Abstract
Cancer health disparities remain stubbornly entrenched in the US health care system. The Affordable Care Act was legislation to target these disparities in health outcomes. Expanded access to health care, reduction in tobacco use, uptake of other preventive measures and cancer screening, and improved cancer therapies greatly reduced cancer mortality among women and men and underserved communities in this country. Yet, disparities in cancer outcomes remain. Underserved populations continue to experience an excessive cancer burden. This burden is largely explained by health care disparities, lifestyle factors, cultural barriers, and disparate exposures to carcinogens and pathogens, as exemplified by the COVID-19 epidemic. However, research also shows that comorbidities, social stress, ancestral and immunobiological factors, and the microbiome, may contribute to health disparities in cancer risk and survival. Recent studies revealed that comorbid conditions can induce an adverse tumor biology, leading to a more aggressive disease and decreased patient survival. In this review, we will discuss unanswered questions and new opportunities in cancer health disparity research related to comorbid chronic diseases, stress signaling, the immune response, and the microbiome, and what contribution these factors may have as causes of cancer health disparities.
Introduction
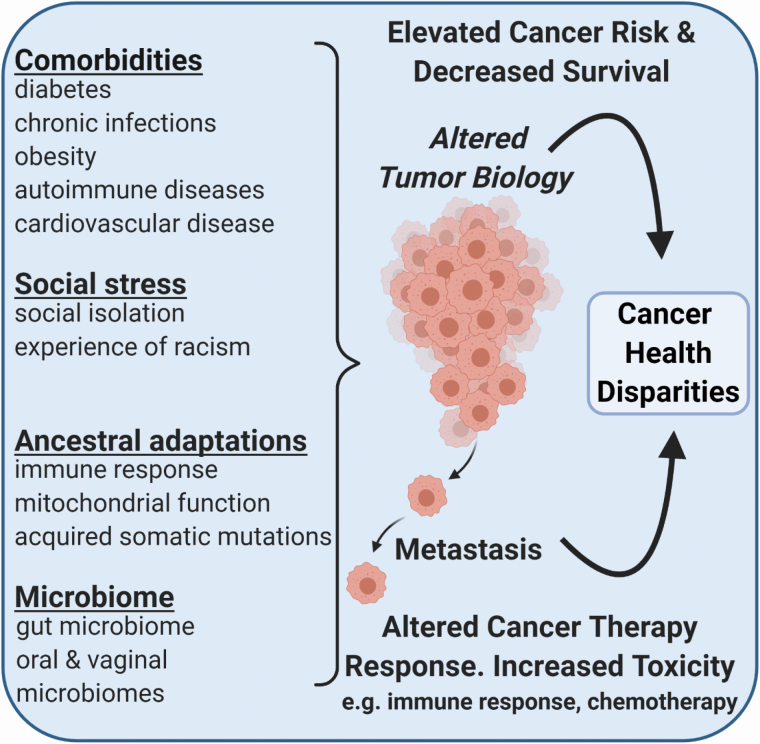
Cancer death rates in the United States (U.S.) reached their high point in the 1990s (1). They have been declining from that time on because of reduced tobacco use among adults, more widespread cancer screening and early detection, and improved cancer therapies (1). Declines in deaths from lung cancer, melanoma, and other leading cancers, like breast, colorectal, and prostate cancer, account for much of the advances in reducing the U.S. cancer mortality. These improvements are more pronounced among younger than older Americans (2). Nevertheless, cancer health disparities persevere. In this review, we will first summarize our understanding of cancer health disparities in the U.S. and abroad and then evaluate the contribution that comorbid chronic diseases, chronic stress exposure, population differences in immune response, and a dysbiosis may have as causes of these disparities (Figure 1). The advent of COVID-19 infections reinforced the notion that diseases other than cancer influence cancer survival and may contribute to an excessive mortality in underserved communities.
Figure 1.
Comorbid chronic diseases, stress exposure, population differences in immune response, and dysbiosis are factors that contribute to cancer health disparities.
Cancer health disparities in the United States and globally
Cancer disparities continue to persist across geographic areas, socioeconomic strata, and different racial and ethnic groups. Rural communities experience higher death rates from lung, cervical, and colorectal cancers than urban communities because of poverty, health risk behavior, and lower vaccination and screening rates (3), consistent with the widening disparity in life expectancy between rural and urban areas (4).
Low educational attainment is an indicator of socioeconomic deprivation and strongly correlates with elevated all-cause death rates in the general population. 40–50% of all pre-mature deaths might not occur if all segments of the U.S. population would experience the death rates of college graduates (5). Socioeconomic status is a key determinant of cancer mortality as well. About a quarter of all cancer deaths may not occur if all Americans were college-educated (6). Cancer survival increases with higher socioeconomic status for all U.S. racial and ethnic groups (7). Yet, socioeconomic patterns in cancer mortality have changed markedly over time (8). Into the 1980s, socioeconomic status positively correlated with U.S. cancer mortality rates, showing a higher risk of cancer deaths among the affluent. This correlation has now turned into the opposite direction, with affluent Americans being less likely of dying from cancer because of advances in disease prevention, early cancer detection, and cancer therapy that benefit patients with private health insurance more so than others. Presently, socioeconomic inequalities contribute most strongly to the excess mortality from lung, colorectal, cervical, stomach, and liver cancer among Americans who live in deprived areas (8). While the prostate cancer mortality did not vary much by socioeconomic status in the past, an inverse socioeconomic gradient appears now to exist (8,9). Neighborhood socioeconomic deprivation can further be linked to shortened telomere length, an indicator of pre-mature aging, and lethal cancer (10–12).
Global disparities in cancer incidence and mortality rates are evident for most cancer sites and indicate socioeconomic inequalities and significant differences in risk factor exposure (13). Rates of cancers including breast, colorectal, and prostate vary greatly between high-income and low-income countries, geographic areas, and race/ethnic groups. Differences in health care and modifiable risk factor exposure are major drivers of these global disparities, as shown by migration studies for breast and other cancers (14–16). Lung cancer is the leading cause of cancer death worldwide but is prominently under-represented in sub-Saharan Africa because of a low smoking prevalence. Prostate cancer is the most common cancer among men worldwide but shows large geographical differences in occurrence, with low incidence rates in East Asia and high rates in Western countries. With the westernization of lifestyles in East Asia, the incidence difference has narrowed (17). Notably, prostate cancer is the leading cause of cancer death among men in sub-Saharan Africa and the Caribbean (18), which led to the hypothesis that genetic ancestral factors may predispose men of sub-Saharan African ancestry to prostate cancer and a more aggressive disease. Recent findings are consistent with this hypothesis (19–22). Cervical cancer is a major cause of cancer deaths among women in sub-Saharan Africa and South-East Asia because of human papillomavirus infections and delayed disease detection. Stomach and esophageal cancer are two other cancers with high incidence and mortality rates in Eastern Asia. Helicobacter pylori and salted foods are major risk factors for stomach cancer. This cancer is particularly common on the Korean peninsula due to a combination of regional dietary risk factors and chronic H. pylori infections whereas Malawi in Eastern African is especially impacted by esophageal cancers, having the highest global disease rates due to factors that have yet to be identified. Lastly, the burden of liver cancer is greatest in Northern and Western Africa and South East Asia and is a primary cause of cancer death in Mongolia. Chronic hepatitis B & C virus infections and exposure to aflatoxin are key causes of the disease in these areas while heavy alcohol use and non-alcoholic fatty liver disease are drivers of the increasing liver cancer incidence in many high-income countries.
Cancer health disparities between population groups in the United States
Large differences in cancer incidence and mortality do also exist between U.S. population groups (1,2). These disparities are largely explained by differences in access to health care, diet, lifestyle, cultural barriers, and disparate exposures to pathogens and carcinogens (23,24). Disparities in liver cancer occur across U.S. states and race/ethnic groups (25,26). This cancer affects American Indians/Alaska Natives, American Asians, and Hispanic Americans more so than African Americans and European Americans. American Indians/Alaska Natives have the lowest 5-year cancer survival across all cancer types and experience elevated rates for many malignancies and major risk factors, like comorbid conditions, when compared to European Americans (1,27,28). In contrast, Hispanics/Latinos and Asian Americans tend to have lower cancer incidence rates than other U.S. population groups. Asian Americans, by themselves a rather heterogenous population group, have the lowest cancer-specific mortality by reasons that are yet unclear but may relate to better treatment responses (29). Among Hispanics/Latinos, infection-related cancers are over-represented and women and men are more likely to be diagnosed with late stage cancer when compared to U.S. European Americans (25). While prostate cancer is generally less common among Hispanic/Latino men, it is the leading cause of cancer death among men in Puerto Rico, indicating heterogeneity in cancer risk within the Hispanic population. African Americans disproportionately bear the cancer burden and have the highest death rates from malignancies of the breast, gastrointestinal tract, lung, and prostate, and develop multiple myeloma more commonly than other population groups (23,30). Reasons of why these specific cancer disparities exist have been extensively reviewed (31–37). Therefore, they will not be the focus of this review. Nonetheless, cancer risk profiles among African Americans are not uniform and vary whether they are Sub-Saharan African-, Caribbean-, or U.S.-born (38,39). African Americans have an excess risk of developing early-onset cancer, which is reminiscent of disease presentation in Africa (40); however, African populations and African Americans in the U.S. are generally younger than the U.S. European American population which may bias cancer-onset comparisons (41). In recent years, cancer incidence and death rates declined faster among African Americans than European Americans, a very positive development that is mainly due to reductions in lung, colorectal, and prostate incidence and mortality (2,30). Barriers still exist and current lung cancer screening guidelines may often exclude African American smokers at increased risk of lung cancer (42). Moreover, men of African ancestry continue to have 2–3-times higher absolute rates of fatal prostate cancer in both the U.S. and England (43).
