Abstract
Rivaroxaban (RVX) was suggested to possess anti-inflammatory and vascular tone modulatory effects. The goal of this study was to investigate whether RVX impacts lipopolysaccharide (LPS)-induced acute vascular inflammatory response. Male rats were treated with 5 mg/kg RVX (oral gavage) followed by 10 mg/kg LPS i.p injection. Circulating levels of IL-6, MCP-1, VCAM-1, and ICAM-1 were measured in plasma 6 and 24 hours after LPS injection, while isolated aorta was used for gene expression analysis, immunohistochemistry, and vascular tone evaluation. RVX pre-treatment significantly reduced LPS mediated increase after 6h and 24h for IL-6 (4.4±2.2 and 2.8±1.7 fold), MCP-1 (1.4±1.5 and 1.3±1.4 fold) VCAM-1 (1.8±2.0 and 1.7±2.1 fold). A similar trend was observed in the aorta for iNOS (5.5±3.3 and 3.3±1.9 folds reduction, P<0.01 and P<0.001, respectively), VCAM-1 (1.3±1.2 and 1.4±1.3 fold reduction, P<0.05), and MCP-1 (3.9±2.2 and 1.9±1.6 fold reduction, P<0.01). Moreover, RVX pre-treatment, improved LPS-induced PE contractile dysfunction in aortic rings (Control vs LPS, Emax reduction = 35.4 and 31.19%, P<0.001; Control vs LPS+RVX, Emax reduction = 10.83 and 11.48%, P>0.05, respectively), resulting in 24.5% and 19.7% change in maximal constriction in LPS and LPS+RVX respectively. These data indicate that RVX pre-treatment attenuates LPS-induced acute vascular inflammation and contractile dysfunction.
Introduction
Coagulation plays a key role in cardiovascular disorders [1] and interfering with coagulation factors represents one of the main pharmacological approaches in CVD [2]. Coagulation factors, not only participate to the activation of the coagulation cascade but also impact vascular function; this is the case for factor X (FXa), one of the main components in the coagulation process [3], which, through the activation of protease-activated receptors (PAR) [4], affects, vasomotor responses, inflammation, endothelial function, vascular proliferation, cellular hypertrophy, atherosclerosis, and thrombosis [5,6].
As hemostatic and inflammatory pathways are highly interconnected [7,8], the approval of novel oral anticoagulants (NOAC), affecting FXa activity and prothrombin complexes (rivaroxaban, apixaban, betrixaban, and edoxaban) [9], have raised interest in the interplay between haemostasis and inflammation linking FXa blockade to PAR inhibition [10,11], and potentially to improved vascular function.
Clinical studies with NOACS have shown that these drugs reduce the incidence of cardiovascular events including coronary and peripheral artery disease, cerebral ischemia, thrombosis, thromboembolic events, and atherosclerosis [12,13]. In addition to this, experimental studies proposed a series of vascular protective properties of NOAC via inhibition of FXa [14–26]. These include potential anti-inflammatory effects [15,19–21,23,24,27], that might perhaps impact vascular function and pathology [14,25]. Indeed inflammation is one of the main contributing factors in coronary artery disease leading to the development of atherosclerosis [28]. Moreover, acute exposure to lipopolysaccharide endotoxin (LPS) has been shown to induce an inflammatory response that in turn supports vascular injury and dysfunction [29,30].
This raises the intriguing possibility that the impact on NOACS on vasomotor function [17,31] might depend also on the ability to control vascular function under acute inflammatory conditions.
To this aim, we used isolated rat aorta, to test the hypothesis that pre-treatment with Rivaroxaban (RVX) might mitigate LPS-induced acute vascular inflammation with a focus on pro-inflammatory, pro-adhesive, and contractile responses under LPS-induced vascular inflammatory conditions.
Material and methods
Animals and treatment
Wistar rats between 10–12 weeks of age (220–260 g) were used in our study. All rats were fed with a normal chow diet during the period of our study. Animals were accommodated in normal rat cages with automatically controlled 12-hours light/12-hour dark cycle and the standard temperature-humidity environment with ad libitum water and food intake. Acute inflammation was induced by a single intraperitoneal (i.p) injection of LPS (10 mg/kg body weight) [32]. RVX (5 mg/kg body weight; supplied by Bayer Pharma AG) was administered via oral gavage 2 hours before LPS injection, the non-RVX groups (control and LPS only) received oral gavage of RVX vehicle (Carboxymethylcellulose Sodium 0.5%). RVX dose and interval used in our study was previously shown to inhibit the in vivo Factor Xa in rat arteriovenous shunt model [33] or thrombus formation [34] and was chosen based on previous in-vivo related mice and rat animal studies [14,17,35,36], which are specific in the previously reported single p.o administration of the RVX pharmacokinetic profile as well [37,38]. Our study protocol has been approved by the Ethical Committee of Medical Faculty–University of Prishtina (Nr. 4962), and all procedures for animal experiments were performed in compliance with guidelines for care and use of animals during whole experimentation procedures.
ELISA
Rats were sacrificed with an i.p overdose of sodium thiopental injection (50 mg/kg body weight) at 6 hours and 24 hours after LPS injection. Blood was collected from the left ventricle (EDTA containing tubes) and plasma isolated following centrifugation (4000 rpm for 10 minutes) and stored at -80°C. IL-6, MCP-1, VCAM-1, and ICAM-1 plasma levels were measured by enzyme-linked immunoassay kit (Abcam, Cambridge, MA) according to manufacturer’s protocol instructions.
Aorta preparation
After blood collection, the whole rat aorta was isolated and cleaned immediately from adhering perivascular adipose and connective tissues. Aortic rings of 4–6 mm were cut from the ascending aorta and fixed in 10% neutral buffered formalin for further immunohistochemical analysis. Subsequently, aortic rings of 5–7 mm were cut from the remaining part of the ascending aorta and descending aorta and snap-frozen in liquid nitrogen for gene expression analysis. Finally, aortic rings of 3–5 mm were cut from the remaining part of the thoracic aorta and used for testing vascular reactivity in the tissue organ bath.
Immunohistochemistry
Formalin-fixed aortic rings were embedded in paraffin and sectioned in 2.5 μm sections. Tissue sections were deparaffinized as described [39], rehydrated and the heat mediated antigens retrieval was performed by placing the slides in 10 mM sodium citrate buffer (pH 6.0) for 45 minutes at 95–98°C. Blocking of endogenous peroxidase activity and non-specific staining was done with hydrogen peroxide and protein block. Subsequently, sections were incubated for 30 minutes with primary antibodies at the following dilution: 1:200 for anti-VCAM-1, 1:200 for anti-MCP-1, or 1:100 for Anti-iNOS. After washing steps, sections were incubated with a biotinylated secondary antibody (goat anti-polyvalent, Mouse, and Rabbit Specific HRP/DAB (ABC) Detection IHC kit, Abcam, Cambridge MA) for additional 15 minutes followed with streptavidin peroxidase 10’ incubation. Peroxidase activity was detected in fixed tissues with DAB substrate chromogen for detection of HRP-conjugated antibody and followed under a microscope to determine staining development. Finally, after the tipping DAB and rinsing in water, the slides were counterstained with hematoxylin histological staining reagent as described [40]. Images were acquired with Olympus CX41 microscope (Olympus America) with Olympus SC100 Digital camera and cell Sens Imaging Software. Relative quantification of IHC staining has been done with Image J (NIH, https://imagej.nih.gov/ij/).
