Abstract
The principal source of vitamin D in humans is its biosynthesis in the skin through a chemical reaction dependent on sun exposure. In lesser amounts, the vitamin can be obtained from the diet, mostly from fatty fish, fish liver oil and mushrooms. Individuals with vitamin D deficiency, defined as a serum level of 25 hydroxyvitamin D < 20 ng/dl, should be supplemented. Vitamin D deficiency is a prevalent global problem caused mainly by low exposure to sunlight. The main role of 1,25 dihydroxyvitamin D is the maintenance of calcium and phosphorus homeostasis. However, vitamin D receptors are found in most human cells and tissues, indicating many extra-skeletal effects of the vitamin, particularly in the immune and cardiovascular (CV) systems. Vitamin D regulates blood pressure by acting on endothelial cells and smooth muscle cells. Its deficiency has been associated with various CV risk factors and appears to be linked to a higher mortality and incidence of CV disease (CVD). Several mechanisms have been proposed relating vitamin D deficiency to CV risk factors such as renin-angiotensin-aldosterone system activation, abnormal nitric oxide regulation, oxidative stress or altered inflammatory pathways. However, in the latest randomized controlled trials no benefits of vitamin D supplementation for CVD have been confirmed. Although more work is needed to establish the protective role of vitamin D in this setting, according to current evidences vitamin D supplements should not be recommended for CVD prevention.
Keywords: Supplements, Cardiovascular disease, Hypertension, Endothelial function, Coronary heart disease
Abbreviations list
- 25(OH)D
25-hydroxyvitamin D
- 7-DHC
7-dehydrocholesterol
- BP
blood pressure
- CHD
coronary heart disease
- COVID-19
coronavirus disease 2019
- CV
cardiovascular
- CVD
cardiovascular disease
- DBP
vitamin D binding protein
- HF
heart failure
- HTN
hypertension
- IL
interleukin
- IOM
Institute of Medicine
- LV
left ventricular
- NFκB
nuclear factor kappa beta
- NO
nitric oxide
- RAAS
renin–angiotensin–aldosterone system
- RCT
random controlled trials
- ROS
reactive oxygen species
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SZA
solar zenith angle
- T2DM
type 2 diabetes mellitus
- UVR
ultraviolet radiation
- VDR
vitamin D receptors
1. Introduction
Cardiovascular (CV) diseases (CVD) are the most common cause of death globally. In 2016, it was estimated that 17.9 million people died from a CVD. In the United States (US), the prevalence of CVD in adults over 20 years of age was 48.0% over the period 2013–2016 (121.5 million in 2016). The annual direct and indirect costs of CVD have been estimated at $351.3 billion (2014–2015) [1]. The main source of vitamin D in humans is skin synthesis dependent on sunlight exposure. The amount of sunlight needed to satisfy our vitamin D requirements is difficult to estimate as it depends on many factors such as skin pigmentation, age, latitude, season or time of the day [2,3]. As there are very few natural sources of vitamin D in food, diet does not provide adequate amounts of vitamin D for most people [4,5]. Hence, in many situations in which sunlight exposure is inadequate, supplementation with vitamin D may be necessary [3,4,6,7]. An example of this situation is the confinement of people during the current coronavirus disease 2019 (COVID-19) pandemic. This restriction is affecting many people reducing their sunlight exposure and consequently their cutaneous vitamin D synthesis. Vitamin D plays an important role in maintaining bone health, has many extra-skeletal effects and plays an important role in CV health. There are many vitamin D-related components in the heart and blood vessels, and vitamin D deficiency has been linked to several CVDs. However, its relevance is still unknown [7,8]. Therefore, this review aims to elucidate the relation between vitamin D levels and CV health and determine whether vitamin D supplementation is effective in reducing the risk of the main CVD, including high blood pressure (BP), hypertension (HTN), endothelial dysfunction, coronary heart disease (CHD) and heart failure (HF).
2. Vitamin D sources
2.1. Dietary sources of vitamin D
There are two main dietary sources of vitamin D: cholecalciferol or vitamin D3 and ergosterol or vitamin D2. The structures of these two forms differ in their C-17 side chain such that vitamin D2 has an additional C-22 to C-23 double bond and a C-24 methyl group [9]. Vitamin D3 is mainly found in fish liver oil, fatty fish, egg yolks, liver and kidney, and vitamin D2 in fungi and yeast [10] (Table 1 ).
Table 1.
Natural sources of Vitamin D. Adapted from Charoenngam et al. [5].
| Source | Vitamin D content |
|---|---|
| Cod liver oil (1 tsp) | 400–1000 IU D3 |
| Fresh wild salmon (3.5 oz) | 600–1000 IU D3 |
| Canned salmon (3.5 oz) | 300–600 IU D3 |
| Fresh farmed salmon (3.5 oz) | 100–250 IU D2 or D3 |
| Canned sardine (3.5 oz) | 300 IU D3 |
| Canned mackerel (3.5 oz) | 250 IU D3 |
| Canned tuna (3.5 oz) | 230 IU D3 |
| Fresh shiitake mushrooms | 600–1000 IU D2 |
| Sun-dried mushrooms (3.5 oz) | 1600 IU D2 |
| Egg yolk | 20 IU D3 or D2 |
| Beef kidney (1lb) | 20–500 IU D3 |
| Beef muscle (1lb) | 0–180 IU D3 |
| Pork liver (1lb) | 70–220 IU D3 |
| Pork muscle (1lb) | 10–250 IU D3 |
Serum levels of circulating 25-hydroxyvitamin D [25(OH)D] is the recommended measurement to assess vitamin D status. Serum levels of 25(OH)D considered physiologically adequate by the Institute of Medicine (IOM) in 2010 were 20 ng/ml [7]. However, according to some specialists, 30–50 ng/ml or even 40–60 ng/ml should be considered the minimal concentration necessary for human well-being [7]. According to vitamin D requirements for optimal human health, vitamin D deficiency or insufficiency are defined as serum 25(OH)D concentrations lower than 50 nmol/l (20 ng/ml) or 525–725 nmol/l (21–29 ng/ml) respectively [4,11,12].
Before the IOM publication in 2010, the recommended vitamin D daily allowance (RDA) up to the age of 50 years was 200 IU/day. After 2010, the IOM recommended 400 IU/day for infants up to 12 months of age, and 600 IU for children, adolescents and adults up to 70 years of age. In individuals over 70 years of age, the RDA is 800 IU [7]. Nevertheless, many countries have their own guidelines for vitamin D intake [13].
Vitamin D deficiency can be the outcome of different factors such as reduced cutaneous synthesis, reduced absorption and acquired and heritable disorders of metabolism and responsiveness to the deficiency [14]. Accordingly, several different populations should be considered for vitamin D screening.
Vitamin D bioavailability is affected by malabsorption syndromes, such as cystic fibrosis, celiac disease, Chron's disease or intestinal bypass surgery [4]. Vitamin D bioavailability may be also affected in obese subjects. Moreover, certain medications can increase vitamin D catabolism (antiepileptics, antiretrovirals, glucocorticoids, antirejection medication) [14]. This means that patients under treatment with these drugs may need at least two or three times more vitamin D than healthy subjects of the same age [4].
Screening for vitamin D deficiency is important in patients with rickets, osteomalacia, or osteoporosis, in conditions in which D metabolism is affected such as chronic kidney disease or liver failure, or in acquired conditions such as granulomatous disorders, hyperparathyroidism and some lymphomas [4]. Central European guidelines also recommend vitamin D deficiency assessment in patients with a chronic autoimmune disease, CVD or patients admitted to hospital because of certain infections and chronic allergic disease [6].
Vitamin D treatment should be administered in patients with a 25(OH)D concentration lower than 20 ng/ml [7]. The Endocrine Society Clinical Practice Guideline makes the following recommendation for vitamin D supplementation for different age groups in the general population [4]: a) 0–1 year: 2000 IU/day or 50,000 IU/week of vitamin D for 6 weeks to raise blood 25(OH)D levels to above 30 ng/ml followed by maintenance therapy of 400–1000 IU/day; b) 1–18 years: 2000 IU/day or 50,000 IU/week of vitamin D for at least 6 weeks to raise blood 25(OH)D levels to above 30 ng/ml followed by maintenance therapy of 600–1000 IU/day; c) 18 years and over: 6000 IU/day or 50,000 IU/week of vitamin D for 8 weeks to raise blood 25(OH)D levels to above 30 ng/ml followed by maintenance therapy of 1500–2000 IU/day.
Other supplementation guidelines may slightly differ. The Central European guidelines recommend treatment for 1–3 months depending on the severity of the vitamin deficiency [6]. After achieving a concentration between 30 and 50 ng/ml, a maintenance dose may be prescribed. Recommended therapeutic doses are as follows (depending on body weight): neonates 1000 IU/day, infants 1–12 months 1000–3000 IU/day, children and adolescents 1–18 years 3000–5000 IU/day and adults and older adults 7000–10,000 IU/day or 50,000 IU/week.
For patients, vitamin D recommendations should consider specific aspects of their disease including whether it is the cause or consequence of the deficiency. Many supplementation guidelines are disease-specific [7].
Several pharmacological formulations of vitamin D exist. Cholecalciferol and ergocalciferol are the most used compounds; the former is more used in Europe and the latter in the US. The potency of each form of vitamin D is a matter of intense debate. There is evidence to suggest that when taken daily, both forms show a similar potency. However, ergocalciferol is much less efficient when administered intermittently.
Many active metabolites also exist: calcifediol (25-hydroxycholecalferol), calcitriol (1α-25 dihydroxycholecalciferol) and the synthetic calcitriol analogue alfacalcidol (1α-hydroxycholecalciferol) [15]. Selective vitamin D receptor activators such as paricalcitol or maxacalcitol can also be used to treat vitamin D deficiency [16].
Recently, the use of oral calcifediol is on the increase. This treatment has the benefit over oral cholecalciferol and ergocalciferol in that it is more effectively absorbed in the intestine and leads to a faster increase in 25(OH)D levels, thus restoring normal levels of vitamin D more rapidly. This form of supplementation is more useful in patients with malabsorption, obesity or liver dysfunction [7,15].
Calcitriol is the most active form of vitamin D, and alfacalcidol is converted to calcitriol in the liver. However, these forms of supplementation increase the risk of hypercalcemia and hypercalciuria [15] so they are not recommended for the routine treatment of vitamin D deficiency [17] and are usually reserved for patients with chronic kidney disease [7,17].
2.2. Sun exposure as an endogenous vitamin D inducer
In humans, the main pathway generating 1,25-dihydroxyvitamin D3, the most active metabolite of vitamin D [4], occurs via an intriguing physicochemical process that takes place in the epidermis, or outer layer of the skin, when UVR is absorbed. Thus, ~90% of all human requirements of the vitamin arise from this cutaneous production [14].
