Abstract
Protein aggregation is classically considered the main cause of neuronal death in neurodegenerative diseases (NDDs). However, increasing evidence suggests that alteration of RNA metabolism is a key factor in the etiopathogenesis of these complex disorders. Non-coding RNAs are the major contributor to the human transcriptome and are particularly abundant in the central nervous system, where they have been proposed to be involved in the onset and development of NDDs. Interestingly, some ncRNAs (such as lncRNAs, circRNAs and pseudogenes) share a common functionality in their ability to regulate gene expression by modulating miRNAs in a phenomenon known as the competing endogenous RNA mechanism. Moreover, ncRNAs are found in body fluids where their presence and concentration could serve as potential non-invasive biomarkers of NDDs. In this review, we summarize the ceRNA networks described in Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, amyotrophic lateral sclerosis and spinocerebellar ataxia type 7, and discuss their potential as biomarkers of these NDDs. Although numerous studies have been carried out, further research is needed to validate these complex interactions between RNAs and the alterations in RNA editing that could provide specific ceRNET profiles for neurodegenerative disorders, paving the way to a better understanding of these diseases.
Keywords: competing endogenous RNAs (ceRNA), neurodegenerative diseases (NDDs), extracellular/circulating biomarkers, microRNA, long non-coding RNA, circular RNA, pseudogene, mRNA, ceRNA network (ceRNET), RNA editing
1. Introduction
Neurodegenerative diseases (NDDs) are of increasing relevance in public health due to the aging of the global population. These common and complex disorders are characterized by a progressive and selective loss of neurons from specific regions of the central nervous system (CNS), the most prevalent NDDs being Alzheimer’s disease (AD), Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS). Although protein aggregation is a common hallmark for these disorders, there is growing evidence that alterations in RNA metabolism contribute to the etiopathogenesis of NDDs [1,2,3]. Defects at all levels of gene regulation, from RNA synthesis to degradation, have been associated with disease-specific alterations in RNA-binding proteins (RBPs) and non-coding RNAs (ncRNAs) [2,3]. Interestingly, approximately 80% of the human genome is transcribed as non-coding transcripts, whereas only 2% encodes proteins [4], highlighting the potential of ncRNAs as disease modifiers, and, given their particular abundance in the CNS, their potential contribution to NDDs onset and development [5,6].
ncRNAs can be classified into two groups according to their length: small ncRNAs (<200 nucleotides) and long ncRNAs (>200 nucleotides) [7]. Among small ncRNAs, microRNAs (miRNA) stand out, being around 22 nucleotides long and regulating gene expression at the post-transcriptional level in a sequence-specific manner [8]. Approximately 70% of the identified miRNAs are expressed in the brain [9] and have been described as major regulators of neuronal homeostasis, their misregulation being associated with pathological conditions of CNS [8]. The largest class of ncRNAs in the mammalian genome is long ncRNAs (lncRNAs), which can be further grouped into linear RNAs and circular RNAs [7,10]. Linear lncRNAs (hereon referred to as lncRNAs) are similar to protein-coding messenger RNA (mRNA) in sequence length and transcriptional and post-transcriptional behavior [7]. However, lncRNAs play a different cellular role compared to mRNAs. Moreover, they have been described to be involved in brain development, neuronal function, maintenance and differentiation [5]. Circular RNAs (circRNAs) represent a relatively recently discovered class of RNAs that, unlike linear RNAs, are characterized by a covalent bond that joins the 5′ and 3′ ends and confers increased stability (half-life of 48 h vs. 10 h for mRNAs) [11]. circRNAs are highly abundant in the brain, enriched in synaptoneurosomes and upregulated during neuronal differentiation [12], so they could be promising biomarkers in age-associated NDDs.
On the other hand, a considerable number of pseudogenes can be transcribed to ncRNAs, even though they have historically been regarded as inactive gene sequences [13,14]. In fact, there is mounting evidence that pseudogenes may modulate the expression of parental as well as unrelated genes [13,14]. Therefore, alteration of pseudogene transcription could perturb gene expression homeostasis leading to disease [13].
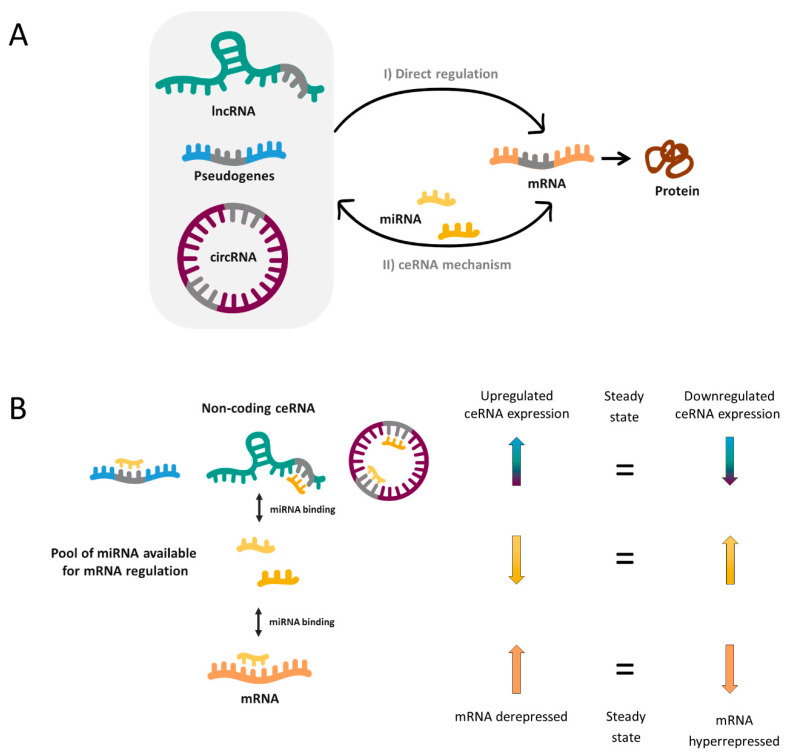
In 2011, Pier Paolo Pandolfi’s group proposed the so-called ceRNA hypothesis [15], which sought to explain how RNAs “talk” to each other, establishing interactions that modify functional genetic information and that may play major roles in pathological conditions. This hypothesis is based on the fact that miRNAs can recognize their specific target sites called miRNA response elements (MRE) in different RNA molecules, causing target repression via miRNA-RISC complex-mediated degradation. Thereby, miRNAs could mediate regulatory crosstalk between the diverse components of the transcriptome, comprising mRNAs and ncRNAs, which include pseudogenes, lncRNAs and circRNAs.
In a simplified manner, when two RNA molecules share the same MRE they potentially compete for the same pool of miRNAs. Thus, when the expression of a ceRNA is upregulated, it will bind and titrate more miRNAs (phenomenon called miRNA sponging), leaving fewer miRNA molecules available for binding the mRNA with shared MRE. Hence, this corresponding mRNA will become derepressed. In reverse, when the ceRNA levels are reduced as a consequence of a biological disturbance, the corresponding mRNA will be downregulated due to hyperrepression (Figure 1).
Figure 1.
(A) Transcriptional and post-transcriptional regulation of messenger RNAs (mRNAs) (orange) can be both influenced by direct and indirect mechanisms involving long non-coding RNAs (lncRNAs) (green), pseudogenes (blue) and circular RNAs (circRNAs) (purple). (I) Direct mechanisms include some processes that act on the transcription rate in the nucleus through the specific RNA-RNA complex and others that help the stability of mRNA molecules in the cytoplasm. (II) Competing endogenous RNA (ceRNA) mechanism is a bidirectional indirect regulation mechanism mediated by microRNAs (miRNAs) (yellow). miRNAs bind lncRNAs, pseudogenes, circRNAs and mRNAs through the miRNA response elements (MRE) (grey). (B) ceRNA hypothesis. Upregulation of a certain ceRNA (pseudogene, lncRNA or circRNA) expression can decrease cellular concentrations of the corresponding miRNA, resulting in the de-repression of other transcripts (mRNA) that contains the same MREs (left arrows). Conversely, the downregulation of a certain ceRNA would lead to increased concentrations of specific miRNAs and thus to hyperrepression of mRNA expression (right arrows).
Without doubt, the reality is more complex and a miRNA can bind more than one mRNA (50% of miRNAs are predicted to target 1–400 mRNAs and some of them up to 1000) [16]. Likewise, most ceRNAs contain 1 to 10 MREs [16] and, as a consequence, complex ceRNA networks involving a large number of RNA molecules are established. Novel bioinformatic and computational tools have enabled to elucidate an increasing number of ceRNA networks, as well as predict the most important enclaves of them. These may provide a valuable global vision to identify new biomarkers, underlying pathways or potential therapeutic targets for complex disorders such as NDDs.
The potential of ceRNAs as biomarkers, the major focus of this review, is augmented by the fact that ncRNAs have been found in body fluids like blood or urine free or inside extracellular vesicles including exosomes, which would allow obtaining new biomarkers in a non-invasive way. On this basis, emerging research on the role of ncRNAs in various diseases has arisen [17,18,19]. In this review, the potential of ceRNA networks as biomarkers in neurodegenerative diseases is discussed.
2. ceRNA Networks and Neurodegenerative Diseases
Over the last years, the ceRNA hypothesis has been corroborated by a large number of experiments. However, investigation of ceRNA mechanisms and their interaction networks has been mainly carried out in cancer research [20,21,22,23]. Nevertheless, some advances have also been made in the field of NDDs. Here, we aim to review the ceRNA networks (ceRNETs) reported to date in experimental (Table 1), and transcriptome profiling and bioinformatic studies (Table 2) in this field, classifying them according to the NDD they have been associated with.
Table 1.
miRNA-ceRNAs networks experimentally validated associated with NDDs.
| Disease | ncRNA | miRNA | mRNA | Sample | Ref. | |
|---|---|---|---|---|---|---|
| AD | lncRNA | BACE1-AS | miR-29, miR-485, miR-761, miR-124 and miR-107 |
BACE1 | Computational analysis from human data and cellular and mouse models | [24] |
| miR-214-3p | - | [25] | ||||
| miR-132-3p | - | [26] | ||||
| XIST | miR-124 | BACE1 | Cellular and mouse models | [27] | ||
| miR-132 | - | [28] | ||||
| NEAT1 | miR-124 | BACE1 | Cellular and mouse models | [29] | ||
| miR-107 | - | [30] | ||||
| SOX21-AS1 | miR-107 | - | Cellular model | [31] | ||
| NEAT1 HOTAIR MALAT1 |
miR-107, miR-103, miR-16, miR-195, miR-15a and miR-15b |
CDK5R1 | Cellular model |
[32] | ||
| MALAT1 | miR-125b |
CDK5, FOXQ1 and PTGS2 |
Cellular and rat models | [33] | ||
| miR-30b | CNR1 | [34] | ||||
| TUG1 | miR-15a | ROCK1 | Cellular and mouse models | [35] | ||
| SNHG1 | miR-137 | KREMEN1 | Cellular model and human primary cell culture | [36] | ||
| miR-361-3p | ZNF217 | [37] | ||||
| lncRNA-ATB | miR-200 | ZNF217 | Cellular model | [38] | ||
| LINC00094 | miR-224-4p miR-497-5p |
SH3GL2 | Cellular model | [39] | ||
| MIAT | miR-150-5p | VEGF | Cellular and mouse models | [40] | ||
| Rpph1 | miR-326 | PKM2 | Cellular and mouse models | [41] | ||
| miR-122 | Wnt1 | [42] | ||||
| miR-330-5p | CDC42 | [43] | ||||
| linc00507 | miR-181c-5p |
MAPT
TTBK1 |
Cellular and mouse models | [44] | ||
| lnc-ANRIL | mir-125a | TNF-α, IL1B IL6 and IL17 | Cellular model | [45] | ||
| circRNA | ciRS-7 | miR-7 | UBE2A | Human brain | [46] | |
| * miR-7 | * NF-Κb/p65 | Cellular models | [47,48,49] | |||
| circ_0000950 | miR-103 | PTGS2 | Cellular models | [50] | ||
| circHDAC9 | miR-138 | Sirt1 | Cellular and mouse models | [51] | ||
| miR-142-5p | - | [52] | ||||
| PD | pseudogene | GBAP1 | miR-22-3p | GBA | Cellular models | [53] |
| lncRNA | SNHG1 | miR-153-3p miR-15b-5p miR-7 miR-221/222 |
PTEN SIAH1, GSK3β NLRP3 CDKN1B (p27) |
Cellular and mouse models | [54] [55,56] [57] [58] |
|
| HAGLROs | miR-100 | ATG10 | Cellular and mouse models | [59] | ||
| HOTAIR | miR-874-5p | ATG10 | Cellular and mouse models | [60] | ||
| miR-126-5p | RAB3IP | [61] | ||||
| NEAT1 | miR-212-5p | RAB3IP | Cellular models | [62] | ||
| miR-1277-5p | ARHGAP26 | [63] | ||||
| miR-124 | - | [64] | ||||
| AL049437 | miR-205-5p | MAPK1 | Cellular and mouse models | [65] | ||
| MALAT1 | miR-205-5p | LRRK2 | Cellular and mouse models | [66] | ||
| miR-124 | DAPK1 | [67,68] | ||||
| miR-129 | SNCA (α-syn) | [69] | ||||
| SNHG14 | miR-133b | SNCA | Cellular and mouse models | [70] | ||
| LincRNA-p21 | miR-1277-5p | SNCA | Cellular and mouse models | [71] | ||
| miR-181 family |
PRKCD (PKC-δ) |
[72] | ||||
| miR-625 | TRPM2 | [73] | ||||
| GAS5 | miR-223-3p | NLRP3 | Cellular and mouse models | [74] | ||
| BDNF-AS | miR-125b-5p | - | Cellular and mouse models | [75] | ||
| Mirt2 | miR-101 | - | Cellular model | [76] | ||
| lncRNA H19 | miR-301b-3p | HPRT1 | Computational analysis from human data and cellular and mouse models | [77] | ||
| miR-585-3p | PIK3R3 | [78] | ||||
| circRNA | * ciRS-7 | miR-7 | SNCA | Cellular and mouse models | [79,80,81,82,83] | |
| circSNCA | miR-7 | SNCA | Cellular model | [84] | ||
| circzip-2 | * miR-60 | M60.4ZK470.2, igeg-2 and idhg-1 | Worm model | [85] | ||
| circDLGAP4 | miR-134-5p | CREB | Cellular and mouse models | [86] | ||
| MS | lncRNA | Gm15575 | miR-686 | CCL7 | Cellular and mouse models | [87] |
| PVT1 | miR-21-5p | SOCS5 | Cellular and mouse models | [88] | ||
| TUG | miR-9-5p | NFKB1 (p50) | Cellular and mouse models | [89] | ||
| HOTAIR | miR-136-5p | AKT2 | Cellular and mouse models | [90] | ||
| GAS5 | miR-137 | - | Human blood | [91] | ||
| circRNA | hsa_circ_0106803 | * miR-149 | * ASIC1a | Human blood (PMBCs) | [92,93] | |
| hsa_circ_0005402 hsa_circ_0035560 |
* 14 miRNAs (miR-1248, miR-766) |
- | Human blood (PMBCs) | [94] | ||
| SCA7 | lncRNA | lnc-SCA7 | miR-124 | ATXN7 | Human samples, and cellular and animal models | [95] |
* Experimental validation is needed.
Table 2.
miRNA-ceRNAs networks identified by transcriptome profiling studies and computational prediction associated with NDDs.
| Disease | ceRNETs | Sample | Outcomes | Representative Networks | Ref. |
|---|---|---|---|---|---|
| AD | lncRNA-miRNA-mRNA | Mouse model (APP/PS1) brain (cortical samples) 12 months |
One ceRNA network that included 4 lncRNAs, 5 miRNAs and 1082 mRNAs mainly related to AD-associated genes. |
|
[43] |
| Mouse model (APP/PS1) brain (cortical samples) 6 and 9 months |
3 ceRNA networks built with lncRNAs, miRNAs and mRNAs differentially expressed according to the age at which they are found deregulated (6, 9 or 6 and 9 months). |
|
[96] | ||
| Human brain (neurons from entorhinal cortex of mid-stage AD cases) |
A neurofibrillary tangles-associated ceRNA network was built with 41 lncRNAs, 630 mRNAs and 2530 edges. |
|
[97] | ||
| Human brain (prefrontal cortex) |
An AD-associated ceRNET containing 6 lncRNAs, 3 miRNAs and 91 mRNAs. |
|
[98] | ||
| circRNA-miRNA-mRNA | Mouse model (SAMP8) brain 7 months |
Two ceRNA networks built with circRNAs, miRNAs and mRNAs found differentially expressed. |
|
[99] | |
| Mouse model (Tg2576) brain 7 and 12 months |
Four ceRNA networks built with circRNAs, miRNAs and mRNAs found differentially expressed. |
|
[100] | ||
| Rat model (Aβ1-42) brain (hippocampal samples) |
A ceRNA network built with 140 circRNAs, 140 miRNAs and 20 mRNAs with 503 relationships. |
|
[101] | ||
| AD | circRNA-miRNA-mRNA | Mouse model (SAMP8) brain (hippocampal samples) 5 and 10 months |
A ceRNA network predicted. |
|
[102] |
| Mouse model (SAMP8) brain (hippocampal) 5 months |
Two ceRNETs built with 2 circRNAs found differentially expressed in PNS-treated mice: mmu_circ_013636, 5 miRNAs and 442 mRNAs, mmu_circ_012180, 5 miRNAs and 631 mRNAs. |
|
[103] | ||
| Mouse model (APP/PS1) brain (cortex) 6 and 9 months |
Five ceRNA networks were constructed based on differentially expressed circRNAs, miRNAs and mRNAs. |
|
[104] | ||
| Mouse model (5xFAD) pineal gland 5 months |
A circRNA-miRNA network was constructed with 10 circRNAs. From it, a complete ceRNA net was predicted. |
|
[105] | ||
| Human brain | An AD-associated ceRNET was constructed with 276 circRNAs, 14 miRNAs and 1117 mRNAs. AD risk ceRNET of KIAA1586 was stablished with 3 miRNAs (hsa-miR-29b, hsa-miR-101 and hsa-miR-15a) and 159 mRNAs. |
|
[106] | ||
| Human brain | A circRNA-mRNA co-expression network was predicted. |
|
[107] | ||
| AD | circRNA-miRNA-mRNA | Human brain | A circRNA-mRNA co-expression network was predicted |
|
[108] |
| Human cerebrospinal fluid | A circRNA-miRNA network was built with the top 5 up- and down-regulated circRNAs (circ-LPAR1, circ-AXL, circ-GPHN, circ-ITPR3, circ-GPI, circ-HAUS4, circ-KIF18B, circ-ATP9A, circ-PCCA and circ-TTC39C). |
|
[109] | ||
| Human blood (PBMCs) | Four nets constructed: (I) circRNA-miRNA network with the top 10 up- and down-regulated circRNAs (II) ceRNA network of hsa_circ_082547 (III) ceRNA network with 3 circRNAs (hsa_circ_101618, hsa_circ_405619, and hsa_circ_000843), 15 miRNAs and 223 mRNAs. (IV) ceRNA network of 4 circRNAs (hsa_circ_402265, hsa_circ_061346, hsa_circ_405836 and hsa_circ_061343), 20 miRNAs and 576 mRNAs. |
|
[110] | ||
| PD | lncRNA-miRNA-mRNA | PD cell model (SY-SH5Y cells treated with α-synuclein oligomer) |
PD-associated ceRNET that included the lncRNAs AC009365.4, RPS14P3 and G046036 together with the mRNAs IRF1, RIMKA, NAV1, SACS and SDC2. | [111] | |
| Human blood | A ceRNA regulatory network, including 7 lncRNAs (XIST1, PART1, MCF2L2, NOP14-AS1, LINC00328, LINC00302 and FAM215A), 3 miRNAs (miR-7, miR-433 and miR-133b), and 55 mRNAs. |
|
[112] | ||
| Human brain (substantia nigra) |
A ceRNA network associated with PD, that included 9 lncRNAs, 18 miRNAs, and 185 mRNA. |
|
[113] | ||
| circRNA-miRNA-mRNA | Mouse model (MPTP) brain |
A ceRNET built with 6 circRNAs (circ_0003292, circ_0001320, circ_0005976, circ_0005388, circ_0012384, and circ_003328), 13 miRNAs and 112 mRNAs. |
|
[114] | |
| Human brain | A circRNA-associated ceRNA network predicted and validated. |
|
[115] | ||
| ALS | circRNA-miRNA-mRNA | Human blood (leukocytes) |
Two networks predicted. |
|
[116,117] |
We searched the PubMed database for articles in English. The main search terms included neurodegenerative diseases, Alzheimer’s disease, AD, Parkinson’s disease, PD, multiple sclerosis, MS, amyotrophic lateral sclerosis, ALS or spinocerebellar ataxia type 7, combined with competing endogenous RNA, ceRNA, long non-coding RNA, lncRNA, circular RNA, circRNA, pseudogene or miRNA. Since this is a relatively new research topic, no filter for the years was applied. We hand-searched the retrieved articles and selected the most relevant articles based on a subjective evaluation of their quality and relevance. Additional articles on specific topics were searched if needed.
2.1. ceRNA and Alzheimer’s Disease
In the context of NDDs, most ceRNA research has been done in AD, probably due to its growing prevalence in the aged population of most developed countries [118]. Such is the case that it is expected to affect 1 in 85 people worldwide by 2050 [119].
At molecular level, dementia associated with AD is characterized by proteinopathy with extracellular deposition of β-amyloid (Aβ) plaques in the brain and the presence of TAU neurofibrillary tangles (NFTs) in neurons. Consequences of AD at cellular level include deregulation of redox homeostasis and low-grade chronic inflammation [120].
2.1.1. LncRNAs
As mentioned above, the deposition of Aβ plaques in the brain is one of the hallmarks of AD. β-secretase 1 (BACE1), one of the enzymes involved in the Aβ formation, is elevated in brains of AD patients [121,122]. Therefore, a misregulation of BACE1 may play an important role in this pathology. Both miRNAs and lncRNAs have been implicated in BACE1 post-transcriptional regulation, one of the most relevant being the lncRNA BACE1-AS [24], an antisense transcript that regulates the expression of the homonym enzyme [123]. Also, BACE1-AS levels are increased in the plasma of AD patients and cell models [25]. Furthermore, Zheng and colleagues found that BACE1-AS shares many MREs with BACE1 in a ceRNA regulation system that involves several miRNAs including miR-29, miR-107 and miR-124 [24]. Indeed, the authors demonstrated in vivo that BACE1-AS overexpression increased Aβ production in transgenic mouse brains. A subsequent computational study from available RNA-seq data published in the GEO database verified that levels of BACE1-AS and implicated miRNAs were also altered in AD clinical samples. Collectively, these data suggest that BACE1-AS acts as ceRNA to sequester miRNAs that target BACE1, thus preventing BACE1 mRNA from degradation. This mechanism complements the one previously reported by which BACE1-AS increases the stability of BACE1 mRNA through the formation of RNA duplexes [123]. BACE1-AS may also aggravate neurotoxicity in AD through other ceRNA mechanisms. It has been shown to sponge miR-214-3p and alter autophagy homeostasis. In fact, miR-214-3p levels are reduced in plasma from AD patients and in cell models [25], being this microRNA an inhibitor of autophagy and neuron apoptosis through transcriptional blockade of Atg12 and having hence a neuroprotective effect [124].