Reverse transcription-quantitative real-time PCR (RT-qPCR) analysis
Total RNA was isolated from aortae by using RNeasy Fibrous Tissue Mini Kit (Qiagen, Hilden, Germany) following the standard protocol. RNA assessed for quality and quantity using absorption measurements (NanoDrop™ 1000 Spectrophotometer, Thermo Fisher Scientific) and retro-transcribed in cDNA with iScriptTM cDNA synthesis kit (BioRad) as described [41]. Gene expression analysis was performed using SYBR Green Supermix (Thermo Fisher Scientific) in CFX connect light cycler (BioRad, Cat#1708841) [42]. Expression was calculated using the ΔΔCt method (Livak and Schmittgen, 2001) and normalized to a housekeeping gene (GAPDH). The sequences are presented in S1 Table and expression levels were expressed with the fold change.
Vascular tone
Aortic ring fragments were mounted in the Tissue Organ Baths (750TOBS, DMT-USA, Ann Arbor, MI, USA) containing 10 mL of Krebs-Henseleit buffer (118.4 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3, 11.1 mM glucose; pH 7.4). The temperature was adjusted at 37°C and the buffer solution was bubble gassed with 5% CO2 and 95% O2 during the whole experiment. Changes in force tension were recorded by isometric force-displacement transducers and continuous mode on a multichannel recorder polygraph model attached with software LabChart7 connected to power lab 4/35 data acquisition system (PowerLab 4/35, ADInstruments Pty Ltd., NSW, Australia).
Each ring was initially stretched to an optimal load (∼2 g). Subsequently, preparations were equilibrated for 60 minutes with changes of fluid every 15 minutes. After the equilibration period, vessel specimen viability was tested with KCl (40 mM) induced contraction, and aortic segment preparations were washed until returning the basal tone. Thereafter, the vascular tone was determined with cumulative concentration-response curves with phenylephrine (10−8 to 10−5 M).
Statistical analysis
All data are presented and calculated with mean ± SEM. The number of rats used in our study was expressed with “n”. A comparison of parameters obtained within the analysis between two groups was performed with unpaired Student’s t-test. Vasoreactivity of the PE contractions was calculated as a percentage of the KCl (40 mM) maximal initial contraction value. The concentration-response curve values were analyzed with two-way ANOVA followed by Tukey’s post hoc test for comparison between groups. P-value < 0.05 was considered to represent a statistically significant group difference. All analyses and graphs were performed using GraphPad PRISM version (6.0).
Results
3.1 Effects of RVX pre-treatment on the acute LPS-induced increase in IL-6, MCP-1, VCAM-1 and ICAM-1 levels
In order to investigate the impact of RVX pre-treatment on LPS-induced pro-inflammatory and proadhesive mediators expression, IL-6, MCP-1, VCAM-1, and ICAM-1 levels were measured in plasma 6 hours and 24 hours post LPS injection. As expected, LPS injection increased plasma levels IL-6, MCP-1, VCAM-1, and ICAM-1(P<0.001) (Fig 1A–1D); an effect which was significantly blunted with RVX pre-treatment (5 mg/kg) for IL-6 (P<0.01), MCP-1 (P<0.05) and VCAM-1 (P<0.05) both after 6 and 24 hours of treatment. These results suggest that RVX pretreatment limits LPS induced inflammatory response (Fig 1A–1C).
Fig 1. Role of RVX pre-treatment on LPS-induced proinflammatory and proadhesive mediator release in rat plasma.
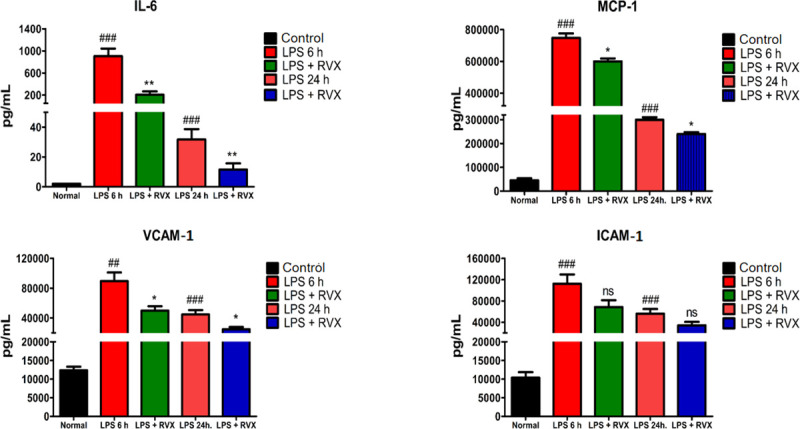
IL-6 (A), MCP-1 (1B), VCAM-1 (C) and ICAM-1 (D) plasma levels (pg/mL) from LPS treated rats in the presence or absence of RVX for 6 hours and 24 hours compared to controls. ##P<0.01 and ###P<0.001 (Student’s t-test) vs. control conditions. *P<0.05, ** P<0.01 and ***P<0.001 (Student’s t-test) vs LPS. Values are expressed as the mean±SEM (n = 6).
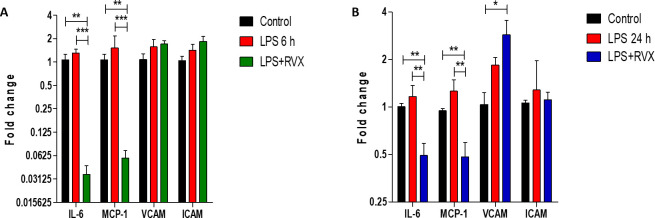
3.2 Effects of RVX pre-treatment on IL-6, MCP-1, VCAM-1 and ICAM-1 gene expression the aorta
Next, we tested whether RVX treatment could improve pro-inflammatory gene expression in the aorta and liver. RVX pre-treatment (5 mg/kg) attenuated IL-6 and MCP-1 mRNA expression in the arterial wall (Fig 2A and 2B), while non-significant changes were observed for VCAM-1 and ICAM-1 expression at this site. Most importantly RVX pre-treatment did not affect LPS induced liver expression of IL-6, MCP-1, VCAM-1, and ICAM-1, by confirming also the liver as a key target of LPS induced acute inflammation (S1 Fig).
Fig 2. Role of RVX pre-treatment on LPS induced proinflammatory and proadhesive mediator’s gene expression in rat aorta.
Comparisons of relative IL-6, MCP-1, VCAM-1 and ICAM-1 gene expression levels normalized to GAPDH in the rat aorta obtained from RVX- or non-treated LPS rats and non-treated control rats 6 hours (A) and 24 hours (B) post LPS exposure. *P<0.05, ** P<0.01 and ***P<0.001 indicates values significantly different (Student’s t-test) vs LPS. Values are expressed as the mean±SEM (n = 6).
3.3 Effects of RVX pre-treatment on LPS-induced iNOS, MCP-1, and VCAM-1 wall expression in the vascular wall
Increased iNOS, MCP-1, and VCAM-1 immunoreactivity were observed in the aortic vascular tissues of LPS treated rats mainly in vascular endothelium, and the subendothelial layer was characterized by smooth muscle cells and perivascular adipose tissues compared to controls (P<0.001) (Fig 3A–3C). RVX pre-treatment (5 mg/kg) reduced LPS-induced iNOS, MCP-1, and VCAM-1 expression both at 6 h and 24 h following LPS injection (Fig 3A–3C).
Fig 3. Role of RVX pre-treatment on LPS induced proinflammatory and proadhesive mediator’s protein expression in rat aorta.
