Abstract
Imaginal exposure, i.e. reducing fear using exposure to mental imagery, is a widely used psychological treatment technique for dysfunctional fears. Yet, little is known about its underlying neural mechanisms. The present study examines the neural basis of imaginal exposure using a novel experimental procedure consisting of repeated exposure to flashpoint mental imagery of phobic (spiders) and neutral (gloves) stimuli. Whether the 10 min long imaginal exposure procedure could reduce fear responses was examined one week later. Thirty participants fearful of spiders underwent the experimental procedure. Neural activity was assessed using functional magnetic resonance imaging (session 1). Subjective fear and skin conductance responses were measured throughout the study (sessions 1 and 2). Imaginal exposure evoked intense fear and heightened skin conductance responses, and indicated robust activation in several brain regions, including amygdala, midcingulate cortex and insula. Findings demonstrate that neural activity in fear-processing brain areas can be elicited solely by generating a mental image of a phobic stimulus, that is, in the absence of the percept. Relevant for treatment development, results reveal that a single 10 min session of brief exposures to flashpoint mental imagery can lead to lasting reductions in phobic fear at both the subjective and physiological levels.
This article is part of the theme issue ‘Offline perception: voluntary and spontaneous perceptual experiences without matching external stimulation'.
Keywords: mental imagery, imaginal exposure, imaginal extinction, functional magnetic resonance imaging, skin conductance responses
1. Introduction
Dysfunctional fear, such as a phobia, can be treated through exposure to the feared object or event (e.g. spider or public speaking). Indeed, exposure therapy is one of our most effective and widely used psychological treatments for anxiety disorders [1,2]. Commonly, exposure is performed in vivo, i.e. to actual feared objects or events (e.g. a real spider). However, in vivo exposure is not always feasible, for example, when feared stimuli cannot be accessed in reality (e.g. attacker in a past trauma). In such cases, mental imagery can be used for exposure: a technique referred to as imaginal exposure [3]. Mental imagery has been described as seeing with the mind's eye, hearing with the mind's ear and so on, referring to percept-like sensory experiences in the absence of external sensory input [4]. Indeed, imaginal exposure is believed to enable emotional processing of fear-provoking stimuli by harnessing the power of mental imagery to evoke similar emotional and physiological responses to direct perception [5–7].
(a). Imaginal exposure
Imaginal exposure is a widely used and effective treatment technique [8] and a key component in evidence-based treatment protocols, such as prolonged exposure (PE) for posttraumatic stress disorder (PTSD) [9]. Imaginal exposure entails producing mental imagery of the fear-provoking stimulus (e.g. trauma memory), usually for prolonged durations. Normally, imaginal exposure is delivered in conjunction with other psychological treatment components [8]. For instance, PE consists of multiple 90 min sessions, including 40–60 min of imaginal exposure followed by 15–20 min of verbal processing of trauma-related thoughts and feelings, and in vivo exposure (homework assignments) between-sessions [9]. Evidence in clinical studies using exposure-based protocols indicates that imaginal exposure alone, without in vivo exposure, can alleviate PTSD symptoms [10,11]. Furthermore, imaginal and in vivo exposure have been observed to produce similar reductions in experimentally induced (conditioned) fear [12] and phobic fear [13,14].
Nevertheless, akin to all exposure therapies, the effects of imaginal exposure-based treatments are many times transient or insufficient, resulting in residual symptoms or relapse after treatment [15,16]. Treatment development is thus needed. An essential step to improve imaginal exposure is to increase our understanding of the mechanisms underlying the reduction of fear within the mind's eye [17]. This step includes dismantling the neural underpinnings of imaginal exposure. Although many studies have investigated the neural mechanisms of in vivo exposure [18], very few have explored its imaginal counterpart. Furthermore, existing neuroimaging studies of imaginal exposure assess the neural correlates associated with entire clinical protocols rather than isolating the specific imaginal exposure component, and brain activity is measured after treatment, rather than during imaginal exposure [19]. These factors make it difficult to isolate the neural underpinnings specifically associated with imaginal exposure.
(b). Current knowledge of the neural basis of imaginal exposure
Several neuroimaging studies on specific phobia have examined neural activity during symptom provocation with phobia-specific (pictorial) stimuli (i.e. in vivo exposure). Meta-analyses of these studies report consistent activations in fear processing areas, such as the amygdala, the insular cortex, the anterior cingulate cortex (ACC) and the anterior midcingulate cortex (MCC) [18,20]. Compared with healthy participants, phobic individuals show hyperactivation of the thalamus, cerebellum and the inferior frontal gyrus during perception of phobic stimuli [18,20]. In addition, some brain-imaging studies of PTSD have used script-driven mental imagery of trauma as symptom provocation. In these studies, results imply that mental imagery of trauma memories in PTSD involves alterations in the medial prefrontal cortex, ACC/MCC, insula and amygdala (for an overview see [21]) However, findings are inconsistent across studies.
The aetiology of specific phobia has been suggested to rely on fear-conditioning processes [22] and there is indeed consistency between brain activations to phobia-specific stimuli and our current neural understanding of fear-conditioning and extinction [18]. Fear extinction refers to the reduction of experimentally induced (conditioned) fear through exposure to the fear-conditioned stimulus. One study, conducted by Reddan et al. [23], has examined the brain mechanisms underlying the reduction of conditioned fear using mental imagery (imaginal extinction). In vivo and imaginal extinction of conditioned fear responses were compared in healthy participants. Results suggested that imaginal and in vivo extinction employed similar core neural circuitry to reduce threat responses, including the ventromedial prefrontal cortex (vmPFC), amygdala and hippocampus. The nucleus accumbens (NAcc) appeared to be more involved in imaginal as compared with in vivo extinction, as this area predicted extinction success in imaginal extinction only [23]. This important study used experimentally induced fear (i.e. a tone paired with an electric shock) to reveal the neural underpinnings of imaginal exposure. In order to bridge the gap between the laboratory and the clinic, the critical next step is to study the neural underpinnings of imaginal exposure to naturally occurring fear (e.g. spider fear).
(c). Developing an experimental procedure to study the neural basis of imaginal exposure
Assessing neural activity of internal processes, such as mental imagery, is methodologically challenging. One method involves script-driven imagery, a procedure used for symptom provocation in neuroimaging studies of PTSD [24,25]. A personalized trauma-script is constructed for each participant. During brain-imaging, participants are asked to produce mental imagery of their specific trauma memory script for 30 s up to 1 min. However, there are some limitations to this method. First, the use of personalized scripts complicates the construction of adequate control conditions, as the content of emotional and neutral scripts may differ in many ways (e.g. traumatic event versus brushing one's teeth) [24]. Also, producing mental imagery for prolonged durations is mentally taxing, as a mental image can generally only be kept in mind for around 250 ms before it has to be actively regenerated [26]. Unbalanced control conditions and individual differences in cognitive resources could thus complicate the interpretation of observed neural activations. Therefore, in order to improve experimental control, and more accurately capture the neural activity tied to emotional processing during imaginal exposure, we designed a novel experimental procedure.
