Key Points
Question
Does therapy with low-dose capecitabine maintenance after standard adjuvant chemotherapy reduce the risk of relapse and death in early-stage triple-negative breast cancer?
Findings
In this randomized clinical trial of 434 women with early-stage triple-negative breast cancer who received standard adjuvant treatment, low-dose capecitabine maintenance therapy for 1 year, compared with observation, resulted in significantly higher 5-year disease-free survival (82.8% vs 73.0%; hazard ratio for risk of recurrence or death, 0.64).
Meaning
Among women with early-stage triple-negative breast cancer, maintenance therapy with low-dose capecitabine significantly improved disease-free survival at 5 years.
Abstract
Importance
Among all subtypes of breast cancer, triple-negative breast cancer has a relatively high relapse rate and poor outcome after standard treatment. Effective strategies to reduce the risk of relapse and death are needed.
Objective
To evaluate the efficacy and adverse effects of low-dose capecitabine maintenance after standard adjuvant chemotherapy in early-stage triple-negative breast cancer.
Design, Setting, and Participants
Randomized clinical trial conducted at 13 academic centers and clinical sites in China from April 2010 to December 2016 and final date of follow-up was April 30, 2020. Patients (n = 443) had early-stage triple-negative breast cancer and had completed standard adjuvant chemotherapy.
Interventions
Eligible patients were randomized 1:1 to receive capecitabine (n = 222) at a dose of 650 mg/m2 twice a day by mouth for 1 year without interruption or to observation (n = 221) after completion of standard adjuvant chemotherapy.
Main Outcomes and Measures
The primary end point was disease-free survival. Secondary end points included distant disease-free survival, overall survival, locoregional recurrence-free survival, and adverse events.
Results
Among 443 women who were randomized, 434 were included in the full analysis set (mean [SD] age, 46 [9.9] years; T1/T2 stage, 93.1%; node-negative, 61.8%) (98.0% completed the trial). After a median follow-up of 61 months (interquartile range, 44-82), 94 events were observed, including 38 events (37 recurrences and 32 deaths) in the capecitabine group and 56 events (56 recurrences and 40 deaths) in the observation group. The estimated 5-year disease-free survival was 82.8% in the capecitabine group and 73.0% in the observation group (hazard ratio [HR] for risk of recurrence or death, 0.64 [95% CI, 0.42-0.95]; P = .03). In the capecitabine group vs the observation group, the estimated 5-year distant disease-free survival was 85.8% vs 75.8% (HR for risk of distant metastasis or death, 0.60 [95% CI, 0.38-0.92]; P = .02), the estimated 5-year overall survival was 85.5% vs 81.3% (HR for risk of death, 0.75 [95% CI, 0.47-1.19]; P = .22), and the estimated 5-year locoregional recurrence-free survival was 85.0% vs 80.8% (HR for risk of locoregional recurrence or death, 0.72 [95% CI, 0.46-1.13]; P = .15). The most common capecitabine-related adverse event was hand-foot syndrome (45.2%), with 7.7% of patients experiencing a grade 3 event.
Conclusions and Relevance
Among women with early-stage triple-negative breast cancer who received standard adjuvant treatment, low-dose capecitabine maintenance therapy for 1 year, compared with observation, resulted in significantly improved 5-year disease-free survival.
Trial Registration
ClinicalTrials.gov Identifier: NCT01112826
This randomized trial compares the effects of low-dose high-frequency capecitabine maintenance therapy vs observation after standard adjuvant chemotherapy on 5-year disease-free survival among women with early-stage triple-negative breast cancer.
Introduction
The poor prognosis of triple-negative breast cancer (TNBC) results from a lack of available targeted treatment options coupled with the aggressive biological behavior of this subtype, which is associated with a high risk of early recurrence, particularly visceral metastasis.1,2 Maintenance endocrine or ERBB2 (formerly HER2)-targeted therapy has been used to reduce the risk of recurrence and death significantly in patients with hormone receptor–positive or ERBB2-overexpressing early-stage breast cancer. However, chemotherapy is the only adjuvant treatment option for patients with early-stage TNBC. Effective maintenance therapies to reduce the risk of relapse and death are needed.
Chemotherapy using lower dosage and higher frequency is thought to exert its anticancer activity by targeting 2 mechanisms of metastasis: angiogenesis and immune escape.3 Therefore, low-dose chemotherapy might prevent TNBC from metastasizing.
Capecitabine, an orally administered chemotherapeutic drug used widely in the treatment of metastatic breast cancer, is a potential candidate therapy for low-dose administration as maintenance to prevent recurrence.4,5,6 Several previous clinical trials added high-dose capecitabine to standard breast cancer adjuvant chemotherapy regimens and have reported conflicting results,7,8,9,10,11 although those trials were not restricted to women with TNBC. The present trial, Sun Yat-sen University Cancer Center 001 (SYSUCC-001), was designed to evaluate the effect of low-dose capecitabine maintenance therapy after completion of standard adjuvant treatment on disease-free and overall survival in women with early-stage TNBC.
Methods
Study Design and Patient Eligibility
The SYSUCC-001 trial (ClinicalTrials.gov: NCT01112826) is an open-label, multicenter, randomized, phase 3 study that compared the efficacy and adverse events of low-dose capecitabine maintenance with observation following standard adjuvant treatment in patients with early-stage TNBC. The trial was sponsored by Sun Yat-sen University and was approved by the SYSUCC ethics committee, together with the ethics committees at each participating institution. All patients provided written informed consent. The study protocol and statistical analysis plan are available in Supplement 1.