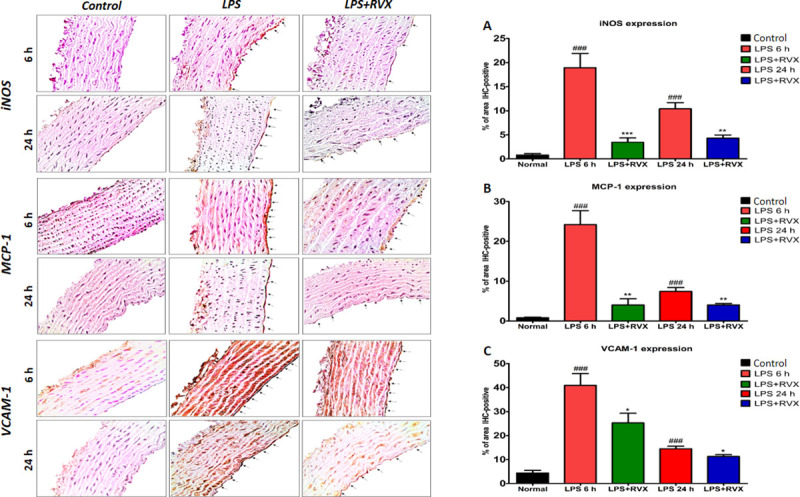
Representative immunohistochemical results for aortic iNOS (A), MCP-1 (B) and VCAM-1 (C) expressions from RVX- or non-treated LPS rats and non-treated control rats as described in the legend. % of IHC positive areas are represented as graphs. ### indicates values significantly different (Student’s t-test) vs. control conditions. *P<0.05, ** P<0.01 and ***P<0.001 indicates values significantly different (Student’s t-test) vs LPS. Values are expressed as the mean±SEM (n = 6).
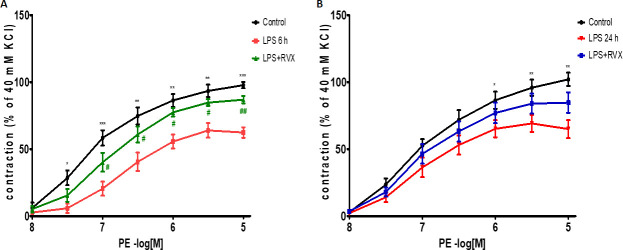
3.4 Effects of RVX pre-treatment on the acute LPS-induced PE contractile dysfunction in aortic rings
Next, we addressed whether improved anti-inflammatory effects of RVX pre-treatment (5 mg/kg) translate into the amelioration of LPS-induced contractile dysfunction to PE. LPS injection deteriorated PE-induced vasoconstriction when compared with the control group [Emax, 62.33±3.8% for LPS (6 h) compared to controls 97.70.±2.30%, P<0.001; and Emax, 71.91±4.81% for LPS (24 h) compared to controls: Emax, 103.1.±3.61%, P<0.01] (Control vs LPS, Emax reduction = 35.4 and 31.19%, P<0.001) (Fig 4A and 4B) (Table 1).
Fig 4.
Role of RVX on the vasoreactivity of aortic rings obtained from A) rats sacrificed 6 hours post LPS and B) rats sacrificed 24 hours post LPS to PE-induced contractions. Comparisons of vascular reactivity to PE in aortic rings from RVX- or non-treated LPS rats and non-treated control rats. *P<0.05, ** P<0.01 and ***P<0.001 indicates statistical significance (Two-Way ANOVA followed Bonferroni correction) vs. control; #P<0.05 and ##P<0.01 indicates values significantly different (Two-Way ANOVA followed Bonferroni correction) vs. LPS. Values are expressed as the ± SEM (n = 6).
Table 1. Role of RVX on the vasoreactivity of aortic rings obstained from rats sacrificed 6 h and 24 h post LPS to PE-induced dose dependent contractions.
| Contractile Agent | Pretreatment | pEC50 | Emax | N |
|---|---|---|---|---|
| Control 6 h | 7.06±0.06 | 97.70±2.30 | 6 | |
| PE | LPS 6 h | 5.92±0.11+++ | 62.33±3.80*** | 6 |
| LPS+RVX 6 h | 6.68±0.06$ $ | 86.87±2.72## | 6 | |
| Control 24 h | 6.96±0.06 | 103.1±3.61 | 6 | |
| LPS 24 h | 6.28±0.12++ | 71.91±4.8** | 6 | |
| LPS+RVX 24 h | 6.74±0.08$ | 91.62±5.8# | 6 |
PE: Phenylephrine. Values are mean ± SEM from (n) different patients. pEC50 and Emax (maximal contraction, % KCl 40 mM) are derived from concentration-response curves presented in Fig 4A and 4B. These values are significantly different:
** P<0.01
*** P<0.001 vs corresponding controls (Control)
# p<0.05
## p<0.01 vs corresponding controls (LPS 6 h)
+++ p<0.001
++ p<0.01
$ $ p<0.01
$ p<0.05, when compared to pEC50 values derived from corresponding controls vasoconstriction.
RVX pre-treatment (5 mg/kg) partially reverted LPS-induced PE contractile dysfunction at both 6 h and 24 h following LPS injection (Emax, 86.87±2.72%, and 91.62±5.83%, for LPS+RVX treatment; vs Emax, 62.33±3.8 and 71.91±4.81 for LPS alone, P<0.01), maximal constriction with RVX+LPS was 24.5 and 19.7% higher compared to LPS alone 6 h and 24 h respectively (Fig 4A and 4B) (Table 1). These results suggest that RVX attenuates contractile dysfunction to PE during acute LPS inflammation.
Discussion
In this study, we demonstrated that a specific inhibitor of FXa, namely rivaroxaban, improves acute inflammation and vascular dysfunction following LPS-induced endotoxin shock.
Besides the role of factor Xa in the coagulation process, this factor contributes also to the pathogenesis of cardiovascular inflammatory disease through PARs and non-PAR receptors signaling mediated response in the vasculature [43,44]. Moreover, previous studies have shown that LPS affects the coagulation cascade by targeting FXa and its intracellular signaling which contributed to the increased inflammatory response and vascular modulation mainly through PAR activated receptors signaling [6,45,46]. For instance, the PAR-2 signaling activation contributes to the activation of macrophages and also to vascular inflammation [47]. Interestingly the time-dependent activation of PAR-2 receptors in the vascular and respiratory tissues obtained from rats is induced from LPS itself [48,49]. Also, in other studies, these response was followed by the activation of the inflammatory pathway, via TL-4/NF-κB signaling [50,51]. Most of these responses were shown to be with RVX (Table 2). Of note LPS causes an inflammatory state characterized by increased proinflammatory and pro adhesive responses [52,53], and this could propagate in septic shock and related major complications such as organ failures e.g. respiratory, heart or kidney failures, or abnormal blood clotting (DIC) [54].
Table 2. Basic experimental studies that investigate the anti-inflammatory properties of pre-treatment and post-treatment with RVX.