The differences in cancer survival between U.S. race/ethnic groups and their underlying causes have been investigated. This research showed that disparities in stage at diagnosis may have the largest contribution to these survival disparities, followed by socioeconomic factors and marital status as other key contributing factors (44,45). The importance of marital status suggests that social isolation and stress may contribute to these racial/ethnic disparities. Still, private insurance provides the single most protective effect against being diagnosed with advanced stage disease, emphasizing the importance of access to health care in reducing the cancer survival health disparity among U.S. population groups (46).
Influence of sex and gender on cancer risk and outcomes
Sex and gender are modifiers of health and contribute to disparities in disease development and outcome (47). Men are at an increased risk of dying from cancer (1,2). Many non-reproductive cancers show a 2:1 male predominance worldwide. Sex hormone signaling and Y chromosome-encoded oncogenes are drivers of sex- and gender-related cancer disparities. Sex differences in cancer genetics have been recognized (48). The androgen receptor has key roles in the progression of liver diseases like fatty liver, cirrhosis, and liver cancer, consistent with a 2:1 to 7:1 male predominance in the liver cancer incidence globally (49). The response to cancer therapy may differ between women and men. For example, the therapy benefit from immune checkpoint inhibitors is sex-dependent and these therapies provide more benefit to men (50). Although sex is a well-established modifier of cancer risk, the biology of sex-related cancer disparities remains incompletely understood. Nonetheless, it has been recommended that clinicians should consider sex and gender in their approach to diagnosis, prevention, and treatment of diseases (47). To end with, there are also cancer health disparities related to sexual behavior. For example, anal cancer incidence rates are increasing in both men and women across the globe and will require population-based preventive measures including advocacy for safe sexual behaviors and human papillomavirus vaccination (51).
Impact of health care access and the Affordable Care Act on cancer health disparities
Access to health care and health insurance coverage are key determinants of receipt of cancer care and cancer survival (52). A survival disparity for African American men with prostate cancer exists in the U.S. population, but is not observed in clinical trials or for men served by the Veteran Affairs equal-access health care system (53), highlighting the importance of equal access to health care in reducing cancer health disparities. Furthermore, insurance status provides the single most protective effect against the diagnosis of metastatic cancer (46). In 2010, the Patient Protection and Affordable Care Act, also termed “the Affordable Care Act”, was signed into law. Its primary goal was to improve health insurance coverage (54). The preliminary impact of this legislation has now been assessed. Disparities in the percentage of uninsured patients have been diminished in Medicaid expansion states under the Affordable Care Act (55–57). Americans living in areas of greater deprivation and rurality still have lower rates of recommended cancer screening than others (58). With the Affordable Care Act, however, colorectal cancer screening uptake seems to have increased, albeit modestly (59), yet race/ethnic disparities persist (60). On the other hand, Medicaid expansion shows consistent relationships with lower odds of having either advanced stage or metastatic cancer at diagnosis among low-income Americans (55,56,61). It also increased care affordability among cancer survivors in Medicare expansion states, but not in nonexpansion states, and increased utilization of cancer surgery by low-income Americans (57,62). Still, race/ethnic disparities remain (62), and Medicaid expansion may not have lowered the disparity in breast cancer mortality between African American and European American women (63). With the continuation of an impact by the Affordable Care Act on both secondary prevention of cancer and cancer care, future analyses of Surveillance, Epidemiology, and End Results program data should provide more clarity to what extent the Affordable Care Act has reduced cancer survival health disparities in low-income communities and across race/ethnic groups.
Chronic diseases modify cancer risk and survival and contribute to health disparities
Comorbidities in cancer patients are chronic diseases that commonly co-occur with cancer because of shared risk factors (64). Common comorbid diseases include obesity, diabetes, and metabolic syndrome, cardiovascular, liver, and autoimmune diseases, chronic infections, but also dysbiosis and neurological and stress-related disorders. They influence cancer diagnosis, tumor biology and metastasis, and the utilization of cancer therapy. Comorbidities do not affect all segments of the US populations equally. American Indians and African Americans have significantly higher rates of comorbidities, when compared to other U.S. population groups (27). Four of these comorbidities, obesity, diabetes, chronic kidney disease, and hypertension, contribute disproportionally to the mortality disparity between African Americans and European Americans. Although not a chronic condition, COVID-19 infections have recently been associated with an excessive mortality among African Americans (65) and cancer patients (66).
Diabetes, hyperinsulinemia, and obesity are closely related comorbid conditions. They are all cancer risk factors (67,68). Because these conditions are more prevalent in underserved and minority populations, one would predict that they contribute to a disproportionate cancer burden in these communities. However, the evidence that link comorbidities to cancer health disparities remains rather sparse, partly because these investigations were either not done or focused on only a few comorbid conditions. Diabetes approximately doubles the risk for liver and pancreas cancer and is additionally associated with the risk of breast, colorectal, endometrial, esophageal, and gallbladder cancer (67,69). Diabetes-related advanced glycation end products have been linked to a cancer health disparity (70). Diabetes is thought to promote cancer development and progression through insulin and insulin-like growth factor signaling, oxidative stress, and excessive inflammation (71). This comorbidity is excessively high among African Americans and in the Hispanic/Latino community (27,72). Insulin resistance and the metabolic syndrome have been found to contribute to disparities in breast cancer outcomes between African American and European American women (73,74). Diabetes also increases the risk of pancreatic cancer in African American and Hispanic/Latino (75), however, the data do not indicate that the conferred risk is higher in these two population groups than in European-Americans.
Comorbidities are associated with an elevated cancer mortality. They impede the participation of cancer patients in clinical trials and adversely affect trial participation (76). Accordingly, clinical trial participation of U.S. minorities remains low (77,78), which may partly relate to barriers in enrollment due to comorbidities. The presence of a comorbidity will influence treatment selection and the use of surgery and chemotherapy (79,80). Cancer patients with a comorbidity are generally less likely to receive curative treatment than those without the comorbidity (81). These deaths are preventable with lifestyle changes and other intervention strategies that target these chronic diseases. Moreover, the negative impact of comorbidities on cancer outcomes tends to increase with the number and severity of the comorbidities. Their impact is generally larger for cancers that have otherwise better survival. Thus, future cancer health disparity research should develop an increased focus on comorbidities and how they contribute to existing U.S. cancer outcome disparities.
Mechanisms linking stress exposure to cancer metastasis and survival and disparate outcomes
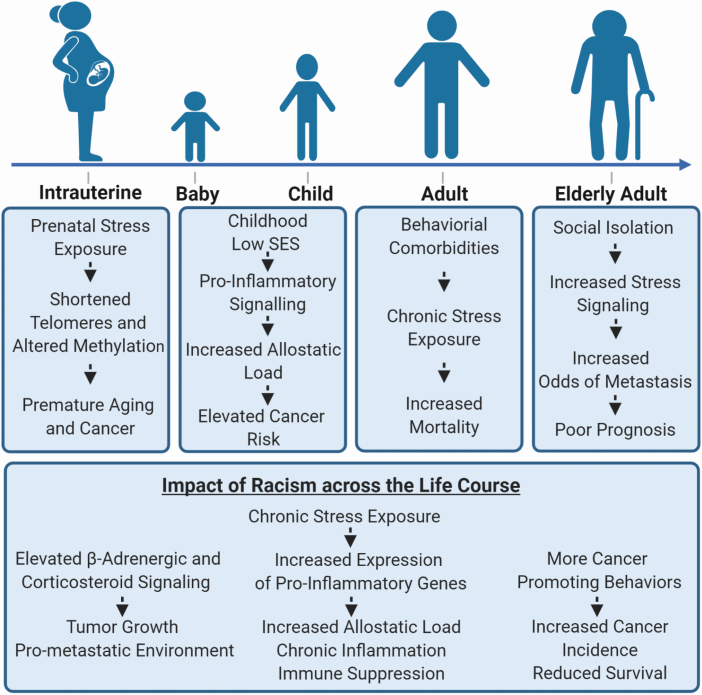
The concept of a public health exposome was developed for targeted community health intervention and includes exposure to stressors, their signaling, and the causes of the stress exposure (82). Posttraumatic stress because of a cancer diagnosis may disproportionally affect minority populations (83). Social adversity in early life can lead to decreased glucocorticoid and increased pro-inflammatory signaling in humans (84). Intrauterine stress exposures associate with a shortened telomere length in young adulthood (85), which may predispose these individuals to premature aging and cancer. Perceived experiences of racism show relationships with breast cancer and cancer-promoting health behaviors, such as increased tobacco and alcohol consumption (86,87). In breast tumors, social isolation may lead to reprogramming of tumor biology (88,89). Thus, stress exposures may alter cancer susceptibility and disproportionally affect socially deprived and minority populations (Figure 2).