In addition, BACE1-AS is a ceRNA for miR-132-3p [26], which is downregulated in AD patients [125] and has been shown to provide neuroprotection in the disease via modulating different target mRNAs and affecting multiple pathways, including regulation of synaptic proteins, tau phosphorylation and amyloid aggregation [126,127,128,129,130].
Besides BACE1-AS, other lncRNAs such as XIST (through miR-124), NEAT1 (which bind miR-124 and miR-107) and SOX21-AS1 (targeting miR-107) may regulate BACE1 mRNA levels in AD cell and mouse models [27,29,30,31]. This highlights the complexity of BACE1 regulation in AD being mediated by several ceRNA networks.
Other members of the miR-15/107 family can also regulate BACE1 expression and the levels of other genes involved in AD [131]. In this line, NEAT1 along with two other lncRNAs, HOTAIR and MALAT1, bind miR-107, miR-103, miR-16, miR-195, miR-15a and miR-15b, exerting a regulatory effect on CDK5R1 levels [32]. CDK5R1 codes for the main activator of cyclin-dependent kinase 5 (CDK5), p35, essential for brain development and functioning so that its deregulation could be implicated in AD onset and progression [32]. Interestingly, in AD cellular models the expression of CDK5 (along with PTGS2 and FOXQ1) is regulated by miR-125b, which in turn is reversely regulated through MALAT1 [33]. Furthermore, MALAT seems to be involved in a third ceRNA network in AD through miR-30b and CNR1, a CNS-enriched cannabinoid receptor associated with learning and memory impairment, and significantly downregulated in AD [34].
Besides miR-124, the lncRNA XIST targets another miRNA and could participate in more than one ceRNET in AD. Wang et al. [28] showed that XIST knockdown inhibits Aβ protein fragment (Aβ25-35)-induced toxicity, oxidative stress and apoptosis in hippocampal neurons by binding miR-132, a miRNA widely reported in AD and known to target SIRT1 [132,133]. Curiously, SIRT1 is involved in neuroinflammation and mitochondrial dysfunction in AD and regulates Aβ production through ROCK1 or ADAM10 [134,135,136,137]. In this line, Li et al. [35] reported that ROCK1 is upregulated in AD cellular and mouse models and silencing of the lncRNA TUG1 depresses apoptosis of hippocampal neurons (like XIST1 knockdown) by elevating miR-15a and repressing ROCK1 expression. ROCK1 is a ubiquitous serine/threonine kinase whose reduction has been reported to diminish Aβ levels by enhancing APP protein degradation in AD [138].
Aβ peptides have also been related to other lncRNAs that have been proposed to act as ceRNA in AD: the lncRNAs SNHG1, lncRNA-ATB, LINC00094, MIAT1 and Rpph1. Small nucleolar RNA host gene 1 (SNHG1) is a recently described lncRNA involved in the development of multiple human tumors, as well as in other types of diseases such as AD or PD [36,37,54,55,56,57,58,139,140,141,142,143,144]. In AD in vitro models, SNHG1 is upregulated and acts as a ceRNA for miR-137, regulating KREMEN1 levels [36] and as a miR-361-3p sponge, modulating ZNF217 [37]. KREMEN1 is a Wnt antagonist that also has pro-apoptotic effects in cells in a Wnt-independent manner [145,146], whereas ZNF217 (zinc finger gene 217) has been described as a potential oncogene [147]. Notably, both studies reported that SNHG1 silencing partially reversed cell injury induced by Aβ25-35. Similar to SNHG1, suppression of the lncRNA-ATB protects cells against Aβ25-35-induced neurotoxicity by modulating ZNF217 [38]. Nevertheless, this lncRNA may not act by regulation of miR-361-3p, but through miR-200.
LncRNA LINC00094 (also known as BRD3OS) has been reported to regulate blood-brain barrier (BBB) permeability in AD microenvironment by sponging miR-224-5p and miR-497-5p, both of which target SH3GL2 mRNA [39]. SH3GL2 codes Endophilin-1, an endocytosis protein markedly increased in the AD brain and involved in Aβ induced postsynaptic dysfunction [148]. Taken that miR-107 has been shown to protect from Aβ-induced BBB disruption and endothelial cell dysfunction by targeting SH3GL2 mRNA [149], it seems possible that BACE1-AS, NEAT1, SOX21-AS1, HOTAIR and MALAT1 may also be involved in Aβ toxicity by regulating Endophilin-1 levels.
LncRNA MIAT (myocardial infarction associate transcript) is aberrantly expressed under neurovascular dysfunction [40], a condition that also aggravates AD pathogenesis by hindering Aβ clearance and, thus, increasing plaque levels in the brain [150]. In this sense, it has been proposed that MIAT modulates neural and vascular cell function and survival through the MIAT/miR-150-5p/VEGF axis, acting as a vascular dysfunction regulator [40,151,152]. Based on these premises, Jiang et al. studied the effect of MIAT knockdown in vivo [40] and observed a decrease in the number of cerebral microvessels, exacerbated neuronal loss, brain β-amyloidosis and neurodegeneration in mice, as well as behavioral deficits with significant impairment in the spatial learning capacity and memory. Thus, this work demonstrated the lncRNA MIAT involvement in the maintenance of adequate microvascular and neuronal function.
In contrast to the lncRNAs discussed above, Rpph1 (ribonuclease P RNA component H1) seems to exert a neuroprotective compensation mechanism in AD pathology through three different ceRNA axes: Rpph1/miR-326/PKM2 [41], Rpph1/miR-122/Wnt1 [42] and Rpph1/miR-330-5p/CD42 [43]. In particular, it has been shown to attenuate Aβ25-35-induced endoplasmic reticulum stress, neuronal injury and apoptosis [41,42], as well as to promote hippocampal neuron dendritic spine formation [43]. Rpph1 was first identified from bioinformatic analysis of whole transcriptome and microRNA sequencing data from a 12-month-old APP/PS1 transgenic mouse model of AD [43], where it was found upregulated in the cortex. This study not only helped to identify Rpph1 as an AD-related ceRNA, but also to establish a whole lncRNA-miRNA-mRNA ceRNET including 4 lncRNAs (C030034L19Rik, Rpph1, A830012C17Rik and Gm15477), 5 miRNAs (miR-182-5p, miR-330-5p, miR-326-3p, miR-132-3p and miR-484), and 1082 mRNAs. It was the first AD-associated ceRNA network based on APP/PS1 mouse model and was found to be enriched in mRNAs related to AD-associated genes, various signaling pathways (MAPK, neurotrophin, insulin/IGF, ErbB), as well as in the regulation of actin cytoskeleton, adherent junction, axon guidance and long-term potentiation.
In continuation to this work, the same laboratory studied the RNA expression profile in cortex samples from the same mouse model at different ages to determine the distinct lncRNA-associated ceRNA network (LncACeNET) that could be participating in the progression of the disease depending on the stage [96]. In general terms, these networks were found to be mainly involved in the cytoskeleton, postsynaptic density, cell–cell adherens junction and dendrite. Interestingly, LncACeNETs differentially expressed in the early stage model (associated with AD pathophysiology) differed from those altered in the advanced stage (more related to AD development), although a few lncACeNETs seemed to be contributing to AD pathology throughout the disease progression. Of all axes raised, authors highlight a series of lncRNAs that were identified as mmu_miR-122-5p and mmu_miR-679-5p ceRNAs, which both target Klf4 (upregulated at the early stage of the disease) and Akap5 (downregulated at late-stage), respectively, and LNC_000033, which acts as a ceRNA of 5 miRNAs that regulate the expression of Synpo. Klf4 and Akap5 are two genes related to Aβ-induced neuroinflammation, and synaptic plasticity and memory, respectively. As for Synpo, it codes for synaptopodin, an essential protein for dendritic spine plasticity of the developing hippocampus that was found upregulated at early stage [96].
Similarly, LncACeNETs have also been constructed based on human samples [97,98]. Wang and coworkers [97] built the first ceRNA network associated with NFTs in AD patients. The network built contained 41 lncRNAs and 630 mRNAs. Of these lncRNAs, three stand out: AP000265.1, RP1-145M9.4 and KB1460A1.5, focused on NFT related biological processes, including JNK cascade, protein phosphorylation and formation and development of neural tube, neural crest cells and epithelial tube morphogenesis. Recently, a second LncACeNET was constructed helping to identify neuroinflammation-related biomarkers for AD [98], including the lncRNA CTB-89H12.4. This lncRNA was shown to competitively bind miR-155-5p and to be significantly co-expressed with a group of genes involved in tau phosphorylation and amyloid formation, intercellular signal transduction and synapse function in addition to neuroinflammation.
As CTB-89H12.4, two other lncRNAs have recently been implicated in AD pathogenesis through tau phosphorylation and neuroinflammation. First, Yan et al. [44] demonstrated in AD cellular and mouse models that linc00507 mediated tau protein hyperphosphorylation by the activation of the p25/p35/GSK3β signaling pathway through regulating MAPT and TTBK1 by sponging miR-181c-5p. As mentioned above, p35 is the main activator of CDK5, a kinase implicated in AD onset and progression [32]. Interestingly, p25 is the cleavage product of p35 that is able to bind to and activate CDK5 and the stability of the p25-CDK5 complex is higher than that of p35-CDK5 [44,153,154]. Hence, linc00507 could also be involved in CDK5 activity like the lncRNAs NEAT1, HOTAIR and MALAT1, but in this case in a more indirect way. Second, Zhou et al. [45] showed that the lnc-ANRIL could regulate neuroinflammation in AD since its knockdown suppressed apoptosis and pro-inflammatory cytokines (TNF-α, Il-1β, Il-6 and Il-17) and promoted neurite outgrowth by targeting miR-125a in an AD cellular model.
2.1.2. CircRNAs
LncRNAs are not the only ncRNAs proposed as AD-associated ceRNAs, as these also include circRNAs. One of the best-studied circRNAs is CDR1as, an antisense circular transcript of the Cerebellar Degeneration-Related protein 1 (CDR1), highly expressed in the brain [155]. This circRNA appears to stabilize the CDR1 mRNA [156] and to act as a miR-7 sponge, thereby being also called ciRS-7 [81]. Interestingly, the levels of ciRS-7/CDR1as are decreased in the hippocampus and neocortex of AD patients, which results in excess ambient miR-7 that downregulates its target mRNAs, such as that of ubiquitin-conjugating enzyme UBE2A that is essential for Aβ clearance [46]. This was the first circRNA-miRNA-mRNA axis reported to be dysregulated in AD [46]. Nevertheless, ciRS-7 could be part of another ceRNA protective network in AD by promoting the expression of ubiquitin C-terminal hydrolase UCHL1 that mediates APP and BACE1 degradation in an NF-κB dependent manner [47]. Although NF-κB p65 subunit mRNA levels were not affected by ciRS-7 here, other studies have shown that the expression of p65 is downregulated by miR-7 [48,49]. Therefore, ciRS-7/miR-7/NF-κB(p65) could represent another potential axis in AD and it might be influenced by APP, since it may reduce the levels of ciRS-7 [47].
Recently, other circRNAs have been found altered in AD, but, unlike ciRS-7, they are less well known. Among these, circ_0000950 promotes neuronal apoptosis, enhances inflammatory cytokine levels and suppresses neurite outgrowth in two cellular models of AD by directly sponging miR-103 and increasing the mRNA expression of PTGS2, a pro-inflammatory gene reversely regulated by miR-103 [50]. Another circRNA altered in AD is circHDAC9, which is decreased in animal and cellular models of this NDD, leading to the hyperrepression of the mRNAs regulated by miR-138 such as Sirt1, since it has been proposed to act as a sponge for this miRNA. As previously mentioned, Sirt1 regulates Aβ production in AD through ROCK1 or ADAM10 [134,135,136,137]. In agreement with the results obtained in animal and cell models, lower levels of circHDAC9 were found in the serum of AD patients [51]. This could be explained by the fact that Aβ downregulates circHDAC9, which, in turn, increases miR-138 expression and leads to a decrease of Sirt1 and ADAM10 levels, thus mediating synaptic function and APP processing in AD [51]. In line with this, Zhang et al. [52] demonstrated that the 42-residue β-amyloid (Aβ42) also triggers a significant downregulation of circHDAC9 in human neuronal cells, acting in this case as a sponge of miR-142-5p. Furthermore, the neuroprotective drug berberine alleviated Aβ42-induced neuronal damage in this cellular model by up-regulating circHDAC9 [52]. Although the study did not establish an mRNA target for miR-142-5p, it revealed a novel circRNA-miRNA axis in AD and opened the door to elucidate the entire ceRNA network. One possibility is that miR-142-5p may affect PSD-95 (a major scaffold postsynaptic protein that has been reported to be downregulated in brains of AD patients) by regulating the expression of AKAP5 and DRD1, which are known to interact with PSD-95 [157]. As previously mentioned, AKAP5 is a gene related to synaptic plasticity and memory and is part of a LncACeNET in AD [96]. As for DRD1, it is a dopamine receptor whose dysregulation could contribute to synaptic injury in AD [158].
The rest of the circRNA-associated ceRNA networks (cirCeNET) described in AD have been identified by transcriptome profiling studies in different animal models and patient samples. In the brain of the senescence-accelerated mouse model (SAMP8), Zhang et al. [99] constructed two AD-related cirCeNETs with circRNAs, miRNAs and mRNAs found differentially expressed. Among these, two ceRNA axes were identified as most likely to be involved in AD pathogenesis. On one hand, six circRNAs were predicted to act as sponges for the miRNA let-7g-3p and regulate a non-histone chromatin protein Hmgb2, involved in the Aβ clearance through up-regulation of the low density lipoprotein receptor-related protein Lrp1 [159,160]. On the other hand, five circRNAs could target miR-122-5p and control iodothyronine deiodinase Dio2 mRNA expression, which is reduced in AD patients and is related to the myelination process [99,161]. In brain samples from the APP Tg2576 mouse model, Lee et al. [100] identified four AD-related cirCeNETs at two different disease stages. No circRNAs were consistently dysregulated at both stages (except for the mmu_circ_29980), suggesting a high time dependence in the regulation of the circRNA expression. One example of ceRNA axis predicted in this study in 12-month-old brain is mmu_circRNA_37345/miR_335-3p/SLY. SLY encodes Silymarin, a natural Aβ aggregation inhibitor with a large potential for the treatment of AD [162,163,164].
Other studies carried out in animal models analyzed specific brain areas such as the hippocampus, cortex or pineal gland. In the hippocampus of Aβ1-42-induced AD rat model [101], Wang et al. predicted an AD-related cirCeNET with 140 circRNAs, 140 miRNAs and 20 mRNAs. For example, circ_101834 and circ_004690/miR-7a-5p/Aqp3, where Aqp3 codes for an aquaporin expressed in astrocytes and neurons, but its role in AD remains scarcely investigated [165]. In hippocampal samples too, but from SAMP8 mice model, Huang et al. [102] established a putative ceRNA network with one of the most significantly dysregulated circRNAs (mmu_circRNA_017963). This circRNA was strongly related to autophagosome assembly, exocytosis, apoptotic process, transport and RNA splicing, and it might potentially interact with 5 miRNAs and 313 mRNAs. Interestingly, in this same mouse model and tissue, Huang et al. studied the AD-associated circRNAs profile after treatment with Panax notoginseng saponins (PNS), used in traditional Chinese medicine [103]. Seven circRNAs were found significantly altered, and ceRNA networks were predicted for two circRNAs (mmu_circRNA_013636 and mmu_circRNA_012180). One example of ceRNA axis predicted was mmu_circRNA_012180/mmu_miRNA_6972-5p/Gsdmd. GSDMD is a key executive protein of pyroptosis, a highly inflammatory form of programmed cell death that has been reported to be an AD therapeutic target [166]. Thus, this axis could help to understand the underlying mechanism of action of PNS, which has been speculated to be a multi-targeted agent with anti-inflammatory properties [167]. In the cortex of APP/PS1 mice, Ma et al. [104] constructed five cirCeNETs. Among them, four were highlighted. Six circRNAs could bind to miR-466b-5p and regulate the expression of Scube2, an epidermal growth factor involved in neural development [168]. Four miRNAs may target Sorbs2, a gene that influences memory and dendritic development and its variants consistently were associated with delayed onset in AD [169,170]. Seven circRNAs could sponge miR-122b-3p and control the expression of Cntnap2, involved in the development of neural systems critical for learning and cross-modal integration [171]. Mmu_circ_0044900 could be a ceRNA for four miRNA that target Creb mRNA. CREB is an important transcriptional factor in the regulation of brain-derived neurotrophic factor (BDNF) and with the CREB-BDNF signaling pathway modulating cognitive status and Aβ toxicity in AD [172]. In the pineal gland of 5xFAD mice, Nam et al. [105] constructed a circRNA-miRNA network with the 10 circRNAs whose expression differences were more significant or whose expression levels were high. From it, a complete ceRNET was established, where circMboat2 and circNlrp5-ps could sponge miR-483 and regulate the expression of Aanat, a critical enzyme for melatonin synthesis [173].
In AD patients, three studies have provided circRNA expression profiling in the brain, and two in body fluids. Using different brain regions, Zhang et al. [106] constructed an AD-related cirCeNET with 276 circRNAs, 14 miRNAs and 1117 mRNAs. KIAA1586 ranked first within the AD risk circRNA-associated ceRNAs. This circRNA was predicted to competitively bind to 3 miRNAs (hsa-miR-29b, hsa-miR-101 and hsa-miR-15a) and regulate 159 mRNAs (some of them from AD-risk genes). Examples of ceRNA axes extracted from this network are KIAA1586/hsa-miR-15a/PSEN2, KIAA1586/hsa-miR-101/UBE2A and KIAA1586/hsa-miR-15a, hsa-miR-29b/BACE1. Accordingly, other studies have demonstrated the relationship between miR-29b and BACE1 in AD [174,175], and the implication of miR-101 and miR-15a in the pathogenesis of the disease through the regulation of other AD-risk genes, such as APP [174,176].
In the brain cortex of patients, Dube et al. [107] identified AD-associated circRNAs through meta-analysis and generated a circRNA and linear mRNA co-expression network in order to infer the biological and pathological relevance of these circRNAs based on the linear transcripts they co-expressed with. circHOMER1 and circCORO1C were pointed out as prognostic and diagnostic biomarkers in AD. CircHOMER1 is derived from HOMER1, a gene involved in Aβ processing whose dysregulation may underlie the early phase of memory loss that occurs in AD [177,178,179]. This circRNA was predicted to have five binding sites for miR-651, which may target the AD-related genes PSEN1 and PSEN2. Likewise, circCORO1C was predicted to contain two binding sites for miR-105 that target APP and SNCA.
Similar to the last study, Lo et al. [108] investigated the circRNA profiles at different AD stages in four brain regions and constructed a circRNA-mRNA co-expressed network in the parahippocampal gyrus. In this net two circRNAs (hsa_circ_0000994 and hsa_circ_0005232) are originated from SLC8A1, a gene that codes for Na+/Ca2+ Exchange Protein 1 (NCX1) which could exert a neuroprotective role in AD [180]. Both circRNAs were predicted to be co-expressed with EPHA4, an emerging Aβ oligomer receptor that could be involved in the synaptic spine alterations found in AD [181]. However, Lo et al. did not predict miRNA binding sites in these circRNAs that could explain this co-expression through a competing endogenous mechanism. Future studies will be needed to elucidate common MREs in these circRNAS and EPHA4 mRNA and confirm this ceRNA network.
In cerebrospinal fluid (CSF) from patients, Li et al. [109] constructed a circRNA-miRNA network with the top five up- and down-regulated circRNAs. Although this study did not predict mRNA targets, it pointed out two potential ceRNA networks. circ-TTC39C could sponge miR-210-3p, which induces dopaminergic neuron damage by reducing BDNF [182]. In turn, circ-PCCA may bind to miR-138-5p, which promotes tau phosphorylation by targeting retinoic acid receptor alpha (RARA) involved in the regulation of GSK-3β activity [183]. Another possible axis is circ-PCCA/miR-138-5p/SIRT1 since miR-138-5p could also bind to SIRT1 and mediate APP processing in AD (see above) [51]. This study also explored the clinical value of these circRNAs, and three of them (circ-AXL, circ-GPHN and circ-PCCA) could be potential biomarkers for predicting disease risk, guiding management and decision-making of AD [109].
In peripheral blood mononuclear cells (PBMCs) of patients, Li et al. [110] constructed a circRNA-miRNA network with the top 10 up- and down-regulated circRNAs, the ceRNA network of hsa_circ_082547 (one of the upregulated circRNAs, which could bind to more than 100 miRNAs and regulate 4 mRNAs), a ceRNA network with 3 circRNAs, 15 miRNAs and 223 mRNAs and a ceRNA network with 4 circRNAs, 20 miRNAs and 576 mRNAs. These networks were predicted to be strongly associated with inflammation, metabolism, and immune responses, which are all AD risk factors. Some examples of ceRNA axes potentially involved in AD pathogenesis are hsa_circ_061346/hsa-miR-5916-3p/APP, hsa_circ_000843/hsa-miR-335-3p/SLC8A1 (involved in neuroprotection) and hsa_circ_061346/hsa-miR-103a-2-5p/HOMER1 (involved in Aβ processing). Interestingly, this study also found that the differentially expressed circRNAs partially overlapped in this network analysis. In this sense, 13 circRNAs contain MRE sequences for hsa-miR-455-3p, which has been confirmed to bind at the 3’UTR of the APP gene and regulates its expression, exerting a protective effect on AD [184].
2.2. ceRNA and Parkinson’s Disease
PD is the second most frequent neurodegenerative disease after AD, and the most prevalent movement disorder, affecting approximately 1% of the population aged over 60 [185]. The etiology of PD is not fully understood, with age being the main risk factor. Clinically, it is characterized by bradykinesia (slow movement and impaired ability to move), rest tremor, muscle rigidity and postural instability [186]. At molecular level, these symptoms are caused by the loss of dopaminergic neurons of the substantia nigra pars compacta (SNpc) and the consequent loss of dopamine levels in the striatum [187]. In addition to this neuronal loss, PD is characterized by the presence of ubiquitinated cytoplasmic protein inclusions in the neurons located in the affected areas of the brain. These inclusions, known as Lewy bodies, are mainly composed of α-synuclein, product of the SNCA gene [188].