| Species | Tissue/Model | Pre-Treatment (1) | Post-Treatment (2) | Response 1 | Response 2 | References |
|---|---|---|---|---|---|---|
| Rat | Lung/LPS | RVX | - | TNF-α, MCP-1, IL-1β, PAR-2, NF-κB ↓ | - | [50] |
| 0.2 or 0.4 mg/g, | ||||||
| 10 days | ||||||
| Rat | Femoral Artery/Atherosclerosis Obliterans | - | RVX | - | IL-1, MCP-1, TNF- α, NF-κB,TLR4 ↓ | [51] |
| 10 mg/kg/day | ||||||
| 4 weeks | ||||||
| Rat | Middle Cerebral Artery/Temporary focal cerebral ischaemia | RVX | RVX | IL-1β, IFN-γ, TNF- α, ICAM-1, CD68 ↓ | [26] | |
| 3 mg/kg/8 hours | 12 mg/kg/ 8 h | |||||
| and 16 h | ||||||
| Mice | Aorta/ApoE -/- | - | RVX | - | TNF- α, IL-6, MCP-1, Egr-1, IFN-γ ↓ | [35] |
| 1 or 5 mg/kg/day | ||||||
| 26 weeks | ||||||
| Mice | Aorta/ApoE -/- | - | RVX | - | TNF- α, COX-2, iNOS, MMP-9, MMP-1 ↓ | [14] |
| 5 mg/kg/day | ||||||
| 20 weeks | ||||||
| Mice | Aorta/ApoE -/- | - | RVX | - | PAR-1,PAR-2, Mac-2, MMP-9 ↓ | [63] |
| 1.2 mg/kg/day | ||||||
| 14 weeks | ||||||
| Mice | EJV/Catheter | RVX | - | MCP-1, MMP-9 ↓ | [15] | |
| Thrombosis | 5 mg/kg/day | |||||
| 21 days | ||||||
| Mice | Atrial/TAC | - | RVX | - | TNF-α, MCP-1, IL-1β, IL-6, PAR-2 ↓ | [21] |
| 30 μg/g/day | ||||||
| 2 weeks | ||||||
| Mice | Left Ventricle/TAC | RVX | IL-1β, IL-6, IFN-γ, NF-κB, TGF-β, CD-45 ↓ | [77] | ||
| 1 or 10 mg/kg/day | ||||||
| 3 weeks | ||||||
| Mice | Left Ventricular/Myocardial Ischaemia-RI and TF | RVX | IL-6, PAR-2, collagen | [16] | ||
| 0.6 or 1.2 g/kg feed/day | 1α2 and 3α1 ↓ | |||||
| 14 days | ||||||
| Mice | Heart/Myocardial Infarction | - | 138.5mg/kg/day chow | TNF-α, PPAR-1, PAR-2, TGF-β, ↓ | [78] | |
| 7 days | ||||||
| Mice | Aortic root, Coronary Arteries/ICM | - | RVX | - | IL-1β, IL-6, NF-κB | [23] |
| 10 mg/kg/day | TNF-α, MMP9, MMP12, TIMP1, TGF-β, PAR-1, PAR-2 ↓ | |||||
| 2 weeks | ||||||
| Mice | Kidney/Ren-TG Hypertensive | - | RVX | - | TNF-α, MCP-1, Pal-1, PAR-2 ↓ | [24] |
| 6 or 12 mg/kg | ||||||
| 1 or 4 months | ||||||
| Mice | Lung/BERKss, vascular Inflammation | - | RVX | - | IL-6, MPO,TAT ↓ | [64] |
| 0.4 mg/g chow | ||||||
| 10 days | ||||||
| Mice | Hind Limb/STZ Diabetes, Ischaemia | RVX | RVX | - | Neovascularisation, CD-31, VEGF ↑ | [17] |
| 1 or 3 mg/kg/day | 1 or 3 mg/kg/day | |||||
| 2 weeks | 3 weeks | |||||
| Mice | Femoral Arteries/Wire-Mediated Vascular Injury | RVX | RVX | - | TNF-α, MCP-1, IL-1β, (TGF)-β1, SDF-1, GM-CSF ↓ | [25] |
| 5 mg/kg/day | 5 mg/kg/day | |||||
| 1 week | 1 week | |||||
| Human | HUVEC/Thrombin | RVX | - | ICAM-1, ELAM-1, IL-8, MCP-1, CXCL1, CXCL2, TF ↓ | - | [19] |
| 0.3–3000 nM | ||||||
| 30 min | ||||||
| Human | HUVEC/Inflammation | RVX | - | TNF-α, IL-6, IL-1β, NF-κB ↓ | - | [79] |
| 1000 nM | ||||||
| 24 hours | ||||||
| Human | HUVEC/FXa Inflammation | - | RVX | - | CCL-2,CCL-5, EDN2, ITGA5, SELE, VCAM-1, TNSF10, MMP-2 ↓ | [80] |
| 50 nM | ||||||
| 12 h | ||||||
| Human | Abdominal Aorta/Aneurysm | - | RVX | - | IL-6, NOS-2, MMP-9 ↓ | [20] |
| 50 nM | ||||||
| Human | Podocytes/Ang-II-induced Inflammation | RVX | - | - | TNF-α, MCP-1, IL-6, PAR-2, NF-κB ↓ | [24] |
| 500 μg/L | ||||||
| 1 hour | ||||||
| Human | Kidney Tubular Cells/AGEs | - | RVX | - | MCP-1, ↓ | [62] |
| 300 nM | ||||||
| 4 hours |
Abbreviations: RVX, Rivaroxaban; LPS, Lipopolysaccharide; AGEs, Advanced glycation end products; RI, Reperfusion Injury; Ang-II, Angiotensin II: STZ, Streptozocin; BERKss, Berkeley Sicle Cell Mice; Ren-TG, Transgenic Ren-2 Mice;TAC, Transverse Aortic Constriction; ICM,Ischaemic Cardiomyopathy; HUVEC, Human Umbilical Vein Endothelial Cells;TNF-α,Tumour Necrosis Factor Alpha;MCP-1, Monocyte Chemottractant Protein;IL-1β,Interleukin-1 Beta;PAR-2, Protease-Activated Receptor 2;NF-κB, Nuclear Factor Kappa Betta;MMP, Matrix metallopeptidase;GM-CSF,Granulocyte-Macrophage Colony-stimulating Factor; SDF-1,Stromal Cell-Derived Factor 1;VEGF,Vascular Endothelial Growth Factor;NOS-2,Nitric Oxide Synthase-2; Egr-1,Early Growth Response Protein 1;IFN-γ,Interferon Gamma; TF,Thrombin Factor; CCL, C–C Motif Chemokine Ligand, EDN2, Endothelin-2, ITGA5, Integrin Alpha-5/Beta-1; SELE, E-Selectin;TNSF10, Tumor Necrosis Factor (Ligand) Superfamily, Member 10; TLR4, Toll Like Receptor 4.
Nowadays, there are different experimental and clinical therapeutic interventions in sepsis [55–57], and targeting the cross-talk between inflammation and coagulation represents an emerging approach for targeting acute conditions as well as improving long term vascular outcomes in inflamed conditions [8,58,59].
Most clinical studies demonstrated that targeting Factor Xa inhibition with NOAC including rivaroxaban prevented systemic thromboembolic disease, reduced cardiovascular events, and death [60]. Moreover, NOAC non-hemostatic cellular effects suggest a potential benefit in inflammation, arterial stiffness, neointima formation, atherosclerosis, and fibrosis [5,61].
Although some anti-inflammatory effects, improvement of hypercoagulable actions such as disseminated intravascular coagulation (DIC), additional acute lung injury from endotoxemia [19,36,50], and additional vasculoprotective properties of RVX have been proposed in different in vitro and tissue models [14,15,20,21,23–25] (see Table 2), a beneficial effect on LPS induced acute vascular inflammatory response in vivo was not investigated yet. In murine macrophages and human tubular cells stimulated with FXa, RVX treatment was shown to reduce the expression of TNF-α, IL-1β, and MCP-1 [14,62]. Similarly, RVX dampened the expression of VCAM-1, ICAM-1, MCP-1, IL-8, CXCL1, CXCL2, TF in thrombin stimulated human endothelial cells [19], as well as IL-6, IL-1β, TNF-α, MMP9, and COL-1 expression in hypoxic cardiac myocytes and fibroblasts [23]. Similarly, also TNF-α, MCP-1, IL-6 expression in angiotensin II-induced inflammatory response in human podocytes was modulated by RVX [24].
Herein, our results extend these findings by demonstrating in vivo that RVX pre-treatment decreased the expression of pro-inflammatory mediators and adhesion molecules namely IL-6, MCP-1, and VCAM-1 induced by LPS in the aorta.
