Abstract
Frailty is a geriatric syndrome defined as a status of extreme vulnerability to stressors, leading to a higher risk of negative health-related outcomes. “Inflammaging”, an age-related state of low-grade chronic inflammation, is characterized by an increased concentration of pro-inflammatory cytokines and acute phase proteins. Inflammaging has been postulated as an underlying mechanism of frailty, and several studies tested the relationship between frailty and concentration of inflammatory mediators. The aim of this systematic review and meta-analysis was to test whether inflammatory mediators are overproduced in frail older adults. Among the 758 articles identified in the literature search, 50 were included in the systematic review, and 39 in the three meta-analyses, i.e., C-reactive protein (CRP), interleukin 6 (IL6), and tumor necrosis factor α. To reduce heterogeneity, meta-analyses were restricted to studies identifying frailty by the Fried et al. [1] [J. Gerontol. A. Biol. Sci. Med. Sci. 56, M146–56] phenotypic criteria. Quantitative analyses measuring the association between frailty and biomarker concentrations showed significant differences when frail subjects were compared to non-frail and pre-frail subjects for CRP and IL6. This work established strong association between inflammatory biomarkers and frailty, confirming the role of age-related chronic inflammation in frailty development.
Electronic supplementary material
The online version of this article (10.1007/s11357-020-00247-4) contains supplementary material, which is available to authorized users.
Keywords: C-reactive protein, Frailty, Inflammaging, Interleukin 6, Tumor necrosis factor alpha, Older adults
Introduction
Populations around the world are rapidly aging because of declining fertility rates and increasing longevity, and this trend is well established from the most developed countries to the lowest income regions [2]. These critical sociodemographic changes require a realignment of health and social systems to better address the unmet needs of older people, independently of their socioeconomic background [3]. Predictions suggest that by the end of the century, people aged 65 years or older will be nearly triple in comparison to 2019 figures, and population aged 80 years or above will grow even faster, increasing nearly six-fold by 2100 [4]. In this context, the obsolete concept of “chronological age” is being replaced by the more accurate and person-tailored parameter of “biological age” to classify older people on the basis of their physiological state [5]. Thus, frailty represents a decline in an individual’s physiological state, constituting a transition phase between successful aging and disability [6].
Frailty is increasingly recognized as an important and common geriatric syndrome. It is defined as a status of extreme vulnerability to endogenous and exogenous stressors leading to a higher risk of negative health-related outcomes, including institutionalization, falls, hospitalization, and mortality [7]. These adverse outcomes constitute a source of considerable healthcare expenditure; therefore, the reduction of aging-related adverse health outcomes would lead to a restraint in medical costs [8]. Frailty is bidirectional and may be prevented, postponed, or even reverted with specific interventions and personalized health strategies [9]. Since frailty is considered a multidimensional syndrome, its causes are complex and generally based on the interplay of genetic, biological, physical, psychological, social, and environmental factors [10].
Due to this multicausal nature, currently there is no international consensus on the definition of frailty; several operational approaches exist for identifying this syndrome. Nowadays, there is a plethora of frailty measurements. A recent systematic review conducted by Dent et al. [11] reported 29 different frailty instruments and concluded that it is necessary to develop a unified criterion to establish a standard measurement for frailty. This would allow comparisons between epidemiological studies, as well as to standardize frailty identification in the clinical practice. The two most commonly used tools to identify frailty are the phenotypic description developed by Fried et al. [1] and the frailty index (FI) based on the cumulative model proposed by Rockwood, Mitnitski, and colleagues [12, 13]. The model proposed by Fried et al. [1] is based on the presence or absence of five specific phenotypic components related to physical fitness and metabolism, namely unintentional weight loss, weakness, exhaustion, low physical activity, and slow walking speed. The FI is based on the accumulation of functional deficits, extending beyond the physical definition of Fried’s model, to include cognitive decline, chronic diseases, environmental risk factors, psycho-social risk factors, geriatric syndromes (e.g., falls, delirium, and urinary incontinence), and even age-related disabilities [14]. The huge amount of modifications of Fried’s frailty phenotype (specifically 264), and in general the large heterogeneity in recognizing the presence of frailty, even when the same tool is used, has been reviewed by Theou et al. [15], who showed as modifying phenotypic criteria may change the estimation of the frailty prevalence, leading to potentially different classifications and results.
Aging-related alterations in the immune response, both humoral and cellular, that compromise the process of generating specific responses to foreign and self-antigens have been defined as “immunosenescence” [16]. This condition comprises a state of low-grade chronic and systemic inflammation in aging, in the absence of overt infection (“sterile” inflammation), called “inflammaging” [17]. Inflammaging is characterized by increases in serum concentrations of pro-inflammatory cytokines such as interleukin 6 (IL6), tumor necrosis factor alpha (TNFα), as well as acute phase proteins such as C-reactive protein (CRP), and decreases in interleukin 10 (IL10), which impair the maintenance of immunological homeostasis.
Inflammaging has been postulated to be an underlying mechanism of frailty, and several studies tested the possible relationship between frailty and altered concentrations of different inflammatory mediators. Indeed, a systematic review and meta-analysis conducted by Soysal et al. [18] reported that frailty and pre-frailty are associated with higher levels of CRP and IL6, but no significant effect was observed for TNFα. Nevertheless, this previous review did not consider that frailty identification criteria used in the original studies is a major source of variability. Moreover, since this meta-analysis was published, several new cross-sectional studies assessing the relationship of different inflammatory mediators with frailty status were published. Hence, in order to provide an updated comprehensive and more homogeneous picture of available data on this topic, a critical review of the new available data has been carried out, and meta-analyses focusing on those studies using Fried’s criteria to identify frail subjects were conducted. Aim of this new systematic review and meta-analysis was to test the hypothesis that inflammatory mediators are overproduced in frail older adults, a result that would confirm the role of age-related chronic inflammation in the development of frailty.
Methods
These systematic review and meta-analyses were conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [19].
Eligibility criteria for the systematic review
Eligible studies embodied in this systematic review were cross-sectional or longitudinal studies conducted in humans, focused on populations of older adults (aged 60 years or above), and written in English or Spanish. In all studies selected for the systematic review, participants had to be classified according to their frailty status following any currently validated scale for frailty identification. Studies evaluating other immunological biomarkers different from inflammatory mediators were excluded (such as vitamin D or standard complete blood count). Studies using frailty as a confounder, employing a non-validated frailty identification tool, or conference abstracts were also excluded from this review. If some papers were published by the same author/s and were carried out on the same population, only the most recent or most complete report was considered.
Search strategy
Studies were identified through an extensive bibliographic search using the PubMed database (National Library of Medicine, National institutes of Health, Bethesda, MD, USA; http://www.ncbi.nih.gov/PubMed), updated to June 2018. Two independent authors (DM-P and VV) conducted the search following a search strategy which comprised two terms that were intersected using the Boolean term “AND”. The search term included as first descriptor was related to frailty (“frail*”), and the second one included descriptors related to immune-inflammatory system and cytokines (“immun*”, “inflamm*”, and “cytokines”). The search filter “humans” was used to retrieve studies conducted only in human subjects. Initial screening was focused on title or abstract.
Data collection process
For each study complying with inclusion criteria, the following information was collected independently by two authors (DM-P and VV): country of origin for the first author, size of the study population and of the different frailty groups, gender distribution, mean age, criteria used to identify frailty, and outcomes (circulating concentrations of inflammatory mediators) in each frailty group.
Studies included in the meta-analysis
A meta-analysis was carried out when an adequate number of studies (8 minimum) analyzing a specific inflammatory biomarker was available, i.e., for CRP, IL6, and TNFα. Other inflammatory biomarkers, such as IL-10, ICAM-1, or MCP-1, were not included in the meta-analysis even though in the systematic review, they were found to be related to frailty status, since they did not reach this minimum required number. Studies included in the meta-analyses were those providing mean ± standard deviation (SD) for one or more of the three biomarkers for each frailty group. For those studies providing parameters different than means, e.g., medians and confidence intervals (CI) or inter-quartile ranges, or using biomarker concentrations as categorical variables, emails were sent to corresponding authors asking for the missing values at least twice. Studies for which authors provided the requested data were included in the meta-analyses. Besides, we decided to restrict each meta-analysis to only those studies employing Fried’s criteria (in original or modified version) to identify frail subjects, in order to reduce heterogeneity. Only cross-sectional data were considered for the meta-analyses; thus, in longitudinal prospective studies, only baseline values of the biomarker concentrations were included (follow-up values were dismissed).
Quality assessment
Quality assessment of studies included in the meta-analyses was carried out independently by DM-P and VV, while a third reviewer was available for mediation (MS-F). The quality score (QS) was calculated for each study to evaluate the standard of study design (Online Resource 1). Each item of the QS scored from 1 to a maximum of 3 points based on reported data and on the balance between frail subjects and controls for most common potential confounders. When a specific information was not available (i.e., age), 0 points were assigned. Seven items were included in the QS, namely (1) population size (1 point for less than 100 subjects, 2 points for 100–300 subjects, and 3 points for more than 300 subjects), (2) age-matching (1 point when the mean age difference was higher than 10 years, 2 points when the difference was 5–10 years, and 3 points when the difference was lower than 5 years, (3) gender-matching (1 point when the rate of one gender was either higher than 0.60 or lower than 0.40, 2 points when this rate was either 0.55–0.60 or 0.40–0.45, and 3 points when the rate was higher than 0.45 and lower than 0.55), (4) frailty balance (in studies with three frailty groups: 3 points when the rate of none of the groups was lower than 0.3, 2 points when the rate of none of the groups was lower than 0.25, and 1 point in the rest of cases; in studies with two frailty groups: rules the same than those applied to gender-matching), (5) frailty measured by geriatricians or specialized personnel (1 point in the absence of description, 2 points if the specialization of the personnel was not specified or they were not geriatricians or nurses, and 3 points if they were geriatricians or nurses), and (6) number of frailty groups (1 point when only non-frail and frail groups were reported, and 2 points when a pre-frail group was also included). The minimum and maximum of total QS possible was 5 and 17, respectively.
Statistical analysis
All analyses were performed using the Comprehensive R Archive Network (https://cran.r-project.org/). Data were expressed as mean value of the biomarker concentration (± SD). Heterogeneity across studies was assessed using the I2 and tested with the Cochran Q Chi-square statistics. Since most meta-analyses conducted showed statistically significant heterogeneity among the studies (I2 value of 50% or greater and P value < 0.05), the pooled standardized mean differences (SMD) with 95%CI were estimated using random-effects models with method according to Dersimonian and Laird [20]. Publication bias was assessed by visually inspecting funnel plots and using the Egger’s bias test. Whenever a significant result (P < 0.05) was found, the trim-and-fill method was used to adjust for any potential unpublished studies. The presence of confounders or effect modifiers was tested with a meta-regression analysis; the final model included year of publication, quality of the study (QS) as a continuous variable as covariates. A sensitivity analysis was performed removing one by one those studies with the most extreme values of SMD [21].