Solar radiation includes a broad spectrum of electromagnetic radiation characterized by specific wavelengths. Among them, UVR in the range 100–400 nm is made up of UV-C (100–280 nm), UV-B (280–315 nm), and UV-A (315–400 nm) [18]. UV-C radiation is fully absorbed by the oxygen and ozone present in the stratospheric layer of the Earth's atmosphere, while UV-B and UV-A rays cross the atmosphere reaching the Earth's surface [19]. However, the amount of ozone present in the lower layer of the atmosphere, the troposphere, determines the level of UV-B and UV-A radiation that reaches the surface. In addition, UV-B radiation may be blocked with glass and plastics objects.
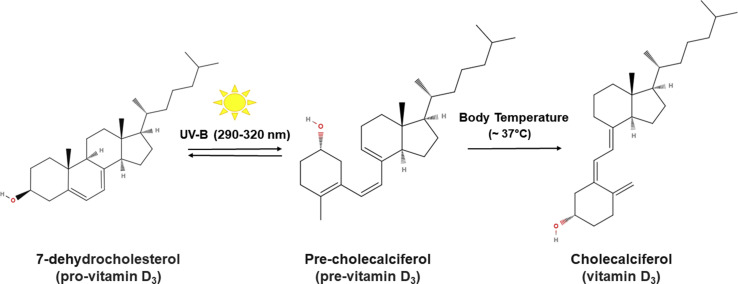
UV-A and B radiation from sunlight can provoke different effects in human cells and molecules depending on the radiation energy, which is inversely proportional to its wavelength. Because of this, UV-A radiation reaches the dermis, while UV-B mainly penetrates the epidermis and barely enters the dermis [20]. UV-B radiation (wavelengths of 290–320 nm) drives the synthesis of cholecalciferol (vitamin D3) in the epidermal plasma membrane keratinocytes in a two-step non enzymatic reaction (Fig. 1 ). In the first step, 7-DHC is converted into 9,10-secosteroid (pre-cholecalciferol) [21], a thermodynamic unstable molecule. In the second step, thermal isomerization takes place at body temperature (~37 °C) converting pre-cholecalciferol into the thermally stable molecule, cholecalciferol. However, prolonged exposure to UV-B induces the conversion of pre-cholecalciferol to lumisterol and tachysterol [22] as well as the photoconversion of cholecalciferol into 5,6 transvitamin D3 and suprasterols I and II [23], thus regulating the epidermal production of vitamin D3.
Fig. 1.
Diagram showing how cholecalciferol (vitamin D3) is synthesized in the human epidermis. In a two-stage process, 7-dehydrocholesterol (7-DHC) present in the cell membranes of keratinocytes is converted into 9,10-secosteroid (pre-cholecalciferol) by UV-B radiation in sunlight (wavelengths of 290–320 nm). Then, through thermal isomerization at body temperature, pre-cholecalciferol is transformed into cholecalciferol (vitamin D3).
Melanin absorbs both UV-A and UV-B radiation. As melanin and 7-DHC absorb UV-B across the same wavelength range, the amount of UV-B that penetrates the inner epidermal layers where 7-DHC is located, depends on the concentration of melanin in the outer stratum of the epidermis [24]. Therefore, the epidermal concentration of melanin determines the quantity of vitamin D3 synthesized, such that skin pigmentation is an important factor regulating vitamin D3 production in the skin [25]. Once cholecalciferol (vitamin D3) is synthesized by epidermal keratinocytes, it reaches the bloodstream through the dermal vascular system, and maximum concentrations of the vitamin occur 24–48 h after UV-B exposure [26].
Solar vitamin D3 production depends on external factors, which regulate the quantity of UV-B reaching the skin, and individual factors, which determine the individual effect of UV-B in the skin [27]. Among the external factors, the solar zenith angle (SZA) is inversely proportional to the height of the sun in the sky. When the SZA is small, as occurs in low-latitude regions, at noon and in summer, the solar radiation pathlength through the atmosphere is low, and UV-B reaches the skin surface with little attenuation. Conversely, a larger SZA, as in high-latitude regions, in the morning, evening and in winter [27], makes the cutaneous production of vitamin D3 more difficult. Another external factor modifying the amount of UV-B radiation that reaches the skin is the ozone level of the atmosphere. The ozone level in the lower atmosphere is increased by pollutants like volatile organic compounds, methane and nitrogen oxides present in densely populated urban regions [28]. Thus, unhealthy levels of ozone at ground-level, besides making it difficult to breath, prevent UV-B radiation reaching the epidermis [2]. In addition, UVR is associated with skin ageing and cancer [29]. The use of sunscreen is an important strategy protecting against UVR from sunlight exposure [30]. Sunscreen attenuates the effects of UVR on the skin, mainly as UVR is absorbed by chemical filters including para-aminobenzoic acid (PABA) derivatives, benzophenones and salycilates [31]. These organic compounds substantially interfere with the skin production of vitamin D3 [32]. Among the individual factors affecting solar vitamin D3 synthesis is the skin pigmentation of an individual. The different world populations can be classified according to their skin phototypes as white (phototype I-IV), brown (phototype V) or black (phototype VI) [33]. These phototypes are dependent on how melanin is synthesized, packaged and distributed [34]. Ageing is another individual factor to consider, as epidermal 7-DHC concentrations and cutaneous vitamin D3 production decrease with age [35,36]. Moreover, obesity (body mass index, BMI, > 30 kg/m2) is also a risk factor for vitamin D deficiency [37], as this lipophilic molecule is stored and isolated in the subcutaneous adipose tissue [4]. Wortsman et al. [38] showed that obese subjects had a serum concentration ~ 50% lower of vitamin D3 than controls after 24 h of whole-body UVR exposure. Further, several epidemiological studies have detected inverse correlation between body mass index (BMI) and blood levels of vitamin D [39,40].
It is estimated that one billion people worldwide have low vitamin D levels thus representing a global public health issue [[41], [42], [43], [44], [45]]. A meta-analysis carried out in 2014 determined that 69.5% of US and 86.4% of European citizens had vitamin D insufficiency (<30 ng/ml) [46]. An observational study has also revealed that ~40% of Europeans are vitamin D deficient (<20 ng/ml) [47].
The prevalence of severe vitamin D deficiency, defined as a serum 25(OH)D concentration <12 ng/ml, has been reported at 5.9% in US, 7.4% in Canada and 13% in Europe, while prevalence estimates of a serum 25(OH)D level <20 ng/ml run at 24% for the US, 37% for Canada and 40% for Europe.
As mentioned earlier, cutaneous derived vitamin D3, as opposed to dietary intake or vitamin D supplements, is the main source of systemic vitamin D [11,48,49], supporting the idea that insufficient sun exposure is the primary cause of vitamin D deficiency [4,50]. The ideal amount of daily sun exposure needed to balance out vitamin D requirements and sun skin damage prevention is therefore difficult to estimate. Many authors consider that sun exposure to the face, arms and legs for 10–15 min twice a week, below the erythemal level [14,[51], [52], [53]], could provide adequate amounts of vitamin D. However, increasing the time or intensity of exposure does not enhance vitamin D synthesis while it does increase the risk of skin cancer [54]. For dark skinned subjects, this could be lengthened to 30 min twice a week. Thus, people with skin phototype V-VI have high epidermal melanin concentrations protecting against sunburn, but require a 3–5 times higher UV-B doses to synthesize a similar quantity of vitamin D3 compared with those with skin phototype I-IV [27,[55], [56], [57], [58]]. In experiments carried out on human skin samples to examine the transformation of 7-DHC into previtamin D3, skin phototype II (Caucasian) was 5–10 fold more efficient than phototype V (Black) [57]. Despite this, however, the most significant factor underlying the increasing prevalence of vitamin D deficiency is scarce exposure to sunlight as a consequence of a change over the years in i) lifestyle, whereby outdoor work has been replaced with more indoor jobs and there are more forms of transport to replace walking or cycling and, ii) sunscreens are employed today to absorb UV-B radiation and avoid the harmful effects of UVR on the skin. For example, the use of a sunscreen with a sun protection factor of 15 could reduce skin vitamin D3 synthesis by more than 97% [32,51].
The skin absorption of UVR can have various adverse effects. Human-related emissions in the last decades of the 20th century led to significant depletion of the stratospheric ozone [59]. Reduced ozone levels in this layer of the atmosphere have resulted in more UVR reaching humans and have been held responsible for a recent increase in the incidence of UV-induced cell injury [60,61]. In addition, sun exposure stimulates the synthesis of melanin which turns the skin darker, and for cosmetic and social reasons, this often provokes abuse of sun exposure. Some of the most significant consequences of overexposure to UVR are skin ageing, sunburn, non-melanoma skin cancer and cutaneous malignant melanoma [62], along with eye diseases like cataract and macular degeneration [63]. Skin photoageing is characterized by loss of color and elasticity and wrinkle formation, resulting from the modification of the cellular and extracellular components of the skin by the UVR formation of reactive oxygen species (ROS) [62]. UV-B radiation is the main responsible factor for erythema and sunburn, resulting from an increase in dermal blood volume associated with erythema, swelling and pain [64,65]. Sunburn has been directly linked to a greater risk of skin ageing and skin cancer [66]. In effect, exposure to UVR is the main risk factor for the progression of skin cancers including non-melanoma skin cancer, basal cell carcinoma, squamous cell carcinoma, and melanoma [[67], [68], [69]]. Melanoma is the most common type of malignant neoplasm in Caucasians, and non-melanoma skin cancer is the 5th most commonly occurring cancer [70]. According to Skin Cancer Foundation Statistics, one in five Americans will develop skin cancer by the age of 70 [71], with solar radiation identified as the most important environmental risk factor [72]. At the molecular level, the adverse effects of UV rays are the consequence of a capacity of proteins and DNA to absorb UVR. Further, it has been reported that UV cell damage is caused by ROS generated when UV light disrupts the enzymes catalase and nitric oxide synthase [73]. UV-A (315–400 nm) photons penetrate the deeper skin or dermis, where they are mainly responsible for free radical and O2 formation, indirectly damaging proteins and DNA through oxidative stress [74]. However, endogenous mechanisms protect against UVR, among which DNA repair machinery, antioxidant systems and melanin synthesis are the most important [75]. Several authors have proposed UV light absorption by the DNA of melanocytes, and ROS accumulation in keratinocytes, as the main mechanisms triggering melanin synthesis [[76], [77], [78]].