2.2.1. Pseudogenes and lncRNAs
The first ceRNA network described in PD involved the regulation of GBA gene and its 96% homology pseudogene GBAP1 by miR-22-3p binding [53]. GBA encodes a lysosomal glucocerebrosidase and its mutations represent the major genetic predisposing factor for PD [189]. Moreover, GBA has been closely related to key processes in this disease, including α-synuclein aggregation, lysosomal and autophagy dysfunction and endoplasmic reticulum stress [190]. However, this is not the best studied ceRNA in Parkinson’s disease.
The lncRNA small nucleolar RNA host gene SNHG1 has been found upregulated in in vitro models of PD from neurons and microglia, as well as murine models [54,55,56,57,58], and it seems to contribute to PD pathogenesis through several complementary ceRNA mechanisms. It decreases viability and increases apoptosis in neurons by inhibiting miR-153-3p via sponging, which in turn regulates PTEN expression [54]. PTEN is an endogenous inhibitor of the PI3K/AKT/mTOR signaling pathway that has been previously demonstrated to be implicated in PD progression [191]. This is consistent with the fact that the PI3K/AKT/mTOR signaling pathway has been shown to prevent PD by promoting the survival and growth of dopaminergic neurons [192] and that miR-153-3p mediates neuroprotective effects against MPP+-induced cytotoxicity. It is worth noting that this signaling pathway has been also associated with the HAGLROS/miR-100/ATG10 LncACeNET [59]. Additionally, SNHG1 promotes α-synuclein aggregation and toxicity by miR-15-5p binding and activating SIAH1 [56], an E3 ubiquitin ligase that promotes α-synuclein aggregation and apoptotic neuronal death [193,194]. miR-15-5p also targets the GSKβ3 gene, establishing a new ceRNA axis implicated in neuron cytotoxicity and reactive oxygen species production [55]. Furthermore, SNHG1 contributes to neuronal death by competitively binding the miR-221/222 cluster and indirectly enhancing the expression of p27/mTOR, thus impairing autophagy [58]. On another note, this lncRNA also promotes microglial activation and neuroinflammation, aggravating PD pathology, through the SNGH1/miR-7/NLRP3 axis [57]. Accordingly, SNHG1 silencing has been shown to promote autophagy [58], attenuate microglial activation and reduce dopaminergic neuron loss in the SNpc of PD mice [57].
LncRNA HOTAIR is overexpressed in both in vitro and in vivo models of PD [60,61,195]. This lncRNA was shown to induce neuronal injury by sponging miR-874-5p and stimulating ATG10 [60], a gene also regulated by the HAGLROS/miR-100 axis [59] that codes for an enzyme essential for autophagosome formation and, therefore, autophagy. Moreover, HOTAIR positively regulates the expression of leucine-rich repeat kinase LRRK2 [195], whose mutations have been linked to both genetic and sporadic forms of PD [196]. HOTAIR knockdown protects against neuronal apoptosis in a PD cell model by repressing caspase 3 activity [195]. This lncRNA also regulates RAB3IP, an important activator of Rab proteins, via miR-126-5p sponging in PD models [61]. The role of RAB3IP in PD is still unknown, although it has been reported to regulate neurite outgrowth and spinal development [197,198]. In this sense, the HOTAIR/miR-126-5p/RAB3IP axis could provide a new pathomechanism and therapeutic target in the disease. To unravel the role of HOTAIR in vivo, PD mice were injected with HOTAIR shRNA, resulting in protection against the initiation and development of PD, including cognitive impairment and bradykinesia. At molecular level, these mice had lower levels of RAB3IP and increased miR-126-5p expression compared to the PD control group. Moreover, at cellular level, shRNA-HOTAIR increased the number of TH-positive cells, reduced α-synuclein-positive cells and protected neurons from apoptosis [61].
RAB3IP is also a part of another ceRNA axis, in this case together with the lncRNA NEAT1 and miR-212-5p [62]. This axis was found unbalanced in a cell model of PD, with NEAT1 and RAB3IP being upregulated and miR-212-5p downregulated. Importantly, NEAT1 is increased in peripheral blood cells of PD patients [199]. Its knockdown or miR-212-5p overexpression in vivo suppresses neuronal apoptosis, inflammation and cytotoxicity [62], supporting the observations that RAB3IP overexpression is detrimental in PD models [61]. NEAT1 also enhances apoptosis, inflammation and cytotoxicity in PD models through two other ceRNA mechanisms, specifically through NEAT1/miR-1277-5p/ARHGAP26 [63] and NEAT1/miR-124 [64] axes.
LncRNAs AL049437, MALAT1, SNHG14, lincRNA-p21, GAS5 and BDNF-AS, upregulated in mouse and in vitro models of PD, have also shown to exert a detrimental role and contribute to the progression of the disease [65,67,70]. AL049437 was demonstrated to act as a regulatory mechanism for the mitogen-activated protein kinase MAPK1 [65], a kinase that has been related to autophagy via miR-205-5p sponging [200,201,202]. AL049437 silencing mitigated neuronal injury in vitro, increasing neuronal viability, reducing cell apoptosis and alleviating neuroinflammation and oxidative stress, and this was reverted by miR-205-5p silencing or MAPK1 overexpression. Likewise, MALAT1 also acts through a ceRNA mechanism involving miR-205-5p but to regulate LRRK2 expression, contributing to cell apoptosis in in vitro and in vivo models of PD [66].
Taken together, these data may point towards a common regulatory network where all MALAT1, AL049437 and HOTAIR, which also regulated LRRK2 [195], sponge miR-205-5p, resulting in the post-transcriptional regulation of the MAPK1 and LRRK2 genes.
Additionally, Liu et al. proposed a second ceRNA mechanism, in which MALAT1 would serve as a molecular sponge of miR-124, although the mRNA downstream this edge was not investigated [67]. Later studies by Lu and colleagues suggest that the downstream piece of this axis may be DAPK1 [68], which encodes for a protein kinase that intervenes in apoptosis and autophagy regulation [203,204]. In this sense, MALAT1 knockdown led to increased miR-124-3p levels and DAPK1 downregulation, alleviating cell apoptosis in vitro and in vivo and improving behavioral changes in PD mice. Alternatively, other mRNA targets of miR-124-3p are likely to play a role in PD. Those include MAP3K3, RELA/p65 [205], Bim [206], SQSTM1, MAPK11 [207], STAT3 [208], and ANXA5 [209], among others [210,211,212,213]. Lastly, MALAT1 was also shown to directly regulate α-synuclein expression via targeting miR-129 [69]. Similar to MALAT1, SNHG14 and lincRNA-p21 modulate α-synuclein levels through miR-133 and miR-1277-5p sponging, respectively [70,71], and inhibition of these two lncRNAs mitigated dopaminergic neuron injury in vitro and in vivo [69,70,71]. LincRNA-p21 also acts at the level of neuroinflammation and microglial activation via the lncRNA p21/miR-181/PRKCD (PKC-δ) family feedback loop [72], and plays a role in dopaminergic neuron death by increasing oxidative stress and neuroinflammation through the lincRNA-p21/miR-625/TRPM2 axis [73]. Finally, GAS5 and BDNF-AS have been shown to regulate NLRP3 expression via competitive sponging of miR-223-3p and miR-125b-5p, enhancing in vivo and in vitro microglial inflammatory response [74] and promoting neuronal apoptosis [75], respectively.
As opposed to the above-mentioned ceRNA networks, lncRNAs can also have a protective role in PD. Such is the case of the lncRNA Mirt2 or lncRNA H19. Mirt2 prevents TNFα-triggered inflammation via the repression of miR-101 [76], whereas lncRNA H19 protects against dopaminergic neuron loss in PD mice via regulating miR-301-3p/HPRT1 [77], and consequently Wnt/β-catenin signaling pathway. LncRNA H19 can also act through miR-585-3p/PIK3R3 axis [78], being PIK3R3 a gene that codes for a regulatory subunit of PI3K associated with increased susceptibility to PD [214].
Besides experimentally validated ceRNA networks, in recent years, bioinformatic predictions have uncovered new disease-associated ceRNAs and their principal mechanisms of action. In 2017, Lin and colleagues [111] established the first LncACeNET from a synthetic cellular model of PD, including three lncRNAs (AC009365.4, RPS14P3, G046036). The most prominent mRNAs involved in these networks were IRF1 and RIMKLA (potentially regulated by AC009365.4), NAV1 (related to RPS14P3) and SACS and SDC2 (G046036), all thought to be related to neurodegenerative processes although their role in PD still awaits clarification. One year later, Chi et al. established a PD-associated network from differentially expressed RNAs in PD patients’ blood versus controls [112]. This network consisted of 7 lncRNAs (including XIST, PART1, MCF2L2, NOP14-AS1, LINC00328, LINC00302 and FAM215A), 3 miRNAs (miR-7, miR-133b and miR-433) and 55 mRNAs especially enriched in the GnRH, insulin and MAPK signaling pathways. More recently, Zhang and colleagues [113] identified a new network in a substantia nigra array from PD patients and matched healthy controls that comprised 9 lncRNAs, 18 miRNAs, and 185 mRNAs functionally related to autophagy, DNA repair and vesicle transport, all critical cellular processes in PD. Based on the most significant relationships, they established a second ceRNA network that was validated using external data. It included four lncRNAs (KCNQ1OT1, LINC00467Z, SOX2-OT and NEAT1), nine miRNAs (miR-3163, miR-424-5p, miR-215-5p, miR-193-3p, miR-195-5p, miR-1-3p, miR-92b-3p, miR-520g-3p and miR-124-3p) and six mRNAs (PTBP1, FBXL7, SRSF1, PTBP2, UBE2Q2 and RBBP6) related to mRNA metabolism, mitochondrial function and injury, DNA damage and protein polyubiquitination. Interestingly, PTBP1 was previously reported in PD [215,216].
2.2.2. CircRNAs
As previously mentioned, miR-7 is part of a PD-related ceRNET where the lncRNA SNHG1 and NLRP3 mRNA could compete for it [57]. However, miR-7 is also capable of binding to SNCA mRNA and repress α-synuclein protein levels [79]. Interestingly, a decrease of miR-7 levels was detected in PD models (MPTP treated mice and MPP+ treated cells) that could contribute to the pathogenesis of the disease [79]. Since CDR1as/ciRS-7 contains over 70 binding sites for miR-7 and has been found to be altered in other NDDs such as AD [46,47] (see above), ciRS-7/miR-7/SNCA axis has been proposed as a possible ceRNET in PD [80,81,82,83]. However, it is not known if the expression of ciRS-7 in PD-affected tissues is altered. What has been experimentally demonstrated [84], though, is that a circRNA from SNCA gene itself (hsa_circ_0127305, also called circSNCA) acts as a ceRNA of miR-7 and upregulates SNCA in PD. Furthermore, pramipexol (PPX), a common treatment for PD, exerts suppressive effects on circSNCA expression. In agreement, Sang and coworkers showed that the inhibition of circSNCA and SNCA reduce apoptosis and promote autophagy, thus attenuating the progression of PD [84]. In light of these findings, miR-7 could be involved in a third ceRNET in PD (circSNCA/miR-7/SNCA), or even ciRS-7, circSNCA and SNHG1 may be part of the same ceRNET (miR-7/SNCA and NLRP3) exerting a cooperative regulation.
In contrast to circSNCA, the circRNAs circzip-2 and circDLGAP4 have been found altered in PD with a protective role. On one hand, Kumar et al. [85] observed in a transgenic C. elegans PD model a significant down-regulation of circzip-2, a circRNA synthesized from zip-2 gene whose human ortholog codes for CCAAT-enhancer-binding protein (C/EBP), a bZIP transcription factor involved in PD by regulating α-synuclein levels [85,217]. Through bioinformatic analysis, it was predicted that circzip-2 could sponge miR-60. Hence, a decrease of circzip-2 may enhance the miR-60 activity, which leads to downregulation of protective genes, including M60.4, ZK470.2, igeg-2 and idhg-1, the ortholog of mitochondrial isocitrate hydrogenase (NAD+). On the other hand, Feng et al. [86] described in PD models (MPTP-induced mice and MPP+-induced cells) a decreased expression of circDLGAP4, which demonstrated attenuate the neurotoxic effects in vitro. The authors predicted that miR-134-5p could be a target of circDLGAP4 both in human and mouse and, in agreement, found this miRNA upregulated in both models. Finally, the same study demonstrated that circDLGAP4/miR-134-5p axis regulates CREB signaling, as well as the transcription of CREB downstream target genes BDNF, Bcl-2 and PGC-1a, all of which are neuroprotective factors involved in many NDDs including AD and PD [218,219,220].
Recently, two studies have provided circRNA expression profiling in PD brain. Jia et al. [114] identified circRNAs differentially expressed in four brain regions of MPTP mice and constructed a ceRNA network with 6 of these circRNAs and its prediction downstream targets that could be involved in the PD-related processes (13 miRNAs and 112 mRNAs). Among them, two circRNAs were highlighted. Mmu_circ_0003292 could act as a sponge of miR-132 and upregulate the expression of Nr4a2, an important transcription factor with a neuroprotective effect in the pathogenesis of NDDs (including PD, AD and MS) [221]. Mmu_circ_0001320 was predicted to sponge miR-124 and regulate the expression of Sox9, a positive regulator of astrogliosis, this miRNA-mRNA interaction being previously reported in PD models [222].
Hanan et al. [115] explored the expression patterns of circRNAs in three different brain regions from PD patients. Unlike the rest of transcriptome profiling studies, this research did not construct ceRNETs with the circRNAs identified. However, seeking differentially expressed circRNAs in all brain tissues from PD and healthy individuals a total of 24 were identified, among them stood out a significant increase of circSLC8A1. This circRNA is derived from the SLC8A1 gene that codes for NCX1, which could be involved in AD (see above) and also PD [223]. circSLC8A1 was significantly upregulated in the substantia nigra of AD patients, was directly modulated by oxidative stress and carries seven binding sites for miR-128. Accordingly, an increase of miR-128 mRNA targets (BMI1, SIRT1 and AXIN1) was reported in PD brains. Therefore, a new ceRNET (circSLC8A1/miR-128/BMI1, SIRT1, and AXIN1) was described that could be involved in PD pathology through oxidative stress [115].
2.3. ceRNA and Multiple Sclerosis
MS is a chronic autoimmune disease, characterized by an immune-cell-mediated attack to the white matter in the CNS causing inflammation, demyelination and axonal loss [224,225]. MS onset usually occurs between 20 and 40 years of age, and results in a life-long physical and cognitive disability [226]. Although MS lacks a preclinical mouse model that could faithfully recapitulate the disease progression, experimental autoimmune encephalomyelitis (EAE) mice are widely used as MS models to investigate and to test therapies targeting the inflammation component.
Lately, a large number of studies support the importance of lncRNAs role in the differentiation, function and misproportion of immune cells [87], as well as autoimmunity and human inflammatory response [227,228], suggesting that they may play a pivotal role in MS. Out of the different classes of immune cells, there is increasing evidence on the pathogenic role of T helper 17 (Th17) cells and the imbalance between these and regulatory T cells in various autoimmune diseases such as MS and neuromyelitis optica [229]. Two lncRNAs (Gm15575 and PVT1) with a ceRNA mechanism of action have been shown to affect the function of Th17 in MS. On one hand, lncRNA Gm15575, which is enriched in Th17 cells and the spleen of EAE mice, was reported to act as a miR-686 sponge. As a consequence, Gm15575 positively regulated the expression of CCL7 [87], a proinflammatory chemokine highly expressed in Th17 cells that in MS patients promotes the infiltration of inflammatory cells into the CNS, stimulating the advance of the disease [230]. This study also showed that Gm15575 promoted IL17 expression and that silencing this lncRNA led to a reduction in IL17 mRNA and protein levels, as well as mRNA of RORϒt, a lineage defining transcription factor required for Th17 cell differentiation and function [87]. These authors postulate that CCL7 secreted by Th17 cells could play a role in promoting the production of other proinflammatory cytokines (such as IL17) and differentiation of CD4+ lymphocytes to Th17, given RORϒt function [231]. This, together with its power to recruit immune cells, would aggravate the disease [87]. On the other hand, lncRNA PVT1 is downregulated in MS patients [232] and in the spinal cord of EAE mice [88]. Wu et al. demonstrated that it acts as a ceRNA for miR-21-5p, thus regulating the expression of SOCS5 [88], a protein from the suppressor of cytokine signaling (SOCS) family, which is also downregulated in MS patients [233]. SOCSs proteins are rapidly transcribed in response to intracellular JAK-STAT signaling [234], regulating the cytokine-induced immune response and therefore playing an important for the progression of multiple sclerosis [233]. Moreover, low levels of PVT1 and SOCS5 in EAE mice were associated with an increased number of Th17 cells and markers of inflammation (IL-17, IL-6, IL-1β and TNF-α) in the spinal cord of this model. These inflammation markers were reduced with the administration of exosomes from M2 microglia, which are enriched in PVT1; and this effect was reversed by the administration of M2 exosomes with shRNA for PVT1. Hence, it was proposed that PVT1 could ultimately inhibit the proinflammatory response of Th17 cells through a JAKs/STAT3-mediated pathway.
The above results indicate that the lncRNA-ceRNA networks influence the inflammatory response in MS. This idea is also supported by the TUG1/miR-9-5p/NFkB1(p50) ceRNET described by Yue et al. in 2019 [89]. TUG1, or lncRNA taurine-upregulated gene 1, was first reported as upregulated in the serum of MS patients by Santoro et al. [227] and last year was proven to regulate p50 through miR-9-5p sponging [89]. Importantly, TUG1 down-regulation in vivo improved mouse behavior, decreased the levels of pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-6 and IL-17, and increased the anti-inflammatory cytokine IL-10 in EAE mice.
Besides inflammation, demyelination is another important MS hallmark, a process that could also be influenced by lncRNAs acting as miRNAs sponges. Sulfasalazine, a drug that promotes remyelination and improves the outcome of MS patients [235,236], exerts its beneficial effect at least partially through the inhibition of the lncRNA HOTAIR. This lncRNA acts as a sponge for miR-136-5p, promoting AKT2-mediated NF-kB activation, thus favoring the microglial shift toward a proinflammatory M1-like phenotype [90], detrimental in MS. Moreover, lncRNA GAS5 has been proposed as a ceRNA for miR-137 participating in the demyelination process [91] based on the following premises: (i) GAS5 is upregulated in MS patients while miR-137 is downregulated, (ii) GAS5 acts as a miR-137 molecular sponge in ischemic stroke [237] and (iii) GAS5 exacerbates demyelination and inhibits microglial M2 polarization in EAE mice and human primary cell culture [238]. Based on their results, they also proposed serum GAS5 and miR-137 as MS biomarkers for negative prediction and severity, respectively [91].
Finally, lncRNA MALAT1, which is also dysregulated in MS, can cause alternative splicing abnormalities of MS-associated genes (e.g., IL7R, SP140) and contribute to back-splicing of approximately 50 circRNAs [239]. Since this lncRNA has been pointed out as a potential MS biomarker [240] and is involved in ceRNETs in other NDDs (such as AD or PD, see above), it is not surprising that MALAT1 could be also involved in a ceRNA axis in MS.
In contrast to lncRNAs, no circRNA-associated ceRNA networks implicated in MS has yet been validated. Nevertheless, novel isoforms and an upregulated circRNA (hsa_circ_0106803) from the GSDMB gene, associated with susceptibility to several autoimmune diseases and involved in pyroptosis [241], have been found in PMBCs of MS patients [92]. Several miRNAs were predicted to contain more than one target site in hsa_circ_0106803 and, among these, miR-1275 and miR-149 are differentially expressed in blood from MS patients [242,243]. It has been reported that miR-149 binds to ASIC1a and reduces its levels [244]. ASIC1a encodes a subunit of acid-sensing ion channel, which is overexpressed in acute MS lesions and could be implicated in the neuronal pathogenesis of this disease [245,246,247,248]. In light of this evidence, a ceRNA network has been proposed where hsa_circ_0106803 could modulate the progression of MS by regulating the expression of ASIC1a mRNA through miR-149 [93]. Another study in PBMCs of MS patients [94] found two downregulated circRNAs (hsa_circ_0005402 and hsa_circ_0035560/hsa_circ_0003452_2) from ANXA2 gene, encoding an annexin related to immune-mediated diseases [249,250]. Through bioinformatic analysis, it was predicted that hsa_circ_0005402 presents a single binding site for 17 miRNAs and two binding sites for miR-1248 and miR-766. Curiously, hsa_circ_0005402 shares 14 common miRNA targets with hsa_circ_0035560, so a cooperative regulation of a circRNA-miRNA-mRNA axis could be involved in MS pathogenesis [93,94]. However, further studies are needed to elucidate all components of this axis and confirm this potential cooperative ceRNA network in MS.
2.4. ceRNA and Amyotrophic Lateral Sclerosis
ALS is the third most common progressive neurodegenerative disease, affecting 150,000 people in the world [251]. It is characterized by atrophy of voluntary muscles and paralysis as a consequence of the progressive and selective loss of motor neurons in the spinal cord and brain [252]. ALS is a complex and multifactorial disease with many dysregulated cellular processes but, in recent years, alterations in genes associated with RNA metabolism have been in the spotlight. This is supported by the fact that two of the most important genes associated with this disease (FUS and TARDBP) are involved in transcription and RNA metabolism [253] and, notably, are able to regulate biogenesis or expression of certain lncRNAs and circRNAs [254,255,256].
In agreement, a potentially pathological paraspeckle enrichment was observed in motor neurons of ALS patients’ brains [257]. Paraspeckles are nuclear bodies formed by a set of specialized RNAs and proteins, among which are the RNA-binding proteins FUS and TDP-43, encoded by the FUS and TARDBP genes, respectively. These specialized RNAs are especially enriched in the lncRNA NEAT1_2, which has been identified predominantly expressed in spinal motor neurons in early phases of ALS pathological process and was shown to directly bind FUS and TDP-43 [257,258]. The frequency of paraspeckle formation is highly increased during the early phases of ALS course, so it has been proposed that NEAT1_2 could act as a scaffold of the RNA-binding proteins in the nucleus [259] by sequestering them and forming aggregates, thus playing a possible important role in RNA metabolism imbalance and ALS pathogenesis. Whether this lncRNA could also act through other pathways such as the ceRNA mechanism, already observed in a cellular PD model [62], remains to be determined. The lncRNA C9ORF72-AS has also recently been related to ALS, although its function in ALS is still unknown. However, it seems that the first exon of this lncRNA has different binding sites for miRNAs [260].
