ABSTRACT
Nuclear pore complexes are multiprotein channels that span the nuclear envelope, which connects the nucleus to the cytoplasm. In addition to their main role in the regulation of nucleocytoplasmic molecule exchange, it has become evident that nuclear pore complexes and their components also have multiple transport-independent functions. In recent years, an increasing number of studies have reported the involvement of nuclear pore complex components in embryogenesis, cell differentiation and tissue-specific processes. Here, we review the findings that highlight the dynamic nature of nuclear pore complexes and their roles in many cell type-specific functions during development and tissue homeostasis.
KEY WORDS: Nuclear pore complex, Nucleocytoplasmic transport, Embryogenesis, Differentiation, Tissue homeostasis
Summary: Nuclear pore complexes and their components have been found to play multiple functions in embryogenesis, cell differentiation and tissue-specific processes. Many of them are performed in a transport-independent manner. This Review highlights the roles of nucleoporins in during development and tissue homeostasis.
Introduction
Nuclear pore complexes (NPCs) are multiprotein aqueous channels that connect the nucleus to the cytoplasm. Although the regulation of nucleocytoplasmic transport has long been considered the main function of NPCs, in the last few decades, many non-transport roles for nuclear pores have also been discovered. These findings outline the functional complexity of these massive multiprotein channels that play a role in practically every cellular process. Thirty-two different proteins termed ‘nucleoporins’ have been identified as the structural components of NPCs. For a long time, NPC composition was considered to be uniform among different cell types and tissues, but several recent studies have uncovered that the expression of NPC components varies in different cell types and that different nucleoporins can play precise roles in the development and homeostasis of specific organs. These specific roles are consistent with the findings that mutations in different nucleoporins result in diseases with remarkably tissue-specific phenotypes. Here, we review the role of nucleoporins in processes ranging from cell differentiation and embryogenesis, to tissue development, homeostasis and aging.
Structure, assembly and cellular functions of the nuclear pore complex
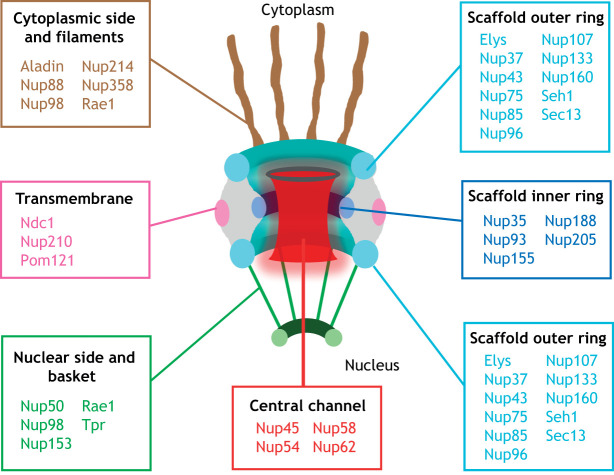
In eukaryotic cells, the genome is stored inside the nucleus and separated from the cytoplasm by the nuclear envelope: a double lipid bilayer that acts as a physical barrier protecting the genetic material (Raices and D'Angelo, 2012). NPCs are multiprotein channels that span the nuclear envelope and allow cells to selectively transport molecules in and out of the nucleus. Thirty-two different NPC proteins, known as nucleoporins or Nups, have been identified (Fig. 1). Although most Nups are ubiquitous components of the NPC, a few have been described to have different stoichiometry at NPCs, to be cell-type specific or to be expressed at different levels in different cell types (Raices and D'Angelo, 2012). The nomenclature of Nups varies between different organisms; for simplicity in this Review, we use the mammalian nomenclature for general description of nucleoporin/NPC properties.
Fig. 1.
Structural organization of the nuclear pore complex. Schematic representation of the nuclear pore complex (NPC) structure and composition in human. A cross-section of a NPC perpendicular to the nuclear envelope is shown. The NPC is embedded into the nuclear envelope and made of nucleoporins (Nups). Nups are grouped and color-coded to indicate that they are part of the same subcomplex or associate with it.
With an estimated molecular mass of 60 MDa in yeast and 90-120 MDa in humans, NPCs are one of the largest protein complexes of eukaryotic cells (Hampoelz et al., 2019a; Lin and Hoelz, 2019; Sun et al., 2019). The overall architecture of the NPC consists of a core scaffold, a central transport channel, eight cytoplasmic filaments and eight nuclear filaments, which are connected to a distal ring to form the nuclear basket (Schwartz, 2016) (Fig. 1). The NPC core is made of an inner ring located between two outer rings: the cytoplasmic ring and the nuclear ring (Hampoelz et al., 2019a; Lin and Hoelz, 2019) (Fig. 1). This scaffold surrounds the central transport channel, which is filled with Nups containing disordered domains rich in phenylalanine-glycine (FG) repeats. FG-Nups weakly associate with each other through hydrophobic interactions, forming a meshwork with gel-like properties and are, therefore, likely to be the first described example of phase-separation condensates (Frey et al., 2006). The central channel Nups define the selective permeability of the NPC and mediate the interaction with transport receptors required for the movement of molecules into and out of the nucleus (Hampoelz et al., 2019a; Lin and Hoelz, 2019) (Box 1). Although NPCs are embedded in the nuclear membranes, only three Nups are transmembrane proteins. These Nups connect the core scaffold to the nuclear envelope membrane. Notably, several nucleoporins have been found to have amphipathic lipid-packing sensor (ALPS) domains, which bind curved membranes and might provide additional tethers to the nuclear envelope (Doucet et al., 2010; Drin et al., 2007; Hampoelz et al., 2019a; Leksa et al., 2009; Lin and Hoelz, 2019; Mészáros et al., 2015). During the course of this Review, we describe the known functions of core scaffold Nups, followed by nuclear basket components and finally cytoplasmic filament proteins.
Box 1. Nucleocytoplasmic transport.
Nuclear pore complexes (NPCs) are the sole gateway into the nucleus and thus mediate the exchange of most molecules between this compartment and the cytoplasm. Although small molecules can freely diffuse into the nucleus, larger molecules (>40-60 kDa) are actively transported through NPCs. Nucleocytoplasmic transport is carried out by a family of transport receptors known as importins and exportins in mammals (karyopherins in yeast) and has been extensively described in recent reviews (Cautain et al., 2015; Fu et al., 2018).
Nuclear import occurs when importins recognize and bind the nuclear localization signal (NLS) on cargo proteins and the resulting complex is transported across the central channel of the NPC by interacting with FG-Nups to reach the nucleoplasm. In the nucleus, the small GTPase Ran, in its GTP-bound form (Ran-GTP), binds to the importin and induces the release of the cargo. During nuclear export, exportins bind Ran-GTP and the nuclear export signal (NES) on cargo proteins, signaling the complex to move across the NPC. Once in the cytoplasm, the Ran-GTPase activating protein 1 (RanGAP1), which forms a complex with two cytoplasmic filaments proteins RanBP1 and Nup358, promotes the hydrolysis of Ran-GTP to Ran-GDP leading to the dissociation of the export complex. The RanGTP gradient (high in the nucleus and low in the cytoplasm) establishes the directionality of transport, and is maintained by the activity of the nuclear transport factor 2 (Ntf2) that binds Ran-GDP in the cytoplasm and transports it back to the nucleus, where RanGEF regulator of chromosome condensation (RCC1) catalyzes its conversion back to Ran-GTP.
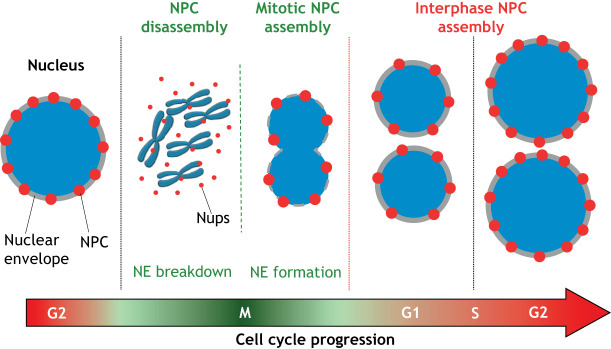
The formation of NPCs is a highly ordered process that involves the stepwise recruitment and assembly of different nucleoporins (Dultz and Ellenberg, 2010; Dultz et al., 2008). In proliferating cells, there are two mechanisms of NPC formation: Post-mitotic NPC assembly, which takes place at the end of mitosis when nuclear envelopes reform around the genomes of daughter cells, and interphase NPC assembly, which occurs continuously from G1 to G2 (Fig. 2) (Otsuka and Ellenberg, 2018). Even though both assembly processes lead to the formation of indistinguishable structures, they have been found to occur through fundamentally different mechanisms (Otsuka and Ellenberg, 2018). Notably, current evidence suggests that NPC biogenesis is mostly restricted to proliferating cells and ceases significantly in post-mitotic cells where NPCs seem to be maintained by the slow replacement of different subcomplexes (D'Angelo et al., 2009; Toyama et al., 2013). Non-dividing quiescent cells, on the other hand, seem to maintain some NPC assembly levels (Toyama et al., 2019). Interestingly, a new mechanism of NPC assembly was recently described in Drosophila embryos, where the formation of NPCs from nucleoporin condensates occurs in the endoplasmic reticulum (ER) and away from the nucleus (Hampoelz et al., 2016; Hampoelz et al., 2019b). This localization leads to the formation of annullate lamellae – stacks of ER membrane with NPCs – that are then used to insert NPCs to support the rapidly growing embryonic nuclei and facilitate fast cell division (Hampoelz et al., 2016; Hampoelz et al., 2019b).
Fig. 2.
Nuclear pore complex assembly in the mammalian cell cycle. When mammalian cells enter mitosis (M phase), nuclear pore complexes (NPCs) are disassembled into subcomplexes during nuclear envelope (NE) breakdown. These subcomplexes are recycled at the end of mitosis to assemble new NPCs in the reforming nuclear envelope around the genomes of the daughter cells (mitotic NPC assembly). From G1 phase to G2 phase, daughter cells double their number of NPCs in preparation for the next round of cell division (interphase NPC assembly).
The canonical function of NPCs has historically been considered the regulation of nucleocytoplasmic transport. However, a significant amount of data accumulated in recent years has highlighted many transport-independent functions of NPCs and nucleoporins (Raices and D'Angelo, 2012). This should not be surprising, considering that this massive protein complex is likely to act as a scaffold where several cellular complexes assemble to regulate specific cellular processes. Some of the best characterized functions of NPCs and nucleoporins are described in Fig. 3 and Table 1.
Fig. 3.
