Abstract
Dietary nutrients have significant effects on the risk of cardiovascular diseases. However, the results were not uniform across different countries. The study aims to determine the relative importance of dietary nutrients associated with coronary artery disease (CAD) among the Nepalese population. A hospital-based matched case-control study was carried out at Shahid Gangalal National Heart Center in Nepal. In the present study, patients with more than seventy percent stenosis in any main coronary artery branch in angiography were defined as cases, while those presenting normal coronary angiography or negative for stressed exercise test were considered controls. Dietary intakes of 612 respondents over the past 12 months were evaluated using a semi-quantitative customized food frequency questionnaire. In conditional regression model, the daily average dietary intake of β-carotene (OR: 0.54; 95%CI: 0.34, 0.87), and vitamin C (OR: 0.96; 95%CI: 0.93, 0.99) were inversely, whereas dietary carbohydrate (OR: 1.16; 95%CI: 1.1, 1.24), total fat/oil (OR: 1.47; 95%CI: 1.27, 1.69), saturated fatty acid (SFA) (OR: 1.2; 95%CI: 1.11, 1.3), cholesterol (OR: 1.01; 95%CI: 1.001, 1.014), and iron intakes (OR: 1.11; 95%CI: 1.03, 1.19) were positively linked with CAD. Moreover, in random forest analysis, the daily average dietary intakes of SFA, vitamin A, total fat/oil, β-carotene, and cholesterol were among the top five nutrients (out of 12 nutrients variables) of relative importance associated with CAD. The nutrients of relative importance imply a reasonable preventive measure in public health nutrients specific intervention to prevent CAD in a resource-poor country like Nepal. The findings are at best suggestive of a possible relationship between these nutrients and the development of CAD, but prospective cohort studies and randomized control trials will need to be performed in the Nepalese population.
Introduction
Coronary artery disease (CAD) is a significant cause of disability and premature death throughout the world. The underlying pathology is atherosclerosis, which develops over many years and is usually advanced by the time symptoms occur, generally in middle age [1]. An estimated 7.4 million people died from CAD in 2015, representing 13% of all global deaths [2]. Primary risk factors are tobacco use, unhealthy diet, physical inactivity, harmful alcohol consumption. These, in turn, show up in people as raised blood pressure, elevated blood glucose, and overweight and obesity risks detrimental to good heart health [3].
A global strategy based on knowledge of the importance of risk factors for cardiovascular disease (CVDs) in different geographic regions and various ethnic groups is needed to prevent diseases effectively [4]. Western people mostly rely on high energy-dense food, substantially not reducing the incidence of obesity and CVDs [5] despite improved medical care and an increase in the cessation of smoking [6]. In low and middle-income countries, fast food, energy-dense food, and diet in high fat are related to an abrupt rise in CAD even in a younger population of high socioeconomic status of an urban area [7].
Dietary habit is considered as one of the potentially modifiable risk factors for CVDs. The quality of dietary carbohydrates plays a significant impact on the development of metabolic diseases and CAD. For instance, refined sugar increases CAD’s risk, while complex carbohydrates lower CAD incidence [8, 9]. Total dietary fat was associated with an increased risk of CVD and all-cause death [10]. After adjustment of some coronary heart disease (CHD) risk factors, higher intakes of polyunsaturated fatty acids (PUFA) and monounsaturated fatty acids (MUFA) were associated with a reduced risk of CHD [11]. Unlike observational studies, randomized control trials suggest that SFAs either do not or only modestly increase the risk for CAD [12, 13]. Evidence supports that industrially-produced trans-fats are linked to increased risk for CVDs [12, 14]. Thus, dietary fat quality contributes to the risk of the leading chronic diseases and is more critical than the quantity of fat/oil in reducing the risk of chronic disease mortality, especially from CVDs.
Dietary fat is rice in energy sources; it also carries fat-soluble vitamins and other nutritious substances, provides essential fatty acids, and aids physiological functions in the body [15]. PUFAs have been of great interest for human health due to their potential anti-inflammatory action that may protect from several chronic-degenerative diseases with inflammatory pathogenesis [16]. In many countries, daily intake of saturated fats exceeds the recommended limit of 10% energy (%E), while intakes of polyunsaturated fats (PUFAs) are often below the recommended range of 6–11%E, and consumption of long-chain ω–3 PUFAs is exceptionally low [17]. The average intake level of fat and carbohydrate varies in different countries, regions, and groups of people across the world. Thus, dietary fat recommendations must consider each country’s dietary fat/oil patterns [17]. However, there are discrepancies in the research findings of the role of vitamin B and antioxidant vitamins for CAD development [18–21].
Like in high-income countries, major traditional risk factors: tobacco use, alcohol consumption, unhealthy diet, and physical inactivity were reported in prevalence studies in the Nepalese population [22]. Although people’s food habits vary with different ethnicities and geography, Nepalese commonly consume rice or bread, pulses, and vegetables, with potatoes prepared mostly in vegetable oil as the main meal. Nonetheless, the daily intakes of animal products, fruits, and vegetables are low in Nepalese [23]. However, analytical studies to determine the strength of association between dietary nutrients and CVDs were scarce in the Nepalese population. Therefore, the present study was carried out to examine the association of energy intake, dietary macronutrients, and micronutrients with CAD.
Materials and methods
A matched case-control analytical study was designed to describe the relation of nutrients to CAD. The data of 612 participants were collected from June 4 to September 4, 2018. The face to face interview was accomplished with patients who visited at highly specialized central-level cardiac treatment center, Shahid Gangalal National Heart Center in Nepal, which provides curative services to patients with CVDs. An ethical review board of the Nepal Health Research Council (308/2017-18) approved the present study. Before conducting a survey, permission was taken for collecting clinical data from the respective hospital. Written consent was taken from the respondent before attending the interview.
Study participants
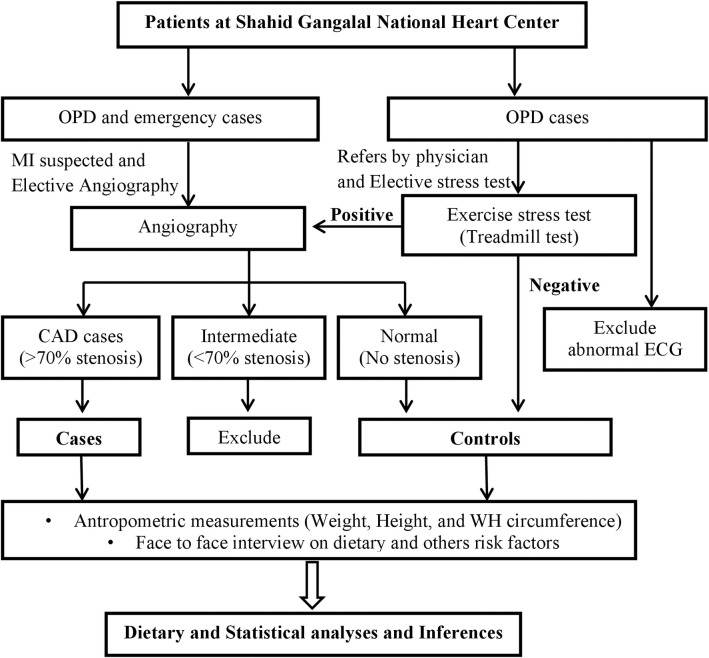
Study cases were selected from admitted patients after suspected myocardial infarction or exercise-induced stress test positive or from those who would electively undergo angiography in the hospital. After angiography, patients with stenosis higher than 70 percent (%) in any main coronary artery branch were defined as study cases. The controls were patients who were either presenting normal coronary angiography or those who were negative for stressed exercise test, also called a treadmill exercise tests (TMT). TMT was carried out for patients who were essentially referred by a physician for the test based on a chief complaint of chest pain and or at least one cardiometabolic risk factor (either hypertension or dyslipidemia or diabetes). Those patients who visited the hospital for an elective check-up or whole body check-up, including TMT test, were also included in the TMT test. A case to control was 1:1; altogether, 306 cases and 306 controls were included in the study. “Sex” and “Age” were matched at the individual level and an interval of five years, respectively. Patients having a report with aortic valve sclerosis on echocardiogram and any abnormality on electrocardiogram were excluded. Severely ill patients like kidney failure, cancer, and heart failure were not included. The participant selection flowchart was presented in Fig 1.
