. Sodium butyrate promotes reassembly of tight junctions in Caco-2 monolayers involving inhibition of MLCK/MLC2 pathway and phosphorylation of PKC beta 2. Int Jf Mol Sci. 2016;17:12. doi: 10.3390/ijms17101696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of amp-activated protein kinase in caco-2 cell monolayers. J Nutr. 2009;139:1619–25. doi: 10.3945/jn.109.104638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, van Goudoever JB, Van Seuningen I, Renes IB. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J. 2009;420:211–219. doi: 10.1042/BJ20082222. [DOI] [PubMed] [Google Scholar]
- 123.Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun. 2002;70:953–963. doi: 10.1128/iai.70.2.953-963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhao Y, Chen F, Wu W, Sun M, Bilotta AJ, Yao S, Xiao Y, Huang X, Eaves-Pyles TD, Golovko G, Fofanov Y, D’Souza W, Zhao Q, Liu Z, Cong Y. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11:752–762. doi: 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Berard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–84. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frankel AE, Coughlin LA, Kim J, Froehlich TW, Xie Y, Frenkel EP, Koh AY. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia. 2017;19:848–855. doi: 10.1016/j.neo.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Geller LT, Barzily-Rokni M, Danino T, Jonas OH, Shental N, Nejman D, Gavert N, Zwang Y, Cooper ZA, Shee K, Thaiss CA, Reuben A, Livny J, Avraham R, Frederick DT, Ligorio M, Chatman K, Johnston SE, Mosher CM, Brandis A, Fuks G, Gurbatri C, Gopalakrishnan V, Kim M, Hurd MW, Katz M, Fleming J, Maitra A, Smith DA, Skalak M, Bu J, Michaud M, Trauger SA, Barshack I, Golan T, Sandbank J, Flaherty KT, Mandinova A, Garrett WS, Thayer SP, Ferrone CR, Huttenhower C, Bhatia SN, Gevers D, Wargo JA, Golub TR, Straussman R. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 130.Patil RS, Bhat SA, Dar AA, Chiplunkar SV. The Jekyll and Hyde story of IL17-producing gamma delta T cells. Front Immunol. 2015;6:37. doi: 10.3389/fimmu.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, Xiao X, Kwong TNY, Tsoi H, Wu WKK, Zeng B, Chan FKL, Sung JJY, Wei H, Yu J. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology. 2017;153:1621–1633. e6. doi: 10.1053/j.gastro.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 132.Shen S, Lim G, You Z, Ding W, Huang P, Ran C, Doheny J, Caravan P, Tate S, Hu K, Kim H, McCabe M, Huang B, Xie Z, Kwon D, Chen L, Mao J. Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat Neuroscie. 2017;20:1213–1216. doi: 10.1038/nn.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fung KY, Brierley GV, Henderson S, Hoffmann P, McColl SR, Lockett T, Head R, Cosgrove L. Butyrate-induced apoptosis in HCT116 colorectal cancer cells includes induction of a cell stress response. J Proteome Res. 2011;10:1860–1869. doi: 10.1021/pr1011125. [DOI] [PubMed] [Google Scholar]
- 134.Hinnebusch BF, Meng SF, Wu JT, Archer SY, Hodin RA. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J Nutr. 2002;132:1012–1017. doi: 10.1093/jn/132.5.1012. [DOI] [PubMed] [Google Scholar]
- 135.Sivaprakasam S, Gurav A, Paschall AV, Coe GL, Chaudhary K, Cai Y, Kolhe R, Martin P, Browning D, Huang L, Shi H, Sifuentes H, Vijay-Kumar M, Thompson SA, Munn DH, Mellor A, McGaha TL, Shiao P, Cutler CW, Liu K, Ganapathy V, Li H, Singh N. An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis. 2016;5:9. doi: 10.1038/oncsis.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tang Y, Chen Y, Jiang H, Robbins GT, Nie D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. International Journal Of Cancer. 2011;128:847–856. doi: 10.1002/ijc.25638. [DOI] [PubMed] [Google Scholar]
- 137.Kim M, Friesen L, Park J, Kim HM, Kim CH. Microbial metabolites, short-chain fatty acids, restrain tissue bacterial load, chronic inflammation, and associated cancer in the colon of mice. Eur J Immunol. 2018;48:1235–1247. doi: 10.1002/eji.201747122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Coutzac C, Jouniaux JM, Paci A, Schmidt J, Mallardo D, Seck A, Asvatourian V, Cassard L, Saulnier P, Lacroix L, Woerther PL, Vozy A, Naigeon M, Nebot-Bral L, Desbois M, Simeone E, Mateus C, Boselli L, Grivel J, Soularue E, Lepage P, Carbonnel F, Ascierto PA, Robert C, Chaput N. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat Commun. 2020;11:2168. doi: 10.1038/s41467-020-16079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406. e1–10. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 140.Hess J, Wang Q, Gould T, Slavin J. Impact of agaricus bisporus mushroom consumption on gut health markers in healthy adults. Nutrients. 2018;10:1402. doi: 10.3390/nu10101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Takagi T, Naito Y, Higashimura Y, Ushiroda C, Mizushima K, Ohashi Y, Yasukawa Z, Ozeki M, Tokunaga M, Okubo T, Katada K, Kamada K, Uchiyama K, Handa O, Itoh Y, Yoshikawa T. Partially hydrolysed guar gum ameliorates murine intestinal inflammation in association with modulating luminal microbiota and SCFA. Br J Nutr. 2016;116:1199–1205. doi: 10.1017/S0007114516003068. [DOI] [PubMed] [Google Scholar]
- 142.Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–672. doi: 10.1038/nrmicro3344. [DOI] [PubMed] [Google Scholar]
- 143.Rey FE, Faith JJ, Bain J, Muehlbauer MJ, Stevens RD, Newgard CB, Gordon JI. Dissecting the in vivo metabolic potential of two human gut acetogens. J Biol Chem. 2010;285:22082–22090. doi: 10.1074/jbc.M110.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]


