Abstract
Neurological heterotopic ossification (NHO) is a debilitating condition where bone forms in soft tissue, such as muscle surrounding the hip and knee, following an injury to the brain or spinal cord. This abnormal formation of bone can result in nerve impingement, pain, contractures and impaired movement. Patients are often diagnosed with NHO after the bone tissue has completely mineralised, leaving invasive surgical resection the only remaining treatment option. Surgical resection of NHO creates potential for added complications, particularly in patients with concomitant injury to the central nervous system (CNS). Although recent work has begun to shed light on the physiological mechanisms involved in NHO, there remains a significant knowledge gap related to the prognostic biomarkers and prophylactic treatments which are necessary to prevent NHO and optimise patient outcomes. This article reviews the current understanding pertaining to NHO epidemiology, pathobiology, biomarkers and treatment options. In particular, we focus on how concomitant CNS injury may drive ectopic bone formation and discuss considerations for treating polytrauma patients with NHO. We conclude that understanding of the pathogenesis of NHO is rapidly advancing, and as such, there is the strong potential for future research to unearth methods capable of identifying patients likely to develop NHO, and targeted treatments to prevent its manifestation.
Subject terms: Diseases, Bone
Introduction
Heterotopic ossification (HO) is the pathological formation of bone in muscles and surrounding joints. The ramifications of this ectopic bone formation in soft tissue include swelling, pain, nerve entrapment, contractures, and in some cases, limited range of movement due to bone fusion in the affected area (i.e. ankylosis).1 Although various forms of hereditary and acquired HO exist, HO that occurs following a neurological insult (i.e. neurological HO; NHO) is of increasing clinical concern due to its rising prevalence in combat and civilian populations.2 Neurological heterotopic ossification (NHO) is particularly common when a neurological insult, such as a traumatic brain injury (TBI) or spinal cord injury (SCI), occurs in the presence of concomitant peripheral injuries (e.g. bone fractures, muscle injuries). Non-surgical interventions include analgesia,3 rest,4 and nerve blockers;5 however, the only cure is surgical excision.6,7 Unfortunately, invasive surgical excision can only occur once the lesion has mineralised, and reoccurs in ~6% of patients.8 Consequently, there is an urgent need to develop prophylactic interventions that can prevent HO and are still appropriate for patients who have sustained severe trauma to the periphery and central nervous system (CNS).9 To date, the development of prophylaxes has been hindered by a limited understanding of how ectopic bone formation is triggered in response to CNS and musculoskeletal trauma. Nonetheless, there has been a recent rise in the number of clinical and pre-clinical studies designed to unearth the cellular and molecular mechanisms of NHO. This article will first review the literature regarding NHO epidemiology, the pathophysiology of CNS injuries, and how they may promote bone formation, mechanisms of endochondral bone formation before presenting new research avenues towards identifying novel mechanisms of NHO, and the limited NHO treatment options. As the literature specific to NHO is relatively limited, we sometimes draw from the broader HO literature and make inferences where relevant.
Epidemiology and risk factors for NHO
It has been reported that at least 10%–20% of patients with a TBI and simultaneous peripheral musculoskeletal injuries (i.e. polytrauma involving substantial muscle injuries and bone fractures) develop NHO.10–13 Following combined SCI and polytrauma, ~15%–30% of patients develop NHO.10–12 This ectopic bone has a tendency to form predominantly in muscle surrounding the hip, although it also frequently affects other joints such as the knee, elbow, and shoulder (see Table 1).10–12 Notably, when NHO is formed posteriorly at the hip, it often entraps the sciatic nerve, resulting in neurological pain and muscle weakness, impaired movement, and difficulty performing everyday tasks (e.g. standing, sitting and getting dressed).10,14 Most studies suggest that NHO prevalence is significantly higher in males than females, which has been attributed to the increased number of males that experience a TBI or SCI.15 However, a recent study employing a mouse model of HO (featuring a dermal burn and Achilles tenotomy) reported that male mice formed ~30% more ectopic bone when compared to female mice, possibly due to increased insulin like growth factor-1 and bone morphogenetic protein (BMP) signalling in males.16 Therefore, males may be predisposed to having increased risk for the development of HO/NHO.
Table 1.
Incidence, demographics and locations of NHO reported across the literature
| Author | Description (total number of patients, age range, mean age, gender, number of patients with NHO) | Type of injury/s | Location of HO and imaging modality used |
|---|---|---|---|
| Reznik et al.165 |
262 TBI patients, 226 M, 36 F TBI-NHO patients: 10, mean: 39.6 years, 10 M 151 SCI patients, 128 M, 23 F 16 SCI-NHO patients, mean: 31.4 years, 13 M, 3 F |
TBI and SCI |
Hip: 17 lesions, shoulder: 1 lesion, elbow: 3 lesions, knee: 5 lesions Bone scintigraphy |
| Dizdar et al.20 |
151 TBI patients, 126 M, 25 F 56 NHO patients, mean: 34.6 years, 48 M, 8 F |
TBI |
Hip: 41 patients, shoulder: 11 patients, elbow: 20 patients, knee: 25 patients, ankle: 2 patients Imaging modality: not reported (NR) |
| Van Kampen et al.22 |
176 TBI patients, 75 M, 22 F 13 TBI-NHO patients, mean: 34.65 years, 10 M, 3 F 79 excluded GCS <8. Coma duration in NHO group: 15 days Coma duration in non-NHO group: 4.18 days Mechanical ventilation: 17.23 days Immobilisation days: 13.46 |
TBI Concomitant bone FX: 8/13 patients |
Hip: 3 lesions, shoulder: 2 lesions, thigh: 2 lesions, elbow: 4 lesions, knee: 5 lesions, femur: 1 lesion, ankle: 1 lesion, iliopsoas muscle: 1 lesion Imaging modality: NR |
| Rigaux et al.166 |
31 TBI patients, 31 M 12 TBI-NHO patients, 33 years,12 M GCS: ≥8 3 months-post-injury |
TBI |
NR X-ray, bone scintigraphy |
| Hurvitz et al.167 |
90 TBI patients, mean: 11.9 years, 67 M, 23 F 13 TBI-NHO patients, 9 M, 4 F 35 patients TBI + extremity FX 30 patients TBI + skull FX |
TBI |
Hip: 4 lesions, shoulder: 3, femur: 3, elbow: 3, knee: 4 lesions, forearm: 2, ischium: 2 X-ray, bone scintigraphy |
| Hendricks et al.168 |
76 TBI patients, 16–84 years, mean: 36.67 years, 47 M 29 F 9 TBI-NHO patients GCS 3: 9 Coma duration of 76 patients: 10.03 (1–61 days) Mechanical ventilation: 8.95 (0–52 days) Diffuse axonal injury: 7 patients |
TBI Concomitant bone FX: 9/9 patients |
Hip: 5 lesions, shoulder: 3 lesions, elbow: 5, knee: 5, ankle: 2 |
| Seipel et al.169 |
1 463 total patients, 17–77 years, mean: 40.4 years, 916 M, 547 F 30 NHO patients, 23 M, 7 F Mean time to HO diagnosis: 7.2 ± 1.2 weeks |
TBI and SCI TBI/SCI: 23 patients Concomitant peripheral trauma: 5 patients Non traumatic: 7 patients |
Hip: 42 lesions, shoulder: 22 lesions, elbow: 7 lesions, knee: 10 lesions, upper ankle joint: 1 lesion, diaphysis of long bones: 3 lesions X-ray |
| Singh et al.170 |
18 SCI patients, 18–54 years, mean: 32 years, 16 M, 2 F 7 SCI-NHO patients, 18–40 years, mean: 30 years Average abbreviated injury scale (AIS): A Average HO score: 1a |
SCI |
Hip: 7 patients X-ray, SPECT |
| Wittenberg et al.171 |
413 SCI patients, mean: 35.4 years, 274 M, 82 F 71 SCI-NHO patients, mean: 33.8 years, 63 M, 8 F 30 tetraplegia, 39 paraplegia 57 excluded from study |
SCI Concomitant head injuries: 23 patients Concomitant extremity injury: 19 patients Concomitant pelvic injury: 1 patient Concomitant injuries (abdominal and thoracic): 33 patients |
Left hip: 70.4%, right hip 57.8% Elbow: 5, knees, 2 X-ray |
| Bravo-Payno et al.172 |
654 SCI patients 85 SCI-NHO patients, 18–56 years, 29.70 years 41 excluded from study |
SCI |
Hip: 36 patients, shoulder: 3 patients, elbow: 1 patient, knee: 4 patients X-ray |
| Orzel and Rudd173 |
50 total patients 43 NHO patients, 18–56 years, 30 M, 13 F |
SCI, TBI, peripheral trauma (cerebral vascular insult, burn) SCI trauma: 27 Paraplegics: 17 Closed head injury: 7 Peripheral trauma: 8 |
Hip: 33 patients, shoulder: 8 patients, thigh: 10 patients, elbow: 8 patients, knee: 3 patients, leg: 1 patient Bone scintigraphy |
F female, M male, NR not reported, TBI traumatic brain injury, SCI spinal cord injury, NHO neurological heterotopic ossification, FX fracture, SPECT single-photon emission computed tomography
aHO grade according to Brooker classification
Whether individuals are genetically predisposed towards NHO formation has not yet been established. Initial investigations have examined the association between human leukocyte antigen (HLA) serotypes and NHO; although, there has been contradicting evidence regarding the role of HLA-B27.17,18 In a clinical study involving 43 patients with a SCI, five of the 21 patients that developed NHO were positive for HLA-B27 compared to none of the 22 patients without NHO.17 However, the hypothesis that HLA-B27 may be a genetic risk factor for NHO was challenged by a separate study that found no differences in the frequency of any HLA-A and HLA-B antigens in 24 NHO patients compared to 740 healthy controls.18 Further large-scale studies are required to determine the link between HLA antigens, and other genetically determined factors, that may contribute to NHO.
Several risk factors have been linked to NHO formation, including coma duration, artificial or mechanical ventilation, duration of immobilisation, elevated serum alkaline phosphatase (ALP) levels, and the presence of TBI featuring diffuse axonal injury.8,19–21 Patients with TBI-induced NHO often have longer mechanical ventilation and coma duration when compared to TBI patients who do not develop NHO; nevertheless, the exact relationship between coma duration and NHO formation is yet to be established.22 It has been proposed that the homoeostatic balance between calcium, oxygen, and pH levels are altered via artificial ventilation, which may result in respiratory alkalosis, contributing to accelerated ectopic bone formation.19 However, as increased coma duration is often the result of more severe injuries, it is difficult to determine the effect it has on NHO. These factors may contribute to the increased prevalence of NHO in patients following a stroke, TBI, or SCI.
Recent studies have identified an increased propensity to develop NHO following combat-related trauma, such as blast-TBI (bTBI) and limb amputations.23 A recent study on Iraq war operations reported that ~80% of injuries were the result of explosive devices (e.g. improvised explosive devices, mortar, or mines).24 Tissue damage caused by bTBI is induced by a combination of shockwaves, supersonic flow and highly heated air flow, resulting in a string of consequences that involve thermal injury, cavitation, and increased intracranial pressure.25 An initial study examined the prevalence of NHO/HO during the recent conflict in Iraq and Afghanistan.26 Approximately 70% of patients exposed to blast injuries requiring at least one orthopaedic procedure developed HO, while 86% of patients who experienced a bTBI and orthopaedic procedures developed NHO.26 Furthermore, univariate analysis demonstrated a significant relationship between HO and TBI severity. These findings demonstrate that the aetiology of polytrauma is incredibly heterogeneous, and as such, NHO may require several therapeutic approaches.27
Pathophysiology of CNS injury
TBI and SCI both induce an array of pathophysiological alterations that may stimulate either formation or resorption of bone.28,29 Briefly, acceleration–deceleration and/or rotational forces at the moment of impact can induce significant damage to neurons, glia and the vasculature, triggering a complex cascade of cellular and molecular changes that may contribute to further damage over the ensuing hours, days and months following injury. Common secondary injury mechanisms involved in TBI/SCI can include excitotoxicity, ionic imbalances, mitochondrial dysfunction, oxidative stress, neuroinflammation, ischaemia and edema.30,31 Notably, damage to the blood–brain barrier (BBB) or blood spinal cord barrier (BSB), the semi-permeable anatomical interfaces that separate the brain and spinal cord from peripheral blood circulation,32 creates potential for abnormal passage of molecules and cells in and out of the brain and spinal cord.33–36 For example, substances that are highly concentrated in the CNS (e.g. neuropeptides) or increased following injury (e.g. inflammatory mediators, growth factors) can migrate into the peripheral circulation, and thereby potentially drive NHO formation. This notion is supported by findings that serum and CSF from TBI patients has been found to increase osteoblastic proliferation.37,38 Furthermore, prolonged pituitary dysfunction is common after TBI,39,40 with alterations to the release of hormones such as parathyroid hormone41 and growth hormone,42 which may influence musculoskeletal tissues and potentially contribute to NHO development.