Results
Seven hundred and fifty-eight articles were initially identified in the literature search. After removal of duplicates, 588 articles were screened for potential eligibility (see flow chart in Fig. 1), and after excluding another 478 articles on the basis of title and abstract, 110 were selected for full-text assessment of eligibility. Thirty-one of those studies fitted with the selection criteria and were selected for analysis. Eighteen additional publications were identified from the references section of these manuscripts and included in the meta-analysis. At the end of this process, 49 studies published between 2002 and 2018 analyzing frailty and inflammation parameters fulfilled the inclusion/exclusion criteria and were included in this review. Among them, 35 studies (70%) associated frailty to CRP, 33 studies (66%) evaluated IL6, 13 studies (26%) reported data for TNFα, and 12 (24%) analyzed other different inflammation biomarkers, including IL10, soluble TNF receptors I (sTNF-RI) and II (sTNF-RII), intercellular adhesion molecule 1 (ICAM-1), monocyte chemoattractant protein-1 (MCP-1), and IL6 receptor (IL6-R).
Fig. 1.
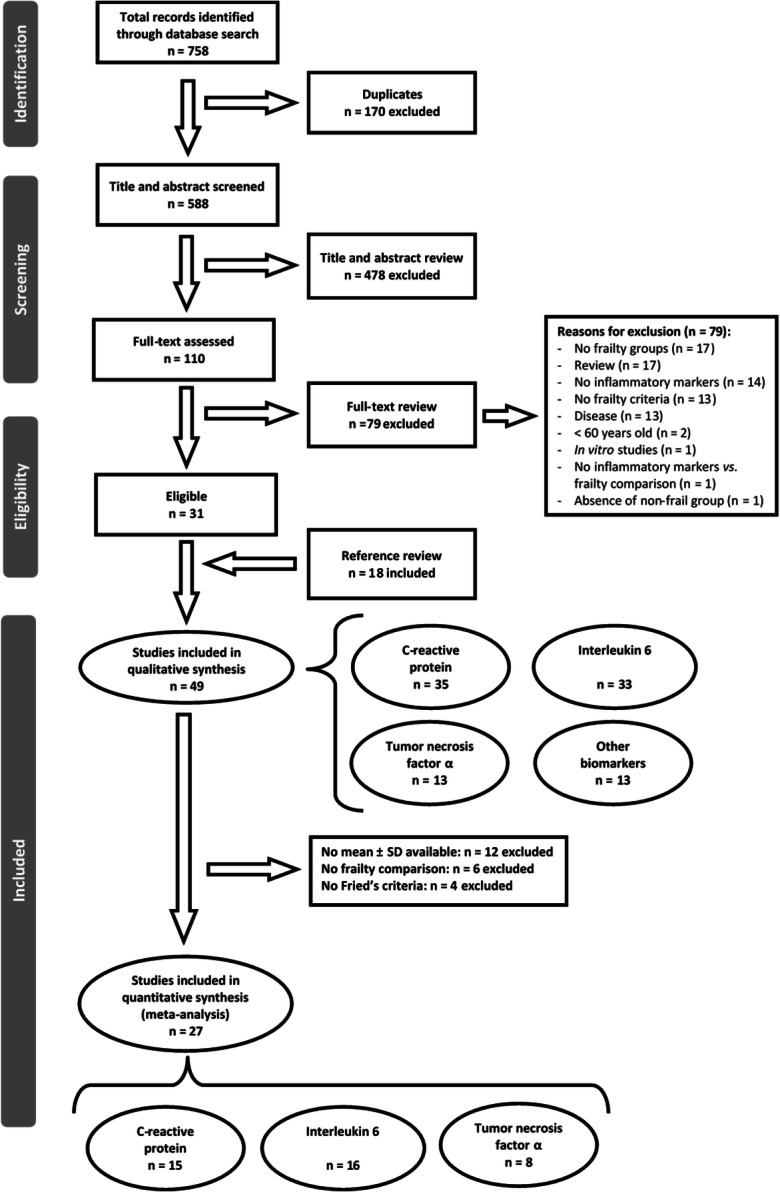
Flow chart: selection of the literature
Table 1 shows the characteristics of the studies included in the systematic review. None of the studies found written in Spanish fulfilled the inclusion criteria. Among the studies included, 48% evaluated more than one of the three main biomarkers (CRP, IL6, TNFα), and 71% of them evaluated CRP and IL6. According to the first author affiliation, 41% of the studies were conducted in the USA, 39% in Europe, 18% in Asia, and 2% in Canada, Australia, and Brazil. The number of individuals analyzed per study ranged from 32 participants [43] to 4735 [67]. The included articles encompassed a total sample of 40,828 individuals; 76% of studies included both men and women, 20% of studies examined only women (5306 individuals) and 4% examined only men (4164 individuals). Overall, 64% of studies considered three frailty groups (non-frail or robust, pre-frail, and frail), and 28% considered only two frailty groups (non-frail and frail), whereas 8% of the studies did not provide any division according to frailty status.
Table 1.
Main characteristics of studies included in the systematic review
| Study | First author country | Study design | Total population (mean age ± SD, years) | Case population (mean age ± SD, years) | Control population (mean age ± SD, years) | Frailty criteria | Outcome | Results |
|---|---|---|---|---|---|---|---|---|
| Aarts et al. [22] | The Netherlands | Cross-sectional | n = 4005 male/female: 1712/2293 |
n = 64 frail (80.0 ± 4.7) male/female: 33/31 |
n = 3941 non-frail (76.0 ± 5.3) male/female: 1679/2262 |
Fried’s phenotype | CRP | ↑ CRP with frailty |
| Almeida et al. [23] | Australia | Cross-sectional | n = 3778 |
n = 196 frail all male age: n.p. |
n = 3582 non-frail male age: n.p. |
FRAIL scale | hsCRP | ↑ hsCRP with frailty |
| Arts et al. [24] | The Netherlands | Cross-sectional | n = 366 |
n = 97 frail (74.0 ± 8.0) male/female: 29/68 |
n = 269 non-frail (69.4 ± 6.7) male/female: 95/174 |
Fried’s phenotype |
CRP IL6 NGAL |
No association of frailty with CRP and IL6 ↑ NGAL with frailty |
| Barzilay et al. [25] | USA | Cross-sectional | n = 2826 |
n = 234 frail (74.7 ± 5.8) male/female: 51/183 n = 1854 pre-frail (72.1 ± 5.1) male/female: 673/1181 |
n = 738 non-frail (70.4 ± 4.0) male/female: 341/397 |
Fried’s phenotype |
CRP IL6 |
↑ CRP and ↑ IL6 with frailty |
| Baylis et al. (2013) | UK | Longitudinal (10-year follow-up) | n = 254 |
n = 153 male (66.9 ± 2.2) n = 101 female (67.3 ± 2.1) |
n.p. | Fried’s phenotype |
IL6 IL1β IL10 CRP TNFα |
Baseline: No association of frailty with any biomarker Follow-up: no significant differences in IL6 and CRP with increased OR of frailty (unadjusted analyses) |
| Blaum et al. [26] | USA | Cross-sectional | n = 599 female (74.0 ± 2.7) |
n = 48 frail n = 258 pre-frail age: n.p. |
n = 293 non-frail age: n.p. |
Fried’s phenotype | CRP | ↑ CRP with frailty |
| Boxer et al. [27] | USA | Cross-sectional |
n = 60 n = 43 male (77 ± 9) n = 17 female (78 ± 12) |
n = 16 frail age: n.p n = 27 pre-frail age: n.p |
n = 17 non-frail age: n.p |
Fried’s phenotype (modified) |
IL6 hsCRP |
↑ hsCRP and ↑ IL6 with frailty |
| Carcaillon et al. [28] | Spain | Cross-sectional | n = 702 female (74.5 ± 5.6) |
n = 69 frail age: n.p. n = 290 pre-frail age: n.p. |
n = 343 non-frail age: n.p. |
Fried’s phenotype | hsCRP |
↑ hsCRP with frailty No differences between frail, pre-frail and non-frail |
| Collerton et al. [29] | UK | Cross-sectional | n = 532 mean 60.1 years |
n = 119 frail male/female: 27/92 n = 333 pre-frail male/female: 140/193 |
n = 100 non-frail male/female: 53/47 |
Fried’s phenotype FI |
TNFα CRP IL6 |
↑ TNFα, ↑ CRP and ↑ IL6 with frailty (both Fried’s phenotype and FI) |
| Darvin et al. [30] | USA | Cross-sectional | n = 65 |
n = 12 frail (85.2 ± 3.8) male/female: 8/4 n = 31 pre-frail (81.8 ± 6.7) male/female: 12/19 |
n = 22 non-frail (76.5 ± 4.7) male/female: 6/16 | Fried’s phenotype | IL6 | ↑ IL6 with frailty |
| Fernández-Garrido et al. [31] | Spain | Cross-sectional | n = 42 female (84.2 ± 6.5) | n.p | n.p | Fried’s phenotype | CRP | ↑ CRP with frailty |
| Fried et al. [32] | USA | Cross-sectional | n = 704 female range 70–79 years |
n = 90 frail age: n.p. n = 330 pre-frail age: n.p. |
n = 284 non-frail age: n.p. |
Fried’s phenotype | IL6 | ↑ IL6 with frailty |
| Gale et al. [33] | UK | Longitudinal (4-year follow-up) | n = 2146 |
Frail: n = 80 male (76.2 ± 1.17) n = 150 female (76.7 ± 0.87) |
Non-frail: n = 908 male (69.2 ± 0.24) n = 1008 female (69.7 ± 0.28) |
Fried’s phenotype (modified) | CRP |
Baseline: ↑ CRP with frailty in male (not significant) and in female (significant). Follow-up: For an SD increase in CRP, the fully-adjusted OR (95%CI) for incident frailty in women was 1.27 (0.96–1.69). In men no significant association was observed with risk of incident frailty |
| Gale et al. [34] | UK | Longitudinal (6-year follow-up) | n = 594 |
n = 85 frail (69.9 ± 0.77) male/female: 41/44 n = 279 pre-frail (69.5 ± 0.81) male/female: 140/139 |
n = 230 non-frail (69.4 ± 0.83) male/female: 122/108 |
Fried’s phenotype (modified) | CRP |
Baseline: ↑ CRP with frailty. Follow-up: no measurement of CRP, only cognitive function |
| Hubbard et al. [35, 36] | UK | Cross-sectional | n = 110 |
n = 30 frail Continuing care (84.9 ± 6.2) male/female: 12/18 n = 40 pre-frail Day hospital (84.2 ± 4.9) male/female: 16/24 |
n = 40 non-frail Independent old (82.7 ± 5.5) male/female: 16/24 |
Fried’s phenotype |
CRP IL6 TNFα |
↑ CRP, ↑ IL6, and ↑ TNFα with increasing frailty (pre-frail and frail) |
| Hubbard et al. [37] | UK | Cross-sectional | n = 110 |
Fried’s phenotype: n = 63 frail (84.8 ± 5.6) male/female: 22/41 n = 25 pre-frail (85.2 ± 4.9) male/female: 11/14 |
Fried’s phenotype: n = 22 non-frail (79.7 ± 3.7) male/female: 12/10 |
Fried’s phenotype FI |
TNFα CRP IL6 |
↑ CRP, ↑ IL6, and ↑ TNFα with Fried’s phenotypic frailty. FI was significantly correlated with ↑ CRP, ↑ IL6, ↑ TNFα |
| Hwang et al. [38] | Taiwan | Cross-sectional | n = 1839 |
n = 125 frail (74.6 ± 9.2 male/female: 60/65 n = 744 pre-frail (66.3 ± 9.3) male/female: 369/375 |
