Abstract
Opportunistic fungal infections are life-threatening conditions with a high rate of morality, mostly occurring in immunocompromised hosts. We reported the case of mixed mold infection in a 69 year-old patient with latent diabetes mellitus. She was initially admitted for right orbital cellulitis. Cerebro-rhino-orbital mucormycosis and aspergillosis coinfection was diagnosed from mycological testing and histology after nasal biopsy sample. The patient received amphotericin B deoxycholate then voriconazole combined to surgical debridement with a favorable outcome.
Keywords: Mucormycosis, Aspergillosis, Invasive fungal infection, Rhinosinusitis, Diabetes
Introduction
Mucormycosis and aspergillosis are the most frequent fungal infections caused by ubiquitous filamentous fungi [1]. Coinfection in the same host is scarce and has a poor prognosis [[2], [3], [4], [5]]. It commonly affects immunocompromised patients. The most common presentation of invasive fungal infection (IFI) is the rhinocerebral involvement [6]. Rapid and efficient microscopic examination is necessary for diagnosis [1]. Treatment can be medical (azoles or polyenes such as deoxycholate and liposomal amphotericin B) and/or surgical (debridement and necrosectomy) depending on the extension of the infection. We reported a case of cerebro-rhino-orbital mucormycosis (caused by Rhizopus species) with an invasive aspergillosis (caused by Aspergillus flavus) in a patient with uncontrolled diabetes mellitus.
Observation
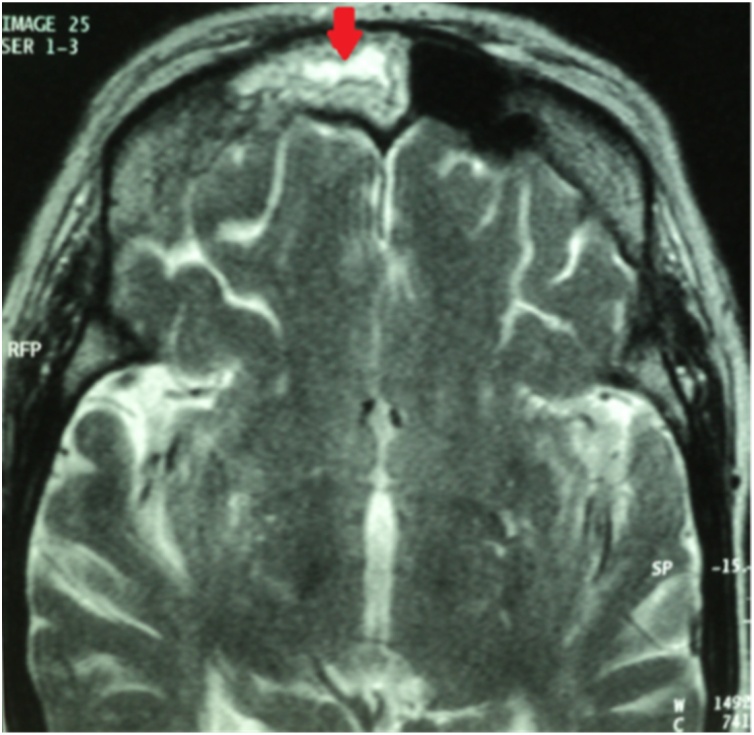
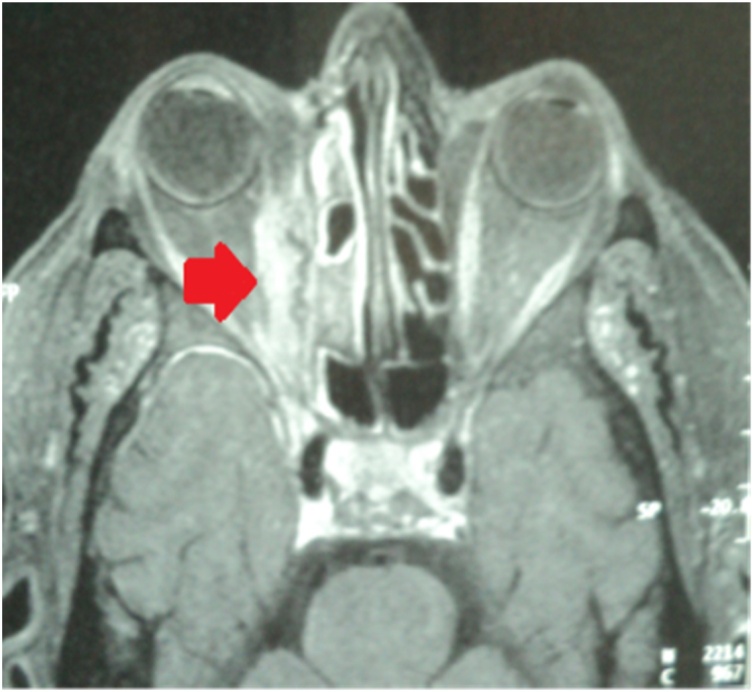
A 69-year-old woman, with a past history of arterial hypertension and diabetes mellitus, presented to emergency room for a right orbital cellulitis. On admission, the patient reported a progressive onset of right periorbital edema associated with ocular pain, chemosis, ophthalmoplegia and fever. Blood cultures were negative. On day 2, brain and facial Computed Tomography (CT) and Brain Magnetic Imaging (MRI) showed right frontal tissue filling (Fig. 1) and disorganized ethmoidal cell architecture with intra-orbital extension (Fig. 2). Treatment was begun empirically with antimicrobial drugs (cefotaxime [6 g IV infusion/d] and ciprofloxacin [500 mg IV 2x/d]) with no recovery occurred. The confirmation of an IFI due to a filamentous fungus was obtained by the anatomopathological examination of a nasal biopsy sample. The histological examination revealed large non-septate mycelial filaments within necrotic and inflammatory material. These filaments were of the Mucorales order as demonstrated by hematoxylin and eosin staining or better by PAS or Gomori-Grocott staining. Rhinosinusitis mucormycosis with intra-orbital and cerebral extension (optic neuritis, infra-temporal and meningeal fossa and cavernous sinus) was diagnosed. The patient was treated with IV amphotericin B deoxycholate (1 mg/kg daily; liposomal amphotericin B was not available in our facility) combined to surgical debridement.