3. Vitamin D metabolism and physiology
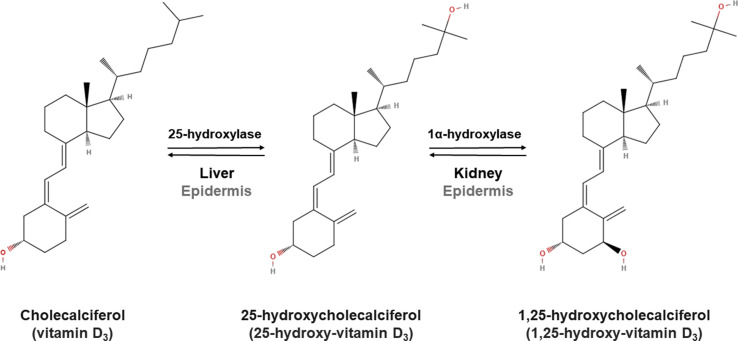
Vitamin D ingested becomes incorporated in chylomicrons which are absorbed into the lymph system and enter the circulation [5,7,79]. Vitamin D3 obtained via skin synthesis and diet, and vitamin D2 from the diet are bound to vitamin D binding protein (DBP) and albumin [80,81]. Vitamin D is transported to the liver where it is converted into 25(OH)D by vitamin-D-25-hydroxylases [79] and inside the liver, it is converted into 1,25(OH)2D by 25-hydroxyvitamin D-1α [79,82]. 1,25(OH)2D circulates in the blood bound to DBP and, after it dissociates from DBP, it binds to the intracellular nuclear vitamin D receptor to exert numerous physiologic functions and regulate its own levels [7,79]. While 25-hydroxycholecalciferol and 1,25-hydroxycholecalciferol are primarily synthesized in the liver and kidney, respectively, in the epidermis, keratinocytes express 25-hydroxylase (CYP27A1) [83] and 1α-hydroxylase (CYP27B1) and 1,25-hydroxycholecalciferol [84]. Nevertheless, the capacity of the skin to produce 1,25(OH)2 D3 is scarce [85], as the skin is the target tissue itself [86] and is where vitamin D produces growth arrest and induces keratinocyte differentiation (Fig. 2 ) [87].
Fig. 2.
Diagram showing the synthesis of 1,25-hydroxy-cholecalciferol (1,25-hydroxyvitamin D3) in humans. Cholecalciferol (vitamin D3) is converted into 25-hydroxycholecalciferol by 25-hydroxylase, and this 25-hydroxyvitamin D3 is then transformed into 1,25-hydroxycholecalciferol (1,25-hydroxyvitamin D3) by 1α-hydroxylase in the liver and kidney, respectively. Both reactions can nevertheless take place in the epidermis.
While highest VDR concentrations are found in tissues that are involved in calcium homeostasis [7,46], VDR have been found in almost all tissues and cells of the human body [5,7,46,88]. Many tissues and cells also feature 25(OH)D1 alpha–hydroxylase activity (CYP27B1) which allows for the elimination of 1,25(OH)2D by the kidney. In these tissues, the enzyme is regulated by specific factors such as inflammatory signaling molecules or cell development stage. Extra-renal tissues can also catabolize 1,25(OH)2D [7]. Vitamin D receptor activation by 1,25(OH)2D leads to extensive biological activations in these tissues through genomic and non-genomic pathways [5,7].
Evidence continues to mount of extra-skeletal vitamin D functions. Vitamin D is known to enhance the immune system and has strong immunomodulatory capacity. Several vitamin D-related components exist in the CV system, suggesting an important role of the vitamin in CV health as discussed below. The vitamin's extraskeletal functions are today being considered as targets to reduce risks of cancer, autoimmune disease, neurocognitive dysfunction and many other diseases and causes of mortality [7]. However, much more work is needed in this area [89].
4. Vitamin D improves CV health
Data from observational studies suggest that low levels of 25(OH)D can negatively affect CV health [90]. Currently, however, there is no consensus regarding the optimal level of the vitamin for possible preventive CVD or cancer benefits. Despite this, serum 25(OH)D levels >30 ng/ml are considered vitamin D optimal levels [4,7,58,91].
Vitamin D is thought to play a protective role against CVD because its receptor, VDR, is intracellular and is able to bind to 1,25(OH)2D3. This event then stimulates VDR to join to the retinoid X receptor (RXR), translocate to the nucleus, and bind to the regulator site in the promotor region of elements of the DNA sequence, promoting the synthesis of proteins that are regulated by vitamin D [92]. Vitamin D receptors have been identified in the main CV cell types (vascular smooth muscle cells, endothelial cells, cardiomyocytes), platelets, macrophages, dendritic cells and other immune cells [93]. An advanced age has been significantly associated with a reduction in VDR expression independently of serum levels of 25(OH)D [94]. Vitamin D crosses the cell membrane and cytoplasm to reach the nucleus where it binds to VDR. When this complex joins to the retinoic acid receptor it modifies gene function, inducing protein synthesis. The vitamin D binding protein is a 58 kDa glycoprotein which is synthesized in the liver and is the main transporter of calcitriol. Its functions are the activation of macrophages, removal of actin and binding of fatty acids, helping vitamin D reach its target tissues. Polymorphisms in vitamin D binding proteins may affect their binding affinity for vitamin D and could be directly related to a risk of vitamin D deficiency or CVD [93]. Vitamin D, thus, has CV pleiotropic effects by activating nuclear VDR in cardiomyocytes and vascular endothelial cells and regulating the renin-angiotensin-aldosterone system, adiposity, energy expense and pancreatic cell activity [94]. In humans, vitamin D deficiency can be linked to vascular dysfunction, arterial stiffness, and left ventricular (LV) hypertrophy. A lack of VDR leads to increased LV mass and increased levels of atrial natriuretic peptide along with imbalance of homeostasis, cardiac metalloproteases and fibroblasts. In turn, this promotes the formation of a fibrotic extracellular matrix and leads to LV dilation and impaired electromechanical coupling [95,96].
In addition, vitamin D deficiency can induce inflammation of epicardial fat and the vessel wall through direct interaction with nuclear factor kappa beta (NFκB) [43], which increases the inflammatory response and promotes atheromatosis and CVD. Accordingly, vitamin D insufficiency gives rise to increased arterial stiffness and endothelial dysfunction in blood vessels, inducing atherogenesis with an important role in regulating blood pressure, while vitamin D deficiency could lead to vascular smooth muscle cell proliferation, endothelial cell dysfunction and increased inflammation [97].
In relation to a proposed anti-diabetic role of vitamin D, VDR are expressed in pancreatic beta cells such that vitamin D induces the secretion of insulin [98,99]. In other studies, vitamin D supplements have shown improved sensitivity to insulin mediated by the increased production of insulin receptors, modulating inflammation [100,101]. Obese subjects have lower vitamin D levels than non-obese individuals, and this is probable related to the storage of vitamin D in body fat. Moreover, obese individuals respond worse to vitamin D supplements and show smaller increases in the vitamin than non-obese controls when the same dose of supplement is used, meaning they need higher doses than recommended [38,102,103].
These findings indicate that vitamin D regulates blood pressure by acting on endothelial and smooth muscle cells and thus plays an important role in CV health.
4.1. Vitamin D deficiency in CVD
Observational studies have detected an inverse relationship between a good vitamin D status and mortality [104]. Further, according to the findings of systematic reviews and meta-analyses of random controlled trials, it seems that vitamin D supplementation could have a slight overall survival benefit [105]. However, the authors of a recent meta-analysis reported that vitamin D supplement intake alone was not in itself associated with a significant reduction in all-cause mortality in adults compared with placebo or no treatment, and neither was there a reduction in death due to CVD, cerebrovascular disease or ischemic heart disease. These authors highlighted that the intake of vitamin D supplements only reduced the risk of cancer-related death by around 16% [106].
Despite these results, vitamin D deficiency has been associated with various CV problems in patients with risk factors such as high blood pressure, type 2 diabetes mellitus (T2DM) and obesity, and a greater frequency of vitamin D deficiency has been detected in subjects with type 1 DM compared to healthy individuals [[107], [108], [109]].
A meta-analysis designed to examine the relationship between vitamin D status or its supplementation and the incidence of T2DM showed that subjects with serum levels of the vitamin >25 ng/ml compared to those with levels <14 ng/ml, had a 43% lower risk of developing type 2 diabetes and that a daily dose of vitamin D supplements above 500 IU, compared to one of <200 IU reduced this risk by 13% [110]. Vitamin D deficiency has also been observed in patients with cardiac failure [111], myocardial infarction (MI) [112], stroke [113] and peripheral arterial disease [114].
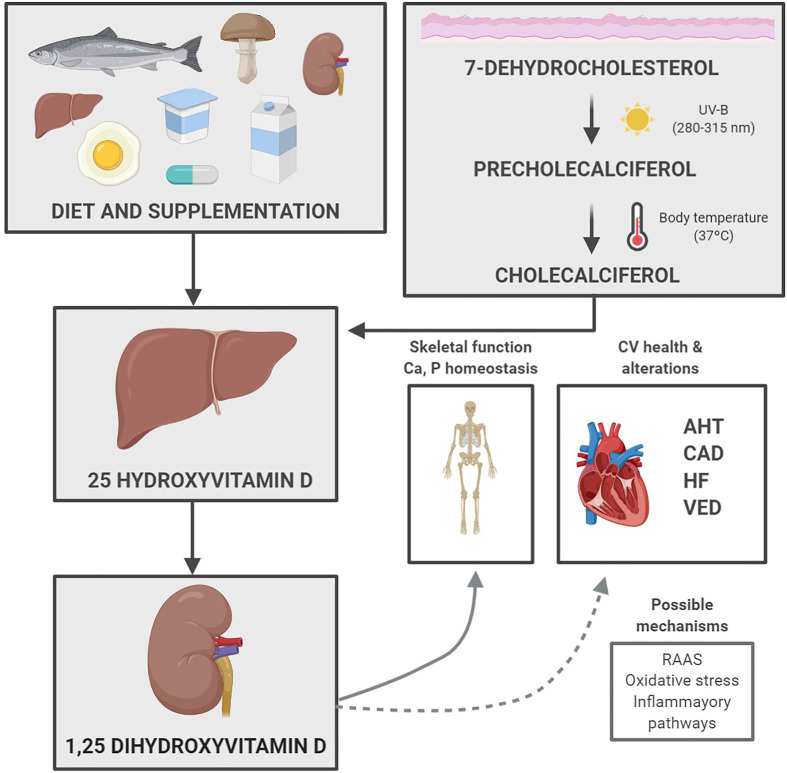
Hence, vitamin D deficiency has been associated with various CV problems. Below we describe the different mechanisms that could help explain this association (Fig. 3 ).
Fig. 3.