Whole transcriptome RNA-seq analyses have revealed differential expression of lncRNAs both in blood PBMCs and iPSC-derived motoneurons from ALS patients [254,261,262], although their possible mechanisms of action have not been addressed yet. Interestingly, at least some of the lncRNAs deregulated in human iPSC-derived motoneurons were conserved between mouse and human, concurring with those of mouse embryonic stem cells with the FUS mutation [254].
Similar to lncRNAs, little is known about circRNA in ALS. However, three circRNAs have shown some potential as diagnostic biomarkers [116]. Dolinar et al. [116] investigated the expression profile of circRNAs and identified 425 differentially expressed in leukocytes from sporadic ALS (SALS) patients. Among these, hsa_circ_0023919, hsa_circ_0063411 and hsa_circ_0088036 showed the highest significance as well as clinical relevance. Curiously, the expression levels of several circRNAs were positively correlated with each other, suggesting that they could be involved in similar biological processes and/or co-regulated [116]. Although possible circRNA interaction networks were not investigated, the authors predicted miRNA targets for two affected circRNAs. Hsa_circ_0023919 was found significantly downregulated in SALS patients and contains two binding sites for hsa-miR-9. Accordingly, two previous studies confirmed the upregulation of miR-9 in both mouse model of ALS [263] and in blood samples of ALS patients [264]. It has been reported that miR-9 directly binds to and reduces the mRNA levels of NEFL [265], which encodes the neurofilament light polypeptide. Interestingly, aggregation of intermediate filament is a characteristic ALS hallmark. In light of this evidence, one potential biomarker based on ceRNA hypothesis has been proposed in ALS (hsa_circ_0023919/miR-9/NEFL), where hsa_circ_0023919 could act as a miR-9 sponge and regulate the metabolism of intermediate filaments (NEFL) observed in ALS [117]. In contrast, hsa_circ_0063411 was found upregulated in patients. This circRNA contains one binding site for miR-647, which has been seen expressed in spinal cords from healthy subjects, but not from SALS patients [266]. However, the study did not establish an mRNA target of hsa_circ_0063411/miR-647 axis, so further studies are necessary to elucidate this potential ALS ceRNA network. One possibility is that hsa_circ_0063411 may bind to miR-647 and increase the expression of PTEN, a direct target of miR-647 [267]. As mentioned above, PTEN has been seen involved in AD- and PD-associated ceRNA networks. Since PTEN has been reported as a potential therapeutic target in motor neuron diseases, including ALS or SMA (spinal muscular atrophy) [268,269,270], it remains possible that PTEN mRNA could also be part of ALS-linked ceRNETs.
It is important to note that, although there are no experimentally confirmed ceRNAs associated with ALS, ceRNA mechanisms can regulate myogenesis [271], an altered process in ALS due to the relevant muscular component in this disease. These mechanisms include the lncRNAs linc-MD1 [272], lncRNA H19 [273], MALAT1 [274], lnc-mg [275], lncMD [276], Yam [277] and Sirt1-AS [278], among others.
2.5. ceRNA and Spinocerebellar Ataxia Type 7
Spinocerebellar Ataxia Type 7 (SCA7) is a rare inherited neurodegenerative disease caused by polyglutamine repeat expansion in Ataxin 7 (ATXN7), a product of the SCA7 gene. This results in formation of protein aggregates and decreased protein activity [279] that contribute to neurodegeneration. The disease comprises a phenotypic spectrum ranging from adolescent- or adult-onset progressive cerebellar ataxia and cone-rod retinal dystrophy to infantile- or early-childhood-onset with multiorgan failure, accelerated course, and early death [280].
In SCA7, crosstalk between ncRNAs have been shown to contribute to cell-specific neurodegeneration. Specifically, lnc-SCA7, a highly conserved lncRNA derived from the retrotransposition of the ATXN7L3 gene (a distant paralog of SCA7), has been shown to regulate the expression of the ATXN7 gene in a miR-124-dependent manner [95]. Tan et al. found that lnc-SCA7 and ATXN7 mRNA levels increased in both patient-derived fibroblasts and SCA7 mouse model brain, while miR-124 levels decreased. Furthermore, in mice, this increase was more prominent in tissues relevant to SCA7, such as the retina and cerebellum. Surprisingly, reduction of lnc-SCA7 levels led to a depletion of both mature and precursor miR-124 levels, generating a novel negative feedback loop involving ATXN7 and miR-124.
3. RNA Editing Alteration and ceRNA Networks in Neurodegenerative Diseases
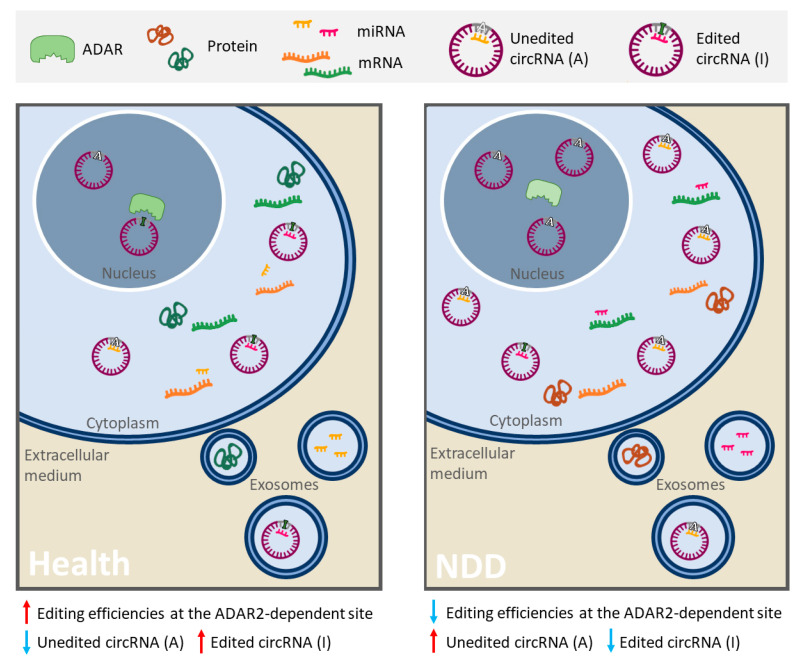
RNA editing is an important mechanism of post-transcriptional processing that can modify RNA molecules by altering its sequences through insertion, deletion, or conversion of a nucleotide [281,282]. Recent discoveries suggest that RNA editing critically regulates neurodevelopment and normal neuronal function, for which some crucial aspects of neurodegenerative diseases may stem from the modification of both coding and non-coding RNA [282,283,284].
The most common type of RNA editing is the conversion of adenosine to inosine (A-to-I), in which enzymes encoded by the adenosine deaminase acting on RNA (ADAR) gene family catalyze the deamination of adenosine (A) nucleotides to inosines (I) [285]. Critical consequences are derived from this modification, since inosine (I) is interpreted by the translation and splicing machineries as guanosine (G) [286]. Editing of pre-mRNA coding regions can lead to codon change that may result in increased diversity of protein isoforms and their respective function [281]. However, most of the RNA editing happens in non-coding RNAs, which can affect their stability, biogenesis and target recognition [281,286,287]. In fact, it has been reported that ADAR is involved in circRNA biogenesis by editing and destabilizing the flanking Alu repeat sequences, which makes circRNA production less favorable [287,288]. Moreover, editing events can affect both the maturation and the expression of miRNAs, but if the modification occurs in MREs or in miRNA seed regions (regions in miRNA sequence that largely determine the binding specificity on its targets), the spectrum of miRNA targets, or “targetome”, shall be changed [289]. Therefore, a single editing site in an RNA molecule could drastically modify its function, resulting in new or different ceRNA networks that regulate gene expression.
Interestingly, A-to-I editing has been reported specifically reduced in SALS motor neurons due to the progressive downregulation of ADAR2 [290,291]. Based on this evidence, Hosaka et al. [292] searched for extracellular RNAs with ADAR2-dependent A-to-I sites that may reflect the intracellular pathological process and thus could be potentially good ALS biomarkers. A total of six RNAs were identified. Among these, a circRNA (hsa_circ_0125620, also called circGRIA2) with an ADAR2-dependent site was detected in human SH-SY5Y neuroblastoma cells as well as in their culture medium [292]. Therefore, variations in RNA editing efficiency in ALS, as a consequence of decreased ADAR2 activity, could be potentially measured in peripheral circRNAs and other relatively stable ncRNAs. In light of this evidence, this editing phenomenon may be considered a very important aspect, since it allows obtain relevant information of disease pathological process from non-coding RNAs.
Other NDDs, such as AD and PD, also present alterations in RNA editing patterns [115,283,293,294]. In fact, a recent study has explored how RNA editing in AD contributes to the regulation of AD-related processes in blood cells in two populations of patients [295]. Results identified differentially edited sites predicted to disrupt miRNA target sites in five genes. In all cases, decreased editing was observed in AD suggesting a greater miRNA-binding affinity relative to controls [295]. In light of this evidence, alterations in RNA editing could result in a specific RNA profile, given by different amount of RNAs, modified interaction networks and editing levels or efficiencies changes in A-to-I sites, that could be useful to identify new robust biomarkers of these NDDs (Figure 2).
Figure 2.
Schematic representation of alterations in RNA editing that could provide a specific RNA profile in neurodegenerative diseases (NDDs). In some linear and circular RNAs, the enzyme ADAR2 deaminates adenosine (A) into inosine (I), resulting in important biological consequences (especially in ncRNAs). On the one hand, a single editing site in MRE or miRNA seed region can drastically change its set of targets. In this image, circ-Purple acts as a miR-Yellow sponge, which regulates the mRNA expression of Orange gene (right panel). Deamination of A into I in circ-Purple could affect its binding site for miR-Yellow. In consequence, circ-Purple stops sponging miR-Yellow, and it may bind to another miRNA (miR-Pink) and promote the expression of Green gene (left panel). Hence, the ceRNA interaction network has changed, emerging a new or different regulatory axis. On the other hand, ADAR editing negatively regulates circRNA biogenesis, resulting in a decrease of circRNA levels (in the left panel there is less circ-Purple expression than in the right panel). In NDDs with a diminution of A-to-I RNA editing (like ALS, AD or PD), a different and opposite profile/pattern could be observed (right panel) with respect to a normal editing efficiency of ADAR (left panel). Therefore, alterations mediated by RNA editing in RNAs and its ceRNA interaction networks may serve as robust biomarkers of these NDDs. This figure is based on a previously published figure [292,296].
4. Conclusions and Future Perspectives
The vast majority of NDDs can be definitively diagnosed only after death or in advanced stage, and their previous diagnosis is based on ruling out other possible causes for the symptoms. For most NDDs, there is no cure or treatment capable of reversing the damage due to neuronal death. Therefore, it is critical to find new biomarkers that would facilitate an early diagnosis, prognosis and efficient monitoring of therapeutic interventions.
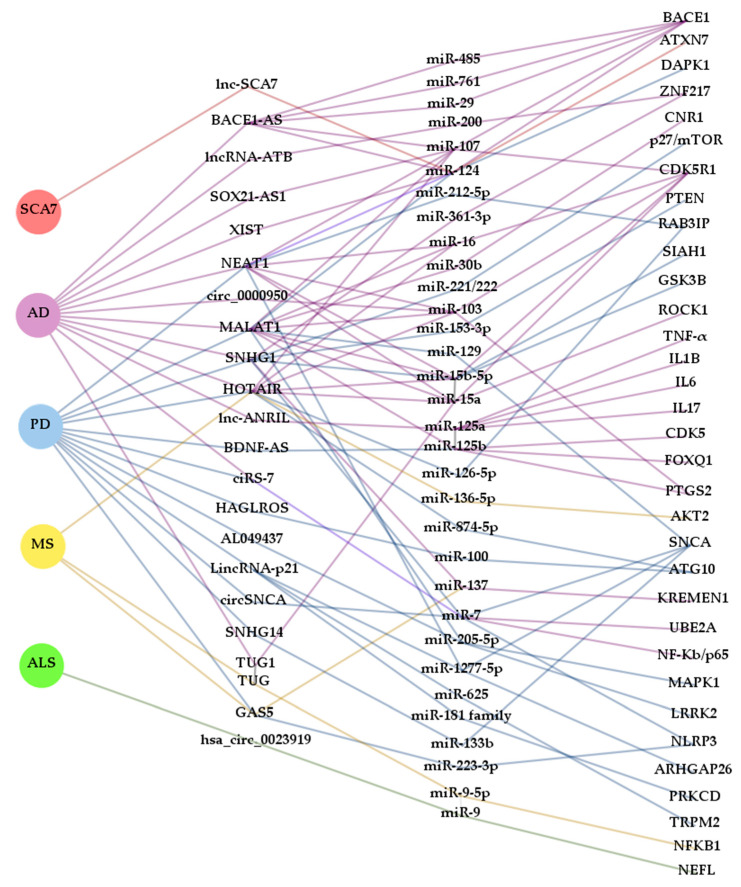
In the search for new biomarkers, non-coding RNAs have been proposed as promising tools for diagnosis and prognosis. Many ncRNAs often arise from genes that cause NDDs or are somehow involved in the development of one of these disorders (like BACE1-AS or circSNCA). Thus, ceRNETs established by these ncRNAs could well be, at least in some cases, disease and even stage-specific. However, as reported in this review, ncRNAs are commonly misregulated in several NDDs (Figure 3). This is the case, for example, of the lncRNAs SNHG1 and HOTAIR, which are altered in AD [36,37] and PD [54,55,56,57,58], and PD [60,61] and MS [90], respectively. However, their miRNA targets may vary depending on cell types affected by the disease and, therefore, the mechanism of action may also differ. Similarly, miR-7 has been shown sponged by ciRS-7/CDR1as and circSNCA in AD [46,47,48,49] and PD [84], respectively, being detrimental in the first case and beneficial in the second, due to regulation of different target mRNAs. The apparent discrepancy between the anti and pro cell death activity of miR-7 reflects the complex regulatory role of miRNAs, so further research is required to clarify their function in different cellular and disease contexts.
Figure 3.
Complexity and interaction of ceRNETs in NDDs. The diagram was constructed with Gephi software from ceRNAs (lncRNAs and circRNAs) that, according to the bibliography cited in this review, contribute to the pathogenesis of more than one neurodegenerative disease and miRNAs that are part of ceRNETs from more than one ceRNA. Interactions between RNA molecules are represented with lines colored in accordance with the NDD background they have been described in: spinocerebellar ataxia type 7 (SCA7) (red), Alzheimer’s disease (AD) (purple), Parkinson’s disease (PD) (blue), multiple sclerosis (MS) (yellow) and amyotrophic lateral sclerosis (ALS) (green).
In this way, by analyzing various elements of the altered ceRNETs, it may be possible to differentiate one NDD from another even if there were common components. Ideally, working with several correlatable molecular targets at the same time (lncRNAs/circRNAs/pseudogenes-miRNA-mRNAs) increases the sensitivity and reliability of ceRNETs as biomarkers. It should be noted that ceRNETs construction also contributes to the identification of new molecular mechanisms of gene regulation that may lead to a better understanding of the etiopathogenesis of the diverse NDDs, as well as to reveal new therapeutic targets and obtain relevant information about the pathological processes of the disease.
In this sense, ceRNETs may also reflect the editing efficiencies of ADAR, a post-transcriptional phenomenon dysregulated in several NDDs. RNA editing can affect the levels and the efficiency of RNA interaction networks, so its alterations could provide a specific RNA fingerprint that helps in the diagnosis or prognosis of NDDs. Finally, the described crosstalk between the RNA molecules in certain ceRNETs is relatively conserved between species, paving the way for translation of data obtained from animal models into clinical practice [297,298].
Among the main advantages of ceRNETs for biomarker research, the fact that these ncRNAs are easily accessible is noteworthy, since they are extremely stable in circulation and may be detected in exosomes. Such is the case for circRNA CDR1as/ciRS-7 and lncRNA MALAT1, found in exosomes. Interestingly, levels of ciRS-7 in these vesicles depend on the intracellular abundance of the miRNA that it sponges (miR-7) [299]. Furthermore, ciRS-7 and MALAT1 may regulate miRNA expression in target cells after exosomal delivery modulating their phenotype, since these ceRNAs retain their biological activity [299,300]. Therefore, ciRS-7 and MALAT1 together with other circulating ncRNAs (e.g., NEAT1, GAS5, hsa_circ_061346, hsa_circ_000843) represent promising candidates for peripheral ceRNA biomarkers of NDDs. Although many of the ncRNAs discussed earlier have not been reported in exosomes to date, some of them are predicted to be detected in human blood exosomes by exoRBase (e.g., circSLC8A1, circCORO1C, SNHG1, BACE1-AS) [301]. Indeed, it has recently been demonstrated that plasma exosomal BACE1-AS levels could serve as a biomarker of AD [302,303].
Because ceRNA interaction networks are multifactorial, they may represent an advantage in studies of these complex neurodegenerative disorders, one being at the level of biomarkers (combined RNA biomarkers panels) and another at the level of therapeutic targets (modulate the levels of multiple disease-associated RNAs at once by just targeting one).
Nevertheless, it must be taken into account that there is still much to do, since these networks are very complex and their interactions must be experimentally defined [297]. In this sense, some “non-canonical” aspects of ncRNAs have also been described: (i) circRNAs that can also sponge or serve as a decoy for RBPs or lncRNAs, (ii) miRNAs that may increase the expression of target genes, (iii) lncRNAs that can be precursors of smaller ncRNAs and can regulate miRNA and circRNA biogenesis, (iv) miRNAs that can direct Ago2 to degrade lncRNA and circRNA, (v) lncRNAs that compete with miRNAs for the target site of mRNA, and (vi) context-specific miRNA function and target identification [304,305,306,307,308,309,310,311].
Although the full extent of ceRNA networks still needs to be still determined, the competition of ncRNA and mRNAs for miRNAs constitutes a key point of gene regulation that could underlie some pathological aspects of neurodegenerative diseases, favoring at the end the identification of specific pathological mechanisms for each disease.