CNS injury may promote bone formation
For quite some time, orthopaedic surgeons have observed that peripheral bone fracture callus formation appears to be significantly enhanced in patients with a TBI. More recently, several clinical studies have supported this anecdotal evidence of increased callus size in TBI patients.43–45 However, human studies of this nature are often are confounded by variations in the location, nature and severity of both the bone fracture and TBI. Rodent studies that control for these variables have since demonstrated that TBI increases volume and strength of newly formed bone within the healing callus at acute and sub-acute time-points.46–51 This phenomenon may also occur following SCI, with a recent study finding that SCI patients with femoral fracture had increased callus volume and accelerated rate of fracture union when compared to patients with an isolated femoral fracture.52
A preliminary study in mice has implicated the involvement of neuronal mechanisms in robust callus formation following TBI.53 It was reported that mice that received a fracture contralateral to the site of TBI had increased callus bone volume at 5 days post injury when compared to fracture-only mice, whereas callus bone volume in mice that were given a TBI ipsilateral to the fracture was comparable to fracture-only mice.53 In contrast to the aforementioned studies,46–51 these differences, however, were not observed at later time-points (i.e. 10 or 14 days post injury).53 These findings led the authors to suggest that neuronal mechanisms play a significant role in increasing bone formation acutely following TBI by causing contralateral activation of fracture healing.53 It still remains unclear as to how TBI/SCI can alter callus formation; however, it appears likely TBI/SCI-induced NHO share a common mechanism.
It is important to acknowledge that in the absence of a peripheral bone fracture, both TBI and SCI have been associated with reduced bone mineral density in rodents,54,55 and humans.29 These findings may indicate that cellular and molecular changes due to tissue damage (e.g. inflammation) at a peripheral site is required to stimulate NHO and fracture callus formation. Overall, the mechanisms responsible for the paradoxical effects that CNS injuries have on bone are yet to be elucidated.
Mechanisms of endochondral HO
The process of ectopic bone formation in trauma induced HO is thought to occur via endochondral (rather than intramembranous) ossification. Although the precise mechanisms are not well characterised, a pool of osteoprogenitor cells (OPCs) residing in skeletal muscle combined with factors that are increased in response to trauma, such as inflammatory cells and molecules, enhanced BMP signalling, and hypoxia are thought to create an environment that together facilitates formation of bone by endochondral ossification.56,57 The formation of ectopic endochondral bone begins with invasion of immune cells, including neutrophils, macrophages, mast cells.2,57 The influx of inflammatory factors to the often hypoxic and acidic peripheral injury site is thought to stimulate the differentiation of OPCs to fibroblasts, which is driven by expression of fibroblast growth factors (FGFs), which form fibrous tissue.58–60 In response to hypoxia, expression of hypoxia-inducible factor-1 alpha (HIF-α) and vascular endothelial growth factor are upregulated to stimulate angiogenesis which provides a conduit for cells to migrate to the injury site.61 Moreover, a hypoxic environment induces expression of transcription factor SOX-9, which promotes the differentiation of chondrocytes by activating SOX-9 in a HIF-1α dependent manner.62 These chondrocytes then undergo hypertrophy and begin to form a cartilaginous matrix.63–65 Subsequent remodelling of this cartilage is mediated by matrix metalloproteinases and results in the release of angiogenic factors that further promote vascular invasion.66 The cartilage is then removed as the lesion begins to mineralise. Over time the initial woven bone is then remodelled to form mature lamellar bone with a marrow cavity. Of interest, sources of OPCs have not yet been established, however it has been suggested that that induction of OPCs from muscle satellite cells cause HO formation.19,67,68 Other studies suggest that the source of OPCs are from fibroadipogenic progenitors that reside within the muscle interstitium, but are not exclusive to muscle.69 It is possible that identification of the exact source of these OPCs may facilitate the design of better therapeutic strategies to prevent HO formation. There is little information regarding whether the mechanisms of NHO differ from HO, but it is important to establish as it has ramifications for identifying potential druggable targets for each condition. For example, although peripheral trauma associated HO is thought to occur exclusively via endochondral ossification, it is not yet known whether the additional presence of neurotrauma may alter the frequency of endochondral vs. intramembranous ossification. Additionally, an initial human study provides evidence that the histological mechanisms of ectopic bone formation were identical in lesions from TBI, SCI and trauma induced HO.70 However, significant heterogeneity existed between the location of HO, furthermore the time-point that the HO was excised was not stated. Therefore, it is difficult to determine whether the mechanisms differ between HO and NHO in the acute phase, (i.e. before mineralisation). For a more in-depth description of the cellular and molecular mechanisms of HO formation, the reader is referred to the following reviews.71–75
Mechanisms of HO formation following CNS injury
Current understanding of the cellular and molecular mechanisms of HO formation specifically in the context of neurotrauma is lacking; however, there are a number of potential mechanisms through which CNS injury may promote formation of ectopic bone at peripheral injury sites (summarised in Fig. 1). Indeed, the past decade has seen the emergence of animal models of polytrauma that have shed some light on the key drivers of HO formation following TBI or SCI. Herein, we outline emerging evidence and hypotheses of the mechanisms of NHO formation, with a particular focus on the potential role of macrophages and neuropeptides.
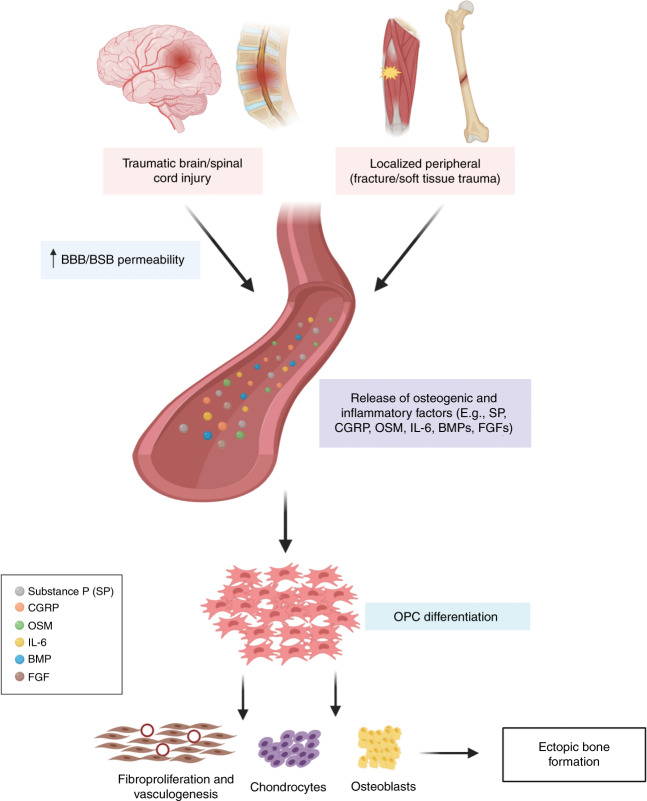
Fig. 1.
Proposed mechanism for NHO development. Simultaneous injury to the CNS and peripheral sites triggers the release of osteogenic and inflammatory factors including; SP, CGRP, OSM, IL-6, BMPs and FGFs. The influx of osteogenic and inflammatory factors, initiates the differentiation of OPCs into fibroblasts which is mediated by fibroblast growth factors (FGFs). This influx also elicits angiogenesis, which results in an increase in oxygen tension, triggering the differentiation of OPCs into chondrocytes which undergo hypertrophy and form a cartilage matrix. This cartilaginous matrix provides a structural framework for the formation of blood vessels, osteoblast proliferation and differentiation and formation of ectopic bone (created with BioRender.com)
Role of inflammatory cells and mediators
Macrophages and neutrophils
Macrophages and neutrophils have been identified as prominent cells of the muscle inflammatory infiltrate in the acute and sub-acute phases post injury and may contribute to NHO. Work by Genet et al. has highlighted the contribution of F4/80+ resident tissue macrophages as key drivers of SCI-induced NHO.12 Following SCI and cardiotoxin induced muscle injury, mice were injected intravenously with clodronate-loaded liposomes to deplete resident tissue macrophages.12 Ablation of these resident tissue macrophages was found to reduce NHO volume by ~90%, and completely prevent NHO development in 3/11 mice.12 These findings indicate that macrophages likely play a prominent role in SCI-induced NHO formation. Resident tissue macrophages have previously been implicated in the development of HO, however it still remains unclear as to whether the contribution of resident tissue macrophages differs between HO and NHO. To the best of our knowledge, to date no studies have compared the effect of resident tissue macrophage depletion in HO and NHO models. However, in a model of HO induced by burn and tenotomy, although injection of mice with clodronate-loaded liposomes decreased total HO volume by ~50%, HO was still observed in all mice.76 Although further studies are required, taken together these findings suggest that the contribution of resident tissue macrophages may differ between HO and NHO.
A number of factors released by macrophages are also released by neutrophils, therefore to determine the role of neutrophils in SCI-induced NHO, Tseng et al. recently examined NHO formation in neutropenic mice.77 Neutrophil depletion had no effect on NHO volumes.77 Nor did treatment with rhG-CSF which significantly increased the number of neutrophils in the blood, bone marrow and injured muscles.77 These findings suggest that macrophage related factors in combination with CNS injury drive NHO.
OncostatinM
Oncostatin M (OSM) is an inflammatory cytokine, derived from activated macrophages, osteoclasts, monocytes, T cells, and neutrophils.78,79 OSM has been reported to stimulate osteoblastic differentiation and hence bone formation by acting on osteoclasts and osteoprogenitors.80 The involvement of OSM in osteogenic differentiation in NHO development suggests that OSM receptor (OSMR) and OSM could be viable therapeutic targets.78
A recent study examined NHO formation using NHO-lesions from 64 patients with CNS injuries (SCI, TBI, stroke, or cerebral anoxia) and a mouse model of SCI-induced NHO.78 Histological analysis of NHO-lesions excised from CNS-injured patients revealed that OSM was expressed by CD68+ macrophages and osteoclasts within NHO sections.78 Of note, OSM plasma protein levels were elevated twofold when compared to healthy donors (i.e. HO negative patients following total hip surgery), suggesting that plasma OSM levels may serve as a biomarker of NHO formation.78 It was also found that muscle-derived stromal cells isolated from NHO-lesions expressed OSMRs, and that treatment with recombinant human OSM increased mineralisation and differentiation.78 In addition, after SCI-induced NHO in mice, immunohistochemistry and mRNA analysis demonstrated that OSM levels were significantly increased in injured muscle post-CDTX injection and SCI, and that OSM is secreted and accumulates at the site of NHO.78 Furthermore, deletion of the OSMR receptor significantly reduced NHO volume (median volume 14.2 mm3 in wild type, 3.2 mm3 in OSMR knockouts). Considered together, these findings suggest that OSM produced by resident tissue macrophages drives NHO formation by stimulating differentiation and mineralisation of muscle stromal cells and that OSM may represent a plasma marker and therapeutic target for preventing/reducing NHO formation.78 The contribution of OSM to TBI-induced NHO is yet to be elucidated in a suitable model, as an animal model of TBI-induced NHO that accurately mimics the combinations of injuries that these patients often present with has only recently been developed.81 Briefly, this model features a concomitant femoral muscle crush injury, femoral fracture and a moderate-severe brain injury in rats, where 70% of rats that underwent these injuries developed ectopic bone at the peripheral injury site.81
Role of neuropeptides
Neuronal injury can trigger neurogenic inflammation, which in the context of moderate-severe TBI or SCI has been shown to exacerbate secondary injury pathologies such as neuronal cell death,82 BBB83 and BSB,82 oedema,83 ischaemia83 and hypoxia.84 Neurogenic inflammation has been associated with the release of neuropeptides, particularly substance P (SP) and calcitonin gene related protein (CGRP) which increases vascular permeability and vasodilation respectively.85 Impairment of the BBB following trauma can promote further propagation of neurogenic inflammatory factors, causing exacerbated neural injury.86 Notably, there is now emerging evidence that release of these neuropeptides into peripheral circulation after neurotrauma may be a key driver of NHO formation.