n = 970 non-frail (60.7 ± 7.5) male/female: 444/526 | Fried’s phenotype | hsCRP | ↑ hsCRP with frailty |
| Kalyani et al. [39] | USA | Longitudinal (14-year follow-up) | n = 73 female |
n = 9 frail (86 ± 3.0) n = 47 pre-frail (87 ± 3.5) |
n = 17 non-frail (86 ± 5.0) | Fried’s phenotype | IL6 |
Baseline: No significant differences in IL6between frailty groups. Follow-up: No differences were seen in adjusted analyses for levels of IL6 |
| Lai et al. [40] | Taiwan | Cross-sectional | n = 386 male |
n = 128 frail (82.2 ± 5.2) n = 228 pre-frail (81.5 ± 4.7) |
n = 30 non-frail (78.7 ± 3.9) | Fried’s phenotype |
IL6 hsCRP TNFα |
↑ IL6 with frailty ↑ hsCRP and ↑ TNFα were not correlated with frailty |
| Langmann et al. [41] | USA | Cross-sectional | n = 178 female (86.5 ± 5.0) |
n = 114 frail (86.3 ± 4.7) n = 53 pre-frail (84.9 ± 4.9) |
n = 11 non-frail (81.6 ± 7.2) | Fried’s phenotype (modified) |
IL6 IL6-R hsCRP TNFα sTNF-RI sTNF-RII IL10 |
↑ hsCRP, ↑ IL6, ↑IL6-R, ↑ sTNF-RI and ↑sTNF-RII levels in frail vs. non-frail participants. ↑ hsCRP, ↑ IL6, ↑ sTNF-RI and ↑ sTNF-RII levels in pre-frail vs. non-frail participants. ↑ hsCRP levels in pre-frail vs. frail subjects. No association of frailty with IL10 |
| Lee et al. [42] | Taiwan | Cross-sectional | n = 946 |
n = 85 frail (74.4 ± 7.9) male/female: 40/45 n = 531 pre-frail (65.5 ± 9.4) male/female: 215/316 |
n = 330 non-frail (63.2 ± 8.2) male/female: 171/159 |
Fried’s phenotype (modified) |
IL6 ICAM-1 |
↑ IL6 in frail subjects but not in pre-frail subjects. ↑ICAM-1 progressively with frailty |
| Leng et al. [43] | USA | Cross-sectional | n = 30 | n = 11 frail (84.9 ± 6.7) male/female: 2/9 | n = 19 non-frail (81.3 ± 4.1) male/female: 5/14 | Fried’s phenotype | IL6 | ↑ IL6 with frailty |
| Leng et al. [44] | USA | Cross-sectional | n = 51 | n = 18 frail (84.9 ± 6.7) male/female: 5/13 |
n = 33 non-frail (81.3 ± 4.6) male/female: 7/26 |
Fried’s phenotype |
IL6 IFG-I |
↑ IL6 not significantly associated with frailty. ↓ IFG-I with frailty |
| Leng et al. [45] | USA | Cross-sectional | n = 22 | n = 11 frail (85.3 ± 6.6) male/female: 3/8 | n = 11 non-frail (85.4 ± 4.8) male/female: 3/8 | Fried’s phenotype |
IL6 IL10 TNFα |
↑ IL6 with frailty. No association of frailty with TNFα and IL10 |
| Leng et al. [46] | USA | Cross-sectional | n = 558 female |
n = 151 frail (80.4 ± 7.6) n = 327 pre-frail (77.0 ± 7.6) |
n = 80 non-frail (72.9 ± 6.1) | Fried’s phenotype | IL6 | ↑ IL6 with frailty |
| Leng et al. [47] | USA | Cross-sectional | n = 558 female |
n = 168 frail (80.0 ± 7.8) n = 314 pre-frail (77.0 ± 7.6) |
n = 76 non-frail (72.8 ± 6.0) | Fried’s phenotype |
IL6 CRP |
↑ IL6 and ↑ CRP with frailty |
| Leng et al. [48] | USA | Cross-sectional | n = 133 |
n = 50 frail (84.4 ± 4.1) male/female: 8/42 n = 32 pre-frail (85.4 ± 4.1) male/female: 10/22 |
n = 51 non-frail (82.7 ± 5.2) male/female: 8/43 |
Fried’s phenotype | IL6 | ↑ IL6 with frailty |
| Lin et al. [49] | Taiwan | Cross-sectional | n = 522 | n = 134 frail (77.3 ± 7.1) | n = 388 non-frail (72.4 ± 5.0) | Fried’s phenotype | hsCRP | ↑ hsCRP with frailty |
| Liu et al. [50] | USA | Cross-sectional | n = 1919 male/female: 884/1035 |
n = 142 frail (77 ± 6) male/female: 65/77 n = 864 pre-frail (72 ± 7) male/female: 369/495 |
n = 913 non-frail (69 ± 6) male/female: 450/463 | Fried’s phenotype |
CRP IL6 ICAM-1 MCP-1 |
↑ CRP, ↑ IL6, ↑ ICAM-1and ↑ MCP-1 with frailty and pre-frailty |
| Marcos-Pérez et al. [51] | Spain | Cross-sectional | n = 259 male/female: 85/174 |
n = 88 frail (85.8 ± 7.9) male/female: 27/13 n = 131 pre-frail (77.05 ± 7.7) male/female: 36/95 |
n = 40 non-frail (73.2 ± 5.5) male/female: 22/66 |
Fried’s phenotype |
IL6 CRP TNFα sTNF-RII |
Progressive increase with frailty severity in all parameters, especially notable for IL6 and sTNF-RII |
| Namioka et al. [52] | Japan | Cross-sectional | n = 140 |
n = 34 frail (82.3 ± 6.1) male/female: 11/23 n = 62 pre-frail (80.5 ± 4.9) male/female: 22/40 |
n = 44 non-frail (78.2 ± 6.0) male/female: 25/19 | Fried’s phenotype (modified) |
IL6 TNFα |
↑ IL6 with frailty No significant↑ TNFα with frailty |
| Pabst et al. [53] | Germany | Cross-sectional |
n = 940 (75.6 ± 6.5 years) male/female: 478/762 |
n = 38 frail n = 351 pre-frail |
n = 551 non-frail | Fried’s phenotype |
IL6 CRP |
n.p. |
| Piggott et al. [54] | USA | Cross-sectional | n = 1326 |
n = 162 frail (50.3 (44.7–53.7)) male/female: 94/68 n = 826 pre-frail (47.5 (42.5–52.3)) male/female: 540/285 |
n = 338 non-frail (47.3 (41.3–52.3)) male/female: 234/104 | Fried’s phenotype | IL6 | ↑IL6 with frailty |
| Puts et al. [55] | The Netherlands | Cross-sectional | n = 1271 | n = 242 frail (79.2 ± 6.2) male/female: 91/151 | n = 1029 non-frail (74.5 ± 6.3) male/female: 531/498 | Nine frailty indicators |
CRP IL6 |
No significant differences in IL6 and CRP with frailty |
| Longitudinal (3-year follow-up) | n = 885 | n = 125 non-frail (78.2 ± 6.2) male/female: 56/69 | n = 760 non-frail (73.4 ± 5.9) male/female: 382/378 |
↑ CRP associated with incident frailty. No significant prospective association of IL6 with incident frailty |
||||
| Qu [56] | USA | Cross-sectional | n = 32 |
n = 16 frail (83 ± 5) male/female: 2/14 |
n = 16 non-frail (83 ± 5) male/female: 2/14 |
Fried’s phenotype |
IL6 TNFα IL1β CXCL-10 |
↑ IL6 and ↑CXCL-10 with frailty. No significant differences in TNFα and IL1β |
| Reiner et al. [57] | USA | Longitudinal (3-year follow-up) | n = 1800 female |
n = 900 frail age: n.p. |
n = 900 non-frail age: n.p. |
Fried’s phenotype (modified) |
CRP IL6 |
Baseline: ↑ IL6 with frailty (model minimally adjusted for hypertension, hormone use, BMI). No significant differences in IL6 (model fully adjusted for hypertension, hormone use, BMI, as well as education, alcohol consumption, arthritis, smoking). No significant differences in CRP (any model). Follow-up: little evidence for association between CRP or IL6 levels and development of frailty |
| Ronning et al. [58] | Norway | Cross-sectional |
n = 137 mean 80.0 years, range 70–94 male/female: 62/75 |
n = 16 frail age: n.p. n = 59 pre-frail age: n.p. |
n = 62 non-frail age: n.p. |
Fried’s phenotype (modified) |
CRP IL6 TNFα |
↑ IL6, ↑ CRP, and ↑ TNFα with frailty. ↑ CRP and ↑ IL6 in frail group vs. pre-frail group |
| Sanchis et al. [59] | Spain | Cross-sectional | n = 342 male/female: 194/138 |
n = 116 frail (81 ± 7) male/female: 47/69 |
n = 226 non-frail (77 ± 7) male/female: 77/149 |
Fried’s phenotype | CRP | No significant differences in CRP between non-frail and frail |
| Saum et al. [60] | USA | Cross-sectional | n = 2518 |
n = 210 frail (73.7 ± 6.0) male/female: 74/136 n = 1463 pre-frail (70.3 ± 6.2) male/female: 643/820 |
n = 845 non-frail (67.8 ± 5.8) male/female: 478/367 |
Fried’s phenotype | CRP | ↑ CRP in the frail group |
| Schmaltz et al. [61] | Canada | Cross-sectional | n = 92 female (73.5 ± 0.3) |
n = 5 frail age: n.p. n = 36 pre-frail age: n.p. |
n = 51 non-frail age: n.p. |
Fried’s phenotype | IL6 | ↑ IL6 with frailty |
| Schoufour et al. [62] | The Netherlands | Cross-sectional |
n = 757 (61.7 ± 8.0) male/female: 396/363 |
n.p. | n.p. | FI (51 items) |
CRP IL6 |
↑ CRP and ↑ IL6 with frailty |
| Serviddio et al. [63] | Italy | Cross-sectional |
n = 62 (76.7 ± 5.1) male/female: 39/23 |
n = 43 frail age: n.p. |
n = 19 non-frail age: n.p. |
Fried’s phenotype | TNFα | ↑ TNFα with frailty |