Abbreviations
| ADAR1 | Adenosine Deaminase Acting on RNA 1 |
| circRNAs | Circular Ribonucleic Acids |
| lncRNAs | Long non-coding Ribonucleic Acids |
| ceRNAs | Competing Endogenous Ribonucleic Acids |
| ceRNET | ceRNA network |
| cirCeNET | circRNA-associated ceRNA networks |
| LncACeNET | lncRNA-associated ceRNA network |
| AD | Alzheimer’s Disease |
| PD | Parkinson’s Disease |
| MS | Multiple Sclerosis |
| SCA7 | Spinocerebellar Ataxia Type 7 |
| ALS | Amyotrophic Lateral Sclerosis |
| CNS | Central Nervous System |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 |
| APP/PS1 | Amyloid Precursor Protein/Presenilin 1 |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-hydrochloride |
| MPP+ | 1-methyl-4-phenylpyridinium |
| EAE | Experimental Autoimmune Encephalomyelitis |
Author Contributions
R.O., P.Z., L.M.-G. and T.L.-R. conceived and designed the structure of the manuscript. L.M.-G. and T.L.-R. wrote the main text. L.M.-M. and M.d.l.T. prepared tables. N.M. and P.A. produced figures. A.C.C., R.M., J.M.T. and R.O. discussed and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Instituto de Salud Carlos III, PI17/00949, and Fondo Europeo de Desarrollo Regional (FEDER) “Una manera de hacer Europa” from the European Union, Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED-612-CB18/05/00037) and Consolidated Groups from Gobierno de Aragón. L.M.-G. was supported by Departamento de Industria e Innovación from Gobierno de Aragón and Fondo Social Europeo. T.L.-R. was supported by Ministerio de Universidades from Gobierno de España.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Neueder A. RNA-Mediated Disease Mechanisms in Neurodegenerative Disorders. J. Mol. Biol. 2019;431:1780–1791. doi: 10.1016/j.jmb.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Liu E.Y., Cali C.P., Lee E.B. RNA metabolism in neurodegenerative disease. Dis. Model. Mech. 2017;10:509–518. doi: 10.1242/dmm.028613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volkening K., Strong M.J. RNA Metabolism in Neurodegenerative Disease. Curr. Chem. Biol. 2011;5:90–98. doi: 10.2174/2212796811105020090. [DOI] [Google Scholar]
- 4.Djebali S., Davis C.A., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F., et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei C.W., Luo T., Zou S.S., Wu A.S. The Role of Long Noncoding RNAs in Central Nervous System and Neurodegenerative Diseases. Front. Behav. Neurosci. 2018;12:175. doi: 10.3389/fnbeh.2018.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salta E., De Strooper B. Noncoding RNAs in neurodegeneration. Nat. Rev. Neurosci. 2017;18:627–640. doi: 10.1038/nrn.2017.90. [DOI] [PubMed] [Google Scholar]
- 7.Amin N., Mcgrath A., Chen Y.P.P. Evaluation of deep learning in non-coding RNA classification. Nat. Mach. Intell. 2019;1:246–256. doi: 10.1038/s42256-019-0051-2. [DOI] [Google Scholar]
- 8.Quinlan S., Kenny A., Medina M., Engel T., Jimenez-Mateos E.M. MicroRNAs in Neurodegenerative Diseases. Int. Rev. Cell Mol. Biol. 2017;334:309–343. doi: 10.1016/bs.ircmb.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Cao X., Yeo G., Muotri A.R., Kuwabara T., Gage F.H. Noncoding RNAs in the Mammalian Central Nervous System. Annu. Rev. Neurosci. 2006;29:77–103. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- 10.Chan J.J., Tay Y. Noncoding RNA: RNA Regulatory Networks in Cancer. Int. J. Mol. Sci. 2018;19:1310. doi: 10.3390/ijms19051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeck W.R., Sorrentino J.A., Wang K., Slevin M.K., Burd C.E., Liu J., Marzluff W.F., Sharpless N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rybak-Wolf A., Stottmeister C., Glažar P., Jens M., Pino N., Giusti S., Hanan M., Behm M., Bartok O., Ashwal-Fluss R., et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell. 2015;58:870–885. doi: 10.1016/j.molcel.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 13.Guo X., Lin M., Rockowitz S., Lachman H.M., Zheng D. Characterization of Human Pseudogene-Derived Non-Coding RNAs for Functional Potential. PLoS ONE. 2014;9:e93972. doi: 10.1371/journal.pone.0093972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pei B., Sisu C., Frankish A., Howald C., Habegger L., Mu X.J., Harte R., Balasubramanian S., Tanzer A., Diekhans M., et al. The GENCODE pseudogene resource. Genome Biol. 2012;13:R51. doi: 10.1186/gb-2012-13-9-r51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmena L., Poliseno L., Tay Y., Kats L., Pandolfi P.P. A ceRNA hypothesis: The rosetta stone of a hidden RNA language? Cell. 2011;146:353–358. doi: 10.1016/j.cell.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ala U., Karreth F.A., Bosia C., Pagnani A., Taulli R., Léopold V., Tay Y., Provero P., Zecchina R., Pandolfi P.P. Integrated transcriptional and competitive endogenous RNA networks are cross-regulated in permissive molecular environments. Proc. Natl. Acad. Sci. USA. 2013;110:7154–7159. doi: 10.1073/pnas.1222509110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slack F.J., Chinnaiyan A.M. The Role of Non-coding RNAs in Oncology. Cell. 2019;179:1033–1055. doi: 10.1016/j.cell.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar S., Vijayan M., Bhatti J.S., Reddy P.H. MicroRNAs as Peripheral Biomarkers in Aging and Age-Related Diseases. Prog. Mol. Biol. Transl. Sci. 2017;146:47–94. doi: 10.1016/bs.pmbts.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Idda M.L., Munk R., Abdelmohsen K., Gorospe M. Noncoding RNAs in Alzheimer’s disease. Wiley Interdiscip Rev. RNA. 2018;9 doi: 10.1002/wrna.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdollahzadeh R., Daraei A., Mansoori Y., Sepahvand M., Amoli M.M., Tavakkoly-Bazzaz J. Competing endogenous RNA (ceRNA) cross talk and language in ceRNA regulatory networks: A new look at hallmarks of breast cancer. J. Cell Physiol. 2019;234:10080–10100. doi: 10.1002/jcp.27941. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Zhu J., Ma X., Wang Y. ceRNA network construction and comparison of gastric cancer with or without Helicobacter pylori infection. J. Cell Physiol. 2019;234:7128–7140. doi: 10.1002/jcp.27467. [DOI] [PubMed] [Google Scholar]
- 22.Xu J., Li Y., Lu J., Pan T., Ding N., Wang Z., Shao T., Zhang J., Wang L., Li X. The mRNA related ceRNA–ceRNA landscape and significance across 20 major cancer types. Nucleic. Acids Res. 2015;43:8169–8182. doi: 10.1093/nar/gkv853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qi X., Zhang D.-H., Wu N., Xiao J.-H., Wang X., Ma W. ceRNA in cancer: Possible functions and clinical implications. J. Med. Genet. 2015;52:710–718. doi: 10.1136/jmedgenet-2015-103334. [DOI] [PubMed] [Google Scholar]
- 24.Zeng T., Ni H., Yu Y., Zhang M., Wu M., Wang Q., Wang L., Xu S., Xu Z., Xu C., et al. BACE1-AS prevents BACE1 mRNA degradation through the sequestration of BACE1-targeting miRNAs. J. Chem. Neuroanat. 2019;98:87–96. doi: 10.1016/j.jchemneu.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 25.He W., Chi S., Jin X., Lu J., Zheng W., Yan J., Zhang D. Long non-coding RNA BACE1-AS modulates isoflurane-induced neurotoxicity to Alzheimer’s Disease through sponging miR-214-3p. Neurochem. Res. 2020;45:2324–2335. doi: 10.1007/s11064-020-03091-2. [DOI] [PubMed] [Google Scholar]
- 26.Ge Y., Song X., Liu J., Liu C., Xu C. The combined therapy of berberine treatment with lncRNA BACE1-AS depletion attenuates Aβ 25–35 induced neuronal injury through regulating the expression of miR -132-3p in neuronal cells. Neurochem. Res. 2020;45:741–751. doi: 10.1007/s11064-019-02947-6. [DOI] [PubMed] [Google Scholar]
- 27.Yue D., Guanqun G., Jingxin L., Sen S., Shuang L., Yan S., Minxue Z., Ping Y., Chong L., Zhuobo Z., et al. Silencing of long noncoding RNA XIST attenuated Alzheimer’s disease-related BACE1 alteration through miR-124. Cell Biol. Int. 2020;44:630–636. doi: 10.1002/cbin.11263. [DOI] [PubMed] [Google Scholar]
- 28.Wang X., Wang C., Geng C., Zhao K. LncRNA XIST knockdown attenuates Aβ 25-35 -induced toxicity, oxidative stress, and apoptosis in primary cultured rat hippocampal neurons by targeting miR-132. Int. J. Clin. Exp. Pathol. 2018;11:3915–3924. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao M.Y., Wang G.Q., Wang N.N., Yu Q.Y., Liu R.L., Shi W.Q. The long-non-coding RNA NEAT1 is a novel target for Alzheimer’s disease progression via miR-124/BACE1 axis. Neurol. Res. 2019;41:489–497. doi: 10.1080/01616412.2018.1548747. [DOI] [PubMed] [Google Scholar]
- 30.Ke S., Yang Z., Yang F., Wang X., Tan J., Liao B. Long noncoding RNA NEAT1 aggravates Aβ-induced neuronal damage by targeting miR-107 in Alzheimer’s disease. Yonsei Med. J. 2019;60:640–650. doi: 10.3349/ymj.2019.60.7.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu W., Li K., Fan Q., Zong B., Han L. Knockdown of long non-coding RNA SOX21-AS1 attenuates amyloid-β-induced neuronal damage by sponging miR-107. Biosci. Rep. 2020;40:1–12. doi: 10.1042/BSR20194295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spreafico M., Grillo B., Rusconi F., Battaglioli E., Venturin M. Multiple Layers of CDK5R1 Regulation in Alzheimer’s Disease Implicate Long Non-Coding RNAs. Int. J. Mol. Sci. 2018;19:2022. doi: 10.3390/ijms19072022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma P., Li Y., Zhang W., Fang F., Sun J., Liu M., Li K., Dong L. Long Non-coding RNA MALAT1 Inhibits Neuron Apoptosis and Neuroinflammation While Stimulates Neurite Outgrowth and Its Correlation With MiR-125b Mediates PTGS2, CDK5 and FOXQ1 in Alzheimer’s Disease. Curr. Alzheimer. Res. 2019;16:596–612. doi: 10.2174/1567205016666190725130134. [DOI] [PubMed] [Google Scholar]
- 34.Li L., Xu Y., Zhao M., Gao Z. Neuro-protective roles of long non-coding RNA MALAT1 in Alzheimer’s disease with the involvement of the microRNA-30b/CNR1 network and the following PI3K/AKT activation. Exp. Mol. Pathol. 2020;117:104545. doi: 10.1016/j.yexmp.2020.104545. [DOI] [PubMed] [Google Scholar]
- 35.Li X., Wang S.W., Li X.L., Yu F.Y., Cong H.M. Knockdown of long non-coding RNA TUG1 depresses apoptosis of hippocampal neurons in Alzheimer’s disease by elevating microRNA-15a and repressing ROCK1 expression. Inflamm. Res. 2020;69:897–910. doi: 10.1007/s00011-020-01364-8. [DOI] [PubMed] [Google Scholar]
- 36.Wang H., Lu B., Chen J. Knockdown of lncRNA SNHG1 attenuated Ab25-35 -inudced neuronal injury via regulating KREMEN1 by acting as a ceRNA of miR-137 in neuronal cells. Biochem. Biophys. Res. Commun. 2019;518:438–444. doi: 10.1016/j.bbrc.2019.08.033. [DOI] [PubMed] [Google Scholar]
- 37.Gao Y., Zhang N., Lv C., Li N., Li X., Li W. LncRNA SNHG1 Knockdown Alleviates Amyloid-B-Induced Neuronal Injury by Regulating ZNF217 via Sponging miR-361-3p in Alzheimer’s Disease. J. Alzheimer Dis. 2020;77:85–98. doi: 10.3233/JAD-191303. [DOI] [PubMed] [Google Scholar]
- 38.Wang J., Zhou T., Wang T., Wang B. Suppression of lncRNA-ATB prevents amyloid-β-induced neurotoxicity in PC12 cells via regulating miR-200/ZNF217 axis. Biomed. Pharmacother. 2018;108:707–715. doi: 10.1016/j.biopha.2018.08.155. [DOI] [PubMed] [Google Scholar]
- 39.Zhu L., Lin M., Ma J., Liu W., Gao L., Wei S., Xue Y., Shang X. The role of LINC00094/miR-224-5p (miR-497-5p)/Endophilin-1 axis in Memantine mediated protective effects on blood-brain barrier in AD microenvironment. J. Cell Mol. Med. 2019;23:3280–3292. doi: 10.1111/jcmm.14214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang Q., Shan K., Qun-Wang X., Zhou R.M., Yang H., Liu C., Li Y.J., Yao J., Li X.M., Shen Y., et al. Long non-coding RNA-MIAT promotes neurovascular remodeling in the eye and brain. Oncotarget. 2016;7:49688–49698. doi: 10.18632/oncotarget.10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu R., Liu R., Wang L., Tang M., Li S.R., Hu X. LncRNA RPPH1 attenuates Aβ25-35-induced endoplasmic reticulum stress and apoptosis in SH-SY5Y cells via miR-326/PKM2. Int. J. Neurosci. 2020:1–8. doi: 10.1080/00207454.2020.1746307. [DOI] [PubMed] [Google Scholar]
- 42.Ran G., Lu W., Man T., Shi-rong L., Rui L., Xiao H. LncRNA Rpph1 Protects Amyloid-β Induced Neuronal Injury in SK-N-SH Cells via miR-122/Wnt1 Axis. Int. J. Neurosci. 2020;130:443–453. doi: 10.1080/00207454.2019.1692834. [DOI] [PubMed] [Google Scholar]
- 43.Cai Y., Sun Z., Jia H., Luo H., Ye X., Wu Q., Xiong Y., Zhang W., Wan J. Rpph1 Upregulates CDC42 Expression and Promotes Hippocampal Neuron Dendritic Spine Formation by Competing with miR-330-5p. Front. Mol. Neurosci. 2017;10:27. doi: 10.3389/fnmol.2017.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan Y., Yan H., Teng Y., Wang Q., Yang P., Zhang L., Cheng H., Fu S. Long non-coding RNA 00507/miRNA-181c-5p/TTBK1/MAPT axis regulates tau hyperphosphorylation in Alzheimer’s disease. J. Gene Med. 2020;5:e3268. doi: 10.1002/jgm.3268. [DOI] [PubMed] [Google Scholar]
- 45.Zhou B., Li L., Qiu X.I.N., Wu J., Xu L.E.I., Shao W. Long non-coding RNA ANRIL knockdown suppresses apoptosis and pro-inflammatory cytokines while enhancing neurite outgrowth via binding microRNA-125a in a cellular model of Alzheimer’s disease. Mol. Med. Rep. 2020;22:1489–1497. doi: 10.3892/mmr.2020.11203. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Zhao Y., Alexandrov P.N., Jaber V., Lukiw W.J. Deficiency in the ubiquitin conjugating enzyme UBE2A in Alzheimer’s Disease (AD) is linked to deficits in a natural circular miRNA-7 sponge (circRNA; ciRS-7) Genes. 2016;7:116. doi: 10.3390/genes7120116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi Z., Chen T., Yao Q., Zheng L., Zhang Z., Wang J., Hu Z., Cui H., Han Y., Han X., et al. The circular RNA ciRS-7 promotes APP and BACE1 degradation in an NF-κB-dependent manner. FEBS J. 2017;284:1096–1109. doi: 10.1111/febs.14045. [DOI] [PubMed] [Google Scholar]
- 48.Ye T., Yang M., Huang D., Wang X., Xue B., Tian N., Xu X., Bao L., Hu H., Lv T., et al. MicroRNA-7 as a potential therapeutic target for aberrant NF-κB-driven distant metastasis of gastric cancer. J. Exp. Clin. Cancer Res. 2019;38:55. doi: 10.1186/s13046-019-1074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi D.C., Chae Y.J., Kabaria S., Chaudhuri A.D., Jain M.R., Li H., Mouradian M.M., Junn E. MicroRNA-7 protects against 1-methyl-4-phenylpyridinium-induced cell death by targeting RelA. J. Neurosci. 2014;34:12725–12737. doi: 10.1523/JNEUROSCI.0985-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang H., Wang H., Shang H., Chen X., Yang S., Qu Y., Ding J., Li X. Circular RNA circ_0000950 promotes neuron apoptosis, suppresses neurite outgrowth and elevates inflammatory cytokines levels via directly sponging miR-103 in Alzheimer’s disease. Cell Cycle. 2019;18:2197–2214. doi: 10.1080/15384101.2019.1629773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Y., Tan L., Wang X. Circular HDAC9/microRNA-138/Sirtuin-1 Pathway Mediates Synaptic and Amyloid Precursor Protein Processing Deficits in Alzheimer’s Disease. Neurosci. Bull. 2019;35:877–888. doi: 10.1007/s12264-019-00361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang N., Gao Y., Yu S., Sun X., Shen K. Berberine attenuates Aβ42-induced neuronal damage through regulating circHDAC9/miR-142-5p axis in human neuronal cells. Life Sci. 2020;252:117637. doi: 10.1016/j.lfs.2020.117637. [DOI] [PubMed] [Google Scholar]
- 53.Straniero L., Rimoldi V., Samarani M., Goldwurm S., Di Fonzo A., Krüger R., Deleidi M., Aureli M., Soldà G., Duga S., et al. The GBAP1 pseudogene acts as a ceRNA for the glucocerebrosidase gene GBA by sponging miR-22-3p. Sci. Rep. 2017;7:12702. doi: 10.1038/s41598-017-12973-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao J., Geng L., Chen Y., Wu C. SNHG1 promotes MPP+- induced cytotoxicity by regulating PTEN/AKT/mTOR signaling pathway in SH-SY5Y cells via sponging miR-153-3p. Biol. Res. 2020;53:1–11. doi: 10.1186/s40659-019-0267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie N., Qi J., Li S., Deng J., Chen Y., Lian Y. Upregulated lncRNA small nucleolar RNA host gene 1 promotes 1-methyl-4-phenylpyridinium ion-induced cytotoxicity and reactive oxygen species production through miR-15b-5p/GSK3β axis in human dopaminergic SH-SY5Y cells. J. Cell Biochem. 2019;120:5790–5801. doi: 10.1002/jcb.27865. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y., Lian Y., Ma Y., Wu C., Zheng Y., Xie N. LncRNA SNHG1 promotes α-synuclein aggregation and toxicity by targeting miR-15b-5p to activate SIAH1 in human neuroblastoma SH-SY5Y cells. Neurotoxicology. 2017;68:212–221. doi: 10.1016/j.neuro.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Cao B., Wang T., Qu Q., Kang T., Yang Q. Long noncoding RNA SNHG1 promotes neuroinflammation in Parkinson’s disease via regulating miR-7/NLRP3 pathway. Neuroscience. 2018;388:118–127. doi: 10.1016/j.neuroscience.2018.07.019. [DOI] [PubMed] [Google Scholar]
- 58.Qian C., Ye Y., Mao H., Yao L., Sun X., Wang B., Zhang H., Xie L., Zhang H., Zhang Y., et al. Downregulated lncRNA-SNHG1 enhances autophagy and prevents cell death through the miR-221/222/p27/mTOR pathway in Parkinson’s disease. Exp. Cell Res. 2019;384:111614. doi: 10.1016/j.yexcr.2019.111614. [DOI] [PubMed] [Google Scholar]
- 59.Peng T., Liu X., Wang J., Liu Y., Fu Z., Ma X., Li J., Sun G., Ji Y., Lu J., et al. Long noncoding RNA HAGLROS regulates apoptosis and autophagy in Parkinson’s disease via regulating miR-100/ATG10 axis and PI3K/Akt/mTOR pathway activation. Artif Cells Nanomed. Biotechnol. 2019;47:2764–2774. doi: 10.1080/21691401.2019.1636805. [DOI] [PubMed] [Google Scholar]
- 60.Zhao J., Li H., Chang N. LncRNA HOTAIR promotes MPP+ -induced neuronal injury in Parkinson’s Disease by regulating the miR-874-5p/ATG10 axis. EXCLI J. 2020;19:1141–1153. doi: 10.17179/excli2020-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin Q., Hou S., Dai Y., Jiang N., Lin Y. LncRNA HOTAIR targets miR-126-5p to promote the progression of Parkinson’s disease through RAB3IP. Biol. Chem. 2019;400:1217–1228. doi: 10.1515/hsz-2018-0431. [DOI] [PubMed] [Google Scholar]
- 62.Liu R., Li F., Zhao W. Long noncoding RNA NEAT1 knockdown inhibits MPP+-induced apoptosis, inflammation and cytotoxicity in SK-N-SH cells by regulating miR-212-5p/RAB3IP axis. Neurosci. Lett. 2020;731:135060. doi: 10.1016/j.neulet.2020.135060. [DOI] [PubMed] [Google Scholar]
- 63.Zhou S., Zhang D., Guo J., Chen Z., Chen Y., Zhang J. Deficiency of NEAT1 prevented MPP + -induced inflammatory response, oxidative stress and apoptosis in dopaminergic SK-N-SH neuroblastoma cells via miR-1277-5p/ARHGAP26 axis. Brain Res. 2020;1750:147156. doi: 10.1016/j.brainres.2020.147156. [DOI] [PubMed] [Google Scholar]
- 64.Xie S., Zhou F., Li J., Duan S. NEAT1 regulates MPP + -induced neuronal injury by targeting miR-124 in neuroblastoma cells. Neurosci. Lett. 2019;708:134340. doi: 10.1016/j.neulet.2019.134340. [DOI] [PubMed] [Google Scholar]
- 65.Zhang L., Wang J., Liu Q., Xiao Z., Dai Q. Knockdown of long non-coding RNA AL049437 mitigates MPP+-induced neuronal injury in SH-SY5Y cells via the microRNA-205-5p/MAPK1 axis. Neurotoxicology. 2020;78:29–35. doi: 10.1016/j.neuro.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 66.Chen Q., Huang X., Li R. lncRNA MALAT1/miR-205-5p axis regulates MPP+-induced cell apoptosis in MN9D cells by directly targeting LRRK2. Am. J. Transl. Res. 2018;10:563–572. [PMC free article] [PubMed] [Google Scholar]
- 67.Liu W., Zhang Q., Zhang J., Pan W., Zhao J., Xu Y. Long non-coding RNA MALAT1 contributes to cell apoptosis by sponging miR-124 in Parkinson disease. Cell Biosci. 2017;7:1–9. doi: 10.1186/s13578-017-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu Y., Gong Z., Wang Z. LncRNA MALAT1 targeting miR-124-3p regulates DAPK1 expression contributes to cell apoptosis in Parkinson’s Disease. J. Cell Biochem. 2020 doi: 10.1002/jcb.29711. Published online. [DOI] [PubMed] [Google Scholar]
- 69.Xia D., Sui R., Zhang Z. Administration of resveratrol improved Parkinson’s disease-like phenotype by suppressing apoptosis of neurons via modulating the MALAT1/miR-129/SNCA signaling pathway. J. Cell Biochem. 2018;120:4942–4951. doi: 10.1002/jcb.27769. [DOI] [PubMed] [Google Scholar]
- 70.Zhang L.M., Wang M.H., Yang H.C., Tian T., Sun G.F., Ji Y.F., Hu W.T., Liu X., Wang J.P., Lu H. Dopaminergic neuron injury in Parkinson’s disease is mitigated by interfering lncRNA SNHG14 expression to regulate the miR-133b/α-synuclein pathway. Aging. 2019;11:9264–9279. doi: 10.18632/aging.102330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu X., Zhuang C., Wu Z., Qiu H., Feng H., Wu J. LincRNA-p21 Inhibits Cell Viability and Promotes Cell Apoptosis in Parkinson’s Disease through Activating α-Synuclein Expression. Biomed. Res. Int. 2018;2018:8181374. doi: 10.1155/2018/8181374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ye Y., He X., Lu F., Mao H., Zhu Z., Yao L., Luo W., Sun X., Wang B., Qian C., et al. A lincRNA-p21/miR-181 family feedback loop regulates microglial activation during systemic LPS- and MPTP- induced neuro inflammation. Cell Death Dis. 2018;9:803. doi: 10.1038/s41419-018-0821-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding X., Zhao L., Qiao H., Wu S., Wang X. Long non-coding RNA-p21 regulates MPP + -induced neuronal injury by targeting miR-625 and derepressing TRPM2 in SH-SY5Y cells. Chem. Biol. Interact. 2019;307:73–81. doi: 10.1016/j.cbi.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 74.Xu W., Zhang L., Geng Y., Liu Y., Zhang N. Long noncoding RNA GAS5 promotes microglial inflammatory response in Parkinson’s disease by regulating NLRP3 pathway through sponging miR-223-3p. Int. Immunopharmacol. 2020;85:106614. doi: 10.1016/j.intimp.2020.106614. [DOI] [PubMed] [Google Scholar]