SubstanceP
SP is a neuropeptide that is distributed throughout the central and peripheral nervous system, with increasing evidence highlighting its role in neurogenic inflammation, bone remodelling and TBI pathology.87,88 SP has previously been identified as a potential therapeutic target that contributes to NHO and HO development.12,89,90 SP possesses a strong affinity to neurokinin-1 receptor (NK-1R) belonging to the tachykinin receptor group.91 Accumulating evidence indicates that SP contributes to NHO development. For instance, several human and animal studies have reported that SP levels are elevated in the blood following TBI and SCI.92,93 Most notably, the role of SP in NHO has been studied in patients a murine model of SCI-induced NHO.12 SP concentrations were significantly higher in plasma from NHO patients compared to healthy volunteers. While in the mouse model, antagonising SP receptor NK-1R with RP67580 reduced NHO volume by ~30%.12 These findings indicate that SP may represent both a prognostic biomarker of NHO and a treatment target, whereby an intervention that downregulates SP is initiated following elevated plasma levels of SP.
Mast cell degranulation has been reported to be essential for SP to induce HO formation.94,95 Further, mast cells also release serotonin, which is known to have dual functions in bone remodelling dependent upon the site of production and it has been proposed that serotonin may drive adipocyte differentiation, creating a further hypoxic microenvironment for NHO formation.96 With respect to TBI-induced NHO, the role of SP has yet to be examined. However, the release of SP following TBI is associated with increased BBB permeability, brain oedema formation, as well as increased intracranial pressure, which contributes to neuronal cell death after the initial trauma.97 Therefore, it is likely that targeting SP may affect NHO either acting locally by preventing ectopic bone formation at the peripheral injury site or by acting centrally where attenuating TBI outcomes may result in reduced NHO volume.
Altogether, these findings propose that SP does play a significant role in both NHO and HO formation. Given the essential role of SP in acute CNS injuries, further studies need to be carried out to potentially use SP as a therapeutic target and blood-based biomarker of NHO.98
Calcitonin gene related protein (CGRP)
CGRP is a sensory neuropeptide that is distributed in both the CNS99 and PNS.100 In the CNS, CGRP is expressed in cerebral cortex, hippocampus and hypothalamus,99 while in the CNS99 and PNS100 can be found in sensory, motor neurons and often colocalizes with SP.100 The upregulation of CGRP following TBI is also thought to contribute to neurogenic inflammation.85 While the precise role of CGRP in the development of NHO remains to be elucidated, in a mouse model of SCI-induced NHO significantly elevated levels of CGRP were found within the injured muscle 14 days post injury.101 In vitro, CGRP was found to promote the differentiation of fibroadipogenic progenitor cells to chondrocytes.101 Interestingly, studies in rodents have revealed that TBI,102–104 or SCI104 concomitant with fracture elevates CGRP serum levels, with these animals having accelerated bone healing. This suggests that increased expression of CGRP following CNS trauma and peripheral injury may contribute to heterotopic bone formation by triggering neurogenic inflammation; however, further studies examining the relationship between SP, CGRP and NHO are required to determine its exact mechanism.
Other neuropeptides
Neurotrophins such as nerve growth factor (NGF), neurotrophin-3 (NT-3), neutrophin-4 (NT-4), and brain-derived neurotrophic factor have all been associated with alterations in bone metabolism. NGF is responsible for the growth and maintenance of neuronal and non-neuronal cells in both PNS and CNS.105 However, a growing body of evidence suggests that NGF and NT-3 may play a role in skeletal development,106 fracture healing107–109 and HO.110 For example, in a rat model of HO which features bilateral midpoint Achilles tenotomy, mRNA expression of NT-3 was significantly (100-fold), while NGF levels were more modestly increased (<20-fold) from 4 weeks to 12-week post-tenotomy.110 The role that these neuropeptides play in NHO is yet to be reported in the literature.
Disrupted neural signalling
There is increasing recognition that bone modelling and remodelling can be regulated by the CNS, with hypothalamic leptin signalling being a key regulator of bone remodelling.111 Although the precise mechanisms are unclear, it has been theorised that central regulation of bone formation occurs via activation of efferent pathways relayed via the brainstem.111,112 As such, damage or alterations in excitability of these neural pathways following TBI or SCI may also be a contributor to NHO formation. Supporting this hypothesis, ventromedial hypothalamic neurons have been identified as playing a key role in bone formation, with chemical lesioning of these neurons resulting in a high bone mass phenotype in mice.113 This was thought to occur via ablation of leptin receptors which are densely populated in this region, thus inhibiting the osteogenic effect of leptin.113 Indeed, future studies are required to elucidate the role of efferent signalling on NHO, as well as the effect that lesions to different structures of the brain have on TBI-induced NHO.
Treatments for NHO
In this section, an overview of existing therapeutic interventions for NHO will be provided. These approaches are summarised in Fig. 2. These strategies include surgical resection of completely mineralised ectopic bone, radiotherapy, non-steroidal anti-inflammatory drugs (NSAIDs) and bisphosphonates. In addition, important considerations for these treatments in the context of polytrauma involving CNS injury are discussed.
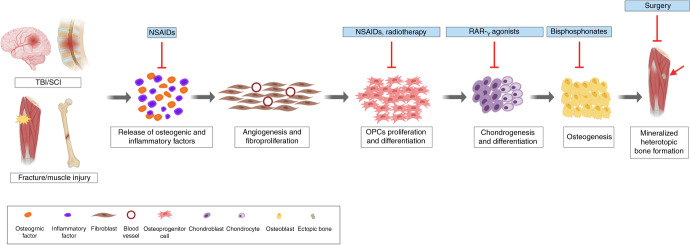
Fig. 2.
Current treatments targeting specific pathways of NHO. NHO development is triggered by a cascade of inflammatory factors. Presently, the preferred prophylactic treatment for NHO/HO involves NSAIDs (e.g. Indomethacin) to downregulate the inflammatory response and prevent OPC differentiation. Radiotherapy is thought to prevent the formation and development of ectopic bone specifically by inhibiting the differentiation of OPCs. RAR-γ agonists have been shown to prevent chondrogenesis and therefore subsequent mineralisation. While, nitrogen-containing bisphosphonates (e.g. sodium etidronate) have been used to inhibit mineralisation, and the formation of ectopic bone. Finally, when bone is completely mineralised, surgical resection is the only remaining intervention. This invasive procedure, however, is accompanied by the risk of recurrence and is associated with complications which include incomplete resection, functional and physiological impairment (created with BioRender.com)
Surgical excision
Presently, invasive surgical resection is the only effective clinical approach to cure NHO.6,7 However, it is recommended that surgery should only be considered if NHO patients fulfil the following criteria: (1) a significant reduction in range of motion (ROM) due to joint ankylosis, (2) an absence of acute inflammatory response and (3) the lesion is sufficiently mineralised (mature) to enable excision.1,114,115 However, several other factors are also to be considered when deciding the timing of surgical intervention. Previous studies have found that to reduce the risk of recurrence, surgical excision is preferred after ectopic bone has fully mineralised.10,116,117 Following SCI and TBI, resection was traditionally performed >12–18 months-post injury.1,118 Over the past decade there has been a shift in the clinical management of NHO to favour earlier resection i.e. surgery is performed as soon as the patient is stable enough to undergo surgery and the lesion is sufficiently mineralised to enable resection.8,119–121 These changes were based on findings that earlier resection of NHO-lesions did not in fact increase the risk of recurrence.8,119–121 Further, recent evidence suggests that early excision may reduce the risk of operative complications (e.g. peri-operative fracture), enhance bone and articular cartilage health, and reduce negative cerebral changes (e.g. atrophy of motor areas) that further inhibit ROM (see Table 2).11,118,122
Table 2.
