ABSTRACT
Background
Antioxidants have been promoted for cardiovascular disease (CVD) risk reduction and for the prevention of cancer. Our preliminary analysis suggested that only when selenium was present were antioxidant mixtures associated with reduced all-cause mortality.
Objective
We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) to determine the effect of selenium supplementation alone and of antioxidant mixtures with or without selenium on the risk of CVD, cancer, and mortality.
Methods
We identified studies using the Cochrane Library, Medline, and Embase for potential CVD outcomes, cancer, and all-cause mortality following selenium supplementation alone or after antioxidant supplement mixtures with and without selenium up to June 5, 2020. RCTs of ≥24 wk were included and data were analyzed using random-effects models and classified by the Grading of Recommendations, Assessment, Development, and Evaluation approach.
Results
The meta-analysis identified 9423 studies, of which 43 were used in the final analysis. Overall, no association of selenium alone or antioxidants was seen with CVD and all-cause mortality. However, a decreased risk with antioxidant mixtures was seen for CVD mortality when selenium was part of the mix (RR: 0.77; 95% CI: 0.62, 0.97; P = 0.02), with no association when selenium was absent. Similarly, when selenium was part of the antioxidant mixture, a decreased risk was seen for all-cause mortality (RR: 0.90; 95% CI: 0.82, 0.98; P = 0.02) as opposed to an increased risk when selenium was absent (RR: 1.09; 95% CI: 1.04, 1.13; P = 0.0002).
Conclusion
The addition of selenium should be considered for supplements containing antioxidant mixtures if they are to be associated with CVD and all-cause mortality risk reduction. This trial was registered at https://www.crd.york.ac.uk/PROSPERO/ as CRD42019138268.
Keywords: supplements, antioxidants, selenium, cardiovascular disease, all-cause mortality, meta-analysis
Introduction
The US Preventive Service Task Force (USPSTF) has warned against the use of vitamin E and β-carotene as single or paired nutrient supplements (1). Yet these vitamins are included in significant quantities in multivitamin supplements that are taken by 33–39% (2) of Western populations. These vitamins have antioxidant properties, and there has been a longstanding interest in antioxidants to reduce the oxidative stress that promotes the aging process (3). Chronic diseases such as cardiovascular disease (CVD), diabetes, and cancer are closely related to aging, but studies using antioxidant supplements have not produced clear evidence of their benefit for these diseases (4–6) and show they may be associated with harm, especially in smokers (7, 8).
However, evidence is emerging that maintaining an active endogenous antioxidant system may be important, including adequate concentrations of selenium-dependent glutathione peroxidase (GPx) (9), which appears to support healthy aging (10). This selenium-dependent GPx reduces lipid hydroperoxides to their corresponding alcohols and water and so maintains intracellular redox status (11).
Selenium is required as a cofactor for the synthesis of this enzyme, and data suggest that reduced blood selenium concentrations are associated with an increased CVD incidence (12), certain cancers (13), and all-cause mortality (14).
We have therefore assessed the effect of selenium alone and in antioxidant supplement mixtures to determine whether its presence, with the potential to maintain endogenous antioxidant activity, resulted in a reduced risk rather than an increased risk of antioxidants for CVD and all-cause mortality, as suggested in a previous more general supplement meta-analysis (15).
Methods
We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) and of existing systematic reviews and meta-analyses up to June 5, 2020. We performed a literature search in the Cochrane Library, Medline, and Embase using search terms for dietary supplements (as well as the individual vitamin or mineral antioxidants of interest—as described in the “Vitamins and minerals assessed” section below), CVD and its components, cancer, and mortality. See Supplementary Table 1 for our full search terms. The search was limited to meta-analyses of RCTs and single RCTs. Meta-analyses were reviewed to identify any further studies not identified from the search. We included RCT studies in human adult populations where oral supplementation (selenium alone and antioxidant mixtures with and without selenium) was provided for a duration of at least 24 wk and where there was a comparable placebo with no other unmatched confounding interventions in either the supplementation or the control group. Only published data on CVD, cancer, and all-cause mortality outcomes were used. Excluded were studies conducted in laboratory animals, children, pregnant and breastfeeding women, and those with chronic infections (e.g., HIV and hepatitis C). Interventions combining other supplements than those specified were excluded as well as studies that were observational, supplements provided intravenously, and studies lasting <24 wk.
Full-article review and data extraction were conducted by 2 independent investigators (MP, SS-P), with all disagreements reconciled through consensus. The extracted data included number of cases and total number of participants for the intervention and the control groups. Where multiple intervention groups existed (e.g., factorial design studies), data from all control groups and all supplement groups were extracted and combined. Data were analyzed using Review Manager (RevMan) version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration), and publication bias analysis was conducted using STATA software, version 16.1 (StataCorp). The methods have been described in detail elsewhere (15). To obtain summary estimates, data were pooled using the Mantel-Haenszel method with data presented using random-effects models. Heterogeneity was assessed using the Cochran Q statistic at P < 0.1 and quantified by the I² statistic. An I² value ≥ 50% indicated substantial heterogeneity (16). Publication bias was investigated by visual inspection of funnel plots and quantitative assessment using Begg's and Egger's tests where P < 0.05 was considered evidence of a significant small study effect (17, 18). If <10 trials were available in a meta-analysis, publication bias analysis was not conducted due to insufficient power. The number needed to treat (NNT) and the number needed to harm (NNH) were calculated by the inverse of the absolute risk reduction or absolute risk increase, respectively (ARR) (NNT = 1/ARR, NNH = 1/ARR) (19). ARR can be calculated either as the difference between the control event rate (CER) and experimental event rate (EER) (ARR = CER – EER) (19) or by multiplying the CER by the relative risk reduction (RRR) (ARR = CER × RRR); RRR is calculated as 1 – RR (20). The latter approach was used in this analysis to avoid infinite NNT values in some situations (Supplementary Table 2, Method A).
Vitamins and minerals assessed
We confined our assessment to combinations of antioxidants (antioxidant mixtures) rather than single antioxidants with the exception of selenium, which was also assessed as a single supplement. The supplements of antioxidant mixtures consisted of combinations of 2 or more of the following: vitamin A, retinol, β-carotene, vitamin C, vitamin E, selenium, zinc, and copper as composite entities. These antioxidants were selected because they were included in the 2012 Cochrane Review (21), to which we added retinol as part of vitamin A as well as zinc and copper as metals associated with the synthesis or activity of the endogenous antioxidant system (22, 23). The antioxidants used in specific studies included in this analysis are shown in Supplementary Table 3.
Outcomes
The primary outcomes of the review are total CVD, CVD mortality, total cancer, cancer mortality, and all-cause mortality. The secondary outcomes of the review are coronary heart disease (CHD), CHD mortality, myocardial infarction (MI), MI mortality, stroke, and stroke mortality.
Dose-response analysis
Post hoc dose-response analysis was completed for all-cause mortality by categories of selenium doses. Linear dose response for selenium use in trials of antioxidants on risk of all-cause mortality was assessed by using random-effects meta-regression. Nonlinear dose-response analysis in the same trials was assessed using a 2-stage multivariate random-effects method with restricted cubic splines and a knot at 50 mcg/d of selenium.
Exploratory analyses
A prespecified exploratory analysis was conducted to determine whether geographic locations affected the outcome, and a further post hoc analysis was conducted stratified by participants’ health or risk status.