The procedure involved (1) non-personalized imagery scripts of a common feared object (e.g. a spider): non-personalized imagery scripts help to reduce variance between individuals, by ensuring the same task across participants. (2) Mental imagery of short durations: here, we use the term ‘flashpoint mental imagery' to refer to evoking mental imagery of short durations (a few seconds) containing emotion-provoking elements (e.g. worst parts, ‘hotspots') [27]. The use of flashpoint imagery for exposure rather than the use of prolonged imagery with more extended durations (e.g. 40 min) has several advantages. First, since it is briefer, it is less mentally taxing, and results should be less dependent on participants' attentional resources. It also readily allows the construction of better-matched control stimuli. For example, for spider phobia, imagery including a spider can then be contrasted with identical imagery of matching complexity where the spider is replaced with a non-fearful object. Moreover, flashpoint mental imagery allows the inclusion of a greater number of trials, which facilitates repeated exposure, a core feature of successful exposure techniques [1].
(d). Primary aim: what is the neural basis of imaginal exposure?
The primary aim of the study was to examine the neural basis of imaginal exposure in individuals fearful of spiders using functional magnetic resonance imaging (fMRI) and skin conductance responses (SCRs). We developed a novel experimental analogue of imaginal exposure, consisting of repeated exposure to flashpoint mental imagery of phobic stimuli (spiders) and neutral stimuli (gloves). The procedure allowed the inclusion of many (13 fearful and 13 neutral) exposure-trials (cf. 1 or 2 trials usually included in script-driven imagery studies). In order to mirror clinical practice, mental imagery was prompted via verbal instructions, and the procedure used graded exposure (i.e. exposure to progressively more fearful stimuli). As neural activity associated with imaginal exposure to naturally occurring fear had not been investigated previously, analyses were largely exploratory, focusing on whole-brain analyses. The pioneering study of Reddan et al. [23] indicated a special role for the NAcc in imaginal extinction of conditioned fear. Thus, a separate region of interest (ROI) analysis was performed for this area.
(e). Secondary aim: can 10 min of exposure to flashpoint mental imagery reduce fear?
In the clinic, imaginal exposure is usually delivered for prolonged durations (ca 40 min) [9]. However, evidence indicates that clinical protocols with briefer exposure sessions (20–30 min) can also be effective [14,28,29]. The experimental procedure used in this study provided an opportunity to explore if an even briefer imaginal exposure session alone (without other treatment components typical of clinical protocols) could influence fear responses. Therefore, a secondary aim was to evaluate possible lasting effects of the experimental procedure (i.e. 10 min of repeated exposure to flashpoint mental imagery) on participants' fear responses. To this end, participants repeated the procedure roughly one week later to enable between-session comparisons of subjective fear ratings and SCRs.
2. Methods and material
(a). Participants
Thirty participants (age: M = 24.0, s.d. = 5.6 years; 22 women and 8 men) fearful of spiders were recruited through advertisement on social media and billboards. The inclusion criterion was significant fear of spiders indicated by a score greater than or equal to 19 on the spider phobia questionnaire (SPQ) [30] in line with SPQ ratings previously reported by individuals with spider phobia [31]. A full clinical diagnostic interview for spider phobia was not conducted. Exclusion criteria included a screening indication of a current psychiatric disorder other than spider phobia, substance abuse, neurological disease, having received psychological treatment or psychotropic medication within six months, and contraindications for magnetic resonance imaging. The Mini International Neuropsychiatric Interview [32] was used to screen for current psychiatric disorders according to the fifth edition of the Diagnostic and statistical manual of mental disorders (DSM5) [33] and was conducted by a licensed clinical psychologist (J.M.H.) or psychology student under supervision (by J.M.H.). Eight participants (27%) included in the study reported a history of psychiatric disorder (major depression: N = 6; panic disorder: N = 1; generalized anxiety disorder: N = 1), and four of these had previously received psychological treatment, although not for spider phobia. Owing to technical problems, SCR data from two participants (session 1) could not be analysed. Furthermore, one participant did not complete session 2. Thus, analyses of SCR included N = 28 for session 1, N = 29 for session 2 and N = 27 for between-session analyses (differences between sessions 1 and 2). Analyses of the subjective fear ratings included N = 30 for session 1, N = 29 for session 2 and N = 29 for between-sessions analyses. Participant characteristics and behavioural data were analysed using IBM SPSS Statistics for Windows, v. 22 (IBM Corp., Armonk, NY). Participants were reimbursed with 500 Swedish kronor for their participation.
(b). Assessments of mental imagery, anxiety and subjective fear
The Spielberger state–trait anxiety inventory (STAI-T) [34] and the Plymouth Sensory Imagery Questionnaire (Psi-Q) [35] were collected at baseline. Self-reported ratings of subjective fear (0 = no fear at all; 100 = extreme fear) and vividness of mental imagery (1 = not at all vivid; 5 = extremely vivid) were collected once per session immediately after participants had completed the experimental procedure. Additionally, after the procedure, participants were asked to estimate to what extent they believed that their feelings were affected (−10, 0, 10; negatively, no change, positively) by imagining the spider scenes (e.g. ‘imagine looking at a spider') when compared with focusing on the verbal content (listening to the sentence describing the scene; focusing on the words and their meaning) to assess demand. For exploratory analyses, participants also completed a one-week diary to register the occurrence (i.e. number) of intrusive mental imagery of spiders, daily, during 7 days (between sessions 1 and 2), and completed the difficulties of emotion regulation scale (DERS) [36].
(c). Design and experimental procedure
Participants performed the experimental procedure twice, approximately 9 days apart (M = 9.5, s.d. = 1.8). The first session was conducted at Uppsala University Hospital, where both fMRI data and SCRs were measured throughout. Session 2 was carried out at the Department of Psychology, Uppsala University, where SCRs, but no fMRI data were collected (electronic supplementary material, figure S1). Prior to entering the magnetic resonance (MR)-scanner, participants underwent general [37] and task-specific mental imagery training (see electronic supplementary material for mental imagery training protocol). Participants were asked to keep their eyes closed throughout the 10 min long experimental procedure. A recorded voice provided short descriptions (2–4 s) of (phobic/neutral) situations at set intervals. After hearing a description, participants produced mental imagery during a 7 s interval. This time interval was adapted to allow measures of SCRs [38]. Participants were asked to try to keep producing mental imagery throughout the whole interval and regenerate the mental image whenever it was lost. Mental imagery was followed by 6 s of rest, during which participants were instructed to ‘relax and wait for the next instruction' (see electronic supplementary material, figure S2 for an overview of the experimental procedure).