Treatments for clinical and radiographical evidence of HO/NHO reported across literature
| Author | Description (total number of patients, age range, mean age, gender, number of patients with NHO) | Injury and imaging modality used to confirm NHO | Location of NHO and treatment/s | Reported recurrence and complications |
|---|---|---|---|---|
| Surgical resection | ||||
| Meiners et al.174 |
29 SCI-NHO patients, 27–68.13 years, mean: 37.87 years, 28 M, 1 F 41 lesions Cervical lesions of spinal cord: 10 patients Thoracic spine lesions: 19 patients |
SCI X-ray |
Hip Dose: average: 9.17 Gy, range: 0.7–12 Gy in 1–5 sessions Mean follow-up: 4.2 years Mean time to surgery: 82.1 months (17–298 months) Indications for surgery: seating problems, loss of functions, pressure sore, pain Preoperative ROM: 21.95° (range: 0–80°) Postoperative ROM: 82.68° (range: 0–120°) |
Recurrence: 3 patients Complications: deep and superficial wound infections, fracture, aneurysm and pressure ulcer |
| Hunt et al.175 |
42 burns patients, 22–62 years, mean: 38 years 42 burn-HO patients, 22–62 years, mean: 38 years 47 lesions Mean TBSA: 55% Mean third degree burn: 37% Average ventilator support: 58 days |
Burn injuries X-ray |
Hip, elbow, forearm Indications for surgery: decreased ROM resulting in loss of functions in daily activities, ulnar nerve entrapment, inability to perform physical therapy Preoperative ROM: 52° Postoperative ROM: 119° |
Recurrence in 6 elbows, 1 hip and 1 forearm Complications: ulnar nerve deficit, numbness weakness, small haematoma, minor wound dehiscence and cellulitis. |
| Radiation therapy | ||||
| Hamid et al.134 |
45 patients with elbow trauma, 18–65 years, mean: 44 years, 25 M, 20 F 20 elbow trauma-HO patients |
Intraarticular distal humeral fracture, Fracture-dislocation with proximal radial and/or ulnar fracture X-ray, CT scan |
Elbow Dose: 700 cGy single fraction dose at 6-MeV photons), N = 21 Mean follow-up: 7.5 months (range: 6–26 months) Mean time to treatment: 72 h Indications for treatment: seating problems, loss of functions, decubitus, pressure sore, pain Preoperative ROM: 21.95° (range: 0–80°) Postoperative ROM: 82.68° (range: 0–120°) |
Trial was terminated early due to high non-union rate observed in the radiation treatment group Recurrence: 0 Complications: infection (2), non-union (8) |
| Stein et al.176 |
11 patients with elbow trauma, 28–78 years, mean: 51 years, 3 M, 8 F 3 elbow trauma-HO patients, 54–78, mean: 63 years, 1 M, 2 F 3 lesions |
Fracture/dislocation of the elbow Radiographs |
11 patients Dose: 700 cGy single non-fractionated at unreported MeV Mean follow-up: 12 months (range: 9–24 months) Mean time to treatment: 5 days (range: 0–16 days) Indications for treatment: NR Preoperative ROM: NR Postoperative ROM: 114.5° (range: 0–135°) |
Recurrence: 0 Complications: decreased sensation along ulnar nerve |
| Müseler et al.177 |
244 SCI-NHO patients, 18–81 years, mean: 46.4 years, 207 M, 37 F, 444 lesions AIS A—12 patients (4 tetraplegic 8 paraplegic) AIS B—1 patient (1 tetraplegic) |
SCI CT scan or MRI |
Radiation therapy (7 Gy, single dose accompanied by 15MV or 6MV) Mean follow-up: 89.4 days Mean time to treatment: 3.7 days Indications for treatment: NR |
Recurrence: 13 patients (26 joints) Complications: NR |
| Cipriano et al.178 |
60 NHO-patients, mean: 36.7 years, 47 M, 13 F 72 lesions |
TBI, SCI, TBI + SCI, TBI + local trauma |
30 patients Dose: 700 cGy dose of radiation Mean follow-up: 12.7 months (range: 6–33 months) Mean time to treatment: 1.18 days (range: 0–4 days) Indications for treatment: limited ROM, nerve impingement, reduced quality of life and functions Preoperative ROM: Postoperative ROM: Hip—4.23°, Hip—67.2° Knees—81.3°, Knees—117.5° Elbows—4.0°, Elbows—140.0° |
Recurrence: 6 joints Complications: NR |
| NSAIDs | ||||
| Banovac et al.138 |
33 SCI-NHO patients AIS A—13 (5 tetraplegics, 7 paraplegic) AIS B—1 (1 tetraplegic) AIS C—2 (2 paraplegic) AIS D—1 (1 tetraplegic) |
SCI Bone scintigraphy (early stage) X-ray (later stage) |
16 patients Oral indomethacin 75 mg daily, IV disodium etidronate, 300 mg daily for 3 days, oral etidronate, 20 mg·kg−1 per day for 6 months Mean follow-up: 1.5 months Mean time to treatment: 21 days Indications for treatment: local erythema, swelling, loss of joint ROM and fever |
Recurrence in 2 patients Complications: upper abdominal discomfort |
| Banovac et al.179 |
76 SCI patients, 65 M, 11 F AIS A—28 patients AIS B—8 patients AIS C—1 patient |
SCI Bone scintigraphy, radiograph |
37 patients Oral rofecoxib 25 mg daily, IV disodium etidronate 300 mg daily for 3 days, oral etidronate, 20 mg·kg−1 per day for 6 months Mean time to treatment: 25 days Indications for treatment: local oedema, fever and decreased joint ROM |
Recurrence in 5/37 patients Complications: NR |
| Romano et al.180 |
400 THA patients, mean: 61.2 years 24 excluded (due to side effects) |
Coxarthrosis, femoral head necrosis Radiograph |
250 patients Rectal indomethacin 50 mg daily for 2 days, a day post-surgery followed by oral indomethacin 50 mg daily for 18 days 150 patients Celecoxib 200 mg daily for 2 days, starting 2 days post-surgery for 20 days Mean follow-up: 12 months Mean time to treatment: 1 and 2 days respectively |
Indomethacin: 40 patients, Celecoxib: 21 patients Complications: (Indomethacin) gastrointestinal side effects, excessive bleeding, mental confusion (Celecoxib), nausea and gastrointestinal pyrosis |
| Schmidt et al.181 | 201 THA patients, 28–89, mean: 67.5 years |
Total hip replacement Radiograph |
102 patients Oral indomethacin 25 mg, thrice daily, for 6 weeks, starting on first postoperative day Mean follow-up: 12 days Mean time to treatment: 1 day |
Recurrence in 13 patients Complications: NR |
| Bedi et al.182 |
616 patients after hip arthroscopy, mean: 31.3 years, 342 M, 274 F 29 HO patients, 15–57 years, mean: 30.6 years, 21 M, 8 F |
Hip arthroscopy Radiographs, CT scan |
277 patients Naproxen (500 mg, twice daily for 30 days, starting a day post-surgery) 339 patients Indomethacin 75 mg daily for 4 days, Naproxen 500 mg, twice daily for 30 days Mean follow-up: 13.2 months (range: 2.9–16.5 months) Mean time to treatment: 1 day 7 patients HO surgical excision, radiation therapy 700 cGy, single dose Mean time to treatment: 11.6 months (range: 5.2–16.2 months) |
Naproxen only: 23 patients have HO Naproxen + Indomethacin: 6 patients Complications: NR |
| Beckmann et al.183 |
106 patients after hip arthroscopy, mean: 35 years, 40 M, 66 F Excluded from study: n = 10 |
Hip arthroscopy Radiographs |
52 patients Naproxen 500 mg, twice daily for 3 weeks, post-surgery Mean time to treatment: 1 day Indications for treatment: pain, radiographic abnormalities and evidence of labral tear on MRI |
Recurrence: 2 Complications: Gastrointestinal discomfort, rash, blood clot, heartburn, headache and pain |
| Neal et al.184 |
2 649 THA patients, mean: 65.5 years, 1 311 M, 1 338 F 601 excluded 627 lesions |
Hip arthroplasty Radiograph |
1 039 patients Aspirin 162 mg·d−1 for 35 days post-surgery Mean follow-up: 22 months |
Recurrence: 627 patients Complications: hip pain (with the need for analgesia), difficulty or restriction of mobility |
| Bisphosphonates | ||||
| Schuetz et al.185 |
7 patients in total, 47–68 years, mean: 54.8 years, 7 M 5 patients with HO, 47–68 years, mean: 54.8 years, 5 M Number of lesions: 8 |
Caisson disease, tetraplegia, e.coli sepsis, osteoarthritis, FOP Radiographs |
IV pamidronate 680 mg/850 mg/1200 mg Mean follow-up: 19.6 months (range: 4–54 months) Indications for treatment: pain, hardening at operation site and decreased ROM |
Recurrence in 1 patient Complications: need for pain medication, lower back pain |
| Orzel and Rudd173 |
50 patients 43 NHO patients, 18–56 years, 30 M, 13 F 81 lesions |
SCI paraplegia, closed head injury, peripheral trauma, cerebral vascular insult, burn Bone scintigraphy |
14 patients Oral etidronate disodium 20 mg·kg−1 for first 2 weeks followed by 10 mg·kg−1 for remainder of study Mean follow-up: 22.5 months Indications for treatment: Radiograph evidence |
No response to therapy in 4/14 Complications: NR |
| Banovac154 |
40 SCI-NHO patients, mean: 23 years, 39 M, 1 F AIS A—37 patients (16 are tetraplegic, 21 are paraplegic) AIS B—3 patients (2 are tetraplegic, 1 is paraplegic) |
SCI Radiograph and bone scintigraphy |
40 patients IV etidronate sodium 300 mg, 3 doses for 3 days followed by oral etidronate sodium 20 mg·kg−1 per day for 6 months Mean follow-up: 35 months Indications for treatment: oedema, reduced ROM, fever, positive scintigraphy |
Recurrence in 2 patients, Complications: NR |
| Banovac et al.152 |
27 SCI patients, 16–54 years, mean: 36 years, 25 M, 2 F 11 SCI-NHO patients 3 excluded |
SCI Bone scintigraphy |
24 patients IV etidronate disodium (300 mg for 3 h, 3 doses for 3 days/5 days) followed by oral etidronate sodium (20 mg·kg−1 per day for 6 months) Indications for treatment: acute swelling, reduced ROM, increased body temperature, laboratory test (increased serum alkaline phosphatase, accelerated erythrocyte sedimentation rate) and positive bone scintigraphy |
Recurrence: 11 patients |
| Garland et al.151 |
75 SCI-patients 14 SCI-NHO patients, 17–30 years, mean: 25 years 5 excluded 14 lesions |
SCI Radiograph, bone scintigraphy |
9 patients Sodium etidronate 20 mg·kg−1 per day for 2 weeks followed by 10 mg·kg−1 per day for 2 years Mean follow-up: 14 months (range: 5–19 months) Mean time to treatment: 26.7 days (range: 0–55 days) Indications for treatment: swelling, reduced ROM |
Recurrence: none Complications: none |
F female, M male, NR not reported, TBI traumatic brain injury, SCI spinal cord injury, NHO neurological heterotopic ossification, ROM range of motion, TBSA total body surface area (%), THA total hip arthroplasty, FOP fibrodysplasia ossificans progressive
Resection has however been associated with a number of complications. For example, complete excision of periarticular NHO is particularly difficult, with patients often left with persistent decreases in ROM. Lesion remnants can result in both functional and physiological impairment due to impingement of neurovascular bundles, ankylosis, and pain.10,118,123–125 Like any other invasive procedures, post- and intra-operative NHO excision is associated with potential blood loss and infection—both complications that may have a substantial effect on TBI or SCI recovery. Moreover, surgical removal can damage adjacent peripheral tissues, and recurrence at the site of excision is common.123,126–129 As such, surgical intervention is not an optimal NHO therapy and should be carefully considered.
Radiation therapy
Radiation therapy is thought to prevent the formation and/or progression of HO by inhibiting the differentiation of OPCs.114 Specifically, in vitro studies have demonstrated that radiotherapy inhibits BMP-2 signalling, reduces osteoblastic proliferation and differentiation, and promotes apoptosis.130,131 In an initial pre-clinical study, adult rats implanted with de-mineralised bone matrix were administered radiation at 2-, 4-, 6-, 8-, 10- and 12 days post-implantation.132 Implanted rats went on to form ectopic bone at 11 days post-implantation.132 It was noted that rats that underwent radiation at 2- or 4 days post-implantation had reduced HO volume by ~60% and 24% respectively. However, when radiation was delayed until 8 days post-implantation, the authors observed no difference in HO volume between rats that were irradiated and controls.132 Several studies have reported beneficial effects of administering radiotherapy to prevent NHO post-TBI and SCI, or to prevent the recurrence of NHO (i.e. post-excision; see Table 2).133–135 For example, in a phase I/II clinical study, 33 SCI patients that underwent radiotherapy observed no further ectopic bone growth, however joint mobility was mildly affected in three patients.133 In some cases, the risk of impaired fracture healing can be prevented in radiotherapy by adequately shielding the surrounding areas of interest; however, this can be difficult when ectopic bone forms close to fractures and around amputation sites. In one particular clinical study where patients with elbow injuries underwent radiation therapy, eight of the 21 patients experienced fracture non-union, whereas for the 24 patients that did not undergo radiotherapy, only one experienced non-union.134 In addition to fracture healing, radiation therapy can also disrupt wound healing, and has been associated with an increased risk of malignancy.136 Therefore, the use of radiotherapy in polytrauma NHO patients is often contraindicated.137
Non-steroidal anti-inflammatory drugs (NSAIDs)
NSAIDs have been successfully used to prevent ectopic bone formation following SCI and hip arthroplasty (see Table 2). While NSAIDs such as celecoxib and meloxicam have been used to prevent NHO, indomethacin, a non-selective COX-1 and COX-2 inhibitor, is currently considered the gold standard for preventing NHO formation and progression.118,138 The effect of indomethacin has been demonstrated in a rat model of HO that features subcutaneous implantation of de-mineralised bone matrix.139 When indomethacin was administered 6 h prior to the implantation, there was a reduction in area of ectopic bone, ALP activity, and calcium content when compared to controls.139 However, when indomethacin was administered at the time of de-mineralised matrix implantation or post-implantation (6 h, 1 d, 2 d, 3 d, and 4 d), there were no differences in ectopic bone area, ALP activity, and calcium content when compared to controls.139
Despite the proven efficacy of NSAIDs for treating NHO, it is important to recognise that there are reports of potentially negative adverse effects of these agents on patients with polytrauma. For example, rofecoxib, a highly selective COX-2 inhibitor was frequently prescribed to prevent HO.140 However, it was withdrawn from the market following a randomised, placebo-controlled, double-blind clinical trial that found chronic use elevated the risk of serious cardiovascular events (i.e. heart attack and stroke) in patients taking it to prevent the recurrence of colorectal polyps.140 Furthermore, evidence suggests that indomethacin may interfere with fracture healing.141,142 A study in rats reported that indomethacin treatment diminished mechanical properties of femoral fracture calluses.141 This finding suggests that indomethacin treatment is problematic, particularly in patients with concomitant fracture. In addition, ibuprofen, a commonly prescribed NSAID, may worsen cognitive outcome after severe TBI in rats.143 Further, rats given a TBI and administered celecoxib, a COX-2 inhibitor often administered to HO patients, had worse motor performance.144 In some circumstances COX-1 inhibitors have been associated with an increased prevalence of gastrointestinal side effects such as bleeding and perforations.145,146 NSAIDs also have a limited therapeutic window and are only effective in the early stages of HO development, prior to the formation of ectopic bone (Fig. 2). Once bone deposition has occurred, NSAIDs are ineffective, hence surgical intervention remains the only option. Overall, despite the efficacy of NSAIDs in preventing/reducing ectopic bone formation, these findings support the notion that careful consideration must be taken before administering NSAIDs to polytrauma patients.