Risk of bias
The Cochrane Risk of Bias Tool, which is based on randomization, allocation concealment, blinding, completeness of follow-up, and an intention-to-treat analysis, was used to assess eligible RCTs (24).
Grading of the evidence
The quality and strength of the evidence was assessed as described previously using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tool (25, 26). Using the GRADE tool, evidence was graded as high-, moderate-, low-, or very low-quality evidence. By default, RCTs are graded as high-quality evidence. Criteria used to downgrade evidence included: study limitations (as assessed by the Cochrane Risk of Bias Tool), inconsistency (substantial) unexplained interstudy heterogeneity, I2 > 50% and P < 0.10; indirectness (presence of factors that limit the generalizability of the results); imprecision [the 95% CI for effect estimates crosses a minimally important difference of 5% (RR, 0.95–1.05) from the line of unity]; and publication bias (significant evidence of small study effects).
Results
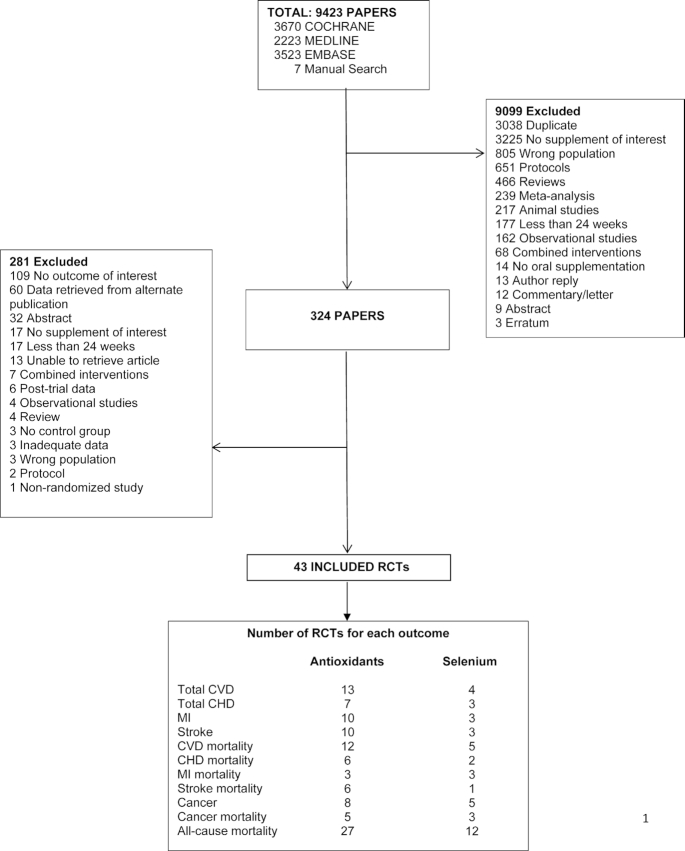
Assessment of the search results identified a total number of 43 single RCTs (27–69). The study flow diagram is presented in Figure 1. Study characteristics, GRADE assessments, search results, and the Cochrane risk of bias are also reported (Supplementary Tables 3–5;Supplementary Figures 1–3).
FIGURE 1.
Search summary. Flow diagram outlining the search strategy used to identify publications that report RCT data on antioxidant supplementation and risk of CVD, cancer, and all-cause mortality. The publications are from database inceptions to June 5, 2020, of single RCTs identified by searching Cochrane, Medline, and Embase and by manual searches. Titles and abstracts were reviewed in the first stage of screening while full-manuscript review was done in the second stage. CHD, coronary heart disease; CVD, cardiovascular disease; MI, myocardial infarction, RCT, randomized controlled trial.
Selenium alone and antioxidant mixtures with and without selenium
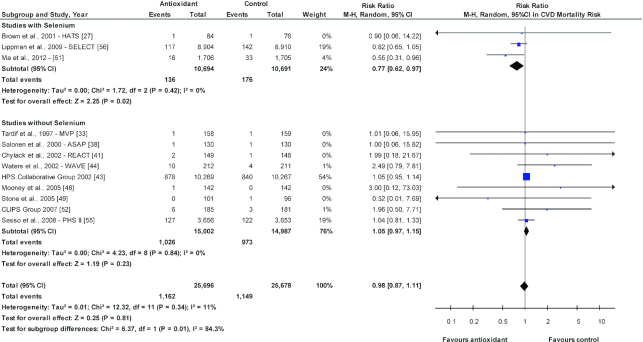
Selenium taken alone (Supplementary Figures 4–15) and combined antioxidants (Supplementary Figures 16–27) were not associated with CVD outcomes, CVD mortality, cancer, cancer mortality, or all-cause mortality. However, when the antioxidant trials were separated into those that included selenium as part of the antioxidant supplement mix compared with those that did not (Supplementary Figures 28–38), a decreased risk was seen for CVD mortality when selenium was part of the antioxidant mix (RR: 0.77; 95% CI: 0.62, 0.97; P = 0.02; I2 = 0%), which was not observed when selenium was not part of the mix (RR: 1.05; 95% CI: 0.97, 1.15; P = 0.23; I2 = 0%). Furthermore, these 2 antioxidant groups (with and without selenium) were significantly different (P = 0.01; I2 = 84.3%; Figure 2). Opposite responses between these 2 antioxidant groups were also observed for all-cause mortality (Figure 3), in which a decreased risk was seen for all-cause mortality when selenium was included (RR: 0.90; 95% CI: 0.82, 0.98; P = 0.02; I2 = 0%), while for antioxidant mixtures without selenium, there was evidence for increased risk (RR: 1.09; 95% CI: 1.04, 1.13; P = 0.0002; I2 = 0%). These 2 antioxidant groups were also significantly different (P = 0.0002; I2 = 92.8%). There was no effect on cancer incidence and mortality. The quality of the evidence was considered moderate for antioxidants and low for selenium supplementation only by GRADE assessment.
FIGURE 2.
Sensitivity analysis of antioxidant supplementation and CVD mortality risk for studies with and without selenium. The diamond represents the pooled risk estimate. Interstudy heterogeneity was tested using the Cochran Q statistic (χ2) at a significance level of P < 0.10 and quantified by the I2 statistic. An I2 value ≥50% is considered to indicate substantial heterogeneity. All results are presented as risk ratios with 95% confidence intervals, using the Mantel-Haenszel method with a random-effects model. NNT for antioxidant supplementation and CVD mortality risk for studies with selenium is 264. The 2 antioxidant groups are significantly different (P = 0.01; I2 = 84.3%). ASAP, Antioxidant Supplementation in Atherosclerosis Prevention; CLIPS, Critical Leg Ischaemia Prevention Study; CVD, cardiovascular disease; HATS, HDL-Atherosclerosis Treatment Study; HPS, Heart Protection Study Collaborative Group; M-H, Mantel-Haenszel; MVP, Multivitamins and Probucol Study Group; NNT, number needed to treat; PHS, Physicians’ Health Study; REACT, Roche European American Cataract Trial; SELECT, Selenium and Vitamin E Cancer Prevention; SIT, Shadong Intervention Trial; WAVE, Women's Angiographic Vitamin and Estrogen.
FIGURE 3.