Diagram showing how vitamin D from the diet and sun exposure can improve skeletal function and prevent several cardiovascular problems. AHT (arterial hypertension), CAD (coronary artery disease), CV (cardiovascular), HF (heart failure), RAAS (renin–angiotensin–aldosterone system), UV (ultraviolet), VED (vascular endothelial dysfunction).
4.1.1. High blood pressure (BP) or hypertension (HTN)
The important role of renin in regulating BP has been well established and several anti-HTN agents act at the level of the renin–angiotensin–aldosterone system (RAAS). A marked increase in renin expression and angiotensin II production has been observed in mice and humans with inactivated VDR. This observation suggests that vitamin D deficiency could activate RAAS, with the consequence of LV hypertrophy and a greater risk of CVD [115,116]. However, data emerging from random controlled trials (RCT) and Mendelian genetic studies have not been able to confirm a causal relationship between vitamin D supplementation and improved CVD risk factors [117]. In addition, the results of RCT designed to assess the impact of the intake of vitamin D supplements on arterial HTN have been inconclusive [118], most likely owing to suboptimal study designs with significant bias. Despite these results, it may be concluded that a deficiency in vitamin D could promote the sustained activation of RAAS, increasing angiotensin with the consequences of arterial hardening, endothelial dysfunction and the development of HTN. Numerous animal model and observational studies in humans strongly support the hypothesis that vitamin D deficiency contributes to high blood pressure. However, it should be noted that the antihypertensive effect of vitamin D was not observed in some trials, which could be due to a sub-optimal study design, the knowledge that a deficiency of vitamin D could promote the sustained activation of RAAS, increasing angiotensin, arterial hardening, and endothelial dysfunction, could support the hypothesis that vitamin D deficiency contributes to the development of HTN.
4.1.2. Endothelial dysfunction
A role has been also ascribed to vitamin D in regulating endothelial function [119]. Endothelial dysfunction is strongly related to the pathogenesis of several CV problems, such as T2DM, HTN, atherosclerosis and peripheral arterial diseases. One of the most significant vasoactive substances, nitric oxide (NO) has a strong vasodilator action and this confers protection against vessel inflammation and the formation of vascular lesions. Some studies have suggested that vitamin D and VDR could play a key role in regulating NO synthesis [118]. Observational studies have identified a link between insufficient vitamin D levels and increased oxidative stress or a lowered antioxidant capacity [120]. Further, circulating serum 25(OH)D concentrations have been inversely correlated with endothelial dysfunction, as assessed by dilation measured through brachial artery flow in patients with chronic non-dialysis renal disease [121]. In the absence of endothelial VDR, endothelial cells show a deteriorated vasodilator response to perfused acetylcholine [122]. This points to a relationship between vitamin D and endothelial function, and an association between deficient serum levels of the vitamin and CVD. However, despite these promising findings supporting a link between the genomic effect of vitamin D and the regulation of endothelial function involving the regulation of NO bioavailability and bioactivity, there is still insufficient evidence that vitamin D supplementation could improve endothelial function in humans. Long term studies are therefore warranted to confirm that the intake of these supplements could be beneficial for human endothelial function [119].
4.1.3. Coronary heart disease (CHD)
Numerous studies have sought to identify a relationship between vitamin D deficiency and CHD. Results so far indicate that patients with reduced concentrations of vitamin D have an elevated risk of experiencing a major adverse CVD event (MACE) [123,124]. Lee et al. [125] examined the prevalence of vitamin D deficiency among patients suffering acute MI, finding that 75% were vitamin D deficient and 21% were vitamin D insufficient. These authors concluded that vitamin D deficiency is present in practically all those with MI.
In a cross-sectional study it was confirmed that patients with T2DM and vitamin D deficiency show a greater frequency of multivessel lesions than individuals without type 2 T2DM, mainly those with serum levels of the vitamin <10 ng/ml. Strong correlation has also been reported between vitamin D deficiency and CHD, and results indicate a significant association between deficiency of the vitamin and disease severity. Moreover, when the risk of CHD was compared between individuals with severe hypovitaminosis (<10 ng/ml) and those with a normal vitamin D status, the adjusted odds ratio was 1.73 (95% CI: 1.18–2.52) [126].
Only a few RCT have been able to identify a beneficial role of vitamin D in the treatment of CHD. In a study in which calcitriol (0.5 μg/day) was administered over 6 months in patients with stable CHD, improvements were noted in the SYNTAX score and cardiometabolic variables, and a significant reduction was observed in CHD and vascular inflammation. These results prompt future studies designed to examine the benefits of potent analogs of vitamin D like calcitriol [127].
Among the studies designed to explore the possible mechanisms explaining the effects of vitamin D in CHD, a study in pigs revealed that vitamin D suppresses the kappa-light-chain-enhancer nuclear factor of the activated B-cell pathway NFκB activation by targeting karyopherin α4 (KPNA4), attenuating the progression of CHD, thus vitamin D deficiency increases the regulation of KPNA4, increasing the activation of NF-κB [43].
Epicardial adipose tissue cells play a major role in atherogenesis progression in the coronary arteries through the synthesis of local inflammatory cytokines [128]. NFκB acts as a transcription factor in the nucleus by binding to different κB elements, which promotes the transcription of inflammatory cytokines such as interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α [129].
Collectively, the available data indicate that adequate levels of intracellular 1,25(OH)2 D3 could have the capacity to reduce the inflammatory response during the atherosclerotic process but it still remains unclear how vitamin D3 suppresses the transcription of KPNA4 [43].
4.1.4. Heart failure (HF)
Vitamin D deficiency could be a predictor of worse survival in patients with HF, who could perhaps benefit from vitamin D supplementation [130]. The results of the National Health and Nutrition Examination Survey (NHANES III) have revealed a growing prevalence of HF in patients with lower serum vitamin D levels [131]. These authors noted that vitamin D therapy rescues tissue sensitivity to angiotensin II in a similar manner to the actions of an angiotensin-converting enzyme inhibitor in obese-HTN subjects, which suggests inhibitory effects of vitamin D on RAAS and a possible regulatory effect of the vitamin D-VDR complex on renin activity [132].
Schleitfoff et al. [133] assessed the effects of vitamin D on survival rate and several biochemical variables in patients with HF. Their findings indicated that vitamin D3 could be useful as a new anti-inflammatory agent and suggested the participation of an altered vitamin D-parathyroid hormone axis in the progression of HF.
The EVITA trial (Effect of Vitamin D on All-Cause Mortality in Heart Failure Patients) is one of the larger studies, with a follow up of 3 years and 400 participants with functional NYHA class HF ≥ II and serum 25(OH)D levels <75 nmol/l. Patients were administered vitamin D3 at a dose of 4000 IU/day. The primary end-point was all-cause mortality. Results revealed a similar mortality among patients given vitamin D or placebo, while the need for a pacemaker was greater in the patients assigned to receive vitamin D. The conclusion of this trial was a need for caution when prescribing moderately high long-term doses of vitamin D in patients with HF [134].
In another study, patients with HF received optimal medical therapy and cholecalciferol at a dose of 100 μg daily for 1 year. Significant improvements were recorded in LV dimensions and LV ejection fraction [135]. However, these results contrast with those obtained in patients with chronic renal disease administered paricalcitol (a synthetic vitamin D analog) over 48 weeks. This intervention led to no structural or functional changes in the left ventricle as determined through cardiac resonance and echocardiography in comparison with subjects receiving placebo [136].
Thus, the results of numerous RCT and meta-analyses examining the possible benefits of vitamin D supplements in patients with CV risk factors have been conflicting. While vitamin D plays roles in modulating RAAS, endothelial function and sympathetic activity, its precise role in this setting remains unclear. Few studies have lent support to the notion that vitamin D could be beneficial in the treatment of CHD. Further work is needed to clarify whether special subgroups (e.g., those with HTN, T2DM, CHD, obesity, or HF) could benefit from vitamin D supplementation.
4.2. Vitamin D to treat CVD
The use of vitamin D supplements has increased widely in the past few years even when there is still debate about the role of these supplements, their doses and which is the optimal vitamin D status. Recent large intervention studies, meta-analyses and RCT have not been able to demonstrate clear benefits of vitamin D supplements at the CV level, although epidemiological studies have related low 25(OH)D levels with CVD risk factors and adverse outcomes [137].
Evidence does exist to suggest that low serum 25(OH)D levels could have detrimental impacts on CV health. Thus, deficient vitamin D activates RAAS and could predispose to LV hypertrophy and high BP. Similarly, vitamin D deficiency causes an increase in parathyroid hormone with the consequence of insulin resistance and T2DM. Further, the results of some observational studies have pointed to a link between low serum 25(OH)D levels and T2DM risk [138]. Thus, it has been postulated whether vitamin D supplementation could have significant health benefits given that an insufficient vitamin D status could be a key factor in the etiology of several chronic diseases such as CVD and glucose intolerance. It should be underscored that these diseases are responsible for 50% of all deaths in middle to high income countries [111].
The likely cardioprotective effect of vitamin D is based on the findings of observational studies that suggest that low vitamin D levels could play a determinant role in various CVD risk factors, such as the development and progression of endothelial dysfunction, vascular and myocardial cell calcification and inflammation (Fig. 3) [139]. Recently, Pittas et al. examined whether vitamin D supplementation could reduce the risk of T2DM in adults fulfilling at least 3 glycemic criteria for prediabetes and no diagnostic criteria for T2DM. The daily intake of 4000 IU of vitamin D3 supplements was found to confer a similar risk of diabetes than placebo after a median follow-up duration of 2.5 years [140].
In view of the uncertain benefits of vitamin D supplementation even among the general non vitamin D-deficient population and the weak cardioprotective effect of vitamin D suggested by the data available, several large RCT have tried to shed light on this topic. The Vitamin D Assessment (ViDA), a double-blind placebo-controlled trial, examined the possible preventive effect on CVD of monthly supplementation with high vitamin D doses in the general population. Thus, more than 5000 people in New Zealand received oral vitamin D3 or placebo at a starting dose of 200,000 IU followed a month later by a monthly dose of 100,000 IU over a median interval of 3.3 years. Around 25% had vitamin D deficiency. The primary outcome measure of CVD was detected in 11.8% of participants in the vitamin D group and 11.5% in the placebo group, regardless of the presence or not of baseline vitamin D deficiency. Accordingly, the conclusion offered was that monthly high-dose vitamin D supplementation does not prevent CVD [141].
