Abstract
Aims
Angiotensin‐converting enzyme‐2 (ACE2) is the receptor for SARS‐CoV‐2. Animal studies suggest that renin–angiotensin–aldosterone system (RAAS) blockers might increase the expression of ACE2 and potentially increase the risk of SARS‐CoV‐2 infection.
Methods and Results
The effect of ACE inhibitor (ACEI) treatment on the pneumonia incidence in non‐COVID‐19 patients (25 studies, 330 780 patients) was associated with a 26% reduction of pneumonia risk (odds ratio [OR]: 0.74, P < .001). Pneumonia‐related death cases in ACEI‐treated non‐COVID‐19 patients were reduced by 27% (OR: 0.73, P = .004). However, angiotensin II receptor blockers (ARB) treatment (10 studies, 275 621 non‐COVID‐19 patients) did not alter pneumonia risk in patients. Pneumonia‐related death cases in ARB‐treated non‐COVID‐19 patients was analysed only in 1 study and was significantly reduced (OR, 0.47; 95% confidence interval, 0.30 to 0.72). Results from 11 studies (8.4 million patients) showed that the risk of getting infected with the SARS‐CoV‐2 virus was reduced by 13% (OR: 0.87, P = .014) in patients treated with ACEI, whereas analysis from 10 studies (8.4 million patients) treated with ARBs showed no effect (OR, 0.92, P = .354). Results from 34 studies in 67 644 COVID‐19 patients showed that RAAS blockade reduces all‐cause mortality by 24% (OR = 0.76, P = .04).
Conclusion
ACEIs reduce the risk of getting infected with the SARS‐CoV‐2 virus. Blocking the RAAS may decrease all‐cause mortality in COVID‐19 patients. ACEIs also reduce the risk of non‐COVID pneumonia. All‐cause mortality due to non‐COVID pneumonia is reduced by ACEI and potentially by ARBs.
Keywords: ACE inhibitors, ACE2, angiotensin II receptor blockers, SARS‐CoV‐2
1. INTRODUCTION
Patients with cardiovascular and renal diseases are frequently treated with drugs interfering with the renin–angiotensin–aldosterone system (RAAS). The clinical benefit of angiotensin‐converting enzyme (ACE) inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) are well established and hence became part of treatment guidelines for these patients worldwide. ACE2 is an isoenzyme of ACE1 (ACE). Both are essential parts of the RAAS (Figure 1). ACE2 is involved in cardiac function, the development of hypertension and diabetes mellitus. Also, ACE2 has been identified as a functional receptor for coronaviruses, including SARS‐CoV and SARS‐CoV‐2. SARS‐CoV‐2 infection is triggered by the binding of the spike protein of the virus to ACE2.
FIGURE 1.
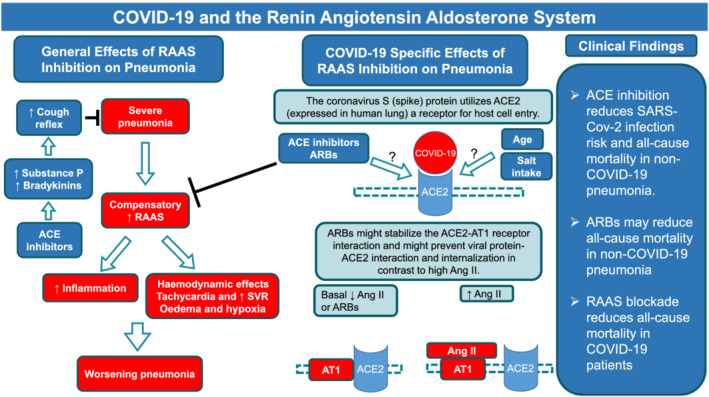
In patients with severe pneumonia, there is likewise a compensatory activation of the RAAS, resulting in tachycardia and an elevation of SVR that may be deleterious in this setting. Tachycardia, which shortens the duration of diastole, impairs the filling of the left ventricle. An elevated SVR increases left ventricular afterload (wall stress), increasing myocardial oxygen demand. These changes can lead to a further increase in left ventricular end‐diastolic pressure and more edema formation. To the degree that pulmonary edema results in hypoxia, there maybe a further worsening of myocardial function. Besides these haemodynamic effects of an activated RAAS in critically ill patients with pneumonia, an activated RAAS promotes also inflammation in the lung and heart likewise contributing to impaired heart and lung function in these patients. This might explain our finding that all‐cause mortality in non‐COVID‐19 pneumonia patients was significantly reduced when blocking the RAAS in general either with ACEI or ARBs. ACEIs do have an additional effect that might be of clinical impact. They increase levels of substance P and bradykinins which can sensitise the sensory nerves of the airways and enhance the cough reflex which may have a protective role on the tracheobronchial tree. ACE2 is expressed in human lungs and COVID‐19 spike (S) protein seems to use it as a cellular entry receptor. It is still a research question whether age and the use of ACE inhibitors and/or ARBs could impact on ACE2 expression and consequently affect the infection pattern of COVID‐19. Another aspect is that ARBs might stabilize the ACE2‐AT1 receptor interaction and might prevent viral S protein‐ACE2 interaction and internalization. Clinical data indicated that SARS‐CoV‐2 infection related myocarditis and heart failure may negatively influence outcome of SARS‐CoV‐2 pneumonia. ACE inhibitor treatment reduces the risk of pneumonia and pneumonia related mortality, whereas ARBs do not reduce the risk of pneumonia in non‐COVID‐19 patients. RAAS blockade reduces severe adverse clinical outcomes and all‐cause mortality in COVID‐19 patients. RAAS = renin–angiotensin–aldosterone system, SVR = systemic vascular resistance; Ang = angiotensin, ACE = angiotensin converting enzyme, AT1 receptors = angiotensin 1 receptors, ARBs = angiotensin receptor blockers, MCRA = mineralocorticoid receptor antagonists, COVID‐19 = coronavirus disease 19
It was suggested that patients with cardiac and renal diseases, hypertension, and diabetes, who are treated with drugs potentially increasing ACE2 expression in the lungs such as ACEIs or angiotensin II receptor blockers are at higher risk for getting infected as well as having severe COVID‐19 infection. 1 , 2 In the current review, we summarize the molecular evidence for this hypothesis (see Figure 1 and supplementary material: Molecular Background); next, we present results of a meta‐analysis of studies analysing the effects of RAAS blocking drugs (ACEIs, ARBs) on the risk of getting and dying from pneumonia in non‐COVID‐19 patients and compare these findings to the so far published evidence coming from COVID‐19 studies. In the COVID‐19 studies, we meta‐analysed the risk of getting infected with the SARS‐CoV‐2 virus, the risk of having severe adverse clinical outcomes and risk of all‐cause mortality in COVID‐19 patients treated with either ACEIs or ARBs.
2. METHODS
2.1. Data sources and search strategy
A systematic literature search was conducted to identify studies investigating the association between ACEIs or ARBs and pneumonia or COVID‐19 in PubMed, Embase (searches using OVID), The Cochrane Central Register of Controlled Trials (CENTRAL), and Clinical trial.gov. The last search was updated on 7 September 2020. The following MeSH terms were used: “angiotensin‐converting enzyme inhibitors” or “angiotensin receptor antagonists” or “mineralocorticoid receptor antagonists” and “pneumonia”. The key words used for the search strategy are listed in the supplementary materials. The references cited in the retrieved studies were hand‐searched for the collection of missing relevant studies.
2.2. Study selection and quality assessment
Two reviewers independently screened titles and abstracts, and further assessed the full text of each potentially relevant study to determine eligibility for inclusion. Reviews, congress reports, case reports, animal experiments and publications in languages other than English were excluded.
We considered the incidence of pneumonia in all adult patients, irrespective of risk factors at baseline, as the primary outcome. Every case of pneumonia considered in our investigation was either a new case, a recurrent case or a hospital‐acquired pneumonia. Diagnosis of pneumonia was based on clinical, radiological or microbiological criteria, or International Classification of Disease codes. We did not consider undefined data or data on upper respiratory tract infections or radiation pneumonitis. The secondary outcome was pneumonia‐related mortality, including fatal pneumonia or in‐hospital death or 30‐day mortality. All of these secondary outcomes had to be caused primarily by pneumonia rather than other co‐existing comorbid conditions. 3 All relevant clinical studies (randomized–controlled trials [RCTs], cohort studies, case–control studies and nested case–control studies, as well as case‐crossover studies) with ACEIs or ARBs as interventions and with an incidence of pneumonia were considered.
The diagnosis of COVID‐19 must be proven by detection of SARS‐CoV‐2 RNA in the patient's upper or lower respiratory tract system. Treatment with RAAS blocking agents was defined as treatment with either an ACEI or an ARB or both (just 3 patients in 1 study). COVID‐19 related adverse severe clinical outcomes are defined as admission to the intensive care unit, the use of assisted ventilation or death. However, we only include peer‐reviewed articles; considering the current situation, some observation studies are online available without careful peer review, and thus the quality might raise concerns.
To avoid considering duplicated published data, we excluded the earlier publications conducted in the same study cohort and only considered the latest publications. In our investigation, the treatment group was defined as being treated with any kind of ARBs or any kind of ACEIs. The control group was defined as being treated with a placebo or any other cardiovascular drug such as calcium‐channel blockers or β‐blockers. Cohort studies had to follow patients to determine pneumonia outcomes. In case–control studies, cases had to be patients with a diagnosis of pneumonia. Controls should be randomly selected to match the cases. A nested case–control study is a variation of a case–control study in which cases and controls are drawn from the population in a fully predefined cohort. In a case–crossover study, as described, 4 the study population consists of subjects who have experienced an episode of pneumonia. Similar to a crossover trial, each study subject serves as their own control.