Sensitivity analysis of antioxidant supplementation and all-cause mortality risk for studies with and without selenium. The diamond represents the pooled risk estimate. Interstudy heterogeneity was tested using the Cochran Q statistic (χ2) at a significance level of P < 0.10 and quantified by the I2 statistic. An I2 value ≥50% is considered to indicate substantial heterogeneity. All results are presented as risk ratios with 95% confidence intervals, using the Mantel-Haenszel method with random-effects model. *Jacobson et al., 2000—data retrieved from meta-analysis (21). NNT for antioxidant supplementation and all-cause mortality risk for studies with selenium is 226. NNH for antioxidant supplementation and all-cause mortality risk for studies without selenium is 115. The 2 antioxidant groups are significantly different (P = 0.0002; I2 = 92.8%). AREDS, Age-Related Eye Disease Study; ASAP, Antioxidant Supplementation in Atherosclerosis Prevention; ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CARET, Carotene and Retinol Efficacy Trial; CLIPS, Critical Leg Ischaemia Prevention Study; HATS, HDL-Atherosclerosis Treatment Study; HPS, Heart Protection Study Collaborative Group; M-H, Mantel-Haenszel; MINVITOAX, Mineral Vitamin Antioxidant; MVP, Multivitamins and Probucol Study Group; NNH, number needed to harm; NNT, number needed to treat; PHS, Physicians’ Health Study; REACT, Roche European American Cataract Trial; SELECT, Selenium and Vitamin E Cancer Prevention; SIT, Shadong Intervention Trial; SU.VI.MAX, Supplémentation en Vitamines et Minéraux AntioXydants study; WACS, Women's Antioxidant Cardiovascular Study; WAVE, Women's Angiographic Vitamin and Estrogen.
Dose-response analysis
Dose-response analysis by category of doses showed no significant findings for selenium-only trials and antioxidant trials containing selenium (Supplementary Figures 39–40). Supplementary Figures 41 and 42 show the linear and nonlinear dose-response relation between selenium and all-cause mortality risk in trials with antioxidants. There was a significant linear (P = 0.004) and nonlinear (P = 0.013) dose response seen for the addition of selenium to antioxidants on all-cause mortality. The addition of selenium to the antioxidant mix was associated with a decrease in the risk for all-cause mortality. However, a dose greater than 50 mcg/d did not seem to reduce the risk further (Supplementary Figure 42).
Exploratory analyses by health or risk status
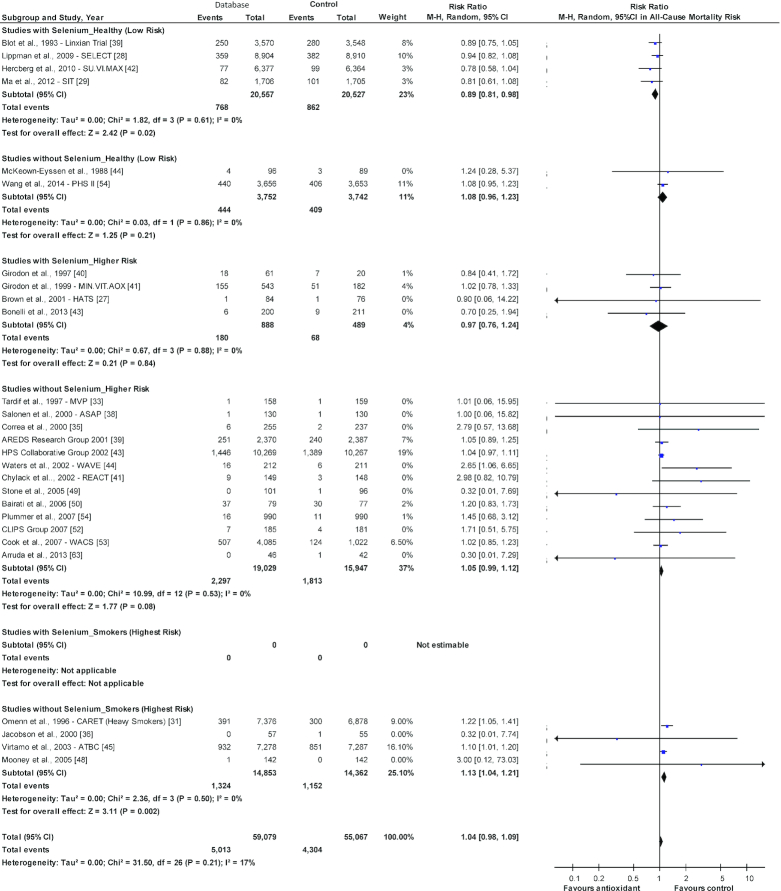
Studies stratified by health or risk status of the population (Supplementary Figures 43–45) showed significant findings for all-cause mortality only. Six trials were identified of healthy individuals who had no preexisting disease or were considered at increased risk (Figure 4). Of those 6 trials, the 4 with selenium in the antioxidant mix showed a decreased risk for all-cause mortality (RR: 0.89; 95% CI: 0.81, 0.98; P = 0.02; I2 = 0%), with no effect seen in the 2 trials without selenium (RR: 1.08; 95% CI: 0.96, 1.23; P = 0.21; I2 = 0%). The difference between the 2 antioxidant groups was significant (P = 0.01; I2 = 83%).
FIGURE 4.
Forest plot showing antioxidants (with and without selenium) by smoking and health status and all-cause mortality. *Jacobson et al., 2000—data retrieved from meta-analysis (21). The diamond represents the pooled risk estimate. Interstudy heterogeneity was tested using the Cochran Q statistic (χ2) at a significance level of P < 0.10 and quantified by the I2 statistic. An I2 value ≥50% is considered to indicate substantial heterogeneity. All results are presented as risk ratios with 95% confidence intervals, using the Mantel-Haenszel method with random-effects model. The difference between studies with and without selenium among the healthy (low-risk) group was significant (P = 0.01; I2 = 83.4%) while the difference within the higher risk group was not significant (P = 0.54; I2 = 0%), and there were no studies in the highest risk category (smokers) with selenium in the antioxidant mix. AREDS, Age-Related Eye Disease Study; ASAP, Antioxidant Supplementation in Atherosclerosis Prevention; ATBC, Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study; CARET, Carotene and Retinol Efficacy Trial; CLIPS, Critical Leg Ischaemia Prevention Study; HATS, HDL-Atherosclerosis Treatment Study; HPS, Heart Protection Study Collaborative Group; M-H, Mantel-Haenszel; MINVITOAX, Mineral Vitamin Antioxidant; MVP, Multivitamins and Probucol Study Group; PHS, Physicians’ Health Study; REACT, Roche European American Cataract Trial; SELECT, Selenium and Vitamin E Cancer Prevention; SIT, Shadong Intervention Trial; SU.VI.MAX, Supplémentation en Vitamines et Minéraux AntioXydants study; WACS, Women's Antioxidant Cardiovascular Study; WAVE, Women's Angiographic Vitamin and Estrogen.
Seventeen trials were identified in higher-risk individuals (e.g., history of CVD, cancer, precancerous lesions, pulmonary artery disease, hypercholesterolemia, institutionalized). In the 4 studies with selenium in the antioxidant mix, there was no association with all-cause mortality, but in the 13 trials without selenium, a possible borderline significant increased risk was seen (RR: 1.05, 95% CI: 0.99, 1.12; P = 0.08; I2 = 0%; Figure 4). The difference between these 2 antioxidant groups was not significant (P = 0.54; I2 = 0%).