- 75.Fan Y., Zhao X., Lu K., Cheng G. LncRNA BDNF-AS promotes autophagy and apoptosis in MPTP-induced Parkinson’s disease via ablating microRNA-125b-5p. Brain Res. Bull. 2020;157:119–127. doi: 10.1016/j.brainresbull.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 76.Han Y., Kang C., Kang M., Quan W., Gao H., Zhong Z. Long non-coding RNA Mirt2 prevents TNF-α-triggered inflammation via the repression of microRNA-101. Int. Immunopharmacol. 2019;76:105878. doi: 10.1016/j.intimp.2019.105878. [DOI] [PubMed] [Google Scholar]
- 77.Jiang J., Piao X., Hu S., Gao J., Bao M. LncRNA H19 diminishes dopaminergic neuron loss by mediating microRNA-301b-3p in Parkinson’s disease via the HPRT1-mediated Wnt/β-catenin signaling pathway. Aging. 2020;12:8820–8836. doi: 10.18632/aging.102877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y., Xia Q., Lin J. LncRNA H19 Attenuates Apoptosis in MPTP-Induced Parkinson’s Disease Through Regulating miR-585-3p/PIK3R3. Neurochem. Res. 2020;45:1700–1710. doi: 10.1007/s11064-020-03035-w. [DOI] [PubMed] [Google Scholar]
- 79.Junn E., Lee K., Jeong B.S., Chan T.W., Im J., Mouradian M.M. Repression of alpha-synuclein expression and toxicity by microRNA-7. Proc. Natl. Acad. Sci. USA. 2009;106:13052–13057. doi: 10.1073/pnas.0906277106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsiao K., Sun H.S., Tsai S. Circular RNA—New member of noncoding RNA with novel functions. Exp. Biol. Med. 2017;242:1136–1141. doi: 10.1177/1535370217708978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hansen T.B., Jensen T.I., Clausen B.H., Bramsen J.B., Finsen B., Damgaard C.K., Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 82.Kumar L., Shamsuzzama Haque R., Baghel T., Nazir A. Circular RNAs: The Emerging Class of Non-coding RNAs and Their Potential Role in Human Neurodegenerative Diseases. Mol. Neurobiol. 2017;54:7224–7234. doi: 10.1007/s12035-016-0213-8. [DOI] [PubMed] [Google Scholar]
- 83.Shao Y., Chen Y. Roles of Circular RNAs in Neurologic Disease. Front. Mol. Neurosci. 2016;9:25. doi: 10.3389/fnmol.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sang Q., Liu X., Wang L., Qi L., Sun W., Wang W., Sun Y., Zhang H. CircSNCA downregulation by pramipexole treatment mediates cell apoptosis and autophagy in Parkinson’s disease by targeting miR-7. Aging. 2018;10:1281–1293. doi: 10.18632/aging.101466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kumar L., Shamsuzzama, Jadiya P., Haque R., Shukla S., Nazir A. Functional Characterization of Novel Circular RNA Molecule, circzip-2 and Its Synthesizing Gene zip-2 in C. elegans Model of Parkinson’s Disease. Mol. Neurobiol. 2018;55:6914–6926. doi: 10.1007/s12035-018-0903-5. [DOI] [PubMed] [Google Scholar]
- 86.Feng Z., Zhang L., Wang S., Hong Q. Circular RNA circDLGAP4 exerts neuroprotective effects via modulating miR-134-5p/CREB pathway in Parkinson’s disease. Biochem. Biophys. Res. Commun. 2020;522:388–394. doi: 10.1016/j.bbrc.2019.11.102. [DOI] [PubMed] [Google Scholar]
- 87.Bian Z., Lei W., Li Q., Xue W., Gao Y., Zeng Y., Wang Y., Tang L., Tang T., Chen C., et al. Gm15575 functions as a ceRNA to up-regulate CCL7 expression through sponging miR-686 in Th17 cells. Mol. Immunol. 2020;125:32–42. doi: 10.1016/j.molimm.2020.06.027. [DOI] [PubMed] [Google Scholar]
- 88.Wu L., Xia J., Li D., Kang Y., Fang W., Huang P. Mechanisms of M2 Macrophage-Derived Exosomal Long Non-coding RNA PVT1 in Regulating Th17 Cell Response in Experimental Autoimmune Encephalomyelitisa. Front. Immunol. 2020;11:1934. doi: 10.3389/fimmu.2020.01934. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Yue P., Jing L., Zhao X., Zhu H., Teng J. Down-regulation of taurine-up-regulated gene 1 attenuates in fl ammation by sponging miR-9-5p via targeting NF- κ B1/p50 in multiple sclerosis. Life Sci. 2019;233:116731. doi: 10.1016/j.lfs.2019.116731. [DOI] [PubMed] [Google Scholar]
- 90.Duan C., Liu Y., Li Y., Chen H., Liu X., Chen X., Yue J., Zhou X., Yang J. Sulfasalazine alters microglia phenotype by competing endogenous RNA effect of miR-136-5p and long non-coding RNA HOTAIR in cuprizone-induced demyelination. Biochem. Pharmacol. 2018;155:110–123. doi: 10.1016/j.bcp.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 91.Senousy M.A., Shaker O.G., Sayed N.H., Fathy N., Kortam M.A. LncRNA GAS5 and miR-137 Polymorphisms and Expression are Associated with Multiple Sclerosis Risk: Mechanistic Insights and Potential Clinical Impact. ACS Chem. Neurosci. 2020;11:1651–1660. doi: 10.1021/acschemneuro.0c00150. [DOI] [PubMed] [Google Scholar]
- 92.Cardamone G., Paraboschi E.M., Rimoldi V., Duga S., Sold G., Asselta R. The Characterization of GSDMB Splicing and Backsplicing Profiles Identifies Novel Isoforms and a Circular RNA That Are Dysregulated in Multiple Sclerosis. Int. J. Mol. Sci. 2017;18:576. doi: 10.3390/ijms18030576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xia X., Tang X., Wang S. Roles of CircRNAs in Autoimmune Diseases. Front. Inmunol. 2019;10:639. doi: 10.3389/fimmu.2019.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iparraguirre L., Muñoz-Culla M., Prada-Luengo I., Castillo-Triviño T., Olascoaga J., Otaegui D. Circular RNA profiling reveals that circular RNAs from ANXA2 can be used as new biomarkers for multiple sclerosis. Hum. Mol. Genet. 2017;26:3564–3572. doi: 10.1093/hmg/ddx243. [DOI] [PubMed] [Google Scholar]
- 95.Tan J.Y., Vance K.W., Varela M.A., Sirey T., Watson L.M., Curtis H.J., Marinello M., Alves S., Steinkraus B., Cooper S., et al. Cross-talking noncoding RNAs contribute to cell-specific neurodegeneration in SCA7. Nat. Struct. Mol. Biol. 2014;21:955–961. doi: 10.1038/nsmb.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ma N., Tie C., Yu B., Zhang W., Wan J. Identifying lncRNA–miRNA–mRNA networks to investigate Alzheimer’s disease pathogenesis and therapy strategy. Aging. 2020;12:2897–2920. doi: 10.18632/aging.102785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang L., Chen X., He D., Li Y., Fu J. Dissection of functional lncRNAs in Alzheimer’s disease by construction and analysis of lncRNA-mRNA networks based on competitive endogenous RNAs. Biochem. Biophys. Res. Commun. 2017;485:569–576. doi: 10.1016/j.bbrc.2016.11.143. [DOI] [PubMed] [Google Scholar]
- 98.Zhou Y., Xu Z., Yu Y., Cao J., Qiao Y. Comprehensive analysis of the lncRNA-associated ceRNA network identifies neuroinflammation biomarkers for Alzheimer’s disease. Mol. Omics. 2019;15:459–469. doi: 10.1039/C9MO00129H. [DOI] [PubMed] [Google Scholar]
- 99.Zhang S., Zhu D., Li H., Li H., Feng C., Zhang W. Characterization of circRNA-Associated-ceRNA Networks in a Senescence-Accelerated Mouse Prone 8 Brain. Mol. Ther. 2017;25:2053–2061. doi: 10.1016/j.ymthe.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee W.J., Moon J., Jeon D., Shin Y.W., Yoo J.S., Park D.K., Lee S.T., Jung K.H., Park K.I., Jung K.Y., et al. Possible epigenetic regulatory effect of dysregulated circular RNAs in Alzheimer’s disease model. Sci. Rep. 2019;9:11956. doi: 10.1038/s41598-019-48471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang Z., Xu P., Chen B., Zhang Z., Zhang C., Zhan Q., Huang S., Xia Z.A., Peng W. Identifying circRNA-associated-ceRNA networks in the hippocampus of Aβ1-42-induced Alzheimer’s disease-like rats using microarray analysis. Aging. 2018;10:775–788. doi: 10.18632/aging.101427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang J.L., Qin M.C., Zhou Y., Xu Z.H., Yang S.M., Zhang F., Zhong J., Liang M.K., Chen B., Zhang W.Y., et al. Comprehensive analysis of differentially expressed profiles of Alzheimer’s disease associated circular RNAs in an Alzheimer’s disease mouse model. Aging. 2018;10:253–265. doi: 10.18632/aging.101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang J.L., Xu Z.H., Yang S.M., Yu C., Zhang F., Qin M.C., Zhou Y., Zhong Z.G., Wu D.P. Identification of Differentially Expressed Profiles of Alzheimer’s Disease Associated Circular RNAs in a Panax Notoginseng Saponins-Treated Alzheimer’s Disease Mouse Model. Comput. Struct. Biotechnol. J. 2018;16:523–531. doi: 10.1016/j.csbj.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ma N., Pan J., Ye X., Yu B., Zhang W., Wan J. Whole-Transcriptome Analysis of APP/PS1 Mouse Brain and Identification of circRNA-miRNA-mRNA Networks to Investigate AD Pathogenesis. Mol. Ther. Nucleic. Acids. 2019;18:1049–1062. doi: 10.1016/j.omtn.2019.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nam K.I., Yoon G., Kim Y.K., Song J. Transcriptome Analysis of Pineal Glands in the Mouse Model of Alzheimer’s Disease. Front. Mol. Neurosci. 2020;12:318. doi: 10.3389/fnmol.2019.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y., Yu F., Bao S., Sun J. Systematic Characterization of Circular RNA-Associated CeRNA Network Identified Novel circRNA Biomarkers in Alzheimer’s Disease. Front. Bioeng. Biotechnol. 2019;7:222. doi: 10.3389/fbioe.2019.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dube U., Del-Aguila J.L., Li Z., Budde J.P., Jiang S., Hsu S., Ibanez L., Fernandez M.V., Farias F., Norton J., et al. An atlas of cortical circular RNA expression in Alzheimer disease brains demonstrates clinical and pathological associations. Nat. Neurosci. 2019;22:1903–1912. doi: 10.1038/s41593-019-0501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lo I., Hill J., Vilhjálmsson B.J., Kjems J. Linking the association between circRNAs and Alzheimer’s disease progression by multi-tissue circular RNA characterization. RNA Biol. 2020;3:1–9. doi: 10.1080/15476286.2020.1783487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li Y., Fan H., Sun J., Ni M., Zhang L., Chen C., Hong X., Fang F., Zhang W., Ma P. Circular RNA expression profile of Alzheimer’s disease and its clinical significance as biomarkers for the disease risk and progression. Int. J. Biochem. Cell Biol. 2020;123:105747. doi: 10.1016/j.biocel.2020.105747. [DOI] [PubMed] [Google Scholar]
- 110.Li Y., Lv Z., Zhang J., Ma Q., Li Q., Song L., Gong L., Zhu Y., Li X., Hao Y., et al. Profiling of differentially expressed circular RNAs in peripheral blood mononuclear cells from Alzheimer’s disease patients. Metab. Brain Dis. 2020;35:201–213. doi: 10.1007/s11011-019-00497-y. [DOI] [PubMed] [Google Scholar]
- 111.Lin D., Liang Y., Jing X., Chen Y., Lei M., Zeng Z., Zhou T., Wu X., Peng S., Zheng D., et al. Microarray analysis of an synthetic a-synuclein induced cellular model reveals the expression profile of long non-coding RNA in Parkinson’s disease. Brain Res. 2018;1678:384–396. doi: 10.1016/j.brainres.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 112.Chi L., Wang L., Jiao D. Identification of Differentially Expressed Genes and Long Noncoding RNAs Associated with Parkinson’s Disease. Parkinsons Dis. 2019;2019:6078251. doi: 10.1155/2019/6078251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang X., Feng S., Fan Y., Luo Y., Jin L., Li S. Identifying a Comprehensive ceRNA Network to Reveal Novel Targets for the Pathogenesis of Parkinson’s Disease. Front. Neurol. 2020;11:810. doi: 10.3389/fneur.2020.00810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jia E., Zhou Y., Liu Z., Wang L., Ouyang T., Pan M., Bai Y., Ge Q. Transcriptomic Profiling of Circular RNA in Different Brain Regions of Parkinson’s Disease in a Mouse Model. Int. J. Mol. Sci. 2020;21:3006. doi: 10.3390/ijms21083006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hanan M., Simchovitz A., Yayon N., Vaknine S., Cohen-Fultheim R., Karmon M., Madrer N., Rohrlich T.M., Maman M., Bennett E.R., et al. A Parkinson’s disease CircRNAs Resource reveals a link between circSLC8A1 and oxidative stress. EMBO Mol. Med. 2020;12:e11942. doi: 10.15252/emmm.202013551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dolinar A., Koritnik B., Glavač D., Ravnik-Glavač M. Circular RNAs as Potential Blood Biomarkers in Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2019;56:8052–8062. doi: 10.1007/s12035-019-1627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ravnik-Glavač M., Glavač D. Circulating RNAs as Potential Biomarkers in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2020;21:1714. doi: 10.3390/ijms21051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lane C.A., Hardy J., Schott J.M. Alzheimer’s disease. Eur. J. Neurol. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 119.Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the Global Burden of Alzheimer’s Disease. Alzheimer Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 120.Reitz C., Brayne C., Mayeux R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li R., Lindholm K., Yang L.B., Yue X., Citron M., Yan R., Beach T., Sue L., Sabbagh M., Cai H., et al. Amyloid β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhao J., Fu Y., Yasvoina M., Shao P., Hitt B., O’Connor T., Logan S., Maus E., Citron M., Berry R., et al. β-site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: Implications for Alzheimer’s disease pathogenesis. J. Neurosci. 2007;27:3639–3649. doi: 10.1523/JNEUROSCI.4396-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Faghihi M.A., Modarresi F., Khalil A.M., Wood D.E., Sahagan B.G., Morgan T.E., Finch C.E., St Laurent G., 3rd, Kenny P.J., Wahlestedt C. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of b-secretase. Nat. Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y., Li Q., Liu C., Gao S., Ping H., Wang J., Wang P. MiR-214-3p attenuates cognition defects via the inhibition of autophagy in SAMP8 mouse model of sporadic Alzheimer’s disease. Neurotoxicology. 2016;56:139–149. doi: 10.1016/j.neuro.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 125.Cha D.J., Mengel D., Mustapic M., Liu W., Selkoe D.J., Kapogiannis D., Galasko D., Rissman R.A., Bennett D.A., Walsh D.M. MiR-212 and miR-132 are downregulated in neurally derived plasma exosomes of Alzheimer’s patients. Front. Neurosci. 2019;13:1208. doi: 10.3389/fnins.2019.01208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao Y., Zhao R., Wu J., Wang Q., Pang K., Shi Q., Gao Q., Hu Y., Dong X., Zhang J., et al. Melatonin protects against Aβ -induced neurotoxicity in primary neurons via miR-132/PTEN/AKT/FOXO3a pathway. Biofactors. 2018;44:609–618. doi: 10.1002/biof.1411. [DOI] [PubMed] [Google Scholar]
- 127.Wang Y., Veremeyko T., Wong A.H., El Fatimy R., Wei Z., Cai W., Krichevsky A.M. Downregulation of miR-132/212 impairs S-nitrosylation balance and induces tau phosphorylation in Alzheimer’s disease. Neurobiol. Aging. 2017;51:156–166. doi: 10.1016/j.neurobiolaging.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 128.Salta E., Sierksma A., Eynden E., De Vanden Strooper B. miR- 132 loss de-represses ITPKB and aggravates amyloid and TAU pathology in Alzheimer’s brain. EMBO Mol. Med. 2016;8:1005–1018. doi: 10.15252/emmm.201606520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xu N., Li A., Ji L., Ye Y., Wang Z., Tong L. miR-132 regulates the expression of synaptic proteins in APP/PS1 transgenic mice through C1q. Eur. J. Histochem. 2019;63:3008. doi: 10.4081/ejh.2019.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.El Fatimy R., Li S., Chen Z., Mushannen T., Gongala S., Wei Z., Balu D.T., Rabinovsky R., Cantlon A., Elkhal A., et al. MicroRNA-132 provides neuroprotection for tauopathies via multiple signaling pathways. Acta Neuropathol. 2018;136:537–555. doi: 10.1007/s00401-018-1880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moncini S., Lunghi M., Valmadre A., Grasso M., Del Vescovo V., Riva P., Denti M.A., Venturin M. The miR-15/107 Family of microRNA Genes Regulates CDK5R1/p35 with Implications for Alzheimer’s Disease Pathogenesis. Mol. Neurobiol. 2017;54:4329–4342. doi: 10.1007/s12035-016-0002-4. [DOI] [PubMed] [Google Scholar]
- 132.Hernandez-Rapp J., Rainone S., Goupil C., Dorval V., Smith P.Y., Saint-Pierre M., Vallée M., Planel E., Droit A., Calon F., et al. microRNA-132/212 deficiency enhances Aβ production and senile plaque deposition in Alzheimer’s disease triple transgenic mice. Sci. Rep. 2016;6:30953. doi: 10.1038/srep30953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hadar A., Milanes E., Walczak M., Kuźnicki J., Squassina A. SIRT1, miR-132 and miR-212 link human longevity to Alzheimer’s Disease. Sci. Rep. 2018;8:8465. doi: 10.1038/s41598-018-26547-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rizzi L., Roriz-Cruz M. Sirtuin 1 and Alzheimer’s disease: An up-to-date review. Neuropeptides. 2018;71:54–60. doi: 10.1016/j.npep.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 135.Lee H.R., Shin H.K., Park S.Y., Kim H.Y., Lee W.S., Rhim B.Y., Hong K.W., Kim C.D. Cilostazol suppresses β-amyloid production by activating a disintegrin and metalloproteinase 10 via the upregulation of SIRT1-coupled retinoic acid receptor-β. J. Neurosci. Res. 2014;92:1581–1590. doi: 10.1002/jnr.23421. [DOI] [PubMed] [Google Scholar]
- 136.Qin W., Yang T., Ho L., Zhao Z., Wang J., Chen L., Zhao W., Thiyagarajan M., MacGrogan D., Rodgers J.T., et al. Neuronal SIRT1 Activation as a Novel Mechanism Underlying the Prevention of Alzheimer Disease Amyloid Neuropathology by Calorie Restriction. J. Biol. Chem. 2006;281:21745–21754. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 137.Feng X., Liang N., Zhu D., Gao Q., Peng L., Dong H., Yue Q., Liu H., Bao L., Zhang J., et al. Resveratrol inhibits β-amyloid-induced neuronal apoptosis through regulation of SIRT1-ROCK1 signaling pathway. PLoS ONE. 2013;8:e59888. doi: 10.1371/journal.pone.0059888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Henderson B.W., Gentry E.G., Rush T., Troncoso J.C., Thambisetty M., Montine T.J., Herskowitz J.H. Rho-associated protein kinase 1 (ROCK1) is increased in Alzheimer’s disease and ROCK1 depletion reduces amyloid-β levels in brain. J. Neurochem. 2016;138:525–531. doi: 10.1111/jnc.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu L., Shi Y., Shi J., Wang H., Sheng Y., Jiang Q., Chen H., Li X., Dong J. The long non-coding RNA SNHG1 promotes glioma progression by competitively binding to miR-194 to regulate PHLDA1 expression. Cell Death Dis. 2019;10:463. doi: 10.1038/s41419-019-1698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xu M., Chen X., Lin K., Zeng K., Liu X., Pan B., Xu X., Xu T., Hu X., Sun L., et al. The long noncoding RNA SNHG1 regulates colorectal cancer cell growth through interactions with EZH2 and miR-154-5p. Mol. Cancer. 2018;17:141. doi: 10.1186/s12943-018-0894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang H., Wang G., Gao Y., Zhao C., Li X., Zhang F., Jiang C., Wu B. Lnc-SNHG1 Activates the TGFBR2/SMAD3 and RAB11A/Wnt/β-Catenin Pathway by Sponging MiR-302/372/373/520 in Invasive Pituitary Tumors. Cell Physiol. Biochem. 2018;48:1291–1303. doi: 10.1159/000492089. [DOI] [PubMed] [Google Scholar]
- 142.Tian M., Gong W., Guo J. Long non-coding RNA SNHG1 indicates poor prognosis and facilitates disease progression in acute myeloid leukemia. Biol. Open. 2019;8 doi: 10.1242/bio.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Q., Li Q., Zhou P., Deng D., Xue L., Shao N., Peng Y., Zhi F. Upregulation of the long non-coding RNA SNHG1 predicts poor prognosis, promotes cell proliferation and invasion, and reduces apoptosis in glioma. Biomed. Pharmacother. 2017;91:906–911. doi: 10.1016/j.biopha.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 144.Lei J., Fu Y., Zhuang Y., Zhang K., Lu D. LncRNA SNHG1 alleviates IL-1β -induced osteoarthritis by inhibiting miR-16-5p-mediated p38 MAPK and NF-κB signaling pathways. Biosci. Rep. 2019;39:BSR20191523. doi: 10.1042/BSR20191523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mulvaney J.F., Thompkins C., Noda T., Nishimura K., Sun W.W., Lin S.Y., Coffin A., Dabdoub A. Kremen1 regulates mechanosensory hair cell development in the mammalian cochlea and the zebrafish lateral line. Sci. Rep. 2016;6:4–13. doi: 10.1038/srep31668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Causeret F., Sumia I., Pierani A. Kremen1 and Dickkopf1 control cell survival in a Wnt-independent manner. Cell Death Differ. 2016;23:323–332. doi: 10.1038/cdd.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Quinlan K.G.R., Verger A., Yaswen P., Crossley M. Amplification of zinc finger gene 217 (ZNF217) and cancer: When good fingers go bad. Biochim. Biophys. Acta. 2007;1775:333–340. doi: 10.1016/j.bbcan.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 148.Yin Y., Cha C., Wu F., Li J., Li S. Endophilin 1 knockdown prevents synaptic dysfunction induced by oligomeric amyloid β. Mol. Med. Rep. 2019;19:4897–4905. doi: 10.3892/mmr.2019.10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu W., Cai H., Lin M., Zhu L., Gao L., Zhong R., Bi S., Xue Y., Shang X. MicroRNA-107 prevents amyloid-beta induced blood-brain barrier disruption and endothelial cell dysfunction by targeting Endophilin-1. Exp. Cell Res. 2016;343:248–257. doi: 10.1016/j.yexcr.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 150.Tanzi R.E., Moir R.D., Wagner S.L. Clearance of Alzheimer’s Aβ peptide: The many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 151.Rodrigues M., Xin X., Jee K., Babapoor-Farrokhran S., Kashiwabuchi F., Ma T., Bhutto I., Hassan S.J., Daoud Y., Baranano D., et al. VEGF secreted by hypoxic Müller cells induces MMP-2 expression and activity in endothelial cells to promote retinal neovascularization in proliferative diabetic retinopathy. Diabetes. 2013;62:3863–3873. doi: 10.2337/db13-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Reichenbanch A., Bringmann A. New functions of Müller cells. Glia. 2013;61:651–678. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- 153.Hagmann H., Taniguchi Y., Pippin J.W., Kauerz H.M., Benzing T., Shankland S.J., Brinkkoetter P.T. Cyclin I and p35 determine the subcellular distribution of Cdk5. Am. J. Physiol. Cell Physiol. 2015;308:C339–C347. doi: 10.1152/ajpcell.00168.2014. [DOI] [PubMed] [Google Scholar]