The methodological quality of RCTs was evaluated using the Cochrane Collaboration's tool for RCTS with the following parameters: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, selective reporting. The quality of included cohort and case control studies was assessed using the Newcastle–Ottawa scale, which has 3 aspects including 8 criteria and yield scores ranging from 0 (high risk of bias) to 9 (low risk of bias). Studies with Newcastle–Ottawa scale scores <6/9 (considered moderate‐to‐high risk of bias) were excluded (Table S1).
Overall quality of evidence was evaluated using the Grading of Recommendations, Assessment, and Evaluation (GRADE) framework. 5
2.3. Data extraction and analysis
Two independent authors extracted the following data from full‐text articles: study design and size, location, population characteristics, primary outcomes, data of relevant outcomes. Data were obtained irrespective of whether they had been reported as predefined outcomes or as adverse effects. We chose the odds ratio (OR) as the measurement estimate for effect because relative estimates are better comparable than absolute effects across studies with different designs, populations, and lengths of follow‐up as described previously. 6 We aimed to extract the maximally adjusted OR that included the greatest number of covariates from the original publication for predescribed outcomes. Otherwise, we used the raw data to convert to crude OR through classic methods, or Peto's method if 1 arm had a zero‐count cell. 7 We used the hazard ratio (HR) when OR was not available nor possible to calculate. To explore differences in estimates for outcomes, we presented the results stratified according to study design. Considering the potential risk of bias, subgroup analysis in which compare results from the adjusted and unadjusted studies was also conducted. Studies that met the inclusion criteria but could not be pooled due to insufficient data were summarized qualitatively. Either fixed‐effects model or, in the presence of heterogeneity, the random‐effects method was used in the pooled results. Data were expressed as OR and 95% confidence intervals (95% CIs). Heterogeneity across studies was assessed by testing with the I2‐statistic, considering 25%, 50% and 75% as an indication of low, moderate, and high variability, respectively. 8
Funnel‐plot analysis and Begg test were performed to evaluate potential publication bias. All analyses were performed using Stata/SE version 14.0 (StataCorp LP, College Station, Texas, USA) and RevMan version5.3.5 (Nordic Cochrane Centre, Cochrane Collaboration, 2014). Tests were 2‐sided, and a P‐value <.05 was considered statistically significant.
3. RESULT
3.1. Literature search & description of studies
We identified 2894 potentially relevant studies using the search terms described in the method section. After removing duplicates and screening titles and abstracts, a total of 2611 publications were excluded. Of the remaining 283 publications, 200 were excluded after full‐text examination based on our in‐ and exclusion criteria. Overall, 83 studies included in qualitative synthesis, and 82 studies included in quantitative synthesis, including 51 cohort studies, 12 RCTs, 14 case–control studies, 3 nested case–control studies and 2 case‐crossover studies (Figure S1).
As for the primary and secondary pneumonia‐related outcomes, totally 36 studies were included. 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 All the RCTs were multicentre, except 1 done by Hou et al. 34 Seven of them compared ACEIs with controls, 9 , 10 , 11 , 12 , 13 , 34 , 35 4 compared ARBs with controls. 41 , 42 , 43 , 44 Regarding demographic distribution, 2 were done worldwide, 13 , 44 4 in Europe 9 , 10 , 12 , 43 (Germany, Czech Republic and Slovakia, Austria, Denmark and Italy), 4 in Asia 11 , 34 , 35 , 42 (China, Japan), and 1 study was located in both Europe and the USA. 41 Among observational studies, 10 were carried out in Asia, 14 , 15 , 16 , 17 , 18 , 20 , 21 , 24 , 32 , 33 10 in the USA and Canada, 19 , 22 , 23 , 28 , 29 , 30 , 31 , 36 , 37 , 40 5 in Europe, 25 , 26 , 27 , 38 , 39 18 studies were retrospective and 7 were prospective. Twenty studies evaluated ACEIs, 6 ARBs. Tables S2–4 summarize the main characteristics of the included studies.
With regard to the COVID‐19‐infected patients, 48 studies 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 published clinical data whether ACEIs and ARBs are associated with COVID‐19 infection or clinical outcomes in patients with COVID‐19 in above mentioned databases. One of them was a global study, 92 however, this study was retracted on 4 June 2020 with concern about the quality of the information in the database, thus we only qualitatively summarized this study.
The included studies consist of 37 cohort studies, 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 85 , 86 , 87 , 88 , 89 , 91 9 case–control studies 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 90 and 1 RCTs. 84 23 of them were conducted in Asia, 46 , 47 , 48 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 58 , 60 , 61 , 64 , 66 , 68 , 71 , 72 , 77 , 79 , 80 , 82 , 83 18 in Europe, 63 , 67 , 69 , 70 , 73 , 74 , 75 , 76 , 78 , 81 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 and 6 in USA 45 , 49 , 57 , 59 , 62 , 65 (main characteristics of the included studies were summarized in Table S5).
3.2. Primary outcomes: Incidence of pneumonia
The effect of ACEI treatment on the incidence of pneumonia was analysed in 25 studies (a total of 330 780 patients coming from 5 RCTs, 9 , 10 , 11 , 12 , 13 7 cohort studies, 14 , 15 , 16 , 17 , 18 , 19 , 20 8 case–control studies, 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 3 nested case–control studies 29 , 30 , 31 and 2 case‐crossover studies 32 , 33 ). Overall, the use of ACEIs was associated with a significant 26% reduction in risk of pneumonia compared with controls (pooled OR, 0.74, 95% CI, 0.65 to 0.85, P < .001; I2 = 76.9%; for further details see Table 1).
TABLE 1.
Renin–angiotensin–aldosterone system inhibitors and risk of non‐SARS‐CoV‐2 pneumonia infection
| Non‐SARS‐CoV‐2 pneumonia infection | OR (95% CI) | P value |
|---|---|---|
| ACE inhibitors (25, n = 330 780) * | 0.74 (0.65, 0.85) | <.0001 |
| Cohort studies (7) | 0.46 (0.35, 0.62) | <.0001 |
| Case–control studies (8) | 0.72 (0.60, 0.88) | .001 |
| Randomized controlled trial (5) | 0.78 (0.54, 1.14) | .200 |
| Nested case–control studies (3) | 1.03 (0.97, 1.10) | .360 |
| Case‐crossover studies (2) | 0.86 (0.67, 1.10) | .234 |
| Adjusted risk factors ORs studies (15) | 0.80 (0.70, 0.92) | .001 |
| Crude ORs studies (5) | 0.39 (0.27, 0.56) | <.0001 |
| ARBs (10, n = 275 621) | 0.90 (0.79, 1.02) | .108 |
| Cohort studies (1) | 0.52 (0.36, 0.76) | .001 |
| Case–control studies (1) | 0.48 (0.17, 1.36) | .168 |
| Randomized controlled trial (4) | 0.84 (0.72, 0.98) | .031 |
| Nested case–control studies (2) | 1.01 (0.93, 1.09) | .794 |
| Case‐crossover studies (2) | 1.01 (0.88, 1.15) | .933 |
| Adjusted risk factors ORs studies (6) | 0.91 (0.77, 1.07) | .265 |
number of studies and population. OR = odds ratio; 95% CI = 95% confidence interval; ACE = angiotensin converting enzyme; ARBs = angiotensin receptor blockers.
The effect of ARB treatment on the incidence of pneumonia was analysed in 10 studies (a total of 275 621 patients from 4 RCTs, 41 , 42 , 43 , 44 1 cohort study, 19 1 case–control study, 28 2 nested case–control studies 30 , 31 and 2 case‐crossover studies. 32 , 33 Pooled results showed that the risk of pneumonia was not significantly different between patients who did or did not use ARBs (pooled OR, 0.90, 95% CI, 0.79 to 1.02, P = .11; I2 = 53.3%). However, 2 individual study types revealed a potential effect of ARBs on the risk of pneumonia. The odds ratios were 0.84 (95% CI, 0.72 to 0.98, P = .03; I2 = 0%) in RCTs and 0.52 (95% CI, 0.36 to 0.76, P = .001) in the cohort study, respectively (Table 1).
3.3. Secondary outcome: Pneumonia‐related mortality
Data of pneumonia‐related deaths were available in 10 studies: 1 comparing ARBs with control summarized qualitatively; 40 9 studies comparing ACEIs with controls (4 RCTs; 11 , 13 , 34 , 35 and 5 cohort studies 37 , 38 , 39 , 40 , 93 ) were included in the meta‐analysis.
Pooled results showed that ACEIs were associated with a significant 27% reduction in risk of pneumonia‐related mortality (OR, 0.73, 95% CI, 0.59 to 0.90, P = .004; I2 = 60.1%) compared with controls (Table 2).
TABLE 2.
Renin–angiotensin–aldosterone system inhibitors and risk of non‐SARS‐CoV‐2 related all‐cause mortality
| Non‐SARS‐CoV‐2 related all‐cause mortality | OR (95% CI) | P value |
|---|---|---|
| ACE inhibitors (9, n = 35 727) * | 0.73 (0.59, 0.90) | .004 |
| Cohort studies (5) | 0.71 (0.57, 0.88) | .002 |
| Randomized controlled trial (4) | 0.83 (0.31, 2.17) | .697 |
| Adjusted risk factors ORs studies (5) | 0.73 (0.59, 0.90) | .002 |
| ARB (1, n = 22 996) | 0.47 (0.30, 0.72) | ‐ |
| Cohort studies (1) | 0.47 (0.30, 0.72) | Not given |
number of studies and population. OR = odds ratio; 95% CI = 95% confidence interval; ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker.
For ARBs, a meta‐analysis for the secondary end‐point mortality was not possible due to the lack of enough eligible studies. Mortensen et al., 40 conducted the only eligible study providing data on ARB and pneumonia‐related mortality. They showed, in a cohort of 22 996 patients where 839 subjects were treated with ARBs, that treatment with ARBs reduced the pneumonia‐related mortality (OR, 0.47, 95% CI, 0.30 to 0.72).