The D-Health Trial assessed the capacity of vitamin D supplements to prevent mortality and cancer in over 21,000 subjects. This RCT examined the benefits of oral monthly doses of 60,000 IU of cholecalciferol or equivalent placebo in an intervention planned for 5 years plus another 5 years of passive follow up through access to health records and death registries. The authors concluded that it was not clear if vitamin D supplementation has any benefits on the objectives analyzed and that data from observational studies should not be used to support the use of the vitamin to prevent disease in healthy persons [142]. Neither have CV improvements emerged following supplementation with cholecalciferol in the results of a recent review [143] and meta-analysis including more than 83,000 individuals, in which no benefits of vitamin D were observed in terms of protection against CVD events [144].
In another double-blind placebo-controlled RCT, it was assessed whether 12 weeks of daily cholecalciferol supplements (800 IU/day) could serve to reduce BP, heart rate and other CVD risk markers in healthy participants. Results indicated that vitamin D failed to improve CVD risk markers although it did improve serum levels of 25(OH)D [145].
The DIMENSION study assessed the impact of daily cholecalciferol supplementation over 16 weeks (2000 IU/day or 4000 IU/day) on endothelial function in patients with T2DM in terms of possible improvements in vascular biomarkers and the reactive hyperemia index. Vitamin D status improved significantly in the intervention arm. However, a multivariate regression analysis revealed no significant impacts on endothelial function [146].
Neither were significant changes observed in markers of heart lesions in response to a cholecalciferol dose of 300,000 IU given prior to percutaneous coronary intervention. In this study, a similar incidence was observed in MACE in the study and control groups [147].
In a setting of primary care, the BEST-D trial investigated the effects of daily supplementation over a year with cholecalciferol (4000 IU or 2000 IU) on disease risk and biochemical markers in healthy individuals. Again, the results of this RCT were not promising as, while vitamin D supplementation improved serum 25(OH)D levels, no significant changes were observed in CVD risk factors, arterial stiffness, blood lipids or BP after the intervention [148].
A recent meta-analysis examined for the first time, the relationship between serum 25(OH)D status and CVD incidence. The studies included were 25 prospective cohort studies conducted from April 2000 to September 2017. No significant relationship was detected, although a reduced vitamin D level was correlated with a 44% greater relative risk of CVD (incidence-mortality combined), and increased CVD-related mortality (RR = 1.54, 95% CI: 1.29–1.84) [149].
The study that has shed most light on this topic is the VITAL trial (VITamin D and Omega-3 triAL) [150]. This trial assessed the prevention of cancer and CVD using vitamin D3 (cholecalciferol) given at a dose of 2000 IU/day and omega-3 fatty acids at 1 g/day in men aged ≥50 years and women ≥55 years. The conclusions were that compared with placebo, vitamin D intake did not confer a reduced risk of any of the primary endpoints examined of invasive cancer of any type and of the major CVD events combined: MI, stroke or CV cause of death. In effect, this has been the most conclusive RCT to date to rule out any cardioprotective or cancer protective effects of vitamin D in the general population.
While acute vitamin D toxicity effects are rare with supplements and require extremely high doses, toxic effects of the vitamin include hypercalcemia and low serum parathyroid levels [151]. Hypercalcemia may cause cardiac arrhythmia (shortened QT interval), or even mimic the effects of acute MI in the electrocardiogram [152].
5. Vitamin D in the coronavirus Disease-19 (COVID-19) era
Vitamin D treatment could reduce the incidence of viral respiratory tract infections, especially in deficiency situations [153]. Vitamin D plays a critical role in the immune system, being an immunomodulatory hormone with antimicrobial and anti-inflammatory effects. This fact could explain the beneficial and protective effect attributed to vitamin D against COVID-19 by being able to prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, reduce virus replication, accelerate viral clearance and/or reduce its spread. Likewise, as abovementioned, vitamin D deficiency impairs vascular function and is associated with CVD. The severity of COVID-19 is worse in those individuals with a history of CVD [154]. In effect, the disease process has been linked to myocardial injury in around 25% of patients, with some developing significant cardiac manifestations, including biventricular heart failure, arrhythmias, and occasionally cardiogenic shock and death [155,156].
On the one hand, vitamin D has anti-inflammatory effects by controlling the adaptive immune system, induces the expression of various molecules involved in the antioxidant defense system, reduces oxidative stress and cellular oxidation, and also shows vasoprotective effects [157]. Besides, vitamin D induces the expression of several molecules involved in the antioxidant defense system, modulates immune function, promotes viral elimination, and reduces inflammatory responses by reducing the generation of inflammatory cytokines such as IL-6, IL -8, IL-12 and IL-17. Importantly, vitamin D levels are associated with lower levels of IL-6, which is involved in the so-called cytokine storm observed in critically ill patients and associated with a worse COVID-19 prognosis. Kox et al., in small sample size, compared cytokine levels in critically ill patients with COVID-19 and patients with other critical illnesses. In this study, plasma concentrations of proinflammatory cytokine TNF, IL-6, and IL-8 were determined, and it was found that the levels of the three cytokines were significantly lower in patients with COVID-19 than in patients with septic shock with acute respiratory distress syndrome (ARDS), which explains these findings to lower severity of the disease despite suffering severe lung injury. Therefore they suggest that the severity of the COVID-19 may not be due to a cytokine storm [158]. Similarly, vitamin D increases the level of angiotensin-converting enzyme 2 (ACE2) in the lungs, a key receptor for the acute respiratory syndrome [159,160].
A recent study investigated the association between vitamin D levels and the incidence of COVID-19 [160]. Individuals with deficient levels of vitamin D at the COVID-19 test had a substantially higher risk of testing positive than those within normal range. Also, those individuals who were supplemented with vitamin D before the pandemic did not have an increased risk of COVID-19 compared to those with normal vitamin D values and not supplemented, which could advocate a likely protective effect of vitamin D supplementation. Taken together, these findings suggested that high-dose vitamin D therapy, in order to restore circulating vitamin D levels rapidly, could reduce the risk of severity and mortality from COVID-19 [161]. People with chronic diseases, i.e., elderly, obese, T2DM, smokers, as well as African Americans, have low concentrations of vitamin D and a more aggressive SARS-CoV-2 infection [162]. Vitamin D deficiency could be a significant risk factor for COVID-19 infection severity. Thus, vitamin D supplementation can be considered in risk groups with low vitamin D levels. Vitamin D supplementation is a relatively cheap, available and safe measure. However, there is no solid scientific evidence to support the routine clinical use of vitamin D in COVID-19 patients [163].
Thus, an interesting debate on the efficacy of high-dose vitamin D supplementation to reduce the risk of COVID-19 infection and severity has recently emerged. Additional clinical trials that provide more solid evidence of vitamin D role in reducing hospitalization rates and mortality, as well as whether vitamin D supplementation reduces the risk of infection by SARS-CoV-2 virus, are still needed.
6. Conclusion
In conclusion, despite a likely association between serum vitamin D deficiency and a greater incidence of CVD and its related mortality, the intake of vitamin D as supplements has not shown any appreciable benefits in terms of reducing the risk of CVD in recent large RCT. This means that no CV benefits can be ascribed to vitamin D supplements even in cases of its insufficiency (<20 ng/ml). These supplements should therefore not be prescribed to prevent CVD until further assessment of the benefits and risks of vitamin D supplements in the primary prevention of CVD and cancer. As there is no categorical evidence so far of vitamin D supplements causing CV damage, their lack of benefits for CV health should not preclude their use for other indications. Pending further insight into this topic, we will continue to recommend the combination of a healthy diet and active lifestyle as the best strategy to improve our vitamin D status and promote good CV health.
Statement of authorship
F.G.G., M.M.F, and N.V. wrote the first draft of the paper; F.S.G., and H.P.G had primary responsibility for final content. F.S.G., C.J.L., and H.P.G revised the final draft of the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by a grant from Universidad Europea de Madrid (#2019/UEM01).