Bisphosphonates
Bisphosphonates are commonly used to treat bone disorders such as osteopenia, osteoporosis, and Paget’s disease by reducing osteoclastic bone resorption.147 Nitrogen-containing bisphosphonates, such as risedronate, sodium etidronate, and alendronate, are often prescribed as prophylaxis due to their ability to effectively prevent mineralisation.148 In vitro, treatment of osteoblasts with nitrogen-containing bisphosphonates, pamidronate and alendronate, at doses of 10−4–10−5 mol·L−1 (therapeutic doses range in humans is between 10−5 and 10−9 mol·L−1) results in osteoblastic apoptosis directly, and/or indirectly via osteoblast cell cycle arrest and cell proliferation inhibition.149,150 These findings were not observed when cells were treated with non-nitrogen-containing bisphosphonates.150 The use of nitrogen-containing etidronate in ectopic bone formation has been well documented.151–154 However, some studies do not recommend sodium etidronate as treatment for NHO.151,153 As bisphosphonates suppress bone resorption and accumulate in the body for an extended period of time, adverse effects associated with high doses include amassed bone microdamage which is frequently observed with old age, hence further contributing to increased skeletal fragility.155,156 In addition, due to the potentially negative impacts on skeletal fragility combined with the high cost of bisphosphonates, it would be beneficial to identify those patients who are at risk of developing NHO, and treat only them with bisphosphonates.157 It is also unknown how bisphosphonates affect TBI and SCI outcomes, which should be considered in NHO patients.
Retinoic acid receptor agonist
Retinoic acid receptors (RAR) are mediators of skeletal development via the Smad complex, that plays an integral role in chondrogenesis.158 An RAR-γ agonist, palovarotene, has shown to be effective in preventing the initial stages of NHO.159 The authors developed a model that mimics the injury combinations and bioburden that occurs in blast related combat injuries.159 Palovarotene significantly decreased NHO by inhibiting the expansion as well as differentiation of OPCs into chondrocytes.159 Further, palovarotene treatment was found to downregulate mRNA expression of chondrocytic (SOX-9 and collagen2α1) and osteoblastic (OC, OPN, BMP-2, BMP-4, POU5FL and RUNX2) genes Fig. 3.159–161
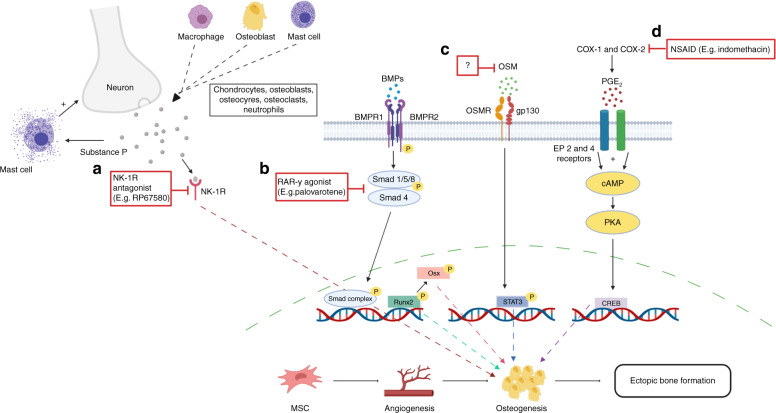
Fig. 3.
Key pathways/mechanisms implicated in the development of NHO highlighting potential and existing therapeutic targets to mitigate NHO. a Substance P receptor (NK-1R) antagonists (e.g. RP67580) have been found to reduce NHO volume in murine models. b Downstream of the BMP pathway, RAR-γ agonists such as palovarotene have been reported to prevent early stages of NHO development by disrupting OPC differentiation, chondrogenesis and osteogenesis by downregulating mRNA expression of SOX-9 and RUNX2. c OSM is a potential therapeutic target and may serve as a biomarker for NHO. Blocking OSM has been found to reduce NHO likely by inhibiting downstream transcription factor, STAT3, which is known to trigger bone formation. d NSAID, indomethacin is currently the preferred prophylaxis for NHO/HO. It targets COX-1 and COX-2 non-selectively, inhibiting the production of prostaglandins and osteogenesis (created with BioRender.com)
RAR-γ activation, however has been shown to delay growth plate development. Therefore, several studies have warned that precautions should be taken when administering an RAR-γ agonist to children.160,162 Whereas in adults, it was proposed that RAR-γ treatment could be given intermittently, henceforth providing sufficient recovery time for the growth plate.160,162 Furthermore, as palovarotene has also been shown to inhibit fracture healing care should be taken when administering to patients with healing fractures.162–164 Nonetheless, activation of RAR-γ still shows promising results as a novel therapeutic agent in preventing progression of NHO.
Conclusion
The development of NHO is relatively common after TBI and SCI, and typically occurs in the presence of concomitant significant peripheral musculoskeletal injuries. The majority of NHO cases are diagnosed following extensive mineralisation, at which stage patients are likely to experience considerable pain and disruption to daily functional activities. Current treatments are limited in effectiveness and not always suitable for NHO patients, and there are no reliable prognostic biomarkers to identify patients at high risk of developing NHO to guide preventative interventions. Fortunately, there are numerous promising avenues for future research to identify new underlying pathophysiological mechanisms related to NHO, prognostic biomarkers, and prophylactic therapies that are suitable for complex trauma patients with CNS injuries. These future studies would benefit from a complementary translational approach that incorporates improved clinically relevant animal models in parallel with more rigorous clinical investigations. In doing so, there is the strong potential to develop biomarkers and prophylactic strategies to improve NHO patient outcomes.
Acknowledgements
R.B. is supported by a grant from NINDS (NINDS RFA-NS-16-012) to T.O.B. and S.S. S.S. is supported by a fellowship from NHMRC.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garland, D. E. A clinical perspective on common forms of acquired heterotopic ossification. Clin. Orthop. Relat. Res.263, 13–29 (1991). [PubMed]
- 2.Hoyt BW, Pavey GJ, Potter BK, Forsberg JA. Heterotopic ossification and lessons learned from fifteen years at war: a review of therapy, novel research, and future directions for military and civilian orthopaedic trauma. Bone. 2018;109:3–11. doi: 10.1016/j.bone.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Citta-Pietrolungo TJ, Alexander MA, Steg NL. Early detection of heterotopic ossification in young patients with traumatic brain injury. Arch. Phys. Med. Rehabil. 1992;73:258–262. [PubMed] [Google Scholar]
- 4.Mavrogenis AF, Soucacos PN, Papagelopoulos PJ. Heterotopic ossification revisited. Orthopedics. 2011;34:177. doi: 10.3928/01477447-20110124-08. [DOI] [PubMed] [Google Scholar]
- 5.Adiguzel E, et al. Knee pain relief with genicular nerve blockage in two brain injured patients with heterotopic ossification. Brain Inj. 2015;29:1736–1739. doi: 10.3109/02699052.2015.1075171. [DOI] [PubMed] [Google Scholar]
- 6.Brady RD, Shultz SR, McDonald SJ, O’Brien TJ. Neurological heterotopic ossification: current understanding and future directions. Bone. 2018;109:35–42. doi: 10.1016/j.bone.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Davis EL, Davis AR, Gugala Z, Olmsted-Davis EA. Is heterotopic ossification getting nervous?: the role of the peripheral nervous system in heterotopic ossification. Bone. 2018;109:22–27. doi: 10.1016/j.bone.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genêt F, et al. Troublesome heterotopic ossification after central nervous system damage: a survey of 570 surgeries. PLoS ONE. 2011;6:e16632. doi: 10.1371/journal.pone.0016632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maheswarappa B, Nair K, Taly A, Shanthi S, Murali T. Heterotopic ossification at unusual site in traumatic brain injury. IJPMR. 2004;15:34–37. [Google Scholar]
- 10.Cipriano CA, Pill SG, Keenan MA. Heterotopic ossification following traumatic brain injury and spinal cord injury. J. Am. Acad. Orthop. Surg. 2009;17:689–697. doi: 10.5435/00124635-200911000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Nauth A, et al. Heterotopic ossification in orthopaedic trauma. J. Orthop. Trauma. 2012;26:684–688. doi: 10.1097/BOT.0b013e3182724624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genêt F, et al. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage‐mediated inflammation in muscle. J. Pathol. 2015;236:229–240. doi: 10.1002/path.4519. [DOI] [PubMed] [Google Scholar]
- 13.Jodoin M, et al. Investigating the incidence and magnitude of heterotopic ossification with and without joints involvement in patients with a limb fracture and mild traumatic brain injury. Bone Rep. 2019;11:100222. doi: 10.1016/j.bonr.2019.100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salga M, et al. Sciatic nerve compression by neurogenic heterotopic ossification: use of CT to determine surgical indications. Skelet. Radiol. 2015;44:233–240. doi: 10.1007/s00256-014-2003-6. [DOI] [PubMed] [Google Scholar]
- 15.Frost RB, Farrer TJ, Primosch M, Hedges DW. Prevalence of traumatic brain injury in the general adult population: a meta-analysis. Neuroepidemiology. 2013;40:154–159. doi: 10.1159/000343275. [DOI] [PubMed] [Google Scholar]
- 16.Ranganathan K, et al. Role of gender in burn-induced heterotopic ossification and mesenchymal cell osteogenic differentiation. Plast. Reconstr. Surg. 2015;135:1631–1641. doi: 10.1097/PRS.0000000000001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larson JM, et al. Increased prevalence of HLA-B27 in patients with ectopic ossification following traumatic spinal cord injury. Rheumatology. 1981;20:193–197. doi: 10.1093/rheumatology/20.4.193. [DOI] [PubMed] [Google Scholar]
- 18.Hunter T, Dubo H, Hildahl C, Smith N, Schroeder M. Histocompatibility antigens in patients with spinal cord injury or cerebral damage complicated by heterotopic ossification. Rheumatology. 1980;19:97–99. doi: 10.1093/rheumatology/19.2.97. [DOI] [PubMed] [Google Scholar]
- 19.Sakellariou V, Grigoriou E, Mavrogenis A, Soucacos P, Papagelopoulos P. Heterotopic ossification following traumatic brain injury and spinal cord injury: insight into the etiology and pathophysiology. J. Musculoskelet. Neuronal Interact. 2012;12:230–240. [PubMed] [Google Scholar]
- 20.Dizdar D, et al. Risk factors for developing heterotopic ossification in patients with traumatic brain injury. Brain Inj. 2013;27:807–811. doi: 10.3109/02699052.2013.775490. [DOI] [PubMed] [Google Scholar]
- 21.Simonsen LL, Sonne-Holm S, Krasheninnikoff M, Engberg AW. Symptomatic heterotopic ossification after very severe traumatic brain injury in 114 patients: incidence and risk factors. Injury. 2007;38:1146–1150. doi: 10.1016/j.injury.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Van Kampen P, Martina J, Vos P, Hoedemaekers C, Hendricks H. Potential risk factors for developing heterotopic ossification in patients with severe traumatic brain injury. J. Head Trauma Rehabil. 2011;26:384–391. doi: 10.1097/HTR.0b013e3181f78a59. [DOI] [PubMed] [Google Scholar]
- 23.Forsberg, J. A. & Potter, B. K. Heterotopic Ossification in Wartime Wounds. J. Surg. Orthop. Adv. Spring.19, 54–61 (2010). [PubMed]
- 24.Taber KH, Warden DL, Hurley RA. Blast-related traumatic brain injury: what is known? J. Neuropsychiatry Clin. Neurosci. 2006;18:141–145. doi: 10.1176/jnp.2006.18.2.141. [DOI] [PubMed] [Google Scholar]
- 25.Rosenfeld JV, et al. Blast-related traumatic brain injury. Lancet Neurol. 2013;12:882–893. doi: 10.1016/S1474-4422(13)70161-3. [DOI] [PubMed] [Google Scholar]
- 26.Forsberg JA, et al. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J. Bone Jt. Surg. Am. 2009;91:1084–1091. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- 27.Clark ME, Scholten JD, Walker RL, Gironda RJ. Assessment and treatment of pain associated with combat-related polytrauma. Pain Med. 2009;10:456–469. doi: 10.1111/j.1526-4637.2009.00589.x. [DOI] [PubMed] [Google Scholar]