Figure 2.
Stress exposure over the life course and its potential impact on socially deprived and minority populations.
Behavioral comorbidities (e.g. depression, fatigue, anxiety, cognitive impairment) are prevalent in cancer patients and a target for therapy (90). Cancer patients have higher rates of depression than most Americans (91). Major depression affects about 5–8% of the U.S. population but approximately 15% of cancer patients. Race- and gender-based discrimination and social isolation of the elderly are common events and create chronic stress exposures in affected individuals. Chronic stress and depressive disorders are associated with an increased cancer mortality (92–94). They are cancer risk factors and have been linked to elevated concentrations of circulating pro-inflammatory cytokines and chemokines (90–92).
Stress exposures and depression transduce their biological effects through the hypothalamic-pituitary-adrenal axis. This signaling pathway is characterized by hypersecretion of the corticotrophin-releasing hormone and activation of the peripheral autonomic and sympathetic nervous system, which has direct effects on tumor biology and immune response, promoting inflammation, angiogenesis, mesenchymal differentiation, and metastasis (95). Chronic stress influences tumor biology through two major pathways involving catecholamines (adrenaline, noradrenaline) and glucocorticoids (96). Socially isolated ovarian cancer patients were found to have elevated tumor noradrenaline levels (97). In mouse models of ovarian and breast cancer, chronic stress promotes invasive tumor growth and metastasis in a β-adrenergic signaling-dependent manner (98–100). Here, catecholamines activate β-adrenergic signaling in cancer cells and tumor-associated macrophages (95,99), leading to a pro-metastatic tumor microenvironment. Consistent with these observations, a pro-metastatic niche has been described for breast tumors from socially isolated women (101) and a decrease in chronic depression may slow metastasis in breast cancer patients (102). In other studies, social stress was found to up-regulate inflammatory gene expression in monocytes through β-adrenergic signaling (103). Likewise, African Americans with exposure to racial discrimination showed up-regulation of these genes (104).
Social isolation may contribute to racial and ethnic differences in cancer survival. Ellis et al. reported that marital status is a contributing factor to these survival disparities (45). Being married provides a survival benefit while being unmarried, a surrogate for social isolation, is a risk factor. There are other studies that link stress exposure and β-adrenergic signaling to cancer survival. β-adrenergic receptor expression may predict a poor prognosis for breast cancer patients (105). β-blocker use after a disease diagnosis reduces disease recurrence and improves survival of breast cancer patients (106), while regular users of the β-blocker, propranolol, are less likely to develop advanced breast cancer and have a reduced breast cancer-specific mortality (107). Beta-blocker use has been associated with improved recurrence-free survival in triple-negative breast cancer as well (108). Together, these data indicate that stress may alter breast cancer biology through activation of the pro-metastatic catecholamine pathway, leading to an aggressive disease in a subpopulation of patients who would benefit from stress management. Lastly, a high prevalence of major depression has been reported for African American men with prostate cancer (109). This condition and other social stress exposures may predispose these men to aggressive disease as it has recently been shown that stress-related signaling pathways are up-regulated in prostate tumors that progressed into lethal disease (110). In summary, it is well documented that stress exposures, which impact underserved and minority communities more so than affluent communities, can adversely affect tumor biology, cancer survival, and quality of life of cancer patients (Figure 2). Yet, a knowledge gap persists. Still few studies have examined the impact of various stress exposures in minority and socially deprived communities using large and well-designed studies. These studies should be conducted as the detrimental impact of chronic stress and depression in cancer patients is preventable using community engagement, psychosocial support, and therapies like β-adrenergic blocking agents.
Ancestry and population differences in immune response as underlying factors of cancer health disparities
Differences in pan-cancer mitochondrial function were found to distinguish African American from European American cancer patients, suggesting an ancestral link (111). Recent observations have shown that population differences in genetic ancestry can contribute to population differences in cancer susceptibility (19,20,112–114). Genetic ancestry and natural selection are underlying causes of population differences in immune response to pathogens (115,116). Those differences may relate to cancer (37,117). Relationships of ancestry with expression levels of inflammatory cytokines are evident in human populations (118,119). These differences may contribute to lung cancer disparities (120,121). Two studies investigated gene expression variations between subjects of European and West African ancestry using lymphoblastoid cell lines (122,123) and observed that these variations can cluster in cancer-related pathways and influence pathway signaling. Thus, genetic differences among population groups may lead to population-specific susceptibilities for common diseases, like certain cancers, because of their effect on the transcriptome (114,124).
One mechanism by which ancestry-related factors affect cancer outcomes is by inducing an adverse tumor biology (125). Research has now documented that tumors from patients of either African, Asian, or European descent show notable differences in acquired somatic mutations (126). Two large studies investigated the relationship of African and European ancestry with mutational signatures and gene expression across 33 cancer sites in the Cancer Genome Atlas (TCGA) database and reported associations of African ancestry with somatic mutations that tended to be cancer type-specific (127,128). At a pan-cancer level, the mutational burden of tumors and associated signatures were not significantly different between patients from these two ancestries, nor were there significant differences in chromosome arm-level copy number alterations. TP53 mutations were enriched in African American patients in a subset of cancers, most notable in breast cancer, whereas genomic alterations in genes of the phosphatidylinositol 3-kinase pathway were less frequent in this patient group. After adjusting for tumor subtype differences between African American and European American patients, few significant associations between ancestry and either tumor somatic mutations or chromosomal aberrations remained (128). Notably, mutations in the gene, FBXW7, showed a pan-cancer association with African ancestry. FBXW7 is a tumor suppressor gene that is involved in the proteasome-mediated degradation of many oncoproteins such as cyclin E, c-Myc, Mcl-1, mTOR, Jun, Notch, and AURKA (129). Mutations in other genes, such as VHL, PBRM1, HRAS, and NFE2L2, showed only cancer-specific associations with ancestry.
Other investigators focused on specific cancer types, such as breast, colorectal, lung, and prostate cancer. The breast cancer studies reported an overall increased mutation frequency, and specifically for TP53, and fewer PIK3CA mutations in African American and Nigerian women, together with an over-representation of triple negative breast tumors among these women (130,131). The latter is consistent with many previous reports (40,132). Breast tumors from Nigerian women were also characterized by the occurrence of GATA3 mutations and a homologous recombination deficiency signature. A smaller study of triple-negative breast tumors that applied whole genome sequencing identified the over-representation of CTNNA1 deletions in African American patients (133). Among patients with colorectal cancer, African Americans seem to acquire KRAS, EPHA6, and FLCN mutations more frequently than other patients whereas APC loss-of-function and oncogenic BRAF mutations may manifest less frequently in their tumors (33,134–136). Lung cancer is the most fatal cancer and is highly heterogenous as a disease and presents with geographic differences in acquired mutations and the therapeutic response of lung cancer patients (31). Mutations in the gene encoding the epidermal growth factor (EGFR) are generally more prevalent in non-small cell lung tumors from smokers and nonsmokers of East Asian ancestry (137,138) whereas mutations in KEAP1 and CDC27 are over-represented in lung adenocarcinomas from patients of European ancestry when compared to East Asian patients, independent of smoking history (138). Furthermore, lung adenocarcinomas from European ancestry patients featured a comparatively high genomic instability score, perhaps explaining some of the reported ethnicity-related differences in survival outcome among non-small cell lung cancer patients (139). Research into racial/ethnic differences in lung cancer mutational profiles has been extended to African Americans. While one study did not find significant differences between African American and European American lung cancer patients (140), another study discovered the distinct occurrence of PTPRT and JAK2 mutations in lung adenocarcinomas among African Americans and their association with increased STAT3 signaling (141).