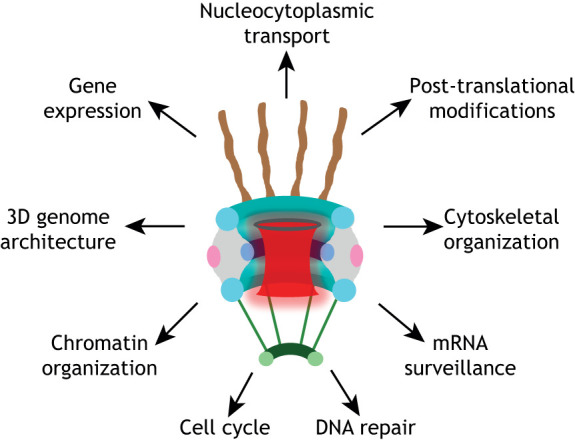
Cellular functions of the nuclear pore complex. The nuclear pore complex and its multiple cellular functions: nucleocytoplasmic transport, regulation of gene expression and 3D genome architecture (Buchwalter et al., 2019; Raices and D'Angelo, 2017; Strambio-De-Castillia et al., 2010), chromatin organization (Kuhn and Capelson, 2019), DNA repair (Freudenreich and Su, 2016; Géli and Lisby, 2015), mRNA surveillance (Géli and Lisby, 2015), post-translational protein modifications (Texari and Stutz, 2015), cytoskeletal organization (Joseph and Dasso, 2008; Joseph et al., 2004), and cell cycle regulation (Chatel and Fahrenkrog, 2011; Imamoto and Funakoshi, 2012).
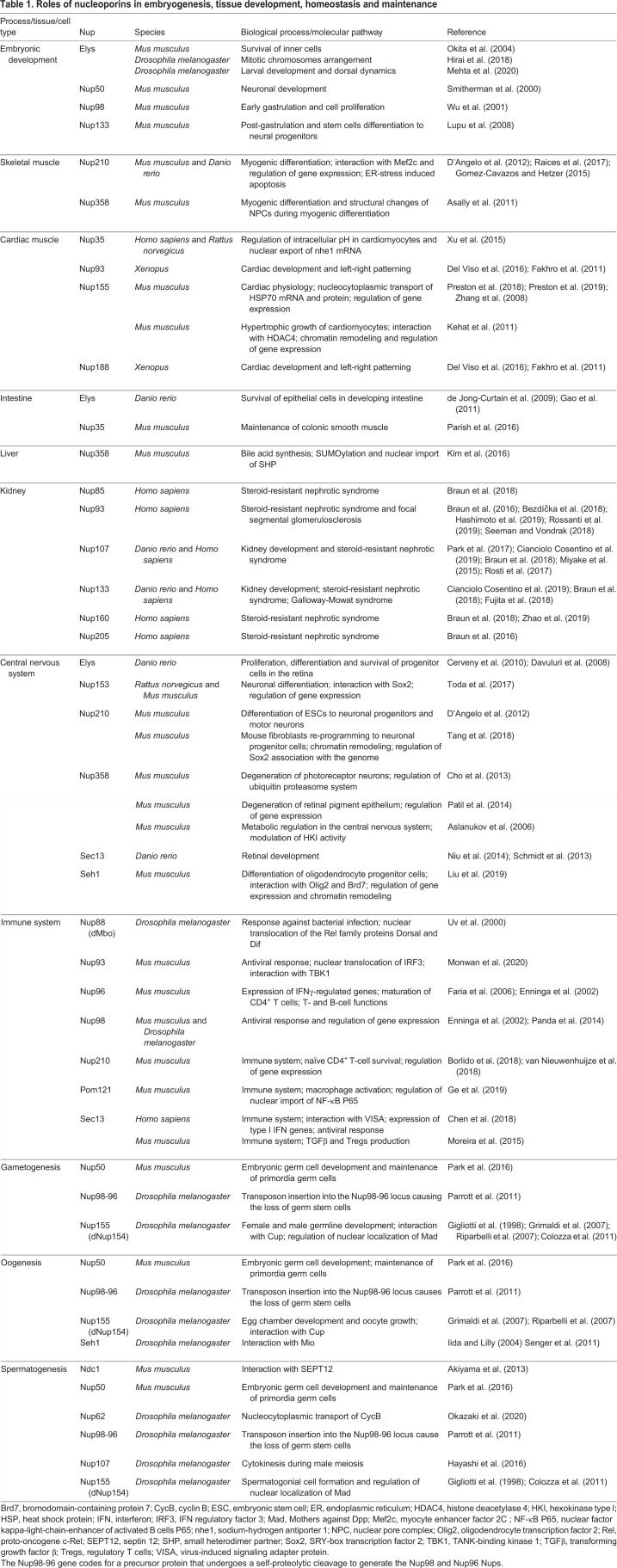
Table 1.
Roles of nucleoporins in embryogenesis, tissue development, homeostasis and maintenance
The role of NPCs during embryonic development
Embryonic development is a complex biological process that requires a coordinated spatiotemporal expression of genes and controlled activation of signaling pathways to regulate the rapid cell proliferation and differentiation that occur during this stage (Molè et al., 2020). As NPCs are essential cellular structures that assemble during cell proliferation, alterations that affect their formation can be expected to have a negative impact on embryonic development, where fast cell proliferation requires the assembly of large numbers of these channels. Thus, constitutive knockouts of several NPC components have been found to be embryonic lethal (Sakuma and D'Angelo, 2017). Surprisingly, increasing evidence also shows that depletion or mutations of various Nups results in tissue-specific defects (Fig. 4 and Table 1). Our goal here is to discuss findings linking nucleoporins to specific developmental processes or tissue functions, but it is important to bear in mind that mutations and alterations that affect the biogenesis or integrity of NPCs could indirectly affect many of the same processes.
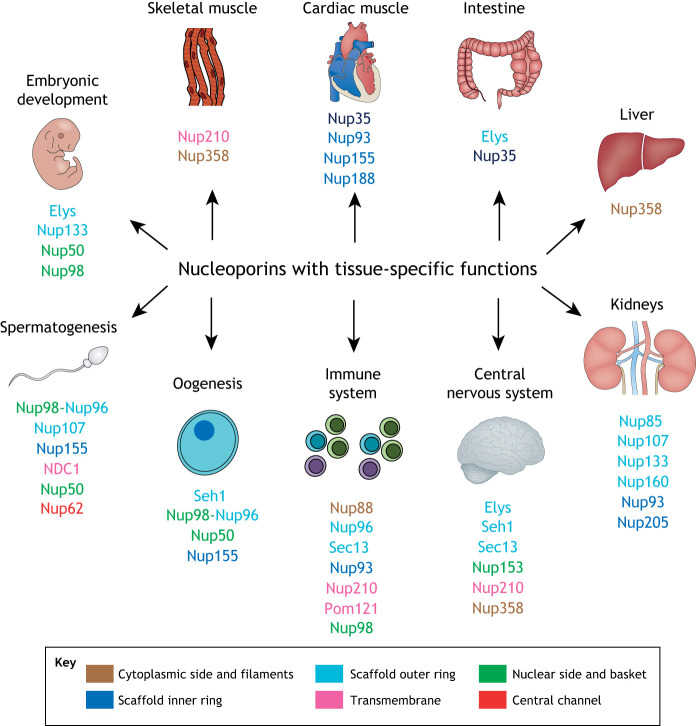
Fig. 4.
Nucleoporins with tissue-specific functions. Summary of nucleoporins involved in embryogenesis, gametogenesis, tissue development and homeostasis. Nucleoporins from different substructures of the NPC are color-coded as in Fig. 1. See Table 1 for more details.
The scaffold Nup107-160 complex is a crucial component of the NPC and probably one the most studied subcomplexes of this structure. Elys, a member of this complex that has also been reported to act as a transcription factor (Kimura et al., 2002), has been found to play a role in early embryogenesis. Mice deficient for Elys die before E5.5, and even though Elys-null E3.5 blastocysts appear morphologically normal, when cultured in vitro they show increased apoptosis and decreased proliferation of inner cells (Okita et al., 2004). In Drosophila, one study has shown that loss-of-function mutation of Elys results in embryonic lethality, with embryos dying after the first cleavage division due to abnormalities in mitotic chromosome arrangement and centrosomes behavior (Hirai et al., 2018). A different study using RNAi-mediated depletion of Elys has found that, although embryos were largely unaffected by downregulation of this nucleoporin, there was major lethality at the larval and pupal stage (Mehta et al., 2020). Loss of Elys results in several alterations in cells, including abnormal NPC assembly, nuclear transport defects, alterations in the organization of the nuclear lamina and re-activation of the Dorsal (NF-κB) pathway, leading to the expression pro-apoptotic genes in late larval stages.
Nup133 is another component of the scaffold Nup107-160 complex. Mice carrying the mermaid (merm) mutation that leads to the loss of Nup133 component undergo developmental arrest and die at mid-gestation E9.5-E10.5, indicating that this nucleoporin is required for embryonic development post-gastrulation (Lupu et al., 2008). Unexpectedly, Nup133 has been found to have a cell type- and stage-specific expression pattern in developing embryos, with highest expression levels in the neuroepithelium and paraxial mesoderm/somites. Consistent with a function in neurodevelopment, the embryonic neural progenitors of merm mice aberrantly maintain hallmarks of pluripotent early epiblast/ES cells and have an impaired ability to differentiate into post-mitotic neurons. How Nup133 regulates ES differentiation is still poorly understood, but merm mutant cells and ES cells depleted of this nucleoporin do not show alterations in NPC assembly. Instead, loss of Nup133 in ES cells has been found to affect the formation of the nuclear basket and the dynamics of the basket nucleoporins Tpr and Nup153 (Souquet et al., 2018). These findings oppose studies using cancer cells, where Nup133 downregulation reduces NPC numbers and inhibit interphase NPC assembly in particular (Doucet et al., 2010).
Targeted ablation of the nuclear basket nucleoporin Nup50 in the mouse germline results in intrauterine growth retardation and late embryonic lethality. Analyses of E8.5 embryos have shown that Nup50 is required for cranial neural tube closure during development. Interestingly, although Nup50 is found ubiquitously expressed in embryonic and adult tissues, the phenotypic alterations due to Nup50 deletion are more severe in the central nervous system (CNS) (Smitherman et al., 2000). Another nuclear basket-associated nucleoporin, the FG-rich Nup98 protein (which also localizes to the cytoplasmic side of the NPC), has been found to be required for the normal development of mice and zebrafish. Homozygous disruption of Nup98 in mice is lethal and impairs early gastrulation, with embryos showing morphological alterations and defects in cell proliferation (Wu et al., 2001). Despite possessing normal numbers of NPCs, in Nup98−/− cells NPCs lack some cytoplasmic nucleoporins, including Nup358, Nup214 and Nup88, and display impaired nuclear import of proteins. These findings highlight how important the integrity of NPCs and the nucleocytoplasmic transport system are during embryonic development.
NPC functions during tissue development and homeostasis
Recent studies in several model organisms have revealed that NPCs are compositionally, structurally and functionally regulated during the development, and maintenance, of many tissues and organs. By performing cell- and time-specific functions (Table 1), NPC components are required for the proper and timely execution of different physiological processes that ensure the homeostasis of different tissues. As described below, Nups can play very different functions, often independently of their role in NPC formation.
Skeletal muscle
Several recent studies have uncovered a role for Nups in muscle development and physiology. For example, Nup210 is an integral transmembrane nucleoporin dispensable for NPC formation or stability and was the first tissue-specific Nup described (Eriksson et al., 2004; Olsson et al., 2004; Upla et al., 2017). Interestingly, Nup210 is absent in proliferating myoblasts, but its expression is induced during myogenesis, and shRNA knockdown of this Nup prevents myogenic differentiation (D'Angelo et al., 2012). These findings have exposed for first time that changes in NPC composition can be exploited to regulate a cellular process. In zebrafish, Nup210 is required for the maturation and growth of differentiated muscle fibers (Raices et al., 2017). Mechanistically, Nup210 has been identified to regulate the expression of genes and microRNAs involved in muscle growth, myofiber maturation and muscle cell survival through its interaction with the transcription factor myocyte enhancer factor 2C (Mef2C). It has also been reported that Nup210 plays a role in muscle cell differentiation independent from its localization at the NPC, by inhibiting endoplasmic reticulum stress-induced apoptosis during myogenesis (Gomez-Cavazos and Hetzer, 2015).