Fig 1. Flowchart diagram for selection of study participants for cases and controls.
OPD: Outpatient department; CAD: Coronary artery disease; ECG: Electrocardiogram, WH: Waist hip.
Data collection technique and tools
Data was collected through face to face interviews and observation, using a semi-structured food frequency questionnaire (FFQ) tool and observation checklist, respectively. The questionnaire set comprised of three-part, namely i. General socio-demographic characteristic ii. Cardio-metabolic and behavioral risk factors and iii. Food frequency questionnaire. Blood pressure, weight and height, waist and hip circumference of cases and controls were measured and recorded using the observation checklist. Data for cases were collected on the second day of angiography after patients became stable. The average time for an interview in FFQ for cases and control was 42±7 minutes and 39±6 minutes, respectively.
Assessment of covariates
A standardized protocol was used to measure the height, weight, and waist and hip circumferences. A wall-mounted stadiometer measured height to the nearest centimeter. We asked respondents to stand upright without shoes, with their back against the wall, heels together, and eyes directed forward. Their weight was measured with a portable electronic weighing scale placed on a firm horizontal surface. Waist and hip circumferences were measured with a non-stretchable standard measuring tape. Waist measurements were obtained over a lightly clothed abdomen at the narrowest point between the costal margin and iliac crest, and hip circumference was measured over light clothing at the level of the widest diameter around the buttocks. Body mass index (BMI) was categorized as normal (<23.0 kg/m2), overweight (23.0 to < 27.5.0 kg/m2), and obese (≥27.5.0 kg/m2) [24]. Abdominal obesity was defined as waist circumference ≥ 90 centimeters in males and ≥ 80 centimeters in females [25]. Dyslipidaemia was defined as hypercholesterolemia or hypertriglyceridemia; if the high-density lipoprotein (HDL) level was below 30 mg/dl, dyslipidemia was considered. Hypertriglyceridemia and hypercholesterolemia were defined as triglyceride (TG) serum and total cholesterol (TC) levels greater than 150 and 200 mg/dl, respectively, or if hypolipidemic treatment was administered [26]. Diabetic individuals were those with fasting blood glucose equal to or greater than 126 mg/dl throughout two tests or those taking diabetes medications [26]. Patients whose blood pressure was greater or equal to 140/90 mmHg or those taking antihypertensive medication according to their medical records were classified as hypertensive [27].
Persons who smoked until within one year of the interview were considered current daily smokers. Never smokers were those who responded with “occasionally” or “not at all” on the questionnaire, and ex-smokers were those who smoked daily before one year of interview. Current alcohol drinkers were categorized as those who engaged in alcohol drinking within the last year. A total of 13.6 g of pure alcohol was considered one standard drink, equivalent to consuming 43 ml of local alcohol (Raksi) and 341 ml of beer, Zaand, or Tongba [28].
Physical activity levels related to work were categorized as vigorous, moderate, or low. Vigorous physical activity was considered any activity that caused a substantial rise in heart and breathing rates, such as digging or plowing fields, lifting heavy weights, etc. Continuous engagement in such activity for at least 10 minutes was considered involvement in vigorous activity. Similarly, moderate physical activity was defined as any activity that caused a moderate increase in heart and breathing rates (examples include domestic chores, gardening, lifting light weights, etc.). Continuously engaging in such activity for at least 30 minutes was considered involvement in moderate activity. Physical activity related to transport was not considered in this study. The recreational activity was also called physical exercise which included two types of activities, vigorous and moderate, based on exertion. The vigorous recreational activity was defined as any recreational activity that significantly increased heart and breathing rates, such as football, fast swimming, and rapid cycling. Ten minutes of such activity was considered involvement in vigorous recreational activity. The moderate recreational activity was defined as any recreational activity that causes a moderate increase in heart and breathing rates; examples include yoga, playing basketball, brisk walking, and regular cycling [29]. During analysis, total physical activity (related to work and recreational) were categorized as “Yes” (includes moderate and vigorous) and “No.”
Dietary assessment
The FFQ was semi-structured, modified, and validated European Prospective Investigation of Cancer FFQ, customized to Nepalese day to day food items for obtaining detailed information regarding dietary nutrients and edible fat and oil intake from study participants. The list of fifty-nine food items (S1 Table), which were frequently consumed by the Nepalese population, was included. For testing internal validity, and intra-class correlation analyses were performed between FFQ1 and FFQ2, criterion validity was measured by Pearson’s correlation of FFQ2 with a "24-h recall diet survey" as a gold standard (S2 Table). An average of at least three days (two weekdays and one weekend) 24-h dietary recall can estimate about mean dietary intake for a day on the population or large-group level [30]. It is an intensive method for assessing dietary intake and is commonly used as a comparison method for validation/calibration studies of structured assessments such as FFQ [31]. However, it does not accurately determine the usual intake over time due to the large intra-individual variation in dietary intakes [30]. As FFQ can provide important information about dietary patterns, it is the most commonly used instrument to assess past dietary intake in epidemiological studies on the relationship between dietary factors and diseases [31].
Intake frequencies for the food items consisted of nine categories ranging from never to more than six times per day. Participants were asked how often they had consumed each food item listed on the FFQ during the past year. After being diagnosed as having any one of CAD's risk factors (obesity/hypertension/diabetes/dyslipidemia), patients who modified their dietary habits and those taking dietary supplements and vitamins were excluded from the study. The quantity of each food consumed by a subject was calculated by multiplying its consumption frequency by the usual amount consumed. Dietary intakes were then calculated using a programmed nutrition calculator based on the value of nutrients per 100 g food consumed per day for each food item with the use of the Nepal food composition table 2017.