Earlier studies in ApoE-deficient mice showed that chronic administration of RVX reduced gene and protein expression for IL-6, TNF-α, MCP-1, i-NOS, COX-2, MMP9 in thoracic and abdominal aortas, attenuated macrophage activation, necrotic core formation, collagen loss, and promoted the stabilization of the atherosclerotic plaque [14,35,63].
Interestingly, a recent study has shown a cardioprotective effect of RVX pre-treatment in ischaemic cardiomyopathy in mice model with diet-induced myocardial infarction [23]. This study has shown that attenuation of cardiac remodeling, fibrosis, alleviation of the aortic root and coronary arteries atherosclerosis is dependent on the reduction of IL-1β, TNF-α, IL-6 cardiac mRNA expression, and nuclear factor kappa B (NF-κB) activation pathway in RVX pre-treated group. Also in the myocardial reperfusion injury mice model, RVX improved survival rates, cardiac function, and reduced IL-6, collagen 1α2, and 3α1 cardiac mRNA expression [16]. RVX protective effects were shown also in a rat model of brain ischemia/reperfusion injury where it reduced VCAM-1 protein expression, macrophage activation, and thrombin mediated thrombus formation [26], and also in pressure overload-induced atrial remodeling with transverse aortic constriction mice model where a reduced macrophage infiltration associated to a decreased expression of MCP-1, IL-6, IL-1β, TNF-α was observed [21].
Moreover, additional studies showed that RVX pre-treatment prevented the development of mechanical femoral vascular injury-induced neointima hyperplasia in mice, again by affecting IL-1β and TNF-α gene expression [25]. Similarly, RVX treatment decreased MCP-1 plasma levels and MMP-9 protein levels in the external jugular vein of mice following catheter thrombosis [15], as well as IL-6 plasma level and neutrophil levels in a mouse model of sickle cell disease [64]. Incubation, ex-vivo, of human abdominal aortic aneurysmal tissues, resulted in the reduction of IL-6 release and NOS-2, MMP9 protein expression [20].
Also, hypertensive renal damage resulted to be ameliorated by RVX chronic pre-treatment of renin overexpressing mice via specifically targeting of TNF-α, MCP-1, and IL-6 [24]. Thus paving the way also to the other newer FXa inhibitor, which recently demonstrated to affect VCAM-1 and ICAM-1 in uremia induced vascular dysfunction [65].
In addition to vascular inflammation, the acute inflammatory response from LPS induces vascular hyporeactivity and hypotension which were shown to be also time-dependent [66,67], thus displaying the highest level of vascular hyperresponsiveness and iNOS expression 6 hours post-exposure to LPS injection [32,68], as observed in our study experimental model. Moreover, FXa has been found to induce hypotension and inflammation response in vascular endothelial cells [43,44], whereas endotoxin activation of FXa and its intracellular signaling have been shown to trigger vascular tone reduction and hypotension [49,69–71]. This effect was shown to depend on factor Xa induced dilation of the rat aorta through the PAR-2 signaling pathway, a contribute pathway which was implicated also in severe hypotension following septic shock [72].
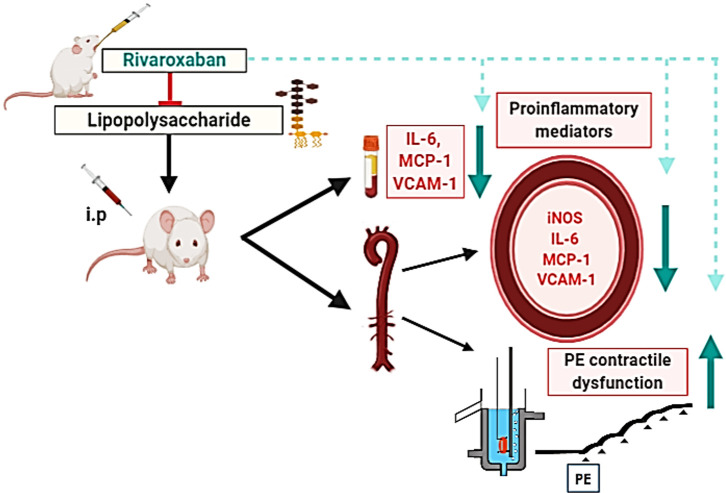
In this study, we demonstrate that RVX pre-treatment improves aortic hyporesponsiveness to PE under inflammatory conditions (Fig 5).
Fig 5. Schematic diagram for the protection of RVX against acute inflammation and vascular dysfunction following LPS-induced endotoxin shock.
RVX pretreatment decreased the expression of pro-inflammatory mediators and adhesion molecules and improved aortic hypo responsiveness to PE induced by LPS in the aorta. The figure was prepared with BioRender (biorender.com).
Previous studies tested in vitro the protective role of FXa inhibitors on the vascular tone of control rat aorta [73,74], mesenteric and basilar arteries [31], and streptozotocin-induced diabetic mice [17,75]. We now translate these findings in vivo by showing an improvement of vascular tone in endotoxin-induced hypotension and proinflammatory response following RVX pre-treatment. The effect could rely on the control of FXa-PAR-2 [47–49,69,76] and TL-4 /NF-κB signaling [24,50,51,77].
Although future additional studies are needed to better delineate the mechanisms beyond these effects, the currently available findings set the stage for investigating the additional molecular effects and also clinical benefit of RVX treatment in inflammation and hypotension associated with endotoxin shock.
Supporting information
Comparisons of relative IL-6, MCP-1, VCAM-1 and ICAM-1 gene expression levels normalized to GAPDH in the rat liver samples obtained from RVX- or non-treated LPS rats and non-treated control rats. *P<0.05, ** P<0.01 and ***P<0.001 indicates values significantly different (Student’s t-test) vs LPS. Values are expressed as the mean±SEM (n = 6).