Descriptions consisted of 13 different situations including spiders, and 13 corresponding situations containing gloves. Importantly, neutral situations were as similar as possible to fearful situations, with the exception that the spider was replaced with a glove (e.g. phobic: ‘see a spider in front of you', ‘you touch a spider’; neutral: ‘see a glove in front of you’, ‘you pick up a glove'; see electronic supplementary material, table S1 for the complete list of situations). Phobic mental imagery and the corresponding neutral mental imagery were presented in pairs, where phobic imagery always preceded neutral imagery in order to minimize expectancy effects (e.g. eliciting fear/imagery of spiders during neutral imagery because participants may immediately start to imagine the same situation including spiders). After trials 3, 6 and 9, participants were reminded through recorded instructions to imagine the scenes as vividly and with as much detail as possible and to regenerate the mental image if lost. Graded exposure was used, i.e. imagery grew progressively more fearful during the experimental procedure (e.g. ‘looking at a spider,’ ‘touching a spider,' ‘a spider crawling in under your shirt'). The experimental procedure was programmed in Eprime 2.0 (Psychology Software Tools, Pittsburgh, PA).
(d). Brain-imaging—acquisition and preprocessing
Data were acquired using a 3 T whole-body MR scanner (Philips Achieva 3.0 T TX, Philips Medical Systems, Best, The Netherlands) with a 32-channel head coil. First, an anatomical T1-weighted reference image (voxel size 0.938 × 0.938 × 1; matrix 256 × 256; 220 slices) was collected. During the experimental session, blood oxygen level-dependent (BOLD) imaging was performed using a single-shot echo-planar imaging (EPI) sequence with whole-brain coverage (repetition time (TR)/echo time (TE) = 35/3000 ms; flip angle 90°; acquired voxel size 1.5 × 1.5 × 3.0, matrix 160 × 160, 45 slices with no gap in ascending order). BOLD images were normalized to the Montreal Neurological Institute (MNI) standard space, slice time corrected and spatially smoothed using an 8 mm full width at half maximum kernel (voxel size 2 × 2 × 2). Preprocessing and all analyses of fMRI data were performed using SPM12 (Wellcome Department of Cognitive Neurology, University College, London (http://www.fil.ion.ucl.ac.uk).
(e). fMRI data analysis
First-level analyses used event-related modelling and included the following regressors. Phobic imagery (7 s per trial) and neutral imagery (7 s per trial) were modelled with one regressor each. In order to pinpoint emotional processing from imagery only, and exclude possible emotional responses to the verbal instructions, phobic and neutral instructions were modelled with one regressor each (variable length depending on the length of instructions audio; about 2–4 s per trial). In order to remove visual processing from baseline, the text instruction used at the start of the sequel was also modelled with a regressor (4 s). Moreover, six regressors measuring head movement were also included. Second-level analysis focused on contrasting phobic imagery over neutral imagery (phobic > neutral), pinpointing emotional processing tied to imaginal exposure, and neutral imagery over baseline (neutral > baseline), revealing activity related to the production of mental imagery. The association between brain activity (BOLD; phobic > neutral) and SCR during imaginal exposure (session 1) was assessed by including mean SCRs for each participant (phobic > neutral) into a regression analysis within SPM12. Likewise, difference scores (session 1 minus session 2) of mean SCRs were included in a regression analysis to explore associations between brain activity and change in SCR between sessions. Corresponding analyses were performed to assess the association between brain activity (phobic > neutral) and subjective fear ratings (phobic > neutral). The statistical threshold was set at p < 0.05 family-wise error correction (FWE) in all analyses. The automated anatomical labelling library from the Wake Forest University Pickatlas (aal) [39] was used to map neural activation results to brain regions. The NAcc ROI was defined using the Hammersmith atlas [40], as a mask for this area is not available in the aal atlas.
(f). Skin conductance response—acquisition and analysis
SCRs were collected with a BIOPAC MP 160 system. An isotonic recording electrode gel was applied to the electrodes before they were placed on the hypothenar eminence of the participant's left hand. The SCR signal passed through a high-pass hardware filter of 0.05 Hz and was analysed with the Ledalab software package using continuous decomposition analysis [41] implemented in Matlab (Mathworks Inc., Natick, MA). SCR was scored using the maximum phasic driver amplitude (Max.SCR) 1–7 s after each stimulus onset. For the simplest possible interpretation of data, and in order to maximize inter-individual variability, correlation analyses between SCR and fMRI data were performed using raw SCR data. For consistency, and because no between-individual analyses were made, SCR raw data were used throughout all SCR analyses. However, square root transformed range-corrected data are presented in the electronic supplementary material. For within-session SCR analyses, the 13 trials of each stimulus category were collected into four bins (trials 1–3, 4–6, 7–9, 10–13), and the mean value for each bin was used.
3. Results
(a). Behavioural data
(i). Session 1
Descriptive statistics regarding participant characteristics are presented in table 1. SPQ ratings were in line with mean scores previously reported for spider phobia [31]. STAI-T and Psi-Q mean scores, respectively, were consistent with levels of trait anxiety [34] and vividness of mental imagery [35] in the general population.
Table 1.
Participant characteristics. SPQ, spider phobia questionnaire; STAI-T, Spielberger state–trait anxiety inventory; Psi-Q, Plymouth Sensory Imagery Questionnaire. No significant gender differences were observed on any measures (all p > 0.05).
| all participants (N = 30) |
women (N = 22) |
men (N = 8) |
||||
|---|---|---|---|---|---|---|
| mean | s.d. | mean | s.d. | mean | s.d. | |
| age | 24.0 | 5.6 | 24.7 | 6.0 | 22.0 | 3.5 |
| SPQ | 23.5 | 2.7 | 23.2 | 2.6 | 24.2 | 3.0 |
| STAI-T | 34.8 | 5.7 | 34.9 | 5.1 | 34.9 | 7.7 |
| Psi-Q | 7.6 | 1.0 | 7.6 | 1.0 | 7.8 | 1.1 |
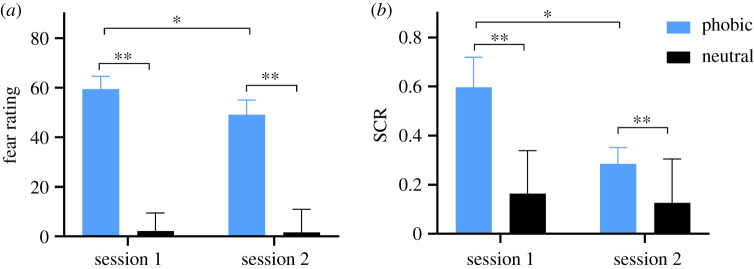
Participants reported much higher levels of subjective fear during mental imagery of spiders (M = 60.2, s.d. = 27.1) compared with neutral imagery (M = 2.0, s.d. = 7.3; t29 = 12.08, p < 0.001, Cohen's d = 2.20). Subjective fear ratings were positively correlated with vividness of phobic imagery (rS = 0.48, p = 0.008). The number of intrusive images of spiders reported in the one-week diary was positively correlated with spider fear (SPQ: N = 25, r = 0.48, p = 0.016), but was not significantly associated with more general trait anxiety (STAI-T: N = 25 r = −0.011, p = 0.96). Participants estimated that imagining the scenes would increase their negative feelings when compared with just listening to the scenes, or focus on the verbal content of the scenes (imagining versus listening: M = −7.0, s.d. = 2.5; imagining versus words: M = −6.0, s.d. = 3.4). Self-rated difficulties in emotion regulation were not associated with reduction in subjective fear between sessions (DERS: N = 23, r = 0.003, p = 0.99).