References
- 1.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., et al. vol. 141. 2020. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. [DOI] [PubMed] [Google Scholar]
- 2.Holick M.F. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61:638–645. doi: 10.1093/ajcn/61.3.638S. [DOI] [PubMed] [Google Scholar]
- 3.Terushkin V., Bender A., Psaty E.L., Engelsen O., Wang S.Q., Halpern A.C. Estimated equivalency of vitamin D production from natural sun exposure versus oral vitamin D supplementation across seasons at two US latitudes. J Am Acad Dermatol. 2010;62 doi: 10.1016/j.jaad.2009.07.028. 929.e1-929.e9. [DOI] [PubMed] [Google Scholar]
- 4.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., et al. Evaluation, treatment, and prevention of vitamin D deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 5.Charoenngam N., Shirvani A., Holick M.F. Vitamin D for skeletal and non-skeletal health: what we should know. J Clin Orthop Trauma. 2019;10:1082–1093. doi: 10.1016/j.jcot.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Płudowski P., Karczmarewicz E., Bayer M., Carter G., Chlebna-Sokół D., Czech-Kowalska J., et al. The role of EBV in thyroid disease. Endokrynol Pol. 2013;64:319–327. doi: 10.5603/EP. [DOI] [PubMed] [Google Scholar]
- 7.Pludowski P., Holick M.F., Grant W.B., Konstantynowicz J., Mascarenhas M.R., Haq A., et al. Vitamin D supplementation guidelines. J Steroid Biochem Mol Biol. 2018;175:125–135. doi: 10.1016/j.jsbmb.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Bouillon R. Vitamin D and cardiovascular disorders. Osteoporos Int. 2019;30:2167–2181. doi: 10.1007/s00198-019-05098-0. [DOI] [PubMed] [Google Scholar]
- 9.Schmid A., Walther B. Natural vitamin D content in animal products. Adv Nutr. 2013;4:453–462. doi: 10.3945/an.113.003780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardwell G., Bornman J.F., James A.P., Black L.J. A review of mushrooms as a potential source of dietary vitamin D. Nutrients. 2018;10:1–11. doi: 10.3390/nu10101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouillon R., Van Schoor N.M., Gielen E., Boonen S., Mathieu C., Vanderschueren D., et al. Optimal vitamin D status: a critical analysis on the basis of evidence-based medicine. J Clin Endocrinol Metab. 2013;98:E1283–E1304. doi: 10.1210/jc.2013-1195. [DOI] [PubMed] [Google Scholar]
- 12.Manson J.A.E., Brannon P.M., Rosen C.J., Taylor C.L. Vitamin D deficiency - is there really a pandemic? N Engl J Med. 2016;375:1817–1820. doi: 10.1056/NEJMp1608005. [DOI] [PubMed] [Google Scholar]
- 13.Bouillon R. Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol. 2017;13:466–479. doi: 10.1038/nrendo.2017.31. [DOI] [PubMed] [Google Scholar]
- 14.Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 15.O'Donnell S., Moher D., Thomas K., Hanley D.A., Cranney A. Systematic review of the benefits and harms of calcitriol and alfacalcidol for fractures and falls. J Bone Miner Metabol. 2008;26:531–542. doi: 10.1007/s00774-008-0868-y. [DOI] [PubMed] [Google Scholar]
- 16.Bover J., Egido J., Fernández-Giráldez E., Praga M., Solozábal-Campos C., Torregrosa J.V., et al. Vitamina D, receptor de la vitamina D e importancia de su activación en el paciente con enfermedad renal crónica. Nefrologia. 2015;35:28–41. doi: 10.3265/Nefrologia.pre2014.Sep.11796. [DOI] [PubMed] [Google Scholar]
- 17.Cesareo R., Attanasio R., Caputo M., Castello R., Chiodini I., Falchetti A., et al. Italian association of clinical endocrinologists (AME) and Italian chapter of the American association of clinical endocrinologists (AACE) position statement: clinical management of vitamin D deficiency in adults. Nutrients. 2018;10:1–22. doi: 10.3390/nu10050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feldman S.H., Easton D.N. Second. Elsevier; 2006. Occupational health and safety. Lab. Rat; pp. 565–586. [DOI] [Google Scholar]
- 19.McKenzie R., Madronich S. 2nd ed. vol. 6. Elsevier; 2003. Ultraviolet, surface. Encycl. Atmos. Sci; pp. 45–50. 2nd ed. [DOI] [Google Scholar]
- 20.D'Orazio J., Jarrett S., Amaro-Ortiz A., Scott T. UV radiation and the skin. Int J Mol Sci. 2013;14:12222–12248. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wacker M., Holick M.F. Sunlight and Vitamin D: a global perspective for health. Dermato-Endocrinology. 2013;5:51–108. doi: 10.4161/derm.24494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikle D. MDText.com, Inc.; 2000. Vitamin D: production, metabolism, and mechanisms of action. [Google Scholar]
- 23.Webb A.R., Decosta B.R., Holick M.F. Sunlight regulates the cutaneous production of vitamin D3 by causing its photodegradation. J Clin Endocrinol Metab. 1989;68:882–887. doi: 10.1210/jcem-68-5-882. [DOI] [PubMed] [Google Scholar]
- 24.Merghoub T., Polsky D., Houghton A.N. Molecular biology of melanoma. Mol Basis Canc. 2008:463–470. doi: 10.1016/B978-141603703-3.10036-6. [DOI] [Google Scholar]
- 25.Holick M.F., MacLaughlin J.A., Doppelt S.H. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211(80):590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 26.Krause R., Bühring M., Hopfenmüller W., Holick M.F., Sharma A.M. Ultraviolet B and blood pressure. Lancet. 1998;352:709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 27.Webb A.R. Who, what, where and when-influences on cutaneous vitamin D synthesis. Prog Biophys Mol Biol. 2006;92:17–25. doi: 10.1016/j.pbiomolbio.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Amann M., Derwent D., Forsberg B., Hänninen O., Hurley F., Krzyzanowski M., et al. 2008. Health risks of ozone from long-range transboundary air pollution. Copenhagen, Denmark. [Google Scholar]
- 29.Armstrong B.K., Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B Biol. 2001;63:8–18. doi: 10.1016/S1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 30.saes da Silva E., Tavares R., da silva Paulitsch F., Zhang L. Use of sunscreen and risk of melanoma and non-melanoma skin cancer: a systematic review and meta-analysis. Eur J Dermatol. 2018;28:186–201. doi: 10.1684/ejd.2018.3251. [DOI] [PubMed] [Google Scholar]
- 31.Perugini P., Bonetti M., Cozzi A.C., Colombo G.L. Topical sunscreen application preventing skin cancer: systematic review. Cosmetics. 2019;6:42. doi: 10.3390/cosmetics6030042. [DOI] [Google Scholar]
- 32.Matsuoka L.Y., Ide L., Worstman J., Maclaughlin J.A., Holick M.F. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64:1165–1168. doi: 10.1210/jcem-64-6-1165. [DOI] [PubMed] [Google Scholar]
- 33.Fitzpatrick T.B. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 34.Stamatas G.N., Zmudzka B.Z., Kollias N., Beer J.Z. Non-invasive measurements of skin pigmentation in situ. Pigm Cell Res. 2004;17:618–626. doi: 10.1111/j.1600-0749.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 35.MacLaughlin J., Holick M.F. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holick M.F., Matsuoka L.Y., Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;334:1104–1105. doi: 10.1016/S0140-6736(89)91124-0. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization W . 2020. Obesity and overweight.https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight [Google Scholar]
- 38.Wortsman J., Matsuoka L.Y., Chen T.C., Lu Z., Holick M.F. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 39.Looker A.C. Body fat and vitamin D status in black versus white women. J Clin Endocrinol Metab. 2005;90:635–640. doi: 10.1210/jc.2004-1765. [DOI] [PubMed] [Google Scholar]
- 40.Cheng S., Massaro J.M., Fox C.S., Larson M.G., Keyes M.J., McCabe E.L., et al. Adiposity, cardiometabolic risk, and vitamin D status: the framingham heart study. Diabetes. 2010;59:242–248. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holick M.F., Chen T.C. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080–1086. doi: 10.1093/ajcn/87.4.1080s. [DOI] [PubMed] [Google Scholar]
- 42.Palacios C., Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144:138–145. doi: 10.1016/j.jsbmb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen S., Swier V.J., Boosani C.S., Radwan M.M., Agrawal D.K. Vitamin D deficiency accelerates coronary artery disease progression in swine. Arterioscler Thromb Vasc Biol. 2016;36:1651–1659. doi: 10.1161/ATVBAHA.116.307586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lips P., Hosking D., Lippuner K., Norquist J.M., Wehren L., Maalouf G., et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–254. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 45.Holick M.F. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 46.Chowdhury R., Kunutsor S., Vitezova A., Oliver-Williams C., Chowdhury S., Kiefte-De-Jong J.C., et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903. doi: 10.1136/bmj.g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cashman K.D., Dowling K.G., Škrabáková Z., Gonzalez-Gross M., Valtueña J., De Henauw S., et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103:1033–1044. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holick M.F. The vitamin D epidemic and its health consequences. J Nutr. 2005;135:2739S–2748S. doi: 10.1093/jn/135.11.2739S. [DOI] [PubMed] [Google Scholar]
- 49.Moan J., Porojnicu A.C., Dahlback A., Setlow R.B. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proc Natl Acad Sci U S A. 2008;105:668–673. doi: 10.1073/pnas.0710615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoel D.G., Berwick M., de Gruijl F.R., Holick M.F. The risks and benefits of sun exposure 2016. Dermato-Endocrinology. 2016;8 doi: 10.1080/19381980.2016.1248325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holick M. Vitamin D : importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004:362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 52.Brender E., Burke A., Glass R.M. Vitamin D. J Am Med Assoc. 2005;294:2386. doi: 10.1001/jama.294.18.2386. [DOI] [PubMed] [Google Scholar]
- 53.Wolpowitz D., Gilchrest B.A. The vitamin D questions: how much do you need and how should you get it? J Am Acad Dermatol. 2006;54:301–317. doi: 10.1016/j.jaad.2005.11.1057. [DOI] [PubMed] [Google Scholar]
- 54.IARC . vol. 5. International Agency for research on Cancer; Lyon, France: 2008. Vitamin D and cancer: IARC working group reports. [DOI] [Google Scholar]
- 55.Clemens T.L., Henderson S.L., Adams J.S., Holick M.F. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;319:74–76. doi: 10.1016/S0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 56.Holick M.F. McCollum award lecture, 1994: vitamin D—new horizons for the 21st century | the American journal of clinical nutrition | oxford academic. Am J Clin Nutr. 1994;60:619–630. doi: 10.1093/ajcn/60.4.619. [DOI] [PubMed] [Google Scholar]
- 57.Chen T.C., Chimeh F., Lu Z., Mathieu J., Person K.S., Zhang A., et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460:213–217. doi: 10.1016/j.abb.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hintzpeter B., Mensink G.B.M., Thierfelder W., Müller M.J., Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur J Clin Nutr. 2008;62:1079–1089. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 59.World Meteorological Organization W. 2018. Scientific assessment of ozone depletion: 2018. Geneva, Switzerland. [Google Scholar]