A food atlas with color photographs of three portions sized- small, medium, and large- for various food items was developed and displayed to respondents to minimize the recall bias. The food items that did not have natural units or applicable household measures were photographed. Different sizes of glasses or bowls were displayed to estimate the number of liquids. Other items were asked as several specified units (slice, number, spoon, etc.). As people usually consumed seasonal fruits and vegetables, we grouped them into “leafy vegetables” and “other vegetables.” Several vegetables are cooked in combination, including potato, onion, and tomato. Some types of vegetables are available in only one season, while others are consumed more than one season. To address the overestimation of these above problems, we calculated average nutrients per 100 g that were allotting more weight to those vegetable items (cauliflower, mustard leaves, tomatoes), which are consumed more extended periods. Liquid oils are used during food preparation, so it is not easy to estimate the actual amount of oil consumed for an individual. Therefore, we asked separately the average number of days that would be sufficient to cook the foods from one liter of oil or ghee. Then, we calculated the total average amount of oil intake per person per day. Finally, fifty-nine food items were grouped into twenty-five food items with specific food ratios from Nepal’s food composition table and translated into particular dietary nutrients value.
Statistical analyses
Firstly, the Mcnemar test for categorical variables and Wilcoxon sign ranked test for continuous variables were executed to find the association between variables and CAD. After that, a conditional logistic regression model was constructed to test the strength of the association between dietary nutrients and CAD. By stepwise backward deletion process, we developed an energy-adjusted parsimonious model. To adjust the collinearity problem among our data variables, we performed random forest (RF) analysis, explaining the nutrients variability associated with CAD. RF consists of many individual de-correlated decision trees by sampling the random set of original data operating as an ensemble. Each tree gives a classification, and the forest selects the classification having the most votes across all the trees in the forest. RF is also common to perform the prediction task in the medical domain [32, 33]. The receiver operating characteristic (ROC) curve was constructed to assess the logistic regression and RF performance. Data analysis was performed with R packages in version 3.6.2, and two-tailed tests with p-value <0.05 were considered significant.
Results
Proportions of socio-demographic, cardio-metabolic, and behavioral characteristics of respondents between cases and controls in the study are presented in Table 1. The median age was 58 years, which was the same in both case and control groups. Because “Age” was matched at an interval of five years in the study, it was significantly associated with CAD (p-value = 0.001) in bivariate analysis. As CAD incidence is lower in the female population, fewer female CAD cases were admitted to the hospital, resulting in the ratio of male-to-female cases of 3:1. Similarly, diabetes mellitus, dyslipidemia, and general and abdominal obesity were significantly related to CAD (p-value <0.001). Regarding behavioral factors, smoking (p-value < 0.001), alcohol use (p-value = 0.019), and physical activity (p-value < 0.001) were found to be statistically significant linked with the disease.
Table 1. Socio-demographic, cardio-metabolic, and behavioral characteristics of respondents between cases and controls in the study.
| Variables | Control (%) n = 306 | Case (%) n = 306 | p-valuea | |
|---|---|---|---|---|
| A. Demographic risk factors | ||||
| Age | 58 (50, 65)b | 58 (50, 65) | <0.001*** | |
| Sex | Female | 73 (23.9) | 73 (23.9) | 1.000 |
| B. Cardiovascular Risk factors | ||||
| Diabetes | Yes | 40 (13.1) | 86 (28.1) | <0.001*** |
| Hypertension | Yes | 143 (46.7) | 142 (46.4) | 0.938 |
| Dyslipidemia | Yes | 32 (10.5) | 90 (29.4) | <0.001*** |
| General obesity | Yesc | 63 (20.6) | 134 (43.8) | 0.001*** |
| Abdominal obesity | Yesd | 160 (52.3) | 240 (78.4) | <0.001*** |
| C. Behavioral Risk Factors | ||||
| Alcohol use | Yes | 66 (21.6) | 91 (29.7) | 0.019* |
| Smoking | Never | 196 (64.1) | 113 (36.9) | <0.001*** |
| Ex-smoker | 70 (22.9) | 73 (23.9) | ||
| Current smoker | 40 (13.1) | 120 (39.2) | ||
| Physical activity | Moderate/ vigorous | 241 (78.8) | 202 (66) | <0.001*** |
aMcnemar test (categorical variables) and Wilcoxon sign ranked test (continuous variables).
bMedian and interquartile value.
cGeneral obesity: Body mass index ≥ 27.5 kg/m2 (for Asian).
dAbdominal obesity: Waist hip ratio ≥ 0.8 for females and ≥ 0.95 for males (for Asian).
*p ≤ .05
**p≤ .01
***p ≤ .001.
As the data of most of the variables were not normally distributed, median and interquartile values of nutrients intake are presented (Table 2). The median energy intake per day was 2674 (2445, 2909) and 2622 (2373, 2963) in control and case groups showing no statistically significant difference (p-value = 0.679). Total fat/oil, fiber, vitamin C, beta (β)-carotene, vitamin A, MUFA, SFA, and cholesterol intake per day were showing significant association with CAD (p-value < 0.001). In contrast, carbohydrate and PUFA intakes were significantly linked with CAD (p-value = 0.002). Also, a significant association of dietary zinc (p-value = 0.023), iron (p-value = 0.003), thiamine (p-value = 0.013), and riboflavin (p-value = 0.016) with CAD was reported. However, dietary intakes of protein, calcium, phosphorous, and niacin were not significantly associated with the disease.
Table 2. Distribution of nutritional factors associated with coronary artery disease between case and control groups in the study.
| Nutrients intake/day | Control (n = 306) | Case (n = 306) | p-valuea |
|---|---|---|---|
| Food energy Kcal | 2674 (2445, 2909)b | 2622 (2373, 2963) | 0.679 |
| Protein g | 70 (61, 78) | 67 (59, 77) | 0.169 |
| Total fat/oil g | 56 (47, 64) | 61 (52, 72) | <0.001*** |
| Carbohydrate g | 433 (388, 481) | 409 (368, 456) | 0.002** |
| Fiber g | 11.2 (9.8, 12.9) | 10.1 (8.7, 11.6) | <0.001*** |
| Calcium mg | 683 (434, 929) | 648 (363, 930) | 0.244 |
| Phosphorus mg | 1431 (1287, 1639) | 1407 (1210, 1615) | 0.146 |
| Iron mg | 21.3 (18.6, 24) | 20 (17.4, 23.5) | 0.003** |
| Zinc mg | 14.1 (12.2, 16.2) | 13.8 (11.7, 15.5) | 0.023* |
| Thiamine mg | 1.2 (1, 1.4) | 1.2 (0.97, 1.3) | 0.013* |
| Riboflavin mg | 1.1 (0.89, 1.3) | 1.04 (0.79, 1.3) | 0.016* |
| Niacin mg | 13.6 (11.9, 16.6) | 13.8 (11.4, 16) | 0.671 |
| Vitamin C mg | 45.3 (38, 52.9) | 39.5 (33, 48.7) | <0.001*** |
| β-carotene mcg | 2579 (2162, 3352) | 2227 (1863, 2698) | <0.001*** |
| Vitamin A R.E. | 698 (546, 836) | 622 (506, 728) | <0.001*** |
| PUFA g | 18.7 (12.5, 23.5) | 19.6 (12.4, 25.7) | 0.002** |
| MUFA g | 17.2 (12.4, 23) | 19.2 (14, 25.6) | 0.001*** |
| SFA g | 15.5 (10.6, 19.2) | 19 (13.9, 23.6) | <0.001*** |
| Cholesterol mg | 108 (71,163) | 129 (94,181) | <0.001*** |
Kcal: kilocalorie; g: gram; mg: milligram; mcg: microgram; R.E.: retinol equivalent; PUFA: polyunsaturated fatty acid; MUFA: monounsaturated fatty acid; SFA: saturated fatty acid.
aWilcoxon sign ranked test.
bMedian and interquartile value.