Within-session SCRs were analysed using a 2 × 4 repeated measures analyses of variance (ANOVA), with stimulus (spiders, gloves) and trial bins (trials 1–3, 4–6, 7–9, 10–13) as within-group variables. There was a main effect of stimulus (F1,27 = 18.36, p < 0.001, ), indicating that SCRs were stronger to phobic imagery (M = 0.59, s.e. = 0.12) compared with neutral imagery (M = 0.16, s.e. = 0.03; t27 = 4.39, p < 0.001, Cohen's d = 0.83). There was no effect of trial bins (F3,81 = 1.68, p = 0.19; ), nor a stimulus × trial bins effect (F3,81 = 0.52, p = 0.62, ). See electronic supplementary material, table S2 for descriptive SCR data.
(ii). Session 2
Subjective fear ratings continued to be higher during phobic (M = 49.7, s.d. = 32.2) compared with neutral imagery (M = 1.7, s.d. = 9.3; t28 = 8.09, p < 0.001; Cohen's d = 1.50). Subjective fear ratings were still positively correlated with vividness (rS = 0.37, p = 0.049). Within-session SCRs were analysed with a 2 × 4 repeated measures ANOVA with stimulus (spiders, gloves) and trial bins (trials 1–3, 4–6, 7–9, 10–13) as within-group variables. Again, a main effect of stimulus was found (F1,28 = 19.41, p < 0.001, ), demonstrating that SCRs were higher during phobic (M = 0.27, s.e. = 0.06) compared with neutral imagery (M = 0.12, s.e. = 0.03; t28 = 4.34, p < 0.001, Cohen's d = 0.81). Similar to session 1, there was no effect of trial bins (F3,84 = 0.36, p = 0.72), and no stimulus × trial bins effect (F3,84 = 0.38, p = 0.77). See electronic supplementary material, table S3 for descriptive SCR data.
(b). Between-sessions differences
Between-sessions differences in SCR and subjective fear ratings, respectively, were examined using 2 × 2 repeated measures ANOVA, with stimulus (phobic, neutral) and session (session 1 and session 2) as within-group variables. For subjective fear ratings, a significant session × stimulus interaction was observed (F1,28 = 17.0, p < 0.001, ; figure 1), showing attenuation of fear rating towards phobic imagery from session 1 to session 2 (t28 = 3.60, p < 0.001, Cohen's d = 0.67), but no change in fear ratings towards neutral imagery between sessions (t28 = 0.23, p = 0.82). In addition, significant main effects were found for both session (F1,28 = 7.3, p = 0.012, ) and stimulus (F1,28 = 98.8, p < 0.001, ). Similarly, a significant stimulus × session interaction (F1,26 = 8.3, p = 0.008, ; figure 1) was observed for mean SCR, showing decreased SCR to phobic (t26 = 2.62, p = 0.014, Cohen's d = 0.51), but not to neutral imagery (t26 = 0.87, p = 0.39) between sessions. Again, a significant main effect was found for session (F1,26 = 5.3, p = 0.029, ), showing a general habituation of SCRs between sessions, as well as a main effect for stimulus (F1,26 = 25.5, p < 0.001, ). See electronic supplementary material, tables S2 and S3 for descriptive SCR data. Square root transformed range-corrected SCR data generally produced larger effects (see electronic supplementary materials for results, tables S4 and S5 for descriptive data). Vividness ratings for mental imagery of spiders did not change significantly between sessions (p > 0.05, Wilcoxon signed rank test).
Figure 1.
Bar charts depict (a) subjective fear ratings (N = 29) and (b) mean skin conductance responses (N = 27) to mental imagery of phobic stimuli (spiders) and neutral stimuli (gloves) during the experimental procedure in sessions 1 and 2. Error bars represent standard error of mean. *p < 0.05, **p < 0.001.
(c). Brain activity: whole-brain analyses
(i). Imaginal exposure to phobic stimuli (phobic > neutral)
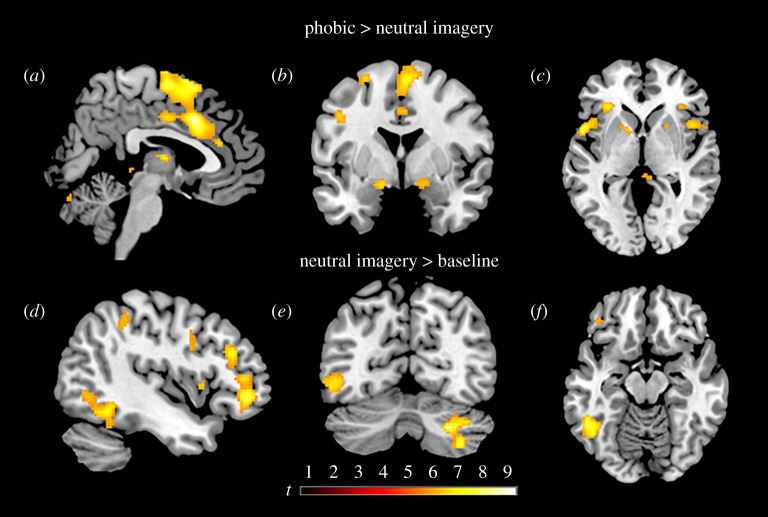
Whole-brain analyses revealed that imaginal exposure to phobic stimuli (phobic > neutral) activated multiple brain areas, including the insula, thalamus, amygdala (extending into the hippocampus), midcingulate cortex (MCC), striatum, supplementary motor area (SMA) and cerebellum (table 2 and figure 2). Neutral imagery (neutral > phobic) did not produce higher activity than phobic imagery in any brain area. To limit the length of the table, only results with a cluster size of greater than or equal to 5 voxels were reported (for significant results with a cluster size of less than or equal to 5, see electronic supplementary material, table S6).
Table 2.