| Silva et al. [64] | Brazil | Cross-sectional | n = 255 male/female: 82/173 | n.p. | n.p. | Fried’s phenotype |
hsCRP IL6 IL1-RA |
↑ hsCRP with frailty. No significant association of IL6 and IL1-RA with frailty |
| Tsai et al. [65] | Taiwan | Cross-sectional | n = 168 |
n = 34 frail (79.06 ± 4.86) male/female: 14/20 n = 92 pre-frail (77.04 ± 5.97) male/female: 47/45 |
n = 42 non-frail (74.69 ± 6.68) male/female: 22/20 | Fried’s phenotype (modified) |
TNFα CRP |
No differences between TNFα, CRP and frailty |
| van Epps et al. [66] | USA | Cross-sectional | n = 117 male/female: 112/5 |
n = 44 frail (median 82, range 62–92 years) n = 50 pre-frail (median 80, range 62–92 years) |
n = 23 non-frail (median 68, range 62–90 years) | Fried’s phenotype |
IL6 IL18 sTNF-RI sTNF-RII CRP IL10 sCD14 Serum amyloid A |
↑ IL6 and ↑ sTNF-RII in frail and pre-frail subjects vs. non-frail subjects. ↑ sTNF-RI and ↑ serum amyloid A in frail vs. non-frail subjects. No significant differences in CRP, IL10, IL18 and sCD14 levels with frailty |
| Walston [67] | USA | Cross-sectional | n = 4735 male/female: 2025/2710 |
n = 299 frail (73.4 ± 6.4) n = 2147 pre-frail (73.4 ± 5.9) |
n = 2289 non-frail (71.5 ± 4.6) | Fried phenotype | CRP | ↑ CRP with frailty |
| Wu et al. [68] | Taiwan | Cross-sectional | n = 90 male/female: 46/44 |
n = 21 frail (79.9 ± 5.8) n = 56 pre-frail (76.8 ± 5.8) |
n = 13 non-frail (73.1 ± 5.3) | Fried’s phenotype | hsCRP | ↑ hsCRP with frailty |
| Yang et al. [69] | China | Cross-sectional | n = 181 |
n = 58 frail (85.9 ± 6.2) male/female: 44/14 |
n = 123 non-frail (78.1 ± 9.4) male/female: 87/36 |
FRAIL scale |
IL6 CRP |
Non-adjusted analysis: ↑ IL6 with frailty. Adjusted analysis (age, gender, smoking, alcohol use, HB, ALB, WBC, NEU and CR): no differences in IL6 and CRP with frailty |
| Zhu et al. [70] | China | Cross-sectional |
n = 1478 (75.3 ± 3.9) male/female: 695/783 |
n = 177 frail n = 634 pre-frail |
n = 667 non-frail | Fried’s phenotype | hsCRP | ↑ hsCRP with frailty |
Statistically significant P values are indicated in italics. Studies in italics letter were included in meta-analysis; ↑ indicates significant increase (although indicated otherwise); ↓ indicates significant decrease (although indicated otherwise)
CD cluster of differentiation, CR creatinine, CRP C-reactive protein, CXCL-10 CXC chemokine ligand 10, FI frailty index, FRAIL scale fatigue, resistance, ambulation, illnesses, and loss of weight scale, hsCRP high sensitivity C-reactive protein, ICAM-1 intercellular adhesive molecule-1, IFG-1 insulin-like growth factor-1, IL interleukin, IL1-RA interleukin 1 receptor antagonist, IL6-R interleukin 6 receptor, MCP-1 monocyte chemotactic protein 1, NGAL neutrophil gelatinase-associated lipocalin, n.p. not provided, OR odds ratio, sTNF-R soluble receptor of tumor necrosis factor alpha, TNFα tumor necrosis factor alpha
As regards the frailty identification, in almost all studies (92%) Fried’s frailty criteria were employed; among these, nine studies (20%) used at least one modified phenotypic criterion. The frailty index developed by Rockwood and Mitnitski was used in 6% of the studies [29, 37, 62]. The remaining studies employed a set of different tools such as FRAIL scale [23, 69] and the Nine frailty indicators [55].
The minimum and maximum QS obtained in the studies included in the meta-analyses were 8 and 13, respectively (Online Resource 1). More than half of the studies included more than 300 participants and the same rate did not match for gender (male/female ratio either higher than 0.60 or lower than 0.40). Almost half of the studies matched for age (difference between groups lower than 5 years), in only two studies frailty groups were balanced (both including only two frailty groups), nearly 80% of the studies did not specify whether frailty was measured by geriatricians or specialized personnel, and nearly 70% of the studies included a group classified as pre-frail.
C-reactive protein
Higher CRP concentrations were significantly related to frailty status in 77% of studies included in this review (Table 1). One study found a significant association of frailty status with CRP in women, but this relationship was not significant in men [33]. Moreover, three studies found significant differences in CRP levels between pre-frail and frail subjects, in addition to the differences between non-frail and frail participants [41, 50, 58]. Among studies reporting significant association, Fried’s original and modified frailty criteria were used, except for four studies (15%) in which only FI [62], FI in combination with Fried’s phenotype [29, 37], or FRAIL scale [23] were employed to identify frailty. On the other hand, 26% of studies which did not find significant associations between frailty and CRP concentration employed different tools to identify frailty, namely, Puts et al. [55] used the Nine frailty indicator method, Yang et al. [69] used the FRAIL scale, and Tsai et al. [65] employed a combination of Fried’s phenotype and Frailty score.
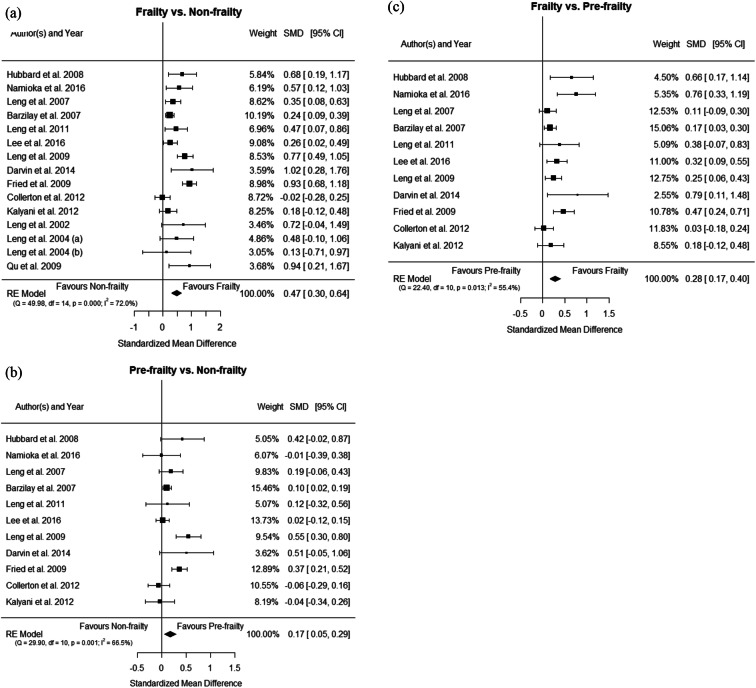
Meta-analysis comparing frailty group vs. non-frailty group included 15 studies. The forest plot is shown in Online Resource 2a. Significant heterogeneity was found among studies (I2 = 99.14%, P < 0.001). Results of the meta-regression showed that difference of CRP concentration between the frail and non-frail individuals was statistically significant (SMD = 0.99, 95%CI = 0.43–1.56, P = 0.0006). The Egger’s regression test revealed the presence of a publication bias (z = 3.0624, P = 0.0022), and therefore, the SMD was re-estimated after adjusting with the trim-and-fill method (SMD = 0.66, 95%CI = 0.45–0.88, P < 0.0001).
Twelve studies were considered for the meta-analysis of pre-frailty group vs. non-frailty group (Online Resource 2b). Significant heterogeneity was observed among studies (I2 = 48.70%, P < 0.001). Results showed that CRP levels were significantly higher in pre-frailty when compared to non-frailty groups (SMD = 0.14, 95%CI = 0.09–0.19, P < 0.0001), and no significant publication bias was observed (z = 1.7730, P = 0.08).
Another group of 12 studies compared frail vs. pre-frail subjects (Online Resource 2c). Heterogeneity was significant (I2 = 99.28%, P < 0.0001). Results showed that difference of CRP concentrations between frail and pre-frail subjects was statistically significant (SMD = 1.12, 95%CI = 0.46–1.78, P < 0.0001). The Egger’s regression test revealed the presence of publication bias (z = 4.6042, P < 0.0001), and the trim-and-fill method confirmed the significant increase in frailty groups (SMD = 1.50, 95%CI = 0.76–2.25, P < 0.0001).
In view of the high heterogeneity among studies, especially in the comparisons of the frailty group vs. non-frailty and pre-frailty groups, we performed sensitivity analyses excluding the studies of Hwang et al. [38] and Marcos-Pérez et al. [51] which showed the most extreme results, to investigate how much of the overall effect was attributable to them (see forest plots in Fig. 2). The heterogeneity was indeed reduced, and significance was removed for the comparison between pre-frailty vs. non-frailty groups (Table 2). Furthermore, SMD estimations were still significant although their values were slightly lower. Egger’s test for publication bias was not significant in any case (see funnel plots in Online Resource 3).