Four trials were identified in smokers (possibly the highest risk group) (Figure 4). Unfortunately, no trials were identified with selenium in the antioxidant mix, but the 4 trials without selenium were associated with the greatest increase in risk for all-cause mortality (RR: 1.13, 95% CI: 1.04, 1.21; P = 0.002; I2 = 0%).
These assessments yielded no significant effects for CVD or CVD mortality probably due to the limited numbers in each group (Supplementary Figures 43–44).
Sequential removal of nonselenium antioxidants
Removal of other specific antioxidants (e.g., vitamins A, C, and E; β-carotene; retinol; and zinc) singly did not influence the outcomes (Supplementary Figures 46–111)at the significance level P < 0.05, with the exception of zinc. Although studies with zinc were not associated with any change in all-cause mortality (RR: 0.94; 95% CI: 0.85, 1.04; P = 0.25; I2 = 0%), antioxidant mixtures that lacked zinc increased the risk of all-cause mortality (RR: 1.07; 95% CI: 1.01, 1.13; P = 0.02; I2 = 14%; Supplementary Figure 100) with a significant difference between these antioxidant groups (P = 0.03; I2 = 78.6%). In addition the Blot trial (30) in Linxian, China, which included zinc, showed a borderline reduction in risk for stroke mortality (RR: 0.71; 95% CI: 0.5, 1.00; P = 0.05; Supplementary Figure 97).
Retinol was borderline significant for reducing risk from stroke mortality (RR: 0.71; 95% CI: 0.5, 1.00; P = 0.05) and cancer mortality (RR: 0.75, 95% CI: 0.57, 1.00; P = 0.05) (Supplementary Figures 108 and 110). The caveat is that these associations are based on a single Chinese trial, the Linxian trial, with different baseline vitamin intake concentrations from North American and European trials (30).
Antioxidants trials from areas with high or low soil selenium
Dividing the studies into areas of higher soil selenium content (the Americas) and lower soil selenium content (Europe and Asia) (70), the 2 studies with higher soil selenium content showed no significant risk reduction when selenium was included in the antioxidant mix, but for the 14 studies in which there was no selenium in the antioxidant supplement, this antioxidant mix was associated with a significantly increased risk of all-cause mortality (RR: 1.13, 95% CI: 1.05, 1.22; P = 0.0006; I2 = 0%; Supplementary Figure 112). A similar pattern was seen in studies with low soil selenium; studies with selenium in the antioxidant mix were associated with a decreased risk (RR: 0.88; 95% CI: 0.78, 0.98; P = 0.02; I2 = 0) while those without selenium were associated with an increased risk of all-cause mortality (RR: 1.06, 95% CI: 1.01, 1.12; P = 0.03; I2 = 0%; Supplementary Figure 112). Again, no differences between antioxidant groups were seen for CVD or CVD mortality due to the limited data (Supplementary Figures 113–114).
Discussion
Our findings suggest that inclusion of selenium, as part of the antioxidant mix, is important to allow the associated decreased risk of antioxidants on CVD and all-cause mortality to be seen. Although definitive benefits of antioxidants have proved elusive (71), there is a long history of interest in mechanisms by which antioxidants might reduce the risk of chronic diseases, especially CVD and cancer—for example, reducing concentrations of oxidized LDL (4) and reducing oxidative damage to DNA (and the formation of 8-OH-2 deoxyguanosine) (72). Our analysis of trials that have included selenium in the antioxidant mix support continued interest in the relation between antioxidant activity and disease. However, our demonstration of significantly increased CVD and all-cause mortality risk when selenium was absent from the antioxidant mix suggests that this process is finely balanced and selenium should be added to any supplement mix that includes antioxidants.
This antioxidant selenium phenomenon did not appear to be influenced by differences in soil and therefore dietary selenium concentrations, although we have no studies from areas with truly low or high concentrations of soil selenium as found in China (73).
Of major relevance to the present study is the 2013 conclusion of the USPSTF (74) “that the current evidence is insufficient to assess the balance of benefits and harms of single or paired nutrient supplements (except for β-carotene and vitamin E, that are recommended against) for the prevention of CVD and cancer.”
Sequential exclusion of antioxidants other than selenium failed to produce the same increased risk of health effects seen with the exclusion of selenium, emphasizing the unique antioxidant role of selenium. An exception to this conclusion may be zinc, which is also part of the endogenous antioxidant system as a component of superoxide dismutase (SOD) (75). However, trials of selenium or zinc taken in isolation have not shown any generally agreed-on CVD or total mortality benefit (15), although diet, blood, and toenail selenium concentrations have been associated with a reduced risk of both overall (76) and aggressive prostate cancer (77).
The possibly differing effects of antioxidants in individuals at different levels of chronic disease risk have been raised (71). Our analyses indicate a potential benefit of antioxidants with selenium in healthy trial participants, no effect in trial participants with a range of conditions or risk factors, and an increased risk of antioxidants without selenium with total mortality in smokers, as perhaps the highest risk group. These data support the concept that antioxidant supplementation may affect those with different levels of risk differently (71).
The antioxidant effects of selenium, zinc, and copper are not direct. They act through the endogenous antioxidant system that also involves SOD, GPx, and catalase. Selenium is essential for the synthesis of GPx and is part of the molecular structure (78) of GPx and other seleno-proteins (79). Likewise, zinc and copper are part of the structure of copper, zinc, and SOD (80, 81). These metals are therefore essential for the activity of the endogenous antioxidant system responsible for quenching the free radicals generated by metabolic processes. The system is finely balanced since the creation of oxidative damage by WBCs is the mechanism by which invading pathogens are destroyed and abnormal or transformed cells removed as part of immune surveillance (80). It therefore appears that a balancing process may exist such that when dietary antioxidants are provided in excess amounts as supplements, the large rise in serum antioxidant activity will suppress the expression of the endogenous antioxidant system (e.g., SOD and GPx) (Figure 5). The presence of higher concentrations of selenium and zinc may promote the rapid and necessary rebound in the endogenous antioxidant system to reverse the suppression of endogenous antioxidant activity that would otherwise leave tissues vulnerable to pro-oxidant stresses, such as postprandial events and smoking (82, 83) (Figure 5). Over time, vulnerable populations such as the elderly and those with preexisting CVD or transformed cells in precancerous lesions may be more vulnerable to the effects of oxidative stress. The adverse effects of antioxidants in smokers, for example, in the Carotene and Retinol Efficacy Trial and Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study that resulted in increased rather than reduced lung cancer incidence (7, 8) might have been due to suppression of the endogenous antioxidant system by the antioxidant supplements provided and may have made trial populations of smokers more vulnerable to the pro-oxidant activity of tobacco smoke (83).
FIGURE 5.
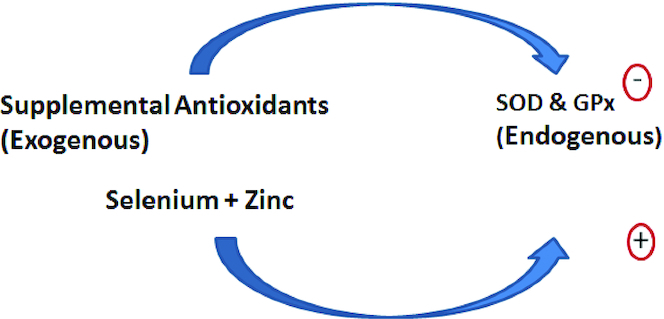
The opposing activity of supplemental antioxidants and selenium and zinc on the endogenous antioxidant system. GPx, glutathione peroxidase; SOD, superoxide dismutase.