Neural activity during exposure to phobic imagery (phobic > neutral imagery). L, left hemisphere; R, right hemisphere; n.a., region not defined in the automated anatomical labelling brain atlas.
| MNI coordinates |
pFWE | Z | no. voxels | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| frontal lobe | ||||||
| middle frontal gyrus L | −30 | 46 | 18 | 0.003 | 5.31 | 91 |
| −32 | 32 | 36 | 0.030 | 4.76 | 5 | |
| inferior frontal gyrus L (triangular) | −42 | 12 | 24 | 0.009 | 5.03 | 64 |
| precentral R | 42 | −6 | 44 | 0.014 | 4.94 | 26 |
| precentral L | −36 | −10 | 50 | 0.002 | 5.34 | 147 |
| −26 | 0 | 64 | 0.007 | 5.10 | 44 | |
| limbic areas | ||||||
| midcingulate cortex L (extending into supplementary motor area) | −4 | 16 | 36 | <0.001 | 6.38 | 1883 |
| insula L | −30 | 24 | 8 | <0.001 | 6.12 | 488 |
| insula R | 46 | 12 | −2 | 0.003 | 5.31 | 194 |
| amygdala L (extending into putamen, pallidum, hippocampus olfactory and caudate) | −16 | −2 | −14 | 0.004 | 5.23 | 109 |
| amygdala R | 16 | 2 | −14 | 0.007 | 5.09 | 43 |
| basal ganglia | ||||||
| thalamus L | −2 | −10 | 10 | 0.001 | 5.52 | 74 |
| putamen R | 22 | 12 | 0 | 0.029 | 4.76 | 9 |
| parietal lobe | ||||||
| precuneus R | 10 | −38 | 6 | 0.012 | 4.98 | 32 |
| precuneus L | −12 | −72 | 54 | 0.033 | 4.73 | 6 |
| supramarginal | −62 | −38 | 26 | 0.039 | 4.69 | 10 |
| cerebellum | ||||||
| R | 26 | −62 | −28 | <0.001 | 5.97 | 432 |
| 16 | −60 | −46 | 0.001 | 5.45 | 81 | |
| 12 | −70 | −22 | 0.017 | 4.90 | 42 | |
| 6 | −84 | −22 | 0.018 | 4.88 | 24 | |
| L | −38 | −56 | −30 | 0.004 | 5.21 | 115 |
| n.a. | 20 | −16 | −12 | 0.014 | 4.93 | 12 |
Figure 2.
Results from whole-brain statistical parametrical mapping overlaid on a standard brain. Images are displayed at p < 0.05 family-wise error-corrected statistical threshold. Top panel depicts increased activity during imaginal exposure to phobic stimuli (phobic > neutral imagery) including the (a) left midcingulate gyrus, supplementary motor area, (b) bilateral amygdala and (c) bilateral insula. Bottom panel shows activity in areas associated with mental imagery production (neutral imagery > baseline) including (d) left dorsolateral and orbitofrontal cortex, (e) right cerebellum and (f) left inferior temporal gyrus. (Online version in colour.)
(ii). Mental imagery production (neutral > baseline)
Mental imagery production (neutral > baseline) activated visual areas in temporal and occipital cortices, as well as areas associated with motor control, language and attention (figure 2; electronic supplementary material, table S7).
(d). Associations between brain activity, skin conductance responses and subjective fear ratings
SCRs to phobic imagery (phobic > neutral) were positively associated with activity in left middle frontal gyrus (MNI x, y, z: −34, 46, 6; Z = 4.92, pFWE = 0.017, 56 mm3). Increased activity in an overlapping area was associated with greater reductions in SCRs to phobic stimuli from session 1 to session 2 (MNI x, y, z: −34, 46, 6; Z = 4.68, pFWE = 0.044, 16 mm3).
A region in the medial orbitofrontal cortex (mOFC) was negatively associated with SCR during phobic imagery (MNI x, y, z: −20, 38, −12; Z = 4.85, pFWE = 0.023, 40 mm3), but did not predict between-sessions change in SCR. Finally, subjective fear ratings were positively correlated with activity within the cerebellum (left: MNI x, y, z: −14, −44, −16; Z = 4.97, pFWE = 0.014, 72 mm3; right: MNI x, y, z: 14, −46, −24; Z = 4.87, pFWE = 0.021, 64 mm3). Change in subjective fear ratings from session 1 to session 2 was not associated with any brain region in whole-brain analyses.
(e). Brain activity: nucleus accumbens region of interest analysis
The NAcc was more extensively activated during phobic than neutral imagery (left: MNI x, y, z: −12, 6, −12; Z = 4.85, pFWE < 0.001, 416 mm3; right: MNI x, y, z: 10, 6, −12; Z = 3.05, pFWE = 0.02, 8 mm3). In addition, NAcc activity during exposure to phobic imagery (phobic > neutral) was positively associated with SCR (MNI x, y, z: −6, 8, −12; Z = 3.16, pFWE = 0.0015, 88 mm3) and to change in SCR (phobic > neutral) between sessions (left: MNI x, y, z: −6, 6, −12; Z = 2.98, pFWE = 0.024, 72 mm3; right: MNI x, y, z: 4, 10, −6; Z=2.82, pFWE = 0.037, 24 mm3).
4. Discussion
The current study explored the neural basis of imaginal exposure with individuals fearful of spiders, using a novel experimental procedure consisting of repeated exposure to flashpoint mental imagery to phobic and neutral stimuli. Results revealed that generating internal mental images of phobic stimuli evoked intense subjective fear, elevated SCRs, and activated fear-processing brain regions. In line with the notion that mental imagery was driving emotion, the vividness of mental imagery and subjective fear during the procedure were positively associated. Importantly, the present study showed that the single 10 min session promoted long-term reductions (ca one week) in fear at both the subjective and physiological levels. That is, current findings indicate that the novel experimental procedure was a successful experimental analogue of imaginal exposure.
(a). The neural basis of imaginal exposure
Whole-brain analyses showed that exposure to phobic imagery (phobic > neutral) activated so-called emotion-processing brain areas, including the amygdala, hippocampus, insula, MCC and thalamus. Notably, even though no direct comparisons were performed, imaginal exposure to phobic stimuli (i.e. mental imagery of spiders) activated similar brain areas to those previously shown to be activated during in vivo exposure (i.e. the direct perception of spiders) [18,20]. Current findings are also in line with emerging evidence on reduction of conditioned fear (i.e. laboratory-acquired fear), where imaginal and in vivo extinction appear to involve overlapping neural circuity [23], and partly extend such findings to naturally occurring phobic fear. Our results of increased activity in insula and cingulate cortex during phobic imagery are consistent with studies of specific phobia [18,20], script-driven-imagery studies of PTSD (e.g. [21]), and also neural activity observed in healthy individuals during mental imagery of emotional pictures [42].
The brain area most extensively activated by phobic imagery was the anterior part of the MCC. The MCC has widespread connections across cortical and subcortical areas [43,44] and seems to subserve several emotion-related functions [45], including the appraisal of emotional stimuli and initiation of associated attentional and motor responses [46,47]. The MCC has been suggested to integrate interoceptive information from the insula and external sensory information from thalamic and amygdala projections, in order to direct attention and motor responses via its connections to lateral prefrontal cortices and SMA [46,47]. Although no causal conclusions can be drawn from the present data, the large activation observed in the MCC and accompanying activations in the insula, thalamus, amygdala, middle frontal gyrus and the SMA is in line with this proposed neural circuitry, and consistent with the idea that the emotional processing of external and internal (i.e. imagined) stimuli shares similar neural circuitry [23,48]. The MCC has also been associated with top-down emotional processing, including conscious threat appraisal [49,50] and effortful emotion regulation [47,51]. For instance, the MCC is consistently activated during instructed fear-conditioning [50] and is reliably activated during reappraisal, a cognitive emotion regulation strategy [51]. It is possible that the observed activation in this area could also represent the conscious appraisal of phobic imagery and/or top-down emotion regulation during imaginal exposure.