Eligible trial participants were women who had pathologically confirmed invasive breast ductal carcinoma that was hormone receptor negative (<1% positive cells by immunohistochemistry staining) and ERBB2 negative. Participants had early-stage tumors that were stage T1b-3N0-3cM0, without positive supraclavicular or internal mammary lymph node involvement based on the American Joint Committee on Cancer (AJCC 2010, seventh edition) staging criteria, and they received standard treatments, including modified radical mastectomy or breast-conserving surgery, neo-/adjuvant chemotherapy, and radiotherapy according to institutional guidelines. Key exclusion criteria included inflammatory or bilateral breast cancer; a history of invasive breast cancer or other malignancies; receipt of other biologic agents or immunotherapy; lactation or pregnancy; or severe coexisting illness.
Intervention
Eligible patients were randomly assigned in a 1:1 ratio to receive either low-dose capecitabine maintenance (intervention group) or observation (control group) within 4 weeks after completion of standard adjuvant chemotherapy, with a block size of 6 (known to the statistician [Y.G.]) and participants were stratified by lymph node status (negative vs positive). Random assignment was performed using a computer-generated code generated using SAS software version 8.01 (SAS Institute) with a random seed of 37 277 generated centrally at the Clinical Trials Centre of Sun Yat-sen University Cancer Center. Details of the random allocations were contained in sequentially numbered, opaque, sealed envelopes, which were prepared by the study statistician (Y.G.).
Study treatment was initiated at randomization. The capecitabine maintenance group received oral capecitabine at 650 mg/m2, twice daily continuously for 1 year. Collection of capecitabine dose received and adverse events were assessed monthly during capecitabine maintenance in the capecitabine group. In both groups, physical examination, assessment of menopausal status, breast ultrasound, and abdominal ultrasound were performed every 3 months during years 1 to 2, every 6 months during years 3 to 5, and yearly thereafter; mammography and chest x-ray were performed yearly. Patients who had not experienced recurrence or death at the time of data analysis were censored as alive and event-free at the date of last follow-up.
Outcome Measures
The primary end point of the study was 5-year disease-free survival, defined as the time from randomization to the first occurrence of the following events: local relapse, distant metastasis, contralateral breast cancer, or death from any cause. Secondary end points included distant disease-free survival (the time from randomization to distant recurrence, contralateral invasive breast cancer, or death from any cause), overall survival (the time from randomization to death from any cause), locoregional recurrence-free survival (the time from randomization to locoregional invasive recurrence or death), and adverse events. Adverse events were assessed and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0. Hand-foot syndrome (a common adverse effect of the fluoropyrimidine chemotherapy agent capecitabine) events were graded from 1 to 3 (Supplement 1).12
Statistical Analysis
The estimated 5-year disease-free survival for patients with TNBC was 68%.13,14 To detect an absolute improvement of 12%15 in 5-year disease-free survival from 68.0% in the control group to 80.0% in the capecitabine maintenance group, approximately 109 disease-free survival events would be required to achieve 80% power at a 2-sided significance level of 5%. The period of enrollment and follow-up required were estimated at 60 and 36 months, respectively. After considering a 9% dropout rate, approximately 424 patients (212 patients in each group) were required to detect an absolute 12% improvement in 5-year disease-free survival to be enrolled in the study. Originally, the study planned to enroll 684 participants under the assumption of 20% dropout with power of 90%; however, dropout was less than anticipated and 80% power was deemed acceptable such that the independent data monitoring committee recommended revising the sample size calculations as above.
The number of disease-free survival events was lower than expected at the prespecified 3-year follow-up performed in December 2019. Although the required number of events had not been reached, the independent data monitoring committee recommended completion of the final analysis. The study investigators followed the independent data monitoring committee’s advice and performed the final analysis with 3 years of follow-up and 94 events, rather than the projected 109 events. This final analysis was performed with a cutoff date of April 30, 2020.
Cumulative survival probabilities were estimated using the Kaplan-Meier analysis method and compared using log-rank tests in the full analysis set, defined as all randomized patients except for those who withdrew informed consent before starting protocol treatment or who had no follow-up after randomization. Hazard ratios (HRs) with 95% CIs were estimated using the Cox proportional hazards model. The proportional hazards assumption was confirmed based on the Schoenfeld residuals.16 The last observation carried forward method was used for handling missing outcome data.
The prespecified exploratory subgroup analyses were conducted according to prognostic factors including age at random assignment, tumor size at diagnosis, histological grade, nodal status, lymphovascular invasion, Ki-67 index, and neo-/adjuvant chemotherapy regimens. The consistency of the treatment effect was measured for each prespecified subgroup and evaluated using an unadjusted Cox proportional hazard model. Treatment effects were evaluated among subgroups by adding interaction terms to Cox proportional hazards models. This study was designed as center randomization and heterogeneity between centers was evaluated using the Breslow-Day test.
The differences between treatment groups were compared using a χ2 test or Fisher exact test for categorical variables, and t test or the Mann-Whitney test for continuous variables, when appropriate.