Fig. 2.
Forest plots of CRP concentration (sensitivity analyses excluding Hwang et al. [38] and Marcos-Pérez et al. [51]): a frailty vs. non-frailty groups; b pre-frailty vs. non-frailty groups; c frailty vs. pre-frailty groups. In order to respect original data provided by Walston et al. (2002), two populations from this study were included in these meta-analyses, which differ in the inclusion (a) or exclusion (b) of participants with cardiovascular diseases history and diabetes
Table 2.
Summary of meta-analyses comparing frailty groups with assessment of publication bias [sensitivity analyses excluding Hwang et al. [38] and Marcos-Pérez et al. [51] for CRP, and excluding Marcos-Pérez et al. [51] for IL6 and TNFα]
| Biomarker and comparison | No. of studies | Heterogeneity | Meta-analysis | Publication bias (Egger’s test) | Trim-and-fill | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I2 | P value | SMD | 95%CI | P value | Z | P value | SMD | 95%CI | P value | ||
| CRP | |||||||||||
| Frailty vs. non-frailty | 13 | 72.81 | < 0.001 | 0.31 | 0.20–0.42 | < 0.0001 | 0.4480 | 0.6542 | |||
| Pre-frailty vs. non-frailty | 10 | 20.78 | 0.2518 | 0.15 | 0.11–0.19 | < 0.0001 | 1.1440 | 0.2524 | |||
| Frailty vs. pre-frailty | 10 | 64.23 | 0.0028 | 0.14 | 0.04–0.24 | 0.0046 | 0.9365 | 0.3490 | |||
| IL6 | |||||||||||
| Frailty vs. non-frailty | 15 | 71.99 | < 0.0001 | 0.47 | 0.30–0.64 | < 0.0001 | 0.1334 | 0.1821 | |||
| Pre-frailty vs. non-frailty | 11 | 66.55 | 0.0009 | 0.17 | 0.05–0.29 | 0.0048 | 0.7731 | 0.4395 | |||
| Frailty vs. pre-frailty | 11 | 55.36 | 0.0132 | 0.28 | 0.17–0.40 | < 0.0001 | 3.1343 | 0.0017 | 0.22 | 0.09–0.35 | 0.0007 |
| TNFα | |||||||||||
| Frailty vs. non-frailty | 7 | 82.76 | < 0.0001 | 0.32 | − 0.11–0.76 | 0.15 | 0.8083 | 0.4189 | |||
| Pre-frailty vs. non-frailty | 5 | 59.20 | 0.0439 | − 0.017 | − 0.27–0.23 | 0.90 | 2.8688 | 0.041 | − 0.24 | − 0.51–0.02 | 0.07 |
| Frailty vs. pre-frailty | 5 | 72.97 | 0.0051 | 0.24 | − 0.09–0.56 | 0.15 | 0.9306 | 0.3520 | |||
Statistically significant P values for the meta-analysis are indicated in italics
CI confidence interval, CRP C-reactive protein, IL6 interleukin 6, SMD standardized mean difference, TNFα tumor necrosis factor alpha
Meta-regression analyses of CRP did not show the presence of any significant effect modification or confounding.
Interleukin 6
Seventy-nine percent of studies encompassed in this literature search which analyzed IL6 found significant associations between increase in its concentration and frailty status (Table 1). Among them, five studies used frailty identification tools different from Fried’s criteria, namely Schoufour et al. [62] employed FI (51 items), Collerton et al. [29] and Hubbard et al. [37] used both FI and Fried’s phenotype, and Puts et al. [55] and Yang et al. [69] employed FRAIL scale and the Nine frailty indicators to classify their populations, respectively. Significant differences in IL6 levels between frail and pre-frail subjects were also reported in three studies [41, 50, 58, 66].
On the contrary, seven out of 33 studies (21%) did not find significant differences in the levels of this biomarker between frailty groups. Five of these studies employed Fried’s phenotype [24, 39, 44, 57, 71], and the others used the Nine frailty indicators [55] and FRAIL scale [69].
The meta-analysis comparing frail vs non-frail individuals included 16 studies; Online Resource 4a displays the forest plot of this comparison. Significant heterogeneity among the studies was found (I2 = 88.7%, P < 0.0001), and a statistically significant difference of the IL6 concentration between the frailty group and the non-frailty group was revealed by the meta-regression (SMD = 0.63, 95%CI = 0.38–0.89, P < 0.0001). The test for publication bias was not significant (Egger’s regression z = 1.5077, P = 0.1316).
Meta-analysis comparing pre-frailty group vs. non-frailty group analyzed 12 studies (Online Resource 4b) and was affected by a high degree of heterogeneity (I2 = 94.30%, P < 0.0001). Results showed that difference of IL6 concentrations between pre-frail and non-frail participants was statistically significant (SMD = 0.43, 95%CI = 0.16–0.70, P = 0.0017). The presence of publication bias (z = 3.4778, P = 0.0005) required effect estimates adjusted with the trim-and-fill method (SMD = 0.81, 95%CI = 0.42–1.21, P < 0.0001).
And 12 studies were included in the meta-analysis of frailty group vs. pre-frailty group (Online Resource 4c). A significantly higher concentration of IL6 was found in frail older adults when compared to pre-frail (SMD = 0.60, 95%CI = 0.26–0.93, P < 0.0001), although affected by heterogeneity among studies (I2 = 95.10%, P = 0.0005). Egger’s test for publication bias was significant (z = 2.3613, P = 0.0182), and a trim-and-fill adjusted estimate of SMD was produced (SMD = 0.95, 95%CI = 0.54–1.37, P < 0.0001).
In an attempt to reduce heterogeneity, sensitivity analyses were conducted excluding the study of Marcos-Pérez et al. [51] which showed the most extreme results (see forest plots in Fig. 3). In the three comparisons carried out for IL6 heterogeneity shifted from high to moderate (lower than 72% in all cases; Table 2). The new SMDs estimates were mildly reduced but still significant, while the effect of the publication bias test was removed in the comparison of pre-frailty vs. non-frailty groups (Table 2). Publication bias was only present in the scenario frailty vs. pre-frailty, where new SMD estimates slightly changed after adjusting with the trim-and-fill method (see funnel plots in Online Resource 5).
Fig. 3.
Forest plots of IL6 concentration (sensitivity analyses excluding Marcos-Pérez et al. [51]): a frailty vs. non-frailty groups; b pre-frailty vs. non-frailty groups; c frailty vs. pre-frailty groups
No presence of significant effect modification or confounding was observed in the meta-regression analyses of IL6.
Tumor necrosis factor alpha
Frailty was significantly related with increasing TNFα concentrations in 46% of the studies, two of which identified frailty through a combination of FI and Fried’s criteria [29, 37] (Table 1). The remaining four studies employed the original or modified versions of Fried’s criteria. One of them found also significant differences in TNFα concentration in pre-frail subjects when compared with the frail group [35]. On the contrary, seven studies failed to observe a significant association between frailty status and TNFα levels [45, 52, 56, 65, 72]; among them, Tsai et al. [65] used Fried’s frailty phenotype and frailty score to differentiate frailty groups.
Meta-analysis of frailty group vs. non-frailty group included eight studies, as shown in the forest plot (Online Resource 6a). Results were affected by heterogeneity among studies (I2 = 92.18%, P < 0.001). Difference of TNFα concentration between frailty and non-frailty groups resulted statistically significant in the original analysis (SMD = 0.70, 95%CI = 0.06–1.34, P = 0.03), and after adjusting with the trim-and-fill method (SMD = 1.04, 95%CI = 0.26–1.82, P = 0.009), to take into consideration the publication bias (z = 2.7656, P = 0.005).
Six studies were analyzed in the meta-analysis comparing pre-frailty group vs. non-frailty group (forest plot in Online Resource 6b). A significant heterogeneity was observed among studies (I2 = 89.79%, P < 0.0001). Results showed no significant difference of TNFα concentrations between pre-frail and non-frail individuals (SMD = 0.31, 95%CI = -0.18–0.80, P = 0.22). After taking into account the publication bias (z = 4.4001, P < 0.0001), SMD trim-and-fill adjusted estimates approached significance (SMD = 0.61, 95%CI = − 0.22–1.25, P = 0.06).
Finally, meta-analysis comparing frailty and pre-frailty groups included six studies (Online Resource 6c). Results showed that difference of TNFα levels between frail and pre-frail subjects was statistically significant (SMD = 0.64, 95%CI = 0.11–1.18, P = 0.02), despite the presence of significant heterogeneity (I2 = 90.66%, P < 0.0001). The Egger’s regression test revealed the presence of a publication bias (z = 2.3897, P = 0.0169), and the estimates of SMD were only slightly changed after adjusting with the trim-and-fill method (SMD = 0.80, 95%CI = 0.22–1.38, P = 0.007).
A considerable reduction in heterogeneity was observed after removing the outlier study of Marcos-Pérez et al. [51] (Fig. 4). SMD estimations were not significant in any of the three comparisons. Publication bias was only observed in the comparison between pre-frailty vs. non-frailty groups (see Online Resource 7). In this latter case, adjustment with the trim-and-fill method did not change notably the estimate of the SMD.
Fig. 4.
Forest plots of TNFα concentration (sensitivity analyses excluding Marcos-Pérez et al. [51]): a frailty vs. non-frailty groups; b pre-frailty vs. non-frailty groups; c frailty vs. pre-frailty groups
Meta-regression analyses of TNFα did not show any significant effect modification or confounding.
Other immunological biomarkers
In addition to the three immunological markers included in the meta-analyses, 11 papers analyzed other immunological parameters in association with frailty, mostly interleukin subclasses (Table 1). IL10 was evaluated in four studies, showing negative results in all of them [41, 45, 66, 71]. Soluble TNF receptors I (sTNF-RI) and II (sTNF-RII) were measured in three studies, all of them reporting general increases of both parameters in pre-frail and frail subjects vs. non-frail individuals [41, 51, 66]. Two studies evaluated IL1β [56, 71], and two others intercellular adhesion molecule 1 (ICAM-1) [42, 50], all of them showing association with frailty status. Other immune parameters were reported in only one study, such as IL6 receptor (IL6-R) (increase in frail vs. non-frail group) [41] and monocyte chemoattractant protein-1 (MCP-1) (increase in pre-frail and frail groups) [50].