A role of tumor biology and the immune response in cancer health disparity: the example of prostate cancer
The most prominent population differences in tumor biology have been reported for prostate cancer. This disease can be classified into subtypes, such as those with ETS-fusion gene arrangements and other subtypes that are negative for ETS-fusion gene arrangements and either overexpress the SPINK1 oncogene or carry a SPOP mutation (142,143). Localized prostate cancer contains few recurrent mutations in oncogenes or tumor suppressor genes (144,145). Instead, prostate tumors are characterized by gene fusions (e.g. ETS gene fusions), allelic gains of the MYC gene, and deletions of the PTEN, TP53, and NKX3-1 tumor suppressors, with additional common changes in DNA methylation that increase aggressiveness (146,147). Multiple reports have now shown that prostate tumors from patients of either European, African, or Asian descent exhibit notable differences in acquired chromosomal aberrations (e.g. ERG fusion events and PTEN loss) and subtype distribution (143,148–150), indicating disparities in disease etiology and mutational events among these population groups. Chinese prostate cancer patients were found to acquire mutations in FOXA1 at a high frequency (41%) (150). By contrast, this gene is mutated at <10% in European-ancestry populations. Comparing African American with European American patients in TCGA, significant differences were observed in the frequency of TMPRSS2-ERG fusions (29.3% African American versus 39.6% European American), SPOP mutations (20.3% African American versus 10% European American), and PTEN deletions (11.5% African American versus 30.2% European American), consistent with other studies in the United States and Africa (143,151–153). The application of whole genome sequencing to the disease in African men, currently performed on only few tumors (154), should provide further insight into the etiology of prostate cancer in Africa. Currently, we do not know how the disease in Sub-Saharan Africa relates to the disease in men of African ancestry in the United States, the Caribbean, or in European and South American countries. However, whole genome sequencing already revealed an elevated tumor mutational burden in prostate cancer patients from South Africa and the frequent loss of the LSAMP locus in African American patients (154,155).
As a key discovery of the study of prostate tumors in African American men, Wallace et al. was the first to describe a prevalent immune-inflammation signature in prostate tumors of African American patients (156), followed by others (157). This finding has been validated in TCGA (127). The signature contains elements of a viral mimicry signature and could be functionally related to the previously describe interferon-related DNA damage resistance signature, also termed IRDS (158,159). Thus, tumors with this signature may not respond as well to radiation and chemotherapy as tumors without the signature, as was shown for breast cancer (159). Yet, these tumors may have an improved response to immunotherapies, and specifically to cancer vaccines, and perhaps ADAR1 inhibitors (160). In agreement with our hypothesis, Sartor et al. recently reported that African American men with metastatic castration-resistant prostate cancer who were treated with the cancer vaccine, Sipuleucel-T, in the PROCEED trial had significantly better survival than the European American patients (161). Our group explored the link between regular use of aspirin and prostate cancer in African American men and found that regular aspirin use significantly reduces the risk of both advanced prostate cancer and disease recurrence in these men (162). The finding is consistent with a similar observation in a previous study (163) and the hypothesis that inflammation is a driver of tumor biology in African American men. There is only a weak association of the immune-inflammation signature with previously described germline genetic risk loci for prostate cancer (127); however, we described a significant relationship with the presence of the interferon-λ4 ΔG genotype that is common in West African ancestry populations and influences the host viral response (124,158). The precise origin of the signature remains poorly understood and may include an infection history in the context of the interferon-λ4 ΔG genotype (164), dietary factors (165), or changes to the epigenome, manifesting in the re-activation of endogenous retroviral sequences (166,167). We described up-regulation of HERV-K retroviral sequences in African American prostate cancer patients (166). In addition, a pro-inflammatory diet that associates with high-grade prostate cancer is more commonly consumed by African American than European American men (165). Others described the up-regulation of the transcription factor, Kaiso, in prostate tumors of African-American men (168). Kaiso regulates pathways related to epithelial-to-mesenchymal transition, apoptosis, and inflammation and may have a significant role in the cancer biology of prostate and breast cancer patients of African descent.
The presence of a distinct immune-inflammation signature has been reported for breast tumors in African American patients as well. Such a signature describes a subset of triple-negative breast tumors (169). Recruitment of tumor-associated macrophages is elevated in breast tumors of African and African-American women, as described by us and others (170–173). Moreover, Martin et al. observed an increased microvessel density in these tumors (170). An elevated tumor vascularization in African-American breast cancer patients was confirmed by Lindner et al. (174). Tumor angiogenesis correlates with breast cancer metastasis and poor survival (175). In Nigerian breast cancer patients, a prominent interferon signature was detected in luminal-type tumors whereas macrophage infiltration was more commonly observed in the basal subtype tumors (131). Hence, current data suggest that inflammation-induced breast cancer progression could be more prevalent in patients of African descent and may relate to increased inflammatory cytokine levels in these women (119,125).
Microbiome and cancer health disparities: impact of geography, ethnicity, and genetics on the human microbiome composition
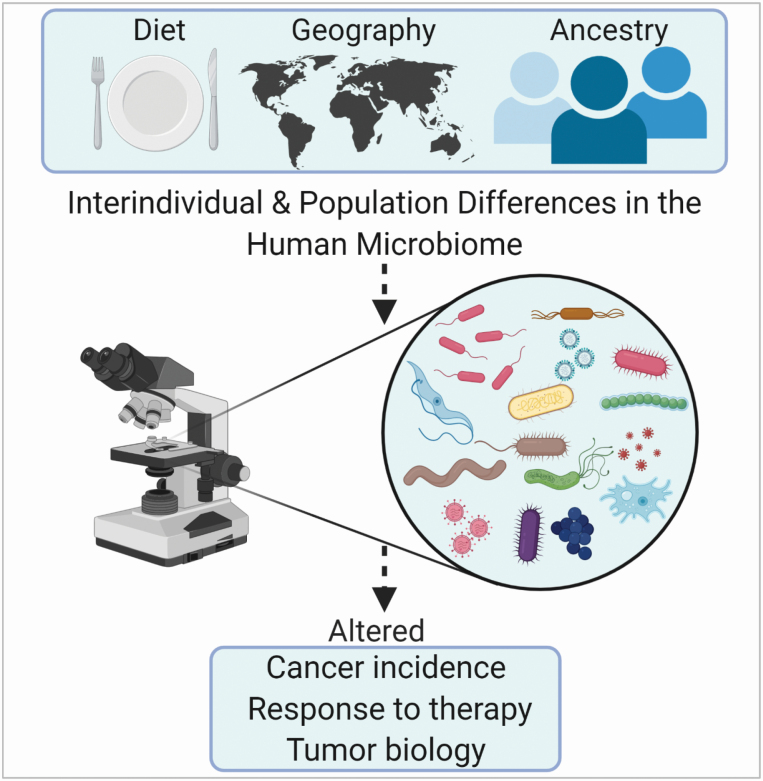
The gut microbiome affects human health (176,177). A dysbiosis can increase cancer risk and modify the cancer therapy response (178–181). Diet and genetics shape the gut microbiome (182–184) and may contribute to cancer health disparities through their effects on the gut microbiome (Figure 3). Likewise, comorbidities may confer their cancer risk through effects on the gut microbiome (185,186). Hence, there is evidence that a dysbiosis can be a cause of cancer (179). An altered microbiome and the accumulation of microbiome-derived metabolites have been reported for various human cancers (187–189). Alterations to the human microbiome can induce an aggressive tumor biology (190), linking the microbiome to cancer survival outcomes.
Figure 3.
Diet, geographic location, and ethnicity strongly associate with the diversity of the gut microbiome and may increase the risk of dysbiosis, a cancer risk factor.
Geographic location and ethnicity strongly associate with the diversity of the gut microbiome (191,192) although geography (e.g. rural versus urban) usually confers a larger effect than ethnicity (193,194). Dissimilarities in the gut microbiota among ethnic groups with a shared environment have been reported, as shown for Amsterdam, a city in the Netherlands (192). Here, the gut microbiome diversity was significantly associated with ethnicity. Other factors, besides ethnicity, influenced the microbiome diversity. Nevertheless, ethnicity was the strongest determinant of gut microbiome diversity in models that included other non-dietary and dietary factors. Similarly, a U.S. study reported that ethnicity captures the gut microbiome with a stronger effect size than body mass, age, and sex, albeit the effect of all these factors was not as impactful as geographic location (194). Microbial community richness was greatest in Hispanics and decreased further from European Americans to Asian-Pacific Islanders to African Americans. However, the authors pointed out that there is more similarity than dissimilarity in the gut microbiome between the four studied U.S. population groups, thus the differences were comparably small. In addition, ethnicity may influence only a subset of the gut microbiome while other microbiome components remained unrelated to the ancestral background. Lastly, immigrants into the United States acquire a “westernized” gut microbiome (195), which is reminiscent of findings from migration studies that immigrants tend to acquire cancer rates of their new home country within two generations (15,196).
Cancer health disparity research has just begun to investigate the contribution of the microbiome to disparities in cancer risk and survival. Observations are sparse and validation of findings is non-existent. Differences in both the oral and vaginal microbiome have been reported comparing subjects of African and European descent (197,198). These studies did not include cancer patients. An exploratory investigation reported a rich bacterial content in high-risk prostate tumors from 6 men of South African ancestry when compared to 16 Australian men (199). In a study of breast cancer, differences in the breast tumor microbiome were observed comparing African American with European American women. Only 12 of the 64 tumors in the study came from African American women. Previously, the microbiome of breast tumors has been described from TCGA data but a separate analysis of African American tumors was not performed (200). Lastly, a large study of the non-cancerous colonic mucosa from 197 African Americans and 132 European Americans with or without colorectal cancer described a robust association of sulfidogenic bacteria with being African American, regardless of disease status (201). Abundance of these bacteria has previously been linked to diet (202) and the up-regulation of these bacteria in the African American study participants might have been related to their high intake of dietary fat and protein, as the authors concluded.