*p ≤ .05
**p≤ .01
***p ≤ .001.
Those micronutrients that showed potential associations with CAD (p-value < 0.05) (Table 2) were then tested in the conditional logistic regression analysis (Table 3). In this analysis, we developed a parsimonious energy-adjusted model by stepwise backward deletion. The final model was adjusted with dyslipidemia, diabetes mellitus, smoking, and BMI. In this model, those nutrients inversely related to CAD were β-carotene (OR: 0.54; 95%CI: 0.34, 0.87) and vitamin C (OR: 0.96; 95%CI: 0.93, 0.99) indicating possible protective factors. However, dietary carbohydrate (OR: 1.16; 95%CI: 1.1, 1.24), total fat/oil intake (OR: 1.47; 95%CI: 1.27, 1.69), SFA (OR: 1.2; 95%CI: 1.11, 1.31), cholesterol (OR: 1.01; 95%CI: 1.001, 1.014), and iron intakes (OR: 1.11; 95%CI: 1.03, 1.19) were shown proportionately linked with CAD indicating probable risk factors.
Table 3. A model based on conditional logistic regression analysis showing the effect of nutrients intake on coronary artery disease.
| Nutrients intake/day | OR (95%CI) | p-value |
|---|---|---|
| Carbohydrate g | 1.16 (1.1, 1.24) | <0.001*** |
| Total fat/oil g | 1.47 (1.27, 1.69) | <0.001*** |
| SFA g | 1.2 (1.11, 1.31) | <0.001*** |
| Cholesterol g | 1.01 (1.001, 1.014) | 0.016* |
| β-carotene mg | 0.54 (0.34, 0.87) | 0.011* |
| Vitamin C mg | 0.96 (0.93, 99) | 0.018* |
| Iron mg | 1.11 (1.03, 1.19) | 0.005** |
g: gram; mg: milligram; SFA: saturated fatty acid; OR: odds ratio; CI: confidence interval.
*p ≤ .05
**p≤ .01
***p ≤ .001.
The model was adjusted daily energy intake and with cardio-metabolic risk factors (Diabetes mellitus (type II), dyslipidemia and body mass index), and smoking.
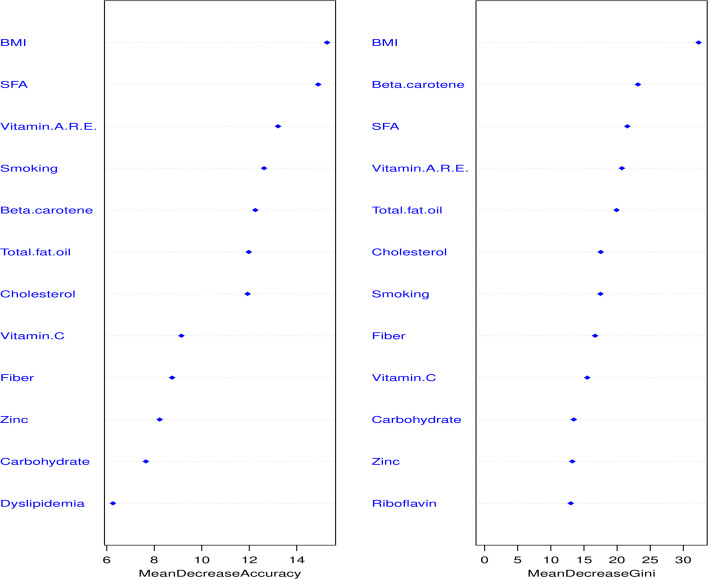
We performed random forest analysis to evaluate the important dietary nutrients linked with CAD. In this RF model, we incorporated all the variables in the first step of conditional logistic regression analysis. The top twelve variables were presented in Fig 2; the five topmost important dietary nutrients linked with CAD were SFA, vitamin A RE, total fat/oil, β-carotene, and cholesterol. In this RF analysis, 250 trees and four variables were tried in each split, where the out-of-bag (OOB) estimate of error rate was 16%.
Fig 2. The essential nutritional and traditional variables in random forest regression analysis.
WH ratio: Waist-Hip ratio; g: gram; T2DM: Type II Diabetes mellitus; mg: milligram; mcg: microgram; RE: Retinol equivalent; PUFA: polyunsaturated fatty acid; MUFA: monounsaturated fatty acid; SFA: saturated fatty acid; kcal: kilocalorie.
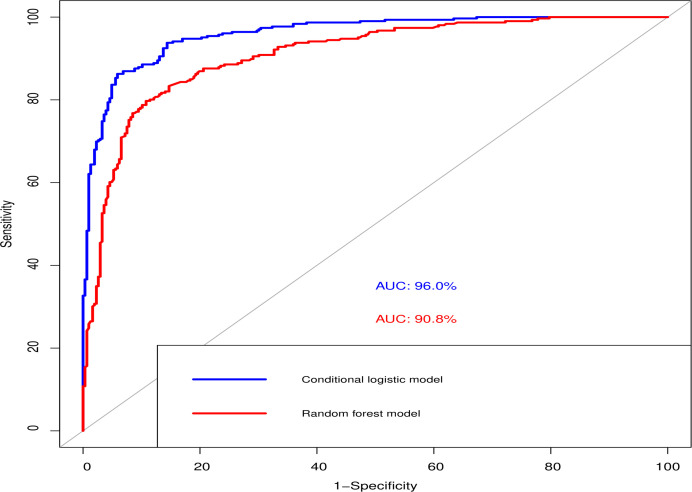
Fig 3 reveals the area under curve (AUC) that evaluated the logistic regression model and RF model. Although the RF model had a lower AUC (90%) and other performance parameters (Table 4) than the logistic model (AUC—96%), RF adjusted the effect of multi-collinearity so that the interaction between correlates was independent.
Fig 3. Receiver operating curve (ROC) to compare the logistic regression model and in the random forest model.
AUC: area under curve.