3.4. Mineralocorticoid receptor antagonists
We did not find any eligible studies addressing the effects of mineralocorticoid receptor antagonists on pneumonia or pneumonia‐related death.
3.5. RAAS inhibitors and risk of COVID‐19 infection
Pooled result from 11 studies, 45 , 49 , 60 , 73 , 76 , 78 , 79 , 80 , 84 , 90 , 91 12 cohorts (143 696 patients) showed that the risk of getting infected (whatever degree of disease—from no symptoms to severe adverse clinical outcomes) was not associated with the treatment of overall RAAS blockade (ACEIs or ARBs; OR, 1.04, 95% CI, 0.94 to 1.14, P = .47; I2 = 71.0%; Figure 2).
FIGURE 2.
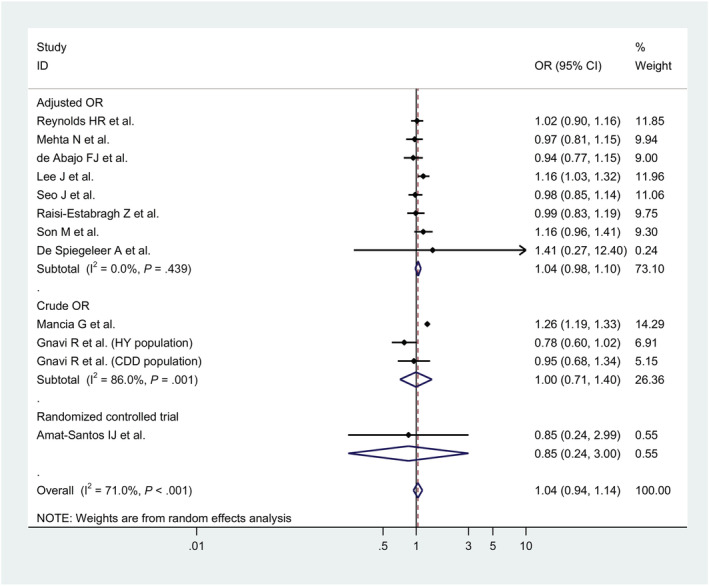
Forest plots for association between renin–angiotensin–aldosterone system inhibitors and risk of COVID‐19 infection
However, use of ACEIs alone was associated with a significant 13% reduction in risk of COVID‐19 positive compared with controls (OR, 0.87, 95% CI, 0.78 to 0.97, P = .014; I2 = 73.5%). Similar results were obtained from subgroup of cohort study (OR, 0.82, 95% CI, 0.70 to 0.94, P = .006; I2 = 67.8%) and studies with adjusted odd ratio (OR, 0.87, 95% CI, 0.77 to 0.98, P = .026; I2 = 80.8%; Table 3).
TABLE 3.
Renin–angiotensin–aldosterone system (RAAS) inhibitors and risk of COVID‐19 infection
| COVID‐19 infection | OR (95% CI) | P value |
|---|---|---|
| RAAS inhibitors (11, n = 143 696) * | 1.04 (0.94, 1.14) | .467 |
| Cohort studies (5) | 1.05 (0.98, 1.13) | .170 |
| Case–control studies (5) | 1.02 (0.87, 1.20) | .787 |
| Randomized controlled trial (1) | 0.85 (0.24, 3.00) | .801 |
| Adjusted risk factors ORs studies (8) | 1.04 (0.98, 1.10) | .196 |
| Crude ORs studies (2) | 1.00 (0.71, 1.40) | .988 |
| ACE inhibitors (10, n = 8 405 242) | 0.87 (0.78, 0.97) | .014 |
| Cohort studies (4) | 0.82 (0.70, 0.94) | .006 |
| Case–control studies (5) | 0.94 (0.87, 1.01) | .081 |
| Randomized controlled trial (1) | 0.85 (0.24, 3.00) | .801 |
| Adjusted risk factors ORs studies (8) | 0.87 (0.77, 0.98) | .026 |
| Crude ORs studies (1) | 0.90 (0.73, 1.10) | .310 |
| ARBs (9, n = 8 405 326) | 0.92 (0.77, 1.10) | .354 |
| Cohort studies (4) | 0.85 (0.59, 1.22) | .371 |
| Case–control studies (5) | 0.98 (0.89, 1.06) | .569 |
| Adjusted risk factors ORs studies (8) | 0.94 (0.76, 1.15) | .549 |
| Crude ORs studies (1) | 0.84 (0.70, 1.01) | .064 |
number of studies and population. COVID‐19 = coronavirus disease 19; OR = odds ratio; 95% CI = 95% confidence interval; ACE = angiotensin converting enzyme; ARBs = angiotensin receptor blockers.
It is of interest to mention that even if we excluded a huge population based cohort study 87 (n = 8.3 million), which showed a significant beneficial effect of ACEIs or ARB treatment on COVID‐19 positive, the pooled odd ratio for the treatment of ACEIs showed the consistent result even when we excluded this study (OR, 0.92, 95% CI, 0.87 to 0.98, P = .012; I2 = 0.0%).
3.6. RAAS inhibitors and risk of all‐cause mortality in COVID‐19 patients
Furthermore, 34 studies 46 , 47 , 48 , 49 , 51 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 77 , 79 , 80 , 82 , 83 , 84 including 67 644 patients showed that the risk of all‐cause mortality among ACEIs/ARBs users was significantly reduced when compared to COVID‐19 patients without ACEIs/ARBs treatment (OR 0.76, 95% CI, 0.59 to 0.99, P = .04; I2 = 88%; Figure 3). When we only considered studies with adjusted odd ratios, treatment with RAAS inhibitors was associated with a significant 31% reduction in risk of COVID‐19 related mortality compared with controls (OR, 0.81, 95% CI, 0.65 to 0.99, P = .04; I2 = 73.1%; Table 4).
FIGURE 3.
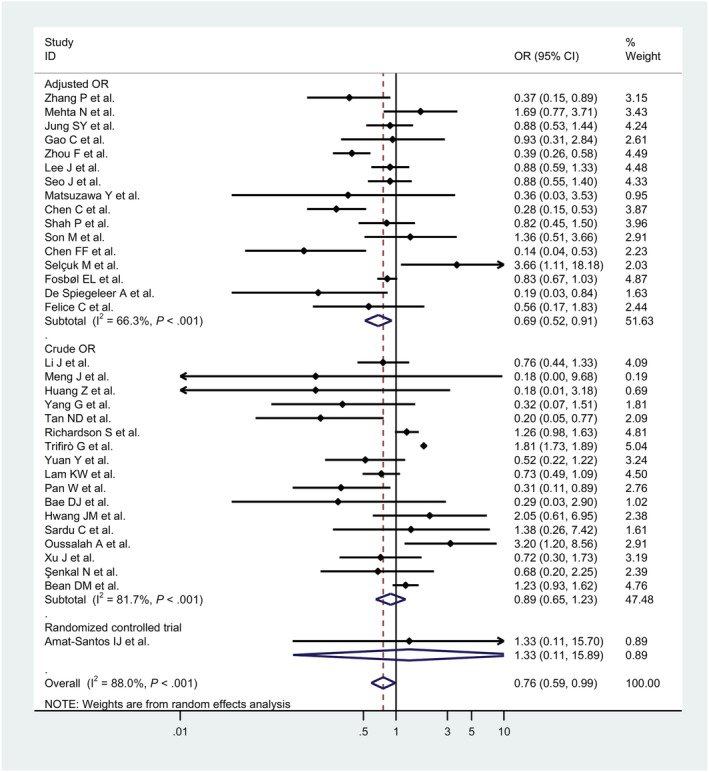
Forest plots for association between renin–angiotensin–aldosterone system inhibitors and risk of all‐cause mortality in COVID‐19 patients
TABLE 4.
Renin–angiotensin–aldosterone system (RAAS) inhibitors and COVID‐19 all‐cause mortality
| COVID‐19 all‐cause mortality | OR (95% CI) | P value |
|---|---|---|
| RAAS inhibitors (34, n = 67 644) | 0.76 (0.59, 0.99) | .040 |
| Cohort studies (28) | 0.75 (0.57, 1.00) | .047 |
| Case–control studies (5) | 0.80 (0.40, 1.60) | .531 |
| Randomized controlled trial (1) | 1.33 (0.11, 15.89) | .822 |
| Adjusted risk factors ORs studies (16) | 0.69 (0.52, 0.91) | .010 |
| Crude ORs studies (18) | 0.89 (0.65, 1.23) | .488 |
| ACE inhibitors (16, n = 21 163) | 1.03 (0.90, 1.16) | .683 |
| Cohort studies (13) | 1.04 (0.91, 1.18) | .598 |
| Case–control studies (2) | 0.63 (0.30, 1.33) | .225 |
| Randomized controlled trial (1) | 1.33 (0.11, 15.89) | .822 |
| Adjusted risk factors ORs studies (7) | 1.02 (0.85, 1.22) | .817 |
| Crude ORs studies (9) | 0.97 (0.76, 1.23) | .791 |
| ARBs (14, n = 20 283) | 0.90 (0.70, 1.16) | .416 |
| Cohort studies (12) | 0.85 (0.65, 1.12) | .250 |
| Case–control studies (2) | 1.31 (0.66, 2.58) | .442 |
| Adjusted risk factors ORs studies (7) | 0.92 (0.72, 1.19) | .526 |
| Crude ORs studies (7) | 0.91 (0.51, 1.60) | .734 |
number of studies and population. COVID‐19 = coronavirus disease 19; OR = odds ratio; 95% CI = 95% confidence interval; ACE = angiotensin converting enzyme; ARBs = angiotensin receptor blockers.
3.7. RAAS inhibitors and risk of COVID‐19 related severe adverse clinical outcomes
Pooled result from 40 studies, 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 a total of 78 960 patients, showed a nonsignificant 11% reduction in risk of COVID‐19 related severe adverse clinical outcomes (admission to the intensive care unit, the use of assisted ventilation or death) associated with use of RAAS inhibitors (OR, 0.89, 95% CI, 0.78 to 1.01, P = .076; I2 = 71.0%; Figure 4).