Nup358, a large Nup composed of multiple structural and functional domains, and a major component of NPC cytoplasmic filaments (Wu et al., 1995), has also been implicated in skeletal muscle differentiation (Asally et al., 2011). In interphase cells, Nup358 localizes to the NPC cytoplasmic filaments and forms a stable complex with Ran GTPase-activating protein 1 (RanGAP1) and the SUMO-conjugating enzyme Ubc9 (Joseph et al., 2002; Reverter and Lima, 2005). Like Nup210, Nup358 expression has been found to increase during the differentiation of C2C12 myoblasts and its downregulation inhibits myotube formation. The upregulation of Nup358 during differentiation is accompanied by a higher number of Nup358 molecules at the NPC and correlates with an increased diameter of the NPC cytoplasmic rim, which has been proposed to augment nuclear export rates of myotubes without changing the NPC passive diffusion or nuclear import rates.
Cardiac muscle
Modifications in NPC architecture and remodeling of nuclear transport factors have also been observed during the differentiation of embryonic stem cells (ESCs) into the cardiac lineage. Similar to the findings in skeletal muscle, the acquisition of the cardiac molecular profile is accompanied by an increased NPC density and by the expansion of the nuclear pore diameter. These changes have been suggested to modify the translocation of cardiac-specific transcription factors required for the execution of the cardiogenic program (Perez-Terzic et al., 2003, 2007).
Four different members of the Nup93-205 nuclear pore scaffold subcomplex have been shown to play a role in heart development and physiology. Morpholino knockdown of Nup188, and to a lesser extent Nup93, in Xenopus have been shown to cause defects in left-right patterning that alter heart morphology (Del Viso et al., 2016; Fakhro et al., 2011). The impairment of left-right patterning is known to be the cause of heterotaxy (Htx): a disease characterized by malformation of organs, including the heart, that leads to a severe form of congenital heart disease (Del Viso et al., 2016). Remarkably, Nup188 and Nup93 have both been found to localize to cilia and depletion of either nucleoporin results in the loss of cilia in mammalian cells and in Xenopus. As cilia are organelles crucial for left-right patterning during embryonic development, these findings explain the cardiac defects observed by depletion of these nucleoporins in vivo. Homozygous missense mutations in the Nup155 gene, another component of the Nup93-205 complex, have been linked to atrial fibrillation (AF) and early sudden death (Zhang et al., 2008). Consistent with its role in the heart, atrial myocytes from Nup155+/− mice display electrophysiological alterations and these animals develop an AF phenotype. Mechanistically, cardiomyocytes from Nup155+/− mice have been found to have a reduced nuclear export of Hsp70 mRNA and nuclear import of Hsp70 protein. To explain the observed electrophysiological abnormalities, it has been proposed that the Hsp70 nuclear transport alterations would impair the folding and trafficking of calcium channels and proteins involved in calcium homeostasis. A more recent study, has shown that Nup155+/− ESCs spontaneously form embryoid body-derived cardiomyocytes, with dysregulated electrical function mimicking the previously reported arrhythmogenic phenotype (Preston et al., 2018). Nup155 insufficiency in these cells results in alterations of transcriptome networks involved in cardiac innervation and fibrosis, as well as the deregulation of non-coding RNAs involved in pluripotency maintenance (Preston et al., 2019, 2018). The transcriptome changes described by these studies highlight a role for Nup155 in regulating gene expression and supports previous studies in which Nup155 has been found to regulate the expression of sarcomeric and Ca2+-handling genes through its interaction with histone deacetylase 4 (HDAC4) (Kehat et al., 2011). Nup35, another member of the Nup93-205 complex, has been found to indirectly regulate intracellular pH levels in cardiomyocytes by binding to the 5′ UTR of the Na+-H+ exchanger 1 (NHE1) mRNA and modulating its nuclear export (Xu et al., 2015). As NHE1 is expressed in multiple cell lines, whether these findings are specific to cardiomyocytes remains to be investigated.
Intestine
Growing evidence supports the involvement of specific Nups in the development and homeostasis of the gastrointestinal system. Studies performed in zebrafish have identified that the flotte lotte (flo) mutant, carrying a nonsense mutation in the Elys Nup, has abnormalities in intestine, liver and pancreas development, which are associated with reduced animal survival. flo embryos show severe defects in the formation of NPCs in the intestinal epithelium and increased apoptosis of epithelial cells (de Jong-Curtain et al., 2009). Disruption of Elys in the developing intestinal epithelium has been found to lead to alterations in the architecture of small intestinal epithelial cells, causing crypt distortion and apoptosis of crypt progenitor cells (Gao et al., 2011).
In mice, a point mutation in the Nup35 gene results in the development of severe megacolon and reduces animal lifespan. Detailed tissue examination has revealed that Nup35 mutations specifically affect colonic smooth muscle, leading to a degenerative myopathy with several characteristics of chronic intestinal pseudo-obstruction (CIPO) disease – a rare, but severe, digestive syndrome characterized by abnormalities in involuntary intestinal muscular contractions (Parish et al., 2016).
Liver
In mice, the cytoplasmic filament protein Nup358 has been found to regulate bile acid production through SUMOylation of the orphan nuclear receptor small heterodimer partner (SHP) (Kim et al., 2016). Nup358 has been found to be involved in the SUMO2 modification of SHP, which promotes its nuclear import and the recruitment of the repressive histone-modifying enzymes HDAC1 and LSD1 to downregulate genes involved in bile acid synthesis. Notably, downregulation of Nup358 in mice leads to a remarkable reduction of SUMO2-SHP levels and decreases nuclear SHP, which increases liver bile acid levels in response to liver injury and inflammation.
Kidney
In the past few years, mutations in many genes encoding Nups have been reported to cause diverse kidney diseases, including steroid-resistant nephrotic syndrome, Galloway-Mowat syndrome and focal segmental glomerulosclerosis (Bezdíčka et al., 2018; Braun et al., 2018, 2016; Fujita et al., 2018; Hashimoto et al., 2019; Miyake et al., 2015; Park et al., 2017; Rossanti et al., 2019; Rosti et al., 2017; Seeman and Vondrak, 2018; Zhao et al., 2019). The mechanisms through which Nup mutations affect kidney development or function are still poorly understood. Studies on Nup133 and Nup107, both members of the same scaffold subcomplex, have provided some insights into the role of Nups in kidney physiology. Spatiotemporal analysis of Nup133 expression during zebrafish development has shown that, by 5 days post-fertilization, this Nup is mainly expressed in liver, but also in pronephric proximal tubules and the glomerulus at lower levels (Cianciolo Cosentino et al., 2019). Morpholino-mediated knockdown of Nup133 results in glomerular expansion, formation of kidney cysts, and liver misplacement. Lack of Nup133 does not affect the development of pronephric tubules and glomerulus, but leads to structural and functional abnormalities of the glomerulus filtration barrier. Stronger reduction of Nup133 levels in this organism has also been associated with microcephaly in addition to glomerular abnormalities, a phenotype reminiscent of Galloway-Mowat syndrome – a human disease that has been linked to mutations in Nup133 and its interacting protein Nup107 (Fujita et al., 2018; Rosti et al., 2017).
CNS
Increasing evidence shows that Nups have crucial functions in the CNS. Moreover, several studies in the past few years have uncovered that alterations in the function of Nups and nuclear pore complexes are present in multiple neurodegenerative diseases, suggesting that the deterioration of the nuclear transport machinery is a common feature of neurodegeneration (Box 2).
Box 2. NPCs aging and neurodegeneration.
Over a decade ago, nuclear pore complexes (NPCs) were identified as one of the most stable protein complexes of post-mitotic cells (D'Angelo et al., 2009). Experiments performed in C. elegans and muscle cells revealed that scaffold nucleoporins (Nups) are downregulated when cells exit the cell cycle and do not turn over during adulthood, indicating that these proteins can last the entire lifespan of the organism. The very low turnover of these structures in post-mitotic tissues was further confirmed by several studies (Savas et al., 2012; Toyama et al., 2019; Toyama et al., 2013). The long life of NPCs in old mouse brains was associated with the loss of the nuclear permeability barrier and the leakiness of proteins through the nuclear envelope. Because the abnormal localization of proteins had been reported in several age-associated neurodegenerative diseases, these findings suggested that the deterioration of NPCs could be a key aging event contributing to neurodegeneration (D'Angelo et al., 2009). Consistent with this idea, recent work from several groups has shown that alterations in NPCs and in nucleocytoplasmic transport are a common feature of neurodegenerative diseases including Alzheimer's disease (Eftekharzadeh et al., 2018; Lee et al., 2006; Leone et al., 2019; Sheffield et al., 2006), Parkinson's disease (Cho et al., 2012), amyotrophic lateral sclerosis and frontotemporal dementia (Freibaum et al., 2015; Hayes et al., 2020; Jovičić et al., 2015; Kramer et al., 2018; Neumann et al., 2012; Nishimura et al., 2010; Renton et al., 2011; Zhang et al., 2018, 2015), and Huntington's disease (Grima et al., 2017). The discovery that maintaining NPC integrity is crucial for proper neuronal function during aging may be the beginning of a broader shift in our understanding of how the deterioration of long-lived cellular structures alters organismal aging. The low turnover of NPCs in differentiated cells also exposes other post-mitotic tissues, such as cardiac and skeletal muscle, to a higher risk of damage during aging, and it is foreseeable that future studies will unveil a more complete picture of the involvement of nuclear pores in the pathogenesis of age-related diseases affecting other tissues and organs.
Three members of the Nup107-160 complex, Elys, Sec13 and Seh1, have recently been reported to play a role in the CNS. Studies performed in zebrafish have revealed that Elys-null flo embryos have small eyes and degeneration of the optic tectum (Davuluri et al., 2008). Lack of Elys in fish has been shown to affect the proliferation, differentiation and survival of progenitor cells in the retina that might be attributed, in part, to the reduced number of NPCs (Cerveny et al., 2010; Davuluri et al., 2008). Besides being a component of the Nup107-160 complex, Sec13 is a core member of the coat protein complex II (COPII) complex that mediates protein trafficking from the ER to the Golgi. Recently, a mutation in Sec13 (sq198) that affects only its NPC functions has been described. As in Elys mutants, zebrafish Sec13sq198 embryos have been found to have small eyes, and also display alterations in retinal lamination (Niu et al., 2014). The phenotype has also been attributed to disrupted NPC formation and the abnormal nuclear accumulation of mRNA. Similar alterations in retina development have been observed with morpholino depletion of Sec13 (Schmidt et al., 2013). Seh1, on the other hand, is required for oligodendrocyte (OLs) differentiation and myelination in the CNS (Liu et al., 2019). Upregulation of Seh1 protein levels have been detected during in vitro differentiation of rat OLs and selective ablation of Seh1 in mice prevents the differentiation of oligodendrocyte progenitor cells (OPCs), leading to defects in myelination during CNS development, adult myelinogenesis and OL remyelination after demyelinating damage. Lack of Seh1 has not been found to affect NPC assembly, but instead alters the expression of genes involved in OPC differentiation and myelination. Seh1 has been shown to directly interact with promoters of genes involved in axon guidance and OL differentiation, and to be required for chromatin remodeling that facilitates the transcription of genes involved in these processes. Like Nup210 in muscle, Seh1 is required for the formation of a transcriptional complex at the nuclear periphery. In this case, Seh1 association with the transcription factor oligodendrocyte transcription factor 2 (Olig2) and the chromatin remodeler bromodomain-containing protein 7 (Brd7) promotes the transcription of myelination-related genes.