Table 4. Comparison of performance for logistic regression and random forest.
| Characteristics | Logistic regression | Random forest |
|---|---|---|
| Accuracy | 0.902 | 0.843 |
| Positive predictive rate | 0.936 | 0.84 |
| Negative predictive rate | 0.873 | 0.846 |
| No. of true positive/false negative | 264/18 | 257/49 |
| No. of true negative/false positive | 288/42 | 259/47 |
| Sensitivity | 0.863 | 0.845 |
| Specificity | 0.941 | 0.841 |
| F1 score | 0.898 | 0.843 |
| Out of bag error estimate (%) | - | 15.69 |
Discussion
The present hospital-based matched case-control study was designed to determine the association of dietary nutrients with CAD in the Nepalese population. Dietary intakes of carbohydrate, total fat, fiber, riboflavin, thiamine, vitamin C, β-carotene, vitamin A, vitamin C, PUFA, MUFA, SFA, and cholesterol were significantly associated with CAD in bivariate analysis. Furthermore, in multivariable conditional logistic regression analyses, we developed an energy-adjusted parsimonious model by stepwise backward deletion process where we adjusted three cardio-metabolic factors (diabetes, dyslipidemia, and BMI) and smoking. However, even though hypertension is a well-established risk factor, we did not observe a significant association; therefore, we did not include it in the model construction. The reason for the insignificant association of hypertension might be selecting the control groups from the same hospital’s outdoor patients, where more hypertensive patients come for their heart check-up. In the final model, we observed that dietary β-carotene and vitamin C were inversely associated with CAD, whereas higher dietary carbohydrate, total fat and oil, SFA, cholesterol, and iron intake were directly associated with CAD. We also performed random forest analysis to adjust the collinearity problem and then identify the topmost nutritional variables. Random forest analysis revealed that dietary intake of SFA, vitamin A, total fat and oil, β-carotene, and cholesterol were the topmost important nutrients associated with CAD. Even though dietary vitamin A was not significantly associated with CAD in conditional regression analysis, it remained important nutrients related to CAD in the random forest analysis.
The quantity and type of dietary fat/oil and carbohydrate consumption, fiber, protein, and alcohol intakes strongly impact blood lipids and lipoprotein metabolism, thereby developing CVDs [34]. Energy from complex carbohydrates has many benefits compared to refined sugars [34]. For example, a low glycemic index diet could improve blood lipids and blood pressure [35], thus reducing CAD [36]. Short term carbohydrate diet reduces the weight and atherosclerotic plaque in CAD, but the long term effect is still controversial [37]. Besides, a low carbohydrate diet is not associated with coronary artery incidence and progression [38]. The present study showed that an increased intake of dietary carbohydrates had a risk of CAD. However, we were unable to differentiate the dietary carbohydrate into refined and complex carbohydrates. A higher intake of fat without replacing protein and carbohydrates causes metabolic disorders related to CAD [39]. We found that total fat intake was proportionately associated with increased risk of CAD. However, dietary fat types, but not total fat intake, are an important determinant of CVDs [40]. Specifically, intakes of PUFAs and MUFAs are associated with a lower risk of CVDs and death, whereas SFA and trans-fat intakes are linked with a higher risk of CVDs [39]. Consistently, we reported significantly higher odds of CAD with increased SFA intake; PUFA and MUFA intakes were inversely but not significantly associated with CAD. The higher intake of carbohydrates, total fat, and SFA indicated an unhealthy eating pattern among the Nepalese population that might increase CAD prevalence.
Nevertheless, previous literature demonstrated a discrepancy in results among these specific fat intake groups. For example, SFA is proportionately associated with CAD [41], but not in the Kuopio Ischemic Heart Disease Risk Factor Study [42] and meta-analysis [43]. Likewise, in a recent meta-analysis, dietary intake of PUFA was not found significantly associated with CAD [44]. Besides, cholesterol is also independent risk factors of CAD according to lipid theory [45]. Incongruous with this study, we also reported a significant relationship between high dietary cholesterol and CAD’s risk; but, the minimal effect size of the risk was observed. A recent study [46], meta-analysis, and systemic reviews [47] do not conclude the dietary cholesterol as CAD’s risk.
Several epidemiological studies reported that higher dietary fiber is associated with a reduced CAD risk [48]. Mechanistically, dietary fibers could lower atherosclerosis [49] and alter microbiota that modulates the immune system [50]. Besides, high fiber consumption is related to a higher intake of vitamins and minerals [51]. In divergence with the findings mentioned above, we could not observe that a higher fiber intake could lower CAD’s risk. Moreover, the daily fiber intake amount was lower than the daily recommended allowance in both case and control groups, possibly because of specific dietary patterns in the Nepalese population. A prospective cohort study in Japan reported an inverse association of CHD with dietary intake of folic acid, vitamin B6, and vitamin B12 [18]. Recent dose-response meta-analysis shows that a higher intake of folate and vitamin B6 are associated with a lower risk of CAD [19]. Niacin intake has more enormous benefits in lowering LDL and increasing HDL in the blood in dyslipidemia patients; it could reduce the risk of CAD [52]. However, the Umbrella study concludes that nutritional supplements, such as folic acid, vitamin B6, vitamin B12 had no significant effect on mortality or CVDs outcomes [20]. In the present study, we did not observe any significant likelihood of CAD with dietary B vitamins. Vitamin A is associated with CAD severity, and β-carotene level diminish disease severity [21]. The present study revealed that vitamin C, vitamin A, and β-carotene intake were linked with CAD; a significant inverse association was reported with the intake of β-carotene but not with Vitamin A in the logistic model. This finding indicated antioxidant vitamins could be protective factors for the prevention of CAD in the Nepalese people. However, further large cohort or RCTs are required to confirm the present findings. A recent study in the Chinese population reported that β-carotene and vitamin C intake from the diet was inversely associated with deaths from all causes and CVDs in middle-aged or older people [53]. However, an inconsistent association of an antioxidant vitamin with CAD was reported in epidemiological studies [54].
Dietary calcium intake is inversely associated with CVDs [55]. Although a longer-term calcium intake is associated with a reduced risk of atherosclerosis, calcium supplementation may increase the risk of coronary artery calcification [56]. Dietary zinc intake was inversely associated with mortality from CHD in men but not women [57]. In contrast to these findings, our results did not reveal increased dietary calcium and zinc could reduce CAD’s risk. In the present study, we observed a significant result of higher dietary iron intake associated with CAD’s risk. Nonetheless, iron intake was not the topmost factors related to CAD in random forest analysis. The significant association of iron intake in the logistic model might be because of the multi-collinearity problem, which disguised the result. Simultaneously, heme iron intake was positively associated with CHD’s risk in Western populations [58], where red meat is a primary dietary source of iron. In contrast, this relationship was negative in the Japanese, who receive heme iron from fish and shellfish [59]. Several studies have reported that CHD incidence was positively associated with ferritin levels and inversely associated with serum Fe and transferrin saturation [60].
In the present study, the data were only taken from non-fatal CAD, and the sample size (306 cases and 306 control)) was quite small. We excluded the patients with equal or more than 50% and less than 70% stenosis in any one main coronary artery branch. As TMT negative patients and patients with normal coronary angiography were included as a control group, they could have a possibility of microvascular angina. Moreover, the control group was selected from the same hospital with or without having cardiovascular risk factors; there was a possibility of selection bias in the study. Thus, generalization is the drawback of the present study. Moreover, since the study was a case-control study, it might have many inherent biases that influence the causality link between the associated nutrients and the disease. Furthermore, our study observed dietary nutrients’ association based on the food list commonly consumed by the Nepalese population. Another limitation was that we calculated only the values of eighteen nutrients available in Nepal food composition table 2017.