FIGURE 4.
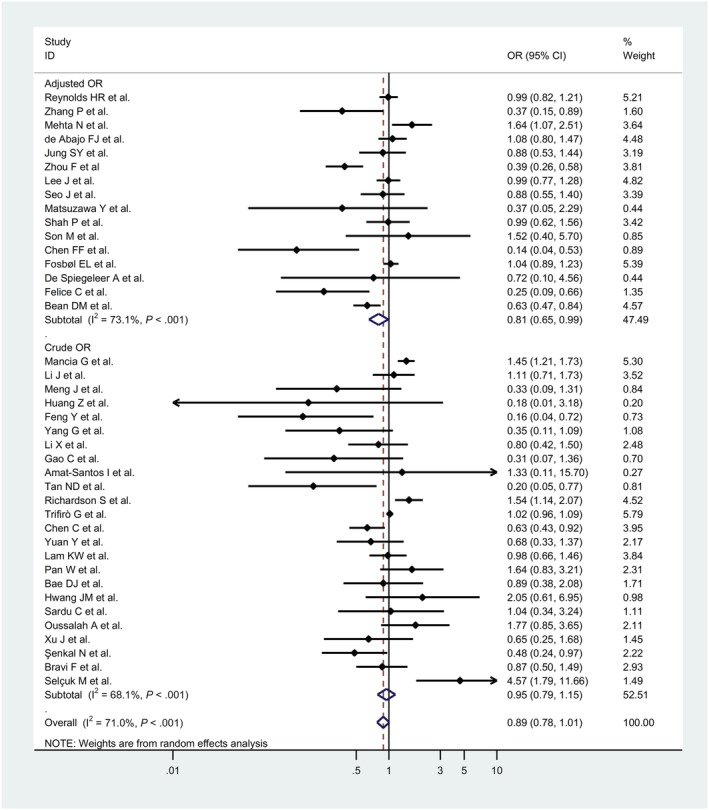
Forest plots for association between renin–angiotensin–aldosterone system inhibitors and COVID‐19 related severe adverse clinical outcomes defined as admission to the intensive care unit, the use of assisted ventilation, or death
In the subgroup analysis, subgroup of cohort studies (69 091 patients) revealed that RAAS inhibitors significantly reduced the risk of COVID‐19 related adverse clinical outcome (OR, 0.82, 95% CI, 0.71 to 0.95, P = .008; I2 = 69.7%). Moreover, the subgroup which only considered adjusted odd ratios (25 096 patients) also showed a significant reduction (OR, 0.81, 95% CI, 0.65 to 0.99, P = .04; I2 = 73.1%; Table S6).
The differentiation analysis of ACEIs and ARBs treatment showed the same trend as RAAS inhibitors, ACEIs (OR, 0.95, 95% CI, 0.85 to 1.06, P = .34; I2 = 53.0%) and ARB (OR, 0.93, 95% CI, 0.82 to 1.05, P = .24; I2 = 59.4%). However, these associations were not significant.
3.8. Publication bias
For the primary outcome of pneumonia referring to RAAS inhibitors, funnel plots did not show apparent visual asymmetry. The Begg tests showed no evidence of publication bias (ACEI, P = .66; ARB, P = .21). For the secondary outcome, funnel plot also did not show any visual asymmetry; testing for publication bias showed no statistic significant result (Begg's P‐value for asymmetry >.999).
As for the COVID‐19 studies referring to RAAS inhibitors, funnel‐plot showed a qualitatively asymmetrical shape for mortality, but not for severity and infection. The Begg test showed a borderline significance of publication bias for COVID‐19‐related severity outcome (P = .05), and a significant publication bias for mortality (P = .02), but no indication of publication bias for infection (P = .84). Referring to ACEIs, funnel‐plot showed the same direction as RAAS inhibitors. The Begg test showed no indication of publication bias for severity outcome, mortality and infection (P = .29; 0.10; 0.84, respectively). For the COVID‐19 studies referring to ARBs, the funnel‐plot showed a qualitatively asymmetrical shape for infection. The Begg test showed no indication of publication bias for severity outcome, mortality and infection (P = .26; 0.66; 0.28, respectively). There are more studies contributing to the favourable effect, indicating possibility of publication bias.
3.9. Quality/certainty of the evidence
The quality/certainty of the evidence regarding the impact of RAAS blockers in patients with SARs‐CoV‐2 and non‐SARS‐CoV‐2 lung infection is summarized in Table 5, according to certainty of the evidence (GRADE). 5
TABLE 5.
Summary of renin–angiotensin–aldosterone system (RAAS) inhibitors treatment effects for each outcome measure and GRADE quality of evidence
| Outcomes | No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Relative effect, OR (95% CI) | Certainty of evidence (GRADE) |
|---|---|---|---|---|---|---|---|---|---|
| Non‐SARS‐CoV‐2 pneumonia infection | |||||||||
| ACEIs | 20 | Observational studies | Not serious | Very serious a | Not serious | Not serious | All plausible residual confounding would reduce the demonstrated effect. | 0.74 (0.64, 0.85) | ⊕◯◯◯ VERY LOW |
| 5 | Randomized controlled trial | Not serious | Serious b | Serious | Serious c | ‐ | 0.78 (0.54, 1.14) | ⊕◯◯◯ VERY LOW | |
| ARBs | 6 | Observational studies | Not serious | Serious b | Not serious | Not serious | All plausible residual confounding would reduce the demonstrated effect. | 0.91 (0.77, 1.07) | ⊕⊕◯◯ LOW |
| 4 | Randomized controlled trial | Not serious | Not serious | Serious | Not serious | ‐ | 0.84 (0.72, 0.98) | ⊕⊕⊕◯ MODERATE | |
| All‐cause mortality in non‐SARS‐CoV‐2 pneumonia patients | |||||||||
| ACEIs | 5 | Observational studies | Not serious | Serious b | Not serious | Not serious | All plausible residual confounding would reduce the demonstrated effect. | 0.71 (0.57, 0.88) | ⊕⊕◯◯ LOW |
| 4 | Randomized controlled trial | Not serious | Serious b | Serious | Very serious c | ‐ | 0.83 (0.31, 2.71) | ⊕◯◯◯ VERY LOW | |
| SARS‐CoV‐2 infection | |||||||||
| RAAS blockers | 10 | Observational studies | Not serious | Serious b | Not serious | Not serious | All plausible residual confounding would reduce the demonstrated effect. | 1.04 (0.94, 1.14) | ⊕◯◯◯ VERY LOW |
| 1 | Randomized controlled trial | Not serious | Not serious | Not serious | Very serious c | ‐ | 0.85 (0.24, 3.00) | ⊕⊕◯◯ LOW | |
| ACEIs | 9 | Observational studies | Not serious | Serious b | Not serious | Not serious | All plausible residual confounding would reduce the demonstrated effect. | 0.87 (0.78, 0.97) | ⊕⊕◯◯ LOW |
| 1 | Randomized controlled trial | Not serious | Not serious | Not serious | Very serious c | ‐ | 0.85 (0.24, 3.00) | ⊕⊕◯◯ LOW | |
| ARBs | 9 | Observational studies | Not serious | Very serious a | Not serious | Not serious | All plausible residual confounding would reduce the demonstrated effect. | 0.92 (0.77, 1.10) | ⊕◯◯◯ VERY LOW |
| All‐cause mortality in SARS‐CoV‐2 infection patients | |||||||||
| RAAS blockers | 33 | Observational studies | Serious d | Very serious a | Not serious | Not serious | All plausible residual confounding would reduce the demonstrated effect. | 0.76 (0.59, 0.98) | ⊕◯◯◯ VERY LOW |
| 1 | Randomized controlled trial | Not serious | Not serious | Not serious | Very serious c | ‐ | 1.33 (0.11, 15.89) | ⊕⊕◯◯ LOW | |
| ACEIs | 15 | Observational studies | Serious d | Not serious | Not serious | Not serious | All plausible residual confounding would reduce the demonstrated effect. | 1.01 (0.89, 1.16) | ⊕⊕◯◯ LOW |
| 1 | Randomized controlled trial | Not serious | Not serious | Not serious | Very serious c | ‐ | 1.33 (0.11, 15.89) | ⊕⊕◯◯ LOW | |
| ARBs | 14 | Observational studies | Serious d | Very serious a | Not serious | Serious c | All plausible residual confounding would reduce the demonstrated effect | 0.90 (0.70, 1.16) | ⊕◯◯◯ VERY LOW |
| SARS‐CoV‐2 infection related severe adverse clinical outcomes | |||||||||
| RAAS blockers | 39 | Observational studies | Serious d | Serious b | Not serious | Not serious | All plausible residual confounding would reduce the demonstrated effect | 0.89 (0.78, 1.01) | ⊕◯◯◯ VERY LOW |
| 1 | Randomized controlled trial | Not serious | Not serious | Not serious | Very serious c | ‐ | 1.33 (0.11, 15.89) | ⊕⊕◯◯ LOW | |
| ACEIs | 22 | Observational studies | Serious d | Serious b | Not serious | Not serious | All plausible residual confounding would reduce the demonstrated effect | 0.95 (0.85, 1.06) | ⊕◯◯◯ VERY LOW |
| 1 | Randomized controlled trial | Not serious | Not serious | Not serious | Very serious c | ‐ | 1.33 (0.11, 15.89) | ⊕⊕◯◯ LOW | |
| ARBs | 21 | Observational studies | Serious d | Serious b | Not serious | Not serious | All plausible residual confounding would reduce the demonstrated effect | 0.93 (0.82, 1.05) | ⊕◯◯◯ VERY LOW |
High heterogeneity;
moderate heterogeneity;
95% confidence interval around the pooled estimate of effect includes both no effect and appreciable benefit or appreciable harm (serious level was considered for downgrading is a relative risk reduction or relative risk increase >25%, Very serious level was considered >100%);
failure to adequately control confounding. ACEIs = angiotensin‐converting enzyme inhibitors; ARBs = angiotensin receptor blockers.