The frequency and severity of each adverse event was recorded according to the National Cancer Institute’s Common Toxicity Criteria for Adverse Events version 4.0. The most severe adverse event grade per category for each patient was reported. A preplanned interim analysis was canceled by the independent data monitoring committee in January 2017 and was reviewed by the SYSUCC Ethics Committee based on the low number of events. A significance level of a 2-tailed P value at .047 (according to the O’Brien-Fleming method) for the final disease-free survival analysis was used; all other statistical tests were 2-tailed at a significance level of .05. The findings for the analyses of secondary end points should be interpreted as exploratory because of the potential for type I error resulting from multiple comparisons. All statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
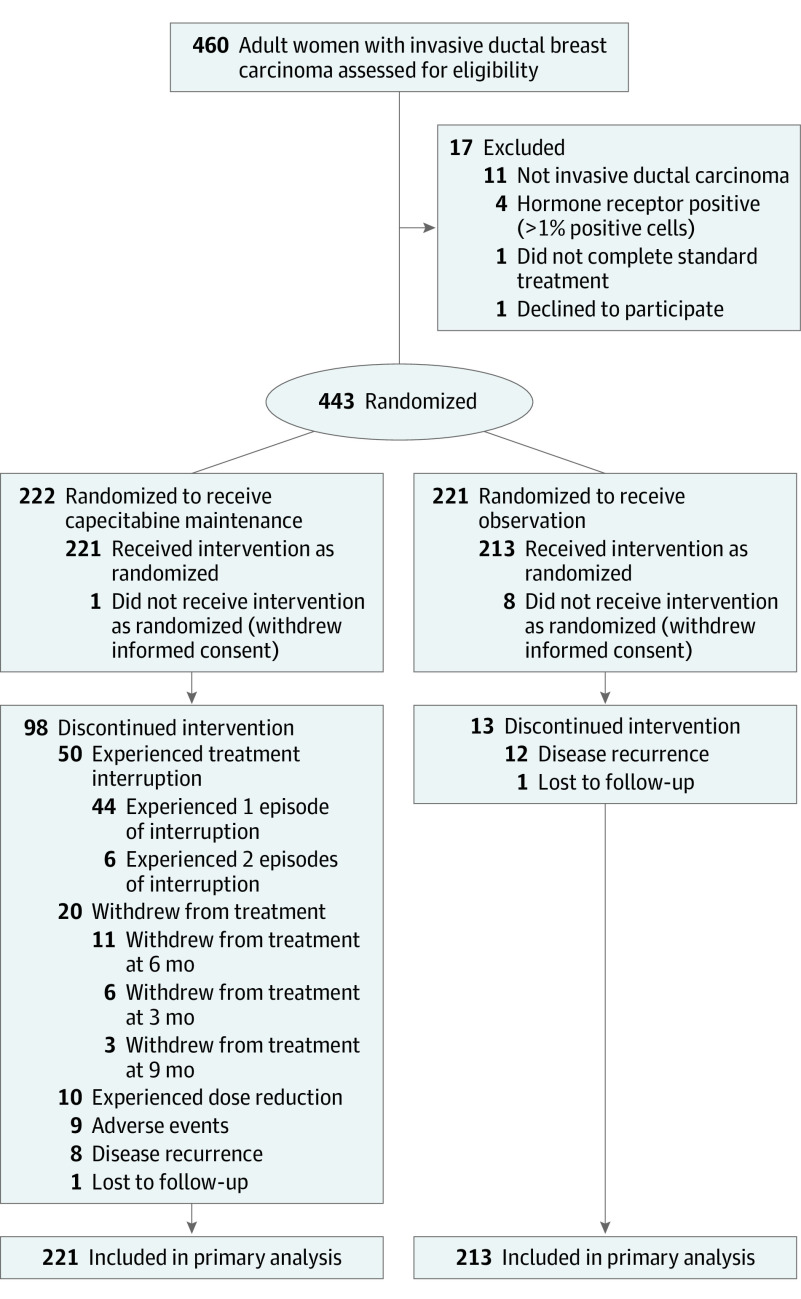
From April 2010 to December 2016, 443 patients from 13 Chinese study sites were enrolled and randomly assigned to the capecitabine group (222 patients) or the observational group (221 patients). Nine patients were excluded because of withdrawal of informed consent before starting the intervention. A total of 434 patients were included in the primary analyses (221 patients in the capecitabine group and 213 in the observation group) (Figure 1).
Figure 1. Flow of Patients Through the SYSUCC-001 Trial of Capecitabine for Triple-Negative Breast Cancer.
Two patients were lost to follow-up, and both had been followed up for more than the preplanned 3 years (1 in the capecitabine group for 49 months, 1 in the observation group for 51 months). Patient, disease, and treatment characteristics at baseline were well balanced between the 2 groups (Table 1). The median (SD) age at randomization was 46 (9.9) years (range, 24-70), and 66.8% were premenopausal. Most patients had undergone a mastectomy (86.4%) and had received anthracycline- and taxane-based regimens as neoadjuvant chemotherapy (5.8%) or adjuvant chemotherapy (78.8%). Most tumors were of T1/T2 (93.1%), node-negative (61.8%), and grade 3 (72.8%).
Table 1. Patient and Tumor Characteristics at Baseline.
| Variable | No. (%) | |
|---|---|---|
| Capecitabine group (n = 221) | Observation group (n = 213) | |
| Age, y | ||
| ≤40 | 62 (28.1) | 49 (23.0) |
| 41-50 | 85 (38.5) | 72 (33.8) |
| ≥51 | 74 (33.5) | 92 (43.2) |
| Median, IQR | 45 (39-53) | 48 (40-57) |
| Premenopausal | 157 (71.0) | 133 (62.4) |
| Tumor size, cma | ||
| ≤2 | 79 (35.7) | 79 (37.1) |
| >2 to ≤5 | 122 (55.2) | 124 (58.2) |
| >5 | 20 (9.1) | 10 (4.7) |
| Node status | ||
| Negative | 135 (61.1) | 133 (62.4) |
| 1-3 positive nodes | 46 (20.8) | 41 (19.2) |
| 4-9 positive nodes | 14 (6.3) | 25 (11.7) |
| ≥10 positive nodes | 26 (11.8) | 14 (6.6) |
| Stage at diagnosis (AJCC 2010)b | ||
| I | 56 (25.3) | 59 (27.7) |
| II | 120 (54.3) | 116 (54.5) |
| III | 45 (20.4) | 38 (17.8) |
| Histological gradec | ||
| 1 | 5 (2.3) | 3 (1.4) |
| 2 | 52 (23.5) | 58 (27.2) |
| 3 | 164 (74.2) | 152 (71.4) |
| Ki-67 index at diagnosis <30%d | 44 (19.9) | 57 (26.8) |
| Lymphovascular invasion | 42 (19.0) | 23 (10.8) |
| Type of surgery | ||
| Mastectomy | 186 (84.2) | 189 (88.7) |
| Lumpectomy | 35 (15.8) | 24 (11.3) |
| Lymph node dissection | ||
| Axillary-node dissection | 176 (79.6) | 169 (79.3) |
| Sentinel-node biopsy only | 45 (20.4) | 44 (20.7) |
| Chemotherapy received | ||
| Adjuvant only | 209 (94.5) | 195 (91.5) |
| Neoadjuvant only | 7 (3.2) | 11 (5.2) |
| Adjuvant and neoadjuvant | 5 (2.3) | 7 (3.3) |
| Chemotherapy regimen | ||
| Anthracyclines and taxane | 198 (89.6) | 189 (88.7) |
| Taxane only | 20 (9.0) | 21 (9.9) |
| Anthracyclines only | 3 (1.4) | 3 (1.4) |
| Received radiotherapy | 111 (50.2) | 86 (40.6) |
Abbreviations: AJCC, American Joint Committee on Cancer; IQR, interquartile range.