- 28.Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 2019;10:282. doi: 10.3389/fneur.2019.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith E, Comiskey C, Carroll A. Prevalence of and risk factors for osteoporosis in adults with acquired brain injury. Ir. J. Med. Sci. 2016;185:473–481. doi: 10.1007/s11845-016-1399-5. [DOI] [PubMed] [Google Scholar]
- 30.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 31.Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76:886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Alves JL. Blood-brain barrier and traumatic brain injury. J. Neurosci. Res. 2014;92:141–147. doi: 10.1002/jnr.23300. [DOI] [PubMed] [Google Scholar]
- 33.Stamatovic SM, Keep RF, Andjelkovic AV. Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Curr. Neuropharmacol. 2008;6:179–192. doi: 10.2174/157015908785777210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson EH, Weninger W, Hunter CA. Trafficking of immune cells in the central nervous system. J. Clin. Investig. 2010;120:1368–1379. doi: 10.1172/JCI41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolburg, H., Wolburg-Buchholz, K. & Engelhardt, B. Involvement of tight junctions during transendothelial migration of mononuclear cells in experimental autoimmune encephalomyelitis. Ernst Schering Res. Found. Workshop.47,17–38 (2004). [DOI] [PubMed]
- 36.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bidner SM, Rubins IM, Desjardins JV, Zukor DJ, Goltzman D. Evidence for a humoral mechanism for enhanced osteogenesis after head injury. J. Bone Jt. Surg. Am. 1990;72:1144–1149. doi: 10.2106/00004623-199072080-00004. [DOI] [PubMed] [Google Scholar]
- 38.Gautschi OP, et al. Osteoinductive effect of cerebrospinal fluid from brain-injured patients. J. Neurotrauma. 2007;24:154–162. doi: 10.1089/neu.2006.0166. [DOI] [PubMed] [Google Scholar]
- 39.Quinn M, Agha A. Post-traumatic hypopituitarism—who should be screened, when, and how? Front. Endocrinol. 2018;9:8. doi: 10.3389/fendo.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan CL, et al. The screening and management of pituitary dysfunction following traumatic brain injury in adults: British Neurotrauma Group guidance. J. Neurol. Neurosurg. Psychiatry. 2017;88:971–981. doi: 10.1136/jnnp-2016-315500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015;22:41–50. doi: 10.1016/j.coph.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindsey RC, Mohan S. Skeletal effects of growth hormone and insulin-like growth factor-I therapy. Mol. Cell. Endocrinol. 2016;432:44–55. doi: 10.1016/j.mce.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cadosch D, et al. Humoral factors enhance fracture-healing and callus formation in patients with traumatic brain injury. J. Bone Jt. Surg. Am. 2009;91:282–288. doi: 10.2106/JBJS.G.01613. [DOI] [PubMed] [Google Scholar]
- 44.Giannoudis PV, et al. Accelerated bone healing and excessive callus formation in patients with femoral fracture and head injury. Injury. 2006;37:S18–S24. doi: 10.1016/j.injury.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 45.Perkins R, Skirving A. Callus formation and the rate of healing of femoral fractures in patients with head injuries. J. Bone Jt. Surg. Br. 1987;69:521–524. doi: 10.1302/0301-620X.69B4.3611150. [DOI] [PubMed] [Google Scholar]
- 46.Locher RJ, et al. Traumatic brain injury and bone healing: radiographic and biomechanical analyses of bone formation and stability in a combined murine trauma model. J. Musculoskelet. Neuronal Interact. 2015;15:309–315. [PMC free article] [PubMed] [Google Scholar]
- 47.Tsitsilonis S, et al. The effect of traumatic brain injury on bone healing: an experimental study in a novel in vivo animal model. Injury. 2015;46:661–665. doi: 10.1016/j.injury.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, et al. Effect of leptin on bone metabolism in rat model of traumatic brain injury and femoral fracture. Chin. J. Traumatol. 2011;14:7–13. [PubMed] [Google Scholar]
- 49.Wei Y, Wang L, Clark JC, Dass CR, Choong PF. Elevated leptin expression in a rat model of fracture and traumatic brain injury. J. Pharm. Pharmacol. 2008;60:1667–1672. doi: 10.1211/jpp.60.12.0013. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, et al. SDF-1 promotes endochondral bone repair during fracture healing at the traumatic brain injury condition. PloS ONE. 2013;8:e54077. doi: 10.1371/journal.pone.0054077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brady RD, et al. Closed head experimental traumatic brain injury increases size and bone volume of callus in mice with concomitant tibial fracture. Sci. Rep. 2016;6:34491. doi: 10.1038/srep34491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L, et al. The effects of spinal cord injury on bone healing in patients with femoral fractures. J. Spinal Cord Med. 2014;37:414–419. doi: 10.1179/2045772313Y.0000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morioka K, et al. Differential fracture response to traumatic brain injury suggests dominance of neuroinflammatory response in polytrauma. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-018-37186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brady RD, et al. Experimental traumatic brain injury induces bone loss in rats. J. Neurotrauma. 2016;33:2154–2160. doi: 10.1089/neu.2014.3836. [DOI] [PubMed] [Google Scholar]
- 55.Brady R, et al. Sodium selenate treatment mitigates reduction of bone volume following traumatic brain injury in rats. J. Musculoskelet. Neuronal Interact. 2016;16:369–376. [PMC free article] [PubMed] [Google Scholar]
- 56.Kaplan FS, Glaser DL, Hebela N, Shore EM. Heterotopic ossification. J. Am. Acad. Orthop. Surg. 2004;12:116–125. doi: 10.5435/00124635-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Shimono K, Uchibe K, Kuboki T, Iwamoto M. The pathophysiology of heterotopic ossification: current treatment considerations in dentistry. Jpn. Dent. Sci. Rev. 2014;50:1–8. doi: 10.1016/j.jdsr.2013.07.003. [DOI] [Google Scholar]
- 58.Edwards DS, Clasper J. Heterotopic ossification: a systematic review. J. R. Army Med. Corps. 2015;161:315–321. doi: 10.1136/jramc-2014-000277. [DOI] [PubMed] [Google Scholar]
- 59.Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat. Rev. Rheumatol. 2010;6:518–527. doi: 10.1038/nrrheum.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kan C, et al. Conserved signaling pathways underlying heterotopic ossification. Bone. 2018;109:43–48. doi: 10.1016/j.bone.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang C, et al. Mesenchymal VEGFA induces aberrant differentiation in heterotopic ossification. Bone Res. 2019;7:1–17. doi: 10.1038/s41413-019-0075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robins JC, et al. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone. 2005;37:313–322. doi: 10.1016/j.bone.2005.04.040. [DOI] [PubMed] [Google Scholar]
- 63.Stegen S, van Gastel N, Carmeliet G. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone. 2015;70:19–27. doi: 10.1016/j.bone.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 64.Qureshi AT, et al. Early characterization of blast-related heterotopic ossification in a rat model. Clin. Orthop. Relat. Res. 2015;473:2831–2839. doi: 10.1007/s11999-015-4240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agarwal S, et al. Diminished chondrogenesis and enhanced osteoclastogenesis in leptin-deficient diabetic mice (ob/ob) impair pathologic, trauma-induced heterotopic ossification. Stem Cells Dev. 2015;24:2864–2872. doi: 10.1089/scd.2015.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Allen, M. R. & Burr, D. B. in Basic and Applied Bone Biology (eds David, B. Burr & Matthew, R. Allen) 75–90 (Academic Press, 2014).
- 67.Chalmers J, Gray D, Rush J. Observations on the induction of bone in soft tissues. J. Bone Jt. Surg. Br. 1975;57:36–45. doi: 10.1302/0301-620X.57B1.36. [DOI] [PubMed] [Google Scholar]
- 68.Davies OG, Grover LM, Eisenstein N, Lewis MP, Liu Y. Identifying the cellular mechanisms leading to heterotopic ossification. Calcif. Tissue Int. 2015;97:432–444. doi: 10.1007/s00223-015-0034-1. [DOI] [PubMed] [Google Scholar]
- 69.Lees-Shepard JB, Goldhamer DJ. Stem cells and heterotopic ossification: lessons from animal models. Bone. 2018;109:178–186. doi: 10.1016/j.bone.2018.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foley KL, Hebela N, Keenan MA, Pignolo RJ. Histopathology of periarticular non-hereditary heterotopic ossification. Bone. 2018;109:65–70. doi: 10.1016/j.bone.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 71.Dey D, et al. The traumatic bone: trauma-induced heterotopic ossification. Transl. Res. 2017;186:95–111. doi: 10.1016/j.trsl.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meyers C, et al. Heterotopic ossification: a comprehensive review. JBMR Plus. 2019;3:e10172. doi: 10.1002/jbm4.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Łęgosz P, Drela K, Pulik Ł, Sarzyńska S, Małdyk P. Challenges of heterotopic ossification—molecular background and current treatment strategies. Clin. Exp. Pharmacol. Physiol. 2018;45:1229–1235. doi: 10.1111/1440-1681.13025. [DOI] [PubMed] [Google Scholar]
- 74.Ranganathan K, et al. Heterotopic ossification: basic-science principles and clinical correlates. J. Bone Jt. Surg. Br. 2015;97:1101–1111. doi: 10.2106/JBJS.N.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu R, Hu J, Zhou X, Yang Y. Heterotopic ossification: mechanistic insights and clinical challenges. Bone. 2018;109:134–142. doi: 10.1016/j.bone.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 76.Sorkin M, et al. Regulation of heterotopic ossification by monocytes in a mouse model of aberrant wound healing. Nat. Commun. 2020;11:1–17. doi: 10.1038/s41467-019-14172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tseng, H. W. et al. Neurogenic heterotopic ossifications develop independently of granulocyte‐colony stimulating factor and neutrophils. J. Bone Miner. Res. 1–10 (2020). [DOI] [PubMed]
- 78.Torossian, F. et al. Macrophage-derived oncostatin M contributes to human and mouse neurogenic heterotopic ossifications. JCI Insight2, e96034 (2017). [DOI] [PMC free article] [PubMed]
- 79.Suda RK, et al. Circulating osteogenic precursor cells in heterotopic bone formation. Stem Cells. 2009;27:2209–2219. doi: 10.1002/stem.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sims, N. A. & Quinn, J. M. Osteoimmunology: oncostatin M as a pleiotropic regulator of bone formation and resorption in health and disease. Bonekey Rep.3, 527 (2014). [DOI] [PMC free article] [PubMed]
- 81.Brady RD, et al. A novel rat model of heterotopic ossification after polytrauma with traumatic brain injury. Bone. 2020;133:115263. doi: 10.1016/j.bone.2020.115263. [DOI] [PubMed] [Google Scholar]