Fig. 1.
Facial MRI (sagittal section) showing right frontal tissue filling in heterogeneous T2 hypersignal (red arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Fig. 2.
Facial MRI (sagittal Section) showing flling of the ethmoidal cells with an intra-orbital contrast grafting (red arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
On day 15, the patient presented with a foul purulent rhinorrhea. Anterior rhinoscopy revealed nasal necrotic lesions in the right nasal cavity. A second nasal biopsy was performed. Direct bacteriological exams and culture were negative. A mycological examination with culture isolated Aspergillus flavus. The patient was treated with IV amphotericin B deoxycholate (1 mg/kg daily) in combination with nasal endoscopic lavage (2x/week). On day 21, the patient developed an acute kidney failure and amphotericin B deoxycholate was replaced with voriconazole (loading dose 400 mg [6 mg/kg] twice first day orally then 200 mg [3 mg/kg] twice daily) for 12 weeks. The clinical course was rapidly favorable with the resolution of symptoms. The patient had undergone subsequent brain MRI (on day 90) showing complete resolution of radiological abnormalities. The patient was discharged with a complete recovery.
Discussion
Mucormycosis and aspergillosis are two opportunistic and IFIs that have clinical and para-clinical similarities. They are rarely associated and their diagnosis is challenging, often misleading practitioners [[2], [3], [4], [5]]. These severe infections could be disseminated involving multiple organs and are frequently fatal in immunocompromised patients [1,2,4,7]. This coinfection have commonly been described in patients with hematologic malignancies and severe neutropenia [2,7].
Individuals with diabetes mellitus often develop fungal infections. Aspergillus and Mucorales rapidly invade tissues and spread both locally and systemically in these patients [4,8]. Both pathogens are filamentous fungi. They are ubiquitous in the environment, commonly found in decaying organic substrates including vegetable and animal excreta. There are different species of Aspergillus and Mucorales. The most prevalent ones are Aspergillus fumigatus and Rhizopus species respectively [1]. Contamination is primarly through spores’ inhalation. Acquisition through the cutaneous or percutaneous route also occurs with traumatic disruption of skin barriers, burns, direct injection or catheters. The damage from Aspergillus spp. results from tissue invasion or from inflammatory cells recruited to sites of infection [9]. Whereas vessels’ invasion with tissue necrosis is the key pathophysiological feature of human Mucorales infection [9].