There is a non-CVD caveat for the use of selenium with antioxidants from the Selenium and Vitamin E Cancer Prevention trial (n = 35,533) that demonstrated possible increased risk for high-grade prostate cancer and type 2 diabetes in participants taking vitamin E and selenium (56, 84). Weak evidence for this association was also found in a large Mendelian randomization study (85). Our data suggest that selenium and zinc supplementation should be part of any antioxidant supplement mixture aimed at reducing CVD risk. However, until we clarify the long-term selenium effect on antioxidant supplement use, a balanced diet rich in antioxidant foods, fruit, vegetables, legumes, whole-grain cereals, and nuts and seeds (86, 87) may be the safer approach (88), as selenium and zinc will come naturally as components of antioxidant-rich foods.
Strengths and Limitations
The study strength lies in having a sufficient number of trials with and without selenium to allow the effect on CVD and all-cause mortality to be determined and the ability to assess all-cause mortality in different geographic areas with different soil selenium contents and in individuals with differing risk factors for chronic disease, especially smokers.
The weaknesses of this study include the inclusion of trials with low event rates and where CVD and all-cause mortality were not the primary outcomes. A major deficiency was the lack of trials in smokers with selenium in the antioxidant mix that might have more clearly demonstrated the potential value of selenium addition. Finally, for selenium taken alone, there is a lack of consistency in the few long-term studies (42, 51, 56) and a relative lack of long-term dose-response studies. However, 1 study demonstrated that selenium consumed alone at doses of 300 µg had adverse effects on all-cause mortality in the 10-y follow-up after a 5-y supplementation. However, the nonsignificant increase in CVD and cancer mortality was not seen at 100-µg and 200-µg doses (89). These data may help inform the safe upper level for selenium inclusion in antioxidant mixtures, especially as our dose-response data show no added benefit above 50 µg/d.
Conclusion
We conclude that if antioxidant supplements are to have a benefit in reducing CVD and all-cause mortality, then inclusion of selenium and possibly zinc may be important to maintain endogenous antioxidant activity.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows–––DJAJ: conceptualized the meta-analysis and designed the overall research plan; MP and SS-P: conducted the database search, screened studies for inclusion, extracted and analyzed the data, and revised the manuscript; DJAJ, DK, ELG, and JLS: drafted and revised the manuscript; SBM and TT: analyzed the data and revised the manuscript; DP, MK, CK, and SCP: revised the manuscript; DJAJ: is the study guarantor; and all authors: reviewed and approved the final version of the manuscript.
DJAJ has received research grants from Loblaw Companies Ltd, the Almond Board of California, Soy Nutrition Institute (SNI), and the Canadian Institutes of Health Research (CIHR). He has received in-kind supplies for trials as a research support from the Almond Board of California, Walnut Council of California, American Peanut Council, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, and WhiteWave Foods. He has been on the speakers’ panel, served on the scientific advisory board, and/or received travel support and/or honoraria from the Loblaw Companies Ltd, Diet Quality Photo Navigation (DQPN), Better Therapeutics (FareWell), Verywell, True Health Initiative (THI), Heali AI Corp, Institute of Food Technologists (IFT), Soy Nutrition Institure (SNI), Herbalife Nutrition Institute (HNI), Herbalife International, Pacific Health Laboratories, Nutritional Fundamentals for Health (NFH), the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), the Toronto Knowledge Translation Group (St. Michael's Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), and the American Society of Nutrition (ASN). He received an honorarium from the USDA to present the 2013 W. O. Atwater Memorial Lecture. He is a member of the International Carbohydrate Quality Consortium (ICQC). His wife, Alexandra L Jenkins, is a director and partner of INQUIS Clinical Research for the Food Industry; his 2 daughters, Wendy Jenkins and Amy Jenkins, have published a vegetarian book that promotes the use of the plant foods advocated here, The Portfolio Diet for Cardiovascular Risk Reduction; and his sister, Caroline Brydson, received funding through a grant from the St. Michael's Hospital Foundation to develop a cookbook for one of his studies. CWCK has received grants or research support from the Advanced Food Materials Network, Agriculture and Agri-Foods Canada (AAFC), Almond Board of California, American Peanut Council, Barilla, Canadian Institutes of Health Research (CIHR), Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands Ltd, Pulse Canada, and Unilever. He has received in-kind research support from the Almond Board of California, American Peanut Council, Barilla, California Walnut Commission, Kellogg Canada, Loblaw Companies, Quaker (PepsiCo), Primo, Unico, Unilever, and WhiteWave Foods/Danone. He has received travel support and/or honoraria from the American Peanut Council, Barilla, California Walnut Commission, Canola Council of Canada, General Mills, International Nut and Dried Fruit Council, International Pasta Organization, Loblaw Brands Ltd, Nutrition Foundation of Italy, Oldways Preservation Trust, Paramount Farms, Peanut Institute, Pulse Canada, Sun-Maid, Tate & Lyle, Unilever, and White Wave Foods/Danone. He has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organization, McCormick Science Institute, and Oldways Preservation Trust. He is a member of the International Carbohydrate Quality Consortium (ICQC), is Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD), is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD and a director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. JLS has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of Health Research (CIHR), Diabetes Canada, PSI Foundation, Banting and Best Diabetes Centre (BBDC), American Society for Nutrition (ASN), INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), and the Nutrition Trialists Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received in-kind food donations to support a randomized controlled trial from the Almond Board of California, California Walnut Commission, American Peanut Council, Barilla, Unilever, Upfield, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, WhiteWave Foods, and Nutrartis. He has received travel support, speaker fees, and/or honoraria from Diabetes Canada, Dairy Farmers of Canada, FoodMinds LLC, International Sweeteners Association, Nestlé, Pulse Canada, Canadian Society for Endocrinology and Metabolism (CSEM), GI Foundation, Abbott, Biofortis, ASN, Northern Ontario School of Medicine, INC Nutrition Research & Education Foundation, European Food Safety Authority (EFSA), Comité Européen des Fabricants de Sucre (CEFS), and Physicians Committee for Responsible Medicine. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, Wirtschaftliche Vereinigung Zucker e.V., and Inquis Clinical Research. He is a member of the European Fruit Juice Association Scientific Expert Panel and Soy Nutrition Institute (SNI) Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the Study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada. He serves or has served as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of the International Life Science Institute (ILSI) North America. He is a member of the International Carbohydrate Quality Consortium (ICQC), executive board member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His wife is an employee of AB InBev. DK, ELG, SS-P, MP, SBM, DP, MK, TT, and SCP have no conflicts of interest to declare.
Notes
Sources of support: Canada Research Chair's Endowment provides discretionary funding to DJAJ. The funder had no role in the design, manuscript drafting, or decision to submit for publication.
Data sharing: Data described in the manuscript and supplementary material will be made available to those who request it for verification or for collaborative purposes.
Disclaimer: The authors have no relevant conflicts of interest over the past 4 y. DJAJ has received funds for dietary studies from Loblaws, which, during the course of his funding, acquired Shopper's Drugmart, which is a pharmaceutical company that also sells supplements.