- 82.Hausmann OB. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 83.Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood–brain barrier pathophysiology in traumatic brain injury. Transl. Stroke Res. 2011;2:492–516. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. BJA Br. J. Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 85.Nimmo A, et al. Neurogenic inflammation is associated with development of edema and functional deficits following traumatic brain injury in rats. Neuropeptides. 2004;38:40–47. doi: 10.1016/j.npep.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 86.Bajwa NM, Kesavan C, Mohan S. Long-term consequences of traumatic brain injury in bone metabolism. Front. Neurol. 2018;9:115. doi: 10.3389/fneur.2018.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Payan DG. Neuropeptides and inflammation: the role of substance P. Annu. Rev. Med. 1989;40:341–352. doi: 10.1146/annurev.me.40.020189.002013. [DOI] [PubMed] [Google Scholar]
- 88.Azuma H, Kido Ji,, Ikedo D, Kataoka M, Nagata T. Substance P enhances the inhibition of osteoblastic cell differentiation induced by lipopolysaccharide from porphyromonas gingivalis. J. Periodontol. 2004;75:974–981. doi: 10.1902/jop.2004.75.7.974. [DOI] [PubMed] [Google Scholar]
- 89.Kan L, et al. Substance P signaling mediates BMP‐dependent heterotopic ossification. J. Cell. Biochem. 2011;112:2759–2772. doi: 10.1002/jcb.23259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Salisbury E, et al. Sensory nerve induced inflammation contributes to heterotopic ossification. J. Cell. Biochem. 2011;112:2748–2758. doi: 10.1002/jcb.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goto T, et al. Substance P stimulates late-stage rat osteoblastic bone formation through neurokinin-1 receptors. Neuropeptides. 2007;41:25–31. doi: 10.1016/j.npep.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 92.Sharma HS, Nyberg F, Olsson Y, Dey PK. Alteration of substance P after trauma to the spinal cord: an experimental study in the rat. Neuroscience. 1990;38:205–212. doi: 10.1016/0306-4522(90)90386-I. [DOI] [PubMed] [Google Scholar]
- 93.Donkin JJ, Turner RJ, Hassan I, Vink R. Substance P in traumatic brain injury. Prog. Brain Res. 2007;161:97–109. doi: 10.1016/S0079-6123(06)61007-8. [DOI] [PubMed] [Google Scholar]
- 94.Medici D, Olsen BR. The role of endothelial‐mesenchymal transition in heterotopic ossification. J. Bone Miner. Res. 2012;27:1619–1622. doi: 10.1002/jbmr.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reichel LM, Salisbury E, Moustoukas MJ, Davis AR, Olmsted-Davis E. Molecular mechanisms of heterotopic ossification. J. Hand Surg. 2014;39:563–566. doi: 10.1016/j.jhsa.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Salisbury, E., Sonnet, C., Heggeness, M., Davis, A. R. & Olmsted-Davis, E. Heterotopic ossification has some nerve. Crit. Rev. Eukaryot. Gene Expr. 20, 313–324 (2010). [DOI] [PMC free article] [PubMed]
- 97.Pearn ML, et al. Pathophysiology associated with traumatic brain injury: current treatments and potential novel therapeutics. Cell. Mol. Neurobiol. 2017;37:571–585. doi: 10.1007/s10571-016-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vink R, van den Heuvel C. Substance P antagonists as a therapeutic approach to improving outcome following traumatic brain injury. Neurotherapeutics. 2010;7:74–80. doi: 10.1016/j.nurt.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.González-Hernández, A. et al. Heteroreceptors modulating CGRP release at neurovascular junction: potential therapeutic implications on some vascular-related diseases. Biomed Res. Int.2016, 2056786 (2016). [DOI] [PMC free article] [PubMed]
- 100.Irie K, Hara‐Irie F, Ozawa H, Yajima T. Calcitonin gene‐related peptide (CGRP)‐containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc. Res. Tech. 2002;58:85–90. doi: 10.1002/jemt.10122. [DOI] [PubMed] [Google Scholar]
- 101.Sang X, Wang Z, Shi P, Li Y, Cheng L. CGRP accelerates the pathogenesis of neurological heterotopic ossification following spinal cord injury. Artif. Cells Nanomed. Biotechnol. 2019;47:2569–2574. doi: 10.1080/21691401.2019.1626865. [DOI] [PubMed] [Google Scholar]
- 102.Song Y, et al. Increased levels of calcitonin gene-related peptide in serum accelerate fracture healing following traumatic brain injury. Mol. Med. Rep. 2012;5:432–438. doi: 10.3892/mmr.2011.645. [DOI] [PubMed] [Google Scholar]
- 103.Song Y, et al. The role of the hippocampus and the function of calcitonin gene-related peptide in the mechanism of traumatic brain injury accelerating fracture-healing. Eur. Rev. Med. Pharmacol. Sci. 2017;21:1522–1531. [PubMed] [Google Scholar]
- 104.Zhang D, et al. The influence of brain injury or peripheral nerve injury on calcitonin gene-related peptide concentration variation and fractures healing process. Artif. Cells Blood Substit. Biotechnol. 2009;37:85–91. doi: 10.1080/10731190902743149. [DOI] [PubMed] [Google Scholar]
- 105.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 106.Tomlinson RyanE, et al. NGF-TrkA signaling by sensory nerves coordinates the vascularization and ossification of developing endochondral bone. Cell Rep. 2016;16:2723–2735. doi: 10.1016/j.celrep.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grills BL, Schuijers JA. Immunohistochemical localization of nerve growth factor in fractured and unfractured rat bone. Acta Orthop. Scand. 1998;69:415–419. doi: 10.3109/17453679808999059. [DOI] [PubMed] [Google Scholar]
- 108.Li X, Sun D, Li Y, Wu X. Neurotrophin-3 improves fracture healing in rats. Eur. Rev. Med. Pharmacol. Sci. 2018;22:2439–2446. doi: 10.26355/eurrev_201804_14837. [DOI] [PubMed] [Google Scholar]
- 109.Asaumi K, Nakanishi T, Asahara H, Inoue H, Takigawa M. Expression of neurotrophins and their receptors (TRK) during fracture healing. Bone. 2000;26:625–633. doi: 10.1016/S8756-3282(00)00281-7. [DOI] [PubMed] [Google Scholar]
- 110.Zhang, J. et al. Macrophage-derived neurotrophin-3 promotes heterotopic ossification in rats. Lab. Investig.100, 762–776 (2020). [DOI] [PubMed]
- 111.Corr A, Smith J, Baldock P. Neuronal control of bone remodeling. Toxicol. Pathol. 2017;45:894–903. doi: 10.1177/0192623317738708. [DOI] [PubMed] [Google Scholar]
- 112.Otto E, et al. Crosstalk of brain and bone—clinical observations and their molecular bases. Int. J. Mol. Sci. 2020;21:4946. doi: 10.3390/ijms21144946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takeda S, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/S0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 114.Chao ST, Joyce MJ, Suh JH. Treatment of heterotopic ossification. Orthopedics. 2007;30:457–464. doi: 10.3928/01477447-20070601-18. [DOI] [PubMed] [Google Scholar]
- 115.Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J. Nucl. Med. 2002;43:346–353. [PubMed] [Google Scholar]
- 116.Ebinger T, Roesch M, Kiefer H, Kinzl L, Schulte M. Influence of etiology in heterotopic bone formation of the hip. J. Trauma Acute Care Surg. 2000;48:1058–1062. doi: 10.1097/00005373-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 117.Subbarao JV, Garrison SJ. Heterotopic ossification: diagnosis and management current concepts and controversies. J. Spinal Cord. Med. 1999;22:273–283. doi: 10.1080/10790268.1999.11719580. [DOI] [PubMed] [Google Scholar]
- 118.Sullivan M, Torres S, Mehta S, Ahn J. Heterotopic ossification after central nervous system trauma: a current review. Bone Jt. Res. 2013;2:51–57. doi: 10.1302/2046-3758.23.2000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Almangour W, et al. Recurrence of heterotopic ossification after removal in patients with traumatic brain injury: a systematic review. Ann. Phys. Rehabil. Med. 2016;59:263–269. doi: 10.1016/j.rehab.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 120.Genet F, et al. Impact of the operative delay and the degree of neurologic sequelae on recurrence of excised heterotopic ossification in patients with traumatic brain injury. J. Head Trauma Rehabil. 2012;27:443–448. doi: 10.1097/HTR.0b013e31822b54ba. [DOI] [PubMed] [Google Scholar]
- 121.Genet F, et al. Beliefs relating to recurrence of heterotopic ossification following excision in patients with spinal cord injury: a review. Spinal Cord. 2015;53:340–344. doi: 10.1038/sc.2015.20. [DOI] [PubMed] [Google Scholar]
- 122.Genêt F, et al. Impact of late surgical intervention on heterotopic ossification of the hip after traumatic neurological injury. J. Bone Jt. Surg. Br. 2009;91:1493–1498. doi: 10.1302/0301-620X.91B11.22305. [DOI] [PubMed] [Google Scholar]
- 123.Agarwal S, et al. Surgical excision of heterotopic ossification leads to re-emergence of mesenchymal stem cell populations responsible for recurrence. Stem Cells Transl. Med. 2017;6:799–806. doi: 10.5966/sctm.2015-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Melamed E, et al. Brain injury-related heterotopic bone formation: treatment strategy and results. Am. J. Phys. Med. Rehabil. 2002;81:670–674. doi: 10.1097/00002060-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 125.van Kuijk AA, Geurts ACH, van Kuppevelt HJM. Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord. 2002;40:313–326. doi: 10.1038/sj.sc.3101309. [DOI] [PubMed] [Google Scholar]
- 126.Pavey GJ, et al. What risk factors predict recurrence of heterotopic ossification after excision in combat-related amputations? Clin. Orthop. Relat. Res. 2015;473:2814–2824. doi: 10.1007/s11999-015-4266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barfield WR, Holmes RE, Hartsock LA. Heterotopic ossification in trauma. Orthop. Clin. 2017;48:35–46. doi: 10.1016/j.ocl.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 128.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J. Bone Jt. Surg. Am. 2007;89:476–486. doi: 10.2106/00004623-200703000-00003. [DOI] [PubMed] [Google Scholar]
- 129.Tsionos I, Leclercq C, Rochet JM. Heterotopic ossification of the elbow in patients with burns. J. Bone Jt. Surg. Br. 2004;86-B:396–403. doi: 10.1302/0301-620X.86B3.14480. [DOI] [PubMed] [Google Scholar]
- 130.Pohl F, et al. Radiation-induced suppression of the Bmp2 signal transduction pathway in the pluripotent mesenchymal cell line C2C12: an in vitro model for prevention of heterotopic ossification by radiotherapy. Radiat. Res. 2003;159:345–350. doi: 10.1667/0033-7587(2003)159[0345:RISOTB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 131.Szymczyk K, Shapiro I, Adams CS. Ionizing radiation sensitizes bone cells to apoptosis. Bone. 2004;34:148–156. doi: 10.1016/j.bone.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 132.Craven PL, URIsT MR. Osteogenesis by radioisotope labelled cell populations in implants of bone matrix under the influence of ionizing radiation. Clin. Orthop. Relat. Res. 1971;76:231–243. doi: 10.1097/00003086-197105000-00030. [DOI] [PubMed] [Google Scholar]
- 133.Sautter-Bihl M, Liebermeister E, Nanassy A. Radiotherapy as a local treatment option for heterotopic ossifications in patients with spinal cord injury. Spinal Cord. 2000;38:33–36. doi: 10.1038/sj.sc.3100847. [DOI] [PubMed] [Google Scholar]
- 134.Hamid N, et al. Radiation therapy for heterotopic ossification prophylaxis acutely after elbow trauma: a prospective randomized study. J. Bone Jt. Surg. Am. 2010;92:2032–2038. doi: 10.2106/JBJS.I.01435. [DOI] [PubMed] [Google Scholar]