Despite these limitations, we tried to minimize the possible bias, such as recall bias, which was minimized by displaying food atlas for portion size and verifying the information with hospital data and patients’ sick book as possible. Notably, we matched “Sex” individually and “Age” at the interval of five years to limit the confounders’ effect. Moreover, patients who modified their dietary intake habit after being diagnosed as having any one of CAD’s risk factors (obesity/hypertension/diabetes/dyslipidemia) were excluded to avoid possible unequal distribution of dietary nutrients due to predetermined health condition. Furthermore, the distribution of dietary nutrients in the control group was within the range of findings in a previous study conducted among Nepalese [61], which assured our control group represented the base/source population. Our study was unable to link the reverse causality between CAD and the dietary intake, which were likely altered as a result of a cardiovascular event; therefore, we used validated FFQ, which takes the information for recent one year, and also is considered elsewhere for research on nutrition concerning disease, so that we can compare the data with other analyses conveniently. We executed conditional logistic regression for model construction and random forest analysis to select nutrients variables, adjusting the collinearity problem to make the results valid.
Conclusions
Diet plays a crucial role in the development of several non-communicable diseases, including CVDs. Resource-poor countries like Nepal, extracting the dietary nutrients of relative importance, could have rational and promising strategies for CAD prevention through dietary intervention. A combination of multiple factors rather than a single factor is more potent for nutrients intervention. Thus, a dietary intervention approach in CVDs is an effective strategy to reduce the public health burden. We conclude that dietary SFA, vitamin A RE, dietary total fat and oil, β-carotene, and cholesterol are the topmost five essential dietary nutrients associated with CAD development. Consistent with most of the studies, we report dietary SFA, total oil and fat, and cholesterol intakes are proportionately related, whereas β-carotene and vitamin C intakes are inversely related to CAD. Our study suggests a higher dietary intake of β-carotene and vitamin C are possible protective dietary nutrients, while an increased intake of dietary SFA, total fat and oil, and cholesterol are potential risk factors for CAD development. However, prospective cohort and RCTs studies with a large sample size are needed to explore the causal link of these nutrients for the risk of CAD development in the Nepalese population.
Supporting information
(DOCX)
ICC: Intraclass correlation coefficient; aData are shown as mean ± standard deviation (SD)
(DOCX)
kcal: kilocalorie; g: gram; mg: milligram; mcg: microgram; R.E.: retinol equivalent; PUFA: polyunsaturated fatty acid; MUFA: monounsaturated fatty acid; SFA: saturated fatty acid. aPaired t-test. bMean and standard deviation (SD) value. *p ≤ .05; **p≤ .01; ***p ≤ .001.
(DOCX)
PUFA: polyunsaturated fatty acid; MUFA: monounsaturated fatty acid; SFA: saturated fatty acid.
(DOCX)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(CSV)
(R)
Acknowledgments
The authors are grateful to Shahid Gangalal National Heart Center in Nepal, where the hospital management assisted with the set-up and data collection. We also acknowledge Professor Aihua Gu and Dr. Xu Cheng (Nanjing Medical University) for directing the entire research work. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Harrison TR. The cardiovascular system diseases Principles of Internal Medicine. Vol. 2, 16th ed, McGraw Hill, 2005. ISBN 88-386-2999-4. [Google Scholar]
- 2.WHO. Cardiovascular diseases fact sheet. World Health Organization; 2017. [retrieved 2020-7-30]; Available from: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). [Google Scholar]
- 3.WHO. Non-communicable Diseases Progress Monitor. Geneva: World Health Organization, 2017. [Google Scholar]
- 4.Al-Attas O, Al-Daghri N, Alokail M, Abd-Alrahman S, Vinodson B, Sabico S. Metabolic Benefits of Six-month Thiamine Supplementation in Patients With and Without Diabetes Mellitus Type 2. Clin Med Insights Endocrinol Diabetes. 2014;7:1–6. Epub 2014/02/20. 10.4137/CMED.S13573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oikonomou E, Psaltopoulou T, Georgiopoulos G, Siasos G, Kokkou E, Antonopoulos A, et al. Western Dietary Pattern Is Associated With Severe Coronary Artery Disease. Angiology. 2018;69(4):339–46. Epub 2017/07/22. 10.1177/0003319717721603 . [DOI] [PubMed] [Google Scholar]
- 6.Mensah GA, Wei GS, Sorlie PD, Fine LJ, Rosenberg Y, Kaufmann PG, et al. Decline in Cardiovascular Mortality: Possible Causes and Implications. Circulation research. 2017;120(2):366–80. Epub 2017/01/21. 10.1161/CIRCRESAHA.116.309115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayen AL, Marques-Vidal P, Paccaud F, Bovet P, Stringhini S. Socioeconomic determinants of dietary patterns in low- and middle-income countries: a systematic review. The American journal of clinical nutrition. 2014;100(6):1520–31. Epub 2014/11/21. 10.3945/ajcn.114.089029 . [DOI] [PubMed] [Google Scholar]
- 8.Loewen OK, Ekwaru JP, Ohinmmaa A, Veugelers PJ. Economic Burden of Not Complying with Canadian Food Recommendations in 2018. 2019;11(10). 10.3390/nu11102529 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang Y, Lee JH, Kim OY, Park HY, Lee SY. Consumption of whole grain and legume powder reduces insulin demand, lipid peroxidation, and plasma homocysteine concentrations in patients with coronary artery disease: randomized controlled clinical trial. Arteriosclerosis, thrombosis, and vascular biology. 2001;21(12):2065–71. Epub 2001/12/18. 10.1161/hq1201.100258 . [DOI] [PubMed] [Google Scholar]
- 10.Iyengar SS, Gupta R, Ravi S, Thangam S, Alexander T, Manjunath CN, et al. Premature coronary artery disease in India: coronary artery disease in the young (CADY) registry. Indian Heart J. 2017;69(2):211–6. Epub 2017/05/04. 10.1016/j.ihj.2016.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fazeli Moghadam E, Tadevosyan A, Fallahi E, Goodarzi R. Nutritional factors and metabolic variables in relation to the risk of coronary heart disease: A case control study in Armenian adults. Diabetes & metabolic syndrome. 2017;11(1):7–11. Epub 2016/06/25. 10.1016/j.dsx.2016.06.013 . [DOI] [PubMed] [Google Scholar]