Nup210 and Nup155 have also been shown to play a role in neuronal differentiation, although their physiological functions in the CNS have not yet been investigated. Nup210 expression has been found to be upregulated during the differentiation of ESCs to neuroprogenitors and motoneurons, and preventing its expression inhibits neuronal differentiation (D'Angelo et al., 2012). Consistent with this role in neurodifferentiation, Nup210 has been found to be crucial for the reprogramming of mouse fibroblasts into NPCs, where it is necessary to induce the expression of SoxB1, a transcription factor required for neural stem cell conversion (Tang et al., 2018). Contrarily, the Nup153 nuclear basket nucleoporin has been found to repress neuronal differentiation: downregulation of Nup153 in neuroprogenitors induces chromatin remodeling at specific target genes, impairs the genomic association of Sox2, and promotes neuronal differentiation (Toda et al., 2017). However, Nup153 might have a more general function in the maintenance of the undifferentiated state, because its depletion from mouse ESCs also induces early cell differentiation by recruiting the epigenetic regulator polycomb-repressive complex 1 (PRC1) to developmental genes to keep them in a transcriptionally silent state (Jacinto et al., 2015).
Nup358 has been found to play roles in retina and brain physiology. Conditional ablation of Nup358 in cone photoreceptors has been found to induce cell autonomous non-apoptotic death of cones and non-cell autonomous apoptosis in rods (Cho et al., 2013), while selective ablation in the mouse retinal pigment epithelium leads to degeneration characterized by increased cell permeability, pigmentation alterations, morphological abnormalities and non-apoptotic cell death (Patil et al., 2014). Mice lacking Nup358 in Thy1-positive motoneurons have been shown to display amyotrophic lateral sclerosis-like syndromes characterized by hindlimb paralysis, respiratory distress and premature death (Cho et al., 2017). Although the absence of Nup358 causes disruption of nucleocytoplasmic transport, motoneuron degeneration has not been observed in this model. The absence of this pathological hallmark of amyotrophic lateral sclerosis prevents any conclusions from being drawn about the involvement of Nup358 in motoneuron maintenance.
Nup358 has also been proposed to play a role in regulating energy balance in the brain and retina. Nup358+/− mice show decreased ability to use glucose, and haploinsufficiency of this Nup decreases the levels of the glycolytic enzyme hexokinase type I (HKI) and ATP selectively in the CNS (Aslanukov et al., 2006). These alterations have been linked to abnormalities in the electrophysiological activity of photosensory and postreceptoral neurons. Mechanistically, Nup358 has been shown to directly interact and modulate the levels of the metabolic enzymes HKI and cytochrome C oxidase copper chaperone 11 (Cox11) by acting as a protein chaperone for them.
Immune system
The first links between NPCs and immunity emerged with the observation that the expression of the Nups Nup98-96 is upregulated by interferons (IFNs) (Enninga et al., 2002). The Nup98-96 gene codes for a large protein that undergoes a self-proteolytic cleavage to generate two Nups, Nup98 and Nup96 (Fontoura et al., 1999). Whereas Nup96 is a stable member of the Nup107-160 scaffold complex, Nup98 is a dynamic peripheral nucleoporin with transcription regulation functions (Chatel and Fahrenkrog, 2011; Griffis et al., 2002). As IFNγ treatment as well as overexpression of Nup96 and Nup98 have been identified to reverse the vesicular stomatitis virus (VSV)-induced mRNA nuclear export inhibition by the M protein, it has been proposed that this protein interferes with Nup98 function in mRNA export. Nup98 has also been found to play a role in the antiviral response in Drosophila (Panda et al., 2014). In this case, Nup98 promotes antiviral activity by inducing the transcription of genes involved in the immune response to infection. In particular, Nup98 has been shown to directly bind to the promoter of target genes and recruit RNA polymerase II upon viral infection. As ablation of Nup96 in mice is embryonically lethal, the role of Nup96 in immunity has been investigated in Nup96+/− mice, which have reduced protein levels (Faria et al., 2006). Consistent with a role in the response to viral infection, Nup96+/− mice are more susceptible to VSV infection. Furthermore, Nup96+/− cells express viral proteins earlier than control cells, which makes them more prone to cell death. Nup96+/− mice displayed decreased levels of IFNγ-regulated genes in macrophages and dendritic cells, and have been characterized by low levels of major histocompatibility complex (MHC) I and II proteins. Interestingly, the reduced MHCI and MHCII protein levels results from nuclear retention of their corresponding transcripts, supporting a function for Nup96 in the nuclear export of specific mRNAs. Nup96+/− mice also show defects in T- and B-cell function that have been, in part, attributed to reduced expression of MHCI and MHCII by antigen-presenting cells.
Mice expressing low levels of Sec13 show reduced expression of MHCI and MHCII in macrophages, low expression of IFNγ and IL6 in stimulated T cells, and decreased frequencies of specific T- and B-cell populations (Moreira et al., 2015). These mice also have higher numbers of regulatory T cells (Tregs) producing TGFβ and increased serum immunoglobulins. Interestingly, many of the alterations in T- and B-cell function recover with activation or immunization, suggesting that animals with low levels of Sec13 expression can still generate normal immune responses. Sec13 has also been shown to be involved in the RIG-I-like receptor (RLR)-mediated antiviral cellular response (Chen et al., 2018; Monwan et al., 2020). The RLR signaling cascade starts with the recognition and binding of viral RNA by the cytoplasmic RLRs, which activate a downstream signaling cascade leading to the transcription of pro-inflammatory cytokine genes and type I IFN genes that mediate the antiviral response (Loo and Gale, 2011). Sec13 has been found to associate with the virus-induced signaling adapter protein (VISA), which is required to form a signaling scaffold downstream of RLR and for the activation of IFN regulatory factor 3 (IRF3, a key effector of RLR signaling), and to regulate its aggregation (Chen et al., 2018). Interestingly, even though previous studies on Sec13+/− fibroblasts found no abnormalities in the activation of RIG-I signaling pathway, when these cells are transfected with synthetic RNAs that mimic a viral infection (Moreira et al., 2015), knockdown of Sec13 in 293T cells has been shown to reduce the Sendai virus-induced activation of IRF3 by VISA, as well as the production of IFNβ (Chen et al., 2018). Conversely, overexpression of Sec13 in these cells increases VISA aggregation, and enhances the phosphorylation and dimerization of IRF3 (Chen et al., 2018). Nup93 has also been linked to this same pathway. In this case, ablation of Nup93 has been shown to impair the phosphorylation and nuclear translocation of IRF3, and to decrease the production of IFNβ and CXCL10 (Monwan et al., 2020).
Recently, two independent studies have revealed a role for the transmembrane Nup Nup210 in T-cell homeostasis (Borlido et al., 2018; van Nieuwenhuijze et al., 2018). Loss of Nup210 has been found to cause a severe decrease in the naïve CD4+ T-cell population. Although Nup210-deficient CD4+ T cells develop normally, they are unable to survive in the periphery. Mechanistically, it has been demonstrated that, in T cells, Nup210 is required for the expression of genes that ensure the proper transmission of T-cell receptor (TCR) signals, and the repression of the cell death receptor Fas (Borlido et al., 2018). The transmembrane nucleoporin Pom121, on the other hand, has been found to modulate macrophage activation (Ge et al., 2019). It has been shown that LPS-stimulated macrophages progressively downregulate Pom121, and that depleting this Nup results in a higher inflammatory response to LPS. Consistent with these findings, mice lacking Pom121 in macrophages have been found to be more susceptible to LPS-induced acute lung injury. The way Pom121 has been proposed to modulate macrophage activation is by regulating the nuclear import of NF-κB P65, which is involved in the transcriptional regulation of pro-inflammatory cytokines.
At the NPC cytoplasmic filaments, Nup358 forms a multisubunit complex with RanGAP1, SUMO and the SUMO E2-conjugating enzyme Ubc9, which has SUMO E3 ligase activity (Joseph et al., 2002; Reverter and Lima, 2005). Notably, during T-cell activation, part of the immune adaptor lymphocyte cytosolic protein 2 (SLP-76) has been shown to relocalize to the cytoplasmic filaments of the NPC through its interaction with SUMO-RanGAP1 (Liu et al., 2015). The association of SLP-76 with NPCs regulates the nuclear import of the transcription factors nuclear factor of activated T cells, cytoplasmic 1 (NFATc1) and RelA/p65, and is required for proper TCR signaling. This work indicates that T-cell receptor activation can modulate the activity of NPCs, while the Nup210 findings show that NPCs play a role in the regulation TCR signaling, suggesting a key role for nuclear pores in T-cell activation.
In Drosophila, another cytoplasmic filament protein Mbo (the homolog of human Nup88) is required for the immune response against bacterial infection. Besides leading to alterations in the CNS, oogenesis and tracheal development, ablation of mbo results in a severe immune deficiency, owing to alterations in the nuclear import of factors key for the activation of the immune response (Uv et al., 2000).
Gametogenesis
In Drosophila, loss-of-function mutation in Nup154, the homolog of human scaffold nucleoporin Nup155, is lethal, whereas hypomorphic mutations impair gametogenesis, leading to both female and male infertility (discussed below) (Gigliotti et al., 1998). In Drosophila, transposon insertion into the Nup98-96 locus has been found to cause severe defects in the progression of germline cells through gametogenesis, in both males and females, due to the loss of germ stem cells (GSCs) that prematurely undergo differentiation (Parrott et al., 2011). How the disruption of Nup98-96 expression leads to the loss of GSCs remains unknown.
In mice, ablation of Nup50 leads to a reduction in the number of primordial germ cells (PGCs) in early embryos (E8.5) and no germ cells develop at later stages (Park et al., 2016). Gonads from Nup50-deficient embryos have been found to have a high level of apoptosis, but a normal rate of proliferation during the migratory and proliferative phases – defects that ultimately result in the progressive loss of germ cells.
Oogenesis
During female gamete development, Nup154 has been found to play a crucial role in egg chamber development and oocyte growth in ovaries (Gigliotti et al., 1998). Moreover, nurse cells from Nup154 mutant flies show abnormalities in the chromosome separation process. The role of Nup154 in oogenesis is performed, at least in part, through its interaction with Cup, a protein with key functions in many stages of female gametogenesis, including regulation of the nurse cell chromosome structure and the control of actin cytoskeletal dynamics (Grimaldi et al., 2007; Riparbelli et al., 2007). Notably, Nup154 deletion in these animals results in several other alterations including the mislocalization of several Nups that could also contribute to the observed phenotype. Consistent with this idea, Nup154 mutant egg chambers also display alterations in F-actin organization in the early stages of oogenesis, as well as abnormalities in the apoptosis process (Riparbelli et al., 2007).