Supplemental Tables 1–5 and Supplemental Figures 1–114 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: ARR, absolute risk reduction; CVD, cardiovascular disease; CER, control event rate; CHD, coronary heart disease; EER, experimental event rate; GPx, glutathione peroxidase; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MI, myocardial infarction; NNH, number needed to harm; NNT, number needed to treat; RCT, randomized controlled trial; RRR, relative risk reduction; SOD, superoxide dismutase; USPSTF, US Preventive Services Task Force.
Contributor Information
David J A Jenkins, Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Li Ka Shing Knowledge Institute, St. Michael's Hospital, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Toronto, Ontario, Canada; Clinical Nutrition Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada; Division of Endocrinology and Metabolism, St. Michael's Hospital, Toronto, Ontario, Canada.
David Kitts, Food Nutrition and Health, Faculty of Land and Food Systems, University of British Columbia, Vancouver, British Columbia, Canada.
Edward L Giovannucci, Department of Nutrition and Epidemiology, Harvard TH Chan School of Public Health, Boston, MA, USA.
Sandhya Sahye-Pudaruth, Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Clinical Nutrition Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Melanie Paquette, Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Clinical Nutrition Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Sonia Blanco Mejia, Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Toronto, Ontario, Canada; Clinical Nutrition Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Darshna Patel, Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Clinical Nutrition Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Meaghan Kavanagh, Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Clinical Nutrition Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Tom Tsirakis, Clinical Nutrition Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada.
Cyril W C Kendall, Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Toronto, Ontario, Canada; Clinical Nutrition Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada; College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
Sathish C Pichika, Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Department of Mathematics and Statistics, University of Windsor, Windsor, Canada.
John L Sievenpiper, Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; Li Ka Shing Knowledge Institute, St. Michael's Hospital, Toronto, Ontario, Canada; Toronto 3D Knowledge Synthesis and Clinical Trials Unit, Toronto, Ontario, Canada; Clinical Nutrition Risk Factor Modification Centre, St. Michael's Hospital, Toronto, Ontario, Canada; Division of Endocrinology and Metabolism, St. Michael's Hospital, Toronto, Ontario, Canada.
References
- 1. Moyer VA. Vitamin, mineral, and multivitamin supplements for the primary prevention of cardiovascular disease and cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(8):558–64. [DOI] [PubMed] [Google Scholar]
- 2. Gahche J, Bailey R, Burt V, Hughes J, Yetley E, Dwyer J, Picciano MF, McDowell M, Sempos C. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994). NCHS Data Brief. 2011;(61):1–8. [PubMed] [Google Scholar]
- 3. Finkel T, Holbrook NJ, Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–47. [DOI] [PubMed] [Google Scholar]
- 4. Steinberg D. Antioxidant vitamins and coronary heart disease. N Engl J Med. 1993;328(20):1487–9. [DOI] [PubMed] [Google Scholar]
- 5. Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. N Engl J Med. 1996;334(18):1156–62. [DOI] [PubMed] [Google Scholar]
- 6. Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin E supplementation and cardiovascular events in high-risk patients. N Engl J Med. 2000;342(3):154–60. [DOI] [PubMed] [Google Scholar]
- 7. The Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330(15):1029–35. [DOI] [PubMed] [Google Scholar]
- 8. Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL Jr, Valanis B, Williams JH Jr et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88(21):1550–9. [DOI] [PubMed] [Google Scholar]
- 9. Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349(17):1605–13. [DOI] [PubMed] [Google Scholar]
- 10. Ristow M, Schmeisser S.. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51(2):327–36. [DOI] [PubMed] [Google Scholar]
- 11. Bakan N, Taysi S, Yilmaz O, Bakan E, Kuskay S, Uzun N, Gundogdu M. Glutathione peroxidase, glutathione reductase, Cu-Zn superoxide dismutase activities, glutathione, nitric oxide, and malondialdehyde concentrations in serum of patients with chronic lymphocytic leukemia. Clin Chim Acta. 2003;338(1–2):143–9. [DOI] [PubMed] [Google Scholar]
- 12. Lubos E, Sinning CR, Schnabel RB, Wild PS, Zeller T, Rupprecht HJ, Bickel C, Lackner KJ, Peetz D, Loscalzo J et al. Serum selenium and prognosis in cardiovascular disease: results from the AtheroGene study. Atherosclerosis. 2010;209(1):271–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168(4):404–10. [DOI] [PubMed] [Google Scholar]
- 14. Akbaraly NT, Arnaud J, Hininger-Favier I, Gourlet V, Roussel AM, Berr C. Selenium and mortality in the elderly: results from the EVA study. Clin Chem. 2005;51(11):2117–23. [DOI] [PubMed] [Google Scholar]
- 15. Jenkins DJA, Spence JD, Giovannucci EL, Kim YI, Josse R, Vieth R, Blanco Mejia S, Viguiliouk E, Nishi S, Sahye-Pudaruth S et al. Supplemental vitamins and minerals for CVD prevention and treatment. J Am Coll Cardiol. 2018;71(22):2570–84. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Begg CB, Mazumdar M.. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 18. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Centre for Evidence-Based Medicine Number needed to treat (NNT) [Internet] [accessed on Mar 13, 2018). Available from: https://www.cebm.net/2014/03/number-needed-to-treat-nnt/. [Google Scholar]
- 20. Smeeth L, Haines A, Ebrahim S. Numbers needed to treat derived from meta-analyses—sometimes informative, usually misleading. BMJ. 1999;318(7197):1548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012(3):CD007176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Little PJ, Bhattacharya R, Moreyra AE, Korichneva IL. Zinc and cardiovascular disease. Nutrition. 2010;26(11–12):1050–7. [DOI] [PubMed] [Google Scholar]
- 23. Al-Bayati MA, Jamil DA, Al-Aubaidy HA. Cardiovascular effects of copper deficiency on activity of superoxide dismutase in diabetic nephropathy. N Am J Med Sci. 2015;7(2):41–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JPT, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. [DOI] [PubMed] [Google Scholar]
- 26. Schünemann H, Brożek J, Guyatt G, Oxman A, GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. Hamilton, Canada: The GRADE Working Group; 2013. Available from: guidelinedevelopment.org/handbook. [Google Scholar]
- 27. McKeown-Eyssen G, Holloway C, Jazmaji V, Bright-See E, Dion P, Bruce WR. A randomized trial of vitamins C and E in the prevention of recurrence of colorectal polyps. Cancer Res. 1988;48(16):4701–5. [PubMed] [Google Scholar]
- 28. DeCosse JJ, Miller HH, Lesser ML. Effect of wheat fiber and vitamins C and E on rectal polyps in patients with familial adenomatous polyposis. J Natl Cancer Inst. 1989;81(17):1290–7. [DOI] [PubMed] [Google Scholar]