(TIF)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research project was supported by the Research Fund of Ministry of Education, Science and Technology-Republic of Kosovo. Project No. 2616. Facilities and infrastructure were provided by Kosovo Interdisciplinary Knowledge Triangle Center (KIKTC, Tempus IV grant). GDN is supported by Fondazione Cariplo 2016-0852; EFSD/Lilly European Diabetes Research Programme 2018, Fondazione Telethon GGP19146 and PRIN 2017K55HLC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Olie RH, van der Meijden PEJ, ten Cate H. The coagulation system in atherothrombosis: Implications for new therapeutic strategies. Res Pract Thromb Haemost. 2018;2: 188–198. 10.1002/rth2.12080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weitz JI, Fredenburgh JC. Factors XI and XII as targets for new anticoagulants. Frontiers in Medicine. Frontiers Media S.A.; 2017. 10.3389/fmed.2017.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rupprecht HJ, Blank R. Clinical pharmacology of direct and indirect factor xa inhibitors. Drugs. 2010. pp. 2153–2170. 10.2165/11538030-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 4.Gieseler F, Ungefroren H, Settmacher U, Hollenberg MD, Kaufmann R. Proteinase-activated receptors (PARs)—Focus on receptor-receptor- interactions and their physiological and pathophysiological impact. Cell Communication and Signaling. 2013. 10.1186/1478-811X-11-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papadaki S, Tselepis AD. Nonhemostatic Activities of Factor Xa: Are There Pleiotropic Effects of Anti-FXa Direct Oral Anticoagulants? Angiology. SAGE Publications Inc; 2019. pp. 896–907. 10.1177/0003319719840861 [DOI] [PubMed] [Google Scholar]
- 6.Ebrahimi S, Rezaei S, Seiri P, Ryzhikov M, Hashemy SI, Hassanian SM. Factor Xa Signaling Contributes to the Pathogenesis of Inflammatory Diseases. J Cell Physiol. 2017;232: 1966–1970. 10.1002/jcp.25714 [DOI] [PubMed] [Google Scholar]
- 7.Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. 2005;131: 417–430. 10.1111/j.1365-2141.2005.05753.x [DOI] [PubMed] [Google Scholar]
- 8.Foley JH, Conway EM. Cross Talk Pathways between Coagulation and Inflammation Circulation Research. Lippincott Williams and Wilkins; 2016. pp. 1392–1408. 10.1161/CIRCRESAHA.116.306853 [DOI] [PubMed] [Google Scholar]
- 9.Barnes GD, Kurtz B. Direct oral anticoagulants: Unique properties and practical approaches to management Heart. BMJ Publishing Group; 2016. pp. 1620–1626. 10.1136/heartjnl-2015-309075 [DOI] [PubMed] [Google Scholar]
- 10.Milesi V, Rebolledo A, Gomez Alvis A, Sanz N, Tommasi J, Drago A, et al. Aspectos estructurales y funcionales de la vena safena humana utilizada como puente aorto-coronario en la cirugia de revascularizacion miocardica. Medicina (B Aires). 2001. [PubMed] [Google Scholar]
- 11.Gómez-Outes A, Suárez-Gea ML, Lecumberri R, Terleira-Fernández AI, Vargas-Castrillón E. Direct-acting oral anticoagulants: pharmacology, indications, management, and future perspectives. Eur J Haematol. 2015;95: 389–404. 10.1111/ejh.12610 [DOI] [PubMed] [Google Scholar]
- 12.Al Said S, Bode C, Duerschmied D. Anticoagulation in Atherosclerotic Disease Hamostaseologie. Georg Thieme Verlag; 2018. pp. 240–246. 10.1055/s-0038-1673412 [DOI] [PubMed] [Google Scholar]
- 13.Turgeon RD, Ackman ML, Babadagli HE, Basaraba JE, Chen JW, Omar M, et al. The Role of Direct Oral Anticoagulants in Patients With Coronary Artery Disease. J Cardiovasc Pharmacol Ther. 2018; 1074248418795889 10.1177/1074248418795889 [DOI] [PubMed] [Google Scholar]
- 14.Hara T, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Nishimoto S, et al. Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in ApoE-deficient mice. Atherosclerosis. 2015;242: 639–646. 10.1016/j.atherosclerosis.2015.03.023 [DOI] [PubMed] [Google Scholar]
- 15.Terry CM, He Y, Cheung AK. Rivaroxaban improves patency and decreases inflammation in a mouse model of catheter thrombosis. Thromb Res. 2016;144: 106–112. 10.1016/j.thromres.2016.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goto M, Sichiro Miura, Suematsu Y, Idemoto Y, Takata K, Imaizumi S, et al. Rivaroxaban, a factor Xa inhibitor, induces the secondary prevention of cardiovascular events after myocardial ischemia reperfusion injury in mice. Int J Cardiol. 2016;220: 602–607. 10.1016/j.ijcard.2016.06.212 [DOI] [PubMed] [Google Scholar]
- 17.Wu T-C, Chan J-S, Lee C-Y, Leu H-B, Huang P-H, Chen J-S, et al. Rivaroxaban, a factor Xa inhibitor, improves neovascularization in the ischemic hindlimb of streptozotocin-induced diabetic mice. Cardiovasc Diabetol. 2015;14: 81 10.1186/s12933-015-0243-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akkaya G, Bilen Ç, Gençpınar T, Akokay P, Uğurlu B. Effects of rivaroxaban on intimal hyperplasia and smooth muscle cell proliferation at the carotid artery anastomosis site in rabbits. Anatol J Cardiol. 2017;18: 261–265. 10.14744/AnatolJCardiol.2017.7898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellinghaus P, Perzborn E, Hauenschild P, Gerdes C, Heitmeier S, Visser M, et al. Expression of pro-inflammatory genes in human endothelial cells: Comparison of rivaroxaban and dabigatran. Thromb Res. 2016;142: 44–51. 10.1016/j.thromres.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 20.Moñux G, Zamorano-León JJ, Marqués P, Sopeña B, García-García JM, Laich de Koller G, et al. FXa inhibition by rivaroxaban modifies mechanisms associated with the pathogenesis of human abdominal aortic aneurysms. Br J Clin Pharmacol. 2017;83: 2661–2670. 10.1111/bcp.13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo H, Abe I, Fukui A, Saito S, Miyoshi M, Aoki K, et al. Possible role of rivaroxaban in attenuating pressure-overload-induced atrial fibrosis and fibrillation. J Cardiol. 2018;71: 310–319. 10.1016/j.jjcc.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 22.Bode MF, Auriemma AC, Grover SP, Hisada Y, Rennie A, Bode WD, et al. The factor Xa inhibitor rivaroxaban reduces cardiac dysfunction in a mouse model of myocardial infarction. Thromb Res. 2018;167: 128–134. 10.1016/j.thromres.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Nishida M, Inui H, Chang J, Zhu Y, Kanno K, et al. Rivaroxaban Suppresses the Progression of Ischemic Cardiomyopathy in a Murine Model of Diet-Induced Myocardial Infarction. J Atheroscler Thromb. 2019;26: 915–930. 10.5551/jat.48405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ichikawa H, Shimada M, Narita M, Narita I, Kimura Y, Tanaka M, et al. Rivaroxaban, a Direct Factor Xa Inhibitor, Ameliorates Hypertensive Renal Damage Through Inhibition of the Inflammatory Response Mediated by Protease-Activated Receptor Pathway. J Am Heart Assoc. 2019;8: e012195 10.1161/JAHA.119.012195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hara T, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Yagi S, et al. Inhibition of activated factor X by rivaroxaban attenuates neointima formation after wire-mediated vascular injury. Eur J Pharmacol. 2018;820: 222–228. 10.1016/j.ejphar.2017.12.037 [DOI] [PubMed] [Google Scholar]
- 26.Dittmeier M, Kraft P, Schuhmann MK, Fluri F, Kleinschnitz C. Pretreatment with rivaroxaban attenuates stroke severity in rats by a dual antithrombotic and anti-inflammatory mechanism. Thromb Haemost. 2016;115: 835–843. 10.1160/TH15-08-0631 [DOI] [PubMed] [Google Scholar]