- 60.De Fabo E.C. Arctic stratospheric ozone depletion and increased UVB radiation: potential impacts to human health. Int J Circumpolar Health. 2005;64:509–522. doi: 10.3402/ijch.v64i5.18032. [DOI] [PubMed] [Google Scholar]
- 61.Lucas R.M., Yazar S., Young A.R., Norval M., De Gruijl F.R., Takizawa Y., et al. Human health in relation to exposure to solar ultraviolet radiation under changing stratospheric ozone and climate. Photochem Photobiol Sci. 2019;18:641–680. doi: 10.1039/C8PP90060D. [DOI] [PubMed] [Google Scholar]
- 62.Balk S.J. Council on Environmental Health, Dermatology S on. Ultraviolet radiation: a hazard to children and adolescents. Pediatrics. 2011;127:588–597. doi: 10.1542/peds.2010-3501. [DOI] [PubMed] [Google Scholar]
- 63.Flaxman S.R., Bourne R.R.A., Resnikoff S., Ackland P., Braithwaite T., Cicinelli M.V., et al. 2017. Articles Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. [DOI] [PubMed] [Google Scholar]
- 64.Gilchrest B.A., Eller M.S., Geller A.C., Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341–1348. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- 65.Woo D.K., Eide M.J. Tanning beds, skin cancer, and vitamin D: an examination of the scientific evidence and public health implications. Dermatol Ther. 2010;23:61–71. doi: 10.1111/j.1529-8019.2009.01291.x. [DOI] [PubMed] [Google Scholar]
- 66.Saraff V., Shaw N. Sunshine and vitamin D. Arch Dis Child. 2016;101:190–192. doi: 10.1136/archdischild-2014-307214. [DOI] [PubMed] [Google Scholar]
- 67.Neale R.E., Davis M., Pandeya N., Whiteman D.C., Green A.C. Basal cell carcinoma on the trunk is associated with excessive sun exposure. J Am Acad Dermatol. 2007;56:380–386. doi: 10.1016/j.jaad.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 68.Arnold M., de Vries E., Whiteman D.C., Jemal A., Bray F., Parkin D.M., et al. Global burden of cutaneous melanoma attributable to ultraviolet radiation in 2012. Int J Canc. 2018;143:1305–1314. doi: 10.1002/ijc.31527. [DOI] [PubMed] [Google Scholar]
- 69.Howell J.Y., Ramsey M.L. StatPearls Publishing; 2020. Cancer, squamous cell of the skin. [Google Scholar]
- 70.World Cancer Research Fund, American Institute for Cancer Research Diet , nutrition , physical activity and skin cancer. Expert Rep. 2018 http://dietandcancerreport.org Available at: [Google Scholar]
- 71.Stern R.S. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146:279–282. doi: 10.1001/archdermatol.2010.4. [DOI] [PubMed] [Google Scholar]
- 72.Reichrath J. Skin cancer prevention and UV-protection: how to avoid vitamin D-deficiency? Br J Dermatol. 2009;161:54–60. doi: 10.1111/j.1365-2133.2009.09450.x. [DOI] [PubMed] [Google Scholar]
- 73.de Jager T.L., Cockrell A.E., Du Plessis S.S. Ultraviolet light induced generation of reactive oxygen species. Adv Exp Med Biol. 2017;996:15–23. doi: 10.1007/978-3-319-56017-5_2. Springer New York LLC. [DOI] [PubMed] [Google Scholar]
- 74.Pattison D.I., Davies M.J. Exp. Suppl., Birkhäuser Basel; 2006. Actions of ultraviolet light on cellular structures; pp. 131–157. [DOI] [PubMed] [Google Scholar]
- 75.Brenner M., Hearing V.J. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84:539–549. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eller M.S., Gilchrest B.A. Tanning as part of the eukaryotic SOS response. Pigm Cell Res. 2000;13:94–97. doi: 10.1034/j.1600-0749.13.s8.17.x. [DOI] [PubMed] [Google Scholar]
- 77.Maddodi N., Jayanthy A., Setaluri V. Shining light on skin pigmentation: the darker and the brighter side of effects of UV radiation. Photochem Photobiol. 2012;88:1075–1082. doi: 10.1111/j.1751-1097.2012.01138.x. Photochem Photobiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.D'Mello S.A.N., Finlay G.J., Baguley B.C., Askarian-Amiri M.E. Signaling pathways in melanogenesis. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Holick M.F. Science in medicine Resurrection of vitamin D deficiency and rickets. Sci Med. 2006;116:2062–2072. doi: 10.1172/JCI29449.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bikle D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cooke N.E., Haddad J.G. Vitamin d binding protein (gc-globulin) Endocr Rev. 1989;10:294–307. doi: 10.1210/edrv-10-3-294. [DOI] [PubMed] [Google Scholar]
- 82.Valero Zanuy M.Á., Hawkins Carranza F. Metabolismo, fuentes endógenas y exógenas de vitamina D. Rev Esp Enfermedades Metab Oseas. 2007;16:63–70. doi: 10.1016/S1132-8460(07)73506-7. [DOI] [Google Scholar]
- 83.Lehmann B., Tiebel O., Meurer M. Expression of vitamin D3 25-hydroxylase (CYP27) mRNA after induction by vitamin D3 or UVB radiation in keratinocytes of human skin equivalents: a preliminary study. Arch Dermatol Res. 1999;291:507–510. doi: 10.1007/s004030050445. [DOI] [PubMed] [Google Scholar]
- 84.Bikle D.D., Nemanic M.K., Whitney J.O., Elias P.W. Neonatal human foreskin keratinocytes produce 1,25-dihydroxyvitamin D3. Biochemistry. 1986;25:1545–1548. doi: 10.1021/bi00355a013. [DOI] [PubMed] [Google Scholar]
- 85.Vantieghem K., Kissmeyer A.M., De Haes P., Bouillon R., Segaert S. UVB-induced production of 1,25-dihydroxyvitamin D3 and vitamin D activity in human keratinocytes pretreated with a sterol Δ7- reductase inhibitor. J Cell Biochem. 2006;98:81–92. doi: 10.1002/jcb.20756. [DOI] [PubMed] [Google Scholar]
- 86.Segaert S., Simonart T. The epidermal vitamin D system and innate immunity: some more light shed on this unique photoendocrine system? Dermatology. 2008;217:7–11. doi: 10.1159/000118506. [DOI] [PubMed] [Google Scholar]
- 87.Bikle D.D. Vitamin D regulated keratinocyte differentiation. J Cell Biochem. 2004;92:436–444. doi: 10.1002/jcb.20095. [DOI] [PubMed] [Google Scholar]
- 88.Bikle D., Christakos S. New aspects of vitamin D metabolism and action — addressing the skin as source and target. Nat Rev Endocrinol. 2020;16:234–252. doi: 10.1038/s41574-019-0312-5. [DOI] [PubMed] [Google Scholar]
- 89.Maretzke F., Bechthold A., Egert S., Ernst J.B., van Lent D.M., Pilz S., et al. Role of vitamin D in preventing and treating selected extraskeletal diseases—an umbrella review. Nutrients. 2020;12:1–36. doi: 10.3390/nu12040969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee J.H., O'Keefe J.H., Bell D., Hensrud D.D., Holick M.F. Vitamin D deficiency. An important, common, and easily treatable cardiovascular risk factor? J Am Coll Cardiol. 2008;52:1949–1956. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 91.Bischoff-Ferrari H.A., Giovannucci E., Willett W.C., Dietrich T., Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 92.Baeke F., Takiishi T., Korf H., Gysemans C., Mathieu C. Vitamin D: modulator of the immune system. Curr Opin Pharmacol. 2010;10:482–496. doi: 10.1016/j.coph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 93.Trehan N., Afonso L., Levine D.L., Levy P.D. Vitamin D deficiency, supplementation, and cardiovascular health. Crit Pathw Cardiol. 2017;16:109–118. doi: 10.1097/HPC.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 94.Al Mheid I., Quyyumi A.A. Vitamin D and cardiovascular disease: controversy unresolved. J Am Coll Cardiol. 2017;70:89–100. doi: 10.1016/j.jacc.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 95.Weishaar R.E., Simpson R.U. Involvement of vitamin D3 with cardiovascular function. II. Direct and indirect effects. Am J Physiol Endocrinol Metab. 1987;253:E134–E142. doi: 10.1152/ajpendo.1987.253.6.e675. [DOI] [PubMed] [Google Scholar]
- 96.Mancuso P., Rahman A., Hershey S.D., Dandu L., Nibbelink K.A., Simpson R.U. 1,25-dihydroxyvitamin-D3 treatment reduces cardiac hypertrophy and left ventricular diameter in spontaneously hypertensive heart failure-prone (cp/+) rats independent of changes in serum leptin. J Cardiovasc Pharmacol. 2008;51:559–564. doi: 10.1097/FJC.0b013e3181761906. [DOI] [PubMed] [Google Scholar]
- 97.Antonopoulos A.S., Sanna F., Sabharwal N., Thomas S., Oikonomou E.K., Herdman L., et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal2658. [DOI] [PubMed] [Google Scholar]
- 98.Boucher B.J. Vitamin D insufficiency and diabetes risks. Curr Drug Targets. 2010;12:61–87. doi: 10.2174/138945011793591653. [DOI] [PubMed] [Google Scholar]
- 99.Wolden-Kirk H., Overbergh L., Christesen H.T., Brusgaard K., Mathieu C. Vitamin D and diabetes: its importance for beta cell and immune function. Mol Cell Endocrinol. 2011;347:106–120. doi: 10.1016/j.mce.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 100.Harris S.S., Pittas A.G., Palermo N.J. A randomized, placebo-controlled trial of vitamin D supplementation to improve glycaemia in overweight and obese African Americans. Diabetes Obes Metabol. 2012;14:789–794. doi: 10.1111/j.1463-1326.2012.01605.x. [DOI] [PubMed] [Google Scholar]
- 101.Holick M.F. The death D-fying vitamin. Mayo Clin Proc. 2018;93:679–681. doi: 10.1016/j.mayocp.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 102.Kamycheva E., Berg V., Jorde R. Insulin-like growth factor I, growth hormone, and insulin sensitivity: the effects of a one-year cholecalciferol supplementation in middle-aged overweight and obese subjects. Endocrine. 2013;43:412–418. doi: 10.1007/s12020-012-9825-6. [DOI] [PubMed] [Google Scholar]
- 103.Castaneda R.A., Nader N., Weaver A., Singh R., Kumar S. Response to vitamin D3 supplementation in obese and non-obese caucasian adolescents. Horm Res Paediatr. 2012;78:226–231. doi: 10.1159/000343446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Durazo-Arvizu R.A., Dawson-Hughes B., Kramer H., Cao G., Merkel J., Coates P.M., et al. The reverse J-shaped association between serum total 25-hydroxyvitamin D concentration and all-cause mortality: the impact of assay standardization. Am J Epidemiol. 2017;185:720–726. doi: 10.1093/aje/kww244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bolland M.J., Grey A., Gamble G.D., Reid I.R. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes - authors' reply. Lancet Diabetes Endocrinol. 2014;2:364–365. doi: 10.1016/S2213-8587(14)70100-7. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y., Fang F., Tang J., Jia L., Feng Y., Xu P., et al. Association between Vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366 doi: 10.1136/bmj.l4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martins D., Wolf M., Pan D., Zadshir A., Tareen N., Thadhani R., et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the third national health and nutrition examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 108.Paschou S.A., Kosmopoulos M., Nikas I.P., Spartalis M., Kassi E., Goulis D.G., et al. The impact of obesity on the association between vitamin D deficiency and cardiovascular disease. Nutrients. 2019;11 doi: 10.3390/nu11102458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Federico G., Genoni A., Puggioni A., Saba A., Gallo D., Randazzo E., et al. Vitamin D status, enterovirus infection, and type 1 diabetes in Italian children/adolescents. Pediatr Diabetes. 2018;19:923–929. doi: 10.1111/pedi.12673. [DOI] [PubMed] [Google Scholar]
- 110.Mitri J., Muraru M.D., Pittas A.G. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65:1005–1015. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 112.Scragg R., Jackson R., Holdaway I.M., Lim T., Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19:559–563. doi: 10.1093/ije/19.3.559. [DOI] [PubMed] [Google Scholar]