Tumor size at diagnosis was based on pathological assessment.
Stage at diagnosis was based on AJCC 2010, seventh edition.
Histological grade at diagnosis was based on the degree of the tumor’s histologic differentiation.
Ki-67 index at diagnosis indicates DNA synthetic activity as measured by immunocytochemistry.
Primary Outcome
After a median follow-up of 61 months (interquartile range, 44-82), 94 events were observed, of which 38 events (37 recurrences and 32 deaths) were in the capecitabine group and 56 events (56 recurrences and 40 deaths) were in the observation group. The primary outcome of estimated 5-year disease-free survival in the capecitabine group vs the observation group was 82.8% vs 73.0% (HR for risk of recurrence or death, 0.64 [95% CI, 0.42-0.95]; P = .03) (Figure 2A).
Figure 2. Kaplan-Meier Estimates of Disease-Specific Mortality, Total Mortality, Distant Disease-Specific Mortality, and Locoregional Recurrence-Specific Mortality in 434 Patients With Triple-Negative Breast Cancer.
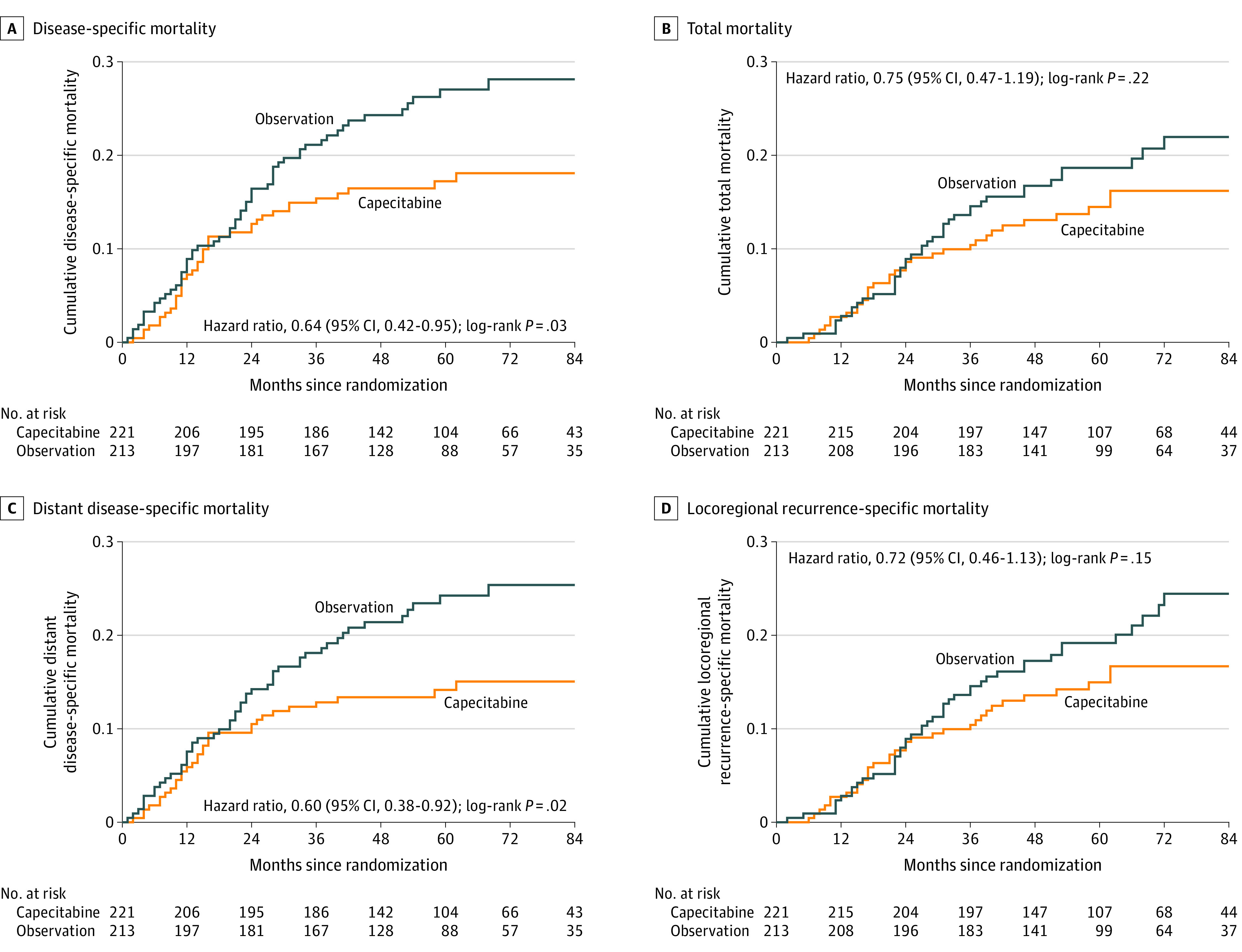
Median observation for all curves was 61 months (interquartile range, 44-82). Cumulative survival probabilities were estimated using the Kaplan-Meier analysis method and compared using log-rank tests. Hazard ratios with 95% CIs were estimated using the Cox proportional hazards model.