- 27.Rosenkranz AC, Schrör K, Rauch BH. Direct inhibitors of thrombin and factor Xa attenuate clot-induced mitogenesis and inflammatory gene expression in human vascular smooth muscle cells. Thromb Haemost. 2011;106: 561–2. 10.1160/TH11-04-0275 [DOI] [PubMed] [Google Scholar]
- 28.Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs Oncotarget. Impact Journals LLC; 2018. pp. 7204–7218. 10.18632/oncotarget.23208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami A, Nakamura Y, Torikai K, Tanaka T, Koshiba T, Koshimizu K, et al. Inhibitory effect of citrus nobiletin on phorbol ester-induced skin inflammation, oxidative stress, and tumor promotion mice. Cancer Res. 2000;60: 5059–5066. [PubMed] [Google Scholar]
- 30.Linghu K-G, Ma QS, Zhao GD, Xiong W, Lin L, Zhang Q-W, et al. Leocarpinolide B attenuates LPS-induced inflammation on RAW264.7 macrophages by mediating NF-κB and Nrf2 pathways. Eur J Pharmacol. 2019; 172854 10.1016/j.ejphar.2019.172854 [DOI] [PubMed] [Google Scholar]
- 31.Villari A, Giurdanella G, Bucolo C, Drago F, Salomone S. Apixaban enhances vasodilatation mediated by protease-activated receptor 2 in isolated rat arteries. Front Pharmacol. 2017;8 10.3389/fphar.2017.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Da Silva-Santos JE, Chiao CW, Leite R, Webb RC. The Rho-A/Rho-kinase pathway is up-regulated but remains inhibited by cyclic guanosine monophosphate-dependent mechanisms during endotoxemia in small mesenteric arteries. Crit Care Med. 2009. 10.1097/CCM.0b013e31819efb43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perzborn E, Strassburger J, Wilmen A, Pohlmann J, Roehrig S, Schlemmer KH, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59–7939—An oral, direct Factor Xa inhibitor. J Thromb Haemost. 2005. 10.1111/j.1538-7836.2005.01166.x [DOI] [PubMed] [Google Scholar]
- 34.Fujiwara Y, Ando H, Ushijima K, Horiguchi M, Yamashita C, Fujimura A. Dosing-time-dependent effect of rivaroxaban on coagulation activity in rats. J Pharmacol Sci. 2017. 10.1016/j.jphs.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 35.Blessing E, Zhou Q, Bea F, Preusch M, Wang H, Isermann B, et al. Evaluation of plaque stability of advanced atherosclerotic lesions in Apo E-deficient mice after treatment with the oral factor Xa inhibitor rivaroxaban. Mediators Inflamm. 2011;2011 10.1155/2011/432080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perzborn E, Hirth-Dietrich C, Fischer E, Groth M, Hartmann E, Sperlich-Wulf K. Rivaroxaban Has Protective Effects in a Model of Disseminated Intravascular Coagulation (DIC) in Rats. Blood. 2007. 10.1182/blood.v110.11.935.935 [DOI] [Google Scholar]
- 37.Weinz C, Buetehorn U, Daehler HP, Kohlsdorfer C, Pleiss U, Sandmann S, et al. Pharmacokinetics of BAY 59–7939—An oral, direct Factor Xa inhibitor—In rats and dogs. Xenobiotica. 2005. 10.1080/00498250500250493 [DOI] [PubMed] [Google Scholar]
- 38.Kim M, Son H, Noh K, Kim E, Shin B, Kang W. Effects of verapamil and diltiazem on the pharmacokinetics and pharmacodynamics of rivaroxaban. Pharmaceutics. 2019. 10.3390/pharmaceutics11030133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonacina F, Moregola A, Porte R, Baragetti A, Bonavita E, Salatin A, et al. Pentraxin 3 deficiency protects from the metabolic inflammation associated to diet-induced obesity. Cardiovasc Res. 2019. 10.1093/cvr/cvz068 [DOI] [PubMed] [Google Scholar]
- 40.Bonacina F, Barbieri SS, Cutuli L, Amadio P, Doni A, Sironi M, et al. Vascular pentraxin 3 controls arterial thrombosis by targeting collagen and fibrinogen induced platelets aggregation. Biochim Biophys Acta—Mol Basis Dis. 2016. 10.1016/j.bbadis.2016.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pulakazhi Venu VK, Uboldi P, Dhyani A, Patrini A, Baetta R, Ferri N, et al. Fibronectin extra domain A stabilises atherosclerotic plaques in apolipoprotein E and in LDL-receptor-deficient mice. Thromb Haemost. 2015. 10.1160/TH14-09-0790 [DOI] [PubMed] [Google Scholar]
- 42.Bonacina F, Coe D, Wang G, Longhi MP, Baragetti A, Moregola A, et al. Myeloid apolipoprotein E controls dendritic cell antigen presentation and T cell activation. Nat Commun. 2018. 10.1038/s41467-018-05322-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Senden NH, Jeunhomme TM, Heemskerk JW, Wagenvoord R, van’t Veer C, Hemker HC, et al. Factor Xa induces cytokine production and expression of adhesion molecules by human umbilical vein endothelial cells. J Immunol. 1998. [PubMed] [Google Scholar]
- 44.Papapetropoulos A, Piccardoni P, Cirino G, Bucci M, Sorrentino R, Cicala C, et al. Hypotension and inflammatory cytokine gene expression triggered by factor Xa-nitric oxide signaling. Proc Natl Acad Sci U S A. 1998;95: 4738–42. 10.1073/pnas.95.8.4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schöchl H, van Griensven M, Heitmeier S, Laux V, Kipman U, Roodt J, et al. Dual inhibition of thrombin and activated factor X attenuates disseminated intravascular coagulation and protects organ function in a baboon model of severe Gram-negative sepsis. Crit Care. 2017. 10.1186/s13054-017-1636-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fiusa MML, Carvalho-Filho MA, Annichino-Bizzacchi JM, De Paula E V. Causes and consequences of coagulation activation in sepsis: An evolutionary medicine perspective. BMC Med. 2015. 10.1186/s12916-015-0327-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hara T, Phuong PT, Fukuda D, Yamaguchi K, Murata C, Nishimoto S, et al. Protease-activated receptor-2 plays a critical role in vascular inflammation and atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2018;138: 1706–1719. 10.1161/CIRCULATIONAHA.118.033544 [DOI] [PubMed] [Google Scholar]
- 48.Jesmin S, Gando S, Zaedi S, Sakuraya F. Differential expression, time course and distribution of four PARs in rats with endotoxin-induced acute lung injury. Inflammation. 2007. 10.1007/s10753-006-9017-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cicala C, Pinto A, Bucci M, Sorrentino R, Walker B, Harriot P, et al. Protease-activated receptor-2 involvement in hypotension in normal and endotoxemic rats in vivo. Circulation. 1999;99: 2590–2597. 10.1161/01.cir.99.19.2590 [DOI] [PubMed] [Google Scholar]
- 50.Shi M, Wang L, Zhou J, Ji S, Wang N, Tong L, et al. Direct factor Xa inhibition attenuates acute lung injury progression via modulation of the PAR-2/NF-κB signaling pathway. Am J Transl Res. 2018. [PMC free article] [PubMed] [Google Scholar]
- 51.Lou X, Yu Z, Yang X, Chen J. Protective effect of rivaroxaban on arteriosclerosis obliterans in rats through modulation of the toll‑like receptor 4/NF‑κB signaling pathway. Exp Ther Med. 2019. 10.3892/etm.2019.7726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burris RL, Ng H-P, Nagarajan S. Soy protein inhibits inflammation-induced VCAM-1 and inflammatory cytokine induction by inhibiting the NF-κB and AKT signaling pathway in apolipoprotein E-deficient mice. Eur J Nutr. 2014;53: 135–48. 10.1007/s00394-013-0509-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.J.E. DS-S, C.-W. C, R. L, R.C. W. The Rho-A/Rho-kinase pathway is up-regulated but remains inhibited by cyclic guanosine monophosphate-dependent mechanisms during endotoxemia in small mesenteric arteries. Critical Care Medicine. 2009. 10.1097/CCM.0b013e31819efb43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fink MP. Animal models of sepsis and its complications. Kidney International. 2008. 10.1038/ki.2008.442 [DOI] [PubMed] [Google Scholar]