- 12.de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ (Clinical research ed). 2015;351:h3978 Epub 2015/08/14. 10.1136/bmj.h3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, Moore H, et al. Reduced or modified dietary fat for preventing cardiovascular disease. The Cochrane database of systematic reviews. 2011;(7):Cd002137 Epub 2011/07/08. 10.1002/14651858.CD002137.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mozaffarian D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation. 2016;133(2):187–225. Epub 2016/01/10. 10.1161/CIRCULATIONAHA.115.018585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenna JT, Lapillonne A. Background paper on fat and fatty acid requirements during pregnancy and lactation. Ann Nutr Metab. 2009;55(1–3):97–122. Epub 2009/09/16. 10.1159/000228998 . [DOI] [PubMed] [Google Scholar]
- 16.Marventano S, Kolacz P, Castellano S, Galvano F, Buscemi S, Mistretta A, et al. A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: does the ratio really matter? International journal of food sciences and nutrition. 2015;66(6):611–22. Epub 2015/08/27. 10.3109/09637486.2015.1077790 . [DOI] [PubMed] [Google Scholar]
- 17.Nettleton JA, Villalpando S, Cassani RS, Elmadfa I. Health significance of fat quality in the diet. Ann Nutr Metab. 2013;63(1–2):96–102. 10.1159/000353207 . [DOI] [PubMed] [Google Scholar]
- 18.Ishihara J, Iso H, Inoue M, Iwasaki M, Okada K, Kita Y, et al. Intake of folate, vitamin B6 and vitamin B12 and the risk of CHD: the Japan Public Health Center-Based Prospective Study Cohort I. Journal of the American College of Nutrition. 2008;27(1):127–36. Epub 2008/05/08. 10.1080/07315724.2008.10719684 . [DOI] [PubMed] [Google Scholar]
- 19.WHO Global Report: Mortality Attributable to Tobacco. Geneva: World Health Organization, 2012. [Google Scholar]
- 20.La Torre G, Saulle R, Di Murro F, Siliquini R, Firenze A, Maurici M, et al. Mediterranean diet adherence and synergy with acute myocardial infarction and its determinants: A multicenter case-control study in Italy. PloS one. 2018;13(3):e0193360 Epub 2018/03/16. 10.1371/journal.pone.0193360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matos A, Goncalves V, Souza G, Cruz SPD, Cruz S, Ramalho A. Vitamin A nutritional status in patients with coronary artery disease and its correlation with the severity of the disease. Nutr Hosp. 2018;35(5):1215–20. Epub 2018/10/12. 10.20960/nh.1804 . [DOI] [PubMed] [Google Scholar]
- 22.Koju R, Humagain S, Khanal K. Association of cardiovascular risk factors and coronary artery lesion among coronary artery disease patients. Kathmandu Univ Med J (KUMJ). 2014;12(46):137–40. Epub 2015/01/02. 10.3126/kumj.v12i2.13661 . [DOI] [PubMed] [Google Scholar]
- 23.Campbell RK, Talegawkar SA, Christian P, Leclerq SC, Khatry SK, Wu LS, et al. Evaluation of a Novel Single-administration Food Frequency Questionnaire for Assessing Seasonally Varied Dietary Patterns among Women in Rural Nepal. Ecol Food Nutr. 2015;54(4):314–27. Epub 2015/02/14. 10.1080/03670244.2014.990635 . [DOI] [PubMed] [Google Scholar]
- 24.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England). 2004;363(9403):157–63. Epub 2004/01/17. 10.1016/S0140-6736(03)15268-3 . [DOI] [PubMed] [Google Scholar]
- 25.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. 2000 0512–3054. [PubMed]
- 26.Chagas P, Mazocco L, Piccoli J, Ardenghi TM, Badimon L, Caramori PRA, et al. Association of alcohol consumption with coronary artery disease severity. Clinical nutrition (Edinburgh, Scotland). 2017;36(4):1036–9. Epub 2016/07/13. 10.1016/j.clnu.2016.06.017 . [DOI] [PubMed] [Google Scholar]
- 27.Aidinoff E, Bluvshtein V, Bierman U, Gelernter I, Front L, Catz A. Coronary artery disease and hypertension in a non-selected spinal cord injury patient population. Spinal Cord. 2017;55(3):321–6. Epub 2016/07/20. 10.1038/sc.2016.109 . [DOI] [PubMed] [Google Scholar]
- 28.Doi-Kanno M, Fukahori H. Predictors of Depression in Patients Diagnosed with Myocardial Infarction after Undergoing Percutaneous Coronary Intervention: A literature review. J Med Dent Sci. 2016;63(2–3):37–43. Epub 2016/10/25. 10.11480/jmds.630301 [DOI] [PubMed] [Google Scholar]
- 29.WHO. STEPS surveillance manual: The WHO STEP wise approach to chronic disease risk factor surveillance. Geneva: World Health Organization; 2005. [Google Scholar]
- 30.Murphy SP, Barr SI. Practice paper of the American Dietetic Association: using the Dietary Reference Intakes. Journal of the American Dietetic Association. 2011;111(5):762–70. Epub 2011/04/26. 10.1016/j.jada.2011.03.022 . [DOI] [PubMed] [Google Scholar]
- 31.Zang J, Luo B, Chang S, Jin S, Shan C, Ma L, et al. Validity and reliability of a food frequency questionnaire for assessing dietary intake among Shanghai residents. 2019;18(1):30 10.1186/s12937-019-0454-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beunza JJ, Puertas E, García-Ovejero E, Villalba G, Condes E, Koleva G, et al. Comparison of machine learning algorithms for clinical event prediction (risk of coronary heart disease). Journal of biomedical informatics. 2019;97:103257 Epub 2019/08/03. 10.1016/j.jbi.2019.103257 . [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Dai Z, Lau EHY, Cui C, Lin H, Qi J, et al. Prevalence of bone mineral density loss and potential risk factors for osteopenia and osteoporosis in rheumatic patients in China: logistic regression and random forest analysis. Annals of translational medicine. 2020;8(5):226 Epub 2020/04/21. 10.21037/atm.2020.01.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fan J, Song Y, Wang Y, Hui R, Zhang W. Dietary glycemic index, glycemic load, and risk of coronary heart disease, stroke, and stroke mortality: a systematic review with meta-analysis. PLoS One. 2012;7(12):e52182 Epub 2013/01/04. 10.1371/journal.pone.0052182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clar C, Al-Khudairy L, Loveman E, Kelly SA, Hartley L, Flowers N, et al. Low glycaemic index diets for the prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;7:Cd004467 Epub 2017/08/02. 10.1002/14651858.CD004467.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi Y, Chang Y, Ryu S, Cho J, Kim MK, Ahn Y, et al. Relation of Dietary Glycemic Index and Glycemic Load to Coronary Artery Calcium in Asymptomatic Korean Adults. Am J Cardiol. 2015;116(4):520–6. Epub 2015/06/16. 10.1016/j.amjcard.2015.05.005 . [DOI] [PubMed] [Google Scholar]
- 37.Hu T, Bazzano LA. The low-carbohydrate diet and cardiovascular risk factors: evidence from epidemiologic studies. Nutr Metab Cardiovasc Dis. 2014;24(4):337–43. Epub 2014/03/13. 10.1016/j.numecd.2013.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu T, Jacobs DR. Low-carbohydrate diets and prevalence, incidence and progression of coronary artery calcium in the Multi-Ethnic Study of Atherosclerosis (MESA). 2019:1–8. 10.1017/s0007114518003513 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guasch-Ferre M, Babio N, Martinez-Gonzalez MA, Corella D, Ros E, Martin-Pelaez S, et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. 2015;102(6):1563–73. 10.3945/ajcn.115.116046 . [DOI] [PubMed] [Google Scholar]