4. DISCUSSION
Patients treated with RAAS blocking drugs due to cardiac and renal diseases certainly belong to the patients with the highest risk for SARS‐CoV‐2 related nonfatal and fatal pneumonia. RAAS blocking drugs belong to drug classes with the clearest proven clinical benefit to patients with cardiac and renal diseases. Given that ACE2 is the receptor for the SARS‐CoV‐2 virus mediating entry to human cells, drugs that might affect the expression of ACE2 in humans and hence potentially disease severity are of major concern. The definitive answer whether RAAS blocking drugs are beneficial, neutral or even harmful can only come from adequately powered clinical trials that are currently initiated (NCT04311177 [Losartan for Patients With COVID‐19 Not Requiring Hospitalization] and NCT04312009 [Losartan for Patients With COVID‐19 Requiring Hospitalization]). This will take some time. Only 1 placebo‐controlled trial with RAAS blocking agents has been reported so far. 84 However, this study is too small and had too few fatal events to allow firm conclusions.
Our data (Tables 1, 2, 3, 4) would rather suggest to study in particular ACEIs instead of ARBs in patients with SARS‐CoV‐2 infection. In any case, general practitioners and physicians in hospitals, especially on intensive care units, treating patients with SARS‐CoV‐2 infection ask for guidance now. As is often the case in everyday clinical medicine, one has to critically summarize and weigh the existing facts and then make clinical decisions. This is, therefore, the ultimate goal of our investigation and below the key points for decision making are summarized:
There is currently no doubt among scientists that ACE2 is the functional receptor of SARS‐CoV‐mediated upper and lower respiratory tract infections. 94 Wrapp et al. 95 showed that the COVID‐19 S protein binds ACE2 with a much higher affinity than SARS‐CoV‐2. This could partly explain the high infection rate of this virus.
Some animal studies do suggest that treatment with either ACEIs or ARBs might increase ACE2 expression in the cardiovascular system. Few animal data exist on the pulmonary expression of ACE2 under RAAS blockade. It should also be emphasized that some animal studies found no effect or even an opposite effect on ACE2 expression. 96 , 97 , 98 , 99 , 100 , 101 It is worth mentioning that 1 of these studies showed beneficial effects of ACE inhibition even though pulmonary ACE2 expression increased 101 (for more details see supplementary file Molecular Background).
The human heart and kidney express ACE2, the receptor for SARS‐CoV‐2. Several independent studies have reported that the heart and potentially the kidney seem to be—in addition to the respiratory tract—a primary target of SARS‐CoV‐2 infections leading to clinical signs of myocarditis and heart failure 102 , 103 , 104 , 105 and potentially renal failure. 106 , 107 The RAAS is activated in heart failure patients and RAAS blockade is a clinical mainstay in the treatment of heart failure. 108 , 109 , 110
In patients with severe pneumonia requiring intensive care treatment, there is a compensatory activation of the RAAS and sympathetic nervous systems, resulting in tachycardia and an elevation of systemic vascular resistance that may be deleterious in this setting. 7 Tachycardia, which shortens the duration of diastole, impairs the filling of the left ventricle. An elevated systemic vascular resistance increases left ventricular afterload (wall stress), increasing myocardial oxygen demand. These changes can lead to a further increase in left ventricular end‐diastolic pressure and more oedema formation. To the degree that pulmonary oedema results in hypoxia, there may be a further worsening of myocardial function. Besides these haemodynamic effects of an activated RAAS in critically ill patients with pneumonia, an activated RAAS also promotes inflammation in the lung and heart likewise contributing to impaired heart and lung function in these patients. This might explain our finding that all‐cause mortality in non‐COVID‐19 pneumonia patients (Table 2) was significantly reduced when blocking the RAAS in general either with ACEIs or ARBs. Similar findings were also seen in COVID‐19 patients (Table 4) when analysing both drug classes together. However, when analysing the effects of ACEIs and ARBs separately, a reduction of all‐cause mortality was not seen. This might be explained by the substantially lower number of analysed patients in the ACEI or ARB group, as compared to studies were RAAS blockade was analysed together (Table 4). ACEIs have an additional effect that might be of clinical impact. They increase levels of substance P and bradykinins. Basic science studies showed that bradykinin and substance P sensitize the sensory nerves of the airways and enhance the cough reflex, 111 , 112 , 113 , 114 , 115 which may have a protective role on the tracheobronchial tree. These mechanisms also improve swallowing by avoiding the exposure of the respiratory tree to oropharynx secretions. 115 , 116 Taken together, the pleiotropic effects of ACEIs were suggested to reduce the incidence of pneumonia. This hypothesis is further supported by a meta‐analysis indicating markedly higher pneumonia incidence in subjects with a specific polymorphism in ACE that reduces substance P and bradykinin, both of which drive the cough reflex, 117 also consistent with the notion that airway reflex sensitivity contributes to pulmonary definitions. ARBs do not increase the levels of Substance P or bradykinin and hence do not have this protective effect. This might at least be 1 reason why ACEIs do reduce the risk of getting non‐COVID‐19 pneumonia (Table 1) or being infected with SARS CoV‐2 (Table 3)
Our findings in COVID‐19‐infected patients with regard to RAAS blocking agents are thus in good agreement with findings in non‐COVID‐19‐infected patients with pneumonias. The smaller numbers of analysed COVID‐19 positive patients and the study design of these studies do not allow a differentiation of ACEIs and ARBs effects with regard to severe adverse clinical outcomes and all‐cause mortality. The findings in COVID‐19 patients are for sure less robust compared to the non‐COVID‐19 studies.
Based on the data in non‐COVID‐19 and COVID‐19 patients, we strongly suggest that ACEIs in particular should not be discontinued in patients who need them due to cardiac and/or renal diseases. The evidence that ACEIs might affect the expression of the molecular target in humans for the SARS CoV‐2 virus (ACE2) is controversial and comes from animal experiments. Moreover, the potential implication of an up‐ or downregulation (except for ACE2 knockout mice) of ACE2 on the risk of infection of the lungs with SARS CoV‐2 is not established so far.
The meta‐analysis of all available clinical studies in non‐COVID‐19 patients provides clear‐cut results. ACEIs are beneficial for patients with pneumonia. They reduce the odds for pneumonia and also pneumonia‐related death. The limited experience so far in COVID‐19 patients clearly supports this interpretation.
For ARBs, the situation is less clear. The overall effect in non‐COVID‐19 pneumonia outcomes were neutral (Figure 3). The data in COVID‐19 patients are clear: RAAS blockade—whatever drug class was used—reduces severe adverse clinical outcomes and all‐cause mortality. We do not know for the time being whether ACEIs or ARBs or both compound classes were the drivers of these beneficial effects. Final conclusions can only be made when we have enough studies on this topic separating the effects of ACEIs from ARBs in COVID‐19 patients.
To ensure the quality of our study, we just accepted studies in international journals after successful peer review, preprints were not considered. We even excluded 1 COVID‐19 study published in the New England Journal of Medicine, 92 because the quality of the data was questioned after publication by the senior editor of this journal. 118 , 119
This represents a study limitation that the vast majority of analysed cases of pneumonia in our meta‐analysis had not been caused by SARS‐CoV‐2 infections. Given the current COVID‐19 pandemic, some of the COVID‐19 studies were done under huge time pressure for designing, conducting and also peer reviewing these studies. This explains at least partially some quality concerns (Table 5). The higher heterogeneity in our analysis—in particular in the COVID‐19 studies—is due to differences in the design of the individual studies, different population analysed and the way of statistical analysis of the original studies. Moreover, there was some heterogeneity regarding the controls used in the meta‐analysis due to the definition of control group (the control group was defined as being treated with a placebo or any other cardiovascular drug such as calcium‐channel blockers or β‐blockers) used in our study. In addition, the secondary outcome of the meta‐analysis in non‐COVID‐19 patients was defined as pneumonia‐related mortality. We accepted the following definitions in the individual studies as pneumonia related mortality: fatal pneumonia or in‐hospital death or 30‐day mortality in patients with pneumonia. For COVID‐19, outcome severe adverse clinical events, we accepted admission to the intensive care unit, the use of assisted ventilation, or death or combinations as severe adverse clinical event. These differences due to the particular outcome definitions in the individual studies may likewise increase heterogeneity. Moreover, the baseline morbidity and mortality risk of the patients was different in the individual studies furthermore increasing heterogeneity. Finally, we had no information on the dosages of the used RASS blocking agents—so any analysis of dose‐dependency of the observed effects was not possible.
An investigation of this topic in a meta‐analysis of all so far available studies in non‐SARS‐CoV‐2 pneumonia combined with an analysis of the available studies in COVID‐19 patients is the as of today the best available approach to obtain clinical evidence on this extremely important clinical question unless adequately powered, placebo‐controlled studies addressing this topic as primary outcome in COVID‐19 patients are available. As of today, only 1 RCT was reported. However, this study had by far to less clinical events to be informative. 84
Furthermore, it is justified to assume that factors that determine the progression, severity and course of pneumonia in general are factors that also determine the course of SARS‐CoV‐2 pneumonia. 120 , 121 , 122 This hypothesis is supported by findings of pathological lung alterations in early disease stages of SARS‐CoV‐2 infection. Early histopathological features were non‐specific and included oedema, pneumocyte hyperplasia, focal inflammation and multinucleated giant‐cell formation; findings also seen in early stages of non‐SARS‐CoV‐2 pneumonias. 123 Figure 1 illustrates the basic science and clinical points discussed above.