- 154.Patrick G.N., Zukerberg L., Nikolic M., De La Monte S., Dikkes P., Tsai L.H. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 155.Guo Z., Cao Q., Zhao Z., Song C. Biogenesis, Features, Functions, and Disease Relationships of a Specific Circular RNA: CDR1as. Aging Dis. 2020;11:1009–1020. doi: 10.14336/AD.2019.0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hansen T.B., Wiklund E.D., Bramsen J.B., Villadsen S.B., Statham A.L., Clark S.J., Kjems J. miRNA-dependent gene silencing involving Ago2-mediated cleavage of a circular antisense RNA. EMBO J. 2011;30:4414–4422. doi: 10.1038/emboj.2011.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Song J., Kim Y.K. Identification of the Role of miR-142-5p in Alzheimer’s Disease by Comparative Bioinformatics and Cellular Analysis. Front. Mol. Neurosci. 2017;10:227. doi: 10.3389/fnmol.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tian J., Guo L., Sui S., Driskill C., Phensy A., Wang Q., Gauba E., Zigman J.M., Swerdlow R.H., Kroener S., et al. Disrupted hippocampal growth hormone secretagogue receptor 1α interaction with dopamine receptor D1 plays a role in Alzheimer’s disease. Sci. Transl. Med. 2019;11:eaav6278. doi: 10.1126/scitranslmed.aav6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yamanaka Y., Faghihi M.A., Magistri M., Alvarez-Garcia O., Lotz M., Wahlestedt C. Antisense RNA controls LRP1 sense transcript expression through interaction with a chromatin-associated protein, HMGB2. Cell Rep. 2015;11:967–976. doi: 10.1016/j.celrep.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martiskainen H., Haapasalo A., Kurkinen K.M., Pihlajamäki J., Soininen H., Hiltunen M. Targeting ApoE4/ApoE receptor LRP1 in Alzheimer’s disease. Expert Opin. Ther. Targets. 2013;17:781–794. doi: 10.1517/14728222.2013.789862. [DOI] [PubMed] [Google Scholar]
- 161.Humphries C.E., Kohli M.A., Nathanson L., Whitehead P., Beecham G., Martin E., Mash D.C., Pericak-Vance M.A., Gilbert J. Integrated whole transcriptome and DNA methylation analysis identifies gene networks specific to late-onset Alzheimer’s disease. J. Alzheimers Dis. 2015;44:977–987. doi: 10.3233/JAD-141989. [DOI] [PubMed] [Google Scholar]
- 162.Murata N., Murakami K., Ozawa Y., Kinoshita N., Irie K., Shirasawa T., Shimizu T. Silymarin attenuated the amyloid β plaque burden and improved behavioral abnormalities in an Alzheimer’s disease mouse model. Biosci. Biotechnol. Biochem. 2010;74:2299–2306. doi: 10.1271/bbb.100524. [DOI] [PubMed] [Google Scholar]
- 163.Guo H., Cao H., Cui X., Zheng W., Wang S., Yu J., Chen Z. Silymarin’s Inhibition and Treatment Effects for Alzheimer’s Disease. Molecules. 2019;24:1748. doi: 10.3390/molecules24091748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Singh A., Kumar A., Verma R.K., Shukla R. Silymarin encapsulated nanoliquid crystals for improved activity against beta amyloid induced cytotoxicity. Int. J. Biol. Macromol. 2020;149:1198–1206. doi: 10.1016/j.ijbiomac.2020.02.041. [DOI] [PubMed] [Google Scholar]
- 165.Xu M., Xiao M., Li S., Yang B. Aquaporins in Nervous System. Adv. Exp. Med. Biol. 2017;969:81–103. doi: 10.1007/978-94-024-1057-0. [DOI] [PubMed] [Google Scholar]
- 166.Han C., Yang Y., Guan Q., Zhang X., Shen H., Sheng Y., Wang J., Zhou X., Li W., Guo L., et al. New mechanism of nerve injury in Alzheimer’s disease: β-amyloid-induced neuronal pyroptosis. J. Cell Mol. Med. 2020;24:8078–8090. doi: 10.1111/jcmm.15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xu Y., Tan H.Y., Li S., Wang N., Feng Y. Panax notoginseng for Inflammation-Related Chronic Diseases: A Review on the Modulations of Multiple Pathways. Am. J. Chin. Med. 2018;46:971–996. doi: 10.1142/S0192415X18500519. [DOI] [PubMed] [Google Scholar]
- 168.Grimmond S., Larder R., Van Hateren N., Siggers P., Morse S., Hacker T., Arkell R., Greenfield A. Expression of a novel mammalian epidermal growth factor-related gene during mouse neural development. Mech. Dev. 2001;102:209–211. doi: 10.1016/S0925-4773(00)00586-4. [DOI] [PubMed] [Google Scholar]
- 169.Zhang Q., Gao X., Li C., Feliciano C., Wang D., Zhou D., Mei Y., Monteiro P., Anand M., Itohara S., et al. Impaired Dendritic Development and Memory in Sorbs2 Knock-Out Mice. J. Neurosci. 2016;36:2247–2260. doi: 10.1523/JNEUROSCI.2528-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee J.H., Cheng R., Vardarajan B., Lantigua R., Reyes-Dumeyer D., Ortmann W., Graham R.R., Bhangale T., Behrens T.W., Medrano M., et al. Genetic Modifiers of Age at Onset in Carriers of the G206A Mutation in PSEN1 With Familial Alzheimer Disease Among Caribbean Hispanics. JAMA Neurol. 2015;72:1043–1051. doi: 10.1001/jamaneurol.2015.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Canali G., Garcia M., Hivert B., Pinatel D., Goullancourt A., Oguievetskaia K., Saint-Martin M., Girault J.A., Faivre-Sarrailh C., Goutebroze L. Genetic variants in autism-related CNTNAP2 impair axonal growth of cortical neurons. Hum. Mol. Genet. 2018;27:1941–1954. doi: 10.1093/hmg/ddy102. [DOI] [PubMed] [Google Scholar]
- 172.Amidfar M., de Oliveira J., Kucharska E., Budni J., Kim Y.K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020;257:118020. doi: 10.1016/j.lfs.2020.118020. [DOI] [PubMed] [Google Scholar]
- 173.Clokie S.J., Lau P., Kim H.H., Coon S.L., Klein D.C. MicroRNAs in the pineal gland: miR-483 regulates melatonin synthesis by targeting arylalkylamine N-acetyltransferase. J. Biol. Chem. 2012;287:25312–25324. doi: 10.1074/jbc.M112.356733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hébert S.S., Horré K., Nicolaï L., Papadopoulou A.S., Mandemakers W., Silahtaroglu A.N., Kauppinen S., Delacourte A., De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc. Natl. Acad. Sci. USA. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pereira P.A., Tomás J.F., Queiroz J.A., Figueiras A.R., Sousa F. Recombinant pre-miR-29b for Alzheimer´s disease therapeutics. Sci. Rep. 2016;6:19946. doi: 10.1038/srep19946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Long J.M., Lahiri D.K. MicroRNA-101 downregulates Alzheimer’s amyloid-β precursor protein levels in human cell cultures and is differentially expressed. Biochem. Biophys. Res. Commun. 2011;404:889–895. doi: 10.1016/j.bbrc.2010.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Luo P., Li X., Fei Z., Poon W. Scaffold protein Homer 1: Implications for neurological diseases. Neurochem. Int. 2012;61:731–738. doi: 10.1016/j.neuint.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 178.Dickey C.A., Loring J.F., Montgomery J., Gordon M.N., Eastman P.S., Morgan D. Selectively reduced expression of synaptic plasticity-related genes in amyloid precursor protein + presenilin-1 transgenic mice. J. Neurosci. 2003;23:5219–5226. doi: 10.1523/JNEUROSCI.23-12-05219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fjell A.M., Sederevicius D., Sneve M.H., de Lange A.G., Bråthen A.C., Idland A.V., Watne L.O., Wang Y., Reinbold C., Dobricic V., et al. Self-reported Sleep Problems Related to Amyloid Deposition in Cortical Regions with High HOMER1 Gene Expression. Cereb. Cortex. 2020;30:2144–2156. doi: 10.1093/cercor/bhz228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pannaccione A., Piccialli I., Secondo A., Ciccone R., Molinaro P., Boscia F., Annunziato L. The Na+/Ca2+exchanger in Alzheimer’s disease. Cell Calcium. 2020;87:102190. doi: 10.1016/j.ceca.2020.102190. [DOI] [PubMed] [Google Scholar]
- 181.Vargas L.M., Cerpa W., Muñoz F.J., Zanlungo S., Alvarez A.R. Amyloid-β oligomers synaptotoxicity: The emerging role of EphA4/c-Abl signaling in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:1148–1159. doi: 10.1016/j.bbadis.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 182.Zhang S., Chen S., Liu A., Wan J., Tang L., Zheng N., Xiong Y. Inhibition of BDNF production by MPP+ through up-regulation of miR-210-3p contributes to dopaminergic neuron damage in MPTP model. Neurosci. Lett. 2018;675:133–139. doi: 10.1016/j.neulet.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 183.Wang X., Tan L., Lu Y., Peng J., Zhu Y., Zhang Y., Sun Z. MicroRNA-138 promotes tau phosphorylation by targeting retinoic acid receptor alpha. FEBS Lett. 2015;589:726–729. doi: 10.1016/j.febslet.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 184.Kumar S., Reddy A.P., Yin X., Reddy P.H. Novel MicroRNA-455-3p and its protective effects against abnormal APP processing and amyloid beta toxicity in Alzheimer’s Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2019:1–28. doi: 10.1016/j.bbadis.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.De Lau L.M.L., Breteler M.M.B. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 186.Jankovic J. Parkinson’s disease: Clinical features and diagnosis. J. Neurosurg. Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 187.Beitz J.M. Parkinson’s disease: A review. Front. Biosci. 2014;6:65–74. doi: 10.2741/S415. [DOI] [PubMed] [Google Scholar]
- 188.Dauer W., Przedborski S. Parkinson’s disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 189.Nichols W.C., Pankratz N., Marek D.K., Pauciulo M.W., Elsaesser V.E., Halter C.A., Rudolph A., Wojcieszek J., Pfeiffer R.F., Foroud T., et al. Mutations in GBA are associated with familial Parkinson disease susceptibility and age at onset. Neurology. 2009;72:210–316. doi: 10.1212/01.wnl.0000327823.81237.d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Balestrino R., Schapira A.H.V. Glucocerebrosidase and Parkinson Disease: Molecular, Clinical, and Therapeutic Implications. Neuroscientist. 2018;24:540–559. doi: 10.1177/1073858417748875. [DOI] [PubMed] [Google Scholar]
- 191.Li W., Jiang Y., Wang Y., Yang S., Bi X., Pan X., Ma A., Li W. MiR-181b regulates autophagy in a model of Parkinson’s disease by targeting the PTEN/Akt/mTOR signaling pathway. Neurosci. Lett. 2018;675:83–88. doi: 10.1016/j.neulet.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 192.Leikas J.V., Kohtala S., Theilmann W., Jalkanen A.J., Forsberg M.M., Rantamäki T. Brief isoflurane anesthesia regulates striatal AKT-GSK3b signaling and ameliorates motor deficits in a rat model of early-stage Parkinson’s disease. J. Neurochem. 2017;142:456–463. doi: 10.1111/jnc.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Cai Z.L., Xu J., Xue S.R., Liu Y.Y., Zhang Y.J., Zhang X.Z., Wang X., Wu F.P., Li X.M. The E3 ubiquitin ligase seven in absentia homolog 1 may be a potential new therapeutic target for Parkinson’s disease. Neural. Regen. Res. 2015;10:1286–1291. doi: 10.4103/1673-5374.162763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lee J.T., Wheeler T.C., Li L., Chin L.-S. Ubiquitination of alpha-synuclein by Siah-1 promotes alpha-synuclein aggregation and apoptotic cell death. Hum. Mol. Genet. 2008;17:906–917. doi: 10.1093/hmg/ddm363. [DOI] [PubMed] [Google Scholar]
- 195.Wang S., Zhang X., Guo Y., Rong H., Liu T. The long noncoding RNA HOTAIR promotes Parkinson’s disease by upregulating LRRK2 expression. Oncotarget. 2017;8:24449–24456. doi: 10.18632/oncotarget.15511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kluss J.H., Mamais A., Cookson M.R., Biology C., Section G.E. LRRK2 links genetic and sporadic Parkinson’s disease. Biochem. Soc. Trans. 2019;47:651–661. doi: 10.1042/BST20180462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ultanir S.K., Hertz N.T., Li G., Ge W.P., Burlingame A.L., Pleasure S.J., Shokat K.M., Jan L.Y., Jan Y.N. Chemical genetic identification of NDR1/2 kinase substrates AAK1 and Rabin8 uncovers their roles in controlling dendrite arborization and spine development. Neuron. 2012;73:1127–1142. doi: 10.1016/j.neuron.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Homma Y., Fukuda M. Rabin8 regulates neurite outgrowth in both GEF activity–dependent and –independent manners. Mol. Biol. Cell. 2016;27:2107–2118. doi: 10.1091/mbc.E16-02-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Boros F.A., Maszlag-Török R., Vécsei L., Klivényi P. Increased level of NEAT1 long non-coding RNA is detectable in peripheral blood cells of patients with Parkinson’s disease. Brain Res. 2020;1730:146672. doi: 10.1016/j.brainres.2020.146672. [DOI] [PubMed] [Google Scholar]
- 200.Hirota Y., Yamashita S., Kurihara Y., Jin X., Aihara M., Saigusa T., Kan D., Kanki T. Mitophagy is primarily due to alternative autophagy and requires the MAPK1 and MAPK14 signaling pathways. Autophagy. 2015;11:332–343. doi: 10.1080/15548627.2015.1023047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xiao Y., Liu H., Yu J., Zhao Z., Xiao F., Xia T., Wang C., Li K., Deng J., Guo Y., et al. MAPK1/3 regulate hepatic lipid metabolism via ATG7-dependent autophagy. Autophagy. 2016;12:592–593. doi: 10.1080/15548627.2015.1135282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kim J., Ko A., Hyun H., Min S., Kang T. P2RX7-MAPK1/2-SP1 axis inhibits MTOR independent HSPB1-mediated astroglial autophagy. Cell Death Dis. 2018;9:546. doi: 10.1038/s41419-018-0586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Singh P., Ravanan P., Talwar P. Death Associated Protein Kinase 1 (DAPK1): A Regulator of Apoptosis and Autophagy. Front. Mol. Neurosci. 2016;9:46. doi: 10.3389/fnmol.2016.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.You M., Kim B.M., Chen C., Begley M.J., Cantley L.C., Lee T.H. Death-associated protein kinase 1 phosphorylates NDRG2 and induces neuronal cell death. Cell Death Differ. 2017;24:238–250. doi: 10.1038/cdd.2016.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yao L., Ye Y., Mao H., Lu F., He X., Lu G., Zhang S. MicroRNA-124 regulates the expression of MEKK3 in the inflammatory pathogenesis of Parkinson’s disease. J. Neuroinflammation. 2018;15:13. doi: 10.1186/s12974-018-1053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang H., Ye Y., Zhu Z., Mo L., Lin C., Wang Q., Wang H., Gong X., He X., Lu G., et al. MiR-124 Regulates Apoptosis and Autophagy Process in MPTP Model of Parkinson’s Disease by Targeting to Bim. Brain Pathol. 2016;26:167–176. doi: 10.1111/bpa.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yao L., Zhu Z., Wu J., Zhang Y., Zhang H., Sun X., Qian C., Wang B., Xie L., Zhang S., et al. MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson’s disease. FASEB J. 2019;33:8648–8665. doi: 10.1096/fj.201900363R. [DOI] [PubMed] [Google Scholar]
- 208.Geng L., Liu W., Chen Y. miR-124-3p attenuates MPP þ-induced neuronal injury by targeting STAT3 in SH-SY5Y cells. Exp. Biol. Med. 2017;242:1757–1764. doi: 10.1177/1535370217734492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Dong R., Zhang B., Tai L., Liu H., Shi F.-K., Liu N.-N. The Neuroprotective Role of miR-124-3p in a 6-Hydroxydopamine- Induced Cell Model of Parkinson’s Disease via the Regulation of ANAX5. J. Cell Biochem. 2018;119:269–277. doi: 10.1002/jcb.26170. [DOI] [PubMed] [Google Scholar]
- 210.Tay S.S.W. Downregulation of miR-124 in MPTP-treated mouse model of Parkinson’s Disease and MPP iodide-treated MN9D cells modulates the expression of the calpain/CDK5 pathway proteins. Neuroscience. 2014;272:167–179. doi: 10.1016/j.neuroscience.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 211.Gong X., Wang H., Ye Y., Shu Y., Deng Y., He X., Lu G., Zhang S. miR-124 regulates cell apoptosis and autophagy in dopaminergic neurons and protects them by regulating AMPK/mTOR pathway in Parkinson’s disease. Am. J. Transl. Res. 2016;8:2127–2137. [PMC free article] [PubMed] [Google Scholar]
- 212.Xing R., Li L. Down regulation of miR-218, miR-124, and miR-144 relates to Parkinson’s disease via activating NF-κB signaling. Kaohsiung J. Med. Sci. 2020;36:786–792. doi: 10.1002/kjm2.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang J., Wang W., Zhai H. MicroRNA-124 Enhances Dopamine Receptor Expression and Neuronal Proliferation in Mouse Models of Parkinson’s Disease via the Hedgehog Signaling Pathway by Targeting EDN2. Neuroimmunomodulation. 2019;26:174–187. doi: 10.1159/000501339. [DOI] [PubMed] [Google Scholar]
- 214.Frahm C., Srivastava A., Schmidt S., Mueller J., Groth M., Guenther M., Ji Y., Priebe S., Platzer M., Witte O.W. Transcriptional profiling reveals protective mechanisms in brains of long-lived mice. Neurobiol. Aging. 2017;52:23–31. doi: 10.1016/j.neurobiolaging.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 215.Santiago J.A., Potashkin J.A. Network-based metaanalysis identifies HNF4A and PTBP1 as longitudinally dynamic biomarkers for Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2015;112:2257–2262. doi: 10.1073/pnas.1423573112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Santiago J.A., Potashkin J.A. Blood biomarkers associated with cognitive decline in early stage and drug-naïve Parkinson’s disease patients. PLoS ONE. 2015;10:e0142582. doi: 10.1371/journal.pone.0142582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Valente T., Dentesano G., Ezquerra M., Fernandez-Santiago R., Martinez-Martin J., Gallastegui E., Domuro C., Compta Y., Martí M.J., Bachs O., et al. CCAAT/enhancer binding protein δ is a transcriptional repressor of α-synuclein. Cell Death Differ. 2020;27:509–524. doi: 10.1038/s41418-019-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bawari S., Tewari D., Argüelles S., Sah A.N., Nabavi S.F., Xu S., Vacca R.A., Nabavi S.M., Shirooie S. Targeting BDNF signaling by natural products: Novel synaptic repair therapeutics for neurodegeneration and behavior disorders. Pharmacol. Res. 2019;148:104458. doi: 10.1016/j.phrs.2019.104458. [DOI] [PubMed] [Google Scholar]
- 219.D’Orsi B., Mateyka J., Prehn J.H.M. Neurochemistry International Control of mitochondrial physiology and cell death by the Bcl-2 family proteins Bax and Bok. Neurochem. Int. 2017;109:162–170. doi: 10.1016/j.neuint.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 220.Lv J., Jiang S., Yang Z., Hu W., Wang Z., Li T., Yang Y. PGC-1α sparks the fire of neuroprotection against neurodegenerative disorders. Ageing Res. Rev. 2018;44:8–21. doi: 10.1016/j.arr.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 221.Jakaria M., Haque M.E., Cho D.Y., Azam S., Kim I.S., Choi D.K. Molecular Insights into NR4A2(Nurr1): An Emerging Target for Neuroprotective Therapy Against Neuroinflammation and Neuronal Cell Death. Mol. Neurobiol. 2019;56:5799–5814. doi: 10.1007/s12035-019-1487-4. [DOI] [PubMed] [Google Scholar]
- 222.Saraiva C., Paiva J., Santos T., Ferreira L., Bernardino L. MicroRNA-124 loaded nanoparticles enhance brain repair in Parkinson’s disease. J. Control Release. 2016;235:291–305. doi: 10.1016/j.jconrel.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 223.Sirabella R., Sisalli M.J., Costa G., Omura K., Ianniello G., Pinna A., Morelli M., Di Renzo G.M., Annunziato L., Scorziello A. NCX1 and NCX3 as potential factors contributing to neurodegeneration and neuroinflammation in the A53T transgenic mouse model of Parkinson’s Disease. Cell Death Dis. 2018 doi: 10.1038/s41419-018-0775-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Cotsapas C., Mitrovic M., Hafler D. Multiple Sclerosis. In: Geschwind D.H., Paulsori H.L., Klein C., editors. Handbook of Clinical Neurology. 3rd ed. Volume 148. Elsevier; Amsterdam, The Netherlands: 2018. pp. 723–730. [DOI] [PubMed] [Google Scholar]
- 225.Oh J., Vidal-jordana A., Montalban X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018;31:752–759. doi: 10.1097/WCO.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 226.Bjelobaba I., Savic D., Lavrnja I. Multiple Sclerosis and neuroinflammation: The overview of current and prospective therapies. Curr. Pharm. Des. 2017;23:693–730. doi: 10.2174/1381612822666161214153108. [DOI] [PubMed] [Google Scholar]
- 227.Santoro M., Nociti V., Lucchini M., De Fino C., Losavio F.A., Mirabella M. Expression Profile of Long Non-Coding RNAs in Serum of Patients with Multiple Sclerosis. J. Mol. Neurosci. 2016;59:18–23. doi: 10.1007/s12031-016-0741-8. [DOI] [PubMed] [Google Scholar]
- 228.Sigdel K.R., Cheng A., Wang Y., Duan L., Zhang Y. The Emerging Functions of Long Noncoding RNA in Immune Cells: Autoimmune Diseases. J. Immunol. Res. 2015;2015:848790. doi: 10.1155/2015/848790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Dos Passos G.R., Sato D.K., Becker J., Fujihara K. Th17 Cells Pathways in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders: Pathophysiological and Therapeutic Implications. Mediat. Inflamm. 2016 doi: 10.1155/2016/5314541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lindner M., Thümmler K., Arthur A., Brunner S., Elliott C., McElroy D., Mohan H., Williams A., Edgar J.M., Schuh C., et al. Fibroblast growth factor signalling in multiple sclerosis: Inhibition of myelination and induction of pro-inflammatory environment by FGF9. Brain. 2015;138:1875–1893. doi: 10.1093/brain/awv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Ivanov I.I., Mckenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T Helper Cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 232.Eftekharian M.M., Ghafouri-Fard S., Soudyab M., Omrani M.D., Rahimi M., Sayad A., Komaki A., Mazdeh M., Taheri M. Expression Analysis of Long Non-coding RNAs in the Blood of Multiple Sclerosis Patients. J. Mol. Neurosci. 2017;63:333–341. doi: 10.1007/s12031-017-0982-1. [DOI] [PubMed] [Google Scholar]