Secondary Outcomes
For the secondary end points, the estimated 5-year distant disease-free survival in the capecitabine group vs the observation group was 85.8% vs 75.8% (HR for risk of distant metastasis or death, 0.60 [95% CI, 0.38-0.92]; P = .02) (Figure 2C). The estimated 5-year overall survival in the capecitabine group vs the observation group was 85.5% vs 81.3% (HR for risk of death, 0.75 [95% CI, 0.47-1.19]; P = .22) (Figure 2B). The estimated 5-year locoregional recurrence-free survival in the capecitabine group vs the observation group was 85.0% vs 80.8% (HR for risk of locoregional recurrence or death, 0.72 [95% CI, 0.46-1.13]; P = .15) (Figure 2D).
Prespecified Exploratory Analysis
A prespecified exploratory subgroup analysis was conducted, and no significant interactions were observed between the treatment groups and the subgroups. The effect of capecitabine on disease-free survival was consistent across all patient subgroups (Figure 3). For patients with node-negative disease, the HR for risk of recurrence or death was 0.37 (95% CI, 0.17-0.79; P = .008). For patients with node-positive disease, the HR for risk of recurrence or death was 0.82 (95% CI, 0.50-1.34; P = .42) (eFigure in Supplement 2). However, the interaction analysis suggested no interaction between capecitabine and nodal status on disease-free survival (P for interaction = .07).
Figure 3. Subgroup Analysis of Disease-Specific Mortality in 434 Patients With Triple-Negative Breast Cancer.
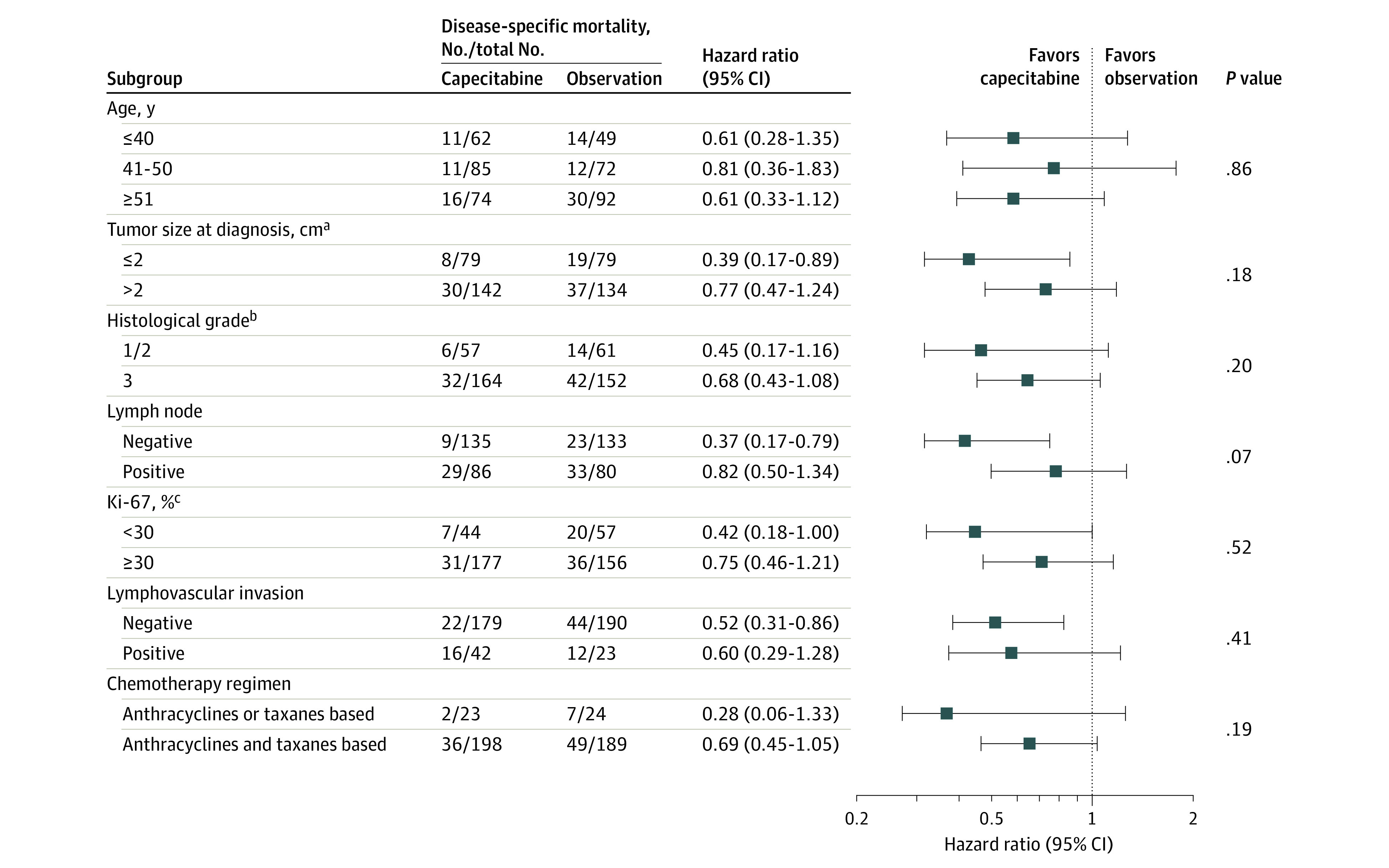
Using the unadjusted Cox model, exploratory subgroup analyses were conducted to estimate hazard ratios with 95% CIs and to test for interactions among subgroups using 2-sided P values. The median observation was 61 months.
aTumor size at diagnosis was based on pathological assessment.
bHistological grade at diagnosis was based on the degree of tumor’s histologic differentiation.
cKi-67 index at diagnosis indicates DNA synthetic activity as measured by immunocytochemistry.