As shown by these few studies, cancer disparity-related differences in the gut, oral, and vaginal microbiome may exist. Future investigations are needed to assess the microbiome as an underlying factor or potential driver of cancer health disparities.
Conclusions and outlook
Minority, immigrant, and other underserved populations continue to experience an excessive cancer burden not only due to barriers in access to health care, but also because of disparate exposure to carcinogens, pathogens, co-morbidities, environmentally induced stress, and ancestry-related risk factors (Figure 1). These factors, singularly or in combination, are the likely causes of cancer health disparities in the U.S. and globally. There is convincing evidence from migration and epidemiological studies that the environment defines cancer risk but there is also indication that population differences in genetic ancestry can lead to population differences in cancer susceptibility.
Genetic ancestry and natural selection are underlying causes of population differences in immune response. Those differences may relate to cancer risk and therapy response. Current data suggest that inflammation-induced cancer progression could be more prevalent in patients of African descent, manifesting in a distinct tumor immune environment. Inflammation-induced cancer progression can be targeted by therapy. Tumors with an immune-inflammation signature may respond favorably to immune therapy.
Comorbidities influence cancer diagnosis, tumor biology and metastasis, and the utilization of cancer therapy. Many comorbidities are cancer risk factors. They do not affect all segments of the US populations equally. Because these conditions are more prevalent in underserved and minority populations, one would predict that they contribute to a disproportionate cancer burden in these communities. Yet, the evidence that link comorbidities to cancer health disparities remains sparse. Thus, future cancer health disparity research should develop an increased focus on cancer comorbidities.
Chronic stress and depressive disorders are associated with an increased cancer mortality and directly influence tumor biology (Figure 2). Chronic stress after a cancer diagnosis may disproportionally affect minority populations. Likewise, social isolation and perceived experiences of racism show relationships with cancer-promoting health behaviors and cancer development. Thus, stress exposures may alter cancer susceptibility and disproportionally affect socially deprived and minority populations. Still, few studies have examined the impact of these exposures in minority and socially deprived communities using large and well-designed studies. These studies should be conducted as the detrimental impact of chronic stress and depression in cancer patients is preventable using community engagement, psychosocial support, and therapeutic approaches. RESPOND is such study that focuses on prostate cancer among African American men and investigates the impact of social stress (https://respondstudy.org/).
Geographic location and ethnicity strongly associate with the diversity of the gut microbiome (Figure 3). Recent advances have shown that the microbiome is causatively linked to cancer. A dysbiosis can increase cancer risk and modify cancer therapy response. Diet and genetics shape the gut microbiome and may contribute to cancer health disparities through their effects on the gut microbiome. Cancer disparity-related differences in the gut, oral, and vaginal microbiome may exist. Future investigations are needed to assess the microbiome as an underlying factor or potential driver of cancer health disparities.
Acknowledgements
Maeve Bailey-Whyte is an NCI Cancer Prevention Fellow funded by the NCI Division of Cancer Prevention. Anuoluwapo Ajao is a Post-baccalaureate Fellow sponsored by the NIH Academy Fellowship Program and by a National Institute of Minority Health and Health Disparities Fellowship. Figures were generated using the software BioRender.
Funding
Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute (NCI), Center for Cancer Research (ZIA BC 010499, ZIA BC 010624, and ZIA BC 010887); U.S. Department of Defense award (W81XWH1810588), and National Institute of Minority Health and Health Disparities.
Conflict of interest statement: None declared.
References
- 1. Siegel, R.L. et al. (2020) Cancer statistics, 2020. CA Cancer J. Clin., 70, 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Zeng, C. et al. (2015) Disparities by race, age, and sex in the improvement of survival for major cancers: results from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol., 1, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Henley, S.J. et al. (2018) Rural cancer control: bridging the chasm in geographic health inequity. Cancer Epidemiol. Biomarkers Prev., 27, 1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Singh, G.K. et al. (2014) Widening rural-urban disparities in life expectancy, U.S., 1969–2009. Am. J. Prev. Med., 46, e19–e29. [DOI] [PubMed] [Google Scholar]
- 5. Jemal, A. et al. (2008) Mortality from leading causes by education and race in the United States, 2001. Am. J. Prev. Med., 34, 1–8. [DOI] [PubMed] [Google Scholar]
- 6. Siegel, R.L. et al. (2018) An assessment of progress in cancer control. CA Cancer J. Clin., 68, 329–339. [DOI] [PubMed] [Google Scholar]
- 7. Kish, J.K. et al. (2014) Racial and ethnic disparities in cancer survival by neighborhood socioeconomic status in Surveillance, Epidemiology, and End Results (SEER) Registries. J. Natl. Cancer Inst. Monogr., 2014, 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh, G.K. et al. (2017) Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: over six decades of changing patterns and widening inequalities. J. Environ. Public Health, 2017, 2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng, I. et al. (2009) Socioeconomic status and prostate cancer incidence and mortality rates among the diverse population of California. Cancer Causes Control, 20, 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Powell-Wiley, T.M. et al. (2020) The relationship between neighborhood socioeconomic deprivation and telomere length: the 1999–2002 National Health and Nutrition Examination Survey. SSM Popul. Health, 10, 100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsai, C.W. et al. (2020) Leukocyte telomere length is associated with aggressive prostate cancer in localized African American prostate cancer patients. Carcinogenesis, 41, 1213–1218. [DOI] [PubMed] [Google Scholar]
- 12. Zhang, C. et al. (2015) The association between telomere length and cancer prognosis: evidence from a meta-analysis. PLoS One, 10, e0133174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bray, F. et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin., 68, 394–424. [DOI] [PubMed] [Google Scholar]
- 14. Buell, P. (1973) Changing incidence of breast cancer in Japanese-American women. J. Natl. Cancer Inst., 51, 1479–1483. [DOI] [PubMed] [Google Scholar]
- 15. Thomas, D.B. et al. (1987) Cancer in first and second generation Americans. Cancer Res., 47, 5771–5776. [PubMed] [Google Scholar]
- 16. Ziegler, R.G. et al. (1993) Migration patterns and breast cancer risk in Asian-American women. J. Natl. Cancer Inst., 85, 1819–1827. [DOI] [PubMed] [Google Scholar]
- 17. Zhang, J. et al. (2012) Trends in mortality from cancers of the breast, colon, prostate, esophagus, and stomach in East Asia: role of nutrition transition. Eur. J. Cancer Prev., 21, 480–489. [DOI] [PubMed] [Google Scholar]
- 18. Ragin, C. et al. (2017) Cancer in populations of African Ancestry: studies of the African Caribbean Cancer Consortium. Cancer Causes Control, 28, 1173–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freedman, M.L. et al. (2006) Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc. Natl. Acad. Sci. U. S. A., 103, 14068–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lachance, J. et al. (2018) Genetic hitchhiking and population bottlenecks contribute to prostate cancer disparities in men of African descent. Cancer Res., 78, 2432–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maruthappu, M. et al. (2015) Incidence of prostate and urological cancers in England by ethnic group, 2001–2007: a descriptive study. BMC Cancer, 15, 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petersen, D.C. et al. (2019) African KhoeSan ancestry linked to high-risk prostate cancer. BMC Med. Genomics, 12, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wallace, T.A. et al. (2011) Interactions among genes, tumor biology and the environment in cancer health disparities: examining the evidence on a national and global scale. Carcinogenesis, 32, 1107–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nguyen, V.K. et al. (2020) A comprehensive analysis of racial disparities in chemical biomarker concentrations in United States women, 1999–2014. Environ. Int., 137, 105496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Islami, F. et al. (2017) Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA Cancer J. Clin., 67, 273–289. [DOI] [PubMed] [Google Scholar]
- 26. El-Serag, H.B. et al. (2020) Texas has the highest hepatocellular carcinoma incidence rates in the USA. Dig Dis Sci., doi: 10.1007/s10620-020-06231-4. [DOI] [PubMed] [Google Scholar]
- 27. Daw, J. (2017) Contribution of four comorbid conditions to racial/ethnic disparities in mortality risk. Am. J. Prev. Med., 52(1S1), S95–S102. [DOI] [PubMed] [Google Scholar]
- 28. Melkonian, S.C. et al. (2019) Disparities in cancer incidence and trends among American Indians and Alaska natives in the United States, 2010–2015. Cancer Epidemiol. Biomarkers Prev., 28, 1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trinh, Q.D. et al. (2015) Cancer-specific mortality of Asian Americans diagnosed with cancer: a nationwide population-based assessment. J. Natl. Cancer Inst., 107, djv054. [DOI] [PubMed] [Google Scholar]
- 30. DeSantis, C.E. et al. (2019) Cancer statistics for African Americans, 2019. CA Cancer J. Clin., 69, 211–233. [DOI] [PubMed] [Google Scholar]
- 31. Ryan, B.M. (2018) Lung cancer health disparities. Carcinogenesis, 39, 741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Augustus, G.J. et al. (2018) Colorectal cancer disparity in African Americans: risk factors and carcinogenic mechanisms. Am. J. Pathol., 188, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ashktorab, H. et al. (2017) Racial disparity in gastrointestinal cancer risk. Gastroenterology, 153, 910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Daly, B. et al. (2015) A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J. Clin., 65, 221–238. [DOI] [PubMed] [Google Scholar]