- 29. Korpela H, Kumpulainen J, Jussila E, Kemilä S, Kääriäinen M, Kääriäinen T, Sotaniemi EA. Effect of selenium supplementation after acute myocardial infarction. Res Commun Chem Pathol Pharmacol. 1989;65(2):249–52. [PubMed] [Google Scholar]
- 30. Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ, Yang CS, Zheng SF, Gail M, Li GY et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85(18):1483–92. [DOI] [PubMed] [Google Scholar]
- 31. Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH et al. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med. 1996;334(18):1150–5. [DOI] [PubMed] [Google Scholar]
- 32. Girodon F, Lombard M, Galan P, Brunet-Lecomte P, Monget AL, Arnaud J, Preziosi P, Hercberg S. Effect of micronutrient supplementation on infection in institutionalized elderly subjects: a controlled trial. Ann Nutr Metab. 1997;41(2):98–107. [DOI] [PubMed] [Google Scholar]
- 33. Tardif JC, Cote G, Lesperance J, Bourassa M, Lambert J, Doucet S, Bilodeau L, Nattel S, de Guise P. Probucol and multivitamins in the prevention of restenosis after coronary angioplasty. Multivitamins and Probucol Study Group. N Engl J Med. 1997;337(6):365–72. [DOI] [PubMed] [Google Scholar]
- 34. Girodon F, Galan P, Monget AL, Boutron-Ruault MC, Brunet-Lecomte P, Preziosi P, Arnaud J, Manuguerra JC, Herchberg S. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. MIN. VIT. AOX. geriatric network. Arch Intern Med. 1999;159(7):748–54. [DOI] [PubMed] [Google Scholar]
- 35. Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, Realpe JL, Malcom GT, Li D, Johnson WD et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti–H elicobacter pylori therapy. J Natl Cancer Inst. 2000;92(23):1881–8. [DOI] [PubMed] [Google Scholar]
- 36. Jacobson JS, Begg MD, Wang LW, Wang Q, Agarwal M, Norkus E, Singh VN, Young TL, Yang D, Santella RM. Effects of a 6-month vitamin intervention on DNA damage in heavy smokers. Cancer Epidemiol Biomarkers Prevent. 2000;9(12):1303–11. [PubMed] [Google Scholar]
- 37. Leppala JM, Virtamo J, Fogelholm R, Albanes D, Taylor PR, Heinonen OP. Vitamin E and beta carotene supplementation in high risk for stroke: a subgroup analysis of the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Arch Neurol. 2000;57(10):1503–9. [DOI] [PubMed] [Google Scholar]
- 38. Salonen JT, Nyyssonen K, Salonen R, Lakka HM, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Lakka TA, Rissanen T, Leskinen L et al. Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) study: a randomized trial of the effect of vitamins E and C on 3-year progression of carotid atherosclerosis. J Intern Med. 2000;248(5):377–86. [DOI] [PubMed] [Google Scholar]
- 39. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119(10):1439–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583–92. [DOI] [PubMed] [Google Scholar]
- 41. Chylack LT Jr, Brown NP, Bron A, Hurst M, Kopcke W, Thien U, Schalch W. The Roche European American Cataract Trial (REACT): a randomized clinical trial to investigate the efficacy of an oral antioxidant micronutrient mixture to slow progression of age-related cataract. Ophthalmic Epidemiol. 2002;9(1):49–80. [DOI] [PubMed] [Google Scholar]
- 42. Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF Jr, Slate EH, Fischbach LA, Marshall JR, Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prevent. 2002;11(7):630–9. [PubMed] [Google Scholar]
- 43. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet North Am Ed. 2002;360(9326):23–33. [DOI] [PubMed] [Google Scholar]
- 44. Waters DD, Alderman EL, Hsia J, Howard BV, Cobb FR, Rogers WJ, Ouyang P, Thompson P, Tardif JC, Higginson L et al. Effects of hormone replacement therapy and antioxidant vitamin supplements on coronary atherosclerosis in postmenopausal women: a randomized controlled trial. JAMA. 2002;288(19):2432–40. [DOI] [PubMed] [Google Scholar]
- 45. Virtamo J, Pietinen P, Huttunen JK, Korhonen P, Malila N, Virtanen MJ, Albanes D, Taylor PR, Albert P. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA. 2003;290(4):476–85. [DOI] [PubMed] [Google Scholar]
- 46. Tornwall ME, Virtamo J, Korhonen PA, Virtanen MJ, Taylor PR, Albanes D, Huttunen JK. Effect of alpha-tocopherol and beta-carotene supplementation on coronary heart disease during the 6-year post-trial follow-up in the ATBC study. Eur Heart J. 2004;25(13):1171–8. [DOI] [PubMed] [Google Scholar]
- 47. Limburg PJ, Wei W, Ahnen DJ, Qiao Y, Hawk ET, Wang G, Giffen CA, Wang G, Roth MJ, Lu N et al. Randomized, placebo-controlled, esophageal squamous cell cancer chemoprevention trial of selenomethionine and celecoxib. Gastroenterology. 2005;129(3):863–73. [DOI] [PubMed] [Google Scholar]
- 48. Mooney LA, Madsen AM, Tang D, Orjuela MA, Tsai WY, Garduno ER, Perera FP. Antioxidant vitamin supplementation reduces benzo(a)pyrene-DNA adducts and potential cancer risk in female smokers. Cancer Epidemiol Biomarkers Prevent. 2005;14(1):237–42. [PubMed] [Google Scholar]
- 49. Stone PH, Lloyd-Jones DM, Kinlay S, Frei B, Carlson W, Rubenstein J, Andrews TC, Johnstone M, Sopko G, Cole H et al. Effect of intensive lipid lowering, with or without antioxidant vitamins, compared with moderate lipid lowering on myocardial ischemia in patients with stable coronary artery disease: the Vascular Basis for the Treatment of Myocardial Ischemia Study. Circulation. 2005;111(14):1747–55. [DOI] [PubMed] [Google Scholar]
- 50. Bairati I, Meyer F, Jobin E, Gelinas M, Fortin A, Nabid A, Brochet F, Tetu B. Antioxidant vitamins supplementation and mortality: a randomized trial in head and neck cancer patients. Int J Cancer. 2006;119(9):2221–4. [DOI] [PubMed] [Google Scholar]
- 51. Stranges S, Marshall JR, Trevisan M, Natarajan R, Donahue RP, Combs GF, Farinaro E, Clark LC, Reid ME. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. Am J Epidemiol. 2006;163(8):694–9. [DOI] [PubMed] [Google Scholar]
- 52. Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261(3):276–84. [DOI] [PubMed] [Google Scholar]
- 53. Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167(15):1610–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Plummer M, Vivas J, Lopez G, Bravo JC, Peraza S, Carillo E, Cano E, Castro D, Andrade O, Sanchez V et al. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: a randomized trial in a high-risk population. J Natl Cancer Inst. 2007;99(2):137–46. [DOI] [PubMed] [Google Scholar]
- 55. Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300(18):2123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2009;301(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hercberg S, Kesse-Guyot E, Druesne-Pecollo N, Touvier M, Favier A, Latino-Martel P, Briancon S, Galan P. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: a postintervention follow-up in the SU.VI.MAX Study. Int J Cancer. 2010;127(8):1875–81. [DOI] [PubMed] [Google Scholar]
- 58. Stratton MS, Algotar AM, Ranger-Moore J, Stratton SP, Slate EH, Hsu CH, Thompson PA, Clark LC, Ahmann FR. Oral selenium supplementation has no effect on prostate-specific antigen velocity in men undergoing active surveillance for localized prostate cancer. Cancer Prev Res. 2010;3(8):1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Marshall JR, Tangen CM, Sakr WA, Wood DP Jr, Berry DL, Klein EA, Lippman SM, Parnes HL, Alberts DS, Jarrard DF et al. Phase III trial of selenium to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prev Res. 2011;4(11):1761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rayman MP, Stranges S, Griffin BA, Pastor-Barriuso R, Guallar E. Effect of supplementation with high-selenium yeast on plasma lipids: a randomized trial. Ann Intern Med. 2011;154(10):656–65. [DOI] [PubMed] [Google Scholar]