Effective immunological response to infections in patients with uncontrolled diabetes mellitus is compromised. These patients have low granulocyte phagocyte activity with an impaired polymorphonuclear leukocyte cell response. Studies have shown that the capacity of immunocompromised patient’ serum to inhibit Rhizopus in vitro is reduced. On the other hand, acidosis and hyperglycemia constitute an adequate environment for the growth of the fungus. [4]. Co-infection usually occurs in the orofacial area or sinuses. In fact, rhino-cerebral localization predominates in patients with diabetes, pulmonary localization in those with hematologic malignancies, and skin forms in trauma patients [10]. Initial symptoms in rhino-cerebral and oro-facial are similar to common causes of sinusitis. The underlying disease of patients such as diabetes often masks the symptoms.
Direct mycological and histological examinations remain the gold standard for diagnosis. Mucor presents large, broad non-septate hyphae with right-angle branching, and Aspergillus spp. shows septate hyphae that branch at 45° angles [11]. A CT scan is crucial for identification of the bony destruction. MRI provides early detection of meningeal or intra-parenchymatous spread and intracranial vascular occlusion. Delayed diagnosis or inappropriate treatment may result in massive tissue destruction and possible extension into the cranial base and/or vault and orbit [5]. The principles of management are complete treatment of underlying medical disease, correction of hypoxia, acidosis, hyperglycemia, and electrolyte abnormalities [4].
Treatments of these IFIs include surgical resection of the affected tissues in association with intravenous antifungal agents. Amphotericin B is active against most agents of mucormycosis and also against aspergillosis [12,13]. ‘Posaconazole can also be used as one of currently available azoles with activity against several agents of mucormycosis’ [13]. Moreover, it has less adverse effects than a long-term voriconazole therapy [12,13]. Isavuconazole, an other ‘somewhat new’ azole with mold activity could be an excellent alternative in both invasive mucor and aspergillus infection. Isavuconazole was non-inferior to voriconazole for the primary treatment of suspected invasive mold disease and was well tolerated [14]. This triazole with an oral and/or IV formulation also showed activity against mucormycosis with efficacy similar to amphotericin B [15].
In rhinosinusitis, surgical debridement of infected tissues is essential and should be urgently performed to limit the aggressive spread of infection to contiguous structures. In our case, the patient was treated with IV amphotericin B replaced with voriconazole due to acute kidney failure after 3 weeks. Voriconazole is drug of choice for aspergillosis; however, it has zero mold activity and therefore would not have treated the mucor. This speaks to the importance of surgical debridement in mucormycosis compared to antifungals alone.
Prolonged, and in some cases, lifelong, secondary prophylaxis may also be necessary. Relapses could occur especially if there is a tissue sequestrum.
Conclusion
There are only a few case reports in medical literature reporting a concomitant rhinosinusitis mucormycosis and aspergillosis infection. Treatment usually combines medical and surgical approaches, often including extended necrosectomies, although the prognosis of generalized fungal infections is very poor.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
No written informed consent was requested in this case report as there are no identifying marks or features and no patient identifiers in the images or accompanying text
Contributors
SZ, AZ collected clinical data and drafted the manuscript. LA, BK and HTB revised the final manuscript.
Declaration of Competing Interest
None.
Acknowledgement
None.