- 135.Burd TA, Lowry KJ, Anglen JO. Indomethacin compared with localized irradiation for the prevention of heterotopic ossification following surgical treatment of acetabular fractures. J. Bone Jt. Surg. Am. 2001;83:1783–1788. doi: 10.2106/00004623-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 136.Haubner F, Ohmann E, Pohl F, Strutz J, Gassner HG. Wound healing after radiation therapy: review of the literature. Radiat. Oncol. 2012;7:162. doi: 10.1186/1748-717X-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Farris MK, Chowdhry VK, Lemke S, Kilpatrick M, Lacombe M. Osteosarcoma following single fraction radiation prophylaxis for heterotopic ossification. Radiat. Oncol. 2012;7:1–6. doi: 10.1186/1748-717X-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Banovac K, Williams JM, Patrick LD, Haniff YM. Prevention of heterotopic ossification after spinal cord injury with indomethacin. Spinal Cord. 2001;39:370–374. doi: 10.1038/sj.sc.3101166. [DOI] [PubMed] [Google Scholar]
- 139.DiCesare PE, Nimni ME, Peng L, Yazdi M, Cheung DT. Effects of indomethacin on demineralized bone‐induced heterotopic ossification in the rat. J. Orthop. Res. 1991;9:855–861. doi: 10.1002/jor.1100090611. [DOI] [PubMed] [Google Scholar]
- 140.Sibbald B. Drug Regulation: Rofecoxib (Vioxx) voluntarily withdrawn from market. Can. Med. Assoc. J. 2004;171:1027–1028. doi: 10.1503/cmaj.1041606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rø J, Sudmann E, Marton PF. Effect of indomethacin on fracture healing in rats. Acta Orthop. Scand. 1976;47:588–599. doi: 10.3109/17453677608988744. [DOI] [PubMed] [Google Scholar]
- 142.Baird EO, Kang QK. Prophylaxis of heterotopic ossification–an updated review. J. Orthop. Surg. Res. 2009;4:12. doi: 10.1186/1749-799X-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Browne KD, Iwata A, Putt ME, Smith DH. Chronic ibuprofen administration worsens cognitive outcome following traumatic brain injury in rats. Exp. Neurol. 2006;201:301–307. doi: 10.1016/j.expneurol.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 144.Dash PK, Mach SA, Moore A. N. Regional expression and role of cyclooxygenase-2 following experimental traumatic brain injury. J. Neurotrauma. 2000;17:69–81. doi: 10.1089/neu.2000.17.69. [DOI] [PubMed] [Google Scholar]
- 145.Suleyman H, Demircan B, Karagoz Y. Anti-inflammatory and side effects of cyclo-oxygenase inhibitors. Pharmacol. Rep. 2007;59:247–258. [PubMed] [Google Scholar]
- 146.Green GA. Understanding NSAIDs: from aspirin to COX-2. Clin. Cornerstone. 2001;3:50–59. doi: 10.1016/S1098-3597(01)90069-9. [DOI] [PubMed] [Google Scholar]
- 147.Drake, M. T., Clarke, B. L. & Khosla, S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin. Proc. 83, 1032–1045 (2008). [DOI] [PMC free article] [PubMed]
- 148.Russell RGG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119:S150–S162. doi: 10.1542/peds.2006-2023H. [DOI] [PubMed] [Google Scholar]
- 149.Manzano-Moreno FJ, Ramos-Torrecillas J, De Luna-Bertos E, Ruiz C, García-Martínez O. High doses of bisphosphonates reduce osteoblast-like cell proliferation by arresting the cell cycle and inducing apoptosis. J. Cranio-Maxillofac. Surg. 2015;43:396–401. doi: 10.1016/j.jcms.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 150.Idris AI, Rojas J, Greig IR, van’t Hof RJ, Ralston SH. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif. Tissue Int. 2008;82:191–201. doi: 10.1007/s00223-008-9104-y. [DOI] [PubMed] [Google Scholar]
- 151.Garland, D. E., Alday, B., Venos, K. G. & Vogt, J. C. Diphosphonate treatment for heterotopic ossification in spinal cord injury patients. Clin. Orthop. Relat. Res.176, 197–200 (1983). [PubMed]
- 152.Banovac K, Gonzalez F, Wade N, Bowker JJ. Intravenous disodium etidronate therapy in spinal cord injury patients with heterotopic ossification. Spinal Cord. 1993;31:660–666. doi: 10.1038/sc.1993.106. [DOI] [PubMed] [Google Scholar]
- 153.Banovac K, Gonzalez F, Renfree KJ. Treatment of heterotopic ossification after spinal cord injury. J. Spinal Cord Med. 1997;20:60–65. doi: 10.1080/10790268.1997.11719457. [DOI] [PubMed] [Google Scholar]
- 154.Banovac K. The effect of etidronate on late development of heterotopic ossification after spinal cord injury. J. Spinal Cord Med. 2000;23:40–44. doi: 10.1080/10790268.2000.11753507. [DOI] [PubMed] [Google Scholar]
- 155.Mashiba T, et al. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J. Bone Miner. Res. 2000;15:613–620. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 156.Burr DB, et al. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J. Bone Miner. Res. 1997;12:6–15. doi: 10.1359/jbmr.1997.12.1.6. [DOI] [PubMed] [Google Scholar]
- 157.Vasileiadis G, et al. Prevention of heterotopic ossification in cases of hypertrophic osteoarthritis submitted to total hip arthroplasty. Etidronate or Indomethacin. J. Musculoskelet. Neuronal Interact. 2010;10:159–165. [PubMed] [Google Scholar]
- 158.Williams JA, et al. Retinoic acid receptors are required for skeletal growth, matrix homeostasis and growth plate function in postnatal mouse. Dev. Biol. 2009;328:315–327. doi: 10.1016/j.ydbio.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pavey GJ, et al. Targeted stimulation of retinoic acid receptor-γ mitigates the formation of heterotopic ossification in an established blast-related traumatic injury model. Bone. 2016;90:159–167. doi: 10.1016/j.bone.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shimono K, et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-γ agonists. Nat. Med. 2011;17:454–460. doi: 10.1038/nm.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yasuhara R, et al. Wnt/β-catenin and retinoic acid receptor signaling pathways interact to regulate chondrocyte function and matrix turnover. J. Biol. Chem. 2010;285:317–327. doi: 10.1074/jbc.M109.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.De Luca, F. et al. Retinoic acid is a potent regulator of growth plate chondrogenesis. Endocrinology141, 346–353 (2000). [DOI] [PubMed]
- 163.Shimono K, et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-gamma agonists. Nat. Med. 2011;17:454–460. doi: 10.1038/nm.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pavey GJ, et al. Targeted stimulation of retinoic acid receptor-gamma mitigates the formation of heterotopic ossification in an established blast-related traumatic injury model. Bone. 2016;90:159–167. doi: 10.1016/j.bone.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reznik J, et al. Prevalence and risk-factors of neurogenic heterotopic ossification in traumatic spinal cord and traumatic brain injured patients admitted to specialised units in Australia. J. Musculoskelet. Neuronal Interact. 2014;14:19–28. [PubMed] [Google Scholar]
- 166.Rigaux P, et al. Study of serum factors potentially involved in the pathogenesis of heterotopic bone formation after severe brain injury. Jt. Bone Spine. 2005;72:146–149. doi: 10.1016/j.jbspin.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 167.Hurvitz EA, Mandac BR, Davidoff G, Johnson JH, Nelson VS. Risk factors for heterotopic ossification in children and adolescents with severe traumatic brain injury. Arch. Phys. Med. Rehabil. 1992;73:459–462. [PubMed] [Google Scholar]
- 168.Hendricks HT, Geurts A, van Ginneken BC, Heeren AJ, Vos PE. Brain injury severity and autonomic dysregulation accurately predict heterotopic ossification in patients with traumatic brain injury. Clin. Rehabil. 2007;21:545–553. doi: 10.1177/0269215507075260. [DOI] [PubMed] [Google Scholar]
- 169.Seipel R, et al. Neurogenic heterotopic ossification: epidemiology and morphology on conventional radiographs in an early neurological rehabilitation population. Skelet. Radiol. 2012;41:61–66. doi: 10.1007/s00256-011-1115-5. [DOI] [PubMed] [Google Scholar]
- 170.Singh RS, Craig MC, Katholi CR, Jackson AB, Mountz JM. The predictive value of creatine phosphokinase and alkaline phosphatase in identification of heterotopic ossification in patients after spinal cord injury. Arch. Phys. Med. Rehabil. 2003;84:1584–1588. doi: 10.1053/S0003-9993(03)00347-2. [DOI] [PubMed] [Google Scholar]
- 171.Wittenberg R, Peschke U, Botel U. Heterotopic ossification after spinal cord injury. Epidemiology and risk factors. J. Bone Jt. Surg. Br. 1992;74:215–218. doi: 10.1302/0301-620X.74B2.1544955. [DOI] [PubMed] [Google Scholar]
- 172.Bravo-Payno P, Esclarin A, Arzoz T, Arroyo O, Labarta C. Incidence and risk factors in the appearance of heterotopic ossification in spinal cord injury. Spinal Cord. 1992;30:740–745. doi: 10.1038/sc.1992.142. [DOI] [PubMed] [Google Scholar]
- 173.Orzel JA, Rudd TG. Heterotopic bone formation: clinical, laboratory, and imaging correlation. J. Nucl. Med. 1985;26:125–132. [PubMed] [Google Scholar]
- 174.Meiners T, Abel R, Böhm V, Gerner H. Resection of heterotopic ossification of the hip in spinal cord injured patients. Spinal Cord. 1997;35:443–445. doi: 10.1038/sj.sc.3100415. [DOI] [PubMed] [Google Scholar]
- 175.Hunt JL, Arnoldo BD, Kowalske K, Helm P, Purdue GF. Heterotopic ossification revisited: a 21-year surgical experience. J. Burn Care Res. 2006;27:535–540. doi: 10.1097/01.BCR.0000226023.58438.14. [DOI] [PubMed] [Google Scholar]
- 176.Stein DA, et al. Prevention of heterotopic ossification at the elbow following trauma using radiation therapy. Bull. Hosp. Jt. Dis. N.Y. 2003;61:151–154. [PubMed] [Google Scholar]
- 177.Müseler A, et al. In-hospital outcomes following single-dose radiation therapy in the treatment of heterotopic ossification of the hip following spinal cord injury—an analysis of 444 cases. Spinal Cord. 2017;55:244–246. doi: 10.1038/sc.2016.112. [DOI] [PubMed] [Google Scholar]
- 178.Cipriano, C., Pill, S. G., Rosenstock, J. & Keenan, M. A. Radiation therapy for preventing recurrence of neurogenic heterotopic ossification. Orthopedics32, 685–689 (2009). [DOI] [PubMed]
- 179.Banovac K, Williams J, Patrick L, Levi A. Prevention of heterotopic ossification after spinal cord injury with COX-2 selective inhibitor (rofecoxib) Spinal Cord. 2004;42:707–710. doi: 10.1038/sj.sc.3101628. [DOI] [PubMed] [Google Scholar]
- 180.Romanò CL, Duci D, Romanò D, Mazza M, Meani E. Celecoxib versus indomethacin in the prevention of heterotopic ossification after total hip arthroplasty. J. Arthroplast. 2004;19:14–18. doi: 10.1016/S0883-5403(03)00279-1. [DOI] [PubMed] [Google Scholar]
- 181.Schmidt SA, et al. The use of indomethacin to prevent the formation of heterotopic bone after total hip replacement. A randomized, double-blind clinical trial. J. Bone Jt. Surg. Am. 1988;70:834–838. doi: 10.2106/00004623-198870060-00005. [DOI] [PubMed] [Google Scholar]
- 182.Bedi A, et al. The incidence of heterotopic ossification after hip arthroscopy. Am. J. Sports Med. 2012;40:854–863. doi: 10.1177/0363546511434285. [DOI] [PubMed] [Google Scholar]
- 183.Beckmann JT, et al. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J. Bone Jt. Surg. Am. 2015;97:2032–2037. doi: 10.2106/JBJS.N.01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Neal BC, et al. No effect of low-dose aspirin for the prevention of heterotopic bone formation after total hip replacement: a randomized trial of 2 649 patients. Acta Orthop. Scand. 2000;71:129–134. doi: 10.1080/000164700317413085. [DOI] [PubMed] [Google Scholar]
- 185.Schuetz P, Mueller B, Christ-Crain M, Dick W, Haas H. Amino-bisphosphonates in heterotopic ossification: first experience in five consecutive cases. Spinal Cord. 2005;43:604–610. doi: 10.1038/sj.sc.3101761. [DOI] [PubMed] [Google Scholar]