- 35. Rebbeck, T.R. et al. (2013) Global patterns of prostate cancer incidence, aggressiveness, and mortality in men of african descent. Prostate Cancer, 2013, 560857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McGinley, K.F. et al. (2016) Prostate cancer in men of African origin. Nat. Rev. Urol., 13, 99–107. [DOI] [PubMed] [Google Scholar]
- 37. Smith, C.J. et al. (2018) Biological determinants of health disparities in multiple myeloma. Blood Cancer J., 8, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Medhanie, G.A. et al. (2017) Cancer incidence profile in sub-Saharan African-born blacks in the United States: similarities and differences with US-born non-Hispanic blacks. Cancer, 123, 3116–3124. [DOI] [PubMed] [Google Scholar]
- 39. Pinheiro, P.S. et al. (2020) Cancer mortality among US blacks: variability between African Americans, Afro-Caribbeans, and Africans. Cancer Epidemiol., 66, 101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huo, D. et al. (2009) Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J. Clin. Oncol., 27, 4515–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robbins, H.A. et al. (2015) Age at cancer diagnosis for blacks compared with whites in the United States. J Natl Cancer Inst., 107, dju489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aldrich, M.C. et al. (2019) Evaluation of USPSTF lung cancer screening guidelines among African American adult smokers. JAMA Oncol., 5, 1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Butler, E.N. et al. (2020) Fatal prostate cancer incidence trends in the United States and England by race, stage, and treatment. Br. J. Cancer, 123, 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Silber, J.H. et al. (2013) Characteristics associated with differences in survival among black and white women with breast cancer. JAMA, 310, 389–397. [DOI] [PubMed] [Google Scholar]
- 45. Ellis, L. et al. (2018) Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J. Clin. Oncol., 36, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aghdam, N. et al. (2020) Ethnicity and insurance status predict metastatic disease presentation in prostate, breast, and non-small cell lung cancer. Cancer Med., 9, 5362–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mauvais-Jarvis, F. et al. (2020) Sex and gender: modifiers of health, disease, and medicine. Lancet, 396, 565–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li, C.H. et al. (2018) Sex Differences in cancer driver genes and biomarkers. Cancer Res., 78, 5527–5537. [DOI] [PubMed] [Google Scholar]
- 49. Ma, W.L. et al. (2014) Androgen receptor roles in hepatocellular carcinoma, fatty liver, cirrhosis and hepatitis. Endocr. Relat. Cancer, 21, R165–R182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Conforti, F. et al. (2018) Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet. Oncol., 19, 737–746. [DOI] [PubMed] [Google Scholar]
- 51. Islami, F. et al. (2017) International trends in anal cancer incidence rates. Int. J. Epidemiol., 46, 924–938. [DOI] [PubMed] [Google Scholar]
- 52. Yabroff, K.R. et al. (2020) Health insurance coverage disruptions and cancer care and outcomes: systematic review of published research. J. Natl. Cancer Inst., 112, 671–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dess, R.T. et al. (2019) Association of black race with prostate cancer-specific and other-cause mortality. JAMA Oncol., 5, 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao, J. et al. (2020) The Affordable Care Act and access to care across the cancer control continuum: a review at 10 years. CA Cancer J. Clin., 70, 165–181. [DOI] [PubMed] [Google Scholar]
- 55. Han, X. et al. (2018) Comparison of insurance status and diagnosis stage among patients with newly diagnosed cancer before vs after implementation of the Patient Protection and Affordable Care Act. JAMA Oncol., 4, 1713–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Takvorian, S.U. et al. (2020) Association of medicaid expansion under the Affordable Care Act with insurance status, cancer stage, and timely treatment among patients with breast, colon, and lung cancer. JAMA Netw. Open, 3, e1921653. [DOI] [PubMed] [Google Scholar]
- 57. Han, X. et al. (2020) Changes in noninsurance and care unaffordability among cancer survivors following the Affordable Care Act. J. Natl. Cancer Inst., 112, 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kurani, S.S. et al. (2020) Association of neighborhood measures of social determinants of health with breast, cervical, and colorectal cancer screening rates in the US Midwest. JAMA Netw. Open, 3, e200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu, M.R. et al. (2020) Impact of the affordable care act on colorectal cancer outcomes: a systematic review. Am. J. Prev. Med., 58, 596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. May, F.P. et al. (2020) Disparities in colorectal cancer screening in the United States before and after implementation of the Affordable Care Act. Clin. Gastroenterol. Hepatol., 18, 1796–1804.e2. [DOI] [PubMed] [Google Scholar]
- 61. Kim, U. et al. (2020) The effect of Medicaid expansion among adults from low-income communities on stage at diagnosis in those with screening-amenable cancers. Cancer, 126, 4209–4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Crocker, A.B. et al. (2019) Expansion coverage and preferential utilization of cancer surgery among racial and ethnic minorities and low-income groups. Surgery, 166, 386–391. [DOI] [PubMed] [Google Scholar]
- 63. Semprini, J. et al. (2020) Evaluating the effect of medicaid expansion on black/white breast cancer mortality disparities: a difference-in-difference analysis. JCO Glob. Oncol., 6, 1178–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Renzi, C. et al. (2019) Comorbid chronic diseases and cancer diagnosis: disease-specific effects and underlying mechanisms. Nat. Rev. Clin. Oncol., 16, 746–761. [DOI] [PubMed] [Google Scholar]
- 65. Price-Haywood, E.G. et al. (2020) Hospitalization and mortality among black patients and white patients with Covid-19. N. Engl. J. Med., 382, 2534–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mehta, V. et al. (2020) Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov., 10, 935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Klil-Drori, A.J. et al. (2017) Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nat. Rev. Clin. Oncol., 14, 85–99. [DOI] [PubMed] [Google Scholar]
- 68. Gallagher, E.J. et al. (2020) Hyperinsulinaemia in cancer. Nat. Rev. Cancer, 20, 629–644. [DOI] [PubMed] [Google Scholar]
- 69. Tsilidis, K.K. et al. (2015) Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ, 350, g7607. [DOI] [PubMed] [Google Scholar]
- 70. Foster, D. et al. (2014) AGE metabolites: a biomarker linked to cancer disparity? Cancer Epidemiol. Biomarkers Prev., 23, 2186–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Giovannucci, E. et al. (2010) Diabetes and cancer: a consensus report. Diabetes Care, 33, 1674–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Geiss, L.S. et al. (2014) Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980–2012. JAMA, 312, 1218–1226. [DOI] [PubMed] [Google Scholar]
- 73. Tammemagi, C.M. et al. (2005) Comorbidity and survival disparities among black and white patients with breast cancer. JAMA, 294, 1765–1772. [DOI] [PubMed] [Google Scholar]
- 74. Gallagher, E.J. et al. (2020) Insulin resistance contributes to racial disparities in breast cancer prognosis in US women. Breast Cancer Res., 22, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Setiawan, V.W. et al. (2019) Pancreatic cancer following incident diabetes in African Americans and Latinos: the multiethnic cohort. J. Natl. Cancer Inst., 111, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Unger, J.M. et al. (2019) Association of patient comorbid conditions with cancer clinical trial participation. JAMA Oncol., 5, 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Duma, N. et al. (2018) Representation of minorities and women in oncology clinical trials: review of the past 14 years. J. Oncol. Pract., 14, e1–e10. [DOI] [PubMed] [Google Scholar]
- 78. Loree, J.M. et al. (2019) Disparity of race reporting and representation in clinical trials leading to cancer drug approvals from 2008 to 2018. JAMA Oncol., 5, e191870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Berndt, S.I. et al. (2007) Disparities in treatment and outcome for renal cell cancer among older black and white patients. J. Clin. Oncol., 25, 3589–3595. [DOI] [PubMed] [Google Scholar]
- 80. Lee, L. et al. (2011) Impact of comorbidity on chemotherapy use and outcomes in solid tumors: a systematic review. J. Clin. Oncol., 29, 106–117. [DOI] [PubMed] [Google Scholar]
- 81. Sarfati, D. et al. (2016) The impact of comorbidity on cancer and its treatment. CA Cancer J. Clin., 66, 337–350. [DOI] [PubMed] [Google Scholar]
- 82. Juarez, P.D. et al. (2014) The public health exposome: a population-based, exposure science approach to health disparities research. Int. J. Environ. Res. Public Health, 11, 12866–12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vin-Raviv, N. et al. (2013) Racial disparities in posttraumatic stress after diagnosis of localized breast cancer: the BQUAL study. J. Natl. Cancer Inst., 105, 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Miller, G.E. et al. (2009) Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc. Natl. Acad. Sci. U. S. A., 106, 14716–14721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Entringer, S. et al. (2011) Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc. Natl. Acad. Sci. U. S. A., 108, E513–E518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Taylor, T.R. et al. (2007) Racial discrimination and breast cancer incidence in US Black women: the Black Women’s Health Study. Am. J. Epidemiol., 166, 46–54. [DOI] [PubMed] [Google Scholar]