In whole-brain analyses, the only area associated with a reduction in SCR between sessions was located in the middle frontal gyrus, in the ventral part of the dorsolateral prefrontal cortex (dlPFC; BA 46). This structure is recruited during top-down emotion regulation [52,53]. In line with this finding, a previous study found that activity in the dlPFC during an imagery-based emotion regulation task was associated with emotion regulation success [53]. Another structure that may have a regulatory influence on SCR is the mOFC, as activity in this area was negatively associated with SCRs during session 1. However, activation in mOFC was not associated with a change in physiological response between sessions.
The only brain area associated with subjective fear ratings found in whole-brain analyses was located in the cerebellum. The positive association between subjective fear and cerebellar activity is consistent with findings indicating a role for the cerebellum in emotion [54]. For instance, lesions in the cerebellum have been associated with blunting of affect and deficiencies in the subjective evaluation of affective states [55].
ROI analyses revealed increased activity in the NAcc during imaginal exposure (phobic > neutral), which was associated with concurrent SCRs, and also predicted the change in SCRs between sessions, i.e. higher activity during exposure was associated with larger reductions in SCR. These findings are in good agreement with results from Reddan et al. [23] suggesting the involvement of the NAcc in the reduction of fear achieved through imaginal exposure, and extend their findings from conditioned fear to phobic fear.
In summary, brain-imaging findings revealed that imaginal exposure recruits brain areas that have been associated with emotion processing [46]. Results are in line with previous findings on conditioned fear, which indicated that imaginal and in vivo extinction may employ similar neurocircuitry [23]. In addition, they suggest that imaginal exposure might recruit brain areas associated with top-down emotional processes, such as the MCC [50] and dlPFC [52]. However, further research is required to disentangle the specific role that these brain areas play in imaginal exposure and the reduction of fear.
(b). The effects of 10 min of exposure to flashpoint mental imagery on fear responses
Imaginal exposure in clinical practice is usually delivered for prolonged durations (e.g. 40 min) [9]. Some evidence indicates that briefer exposure sessions may be as effective (20–30 min) [14,28,29]. The findings of the current study demonstrated that even a single and short (10 min) session containing brief exposures to flashpoint mental imagery alone (without other treatment components) was accompanied by longer-term reductions in fear responses over one week.
No within-session extinction was observed, presumably because imagery grew progressively more fearful throughout the procedure (i.e. was graded within that session). This is perhaps unsurprising since extensive evidence shows that within-session extinction does not necessarily predict therapeutic outcome between sessions, i.e. long-term fear reduction does not require that patients' fear responses are attenuated within an exposure session [56]. Current results thus are consistent with the idea that briefer imaginal exposure sessions can reduce symptoms and that within-session habituation is not necessary for treatment effects in anxiety disorders. Between-sessions fear reduction to phobic stimuli could, to some extent, reflect a repetition effect, as participants had gone through the same experimental procedure once before. The change in the experimental context between sessions 1 and 2 (MR scanner versus laboratory) could also have influenced the change in fear responses—fear responses can increase if the feared stimulus is presented in a context different from that where extinction took place (renewal) [57]. In the present study, because there was a decrease in fear responses between sessions, no renewal effect could be noted. However, we cannot exclude that a renewal effect was partly counteracting the fear reduction.
The present findings could hold clinical implications. The use of short exposure sessions of repeated flashpoint (7 s) imagery could potentially add a way to expand imaginal exposure treatment techniques. For instance, it could make imaginal exposure more accessible to patients, by demanding a lower level of attentional resources/mental imagery skills compared with prolonged imagery of longer durations. Besides, the use of flashpoint imagery facilitates repeated exposure to more numerous (imagined) instances of the feared object/event (e.g. different types of spiders) as well as contextual modifications (encountering spider at home/on the lawn/shower). Exposure to multiple contexts may aid retention [58] and generalization of treatment effects, as these effects are often context-specific [1]. Brief sessions of repeated exposure to flashpoint imagery could potentially also improve scalability by facilitating the incorporation of the mechanistically important imaginal exposure component within internet treatment protocols and could expand patient choice in terms of personalized approaches [17]. Future studies should seek to test the replication of our findings and explore implications. It would be of future interest to explore the clinical viability of flashpoint imagery in imaginal exposure, e.g. dose–response effects, generalizability, tolerability and long-term effects.
(c). Limitations
The present study has several limitations that should be considered. Because the primary aim of the study was to characterize neural activations of imaginal exposure, we did not include an in vivo exposure control condition, which precluded direct comparisons between these exposure types. An interesting future direction would be to include an in vivo contrast to compare the impact of imaginal and in vivo exposure on neural activity, as well as contrast the effects of the brief exposure session using visual or imagined phobic stimuli. In the present study, neural activity (fMRI) was only measured in session 1. Hence, it was not possible to examine whether fear reduction observed between sessions in behavioural measures was accompanied by a change in neural activity a week later. Future studies should also examine brain activity in a follow-up session. Finally, a test of replication of the current findings is needed using preregistration, a priori sample size calculation based on results from the current study, and so forth.
5. Conclusion
This is to our knowledge the first experimental study that has investigated neural processing during (not after) imaginal exposure to phobic fear, revealing the neural underpinnings of naturally occurring fear created internally within the mind's eye. Critically, results showed that, solely by generating an internal mental image of a phobic stimulus and in the absence of the percept (i.e. imaginal rather than in vivo exposure), neural activity can be elicited in brain areas associated with fear-processing. A second key finding relates to the nature of exposure duration, that is, how long the treatment technique needs to last to have an impact on fear responses. In the clinic, imaginal exposure is usually delivered for prolonged durations (ca 40 min). This study demonstrates that a single 10 min session of brief exposures to flashpoint mental imagery (7 s) can lead to lasting reductions (one week) in phobic fear at both the subjective and physiological levels.
Supplementary Material
Acknowledgements
We thank Staffan Holmlöv, Isabel Sörensen and Wilhem Duse for their contribution to data collection.
Ethics
Ethical approval was granted by the Swedish Ethical Review Authority – Uppsala (2017/419) to T. A. Written and informed consent was obtained from all participants.
Data accessibility
Data and training protocols are available at Open Science Framework (https://osf.io/6dc5h/).
Authors' contributions
J.M.H. made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, as well as drafting the article. E.A.H. made substantial contributions to conception and design, and interpretation of data, and revised the manuscript critically for important intellectual content. T.A. made substantial contributions to conception and design, acquisition of data, analysis, and interpretation of data, and revised the manuscript critically for important intellectual content. All authors approved the final version of the manuscript.
Competing interests
The other authors declare no conflict of interest.