Seh1, has also been described to play a role during Drosophila oogenesis (Senger et al., 2011). It has been shown that a fraction of oocytes in the ovaries of Seh1 mutant flies fail to properly undergo meiosis and maintain their oocyte identity, ultimately developing as pseudo-nurse cells. Mechanistically, Seh1 has been reported to function through its interaction with Mio (the Drosophila homolog of carbohydrate response element-binding protein, ChREBP) to regulate meiosis and oocyte fate in the later stages of oogenesis (Iida and Lilly, 2004; Senger et al., 2011).
Spermatogenesis
The scaffold nucleoporin Nup107 has recently been reported to be involved in male meiosis I (Hayashi et al., 2016). It has been observed that Nup107 colocalizes with lamins at the spindle envelope during meiosis I and that spermatocyte-specific depletion of Nup107 results in a higher frequency of cytokinesis failure in male meiosis, abnormalities in the central spindle microtubules and impaired the recruitment of contractile ring components to cleavage sites. These observations suggest that Nup107 is necessary for the formation and reinforcement of the nuclear envelope and spindle envelope in Drosophila male meiosis.
Nup154 mutations in Drosophila have been found to more strongly affect spermatogenesis than oogenesis. Nup154 mutants have small testes and contain cysts that lacked developed germ cells and meiotic figures (Gigliotti et al., 1998). A more recent report has proposed that the decreased number of spermatogonial cells that result from Nup154 mutation is due to increased cell death and reduced cell proliferation (Colozza et al., 2011). Mechanistically, Nup154 has been found to be required for the nuclear accumulation of the transcription factor Mothers against Dpp (Mad), which is involved in regulation of germ cell survival and proliferation.
Ndc1, also known as Tmem48, is a transmembrane Nup, which is also part of the spindle pole body in yeast. Despite previous studies showing that Ndc1 is required for NPC assembly (Mansfeld et al., 2006), a mutation in this Nup that results in protein loss does not affect mouse viability but leads to spermatogenesis defects and skeletal malformations (Akiyama et al., 2013). Interestingly, Ndc1 has been shown to function through its interaction with septin 12 (SEPT12), a factor crucial for sperm formation (Lai et al., 2016).
A role for the FG-rich nucleoporin Nup62 in Drosophila spermatogenesis has also been reported (Okazaki et al., 2020). Spermatocyte-specific depletion of Nup62 results in cell cycle arrest before the first meiotic division in spermatocytes. In Nup62-depleted cells, the failure of meiotic entry is due to the inhibition of CDK1 activation prior to meiosis as a result of the nuclear accumulation of cyclin B (CycB), the regulatory subunit of CDK1 (Okazaki et al., 2020). This study suggests that the nuclear export of CycB through the NPCs is required to initiate male meiosis in Drosophila.
Conclusions
In the past two decades, NPCs and their components have been found to regulate many physiological processes throughout the entire life of an organism. These findings have exposed the dynamic nature of this massive multiprotein complex and indicate that NPCs should be considered crucial players in the development and function of specific tissues and organs. In this Review, we provide a comprehensive overview of the current knowledge of the role of NPCs and Nups in embryogenesis and tissue development, as well as in the maintenance of adult tissues. One clear conclusion that can be drawn from these findings is that NPCs and Nups can have cell type- and tissue-specific functions, which explains why mutations in many distinct Nups result in human diseases with strikingly tissue-specific phenotypes (Sakuma and D'Angelo, 2017).
As described here, a significant amount of data show that specific NPC components play crucial roles in different aspects of embryonic development. Yet the number of Nups studied so far is limited. One constraint in addressing this gap in knowledge has been the lack of animal models for several NPC components. The recent development of simple and fast genetic modifying methods, such as CRISPR technology, is likely to result in the generation of more Nup animal models that will allow us to gain additional insights into the role of other pore components in embryonic development.
In the past few years, we have also begun to recognize Nups as key players in the maintenance and function of adult tissues. The discovery that several Nups, including those that have historically been considered essential members of this structure and have remarkable tissue-specific phenotypes when depleted, has revolutionized our view of how nuclear pores function. Although previously the identification of NPC components in screens for diverse processes generally warranted no further studies due to the assumption that the phenotypes resulted from alterations of housekeeping processes, these components now represent exciting candidates for the regulation of cell and tissue physiology.
Despite the increasing number of studies showing crucial functions for NPCs in different tissues, and at different stages of development, our understanding of the functional complexity of these multiprotein channels is still far from complete. Considering the data accumulated so far, the most likely scenario is that NPCs act as molecular hubs for a plethora of essential cellular activities. We envision that, in the next decade, many more cell type-specific functions for Nups will be discovered, further exposing the multifunctional nature of NPCs, and establishing their significance for the regulation of organismal physiology.
Acknowledgements
We apologize to all colleagues whose work could not be cited directly owing to space limitation.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
M.A.D. is supported by a Research Scholar Grant from the American Cancer Society. This work was also supported by the National Institutes of Health and U. S. Department of Defense. Deposited in PMC for release after 12 months.
References
- Akiyama K., Noguchi J., Hirose M., Kajita S., Katayama K., Khalaj M., Tsuji T., Fairfield H., Byers C., Reinholdt L. et al. (2013). A mutation in the nuclear pore complex gene Tmem48 causes gametogenesis defects in skeletal fusions with sterility (sks) mice. J. Biol. Chem. 288, 31830-31841. 10.1074/jbc.M113.492306.E [DOI] [PMC free article] [PubMed]
- Asally M., Yasuda Y., Oka M., Otsuka S., Yoshimura S. H., Takeyasu K. and Yoneda Y. (2011). Nup358, a nucleoporin, functions as a key determinant of the nuclear pore complex structure remodeling during skeletal myogenesis. FEBS J. 278, 610-621. 10.1111/j.1742-4658.2010.07982.x [DOI] [PubMed] [Google Scholar]
- Aslanukov A., Bhowmick R., Guruju M., Oswald J., Raz D., Bush R. A., Sieving P. A., Lu X., Bock C. B. and Ferreira P. A. (2006). RanBP2 modulates Cox11 and hexokinase I activities and haploinsufficiency of RanBP2 causes deficits in glucose metabolism. PLoS Genet. 2, e177 10.1371/journal.pgen.0020177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezdíčka M., Štolbová Š., Seeman T., Cinek O., Malina M., Šimánková N., Průhová Š. and Zieg J. (2018). Genetic diagnosis of steroid-resistant nephrotic syndrome in a longitudinal collection of Czech and Slovak patients: a high proportion of causative variants in NUP93. Pediatr. Nephrol. 33, 1347-1363. 10.1007/s00467-018-3950-2 [DOI] [PubMed] [Google Scholar]
- Borlido J., Sakuma S., Raices M., Carrette F., Tinoco R., Bradley L. M. and D'Angelo M. A. (2018). Nuclear pore complex-mediated modulation of TCR signaling is required for naïve CD4(+) T cell homeostasis. Nat. Immunol. 19, 594-605. 10.1038/s41590-018-0103-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D. A., Sadowski C. E., Kohl S., Lovric S., Astrinidis S. A., Pabst W. L., Gee H. Y., Ashraf S., Lawson J. A., Shril S. et al. (2016). Mutations in nuclear pore genes NUP93, NUP205 and XPO5 cause steroid-resistant nephrotic syndrome. Nat. Genet. 48, 457-465. 10.1038/ng.3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D. A., Lovric S., Schapiro D., Schneider R., Marquez J., Asif M., Hussain M. S., Daga A., Widmeier E., Rao J. et al. (2018). Mutations in multiple components of the nuclear pore complex cause nephrotic syndrome. J. Clin. Invest. 128, 4313-4328. 10.1172/JCI98688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwalter A., Kaneshiro J. M. and Hetzer M. W. (2019). Coaching from the sidelines: the nuclear periphery in genome regulation. Nat. Rev. Genet. 20, 39-50. 10.1038/s41576-018-0063-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cautain B., Hill R., de Pedro N. and Link W. (2015). Components and regulation of nuclear transport processes. FEBS J. 282, 445-462. 10.1111/febs.13163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerveny K. L., Cavodeassi F., Turner K. J., de Jong-Curtain T. A., Heath J. K. and Wilson S. W. (2010). The zebrafish flotte lotte mutant reveals that the local retinal environment promotes the differentiation of proliferating precursors emerging from their stem cell niche. Development 137, 2107-2115. 10.1242/dev.047753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatel G. and Fahrenkrog B. (2011). Nucleoporins: leaving the nuclear pore complex for a successful mitosis. Cell. Signal. 23, 1555-1562. 10.1016/j.cellsig.2011.05.023 [DOI] [PubMed] [Google Scholar]
- Chen T., Wang D., Xie T. and Xu L.-G. (2018). Sec13 is a positive regulator of VISA-mediated antiviral signaling. Virus Genes 54, 514-526. 10.1007/s11262-018-1581-0 [DOI] [PubMed] [Google Scholar]
- Cho K.-I., Searle K., Webb M., Yi H. and Ferreira P. A. (2012). Ranbp2 haploinsufficiency mediates distinct cellular and biochemical phenotypes in brain and retinal dopaminergic and glia cells elicited by the Parkinsonian neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Cell. Mol. Life Sci. 69, 3511-3527. 10.1007/s00018-012-1071-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K.-I., Haque M. E., Wang J., Yu M., Hao Y., Qiu S., Pillai I. C. L., Peachey N. S. and Ferreira P. A. (2013). Distinct and atypical intrinsic and extrinsic cell death pathways between photoreceptor cell types upon specific ablation of Ranbp2 in cone photoreceptors. PLoS Genet. 9, e1003555 10.1371/journal.pgen.1003555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K.-I., Yoon D., Qiu S., Danziger Z., Grill W. M., Wetsel W. C. and Ferreira P. A. (2017). Loss of Ranbp2 in motoneurons causes disruption of nucleocytoplasmic and chemokine signaling, proteostasis of hnRNPH3 and Mmp28, and development of amyotrophic lateral sclerosis-like syndromes. Dis. Model. Mech. 10, 559-579. 10.1242/dmm.027730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciolo Cosentino C., Berto A., Pelletier S., Hari M., Loffing J., Neuhauss S. C. F. and Doye V. (2019). Moderate Nucleoporin 133 deficiency leads to glomerular damage in zebrafish. Sci. Rep. 9, 4750 10.1038/s41598-019-41202-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colozza G., Montembault E., Quénerch'du E., Riparbelli M. G., D'Avino P. P. and Callaini G. (2011). Drosophila nucleoporin Nup154 controls cell viability, proliferation and nuclear accumulation of Mad transcription factor. Tissue Cell 43, 254-261. 10.1016/j.tice.2011.05.001 [DOI] [PubMed] [Google Scholar]