References
- 1.Boroujeni Z.B., Shamsaei S., Yarahmadi M., Getso M.I., Salimi-Khorashad A., Haghighi L. Distribution of invasive fungal infections: molecular epidemiology, etiology, clinical conditions, diagnosis and risk factors: a 3-year experience with 490 patients under intensive care. Microb Pathog. 2020;104616 doi: 10.1016/j.micpath.2020.104616. [DOI] [PubMed] [Google Scholar]
- 2.Boras Vv, Jurlina M., Brailo V., Vuković K.Đ, Rončević P., Kinda Sb. Oral mucormycosis and aspergillosis in the patient with acute leukemia. Acta Stomatol Croat. 2019;53(3):274. doi: 10.15644/asc53/3/9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davoudi S., Anderlini P., Fuller G.N., Kontoyiannis D.P. A long-term survivor of disseminated Aspergillus and Mucorales infection: an instructive case. Mycopathologia. 2014;178(5–6):465–470. doi: 10.1007/s11046-014-9785-x. [DOI] [PubMed] [Google Scholar]
- 4.Rit K., Saha R., Dey R., Barik G. Rhino-oculo-cerebral aspergillus and mucor co-infections in an immunocompromised patient with type 2 diabetes mellitus. Med J Dr Patil Univ. 2014;7(4):486. [Google Scholar]
- 5.Maiorano E., Favia G., Capodiferro S., Montagna Mt, Lo Muzio L. Combined mucormycosis and aspergillosis of the oro-sinonasal region in a patient affected by Castleman disease. Virchows Arch Int J Pathol. 2005;446(1):28–33. doi: 10.1007/s00428-004-1126-x. [DOI] [PubMed] [Google Scholar]
- 6.Candoni A., Klimko N., Busca A., Di Blasi R., Shadrivova O., Cesaro S. Fungal infections of the central nervous system and paranasal sinuses in onco-haematologic patients. Epidemiological study reporting the diagnostic-therapeutic approach and outcome in 89 cases. Mycoses. 2019;62(3):252–260. doi: 10.1111/myc.12884. [DOI] [PubMed] [Google Scholar]
- 7.Safai Nodeh S., Dehghan Manshadi S., Jahanbin B., Khodaveisi S., Giasvand F., Seifi A. Invasive fungal consecutive infections in a patient with acute myeloid leukaemia. Niger J Clin Pract. 2019;22(4):582. doi: 10.4103/njcp.njcp_359_17. [DOI] [PubMed] [Google Scholar]
- 8.Mahadevaiah A.H., Rajagopalan N., Patil M. Coinfection of pulmonary mucormycosis and aspergillosis presenting as bilateral vocal cord palsy. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-009615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chermetz M., Gobbo M., Rupel K., Ottaviani G., Tirelli G., Bussani R. Combined orofacial aspergillosis and mucormycosis: fatal complication of a recurrent paediatric glioma-case report and review of literature. Mycopathologia. 2016;181(9–10):723–733. doi: 10.1007/s11046-016-0021-8. [DOI] [PubMed] [Google Scholar]
- 10.Pozo Laderas J.C., Pontes Moreno A., Pozo Salido C., Robles Arista J.C., Linares Sicilia M.J. [Disseminated mucormycosis in immunocompetent patients: a disease that also exists] Rev Iberoam Micol. 2015;32(2):63–70. doi: 10.1016/j.riam.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Arndt S., Aschendorff A., Echternach M., Daemmrich T.D., Maier W. Rhino-orbital-cerebral mucormycosis and aspergillosis: differential diagnosis and treatment. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2009;266(1):71–76. doi: 10.1007/s00405-008-0692-y. [DOI] [PubMed] [Google Scholar]
- 12.Maiorano E., Favia G., Capodiferro S., Montagna Mt, Lo Muzio L. Combined mucormycosis and aspergillosis of the oro-sinonasal region in a patient affected by Castleman disease. Virchows Arch. 2005;446(1):28–33. doi: 10.1007/s00428-004-1126-x. [DOI] [PubMed] [Google Scholar]
- 13.Guinea J., Verweij P.E., Meletiadis J., Mouton J.W., Barchiesi F., Arendrup M.C. How to: EUCAST recommendations on the screening procedure E.DEf 10.1 for the detection of azole resistance in Aspergillus fumigatus isolates using four-well azole-containing agar plates. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2019;25(6):681–687. doi: 10.1016/j.cmi.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Maertens J.A., Raad I.I., Marr K.A., Patterson T.F., Kontoyiannis D.P., Cornely O.A. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet Lond Engl. 2016;387(10020):760–769. doi: 10.1016/S0140-6736(15)01159-9. [DOI] [PubMed] [Google Scholar]
- 15.Marty F.M., Ostrosky-Zeichner L., Cornely O.A., Mullane K.M., Perfect J.R., Thompson G.R. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16(7):828–837. doi: 10.1016/S1473-3099(16)00071-2. [DOI] [PubMed] [Google Scholar]