Discussion
Results of the meta-analyses conducted clearly show the presence of significant association between CRP and IL6 concentration and frailty in older adults. To take into account those studies reporting a much stronger association than the others (Hwang et al. [38] and Marcos-Pérez et al. [51] for CRP; and Marcos-Pérez et al. [51] for IL6 and TNFα) and in the attempt to reduce heterogeneity, we applied a conservative approach excluding those studies. As a result of this approach, heterogeneity was notably reduced in the sensitivity analyses, and the distribution of studies in the funnel plots, confirmed by the Egger’s test, indicated absence of publication bias (except in the case of frailty vs. pre-frailty comparison for IL6). The difference between frailty groups in comparison with non-frail individuals was still highly significant, confirming without doubt the link between frailty status and CRP and IL6.
The globally accepted theory of “inflammaging” [73] reports an age-related increased level of some inflammatory markers such as IL6 [74, 75], TNFα [76, 77] and its soluble receptors [76, 78], acute phase protein CRP [74, 75], certain lymphocyte subpopulations [79, 80], and decreased concentration of IL10 [81]. Inflammaging is associated with increased morbidity and mortality in older adults [82, 83].
CRP, a member of “pentraxin” family of proteins, was the first acute-phase protein to be described. It is produced in the liver only by hepatocytes mainly in response to increased levels of IL6 [84]. Its main function is to recognize pathogens and host’s damaged cells in order to mediate their elimination by recruiting the complement system and phagocytic cells; thus, it is a very useful non-specific biochemical marker of inflammation [85]. CRP concentration increases during aging [86] and this situation may become chronic, taking part in the pathogenesis of several age-related diseases such as cardiovascular diseases [87] and type 2 diabetes [88], among others. A previous meta-analysis [18] described the presence of significant association between CRP concentration and frailty severity, for both frail and pre-frail participants. In agreement with these results, the current study showed that CRP levels progressively increase with frailty severity, classified according to Fried’s criteria (even in the conservative sensitivity analyses), shedding more conscious light on previous results, which included studies using different frailty identification tools [18].
IL6 is a pleiotropic inflammatory cytokine which is produced by a wide variety of cells. It plays an important role in the acute inflammatory response cascade, inhibiting the production of TNFα and IL1β, and inducing the production of CRP, as well as other acute phase reactants, such as the fibrinogen. IL6 concomitantly regulates pro-inflammatory and anti-inflammatory activities and contributes to both development and resolution of the acute inflammatory response. It is well known that IL6 levels increase with age [74, 75, 89, 90], having an important role in the development of several age-related diseases, including type 2 diabetes [91], and several cancers [92]. Increased IL6 concentrations have been suggested to predict the risk of developing reduced muscle strength and physical disability; both features are key components of the frailty syndrome [93]. Present results showed a clear association of frailty and pre-frailty status with increased levels of IL6 in older adults, thus providing further support to the involvement of inflammaging in the pathophysiology of frailty. These results are in agreement with the previous reviews from Ramakrishnan et al. [94] and Soysal et al. [18]. The former one reviewed the relationship of frailty with a heterogeneous battery of biomarkers (endocrine, genetic, inflammation, and nutritional biomarkers), while the latter found similar associations in their meta-analysis, but they were based on a lower number of studies and showed higher heterogeneity, since those studies used diverse criteria to assess frailty phenotype.
TNFα is a polypeptide cytokine mainly produced by stimulated monocytes, macrophages, and T lymphocyte subsets. TNFα contributes to the production of IL6 through activation of several pathways and plays a key role during the immune response, mediating inflammatory mechanisms both in normal immune surveillance and in pathologic conditions [95]. Plasma levels of TNFα were reported to be linearly related with IL6 and CRP in centenarians, indicating an interrelated activation of the entire inflammatory cascade in the oldest old [76]. However, no significant differences emerged from Soysal et al. [18] meta-analyses regarding the association between TNFα concentration and frailty status in older subjects, although a scarce number of studies were included (3 for frail vs. pre-frail/non-frail, and 4 for frail vs. non-frail and pre-frail). Our initial results including all studies showed a border line association between frailty and TNFα concentration after meta-regression analysis both in the comparisons frail vs. non-frail subjects (eight studies) and frail vs. pre-frail subjects (6 studies). A non-significant result was obtained comparing pre-frail and non-frail participants (six studies). Nevertheless, results from the sensitivity analyses exhibited a quantitatively feebler association which was no longer significant. These results suggest that there may be an association between frailty and TNFα, but the link is clearly weaker than for CRP and IL6, and further studies are necessary to confirm this potential relationship.
Among all studies analyzing IL10 concentration included in this review, only one showed a trend to decrease in frail subjects as compared with non-frail subjects (not significant) [45]; all other studies found negative results. And no associations between frailty and IL1β levels were found in any of the two studies determining this biomarker [56, 96]. The latter finding could be due to the promotion induced by IL1β and TNFα of IL6 secretion, which in turn inhibits the synthesis of TNFα and IL1β and stimulates the expression and release of soluble TNF receptors [97]. Indeed, higher concentrations of sTNF-RI and sTNF-RII associated with frailty status were reported in several of the revised studies [41, 51, 66], and sTNF-RII was recently suggested to be an accurate predictive biomarker to identify frail subjects [51].
Among the other biomarkers, briefly discussed in this systematic review, ICAM-1, an immunoglobulin-like cell adhesion molecule expressed by several cell types, including leukocytes and endothelial cells, plays a role in leukocyte migration [15] and activates pro-inflammatory cascades [98]. Changes in ICAM-1 concentration are shown to be associated with several age-related diseases and mortality [42]. Moreover, results obtained in the two studies included in this systematic review which analyzed this biomarker clearly showed increased levels of ICAM-1 with frailty, suggesting that the activation of inflammatory processes through leukocyte migration induced by ICAM-1 contributes, at least partially, to frailty development.
Inflammaging is currently accepted as a pathogenic feature in the development of several age-related diseases, such as cardiovascular disease, cancer, osteoporosis, and Alzheimer’s disease [99]. Interestingly, a very recent review by Pansarasa et al. [100] analyzed the role of genetic factors in frailty onset and the impact of diet on inflammation and, in turn, on frailty. In this regard, evidence gathered suggests that nutritional status, through its effects on cellular metabolism, may influence the immune system, i.e., cytokine levels, and immune cell populations and function, increasing inflammation and contributing to frailty. In the present meta-analyses, we described the presence of quantitative and significant associations between frailty and pre-frailty with two inflammatory biomarkers, i.e., CRP and IL6, obtaining statistical significance for all comparisons. Based on these results, the involvement of inflammaging in the pathophysiology of frailty in older adults is further confirmed, paving the way to studies validating the use of markers of inflammaging to predict frailty [96].
One possible limitation of this study is the high level of heterogeneity found in most of the comparisons performed, even after restriction to those studies that used Fried’s criteria to identify frailty, which considerably reduced heterogeneity with regard to previous meta-analyses [18]. Further decrease in heterogeneity was obtained in the sensitivity analyses. Nevertheless, several studies included in the analyses used criteria modified from Fried’s original description, possibly influencing the definition of frailty and, as a consequence, the results of its relationship with the biomarkers evaluated. Besides, as Fried’s criteria do not consider cognitive status, it is still to be assessed whether associations found for physical frailty can be also extrapolated to cognitive frailty, or cognitive decline in frail older adults is driven by different physiological alterations. On the other hand, and despite the wide range of the quality score in the studies analyzed, this parameter was not found to influence the results of the meta-regression analyses in any case.
In conclusion, this work established strong and quantitative associations between CRP and IL6 inflammatory biomarkers and frailty in older adults, a condition homogeneously identified by the most commonly used tool for frailty assessment (Fried’s frailty phenotype). These results are mostly based on cross-sectional studies, while longitudinal studies were scarce and it was not possible to conduct meta-regression analysis with these data. To evaluate and address this limit, further investigations should be done in longitudinal prospective studies, to assess (i) the possibility to predict frailty with inflammatory biomarkers, (ii) the directionality of the causal relationship between frailty and inflammaging, and (iii) the possible association of frailty status with other immunological biomarkers.
Electronic supplementary material
(PDF 58 kb)
(PDF 684 kb)
(PDF 136 kb)
(PDF 557 kb)
(PDF 138 kb)
(PDF 404 kb)
(PDF 156 kb)
Acknowledgments
We want to express our gratitude to Brenner, H. (Division of Clinical Epidemiology and Aging Research, German Cancer Research Center); Collerton, J. (Institute of Ageing and Health and Institute of Cellular Medicine, Newcastle University); Gale, C.R. (HRC Lifecourse Epidemiology Unit, University of Southampton); Sanchís, J. (Department of Cardiology, Hospital Clínico Universitario, University of Valencia); and Wang, X. (Unit of Epidemiology, Fudan University) for sending us their data.
Code availability
Not applicable.
Authors’ contributions
EP, BL, JPT, and JF-T designed the study. DM-P, VV, and MS-F conducted the literature search and screening, data collection, and contacts with authors. SP, SC, and SB carried out the statistical analyses. DM-P, BL, and VV wrote the manuscript draft. All authors reviewed and approved the manuscript draft.
Funding information
This research was funded by Xunta de Galicia [ED431B 2019/02]; Ministerio de Educación, Cultura y Deporte [BEAGAL18/00142 to V.V, PRX19/00353 to B.L.]; and Deputación Provincial de A Coruña [to D.M.-P. and M.S.-F.].
Data availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Data for the present meta-analysis were acquired through previously published articles, and the study did not involve participants or patients.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Blanca Laffon and Vanessa Valdiglesias contributed equally to the senior authorship of this manuscript.