- D'Angelo M. A., Raices M., Panowski S. H. and Hetzer M. W. (2009). Age-dependent deterioration of nuclear pore complexes causes a loss of nuclear integrity in postmitotic cells. Cell 136, 284-295. 10.1016/j.cell.2008.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo M. A., Gomez-Cavazos J. S., Mei A., Lackner D. H. and Hetzer M. W. (2012). A change in nuclear pore complex composition regulates cell differentiation. Dev. Cell 22, 446-458. 10.1016/j.devcel.2011.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davuluri G., Gong W., Yusuff S., Lorent K., Muthumani M., Dolan A. C. and Pack M. (2008). Mutation of the zebrafish nucleoporin elys sensitizes tissue progenitors to replication stress. PLoS Genet. 4, e1000240 10.1371/journal.pgen.1000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong-Curtain T. A., Parslow A. C., Trotter A. J., Hall N. E., Verkade H., Tabone T., Christie E. L., Crowhurst M. O., Layton J. E., Shepherd I. T. et al. (2009). Abnormal nuclear pore formation triggers apoptosis in the intestinal epithelium of elys-deficient zebrafish. Gastroenterology 136, 902-911.e7. 10.1053/j.gastro.2008.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Viso F., Huang F., Myers J., Chalfant M., Zhang Y., Reza N., Bewersdorf J., Lusk C. P. and Khokha M. K. (2016). Congenital heart disease genetics uncovers context-dependent organization and function of nucleoporins at cilia. Dev. Cell 38, 478-492. 10.1016/j.devcel.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet C. M., Talamas J. A. and Hetzer M. W. (2010). Cell cycle-dependent differences in nuclear pore complex assembly in metazoa. Cell 141, 1030-1041. 10.1016/j.cell.2010.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drin G., Casella J.-F., Gautier R., Boehmer T., Schwartz T. U. and Antonny B. (2007). A general amphipathic α-helical motif for sensing membrane curvature. Nat. Struct. Mol. Biol. 14, 138-146. 10.1038/nsmb1194 [DOI] [PubMed] [Google Scholar]
- Dultz E. and Ellenberg J. (2010). Live imaging of single nuclear pores reveals unique assembly kinetics and mechanism in interphase. J. Cell Biol. 191, 15-22. 10.1083/jcb.201007076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dultz E., Zanin E., Wurzenberger C., Braun M., Rabut G., Sironi L. and Ellenberg J. (2008). Systematic kinetic analysis of mitotic dis- and reassembly of the nuclear pore in living cells. J. Cell Biol. 180, 857-865. 10.1083/jcb.200707026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekharzadeh B., Daigle J. G., Kapinos L. E., Coyne A., Schiantarelli J., Carlomagno Y., Cook C., Miller S. J., Dujardin S., Amaral A. S. et al. (2018). Tau protein disrupts nucleocytoplasmic transport in Alzheimer's disease. Neuron 99, 925-940.e927. 10.1016/j.neuron.2018.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enninga J., Levy D. E., Blobel G. and Fontoura B. M. (2002). Role of nucleoporin induction in releasing an mRNA nuclear export block. Science 295, 1523-1525. 10.1126/science.1067861 [DOI] [PubMed] [Google Scholar]
- Eriksson C., Rustum C. and Hallberg E. (2004). Dynamic properties of nuclear pore complex proteins in gp210 deficient cells. FEBS Lett. 572, 261-265. 10.1016/j.febslet.2004.07.044 [DOI] [PubMed] [Google Scholar]
- Fakhro K. A., Choi M., Ware S. M., Belmont J. W., Towbin J. A., Lifton R. P., Khokha M. K. and Brueckner M. (2011). Rare copy number variations in congenital heart disease patients identify unique genes in left-right patterning. Proc. Natl. Acad. Sci. USA 108, 2915-2920. 10.1073/pnas.1019645108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria A. M. C., Levay A., Wang Y., Kamphorst A. O., Rosa M. L. P., Nussenzveig D. R., Balkan W., Chook Y. M., Levy D. E. and Fontoura B. M. A. (2006). The nucleoporin Nup96 is required for proper expression of interferon-regulated proteins and functions. Immunity 24, 295-304. 10.1016/j.immuni.2006.01.014 [DOI] [PubMed] [Google Scholar]
- Fontoura B. M. A., Blobel G. and Matunis M. J. (1999). A conserved biogenesis pathway for nucleoporins: proteolytic processing of a 186-kilodalton precursor generates Nup98 and the novel nucleoporin, Nup96. J. Cell Biol. 144, 1097-1112. 10.1083/jcb.144.6.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freibaum B. D., Lu Y., Lopez-Gonzalez R., Kim N. C., Almeida S., Lee K.-H., Badders N., Valentine M., Miller B. L., Wong P. C. et al. (2015). GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature 525, 129-133. 10.1038/nature14974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenreich C. H. and Su X. A. (2016). Relocalization of DNA lesions to the nuclear pore complex. FEMS Yeast Res. 16, fow095 10.1093/femsyr/fow095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S., Richter R. P. and Görlich D. (2006). FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science 314, 815-817. 10.1126/science.1132516 [DOI] [PubMed] [Google Scholar]
- Fu X., Liang C., Li F., Wang L., Wu X., Lu A., Xiao G. and Zhang G. (2018). The rules and functions of nucleocytoplasmic shuttling proteins. Int. J. Mol. Sci. 19, 1445 10.3390/ijms19051445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A., Tsukaguchi H., Koshimizu E., Nakazato H., Itoh K., Kuraoka S., Komohara Y., Shiina M., Nakamura S., Kitajima M. et al. (2018). Homozygous splicing mutation in NUP133 causes Galloway-Mowat syndrome. Ann. Neurol. 84, 814-828. 10.1002/ana.25370 [DOI] [PubMed] [Google Scholar]
- Gao N., Davuluri G., Gong W., Seiler C., Lorent K., Furth E. E., Kaestner K. H. and Pack M. (2011). The nuclear pore complex protein Elys is required for genome stability in mouse intestinal epithelial progenitor cells. Gastroenterology 140, 1547-1555.e1510. 10.1053/j.gastro.2011.01.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W., Yue Y. and Xiong S. (2019). POM121 inhibits the macrophage inflammatory response by impacting NF-κB P65 nuclear accumulation. Exp. Cell Res. 377, 17-23. 10.1016/j.yexcr.2019.02.021 [DOI] [PubMed] [Google Scholar]
- Géli V. and Lisby M. (2015). Recombinational DNA repair is regulated by compartmentalization of DNA lesions at the nuclear pore complex. BioEssays 37, 1287-1292. 10.1002/bies.201500084 [DOI] [PubMed] [Google Scholar]
- Gigliotti S., Callaini G., Andone S., Riparbelli M. G., Pernas-Alonso R., Hoffmann G., Graziani F. and Malva C. (1998). Nup154, a new Drosophila gene essential for male and female gametogenesis is related to the nup155 vertebrate nucleoporin gene. J. Cell Biol. 142, 1195-1207. 10.1083/jcb.142.5.1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Cavazos J. S. and Hetzer M. W. (2015). The nucleoporin gp210/Nup210 controls muscle differentiation by regulating nuclear envelope/ER homeostasis. J. Cell Biol. 208, 671-681. 10.1083/jcb.201410047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis E. R., Altan N., Lippincott-Schwartz J. and Powers M. A. (2002). Nup98 is a mobile nucleoporin with transcription-dependent dynamics. Mol. Biol. Cell 13, 1282-1297. 10.1091/mbc.01-11-0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima J. C., Daigle J. G., Arbez N., Cunningham K. C., Zhang K., Ochaba J., Geater C., Morozko E., Stocksdale J., Glatzer J. C. et al. (2017). Mutant Huntingtin disrupts the nuclear pore complex. Neuron 94, 93-107.e106. 10.1016/j.neuron.2017.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi M. R., Cozzolino L., Malva C., Graziani F. and Gigliotti S. (2007). nup154 genetically interacts with cup and plays a cell-type-specific function during Drosophila melanogaster egg-chamber development. Genetics 175, 1751-1759. 10.1534/genetics.106.062844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampoelz B., Mackmull M.-T., Machado P., Ronchi P., Bui K. H., Schieber N., Santarella-Mellwig R., Necakov A., Andrés-Pons A., Philippe J. M. et al. (2016). Pre-assembled nuclear pores insert into the nuclear envelope during early development. Cell 166, 664-678. 10.1016/j.cell.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampoelz B., Andres-Pons A., Kastritis P. and Beck M. (2019a). Structure and assembly of the nuclear pore complex. Annu. Rev. Biophys. 48, 515-536. 10.1146/annurev-biophys-052118-115308 [DOI] [PubMed] [Google Scholar]
- Hampoelz B., Schwarz A., Ronchi P., Bragulat-Teixidor H., Tischer C., Gaspar I., Ephrussi A., Schwab Y. and Beck M. (2019b). Nuclear pores assemble from nucleoporin condensates during oogenesis. Cell 179, 671-686.e617. 10.1016/j.cell.2019.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T., Harita Y., Takizawa K., Urae S., Ishizuka K., Miura K., Horita S., Ogino D., Tamiya G., Ishida H. et al. (2019). In vivo expression of NUP93 and its alteration by NUP93 mutations causing focal segmental glomerulosclerosis. Kidney Int. Rep. 4, 1312-1322. 10.1016/j.ekir.2019.05.1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi D., Tanabe K., Katsube H. and Inoue Y. H. (2016). B-type nuclear lamin and the nuclear pore complex Nup107-160 influences maintenance of the spindle envelope required for cytokinesis in Drosophila male meiosis. Biol. Open 5, 1011-1021. 10.1242/bio.017566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes L. R., Duan L., Bowen K., Kalab P. and Rothstein J. D. (2020). C9orf72 arginine-rich dipeptide repeat proteins disrupt karyopherin-mediated nuclear import. eLife 9, e51685 10.7554/eLife.51685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Wang Z., Miura K., Hayashi T., Awasaki T., Wada M., Keira Y., Ishikawa H. O. and Sawamura K. (2018). Genetic analyses of Elys mutations in Drosophila show maternal-effect lethality and interactions with nucleoporin genes. G3 (Bethesda) 8, 2421-2431. 10.1534/g3.118.200361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida T. and Lilly M. A. (2004). Missing oocyte encodes a highly conserved nuclear protein required for the maintenance of the meiotic cycle and oocyte identity in Drosophila. Development 131, 1029-1039. 10.1242/dev.01001 [DOI] [PubMed] [Google Scholar]