- 61. Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, Liu WD, Hu Y, Han ZX, Crystal-Mansour S et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104(6):488–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Algotar AM, Stratton MS, Ahmann FR, Ranger-Moore J, Nagle RB, Thompson PA, Slate E, Hsu CH, Dalkin BL, Sindhwani P et al. Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer. Prostate. 2013;73(3):328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Arruda MM, Mecabo G, Rodrigues CA, Matsuda SS, Rabelo IB, Figueiredo MS. Antioxidant vitamins C and E supplementation increases markers of haemolysis in sickle cell anaemia patients: a randomized, double-blind, placebo-controlled trial. Br J Haematol. 2013;160(5):688–700. [DOI] [PubMed] [Google Scholar]
- 64. Bonelli L, Puntoni M, Gatteschi B, Massa P, Missale G, Munizzi F, Turbino L, Villanacci V, De Censi A, Bruzzi P. Antioxidant supplement and long-term reduction of recurrent adenomas of the large bowel: a double-blind randomized trial. J Gastroenterol. 2013;48(6):698–705. [DOI] [PubMed] [Google Scholar]
- 65. Karp DD, Lee SJ, Keller SM, Wright GS, Aisner S, Belinsky SA, Johnson DH, Johnston MR, Goodman G, Clamon G et al. Randomized, double-blind, placebo-controlled, phase III chemoprevention trial of selenium supplementation in patients with resected stage I non-small-cell lung cancer: ECOG 5597. J Clin Oncol. 2013;31(33):4179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang L, Sesso HD, Glynn RJ, Christen WG, Bubes V, Manson JE, Buring JE, Gaziano JM. Vitamin E and C supplementation and risk of cancer in men: posttrial follow-up in the Physicians' Health Study II randomized trial. Am J Clin Nutr. 2014;100(3):915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Goossens ME, Zeegers MP, van Poppel H, Joniau S, Ackaert K, Ameye F, Billiet I, Braeckman J, Breugelmans A, Darras J et al. Phase III randomised chemoprevention study with selenium on the recurrence of non-invasive urothelial carcinoma. The SELEnium and BLAdder cancer Trial. Eur J Cancer. 2016;69:9–18. [DOI] [PubMed] [Google Scholar]
- 68. Thompson PA, Ashbeck EL, Roe DJ, Fales L, Buckmeier J, Wang F, Bhattacharyya A, Hsu CH, Chow HH, Ahnen DJ et al. Selenium supplementation for prevention of colorectal adenomas and risk of associated type 2 diabetes. J Natl Cancer Inst. 2016;108(12):djw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Rayman MP, Winther KH, Pastor-Barriuso R, Cold F, Thvilum M, Stranges S, Guallar E, Cold S. Effect of long-term selenium supplementation on mortality: results from a multiple-dose, randomised controlled trial. Free Radic Biol Med. 2018;127:46–54. [DOI] [PubMed] [Google Scholar]
- 70. Lopes G, Avila FW, Guilherme LRG. Selenium behavior in the soil environment and its implication for human health. Ciencia Agrotec. 2017;41(6):605–15. [Google Scholar]
- 71. Biesalski HK, Grune T, Tinz J, Zollner I, Blumberg JB. Reexamination of a meta-analysis of the effect of antioxidant supplementation on mortality and health in randomized trials. Nutrients. 2010;2(9):929–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Guo C, Li X, Wang R, Yu J, Ye M, Mao L, Zhang S, Zheng S. Association between oxidative DNA damage and risk of colorectal cancer: sensitive determination of urinary 8-hydroxy-2'-deoxyguanosine by UPLC-MS/MS analysis. Sci Rep. 2016;6:32581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gao J, Liu Y, Huang Y, Lin ZQ, Banuelos GS, Lam MHW, Yin X. Daily selenium intake in a moderate selenium deficiency area of Suzhou, China. Food Chem. 2011;126(3):1088–93. [Google Scholar]
- 74. Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159(12):824–34. [DOI] [PubMed] [Google Scholar]
- 75. Petersen SV, Oury TD, Valnickova Z, Thogersen IB, Hojrup P, Crapo JD, Enghild JJ. The dual nature of human extracellular superoxide dismutase: one sequence and two structures. Proc Natl Acad Sci. 2003;100(24):13875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Etminan M, FitzGerald JM, Gleave M, Chambers K. Intake of selenium in the prevention of prostate cancer: a systematic review and meta-analysis. Cancer Causes Control. 2005;16(9):1125–31. [DOI] [PubMed] [Google Scholar]
- 77. Allen NE, Travis RC, Appleby PN, Albanes D, Barnett MJ, Black A, Bueno-de-Mesquita HB, Deschasaux M, Galan P, Goodman GE et al. Selenium and prostate cancer: analysis of individual participant data from fifteen prospective studies. J Natl Cancer Inst. 2016;108(11):djw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Arthur JR. The glutathione peroxidases. Cell Mol Life Sci. 2000;57(13–14):1825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hatfield DL, Tsuji PA, Carlson BA, Gladyshev VN. Selenium and selenocysteine: roles in cancer, health, and development. Trends Biochem Sci. 2014;39(3):112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ceriello A. Impaired glucose tolerance and cardiovascular disease: the possible role of post-prandial hyperglycemia. Am Heart J. 2004;147(5):803–7. [DOI] [PubMed] [Google Scholar]
- 81. Tainer JA, Getzoff ED, Richardson JS, Richardson DC. Structure and mechanism of copper, zinc superoxide dismutase. Nature. 1983;306(5940):284–7. [DOI] [PubMed] [Google Scholar]
- 82. Ceriello A, Motz E.. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24(5):816–23. [DOI] [PubMed] [Google Scholar]
- 83. Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health. 2009;6(2):445–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011;306(14):1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yarmolinsky J, Bonilla C, Haycock PC, Langdon RJQ, Lotta LA, Langenberg C, Relton CL, Lewis SJ, Evans DM, PRACTICAL Consortium, Davey Smith G, Martin RM. Circulating Selenium and Prostate Cancer Risk: A Mendelian Randomization Analysis. J Natl Cancer Inst. 2018;110(9):1035–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen CY, Kamil A, Blumberg JB. Phytochemical composition and antioxidant capacity of whole wheat products. Int J Food Sci Nutr. 2015;66(1):63–70. [DOI] [PubMed] [Google Scholar]
- 87. Bolling BW, Blumberg JB, Chen CO. The influence of roasting, pasteurisation, and storage on the polyphenol content and antioxidant capacity of California almond skins. Food Chem. 2010;123(4):1040–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wallace TC, Bailey RL, Blumberg JB, Burton-Freeman B, Chen CO, Crowe-White KM, Drewnowski A, Hooshmand S, Johnson E, Lewis R et al. Fruits, vegetables, and health: a comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit Rev Food Sci Nutr. 2019;60(13):2174–211. [DOI] [PubMed] [Google Scholar]
- 89. Rayman MPW, Winther KH, Pastor-Barriuso R, Cold F, Thvilum M, Stranges S, Guallar E, Cold S. Effect of long-term selenium supplementation on mortality: results from a multiple-dose, randomised controlled trial. Free Radic Biol Med. 2018;127:46–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.