Funding
The study was funded with support from the Swedish Research Council (grant no. 2014-01335). E.A.H. received support from the Swedish Research Council (VR 2017-00957), the Lupina Foundation and the Oak Foundation (OCAY-18-442) during the conduct of the study.
References
- 1.Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. 2014. Maximizing exposure therapy: an inhibitory learning approach. Behav. Res. Ther. 58, 10–23. ( 10.1016/j.brat.2014.04.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofmann SG, Smits JAJ. 2008. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. J. Clin. Psychiatry 69, 621–632. ( 10.4088/JCP.v69n0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolpe J. 1968. Psychotherapy by reciprocal inhibition. Cond. Reflex 3, 234–240. ( 10.1007/BF03000093) [DOI] [PubMed] [Google Scholar]
- 4.Kosslyn SM, Ganis G, Thompson WL. 2001. Neural foundations of imagery. Nat. Rev. Neurosci. 2, 635–642. ( 10.1038/35090055) [DOI] [PubMed] [Google Scholar]
- 5.Foa EB, Kozak MJ. 1986. Emotional processing of fear. Exposure to corrective information. Psychol. Bull. 99, 20–35. ( 10.1037/0033-2909.99.1.20) [DOI] [PubMed] [Google Scholar]
- 6.Lang PJ. 1979. A bio-informational theory of emotional imagery. Psychophysiology 16, 495–512. ( 10.1111/j.1469-8986.1979.tb01511.x) [DOI] [PubMed] [Google Scholar]
- 7.Hackmann A, Bennett-Levy J, Holmes E. 2011. Oxford guide to imagery in cognitive therapy. Oxford, UK: Oxford University Press. [Google Scholar]
- 8.Foa EB, McLean CP. 2016. The efficacy of exposure therapy for anxiety-related disorders and its underlying mechanisms: the case of OCD and PTSD. Annu. Rev. Clin. Psychol. 12, 1–28. ( 10.1146/annurev-clinpsy-021815-093533) [DOI] [PubMed] [Google Scholar]
- 9.Foa EB, Hembree EA, Rothbaum BO. 2007. Prolonged exposure therapy for PTSD : emotional processing of traumatic experiences: therapist guide. Oxford, UK: Oxford University Press. [Google Scholar]
- 10.Bryant RA, Moulds ML, Guthrie RM, Dang ST, Nixon RDV. 2003. Imaginal exposure alone and imaginal exposure with cognitive restructuring in treatment of posttraumatic stress disorder. J. Consult. Clin. Psychol. 71, 706–712. ( 10.1037/0022-006X.71.4.706) [DOI] [PubMed] [Google Scholar]
- 11.Zoellner LA, Telch M, Foa EB, Farach FJ, McLean CP, Gallop R, Bluett EJ, Cobb A, Gonzalez-Lima F. 2017. Enhancing extinction learning in posttraumatic stress disorder with brief daily imaginal exposure and methylene blue: a randomized controlled trial. J. Clin. Psychiatry 78, e782–e789. ( 10.4088/JCP.16m10936) [DOI] [PubMed] [Google Scholar]
- 12.Agren T, Björkstrand J, Fredrikson M. 2017. Disruption of human fear reconsolidation using imaginal and in vivo extinction. Behav. Brain Res. 319, 9–15. ( 10.1016/j.bbr.2016.11.014) [DOI] [PubMed] [Google Scholar]
- 13.James JE. 1986. Review of the relative efficacy of imaginal and in vivo flooding in the treatment of clinical fear. Behav. Psychother. 14, 183–191. ( 10.1017/S0141347300014725) [DOI] [Google Scholar]
- 14.Hecker JE. 1990. Emotional processing in the treatment of simple phobia: a comparison of imaginal and in vivo exposure. Behav. Psychother. 18, 21–34. ( 10.1017/S0141347300017961) [DOI] [Google Scholar]
- 15.Vervliet B, Craske MG, Hermans D. 2013. Fear extinction and relapse: state of the art. Annu. Rev. Clin. Psychol. 9, 215–248. ( 10.1146/annurev-clinpsy-050212-185542) [DOI] [PubMed] [Google Scholar]
- 16.Bradley R, Greene J, Russ E, Dutra L, Westen D. 2005. A multidimensional meta-analysis of psychotherapy for PTSD. Am. J. Psychiatry 162, 214–227. ( 10.1176/appi.ajp.162.2.214) [DOI] [PubMed] [Google Scholar]
- 17.Holmes EA, et al. 2018. The Lancet Psychiatry Commission on psychological treatments research in tomorrow's science. Lancet Psychiatry 5, 237–286. ( 10.1016/S2215-0366(17)30513-8) [DOI] [PubMed] [Google Scholar]
- 18.Ipser JC, Singh L, Stein DJ. 2013. Meta-analysis of functional brain imaging in specific phobia. Psychiatry Clin. Neurosci. 67, 311–322. ( 10.1111/pcn.12055) [DOI] [PubMed] [Google Scholar]
- 19.Helpman L, et al. 2016. Neural changes in extinction recall following prolonged exposure treatment for PTSD: a longitudinal fMRI study. NeuroImage Clin. 12, 715–723. ( 10.1016/j.nicl.2016.10.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentili C, Messerotti Benvenuti S, Lettieri G, Costa C, Cecchetti L. 2019. ROI and phobias: the effect of ROI approach on an ALE meta-analysis of specific phobias. Hum. Brain Mapp. 40, 1814–1828. ( 10.1002/hbm.24492) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanius RA, Frewen PA, Girotti M, Neufeld RWJJ, Stevens TK, Densmore M. 2007. Neural correlates of trauma script-imagery in posttraumatic stress disorder with and without comorbid major depression: a functional MRI investigation. Psychiatry Res. Neuroimaging 155, 45–56. ( 10.1016/j.pscychresns.2006.11.006) [DOI] [PubMed] [Google Scholar]
- 22.Mineka S, Oehlberg K. 2008. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol. 127, 567–580. ( 10.1016/j.actpsy.2007.11.007) [DOI] [PubMed] [Google Scholar]
- 23.Reddan MC, Wager TD, Schiller D. 2018. Attenuating neural threat expression with imagination. Neuron 100, 994–1005.e4. ( 10.1016/j.neuron.2018.10.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rauch SL, Van Der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. 1996. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch. Gen. Psychiatry 53, 380–387. ( 10.1001/archpsyc.1996.01830050014003) [DOI] [PubMed] [Google Scholar]
- 25.Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. 2005. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol. Psychiatry 57, 832–840. ( 10.1016/j.biopsych.2004.12.025) [DOI] [PubMed] [Google Scholar]
- 26.Pearson DG, Deeprose C, Wallace-Hadrill SMA, Heyes SB, Holmes EA. 2013. Assessing mental imagery in clinical psychology: a review of imagery measures and a guiding framework. Clin. Psychol. Rev. 33, 1–23. ( 10.1016/j.cpr.2012.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes EA, Grey N, Young KAD. 2005. Intrusive images and ‘hotspots’ of trauma memories in posttraumatic stress disorder: an exploratory investigation of emotions and cognitive themes. J. Behav. Ther. Exp. Psychiatry 36, 3–17. ( 10.1016/j.jbtep.2004.11.002) [DOI] [PubMed] [Google Scholar]