- 87. Shariff-Marco, S. et al. (2010) Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am. J. Public Health, 100, 364–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Williams, J.B. et al. (2009) A model of gene-environment interaction reveals altered mammary gland gene expression and increased tumor growth following social isolation. Cancer Prev. Res. (Phila)., 2, 850–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Volden, P.A. et al. (2013) Chronic social isolation is associated with metabolic gene expression changes specific to mammary adipose tissue. Cancer Prev. Res. (Phila)., 6, 634–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bortolato, B. et al. (2017) Depression in cancer: the many biobehavioral pathways driving tumor progression. Cancer Treat. Rev., 52, 58–70. [DOI] [PubMed] [Google Scholar]
- 91. Currier, M.B. et al. (2014) Depression as a risk factor for cancer: from pathophysiological advances to treatment implications. Annu. Rev. Med., 65, 203–221. [DOI] [PubMed] [Google Scholar]
- 92. Chida, Y. et al. (2008) Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol., 5, 466–475. [DOI] [PubMed] [Google Scholar]
- 93. Satin, J.R. et al. (2009) Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer, 115, 5349–5361. [DOI] [PubMed] [Google Scholar]
- 94. Machado, M.O. et al. (2018) The association of depression and all-cause and cause-specific mortality: an umbrella review of systematic reviews and meta-analyses. BMC Med., 16, 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cole, S.W. et al. (2015) Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer, 15, 563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Antoni, M.H. et al. (2006) The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat. Rev. Cancer, 6, 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lutgendorf, S.K. et al. (2011) Social isolation is associated with elevated tumor norepinephrine in ovarian carcinoma patients. Brain. Behav. Immun., 25, 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Thaker, P.H. et al. (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med., 12, 939–944. [DOI] [PubMed] [Google Scholar]
- 99. Sloan, E.K. et al. (2010) The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res., 70, 7042–7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Le, C.P. et al. (2016) Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun., 7, 10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bower, J.E. et al. (2018) Prometastatic molecular profiles in breast tumors from socially isolated women. JNCI Cancer Spectr., 2, pky029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Giese-Davis, J. et al. (2011) Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J. Clin. Oncol., 29, 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Powell, N.D. et al. (2013) Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via β-adrenergic induction of myelopoiesis. Proc. Natl. Acad. Sci. U. S. A., 110, 16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Thames, A.D. et al. (2019) Experienced discrimination and racial differences in leukocyte gene expression. Psychoneuroendocrinology, 106, 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kurozumi, S. et al. (2019) β2-Adrenergic receptor expression is associated with biomarkers of tumor immunity and predicts poor prognosis in estrogen receptor-negative breast cancer. Breast Cancer Res. Treat., 177, 603–610. [DOI] [PubMed] [Google Scholar]
- 106. Powe, D.G. et al. (2010) Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget, 1, 628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Barron, T.I. et al. (2011) Beta blockers and breast cancer mortality: a population- based study. J. Clin. Oncol., 29, 2635–2644. [DOI] [PubMed] [Google Scholar]
- 108. Melhem-Bertrandt, A. et al. (2011) Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol., 29, 2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kinlock, B.L. et al. (2017) Prevalence and correlates of major depressive symptoms among black men with prostate cancer. Ethn. Dis., 27, 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lu, D. et al. (2016) Stress-related signaling pathways in lethal and nonlethal prostate cancer. Clin. Cancer Res., 22, 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Piyarathna, D.W.B. et al. (2019) ERR1 and PGC1α associated mitochondrial alterations correlate with pan-cancer disparity in African Americans. J. Clin. Invest., 129, 2351–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Fejerman, L. et al. (2008) Genetic ancestry and risk of breast cancer among U.S. Latinas. Cancer Res., 68, 9723–9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fejerman, L. et al. (2010) European ancestry is positively associated with breast cancer risk in Mexican women. Cancer Epidemiol. Biomarkers Prev., 19, 1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Oak, N. et al. ; TCGA Analysis Network. (2020) Ancestry-specific predisposing germline variants in cancer. Genome Med., 12, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Nédélec, Y. et al. (2016) Genetic ancestry and natural selection drive population differences in immune responses to pathogens. Cell, 167, 657–669.e21. [DOI] [PubMed] [Google Scholar]
- 116. Barreiro, L.B. et al. (2020) Evolutionary and population (epi)genetics of immunity to infection. Hum. Genet., 139, 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Koshiol, J. et al. (2011) Racial differences in chronic immune stimulatory conditions and risk of non-Hodgkin’s lymphoma in veterans from the United States. J. Clin. Oncol., 29, 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Coe, C.L. et al. (2011) Population differences in proinflammatory biology: Japanese have healthier profiles than Americans. Brain. Behav. Immun., 25, 494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yao, S. et al. (2018) Genetic ancestry and population differences in levels of inflammatory cytokines in women: role for evolutionary selection and environmental factors. PLoS Genet., 14, e1007368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pine, S.R. et al. (2016) Differential serum cytokine levels and risk of lung cancer between African and European Americans. Cancer Epidemiol. Biomarkers Prev., 25, 488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Meaney, C.L. et al. (2019) Circulating inflammation proteins associated with lung cancer in African Americans. J. Thorac. Oncol., 14, 1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Storey, J.D. et al. (2007) Gene-expression variation within and among human populations. Am. J. Hum. Genet., 80, 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Zhang, W. et al. (2008) Evaluation of genetic variation contributing to differences in gene expression between populations. Am. J. Hum. Genet., 82, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Prokunina-Olsson, L. et al. (2013) A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat. Genet., 45, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jenkins, B.D. et al. (2019) Atypical Chemokine Receptor 1 (DARC/ACKR1) in breast tumors is associated with survival, circulating chemokines, tumor-infiltrating immune cells, and African Ancestry. Cancer Epidemiol. Biomarkers Prev., 28, 690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tan, D.S. et al. (2016) Cancer genomics: diversity and disparity across ethnicity and geography. J. Clin. Oncol., 34, 91–101. [DOI] [PubMed] [Google Scholar]
- 127. Yuan, J. et al. (2018) Integrated analysis of genetic ancestry and genomic alterations across cancers. Cancer Cell, 34, 549–560.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Carrot-Zhang, J. et al. ; Cancer Genome Atlas Analysis Network. (2020) Comprehensive analysis of genetic ancestry and its molecular correlates in cancer. Cancer Cell, 37, 639–654.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yeh, C.H. et al. (2018) FBXW7: a critical tumor suppressor of human cancers. Mol. Cancer, 17, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Keenan, T. et al. (2015) Comparison of the genomic landscape between primary breast cancer in african american versus white women and the association of racial differences with tumor recurrence. J. Clin. Oncol., 33, 3621–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Pitt, J.J. et al. (2018) Characterization of Nigerian breast cancer reveals prevalent homologous recombination deficiency and aggressive molecular features. Nat. Commun., 9, 4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Carey, L.A. et al. (2006) Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA, 295, 2492–2502. [DOI] [PubMed] [Google Scholar]
- 133. Craig, D.W. et al. (2013) Genome and transcriptome sequencing in prospective metastatic triple-negative breast cancer uncovers therapeutic vulnerabilities. Mol. Cancer Ther., 12, 104–116. [DOI] [PubMed] [Google Scholar]
- 134. Guda, K. et al. (2015) Novel recurrently mutated genes in African American colon cancers. Proc. Natl. Acad. Sci. U. S. A., 112, 1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Yoon, H.H. et al. (2015) Racial differences in BRAF/KRAS mutation rates and survival in stage III colon cancer patients. J Natl Cancer Inst., 107, djv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Xicola, R.M. et al. (2018) Lack of APC somatic mutation is associated with early-onset colorectal cancer in African Americans. Carcinogenesis, 39, 1331–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Shigematsu, H. et al. (2005) Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J. Natl. Cancer Inst., 97, 339–346. [DOI] [PubMed] [Google Scholar]
- 138. Chen, J. et al. (2020) Genomic landscape of lung adenocarcinoma in East Asians. Nat. Genet., 52, 177–186. [DOI] [PubMed] [Google Scholar]
- 139. Soo, R.A. et al. (2011) Ethnic differences in survival outcome in patients with advanced stage non-small cell lung cancer: results of a meta-analysis of randomized controlled trials. J. Thorac. Oncol., 6, 1030–1038. [DOI] [PubMed] [Google Scholar]
- 140. Campbell, J.D. et al. (2017) Comparison of prevalence and types of mutations in lung cancers among Black and White populations. JAMA Oncol., 3, 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Mitchell, K.A. et al. (2019) Recurrent PTPRT/JAK2 mutations in lung adenocarcinoma among African Americans. Nat. Commun., 10, 5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Attard, G. et al. (2016) Prostate cancer. Lancet, 387, 70–82. [DOI] [PubMed] [Google Scholar]
- 143. Faisal, F.A. et al. (2016) Racial variations in prostate cancer molecular subtypes and androgen receptor signaling reflect anatomic tumor location. Eur. Urol., 70, 14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Berger, M.F. et al. (2011) The genomic complexity of primary human prostate cancer. Nature, 470, 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Fraser, M. et al. (2017) Genomic hallmarks of localized, non-indolent prostate cancer. Nature, 541, 359–364. [DOI] [PubMed] [Google Scholar]