- 113.Poole K.E.S., Loveridge N., Barker P.J., Halsall D.J., Rose C., Reeve J., et al. Reduced vitamin D in acute stroke. Stroke. 2006;37:243–245. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- 114.Melamed M.L., Muntner P., Michos E.D., Uribarri J., Weber C., Sharma J., et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28:1179–1185. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Norman P.E., Powell J.T. Vitamin D and cardiovascular disease. Circ Res. 2014;114:379–393. doi: 10.1161/CIRCRESAHA.113.301241. [DOI] [PubMed] [Google Scholar]
- 116.Al-Ishaq R.K., Kubatka P., Brozmanova M., Gazdikova K., Caprnda M., Büsselberg D. Health implication of vitamin D on the cardiovascular and the renal system. Arch Physiol Biochem. 2019;1–15 doi: 10.1080/13813455.2019.1628064. [DOI] [PubMed] [Google Scholar]
- 117.Pilz S., Verheyen N., Grübler M.R., Tomaschitz A., März W. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. 2016;13:404–417. doi: 10.1038/nrcardio.2016.73. [DOI] [PubMed] [Google Scholar]
- 118.Legarth C., Grimm D., Wehland M., Bauer J., Krüger M. The impact of vitamin d in the treatment of essential hypertension. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim D.H., Meza C.A., Clarke H., Kim J.S., Hickner R.C. Vitamin D and endothelial function. Nutrients. 2020;12 doi: 10.3390/nu12020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Victor V.M., Rocha M., Solá E., Bañuls C., Garcia-Malpartida K., Hernández-Mijares A. Oxidative stress, endothelial dysfunction and atherosclerosis. Curr Pharmaceut Des. 2009;15:2988–3002. doi: 10.2174/138161209789058093. [DOI] [PubMed] [Google Scholar]
- 121.Zhang Q yan, Jiang C ming, Sun C., Tang T feng, Jin B., Cao D wei, et al. Hypovitaminosis D is associated with endothelial dysfunction in patients with non-dialysis chronic kidney disease. J Nephrol. 2015;28:471–476. doi: 10.1007/s40620-014-0167-8. [DOI] [PubMed] [Google Scholar]
- 122.Napoli C., Nigris F., Williams-Ignarro S., Pignalosa O., Sica V., Ignarro L.J. Nitric oxide and atherosclerosis: an update. Nitric Oxide Biol Chem. 2006;15:265–279. doi: 10.1016/j.niox.2006.03.011. 2006 SRC. [DOI] [PubMed] [Google Scholar]
- 123.Siasos G., Tousoulis D., Oikonomou E., Maniatis K., Kioufis S., Kokkou E., et al. Vitamin D3, D2 and arterial wall properties in coronary artery disease. Curr Pharmaceut Des. 2014;20:5914–5918. doi: 10.2174/1381612820666140619122937. [DOI] [PubMed] [Google Scholar]
- 124.Giovannucci E., Liu Y., Hollis B.W., Rimm E.B. 25-Hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee J.H., Gadi R., Spertus J.A., Tang F., O'Keefe J.H. Prevalence of vitamin D deficiency in patients with acute myocardial infarction. Am J Cardiol. 2011;107:1636–1638. doi: 10.1016/j.amjcard.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Verdoia M., Schaffer A., Sartori C., Barbieri L., Cassetti E., Marino P., et al. Vitamin D deficiency is independently associated with the extent of coronary artery disease. Eur J Clin Invest. 2014;44:634–642. doi: 10.1111/eci.12281. [DOI] [PubMed] [Google Scholar]
- 127.Wu Z., Wang T., Zhu S., Li L. Effects of Vitamin D supplementation as an adjuvant therapy in coronary artery disease patients. Scand Cardiovasc J. 2016;50:9–16. doi: 10.3109/14017431.2015.1103893. [DOI] [PubMed] [Google Scholar]
- 128.Fatkhullina A.R., Peshkova I.O., Koltsova E.K. The role of cytokines in the development of atherosclerosis. Biochem. 2016;81:1358–1370. doi: 10.1134/S0006297916110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sun X., Icli B., Wara A.K., Belkin N., He S., Kobzik L., et al. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J Clin Invest. 2012;122:2012. doi: 10.1172/JCI61495. SRC:1973–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gotsman I., Shauer A., Zwas D.R., Hellman Y., Keren A., Lotan C., et al. Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; Vitamin D supplementation improves outcome. Eur J Heart Fail. 2012;14:357–366. doi: 10.1093/eurjhf/hfr175. [DOI] [PubMed] [Google Scholar]
- 131.Kim D.H., Sabour S., Sagar U.N., Adams S., Whellan D.J. Prevalence of hypovitaminosis D in cardiovascular diseases (from the national health and nutrition examination Survey 2001 to 2004) Am J Cardiol. 2008;102:1540–1544. doi: 10.1016/j.amjcard.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 132.Vaidya A., Sun B., Larson C., Forman J.P., Williams J.S. Vitamin D3 therapy corrects the tissue sensitivity to angiotensin II akin to the action of a converting enzyme inhibitor in obese hypertensives: an interventional study. J Clin Endocrinol Metab. 2012;97:2456–2465. doi: 10.1210/jc.2012-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schleithoff S.S., Zittermann A., Tenderich G., Berthold H.K., Stehle P., Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 134.Zittermann A., Ernst J.B., Prokop S., Fuchs U., Dreier J., Kuhn J., et al. Effect of Vitamin D on all-cause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU Vitamin D daily. Eur Heart J. 2017;38:2279–2286. doi: 10.1093/eurheartj/ehx235. [DOI] [PubMed] [Google Scholar]
- 135.Witte K.K., Byrom R., Gierula J., Paton M.F., Jamil H.A., Lowry J.E., et al. Effects of vitamin D on cardiac function in patients with chronic HF: the VINDICATE study. J Am Coll Cardiol. 2016;67:2593–2603. doi: 10.1016/j.jacc.2016.03.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thadhani R., Appelbaum E., Pritchett Y., Chang Y., Wenger J., Tamez H., et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA, J Am Med Assoc. 2012;307:674–684. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 137.Wang T.J., Pencina M.J., Booth S.L., Jacques P.F., Ingelsson E., Lanier K., et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Song Y., Wang L., Pittas A.G., Del Gobbo L.C., Zhang C., Manson J.E., et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36:1422–1428. doi: 10.2337/dc12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liss Y., Frishman W.H. Vitamin D: a cardioprotective agent? Cardiol Rev. 2012;20:38–44. doi: 10.1097/CRD.0b013e31822c5380. [DOI] [PubMed] [Google Scholar]
- 140.Pittas A.G., Dawson-Hughes B., Sheehan P., Ware J.H., Knowler W.C., Aroda V.R., et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019;381:520–530. doi: 10.1056/NEJMoa1900906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Scragg R., Stewart A.W., Waayer D., Lawes C.M.M., Toop L., Sluyter J., et al. Effect of monthly high-dose vitamin D supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. JAMA Cardiol. 2017;2:608–616. doi: 10.1001/jamacardio.2017.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neale R.E., Armstrong B.K., Baxter C., Duarte Romero B., Ebeling P., English D.R., et al. The D-Health Trial: a randomized trial of vitamin D for prevention of mortality and cancer. Contemp Clin Trials. 2016;48:83–90. doi: 10.1016/j.cct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 143.Legarth C., Grimm D., Krüger M., Wehland M., Infanger M. Potential beneficial effects of vitamin d in coronary artery disease. Nutrients. 2020;12 doi: 10.3390/nu12010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barbarawi M., Kheiri B., Zayed Y., Barbarawi O., Dhillon H., Swaid B., et al. Vitamin D supplementation and cardiovascular disease risks in more than 83000 individuals in 21 randomized clinical trials: a meta-analysis. JAMA Cardiol. 2019;4:765–775. doi: 10.1001/jamacardio.2019.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Seibert E., Lehmann U., Riedel A., Ulrich C., Hirche F., Brandsch C., et al. Vitamin D3 supplementation does not modify cardiovascular risk profile of adults with inadequate vitamin D status. Eur J Nutr. 2017;56:621–634. doi: 10.1007/s00394-015-1106-8. [DOI] [PubMed] [Google Scholar]
- 146.Dalan R., Liew H., Assam P.N., Chan E.S.Y., Siddiqui F.J., Tan A.W.K., et al. A randomised controlled trial evaluating the impact of targeted Vitamin D supplementation on endothelial function in type 2 diabetes mellitus: the DIMENSION trial. Diabetes Vasc Dis Res. 2016;13:192–200. doi: 10.1177/1479164115621667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aslanabadi N., Jafaripor I., Sadeghi S., Hamishehkar H., Ghaffari S., Toluey M., et al. Effect of vitamin D in the prevention of myocardial injury following elective percutaneous coronary intervention: a pilot randomized clinical trial. J Clin Pharmacol. 2018;58:144–151. doi: 10.1002/jcph.989. [DOI] [PubMed] [Google Scholar]
- 148.Hin H., Tomson J., Newman C., Kurien R., Lay M., Cox J., et al. Optimum dose of vitamin D for disease prevention in older people: BEST-D trial of vitamin D in primary care. Osteoporos Int. 2017;28:841–851. doi: 10.1007/s00198-016-3833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gholami F., Moradi G., Zareei B., Rasouli M.A., Nikkhoo B., Roshani D., et al. The association between circulating 25-hydroxyvitamin D and cardiovascular diseases: a meta-analysis of prospective cohort studies. BMC Cardiovasc Disord. 2019;19:248. doi: 10.1186/s12872-019-1236-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Manson J.A.E., Cook N.R., Lee I.M., Christen W., Bassuk S.S., Mora S., et al. Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med. 2019;380:33–44. doi: 10.1056/NEJMoa1809944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr. 1999;69:842–856. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 152.Turhan S., Kilickap M., Kilinc S. Images in cardiology: ST segment elevation mimicking acute myocardial infarction in hypercalcaemia. Heart. 2005;91:999. doi: 10.1136/hrt.2003.030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356 doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aggarwal G., Cheruiyot I., Aggarwal S., Wong J., Lippi G., Lavie C.J., et al. Association of cardiovascular disease with coronavirus disease 2019 (COVID-19) severity: a meta-analysis. Curr Probl Cardiol. 2020;45 doi: 10.1016/j.cpcardiol.2020.100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G., et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Beltrán-García J., Osca-Verdegal R., Pallardó F.V., Ferreres J., Rodríguez M., Mulet S., et al. vol. 9. Antioxidants; Basel, Switzerland: 2020. Oxidative stress and inflammation in COVID-19-associated sepsis: the potential role of anti-oxidant therapy in avoiding disease progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kox M., Waalders N.J.B., Kooistra E.J., Gerretsen J., Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA, J Am Med Assoc. 2020;324:1565–1567. doi: 10.1001/jama.2020.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aygun H. Vitamin D can prevent COVID-19 infection-induced multiple organ damage. Naunyn-Schmiedeberg’s Arch Pharmacol. 2020;393:1157–1160. doi: 10.1007/s00210-020-01911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V., Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ebadi M., Montano-Loza A.J. Perspective: improving vitamin D status in the management of COVID-19. Eur J Clin Nutr. 2020;74:856–859. doi: 10.1038/s41430-020-0661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yancy C.W. COVID-19 and african Americans. JAMA, J Am Med Assoc. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 163.Mohan M., Cherian J.J., Sharma A. Exploring links between vitamin D deficiency and COVID-19. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008874. [DOI] [PMC free article] [PubMed] [Google Scholar]