- 233.Toghi M., Taheri M., Arsang-jang S., Ohadi M., Mirfakhraie R. SOCS gene family expression profile in the blood of multiple sclerosis patients. J. Neurol. Sci. 2017;375:481–485. doi: 10.1016/j.jns.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 234.Fitzgerald J.S., Toth B., Jeschke U., Schleussner E., Markert U.R. Knocking off the suppressors of cytokine signaling (SOCS): Their roles in mammalian pregnancy. J. Reprod. Immunol. 2009;83:117–123. doi: 10.1016/j.jri.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 235.Kim S., Lee Y.I., Chang K.Y., Lee D.W., Cho S.C., Ha Y.W., Na J.E., Rhyu I.J., Park S.C., Park H.C. Promotion of remyelination by sulfasalazine in a transgenic zebrafish model of demyelination. Mol. Cells. 2015;38:1013–1021. doi: 10.14348/molcells.2015.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Prosiegel M., Neu I., Vogl S., Hoffmann G., Wildfeuer A., Ruhenstroth-Bauer G. Suppression of experimental autoimmune encephalomyelitis by sulfasalazine. Acta Neurol. Scand. 1990;81:237–238. doi: 10.1111/j.1600-0404.1990.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 237.Chen F., Zhang L., Wang E., Zhang C., Li X. LncRNA GAS5 regulates ischemic stroke as a competing endogenous RNA for miR-137 to regulate the Notch1 signaling pathway. Biochem. Biophys. Res. Commun. 2018;496:184–190. doi: 10.1016/j.bbrc.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 238.Sun D., Yu Z., Fang X., Liu M., Pu Y., Shao Q., Wang D., Zhao X., Huang A., Xiang Z., et al. LncRNA GAS 5 inhibits microglial M2 polarization and exacerbates demyelination. EMBO Rep. 2017;18:1801–1816. doi: 10.15252/embr.201643668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cardamone G., Paraboschi E.M., Soldà G., Cantoni C., Supino D., Piccio L., Duga S., Asselta R. Not only cancer: The long non-coding RNA MALAT1 affects the repertoire of alternatively spliced transcripts and circular RNAs in multiple sclerosis. Hum. Mol. Genet. 2019;28:1414–1428. doi: 10.1093/hmg/ddy438. [DOI] [PubMed] [Google Scholar]
- 240.Shaker O.G., Mahmoud R.H., Abdelaleem O.O., Ibrahem E.G., Mohamed A.A., Zaki O.M., Abdelghaffar N.K., Ahmed T.I., Hemeda N.F., Ahmed N.A., et al. LncRNAs, MALAT1 and lnc-DC as potential biomarkers for multiple sclerosis diagnosis. Biosci. Rep. 2019;39 doi: 10.1042/BSR20181335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Das S., Miller M., Broide D.H. Chromosome 17q21 Genes ORMDL3 and GSDMB in Asthma and Immune Diseases. Adv. Immunol. 2017;135:1–52. doi: 10.1016/bs.ai.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 242.Keller A., Leidinger P., Lange J., Borries A., Schroers H., Scheffler M., Lenhof H.P., Ruprecht K., Meese E. Multiple sclerosis: microRNA expression profiles accurately differentiate patients with relapsing-remitting disease from healthy controls. PLoS ONE. 2009;4:e7440. doi: 10.1371/journal.pone.0007440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hecker M., Thamilarasan M., Koczan D., Schröder I., Flechtner K., Freiesleben S., Füllen G., Thiesen H.J., Zettl U.K. MicroRNA expression changes during interferon-beta treatment in the peripheral blood of multiple sclerosis patients. Int. J. Mol. Sci. 2013;14:16087–16110. doi: 10.3390/ijms140816087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Jiang Y., Zha X. miR-149 reduces while let-7 elevates ASIC1a expression in vitro. Int. J. Physiol. Pathophysiol. Pharmacol. 2017;9:147–152. [PMC free article] [PubMed] [Google Scholar]
- 245.Friese M.A., Craner M.J., Etzensperger R., Vergo S., Wemmie J.A., Welsh M.J., Vincent A., Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat. Med. 2007;13:1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 246.Arun T., Tomassini V., Sbardella E., de Ruiter M.B., Matthews L., Leite M.I., Gelineau-Morel R., Cavey A., Vergo S., Craner M., et al. Targeting ASIC1 in primary progressive multiple sclerosis: Evidence of neuroprotection with amiloride. Brain. 2015;136:106–115. doi: 10.1093/brain/aws325. [DOI] [PubMed] [Google Scholar]
- 247.Boiko N., Kucher V., Eaton B.A., Stockand J.D. Inhibition of neuronal degenerin/epithelial Na+ channels by the multiple sclerosis drug 4-aminopyridine. J. Biol. Chem. 2013;288:9418–9427. doi: 10.1074/jbc.M112.449413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Vergo S., Craner M.J., Etzensperger R., Attfield K., Friese M.A., Newcombe J., Esiri M., Fugger L. Acid-sensing ion channel 1 is involved in both axonal injury and demyelination in multiple sclerosis and its animal model. Brain. 2011;134:571–584. doi: 10.1093/brain/awq337. [DOI] [PubMed] [Google Scholar]
- 249.Pianta A., Drouin E.E., Crowley J.T., Arvikar S., Strle K., Costello C.E., Steere A.C. Annexin A2 is a target of autoimmune T and B cell responses associated with synovial fi broblast proliferation in patients with antibiotic-refractory Lyme arthritis. Clin. Immunol. 2015;160:336–341. doi: 10.1016/j.clim.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cañas F., Simonin L., Couturaud F., Renaudineau Y. Annexin A2 autoantibodies in thrombosis and autoimmune diseases. Thromb. Res. 2015;135:226–230. doi: 10.1016/j.thromres.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 251.Talbott E.O., Malek A.M., Lacomis D. The Epidemiology of Amyotrophic Lateral Sclerosis. 1st ed. Volume 138. Elsevier, B.V.; Amsterdam, The Netherlands: 2016. [DOI] [PubMed] [Google Scholar]
- 252.Zarei S., Carr K., Reiley L., Diaz K., Guerra O., Fernandez Altamirano P., Pagani W., Lodin D., Orozco G., Chinea A. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 2015;6:171. doi: 10.4103/2152-7806.169561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Strong M.J. The evidence for altered RNA metabolism in amyotrophic lateral sclerosis (ALS) J. Neurol. Sci. 2010;288:1–12. doi: 10.1016/j.jns.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 254.Biscarini S., Capauto D., Peruzzi G., Lu L., Colantoni A., Santini T., Shneider N.A., Caffarelli E., Laneve P., Bozzoni I. Characterization of the lncRNA transcriptome in mESC-derived motor neurons: Implications for FUS-ALS. Stem. Cell Res. 2018;27:172–179. doi: 10.1016/j.scr.2018.01.037. [DOI] [PubMed] [Google Scholar]
- 255.Errichelli L., Dini Modigliani S., Laneve P., Colantoni A., Legnini I., Capauto D., Rosa A., De Santis R., Scarfò R., Peruzzi G., et al. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat. Commun. 2017;8:1–11. doi: 10.1038/ncomms14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Wu L., Cheng W., Chen C., Wu M., Wang Y., Tseng Y., Chuang T., Shen C.J. Transcriptomopathies of pre- and post- symptomatic frontotemporal dementia-like mice with TDP-43 depletion in forebrain neurons. Acta Neuropathol. Commun. 2019;7:50. doi: 10.1186/s40478-019-0674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Nishimoto Y., Nakagawa S., Hirose T., Okano H.J., Takao M., Shibata S., Suyama S., Kuwako K., Imai T., Murayama S., et al. The long non-coding RNA nuclear-enriched abundant transcript 1-2 induces paraspeckle formation in the motor neuron during the early phase of amyotrophic lateral sclerosis. Mol. Brain. 2013;6:31. doi: 10.1186/1756-6606-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tollervey J.R., Curk T., Rogelj B., Briese M., Cereda M., Kayikci M., König J., Hortobágyi T., Nishimura A.L., Zupunski V., et al. Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat. Neurosci. 2011;14:452–458. doi: 10.1038/nn.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Hutchinson J.N., Ensminger A.W., Clemson C.M., Lynch C.R., Lawrence J.B., Chess A. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genom. 2007;8:39. doi: 10.1186/1471-2164-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gagliardi S., Pandini C., Garofalo M., Bordoni M., Pansarasa O., Cereda C. Long non coding RNAs and ALS: Still much to do. Non Coding RNA Res. 2018;3:226–231. doi: 10.1016/j.ncrna.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gagliardi S., Zucca S., Pandini C., Diamanti L., Bordoni M., Sproviero D., Arigoni M., Olivero M., Pansarasa O., Ceroni M., et al. Long non-coding and coding RNAs characterization in Peripheral Blood Mononuclear Cells and Spinal Cord from Amyotrophic Lateral Sclerosis patients. Sci. Rep. 2018;8:2378. doi: 10.1038/s41598-018-20679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zucca S., Gagliardi S., Pandini C., Diamanti L., Bordoni M., Sproviero D., Arigoni M., Olivero M., Pansarasa O., Ceroni M., et al. RNA-Seq profiling in peripheral blood mononuclear cells of amyotrophic lateral sclerosis patients and controls. Sci. Data. 2019;6:190006. doi: 10.1038/sdata.2019.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhou F., Guan Y., Chen Y., Zhang C., Yu L., Gao H., Du H., Liu B., Wang X. miRNA-9 expression is upregulated in the spinal cord of G93A-SOD1 transgenic mice. Int. J. Clin. Exp. Pathol. 2013;6:1826–1838. [PMC free article] [PubMed] [Google Scholar]
- 264.Vrabec K., Boštjančič E., Koritnik B., Leonardis L., Grošelj L.D., Zidar J., Rogelj B., Glavač D., Ravnik-Glavač M. Differential Expression of Several miRNAs and the Host Genes AATK and DNM2 in Leukocytes of Sporadic ALS Patients. Front. Mol. Neurosci. 2018;11:106. doi: 10.3389/fnmol.2018.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Hawley Z.C.E., Campos-Melo D., Strong M.J. MiR-105 and miR-9 regulate the mRNA stability of neuronal intermediate filaments. Implications for the pathogenesis of amyotrophic lateral sclerosis (ALS) Brain Res. 2019;1706:93–100. doi: 10.1016/j.brainres.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 266.Campos-Melo D., Droppelmann C.A., He Z., Volkening K., Strong M.J. Altered microRNA expression profile in amyotrophic lateral sclerosis: A role in the regulation of NFL mRNA levels. Mol. Brain. 2013;6:26. doi: 10.1186/1756-6606-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Xu C., Xu L., Peng F., Cai Y., Wang Y. MiR-647 promotes proliferation and migration of ox-LDL-treated vascular smooth muscle cells through regulating PTEN/PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23:7110–7119. doi: 10.26355/eurrev_201908_18756. [DOI] [PubMed] [Google Scholar]
- 268.Yang D., Wang X., Ismail A., Ashman C.J., Valori C.F., Wang G., Gao S., Higginbottom A., Ince P.G., Azzouz M., et al. PTEN regulates AMPA receptor-mediated cell viability in iPS-derived motor neurons. Cell Death Dis. 2014;5:e1096. doi: 10.1038/cddis.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kirby J., Ning K., Ferraiuolo L., Heath P.R., Ismail A., Kuo S., Valori C.F., Cox L., Sharrack B., Wharton S.B., et al. Phosphatase and tensin homologue/protein kinase B pathway linked to motor neuron survival in human superoxide dismutase 1-related amyotrophic lateral sclerosis. Brain. 2011;134:506–517. doi: 10.1093/brain/awq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Ning K., Drepper C., Valori C.F., Ahsan M., Wyles M., Higginbottom A., Herrmann T., Shaw P., Azzouz M., Sendtner M. PTEN depletion rescues axonal growth defect and improves survival in SMN-deficient motor neurons. Hum. Mol. Genet. 2010;19:3159–3168. doi: 10.1093/hmg/ddq226. [DOI] [PubMed] [Google Scholar]
- 271.Li Y., Chen X., Sun H., Wang H. Long non-coding RNAs in the regulation of skeletal myogenesis and muscle diseases. Cancer Lett. 2018;417:58–64. doi: 10.1016/j.canlet.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 272.Cesana M., Cacchiarelli D., Legnini I., Santini T., Sthandier O., Chinappi M., Tramontano A., Bozzoni I. A Long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147:358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kallen A.N., Zhou X., Xu J., Qiao C., Mai J., Yan L., Lu L., Liu C., Yi J., Zhang H., et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol. Cell. 2013;52:101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Han X., Yang F., Cao H., Liang Z. Malat1 regulates serum response factor through miR-133 as a competing endogenous RNA in myogenesis. FASEB J. 2015;29:3054–3064. doi: 10.1096/fj.14-259952. [DOI] [PubMed] [Google Scholar]
- 275.Zhu M., Liu J., Xiao J., Yang L., Cai M., Shen H., Chen X., Ma Y., Hu S., Wang Z., et al. Lnc-mg is a long non-coding RNA that promotes myogenesis. Nat. Commun. 2017;8:14718. doi: 10.1038/ncomms14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Sun X., Li M., Sun Y., Cai H., Lan X., Huang Y., Bai Y., Qi X., Chen H. The developmental transcriptome sequencing of bovine skeletal muscle reveals a long noncoding RNA, lncMD, promotes muscle differentiation by sponging miR-125b. BBA Mol. Cell Res. 2016;1863:2835–2845. doi: 10.1016/j.bbamcr.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 277.Lu L., Sun K., Chen X., Zhao Y., Wang L., Zhou L., Sun H., Wang H. Genome-wide survey by ChIP-seq reveals YY1 regulation of lincRNAs in skeletal myogenesis. EMBO J. 2013;32:2575–2588. doi: 10.1038/emboj.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Pardo P.S., Boriek A.M. The physiological roles of Sirt1 in skeletal muscle. Aging. 2011;3:430–437. doi: 10.18632/aging.100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Holmberg M., Duyckaerts C., Dürr A., Cancel G., Gourfinkel-An I., Damier P., Faucheux B., Trottier Y., Hirsch E.C., Agid Y., et al. Spinocerebellar ataxia type 7 (SCA7): A neurodegenerative disorder with neuronal intranuclear inclusions. Hum. Mol. Genet. 1998;7:913–918. doi: 10.1093/hmg/7.5.913. [DOI] [PubMed] [Google Scholar]
- 280.Matsuura T., Ashizawa T. Spinocerebellar Ataxia Type 10. GeneReviews®. 2019:1–18. [Google Scholar]
- 281.Yang Y., Zhou X., Jin Y. ADAR-mediated RNA editing in non-coding RNA sequences. Sci. China Life Sci. 2013;56:944–952. doi: 10.1007/s11427-013-4546-5. [DOI] [PubMed] [Google Scholar]
- 282.Zipeto M.A., Jiang Q., Melese E., Jamieson C.H.M. RNA rewriting, recoding, and rewiring in human disease. Trends Mol. Med. 2015;21:549–559. doi: 10.1016/j.molmed.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 283.Lorenzini I., Moore S., Sattler R. RNA Editing Deficiency in Neurodegeneration. Adv. Neurobiol. 2018;20:63–83. doi: 10.1007/978-3-319-89689-2. [DOI] [PubMed] [Google Scholar]
- 284.Breen M.S., Dobbyn A., Li Q., Roussos P., Hoffman G.E., Stahl E., Chess A., Sklar P., Li J.B., Devlin B., et al. Global landscape and genetic regulation of RNA editing in cortical samples from individuals with schizophrenia. Nat. Neurosci. 2019;22:1402–1412. doi: 10.1038/s41593-019-0463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Eisenberg E., Levanon E.Y. A-to-I RNA editing—immune protector and transcriptome diversifier. Nat. Rev. Genet. 2018;19:473–490. doi: 10.1038/s41576-018-0006-1. [DOI] [PubMed] [Google Scholar]
- 286.Nigita G., Veneziano D., Ferro A. A-to-I RNA Editing: Current Knowledge Sources and Computational Approaches with Special Emphasis on Non-Coding RNA Molecules. Front. Bioeng. Biotechnol. 2015;3:37. doi: 10.3389/fbioe.2015.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Daniel C., Lagergren J., Öhman M. RNA editing of non-coding RNA and its role in gene regulation. Biochimie. 2015;117:22–27. doi: 10.1016/j.biochi.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 288.Shevchenko G., Morris K.V. All I’s on the RADAR: Role of ADAR in gene regulation. FEBS Lett. 2018;592:2860–2873. doi: 10.1002/1873-3468.13093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Nigita G., Distefano R., Veneziano D., Romano G., Rahman M., Wang K., Pass H., Croce C.M., Acunzo M., Nana-Sinkam P. Tissue and exosomal miRNA editing in Non-Small Cell Lung Cancer. Sci. Rep. 2018;8:10222. doi: 10.1038/s41598-018-28528-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hideyama T., Yamashita T., Aizawa H., Tsuji S., Kakita A., Takahashi H., Kwak S. Profound downregulation of the RNA editing enzyme ADAR2 in ALS spinal motor neurons. Neurobiol. Dis. 2012;45:1121–1128. doi: 10.1016/j.nbd.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 291.Aizawa H., Hideyama T., Yamashita T., Kimura T., Suzuki N., Aoki M., Kwak S. Deficient RNA-editing enzyme ADAR2 in an amyotrophic lateral sclerosis patient with a FUS(P525L) mutation. J. Clin. Neurosci. 2016;32:128–129. doi: 10.1016/j.jocn.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 292.Hosaka T., Yamashita T., Teramoto S., Hirose N., Tamaoka A., Kwak S. ADAR2-dependent A-to-I RNA editing in the extracellular linear and circular RNAs. Neurosci. Res. 2019;147:48–57. doi: 10.1016/j.neures.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 293.Khermesh K., D’Erchia A.M., Barak M., Annese A., Wachtel C., Levanon E.Y., Picardi E., Eisenberg E. Reduced levels of protein recoding by A-to-I RNA editing in Alzheimer’s disease. RNA. 2016;22:290–302. doi: 10.1261/rna.054627.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Singh M. Dysregulated A to I RNA editing and non-coding RNAs in neurodegeneration. Front. Genet. 2013;3:326. doi: 10.3389/fgene.2012.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Gardner O.K., Wang L., Van Booven D., Whitehead P.L., Hamilton-Nelson K.L., Adams L.D., Starks T.D., Hofmann N.K., Vance J.M., Cuccaro M.L., et al. RNA editing alterations in a multi-ethnic Alzheimer disease cohort converge on immune and endocytic molecular pathways. Hum. Mol. Genet. 2019;28:3053–3061. doi: 10.1093/hmg/ddz110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hosaka T., Yamashita T., Tamaoka A., Kwak S. Extracellular RNAs as Biomarkers of Sporadic Amyotrophic Lateral Sclerosis and Other Neurodegenerative Diseases. Int. J. Mol. Sci. 2019;20:3148. doi: 10.3390/ijms20133148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tay Y., Rinn J., Pandolfi P.P. The multilayered complexity of ceRNA crosstalk and competition. Nature. 2014;505:344–352. doi: 10.1038/nature12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Xu J., Feng L., Han Z., Li Y., Wu A., Shao T., Ding N., Li L., Deng W., Di X., et al. Extensive ceRNA—ceRNA interaction networks mediated by miRNAs regulate development in multiple rhesus tissues. Nucleic Acids Res. 2016;44:9438–9451. doi: 10.1093/nar/gkw587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Li Y., Zheng Q., Bao C., Li S., Guo W., Zhao J., Chen D., Gu J., He X., Huang S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–984. doi: 10.1038/cr.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Patel N.A., Moss L.D., Lee J., Tajiri N., Acosta S., Hudson C., Parag S., Cooper D.R., Borlongan C.V., Bickford P.C. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J. Neuroinflamm. 2018;15:204. doi: 10.1186/s12974-018-1240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Li S., Li Y., Chen B., Zhao J., Yu S., Tang Y., Zheng Q., Li Y., Wang P., He X., et al. exoRBase: A database of circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids Res. 2018;46:D106–D112. doi: 10.1093/nar/gkx891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Wang D., Wang P., Bian X., Xu S., Zhou Q., Zhang Y., Ding M., Han M., Huang L., Bi J., et al. Elevated plasma levels of exosomal BACE1-AS combined with the volume and thickness of the right entorhinal cortex may serve as a biomarker for the detection of Alzheimer’s disease. Mol. Med. Rep. 2020;22:227–238. doi: 10.3892/mmr.2020.11118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Fotuhi S.N., Khalaj-Kondori M., Hoseinpour Feizi M.A., Talebi M. Long Non-coding RNA BACE1-AS May Serve as an Alzheimer’s Disease Blood-Based Biomarker. J. Mol. Neurosci. 2019;69:351–359. doi: 10.1007/s12031-019-01364-2. [DOI] [PubMed] [Google Scholar]
- 304.Adams B.D., Parsons C., Walker L., Zhang W.C., Slack F.J. Targeting noncoding RNAs in disease. J. Clin. Investig. 2017;127:761–771. doi: 10.1172/JCI84424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yao C., Yu B. Role of Long Noncoding RNAs and Circular RNAs in Nerve Regeneration. Front. Mol. Neurosci. 2019;12:165. doi: 10.3389/fnmol.2019.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Carroll A.P., Tooney P.A., Cairns M.J. Context-specific microRNA function in developmental complexity. J. Mol. Cell Biol. 2013;5:73–84. doi: 10.1093/jmcb/mjt004. [DOI] [PubMed] [Google Scholar]
- 307.Stavast C.J., Erkeland S.J. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells. 2019;8:1465. doi: 10.3390/cells8111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Fernandes J.C.R., Acuña S.M., Aoki J.I., Floeter-Winter L.M., Muxel S.M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA. 2019;5:17. doi: 10.3390/ncrna5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ørom U.A., Nielsen F.C., Lund A.H. MicroRNA-10a binds the 5’UTR of ribosomal protein mRNAs and enhances their translation. Mol. Cell. 2008;30:460–471. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 310.Vasudevan S., Tong Y., Steitz J. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 311.Xiao M., Li J., Li W., Wang Y., Wu F., Xi Y., Zhang L., Ding C., Luo H., Li Y., et al. MicroRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 2017;14:1326–1334. doi: 10.1080/15476286.2015.1112487. [DOI] [PMC free article] [PubMed] [Google Scholar]