Of the 221 patients who were assigned to the capecitabine group, 183 patients (82.8%) completed 1 year of treatment according to the treatment protocol. Of these, 44 patients experienced 1 episode of treatment interruption, 6 had 2 episodes of treatment interruption, and 10 required dose reduction. Thirty-seven patients (16.7%) discontinued capecitabine before completing 1 year of treatment: 20 (9.0%) because of the patient’s desire to withdraw from the study, 9 (4.1%) because of unacceptable toxicity of hand-foot syndrome, and 8 (3.6%) because of disease recurrence. The median relative dose intensity for capecitabine was 84.7%, and the median cumulative dose of capecitabine was 480 000 mg/m2 (minimum-maximum, 33 000-480 000 mg/m2).
In addition, the number and type of disease-free events were analyzed (eTable in Supplement 2). The median time from randomization to the first recurrence was 18.5 months (range, 4-68). Distant recurrence, particularly lung and bone metastasis, was more frequent in the observation group than in the capecitabine group (lung: 12 in the capecitabine group and 25 in the observation group, P = .02; bone: 7 in the capecitabine group and 19 in the observation group; P = .01). Testing for heterogeneity between treatment sites revealed consistent effects across centers (P = .49).
Adverse Events
In the capecitabine group, hand-foot syndrome was the most frequent adverse event, occurring in 100 patients (45.2%), including 17 patients (7.7%) with a grade 3 event. Other common adverse events in the capecitabine group were leucopenia (23.5%), elevated bilirubin (12.7%), abdominal pain/diarrhea (6.8%), and elevated alanine aminotransferase/aspartate transaminase levels (5.0%). All these adverse events were grade 1 or 2 in severity (Table 2).
Table 2. Adverse Events.
| Eventa | No. (%) | ||||
|---|---|---|---|---|---|
| Capecitabine group (n = 221) | Observation group (n = 213)b | ||||
| Mild (grade 1) | Moderate (grade 2) | Severe (grade 3) | Mild (grade 1) | Moderate (grade 2) | |
| Hand-foot syndromec | 41 (18.6) | 42 (19.0) | 17 (7.7) | 0 | 0 |
| Leukocytes <4000/μL | 40 (18.1) | 12 (5.4) | 0 | 12 (5.6) | 5 (2.3) |
| Bilirubin ≥1.5 times high normal value | 17 (7.7) | 11 (5.0) | 0 | 3 (1.4) | 0 |
| Abdominal pain/diarrhea | 7 (3.2) | 8 (3.6) | 0 | 2 (0.9) | 0 |
| ALT/AST >2.5 times high normal value | 8 (3.6) | 3 (1.4) | 0 | 11 (5.2) | 2 (0.9) |
| Fatigue | 4 (1.9) | 0 | 0 | 3 (1.4) | 0 |
| Nausea | 3 (1.4) | 0 | 0 | 3 (1.4) | 0 |
| Vomiting | 1 (0.5) | 0 | 0 | 0 | 0 |
| Stomatitis | 1 (0.5) | 0 | 0 | 2 (0.9) | 0 |
| Patients with ≥1 adverse events | 145 (65.6) | 41 (19.2) | |||
Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase.
Severity of adverse events was graded by investigators according to the National Cancer Institute’s (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. For events not listed in NCI CTCAE version 4.0, mild, moderate, or severe were determined based on any, mild, or significant effect on the daily activities of patients.
No patients in the observation group experienced severe adverse events.
Hand-foot syndrome events were graded using the following criteria: mild (grade 1) was defined as numbness, tingling sensation, or erythema of hands and/or feet that cause painless swelling or discomfort without affecting daily activities; moderate (grade 2) as painful erythema or swelling of hands and/or feet that affect daily activities; and severe (grade 3) as wet desquamation, ulceration, blistering, severe pain of hands and/or feet, and/or unable to work or perform daily activities12 (as detailed in the study protocol, see Supplement 1).
Discussion
In this randomized clinical trial conducted among Chinese women with early-stage TNBC, the addition of low-dose capecitabine as maintenance therapy for 1 year following standard adjuvant treatment, compared with placebo, significantly improved disease-free survival. The treatment effects on disease-free survival were consistent across all patient subgroups. Capecitabine was associated with significant improvement in distant disease-free survival but not significant improvement in overall survival or locoregional recurrence-free survival.
Chemotherapy with low-dose capecitabine may reduce recurrence for women with TNBC by targeting 2 mechanisms of metastasis: angiogenesis and immune escape.3 However, there has been uncertainty regarding both the efficacy and acceptability of prolonged treatment necessary to reduce recurrence. The current trial demonstrated that a year of capecitabine was tolerable for most women without significant treatment discontinuation due to toxicity. More than 80% of participants completed a year of treatment and less than a quarter required any treatment interruption.