- Imamoto N. and Funakoshi T. (2012). Nuclear pore dynamics during the cell cycle. Curr. Opin. Cell Biol. 24, 453-459. 10.1016/j.ceb.2012.06.004 [DOI] [PubMed] [Google Scholar]
- Jacinto F. V., Benner C. and Hetzer M. W. (2015). The nucleoporin Nup153 regulates embryonic stem cell pluripotency through gene silencing. Genes Dev. 29, 1224-1238. 10.1101/gad.260919.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J. and Dasso M. (2008). The nucleoporin Nup358 associates with and regulates interphase microtubules. FEBS Lett. 582, 190-196. 10.1016/j.febslet.2007.11.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J., Tan S.-H., Karpova T. S., McNally J. G. and Dasso M. (2002). SUMO-1 targets RanGAP1 to kinetochores and mitotic spindles. J. Cell Biol. 156, 595-602. 10.1083/jcb.200110109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph J., Liu S.-T., Jablonski S. A., Yen T. J. and Dasso M. (2004). The RanGAP1-RanBP2 complex is essential for microtubule-kinetochore interactions in vivo. Curr. Biol. 14, 611-617. 10.1016/j.cub.2004.03.031 [DOI] [PubMed] [Google Scholar]
- Jovičić A., Mertens J., Boeynaems S., Bogaert E., Chai N., Yamada S. B., Paul J. W. III, Sun S., Herdy J. R., Bieri G. et al. (2015). Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat. Neurosci. 18, 1226-1229. 10.1038/nn.4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I., Accornero F., Aronow B. J. and Molkentin J. D. (2011). Modulation of chromatin position and gene expression by HDAC4 interaction with nucleoporins. J. Cell Biol. 193, 21-29. 10.1083/jcb.201101046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-H., Kwon S., Byun S., Xiao Z., Park S., Wu S.-Y., Chiang C.-M., Kemper B. and Kemper J. K. (2016). Critical role of RanBP2-mediated SUMOylation of Small Heterodimer Partner in maintaining bile acid homeostasis. Nat. Commun. 7, 12179-12179 10.1038/ncomms12179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N., Takizawa M., Okita K., Natori O., Igarashi K., Ueno M., Nakashima K.-I., Nobuhisa I. and Taga T. (2002). Identification of a novel transcription factor, ELYS, expressed predominantly in mouse foetal haematopoietic tissues. Genes Cells 7, 435-446. 10.1046/j.1365-2443.2002.00529.x [DOI] [PubMed] [Google Scholar]
- Kramer N. J., Haney M. S., Morgens D. W., Jovičić A., Couthouis J., Li A., Ousey J., Ma R., Bieri G., Tsui C. K. et al. (2018). CRISPR-Cas9 screens in human cells and primary neurons identify modifiers of C9ORF72 dipeptide-repeat-protein toxicity. Nat. Genet. 50, 603-612. 10.1038/s41588-018-0070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn T. M. and Capelson M. (2019). Nuclear pore proteins in regulation of chromatin state. Cells 8, 1414 10.3390/cells8111414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai T.-H., Wu Y.-Y., Wang Y.-Y., Chen M.-F., Wang P., Chen T.-M., Wu Y.-N., Chiang H.-S., Kuo P.-L. and Lin Y.-H. (2016). SEPT12-NDC1 complexes are required for mammalian spermiogenesis. Int. J. Mol. Sci. 17, 1911 10.3390/ijms17111911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-G., Ueda M., Miyamoto Y., Yoneda Y., Perry G., Smith M. A. and Zhu X. (2006). Aberrant localization of importin α1 in hippocampal neurons in Alzheimer disease. Brain Res. 1124, 1-4. 10.1016/j.brainres.2006.09.084 [DOI] [PubMed] [Google Scholar]
- Leksa N. C., Brohawn S. G. and Schwartz T. U. (2009). The structure of the scaffold nucleoporin Nup120 reveals a new and unexpected domain architecture. Structure 17, 1082-1091. 10.1016/j.str.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone L., Colussi C., Gironi K., Longo V., Fusco S., Li Puma D. D., D'Ascenzo M. and Grassi C. (2019). Altered Nup153 expression impairs the function of cultured hippocampal neural stem cells isolated from a mouse model of Alzheimer's disease. Mol. Neurobiol. 56, 5934-5949. 10.1007/s12035-018-1466-1 [DOI] [PubMed] [Google Scholar]
- Lin D. H. and Hoelz A. (2019). The structure of the nuclear pore complex (an update). Annu. Rev. Biochem. 88, 725-783. 10.1146/annurev-biochem-062917-011901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Schneider H., Recino A., Richardson C., Goldberg M. W. and Rudd C. E. (2015). The immune adaptor SLP-76 binds to SUMO-RANGAP1 at nuclear pore complex filaments to regulate nuclear import of transcription factors in T cells. Mol. Cell 59, 840-849. 10.1016/j.molcel.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Yan M., Liang Y., Liu M., Zhang K., Shao D., Jiang R., Li L., Wang C., Nussenzveig D. R. et al. (2019). Nucleoporin Seh1 interacts with Olig2/Brd7 to promote oligodendrocyte differentiation and myelination. Neuron 102, 587-601.e587. 10.1016/j.neuron.2019.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo Y.-M. and Gale M. Jr (2011). Immune signaling by RIG-I-like receptors. Immunity 34, 680-692. 10.1016/j.immuni.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupu F., Alves A., Anderson K., Doye V. and Lacy E. (2008). Nuclear pore composition regulates neural stem/progenitor cell differentiation in the mouse embryo. Dev. Cell 14, 831-842. 10.1016/j.devcel.2008.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfeld J., Güttinger S., Hawryluk-Gara L. A., Panté N., Mall M., Galy V., Haselmann U., Mühlhäusser P., Wozniak R. W., Mattaj I. W. et al. (2006). The conserved transmembrane nucleoporin NDC1 is required for nuclear pore complex assembly in vertebrate cells. Mol. Cell 22, 93-103. 10.1016/j.molcel.2006.02.015 [DOI] [PubMed] [Google Scholar]
- Mehta S. J. K., Kumar V. and Mishra R. K. (2020). Drosophila ELYS regulates Dorsal dynamics during development. J. Biol. Chem. 295, 2421-2437. 10.1074/jbc.RA119.009451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mészáros N., Cibulka J., Mendiburo M. J., Romanauska A., Schneider M. and Köhler A. (2015). Nuclear pore basket proteins are tethered to the nuclear envelope and can regulate membrane curvature. Dev. Cell 33, 285-298. 10.1016/j.devcel.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake N., Tsukaguchi H., Koshimizu E., Shono A., Matsunaga S., Shiina M., Mimura Y., Imamura S., Hirose T., Okudela K. et al. (2015). Biallelic mutations in nuclear pore complex subunit NUP107 cause early-childhood-onset steroid-resistant Nephrotic syndrome. Am. J. Hum. Genet. 97, 555-566. 10.1016/j.ajhg.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molè M. A., Weberling A. and Zernicka-Goetz M. (2020). Comparative analysis of human and mouse development: from zygote to pre-gastrulation. Curr. Top. Dev. Biol. 136, 113-138. 10.1016/bs.ctdb.2019.10.002 [DOI] [PubMed] [Google Scholar]
- Monwan W., Kawasaki T., Hasan M. Z., Ori D. and Kawai T. (2020). Identification of nucleoporin 93 (Nup93) that mediates antiviral innate immune responses. Biochem. Biophys. Res. Commun. 521, 1077-1082. 10.1016/j.bbrc.2019.11.035 [DOI] [PubMed] [Google Scholar]
- Moreira T. G., Zhang L., Shaulov L., Harel A., Kuss S. K., Williams J., Shelton J., Somatilaka B., Seemann J., Yang J. et al. (2015). Sec13 regulates expression of specific immune factors involved in inflammation in vivo. Sci. Rep. 5, 17655 10.1038/srep17655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M., Valori C. F., Ansorge O., Kretzschmar H. A., Munoz D. G., Kusaka H., Yokota O., Ishihara K., Ang L.-C., Bilbao J. M. et al. (2012). Transportin 1 accumulates specifically with FET proteins but no other transportin cargos in FTLD-FUS and is absent in FUS inclusions in ALS with FUS mutations. Acta Neuropathol. 124, 705-716. 10.1007/s00401-012-1020-6 [DOI] [PubMed] [Google Scholar]
- Nishimura A. L., Župunski V., Troakes C., Kathe C., Fratta P., Howell M., Gallo J. M., Hortobágyi T., Shaw C. E. and Rogelj B. (2010). Nuclear import impairment causes cytoplasmic trans-activation response DNA-binding protein accumulation and is associated with frontotemporal lobar degeneration. Brain 133, 1763-1771. 10.1093/brain/awq111 [DOI] [PubMed] [Google Scholar]
- Niu X., Hong J., Zheng X., Melville D. B., Knapik E. W., Meng A. and Peng J. (2014). The nuclear pore complex function of Sec13 protein is required for cell survival during retinal development. J. Biol. Chem. 289, 11971-11985. 10.1074/jbc.M114.547190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Yamazoe K. and Inoue Y. H. (2020). Nuclear export of Cyclin B mediated by the Nup62 complex is required for meiotic initiation in Drosophila males. Cells 9, 270 10.3390/cells9020270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K., Kiyonari H., Nobuhisa I., Kimura N., Aizawa S. and Taga T. (2004). Targeted disruption of the mouse ELYS gene results in embryonic death at peri-implantation development. Genes Cells 9, 1083-1091. 10.1111/j.1365-2443.2004.00791.x [DOI] [PubMed] [Google Scholar]
- Olsson M., Schéele S. and Ekblom P. (2004). Limited expression of nuclear pore membrane glycoprotein 210 in cell lines and tissues suggests cell-type specific nuclear pores in metazoans. Exp. Cell Res. 292, 359-370. 10.1016/j.yexcr.2003.09.014 [DOI] [PubMed] [Google Scholar]
- Otsuka S. and Ellenberg J. (2018). Mechanisms of nuclear pore complex assembly - two different ways of building one molecular machine. FEBS Lett. 592, 475-488. 10.1002/1873-3468.12905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda D., Pascual-Garcia P., Dunagin M., Tudor M., Hopkins K. C., Xu J., Gold B., Raj A., Capelson M. and Cherry S. (2014). Nup98 promotes antiviral gene expression to restrict RNA viral infection in Drosophila. Proc. Natl. Acad. Sci. USA 111, E3890-E3899. 10.1073/pnas.1410087111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish I. A., Stamp L. A., Lorenzo A. M. D., Fowler S. M., Sontani Y., Miosge L. A., Howard D. R., Goodnow C. C., Young H. M. and Furness J. B. (2016). A novel mutation in nucleoporin 35 causes murine degenerative colonic smooth muscle myopathy. Am. J. Pathol. 186, 2254-2261. 10.1016/j.ajpath.2016.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Lee B., Clurman B. E. and Lee K. (2016). NUP50 is necessary for the survival of primordial germ cells in mouse embryos. Reproduction 151, 51-58. 10.1530/REP-14-0649 [DOI] [PubMed] [Google Scholar]
- Park E., Ahn Y. H., Kang H. G., Miyake N., Tsukaguchi H. and Cheong H. I. (2017). NUP107 mutations in children with steroid-resistant nephrotic syndrome. Nephrol. Dial. Transplant. 32, 1013-1017. 10.1093/ndt/gfw103 [DOI] [PubMed] [Google Scholar]