References
- 1.Fried L, Tangen C, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 2.United Nations . World Population Ageing Report. 2015. pp. 1–164. [Google Scholar]
- 3.Cesari M, Prince M, Thiyagarajan JA, de Carvalho IA, Bernabei R, Chan P, Gutierrez-Robledo LM, Michel JP, Morley JE, Ong P, Rodriguez Manas L, Sinclair A, Won CW, Beard J, Vellas B. Frailty: an emerging public health priority. JAMDA. 2016;17:188–192. doi: 10.1016/j.jamda.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 4.United Nations . World population prospects. 2019. p. 2019. [Google Scholar]
- 5.Cesari M, Gambassi G, Van Kan GA, Vellas B. The frailty phenotype and the frailty index: different instruments for different purposes. Age Ageing. 2014;43:10–12. doi: 10.1093/ageing/aft160. [DOI] [PubMed] [Google Scholar]
- 6.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morley JE, Vellas B, Abellan van Kan G, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, Walston J. Frailty consensus: a call to action. JAMDA. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutler DM. Declining disability among the elderly. Health Aff. 2001;20:10–27. doi: 10.1377/hlthaff.20.6.11. [DOI] [PubMed] [Google Scholar]
- 9.Gill T, Gahbauer E, Allore H, Han L. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/.418. [DOI] [PubMed] [Google Scholar]
- 10.Mulero J, Zafrilla P, Martinez-Cacha A. Oxidative stress, frailty and cognitive decline. J Nutr Health Aging. 2011;15:756–760. doi: 10.1007/s12603-011-0130-5. [DOI] [PubMed] [Google Scholar]
- 11.Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Mitnitski A, Mogilner A, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rockwood K, Song X, Macknight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:9–13. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulop T, McElhaney J, Pawelec G, et al. Frailty, inflammation and immunosenescence. Interdiscip Top Gerontol Geriatr. 2015;41:26. doi: 10.1159/000381134. [DOI] [PubMed] [Google Scholar]
- 15.Theou O, Cann L, Blodgett J, Wallace LMK, Brothers TD, Rockwood K. Modifications to the frailty phenotype criteria: systematic review of the current literature and investigation of 262 frailty phenotypes in the survey of health, ageing, and retirement in Europe. Ageing Res Rev. 2015;21:78–94. doi: 10.1016/j.arr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Castelo-Branco C, Soveral I. The immune system and aging: a review. Gynecol Endocrinol. 2014;30:16–22. doi: 10.3109/09513590.2013.852531. [DOI] [PubMed] [Google Scholar]
- 17.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69:S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 18.Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, Sergi G, Isik AT, Manzato E, Maggi S, Maggio M, Prina AM, Cosco TD, Wu YT, Veronese N. Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev. 2016;31:1–8. doi: 10.1016/j.arr.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.3736/jcim20090918. [DOI] [PubMed] [Google Scholar]
- 20.Dersimonian R, Laird N. Meta-analysis in clinical trials *. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 22.Aarts S, Patel KV, Garcia ME, et al. Co-presence of multimorbidity and disability with frailty: an examination of heterogeneity in the frail older people. J Frailty Aging. 2015;4:131–138. doi: 10.14283/jfa.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida OP, Norman PE, van Bockxmeer FM, Hankey GJ, Flicker L. CRP 1846G>a polymorphism increases risk of frailty. Maturitas. 2012;71:261–266. doi: 10.1016/j.maturitas.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Arts MHL, Collard RM, Comijs HC, Naudé PJW, Risselada R, Naarding P, Oude Voshaar RC. Relationship between physical frailty and low-grade inflammation in late-life depression. J Am Geriatr Soc. 2015;63:1652–1657. doi: 10.1111/jgs.13528. [DOI] [PubMed] [Google Scholar]
- 25.Barzilay JI, Blaum C, Moore T, et al. Insulin resistance and inflammation as precursors of frailty: the cardiovascular health study. Arch Intern Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- 26.Blaum CS, Qian Ã, Xue L, et al. The association between obesity and the frailty syndrome in older women : the women’s health and aging. Studies. 2005;53:927–934. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- 27.Boxer RS, Dauser DA, Walsh SJ, Hager WD, Kenny AM. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J Am Geriatr Soc. 2008;56:454–461. doi: 10.1111/j.1532-5415.2007.01601.x. [DOI] [PubMed] [Google Scholar]
- 28.Carcaillon L, García-García FJ, Tresguerres JAF, Gutiérrez Avila G, Kireev R, Rodríguez-Mañas L. Higher levels of endogenous estradiol are associated with frailty in postmenopausal women from the Toledo study for healthy aging. J Clin Endocrinol Metab. 2012;97:2898–2906. doi: 10.1210/jc.2012-1271. [DOI] [PubMed] [Google Scholar]
- 29.Collerton J, Martin-Ruiz C, Davies K, Hilkens CM, Isaacs J, Kolenda C, Parker C, Dunn M, Catt M, Jagger C, von Zglinicki T, Kirkwood TBL. Frailty and the role of inflammation, immunosenescence and cellular ageing in the very old: cross-sectional findings from the Newcastle 85+ study. Mech Ageing Dev. 2012;133:456–466. doi: 10.1016/j.mad.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Darvin K, Randolph A, Ovalles S, Halade D, Breeding L, Richardson A, Espinoza SE. Plasma protein biomarkers of the geriatric syndrome of frailty. Journals Gerontol - Ser A Biol Sci Med Sci. 2014;69(A):182–186. doi: 10.1093/gerona/glt183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Garrido J, Navarro-Martínez R, Buigues-González C, Martínez-Martínez M, Ruiz-Ros V, Cauli O. The value of neutrophil and lymphocyte count in frail older women. Exp Gerontol. 2014;54:35–41. doi: 10.1016/j.exger.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Fried L, Xue Q, Cappola A, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Med Sci. 2009;64:1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gale CR, Baylis D, Cooper C, Sayer AA. Inflammatory markers and incident frailty in men and women: the english longitudinal study of ageing. Age (Omaha) 2013;35:2493–2501. doi: 10.1007/s11357-013-9528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gale CR, Ritchie SJ, Cooper C, Starr JM, Deary IJ. Cognitive ability in late life and onset of physical frailty: the Lothian birth cohort 1936. J Am Geriatr Soc. 2017;65:1289–1295. doi: 10.1111/jgs.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hubbard R, O’Mahony M, Calver B, Woodhouse K. Plasma esterases and inflammation in ageing and frailty. Eur J Clin Pharmacol. 2008;64:895–900. doi: 10.1007/s00228-008-0499-1. [DOI] [PubMed] [Google Scholar]
- 36.Hubbard RE, O’Mahony MS, Calver BL, Woodhouse KW. Nutrition, inflammation, and leptin levels in aging and frailty. J Am Geriatr Soc. 2008;56:279–284. doi: 10.1111/j.1532-5415.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 37.Hubbard R, O’Mahony M, Savva G, et al. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13:3103–3109. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang A-C, Liu L-K, Lee W-J, Chen LY, Peng LN, Lin MH, Chen LK. Association of frailty and cardiometabolic risk among community-dwelling middle-aged and older people: results from the I-Lan longitudinal aging study. Rejuvenation Res. 2015;18:564–572. doi: 10.1089/rej.2015.1699. [DOI] [PubMed] [Google Scholar]
- 39.Kalyani RR, VaRadhan R, Weiss CO, et al. Frailty status and altered dynamics of circulating energy metabolism hormones after oral glucose in older women. J Nutr Health Aging. 2012;16:679–686. doi: 10.1007/s12603-012-0066-4. [DOI] [PubMed] [Google Scholar]
- 40.Lai H-Y, Chang H, Lee YL, Hwang S-J. Association between inflammatory markers and frailty in institutionalized older men. Maturitas. 2014;79:329–333. doi: 10.1016/j.maturitas.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 41.Langmann G, Perera S, Ferchak M, et al. Inflammatory markers and frailty in long-term care residents. J Am Geriatr Soc. 2017;65:1777–1783. doi: 10.1111/jgs.14876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee WJ, Chen LK, Liang CK, Peng LN, Chiou ST, Chou P. Soluble ICAM-1, independent of IL-6, is associated with prevalent frailty in community-dwelling elderly Taiwanese people. PLoS One. 2016;11:1–9. doi: 10.1371/journal.pone.0157877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50:1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 44.Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16:153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 45.Leng SX, Yang H, Walston JD. Decreased cell proliferation and altered cytokine production in frail older adults. Aging Clin Exp Res. 2004;16:249–252. doi: 10.1007/BF03327392. [DOI] [PubMed] [Google Scholar]
- 46.Leng SX, Xue Q-L, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 47.Leng SX, Xue Q-L, Tian J, Huang Y, Yeh SH, Fried LP. Associations of neutrophil and monocyte counts with frailty in community-dwelling disabled older women: results from the Women’s health and aging studies I. Exp Gerontol. 2009;44:511–516. doi: 10.1016/j.exger.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Leng SX, Tian X, Matteini A, Li H, Hughes J, Jain A, Walston JD, Fedarko NS. IL-6-independent association of elevated serum neopterin levels with prevalent frailty in community-dwelling older adults. Age Ageing. 2011;40:475–481. doi: 10.1093/ageing/afr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin CC, Wu FY, Liao LN, Li CI, Lin CH, Yang CW, Meng NH, Chang CK, Lin WY, Liu CS, Li TC. Association of CRP gene polymorphisms with serum CRP level and handgrip strength in community-dwelling elders in Taiwan: TAICHUNG Community Health Study for Elders (TCHS-E) Exp Gerontol. 2014;57:141–148. doi: 10.1016/j.exger.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Liu C, Lyass A, Larson M, et al. Biomarkers of oxidative stress are associated with frailty: the Framingham offspring study. Age (Omaha) 2016;38:1. doi: 10.1007/s11357-015-9864-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marcos-Pérez D, Sánchez-Flores M, Maseda A, Lorenzo-López L, Millán-Calenti JC, Gostner JM, Fuchs D, Pásaro E, Laffon B, Valdiglesias V. Frailty in older adults is associated with plasma concentrations of inflammatory mediators but not with lymphocyte subpopulations. Front Immunol. 2018;9:1–9. doi: 10.3389/fimmu.2018.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Namioka N, Hanyu H, Hirose D, Hatanaka H, Sato T, Shimizu S. Oxidative stress and inflammation are associated with physical frailty in patients with Alzheimer’s disease. Geriatr Gerontol Int. 2016;17:1–6. doi: 10.1111/ggi.12804. [DOI] [PubMed] [Google Scholar]
- 53.Pabst G, Zimmermann AK, Huth C, Koenig W, Ludwig T, Zierer A, Peters A, Thorand B. Association of low 25-hydroxyvitamin D levels with the frailty syndrome in an aged population: results from the KORA-age Augsburg study. J Nutr Health Aging. 2015;19:258–264. doi: 10.1007/s12603-014-0546-9. [DOI] [PubMed] [Google Scholar]