5. CONCLUSION
Our study provides evidence that the use of ACEIs but not ARBs reduces the risk of getting infected with the SARS CoV‐2 virus. Blocking the RAAS with either ACEI or ARBs may decrease all‐cause mortality in COVID‐19 patients. The lack of adequately powered controlled clinical COVID‐19 studies, however, limits the power of these conclusions.
ACEIs (but not ARBs) reduce the risk of non‐COVID‐19 pneumonia. All‐cause mortality due to non‐COVID‐19 pneumonia is reduced by ACEI and may be reduced by ARBs (the statement for all‐cause mortality for ARBs is just based on 1 study).
Considering the findings in our meta‐analysis as summarized above and the overall very weak evidence in animal studies that the RAAS blockade‐related alterations of the pulmonary ACE2 expression is linked to disease severity, RAAS‐blocking drugs should not be withdrawn in clinical practice. Our findings provide a stimulus for the initiation of further randomized clinical trials investigating ACEIs in comparison to ARBs in COVID‐19 patients to dissect ACEI effects from ARB‐related effects in this population.
COMPETING INTERESTS
The authors have no conflicts of interest to declare.
CONTRIBUTORS
B.H. and B.K.K. conceived and designed the study. C.C., S.Z. and C.F.H. searched the articles and retrieved the data. C.C., S.Z., A.A.H. and C.F.H. performed the statistical analysis. C.C., A.A.H. and B.H. drafted the article. B.H. and B.K.K. supervised the systematic review and meta‐analysis and significantly amended the manuscript. All authors took part in the interpretation of the results and approved the version of the manuscript.
Supporting information
TABLE S1 Newcastle–Ottawa scale of included studies.
TABLE S2 Main characteristics of randomized–controlled trials in the meta‐analysis.
TABLE S3 Main characteristics of cohort studies in the meta‐analysis.
TABLE S4 Main characteristics of case–control studies, nested case–control studies and case–crossover studies in the meta‐analysis.
TABLE S5 Main characteristics of COVID‐19 clinical studies.
TABLE S6 Renin–angiotensin system inhibitors and COVID‐19 severe adverse clinical outcomes.
FIGURE S1 PRISMA 2009 flow diagram of studies through review.
ACKNOWLEDGEMENTS
China Scholarship Council supported C.C. and S.Z.
Open access funding enabled and organized by Projekt DEAL.
Chu C, Zeng S, Hasan AA, Hocher C‐F, Krämer BK, Hocher B. Comparison of infection risks and clinical outcomes in patients with and without SARS‐CoV‐2 lung infection under renin–angiotensin–aldosterone system blockade: Systematic review and meta‐analysis. Br J Clin Pharmacol. 2021;87:2475–2492. 10.1111/bcp.14660
REFERENCES
- 1. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection?. Lancet Respir Med. 2020;8(4):e21. 10.1016/s2213-2600(20)30116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mortensen EM, Coley CM, Singer DE, et al. Causes of death for patients with community‐acquired pneumonia: results from the pneumonia patient outcomes research team cohort study. Arch Intern Med. 2002;162(9):1059‐1064. [DOI] [PubMed] [Google Scholar]
- 4. Maclure M. The case‐crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 2017;185(11):1174‐1183. [DOI] [PubMed] [Google Scholar]
- 5. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Stat Med. 2002;21(11):1575‐1600. [DOI] [PubMed] [Google Scholar]
- 7. Caldeira D, Alarcao J, Vaz‐Carneiro A, Costa J.. Risk of pneumonia associated with use of angiotensin converting enzyme inhibitors and angiotensin receptor blockers: systematic review and meta‐analysis. BMJ. 2012;345:e4260. 10.1136/bmj.e4260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Widimsky J, Kremer HJ, Jerie P, Uhlir O. Czech and Slovak spirapril intervention study (CASSIS). A randomized, placebo and active‐controlled, double‐blind multicentre trial in patients with congestive heart failure. Eur J Clin Pharmacol. 1995;49(1–2):95‐102. [DOI] [PubMed] [Google Scholar]
- 10. Kober L, Torp‐Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin‐converting‐enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril cardiac evaluation (TRACE) study group. N Engl J Med. 1995;333(25):1670‐1676. [DOI] [PubMed] [Google Scholar]
- 11. Lee JS, Chui PY, Ma HM, et al. Does low dose angiotensin converting enzyme inhibitor prevent pneumonia in older people with neurologic dysphagia‐‐a randomized placebo‐controlled trial. J Am Med Dir Assoc. 2015;16(8):702‐707. [DOI] [PubMed] [Google Scholar]
- 12. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) . Randomised placebo‐controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non‐diabetic nephropathy. Lancet. 1997;349(9069):1857‐1863. [PubMed] [Google Scholar]
- 13. Ohkubo T, Chapman N, Neal B, et al. Effects of an angiotensin‐converting enzyme inhibitor‐based regimen on pneumonia risk. Am J Respir Crit Care Med. 2004;169(9):1041‐1045. [DOI] [PubMed] [Google Scholar]
- 14. Sekizawa K, Matsui T, Nakagawa T, Nakayama K, Sasaki H. ACE inhibitors and pneumonia. The Lancet. 1998;352(9133):1069. 10.1016/s0140-6736(05)60114-6 [DOI] [PubMed] [Google Scholar]
- 15. Teramoto S, Ouchi Y. ACE inhibitors and prevention of aspiration pneumonia in elderly hypertensives. The Lancet. 1999;353(9155):843. 10.1016/s0140-6736(05)76506-5 [DOI] [PubMed] [Google Scholar]
- 16. Arai T, Sekizawa K, Ohrui T, et al. ACE inhibitors and protection against pneumonia in elderly patients with stroke. Neurology. 2005;64(3):573‐574. [DOI] [PubMed] [Google Scholar]
- 17. Arai T, Yasuda Y, Takaya T, et al. Angiotensin‐converting enzyme inhibitors, angiotensin II receptor antagonists, and symptomless dysphagia. Chest. 2000;117(6):1819‐1820. [DOI] [PubMed] [Google Scholar]
- 18. Harada J, Sekizawa K. Angiotensin‐converting enzyme inhibitors and pneumonia in elderly patients with intracerebral hemorrhage. J Am Geriatr Soc. 2006;54(1):175‐176. [DOI] [PubMed] [Google Scholar]
- 19. Shah S, McArthur E, Farag A, et al. Risk of hospitalization for community acquired pneumonia with renin‐angiotensin blockade in elderly patients: a population‐based study. PLoS ONE. 2014;9(10):e110165. 10.1371/journal.pone.0110165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishifuji T, Sando E, Kaneko N, et al. Recurrent pneumonia among Japanese adults: disease burden and risk factors. BMC Pulm Med. 2017;17(1):12. 10.1186/s12890-016-0359-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okaishi K, Morimoto S, Fukuo K, et al. Reduction of risk of pneumonia associated with use of angiotensin I converting enzyme inhibitors in elderly inpatients. Am J Hypertens. 1999;12(8 Pt 1):778‐783. [DOI] [PubMed] [Google Scholar]
- 22. El Solh AA, Brewer T, Okada M, Bashir O, Gough M. Indicators of recurrent hospitalization for pneumonia in the elderly. J Am Geriatr Soc. 2004;52(12):2010‐2015. [DOI] [PubMed] [Google Scholar]
- 23. Marciniak C, Korutz AW, Lin E, Roth E, Welty L, Lovell L. Examination of selected clinical factors and medication use as risk factors for pneumonia during stroke rehabilitation: a case‐control study. Am J Phys Med Rehabil. 2009;88(1):30‐38. [DOI] [PubMed] [Google Scholar]
- 24. Takahashi T, Morimoto S, Okaishi K, et al. Reduction of pneumonia risk by an angiotensin I‐converting enzyme inhibitor in elderly Japanese inpatients according to insertion/deletion polymorphism of the angiotensin I‐converting enzyme gene. Am J Hypertens. 2005;18(10):1353‐1359. [DOI] [PubMed] [Google Scholar]
- 25. van de Garde EM, Souverein PC, Hak E, Deneer VH, van den Bosch JM, Leufkens HG. Angiotensin‐converting enzyme inhibitor use and protection against pneumonia in patients with diabetes. J Hypertens. 2007;25(1):235‐239. [DOI] [PubMed] [Google Scholar]
- 26. van de Garde EM, Souverein PC, van den Bosch JM, Deneer VH, Leufkens HG. Angiotensin‐converting enzyme inhibitor use and pneumonia risk in a general population. Eur Respir J. 2006;27(6):1217‐1222. [DOI] [PubMed] [Google Scholar]
- 27. Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community‐acquired pneumonia and the use of statins, ace inhibitors and gastric acid suppressants: a population‐based case‐control study. Pharmacoepidemiol Drug Saf. 2009;18(4):269‐275. [DOI] [PubMed] [Google Scholar]
- 28. Henry C, Zaizafoun M, Stock E, Ghamande S, Arroliga AC, White HD. Impact of angiotensin‐converting enzyme inhibitors and statins on viral pneumonia. Proc (Bayl Univ Med Cent). 2018;31(4):419‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dublin S, Walker RL, Jackson ML, Nelson JC, Weiss NS, Jackson LA. Angiotensin‐converting enzyme inhibitor use and pneumonia risk in community‐dwelling older adults: results from a population‐based case‐control study. Pharmacoepidemiol Drug Saf. 2012;21(11):1173‐1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Etminan M, Zhang B, Fitzgerald M, Brophy JM. Do angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers decrease the risk of hospitalization secondary to community‐acquired pneumonia? A nested case‐control study. Pharmacotherapy. 2006;26(4):479‐482. [DOI] [PubMed] [Google Scholar]
- 31. Mukamal KJ, Ghimire S, Pandey R, O'Meara ES, Gautam S. Antihypertensive medications and risk of community‐acquired pneumonia. J Hypertens. 2010;28(2):401‐405. [DOI] [PubMed] [Google Scholar]
- 32. Liu CL, Shau WY, Chang CH, Wu CS, Lai MS. Pneumonia risk and use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers. J Epidemiol. 2013;23(5):344‐350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu CL, Shau WY, Wu CS, Lai MS. Angiotensin‐converting enzyme inhibitor/angiotensin II receptor blockers and pneumonia risk among stroke patients. J Hypertens. 2012;30(11):2223‐2229. [DOI] [PubMed] [Google Scholar]
- 34. Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354(2):131‐140. [DOI] [PubMed] [Google Scholar]
- 35. Kanda A, Ebihara S, Yasuda H, Takashi O, Sasaki T, Sasaki H. A combinatorial therapy for pneumonia in elderly people. J Am Geriatr Soc. 2004;52(5):846‐847. [DOI] [PubMed] [Google Scholar]