- 28.Van Minnen A, Foa EB. 2006. The effect of imaginal exposure length on outcome of treatment for PTSD. J. Traum. Stress 19, 427–438. ( 10.1002/jts.20146) [DOI] [PubMed] [Google Scholar]
- 29.Nacasch N, Huppert JD, Su YJ, Kivity Y, Dinshtein Y, Yeh R, Foa EB. 2015. Are 60-minute prolonged exposure sessions with 20-minute imaginal exposure to traumatic memories sufficient to successfully treat PTSD? A randomized noninferiority clinical trial. Behav. Ther. 46, 328–341. ( 10.1016/j.beth.2014.12.002) [DOI] [PubMed] [Google Scholar]
- 30.Klorman R, Weerts TC, Hastings JE, Melamed BG, Lang PJ. 1974. Psychometric description of some specific-fear questionnaires. Behav. Ther. 5, 401–409. ( 10.1016/S0005-7894(74)80008-0) [DOI] [Google Scholar]
- 31.Fredrikson M. 1983. Reliability and validity of some specific fear questionnaires. Scand. J. Psychol. 24, 331–334. ( 10.1111/j.1467-9450.1983.tb00507.x) [DOI] [PubMed] [Google Scholar]
- 32.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, 22–33. [PubMed] [Google Scholar]
- 33.American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders, 5th edn. Arlington, VA: American Psychiatric Association. [Google Scholar]
- 34.Spielberger CD, et al. 1983. Manual for the state-trait anxiety inventory. Palo Alto, CA: Mind Garden. [Google Scholar]
- 35.Andrade J, May J, Deeprose C, Baugh SJ, Ganis G. 2014. Assessing vividness of mental imagery: the Plymouth Sensory Imagery Questionnaire. Br. J. Psychol. 105, 547–563. ( 10.1111/bjop.12050) [DOI] [PubMed] [Google Scholar]
- 36.Gratz KL, Roemer L. 2004. Multidimensional assessment of emotion regulation and dysregulation. J. Psychopathol. Behav. Assess. 26, 41–54. ( 10.1023/B:JOBA.0000007455.08539.94) [DOI] [Google Scholar]
- 37.Holmes EA, Mathews A. 2005. Mental imagery and emotion: a special relationship? Emotion 5, 489–497. ( 10.1037/1528-3542.5.4.489) [DOI] [PubMed] [Google Scholar]
- 38.Boucsein W. 2012. Electrodermal activity. New York, NY: Springer Science & Business Media. [Google Scholar]
- 39.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19, 1233–1239. ( 10.1016/S1053-8119(03)00169-1) [DOI] [PubMed] [Google Scholar]
- 40.Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, Mitchell TN, Brooks DJ, Duncan JS. 2003. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 19, 224–247. ( 10.1002/hbm.10123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benedek M, Kaernbach C. 2010. A continuous measure of phasic electrodermal activity. J. Neurosci. Methods 190, 80–91. ( 10.1016/j.jneumeth.2010.04.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kosslyn SM, Shin LM, Thompson WL, McNally RJ, Rauch SL, Pitman RK, Alpert NM. 1996. Neural effects of visualizing and perceiving aversive stimuli: a PET investigation. Neuroreport 7, 1569–1576. ( 10.1097/00001756-199607080-00007) [DOI] [PubMed] [Google Scholar]
- 43.Vogt BA. 2016. Midcingulate cortex: structure, connections, homologies, functions and diseases. J. Chem. Neuroanat. 74, 28–46. ( 10.1016/j.jchemneu.2016.01.010) [DOI] [PubMed] [Google Scholar]
- 44.Ray RD, Zald DH. 2012. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev. 36, 479–501. ( 10.1016/j.neubiorev.2011.08.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Etkin A, Egner T, Kalisch R. 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 15, 85–93. ( 10.1016/j.tics.2010.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF. 2012. The brain basis of emotion: a meta-analytic review. Behav. Brain Sci. 35, 121–143. ( 10.1017/S0140525X11000446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Etkin A, Büchel C, Gross JJ. 2015. The neural bases of emotion regulation. Nat. Rev. Neurosci. 16, 693–700. ( 10.1038/nrn4044) [DOI] [PubMed] [Google Scholar]
- 48.Pearson J, Naselaris T, Holmes EA, Kosslyn SM. 2015. Mental imagery: functional mechanisms and clinical applications. Trends Cogn. Sci. 19, 590–602. ( 10.1016/j.tics.2015.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalisch R, Wiech K, Critchley HD, Dolan RJ. 2006. Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. Neuroimage 30, 1458–1466. ( 10.1016/j.neuroimage.2005.11.011) [DOI] [PubMed] [Google Scholar]
- 50.Mechias M-L, Etkin A, Kalisch R. 2010. A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage 49, 1760–1768. ( 10.1016/j.neuroimage.2009.09.040) [DOI] [PubMed] [Google Scholar]
- 51.Kalisch R. 2009. The functional neuroanatomy of reappraisal: time matters. Neurosci. Biobehav. Rev. 33, 1215–1226. ( 10.1016/j.neubiorev.2009.06.003) [DOI] [PubMed] [Google Scholar]
- 52.Gyurak A, Gross JJ, Etkin A. 2011. Explicit and implicit emotion regulation: a dual-process framework. Cogn. Emot. 25, 400–412. ( 10.1080/02699931.2010.544160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delgado MR, Nearing KI, LeDoux JE, Phelps EA. 2008. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 59, 829–838. ( 10.1016/j.neuron.2008.06.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stoodley CJ, Schmahmann JD. 2010. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46, 831–844. ( 10.1016/j.cortex.2009.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmahmann JD, Sherman JC. 1998. The cerebellar cognitive affective syndrome. Brain 121, 561–579. ( 10.1093/brain/121.4.561) [DOI] [PubMed] [Google Scholar]
- 56.Craske MG, Kircanski K, Zelikowsky M, Mystkowski J, Chowdhury N, Baker A. 2008. Optimizing inhibitory learning during exposure therapy. Behav. Res. Ther. 46, 5–27. ( 10.1016/j.brat.2007.10.003) [DOI] [PubMed] [Google Scholar]
- 57.Bouton ME, Bolles RC. 1979. Contextual control of the extinction of conditioned fear. Learn. Motiv. 10, 445–466. ( 10.1016/0023-9690(79)90057-2) [DOI] [Google Scholar]
- 58.Foa EB, Steketee G, Turner RM, Fischer SC. 1980. Effects of imaginal exposure to feared disasters in obsessive-compulsive checkers. Behav. Res. Ther. 18, 449–455. ( 10.1016/0005-7967(80)90010-8) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and training protocols are available at Open Science Framework (https://osf.io/6dc5h/).