- 54.Piggott DA, Varadhan R, Mehta SH, Brown TT, Li H, Walston JD, Leng SX, Kirk GD. Frailty, inflammation, and mortality among persons aging with HIV infection and injection drug use. J Gerontol - Ser A Biol Sci Med Sci. 2015;70:1542–1547. doi: 10.1093/gerona/glv107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puts MTE, Visser M, Twisk JWR, Deeg DJH, Lips P. Endocrine and inflammatory markers as predictors of frailty. Clin Endocrinol. 2005;63:403–411. doi: 10.1111/j.1365-2265.2005.02355.x. [DOI] [PubMed] [Google Scholar]
- 56.Qu T, Yang H, Walston J, et al. Upregulated monocytic expression of CXC chemokine ligand 10 (CXCL-10) and its relationship with serum interleukin-6 levels in the syndrome of frailty. Cytokine. 2009;46:319–324. doi: 10.1136/bjo.45.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reiner A, Aragaki A, Gray S, et al. Inflammation and thrombosis biomarkers and. J Am Geriatr Soc. 2009;122:947–954. doi: 10.1016/j.amjmed.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ronning B, Wyller T, Seljeflot I, et al. Frailty measures, inflammatory biomarkers and post-operative complications in older surgical patients. Age Ageing. 2010;39:755–758. doi: 10.1093/ageing/afq121. [DOI] [PubMed] [Google Scholar]
- 59.Sanchis J, Núñez E, Ruiz V, Bonanad C, Fernández J, Cauli O, García-Blas S, Mainar L, Valero E, Rodríguez-Borja E, Chorro FJ, Hermenegildo C, Núñez J. Usefulness of clinical data and biomarkers for the identification of frailty after acute coronary syndromes. Can J Cardiol. 2015;31:1–7. doi: 10.1016/j.cjca.2015.07.737. [DOI] [PubMed] [Google Scholar]
- 60.Saum K, Dieffenbach A, Jansen E, et al. Association between oxidative stress and frailty in an elderly German population: results from the esther cohort study. Gerontology. 2015;61:407–415. doi: 10.1159/000380881. [DOI] [PubMed] [Google Scholar]
- 61.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532.5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 62.Schoufour JD, Echteld MA, Boonstra A, Groothuismink ZMA, Evenhuis HM. Biochemical measures and frailty in people with intellectual disabilities. Age Ageing. 2016;45:142–148. doi: 10.1093/ageing/afv152. [DOI] [PubMed] [Google Scholar]
- 63.Serviddio G, Romano A, Greco A, et al. Frailty syndrome is associated with altered circulating redox balance and increased markers of oxidative stress. Int J Immunopathol Pharmacol. 2009;22:819–827. doi: 10.1177/039463200902200328. [DOI] [PubMed] [Google Scholar]
- 64.Silva J, de Moraes Z, da Silva C, et al. Understanding red blood cell parameters in the context of the frailty phenotype: interpretations of the FIBRA (frailty in Brazilian seniors) study. Arch Gerontol Geriatr. 2014;59:636–641. doi: 10.1016/j.archger.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 65.Tsai J, Wu C, Chen S, et al. Plasma Adiponectin levels correlate positively with an increasing number of components of frailty in male elders. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0056250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van Epps P, Oswald D, Higgins PA, Hornick TR, Aung H, Banks RE, Wilson BM, Burant C, Gravenstein S, Canaday DH. Frailty has a stronger association with inflammation than age in older veterans. Immun Ageing. 2016;13:1–9. doi: 10.1186/s12979-016-0082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walston J. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities. Results from the cardiovascular health study. Arch Intern Med. 2002;162:2333. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 68.Wu I, Shiesh S, Kuo P, Lin X. High oxidative stress is correlated with frailty in elderly Chinese. J Gerontol A Biol Med Sci. 2009;57:1666–1671. doi: 10.1111/j.1532-5415.2009.02392.x. [DOI] [PubMed] [Google Scholar]
- 69.Yang Y, Hao Q, Flaherty J, et al. Comparison of procalcitonin, a potentially new inflammatory biomarker of frailty, to interleukin-6 and C-reactive protein among older Chinese hospitalized patients. Aging Clin Exp Res. 2018;30:1459–1464. doi: 10.1007/s40520-018-0964-3. [DOI] [PubMed] [Google Scholar]
- 70.Zhu Y, Liu Z, Wang Y, Wang Z, Shi J, Xie X, Jin L, Chu X, Wang X. C-reactive protein, frailty and overnight hospital admission in elderly individuals: a population-based study. Arch Gerontol Geriatr. 2016;64:1–5. doi: 10.1016/j.archger.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 71.Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging. Longev Heal. 2013;2:8. doi: 10.1186/2046-2395-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lai S, Thomas P, Fenech M. Genome instability biomarkers and blood micronutrient risk profiles associated with mild cognitive impairment and Alzheimer ’ s disease. Mutat Res Fundam Mol Mech Mutagen. 2015;776:54–83. doi: 10.1016/j.mrfmmm.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 73.Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sc. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 74.Capuron L, Schroecksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, Layé S, Fuchs D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70:175–182. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proin ammatory state. Aging (Albany NY) 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhoj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol A Biol Sci Med Sci. 1999;54:M357–M364. doi: 10.1093/gerona/54.7.M357. [DOI] [PubMed] [Google Scholar]
- 77.Bruunsgaard H, Skinhøj P, Pedersen AN, et al. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121:255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hasegawa Y, Sawada M, Ozaki N, et al. Increased soluble tumor necrosis factor receptor levels in the serum of elderly people. Gerontology. 2000;46:185–188. doi: 10.1159/000022157. [DOI] [PubMed] [Google Scholar]
- 79.García-Dabrio MC, Pujol-Moix N, Martinez-Perez Á, Fontcuberta J, Souto JC, Soria JM, Nomdedéu JF. Influence of age, gender and lifestyle in lymphocyte subsets: report from the Spanish gait-2 study. Acta Haematol. 2012;127:244–249. doi: 10.1159/000337051. [DOI] [PubMed] [Google Scholar]
- 80.Valdiglesias V, Sánchez-Flores M, Maseda A, Marcos-Pérez D, Millán-Calenti JC, Pásaro E, Lorenzo-López L, Laffon B. Lymphocyte subsets in a population of nonfrail elderly individuals. J Toxicol Env Heal A. 2015;78:790–804. doi: 10.1080/15287394.2015.1051170. [DOI] [PubMed] [Google Scholar]
- 81.Bartlett DB, Firth CM, Phillips AC, Moss P, Baylis D, Syddall H, Sayer AA, Cooper C, Lord JM. The age-related increase in low-grade systemic inflammation (Inflammaging) is not driven by cytomegalovirus infection. Aging Cell. 2012;11:912–915. doi: 10.1111/j.1474-9726.2012.00849.x. [DOI] [PubMed] [Google Scholar]
- 82.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 83.Roubenoff R, Parise H, Payette HA, Abad LW, D'Agostino R, Jacques PF, Wilson PWF, Dinarello CA, Harris TB. Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: the Framingham heart study. Am J Med. 2003;115:429–435. doi: 10.1016/j.amjmed.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 84.Pepys MB, Hirschfield GM. C-reactive protein : a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI200318921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Volanakis J. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001;38:189–197. doi: 10.1016/S0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 86.Hutchinson WL, Koenig W, Fröhlich M, et al. Immunoradiometric assay of circulating C-reactive protein: age-related values in the adult general population. Clin Chem. 2000;46:934–938. doi: 10.1093/clinchem/46.7.934. [DOI] [PubMed] [Google Scholar]
- 87.de Ferranti S, Rifai N. C-reactive protein and cardiovascular disease: a review of risk prediction and interventions. Clin Chim Acta. 2002;317:1–15. doi: 10.1016/S0009-8981(01)00797-5. [DOI] [PubMed] [Google Scholar]
- 88.Freeman DJ, Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GD, O'Reilly DS, Packard CJ, Sattar N, West of Scotland Coronary Prevention Study C-reactive protein is an independent predictor of risk for the development of diabetes in the west of Scotland coronary prevention study. Diabetes. 2002;51:1596–1600. doi: 10.2337/diabetes.51.5.1596. [DOI] [PubMed] [Google Scholar]
- 89.Cohen HJ, Pieper CF, Harris T, Rao KMK, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52:M201–M208. doi: 10.1093/gerona/52A.4.M201. [DOI] [PubMed] [Google Scholar]
- 90.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated Interleukin-6 and C-reactive protein levels with mortality in the elderly∗ ∗ access the “journal Club” discussion of this paper at http:/www.elsevier.com/locate/ajmselect/ Am J Med. 1999;106:506–512. doi: 10.1016/S0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 91.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 92.Il’yasova, Colbert LH, Harris TB, et al. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Biomarkers. 2005;14:2413–2418. doi: 10.1158/1055-9965.EPI-05-0316. [DOI] [PubMed] [Google Scholar]
- 93.Ferrucci L, Penninx BWJH, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, Leveille SG, Fried LP, Md JMG. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 94.Ramakrishnan P, Alyousefi N, Abdul-Rahman PS, Kamaruzzaman SB, Chin AV, Tan MP. A systematic review of studies comparing potential biochemical biomarkers of frailty with frailty assessments. Eur Geriatr Med. 2017;8:397–407. doi: 10.1016/j.eurger.2017.07.010. [DOI] [Google Scholar]
- 95.Diez-Ruiz A, Tilz G, Zangerle R, et al. Soluble receptors for tumour necrosis factor in clinical laboratory diagnosis. Eur J Haematol. 1995;54:1–8. doi: 10.1111/j.1600-0609.1995.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 96.Baylis D, Bartlett DB, Syddall HE, Ntani G, Gale CR, Cooper C, Lord JM, Sayer AA. Immune-endocrine biomarkers as predictors of frailty and mortality: a 10-year longitudinal study in community-dwelling older people. Age (Omaha) 2013;35:963–971. doi: 10.1007/s11357-012-9396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Gonzalo-Calvo D, Neitzert K, Fernández M, Vega-Naredo I, Caballero B, García-Macía M, Suárez FM, Rodríguez-Colunga MJ, Solano JJ, Coto-Montes A. Differential inflammatory responses in aging and disease: TNF-α and IL-6 as possible biomarkers. Free Radic Biol Med. 2010;49:733–737. doi: 10.1016/j.freeradbiomed.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 98.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacol Rep. 2009;61:22–32. doi: 10.1016/S1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 99.DeAraújo AL, Silva LCR, Fernandes JR, Benard G. Preventing or reversing immunosenescence: can exercise be an immunotherapy? Immunotherapy. 2013;5:879–893. doi: 10.2217/imt.13.77. [DOI] [PubMed] [Google Scholar]
- 100.Pansarasa O, Pistono C, Davin A, Bordoni M, Mimmi MC, Guaita A, Cereda C. Altered immune system in frailty: genetics and diet may influence inflammation. Ageing Res Rev. 2019;54:100935. doi: 10.1016/j.arr.2019.100935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 58 kb)
(PDF 684 kb)
(PDF 136 kb)
(PDF 557 kb)
(PDF 138 kb)
(PDF 404 kb)
(PDF 156 kb)
Data Availability Statement
Not applicable.