- 146. Zhao, S. et al. (2017) Epigenome-wide tumor DNA methylation profiling identifies novel prognostic biomarkers of metastatic-lethal progression in men diagnosed with clinically localized prostate cancer. Clin. Cancer Res., 23, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Mundbjerg, K. et al. (2017) Identifying aggressive prostate cancer foci using a DNA methylation classifier. Genome Biol., 18, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Magi-Galluzzi, C. et al. (2011) TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of Caucasian, African-American and Japanese patients. Prostate, 71, 489–497. [DOI] [PubMed] [Google Scholar]
- 149. Rosen, P. et al. (2012) Clinical potential of the ERG oncoprotein in prostate cancer. Nat. Rev. Urol., 9, 131–137. [DOI] [PubMed] [Google Scholar]
- 150. Li, J. et al. (2020) A genomic and epigenomic atlas of prostate cancer in Asian populations. Nature, 580, 93–99. [DOI] [PubMed] [Google Scholar]
- 151. Khani, F. et al. (2014) Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clin. Cancer Res., 20, 4925–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Blackburn, J. et al. (2019) TMPRSS2-ERG fusions linked to prostate cancer racial health disparities: a focus on Africa. Prostate, 79, 1191–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Koga, Y. et al. (2020) Genomic profiling of prostate cancers from men with African and European ancestry. Clin. Cancer Res., 26, 4651–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Jaratlerdsiri, W. et al. (2018) Whole-genome sequencing reveals elevated tumor mutational burden and initiating driver mutations in African men with treatment-naïve, high-risk prostate cancer. Cancer Res., 78, 6736–6746. [DOI] [PubMed] [Google Scholar]
- 155. Petrovics, G. et al. (2015) A novel genomic alteration of LSAMP associates with aggressive prostate cancer in African American men. EBioMedicine, 2, 1957–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Wallace, T.A. et al. (2008) Tumor immunobiological differences in prostate cancer between African-American and European-American men. Cancer Res., 68, 927–936. [DOI] [PubMed] [Google Scholar]
- 157. Reams, R.R. et al. (2009) Microarray comparison of prostate tumor gene expression in African-American and Caucasian American males: a pilot project study. Infect. Agent. Cancer, 4 Suppl 1, S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Tang, W. et al. (2018) IFNL4-ΔG Allele is associated with an interferon signature in tumors and survival of African-American men with prostate cancer. Clin. Cancer Res., 24, 5471–5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Weichselbaum, R.R. et al. (2008) An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc. Natl. Acad. Sci. U. S. A., 105, 18490–18495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gannon, H.S. et al. (2018) Identification of ADAR1 adenosine deaminase dependency in a subset of cancer cells. Nat. Commun., 9, 5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Sartor, O. et al. (2020) Survival of African-American and Caucasian men after sipuleucel-T immunotherapy: outcomes from the PROCEED registry. Prostate Cancer Prostatic Dis., 23, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Smith, C.J. et al. (2017) Aspirin use reduces the risk of aggressive prostate cancer and disease recurrence in African-American men. Cancer Epidemiol. Biomarkers Prev., 26, 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Osborn, V.W. et al. (2016) Impact of aspirin on clinical outcomes for African American men with prostate cancer undergoing radiation. Tumori, 102, 65–70. [DOI] [PubMed] [Google Scholar]
- 164. Minas, T.Z. et al. (2018) IFNL4-ΔG is associated with prostate cancer among men at increased risk of sexually transmitted infections. Commun. Biol., 1, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Vidal, A.C. et al. (2019) Dietary inflammatory index (DII) and risk of prostate cancer in a case-control study among Black and White US Veteran men. Prostate Cancer Prostatic Dis., 22, 580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wallace, T.A. et al. (2014) Elevated HERV-K mRNA expression in PBMC is associated with a prostate cancer diagnosis particularly in older men and smokers. Carcinogenesis, 35, 2074–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Chiappinelli, K.B. et al. (2015) Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell, 162, 974–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Pierre, C.C. et al. (2019) Dancing from bottoms up - roles of the POZ-ZF transcription factor Kaiso in Cancer. Biochim. Biophys. Acta. Rev. Cancer, 1871, 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Lehmann, B.D. et al. (2011) Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest., 121, 2750–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Martin, D.N. et al. (2009) Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS One, 4, e4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Mukhtar, R.A. et al. (2011) Elevated PCNA+ tumor-associated macrophages in breast cancer are associated with early recurrence and non-Caucasian ethnicity. Breast Cancer Res. Treat., 130, 635–644. [DOI] [PubMed] [Google Scholar]
- 172. Adisa, C.A. et al. (2012) Biology of breast cancer in Nigerian women: a pilot study. Ann. Afr. Med., 11, 169–175. [DOI] [PubMed] [Google Scholar]
- 173. Sawe, R.T. et al. (2016) Aggressive breast cancer in western Kenya has early onset, high proliferation, and immune cell infiltration. BMC Cancer, 16, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Lindner, R. et al. (2013) Molecular phenotypes in triple negative breast cancer from African American patients suggest targets for therapy. PLoS One, 8, e71915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Weidner, N. (1995) Intratumor microvessel density as a prognostic factor in cancer. Am. J. Pathol., 147, 9–19. [PMC free article] [PubMed] [Google Scholar]
- 176. Zackular, J.P. et al. (2013) The gut microbiome modulates colon tumorigenesis. mBio, 4, e00692–e00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Zhernakova, D.V. et al. ; LifeLines cohort study; BIOS consortium. (2018) Individual variations in cardiovascular-disease-related protein levels are driven by genetics and gut microbiome. Nat. Genet., 50, 1524–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. O’Keefe, S.J. (2016) Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol., 13, 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Scott, A.J. et al. (2019) International Cancer Microbiome Consortium consensus statement on the role of the human microbiome in carcinogenesis. Gut, 68, 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Iida, N. et al. (2013) Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science, 342, 967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Routy, B. et al. (2018) Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science, 359, 91–97. [DOI] [PubMed] [Google Scholar]
- 182. David, L.A. et al. (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505, 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Goodrich, J.K. et al. (2014) Human genetics shape the gut microbiome. Cell, 159, 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Schulz, M.D. et al. (2014) High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature, 514, 508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Yoshimoto, S. et al. (2013) Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature, 499, 97–101. [DOI] [PubMed] [Google Scholar]
- 186. Forslund, K. et al. ; MetaHIT consortium. (2015) Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature, 528, 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Kostic, A.D. et al. (2013) Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe, 14, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Greathouse, K.L. et al. (2018) Interaction between the microbiome and TP53 in human lung cancer. Genome Biol., 19, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Tang, W. et al. (2019) Liver- and microbiome-derived bile acids accumulate in human breast tumors and inhibit growth and improve patient survival. Clin. Cancer Res., 25, 5972–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Zitvogel, L. et al. (2015) Cancer and the gut microbiota: an unexpected link. Sci. Transl. Med., 7, 271ps1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Yatsunenko, T. et al. (2012) Human gut microbiome viewed across age and geography. Nature, 486, 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Deschasaux, M. et al. (2018) Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med., 24, 1526–1531. [DOI] [PubMed] [Google Scholar]
- 193. He, Y. et al. (2018) Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med., 24, 1532–1535. [DOI] [PubMed] [Google Scholar]
- 194. Brooks, A.W. et al. (2018) Gut microbiota diversity across ethnicities in the United States. PLoS Biol., 16, e2006842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Vangay, P. et al. (2018) US immigration westernizes the human gut microbiome. Cell, 175, 962–972.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Maskarinec, G. et al. (2004) The effect of migration on cancer incidence among Japanese in Hawaii. Ethn. Dis., 14, 431–439. [PubMed] [Google Scholar]
- 197. Fettweis, J.M. et al. (2014) Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology (Reading), 160(Pt 10), 2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Yang, Y. et al. (2019) Racial differences in the oral microbiome: data from low-income populations of African Ancestry and European Ancestry. mSystems, 26, E00639– 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Feng, Y. et al. (2019) Metagenomic analysis reveals a rich bacterial content in high-risk prostate tumors from African men. Prostate, 79, 1731–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Thompson, K.J. et al. (2017) A comprehensive analysis of breast cancer microbiota and host gene expression. PLoS One, 12, e0188873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Yazici, C. et al. (2017) Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut, 66, 1983–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. O’Keefe, S.J. et al. (2015) Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun., 6, 6342. [DOI] [PMC free article] [PubMed] [Google Scholar]