Four previous randomized trials in women with early-stage breast cancer, the FinXX trial,9 the USO01062 trial,10 the GEICAM-CIBOMA trial,11 and the GEICAM/2003-10 trial,17 each failed to demonstrate that the addition of capecitabine to standard adjuvant chemotherapy improved either disease-free or overall survival. There are 2 potential explanations for the conflicting results between these previous trials and the current study. First, the benefit of maintenance capecitabine may be limited to TNBC.9,10 The FinXX, USO01062, GEICAM-COBOMA, and GEICAM/2003-10 studies included patients with breast cancer subtypes other than TNBC. Subgroup analyses of these studies suggested benefit for participants with TNBC. The CBCSG010 trial was restricted to early-stage TNBC and showed that the addition of capecitabine to standard adjuvant regimens improved 5-year disease-free survival rates by 6% compared with standard adjuvant therapy without capecitabine.
The present study result is also consistent with findings from the CREATE-X trial,8 which compared adjuvant capecitabine vs no adjuvant treatment in patients with ERBB2-negative breast cancer who had completed neoadjuvant chemotherapy but had residual invasive tumor identified on surgical pathology. The CREATE-X trial included women with hormone receptor–positive tumors as well as women with TNBC, but showed that the TNBC subgroup derived greater benefit from the addition of capecitabine. A meta-analysis of neoadjuvant and adjuvant capecitabine trials also suggested that the benefit of adding capecitabine to standard chemotherapy was observed only in the subgroup of patients with TNBC.18
Second, the extended duration of capecitabine may influence the efficacy of maintenance treatment. The GEICAM-CIBOMA trial randomized participants with TNBC to 8 cycles of capecitabine (24 weeks) or to no further treatment and the results showed no statistically significant increase in disease-free survival. Because the study designs and patient characteristics were otherwise similar, the longer duration of capecitabine maintenance of 52 weeks in the present study vs 24 weeks in the GEICAM-CIBOMA trial may explain the discrepant findings.
Low-dose capecitabine maintenance was generally well tolerated and most participants completed a year of treatment without requiring treatment interruptions. The most common adverse event of capecitabine monotherapy was hand-foot syndrome, which occurred in 45.2% of patients. For 7.7%, hand-foot syndrome was grade 3, which involves blistering and interference with the ability to function in routine daily activities. Other adverse events included diarrhea, hyperbilirubinemia, and leucopenia but events were not severe. All toxicity and adverse events other than hand-foot syndrome were grade 1 or 2.
Limitations
This study has several limitations. First, the optimal dose and duration of low-dose capecitabine maintenance merits further exploration. The dose selected for this trial was 650 mg/m2 twice daily based on the dose used in a randomized trial of frail women with metastatic breast cancer that demonstrated fewer adverse effects and better quality of life when compared with higher doses given on a 2 week on, 1 week off schedule.19 Various dosages of low-dose capecitabine ranging from a fixed dose of 500 mg 3 times daily to 800 mg/m2 twice daily20,21,22 have been evaluated. However, there is residual uncertainty about the optimal dose that minimizes toxicity without compromising efficacy. The treatment duration of 1 year was selected because that is the duration of trastuzumab used in the HERA study23 for maintenance treatment of women with ERBB2-positive breast cancer. The long duration was also justified based on the high rates of recurrence for patients with TNBC. Whether 1 year of capecitabine therapy is sufficient for patients with early-stage TNBC remains uncertain because the peak risk of recurrence is within the first 2 years of diagnosis.24
Second, the trial was limited to recruitment at 13 Chinese hospitals and, therefore, results may not be generalizable to patients with breast cancer from other geographic regions and from other racial/ethnic backgrounds. For example, the tolerability of capecitabine may vary based on different genetic backgrounds and/or dietary folate intake.25,26
Third, in the current study, only 5.8% of patients received neoadjuvant chemotherapy, which is the current standard of care for patients with node-positive TNBC in Europe and North America.27 Fourth, there was some imbalance in the randomization, with a higher proportion of older women assigned to receive placebo, which could have favored the capecitabine group.
Conclusions
Among women with early-stage TNBC who received standard adjuvant treatment, low-dose capecitabine maintenance therapy for 1 year, compared with observation, resulted in significantly improved 5-year disease-free survival.