- 55.Shukla P, Rao GM, Pandey G, Sharma S, Mittapelly N, Shegokar R, et al. Therapeutic interventions in sepsis: current and anticipated pharmacological agents. Br J Pharmacol. 2014;171: 5011–31. 10.1111/bph.12829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shih CC, Liao MH, Hsiao TS, Hii HP, Shen CH, Chen SJ, et al. Procainamide inhibits DNA methylation and alleviates multiple organ dysfunction in rats with endotoxic shock. PLoS One. 2016. 10.1371/journal.pone.0163690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen CL, Chen JT, Liang CM, Tai MC, Lu DW, Chen YH. Silibinin treatment prevents endotoxin-induced uveitis in rats in vivo and in vitro. PLoS One. 2017. 10.1371/journal.pone.0174971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levi M, van der Poll T. Coagulation and sepsis Thrombosis Research. Elsevier Ltd; 2017. pp. 38–44. 10.1016/j.thromres.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 59.Jones DP, Patel J. Therapeutic approaches targeting inflammation in cardiovascular disorders. Biology. MDPI AG; 2018. 10.3390/biology7040049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shantsila E, Lip GY. Factor Xa Inhibitors Non-Vitamin K Antagonist Oral Anticoagulants. Cham: Springer International Publishing; 2016. pp. 25–71. 10.1007/978-3-319-25460-9_3 [DOI] [Google Scholar]
- 61.Sanmartín M, Bellmunt S, Cosín-Sales J, García-Moll X, Riera-Mestre A, Almendro-Delia M, et al. Role of rivaroxaban in the prevention of atherosclerotic events. Expert Rev Clin Pharmacol. 2019;12: 771–780. 10.1080/17512433.2019.1637732 [DOI] [PubMed] [Google Scholar]
- 62.Ishibashi Y, Matsui T, Fukami K, Ueda S, Okuda S, Yamagishi S. Rivaroxaban inhibits oxidative and inflammatory reactions in advanced glycation end product-exposed tubular cells by blocking thrombin/protease-activated receptor-2 system. Thromb Res. 2015;135: 770–3. 10.1016/j.thromres.2015.01.023 [DOI] [PubMed] [Google Scholar]
- 63.Posthuma JJ, Posma JJN, van Oerle R, Leenders P, van Gorp RH, Jaminon AMG, et al. Targeting Coagulation Factor Xa Promotes Regression of Advanced Atherosclerosis in Apolipoprotein-E Deficient Mice. Sci Rep. 2019;9 10.1038/s41598-018-36956-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sparkenbaugh EM, Chantrathammachart P, Mickelson J, Van Ryn J, Hebbel RP, Monroe DM, et al. Differential contribution of FXa and thrombin to vascular inflammation in a mouse model of sickle cell disease. Blood. 2014;123: 1747–1756. 10.1182/blood-2013-08-523936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torramade-Moix S, Palomo M, Vera M, Jerez D, Moreno-Castaño AB, Zafar MU, et al. Apixaban Downregulates Endothelial Inflammatory and Prothrombotic Phenotype in an In Vitro Model of Endothelial Dysfunction in Uremia. Cardiovasc Drugs Ther. 2020. 10.1007/s10557-020-07010-z [DOI] [PubMed] [Google Scholar]
- 66.Öztürk OH, Çetin A, Özdem SS, Uysal N, Kayişli ÜA, Şentürk ÜK, et al. Plasma levels of nitrites, PGF1α and nitrotyrosine in LPS-treated rats: Functional and histochemical implications in aorta. J Physiol Biochem. 2006;62: 27–34. 10.1007/BF03165803 [DOI] [PubMed] [Google Scholar]
- 67.Bermejo A, Zarzuelo A, Duarte J. In vivo vascular effects of genistein on a rat model of septic shock induced by lipopolysaccharide. J Cardiovasc Pharmacol. 2003;42: 329–38. 10.1097/00005344-200309000-00003 [DOI] [PubMed] [Google Scholar]
- 68.Liao MH, Shih CC, Tsao CM, Chen SJ, Wu CC. RhoA/Rho-Kinase and Nitric Oxide in Vascular Reactivity in Rats with Endotoxaemia. PLoS One. 2013. 10.1371/journal.pone.0056331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akahane K, Okamoto K, Kikuchi M, Todoroki H, Higure A, Ohuchida T, et al. Inhibition of factor Xa suppresses the expression of tissue factor in human monocytes and lipopolysaccharide-induced endotoxemia in rats. Surgery. 2001;130: 809–18. 10.1067/msy.2001.116452 [DOI] [PubMed] [Google Scholar]
- 70.Saifeddine M, Al-ani B, Cheng CH, Wang L, Hollenberg MD. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br J Pharmacol. 1996;118: 521–530. 10.1111/j.1476-5381.1996.tb15433.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Damiano BP, Cheung WM, Santulli RJ, Fung-Leung WP, Ngo K, Ye RD, et al. Cardiovascular responses mediated by protease-activated receptor-2 (PAR- 2) and thrombin receptor (PAR-1) are distinguished in mice deficient in PAR- 2 or PAR-1. J Pharmacol Exp Ther. 1999;288: 671–678. [PubMed] [Google Scholar]
- 72.Kawabata A, Kuroda R, Nakaya Y, Kawai K, Nishikawa H, Kawao N. Factor Xa-evoked relaxation in rat aorta: involvement of PAR-2. Biochem Biophys Res Commun. 2001;282: 432–5. 10.1006/bbrc.2001.4597 [DOI] [PubMed] [Google Scholar]
- 73.Schaeffer P, Mares AM, Dol F, Bono F, Herbert JM. Coagulation factor Xa induces endothelium-dependent relaxations in rat aorta. Circ Res. 1997. 10.1161/01.res.81.5.824 [DOI] [PubMed] [Google Scholar]
- 74.Mabley J, Patel JP, Sayed A, Arya R, Scutt G. Direct oral anticoagulant (DOAC)-mediated vasodilation: Role of nitric oxide. Thrombosis Research. 2019. 10.1016/j.thromres.2019.02.014 [DOI] [PubMed] [Google Scholar]
- 75.Pham PT, Fukuda D, Yagi S, Kusunose K, Yamada H, Soeki T, et al. Rivaroxaban, a specific FXa inhibitor, improved endothelium-dependent relaxation of aortic segments in diabetic mice. Sci Rep. 2019. 10.1038/s41598-019-47474-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oe Y, Hayashi S, Fushima T, Sato E, Kisu K, Sato H, et al. Coagulation Factor Xa and Protease-Activated Receptor 2 as Novel Therapeutic Targets for Diabetic Nephropathy. Arterioscler Thromb Vasc Biol. 2016. 10.1161/ATVBAHA.116.307883 [DOI] [PubMed] [Google Scholar]
- 77.Guo X, Kolpakov MA, Hooshdaran B, Schappell W, Wang T, Eguchi S, et al. Cardiac Expression of Factor X Mediates Cardiac Hypertrophy and Fibrosis in Pressure Overload. JACC Basic to Transl Sci. 2020. 10.1016/j.jacbts.2019.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Nakanishi N, Kaikita K, Ishii M, Mitsuse T, Oimatsu Y, Tsujita K. Effects of rivaroxaban on cardiac remodeling after experimental myocardial infarction. Circ Conf. 2018. [Google Scholar]
- 79.Meng S, Jiechun H, Xiaotian S, Fangrui W, Xianglin C, Rongrong J, et al. Effect of rivaroxaban on the injury during endotoxin-induced damage to human umbilical vein endothelial cells. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31: 468–473. 10.3760/cma.j.issn.2095-4352.2019.04.019 [DOI] [PubMed] [Google Scholar]
- 80.Álvarez E, Paradela-Dobarro B, Raposeiras-Roubín S, González-Juanatey JR. Protective, repairing and fibrinolytic effects of rivaroxaban on vascular endothelium. Br J Clin Pharmacol. 2018. 10.1111/bcp.13440 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparisons of relative IL-6, MCP-1, VCAM-1 and ICAM-1 gene expression levels normalized to GAPDH in the rat liver samples obtained from RVX- or non-treated LPS rats and non-treated control rats. *P<0.05, ** P<0.01 and ***P<0.001 indicates values significantly different (Student’s t-test) vs LPS. Values are expressed as the mean±SEM (n = 6).
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.