- 40.Zock PL, Blom WA, Nettleton JA, Hornstra G. Progressing Insights into the Role of Dietary Fats in the Prevention of Cardiovascular Disease. Current cardiology reports. 2016;18(11):111 Epub 2016/09/22. 10.1007/s11886-016-0793-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zong G, Li Y, Wanders AJ, Alssema M, Zock PL, Willett WC, et al. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: two prospective longitudinal cohort studies. BMJ (Clinical research ed). 2016;355:i5796 Epub 2016/11/25. 10.1136/bmj.i5796 www.icmje.org/coi_disclosure.pdf and declare: support from the National Institutes of Health for the submitted work; GZ is supported by a postdoctoral fellowship funded by Unilever R&D, Vlaardingen, Netherlands; AJW, MA, and PLZ are employees of Unilever R&D (Unilever is a producer of food consumer products); FBH has received research support from California Walnut Commission and Metagenics; no other relationships or activities that could appear to have influenced the submitted work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virtanen JK, Mursu J, Tuomainen TP, Voutilainen S. Dietary fatty acids and risk of coronary heart disease in men: the Kuopio Ischemic Heart Disease Risk Factor Study. Arterioscler Thromb Vasc Biol. 2014;34(12). 10.1161/ATVBAHA.114.304082 [DOI] [PubMed] [Google Scholar]
- 43.Farvid MS, Ding M, Pan A, Sun Q, Chiuve SE, Steffen LM, et al. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130(18):1568–78. Epub 2014/08/28. 10.1161/CIRCULATIONAHA.114.010236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Annals of internal medicine. 2014;160(6):398–406. Epub 2014/04/12. 10.7326/M13-1788 . [DOI] [PubMed] [Google Scholar]
- 45.Ruiz-Nunez B, Dijck-Brouwer DA, Muskiet FA. The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J Nutr Biochem. 2016;36:1–20. Epub 2016/10/04. 10.1016/j.jnutbio.2015.12.007 . [DOI] [PubMed] [Google Scholar]
- 46.Virtanen JK, Mursu J, Virtanen HE, Fogelholm M, Salonen JT, Koskinen TT, et al. Associations of egg and cholesterol intakes with carotid intima-media thickness and risk of incident coronary artery disease according to apolipoprotein E phenotype in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. The American journal of clinical nutrition. 2016;103(3):895–901. Epub 2016/02/13. 10.3945/ajcn.115.122317 . [DOI] [PubMed] [Google Scholar]
- 47.Berger S, Raman G. Dietary cholesterol and cardiovascular disease: a systematic review and meta-analysis. 2015;102(2):276–94. 10.3945/ajcn.114.100305 . [DOI] [PubMed] [Google Scholar]
- 48.Wu Y, Qian Y, Pan Y, Li P, Yang J, Ye X, et al. Association between dietary fiber intake and risk of coronary heart disease: A meta-analysis. Clin Nutr. 2015;34(4):603–11. Epub 2014/06/16. 10.1016/j.clnu.2014.05.009 . [DOI] [PubMed] [Google Scholar]
- 49.North CJ, Venter CS, Jerling JC. The effects of dietary fibre on C-reactive protein, an inflammation marker predicting cardiovascular disease. European journal of clinical nutrition. 2009;63(8):921–33. Epub 2009/02/19. 10.1038/ejcn.2009.8 . [DOI] [PubMed] [Google Scholar]
- 50.Kuo SM. The interplay between fiber and the intestinal microbiome in the inflammatory response. Advances in nutrition (Bethesda, Md). 2013;4(1):16–28. Epub 2013/01/16. 10.3945/an.112.003046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaluza J, Orsini N, Levitan EB, Brzozowska A, Roszkowski W, Wolk A. Dietary calcium and magnesium intake and mortality: a prospective study of men. American journal of epidemiology. 2010;171(7):801–7. Epub 2010/02/23. 10.1093/aje/kwp467 . [DOI] [PubMed] [Google Scholar]
- 52.Superko HR, Zhao XQ, Hodis HN, Guyton JR. Niacin and heart disease prevention: Engraving its tombstone is a mistake. J Clin Lipidol. 2017;11(6):1309–17. Epub 2017/09/21. 10.1016/j.jacl.2017.08.005 . [DOI] [PubMed] [Google Scholar]
- 53.Zhao LG, Shu XO, Li HL, Zhang W, Gao J, Sun JW, et al. Dietary antioxidant vitamins intake and mortality: A report from two cohort studies of Chinese adults in Shanghai. J Epidemiol. 2017;27(3):89–97. Epub 2017/02/01. 10.1016/j.je.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alissa EM, Bahjri SM, Al-Ama N, Ahmed WH, Starkey B, Ferns GA. Dietary vitamin A may be a cardiovascular risk factor in a Saudi population. Asia Pacific journal of clinical nutrition. 2005;14(2):137–44. Epub 2005/06/02. . [PubMed] [Google Scholar]
- 55.Kong SH, Kim JH. Dietary calcium intake and risk of cardiovascular disease, stroke, and fracture in a population with low calcium intake. 2017;106(1):27–34. 10.3945/ajcn.116.148171 . [DOI] [PubMed] [Google Scholar]
- 56.Anderson JJ, Kruszka B, Delaney JA, He K, Burke GL, Alonso A, et al. Calcium Intake From Diet and Supplements and the Risk of Coronary Artery Calcification and its Progression Among Older Adults: 10-Year Follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA). Journal of the American Heart Association. 2016;5(10). Epub 2016/10/13. 10.1161/jaha.116.003815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eshak ES, Iso H, Yamagishi K, Maruyama K, Umesawa M, Tamakoshi A. Associations between copper and zinc intakes from diet and mortality from cardiovascular disease in a large population-based prospective cohort study. Journal of Nutritional Biochemistry. 2018;56:126–32. 10.1016/j.jnutbio.2018.02.008 WOS:000435747700014. [DOI] [PubMed] [Google Scholar]
- 58.de Oliveira Otto MC, Alonso A, Lee DH, Delclos GL, Bertoni AG, Jiang R, et al. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. The Journal of nutrition. 2012;142(3):526–33. Epub 2012/01/20. 10.3945/jn.111.149781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Q, Shi L, Rimm EB, Giovannucci EL, Hu FB, Manson JE, et al. Vitamin D intake and risk of cardiovascular disease in US men and women. The American journal of clinical nutrition. 2011;94(2):534–42. Epub 2011/06/10. 10.3945/ajcn.110.008763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hunnicutt J, He K, Xun P. Dietary iron intake and body iron stores are associated with risk of coronary heart disease in a meta-analysis of prospective cohort studies. J Nutr. 2014;144(3):359–66. Epub 2014/01/10. 10.3945/jn.113.185124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shrestha A, Koju RP, Beresford SAA, Chan KCG, Connell FA, Karmacharya BM, et al. Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for Nepalese diet. International journal of food sciences and nutrition. 2017;68(5):605–12. Epub 2017/01/18. 10.1080/09637486.2016.1268099 . [DOI] [PubMed] [Google Scholar]