Trial Protocol
eFigure. Effect of Different Node Status on Outcomes
eTable. Disease-Free Survival Events in 434 Patients With TNBC
Data Sharing Statement
References
- 1.Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115(2):423-428. doi: 10.1007/s10549-008-0086-2 [DOI] [PubMed] [Google Scholar]
- 2.Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113(10):2638-2645. doi: 10.1002/cncr.23930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7(8):455-465. doi: 10.1038/nrclinonc.2010.82 [DOI] [PubMed] [Google Scholar]
- 4.Blum JL, Jones SE, Buzdar AU, et al. . Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17(2):485-493. doi: 10.1200/JCO.1999.17.2.485 [DOI] [PubMed] [Google Scholar]
- 5.Reichardt P, Von Minckwitz G, Thuss-Patience PC, et al. . Multicenter phase II study of oral capecitabine (Xeloda(“)) in patients with metastatic breast cancer relapsing after treatment with a taxane-containing therapy. Ann Oncol. 2003;14(8):1227-1233. doi: 10.1093/annonc/mdg346 [DOI] [PubMed] [Google Scholar]
- 6.Zielinski C, Gralow J, Martin M. Optimising the dose of capecitabine in metastatic breast cancer: confused, clarified or confirmed? Ann Oncol. 2010;21(11):2145-2152. doi: 10.1093/annonc/mdq069 [DOI] [PubMed] [Google Scholar]
- 7.Li J, Yu K, Pang D, et al. ; CBCSG010 Study Group . Adjuvant capecitabine with docetaxel and cyclophosphamide plus epirubicin for triple-negative breast cancer (CBCSG010): an open-label, randomized, multicenter, phase III trial. J Clin Oncol. 2020;38(16):1774-1784. doi: 10.1200/JCO.19.02474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masuda N, Lee SJ, Ohtani S, et al. . Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376(22):2147-2159. doi: 10.1056/NEJMoa1612645 [DOI] [PubMed] [Google Scholar]
- 9.Joensuu H, Kellokumpu-Lehtinen PL, Huovinen R, et al. . Adjuvant capecitabine, docetaxel, cyclophosphamide, and epirubicin for early breast cancer: final analysis of the randomized FinXX trial. J Clin Oncol. 2012;30(1):11-18. doi: 10.1200/JCO.2011.35.4639 [DOI] [PubMed] [Google Scholar]
- 10.O’Shaughnessy J, Koeppen H, Xiao Y, et al. . Patients with slowly proliferative early breast cancer have low five-year recurrence rates in a phase III adjuvant trial of capecitabine. Clin Cancer Res. 2015;21(19):4305-4311. doi: 10.1158/1078-0432.CCR-15-0636 [DOI] [PubMed] [Google Scholar]
- 11.Lluch A, Barrios CH, Torrecillas L, et al. ; GEICAM Spanish Breast Cancer Group; CIBOMA (Iberoamerican Coalition for Research in Breast Oncology); LACOG (Latin American Cooperative Oncology Group) . Phase III trial of adjuvant capecitabine after standard neo-/adjuvant chemotherapy in patients with early triple-negative breast cancer (GEICAM/2003-11_CIBOMA/2004-01). J Clin Oncol. 2020;38(3):203-213. doi: 10.1200/JCO.19.00904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gressett SM, Stanford BL, Hardwicke F. Management of hand-foot syndrome induced by capecitabine. J Oncol Pharm Pract. 2006;12(3):131-141. doi: 10.1177/1078155206069242 [DOI] [PubMed] [Google Scholar]
- 13.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7(1-2):4-13. doi: 10.3121/cmr.2008.825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liedtke C, Mazouni C, Hess KR, et al. . Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275-1281. doi: 10.1200/JCO.2007.14.4147 [DOI] [PubMed] [Google Scholar]
- 15.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687-1717. doi: 10.1016/S0140-6736(05)66544-0 [DOI] [PubMed] [Google Scholar]
- 16.Wileyto EP, Li Y, Chen J, Heitjan DF. Assessing the fit of parametric cure models. Biostatistics. 2013;14(2):340-350. doi: 10.1093/biostatistics/kxs043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martín M, Ruiz Simón A, Ruiz Borrego M, et al. . Epirubicin plus cyclophosphamide followed by docetaxel versus epirubicin plus docetaxel followed by capecitabine as adjuvant therapy for node-positive early breast cancer: results from the GEICAM/2003-10 Study. J Clin Oncol. 2015;33(32):3788-3795. doi: 10.1200/JCO.2015.61.9510 [DOI] [PubMed] [Google Scholar]
- 18.Simsek C, Esin E, Yalcin S. Metronomic chemotherapy: a systematic review of the literature and clinical experience. J Oncol. 2019;2019:5483791. doi: 10.1155/2019/5483791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stockler MR, Harvey VJ, Francis PA, et al. . Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol. 2011;29(34):4498-4504. doi: 10.1200/JCO.2010.33.9101 [DOI] [PubMed] [Google Scholar]
- 20.Fedele P, Marino A, Orlando L, et al. . Efficacy and safety of low-dose metronomic chemotherapy with capecitabine in heavily pretreated patients with metastatic breast cancer. Eur J Cancer. 2012;48(1):24-29. doi: 10.1016/j.ejca.2011.06.040 [DOI] [PubMed] [Google Scholar]
- 21.Montagna E, Palazzo A, Maisonneuve P, et al. . Safety and efficacy study of metronomic vinorelbine, cyclophosphamide plus capecitabine in metastatic breast cancer: a phase II trial. Cancer Lett. 2017;400:276-281. doi: 10.1016/j.canlet.2017.01.027 [DOI] [PubMed] [Google Scholar]
- 22.Martín M, Martínez N, Ramos M, et al. . Standard versus continuous administration of capecitabine in metastatic breast cancer (GEICAM/2009-05): a randomized, noninferiority phase II trial with a pharmacogenetic analysis. Oncologist. 2015;20(2):111-112. doi: 10.1634/theoncologist.2014-0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. ; Herceptin Adjuvant (HERA) Trial Study Team . Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659-1672. doi: 10.1056/NEJMoa052306 [DOI] [PubMed] [Google Scholar]
- 24.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938-1948. doi: 10.1056/NEJMra1001389 [DOI] [PubMed] [Google Scholar]
- 25.Ma Y, Tang L, Wang HX, Xu YC, Ma Y, Zhang FC. Capecitabine for the treatment for advanced gastric cancer: efficacy, safety and ethnicity. J Clin Pharm Ther. 2012;37(3):266-275. doi: 10.1111/j.1365-2710.2011.01289.x [DOI] [PubMed] [Google Scholar]
- 26.Haller DG, Cassidy J, Clarke SJ, et al. . Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol. 2008;26(13):2118-2123. doi: 10.1200/JCO.2007.15.2090 [DOI] [PubMed] [Google Scholar]
- 27.Burstein HJ, Curigliano G, Loibl S, et al. ; Members of the St. Gallen International Consensus Panel on the Primary Therapy of Early Breast Cancer 2019 . Estimating the benefits of therapy for early-stage breast cancer: the St Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol. 2019;30(10):1541-1557. doi: 10.1093/annonc/mdz235 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. Effect of Different Node Status on Outcomes
eTable. Disease-Free Survival Events in 434 Patients With TNBC
Data Sharing Statement