- 36. Mortensen EM, Pugh MJ, Copeland LA, et al. Impact of statins and angiotensin‐converting enzyme inhibitors on mortality of subjects hospitalised with pneumonia. Eur Respir J. 2008;31(3):611‐617. [DOI] [PubMed] [Google Scholar]
- 37. Mortensen EM, Restrepo MI, Anzueto A, Pugh J. The impact of prior outpatient ACE inhibitor use on 30‐day mortality for patients hospitalized with community‐acquired pneumonia. BMC Pulm Med. 2005;5(1):12. 10.1186/1471-2466-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chalmers JD, Singanayagam A, Murray MP, Hill AT. Prior statin use is associated with improved outcomes in community‐acquired pneumonia. Am J Med. 2008;121(11):1002‐1007. e1001 [DOI] [PubMed] [Google Scholar]
- 39. Myles PR, Hubbard RB, Gibson JE, Pogson Z, Smith CJ, McKeever TM. The impact of statins, ACE inhibitors and gastric acid suppressants on pneumonia mortality in a UK general practice population cohort. Pharmacoepidemiol Drug Saf. 2009;18(8):697‐703. [DOI] [PubMed] [Google Scholar]
- 40. Mortensen EM, Nakashima B, Cornell J, et al. Population‐based study of statins, angiotensin II receptor blockers, and angiotensin‐converting enzyme inhibitors on pneumonia‐related outcomes. Clin Infect Dis. 2012;55(11):1466‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dahlof B, Devereux RB, Kjeldsen SE, et al. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995‐1003. [DOI] [PubMed] [Google Scholar]
- 42. Kasanuki H, Hagiwara N, Hosoda S, et al. Angiotensin II receptor blocker‐based vs. non‐angiotensin II receptor blocker‐based therapy in patients with angiographically documented coronary artery disease and hypertension: the heart Institute of Japan Candesartan Randomized Trial for evaluation in coronary artery disease (HIJ‐CREATE). Eur Heart J. 2009;30(10):1203‐1212. [DOI] [PubMed] [Google Scholar]
- 43. Schrader J, Luders S, Kulschewski A, et al. Morbidity and mortality after stroke, Eprosartan compared with Nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (MOSES). Stroke. 2005;36(6):1218‐1226. [DOI] [PubMed] [Google Scholar]
- 44. Weber M. Clinical safety and tolerability of losartan. Clin Ther. 1997;19(4):604‐616. discussion 603 [DOI] [PubMed] [Google Scholar]
- 45. Reynolds HR, Adhikari S, Pulgarin C, et al. Renin‐angiotensin‐aldosterone system inhibitors and risk of Covid‐19. N Engl J Med. 2020;382(25):2441‐2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li J, Wang X, Chen J, Zhang H, Deng A. Association of Renin‐Angiotensin System Inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Meng J, Xiao G, Zhang J, et al. Renin‐angiotensin system inhibitors improve the clinical outcomes of COVID‐19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757‐760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang Z, Cao J, Yao Y, et al. The effect of RAS blockers on the clinical characteristics of COVID‐19 patients with hypertension. Ann Transl Med. 2020;8(7):430. 10.21037/atm.2020.03.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mehta N, Kalra A, Nowacki AS, et al. Association of use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(9):1020‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Feng Y, Ling Y, Bai T, et al. COVID‐19 with different severity: a multi‐center study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang G, Tan Z, Zhou L, et al. Effects of ARBs and ACEIs on virus infection, inflammatory status and clinical outcomes in COVID‐19 patients with hypertension: a single center retrospective study. Hypertension. 2020;76(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 52. Li XC, Zhang J, Zhuo JL. The vasoprotective axes of the renin‐angiotensin system: physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res. 2017;125(Pt A):21‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jung SY, Choi JC, You SH, Kim WY. Association of renin‐angiotensin‐aldosterone system inhibitors with COVID‐19‐related outcomes in Korea: a nationwide population‐based cohort study. Clin Infect Dis. 2020;71(16):2121‐2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gao C, Cai Y, Zhang K, et al. Association of hypertension and antihypertensive treatment with COVID‐19 mortality: a retrospective observational study. Eur Heart J. 2020;41(22):2058‐2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou F, Liu YM, Xie J, et al. Comparative impacts of angiotensin converting enzyme inhibitors versus angiotensin II receptor blockers on the risk of COVID‐19 mortality. Hypertension. 2020;76(2):e15‐e17. [DOI] [PubMed] [Google Scholar]
- 56. Tan ND, Qiu Y, Xing XB, Ghosh S, Chen MH, Mao R. Associations between angiotensin converting enzyme inhibitors and angiotensin II receptor blocker use, gastrointestinal symptoms, and mortality among patients with COVID‐19. Gastroenterology. 2020;159(3):1170‐1172.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen C, Wang F, Chen P, et al. Mortality and Pre‐Hospitalization use of Renin‐Angiotensin System Inhibitors in Hypertensive COVID‐19 Patients. J Am Heart Assoc. 2020;9(21):e017736. 10.1161/jaha.120.017736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lam KW, Chow KW, Vo J, et al. Continued in‐hospital angiotensin‐converting enzyme inhibitor and angiotensin II receptor blocker use in hypertensive COVID‐19 patients is associated with positive clinical outcome. J Infect Dis. 2020;222(8):1256‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee J, Jo SJ, Cho Y, et al. Effects of renin‐angiotensin system blockers on the risk and outcomes of SARS‐CoV‐2 infection in patients with hypertension. Korean J Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Matsuzawa Y, Ogawa H, Kimura K, et al. Renin‐angiotensin system inhibitors and the severity of coronavirus disease 2019 in Kanagawa, Japan: a retrospective cohort study. Hypertens Res. 2020;43(11):1257‐1266. [DOI] [PubMed] [Google Scholar]
- 62. Shah P, Owens J, Franklin J, Jani Y, Kumar A, Doshi R. Baseline use of angiotensin‐converting enzyme inhibitor/AT1 blocker and outcomes in hospitalized coronavirus disease 2019 African‐American patients. J Hypertens. 2020;38(12):2537‐2541. [DOI] [PubMed] [Google Scholar]
- 63. Trifirò G, Massari M, Da Cas R, et al. Renin–angiotensin–aldosterone system inhibitors and risk of death in patients hospitalised with COVID‐19: a retrospective italian cohort study of 43,000 patients. Drug Saf. 2020;43(12):1297‐1308. 10.1007/s40264-020-00994-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yuan Y, Liu D, Zeng S, et al. In‐hospital use of ACEI/ARB is associated with lower risk of mortality and critic illness in COVID‐19 patients with hypertension. J Infect. 2020;81(5):816‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bae DJ, Tehrani DM, Rabadia SV, et al. Angiotensin converting enzyme inhibitor and angiotensin II receptor blocker use among outpatients diagnosed with COVID‐19. Am J Cardiol. 2020;132:150‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chen FF, Zhong M, Liu Y, et al. The characteristics and outcomes of 681 severe cases with COVID‐19 in China. J Crit Care. 2020;60:32‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fosbol EL, Butt JH, Ostergaard L, et al. Association of Angiotensin‐Converting Enzyme Inhibitor or angiotensin receptor blocker use with COVID‐19 diagnosis and mortality. JAMA. 2020;324(2):168‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hwang JM, Kim JH, Park JS, Chang MC, Park D. Neurological diseases as mortality predictive factors for patients with COVID‐19: a retrospective cohort study. Neurol Sci. 2020;41(9):2317‐2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Oussalah A, Gleye S, Clerc Urmes I, et al. Long‐term ACE inhibitor/ARB use is associated with severe renal dysfunction and acute kidney injury in patients with severe COVID‐19: results from a referral center cohort in the north east of France. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sardu C, Maggi P, Messina V, et al. Could anti‐hypertensive drug therapy affect the clinical prognosis of hypertensive patients with COVID‐19 infection? data from centers of southern Italy. J Am Heart Assoc. 2020;9(17):e016948. 10.1161/jaha.120.016948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Senkal N, Meral R, Medetalibeyoglu A, Konyaoglu H, Kose M, Tukek T. Association between chronic ACE inhibitor exposure and decreased odds of severe disease in patients with COVID‐19. Anatol J Cardiol. 2020;24(1):21‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xu J, Huang C, Fan G, et al. Use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers in context of COVID‐19 outbreak: a retrospective analysis. Front Med. 2020;14(5):601‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. De Spiegeleer A, Bronselaer A, Teo JT, et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID‐19 infection among nursing home residents. J Am Med Dir Assoc. 2020;21(7):909‐914. e902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bean DM, Kraljevic Z, Searle T, et al. Angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers are not associated with severe COVID‐19 infection in a multi‐site UK acute hospital trust. Eur J Heart Fail. 2020;22(6):967‐974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Felice C, Nardin C, Di Tanna GL, et al. Use of RAAS inhibitors and risk of clinical deterioration in COVID‐19: results from an Italian cohort of 133 hypertensives. Am J Hypertens. 2020;33(10):944‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin‐angiotensin‐aldosterone system blockers and the risk of Covid‐19. N Engl J Med. 2020;382(25):2431‐2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin‐converting enzyme inhibitors and angiotensin ii receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res. 2020;126(12):1671‐1681. 10.1161/circresaha.120.317134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. de Abajo FJ, Rodriguez‐Martin S, Lerma V, et al. Use of renin‐angiotensin‐aldosterone system inhibitors and risk of COVID‐19 requiring admission to hospital: a case‐population study. Lancet. 2020;395:1705‐1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Seo J, Son M. Update on association between exposure to renin‐angiotensin‐aldosterone system inhibitors and coronavirus disease 2019 in South Korea. Korean J Intern Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Son M, Seo J, Yang S. Association between renin‐angiotensin‐aldosterone system inhibitors and COVID‐19 infection in South Korea. Hypertension. 2020;76(3):742‐749. [DOI] [PubMed] [Google Scholar]