- Parrott B. B., Chiang Y., Hudson A., Sarkar A., Guichet A. and Schulz C. (2011). Nucleoporin98-96 function is required for transit amplification divisions in the germ line of Drosophila melanogaster. PLoS ONE 6, e25087 10.1371/journal.pone.0025087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil H., Saha A., Senda E., Cho K.-I., Haque M. E., Yu M., Qiu S., Yoon D., Hao Y., Peachey N. S. et al. (2014). Selective impairment of a subset of Ran-GTP-binding domains of ran-binding protein 2 (Ranbp2) suffices to recapitulate the degeneration of the retinal pigment epithelium (RPE) triggered by Ranbp2 ablation. J. Biol. Chem. 289, 29767-29789. 10.1074/jbc.M114.586834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Terzic C., Behfar A., Méry A., van Deursen J. M. A., Terzic A. and Pucéat M. (2003). Structural adaptation of the nuclear pore complex in stem cell-derived cardiomyocytes. Circ. Res. 92, 444-452. 10.1161/01.RES.0000059415.25070.54 [DOI] [PubMed] [Google Scholar]
- Perez-Terzic C., Faustino R. S., Boorsma B. J., Arrell D. K., Niederländer N. J., Behfar A. and Terzic A. (2007). Stem cells transform into a cardiac phenotype with remodeling of the nuclear transport machinery. Nat. Clin. Pract. Cardiovasc. Med. 4 Suppl. 1, S68-S76. 10.1038/ncpcardio0763 [DOI] [PubMed] [Google Scholar]
- Preston C. C., Wyles S. P., Reyes S., Storm E. C., Eckloff B. W. and Faustino R. S. (2018). NUP155 insufficiency recalibrates a pluripotent transcriptome with network remodeling of a cardiogenic signaling module. BMC Syst. Biol. 12, 62 10.1186/s12918-018-0590-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston C. C., Storm E. C., Burdine R. D., Bradley T. A., Uttecht A. D. and Faustino R. S. (2019). Nucleoporin insufficiency disrupts a pluripotent regulatory circuit in a pro-arrhythmogenic stem cell line. Sci. Rep. 9, 12691 10.1038/s41598-019-49147-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raices M. and D'Angelo M. A. (2012). Nuclear pore complex composition: a new regulator of tissue-specific and developmental functions. Nat. Rev. Mol. Cell Biol. 13, 687-699. 10.1038/nrm3461 [DOI] [PubMed] [Google Scholar]
- Raices M. and D'Angelo M. A. (2017). Nuclear pore complexes and regulation of gene expression. Curr. Opin. Cell Biol. 46, 26-32. 10.1016/j.ceb.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raices M., Bukata L., Sakuma S., Borlido J., Hernandez L. S., Hart D. O. and D'Angelo M. A. (2017). Nuclear pores regulate muscle development and maintenance by assembling a localized Mef2C complex. Dev. Cell 41, 540-554.e547. 10.1016/j.devcel.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton A. E., Majounie E., Waite A., Simón-Sánchez J., Rollinson S., Gibbs J. R., Schymick J. C., Laaksovirta H., van Swieten J. C., Myllykangas L. et al. (2011). A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 72, 257-268. 10.1016/j.neuron.2011.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverter D. and Lima C. D. (2005). Insights into E3 ligase activity revealed by a SUMO-RanGAP1-Ubc9-Nup358 complex. Nature 435, 687-692. 10.1038/nature03588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riparbelli M. G., Gigliotti S. and Callaini G. (2007). The Drosophila nucleoporin gene nup154 is required for correct microfilament dynamics and cell death during oogenesis. Cell Motil. Cytoskeleton 64, 590-604. 10.1002/cm.20206 [DOI] [PubMed] [Google Scholar]
- Rossanti R., Shono A., Miura K., Hattori M., Yamamura T., Nakanishi K., Minamikawa S., Fujimura J., Horinouchi T., Nagano C. et al. (2019). Molecular assay for an intronic variant in NUP93 that causes steroid resistant nephrotic syndrome. J. Hum. Genet. 64, 673-679. 10.1038/s10038-019-0606-4 [DOI] [PubMed] [Google Scholar]
- Rosti R. O., Sotak B. N., Bielas S. L., Bhat G., Silhavy J. L., Aslanger A. D., Altunoglu U., Bilge I., Tasdemir M., Yzaguirrem A. D. et al. (2017). Homozygous mutation in NUP107 leads to microcephaly with steroid-resistant nephrotic condition similar to Galloway-Mowat syndrome. J. Med. Genet. 54, 399-403. 10.1136/jmedgenet-2016-104237 [DOI] [PubMed] [Google Scholar]
- Sakuma S. and D'Angelo M. A. (2017). The roles of the nuclear pore complex in cellular dysfunction, aging and disease. Semin. Cell Dev. Biol. 68, 72-84. 10.1016/j.semcdb.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savas J. N., Toyama B. H., Xu T., Yates J. R. III and Hetzer M. W. (2012). Extremely long-lived nuclear pore proteins in the rat brain. Science 335, 942 10.1126/science.1217421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K., Cavodeassi F., Feng Y. and Stephens D. J. (2013). Early stages of retinal development depend on Sec13 function. Biol Open 2, 256-266. 10.1242/bio.20133251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz T. U. (2016). The structure inventory of the nuclear pore complex. J. Mol. Biol. 428, 1986-2000. 10.1016/j.jmb.2016.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T. and Vondrak K. (2018). First report of recurrent nephrotic syndrome after kidney transplantation in a patient with NUP93 gene mutations: a case report. Transplant. Proc. 50, 3954-3956. 10.1016/j.transproceed.2018.07.010 [DOI] [PubMed] [Google Scholar]
- Senger S., Csokmay J., Akbar T., Jones T. I., Sengupta P. and Lilly M. A. (2011). The nucleoporin Seh1 forms a complex with Mio and serves an essential tissue-specific function in Drosophila oogenesis. Development 138, 2133-2142. 10.1242/dev.057372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield L. G., Miskiewicz H. B., Tannenbaum L. B. and Mirra S. S. (2006). Nuclear pore complex proteins in Alzheimer disease. J. Neuropathol. Exp. Neurol. 65, 45-54. 10.1097/01.jnen.0000195939.40410.08 [DOI] [PubMed] [Google Scholar]
- Smitherman M., Lee K., Swanger J., Kapur R. and Clurman B. E. (2000). Characterization and targeted disruption of murine Nup50, a p27(Kip1)-interacting component of the nuclear pore complex. Mol. Cell. Biol. 20, 5631-5642. 10.1128/MCB.20.15.5631-5642.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souquet B., Freed E., Berto A., Andric V., Audugé N., Reina-San-Martin B., Lacy E. and Doye V. (2018). Nup133 is required for proper nuclear pore basket assembly and dynamics in embryonic stem cells. Cell Rep. 23, 2443-2454. 10.1016/j.celrep.2018.04.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strambio-De-Castillia C., Niepel M. and Rout M. P. (2010). The nuclear pore complex: bridging nuclear transport and gene regulation. Nat. Rev. Mol. Cell Biol. 11, 490-501. 10.1038/nrm2928 [DOI] [PubMed] [Google Scholar]
- Sun J., Shi Y. and Yildirim E. (2019). The nuclear pore complex in cell type-specific chromatin structure and gene regulation. Trends Genet. 35, 579-588. 10.1016/j.tig.2019.05.006 [DOI] [PubMed] [Google Scholar]
- Tang Y., Xiong S., Yu P., Liu F. and Cheng L. (2018). direct conversion of mouse fibroblasts into neural stem cells by chemical cocktail requires stepwise activation of growth factors and Nup210. Cell Rep. 24, 1355-1362.e1353. 10.1016/j.celrep.2018.06.116 [DOI] [PubMed] [Google Scholar]
- Texari L. and Stutz F. (2015). Sumoylation and transcription regulation at nuclear pores. Chromosoma 124, 45-56. 10.1007/s00412-014-0481-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Hsu J. Y., Linker S. B., Hu L., Schafer S. T., Mertens J., Jacinto F. V., Hetzer M. W. and Gage F. H. (2017). Nup153 interacts with Sox2 to enable bimodal gene regulation and maintenance of neural progenitor cells. Cell Stem Cell 21, 618-634.e617. 10.1016/j.stem.2017.08.012 [DOI] [PubMed] [Google Scholar]
- Toyama B. H., Savas J. N., Park S. K., Harris M. S., Ingolia N. T., Yates J. R. III and Hetzer M. W. (2013). Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell 154, 971-982. 10.1016/j.cell.2013.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama B. H., Arrojo E. D. R., Lev-Ram V., Ramachandra R., Deerinck T. J., Lechene C., Ellisman M. H. and Hetzer M. W. (2019). Visualization of long-lived proteins reveals age mosaicism within nuclei of postmitotic cells. J. Cell Biol. 218, 433-444. 10.1083/jcb.201809123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upla P., Kim S. J., Sampathkumar P., Dutta K., Cahill S. M., Chemmama I. E., Williams R., Bonanno J. B., Rice W. J., Stokes D. L. et al. (2017). Molecular architecture of the major membrane ring component of the nuclear pore complex. Structure 25, 434-445. 10.1016/j.str.2017.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uv A. E., Roth P., Xylourgidis N., Wickberg A., Cantera R. and Samakovlis C. (2000). members only encodes a Drosophila nucleoporin required for rel protein import and immune response activation. Genes Dev. 14, 1945-1957. [PMC free article] [PubMed] [Google Scholar]
- van Nieuwenhuijze A., Burton O., Lemaitre P., Denton A. E., Cascalho A., Goodchild R. E., Malengier-Devlies B., Cauwe B., Linterman M. A., Humblet-Baron S. et al. (2018). Mice deficient in nucleoporin Nup210 develop peripheral T cell alterations. Front. Immunol. 9, 2234-2234 10.3389/fimmu.2018.02234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Matunis M. J., Kraemer D., Blobel G. and Coutavas E. (1995). Nup358, a cytoplasmically exposed nucleoporin with peptide repeats, Ran-GTP binding sites, zinc fingers, a cyclophilin A homologous domain, and a leucine-rich region. J. Biol. Chem. 270, 14209-14213. 10.1074/jbc.270.23.14209 [DOI] [PubMed] [Google Scholar]
- Wu X., Kasper L. H., Mantcheva R. T., Mantchev G. T., Springett M. J. and van Deursen J. M. A. (2001). Disruption of the FG nucleoporin NUP98 causes selective changes in nuclear pore complex stoichiometry and function. Proc. Natl. Acad. Sci. USA 98, 3191-3196. 10.1073/pnas.051631598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Pan L., Li J., Huang B., Feng J., Li C., Wang S., The E., Liu Y., Yuan T. et al. (2015). Nucleoporin 35 regulates cardiomyocyte pH homeostasis by controlling Na+-H+ exchanger-1 expression. J. Mol. Cell Biol. 7, 476-485. 10.1093/jmcb/mjv054 [DOI] [PubMed] [Google Scholar]
- Zhang X., Chen S., Yoo S., Chakrabarti S., Zhang T., Ke T., Oberti C., Yong S. L., Fang F., Li L. et al. (2008). Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell 135, 1017-1027. 10.1016/j.cell.2008.10.022 [DOI] [PubMed] [Google Scholar]
- Zhang K., Donnelly C. J., Haeusler A. R., Grima J. C., Machamer J. B., Steinwald P., Daley E. L., Miller S. J., Cunningham K. M., Vidensky S. et al. (2015). The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature 525, 56-61. 10.1038/nature14973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Daigle J. G., Cunningham K. M., Coyne A. N., Ruan K., Grima J. C., Bowen K. E., Wadhwa H., Yang P., Rigo F. et al. (2018). Stress granule assembly disrupts nucleocytoplasmic transport. Cell 173, 958-971.e917. 10.1016/j.cell.2018.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Zhu J.-Y., Richman A., Fu Y., Huang W., Chen N., Pan X., Yi C., Ding X., Wang S. et al. (2019). Mutations in NUP160 are implicated in steroid-resistant Nephrotic syndrome. J. Am. Soc. Nephrol. 30, 840-853. 10.1681/ASN.2018080786 [DOI] [PMC free article] [PubMed] [Google Scholar]