- 81. Bravi F, Flacco ME, Carradori T, et al. Predictors of severe or lethal COVID‐19, including angiotensin converting enzyme inhibitors and angiotensin ii receptor blockers, in a sample of infected italian citizens. PLOS ONE. 2020;15(6):e0235248. 10.1371/journal.pone.0235248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pan W, Zhang J, Wang M, et al. Clinical features of COVID‐19 in patients with essential hypertension and the impacts of renin‐angiotensin‐aldosterone system inhibitors on the prognosis of COVID‐19 patients. Hypertension. 2020;76(3):732‐741. [DOI] [PubMed] [Google Scholar]
- 83. Selcuk M, Cinar T, Keskin M, et al. Is the use of ACE inb/ARBs associated with higher in‐hospital mortality in Covid‐19 pneumonia patients? Clin Exp Hypertens. 2020;42(8):738‐742. [DOI] [PubMed] [Google Scholar]
- 84. Amat‐Santos IJ, Santos‐Martinez S, Lopez‐Otero D, et al. Ramipril in high risk patients with COVID‐19. J Am Coll Cardiol. 2020;76(3):268‐276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Khan KS, Reed‐Embleton H, Lewis J, Bain P, Mahmud S. Angiotensin converting enzyme inhibitors do not increase the risk of poor outcomes in COVID‐19 disease. A multi‐Centre observational study. Scott Med J. 2020;65(4):149‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Giorgi Rossi P, Marino M, Formisano D, et al. Characteristics and outcomes of a cohort of COVID‐19 patients in the Province of Reggio Emilia, Italy. PLOS ONE. 2020;15(8):e0238281. 10.1371/journal.pone.0238281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hippisley‐Cox J, Young D, Coupland C, et al. Risk of severe COVID‐19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106(19):1503‐1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Grasselli G, Greco M, Zanella A, et al. Risk factors associated with mortality among patients with COVID‐19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Vila‐Corcoles A, Satue‐Gracia E, Ochoa‐Gondar O, et al. Use of distinct anti‐hypertensive drugs and risk for COVID‐19 among hypertensive people: a population‐based cohort study in southern Catalonia, Spain. J Clin Hypertens (Greenwich). 2020;22(8):1379‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gnavi R, Demaria M, Picariello R, Dalmasso M, Ricceri F, Costa G. Therapy with agents acting on the renin‐angiotensin system and risk of SARS‐CoV‐2 infection. Clin Infect Dis. 2020;71(16):2291‐2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Raisi‐Estabragh Z, McCracken C, Ardissino M, et al. Renin‐angiotensin‐aldosterone system blockers are not associated with coronavirus disease 2019 (COVID‐19) hospitalization: study of 1,439 uk biobank cases. Front Cardiovasc Med. 2020;7:138. 10.3389/fcvm.2020.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular Disease, Drug Therapy, and Mortality in Covid‐19. N Engl J Med. 2020;382(25):e102. 10.1056/nejmoa2007621 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93. Mortensen EM, Restrepo MI, Copeland LA, Pugh JA, Anzueto A. Association of hydrophilic versus lipophilic angiotensin‐converting enzyme inhibitor use on pneumonia‐related mortality. Am J Med Sci. 2008;336(6):462‐466. [DOI] [PubMed] [Google Scholar]
- 94. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N Engl J Med. 2020;382(17):1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111(20):2605‐2610. [DOI] [PubMed] [Google Scholar]
- 97. Ocaranza MP, Godoy I, Jalil JE, et al. Enalapril attenuates downregulation of angiotensin‐converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension. 2006;48(4):572‐578. [DOI] [PubMed] [Google Scholar]
- 98. Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin‐converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43(5):970‐976. [DOI] [PubMed] [Google Scholar]
- 99. Burchill LJ, Velkoska E, Dean RG, Griggs K, Patel SK, Burrell LM. Combination renin‐angiotensin system blockade and angiotensin‐converting enzyme 2 in experimental myocardial infarction: implications for future therapeutic directions. Clin Sci (Lond). 2012;123(11):649‐658. [DOI] [PubMed] [Google Scholar]
- 100. Burrell LM, Risvanis J, Kubota E, et al. Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J. 2005;26(4):369‐375. discussion 322–364 [DOI] [PubMed] [Google Scholar]
- 101. Li Y, Zeng Z, Huang W, Zhou M, Zhang X, Jiang W. Angiotensin‐converting enzyme inhibition attenuates lipopolysaccharide‐induced lung injury by regulating the balance between angiotensin‐converting enzyme and angiotensin‐converting enzyme 2 and inhibiting mitogen‐activated protein kinase activation. Shock. 2015;43(4):395‐404. [DOI] [PubMed] [Google Scholar]
- 102. Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS‐CoV‐2. Cardiovasc Res. 2020;116(6):1097‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020:m1091. 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Han H, Xie L, Liu R, et al. Analysis of heart injury laboratory parameters in 273 COVID‐19 patients in 1 hospital in Wuhan, China. J Med Virol. 2020;92(7):819‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Madjid M, Safavi‐Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831‐840. [DOI] [PubMed] [Google Scholar]
- 106. Goicoechea M, Sanchez Camara LA, Macias N, et al. COVID‐19: clinical course and outcomes of 36 maintenance hemodialysis patients from a single center in Spain. Kidney Int. 2020;98(1):27‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID‐19 multiorgan impact. Kidney Int. 2020;98(2):314‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bayes‐Genis A. Highlights of the 2016 European Society of Cardiology Guidelines on heart failure. Eur Cardiol. 2017;12(2):76‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. de Frutos F, Mirabet S, Ortega‐Paz L, et al. Management of Heart Failure with reduced ejection fraction after ESC 2016 heart failure guidelines: the Linx registry. ESC Heart Fail. 2020;7(1):25‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Solomon SD, Rizkala AR, Gong J, et al. Angiotensin receptor Neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON‐HF trial. JACC Heart Failure. 2017;5(7):471‐482. [DOI] [PubMed] [Google Scholar]
- 111. Fox AJ, Lalloo UG, Belvisi MG, Bernareggi M, Chung KF, Barnes PJ. Bradykinin‐evoked sensitization of airway sensory nerves: a mechanism for ACE‐inhibitor cough. Nat Med. 1996;2(7):814‐817. [DOI] [PubMed] [Google Scholar]
- 112. Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124(1):328‐336. [DOI] [PubMed] [Google Scholar]
- 113. Morice AH, Lowry R, Brown MJ, Higenbottam T. Angiotensin‐converting enzyme and the cough reflex. Lancet. 1987;2(8568):1116‐1118. [DOI] [PubMed] [Google Scholar]
- 114. Sekizawa K, Ujiie Y, Itabashi S, Sasaki H, Takishima T. Lack of cough reflex in aspiration pneumonia. Lancet. 1990;335(8699):1228‐1229. [DOI] [PubMed] [Google Scholar]
- 115. Tomaki M, Ichinose M, Miura M, et al. Angiotensin converting enzyme (ACE) inhibitor‐induced cough and substance P. Thorax. 1996;51(2):199‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hoesch RE, Lin E, Young M, et al. Acute lung injury in critical neurological illness. Crit Care Med. 2012;40(2):587‐593. [DOI] [PubMed] [Google Scholar]
- 117. Wang H, Zhang K, Qin H, Yang L, Zhang L, Cao Y. Genetic Association Between CD143 rs4340 Polymorphism and Pneumonia risk. Medicine. 2015;94(30):e883. 10.1097/md.0000000000000883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Retraction: Cardiovascular Disease, Drug Therapy, and Mortality in Covid‐19. N Engl J Med. DOI: 10.1056/NEJMoa2007621.. N Engl J Med. 2020;382(26):2582. 10.1056/nejmc2021225 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119. Rubin EJ. Expression of concern: Mehra MR et al. cardiovascular disease, drug therapy, and mortality in Covid‐19. N Engl J Med. 2020;382(25):2464. 10.1056/nejme2020822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mizgerd JP. Pathogenesis of severe pneumonia: advances and knowledge gaps. Curr Opin Pulm Med. 2017;23(3):193‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hendrickson CM, Matthay MA. Viral pathogens and acute lung injury: investigations inspired by the SARS epidemic and the 2009 H1N1 influenza pandemic. Semin Respir Crit Care Med. 2013;34(4):475‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Tsukagoshi H, Ishioka T, Noda M, Kozawa K, Kimura H. Molecular epidemiology of respiratory viruses in virus‐induced asthma. Front Microbiol. 2013;4:278. 10.3389/fmicb.2013.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early‐phase 2019 novel coronavirus (COVID‐19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1 Newcastle–Ottawa scale of included studies.
TABLE S2 Main characteristics of randomized–controlled trials in the meta‐analysis.
TABLE S3 Main characteristics of cohort studies in the meta‐analysis.
TABLE S4 Main characteristics of case–control studies, nested case–control studies and case–crossover studies in the meta‐analysis.
TABLE S5 Main characteristics of COVID‐19 clinical studies.
TABLE S6 Renin–angiotensin system inhibitors and COVID‐19 severe adverse clinical outcomes.
FIGURE S1 PRISMA 2009 flow diagram of studies through review.


